User login
Fat shaming interferes with patients’ medical care, experts say
WASHINGTON – Stereotypical views held by physicians, psychologists, nurses, and other medical professionals about overweight and obese patients need to change, panelists said at the annual convention of the American Psychological Association.
“We have a lot of negative attitudes toward heavy-weight people. Judging patients as too big or too fat produces physical and mental health effects,” said Joan C. Chrisler, PhD, the Class of ’43 Professor of Psychology at Connecticut College, New London. “Shame and disrespectful treatment can lead to delay in seeking health care, reluctance to return visits, or lower trust in the providers and their recommendations.”
A study of more than 300 autopsy reports showed that obese patients were 1.65 times more likely than normal weight and underweight groups combined were to have medical conditions such as endocarditis, ischemic bowel disease, and lung cancer that were not diagnosed, Dr. Chrisler said in a press release (Am J Clin Pathol. 2006 Jan;125[1]:127-31).
In addition to preventing patients from seeking care, Dr. Chrisler said in the release, unfamiliarity with the dosing adjustments sometimes required on medications based on the body mass index of patients affects the quality of care. She cited a retrospective study showing that emergency physicians frequently underdose common antibiotics in the emergency department (Am J Emerg Med. 2012 Sep;30[7]:1212-4).
“People aren’t born repulsed by fat people,” said Dr. McHugh, professor of psychology at Indiana University of Pennsylvania. “Fat hate is learned within the family.”
They encouraged more sensitivity from medical professionals when it comes to treating overweight and obese patients, such as providing changing gowns of different sizes in examination rooms and weighing patients in private areas of the medical office.
Dr. Chrisler and Dr. McHugh said weight stigma should be addressed in medicine and psychology through training and research, and in working with patients who are obese. “Treatments should focus on mental and physical health as the desired outcomes for therapy rather than weight,” Dr. McHugh said.
Dr. Chrisler, coauthor of a recent article on sizeism (Fat Studies. 2017 Aug;6[1]:38-53), had no disclosures. Dr. McHugh also had no disclosures.
WASHINGTON – Stereotypical views held by physicians, psychologists, nurses, and other medical professionals about overweight and obese patients need to change, panelists said at the annual convention of the American Psychological Association.
“We have a lot of negative attitudes toward heavy-weight people. Judging patients as too big or too fat produces physical and mental health effects,” said Joan C. Chrisler, PhD, the Class of ’43 Professor of Psychology at Connecticut College, New London. “Shame and disrespectful treatment can lead to delay in seeking health care, reluctance to return visits, or lower trust in the providers and their recommendations.”
A study of more than 300 autopsy reports showed that obese patients were 1.65 times more likely than normal weight and underweight groups combined were to have medical conditions such as endocarditis, ischemic bowel disease, and lung cancer that were not diagnosed, Dr. Chrisler said in a press release (Am J Clin Pathol. 2006 Jan;125[1]:127-31).
In addition to preventing patients from seeking care, Dr. Chrisler said in the release, unfamiliarity with the dosing adjustments sometimes required on medications based on the body mass index of patients affects the quality of care. She cited a retrospective study showing that emergency physicians frequently underdose common antibiotics in the emergency department (Am J Emerg Med. 2012 Sep;30[7]:1212-4).
“People aren’t born repulsed by fat people,” said Dr. McHugh, professor of psychology at Indiana University of Pennsylvania. “Fat hate is learned within the family.”
They encouraged more sensitivity from medical professionals when it comes to treating overweight and obese patients, such as providing changing gowns of different sizes in examination rooms and weighing patients in private areas of the medical office.
Dr. Chrisler and Dr. McHugh said weight stigma should be addressed in medicine and psychology through training and research, and in working with patients who are obese. “Treatments should focus on mental and physical health as the desired outcomes for therapy rather than weight,” Dr. McHugh said.
Dr. Chrisler, coauthor of a recent article on sizeism (Fat Studies. 2017 Aug;6[1]:38-53), had no disclosures. Dr. McHugh also had no disclosures.
WASHINGTON – Stereotypical views held by physicians, psychologists, nurses, and other medical professionals about overweight and obese patients need to change, panelists said at the annual convention of the American Psychological Association.
“We have a lot of negative attitudes toward heavy-weight people. Judging patients as too big or too fat produces physical and mental health effects,” said Joan C. Chrisler, PhD, the Class of ’43 Professor of Psychology at Connecticut College, New London. “Shame and disrespectful treatment can lead to delay in seeking health care, reluctance to return visits, or lower trust in the providers and their recommendations.”
A study of more than 300 autopsy reports showed that obese patients were 1.65 times more likely than normal weight and underweight groups combined were to have medical conditions such as endocarditis, ischemic bowel disease, and lung cancer that were not diagnosed, Dr. Chrisler said in a press release (Am J Clin Pathol. 2006 Jan;125[1]:127-31).
In addition to preventing patients from seeking care, Dr. Chrisler said in the release, unfamiliarity with the dosing adjustments sometimes required on medications based on the body mass index of patients affects the quality of care. She cited a retrospective study showing that emergency physicians frequently underdose common antibiotics in the emergency department (Am J Emerg Med. 2012 Sep;30[7]:1212-4).
“People aren’t born repulsed by fat people,” said Dr. McHugh, professor of psychology at Indiana University of Pennsylvania. “Fat hate is learned within the family.”
They encouraged more sensitivity from medical professionals when it comes to treating overweight and obese patients, such as providing changing gowns of different sizes in examination rooms and weighing patients in private areas of the medical office.
Dr. Chrisler and Dr. McHugh said weight stigma should be addressed in medicine and psychology through training and research, and in working with patients who are obese. “Treatments should focus on mental and physical health as the desired outcomes for therapy rather than weight,” Dr. McHugh said.
Dr. Chrisler, coauthor of a recent article on sizeism (Fat Studies. 2017 Aug;6[1]:38-53), had no disclosures. Dr. McHugh also had no disclosures.
EXPERT ANALYSIS FROM THE 2017 APA CONVENTION
Hormonal IUD is most cost-effective menorrhagia management
Quality of life was higher, and costs were lower, with the levonorgestrel-releasing intrauterine system for treatment of heavy menstrual bleeding than with three other common treatments, according to data from a model and a hypothetical population of 100,000 premenopausal women. The findings were published online in the American Journal of Obstetrics and Gynecology.
“As health systems and policies continue to emphasize value-based treatment decisions, it is important to give physicians and patients the tools to understand the health and economic trade-offs associated with each of these options,” Jennifer C. Spencer of the University of North Carolina, Chapel Hill, and her colleagues wrote (Am J Obstet Gynecol. 2017 Jul 25. doi: 10.1016/j.ajog.2017.07.024).
Overall, LNG-IUS was superior to hysterectomy and both types of endometrial ablation in terms of cost and quality of life, although quality of life scores were similar across all four treatments.
LNG-IUS was cost effective, compared with hysterectomy, in 90% of scenarios. Both types of ablation were similarly more cost effective, compared with hysterectomy; resectoscopic endometrial ablation was more cost effective in 44% of scenarios, nonresectoscopic endometrial ablation was more cost effective in 53% of scenarios.
“The 5-year cost of women undergoing LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500),” the researchers noted.
“Our analysis finds strong evidence in favor of LNG-IUS as a cost-saving, dominant alternative to hysterectomy for women with heavy menstrual bleeding,” they wrote.
If LNG-IUS is not an option, the model shows that hysterectomy resulted in better quality of life in the majority of simulations but is cost effective in just over half of the simulations, compared with either resectoscopic or nonresectoscopic ablation.
“The comparative cost effectiveness of endometrial ablation and hysterectomy highlights important trade-offs for patients and providers to consider when selecting between treatment options, such as the need for future procedures or the potential for rare, but serious, complications,” the researchers wrote.
No other studies on this topic have been conducted in the United States, but the findings are consistent with results from studies conducted outside the United States, the researchers wrote.
The study was limited by the short follow-up period and the inability to extend the model to women with large fibroids, polyps, or other uterine pathologies.
Two of the authors reported receiving grant funding from Pfizer for an unrelated study. Other authors reported serving as consultants for Teleflex Medical, Applied Medical, and Olympus.
Quality of life was higher, and costs were lower, with the levonorgestrel-releasing intrauterine system for treatment of heavy menstrual bleeding than with three other common treatments, according to data from a model and a hypothetical population of 100,000 premenopausal women. The findings were published online in the American Journal of Obstetrics and Gynecology.
“As health systems and policies continue to emphasize value-based treatment decisions, it is important to give physicians and patients the tools to understand the health and economic trade-offs associated with each of these options,” Jennifer C. Spencer of the University of North Carolina, Chapel Hill, and her colleagues wrote (Am J Obstet Gynecol. 2017 Jul 25. doi: 10.1016/j.ajog.2017.07.024).
Overall, LNG-IUS was superior to hysterectomy and both types of endometrial ablation in terms of cost and quality of life, although quality of life scores were similar across all four treatments.
LNG-IUS was cost effective, compared with hysterectomy, in 90% of scenarios. Both types of ablation were similarly more cost effective, compared with hysterectomy; resectoscopic endometrial ablation was more cost effective in 44% of scenarios, nonresectoscopic endometrial ablation was more cost effective in 53% of scenarios.
“The 5-year cost of women undergoing LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500),” the researchers noted.
“Our analysis finds strong evidence in favor of LNG-IUS as a cost-saving, dominant alternative to hysterectomy for women with heavy menstrual bleeding,” they wrote.
If LNG-IUS is not an option, the model shows that hysterectomy resulted in better quality of life in the majority of simulations but is cost effective in just over half of the simulations, compared with either resectoscopic or nonresectoscopic ablation.
“The comparative cost effectiveness of endometrial ablation and hysterectomy highlights important trade-offs for patients and providers to consider when selecting between treatment options, such as the need for future procedures or the potential for rare, but serious, complications,” the researchers wrote.
No other studies on this topic have been conducted in the United States, but the findings are consistent with results from studies conducted outside the United States, the researchers wrote.
The study was limited by the short follow-up period and the inability to extend the model to women with large fibroids, polyps, or other uterine pathologies.
Two of the authors reported receiving grant funding from Pfizer for an unrelated study. Other authors reported serving as consultants for Teleflex Medical, Applied Medical, and Olympus.
Quality of life was higher, and costs were lower, with the levonorgestrel-releasing intrauterine system for treatment of heavy menstrual bleeding than with three other common treatments, according to data from a model and a hypothetical population of 100,000 premenopausal women. The findings were published online in the American Journal of Obstetrics and Gynecology.
“As health systems and policies continue to emphasize value-based treatment decisions, it is important to give physicians and patients the tools to understand the health and economic trade-offs associated with each of these options,” Jennifer C. Spencer of the University of North Carolina, Chapel Hill, and her colleagues wrote (Am J Obstet Gynecol. 2017 Jul 25. doi: 10.1016/j.ajog.2017.07.024).
Overall, LNG-IUS was superior to hysterectomy and both types of endometrial ablation in terms of cost and quality of life, although quality of life scores were similar across all four treatments.
LNG-IUS was cost effective, compared with hysterectomy, in 90% of scenarios. Both types of ablation were similarly more cost effective, compared with hysterectomy; resectoscopic endometrial ablation was more cost effective in 44% of scenarios, nonresectoscopic endometrial ablation was more cost effective in 53% of scenarios.
“The 5-year cost of women undergoing LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500),” the researchers noted.
“Our analysis finds strong evidence in favor of LNG-IUS as a cost-saving, dominant alternative to hysterectomy for women with heavy menstrual bleeding,” they wrote.
If LNG-IUS is not an option, the model shows that hysterectomy resulted in better quality of life in the majority of simulations but is cost effective in just over half of the simulations, compared with either resectoscopic or nonresectoscopic ablation.
“The comparative cost effectiveness of endometrial ablation and hysterectomy highlights important trade-offs for patients and providers to consider when selecting between treatment options, such as the need for future procedures or the potential for rare, but serious, complications,” the researchers wrote.
No other studies on this topic have been conducted in the United States, but the findings are consistent with results from studies conducted outside the United States, the researchers wrote.
The study was limited by the short follow-up period and the inability to extend the model to women with large fibroids, polyps, or other uterine pathologies.
Two of the authors reported receiving grant funding from Pfizer for an unrelated study. Other authors reported serving as consultants for Teleflex Medical, Applied Medical, and Olympus.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Key clinical point:
Major finding: LNG-IUS is the most cost-effective option in 90% of scenarios, compared with hysterectomy.
Data source: The data come from a model created using a literature review of four treatment options: resectoscopic ablation, nonresectoscopic ablation, hysterectomy, and LNG-IUS. It included a hypothetical cohort of 100,000 premenopausal women.
Disclosures: Two of the authors reported receiving grant funding from Pfizer for an unrelated study. Other authors reported serving as consultants for Teleflex Medical, Applied Medical, and Olympus.
Aging workforce stresses ob.gyn. field
The number of ob.gyns. nearing retirement age is outpacing the number of younger physicians in the specialty, making it likely that the United States will not have enough women’s health physicians to meet the demand.
New research by the medical social network Doximity finds that 37% of ob.gyns. in the United States are 55 or older, while just 14% are 40 or younger. Furthering the potential for shortage, Doximity cited research from the American Congress of Obstetricians and Gynecologists that most ob.gyns. begin to retire at age 59, with the median retirement age being 64. Doximity’s own research found the average age of ob.gyns. across the nation is 51.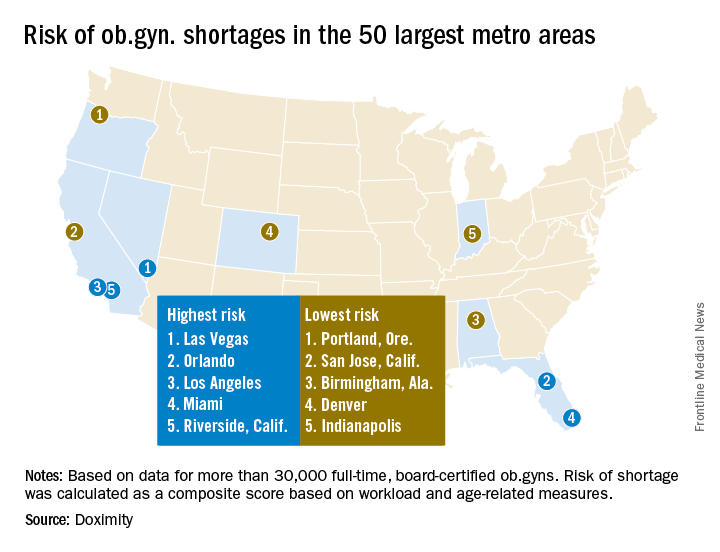
The report notes that after emergency physicians, ob.gyns. “have the highest burn-out rates of all medical specialties. Moreover, due to the nature of obstetrics, and child birth in particular, this job is especially demanding, often requiring ob.gyns. to work at unpredictable hours. This lifestyle can lead ob.gyns. to retire at younger ages than physicians in other specialties.”
At the same time, the ob.gyn. workforce remains stagnant. “According to ACOG, there are now 1,287 first year ob.gyn. residency positions, a number that has increased only minimally in the past few decades, while the number of adult. U.S. women has increased much more significantly, stretching the ratio of ob.gyns. to patients,” the Doximity report said.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, agreed with the findings.
Specifically, more general ob.gyns. are gravitating to metropolitan areas and are employed by health systems or large group practices, he noted.
Doximity identified the top 10 metropolitan areas that are at highest risk for an ob.gyn. shortage: Las Vegas; Orlando; Los Angeles; Miami; Riverside, Calif.; Detroit; Memphis; Salt Lake City; St. Louis; and Buffalo, N.Y.
In contrast, the metropolitan areas with the lowest risk of shortages are Portland, Ore.; San Jose, Calif.; Birmingham, Ala.; Denver; Indianapolis; Cleveland; San Francisco; Richmond, Va.; Louisville, Ky.; and Columbus, Ohio.
This is likely to translate to access to care problems in smaller communities, Dr. Rayburn said. “What we are going to be seeing is more graduates from nonphysician clinician programs who are going to be hired, such as nurse practitioners, to fill the gaps.”
The Doximity analysis is based on data from the Centers for Medicare and Medicaid Services, board certification, and self-reported data from more than 30,000 ob.gyns.
gtwachtman@frontlinemedcom.com
On Twitter @ObGynNews
The number of ob.gyns. nearing retirement age is outpacing the number of younger physicians in the specialty, making it likely that the United States will not have enough women’s health physicians to meet the demand.
New research by the medical social network Doximity finds that 37% of ob.gyns. in the United States are 55 or older, while just 14% are 40 or younger. Furthering the potential for shortage, Doximity cited research from the American Congress of Obstetricians and Gynecologists that most ob.gyns. begin to retire at age 59, with the median retirement age being 64. Doximity’s own research found the average age of ob.gyns. across the nation is 51.
The report notes that after emergency physicians, ob.gyns. “have the highest burn-out rates of all medical specialties. Moreover, due to the nature of obstetrics, and child birth in particular, this job is especially demanding, often requiring ob.gyns. to work at unpredictable hours. This lifestyle can lead ob.gyns. to retire at younger ages than physicians in other specialties.”
At the same time, the ob.gyn. workforce remains stagnant. “According to ACOG, there are now 1,287 first year ob.gyn. residency positions, a number that has increased only minimally in the past few decades, while the number of adult. U.S. women has increased much more significantly, stretching the ratio of ob.gyns. to patients,” the Doximity report said.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, agreed with the findings.
Specifically, more general ob.gyns. are gravitating to metropolitan areas and are employed by health systems or large group practices, he noted.
Doximity identified the top 10 metropolitan areas that are at highest risk for an ob.gyn. shortage: Las Vegas; Orlando; Los Angeles; Miami; Riverside, Calif.; Detroit; Memphis; Salt Lake City; St. Louis; and Buffalo, N.Y.
In contrast, the metropolitan areas with the lowest risk of shortages are Portland, Ore.; San Jose, Calif.; Birmingham, Ala.; Denver; Indianapolis; Cleveland; San Francisco; Richmond, Va.; Louisville, Ky.; and Columbus, Ohio.
This is likely to translate to access to care problems in smaller communities, Dr. Rayburn said. “What we are going to be seeing is more graduates from nonphysician clinician programs who are going to be hired, such as nurse practitioners, to fill the gaps.”
The Doximity analysis is based on data from the Centers for Medicare and Medicaid Services, board certification, and self-reported data from more than 30,000 ob.gyns.
gtwachtman@frontlinemedcom.com
On Twitter @ObGynNews
The number of ob.gyns. nearing retirement age is outpacing the number of younger physicians in the specialty, making it likely that the United States will not have enough women’s health physicians to meet the demand.
New research by the medical social network Doximity finds that 37% of ob.gyns. in the United States are 55 or older, while just 14% are 40 or younger. Furthering the potential for shortage, Doximity cited research from the American Congress of Obstetricians and Gynecologists that most ob.gyns. begin to retire at age 59, with the median retirement age being 64. Doximity’s own research found the average age of ob.gyns. across the nation is 51.
The report notes that after emergency physicians, ob.gyns. “have the highest burn-out rates of all medical specialties. Moreover, due to the nature of obstetrics, and child birth in particular, this job is especially demanding, often requiring ob.gyns. to work at unpredictable hours. This lifestyle can lead ob.gyns. to retire at younger ages than physicians in other specialties.”
At the same time, the ob.gyn. workforce remains stagnant. “According to ACOG, there are now 1,287 first year ob.gyn. residency positions, a number that has increased only minimally in the past few decades, while the number of adult. U.S. women has increased much more significantly, stretching the ratio of ob.gyns. to patients,” the Doximity report said.
William F. Rayburn, MD, distinguished professor and emeritus chair of obstetrics and gynecology at the University of New Mexico, Albuquerque, agreed with the findings.
Specifically, more general ob.gyns. are gravitating to metropolitan areas and are employed by health systems or large group practices, he noted.
Doximity identified the top 10 metropolitan areas that are at highest risk for an ob.gyn. shortage: Las Vegas; Orlando; Los Angeles; Miami; Riverside, Calif.; Detroit; Memphis; Salt Lake City; St. Louis; and Buffalo, N.Y.
In contrast, the metropolitan areas with the lowest risk of shortages are Portland, Ore.; San Jose, Calif.; Birmingham, Ala.; Denver; Indianapolis; Cleveland; San Francisco; Richmond, Va.; Louisville, Ky.; and Columbus, Ohio.
This is likely to translate to access to care problems in smaller communities, Dr. Rayburn said. “What we are going to be seeing is more graduates from nonphysician clinician programs who are going to be hired, such as nurse practitioners, to fill the gaps.”
The Doximity analysis is based on data from the Centers for Medicare and Medicaid Services, board certification, and self-reported data from more than 30,000 ob.gyns.
gtwachtman@frontlinemedcom.com
On Twitter @ObGynNews
FDA committee recommends approval of tofacitinib for PsA
Convinced largely by encouraging efficacy data, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly in favor of approval of tofacitinib for the treatment of adult patients with active psoriatic arthritis.
If approved by the FDA, which usually adheres to advisory committee recommendations, the oral inhibitor of Janus-associated kinases (JAK) would be the first JAK inhibitor approved for the treatment of psoriatic arthritis (PsA). Pfizer submitted supplemental new drug applications (sNDAs) for both tofacitinib tablets (Xeljanz) and tofacitinib extended-release tablets (Xeljanz XR) at a dose of 5 mg twice daily and 11 mg once daily, respectively and, despite some reservations with respect to adverse events and lack of evidence regarding inhibition of radiographic progression, the committee voted 10-1 in favor of approval at an Aug. 3 meeting.
“I voted yes and, although there are safety concerns, I feel like it’s nothing different than what we see with other biologics, and I want to make sure that patients have options,” said Jennifer Horonjeff, PhD, a research fellow and patient advocate with the Center for Immune Disease with Onset in Childhood at Columbia University Medical Center, New York, and a consumer representative on the committee.
Dr. Horonjeff added that she hopes there is continued conversation between the sponsor and the FDA on “what we can do to make patients aware of these risks.”
Similarly, committee member Daniel H. Solomon, MD, a professor of medicine at Harvard Medical School and chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, both in Boston, said he sees a “great opportunity for risk mitigation that the sponsor and the [FDA] can take together.”
“We have a clear risk, we have a clear strategy for mitigating the risk, and there are going to be a lot more people exposed to this drug with a known risk, so let’s do something about it,” Dr. Solomon said about a plan put forward by Pfizer, and discussed at some length, to mitigate risks through measures such as vaccination against herpes zoster and additional study.
Temporary voting member Steven Meisel, PharmD, system director of patient safety at Fairview Health Services in Minneapolis added: “These are nasty drugs, but I think those who use them understand that, and this is no different than any of the other nasty drugs in these categories.”
Diane Aronson, a patient representative and temporary voting member on the committee, cast the only vote against approval, citing concerns about the lack of inhibition of radiographic progression of the disease and about the infection risks in a vulnerable population.
Tofacitinib was initially approved in 2012 at a dose of 5 mg, twice daily, for the treatment of adults with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to methotrexate. The extended-release formulation was approved in 2016 at a dose of 11 mg daily.
With respect to the current sNDAs, Pfizer presented data from two placebo-controlled phase 3 trials in patients with psoriatic arthritis. The FDA deemed these trials to be adequate and well-controlled, providing “corroborating evidence of the efficacy of tofacitinib for reducing signs and symptoms of PsA, based on the proportion of patients experiencing the American College of Rheumatology (ACR) 20% response criteria,” according to a report presented to the committee. The report also noted that both phase 3 trials provided evidence of improvement in physical function, but did not provide sufficient evidence that tofacitinib inhibits radiographic progression in PsA.
The report also stated that the safety profile of tofacitinib in PsA was consistent with that established in RA; risks include serious infections, opportunistic infections, malignancy, gastrointestinal perforation, and laboratory abnormalities, including elevations in low-density lipoprotein and triglycerides.
“No new safety signals were identified in PsA,” the report states.
Of note, the sNDAs do not include an indication for generalized psoriasis; an application for that indication was withdrawn in 2016, and Dr. Meisel cautioned against any “unintentional leakage of the use of this drug for generalized psoriasis.”
He and others also cautioned against any implied endorsement in labeling that the drug inhibits radiographic progression of PsA.
Two individuals who participated in the open public hearing portion of the meeting each urged the committee to recommend approval of the sNDAs, with one, Stephen Marmaras, manager of state and national advocacy for the Global Healthy Living Foundation, noting that the joint pain and stiffness associated with PsA are a primary concern of patients.
“Our members with psoriatic arthritis overwhelmingly prioritize joint pain and stiffness as the most bothersome symptoms they experience,” Mr. Marmaras said. “With that in mind, we were encouraged to read that tofacitinib has particularly notable efficacy in treating the joint symptoms of the disease in clinical trials.”
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Convinced largely by encouraging efficacy data, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly in favor of approval of tofacitinib for the treatment of adult patients with active psoriatic arthritis.
If approved by the FDA, which usually adheres to advisory committee recommendations, the oral inhibitor of Janus-associated kinases (JAK) would be the first JAK inhibitor approved for the treatment of psoriatic arthritis (PsA). Pfizer submitted supplemental new drug applications (sNDAs) for both tofacitinib tablets (Xeljanz) and tofacitinib extended-release tablets (Xeljanz XR) at a dose of 5 mg twice daily and 11 mg once daily, respectively and, despite some reservations with respect to adverse events and lack of evidence regarding inhibition of radiographic progression, the committee voted 10-1 in favor of approval at an Aug. 3 meeting.
“I voted yes and, although there are safety concerns, I feel like it’s nothing different than what we see with other biologics, and I want to make sure that patients have options,” said Jennifer Horonjeff, PhD, a research fellow and patient advocate with the Center for Immune Disease with Onset in Childhood at Columbia University Medical Center, New York, and a consumer representative on the committee.
Dr. Horonjeff added that she hopes there is continued conversation between the sponsor and the FDA on “what we can do to make patients aware of these risks.”
Similarly, committee member Daniel H. Solomon, MD, a professor of medicine at Harvard Medical School and chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, both in Boston, said he sees a “great opportunity for risk mitigation that the sponsor and the [FDA] can take together.”
“We have a clear risk, we have a clear strategy for mitigating the risk, and there are going to be a lot more people exposed to this drug with a known risk, so let’s do something about it,” Dr. Solomon said about a plan put forward by Pfizer, and discussed at some length, to mitigate risks through measures such as vaccination against herpes zoster and additional study.
Temporary voting member Steven Meisel, PharmD, system director of patient safety at Fairview Health Services in Minneapolis added: “These are nasty drugs, but I think those who use them understand that, and this is no different than any of the other nasty drugs in these categories.”
Diane Aronson, a patient representative and temporary voting member on the committee, cast the only vote against approval, citing concerns about the lack of inhibition of radiographic progression of the disease and about the infection risks in a vulnerable population.
Tofacitinib was initially approved in 2012 at a dose of 5 mg, twice daily, for the treatment of adults with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to methotrexate. The extended-release formulation was approved in 2016 at a dose of 11 mg daily.
With respect to the current sNDAs, Pfizer presented data from two placebo-controlled phase 3 trials in patients with psoriatic arthritis. The FDA deemed these trials to be adequate and well-controlled, providing “corroborating evidence of the efficacy of tofacitinib for reducing signs and symptoms of PsA, based on the proportion of patients experiencing the American College of Rheumatology (ACR) 20% response criteria,” according to a report presented to the committee. The report also noted that both phase 3 trials provided evidence of improvement in physical function, but did not provide sufficient evidence that tofacitinib inhibits radiographic progression in PsA.
The report also stated that the safety profile of tofacitinib in PsA was consistent with that established in RA; risks include serious infections, opportunistic infections, malignancy, gastrointestinal perforation, and laboratory abnormalities, including elevations in low-density lipoprotein and triglycerides.
“No new safety signals were identified in PsA,” the report states.
Of note, the sNDAs do not include an indication for generalized psoriasis; an application for that indication was withdrawn in 2016, and Dr. Meisel cautioned against any “unintentional leakage of the use of this drug for generalized psoriasis.”
He and others also cautioned against any implied endorsement in labeling that the drug inhibits radiographic progression of PsA.
Two individuals who participated in the open public hearing portion of the meeting each urged the committee to recommend approval of the sNDAs, with one, Stephen Marmaras, manager of state and national advocacy for the Global Healthy Living Foundation, noting that the joint pain and stiffness associated with PsA are a primary concern of patients.
“Our members with psoriatic arthritis overwhelmingly prioritize joint pain and stiffness as the most bothersome symptoms they experience,” Mr. Marmaras said. “With that in mind, we were encouraged to read that tofacitinib has particularly notable efficacy in treating the joint symptoms of the disease in clinical trials.”
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Convinced largely by encouraging efficacy data, the Food and Drug Administration’s Arthritis Advisory Committee voted overwhelmingly in favor of approval of tofacitinib for the treatment of adult patients with active psoriatic arthritis.
If approved by the FDA, which usually adheres to advisory committee recommendations, the oral inhibitor of Janus-associated kinases (JAK) would be the first JAK inhibitor approved for the treatment of psoriatic arthritis (PsA). Pfizer submitted supplemental new drug applications (sNDAs) for both tofacitinib tablets (Xeljanz) and tofacitinib extended-release tablets (Xeljanz XR) at a dose of 5 mg twice daily and 11 mg once daily, respectively and, despite some reservations with respect to adverse events and lack of evidence regarding inhibition of radiographic progression, the committee voted 10-1 in favor of approval at an Aug. 3 meeting.
“I voted yes and, although there are safety concerns, I feel like it’s nothing different than what we see with other biologics, and I want to make sure that patients have options,” said Jennifer Horonjeff, PhD, a research fellow and patient advocate with the Center for Immune Disease with Onset in Childhood at Columbia University Medical Center, New York, and a consumer representative on the committee.
Dr. Horonjeff added that she hopes there is continued conversation between the sponsor and the FDA on “what we can do to make patients aware of these risks.”
Similarly, committee member Daniel H. Solomon, MD, a professor of medicine at Harvard Medical School and chief of the section of clinical sciences in the divisions of rheumatology and pharmacoepidemiology at Brigham and Women’s Hospital, both in Boston, said he sees a “great opportunity for risk mitigation that the sponsor and the [FDA] can take together.”
“We have a clear risk, we have a clear strategy for mitigating the risk, and there are going to be a lot more people exposed to this drug with a known risk, so let’s do something about it,” Dr. Solomon said about a plan put forward by Pfizer, and discussed at some length, to mitigate risks through measures such as vaccination against herpes zoster and additional study.
Temporary voting member Steven Meisel, PharmD, system director of patient safety at Fairview Health Services in Minneapolis added: “These are nasty drugs, but I think those who use them understand that, and this is no different than any of the other nasty drugs in these categories.”
Diane Aronson, a patient representative and temporary voting member on the committee, cast the only vote against approval, citing concerns about the lack of inhibition of radiographic progression of the disease and about the infection risks in a vulnerable population.
Tofacitinib was initially approved in 2012 at a dose of 5 mg, twice daily, for the treatment of adults with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to methotrexate. The extended-release formulation was approved in 2016 at a dose of 11 mg daily.
With respect to the current sNDAs, Pfizer presented data from two placebo-controlled phase 3 trials in patients with psoriatic arthritis. The FDA deemed these trials to be adequate and well-controlled, providing “corroborating evidence of the efficacy of tofacitinib for reducing signs and symptoms of PsA, based on the proportion of patients experiencing the American College of Rheumatology (ACR) 20% response criteria,” according to a report presented to the committee. The report also noted that both phase 3 trials provided evidence of improvement in physical function, but did not provide sufficient evidence that tofacitinib inhibits radiographic progression in PsA.
The report also stated that the safety profile of tofacitinib in PsA was consistent with that established in RA; risks include serious infections, opportunistic infections, malignancy, gastrointestinal perforation, and laboratory abnormalities, including elevations in low-density lipoprotein and triglycerides.
“No new safety signals were identified in PsA,” the report states.
Of note, the sNDAs do not include an indication for generalized psoriasis; an application for that indication was withdrawn in 2016, and Dr. Meisel cautioned against any “unintentional leakage of the use of this drug for generalized psoriasis.”
He and others also cautioned against any implied endorsement in labeling that the drug inhibits radiographic progression of PsA.
Two individuals who participated in the open public hearing portion of the meeting each urged the committee to recommend approval of the sNDAs, with one, Stephen Marmaras, manager of state and national advocacy for the Global Healthy Living Foundation, noting that the joint pain and stiffness associated with PsA are a primary concern of patients.
“Our members with psoriatic arthritis overwhelmingly prioritize joint pain and stiffness as the most bothersome symptoms they experience,” Mr. Marmaras said. “With that in mind, we were encouraged to read that tofacitinib has particularly notable efficacy in treating the joint symptoms of the disease in clinical trials.”
All voting advisory committee members were screened and cleared with respect to potential conflicts of interest.
Landmark women’s health care remains law of the land
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
Starting in 2010 with the Patient Protection and Affordable Care Act (ACA), our patients have had insurance that provides maternity care coverage, no-deductible or copay contraceptives, and access to breast cancer screening. They also have been protected from predatory insurance practices—such as preexisting condition exclusions, arbitrary rescission, and annual or lifetime coverage limits—which had previously and regularly been used to deny coverage. These landmark protections apply to all our patients, regardless of where they live, how much they earn, who their employers are, and which insurance plan they use. They have become part of the fabric of our society.
Between 2008 and 2010, members of the American College of Obstetricians and Gynecologists (ACOG) worked hard to define and help enact these provisions, which we considered the women’s piece of the health care reform puzzle. We also worked with a broad community of clinicians to try to make sure reform would benefit them too. That effort did not go as well, and ACOG ultimately did not endorse the ACA.
Early ACA troubles, misguided solutions
Since the ACA was signed into law 7 years ago, insurers have raised premiums and deductibles and narrowed their provider networks—putting needed care out of the reach of many patients. In addition, skyrocketing prescription drug prices have driven health care costs even higher. Against this backdrop, Congress in 2017 started trying to pass bills that would undo the ACA.
ACOG and our medical colleague organizations stepped up. We brought many ideas to House and Senate Republicans and Democrats and sought opportunities to work with them to improve the ACA for our physicians and patients. Unfortunately, the statute was so polarizing that few in Congress wanted to amend or revise it; most wanted it repealed or left as is.
Throughout these proceedings, ACOG remained committed to ensuring that no one with health insurance coverage would lose it and that Congress would not turn back the clock on women’s health. As long as these 2 principles were assured, we would work with anyone on improving health insurance.
Path to a better way
We delivered our message repeatedly. ACOG President Haywood Brown, MD, often accompanied by his American College of Physicians, American Academy of Pediatrics, American Academy of Family Physicians, American Psychiatric Association, and American Osteopathic Association counterparts, attended high-level meetings with Congressional Republicans and Democrats. Dr. Brown also led fly-ins of our members. In addition, ACOG Past President Tom Gellhaus, MD, together with all 600 ObGyns at the 2017 ACOG Congressional Leadership Conference, spoke out.
Somehow, though, the proposed bills kept getting worse—more patients would be losing coverage, and women’s health protections would be stripped away—and Congress was not seeking or including physician input. None at all.
The ACOG teleconference
In response, ACOG set up a member teleconference headed by Dr. Brown, Dr. Gellhaus, Incoming President Lisa Hollier, MD, Past President and ObGyn PAC Chair Mark DeFrancesco, MD, and Executive Vice President and CEO Hal Lawrence, MD. Discussing our concerns, we focused on the Senate’s Better Care Reconciliation Act (BCRA) and its potential impact on maternity care coverage, preexisting condition coverage, Medicaid, Planned Parenthood (PP), and the opioid epidemic.
BCRA
Dr. Brown led off the teleconference with this assessment: “Without a doubt, the BCRA would not result in better care for our patients. This legislation would pull the rug out from under women and families. The nonpartisan Congressional Budget Office estimated that 22 million Americans, more than half of them women, would lose coverage. More than $770 billion would be cut from Medicaid, the program that covers nearly half of all births nationwide as well as primary and preventive care for low-income patients.”
Coverage for maternity care and preexisting conditions
Dr. Gellhaus discussed how the BCRA would gut maternity care coverage and hurt patients with preexisting conditions. Under this bill, states would be able to drop the requirement for such coverage, thereby creating an enormous hole in patient care. He asked an important question: “If your state opted out and allowed private insurers not to offer maternity care or preventive care, what would this mean for your patients?”
His answer: “It would take us back to a time when only 9 states required insurers cover maternity care, and when only 12% of plans included such coverage; a time when patients had to buy expensive riders, sometimes with 12-month waiting periods, if they wanted maternity coverage; a time when expecting families faced thousands of dollars in out-of-pocket costs. Do we want to go back to that time? It is also important to note that roughly half of all pregnancies are unplanned. Pregnancy should not leave patients fearing bankruptcy and unable to afford the full range of prenatal and postnatal care.
“States that opt out of covering preventive care would discontinue no-copay coverage for women’s preventive services, including contraception. Fifty-five million American women currently have this coverage, and as a result the country’s unintended pregnancy rate is at a 30-year low, and its teen pregnancy rate the lowest in recorded history. We cannot go back.”
Medicaid
Dr. Hollier pointed out that the BCRA would cut $772 billion from Medicaid, ending the program as we know it and shifting costs to states. “This section alone would devastate our patients in every state,” she said.
ACOG is a strong supporter of Medicaid expansion, which increased access to primary and preventive care, including contraception, for low-income women who otherwise would not see a physician until they became pregnant. Thirty-two states and the District of Columbia expanded their Medicaid programs, and other states have expressed interest in doing the same.
Medicaid expansion was a major factor in the almost 50% decrease in the rate of uninsured women since 2010. The BCRA would roll back coverage for essential health benefits beginning in 2020 and end federal expansion funding by 2023.
Dr. Hollier continued, “Regular Medicaid would be threatened, too. The Senate bill would limit, for the first time ever, federal funding for Medicaid services per beneficiary. This would jeopardize the ability of the United States to respond to disasters and public health crises and pose a threat to health care coverage and benefits for tens of millions of Americans.”
“Given that nearly half of US births are covered by Medicaid, cutting this program would have a huge impact on our practices and on our patients with high-risk and expensive pregnancies. What happens when a low-income pregnant patient with hypertension, gestational diabetes, or preeclampsia reaches her Medicaid cap? What happens to a patient with an opioid use disorder or a patient who may have been exposed to the Zika virus? In all likelihood, physicians would have to continue providing care, regardless of coverage, or states would have to reduce physician payments to fill the gap in federal funding. I am sure you are as horrified as I am by these scenarios,” said Dr. Hollier.
Planned Parenthood
Dr. DeFrancesco discussed the threat to PP. First, he explained what defunding the organization would mean. “Planned Parenthood does not just receive a check from the government each year. Like other qualified providers, like us, PP health centers receive federal reimbursement for primary and preventive services provided to patients with Medicaid coverage. Fifty-four percent of these centers are located in rural and medically underserved communities.”
The BCRA would exclude PP health centers from the Medicaid program, which means Medicaid patients would be denied primary and preventive care at these centers. Within the first year, up to 1 million women would find their access to care restricted. In addition, about half of all PP centers would have to close, and most would not reopen. Dr. DeFrancesco asked, “How would this move help our patients? It wouldn’t.”
Two examples shed light on the situation. First, when PP was excluded from a Texas program serving low-income patients, the number of women using the most effective birth control methods decreased by 35%, and the number of Medicaid-covered births increased by 27%. Second, when public health funding cuts forced many Indiana clinics to close, rural areas of the state experienced one of the fastest and largest HIV outbreaks ever to occur in the United States.
Dr. DeFrancesco said, “Excluding Planned Parenthood from the Medicaid program interferes with the patient–physician relationship and sets a dangerous precedent of targeting qualified providers for political purposes.”
Opioid epidemic
Dr. Brown indicated that the BCRA would cripple attempts to address our very serious national opioid epidemic. The $2 billion the bill would allocate for opioid use disorder treatment for 1 year would replace funding lost by Medicaid and would pay for only a fraction of what is needed. Dr. Brown called this measure a “token, not a commitment, and a big step back in the progress we have made to address this public health crisis.”
The Hippocratic oath
While preparing for the teleconference, I kept thinking about the Hippocratic oath and our deep obligation to our patients. Every physician I know goes beyond the exam room to care for patients. We lose sleep not only when we get up to deliver babies, but when we worry about the ailing mother of four we saw yesterday, or the scared teenager who missed last week’s appointment. We care for our patients because it is the right thing to do, and it is our calling. Well, this year, our patients needed us more than ever. We had to step up, speak out, do everything we could to stop BCRA from passing. The stakes could not have been higher.
The vote, and the road ahead
The morning after Senators Collins, Murkowski, and McCain joined Senate Democrats to end the bill, Dr. Brown wrote the following to ACOG members and the US Congress:
“This was a battle we simply had to win to protect our patients. Thanks to your tireless advocacy, landmark women’s health care protections remain law, and millions of our patients will continue to get the care they need. And our work continues. The ACA is not perfect and needs major reform. ACOG is ready, willing, and able to work with Republicans and Democrats in the US House and Senate to reform the law, through an open and collaborative process. We hope it is clear to everyone in Congress that physicians must be part of the conversation and the solution.”
CGRP Monoclonal Antibodies May Be Beneficial for Migraine Prevention
BOSTON—A new class of drugs for the prevention of chronic and episodic migraine demonstrated promising results in recent phase II and phase III trials. Data for four humanized calcitonin gene–related peptide (CGRP) monoclonal antibodies—eptinezumab, erenumab, fremanezumab, and galcanezumab—were presented at the 59th Annual Scientific Meeting of the American Headache Society. Previous research has long implicated CGRP (which is elevated in jugular vein blood during acute migraine and cluster headaches) in disease pathophysiology. Recent advances in molecular neuroscience have helped shed further light on the pathogenic mechanisms and possible targets for treatment.
Eptinezumab
In a phase II study, a single infusion of eptinezumab (which Alder BioPharmaceuticals is developing under the name ALD403) reduced the number of migraine days per month, as well as the number of migraines classified as severe, in patients with chronic migraine. Of the four drugs presented at the meeting, eptinezumab was the only one to have an IV mode of delivery.
“We used International Classification of Headache Disorders 3 criteria to define chronic migraine, which is at least 15 headache days per month, of which eight must be migraine-like,” said Jeffrey T. L. Smith, MD, Senior Vice President of Translational Medicine at Alder BioPharmaceuticals in Bothell, Washington. “Patients also had to have a history of migraine for a year or more.”
On average, the patients included in this multisite study were slightly younger than 40. Most patients were women, and the average number of migraine days was between 16.2 and 16.5. After a one-month run-in period, patients were randomized to receive placebo or one of four doses of eptinezumab (ie, 300 mg, 100 mg, 30 mg, or 10 mg).
Reporting 12-week data from the 48-week trial, Dr. Smith and colleagues found that significantly more patients taking the 300-mg (33%) and 100-mg (31%) doses of eptinezumab reached the primary end point of a 75% reduction from baseline in migraine days, compared with patients who received placebo (21%). Differences in patients taking the 30-mg and 10-mg doses (28% and 27%, respectively) were not statistically significant. “Overall, approximately half of patients treated with eptinezumab experienced a 50% reduction in migraine days versus 41% of patients on placebo,” Dr. Smith added. Eptinezumab was well tolerated, and no serious adverse events were reported.
Post hoc analyses indicated that of the migraines that were not eliminated as a result of treatment, the percentage classified as severe was significantly reduced from baseline in each of the eptinezumab groups, compared with placebo. Also, fewer patients in the eptinezumab groups than in the placebo group experienced a migraine within the first 24 to 48 hours after infusion. “Although the post hoc results are exploratory, these findings are important as well,” said Dr. Smith. “Other things are happening besides the reduction in migraine frequency that may be of benefit.”
Erenumab
A registration, pivotal study on erenumab for chronic migraine prevention was reported at the meeting. “Unlike eptinezumab, fremanezumab, and galcanezumab, which are monoclonal antibodies against the CGRP ligand, erenumab is an anti-CGRP receptor monoclonal antibody,” said Stewart J. Tepper, MD, Professor of Neurology at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire. Amgen is developing this antibody.
In the 12-week randomized controlled trial, patients were enrolled in the US and around the world, Dr. Tepper noted. He and his colleagues included patients who had 15 or more headache days per month with at least eight days of migraine per month. Patients were excluded if they had experienced treatment failure with more than three classes of preventive agents. Mean age was 40, about 80% of patients were women, and roughly half of patients had previously experienced treatment failure with at least two preventive agents.
A total of 667 patients were randomized 3:2:2 to placebo, erenumab (70 mg), or erenumab (140 mg). Treatment was given monthly via subcutaneous injection. The primary end point was change from baseline in monthly migraine days (MMD). Secondary end points included at least a 50% reduction in MMD, change from baseline in acute migraine-specific medication days, and cumulative headache hours.
In both erenumab groups, patients experienced a mean 6.6-day reduction in MMD from a baseline of 18.0 days, which was significantly different, compared with the 4.2-day reduction seen in the placebo group. Furthermore, a significantly greater proportion of patients in the 70-mg and 140-mg groups (40% and 41%, respectively) experienced at least a 50% reduction in MMD, compared with the placebo group (23%). For the secondary end points, both erenumab groups had significantly greater reductions in acute migraine-specific medication days versus placebo. However, the difference in the reduction of cumulative headache hours did not reach statistical significance. “We speculated that this was because headache hours is a relatively variable end point, and it is collected in differing ways, with wide confidence intervals,” Dr. Tepper said.
The safety profile for both erenumab groups and the placebo group was similar. “No serious adverse events were reported by more than one patient in any treatment arm, and no serious adverse events were considered related to treatment,” he said.
Another erenumab trial enrolled patients with episodic migraine. Randomization was stratified by region and current and prior migraine preventive medication, said Daniel Mikol, MD, PhD, Executive Medical Director of Neuroscience Development at Amgen in Thousand Oaks, California. Patients included in the phase III study could be taking a single concomitant migraine preventive therapy. “Patients could have also failed previous preventive therapies due to poor tolerability or lack of efficacy,” he said. “There was no limit regarding the number of previous failures due to poor tolerability. With regard to lack of efficacy, patients could have no therapeutic response to two or fewer preventive therapies.”
Adults with episodic migraine (n = 955) were randomized 1:1:1 to monthly subcutaneous injections of placebo, erenumab (70 mg), or erenumab (140 mg) for 24 weeks. The primary end point was change from baseline in MMD from weeks 13 to 24. Secondary end points included achievement of 50% or more reduction in MMD, change in mean acute migraine-specific medication days, and changes in mean physical impairment and impact on everyday activity scores, as measured by the Migraine Physical Function Impact Diary, a novel patient-reported instrument, Dr. Mikol explained.
Subjects had a mean of 8.3 MMD at baseline. This measurement was reduced by 3.2, 3.7, and 1.8 days in the 70-mg, 140-mg, and placebo groups, respectively—a statistically significant difference in favor of the erenumab groups. Statistically superior results for erenumab 70 mg and 140 mg over placebo also were seen in the secondary end points of > 50% response (43%, 50%, and 27%, respectively), reduction from baseline in monthly acute migraine-specific medication days (1.1, 1.6, and 0.2), improved physical impairment score from baseline (4.2, 4.8, and 2.5), and improved everyday activity scores from baseline (5.5, 5.9, and 3.3).
“The safety profiles for erenumab were similar to that of placebo,” Dr. Mikol said. “Specifically, 63% of patients on placebo had adverse events, compared with 56% to 57% of patients in the erenumab groups. Serious adverse events and adverse events leading to treatment discontinuation were low in frequency and balanced across the groups.”
Galcanezumab
The efficacy, tolerability, and safety of galcanezumab, an investigational drug for the treatment of episodic migraine, was assessed in a six-month phase III study called EVOLVE-2. In this study, patients with episodic migraine experienced four to 14 migraine headache days per month with or without aura. “Our patient population had a mean age of about 40, and most [participants] were Caucasian women,” said Robert Conley, MD, Distinguished Lilly Scholar of Neuroscience at Eli Lilly and Company (the developer of galcanezumab) in Indianapolis.
“Half of our subjects were from North America, a quarter from Europe, and a quarter from the rest of the world.” Subjects had a 20-year migraine history on average and a mean of nine headache days per month at baseline, he noted. “In addition, two-thirds had been on prior preventive treatment, and they had relatively severe levels of disease.”
After screening 1,700 patients for the study, Dr. Conley and colleagues randomized 914 patients 1:1:2 to subcutaneous injection of galcanezumab (120 mg/month), galcanezumab (240 mg/month), or placebo. “There was about an 87% completion rate for the galcanezumab groups and an 83% completion rate in the placebo group. The higher number of dropouts in the placebo group was due to lack of efficacy,” said Dr. Conley. The primary end point was change from baseline in MMD at the end of six months. Secondary end points were percentages of patients with 50%, 75%, and 100% response rates; reduction in MMD requiring acute migraine medication; change in function score on the Migraine-Specific Quality of Life Questionnaire (MSQ); and change in the Patient Global Impression of Severity (PGI-S) rating.
After adjusting for multiplicity, the investigators found statistically significant differences between placebo and the galcanezumab arms in the primary end point. Mean reductions in headache days were 4.29 in the 120-mg group and 4.18 in the 240-mg group. Patients in the placebo group experienced a reduction of 2.28 days. “We looked at the early effects of treatment and found statistically significant improvements within a few days of injection that persisted over time,” Dr. Conley said.
In addition, statistically significant differences between galcanezumab and placebo were observed for all secondary end points. “In the 120-mg and 240-mg treatment groups, 59.3% and 56.5%, respectively, had at least a 50% reduction in monthly headache days, which was significantly different from placebo, but not different from each other,” said Dr. Conley. “We also saw that approximately one-third of patients in the galcanezumab arms experienced 75% response rates, which is double that seen in the placebo group. The 100% response rate was 11.5% to 13.8% in the treated groups—again, not a significant difference between them, but significantly different from placebo.” The change in the MSQ function score and the PGI-S rating averaged for months 4, 5, and 6 were significantly improved in the galcanezumab arms, compared with the placebo arm.
For most of the safety parameters, there were no significant differences between either dose of galcanezumab and placebo. However, the rates of injection-site reactions and pruritus were significantly higher for both drug doses, compared with placebo. Injection-site erythema was significantly higher in the 240-mg dose only.
Fremanezumab
“The phase III trial for fremanezumab in chronic migraine investigated a flexible dose for a quarterly and a monthly regimen,” said Ernesto Aycardi, MD, Vice President and Therapeutic Area Head for Migraine and Headache at Teva Pharmaceuticals
The primary end point of this multicenter trial was the mean change from baseline in monthly days of headache of at least moderate severity. The four main secondary end points were the proportion of patients with at least a 50% reduction in headache days, mean change in the number of days using acute headache medication at the end of the 12-week period of the trial, mean change from baseline in the days of at least moderate severity in the first four weeks of the trial, and change in disability scale (HIT-6) score from baseline. In this trial, patients treated with fremanezumab experienced significant improvement, compared with placebo on all primary and secondary end points for both monthly and quarterly dosing regimens.
“The average age of the study population was 41, with an average history of migraine of about 20 years,” Dr. Aycardi said. “All demographic characteristics, including weight, height, and ethnicity, were well balanced between the groups.”
Patients on the monthly regimen had a mean reduction of 4.6 headache days of at least moderate severity, and patients on the quarterly regimen had a reduction of 4.3 headache days of at least moderate severity, which was significantly different from the placebo group (2.5 headache days). Researchers also observed significant differences for each of the four secondary end points in favor of the monthly and quarterly regimens.
“Fremanezumab was well tolerated, compared with placebo,” Dr. Aycardi said. “The most commonly reported adverse event in the study was injection-site pain, with similar rates in the placebo and active groups.”
Dr. Aycardi highlighted how phase II results validated that target in chronic migraine. “With these phase III results, this is confirmed, bringing hope for patients with this disabling condition.”
—Adriene Marshall
Suggested Reading
Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8.
Reuter U. Anti-CGRP antibodies: a new approach to migraine prevention. Lancet Neurol. 2014;13(9):857-859.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
BOSTON—A new class of drugs for the prevention of chronic and episodic migraine demonstrated promising results in recent phase II and phase III trials. Data for four humanized calcitonin gene–related peptide (CGRP) monoclonal antibodies—eptinezumab, erenumab, fremanezumab, and galcanezumab—were presented at the 59th Annual Scientific Meeting of the American Headache Society. Previous research has long implicated CGRP (which is elevated in jugular vein blood during acute migraine and cluster headaches) in disease pathophysiology. Recent advances in molecular neuroscience have helped shed further light on the pathogenic mechanisms and possible targets for treatment.
Eptinezumab
In a phase II study, a single infusion of eptinezumab (which Alder BioPharmaceuticals is developing under the name ALD403) reduced the number of migraine days per month, as well as the number of migraines classified as severe, in patients with chronic migraine. Of the four drugs presented at the meeting, eptinezumab was the only one to have an IV mode of delivery.
“We used International Classification of Headache Disorders 3 criteria to define chronic migraine, which is at least 15 headache days per month, of which eight must be migraine-like,” said Jeffrey T. L. Smith, MD, Senior Vice President of Translational Medicine at Alder BioPharmaceuticals in Bothell, Washington. “Patients also had to have a history of migraine for a year or more.”
On average, the patients included in this multisite study were slightly younger than 40. Most patients were women, and the average number of migraine days was between 16.2 and 16.5. After a one-month run-in period, patients were randomized to receive placebo or one of four doses of eptinezumab (ie, 300 mg, 100 mg, 30 mg, or 10 mg).
Reporting 12-week data from the 48-week trial, Dr. Smith and colleagues found that significantly more patients taking the 300-mg (33%) and 100-mg (31%) doses of eptinezumab reached the primary end point of a 75% reduction from baseline in migraine days, compared with patients who received placebo (21%). Differences in patients taking the 30-mg and 10-mg doses (28% and 27%, respectively) were not statistically significant. “Overall, approximately half of patients treated with eptinezumab experienced a 50% reduction in migraine days versus 41% of patients on placebo,” Dr. Smith added. Eptinezumab was well tolerated, and no serious adverse events were reported.
Post hoc analyses indicated that of the migraines that were not eliminated as a result of treatment, the percentage classified as severe was significantly reduced from baseline in each of the eptinezumab groups, compared with placebo. Also, fewer patients in the eptinezumab groups than in the placebo group experienced a migraine within the first 24 to 48 hours after infusion. “Although the post hoc results are exploratory, these findings are important as well,” said Dr. Smith. “Other things are happening besides the reduction in migraine frequency that may be of benefit.”
Erenumab
A registration, pivotal study on erenumab for chronic migraine prevention was reported at the meeting. “Unlike eptinezumab, fremanezumab, and galcanezumab, which are monoclonal antibodies against the CGRP ligand, erenumab is an anti-CGRP receptor monoclonal antibody,” said Stewart J. Tepper, MD, Professor of Neurology at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire. Amgen is developing this antibody.
In the 12-week randomized controlled trial, patients were enrolled in the US and around the world, Dr. Tepper noted. He and his colleagues included patients who had 15 or more headache days per month with at least eight days of migraine per month. Patients were excluded if they had experienced treatment failure with more than three classes of preventive agents. Mean age was 40, about 80% of patients were women, and roughly half of patients had previously experienced treatment failure with at least two preventive agents.
A total of 667 patients were randomized 3:2:2 to placebo, erenumab (70 mg), or erenumab (140 mg). Treatment was given monthly via subcutaneous injection. The primary end point was change from baseline in monthly migraine days (MMD). Secondary end points included at least a 50% reduction in MMD, change from baseline in acute migraine-specific medication days, and cumulative headache hours.
In both erenumab groups, patients experienced a mean 6.6-day reduction in MMD from a baseline of 18.0 days, which was significantly different, compared with the 4.2-day reduction seen in the placebo group. Furthermore, a significantly greater proportion of patients in the 70-mg and 140-mg groups (40% and 41%, respectively) experienced at least a 50% reduction in MMD, compared with the placebo group (23%). For the secondary end points, both erenumab groups had significantly greater reductions in acute migraine-specific medication days versus placebo. However, the difference in the reduction of cumulative headache hours did not reach statistical significance. “We speculated that this was because headache hours is a relatively variable end point, and it is collected in differing ways, with wide confidence intervals,” Dr. Tepper said.
The safety profile for both erenumab groups and the placebo group was similar. “No serious adverse events were reported by more than one patient in any treatment arm, and no serious adverse events were considered related to treatment,” he said.
Another erenumab trial enrolled patients with episodic migraine. Randomization was stratified by region and current and prior migraine preventive medication, said Daniel Mikol, MD, PhD, Executive Medical Director of Neuroscience Development at Amgen in Thousand Oaks, California. Patients included in the phase III study could be taking a single concomitant migraine preventive therapy. “Patients could have also failed previous preventive therapies due to poor tolerability or lack of efficacy,” he said. “There was no limit regarding the number of previous failures due to poor tolerability. With regard to lack of efficacy, patients could have no therapeutic response to two or fewer preventive therapies.”
Adults with episodic migraine (n = 955) were randomized 1:1:1 to monthly subcutaneous injections of placebo, erenumab (70 mg), or erenumab (140 mg) for 24 weeks. The primary end point was change from baseline in MMD from weeks 13 to 24. Secondary end points included achievement of 50% or more reduction in MMD, change in mean acute migraine-specific medication days, and changes in mean physical impairment and impact on everyday activity scores, as measured by the Migraine Physical Function Impact Diary, a novel patient-reported instrument, Dr. Mikol explained.
Subjects had a mean of 8.3 MMD at baseline. This measurement was reduced by 3.2, 3.7, and 1.8 days in the 70-mg, 140-mg, and placebo groups, respectively—a statistically significant difference in favor of the erenumab groups. Statistically superior results for erenumab 70 mg and 140 mg over placebo also were seen in the secondary end points of > 50% response (43%, 50%, and 27%, respectively), reduction from baseline in monthly acute migraine-specific medication days (1.1, 1.6, and 0.2), improved physical impairment score from baseline (4.2, 4.8, and 2.5), and improved everyday activity scores from baseline (5.5, 5.9, and 3.3).
“The safety profiles for erenumab were similar to that of placebo,” Dr. Mikol said. “Specifically, 63% of patients on placebo had adverse events, compared with 56% to 57% of patients in the erenumab groups. Serious adverse events and adverse events leading to treatment discontinuation were low in frequency and balanced across the groups.”
Galcanezumab
The efficacy, tolerability, and safety of galcanezumab, an investigational drug for the treatment of episodic migraine, was assessed in a six-month phase III study called EVOLVE-2. In this study, patients with episodic migraine experienced four to 14 migraine headache days per month with or without aura. “Our patient population had a mean age of about 40, and most [participants] were Caucasian women,” said Robert Conley, MD, Distinguished Lilly Scholar of Neuroscience at Eli Lilly and Company (the developer of galcanezumab) in Indianapolis.
“Half of our subjects were from North America, a quarter from Europe, and a quarter from the rest of the world.” Subjects had a 20-year migraine history on average and a mean of nine headache days per month at baseline, he noted. “In addition, two-thirds had been on prior preventive treatment, and they had relatively severe levels of disease.”
After screening 1,700 patients for the study, Dr. Conley and colleagues randomized 914 patients 1:1:2 to subcutaneous injection of galcanezumab (120 mg/month), galcanezumab (240 mg/month), or placebo. “There was about an 87% completion rate for the galcanezumab groups and an 83% completion rate in the placebo group. The higher number of dropouts in the placebo group was due to lack of efficacy,” said Dr. Conley. The primary end point was change from baseline in MMD at the end of six months. Secondary end points were percentages of patients with 50%, 75%, and 100% response rates; reduction in MMD requiring acute migraine medication; change in function score on the Migraine-Specific Quality of Life Questionnaire (MSQ); and change in the Patient Global Impression of Severity (PGI-S) rating.
After adjusting for multiplicity, the investigators found statistically significant differences between placebo and the galcanezumab arms in the primary end point. Mean reductions in headache days were 4.29 in the 120-mg group and 4.18 in the 240-mg group. Patients in the placebo group experienced a reduction of 2.28 days. “We looked at the early effects of treatment and found statistically significant improvements within a few days of injection that persisted over time,” Dr. Conley said.
In addition, statistically significant differences between galcanezumab and placebo were observed for all secondary end points. “In the 120-mg and 240-mg treatment groups, 59.3% and 56.5%, respectively, had at least a 50% reduction in monthly headache days, which was significantly different from placebo, but not different from each other,” said Dr. Conley. “We also saw that approximately one-third of patients in the galcanezumab arms experienced 75% response rates, which is double that seen in the placebo group. The 100% response rate was 11.5% to 13.8% in the treated groups—again, not a significant difference between them, but significantly different from placebo.” The change in the MSQ function score and the PGI-S rating averaged for months 4, 5, and 6 were significantly improved in the galcanezumab arms, compared with the placebo arm.
For most of the safety parameters, there were no significant differences between either dose of galcanezumab and placebo. However, the rates of injection-site reactions and pruritus were significantly higher for both drug doses, compared with placebo. Injection-site erythema was significantly higher in the 240-mg dose only.
Fremanezumab
“The phase III trial for fremanezumab in chronic migraine investigated a flexible dose for a quarterly and a monthly regimen,” said Ernesto Aycardi, MD, Vice President and Therapeutic Area Head for Migraine and Headache at Teva Pharmaceuticals
The primary end point of this multicenter trial was the mean change from baseline in monthly days of headache of at least moderate severity. The four main secondary end points were the proportion of patients with at least a 50% reduction in headache days, mean change in the number of days using acute headache medication at the end of the 12-week period of the trial, mean change from baseline in the days of at least moderate severity in the first four weeks of the trial, and change in disability scale (HIT-6) score from baseline. In this trial, patients treated with fremanezumab experienced significant improvement, compared with placebo on all primary and secondary end points for both monthly and quarterly dosing regimens.
“The average age of the study population was 41, with an average history of migraine of about 20 years,” Dr. Aycardi said. “All demographic characteristics, including weight, height, and ethnicity, were well balanced between the groups.”
Patients on the monthly regimen had a mean reduction of 4.6 headache days of at least moderate severity, and patients on the quarterly regimen had a reduction of 4.3 headache days of at least moderate severity, which was significantly different from the placebo group (2.5 headache days). Researchers also observed significant differences for each of the four secondary end points in favor of the monthly and quarterly regimens.
“Fremanezumab was well tolerated, compared with placebo,” Dr. Aycardi said. “The most commonly reported adverse event in the study was injection-site pain, with similar rates in the placebo and active groups.”
Dr. Aycardi highlighted how phase II results validated that target in chronic migraine. “With these phase III results, this is confirmed, bringing hope for patients with this disabling condition.”
—Adriene Marshall
Suggested Reading
Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8.
Reuter U. Anti-CGRP antibodies: a new approach to migraine prevention. Lancet Neurol. 2014;13(9):857-859.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
BOSTON—A new class of drugs for the prevention of chronic and episodic migraine demonstrated promising results in recent phase II and phase III trials. Data for four humanized calcitonin gene–related peptide (CGRP) monoclonal antibodies—eptinezumab, erenumab, fremanezumab, and galcanezumab—were presented at the 59th Annual Scientific Meeting of the American Headache Society. Previous research has long implicated CGRP (which is elevated in jugular vein blood during acute migraine and cluster headaches) in disease pathophysiology. Recent advances in molecular neuroscience have helped shed further light on the pathogenic mechanisms and possible targets for treatment.
Eptinezumab
In a phase II study, a single infusion of eptinezumab (which Alder BioPharmaceuticals is developing under the name ALD403) reduced the number of migraine days per month, as well as the number of migraines classified as severe, in patients with chronic migraine. Of the four drugs presented at the meeting, eptinezumab was the only one to have an IV mode of delivery.
“We used International Classification of Headache Disorders 3 criteria to define chronic migraine, which is at least 15 headache days per month, of which eight must be migraine-like,” said Jeffrey T. L. Smith, MD, Senior Vice President of Translational Medicine at Alder BioPharmaceuticals in Bothell, Washington. “Patients also had to have a history of migraine for a year or more.”
On average, the patients included in this multisite study were slightly younger than 40. Most patients were women, and the average number of migraine days was between 16.2 and 16.5. After a one-month run-in period, patients were randomized to receive placebo or one of four doses of eptinezumab (ie, 300 mg, 100 mg, 30 mg, or 10 mg).
Reporting 12-week data from the 48-week trial, Dr. Smith and colleagues found that significantly more patients taking the 300-mg (33%) and 100-mg (31%) doses of eptinezumab reached the primary end point of a 75% reduction from baseline in migraine days, compared with patients who received placebo (21%). Differences in patients taking the 30-mg and 10-mg doses (28% and 27%, respectively) were not statistically significant. “Overall, approximately half of patients treated with eptinezumab experienced a 50% reduction in migraine days versus 41% of patients on placebo,” Dr. Smith added. Eptinezumab was well tolerated, and no serious adverse events were reported.
Post hoc analyses indicated that of the migraines that were not eliminated as a result of treatment, the percentage classified as severe was significantly reduced from baseline in each of the eptinezumab groups, compared with placebo. Also, fewer patients in the eptinezumab groups than in the placebo group experienced a migraine within the first 24 to 48 hours after infusion. “Although the post hoc results are exploratory, these findings are important as well,” said Dr. Smith. “Other things are happening besides the reduction in migraine frequency that may be of benefit.”
Erenumab
A registration, pivotal study on erenumab for chronic migraine prevention was reported at the meeting. “Unlike eptinezumab, fremanezumab, and galcanezumab, which are monoclonal antibodies against the CGRP ligand, erenumab is an anti-CGRP receptor monoclonal antibody,” said Stewart J. Tepper, MD, Professor of Neurology at the Geisel School of Medicine at Dartmouth in Hanover, New Hampshire. Amgen is developing this antibody.
In the 12-week randomized controlled trial, patients were enrolled in the US and around the world, Dr. Tepper noted. He and his colleagues included patients who had 15 or more headache days per month with at least eight days of migraine per month. Patients were excluded if they had experienced treatment failure with more than three classes of preventive agents. Mean age was 40, about 80% of patients were women, and roughly half of patients had previously experienced treatment failure with at least two preventive agents.
A total of 667 patients were randomized 3:2:2 to placebo, erenumab (70 mg), or erenumab (140 mg). Treatment was given monthly via subcutaneous injection. The primary end point was change from baseline in monthly migraine days (MMD). Secondary end points included at least a 50% reduction in MMD, change from baseline in acute migraine-specific medication days, and cumulative headache hours.
In both erenumab groups, patients experienced a mean 6.6-day reduction in MMD from a baseline of 18.0 days, which was significantly different, compared with the 4.2-day reduction seen in the placebo group. Furthermore, a significantly greater proportion of patients in the 70-mg and 140-mg groups (40% and 41%, respectively) experienced at least a 50% reduction in MMD, compared with the placebo group (23%). For the secondary end points, both erenumab groups had significantly greater reductions in acute migraine-specific medication days versus placebo. However, the difference in the reduction of cumulative headache hours did not reach statistical significance. “We speculated that this was because headache hours is a relatively variable end point, and it is collected in differing ways, with wide confidence intervals,” Dr. Tepper said.
The safety profile for both erenumab groups and the placebo group was similar. “No serious adverse events were reported by more than one patient in any treatment arm, and no serious adverse events were considered related to treatment,” he said.
Another erenumab trial enrolled patients with episodic migraine. Randomization was stratified by region and current and prior migraine preventive medication, said Daniel Mikol, MD, PhD, Executive Medical Director of Neuroscience Development at Amgen in Thousand Oaks, California. Patients included in the phase III study could be taking a single concomitant migraine preventive therapy. “Patients could have also failed previous preventive therapies due to poor tolerability or lack of efficacy,” he said. “There was no limit regarding the number of previous failures due to poor tolerability. With regard to lack of efficacy, patients could have no therapeutic response to two or fewer preventive therapies.”
Adults with episodic migraine (n = 955) were randomized 1:1:1 to monthly subcutaneous injections of placebo, erenumab (70 mg), or erenumab (140 mg) for 24 weeks. The primary end point was change from baseline in MMD from weeks 13 to 24. Secondary end points included achievement of 50% or more reduction in MMD, change in mean acute migraine-specific medication days, and changes in mean physical impairment and impact on everyday activity scores, as measured by the Migraine Physical Function Impact Diary, a novel patient-reported instrument, Dr. Mikol explained.
Subjects had a mean of 8.3 MMD at baseline. This measurement was reduced by 3.2, 3.7, and 1.8 days in the 70-mg, 140-mg, and placebo groups, respectively—a statistically significant difference in favor of the erenumab groups. Statistically superior results for erenumab 70 mg and 140 mg over placebo also were seen in the secondary end points of > 50% response (43%, 50%, and 27%, respectively), reduction from baseline in monthly acute migraine-specific medication days (1.1, 1.6, and 0.2), improved physical impairment score from baseline (4.2, 4.8, and 2.5), and improved everyday activity scores from baseline (5.5, 5.9, and 3.3).
“The safety profiles for erenumab were similar to that of placebo,” Dr. Mikol said. “Specifically, 63% of patients on placebo had adverse events, compared with 56% to 57% of patients in the erenumab groups. Serious adverse events and adverse events leading to treatment discontinuation were low in frequency and balanced across the groups.”
Galcanezumab
The efficacy, tolerability, and safety of galcanezumab, an investigational drug for the treatment of episodic migraine, was assessed in a six-month phase III study called EVOLVE-2. In this study, patients with episodic migraine experienced four to 14 migraine headache days per month with or without aura. “Our patient population had a mean age of about 40, and most [participants] were Caucasian women,” said Robert Conley, MD, Distinguished Lilly Scholar of Neuroscience at Eli Lilly and Company (the developer of galcanezumab) in Indianapolis.
“Half of our subjects were from North America, a quarter from Europe, and a quarter from the rest of the world.” Subjects had a 20-year migraine history on average and a mean of nine headache days per month at baseline, he noted. “In addition, two-thirds had been on prior preventive treatment, and they had relatively severe levels of disease.”
After screening 1,700 patients for the study, Dr. Conley and colleagues randomized 914 patients 1:1:2 to subcutaneous injection of galcanezumab (120 mg/month), galcanezumab (240 mg/month), or placebo. “There was about an 87% completion rate for the galcanezumab groups and an 83% completion rate in the placebo group. The higher number of dropouts in the placebo group was due to lack of efficacy,” said Dr. Conley. The primary end point was change from baseline in MMD at the end of six months. Secondary end points were percentages of patients with 50%, 75%, and 100% response rates; reduction in MMD requiring acute migraine medication; change in function score on the Migraine-Specific Quality of Life Questionnaire (MSQ); and change in the Patient Global Impression of Severity (PGI-S) rating.
After adjusting for multiplicity, the investigators found statistically significant differences between placebo and the galcanezumab arms in the primary end point. Mean reductions in headache days were 4.29 in the 120-mg group and 4.18 in the 240-mg group. Patients in the placebo group experienced a reduction of 2.28 days. “We looked at the early effects of treatment and found statistically significant improvements within a few days of injection that persisted over time,” Dr. Conley said.
In addition, statistically significant differences between galcanezumab and placebo were observed for all secondary end points. “In the 120-mg and 240-mg treatment groups, 59.3% and 56.5%, respectively, had at least a 50% reduction in monthly headache days, which was significantly different from placebo, but not different from each other,” said Dr. Conley. “We also saw that approximately one-third of patients in the galcanezumab arms experienced 75% response rates, which is double that seen in the placebo group. The 100% response rate was 11.5% to 13.8% in the treated groups—again, not a significant difference between them, but significantly different from placebo.” The change in the MSQ function score and the PGI-S rating averaged for months 4, 5, and 6 were significantly improved in the galcanezumab arms, compared with the placebo arm.
For most of the safety parameters, there were no significant differences between either dose of galcanezumab and placebo. However, the rates of injection-site reactions and pruritus were significantly higher for both drug doses, compared with placebo. Injection-site erythema was significantly higher in the 240-mg dose only.
Fremanezumab
“The phase III trial for fremanezumab in chronic migraine investigated a flexible dose for a quarterly and a monthly regimen,” said Ernesto Aycardi, MD, Vice President and Therapeutic Area Head for Migraine and Headache at Teva Pharmaceuticals
The primary end point of this multicenter trial was the mean change from baseline in monthly days of headache of at least moderate severity. The four main secondary end points were the proportion of patients with at least a 50% reduction in headache days, mean change in the number of days using acute headache medication at the end of the 12-week period of the trial, mean change from baseline in the days of at least moderate severity in the first four weeks of the trial, and change in disability scale (HIT-6) score from baseline. In this trial, patients treated with fremanezumab experienced significant improvement, compared with placebo on all primary and secondary end points for both monthly and quarterly dosing regimens.
“The average age of the study population was 41, with an average history of migraine of about 20 years,” Dr. Aycardi said. “All demographic characteristics, including weight, height, and ethnicity, were well balanced between the groups.”
Patients on the monthly regimen had a mean reduction of 4.6 headache days of at least moderate severity, and patients on the quarterly regimen had a reduction of 4.3 headache days of at least moderate severity, which was significantly different from the placebo group (2.5 headache days). Researchers also observed significant differences for each of the four secondary end points in favor of the monthly and quarterly regimens.
“Fremanezumab was well tolerated, compared with placebo,” Dr. Aycardi said. “The most commonly reported adverse event in the study was injection-site pain, with similar rates in the placebo and active groups.”
Dr. Aycardi highlighted how phase II results validated that target in chronic migraine. “With these phase III results, this is confirmed, bringing hope for patients with this disabling condition.”
—Adriene Marshall
Suggested Reading
Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8.
Reuter U. Anti-CGRP antibodies: a new approach to migraine prevention. Lancet Neurol. 2014;13(9):857-859.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
Hidden CABG costs will disrupt bundled payment systems
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.
For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.
For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
COLORADO SPRINGS – With bundled payment models for coronary artery bypass graft surgery looming ahead, it’s vital that cardiac surgeons take a hard look at the procedure’s hidden costs – namely, the steep price tag for postoperative complications, James H. Mehaffey, MD, said at the annual meeting of the Western Thoracic Surgical Association.
He presented a retrospective study of the 30-day hospital costs for all 36,588 patients who underwent isolated CABG during 2006-2015 at the 19 Virginia centers where the surgery is performed. This was a typical CABG population, with an average predicted risk of mortality of 1.9%. The actual 30-day mortality was 0.6%, so the surgical performance was better than expected.
“The population of patients experiencing one or more major comorbidities demonstrated a significant and dramatic increase in total hospital costs. It was an exponential increase with each additional major morbidity,” reported Dr. Mehaffey of the University of Virginia, Charlottesville.
Indeed, the average cost jumped from $36,580 for uncomplicated surgery to $64,542 with one major complication, $111,239 with two, and $194,043 with three.
The two most frequent major complications were postoperative atrial fibrillation, which occurred in 18.4% of patients, and prolonged ventilation for longer than 24 hours, which occurred in 9%. Over the course of the decade-long study period, the 19 medical centers in the Virginia Cardiac Surgery Quality Initiative collectively spent roughly $59 million on prolonged ventilation and $27 million for postoperative atrial fibrillation.
The cost of CABG during the study years outpaced the CMS health care–specific inflation rate, and this escalating cost was driven primarily by postoperative complications.
For the Virginia cardiac surgery collaborative, these data on the cost of postoperative complications will be utilized to prioritize quality improvement projects.
For example, during the past decade, the Virginia collaborative made reduction in the rate of postoperative atrial fibrillation a priority. Toward that end, the collaborative developed a protocol for routine perioperative prophylactic amiodarone therapy.
“At the beginning of the study decade we had postoperative atrial fibrillation rates above 25%. The average for the entire decade was just over 18%, and in the last couple years we’ve been in the 15%-16% range. So I think we are moving the needle on this. We are making a meaningful impact,” Dr. Mehaffey said.
“We’ve already used the complication cost data to do a cost-effectiveness analysis of our prophylactic amiodarone innovation. We showed we saved an average of $250 per patient, even though we’re treating a bunch of patients who’d never get that complication,” he continued.
This sort of data on the cost of adverse events is also critical to accurately risk-adjust bundled payment models.
Discussant Richard J. Shemin, MD, asked if there was much variability in postoperative complication costs between the CABG centers in the Virginia collaborative.
The variability is enormous, Dr. Mehaffey replied. Investigators recently plugged the last 5 years worth of hospital cost and complication rate data into a proposed CABG bundled payment model and extrapolated what that would mean over the next 5 years.
“There were some institutions that would be positive by a couple million dollars from this payment system and some that were losing more than $20 million, just because of the cost variability,” said Dr. Mehaffey.
Dr. Shemin also noted that the Virginia collaborative was able to collect 30-day outcome data only through the STS database, yet the bundled payment programs are based on the 90-day postoperative experience.
“How do we capture the costs in that full 90 days that we’ll be responsible for?” asked Dr. Shemin, professor of surgery and codirector of the UCLA Cardiovascular Center.
Dr. Mehaffey said that’s indeed an important question, since a major complication such as stroke or deep sternal wound infection typically entails considerable long-term costs and repeated hospital admissions beyond the 30-day window. In Virginia, the cardiac surgery collaborative is working with payers to gain access to the 90 days worth of patient data.
He reported having no financial conflicts regarding his study.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: The average 30-day total hospital cost of an isolated CABG procedure during 2006-2015 in Virginia was $36,580 if there were no postoperative complications, jumping to $64,542 with one major complication and $111,239 with two.
Data source: A retrospective study of the 30-day total hospital costs for all isolated CABG procedures performed in Virginia during 2006-2015.
Disclosures: The study presenter reported having no financial conflicts.
Medicare Part D premiums dip as drug costs continue to rise
As insurance premiums in other sectors of the health care industry continue to soar, seniors enrolled in the Medicare Part D prescription drug plan will see a slight decrease in the average premium for their drug coverage.
The Centers for Medicare & Medicaid Services announced Aug. 2 that the average basic premium for drug coverage is projected to drop to $33.50 per month in 2018, down $1.20 from 2017’s average monthly premium of $34.70.
Average premiums are falling despite the rise in drug spending, according to the 2017 Medicare Trustees report, which details the solvency of the Medicare program.
The report notes, however, that drug costs are lower than previous reports due to higher manufacturer rebates and decreased use of direct acting antiviral therapies for hepatitis C virus. Overall, Medicare Part D expenditures per enrollee are estimated to increase by an average of 4.7% annually from 2017 through 2026.
As insurance premiums in other sectors of the health care industry continue to soar, seniors enrolled in the Medicare Part D prescription drug plan will see a slight decrease in the average premium for their drug coverage.
The Centers for Medicare & Medicaid Services announced Aug. 2 that the average basic premium for drug coverage is projected to drop to $33.50 per month in 2018, down $1.20 from 2017’s average monthly premium of $34.70.
Average premiums are falling despite the rise in drug spending, according to the 2017 Medicare Trustees report, which details the solvency of the Medicare program.
The report notes, however, that drug costs are lower than previous reports due to higher manufacturer rebates and decreased use of direct acting antiviral therapies for hepatitis C virus. Overall, Medicare Part D expenditures per enrollee are estimated to increase by an average of 4.7% annually from 2017 through 2026.
As insurance premiums in other sectors of the health care industry continue to soar, seniors enrolled in the Medicare Part D prescription drug plan will see a slight decrease in the average premium for their drug coverage.
The Centers for Medicare & Medicaid Services announced Aug. 2 that the average basic premium for drug coverage is projected to drop to $33.50 per month in 2018, down $1.20 from 2017’s average monthly premium of $34.70.
Average premiums are falling despite the rise in drug spending, according to the 2017 Medicare Trustees report, which details the solvency of the Medicare program.
The report notes, however, that drug costs are lower than previous reports due to higher manufacturer rebates and decreased use of direct acting antiviral therapies for hepatitis C virus. Overall, Medicare Part D expenditures per enrollee are estimated to increase by an average of 4.7% annually from 2017 through 2026.
How to Keep Pace With Genetic Advances in Movement Disorders
VANCOUVER—Researchers’ understanding of genetic causes of movement disorders is advancing rapidly. “The ability to generate data has almost surpassed our ability to understand and use it in the clinic,” said Buz Jinnah, MD, PhD, at the 21st International Congress of Parkinson’s Disease and Movement Disorders.
A recent study underscores the swift evolution of the field. Investigators compared the diagnostic ability of a dystonia gene panel with that of experts who formulated a diagnostic workup without the use of gene panel analysis. In a cohort of 61 patients, the gene panel led to more genetic diagnoses and did so faster and at less cost on average, compared with experts using traditional methods.
Neurologists face practical questions, such as which patients need genetic testing, which tests they should perform, and how the results influence clinical management, said Dr. Jinnah, Professor of Neurology, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta. Neurologists once considered Wilson’s disease to have the only genetic cause of movement disorders that significantly changed treatment. Now, researchers have identified more than 30 genetic movement disorders with specific, effective treatments.
Does a Patient Need Genetic Testing?
Not all patients need genetic testing. In some cases, the need for genetic testing is obvious, but in other cases, it is not so clear, Dr. Jinnah said. Certain features increase suspicion for a genetic disorder, such as family history, unusually young age of onset, and combinations of features that fit a recognized syndrome.
Genetic testing also may be warranted in presumed acquired cases. “Methodical studies of these so-called acquired cases reveal a high proportion of genetic explanation for them,” he said. “We typically think of cerebral palsy as an acquired disorder…. But the studies are now showing that about a third of these cases have a genetic explanation.”
Single-gene sequencing, chromosomal microarray, and tests for deletions or insertions are available, as well as next-generation sequencing methods with broader reach. Next-generation techniques include gene panels that can test hundreds of genes associated with a disease, whole-exome sequencing, and whole-genome sequencing.
Choosing a Test
Experts tend to use one of three strategies to select which genetic test to perform. One approach entails identifying red flags—“telltale clinical features that point to a specific diagnosis or group of diagnoses,” said Dr. Jinnah.
Another approach involves pattern recognition. Syndromic combinations may lead a neurologist “straight to a single gene test to confirm the diagnosis” if a patient has a classic presentation of a genetic disease.
The third approach uses diagnostic algorithms. “Depending on the presence or absence of various clinical features … [an algorithm] tells you which gene or collection of genes you need to test for,” he said.
Each strategy has limitations, however. It is difficult to remember all of the red flags and syndromic patterns, and many disorders lack red flags. Atypical syndromes can be misleading, and atypical presentations that have been reported in the literature for decades “continue to surprise us,” Dr. Jinnah said. Researchers have published various diagnostic algorithms for dystonia, parkinsonism, and ataxia, and none of them is perfect.
Several studies have shown the potential of next-generation genetic testing. A report from the NIH’s Undiagnosed Diseases Program found that 25% of patients in the program reached a diagnosis within a few weeks using whole-exome sequencing. “Their conclusion was that these agnostic methods need to be used earlier in the diagnostic process,” he said.
A similar study in Canada evaluated the use of whole-exome sequencing in 362 children with disorders that experts had not been able to diagnose. About 30% of the patients received a definitive diagnosis using whole-exome sequencing.
Clinical Implications
Genetic testing may be important for patient counseling. For instance, if a patient has a family history of Huntington’s disease and develops symptoms suggestive of Huntington’s disease, a normal test result is therapeutic, Dr. Jinnah said. Normal results may be false negatives, however. For example, next-generation sequencing methods are not suitable for detecting certain problems like large insertions, duplications, triplet disorders, and certain genes, and neurologists should consider whether other tests may be useful.
If a genetic test yields a pathologic result, it can inform prognosis and guide family counseling and planning. Furthermore, some inherited disorders have life-altering treatments.
Neurologists are familiar with typical and atypical presentations of Wilson’s disease because the disorder has highly effective treatments. But recent studies have identified lesser-known disorders that may be equally important to recognize. “How many of us are routinely testing these same cases for disorders of manganese transporters?” Dr. Jinnah asked. “It is relatively new information, but the clinical scenario is virtually identical.”
The International Parkinson and Movement Disorder Society’s Rare Movement Disorders Task Force sought to determine how many treatable genetic movement disorders have been identified. The task force found more than 30 disorders with specific treatments—including vitamins, dietary interventions, trigger avoidance, and drugs—that can have “a dramatic impact on the patient’s clinical course,” Dr. Jinnah said. “All of them are quite rare, but we do not want to miss them, because they are treatable.” Investigators identify more treatable disorders each year.
Interpreting Ambiguous Results
Another practical concern for neurologists is the interpretation of variants of unknown significance (VOUS) in genetic test results. “It means that a change or variant in the normal gene was discovered by the laboratory, but the change has never been described in association with the disease that you are interested in,” Dr. Jinnah said. “As a result, the laboratory cannot tell you whether it is relevant or not.”
Neurologists might be inclined to tell a patient that they “found something weird in their DNA,” but this approach “consolidates the patient’s concern that they have some mysterious disorder that none of the doctors can figure out, even the experts,” he said. Nor should neurologists tell a patient that the VOUS is not significant, because that is not what VOUS means.
A VOUS is comparable to a radiology report that says, “Clinical correlation is advised.” That phrase leads neurologists to compare neuroimaging to the clinical phenotype and ask whether the imaging findings make sense. “A VOUS is no different from this,” he said. Neurologists cannot explain all genetic variants, just as neurologists cannot explain all of the spots on a brain scan. “We have to get comfortable with the fact that we do not have answers for everything and that there will be some ambiguities when we are done.”
Genetics is logical and straightforward, and educational sessions are available at conferences for neurologists who are interested in learning more about genetic testing, Dr. Jinnah said. Neurologists also can refer patients to experts. “This referral must be a collaboration,” he said. “People in human genetics are not trained in movement disorders, and many of them have no formal training in neurology. They need your careful phenotypic exam to point them in th
Genetic testing often is expensive, not readily available, and not covered by insurance, but this situation may change, Dr. Jinnah said. He likened the situation of genetic testing today to that of MRI in the 1980s. “Only a few centers in the world had [MRI]. It was extremely expensive. Now, MRI scanners are everywhere, and everybody uses it because it is so valuable in clinical medicine,” he said. “I suspect that in a few years, we will see the same with genetic tests.”
—Jake Remaly
Suggested Reading
Charlesworth G, Bhatia KP, Wood NW. The genetics of dystonia: new twists in an old tale. Brain. 2013;136(Pt 7):2017-2037.
Gahl WA, Tifft CJ. The NIH Undiagnosed Diseases Program: lessons learned. JAMA. 2011;305(18):1904-1905.
Olgiati S, Quadri M, Bonifati V. Genetics of movement disorders in the next-generation sequencing era. Mov Disord. 2016;31(4):458-470.
Sawyer SL, Hartley T, Dyment DA, et al. Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin Genet. 2016;89(3):275-284.
van Egmond ME, Lugtenberg CHA, Brouwer OF, et al. A post hoc study on gene panel analysis for the diagnosis of dystonia. Mov Disord. 2017;32(4):569-575.
VANCOUVER—Researchers’ understanding of genetic causes of movement disorders is advancing rapidly. “The ability to generate data has almost surpassed our ability to understand and use it in the clinic,” said Buz Jinnah, MD, PhD, at the 21st International Congress of Parkinson’s Disease and Movement Disorders.
A recent study underscores the swift evolution of the field. Investigators compared the diagnostic ability of a dystonia gene panel with that of experts who formulated a diagnostic workup without the use of gene panel analysis. In a cohort of 61 patients, the gene panel led to more genetic diagnoses and did so faster and at less cost on average, compared with experts using traditional methods.
Neurologists face practical questions, such as which patients need genetic testing, which tests they should perform, and how the results influence clinical management, said Dr. Jinnah, Professor of Neurology, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta. Neurologists once considered Wilson’s disease to have the only genetic cause of movement disorders that significantly changed treatment. Now, researchers have identified more than 30 genetic movement disorders with specific, effective treatments.
Does a Patient Need Genetic Testing?
Not all patients need genetic testing. In some cases, the need for genetic testing is obvious, but in other cases, it is not so clear, Dr. Jinnah said. Certain features increase suspicion for a genetic disorder, such as family history, unusually young age of onset, and combinations of features that fit a recognized syndrome.
Genetic testing also may be warranted in presumed acquired cases. “Methodical studies of these so-called acquired cases reveal a high proportion of genetic explanation for them,” he said. “We typically think of cerebral palsy as an acquired disorder…. But the studies are now showing that about a third of these cases have a genetic explanation.”
Single-gene sequencing, chromosomal microarray, and tests for deletions or insertions are available, as well as next-generation sequencing methods with broader reach. Next-generation techniques include gene panels that can test hundreds of genes associated with a disease, whole-exome sequencing, and whole-genome sequencing.
Choosing a Test
Experts tend to use one of three strategies to select which genetic test to perform. One approach entails identifying red flags—“telltale clinical features that point to a specific diagnosis or group of diagnoses,” said Dr. Jinnah.
Another approach involves pattern recognition. Syndromic combinations may lead a neurologist “straight to a single gene test to confirm the diagnosis” if a patient has a classic presentation of a genetic disease.
The third approach uses diagnostic algorithms. “Depending on the presence or absence of various clinical features … [an algorithm] tells you which gene or collection of genes you need to test for,” he said.
Each strategy has limitations, however. It is difficult to remember all of the red flags and syndromic patterns, and many disorders lack red flags. Atypical syndromes can be misleading, and atypical presentations that have been reported in the literature for decades “continue to surprise us,” Dr. Jinnah said. Researchers have published various diagnostic algorithms for dystonia, parkinsonism, and ataxia, and none of them is perfect.
Several studies have shown the potential of next-generation genetic testing. A report from the NIH’s Undiagnosed Diseases Program found that 25% of patients in the program reached a diagnosis within a few weeks using whole-exome sequencing. “Their conclusion was that these agnostic methods need to be used earlier in the diagnostic process,” he said.
A similar study in Canada evaluated the use of whole-exome sequencing in 362 children with disorders that experts had not been able to diagnose. About 30% of the patients received a definitive diagnosis using whole-exome sequencing.
Clinical Implications
Genetic testing may be important for patient counseling. For instance, if a patient has a family history of Huntington’s disease and develops symptoms suggestive of Huntington’s disease, a normal test result is therapeutic, Dr. Jinnah said. Normal results may be false negatives, however. For example, next-generation sequencing methods are not suitable for detecting certain problems like large insertions, duplications, triplet disorders, and certain genes, and neurologists should consider whether other tests may be useful.
If a genetic test yields a pathologic result, it can inform prognosis and guide family counseling and planning. Furthermore, some inherited disorders have life-altering treatments.
Neurologists are familiar with typical and atypical presentations of Wilson’s disease because the disorder has highly effective treatments. But recent studies have identified lesser-known disorders that may be equally important to recognize. “How many of us are routinely testing these same cases for disorders of manganese transporters?” Dr. Jinnah asked. “It is relatively new information, but the clinical scenario is virtually identical.”
The International Parkinson and Movement Disorder Society’s Rare Movement Disorders Task Force sought to determine how many treatable genetic movement disorders have been identified. The task force found more than 30 disorders with specific treatments—including vitamins, dietary interventions, trigger avoidance, and drugs—that can have “a dramatic impact on the patient’s clinical course,” Dr. Jinnah said. “All of them are quite rare, but we do not want to miss them, because they are treatable.” Investigators identify more treatable disorders each year.
Interpreting Ambiguous Results
Another practical concern for neurologists is the interpretation of variants of unknown significance (VOUS) in genetic test results. “It means that a change or variant in the normal gene was discovered by the laboratory, but the change has never been described in association with the disease that you are interested in,” Dr. Jinnah said. “As a result, the laboratory cannot tell you whether it is relevant or not.”
Neurologists might be inclined to tell a patient that they “found something weird in their DNA,” but this approach “consolidates the patient’s concern that they have some mysterious disorder that none of the doctors can figure out, even the experts,” he said. Nor should neurologists tell a patient that the VOUS is not significant, because that is not what VOUS means.
A VOUS is comparable to a radiology report that says, “Clinical correlation is advised.” That phrase leads neurologists to compare neuroimaging to the clinical phenotype and ask whether the imaging findings make sense. “A VOUS is no different from this,” he said. Neurologists cannot explain all genetic variants, just as neurologists cannot explain all of the spots on a brain scan. “We have to get comfortable with the fact that we do not have answers for everything and that there will be some ambiguities when we are done.”
Genetics is logical and straightforward, and educational sessions are available at conferences for neurologists who are interested in learning more about genetic testing, Dr. Jinnah said. Neurologists also can refer patients to experts. “This referral must be a collaboration,” he said. “People in human genetics are not trained in movement disorders, and many of them have no formal training in neurology. They need your careful phenotypic exam to point them in th
Genetic testing often is expensive, not readily available, and not covered by insurance, but this situation may change, Dr. Jinnah said. He likened the situation of genetic testing today to that of MRI in the 1980s. “Only a few centers in the world had [MRI]. It was extremely expensive. Now, MRI scanners are everywhere, and everybody uses it because it is so valuable in clinical medicine,” he said. “I suspect that in a few years, we will see the same with genetic tests.”
—Jake Remaly
Suggested Reading
Charlesworth G, Bhatia KP, Wood NW. The genetics of dystonia: new twists in an old tale. Brain. 2013;136(Pt 7):2017-2037.
Gahl WA, Tifft CJ. The NIH Undiagnosed Diseases Program: lessons learned. JAMA. 2011;305(18):1904-1905.
Olgiati S, Quadri M, Bonifati V. Genetics of movement disorders in the next-generation sequencing era. Mov Disord. 2016;31(4):458-470.
Sawyer SL, Hartley T, Dyment DA, et al. Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin Genet. 2016;89(3):275-284.
van Egmond ME, Lugtenberg CHA, Brouwer OF, et al. A post hoc study on gene panel analysis for the diagnosis of dystonia. Mov Disord. 2017;32(4):569-575.
VANCOUVER—Researchers’ understanding of genetic causes of movement disorders is advancing rapidly. “The ability to generate data has almost surpassed our ability to understand and use it in the clinic,” said Buz Jinnah, MD, PhD, at the 21st International Congress of Parkinson’s Disease and Movement Disorders.
A recent study underscores the swift evolution of the field. Investigators compared the diagnostic ability of a dystonia gene panel with that of experts who formulated a diagnostic workup without the use of gene panel analysis. In a cohort of 61 patients, the gene panel led to more genetic diagnoses and did so faster and at less cost on average, compared with experts using traditional methods.
Neurologists face practical questions, such as which patients need genetic testing, which tests they should perform, and how the results influence clinical management, said Dr. Jinnah, Professor of Neurology, Human Genetics, and Pediatrics at Emory University School of Medicine in Atlanta. Neurologists once considered Wilson’s disease to have the only genetic cause of movement disorders that significantly changed treatment. Now, researchers have identified more than 30 genetic movement disorders with specific, effective treatments.
Does a Patient Need Genetic Testing?
Not all patients need genetic testing. In some cases, the need for genetic testing is obvious, but in other cases, it is not so clear, Dr. Jinnah said. Certain features increase suspicion for a genetic disorder, such as family history, unusually young age of onset, and combinations of features that fit a recognized syndrome.
Genetic testing also may be warranted in presumed acquired cases. “Methodical studies of these so-called acquired cases reveal a high proportion of genetic explanation for them,” he said. “We typically think of cerebral palsy as an acquired disorder…. But the studies are now showing that about a third of these cases have a genetic explanation.”
Single-gene sequencing, chromosomal microarray, and tests for deletions or insertions are available, as well as next-generation sequencing methods with broader reach. Next-generation techniques include gene panels that can test hundreds of genes associated with a disease, whole-exome sequencing, and whole-genome sequencing.
Choosing a Test
Experts tend to use one of three strategies to select which genetic test to perform. One approach entails identifying red flags—“telltale clinical features that point to a specific diagnosis or group of diagnoses,” said Dr. Jinnah.
Another approach involves pattern recognition. Syndromic combinations may lead a neurologist “straight to a single gene test to confirm the diagnosis” if a patient has a classic presentation of a genetic disease.
The third approach uses diagnostic algorithms. “Depending on the presence or absence of various clinical features … [an algorithm] tells you which gene or collection of genes you need to test for,” he said.
Each strategy has limitations, however. It is difficult to remember all of the red flags and syndromic patterns, and many disorders lack red flags. Atypical syndromes can be misleading, and atypical presentations that have been reported in the literature for decades “continue to surprise us,” Dr. Jinnah said. Researchers have published various diagnostic algorithms for dystonia, parkinsonism, and ataxia, and none of them is perfect.
Several studies have shown the potential of next-generation genetic testing. A report from the NIH’s Undiagnosed Diseases Program found that 25% of patients in the program reached a diagnosis within a few weeks using whole-exome sequencing. “Their conclusion was that these agnostic methods need to be used earlier in the diagnostic process,” he said.
A similar study in Canada evaluated the use of whole-exome sequencing in 362 children with disorders that experts had not been able to diagnose. About 30% of the patients received a definitive diagnosis using whole-exome sequencing.
Clinical Implications
Genetic testing may be important for patient counseling. For instance, if a patient has a family history of Huntington’s disease and develops symptoms suggestive of Huntington’s disease, a normal test result is therapeutic, Dr. Jinnah said. Normal results may be false negatives, however. For example, next-generation sequencing methods are not suitable for detecting certain problems like large insertions, duplications, triplet disorders, and certain genes, and neurologists should consider whether other tests may be useful.
If a genetic test yields a pathologic result, it can inform prognosis and guide family counseling and planning. Furthermore, some inherited disorders have life-altering treatments.
Neurologists are familiar with typical and atypical presentations of Wilson’s disease because the disorder has highly effective treatments. But recent studies have identified lesser-known disorders that may be equally important to recognize. “How many of us are routinely testing these same cases for disorders of manganese transporters?” Dr. Jinnah asked. “It is relatively new information, but the clinical scenario is virtually identical.”
The International Parkinson and Movement Disorder Society’s Rare Movement Disorders Task Force sought to determine how many treatable genetic movement disorders have been identified. The task force found more than 30 disorders with specific treatments—including vitamins, dietary interventions, trigger avoidance, and drugs—that can have “a dramatic impact on the patient’s clinical course,” Dr. Jinnah said. “All of them are quite rare, but we do not want to miss them, because they are treatable.” Investigators identify more treatable disorders each year.
Interpreting Ambiguous Results
Another practical concern for neurologists is the interpretation of variants of unknown significance (VOUS) in genetic test results. “It means that a change or variant in the normal gene was discovered by the laboratory, but the change has never been described in association with the disease that you are interested in,” Dr. Jinnah said. “As a result, the laboratory cannot tell you whether it is relevant or not.”
Neurologists might be inclined to tell a patient that they “found something weird in their DNA,” but this approach “consolidates the patient’s concern that they have some mysterious disorder that none of the doctors can figure out, even the experts,” he said. Nor should neurologists tell a patient that the VOUS is not significant, because that is not what VOUS means.
A VOUS is comparable to a radiology report that says, “Clinical correlation is advised.” That phrase leads neurologists to compare neuroimaging to the clinical phenotype and ask whether the imaging findings make sense. “A VOUS is no different from this,” he said. Neurologists cannot explain all genetic variants, just as neurologists cannot explain all of the spots on a brain scan. “We have to get comfortable with the fact that we do not have answers for everything and that there will be some ambiguities when we are done.”
Genetics is logical and straightforward, and educational sessions are available at conferences for neurologists who are interested in learning more about genetic testing, Dr. Jinnah said. Neurologists also can refer patients to experts. “This referral must be a collaboration,” he said. “People in human genetics are not trained in movement disorders, and many of them have no formal training in neurology. They need your careful phenotypic exam to point them in th
Genetic testing often is expensive, not readily available, and not covered by insurance, but this situation may change, Dr. Jinnah said. He likened the situation of genetic testing today to that of MRI in the 1980s. “Only a few centers in the world had [MRI]. It was extremely expensive. Now, MRI scanners are everywhere, and everybody uses it because it is so valuable in clinical medicine,” he said. “I suspect that in a few years, we will see the same with genetic tests.”
—Jake Remaly
Suggested Reading
Charlesworth G, Bhatia KP, Wood NW. The genetics of dystonia: new twists in an old tale. Brain. 2013;136(Pt 7):2017-2037.
Gahl WA, Tifft CJ. The NIH Undiagnosed Diseases Program: lessons learned. JAMA. 2011;305(18):1904-1905.
Olgiati S, Quadri M, Bonifati V. Genetics of movement disorders in the next-generation sequencing era. Mov Disord. 2016;31(4):458-470.
Sawyer SL, Hartley T, Dyment DA, et al. Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin Genet. 2016;89(3):275-284.
van Egmond ME, Lugtenberg CHA, Brouwer OF, et al. A post hoc study on gene panel analysis for the diagnosis of dystonia. Mov Disord. 2017;32(4):569-575.
Liposomal daunorubicin and cytarabine approved for t-AML, AML-MRC
, the Food and Drug Administration announced on Aug. 3.
Vyxeos is the first FDA-approved treatment specifically for patients with t-AML or AML-MRC, the FDA said in its press release announcing the approval.
“Vyxeos is the first chemotherapy to demonstrate an overall survival advantage over the standard of care in a phase 3 randomized study of older adults with newly-diagnosed therapy-related AML or AML with myelodysplasia-related changes,” Jeffrey E. Lancet, MD, an investigator in the clinical trials of Vyxeos and chair of the department of malignant hematology at the H. Lee Moffitt Cancer Center in Tampa, Fla., said in a press release.
Vyxeos was associated with a median overall survival of 9.6 months and a standard combination of cytarabine and daunorubicin (7+3) was associated with a median survival of 5.9 months in a randomized, multicenter, open-label trial of 309 patients aged 60-75 years with newly-diagnosed t-AML or AML-MRC. Data from the study, which is NCT01696084, was the basis for the drug’s approval.
Vyxeos is a fixed-dose combination with each Vyxeos vial containing 44 mg daunorubicin and 100 mg cytarabine encapsulated together in liposomes. As dosing is based on the daunorubicin component, the corresponding cytarabine dose does not need to be calculated. Daunorubicin dosing is calculated on the basis of body surface area (mg/m2).
For the first induction cycle, the recommended Vyxeos dose is daunorubicin 44 mg/m2 (cytarabine 100 mg/m2) infused intravenously over 90 minutes on days 1, 3, and 5. If a second induction cycle is needed, the same dose is administered on days 1 and 3. The recommended dose of Vyxeos for each cycle of consolidation therapy is daunorubicin 29 mg/m2 (cytarabine 65 mg/m2) liposome via intravenous infusion over 90 minutes on days 1 and 3.
Adverse reactions occurring in at least 25% of treated patients in the clinical trial included hemorrhage, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting.
The prescribing information includes a boxed warning not to substitute Vyxeos with other daunorubicin- or cytarabine-containing products. Full prescribing information is available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/209401s000lbl.pdf
The maker of Vyxeos is Jazz Pharmaceuticals.
mdales@frontlinemedcom.com
On Twitter @maryjodales
, the Food and Drug Administration announced on Aug. 3.
Vyxeos is the first FDA-approved treatment specifically for patients with t-AML or AML-MRC, the FDA said in its press release announcing the approval.
“Vyxeos is the first chemotherapy to demonstrate an overall survival advantage over the standard of care in a phase 3 randomized study of older adults with newly-diagnosed therapy-related AML or AML with myelodysplasia-related changes,” Jeffrey E. Lancet, MD, an investigator in the clinical trials of Vyxeos and chair of the department of malignant hematology at the H. Lee Moffitt Cancer Center in Tampa, Fla., said in a press release.
Vyxeos was associated with a median overall survival of 9.6 months and a standard combination of cytarabine and daunorubicin (7+3) was associated with a median survival of 5.9 months in a randomized, multicenter, open-label trial of 309 patients aged 60-75 years with newly-diagnosed t-AML or AML-MRC. Data from the study, which is NCT01696084, was the basis for the drug’s approval.
Vyxeos is a fixed-dose combination with each Vyxeos vial containing 44 mg daunorubicin and 100 mg cytarabine encapsulated together in liposomes. As dosing is based on the daunorubicin component, the corresponding cytarabine dose does not need to be calculated. Daunorubicin dosing is calculated on the basis of body surface area (mg/m2).
For the first induction cycle, the recommended Vyxeos dose is daunorubicin 44 mg/m2 (cytarabine 100 mg/m2) infused intravenously over 90 minutes on days 1, 3, and 5. If a second induction cycle is needed, the same dose is administered on days 1 and 3. The recommended dose of Vyxeos for each cycle of consolidation therapy is daunorubicin 29 mg/m2 (cytarabine 65 mg/m2) liposome via intravenous infusion over 90 minutes on days 1 and 3.
Adverse reactions occurring in at least 25% of treated patients in the clinical trial included hemorrhage, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting.
The prescribing information includes a boxed warning not to substitute Vyxeos with other daunorubicin- or cytarabine-containing products. Full prescribing information is available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/209401s000lbl.pdf
The maker of Vyxeos is Jazz Pharmaceuticals.
mdales@frontlinemedcom.com
On Twitter @maryjodales
, the Food and Drug Administration announced on Aug. 3.
Vyxeos is the first FDA-approved treatment specifically for patients with t-AML or AML-MRC, the FDA said in its press release announcing the approval.
“Vyxeos is the first chemotherapy to demonstrate an overall survival advantage over the standard of care in a phase 3 randomized study of older adults with newly-diagnosed therapy-related AML or AML with myelodysplasia-related changes,” Jeffrey E. Lancet, MD, an investigator in the clinical trials of Vyxeos and chair of the department of malignant hematology at the H. Lee Moffitt Cancer Center in Tampa, Fla., said in a press release.
Vyxeos was associated with a median overall survival of 9.6 months and a standard combination of cytarabine and daunorubicin (7+3) was associated with a median survival of 5.9 months in a randomized, multicenter, open-label trial of 309 patients aged 60-75 years with newly-diagnosed t-AML or AML-MRC. Data from the study, which is NCT01696084, was the basis for the drug’s approval.
Vyxeos is a fixed-dose combination with each Vyxeos vial containing 44 mg daunorubicin and 100 mg cytarabine encapsulated together in liposomes. As dosing is based on the daunorubicin component, the corresponding cytarabine dose does not need to be calculated. Daunorubicin dosing is calculated on the basis of body surface area (mg/m2).
For the first induction cycle, the recommended Vyxeos dose is daunorubicin 44 mg/m2 (cytarabine 100 mg/m2) infused intravenously over 90 minutes on days 1, 3, and 5. If a second induction cycle is needed, the same dose is administered on days 1 and 3. The recommended dose of Vyxeos for each cycle of consolidation therapy is daunorubicin 29 mg/m2 (cytarabine 65 mg/m2) liposome via intravenous infusion over 90 minutes on days 1 and 3.
Adverse reactions occurring in at least 25% of treated patients in the clinical trial included hemorrhage, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting.
The prescribing information includes a boxed warning not to substitute Vyxeos with other daunorubicin- or cytarabine-containing products. Full prescribing information is available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/209401s000lbl.pdf
The maker of Vyxeos is Jazz Pharmaceuticals.
mdales@frontlinemedcom.com
On Twitter @maryjodales

















