User login
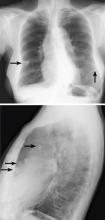
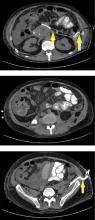
Breast calcifications mimicking pulmonary nodules
On examination, her lung fields were clear, with no audible murmurs, and she had no lower-extremity edema. Her oxygen saturation was 98% on room air.
BREAST CALCIFICATIONS CAN MIMIC PULMONARY NODULES
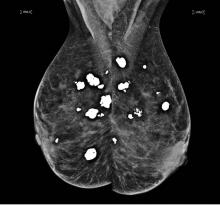
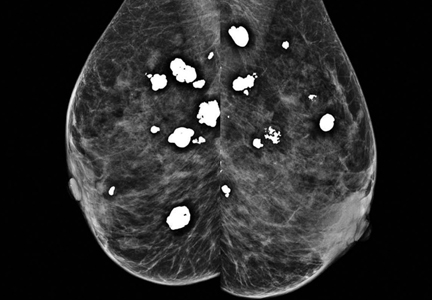
Diffuse bilateral calcifications on mammography are typically benign and represent either dermal calcification (spherical lucent- centered calcification that develops from a degenerative metaplastic process) or fibrocystic changes.1 Up to 10% of women have fibroadenomas, and 19% of fibroadenomas have microcalcifications.2–4 Therefore, given the high prevalence, calcified breast masses should be considered in the differential diagnosis when evaluating initial chest radiographs in women.
Calcifications in the breast can overlie the lung fields and mimic pulmonary nodules. When assessing pulmonary nodules, prior imaging of the chest should always be assessed if available to determine if a lesion is new or has remained stable.
Given our patient’s age and 35-pack-year history of smoking, apparent pulmonary lesions caused concern and prompted chest CT to clarify the diagnosis. However, if the patient has no risk factors for lung malignancy, it can be safe to proceed with mammography.
By including breast calcifications in the differential diagnosis of apparent pulmonary nodules on chest radiography, the clinician can approach the case differently and inquire about a history of fibroadenomas and prior mammograms before pursuing a further workup. This can avoid unnecessary radiation exposure, the costs of CT, and apprehension in the patient raised by unwarranted concern for malignancy.
- Sitzman SB. A useful sign for distinguishing clustered skin calcifications from calcifications within the breast on mammograms. AJR Am J Roentgenol 1992; 158:1407–1408.
- Anastassiades OT, Bouropoulou V, Kontogeorgos G, Rachmanides M, Gogas I. Microcalcifications in benign breast diseases. A histological and histochemical study. Pathol Res Pract 1984; 178:237–242.
- Millis RR, Davis R, Stacey AJ. The detection and significance of calcifications in the breast: a radiological and pathological study. Br J Radiol 1976; 49:12–26.
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353:275–285.
On examination, her lung fields were clear, with no audible murmurs, and she had no lower-extremity edema. Her oxygen saturation was 98% on room air.
BREAST CALCIFICATIONS CAN MIMIC PULMONARY NODULES
Diffuse bilateral calcifications on mammography are typically benign and represent either dermal calcification (spherical lucent- centered calcification that develops from a degenerative metaplastic process) or fibrocystic changes.1 Up to 10% of women have fibroadenomas, and 19% of fibroadenomas have microcalcifications.2–4 Therefore, given the high prevalence, calcified breast masses should be considered in the differential diagnosis when evaluating initial chest radiographs in women.
Calcifications in the breast can overlie the lung fields and mimic pulmonary nodules. When assessing pulmonary nodules, prior imaging of the chest should always be assessed if available to determine if a lesion is new or has remained stable.
Given our patient’s age and 35-pack-year history of smoking, apparent pulmonary lesions caused concern and prompted chest CT to clarify the diagnosis. However, if the patient has no risk factors for lung malignancy, it can be safe to proceed with mammography.
By including breast calcifications in the differential diagnosis of apparent pulmonary nodules on chest radiography, the clinician can approach the case differently and inquire about a history of fibroadenomas and prior mammograms before pursuing a further workup. This can avoid unnecessary radiation exposure, the costs of CT, and apprehension in the patient raised by unwarranted concern for malignancy.
On examination, her lung fields were clear, with no audible murmurs, and she had no lower-extremity edema. Her oxygen saturation was 98% on room air.
BREAST CALCIFICATIONS CAN MIMIC PULMONARY NODULES
Diffuse bilateral calcifications on mammography are typically benign and represent either dermal calcification (spherical lucent- centered calcification that develops from a degenerative metaplastic process) or fibrocystic changes.1 Up to 10% of women have fibroadenomas, and 19% of fibroadenomas have microcalcifications.2–4 Therefore, given the high prevalence, calcified breast masses should be considered in the differential diagnosis when evaluating initial chest radiographs in women.
Calcifications in the breast can overlie the lung fields and mimic pulmonary nodules. When assessing pulmonary nodules, prior imaging of the chest should always be assessed if available to determine if a lesion is new or has remained stable.
Given our patient’s age and 35-pack-year history of smoking, apparent pulmonary lesions caused concern and prompted chest CT to clarify the diagnosis. However, if the patient has no risk factors for lung malignancy, it can be safe to proceed with mammography.
By including breast calcifications in the differential diagnosis of apparent pulmonary nodules on chest radiography, the clinician can approach the case differently and inquire about a history of fibroadenomas and prior mammograms before pursuing a further workup. This can avoid unnecessary radiation exposure, the costs of CT, and apprehension in the patient raised by unwarranted concern for malignancy.
- Sitzman SB. A useful sign for distinguishing clustered skin calcifications from calcifications within the breast on mammograms. AJR Am J Roentgenol 1992; 158:1407–1408.
- Anastassiades OT, Bouropoulou V, Kontogeorgos G, Rachmanides M, Gogas I. Microcalcifications in benign breast diseases. A histological and histochemical study. Pathol Res Pract 1984; 178:237–242.
- Millis RR, Davis R, Stacey AJ. The detection and significance of calcifications in the breast: a radiological and pathological study. Br J Radiol 1976; 49:12–26.
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353:275–285.
- Sitzman SB. A useful sign for distinguishing clustered skin calcifications from calcifications within the breast on mammograms. AJR Am J Roentgenol 1992; 158:1407–1408.
- Anastassiades OT, Bouropoulou V, Kontogeorgos G, Rachmanides M, Gogas I. Microcalcifications in benign breast diseases. A histological and histochemical study. Pathol Res Pract 1984; 178:237–242.
- Millis RR, Davis R, Stacey AJ. The detection and significance of calcifications in the breast: a radiological and pathological study. Br J Radiol 1976; 49:12–26.
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353:275–285.
Necrotizing pancreatitis: Diagnose, treat, consult
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
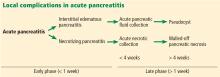
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
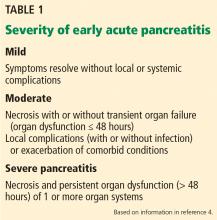
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
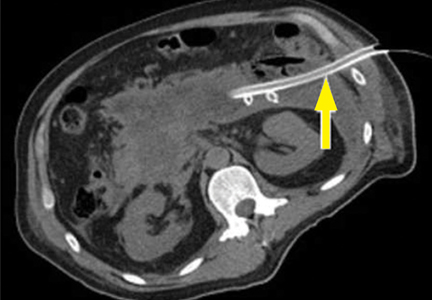
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
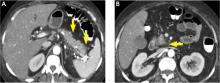
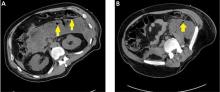
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
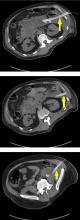
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
KEY POINTS
- Selective and appropriate timing of radiologic imaging is vital in managing necrotizing pancreatitis. Protocols are valuable tools.
- While the primary indication for debridement and drainage in necrotizing pancreatitis is infection, other indications are symptomatic walled-off pancreatic necrosis, intractable abdominal pain, bowel obstruction, and failure to thrive.
- Open surgical necrosectomy remains an important treatment for infected pancreatic necrosis or intractable symptoms.
- A “step-up” approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
Military Brats: Members of a Lost Tribe
Some of you who are reading this column likely are military brats from one branch or another. Many of us felt the call to give back by either joining the military, PHS, or working in organizations like the VA, treating former service members. That certainly was a huge motivation for me to become a VA physician. I always felt more welcomed, even felt at home, at the VA or at a military hospital than I did at any civilian health care facility. And many of my colleagues feel the same way. Other brats have never interacted much with the military except for their being raised by family members in the armed forces; yet this designation is still a part of their identity,
The percentage of adults > 50 years old who have an immediate family member who served in the military is 77%; the percentage of those aged 30 to 49 years is 57%; and aged < 30 years, only 33%.1 Almost 5% of adult Americans are military brats. This demographic trend brings with it an increasing chance that current and former service members may feel socially isolated and that many health care professionals will struggle to relate to, and appreciate, their unique cultural background.
Authors always should acknowledge any material conflict of interest, and as a double Army brat, I am far from objective on this subject. I was born and raised on an army base. My father was a career military physician, and my mother, albeit briefly, was an army nurse. Some of my earliest memories are of being with my father and driving around Fort Sam Houston when everyone and everything stopped upon hearing the sound of a bugle (at the time, it was still a real bugle). My father and I would get out of the car. He would salute, and I would stand as still as a small active child can while we turned toward the flag being lowered over the base.
In reading about army brats, this memory seems to be a common one. Many individuals have commented on how this repeated experience from their youth instilled in them a sense of respect for our flag and country and an appreciation of order and discipline that stayed with them long after they became adults.
Obviously, while those of us claiming this identity use it positively as a phrase of winsome nostalgia and civic pride, in everyday language a brat is a pejorative reference. The online magazine Military Brat Life, defines the term as “someone, who, as a child, grows up in a family where one or more parents are ‘career’ military, and where the children move from base to base, experiencing life in several different places and possibly different countries.”2 The phrase denotes an individual whose parents at some point served full-time in the military, no duration is specified or whether the parents had to be active duty, reserve or National Guard members. The prefix for the label comes from the military branch in which the parents primarily served, though like hyphenated names some younger generations will introduce themselves as a Navy-Air Force brat. Other sites suggest that it doesn’t refer to a spoiled child at all but actually is yet another of the acronyms that proliferate in military environments. Although after I read these possible theories, many seemed retrospective attempts to jettison the negative connotations.
I learned that like others sharing similar formative experiences, military brats are considered a subculture or a third culture, in some of the literature. There is a dearth of scholarly data about the phenomenology and social psychology of adults who spent some of their formative years under the auspices of military culture. As in any foray into cultural competence, avoiding stereotypes is crucial. However, research has shown that the experience of growing up in the military is one that bestows resilience and risk.3 It is also an important piece of a patient’s narrative that health care professionals in and out of the federal system should consider to provide patient-centered care.
A childhood in a military environment is often romanticized as shaping an adult who is worldly, cosmopolitan, resilient, and tolerant. Although these are adaptive traits that children of military personnel develop, there also is a far darker side emerging in the research.4 We are all too aware of the epidemic of suicide, opioid use, and posttraumatic stress disorder that has developed in the wake of our country’s latest and lasting conflicts. The reverberations of these mental health problems are felt by the children who lived through them or who lost loved ones to war or suicide. The DoD has begun recognizing this collateral damage and is developing innovative programs to help children and adolescents.
We need to do more though, not just in this arena when the wounds occur, but also later when those wounded come to nurse practitioners and psychologists, social workers, and physicians. Our growing number of community partners through Choice and other programs also need to be aware of the potential mental health impacts of being a military brat or family member.
In the introduction to one of the best books written on the subject, Military Brats: Legacies of Childhood Inside the Fortress, author Pat Conroy wrote, “I thought I was singular in all this, one of a kind.... I discovered that I speak in the multitongued, deep-throated voice of my tribe. It’s a language I was not even aware I spoke... a secret family I did not know I had.... Military brats, my lost tribe, spent their entire youth in service to this country, and no one even knew we were there.”5
1. Pew Research Center. The military-civilian gap: fewer family connections. http://www.pewsocialtrends.org/2011/11/23/the-military-civilian-gap-fewer-family-connections. Published November 23, 2011. Accessed July 12, 2017.
2. Baker V. What is a military brat? http://militarybratlife.com/what-is-a-military-brat. Published January 22, 2015. Accessed July 13, 2017.
3. Park N. Military children and families: strengths and challenges in war and peace. Am Psychol. 2011;66(1):65-72.
4. McGuire AC, Kanesarajah J, Runge CE, Ireland R, Waller M, Dobson AJ. Effect of multiple deployments on military families: a cross-sectional study of health and well-being of partners and children. Mill Med. 2016;181(4):319-327.
5. Wertsch ME. Military Brats: Legacies of Childhood Inside the Fortress. St. Louis, MO: Brightwell Publishing; 2011.
Some of you who are reading this column likely are military brats from one branch or another. Many of us felt the call to give back by either joining the military, PHS, or working in organizations like the VA, treating former service members. That certainly was a huge motivation for me to become a VA physician. I always felt more welcomed, even felt at home, at the VA or at a military hospital than I did at any civilian health care facility. And many of my colleagues feel the same way. Other brats have never interacted much with the military except for their being raised by family members in the armed forces; yet this designation is still a part of their identity,
The percentage of adults > 50 years old who have an immediate family member who served in the military is 77%; the percentage of those aged 30 to 49 years is 57%; and aged < 30 years, only 33%.1 Almost 5% of adult Americans are military brats. This demographic trend brings with it an increasing chance that current and former service members may feel socially isolated and that many health care professionals will struggle to relate to, and appreciate, their unique cultural background.
Authors always should acknowledge any material conflict of interest, and as a double Army brat, I am far from objective on this subject. I was born and raised on an army base. My father was a career military physician, and my mother, albeit briefly, was an army nurse. Some of my earliest memories are of being with my father and driving around Fort Sam Houston when everyone and everything stopped upon hearing the sound of a bugle (at the time, it was still a real bugle). My father and I would get out of the car. He would salute, and I would stand as still as a small active child can while we turned toward the flag being lowered over the base.
In reading about army brats, this memory seems to be a common one. Many individuals have commented on how this repeated experience from their youth instilled in them a sense of respect for our flag and country and an appreciation of order and discipline that stayed with them long after they became adults.
Obviously, while those of us claiming this identity use it positively as a phrase of winsome nostalgia and civic pride, in everyday language a brat is a pejorative reference. The online magazine Military Brat Life, defines the term as “someone, who, as a child, grows up in a family where one or more parents are ‘career’ military, and where the children move from base to base, experiencing life in several different places and possibly different countries.”2 The phrase denotes an individual whose parents at some point served full-time in the military, no duration is specified or whether the parents had to be active duty, reserve or National Guard members. The prefix for the label comes from the military branch in which the parents primarily served, though like hyphenated names some younger generations will introduce themselves as a Navy-Air Force brat. Other sites suggest that it doesn’t refer to a spoiled child at all but actually is yet another of the acronyms that proliferate in military environments. Although after I read these possible theories, many seemed retrospective attempts to jettison the negative connotations.
I learned that like others sharing similar formative experiences, military brats are considered a subculture or a third culture, in some of the literature. There is a dearth of scholarly data about the phenomenology and social psychology of adults who spent some of their formative years under the auspices of military culture. As in any foray into cultural competence, avoiding stereotypes is crucial. However, research has shown that the experience of growing up in the military is one that bestows resilience and risk.3 It is also an important piece of a patient’s narrative that health care professionals in and out of the federal system should consider to provide patient-centered care.
A childhood in a military environment is often romanticized as shaping an adult who is worldly, cosmopolitan, resilient, and tolerant. Although these are adaptive traits that children of military personnel develop, there also is a far darker side emerging in the research.4 We are all too aware of the epidemic of suicide, opioid use, and posttraumatic stress disorder that has developed in the wake of our country’s latest and lasting conflicts. The reverberations of these mental health problems are felt by the children who lived through them or who lost loved ones to war or suicide. The DoD has begun recognizing this collateral damage and is developing innovative programs to help children and adolescents.
We need to do more though, not just in this arena when the wounds occur, but also later when those wounded come to nurse practitioners and psychologists, social workers, and physicians. Our growing number of community partners through Choice and other programs also need to be aware of the potential mental health impacts of being a military brat or family member.
In the introduction to one of the best books written on the subject, Military Brats: Legacies of Childhood Inside the Fortress, author Pat Conroy wrote, “I thought I was singular in all this, one of a kind.... I discovered that I speak in the multitongued, deep-throated voice of my tribe. It’s a language I was not even aware I spoke... a secret family I did not know I had.... Military brats, my lost tribe, spent their entire youth in service to this country, and no one even knew we were there.”5
Some of you who are reading this column likely are military brats from one branch or another. Many of us felt the call to give back by either joining the military, PHS, or working in organizations like the VA, treating former service members. That certainly was a huge motivation for me to become a VA physician. I always felt more welcomed, even felt at home, at the VA or at a military hospital than I did at any civilian health care facility. And many of my colleagues feel the same way. Other brats have never interacted much with the military except for their being raised by family members in the armed forces; yet this designation is still a part of their identity,
The percentage of adults > 50 years old who have an immediate family member who served in the military is 77%; the percentage of those aged 30 to 49 years is 57%; and aged < 30 years, only 33%.1 Almost 5% of adult Americans are military brats. This demographic trend brings with it an increasing chance that current and former service members may feel socially isolated and that many health care professionals will struggle to relate to, and appreciate, their unique cultural background.
Authors always should acknowledge any material conflict of interest, and as a double Army brat, I am far from objective on this subject. I was born and raised on an army base. My father was a career military physician, and my mother, albeit briefly, was an army nurse. Some of my earliest memories are of being with my father and driving around Fort Sam Houston when everyone and everything stopped upon hearing the sound of a bugle (at the time, it was still a real bugle). My father and I would get out of the car. He would salute, and I would stand as still as a small active child can while we turned toward the flag being lowered over the base.
In reading about army brats, this memory seems to be a common one. Many individuals have commented on how this repeated experience from their youth instilled in them a sense of respect for our flag and country and an appreciation of order and discipline that stayed with them long after they became adults.
Obviously, while those of us claiming this identity use it positively as a phrase of winsome nostalgia and civic pride, in everyday language a brat is a pejorative reference. The online magazine Military Brat Life, defines the term as “someone, who, as a child, grows up in a family where one or more parents are ‘career’ military, and where the children move from base to base, experiencing life in several different places and possibly different countries.”2 The phrase denotes an individual whose parents at some point served full-time in the military, no duration is specified or whether the parents had to be active duty, reserve or National Guard members. The prefix for the label comes from the military branch in which the parents primarily served, though like hyphenated names some younger generations will introduce themselves as a Navy-Air Force brat. Other sites suggest that it doesn’t refer to a spoiled child at all but actually is yet another of the acronyms that proliferate in military environments. Although after I read these possible theories, many seemed retrospective attempts to jettison the negative connotations.
I learned that like others sharing similar formative experiences, military brats are considered a subculture or a third culture, in some of the literature. There is a dearth of scholarly data about the phenomenology and social psychology of adults who spent some of their formative years under the auspices of military culture. As in any foray into cultural competence, avoiding stereotypes is crucial. However, research has shown that the experience of growing up in the military is one that bestows resilience and risk.3 It is also an important piece of a patient’s narrative that health care professionals in and out of the federal system should consider to provide patient-centered care.
A childhood in a military environment is often romanticized as shaping an adult who is worldly, cosmopolitan, resilient, and tolerant. Although these are adaptive traits that children of military personnel develop, there also is a far darker side emerging in the research.4 We are all too aware of the epidemic of suicide, opioid use, and posttraumatic stress disorder that has developed in the wake of our country’s latest and lasting conflicts. The reverberations of these mental health problems are felt by the children who lived through them or who lost loved ones to war or suicide. The DoD has begun recognizing this collateral damage and is developing innovative programs to help children and adolescents.
We need to do more though, not just in this arena when the wounds occur, but also later when those wounded come to nurse practitioners and psychologists, social workers, and physicians. Our growing number of community partners through Choice and other programs also need to be aware of the potential mental health impacts of being a military brat or family member.
In the introduction to one of the best books written on the subject, Military Brats: Legacies of Childhood Inside the Fortress, author Pat Conroy wrote, “I thought I was singular in all this, one of a kind.... I discovered that I speak in the multitongued, deep-throated voice of my tribe. It’s a language I was not even aware I spoke... a secret family I did not know I had.... Military brats, my lost tribe, spent their entire youth in service to this country, and no one even knew we were there.”5
1. Pew Research Center. The military-civilian gap: fewer family connections. http://www.pewsocialtrends.org/2011/11/23/the-military-civilian-gap-fewer-family-connections. Published November 23, 2011. Accessed July 12, 2017.
2. Baker V. What is a military brat? http://militarybratlife.com/what-is-a-military-brat. Published January 22, 2015. Accessed July 13, 2017.
3. Park N. Military children and families: strengths and challenges in war and peace. Am Psychol. 2011;66(1):65-72.
4. McGuire AC, Kanesarajah J, Runge CE, Ireland R, Waller M, Dobson AJ. Effect of multiple deployments on military families: a cross-sectional study of health and well-being of partners and children. Mill Med. 2016;181(4):319-327.
5. Wertsch ME. Military Brats: Legacies of Childhood Inside the Fortress. St. Louis, MO: Brightwell Publishing; 2011.
1. Pew Research Center. The military-civilian gap: fewer family connections. http://www.pewsocialtrends.org/2011/11/23/the-military-civilian-gap-fewer-family-connections. Published November 23, 2011. Accessed July 12, 2017.
2. Baker V. What is a military brat? http://militarybratlife.com/what-is-a-military-brat. Published January 22, 2015. Accessed July 13, 2017.
3. Park N. Military children and families: strengths and challenges in war and peace. Am Psychol. 2011;66(1):65-72.
4. McGuire AC, Kanesarajah J, Runge CE, Ireland R, Waller M, Dobson AJ. Effect of multiple deployments on military families: a cross-sectional study of health and well-being of partners and children. Mill Med. 2016;181(4):319-327.
5. Wertsch ME. Military Brats: Legacies of Childhood Inside the Fortress. St. Louis, MO: Brightwell Publishing; 2011.
Studies support early use of genetic tests in early childhood disorders
Results from two new studies suggest that genetic testing early in the diagnostic pathway may allow for earlier and more precise diagnoses in early-life epilepsies and a range of other childhood-onset disorders, and potentially limit costs associated with a long diagnostic course.
Both papers, published online July 31 in JAMA Pediatrics, showed the diagnostic yield of genetic testing approaches, including whole-exome sequencing (WES), to be high.
The results also argue for the incorporation of genetic testing into the first diagnostic assessments; not limiting it to severe presentations only; and for broad testing methods to be employed in lieu of narrower ones.
Of these patients, just under half (n = 327) underwent various forms of genetic testing at the discretion of the treating physician, including karyotyping, microarrays, epilepsy gene panels, WES, mitochondrial panels, and other tests. Pathogenic variants were discovered in 132 children, or 40% of those receiving genetic testing (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1743).
Of all the genetic testing methods employed in the study, diagnostic yields were significantly greater for epilepsy gene panels (29.2%) and WES (27.8%), compared with chromosome microarray (7.9%).
The results, the investigators said, provide “added impetus to move the diagnosis of the specific cause to the point of initial presentation ... it is time to provide greater emphasis on and support for thorough genetic evaluations, particularly sequencing-based evaluations, for children with newly presenting epilepsies in the first few years of life.”
In addition to aiding management decisions, early genetic testing “ends the diagnostic odyssey during which parents and physicians spend untold amounts of time searching for an explanation for a child’s epilepsy and reduces associated costs,” Dr. Berg and her colleagues concluded.
In a separate study led by Tiong Yang Tan, MBBS, PhD, of Victorian Clinical Genetics Services in Melbourne, Australia, and his colleagues, singleton WES was used in 44 children recruited at outpatient clinics of a Melbourne hospital system (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1755).
Children in the study were aged 2-18 years (with mean age at presentation 28 months) and had a wide variety of suspected genetic disorders, including skeletal, skin, neurometabolic, and intellectual disorders. Some of these had features overlapping several conditions. The children in the cohort had not received prior genetic testing before undergoing WES.
The molecular test resulted in a diagnosis in 52% (n = 23) of the children, including unexpected diagnoses in eight of these. Clinical management was altered as result of sequencing findings in six children.
“Although phenotyping is critical, 35% of children had a diagnosis caused by a gene outside the initially prioritized gene list. This finding not only possibly reflects lack of clinical recognition but also underscores the utility of WES in achieving a diagnosis even when the a priori hypothesis is imprecise,” Dr. Tan and his associates wrote in their analysis.
Dr. Tan and his colleagues conducted a cost analysis that found WES performed at initial tertiary presentation resulted in a cost savings of U.S. $6,838 per additional diagnosis (95% confidence interval, U.S. $3,263-$11,678), compared with the standard diagnostic pathway. The figures reflect costs in an Australian care setting.
The children in the study had a mean diagnostic odyssey of 6 years, including a mean of 19 tests and four clinical genetics and four non–genetics specialist consultations. A quarter of them had undergone at least one diagnostic procedure under general anesthesia.
“The diagnostic odyssey of children suspected of having monogenic disorders is protracted and painful and may not provide a precise diagnosis,” Dr. Tan and his colleagues wrote in their analysis. “This paradigm has markedly shifted with the advent of WES.”
WES is best targeted to children “with genetically heterogeneous disorders or features overlapping several conditions,” the investigators concluded. “Our findings suggest that these children are best served by early recognition by their pediatrician and expedited referral to clinical genetics with WES applied after chromosomal microarray but before an extensive diagnostic process.”
Dr. Tan and his colleagues’ study was funded by the Melbourne Genomics Health Alliance and state and national governments in Australia. None of the authors declared conflicts of interest. Dr. Berg and her colleagues’ study was funded by the Pediatric Epilepsy Research Foundation, and none of its authors disclosed commercial conflicts of interest.
The studies by Tan et al. and Berg et al. demonstrate the dramatic effect of the diagnostic yield of different genetic testing approaches on cost-effectiveness and the potential design of testing strategies in children with suspected monogenic conditions. Both studies emphasize the effect of the results of genetic testing. Whereas Tan et al. showed that, in 26% of cases, the result enabled a specific modification of patient care, Berg et al. also demonstrated that there is no basis for identifying optimal, targeted treatments, when testing is not performed and genetic diagnoses are not made.
However, in the absence of targeted treatments, a genetic diagnosis is of high value for the patients, their families, and treating physicians. A clear diagnosis may not only be of prognostic value but also put an end to a possibly stressful and demanding diagnostic odyssey. It may enable patient care that is explicitly focused on the individual needs of the patient. A clear diagnosis usually also allows a better assessment of the risks of recurrence in the family and possibly enables prenatal testing in relatives. Finally, it enables research and a better scientific understanding of the underlying pathophysiology, which may ideally lead to the identification of novel therapeutic prospects. Seven years ago, an international consensus statement endorsed the replacement of classic cytogenetic karyotype analysis by chromosomal microarrays as a first-tier diagnostic test in individuals with developmental disabilities or congenital anomalies. The studies add to the growing evidence that this consensus may already be outdated, as high-throughput sequencing techniques may achieve even higher diagnostic yields and, thus, are capable to become the new first-tier diagnostic test in congenital and early-onset disorders.
Johannes R. Lemke, MD, is with the Institute of Human Genetics at the University of Leipzig (Germany) Hospitals and Clinics. He reports no conflicts of interest associated with his editorial, which accompanied the JAMA Pediatrics reports (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1743).
The studies by Tan et al. and Berg et al. demonstrate the dramatic effect of the diagnostic yield of different genetic testing approaches on cost-effectiveness and the potential design of testing strategies in children with suspected monogenic conditions. Both studies emphasize the effect of the results of genetic testing. Whereas Tan et al. showed that, in 26% of cases, the result enabled a specific modification of patient care, Berg et al. also demonstrated that there is no basis for identifying optimal, targeted treatments, when testing is not performed and genetic diagnoses are not made.
However, in the absence of targeted treatments, a genetic diagnosis is of high value for the patients, their families, and treating physicians. A clear diagnosis may not only be of prognostic value but also put an end to a possibly stressful and demanding diagnostic odyssey. It may enable patient care that is explicitly focused on the individual needs of the patient. A clear diagnosis usually also allows a better assessment of the risks of recurrence in the family and possibly enables prenatal testing in relatives. Finally, it enables research and a better scientific understanding of the underlying pathophysiology, which may ideally lead to the identification of novel therapeutic prospects. Seven years ago, an international consensus statement endorsed the replacement of classic cytogenetic karyotype analysis by chromosomal microarrays as a first-tier diagnostic test in individuals with developmental disabilities or congenital anomalies. The studies add to the growing evidence that this consensus may already be outdated, as high-throughput sequencing techniques may achieve even higher diagnostic yields and, thus, are capable to become the new first-tier diagnostic test in congenital and early-onset disorders.
Johannes R. Lemke, MD, is with the Institute of Human Genetics at the University of Leipzig (Germany) Hospitals and Clinics. He reports no conflicts of interest associated with his editorial, which accompanied the JAMA Pediatrics reports (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1743).
The studies by Tan et al. and Berg et al. demonstrate the dramatic effect of the diagnostic yield of different genetic testing approaches on cost-effectiveness and the potential design of testing strategies in children with suspected monogenic conditions. Both studies emphasize the effect of the results of genetic testing. Whereas Tan et al. showed that, in 26% of cases, the result enabled a specific modification of patient care, Berg et al. also demonstrated that there is no basis for identifying optimal, targeted treatments, when testing is not performed and genetic diagnoses are not made.
However, in the absence of targeted treatments, a genetic diagnosis is of high value for the patients, their families, and treating physicians. A clear diagnosis may not only be of prognostic value but also put an end to a possibly stressful and demanding diagnostic odyssey. It may enable patient care that is explicitly focused on the individual needs of the patient. A clear diagnosis usually also allows a better assessment of the risks of recurrence in the family and possibly enables prenatal testing in relatives. Finally, it enables research and a better scientific understanding of the underlying pathophysiology, which may ideally lead to the identification of novel therapeutic prospects. Seven years ago, an international consensus statement endorsed the replacement of classic cytogenetic karyotype analysis by chromosomal microarrays as a first-tier diagnostic test in individuals with developmental disabilities or congenital anomalies. The studies add to the growing evidence that this consensus may already be outdated, as high-throughput sequencing techniques may achieve even higher diagnostic yields and, thus, are capable to become the new first-tier diagnostic test in congenital and early-onset disorders.
Johannes R. Lemke, MD, is with the Institute of Human Genetics at the University of Leipzig (Germany) Hospitals and Clinics. He reports no conflicts of interest associated with his editorial, which accompanied the JAMA Pediatrics reports (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1743).
Results from two new studies suggest that genetic testing early in the diagnostic pathway may allow for earlier and more precise diagnoses in early-life epilepsies and a range of other childhood-onset disorders, and potentially limit costs associated with a long diagnostic course.
Both papers, published online July 31 in JAMA Pediatrics, showed the diagnostic yield of genetic testing approaches, including whole-exome sequencing (WES), to be high.
The results also argue for the incorporation of genetic testing into the first diagnostic assessments; not limiting it to severe presentations only; and for broad testing methods to be employed in lieu of narrower ones.
Of these patients, just under half (n = 327) underwent various forms of genetic testing at the discretion of the treating physician, including karyotyping, microarrays, epilepsy gene panels, WES, mitochondrial panels, and other tests. Pathogenic variants were discovered in 132 children, or 40% of those receiving genetic testing (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1743).
Of all the genetic testing methods employed in the study, diagnostic yields were significantly greater for epilepsy gene panels (29.2%) and WES (27.8%), compared with chromosome microarray (7.9%).
The results, the investigators said, provide “added impetus to move the diagnosis of the specific cause to the point of initial presentation ... it is time to provide greater emphasis on and support for thorough genetic evaluations, particularly sequencing-based evaluations, for children with newly presenting epilepsies in the first few years of life.”
In addition to aiding management decisions, early genetic testing “ends the diagnostic odyssey during which parents and physicians spend untold amounts of time searching for an explanation for a child’s epilepsy and reduces associated costs,” Dr. Berg and her colleagues concluded.
In a separate study led by Tiong Yang Tan, MBBS, PhD, of Victorian Clinical Genetics Services in Melbourne, Australia, and his colleagues, singleton WES was used in 44 children recruited at outpatient clinics of a Melbourne hospital system (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1755).
Children in the study were aged 2-18 years (with mean age at presentation 28 months) and had a wide variety of suspected genetic disorders, including skeletal, skin, neurometabolic, and intellectual disorders. Some of these had features overlapping several conditions. The children in the cohort had not received prior genetic testing before undergoing WES.
The molecular test resulted in a diagnosis in 52% (n = 23) of the children, including unexpected diagnoses in eight of these. Clinical management was altered as result of sequencing findings in six children.
“Although phenotyping is critical, 35% of children had a diagnosis caused by a gene outside the initially prioritized gene list. This finding not only possibly reflects lack of clinical recognition but also underscores the utility of WES in achieving a diagnosis even when the a priori hypothesis is imprecise,” Dr. Tan and his associates wrote in their analysis.
Dr. Tan and his colleagues conducted a cost analysis that found WES performed at initial tertiary presentation resulted in a cost savings of U.S. $6,838 per additional diagnosis (95% confidence interval, U.S. $3,263-$11,678), compared with the standard diagnostic pathway. The figures reflect costs in an Australian care setting.
The children in the study had a mean diagnostic odyssey of 6 years, including a mean of 19 tests and four clinical genetics and four non–genetics specialist consultations. A quarter of them had undergone at least one diagnostic procedure under general anesthesia.
“The diagnostic odyssey of children suspected of having monogenic disorders is protracted and painful and may not provide a precise diagnosis,” Dr. Tan and his colleagues wrote in their analysis. “This paradigm has markedly shifted with the advent of WES.”
WES is best targeted to children “with genetically heterogeneous disorders or features overlapping several conditions,” the investigators concluded. “Our findings suggest that these children are best served by early recognition by their pediatrician and expedited referral to clinical genetics with WES applied after chromosomal microarray but before an extensive diagnostic process.”
Dr. Tan and his colleagues’ study was funded by the Melbourne Genomics Health Alliance and state and national governments in Australia. None of the authors declared conflicts of interest. Dr. Berg and her colleagues’ study was funded by the Pediatric Epilepsy Research Foundation, and none of its authors disclosed commercial conflicts of interest.
Results from two new studies suggest that genetic testing early in the diagnostic pathway may allow for earlier and more precise diagnoses in early-life epilepsies and a range of other childhood-onset disorders, and potentially limit costs associated with a long diagnostic course.
Both papers, published online July 31 in JAMA Pediatrics, showed the diagnostic yield of genetic testing approaches, including whole-exome sequencing (WES), to be high.
The results also argue for the incorporation of genetic testing into the first diagnostic assessments; not limiting it to severe presentations only; and for broad testing methods to be employed in lieu of narrower ones.
Of these patients, just under half (n = 327) underwent various forms of genetic testing at the discretion of the treating physician, including karyotyping, microarrays, epilepsy gene panels, WES, mitochondrial panels, and other tests. Pathogenic variants were discovered in 132 children, or 40% of those receiving genetic testing (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1743).
Of all the genetic testing methods employed in the study, diagnostic yields were significantly greater for epilepsy gene panels (29.2%) and WES (27.8%), compared with chromosome microarray (7.9%).
The results, the investigators said, provide “added impetus to move the diagnosis of the specific cause to the point of initial presentation ... it is time to provide greater emphasis on and support for thorough genetic evaluations, particularly sequencing-based evaluations, for children with newly presenting epilepsies in the first few years of life.”
In addition to aiding management decisions, early genetic testing “ends the diagnostic odyssey during which parents and physicians spend untold amounts of time searching for an explanation for a child’s epilepsy and reduces associated costs,” Dr. Berg and her colleagues concluded.
In a separate study led by Tiong Yang Tan, MBBS, PhD, of Victorian Clinical Genetics Services in Melbourne, Australia, and his colleagues, singleton WES was used in 44 children recruited at outpatient clinics of a Melbourne hospital system (JAMA Pediatr. 2017 July 31. doi: 10.1001/jamapediatrics.2017.1755).
Children in the study were aged 2-18 years (with mean age at presentation 28 months) and had a wide variety of suspected genetic disorders, including skeletal, skin, neurometabolic, and intellectual disorders. Some of these had features overlapping several conditions. The children in the cohort had not received prior genetic testing before undergoing WES.
The molecular test resulted in a diagnosis in 52% (n = 23) of the children, including unexpected diagnoses in eight of these. Clinical management was altered as result of sequencing findings in six children.
“Although phenotyping is critical, 35% of children had a diagnosis caused by a gene outside the initially prioritized gene list. This finding not only possibly reflects lack of clinical recognition but also underscores the utility of WES in achieving a diagnosis even when the a priori hypothesis is imprecise,” Dr. Tan and his associates wrote in their analysis.
Dr. Tan and his colleagues conducted a cost analysis that found WES performed at initial tertiary presentation resulted in a cost savings of U.S. $6,838 per additional diagnosis (95% confidence interval, U.S. $3,263-$11,678), compared with the standard diagnostic pathway. The figures reflect costs in an Australian care setting.
The children in the study had a mean diagnostic odyssey of 6 years, including a mean of 19 tests and four clinical genetics and four non–genetics specialist consultations. A quarter of them had undergone at least one diagnostic procedure under general anesthesia.
“The diagnostic odyssey of children suspected of having monogenic disorders is protracted and painful and may not provide a precise diagnosis,” Dr. Tan and his colleagues wrote in their analysis. “This paradigm has markedly shifted with the advent of WES.”
WES is best targeted to children “with genetically heterogeneous disorders or features overlapping several conditions,” the investigators concluded. “Our findings suggest that these children are best served by early recognition by their pediatrician and expedited referral to clinical genetics with WES applied after chromosomal microarray but before an extensive diagnostic process.”
Dr. Tan and his colleagues’ study was funded by the Melbourne Genomics Health Alliance and state and national governments in Australia. None of the authors declared conflicts of interest. Dr. Berg and her colleagues’ study was funded by the Pediatric Epilepsy Research Foundation, and none of its authors disclosed commercial conflicts of interest.
FROM JAMA PEDIATRICS
Which Acute Myeloid Leukemia Patients are Good Immunotherapy Candidates?
Some patients with acute myeloid leukemia (AML) may have trouble with immunotherapy following chemotherapy. Researchers from the National Heart, Lung and Blood Institute may have found a reason why.
Related: Novel Treatment Shows Promise for Acute Lymphoblastic Leukemia
The researchers wanted to perform a “deep assessment” of the state of the adaptive immune system in AML patients in remission after chemotherapy. They used these patients’ response to seasonal influenza vaccination as a surrogate for the robustness of the immune system. The researchers say their approach was unique in that they established a comprehensive picture of the adaptive “immunome” by simultaneously examining the genetic, phenotypic, and functional consequences of chemotherapy.
Their assessment revealed a “dramatic impact” in the B-cell compartment, which appeared slower to recover than the T-cell compartment. Of 10 patients in the study, only 2 generated protective titers in response to vaccination. Most had abnormal frequencies of transitional and memory B-cells. The researchers say the inability of AML patients to produce protective antibody titers in response to influenza vaccination is likely due to multiple B-cell abnormalities.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
The researchers “strikingly” found similar patterns of immune dysfunction across all the patients in the study. When they ranked patients based on time elapsed since chemotherapy, the degree of dysfunction was shown to be less in patients who had the most time elapsed form their chemotherapy treatment.
The researchers conclude the “consistent finding” of a reduction of memory B-cells in all the AML patients suggests that humoral immunity reconstitution is “a very long process.” They add that a better understanding of the changes in adaptive immune cell subsets after chemotherapy will be useful in designing immunotherapies that can work with existing immune capacity.
Source:
Goswami M, Prince G, Biancotto A, et al. J Transl Med. 2017;15:155.
doi: 10.1186/s12967-017-1252-2
Some patients with acute myeloid leukemia (AML) may have trouble with immunotherapy following chemotherapy. Researchers from the National Heart, Lung and Blood Institute may have found a reason why.
Related: Novel Treatment Shows Promise for Acute Lymphoblastic Leukemia
The researchers wanted to perform a “deep assessment” of the state of the adaptive immune system in AML patients in remission after chemotherapy. They used these patients’ response to seasonal influenza vaccination as a surrogate for the robustness of the immune system. The researchers say their approach was unique in that they established a comprehensive picture of the adaptive “immunome” by simultaneously examining the genetic, phenotypic, and functional consequences of chemotherapy.
Their assessment revealed a “dramatic impact” in the B-cell compartment, which appeared slower to recover than the T-cell compartment. Of 10 patients in the study, only 2 generated protective titers in response to vaccination. Most had abnormal frequencies of transitional and memory B-cells. The researchers say the inability of AML patients to produce protective antibody titers in response to influenza vaccination is likely due to multiple B-cell abnormalities.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
The researchers “strikingly” found similar patterns of immune dysfunction across all the patients in the study. When they ranked patients based on time elapsed since chemotherapy, the degree of dysfunction was shown to be less in patients who had the most time elapsed form their chemotherapy treatment.
The researchers conclude the “consistent finding” of a reduction of memory B-cells in all the AML patients suggests that humoral immunity reconstitution is “a very long process.” They add that a better understanding of the changes in adaptive immune cell subsets after chemotherapy will be useful in designing immunotherapies that can work with existing immune capacity.
Source:
Goswami M, Prince G, Biancotto A, et al. J Transl Med. 2017;15:155.
doi: 10.1186/s12967-017-1252-2
Some patients with acute myeloid leukemia (AML) may have trouble with immunotherapy following chemotherapy. Researchers from the National Heart, Lung and Blood Institute may have found a reason why.
Related: Novel Treatment Shows Promise for Acute Lymphoblastic Leukemia
The researchers wanted to perform a “deep assessment” of the state of the adaptive immune system in AML patients in remission after chemotherapy. They used these patients’ response to seasonal influenza vaccination as a surrogate for the robustness of the immune system. The researchers say their approach was unique in that they established a comprehensive picture of the adaptive “immunome” by simultaneously examining the genetic, phenotypic, and functional consequences of chemotherapy.
Their assessment revealed a “dramatic impact” in the B-cell compartment, which appeared slower to recover than the T-cell compartment. Of 10 patients in the study, only 2 generated protective titers in response to vaccination. Most had abnormal frequencies of transitional and memory B-cells. The researchers say the inability of AML patients to produce protective antibody titers in response to influenza vaccination is likely due to multiple B-cell abnormalities.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
The researchers “strikingly” found similar patterns of immune dysfunction across all the patients in the study. When they ranked patients based on time elapsed since chemotherapy, the degree of dysfunction was shown to be less in patients who had the most time elapsed form their chemotherapy treatment.
The researchers conclude the “consistent finding” of a reduction of memory B-cells in all the AML patients suggests that humoral immunity reconstitution is “a very long process.” They add that a better understanding of the changes in adaptive immune cell subsets after chemotherapy will be useful in designing immunotherapies that can work with existing immune capacity.
Source:
Goswami M, Prince G, Biancotto A, et al. J Transl Med. 2017;15:155.
doi: 10.1186/s12967-017-1252-2
August 2017 Digital Edition
Click here to access the August 2017 Digital Edition.
Table of Contents
- Collaboration of the NIH and PHS Commissioned Corps in the International Ebola Clinical Research Response
- Parkinsonism and Vitamin C Deficiency
- Right Paraduodenal Hernia
- Military Brats: Members of a Lost Tribe
- Practitioner Cognitive Reframing: Working More Effectively in Addictions
- The Role of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swabs in Clinical Decision Making
- Improving Veteran Engagement With Mental Health Care

Click here to access the August 2017 Digital Edition.
Table of Contents
- Collaboration of the NIH and PHS Commissioned Corps in the International Ebola Clinical Research Response
- Parkinsonism and Vitamin C Deficiency
- Right Paraduodenal Hernia
- Military Brats: Members of a Lost Tribe
- Practitioner Cognitive Reframing: Working More Effectively in Addictions
- The Role of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swabs in Clinical Decision Making
- Improving Veteran Engagement With Mental Health Care

Click here to access the August 2017 Digital Edition.
Table of Contents
- Collaboration of the NIH and PHS Commissioned Corps in the International Ebola Clinical Research Response
- Parkinsonism and Vitamin C Deficiency
- Right Paraduodenal Hernia
- Military Brats: Members of a Lost Tribe
- Practitioner Cognitive Reframing: Working More Effectively in Addictions
- The Role of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swabs in Clinical Decision Making
- Improving Veteran Engagement With Mental Health Care

Post-Treatment Follow-Up by Oncologic Specialists as a Relevant Component of Cancer Survivorship for Veteran Patients Living in Rural Area
Purpose: To present a lesson learned from a pilot project aiming to improve post-radiotherapy (RT) followup (FU) care for Veterans living in rural area within our VISN, thereby questioning if FU care as dictated by oncologic specialists would be beneficial in a rural Veteran’s cancer survivorship.
Methods: A team of radiation oncology (RO) specialists was assembled to include clinical providers and medical physicists. A 2-pronged approach was employed: 1 by inperson visit at selected rural community-based outpatient clinic (rCBOC), the other via telehealth link. Target population included rural Veterans who had received RT at either a VA or Non-VA Care Center (NVCC) facility. On-site visits were done by RO specialists at each rCBOC. Patient satisfaction was evaluated via feedback survey. Mileage and time saved were calculated for each Veteran who might otherwise travel to see a VA RO specialist.
Results: In a span of 14 months, 9 separate rCBOC visits were made for 3 sites and a total of 49 Veteran visits. Excellent patient satisfaction was obtained, and the average mileage and time saved per Veteran visit was 217.2 miles and 201 min (off-traffic peak), respectively. However, 4 of 5 NVCC treatment plans encountered contained physics quality assurance (QA) data not considered to have met professional standards. Dedicated telehealth equipment was acquired and connections validated. Challenges faced included: soliciting timely assistance of administrative leadership, identifying patients to be seen and accessing their records, and obtaining clinical privilege and EHR access at rCBOCs.
Implications: Access to post-treatment cancer care for rural Veterans can be improved with in-person visits by VA oncologic specialists at corresponding rCBOCs. Barriers due to distance and time can be reduced significantly, with excellent patient satisfaction outcome. The efficacy of telehealth link requires further clinical testing. Furthermore, the inadvertent finding of physics QA deficiencies at NVCC sites raised plausible concern for overall quality of RT care, reflecting the probable need for future oversight by VA specialists. By reaching out to rural Veterans proactively, VA oncologic specialists can enhance their post-treatment cancer care, thereby improving their cancer survivorship.
Purpose: To present a lesson learned from a pilot project aiming to improve post-radiotherapy (RT) followup (FU) care for Veterans living in rural area within our VISN, thereby questioning if FU care as dictated by oncologic specialists would be beneficial in a rural Veteran’s cancer survivorship.
Methods: A team of radiation oncology (RO) specialists was assembled to include clinical providers and medical physicists. A 2-pronged approach was employed: 1 by inperson visit at selected rural community-based outpatient clinic (rCBOC), the other via telehealth link. Target population included rural Veterans who had received RT at either a VA or Non-VA Care Center (NVCC) facility. On-site visits were done by RO specialists at each rCBOC. Patient satisfaction was evaluated via feedback survey. Mileage and time saved were calculated for each Veteran who might otherwise travel to see a VA RO specialist.
Results: In a span of 14 months, 9 separate rCBOC visits were made for 3 sites and a total of 49 Veteran visits. Excellent patient satisfaction was obtained, and the average mileage and time saved per Veteran visit was 217.2 miles and 201 min (off-traffic peak), respectively. However, 4 of 5 NVCC treatment plans encountered contained physics quality assurance (QA) data not considered to have met professional standards. Dedicated telehealth equipment was acquired and connections validated. Challenges faced included: soliciting timely assistance of administrative leadership, identifying patients to be seen and accessing their records, and obtaining clinical privilege and EHR access at rCBOCs.
Implications: Access to post-treatment cancer care for rural Veterans can be improved with in-person visits by VA oncologic specialists at corresponding rCBOCs. Barriers due to distance and time can be reduced significantly, with excellent patient satisfaction outcome. The efficacy of telehealth link requires further clinical testing. Furthermore, the inadvertent finding of physics QA deficiencies at NVCC sites raised plausible concern for overall quality of RT care, reflecting the probable need for future oversight by VA specialists. By reaching out to rural Veterans proactively, VA oncologic specialists can enhance their post-treatment cancer care, thereby improving their cancer survivorship.
Purpose: To present a lesson learned from a pilot project aiming to improve post-radiotherapy (RT) followup (FU) care for Veterans living in rural area within our VISN, thereby questioning if FU care as dictated by oncologic specialists would be beneficial in a rural Veteran’s cancer survivorship.
Methods: A team of radiation oncology (RO) specialists was assembled to include clinical providers and medical physicists. A 2-pronged approach was employed: 1 by inperson visit at selected rural community-based outpatient clinic (rCBOC), the other via telehealth link. Target population included rural Veterans who had received RT at either a VA or Non-VA Care Center (NVCC) facility. On-site visits were done by RO specialists at each rCBOC. Patient satisfaction was evaluated via feedback survey. Mileage and time saved were calculated for each Veteran who might otherwise travel to see a VA RO specialist.
Results: In a span of 14 months, 9 separate rCBOC visits were made for 3 sites and a total of 49 Veteran visits. Excellent patient satisfaction was obtained, and the average mileage and time saved per Veteran visit was 217.2 miles and 201 min (off-traffic peak), respectively. However, 4 of 5 NVCC treatment plans encountered contained physics quality assurance (QA) data not considered to have met professional standards. Dedicated telehealth equipment was acquired and connections validated. Challenges faced included: soliciting timely assistance of administrative leadership, identifying patients to be seen and accessing their records, and obtaining clinical privilege and EHR access at rCBOCs.
Implications: Access to post-treatment cancer care for rural Veterans can be improved with in-person visits by VA oncologic specialists at corresponding rCBOCs. Barriers due to distance and time can be reduced significantly, with excellent patient satisfaction outcome. The efficacy of telehealth link requires further clinical testing. Furthermore, the inadvertent finding of physics QA deficiencies at NVCC sites raised plausible concern for overall quality of RT care, reflecting the probable need for future oversight by VA specialists. By reaching out to rural Veterans proactively, VA oncologic specialists can enhance their post-treatment cancer care, thereby improving their cancer survivorship.
Update on the VA Precision Oncology Program
Purpose: To inform VA stakeholders of the availability of precision oncology (PO) services for Veterans with advanced cancer.
Background: PO offers the promise of effective, low-toxicity targeted therapies tailored to individual tumor genomics but is unequally available within VHA. A systemwide PO program (POP), including patients in rural areas, launched in July 2016.
Methods: Patients tested with multigene next generation sequencing (NGS) tumor testing through 2 contracted vendors were identified from POP records and cancer characteristics were extracted from POP and medical records. Drug use data were obtained from the VA Corporate Data Warehouse. NGS testing results, and annotations were extracted from POP records.
Results: 1,442 tumor samples were sent for NGS testing as of 5/21/17 from 61 facilities. Rural patient testing (35%) was similar to VHA rurality (33%) and more than twice the US rate (14%). Most common diagnoses: lung (688: adeno 482, squamous 134), unknown (114), colorectal (103), skin (96), prostate (76), and H&N (66). Sample test requests increased rapidly after national implementation in July 2016 (23 samples/month prior to implementation to mean 126 samples/month 3 months later) as did the number of participating facilities (10/quarter to 39/month). Sequencing success rate increased from 68% to 71% over the same interval, while mean turn around time remained similar at 19.7 and 19.1 days, respectively. To date, 26 patients received a recommended drug outside a clinical trial, some more than 9 months after NGS. 5 additional patients had received an NGS-recommended drug prior to testing. NGS results are available for a cohort of 344 patients including: lung 200 (adeno 138, squamous 51), skin 28, LN 20, liver 19, GI 16. 979 variants were found most commonly in TP53, KRAS, STK11, APC, PIK3CA, and CDKN2A. 228 patients (66%) had actionable results (on-label drug 24, off-label drug 165, clinical trial 213). A PO consultation service (available by IFC) and a liquid biopsy are now available nationally.
Conclusions: Implementation of tumor NGS testing in VHA has been successful. Further program expansion, addition of hematological malignancies, deployment of informatics tools and efforts to expand access to appropriate drugs are ongoing.
Purpose: To inform VA stakeholders of the availability of precision oncology (PO) services for Veterans with advanced cancer.
Background: PO offers the promise of effective, low-toxicity targeted therapies tailored to individual tumor genomics but is unequally available within VHA. A systemwide PO program (POP), including patients in rural areas, launched in July 2016.
Methods: Patients tested with multigene next generation sequencing (NGS) tumor testing through 2 contracted vendors were identified from POP records and cancer characteristics were extracted from POP and medical records. Drug use data were obtained from the VA Corporate Data Warehouse. NGS testing results, and annotations were extracted from POP records.
Results: 1,442 tumor samples were sent for NGS testing as of 5/21/17 from 61 facilities. Rural patient testing (35%) was similar to VHA rurality (33%) and more than twice the US rate (14%). Most common diagnoses: lung (688: adeno 482, squamous 134), unknown (114), colorectal (103), skin (96), prostate (76), and H&N (66). Sample test requests increased rapidly after national implementation in July 2016 (23 samples/month prior to implementation to mean 126 samples/month 3 months later) as did the number of participating facilities (10/quarter to 39/month). Sequencing success rate increased from 68% to 71% over the same interval, while mean turn around time remained similar at 19.7 and 19.1 days, respectively. To date, 26 patients received a recommended drug outside a clinical trial, some more than 9 months after NGS. 5 additional patients had received an NGS-recommended drug prior to testing. NGS results are available for a cohort of 344 patients including: lung 200 (adeno 138, squamous 51), skin 28, LN 20, liver 19, GI 16. 979 variants were found most commonly in TP53, KRAS, STK11, APC, PIK3CA, and CDKN2A. 228 patients (66%) had actionable results (on-label drug 24, off-label drug 165, clinical trial 213). A PO consultation service (available by IFC) and a liquid biopsy are now available nationally.
Conclusions: Implementation of tumor NGS testing in VHA has been successful. Further program expansion, addition of hematological malignancies, deployment of informatics tools and efforts to expand access to appropriate drugs are ongoing.
Purpose: To inform VA stakeholders of the availability of precision oncology (PO) services for Veterans with advanced cancer.
Background: PO offers the promise of effective, low-toxicity targeted therapies tailored to individual tumor genomics but is unequally available within VHA. A systemwide PO program (POP), including patients in rural areas, launched in July 2016.
Methods: Patients tested with multigene next generation sequencing (NGS) tumor testing through 2 contracted vendors were identified from POP records and cancer characteristics were extracted from POP and medical records. Drug use data were obtained from the VA Corporate Data Warehouse. NGS testing results, and annotations were extracted from POP records.
Results: 1,442 tumor samples were sent for NGS testing as of 5/21/17 from 61 facilities. Rural patient testing (35%) was similar to VHA rurality (33%) and more than twice the US rate (14%). Most common diagnoses: lung (688: adeno 482, squamous 134), unknown (114), colorectal (103), skin (96), prostate (76), and H&N (66). Sample test requests increased rapidly after national implementation in July 2016 (23 samples/month prior to implementation to mean 126 samples/month 3 months later) as did the number of participating facilities (10/quarter to 39/month). Sequencing success rate increased from 68% to 71% over the same interval, while mean turn around time remained similar at 19.7 and 19.1 days, respectively. To date, 26 patients received a recommended drug outside a clinical trial, some more than 9 months after NGS. 5 additional patients had received an NGS-recommended drug prior to testing. NGS results are available for a cohort of 344 patients including: lung 200 (adeno 138, squamous 51), skin 28, LN 20, liver 19, GI 16. 979 variants were found most commonly in TP53, KRAS, STK11, APC, PIK3CA, and CDKN2A. 228 patients (66%) had actionable results (on-label drug 24, off-label drug 165, clinical trial 213). A PO consultation service (available by IFC) and a liquid biopsy are now available nationally.
Conclusions: Implementation of tumor NGS testing in VHA has been successful. Further program expansion, addition of hematological malignancies, deployment of informatics tools and efforts to expand access to appropriate drugs are ongoing.
DDSEP® 8 Quick quiz - August 2017 Question 2
Q2: Answer: D
The history of weight loss, intermittent diarrhea, and bloating are suspicious for celiac disease. While lactose intolerance can explain the pain, diarrhea, and bloating, there does not appear to be any correlation with the ingestion of particular foods, nor should there be any weight loss. While inflammatory bowel disease is certainly a possible explanation for his symptoms, it would be premature to jump to upper and lower endoscopy as initial evaluations.
Tissue transglutaminase antibodies are a sensitive and specific screening test for celiac disease, with published sensitivities and specificities greater than 95%. Obtaining a total serum IgA level at the time of screening is recommended to exclude IgA deficiency, which may result in a false-negative test.
Reference
1. Husby S., Koletzko S., Korponay-Szabo I.R., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-60.
2. Olen O., Gudjonsdottir A., Browaldh L., et al. Antibodies against deamindated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:695-700.
Q2: Answer: D
The history of weight loss, intermittent diarrhea, and bloating are suspicious for celiac disease. While lactose intolerance can explain the pain, diarrhea, and bloating, there does not appear to be any correlation with the ingestion of particular foods, nor should there be any weight loss. While inflammatory bowel disease is certainly a possible explanation for his symptoms, it would be premature to jump to upper and lower endoscopy as initial evaluations.
Tissue transglutaminase antibodies are a sensitive and specific screening test for celiac disease, with published sensitivities and specificities greater than 95%. Obtaining a total serum IgA level at the time of screening is recommended to exclude IgA deficiency, which may result in a false-negative test.
Reference
1. Husby S., Koletzko S., Korponay-Szabo I.R., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-60.
2. Olen O., Gudjonsdottir A., Browaldh L., et al. Antibodies against deamindated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:695-700.
Q2: Answer: D
The history of weight loss, intermittent diarrhea, and bloating are suspicious for celiac disease. While lactose intolerance can explain the pain, diarrhea, and bloating, there does not appear to be any correlation with the ingestion of particular foods, nor should there be any weight loss. While inflammatory bowel disease is certainly a possible explanation for his symptoms, it would be premature to jump to upper and lower endoscopy as initial evaluations.
Tissue transglutaminase antibodies are a sensitive and specific screening test for celiac disease, with published sensitivities and specificities greater than 95%. Obtaining a total serum IgA level at the time of screening is recommended to exclude IgA deficiency, which may result in a false-negative test.
Reference
1. Husby S., Koletzko S., Korponay-Szabo I.R., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-60.
2. Olen O., Gudjonsdottir A., Browaldh L., et al. Antibodies against deamindated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:695-700.
A 10-year-old boy is referred after he was noted to have lost weight over the past year during a routine physical exam. He denies trying to lose weight. He has occasional abdominal pain and intermittent watery nonbloody diarrhea, which do not seem associated with particular foods. He also complains of feeling bloated and his mother reports that “his belly always looks swollen.” He has had no other symptoms of illness. On physical exam, he is slender and has a mildly distended and tympanitic abdomen.