User login
P falciparum malaria existed 2000 years ago, team says

individual from Velia, Italy
Photo courtesy of Luca
Bandioli, Pigorini Museum
An analysis of 2000-year-old human remains from several regions across the Italian peninsula has confirmed the presence of Plasmodium falciparum malaria during the Roman Empire, according to researchers.
The team found mitochondrial genomic evidence of P falciparum malaria, coaxed from the teeth of bodies buried in 3 Italian cemeteries, dating back to the Imperial period.
The researchers said these finding provide a key reference point for when and where the malaria parasite existed in humans, as well as more information about the evolution of human disease.
The team reported these findings in Current Biology.
“There is extensive written evidence describing fevers that sound like malaria in ancient Greece and Rome, but the specific malaria species responsible is unknown,” said study author Stephanie Marciniak, PhD, of Pennsylvania State University in University Park.
“Our data confirm that the species was likely Plasmodium falciparum and that it affected people in different ecological and cultural environments. These results open up new questions to explore, particularly how widespread this parasite was and what burden it placed upon communities in Imperial Roman Italy.”
Dr Marciniak and her colleagues sampled teeth taken from 58 adults interred at 3 Imperial period Italian cemeteries: Isola Sacra, Velia, and Vagnari.
Located on the coast, Velia and Isola Sacra were known as important port cities and trading centers. Vagnari is located further inland and believed to be the burial site of laborers who would have worked on a Roman rural estate.
The researchers mined tiny DNA fragments from dental pulp. They were able to extract, purify, and enrich specifically for the Plasmodium species known to infect humans.
The team noted that usable DNA is challenging to extract because the parasites primarily dwell within the bloodstream and organs, which decompose and break down over time—in this instance, over the course of 2 millennia.
However, the researchers recovered more than half of the P falciparum mitochondrial genome from 2 individuals from Velia and Vagnari. ![]()

individual from Velia, Italy
Photo courtesy of Luca
Bandioli, Pigorini Museum
An analysis of 2000-year-old human remains from several regions across the Italian peninsula has confirmed the presence of Plasmodium falciparum malaria during the Roman Empire, according to researchers.
The team found mitochondrial genomic evidence of P falciparum malaria, coaxed from the teeth of bodies buried in 3 Italian cemeteries, dating back to the Imperial period.
The researchers said these finding provide a key reference point for when and where the malaria parasite existed in humans, as well as more information about the evolution of human disease.
The team reported these findings in Current Biology.
“There is extensive written evidence describing fevers that sound like malaria in ancient Greece and Rome, but the specific malaria species responsible is unknown,” said study author Stephanie Marciniak, PhD, of Pennsylvania State University in University Park.
“Our data confirm that the species was likely Plasmodium falciparum and that it affected people in different ecological and cultural environments. These results open up new questions to explore, particularly how widespread this parasite was and what burden it placed upon communities in Imperial Roman Italy.”
Dr Marciniak and her colleagues sampled teeth taken from 58 adults interred at 3 Imperial period Italian cemeteries: Isola Sacra, Velia, and Vagnari.
Located on the coast, Velia and Isola Sacra were known as important port cities and trading centers. Vagnari is located further inland and believed to be the burial site of laborers who would have worked on a Roman rural estate.
The researchers mined tiny DNA fragments from dental pulp. They were able to extract, purify, and enrich specifically for the Plasmodium species known to infect humans.
The team noted that usable DNA is challenging to extract because the parasites primarily dwell within the bloodstream and organs, which decompose and break down over time—in this instance, over the course of 2 millennia.
However, the researchers recovered more than half of the P falciparum mitochondrial genome from 2 individuals from Velia and Vagnari. ![]()

individual from Velia, Italy
Photo courtesy of Luca
Bandioli, Pigorini Museum
An analysis of 2000-year-old human remains from several regions across the Italian peninsula has confirmed the presence of Plasmodium falciparum malaria during the Roman Empire, according to researchers.
The team found mitochondrial genomic evidence of P falciparum malaria, coaxed from the teeth of bodies buried in 3 Italian cemeteries, dating back to the Imperial period.
The researchers said these finding provide a key reference point for when and where the malaria parasite existed in humans, as well as more information about the evolution of human disease.
The team reported these findings in Current Biology.
“There is extensive written evidence describing fevers that sound like malaria in ancient Greece and Rome, but the specific malaria species responsible is unknown,” said study author Stephanie Marciniak, PhD, of Pennsylvania State University in University Park.
“Our data confirm that the species was likely Plasmodium falciparum and that it affected people in different ecological and cultural environments. These results open up new questions to explore, particularly how widespread this parasite was and what burden it placed upon communities in Imperial Roman Italy.”
Dr Marciniak and her colleagues sampled teeth taken from 58 adults interred at 3 Imperial period Italian cemeteries: Isola Sacra, Velia, and Vagnari.
Located on the coast, Velia and Isola Sacra were known as important port cities and trading centers. Vagnari is located further inland and believed to be the burial site of laborers who would have worked on a Roman rural estate.
The researchers mined tiny DNA fragments from dental pulp. They were able to extract, purify, and enrich specifically for the Plasmodium species known to infect humans.
The team noted that usable DNA is challenging to extract because the parasites primarily dwell within the bloodstream and organs, which decompose and break down over time—in this instance, over the course of 2 millennia.
However, the researchers recovered more than half of the P falciparum mitochondrial genome from 2 individuals from Velia and Vagnari. ![]()
Partnerships with pediatric tertiary care centers improve community ED asthma treatment
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
FROM PEDIATRICS
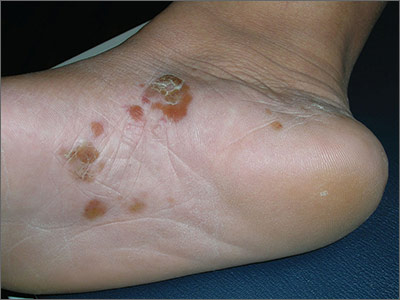
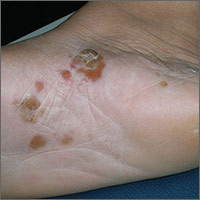
Itchy blisters on feet
The patient was given a diagnosis of vesicular (vesiculobullous) tinea pedis, based on the vesicles and bullae over the arch region of the foot. The arch is a typical location for vesiculobullous tinea pedis. There are 3 main types of tinea pedis: interdigital, moccasin distribution, and vesiculobullous (which is the least common type). Interdigital tinea pedis is the most common type and is typically seen between the fourth and fifth digits. The moccasin distribution tends to involve more erythema and scale—especially on the sides of the feet—giving the appearance of moccasins.
In general, tinea pedis is most commonly caused by Trichophyton rubrum. Other causative organisms include Trichophyton mentagrophytes and Epidermophyton floccosum. The vesiculobullous type of tinea pedis is usually caused by T mentagrophytes. The FP made this diagnosis clinically and did not perform a potassium hydroxide (KOH) preparation, as this type of tinea pedis has minimal scale and the dermatophyte may be harder to identify with the superficial scraping.
The FP prescribed 2 weeks of oral terbinafine 250 mg/d instead of relying on topical antifungal medicine because a better response can be expected with it. One month later, the vesiculobullous tinea pedis was fully resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Reppa R. Tinea pedis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:799-804.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The patient was given a diagnosis of vesicular (vesiculobullous) tinea pedis, based on the vesicles and bullae over the arch region of the foot. The arch is a typical location for vesiculobullous tinea pedis. There are 3 main types of tinea pedis: interdigital, moccasin distribution, and vesiculobullous (which is the least common type). Interdigital tinea pedis is the most common type and is typically seen between the fourth and fifth digits. The moccasin distribution tends to involve more erythema and scale—especially on the sides of the feet—giving the appearance of moccasins.
In general, tinea pedis is most commonly caused by Trichophyton rubrum. Other causative organisms include Trichophyton mentagrophytes and Epidermophyton floccosum. The vesiculobullous type of tinea pedis is usually caused by T mentagrophytes. The FP made this diagnosis clinically and did not perform a potassium hydroxide (KOH) preparation, as this type of tinea pedis has minimal scale and the dermatophyte may be harder to identify with the superficial scraping.
The FP prescribed 2 weeks of oral terbinafine 250 mg/d instead of relying on topical antifungal medicine because a better response can be expected with it. One month later, the vesiculobullous tinea pedis was fully resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Reppa R. Tinea pedis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:799-804.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The patient was given a diagnosis of vesicular (vesiculobullous) tinea pedis, based on the vesicles and bullae over the arch region of the foot. The arch is a typical location for vesiculobullous tinea pedis. There are 3 main types of tinea pedis: interdigital, moccasin distribution, and vesiculobullous (which is the least common type). Interdigital tinea pedis is the most common type and is typically seen between the fourth and fifth digits. The moccasin distribution tends to involve more erythema and scale—especially on the sides of the feet—giving the appearance of moccasins.
In general, tinea pedis is most commonly caused by Trichophyton rubrum. Other causative organisms include Trichophyton mentagrophytes and Epidermophyton floccosum. The vesiculobullous type of tinea pedis is usually caused by T mentagrophytes. The FP made this diagnosis clinically and did not perform a potassium hydroxide (KOH) preparation, as this type of tinea pedis has minimal scale and the dermatophyte may be harder to identify with the superficial scraping.
The FP prescribed 2 weeks of oral terbinafine 250 mg/d instead of relying on topical antifungal medicine because a better response can be expected with it. One month later, the vesiculobullous tinea pedis was fully resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Reppa R. Tinea pedis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:799-804.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
15 and Going Gray
ANSWER
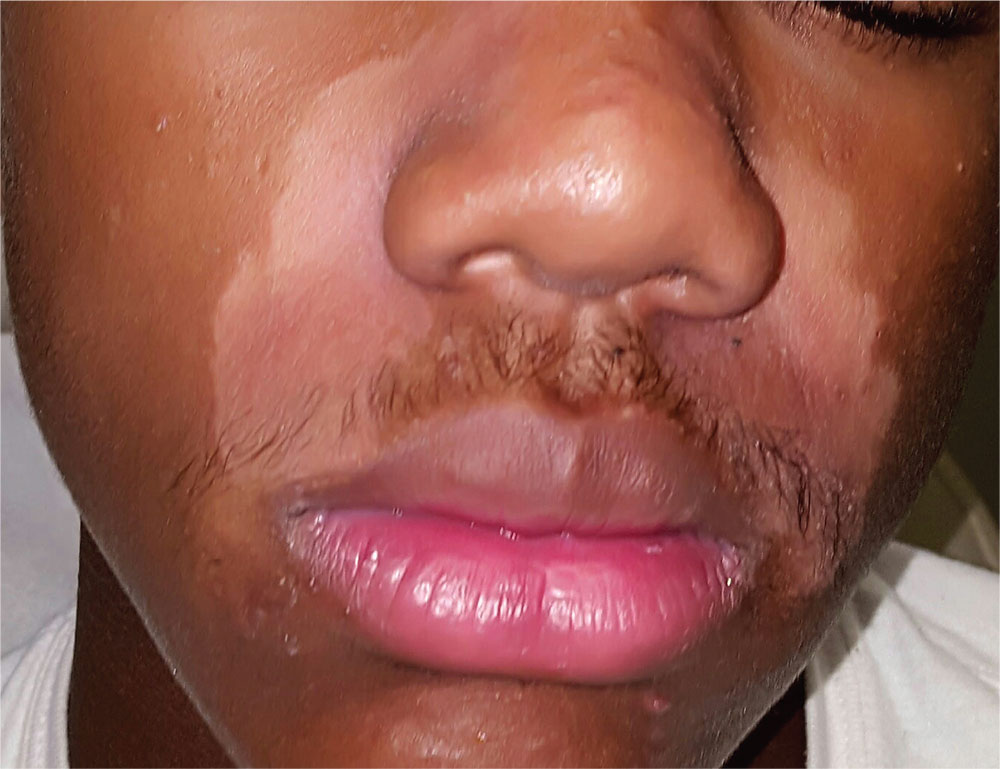
The correct answer is seborrheic dermatitis (choice “d”).
Psoriasis (choice “a”) can manifest in a similar fashion but would also appear elsewhere (eg, the elbows, knees, or nails). The scaling seen with psoriasis is usually white on a salmon-colored base and much coarser.
Vitiligo (choice “b”) presents with total, porcelain-white depigmentation and is not scaly at all; this patient’s loss of pigment was only partial.
Rashes like this are often thought to be fungal, but a fungal infection (choice “c”) is rarely seen on the face and would not demonstrate this exact pattern.
DISCUSSION
Seborrheic dermatitis (SD) is an extremely common papulosquamous skin condition that manifests primarily in oil-rich areas, such as the central face, scalp (dandruff), and in and behind the ears. On white skin, it yields a pinkish-brown scaly rash, but on darker skin, SD causes partial depigmentation—sometimes to an alarming extent. The distribution in this case is typical, with nasolabial, glabellar, ear, and brow involvement.
SD might be caused by a number of factors, some related to climate or genetics. One prevailing theory is that it involves an inflammatory reaction resulting from commensal yeast organisms (Malassezia furfur) feeding on sebum. In this case, the contribution of stress was clear.
TREATMENT/PROGNOSIS
Treatment comprised daily use of ketoconazole shampoo on the scalp and face and a topical ketoconazole cream. The condition cleared within two weeks. Although the chances of a recurrence are quite high, the patient is now armed with information and products to avoid the worst effects.
ANSWER
The correct answer is seborrheic dermatitis (choice “d”).
Psoriasis (choice “a”) can manifest in a similar fashion but would also appear elsewhere (eg, the elbows, knees, or nails). The scaling seen with psoriasis is usually white on a salmon-colored base and much coarser.
Vitiligo (choice “b”) presents with total, porcelain-white depigmentation and is not scaly at all; this patient’s loss of pigment was only partial.
Rashes like this are often thought to be fungal, but a fungal infection (choice “c”) is rarely seen on the face and would not demonstrate this exact pattern.
DISCUSSION
Seborrheic dermatitis (SD) is an extremely common papulosquamous skin condition that manifests primarily in oil-rich areas, such as the central face, scalp (dandruff), and in and behind the ears. On white skin, it yields a pinkish-brown scaly rash, but on darker skin, SD causes partial depigmentation—sometimes to an alarming extent. The distribution in this case is typical, with nasolabial, glabellar, ear, and brow involvement.
SD might be caused by a number of factors, some related to climate or genetics. One prevailing theory is that it involves an inflammatory reaction resulting from commensal yeast organisms (Malassezia furfur) feeding on sebum. In this case, the contribution of stress was clear.
TREATMENT/PROGNOSIS
Treatment comprised daily use of ketoconazole shampoo on the scalp and face and a topical ketoconazole cream. The condition cleared within two weeks. Although the chances of a recurrence are quite high, the patient is now armed with information and products to avoid the worst effects.
ANSWER
The correct answer is seborrheic dermatitis (choice “d”).
Psoriasis (choice “a”) can manifest in a similar fashion but would also appear elsewhere (eg, the elbows, knees, or nails). The scaling seen with psoriasis is usually white on a salmon-colored base and much coarser.
Vitiligo (choice “b”) presents with total, porcelain-white depigmentation and is not scaly at all; this patient’s loss of pigment was only partial.
Rashes like this are often thought to be fungal, but a fungal infection (choice “c”) is rarely seen on the face and would not demonstrate this exact pattern.
DISCUSSION
Seborrheic dermatitis (SD) is an extremely common papulosquamous skin condition that manifests primarily in oil-rich areas, such as the central face, scalp (dandruff), and in and behind the ears. On white skin, it yields a pinkish-brown scaly rash, but on darker skin, SD causes partial depigmentation—sometimes to an alarming extent. The distribution in this case is typical, with nasolabial, glabellar, ear, and brow involvement.
SD might be caused by a number of factors, some related to climate or genetics. One prevailing theory is that it involves an inflammatory reaction resulting from commensal yeast organisms (Malassezia furfur) feeding on sebum. In this case, the contribution of stress was clear.
TREATMENT/PROGNOSIS
Treatment comprised daily use of ketoconazole shampoo on the scalp and face and a topical ketoconazole cream. The condition cleared within two weeks. Although the chances of a recurrence are quite high, the patient is now armed with information and products to avoid the worst effects.
A 15-year-old African-American boy is brought in by his family for evaluation of a condition that began several months ago. Despite multiple attempts at treatment, the uniform but partial depigmentation of the skin around his nose persists. It is made obvious by his type V skin, extending up into the glabellar area and into both eyebrows.
Fine but definite scaling is confined to the depigmented areas. There is heavier white to gray scaling on his frontal scalp, and light scaling in and behind both ears. His elbows, knees, and nails appear normal. He is asymptomatic and in excellent health otherwise, but he considers his condition “unsightly and embarrassing.”
More history-taking reveals that around the time this problem manifested, he was facing the threat of a one-year suspension from school for disciplinary reasons.
Group estimates global cancer cases, deaths in 2015

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()
Data suggest one BTK inhibitor could replace another

ASH Annual Meeting
SAN DIEGO—When patients with chronic lymphocytic leukemia (CLL) cannot tolerate one Bruton’s tyrosine kinase (BTK) inhibitor, they may do well on another, according to a presentation at the 2016 ASH Annual Meeting.
Researchers conducting a phase 1/2 study found that acalabrutinib was “well-tolerated” and demonstrated “promising activity” in patients intolerant to ibrutinib.
Seventy-nine percent of patients responded to acalabrutinib.
And although 36% of patients had a recurrence of an adverse event (AE) they experienced while on ibrutinib, none of the patients discontinued acalabrutinib due to AE recurrence.
Farrukh T. Awan, MD, of The Ohio State University in Columbus, Ohio, presented these results at the meeting as abstract 638.*
Dr Awan noted that integrating ibrutinib into standard CLL therapy has improved patient outcomes, but a lack of tolerability observed in some patients suggests that more selective BTK inhibition may be desirable.
“We know that around 10% to 20% of patients who are treated with ibrutinib would have to stop therapy because of an adverse event,” Dr Awan said. “Acalabrutinib is a highly selective, potent BTK inhibitor that has shown promising efficacy, and that [research] was published last year.”
In this ongoing, phase 1/2 study, Dr Awan and his colleagues are testing acalabrutinib in patients with CLL/small lymphocytic leukemia. The study has enrolled multiple cohorts of patients—relapsed/refractory, treatment-naïve, Richter’s transformation/prolymphocytic leukemia, and ibrutinib intolerant.
At this year’s ASH meeting, Dr Awan presented data on the 33 CLL patients who were ibrutinib intolerant. The patients’ median age was 64 (range, 50-82), 61% were male, 97% had an ECOG performance status of 0-1, 52% had Rai stage III-IV, and 31% had bulky disease.
The median number of prior therapies was 4 (range, 2-13), and 91% of patients had ibrutinib as their last therapy. The median duration of prior ibrutinib treatment was 11.5 months (range, 1-62), and the median time from ending ibrutinib to starting acalabrutinib was 47 days (range, 3-331 days).
Treatment and safety
Patients received acalabrutinib at 100 mg twice daily (n=30) or 200 mg daily (n=3) until disease progression or discontinuation for another reason. The patients’ median time on therapy was 12.2 months (range, 0.2-23.6 months).
Nine patients discontinued treatment—3 due to disease progression, 3 due to AEs, 2 due to an increase in BTK C481S mutation frequency in the peripheral blood and central nervous system involvement, and 1 due to physician decision (because the patient had concurrent hemophilia).
The 3 AEs that led to treatment discontinuation were fatal hemorrhagic stroke, fatal fungal infection, and metastatic endometrial cancer. All 3 events were considered unrelated to acalabrutinib.
Serious AEs occurred in 11 patients (33%). A serious AE that occurred in more than 1 patient was pneumonia (n=2).
The most common AEs were diarrhea (52%, grade 1-2), headache (39%, grade 1-2), cough (24%, grade 1-2), increased weight (24%, grade 1-2), nausea (21%, grade 1-2), contusion (18%, grade 1-2), ecchymosis (18%, grade 1-2), fatigue (18%, grade 1-2), hypertension (18% overall, 6% ≥ grade 3), pyrexia (18% overall, 3% ≥ grade 3), vomiting (18%, grade 1-2), myalgia (15% overall, 3% ≥ grade 3), rash (15%, grade 1-2), stomatitis (15%, grade 1-2), upper respiratory tract infection (15%, grade 1-2), and urinary tract infection (15%, grade 1-2).
AE recurrence
Twelve patients (36%) had a recurrence of ibrutinib-related AEs—a total of 16 events. Fourteen of these events either decreased in severity or were unchanged with acalabrutinib treatment.
The events without a change in severity were atrial fibrillation (n=1), fatigue (n=1), muscle spasms (n=1), myalgia (n=1), peripheral edema (n=1), panniculitis (n=1), and rash (n=1).
The events that decreased in severity were diarrhea (n=2), arthralgia (n=1), ecchymosis (n=1), fatigue (n=1), panniculitis (n=1), and rash (n=1).
The events that increased in severity were contusion (n=1, grade 1 to 2) and fatigue (n=1, grade 1 to 2).
None of the patients discontinued acalabrutinib due to AE recurrence.
Efficacy
Twenty-nine patients were evaluable for efficacy.
The overall response rate was 79% (n=23). One patient had a complete response (3%), 15 had a partial response (52%), and 7 had a partial response with lymphocytosis (24%). Six patients had stable disease (21%).
The median time to response was 1.9 months. Eighty-one percent of responding patients have a response duration of 12 months or longer.
The median progression-free survival has not been reached.
The research is sponsored by Acerta Pharma.![]()
*Information presented at the meeting differs from the abstract.

ASH Annual Meeting
SAN DIEGO—When patients with chronic lymphocytic leukemia (CLL) cannot tolerate one Bruton’s tyrosine kinase (BTK) inhibitor, they may do well on another, according to a presentation at the 2016 ASH Annual Meeting.
Researchers conducting a phase 1/2 study found that acalabrutinib was “well-tolerated” and demonstrated “promising activity” in patients intolerant to ibrutinib.
Seventy-nine percent of patients responded to acalabrutinib.
And although 36% of patients had a recurrence of an adverse event (AE) they experienced while on ibrutinib, none of the patients discontinued acalabrutinib due to AE recurrence.
Farrukh T. Awan, MD, of The Ohio State University in Columbus, Ohio, presented these results at the meeting as abstract 638.*
Dr Awan noted that integrating ibrutinib into standard CLL therapy has improved patient outcomes, but a lack of tolerability observed in some patients suggests that more selective BTK inhibition may be desirable.
“We know that around 10% to 20% of patients who are treated with ibrutinib would have to stop therapy because of an adverse event,” Dr Awan said. “Acalabrutinib is a highly selective, potent BTK inhibitor that has shown promising efficacy, and that [research] was published last year.”
In this ongoing, phase 1/2 study, Dr Awan and his colleagues are testing acalabrutinib in patients with CLL/small lymphocytic leukemia. The study has enrolled multiple cohorts of patients—relapsed/refractory, treatment-naïve, Richter’s transformation/prolymphocytic leukemia, and ibrutinib intolerant.
At this year’s ASH meeting, Dr Awan presented data on the 33 CLL patients who were ibrutinib intolerant. The patients’ median age was 64 (range, 50-82), 61% were male, 97% had an ECOG performance status of 0-1, 52% had Rai stage III-IV, and 31% had bulky disease.
The median number of prior therapies was 4 (range, 2-13), and 91% of patients had ibrutinib as their last therapy. The median duration of prior ibrutinib treatment was 11.5 months (range, 1-62), and the median time from ending ibrutinib to starting acalabrutinib was 47 days (range, 3-331 days).
Treatment and safety
Patients received acalabrutinib at 100 mg twice daily (n=30) or 200 mg daily (n=3) until disease progression or discontinuation for another reason. The patients’ median time on therapy was 12.2 months (range, 0.2-23.6 months).
Nine patients discontinued treatment—3 due to disease progression, 3 due to AEs, 2 due to an increase in BTK C481S mutation frequency in the peripheral blood and central nervous system involvement, and 1 due to physician decision (because the patient had concurrent hemophilia).
The 3 AEs that led to treatment discontinuation were fatal hemorrhagic stroke, fatal fungal infection, and metastatic endometrial cancer. All 3 events were considered unrelated to acalabrutinib.
Serious AEs occurred in 11 patients (33%). A serious AE that occurred in more than 1 patient was pneumonia (n=2).
The most common AEs were diarrhea (52%, grade 1-2), headache (39%, grade 1-2), cough (24%, grade 1-2), increased weight (24%, grade 1-2), nausea (21%, grade 1-2), contusion (18%, grade 1-2), ecchymosis (18%, grade 1-2), fatigue (18%, grade 1-2), hypertension (18% overall, 6% ≥ grade 3), pyrexia (18% overall, 3% ≥ grade 3), vomiting (18%, grade 1-2), myalgia (15% overall, 3% ≥ grade 3), rash (15%, grade 1-2), stomatitis (15%, grade 1-2), upper respiratory tract infection (15%, grade 1-2), and urinary tract infection (15%, grade 1-2).
AE recurrence
Twelve patients (36%) had a recurrence of ibrutinib-related AEs—a total of 16 events. Fourteen of these events either decreased in severity or were unchanged with acalabrutinib treatment.
The events without a change in severity were atrial fibrillation (n=1), fatigue (n=1), muscle spasms (n=1), myalgia (n=1), peripheral edema (n=1), panniculitis (n=1), and rash (n=1).
The events that decreased in severity were diarrhea (n=2), arthralgia (n=1), ecchymosis (n=1), fatigue (n=1), panniculitis (n=1), and rash (n=1).
The events that increased in severity were contusion (n=1, grade 1 to 2) and fatigue (n=1, grade 1 to 2).
None of the patients discontinued acalabrutinib due to AE recurrence.
Efficacy
Twenty-nine patients were evaluable for efficacy.
The overall response rate was 79% (n=23). One patient had a complete response (3%), 15 had a partial response (52%), and 7 had a partial response with lymphocytosis (24%). Six patients had stable disease (21%).
The median time to response was 1.9 months. Eighty-one percent of responding patients have a response duration of 12 months or longer.
The median progression-free survival has not been reached.
The research is sponsored by Acerta Pharma.![]()
*Information presented at the meeting differs from the abstract.

ASH Annual Meeting
SAN DIEGO—When patients with chronic lymphocytic leukemia (CLL) cannot tolerate one Bruton’s tyrosine kinase (BTK) inhibitor, they may do well on another, according to a presentation at the 2016 ASH Annual Meeting.
Researchers conducting a phase 1/2 study found that acalabrutinib was “well-tolerated” and demonstrated “promising activity” in patients intolerant to ibrutinib.
Seventy-nine percent of patients responded to acalabrutinib.
And although 36% of patients had a recurrence of an adverse event (AE) they experienced while on ibrutinib, none of the patients discontinued acalabrutinib due to AE recurrence.
Farrukh T. Awan, MD, of The Ohio State University in Columbus, Ohio, presented these results at the meeting as abstract 638.*
Dr Awan noted that integrating ibrutinib into standard CLL therapy has improved patient outcomes, but a lack of tolerability observed in some patients suggests that more selective BTK inhibition may be desirable.
“We know that around 10% to 20% of patients who are treated with ibrutinib would have to stop therapy because of an adverse event,” Dr Awan said. “Acalabrutinib is a highly selective, potent BTK inhibitor that has shown promising efficacy, and that [research] was published last year.”
In this ongoing, phase 1/2 study, Dr Awan and his colleagues are testing acalabrutinib in patients with CLL/small lymphocytic leukemia. The study has enrolled multiple cohorts of patients—relapsed/refractory, treatment-naïve, Richter’s transformation/prolymphocytic leukemia, and ibrutinib intolerant.
At this year’s ASH meeting, Dr Awan presented data on the 33 CLL patients who were ibrutinib intolerant. The patients’ median age was 64 (range, 50-82), 61% were male, 97% had an ECOG performance status of 0-1, 52% had Rai stage III-IV, and 31% had bulky disease.
The median number of prior therapies was 4 (range, 2-13), and 91% of patients had ibrutinib as their last therapy. The median duration of prior ibrutinib treatment was 11.5 months (range, 1-62), and the median time from ending ibrutinib to starting acalabrutinib was 47 days (range, 3-331 days).
Treatment and safety
Patients received acalabrutinib at 100 mg twice daily (n=30) or 200 mg daily (n=3) until disease progression or discontinuation for another reason. The patients’ median time on therapy was 12.2 months (range, 0.2-23.6 months).
Nine patients discontinued treatment—3 due to disease progression, 3 due to AEs, 2 due to an increase in BTK C481S mutation frequency in the peripheral blood and central nervous system involvement, and 1 due to physician decision (because the patient had concurrent hemophilia).
The 3 AEs that led to treatment discontinuation were fatal hemorrhagic stroke, fatal fungal infection, and metastatic endometrial cancer. All 3 events were considered unrelated to acalabrutinib.
Serious AEs occurred in 11 patients (33%). A serious AE that occurred in more than 1 patient was pneumonia (n=2).
The most common AEs were diarrhea (52%, grade 1-2), headache (39%, grade 1-2), cough (24%, grade 1-2), increased weight (24%, grade 1-2), nausea (21%, grade 1-2), contusion (18%, grade 1-2), ecchymosis (18%, grade 1-2), fatigue (18%, grade 1-2), hypertension (18% overall, 6% ≥ grade 3), pyrexia (18% overall, 3% ≥ grade 3), vomiting (18%, grade 1-2), myalgia (15% overall, 3% ≥ grade 3), rash (15%, grade 1-2), stomatitis (15%, grade 1-2), upper respiratory tract infection (15%, grade 1-2), and urinary tract infection (15%, grade 1-2).
AE recurrence
Twelve patients (36%) had a recurrence of ibrutinib-related AEs—a total of 16 events. Fourteen of these events either decreased in severity or were unchanged with acalabrutinib treatment.
The events without a change in severity were atrial fibrillation (n=1), fatigue (n=1), muscle spasms (n=1), myalgia (n=1), peripheral edema (n=1), panniculitis (n=1), and rash (n=1).
The events that decreased in severity were diarrhea (n=2), arthralgia (n=1), ecchymosis (n=1), fatigue (n=1), panniculitis (n=1), and rash (n=1).
The events that increased in severity were contusion (n=1, grade 1 to 2) and fatigue (n=1, grade 1 to 2).
None of the patients discontinued acalabrutinib due to AE recurrence.
Efficacy
Twenty-nine patients were evaluable for efficacy.
The overall response rate was 79% (n=23). One patient had a complete response (3%), 15 had a partial response (52%), and 7 had a partial response with lymphocytosis (24%). Six patients had stable disease (21%).
The median time to response was 1.9 months. Eighty-one percent of responding patients have a response duration of 12 months or longer.
The median progression-free survival has not been reached.
The research is sponsored by Acerta Pharma.![]()
*Information presented at the meeting differs from the abstract.
Serious mental illness gets serious attention in 21st Century Cures Act
WASHINGTON – Bipartisan legislation to improve care of patients with serious mental illness is just a pen stroke from passage, now that the 21st Century Cures Act is headed for the President’s desk.
While the mental health care reform provisions in the legislation (H.R. 34) will impact mental health services broadly, the bulk of the reform language comes from H.R. 2646, a bill sponsored by Rep. Tim Murphy (R-Penn.), which was intended to reverse decades of fragmented – and at times nonexistent – care for persons with serious mental illness.
The legislation calls for the expanded use of assisted outpatient treatment, federal guidance on how states can be reimbursed for offering inpatient psychiatric services, better data collection on mental health services paid for with state and federal dollars, new oversight of the federal agency in charge of mental health, and clarification of HIPAA laws, among other provisions.
“This is big,” Rep. Murphy said at a press conference Dec. 5. “After all the tears and suffering … know that those times when you felt no one was willing to listen, and you were ready to give up, now there is hope.”
Treatment vs. incarceration
Currently, states and communities can draw from $80 million dollars in federal grants for assisted outpatient treatment for patients with serious mental illness. The new legislation adds another $20 million and extends availability of the funding until 2022. It also allows state criminal justice systems to use assisted outpatient treatment grants to treat convicted criminals with serious mental illness instead of incarcerating them.
“[In prison], their illness gets worse, not better, creating a turnstile through which they will return time and again through our criminal justice system,” Senate Majority Whip John Cornyn (R.-Tex.) said during the press conference.
Paying for inpatient psychiatric care
The Cures Act also strengthens momentum for overhauling the Medicaid Institutions of Mental Diseases (IMD) exclusion, long a focus of mental health care advocates who view it as a governor on the number of psychiatric beds available nationally.
The exclusion prohibits any medical facility with more than 16 beds from using federal funds to pay for inpatient mental health care for adults under age 65 years. The Cures Act requires the Centers for Medicare & Medicaid Services to offer guidance on how states can use Medicaid demonstration projects to provide innovative inpatient psychiatric services using these waivers.
This follows an April 2016 CMS final rule that matches state funding dollar-per-dollar for up to 15 days of inpatient psychiatric treatment given to Medicaid managed care patients.
Although Rep. Murphy’s original bill called for abolishing the exclusion, he and other advocates are not disappointed in the progress made so far. “We didn’t get everything we wanted, but we needed everything we got,” he said.
“That 15 days was a massive win,” Frankie Berger, advocacy director of the Treatment Advocacy Center in Arlington, Va., said in an interview. “Now we have this legislation that requires CMS to tell state Medicaid directors what their wiggle room is when solving the problem on how to get care to these patients. If you read between the lines, this is care that is going to be provided by an IMD.”
The legislation also calls for more data collection and evaluation of IMDs, something that until now has been missing, Ms. Berger said. “A lot of us who work on this issue have found there is absolutely no data on where IMDs are, or who the folks are that are getting treatment in them. We need that information to make good choices,” she said.
“I think we will find savings as we expand the number of psychiatric beds … [and persons with serious mental illness] do not end up in jail cells, homeless, or in the morgue,” Sen. Bill Cassidy (R-La.) said at the press conference. He predicted that for each additional psychiatric bed introduced, savings would amount to between $30,000 and $50,000.
Restructuring, clarifying
The Cures Act reforms also restructure the Substance Abuse and Mental Health Services Administration, putting it under the authority of a new assistant secretary of Health and Human Services and giving it a chief medical officer. The law calls for the new CMO to have “real-world” experience treating patients with serious mental illness. The reformed SAMHSA will have responsibility for centralizing data collection to be used in creating best practices in mental health care. These reforms are particular victories for Rep. Murphy who has railed against the agency for years, citing its lack of medically trained leadership and lack of evidence-based approaches to care.
HIPAA requirements in serious mental illness are also clarified.
“Existing HIPAA laws are badly misunderstood,” said Sen. Chris Murphy (D-Conn.) at the briefing. “Many physicians think there is an outright ban on sharing [patient health] information.”
The legislation calls for the U.S. Department of Health and Human Services secretary to clarify when providers can disclose protected health information related to the treatment of an adult with a mental illness or substance use disorder.
“I would argue that [HIPAA] may be an area we will have to work on down the line, if the education campaign doesn’t work, but we will give this a try,” said Sen. Murphy, who has cosponsored legislation to do so.
As passed, the mental health provisions of the 21st Century Cures Act are an amalgamation of several bills introduced during the 114th Congress and are unique in that they largely were written by health care professionals with direct, relevant experience. Rep. Murphy is a clinical psychologist, while Rep. Cassidy is a physician with experience treating uninsured and incarcerated patients. Rep. Eddie Bernice Johnson (D-Tex.), who cosponsored Rep. Murphy’s bill, previously served as chief psychiatric nurse at the Dallas Veterans Affairs Medical Center.
WASHINGTON – Bipartisan legislation to improve care of patients with serious mental illness is just a pen stroke from passage, now that the 21st Century Cures Act is headed for the President’s desk.
While the mental health care reform provisions in the legislation (H.R. 34) will impact mental health services broadly, the bulk of the reform language comes from H.R. 2646, a bill sponsored by Rep. Tim Murphy (R-Penn.), which was intended to reverse decades of fragmented – and at times nonexistent – care for persons with serious mental illness.
The legislation calls for the expanded use of assisted outpatient treatment, federal guidance on how states can be reimbursed for offering inpatient psychiatric services, better data collection on mental health services paid for with state and federal dollars, new oversight of the federal agency in charge of mental health, and clarification of HIPAA laws, among other provisions.
“This is big,” Rep. Murphy said at a press conference Dec. 5. “After all the tears and suffering … know that those times when you felt no one was willing to listen, and you were ready to give up, now there is hope.”
Treatment vs. incarceration
Currently, states and communities can draw from $80 million dollars in federal grants for assisted outpatient treatment for patients with serious mental illness. The new legislation adds another $20 million and extends availability of the funding until 2022. It also allows state criminal justice systems to use assisted outpatient treatment grants to treat convicted criminals with serious mental illness instead of incarcerating them.
“[In prison], their illness gets worse, not better, creating a turnstile through which they will return time and again through our criminal justice system,” Senate Majority Whip John Cornyn (R.-Tex.) said during the press conference.
Paying for inpatient psychiatric care
The Cures Act also strengthens momentum for overhauling the Medicaid Institutions of Mental Diseases (IMD) exclusion, long a focus of mental health care advocates who view it as a governor on the number of psychiatric beds available nationally.
The exclusion prohibits any medical facility with more than 16 beds from using federal funds to pay for inpatient mental health care for adults under age 65 years. The Cures Act requires the Centers for Medicare & Medicaid Services to offer guidance on how states can use Medicaid demonstration projects to provide innovative inpatient psychiatric services using these waivers.
This follows an April 2016 CMS final rule that matches state funding dollar-per-dollar for up to 15 days of inpatient psychiatric treatment given to Medicaid managed care patients.
Although Rep. Murphy’s original bill called for abolishing the exclusion, he and other advocates are not disappointed in the progress made so far. “We didn’t get everything we wanted, but we needed everything we got,” he said.
“That 15 days was a massive win,” Frankie Berger, advocacy director of the Treatment Advocacy Center in Arlington, Va., said in an interview. “Now we have this legislation that requires CMS to tell state Medicaid directors what their wiggle room is when solving the problem on how to get care to these patients. If you read between the lines, this is care that is going to be provided by an IMD.”
The legislation also calls for more data collection and evaluation of IMDs, something that until now has been missing, Ms. Berger said. “A lot of us who work on this issue have found there is absolutely no data on where IMDs are, or who the folks are that are getting treatment in them. We need that information to make good choices,” she said.
“I think we will find savings as we expand the number of psychiatric beds … [and persons with serious mental illness] do not end up in jail cells, homeless, or in the morgue,” Sen. Bill Cassidy (R-La.) said at the press conference. He predicted that for each additional psychiatric bed introduced, savings would amount to between $30,000 and $50,000.
Restructuring, clarifying
The Cures Act reforms also restructure the Substance Abuse and Mental Health Services Administration, putting it under the authority of a new assistant secretary of Health and Human Services and giving it a chief medical officer. The law calls for the new CMO to have “real-world” experience treating patients with serious mental illness. The reformed SAMHSA will have responsibility for centralizing data collection to be used in creating best practices in mental health care. These reforms are particular victories for Rep. Murphy who has railed against the agency for years, citing its lack of medically trained leadership and lack of evidence-based approaches to care.
HIPAA requirements in serious mental illness are also clarified.
“Existing HIPAA laws are badly misunderstood,” said Sen. Chris Murphy (D-Conn.) at the briefing. “Many physicians think there is an outright ban on sharing [patient health] information.”
The legislation calls for the U.S. Department of Health and Human Services secretary to clarify when providers can disclose protected health information related to the treatment of an adult with a mental illness or substance use disorder.
“I would argue that [HIPAA] may be an area we will have to work on down the line, if the education campaign doesn’t work, but we will give this a try,” said Sen. Murphy, who has cosponsored legislation to do so.
As passed, the mental health provisions of the 21st Century Cures Act are an amalgamation of several bills introduced during the 114th Congress and are unique in that they largely were written by health care professionals with direct, relevant experience. Rep. Murphy is a clinical psychologist, while Rep. Cassidy is a physician with experience treating uninsured and incarcerated patients. Rep. Eddie Bernice Johnson (D-Tex.), who cosponsored Rep. Murphy’s bill, previously served as chief psychiatric nurse at the Dallas Veterans Affairs Medical Center.
WASHINGTON – Bipartisan legislation to improve care of patients with serious mental illness is just a pen stroke from passage, now that the 21st Century Cures Act is headed for the President’s desk.
While the mental health care reform provisions in the legislation (H.R. 34) will impact mental health services broadly, the bulk of the reform language comes from H.R. 2646, a bill sponsored by Rep. Tim Murphy (R-Penn.), which was intended to reverse decades of fragmented – and at times nonexistent – care for persons with serious mental illness.
The legislation calls for the expanded use of assisted outpatient treatment, federal guidance on how states can be reimbursed for offering inpatient psychiatric services, better data collection on mental health services paid for with state and federal dollars, new oversight of the federal agency in charge of mental health, and clarification of HIPAA laws, among other provisions.
“This is big,” Rep. Murphy said at a press conference Dec. 5. “After all the tears and suffering … know that those times when you felt no one was willing to listen, and you were ready to give up, now there is hope.”
Treatment vs. incarceration
Currently, states and communities can draw from $80 million dollars in federal grants for assisted outpatient treatment for patients with serious mental illness. The new legislation adds another $20 million and extends availability of the funding until 2022. It also allows state criminal justice systems to use assisted outpatient treatment grants to treat convicted criminals with serious mental illness instead of incarcerating them.
“[In prison], their illness gets worse, not better, creating a turnstile through which they will return time and again through our criminal justice system,” Senate Majority Whip John Cornyn (R.-Tex.) said during the press conference.
Paying for inpatient psychiatric care
The Cures Act also strengthens momentum for overhauling the Medicaid Institutions of Mental Diseases (IMD) exclusion, long a focus of mental health care advocates who view it as a governor on the number of psychiatric beds available nationally.
The exclusion prohibits any medical facility with more than 16 beds from using federal funds to pay for inpatient mental health care for adults under age 65 years. The Cures Act requires the Centers for Medicare & Medicaid Services to offer guidance on how states can use Medicaid demonstration projects to provide innovative inpatient psychiatric services using these waivers.
This follows an April 2016 CMS final rule that matches state funding dollar-per-dollar for up to 15 days of inpatient psychiatric treatment given to Medicaid managed care patients.
Although Rep. Murphy’s original bill called for abolishing the exclusion, he and other advocates are not disappointed in the progress made so far. “We didn’t get everything we wanted, but we needed everything we got,” he said.
“That 15 days was a massive win,” Frankie Berger, advocacy director of the Treatment Advocacy Center in Arlington, Va., said in an interview. “Now we have this legislation that requires CMS to tell state Medicaid directors what their wiggle room is when solving the problem on how to get care to these patients. If you read between the lines, this is care that is going to be provided by an IMD.”
The legislation also calls for more data collection and evaluation of IMDs, something that until now has been missing, Ms. Berger said. “A lot of us who work on this issue have found there is absolutely no data on where IMDs are, or who the folks are that are getting treatment in them. We need that information to make good choices,” she said.
“I think we will find savings as we expand the number of psychiatric beds … [and persons with serious mental illness] do not end up in jail cells, homeless, or in the morgue,” Sen. Bill Cassidy (R-La.) said at the press conference. He predicted that for each additional psychiatric bed introduced, savings would amount to between $30,000 and $50,000.
Restructuring, clarifying
The Cures Act reforms also restructure the Substance Abuse and Mental Health Services Administration, putting it under the authority of a new assistant secretary of Health and Human Services and giving it a chief medical officer. The law calls for the new CMO to have “real-world” experience treating patients with serious mental illness. The reformed SAMHSA will have responsibility for centralizing data collection to be used in creating best practices in mental health care. These reforms are particular victories for Rep. Murphy who has railed against the agency for years, citing its lack of medically trained leadership and lack of evidence-based approaches to care.
HIPAA requirements in serious mental illness are also clarified.
“Existing HIPAA laws are badly misunderstood,” said Sen. Chris Murphy (D-Conn.) at the briefing. “Many physicians think there is an outright ban on sharing [patient health] information.”
The legislation calls for the U.S. Department of Health and Human Services secretary to clarify when providers can disclose protected health information related to the treatment of an adult with a mental illness or substance use disorder.
“I would argue that [HIPAA] may be an area we will have to work on down the line, if the education campaign doesn’t work, but we will give this a try,” said Sen. Murphy, who has cosponsored legislation to do so.
As passed, the mental health provisions of the 21st Century Cures Act are an amalgamation of several bills introduced during the 114th Congress and are unique in that they largely were written by health care professionals with direct, relevant experience. Rep. Murphy is a clinical psychologist, while Rep. Cassidy is a physician with experience treating uninsured and incarcerated patients. Rep. Eddie Bernice Johnson (D-Tex.), who cosponsored Rep. Murphy’s bill, previously served as chief psychiatric nurse at the Dallas Veterans Affairs Medical Center.
KRAS-targeted T-cell therapy derails metastatic cancer
Adoptive transfer of cytotoxic T cells targeting a specific KRAS mutation (KRAS G12D) expressed by a metastatic colorectal cancer induced the regression of all seven lung metastases in a single patient, investigators report in the New England Journal of Medicine.
Mutations of the KRAS oncogene are frequent and drive the formation and progression of many human cancers. “The high incidence of KRAS G12D expression in [colorectal, pancreatic, and multiple other] cancers means that in the United States alone, thousands of patients per year would be potentially eligible for T-cell–based immunotherapy targeting KRAS G12D,” said Eric Tran, PhD, and his associates in the surgery branch, National Cancer Institute, Bethesda, Md.
They described the case of a 50-year-old woman participating in an ongoing phase II clinical trial assessing adoptive transfer of tumor-infiltrating lymphocytes targeting personalized cancer neoepitopes (individualized T-cell therapy). This patient was the only participant to date in whom the treatment induced tumor regression.
A total of 3 of her 10 lung metastases were resected to identify mutations expressed by the tumors, and these were found to express mutant KRAS G12D and HLA-C*08:02. The lymphocyte cultures that reacted most strongly against these antigens were expanded and made into a single infusion, which was administered after the patient had undergone nonmyeloablative conditioning using cyclophosphamide and fludarabine. The patient then received five doses of interleukin-2, which produced the only adverse effect of the entire regimen, fatigue.
At 40-day follow-up, all seven remaining lung metastases showed marked regression. One lesion showed progression at 9-month follow-up and was resected, and the patient has remained cancer free for the subsequent 4 months (N Engl J Med. 2016 Dec 8. doi:10.1056/NEJMoa1609279).
Laboratory analysis of the one metastasis that progressed showed that it had mutated further, losing heterozygosity of the copy of chromosome 6 that encoded HLA-C*08:02. “The presence of this molecule is required for direct tumor recognition by the transferred KRAS G12D-specific T cells. ... [Such a loss] has been observed in human cancers and may represent a hurdle that needs to be overcome to enhance immunotherapeutic efficacy in some patients,” Dr. Tran and his associates wrote.
“In such cases, targeting antigens that are presented by the remaining HLA class I alleles or exploiting the CD4+ T-cell response or the innate immune system against cancer may be necessary to induce a durable clinical response,” they added.
Adoptive transfer of cytotoxic T cells targeting a specific KRAS mutation (KRAS G12D) expressed by a metastatic colorectal cancer induced the regression of all seven lung metastases in a single patient, investigators report in the New England Journal of Medicine.
Mutations of the KRAS oncogene are frequent and drive the formation and progression of many human cancers. “The high incidence of KRAS G12D expression in [colorectal, pancreatic, and multiple other] cancers means that in the United States alone, thousands of patients per year would be potentially eligible for T-cell–based immunotherapy targeting KRAS G12D,” said Eric Tran, PhD, and his associates in the surgery branch, National Cancer Institute, Bethesda, Md.
They described the case of a 50-year-old woman participating in an ongoing phase II clinical trial assessing adoptive transfer of tumor-infiltrating lymphocytes targeting personalized cancer neoepitopes (individualized T-cell therapy). This patient was the only participant to date in whom the treatment induced tumor regression.
A total of 3 of her 10 lung metastases were resected to identify mutations expressed by the tumors, and these were found to express mutant KRAS G12D and HLA-C*08:02. The lymphocyte cultures that reacted most strongly against these antigens were expanded and made into a single infusion, which was administered after the patient had undergone nonmyeloablative conditioning using cyclophosphamide and fludarabine. The patient then received five doses of interleukin-2, which produced the only adverse effect of the entire regimen, fatigue.
At 40-day follow-up, all seven remaining lung metastases showed marked regression. One lesion showed progression at 9-month follow-up and was resected, and the patient has remained cancer free for the subsequent 4 months (N Engl J Med. 2016 Dec 8. doi:10.1056/NEJMoa1609279).
Laboratory analysis of the one metastasis that progressed showed that it had mutated further, losing heterozygosity of the copy of chromosome 6 that encoded HLA-C*08:02. “The presence of this molecule is required for direct tumor recognition by the transferred KRAS G12D-specific T cells. ... [Such a loss] has been observed in human cancers and may represent a hurdle that needs to be overcome to enhance immunotherapeutic efficacy in some patients,” Dr. Tran and his associates wrote.
“In such cases, targeting antigens that are presented by the remaining HLA class I alleles or exploiting the CD4+ T-cell response or the innate immune system against cancer may be necessary to induce a durable clinical response,” they added.
Adoptive transfer of cytotoxic T cells targeting a specific KRAS mutation (KRAS G12D) expressed by a metastatic colorectal cancer induced the regression of all seven lung metastases in a single patient, investigators report in the New England Journal of Medicine.
Mutations of the KRAS oncogene are frequent and drive the formation and progression of many human cancers. “The high incidence of KRAS G12D expression in [colorectal, pancreatic, and multiple other] cancers means that in the United States alone, thousands of patients per year would be potentially eligible for T-cell–based immunotherapy targeting KRAS G12D,” said Eric Tran, PhD, and his associates in the surgery branch, National Cancer Institute, Bethesda, Md.
They described the case of a 50-year-old woman participating in an ongoing phase II clinical trial assessing adoptive transfer of tumor-infiltrating lymphocytes targeting personalized cancer neoepitopes (individualized T-cell therapy). This patient was the only participant to date in whom the treatment induced tumor regression.
A total of 3 of her 10 lung metastases were resected to identify mutations expressed by the tumors, and these were found to express mutant KRAS G12D and HLA-C*08:02. The lymphocyte cultures that reacted most strongly against these antigens were expanded and made into a single infusion, which was administered after the patient had undergone nonmyeloablative conditioning using cyclophosphamide and fludarabine. The patient then received five doses of interleukin-2, which produced the only adverse effect of the entire regimen, fatigue.
At 40-day follow-up, all seven remaining lung metastases showed marked regression. One lesion showed progression at 9-month follow-up and was resected, and the patient has remained cancer free for the subsequent 4 months (N Engl J Med. 2016 Dec 8. doi:10.1056/NEJMoa1609279).
Laboratory analysis of the one metastasis that progressed showed that it had mutated further, losing heterozygosity of the copy of chromosome 6 that encoded HLA-C*08:02. “The presence of this molecule is required for direct tumor recognition by the transferred KRAS G12D-specific T cells. ... [Such a loss] has been observed in human cancers and may represent a hurdle that needs to be overcome to enhance immunotherapeutic efficacy in some patients,” Dr. Tran and his associates wrote.
“In such cases, targeting antigens that are presented by the remaining HLA class I alleles or exploiting the CD4+ T-cell response or the innate immune system against cancer may be necessary to induce a durable clinical response,” they added.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adoptive transfer of cytotoxic T cells targeting a specific KRAS mutation expressed by a metastatic colorectal cancer induced the regression of all seven lung metastases in a single patient.
Major finding: At 40-day follow-up, all seven lung metastases showed marked regression; the single metastasis that showed progression at 9-month follow-up was resected, and the patient has remained cancer free for the subsequent 4 months.
Data source: A single case study involving a woman with metastatic colorectal cancer treated with a experimental immunotherapy and followed for 13 months.
Disclosures: This work was supported by the National Cancer Institute. Dr. Tran and some of his associates reported holding a pending patent related to anti-KRAS G12D T-cell receptors.
Senate sends 21st Century Cures bill to president
With little debate and less dissent, the Senate passed the 21st Century Cures bill and sent it to President Obama for his promised signature.
The legislation passed the House by an overwhelming 392-26 vote on Nov. 30. Key provisions of the bill (H.R. 34) include:
• Food and Drug Administration reforms, including expedited review for certain medical devices, streamlined review for drug/device combinations, and increased patient involvement in the drug approval process, with $500 million to implement the reforms.
• $4.8 billion over 10 years for key biomedical research efforts including the BRAIN Initiative, the Cancer Moonshot, and the Precision Medicine Initiative.
• $1 billion in grants to states over a 2-year period to help supplement opioid abuse prevention and treatment activities.
• Provisions to improve the interoperability of EHRs.
• Provisions to improve treatment of serious mental illness.
“This bipartisan legislation – which [Senate] Majority Leader Mitch McConnell [R-Ky.] has called ‘the most important legislation Congress will pass this year’ – will help us take advantage of the breathtaking advances in biomedical research and bring those innovations to doctors’ offices and patients’ medicine cabinets around the county,” Sen. Lamar Alexander (R-Tenn.), chair of the Senate Health, Education, Labor, and Pensions Committee, said in a statement.
“The most important prescription drug-related crisis facing our country right now is the skyrocketing price of prescription drugs. This bill does not even deal with that issue,” Sen. Sanders said on the Senate floor. “How can we talk about a bill dealing with the pharmaceutical industry without addressing the elephant in the room, which is the fact that we pay the highest prices in the world for medicine?”
In his weekly address on Dec. 3, President Obama promised to “sign [the bill] as soon as it reaches my desk.”
Gregory Twachtman contributed to this report.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
With little debate and less dissent, the Senate passed the 21st Century Cures bill and sent it to President Obama for his promised signature.
The legislation passed the House by an overwhelming 392-26 vote on Nov. 30. Key provisions of the bill (H.R. 34) include:
• Food and Drug Administration reforms, including expedited review for certain medical devices, streamlined review for drug/device combinations, and increased patient involvement in the drug approval process, with $500 million to implement the reforms.
• $4.8 billion over 10 years for key biomedical research efforts including the BRAIN Initiative, the Cancer Moonshot, and the Precision Medicine Initiative.
• $1 billion in grants to states over a 2-year period to help supplement opioid abuse prevention and treatment activities.
• Provisions to improve the interoperability of EHRs.
• Provisions to improve treatment of serious mental illness.
“This bipartisan legislation – which [Senate] Majority Leader Mitch McConnell [R-Ky.] has called ‘the most important legislation Congress will pass this year’ – will help us take advantage of the breathtaking advances in biomedical research and bring those innovations to doctors’ offices and patients’ medicine cabinets around the county,” Sen. Lamar Alexander (R-Tenn.), chair of the Senate Health, Education, Labor, and Pensions Committee, said in a statement.
“The most important prescription drug-related crisis facing our country right now is the skyrocketing price of prescription drugs. This bill does not even deal with that issue,” Sen. Sanders said on the Senate floor. “How can we talk about a bill dealing with the pharmaceutical industry without addressing the elephant in the room, which is the fact that we pay the highest prices in the world for medicine?”
In his weekly address on Dec. 3, President Obama promised to “sign [the bill] as soon as it reaches my desk.”
Gregory Twachtman contributed to this report.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
With little debate and less dissent, the Senate passed the 21st Century Cures bill and sent it to President Obama for his promised signature.
The legislation passed the House by an overwhelming 392-26 vote on Nov. 30. Key provisions of the bill (H.R. 34) include:
• Food and Drug Administration reforms, including expedited review for certain medical devices, streamlined review for drug/device combinations, and increased patient involvement in the drug approval process, with $500 million to implement the reforms.
• $4.8 billion over 10 years for key biomedical research efforts including the BRAIN Initiative, the Cancer Moonshot, and the Precision Medicine Initiative.
• $1 billion in grants to states over a 2-year period to help supplement opioid abuse prevention and treatment activities.
• Provisions to improve the interoperability of EHRs.
• Provisions to improve treatment of serious mental illness.
“This bipartisan legislation – which [Senate] Majority Leader Mitch McConnell [R-Ky.] has called ‘the most important legislation Congress will pass this year’ – will help us take advantage of the breathtaking advances in biomedical research and bring those innovations to doctors’ offices and patients’ medicine cabinets around the county,” Sen. Lamar Alexander (R-Tenn.), chair of the Senate Health, Education, Labor, and Pensions Committee, said in a statement.
“The most important prescription drug-related crisis facing our country right now is the skyrocketing price of prescription drugs. This bill does not even deal with that issue,” Sen. Sanders said on the Senate floor. “How can we talk about a bill dealing with the pharmaceutical industry without addressing the elephant in the room, which is the fact that we pay the highest prices in the world for medicine?”
In his weekly address on Dec. 3, President Obama promised to “sign [the bill] as soon as it reaches my desk.”
Gregory Twachtman contributed to this report.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
VIDEO: Angiogenesis has a role to play in NASH and NAFLD
BOSTON – A multimodal research process has provided clues to the role of angiogenesis in nonalcoholic fatty liver disease (NAFLD) and its more serious cousin, nonalcoholic steatohepatitis (NASH).
In constructing a protocol that began with patients, moved on to bioinformatics and then performed final validation in the petri dish, Savneet Kaur, PhD, and her colleagues were able to identify several angiogenesis genes likely to contribute to the development of NAFLD and NASH.
“We have seen angiogenic mechanisms and angiogenic genes in the pathophysiology of nonalcoholic fatty liver disease,” said Dr. Kaur, professor of biotechnology at Gautam Buddha Medical School, Greater Noida, India, in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Kaur said that she and her coinvestigators began with a small group of eight patients with NASH, and seven patients with NAFLD, and also with seven healthy control participants. Genetic analysis and comparison of these patients yielded differential expression of certain genes already known to be associated with angiogenesis in the NASH and NAFLD, but not the healthy control participants.
“We did a microarray analysis, a high throughput gene expression study, and then we selected around 25 to 26 genes which were associated with oxidative stress and angiogenesis,” said Dr. Kaur.
For validation of these genes, Dr. Kaur and her associates used a larger study group of about 150 participants, again approximately evenly divided between those with mild NAFLD, those with more severe steatohepatitis, and healthy controls. “We validated the angiogenic genes in the subject group, and found that around 13 genes are preferentially expressed.”
“About 13 genes including VEGFR1, PIK3CA, CXCL8, NOS3, EREG, CCL2, PRKCE, PPar-gamma, PROK2, RUNX1, SIRT1, HMOX1 and CXCR4 showed significantly different gene expression in the [reverse transcription–polymerase chain reaction] analysis in Gr3 as compared to Gr1 (P less than .05 for each), whereas genes such as PIK3CA, CXCL8, NOS3, CCL2, PROK2, RUNX1, and HMOX1 were differentially expressed in Gr3 in comparison to Gr2 (P less than .05 each). A few genes, PPar-gamma, SIRT1, VEGFR1, HMOX1, PIK3CA, CXCR4, PROK2, and CCL2, showed correlations with fibrosis scores, angiogenesis scores, and NAFLD activity scores of the patients,” wrote Dr. Kaur and her colleagues in the abstract accompanying the poster presentation.
Taking these candidate genes, Dr. Kaur and her colleagues conducted a bioinformatics analysis to determine which transcription factors were controlling the genes. “We wanted to study the pathway – the mechanisms – to determine the upregulation and downregulation of these genes,” said Dr. Kaur.
Finally, Dr. Kaur and her associates took an in vitro approach, using human steatotic hepatocytes and endothelial cells, since “angiogenesis is a property of endothelial cells.” The two types of cells were cultured together, and angiogenic function and gene expression were examined, and checked against the genes and pathways identified in the first two steps. They again saw expression of the angiogenic pathways in the cell culture model. This was consistent with what is seen in patients with NAFLD and NASH: “Definitely, there’s an increase in angiogenesis. There’s an increase in the endothelial cell proliferation, with more fat, more steatosis in the patients,” said Dr. Kaur. Some genes, said Dr. Kaur, are “really important” to this process. Her group is now investigating how the genes are regulated, in order to understand better the precise role of angiogenesis in steatohepatitis.
The study was part of a joint Indo-German project, and sponsored by the Indian Council for Medical Research and the German Federal Ministry of Education and Research. Dr. Kaur reported no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
BOSTON – A multimodal research process has provided clues to the role of angiogenesis in nonalcoholic fatty liver disease (NAFLD) and its more serious cousin, nonalcoholic steatohepatitis (NASH).
In constructing a protocol that began with patients, moved on to bioinformatics and then performed final validation in the petri dish, Savneet Kaur, PhD, and her colleagues were able to identify several angiogenesis genes likely to contribute to the development of NAFLD and NASH.
“We have seen angiogenic mechanisms and angiogenic genes in the pathophysiology of nonalcoholic fatty liver disease,” said Dr. Kaur, professor of biotechnology at Gautam Buddha Medical School, Greater Noida, India, in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Kaur said that she and her coinvestigators began with a small group of eight patients with NASH, and seven patients with NAFLD, and also with seven healthy control participants. Genetic analysis and comparison of these patients yielded differential expression of certain genes already known to be associated with angiogenesis in the NASH and NAFLD, but not the healthy control participants.
“We did a microarray analysis, a high throughput gene expression study, and then we selected around 25 to 26 genes which were associated with oxidative stress and angiogenesis,” said Dr. Kaur.
For validation of these genes, Dr. Kaur and her associates used a larger study group of about 150 participants, again approximately evenly divided between those with mild NAFLD, those with more severe steatohepatitis, and healthy controls. “We validated the angiogenic genes in the subject group, and found that around 13 genes are preferentially expressed.”
“About 13 genes including VEGFR1, PIK3CA, CXCL8, NOS3, EREG, CCL2, PRKCE, PPar-gamma, PROK2, RUNX1, SIRT1, HMOX1 and CXCR4 showed significantly different gene expression in the [reverse transcription–polymerase chain reaction] analysis in Gr3 as compared to Gr1 (P less than .05 for each), whereas genes such as PIK3CA, CXCL8, NOS3, CCL2, PROK2, RUNX1, and HMOX1 were differentially expressed in Gr3 in comparison to Gr2 (P less than .05 each). A few genes, PPar-gamma, SIRT1, VEGFR1, HMOX1, PIK3CA, CXCR4, PROK2, and CCL2, showed correlations with fibrosis scores, angiogenesis scores, and NAFLD activity scores of the patients,” wrote Dr. Kaur and her colleagues in the abstract accompanying the poster presentation.
Taking these candidate genes, Dr. Kaur and her colleagues conducted a bioinformatics analysis to determine which transcription factors were controlling the genes. “We wanted to study the pathway – the mechanisms – to determine the upregulation and downregulation of these genes,” said Dr. Kaur.
Finally, Dr. Kaur and her associates took an in vitro approach, using human steatotic hepatocytes and endothelial cells, since “angiogenesis is a property of endothelial cells.” The two types of cells were cultured together, and angiogenic function and gene expression were examined, and checked against the genes and pathways identified in the first two steps. They again saw expression of the angiogenic pathways in the cell culture model. This was consistent with what is seen in patients with NAFLD and NASH: “Definitely, there’s an increase in angiogenesis. There’s an increase in the endothelial cell proliferation, with more fat, more steatosis in the patients,” said Dr. Kaur. Some genes, said Dr. Kaur, are “really important” to this process. Her group is now investigating how the genes are regulated, in order to understand better the precise role of angiogenesis in steatohepatitis.
The study was part of a joint Indo-German project, and sponsored by the Indian Council for Medical Research and the German Federal Ministry of Education and Research. Dr. Kaur reported no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
BOSTON – A multimodal research process has provided clues to the role of angiogenesis in nonalcoholic fatty liver disease (NAFLD) and its more serious cousin, nonalcoholic steatohepatitis (NASH).
In constructing a protocol that began with patients, moved on to bioinformatics and then performed final validation in the petri dish, Savneet Kaur, PhD, and her colleagues were able to identify several angiogenesis genes likely to contribute to the development of NAFLD and NASH.
“We have seen angiogenic mechanisms and angiogenic genes in the pathophysiology of nonalcoholic fatty liver disease,” said Dr. Kaur, professor of biotechnology at Gautam Buddha Medical School, Greater Noida, India, in a video interview at the annual meeting of the American Association for the Study of Liver Diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Kaur said that she and her coinvestigators began with a small group of eight patients with NASH, and seven patients with NAFLD, and also with seven healthy control participants. Genetic analysis and comparison of these patients yielded differential expression of certain genes already known to be associated with angiogenesis in the NASH and NAFLD, but not the healthy control participants.
“We did a microarray analysis, a high throughput gene expression study, and then we selected around 25 to 26 genes which were associated with oxidative stress and angiogenesis,” said Dr. Kaur.
For validation of these genes, Dr. Kaur and her associates used a larger study group of about 150 participants, again approximately evenly divided between those with mild NAFLD, those with more severe steatohepatitis, and healthy controls. “We validated the angiogenic genes in the subject group, and found that around 13 genes are preferentially expressed.”
“About 13 genes including VEGFR1, PIK3CA, CXCL8, NOS3, EREG, CCL2, PRKCE, PPar-gamma, PROK2, RUNX1, SIRT1, HMOX1 and CXCR4 showed significantly different gene expression in the [reverse transcription–polymerase chain reaction] analysis in Gr3 as compared to Gr1 (P less than .05 for each), whereas genes such as PIK3CA, CXCL8, NOS3, CCL2, PROK2, RUNX1, and HMOX1 were differentially expressed in Gr3 in comparison to Gr2 (P less than .05 each). A few genes, PPar-gamma, SIRT1, VEGFR1, HMOX1, PIK3CA, CXCR4, PROK2, and CCL2, showed correlations with fibrosis scores, angiogenesis scores, and NAFLD activity scores of the patients,” wrote Dr. Kaur and her colleagues in the abstract accompanying the poster presentation.
Taking these candidate genes, Dr. Kaur and her colleagues conducted a bioinformatics analysis to determine which transcription factors were controlling the genes. “We wanted to study the pathway – the mechanisms – to determine the upregulation and downregulation of these genes,” said Dr. Kaur.
Finally, Dr. Kaur and her associates took an in vitro approach, using human steatotic hepatocytes and endothelial cells, since “angiogenesis is a property of endothelial cells.” The two types of cells were cultured together, and angiogenic function and gene expression were examined, and checked against the genes and pathways identified in the first two steps. They again saw expression of the angiogenic pathways in the cell culture model. This was consistent with what is seen in patients with NAFLD and NASH: “Definitely, there’s an increase in angiogenesis. There’s an increase in the endothelial cell proliferation, with more fat, more steatosis in the patients,” said Dr. Kaur. Some genes, said Dr. Kaur, are “really important” to this process. Her group is now investigating how the genes are regulated, in order to understand better the precise role of angiogenesis in steatohepatitis.
The study was part of a joint Indo-German project, and sponsored by the Indian Council for Medical Research and the German Federal Ministry of Education and Research. Dr. Kaur reported no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT THE LIVER MEETING 2016
Key clinical point:
Major finding: PPar-gamma, SIRT1, VEGFR1, and other genes are associated with fibrosis, angiogenesis, and steatosis.
Data source: Human bioinformatics–in vitro exploration of genetic pathways associated with angiogenesis in NASH and nonalcoholic fatty liver disease (NAFLD).
Disclosures: The study was part of a joint Indo-German project, and funded by the Indian Council for Medical Research and the German Federal Ministry of Education and Research. Dr. Kaur reported no relevant financial disclosures.