User login
Prevalence of Antibiotic Allergy at a Spinal Cord Injury Center
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described asa threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
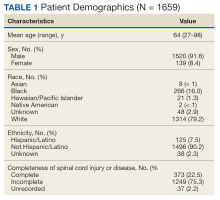
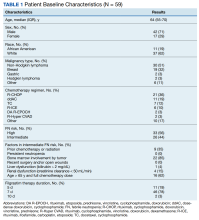
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
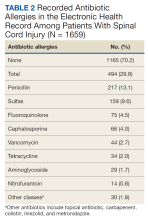
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described asa threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
Infectious diseases are the most common reason for rehospitalization among patients with spinal cord injuries (SCI), regardless of the number of years postinjury.1 The appropriate use and selection of antibiotics for properly diagnosed infectious diseases is especially important for this population. This principle helps to avoid the development of drug-resistant organisms and reduces the risk of recurrent infections, aligning with antibiotic stewardship.
Antibiotics are the most common class of drug allergies in the general population, and penicillin is the most frequently reported allergen (up to 10%).2 Prescription drug–induced anaphylaxis is severe and life threatening with a reported frequency of 1.1%. Penicillin and sulfonamide (46 and 15 per 10,000 patients, respectively) are the most common allergens.3 Although there is a significant difference between an adverse drug reaction (ADR) and true hypersensitivity, once documented in the electronic health record (EHR) as an allergy, this information deters use of the listed drugs.
Genitourinary, skin, and respiratory diseases are the leading causes for rehospitalization in patients with SCI.1 A large proportion of these are infectious in etiology and require antibiotic treatment. In fact, persons with SCI are at high risk for antibiotic overuse and hospital-acquired infection due to chronic bacteriuria, frequent health care exposure, implanted medical devices, and other factors.4 Concurrently, there is a crisis of antibiotic-resistant bacteria proliferation, described asa threat to patient safety and public health.5,6 Its severity is illustrated by the report that 38% of the cultures from patients with spinal cord injury are multidrug resistant gram-negative organisms.7
The SCI center at James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, serves a high concentration of active-duty military members and veterans with SCI. A study that reviews the exact frequency of antibiotic drug allergies listed on the EHR would be a key first step to identify the magnitude of this issue. The results could guide investigation into differentiating true allergies from ADRs, thereby widening the options for potentially life-saving antibiotic treatment.
Methods
We performed a retrospective chart review of patients included in the local SCI registry between October 1, 2015, and September 30, 2017. We collected data on patient demographics (age, sex, race and ethnicity) and a description of patients’ injuries (International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and etiology of injury [traumatic vs atraumatic]). The outcomes included antibiotic allergy and ADRs.
In the EHR, allergies can be listed toward an antibiotic class or a specific antibiotic. An allergy to each specific antibiotic would be recorded separately; however, overlap among antibiotic classes was not duplicated. For example, if a subject has a listed antibiotic allergy to ceftriaxone and cefepime with listed reactions, we would record allergies to each of these antibiotics but would only report a single allergy to the cephalosporin subclass.
Since we did not differentiate hypersensitivity reactions (HSRs) from other ADRs, the reported reactions were grouped by signs and symptoms. There is a variety of terms used to report similar reactions, and best efforts were made to record the data as accurately as possible. Patient-reported history for risk stratification is a tool we used to group these historical reactions into high- vs low-risk for severe reactions. High-risk signs are those listed as anaphylaxis; anaphylactic reactions; angioedema presenting as swelling of mouth, eyes, lips, or tongue; blisters or ulcers involving the lips, mouth, eyes, urethra, vagina, or peeling skin; respiratory changes; shortness of breath; dyspnea; hypotension; or organ involvement (kidneys, lungs, liver).6
Inclusion criteria were all veterans who were diagnosed with tetraplegia or paraplegia and received annual evaluation between October 1, 2015, and September 30, 2017. We chose this period because it was the beginning of a financial year at the JAHVH SCI department using the SCI registry. The SCI annual evaluation is a routine practitioner encounter with the veteran, along with appropriate laboratory testing and imaging to follow up potential chronic health issues specific to patients with SCI. Annual evaluations provide an opportunity to maintain routine health screening and preventive care. Patients who had significant portions of data missing or missing elements of primary outcomes were excluded from analysis. The study was reviewed and approved by the University of South Florida Institutional Review Board (VA IRBNet #1573370-4 on September 9, 2019).
Results
Of 1866 patients reviewed, 207 (11.1%) were excluded due to missing data, resulting in 1659 records that were analyzed. Mean age was 64 years, and male to female ratio was about 10 to 1. Most of the SCI or diseases were classified as incomplete (n = 1249) per ISNCSCI (absence of sensory and motor function in the lowest sacral segments) compared with 373 classified as complete.
Of the 1659 patients, 494 (29.8%) had a recorded allergy to antibiotics. The most frequently recorded were 217 penicillin (13.1%), 159 sulfa drugs (9.6%), 75 fluoroquinolone (4.5%), 66 cephalosporin (4.0%), and 44 vancomycin (2.7%) allergies.
Discussion
In this study, we evaluated the frequency and characteristics of antibiotic allergies at a single SCI center to better identify potential areas for quality improvement when recording drug allergies. A study in the general population used self-reported methods to collect such information found about a 15% prevalence of antibiotic allergy, which was lower than the 29.8% prevalence noted in our study.8
Regarding the most common antibiotic allergies, one study reported allergy to penicillin in the EHR in 12.8% of patients at a major US regional health care system, while 13.1% of patients with SCI had documented allergy to penicillin in our study.9 Regarding the other antibiotic classes, the percentage of allergies were higher than those reported in the general population: sulfonamide (9.6% vs 7.4%), fluoroquinolones (4.5% vs 1.3%), and cephalosporins (4.0% vs 1.7%).10 The EHR appears to capture a much higher rate of antibiotic allergies than that in self-reported studies, such as a study of self-reported allergy in the general adult population in Portugal, where only 4.5% of patients reported allergy to any β-lactam medications.10
The prevalence of an antibiotic allergy could be affected by the health care setting and sex distribution. For example, the Zhou and colleagues’ study conducted in the Greater Boston area showed higher reported antibiotic rates than those in a study from a Southern California medical group. The higher proportion of tertiary referral patients in that specific network was suggested to be the cause of the difference.8,9 Our results in the SCI population are more comparable to that in a tertiary setting. This is consistent with the fact that persons with SCI generally have more exposure to antibiotics and consequently a higher reported rate of allergic reactions to antibiotics.
Similarly, the same study in Southern California noted that female patients use more antibiotics than do male patients, thus potentially contributing to higher rates of reported allergy toward all classes of antibiotics.8 Our study did not investigate antibiotic allergy by sex; however, the significantly higher proportion of male sex among the veteran population would have impacted these results.
Limitations
Our study was limited as a single-center retrospective study. However, our center is one of the major SCI specialty hubs, and the results should be somewhat reflective of those in the veterans with SCI population. Veterans under the US Department of Veterans Affairs (VA) medical care have the option to seek care or procedures in non-VA facilities. If allergies to antibiotics occurred outside of the VA system, there is no mechanism to automatically merge with the VA EHR allergy list, unless they are later recorded and added to the VA EHR. Thus, there is potential for underreporting.
Drug anaphylaxis incidence was noted to change over time.4,8,9 For example, a downtrend of reported antibiotic allergy was reported between 1990 and 2013.10 Our study only reflects an overall prevalence of a single cohort, without demonstration of relationship to time.
Lastly, this study did not aim to differentiate HSRs from other ADRs. This is exactly the point of the study, which investigated the frequency of EHR-recorded antibiotic allergies in our SCI population and reflects the issue with indiscriminate recording of ADRs and HSRs under the umbrella of allergy in the EHR. Further diagnosing true allergies should be considered in the SCI population after weighing the risks and benefits of assessment, aligning with the wishes of the veteran, obtaining informed consent, and addressing the cost-effectiveness of specific tests. We suggest that primary care practitioners work closely with allergy specialists to formulate a mechanism to diagnose various antibiotic allergic reactions, including serum tryptase, epicutaneous skin testing, intradermal skin testing, patch testing, delayed intradermal testing, and drug challenge as appropriate. It is also possible that in cases where very mild reactions/adverse effects of antibiotics were recorded in the EHR, the clinicians and veterans may discuss reintroducing the same antibiotics or proceeding with further testing if necessary. In contrast, the 12% of those with a high risk of severe allergic reactions to penicillin in our study would benefit from allergist evaluation and access to epinephrine auto-injectors at all times. Differentiating true allergy is the only clear way to deter unnecessary avoidance of first-line therapies for antibiotic treatment and avoid promotion of antibiotic resistance.
Future studies can analyze antibiotic allergy based on demographics, including sex and age difference, as well as exploring outpatient vs inpatient settings. Aside from prevalence, we hope to demonstrate antibiotic allergy over time, especially after integration of diagnostic allergy testing, to evaluate the impact to EHR-recorded allergies.
Conclusions
Almost 30% of patients with SCI had a recorded allergy to at least 1 antibiotic. The most common allergy was to penicillin, which is similar to what has previously been reported for the general adult US population. However, only 12% of those with a penicillin allergy were considered high risk of true allergic reactions. Consequently, there are opportunities to examine whether approaches to confirm true reactions (such as skin testing) would help to mitigate unnecessary avoidance of certain antibiotic classes due to mild ADRs, rather than a true allergy, in persons with SCI. This would be an important effort to combat both individual safety concerns and the public health crisis of antibiotic resistance. Given the available evidence, it is reasonable for SCI health care practitioners to discuss the potential risks and benefits of allergy testing with patients with SCI; this maintains a patient-centered approach that can ensure judicious use of antibiotics when necessary.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
References
1. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Model Systems. 2016 Annual Report –Complete Public Version. University of Alabama at Birmingham. Accessed March 20, 2023. https://www.nscisc.uab.edu/Public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
2. Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997;100(5):586-591. doi:10.1016/s0091-6749(97)70159-3 3. Dhopeshwarkar N, Sheikh A, Doan R, et al. Drug-induced anaphylaxis documented in electronic health records. J Allergy Clin Immunol Pract. 2019;7(1):103-111. doi:10.1016/j.jaip.2018.06.010
4. Evans CT, LaVela SL, Weaver FM, et al. Epidemiology of hospital-acquired infections in veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol. 2008;29(3):234-242. doi:10.1086/527509
5. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol. 2018;39(2):210-213. doi:10.1017/ice.2017.291
6. Blumenthal KG, Peter JG, Trubiano JA, Phllips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183-198. doi:10.1016/S0140-6736(18)32218-9 7. Evans CT, Fitzpatrick MA, Jones MM, et al. Prevalence and factors associated with multidrug-resistant gram-negative organisms in patients with spinal cord injury. Infect Control Hosp Epidemiol. 2017;38(12):1464-1471. doi:10.1017/ice.2017.238 8. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e1-778.e7. doi:10.1016/j.amjmed.2009.01.034
9. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305-1313. doi:10.1111/all.12881
10. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. doi:10.1111/j.1365-2222.2004.02070.x
Open Clinical Trials for Patients With Cancer
Prostate Cancer
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor: VA Greater Los Angeles Healthcare System
Location: VA Greater Los Angeles Healthcare System
Patient Decision-Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This project proposes to understand and improve veterans’ decision-making in precision oncology (germline testing, somatic tumor testing, and targeted therapy) for advanced prostate cancer. As precision oncology expands, a comprehensive strategy to support patient informed decision-making has not been developed.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; US Department of Defense
Location: San Francisco VA Medical Center
Intramuscular Mechanisms of Androgen Deprivation-Related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes lowering the levels of the hormone testosterone as much as possible, which is called androgen deprivation therapy (ADT). Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as sarcopenia, and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because investigators do not understand the mechanisms that cause it. The mitochondria is the part of the cells responsible for providing energy to muscles but to date the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT. The overall goal of this proposal is to establish if the mitochondria is responsible for sarcopenia in patients with prostate cancer receiving ADT. The investigators will measure mitochondrial function, muscle mass and strength, and feelings of fatigue and quality of life in patients with prostate cancer before starting and after 6 months of ADT.
ID: NCT03867357
Sponsor; Collaborator: Seattle Institute for Biomedical and Clinical Research; US Department of Defense
Location: VA Puget Sound Health Care System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for OligoRecurrent Prostate Cancer (VA STARPORT)
The primary goal of this study is to determine if adding PET-directed local therapy improves disease control compared to standard systemic therapy alone (SST) in veterans with oligorecurrent prostate cancer on PET/CT. The investigators will conduct a multi-institutional phase II/III randomized trial comparing SST with or without PET-directed local therapy using radiation or surgery to all metastases and if a local recurrence is present.
ID: NCT04787744
Sponsor: VA Office of Research and Development
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VA Medical Center, St. Louis VA Medical Center John Cochran Division, East Orange Campus of the VA New Jersey Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, Clement J. Zablocki VA Medical Center
Standard Systemic Therapy With or Without Definitive Treatment in Treating Participants With Metastatic Prostate Cancer
This phase III trial studies how well standard systemic therapy with or without definitive treatment (prostate removal surgery or radiation therapy) works in treating participants with prostate cancer that has spread to other places in the body. The addition of prostate removal surgery or radiation therapy to standard systemic therapy for prostate cancer may lower the chance of the cancer growing or spreading.
ID: NCT03678025
Sponsor; Collaborator: Southwest Oncology Group; National Cancer Institute (NCI)
Locations: 328 sites, including Tibor Rubin VA Medical Center, Atlanta VA Medical Center, James J. Peters VA Medical Center, Michael E. DeBakey VA Medical Center, and Audie L. Murphy VA Hospital
A Clinical Study Evaluating the Benefit of Adding Rucaparib to Enzalutamide for Men With Metastatic Prostate Cancer That Has Become Resistant to Testosterone-Deprivation Therapy (CASPAR)
This randomized, placebo-controlled, phase III trial is evaluating the benefit of rucaparib and enzalutamide combination therapy vs enzalutamide alone for the treatment of men with prostate cancer that has spread to other places in the body (metastatic) and has become resistant to testosterone-deprivation therapy (castration-resistant). Enzalutamide helps fight prostate cancer by blocking the use of testosterone by the tumor cells for growth. Poly adenosine diphosphate (ADP)-ribose polymerase (PARP) inhibitors, such as rucaparib, fight prostate cancer by prevent tumor cells from repairing their DNA. Giving enzalutamide and rucaparib may make patients live longer or prevent their cancer from growing or spreading for a longer time, or both. It may also help doctors learn if a mutation in any of the homologous recombination DNA repair genes is helpful to decide which treatment is best for the patient.
ID: NCT04455750
Sponsor; Collaborator: Alliance for Clinical Trials in Oncology; National Cancer Institute (NCI)
Locations: 413 sites
Digitally Captured Activity Data and PROs to Monitor Physical Function in Prostate Cancer Patients (DigiPRO)
Physical function is a known predictor of quality of life in advanced prostate cancer patients and key measure of treatment tolerability. While treatment with androgen deprivation therapy (ADT) improves survival, it is associated with significant toxicities that lead to physical function (PF) decline. The average age of incident prostate cancer is 66 years, and in this older group of men, chronic comorbid conditions often co-occur with diagnosis, further adding to the risk for PF decline. With over 2.9 million prostate cancer survivors in the US, there is an increasing demand for adequate symptom monitoring and PF assessment throughout cancer care. However, there are currently no validated methods to systematically evaluate and predict PF decline. Thus, the overarching objective of this proposal is to determine whether the use of wearable technology to monitor objective daily activity combined with routine symptom reporting can predict PF decline. To accomplish this, we propose a mixed-methods approach that will provide quantitative information to help identify PC survivors at higher risk for PF decline as well as a qualitative aim gain a deeper understanding of the perceived relationships that PC survivors have with their physical activity levels and treatment symptoms.
ID: NCT04575402
Sponsor; Collaborator: Cedars-Sinai Medical Center; US Department of Defense
Location: Cedars Sinai Medical Center
The BurnAlong Pilot Study for Adolescent and Young Adult Cancer Survivors
The purpose of this prospective, interventional, single-arm pilot study is to evaluate whether virtually delivered group-based physical activity is feasible for adolescent and young adult (AYA) cancer survivors. AYAs who were diagnosed with cancer and have completed cancer treatment will be recruited for this study. This study will enroll 20 participants in total and will last approximately 3 months.
ID: NCT05131815
Sponsor; Collaborator: Cedars-Sinai Medical Center; Walter Reed National Military Medical Center
Location: Cedars-Sinai Medical Center
Lung Cancer
DECAMP 1 PLUS: Prediction of Lung Cancer Using Noninvasive Biomarkers
The Detection of Early lung Cancer Among Military Personnel (DECAMP) consortium is a multidisciplinary and translational research program for lung cancer early detection. DECAMP 1 PLUS aims to improve the efficiency of the diagnostic evaluation of patients with indeterminate pulmonary nodules (8-25 mm). Molecular biomarkers for lung cancer diagnosis measured in minimally invasive and noninvasive biospecimens may be able to distinguish between malignant or benign indeterminate pulmonary nodules in high-risk smokers. Ultimately, this study aims to validate molecular as well as clinical and imaging biomarkers of lung cancer in individuals with indeterminate lung nodules.
ID: NCT04165564
Sponsor: Boston University
Locations: 3 VA medical centers (VA Greater LA Healthcare System, VA Boston Healthcare System, and VA Tennessee Valley Healthcare System), 3 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, and Naval Medical Center Portsmouth) and 12 academic hospitals
DECAMP-2: Screening of Patients With Early Stage Lung Cancer or at High Risk for Developing Lung Cancer (DECAMP-2)
The goal of this project is to improve lung cancer screening in high-risk individuals by identifying biomarkers of preclinical disease and disease risk that are measured in minimally invasive and noninvasive biospecimens. Existing biomarkers for lung cancer diagnosis as well as new biomarkers discovered specifically in this clinical setting will be examined. Biomarkers that identify individuals at highest risk for being diagnosed with lung cancer prior to the appearance of concerning symptoms could increase the utility of lung cancer surveillance and the efficiency of lung cancer chemoprevention clinical trials. Achieving these goals would improve the detection and treatment of early-stage and incipient lung cancer, while restricting the risk of these procedures to those individuals who currently exhibit the early molecular warning signs of impending disease.
ID: NCT02504697
Sponsor: Boston University
Locations: VA medical centers (including Los Angeles VA Healthcare System, Boston VA Research Institute, Inc, Philadelphia VA Medical Center, Veterans Research Foundation of Pittsburgh, and VA North Texas Health Care System), 4 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, San Antonio Military Medical Center, and Naval Medical Center Portsmouth), and 4 academic hospitals
Improving Decision-Making Encounters in Lung Cancer Using a Low-Literacy Conversation Tool (iDECIDE)
This clinical trial evaluates the effectiveness of a conversation tool on patient-centered health and decision-making outcomes in patients with lung cancer making treatment decisions. This research is being conducted to help doctors understand the information patients need to participate in shared decision-making about their lung cancer treatment options. The focus of this research is to study how patients choose lung cancer treatment options and the information needed to make that choice, with a focus on patients with lower health literacy.
ID: NCT05407168
Sponsor: Oregon Health & Science University Knight Cancer Institute
Locations: Portland VA Medical Center and Oregon Health & Science University Knight Cancer Institute
VA Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non–small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggest that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of 2 incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these 2 treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life expectancy and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
This study is designed to open at VA medical centers with expertise in both treatments. The recruitment process includes shared decision making and multidisciplinary evaluations with lung cancer specialists. Mandatory evaluations before randomization include tissue confirmation of NSCLC, staging with FDG-PET/CT, and biopsies of all hilar and/or mediastinal lymph nodes > 10 mm that have a SUV > 2.5. Prerandomization elective lymph node sampling is strongly encouraged, but not required. Following treatment, patients will be followed for a minimum of 5 years.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: 17 VA medical centers, including VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Miami VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, Baltimore VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Corporal Micheal J. Crescenz VA Medical Center, VA Pittsburgh Healthcare System University Drive Division, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, and Clement J. Zablocki VA Medical Center
Utility of CAML as Diagnostic for Early Stage Lung Cancer
The primary objective of this study is to determine the prevalence of cancer associated macrophage-like cells (CAMLS) in patients with pulmonary nodules. Secondary objectives include the following: determine the positive and negative predictive value of CAMLS in patients with pulmonary nodules who undergo biopsy; model combinations of clinical factors with the presence/absence of CAMLS to refine strategies for assessment of patients with pulmonary nodules; and evaluate whether these measures result in enhanced T-cell activity and/or natural killer cell function and number.
ID: NCT03992183
Sponsor; Collaborators: Fox Chase Cancer Center; US Department of Defense
Locations: Corporal Michael J. Crescenz VA Medical Center and Fox Chase Cancer Center
PROSPECT - Profiling of Resistance Patterns & Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax and Therapeutic Target Identification
This study will use therapeutic target-focused (TTF) profiling, genome-wide mRNA profiling, and assessments of tumor phosphopeptides and DNA that are shed into the bloodstream to define how various molecular factors alone and in combination relate to resistance to therapy, to prognosis, and to metastatic patterns at relapse. This study will examine how the presence of factors that drive cell growth, antagonize apoptosis, or confer resistance in other ways may counter the effect of systemic therapies and/or promote rapid tumor recurrence. In this way, the investigators will identify new, previously unappreciated potential therapeutic targets while also identifying which targets are most likely to increase resistance to therapy and worsen prognosis.
ID: NCT05049837
Sponsor; Collaborators: MD Anderson Cancer Center; US Department of Defense, National Institutes of Health (NIH), and National Cancer Institute (NCI)
Location: MD Anderson Cancer Center
Tribally Engaged Approaches to Lung Screening (TEALS)
Lung cancer is the leading cause of cancer mortality among American Indians and Alaska Natives (AI/AN), and AI/AN have worse lung cancer incidence rates, survival, and death compared to the general population. Because lung cancer screening (LCS) with low-dose computed tomography (LDCT) has been shown to reduce lung cancer mortality by roughly 20%, the US Preventive Services Task Force now recommends LCS for persons aged 55 to 80 years who meet specific eligibility criteria (grade-B evidence), and subsequently the Center for Medicare and Medicaid Services (CMS) opted to cover this test. However, the uptake of LCS implementation has been slow in most health care systems, and LCS implementation among AI/AN has never been studied.
To address this knowledge, the Tribally Engaged Approaches to Lung Screening (TEALS) study, a collaborative effort between the Choctaw Nation of Oklahoma, the Stephenson Cancer Center, and the University of Oklahoma Health Sciences Center, will address the following over the course of 5 years: conduct focus groups and semistructured interviews with Choctaw Nation Health Services Authority (CNHSA) patients, clinicians, and health administrators to elucidate individual- and system-level barriers and facilitators that affect the implementation of LCS; develop an LCS care coordination intervention that will identify eligible persons for LCS, help these patients navigate the screening process, and link them with smoking cessation services, when applicable; measure the impact of the TEALS intervention on the receipt of screening and a set of patient- and practice-level outcomes by conducting a cluster-randomized clinical trial of LCS implementation; and disseminate the TEALS program to other researchers and healthcare systems that serve AI/AN patients. TEALS will bridge the gap between evidence and clinical practice for LCS in a high-need, low-resource setting by intervening at the level of the healthcare system.
System-level interventions for guideline implementation tend to be understudied compared to those evaluating individual-level, behavioral interventions. However, the careful development and evaluation of an LCS screening program at the level of the healthcare system would be critical to ensure that more patients can receive LCS. Our research will create a critically needed platform from which future studies could be launched that will examine how to tailor the application of the LCS guideline to the individual preferences of AI/AN patients. TEALS will establish an effective LCS program in a tribal system and thus provide a direct benefit to the Choctaw Nation by increasing LCS participation. TEALS will serve as a blueprint for establishing a sustainable and accessible infrastructure for LCS in AI/AN and other community health systems. By increasing screening for early stage lung cancer, TEALS could ultimately reduce lung cancer mortality in AI/AN communities.
ID: NCT04948060
Sponsor; Collaborator: University of Oklahoma; Choctaw Nation of Oklahoma
Location: University of Oklahoma Health Sciences Center
Prostate Cancer
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor: VA Greater Los Angeles Healthcare System
Location: VA Greater Los Angeles Healthcare System
Patient Decision-Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This project proposes to understand and improve veterans’ decision-making in precision oncology (germline testing, somatic tumor testing, and targeted therapy) for advanced prostate cancer. As precision oncology expands, a comprehensive strategy to support patient informed decision-making has not been developed.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; US Department of Defense
Location: San Francisco VA Medical Center
Intramuscular Mechanisms of Androgen Deprivation-Related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes lowering the levels of the hormone testosterone as much as possible, which is called androgen deprivation therapy (ADT). Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as sarcopenia, and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because investigators do not understand the mechanisms that cause it. The mitochondria is the part of the cells responsible for providing energy to muscles but to date the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT. The overall goal of this proposal is to establish if the mitochondria is responsible for sarcopenia in patients with prostate cancer receiving ADT. The investigators will measure mitochondrial function, muscle mass and strength, and feelings of fatigue and quality of life in patients with prostate cancer before starting and after 6 months of ADT.
ID: NCT03867357
Sponsor; Collaborator: Seattle Institute for Biomedical and Clinical Research; US Department of Defense
Location: VA Puget Sound Health Care System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for OligoRecurrent Prostate Cancer (VA STARPORT)
The primary goal of this study is to determine if adding PET-directed local therapy improves disease control compared to standard systemic therapy alone (SST) in veterans with oligorecurrent prostate cancer on PET/CT. The investigators will conduct a multi-institutional phase II/III randomized trial comparing SST with or without PET-directed local therapy using radiation or surgery to all metastases and if a local recurrence is present.
ID: NCT04787744
Sponsor: VA Office of Research and Development
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VA Medical Center, St. Louis VA Medical Center John Cochran Division, East Orange Campus of the VA New Jersey Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, Clement J. Zablocki VA Medical Center
Standard Systemic Therapy With or Without Definitive Treatment in Treating Participants With Metastatic Prostate Cancer
This phase III trial studies how well standard systemic therapy with or without definitive treatment (prostate removal surgery or radiation therapy) works in treating participants with prostate cancer that has spread to other places in the body. The addition of prostate removal surgery or radiation therapy to standard systemic therapy for prostate cancer may lower the chance of the cancer growing or spreading.
ID: NCT03678025
Sponsor; Collaborator: Southwest Oncology Group; National Cancer Institute (NCI)
Locations: 328 sites, including Tibor Rubin VA Medical Center, Atlanta VA Medical Center, James J. Peters VA Medical Center, Michael E. DeBakey VA Medical Center, and Audie L. Murphy VA Hospital
A Clinical Study Evaluating the Benefit of Adding Rucaparib to Enzalutamide for Men With Metastatic Prostate Cancer That Has Become Resistant to Testosterone-Deprivation Therapy (CASPAR)
This randomized, placebo-controlled, phase III trial is evaluating the benefit of rucaparib and enzalutamide combination therapy vs enzalutamide alone for the treatment of men with prostate cancer that has spread to other places in the body (metastatic) and has become resistant to testosterone-deprivation therapy (castration-resistant). Enzalutamide helps fight prostate cancer by blocking the use of testosterone by the tumor cells for growth. Poly adenosine diphosphate (ADP)-ribose polymerase (PARP) inhibitors, such as rucaparib, fight prostate cancer by prevent tumor cells from repairing their DNA. Giving enzalutamide and rucaparib may make patients live longer or prevent their cancer from growing or spreading for a longer time, or both. It may also help doctors learn if a mutation in any of the homologous recombination DNA repair genes is helpful to decide which treatment is best for the patient.
ID: NCT04455750
Sponsor; Collaborator: Alliance for Clinical Trials in Oncology; National Cancer Institute (NCI)
Locations: 413 sites
Digitally Captured Activity Data and PROs to Monitor Physical Function in Prostate Cancer Patients (DigiPRO)
Physical function is a known predictor of quality of life in advanced prostate cancer patients and key measure of treatment tolerability. While treatment with androgen deprivation therapy (ADT) improves survival, it is associated with significant toxicities that lead to physical function (PF) decline. The average age of incident prostate cancer is 66 years, and in this older group of men, chronic comorbid conditions often co-occur with diagnosis, further adding to the risk for PF decline. With over 2.9 million prostate cancer survivors in the US, there is an increasing demand for adequate symptom monitoring and PF assessment throughout cancer care. However, there are currently no validated methods to systematically evaluate and predict PF decline. Thus, the overarching objective of this proposal is to determine whether the use of wearable technology to monitor objective daily activity combined with routine symptom reporting can predict PF decline. To accomplish this, we propose a mixed-methods approach that will provide quantitative information to help identify PC survivors at higher risk for PF decline as well as a qualitative aim gain a deeper understanding of the perceived relationships that PC survivors have with their physical activity levels and treatment symptoms.
ID: NCT04575402
Sponsor; Collaborator: Cedars-Sinai Medical Center; US Department of Defense
Location: Cedars Sinai Medical Center
The BurnAlong Pilot Study for Adolescent and Young Adult Cancer Survivors
The purpose of this prospective, interventional, single-arm pilot study is to evaluate whether virtually delivered group-based physical activity is feasible for adolescent and young adult (AYA) cancer survivors. AYAs who were diagnosed with cancer and have completed cancer treatment will be recruited for this study. This study will enroll 20 participants in total and will last approximately 3 months.
ID: NCT05131815
Sponsor; Collaborator: Cedars-Sinai Medical Center; Walter Reed National Military Medical Center
Location: Cedars-Sinai Medical Center
Lung Cancer
DECAMP 1 PLUS: Prediction of Lung Cancer Using Noninvasive Biomarkers
The Detection of Early lung Cancer Among Military Personnel (DECAMP) consortium is a multidisciplinary and translational research program for lung cancer early detection. DECAMP 1 PLUS aims to improve the efficiency of the diagnostic evaluation of patients with indeterminate pulmonary nodules (8-25 mm). Molecular biomarkers for lung cancer diagnosis measured in minimally invasive and noninvasive biospecimens may be able to distinguish between malignant or benign indeterminate pulmonary nodules in high-risk smokers. Ultimately, this study aims to validate molecular as well as clinical and imaging biomarkers of lung cancer in individuals with indeterminate lung nodules.
ID: NCT04165564
Sponsor: Boston University
Locations: 3 VA medical centers (VA Greater LA Healthcare System, VA Boston Healthcare System, and VA Tennessee Valley Healthcare System), 3 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, and Naval Medical Center Portsmouth) and 12 academic hospitals
DECAMP-2: Screening of Patients With Early Stage Lung Cancer or at High Risk for Developing Lung Cancer (DECAMP-2)
The goal of this project is to improve lung cancer screening in high-risk individuals by identifying biomarkers of preclinical disease and disease risk that are measured in minimally invasive and noninvasive biospecimens. Existing biomarkers for lung cancer diagnosis as well as new biomarkers discovered specifically in this clinical setting will be examined. Biomarkers that identify individuals at highest risk for being diagnosed with lung cancer prior to the appearance of concerning symptoms could increase the utility of lung cancer surveillance and the efficiency of lung cancer chemoprevention clinical trials. Achieving these goals would improve the detection and treatment of early-stage and incipient lung cancer, while restricting the risk of these procedures to those individuals who currently exhibit the early molecular warning signs of impending disease.
ID: NCT02504697
Sponsor: Boston University
Locations: VA medical centers (including Los Angeles VA Healthcare System, Boston VA Research Institute, Inc, Philadelphia VA Medical Center, Veterans Research Foundation of Pittsburgh, and VA North Texas Health Care System), 4 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, San Antonio Military Medical Center, and Naval Medical Center Portsmouth), and 4 academic hospitals
Improving Decision-Making Encounters in Lung Cancer Using a Low-Literacy Conversation Tool (iDECIDE)
This clinical trial evaluates the effectiveness of a conversation tool on patient-centered health and decision-making outcomes in patients with lung cancer making treatment decisions. This research is being conducted to help doctors understand the information patients need to participate in shared decision-making about their lung cancer treatment options. The focus of this research is to study how patients choose lung cancer treatment options and the information needed to make that choice, with a focus on patients with lower health literacy.
ID: NCT05407168
Sponsor: Oregon Health & Science University Knight Cancer Institute
Locations: Portland VA Medical Center and Oregon Health & Science University Knight Cancer Institute
VA Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non–small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggest that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of 2 incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these 2 treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life expectancy and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
This study is designed to open at VA medical centers with expertise in both treatments. The recruitment process includes shared decision making and multidisciplinary evaluations with lung cancer specialists. Mandatory evaluations before randomization include tissue confirmation of NSCLC, staging with FDG-PET/CT, and biopsies of all hilar and/or mediastinal lymph nodes > 10 mm that have a SUV > 2.5. Prerandomization elective lymph node sampling is strongly encouraged, but not required. Following treatment, patients will be followed for a minimum of 5 years.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: 17 VA medical centers, including VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Miami VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, Baltimore VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Corporal Micheal J. Crescenz VA Medical Center, VA Pittsburgh Healthcare System University Drive Division, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, and Clement J. Zablocki VA Medical Center
Utility of CAML as Diagnostic for Early Stage Lung Cancer
The primary objective of this study is to determine the prevalence of cancer associated macrophage-like cells (CAMLS) in patients with pulmonary nodules. Secondary objectives include the following: determine the positive and negative predictive value of CAMLS in patients with pulmonary nodules who undergo biopsy; model combinations of clinical factors with the presence/absence of CAMLS to refine strategies for assessment of patients with pulmonary nodules; and evaluate whether these measures result in enhanced T-cell activity and/or natural killer cell function and number.
ID: NCT03992183
Sponsor; Collaborators: Fox Chase Cancer Center; US Department of Defense
Locations: Corporal Michael J. Crescenz VA Medical Center and Fox Chase Cancer Center
PROSPECT - Profiling of Resistance Patterns & Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax and Therapeutic Target Identification
This study will use therapeutic target-focused (TTF) profiling, genome-wide mRNA profiling, and assessments of tumor phosphopeptides and DNA that are shed into the bloodstream to define how various molecular factors alone and in combination relate to resistance to therapy, to prognosis, and to metastatic patterns at relapse. This study will examine how the presence of factors that drive cell growth, antagonize apoptosis, or confer resistance in other ways may counter the effect of systemic therapies and/or promote rapid tumor recurrence. In this way, the investigators will identify new, previously unappreciated potential therapeutic targets while also identifying which targets are most likely to increase resistance to therapy and worsen prognosis.
ID: NCT05049837
Sponsor; Collaborators: MD Anderson Cancer Center; US Department of Defense, National Institutes of Health (NIH), and National Cancer Institute (NCI)
Location: MD Anderson Cancer Center
Tribally Engaged Approaches to Lung Screening (TEALS)
Lung cancer is the leading cause of cancer mortality among American Indians and Alaska Natives (AI/AN), and AI/AN have worse lung cancer incidence rates, survival, and death compared to the general population. Because lung cancer screening (LCS) with low-dose computed tomography (LDCT) has been shown to reduce lung cancer mortality by roughly 20%, the US Preventive Services Task Force now recommends LCS for persons aged 55 to 80 years who meet specific eligibility criteria (grade-B evidence), and subsequently the Center for Medicare and Medicaid Services (CMS) opted to cover this test. However, the uptake of LCS implementation has been slow in most health care systems, and LCS implementation among AI/AN has never been studied.
To address this knowledge, the Tribally Engaged Approaches to Lung Screening (TEALS) study, a collaborative effort between the Choctaw Nation of Oklahoma, the Stephenson Cancer Center, and the University of Oklahoma Health Sciences Center, will address the following over the course of 5 years: conduct focus groups and semistructured interviews with Choctaw Nation Health Services Authority (CNHSA) patients, clinicians, and health administrators to elucidate individual- and system-level barriers and facilitators that affect the implementation of LCS; develop an LCS care coordination intervention that will identify eligible persons for LCS, help these patients navigate the screening process, and link them with smoking cessation services, when applicable; measure the impact of the TEALS intervention on the receipt of screening and a set of patient- and practice-level outcomes by conducting a cluster-randomized clinical trial of LCS implementation; and disseminate the TEALS program to other researchers and healthcare systems that serve AI/AN patients. TEALS will bridge the gap between evidence and clinical practice for LCS in a high-need, low-resource setting by intervening at the level of the healthcare system.
System-level interventions for guideline implementation tend to be understudied compared to those evaluating individual-level, behavioral interventions. However, the careful development and evaluation of an LCS screening program at the level of the healthcare system would be critical to ensure that more patients can receive LCS. Our research will create a critically needed platform from which future studies could be launched that will examine how to tailor the application of the LCS guideline to the individual preferences of AI/AN patients. TEALS will establish an effective LCS program in a tribal system and thus provide a direct benefit to the Choctaw Nation by increasing LCS participation. TEALS will serve as a blueprint for establishing a sustainable and accessible infrastructure for LCS in AI/AN and other community health systems. By increasing screening for early stage lung cancer, TEALS could ultimately reduce lung cancer mortality in AI/AN communities.
ID: NCT04948060
Sponsor; Collaborator: University of Oklahoma; Choctaw Nation of Oklahoma
Location: University of Oklahoma Health Sciences Center
Prostate Cancer
18F-DCFPyL PET/CT Impact on Treatment Strategies for Patients With Prostate Cancer (PROSPYL)
The main purpose of this phase II trial study is to determine whether a positron emission tomography (PET)/computed tomography (CT) scan using 18F-DCFPyL affects the clinical management plan in veterans. In this study, the management plan prior to and after 18F-DCFPyL PET/CT will be recorded by specific questionnaires and corresponding changes in management will be analyzed. The scan will be used to see how the disease has spread. Both the treatment strategies and probable disease outcomes as relevant to clinical endpoints will be assessed. This study is open to veterans only.
ID: NCT04390880
Sponsor: VA Greater Los Angeles Healthcare System
Location: VA Greater Los Angeles Healthcare System
Patient Decision-Making About Precision Oncology in Veterans With Advanced Prostate Cancer
This project proposes to understand and improve veterans’ decision-making in precision oncology (germline testing, somatic tumor testing, and targeted therapy) for advanced prostate cancer. As precision oncology expands, a comprehensive strategy to support patient informed decision-making has not been developed.
ID: NCT05396872
Sponsor; Collaborator: University of California, San Francisco; US Department of Defense
Location: San Francisco VA Medical Center
Intramuscular Mechanisms of Androgen Deprivation-Related Sarcopenia
Prostate cancer is the most common cancer among men and is even more common in the military and veteran population. For patients with advanced prostate cancer, the most common treatment includes lowering the levels of the hormone testosterone as much as possible, which is called androgen deprivation therapy (ADT). Unfortunately, ADT also causes patients to be fatigued, weak, and to lose muscle. This is often referred to as sarcopenia, and it leads to falls, poor quality of life, and higher risk of death. Currently, there is no treatment for sarcopenia because investigators do not understand the mechanisms that cause it. The mitochondria is the part of the cells responsible for providing energy to muscles but to date the investigators do not know if it is affected in prostate cancer patients with sarcopenia due to ADT. The overall goal of this proposal is to establish if the mitochondria is responsible for sarcopenia in patients with prostate cancer receiving ADT. The investigators will measure mitochondrial function, muscle mass and strength, and feelings of fatigue and quality of life in patients with prostate cancer before starting and after 6 months of ADT.
ID: NCT03867357
Sponsor; Collaborator: Seattle Institute for Biomedical and Clinical Research; US Department of Defense
Location: VA Puget Sound Health Care System
VA Seamless Phase II/III Randomized Trial of Standard Systemic Therapy With or Without PET-Directed Local Therapy for OligoRecurrent Prostate Cancer (VA STARPORT)
The primary goal of this study is to determine if adding PET-directed local therapy improves disease control compared to standard systemic therapy alone (SST) in veterans with oligorecurrent prostate cancer on PET/CT. The investigators will conduct a multi-institutional phase II/III randomized trial comparing SST with or without PET-directed local therapy using radiation or surgery to all metastases and if a local recurrence is present.
ID: NCT04787744
Sponsor: VA Office of Research and Development
Locations: VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Kansas City VA Medical Center, St. Louis VA Medical Center John Cochran Division, East Orange Campus of the VA New Jersey Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, Clement J. Zablocki VA Medical Center
Standard Systemic Therapy With or Without Definitive Treatment in Treating Participants With Metastatic Prostate Cancer
This phase III trial studies how well standard systemic therapy with or without definitive treatment (prostate removal surgery or radiation therapy) works in treating participants with prostate cancer that has spread to other places in the body. The addition of prostate removal surgery or radiation therapy to standard systemic therapy for prostate cancer may lower the chance of the cancer growing or spreading.
ID: NCT03678025
Sponsor; Collaborator: Southwest Oncology Group; National Cancer Institute (NCI)
Locations: 328 sites, including Tibor Rubin VA Medical Center, Atlanta VA Medical Center, James J. Peters VA Medical Center, Michael E. DeBakey VA Medical Center, and Audie L. Murphy VA Hospital
A Clinical Study Evaluating the Benefit of Adding Rucaparib to Enzalutamide for Men With Metastatic Prostate Cancer That Has Become Resistant to Testosterone-Deprivation Therapy (CASPAR)
This randomized, placebo-controlled, phase III trial is evaluating the benefit of rucaparib and enzalutamide combination therapy vs enzalutamide alone for the treatment of men with prostate cancer that has spread to other places in the body (metastatic) and has become resistant to testosterone-deprivation therapy (castration-resistant). Enzalutamide helps fight prostate cancer by blocking the use of testosterone by the tumor cells for growth. Poly adenosine diphosphate (ADP)-ribose polymerase (PARP) inhibitors, such as rucaparib, fight prostate cancer by prevent tumor cells from repairing their DNA. Giving enzalutamide and rucaparib may make patients live longer or prevent their cancer from growing or spreading for a longer time, or both. It may also help doctors learn if a mutation in any of the homologous recombination DNA repair genes is helpful to decide which treatment is best for the patient.
ID: NCT04455750
Sponsor; Collaborator: Alliance for Clinical Trials in Oncology; National Cancer Institute (NCI)
Locations: 413 sites
Digitally Captured Activity Data and PROs to Monitor Physical Function in Prostate Cancer Patients (DigiPRO)
Physical function is a known predictor of quality of life in advanced prostate cancer patients and key measure of treatment tolerability. While treatment with androgen deprivation therapy (ADT) improves survival, it is associated with significant toxicities that lead to physical function (PF) decline. The average age of incident prostate cancer is 66 years, and in this older group of men, chronic comorbid conditions often co-occur with diagnosis, further adding to the risk for PF decline. With over 2.9 million prostate cancer survivors in the US, there is an increasing demand for adequate symptom monitoring and PF assessment throughout cancer care. However, there are currently no validated methods to systematically evaluate and predict PF decline. Thus, the overarching objective of this proposal is to determine whether the use of wearable technology to monitor objective daily activity combined with routine symptom reporting can predict PF decline. To accomplish this, we propose a mixed-methods approach that will provide quantitative information to help identify PC survivors at higher risk for PF decline as well as a qualitative aim gain a deeper understanding of the perceived relationships that PC survivors have with their physical activity levels and treatment symptoms.
ID: NCT04575402
Sponsor; Collaborator: Cedars-Sinai Medical Center; US Department of Defense
Location: Cedars Sinai Medical Center
The BurnAlong Pilot Study for Adolescent and Young Adult Cancer Survivors
The purpose of this prospective, interventional, single-arm pilot study is to evaluate whether virtually delivered group-based physical activity is feasible for adolescent and young adult (AYA) cancer survivors. AYAs who were diagnosed with cancer and have completed cancer treatment will be recruited for this study. This study will enroll 20 participants in total and will last approximately 3 months.
ID: NCT05131815
Sponsor; Collaborator: Cedars-Sinai Medical Center; Walter Reed National Military Medical Center
Location: Cedars-Sinai Medical Center
Lung Cancer
DECAMP 1 PLUS: Prediction of Lung Cancer Using Noninvasive Biomarkers
The Detection of Early lung Cancer Among Military Personnel (DECAMP) consortium is a multidisciplinary and translational research program for lung cancer early detection. DECAMP 1 PLUS aims to improve the efficiency of the diagnostic evaluation of patients with indeterminate pulmonary nodules (8-25 mm). Molecular biomarkers for lung cancer diagnosis measured in minimally invasive and noninvasive biospecimens may be able to distinguish between malignant or benign indeterminate pulmonary nodules in high-risk smokers. Ultimately, this study aims to validate molecular as well as clinical and imaging biomarkers of lung cancer in individuals with indeterminate lung nodules.
ID: NCT04165564
Sponsor: Boston University
Locations: 3 VA medical centers (VA Greater LA Healthcare System, VA Boston Healthcare System, and VA Tennessee Valley Healthcare System), 3 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, and Naval Medical Center Portsmouth) and 12 academic hospitals
DECAMP-2: Screening of Patients With Early Stage Lung Cancer or at High Risk for Developing Lung Cancer (DECAMP-2)
The goal of this project is to improve lung cancer screening in high-risk individuals by identifying biomarkers of preclinical disease and disease risk that are measured in minimally invasive and noninvasive biospecimens. Existing biomarkers for lung cancer diagnosis as well as new biomarkers discovered specifically in this clinical setting will be examined. Biomarkers that identify individuals at highest risk for being diagnosed with lung cancer prior to the appearance of concerning symptoms could increase the utility of lung cancer surveillance and the efficiency of lung cancer chemoprevention clinical trials. Achieving these goals would improve the detection and treatment of early-stage and incipient lung cancer, while restricting the risk of these procedures to those individuals who currently exhibit the early molecular warning signs of impending disease.
ID: NCT02504697
Sponsor: Boston University
Locations: VA medical centers (including Los Angeles VA Healthcare System, Boston VA Research Institute, Inc, Philadelphia VA Medical Center, Veterans Research Foundation of Pittsburgh, and VA North Texas Health Care System), 4 military treatment facilities (Naval Medical Center San Diego, Walter Reed National Military Medical Center, San Antonio Military Medical Center, and Naval Medical Center Portsmouth), and 4 academic hospitals
Improving Decision-Making Encounters in Lung Cancer Using a Low-Literacy Conversation Tool (iDECIDE)
This clinical trial evaluates the effectiveness of a conversation tool on patient-centered health and decision-making outcomes in patients with lung cancer making treatment decisions. This research is being conducted to help doctors understand the information patients need to participate in shared decision-making about their lung cancer treatment options. The focus of this research is to study how patients choose lung cancer treatment options and the information needed to make that choice, with a focus on patients with lower health literacy.
ID: NCT05407168
Sponsor: Oregon Health & Science University Knight Cancer Institute
Locations: Portland VA Medical Center and Oregon Health & Science University Knight Cancer Institute
VA Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR)
The standard of care for stage I non–small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Recent data now suggest that stereotactic radiotherapy may be a suitable alternative. This includes the results from a pooled analysis of 2 incomplete phase III studies that reported a 15% overall survival advantage with stereotactic radiotherapy at 3 years. While these data are promising, the median follow-up period was short, the results underpowered, and the findings were in contradiction to multiple retrospective studies that demonstrate the outcomes with surgery are likely equal or superior. Therefore, the herein trial aims to evaluate these 2 treatments in a prospective randomized fashion with a goal to compare the overall survival beyond 5 years. It has been designed to enroll patients who have a long life expectancy and are fit enough to tolerate an anatomic pulmonary resection with intraoperative lymph node sampling.
This study is designed to open at VA medical centers with expertise in both treatments. The recruitment process includes shared decision making and multidisciplinary evaluations with lung cancer specialists. Mandatory evaluations before randomization include tissue confirmation of NSCLC, staging with FDG-PET/CT, and biopsies of all hilar and/or mediastinal lymph nodes > 10 mm that have a SUV > 2.5. Prerandomization elective lymph node sampling is strongly encouraged, but not required. Following treatment, patients will be followed for a minimum of 5 years.
ID: NCT02984761
Sponsor: VA Office of Research and Development
Locations: 17 VA medical centers, including VA Long Beach Healthcare System, VA Greater Los Angeles Healthcare System, Bay Pines VA Healthcare System, Miami VA Healthcare System, Edward Hines Jr. VA Hospital, Richard L. Roudebush VA Medical Center, Baltimore VA Medical Center, VA Boston Healthcare System Jamaica Plain Campus, VA Ann Arbor Healthcare System, Minneapolis VA Health Care System, Durham VA Medical Center, Louis Stokes VA Medical Center, Corporal Micheal J. Crescenz VA Medical Center, VA Pittsburgh Healthcare System University Drive Division, Michael E. DeBakey VA Medical Center, Hunter Holmes McGuire VA Medical Center, and Clement J. Zablocki VA Medical Center
Utility of CAML as Diagnostic for Early Stage Lung Cancer
The primary objective of this study is to determine the prevalence of cancer associated macrophage-like cells (CAMLS) in patients with pulmonary nodules. Secondary objectives include the following: determine the positive and negative predictive value of CAMLS in patients with pulmonary nodules who undergo biopsy; model combinations of clinical factors with the presence/absence of CAMLS to refine strategies for assessment of patients with pulmonary nodules; and evaluate whether these measures result in enhanced T-cell activity and/or natural killer cell function and number.
ID: NCT03992183
Sponsor; Collaborators: Fox Chase Cancer Center; US Department of Defense
Locations: Corporal Michael J. Crescenz VA Medical Center and Fox Chase Cancer Center
PROSPECT - Profiling of Resistance Patterns & Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax and Therapeutic Target Identification
This study will use therapeutic target-focused (TTF) profiling, genome-wide mRNA profiling, and assessments of tumor phosphopeptides and DNA that are shed into the bloodstream to define how various molecular factors alone and in combination relate to resistance to therapy, to prognosis, and to metastatic patterns at relapse. This study will examine how the presence of factors that drive cell growth, antagonize apoptosis, or confer resistance in other ways may counter the effect of systemic therapies and/or promote rapid tumor recurrence. In this way, the investigators will identify new, previously unappreciated potential therapeutic targets while also identifying which targets are most likely to increase resistance to therapy and worsen prognosis.
ID: NCT05049837
Sponsor; Collaborators: MD Anderson Cancer Center; US Department of Defense, National Institutes of Health (NIH), and National Cancer Institute (NCI)
Location: MD Anderson Cancer Center
Tribally Engaged Approaches to Lung Screening (TEALS)
Lung cancer is the leading cause of cancer mortality among American Indians and Alaska Natives (AI/AN), and AI/AN have worse lung cancer incidence rates, survival, and death compared to the general population. Because lung cancer screening (LCS) with low-dose computed tomography (LDCT) has been shown to reduce lung cancer mortality by roughly 20%, the US Preventive Services Task Force now recommends LCS for persons aged 55 to 80 years who meet specific eligibility criteria (grade-B evidence), and subsequently the Center for Medicare and Medicaid Services (CMS) opted to cover this test. However, the uptake of LCS implementation has been slow in most health care systems, and LCS implementation among AI/AN has never been studied.
To address this knowledge, the Tribally Engaged Approaches to Lung Screening (TEALS) study, a collaborative effort between the Choctaw Nation of Oklahoma, the Stephenson Cancer Center, and the University of Oklahoma Health Sciences Center, will address the following over the course of 5 years: conduct focus groups and semistructured interviews with Choctaw Nation Health Services Authority (CNHSA) patients, clinicians, and health administrators to elucidate individual- and system-level barriers and facilitators that affect the implementation of LCS; develop an LCS care coordination intervention that will identify eligible persons for LCS, help these patients navigate the screening process, and link them with smoking cessation services, when applicable; measure the impact of the TEALS intervention on the receipt of screening and a set of patient- and practice-level outcomes by conducting a cluster-randomized clinical trial of LCS implementation; and disseminate the TEALS program to other researchers and healthcare systems that serve AI/AN patients. TEALS will bridge the gap between evidence and clinical practice for LCS in a high-need, low-resource setting by intervening at the level of the healthcare system.
System-level interventions for guideline implementation tend to be understudied compared to those evaluating individual-level, behavioral interventions. However, the careful development and evaluation of an LCS screening program at the level of the healthcare system would be critical to ensure that more patients can receive LCS. Our research will create a critically needed platform from which future studies could be launched that will examine how to tailor the application of the LCS guideline to the individual preferences of AI/AN patients. TEALS will establish an effective LCS program in a tribal system and thus provide a direct benefit to the Choctaw Nation by increasing LCS participation. TEALS will serve as a blueprint for establishing a sustainable and accessible infrastructure for LCS in AI/AN and other community health systems. By increasing screening for early stage lung cancer, TEALS could ultimately reduce lung cancer mortality in AI/AN communities.
ID: NCT04948060
Sponsor; Collaborator: University of Oklahoma; Choctaw Nation of Oklahoma
Location: University of Oklahoma Health Sciences Center
Oropharyngeal Squamous Cell Carcinoma Outcomes by p16 INK4a Antigen Status in a Veteran Population
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
Since 1983, the correlation between head and neck squamous cell carcinoma (SCC) and human papillomavirus (HPV) has been of great interest to head and neck oncologists.1 In 1998, Smith and colleagues provided evidence of HPV as an independent risk factor for the development of head and neck SCC.2 HPV-associated head and neck SCC accounts for between 30% and 64% of oropharyngeal SCC, depending on the published study; tonsil primaries account for the majority of these cancers.3,4
The presence of HPV E6 and E7 oncoproteins leads to the inactivation of p53 and pRb tumor suppressors. Furthermore, Ragin and colleagues discussed a distinct molecular pathway specific to HPV-associated head and neck SCC, which was different from non–HPV-associated head and neck SCC, involving genetic mutations in CDKN2A/p16.5
Current methods in correlating the presence of HPV infection in head and neck SCC have centered on p16INK4a (p16) immunohistochemistry (IHC) staining and DNA in situ hybridization (ISH) for specific HPV DNA types. IHC staining for p16 involves a monoclonal antibody specific to p16. The usefulness of this test relies on p16 overexpression due to the inactivation of pRb by the HPV E7 oncoprotein. This test is readily performed on archived tissue and has a documented sensitivity and specificity of 100% and 79%, respectively, as reported by Singhi and Westra in 2010.6 HPV DNA fluorescence in situ hybridization is the gold standard for determining the presence of specific types of HPV DNA; however, p16IHC can serve as a rapid, less costly means of studying archived tissue, lending its utility to retrospective population-based studies.
METHODS
A retrospective study was designed to determine the proportion of HPV-associated oropharyngeal SCC in a US Department of Veterans Affairs (VA) population, using p16antigen IHC on paraffin-embedded tissue as the surrogate marker for the presence of HPV infection. Patients consisted of veterans who were treated for oropharyngeal SCC at Veterans Affairs Memphis Healthcare System (VAMHS) in Tennessee between January 1, 2000, and December 31, 2008. This data range allowed for at least 5 years of follow-up. Patients were excluded who lacked enough tissue specimens for analysis. Measurement outcomes included p16expression, with subset analysis by race and ethnicity, degree of tobacco and alcohol use, tumor location, stage, age at diagnosis, and survival outcome. Microsoft Excel was used to calculate Fisher exact test, Student t test, and χ2 statistics. Significance was set at P < .05. This study received institutional review board approval from the University of Tennessee Health Science Center and the VAMHS.
RESULTS
We identified 66 total cases of oropharyngeal SCC; 19 cases (29%) were positive for p16. The mean age at diagnosis for the p16-positive cohort was 59 years vs 61 years for the p16-negative cohort (P = .22; Table 1).
Although the tonsil was the most common site of tumor origin in both the p16-positive and negative cohorts (63% vs 51%, respectively), our analysis showed no statistically significant difference in sites of origin (P = .69) (Table 2).
DISCUSSION
The VAMHS population in our study had a lower proportion of HPV-associated oropharyngeal SCC compared with studies on nonveteran populations (29% vs 40%-80%, respectively).5,6 This disparity may indicate a true difference in these populations or may be related to a decreased prevalence of HPV infection in the population served by the VAMHS. This single-institution population did not completely correlate with previous population studies. Specifically, age at presentation (equivalent to patients with p16-negative status rather than earlier age at onset), disease stage at presentation (lower stage for patients with p16-positive status), and disease-specific survival (not improved compared with patients with p16-negative status in other studies) were dissimilar to previous investigations.2,3
The increased age and staging at presentation could be related in these patients with p16-positive status, which may further account for the lack of improved survival. Furthermore, both groups tended to use alcohol at a high proportion; whereas other populations have had a lesser degree of alcohol intake with p16 positivity.1-4 These differences may be due to variations in the habits and behavior of VA patients compared with non-VA patients.3,4
HPV-associated oropharyngeal SCC in published data has been associated with high-risk sexual behavior, lower age, and less tobacco and alcohol use.5,6 No difference was noted in tumor site predilection; however, the small size of our study could explain the lack of finding site preference shown in previous studies.2,3Other veteran-specific factors are absent in the at-large population, such as Agent Orange exposure. More than 8 million veterans (22%) from the Vietnam era self-reported Agent Orange exposure.7 Agent Orange exposure significantly predicted developing upper aerodigestive tract cancer. Oropharyngeal, nasopharyngeal, laryngeal, and thyroid cancers were significantly associated with Agent Orange exposure. Interestingly, these patients experienced an improved 10-year survival rate compared with patients not exposed to Agent Orange. This finding contrasts with our patients, who did not experience improved outcomes vs nonveteran patients with head and neck cancer.7
Suicide in veterans with head and neck cancer has been evaluated and was found at an incidence of 0.7%. Survivors of head and neck cancer are almost twice as likely to die by suicide compared with other cancer survivors. These patients have a higher rate of mental health disorders, substance misuse, and use of palliative care services.8 Sixty-five of 66 of our patients died during the 5-year observation period, although none died by suicide.
In a 2022 cohort study by Sun and colleagues, upfront surgical treatment was associated with a 23% reduced risk of stroke compared with definitive chemoradiotherapy in US veterans with oropharyngeal carcinoma.9 In our study, 58 of 66 patients (88%) received concurrent chemoradiation, possibly reflecting the more advanced stage of diagnosis in our study population. This was due to comorbidities and other health and economic factors. In our study, 43 patients (65%) died of factors not related to the disease, reflecting the overall comorbidity burden of this population. Seven patients (11%) in our 5-year study died of a documented stroke. In the study of veterans by Sun and colleagues, the 10-year cumulative incidence of stroke was 12.5% and death was 57.3%.9 Our veteran population experienced a similar incidence of strokes. These findings may need to be included when discussing the risk-benefit aspects of different treatment options with our veteran patients with oropharyngeal cancer.
To understand the influence of HPV infection on the course of oropharyngeal SCC in the VA patient population and to apply this understanding to future individualized treatment paradigms, this study can be expanded to a greater number of VA patients. p16 immunoexpression appears to be a useful surrogate for high-risk HPV infection in oropharyngeal SCC, and its ease of use supports its feasibility in further VA population analysis.10 While realizing that the veteran HPV-associated oropharyngeal SCC population differs from the civilian HPV-associated oropharyngeal SCC population, we also have realized that other unique considerations in the veteran population, such as chemical warfare exposure, mental illness, and vascular disease, complicate treatment decisions in these patients.
CONCLUSIONS
Disparities in racial distribution and tobacco use between patients with p16-positive and p16-negative status are similar to those reported in non-VA populations. In contrast, the frequently reported younger age at presentation and better disease outcomes seen in non-VA patients were not observed, perhaps due to the lower percentage of p16expression in VA patients with oropharyngeal SCC. Whereas de-intensification of therapy may be considered for many patients with oropharygeal cancer that is HPV-associated because of improved prognosis, this approach should be undertaken with great care in this group of patients. Personalization of therapy for these HPV-associated oropharyngeal SCC in the veteran population must be adapted to mitigate this critical disparity.
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418-424. doi:10.1016/s0300-9785(83)80033-7
2. Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108(7):1098-1103. doi:10.1097/00005537-199807000-00027
3. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi:10.1056/NEJMoa0912217
4. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi:10.1002/ijc.22851
5. Ragin CC, Taioli E, Weissfeld JL, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer. 2006;95(10):1432-1438. doi:10.1038/sj.bjc.6603394
6. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166-2173. doi:10.1002/cncr.25033
7. Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral Oncol. 2020;100:104483. doi:10.1016/j.oraloncology.2019.104483
8. Nugent SM, Morasco BJ, Handley R, et al. Risk of suicidal self-directed violence among US veteran survivors of head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(11):981-989. doi:10.1001/jamaoto.2021.2625
9. Sun L, Brody R, Candelieri D, et al. Association between up-front surgery and risk of stroke in US veterans with oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2022;148(8):740-747. doi:10.1001/jamaoto.2022.1327
10. El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34(4):459-461. doi:10.1002/hed.21974
Outcomes in Patients With Curative Malignancies Receiving Filgrastim as Primary Prophylaxis
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
Contralateral Constrictor Dose Predicts Swallowing Function After Radiation for Head and Neck Cancer
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
Radiation therapy can cause long-term dysphagia that seriously affects quality of life for survivors of head and neck cancer. 1-3 Numerous studies have linked pharyngeal constrictor dose to long-term dysphagia, but conclusions about the dose distribution that can be safely tolerated have been inconsistent. For example, a group from the Netherlands found that the mean dose to the superior pharyngeal constrictor muscle and the supraglottic larynx were each predictive of dysphagia. 4 A subsequent Vanderbilt study refuted these findings, reporting that these structures were not predictive but that dose to the inferior pharyngeal constrictor muscle was. 5 Other studies have connected late dysphagia with dose to the middle pharyngeal constrictor muscle, total larynx, oral cavity, contralateral submandibular gland, contralateral parotid gland, or a combination of these structures. 6-14 NRG Oncology trials commonly evaluate dose to the “uninvolved pharynx,” which is the total pharyngeal constrictor muscle volume minus the planning target volume (PTV) for the lowest dose target volume. NRG head and neck trials 3, 4, 5, 6, 8, and 9 all use uninvolved pharynx mean dose ≤ 45 Gy as a constraint to judge radiation plan quality.
Differences in methodology or patient population may explain the inconsistency of prior studies on dosimetric predictors of dysphagia, but it is possible that these studies did not evaluate the optimal metric for dysphagia. This study evaluates a novel organ at risk, the contralateral pharyngeal constrictor muscle, to determine whether dose to this structure is predictive of late swallowing function. The study also compares a constraint based on this structure to the NRG uninvolved pharynx constraint mentioned earlier.
Methods
This study is a retrospective review of patients treated at the Richard L. Roudebush Veterans Affairs (VA) Medical Center in Indianapolis, Indiana. Patients were identified by searching the VA Cancer Registry for patients treated for head and neck squamous cell carcinoma between September 1, 2016, and August 30, 2019. Eligible sites included cancers of the nasopharynx, oropharynx, hypopharynx, larynx and oral cavity, as well as head and neck cancer of an unknown primary site. Only patients treated with primary radiation with concurrent systemic therapy were included. Patients were excluded if they had prior surgery or radiation to the head and neck.
The pharyngeal constrictor muscles were contoured per the techniques described by Bhide and colleagues.11 The contralateral constrictor was defined as the half of the constrictor volume contralateral to the primary site. For midline tumors, the side of the neck with a lower volume of lymph node metastases was judged to be the contralateral side.
One-year dysphagia was defined as having a gastronomy tube (G-tube) in place or an abnormal modified barium swallow (MBS) ≥ 12 months after the completion of radiation. At the study institution, MBS is not routinely done after therapy but is ordered if a patient or clinician has concerns about swallowing function. MBS was considered abnormal if there was laryngeal penetration that reached the level of the glottis or was not ejected from the larynx.
Results
The VA Cancer Registry identified 113 patients treated for head and neck cancer during the study period. Of these, 55 patients met the inclusion criteria. No patients were lost to follow-up. The median follow-up was 29 months. The median age was 67 years (range, 41-83) (Table 1).
All patients were treated with intensity-modulated radiotherapy. Patients treated with a sequential boost had an initial dose of 54 Gy and/or 50 Gy, followed by a boost to a total of 70 Gy at 2 Gy per fraction. Patients treated with a simultaneous integrated boost (SIB) technique received 70.0 Gy in 33 fractions, with elective volumes treated to 54.5 Gy in 33 fractions. Both patients with nasopharyngeal cancer were treated with SIB plans and had an intermediate dose volume of 59.4 Gy.
Systemic therapy was weekly cisplatin in 41 patients (75%) and cetuximab in 14 (25%). Twenty percent of patients receiving cisplatin switched to an alternative agent during treatment, most commonly carboplatin.
Forty-nine patients (89%) had a G-tube placed before starting radiation. G-tubes were in place for an interval of 0 to 47 months (mean, 8.6); 12 (22%) had a G-tube > 12 months. After completion of radiation, 18 patients (33%) had an abnormal MBS. These were done 1 to 50 months (mean, 14.8) after completion of radiation. Abnormal MBS occurred ≥ 12 months after radiation in 9 patients, 5 of whom had their G-tube in place for less than a year.
Forty-six patients (84%) survived more than 1 year and could be evaluated for late swallowing function. One-year dysphagia was seen in 17 (37%) of these patients. Recurrence was seen in 20 patients (36%), with locoregional recurrence in 12 (60%) of these cases. Recurrence occurred at a range of 0 to 15 months (mean, 5.6). Neither recurrence (P = .69) nor locoregional recurrence (P = .11) was associated with increased dysphagia at 1 year.
In patients who could be evaluated for long-term swallowing function, contralateral constrictor V60 ranged from 0% to 100% (median, 51%). V60 was < 40% in 18 patients (39%). With V60 < 40%, there was a 6% rate of 1-year dysphagia compared with 57% for V60 ≥ 40% (P < .001).
Patients with contralateral constrictor V60 < 40 and V60 ≥ 40 both had a mean age of 65 years. χ2 analysis did not show a difference in T stage or systemic treatment but did show that patients with V60 < 40% were more likely to have N1 disease (P = .01), and less likely to have N2 disease (P = .01) compared with patients with V60 ≥ 40%. The difference in 1-year dysphagia between N0 to N1 patients (27%) and N2 to N3 patients (46%) was not statistically significant (P = .19).
In patients who could be evaluated for long-term swallowing function, the uninvolved pharynx volume median of the total constrictor volume was 32% (range, < 1%-62%). The uninvolved pharynx mean dose ranged from 28 to 68 Gy (median, 45). When the uninvolved pharynx mean dose was < 45 Gy, 1-year dysphagia was 22% compared with 52% with a dose ≥ 45 Gy (P = .03).
Air cavity editing was performed in 27 patients (49%). One-year survival was 93% with air cavity editing, and 75% without, which was not statistically significant. Locoregional recurrence occurred in 3 patients (11%) with air cavity editing, and 9 (32%) without, which was not statistically significant. In patients surviving at least 1 year, contralateral constrictor V60 averaged 33% with editing and 62% without editing (P < .001). One-year dysphagia was 12% with air cavity editing and 67% without editing (P < .001).
An SIB technique was done in 26 patients (47%). One-year survival was 85% (n = 22) with SIB and 83% (n = 24) with sequential boost, which was not statistically significant. Locoregional recurrence occurred in 19% with SIB, and 32% with sequential boost, which was not statistically significant. For SIB patients alive at 1 year, the median contralateral V60 was 28%, compared with 66% for patients treated with sequential technique. Seventeen patients (77%) with SIB had V60 < 40%. Nineteen (86%) of SIB plans also had air cavity editing. One patient (5%) with SIB had dysphagia at 1 year compared with 16 (67%) sequential patients (P < .001).
Discussion
This is the first study to link contralateral constrictor dose to long-term dysphagia in patients treated with radiation for head and neck cancer. Editing the boost volume off air cavities was associated with lower contralateral constrictor V60 and with less long-term dysphagia. This may indicate that optimizing plans to meet a contralateral constrictor constraint can reduce rates of long-term dysphagia.
The most useful clinical predictors are those that identify a patient at low risk for toxicity. These constraints are useful because they reassure physicians that treatments will have a favorable risk/benefit ratio while identifying plans that may need modification before starting treatment.
The contralateral constrictor outperformed the uninvolved pharynx in identifying patients at low risk for long-term dysphagia. This difference could not be overcome by decreasing the threshold of the pharynx constraint, as 17% of patients with dysphagia had a mean dose of < 40 Gy to the uninvolved pharynx, which was not statistically significant. An advantage of contralateral constrictor is that it is independent of PTV size. The uninvolved pharynx structure depends on the PTV contour, so it may obscure a connection between PTV size and dysphagia.
In the context of a clinical trial, only measuring dose to the uninvolved pharynx may allow more plans to meet constraints, but even in NRG trials, physicians have some control over target volumes. For example, NRG HN009, a national trial for patients with head and neck cancer, recommends editing the CTV_7000 (clinical target volume treated to 70 Gy) off air cavities but does not define how much the volume should be cropped or specify protocol violations if the volume is not cropped.15 Furthermore, constraints used in clinical trials are often adopted for use outside the trial, where physicians have extensive control over target volumes.
The broad range of uninvolved pharynx volume relative to total constrictor volume confounds predictions using this variable. For example, according to the NRG constraint, a patient with an uninvolved pharynx mean dose of 44 Gy will have a low risk of dysphagia even if this structure is only 1% of the total constrictor. The contralateral constrictor is always about 50% of the total constrictor volume, which means that predictions using this structure will not be confounded by the same variation in volume size.
Figure 2 shows a representative patient who met the NRG uninvolved pharynx constraint but developed long-term dysphagia.
Pharyngoesophageal stricture is a common cause of dysphagia after intensity-modulated radiotherapy for head and neck cancer.16 Radiation has been shown to decrease pharyngeal function in patients with head and neck cancer.17 Sparing one side of the pharynx may allow for better pharyngeal compliance throughout the length of the pharynx, possibly decreasing the rate of pharyngoesophageal stricture. Additionally, constraining the contralateral constrictor may preserve strength on this side, allowing it to compensate for weakness on the side of the primary cancer. An exercise sometimes used for dysphagia involves head rotation toward the affected side during swallowing. This technique has been shown to cause food to move to the unaffected side.18 Sparing the contralateral constrictor may help such techniques work better in patients with head and neck cancer.
Few studies have commented specifically on dose to swallowing structures contralateral to the primary tumor. Two studies have proposed contralateral submandibular gland constraints for dysphagia (not xerostomia), but neither measured the dose to the contralateral constrictor muscle.9,10 Although the contralateral submandibular dose may correlate with dose to the constrictor on that side, the submandibular gland may have a less direct impact on swallowing than the constrictor muscle, and its limited dimensions may make constraints based on the gland less robust for cancers outside the oropharynx.
Another study reported improved quality of life in patients who were not treated with elective contralateral retropharyngeal radiation.19 Although it is likely that doses to the contralateral constrictor were lower in patients who did not receive elective radiation to this area, this study did not measure or constrain doses to the contralateral constrictors.
Limitations
This study is limited by its single institution, retrospective design, small sample size, and by all patients being male. The high correlation between air cavity editing and the use of SIB makes it impossible to assess the impact of each technique individually. Patients with contralateral constrictor V60 < 40% were less likely to have N2 disease, but N2 to N3 disease did not predict higher 1-year dysphagia, so the difference in N-category cannot fully explain the difference in 1-year dysphagia. It is possible that unreported factors, such as CTV, may contribute significantly to swallowing function. Nevertheless, within the study population, contralateral constrictor dose was able to identify a group with a low rate of long-term dysphagia.
Conclusions
Contralateral constrictor dose is a promising predictor of late dysphagia for patients with head and neck cancer treated with radiation with concurrent systemic therapy. Contralateral constrictor V60 < 40% was able to identify a group of patients with a low rate of 1-year dysphagia in this single-center retrospective study. The correlation between air cavity editing and contralateral constrictor V60 suggests that contralateral constrictor dose may depend partly on technique. Further studies are needed to see if the contralateral constrictor dose can be used to predict long-term dysphagia prospectively and in other patient populations.
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
1. Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi:10.1200/JCO.2007.14.6647
2. Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772-778. doi:10.1016/j.ijrobp.2004.06.017
3. Ramaekers BLT, Joore MA, Grutters JPC, et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011;47(8):768-774. doi:10.1016/j.oraloncology.2011.05.012
4. Christianen MEMC, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107-114. doi:10.1016/j.radonc.2011.08.009
5. Vlachich G, Spratt DE, Diaz R, et al. Dose to inferior pharyngeal conctrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110(3):435-440. doi:10.1016/j.radonc.2013.12.007
6. Mogadas S, Busch CJ, Pflug Cet al. Influence of radiation dose to pharyngeal constrictor muscles on late dysphagia and quality of life in patients with locally advanced oropharyngeal carcinoma. Strahlenther Onkol. 2020;196(6):522-529. doi:10.1007/s00066-019-01572-0
7. Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts of swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110-1118. doi:10.1016/j.ijrobp.2008.02.048
8. Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(5):1356-1365. doi:10.1016/j.ijrobp.2009.10.002
9. Gensheimer MF, Nyflot M, Laramore GE, Laio JL, Parvathaneni U. Contribution of submandibular gland and swallowing structure sparing to post-radiation therapy peg dependence in oropharynx cancer patients treated with split-neck IMRT technique. Radiat Oncol. 2015;11(1):1-7. doi:10.1186/s13014-016-0726-3
10. Hedström J, Tuomi L, Finizia C, Olsson C. Identifying organs at risk for radiation-induced late dysphagia in head and neck cancer patients. Clin Transl Radiat Oncol. 2019;19:87-95. doi:10.1016/j.ctro.2019.08.005
11. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539-544. doi:10.1016/j.radonc.2009.09.017
12. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403-409. doi:10.1016/j.ijrobp.2009.02.017
13. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64-73. doi:10.1016/j.radonc.2007.07.009
14. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60(5):1425-1439. doi:10.1016/j.ijrobp.2004.05.050
15. Harari PM; NRG Oncology. Comparing high-dose cisplatin every three weeks to low-dose cisplatin weekly when combined with radiation for patients with advanced head and neck cancer. ClinicalTrials.gov identifier: NCT05050162. Updated November 25, 2022. Accessed December 7, 2022. https://clinicaltrials.gov/ct2/show/NCT05050162
16. Wang JJ, Goldsmith TA, Holman AS, Cianchetti M, Chan AW. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2011;34(7):967-973. doi:10.1002/hed.21842
17. Kendall KA, McKenzie SW, Leonard RJ, Jones CU. Timing of swallowing events after single-modality treatment of head and neck carcinoma with radiotherapy. Ann Otol Rhinol Laryngol. 2000;109(8, pt 1):767-775. doi:10.1177/000348940010900812
18. Ohmae Y, Ogura M, Kitahara S. Effects of head rotation on pharyngeal function during normal swallow. Ann Otol Rhinol Laryngol. 1998;107(4):344-348. doi:10.1177/000348949810700414
19. Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994-4002. doi:10.1002/cncr.28938
Sybil – Prophecies for lung cancer risk prediction?
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large
Thoracic Oncology and Chest Procedures Network
Lung Cancer Section
The mortality benefit associated with lung cancer screening (LCS) using low dose CT (LDCT) relies, in large part, on adherence rates to annual screening of ≥90%. However, the first 1 million “real world” patients screened in the US had very low (22%) annual adherence (Silvestri, et al. Chest. 2023;S0012-3692[23]00175-7). Refining how we estimate future lung cancer risk is an important opportunity for personalized medicine to bolster adherence to follow-up after initial LDCT.
(Mikhael, et al. J Clin Oncol. 2023;JCO2201345). The model was developed, trained, and tested in a total of 14,185 National Lung Screening Trial (NLST) participants including all cancer diagnoses. Within these data, Sybil’s accuracy in predicting 1-year lung cancer risk had AUC 0.92 (95% CI, 0.88-0.95) and at 6 years, AUC 0.75 (95% CI, 0.72-0.78).
The model was validated in two large independent LCS datasets, one in the US and one in Taiwan, where an LDCT can be obtained regardless of a personal smoking history. The cancer prevalence in these datasets was 3.4% and 0.9%, respectively. Reassuringly, Sybil’s performance was similar to the NLST data and was maintained in relevant subgroups such as sex, age and smoking history. Furthermore, Sybil reduced the false positive rate in the NLST to 8% at baseline scan, compared with 14% for Lung-RADS 1.0. Sybil’s algorithm, unlike others, has been made publicly available and hopefully will spur further validation and prospective study.
Robert Smyth, MD
Member-at-Large
Primary Hepatic Lymphoma: A Rare Form of Diffuse Large B-Cell Lymphoma of the Liver
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Watching feasible for asymptomatic kidney stones
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Diagnosis of Indolent Clonorchis sinensis and Opisthorchis viverrini Infections as Risk Factors for Cholangiocarcinoma: An Unmet Medical Need
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.