User login
New-Onset Pemphigoid Gestationis Following COVID-19 Vaccination
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
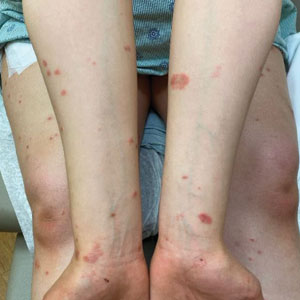
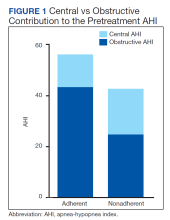
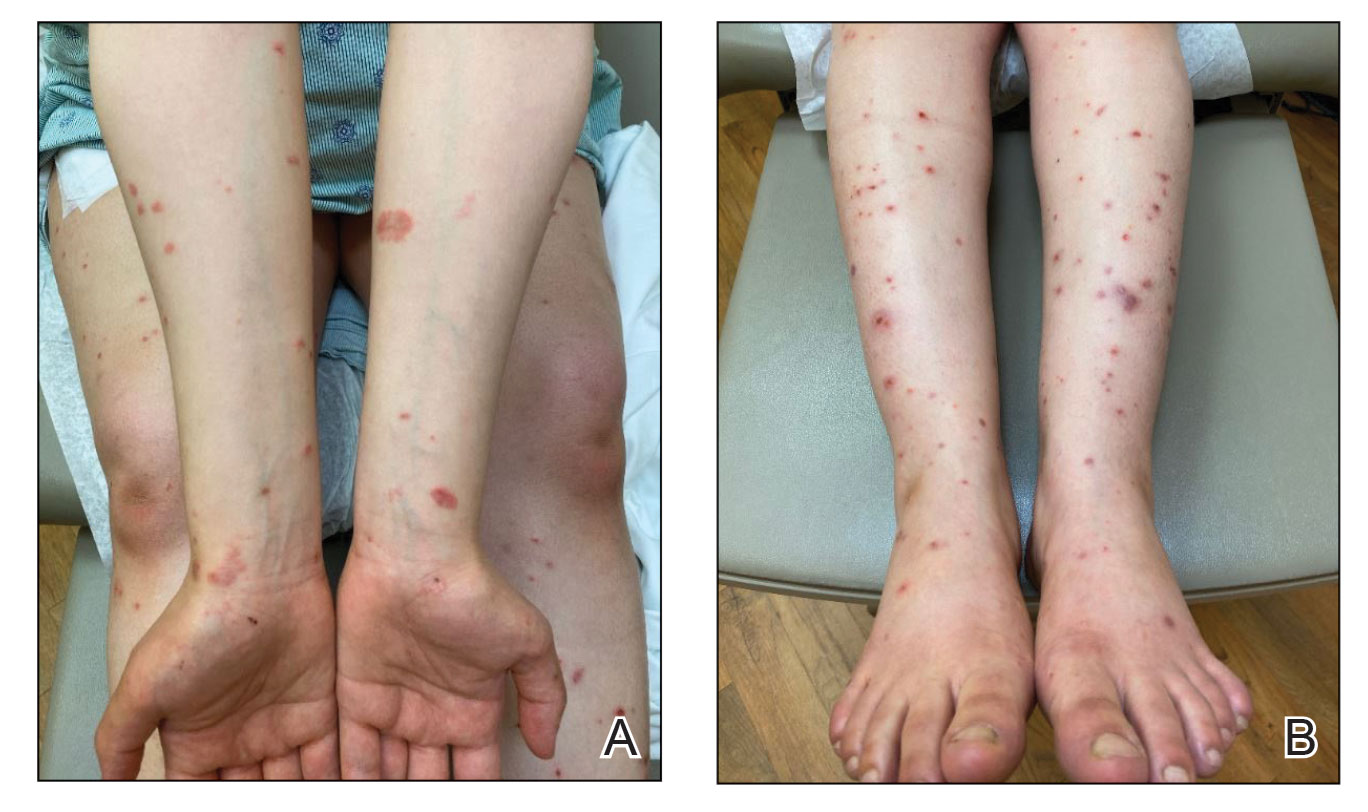
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.
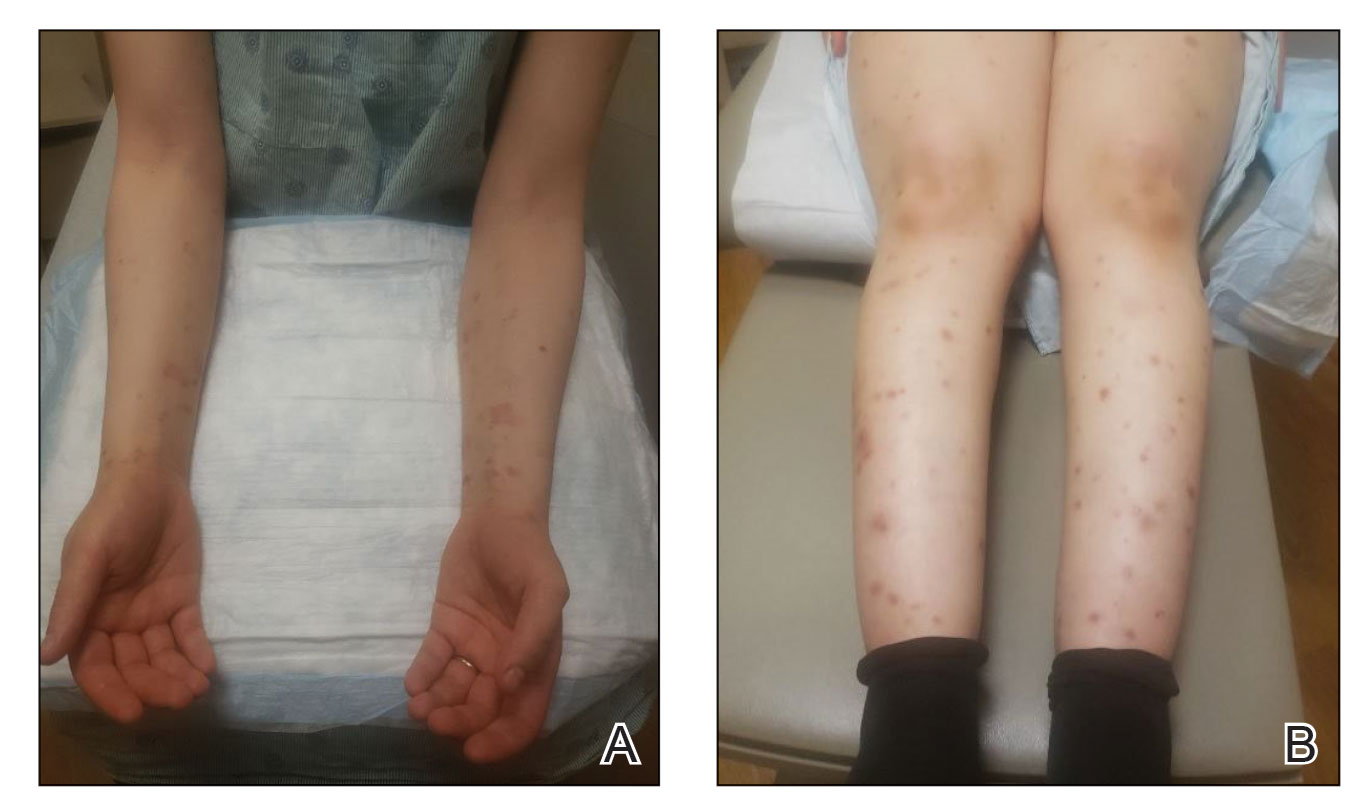
The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.
Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.

The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.

Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.

The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.

Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
Practice Points
- Dermatologists should be aware that COVID-19 messenger RNA vaccinations may present with various cutaneous complications.
- Pemphigoid gestationis should be considered in a pregnant or postpartum woman with an unexplained eruption of persistent, pruritic, urticarial lesions and blisters occurring postvaccination. Treatments include high-potency topical steroids and frequently systemic corticosteroids, along with steroid-sparing agents in severe cases.
Papular Reticulated Rash
The Diagnosis: Prurigo Pigmentosa
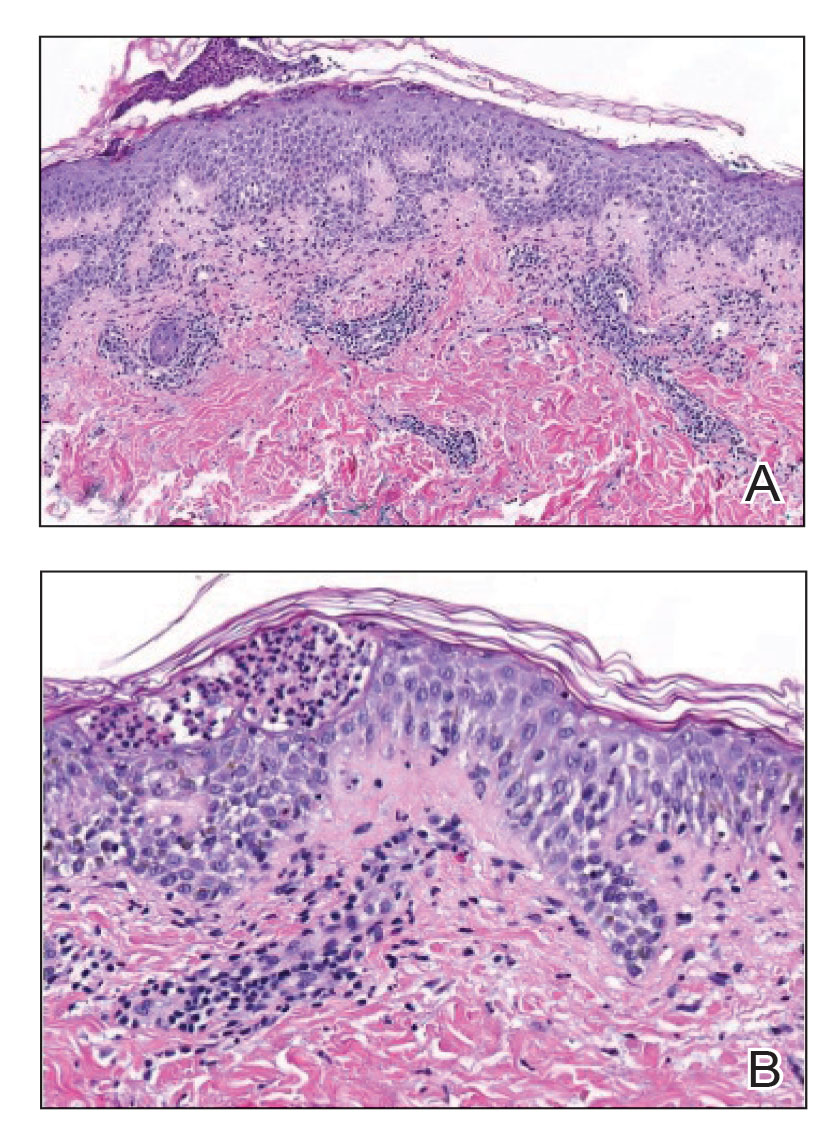
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).
Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).

Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).

Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
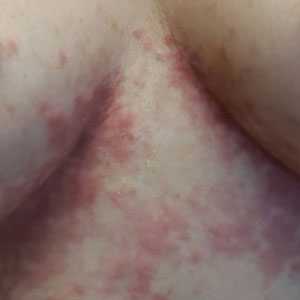
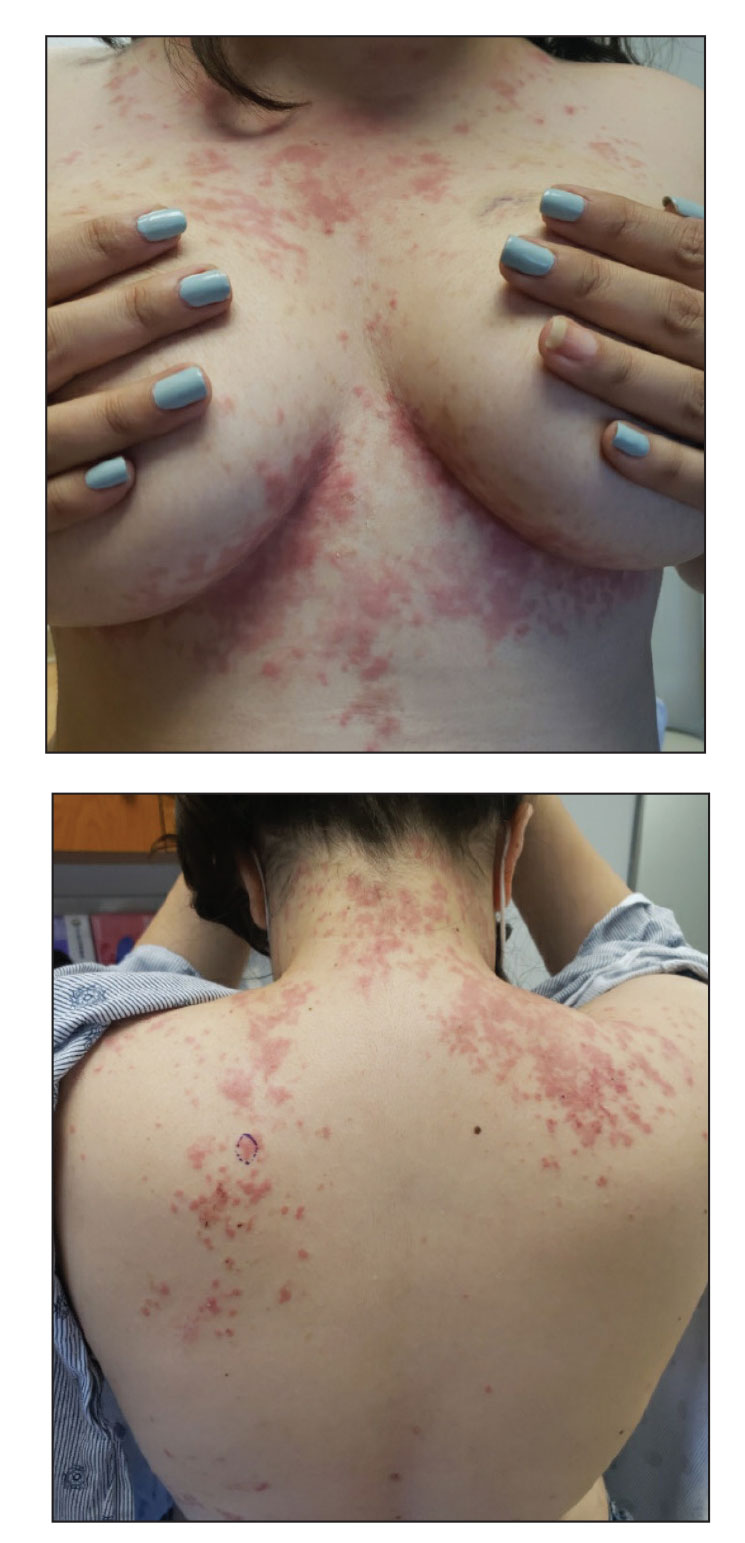
An otherwise healthy 22-year-old woman presented with a painful eruption with burning and pruritus that had been slowly worsening as it spread over the last 4 weeks. The rash first appeared on the lower chest and inframammary folds (top) and spread to the upper chest, neck, back (bottom), arms, and lower face. Physical examination revealed multiple illdefined, erythematous papules, patches, and plaques on the chest, back, neck, and upper abdomen. Individual lesions coalesced into plaques that displayed a reticular configuration. There were no lesions in the axillae. The patient had been following a low-carbohydrate diet for 4 months. A punch biopsy was performed.
Low disease state for childhood lupus approaches validation
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT BSR 2023
Part-time physician: Is it a viable career choice?
On average, physicians reported in the Medscape Physician Compensation Report 2023 that they worked 50 hours per week. Five specialties, including critical care, cardiology, and general surgery reported working 55 or more hours weekly.
In 2011, The New England Journal of Medicine reported that part-time physician careers were rising. At the time, part-time doctors made up 21% of the physician workforce, up from 13% in 2005.
In a more recent survey from the California Health Care Foundation, only 12% of California physicians said they devoted 20-29 hours a week to patient care.
Amy Knoup, a senior recruitment adviser with Provider Solutions & Development), has been helping doctors find jobs for over a decade, and she’s noticed a trend.
“Not only are more physicians seeking part-time roles than they were 10 years ago, but more large health care systems are also offering part time or per diem as well,” said Ms. Knoup.
Who’s working part time, and why?
Ten years ago, the fastest growing segment of part-timers were men nearing retirement and early- to mid-career women.
Pediatricians led the part-time pack in 2002, according to an American Academy of Pediatrics study. At the time, 15% of pediatricians reported their hours as part time. However, the numbers may have increased over the years. For example, a 2021 study by the department of pediatrics, Boston Medical Center, and Boston University found that almost 30% of graduating pediatricians sought part-time work at the end of their training.
At PS&D, Ms. Knoup said she has noticed a trend toward part-timers among primary care, behavioral health, and outpatient specialties such as endocrinology. “We’re also seeing it with the inpatient side in roles that are more shift based like hospitalists, radiologists, and critical care and ER doctors.”
Another trend Ms. Knoup has noticed is with early-career doctors. “They have a different mindset,” she said. “Younger generations are acutely aware of burnout. They may have experienced it in residency or during the pandemic. They’ve had a taste of that and don’t want to go down that road again, so they’re seeking part-time roles. It’s an intentional choice.”
Tracey O’Connell, MD, a radiologist, always knew that she wanted to work part time. “I had a baby as a resident, and I was pregnant with my second child as a fellow,” she said. “I was already feeling overwhelmed with medical training and having a family.”
Dr. O’Connell worked in private practice for 16 years on Mondays, Wednesdays, and Fridays, with no nights or weekends.
“I still found it completely overwhelming,” she said. “Even though I had more days not working than working, I felt like the demands of medical life had advanced faster than human beings could adapt, and I still feel that way.”
Today she runs a part-time teleradiology practice from home but spends more time on her second career as a life coach. “Most of my clients are physicians looking for more fulfillment and sustainable ways of practicing medicine while maintaining their own identity as human beings, not just the all-consuming identity of ‘doctor,’ ” she said.
On the other end of the career spectrum is Lois Goodman, MD, an ob.gyn. in her late 70s. After 42 years in a group practice, she started her solo practice at 72, seeing patients 3 days per week. “I’m just happy to be working. That’s a tremendous payoff for me. I need to keep working for my mental health.”
How does part-time work affect physician shortages and care delivery?
Reducing clinical effort is one of the strategies physicians use to scale down overload. Still, it’s not viable as a long-term solution, said Christine Sinsky, MD, AMA’s vice president of professional satisfaction and a nationally regarded researcher on physician burnout.
“If all the physicians in a community went from working 100% FTE clinical to 50% FTE clinical, then the people in that community would have half the access to care that they had,” said Dr. Sinsky. “There’s less capacity in the system to care for patients.”
Some could argue, then, that part-time physician work may contribute to physician shortage predictions. An Association of American Medical Colleges report estimates there will be a shortage of 37,800 to 124,000 physicians by 2034.
But physicians working part-time express a contrasting point of view. “I don’t believe that part-time workers are responsible for the health care shortage but rather, a great solution,” said Dr. O’Connell. “Because in order to continue working for a long time rather than quitting when the demands exceed human capacity, working part time is a great compromise to offer a life of more sustainable well-being and longevity as a physician, and still live a wholehearted life.”
Pros and cons of being a part-time physician
Pros
Less burnout: The American Medical Association has tracked burnout rates for 22 years. By the end of 2021, nearly 63% of physicians reported burnout symptoms, compared with 38% the year before. Going part time appears to reduce burnout, suggests a study published in Mayo Clinic Proceedings.
Better work-life balance: Rachel Miller, MD, an ob.gyn., worked 60-70 hours weekly for 9 years. In 2022, she went to work as an OB hospitalist for a health care system that welcomes part-time clinicians. Since then, she has achieved a better work-life balance, putting in 26-28 hours a week. Dr. Miller now spends more time with her kids and in her additional role as an executive coach to leaders in the medical field.
More focus: “When I’m at work, I’m 100% mentally in and focused,” said Dr. Miller. “My interactions with patients are different because I’m not burned out. My demeanor and my willingness to connect are stronger.”
Better health: Mehmet Cilingiroglu, MD, with CardioSolution, traded full-time work for part time when health issues and a kidney transplant sidelined his 30-year career in 2018. “Despite my significant health issues, I’ve been able to continue working at a pace that suits me rather than having to retire,” he said. “Part-time physicians can still enjoy patient care, research, innovation, education, and training while balancing that with other areas of life.”
Errin Weisman, a DO who gave up full-time work in 2016, said cutting back makes her feel healthier, happier, and more energized. “Part-time work helps me to bring my A game each day I work and deliver the best care.” She’s also a life coach encouraging other physicians to find balance in their professional and personal lives.
Cons
Cut in pay: Obviously, the No. 1 con is you’ll make less working part time, so adjusting to a salary decrease can be a huge issue, especially if you don’t have other sources of income. Physicians paying off student loans, those caring for children or elderly parents, or those in their prime earning years needing to save for retirement may not be able to go part time.
Diminished career: The chance for promotions or being well known in your field can be diminished, as well as a loss of proficiency if you’re only performing surgery or procedures part time. In some specialties, working part time and not keeping up with (or being able to practice) newer technology developments can harm your career or reputation in the long run.
Missing out: While working part time has many benefits, physicians also experience a wide range of drawbacks. Dr. Goodman, for example, said she misses delivering babies and doing surgeries. Dr. Miller said she gave up some aspects of her specialty, like performing hysterectomies, participating in complex cases, and no longer having an office like she did as a full-time ob.gyn.
Loss of fellowship: Dr. O’Connell said she missed the camaraderie and sense of belonging when she scaled back her hours. “I felt like a fish out of water, that my values didn’t align with the group’s values,” she said. This led to self-doubt, frustrated colleagues, and a reduction in benefits.
Lost esteem: Dr. O’Connell also felt she was expected to work overtime without additional pay and was no longer eligible for bonuses. “I was treated as a team player when I was needed, but not when it came to perks and benefits and insider privilege,” she said. There may be a loss of esteem among colleagues and supervisors.
Overcoming stigma: Because part-time physician work is still not prevalent among colleagues, some may resist the idea, have less respect for it, perceive it as not being serious about your career as a physician, or associate it with being lazy or entitled.
Summing it up
Every physician must weigh the value and drawbacks of part-time work, but the more physicians who go this route, the more part-time medicine gains traction and the more physicians can learn about its values versus its drawbacks.
A version of this article first appeared on Medscape.com.
On average, physicians reported in the Medscape Physician Compensation Report 2023 that they worked 50 hours per week. Five specialties, including critical care, cardiology, and general surgery reported working 55 or more hours weekly.
In 2011, The New England Journal of Medicine reported that part-time physician careers were rising. At the time, part-time doctors made up 21% of the physician workforce, up from 13% in 2005.
In a more recent survey from the California Health Care Foundation, only 12% of California physicians said they devoted 20-29 hours a week to patient care.
Amy Knoup, a senior recruitment adviser with Provider Solutions & Development), has been helping doctors find jobs for over a decade, and she’s noticed a trend.
“Not only are more physicians seeking part-time roles than they were 10 years ago, but more large health care systems are also offering part time or per diem as well,” said Ms. Knoup.
Who’s working part time, and why?
Ten years ago, the fastest growing segment of part-timers were men nearing retirement and early- to mid-career women.
Pediatricians led the part-time pack in 2002, according to an American Academy of Pediatrics study. At the time, 15% of pediatricians reported their hours as part time. However, the numbers may have increased over the years. For example, a 2021 study by the department of pediatrics, Boston Medical Center, and Boston University found that almost 30% of graduating pediatricians sought part-time work at the end of their training.
At PS&D, Ms. Knoup said she has noticed a trend toward part-timers among primary care, behavioral health, and outpatient specialties such as endocrinology. “We’re also seeing it with the inpatient side in roles that are more shift based like hospitalists, radiologists, and critical care and ER doctors.”
Another trend Ms. Knoup has noticed is with early-career doctors. “They have a different mindset,” she said. “Younger generations are acutely aware of burnout. They may have experienced it in residency or during the pandemic. They’ve had a taste of that and don’t want to go down that road again, so they’re seeking part-time roles. It’s an intentional choice.”
Tracey O’Connell, MD, a radiologist, always knew that she wanted to work part time. “I had a baby as a resident, and I was pregnant with my second child as a fellow,” she said. “I was already feeling overwhelmed with medical training and having a family.”
Dr. O’Connell worked in private practice for 16 years on Mondays, Wednesdays, and Fridays, with no nights or weekends.
“I still found it completely overwhelming,” she said. “Even though I had more days not working than working, I felt like the demands of medical life had advanced faster than human beings could adapt, and I still feel that way.”
Today she runs a part-time teleradiology practice from home but spends more time on her second career as a life coach. “Most of my clients are physicians looking for more fulfillment and sustainable ways of practicing medicine while maintaining their own identity as human beings, not just the all-consuming identity of ‘doctor,’ ” she said.
On the other end of the career spectrum is Lois Goodman, MD, an ob.gyn. in her late 70s. After 42 years in a group practice, she started her solo practice at 72, seeing patients 3 days per week. “I’m just happy to be working. That’s a tremendous payoff for me. I need to keep working for my mental health.”
How does part-time work affect physician shortages and care delivery?
Reducing clinical effort is one of the strategies physicians use to scale down overload. Still, it’s not viable as a long-term solution, said Christine Sinsky, MD, AMA’s vice president of professional satisfaction and a nationally regarded researcher on physician burnout.
“If all the physicians in a community went from working 100% FTE clinical to 50% FTE clinical, then the people in that community would have half the access to care that they had,” said Dr. Sinsky. “There’s less capacity in the system to care for patients.”
Some could argue, then, that part-time physician work may contribute to physician shortage predictions. An Association of American Medical Colleges report estimates there will be a shortage of 37,800 to 124,000 physicians by 2034.
But physicians working part-time express a contrasting point of view. “I don’t believe that part-time workers are responsible for the health care shortage but rather, a great solution,” said Dr. O’Connell. “Because in order to continue working for a long time rather than quitting when the demands exceed human capacity, working part time is a great compromise to offer a life of more sustainable well-being and longevity as a physician, and still live a wholehearted life.”
Pros and cons of being a part-time physician
Pros
Less burnout: The American Medical Association has tracked burnout rates for 22 years. By the end of 2021, nearly 63% of physicians reported burnout symptoms, compared with 38% the year before. Going part time appears to reduce burnout, suggests a study published in Mayo Clinic Proceedings.
Better work-life balance: Rachel Miller, MD, an ob.gyn., worked 60-70 hours weekly for 9 years. In 2022, she went to work as an OB hospitalist for a health care system that welcomes part-time clinicians. Since then, she has achieved a better work-life balance, putting in 26-28 hours a week. Dr. Miller now spends more time with her kids and in her additional role as an executive coach to leaders in the medical field.
More focus: “When I’m at work, I’m 100% mentally in and focused,” said Dr. Miller. “My interactions with patients are different because I’m not burned out. My demeanor and my willingness to connect are stronger.”
Better health: Mehmet Cilingiroglu, MD, with CardioSolution, traded full-time work for part time when health issues and a kidney transplant sidelined his 30-year career in 2018. “Despite my significant health issues, I’ve been able to continue working at a pace that suits me rather than having to retire,” he said. “Part-time physicians can still enjoy patient care, research, innovation, education, and training while balancing that with other areas of life.”
Errin Weisman, a DO who gave up full-time work in 2016, said cutting back makes her feel healthier, happier, and more energized. “Part-time work helps me to bring my A game each day I work and deliver the best care.” She’s also a life coach encouraging other physicians to find balance in their professional and personal lives.
Cons
Cut in pay: Obviously, the No. 1 con is you’ll make less working part time, so adjusting to a salary decrease can be a huge issue, especially if you don’t have other sources of income. Physicians paying off student loans, those caring for children or elderly parents, or those in their prime earning years needing to save for retirement may not be able to go part time.
Diminished career: The chance for promotions or being well known in your field can be diminished, as well as a loss of proficiency if you’re only performing surgery or procedures part time. In some specialties, working part time and not keeping up with (or being able to practice) newer technology developments can harm your career or reputation in the long run.
Missing out: While working part time has many benefits, physicians also experience a wide range of drawbacks. Dr. Goodman, for example, said she misses delivering babies and doing surgeries. Dr. Miller said she gave up some aspects of her specialty, like performing hysterectomies, participating in complex cases, and no longer having an office like she did as a full-time ob.gyn.
Loss of fellowship: Dr. O’Connell said she missed the camaraderie and sense of belonging when she scaled back her hours. “I felt like a fish out of water, that my values didn’t align with the group’s values,” she said. This led to self-doubt, frustrated colleagues, and a reduction in benefits.
Lost esteem: Dr. O’Connell also felt she was expected to work overtime without additional pay and was no longer eligible for bonuses. “I was treated as a team player when I was needed, but not when it came to perks and benefits and insider privilege,” she said. There may be a loss of esteem among colleagues and supervisors.
Overcoming stigma: Because part-time physician work is still not prevalent among colleagues, some may resist the idea, have less respect for it, perceive it as not being serious about your career as a physician, or associate it with being lazy or entitled.
Summing it up
Every physician must weigh the value and drawbacks of part-time work, but the more physicians who go this route, the more part-time medicine gains traction and the more physicians can learn about its values versus its drawbacks.
A version of this article first appeared on Medscape.com.
On average, physicians reported in the Medscape Physician Compensation Report 2023 that they worked 50 hours per week. Five specialties, including critical care, cardiology, and general surgery reported working 55 or more hours weekly.
In 2011, The New England Journal of Medicine reported that part-time physician careers were rising. At the time, part-time doctors made up 21% of the physician workforce, up from 13% in 2005.
In a more recent survey from the California Health Care Foundation, only 12% of California physicians said they devoted 20-29 hours a week to patient care.
Amy Knoup, a senior recruitment adviser with Provider Solutions & Development), has been helping doctors find jobs for over a decade, and she’s noticed a trend.
“Not only are more physicians seeking part-time roles than they were 10 years ago, but more large health care systems are also offering part time or per diem as well,” said Ms. Knoup.
Who’s working part time, and why?
Ten years ago, the fastest growing segment of part-timers were men nearing retirement and early- to mid-career women.
Pediatricians led the part-time pack in 2002, according to an American Academy of Pediatrics study. At the time, 15% of pediatricians reported their hours as part time. However, the numbers may have increased over the years. For example, a 2021 study by the department of pediatrics, Boston Medical Center, and Boston University found that almost 30% of graduating pediatricians sought part-time work at the end of their training.
At PS&D, Ms. Knoup said she has noticed a trend toward part-timers among primary care, behavioral health, and outpatient specialties such as endocrinology. “We’re also seeing it with the inpatient side in roles that are more shift based like hospitalists, radiologists, and critical care and ER doctors.”
Another trend Ms. Knoup has noticed is with early-career doctors. “They have a different mindset,” she said. “Younger generations are acutely aware of burnout. They may have experienced it in residency or during the pandemic. They’ve had a taste of that and don’t want to go down that road again, so they’re seeking part-time roles. It’s an intentional choice.”
Tracey O’Connell, MD, a radiologist, always knew that she wanted to work part time. “I had a baby as a resident, and I was pregnant with my second child as a fellow,” she said. “I was already feeling overwhelmed with medical training and having a family.”
Dr. O’Connell worked in private practice for 16 years on Mondays, Wednesdays, and Fridays, with no nights or weekends.
“I still found it completely overwhelming,” she said. “Even though I had more days not working than working, I felt like the demands of medical life had advanced faster than human beings could adapt, and I still feel that way.”
Today she runs a part-time teleradiology practice from home but spends more time on her second career as a life coach. “Most of my clients are physicians looking for more fulfillment and sustainable ways of practicing medicine while maintaining their own identity as human beings, not just the all-consuming identity of ‘doctor,’ ” she said.
On the other end of the career spectrum is Lois Goodman, MD, an ob.gyn. in her late 70s. After 42 years in a group practice, she started her solo practice at 72, seeing patients 3 days per week. “I’m just happy to be working. That’s a tremendous payoff for me. I need to keep working for my mental health.”
How does part-time work affect physician shortages and care delivery?
Reducing clinical effort is one of the strategies physicians use to scale down overload. Still, it’s not viable as a long-term solution, said Christine Sinsky, MD, AMA’s vice president of professional satisfaction and a nationally regarded researcher on physician burnout.
“If all the physicians in a community went from working 100% FTE clinical to 50% FTE clinical, then the people in that community would have half the access to care that they had,” said Dr. Sinsky. “There’s less capacity in the system to care for patients.”
Some could argue, then, that part-time physician work may contribute to physician shortage predictions. An Association of American Medical Colleges report estimates there will be a shortage of 37,800 to 124,000 physicians by 2034.
But physicians working part-time express a contrasting point of view. “I don’t believe that part-time workers are responsible for the health care shortage but rather, a great solution,” said Dr. O’Connell. “Because in order to continue working for a long time rather than quitting when the demands exceed human capacity, working part time is a great compromise to offer a life of more sustainable well-being and longevity as a physician, and still live a wholehearted life.”
Pros and cons of being a part-time physician
Pros
Less burnout: The American Medical Association has tracked burnout rates for 22 years. By the end of 2021, nearly 63% of physicians reported burnout symptoms, compared with 38% the year before. Going part time appears to reduce burnout, suggests a study published in Mayo Clinic Proceedings.
Better work-life balance: Rachel Miller, MD, an ob.gyn., worked 60-70 hours weekly for 9 years. In 2022, she went to work as an OB hospitalist for a health care system that welcomes part-time clinicians. Since then, she has achieved a better work-life balance, putting in 26-28 hours a week. Dr. Miller now spends more time with her kids and in her additional role as an executive coach to leaders in the medical field.
More focus: “When I’m at work, I’m 100% mentally in and focused,” said Dr. Miller. “My interactions with patients are different because I’m not burned out. My demeanor and my willingness to connect are stronger.”
Better health: Mehmet Cilingiroglu, MD, with CardioSolution, traded full-time work for part time when health issues and a kidney transplant sidelined his 30-year career in 2018. “Despite my significant health issues, I’ve been able to continue working at a pace that suits me rather than having to retire,” he said. “Part-time physicians can still enjoy patient care, research, innovation, education, and training while balancing that with other areas of life.”
Errin Weisman, a DO who gave up full-time work in 2016, said cutting back makes her feel healthier, happier, and more energized. “Part-time work helps me to bring my A game each day I work and deliver the best care.” She’s also a life coach encouraging other physicians to find balance in their professional and personal lives.
Cons
Cut in pay: Obviously, the No. 1 con is you’ll make less working part time, so adjusting to a salary decrease can be a huge issue, especially if you don’t have other sources of income. Physicians paying off student loans, those caring for children or elderly parents, or those in their prime earning years needing to save for retirement may not be able to go part time.
Diminished career: The chance for promotions or being well known in your field can be diminished, as well as a loss of proficiency if you’re only performing surgery or procedures part time. In some specialties, working part time and not keeping up with (or being able to practice) newer technology developments can harm your career or reputation in the long run.
Missing out: While working part time has many benefits, physicians also experience a wide range of drawbacks. Dr. Goodman, for example, said she misses delivering babies and doing surgeries. Dr. Miller said she gave up some aspects of her specialty, like performing hysterectomies, participating in complex cases, and no longer having an office like she did as a full-time ob.gyn.
Loss of fellowship: Dr. O’Connell said she missed the camaraderie and sense of belonging when she scaled back her hours. “I felt like a fish out of water, that my values didn’t align with the group’s values,” she said. This led to self-doubt, frustrated colleagues, and a reduction in benefits.
Lost esteem: Dr. O’Connell also felt she was expected to work overtime without additional pay and was no longer eligible for bonuses. “I was treated as a team player when I was needed, but not when it came to perks and benefits and insider privilege,” she said. There may be a loss of esteem among colleagues and supervisors.
Overcoming stigma: Because part-time physician work is still not prevalent among colleagues, some may resist the idea, have less respect for it, perceive it as not being serious about your career as a physician, or associate it with being lazy or entitled.
Summing it up
Every physician must weigh the value and drawbacks of part-time work, but the more physicians who go this route, the more part-time medicine gains traction and the more physicians can learn about its values versus its drawbacks.
A version of this article first appeared on Medscape.com.
Mohs surgery improves survival in early-stage Merkel cell carcinoma
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
Surprisingly more nonsustained VT shown in HCM using extended ECG monitoring
BARCELONA – , suggests a study that questions current risk stratification practices in HCM.
In the registry study, such arrythmias were observed in about six times as many HCM patients during 30 days of ambulatory electrocardiographic monitoring as would have been identified based on the first 24 hours of the monitoring period: 65% vs. 11% of the cohort.
Also, about 62% of the patients showed NSVT at “extended” 30-day monitoring, compared with an 8% prevalence of the arrhythmia based on the more conventional ECG monitoring period of 24 hours.
Nonsustained ventricular tachycardia, an important arrhythmia used every day in clinical practice to make decisions, is “much, much more prevalent than we thought” in patients with HCM, Juan Caro Codón, MD, the study’s principal investigator, said in an interview. “We should invest in further research regarding extended ECG monitoring in these patients.”
Dr. Caro Codón, of La Paz University Hospital, Madrid, presented the findings from the TEMPO-HCM study at the European Heart Rhythm Association 2023 Congress, held in Barcelona and virtually.
Its results, he said, have implications for stratifying HCM patients according to their risk for sudden cardiac death in deciding who should be offered an implantable cardioverter-defibrillator (ICD).
The life-incidence of atrial fibrillation (AF) in patients like those in the current analysis has previously been found to be about 20%, and the life-prevalence of NSVT about 20%-30%, using traditional 24- or 48-hour Holter monitoring, Dr. Caro Codón said.
“These arrhythmias are clinically relevant events because they are linked to very meaningful clinical endpoints,” including stroke and thromboembolism, he said, “but also for sudden cardiac death.”
Extended ECG monitoring has been shown useful in the setting of cryptogenic stroke and after AF ablation, but similar findings have been scarce in HCM. Patients using personal wearable monitors such as smart watches, Dr. Caro Codón said, have come to his clinic with concerns that the devices may have signaled a problem. But the lack of relevant data leaves them without a sufficient answer.
In other findings, invited discussant Isabelle van Gelder, MD, PhD, observed after Dr. Caro Codón’s presentation that the number of patients with AF almost doubled based on extended monitoring, compared with the first 24 hours of monitoring.
Based on European Society of Cardiology guidelines from 2020, “Once clinical AF has been documented, there is a class IIA recommendation to start anticoagulation,” said Dr. van Gelder, University of Groningen, the Netherlands. “Therefore, your data really are a call for more data on screening for AF in hypertrophic cardiomyopathy patients.”
Prospective multicenter registry
The TEMPO-HCM registry includes patients with HCM and a clinical indication for standard Holter monitoring at five hospitals in Spain. It excludes patients with an HCM-like phenotype but who lack the telltale genotype, as well as those already implanted with an ICD.
Those in the current analysis underwent 30-day ECG monitoring with a small, wearable device that Dr. Caro Codón described as about 7 cm long, worn in what is essentially a T-shirt with a pocket. Patients could remove the shirt and device to bathe or go swimming, for example, and still be monitored for most of the day.
The analysis included the registry’s first 100 patients (mean age, 57 years; 78% male). Hypertension was present in 47%, 58% were on beta-blockers, 16% had prior AF or atrial flutter, and 19% were taking anticoagulants. Only 8% were on antiarrhythmic drugs, Dr. Caro Codón reported.
The patients had good functional status (68% and 29% were in NYHA class 1 and 2, respectively) and their left ventricular ejection fraction averaged 66%. Of the 71 patients who underwent MRI, 28.2% showed late gadolinium enhancement suggesting myocardial scarring.
More arrhythmias on 30-day monitoring
The primary endpoint of clinically relevant arrhythmia (AF, atrial flutter, or NSVT) was identified during the first 24 hours of monitoring in 11% of patients. The prevalence rose to 65% (P < .001) based on 30-day monitoring.
Similarly, prevalences of the composite primary endpoint components grew on extended monitoring, but the increases reached statistical significance only for NSVT; its prevalence went from 8% to 62% (P < .001). Prevalences rose nonsignificantly from 6% to 10% for AF and 0% to 1% for sustained ventricular tachycardia.
The incidence of NSVT during monitoring climbed fastest from day 0 through about day 19 and then rose more slowly through day 30, Dr. Caro Codón said. “It actually didn’t reach a plateau during this time period, so there is the possibility that if we had continued monitoring patients, the difference between both periods may have been even higher.”
Three variables predicted the incidence of nonsustained VT during monitoring, he said: age, atrial wall thickness, and whether there was late gadolinium enhancement at MRI.
An exploratory analysis looked at the 5-year risk of sudden cardiac death using the European Society of Cardiology HCM-SCD risk calculator recommended in guidelines. Risk assessment based on the 30-day extended monitoring period, compared with the first 24 hours of monitoring alone, predicted a significantly higher 5-year risk of sudden death, Dr. Caro Codón said.
“Even more importantly,” he added, “over 20%” of patients would have been reclassified into a higher-risk group and possibly considered for an ICD based on extended monitoring, compared to 24-hour monitoring.
However, given that more than 50% of patients were found to have NSVT during extended monitoring, Dr. Caro Codón proposed that decisions on whether to implant an ICD should not be so “binary” based on the presence or absence of symptoms, and proposed further investigations be conducted into the complete phenotype of these arrhythmias.
The study has limitations, he observed, including a relatively small size; but it was able to detect important differences between 24-hour and 30-day monitoring outcomes even with only 100 patients. It was also limited by a lack of clinical follow-up for information on endpoints like stroke, thromboembolism, and sudden cardiac death.
Extended monitoring detected more cases of NSVT in the study’s relatively low-risk HCM patients who would not generally have an indication for ICD implantation, observed Dr. van Gelder. Also, at present the prognostic value of NSVT for SCD “seems to be more important at younger age” – that is, younger than 30 years – in patients with HCM.
Dr. van Gelder echoed Dr. Caro Codón’s call for more data from prolonged monitoring to help stratify patients according to risk; she proposed NSVT frequency, duration, and rate as possible targets.
The study was supported by an unrestricted grant from Nuubo, which provided the ECG monitoring systems. Dr. Caro Codón and Dr. van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – , suggests a study that questions current risk stratification practices in HCM.
In the registry study, such arrythmias were observed in about six times as many HCM patients during 30 days of ambulatory electrocardiographic monitoring as would have been identified based on the first 24 hours of the monitoring period: 65% vs. 11% of the cohort.
Also, about 62% of the patients showed NSVT at “extended” 30-day monitoring, compared with an 8% prevalence of the arrhythmia based on the more conventional ECG monitoring period of 24 hours.
Nonsustained ventricular tachycardia, an important arrhythmia used every day in clinical practice to make decisions, is “much, much more prevalent than we thought” in patients with HCM, Juan Caro Codón, MD, the study’s principal investigator, said in an interview. “We should invest in further research regarding extended ECG monitoring in these patients.”
Dr. Caro Codón, of La Paz University Hospital, Madrid, presented the findings from the TEMPO-HCM study at the European Heart Rhythm Association 2023 Congress, held in Barcelona and virtually.
Its results, he said, have implications for stratifying HCM patients according to their risk for sudden cardiac death in deciding who should be offered an implantable cardioverter-defibrillator (ICD).
The life-incidence of atrial fibrillation (AF) in patients like those in the current analysis has previously been found to be about 20%, and the life-prevalence of NSVT about 20%-30%, using traditional 24- or 48-hour Holter monitoring, Dr. Caro Codón said.
“These arrhythmias are clinically relevant events because they are linked to very meaningful clinical endpoints,” including stroke and thromboembolism, he said, “but also for sudden cardiac death.”
Extended ECG monitoring has been shown useful in the setting of cryptogenic stroke and after AF ablation, but similar findings have been scarce in HCM. Patients using personal wearable monitors such as smart watches, Dr. Caro Codón said, have come to his clinic with concerns that the devices may have signaled a problem. But the lack of relevant data leaves them without a sufficient answer.
In other findings, invited discussant Isabelle van Gelder, MD, PhD, observed after Dr. Caro Codón’s presentation that the number of patients with AF almost doubled based on extended monitoring, compared with the first 24 hours of monitoring.
Based on European Society of Cardiology guidelines from 2020, “Once clinical AF has been documented, there is a class IIA recommendation to start anticoagulation,” said Dr. van Gelder, University of Groningen, the Netherlands. “Therefore, your data really are a call for more data on screening for AF in hypertrophic cardiomyopathy patients.”
Prospective multicenter registry
The TEMPO-HCM registry includes patients with HCM and a clinical indication for standard Holter monitoring at five hospitals in Spain. It excludes patients with an HCM-like phenotype but who lack the telltale genotype, as well as those already implanted with an ICD.
Those in the current analysis underwent 30-day ECG monitoring with a small, wearable device that Dr. Caro Codón described as about 7 cm long, worn in what is essentially a T-shirt with a pocket. Patients could remove the shirt and device to bathe or go swimming, for example, and still be monitored for most of the day.
The analysis included the registry’s first 100 patients (mean age, 57 years; 78% male). Hypertension was present in 47%, 58% were on beta-blockers, 16% had prior AF or atrial flutter, and 19% were taking anticoagulants. Only 8% were on antiarrhythmic drugs, Dr. Caro Codón reported.
The patients had good functional status (68% and 29% were in NYHA class 1 and 2, respectively) and their left ventricular ejection fraction averaged 66%. Of the 71 patients who underwent MRI, 28.2% showed late gadolinium enhancement suggesting myocardial scarring.
More arrhythmias on 30-day monitoring
The primary endpoint of clinically relevant arrhythmia (AF, atrial flutter, or NSVT) was identified during the first 24 hours of monitoring in 11% of patients. The prevalence rose to 65% (P < .001) based on 30-day monitoring.
Similarly, prevalences of the composite primary endpoint components grew on extended monitoring, but the increases reached statistical significance only for NSVT; its prevalence went from 8% to 62% (P < .001). Prevalences rose nonsignificantly from 6% to 10% for AF and 0% to 1% for sustained ventricular tachycardia.
The incidence of NSVT during monitoring climbed fastest from day 0 through about day 19 and then rose more slowly through day 30, Dr. Caro Codón said. “It actually didn’t reach a plateau during this time period, so there is the possibility that if we had continued monitoring patients, the difference between both periods may have been even higher.”
Three variables predicted the incidence of nonsustained VT during monitoring, he said: age, atrial wall thickness, and whether there was late gadolinium enhancement at MRI.
An exploratory analysis looked at the 5-year risk of sudden cardiac death using the European Society of Cardiology HCM-SCD risk calculator recommended in guidelines. Risk assessment based on the 30-day extended monitoring period, compared with the first 24 hours of monitoring alone, predicted a significantly higher 5-year risk of sudden death, Dr. Caro Codón said.
“Even more importantly,” he added, “over 20%” of patients would have been reclassified into a higher-risk group and possibly considered for an ICD based on extended monitoring, compared to 24-hour monitoring.
However, given that more than 50% of patients were found to have NSVT during extended monitoring, Dr. Caro Codón proposed that decisions on whether to implant an ICD should not be so “binary” based on the presence or absence of symptoms, and proposed further investigations be conducted into the complete phenotype of these arrhythmias.
The study has limitations, he observed, including a relatively small size; but it was able to detect important differences between 24-hour and 30-day monitoring outcomes even with only 100 patients. It was also limited by a lack of clinical follow-up for information on endpoints like stroke, thromboembolism, and sudden cardiac death.
Extended monitoring detected more cases of NSVT in the study’s relatively low-risk HCM patients who would not generally have an indication for ICD implantation, observed Dr. van Gelder. Also, at present the prognostic value of NSVT for SCD “seems to be more important at younger age” – that is, younger than 30 years – in patients with HCM.
Dr. van Gelder echoed Dr. Caro Codón’s call for more data from prolonged monitoring to help stratify patients according to risk; she proposed NSVT frequency, duration, and rate as possible targets.
The study was supported by an unrestricted grant from Nuubo, which provided the ECG monitoring systems. Dr. Caro Codón and Dr. van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – , suggests a study that questions current risk stratification practices in HCM.
In the registry study, such arrythmias were observed in about six times as many HCM patients during 30 days of ambulatory electrocardiographic monitoring as would have been identified based on the first 24 hours of the monitoring period: 65% vs. 11% of the cohort.
Also, about 62% of the patients showed NSVT at “extended” 30-day monitoring, compared with an 8% prevalence of the arrhythmia based on the more conventional ECG monitoring period of 24 hours.
Nonsustained ventricular tachycardia, an important arrhythmia used every day in clinical practice to make decisions, is “much, much more prevalent than we thought” in patients with HCM, Juan Caro Codón, MD, the study’s principal investigator, said in an interview. “We should invest in further research regarding extended ECG monitoring in these patients.”
Dr. Caro Codón, of La Paz University Hospital, Madrid, presented the findings from the TEMPO-HCM study at the European Heart Rhythm Association 2023 Congress, held in Barcelona and virtually.
Its results, he said, have implications for stratifying HCM patients according to their risk for sudden cardiac death in deciding who should be offered an implantable cardioverter-defibrillator (ICD).
The life-incidence of atrial fibrillation (AF) in patients like those in the current analysis has previously been found to be about 20%, and the life-prevalence of NSVT about 20%-30%, using traditional 24- or 48-hour Holter monitoring, Dr. Caro Codón said.
“These arrhythmias are clinically relevant events because they are linked to very meaningful clinical endpoints,” including stroke and thromboembolism, he said, “but also for sudden cardiac death.”
Extended ECG monitoring has been shown useful in the setting of cryptogenic stroke and after AF ablation, but similar findings have been scarce in HCM. Patients using personal wearable monitors such as smart watches, Dr. Caro Codón said, have come to his clinic with concerns that the devices may have signaled a problem. But the lack of relevant data leaves them without a sufficient answer.
In other findings, invited discussant Isabelle van Gelder, MD, PhD, observed after Dr. Caro Codón’s presentation that the number of patients with AF almost doubled based on extended monitoring, compared with the first 24 hours of monitoring.
Based on European Society of Cardiology guidelines from 2020, “Once clinical AF has been documented, there is a class IIA recommendation to start anticoagulation,” said Dr. van Gelder, University of Groningen, the Netherlands. “Therefore, your data really are a call for more data on screening for AF in hypertrophic cardiomyopathy patients.”
Prospective multicenter registry
The TEMPO-HCM registry includes patients with HCM and a clinical indication for standard Holter monitoring at five hospitals in Spain. It excludes patients with an HCM-like phenotype but who lack the telltale genotype, as well as those already implanted with an ICD.
Those in the current analysis underwent 30-day ECG monitoring with a small, wearable device that Dr. Caro Codón described as about 7 cm long, worn in what is essentially a T-shirt with a pocket. Patients could remove the shirt and device to bathe or go swimming, for example, and still be monitored for most of the day.
The analysis included the registry’s first 100 patients (mean age, 57 years; 78% male). Hypertension was present in 47%, 58% were on beta-blockers, 16% had prior AF or atrial flutter, and 19% were taking anticoagulants. Only 8% were on antiarrhythmic drugs, Dr. Caro Codón reported.
The patients had good functional status (68% and 29% were in NYHA class 1 and 2, respectively) and their left ventricular ejection fraction averaged 66%. Of the 71 patients who underwent MRI, 28.2% showed late gadolinium enhancement suggesting myocardial scarring.
More arrhythmias on 30-day monitoring
The primary endpoint of clinically relevant arrhythmia (AF, atrial flutter, or NSVT) was identified during the first 24 hours of monitoring in 11% of patients. The prevalence rose to 65% (P < .001) based on 30-day monitoring.
Similarly, prevalences of the composite primary endpoint components grew on extended monitoring, but the increases reached statistical significance only for NSVT; its prevalence went from 8% to 62% (P < .001). Prevalences rose nonsignificantly from 6% to 10% for AF and 0% to 1% for sustained ventricular tachycardia.
The incidence of NSVT during monitoring climbed fastest from day 0 through about day 19 and then rose more slowly through day 30, Dr. Caro Codón said. “It actually didn’t reach a plateau during this time period, so there is the possibility that if we had continued monitoring patients, the difference between both periods may have been even higher.”
Three variables predicted the incidence of nonsustained VT during monitoring, he said: age, atrial wall thickness, and whether there was late gadolinium enhancement at MRI.
An exploratory analysis looked at the 5-year risk of sudden cardiac death using the European Society of Cardiology HCM-SCD risk calculator recommended in guidelines. Risk assessment based on the 30-day extended monitoring period, compared with the first 24 hours of monitoring alone, predicted a significantly higher 5-year risk of sudden death, Dr. Caro Codón said.
“Even more importantly,” he added, “over 20%” of patients would have been reclassified into a higher-risk group and possibly considered for an ICD based on extended monitoring, compared to 24-hour monitoring.
However, given that more than 50% of patients were found to have NSVT during extended monitoring, Dr. Caro Codón proposed that decisions on whether to implant an ICD should not be so “binary” based on the presence or absence of symptoms, and proposed further investigations be conducted into the complete phenotype of these arrhythmias.
The study has limitations, he observed, including a relatively small size; but it was able to detect important differences between 24-hour and 30-day monitoring outcomes even with only 100 patients. It was also limited by a lack of clinical follow-up for information on endpoints like stroke, thromboembolism, and sudden cardiac death.
Extended monitoring detected more cases of NSVT in the study’s relatively low-risk HCM patients who would not generally have an indication for ICD implantation, observed Dr. van Gelder. Also, at present the prognostic value of NSVT for SCD “seems to be more important at younger age” – that is, younger than 30 years – in patients with HCM.
Dr. van Gelder echoed Dr. Caro Codón’s call for more data from prolonged monitoring to help stratify patients according to risk; she proposed NSVT frequency, duration, and rate as possible targets.
The study was supported by an unrestricted grant from Nuubo, which provided the ECG monitoring systems. Dr. Caro Codón and Dr. van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EHRA
Clinical trials: Top priority for long COVID
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
Colchicine’s 2010 price spike had major impact on gout care
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Experience With Adaptive Servo-Ventilation Among Veterans in the Post-SERVE-HF Era
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
Pharmacist-Led Antimicrobial Stewardship and Antibiotic Use in Hospitalized Patients With COVID-19
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
The inappropriate use of antibiotics is associated with an increased risk of antibiotic resistance, health care costs, and risk of adverse drug reactions.1 According to the Centers for Disease Control and Prevention (CDC), a 10% decrease in overall antibiotic use across different wards was associated with a 34% decrease in Clostridioides difficile (C difficile) infections.2 In addition, antimicrobial resistance accounts for > 2.8 million infections and > 35,000 deaths each year.3 The estimated total economic costs of antibiotic resistance to the US economy have ranged as high as $20 billion in excess direct health care costs.4 A main goal of an antimicrobial stewardship program (ASP) is to optimize antibiotic use to prevent the adverse consequences of inappropriate antibiotic prescribing.
During the COVID-19 pandemic, increased use of empiric antibiotic therapy has been observed. According to the CDC, almost 80% of patients hospitalized with COVID-19 received an antibiotic from March 2020 to October 2020.5 Studies were conducted to investigate the prevalence of bacterial coinfection in patients with COVID-19 and whether antibiotics were indicated in this patient population. A United Kingdom multicenter, prospective cohort study showed a high proportion of patients hospitalized with COVID-19 received antimicrobials despite microbiologically confirmed bacterial infections being rare and more likely to be secondary infections.6
Many other studies have reported similar findings. Langord and colleagues found the prevalence of bacterial coinfection in patients with COVID-19 was 3.5% but that 71.9% received antibiotics.7 Coenen and colleagues identified 12.4% of the patients with possible and 1.1% of patients with probable bacterial coinfection, while 81% of the study population and 78% of patients were classified as unlikely bacterial coinfection received antibiotics.8
At Veterans Affairs Southern Nevada Healthcare System (VASNHS), an ASP team consisting of an infectious disease (ID) physician and 2 pharmacists provide daily prospective audits with intervention and feedback along with other interventions, such as providing restricted order menus, institutional treatment guidelines, and staff education to help improve antibiotic prescribing. The ASP pharmacists have a scope of practice to make changes to anti-infective therapies. The purpose of this study was to describe antibiotic prescribing in patients hospitalized with COVID-19 from November 1, 2020, to January 31, 2021, in an ASP setting led by pharmacists.
Methods
This retrospective descriptive study included patients who were hospitalized for the treatment of laboratory-confirmed COVID-19 infection. The Theradoc clinical surveillance system was used to retrieve a list of patients who were admitted to VASNHS from November 1, 2020, to January 31, 2021, and tested positive for COVID-19. Patients with incidental positive COVID-19 test results or those who received antibiotics for extrapulmonary indications on hospital admission were excluded.
Each patient chart was reviewed and data, including clinical presentations, procalcitonin (PCT), the requirement of supplemental oxygen, vital signs, imaging findings, antibiotic orders on admission, ASP interventions such as discontinuation or changes to antibiotic therapy during the first 72 hours of hospital admission, clinical outcomes, culture results, and readmission rate, defined as any hospital admission related to COVID-19 or respiratory tract infection within 30 days from the previous discharge, were collected.
The primary objective of the study was to describe antibiotic prescribing in patients hospitalized with COVID-19. The secondary outcomes included the prevalence of bacterial coinfection and nosocomial bacterial infection in patients hospitalized with COVID-19.
Results
A total of 199 patients were admitted to the hospital for laboratory-confirmed COVID-19 infection from November 1, 2020, to January 31, 2021. Sixty-one patients (31%) received at least 1 antibiotic on hospital admission. Among those patients who received empiric antibiotic treatment, 29 patients (48%) met the Systemic Inflammatory Response Syndrome (SIRS) criteria. Fifty-six patients (92%) had ≥ 1 PCT level obtained, and 26 of those (46%) presented with elevated PCT levels (PCT > 0.25). Fifty patients (82%) required oxygen supplement and 49 (80%) presented with remarkable imaging findings. Of 138 patients who did not receive empiric antibiotic therapy within 72 hours of hospital admission, 56 (41%) met the SIRS criteria, 31 (29%) had elevated PCT levels, 100 (72%) required oxygen supplement, and 79 (59%) presented with remarkable imaging findings.
Antibiotic Prescribing
Forty-six of 61 patients (75%) received antibiotic treatment for community-acquired pneumonia (CAP) that included ceftriaxone and azithromycin. Three patients (5%) received ≥ 1 broad-spectrum antibiotic (4th generation cephalosporin [cefepime] or piperacillin-tazobactam), 2 (3%) received vancomycin, and 1 (2%) received a fluoroquinolone (levofloxacin) on admission.
Among 61 patients who received empiric antibiotics, the readmission rate was 6%. The mortality rate was 20%, and the mean (SD) duration of hospital stay was 13.1 (12.5) days.
Six of 199 patients (3%) had microbiologically confirmed bacterial coinfection on hospital admission: 3 were Pseudomonas aeruginosa (P aeruginosa) and 2 were Klebsiella oxytoca (Table 1).
Discussion
Prospective audit and feedback and preauthorization are recommended in guidelines as “core components of any stewardship program.”9 At VASNHS, the ASP performs daily prospective audits with intervention and feedback. Efforts have been made to maintain daily ASP activities during the pandemic. This study aimed to describe antibiotic prescribing for patients hospitalized with COVID-19 in a pharmacist-led ASP setting. It was found that up to 31% of the patients received ≥ 1 antibiotic on admission for empiric treatment of bacterial coinfection. About half of these patients met the SIRS criteria. Most of these patients received ceftriaxone and azithromycin for concern of CAP. ASP discontinued antibiotics within 72 hours in most of the patients. Chart review and discussion with ID physicians and/or hospitalists determined the probability of bacterial coinfection as well as any potential complication or patient-specific risk factor. It is important to note that most patients who received antibiotics on admission had ≥ 1 PCT level and up to 46% of them had a PCT level > 0.25. However, according to Relph and colleagues, PCT may not be a reliable indicator of bacterial infection in severe viral diseases with raised interleukin-6 levels.10 An elevated PCT level should not be the sole indicator for empiric antibiotic treatment.
Study findings confirmed the low prevalence of bacterial coinfection in patients hospitalized with COVID-19. The overuse of empiric antibiotics in a patient population unlikely to present with bacterial coinfection is concerning. It is essential to continue promoting antimicrobial stewardship during the COVID-19 pandemic to ensure appropriate and responsible antimicrobial prescribing. A thorough clinical assessment consisting of comorbidities, clinical symptoms, radiologic and microbiologic findings, as well as other relevant workup or biomarker results is crucial to determine whether the antibiotic is strongly indicated in patients hospitalized with COVID-19. Empiric antibiotic therapy should be considered only in patients with clinical findings suggestive of bacterial coinfection.
Limitations
Limitations of our study included the study design (single-center, retrospective review, lack of comparative group) and small sample size with a 3-month study period. In addition, respiratory cultures are not commonly obtained in patients who present with mild-to-moderate CAP. Using culture results solely to confirm bacterial coinfection in patients with COVID-19 could have underestimated the prevalence of bacterial infection. Developing diagnostic criteria that include clinical signs and symptoms, imaging findings, and laboratory results as well as culture results would help to better assess the presence of bacterial coinfection in this patient population.
Conclusions
The study findings showed that up to 30% of patients hospitalized for COVID-19 infection received empiric antibiotic treatment for concern of bacterial coinfection. A pharmacist-led ASP provided interventions, including early discontinuation of antibiotics in 77% of these patients.
A low prevalence of bacterial coinfection (3%) in patients hospitalized with COVID-19 also was reported. A thorough clinical workup to determine the risk of bacterial coinfection in patients with COVID-19 is important before starting empiric antibiotic therapy. Continuing to promote the ASP during the COVID-19 pandemic to ensure responsible antibiotic use and prevent antimicrobial resistance is essential.
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179
1. Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC grand rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 2015;64(32):871-873. doi:10.15585/mmwr.mm6432a3
2. Nearly half a million Americans suffered from Clostridium difficile infections in a single year. Centers for Disease Control and Prevention. Updated March 22, 2017. Accessed March 21, 2023. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html
3. Centers for Disease Control and Prevention. About antimicrobial resistance. Updated October 5, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/about.html
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
5. Centers for Disease Control and Prevention. COVID-19 & antibiotic resistance. Updated February 25, 2022. Accessed March 21, 2023. https://www.cdc.gov/drugresistance/covid19.html
6. Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354-e365. doi:10.1016/S2666-5247(21)00090-2
7. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622-1629. doi:10.1016/j.cmi.2020.07.016
8. Coenen S, de la Court JR, Buis DTP, et al. Low frequency of community-acquired bacterial co-infection in patients hospitalized for COVID-19 based on clinical, radiological and microbiological criteria: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10(1):155. doi:10.1186/s13756-021-01024-4
9. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs: 2019. Accessed March 21, 2023. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
10. Relph KA, Russell CD, Fairfield CJ, et al; International Severe Acute Respiratory and Emerging Infections Consortium; Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with Coronavirus Disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi:10.1093/ofid/ofac179