User login
Swab, spit, stay home? College coronavirus testing plans are all over the map
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.
What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.
What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
Yousuf El-Jayyousi, a junior engineering student at the University of Missouri, wanted guidance and reassurance that it would be safe to go back to school for the fall semester. He tuned into a pair of online town halls organized by the university hoping to find that.
He did not.
What he got instead from those town halls last month was encouragement to return to class at the institution affectionately known as Mizzou. The university, in Columbia, would be testing only people with symptoms, and at that point, the university said people who test positive off campus were under no obligation to inform the school.
“It feels like the university doesn’t really care whether we get sick or not,” said El-Jayyousi, who is scheduled for two in-person classes, and lives at home with his parents and 90-year-old grandmother.
He’s seen the studies from researchers at Yale and Harvard that suggest testing needs to be much more widespread. He asked his instructors if he could join lectures remotely once classes begin Monday. One was considering it; the other rejected it.
“It was kind of very dismissive, like ‘so what?’ ” El-Jayyousi said.
But it’s an enormous “so what?” packed with fear and unknowns for Jayyousi and some 20 million other students enrolled in some level of postsecondary education in America, if they are not already online only.
Policies for reentry onto campuses that were abruptly shut in March are all over the map.
Hundreds Undecided
According to the College Crisis Initiative, or C2i, a project of Davidson College that monitors how higher ed is responding to the pandemic, there is nothing resembling a common approach. Of 2,958 institutions it follows, 151 were planning to open fully online, 729 were mostly online and 433 were taking a hybrid approach. Just 75 schools were insisting on students attending fully in person, and 614 were aiming to be primarily in-person. Some 800 others were still deciding, just weeks before instruction was to start.
The decisions often have little correlation with the public health advisories in the region. Mizzou, which is in an area with recent COVID spikes, is holding some in-person instruction and has nearly 7,000 students signed up to live in dorms and other university-owned housing. Harvard, in a region with extremely low rates of viral spread, has opted to go all online and allowed students to defer a year.
The specific circumstances colleges and universities face are as much determined by local fiscal and political dictates as by medicine and epidemiology. It is often unclear who is making the call. So it’s every student for herself to chart these unknown waters, even as students (or their families) have written tuition checks for tens of thousands of dollars and signed leases for campus and off-campus housing.
And the risks – health, educational and financial – boomerang back on individual students: Two weeks after University of North Carolina students, as instructed, returned to the flagship campus in Chapel Hill with the promise of at least some in-person learning, all classes went online. Early outbreaks surged from a few students to more than 130 in a matter of days. Most undergrads have about a week to clear out of their dorms.
“It’s really tough,” said neuroscience major Luke Lawless, 20. “Chapel Hill is an amazing place, and as a senior it’s tough to know that my time’s running out – and the virus only adds to that.”
Location, location, location
C2i’s creator, Davidson education Assistant Professor Chris Marsicano, said the extreme diversity of approaches comes from the sheer diversity of schools, the penchant of many to follow the leads of more prestigious peers, and local politics.
“Some states have very strong and stringent mask requirements. Some have stronger stay-at-home orders. Others are sort of leaving it up to localities. So the confluence of politics, institutional isomorphism – that imitation – and different needs that the institutions have are driving the differences,” Marsicano said.
Location matters a lot, too, Marsicano said, pointing to schools like George Washington University and Boston University in urban settings where the environment is beyond the control of the school, versus a place like the University of the South in remote, rural Sewanee, Tennessee, where 90% of students will return to campus.
“It’s a lot easier to control an outbreak if you are a fairly isolated college campus than if you are in the middle of a city,” Marsicano said.
Student behavior is another wild card, Marsicano said, since even the best plans will fail if college kids “do something stupid, like have a massive frat party without masks.”
“You’ve got student affairs professionals across the country who are screaming at the top of their lungs, ‘We can’t control student behavior when they go off campus’” Marsicano said.
Another factor is a vacuum at the federal level. Although the Department of Education says Secretary Betsy DeVos has held dozens of calls with governors and state education superintendents, there’s no sign of an attempt to offer unified guidance to colleges beyond a webpage that links to relaxed regulatory requirements and anodyne fact sheets from the Centers for Disease Control and Prevention on preventing viral spread.
Even the money that the department notes it has dispensed – $30 billion from Congress’ CARES Act – is weighted toward K-12 schools, with about $13 billion for higher education, including student aid.
The U.S. Senate adjourned last week until Sept. 8, having never taken up a House-passed relief package that included some $30 billion for higher education. A trio of Democratic senators, including Sen. Elizabeth Warren, is calling for national reporting standards on college campuses.
No benchmarks
Campus communities with very different levels of contagion are making opposite calls about in-person learning. Mizzou’s Boone County has seen more than 1,400 confirmed COVID cases after a spike in mid-July. According to the Harvard Global Health Institute’s COVID risk map, Boone has accelerated spread, with 14 infections per day per 100,000 people. The institute advises stay-at-home orders or rigorous testing and tracing at such rates of infection. Two neighboring counties were in the red zone recently, with more than 25 cases per day per 100,000 people. Mizzou has left it up to deans whether classes will meet in person, making a strong argument for face-to-face instruction.
Meanwhile, Columbia University in New York City opted for all online instruction, even though the rate of infection there is a comparatively low 3.8 cases per day per 100,000 people.
Administrators at Mizzou considered and rejected mandatory testing. “All that does is provide one a snapshot of the situation,” University of Missouri system President Mun Choi said in one of the town halls.
Mizzou has an in-house team that will carry out case investigation and contact tracing with the local health department. This week, following questions from the press and pressure from the public, the university announced students will be required to report any positive COVID test to the school.
Who do you test? When?
CDC guidance for higher education suggests there’s not enough data to know whether testing everyone is effective, but some influential researchers, such as those at Harvard and Yale, disagree.
“This virus is subject to silent spreading and asymptomatic spreading, and it’s very hard to play catch-up,” said Yale professor David Paltiel, who studies public health policy. “And so thinking that you can keep your campus safe by simply waiting until students develop symptoms before acting, I think, is a very dangerous game.”
Simulation models conducted by Paltiel and his colleagues show that, of all the factors university administrators can control – including the sensitivity and specificity of COVID-19 tests – the frequency of testing is most important.
He’s “painfully aware” that testing everyone on campus every few days sets a very high bar – logistically, financially, behaviorally – that may be beyond what most schools can reach. But he says the consequences of reopening campuses without those measures are severe, not just for students, but for vulnerable populations among school workers and in the surrounding community.
“You really have to ask yourself whether you have any business reopening if you’re not going to commit to an aggressive program of high-frequency testing,” he said.
The fighting – and testing – Illini
Some institutions that desperately want students to return to campus are backing the goal with a maximal approach to safety and testing.
About a 4-hour drive east along the interstates from Mizzou is the University of Illinois at Urbana-Champaign, whose sports teams are known as the Fighting Illini.
Weeks ago, large white tents with signs reading “Walk-Up COVID-19 Testing” have popped up across campus; there students take a simple saliva test.
“This seems to be a lot easier than sticking a cotton swab up your nose,” graduate student Kristen Muñoz said after collecting a bit of her saliva in a plastic tube and sealing it in a bag labeled “Biohazard.”
In just a few hours, she got back her result: negative.
The school plans to offer free tests to the 50,000 students expected to return this month, as well as some 11,000 faculty and staff members.
“The exciting thing is, because we can test up to 10,000 per day, it allows the scientist to do what’s really the best for trying to protect the community as opposed to having to cut corners, because of the limitations of the testing,” said University of Illinois chemist Martin Burke, who helped develop the campus’s saliva test, which received emergency use authorization from the federal Food and Drug Administration this week.
The test is similar to one designed by Yale and funded by the NBA that cleared the FDA hurdle just before the Illinois test. Both Yale and Illinois hope aggressive testing will allow most undergraduate students to live on campus, even though most classes will be online.
University of Illinois epidemiologist Becky Smith said they are following data that suggest campuses need to test everyone every few days because the virus is not detectable in infected people for 3 or 4 days.
“But about two days after that, your infectiousness peaks,” she said. “So, we have a very small window of time in which to catch people before they have done most of the infection that they’re going to be doing.”
Campus officials accepted Smith’s recommendation that all faculty, staffers and students participating in any on-campus activities be required to get tested twice a week.
Illinois can do that because its test is convenient and not invasive, which spares the campus from using as much personal protective equipment as the more invasive tests require, Burke said. And on-site analysis avoids backlogs at public health and commercial labs.
Muddled in the middle
Most other colleges fall somewhere between the approaches of Mizzou and the University of Illinois, and many of their students still are uncertain how their fall semester will go.
At the University of Southern California, a private campus of about 48,500 students in Los Angeles, officials had hoped to have about 20% of classes in person – but the county government scaled that back, insisting on tougher rules for reopening than the statewide standards.
If students eventually are allowed back, they will have to show a recent coronavirus test result that they obtained on their own, said Dr. Sarah Van Orman, chief health officer of USC Student Health.
They will be asked to do daily health assessments, such as fever checks, and those who have been exposed to the virus or show symptoms will receive a rapid test, with about a 24-hour turnaround through the university medical center’s lab. “We believe it is really important to have very rapid access to those results,” Van Orman said.
At California State University – the nation’s largest 4-year system, with 23 campuses and nearly a half-million students – officials decided back in May to move nearly all its fall courses online.
“The first priority was really the health and safety of all of the campus community,” said Mike Uhlenkamp, spokesperson for the CSU Chancellor’s Office. About 10% of CSU students are expected to attend some in-person classes, such as nursing lab courses, fine art and dance classes, and some graduate classes.
Uhlenkamp said testing protocols are being left up to each campus, though all are required to follow local safety guidelines. And without a medical campus in the system, CSU campuses do not have the same capacity to take charge of their own testing, as the University of Illinois is doing.
For students who know they won’t be on campus this fall, there is regret at lost social experiences, networking and hands-on learning so important to college.
But the certainty also brings relief.
“I don’t think I would want to be indoors with a group of, you know, even just a handful of people, even if we have masks on,” said Haley Gray, a 28-year-old graduate student at the University of California-Berkeley starting the second year of her journalism program.
She knows she won’t have access to Berkeley’s advanced media labs or the collaborative sessions students experience there. And she said she realized the other day she probably won’t just sit around the student lounge and strike up unexpected friendships.
“That’s a pretty big bummer but, you know, I think overall we’re all just doing our best, and given the circumstances, I feel pretty OK about it,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This story is part of a partnership that includes KBIA, Illinois Public Media, Side Effects Public Media, NPR and Kaiser Health News.
Population health can improve postdischarge care
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
With the United States spending the most per capita on health care among industrialized nations but having the worst aggregate health outcomes, there’s a stark need for improvement, according to an expert at HM20 Virtual, hosted by the Society of Hospital Medicine.
Broadening the focus beyond the four walls of the hospital can bring better results while also saving money, said Adam Myers, MD, chief of population health at Cleveland Clinic. Dr. Myers described the way his health system has begun to pay more careful attention to the needs of specific kinds of patients and tailoring posthospitalization care accordingly, with in-person and virtual home visits, and postdischarge clinics.
With an increasing attention to value, health care organizations have to change their structure or risk going the way of the Choluteca Bridge in Honduras, Dr. Myers said. The Choluteca Bridge was built to be hurricane proof, but was nonetheless rendered useless in 1998 after Hurricane Mitch shifted the very course of the river beneath it.
Similarly, the way health care is delivered often does not meet the needs of the population.
“Our national system has been focused almost entirely on inpatient care,” Dr. Myers said. “A lot of the transition in care is outside of facilities and outside the walls of our inpatient settings.”
Instead, he said a focus on population health – understanding and tending to the needs of people rather than just treating them when they show up at clinics – should involve more outpatient care that is less centralized, fees based on outcomes and patient experience rather than simply volume of services, team approaches rather than single-provider care, and a general attention to preserving health rather than treating sickness.
At Cleveland Clinic, care teams try to understand not just the care that is medically necessary, but what is wanted and justified, as well as how to deliver that care safely, reliably, and affordably with outcomes that patients and families desire.
The results are striking. After increasing the number of ambulatory patient “touches” for those with chronic disease, inpatient care – disliked by patients and costly to health centers – decreased. From the first quarter of 2018, outpatient visits increased 9%, while inpatient visits dropped 7.4%, Dr. Myers said.
“As we managed patients more effectively on an outpatient basis, their need for inpatient care diminished,” he said. “It works.”
Cleveland Clinic has also made changes designed to reduce costly readmissions, using virtual visits, house calls, time reserved for team meetings to identify patients with gaps in their care, and attention to nonmedical determinants of health, such as assessing fall risk at home and addressing lack of nutritious food options in a community.
The health system has seen a 28% reduction in the cost of care attributed to house calls, 12% cost reduction attributed to better care coordination, and a 49% decrease in hospital days for “superutilizers” of the ED, Dr. Myers said.
Postdischarge clinics – where patients can be seen for the first few visits after hospitalization – have also been valuable for many health systems, because they are closely in tune with what happened during the inpatient stay. These clinics are staffed by hospitalists, interns, residents, or ambulatory clinicians. Dr. Myers said hospitalists tend to have an improved perspective after working in a discharge clinic, with more concern about a patient’s needs once they leave the hospital bed.
“Those hospitalists that I know who have participated in programs like this start to act a bit more like primary care physicians,” he said.
In a Q&A session after Dr. Myers’ presentation, he discussed how hospitalists can affect the many layers of health care policy, factors that often overlap with population health.
He noted that medical care accounts for only about 20% of patient outcomes – the rest involve social and environmental factors.
“I don’t know about you , but I’m not satisfied only impacting 20% of health outcomes,” he said. First, physicians need to understand what is happening in their communities, and the health policies that are preventing improvement. Then, build partnerships to help fix these problems. He pointed to lead poisoning as an example.
“If you think about it, lead poisoning is a social housing problem that shows up as a health care issue. Unless we are getting out into the community and mitigating the root problem, we will have to treat it over and over again,” he said.
Dr. Myers reported no relevant financial disclosures.
Managing acute pain in inpatients on OUD therapy
“This is something we’re going to see more frequently, and many of us already have,” Theresa E. Vettese, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
The drastic drop in prescriptions for opioid pain medications in the last several years hasn’t curtailed the current opioid epidemic. Instead, the epidemic has to a great extent morphed into expanded use of illicit heroin and fentanyl, noted Dr. Vettese, an internist, hospitalist, and palliative care physician at Emory University and Grady Memorial Hospital in Atlanta.
Mythbusting
Treatment of acute pain in hospitalized patients on opioid agonist therapy for opioid use disorder (OUD) is actually pretty straightforward once a few common myths have been dispelled, she said.
One of these myths –common among both physicians and patients in treatment for OUD – is that prescribing opioids for management of acute pain will place such patients at risk for OUD relapse.
“In fact, the data really strongly suggest this is not the case,” Dr. Vettese said. “It will not worsen addiction. But if we don’t aggressively treat these patients’ acute pain, it puts them at higher risk for bad outcomes.”
Another myth – this one not uncommon among hospital pharmacy departments – is that only physicians with a special certification can prescribe methadone for inpatients.
“The federal laws are clear: Any physician who has a DEA license can prescribe methadone in the hospital acute care setting, not only for pain management, but also for treatment of opioid withdrawal. You can’t prescribe it in the outpatient setting for opioid withdrawal – that has to be dispensed through a federally regulated methadone outpatient treatment program. But in the hospital, we can feel safe that we can do so. You may need to educate your pharmacist about this,” she said.
Hospitalists also can prescribe buprenorphine in the acute care inpatient setting, both for pain and treatment of opioid withdrawal, without need for a DEA waiver.
“It’s useful to get some skills in using buprenorphine in the inpatient setting. You don’t need an X waiver, but I encourage everyone to do the X-waiver training because it’s a terrific educational session. It’s 8 hours for physicians and well worth it,” Dr. Vettese noted.
By federal law the inpatient physician also can prescribe 3 days of buprenorphine at discharge to get the patient to an outpatient provider.
Misconceptions also abound about NSAIDs as a nonopioid component of acute pain management in hospitalized patients. They actually are extremely effective for the treatment of musculoskeletal, orthopedic, procedural, migraine, and some types of cancer pain. The number needed to treat (NNT) for postoperative pain relief for ibuprofen or celecoxib is 2.5, and when used in conjunction with acetaminophen at 325 mg every 4 hours, that NNT drops to 1.5, similar to the NNT of 1.7 for oxycodone at 15 mg. It should be noted, however, that the bar defining effective pain relief in randomized studies is set rather low: A 50% greater reduction in pain than achieved with placebo.
Many hospitalists would like to use NSAIDs more often, but they’re leery of the associated risks of GI bleeding, ischemic cardiovascular events, and worsening kidney function. Dr. Vettese offered several risk-mitigation strategies to increase the use of NSAIDs as opioid-sparing agents for acute pain management.
She has changed her own clinical practice with regard to using NSAIDs in patients with chronic kidney disease in response to a 2019 systematic review by investigators at the University of Ottawa.
“This was a game changer for me because in this review, low-dose NSAIDs were safe in that they didn’t significantly increase the risk of worsening kidney failure even in patients with stage 3 chronic kidney disease. So this has expanded my use of NSAIDs in this population through stage 3 CKD. With a creatinine clearance below 30, however, kidney failure worsened rapidly, so I don’t do it in patients with CKD stage 4,” Dr. Vettese said.
Gastroenterologists categorize patients as being at high risk of GI bleeding related to NSAID use if they have a history of a complicated ulcer or they have at least three of the following risk factors: Age above 65 years, history of an uncomplicated ulcer, being on high-dose NSAID therapy, or concurrent use of aspirin, glucocorticoids, or anticoagulants. Patients are considered at moderate risk if they have one or two of the risk factors, and low risk if they have none. Dr. Vettese said that, while NSAIDs clearly should be avoided in the high-risk group, moderate-risk patients are a different matter.
“Many avoid the use of NSAIDs with moderate risk, but I think we can expand their use if we use the right NSAID and we use protective strategies,” Dr. Vettese said.
Celecoxib is the safest drug in terms of upper GI bleeding risk, but ibuprofen is close. They are associated with a 2.2-fold increased risk of bleeding when compared with risk in patients not on an NSAID. Naproxen or indeomethacin use carries a fourfold to fivefold increased risk.
“Celecoxib with a proton pump inhibitor is safest, followed by celecoxib alone, followed by ibuprofen with a proton pump inhibitor. So I advocate using NSAIDs more frequently in people who are at moderate risk by using them with a PPI,” she said.
There is persuasive evidence of increased cardiovascular risk in association with even short-duration NSAIDs, as the drugs are utilized in the treatment of acute pain in hospitalized patients. That being said, Dr. Vettese believes hospitalists can use these drugs safely in more patients by following a thoughtful cardiovascular risk-mitigation strategy developed by Italian investigators.
Communicating about pain management
“Communication is always the key to effective pain management in every situation,” Dr. Vettese emphasized.
“I talk to the patient about the goals of effective pain management. I’ll discourage the use of the 1-10 pain scale, and instead, I’ll be honest about expectations, saying, ‘You have a problem that will cause acute pain, and it’s unlikely that I will be able to completely relieve your pain. The goal is to improve your function so that you can get up and go the bathroom by yourself, and so that you can sleep for a few hours. That’s how we’re going to measure the efficacy of our pain-management program.’ ”
She explains to the patient that she’ll be using nonopioid medications and nondrug therapies along with oral opioid pain medications, which are less risky than IV opioids. She offers reassurance that this treatment strategy won’t cause an OUD relapse. She lets the patient know up-front that the opioids will be tapered as the acute pain improves.
For the patient who comes into the hospital on buprenorphine for OUD, she immediately checks with the state prescription drug monitoring program to make sure everything is above board and there’s no indication of doctor shopping for prescriptions. For in-hospital acute pain, it’s safe and effective to continue the outpatient dose. On an outpatient basis, however, the drug is given once daily. On that dosing schedule both the euphoric effect as well as the analgesic effect are lost, so for acute pain management in the hospital it’s recommended to split the dose into twice- or thrice-daily doses to achieve an analgesic effect.
Oral NSAIDs are part of the treatment strategy whenever possible. For severe acute pain, Dr. Vettese will prescribe an immediate-release opioid having a high affinity to the mu opioid receptor, such as oral hydromorphone, on an as-needed basis. The drug has onset of effect in 30 minutes, peak effect in 1 hour, and a duration of effect of 4-6 hours, although she recommends going with 4 hours to provide adequate analgesia.
“These patients will require much higher doses than the patients who are opioid naive,” she advised.
For the patient with acute pain who is admitted while on methadone for OUD, it’s important to call the outpatient treatment program to verify the dosage.
“You can split the dose of methadone to try to get better analgesia, although I can tell you that patients who are treated with methadone for OUD frequently don’t want to do that. And if they don’t want to, then I don’t,” the hospitalist said.
As with the patient on buprenorphine for OUD, she’ll use additional oral immediate-release opioids as needed for acute severe pain in a patient on methadone for medication-assisted OUD treatment.
Dr. Vettese reported having no financial conflicts regarding her presentation.
“This is something we’re going to see more frequently, and many of us already have,” Theresa E. Vettese, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
The drastic drop in prescriptions for opioid pain medications in the last several years hasn’t curtailed the current opioid epidemic. Instead, the epidemic has to a great extent morphed into expanded use of illicit heroin and fentanyl, noted Dr. Vettese, an internist, hospitalist, and palliative care physician at Emory University and Grady Memorial Hospital in Atlanta.
Mythbusting
Treatment of acute pain in hospitalized patients on opioid agonist therapy for opioid use disorder (OUD) is actually pretty straightforward once a few common myths have been dispelled, she said.
One of these myths –common among both physicians and patients in treatment for OUD – is that prescribing opioids for management of acute pain will place such patients at risk for OUD relapse.
“In fact, the data really strongly suggest this is not the case,” Dr. Vettese said. “It will not worsen addiction. But if we don’t aggressively treat these patients’ acute pain, it puts them at higher risk for bad outcomes.”
Another myth – this one not uncommon among hospital pharmacy departments – is that only physicians with a special certification can prescribe methadone for inpatients.
“The federal laws are clear: Any physician who has a DEA license can prescribe methadone in the hospital acute care setting, not only for pain management, but also for treatment of opioid withdrawal. You can’t prescribe it in the outpatient setting for opioid withdrawal – that has to be dispensed through a federally regulated methadone outpatient treatment program. But in the hospital, we can feel safe that we can do so. You may need to educate your pharmacist about this,” she said.
Hospitalists also can prescribe buprenorphine in the acute care inpatient setting, both for pain and treatment of opioid withdrawal, without need for a DEA waiver.
“It’s useful to get some skills in using buprenorphine in the inpatient setting. You don’t need an X waiver, but I encourage everyone to do the X-waiver training because it’s a terrific educational session. It’s 8 hours for physicians and well worth it,” Dr. Vettese noted.
By federal law the inpatient physician also can prescribe 3 days of buprenorphine at discharge to get the patient to an outpatient provider.
Misconceptions also abound about NSAIDs as a nonopioid component of acute pain management in hospitalized patients. They actually are extremely effective for the treatment of musculoskeletal, orthopedic, procedural, migraine, and some types of cancer pain. The number needed to treat (NNT) for postoperative pain relief for ibuprofen or celecoxib is 2.5, and when used in conjunction with acetaminophen at 325 mg every 4 hours, that NNT drops to 1.5, similar to the NNT of 1.7 for oxycodone at 15 mg. It should be noted, however, that the bar defining effective pain relief in randomized studies is set rather low: A 50% greater reduction in pain than achieved with placebo.
Many hospitalists would like to use NSAIDs more often, but they’re leery of the associated risks of GI bleeding, ischemic cardiovascular events, and worsening kidney function. Dr. Vettese offered several risk-mitigation strategies to increase the use of NSAIDs as opioid-sparing agents for acute pain management.
She has changed her own clinical practice with regard to using NSAIDs in patients with chronic kidney disease in response to a 2019 systematic review by investigators at the University of Ottawa.
“This was a game changer for me because in this review, low-dose NSAIDs were safe in that they didn’t significantly increase the risk of worsening kidney failure even in patients with stage 3 chronic kidney disease. So this has expanded my use of NSAIDs in this population through stage 3 CKD. With a creatinine clearance below 30, however, kidney failure worsened rapidly, so I don’t do it in patients with CKD stage 4,” Dr. Vettese said.
Gastroenterologists categorize patients as being at high risk of GI bleeding related to NSAID use if they have a history of a complicated ulcer or they have at least three of the following risk factors: Age above 65 years, history of an uncomplicated ulcer, being on high-dose NSAID therapy, or concurrent use of aspirin, glucocorticoids, or anticoagulants. Patients are considered at moderate risk if they have one or two of the risk factors, and low risk if they have none. Dr. Vettese said that, while NSAIDs clearly should be avoided in the high-risk group, moderate-risk patients are a different matter.
“Many avoid the use of NSAIDs with moderate risk, but I think we can expand their use if we use the right NSAID and we use protective strategies,” Dr. Vettese said.
Celecoxib is the safest drug in terms of upper GI bleeding risk, but ibuprofen is close. They are associated with a 2.2-fold increased risk of bleeding when compared with risk in patients not on an NSAID. Naproxen or indeomethacin use carries a fourfold to fivefold increased risk.
“Celecoxib with a proton pump inhibitor is safest, followed by celecoxib alone, followed by ibuprofen with a proton pump inhibitor. So I advocate using NSAIDs more frequently in people who are at moderate risk by using them with a PPI,” she said.
There is persuasive evidence of increased cardiovascular risk in association with even short-duration NSAIDs, as the drugs are utilized in the treatment of acute pain in hospitalized patients. That being said, Dr. Vettese believes hospitalists can use these drugs safely in more patients by following a thoughtful cardiovascular risk-mitigation strategy developed by Italian investigators.
Communicating about pain management
“Communication is always the key to effective pain management in every situation,” Dr. Vettese emphasized.
“I talk to the patient about the goals of effective pain management. I’ll discourage the use of the 1-10 pain scale, and instead, I’ll be honest about expectations, saying, ‘You have a problem that will cause acute pain, and it’s unlikely that I will be able to completely relieve your pain. The goal is to improve your function so that you can get up and go the bathroom by yourself, and so that you can sleep for a few hours. That’s how we’re going to measure the efficacy of our pain-management program.’ ”
She explains to the patient that she’ll be using nonopioid medications and nondrug therapies along with oral opioid pain medications, which are less risky than IV opioids. She offers reassurance that this treatment strategy won’t cause an OUD relapse. She lets the patient know up-front that the opioids will be tapered as the acute pain improves.
For the patient who comes into the hospital on buprenorphine for OUD, she immediately checks with the state prescription drug monitoring program to make sure everything is above board and there’s no indication of doctor shopping for prescriptions. For in-hospital acute pain, it’s safe and effective to continue the outpatient dose. On an outpatient basis, however, the drug is given once daily. On that dosing schedule both the euphoric effect as well as the analgesic effect are lost, so for acute pain management in the hospital it’s recommended to split the dose into twice- or thrice-daily doses to achieve an analgesic effect.
Oral NSAIDs are part of the treatment strategy whenever possible. For severe acute pain, Dr. Vettese will prescribe an immediate-release opioid having a high affinity to the mu opioid receptor, such as oral hydromorphone, on an as-needed basis. The drug has onset of effect in 30 minutes, peak effect in 1 hour, and a duration of effect of 4-6 hours, although she recommends going with 4 hours to provide adequate analgesia.
“These patients will require much higher doses than the patients who are opioid naive,” she advised.
For the patient with acute pain who is admitted while on methadone for OUD, it’s important to call the outpatient treatment program to verify the dosage.
“You can split the dose of methadone to try to get better analgesia, although I can tell you that patients who are treated with methadone for OUD frequently don’t want to do that. And if they don’t want to, then I don’t,” the hospitalist said.
As with the patient on buprenorphine for OUD, she’ll use additional oral immediate-release opioids as needed for acute severe pain in a patient on methadone for medication-assisted OUD treatment.
Dr. Vettese reported having no financial conflicts regarding her presentation.
“This is something we’re going to see more frequently, and many of us already have,” Theresa E. Vettese, MD, said at HM20 Virtual, hosted by the Society of Hospital Medicine.
The drastic drop in prescriptions for opioid pain medications in the last several years hasn’t curtailed the current opioid epidemic. Instead, the epidemic has to a great extent morphed into expanded use of illicit heroin and fentanyl, noted Dr. Vettese, an internist, hospitalist, and palliative care physician at Emory University and Grady Memorial Hospital in Atlanta.
Mythbusting
Treatment of acute pain in hospitalized patients on opioid agonist therapy for opioid use disorder (OUD) is actually pretty straightforward once a few common myths have been dispelled, she said.
One of these myths –common among both physicians and patients in treatment for OUD – is that prescribing opioids for management of acute pain will place such patients at risk for OUD relapse.
“In fact, the data really strongly suggest this is not the case,” Dr. Vettese said. “It will not worsen addiction. But if we don’t aggressively treat these patients’ acute pain, it puts them at higher risk for bad outcomes.”
Another myth – this one not uncommon among hospital pharmacy departments – is that only physicians with a special certification can prescribe methadone for inpatients.
“The federal laws are clear: Any physician who has a DEA license can prescribe methadone in the hospital acute care setting, not only for pain management, but also for treatment of opioid withdrawal. You can’t prescribe it in the outpatient setting for opioid withdrawal – that has to be dispensed through a federally regulated methadone outpatient treatment program. But in the hospital, we can feel safe that we can do so. You may need to educate your pharmacist about this,” she said.
Hospitalists also can prescribe buprenorphine in the acute care inpatient setting, both for pain and treatment of opioid withdrawal, without need for a DEA waiver.
“It’s useful to get some skills in using buprenorphine in the inpatient setting. You don’t need an X waiver, but I encourage everyone to do the X-waiver training because it’s a terrific educational session. It’s 8 hours for physicians and well worth it,” Dr. Vettese noted.
By federal law the inpatient physician also can prescribe 3 days of buprenorphine at discharge to get the patient to an outpatient provider.
Misconceptions also abound about NSAIDs as a nonopioid component of acute pain management in hospitalized patients. They actually are extremely effective for the treatment of musculoskeletal, orthopedic, procedural, migraine, and some types of cancer pain. The number needed to treat (NNT) for postoperative pain relief for ibuprofen or celecoxib is 2.5, and when used in conjunction with acetaminophen at 325 mg every 4 hours, that NNT drops to 1.5, similar to the NNT of 1.7 for oxycodone at 15 mg. It should be noted, however, that the bar defining effective pain relief in randomized studies is set rather low: A 50% greater reduction in pain than achieved with placebo.
Many hospitalists would like to use NSAIDs more often, but they’re leery of the associated risks of GI bleeding, ischemic cardiovascular events, and worsening kidney function. Dr. Vettese offered several risk-mitigation strategies to increase the use of NSAIDs as opioid-sparing agents for acute pain management.
She has changed her own clinical practice with regard to using NSAIDs in patients with chronic kidney disease in response to a 2019 systematic review by investigators at the University of Ottawa.
“This was a game changer for me because in this review, low-dose NSAIDs were safe in that they didn’t significantly increase the risk of worsening kidney failure even in patients with stage 3 chronic kidney disease. So this has expanded my use of NSAIDs in this population through stage 3 CKD. With a creatinine clearance below 30, however, kidney failure worsened rapidly, so I don’t do it in patients with CKD stage 4,” Dr. Vettese said.
Gastroenterologists categorize patients as being at high risk of GI bleeding related to NSAID use if they have a history of a complicated ulcer or they have at least three of the following risk factors: Age above 65 years, history of an uncomplicated ulcer, being on high-dose NSAID therapy, or concurrent use of aspirin, glucocorticoids, or anticoagulants. Patients are considered at moderate risk if they have one or two of the risk factors, and low risk if they have none. Dr. Vettese said that, while NSAIDs clearly should be avoided in the high-risk group, moderate-risk patients are a different matter.
“Many avoid the use of NSAIDs with moderate risk, but I think we can expand their use if we use the right NSAID and we use protective strategies,” Dr. Vettese said.
Celecoxib is the safest drug in terms of upper GI bleeding risk, but ibuprofen is close. They are associated with a 2.2-fold increased risk of bleeding when compared with risk in patients not on an NSAID. Naproxen or indeomethacin use carries a fourfold to fivefold increased risk.
“Celecoxib with a proton pump inhibitor is safest, followed by celecoxib alone, followed by ibuprofen with a proton pump inhibitor. So I advocate using NSAIDs more frequently in people who are at moderate risk by using them with a PPI,” she said.
There is persuasive evidence of increased cardiovascular risk in association with even short-duration NSAIDs, as the drugs are utilized in the treatment of acute pain in hospitalized patients. That being said, Dr. Vettese believes hospitalists can use these drugs safely in more patients by following a thoughtful cardiovascular risk-mitigation strategy developed by Italian investigators.
Communicating about pain management
“Communication is always the key to effective pain management in every situation,” Dr. Vettese emphasized.
“I talk to the patient about the goals of effective pain management. I’ll discourage the use of the 1-10 pain scale, and instead, I’ll be honest about expectations, saying, ‘You have a problem that will cause acute pain, and it’s unlikely that I will be able to completely relieve your pain. The goal is to improve your function so that you can get up and go the bathroom by yourself, and so that you can sleep for a few hours. That’s how we’re going to measure the efficacy of our pain-management program.’ ”
She explains to the patient that she’ll be using nonopioid medications and nondrug therapies along with oral opioid pain medications, which are less risky than IV opioids. She offers reassurance that this treatment strategy won’t cause an OUD relapse. She lets the patient know up-front that the opioids will be tapered as the acute pain improves.
For the patient who comes into the hospital on buprenorphine for OUD, she immediately checks with the state prescription drug monitoring program to make sure everything is above board and there’s no indication of doctor shopping for prescriptions. For in-hospital acute pain, it’s safe and effective to continue the outpatient dose. On an outpatient basis, however, the drug is given once daily. On that dosing schedule both the euphoric effect as well as the analgesic effect are lost, so for acute pain management in the hospital it’s recommended to split the dose into twice- or thrice-daily doses to achieve an analgesic effect.
Oral NSAIDs are part of the treatment strategy whenever possible. For severe acute pain, Dr. Vettese will prescribe an immediate-release opioid having a high affinity to the mu opioid receptor, such as oral hydromorphone, on an as-needed basis. The drug has onset of effect in 30 minutes, peak effect in 1 hour, and a duration of effect of 4-6 hours, although she recommends going with 4 hours to provide adequate analgesia.
“These patients will require much higher doses than the patients who are opioid naive,” she advised.
For the patient with acute pain who is admitted while on methadone for OUD, it’s important to call the outpatient treatment program to verify the dosage.
“You can split the dose of methadone to try to get better analgesia, although I can tell you that patients who are treated with methadone for OUD frequently don’t want to do that. And if they don’t want to, then I don’t,” the hospitalist said.
As with the patient on buprenorphine for OUD, she’ll use additional oral immediate-release opioids as needed for acute severe pain in a patient on methadone for medication-assisted OUD treatment.
Dr. Vettese reported having no financial conflicts regarding her presentation.
FROM HM20 VIRTUAL
NBA star Mason Plumlee on COVID and life inside the Orlando ‘bubble’
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.
Editor’s Note: This transcript from the August 20 episode of the Blood & Cancer podcast has been edited for clarity. Click this link to listen to the full episode.
David Henry, MD: Welcome to this Blood & Cancer podcast. I’m your host, Dr. David Henry. This podcast airs on Thursday morning each week. This interview and others are archived with show notes from our residents at Pennsylvania Hospital at this link.
Each week we interview key opinion leaders involved in various aspects of blood and cancer. Mason was a first round pick in the NBA, a gold medalist for the U.S. men’s national team, and NBA All-Rookie first team honoree. He’s one of the top playmaking forwards in the country, if not the world, in my opinion. In his four-year college career at Duke University, he helped lead the Blue Devils to a National Collegiate Athletic Association (NCAA) championship and twice earned All-America first team academic honors at Duke. So he’s not just a basketball star, but an academic star as well. Mason, thanks so much for taking some time out from the bubble in Florida to talk with us today.
Mason Plumlee: Thanks for having me on. I’m happy to be here.
Henry: Beginning in March, the NBA didn’t know what to do about the COVID pandemic but finally decided to put you professional players in a ‘bubble.’ What did you have to go through to get there? You, your teammates, coaches, trainers, etc. And what’s the ongoing plan to be sure you continue to be safe?
Plumlee: Back to when the season shut down in March, the NBA shut down the practice facilities at the same time. Most people went home. I went back to Indiana. And then, as the idea of this bubble came up and the NBA formalized a plan to start the season again, players started to go back to market. I went back to Denver and was working out there.
About two weeks before we were scheduled to arrive in Orlando, they started testing us every other day. They used the deep nasal swab as well as the throat swab. But they were also taking two to three blood tests in that time period. You needed a certain number of consecutive negative tests before they would allow you to fly on the team plane down to Orlando. So there was an incredible amount of testing in the market. Once you got to Orlando, you went into a 48-hour quarantine. You had to have two negative tests with 48 hours between them before you could leave your hotel room.
Since then, it’s been quite strict down here. And although it’s annoying in a lot of ways, I think it’s one of the reasons our league has been able to pull this off. We’ve had no positive tests within the bubble and we are tested every day. A company called BioReference Laboratories has a setup in one of the meeting rooms here, and it’s like clockwork—we go in, we get our tests. One of my teammates missed a test and they made him stay in his room until he could get another test and get the results, so he missed a game because of that.
Henry: During this bubble time, no one has tested positive—players, coaches, staff?
Plumlee: Correct.
Henry: That’s incredible, and it’s allowed those of us who want to watch the NBA and those of you who are in it professionally to continue the sport. It must be a real nuisance for you and your family and friends, because no one can visit you, right?
Plumlee: Right. There’s no visitation. We had one false positive. It was our media relations person and the actions they took when that positive test came in -- they quarantined him in his room and interviewed everybody he had talked to; they tested anyone who had any interaction with him and those people had to go into quarantine. They’re on top of things down here. In addition to the testing, we each have a pulse oximeter and a thermometer, and we use these to check in everyday on an app. So, they’re getting all the insight they need. After the first round of the playoffs, they’re going to open the bubble to friends and family, but those friends and family will be subject to all the same protocols that we were coming in and once they’re here as well.
Henry: I’m sure you’ve heard about the Broadway star [Nick Cordero] who was healthy and suddenly got sick, lost a leg, and then lost his life. There have been some heart attacks that surprised us. Have your colleagues—players, coaches, etc.—been worried? Or are they thinking, what’s the big deal? Has the sense of how serious this is permeated through this sport?
Plumlee: The NBA is one of the groups that has heightened the understanding and awareness of this by shutting down. I think a lot of people were moving forward as is, and then, when the NBA decided to cancel the season, it let the world know, look, this is to be taken seriously.
Henry: A couple of players did test positive early on.
Plumlee: Exactly. A couple of people tested positive. I think at the outset, the unknown is always scarier. As we’ve learned more about the virus, the guys have become more comfortable. You know, I tested positive back in March. At the time, a loss of taste and smell was not a reported symptom.
Henry: And you had that?
Plumlee: I did have that, but I didn’t know what to think. More research has come out and we have a better understanding of that. I think most of the players are comfortable with the virus. We’re at a time in our lives where we’re healthy, we’re active, and we should be able to fight it off. We know the numbers for our age group. Even still, I think nobody wants to get it. Nobody wants to have to go through it. So why chance it?
Henry: Hats off to you and your sport. Other sports such as Major League Baseball haven’t been quite so successful. Of course, they’re wrestling with the players testing positive, and this has stopped games this season.
I was looking over your background prior to the interview and learned that your mother and father have been involved in the medical arena. Can you tell us about that and how it’s rubbed off on you?
Plumlee: Definitely. My mom is a pharmacist, so I spent a lot of time as a kid going to see her at work. And my dad is general counsel for an orthopedic company. My hometown is Warsaw, Ind. Some people refer to it as the “Orthopedic Capital of the World.” Zimmer Biomet is headquartered there. DePuy Synthes is there. Medtronic has offices there, as well as a lot of cottage businesses that support the orthopedic industry. In my hometown, the rock star was Dane Miller, who founded Biomet. I have no formal education in medicine or health care, but I’ve seen the impact of it. From my parents and some cousins, uncles who are doctors and surgeons, it’s been interesting to see their work and learn about what’s the latest and greatest in health care.
Henry: What’s so nice about you in particular is, with that background of interests from your family and your celebrity and accomplishments in professional basketball, you have used that to explore and promote ways to make progress in health care and help others who are less fortunate. For example, you’re involved in a telehealth platform for all-in-one practice management; affordable telehealth for pediatrics; health benefits for small businesses; prior authorization—if you can help with prior authorization, we will be in the stands for you at every game because it’s the bane of our existence; radiotherapy; and probably from mom’s background, pharmacy benefit management. Pick any of those you’d like to talk about, and tell us about your involvement and how it’s going.
Plumlee: My ticket into the arena is investment. Nobody’s calling me, asking for my expertise. But a lot of these visionary founders need financial support, and that’s where I get involved. Then also, with the celebrity angle from being an athlete, sometimes you can open doors for a start-up founder that they may not be able to open themselves.
I’m happy to speak about any of those companies. I am excited about the relaxed regulation that’s come from the pandemic; not that it’s like the Wild West out here, but I think it has allowed companies to implement solutions or think about problems in a way that they couldn’t before the pandemic. Take the prior authorization play, for example, and a company called Banjo Health, with one of my favorite founders, a guy named Saar Mahna. Medicare mandates that you turn around prior authorizations within three days. This company has an artificial intelligence and machine-learning play on prior authorizations that can deliver on that.
So efficiencies, things that increase access or affordability, better outcomes, those are the things that attract me. I lean on other people for the due diligence. The pediatric play that you referenced is a company called Blueberry Pediatrics. You have a monthly subscription for $15 that can be reimbursed by Medicaid. They send two devices to your home—an otoscope and an oximeter. The company is live in Florida right now, and it’s diverting a ton of emergency room (ER) visits. From home, for $15 a month, a mom has an otoscope and an oximeter, and she can chat or video conference with a pediatrician. There’s no additional fee. So that’s saving everyone time and saving the system money. Those are the kinds of things I’m attracted to.
Henry: You’ve touched on a couple of hot button issues for us. In oncology, unfortunately, most of our patients have pain. I am mystified every time I try to get a narcotic or a strong painkiller for a patient on a Friday night and I’m told it requires prior authorization and they’ll open up again on Monday. Well, that’s insane. These patients need something right away. So if you have a special interest in helping all of us with prior authorization, the artificial intelligence is a no brainer. If this kind of computer algorithm could happen overnight, that would be wonderful.
You mentioned the ER. Many people go to the ER as a default. They don’t know what else to do. In the COVID era, we’re trying to dial that down because we want to be able to see the sickest and have the non-sick get care elsewhere. If this particular person or people don’t know what to do, they go to the ER, it costs money, takes a lot of time, and others who may be sick are diverted from care. Families worry terribly about their children, so a device for mom and access to a pediatrician for $15 a month is another wonderful idea. These are both very interesting. Another company is in the pharmacy benefit management (PBM) space. Anything you could say about how that works?
Plumlee: I can give an overview of how I look at this as an investor in the PBM space. Three companies control about 75% of a multibillion dollar market. Several initiatives have been pursued politically to provide transparent pricing between these PBMs and pharmaceutical companies, and a lot of people are pointing fingers, but ultimately, drug prices just keep going up. Everybody knows it.
A couple of start-up founders are really set on bringing a competitive marketplace back to the pharmacy benefit manager. As an investor, when you see three people controlling a market, and you have small or medium PBMs that depend on aggregators to get competitive pricing with those big three, you get interested. It’s an interesting industry. My feeling is that somebody is going to disrupt it and bring competition back to that space. Ultimately, drug prices will come down because it’s not sustainable. The insurance companies just accommodate whatever the drug pricing is. If the drug prices go up, your premiums go up. I think these new companies will be level-setting.
Henry: In my world of oncology, we’re just a little more than halfway through 2020 and we’ve had five, six, seven new drugs approved. They all will be very expensive. One of the nicer things that’s happening and may help to tamp this down involves biosimilars. When you go to CVS or Rite Aid, you go down the aspirin aisle and see the generics, and they’re identical to the brand name aspirin. Well, these very complex molecules we used to treat cancer are antibodies or proteins, and they’re made in nature’s factories called cells. They’re not identical to the brand name drugs, but they’re called biosimilars. They work exactly the same as the branded drugs with exactly the same safety–our U.S. FDA has done a nice job of vetting that, to be sure. X, Y, Z Company has copied the brand drug after the patent expires. They were hoping for about a 30% discount in price but we’re seeing more like 15%. Nothing’s ever easy. So you make a very good point. This is not sustainable and the competition will be wonderful to tamp down these prices.
Plumlee: My hope is that those biosimilars and generics get placement in these formularies because the formularies are what’s valuable to the drug manufacturers. But they have to accommodate what the Big Three want in the PBM space. To me, making things affordable and accessible is what a lot of these startups are trying to do. And hopefully they will win.
Henry: What have you been going through, in terms of COVID? Have you recovered fully? Have your taste and smell returned, and you’re back to normal?
Plumlee: I’m all good. It caught me off guard but the symptoms weren’t too intense. For me, it was less than a flu, but more than a cold. And I’m all good today.
Henry: We’re so glad and wish you the best of luck.
Dr. Henry is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia and the host of the Blood & Cancer podcast. He has no relevant financial conflicts.
Mr. Plumlee is a board advisor to both Formsense and the Prysm Institute and a board observer with Voiceitt.
Performance status, molecular testing key to metastatic cancer prognosis
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.
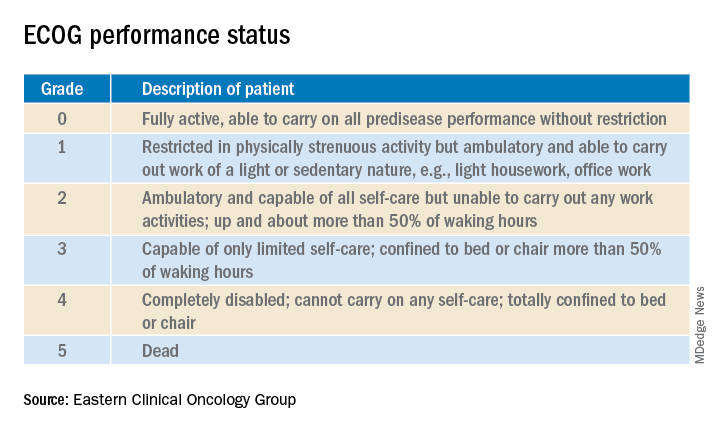
Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.

Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.

Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
FROM HM20 VIRTUAL
Oleander extract for COVID-19? That’s a hard ‘no’ experts say
“Though renowned for its beauty and use in landscaping, this Mediterranean shrub is responsible for cases of accidental poisoning across the globe. All parts of the plant are poisonous,” Cassandra Quave, PhD, ethnobotanist and herbarium curator at Emory University, Atlanta, cautioned in an article in The Conversation, an independent, not-for-profit publication.
Oleandrin has properties similar to digoxin; the onset of toxicity occurs several hours after consumption.
The first symptoms of oleandrin poisoning may be gastrointestinal, such as nausea, vomiting, abdominal pain, diarrhea (which may contain blood), and loss of appetite.
After these first symptoms, the heart may be affected by tachyarrhythmia, bradyarrhythmia, premature ventricular contractions, or atrioventricular blockage. Xanthopsia (yellow vision), a burning sensation in the eyes, paralysis of the gastrointestinal tract, and respiratory symptoms may also occur.
Oleandrin poisoning may affect the central nervous system, as evidenced by drowsiness, tremors, seizures, collapse, and coma leading to death. When applied to the skin, oleander sap can cause skin irritations and allergic reactions characterized by dermatitis.
Diagnosis of oleandrin poisoning is mainly made on the basis of a description of the plant, how much of it was ingested, how much time has elapsed since ingestion, and symptoms. Confirmation of oleandrin in blood involves fluorescence polarization immunoassay, digoxin immunoassay, or liquid chromatography-electrospray tandem mass spectrometry.
Neither oleander nor oleandrin is approved by regulatory agencies as a prescription drug or dietary supplement.
In vitro study
Oleandrin for COVID-19 made headlines after President Trump met in the Oval Office with Andrew Whitney, vice chairman and director of Phoenix Biotechnology, along with Housing and Urban Development Secretary Ben Carson, MD, and MyPillow founder/CEO Mike Lindell, a strong supporter of Trump and an investor in the biotech company, to learn about oleandrin, which Whitney called a “cure” for COVID-19, Axios reported.
In an in vitro study, researchers from Phoenix Biotechnology and the University of Texas Medical Branch, Galveston, tested oleandrin against SARS-CoV-2 in cultured Vero cells.
“When administered both before and after virus infection, nanogram doses of oleandrin significantly inhibited replication by 45 to 3000-fold,” the researchers said in an article posted on bioRxiv, a free online archive and distribution service for unpublished preprints in the life sciences. The study has not been peer reviewed.
On the basis of these in vitro findings, the researchers said the plant extract has “potential to prevent disease and virus spread in persons recently exposed to SARS-CoV-2, as well as to prevent severe disease in persons at high risk.”
But it’s a far cry from test tube to human, one expert cautioned.
“This is an understatement: Care must be taken when inferring potential therapeutic benefits from in vitro antiviral effects,” Harlan Krumholz, MD, cardiologist and director, Yale New Haven Hospital Center for Outcomes Research and Evaluation, New Haven, Connecticut, told Medscape Medical News.
“There is a chasm between a single in vitro study and any use in humans outside of a protocol. People should be cautioned about that distance and the need [to] avoid such remedies unless part of a credible research project,” said Krumholz.
Yet Lindell told Axios that, in the Oval Office meeting, Trump expressed enthusiasm for the Food and Drug Administration to allow oleandrin to be marketed as a dietary supplement or approved for COVID-19.
“This is really just nonsense and a distraction,” Jonathan Reiner, MD, of George Washington University Medical Center, Washington, DC, said on CNN.
This article first appeared on Medscape.com.
“Though renowned for its beauty and use in landscaping, this Mediterranean shrub is responsible for cases of accidental poisoning across the globe. All parts of the plant are poisonous,” Cassandra Quave, PhD, ethnobotanist and herbarium curator at Emory University, Atlanta, cautioned in an article in The Conversation, an independent, not-for-profit publication.
Oleandrin has properties similar to digoxin; the onset of toxicity occurs several hours after consumption.
The first symptoms of oleandrin poisoning may be gastrointestinal, such as nausea, vomiting, abdominal pain, diarrhea (which may contain blood), and loss of appetite.
After these first symptoms, the heart may be affected by tachyarrhythmia, bradyarrhythmia, premature ventricular contractions, or atrioventricular blockage. Xanthopsia (yellow vision), a burning sensation in the eyes, paralysis of the gastrointestinal tract, and respiratory symptoms may also occur.
Oleandrin poisoning may affect the central nervous system, as evidenced by drowsiness, tremors, seizures, collapse, and coma leading to death. When applied to the skin, oleander sap can cause skin irritations and allergic reactions characterized by dermatitis.
Diagnosis of oleandrin poisoning is mainly made on the basis of a description of the plant, how much of it was ingested, how much time has elapsed since ingestion, and symptoms. Confirmation of oleandrin in blood involves fluorescence polarization immunoassay, digoxin immunoassay, or liquid chromatography-electrospray tandem mass spectrometry.
Neither oleander nor oleandrin is approved by regulatory agencies as a prescription drug or dietary supplement.
In vitro study
Oleandrin for COVID-19 made headlines after President Trump met in the Oval Office with Andrew Whitney, vice chairman and director of Phoenix Biotechnology, along with Housing and Urban Development Secretary Ben Carson, MD, and MyPillow founder/CEO Mike Lindell, a strong supporter of Trump and an investor in the biotech company, to learn about oleandrin, which Whitney called a “cure” for COVID-19, Axios reported.
In an in vitro study, researchers from Phoenix Biotechnology and the University of Texas Medical Branch, Galveston, tested oleandrin against SARS-CoV-2 in cultured Vero cells.
“When administered both before and after virus infection, nanogram doses of oleandrin significantly inhibited replication by 45 to 3000-fold,” the researchers said in an article posted on bioRxiv, a free online archive and distribution service for unpublished preprints in the life sciences. The study has not been peer reviewed.
On the basis of these in vitro findings, the researchers said the plant extract has “potential to prevent disease and virus spread in persons recently exposed to SARS-CoV-2, as well as to prevent severe disease in persons at high risk.”
But it’s a far cry from test tube to human, one expert cautioned.
“This is an understatement: Care must be taken when inferring potential therapeutic benefits from in vitro antiviral effects,” Harlan Krumholz, MD, cardiologist and director, Yale New Haven Hospital Center for Outcomes Research and Evaluation, New Haven, Connecticut, told Medscape Medical News.
“There is a chasm between a single in vitro study and any use in humans outside of a protocol. People should be cautioned about that distance and the need [to] avoid such remedies unless part of a credible research project,” said Krumholz.
Yet Lindell told Axios that, in the Oval Office meeting, Trump expressed enthusiasm for the Food and Drug Administration to allow oleandrin to be marketed as a dietary supplement or approved for COVID-19.
“This is really just nonsense and a distraction,” Jonathan Reiner, MD, of George Washington University Medical Center, Washington, DC, said on CNN.
This article first appeared on Medscape.com.
“Though renowned for its beauty and use in landscaping, this Mediterranean shrub is responsible for cases of accidental poisoning across the globe. All parts of the plant are poisonous,” Cassandra Quave, PhD, ethnobotanist and herbarium curator at Emory University, Atlanta, cautioned in an article in The Conversation, an independent, not-for-profit publication.
Oleandrin has properties similar to digoxin; the onset of toxicity occurs several hours after consumption.
The first symptoms of oleandrin poisoning may be gastrointestinal, such as nausea, vomiting, abdominal pain, diarrhea (which may contain blood), and loss of appetite.
After these first symptoms, the heart may be affected by tachyarrhythmia, bradyarrhythmia, premature ventricular contractions, or atrioventricular blockage. Xanthopsia (yellow vision), a burning sensation in the eyes, paralysis of the gastrointestinal tract, and respiratory symptoms may also occur.
Oleandrin poisoning may affect the central nervous system, as evidenced by drowsiness, tremors, seizures, collapse, and coma leading to death. When applied to the skin, oleander sap can cause skin irritations and allergic reactions characterized by dermatitis.
Diagnosis of oleandrin poisoning is mainly made on the basis of a description of the plant, how much of it was ingested, how much time has elapsed since ingestion, and symptoms. Confirmation of oleandrin in blood involves fluorescence polarization immunoassay, digoxin immunoassay, or liquid chromatography-electrospray tandem mass spectrometry.
Neither oleander nor oleandrin is approved by regulatory agencies as a prescription drug or dietary supplement.
In vitro study
Oleandrin for COVID-19 made headlines after President Trump met in the Oval Office with Andrew Whitney, vice chairman and director of Phoenix Biotechnology, along with Housing and Urban Development Secretary Ben Carson, MD, and MyPillow founder/CEO Mike Lindell, a strong supporter of Trump and an investor in the biotech company, to learn about oleandrin, which Whitney called a “cure” for COVID-19, Axios reported.
In an in vitro study, researchers from Phoenix Biotechnology and the University of Texas Medical Branch, Galveston, tested oleandrin against SARS-CoV-2 in cultured Vero cells.
“When administered both before and after virus infection, nanogram doses of oleandrin significantly inhibited replication by 45 to 3000-fold,” the researchers said in an article posted on bioRxiv, a free online archive and distribution service for unpublished preprints in the life sciences. The study has not been peer reviewed.
On the basis of these in vitro findings, the researchers said the plant extract has “potential to prevent disease and virus spread in persons recently exposed to SARS-CoV-2, as well as to prevent severe disease in persons at high risk.”
But it’s a far cry from test tube to human, one expert cautioned.
“This is an understatement: Care must be taken when inferring potential therapeutic benefits from in vitro antiviral effects,” Harlan Krumholz, MD, cardiologist and director, Yale New Haven Hospital Center for Outcomes Research and Evaluation, New Haven, Connecticut, told Medscape Medical News.
“There is a chasm between a single in vitro study and any use in humans outside of a protocol. People should be cautioned about that distance and the need [to] avoid such remedies unless part of a credible research project,” said Krumholz.
Yet Lindell told Axios that, in the Oval Office meeting, Trump expressed enthusiasm for the Food and Drug Administration to allow oleandrin to be marketed as a dietary supplement or approved for COVID-19.
“This is really just nonsense and a distraction,” Jonathan Reiner, MD, of George Washington University Medical Center, Washington, DC, said on CNN.
This article first appeared on Medscape.com.
Pulmonary rehab reduces COPD readmissions
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
PHM20 Virtual: Can’t miss heart disease for hospitalists
PHM20 Virtual session title
Can’t Miss Heart Disease for Hospitalists
Presenter
Erich Maul, DO, MPH, FAAP, SFHM
Session summary
Dr. Erich Maul, professor of pediatrics, medical director for progressive care and acute care, and chief of hospital pediatrics at Kentucky Children’s Hospital, Lexington, presented an engaging, case-based approach to evaluate heart disease when “on call.” He iterated the importance of recognizing congenital heart disease, especially since 25% of these patients usually need surgical intervention within the first month of diagnosis and about 50% of congenital heart disease patients do not have a murmur.
Presenting cases seen during a busy hospitalist call night, Dr. Maul highlighted that patients can present with signs of heart failure, cyanosis, sepsis or hypoperfusion, failure to thrive, and respiratory distress or failure. He discussed the presentation, epidemiology, diagnosis, treatment, and prognosis. He also provided examples of common arrhythmias and provided refreshers on management using basic life support (BLS) and pediatric advanced life support.
Key takeaways
- Always start with the nine steps to resuscitation: ABC (airway, breathing, circulation), ABC, oxygen, access, monitoring.
- Early BLS is important.
- Congenital heart disease often presents with either cyanosis, hypoperfusion, failure to thrive, or respiratory distress.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
PHM20 Virtual session title
Can’t Miss Heart Disease for Hospitalists
Presenter
Erich Maul, DO, MPH, FAAP, SFHM
Session summary
Dr. Erich Maul, professor of pediatrics, medical director for progressive care and acute care, and chief of hospital pediatrics at Kentucky Children’s Hospital, Lexington, presented an engaging, case-based approach to evaluate heart disease when “on call.” He iterated the importance of recognizing congenital heart disease, especially since 25% of these patients usually need surgical intervention within the first month of diagnosis and about 50% of congenital heart disease patients do not have a murmur.
Presenting cases seen during a busy hospitalist call night, Dr. Maul highlighted that patients can present with signs of heart failure, cyanosis, sepsis or hypoperfusion, failure to thrive, and respiratory distress or failure. He discussed the presentation, epidemiology, diagnosis, treatment, and prognosis. He also provided examples of common arrhythmias and provided refreshers on management using basic life support (BLS) and pediatric advanced life support.
Key takeaways
- Always start with the nine steps to resuscitation: ABC (airway, breathing, circulation), ABC, oxygen, access, monitoring.
- Early BLS is important.
- Congenital heart disease often presents with either cyanosis, hypoperfusion, failure to thrive, or respiratory distress.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
PHM20 Virtual session title
Can’t Miss Heart Disease for Hospitalists
Presenter
Erich Maul, DO, MPH, FAAP, SFHM
Session summary
Dr. Erich Maul, professor of pediatrics, medical director for progressive care and acute care, and chief of hospital pediatrics at Kentucky Children’s Hospital, Lexington, presented an engaging, case-based approach to evaluate heart disease when “on call.” He iterated the importance of recognizing congenital heart disease, especially since 25% of these patients usually need surgical intervention within the first month of diagnosis and about 50% of congenital heart disease patients do not have a murmur.
Presenting cases seen during a busy hospitalist call night, Dr. Maul highlighted that patients can present with signs of heart failure, cyanosis, sepsis or hypoperfusion, failure to thrive, and respiratory distress or failure. He discussed the presentation, epidemiology, diagnosis, treatment, and prognosis. He also provided examples of common arrhythmias and provided refreshers on management using basic life support (BLS) and pediatric advanced life support.
Key takeaways
- Always start with the nine steps to resuscitation: ABC (airway, breathing, circulation), ABC, oxygen, access, monitoring.
- Early BLS is important.
- Congenital heart disease often presents with either cyanosis, hypoperfusion, failure to thrive, or respiratory distress.
Dr. Tantoco is an academic med-peds hospitalist practicing at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
COVID-19 child case count now over 400,000
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
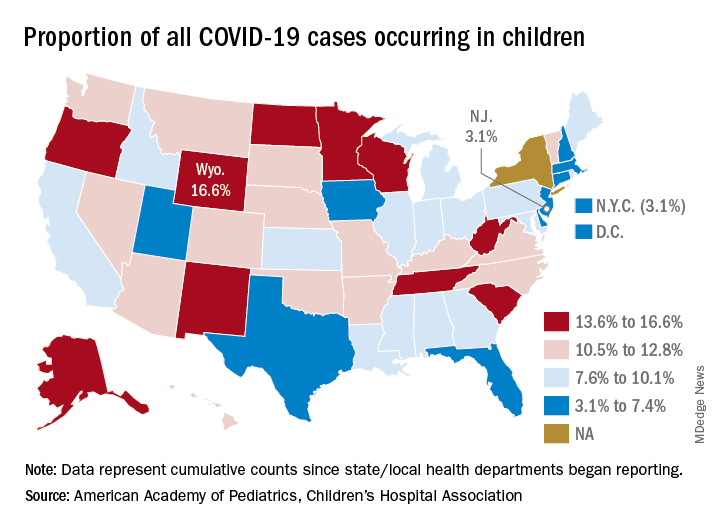
The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

The 406,000 children who have tested positive for COVID-19 represent 9.1% of all cases reported so far by 49 states (New York does not provide age distribution), New York City, the District of Columbia, Puerto Rico, and Guam. Since the proportion of child cases also was 9.1% on Aug. 6, the most recent week is the first without an increase since tracking began in mid-April, the report shows.
State-level data show that Wyoming has the highest percentage of child cases (16.6%) after Alabama changed its “definition of child case from 0-24 to 0-17 years, resulting in a downward revision of cumulative child cases,” the AAP and the CHA said. Alabama’s proportion of such cases dropped from 22.5% to 9.0%.
New Jersey had the lowest rate (3.1%) again this week, along with New York City, but both were up slightly from the week before, when New Jersey was at 2.9% and N.Y.C. was 3.0%. The only states, other than Alabama, that saw declines over the last week were Arkansas, Massachusetts, Mississippi, South Dakota, Texas, and West Virginia. Texas, however, has reported age for only 8% of its confirmed cases, the report noted.
The overall rate of child COVID-19 cases as of Aug. 13 was 538 per 100,000 children, up from 500.7 per 100,000 a week earlier. Arizona was again highest among the states with a rate of 1,254 per 100,000 (up from 1,206) and Vermont was lowest at 121, although Puerto Rico (114) and Guam (88) were lower still, the AAP/CHA data indicate.
For the nine states that report testing information for children, Arizona has the highest positivity rate at 18.3% and West Virginia has the lowest at 3.6%. Data on hospitalizations – available from 21 states and N.Y.C. – show that 3,849 children have been admitted, with rates varying from 0.2% of children in Hawaii to 8.8% in the Big Apple, according to the report.
More specific information on child cases, such as symptoms or underlying conditions, is not being provided by states at this time, the AAP and CHA pointed out.
HFNC more comfortable for posthypercapnic patients with COPD
Following invasive ventilation for severe hypercapnic respiratory failure, patients with chronic obstructive pulmonary disease had similar levels of treatment failure if they received high-flow nasal cannula oxygen therapy or noninvasive ventilation, recent research in Critical Care has suggested.
However, for patients with COPD weaned off invasive ventilation, high-flow nasal cannula (HFNC) oxygen therapy was “more comfortable and better tolerated,” compared with noninvasive ventilation (NIV). In addition, “airway care interventions and the incidence of nasofacial skin breakdown associated with HFNC were significantly lower than in NIV,” according to Dingyu Tan of the Clinical Medical College of Yangzhou (China) University, Northern Jiangsu People’s Hospital, and colleagues. “HFNC appears to be an effective means of respiratory support for COPD patients extubated after severe hypercapnic respiratory failure,” they said.
The investigators screened patients with COPD and hypercapnic respiratory failure for enrollment, including those who met Global Initiative for Obstructive Lung Disease (GOLD) criteria, were 85 years old or younger and caring for themselves, had bronchopulmonary infection–induced respiratory failure, and had achieved pulmonary infection control criteria. Exclusion criteria were:
- Patients under age 18 years.
- Presence of oral or facial trauma.
- Poor sputum excretion ability.
- Hemodynamic instability that would contraindicate use of NIV.
- Poor cough during PIC window.
- Poor short-term prognosis.
- Failure of the heart, brain, liver or kidney.
- Patients who could not consent to treatment.
Patients were determined to have failed treatment if they returned to invasive mechanical ventilation or switched from one treatment to another (HFNC to NIV or NIV to HFNC). Investigators also performed an arterial blood gas analysis, recorded the number of duration of airway care interventions, and monitored vital signs at 1 hour, 24 hours, and 48 hours after extubation as secondary analyses.
Overall, 44 patients randomized to receive HFNC and 42 patients randomized for NIV were available for analysis. The investigators found 22.7% of patients in the HFNC group and 28.6% in the NIV group experienced treatment failure (risk difference, –5.8%; 95% confidence interval, −23.8 to 12.4%; P = .535), with patients in the HFNC group experiencing a significantly lower level of treatment intolerance, compared with patients in the NIV group (risk difference, –50.0%; 95% CI, −74.6 to −12.9%; P = .015). There were no significant differences between either group regarding intubation (−0.65%; 95% CI, −16.01 to 14.46%), while rate of switching treatments was lower in the HFNC group but not significant (−5.2%; 95% CI, −19.82 to 9.05%).
Patients in both the HFNC and NIV groups had faster mean respiratory rates 1 hour after extubation (P < .050). After 24 hours, the NIV group had higher-than-baseline respiratory rates, compared with the HFNC group, which had returned to normal (20 vs. 24.5 breaths per minute; P < .050). Both groups had returned to baseline by 48 hours after extubation. At 1 hour after extubation, patients in the HFNC group had lower PaO2/FiO2 (P < .050) and pH values (P < .050), and higher PaCO2 values (P less than .050), compared with baseline. There were no statistically significant differences in PaO2/FiO2, pH, and PaCO2 values in either group at 24 hours or 48 hours after extubation.
Daily airway care interventions were significantly higher on average in the NIV group, compared with the HFNC group (7 vs. 6; P = .0006), and the HFNC group also had significantly better comfort scores (7 vs. 5; P < .001) as measured by a modified visual analog scale, as well as incidence of nasal and facial skin breakdown (0 vs. 9.6%; P = .027), compared with the NIV group.
Results difficult to apply to North American patients
David L. Bowton, MD, FCCP, a professor specializing in critical care at Wake Forest University, Winston-Salem, N.C., said in an interview the results of this trial may not be applicable for patients with infection-related respiratory failure and COPD in North America “due to the differences in common weaning practices between North America and China.”
For example, the trial used the pulmonary infection control (PIC) window criteria for extubation, which requires a significant decrease in radiographic infiltrates, improvement in quality and quantity of sputum, normalizing of leukocyte count, a synchronized intermittent mandatory ventilation (SIMV) rate of 10-12 breaths per minute, and pressure support less than 10-12 cm/H2O (Int J Chron Obstruct Pulmon Dis. 2017;12:1255-67).
“The process used to achieve these measures is not standardized. In North America, daily awakening and screening for spontaneous breathing trials would be usual, but this was not reported in the current trial,” he explained.
Differences in patient population also make the application of the results difficult, Dr. Bowton said. “Only 60% of the patients had spirometrically confirmed COPD and fewer than half were on at least dual inhaled therapy prior to hospitalization with only one-third taking beta agonists or anticholinergic agents,” he noted. “The cause of respiratory failure was infectious, requiring an infiltrate on chest radiograph; thus, patients with hypercarbic respiratory failure without a new infiltrate were excluded from the study. On average, patients were hypercarbic, yet alkalemic at the time of extubation; the PaCO2 and pH at the time of intubation were not reported.
“This study suggests that in some patients with COPD and respiratory failure requiring invasive mechanical ventilation, HFO [high-flow oxygen] may be better tolerated and equally effective as NIPPV [noninvasive positive-pressure ventilation] at mitigating the need for reintubation following extubation. In this patient population where hypoxemia prior to extubation was not severe, the mechanisms by which HFO is beneficial remain speculative,” he said.
This study was funded by the Rui E special fund for emergency medicine research and the Yangzhou Science and Technology Development Plan. The authors report no relevant conflicts of interest. Dr. Bowton reports no relevant conflicts of interest.
SOURCE: Tan D et al. Crit Care. 2020 Aug 6. doi: 10.1186/s13054-020-03214-9.
Following invasive ventilation for severe hypercapnic respiratory failure, patients with chronic obstructive pulmonary disease had similar levels of treatment failure if they received high-flow nasal cannula oxygen therapy or noninvasive ventilation, recent research in Critical Care has suggested.
However, for patients with COPD weaned off invasive ventilation, high-flow nasal cannula (HFNC) oxygen therapy was “more comfortable and better tolerated,” compared with noninvasive ventilation (NIV). In addition, “airway care interventions and the incidence of nasofacial skin breakdown associated with HFNC were significantly lower than in NIV,” according to Dingyu Tan of the Clinical Medical College of Yangzhou (China) University, Northern Jiangsu People’s Hospital, and colleagues. “HFNC appears to be an effective means of respiratory support for COPD patients extubated after severe hypercapnic respiratory failure,” they said.
The investigators screened patients with COPD and hypercapnic respiratory failure for enrollment, including those who met Global Initiative for Obstructive Lung Disease (GOLD) criteria, were 85 years old or younger and caring for themselves, had bronchopulmonary infection–induced respiratory failure, and had achieved pulmonary infection control criteria. Exclusion criteria were:
- Patients under age 18 years.
- Presence of oral or facial trauma.
- Poor sputum excretion ability.
- Hemodynamic instability that would contraindicate use of NIV.
- Poor cough during PIC window.
- Poor short-term prognosis.
- Failure of the heart, brain, liver or kidney.
- Patients who could not consent to treatment.
Patients were determined to have failed treatment if they returned to invasive mechanical ventilation or switched from one treatment to another (HFNC to NIV or NIV to HFNC). Investigators also performed an arterial blood gas analysis, recorded the number of duration of airway care interventions, and monitored vital signs at 1 hour, 24 hours, and 48 hours after extubation as secondary analyses.
Overall, 44 patients randomized to receive HFNC and 42 patients randomized for NIV were available for analysis. The investigators found 22.7% of patients in the HFNC group and 28.6% in the NIV group experienced treatment failure (risk difference, –5.8%; 95% confidence interval, −23.8 to 12.4%; P = .535), with patients in the HFNC group experiencing a significantly lower level of treatment intolerance, compared with patients in the NIV group (risk difference, –50.0%; 95% CI, −74.6 to −12.9%; P = .015). There were no significant differences between either group regarding intubation (−0.65%; 95% CI, −16.01 to 14.46%), while rate of switching treatments was lower in the HFNC group but not significant (−5.2%; 95% CI, −19.82 to 9.05%).
Patients in both the HFNC and NIV groups had faster mean respiratory rates 1 hour after extubation (P < .050). After 24 hours, the NIV group had higher-than-baseline respiratory rates, compared with the HFNC group, which had returned to normal (20 vs. 24.5 breaths per minute; P < .050). Both groups had returned to baseline by 48 hours after extubation. At 1 hour after extubation, patients in the HFNC group had lower PaO2/FiO2 (P < .050) and pH values (P < .050), and higher PaCO2 values (P less than .050), compared with baseline. There were no statistically significant differences in PaO2/FiO2, pH, and PaCO2 values in either group at 24 hours or 48 hours after extubation.
Daily airway care interventions were significantly higher on average in the NIV group, compared with the HFNC group (7 vs. 6; P = .0006), and the HFNC group also had significantly better comfort scores (7 vs. 5; P < .001) as measured by a modified visual analog scale, as well as incidence of nasal and facial skin breakdown (0 vs. 9.6%; P = .027), compared with the NIV group.
Results difficult to apply to North American patients
David L. Bowton, MD, FCCP, a professor specializing in critical care at Wake Forest University, Winston-Salem, N.C., said in an interview the results of this trial may not be applicable for patients with infection-related respiratory failure and COPD in North America “due to the differences in common weaning practices between North America and China.”
For example, the trial used the pulmonary infection control (PIC) window criteria for extubation, which requires a significant decrease in radiographic infiltrates, improvement in quality and quantity of sputum, normalizing of leukocyte count, a synchronized intermittent mandatory ventilation (SIMV) rate of 10-12 breaths per minute, and pressure support less than 10-12 cm/H2O (Int J Chron Obstruct Pulmon Dis. 2017;12:1255-67).
“The process used to achieve these measures is not standardized. In North America, daily awakening and screening for spontaneous breathing trials would be usual, but this was not reported in the current trial,” he explained.
Differences in patient population also make the application of the results difficult, Dr. Bowton said. “Only 60% of the patients had spirometrically confirmed COPD and fewer than half were on at least dual inhaled therapy prior to hospitalization with only one-third taking beta agonists or anticholinergic agents,” he noted. “The cause of respiratory failure was infectious, requiring an infiltrate on chest radiograph; thus, patients with hypercarbic respiratory failure without a new infiltrate were excluded from the study. On average, patients were hypercarbic, yet alkalemic at the time of extubation; the PaCO2 and pH at the time of intubation were not reported.
“This study suggests that in some patients with COPD and respiratory failure requiring invasive mechanical ventilation, HFO [high-flow oxygen] may be better tolerated and equally effective as NIPPV [noninvasive positive-pressure ventilation] at mitigating the need for reintubation following extubation. In this patient population where hypoxemia prior to extubation was not severe, the mechanisms by which HFO is beneficial remain speculative,” he said.
This study was funded by the Rui E special fund for emergency medicine research and the Yangzhou Science and Technology Development Plan. The authors report no relevant conflicts of interest. Dr. Bowton reports no relevant conflicts of interest.
SOURCE: Tan D et al. Crit Care. 2020 Aug 6. doi: 10.1186/s13054-020-03214-9.
Following invasive ventilation for severe hypercapnic respiratory failure, patients with chronic obstructive pulmonary disease had similar levels of treatment failure if they received high-flow nasal cannula oxygen therapy or noninvasive ventilation, recent research in Critical Care has suggested.
However, for patients with COPD weaned off invasive ventilation, high-flow nasal cannula (HFNC) oxygen therapy was “more comfortable and better tolerated,” compared with noninvasive ventilation (NIV). In addition, “airway care interventions and the incidence of nasofacial skin breakdown associated with HFNC were significantly lower than in NIV,” according to Dingyu Tan of the Clinical Medical College of Yangzhou (China) University, Northern Jiangsu People’s Hospital, and colleagues. “HFNC appears to be an effective means of respiratory support for COPD patients extubated after severe hypercapnic respiratory failure,” they said.
The investigators screened patients with COPD and hypercapnic respiratory failure for enrollment, including those who met Global Initiative for Obstructive Lung Disease (GOLD) criteria, were 85 years old or younger and caring for themselves, had bronchopulmonary infection–induced respiratory failure, and had achieved pulmonary infection control criteria. Exclusion criteria were:
- Patients under age 18 years.
- Presence of oral or facial trauma.
- Poor sputum excretion ability.
- Hemodynamic instability that would contraindicate use of NIV.
- Poor cough during PIC window.
- Poor short-term prognosis.
- Failure of the heart, brain, liver or kidney.
- Patients who could not consent to treatment.
Patients were determined to have failed treatment if they returned to invasive mechanical ventilation or switched from one treatment to another (HFNC to NIV or NIV to HFNC). Investigators also performed an arterial blood gas analysis, recorded the number of duration of airway care interventions, and monitored vital signs at 1 hour, 24 hours, and 48 hours after extubation as secondary analyses.
Overall, 44 patients randomized to receive HFNC and 42 patients randomized for NIV were available for analysis. The investigators found 22.7% of patients in the HFNC group and 28.6% in the NIV group experienced treatment failure (risk difference, –5.8%; 95% confidence interval, −23.8 to 12.4%; P = .535), with patients in the HFNC group experiencing a significantly lower level of treatment intolerance, compared with patients in the NIV group (risk difference, –50.0%; 95% CI, −74.6 to −12.9%; P = .015). There were no significant differences between either group regarding intubation (−0.65%; 95% CI, −16.01 to 14.46%), while rate of switching treatments was lower in the HFNC group but not significant (−5.2%; 95% CI, −19.82 to 9.05%).
Patients in both the HFNC and NIV groups had faster mean respiratory rates 1 hour after extubation (P < .050). After 24 hours, the NIV group had higher-than-baseline respiratory rates, compared with the HFNC group, which had returned to normal (20 vs. 24.5 breaths per minute; P < .050). Both groups had returned to baseline by 48 hours after extubation. At 1 hour after extubation, patients in the HFNC group had lower PaO2/FiO2 (P < .050) and pH values (P < .050), and higher PaCO2 values (P less than .050), compared with baseline. There were no statistically significant differences in PaO2/FiO2, pH, and PaCO2 values in either group at 24 hours or 48 hours after extubation.
Daily airway care interventions were significantly higher on average in the NIV group, compared with the HFNC group (7 vs. 6; P = .0006), and the HFNC group also had significantly better comfort scores (7 vs. 5; P < .001) as measured by a modified visual analog scale, as well as incidence of nasal and facial skin breakdown (0 vs. 9.6%; P = .027), compared with the NIV group.
Results difficult to apply to North American patients
David L. Bowton, MD, FCCP, a professor specializing in critical care at Wake Forest University, Winston-Salem, N.C., said in an interview the results of this trial may not be applicable for patients with infection-related respiratory failure and COPD in North America “due to the differences in common weaning practices between North America and China.”
For example, the trial used the pulmonary infection control (PIC) window criteria for extubation, which requires a significant decrease in radiographic infiltrates, improvement in quality and quantity of sputum, normalizing of leukocyte count, a synchronized intermittent mandatory ventilation (SIMV) rate of 10-12 breaths per minute, and pressure support less than 10-12 cm/H2O (Int J Chron Obstruct Pulmon Dis. 2017;12:1255-67).
“The process used to achieve these measures is not standardized. In North America, daily awakening and screening for spontaneous breathing trials would be usual, but this was not reported in the current trial,” he explained.
Differences in patient population also make the application of the results difficult, Dr. Bowton said. “Only 60% of the patients had spirometrically confirmed COPD and fewer than half were on at least dual inhaled therapy prior to hospitalization with only one-third taking beta agonists or anticholinergic agents,” he noted. “The cause of respiratory failure was infectious, requiring an infiltrate on chest radiograph; thus, patients with hypercarbic respiratory failure without a new infiltrate were excluded from the study. On average, patients were hypercarbic, yet alkalemic at the time of extubation; the PaCO2 and pH at the time of intubation were not reported.
“This study suggests that in some patients with COPD and respiratory failure requiring invasive mechanical ventilation, HFO [high-flow oxygen] may be better tolerated and equally effective as NIPPV [noninvasive positive-pressure ventilation] at mitigating the need for reintubation following extubation. In this patient population where hypoxemia prior to extubation was not severe, the mechanisms by which HFO is beneficial remain speculative,” he said.
This study was funded by the Rui E special fund for emergency medicine research and the Yangzhou Science and Technology Development Plan. The authors report no relevant conflicts of interest. Dr. Bowton reports no relevant conflicts of interest.
SOURCE: Tan D et al. Crit Care. 2020 Aug 6. doi: 10.1186/s13054-020-03214-9.
FROM CRITICAL CARE







