User login
Evidence mounts for COVID-19 effects on thyroid gland
Rates of thyrotoxicosis are significantly higher among patients who are critically ill with COVID-19 than among patients who are critically ill but who do not not have COVID-19, suggesting an atypical form of thyroiditis related to the novel coronavirus infection, according to new research.
“We suggest routine assessment of thyroid function in patients with COVID-19 requiring high-intensity care because they frequently present with thyrotoxicosis due to a form of subacute thyroiditis related to SARS-CoV-2,” the authors wrote in correspondence published online in The Lancet Diabetes and Endocrinology.
However, notably, the study – which compared critically ill ICU patients who had COVID-19 with those who did not have COVID-19 or who had milder cases of COVID-19 – indicates that thyroid disorders do not appear to increase the risk of developing COVID-19, first author Ilaria Muller, MD, PhD, of the department of endocrinology, IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico, Milan, said in an interview.
“It is important to highlight that we did not find an increased prevalence of preexisting thyroid disorders in COVID-19 patients (contrary to early media reports),” she said. “So far, clinical observations do not support this fear, and we need to reassure people with thyroid disorders, since such disorders are very common among the general population.”
Yet the findings add to emerging evidence of a COVID-19/thyroid relationship, Angela M. Leung, MD, said in an interview.
“Given the health care impacts of the current COVID-19 pandemic worldwide, this study provides some insight on the potential systemic inflammation, as well as thyroid-specific inflammation, of the SARS-Cov-2 virus that is described in some emerging reports,” she said.
“This study joins at least six others that have reported a clinical presentation resembling subacute thyroiditis in critically ill patients with COVID-19,” noted Dr. Leung, of the division of endocrinology, diabetes, and metabolism in the department of medicine at the University of California, Los Angeles.
Thyroid function analysis in those with severe COVID-19
Dr. Muller explained that preliminary data from her institution showed thyroid abnormalities in patients who were severely ill with COVID-19. She and her team extended the evaluation to include thyroid data and other data on 93 patients with COVID-19 who were admitted to high-intensity care units (HICUs) in Italy during the 2020 pandemic.
Those data were compared with data on 101 critically ill patients admitted to the same HICUs in 2019 who did not have COVID-19. A third group of 52 patients with COVID-19 who were admitted to low-intensity care units (LICUs) in Italy in 2020 were also included in the analysis.
The mean age of the patients in the HICU 2020 group was 65.3 years; in the HICU 2019 group, it was 73 years; and in the LICU group, it was 70 years (P = .001). In addition, the HICU 2020 group included more men than the other two groups (69% vs. 56% and 48%; P = .03).
Of note, only 9% of patients in the HICU 2020 group had preexisting thyroid disorders, compared with 21% in the LICU group and 23% in the HICU 2019 group (P = .017).
These findings suggest that “such conditions are not a risk factor for SARS-CoV-2 infection or severity of COVID-19,” the authors wrote.
The patients with the preexisting thyroid conditions were excluded from the thyroid function analysis.
A significantly higher proportion of patients in the HICU 2020 group (13; 15%) were thyrotoxic upon admission, compared with just 1 (1%) of 78 patients in the HICU 2019 group (P = .002) and one (2%) of 41 patients in the LICU group (P = .025).
Among the 14 patients in the two COVID-19 groups who had thyrotoxicosis, the majority were male (9; 64%)
Among those in the HICU 2020 group, serum thyroid-stimulating hormone concentrations were lower than in either of the other two groups (P = .018), and serum free thyroxine (free T4) concentrations were higher than in the LICU group (P = .016) but not the HICU 2019 group.
Differences compared with other infection-related thyroiditis
Although thyrotoxicosis relating to subacute viral thyroiditis can result from a wide variety of viral infections, there are some key differences with COVID-19, Dr. Muller said.
“Thyroid dysfunction related to SARS-CoV-2 seems to be milder than that of classic subacute thyroiditis due to other viruses,” she explained. Furthermore, thyroid dysfunction associated with other viral infections is more common in women, whereas there were more male patients with the COVID-19–related atypical thyroiditis.
In addition, the thyroid effects developed early with COVID-19, whereas they usually emerge after the infections by other viruses.
Patients did not demonstrate the neck pain that is common with classic viral thyroiditis, and the thyroid abnormalities appear to correlate with the severity of COVID-19, whereas they are seen even in patients with mild symptoms when other viral infections are the cause.
In addition to the risk for subacute viral thyroiditis, critically ill patients in general are at risk of developing nonthyroidal illness syndrome, with alterations in thyroid function. However, thyroid hormone measures in the patients severely ill with COVID-19 were not consistent with that syndrome.
A subanalysis of eight HICU 2020 patients with thyroid dysfunction who were followed for 55 days after discharge showed that two experienced hyperthyroidism but likely not from COVID-19; in the remaining six, thyroid function normalized.
Muller speculated that, when ill with COVID-19, the patients likely had a combination of SARS-CoV-2–related atypical thyroiditis and nonthyroidal illness syndrome, known as T4 toxicosis.
Will there be any long-term effects?
Importantly, it remains unknown whether the novel coronavirus has longer-term effects on the thyroid, Dr. Muller said.
“We cannot predict what will be the long-lasting thyroid effects after COVID-19,” she said.
With classic subacute viral thyroiditis, “After a few years ... 5%-20% of patients develop permanent hypothyroidism, [and] the same might happen in COVID-19 patients,” she hypothesized. “We will follow our patients long term to answer this question – this study is already ongoing.”
In the meantime, diagnosis of thyroid dysfunction in patients with COVID-19 is important, inasmuch as it could worsen the already critical conditions of patients, Muller stressed.
“The gold-standard treatment for thyroiditis is steroids, so the presence of thyroid dysfunction might represent an additional indication to such treatment in COVID-19 patients, to be verified in properly designed clinical trials,” she advised.
ACE2 cell receptors highly expressed in thyroid
Dr. Muller and colleagues also noted recent research showing that ACE2 – demonstrated to be a key host-cell entry receptor for both SARS-CoV and SARS-CoV-2 – is expressed in even higher levels in the thyroid than the lungs, where it causes COVID-19’s notorious pulmonary effects.
Dr. Muller said the implications of ACE2 expression in the thyroid remain to be elucidated.
“If ACE2 is confirmed to be expressed at higher levels, compared with the lungs in the thyroid gland and other tissues, i.e., small intestine, testis, kidney, heart, etc, dedicated studies will be needed to correlate ACE2 expression with the organs’ susceptibility to SARS-CoV-2 reflected by clinical presentation,” she said.
Dr. Leung added that, as a take-home message from these and the other thyroid/COVID-19 studies, “data are starting to show us that COVID-19 infection may cause thyrotoxicosis that is possibly related to thyroid and systemic inflammation. However, the serum thyroid function test abnormalities seen in COVID-19 patients with subacute thyroiditis are also likely exacerbated to a substantial extent by nonthyroidal illness physiology.”
The authors have disclosed no relevant financial relationships. Dr. Leung is on the advisory board of Medscape Diabetes and Endocrinology.
A version of this article originally appeared on Medscape.com.
Rates of thyrotoxicosis are significantly higher among patients who are critically ill with COVID-19 than among patients who are critically ill but who do not not have COVID-19, suggesting an atypical form of thyroiditis related to the novel coronavirus infection, according to new research.
“We suggest routine assessment of thyroid function in patients with COVID-19 requiring high-intensity care because they frequently present with thyrotoxicosis due to a form of subacute thyroiditis related to SARS-CoV-2,” the authors wrote in correspondence published online in The Lancet Diabetes and Endocrinology.
However, notably, the study – which compared critically ill ICU patients who had COVID-19 with those who did not have COVID-19 or who had milder cases of COVID-19 – indicates that thyroid disorders do not appear to increase the risk of developing COVID-19, first author Ilaria Muller, MD, PhD, of the department of endocrinology, IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico, Milan, said in an interview.
“It is important to highlight that we did not find an increased prevalence of preexisting thyroid disorders in COVID-19 patients (contrary to early media reports),” she said. “So far, clinical observations do not support this fear, and we need to reassure people with thyroid disorders, since such disorders are very common among the general population.”
Yet the findings add to emerging evidence of a COVID-19/thyroid relationship, Angela M. Leung, MD, said in an interview.
“Given the health care impacts of the current COVID-19 pandemic worldwide, this study provides some insight on the potential systemic inflammation, as well as thyroid-specific inflammation, of the SARS-Cov-2 virus that is described in some emerging reports,” she said.
“This study joins at least six others that have reported a clinical presentation resembling subacute thyroiditis in critically ill patients with COVID-19,” noted Dr. Leung, of the division of endocrinology, diabetes, and metabolism in the department of medicine at the University of California, Los Angeles.
Thyroid function analysis in those with severe COVID-19
Dr. Muller explained that preliminary data from her institution showed thyroid abnormalities in patients who were severely ill with COVID-19. She and her team extended the evaluation to include thyroid data and other data on 93 patients with COVID-19 who were admitted to high-intensity care units (HICUs) in Italy during the 2020 pandemic.
Those data were compared with data on 101 critically ill patients admitted to the same HICUs in 2019 who did not have COVID-19. A third group of 52 patients with COVID-19 who were admitted to low-intensity care units (LICUs) in Italy in 2020 were also included in the analysis.
The mean age of the patients in the HICU 2020 group was 65.3 years; in the HICU 2019 group, it was 73 years; and in the LICU group, it was 70 years (P = .001). In addition, the HICU 2020 group included more men than the other two groups (69% vs. 56% and 48%; P = .03).
Of note, only 9% of patients in the HICU 2020 group had preexisting thyroid disorders, compared with 21% in the LICU group and 23% in the HICU 2019 group (P = .017).
These findings suggest that “such conditions are not a risk factor for SARS-CoV-2 infection or severity of COVID-19,” the authors wrote.
The patients with the preexisting thyroid conditions were excluded from the thyroid function analysis.
A significantly higher proportion of patients in the HICU 2020 group (13; 15%) were thyrotoxic upon admission, compared with just 1 (1%) of 78 patients in the HICU 2019 group (P = .002) and one (2%) of 41 patients in the LICU group (P = .025).
Among the 14 patients in the two COVID-19 groups who had thyrotoxicosis, the majority were male (9; 64%)
Among those in the HICU 2020 group, serum thyroid-stimulating hormone concentrations were lower than in either of the other two groups (P = .018), and serum free thyroxine (free T4) concentrations were higher than in the LICU group (P = .016) but not the HICU 2019 group.
Differences compared with other infection-related thyroiditis
Although thyrotoxicosis relating to subacute viral thyroiditis can result from a wide variety of viral infections, there are some key differences with COVID-19, Dr. Muller said.
“Thyroid dysfunction related to SARS-CoV-2 seems to be milder than that of classic subacute thyroiditis due to other viruses,” she explained. Furthermore, thyroid dysfunction associated with other viral infections is more common in women, whereas there were more male patients with the COVID-19–related atypical thyroiditis.
In addition, the thyroid effects developed early with COVID-19, whereas they usually emerge after the infections by other viruses.
Patients did not demonstrate the neck pain that is common with classic viral thyroiditis, and the thyroid abnormalities appear to correlate with the severity of COVID-19, whereas they are seen even in patients with mild symptoms when other viral infections are the cause.
In addition to the risk for subacute viral thyroiditis, critically ill patients in general are at risk of developing nonthyroidal illness syndrome, with alterations in thyroid function. However, thyroid hormone measures in the patients severely ill with COVID-19 were not consistent with that syndrome.
A subanalysis of eight HICU 2020 patients with thyroid dysfunction who were followed for 55 days after discharge showed that two experienced hyperthyroidism but likely not from COVID-19; in the remaining six, thyroid function normalized.
Muller speculated that, when ill with COVID-19, the patients likely had a combination of SARS-CoV-2–related atypical thyroiditis and nonthyroidal illness syndrome, known as T4 toxicosis.
Will there be any long-term effects?
Importantly, it remains unknown whether the novel coronavirus has longer-term effects on the thyroid, Dr. Muller said.
“We cannot predict what will be the long-lasting thyroid effects after COVID-19,” she said.
With classic subacute viral thyroiditis, “After a few years ... 5%-20% of patients develop permanent hypothyroidism, [and] the same might happen in COVID-19 patients,” she hypothesized. “We will follow our patients long term to answer this question – this study is already ongoing.”
In the meantime, diagnosis of thyroid dysfunction in patients with COVID-19 is important, inasmuch as it could worsen the already critical conditions of patients, Muller stressed.
“The gold-standard treatment for thyroiditis is steroids, so the presence of thyroid dysfunction might represent an additional indication to such treatment in COVID-19 patients, to be verified in properly designed clinical trials,” she advised.
ACE2 cell receptors highly expressed in thyroid
Dr. Muller and colleagues also noted recent research showing that ACE2 – demonstrated to be a key host-cell entry receptor for both SARS-CoV and SARS-CoV-2 – is expressed in even higher levels in the thyroid than the lungs, where it causes COVID-19’s notorious pulmonary effects.
Dr. Muller said the implications of ACE2 expression in the thyroid remain to be elucidated.
“If ACE2 is confirmed to be expressed at higher levels, compared with the lungs in the thyroid gland and other tissues, i.e., small intestine, testis, kidney, heart, etc, dedicated studies will be needed to correlate ACE2 expression with the organs’ susceptibility to SARS-CoV-2 reflected by clinical presentation,” she said.
Dr. Leung added that, as a take-home message from these and the other thyroid/COVID-19 studies, “data are starting to show us that COVID-19 infection may cause thyrotoxicosis that is possibly related to thyroid and systemic inflammation. However, the serum thyroid function test abnormalities seen in COVID-19 patients with subacute thyroiditis are also likely exacerbated to a substantial extent by nonthyroidal illness physiology.”
The authors have disclosed no relevant financial relationships. Dr. Leung is on the advisory board of Medscape Diabetes and Endocrinology.
A version of this article originally appeared on Medscape.com.
Rates of thyrotoxicosis are significantly higher among patients who are critically ill with COVID-19 than among patients who are critically ill but who do not not have COVID-19, suggesting an atypical form of thyroiditis related to the novel coronavirus infection, according to new research.
“We suggest routine assessment of thyroid function in patients with COVID-19 requiring high-intensity care because they frequently present with thyrotoxicosis due to a form of subacute thyroiditis related to SARS-CoV-2,” the authors wrote in correspondence published online in The Lancet Diabetes and Endocrinology.
However, notably, the study – which compared critically ill ICU patients who had COVID-19 with those who did not have COVID-19 or who had milder cases of COVID-19 – indicates that thyroid disorders do not appear to increase the risk of developing COVID-19, first author Ilaria Muller, MD, PhD, of the department of endocrinology, IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico, Milan, said in an interview.
“It is important to highlight that we did not find an increased prevalence of preexisting thyroid disorders in COVID-19 patients (contrary to early media reports),” she said. “So far, clinical observations do not support this fear, and we need to reassure people with thyroid disorders, since such disorders are very common among the general population.”
Yet the findings add to emerging evidence of a COVID-19/thyroid relationship, Angela M. Leung, MD, said in an interview.
“Given the health care impacts of the current COVID-19 pandemic worldwide, this study provides some insight on the potential systemic inflammation, as well as thyroid-specific inflammation, of the SARS-Cov-2 virus that is described in some emerging reports,” she said.
“This study joins at least six others that have reported a clinical presentation resembling subacute thyroiditis in critically ill patients with COVID-19,” noted Dr. Leung, of the division of endocrinology, diabetes, and metabolism in the department of medicine at the University of California, Los Angeles.
Thyroid function analysis in those with severe COVID-19
Dr. Muller explained that preliminary data from her institution showed thyroid abnormalities in patients who were severely ill with COVID-19. She and her team extended the evaluation to include thyroid data and other data on 93 patients with COVID-19 who were admitted to high-intensity care units (HICUs) in Italy during the 2020 pandemic.
Those data were compared with data on 101 critically ill patients admitted to the same HICUs in 2019 who did not have COVID-19. A third group of 52 patients with COVID-19 who were admitted to low-intensity care units (LICUs) in Italy in 2020 were also included in the analysis.
The mean age of the patients in the HICU 2020 group was 65.3 years; in the HICU 2019 group, it was 73 years; and in the LICU group, it was 70 years (P = .001). In addition, the HICU 2020 group included more men than the other two groups (69% vs. 56% and 48%; P = .03).
Of note, only 9% of patients in the HICU 2020 group had preexisting thyroid disorders, compared with 21% in the LICU group and 23% in the HICU 2019 group (P = .017).
These findings suggest that “such conditions are not a risk factor for SARS-CoV-2 infection or severity of COVID-19,” the authors wrote.
The patients with the preexisting thyroid conditions were excluded from the thyroid function analysis.
A significantly higher proportion of patients in the HICU 2020 group (13; 15%) were thyrotoxic upon admission, compared with just 1 (1%) of 78 patients in the HICU 2019 group (P = .002) and one (2%) of 41 patients in the LICU group (P = .025).
Among the 14 patients in the two COVID-19 groups who had thyrotoxicosis, the majority were male (9; 64%)
Among those in the HICU 2020 group, serum thyroid-stimulating hormone concentrations were lower than in either of the other two groups (P = .018), and serum free thyroxine (free T4) concentrations were higher than in the LICU group (P = .016) but not the HICU 2019 group.
Differences compared with other infection-related thyroiditis
Although thyrotoxicosis relating to subacute viral thyroiditis can result from a wide variety of viral infections, there are some key differences with COVID-19, Dr. Muller said.
“Thyroid dysfunction related to SARS-CoV-2 seems to be milder than that of classic subacute thyroiditis due to other viruses,” she explained. Furthermore, thyroid dysfunction associated with other viral infections is more common in women, whereas there were more male patients with the COVID-19–related atypical thyroiditis.
In addition, the thyroid effects developed early with COVID-19, whereas they usually emerge after the infections by other viruses.
Patients did not demonstrate the neck pain that is common with classic viral thyroiditis, and the thyroid abnormalities appear to correlate with the severity of COVID-19, whereas they are seen even in patients with mild symptoms when other viral infections are the cause.
In addition to the risk for subacute viral thyroiditis, critically ill patients in general are at risk of developing nonthyroidal illness syndrome, with alterations in thyroid function. However, thyroid hormone measures in the patients severely ill with COVID-19 were not consistent with that syndrome.
A subanalysis of eight HICU 2020 patients with thyroid dysfunction who were followed for 55 days after discharge showed that two experienced hyperthyroidism but likely not from COVID-19; in the remaining six, thyroid function normalized.
Muller speculated that, when ill with COVID-19, the patients likely had a combination of SARS-CoV-2–related atypical thyroiditis and nonthyroidal illness syndrome, known as T4 toxicosis.
Will there be any long-term effects?
Importantly, it remains unknown whether the novel coronavirus has longer-term effects on the thyroid, Dr. Muller said.
“We cannot predict what will be the long-lasting thyroid effects after COVID-19,” she said.
With classic subacute viral thyroiditis, “After a few years ... 5%-20% of patients develop permanent hypothyroidism, [and] the same might happen in COVID-19 patients,” she hypothesized. “We will follow our patients long term to answer this question – this study is already ongoing.”
In the meantime, diagnosis of thyroid dysfunction in patients with COVID-19 is important, inasmuch as it could worsen the already critical conditions of patients, Muller stressed.
“The gold-standard treatment for thyroiditis is steroids, so the presence of thyroid dysfunction might represent an additional indication to such treatment in COVID-19 patients, to be verified in properly designed clinical trials,” she advised.
ACE2 cell receptors highly expressed in thyroid
Dr. Muller and colleagues also noted recent research showing that ACE2 – demonstrated to be a key host-cell entry receptor for both SARS-CoV and SARS-CoV-2 – is expressed in even higher levels in the thyroid than the lungs, where it causes COVID-19’s notorious pulmonary effects.
Dr. Muller said the implications of ACE2 expression in the thyroid remain to be elucidated.
“If ACE2 is confirmed to be expressed at higher levels, compared with the lungs in the thyroid gland and other tissues, i.e., small intestine, testis, kidney, heart, etc, dedicated studies will be needed to correlate ACE2 expression with the organs’ susceptibility to SARS-CoV-2 reflected by clinical presentation,” she said.
Dr. Leung added that, as a take-home message from these and the other thyroid/COVID-19 studies, “data are starting to show us that COVID-19 infection may cause thyrotoxicosis that is possibly related to thyroid and systemic inflammation. However, the serum thyroid function test abnormalities seen in COVID-19 patients with subacute thyroiditis are also likely exacerbated to a substantial extent by nonthyroidal illness physiology.”
The authors have disclosed no relevant financial relationships. Dr. Leung is on the advisory board of Medscape Diabetes and Endocrinology.
A version of this article originally appeared on Medscape.com.
Non-COVID-19 clinical trials grind to a halt during pandemic
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic has created unique and unprecedented challenges for the clinical research world, with potentially long-lasting consequences.
A new analysis of the extent of disruption shows that the average rate of stopped trials nearly doubled during the first 5 months of 2020, compared with the 2 previous years.
“Typically, clinical research precedes clinical practice by several years, so this disruption we’re seeing now will be felt for many years to come,” said Mario Guadino, MD, of Weill Cornell Medicine, New York.
The analysis was published online July 31 in the Journal of the American College of Cardiology.
The researchers used Python software to query meta-data from all trials reported on ClinicalTrials.gov. Of 321,218 non-COVID-19 trials queried, 28,672 (8.9%) were reported as stopped, defined as a switch in trial status from “recruiting” to “active and not recruiting,” “completed,” “suspended,” “terminated,” or “withdrawn.”
The average rate of discontinuation was 638 trials/month from January 2017 to December 2019, rising to 1,147 trials/month between January 2020 and May 2020 (P < .001 for trend).
Once stopped (as opposed to paused), restarting a trial is a tricky prospect, said Dr. Guadino. “You can’t stop and restart a trial because it creates a lot of issues, so we should expect many of these stopped trials to never be completed.”
He said these figures likely represent an underestimate of the true impact of the pandemic because there is typically a delay in the updating of the status of a trial on ClinicalTrials.gov.
“We are likely looking only at the tip of the iceberg,” he added. “My impression is that the number of trials that will be affected and even canceled will be very high.”
As for cardiology trials, one of the report’s authors, Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, without naming specific trials, had this to say: “Several cardiovascular trials were paused, and some were permanently discontinued. It may be a while before we fully appreciate just how much information was lost and how much might be salvaged.”
He’s not worried, however, that upcoming cardiology meetings, which have moved online for the foreseeable future, might get a bit boring. “Fortunately, there is enough good work going on in the cardiovascular and cardiometabolic space that I believe there will still be ample randomized and observational data of high quality to present at the major meetings,” Dr. Bhatt said in an email.
The researchers found a weak correlation between the national population-adjusted numbers of COVID-19 cases and the proportion of non-COVID-19 trials stopped by country.
Even for trials that stopped recruiting for a period of time but are continuing, there are myriad issues involving compliance, data integrity, statistical interpretability, etc.
“Even if there is just a temporary disruption, that will most likely lead to reduced enrollment, missing follow-up visits, and protocol deviations, all things that would be red flags during normal times and impact the quality of the clinical trial,” said Dr. Guadino.
“And if your outcome of interest is mortality, well, how exactly do you measure that during a pandemic?” he added.
Stopped for lack of funding
Besides the logistical issues, another reason trials may be in jeopardy is funding. A warning early in the pandemic from the research community in Canada that funding was quickly drying up, leaving both jobs and data at risk, led to an aid package from the government to keep the lights on.
The National Institutes of Health (NIH), the Canadian Institutes of Health Research, and similar groups “have devoted large sums of money to research in COVID, which is of course very appropriate, but that clearly reduces the amount of funding that is available for other researchers,” said Dr. Guadino.
Some funding agencies around the world have canceled or put on hold all non-COVID-19 clinical trials still at the design state, Dr. Guadino said in an interview.
The NIH, he stressed, has not canceled funding and has been “extremely open and cooperative” in trying to help trialists navigate the many COVID-generated issues. They’ve even issued guidance on how to manage trials during COVID-19.
Of note, in the survey, the majority of the trials stopped (95.4%) had nongovernmental funding.
“The data are not very granular, so we’re only able to make some very simple, descriptive comments, but it does seem like the more fragile trials – those that are smaller and industry-funded – are the ones more likely to be disrupted,” said Dr. Guadino.
In some cases, he said, priorities have shifted to COVID-19. “If a small company is sponsoring a trial and they decide they want to sponsor something related to COVID, or they realize that because of the slow enrollment, the trial becomes too expensive to complete, they may opt to just abandon it,” said Dr. Guadino.
At what cost? It will take years to sort that out, he said.
This study received no funding. Dr. Guadino and Dr. Bhatt are both active trialists, participating in both industry- and government-sponsored clinical research.
A version of this article originally appeared on Medscape.com.
Severe obesity ups risk for death in younger men with COVID-19
In a large California health care plan, among patients with COVID-19, men aged 60 years and younger had a much higher risk of dying within 3 weeks of diagnosis if they had severe obesity as opposed to being of normal weight, independently of other risk factors.
reported Sara Y. Tartof, PhD, MPH, Kaiser Permanente Southern California, Pasadena, Calif., and coauthors.
The data “highlight the leading role of severe obesity over correlated risk factors, providing a target for early intervention,” they concluded in an article published online Aug. 12 in Annals of Internal Medicine.
This work adds to nearly 300 articles that have shown that severe obesity is associated with an increased risk for morbidity and mortality from COVID-19.
In an accompanying editorial, David A. Kass, MD, said: “Consistency of this new study and prior research should put to rest the contention that obesity is common in severe COVID-19 because it is common in the population.”
Rather, these findings show that “obesity is an important independent risk factor for serious COVID-19 disease,” he pointed out.
On the basis of this evidence, “arguably the hardest question to answer is: What is to be done?” wondered Kass, of Johns Hopkins University, Baltimore.
Although data consistently show that a body mass index >35 kg/m2 is predictive of major health risks, “weight reduction at that level of obesity is difficult and certainly is not achieved rapidly,” Dr. Kass stressed.
“Therefore ... social distancing; altering behaviors to reduce viral exposure and transmission, such as wearing masks; and instituting policies and health care approaches that recognize the potential effects of obesity should be implemented,” he emphasized. “These actions should help and are certainly doable.”
Similarly, Dr. Tartof and colleagues said their “findings also reveal the distressing collision of two pandemics: COVID-19 and obesity.
“As COVID-19 continues to spread unabated, we must focus our immediate efforts on containing the crisis at hand,” they urged.
However, the findings also “underscore the need for future collective efforts to combat the equally devastating, and potentially synergistic, force of the obesity epidemic.”
COVID-19 pandemic collides with obesity epidemic
Previous studies of obesity and COVID-19 were small, did not adjust for multiple confounders, or did not include nonhospitalized patients, Dr. Tartof and coauthors wrote.
Their study included 6,916 members of the Kaiser Permanente Southern California health care plan who were diagnosed with COVID-19 from Feb. 13 to May 2, 2020.
The researchers calculated the risk for death at 21 days after a COVID-19 diagnosis; findings were corrected for age, sex, race/ethnicity, smoking, myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, renal disease, metastatic tumor or malignancy, other immune disease, hyperlipidemia, hypertension, asthma, organ transplant, and diabetes status.
On the basis of BMI, the patients were classified as being underweight, of normal weight, overweight, or as having class 1, 2, or 3 obesity. BMI of 18.5 to 24 kg/m2 is defined as normal weight.
Class 3 obesity, also called severe obesity, included moderately severe obesity (BMI, 40-44 kg/m2) and extremely severe obesity (≥45 kg/m2).
A little more than half of the patients were women (55%), and more than 50% were Hispanic (54%).
A total of 206 patients (3%) died within 21 days of being diagnosed with COVID-19; of these, 67% had been hospitalized, and 43% had been intubated.
Overall, the COVID-19 patients with moderately severe or extremely severe obesity had a 2.7-fold and 4.2-fold increased risk for death, respectively, within 3 weeks compared with patients of normal weight.
Patients in the other BMI categories did not have a significantly higher risk of dying during follow-up.
However, each decade of increasing age after age 40 was associated with a stepwise increased risk for death within 3 weeks of the COVID-19 diagnosis.
Risk stratified by age and sex
Further analysis showed that, “most strikingly,” among patients aged 60 and younger, those with moderately severe obesity and extremely severe obesity had significant 17-fold and 12-fold higher risks of dying during follow-up, respectively, compared with patients of normal weight, the researchers reported.
In patients older than 60, moderately severe obesity did not confer a significant increased risk for imminent death from COVID-19; extremely severe obesity conferred a smaller, threefold increased risk for this.
“Our finding that severe obesity, particularly among younger patients, eclipses the mortality risk posed by other obesity-related conditions, such as history of myocardial infarction (MI), diabetes, hypertension, or hyperlipidemia, suggests a significant pathophysiologic link between excess adiposity and severe COVID-19 illness,” the researchers noted.
This independent increased risk for death with severe obesity was seen in men but not in women.
Men with moderately severe and extremely severe obesity had significant 4.8-fold and 10-fold higher risks of dying within 3 weeks, respectively, compared with men of normal weight.
“That the risks are higher in younger patients is probably not because obesity is particularly damaging in this age group; it is more likely that other serious comorbidities that evolve later in life take over as dominant risk factors,” Dr. Kass suggested in his editorial.
“That males are particularly affected may reflect their greater visceral adiposity over females, given that this fat is notably proinflammatory and contributes to metabolic and vascular disease,” he added.
“As a cardiologist who studies heart failure,” Dr. Kass wrote, “I am struck by how many of the mechanisms that are mentioned in reviews of obesity risk and heart disease are also mentioned in reviews of obesity and COVID-19.”
The study was funded by Roche-Genentech. Kass has disclosed no relevant financial relationships. Disclosures of the authors are listed in the article.
A version of this article originally appeared on Medscape.com.
In a large California health care plan, among patients with COVID-19, men aged 60 years and younger had a much higher risk of dying within 3 weeks of diagnosis if they had severe obesity as opposed to being of normal weight, independently of other risk factors.
reported Sara Y. Tartof, PhD, MPH, Kaiser Permanente Southern California, Pasadena, Calif., and coauthors.
The data “highlight the leading role of severe obesity over correlated risk factors, providing a target for early intervention,” they concluded in an article published online Aug. 12 in Annals of Internal Medicine.
This work adds to nearly 300 articles that have shown that severe obesity is associated with an increased risk for morbidity and mortality from COVID-19.
In an accompanying editorial, David A. Kass, MD, said: “Consistency of this new study and prior research should put to rest the contention that obesity is common in severe COVID-19 because it is common in the population.”
Rather, these findings show that “obesity is an important independent risk factor for serious COVID-19 disease,” he pointed out.
On the basis of this evidence, “arguably the hardest question to answer is: What is to be done?” wondered Kass, of Johns Hopkins University, Baltimore.
Although data consistently show that a body mass index >35 kg/m2 is predictive of major health risks, “weight reduction at that level of obesity is difficult and certainly is not achieved rapidly,” Dr. Kass stressed.
“Therefore ... social distancing; altering behaviors to reduce viral exposure and transmission, such as wearing masks; and instituting policies and health care approaches that recognize the potential effects of obesity should be implemented,” he emphasized. “These actions should help and are certainly doable.”
Similarly, Dr. Tartof and colleagues said their “findings also reveal the distressing collision of two pandemics: COVID-19 and obesity.
“As COVID-19 continues to spread unabated, we must focus our immediate efforts on containing the crisis at hand,” they urged.
However, the findings also “underscore the need for future collective efforts to combat the equally devastating, and potentially synergistic, force of the obesity epidemic.”
COVID-19 pandemic collides with obesity epidemic
Previous studies of obesity and COVID-19 were small, did not adjust for multiple confounders, or did not include nonhospitalized patients, Dr. Tartof and coauthors wrote.
Their study included 6,916 members of the Kaiser Permanente Southern California health care plan who were diagnosed with COVID-19 from Feb. 13 to May 2, 2020.
The researchers calculated the risk for death at 21 days after a COVID-19 diagnosis; findings were corrected for age, sex, race/ethnicity, smoking, myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, renal disease, metastatic tumor or malignancy, other immune disease, hyperlipidemia, hypertension, asthma, organ transplant, and diabetes status.
On the basis of BMI, the patients were classified as being underweight, of normal weight, overweight, or as having class 1, 2, or 3 obesity. BMI of 18.5 to 24 kg/m2 is defined as normal weight.
Class 3 obesity, also called severe obesity, included moderately severe obesity (BMI, 40-44 kg/m2) and extremely severe obesity (≥45 kg/m2).
A little more than half of the patients were women (55%), and more than 50% were Hispanic (54%).
A total of 206 patients (3%) died within 21 days of being diagnosed with COVID-19; of these, 67% had been hospitalized, and 43% had been intubated.
Overall, the COVID-19 patients with moderately severe or extremely severe obesity had a 2.7-fold and 4.2-fold increased risk for death, respectively, within 3 weeks compared with patients of normal weight.
Patients in the other BMI categories did not have a significantly higher risk of dying during follow-up.
However, each decade of increasing age after age 40 was associated with a stepwise increased risk for death within 3 weeks of the COVID-19 diagnosis.
Risk stratified by age and sex
Further analysis showed that, “most strikingly,” among patients aged 60 and younger, those with moderately severe obesity and extremely severe obesity had significant 17-fold and 12-fold higher risks of dying during follow-up, respectively, compared with patients of normal weight, the researchers reported.
In patients older than 60, moderately severe obesity did not confer a significant increased risk for imminent death from COVID-19; extremely severe obesity conferred a smaller, threefold increased risk for this.
“Our finding that severe obesity, particularly among younger patients, eclipses the mortality risk posed by other obesity-related conditions, such as history of myocardial infarction (MI), diabetes, hypertension, or hyperlipidemia, suggests a significant pathophysiologic link between excess adiposity and severe COVID-19 illness,” the researchers noted.
This independent increased risk for death with severe obesity was seen in men but not in women.
Men with moderately severe and extremely severe obesity had significant 4.8-fold and 10-fold higher risks of dying within 3 weeks, respectively, compared with men of normal weight.
“That the risks are higher in younger patients is probably not because obesity is particularly damaging in this age group; it is more likely that other serious comorbidities that evolve later in life take over as dominant risk factors,” Dr. Kass suggested in his editorial.
“That males are particularly affected may reflect their greater visceral adiposity over females, given that this fat is notably proinflammatory and contributes to metabolic and vascular disease,” he added.
“As a cardiologist who studies heart failure,” Dr. Kass wrote, “I am struck by how many of the mechanisms that are mentioned in reviews of obesity risk and heart disease are also mentioned in reviews of obesity and COVID-19.”
The study was funded by Roche-Genentech. Kass has disclosed no relevant financial relationships. Disclosures of the authors are listed in the article.
A version of this article originally appeared on Medscape.com.
In a large California health care plan, among patients with COVID-19, men aged 60 years and younger had a much higher risk of dying within 3 weeks of diagnosis if they had severe obesity as opposed to being of normal weight, independently of other risk factors.
reported Sara Y. Tartof, PhD, MPH, Kaiser Permanente Southern California, Pasadena, Calif., and coauthors.
The data “highlight the leading role of severe obesity over correlated risk factors, providing a target for early intervention,” they concluded in an article published online Aug. 12 in Annals of Internal Medicine.
This work adds to nearly 300 articles that have shown that severe obesity is associated with an increased risk for morbidity and mortality from COVID-19.
In an accompanying editorial, David A. Kass, MD, said: “Consistency of this new study and prior research should put to rest the contention that obesity is common in severe COVID-19 because it is common in the population.”
Rather, these findings show that “obesity is an important independent risk factor for serious COVID-19 disease,” he pointed out.
On the basis of this evidence, “arguably the hardest question to answer is: What is to be done?” wondered Kass, of Johns Hopkins University, Baltimore.
Although data consistently show that a body mass index >35 kg/m2 is predictive of major health risks, “weight reduction at that level of obesity is difficult and certainly is not achieved rapidly,” Dr. Kass stressed.
“Therefore ... social distancing; altering behaviors to reduce viral exposure and transmission, such as wearing masks; and instituting policies and health care approaches that recognize the potential effects of obesity should be implemented,” he emphasized. “These actions should help and are certainly doable.”
Similarly, Dr. Tartof and colleagues said their “findings also reveal the distressing collision of two pandemics: COVID-19 and obesity.
“As COVID-19 continues to spread unabated, we must focus our immediate efforts on containing the crisis at hand,” they urged.
However, the findings also “underscore the need for future collective efforts to combat the equally devastating, and potentially synergistic, force of the obesity epidemic.”
COVID-19 pandemic collides with obesity epidemic
Previous studies of obesity and COVID-19 were small, did not adjust for multiple confounders, or did not include nonhospitalized patients, Dr. Tartof and coauthors wrote.
Their study included 6,916 members of the Kaiser Permanente Southern California health care plan who were diagnosed with COVID-19 from Feb. 13 to May 2, 2020.
The researchers calculated the risk for death at 21 days after a COVID-19 diagnosis; findings were corrected for age, sex, race/ethnicity, smoking, myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, renal disease, metastatic tumor or malignancy, other immune disease, hyperlipidemia, hypertension, asthma, organ transplant, and diabetes status.
On the basis of BMI, the patients were classified as being underweight, of normal weight, overweight, or as having class 1, 2, or 3 obesity. BMI of 18.5 to 24 kg/m2 is defined as normal weight.
Class 3 obesity, also called severe obesity, included moderately severe obesity (BMI, 40-44 kg/m2) and extremely severe obesity (≥45 kg/m2).
A little more than half of the patients were women (55%), and more than 50% were Hispanic (54%).
A total of 206 patients (3%) died within 21 days of being diagnosed with COVID-19; of these, 67% had been hospitalized, and 43% had been intubated.
Overall, the COVID-19 patients with moderately severe or extremely severe obesity had a 2.7-fold and 4.2-fold increased risk for death, respectively, within 3 weeks compared with patients of normal weight.
Patients in the other BMI categories did not have a significantly higher risk of dying during follow-up.
However, each decade of increasing age after age 40 was associated with a stepwise increased risk for death within 3 weeks of the COVID-19 diagnosis.
Risk stratified by age and sex
Further analysis showed that, “most strikingly,” among patients aged 60 and younger, those with moderately severe obesity and extremely severe obesity had significant 17-fold and 12-fold higher risks of dying during follow-up, respectively, compared with patients of normal weight, the researchers reported.
In patients older than 60, moderately severe obesity did not confer a significant increased risk for imminent death from COVID-19; extremely severe obesity conferred a smaller, threefold increased risk for this.
“Our finding that severe obesity, particularly among younger patients, eclipses the mortality risk posed by other obesity-related conditions, such as history of myocardial infarction (MI), diabetes, hypertension, or hyperlipidemia, suggests a significant pathophysiologic link between excess adiposity and severe COVID-19 illness,” the researchers noted.
This independent increased risk for death with severe obesity was seen in men but not in women.
Men with moderately severe and extremely severe obesity had significant 4.8-fold and 10-fold higher risks of dying within 3 weeks, respectively, compared with men of normal weight.
“That the risks are higher in younger patients is probably not because obesity is particularly damaging in this age group; it is more likely that other serious comorbidities that evolve later in life take over as dominant risk factors,” Dr. Kass suggested in his editorial.
“That males are particularly affected may reflect their greater visceral adiposity over females, given that this fat is notably proinflammatory and contributes to metabolic and vascular disease,” he added.
“As a cardiologist who studies heart failure,” Dr. Kass wrote, “I am struck by how many of the mechanisms that are mentioned in reviews of obesity risk and heart disease are also mentioned in reviews of obesity and COVID-19.”
The study was funded by Roche-Genentech. Kass has disclosed no relevant financial relationships. Disclosures of the authors are listed in the article.
A version of this article originally appeared on Medscape.com.
FDA authorizes new saliva COVID-19 test
The FDA authorized a new type of saliva-based coronavirus test on August 15 that could cut down on the cost of testing and the time it takes to process results.
The emergency use authorization is for SalivaDirect, a diagnostic test created by the Yale School of Public Health. The test doesn’t require a special type of swab or collection tube — saliva can be collected in any sterile container, according to the FDA announcement.
The new test is “yet another testing innovation game changer that will reduce the demand for scarce testing resources,” Admiral Brett Giroir, MD, the assistant secretary for health and the COVID-19 testing coordinator, said in the statement.
The test also doesn’t require a special type of extractor, which is helpful because the extraction kits used to process other tests have faced shortages during the pandemic. The test can be used with different types of reagents and instruments already found in labs.
“Providing this type of flexibility for processing saliva samples to test for COVID-19 infection is groundbreaking in terms of efficiency and avoiding shortages of crucial test components like reagents,” Stephen Hahn, MD, the FDA commissioner, also said in the statement.
Yale will provide the instructions to labs as an “open source” protocol. The test doesn’t require any proprietary equipment or testing components, so labs across the country can assemble and use it based on the FDA guidelines. The testing method is available immediately and could be scaled up quickly in the next few weeks, according to a statement from Yale.
“This is a huge step forward to make testing more accessible,” Chantal Vogels, a postdoctoral fellow at Yale who led the lab development and test validation efforts, said in the statement.
The Yale team is further testing whether the saliva method can be used to find coronavirus cases among people who don’t have any symptoms and has been working with players and staff from the NBA. So far, the results have been accurate and similar to the nasal swabs for COVID-19, according to a preprint study published on medRxiv.
The research team wanted to get rid of the expensive collection tubes that other companies use to preserve the virus during processing, according to the Yale statement. They found that the virus is stable in saliva for long periods of time at warm temperatures and that special tubes aren’t necessary.
The FDA has authorized other saliva-based tests, according to ABC News, but SalivaDirect is the first that doesn’t require the extraction process used to test viral genetic material. Instead, the Yale process breaks down the saliva with an enzyme and applied heat. This type of testing could cost about $10, the Yale researchers said, and people can collect the saliva themselves under supervision.
“This, I hope, is a turning point,” Anne Wyllie, PhD, one of the lead researchers at Yale, told the news station.* “Expand testing capacity, inspire creativity and we can take competition to those labs charging a lot and bring prices down.”
This article first appeared on WebMD.com.
Correction, 8/25/20: An earlier version of this article misstated Dr. Wylie's academic degree.
The FDA authorized a new type of saliva-based coronavirus test on August 15 that could cut down on the cost of testing and the time it takes to process results.
The emergency use authorization is for SalivaDirect, a diagnostic test created by the Yale School of Public Health. The test doesn’t require a special type of swab or collection tube — saliva can be collected in any sterile container, according to the FDA announcement.
The new test is “yet another testing innovation game changer that will reduce the demand for scarce testing resources,” Admiral Brett Giroir, MD, the assistant secretary for health and the COVID-19 testing coordinator, said in the statement.
The test also doesn’t require a special type of extractor, which is helpful because the extraction kits used to process other tests have faced shortages during the pandemic. The test can be used with different types of reagents and instruments already found in labs.
“Providing this type of flexibility for processing saliva samples to test for COVID-19 infection is groundbreaking in terms of efficiency and avoiding shortages of crucial test components like reagents,” Stephen Hahn, MD, the FDA commissioner, also said in the statement.
Yale will provide the instructions to labs as an “open source” protocol. The test doesn’t require any proprietary equipment or testing components, so labs across the country can assemble and use it based on the FDA guidelines. The testing method is available immediately and could be scaled up quickly in the next few weeks, according to a statement from Yale.
“This is a huge step forward to make testing more accessible,” Chantal Vogels, a postdoctoral fellow at Yale who led the lab development and test validation efforts, said in the statement.
The Yale team is further testing whether the saliva method can be used to find coronavirus cases among people who don’t have any symptoms and has been working with players and staff from the NBA. So far, the results have been accurate and similar to the nasal swabs for COVID-19, according to a preprint study published on medRxiv.
The research team wanted to get rid of the expensive collection tubes that other companies use to preserve the virus during processing, according to the Yale statement. They found that the virus is stable in saliva for long periods of time at warm temperatures and that special tubes aren’t necessary.
The FDA has authorized other saliva-based tests, according to ABC News, but SalivaDirect is the first that doesn’t require the extraction process used to test viral genetic material. Instead, the Yale process breaks down the saliva with an enzyme and applied heat. This type of testing could cost about $10, the Yale researchers said, and people can collect the saliva themselves under supervision.
“This, I hope, is a turning point,” Anne Wyllie, PhD, one of the lead researchers at Yale, told the news station.* “Expand testing capacity, inspire creativity and we can take competition to those labs charging a lot and bring prices down.”
This article first appeared on WebMD.com.
Correction, 8/25/20: An earlier version of this article misstated Dr. Wylie's academic degree.
The FDA authorized a new type of saliva-based coronavirus test on August 15 that could cut down on the cost of testing and the time it takes to process results.
The emergency use authorization is for SalivaDirect, a diagnostic test created by the Yale School of Public Health. The test doesn’t require a special type of swab or collection tube — saliva can be collected in any sterile container, according to the FDA announcement.
The new test is “yet another testing innovation game changer that will reduce the demand for scarce testing resources,” Admiral Brett Giroir, MD, the assistant secretary for health and the COVID-19 testing coordinator, said in the statement.
The test also doesn’t require a special type of extractor, which is helpful because the extraction kits used to process other tests have faced shortages during the pandemic. The test can be used with different types of reagents and instruments already found in labs.
“Providing this type of flexibility for processing saliva samples to test for COVID-19 infection is groundbreaking in terms of efficiency and avoiding shortages of crucial test components like reagents,” Stephen Hahn, MD, the FDA commissioner, also said in the statement.
Yale will provide the instructions to labs as an “open source” protocol. The test doesn’t require any proprietary equipment or testing components, so labs across the country can assemble and use it based on the FDA guidelines. The testing method is available immediately and could be scaled up quickly in the next few weeks, according to a statement from Yale.
“This is a huge step forward to make testing more accessible,” Chantal Vogels, a postdoctoral fellow at Yale who led the lab development and test validation efforts, said in the statement.
The Yale team is further testing whether the saliva method can be used to find coronavirus cases among people who don’t have any symptoms and has been working with players and staff from the NBA. So far, the results have been accurate and similar to the nasal swabs for COVID-19, according to a preprint study published on medRxiv.
The research team wanted to get rid of the expensive collection tubes that other companies use to preserve the virus during processing, according to the Yale statement. They found that the virus is stable in saliva for long periods of time at warm temperatures and that special tubes aren’t necessary.
The FDA has authorized other saliva-based tests, according to ABC News, but SalivaDirect is the first that doesn’t require the extraction process used to test viral genetic material. Instead, the Yale process breaks down the saliva with an enzyme and applied heat. This type of testing could cost about $10, the Yale researchers said, and people can collect the saliva themselves under supervision.
“This, I hope, is a turning point,” Anne Wyllie, PhD, one of the lead researchers at Yale, told the news station.* “Expand testing capacity, inspire creativity and we can take competition to those labs charging a lot and bring prices down.”
This article first appeared on WebMD.com.
Correction, 8/25/20: An earlier version of this article misstated Dr. Wylie's academic degree.
PPE shortage could last years without strategic plan, experts warn
Officials said logistical challenges continue 7 months after the coronavirus reached the United States, as the flu season approaches and as some state emergency management agencies prepare for a fall surge in COVID-19 cases.
Although the disarray is not as widespread as it was this spring, hospitals said rolling shortages of supplies range from specialized beds to disposable isolation gowns to thermometers.
“A few weeks ago, we were having a very difficult time getting the sanitary wipes. You just couldn’t get them,” said Bernard Klein, MD, chief executive of Providence Holy Cross Medical Center in Mission Hills, Calif., near Los Angeles. “We actually had to manufacture our own.”
This same dynamic has played out across a number of critical supplies in his hospital. First masks, then isolation gowns and now a specialized bed that allows nurses to turn COVID-19 patients onto their bellies – equipment that helps workers with what can otherwise be a six-person job.
“We’ve seen whole families come to our hospital with COVID, and several members hospitalized at the same time,” said Dr. Klein. “It’s very, very sad.”
Testing supplies ran short as the predominantly Latino community served by Providence Holy Cross was hit hard by COVID, and even as nearby hospitals could process 15-minute tests.
“If we had a more coordinated response with a partnership between the medical field, the government and the private industry, it would help improve the supply chain to the areas that need it most,” Dr. Klein said.
Dr. Klein said he expected to deal with equipment and supply shortages throughout 2021, especially as flu season approaches.
“Most people focus on those N95 respirators,” said Carmela Coyle, CEO of the California Hospital Association, an industry group that represents more than 400 hospitals across one of America’s hardest-hit states.
She said she believed COVID-19-related supply challenges will persist through 2022.
“We have been challenged with shortages of isolation gowns, face shields, which you’re now starting to see in public places. Any one piece that’s in shortage or not available creates risk for patients and for health care workers,” said Ms. Coyle.
At the same time, trade associations representing manufacturers said persuading customers to shift to American suppliers had been difficult.
“I also have industry that’s working only at 10-20% capacity, who can make PPE in our own backyard, but have no orders,” said Kim Glas, CEO of the National Council of Textile Organizations, whose members make reusable cloth gowns.
Manufacturers in her organization have made “hundreds of millions of products,” but, without long-term government contracts, many are apprehensive to invest in the equipment needed to scale up the business and eventually lower prices.
“If there continues to be an upward trajectory of COVID-19 cases, not just in the U.S. but globally, you can see those supply chains breaking down again,” Ms. Glas said. “It is a health care security issue.”
For the past 2 decades, personal protective equipment was supplied to health care institutions in lean supply chains in the same way toilet paper was to grocery stores. Chains between major manufacturers and end users were so efficient, there was no need to stockpile goods.
But in March, the supply chain broke when major Asian PPE exporters embargoed materials or shut down just as demand increased exponentially. Thus, health care institutions were in much the same position as regular grocery shoppers, who were trying to buy great quantities of a product they never needed to stockpile before.
“I am very concerned about long-term PPE shortages for the foreseeable future,” said Susan Bailey, MD, president of the American Medical Association.
“There’s no question the situation is better than it was a couple of months ago,” said Bailey. However, many health care organizations, including her own, have struggled to obtain PPE. Bailey practices at a 10-doctor allergy clinic and was met with a 10,000-mask minimum when they tried to order N95 respirators.
“We have not seen evidence of a long-term strategic plan for the manufacture, acquisition and distribution of PPE” from the government, said Dr. Bailey. “The supply chain needs to be strengthened dramatically, and we need less dependence on foreign goods to manufacture our own PPE in the U.S.”
Some products have now come back to be made in the United States – although factories are not expected to be able to reach demand until mid-2021.
“A lot has been done in the last 6 months. We are largely out of the hole, and we have planted the seeds to render the United States self-sufficient,” said Dave Rousse, president of the Association of the Nonwoven Fabrics Industry.
In 2019, 850 tons of the material used in disposable masks was made in the United States. Around 10,000 tons is expected to be made in 2021, satisfying perhaps 80% of demand. But PPE is a suite of items – including gloves, gowns and face shields – not all of which have seen the same success.
“Thermometers are becoming a real issue,” said Cindy Juhas, chief strategy officer of CME, an American health care product distributor. “They’re expecting even a problem with needles and syringes for the amount of vaccines they have to make.”
Federal government efforts to address the supply chain have foundered. The Federal Emergency Management Agency, in charge of the COVID-19 response, told congressional interviewers in June it had “no involvement” in distributing PPE to hot spots.
Project Airbridge, an initiative headed by Jared Kushner, President Donald Trump’s son-in-law, flew PPE from international suppliers to the U.S. at taxpayer expense but was phased out. And the government has not responded to the AMA’s calls for more distribution data.
Arguably, Dr. Klein is among the best placed to weather such disruptions. He is part of a 51-hospital chain with purchasing power, and among the institutions that distributors prioritize when selling supplies. But tribulations continue even in hospitals, as shortages have pushed buyers to look directly for manufacturers, often through a swamp of companies that have sprung up overnight.
Now distributors are being called upon not just by their traditional customers – hospitals and long-term care homes – but by nearly every segment of society. First responders, schools, clinics and even food businesses are all buying medical equipment now.
“There’s going to be lots of other shortages we haven’t even thought about,” said Ms. Juhas.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Officials said logistical challenges continue 7 months after the coronavirus reached the United States, as the flu season approaches and as some state emergency management agencies prepare for a fall surge in COVID-19 cases.
Although the disarray is not as widespread as it was this spring, hospitals said rolling shortages of supplies range from specialized beds to disposable isolation gowns to thermometers.
“A few weeks ago, we were having a very difficult time getting the sanitary wipes. You just couldn’t get them,” said Bernard Klein, MD, chief executive of Providence Holy Cross Medical Center in Mission Hills, Calif., near Los Angeles. “We actually had to manufacture our own.”
This same dynamic has played out across a number of critical supplies in his hospital. First masks, then isolation gowns and now a specialized bed that allows nurses to turn COVID-19 patients onto their bellies – equipment that helps workers with what can otherwise be a six-person job.
“We’ve seen whole families come to our hospital with COVID, and several members hospitalized at the same time,” said Dr. Klein. “It’s very, very sad.”
Testing supplies ran short as the predominantly Latino community served by Providence Holy Cross was hit hard by COVID, and even as nearby hospitals could process 15-minute tests.
“If we had a more coordinated response with a partnership between the medical field, the government and the private industry, it would help improve the supply chain to the areas that need it most,” Dr. Klein said.
Dr. Klein said he expected to deal with equipment and supply shortages throughout 2021, especially as flu season approaches.
“Most people focus on those N95 respirators,” said Carmela Coyle, CEO of the California Hospital Association, an industry group that represents more than 400 hospitals across one of America’s hardest-hit states.
She said she believed COVID-19-related supply challenges will persist through 2022.
“We have been challenged with shortages of isolation gowns, face shields, which you’re now starting to see in public places. Any one piece that’s in shortage or not available creates risk for patients and for health care workers,” said Ms. Coyle.
At the same time, trade associations representing manufacturers said persuading customers to shift to American suppliers had been difficult.
“I also have industry that’s working only at 10-20% capacity, who can make PPE in our own backyard, but have no orders,” said Kim Glas, CEO of the National Council of Textile Organizations, whose members make reusable cloth gowns.
Manufacturers in her organization have made “hundreds of millions of products,” but, without long-term government contracts, many are apprehensive to invest in the equipment needed to scale up the business and eventually lower prices.
“If there continues to be an upward trajectory of COVID-19 cases, not just in the U.S. but globally, you can see those supply chains breaking down again,” Ms. Glas said. “It is a health care security issue.”
For the past 2 decades, personal protective equipment was supplied to health care institutions in lean supply chains in the same way toilet paper was to grocery stores. Chains between major manufacturers and end users were so efficient, there was no need to stockpile goods.
But in March, the supply chain broke when major Asian PPE exporters embargoed materials or shut down just as demand increased exponentially. Thus, health care institutions were in much the same position as regular grocery shoppers, who were trying to buy great quantities of a product they never needed to stockpile before.
“I am very concerned about long-term PPE shortages for the foreseeable future,” said Susan Bailey, MD, president of the American Medical Association.
“There’s no question the situation is better than it was a couple of months ago,” said Bailey. However, many health care organizations, including her own, have struggled to obtain PPE. Bailey practices at a 10-doctor allergy clinic and was met with a 10,000-mask minimum when they tried to order N95 respirators.
“We have not seen evidence of a long-term strategic plan for the manufacture, acquisition and distribution of PPE” from the government, said Dr. Bailey. “The supply chain needs to be strengthened dramatically, and we need less dependence on foreign goods to manufacture our own PPE in the U.S.”
Some products have now come back to be made in the United States – although factories are not expected to be able to reach demand until mid-2021.
“A lot has been done in the last 6 months. We are largely out of the hole, and we have planted the seeds to render the United States self-sufficient,” said Dave Rousse, president of the Association of the Nonwoven Fabrics Industry.
In 2019, 850 tons of the material used in disposable masks was made in the United States. Around 10,000 tons is expected to be made in 2021, satisfying perhaps 80% of demand. But PPE is a suite of items – including gloves, gowns and face shields – not all of which have seen the same success.
“Thermometers are becoming a real issue,” said Cindy Juhas, chief strategy officer of CME, an American health care product distributor. “They’re expecting even a problem with needles and syringes for the amount of vaccines they have to make.”
Federal government efforts to address the supply chain have foundered. The Federal Emergency Management Agency, in charge of the COVID-19 response, told congressional interviewers in June it had “no involvement” in distributing PPE to hot spots.
Project Airbridge, an initiative headed by Jared Kushner, President Donald Trump’s son-in-law, flew PPE from international suppliers to the U.S. at taxpayer expense but was phased out. And the government has not responded to the AMA’s calls for more distribution data.
Arguably, Dr. Klein is among the best placed to weather such disruptions. He is part of a 51-hospital chain with purchasing power, and among the institutions that distributors prioritize when selling supplies. But tribulations continue even in hospitals, as shortages have pushed buyers to look directly for manufacturers, often through a swamp of companies that have sprung up overnight.
Now distributors are being called upon not just by their traditional customers – hospitals and long-term care homes – but by nearly every segment of society. First responders, schools, clinics and even food businesses are all buying medical equipment now.
“There’s going to be lots of other shortages we haven’t even thought about,” said Ms. Juhas.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Officials said logistical challenges continue 7 months after the coronavirus reached the United States, as the flu season approaches and as some state emergency management agencies prepare for a fall surge in COVID-19 cases.
Although the disarray is not as widespread as it was this spring, hospitals said rolling shortages of supplies range from specialized beds to disposable isolation gowns to thermometers.
“A few weeks ago, we were having a very difficult time getting the sanitary wipes. You just couldn’t get them,” said Bernard Klein, MD, chief executive of Providence Holy Cross Medical Center in Mission Hills, Calif., near Los Angeles. “We actually had to manufacture our own.”
This same dynamic has played out across a number of critical supplies in his hospital. First masks, then isolation gowns and now a specialized bed that allows nurses to turn COVID-19 patients onto their bellies – equipment that helps workers with what can otherwise be a six-person job.
“We’ve seen whole families come to our hospital with COVID, and several members hospitalized at the same time,” said Dr. Klein. “It’s very, very sad.”
Testing supplies ran short as the predominantly Latino community served by Providence Holy Cross was hit hard by COVID, and even as nearby hospitals could process 15-minute tests.
“If we had a more coordinated response with a partnership between the medical field, the government and the private industry, it would help improve the supply chain to the areas that need it most,” Dr. Klein said.
Dr. Klein said he expected to deal with equipment and supply shortages throughout 2021, especially as flu season approaches.
“Most people focus on those N95 respirators,” said Carmela Coyle, CEO of the California Hospital Association, an industry group that represents more than 400 hospitals across one of America’s hardest-hit states.
She said she believed COVID-19-related supply challenges will persist through 2022.
“We have been challenged with shortages of isolation gowns, face shields, which you’re now starting to see in public places. Any one piece that’s in shortage or not available creates risk for patients and for health care workers,” said Ms. Coyle.
At the same time, trade associations representing manufacturers said persuading customers to shift to American suppliers had been difficult.
“I also have industry that’s working only at 10-20% capacity, who can make PPE in our own backyard, but have no orders,” said Kim Glas, CEO of the National Council of Textile Organizations, whose members make reusable cloth gowns.
Manufacturers in her organization have made “hundreds of millions of products,” but, without long-term government contracts, many are apprehensive to invest in the equipment needed to scale up the business and eventually lower prices.
“If there continues to be an upward trajectory of COVID-19 cases, not just in the U.S. but globally, you can see those supply chains breaking down again,” Ms. Glas said. “It is a health care security issue.”
For the past 2 decades, personal protective equipment was supplied to health care institutions in lean supply chains in the same way toilet paper was to grocery stores. Chains between major manufacturers and end users were so efficient, there was no need to stockpile goods.
But in March, the supply chain broke when major Asian PPE exporters embargoed materials or shut down just as demand increased exponentially. Thus, health care institutions were in much the same position as regular grocery shoppers, who were trying to buy great quantities of a product they never needed to stockpile before.
“I am very concerned about long-term PPE shortages for the foreseeable future,” said Susan Bailey, MD, president of the American Medical Association.
“There’s no question the situation is better than it was a couple of months ago,” said Bailey. However, many health care organizations, including her own, have struggled to obtain PPE. Bailey practices at a 10-doctor allergy clinic and was met with a 10,000-mask minimum when they tried to order N95 respirators.
“We have not seen evidence of a long-term strategic plan for the manufacture, acquisition and distribution of PPE” from the government, said Dr. Bailey. “The supply chain needs to be strengthened dramatically, and we need less dependence on foreign goods to manufacture our own PPE in the U.S.”
Some products have now come back to be made in the United States – although factories are not expected to be able to reach demand until mid-2021.
“A lot has been done in the last 6 months. We are largely out of the hole, and we have planted the seeds to render the United States self-sufficient,” said Dave Rousse, president of the Association of the Nonwoven Fabrics Industry.
In 2019, 850 tons of the material used in disposable masks was made in the United States. Around 10,000 tons is expected to be made in 2021, satisfying perhaps 80% of demand. But PPE is a suite of items – including gloves, gowns and face shields – not all of which have seen the same success.
“Thermometers are becoming a real issue,” said Cindy Juhas, chief strategy officer of CME, an American health care product distributor. “They’re expecting even a problem with needles and syringes for the amount of vaccines they have to make.”
Federal government efforts to address the supply chain have foundered. The Federal Emergency Management Agency, in charge of the COVID-19 response, told congressional interviewers in June it had “no involvement” in distributing PPE to hot spots.
Project Airbridge, an initiative headed by Jared Kushner, President Donald Trump’s son-in-law, flew PPE from international suppliers to the U.S. at taxpayer expense but was phased out. And the government has not responded to the AMA’s calls for more distribution data.
Arguably, Dr. Klein is among the best placed to weather such disruptions. He is part of a 51-hospital chain with purchasing power, and among the institutions that distributors prioritize when selling supplies. But tribulations continue even in hospitals, as shortages have pushed buyers to look directly for manufacturers, often through a swamp of companies that have sprung up overnight.
Now distributors are being called upon not just by their traditional customers – hospitals and long-term care homes – but by nearly every segment of society. First responders, schools, clinics and even food businesses are all buying medical equipment now.
“There’s going to be lots of other shortages we haven’t even thought about,” said Ms. Juhas.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The evidence is not clear: Rheumatic diseases, drugs, and COVID-19
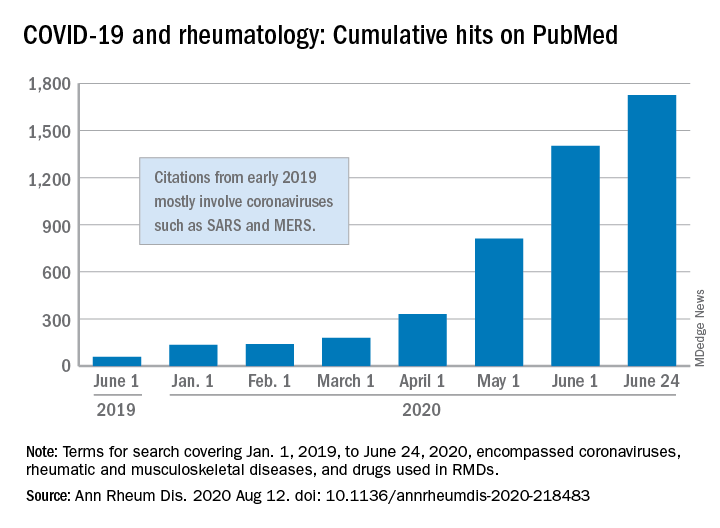
“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.

“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.

“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.
FROM ANNALS OF THE RHEUMATIC DISEASES
Pooled COVID-19 testing feasible, greatly reduces supply use
‘Straightforward, cost effective, and efficient’
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
‘Straightforward, cost effective, and efficient’
‘Straightforward, cost effective, and efficient’
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
How to truly connect with your patients
Introducing the ‘6H model’
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.
Introducing the ‘6H model’
Introducing the ‘6H model’
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.
I vividly remember the conversation that changed the way I practice medicine today.
During my medicine residency rounds, my attending at a Veterans Affairs hospital stated: “Remember Swati, there are three simple steps to gain your patients’ trust. The three questions they have are: No. 1, who are you? No. 2, are you any good? No. 3, do you really care about me?”
The first two questions are easier to address. The third question requires us bare our authentic human self often hiding behind our white coat and medical degree.
Who are you?
- Introduce yourself (everyone is wearing scrubs/white coats – state your full name and title)
- Describe your role in patient’s care plan
- Hand them your card (your name, photo, and a short description of the role of a hospitalist)
Are you any good?
- Briefly address your professional experience
- Explicitly state all the hard work you have done prior to entering the patient’s room (reviewing past medical records, hand off from ED provider or prior hospitalist)
- State your aim to collaborate with all people involved – their primary care provider, nurse, consultant
“Hello Mrs. Jones, my name is Dr. Swati Mehta. I will be your physician today. As a hospitalist, my role is to take care of your medical needs & worries. I will coordinate with your consultants, primary care physician, and other care teams to get you the answers you need. I have been working at XYZ Hospital for 6 years and have over 12 years of experience in medicine taking care of patients. I have reviewed your medical records, blood work, and x-rays before coming in. How are you feeling today? Do you mind if I ask you a few questions?”
Addressing the third question – Do you really care about me? – is the foundation of every human interaction. Answering this question involves addressing our patients’ many fears: Do you care about what I think is going on with my disease? Will you judge me by my socioeconomic status, gender, color of my skin, or addictions? Am I safe to open up and trust you? Are we equal partners in my health care journey? Do you really care?
A successful connection is achieved when we create a space of psychological safety and mutual respect. Once that happens, our patients open up to let us in their world and become more amenable to our opinion and recommendations. That is when true healing begins.
The “6H model” is an aide to form a strong human-centric connection.
The 6H model: Human connection with patients
Looking back at each patient interaction, good or bad, I have had in my almost 2 decades of practicing clinical medicine, the 6H model has brought me closer to my patients. We have formed a bond which has helped them navigate their arduous hospital journey, including medical and financial burdens, social and emotional needs. Utilizing this model, we were fortunate to receive the highest HCAHPS (Hospital Consumer Assessment of Healthcare Providers and Systems) Survey scores for 3 consecutive years while I served as the medical director of a 40-provider hospitalist program in a busy 450-bed hospital in Oregon.
In 2020, we are in the process of embedding the 6H model in several hospitalist programs across California. We are optimistic this intuitive approach will strengthen patient-provider relationships and ultimately improve HCAHPS scores.
To form an authentic connection with our patients doesn’t necessary require a lot of our time. Hardwiring the 6H approach when addressing our patients’ three questions is the key. The answers can change slightly, but the core message remains the same.
While we might not have much influence on all the factors that make or break our patients’ experience, the patient encounter is where we can truly make a difference. Consider using this 6H model in your next clinical shift. Human connection in health care is the need of the hour. Let’s bring “care” back to health care.
Dr. Mehta is director of quality & performance and patient experience at Vituity in Emeryville, Calif., and vice chair of the SHM patient experience committee.
CDC data confirm mental health is suffering during COVID-19
The ongoing COVID-19 pandemic continues to exact a huge toll on mental health in the United States, according to results of a survey released Aug. 13 by the Centers for Disease Control and Prevention.
During late June, about two in five U.S. adults surveyed said they were struggling with mental health or substance use. Younger adults, racial/ethnic minorities, essential workers, and those with preexisting psychiatric conditions were suffering the most.
“Addressing mental health disparities and preparing support systems to mitigate mental health consequences as the pandemic evolves will continue to be needed urgently,” write Rashon Lane, with the CDC COVID-19 Response Team, and colleagues in an article published online in the CDC’s Morbidity and Mortality Weekly Report.
During the period of June 24-30, 2020, 5,412 U.S. adults aged 18 and older completed online surveys that gauged mental health, substance use, and suicidal ideation.
Overall, 40.9% of respondents reported having at least one adverse mental or behavioral health condition; 31% reported symptoms of anxiety or depressive disorder; and 26% reported symptoms of a trauma- and stressor-related disorder related to the pandemic.
The prevalence of symptoms of anxiety disorder alone was roughly three times that reported in the second quarter of 2019, the authors noted.
In addition, , and nearly 11% reported having seriously considered suicide in the preceding 30 days.
Approximately twice as many respondents reported seriously considering suicide in the prior month compared with adults in the United States in 2018 (referring to the previous 12 months), the authors noted.
Suicidal ideation was significantly higher among younger respondents (aged 18-24 years, 26%), Hispanic persons (19%), non-Hispanic Black persons (15%), unpaid caregivers for adults (31%), and essential workers (22%).
The survey results are in line with recent data from Mental Health America, which indicate dramatic increases in depression, anxiety, and suicidality since the start of the COVID-19 pandemic.
The “markedly elevated” prevalence of adverse mental and behavioral health conditions associated with the COVID-19 pandemic highlights the “broad impact of the pandemic and the need to prevent and treat these conditions,” the researchers wrote.
The survey also highlights populations at increased risk for psychological distress and unhealthy coping.
“Future studies should identify drivers of adverse mental and behavioral health during the COVID-19 pandemic and whether factors such as social isolation, absence of school structure, unemployment and other financial worries, and various forms of violence (e.g., physical, emotional, mental, or sexual abuse) serve as additional stressors,” they suggested.
A version of this article originally appeared on Medscape.com.
The ongoing COVID-19 pandemic continues to exact a huge toll on mental health in the United States, according to results of a survey released Aug. 13 by the Centers for Disease Control and Prevention.
During late June, about two in five U.S. adults surveyed said they were struggling with mental health or substance use. Younger adults, racial/ethnic minorities, essential workers, and those with preexisting psychiatric conditions were suffering the most.
“Addressing mental health disparities and preparing support systems to mitigate mental health consequences as the pandemic evolves will continue to be needed urgently,” write Rashon Lane, with the CDC COVID-19 Response Team, and colleagues in an article published online in the CDC’s Morbidity and Mortality Weekly Report.
During the period of June 24-30, 2020, 5,412 U.S. adults aged 18 and older completed online surveys that gauged mental health, substance use, and suicidal ideation.
Overall, 40.9% of respondents reported having at least one adverse mental or behavioral health condition; 31% reported symptoms of anxiety or depressive disorder; and 26% reported symptoms of a trauma- and stressor-related disorder related to the pandemic.
The prevalence of symptoms of anxiety disorder alone was roughly three times that reported in the second quarter of 2019, the authors noted.
In addition, , and nearly 11% reported having seriously considered suicide in the preceding 30 days.
Approximately twice as many respondents reported seriously considering suicide in the prior month compared with adults in the United States in 2018 (referring to the previous 12 months), the authors noted.
Suicidal ideation was significantly higher among younger respondents (aged 18-24 years, 26%), Hispanic persons (19%), non-Hispanic Black persons (15%), unpaid caregivers for adults (31%), and essential workers (22%).
The survey results are in line with recent data from Mental Health America, which indicate dramatic increases in depression, anxiety, and suicidality since the start of the COVID-19 pandemic.
The “markedly elevated” prevalence of adverse mental and behavioral health conditions associated with the COVID-19 pandemic highlights the “broad impact of the pandemic and the need to prevent and treat these conditions,” the researchers wrote.
The survey also highlights populations at increased risk for psychological distress and unhealthy coping.
“Future studies should identify drivers of adverse mental and behavioral health during the COVID-19 pandemic and whether factors such as social isolation, absence of school structure, unemployment and other financial worries, and various forms of violence (e.g., physical, emotional, mental, or sexual abuse) serve as additional stressors,” they suggested.
A version of this article originally appeared on Medscape.com.
The ongoing COVID-19 pandemic continues to exact a huge toll on mental health in the United States, according to results of a survey released Aug. 13 by the Centers for Disease Control and Prevention.
During late June, about two in five U.S. adults surveyed said they were struggling with mental health or substance use. Younger adults, racial/ethnic minorities, essential workers, and those with preexisting psychiatric conditions were suffering the most.
“Addressing mental health disparities and preparing support systems to mitigate mental health consequences as the pandemic evolves will continue to be needed urgently,” write Rashon Lane, with the CDC COVID-19 Response Team, and colleagues in an article published online in the CDC’s Morbidity and Mortality Weekly Report.
During the period of June 24-30, 2020, 5,412 U.S. adults aged 18 and older completed online surveys that gauged mental health, substance use, and suicidal ideation.
Overall, 40.9% of respondents reported having at least one adverse mental or behavioral health condition; 31% reported symptoms of anxiety or depressive disorder; and 26% reported symptoms of a trauma- and stressor-related disorder related to the pandemic.
The prevalence of symptoms of anxiety disorder alone was roughly three times that reported in the second quarter of 2019, the authors noted.
In addition, , and nearly 11% reported having seriously considered suicide in the preceding 30 days.
Approximately twice as many respondents reported seriously considering suicide in the prior month compared with adults in the United States in 2018 (referring to the previous 12 months), the authors noted.
Suicidal ideation was significantly higher among younger respondents (aged 18-24 years, 26%), Hispanic persons (19%), non-Hispanic Black persons (15%), unpaid caregivers for adults (31%), and essential workers (22%).
The survey results are in line with recent data from Mental Health America, which indicate dramatic increases in depression, anxiety, and suicidality since the start of the COVID-19 pandemic.
The “markedly elevated” prevalence of adverse mental and behavioral health conditions associated with the COVID-19 pandemic highlights the “broad impact of the pandemic and the need to prevent and treat these conditions,” the researchers wrote.
The survey also highlights populations at increased risk for psychological distress and unhealthy coping.
“Future studies should identify drivers of adverse mental and behavioral health during the COVID-19 pandemic and whether factors such as social isolation, absence of school structure, unemployment and other financial worries, and various forms of violence (e.g., physical, emotional, mental, or sexual abuse) serve as additional stressors,” they suggested.
A version of this article originally appeared on Medscape.com.
Does metformin reduce risk for death in COVID-19?
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.