User login
Prior beta-blockers predict extra burden of heart failure in women with ACS
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the analysis of more than 13,000 patients with ACS and no history of cardiovascular (CV) disease, the women who had taken beta-blockers for hypertension showed about a one-third increased risk for heart failure (HF) at the time of their ACS presentation.
The difference between women and men was especially pronounced among patients with ST-segment elevation MI, compared with those with non-STEMI.
No such relationship between sex and risk for HF with ACS was observed among the larger portion of the cohort that had not previously been treated with beta-blockers, according to a report published July 13 in Hypertension, with lead author Raffaele Bugiardini, MD, University of Bologna (Italy).
Mortality at 30 days was sharply higher for patients with than without HF at their ACS presentation, by more than 600% for women and more than 800% for men.
“Our study provides robust evidence of an interaction between sex and beta-blocker therapy and suggests an increased risk of HF among women presenting with incident myocardial infarction,” Dr. Bugiardini said in an interview.
Given their novelty, “our findings raise strong concern about the appropriate role of beta-blockers in the therapy of hypertension in women with no prior history of cardiovascular diseases. Beta-blocker use may be an acute precipitant of heart failure in women presenting with incident ACS as first manifestation of coronary heart disease.” Dr. Bugiardini and colleagues wrote.
“There is one main implication for clinical practice. Discontinuing a beta-blocker in an otherwise healthy woman with hypertension and no prior CV disease is not harmful and could be wise,” Dr. Bugiardini said. “Blood pressure in women may be regulated in a safer way, such as using other medications and, of course, through diet and exercise.”
Rationale for the study
Men and women “differ with respect to the risk, causes, and prognosis of HF,” Dr. Bugiardini and colleagues wrote, and current guidelines “do not differentiate between the use of beta-blockers in men and in women.”
However, they proposed, “because prior trials and meta-analyses enrolled nearly five men for every woman, any differences in the effect of beta-blockers among women would have been concealed by the effect of beta-blocker therapy among men.”
The current study looked at data from October 2010 to July 2018 in the ISACS ARCHIVES, ISACS-TC, and the EMMACE-3X registries, covering 13,764 patients from 12 European countries who had a history of hypertension and presented with confirmed ACS.
Of the combined cohort, 2,590 (19%) had been treated with beta-blockers prior to their ACS presentation. They were similar to those without a history of beta-blocker use with respect to baseline features and use of other medications in an adjusted analysis.
In the group with prior beta-blocker use, 21.3% of the women and 16.7% of the men had HF of Killip class 2 or higher, a 4.6% absolute difference that worked out to a relative risk of 1.35 (95% confidence interval, 1.10-1.65).
The corresponding rates for women and men without prior beta-blocker use were 17.2% and 16.1%, respectively, for an absolute difference of only 1.1% and an RR of1.09 (95% CI, 0.97-1.21).
The interaction between sex and beta-blocker therapy for the HF outcome was significant (P < .034). An analysis that excluded patients in cardiogenic shock at their ACS presentation produced similar results.
In an analysis only of patients with STEMI, the RR for HF in women versus men was 1.44 (95% CI, 1.12-1.84) among those with a history of beta-blocker use, and 1.11 (95% CI, 0.98-1.26) among those who hadn’t used the drugs. The interaction between sex and beta-blocker use was significant (P = .033).
No such significant interaction was seen for the subgroup with non-STEMI as their index ACS (P = .14).
Heart failure at ACS was the most powerful observed predictor of 30-day mortality in women and in men in multivariate analysis; the odds ratios were 7.54 (95% CI, 5.78-9.83) and 9.62 (95% CI, 7.67-12.07), respectively.
“Our study underscores the importance of sex analyses in clinical research studies, which may provide further actionable data,” Dr. Bugiardini stated. “Failure to include both sexes in therapeutic studies is a missed opportunity to uncover underlying sex-specific risks. The adverse effect of beta-blocker therapy in women with hypertension is a sex-specific risk.”
Not just a male disease
Part of the study’s conclusions are “really not that surprising, because we have known for a long time that women who have an MI are much more likely to develop HF than men, and we also know that HF raises mortality after MI,” Ileana L. Pina, MD, MPH, Wayne State University, Detroit, said in an interview.
But what surprised her was that women taking beta-blockers were at greater risk for HF. “This association needs to be proven in a prospective study and confirmed in another dataset,” said Dr. Pina, who was not involved with the current study. “The most important message is to remember that HF is not just a ‘male’ disease and to pay attention to the symptoms of women and not discount or relegate them to anxiety or gastric problems.”
The study was observational, Dr. Bugiardini noted, so “the results may have some variance and need confirmation. However, a sex-stratified, randomized, controlled trial of beta-blocker therapy in patients with hypertension but no prior history of coronary heart disease or HF may not be considered ethical, since it would be designed to confirm risk … and not benefit.”
“Further observational studies may give confirmation,” he added. “In the meantime, the Food and Drug Administration should alert health care professionals of the adverse events associated with beta-blocker use in women with hypertension and no prior history of CV disease, [because] prescribing beta-blockers to a woman with hypertension means exposing her to unnecessary risk.”
Dr. Bugiardini and the other authors had no disclosures. Dr. Pina reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Proton pump inhibitors tied to COVID-19 risk
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, AGAF, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, AGAF, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In light of this finding, physicians should consider which patients truly need these powerful acid-lowering drugs, said Brennan Spiegel, MD, MSHS, AGAF, professor of medicine and public health at Cedars Sinai Medical Center in Los Angeles, Calif.
“All it means is that we’re going to have a conversation with our patients,” he said in an interview. “We don’t normally have that conversation because we don’t live in an environment with a high risk of enteric infection. But now we’re in a pandemic.”
The study by Dr. Spiegel and his colleagues was published online on July 7 in the American Journal of Gastroenterology.
Use of PPIs has skyrocketed over the past 2 decades. For ambulatory care visits, their use increased from 1.6% in 1998 to 7.6% in 2015. The increase raised questions about overprescription.
Although studies have not borne out many of the other concerns raised about adverse reactions, they have shown that the drugs increase the risk for enteric infections, including infections by SARS-CoV-1, a virus that is related to the COVID-19 virus, SARS-CoV-2, Dr. Spiegel said.
SARS-CoV-2 uses the angiotensin-converting enzyme–2 receptor to invade enterocytes. Dr. Spiegel theorized that an increase in stomach pH above 3 as a result of use of PPIs might allow the virus to enter the GI tract more easily, leading to enteritis, colitis, and systemic spread to other organs, including the lungs. “There is a reason we have acid in our stomachs,” Dr. Spiegel said.
To see how PPI use relates to COVID-19 infections, Dr. Spiegel and his colleagues surveyed online a nationally representative sample of Americans between May 3 and June 24, 2020, as part of a larger survey on gastroenterologic health.
Participants answered questions about gastrointestinal symptoms, current use of PPIs, and COVID-19 test results. They also answered questions about histamine-2 receptor agonists (H2RAs), also known as H2 blockers, which are used to treat some of the same conditions as PPIs but that do not reduce stomach acid as much.
The surveying firm, Cint, contacted 264,058 people. Of the 86,602 eligible participants who completed the survey, 53,130 said they had experienced abdominal discomfort, acid reflux, heartburn, or regurgitation. These survey participants were subsequently asked about PPI and H2RA use.
Of these, 6.4% reported testing positive for SARS-CoV-2. The researchers adjusted for age, sex, race/ethnicity, education, marital status, household income, body mass index, smoking, alcohol consumption, U.S. region, insurance status, and the presence of irritable bowel syndrome, celiac disease, gastroesophageal reflux disease, liver cirrhosis, Crohn’s disease, ulcerative colitis, diabetes, and HIV/AIDS.
After adjusting for these factors, the researchers found that those who took PPIs up to once a day were twice as likely to have had a positive COVID-19 test result than those who did not take the drugs (odds ratio, 2.15; 95% confidence interval, 1.90-2.44).
Those who took PPIs twice a day were almost four times as likely to have tested positive for the disease (OR, 3.67; 95% CI, 2.93-4.60).
By contrast, those taking H2RA drugs once daily were 15% less likely to report a positive COVID-19 test result (OR, 0.85; 95% CI, 0.74-0.99). Research is currently underway to determine whether H2RAs might protect against the disease for reasons unrelated to pH balance.
Dr. Spiegel cautioned that the current data show only an association between PPI use and COVID-19 positivity; it cannot prove cause and effect.
Nevertheless, Dr. Spiegel said the findings should encourage physicians to prescribe PPIs only when clearly indicated. “If somebody is not yet on a PPI and you’re considering whether to start them on a PPI, it’s a good idea to consider H2 blockers,” he said.
People who need a daily dose of a PPI to control a severe condition can safely continue doing so, but such patients should take care to follow standard public health recommendations for avoiding exposure to the virus. These recommendations include wearing a mask, maintaining social distance, and washing hands frequently.
“People who are older, comorbid, or smokers – if they get infected, it could be severe,” he said. “[For] someone like that, it’s reasonable to ask, do we really need to be on twice-daily PPIs? There is good evidence that they are no better off than if they are taking once-daily doses.”
Brian Lacy, MD, PhD, a professor of medicine at the Mayo Clinic in Jacksonville, Fla., agreed that the study should prompt physicians to take a second look at their patients’ PPI prescriptions. “My view is that PPIs are frequently overused, and maybe this is one more piece of data that, if someone is on PPIs, maybe they don’t need to be on this medication.”
On the other hand, the drugs are important for treating conditions such as erosive esophagitis and healing ulcers, he said. The overall risk of contracting COVID-19 is low, so even this finding of a 3.7-fold increased risk should not lead patients or providers to stop taking or prescribing PPIs.
The study also lends support to the idea that the gastrointestinal tract could be involved in SARS-CoV-2 transmission, and it supports warnings about aerosols emitted from flushing toilets and through exhalation, Dr. Spiegel said. There is less evidence of the virus being transmitted through food. “It may not be fecal-oral; it may be fecal-respiratory,” he said.
The study was part of a larger project funded by Ironwood Pharmaceuticals. Dr. Spiegel reported relationships with Alnylam Pharmaceuticals, Arena Pharmaceuticals, Ironwood Pharmaceuticals, Salix Pharmaceuticals, Shire Pharmaceuticals, Synergy Pharmaceuticals, and Takeda Pharmaceuticals. Dr. Lacy has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Provide support in uncertain times
A sense of safety and stability, both emotional and physical, is crucial in promoting the healthy development of youth. Between the global pandemic, need for social distancing, economic downturn, and increased awareness of racial disparities, for many this sense of stability has been rattled.
School closures have led to a loss of social interaction, challenges to continued academic growth, and, for some students, lack of access to nutrition and increased food insecurity. For students with learning or mental health challenges, closures may have eliminated or significantly reduced desperately needed supports received in school.1 While these trying circumstances have been difficult for many, the transition back to school in the fall also may be challenging because of the uncertainty about what this will look like and possible change in routine. Some students or their families may have anxiety about returning, either because of a history of adverse experiences at school such as bullying, or because of fears about exposure for themselves or others to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The past several months also brought about greater awareness of systemic racial disparities, whether as reflected in health care, education, or the criminal justice system. According to the Centers for Disease Control and Prevention data, Latinx and African-American individuals in the United States have had a threefold greater chance of contracting SARS-CoV-2 and have a twofold greater risk of death, compared with white people in the same communities.2 Other social determinants of health – economic stability, education, social factors such as incarceration and discrimination, and neighborhood factors including access to healthy food – play a role in this vulnerability.
The pandemic has resulted in a need for social distancing, and as a result, isolation. Children and teens exposed to the news may have anxiety about what they see or hear. Additional pressures in the family can include economic uncertainty, loss of employment for the primary wage earner of the household, or stress related to family members being first responders.
Any one of these factors is a potentially significant stressor, so how do we best support youth to help them survive and hopefully thrive during this time?
- It is important to establish a sense of routine; this can help create a sense of stability and safety. Recognizing that circumstances are not the same as they were 5 or 6 months ago, encouraging structure should not come at the cost of preserving connection.
- Note positive behavior and choices made by children and make sure they know it was observed.
- Many children have experienced increased screen time with the lack of structure of the traditional school day or summer camp and extracurricular activities. Limiting screen time and being mindful of its potential impact on mood is prudent.
- Self-care for parents and guardians is important. This time is stressful for the adults of the household, let alone children who are learning self-regulation skills.
- Listen to children’s or teens’ concerns and share information in developmentally appropriate ways. It is okay to not have all of the answers.
- Balance fostering a sense of gratitude with not invalidating a child’s or teen’s experience. Showing empathy during this time is vital. While there may be other soccer seasons, it is normal to experience grief about the loss of experiences during this time.
- Parents and guardians know their children best, so it is prudent for them to be mindful of concerning changes such as an increase in sadness, anxiety, or irritability that negatively impacts daily functioning such as sleeping, eating, or relationships with family and friends.
- Promote social interactions with appropriate safeguards in place. Unfortunately, the number of SARS-CoV-2 infections is increasing in multiple states, and there is the potential to return to some of the previous restrictions. However, encouraging social interaction while following local guidelines and with cautions such as limiting the number of people present, meeting outside, or considering interacting with others who are similarly social distancing can help foster social connection and development.
- Maintain connection digitally when in-person contact is not an option.3 Social groups, places of worship, and other activities have been agile in developing virtual communities. Communication by voice and/or video is thought to be more powerful than by written communication (text, email) alone.4 However, it is important to consider those who may have limited to no access to electronic methods.
- Encourage open communication with children about diversity and bias, and consider how our interactions with others may affect our children’s perspectives.5
- As providers, it is crucial that we address structural and institutional systems that negatively impact the health, safety, and access to care including our Black, indigenous, and people of color (BIPOC) and lesbian, gay, bisexual, transgender/transsexual, queer/questioning, intersex, and allied/asexual/aromantic/agender (LGBTQIA) patients.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. Dr. Strange has no relevant financial disclosures. Email her at pdnews@mdedge.com.
Online resources for parents and families
- Child Mind Institute: Coping With the Coronavirus Crisis: Supporting Your Kids.
- American Psychological Association: Talking with children about discrimination.
- Common Sense Media: Help with determining appropriateness of media for children.
Hotlines
- National Suicide Prevention Hotline: 1-800-273-8255
- GLBT National Hotline: 888-843-4564
- The California Peer-Run Warm Line: 1-855-845-7415
- Trevor Project: 866-488-7386 or text TREVOR to 1-202-304-1200
- Trans Lifeline: 877-565-8860
- Crisis Text Line: Text HOME to 741741
References
1. JAMA Pediatr. 2020 Apr 14. doi: 10.1001/jamapediatrics.2020.1456.
2. CDC: COVID-19 in Racial and Ethnic Minority Groups.
3. JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4469.
4. JAMA Intern Med. 2020 Apr 10. doi: 10.1001/jamainternmed.2020.1562.
5. American Psychological Association: Talking with children about discrimination.
A sense of safety and stability, both emotional and physical, is crucial in promoting the healthy development of youth. Between the global pandemic, need for social distancing, economic downturn, and increased awareness of racial disparities, for many this sense of stability has been rattled.
School closures have led to a loss of social interaction, challenges to continued academic growth, and, for some students, lack of access to nutrition and increased food insecurity. For students with learning or mental health challenges, closures may have eliminated or significantly reduced desperately needed supports received in school.1 While these trying circumstances have been difficult for many, the transition back to school in the fall also may be challenging because of the uncertainty about what this will look like and possible change in routine. Some students or their families may have anxiety about returning, either because of a history of adverse experiences at school such as bullying, or because of fears about exposure for themselves or others to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The past several months also brought about greater awareness of systemic racial disparities, whether as reflected in health care, education, or the criminal justice system. According to the Centers for Disease Control and Prevention data, Latinx and African-American individuals in the United States have had a threefold greater chance of contracting SARS-CoV-2 and have a twofold greater risk of death, compared with white people in the same communities.2 Other social determinants of health – economic stability, education, social factors such as incarceration and discrimination, and neighborhood factors including access to healthy food – play a role in this vulnerability.
The pandemic has resulted in a need for social distancing, and as a result, isolation. Children and teens exposed to the news may have anxiety about what they see or hear. Additional pressures in the family can include economic uncertainty, loss of employment for the primary wage earner of the household, or stress related to family members being first responders.
Any one of these factors is a potentially significant stressor, so how do we best support youth to help them survive and hopefully thrive during this time?
- It is important to establish a sense of routine; this can help create a sense of stability and safety. Recognizing that circumstances are not the same as they were 5 or 6 months ago, encouraging structure should not come at the cost of preserving connection.
- Note positive behavior and choices made by children and make sure they know it was observed.
- Many children have experienced increased screen time with the lack of structure of the traditional school day or summer camp and extracurricular activities. Limiting screen time and being mindful of its potential impact on mood is prudent.
- Self-care for parents and guardians is important. This time is stressful for the adults of the household, let alone children who are learning self-regulation skills.
- Listen to children’s or teens’ concerns and share information in developmentally appropriate ways. It is okay to not have all of the answers.
- Balance fostering a sense of gratitude with not invalidating a child’s or teen’s experience. Showing empathy during this time is vital. While there may be other soccer seasons, it is normal to experience grief about the loss of experiences during this time.
- Parents and guardians know their children best, so it is prudent for them to be mindful of concerning changes such as an increase in sadness, anxiety, or irritability that negatively impacts daily functioning such as sleeping, eating, or relationships with family and friends.
- Promote social interactions with appropriate safeguards in place. Unfortunately, the number of SARS-CoV-2 infections is increasing in multiple states, and there is the potential to return to some of the previous restrictions. However, encouraging social interaction while following local guidelines and with cautions such as limiting the number of people present, meeting outside, or considering interacting with others who are similarly social distancing can help foster social connection and development.
- Maintain connection digitally when in-person contact is not an option.3 Social groups, places of worship, and other activities have been agile in developing virtual communities. Communication by voice and/or video is thought to be more powerful than by written communication (text, email) alone.4 However, it is important to consider those who may have limited to no access to electronic methods.
- Encourage open communication with children about diversity and bias, and consider how our interactions with others may affect our children’s perspectives.5
- As providers, it is crucial that we address structural and institutional systems that negatively impact the health, safety, and access to care including our Black, indigenous, and people of color (BIPOC) and lesbian, gay, bisexual, transgender/transsexual, queer/questioning, intersex, and allied/asexual/aromantic/agender (LGBTQIA) patients.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. Dr. Strange has no relevant financial disclosures. Email her at pdnews@mdedge.com.
Online resources for parents and families
- Child Mind Institute: Coping With the Coronavirus Crisis: Supporting Your Kids.
- American Psychological Association: Talking with children about discrimination.
- Common Sense Media: Help with determining appropriateness of media for children.
Hotlines
- National Suicide Prevention Hotline: 1-800-273-8255
- GLBT National Hotline: 888-843-4564
- The California Peer-Run Warm Line: 1-855-845-7415
- Trevor Project: 866-488-7386 or text TREVOR to 1-202-304-1200
- Trans Lifeline: 877-565-8860
- Crisis Text Line: Text HOME to 741741
References
1. JAMA Pediatr. 2020 Apr 14. doi: 10.1001/jamapediatrics.2020.1456.
2. CDC: COVID-19 in Racial and Ethnic Minority Groups.
3. JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4469.
4. JAMA Intern Med. 2020 Apr 10. doi: 10.1001/jamainternmed.2020.1562.
5. American Psychological Association: Talking with children about discrimination.
A sense of safety and stability, both emotional and physical, is crucial in promoting the healthy development of youth. Between the global pandemic, need for social distancing, economic downturn, and increased awareness of racial disparities, for many this sense of stability has been rattled.
School closures have led to a loss of social interaction, challenges to continued academic growth, and, for some students, lack of access to nutrition and increased food insecurity. For students with learning or mental health challenges, closures may have eliminated or significantly reduced desperately needed supports received in school.1 While these trying circumstances have been difficult for many, the transition back to school in the fall also may be challenging because of the uncertainty about what this will look like and possible change in routine. Some students or their families may have anxiety about returning, either because of a history of adverse experiences at school such as bullying, or because of fears about exposure for themselves or others to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The past several months also brought about greater awareness of systemic racial disparities, whether as reflected in health care, education, or the criminal justice system. According to the Centers for Disease Control and Prevention data, Latinx and African-American individuals in the United States have had a threefold greater chance of contracting SARS-CoV-2 and have a twofold greater risk of death, compared with white people in the same communities.2 Other social determinants of health – economic stability, education, social factors such as incarceration and discrimination, and neighborhood factors including access to healthy food – play a role in this vulnerability.
The pandemic has resulted in a need for social distancing, and as a result, isolation. Children and teens exposed to the news may have anxiety about what they see or hear. Additional pressures in the family can include economic uncertainty, loss of employment for the primary wage earner of the household, or stress related to family members being first responders.
Any one of these factors is a potentially significant stressor, so how do we best support youth to help them survive and hopefully thrive during this time?
- It is important to establish a sense of routine; this can help create a sense of stability and safety. Recognizing that circumstances are not the same as they were 5 or 6 months ago, encouraging structure should not come at the cost of preserving connection.
- Note positive behavior and choices made by children and make sure they know it was observed.
- Many children have experienced increased screen time with the lack of structure of the traditional school day or summer camp and extracurricular activities. Limiting screen time and being mindful of its potential impact on mood is prudent.
- Self-care for parents and guardians is important. This time is stressful for the adults of the household, let alone children who are learning self-regulation skills.
- Listen to children’s or teens’ concerns and share information in developmentally appropriate ways. It is okay to not have all of the answers.
- Balance fostering a sense of gratitude with not invalidating a child’s or teen’s experience. Showing empathy during this time is vital. While there may be other soccer seasons, it is normal to experience grief about the loss of experiences during this time.
- Parents and guardians know their children best, so it is prudent for them to be mindful of concerning changes such as an increase in sadness, anxiety, or irritability that negatively impacts daily functioning such as sleeping, eating, or relationships with family and friends.
- Promote social interactions with appropriate safeguards in place. Unfortunately, the number of SARS-CoV-2 infections is increasing in multiple states, and there is the potential to return to some of the previous restrictions. However, encouraging social interaction while following local guidelines and with cautions such as limiting the number of people present, meeting outside, or considering interacting with others who are similarly social distancing can help foster social connection and development.
- Maintain connection digitally when in-person contact is not an option.3 Social groups, places of worship, and other activities have been agile in developing virtual communities. Communication by voice and/or video is thought to be more powerful than by written communication (text, email) alone.4 However, it is important to consider those who may have limited to no access to electronic methods.
- Encourage open communication with children about diversity and bias, and consider how our interactions with others may affect our children’s perspectives.5
- As providers, it is crucial that we address structural and institutional systems that negatively impact the health, safety, and access to care including our Black, indigenous, and people of color (BIPOC) and lesbian, gay, bisexual, transgender/transsexual, queer/questioning, intersex, and allied/asexual/aromantic/agender (LGBTQIA) patients.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. Dr. Strange has no relevant financial disclosures. Email her at pdnews@mdedge.com.
Online resources for parents and families
- Child Mind Institute: Coping With the Coronavirus Crisis: Supporting Your Kids.
- American Psychological Association: Talking with children about discrimination.
- Common Sense Media: Help with determining appropriateness of media for children.
Hotlines
- National Suicide Prevention Hotline: 1-800-273-8255
- GLBT National Hotline: 888-843-4564
- The California Peer-Run Warm Line: 1-855-845-7415
- Trevor Project: 866-488-7386 or text TREVOR to 1-202-304-1200
- Trans Lifeline: 877-565-8860
- Crisis Text Line: Text HOME to 741741
References
1. JAMA Pediatr. 2020 Apr 14. doi: 10.1001/jamapediatrics.2020.1456.
2. CDC: COVID-19 in Racial and Ethnic Minority Groups.
3. JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4469.
4. JAMA Intern Med. 2020 Apr 10. doi: 10.1001/jamainternmed.2020.1562.
5. American Psychological Association: Talking with children about discrimination.
Guidance addresses elders with diabetes during COVID-19
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Creating a student-staffed family call line to alleviate clinical burden
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
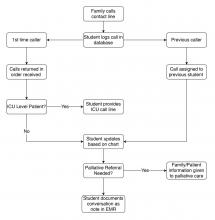
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
COVID-19 symptoms can linger for months
Clinicians and researchers have focused on the acute phase of COVID-19 infection, but it’s increasingly clear that some recovered patients discharged from acute care need continued monitoring for long-lasting effects, a study has found.
In a research letter published online July 9 in JAMA, Angelo Carfi, MD, and colleagues from the Gemelli Against COVID-19 Post–Acute Care Study Group in Rome, report that
Postdischarge assessments of patients who met criteria for SARS-CoV-2 negativity, including a reverse transcriptase–polymerase chain reaction test, were conducted from April 21 to May 29. Among the results:
- Only 12.6% of the 143 patients were completely free of any COVID-19 symptom
- About 32% of patients had one or two symptoms and 55% had three or more
- None had fever or other signs and symptoms of acute illness
- About 53% of patients still had fatigue, 43.4% had dyspnea, 27.3% had joint pain, and had 21.7% chest pain
- About 44% reported worsened quality of life on the EuroQol visual analog scale.
The sample cohort, assessed in a COVID-19 patient service recently established at the Fondazione Policlinico Universitario Agostino Gemelli had a mean age of 56.5 years and 37% were women. The mean length of hospital stay was 13.5 days. During their hospitalization, 72.7% of patients showed evidence of interstitial pneumonia. Noninvasive ventilation was given to 14.7% of patients and 4.9% received invasive ventilation.
The reality of lingering symptoms has led Dr. Carfi’s clinic to schedule a final “wrap-up visit” for patients after full assessment. “On that occasion the doctor prescribes anything necessary to correct the anomalies found during the full evaluation,” Dr. Carfi, a geriatrician at the Gemelli clinic, said in an interview. “These usually include vitamin supplementation and, in selected cases, a new drug prescription such as a blood thinner if necessary.”
Patients can also enroll in a training program in which breathing status is monitored.
In North America, doctors are also addressing the reality that the road to recovery can be a long and upward one, with persistent symptoms worse than those seen with acute influenza infection. “We see patients who were first diagnosed in March or April and still have symptoms in July,” said Zijian Chen, MD, an endocrinologist and medical director of Mount Sinai Health System’s Center for Post-COVID Care in New York.
“Persistent symptoms are much worse for COVID patients than flu patients. Even flu patients who spent time in the intensive care unit recover fully, and we can optimize their breathing before discharge,” Dr. Chen said in an interview.
As in the Italian study, Dr. Chen sees patients with COVID-19 who have ongoing shortness of breath, some requiring supplemental oxygen, or with persistent chest pain on exertion, blood clotting problems, poor concentration, gastrointestinal distress, and reduced muscle strength and impaired grasping power. He doesn’t rule out permanent lung damage in some. “Even asymptomatic individuals already show lung scarring on imaging,” he said.
The Mount Sinai program provides specialized interdisciplinary management that may include CT scans, endoscopy, and drugs such as respiratory medications or anticoagulants. It also offers training to combat the fatigue and deconditioning caused by the infection, symptoms that are not medically treatable but impact quality of life.
“These patients do get better, but I expect they may still have symptoms requiring monitoring after a year,” Dr. Chen said.
The study received no specific funding. Dr. Carfi and colleagues have disclosed no relevant financial relationships. Dr. Chen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Clinicians and researchers have focused on the acute phase of COVID-19 infection, but it’s increasingly clear that some recovered patients discharged from acute care need continued monitoring for long-lasting effects, a study has found.
In a research letter published online July 9 in JAMA, Angelo Carfi, MD, and colleagues from the Gemelli Against COVID-19 Post–Acute Care Study Group in Rome, report that
Postdischarge assessments of patients who met criteria for SARS-CoV-2 negativity, including a reverse transcriptase–polymerase chain reaction test, were conducted from April 21 to May 29. Among the results:
- Only 12.6% of the 143 patients were completely free of any COVID-19 symptom
- About 32% of patients had one or two symptoms and 55% had three or more
- None had fever or other signs and symptoms of acute illness
- About 53% of patients still had fatigue, 43.4% had dyspnea, 27.3% had joint pain, and had 21.7% chest pain
- About 44% reported worsened quality of life on the EuroQol visual analog scale.
The sample cohort, assessed in a COVID-19 patient service recently established at the Fondazione Policlinico Universitario Agostino Gemelli had a mean age of 56.5 years and 37% were women. The mean length of hospital stay was 13.5 days. During their hospitalization, 72.7% of patients showed evidence of interstitial pneumonia. Noninvasive ventilation was given to 14.7% of patients and 4.9% received invasive ventilation.
The reality of lingering symptoms has led Dr. Carfi’s clinic to schedule a final “wrap-up visit” for patients after full assessment. “On that occasion the doctor prescribes anything necessary to correct the anomalies found during the full evaluation,” Dr. Carfi, a geriatrician at the Gemelli clinic, said in an interview. “These usually include vitamin supplementation and, in selected cases, a new drug prescription such as a blood thinner if necessary.”
Patients can also enroll in a training program in which breathing status is monitored.
In North America, doctors are also addressing the reality that the road to recovery can be a long and upward one, with persistent symptoms worse than those seen with acute influenza infection. “We see patients who were first diagnosed in March or April and still have symptoms in July,” said Zijian Chen, MD, an endocrinologist and medical director of Mount Sinai Health System’s Center for Post-COVID Care in New York.
“Persistent symptoms are much worse for COVID patients than flu patients. Even flu patients who spent time in the intensive care unit recover fully, and we can optimize their breathing before discharge,” Dr. Chen said in an interview.
As in the Italian study, Dr. Chen sees patients with COVID-19 who have ongoing shortness of breath, some requiring supplemental oxygen, or with persistent chest pain on exertion, blood clotting problems, poor concentration, gastrointestinal distress, and reduced muscle strength and impaired grasping power. He doesn’t rule out permanent lung damage in some. “Even asymptomatic individuals already show lung scarring on imaging,” he said.
The Mount Sinai program provides specialized interdisciplinary management that may include CT scans, endoscopy, and drugs such as respiratory medications or anticoagulants. It also offers training to combat the fatigue and deconditioning caused by the infection, symptoms that are not medically treatable but impact quality of life.
“These patients do get better, but I expect they may still have symptoms requiring monitoring after a year,” Dr. Chen said.
The study received no specific funding. Dr. Carfi and colleagues have disclosed no relevant financial relationships. Dr. Chen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Clinicians and researchers have focused on the acute phase of COVID-19 infection, but it’s increasingly clear that some recovered patients discharged from acute care need continued monitoring for long-lasting effects, a study has found.
In a research letter published online July 9 in JAMA, Angelo Carfi, MD, and colleagues from the Gemelli Against COVID-19 Post–Acute Care Study Group in Rome, report that
Postdischarge assessments of patients who met criteria for SARS-CoV-2 negativity, including a reverse transcriptase–polymerase chain reaction test, were conducted from April 21 to May 29. Among the results:
- Only 12.6% of the 143 patients were completely free of any COVID-19 symptom
- About 32% of patients had one or two symptoms and 55% had three or more
- None had fever or other signs and symptoms of acute illness
- About 53% of patients still had fatigue, 43.4% had dyspnea, 27.3% had joint pain, and had 21.7% chest pain
- About 44% reported worsened quality of life on the EuroQol visual analog scale.
The sample cohort, assessed in a COVID-19 patient service recently established at the Fondazione Policlinico Universitario Agostino Gemelli had a mean age of 56.5 years and 37% were women. The mean length of hospital stay was 13.5 days. During their hospitalization, 72.7% of patients showed evidence of interstitial pneumonia. Noninvasive ventilation was given to 14.7% of patients and 4.9% received invasive ventilation.
The reality of lingering symptoms has led Dr. Carfi’s clinic to schedule a final “wrap-up visit” for patients after full assessment. “On that occasion the doctor prescribes anything necessary to correct the anomalies found during the full evaluation,” Dr. Carfi, a geriatrician at the Gemelli clinic, said in an interview. “These usually include vitamin supplementation and, in selected cases, a new drug prescription such as a blood thinner if necessary.”
Patients can also enroll in a training program in which breathing status is monitored.
In North America, doctors are also addressing the reality that the road to recovery can be a long and upward one, with persistent symptoms worse than those seen with acute influenza infection. “We see patients who were first diagnosed in March or April and still have symptoms in July,” said Zijian Chen, MD, an endocrinologist and medical director of Mount Sinai Health System’s Center for Post-COVID Care in New York.
“Persistent symptoms are much worse for COVID patients than flu patients. Even flu patients who spent time in the intensive care unit recover fully, and we can optimize their breathing before discharge,” Dr. Chen said in an interview.
As in the Italian study, Dr. Chen sees patients with COVID-19 who have ongoing shortness of breath, some requiring supplemental oxygen, or with persistent chest pain on exertion, blood clotting problems, poor concentration, gastrointestinal distress, and reduced muscle strength and impaired grasping power. He doesn’t rule out permanent lung damage in some. “Even asymptomatic individuals already show lung scarring on imaging,” he said.
The Mount Sinai program provides specialized interdisciplinary management that may include CT scans, endoscopy, and drugs such as respiratory medications or anticoagulants. It also offers training to combat the fatigue and deconditioning caused by the infection, symptoms that are not medically treatable but impact quality of life.
“These patients do get better, but I expect they may still have symptoms requiring monitoring after a year,” Dr. Chen said.
The study received no specific funding. Dr. Carfi and colleagues have disclosed no relevant financial relationships. Dr. Chen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intubation boxes may do more harm than good in COVID-19 risk
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Wave, surge, or tsunami
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Different COVID-19 models and predicting inpatient bed capacity
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Hep C sofosbuvir/daclatasvir combo promising for COVID-19
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
Medical societies advise on vitamin D in midst of COVID-19
Six medical societies from across the globe are emphasizing the importance of individuals obtaining the daily recommended dose of vitamin D, especially given the impact of the COVID-19 pandemic on outdoor time.
The statement, “Joint Guidance on Vitamin D in the Era of COVID-19,” is supported by the American Society for Bone and Mineral Research, the Endocrine Society, and the American Association of Clinical Endocrinologists, among others.
They felt the need to clarify the recommendations for clinicians. Central to the guidance is the recommendation to directly expose the skin to sunlight for 15-30 minutes per day, while taking care to avoid sunburn.
The statement noted that “vitamin D is very safe when taken at reasonable dosages and is important for musculoskeletal health. Levels are likely to decline as individuals reduce outside activity (sun exposure) during the pandemic.”
It added that “most older and younger adults can safely take 400-1000 IU daily to keep vitamin D levels within the optimal range as recommended by [the US] Institute of Medicine guidelines.”
The statement also noted that the scientific evidence clearly supports the benefits that vitamin D (in combination with calcium intake) plays in building a strong skeleton and preventing bone loss.
Other societies supporting the statement are the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
What role for vitamin D in COVID-19?
Over recent months, the role of vitamin D in relation to prevention of COVID-19 has been the subject of intense debate. Now, these societies have joined forces and endorsed evidence-based guidance to clarify the issue around obtaining the daily recommended dosage of vitamin D.
During the pandemic, orders to stay at home meant individuals were likely to spend less time outdoors and have less opportunity to draw their vitamin D directly from sunlight, which is its main source, other than a limited number of foods or as a dietary supplement, the societies explained.
However, they acknowledged that the role of vitamin D in COVID-19 remains unclear.
“The current data do not provide any evidence that vitamin D supplementation will help prevent or treat COVID-19 infection; however, our guidance does not preclude further study of the potential effects of vitamin D on COVID-19,” the joint statement said.
Research to date suggests that vitamin D may play a role in enhancing the immune response, and given prior work demonstrating a role for the activated form of vitamin D – 1,25(OH)2D – in immune responses, “further research into vitamin D supplementation in COVID-19 disease is warranted,” it added. “Trials to date have been observational and there have been no randomized, controlled trials from which firm conclusions about causal relationships can be drawn. Observational studies suggest associations between low vitamin D concentrations and higher rates of COVID-19 infection.”
Medscape Medical News previously reported on the existing observational data regarding vitamin D in COVID-19. A recent rapid evidence review by the National Institute for Health and Care Excellence failed to find any evidence that vitamin D supplementation reduces the risk or severity of COVID-19.
A version of this article originally appeared on Medscape.com.
Six medical societies from across the globe are emphasizing the importance of individuals obtaining the daily recommended dose of vitamin D, especially given the impact of the COVID-19 pandemic on outdoor time.
The statement, “Joint Guidance on Vitamin D in the Era of COVID-19,” is supported by the American Society for Bone and Mineral Research, the Endocrine Society, and the American Association of Clinical Endocrinologists, among others.
They felt the need to clarify the recommendations for clinicians. Central to the guidance is the recommendation to directly expose the skin to sunlight for 15-30 minutes per day, while taking care to avoid sunburn.
The statement noted that “vitamin D is very safe when taken at reasonable dosages and is important for musculoskeletal health. Levels are likely to decline as individuals reduce outside activity (sun exposure) during the pandemic.”
It added that “most older and younger adults can safely take 400-1000 IU daily to keep vitamin D levels within the optimal range as recommended by [the US] Institute of Medicine guidelines.”
The statement also noted that the scientific evidence clearly supports the benefits that vitamin D (in combination with calcium intake) plays in building a strong skeleton and preventing bone loss.
Other societies supporting the statement are the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
What role for vitamin D in COVID-19?
Over recent months, the role of vitamin D in relation to prevention of COVID-19 has been the subject of intense debate. Now, these societies have joined forces and endorsed evidence-based guidance to clarify the issue around obtaining the daily recommended dosage of vitamin D.
During the pandemic, orders to stay at home meant individuals were likely to spend less time outdoors and have less opportunity to draw their vitamin D directly from sunlight, which is its main source, other than a limited number of foods or as a dietary supplement, the societies explained.
However, they acknowledged that the role of vitamin D in COVID-19 remains unclear.
“The current data do not provide any evidence that vitamin D supplementation will help prevent or treat COVID-19 infection; however, our guidance does not preclude further study of the potential effects of vitamin D on COVID-19,” the joint statement said.
Research to date suggests that vitamin D may play a role in enhancing the immune response, and given prior work demonstrating a role for the activated form of vitamin D – 1,25(OH)2D – in immune responses, “further research into vitamin D supplementation in COVID-19 disease is warranted,” it added. “Trials to date have been observational and there have been no randomized, controlled trials from which firm conclusions about causal relationships can be drawn. Observational studies suggest associations between low vitamin D concentrations and higher rates of COVID-19 infection.”
Medscape Medical News previously reported on the existing observational data regarding vitamin D in COVID-19. A recent rapid evidence review by the National Institute for Health and Care Excellence failed to find any evidence that vitamin D supplementation reduces the risk or severity of COVID-19.
A version of this article originally appeared on Medscape.com.
Six medical societies from across the globe are emphasizing the importance of individuals obtaining the daily recommended dose of vitamin D, especially given the impact of the COVID-19 pandemic on outdoor time.
The statement, “Joint Guidance on Vitamin D in the Era of COVID-19,” is supported by the American Society for Bone and Mineral Research, the Endocrine Society, and the American Association of Clinical Endocrinologists, among others.
They felt the need to clarify the recommendations for clinicians. Central to the guidance is the recommendation to directly expose the skin to sunlight for 15-30 minutes per day, while taking care to avoid sunburn.
The statement noted that “vitamin D is very safe when taken at reasonable dosages and is important for musculoskeletal health. Levels are likely to decline as individuals reduce outside activity (sun exposure) during the pandemic.”
It added that “most older and younger adults can safely take 400-1000 IU daily to keep vitamin D levels within the optimal range as recommended by [the US] Institute of Medicine guidelines.”
The statement also noted that the scientific evidence clearly supports the benefits that vitamin D (in combination with calcium intake) plays in building a strong skeleton and preventing bone loss.
Other societies supporting the statement are the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
What role for vitamin D in COVID-19?
Over recent months, the role of vitamin D in relation to prevention of COVID-19 has been the subject of intense debate. Now, these societies have joined forces and endorsed evidence-based guidance to clarify the issue around obtaining the daily recommended dosage of vitamin D.
During the pandemic, orders to stay at home meant individuals were likely to spend less time outdoors and have less opportunity to draw their vitamin D directly from sunlight, which is its main source, other than a limited number of foods or as a dietary supplement, the societies explained.
However, they acknowledged that the role of vitamin D in COVID-19 remains unclear.
“The current data do not provide any evidence that vitamin D supplementation will help prevent or treat COVID-19 infection; however, our guidance does not preclude further study of the potential effects of vitamin D on COVID-19,” the joint statement said.
Research to date suggests that vitamin D may play a role in enhancing the immune response, and given prior work demonstrating a role for the activated form of vitamin D – 1,25(OH)2D – in immune responses, “further research into vitamin D supplementation in COVID-19 disease is warranted,” it added. “Trials to date have been observational and there have been no randomized, controlled trials from which firm conclusions about causal relationships can be drawn. Observational studies suggest associations between low vitamin D concentrations and higher rates of COVID-19 infection.”
Medscape Medical News previously reported on the existing observational data regarding vitamin D in COVID-19. A recent rapid evidence review by the National Institute for Health and Care Excellence failed to find any evidence that vitamin D supplementation reduces the risk or severity of COVID-19.
A version of this article originally appeared on Medscape.com.