User login
QI initiative can decrease unnecessary IV treatment of asymptomatic hypertension
Background: Limited research suggests IV treatment of asymptomatic hypertension may be widespread and unhelpful. There is potential for unnecessary treatment to have adverse outcomes, such as hypotension.
Study design: Retrospective cohort study.
Setting: A single academic hospital.
Synopsis: Of 2,306 inpatients with asymptomatic hypertension, 11% were treated with IV medications to lower their blood pressure. Patients with indications for stricter blood pressure control (such as stroke, intracranial hemorrhage, aortic dissection) were excluded from the study. Following the baseline period, an education intervention was employed that included presentations, handouts, and posters. A second phase of quality improvement intervention included adjustment of the electronic medical record blood pressure alert parameters from more than 160/90 to more than 180/90. After these interventions, a lower percentage of patients received IV blood pressure medications for asymptomatic hypertension without a significant change in the number of rapid response calls, ICU transfers, or code blues. Limitations include that this is a single-center study and it is unclear if the performance improvement seen will be maintained over time.
Bottom line: IV antihypertensive use for asymptomatic hypertension is common despite lack of data to support its use, and reduced use is possible using quality improvement interventions.
Citation: Jacobs Z et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019 Mar;14(3):144-50.
Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
Background: Limited research suggests IV treatment of asymptomatic hypertension may be widespread and unhelpful. There is potential for unnecessary treatment to have adverse outcomes, such as hypotension.
Study design: Retrospective cohort study.
Setting: A single academic hospital.
Synopsis: Of 2,306 inpatients with asymptomatic hypertension, 11% were treated with IV medications to lower their blood pressure. Patients with indications for stricter blood pressure control (such as stroke, intracranial hemorrhage, aortic dissection) were excluded from the study. Following the baseline period, an education intervention was employed that included presentations, handouts, and posters. A second phase of quality improvement intervention included adjustment of the electronic medical record blood pressure alert parameters from more than 160/90 to more than 180/90. After these interventions, a lower percentage of patients received IV blood pressure medications for asymptomatic hypertension without a significant change in the number of rapid response calls, ICU transfers, or code blues. Limitations include that this is a single-center study and it is unclear if the performance improvement seen will be maintained over time.
Bottom line: IV antihypertensive use for asymptomatic hypertension is common despite lack of data to support its use, and reduced use is possible using quality improvement interventions.
Citation: Jacobs Z et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019 Mar;14(3):144-50.
Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
Background: Limited research suggests IV treatment of asymptomatic hypertension may be widespread and unhelpful. There is potential for unnecessary treatment to have adverse outcomes, such as hypotension.
Study design: Retrospective cohort study.
Setting: A single academic hospital.
Synopsis: Of 2,306 inpatients with asymptomatic hypertension, 11% were treated with IV medications to lower their blood pressure. Patients with indications for stricter blood pressure control (such as stroke, intracranial hemorrhage, aortic dissection) were excluded from the study. Following the baseline period, an education intervention was employed that included presentations, handouts, and posters. A second phase of quality improvement intervention included adjustment of the electronic medical record blood pressure alert parameters from more than 160/90 to more than 180/90. After these interventions, a lower percentage of patients received IV blood pressure medications for asymptomatic hypertension without a significant change in the number of rapid response calls, ICU transfers, or code blues. Limitations include that this is a single-center study and it is unclear if the performance improvement seen will be maintained over time.
Bottom line: IV antihypertensive use for asymptomatic hypertension is common despite lack of data to support its use, and reduced use is possible using quality improvement interventions.
Citation: Jacobs Z et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019 Mar;14(3):144-50.
Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
COVID-19-related inflammatory condition more common in black children in small study
More evidence has linked the Kawasaki-like multisystem inflammatory syndrome in children to COVID-19 and suggests that black children have a greater risk of the condition, according to a study published in the BMJ.
A small observational study in Paris found more than half of the 21 children who were admitted for the condition at the city’s pediatric hospital for COVID-19 patients were of African ancestry.
“The observation of a higher proportion of patients of African ancestry is consistent with recent findings, suggesting an effect of either social and living conditions or genetic susceptibility,” wrote Julie Toubiana, MD, PhD, of the University of Paris and the Pasteur Institute, and colleagues.
The findings did not surprise Edward M. Behrens, MD, chief of the division of rheumatology at Children’s Hospital of Philadelphia, whose institution has seen similar disparities that he attributes to social disadvantages.
“Infection rate will be higher in vulnerable populations that are less able to socially distance, have disproportionate numbers of essential workers, and have less access to health care and other resources,” Dr. Behrens said in an interview. “While there may be a role for genetics, environment – including social disparities – is almost certainly playing a role.”
Although the study’s small size is a limitation, he said, “the features described seem to mirror the experience of our center and what has been discussed more broadly amongst U.S. physicians.”
Byron Whyte, MD, a pediatrician in private practice in southeast Washington, found the differences in race interesting, but said the study was too small to draw any conclusions or generalize to the United States. But social disparities related to race are likely similar in France as they are in the United States, he said.
The prospective observational study assessed the clinical and demographic characteristics of all patients under age 18 who met the criteria for Kawasaki disease and were admitted between April 27 and May 20 to the Necker Hospital for Sick Children in Paris.
The 21 children had an average age of 8 years (ranging from 3 to 16), and 57% had at least one parent from sub-Saharan Africa or a Caribbean island; 14% had parents from Asia (two from China and one from Sri Lanka). The authors noted in their discussion that past U.S. and U.K. studies of Kawasaki disease have found a 2.5 times greater risk in Asian-American children and 1.5 times greater risk in African-American children compared with children with European ancestry.
Most of the patients (81%) needed intensive care, with 57% presenting with Kawasaki disease shock syndrome and 67% with myocarditis. Dr. Toubiana and associates also noted that “gastrointestinal symptoms were also unusually common, affecting all of our 21 patients.”
Only nine of the children reported having symptoms of a viral-like illness when they were admitted, primarily headache, cough, coryza, and fever, plus anosmia in one child. Among those children, the Kawasaki symptoms began a median 45 days after onset of the viral symptoms (range 18-79 days).
Only two children showed no positive test result for current COVID-19 infection or antibodies. Eight (38%) of the children had positive PCR tests for SARS-CoV2, and 19 (90%) had positive tests for IgG antibodies. The two patients with both negative tests did not require intensive care and did not have myocarditis.
About half the patients (52%) met all the criteria of Kawasaki disease, and the other 10 had “incomplete Kawasaki disease.” The most common Kawasaki symptoms were the polymorphous skin rash, occurring in 76% of the patients, changes to the lips and oral cavity (76%), and bilateral bulbar conjunctival injection (81%). Three patients (14%) had pleural effusion, and 10 of them (48%) had pericardial effusion, Dr. Toubiana and associates reported.
But Dr. Behrens said he disagrees with the assertion that the illness described in the paper and what he is seeing at Children’s Hospital of Philadelphia is related to Kawasaki disease.
“Most experts here in the U.S. seem to agree this is not Kawasaki disease, but a distinct clinical syndrome called multisystem inflammatory syndrome in children, or MIS-C, that seems to have some overlap with the most nonspecific features of Kawasaki disease,” said Dr. Behrens, who is the Joseph Lee Hollander Chair in Pediatric Rheumatology at Children’s Hospital of Philadelphia. He has coauthored a study currently under review and available as a preprint soon that examines the biologic mechanisms underlying MIS-C.
Neither Dr. Behrens nor Dr. Whyte believed the findings had clinical implications that might change practice, but Dr. Whyte said he will be paying closer attention to the black children he treats – 99% of his practice – who are recovering from COVID-19.
“And, because we know that the concerns of African Americans are often overlooked in health care,” Dr. Whyte said, physicians should “pay a little more attention to symptom reporting on those kids, since there is a possibility that those kids would need hospitalization.”
All the patients in the study were treated with intravenous immunoglobulin, and corticosteroids were administered to 10 of them (48%). Their median hospital stay was 8 days (5 days in intensive care), and all were discharged without any deaths.
“Only one patient had symptoms suggestive of acute covid-19 and most had positive serum test results for IgG antibodies, suggesting that the development of Kawasaki disease in these patients is more likely to be the result of a postviral immunological reaction,” Dr. Toubiana and associates said.
The research received no external funding, and neither the authors nor other quoted physicians had any relevant financial disclosures.
SOURCE: Toubiana J et al. BMJ. 2020 Jun 3, doi: 10.1136 bmj.m2094.
More evidence has linked the Kawasaki-like multisystem inflammatory syndrome in children to COVID-19 and suggests that black children have a greater risk of the condition, according to a study published in the BMJ.
A small observational study in Paris found more than half of the 21 children who were admitted for the condition at the city’s pediatric hospital for COVID-19 patients were of African ancestry.
“The observation of a higher proportion of patients of African ancestry is consistent with recent findings, suggesting an effect of either social and living conditions or genetic susceptibility,” wrote Julie Toubiana, MD, PhD, of the University of Paris and the Pasteur Institute, and colleagues.
The findings did not surprise Edward M. Behrens, MD, chief of the division of rheumatology at Children’s Hospital of Philadelphia, whose institution has seen similar disparities that he attributes to social disadvantages.
“Infection rate will be higher in vulnerable populations that are less able to socially distance, have disproportionate numbers of essential workers, and have less access to health care and other resources,” Dr. Behrens said in an interview. “While there may be a role for genetics, environment – including social disparities – is almost certainly playing a role.”
Although the study’s small size is a limitation, he said, “the features described seem to mirror the experience of our center and what has been discussed more broadly amongst U.S. physicians.”
Byron Whyte, MD, a pediatrician in private practice in southeast Washington, found the differences in race interesting, but said the study was too small to draw any conclusions or generalize to the United States. But social disparities related to race are likely similar in France as they are in the United States, he said.
The prospective observational study assessed the clinical and demographic characteristics of all patients under age 18 who met the criteria for Kawasaki disease and were admitted between April 27 and May 20 to the Necker Hospital for Sick Children in Paris.
The 21 children had an average age of 8 years (ranging from 3 to 16), and 57% had at least one parent from sub-Saharan Africa or a Caribbean island; 14% had parents from Asia (two from China and one from Sri Lanka). The authors noted in their discussion that past U.S. and U.K. studies of Kawasaki disease have found a 2.5 times greater risk in Asian-American children and 1.5 times greater risk in African-American children compared with children with European ancestry.
Most of the patients (81%) needed intensive care, with 57% presenting with Kawasaki disease shock syndrome and 67% with myocarditis. Dr. Toubiana and associates also noted that “gastrointestinal symptoms were also unusually common, affecting all of our 21 patients.”
Only nine of the children reported having symptoms of a viral-like illness when they were admitted, primarily headache, cough, coryza, and fever, plus anosmia in one child. Among those children, the Kawasaki symptoms began a median 45 days after onset of the viral symptoms (range 18-79 days).
Only two children showed no positive test result for current COVID-19 infection or antibodies. Eight (38%) of the children had positive PCR tests for SARS-CoV2, and 19 (90%) had positive tests for IgG antibodies. The two patients with both negative tests did not require intensive care and did not have myocarditis.
About half the patients (52%) met all the criteria of Kawasaki disease, and the other 10 had “incomplete Kawasaki disease.” The most common Kawasaki symptoms were the polymorphous skin rash, occurring in 76% of the patients, changes to the lips and oral cavity (76%), and bilateral bulbar conjunctival injection (81%). Three patients (14%) had pleural effusion, and 10 of them (48%) had pericardial effusion, Dr. Toubiana and associates reported.
But Dr. Behrens said he disagrees with the assertion that the illness described in the paper and what he is seeing at Children’s Hospital of Philadelphia is related to Kawasaki disease.
“Most experts here in the U.S. seem to agree this is not Kawasaki disease, but a distinct clinical syndrome called multisystem inflammatory syndrome in children, or MIS-C, that seems to have some overlap with the most nonspecific features of Kawasaki disease,” said Dr. Behrens, who is the Joseph Lee Hollander Chair in Pediatric Rheumatology at Children’s Hospital of Philadelphia. He has coauthored a study currently under review and available as a preprint soon that examines the biologic mechanisms underlying MIS-C.
Neither Dr. Behrens nor Dr. Whyte believed the findings had clinical implications that might change practice, but Dr. Whyte said he will be paying closer attention to the black children he treats – 99% of his practice – who are recovering from COVID-19.
“And, because we know that the concerns of African Americans are often overlooked in health care,” Dr. Whyte said, physicians should “pay a little more attention to symptom reporting on those kids, since there is a possibility that those kids would need hospitalization.”
All the patients in the study were treated with intravenous immunoglobulin, and corticosteroids were administered to 10 of them (48%). Their median hospital stay was 8 days (5 days in intensive care), and all were discharged without any deaths.
“Only one patient had symptoms suggestive of acute covid-19 and most had positive serum test results for IgG antibodies, suggesting that the development of Kawasaki disease in these patients is more likely to be the result of a postviral immunological reaction,” Dr. Toubiana and associates said.
The research received no external funding, and neither the authors nor other quoted physicians had any relevant financial disclosures.
SOURCE: Toubiana J et al. BMJ. 2020 Jun 3, doi: 10.1136 bmj.m2094.
More evidence has linked the Kawasaki-like multisystem inflammatory syndrome in children to COVID-19 and suggests that black children have a greater risk of the condition, according to a study published in the BMJ.
A small observational study in Paris found more than half of the 21 children who were admitted for the condition at the city’s pediatric hospital for COVID-19 patients were of African ancestry.
“The observation of a higher proportion of patients of African ancestry is consistent with recent findings, suggesting an effect of either social and living conditions or genetic susceptibility,” wrote Julie Toubiana, MD, PhD, of the University of Paris and the Pasteur Institute, and colleagues.
The findings did not surprise Edward M. Behrens, MD, chief of the division of rheumatology at Children’s Hospital of Philadelphia, whose institution has seen similar disparities that he attributes to social disadvantages.
“Infection rate will be higher in vulnerable populations that are less able to socially distance, have disproportionate numbers of essential workers, and have less access to health care and other resources,” Dr. Behrens said in an interview. “While there may be a role for genetics, environment – including social disparities – is almost certainly playing a role.”
Although the study’s small size is a limitation, he said, “the features described seem to mirror the experience of our center and what has been discussed more broadly amongst U.S. physicians.”
Byron Whyte, MD, a pediatrician in private practice in southeast Washington, found the differences in race interesting, but said the study was too small to draw any conclusions or generalize to the United States. But social disparities related to race are likely similar in France as they are in the United States, he said.
The prospective observational study assessed the clinical and demographic characteristics of all patients under age 18 who met the criteria for Kawasaki disease and were admitted between April 27 and May 20 to the Necker Hospital for Sick Children in Paris.
The 21 children had an average age of 8 years (ranging from 3 to 16), and 57% had at least one parent from sub-Saharan Africa or a Caribbean island; 14% had parents from Asia (two from China and one from Sri Lanka). The authors noted in their discussion that past U.S. and U.K. studies of Kawasaki disease have found a 2.5 times greater risk in Asian-American children and 1.5 times greater risk in African-American children compared with children with European ancestry.
Most of the patients (81%) needed intensive care, with 57% presenting with Kawasaki disease shock syndrome and 67% with myocarditis. Dr. Toubiana and associates also noted that “gastrointestinal symptoms were also unusually common, affecting all of our 21 patients.”
Only nine of the children reported having symptoms of a viral-like illness when they were admitted, primarily headache, cough, coryza, and fever, plus anosmia in one child. Among those children, the Kawasaki symptoms began a median 45 days after onset of the viral symptoms (range 18-79 days).
Only two children showed no positive test result for current COVID-19 infection or antibodies. Eight (38%) of the children had positive PCR tests for SARS-CoV2, and 19 (90%) had positive tests for IgG antibodies. The two patients with both negative tests did not require intensive care and did not have myocarditis.
About half the patients (52%) met all the criteria of Kawasaki disease, and the other 10 had “incomplete Kawasaki disease.” The most common Kawasaki symptoms were the polymorphous skin rash, occurring in 76% of the patients, changes to the lips and oral cavity (76%), and bilateral bulbar conjunctival injection (81%). Three patients (14%) had pleural effusion, and 10 of them (48%) had pericardial effusion, Dr. Toubiana and associates reported.
But Dr. Behrens said he disagrees with the assertion that the illness described in the paper and what he is seeing at Children’s Hospital of Philadelphia is related to Kawasaki disease.
“Most experts here in the U.S. seem to agree this is not Kawasaki disease, but a distinct clinical syndrome called multisystem inflammatory syndrome in children, or MIS-C, that seems to have some overlap with the most nonspecific features of Kawasaki disease,” said Dr. Behrens, who is the Joseph Lee Hollander Chair in Pediatric Rheumatology at Children’s Hospital of Philadelphia. He has coauthored a study currently under review and available as a preprint soon that examines the biologic mechanisms underlying MIS-C.
Neither Dr. Behrens nor Dr. Whyte believed the findings had clinical implications that might change practice, but Dr. Whyte said he will be paying closer attention to the black children he treats – 99% of his practice – who are recovering from COVID-19.
“And, because we know that the concerns of African Americans are often overlooked in health care,” Dr. Whyte said, physicians should “pay a little more attention to symptom reporting on those kids, since there is a possibility that those kids would need hospitalization.”
All the patients in the study were treated with intravenous immunoglobulin, and corticosteroids were administered to 10 of them (48%). Their median hospital stay was 8 days (5 days in intensive care), and all were discharged without any deaths.
“Only one patient had symptoms suggestive of acute covid-19 and most had positive serum test results for IgG antibodies, suggesting that the development of Kawasaki disease in these patients is more likely to be the result of a postviral immunological reaction,” Dr. Toubiana and associates said.
The research received no external funding, and neither the authors nor other quoted physicians had any relevant financial disclosures.
SOURCE: Toubiana J et al. BMJ. 2020 Jun 3, doi: 10.1136 bmj.m2094.
FROM BMJ
COVID-19 neurologic effects: Does the virus directly attack the brain?
A new review article summarizes what is known so far, and what clinicians need to look out for.
“We frequently see neurological conditions in people with COVID-19, but we understand very little about these effects. Is it the virus entering the brain/nerves or are they a result of a general inflammation or immune response – a bystander effect of people being severely ill. It is probably a combination of both,” said senior author Serena Spudich, MD, Gilbert H. Glaser Professor of Neurology; division chief of neurological infections & global neurology; and codirector of the Center for Neuroepidemiology and Clinical Neurological Research at Yale University, New Haven, Conn.
“Our message is that there are fairly frequent neurological sequelae of COVID-19 and we need to be alert to these, and to try to understand the potential long-term consequences,” she said.
The review was published online May 29 in JAMA Neurology.
Brain changes linked to loss of smell
In a separate article also published online in JAMA Neurology the same day, an Italian group describes a COVID-19 patient with anosmia (loss of sense of smell) who showed brain abnormalities on MRI in the areas associated with smell – the right gyrus rectus and the olfactory bulbs. These changes were resolved on later scan and the patient recovered her sense of smell.
“Based on the MRI findings, we can speculate that SARS-CoV-2 might invade the brain through the olfactory pathway,” conclude the researchers, led by first author Letterio S. Politi, MD, of the department of neuroradiology at IRCCS Istituto Clinico Humanitas and Humanitas University, Milan, Italy.
Can coronaviruses enter the CNS?
Dr. Spudich described this case report as “compelling evidence suggesting that loss of smell is a neurologic effect.”
“Loss of smell and/or taste is a common symptom in COVID-19, so this may suggest that an awful lot of people have some neurological involvement,” Dr. Spudich commented. “While a transient loss of smell or taste is not serious, if the virus has infected brain tissue the question is could this then spread to other parts of the brain and cause other more serious neurological effects,” she added.
In their review article, Dr. Spudich and colleagues present evidence showing that coronaviruses can enter the CNS.
“We know that SARS-1 and MERS have been shown to enter the nervous system and several coronaviruses have been shown to cause direct brain effects,” she said. “There is also some evidence that SARS-CoV-2 can do this too. As well as these latest MRI findings linked to loss of smell, there is a report of the virus being found in endothelial cells in the brain and a French autopsy study has also detected virus in the brain.”
Complications of other systemic effects?
Dr. Spudich is a neurologist specializing in neurologic consequences of infectious disease. “We don’t normally have such vast numbers of patients but in the last 3 months there has been an avalanche,” she says. From her personal experience, she believes the majority of neurologic symptoms in COVID-19 patients are most probably complications of other systemic effects, such as kidney, heart, or liver problems. But there is likely also a direct viral effect on the CNS in some patients.
“Reports from China suggested that serious neurologic effects were present in about one-third of hospitalized COVID-19 patients. I would say in our experience the figure would be less than that – maybe around 10%,” she noted.
Some COVID-19 patients are presenting with primary neurologic symptoms. For example, an elderly person may first develop confusion rather than a cough or shortness of breath; others have had severe headache as an initial COVID-19 symptom, Dr. Spudich reported. “Medical staff need to be aware of this – a severe headache in a patient who doesn’t normally get headaches could be a sign of the virus.”
Some of the neurologic symptoms could be caused by autoimmunity. Dr. Spudich explained that, in acute HIV infection a small proportion of patients can first present with autoimmune neurologic effects such as Guillain-Barré syndrome, an autoimmune condition of the nerves which causes a tingling sensation in the hands and feet. “This is well described in HIV, but we are also now seeing this in COVID-19 patients too,” she said. “A panoply of conditions can be caused by autoimmunity.”
On the increase in strokes that has been reported in COVID-19 patients, Dr. Spudich said, “this could be due to direct effects of the virus (e.g., causing an increase in coagulation or infecting the endothelial cells in the brain) or it could just be the final trigger for patients who were at risk of stroke anyway.”
There have been some very high-profile reports of younger patients with major strokes, she said, “but we haven’t seen that in our hospital. For the most part in my experience, strokes are happening in older COVID-19 patients with stroke risk factors such as AF [atrial fibrillation], hypertension, and diabetes. We haven’t seen a preponderance of strokes in young, otherwise healthy people.”
Even in patients who have neurologic effects as the first sign of COVID-19 infection, it is not known whether these symptoms are caused directly by the virus.
“We know that flu can cause people to have headaches, but that is because of an increase in inflammatory cytokines. On the other hand, patients with acute HIV infection often have headaches as a result of the virus getting into the brain. We don’t know where in this [cluster] COVID-19 virus falls,” Dr. Spudich said.
Much is still unknown
“The information we have is very sparse at this point. We need far more systematic information on this from CSF samples and imaging.” Dr. Spudich urged clinicians to try to collect such information in patients with neurologic symptoms.
Acknowledging that fewer such tests are being done at present because of concerns over infection risk, Dr. Spudich suggested that some changes in procedure may help. “In our hospital we have a portable MRI scanner which can be brought to the patient. This means the patient does not have to move across the hospital for a scan. This helps us to decide whether the patient has had a stroke, which can be missed when patients are on a ventilator.”
It is also unclear whether the neurologic effects seen during COVID-19 infection will last long term.
Dr. Spudich noted that there have been reports of COVID-19 patients discharged from intensive care having difficulty with higher cognitive function for some time thereafter. “This can happen after being in ICU but is it more pronounced in COVID-19 patients? An ongoing study is underway to look at this,” she said.
This article first appeared on Medscape.com.
A new review article summarizes what is known so far, and what clinicians need to look out for.
“We frequently see neurological conditions in people with COVID-19, but we understand very little about these effects. Is it the virus entering the brain/nerves or are they a result of a general inflammation or immune response – a bystander effect of people being severely ill. It is probably a combination of both,” said senior author Serena Spudich, MD, Gilbert H. Glaser Professor of Neurology; division chief of neurological infections & global neurology; and codirector of the Center for Neuroepidemiology and Clinical Neurological Research at Yale University, New Haven, Conn.
“Our message is that there are fairly frequent neurological sequelae of COVID-19 and we need to be alert to these, and to try to understand the potential long-term consequences,” she said.
The review was published online May 29 in JAMA Neurology.
Brain changes linked to loss of smell
In a separate article also published online in JAMA Neurology the same day, an Italian group describes a COVID-19 patient with anosmia (loss of sense of smell) who showed brain abnormalities on MRI in the areas associated with smell – the right gyrus rectus and the olfactory bulbs. These changes were resolved on later scan and the patient recovered her sense of smell.
“Based on the MRI findings, we can speculate that SARS-CoV-2 might invade the brain through the olfactory pathway,” conclude the researchers, led by first author Letterio S. Politi, MD, of the department of neuroradiology at IRCCS Istituto Clinico Humanitas and Humanitas University, Milan, Italy.
Can coronaviruses enter the CNS?
Dr. Spudich described this case report as “compelling evidence suggesting that loss of smell is a neurologic effect.”
“Loss of smell and/or taste is a common symptom in COVID-19, so this may suggest that an awful lot of people have some neurological involvement,” Dr. Spudich commented. “While a transient loss of smell or taste is not serious, if the virus has infected brain tissue the question is could this then spread to other parts of the brain and cause other more serious neurological effects,” she added.
In their review article, Dr. Spudich and colleagues present evidence showing that coronaviruses can enter the CNS.
“We know that SARS-1 and MERS have been shown to enter the nervous system and several coronaviruses have been shown to cause direct brain effects,” she said. “There is also some evidence that SARS-CoV-2 can do this too. As well as these latest MRI findings linked to loss of smell, there is a report of the virus being found in endothelial cells in the brain and a French autopsy study has also detected virus in the brain.”
Complications of other systemic effects?
Dr. Spudich is a neurologist specializing in neurologic consequences of infectious disease. “We don’t normally have such vast numbers of patients but in the last 3 months there has been an avalanche,” she says. From her personal experience, she believes the majority of neurologic symptoms in COVID-19 patients are most probably complications of other systemic effects, such as kidney, heart, or liver problems. But there is likely also a direct viral effect on the CNS in some patients.
“Reports from China suggested that serious neurologic effects were present in about one-third of hospitalized COVID-19 patients. I would say in our experience the figure would be less than that – maybe around 10%,” she noted.
Some COVID-19 patients are presenting with primary neurologic symptoms. For example, an elderly person may first develop confusion rather than a cough or shortness of breath; others have had severe headache as an initial COVID-19 symptom, Dr. Spudich reported. “Medical staff need to be aware of this – a severe headache in a patient who doesn’t normally get headaches could be a sign of the virus.”
Some of the neurologic symptoms could be caused by autoimmunity. Dr. Spudich explained that, in acute HIV infection a small proportion of patients can first present with autoimmune neurologic effects such as Guillain-Barré syndrome, an autoimmune condition of the nerves which causes a tingling sensation in the hands and feet. “This is well described in HIV, but we are also now seeing this in COVID-19 patients too,” she said. “A panoply of conditions can be caused by autoimmunity.”
On the increase in strokes that has been reported in COVID-19 patients, Dr. Spudich said, “this could be due to direct effects of the virus (e.g., causing an increase in coagulation or infecting the endothelial cells in the brain) or it could just be the final trigger for patients who were at risk of stroke anyway.”
There have been some very high-profile reports of younger patients with major strokes, she said, “but we haven’t seen that in our hospital. For the most part in my experience, strokes are happening in older COVID-19 patients with stroke risk factors such as AF [atrial fibrillation], hypertension, and diabetes. We haven’t seen a preponderance of strokes in young, otherwise healthy people.”
Even in patients who have neurologic effects as the first sign of COVID-19 infection, it is not known whether these symptoms are caused directly by the virus.
“We know that flu can cause people to have headaches, but that is because of an increase in inflammatory cytokines. On the other hand, patients with acute HIV infection often have headaches as a result of the virus getting into the brain. We don’t know where in this [cluster] COVID-19 virus falls,” Dr. Spudich said.
Much is still unknown
“The information we have is very sparse at this point. We need far more systematic information on this from CSF samples and imaging.” Dr. Spudich urged clinicians to try to collect such information in patients with neurologic symptoms.
Acknowledging that fewer such tests are being done at present because of concerns over infection risk, Dr. Spudich suggested that some changes in procedure may help. “In our hospital we have a portable MRI scanner which can be brought to the patient. This means the patient does not have to move across the hospital for a scan. This helps us to decide whether the patient has had a stroke, which can be missed when patients are on a ventilator.”
It is also unclear whether the neurologic effects seen during COVID-19 infection will last long term.
Dr. Spudich noted that there have been reports of COVID-19 patients discharged from intensive care having difficulty with higher cognitive function for some time thereafter. “This can happen after being in ICU but is it more pronounced in COVID-19 patients? An ongoing study is underway to look at this,” she said.
This article first appeared on Medscape.com.
A new review article summarizes what is known so far, and what clinicians need to look out for.
“We frequently see neurological conditions in people with COVID-19, but we understand very little about these effects. Is it the virus entering the brain/nerves or are they a result of a general inflammation or immune response – a bystander effect of people being severely ill. It is probably a combination of both,” said senior author Serena Spudich, MD, Gilbert H. Glaser Professor of Neurology; division chief of neurological infections & global neurology; and codirector of the Center for Neuroepidemiology and Clinical Neurological Research at Yale University, New Haven, Conn.
“Our message is that there are fairly frequent neurological sequelae of COVID-19 and we need to be alert to these, and to try to understand the potential long-term consequences,” she said.
The review was published online May 29 in JAMA Neurology.
Brain changes linked to loss of smell
In a separate article also published online in JAMA Neurology the same day, an Italian group describes a COVID-19 patient with anosmia (loss of sense of smell) who showed brain abnormalities on MRI in the areas associated with smell – the right gyrus rectus and the olfactory bulbs. These changes were resolved on later scan and the patient recovered her sense of smell.
“Based on the MRI findings, we can speculate that SARS-CoV-2 might invade the brain through the olfactory pathway,” conclude the researchers, led by first author Letterio S. Politi, MD, of the department of neuroradiology at IRCCS Istituto Clinico Humanitas and Humanitas University, Milan, Italy.
Can coronaviruses enter the CNS?
Dr. Spudich described this case report as “compelling evidence suggesting that loss of smell is a neurologic effect.”
“Loss of smell and/or taste is a common symptom in COVID-19, so this may suggest that an awful lot of people have some neurological involvement,” Dr. Spudich commented. “While a transient loss of smell or taste is not serious, if the virus has infected brain tissue the question is could this then spread to other parts of the brain and cause other more serious neurological effects,” she added.
In their review article, Dr. Spudich and colleagues present evidence showing that coronaviruses can enter the CNS.
“We know that SARS-1 and MERS have been shown to enter the nervous system and several coronaviruses have been shown to cause direct brain effects,” she said. “There is also some evidence that SARS-CoV-2 can do this too. As well as these latest MRI findings linked to loss of smell, there is a report of the virus being found in endothelial cells in the brain and a French autopsy study has also detected virus in the brain.”
Complications of other systemic effects?
Dr. Spudich is a neurologist specializing in neurologic consequences of infectious disease. “We don’t normally have such vast numbers of patients but in the last 3 months there has been an avalanche,” she says. From her personal experience, she believes the majority of neurologic symptoms in COVID-19 patients are most probably complications of other systemic effects, such as kidney, heart, or liver problems. But there is likely also a direct viral effect on the CNS in some patients.
“Reports from China suggested that serious neurologic effects were present in about one-third of hospitalized COVID-19 patients. I would say in our experience the figure would be less than that – maybe around 10%,” she noted.
Some COVID-19 patients are presenting with primary neurologic symptoms. For example, an elderly person may first develop confusion rather than a cough or shortness of breath; others have had severe headache as an initial COVID-19 symptom, Dr. Spudich reported. “Medical staff need to be aware of this – a severe headache in a patient who doesn’t normally get headaches could be a sign of the virus.”
Some of the neurologic symptoms could be caused by autoimmunity. Dr. Spudich explained that, in acute HIV infection a small proportion of patients can first present with autoimmune neurologic effects such as Guillain-Barré syndrome, an autoimmune condition of the nerves which causes a tingling sensation in the hands and feet. “This is well described in HIV, but we are also now seeing this in COVID-19 patients too,” she said. “A panoply of conditions can be caused by autoimmunity.”
On the increase in strokes that has been reported in COVID-19 patients, Dr. Spudich said, “this could be due to direct effects of the virus (e.g., causing an increase in coagulation or infecting the endothelial cells in the brain) or it could just be the final trigger for patients who were at risk of stroke anyway.”
There have been some very high-profile reports of younger patients with major strokes, she said, “but we haven’t seen that in our hospital. For the most part in my experience, strokes are happening in older COVID-19 patients with stroke risk factors such as AF [atrial fibrillation], hypertension, and diabetes. We haven’t seen a preponderance of strokes in young, otherwise healthy people.”
Even in patients who have neurologic effects as the first sign of COVID-19 infection, it is not known whether these symptoms are caused directly by the virus.
“We know that flu can cause people to have headaches, but that is because of an increase in inflammatory cytokines. On the other hand, patients with acute HIV infection often have headaches as a result of the virus getting into the brain. We don’t know where in this [cluster] COVID-19 virus falls,” Dr. Spudich said.
Much is still unknown
“The information we have is very sparse at this point. We need far more systematic information on this from CSF samples and imaging.” Dr. Spudich urged clinicians to try to collect such information in patients with neurologic symptoms.
Acknowledging that fewer such tests are being done at present because of concerns over infection risk, Dr. Spudich suggested that some changes in procedure may help. “In our hospital we have a portable MRI scanner which can be brought to the patient. This means the patient does not have to move across the hospital for a scan. This helps us to decide whether the patient has had a stroke, which can be missed when patients are on a ventilator.”
It is also unclear whether the neurologic effects seen during COVID-19 infection will last long term.
Dr. Spudich noted that there have been reports of COVID-19 patients discharged from intensive care having difficulty with higher cognitive function for some time thereafter. “This can happen after being in ICU but is it more pronounced in COVID-19 patients? An ongoing study is underway to look at this,” she said.
This article first appeared on Medscape.com.
FDA approves new antibiotic for HABP/VABP treatment
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
Lancet, NEJM retract studies on hydroxychloroquine for COVID-19
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
Should healthcare workers wear masks at home?
Wearing a mask at home, even when everyone is feeling fine, might reduce the risk of frontline healthcare workers transmitting SARS-CoV-2 infection to their families, a recent study from China suggests. But the benefits might not outweigh the costs, according to several physicians interviewed.
“My gut reaction is that home mask use for healthcare workers would place an inordinately high burden on those healthcare workers and their families,” said Jeanne Noble, MD, an emergency care physician at the University of California, San Francisco. “Wearing a mask for a 10-hour shift already represents significant physical discomfort, causing sores across the nose and behind the ears. The emotional toll of the physical distance that comes with mask use, with limited facial expression, is also quite real.”
The suggested benefit of home mask use comes from research published online May 28 in BMJ Global Health. To assess predictors of household transmission of SARS-CoV-2 infection, Yu Wang, MD, of the Beijing Center for Disease Prevention and Control and colleagues conducted a retrospective study of 124 families in Beijing in which there was a confirmed case of COVID-19 as of February 21. The researchers surveyed family members by telephone about household hygiene and behaviors during the pandemic to examine risk factors for transmission.
During the 2 weeks following onset of the primary case, secondary transmission occurred in 41 families. Overall, 77 of 335 family members developed COVID-19.
A multivariable logistic regression analysis found that in households in which family members wore masks at home before the first person became ill, there was less likelihood of transmission of disease to a family member, compared with families in which no one wore a mask prior to illness onset.
“Facemasks were 79% effective and disinfection was 77% effective in preventing transmission,” the researchers report, “whilst close frequent contact in the household increased the risk of transmission 18 times, and diarrhea in the index patient increased the risk by four times.
However, wearing masks after symptom onset was not protective, according to the analysis. The findings support “universal face mask use, and also provides guidance on risk reduction for families living with someone in quarantine or isolation, and families of health workers, who may face ongoing risk,” the authors write.
Still, other precautions may be more important, experts say.
“I think by far the best way for healthcare professionals to protect their families is to carefully employ appropriate infection prevention measures at work,” said Mark E. Rupp, MD, chief of the Division of Infectious Diseases at Nebraska Medical Center in Omaha. “The combination of administrative interventions, engineering improvements, and personal protective equipment is very effective in preventing SARS-CoV-2 acquisition in the workplace.”
Many physicians already wear masks at home, and this study “only reemphasized the importance of doing so,” said Raghavendra Tirupathi, MD, medical director of Keystone Infectious Diseases in Chambersburg, Pennsylvania, who recently reviewed studies about masks and COVID-19.
Home mask use provides “one more layer of protection that might help mitigate the risk of transmission to family members,” Tirupathi said. But it does not obviate the need to follow other preventive measures, such as social distancing and proper hygiene.
But Rupp, whose advice on how healthcare workers can protect their families was recently highlighted by the American Medical Association, isn’t convinced. He said he won’t be adding home mask use to his list of recommendations. “It would be intrusive, cumbersome, and impractical to wear a mask in the home setting,” Rupp said in an interview.
However, when out in the community, all family members must protect one another by practicing social distancing, wearing masks, and practicing proper hand hygiene. “I also think that it is a good idea to have some masks on hand in case anyone does develop symptoms in the household and to wear them if a family member falls ill ― at least until testing can confirm COVID-19,” Rupp said. “If a family member does fall ill, masks for the ill person as well as the well persons would be indicated along with other home quarantine measures.”
For her part, Noble, who has provided guidance about proper mask use, said that targeted use of masks at home, such as around older visiting relatives or other more vulnerable family members, may be more realistic than continuous in-home use.
When a household member becomes ill, recommendations for preventing disease spread include having a sick family member sleep in a separate bedroom, using a separate bathroom, and wearing a mask when within 6 feet of other household members. They also should avoid sharing meals. “For a household member who is a medical provider, to follow these self-isolation precautions while at home for months on end would have a significant emotional toll,” Noble said in an email. “With no end in sight for the pandemic, perpetual mask use in both the private and public sphere strikes me as overwhelming ― I write this near the end of my 10-hour shift wearing both an N95 and surgical mask and counting the minutes before I can take them off!”
A limitation of the study was its reliance on telephone interviews, which are subject to recall bias, the authors note.
The study was funded by the Beijing Science and Technology Planning Project. The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Wearing a mask at home, even when everyone is feeling fine, might reduce the risk of frontline healthcare workers transmitting SARS-CoV-2 infection to their families, a recent study from China suggests. But the benefits might not outweigh the costs, according to several physicians interviewed.
“My gut reaction is that home mask use for healthcare workers would place an inordinately high burden on those healthcare workers and their families,” said Jeanne Noble, MD, an emergency care physician at the University of California, San Francisco. “Wearing a mask for a 10-hour shift already represents significant physical discomfort, causing sores across the nose and behind the ears. The emotional toll of the physical distance that comes with mask use, with limited facial expression, is also quite real.”
The suggested benefit of home mask use comes from research published online May 28 in BMJ Global Health. To assess predictors of household transmission of SARS-CoV-2 infection, Yu Wang, MD, of the Beijing Center for Disease Prevention and Control and colleagues conducted a retrospective study of 124 families in Beijing in which there was a confirmed case of COVID-19 as of February 21. The researchers surveyed family members by telephone about household hygiene and behaviors during the pandemic to examine risk factors for transmission.
During the 2 weeks following onset of the primary case, secondary transmission occurred in 41 families. Overall, 77 of 335 family members developed COVID-19.
A multivariable logistic regression analysis found that in households in which family members wore masks at home before the first person became ill, there was less likelihood of transmission of disease to a family member, compared with families in which no one wore a mask prior to illness onset.
“Facemasks were 79% effective and disinfection was 77% effective in preventing transmission,” the researchers report, “whilst close frequent contact in the household increased the risk of transmission 18 times, and diarrhea in the index patient increased the risk by four times.
However, wearing masks after symptom onset was not protective, according to the analysis. The findings support “universal face mask use, and also provides guidance on risk reduction for families living with someone in quarantine or isolation, and families of health workers, who may face ongoing risk,” the authors write.
Still, other precautions may be more important, experts say.
“I think by far the best way for healthcare professionals to protect their families is to carefully employ appropriate infection prevention measures at work,” said Mark E. Rupp, MD, chief of the Division of Infectious Diseases at Nebraska Medical Center in Omaha. “The combination of administrative interventions, engineering improvements, and personal protective equipment is very effective in preventing SARS-CoV-2 acquisition in the workplace.”
Many physicians already wear masks at home, and this study “only reemphasized the importance of doing so,” said Raghavendra Tirupathi, MD, medical director of Keystone Infectious Diseases in Chambersburg, Pennsylvania, who recently reviewed studies about masks and COVID-19.
Home mask use provides “one more layer of protection that might help mitigate the risk of transmission to family members,” Tirupathi said. But it does not obviate the need to follow other preventive measures, such as social distancing and proper hygiene.
But Rupp, whose advice on how healthcare workers can protect their families was recently highlighted by the American Medical Association, isn’t convinced. He said he won’t be adding home mask use to his list of recommendations. “It would be intrusive, cumbersome, and impractical to wear a mask in the home setting,” Rupp said in an interview.
However, when out in the community, all family members must protect one another by practicing social distancing, wearing masks, and practicing proper hand hygiene. “I also think that it is a good idea to have some masks on hand in case anyone does develop symptoms in the household and to wear them if a family member falls ill ― at least until testing can confirm COVID-19,” Rupp said. “If a family member does fall ill, masks for the ill person as well as the well persons would be indicated along with other home quarantine measures.”
For her part, Noble, who has provided guidance about proper mask use, said that targeted use of masks at home, such as around older visiting relatives or other more vulnerable family members, may be more realistic than continuous in-home use.
When a household member becomes ill, recommendations for preventing disease spread include having a sick family member sleep in a separate bedroom, using a separate bathroom, and wearing a mask when within 6 feet of other household members. They also should avoid sharing meals. “For a household member who is a medical provider, to follow these self-isolation precautions while at home for months on end would have a significant emotional toll,” Noble said in an email. “With no end in sight for the pandemic, perpetual mask use in both the private and public sphere strikes me as overwhelming ― I write this near the end of my 10-hour shift wearing both an N95 and surgical mask and counting the minutes before I can take them off!”
A limitation of the study was its reliance on telephone interviews, which are subject to recall bias, the authors note.
The study was funded by the Beijing Science and Technology Planning Project. The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Wearing a mask at home, even when everyone is feeling fine, might reduce the risk of frontline healthcare workers transmitting SARS-CoV-2 infection to their families, a recent study from China suggests. But the benefits might not outweigh the costs, according to several physicians interviewed.
“My gut reaction is that home mask use for healthcare workers would place an inordinately high burden on those healthcare workers and their families,” said Jeanne Noble, MD, an emergency care physician at the University of California, San Francisco. “Wearing a mask for a 10-hour shift already represents significant physical discomfort, causing sores across the nose and behind the ears. The emotional toll of the physical distance that comes with mask use, with limited facial expression, is also quite real.”
The suggested benefit of home mask use comes from research published online May 28 in BMJ Global Health. To assess predictors of household transmission of SARS-CoV-2 infection, Yu Wang, MD, of the Beijing Center for Disease Prevention and Control and colleagues conducted a retrospective study of 124 families in Beijing in which there was a confirmed case of COVID-19 as of February 21. The researchers surveyed family members by telephone about household hygiene and behaviors during the pandemic to examine risk factors for transmission.
During the 2 weeks following onset of the primary case, secondary transmission occurred in 41 families. Overall, 77 of 335 family members developed COVID-19.
A multivariable logistic regression analysis found that in households in which family members wore masks at home before the first person became ill, there was less likelihood of transmission of disease to a family member, compared with families in which no one wore a mask prior to illness onset.
“Facemasks were 79% effective and disinfection was 77% effective in preventing transmission,” the researchers report, “whilst close frequent contact in the household increased the risk of transmission 18 times, and diarrhea in the index patient increased the risk by four times.
However, wearing masks after symptom onset was not protective, according to the analysis. The findings support “universal face mask use, and also provides guidance on risk reduction for families living with someone in quarantine or isolation, and families of health workers, who may face ongoing risk,” the authors write.
Still, other precautions may be more important, experts say.
“I think by far the best way for healthcare professionals to protect their families is to carefully employ appropriate infection prevention measures at work,” said Mark E. Rupp, MD, chief of the Division of Infectious Diseases at Nebraska Medical Center in Omaha. “The combination of administrative interventions, engineering improvements, and personal protective equipment is very effective in preventing SARS-CoV-2 acquisition in the workplace.”
Many physicians already wear masks at home, and this study “only reemphasized the importance of doing so,” said Raghavendra Tirupathi, MD, medical director of Keystone Infectious Diseases in Chambersburg, Pennsylvania, who recently reviewed studies about masks and COVID-19.
Home mask use provides “one more layer of protection that might help mitigate the risk of transmission to family members,” Tirupathi said. But it does not obviate the need to follow other preventive measures, such as social distancing and proper hygiene.
But Rupp, whose advice on how healthcare workers can protect their families was recently highlighted by the American Medical Association, isn’t convinced. He said he won’t be adding home mask use to his list of recommendations. “It would be intrusive, cumbersome, and impractical to wear a mask in the home setting,” Rupp said in an interview.
However, when out in the community, all family members must protect one another by practicing social distancing, wearing masks, and practicing proper hand hygiene. “I also think that it is a good idea to have some masks on hand in case anyone does develop symptoms in the household and to wear them if a family member falls ill ― at least until testing can confirm COVID-19,” Rupp said. “If a family member does fall ill, masks for the ill person as well as the well persons would be indicated along with other home quarantine measures.”
For her part, Noble, who has provided guidance about proper mask use, said that targeted use of masks at home, such as around older visiting relatives or other more vulnerable family members, may be more realistic than continuous in-home use.
When a household member becomes ill, recommendations for preventing disease spread include having a sick family member sleep in a separate bedroom, using a separate bathroom, and wearing a mask when within 6 feet of other household members. They also should avoid sharing meals. “For a household member who is a medical provider, to follow these self-isolation precautions while at home for months on end would have a significant emotional toll,” Noble said in an email. “With no end in sight for the pandemic, perpetual mask use in both the private and public sphere strikes me as overwhelming ― I write this near the end of my 10-hour shift wearing both an N95 and surgical mask and counting the minutes before I can take them off!”
A limitation of the study was its reliance on telephone interviews, which are subject to recall bias, the authors note.
The study was funded by the Beijing Science and Technology Planning Project. The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FLU/SAL inhalers for COPD carry greater pneumonia risk
For well over a decade the elevated risk of pneumonia from inhaled corticosteroids for moderate to very severe COPD has been well documented, although the pneumonia risks from different types of ICSs have not been well understood.
Researchers from Taiwan have taken a step in to investigate this question with a nationwide cohort study that reported inhalers with budesonide and beclomethasone may have a lower pneumonia risk than that of fluticasone propionate/salmeterol inhalers (CHEST. 2020;157:117-29).
The study is the first to include beclomethasone-containing inhalers in a comparison of ICS/long-acting beta2-agonist (LABA) fixed combinations to evaluate pneumonia risk, along with dose and drug properties, wrote Ting-Yu Chang, MS, of the Graduate Institute of Clinical Pharmacology at the College of Medicine, National Taiwan University in Taipei, and colleagues.
The study evaluated 42,393 people with COPD in the National Health Insurance Research Database who got at least two continuous prescriptions for three different types of inhalers:
- Budesonide/formoterol (BUD/FOR).
- Beclomethasone/formoterol (BEC/FOR).
- Fluticasone propionate/salmeterol (FLU/SAL).
The study included patients aged 40 years and older who used a metered-dose inhaler (MDI) or dry-powder inhaler (DPI) between January 2011 and June 2015.
Patient experience with adverse events (AEs) was a factor in risk stratification, Mr. Chang and colleagues noted. “For the comparison between the BEC/FOR MDI and FLU/SAL MDI, the lower risk associated with the BEC/FOR MDI was more prominent in patients without severe AE in the past year,” they wrote.
The study found that BUD/FOR DPI users had a 17% lower risk of severe pneumonia and a 12% lower risk of severe AEs than that of FLU/SAL DPI users. The risk difference in pneumonia remained significant after adjustment for the ICS-equivalent daily dose, but the spread for AEs didn’t.
BEC/FOR MDI users were 31% less likely to get severe pneumonia and 18% less likely to have severe AEs than were FLU/SAL MDI users, but that difference declined and became nonsignificant after adjustment for the ICS-equivalent daily dose.
The study also found that a high average daily dose (> 500 mcg/d) of FLU/SAL MDI carried a 66% greater risk of severe pneumonia, compared with that of low-dose users. Also, medium-dose BEC/FOR MDI users (FLU equivalent 299-499 mcg/d) had a 38% greater risk of severe pneumonia than low-dose (< 200 mcg/d) users.
The variable pneumonia risks may be linked to each ICS’s pharmacokinetics, specifically their distinct lipophilic properties, Mr. Chang and colleagues wrote. Fluticasone propionate is known to be more lipophilic than budesonide, and while beclomethasone is more lipophilic than both, as a prodrug it rapidly converts to lower lipophilicity upon contact with bronchial secretions. “In general, a lipophilic ICS has a longer retention time within the airway or lung tissue to exert local immunosuppression and reduce inflammation,” Mr. Chang and colleagues stated.
The Taiwan Ministry of Science and Technology provided partial support for the study. Mr. Chang and colleagues have no relationships to disclose.
SOURCE: Chang TY et al. CHEST. 2020;157:117-29.
For well over a decade the elevated risk of pneumonia from inhaled corticosteroids for moderate to very severe COPD has been well documented, although the pneumonia risks from different types of ICSs have not been well understood.
Researchers from Taiwan have taken a step in to investigate this question with a nationwide cohort study that reported inhalers with budesonide and beclomethasone may have a lower pneumonia risk than that of fluticasone propionate/salmeterol inhalers (CHEST. 2020;157:117-29).
The study is the first to include beclomethasone-containing inhalers in a comparison of ICS/long-acting beta2-agonist (LABA) fixed combinations to evaluate pneumonia risk, along with dose and drug properties, wrote Ting-Yu Chang, MS, of the Graduate Institute of Clinical Pharmacology at the College of Medicine, National Taiwan University in Taipei, and colleagues.
The study evaluated 42,393 people with COPD in the National Health Insurance Research Database who got at least two continuous prescriptions for three different types of inhalers:
- Budesonide/formoterol (BUD/FOR).
- Beclomethasone/formoterol (BEC/FOR).
- Fluticasone propionate/salmeterol (FLU/SAL).
The study included patients aged 40 years and older who used a metered-dose inhaler (MDI) or dry-powder inhaler (DPI) between January 2011 and June 2015.
Patient experience with adverse events (AEs) was a factor in risk stratification, Mr. Chang and colleagues noted. “For the comparison between the BEC/FOR MDI and FLU/SAL MDI, the lower risk associated with the BEC/FOR MDI was more prominent in patients without severe AE in the past year,” they wrote.
The study found that BUD/FOR DPI users had a 17% lower risk of severe pneumonia and a 12% lower risk of severe AEs than that of FLU/SAL DPI users. The risk difference in pneumonia remained significant after adjustment for the ICS-equivalent daily dose, but the spread for AEs didn’t.
BEC/FOR MDI users were 31% less likely to get severe pneumonia and 18% less likely to have severe AEs than were FLU/SAL MDI users, but that difference declined and became nonsignificant after adjustment for the ICS-equivalent daily dose.
The study also found that a high average daily dose (> 500 mcg/d) of FLU/SAL MDI carried a 66% greater risk of severe pneumonia, compared with that of low-dose users. Also, medium-dose BEC/FOR MDI users (FLU equivalent 299-499 mcg/d) had a 38% greater risk of severe pneumonia than low-dose (< 200 mcg/d) users.
The variable pneumonia risks may be linked to each ICS’s pharmacokinetics, specifically their distinct lipophilic properties, Mr. Chang and colleagues wrote. Fluticasone propionate is known to be more lipophilic than budesonide, and while beclomethasone is more lipophilic than both, as a prodrug it rapidly converts to lower lipophilicity upon contact with bronchial secretions. “In general, a lipophilic ICS has a longer retention time within the airway or lung tissue to exert local immunosuppression and reduce inflammation,” Mr. Chang and colleagues stated.
The Taiwan Ministry of Science and Technology provided partial support for the study. Mr. Chang and colleagues have no relationships to disclose.
SOURCE: Chang TY et al. CHEST. 2020;157:117-29.
For well over a decade the elevated risk of pneumonia from inhaled corticosteroids for moderate to very severe COPD has been well documented, although the pneumonia risks from different types of ICSs have not been well understood.
Researchers from Taiwan have taken a step in to investigate this question with a nationwide cohort study that reported inhalers with budesonide and beclomethasone may have a lower pneumonia risk than that of fluticasone propionate/salmeterol inhalers (CHEST. 2020;157:117-29).
The study is the first to include beclomethasone-containing inhalers in a comparison of ICS/long-acting beta2-agonist (LABA) fixed combinations to evaluate pneumonia risk, along with dose and drug properties, wrote Ting-Yu Chang, MS, of the Graduate Institute of Clinical Pharmacology at the College of Medicine, National Taiwan University in Taipei, and colleagues.
The study evaluated 42,393 people with COPD in the National Health Insurance Research Database who got at least two continuous prescriptions for three different types of inhalers:
- Budesonide/formoterol (BUD/FOR).
- Beclomethasone/formoterol (BEC/FOR).
- Fluticasone propionate/salmeterol (FLU/SAL).
The study included patients aged 40 years and older who used a metered-dose inhaler (MDI) or dry-powder inhaler (DPI) between January 2011 and June 2015.
Patient experience with adverse events (AEs) was a factor in risk stratification, Mr. Chang and colleagues noted. “For the comparison between the BEC/FOR MDI and FLU/SAL MDI, the lower risk associated with the BEC/FOR MDI was more prominent in patients without severe AE in the past year,” they wrote.
The study found that BUD/FOR DPI users had a 17% lower risk of severe pneumonia and a 12% lower risk of severe AEs than that of FLU/SAL DPI users. The risk difference in pneumonia remained significant after adjustment for the ICS-equivalent daily dose, but the spread for AEs didn’t.
BEC/FOR MDI users were 31% less likely to get severe pneumonia and 18% less likely to have severe AEs than were FLU/SAL MDI users, but that difference declined and became nonsignificant after adjustment for the ICS-equivalent daily dose.
The study also found that a high average daily dose (> 500 mcg/d) of FLU/SAL MDI carried a 66% greater risk of severe pneumonia, compared with that of low-dose users. Also, medium-dose BEC/FOR MDI users (FLU equivalent 299-499 mcg/d) had a 38% greater risk of severe pneumonia than low-dose (< 200 mcg/d) users.
The variable pneumonia risks may be linked to each ICS’s pharmacokinetics, specifically their distinct lipophilic properties, Mr. Chang and colleagues wrote. Fluticasone propionate is known to be more lipophilic than budesonide, and while beclomethasone is more lipophilic than both, as a prodrug it rapidly converts to lower lipophilicity upon contact with bronchial secretions. “In general, a lipophilic ICS has a longer retention time within the airway or lung tissue to exert local immunosuppression and reduce inflammation,” Mr. Chang and colleagues stated.
The Taiwan Ministry of Science and Technology provided partial support for the study. Mr. Chang and colleagues have no relationships to disclose.
SOURCE: Chang TY et al. CHEST. 2020;157:117-29.
FROM CHEST
Patients’ perceptions and high hospital use
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
Background: A small proportion of patients accounts for a large proportion of hospital use and readmissions. As hospitals and hospitalists focus efforts to improve transitions of care, there is a paucity of data that incorporates patients’ perspectives into the design of these programs.
Study design: Qualitative research study.
Setting: Northwestern Memorial Hospital, a single urban academic medical center in Chicago.
Synopsis: Eligible patients had two unplanned 30-day readmissions within the prior 12 months in addition to one or more of the following: at least one readmission in the last 6 months; a referral from a patient’s medical provider; or at least three observation visits.
A research coordinator conducted one-on-one semistructured interviews. Each interview was recorded, transcribed, and then coded using a team-based approach; 26 patients completed the interview process. From the analysis, four major themes emerged: Major medical problems were universal but high hospital use onset varied; participants noted that fluctuations in their course were often related to social, economic, and psychological stressors; onset and progression of episodes seemed uncontrollable and unpredictable; participants preferred to avoid hospitalization and sought care when attempts at self-management failed. The major limitation of this study was the small sample size located at one medical center, creating a data pool that is potentially not generalizable to other medical centers. These findings, however, are an important reminder to focus our interventions with patients’ needs and perceptions in mind.
Bottom line: Frequently hospitalized patients have insights into factors contributing to their high hospital use. Engaging patients in this discussion can enable us to create sustainable patient-centered programs that avoid rehospitalization.
Citation: O’Leary KJ et al. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019 Mar 20;14:e1-6.
Dr. Richardson is a hospitalist at Duke University Health System.
More fatalities in heart transplant patients with COVID-19
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Americans avoided emergency departments early in the pandemic
compared with the corresponding period in 2019, according to a report from the Centers for Disease Control and Prevention.
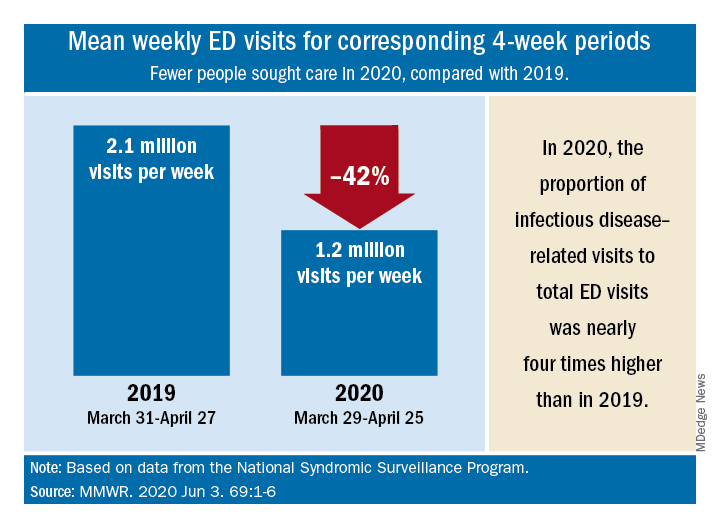
“The striking decline in ED visits nationwide … suggests that the pandemic has altered the use of the ED by the public,” Kathleen P. Hartnett, PhD, and associates at the CDC said June 3 in the Mortality and Morbidity Weekly Report.
The weekly mean was just over 1.2 million ED visits for the 4 weeks from March 29 to April 25, 2020, compared with the nearly 2.2 million visits per week recorded from March 31 to April 27, 2019 – a drop of 42%, based on an analysis of data from the National Syndromic Surveillance Program.
Despite that drop, ED visits for infectious disease–related reasons, taken as a proportion of all 1.2 ED visits during the early pandemic period, were 3.8 times higher than the comparison period in 2019, the investigators reported.
ED visits also were higher in 2020 for specified and unspecified lower respiratory disease not including influenza, pneumonia, asthma, or bronchitis (prevalence ratio of 1.99, compared with 2019), cardiac arrest and ventricular fibrillation (PR, 1.98), and pneumonia not caused by tuberculosis (PR, 1.91), Dr. Hartnett and associates said.
Prevalence ratios for the early pandemic period were down for most other conditions, with some of the largest decreases seen for influenza (PR, 0.16), otitis media (PR, 0.35), and neoplasm-related encounters (PR, 0.40), they said.
Visits have increased each week since reaching their lowest point during April 12-18, but the number for the most recent full week, May 24-30, which was not included in the analysis, was still 26% lower than the corresponding week in 2019, the CDC team pointed out.
“Some persons could be delaying care for conditions that might result in additional mortality if left untreated,” the investigators noted, and those “who use the ED as a safety net because they lack access to primary care and telemedicine might be disproportionately affected if they avoid seeking care because of concerns about the infection risk in the ED.”
SOURCE: Hartnett KP et al. MMWR. 2020 Jun 3. 69:1-6.
compared with the corresponding period in 2019, according to a report from the Centers for Disease Control and Prevention.

“The striking decline in ED visits nationwide … suggests that the pandemic has altered the use of the ED by the public,” Kathleen P. Hartnett, PhD, and associates at the CDC said June 3 in the Mortality and Morbidity Weekly Report.
The weekly mean was just over 1.2 million ED visits for the 4 weeks from March 29 to April 25, 2020, compared with the nearly 2.2 million visits per week recorded from March 31 to April 27, 2019 – a drop of 42%, based on an analysis of data from the National Syndromic Surveillance Program.
Despite that drop, ED visits for infectious disease–related reasons, taken as a proportion of all 1.2 ED visits during the early pandemic period, were 3.8 times higher than the comparison period in 2019, the investigators reported.
ED visits also were higher in 2020 for specified and unspecified lower respiratory disease not including influenza, pneumonia, asthma, or bronchitis (prevalence ratio of 1.99, compared with 2019), cardiac arrest and ventricular fibrillation (PR, 1.98), and pneumonia not caused by tuberculosis (PR, 1.91), Dr. Hartnett and associates said.
Prevalence ratios for the early pandemic period were down for most other conditions, with some of the largest decreases seen for influenza (PR, 0.16), otitis media (PR, 0.35), and neoplasm-related encounters (PR, 0.40), they said.
Visits have increased each week since reaching their lowest point during April 12-18, but the number for the most recent full week, May 24-30, which was not included in the analysis, was still 26% lower than the corresponding week in 2019, the CDC team pointed out.
“Some persons could be delaying care for conditions that might result in additional mortality if left untreated,” the investigators noted, and those “who use the ED as a safety net because they lack access to primary care and telemedicine might be disproportionately affected if they avoid seeking care because of concerns about the infection risk in the ED.”
SOURCE: Hartnett KP et al. MMWR. 2020 Jun 3. 69:1-6.
compared with the corresponding period in 2019, according to a report from the Centers for Disease Control and Prevention.

“The striking decline in ED visits nationwide … suggests that the pandemic has altered the use of the ED by the public,” Kathleen P. Hartnett, PhD, and associates at the CDC said June 3 in the Mortality and Morbidity Weekly Report.
The weekly mean was just over 1.2 million ED visits for the 4 weeks from March 29 to April 25, 2020, compared with the nearly 2.2 million visits per week recorded from March 31 to April 27, 2019 – a drop of 42%, based on an analysis of data from the National Syndromic Surveillance Program.
Despite that drop, ED visits for infectious disease–related reasons, taken as a proportion of all 1.2 ED visits during the early pandemic period, were 3.8 times higher than the comparison period in 2019, the investigators reported.
ED visits also were higher in 2020 for specified and unspecified lower respiratory disease not including influenza, pneumonia, asthma, or bronchitis (prevalence ratio of 1.99, compared with 2019), cardiac arrest and ventricular fibrillation (PR, 1.98), and pneumonia not caused by tuberculosis (PR, 1.91), Dr. Hartnett and associates said.
Prevalence ratios for the early pandemic period were down for most other conditions, with some of the largest decreases seen for influenza (PR, 0.16), otitis media (PR, 0.35), and neoplasm-related encounters (PR, 0.40), they said.
Visits have increased each week since reaching their lowest point during April 12-18, but the number for the most recent full week, May 24-30, which was not included in the analysis, was still 26% lower than the corresponding week in 2019, the CDC team pointed out.
“Some persons could be delaying care for conditions that might result in additional mortality if left untreated,” the investigators noted, and those “who use the ED as a safety net because they lack access to primary care and telemedicine might be disproportionately affected if they avoid seeking care because of concerns about the infection risk in the ED.”
SOURCE: Hartnett KP et al. MMWR. 2020 Jun 3. 69:1-6.
FROM MMWR



