User login
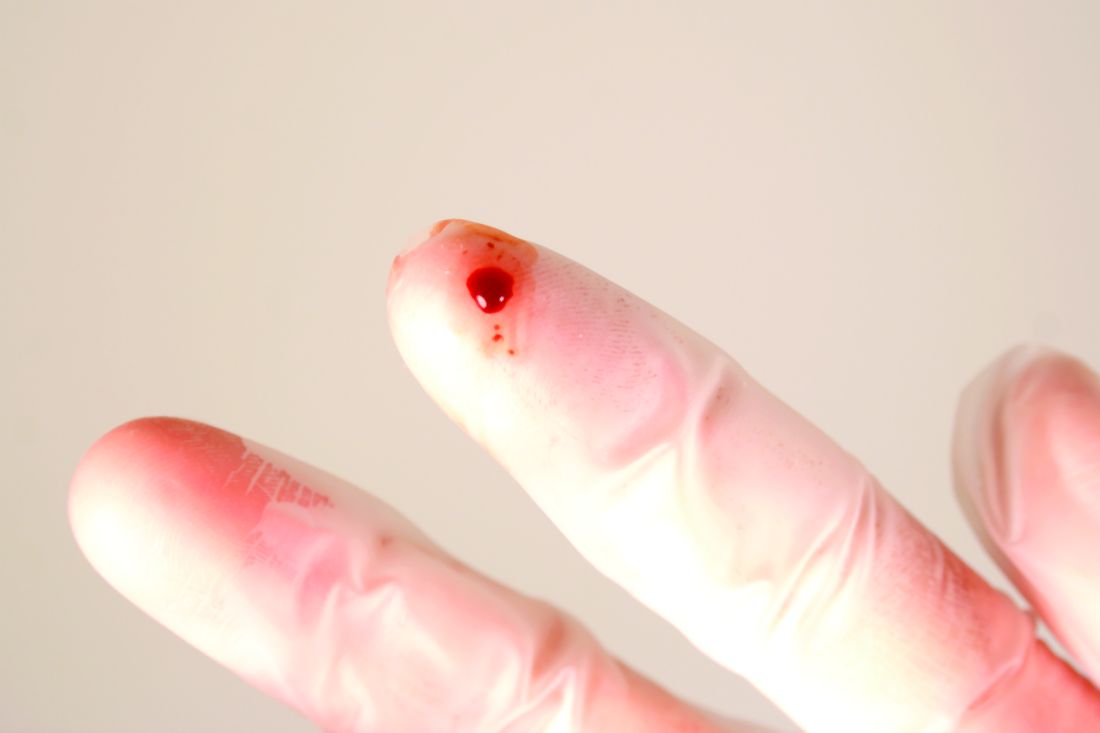
Monthly needlestick rates suggest a steep learning curve
The rate of injuries with needles and other sharp instruments among hospital staff jumped sharply in July, which suggests the need for safety instruction early in the academic year, researchers say.
“The reason this is important is it gives us an idea of when the best time to intervene might be,” said Jonathan Zampella, MD, an assistant professor of dermatology at New York University.
The findings were published online Nov. 4 in a research letter in JAMA Surgery.
Hundreds of thousands of health care workers incur injuries with needles and other sharp instruments every year, which places them at risk for blood-borne infections.
“Especially amongst dermatologists, it’s not a question of if you get stuck, it’s a question of when,” Dr. Zampella said in an interview. “Most have been stuck at some point in their lives.”
Until now, studies of these injuries have mostly depended on surveys, he said. By contrast, for the current study, Dr. Zampella and colleagues used a dataset of injuries reported to NYU Langone Health’s Occupational Health Services.
They identified 5,395 such injuries that occurred between January 2000 and February 2020. The total number was similar among surgical and nonsurgical specialists, but the mean incident rate was 4.7 for every 10 people among the nonsurgical staff versus 9.4 for every 10 people in the surgical staff.
Dr. Zampella and colleagues further found that the highest rate of injury, at 16.0 incidents for every 10 people, occurred among urology house staff, followed by orthopedic surgery staff, with 14.1, and general surgery staff, with 14.0. The lowest staff rates were among psychiatrists (0.3), radiation oncologists (1.1), and neurologists (2.4).
But even some nonsurgical specialties had high rates. For example, the rate was 11.5 for pathology house staff and 11.3 for dermatology house staff.
Dr. Zampella said his first reaction to the data was, “What the heck? What are pathologists doing that they are getting needlestick injuries?
“But it makes sense,” he said. “Sometimes they do biopsies, and they do fine-needle aspirations – these kinds of things that we might not be paying as much attention to as we should.”
The finding suggests that nonsurgical specialists should receive more training in injury prevention, he said.
The training should be in person, and it should not just be for first-year residents. “Everybody needs to have refreshers on preventing needlesticks,” he said. “And we have to make sure everyone in the hospital is playing for the same team. Residents are learning, and if they see poor technique by one of their attendings, that’s something they may imitate.”
The study’s primary conclusion regards the importance of seasonality in needlestick and other injuries from sharp instruments.
Among house staff, 9.4% of the injuries occurred in July. The proportion then gradually rose to 10.5% in October before gradually going back down to a low of 6.2% in June.
The difference from one quarter to the next was statistically significant (P = .02).
July is when internships and residencies start, Dr. Zampella pointed out. Among the nonhouse staff, the rate was consistent throughout the year.
This suggests that the beginning of the academic year for trainees was the key factor driving the uptick in injuries, he said.
He said that residents are receiving instruction in injury prevention, but perhaps not at the right time of year. For example, dermatology residents at NYU are given a lecture in needlestick injury prevention in February.
Dr. Zampella has received personal fees from X4 pharmaceuticals. The other authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The rate of injuries with needles and other sharp instruments among hospital staff jumped sharply in July, which suggests the need for safety instruction early in the academic year, researchers say.
“The reason this is important is it gives us an idea of when the best time to intervene might be,” said Jonathan Zampella, MD, an assistant professor of dermatology at New York University.
The findings were published online Nov. 4 in a research letter in JAMA Surgery.
Hundreds of thousands of health care workers incur injuries with needles and other sharp instruments every year, which places them at risk for blood-borne infections.
“Especially amongst dermatologists, it’s not a question of if you get stuck, it’s a question of when,” Dr. Zampella said in an interview. “Most have been stuck at some point in their lives.”
Until now, studies of these injuries have mostly depended on surveys, he said. By contrast, for the current study, Dr. Zampella and colleagues used a dataset of injuries reported to NYU Langone Health’s Occupational Health Services.
They identified 5,395 such injuries that occurred between January 2000 and February 2020. The total number was similar among surgical and nonsurgical specialists, but the mean incident rate was 4.7 for every 10 people among the nonsurgical staff versus 9.4 for every 10 people in the surgical staff.
Dr. Zampella and colleagues further found that the highest rate of injury, at 16.0 incidents for every 10 people, occurred among urology house staff, followed by orthopedic surgery staff, with 14.1, and general surgery staff, with 14.0. The lowest staff rates were among psychiatrists (0.3), radiation oncologists (1.1), and neurologists (2.4).
But even some nonsurgical specialties had high rates. For example, the rate was 11.5 for pathology house staff and 11.3 for dermatology house staff.
Dr. Zampella said his first reaction to the data was, “What the heck? What are pathologists doing that they are getting needlestick injuries?
“But it makes sense,” he said. “Sometimes they do biopsies, and they do fine-needle aspirations – these kinds of things that we might not be paying as much attention to as we should.”
The finding suggests that nonsurgical specialists should receive more training in injury prevention, he said.
The training should be in person, and it should not just be for first-year residents. “Everybody needs to have refreshers on preventing needlesticks,” he said. “And we have to make sure everyone in the hospital is playing for the same team. Residents are learning, and if they see poor technique by one of their attendings, that’s something they may imitate.”
The study’s primary conclusion regards the importance of seasonality in needlestick and other injuries from sharp instruments.
Among house staff, 9.4% of the injuries occurred in July. The proportion then gradually rose to 10.5% in October before gradually going back down to a low of 6.2% in June.
The difference from one quarter to the next was statistically significant (P = .02).
July is when internships and residencies start, Dr. Zampella pointed out. Among the nonhouse staff, the rate was consistent throughout the year.
This suggests that the beginning of the academic year for trainees was the key factor driving the uptick in injuries, he said.
He said that residents are receiving instruction in injury prevention, but perhaps not at the right time of year. For example, dermatology residents at NYU are given a lecture in needlestick injury prevention in February.
Dr. Zampella has received personal fees from X4 pharmaceuticals. The other authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The rate of injuries with needles and other sharp instruments among hospital staff jumped sharply in July, which suggests the need for safety instruction early in the academic year, researchers say.
“The reason this is important is it gives us an idea of when the best time to intervene might be,” said Jonathan Zampella, MD, an assistant professor of dermatology at New York University.
The findings were published online Nov. 4 in a research letter in JAMA Surgery.
Hundreds of thousands of health care workers incur injuries with needles and other sharp instruments every year, which places them at risk for blood-borne infections.
“Especially amongst dermatologists, it’s not a question of if you get stuck, it’s a question of when,” Dr. Zampella said in an interview. “Most have been stuck at some point in their lives.”
Until now, studies of these injuries have mostly depended on surveys, he said. By contrast, for the current study, Dr. Zampella and colleagues used a dataset of injuries reported to NYU Langone Health’s Occupational Health Services.
They identified 5,395 such injuries that occurred between January 2000 and February 2020. The total number was similar among surgical and nonsurgical specialists, but the mean incident rate was 4.7 for every 10 people among the nonsurgical staff versus 9.4 for every 10 people in the surgical staff.
Dr. Zampella and colleagues further found that the highest rate of injury, at 16.0 incidents for every 10 people, occurred among urology house staff, followed by orthopedic surgery staff, with 14.1, and general surgery staff, with 14.0. The lowest staff rates were among psychiatrists (0.3), radiation oncologists (1.1), and neurologists (2.4).
But even some nonsurgical specialties had high rates. For example, the rate was 11.5 for pathology house staff and 11.3 for dermatology house staff.
Dr. Zampella said his first reaction to the data was, “What the heck? What are pathologists doing that they are getting needlestick injuries?
“But it makes sense,” he said. “Sometimes they do biopsies, and they do fine-needle aspirations – these kinds of things that we might not be paying as much attention to as we should.”
The finding suggests that nonsurgical specialists should receive more training in injury prevention, he said.
The training should be in person, and it should not just be for first-year residents. “Everybody needs to have refreshers on preventing needlesticks,” he said. “And we have to make sure everyone in the hospital is playing for the same team. Residents are learning, and if they see poor technique by one of their attendings, that’s something they may imitate.”
The study’s primary conclusion regards the importance of seasonality in needlestick and other injuries from sharp instruments.
Among house staff, 9.4% of the injuries occurred in July. The proportion then gradually rose to 10.5% in October before gradually going back down to a low of 6.2% in June.
The difference from one quarter to the next was statistically significant (P = .02).
July is when internships and residencies start, Dr. Zampella pointed out. Among the nonhouse staff, the rate was consistent throughout the year.
This suggests that the beginning of the academic year for trainees was the key factor driving the uptick in injuries, he said.
He said that residents are receiving instruction in injury prevention, but perhaps not at the right time of year. For example, dermatology residents at NYU are given a lecture in needlestick injury prevention in February.
Dr. Zampella has received personal fees from X4 pharmaceuticals. The other authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Nearly 10% of hospitalized patients with COVID-19 later readmitted
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with mental illness a priority for COVID vaccine, experts say
With this week’s announcement that Pfizer’s vaccine candidate against SARS-CoV-2 was 90% effective in preventing COVID-19, the world is one step closer to an effective vaccine.
Nevertheless, with a limited supply of initial doses, the question becomes, who should get it first? Individuals with severe mental illness should be a priority group to receive a COVID-19 vaccine, assert the authors of a perspective article published Nov. 1 in World Psychiatry.
Patients with underlying physical conditions, such as cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, obesity, immunodeficiency, and cancer, are particularly vulnerable to developing more severe illness and dying from COVID-19.
In these populations, the risk of a more severe course of infection or early death is significant enough for the U.S. National Academies of Sciences, Engineering, and Medicine to make these patients priority recipients of a vaccine against COVID-19.
Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors argued that those with severe mental illness also fit into this group.
Even without factoring COVID-19 into the calculation, those with severe mental illness have a two- to threefold higher mortality rate than the general population, resulting in reduction in life expectancy of 10-20 years, they noted. This is largely because of physical diseases including cardiovascular disease, type 2 diabetes, and respiratory ailments.
Individuals with severe mental illness also have higher rates of obesity than the general population and obesity is a risk factor for dying from COVID-19.
High-risk population
Like their peers with physical illnesses, recent studies suggest that those with severe mental illness are also at increased risk of morbidity and mortality from COVID-19.
For example, a recent U.S. case-control study with over 61 million adults showed that those recently diagnosed with a mental health disorder had a significantly increased risk for COVID-19 infection, an effect strongest for depression and schizophrenia.
Other recent studies have confirmed these data, including one linking a psychiatric diagnosis in patients hospitalized with COVID-19 to a significantly increased risk for death, as reported by Medscape Medical News.
Dr. De Hert and colleagues put these findings into perspective with this example: In 2017, there were an estimated 11.2 million adults in the United States with severe mental illness. Taking into account the 8.5% death rate in COVID-19 patients recently diagnosed with a severe mental illness, this means that about 1 million patients with severe mental illness in the United States would die if all were infected with the virus.
In light of this knowledge, and taking into account published ethical principles that should guide vaccine allocation, Dr. De Hert and colleagues said it is “paramount” that persons with severe mental illness be prioritized to guarantee that they receive a COVID-19 vaccine during the first phase of its distribution.
“It is our responsibility as psychiatrists in this global health crisis to advocate for the needs of our patients with governments and public health policy bodies,” they wrote.
The authors also encourage public health agencies to develop and implement targeted programs to ensure that patients with severe mental illness and their health care providers “are made aware of these increased risks as well as the benefits of vaccination.”
An argument for fairness
Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, also believes those with severe mental illness should be a priority group for a COVID vaccine.
“When we’re prioritizing groups for a COVID-19 vaccine, let’s not forget that people with serious mental illness have much lower life expectancies, more obesity, and more undiagnosed chronic conditions. They should be a priority group,” Dr. Appelbaum said in an interview.
“The argument for including people with severe mental illnesses among the vulnerable populations who should be prioritized for receipt of a COVID-19 vaccine is an argument for fairness in constructing that group,” he added.
“Like people with other chronic conditions associated with poor outcomes after SARS-CoV-2 infection, people with severe mental illnesses are more likely to be hospitalized and more likely to die. Although they are often systematically ignored when decisions are made about allocation of resources, there is some hope that, with enough public attention to this issue, they can be included this time,” Dr. Appelbaum said.
Dr. De Hert and Dr. Applebaum disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
With this week’s announcement that Pfizer’s vaccine candidate against SARS-CoV-2 was 90% effective in preventing COVID-19, the world is one step closer to an effective vaccine.
Nevertheless, with a limited supply of initial doses, the question becomes, who should get it first? Individuals with severe mental illness should be a priority group to receive a COVID-19 vaccine, assert the authors of a perspective article published Nov. 1 in World Psychiatry.
Patients with underlying physical conditions, such as cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, obesity, immunodeficiency, and cancer, are particularly vulnerable to developing more severe illness and dying from COVID-19.
In these populations, the risk of a more severe course of infection or early death is significant enough for the U.S. National Academies of Sciences, Engineering, and Medicine to make these patients priority recipients of a vaccine against COVID-19.
Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors argued that those with severe mental illness also fit into this group.
Even without factoring COVID-19 into the calculation, those with severe mental illness have a two- to threefold higher mortality rate than the general population, resulting in reduction in life expectancy of 10-20 years, they noted. This is largely because of physical diseases including cardiovascular disease, type 2 diabetes, and respiratory ailments.
Individuals with severe mental illness also have higher rates of obesity than the general population and obesity is a risk factor for dying from COVID-19.
High-risk population
Like their peers with physical illnesses, recent studies suggest that those with severe mental illness are also at increased risk of morbidity and mortality from COVID-19.
For example, a recent U.S. case-control study with over 61 million adults showed that those recently diagnosed with a mental health disorder had a significantly increased risk for COVID-19 infection, an effect strongest for depression and schizophrenia.
Other recent studies have confirmed these data, including one linking a psychiatric diagnosis in patients hospitalized with COVID-19 to a significantly increased risk for death, as reported by Medscape Medical News.
Dr. De Hert and colleagues put these findings into perspective with this example: In 2017, there were an estimated 11.2 million adults in the United States with severe mental illness. Taking into account the 8.5% death rate in COVID-19 patients recently diagnosed with a severe mental illness, this means that about 1 million patients with severe mental illness in the United States would die if all were infected with the virus.
In light of this knowledge, and taking into account published ethical principles that should guide vaccine allocation, Dr. De Hert and colleagues said it is “paramount” that persons with severe mental illness be prioritized to guarantee that they receive a COVID-19 vaccine during the first phase of its distribution.
“It is our responsibility as psychiatrists in this global health crisis to advocate for the needs of our patients with governments and public health policy bodies,” they wrote.
The authors also encourage public health agencies to develop and implement targeted programs to ensure that patients with severe mental illness and their health care providers “are made aware of these increased risks as well as the benefits of vaccination.”
An argument for fairness
Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, also believes those with severe mental illness should be a priority group for a COVID vaccine.
“When we’re prioritizing groups for a COVID-19 vaccine, let’s not forget that people with serious mental illness have much lower life expectancies, more obesity, and more undiagnosed chronic conditions. They should be a priority group,” Dr. Appelbaum said in an interview.
“The argument for including people with severe mental illnesses among the vulnerable populations who should be prioritized for receipt of a COVID-19 vaccine is an argument for fairness in constructing that group,” he added.
“Like people with other chronic conditions associated with poor outcomes after SARS-CoV-2 infection, people with severe mental illnesses are more likely to be hospitalized and more likely to die. Although they are often systematically ignored when decisions are made about allocation of resources, there is some hope that, with enough public attention to this issue, they can be included this time,” Dr. Appelbaum said.
Dr. De Hert and Dr. Applebaum disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
With this week’s announcement that Pfizer’s vaccine candidate against SARS-CoV-2 was 90% effective in preventing COVID-19, the world is one step closer to an effective vaccine.
Nevertheless, with a limited supply of initial doses, the question becomes, who should get it first? Individuals with severe mental illness should be a priority group to receive a COVID-19 vaccine, assert the authors of a perspective article published Nov. 1 in World Psychiatry.
Patients with underlying physical conditions, such as cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, obesity, immunodeficiency, and cancer, are particularly vulnerable to developing more severe illness and dying from COVID-19.
In these populations, the risk of a more severe course of infection or early death is significant enough for the U.S. National Academies of Sciences, Engineering, and Medicine to make these patients priority recipients of a vaccine against COVID-19.
Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors argued that those with severe mental illness also fit into this group.
Even without factoring COVID-19 into the calculation, those with severe mental illness have a two- to threefold higher mortality rate than the general population, resulting in reduction in life expectancy of 10-20 years, they noted. This is largely because of physical diseases including cardiovascular disease, type 2 diabetes, and respiratory ailments.
Individuals with severe mental illness also have higher rates of obesity than the general population and obesity is a risk factor for dying from COVID-19.
High-risk population
Like their peers with physical illnesses, recent studies suggest that those with severe mental illness are also at increased risk of morbidity and mortality from COVID-19.
For example, a recent U.S. case-control study with over 61 million adults showed that those recently diagnosed with a mental health disorder had a significantly increased risk for COVID-19 infection, an effect strongest for depression and schizophrenia.
Other recent studies have confirmed these data, including one linking a psychiatric diagnosis in patients hospitalized with COVID-19 to a significantly increased risk for death, as reported by Medscape Medical News.
Dr. De Hert and colleagues put these findings into perspective with this example: In 2017, there were an estimated 11.2 million adults in the United States with severe mental illness. Taking into account the 8.5% death rate in COVID-19 patients recently diagnosed with a severe mental illness, this means that about 1 million patients with severe mental illness in the United States would die if all were infected with the virus.
In light of this knowledge, and taking into account published ethical principles that should guide vaccine allocation, Dr. De Hert and colleagues said it is “paramount” that persons with severe mental illness be prioritized to guarantee that they receive a COVID-19 vaccine during the first phase of its distribution.
“It is our responsibility as psychiatrists in this global health crisis to advocate for the needs of our patients with governments and public health policy bodies,” they wrote.
The authors also encourage public health agencies to develop and implement targeted programs to ensure that patients with severe mental illness and their health care providers “are made aware of these increased risks as well as the benefits of vaccination.”
An argument for fairness
Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, also believes those with severe mental illness should be a priority group for a COVID vaccine.
“When we’re prioritizing groups for a COVID-19 vaccine, let’s not forget that people with serious mental illness have much lower life expectancies, more obesity, and more undiagnosed chronic conditions. They should be a priority group,” Dr. Appelbaum said in an interview.
“The argument for including people with severe mental illnesses among the vulnerable populations who should be prioritized for receipt of a COVID-19 vaccine is an argument for fairness in constructing that group,” he added.
“Like people with other chronic conditions associated with poor outcomes after SARS-CoV-2 infection, people with severe mental illnesses are more likely to be hospitalized and more likely to die. Although they are often systematically ignored when decisions are made about allocation of resources, there is some hope that, with enough public attention to this issue, they can be included this time,” Dr. Appelbaum said.
Dr. De Hert and Dr. Applebaum disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Treatments for COVID-19: Update for hospitalists
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Search for a snakebite drug might lead to a COVID treatment, too
Matthew Lewin, MD, PhD, founder of the Center for Exploration and Travel Health at the California Academy of Sciences, was researching snakebite treatments in rural locations in preparation for an expedition to the Philippines in 2011.
The story of a renowned herpetologist from the academy, Joseph Slowinski, who was bitten by a highly venomous krait in Myanmar and couldn’t get to a hospital in time to save his life a decade earlier, weighed on the emergency room doctor.
“I concluded that I needed something small and compact and that doesn’t care what kind of snake,” Dr. Lewin said.
It didn’t exist. That set Dr. Lewin in pursuit of a modern snakebite drug, a journey that finds his Corte Madera, Calif., company, Ophirex, nearing a promising oral treatment that fits in a pocket; is stable, easy to use, and affordable; and treats the venom from many species. “That’s the holy grail of snakebite treatment,” he said.
His work has gotten a boost with multimillion-dollar grants from a British charity and the U.S. Army. If it works – and it has been shown to work extremely well in mice and pigs – it could save tens of thousands of lives a year.
Dr. Lewin and Ophirex are not alone in their quest. Snakebites kill nearly 140,000 people a year, overwhelmingly in impoverished rural areas of Asia and Africa without adequate medical infrastructure and knowledge to administer antivenom. Though just a few people die each year in the United States from snakebites, the problem has risen to the top of the list of global health concerns in recent years. Funding has soared, and other research groups have also done promising work on new treatments. Herpetologists say deforestation and climate change are increasing human-snake encounters by forcing snakes to move to new habitats.
Dr. Lewin’s research is centered on a drug called varespladib. The enzyme inhibitor has proven itself in in-vitro lab studies and has effectively saved mice and pigs dosed with venom.
Along the way, Dr. Lewin and his team have come across another potential use for the drug. Varespladib has a positive effect on acute respiratory distress syndrome, associated with COVID-19. Next year, Ophirex will conduct human trials for the possible treatment of the condition funded with $9.9 million from the Army.
The link to a snakebite? The inflammation of the lungs caused by the coronavirus produces the sPLA2 enzyme. A more deadly version of the same enzyme is produced by snake venom.
The other companies that have come up with promising approaches to snakebite aren’t as far along as Ophirex. At the University of California-Irvine, chemist Ken Shea and his team created a nanogel – a kind of polymer used in medical applications – that blocks key proteins in the venom that cause cell destruction. At the Technical University of Denmark, Copenhagen, Andreas Laustsen is looking at engineering bacteria to manufacture anti-venom in fermentation tanks.
The days of incising a snakebite and sucking out the poison are long over, but the current treatment for venomous snakebites remains archaic.
Since the early 1900s, antivenom has been made by injecting horses or other animals with venom milked from snakes and diluted. The animals’ immune systems generate antibodies over several months, and blood plasma is taken from the animals and antibodies extracted from it.
It’s extremely expensive. Hospitals in the United States can charge as much as $15,000 a vial – and a single snakebite might require anywhere from 4 to 50 vials. Moreover, antivenom exists for little more than half the world’s species of venomous snakes.
A major problem is the roughly 2 hours it takes on average for a snakebite victim to reach a hospital and begin treatment. The chemical weapon that is venom starts immediately to destroy cells as it digests its next meal, making fast treatment essential to saving lives and preventing tissue loss.
“The two-hour window between fang and needle is where the most damage occurs,” said Leslie Boyer, director of the University of Arizona’s Venom Immunochemistry, Pharmacology and Emergency Response (VIPER) Institute. “We have a saying, ‘Time is tissue.’ ”
That’s why the search for a new snakebite drug has focused on an inexpensive treatment that can be taken into the field. Dr. Lewin’s drug wouldn’t replace antivenom. Instead, he thinks of it as the first line of defense until the victim can reach a hospital for antivenom treatment.
Dr. Lewin said he expects the drug to be inexpensive, so people in regions where snakebites are common can afford it.
Venom is extremely complicated chemically, and Dr. Lewin began his search by sussing out which of its myriad components to block. He zeroed in on the sPLA2 enzyme.
Surveying the literature about drugs that had been clinically tested for other conditions, he came across varespladib. It had been developed jointly by Eli Lilly and Shionogi, a Japanese pharmaceutical company, as a possible treatment for sepsis. They had never taken it to market.
If it worked, Dr. Lewin could license the right to produce the drug, which had already been thoroughly studied and was shown to be safe.
He placed venom in an array of test tubes. Varespladib and other drugs were added to the venom. He then added a reagent. If the venom was still active, the solution would turn yellow; if it was neutralized, it would remain clear.
The vials with varespladib “came up completely blank,” he said. “It was so stunning I said, ‘I must have made a mistake.’ ”
With a small grant, he sent the drug to the Yale Center for Molecular Discovery and found that varespladib effectively neutralized the venom of snakes found on six continents. The results were published in the journal Toxins and sent ripples through the small community of snakebite researchers.
Dr. Lewin then conducted tests on mice and pigs. Both were successful.
Human clinical trials are next, but they have been delayed by the pandemic. They are scheduled to get underway next spring.
Along the way, Dr. Lewin was fortunate enough to make some good connections that led to funding. In 2012, he attended a party at the Mill Valley, Calif., home of Jerry Harrison, the former guitarist and keyboardist for Talking Heads. Mr. Harrison had long been interested in business and start-ups – he said he was the most careful reader of the ’80s band’s contracts – and at the party he asked “if anyone had any ideas lying fallow,” Mr. Harrison said.
“And Matt pipes up and says, ‘I have this idea how to prevent people from dying from snakebites,’ ” Mr. Harrison said.
The musician said he was a bit taken aback by such an unusual and dire problem, but “I thought if it can save lives we have to do it,” he said. He became an investor and cofounder of Ophirex with Dr. Lewin.
Dr. Lewin met Lt. Col. Rebecca Carter, a biochemist who was assigned to lead the Medical Modernization Division of Air Force Special Operations Command, in 2016 when she attended a Venom Week conference in Greenville, N.C. He was presenting the results of his mouse studies. She told him about her first mission: to find a universal antivenom for medics on special operations teams in Africa. She persuaded the Special Operations Command Biomedical Research Advisory Group, which specializes in getting critical projects to production, to grant Ophirex $148,000 in 2017. She later retired from the Air Force and now works for Ophirex as vice president.
More multimillion-dollar grants followed, including the Army’s COVID grant. Clinical trials are scheduled to begin this winter.
Despite the progress and the sudden cash flow, Dr. Lewin tamps down talk of a universal snakebite cure. “There’s enough evidence to say the drug deserves to have its day in clinical trials,” he said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Matthew Lewin, MD, PhD, founder of the Center for Exploration and Travel Health at the California Academy of Sciences, was researching snakebite treatments in rural locations in preparation for an expedition to the Philippines in 2011.
The story of a renowned herpetologist from the academy, Joseph Slowinski, who was bitten by a highly venomous krait in Myanmar and couldn’t get to a hospital in time to save his life a decade earlier, weighed on the emergency room doctor.
“I concluded that I needed something small and compact and that doesn’t care what kind of snake,” Dr. Lewin said.
It didn’t exist. That set Dr. Lewin in pursuit of a modern snakebite drug, a journey that finds his Corte Madera, Calif., company, Ophirex, nearing a promising oral treatment that fits in a pocket; is stable, easy to use, and affordable; and treats the venom from many species. “That’s the holy grail of snakebite treatment,” he said.
His work has gotten a boost with multimillion-dollar grants from a British charity and the U.S. Army. If it works – and it has been shown to work extremely well in mice and pigs – it could save tens of thousands of lives a year.
Dr. Lewin and Ophirex are not alone in their quest. Snakebites kill nearly 140,000 people a year, overwhelmingly in impoverished rural areas of Asia and Africa without adequate medical infrastructure and knowledge to administer antivenom. Though just a few people die each year in the United States from snakebites, the problem has risen to the top of the list of global health concerns in recent years. Funding has soared, and other research groups have also done promising work on new treatments. Herpetologists say deforestation and climate change are increasing human-snake encounters by forcing snakes to move to new habitats.
Dr. Lewin’s research is centered on a drug called varespladib. The enzyme inhibitor has proven itself in in-vitro lab studies and has effectively saved mice and pigs dosed with venom.
Along the way, Dr. Lewin and his team have come across another potential use for the drug. Varespladib has a positive effect on acute respiratory distress syndrome, associated with COVID-19. Next year, Ophirex will conduct human trials for the possible treatment of the condition funded with $9.9 million from the Army.
The link to a snakebite? The inflammation of the lungs caused by the coronavirus produces the sPLA2 enzyme. A more deadly version of the same enzyme is produced by snake venom.
The other companies that have come up with promising approaches to snakebite aren’t as far along as Ophirex. At the University of California-Irvine, chemist Ken Shea and his team created a nanogel – a kind of polymer used in medical applications – that blocks key proteins in the venom that cause cell destruction. At the Technical University of Denmark, Copenhagen, Andreas Laustsen is looking at engineering bacteria to manufacture anti-venom in fermentation tanks.
The days of incising a snakebite and sucking out the poison are long over, but the current treatment for venomous snakebites remains archaic.
Since the early 1900s, antivenom has been made by injecting horses or other animals with venom milked from snakes and diluted. The animals’ immune systems generate antibodies over several months, and blood plasma is taken from the animals and antibodies extracted from it.
It’s extremely expensive. Hospitals in the United States can charge as much as $15,000 a vial – and a single snakebite might require anywhere from 4 to 50 vials. Moreover, antivenom exists for little more than half the world’s species of venomous snakes.
A major problem is the roughly 2 hours it takes on average for a snakebite victim to reach a hospital and begin treatment. The chemical weapon that is venom starts immediately to destroy cells as it digests its next meal, making fast treatment essential to saving lives and preventing tissue loss.
“The two-hour window between fang and needle is where the most damage occurs,” said Leslie Boyer, director of the University of Arizona’s Venom Immunochemistry, Pharmacology and Emergency Response (VIPER) Institute. “We have a saying, ‘Time is tissue.’ ”
That’s why the search for a new snakebite drug has focused on an inexpensive treatment that can be taken into the field. Dr. Lewin’s drug wouldn’t replace antivenom. Instead, he thinks of it as the first line of defense until the victim can reach a hospital for antivenom treatment.
Dr. Lewin said he expects the drug to be inexpensive, so people in regions where snakebites are common can afford it.
Venom is extremely complicated chemically, and Dr. Lewin began his search by sussing out which of its myriad components to block. He zeroed in on the sPLA2 enzyme.
Surveying the literature about drugs that had been clinically tested for other conditions, he came across varespladib. It had been developed jointly by Eli Lilly and Shionogi, a Japanese pharmaceutical company, as a possible treatment for sepsis. They had never taken it to market.
If it worked, Dr. Lewin could license the right to produce the drug, which had already been thoroughly studied and was shown to be safe.
He placed venom in an array of test tubes. Varespladib and other drugs were added to the venom. He then added a reagent. If the venom was still active, the solution would turn yellow; if it was neutralized, it would remain clear.
The vials with varespladib “came up completely blank,” he said. “It was so stunning I said, ‘I must have made a mistake.’ ”
With a small grant, he sent the drug to the Yale Center for Molecular Discovery and found that varespladib effectively neutralized the venom of snakes found on six continents. The results were published in the journal Toxins and sent ripples through the small community of snakebite researchers.
Dr. Lewin then conducted tests on mice and pigs. Both were successful.
Human clinical trials are next, but they have been delayed by the pandemic. They are scheduled to get underway next spring.
Along the way, Dr. Lewin was fortunate enough to make some good connections that led to funding. In 2012, he attended a party at the Mill Valley, Calif., home of Jerry Harrison, the former guitarist and keyboardist for Talking Heads. Mr. Harrison had long been interested in business and start-ups – he said he was the most careful reader of the ’80s band’s contracts – and at the party he asked “if anyone had any ideas lying fallow,” Mr. Harrison said.
“And Matt pipes up and says, ‘I have this idea how to prevent people from dying from snakebites,’ ” Mr. Harrison said.
The musician said he was a bit taken aback by such an unusual and dire problem, but “I thought if it can save lives we have to do it,” he said. He became an investor and cofounder of Ophirex with Dr. Lewin.
Dr. Lewin met Lt. Col. Rebecca Carter, a biochemist who was assigned to lead the Medical Modernization Division of Air Force Special Operations Command, in 2016 when she attended a Venom Week conference in Greenville, N.C. He was presenting the results of his mouse studies. She told him about her first mission: to find a universal antivenom for medics on special operations teams in Africa. She persuaded the Special Operations Command Biomedical Research Advisory Group, which specializes in getting critical projects to production, to grant Ophirex $148,000 in 2017. She later retired from the Air Force and now works for Ophirex as vice president.
More multimillion-dollar grants followed, including the Army’s COVID grant. Clinical trials are scheduled to begin this winter.
Despite the progress and the sudden cash flow, Dr. Lewin tamps down talk of a universal snakebite cure. “There’s enough evidence to say the drug deserves to have its day in clinical trials,” he said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Matthew Lewin, MD, PhD, founder of the Center for Exploration and Travel Health at the California Academy of Sciences, was researching snakebite treatments in rural locations in preparation for an expedition to the Philippines in 2011.
The story of a renowned herpetologist from the academy, Joseph Slowinski, who was bitten by a highly venomous krait in Myanmar and couldn’t get to a hospital in time to save his life a decade earlier, weighed on the emergency room doctor.
“I concluded that I needed something small and compact and that doesn’t care what kind of snake,” Dr. Lewin said.
It didn’t exist. That set Dr. Lewin in pursuit of a modern snakebite drug, a journey that finds his Corte Madera, Calif., company, Ophirex, nearing a promising oral treatment that fits in a pocket; is stable, easy to use, and affordable; and treats the venom from many species. “That’s the holy grail of snakebite treatment,” he said.
His work has gotten a boost with multimillion-dollar grants from a British charity and the U.S. Army. If it works – and it has been shown to work extremely well in mice and pigs – it could save tens of thousands of lives a year.
Dr. Lewin and Ophirex are not alone in their quest. Snakebites kill nearly 140,000 people a year, overwhelmingly in impoverished rural areas of Asia and Africa without adequate medical infrastructure and knowledge to administer antivenom. Though just a few people die each year in the United States from snakebites, the problem has risen to the top of the list of global health concerns in recent years. Funding has soared, and other research groups have also done promising work on new treatments. Herpetologists say deforestation and climate change are increasing human-snake encounters by forcing snakes to move to new habitats.
Dr. Lewin’s research is centered on a drug called varespladib. The enzyme inhibitor has proven itself in in-vitro lab studies and has effectively saved mice and pigs dosed with venom.
Along the way, Dr. Lewin and his team have come across another potential use for the drug. Varespladib has a positive effect on acute respiratory distress syndrome, associated with COVID-19. Next year, Ophirex will conduct human trials for the possible treatment of the condition funded with $9.9 million from the Army.
The link to a snakebite? The inflammation of the lungs caused by the coronavirus produces the sPLA2 enzyme. A more deadly version of the same enzyme is produced by snake venom.
The other companies that have come up with promising approaches to snakebite aren’t as far along as Ophirex. At the University of California-Irvine, chemist Ken Shea and his team created a nanogel – a kind of polymer used in medical applications – that blocks key proteins in the venom that cause cell destruction. At the Technical University of Denmark, Copenhagen, Andreas Laustsen is looking at engineering bacteria to manufacture anti-venom in fermentation tanks.
The days of incising a snakebite and sucking out the poison are long over, but the current treatment for venomous snakebites remains archaic.
Since the early 1900s, antivenom has been made by injecting horses or other animals with venom milked from snakes and diluted. The animals’ immune systems generate antibodies over several months, and blood plasma is taken from the animals and antibodies extracted from it.
It’s extremely expensive. Hospitals in the United States can charge as much as $15,000 a vial – and a single snakebite might require anywhere from 4 to 50 vials. Moreover, antivenom exists for little more than half the world’s species of venomous snakes.
A major problem is the roughly 2 hours it takes on average for a snakebite victim to reach a hospital and begin treatment. The chemical weapon that is venom starts immediately to destroy cells as it digests its next meal, making fast treatment essential to saving lives and preventing tissue loss.
“The two-hour window between fang and needle is where the most damage occurs,” said Leslie Boyer, director of the University of Arizona’s Venom Immunochemistry, Pharmacology and Emergency Response (VIPER) Institute. “We have a saying, ‘Time is tissue.’ ”
That’s why the search for a new snakebite drug has focused on an inexpensive treatment that can be taken into the field. Dr. Lewin’s drug wouldn’t replace antivenom. Instead, he thinks of it as the first line of defense until the victim can reach a hospital for antivenom treatment.
Dr. Lewin said he expects the drug to be inexpensive, so people in regions where snakebites are common can afford it.
Venom is extremely complicated chemically, and Dr. Lewin began his search by sussing out which of its myriad components to block. He zeroed in on the sPLA2 enzyme.
Surveying the literature about drugs that had been clinically tested for other conditions, he came across varespladib. It had been developed jointly by Eli Lilly and Shionogi, a Japanese pharmaceutical company, as a possible treatment for sepsis. They had never taken it to market.
If it worked, Dr. Lewin could license the right to produce the drug, which had already been thoroughly studied and was shown to be safe.
He placed venom in an array of test tubes. Varespladib and other drugs were added to the venom. He then added a reagent. If the venom was still active, the solution would turn yellow; if it was neutralized, it would remain clear.
The vials with varespladib “came up completely blank,” he said. “It was so stunning I said, ‘I must have made a mistake.’ ”
With a small grant, he sent the drug to the Yale Center for Molecular Discovery and found that varespladib effectively neutralized the venom of snakes found on six continents. The results were published in the journal Toxins and sent ripples through the small community of snakebite researchers.
Dr. Lewin then conducted tests on mice and pigs. Both were successful.
Human clinical trials are next, but they have been delayed by the pandemic. They are scheduled to get underway next spring.
Along the way, Dr. Lewin was fortunate enough to make some good connections that led to funding. In 2012, he attended a party at the Mill Valley, Calif., home of Jerry Harrison, the former guitarist and keyboardist for Talking Heads. Mr. Harrison had long been interested in business and start-ups – he said he was the most careful reader of the ’80s band’s contracts – and at the party he asked “if anyone had any ideas lying fallow,” Mr. Harrison said.
“And Matt pipes up and says, ‘I have this idea how to prevent people from dying from snakebites,’ ” Mr. Harrison said.
The musician said he was a bit taken aback by such an unusual and dire problem, but “I thought if it can save lives we have to do it,” he said. He became an investor and cofounder of Ophirex with Dr. Lewin.
Dr. Lewin met Lt. Col. Rebecca Carter, a biochemist who was assigned to lead the Medical Modernization Division of Air Force Special Operations Command, in 2016 when she attended a Venom Week conference in Greenville, N.C. He was presenting the results of his mouse studies. She told him about her first mission: to find a universal antivenom for medics on special operations teams in Africa. She persuaded the Special Operations Command Biomedical Research Advisory Group, which specializes in getting critical projects to production, to grant Ophirex $148,000 in 2017. She later retired from the Air Force and now works for Ophirex as vice president.
More multimillion-dollar grants followed, including the Army’s COVID grant. Clinical trials are scheduled to begin this winter.
Despite the progress and the sudden cash flow, Dr. Lewin tamps down talk of a universal snakebite cure. “There’s enough evidence to say the drug deserves to have its day in clinical trials,” he said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Moral distress: COVID-19 shortages prompt tough decisions at bedside
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
What happened to melanoma care during COVID-19 sequestration
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
REPORTING FROM THE CUTANEOUS MALIGNANCIES FORUM
FDA grants emergency use authorization to Lilly’s antibody COVID-19 therapy
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
New eGFR equation ‘less biased’ by age, kidney function; some disagree
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
The European Kidney Function Consortium (EKFC) equation surpasses existing equations by “resulting in generally lower bias across the spectrum of age and kidney function,” its developers wrote in an article published online Nov. 9 in Annals of Internal Medicine.
“The new EKFC equation may have helpful properties and perform better in estimating GFR, compared with the current KDIGO [Kidney Disease: Improving Global Outcomes]-recommended equations,” they added.
The primary KDIGO-recommended equation in its most recent guideline was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, designed for adults, and a companion equation, the CKiD, covers children and adolescents.
“Key in our [new] equation is the adjustment for differences in serum creatinine generation between children and adults, or between men and women,” lead author Hans Pottel, PhD, KU Leuven (Belgium), said in an interview.
In an accompanying editorial, Andrew M. Levey, MD, and associates wrote: “We agree that a single eGFR equation that can be used in children and adults and performs well in the transition from adolescence to young adulthood is a worthy goal.”
“But the claim of equivalent or superior performance, compared with the CKD-EPI equation is not conclusive,” claimed Dr. Levey, who led the research team that developed the CKD-EPI equation, and coauthors.
Dr. Levey is professor of medicine at Tufts University, Boston.
What’s new is Q
Dr. Pottel and codevelopers devised what they call Q values: age- and sex-dependent median creatinine levels in normal individuals.
Q values act to “normalize or rescale creatinine before entering it into the equation, because we know that creatinine generation is different” based on factors that include age, sex, and muscle mass.
The EKFC equation extends the CKD-EPI equation and first eGFR equation by using Q values and applying across age ranges, like the full-age spectrum (FAS) equation, first reported in 2016 by a team led by Dr. Pottel.
“Although the FAS equation was designed to overcome the challenge in measuring GFR in patients transitioning from adolescence to adult nephrology care, it also underestimates GFR at low serum creatinine values and in patients with chronic kidney disease,” wrote Dr. Pottel and coauthors.
Hence, their intent to tweak the FAS equation to overcome this limitation and create the EKFC equation.
“The new equation combines the strengths of the CKD-EPI and FAS equations,” they woite.
However, “we acknowledge that lack of precision is still a major problem with all eGFR equations,” including the new EKFC, they added.
Editorialists dispute better performance of EKFC over CKD-EPI
In their editorial, Dr. Levey and coauthors noted the EKFC equations and other adapted equations in development “represent a conceptual advance over the FAS equations,” but they dispute the claims of better performance, compared with the CKD-EPI.
“We compared the performance of the EKFC and CKD-EPI equations in a different, large external validation population of Black and non-Black adults,” the external population used to validate the CKD-EPI equation, the editorialists reported.
The upshot was “our results did not confirm the author’s conclusions” about the EKFC equation.
In response, Dr. Pottel highlighted that the EKFC equation is currently not designed for use in Black patients.
“With its derivation and validation now reported in the new article, the EKFC equation is fully validated and ready for routine use in Whites,” he said. “We plan to evaluate and possibly fine tune our equation for its application in other ethnicities.”
Regarding the inferior performance, compared with the CKD-EPI equation in the non-Black population tested by the editorialists, Dr. Pottel cited “calibration issues for serum creatinine” that some experts have found in the datasets compiled by developers of the CKI-EPI equation that could limit the utility of these data.
Still room for improvement; app hopefully coming next year
Dr. Pottel and coauthors developed and validated the EKFC equation with data from 19,629 patients drawn from 13 cohorts. This included 11,251 patients from seven cohorts for development and internal validation, and 8378 from six cohorts for external validation. The EKFC effort received endorsement from the European Renal Association–European Dialysis and Transplant Association.
However, “We acknowledge that there is still room for improvement,” Dr. Pottel said.
Although the new report presents the EKFC equations (actually two slightly different equations depending on whether a patient’s serum creatinine is higher or lower than the relevant Q value), most potential users will likely find the equations easier to work with once they’re in an app form that allows someone to simply plug in age, sex, and serum creatinine level. That app currently doesn’t exist but is coming soon, promised Dr. Pottel.
“I hope to have an electronic tool by the beginning of 2021,” he said. “I have to find a programmer who can do this for me.”
The EKFC project has received no commercial funding. Dr. Pottel reported no relevant financial relationships. Dr. Levey has reported receiving research funding from AstraZeneca.
A version of this article originally appeared on Medscape.com.
Great Barrington coauthor backs off strict reliance on herd immunity
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.