User login
COVID-19 vaccine standards questioned at FDA advisory meeting
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
The FDA’s Vaccines and Related Biological Products Advisory Committee met for a wide-ranging discussion beginning around 10 am. The FDA did not ask the panel to weigh in on any particular vaccine. Instead, the FDA asked for the panel’s feedback on a series of questions, including considerations for continuing phase 3 trials if a product were to get an interim clearance known as an emergency use authorization (EUA).
Speakers at the hearing made a variety of requests, including asking for data showing COVID-19 vaccines can prevent serious illness and urging transparency about the agency’s deliberations for each product to be considered.
FDA staff are closely tracking the crop of experimental vaccines that have made it into advanced stages of testing, including products from Pfizer Inc, AstraZeneca, Johnson & Johnson, and Moderna.
‘Time for a reset’
Among the speakers at the public hearing was Peter Lurie, MD, who served as an FDA associate commissioner from 2014 to 2017. Now the president of the Center for Science in the Public Interest, Lurie was among the speakers who asked the agency to make its independence clear.
President Donald Trump has for months been making predictions about COVID-19 vaccine approvals that have been overly optimistic. In one example, the president, who is seeking re-election on November 3, last month spoke about being able to begin distributing a vaccine in October.
“Until now the process of developing candidate vaccines has been inappropriately politicized with an eye on the election calendar, rather than the deliberate timeframe science requires,” Lurie told the FDA advisory panel. “Now is the time for a reset. This committee has a unique opportunity to set a new tone for vaccine deliberations going forward.”
Lurie asked the panel to press the FDA to commit to hold an advisory committee meeting on requests by drugmakers for EUAs. He also asked the panel to demand that informed consent forms and minutes from institutional review board (IRB) discussions of COVID-19 vaccines trials be made public.
Also among the speakers at the public hearing was Peter Doshi, PhD, an associate professor at the University of Maryland School of Pharmacy, who argued that the current trials won’t answer the right questions about the COVID-19 vaccines.
“We could end up with approved vaccines that reduce the risk of mild infection, but do not decrease the risk of hospitalization, ICU use, or death — either at all or by a clinically relevant amount,” Doshi told the panel.
In his presentation, he reiterated points he had made previously, including in an October 21 article in the BMJ, for which he is an associate editor. Doshi also raised these concerns in a September opinion article in The New York Times, co-authored with Eric Topol, MD, director of the Scripps Research Translational Institute and editor-in-chief of Medscape.
Risks of a ‘rushed vaccine’
Other complaints about the FDA’s approach included criticism of a 2-month follow-up time after vaccination, which was seen as too short. ECRI, a nonprofit organization that seeks to improve the safety, quality, and cost-effectiveness of medicines, has argued that approving a weak COVID-19 vaccine might worsen the pandemic.
In an October 21 statement, ECRI noted the risk of a partially effective vaccine, which could be welcomed as a means of slowing transmission of the virus. But public response and attitudes over the past 9 months in the United States suggest that people would relax their precautions as soon as a vaccine is available.
“Resulting infections may offset the vaccine’s impact and end up increasing the mortality and morbidity burden,” ECRI said in the brief.
“The risks and consequences of a rushed vaccine could be very severe if the review is anything shy of thorough,” ECRI Chief Executive Officer Marcus Schabacker, MD, PhD, said in a statement prepared for the hearing.
This article first appeared on Medscape.com.
CDC expands definition of COVID-19 exposure from ‘close contact’
New data suggest each close encounter – coming within 6 feet of an infected person – can increase the risk for transmission, CDC director Robert Redfield, MD, said during a media briefing.
“As we get more data and understand the science of COVID, we’re going to continue to incorporate that in our recommendations,” Dr. Redfield said in response to a reporter’s question about a recent study.
Previously, the CDC cautioned against spending 15 minutes or longer in close proximity to an infected person, particularly in enclosed indoor spaces.
In a new report published online Oct. 21 in Morbidity and Mortality Weekly Report, however, investigators “determined that an individual who had a series of shorter contacts that over time added up to more than 15 minutes became infected.”
Beware of brief encounters?
On July 28, a 20-year-old male correctional officer in Vermont had multiple brief encounters with six transferred incarcerated or detained people while their SARS-CoV-2 test results were pending. The six were asymptomatic at the time and were housed in a quarantine unit, reported CDC researcher Julia Pringle, PhD, and colleagues.
The following day, all six inmates tested polymerase chain reaction (PCR) positive for COVID-19. The correctional officer did not spend 15 minutes or more within 6 feet of any of the inmates, according to video surveillance footage, and he continued to work.
On Aug. 4, however, he developed symptoms that included loss of smell and taste, myalgia, runny nose, cough, shortness of breath, headache, loss of appetite, and gastrointestinal symptoms. He stayed home starting the next day and tested PCR positive for COVID-19 on Aug. 11.
Further review of the surveillance video showed that the officer had numerous brief encounters of approximately 1 minute each that cumulatively exceeded 15 minutes over a 24-hour period, the researchers reported.
During all the interactions with inmates, the correctional officer wore a cloth mask, gown, and eye protection. The inmates wore masks while in their cells but did not have them on during brief cell doorway interactions or in the recreation room, according to the report.
No interaction is 100% safe
“We know that every activity that involves interacting with others has some degree of risk right now,” said Jay Butler, MD, CDC deputy director for infectious diseases.
“Unfortunately, we’re seeing a distressing trend here in the United States with COVID-19 cases increasing in nearly 75% of the country,” he said. “We’ve confirmed 8.1 million cases and, sadly, over 220,000 deaths since January.
“I know these are numbers, but these are also people,” Dr. Butler added.
“The pandemic is not over,” Dr. Redfield said. “Earlier this week, COVID virus cases reached over 40 million globally. Here in the United States we are approaching a critical phase.”
Four factors associated with higher risk for transmission are the proximity of each encounter, its duration, whether an interaction takes place indoors or outdoors, and the number of people encountered, Dr. Butler said.
Dr. Butler acknowledged widespread fatigue with adherence to personal protection measures, but added that social distancing, mask-wearing, and other measures are more important now than ever. He noted that more Americans will be spending time indoors with the onset of cooler weather and the upcoming holidays.
A note of optimism
Dr. Redfield remains optimistic about the limited availability of a vaccine or vaccines by year’s end but added that “it’s important for all of us to remain diligent in our efforts to defeat this virus.”
“There is hope on the way, in the form of safe and effective vaccines in a matter of weeks or months. To bridge to that next phase, we have to take steps to keep ourselves, our families, and our communities safe,” said Alex Azar, secretary of the Department of Health & Human Services.
“I know it’s been a difficult year for Americans, but we are going to come through this on the other side,” Dr. Redfield said.
New data suggest each close encounter – coming within 6 feet of an infected person – can increase the risk for transmission, CDC director Robert Redfield, MD, said during a media briefing.
“As we get more data and understand the science of COVID, we’re going to continue to incorporate that in our recommendations,” Dr. Redfield said in response to a reporter’s question about a recent study.
Previously, the CDC cautioned against spending 15 minutes or longer in close proximity to an infected person, particularly in enclosed indoor spaces.
In a new report published online Oct. 21 in Morbidity and Mortality Weekly Report, however, investigators “determined that an individual who had a series of shorter contacts that over time added up to more than 15 minutes became infected.”
Beware of brief encounters?
On July 28, a 20-year-old male correctional officer in Vermont had multiple brief encounters with six transferred incarcerated or detained people while their SARS-CoV-2 test results were pending. The six were asymptomatic at the time and were housed in a quarantine unit, reported CDC researcher Julia Pringle, PhD, and colleagues.
The following day, all six inmates tested polymerase chain reaction (PCR) positive for COVID-19. The correctional officer did not spend 15 minutes or more within 6 feet of any of the inmates, according to video surveillance footage, and he continued to work.
On Aug. 4, however, he developed symptoms that included loss of smell and taste, myalgia, runny nose, cough, shortness of breath, headache, loss of appetite, and gastrointestinal symptoms. He stayed home starting the next day and tested PCR positive for COVID-19 on Aug. 11.
Further review of the surveillance video showed that the officer had numerous brief encounters of approximately 1 minute each that cumulatively exceeded 15 minutes over a 24-hour period, the researchers reported.
During all the interactions with inmates, the correctional officer wore a cloth mask, gown, and eye protection. The inmates wore masks while in their cells but did not have them on during brief cell doorway interactions or in the recreation room, according to the report.
No interaction is 100% safe
“We know that every activity that involves interacting with others has some degree of risk right now,” said Jay Butler, MD, CDC deputy director for infectious diseases.
“Unfortunately, we’re seeing a distressing trend here in the United States with COVID-19 cases increasing in nearly 75% of the country,” he said. “We’ve confirmed 8.1 million cases and, sadly, over 220,000 deaths since January.
“I know these are numbers, but these are also people,” Dr. Butler added.
“The pandemic is not over,” Dr. Redfield said. “Earlier this week, COVID virus cases reached over 40 million globally. Here in the United States we are approaching a critical phase.”
Four factors associated with higher risk for transmission are the proximity of each encounter, its duration, whether an interaction takes place indoors or outdoors, and the number of people encountered, Dr. Butler said.
Dr. Butler acknowledged widespread fatigue with adherence to personal protection measures, but added that social distancing, mask-wearing, and other measures are more important now than ever. He noted that more Americans will be spending time indoors with the onset of cooler weather and the upcoming holidays.
A note of optimism
Dr. Redfield remains optimistic about the limited availability of a vaccine or vaccines by year’s end but added that “it’s important for all of us to remain diligent in our efforts to defeat this virus.”
“There is hope on the way, in the form of safe and effective vaccines in a matter of weeks or months. To bridge to that next phase, we have to take steps to keep ourselves, our families, and our communities safe,” said Alex Azar, secretary of the Department of Health & Human Services.
“I know it’s been a difficult year for Americans, but we are going to come through this on the other side,” Dr. Redfield said.
New data suggest each close encounter – coming within 6 feet of an infected person – can increase the risk for transmission, CDC director Robert Redfield, MD, said during a media briefing.
“As we get more data and understand the science of COVID, we’re going to continue to incorporate that in our recommendations,” Dr. Redfield said in response to a reporter’s question about a recent study.
Previously, the CDC cautioned against spending 15 minutes or longer in close proximity to an infected person, particularly in enclosed indoor spaces.
In a new report published online Oct. 21 in Morbidity and Mortality Weekly Report, however, investigators “determined that an individual who had a series of shorter contacts that over time added up to more than 15 minutes became infected.”
Beware of brief encounters?
On July 28, a 20-year-old male correctional officer in Vermont had multiple brief encounters with six transferred incarcerated or detained people while their SARS-CoV-2 test results were pending. The six were asymptomatic at the time and were housed in a quarantine unit, reported CDC researcher Julia Pringle, PhD, and colleagues.
The following day, all six inmates tested polymerase chain reaction (PCR) positive for COVID-19. The correctional officer did not spend 15 minutes or more within 6 feet of any of the inmates, according to video surveillance footage, and he continued to work.
On Aug. 4, however, he developed symptoms that included loss of smell and taste, myalgia, runny nose, cough, shortness of breath, headache, loss of appetite, and gastrointestinal symptoms. He stayed home starting the next day and tested PCR positive for COVID-19 on Aug. 11.
Further review of the surveillance video showed that the officer had numerous brief encounters of approximately 1 minute each that cumulatively exceeded 15 minutes over a 24-hour period, the researchers reported.
During all the interactions with inmates, the correctional officer wore a cloth mask, gown, and eye protection. The inmates wore masks while in their cells but did not have them on during brief cell doorway interactions or in the recreation room, according to the report.
No interaction is 100% safe
“We know that every activity that involves interacting with others has some degree of risk right now,” said Jay Butler, MD, CDC deputy director for infectious diseases.
“Unfortunately, we’re seeing a distressing trend here in the United States with COVID-19 cases increasing in nearly 75% of the country,” he said. “We’ve confirmed 8.1 million cases and, sadly, over 220,000 deaths since January.
“I know these are numbers, but these are also people,” Dr. Butler added.
“The pandemic is not over,” Dr. Redfield said. “Earlier this week, COVID virus cases reached over 40 million globally. Here in the United States we are approaching a critical phase.”
Four factors associated with higher risk for transmission are the proximity of each encounter, its duration, whether an interaction takes place indoors or outdoors, and the number of people encountered, Dr. Butler said.
Dr. Butler acknowledged widespread fatigue with adherence to personal protection measures, but added that social distancing, mask-wearing, and other measures are more important now than ever. He noted that more Americans will be spending time indoors with the onset of cooler weather and the upcoming holidays.
A note of optimism
Dr. Redfield remains optimistic about the limited availability of a vaccine or vaccines by year’s end but added that “it’s important for all of us to remain diligent in our efforts to defeat this virus.”
“There is hope on the way, in the form of safe and effective vaccines in a matter of weeks or months. To bridge to that next phase, we have to take steps to keep ourselves, our families, and our communities safe,” said Alex Azar, secretary of the Department of Health & Human Services.
“I know it’s been a difficult year for Americans, but we are going to come through this on the other side,” Dr. Redfield said.
FDA approves remdesivir, first treatment for COVID-19
making it the first and only approved treatment for COVID-19, according to a release from drug manufacturer Gilead Sciences.
The FDA’s initial Emergency Use Authorization (EUA) of the antiviral, issued in May, allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who needed oxygen therapy or mechanical ventilation.
An August EUA expanded treatment to include all adult and pediatric hospitalized COVID-19 patients, regardless of the severity of their disease. The FDA also issued a new EUA for remdesivir Oct. 22 allowing treatment of hospitalized pediatric patients younger than 12 weighing at least 3.5 kg.
Today’s approval is based on three randomized controlled trials, according to Gilead.
Final trial results from one of them, the National Institute of Allergy and Infectious Disease–funded ACTT-1 trial, published earlier in October, showed that hospitalized patients with COVID-19 who received remdesivir had a shorter median recovery time than those who received a placebo – 10 days versus 15 days.
This difference and some related secondary endpoints were statistically significant in the randomized trial, but there was not a statistically significant difference in mortality between the treatment and placebo groups.
The other two trials used for the approval, the SIMPLE trials, were open-label phase 3 trials conducted in countries with a high prevalence of COVID-19 infections, according to Gilead.
The SIMPLE-Severe trial was a randomized, multicenter study that evaluated the efficacy and safety of 5-day and 10-day dosing plus standard of care in 397 hospitalized adult patients with severe COVID-19. The primary endpoint was clinical status on day 14 assessed on a 7-point ordinal scale, according to Gilead.
The trial found that a 5-day or a 10-day treatment course of Veklury achieved similar clinical outcomes to the ACTT-1 trial (odds ratio, 0.75; 95% confidence interval, 0.51-1.12).
The SIMPLE-Moderate trial was a randomized, controlled, multicenter study that evaluated the efficacy and safety of 5-day and 10-day dosing durations of Veklury plus standard of care, compared with standard of care alone in 600 hospitalized adult patients with moderate COVID-19, Gilead stated in its release.
The primary endpoint was clinical status on day 11 assessed on a 7-point ordinal scale.
The results showed statistically improved clinical outcomes with a 5-day treatment course of Veklury, compared with standard of care (OR, 1.65; 95% CI, 1.0-2.48; P = .017), according to Gilead.
This article first appeared on Medscape.com.
making it the first and only approved treatment for COVID-19, according to a release from drug manufacturer Gilead Sciences.
The FDA’s initial Emergency Use Authorization (EUA) of the antiviral, issued in May, allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who needed oxygen therapy or mechanical ventilation.
An August EUA expanded treatment to include all adult and pediatric hospitalized COVID-19 patients, regardless of the severity of their disease. The FDA also issued a new EUA for remdesivir Oct. 22 allowing treatment of hospitalized pediatric patients younger than 12 weighing at least 3.5 kg.
Today’s approval is based on three randomized controlled trials, according to Gilead.
Final trial results from one of them, the National Institute of Allergy and Infectious Disease–funded ACTT-1 trial, published earlier in October, showed that hospitalized patients with COVID-19 who received remdesivir had a shorter median recovery time than those who received a placebo – 10 days versus 15 days.
This difference and some related secondary endpoints were statistically significant in the randomized trial, but there was not a statistically significant difference in mortality between the treatment and placebo groups.
The other two trials used for the approval, the SIMPLE trials, were open-label phase 3 trials conducted in countries with a high prevalence of COVID-19 infections, according to Gilead.
The SIMPLE-Severe trial was a randomized, multicenter study that evaluated the efficacy and safety of 5-day and 10-day dosing plus standard of care in 397 hospitalized adult patients with severe COVID-19. The primary endpoint was clinical status on day 14 assessed on a 7-point ordinal scale, according to Gilead.
The trial found that a 5-day or a 10-day treatment course of Veklury achieved similar clinical outcomes to the ACTT-1 trial (odds ratio, 0.75; 95% confidence interval, 0.51-1.12).
The SIMPLE-Moderate trial was a randomized, controlled, multicenter study that evaluated the efficacy and safety of 5-day and 10-day dosing durations of Veklury plus standard of care, compared with standard of care alone in 600 hospitalized adult patients with moderate COVID-19, Gilead stated in its release.
The primary endpoint was clinical status on day 11 assessed on a 7-point ordinal scale.
The results showed statistically improved clinical outcomes with a 5-day treatment course of Veklury, compared with standard of care (OR, 1.65; 95% CI, 1.0-2.48; P = .017), according to Gilead.
This article first appeared on Medscape.com.
making it the first and only approved treatment for COVID-19, according to a release from drug manufacturer Gilead Sciences.
The FDA’s initial Emergency Use Authorization (EUA) of the antiviral, issued in May, allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who needed oxygen therapy or mechanical ventilation.
An August EUA expanded treatment to include all adult and pediatric hospitalized COVID-19 patients, regardless of the severity of their disease. The FDA also issued a new EUA for remdesivir Oct. 22 allowing treatment of hospitalized pediatric patients younger than 12 weighing at least 3.5 kg.
Today’s approval is based on three randomized controlled trials, according to Gilead.
Final trial results from one of them, the National Institute of Allergy and Infectious Disease–funded ACTT-1 trial, published earlier in October, showed that hospitalized patients with COVID-19 who received remdesivir had a shorter median recovery time than those who received a placebo – 10 days versus 15 days.
This difference and some related secondary endpoints were statistically significant in the randomized trial, but there was not a statistically significant difference in mortality between the treatment and placebo groups.
The other two trials used for the approval, the SIMPLE trials, were open-label phase 3 trials conducted in countries with a high prevalence of COVID-19 infections, according to Gilead.
The SIMPLE-Severe trial was a randomized, multicenter study that evaluated the efficacy and safety of 5-day and 10-day dosing plus standard of care in 397 hospitalized adult patients with severe COVID-19. The primary endpoint was clinical status on day 14 assessed on a 7-point ordinal scale, according to Gilead.
The trial found that a 5-day or a 10-day treatment course of Veklury achieved similar clinical outcomes to the ACTT-1 trial (odds ratio, 0.75; 95% confidence interval, 0.51-1.12).
The SIMPLE-Moderate trial was a randomized, controlled, multicenter study that evaluated the efficacy and safety of 5-day and 10-day dosing durations of Veklury plus standard of care, compared with standard of care alone in 600 hospitalized adult patients with moderate COVID-19, Gilead stated in its release.
The primary endpoint was clinical status on day 11 assessed on a 7-point ordinal scale.
The results showed statistically improved clinical outcomes with a 5-day treatment course of Veklury, compared with standard of care (OR, 1.65; 95% CI, 1.0-2.48; P = .017), according to Gilead.
This article first appeared on Medscape.com.
Certain statins linked to lower mortality risk in patients admitted for sepsis
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
FROM CHEST 2020
Rinse and repeat? Mouthwash might mitigate COVID-19 spread
Listerine Antiseptic led the list of most effective mouthwashes for inactivating the coronavirus. Interestingly, a 1% nasal rinse solution of Johnson’s Baby Shampoo also worked, eliminating up to 99.9% of the viral load in the in vitro experiments.
In contrast, use of a neti pot nasal solution yielded no decrease in virus levels.
The study was published in the Journal of Medical Virology.
Because the mouthwash and hydrogen peroxide oral rinses in the study are widely available and easy to use, “I would recommend the use of the rinses on top of wearing mask and social distancing. This could add a layer of protection for yourself and others,” lead study author Craig Meyers, PhD, professor of microbiology and immunology and obstetrics and gynecology, Penn State College of Medicine in Hershey, Pennsylvania, told Medscape Medical News.
Meyers and colleagues found that efficacy aligned with duration of time the cell cultures were exposed to each mouthwash or rinse product. Although it varied, the products required at least 30 seconds to kill most of the virus. Waiting 1 or 2 minutes tended to fortify results.
“This study adds to and further confirms the recently published evidence from virologists in Germany that mouthwashes can inactivate the virus that causes COVID-19 in a test tube,” Valerie O’Donnell, PhD, co-director of the Systems Immunity Research Institute of Cardiff University, Cardiff, Wales, said when asked to comment on the study.
“While this is great to see, what is still lacking is in vivo evidence, since we know the virus will be continually shed in the mouth,” O’Donnell said. “So, the question now becomes, by how much could mouthwashes reduce viral load in the oropharynx of infected people, and if so, then for how long?”
Meyers noted that studies of people positive for COVID-19 using each product would be informative. It remains unknown, for example, if swishing, gargling, and/or spitting out mouthwash would add or decrease the efficacy demonstrated in the lab.
The investigators used the human coronavirus HCoV‐229e as a surrogate for SARS-CoV-2. They noted HCoV-229e is analogous, and SARS-CoV-2 would have been more expensive, less available, and would have required biosafety level 3 laboratory conditions.
Listerine Antiseptic leads the way
“Surprisingly, we found that several of these common products had strong virucidal properties, inactivating from 2 log10 [or 99%] to greater than 4 log10 [or 99.99%] of infectious human coronavirus,” the researchers note.
The researchers added a small amount of organic material (extra protein) to each product to more closely mimic physiologic conditions in the nasopharynx.
Listerine Antiseptic “historically has claimed numerous antimicrobial properties,” the researchers note. Although the label currently only claims to kill germs that cause bad breath, “our tests show that it is highly effective at inactivating human coronavirus in solution. Even at the lowest contact time of 30 seconds, it inactivated greater than 99.99% of human coronavirus.”
Interestingly, the mouthwashes that contained the same active ingredients as Listerine Antiseptic — Listerine Ultra, Equate Antiseptic, and CVS Antiseptic Mouth Wash — were less efficacious. Meyers said the reason remains unclear, but he and colleagues found the same result when they repeated the comparisons.
Timing of the essence?
Meyers and colleagues also tested a nasal rinse solution of 1% baby shampoo because it is sometimes used to treat people with chronic rhinosinusitis. They found 30 seconds led to < 90% to < 99.99% effectiveness, but that, by 2 minutes, efficacy climbed to > 99.9% to > 99.99%.
“Thirty seconds for some products just was not enough time for the efficacy to be observed,” Meyers said. “Whereas, after a minute or two the active ingredient had enough time to work. Thirty seconds may be at the border to see full efficacy.” More research is needed to confirm the timing and determine which active ingredients are driving the findings.
A future trial could test the efficacy of mouthwash products to reduce the viral load in people with COVID-19. “If we are able to get funding to continue, I would like to see a small clinical trial as the next step,” Meyers said.
Meyers and O’Donnell disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Listerine Antiseptic led the list of most effective mouthwashes for inactivating the coronavirus. Interestingly, a 1% nasal rinse solution of Johnson’s Baby Shampoo also worked, eliminating up to 99.9% of the viral load in the in vitro experiments.
In contrast, use of a neti pot nasal solution yielded no decrease in virus levels.
The study was published in the Journal of Medical Virology.
Because the mouthwash and hydrogen peroxide oral rinses in the study are widely available and easy to use, “I would recommend the use of the rinses on top of wearing mask and social distancing. This could add a layer of protection for yourself and others,” lead study author Craig Meyers, PhD, professor of microbiology and immunology and obstetrics and gynecology, Penn State College of Medicine in Hershey, Pennsylvania, told Medscape Medical News.
Meyers and colleagues found that efficacy aligned with duration of time the cell cultures were exposed to each mouthwash or rinse product. Although it varied, the products required at least 30 seconds to kill most of the virus. Waiting 1 or 2 minutes tended to fortify results.
“This study adds to and further confirms the recently published evidence from virologists in Germany that mouthwashes can inactivate the virus that causes COVID-19 in a test tube,” Valerie O’Donnell, PhD, co-director of the Systems Immunity Research Institute of Cardiff University, Cardiff, Wales, said when asked to comment on the study.
“While this is great to see, what is still lacking is in vivo evidence, since we know the virus will be continually shed in the mouth,” O’Donnell said. “So, the question now becomes, by how much could mouthwashes reduce viral load in the oropharynx of infected people, and if so, then for how long?”
Meyers noted that studies of people positive for COVID-19 using each product would be informative. It remains unknown, for example, if swishing, gargling, and/or spitting out mouthwash would add or decrease the efficacy demonstrated in the lab.
The investigators used the human coronavirus HCoV‐229e as a surrogate for SARS-CoV-2. They noted HCoV-229e is analogous, and SARS-CoV-2 would have been more expensive, less available, and would have required biosafety level 3 laboratory conditions.
Listerine Antiseptic leads the way
“Surprisingly, we found that several of these common products had strong virucidal properties, inactivating from 2 log10 [or 99%] to greater than 4 log10 [or 99.99%] of infectious human coronavirus,” the researchers note.
The researchers added a small amount of organic material (extra protein) to each product to more closely mimic physiologic conditions in the nasopharynx.
Listerine Antiseptic “historically has claimed numerous antimicrobial properties,” the researchers note. Although the label currently only claims to kill germs that cause bad breath, “our tests show that it is highly effective at inactivating human coronavirus in solution. Even at the lowest contact time of 30 seconds, it inactivated greater than 99.99% of human coronavirus.”
Interestingly, the mouthwashes that contained the same active ingredients as Listerine Antiseptic — Listerine Ultra, Equate Antiseptic, and CVS Antiseptic Mouth Wash — were less efficacious. Meyers said the reason remains unclear, but he and colleagues found the same result when they repeated the comparisons.
Timing of the essence?
Meyers and colleagues also tested a nasal rinse solution of 1% baby shampoo because it is sometimes used to treat people with chronic rhinosinusitis. They found 30 seconds led to < 90% to < 99.99% effectiveness, but that, by 2 minutes, efficacy climbed to > 99.9% to > 99.99%.
“Thirty seconds for some products just was not enough time for the efficacy to be observed,” Meyers said. “Whereas, after a minute or two the active ingredient had enough time to work. Thirty seconds may be at the border to see full efficacy.” More research is needed to confirm the timing and determine which active ingredients are driving the findings.
A future trial could test the efficacy of mouthwash products to reduce the viral load in people with COVID-19. “If we are able to get funding to continue, I would like to see a small clinical trial as the next step,” Meyers said.
Meyers and O’Donnell disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Listerine Antiseptic led the list of most effective mouthwashes for inactivating the coronavirus. Interestingly, a 1% nasal rinse solution of Johnson’s Baby Shampoo also worked, eliminating up to 99.9% of the viral load in the in vitro experiments.
In contrast, use of a neti pot nasal solution yielded no decrease in virus levels.
The study was published in the Journal of Medical Virology.
Because the mouthwash and hydrogen peroxide oral rinses in the study are widely available and easy to use, “I would recommend the use of the rinses on top of wearing mask and social distancing. This could add a layer of protection for yourself and others,” lead study author Craig Meyers, PhD, professor of microbiology and immunology and obstetrics and gynecology, Penn State College of Medicine in Hershey, Pennsylvania, told Medscape Medical News.
Meyers and colleagues found that efficacy aligned with duration of time the cell cultures were exposed to each mouthwash or rinse product. Although it varied, the products required at least 30 seconds to kill most of the virus. Waiting 1 or 2 minutes tended to fortify results.
“This study adds to and further confirms the recently published evidence from virologists in Germany that mouthwashes can inactivate the virus that causes COVID-19 in a test tube,” Valerie O’Donnell, PhD, co-director of the Systems Immunity Research Institute of Cardiff University, Cardiff, Wales, said when asked to comment on the study.
“While this is great to see, what is still lacking is in vivo evidence, since we know the virus will be continually shed in the mouth,” O’Donnell said. “So, the question now becomes, by how much could mouthwashes reduce viral load in the oropharynx of infected people, and if so, then for how long?”
Meyers noted that studies of people positive for COVID-19 using each product would be informative. It remains unknown, for example, if swishing, gargling, and/or spitting out mouthwash would add or decrease the efficacy demonstrated in the lab.
The investigators used the human coronavirus HCoV‐229e as a surrogate for SARS-CoV-2. They noted HCoV-229e is analogous, and SARS-CoV-2 would have been more expensive, less available, and would have required biosafety level 3 laboratory conditions.
Listerine Antiseptic leads the way
“Surprisingly, we found that several of these common products had strong virucidal properties, inactivating from 2 log10 [or 99%] to greater than 4 log10 [or 99.99%] of infectious human coronavirus,” the researchers note.
The researchers added a small amount of organic material (extra protein) to each product to more closely mimic physiologic conditions in the nasopharynx.
Listerine Antiseptic “historically has claimed numerous antimicrobial properties,” the researchers note. Although the label currently only claims to kill germs that cause bad breath, “our tests show that it is highly effective at inactivating human coronavirus in solution. Even at the lowest contact time of 30 seconds, it inactivated greater than 99.99% of human coronavirus.”
Interestingly, the mouthwashes that contained the same active ingredients as Listerine Antiseptic — Listerine Ultra, Equate Antiseptic, and CVS Antiseptic Mouth Wash — were less efficacious. Meyers said the reason remains unclear, but he and colleagues found the same result when they repeated the comparisons.
Timing of the essence?
Meyers and colleagues also tested a nasal rinse solution of 1% baby shampoo because it is sometimes used to treat people with chronic rhinosinusitis. They found 30 seconds led to < 90% to < 99.99% effectiveness, but that, by 2 minutes, efficacy climbed to > 99.9% to > 99.99%.
“Thirty seconds for some products just was not enough time for the efficacy to be observed,” Meyers said. “Whereas, after a minute or two the active ingredient had enough time to work. Thirty seconds may be at the border to see full efficacy.” More research is needed to confirm the timing and determine which active ingredients are driving the findings.
A future trial could test the efficacy of mouthwash products to reduce the viral load in people with COVID-19. “If we are able to get funding to continue, I would like to see a small clinical trial as the next step,” Meyers said.
Meyers and O’Donnell disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Cardiogenic shock rate soars in COVID-positive ACS
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
COVID-19–positive patients undergoing an invasive strategy for acute coronary syndrome presented hours later than uninfected historical controls, had a far higher incidence of cardiogenic shock, and their in-hospital mortality rate was four- to fivefold greater, according to data from the Global Multicenter Prospective COVID–ACS Registry. These phenomena are probably interrelated, according to Anthony Gershlick, MBBS, who presented the registry results at the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
“We know that increasing ischemic time leads to bigger infarcts. And we know that bigger infarcts lead to cardiogenic shock, with its known higher mortality,” said Dr. Gershlick, professor of interventional cardiology at the University of Leicester (England).
“These data suggest that patients may have presented late, likely due to COVID concerns, and they had worse outcomes. If these data are borne out, future public information strategies need to be reassuring, proactive, simple, and more effective because we think patients stayed away,” the cardiologist added. “There are important public information messages to be taken from these data about getting patients to come to hospital during such pandemics.”
He presented prospectively collected registry data on 144 patients with confirmed ST-elevation MI (STEMI) and 122 with non-ST–elevation MI (NSTEMI), all COVID-19 positive on presentation at 85 hospitals in the United Kingdom, Europe, and North America during March through August of 2020. Since the initial message to the public early in the pandemic in many places was to try to avoid the hospital, the investigators selected for their no-COVID comparison group the data on more than 22,000 STEMI and NSTEMI patients included in two British national databases covering 2018-2019.
The COVID-positive STEMI patients were significantly younger, had more comorbidities, and had a higher mean heart rate and lower systolic blood pressure at admission than the non-COVID STEMI control group. Their median time from symptom onset to admission was 339 minutes, compared with 178 minutes in controls. Their door-to-balloon time averaged 83 minutes, versus 37 minutes in the era before the pandemic.
“I suspect that’s got something to do with the donning and doffing of personal protective equipment,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
The in-hospital mortality rates were strikingly different: 27.1% in COVID-positive STEMI patients versus 5.7% in controls. Bleeding Academic Research Consortium type 3-5 bleeding was increased as well, by a margin of 2.8% to 0.3%. So was stroke, with a 2.1% in-hospital incidence in COVID-positive STEMI patients and a 0.1% rate in the comparator arm.
“But the biggest headline here for me was that the cardiogenic shock rate was 20.1% in the COVID-positive patients versus 8.7% in the non-COVID STEMI patients,” the cardiologist continued.
The same pattern held true among the COVID-positive NSTEMI patients: They were younger, sicker, and slower to present to the hospital than the non-COVID group. The in-hospital mortality rate was 6.6% in the COVID-positive NSTEMI patients, compared with 1.2% in the reference group. The COVID-positive patients had a 2.5% bleeding rate versus 0.1% in the controls. And the incidence of cardiogenic shock was 5%, compared with 1.4% in the controls from before the pandemic.
“Even though NSTEMI is traditionally regarded as lower risk, this is really quite dramatic. These are sick patients,” Dr. Gershlick observed.
Nearly two-thirds of in-hospital deaths in COVID-positive ACS patients were cardiovascular, and three-quarters of those cardiovascular deaths occurred in patients with cardiogenic shock. Thirty-two percent of deaths in COVID-positive ACS patients were of respiratory causes, and 4.9% were neurologic.
Notably, the ischemic time of patients with cardiogenic shock who died – that is, the time from symptom onset to balloon deployment – averaged 1,271 minutes, compared with 441 minutes in those who died without being in cardiogenic shock.
Session comoderator Sahil A. Parikh, MD, director of endovascular services at Columbia University Medical Center in New York, commented, “One of the striking things that is resonating with me is the high incidence of cardiogenic shock and the mortality. It’s akin to what we’ve seen in New York.”
Discussant Valentin Fuster, MD, PhD, said he doubts that the increased in-hospital mortality in the COVID–ACS registry is related to the prolonged time to presentation at the hospital. More likely, it’s related to the greater thrombotic burden various studies have shown accompanies COVID-positive ACS. It might even be caused by a direct effect of the virus on the myocardium, added Dr. Fuster, director of the Zena and Michael A. Wiener Cardiovascular Institute and professor of medicine at the Icahn School of Medicine at Mount Sinai in New York.
“I have to say I absolutely disagree,” responded Dr. Gershlick. “I think it’s important that we try to understand all the mechanisms, but we know that patients with COVID are anxious, and I think one of the messages from this registry is patients took longer to come to hospital, they were sicker, they had more cardiogenic shock, and they died. And I don’t think it’s anything more complicated than that.”
Another discussant, Mamas Mamas, MD, is involved with a 500-patient U.K. pandemic ACS registry nearing publication. The findings, he said, are similar to what Dr. Gershlick reported in terms of the high rate of presentation with cardiogenic shock and elevated in-hospital mortality. The COVID-positive ACS patients were also more likely to present with out-of-hospital cardiac arrest. But like Dr. Fuster, he is skeptical that their worse outcomes can be explained by a delay in seeking care.
“I don’t think the delay in presentation is really associated with the high mortality rate that we see. The delay in our U.K. registry is maybe half an hour for STEMIs and maybe 2-3 hours for NSTEMIs. And I don’t think that can produce a 30%-40% increase in mortality,” asserted Dr. Mamas, professor of cardiology at Keele University in Staffordshire, England.
Dr. Gershlick reported having no financial conflicts regarding his presentation.
FROM TCT 2020
Brazil confirms death of volunteer in COVID-19 vaccine trial
The Brazilian National Health Surveillance Agency (Anvisa) announced Oct. 21 that it is investigating data received on the death of a volunteer in a clinical trial of the COVID-19 vaccine developed by Oxford University and the pharmaceutical company AstraZeneca.
In an email sent to Medscape Medical News, the agency states that it was formally informed of the death on October 19. It has already received data regarding the investigation of the case, which is now being conducted by the Brazilian International Security Assessment Committee.
The identity of the volunteer and cause of death have not yet been confirmed by any official source linked to the study. In the email, Anvisa reiterated that “according to national and international regulations on good clinical practices, data on clinical research volunteers must be kept confidential, in accordance with the principles of confidentiality, human dignity, and protection of participants.”
A report in the Brazilian newspaper O Globo, however, states that the patient who died is a 28-year-old doctor, recently graduated, who worked on the front line of combating COVID-19 in three hospitals in Rio de Janeiro. . Due to the study design, it is impossible to know whether the volunteer received the vaccine or placebo.
It is imperative to wait for the results of the investigations, said Sergio Cimerman, MD, the scientific coordinator of the Brazilian Society of Infectious Diseases (SBI), because death is possible during any vaccine trial, even more so in cases in which the final goal is to immunize the population in record time.
“It is precisely the phase 3 study that assesses efficacy and safety so that the vaccine can be used for the entire population. We cannot let ourselves lose hope, and we must move forward, as safely as possible, in search of an ideal vaccine,” said Cimerman, who works at the Instituto de Infectologia Emílio Ribas and is also an advisor to the Portuguese edition of Medscape.
This article was translated and adapted from the Portuguese edition of Medscape.
The Brazilian National Health Surveillance Agency (Anvisa) announced Oct. 21 that it is investigating data received on the death of a volunteer in a clinical trial of the COVID-19 vaccine developed by Oxford University and the pharmaceutical company AstraZeneca.
In an email sent to Medscape Medical News, the agency states that it was formally informed of the death on October 19. It has already received data regarding the investigation of the case, which is now being conducted by the Brazilian International Security Assessment Committee.
The identity of the volunteer and cause of death have not yet been confirmed by any official source linked to the study. In the email, Anvisa reiterated that “according to national and international regulations on good clinical practices, data on clinical research volunteers must be kept confidential, in accordance with the principles of confidentiality, human dignity, and protection of participants.”
A report in the Brazilian newspaper O Globo, however, states that the patient who died is a 28-year-old doctor, recently graduated, who worked on the front line of combating COVID-19 in three hospitals in Rio de Janeiro. . Due to the study design, it is impossible to know whether the volunteer received the vaccine or placebo.
It is imperative to wait for the results of the investigations, said Sergio Cimerman, MD, the scientific coordinator of the Brazilian Society of Infectious Diseases (SBI), because death is possible during any vaccine trial, even more so in cases in which the final goal is to immunize the population in record time.
“It is precisely the phase 3 study that assesses efficacy and safety so that the vaccine can be used for the entire population. We cannot let ourselves lose hope, and we must move forward, as safely as possible, in search of an ideal vaccine,” said Cimerman, who works at the Instituto de Infectologia Emílio Ribas and is also an advisor to the Portuguese edition of Medscape.
This article was translated and adapted from the Portuguese edition of Medscape.
The Brazilian National Health Surveillance Agency (Anvisa) announced Oct. 21 that it is investigating data received on the death of a volunteer in a clinical trial of the COVID-19 vaccine developed by Oxford University and the pharmaceutical company AstraZeneca.
In an email sent to Medscape Medical News, the agency states that it was formally informed of the death on October 19. It has already received data regarding the investigation of the case, which is now being conducted by the Brazilian International Security Assessment Committee.
The identity of the volunteer and cause of death have not yet been confirmed by any official source linked to the study. In the email, Anvisa reiterated that “according to national and international regulations on good clinical practices, data on clinical research volunteers must be kept confidential, in accordance with the principles of confidentiality, human dignity, and protection of participants.”
A report in the Brazilian newspaper O Globo, however, states that the patient who died is a 28-year-old doctor, recently graduated, who worked on the front line of combating COVID-19 in three hospitals in Rio de Janeiro. . Due to the study design, it is impossible to know whether the volunteer received the vaccine or placebo.
It is imperative to wait for the results of the investigations, said Sergio Cimerman, MD, the scientific coordinator of the Brazilian Society of Infectious Diseases (SBI), because death is possible during any vaccine trial, even more so in cases in which the final goal is to immunize the population in record time.
“It is precisely the phase 3 study that assesses efficacy and safety so that the vaccine can be used for the entire population. We cannot let ourselves lose hope, and we must move forward, as safely as possible, in search of an ideal vaccine,” said Cimerman, who works at the Instituto de Infectologia Emílio Ribas and is also an advisor to the Portuguese edition of Medscape.
This article was translated and adapted from the Portuguese edition of Medscape.
Survey: Acceptance of COVID-19 vaccine dips below 50%
Less than half of Americans now say that they would get a coronavirus vaccine if one became available, according to a survey conducted Oct. 8-10.
the lowest number since the weekly survey began at the end of February, digital media company Morning Consult reported.
Americans’ willingness to receive such a vaccine reached its high point, 72%, in early April but has been steadily dropping. “Overall willingness has hovered around 50% throughout September, fueled primarily by a sharp drop among Democrats since mid-August, around the time reports of White House interference at the Food and Drug Administration and other federal health agencies began to command more public attention,” Morning Consult noted.
Despite that drop, a majority of Democrats (55%) are still willing to get a COVID-19 vaccine, compared with 48% of Republicans and just 41% of independents. The willingness gap between the two parties was quite a bit wider in the previous poll, conducted Oct. 1-4: 60% of Democrats versus 48% for Republicans, the company said.
“Keeping with longstanding trends, the survey also shows women were less likely to say they’d seek a vaccine than men (42% to 55%), as were people with lower education levels and those who live in rural areas,” the news outlet added.
The latest poll results also show that 33% of respondents (43% of Republicans/25% of Democrats) are socializing in public places. The overall number was just 8% in mid-April but was up to 27% by mid-June. The proportion of all adults who believe in the effectiveness of face masks has been around 80% since April, but there is a significant gap between those who strongly approve of President Trump (66%) and those who strongly disapprove (95%), Morning Consult said.
Less than half of Americans now say that they would get a coronavirus vaccine if one became available, according to a survey conducted Oct. 8-10.
the lowest number since the weekly survey began at the end of February, digital media company Morning Consult reported.
Americans’ willingness to receive such a vaccine reached its high point, 72%, in early April but has been steadily dropping. “Overall willingness has hovered around 50% throughout September, fueled primarily by a sharp drop among Democrats since mid-August, around the time reports of White House interference at the Food and Drug Administration and other federal health agencies began to command more public attention,” Morning Consult noted.
Despite that drop, a majority of Democrats (55%) are still willing to get a COVID-19 vaccine, compared with 48% of Republicans and just 41% of independents. The willingness gap between the two parties was quite a bit wider in the previous poll, conducted Oct. 1-4: 60% of Democrats versus 48% for Republicans, the company said.
“Keeping with longstanding trends, the survey also shows women were less likely to say they’d seek a vaccine than men (42% to 55%), as were people with lower education levels and those who live in rural areas,” the news outlet added.
The latest poll results also show that 33% of respondents (43% of Republicans/25% of Democrats) are socializing in public places. The overall number was just 8% in mid-April but was up to 27% by mid-June. The proportion of all adults who believe in the effectiveness of face masks has been around 80% since April, but there is a significant gap between those who strongly approve of President Trump (66%) and those who strongly disapprove (95%), Morning Consult said.
Less than half of Americans now say that they would get a coronavirus vaccine if one became available, according to a survey conducted Oct. 8-10.
the lowest number since the weekly survey began at the end of February, digital media company Morning Consult reported.
Americans’ willingness to receive such a vaccine reached its high point, 72%, in early April but has been steadily dropping. “Overall willingness has hovered around 50% throughout September, fueled primarily by a sharp drop among Democrats since mid-August, around the time reports of White House interference at the Food and Drug Administration and other federal health agencies began to command more public attention,” Morning Consult noted.
Despite that drop, a majority of Democrats (55%) are still willing to get a COVID-19 vaccine, compared with 48% of Republicans and just 41% of independents. The willingness gap between the two parties was quite a bit wider in the previous poll, conducted Oct. 1-4: 60% of Democrats versus 48% for Republicans, the company said.
“Keeping with longstanding trends, the survey also shows women were less likely to say they’d seek a vaccine than men (42% to 55%), as were people with lower education levels and those who live in rural areas,” the news outlet added.
The latest poll results also show that 33% of respondents (43% of Republicans/25% of Democrats) are socializing in public places. The overall number was just 8% in mid-April but was up to 27% by mid-June. The proportion of all adults who believe in the effectiveness of face masks has been around 80% since April, but there is a significant gap between those who strongly approve of President Trump (66%) and those who strongly disapprove (95%), Morning Consult said.
Latest week brings 44,000 more children with COVID-19
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
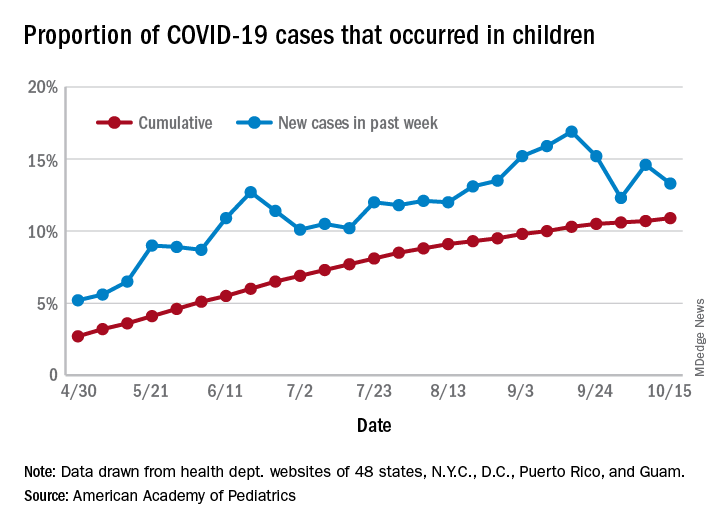
The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases among children was 741,891 as of Oct. 15, which puts the cumulative proportion at 10.9% of the 6.8 million cases reported in all ages by 49 states (New York does not report ages), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
The 44,258 new cases in children represented 13.3% of all cases reported during the week ending Oct. 15, down from 14.6% the previous week (children make up almost 23% of the total U.S. population), the AAP/CHA data show.
Those data also indicate that there have been almost 986 cases of COVID-19 per 100,000 children in the United States. Corresponding rates among the states range from 181 per 100,000 in Vermont to 2,581 per 100,000 in North Dakota. Tennessee (2,277) and South Carolina (2,212) are the only other states above 2,000, according to the report.
California has reported the most child cases, 89,843 (1,010 per 100,000 children), so far, followed by Florida (44,199), Illinois (42,132), and Tennessee (40,137). Seven other states have had over 20,000 cases each, the AAP and CHA noted.
Measures of severe illness continue to be low, although the data are less comprehensive. Children represent only 1.7% of all COVID-19 hospitalizations (24 states and N.Y.C. reporting) and 0.07% of all deaths (42 states and N.Y.C. reporting). Thirteen states and D.C. have had no deaths yet, while Texas has reported three times as many (27) as any other state (Arizona is next with 9, although N.Y.C. has had 15), the AAP/CHA report said.
COVID-19 antibody response not reduced with diabetes
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Neither diabetes per se nor hyperglycemia appear to impair the antibody response to SARS-CoV-2, suggesting that a COVID-19 vaccine would be just as effective in people with diabetes as in those without, new research finds.
Results from a study involving 480 patients with confirmed COVID-19 seen at an Italian hospital between February 25 and April 19 were published online October 8 in Diabetologia by Vito Lampasona, MD, and colleagues.
Antibody responses against multiple SARS-CoV-2 antigens among the 27% of patients with COVID-19 and diabetes (preexisting and newly diagnosed) were similar with regard to timing, titers, and classes to those of patients with COVID-19 and without diabetes, and the results did not differ by glucose levels.
Moreover, positivity for immunoglobulin G (IgG) against the SARS-CoV-2 spike receptor-binding domain (RBD) was associated with improved survival regardless of diabetes status.
And as previously shown, high blood glucose levels were strongly associated with greater COVID-19 mortality even in those without diabetes.
This is the first study of the immunologic humoral response against SARS-CoV-2 in patients with hyperglycemia, the authors say.
“The immunological response to a future SARS-CoV-2 vaccine will be assessed when the vaccine becomes available. However, our data allow a cautious optimism regarding effective immunization in individuals with diabetes, as well as in the general population,” wrote Dr. Lampasona of San Raffaele Diabetes Research Institute, IRCCS Ospedale San Raffaele in Milan, and colleagues.
Diabetes and hyperglycemia worsen COVID-19 outcomes
The investigators analyzed the presence of three types of antibody to multiple SARS-CoV-2 antigens in 509 participants: IgG, which is evidence of past infection; IgM, which indicates more recent or current infection; and IgA, which is involved in the mucosal immune response, for example, in the nose where the virus enters the body.
Overall, 452 (88.8%) patients were hospitalized, 79 (15.5%) patients were admitted to intensive care, and 93 (18.3%) patients died during follow-up.
Of the 139 patients with diabetes, 90 (17.7% of the study cohort) already had a diagnosis of diabetes, and 49 (9.6%) were newly diagnosed.
Those with diabetes were older, had a higher body mass index (BMI), and were more likely to have cardiovascular comorbidities, hypertension, and chronic kidney disease. As has been previously reported for diabetes and COVID-19, diabetes was also associated with increased levels of inflammatory biomarkers, hypercoagulopathy, leukocytosis, and neutrophilia.
In multivariate analysis, diabetes status (hazard ratio, 2.32; P = .001), mean fasting plasma glucose (P < .001), and glucose variability (P = .002) were all independently associated with increased mortality and ICU admission. And fasting plasma glucose was associated with increased mortality risk even among those without diabetes (P < .001).
Antibody response similar in patients with and without diabetes
The humoral response against SARS-CoV-2 in patients with diabetes was present and superimposable in terms of timing and antibody titers to that of patients without diabetes, with marginal differences, and was not influenced by glucose levels.
After adjustment for sex, age, and diabetes status and stratification by symptom duration at time of sampling, the development of SARS-CoV-2 RBD IgG antibodies was associated with improved survival, with an HR for time to death of 0.4 (P = .002).
“Of the measured antibody responses, positivity for IgG against the SARS-CoV-2 spike RBD was predictive of survival rate, both in the presence or absence of diabetes,” the authors stressed, with similar HRs for those with diabetes (0.37; P = .013) and without diabetes (0.43; P = .038).
These data confirm “the relevance for patient survival rate of the specific antigen response against spike RBD even in the presence of diabetes, and it underlines how the mechanism explaining the worse clinical outcome in patients with diabetes is unrelated to the antibody response,” they explain.
They added, “This, together with evidence that increased blood glucose levels do predict a poor prognosis even in nondiabetic individuals and the association with increased levels of inflammatory biomarkers and hypercoagulopathy, as well as leukocytosis and neutrophilia, support the speculation that glucose per se could be an independent biological negative factor, acting as a direct regulator of innate immunity.”
“The observed increased severity and mortality risk of COVID-19 pneumonia in patients with hyperglycemia was not the result of an impaired humoral response against SARS-CoV-2.”
“RBD IgG positivity was associated with a remarkable protective effect, allowing for a cautious optimism about the efficacy of future vaccines against SARS-COV-2 in people with diabetes,” they reiterated.
The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.



