User login
Older age, r/r disease in lymphoma patients tied to increased COVID-19 death rate
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
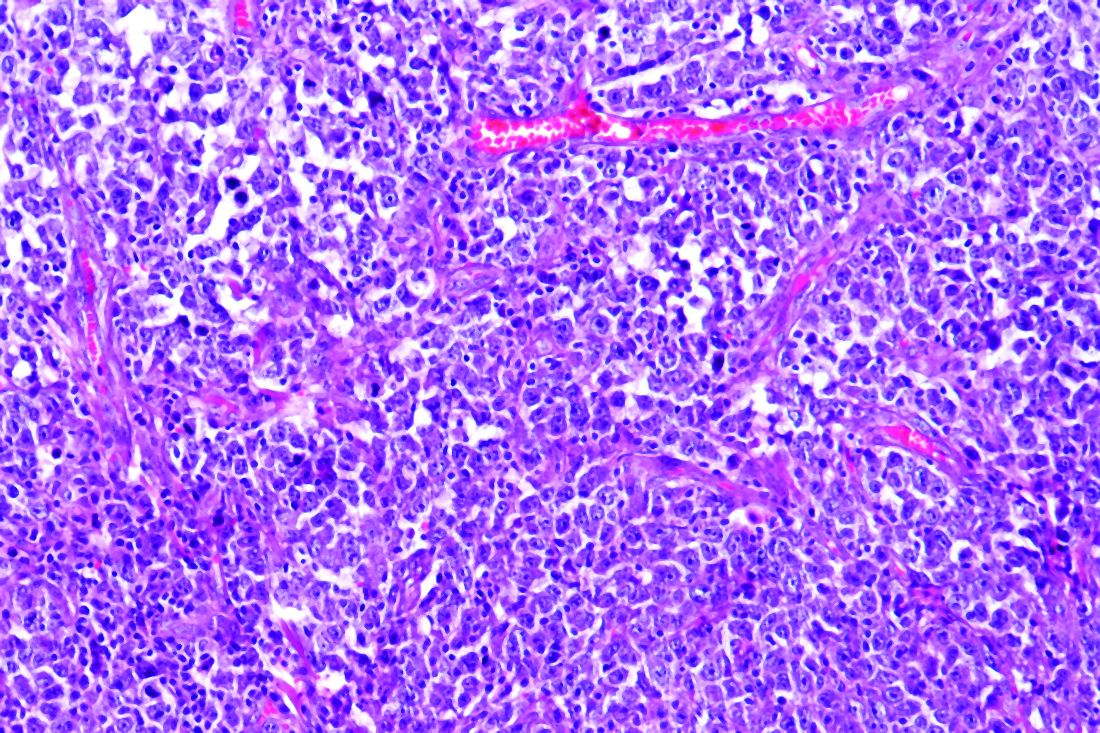
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
FROM ECLINICALMEDICINE
Link between vitamin D and ICU outcomes unclear
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CHEST 2020
Sleepless nights, hair loss, and cracked teeth: Pandemic stress takes its toll
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Survey: Doctors lonely, burned out in COVID-19
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Fauci: Cautious optimism for COVID-19 vaccine by end of 2020
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
FROM CHEST 2020
NACMI: Clear benefit with PCI in STEMI COVID-19 patients
Patients with COVID-19 who present with ST-segment elevation MI (STEMI) represent a unique, high-risk population with greater risks for in-hospital death and stroke, according to initial results from the North American COVID-19 ST-Segment Elevation Myocardial Infarction Registry (NACMI).
Although COVID-19–confirmed patients were less likely to undergo angiography than patients under investigation (PUI) for COVID-19 or historical STEMI activation controls, 71% underwent primary percutaneous coronary intervention (PCI).
“Primary PCI is preferable and feasible in COVID-19–positive patients, with door-to-balloon times similar to PUI or COVID-negative patients, and that supports the updated COVID-specific STEMI guidelines,” study cochair Timothy D. Henry, MD, said in a late-breaking clinical science session at TCT 2020, the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
The multisociety COVID-specific guidelines were initially issued in April, endorsing PCI as the standard of care and allowing for consideration of fibrinolysis-based therapy at non-PCI capable hospitals.
Five previous publications on a total of 174 COVID-19 patients with ST-elevation have shown there are more frequent in-hospital STEMI presentations, more cases without a clear culprit lesion, more thrombotic lesions and microthrombi, and higher mortality, ranging from 12% to 72%. Still, there has been considerable controversy over exactly what to do when COVID-19 patients with ST elevation reach the cath lab, he said at the meeting sponsored by the Cardiovascular Research Foundation.
NACMI represents the largest experience with ST-elevation patients and is a unique collaboration between the Society for Cardiovascular Angiography and Interventions, Canadian Association of Interventional Cardiology, American College of Cardiology, and Midwest STEMI Consortium, noted Dr. Henry, who is medical director of the Lindner Center for Research and Education at the Christ Hospital, Cincinnati.
The registry enrolled any COVID-19–positive patient or person under investigation older than 18 years with ST-segment elevation or new-onset left bundle branch block on electrocardiogram with a clinical correlate of myocardial ischemia such as chest pain, dyspnea, cardiac arrest, shock, or mechanical ventilation. There were no exclusion criteria.
Data from 171 patients with confirmed COVID-19 and 423 PUI from 64 sites were then propensity-matched to a control population from the Midwest STEMI Consortium, a prospective, multicenter registry of consecutive STEMI patients.
The three groups were similar in sex and age but there was a striking difference in race, with 27% of African American and 24% of Hispanic patients COVID-confirmed, compared with 11% and 6% in the PUI group and 4% and 1% in the control group. Likewise, there was a significant increase in diabetes (44% vs. 33% vs. 20%), which has been reported previously with influenza.
COVID-19–positive patients, as compared with PUI and controls, were significantly more likely to present with cardiogenic shock before PCI (20% vs. 14% vs. 5%), but not cardiac arrest (12% vs. 17% vs. 11%), and to have lower left ventricular ejection fractions (45% vs. 45% vs. 50%).
They also presented with more atypical symptoms than PUI patients, particularly infiltrates on chest x-ray (49% vs. 17%) and dyspnea (58% vs. 38%). Data were not available for these outcomes among historic controls.
Importantly, 21% of the COVID-19 patients did not undergo angiography, compared with 5% of PUI patients and 0% of controls (P < .001), “which is much higher than we would expect or have suspected,” Dr. Henry said. Thrombolytic use was very uncommon in those undergoing angiography, likely as a result of the guidelines.
Very surprisingly, there were no differences in door-to-balloon times between the COVID-positive, PUI, and control groups despite the ongoing pandemic (80 min vs. 78 min vs. 86 min).
But there was clear worsening in in-hospital mortality in COVID-19–positive patients (32% vs. 12% and 6%; P < .001), as well as in-hospital stroke (3.4% vs. 2% vs. 0.6%) that reached statistical significance only when compared with historical controls (P = .039). Total length of stay was twice as long in COVID-confirmed patients as in both PUI and controls (6 days vs. 3 days; P < .001).
Following the formal presentation, invited discussant Philippe Gabriel Steg, MD, Imperial College London, said the researchers have provided a great service in reporting the data so quickly but noted that an ongoing French registry of events before, during, and after the first COVID-19 wave has not seen an increased death rate.
“Can you tease out whether the increased death rate is related to cardiovascular deaths or to COVID-related pneumonias, shocks, ARDSs [acute respiratory distress syndromes], and so on and so forth? Because our impression – and that’s what we’ve published in Lancet Public Health – is that the cardiovascular morality rate doesn’t seem that affected by COVID.”
Dr. Henry replied that these are early data but “I will tell you that patients who did get PCI had a mortality rate that was only around 12% or 13%, and the patients who did not undergo angiography or were treated with medical therapy had higher mortality. Now, of course, that’s selected and we need to do a much better matching and look at that, but that’s our goal and we will have that information,” he said.
During a press briefing on the study, discussant Renu Virmani, MD, president and founder of CVPath Institute, noted that, in their analysis of 40 autopsy cases from Bergamot, Italy, small intramyocardial microthrombi were seen in nine patients, whereas epicardial microthrombi were seen in only three or four.
“Some of the cases are being taken as being related to coronary disease but may be more thrombotic than anything else,” she said. “I think there’s a combination, and that’s why the outcomes are so poor. You didn’t show us TIMI flow but that’s something to think about: Was TIMI flow different in the patients who died because you have very high mortality? I think we need to get to the bottom of what is the underlying cause of that thrombosis.”
Future topics of interest include ethnic and regional/country differences; time-to-treatment including chest pain onset-to-arrival; transfer, in-hospital, and no-culprit patients; changes over time during the pandemic; and eventually 1-year outcomes, Dr. Henry said.
Press briefing moderator Ajay Kirtane, MD, director of the cardiac catheterization labs at NewYork-Presbyterian/Columbia University Irving, New York, remarked that “a lot of times people will pooh-pooh observational data, but this is exactly the type of data that we need to try to be able to gather information about what our practices are, how they fit. And I think many of us around the world will see these data, and it will echo their own experience.”
The study was funded by the Society for Cardiovascular Angiography and Interventions and the Canadian Association of Interventional Cardiology. Dr. Henry has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with COVID-19 who present with ST-segment elevation MI (STEMI) represent a unique, high-risk population with greater risks for in-hospital death and stroke, according to initial results from the North American COVID-19 ST-Segment Elevation Myocardial Infarction Registry (NACMI).
Although COVID-19–confirmed patients were less likely to undergo angiography than patients under investigation (PUI) for COVID-19 or historical STEMI activation controls, 71% underwent primary percutaneous coronary intervention (PCI).
“Primary PCI is preferable and feasible in COVID-19–positive patients, with door-to-balloon times similar to PUI or COVID-negative patients, and that supports the updated COVID-specific STEMI guidelines,” study cochair Timothy D. Henry, MD, said in a late-breaking clinical science session at TCT 2020, the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
The multisociety COVID-specific guidelines were initially issued in April, endorsing PCI as the standard of care and allowing for consideration of fibrinolysis-based therapy at non-PCI capable hospitals.
Five previous publications on a total of 174 COVID-19 patients with ST-elevation have shown there are more frequent in-hospital STEMI presentations, more cases without a clear culprit lesion, more thrombotic lesions and microthrombi, and higher mortality, ranging from 12% to 72%. Still, there has been considerable controversy over exactly what to do when COVID-19 patients with ST elevation reach the cath lab, he said at the meeting sponsored by the Cardiovascular Research Foundation.
NACMI represents the largest experience with ST-elevation patients and is a unique collaboration between the Society for Cardiovascular Angiography and Interventions, Canadian Association of Interventional Cardiology, American College of Cardiology, and Midwest STEMI Consortium, noted Dr. Henry, who is medical director of the Lindner Center for Research and Education at the Christ Hospital, Cincinnati.
The registry enrolled any COVID-19–positive patient or person under investigation older than 18 years with ST-segment elevation or new-onset left bundle branch block on electrocardiogram with a clinical correlate of myocardial ischemia such as chest pain, dyspnea, cardiac arrest, shock, or mechanical ventilation. There were no exclusion criteria.
Data from 171 patients with confirmed COVID-19 and 423 PUI from 64 sites were then propensity-matched to a control population from the Midwest STEMI Consortium, a prospective, multicenter registry of consecutive STEMI patients.
The three groups were similar in sex and age but there was a striking difference in race, with 27% of African American and 24% of Hispanic patients COVID-confirmed, compared with 11% and 6% in the PUI group and 4% and 1% in the control group. Likewise, there was a significant increase in diabetes (44% vs. 33% vs. 20%), which has been reported previously with influenza.
COVID-19–positive patients, as compared with PUI and controls, were significantly more likely to present with cardiogenic shock before PCI (20% vs. 14% vs. 5%), but not cardiac arrest (12% vs. 17% vs. 11%), and to have lower left ventricular ejection fractions (45% vs. 45% vs. 50%).
They also presented with more atypical symptoms than PUI patients, particularly infiltrates on chest x-ray (49% vs. 17%) and dyspnea (58% vs. 38%). Data were not available for these outcomes among historic controls.
Importantly, 21% of the COVID-19 patients did not undergo angiography, compared with 5% of PUI patients and 0% of controls (P < .001), “which is much higher than we would expect or have suspected,” Dr. Henry said. Thrombolytic use was very uncommon in those undergoing angiography, likely as a result of the guidelines.
Very surprisingly, there were no differences in door-to-balloon times between the COVID-positive, PUI, and control groups despite the ongoing pandemic (80 min vs. 78 min vs. 86 min).
But there was clear worsening in in-hospital mortality in COVID-19–positive patients (32% vs. 12% and 6%; P < .001), as well as in-hospital stroke (3.4% vs. 2% vs. 0.6%) that reached statistical significance only when compared with historical controls (P = .039). Total length of stay was twice as long in COVID-confirmed patients as in both PUI and controls (6 days vs. 3 days; P < .001).
Following the formal presentation, invited discussant Philippe Gabriel Steg, MD, Imperial College London, said the researchers have provided a great service in reporting the data so quickly but noted that an ongoing French registry of events before, during, and after the first COVID-19 wave has not seen an increased death rate.
“Can you tease out whether the increased death rate is related to cardiovascular deaths or to COVID-related pneumonias, shocks, ARDSs [acute respiratory distress syndromes], and so on and so forth? Because our impression – and that’s what we’ve published in Lancet Public Health – is that the cardiovascular morality rate doesn’t seem that affected by COVID.”
Dr. Henry replied that these are early data but “I will tell you that patients who did get PCI had a mortality rate that was only around 12% or 13%, and the patients who did not undergo angiography or were treated with medical therapy had higher mortality. Now, of course, that’s selected and we need to do a much better matching and look at that, but that’s our goal and we will have that information,” he said.
During a press briefing on the study, discussant Renu Virmani, MD, president and founder of CVPath Institute, noted that, in their analysis of 40 autopsy cases from Bergamot, Italy, small intramyocardial microthrombi were seen in nine patients, whereas epicardial microthrombi were seen in only three or four.
“Some of the cases are being taken as being related to coronary disease but may be more thrombotic than anything else,” she said. “I think there’s a combination, and that’s why the outcomes are so poor. You didn’t show us TIMI flow but that’s something to think about: Was TIMI flow different in the patients who died because you have very high mortality? I think we need to get to the bottom of what is the underlying cause of that thrombosis.”
Future topics of interest include ethnic and regional/country differences; time-to-treatment including chest pain onset-to-arrival; transfer, in-hospital, and no-culprit patients; changes over time during the pandemic; and eventually 1-year outcomes, Dr. Henry said.
Press briefing moderator Ajay Kirtane, MD, director of the cardiac catheterization labs at NewYork-Presbyterian/Columbia University Irving, New York, remarked that “a lot of times people will pooh-pooh observational data, but this is exactly the type of data that we need to try to be able to gather information about what our practices are, how they fit. And I think many of us around the world will see these data, and it will echo their own experience.”
The study was funded by the Society for Cardiovascular Angiography and Interventions and the Canadian Association of Interventional Cardiology. Dr. Henry has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients with COVID-19 who present with ST-segment elevation MI (STEMI) represent a unique, high-risk population with greater risks for in-hospital death and stroke, according to initial results from the North American COVID-19 ST-Segment Elevation Myocardial Infarction Registry (NACMI).
Although COVID-19–confirmed patients were less likely to undergo angiography than patients under investigation (PUI) for COVID-19 or historical STEMI activation controls, 71% underwent primary percutaneous coronary intervention (PCI).
“Primary PCI is preferable and feasible in COVID-19–positive patients, with door-to-balloon times similar to PUI or COVID-negative patients, and that supports the updated COVID-specific STEMI guidelines,” study cochair Timothy D. Henry, MD, said in a late-breaking clinical science session at TCT 2020, the Transcatheter Cardiovascular Therapeutics virtual annual meeting.
The multisociety COVID-specific guidelines were initially issued in April, endorsing PCI as the standard of care and allowing for consideration of fibrinolysis-based therapy at non-PCI capable hospitals.
Five previous publications on a total of 174 COVID-19 patients with ST-elevation have shown there are more frequent in-hospital STEMI presentations, more cases without a clear culprit lesion, more thrombotic lesions and microthrombi, and higher mortality, ranging from 12% to 72%. Still, there has been considerable controversy over exactly what to do when COVID-19 patients with ST elevation reach the cath lab, he said at the meeting sponsored by the Cardiovascular Research Foundation.
NACMI represents the largest experience with ST-elevation patients and is a unique collaboration between the Society for Cardiovascular Angiography and Interventions, Canadian Association of Interventional Cardiology, American College of Cardiology, and Midwest STEMI Consortium, noted Dr. Henry, who is medical director of the Lindner Center for Research and Education at the Christ Hospital, Cincinnati.
The registry enrolled any COVID-19–positive patient or person under investigation older than 18 years with ST-segment elevation or new-onset left bundle branch block on electrocardiogram with a clinical correlate of myocardial ischemia such as chest pain, dyspnea, cardiac arrest, shock, or mechanical ventilation. There were no exclusion criteria.
Data from 171 patients with confirmed COVID-19 and 423 PUI from 64 sites were then propensity-matched to a control population from the Midwest STEMI Consortium, a prospective, multicenter registry of consecutive STEMI patients.
The three groups were similar in sex and age but there was a striking difference in race, with 27% of African American and 24% of Hispanic patients COVID-confirmed, compared with 11% and 6% in the PUI group and 4% and 1% in the control group. Likewise, there was a significant increase in diabetes (44% vs. 33% vs. 20%), which has been reported previously with influenza.
COVID-19–positive patients, as compared with PUI and controls, were significantly more likely to present with cardiogenic shock before PCI (20% vs. 14% vs. 5%), but not cardiac arrest (12% vs. 17% vs. 11%), and to have lower left ventricular ejection fractions (45% vs. 45% vs. 50%).
They also presented with more atypical symptoms than PUI patients, particularly infiltrates on chest x-ray (49% vs. 17%) and dyspnea (58% vs. 38%). Data were not available for these outcomes among historic controls.
Importantly, 21% of the COVID-19 patients did not undergo angiography, compared with 5% of PUI patients and 0% of controls (P < .001), “which is much higher than we would expect or have suspected,” Dr. Henry said. Thrombolytic use was very uncommon in those undergoing angiography, likely as a result of the guidelines.
Very surprisingly, there were no differences in door-to-balloon times between the COVID-positive, PUI, and control groups despite the ongoing pandemic (80 min vs. 78 min vs. 86 min).
But there was clear worsening in in-hospital mortality in COVID-19–positive patients (32% vs. 12% and 6%; P < .001), as well as in-hospital stroke (3.4% vs. 2% vs. 0.6%) that reached statistical significance only when compared with historical controls (P = .039). Total length of stay was twice as long in COVID-confirmed patients as in both PUI and controls (6 days vs. 3 days; P < .001).
Following the formal presentation, invited discussant Philippe Gabriel Steg, MD, Imperial College London, said the researchers have provided a great service in reporting the data so quickly but noted that an ongoing French registry of events before, during, and after the first COVID-19 wave has not seen an increased death rate.
“Can you tease out whether the increased death rate is related to cardiovascular deaths or to COVID-related pneumonias, shocks, ARDSs [acute respiratory distress syndromes], and so on and so forth? Because our impression – and that’s what we’ve published in Lancet Public Health – is that the cardiovascular morality rate doesn’t seem that affected by COVID.”
Dr. Henry replied that these are early data but “I will tell you that patients who did get PCI had a mortality rate that was only around 12% or 13%, and the patients who did not undergo angiography or were treated with medical therapy had higher mortality. Now, of course, that’s selected and we need to do a much better matching and look at that, but that’s our goal and we will have that information,” he said.
During a press briefing on the study, discussant Renu Virmani, MD, president and founder of CVPath Institute, noted that, in their analysis of 40 autopsy cases from Bergamot, Italy, small intramyocardial microthrombi were seen in nine patients, whereas epicardial microthrombi were seen in only three or four.
“Some of the cases are being taken as being related to coronary disease but may be more thrombotic than anything else,” she said. “I think there’s a combination, and that’s why the outcomes are so poor. You didn’t show us TIMI flow but that’s something to think about: Was TIMI flow different in the patients who died because you have very high mortality? I think we need to get to the bottom of what is the underlying cause of that thrombosis.”
Future topics of interest include ethnic and regional/country differences; time-to-treatment including chest pain onset-to-arrival; transfer, in-hospital, and no-culprit patients; changes over time during the pandemic; and eventually 1-year outcomes, Dr. Henry said.
Press briefing moderator Ajay Kirtane, MD, director of the cardiac catheterization labs at NewYork-Presbyterian/Columbia University Irving, New York, remarked that “a lot of times people will pooh-pooh observational data, but this is exactly the type of data that we need to try to be able to gather information about what our practices are, how they fit. And I think many of us around the world will see these data, and it will echo their own experience.”
The study was funded by the Society for Cardiovascular Angiography and Interventions and the Canadian Association of Interventional Cardiology. Dr. Henry has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Diarrhea prevalent among COVID-19 patients with IBD
Diarrhea affected one in every five patients with inflammatory bowel disease (IBD) and COVID-19, compared with only 7%-10% of all patients with COVID-19 in prior studies, researchers reported in Clinical Gastroenterology and Hepatology.
In a systematic review and meta-analysis of 23 studies incorporating data from 449 patients with IBD and COVID-19, their most common symptoms were fever (affecting 48.3% of patients), cough (46.5%), and diarrhea (20.5%), and diarrhea was approximately twice as prevalent as dyspnea, nausea, abdominal pain, and fatigue, wrote Ferdinando D’Amico of Humanitas University in Milan and his associates. “[S]ymptoms experienced by IBD patients with COVID-19 are similar to those occurring in the general population, except for a higher percentage of diarrhea,” they wrote. This increased prevalence might result from IBD itself or from inflammatory effects of viral gut tropism, they noted. “Currently, the diagnostic–therapeutic approach does not differ between IBD and non-IBD patients, but further studies are needed to evaluate whether fecal research of viral RNA and treatment with IBD drugs may play a role in the management of COVID-19 patients.”
To characterize the clinical presentation and course of patients with IBD and COVID-19, the researchers searched PubMed, Embase, Web of Science, and MedRxiv through July 29, 2020, for keywords related to COVID-19, Crohn’s disease, ulcerative colitis, and IBD. They identified 23 studies presenting clinical data from adults or children with a confirmed IBD diagnosis and least one case of COVID-19. Among 243,760 patients with IBD, 1,028 patients had COVID-19 infection, including 509 patients with Crohn’s disease, 428 patients with ulcerative colitis, 49 patients with indeterminate colitis, and 42 patients for whom the IBD subtype was not recorded.
In all, 0.4% of patients with IBD had COVID-19. Nearly all had been diagnosed by polymerase chain reaction of nasopharyngeal swabs, and approximately 40% also had received chest CT scans. Most were male (56.5%), and 43.5% were older than 65 years. Patients were receiving a wide range of IBD therapies, most commonly anti–tumor necrosis factor (TNF) agents, mesalamine, thiopurine (alone or in combination with biologics), vedolizumab, ustekinumab, steroids, methotrexate, and tofacitinib. Results from six studies indicated that patients with IBD were significantly more likely to be diagnosed with COVID-19 if they were older than 66 years (odds ratio, 21.3) or had other comorbidities (OR, 1.24). The most commonly used drugs for managing COVID-19 were hydroxychloroquine, lopinavir/ritonavir, steroids, antibiotics, chloroquine, tofacitinib, and infliximab.
A total of 30.6% of patients with IBD and COVID-19 were hospitalized, 11.4% stayed in the ICU, 3.7% required mechanical ventilation, and 3.8% died from COVID-19. Significant risk factors for death from COVID-19 included older age, active IBD, and a Charlson Comorbidity Index score above 1. Similarly, risk factors for severe COVID-19 included older age, having two or more comorbidities, receiving systemic steroids, and receiving mesalamine/sulfasalazine. In one study, a recent (3-month) history of corticosteroid treatment was associated with a 60% increase in the risk for severe COVID-19. Other immune-mediated therapies did not show this association. Patients with ulcerative colitis were significantly more likely to be seen in the ED or hospitalized, compared with patients with other forms of IBD (adjusted OR, 12.7).
No funding sources were disclosed. Dr. D’Amico reported having no conflicts of interest. Two coinvestigators disclosed ties to AbbVie, MSD, Schering-Plough, UCB Pharma, and several other pharmaceutical companies.
SOURCE: D’Amico F et al. Clin Gastroenterol Hepatol. 2020 Aug 7. doi: 10.1016/j.cgh.2020.08.003.
Diarrhea is one of the hallmark features in inflammatory bowel disease (IBD). The systematic review and meta-analysis by D’Amico and colleagues highlights an increased prevalence of diarrhea in IBD patients with COVID-19. We have learned that SARS-CoV-2 enters the gastrointestinal tract through angiotensin converting enzyme 2, which has been found in absorptive enterocytes of the ileum and colon. The subsequent invasion can cause a change in intestinal microbiota (dysbiosis) and trigger diarrhea. Prior studies also reported SARS-CoV-2 being isolated in the duodenum and rectum while showing RNA shedding in approximately 40% of patients. Clinicians may now face the diagnostic challenge of distinguishing the cause of diarrhea as an exacerbation from underlying IBD versus viral superinfection. The authors astutely hypothesized that having access to fecal polymerase chain reaction tests may be particularly useful to guiding clinical treatment decisions.
Lukasz Kwapisz, MD, FRCPC, is assistant professor of medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
Diarrhea is one of the hallmark features in inflammatory bowel disease (IBD). The systematic review and meta-analysis by D’Amico and colleagues highlights an increased prevalence of diarrhea in IBD patients with COVID-19. We have learned that SARS-CoV-2 enters the gastrointestinal tract through angiotensin converting enzyme 2, which has been found in absorptive enterocytes of the ileum and colon. The subsequent invasion can cause a change in intestinal microbiota (dysbiosis) and trigger diarrhea. Prior studies also reported SARS-CoV-2 being isolated in the duodenum and rectum while showing RNA shedding in approximately 40% of patients. Clinicians may now face the diagnostic challenge of distinguishing the cause of diarrhea as an exacerbation from underlying IBD versus viral superinfection. The authors astutely hypothesized that having access to fecal polymerase chain reaction tests may be particularly useful to guiding clinical treatment decisions.
Lukasz Kwapisz, MD, FRCPC, is assistant professor of medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
Diarrhea is one of the hallmark features in inflammatory bowel disease (IBD). The systematic review and meta-analysis by D’Amico and colleagues highlights an increased prevalence of diarrhea in IBD patients with COVID-19. We have learned that SARS-CoV-2 enters the gastrointestinal tract through angiotensin converting enzyme 2, which has been found in absorptive enterocytes of the ileum and colon. The subsequent invasion can cause a change in intestinal microbiota (dysbiosis) and trigger diarrhea. Prior studies also reported SARS-CoV-2 being isolated in the duodenum and rectum while showing RNA shedding in approximately 40% of patients. Clinicians may now face the diagnostic challenge of distinguishing the cause of diarrhea as an exacerbation from underlying IBD versus viral superinfection. The authors astutely hypothesized that having access to fecal polymerase chain reaction tests may be particularly useful to guiding clinical treatment decisions.
Lukasz Kwapisz, MD, FRCPC, is assistant professor of medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
Diarrhea affected one in every five patients with inflammatory bowel disease (IBD) and COVID-19, compared with only 7%-10% of all patients with COVID-19 in prior studies, researchers reported in Clinical Gastroenterology and Hepatology.
In a systematic review and meta-analysis of 23 studies incorporating data from 449 patients with IBD and COVID-19, their most common symptoms were fever (affecting 48.3% of patients), cough (46.5%), and diarrhea (20.5%), and diarrhea was approximately twice as prevalent as dyspnea, nausea, abdominal pain, and fatigue, wrote Ferdinando D’Amico of Humanitas University in Milan and his associates. “[S]ymptoms experienced by IBD patients with COVID-19 are similar to those occurring in the general population, except for a higher percentage of diarrhea,” they wrote. This increased prevalence might result from IBD itself or from inflammatory effects of viral gut tropism, they noted. “Currently, the diagnostic–therapeutic approach does not differ between IBD and non-IBD patients, but further studies are needed to evaluate whether fecal research of viral RNA and treatment with IBD drugs may play a role in the management of COVID-19 patients.”
To characterize the clinical presentation and course of patients with IBD and COVID-19, the researchers searched PubMed, Embase, Web of Science, and MedRxiv through July 29, 2020, for keywords related to COVID-19, Crohn’s disease, ulcerative colitis, and IBD. They identified 23 studies presenting clinical data from adults or children with a confirmed IBD diagnosis and least one case of COVID-19. Among 243,760 patients with IBD, 1,028 patients had COVID-19 infection, including 509 patients with Crohn’s disease, 428 patients with ulcerative colitis, 49 patients with indeterminate colitis, and 42 patients for whom the IBD subtype was not recorded.
In all, 0.4% of patients with IBD had COVID-19. Nearly all had been diagnosed by polymerase chain reaction of nasopharyngeal swabs, and approximately 40% also had received chest CT scans. Most were male (56.5%), and 43.5% were older than 65 years. Patients were receiving a wide range of IBD therapies, most commonly anti–tumor necrosis factor (TNF) agents, mesalamine, thiopurine (alone or in combination with biologics), vedolizumab, ustekinumab, steroids, methotrexate, and tofacitinib. Results from six studies indicated that patients with IBD were significantly more likely to be diagnosed with COVID-19 if they were older than 66 years (odds ratio, 21.3) or had other comorbidities (OR, 1.24). The most commonly used drugs for managing COVID-19 were hydroxychloroquine, lopinavir/ritonavir, steroids, antibiotics, chloroquine, tofacitinib, and infliximab.
A total of 30.6% of patients with IBD and COVID-19 were hospitalized, 11.4% stayed in the ICU, 3.7% required mechanical ventilation, and 3.8% died from COVID-19. Significant risk factors for death from COVID-19 included older age, active IBD, and a Charlson Comorbidity Index score above 1. Similarly, risk factors for severe COVID-19 included older age, having two or more comorbidities, receiving systemic steroids, and receiving mesalamine/sulfasalazine. In one study, a recent (3-month) history of corticosteroid treatment was associated with a 60% increase in the risk for severe COVID-19. Other immune-mediated therapies did not show this association. Patients with ulcerative colitis were significantly more likely to be seen in the ED or hospitalized, compared with patients with other forms of IBD (adjusted OR, 12.7).
No funding sources were disclosed. Dr. D’Amico reported having no conflicts of interest. Two coinvestigators disclosed ties to AbbVie, MSD, Schering-Plough, UCB Pharma, and several other pharmaceutical companies.
SOURCE: D’Amico F et al. Clin Gastroenterol Hepatol. 2020 Aug 7. doi: 10.1016/j.cgh.2020.08.003.
Diarrhea affected one in every five patients with inflammatory bowel disease (IBD) and COVID-19, compared with only 7%-10% of all patients with COVID-19 in prior studies, researchers reported in Clinical Gastroenterology and Hepatology.
In a systematic review and meta-analysis of 23 studies incorporating data from 449 patients with IBD and COVID-19, their most common symptoms were fever (affecting 48.3% of patients), cough (46.5%), and diarrhea (20.5%), and diarrhea was approximately twice as prevalent as dyspnea, nausea, abdominal pain, and fatigue, wrote Ferdinando D’Amico of Humanitas University in Milan and his associates. “[S]ymptoms experienced by IBD patients with COVID-19 are similar to those occurring in the general population, except for a higher percentage of diarrhea,” they wrote. This increased prevalence might result from IBD itself or from inflammatory effects of viral gut tropism, they noted. “Currently, the diagnostic–therapeutic approach does not differ between IBD and non-IBD patients, but further studies are needed to evaluate whether fecal research of viral RNA and treatment with IBD drugs may play a role in the management of COVID-19 patients.”
To characterize the clinical presentation and course of patients with IBD and COVID-19, the researchers searched PubMed, Embase, Web of Science, and MedRxiv through July 29, 2020, for keywords related to COVID-19, Crohn’s disease, ulcerative colitis, and IBD. They identified 23 studies presenting clinical data from adults or children with a confirmed IBD diagnosis and least one case of COVID-19. Among 243,760 patients with IBD, 1,028 patients had COVID-19 infection, including 509 patients with Crohn’s disease, 428 patients with ulcerative colitis, 49 patients with indeterminate colitis, and 42 patients for whom the IBD subtype was not recorded.
In all, 0.4% of patients with IBD had COVID-19. Nearly all had been diagnosed by polymerase chain reaction of nasopharyngeal swabs, and approximately 40% also had received chest CT scans. Most were male (56.5%), and 43.5% were older than 65 years. Patients were receiving a wide range of IBD therapies, most commonly anti–tumor necrosis factor (TNF) agents, mesalamine, thiopurine (alone or in combination with biologics), vedolizumab, ustekinumab, steroids, methotrexate, and tofacitinib. Results from six studies indicated that patients with IBD were significantly more likely to be diagnosed with COVID-19 if they were older than 66 years (odds ratio, 21.3) or had other comorbidities (OR, 1.24). The most commonly used drugs for managing COVID-19 were hydroxychloroquine, lopinavir/ritonavir, steroids, antibiotics, chloroquine, tofacitinib, and infliximab.
A total of 30.6% of patients with IBD and COVID-19 were hospitalized, 11.4% stayed in the ICU, 3.7% required mechanical ventilation, and 3.8% died from COVID-19. Significant risk factors for death from COVID-19 included older age, active IBD, and a Charlson Comorbidity Index score above 1. Similarly, risk factors for severe COVID-19 included older age, having two or more comorbidities, receiving systemic steroids, and receiving mesalamine/sulfasalazine. In one study, a recent (3-month) history of corticosteroid treatment was associated with a 60% increase in the risk for severe COVID-19. Other immune-mediated therapies did not show this association. Patients with ulcerative colitis were significantly more likely to be seen in the ED or hospitalized, compared with patients with other forms of IBD (adjusted OR, 12.7).
No funding sources were disclosed. Dr. D’Amico reported having no conflicts of interest. Two coinvestigators disclosed ties to AbbVie, MSD, Schering-Plough, UCB Pharma, and several other pharmaceutical companies.
SOURCE: D’Amico F et al. Clin Gastroenterol Hepatol. 2020 Aug 7. doi: 10.1016/j.cgh.2020.08.003.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
COVID-19: A second wave of mental illness 'imminent'
The mental health consequences of COVID-19 deaths are likely to overwhelm an already tattered U.S. mental health system, leading to a lack of access, particularly for the most vulnerable, experts warn.
“A second wave of devastation is imminent, attributable to mental health consequences of COVID-19,” write Naomi Simon, MD, and coauthors with the department of psychiatry, New York University.
In a Viewpoint article published in JAMA on Oct. 12, physicians offer some sobering statistics.
Since February 2020, COVID-19 has taken the lives of more than 214,000 Americans. The number of deaths currently attributed to the virus is nearly four times the number of Americans killed during the Vietnam War. The magnitude of death over a short period is a tragedy on a “historic scale,” wrote Dr. Simon and colleagues.
The surge in mental health problems related to COVID-19 deaths will bring further challenges to individuals, families, and communities, including a spike in deaths from suicide and drug overdoses, they warned.
It’s important to consider, they noted, that each COVID-19 death leaves an estimated nine family members bereaved, which is projected to lead to an estimated 2 million bereaved individuals in the United States.
The necessary social distancing and quarantine measures implemented to fight the virus have amplified emotional turmoil and have disrupted the ability of personal support networks and communities to come together and grieve.
“Of central concern is the transformation of normal grief and distress into prolonged grief and major depressive disorder and symptoms of posttraumatic stress disorder,” Simon and colleagues said.
“Once established, these conditions can become chronic with additional comorbidities such as substance use disorders. Prolonged grief affects approximately 10% of bereaved individuals, but this is likely an underestimate for grief related to deaths from COVID-19,” they wrote.
As with the first COVID-19 wave, the mental health wave will disproportionately affect Black persons, Hispanic persons, older adults, persons in lower socioeconomic groups of all races and ethnicities, and healthcare workers, they note.
The psychological risks for health care and other essential workers are of particular concern, they say. “Supporting the mental health of these and other essential workforce is critical to readiness for managing recurrent waves of the pandemic,” they stated.
How will the United States manage this impending wave of mental health problems?
“The solution will require increased funding for mental health; widespread screening to identify individuals at highest risk including suicide risk; availability of primary care clinicians and mental health professionals trained to treat those with prolonged grief, depression, traumatic stress, and substance abuse; and a diligent focus on families and communities to creatively restore the approaches by which they have managed tragedy and loss over generations,” the authors wrote.
“History has shown that societies recover from such devastation when leaders and members are joined by a shared purpose, acting in a unified way to facilitate recovery. In such societies, there is a shared understanding that its members must care for one another because the loss of one is a loss for all. Above all, this shared understanding must be restored,” they concluded.
Dr. Simon has received personal fees from Vanda Pharmaceuticals Inc, MGH Psychiatry Academy, Axovant Sciences, Springworks, Praxis Therapeutics, Aptinyx, Genomind, and Wiley (deputy editor, Depression and Anxiety). Saxe has received royalties from Guilford Press for the book Trauma Systems Therapy for Children and Teens (2016). Marmar serves on the scientific advisory board and owns equity in Receptor Life Sciences and serves on the PTSD advisory board for Otsuka Pharmaceutical.
A version of this article originally appeared on Medscape.com.
The mental health consequences of COVID-19 deaths are likely to overwhelm an already tattered U.S. mental health system, leading to a lack of access, particularly for the most vulnerable, experts warn.
“A second wave of devastation is imminent, attributable to mental health consequences of COVID-19,” write Naomi Simon, MD, and coauthors with the department of psychiatry, New York University.
In a Viewpoint article published in JAMA on Oct. 12, physicians offer some sobering statistics.
Since February 2020, COVID-19 has taken the lives of more than 214,000 Americans. The number of deaths currently attributed to the virus is nearly four times the number of Americans killed during the Vietnam War. The magnitude of death over a short period is a tragedy on a “historic scale,” wrote Dr. Simon and colleagues.
The surge in mental health problems related to COVID-19 deaths will bring further challenges to individuals, families, and communities, including a spike in deaths from suicide and drug overdoses, they warned.
It’s important to consider, they noted, that each COVID-19 death leaves an estimated nine family members bereaved, which is projected to lead to an estimated 2 million bereaved individuals in the United States.
The necessary social distancing and quarantine measures implemented to fight the virus have amplified emotional turmoil and have disrupted the ability of personal support networks and communities to come together and grieve.
“Of central concern is the transformation of normal grief and distress into prolonged grief and major depressive disorder and symptoms of posttraumatic stress disorder,” Simon and colleagues said.
“Once established, these conditions can become chronic with additional comorbidities such as substance use disorders. Prolonged grief affects approximately 10% of bereaved individuals, but this is likely an underestimate for grief related to deaths from COVID-19,” they wrote.
As with the first COVID-19 wave, the mental health wave will disproportionately affect Black persons, Hispanic persons, older adults, persons in lower socioeconomic groups of all races and ethnicities, and healthcare workers, they note.
The psychological risks for health care and other essential workers are of particular concern, they say. “Supporting the mental health of these and other essential workforce is critical to readiness for managing recurrent waves of the pandemic,” they stated.
How will the United States manage this impending wave of mental health problems?
“The solution will require increased funding for mental health; widespread screening to identify individuals at highest risk including suicide risk; availability of primary care clinicians and mental health professionals trained to treat those with prolonged grief, depression, traumatic stress, and substance abuse; and a diligent focus on families and communities to creatively restore the approaches by which they have managed tragedy and loss over generations,” the authors wrote.
“History has shown that societies recover from such devastation when leaders and members are joined by a shared purpose, acting in a unified way to facilitate recovery. In such societies, there is a shared understanding that its members must care for one another because the loss of one is a loss for all. Above all, this shared understanding must be restored,” they concluded.
Dr. Simon has received personal fees from Vanda Pharmaceuticals Inc, MGH Psychiatry Academy, Axovant Sciences, Springworks, Praxis Therapeutics, Aptinyx, Genomind, and Wiley (deputy editor, Depression and Anxiety). Saxe has received royalties from Guilford Press for the book Trauma Systems Therapy for Children and Teens (2016). Marmar serves on the scientific advisory board and owns equity in Receptor Life Sciences and serves on the PTSD advisory board for Otsuka Pharmaceutical.
A version of this article originally appeared on Medscape.com.
The mental health consequences of COVID-19 deaths are likely to overwhelm an already tattered U.S. mental health system, leading to a lack of access, particularly for the most vulnerable, experts warn.
“A second wave of devastation is imminent, attributable to mental health consequences of COVID-19,” write Naomi Simon, MD, and coauthors with the department of psychiatry, New York University.
In a Viewpoint article published in JAMA on Oct. 12, physicians offer some sobering statistics.
Since February 2020, COVID-19 has taken the lives of more than 214,000 Americans. The number of deaths currently attributed to the virus is nearly four times the number of Americans killed during the Vietnam War. The magnitude of death over a short period is a tragedy on a “historic scale,” wrote Dr. Simon and colleagues.
The surge in mental health problems related to COVID-19 deaths will bring further challenges to individuals, families, and communities, including a spike in deaths from suicide and drug overdoses, they warned.
It’s important to consider, they noted, that each COVID-19 death leaves an estimated nine family members bereaved, which is projected to lead to an estimated 2 million bereaved individuals in the United States.
The necessary social distancing and quarantine measures implemented to fight the virus have amplified emotional turmoil and have disrupted the ability of personal support networks and communities to come together and grieve.
“Of central concern is the transformation of normal grief and distress into prolonged grief and major depressive disorder and symptoms of posttraumatic stress disorder,” Simon and colleagues said.
“Once established, these conditions can become chronic with additional comorbidities such as substance use disorders. Prolonged grief affects approximately 10% of bereaved individuals, but this is likely an underestimate for grief related to deaths from COVID-19,” they wrote.
As with the first COVID-19 wave, the mental health wave will disproportionately affect Black persons, Hispanic persons, older adults, persons in lower socioeconomic groups of all races and ethnicities, and healthcare workers, they note.
The psychological risks for health care and other essential workers are of particular concern, they say. “Supporting the mental health of these and other essential workforce is critical to readiness for managing recurrent waves of the pandemic,” they stated.
How will the United States manage this impending wave of mental health problems?
“The solution will require increased funding for mental health; widespread screening to identify individuals at highest risk including suicide risk; availability of primary care clinicians and mental health professionals trained to treat those with prolonged grief, depression, traumatic stress, and substance abuse; and a diligent focus on families and communities to creatively restore the approaches by which they have managed tragedy and loss over generations,” the authors wrote.
“History has shown that societies recover from such devastation when leaders and members are joined by a shared purpose, acting in a unified way to facilitate recovery. In such societies, there is a shared understanding that its members must care for one another because the loss of one is a loss for all. Above all, this shared understanding must be restored,” they concluded.
Dr. Simon has received personal fees from Vanda Pharmaceuticals Inc, MGH Psychiatry Academy, Axovant Sciences, Springworks, Praxis Therapeutics, Aptinyx, Genomind, and Wiley (deputy editor, Depression and Anxiety). Saxe has received royalties from Guilford Press for the book Trauma Systems Therapy for Children and Teens (2016). Marmar serves on the scientific advisory board and owns equity in Receptor Life Sciences and serves on the PTSD advisory board for Otsuka Pharmaceutical.
A version of this article originally appeared on Medscape.com.
Entresto halves renal events in preserved EF heart failure patients
Patients with heart failure with preserved ejection fraction (HFpEF) who received sacubitril/valsartan in the PARAGON-HF trial had significant protection against progression of renal dysfunction in a prespecified secondary analysis.
The 2,419 patients with HFpEF who received sacubitril/valsartan (Entresto) had half the rate of the primary adverse renal outcome, compared with the 2,403 patients randomized to valsartan alone in the comparator group, a significant difference, according to the results published online Sept. 29 in Circulation by Finnian R. McCausland, MBBCh, and colleagues.
In absolute terms, sacubitril/valsartan treatment, an angiotensin-receptor/neprilysin inhibitor (ARNI), cut the incidence of the combined renal endpoint – renal death, end-stage renal disease, or at least a 50% drop in estimated glomerular filtration rate (eGFR) – from 2.7% in the control group to 1.4% in the sacubitril/valsartan group during a median follow-up of 35 months.
The absolute difference of 1.3% equated to a number needed to treat of 51 to prevent one of these events.
Also notable was that renal protection from sacubitril/valsartan was equally robust across the range of baseline kidney function.
‘An important therapeutic option’
The efficacy “across the spectrum of baseline renal function” indicates treatment with sacubitril/valsartan is “an important therapeutic option to slow renal-function decline in patients with heart failure,” wrote Dr. McCausland, a nephrologist at Brigham and Women’s Hospital in Boston, and colleagues.
The authors’ conclusion is striking because currently no drug class has produced clear evidence for efficacy in HFpEF.
On the other hand, the PARAGON-HF trial that provided the data for this new analysis was statistically neutral for its primary endpoint – a reduction in the combined rate of cardiovascular death and hospitalizations for heart failure – with a P value of .06 and 95% confidence interval of 0.75-1.01.
“Because this difference [in the primary endpoint incidence between the two study group] did not meet the predetermined level of statistical significance, subsequent analyses were considered to be exploratory,” noted the authors of the primary analysis of PARAGON-HF, as reported by Medscape Medical News.
Despite this limitation in interpreting secondary outcomes from the trial, the new report of a significant renal benefit “opens the potential to provide evidence-based treatment for patients with HFpEF,” commented Sheldon W. Tobe, MD, and Stephanie Poon, MD, in an editorial accompanying the latest analysis.
“At the very least, these results are certainly intriguing and suggest that there may be important patient subgroups with HFpEF who might benefit from using sacubitril/valsartan,” they emphasized.
First large trial to show renal improvement in HFpEF
The editorialists’ enthusiasm for the implications of the new findings relate in part to the fact that “PARAGON-HF is the first large trial to demonstrate improvement in renal parameters in HFpEF,” they noted.
“The finding that the composite renal outcome did not differ according to baseline eGFR is significant and suggests that the beneficial effect on renal function was indirect, possibly linked to improved cardiac function,” say Dr. Tobe, a nephrologist, and Dr. Poon, a cardiologist, both at Sunnybrook Health Sciences Centre in Toronto.
PARAGON-HF enrolled 4,822 HFpEF patients at 848 centers in 43 countries, and the efficacy analysis included 4,796 patients.
The composite renal outcome was mainly driven by the incidence of a 50% or greater drop from baseline in eGFR, which occurred in 27 patients (1.1%) in the sacubitril/valsartan group and 60 patients (2.5%) who received valsartan alone.
The annual average drop in eGFR during the study was 2.0 mL/min per 1.73m2 in the sacubitril/valsartan group and 2.7 mL/min per 1.73m2 in the control group.
Although the heart failure community was disappointed that sacubitril/valsartan failed to show a significant benefit for the study’s primary outcome in HFpEF, the combination has become a mainstay of treatment for patients with HFpEF based on its performance in the PARADIGM-HF trial.
And despite the unqualified support sacubitril/valsartan now receives in guidelines and its label as a foundational treatment for HFpEF, the formulation has had a hard time gaining traction in U.S. practice, often because of barriers placed by third-party payers.
PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. McCausland has reported no relevant financial relationships. Dr. Tobe has reported participating on a steering committee for Bayer Fidelio/Figaro studies and being a speaker on behalf of Pfizer and Servier. Dr. Poon has reported being an adviser to Novartis, Boehringer Ingelheim, and Servier.
A version of this article originally appeared on Medscape.com.
Patients with heart failure with preserved ejection fraction (HFpEF) who received sacubitril/valsartan in the PARAGON-HF trial had significant protection against progression of renal dysfunction in a prespecified secondary analysis.
The 2,419 patients with HFpEF who received sacubitril/valsartan (Entresto) had half the rate of the primary adverse renal outcome, compared with the 2,403 patients randomized to valsartan alone in the comparator group, a significant difference, according to the results published online Sept. 29 in Circulation by Finnian R. McCausland, MBBCh, and colleagues.
In absolute terms, sacubitril/valsartan treatment, an angiotensin-receptor/neprilysin inhibitor (ARNI), cut the incidence of the combined renal endpoint – renal death, end-stage renal disease, or at least a 50% drop in estimated glomerular filtration rate (eGFR) – from 2.7% in the control group to 1.4% in the sacubitril/valsartan group during a median follow-up of 35 months.
The absolute difference of 1.3% equated to a number needed to treat of 51 to prevent one of these events.
Also notable was that renal protection from sacubitril/valsartan was equally robust across the range of baseline kidney function.
‘An important therapeutic option’
The efficacy “across the spectrum of baseline renal function” indicates treatment with sacubitril/valsartan is “an important therapeutic option to slow renal-function decline in patients with heart failure,” wrote Dr. McCausland, a nephrologist at Brigham and Women’s Hospital in Boston, and colleagues.
The authors’ conclusion is striking because currently no drug class has produced clear evidence for efficacy in HFpEF.
On the other hand, the PARAGON-HF trial that provided the data for this new analysis was statistically neutral for its primary endpoint – a reduction in the combined rate of cardiovascular death and hospitalizations for heart failure – with a P value of .06 and 95% confidence interval of 0.75-1.01.
“Because this difference [in the primary endpoint incidence between the two study group] did not meet the predetermined level of statistical significance, subsequent analyses were considered to be exploratory,” noted the authors of the primary analysis of PARAGON-HF, as reported by Medscape Medical News.
Despite this limitation in interpreting secondary outcomes from the trial, the new report of a significant renal benefit “opens the potential to provide evidence-based treatment for patients with HFpEF,” commented Sheldon W. Tobe, MD, and Stephanie Poon, MD, in an editorial accompanying the latest analysis.
“At the very least, these results are certainly intriguing and suggest that there may be important patient subgroups with HFpEF who might benefit from using sacubitril/valsartan,” they emphasized.
First large trial to show renal improvement in HFpEF
The editorialists’ enthusiasm for the implications of the new findings relate in part to the fact that “PARAGON-HF is the first large trial to demonstrate improvement in renal parameters in HFpEF,” they noted.
“The finding that the composite renal outcome did not differ according to baseline eGFR is significant and suggests that the beneficial effect on renal function was indirect, possibly linked to improved cardiac function,” say Dr. Tobe, a nephrologist, and Dr. Poon, a cardiologist, both at Sunnybrook Health Sciences Centre in Toronto.
PARAGON-HF enrolled 4,822 HFpEF patients at 848 centers in 43 countries, and the efficacy analysis included 4,796 patients.
The composite renal outcome was mainly driven by the incidence of a 50% or greater drop from baseline in eGFR, which occurred in 27 patients (1.1%) in the sacubitril/valsartan group and 60 patients (2.5%) who received valsartan alone.
The annual average drop in eGFR during the study was 2.0 mL/min per 1.73m2 in the sacubitril/valsartan group and 2.7 mL/min per 1.73m2 in the control group.
Although the heart failure community was disappointed that sacubitril/valsartan failed to show a significant benefit for the study’s primary outcome in HFpEF, the combination has become a mainstay of treatment for patients with HFpEF based on its performance in the PARADIGM-HF trial.
And despite the unqualified support sacubitril/valsartan now receives in guidelines and its label as a foundational treatment for HFpEF, the formulation has had a hard time gaining traction in U.S. practice, often because of barriers placed by third-party payers.
PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. McCausland has reported no relevant financial relationships. Dr. Tobe has reported participating on a steering committee for Bayer Fidelio/Figaro studies and being a speaker on behalf of Pfizer and Servier. Dr. Poon has reported being an adviser to Novartis, Boehringer Ingelheim, and Servier.
A version of this article originally appeared on Medscape.com.
Patients with heart failure with preserved ejection fraction (HFpEF) who received sacubitril/valsartan in the PARAGON-HF trial had significant protection against progression of renal dysfunction in a prespecified secondary analysis.
The 2,419 patients with HFpEF who received sacubitril/valsartan (Entresto) had half the rate of the primary adverse renal outcome, compared with the 2,403 patients randomized to valsartan alone in the comparator group, a significant difference, according to the results published online Sept. 29 in Circulation by Finnian R. McCausland, MBBCh, and colleagues.
In absolute terms, sacubitril/valsartan treatment, an angiotensin-receptor/neprilysin inhibitor (ARNI), cut the incidence of the combined renal endpoint – renal death, end-stage renal disease, or at least a 50% drop in estimated glomerular filtration rate (eGFR) – from 2.7% in the control group to 1.4% in the sacubitril/valsartan group during a median follow-up of 35 months.
The absolute difference of 1.3% equated to a number needed to treat of 51 to prevent one of these events.
Also notable was that renal protection from sacubitril/valsartan was equally robust across the range of baseline kidney function.
‘An important therapeutic option’
The efficacy “across the spectrum of baseline renal function” indicates treatment with sacubitril/valsartan is “an important therapeutic option to slow renal-function decline in patients with heart failure,” wrote Dr. McCausland, a nephrologist at Brigham and Women’s Hospital in Boston, and colleagues.
The authors’ conclusion is striking because currently no drug class has produced clear evidence for efficacy in HFpEF.
On the other hand, the PARAGON-HF trial that provided the data for this new analysis was statistically neutral for its primary endpoint – a reduction in the combined rate of cardiovascular death and hospitalizations for heart failure – with a P value of .06 and 95% confidence interval of 0.75-1.01.
“Because this difference [in the primary endpoint incidence between the two study group] did not meet the predetermined level of statistical significance, subsequent analyses were considered to be exploratory,” noted the authors of the primary analysis of PARAGON-HF, as reported by Medscape Medical News.
Despite this limitation in interpreting secondary outcomes from the trial, the new report of a significant renal benefit “opens the potential to provide evidence-based treatment for patients with HFpEF,” commented Sheldon W. Tobe, MD, and Stephanie Poon, MD, in an editorial accompanying the latest analysis.
“At the very least, these results are certainly intriguing and suggest that there may be important patient subgroups with HFpEF who might benefit from using sacubitril/valsartan,” they emphasized.
First large trial to show renal improvement in HFpEF
The editorialists’ enthusiasm for the implications of the new findings relate in part to the fact that “PARAGON-HF is the first large trial to demonstrate improvement in renal parameters in HFpEF,” they noted.
“The finding that the composite renal outcome did not differ according to baseline eGFR is significant and suggests that the beneficial effect on renal function was indirect, possibly linked to improved cardiac function,” say Dr. Tobe, a nephrologist, and Dr. Poon, a cardiologist, both at Sunnybrook Health Sciences Centre in Toronto.
PARAGON-HF enrolled 4,822 HFpEF patients at 848 centers in 43 countries, and the efficacy analysis included 4,796 patients.
The composite renal outcome was mainly driven by the incidence of a 50% or greater drop from baseline in eGFR, which occurred in 27 patients (1.1%) in the sacubitril/valsartan group and 60 patients (2.5%) who received valsartan alone.
The annual average drop in eGFR during the study was 2.0 mL/min per 1.73m2 in the sacubitril/valsartan group and 2.7 mL/min per 1.73m2 in the control group.
Although the heart failure community was disappointed that sacubitril/valsartan failed to show a significant benefit for the study’s primary outcome in HFpEF, the combination has become a mainstay of treatment for patients with HFpEF based on its performance in the PARADIGM-HF trial.
And despite the unqualified support sacubitril/valsartan now receives in guidelines and its label as a foundational treatment for HFpEF, the formulation has had a hard time gaining traction in U.S. practice, often because of barriers placed by third-party payers.
PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. McCausland has reported no relevant financial relationships. Dr. Tobe has reported participating on a steering committee for Bayer Fidelio/Figaro studies and being a speaker on behalf of Pfizer and Servier. Dr. Poon has reported being an adviser to Novartis, Boehringer Ingelheim, and Servier.
A version of this article originally appeared on Medscape.com.
Blood group O linked to decreased risk of SARS-CoV-2 infection
Blood group O was associated with a decreased risk for contracting SARS-CoV-2 infection, according to the results of large retrospective analysis of the Danish population.
Researchers Mike Bogetofte Barnkob, MD, of the Department of Clinical Immunology, Odense (Denmark) University Hospital, and colleagues performed a retrospective cohort analysis of all Danish individuals with a known ABO blood group who were tested for SARS-CoV-2 between Feb. 27, 2020, and July 30, 2020.
Of the 841,327 people tested, ABO and RhD blood groups could be identified for 473,654 individuals. ABO and RhD data from 2,204,742 (38% of the entire Danish population) were used as a reference, according to the online report in Blood Advances.
The primary outcome was status of ABO and RhD blood groups and test results for SARS-CoV-2. The secondary outcomes followed were hospitalization and death from COVID-19.
Reduced prevalence
The study found that ABO blood groups varied significantly between patients and the reference group, with only 38.41% (95% confidence interval, 37.30%-39.50%) of the patients belonging to blood group O, compared with 41.70% (95% CI, 41.60%-41.80%) in the controls, corresponding to a relative risk of 0.87 (95% CI, 0.83-0.91) for acquiring COVID-19.
There was a slight, but statistically significant, difference in blood group distribution between the SARS-CoV-22 individuals and the reference population (P < .001), according to the authors.
Among the SARS-CoV-2 individuals, fewer group O individuals were found (P < .001); while more A, B, and AB individuals were seen (P < .001, P = .011, and P = .091, respectively). There was no significant difference seen among A, B, and AB blood groups (P = .30). The RR for contracting SARS-CoV-2 were 1.09 (95% CI, 1.04-1.14) for A group individuals; 1.06 (95% CI, 0.99-1.14) for B group; and 1.15 (95% CI, 1.03-1.27) for AB group, respectively.
There was no difference found in the RhD group between positive test cases and the reference population (P = .15). In addition, there was no statistical difference (all P > .40) between ABO blood groups and clinical severity of COVID-19 for nonhospitalized patients versus hospitalized patients or for deceased patients versus living patients, the researchers added.
Possible causes
The authors speculated on two possible causes of the lower prevalence of SARS-CoV-2 infection in the blood group O population. The first is that anti-A and anti-B antibodies may have an effect on neutralizing SARS-CoV viruses and that anti-A and anti-B are present on mucosal surfaces in some individuals lacking the corresponding ABO blood group. The second is that the association between ABO blood groups and levels of von Willebrand factor, which is higher in non-O individuals and is tied to an increased likelihood of arterial and venous thrombosis, could have an indirect or unknown impact on susceptibility to infection, according to the authors.
“Given the known increased risk of thrombosis in non-O individuals and the evolving central role for thrombosis in the pathogenesis of COVID-19, it is important to explore this aspect more closely in larger patient cohorts (e.g., by examining ABO blood type and viral load, the severity of symptoms, and the long-term effects following COVID-19),” the researchers concluded.
One author reported receiving fees from Bristol Myers Squibb, Novartis, and Roche. The remaining authors reported they had no competing financial interests.
SOURCE: Barnkob MB et al. Blood Adv. 2020 Oct 14. doi: 10.1182/bloodadvances.2020002657.
Blood group O was associated with a decreased risk for contracting SARS-CoV-2 infection, according to the results of large retrospective analysis of the Danish population.
Researchers Mike Bogetofte Barnkob, MD, of the Department of Clinical Immunology, Odense (Denmark) University Hospital, and colleagues performed a retrospective cohort analysis of all Danish individuals with a known ABO blood group who were tested for SARS-CoV-2 between Feb. 27, 2020, and July 30, 2020.
Of the 841,327 people tested, ABO and RhD blood groups could be identified for 473,654 individuals. ABO and RhD data from 2,204,742 (38% of the entire Danish population) were used as a reference, according to the online report in Blood Advances.
The primary outcome was status of ABO and RhD blood groups and test results for SARS-CoV-2. The secondary outcomes followed were hospitalization and death from COVID-19.
Reduced prevalence
The study found that ABO blood groups varied significantly between patients and the reference group, with only 38.41% (95% confidence interval, 37.30%-39.50%) of the patients belonging to blood group O, compared with 41.70% (95% CI, 41.60%-41.80%) in the controls, corresponding to a relative risk of 0.87 (95% CI, 0.83-0.91) for acquiring COVID-19.
There was a slight, but statistically significant, difference in blood group distribution between the SARS-CoV-22 individuals and the reference population (P < .001), according to the authors.
Among the SARS-CoV-2 individuals, fewer group O individuals were found (P < .001); while more A, B, and AB individuals were seen (P < .001, P = .011, and P = .091, respectively). There was no significant difference seen among A, B, and AB blood groups (P = .30). The RR for contracting SARS-CoV-2 were 1.09 (95% CI, 1.04-1.14) for A group individuals; 1.06 (95% CI, 0.99-1.14) for B group; and 1.15 (95% CI, 1.03-1.27) for AB group, respectively.
There was no difference found in the RhD group between positive test cases and the reference population (P = .15). In addition, there was no statistical difference (all P > .40) between ABO blood groups and clinical severity of COVID-19 for nonhospitalized patients versus hospitalized patients or for deceased patients versus living patients, the researchers added.
Possible causes
The authors speculated on two possible causes of the lower prevalence of SARS-CoV-2 infection in the blood group O population. The first is that anti-A and anti-B antibodies may have an effect on neutralizing SARS-CoV viruses and that anti-A and anti-B are present on mucosal surfaces in some individuals lacking the corresponding ABO blood group. The second is that the association between ABO blood groups and levels of von Willebrand factor, which is higher in non-O individuals and is tied to an increased likelihood of arterial and venous thrombosis, could have an indirect or unknown impact on susceptibility to infection, according to the authors.
“Given the known increased risk of thrombosis in non-O individuals and the evolving central role for thrombosis in the pathogenesis of COVID-19, it is important to explore this aspect more closely in larger patient cohorts (e.g., by examining ABO blood type and viral load, the severity of symptoms, and the long-term effects following COVID-19),” the researchers concluded.
One author reported receiving fees from Bristol Myers Squibb, Novartis, and Roche. The remaining authors reported they had no competing financial interests.
SOURCE: Barnkob MB et al. Blood Adv. 2020 Oct 14. doi: 10.1182/bloodadvances.2020002657.
Blood group O was associated with a decreased risk for contracting SARS-CoV-2 infection, according to the results of large retrospective analysis of the Danish population.
Researchers Mike Bogetofte Barnkob, MD, of the Department of Clinical Immunology, Odense (Denmark) University Hospital, and colleagues performed a retrospective cohort analysis of all Danish individuals with a known ABO blood group who were tested for SARS-CoV-2 between Feb. 27, 2020, and July 30, 2020.
Of the 841,327 people tested, ABO and RhD blood groups could be identified for 473,654 individuals. ABO and RhD data from 2,204,742 (38% of the entire Danish population) were used as a reference, according to the online report in Blood Advances.
The primary outcome was status of ABO and RhD blood groups and test results for SARS-CoV-2. The secondary outcomes followed were hospitalization and death from COVID-19.
Reduced prevalence
The study found that ABO blood groups varied significantly between patients and the reference group, with only 38.41% (95% confidence interval, 37.30%-39.50%) of the patients belonging to blood group O, compared with 41.70% (95% CI, 41.60%-41.80%) in the controls, corresponding to a relative risk of 0.87 (95% CI, 0.83-0.91) for acquiring COVID-19.
There was a slight, but statistically significant, difference in blood group distribution between the SARS-CoV-22 individuals and the reference population (P < .001), according to the authors.
Among the SARS-CoV-2 individuals, fewer group O individuals were found (P < .001); while more A, B, and AB individuals were seen (P < .001, P = .011, and P = .091, respectively). There was no significant difference seen among A, B, and AB blood groups (P = .30). The RR for contracting SARS-CoV-2 were 1.09 (95% CI, 1.04-1.14) for A group individuals; 1.06 (95% CI, 0.99-1.14) for B group; and 1.15 (95% CI, 1.03-1.27) for AB group, respectively.
There was no difference found in the RhD group between positive test cases and the reference population (P = .15). In addition, there was no statistical difference (all P > .40) between ABO blood groups and clinical severity of COVID-19 for nonhospitalized patients versus hospitalized patients or for deceased patients versus living patients, the researchers added.
Possible causes
The authors speculated on two possible causes of the lower prevalence of SARS-CoV-2 infection in the blood group O population. The first is that anti-A and anti-B antibodies may have an effect on neutralizing SARS-CoV viruses and that anti-A and anti-B are present on mucosal surfaces in some individuals lacking the corresponding ABO blood group. The second is that the association between ABO blood groups and levels of von Willebrand factor, which is higher in non-O individuals and is tied to an increased likelihood of arterial and venous thrombosis, could have an indirect or unknown impact on susceptibility to infection, according to the authors.
“Given the known increased risk of thrombosis in non-O individuals and the evolving central role for thrombosis in the pathogenesis of COVID-19, it is important to explore this aspect more closely in larger patient cohorts (e.g., by examining ABO blood type and viral load, the severity of symptoms, and the long-term effects following COVID-19),” the researchers concluded.
One author reported receiving fees from Bristol Myers Squibb, Novartis, and Roche. The remaining authors reported they had no competing financial interests.
SOURCE: Barnkob MB et al. Blood Adv. 2020 Oct 14. doi: 10.1182/bloodadvances.2020002657.
FROM BLOOD ADVANCES