User login
“Blind” endometrial sampling: A call to end the practice
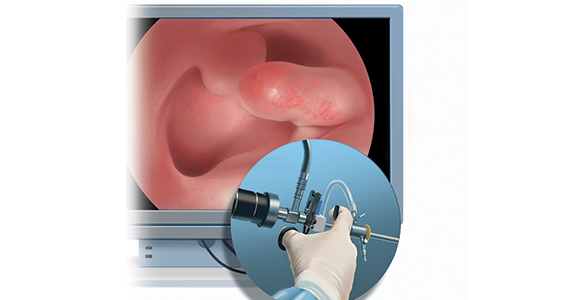
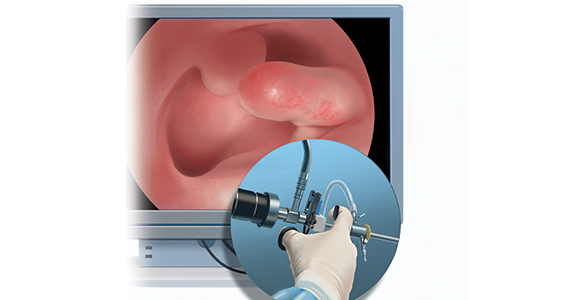
Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
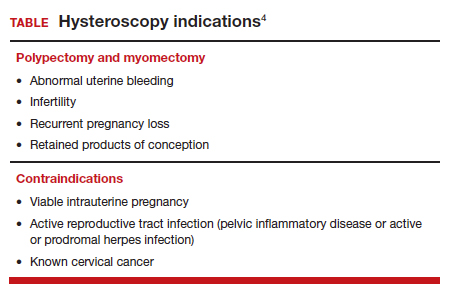
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
Do scare tactics work?
I suspect that you have heard about or maybe read the recent Associated Press story reporting that four daycare workers in Hamilton, Miss., have been charged with felony child abuse for intentionally scaring the children “who didn’t clean up or act good” by wearing a Halloween mask and yelling in their faces. I can have some sympathy for those among us who choose to spend their days tending a flock of sometimes unruly and mischievous toddlers and preschoolers. But, I think one would be hard pressed to find very many adults who would condone the strategy of these misguided daycare providers. Not surprisingly, the parents of some of these children describe their children as traumatized and having disordered sleep.
The news report of this incident in Mississippi doesn’t tell us if these daycare providers had used this tactic in the past. One wonders whether they had found less dramatic verbal threats just weren’t as effective as they had hoped and so decided to go all out.
How effective is fear in changing behavior? Certainly, we have all experienced situations in which a frightening experience has caused us to avoid places, people, and activities. But, is a fear-focused strategy one that health care providers should include in their quiver as we try to mold patient behavior? As luck would have it, 2 weeks before this news story broke I encountered a global study from 84 countries that sought to answer this question (Affect Sci. 2022 Sep. doi: 10.1007/s42761-022-00128-3).
Using the WHO four-point advice about COVID prevention (stay home/avoid shops/use face covering/isolate if exposed) as a model the researchers around the world reviewed the responses of 16,000 individuals. They found that there was no difference in the effectiveness of the message whether it was framed as a negative (“you have so much to lose”) or a positive (“you have so much to gain”). However, investigators observed that the negatively framed presentations generated significantly more anxiety in the respondents. The authors of the paper conclude that if there is no significant difference in the effectiveness, why would we chose a negatively framed presentation that is likely to generate anxiety that we know is associated with increased morbidity and mortality. From a purely public health perspective, it doesn’t make sense and is counterproductive.
I guess if we look back to the old carrot and stick metaphor we shouldn’t be surprised by the findings in this paper. If one’s only goal is to get a group of young preschoolers to behave by scaring the b’geezes out of them with a mask or a threat of bodily punishment, then go for it. Scare tactics will probably work just as well as offering a well-chosen reward system. However, the devil is in the side effects. It’s the same argument that I give to parents who argue that spanking works. Of course it does, but it has a narrow margin for safety and can set up ripples of negative side effects that can destroy healthy parent-child relationships.
The bottom line of this story is the sad truth that somewhere along the line someone failed to effectively train these four daycare workers. But, do we as health care providers need to rethink our training? Have we forgotten our commitment to “First do no harm?” As we craft our messaging have we thought enough about the potential side effects of our attempts at scaring the public into following our suggestions?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I suspect that you have heard about or maybe read the recent Associated Press story reporting that four daycare workers in Hamilton, Miss., have been charged with felony child abuse for intentionally scaring the children “who didn’t clean up or act good” by wearing a Halloween mask and yelling in their faces. I can have some sympathy for those among us who choose to spend their days tending a flock of sometimes unruly and mischievous toddlers and preschoolers. But, I think one would be hard pressed to find very many adults who would condone the strategy of these misguided daycare providers. Not surprisingly, the parents of some of these children describe their children as traumatized and having disordered sleep.
The news report of this incident in Mississippi doesn’t tell us if these daycare providers had used this tactic in the past. One wonders whether they had found less dramatic verbal threats just weren’t as effective as they had hoped and so decided to go all out.
How effective is fear in changing behavior? Certainly, we have all experienced situations in which a frightening experience has caused us to avoid places, people, and activities. But, is a fear-focused strategy one that health care providers should include in their quiver as we try to mold patient behavior? As luck would have it, 2 weeks before this news story broke I encountered a global study from 84 countries that sought to answer this question (Affect Sci. 2022 Sep. doi: 10.1007/s42761-022-00128-3).
Using the WHO four-point advice about COVID prevention (stay home/avoid shops/use face covering/isolate if exposed) as a model the researchers around the world reviewed the responses of 16,000 individuals. They found that there was no difference in the effectiveness of the message whether it was framed as a negative (“you have so much to lose”) or a positive (“you have so much to gain”). However, investigators observed that the negatively framed presentations generated significantly more anxiety in the respondents. The authors of the paper conclude that if there is no significant difference in the effectiveness, why would we chose a negatively framed presentation that is likely to generate anxiety that we know is associated with increased morbidity and mortality. From a purely public health perspective, it doesn’t make sense and is counterproductive.
I guess if we look back to the old carrot and stick metaphor we shouldn’t be surprised by the findings in this paper. If one’s only goal is to get a group of young preschoolers to behave by scaring the b’geezes out of them with a mask or a threat of bodily punishment, then go for it. Scare tactics will probably work just as well as offering a well-chosen reward system. However, the devil is in the side effects. It’s the same argument that I give to parents who argue that spanking works. Of course it does, but it has a narrow margin for safety and can set up ripples of negative side effects that can destroy healthy parent-child relationships.
The bottom line of this story is the sad truth that somewhere along the line someone failed to effectively train these four daycare workers. But, do we as health care providers need to rethink our training? Have we forgotten our commitment to “First do no harm?” As we craft our messaging have we thought enough about the potential side effects of our attempts at scaring the public into following our suggestions?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
I suspect that you have heard about or maybe read the recent Associated Press story reporting that four daycare workers in Hamilton, Miss., have been charged with felony child abuse for intentionally scaring the children “who didn’t clean up or act good” by wearing a Halloween mask and yelling in their faces. I can have some sympathy for those among us who choose to spend their days tending a flock of sometimes unruly and mischievous toddlers and preschoolers. But, I think one would be hard pressed to find very many adults who would condone the strategy of these misguided daycare providers. Not surprisingly, the parents of some of these children describe their children as traumatized and having disordered sleep.
The news report of this incident in Mississippi doesn’t tell us if these daycare providers had used this tactic in the past. One wonders whether they had found less dramatic verbal threats just weren’t as effective as they had hoped and so decided to go all out.
How effective is fear in changing behavior? Certainly, we have all experienced situations in which a frightening experience has caused us to avoid places, people, and activities. But, is a fear-focused strategy one that health care providers should include in their quiver as we try to mold patient behavior? As luck would have it, 2 weeks before this news story broke I encountered a global study from 84 countries that sought to answer this question (Affect Sci. 2022 Sep. doi: 10.1007/s42761-022-00128-3).
Using the WHO four-point advice about COVID prevention (stay home/avoid shops/use face covering/isolate if exposed) as a model the researchers around the world reviewed the responses of 16,000 individuals. They found that there was no difference in the effectiveness of the message whether it was framed as a negative (“you have so much to lose”) or a positive (“you have so much to gain”). However, investigators observed that the negatively framed presentations generated significantly more anxiety in the respondents. The authors of the paper conclude that if there is no significant difference in the effectiveness, why would we chose a negatively framed presentation that is likely to generate anxiety that we know is associated with increased morbidity and mortality. From a purely public health perspective, it doesn’t make sense and is counterproductive.
I guess if we look back to the old carrot and stick metaphor we shouldn’t be surprised by the findings in this paper. If one’s only goal is to get a group of young preschoolers to behave by scaring the b’geezes out of them with a mask or a threat of bodily punishment, then go for it. Scare tactics will probably work just as well as offering a well-chosen reward system. However, the devil is in the side effects. It’s the same argument that I give to parents who argue that spanking works. Of course it does, but it has a narrow margin for safety and can set up ripples of negative side effects that can destroy healthy parent-child relationships.
The bottom line of this story is the sad truth that somewhere along the line someone failed to effectively train these four daycare workers. But, do we as health care providers need to rethink our training? Have we forgotten our commitment to “First do no harm?” As we craft our messaging have we thought enough about the potential side effects of our attempts at scaring the public into following our suggestions?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Past, Present, and Future of Pediatric Atopic Dermatitis Management
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
PRACTICE POINTS
- Pediatric atopic dermatitis (AD) therapeutics have rapidly evolved over the last decade and dermatologists should be aware of new tools in their treatment arsenal.
- New topical nonsteroidal agents serve as useful alternatives to topical corticosteroids through mitigating adverse effects from current standard therapy and potentially simplifying topical regimens.
- Monoclonal antibodies and Janus kinase inhibitors are part of an important set of new systemic therapeutics for pediatric AD.
- Long-term data on these new therapeutics is required to better understand their impact on pediatric AD comorbidities and impact on the longitudinal disease course.
Update on Tinea Capitis Diagnosis and Treatment
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4
Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4

Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4

Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
A 95-year-old White male with hypertension presented with itchy patches and bullae on the trunk and extremities
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
Four methods to chip away at imposter syndrome
Regardless of the setting, one of the most frequently discussed topics in health care is imposter syndrome.
Imposter syndrome was first defined by Clance and Imes as an inability to internalize success, and the tendency to attribute success to external causes such as luck, error, or knowing the appropriate individual.1 This definition is essential because most health care professionals have had a sense of doubt or questioned the full extent of their competencies in various situations. I would argue that this is normal and – within reason – helpful to the practice of medicine. The problem with true imposter syndrome is that the individual does not incorporate success in a way that builds healthy self-esteem and self-efficacy.2
Imposter syndrome has a very nasty way of interacting with burnout. Studies have shown that imposter syndrome can be associated with high levels of emotional exhaustion at work.3 In my experience, this makes clinical sense. Professionals suffering from imposter syndrome can spend a great deal of time and energy trying to maintain a particular image.4 They are acting a part 24/7. Have you ever seriously tried to act? It’s arduous work. A friend once asked me to read a role for a play because “you’d be great; you’re a natural.” By the time I was done with rehearsal, I felt like I had run a 4-by-400-meter relay, by myself, in Victoria, Tex.
And any talk of imposter syndrome must include its running mate, perfectionism. These two conditions exist together so commonly it can be a bit of a chicken or egg question as to which came first.
Imposter syndrome, perfectionism, and burnout can form a deadly triad if not recognized and addressed quickly. In medicine, perfectionism can be a coping strategy that sets up unrelenting standards. Failure to meet unrelenting standards then serves as fuel and validation for imposter syndrome and emotional exhaustion. The consequences of this cycle going unchecked over a health care professional’s career are seismic and can include downstream effects ranging from depression to suicide.
Some readers will relate to this, while others will shrug their shoulders and say that this has never happened in their professional life. I get it. However, I would now ask if you have ever felt like an imposter in your personal life. I’ll make a cup of tea and wait for you to figure out precisely what is the boundary between your personal and professional life. Okay, all done? Great. Now I’ll give you some more time to sincerely reflect if any of the traits of imposter syndrome have described you at times in your personal life. Hmmm, interesting to think about, isn’t it?
I believe that health care professionals frequently use one credit card to pay off another, but the debt remains the same. So even if things are going well at work, we may have just shifted the debt to our personal lives. (At some point in the future, I’ll share my 10 greatest father fails to date to elucidate my point.)
In my work at the GW Resiliency and Well-Being Center, I’ve gravitated toward a few methods supported by evidence that help alleviate imposter syndrome symptoms and potentially serve as protective factors against the future development of imposter syndrome.4 These include but are not limited to:
- Keep a record of small personal success that is yours alone.
- Have a mentor to share failures with.
- Use personal reflection to examine what it means to successfully reach your goals and fulfill your purpose, not a relative value unit target.
- Share experiences with each other, so you know you’re not alone.
The last method is one of my favorites because it involves connecting to others and shining a light on our shared experiences and, coincidentally, our collective strengths. Once this collective strength is realized, the circumstances of that 4-by-400-meter relay change drastically. Be safe and well, everyone.
Lorenzo Norris, MD, is a psychiatrist and chief wellness officer for the George Washington University Medical Enterprise and serves as associate dean of student affairs and administration for the George Washington University School of Medicine and Health Sciences. A version of this article first appeared on Medscape.com.
References
1. Clance PR, Imes SA. The imposter phenomenon in high achieving women: Dynamics and therapeutic intervention. Psychotherapy: Theory, Research & Practice. 1978;15(3): 241-7. doi: 10.1037/h0086006.
2. Thomas M, Bigatti S. Perfectionism, impostor phenomenon, and mental health in medicine: A literature review. Int J Med Educ. 2020 Sep 28;11:201-3. doi: 10.5116/ijme.5f54.c8f8.
3. Liu RQ et al. Impostorism and anxiety contribute to burnout among resident physicians. Med Teach. 2022 Jul;44(7):758-64. doi: 10.1080/0142159X.2022.2028751.
4. Gottlieb M et al. Impostor syndrome among physicians and physicians in training: A scoping review. Med Educ. 2020 Feb;54(2):116-24. doi: 10.1111/medu.13956.
Regardless of the setting, one of the most frequently discussed topics in health care is imposter syndrome.
Imposter syndrome was first defined by Clance and Imes as an inability to internalize success, and the tendency to attribute success to external causes such as luck, error, or knowing the appropriate individual.1 This definition is essential because most health care professionals have had a sense of doubt or questioned the full extent of their competencies in various situations. I would argue that this is normal and – within reason – helpful to the practice of medicine. The problem with true imposter syndrome is that the individual does not incorporate success in a way that builds healthy self-esteem and self-efficacy.2
Imposter syndrome has a very nasty way of interacting with burnout. Studies have shown that imposter syndrome can be associated with high levels of emotional exhaustion at work.3 In my experience, this makes clinical sense. Professionals suffering from imposter syndrome can spend a great deal of time and energy trying to maintain a particular image.4 They are acting a part 24/7. Have you ever seriously tried to act? It’s arduous work. A friend once asked me to read a role for a play because “you’d be great; you’re a natural.” By the time I was done with rehearsal, I felt like I had run a 4-by-400-meter relay, by myself, in Victoria, Tex.
And any talk of imposter syndrome must include its running mate, perfectionism. These two conditions exist together so commonly it can be a bit of a chicken or egg question as to which came first.
Imposter syndrome, perfectionism, and burnout can form a deadly triad if not recognized and addressed quickly. In medicine, perfectionism can be a coping strategy that sets up unrelenting standards. Failure to meet unrelenting standards then serves as fuel and validation for imposter syndrome and emotional exhaustion. The consequences of this cycle going unchecked over a health care professional’s career are seismic and can include downstream effects ranging from depression to suicide.
Some readers will relate to this, while others will shrug their shoulders and say that this has never happened in their professional life. I get it. However, I would now ask if you have ever felt like an imposter in your personal life. I’ll make a cup of tea and wait for you to figure out precisely what is the boundary between your personal and professional life. Okay, all done? Great. Now I’ll give you some more time to sincerely reflect if any of the traits of imposter syndrome have described you at times in your personal life. Hmmm, interesting to think about, isn’t it?
I believe that health care professionals frequently use one credit card to pay off another, but the debt remains the same. So even if things are going well at work, we may have just shifted the debt to our personal lives. (At some point in the future, I’ll share my 10 greatest father fails to date to elucidate my point.)
In my work at the GW Resiliency and Well-Being Center, I’ve gravitated toward a few methods supported by evidence that help alleviate imposter syndrome symptoms and potentially serve as protective factors against the future development of imposter syndrome.4 These include but are not limited to:
- Keep a record of small personal success that is yours alone.
- Have a mentor to share failures with.
- Use personal reflection to examine what it means to successfully reach your goals and fulfill your purpose, not a relative value unit target.
- Share experiences with each other, so you know you’re not alone.
The last method is one of my favorites because it involves connecting to others and shining a light on our shared experiences and, coincidentally, our collective strengths. Once this collective strength is realized, the circumstances of that 4-by-400-meter relay change drastically. Be safe and well, everyone.
Lorenzo Norris, MD, is a psychiatrist and chief wellness officer for the George Washington University Medical Enterprise and serves as associate dean of student affairs and administration for the George Washington University School of Medicine and Health Sciences. A version of this article first appeared on Medscape.com.
References
1. Clance PR, Imes SA. The imposter phenomenon in high achieving women: Dynamics and therapeutic intervention. Psychotherapy: Theory, Research & Practice. 1978;15(3): 241-7. doi: 10.1037/h0086006.
2. Thomas M, Bigatti S. Perfectionism, impostor phenomenon, and mental health in medicine: A literature review. Int J Med Educ. 2020 Sep 28;11:201-3. doi: 10.5116/ijme.5f54.c8f8.
3. Liu RQ et al. Impostorism and anxiety contribute to burnout among resident physicians. Med Teach. 2022 Jul;44(7):758-64. doi: 10.1080/0142159X.2022.2028751.
4. Gottlieb M et al. Impostor syndrome among physicians and physicians in training: A scoping review. Med Educ. 2020 Feb;54(2):116-24. doi: 10.1111/medu.13956.
Regardless of the setting, one of the most frequently discussed topics in health care is imposter syndrome.
Imposter syndrome was first defined by Clance and Imes as an inability to internalize success, and the tendency to attribute success to external causes such as luck, error, or knowing the appropriate individual.1 This definition is essential because most health care professionals have had a sense of doubt or questioned the full extent of their competencies in various situations. I would argue that this is normal and – within reason – helpful to the practice of medicine. The problem with true imposter syndrome is that the individual does not incorporate success in a way that builds healthy self-esteem and self-efficacy.2
Imposter syndrome has a very nasty way of interacting with burnout. Studies have shown that imposter syndrome can be associated with high levels of emotional exhaustion at work.3 In my experience, this makes clinical sense. Professionals suffering from imposter syndrome can spend a great deal of time and energy trying to maintain a particular image.4 They are acting a part 24/7. Have you ever seriously tried to act? It’s arduous work. A friend once asked me to read a role for a play because “you’d be great; you’re a natural.” By the time I was done with rehearsal, I felt like I had run a 4-by-400-meter relay, by myself, in Victoria, Tex.
And any talk of imposter syndrome must include its running mate, perfectionism. These two conditions exist together so commonly it can be a bit of a chicken or egg question as to which came first.
Imposter syndrome, perfectionism, and burnout can form a deadly triad if not recognized and addressed quickly. In medicine, perfectionism can be a coping strategy that sets up unrelenting standards. Failure to meet unrelenting standards then serves as fuel and validation for imposter syndrome and emotional exhaustion. The consequences of this cycle going unchecked over a health care professional’s career are seismic and can include downstream effects ranging from depression to suicide.
Some readers will relate to this, while others will shrug their shoulders and say that this has never happened in their professional life. I get it. However, I would now ask if you have ever felt like an imposter in your personal life. I’ll make a cup of tea and wait for you to figure out precisely what is the boundary between your personal and professional life. Okay, all done? Great. Now I’ll give you some more time to sincerely reflect if any of the traits of imposter syndrome have described you at times in your personal life. Hmmm, interesting to think about, isn’t it?
I believe that health care professionals frequently use one credit card to pay off another, but the debt remains the same. So even if things are going well at work, we may have just shifted the debt to our personal lives. (At some point in the future, I’ll share my 10 greatest father fails to date to elucidate my point.)
In my work at the GW Resiliency and Well-Being Center, I’ve gravitated toward a few methods supported by evidence that help alleviate imposter syndrome symptoms and potentially serve as protective factors against the future development of imposter syndrome.4 These include but are not limited to:
- Keep a record of small personal success that is yours alone.
- Have a mentor to share failures with.
- Use personal reflection to examine what it means to successfully reach your goals and fulfill your purpose, not a relative value unit target.
- Share experiences with each other, so you know you’re not alone.
The last method is one of my favorites because it involves connecting to others and shining a light on our shared experiences and, coincidentally, our collective strengths. Once this collective strength is realized, the circumstances of that 4-by-400-meter relay change drastically. Be safe and well, everyone.
Lorenzo Norris, MD, is a psychiatrist and chief wellness officer for the George Washington University Medical Enterprise and serves as associate dean of student affairs and administration for the George Washington University School of Medicine and Health Sciences. A version of this article first appeared on Medscape.com.
References
1. Clance PR, Imes SA. The imposter phenomenon in high achieving women: Dynamics and therapeutic intervention. Psychotherapy: Theory, Research & Practice. 1978;15(3): 241-7. doi: 10.1037/h0086006.
2. Thomas M, Bigatti S. Perfectionism, impostor phenomenon, and mental health in medicine: A literature review. Int J Med Educ. 2020 Sep 28;11:201-3. doi: 10.5116/ijme.5f54.c8f8.
3. Liu RQ et al. Impostorism and anxiety contribute to burnout among resident physicians. Med Teach. 2022 Jul;44(7):758-64. doi: 10.1080/0142159X.2022.2028751.
4. Gottlieb M et al. Impostor syndrome among physicians and physicians in training: A scoping review. Med Educ. 2020 Feb;54(2):116-24. doi: 10.1111/medu.13956.
Fitness trackers: Useful in sleep medicine?
Who doesn’t love data, especially their own? With that thought in mind, over the years I have owned several activity trackers, including at least two Fitbits, and I frequently check my iPhone to see how far I’ve walked or how many steps I have taken. My most recent acquisition is an Oura (smart ring, third generation), which includes my first sleep tracker.
Sleep trackers are not unique to the Oura Ring; they are included on many of the newer activity trackers and smart watches, but the design and breakdown of daily sleep, activity, and readiness scores are hallmarks of Oura Rings.
The ring generates data for different phases of sleep, movements, oxygen saturation, disturbances in breathing, heart rate, and heart rate variability. I began to wonder how useful this information would be clinically and whether it might be helpful in either the diagnosis or treatment of sleep disorders.
David Neubauer, MD, is a psychiatrist at the Johns Hopkins Sleep Disorders Center. “Sleep tracking devices are more than just toys but less than medical devices. They do have clinical utility and might show findings that warrant further medical workup,” Dr. Neubauer said. “It is impressive that these devices estimate sleep as well as they do, but there is a problem with how they divide sleep stages that can lead people to believe their sleep is worse than it really is.”
For more than 50 years, he explained, sleep researchers and clinicians have categorized sleep as non–rapid eye movement (NREM) sleep stages 1-4 and REM sleep. More recently, sleep was reorganized to N1, N2, and N3 (which combines the older stages 3 and 4, representing “deep sleep” or “slow wave sleep”) and REM sleep. We normally spend more time in N2 than the other stages. However, the device companies often categorize their sleep estimates as “light sleep,” “deep sleep,” or “REM.” With “light sleep,” they are lumping together N1 and N2 sleep, and this is misleading, said Dr. Neubauer. “Understandably, people often think that there is something wrong if their tracker reports they are spending a lot of time in light sleep, when actually their sleep may be entirely normal.”
Sleep tracker validity
A study by Massimiliano de Zambotti, PhD, and colleagues, “The Sleep of the Ring: Comparison of the ŌURA Sleep Tracker Against Polysomnography”, looked at sleep patterns of 41 adolescents and young adults and concluded that the second-generation tracker was accurate in terms of total sleep but underestimated time spent in N3 stage sleep by approximately 20 minutes while overestimating time spent in REM sleep by 17 minutes. They concluded that the ring had potential to be clinically useful but that further studies and validation were needed.
A larger study of the newest, third-generation Oura tracker, conducted by Altini and Kinnunen at Oura Health, found that the added sensors with the newer-generation ring led to improved accuracy, but they noted that the study was done with a healthy population and might not generalize to clinical populations.
Fernando Goes, MD, and Matthew Reid, PhD, both at Johns Hopkins, are working on a multicenter study using the Oura Ring and the mindLAMP app to look at the impact of sleep on mood in people with mood disorders as well as healthy controls. Dr. Reid said that “validation of sleep stages takes a hit when the ring is used in people with insomnia. We find it useful for total sleep time, but when you look at sleep architecture, the concordance is only 60%. And oxygen saturation measures are less accurate in people with dark skin.”
Clinical uses for sleep trackers
More accurate information might prove reassuring to patients. Dr. Goes added, “One use, for example, might be to help patients to limit or come off of long-term hypnotics with a more benign intervention that incorporates passive monitoring such as that in the Oura Ring. Some patients worry excessively about not being able to sleep, and sleep monitoring data can be helpful to reduce some of these concerns so patients can focus on safer interventions, such as cognitive behavioral therapy for insomnia.” Dr. Reid believes that wearable trackers have potential usefulness in monitoring sleep in patients with insomnia. “In insomnia, sleep state misperception is common. They are hyper-aroused, and they perceive that they are awake when in fact they are sleeping.”
Dr. Goes mentioned another use for sleep trackers in clinical settings: “In our inpatient units, the nurses open the door to look in on patients every hour to monitor and document if they are sleeping. If they look in and the patient isn’t moving, they will ask the patient to raise their hand, which of course is not going to help someone to fall back asleep.” Wearable devices might provide data on sleep without the risk of waking patients every hour through the night.
Not medical devices
However, Dr. Neubauer emphasized that current sleep trackers are not medical devices, saying “they may be measuring the same parameters that are measured with medical devices, for example pulse oximetry or sleep states, but there’s no simple answer yet to the question of whether the devices provide reliable data for clinical decision-making.”
Dr. Neubauer is skeptical about the accuracy of some of the measures the device provides. “I would not use the information from a consumer device to rule out obstructive sleep apnea based on good oxygen saturation numbers. So much depends on the history – snoring, gasping awakenings, reports from bed partners, and daytime sleepiness. These devices do not measure respiratory effort or nasal airflow as sleep studies do. But big drops in oxygen saturation from a consumer device certainly warrant attention for further evaluation.” Dr. Neubauer also noted that the parameters on sleep trackers do not differentiate between central or obstructive sleep apnea and that insurers won’t pay for continuous positive airway pressure to treat sleep apnea without a sleep study.
I enjoy looking at the data, even knowing that they are not entirely accurate. and we may find more clinical uses for these devices. For now, I’m off to get more exercise, at the suggestion of my tracker!
Dinah Miller, MD, is assistant professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore.
A version of this article first appeared on Medscape.com.
Who doesn’t love data, especially their own? With that thought in mind, over the years I have owned several activity trackers, including at least two Fitbits, and I frequently check my iPhone to see how far I’ve walked or how many steps I have taken. My most recent acquisition is an Oura (smart ring, third generation), which includes my first sleep tracker.
Sleep trackers are not unique to the Oura Ring; they are included on many of the newer activity trackers and smart watches, but the design and breakdown of daily sleep, activity, and readiness scores are hallmarks of Oura Rings.
The ring generates data for different phases of sleep, movements, oxygen saturation, disturbances in breathing, heart rate, and heart rate variability. I began to wonder how useful this information would be clinically and whether it might be helpful in either the diagnosis or treatment of sleep disorders.
David Neubauer, MD, is a psychiatrist at the Johns Hopkins Sleep Disorders Center. “Sleep tracking devices are more than just toys but less than medical devices. They do have clinical utility and might show findings that warrant further medical workup,” Dr. Neubauer said. “It is impressive that these devices estimate sleep as well as they do, but there is a problem with how they divide sleep stages that can lead people to believe their sleep is worse than it really is.”
For more than 50 years, he explained, sleep researchers and clinicians have categorized sleep as non–rapid eye movement (NREM) sleep stages 1-4 and REM sleep. More recently, sleep was reorganized to N1, N2, and N3 (which combines the older stages 3 and 4, representing “deep sleep” or “slow wave sleep”) and REM sleep. We normally spend more time in N2 than the other stages. However, the device companies often categorize their sleep estimates as “light sleep,” “deep sleep,” or “REM.” With “light sleep,” they are lumping together N1 and N2 sleep, and this is misleading, said Dr. Neubauer. “Understandably, people often think that there is something wrong if their tracker reports they are spending a lot of time in light sleep, when actually their sleep may be entirely normal.”
Sleep tracker validity
A study by Massimiliano de Zambotti, PhD, and colleagues, “The Sleep of the Ring: Comparison of the ŌURA Sleep Tracker Against Polysomnography”, looked at sleep patterns of 41 adolescents and young adults and concluded that the second-generation tracker was accurate in terms of total sleep but underestimated time spent in N3 stage sleep by approximately 20 minutes while overestimating time spent in REM sleep by 17 minutes. They concluded that the ring had potential to be clinically useful but that further studies and validation were needed.
A larger study of the newest, third-generation Oura tracker, conducted by Altini and Kinnunen at Oura Health, found that the added sensors with the newer-generation ring led to improved accuracy, but they noted that the study was done with a healthy population and might not generalize to clinical populations.
Fernando Goes, MD, and Matthew Reid, PhD, both at Johns Hopkins, are working on a multicenter study using the Oura Ring and the mindLAMP app to look at the impact of sleep on mood in people with mood disorders as well as healthy controls. Dr. Reid said that “validation of sleep stages takes a hit when the ring is used in people with insomnia. We find it useful for total sleep time, but when you look at sleep architecture, the concordance is only 60%. And oxygen saturation measures are less accurate in people with dark skin.”
Clinical uses for sleep trackers
More accurate information might prove reassuring to patients. Dr. Goes added, “One use, for example, might be to help patients to limit or come off of long-term hypnotics with a more benign intervention that incorporates passive monitoring such as that in the Oura Ring. Some patients worry excessively about not being able to sleep, and sleep monitoring data can be helpful to reduce some of these concerns so patients can focus on safer interventions, such as cognitive behavioral therapy for insomnia.” Dr. Reid believes that wearable trackers have potential usefulness in monitoring sleep in patients with insomnia. “In insomnia, sleep state misperception is common. They are hyper-aroused, and they perceive that they are awake when in fact they are sleeping.”
Dr. Goes mentioned another use for sleep trackers in clinical settings: “In our inpatient units, the nurses open the door to look in on patients every hour to monitor and document if they are sleeping. If they look in and the patient isn’t moving, they will ask the patient to raise their hand, which of course is not going to help someone to fall back asleep.” Wearable devices might provide data on sleep without the risk of waking patients every hour through the night.
Not medical devices
However, Dr. Neubauer emphasized that current sleep trackers are not medical devices, saying “they may be measuring the same parameters that are measured with medical devices, for example pulse oximetry or sleep states, but there’s no simple answer yet to the question of whether the devices provide reliable data for clinical decision-making.”
Dr. Neubauer is skeptical about the accuracy of some of the measures the device provides. “I would not use the information from a consumer device to rule out obstructive sleep apnea based on good oxygen saturation numbers. So much depends on the history – snoring, gasping awakenings, reports from bed partners, and daytime sleepiness. These devices do not measure respiratory effort or nasal airflow as sleep studies do. But big drops in oxygen saturation from a consumer device certainly warrant attention for further evaluation.” Dr. Neubauer also noted that the parameters on sleep trackers do not differentiate between central or obstructive sleep apnea and that insurers won’t pay for continuous positive airway pressure to treat sleep apnea without a sleep study.
I enjoy looking at the data, even knowing that they are not entirely accurate. and we may find more clinical uses for these devices. For now, I’m off to get more exercise, at the suggestion of my tracker!
Dinah Miller, MD, is assistant professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore.
A version of this article first appeared on Medscape.com.
Who doesn’t love data, especially their own? With that thought in mind, over the years I have owned several activity trackers, including at least two Fitbits, and I frequently check my iPhone to see how far I’ve walked or how many steps I have taken. My most recent acquisition is an Oura (smart ring, third generation), which includes my first sleep tracker.
Sleep trackers are not unique to the Oura Ring; they are included on many of the newer activity trackers and smart watches, but the design and breakdown of daily sleep, activity, and readiness scores are hallmarks of Oura Rings.
The ring generates data for different phases of sleep, movements, oxygen saturation, disturbances in breathing, heart rate, and heart rate variability. I began to wonder how useful this information would be clinically and whether it might be helpful in either the diagnosis or treatment of sleep disorders.
David Neubauer, MD, is a psychiatrist at the Johns Hopkins Sleep Disorders Center. “Sleep tracking devices are more than just toys but less than medical devices. They do have clinical utility and might show findings that warrant further medical workup,” Dr. Neubauer said. “It is impressive that these devices estimate sleep as well as they do, but there is a problem with how they divide sleep stages that can lead people to believe their sleep is worse than it really is.”
For more than 50 years, he explained, sleep researchers and clinicians have categorized sleep as non–rapid eye movement (NREM) sleep stages 1-4 and REM sleep. More recently, sleep was reorganized to N1, N2, and N3 (which combines the older stages 3 and 4, representing “deep sleep” or “slow wave sleep”) and REM sleep. We normally spend more time in N2 than the other stages. However, the device companies often categorize their sleep estimates as “light sleep,” “deep sleep,” or “REM.” With “light sleep,” they are lumping together N1 and N2 sleep, and this is misleading, said Dr. Neubauer. “Understandably, people often think that there is something wrong if their tracker reports they are spending a lot of time in light sleep, when actually their sleep may be entirely normal.”
Sleep tracker validity
A study by Massimiliano de Zambotti, PhD, and colleagues, “The Sleep of the Ring: Comparison of the ŌURA Sleep Tracker Against Polysomnography”, looked at sleep patterns of 41 adolescents and young adults and concluded that the second-generation tracker was accurate in terms of total sleep but underestimated time spent in N3 stage sleep by approximately 20 minutes while overestimating time spent in REM sleep by 17 minutes. They concluded that the ring had potential to be clinically useful but that further studies and validation were needed.
A larger study of the newest, third-generation Oura tracker, conducted by Altini and Kinnunen at Oura Health, found that the added sensors with the newer-generation ring led to improved accuracy, but they noted that the study was done with a healthy population and might not generalize to clinical populations.
Fernando Goes, MD, and Matthew Reid, PhD, both at Johns Hopkins, are working on a multicenter study using the Oura Ring and the mindLAMP app to look at the impact of sleep on mood in people with mood disorders as well as healthy controls. Dr. Reid said that “validation of sleep stages takes a hit when the ring is used in people with insomnia. We find it useful for total sleep time, but when you look at sleep architecture, the concordance is only 60%. And oxygen saturation measures are less accurate in people with dark skin.”
Clinical uses for sleep trackers
More accurate information might prove reassuring to patients. Dr. Goes added, “One use, for example, might be to help patients to limit or come off of long-term hypnotics with a more benign intervention that incorporates passive monitoring such as that in the Oura Ring. Some patients worry excessively about not being able to sleep, and sleep monitoring data can be helpful to reduce some of these concerns so patients can focus on safer interventions, such as cognitive behavioral therapy for insomnia.” Dr. Reid believes that wearable trackers have potential usefulness in monitoring sleep in patients with insomnia. “In insomnia, sleep state misperception is common. They are hyper-aroused, and they perceive that they are awake when in fact they are sleeping.”
Dr. Goes mentioned another use for sleep trackers in clinical settings: “In our inpatient units, the nurses open the door to look in on patients every hour to monitor and document if they are sleeping. If they look in and the patient isn’t moving, they will ask the patient to raise their hand, which of course is not going to help someone to fall back asleep.” Wearable devices might provide data on sleep without the risk of waking patients every hour through the night.
Not medical devices
However, Dr. Neubauer emphasized that current sleep trackers are not medical devices, saying “they may be measuring the same parameters that are measured with medical devices, for example pulse oximetry or sleep states, but there’s no simple answer yet to the question of whether the devices provide reliable data for clinical decision-making.”
Dr. Neubauer is skeptical about the accuracy of some of the measures the device provides. “I would not use the information from a consumer device to rule out obstructive sleep apnea based on good oxygen saturation numbers. So much depends on the history – snoring, gasping awakenings, reports from bed partners, and daytime sleepiness. These devices do not measure respiratory effort or nasal airflow as sleep studies do. But big drops in oxygen saturation from a consumer device certainly warrant attention for further evaluation.” Dr. Neubauer also noted that the parameters on sleep trackers do not differentiate between central or obstructive sleep apnea and that insurers won’t pay for continuous positive airway pressure to treat sleep apnea without a sleep study.
I enjoy looking at the data, even knowing that they are not entirely accurate. and we may find more clinical uses for these devices. For now, I’m off to get more exercise, at the suggestion of my tracker!
Dinah Miller, MD, is assistant professor of psychiatry and behavioral sciences, Johns Hopkins Medicine, Baltimore.
A version of this article first appeared on Medscape.com.
Rheumatic diseases and assisted reproductive technology: Things to consider
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
Is evolution’s greatest triumph its worst blunder?
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
Warning: Watch out for ‘medication substitution reaction’
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages