User login
Love them or hate them, masks in schools work
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The body of evidence for Paxlovid therapy
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Which exercise is best for bone health?
An 18-year-old woman with Crohn’s disease (diagnosed 3 years ago) came to my office for advice regarding management of osteoporosis. Her bone density was low for her age, and she had three low-impact fractures of her long bones in the preceding 4 years.
Loss of weight after the onset of Crohn’s disease, subsequent loss of periods, inflammation associated with her underlying diagnosis, and early treatment with glucocorticoids (known to have deleterious effects on bone) were believed to have caused osteoporosis in this young woman.
A few months previously, she was switched to a medication that doesn’t impair bone health and glucocorticoids were discontinued; her weight began to improve, and her Crohn’s disease was now in remission. Her menses had resumed about 3 months before her visit to my clinic after a prolonged period without periods. She was on calcium and vitamin D supplements, with normal levels of vitamin D.
Many factors determine bone health including (but not limited to) genetics, nutritional status, exercise activity (with mechanical loading of bones), macro- and micronutrient intake, hormonal status, chronic inflammation and other disease states, and medication use.
Exercise certainly has beneficial effects on bone. Bone-loading activities increase bone formation through the activation of certain cells in bone called osteocytes, which serve as mechanosensors and sense bone loading. Osteocytes make a hormone called sclerostin, which typically inhibits bone formation. When osteocytes sense bone-loading activities, sclerostin secretion reduces, allowing for increased bone formation.
Consistent with this, investigators in Canada have demonstrated greater increases in bone density and strength in schoolchildren who engage in moderate to vigorous physical activity, particularly bone-loading exercise, during the school day, compared with those who don’t (J Bone Miner Res. 2007 Mar;22[3]:434-46; J Bone Miner Res. 2017 Jul;32[7]:1525-36). In females, normal levels of estrogen seem necessary for osteocytes to bring about these effects after bone-loading activities. This is probably one of several reasons why athletes who lose their periods (indicative of low estrogen levels) and develop low bone density with an increased risk for fracture even when they are still at a normal weight (J Clin Endocrinol Metab. 2018 Jun 1;103[6]:2392-402; Med Sci Sports Exerc. 2015 Aug;47[8]:1577-86).
One concern around prescribing bone-loading activity or exercise to persons with osteoporosis is whether it would increase the risk for fracture from the impact on fragile bone. The extent of bone loading safe for fragile bone can be difficult to determine. Furthermore, excessive exercise may worsen bone health by causing weight loss or loss of periods in women. Very careful monitoring may be necessary to ensure that energy balance is maintained. Therefore, the nature and volume of exercise should be discussed with one’s doctor or physical therapist as well as a dietitian (if the patient is seeing one).
In patients with osteoporosis, high-impact activities such as jumping; repetitive impact activities such as running or jogging; and bending and twisting activities such as touching one’s toes, golf, tennis, and bowling aren’t recommended because they increase the risk for fracture. Even yoga poses should be discussed, because some may increase the risk for compression fractures of the vertebrae in the spine.
Strength and resistance training are generally believed to be good for bones. Strength training involves activities that build muscle strength and mass. Resistance training builds muscle strength, mass, and endurance by making muscles work against some form of resistance. Such activities include weight training with free weights or weight machines, use of resistance bands, and use of one’s own body to strengthen major muscle groups (such as through push-ups, squats, lunges, and gluteus maximus extension).
Some amount of weight-bearing aerobic training is also recommended, including walking, low-impact aerobics, the elliptical, and stair-climbing. Non–weight-bearing activities, such as swimming and cycling, typically don’t contribute to improving bone density.
In older individuals with osteoporosis, agility exercises are particularly useful to reduce the fall risk (J Am Geriatr Soc. 2004 May;52[5]:657-65; CMAJ. 2002 Oct 29;167[9]:997-1004). These can be structured to improve hand-eye coordination, foot-eye coordination, static and dynamic balance, and reaction time. Agility exercises with resistance training help improve bone density in older women.
An optimal exercise regimen includes a combination of strength and resistance training; weight-bearing aerobic training; and exercises that build flexibility, stability, and balance. A doctor, physical therapist, or trainer with expertise in the right combination of exercises should be consulted to ensure optimal effects on bone and general health.
In those at risk for overexercising to the point that they start to lose weight or lose their periods, and certainly in all women with disordered eating patterns, a dietitian should be part of the decision team to ensure that energy balance is maintained. In this group, particularly in very-low-weight women with eating disorders, exercise activity is often limited until they reach a healthier weight, and ideally after their menses resume.
For my patient with Crohn’s disease, I recommended that she see a physical therapist and a dietitian for guidance about a graded increase in exercise activity and an exercise regimen that would work best for her. I assess her bone density annually using dual-energy x-ray absorptiometry. Her bone density has gradually improved with the combination of weight gain, resumption of menses, medications for Crohn’s disease that do not affect bone deleteriously, remission of Crohn’s disease, and her exercise regimen.
Dr. Misra is chief of the division of pediatric endocrinology at Mass General Hospital for Children and professor in the department of pediatrics at Harvard Medical School, both in Boston. She reported conflicts of interest with AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
An 18-year-old woman with Crohn’s disease (diagnosed 3 years ago) came to my office for advice regarding management of osteoporosis. Her bone density was low for her age, and she had three low-impact fractures of her long bones in the preceding 4 years.
Loss of weight after the onset of Crohn’s disease, subsequent loss of periods, inflammation associated with her underlying diagnosis, and early treatment with glucocorticoids (known to have deleterious effects on bone) were believed to have caused osteoporosis in this young woman.
A few months previously, she was switched to a medication that doesn’t impair bone health and glucocorticoids were discontinued; her weight began to improve, and her Crohn’s disease was now in remission. Her menses had resumed about 3 months before her visit to my clinic after a prolonged period without periods. She was on calcium and vitamin D supplements, with normal levels of vitamin D.
Many factors determine bone health including (but not limited to) genetics, nutritional status, exercise activity (with mechanical loading of bones), macro- and micronutrient intake, hormonal status, chronic inflammation and other disease states, and medication use.
Exercise certainly has beneficial effects on bone. Bone-loading activities increase bone formation through the activation of certain cells in bone called osteocytes, which serve as mechanosensors and sense bone loading. Osteocytes make a hormone called sclerostin, which typically inhibits bone formation. When osteocytes sense bone-loading activities, sclerostin secretion reduces, allowing for increased bone formation.
Consistent with this, investigators in Canada have demonstrated greater increases in bone density and strength in schoolchildren who engage in moderate to vigorous physical activity, particularly bone-loading exercise, during the school day, compared with those who don’t (J Bone Miner Res. 2007 Mar;22[3]:434-46; J Bone Miner Res. 2017 Jul;32[7]:1525-36). In females, normal levels of estrogen seem necessary for osteocytes to bring about these effects after bone-loading activities. This is probably one of several reasons why athletes who lose their periods (indicative of low estrogen levels) and develop low bone density with an increased risk for fracture even when they are still at a normal weight (J Clin Endocrinol Metab. 2018 Jun 1;103[6]:2392-402; Med Sci Sports Exerc. 2015 Aug;47[8]:1577-86).
One concern around prescribing bone-loading activity or exercise to persons with osteoporosis is whether it would increase the risk for fracture from the impact on fragile bone. The extent of bone loading safe for fragile bone can be difficult to determine. Furthermore, excessive exercise may worsen bone health by causing weight loss or loss of periods in women. Very careful monitoring may be necessary to ensure that energy balance is maintained. Therefore, the nature and volume of exercise should be discussed with one’s doctor or physical therapist as well as a dietitian (if the patient is seeing one).
In patients with osteoporosis, high-impact activities such as jumping; repetitive impact activities such as running or jogging; and bending and twisting activities such as touching one’s toes, golf, tennis, and bowling aren’t recommended because they increase the risk for fracture. Even yoga poses should be discussed, because some may increase the risk for compression fractures of the vertebrae in the spine.
Strength and resistance training are generally believed to be good for bones. Strength training involves activities that build muscle strength and mass. Resistance training builds muscle strength, mass, and endurance by making muscles work against some form of resistance. Such activities include weight training with free weights or weight machines, use of resistance bands, and use of one’s own body to strengthen major muscle groups (such as through push-ups, squats, lunges, and gluteus maximus extension).
Some amount of weight-bearing aerobic training is also recommended, including walking, low-impact aerobics, the elliptical, and stair-climbing. Non–weight-bearing activities, such as swimming and cycling, typically don’t contribute to improving bone density.
In older individuals with osteoporosis, agility exercises are particularly useful to reduce the fall risk (J Am Geriatr Soc. 2004 May;52[5]:657-65; CMAJ. 2002 Oct 29;167[9]:997-1004). These can be structured to improve hand-eye coordination, foot-eye coordination, static and dynamic balance, and reaction time. Agility exercises with resistance training help improve bone density in older women.
An optimal exercise regimen includes a combination of strength and resistance training; weight-bearing aerobic training; and exercises that build flexibility, stability, and balance. A doctor, physical therapist, or trainer with expertise in the right combination of exercises should be consulted to ensure optimal effects on bone and general health.
In those at risk for overexercising to the point that they start to lose weight or lose their periods, and certainly in all women with disordered eating patterns, a dietitian should be part of the decision team to ensure that energy balance is maintained. In this group, particularly in very-low-weight women with eating disorders, exercise activity is often limited until they reach a healthier weight, and ideally after their menses resume.
For my patient with Crohn’s disease, I recommended that she see a physical therapist and a dietitian for guidance about a graded increase in exercise activity and an exercise regimen that would work best for her. I assess her bone density annually using dual-energy x-ray absorptiometry. Her bone density has gradually improved with the combination of weight gain, resumption of menses, medications for Crohn’s disease that do not affect bone deleteriously, remission of Crohn’s disease, and her exercise regimen.
Dr. Misra is chief of the division of pediatric endocrinology at Mass General Hospital for Children and professor in the department of pediatrics at Harvard Medical School, both in Boston. She reported conflicts of interest with AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
An 18-year-old woman with Crohn’s disease (diagnosed 3 years ago) came to my office for advice regarding management of osteoporosis. Her bone density was low for her age, and she had three low-impact fractures of her long bones in the preceding 4 years.
Loss of weight after the onset of Crohn’s disease, subsequent loss of periods, inflammation associated with her underlying diagnosis, and early treatment with glucocorticoids (known to have deleterious effects on bone) were believed to have caused osteoporosis in this young woman.
A few months previously, she was switched to a medication that doesn’t impair bone health and glucocorticoids were discontinued; her weight began to improve, and her Crohn’s disease was now in remission. Her menses had resumed about 3 months before her visit to my clinic after a prolonged period without periods. She was on calcium and vitamin D supplements, with normal levels of vitamin D.
Many factors determine bone health including (but not limited to) genetics, nutritional status, exercise activity (with mechanical loading of bones), macro- and micronutrient intake, hormonal status, chronic inflammation and other disease states, and medication use.
Exercise certainly has beneficial effects on bone. Bone-loading activities increase bone formation through the activation of certain cells in bone called osteocytes, which serve as mechanosensors and sense bone loading. Osteocytes make a hormone called sclerostin, which typically inhibits bone formation. When osteocytes sense bone-loading activities, sclerostin secretion reduces, allowing for increased bone formation.
Consistent with this, investigators in Canada have demonstrated greater increases in bone density and strength in schoolchildren who engage in moderate to vigorous physical activity, particularly bone-loading exercise, during the school day, compared with those who don’t (J Bone Miner Res. 2007 Mar;22[3]:434-46; J Bone Miner Res. 2017 Jul;32[7]:1525-36). In females, normal levels of estrogen seem necessary for osteocytes to bring about these effects after bone-loading activities. This is probably one of several reasons why athletes who lose their periods (indicative of low estrogen levels) and develop low bone density with an increased risk for fracture even when they are still at a normal weight (J Clin Endocrinol Metab. 2018 Jun 1;103[6]:2392-402; Med Sci Sports Exerc. 2015 Aug;47[8]:1577-86).
One concern around prescribing bone-loading activity or exercise to persons with osteoporosis is whether it would increase the risk for fracture from the impact on fragile bone. The extent of bone loading safe for fragile bone can be difficult to determine. Furthermore, excessive exercise may worsen bone health by causing weight loss or loss of periods in women. Very careful monitoring may be necessary to ensure that energy balance is maintained. Therefore, the nature and volume of exercise should be discussed with one’s doctor or physical therapist as well as a dietitian (if the patient is seeing one).
In patients with osteoporosis, high-impact activities such as jumping; repetitive impact activities such as running or jogging; and bending and twisting activities such as touching one’s toes, golf, tennis, and bowling aren’t recommended because they increase the risk for fracture. Even yoga poses should be discussed, because some may increase the risk for compression fractures of the vertebrae in the spine.
Strength and resistance training are generally believed to be good for bones. Strength training involves activities that build muscle strength and mass. Resistance training builds muscle strength, mass, and endurance by making muscles work against some form of resistance. Such activities include weight training with free weights or weight machines, use of resistance bands, and use of one’s own body to strengthen major muscle groups (such as through push-ups, squats, lunges, and gluteus maximus extension).
Some amount of weight-bearing aerobic training is also recommended, including walking, low-impact aerobics, the elliptical, and stair-climbing. Non–weight-bearing activities, such as swimming and cycling, typically don’t contribute to improving bone density.
In older individuals with osteoporosis, agility exercises are particularly useful to reduce the fall risk (J Am Geriatr Soc. 2004 May;52[5]:657-65; CMAJ. 2002 Oct 29;167[9]:997-1004). These can be structured to improve hand-eye coordination, foot-eye coordination, static and dynamic balance, and reaction time. Agility exercises with resistance training help improve bone density in older women.
An optimal exercise regimen includes a combination of strength and resistance training; weight-bearing aerobic training; and exercises that build flexibility, stability, and balance. A doctor, physical therapist, or trainer with expertise in the right combination of exercises should be consulted to ensure optimal effects on bone and general health.
In those at risk for overexercising to the point that they start to lose weight or lose their periods, and certainly in all women with disordered eating patterns, a dietitian should be part of the decision team to ensure that energy balance is maintained. In this group, particularly in very-low-weight women with eating disorders, exercise activity is often limited until they reach a healthier weight, and ideally after their menses resume.
For my patient with Crohn’s disease, I recommended that she see a physical therapist and a dietitian for guidance about a graded increase in exercise activity and an exercise regimen that would work best for her. I assess her bone density annually using dual-energy x-ray absorptiometry. Her bone density has gradually improved with the combination of weight gain, resumption of menses, medications for Crohn’s disease that do not affect bone deleteriously, remission of Crohn’s disease, and her exercise regimen.
Dr. Misra is chief of the division of pediatric endocrinology at Mass General Hospital for Children and professor in the department of pediatrics at Harvard Medical School, both in Boston. She reported conflicts of interest with AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
A new ultrabrief screening scale for pediatric OCD
Obsessive-compulsive disorder (OCD) affects 1-2% of the population. The disorder is characterized by recurrent intrusive unwanted thoughts (obsessions) that cause significant distress and anxiety, and behavioral or mental rituals (compulsions) that are performed to reduce distress stemming from obsessions. OCD may onset at any time in life, but most commonly begins in childhood or in early adulthood.
Cognitive behavioral therapy (CBT) with exposure and response prevention is an empirically based and highly effective treatment for OCD. However, most youth with OCD do not receive any treatment, which is related to a shortage of mental health care providers with expertise in assessment and treatment of the disorder, and misdiagnosis of the disorder is all too prevalent.
Aside from the subjective emotional toll associated with OCD, individuals living with this disorder frequently experience interpersonal, academic, and vocational impairments. Nevertheless, OCD is often overlooked or misdiagnosed. This may be more pronounced in youth with OCD, particularly in primary health care settings and large nonspecialized medical institutions. In fact, research indicates that pediatric OCD is often underrecognized even among mental health professionals. This situation is not new, and in fact the National Institute for Health and Care Excellence (NICE) in the United Kingdom stated that there is an urgent need to develop brief reliable screeners for OCD nearly 20 years ago.
Although there were several attempts to develop brief screening scales for adults and youth with OCD, none of them were found to be suitable for use as rapid screening tools in nonspecialized settings. One of the primary reasons is that OCD is associated with different “themes” or dimensions. For example, a child with OCD may engage in cleaning rituals because the context (or dimension) of their obsessions is contamination concerns. Another child with OCD, who may suffer from similar overall symptom severity, may primarily engage in checking rituals which are related with obsessions associated with fear of being responsible for harm. Therefore, one child with OCD may score very high on items assessing one dimension (e.g., contamination concerns), but very low on another dimension (e.g., harm obsessions).
This results in a known challenge in the assessment and psychometrics of self-report (as opposed to clinician administered) measures of OCD. Secondly, development of such measures requires very large carefully screened samples of individuals with OCD, with other disorders, and those without a known psychological disorder – which may be more challenging than requiring adult participants.
To accomplish this, we harmonized data from several sites that included three samples of carefully screened youths with OCD, with other disorders, and without known disorders who completed multiple self-report questionnaires, including the 21-item Obsessive-Compulsive Inventory – Child Version (OCI-CV).
Utilizing psychometric analyses including factor analyses, invariance analyses, and item response theory methodologies, we were able to develop an ultrabrief measure extracted from the OCI-CV: the 5-item Obsessive-Compulsive Inventory – Child Version (OCI-CV-5). This very brief self-report measure was found to have very good psychometric properties including a sensitive and specific clinical cutoff score. Youth who score at or above the cutoff score are nearly 21 times more likely to meet criteria for OCD.
This measure corresponds to a need to rapidly screen for OCD in children in nonspecialized settings, including community mental health clinics, primary care settings, and pediatric treatment facilities. However, it is important to note it is not a diagnostic measure. The measure is intended to identify youth who should be referred to a mental health care professional to conduct a diagnostic interview.
Dr. Abramovitch is a clinical psychologist and neuropsychologist based in Austin, Tex., and an associate professor at Texas State University. Dr. Abramowitz is professor and director of clinical training in the Anxiety and Stress Lab at University of North Carolina at Chapel Hill. Dr. McKay is professor of psychology at Fordham University, Bronx, N.Y.
Obsessive-compulsive disorder (OCD) affects 1-2% of the population. The disorder is characterized by recurrent intrusive unwanted thoughts (obsessions) that cause significant distress and anxiety, and behavioral or mental rituals (compulsions) that are performed to reduce distress stemming from obsessions. OCD may onset at any time in life, but most commonly begins in childhood or in early adulthood.
Cognitive behavioral therapy (CBT) with exposure and response prevention is an empirically based and highly effective treatment for OCD. However, most youth with OCD do not receive any treatment, which is related to a shortage of mental health care providers with expertise in assessment and treatment of the disorder, and misdiagnosis of the disorder is all too prevalent.
Aside from the subjective emotional toll associated with OCD, individuals living with this disorder frequently experience interpersonal, academic, and vocational impairments. Nevertheless, OCD is often overlooked or misdiagnosed. This may be more pronounced in youth with OCD, particularly in primary health care settings and large nonspecialized medical institutions. In fact, research indicates that pediatric OCD is often underrecognized even among mental health professionals. This situation is not new, and in fact the National Institute for Health and Care Excellence (NICE) in the United Kingdom stated that there is an urgent need to develop brief reliable screeners for OCD nearly 20 years ago.
Although there were several attempts to develop brief screening scales for adults and youth with OCD, none of them were found to be suitable for use as rapid screening tools in nonspecialized settings. One of the primary reasons is that OCD is associated with different “themes” or dimensions. For example, a child with OCD may engage in cleaning rituals because the context (or dimension) of their obsessions is contamination concerns. Another child with OCD, who may suffer from similar overall symptom severity, may primarily engage in checking rituals which are related with obsessions associated with fear of being responsible for harm. Therefore, one child with OCD may score very high on items assessing one dimension (e.g., contamination concerns), but very low on another dimension (e.g., harm obsessions).
This results in a known challenge in the assessment and psychometrics of self-report (as opposed to clinician administered) measures of OCD. Secondly, development of such measures requires very large carefully screened samples of individuals with OCD, with other disorders, and those without a known psychological disorder – which may be more challenging than requiring adult participants.
To accomplish this, we harmonized data from several sites that included three samples of carefully screened youths with OCD, with other disorders, and without known disorders who completed multiple self-report questionnaires, including the 21-item Obsessive-Compulsive Inventory – Child Version (OCI-CV).
Utilizing psychometric analyses including factor analyses, invariance analyses, and item response theory methodologies, we were able to develop an ultrabrief measure extracted from the OCI-CV: the 5-item Obsessive-Compulsive Inventory – Child Version (OCI-CV-5). This very brief self-report measure was found to have very good psychometric properties including a sensitive and specific clinical cutoff score. Youth who score at or above the cutoff score are nearly 21 times more likely to meet criteria for OCD.
This measure corresponds to a need to rapidly screen for OCD in children in nonspecialized settings, including community mental health clinics, primary care settings, and pediatric treatment facilities. However, it is important to note it is not a diagnostic measure. The measure is intended to identify youth who should be referred to a mental health care professional to conduct a diagnostic interview.
Dr. Abramovitch is a clinical psychologist and neuropsychologist based in Austin, Tex., and an associate professor at Texas State University. Dr. Abramowitz is professor and director of clinical training in the Anxiety and Stress Lab at University of North Carolina at Chapel Hill. Dr. McKay is professor of psychology at Fordham University, Bronx, N.Y.
Obsessive-compulsive disorder (OCD) affects 1-2% of the population. The disorder is characterized by recurrent intrusive unwanted thoughts (obsessions) that cause significant distress and anxiety, and behavioral or mental rituals (compulsions) that are performed to reduce distress stemming from obsessions. OCD may onset at any time in life, but most commonly begins in childhood or in early adulthood.
Cognitive behavioral therapy (CBT) with exposure and response prevention is an empirically based and highly effective treatment for OCD. However, most youth with OCD do not receive any treatment, which is related to a shortage of mental health care providers with expertise in assessment and treatment of the disorder, and misdiagnosis of the disorder is all too prevalent.
Aside from the subjective emotional toll associated with OCD, individuals living with this disorder frequently experience interpersonal, academic, and vocational impairments. Nevertheless, OCD is often overlooked or misdiagnosed. This may be more pronounced in youth with OCD, particularly in primary health care settings and large nonspecialized medical institutions. In fact, research indicates that pediatric OCD is often underrecognized even among mental health professionals. This situation is not new, and in fact the National Institute for Health and Care Excellence (NICE) in the United Kingdom stated that there is an urgent need to develop brief reliable screeners for OCD nearly 20 years ago.
Although there were several attempts to develop brief screening scales for adults and youth with OCD, none of them were found to be suitable for use as rapid screening tools in nonspecialized settings. One of the primary reasons is that OCD is associated with different “themes” or dimensions. For example, a child with OCD may engage in cleaning rituals because the context (or dimension) of their obsessions is contamination concerns. Another child with OCD, who may suffer from similar overall symptom severity, may primarily engage in checking rituals which are related with obsessions associated with fear of being responsible for harm. Therefore, one child with OCD may score very high on items assessing one dimension (e.g., contamination concerns), but very low on another dimension (e.g., harm obsessions).
This results in a known challenge in the assessment and psychometrics of self-report (as opposed to clinician administered) measures of OCD. Secondly, development of such measures requires very large carefully screened samples of individuals with OCD, with other disorders, and those without a known psychological disorder – which may be more challenging than requiring adult participants.
To accomplish this, we harmonized data from several sites that included three samples of carefully screened youths with OCD, with other disorders, and without known disorders who completed multiple self-report questionnaires, including the 21-item Obsessive-Compulsive Inventory – Child Version (OCI-CV).
Utilizing psychometric analyses including factor analyses, invariance analyses, and item response theory methodologies, we were able to develop an ultrabrief measure extracted from the OCI-CV: the 5-item Obsessive-Compulsive Inventory – Child Version (OCI-CV-5). This very brief self-report measure was found to have very good psychometric properties including a sensitive and specific clinical cutoff score. Youth who score at or above the cutoff score are nearly 21 times more likely to meet criteria for OCD.
This measure corresponds to a need to rapidly screen for OCD in children in nonspecialized settings, including community mental health clinics, primary care settings, and pediatric treatment facilities. However, it is important to note it is not a diagnostic measure. The measure is intended to identify youth who should be referred to a mental health care professional to conduct a diagnostic interview.
Dr. Abramovitch is a clinical psychologist and neuropsychologist based in Austin, Tex., and an associate professor at Texas State University. Dr. Abramowitz is professor and director of clinical training in the Anxiety and Stress Lab at University of North Carolina at Chapel Hill. Dr. McKay is professor of psychology at Fordham University, Bronx, N.Y.
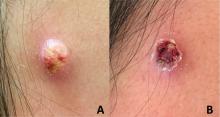
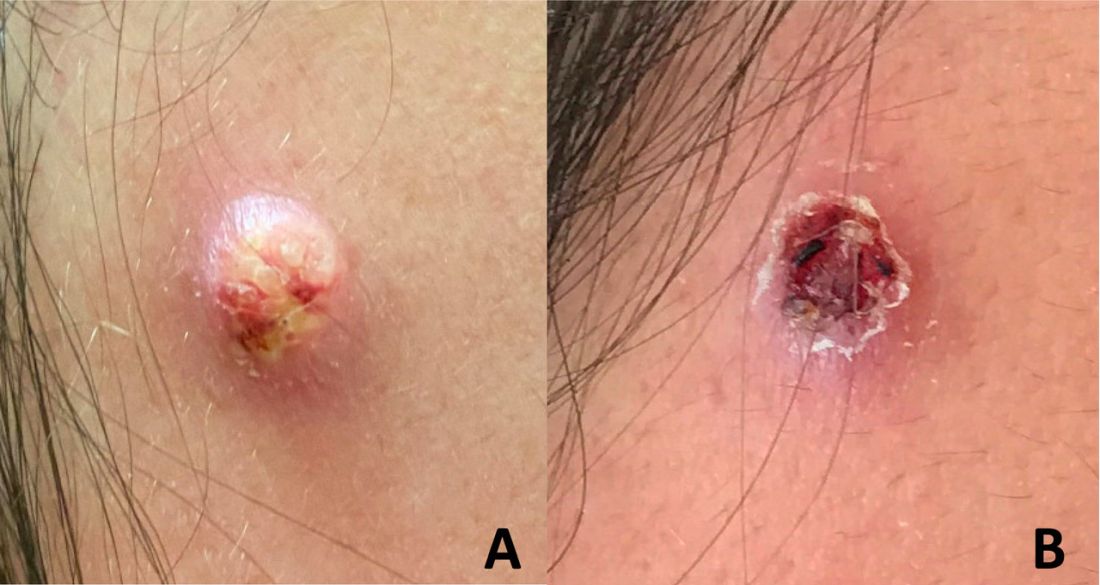
An adolescent male presents with an eroded bump on the temple
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
Will Congress step up to save primary care?
Primary care and family physicians operate on the front lines of health care, working tirelessly to serve patients and their families. However, many primary care practices are operating on tight margins and cannot sustain additional financial hits. As we continue to navigate a pandemic that has altered our health care landscape, we traveled to Capitol Hill to urge Congress to act on two critical issues: Medicare payment reform and streamlining administrative burden for physicians.
The current Medicare system for compensating physicians jeopardizes access to primary care. Family physicians, along with other primary care clinicians, are facing significant cuts in payments and rising inflation that threaten our ability to care for patients.
Each of us has experienced the effects of this pincer in devastating ways – from the independent clinicians who have been forced to sell their practices to hospitals or large health systems, to the physicians who are retiring early, leaving their practices, or even closing them because they can’t afford to keep their doors open.
Practices also struggle to cover the rising costs of staff wages, leasing space, and purchasing supplies and equipment, leaving little room for innovation or investments to transition into new payment models. Meanwhile, hospitals, skilled nursing facilities, ambulatory surgery centers, and other Medicare providers receive annual payment increases to account for rising costs.
Insufficient Medicare payments also challenge practices that serve many publicly insured patients. If practices cannot cover their expenses, they may be forced to turn away new Medicare and Medicaid patients – something that goes against the core tenets of our health care system.
Fortunately, we have some solutions. We’re asking Congress to pass the Supporting Medicare Providers Act of 2022, which calls for a 4.42% positive adjustment to the Medicare Physician Fee Schedule (MPFS) conversion factor for 2023 to offset the statutory reduction triggered by budget neutrality rules.
We also are calling on lawmakers to end the statutory freeze on annual updates to the MPFS and enact a positive annual update to the conversion factor based on the Medicare Economic Index. This critical relief would stave off the most immediate cuts while giving us more time to work with Congress on comprehensive reforms to the Medicare physician payment system.
As many practices struggle to operate, burnout among primary care physicians has also increased, with research showing that 66% of primary care physicians reported frequent burnout symptoms in 2021. Streamlining prior authorizations – a cumbersome process that requires physicians to obtain preapproval for treatments or tests before providing care to patients, and can risk patients’ access to timely care – is one way to reduce burden and alleviate burnout.
According to the American Medical Association, 82% of physicians report that prior authorization can lead to patients abandoning care, and 93% believe that prior authorization delays access to necessary care.
All of us have had patients whose care has been affected by these delays, including difficulty in getting necessary medications filled or having medical procedures postponed. Moreover, primary care physicians and their staff spend hours each week completing paperwork and communicating with insurers to ensure that their patients can access the treatments and services they need.
That is why we’re urging the Senate to pass the Improving Seniors’ Timely Access to Care Act, which would streamline the prior authorization process in the Medicare Advantage program.
As family physicians, we are in a unique position to help improve our patients’ health and their quality of life. But we can’t do this alone. We need the support of policy makers to make patient health and primary care a national priority.
Dr. Iroku-Malize is a family physician in Long Island, New York, and President of the American Academy of Family Physicians. Dr. Ransone is a family physician in Deltaville, Va., and board chair, immediate past president of the AAFP. Dr. Furr is a family physician in Jackson, Ala., and President-elect of the AAFP. They reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Primary care and family physicians operate on the front lines of health care, working tirelessly to serve patients and their families. However, many primary care practices are operating on tight margins and cannot sustain additional financial hits. As we continue to navigate a pandemic that has altered our health care landscape, we traveled to Capitol Hill to urge Congress to act on two critical issues: Medicare payment reform and streamlining administrative burden for physicians.
The current Medicare system for compensating physicians jeopardizes access to primary care. Family physicians, along with other primary care clinicians, are facing significant cuts in payments and rising inflation that threaten our ability to care for patients.
Each of us has experienced the effects of this pincer in devastating ways – from the independent clinicians who have been forced to sell their practices to hospitals or large health systems, to the physicians who are retiring early, leaving their practices, or even closing them because they can’t afford to keep their doors open.
Practices also struggle to cover the rising costs of staff wages, leasing space, and purchasing supplies and equipment, leaving little room for innovation or investments to transition into new payment models. Meanwhile, hospitals, skilled nursing facilities, ambulatory surgery centers, and other Medicare providers receive annual payment increases to account for rising costs.
Insufficient Medicare payments also challenge practices that serve many publicly insured patients. If practices cannot cover their expenses, they may be forced to turn away new Medicare and Medicaid patients – something that goes against the core tenets of our health care system.
Fortunately, we have some solutions. We’re asking Congress to pass the Supporting Medicare Providers Act of 2022, which calls for a 4.42% positive adjustment to the Medicare Physician Fee Schedule (MPFS) conversion factor for 2023 to offset the statutory reduction triggered by budget neutrality rules.
We also are calling on lawmakers to end the statutory freeze on annual updates to the MPFS and enact a positive annual update to the conversion factor based on the Medicare Economic Index. This critical relief would stave off the most immediate cuts while giving us more time to work with Congress on comprehensive reforms to the Medicare physician payment system.
As many practices struggle to operate, burnout among primary care physicians has also increased, with research showing that 66% of primary care physicians reported frequent burnout symptoms in 2021. Streamlining prior authorizations – a cumbersome process that requires physicians to obtain preapproval for treatments or tests before providing care to patients, and can risk patients’ access to timely care – is one way to reduce burden and alleviate burnout.
According to the American Medical Association, 82% of physicians report that prior authorization can lead to patients abandoning care, and 93% believe that prior authorization delays access to necessary care.
All of us have had patients whose care has been affected by these delays, including difficulty in getting necessary medications filled or having medical procedures postponed. Moreover, primary care physicians and their staff spend hours each week completing paperwork and communicating with insurers to ensure that their patients can access the treatments and services they need.
That is why we’re urging the Senate to pass the Improving Seniors’ Timely Access to Care Act, which would streamline the prior authorization process in the Medicare Advantage program.
As family physicians, we are in a unique position to help improve our patients’ health and their quality of life. But we can’t do this alone. We need the support of policy makers to make patient health and primary care a national priority.
Dr. Iroku-Malize is a family physician in Long Island, New York, and President of the American Academy of Family Physicians. Dr. Ransone is a family physician in Deltaville, Va., and board chair, immediate past president of the AAFP. Dr. Furr is a family physician in Jackson, Ala., and President-elect of the AAFP. They reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Primary care and family physicians operate on the front lines of health care, working tirelessly to serve patients and their families. However, many primary care practices are operating on tight margins and cannot sustain additional financial hits. As we continue to navigate a pandemic that has altered our health care landscape, we traveled to Capitol Hill to urge Congress to act on two critical issues: Medicare payment reform and streamlining administrative burden for physicians.
The current Medicare system for compensating physicians jeopardizes access to primary care. Family physicians, along with other primary care clinicians, are facing significant cuts in payments and rising inflation that threaten our ability to care for patients.
Each of us has experienced the effects of this pincer in devastating ways – from the independent clinicians who have been forced to sell their practices to hospitals or large health systems, to the physicians who are retiring early, leaving their practices, or even closing them because they can’t afford to keep their doors open.
Practices also struggle to cover the rising costs of staff wages, leasing space, and purchasing supplies and equipment, leaving little room for innovation or investments to transition into new payment models. Meanwhile, hospitals, skilled nursing facilities, ambulatory surgery centers, and other Medicare providers receive annual payment increases to account for rising costs.
Insufficient Medicare payments also challenge practices that serve many publicly insured patients. If practices cannot cover their expenses, they may be forced to turn away new Medicare and Medicaid patients – something that goes against the core tenets of our health care system.
Fortunately, we have some solutions. We’re asking Congress to pass the Supporting Medicare Providers Act of 2022, which calls for a 4.42% positive adjustment to the Medicare Physician Fee Schedule (MPFS) conversion factor for 2023 to offset the statutory reduction triggered by budget neutrality rules.
We also are calling on lawmakers to end the statutory freeze on annual updates to the MPFS and enact a positive annual update to the conversion factor based on the Medicare Economic Index. This critical relief would stave off the most immediate cuts while giving us more time to work with Congress on comprehensive reforms to the Medicare physician payment system.
As many practices struggle to operate, burnout among primary care physicians has also increased, with research showing that 66% of primary care physicians reported frequent burnout symptoms in 2021. Streamlining prior authorizations – a cumbersome process that requires physicians to obtain preapproval for treatments or tests before providing care to patients, and can risk patients’ access to timely care – is one way to reduce burden and alleviate burnout.
According to the American Medical Association, 82% of physicians report that prior authorization can lead to patients abandoning care, and 93% believe that prior authorization delays access to necessary care.
All of us have had patients whose care has been affected by these delays, including difficulty in getting necessary medications filled or having medical procedures postponed. Moreover, primary care physicians and their staff spend hours each week completing paperwork and communicating with insurers to ensure that their patients can access the treatments and services they need.
That is why we’re urging the Senate to pass the Improving Seniors’ Timely Access to Care Act, which would streamline the prior authorization process in the Medicare Advantage program.
As family physicians, we are in a unique position to help improve our patients’ health and their quality of life. But we can’t do this alone. We need the support of policy makers to make patient health and primary care a national priority.
Dr. Iroku-Malize is a family physician in Long Island, New York, and President of the American Academy of Family Physicians. Dr. Ransone is a family physician in Deltaville, Va., and board chair, immediate past president of the AAFP. Dr. Furr is a family physician in Jackson, Ala., and President-elect of the AAFP. They reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
More children should be getting flu vaccines
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
Cold and flu season came early in 2022.
On Nov. 4, 2022, the Centers for Disease Control and Prevention issued a Health Alert Network Health Advisory about early, elevated respiratory disease incidence caused by multiple viruses other than SARS-CoV-2.
Interseasonal spread of respiratory syncytial virus has continued in 2022, with RSV-associated hospitalizations increasing in the late spring and continuing throughout the summer and into the fall. In October, some regions of the country were seeing RSV activity near the peak seasonal levels typically observed in December and January.
Cases of severe respiratory infection in children who tested positive for rhinovirus or enterovirus spiked in August; further testing confirmed the presence of EV-D68 in some children. Rhinovirus and enterovirus continue to circulate and are isolated in hospitalized children with respiratory illness.
In some parts of the country, influenza cases have rapidly increased ahead of what we normally anticipate. According to preliminary estimates from the CDC, between Oct. 1 and Oct. 22, 880,000 people were sickened with flu, 420,000 people visited a health care provider for flu illness, and 6,900 people were hospitalized for flu. The cumulative hospitalization rate is higher than observed at this time of year in every previous flu season since 2010-2011. Hospitalization rates are highest in children aged 0-4 years and adults 65 years and older.
Of course, this report came as no surprise to pediatric health care providers. Many children’s hospitals had been operating at or over capacity for weeks. While a systematic assessment of the surge on children’s hospitals has not been published, anecdotally, hospitals from around the country have described record emergency department visits and inpatient census numbers. Some have set up tents or other temporary facilities to see ambulatory patients and have canceled elective surgeries because of a lack of beds.
There is no quick or easy solution to stem the tide of RSV-related or enterovirus/rhinovirus admissions, but many flu-related hospitalizations are vaccine preventable. Unfortunately, too few children are receiving influenza vaccine. As of the week ending Oct. 15, only about 22.1% of eligible children had been immunized. The American Academy of Pediatrics and the CDC recommend that all children are vaccinated, preferably by the end of October so they have time to develop immunity before influenza starts circulating. As it stands now, the majority of the nation’s children are facing a flu season without the benefits of vaccine.
There is still time to take steps to prevent this flu season from becoming one of the worst in recent memory. A strong provider recommendation for influenza vaccine is consistently associated with higher rates of vaccine acceptance. We need to recommend influenza vaccine to all eligible patients at every visit and in every setting. It will help if we can say it like we mean it. Some of us are tired of debating the merits of COVID-19 vaccine with families and may be leery of additional debates about flu. Some of us may just be tired, as many practices have already expanded office hours to care for the influx of kids with respiratory illness. On the heels of two atypical flu seasons, a few of us may be quietly complacent about the importance of flu vaccines for children.
Anyone in need of a little motivation should check out a paper recently published in Clinical Infectious Diseases that reinforces the value of flu vaccine, even in a year when there is a poor match between the vaccine and circulating viruses.
The 2019-2020 flu season was a bad flu season for children. Two antigenically drifted influenza viruses predominated and cases of influenza soared, resulting in the largest influenza epidemic in children in the United States since 1992. Pediatric Intensive Care Influenza Study investigators used a test-negative design to estimate the effectiveness of influenza vaccine in preventing critical and life-threatening influenza in children during that season. The good news: vaccination reduced the risk of critical influenza by 78% against H1N1pdm09 viruses that were well-matched to vaccine and by 47% against mismatched viruses. Vaccination was estimated to be 75% protective against antigenically drifted B-Victoria viruses. Overall vaccine effectiveness against critical illness from any influenza virus was 63% (95% confidence interval, 38%-78%).
While it might be tempting to attribute suboptimal immunization rates to vaccine hesitancy, ready availability remains an issue for some families. We need to eliminate barriers to access. While the AAP continues to emphasize immunization in the medical home, especially for the youngest infants, the 2022 policy statement suggests that vaccinating children in schools, pharmacies, and other nontraditional settings could improve immunization rates. To the extent feasible, we need to work with partners to support community-based initiatives and promote these to families who struggle to make it into the office.
Improving access is just one potential way to reduce health disparities related to influenza and influenza vaccination. Over 10 influenza seasons, higher rates of influenza-associated hospitalizations and intensive care unit admissions were observed in Black, Hispanic, and American Indian/Alaska Native people. These disparities were highest in children aged younger than 4 years and influenza-associated in-hospital deaths were three- to fourfold higher in Black, Hispanic, and Asian/Pacific Islander children, compared with White children. The reason for the disparities isn’t completely clear but increasing immunization rates may be part of the solution. During the 2020-2021 influenza season, flu immunization rates in Black children (51.6%) were lower than those seen in White (57.4%) and Hispanic children (58.9%).
The AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023, highlight a variety of evidence-based strategies to increase influenza immunization rates. These may provide a little inspiration for clinicians looking to try a new approach. If you wish to share your experience with increasing influenza immunization rates in your practice setting, please email me at Kristina.bryant@louisville.edu.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022–2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead.
The Long Arc of Justice for Veteran Benefits
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
Dietary Triggers for Atopic Dermatitis in Children
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
Practice Points
- The perception of dietary triggers is so entrenched and widespread that it should be addressed even when thought to be irrelevant.
- It is important not to dismiss food as a factor in atopic dermatitis (AD), as it can play a number of roles in the condition.
- On the other hand, education about the wide range of food reactions and the relative rarity of true food-driven AD along with the potential risks of dietary modification may enhance both rapport and understanding between the clinician and patient.
With a little help from your friends
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.