User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Genotype, need for transfusion predict death in VEXAS syndrome
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
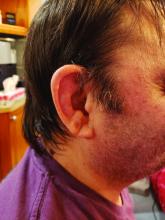
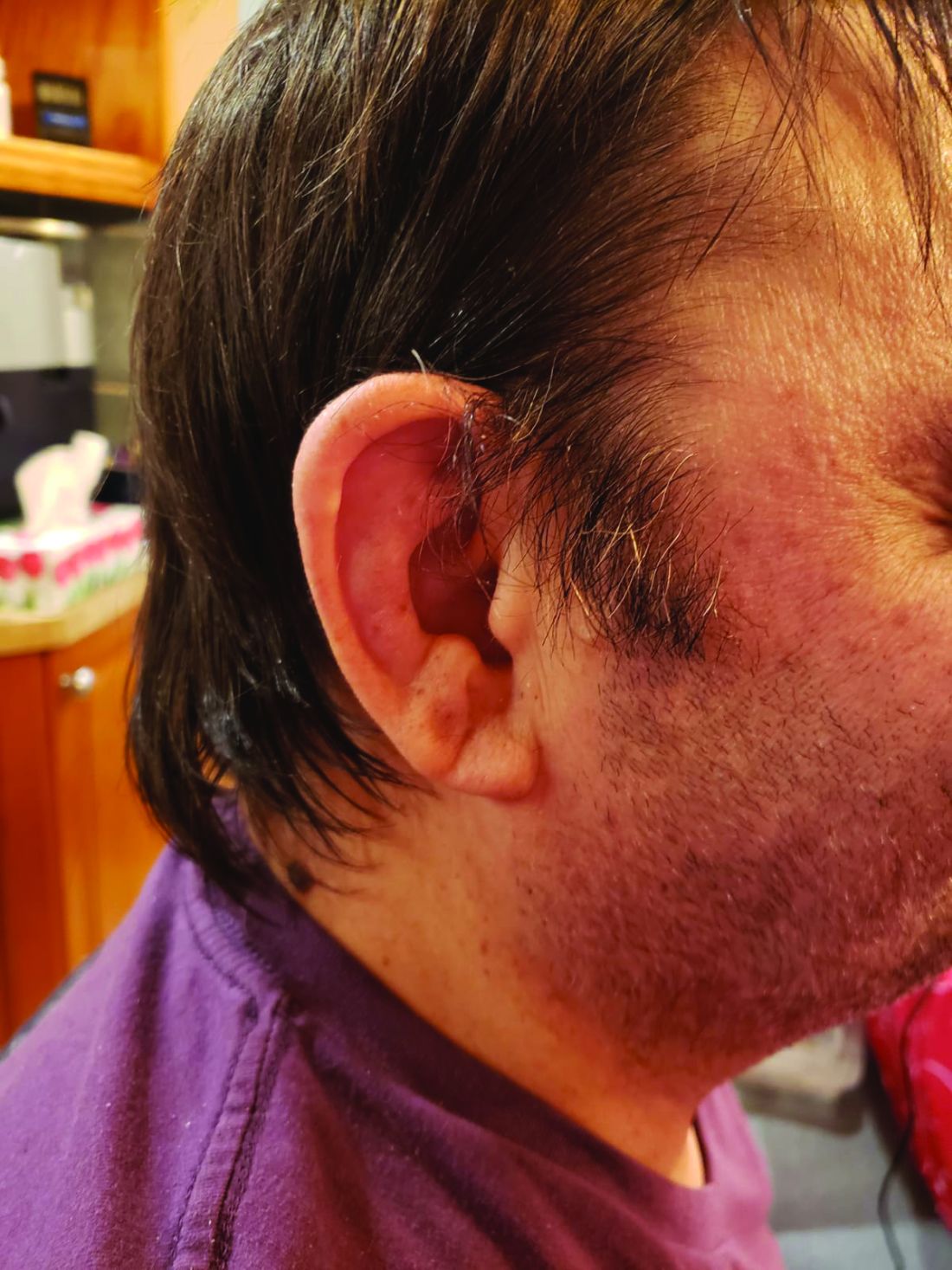
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
Lupus patients in remission see more flares with HCQ reduction, discontinuation
Continuation of hydroxychloroquine (HCQ) when a patient’s systemic lupus erythematosus (SLE) is in remission or has very low disease activity is linked to a lower risk of flares than is reducing or stopping the antimalarial drug, according to new research presented at the virtual annual meeting of the American College of Rheumatology.
“Though HCQ is a cornerstone SLE drug, physicians and patients often consider lowering or stopping the drug during remission or low disease activity in order to limit long-term toxicity,” Sasha Bernatsky, MD, PhD, a professor of rheumatology at McGill University in Montreal, told attendees. Her group’s findings revealed a 20% increased risk of flares in those who reduced their HCQ dose and a 56% greater risk of flares in those who discontinued HCQ, compared with those who continued on a maintenance dose.
“I’m going to be using these results in discussions with my patients regarding what the potential implications are of lowering or stopping hydroxychloroquine,” Dr. Bernatsky told attendees. “I think, in the end, this information should be in their hands so that they can be the ones to make these decisions with us, and, of course, given the significant flare rates even in remission, we need to keep on working on optimizing lupus treatments.”
Study details
The researchers analyzed prospective data from 1,460 patients enrolled in the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) cohort, which includes 33 sites across Europe, Asia, and North America. Patients in this cohort undergo annual follow-ups after enrollment within 15 months of their diagnosis. The study population was 89% female and 52% white. All participants either had low disease activity, defined as a score of 4 or lower on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and/or as a prednisone dose no greater than 7.5 mg/day, or were in complete remission, defined as a 0 on SLEDAI-2K while receiving no therapy, including no prednisone or immunosuppressives in the past year.
In addition to adjusting for sex, race/ethnicity, age, education, and geographic residence, the researchers took into account baseline SLE duration, renal damage, body mass index, smoking status, and use of prednisone, immunosuppressives, and biologics. For the outcome of time to first flare, the researchers analyzed those who discontinued HCQ separately from those who reduced the dose, comparing each to those who continued HCQ maintenance therapy. The researchers defined first flare as either hospitalization because of SLE, increased disease activity (at least 4 points on the SLEDAI-2K), or therapy augmentation with steroids, immunosuppressives, antimalarials, or biologics.
Within each cohort, patients who reduced or stopped HCQ therapy were matched to patients who continued HCQ maintenance therapy based on duration of HCQ since time zero, the point at which participants were considered at risk for SLE flares. In the reduction cohort, time zero was the date of a participant’s first HCQ reduction; in the discontinuation cohort, time zero was the date a participant stopped the therapy. Because of the study’s design and reliance on person-years of exposure, it was possible for a single participant to contribute data to more than one cohort.
Results
The overall cohort examining reduction of HCQ dose included 564 patients who reduced their dose, contributing 1,063 person-years of data, and 778 matched patients who started HCQ at the same time but continued HCQ maintenance therapy without a dose reduction, contributing 1,242 person-years. The average duration of HCQ use since time zero in this cohort was 3.4 years.
Before stratifying for disease activity, the group who reduced their therapy experienced 40 first flares per 100 person-years, compared with 31.9 first flares per 100 person-years on maintenance therapy. Those who reduced HCQ had a 20% greater risk of flares than did those who continued it (adjusted hazard ratio, 1.2). However, when those in remission were compared with those not in remission – independent of disease activity level – patients in remission were twice as likely to experience a flare if they reduced their HCQ dose (aHR, 2.14).
In the discontinuation cohort, 389 patients who stopped HCQ therapy contributed 657 person-years, and 577 matched patients who continued HCQ maintenance therapy contributed 924 person-years. The average duration of HCQ use since time zero in this cohort was 4.2 years. Before stratifying for disease activity, the average number of first flares per 100 person-years was 41.3 in the HCQ discontinuation group and 30 in the HCQ maintenance group, resulting in a 56% higher risk of flares for those who stopped HCQ, compared with patients who continued HCQ (aHR, 1.56). Looking only at those in remission, patients were nearly three times more likely to experience a flare if they stopped HCQ than were patients not in remission who continued a maintenance dose (aHR, 2.77).
Patient age is an important consideration
Overall, these findings are not surprising, said Jill P. Buyon, MD, director of the division of rheumatology and of the Lupus Center at NYU-Langone Health in New York. Dr. Buyon is not involved in the current study but is studying discontinuation of HCQ in older adults with lupus.
“It has been already shown that when lupus patients discontinue HCQ, flares are more likely, but does this apply to all age groups?” Dr. Buyon asked in an interview. “Data are essential to more accurately weigh the balance between accumulating ocular exposure, the explosion of new tools to assess retinal injury, and the risk of disease flare in a population that may have more stable/quiescent disease than younger patients.”
Although HCQ’s track record with infection risk is consistently better than that of more immunosuppressive drugs and is very safe during pregnancy, Dr. Buyon said her “ophthalmology colleagues persistently emphasize the risk of retinal accumulation of drug and ocular toxicity over time.” She referenced a recent case-control study in which overall prevalence of HCQ retinopathy was 7.5%, and greater for patients taking more than 5 mg/kg of HCQ or who used HCQ for more than 10 years.
”Risk escalates with continued use, and evaluation by sensitive approaches such as multifocal electroretinography suggests nearly a third of patients accrue retinal damage,” Dr. Buyon said. “As the longevity of patients improves and comorbidities such as renal insufficiency (which affects HCQ clearance) may increase, the ratio of efficacy to toxicity would be expected to decrease.” Further, the fact that disease activity may wane as people age means that rheumatologists treating older adults need to address a critical question, she said: “Can HCQ be safely withdrawn? This question is important in the context of an even broader concern regarding management of SLE in the elderly population, a topic which has received minimal attention.”
The study is limited by its observational design and the fact that the intervention was not randomly allocated, although the researchers attempted to adjust for confounders. Dr. Bernatsky also noted that mild flares might have been missed, and the researchers did not evaluate HCQ levels or adherence, nor did the data set include physicians’ or patients’ explicitly stated reasons for HCQ reduction or discontinuation.
”We estimated that 5% of patients may have reduced HCQ therapy as result of the AAO [American Academy of Ophthalmology] guidelines, 55% because of low disease activity state, and the remainder (40%) for other reasons, possibly intolerance or patient preference,” the researchers noted in their abstract. “Among those who discontinued HCQ, 4% had retinal changes of concern, 15% were in clinical remission, and the remainder stopped for unknown reasons, possibly intolerance or patient preference.”
Dr. Buyon also pointed out that the cohort was initially intended for studying cardiovascular risk and not designed to capture all visits during each year of follow-up.
“Thus, while hospitalizations would be well captured, not all flares, particularly those not severe, would be captured, and thus we may not have the complete picture,” she said, reiterating Dr. Bernatsky’s point that mild flares may have been missed.
”Clearly, this is a very important topic for the management of our patients, particularly those who are elderly and may have already reaped the benefits of hydroxychloroquine,” Dr. Buyon said. “Of course, we have to be mindful of the potential benefit with regard to blood clotting and lipid lowering. Nevertheless, accumulated ocular toxicity and cardiac issues such as cardiomyopathy may emerge to tip the balance after years of accumulated drug exposure.”
The research was funded by the Canadian Institute of Health Research, the Singer Family Fund for Lupus Research, and the SLICC Group. Dr. Bernatsky had no disclosures. Dr. Buyon noted that she has an R34 NIH planning grant to study the safety of withdrawal of hydroxychloroquine in elderly lupus patients that is relevant to this study.
Continuation of hydroxychloroquine (HCQ) when a patient’s systemic lupus erythematosus (SLE) is in remission or has very low disease activity is linked to a lower risk of flares than is reducing or stopping the antimalarial drug, according to new research presented at the virtual annual meeting of the American College of Rheumatology.
“Though HCQ is a cornerstone SLE drug, physicians and patients often consider lowering or stopping the drug during remission or low disease activity in order to limit long-term toxicity,” Sasha Bernatsky, MD, PhD, a professor of rheumatology at McGill University in Montreal, told attendees. Her group’s findings revealed a 20% increased risk of flares in those who reduced their HCQ dose and a 56% greater risk of flares in those who discontinued HCQ, compared with those who continued on a maintenance dose.
“I’m going to be using these results in discussions with my patients regarding what the potential implications are of lowering or stopping hydroxychloroquine,” Dr. Bernatsky told attendees. “I think, in the end, this information should be in their hands so that they can be the ones to make these decisions with us, and, of course, given the significant flare rates even in remission, we need to keep on working on optimizing lupus treatments.”
Study details
The researchers analyzed prospective data from 1,460 patients enrolled in the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) cohort, which includes 33 sites across Europe, Asia, and North America. Patients in this cohort undergo annual follow-ups after enrollment within 15 months of their diagnosis. The study population was 89% female and 52% white. All participants either had low disease activity, defined as a score of 4 or lower on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and/or as a prednisone dose no greater than 7.5 mg/day, or were in complete remission, defined as a 0 on SLEDAI-2K while receiving no therapy, including no prednisone or immunosuppressives in the past year.
In addition to adjusting for sex, race/ethnicity, age, education, and geographic residence, the researchers took into account baseline SLE duration, renal damage, body mass index, smoking status, and use of prednisone, immunosuppressives, and biologics. For the outcome of time to first flare, the researchers analyzed those who discontinued HCQ separately from those who reduced the dose, comparing each to those who continued HCQ maintenance therapy. The researchers defined first flare as either hospitalization because of SLE, increased disease activity (at least 4 points on the SLEDAI-2K), or therapy augmentation with steroids, immunosuppressives, antimalarials, or biologics.
Within each cohort, patients who reduced or stopped HCQ therapy were matched to patients who continued HCQ maintenance therapy based on duration of HCQ since time zero, the point at which participants were considered at risk for SLE flares. In the reduction cohort, time zero was the date of a participant’s first HCQ reduction; in the discontinuation cohort, time zero was the date a participant stopped the therapy. Because of the study’s design and reliance on person-years of exposure, it was possible for a single participant to contribute data to more than one cohort.
Results
The overall cohort examining reduction of HCQ dose included 564 patients who reduced their dose, contributing 1,063 person-years of data, and 778 matched patients who started HCQ at the same time but continued HCQ maintenance therapy without a dose reduction, contributing 1,242 person-years. The average duration of HCQ use since time zero in this cohort was 3.4 years.
Before stratifying for disease activity, the group who reduced their therapy experienced 40 first flares per 100 person-years, compared with 31.9 first flares per 100 person-years on maintenance therapy. Those who reduced HCQ had a 20% greater risk of flares than did those who continued it (adjusted hazard ratio, 1.2). However, when those in remission were compared with those not in remission – independent of disease activity level – patients in remission were twice as likely to experience a flare if they reduced their HCQ dose (aHR, 2.14).
In the discontinuation cohort, 389 patients who stopped HCQ therapy contributed 657 person-years, and 577 matched patients who continued HCQ maintenance therapy contributed 924 person-years. The average duration of HCQ use since time zero in this cohort was 4.2 years. Before stratifying for disease activity, the average number of first flares per 100 person-years was 41.3 in the HCQ discontinuation group and 30 in the HCQ maintenance group, resulting in a 56% higher risk of flares for those who stopped HCQ, compared with patients who continued HCQ (aHR, 1.56). Looking only at those in remission, patients were nearly three times more likely to experience a flare if they stopped HCQ than were patients not in remission who continued a maintenance dose (aHR, 2.77).
Patient age is an important consideration
Overall, these findings are not surprising, said Jill P. Buyon, MD, director of the division of rheumatology and of the Lupus Center at NYU-Langone Health in New York. Dr. Buyon is not involved in the current study but is studying discontinuation of HCQ in older adults with lupus.
“It has been already shown that when lupus patients discontinue HCQ, flares are more likely, but does this apply to all age groups?” Dr. Buyon asked in an interview. “Data are essential to more accurately weigh the balance between accumulating ocular exposure, the explosion of new tools to assess retinal injury, and the risk of disease flare in a population that may have more stable/quiescent disease than younger patients.”
Although HCQ’s track record with infection risk is consistently better than that of more immunosuppressive drugs and is very safe during pregnancy, Dr. Buyon said her “ophthalmology colleagues persistently emphasize the risk of retinal accumulation of drug and ocular toxicity over time.” She referenced a recent case-control study in which overall prevalence of HCQ retinopathy was 7.5%, and greater for patients taking more than 5 mg/kg of HCQ or who used HCQ for more than 10 years.
”Risk escalates with continued use, and evaluation by sensitive approaches such as multifocal electroretinography suggests nearly a third of patients accrue retinal damage,” Dr. Buyon said. “As the longevity of patients improves and comorbidities such as renal insufficiency (which affects HCQ clearance) may increase, the ratio of efficacy to toxicity would be expected to decrease.” Further, the fact that disease activity may wane as people age means that rheumatologists treating older adults need to address a critical question, she said: “Can HCQ be safely withdrawn? This question is important in the context of an even broader concern regarding management of SLE in the elderly population, a topic which has received minimal attention.”
The study is limited by its observational design and the fact that the intervention was not randomly allocated, although the researchers attempted to adjust for confounders. Dr. Bernatsky also noted that mild flares might have been missed, and the researchers did not evaluate HCQ levels or adherence, nor did the data set include physicians’ or patients’ explicitly stated reasons for HCQ reduction or discontinuation.
”We estimated that 5% of patients may have reduced HCQ therapy as result of the AAO [American Academy of Ophthalmology] guidelines, 55% because of low disease activity state, and the remainder (40%) for other reasons, possibly intolerance or patient preference,” the researchers noted in their abstract. “Among those who discontinued HCQ, 4% had retinal changes of concern, 15% were in clinical remission, and the remainder stopped for unknown reasons, possibly intolerance or patient preference.”
Dr. Buyon also pointed out that the cohort was initially intended for studying cardiovascular risk and not designed to capture all visits during each year of follow-up.
“Thus, while hospitalizations would be well captured, not all flares, particularly those not severe, would be captured, and thus we may not have the complete picture,” she said, reiterating Dr. Bernatsky’s point that mild flares may have been missed.
”Clearly, this is a very important topic for the management of our patients, particularly those who are elderly and may have already reaped the benefits of hydroxychloroquine,” Dr. Buyon said. “Of course, we have to be mindful of the potential benefit with regard to blood clotting and lipid lowering. Nevertheless, accumulated ocular toxicity and cardiac issues such as cardiomyopathy may emerge to tip the balance after years of accumulated drug exposure.”
The research was funded by the Canadian Institute of Health Research, the Singer Family Fund for Lupus Research, and the SLICC Group. Dr. Bernatsky had no disclosures. Dr. Buyon noted that she has an R34 NIH planning grant to study the safety of withdrawal of hydroxychloroquine in elderly lupus patients that is relevant to this study.
Continuation of hydroxychloroquine (HCQ) when a patient’s systemic lupus erythematosus (SLE) is in remission or has very low disease activity is linked to a lower risk of flares than is reducing or stopping the antimalarial drug, according to new research presented at the virtual annual meeting of the American College of Rheumatology.
“Though HCQ is a cornerstone SLE drug, physicians and patients often consider lowering or stopping the drug during remission or low disease activity in order to limit long-term toxicity,” Sasha Bernatsky, MD, PhD, a professor of rheumatology at McGill University in Montreal, told attendees. Her group’s findings revealed a 20% increased risk of flares in those who reduced their HCQ dose and a 56% greater risk of flares in those who discontinued HCQ, compared with those who continued on a maintenance dose.
“I’m going to be using these results in discussions with my patients regarding what the potential implications are of lowering or stopping hydroxychloroquine,” Dr. Bernatsky told attendees. “I think, in the end, this information should be in their hands so that they can be the ones to make these decisions with us, and, of course, given the significant flare rates even in remission, we need to keep on working on optimizing lupus treatments.”
Study details
The researchers analyzed prospective data from 1,460 patients enrolled in the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) cohort, which includes 33 sites across Europe, Asia, and North America. Patients in this cohort undergo annual follow-ups after enrollment within 15 months of their diagnosis. The study population was 89% female and 52% white. All participants either had low disease activity, defined as a score of 4 or lower on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and/or as a prednisone dose no greater than 7.5 mg/day, or were in complete remission, defined as a 0 on SLEDAI-2K while receiving no therapy, including no prednisone or immunosuppressives in the past year.
In addition to adjusting for sex, race/ethnicity, age, education, and geographic residence, the researchers took into account baseline SLE duration, renal damage, body mass index, smoking status, and use of prednisone, immunosuppressives, and biologics. For the outcome of time to first flare, the researchers analyzed those who discontinued HCQ separately from those who reduced the dose, comparing each to those who continued HCQ maintenance therapy. The researchers defined first flare as either hospitalization because of SLE, increased disease activity (at least 4 points on the SLEDAI-2K), or therapy augmentation with steroids, immunosuppressives, antimalarials, or biologics.
Within each cohort, patients who reduced or stopped HCQ therapy were matched to patients who continued HCQ maintenance therapy based on duration of HCQ since time zero, the point at which participants were considered at risk for SLE flares. In the reduction cohort, time zero was the date of a participant’s first HCQ reduction; in the discontinuation cohort, time zero was the date a participant stopped the therapy. Because of the study’s design and reliance on person-years of exposure, it was possible for a single participant to contribute data to more than one cohort.
Results
The overall cohort examining reduction of HCQ dose included 564 patients who reduced their dose, contributing 1,063 person-years of data, and 778 matched patients who started HCQ at the same time but continued HCQ maintenance therapy without a dose reduction, contributing 1,242 person-years. The average duration of HCQ use since time zero in this cohort was 3.4 years.
Before stratifying for disease activity, the group who reduced their therapy experienced 40 first flares per 100 person-years, compared with 31.9 first flares per 100 person-years on maintenance therapy. Those who reduced HCQ had a 20% greater risk of flares than did those who continued it (adjusted hazard ratio, 1.2). However, when those in remission were compared with those not in remission – independent of disease activity level – patients in remission were twice as likely to experience a flare if they reduced their HCQ dose (aHR, 2.14).
In the discontinuation cohort, 389 patients who stopped HCQ therapy contributed 657 person-years, and 577 matched patients who continued HCQ maintenance therapy contributed 924 person-years. The average duration of HCQ use since time zero in this cohort was 4.2 years. Before stratifying for disease activity, the average number of first flares per 100 person-years was 41.3 in the HCQ discontinuation group and 30 in the HCQ maintenance group, resulting in a 56% higher risk of flares for those who stopped HCQ, compared with patients who continued HCQ (aHR, 1.56). Looking only at those in remission, patients were nearly three times more likely to experience a flare if they stopped HCQ than were patients not in remission who continued a maintenance dose (aHR, 2.77).
Patient age is an important consideration
Overall, these findings are not surprising, said Jill P. Buyon, MD, director of the division of rheumatology and of the Lupus Center at NYU-Langone Health in New York. Dr. Buyon is not involved in the current study but is studying discontinuation of HCQ in older adults with lupus.
“It has been already shown that when lupus patients discontinue HCQ, flares are more likely, but does this apply to all age groups?” Dr. Buyon asked in an interview. “Data are essential to more accurately weigh the balance between accumulating ocular exposure, the explosion of new tools to assess retinal injury, and the risk of disease flare in a population that may have more stable/quiescent disease than younger patients.”
Although HCQ’s track record with infection risk is consistently better than that of more immunosuppressive drugs and is very safe during pregnancy, Dr. Buyon said her “ophthalmology colleagues persistently emphasize the risk of retinal accumulation of drug and ocular toxicity over time.” She referenced a recent case-control study in which overall prevalence of HCQ retinopathy was 7.5%, and greater for patients taking more than 5 mg/kg of HCQ or who used HCQ for more than 10 years.
”Risk escalates with continued use, and evaluation by sensitive approaches such as multifocal electroretinography suggests nearly a third of patients accrue retinal damage,” Dr. Buyon said. “As the longevity of patients improves and comorbidities such as renal insufficiency (which affects HCQ clearance) may increase, the ratio of efficacy to toxicity would be expected to decrease.” Further, the fact that disease activity may wane as people age means that rheumatologists treating older adults need to address a critical question, she said: “Can HCQ be safely withdrawn? This question is important in the context of an even broader concern regarding management of SLE in the elderly population, a topic which has received minimal attention.”
The study is limited by its observational design and the fact that the intervention was not randomly allocated, although the researchers attempted to adjust for confounders. Dr. Bernatsky also noted that mild flares might have been missed, and the researchers did not evaluate HCQ levels or adherence, nor did the data set include physicians’ or patients’ explicitly stated reasons for HCQ reduction or discontinuation.
”We estimated that 5% of patients may have reduced HCQ therapy as result of the AAO [American Academy of Ophthalmology] guidelines, 55% because of low disease activity state, and the remainder (40%) for other reasons, possibly intolerance or patient preference,” the researchers noted in their abstract. “Among those who discontinued HCQ, 4% had retinal changes of concern, 15% were in clinical remission, and the remainder stopped for unknown reasons, possibly intolerance or patient preference.”
Dr. Buyon also pointed out that the cohort was initially intended for studying cardiovascular risk and not designed to capture all visits during each year of follow-up.
“Thus, while hospitalizations would be well captured, not all flares, particularly those not severe, would be captured, and thus we may not have the complete picture,” she said, reiterating Dr. Bernatsky’s point that mild flares may have been missed.
”Clearly, this is a very important topic for the management of our patients, particularly those who are elderly and may have already reaped the benefits of hydroxychloroquine,” Dr. Buyon said. “Of course, we have to be mindful of the potential benefit with regard to blood clotting and lipid lowering. Nevertheless, accumulated ocular toxicity and cardiac issues such as cardiomyopathy may emerge to tip the balance after years of accumulated drug exposure.”
The research was funded by the Canadian Institute of Health Research, the Singer Family Fund for Lupus Research, and the SLICC Group. Dr. Bernatsky had no disclosures. Dr. Buyon noted that she has an R34 NIH planning grant to study the safety of withdrawal of hydroxychloroquine in elderly lupus patients that is relevant to this study.
FROM ACR 2021
‘If obesity were diabetes or cancer, how would you approach it?’
“When considering the challenges of obesity, ask yourself: ‘If it were diabetes, cancer, HIV, or Alzheimer’s, how would you discuss it, approach it, assess it, treat it?’” Lee M. Kaplan, MD, PhD, asked the audience of health care professionals during ObesityWeek®, the annual meeting of The Obesity Society.
“And then do it for obesity, using the full spectrum of tools at our disposal,” he advised.
This was the takeaway that Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital and associate professor, Harvard Medical School, Boston, left the audience with at the end of his lecture entitled, “What does the future of obesity care look like?”
Invited to summarize his main points, Dr. Kaplan told this news organization in an interview that practitioners caring for patients with obesity need to first “recognize that obesity is a disease” caused by dysfunction of the metabolic system that regulates body fat – in the same way immune dysregulation can lead to asthma.
Second, “we are finally developing noninvasive therapies that are more effective,” he noted, referring to the recently approved semaglutide, and even more potent weight-loss therapies that could be on the market within 3 years, so that weight-loss outcomes with pharmacotherapy are approaching those with bariatric surgery.
Third, it is important that patients with obesity get “broad and equitable access” to treatment, and health care practitioners need to be on the same page and have a “shared understanding” of which treatments are appropriate for individual patients, “just as we do for other diseases.”
Need for a shared understanding
“Dr. Kaplan really brought home the idea that we all need a shared understanding of what obesity is – and what it is not,” agreed symposium moderator Donna H. Ryan, MD, in an email.
“He underscored the biologic basis of obesity,” noted Dr. Ryan, professor emerita at Pennington Biomedical Research Center in Baton Rouge, Louisiana, and associate editor-in-chief of Obesity, the official journal of The Obesity Society.
“It is a dysregulation of the body’s weight (especially adipose tissue) regulatory system,” she continued. “The body responds to powerful environmental pressures that produce excess energy balance, and we store that as fat and defend our highest fat mass. This makes obesity a disease, a chronic disease that requires a medical approach to reverse. It’s not a cosmetic problem, it’s a medical problem,” she emphasized.
There is so much misinformation out there about obesity, according to Dr. Ryan.
“People think it’s a lack of willpower, and even patients blame themselves for not being able to lose weight and keep it off. It’s not their fault! It’s biology.”
Although the supplement industry and fad diets falsely promise fast results, there is no magic diet, she continued.
“But we have made progress based on understanding the biologic basis of obesity and have new medications that offer real hope for patients.”
“With 42% of U.S. adults having a BMI that qualifies as obesity, we need a concerted and broad effort to address this problem, and that starts with everybody on the same page as to what obesity is ... a shared understanding of the biologic basis of obesity. It’s time to take obesity seriously,” she summarized, echoing Dr. Kaplan.
A question of biology
“Obesity results from inappropriate pathophysiological regulation of body fat mass,” when the body defends adiposity, Dr. Kaplan explained at the start of his lecture.
The treatment strategy for obesity has always been a stepwise approach starting with lifestyle changes, then pharmacotherapy, then possibly bariatric surgery – each step with a potentially greater chance of weight loss. But now, he explained, medicine is on the verge of having an armamentarium of more potent weight-loss medications.
Compared with phentermine/topiramate, orlistat, naltrexone/bupropion, and liraglutide – which roughly might provide 5% to 10% weight loss, the glucagon-like peptide-1 (GLP-1) agonist semaglutide 2.4 mg/week (Wegovy, Novo Nordisk), approved by the U.S. Food and Drug Association in June, provides almost double this potential weight loss.
And two new agents that could provide “never seen before weight loss” of 25% could potentially enter the marketplace by 2025: the amylin agonist cagrilintide (Novo Nordisk) and the twincretin tirzepatide (Eli Lilly) (a combined glucose-dependent insulinotropic polypeptide [GIP] and GLP-1 receptor agonist).
In addition, when liraglutide comes off patent, a generic version could potentially be introduced, and combined generic liraglutide plus generic phentermine/topiramate could be a less expensive weight-loss treatment option in the future, he noted.
One size does not fit all
Importantly, weight loss varies widely among individual patients.
A graph of potential weight loss with different treatments (for example, bariatric surgery or liraglutide) versus the percentage of patients that attain the weight losses is roughly bell-shaped, Dr. Kaplan explained. For example, in the STEP1 trial of semaglutide, roughly 7.1% of patients lost less than 5% of their initial weight, 25% of patients lost 20% to 30%, and 10.8% of patients lost 30% or more; that is, patients at the higher end had weight loss comparable to that seen with bariatric surgery
Adding pharmacotherapy after bariatric surgery could be synergistic. For example, in the GRAVITAS study of patients with type 2 diabetes who had gastric bypass surgery, those who received liraglutide after surgery had augmented weight loss compared with those who received placebo.
People at a cocktail party might come up to him and say, “I’d like to lose 5 pounds, 10 pounds,” Dr. Kaplan related in the Q&A session.
“That’s not obesity,” he emphasized. Obesity is excess body fat that poses a risk to health. A person with obesity may have 50 or more excess pounds, and the body is trying to defend this weight.
“If we want to treat obesity more effectively, we have to fully understand why it is a disease and how that disease differs from the cultural desire for thinness,” he reiterated.
“We have to keep the needs and goals of all people living with obesity foremost in our minds, even if many of them have been previously misled by the bias, stigma, blame, and discrimination that surrounds them.”
“We need to re-evaluate what we think we know about obesity and open our minds to new ideas,” he added.
Dr. Kaplan has reported financial ties to Eli Lilly, Gelesis, GI Dynamics, IntelliHealth, Johnson & Johnson, Novo Nordisk, Pfizer, and Rhythm Pharmaceuticals. Dr. Ryan has ties to numerous Novo Nordisk, Pfizer, and several other pharmaceutical companies, including having an ownership interest in Gila Therapeutics, Xeno Biosciences, Epitomee, Calibrate, Roman, and Scientific Intake.
A version of this article first appeared on Medscape.com.
“When considering the challenges of obesity, ask yourself: ‘If it were diabetes, cancer, HIV, or Alzheimer’s, how would you discuss it, approach it, assess it, treat it?’” Lee M. Kaplan, MD, PhD, asked the audience of health care professionals during ObesityWeek®, the annual meeting of The Obesity Society.
“And then do it for obesity, using the full spectrum of tools at our disposal,” he advised.
This was the takeaway that Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital and associate professor, Harvard Medical School, Boston, left the audience with at the end of his lecture entitled, “What does the future of obesity care look like?”
Invited to summarize his main points, Dr. Kaplan told this news organization in an interview that practitioners caring for patients with obesity need to first “recognize that obesity is a disease” caused by dysfunction of the metabolic system that regulates body fat – in the same way immune dysregulation can lead to asthma.
Second, “we are finally developing noninvasive therapies that are more effective,” he noted, referring to the recently approved semaglutide, and even more potent weight-loss therapies that could be on the market within 3 years, so that weight-loss outcomes with pharmacotherapy are approaching those with bariatric surgery.
Third, it is important that patients with obesity get “broad and equitable access” to treatment, and health care practitioners need to be on the same page and have a “shared understanding” of which treatments are appropriate for individual patients, “just as we do for other diseases.”
Need for a shared understanding
“Dr. Kaplan really brought home the idea that we all need a shared understanding of what obesity is – and what it is not,” agreed symposium moderator Donna H. Ryan, MD, in an email.
“He underscored the biologic basis of obesity,” noted Dr. Ryan, professor emerita at Pennington Biomedical Research Center in Baton Rouge, Louisiana, and associate editor-in-chief of Obesity, the official journal of The Obesity Society.
“It is a dysregulation of the body’s weight (especially adipose tissue) regulatory system,” she continued. “The body responds to powerful environmental pressures that produce excess energy balance, and we store that as fat and defend our highest fat mass. This makes obesity a disease, a chronic disease that requires a medical approach to reverse. It’s not a cosmetic problem, it’s a medical problem,” she emphasized.
There is so much misinformation out there about obesity, according to Dr. Ryan.
“People think it’s a lack of willpower, and even patients blame themselves for not being able to lose weight and keep it off. It’s not their fault! It’s biology.”
Although the supplement industry and fad diets falsely promise fast results, there is no magic diet, she continued.
“But we have made progress based on understanding the biologic basis of obesity and have new medications that offer real hope for patients.”
“With 42% of U.S. adults having a BMI that qualifies as obesity, we need a concerted and broad effort to address this problem, and that starts with everybody on the same page as to what obesity is ... a shared understanding of the biologic basis of obesity. It’s time to take obesity seriously,” she summarized, echoing Dr. Kaplan.
A question of biology
“Obesity results from inappropriate pathophysiological regulation of body fat mass,” when the body defends adiposity, Dr. Kaplan explained at the start of his lecture.
The treatment strategy for obesity has always been a stepwise approach starting with lifestyle changes, then pharmacotherapy, then possibly bariatric surgery – each step with a potentially greater chance of weight loss. But now, he explained, medicine is on the verge of having an armamentarium of more potent weight-loss medications.
Compared with phentermine/topiramate, orlistat, naltrexone/bupropion, and liraglutide – which roughly might provide 5% to 10% weight loss, the glucagon-like peptide-1 (GLP-1) agonist semaglutide 2.4 mg/week (Wegovy, Novo Nordisk), approved by the U.S. Food and Drug Association in June, provides almost double this potential weight loss.
And two new agents that could provide “never seen before weight loss” of 25% could potentially enter the marketplace by 2025: the amylin agonist cagrilintide (Novo Nordisk) and the twincretin tirzepatide (Eli Lilly) (a combined glucose-dependent insulinotropic polypeptide [GIP] and GLP-1 receptor agonist).
In addition, when liraglutide comes off patent, a generic version could potentially be introduced, and combined generic liraglutide plus generic phentermine/topiramate could be a less expensive weight-loss treatment option in the future, he noted.
One size does not fit all
Importantly, weight loss varies widely among individual patients.
A graph of potential weight loss with different treatments (for example, bariatric surgery or liraglutide) versus the percentage of patients that attain the weight losses is roughly bell-shaped, Dr. Kaplan explained. For example, in the STEP1 trial of semaglutide, roughly 7.1% of patients lost less than 5% of their initial weight, 25% of patients lost 20% to 30%, and 10.8% of patients lost 30% or more; that is, patients at the higher end had weight loss comparable to that seen with bariatric surgery
Adding pharmacotherapy after bariatric surgery could be synergistic. For example, in the GRAVITAS study of patients with type 2 diabetes who had gastric bypass surgery, those who received liraglutide after surgery had augmented weight loss compared with those who received placebo.
People at a cocktail party might come up to him and say, “I’d like to lose 5 pounds, 10 pounds,” Dr. Kaplan related in the Q&A session.
“That’s not obesity,” he emphasized. Obesity is excess body fat that poses a risk to health. A person with obesity may have 50 or more excess pounds, and the body is trying to defend this weight.
“If we want to treat obesity more effectively, we have to fully understand why it is a disease and how that disease differs from the cultural desire for thinness,” he reiterated.
“We have to keep the needs and goals of all people living with obesity foremost in our minds, even if many of them have been previously misled by the bias, stigma, blame, and discrimination that surrounds them.”
“We need to re-evaluate what we think we know about obesity and open our minds to new ideas,” he added.
Dr. Kaplan has reported financial ties to Eli Lilly, Gelesis, GI Dynamics, IntelliHealth, Johnson & Johnson, Novo Nordisk, Pfizer, and Rhythm Pharmaceuticals. Dr. Ryan has ties to numerous Novo Nordisk, Pfizer, and several other pharmaceutical companies, including having an ownership interest in Gila Therapeutics, Xeno Biosciences, Epitomee, Calibrate, Roman, and Scientific Intake.
A version of this article first appeared on Medscape.com.
“When considering the challenges of obesity, ask yourself: ‘If it were diabetes, cancer, HIV, or Alzheimer’s, how would you discuss it, approach it, assess it, treat it?’” Lee M. Kaplan, MD, PhD, asked the audience of health care professionals during ObesityWeek®, the annual meeting of The Obesity Society.
“And then do it for obesity, using the full spectrum of tools at our disposal,” he advised.
This was the takeaway that Dr. Kaplan, director of the Obesity, Metabolism, and Nutrition Institute at Massachusetts General Hospital and associate professor, Harvard Medical School, Boston, left the audience with at the end of his lecture entitled, “What does the future of obesity care look like?”
Invited to summarize his main points, Dr. Kaplan told this news organization in an interview that practitioners caring for patients with obesity need to first “recognize that obesity is a disease” caused by dysfunction of the metabolic system that regulates body fat – in the same way immune dysregulation can lead to asthma.
Second, “we are finally developing noninvasive therapies that are more effective,” he noted, referring to the recently approved semaglutide, and even more potent weight-loss therapies that could be on the market within 3 years, so that weight-loss outcomes with pharmacotherapy are approaching those with bariatric surgery.
Third, it is important that patients with obesity get “broad and equitable access” to treatment, and health care practitioners need to be on the same page and have a “shared understanding” of which treatments are appropriate for individual patients, “just as we do for other diseases.”
Need for a shared understanding
“Dr. Kaplan really brought home the idea that we all need a shared understanding of what obesity is – and what it is not,” agreed symposium moderator Donna H. Ryan, MD, in an email.
“He underscored the biologic basis of obesity,” noted Dr. Ryan, professor emerita at Pennington Biomedical Research Center in Baton Rouge, Louisiana, and associate editor-in-chief of Obesity, the official journal of The Obesity Society.
“It is a dysregulation of the body’s weight (especially adipose tissue) regulatory system,” she continued. “The body responds to powerful environmental pressures that produce excess energy balance, and we store that as fat and defend our highest fat mass. This makes obesity a disease, a chronic disease that requires a medical approach to reverse. It’s not a cosmetic problem, it’s a medical problem,” she emphasized.
There is so much misinformation out there about obesity, according to Dr. Ryan.
“People think it’s a lack of willpower, and even patients blame themselves for not being able to lose weight and keep it off. It’s not their fault! It’s biology.”
Although the supplement industry and fad diets falsely promise fast results, there is no magic diet, she continued.
“But we have made progress based on understanding the biologic basis of obesity and have new medications that offer real hope for patients.”
“With 42% of U.S. adults having a BMI that qualifies as obesity, we need a concerted and broad effort to address this problem, and that starts with everybody on the same page as to what obesity is ... a shared understanding of the biologic basis of obesity. It’s time to take obesity seriously,” she summarized, echoing Dr. Kaplan.
A question of biology
“Obesity results from inappropriate pathophysiological regulation of body fat mass,” when the body defends adiposity, Dr. Kaplan explained at the start of his lecture.
The treatment strategy for obesity has always been a stepwise approach starting with lifestyle changes, then pharmacotherapy, then possibly bariatric surgery – each step with a potentially greater chance of weight loss. But now, he explained, medicine is on the verge of having an armamentarium of more potent weight-loss medications.
Compared with phentermine/topiramate, orlistat, naltrexone/bupropion, and liraglutide – which roughly might provide 5% to 10% weight loss, the glucagon-like peptide-1 (GLP-1) agonist semaglutide 2.4 mg/week (Wegovy, Novo Nordisk), approved by the U.S. Food and Drug Association in June, provides almost double this potential weight loss.
And two new agents that could provide “never seen before weight loss” of 25% could potentially enter the marketplace by 2025: the amylin agonist cagrilintide (Novo Nordisk) and the twincretin tirzepatide (Eli Lilly) (a combined glucose-dependent insulinotropic polypeptide [GIP] and GLP-1 receptor agonist).
In addition, when liraglutide comes off patent, a generic version could potentially be introduced, and combined generic liraglutide plus generic phentermine/topiramate could be a less expensive weight-loss treatment option in the future, he noted.
One size does not fit all
Importantly, weight loss varies widely among individual patients.
A graph of potential weight loss with different treatments (for example, bariatric surgery or liraglutide) versus the percentage of patients that attain the weight losses is roughly bell-shaped, Dr. Kaplan explained. For example, in the STEP1 trial of semaglutide, roughly 7.1% of patients lost less than 5% of their initial weight, 25% of patients lost 20% to 30%, and 10.8% of patients lost 30% or more; that is, patients at the higher end had weight loss comparable to that seen with bariatric surgery
Adding pharmacotherapy after bariatric surgery could be synergistic. For example, in the GRAVITAS study of patients with type 2 diabetes who had gastric bypass surgery, those who received liraglutide after surgery had augmented weight loss compared with those who received placebo.
People at a cocktail party might come up to him and say, “I’d like to lose 5 pounds, 10 pounds,” Dr. Kaplan related in the Q&A session.
“That’s not obesity,” he emphasized. Obesity is excess body fat that poses a risk to health. A person with obesity may have 50 or more excess pounds, and the body is trying to defend this weight.
“If we want to treat obesity more effectively, we have to fully understand why it is a disease and how that disease differs from the cultural desire for thinness,” he reiterated.
“We have to keep the needs and goals of all people living with obesity foremost in our minds, even if many of them have been previously misled by the bias, stigma, blame, and discrimination that surrounds them.”
“We need to re-evaluate what we think we know about obesity and open our minds to new ideas,” he added.
Dr. Kaplan has reported financial ties to Eli Lilly, Gelesis, GI Dynamics, IntelliHealth, Johnson & Johnson, Novo Nordisk, Pfizer, and Rhythm Pharmaceuticals. Dr. Ryan has ties to numerous Novo Nordisk, Pfizer, and several other pharmaceutical companies, including having an ownership interest in Gila Therapeutics, Xeno Biosciences, Epitomee, Calibrate, Roman, and Scientific Intake.
A version of this article first appeared on Medscape.com.
FROM OBESITY WEEK 2020
Obesity interventions tied to colon cancer risk reduction
LAS VEGAS – People with obesity may be able to reduce their risk of colorectal cancer with weight loss surgery or medication, researchers say.
“We need to have conversations with our patients in the clinic and educate them that they have these resources available,” said Aakash Desai, MD, a hospitalist at MetroHealth Medical Center, Cleveland, in an interview with this news organization.
Dr. Desai and colleagues found that sleeve gastrectomy and four medications were associated with a reduced risk of colorectal cancer but Roux-en-Y gastrojejunostomy and orlistat were not.
Coauthor Zryan Shwani, MD, a gastroenterology fellow at Sibley Memorial Hospital, Washington, D.C., presented the findings here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
Working with an underserved population with high rates of obesity in northeastern Ohio, the researchers wondered how surgery and medication could affect these patients.
They analyzed data from the IBM Explorys clinical database, which compiles and standardizes data from electronic medical records on about 74 million patients from more than 300 U.S. hospitals. Consistent with previous studies, they determined that patients with obesity in the database were 2.5 times more likely than people with a healthy weight to be diagnosed with colorectal cancer (odds ratio, 2.48; 95% CI, 2.45-2.51).
Zeroing in on people who had weight loss interventions, they included adults aged 18-75 years who had undergone either Roux-en-Y gastrojejunostomy or sleeve gastrectomy, or had taken the medications liraglutide, orlistat, phentermine/topiramate, bupropion/naltrexone, or lorcaserin.
They excluded patients with Lynch syndrome, intestinal polyposis syndrome, a family history of gastrointestinal malignancy, inflammatory bowel disease, or tobacco or alcohol abuse. Patients who had taken one of the weight loss medications and also had type 2 diabetes were excluded. They did not include patients who had undergone gastric banding because it has become less popular.
For the weight loss medication group, they found 117,730 patients who met their criteria. For the surgery group, 43,050 patients met the criteria.
In analyzing the colorectal cancer rates, they included only diagnoses of malignant neoplasms made 2 years after the interventions.
They compared these patients to a control group of 52,540 people matched in age, with a body mass index (BMI) greater than 30 kg/m2 who did not undergo weight loss surgery or take weight loss medication.
Among the 9,370 patients who underwent Roux-en-Y gastrojejunostomy, 50 were diagnosed with colorectal cancer and 400 had benign polyps. Their rate of colorectal cancer was not statistically different from people who didn’t have surgery (OR, 1.09; 95% CI, 0.82-1.43). The rate of benign polyps after Roux-en-Y gastrojejunostomy was greater (OR, 1.72; 95% CI, 1.55-1.90).
On the other hand, among the 33,680 patients who underwent sleeve gastrectomy, 50 were diagnosed with colorectal cancer, a lower rate than in the population who didn’t have surgery (OR, 0.30; 95% CI, 0.22-0.39). Their risk of benign polyps was also reduced (OR, 0.45; 95% CI, 0.40-0.50).
All of the medications were significantly associated with a lower risk of colorectal cancer, except orlistat (OR, 0.94; 95% CI, 0.72-1.25).
The finding on Roux-en-Y gastrojejunostomy agreed with studies from England and Nordic countries showing double the risk of colorectal cancer in those patients but conflicted with a French study showing decreased risk, Dr. Shwani said.
While the study doesn’t establish a reason why Roux-en-Y gastrojejunostomy was less beneficial, other researchers have associated the procedure with biomarkers of inflammation, Dr. Shwani said. “It’s inconsistent, and I don’t think we have a clear answer why.”
As a retrospective analysis, the study could not establish a cause-and-effect relationship between surgery or medication and cancer, or adjust for such factors as diet, exercise, or genes, he acknowledged.
Colorectal cancer is just one outcome to consider when deciding whether to undergo weight loss surgery or take weight loss drugs, said session moderator Mohammad Yaghoobi, MD, an associate professor of medicine at McMaster University, Hamilton, Ont.
“The most important outcome that should be investigated is the survival of the patients after obesity surgery,” he told this news organization. “The second would be the quality of life of those patients. Colon cancer is preventable if you are having regular colonoscopies.”
Other studies have not shown much difference between patients who have weight loss surgery and those who don’t, he added.
The study was funded by Merck. Dr. Desai and Dr. Shwani have reported receiving grant funding from Merck. Dr. Yaghoobi has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAS VEGAS – People with obesity may be able to reduce their risk of colorectal cancer with weight loss surgery or medication, researchers say.
“We need to have conversations with our patients in the clinic and educate them that they have these resources available,” said Aakash Desai, MD, a hospitalist at MetroHealth Medical Center, Cleveland, in an interview with this news organization.
Dr. Desai and colleagues found that sleeve gastrectomy and four medications were associated with a reduced risk of colorectal cancer but Roux-en-Y gastrojejunostomy and orlistat were not.
Coauthor Zryan Shwani, MD, a gastroenterology fellow at Sibley Memorial Hospital, Washington, D.C., presented the findings here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
Working with an underserved population with high rates of obesity in northeastern Ohio, the researchers wondered how surgery and medication could affect these patients.
They analyzed data from the IBM Explorys clinical database, which compiles and standardizes data from electronic medical records on about 74 million patients from more than 300 U.S. hospitals. Consistent with previous studies, they determined that patients with obesity in the database were 2.5 times more likely than people with a healthy weight to be diagnosed with colorectal cancer (odds ratio, 2.48; 95% CI, 2.45-2.51).
Zeroing in on people who had weight loss interventions, they included adults aged 18-75 years who had undergone either Roux-en-Y gastrojejunostomy or sleeve gastrectomy, or had taken the medications liraglutide, orlistat, phentermine/topiramate, bupropion/naltrexone, or lorcaserin.
They excluded patients with Lynch syndrome, intestinal polyposis syndrome, a family history of gastrointestinal malignancy, inflammatory bowel disease, or tobacco or alcohol abuse. Patients who had taken one of the weight loss medications and also had type 2 diabetes were excluded. They did not include patients who had undergone gastric banding because it has become less popular.
For the weight loss medication group, they found 117,730 patients who met their criteria. For the surgery group, 43,050 patients met the criteria.
In analyzing the colorectal cancer rates, they included only diagnoses of malignant neoplasms made 2 years after the interventions.
They compared these patients to a control group of 52,540 people matched in age, with a body mass index (BMI) greater than 30 kg/m2 who did not undergo weight loss surgery or take weight loss medication.
Among the 9,370 patients who underwent Roux-en-Y gastrojejunostomy, 50 were diagnosed with colorectal cancer and 400 had benign polyps. Their rate of colorectal cancer was not statistically different from people who didn’t have surgery (OR, 1.09; 95% CI, 0.82-1.43). The rate of benign polyps after Roux-en-Y gastrojejunostomy was greater (OR, 1.72; 95% CI, 1.55-1.90).
On the other hand, among the 33,680 patients who underwent sleeve gastrectomy, 50 were diagnosed with colorectal cancer, a lower rate than in the population who didn’t have surgery (OR, 0.30; 95% CI, 0.22-0.39). Their risk of benign polyps was also reduced (OR, 0.45; 95% CI, 0.40-0.50).
All of the medications were significantly associated with a lower risk of colorectal cancer, except orlistat (OR, 0.94; 95% CI, 0.72-1.25).
The finding on Roux-en-Y gastrojejunostomy agreed with studies from England and Nordic countries showing double the risk of colorectal cancer in those patients but conflicted with a French study showing decreased risk, Dr. Shwani said.
While the study doesn’t establish a reason why Roux-en-Y gastrojejunostomy was less beneficial, other researchers have associated the procedure with biomarkers of inflammation, Dr. Shwani said. “It’s inconsistent, and I don’t think we have a clear answer why.”
As a retrospective analysis, the study could not establish a cause-and-effect relationship between surgery or medication and cancer, or adjust for such factors as diet, exercise, or genes, he acknowledged.
Colorectal cancer is just one outcome to consider when deciding whether to undergo weight loss surgery or take weight loss drugs, said session moderator Mohammad Yaghoobi, MD, an associate professor of medicine at McMaster University, Hamilton, Ont.
“The most important outcome that should be investigated is the survival of the patients after obesity surgery,” he told this news organization. “The second would be the quality of life of those patients. Colon cancer is preventable if you are having regular colonoscopies.”
Other studies have not shown much difference between patients who have weight loss surgery and those who don’t, he added.
The study was funded by Merck. Dr. Desai and Dr. Shwani have reported receiving grant funding from Merck. Dr. Yaghoobi has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAS VEGAS – People with obesity may be able to reduce their risk of colorectal cancer with weight loss surgery or medication, researchers say.
“We need to have conversations with our patients in the clinic and educate them that they have these resources available,” said Aakash Desai, MD, a hospitalist at MetroHealth Medical Center, Cleveland, in an interview with this news organization.
Dr. Desai and colleagues found that sleeve gastrectomy and four medications were associated with a reduced risk of colorectal cancer but Roux-en-Y gastrojejunostomy and orlistat were not.
Coauthor Zryan Shwani, MD, a gastroenterology fellow at Sibley Memorial Hospital, Washington, D.C., presented the findings here at the American College of Gastroenterology (ACG) 2021 Annual Scientific Meeting.
Working with an underserved population with high rates of obesity in northeastern Ohio, the researchers wondered how surgery and medication could affect these patients.
They analyzed data from the IBM Explorys clinical database, which compiles and standardizes data from electronic medical records on about 74 million patients from more than 300 U.S. hospitals. Consistent with previous studies, they determined that patients with obesity in the database were 2.5 times more likely than people with a healthy weight to be diagnosed with colorectal cancer (odds ratio, 2.48; 95% CI, 2.45-2.51).
Zeroing in on people who had weight loss interventions, they included adults aged 18-75 years who had undergone either Roux-en-Y gastrojejunostomy or sleeve gastrectomy, or had taken the medications liraglutide, orlistat, phentermine/topiramate, bupropion/naltrexone, or lorcaserin.
They excluded patients with Lynch syndrome, intestinal polyposis syndrome, a family history of gastrointestinal malignancy, inflammatory bowel disease, or tobacco or alcohol abuse. Patients who had taken one of the weight loss medications and also had type 2 diabetes were excluded. They did not include patients who had undergone gastric banding because it has become less popular.
For the weight loss medication group, they found 117,730 patients who met their criteria. For the surgery group, 43,050 patients met the criteria.
In analyzing the colorectal cancer rates, they included only diagnoses of malignant neoplasms made 2 years after the interventions.
They compared these patients to a control group of 52,540 people matched in age, with a body mass index (BMI) greater than 30 kg/m2 who did not undergo weight loss surgery or take weight loss medication.
Among the 9,370 patients who underwent Roux-en-Y gastrojejunostomy, 50 were diagnosed with colorectal cancer and 400 had benign polyps. Their rate of colorectal cancer was not statistically different from people who didn’t have surgery (OR, 1.09; 95% CI, 0.82-1.43). The rate of benign polyps after Roux-en-Y gastrojejunostomy was greater (OR, 1.72; 95% CI, 1.55-1.90).
On the other hand, among the 33,680 patients who underwent sleeve gastrectomy, 50 were diagnosed with colorectal cancer, a lower rate than in the population who didn’t have surgery (OR, 0.30; 95% CI, 0.22-0.39). Their risk of benign polyps was also reduced (OR, 0.45; 95% CI, 0.40-0.50).
All of the medications were significantly associated with a lower risk of colorectal cancer, except orlistat (OR, 0.94; 95% CI, 0.72-1.25).
The finding on Roux-en-Y gastrojejunostomy agreed with studies from England and Nordic countries showing double the risk of colorectal cancer in those patients but conflicted with a French study showing decreased risk, Dr. Shwani said.
While the study doesn’t establish a reason why Roux-en-Y gastrojejunostomy was less beneficial, other researchers have associated the procedure with biomarkers of inflammation, Dr. Shwani said. “It’s inconsistent, and I don’t think we have a clear answer why.”
As a retrospective analysis, the study could not establish a cause-and-effect relationship between surgery or medication and cancer, or adjust for such factors as diet, exercise, or genes, he acknowledged.
Colorectal cancer is just one outcome to consider when deciding whether to undergo weight loss surgery or take weight loss drugs, said session moderator Mohammad Yaghoobi, MD, an associate professor of medicine at McMaster University, Hamilton, Ont.
“The most important outcome that should be investigated is the survival of the patients after obesity surgery,” he told this news organization. “The second would be the quality of life of those patients. Colon cancer is preventable if you are having regular colonoscopies.”
Other studies have not shown much difference between patients who have weight loss surgery and those who don’t, he added.
The study was funded by Merck. Dr. Desai and Dr. Shwani have reported receiving grant funding from Merck. Dr. Yaghoobi has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACG 2021
FAVOR III China: QFR-guided PCI shows advantage over angiography
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
Percutaneous coronary intervention (PCI) guided by quantitative flow ratio (QFR) lesion assessment provided better clinical outcomes than visual assessment of the angiogram in the sham-controlled FAVOR III China study.
PCI success rates were about 95% with both strategies; however, QFR guidance was associated with fewer major adverse cardiac events (MACE) at 1 year, use of fewer stents, less contrast medium exposure, and fewer procedural complications.
“The simplicity and safety of QFR compared with wire-based physiologic measurements should facilitate the adoption of physiologic lesion assessment into routine clinical practice,” co–primary investigator Bo Xu, MBBS, Fuwai Hospital, Beijing, said.
The results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2021, held online and in Orlando, and published simultaneously in The Lancet.
Although pressure wire–based physiological assessment with fractional flow reserve (FFR) and instantaneous wave-free ratio (IFR) more accurately identify flow-limiting lesions than standard angiography and have been shown to improve outcomes after PCI, the authors note that it’s underused in practice because of prolonged procedural time, potential pressure wire complications, and side effects from hyperemic agents.
QFR, however, is derived from 3-dimensional coronary artery reconstruction and computational fluid dynamics from the angiogram, so FFR can be estimated without the need for a pressure wire or hyperemic drugs.
FAVOR III China was designed statistically for superiority and enrolled 3,847 patients with stable or unstable angina or a myocardial infarction (MI) at least 72 hours before screening if they had at least one coronary lesion with a diameter stenosis of 50% to 90% and a reference vessel diameter of at least 2.5 mm. The intention-to-treat population included 3,825 patients (mean age, 62.7 years; 29.4% female).
In the QFR group, QFR was measured in all coronary arteries with a lesion but PCI performed only in lesions with a QFR of at least 0.80 or diameter stenosis greater than 90%. Two angiographic imaging runs were taken and the data transmitted to the AngioPlus system (Pulse Medical Imaging Technology) by a local network of sites for QFR calculation.
PCI in the angiography-guided group was performed on the basis of visual angiographic assessment only. A 10-minute delay was used in both groups to preserve masking.
The primary endpoint of 1-year MACE, a composite of all-cause death, MI, or ischemia-driven revascularization, occurred in 5.8% of the QFR-guided group and 8.8% of the angiography-guided group (hazard ratio, 0.65; 95% CI, 0.51-0.83; P = .0004).
The curves separated within 48 hours, driven largely by fewer MIs (3.4% vs. 5.7%; P = .0008) and ischemia-driven revascularizations (2.0% vs. 3.1%; P = .0078) in the QFR-guided group, Mr. Xu said.
The major secondary endpoint of MACE excluding periprocedural MI occurred in 3.1% of QFR-guided patients and 4.8% of angiography-guided patients (HR, 0.64; 95% CI, 0.46-0.89; P = .0073).
The prerandomization revascularization plan was changed in 23.3% of patients with QFR and only 6.2% in the angiography group (P < .0001), mainly due to deferral of treatment of at least one vessel originally planned for PCI (19.6% vs. 5.2%; P < .0001).
“I think in the next guideline they will change the recommendation, not just to include FFR and IFR, but also to include QFR,” Giuseppe Tarantini, MD, PhD, University of Padua, Italy, said during a press briefing on the study.
“This is a milestone in our community, not only because it is easier to use compared to the other lesion-specific indexes like FFR, IFR, but also for the need to expand the use of physiology in the setting of interventional cardiology,” he added.
In an accompanying commentary, Robert A. Byrne, MBBCh, PhD, and Laurna McGovern, MBBCh, both from the Cardiovascular Research Institute Dublin, say the results are “relevant for cardiovascular disease researchers and clinicians and an important step forward for the field of angiography-derived flow measurements for guidance of PCI.”
They point out, however, that the control group did not receive pressure wire–guided PCI, which is the standard of care in contemporary practice and out of step with clinical practice guidelines, thus limiting external validity.
They also note that experiences to date suggest that up to 20% of patients may be unsuitable for the algorithm analysis because of coronary anatomy, presence of overlapping vessels, and insufficient image quality.
Commenting for this news organization, David E. Kandzari, MD, chief of the Piedmont Heart Institute, Atlanta, said “the technology isn’t readily available in catheterization labs today. Could it be assimilated into the cath labs at one point in the near term? I think absolutely, and that would be a welcome addition to expedite the procedure itself.”
Nevertheless, he said the results “need to be externally validated too, with what is the gold standard today of FFR in a larger experience.”
Session moderator Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said FAVOR III China has “advanced our knowledge” but pointed out that the ongoing randomized FAVOR III Europe Japan study is directly comparing QFR with invasive pressure-wire assessed FFR. The estimated primary completion date for that study is Dec. 31.
The study was supported by grants from the Beijing Municipal Science and Technology Commission, Chinese Academy of Medical Sciences, and the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital. Dr. Byrne reported institutional research or educational funding from Abbott Vascular, Biosensors, Biotronik, and Boston Scientific. Ms. McGovern has disclosed no relevant financial relationships. Dr. Kandzari reported minor consulting honoraria from the interventional device industry and institutional research grant support.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV have coronary plaque
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
More than half of people living with HIV and suppressed viral loads nonetheless had imaging-confirmed coronary artery disease – and despite longtime use of HIV drugs that have been associated with cardiovascular trouble, none of those drugs were implicated in disease risk in this study.
“Traditional risk factors and duration of HIV infection were associated with severe coronary artery disease,” said Andreas Knudsen, MD, PhD, an infectious disease provider at Copenhagen University Hospital, Hvidovre, Denmark, during his presentation at the 18th European AIDS Conference. “When we adjusted for time since diagnosis of HIV, none of the drugs remained associated with the severity of coronary artery disease.”
Notably, that included abacavir, which was found in another EACS presentation and in past research to be associated with increased rates of heart attacks. Abacavir is sold individually as a generic as well as a component of Epzicom (abacavir/lamivudine) and the single-drug regimen Triumeq (dolutegravir/abacavir/lamivudine).
The Copenhagen Comorbidity in HIV Infection (COCOMO) study enrolled 1,099 people living with HIV in the Danish capital beginning in 2015, and 705 of them had angiographies via CT available to include in the results. The participants were almost all male (89%), at a healthy weight (BMI of 25), and 96% had undetectable viral loads.
Large minorities of participants also had traditional risk factors for coronary artery disease. More than one in four smoked, one in five had high cholesterol, and 42% had high blood pressure. In addition, many had used drugs that have been associated with cardiovascular trouble, including abacavir, which 26% of participants had used; indinavir, used by 17% of participants; zidovudine/AZT, used by 47%; and didanosine, which 14% used. (While abacavir is still in use, the other three drugs are considered legacy drugs and are not in current use.)
In addition, nearly one in three (29%) were currently using a protease inhibitor, which has been associated with heart failure.
When the investigators looked at participants’ CTs, they found that, by the Coronary Artery Disease-Reporting and Data Systems (CAD-RAMS) scoring system, close to half (46%) had clear arteries with no signs of coronary artery disease. But that also meant that 54% had some blockage or stiffening of the arteries. The good news is that 27% of those people had minimal or mild coronary artery disease.
But a full 17% had confirmed obstructive coronary artery disease, and another 1 in 10 participants had the highest level of blockages. When they broke the data down by traditional and HIV medication–related risk factors for coronary artery disease, they found something interesting. Although obesity was associated with the presence of atherosclerosis, it wasn’t associated with severe disease. But diabetes was the reverse of that: It wasn’t associated with the presence of the disease, but it was associated with more severe disease.
And when they looked at abacavir, they found no relationship between the drug and atherosclerosis. “Abacavir was not associated with the presence of atherosclerosis and was also not associated with severity of disease,” said Dr. Knudsen.
Although past use of AZT, indinavir, and didanosine were associated with severity of atherosclerosis, that association went away when Dr. Knudsen and team adjusted the findings for time since diagnosis. What was associated atherosclerosis was length of time living with HIV itself. For every 5 years a person lived with HIV, the study found the risk of having any atherosclerosis increased 20% and severity increased 23%. In addition, being a man was associated with a nearly 2.5-times increased risk of having any atherosclerosis and a 96% increased chance of having more severe atherosclerosis. Having diabetes was associated with a nearly threefold increased risk of atherosclerosis, as was every additional decade of life for a person who was living with HIV.
The findings confirm the baseline data of the REPRIEVE trial, which recently released data showing similarly high rates of atherosclerotic plaque in people living with HIV who didn’t register as “at risk” for cardiovascular disease using traditional scoring methods.
“It’s important in that it’s a huge study that’s confirmatory [of] what we know, which is that there are high levels of subclinical coronary artery disease in people living with HIV,” said Steven Grinspoon, MD, professor at Harvard Medical School in Boston, Massachusetts, and principal investigator of REPRIEVE.
As for the lack of association between abacavir and cardiovascular risk, he said he’s taking the findings with a grain of salt.
“It’s hard to make a lot out of that,” he said. “It’s hard to know in a cross-sectional study. People put people on different things.”
In Spain, where Jose Ignacio Bernardino, MD, treats people living with HIV at La Paz University Hospital in Madrid, abacavir is mostly a moot point, as clinicians have long since moved away from maintaining people living with HIV on any abacavir-containing regimens. What’s more important in the study, he told this news organization, is that “worrisome” high level of risk. REPRIEVE will test whether statins can reduce heart disease events in people living with HIV. But in the meantime, he said the take-away for clinicians from the study is the primary importance of traditional cardiovascular risk factors.
“We have to acknowledge that the major cardiovascular risk factor is age,” he said. “When patients are approaching their 50s, I usually try to stress a lot about cardiovascular risk factors in general. I stress healthy lifestyle – get physical exercise, hypertension, glucose, lipids – in every single patient.”
Dr. Knudsen and Dr. Bernardino have disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
SBRT on oligoprogressive lesions: Benefit in lung cancer
Patients treated with SBRT had a median PFS of 44 weeks, compared with 9 weeks for those who received standard care.
However, no benefit was observed in patients with metastatic breast cancer. There was no significant difference in PFS between the two groups (18 weeks with SBRT vs. 19 weeks with standard care).
“In this preplanned interim analysis, we demonstrated the benefit of SBRT to sites of oligoprogression on overall progression-free survival, which was the primary endpoint,” said lead author C. Jillian Tsai, MD, PhD, a radiation oncologist and director of metastatic disease radiation oncology research at Memorial Sloan Kettering Cancer Center in New York. “The difference was driven by the substantial response in [this] NSCLC cohort.”
There was no benefit of SBRT seen in the breast cohort, she noted, and most breast patients developed new lesions upon further progression.
Dr. Tsai and colleagues are planning to close the trial early, after the interim analysis established the benefit of SBRT. They are now investigating why SBRT was beneficial in NSCLC but not in breast cancer.
The findings were presented at the American Society for Radiation Oncology (ASTRO) annual meeting.
Dr. Tsai explained that the current standard of care for patients with oligoprogressive metastatic NSCLC is to switch to a different targeted therapy or chemotherapy following progression, but options may be limited. Efficacy for second-line therapy can be poor, with PFS ranging from about 4 months to 10 months for NSCLC, “and after second line, efficacy for third and fourth lines is even poorer,” she said.
Similarly, for breast cancer, PFS ranges from about 9 months to 20 months for estrogen-receptor positive patients. “But for triple negative patients, there really is no standard of care and PFS is poor,” Dr. Tsai said.
SBRT superior to standard of care
The authors hypothesized that there is an oligoprogressive state in metastatic cancer, in which disease control can be improved by applying local therapy to progressive lesions only.
The cohort included 102 patients with metastatic NSCLC or breast cancer who had received one or more lines of systemic therapy and had oligoprogressive lesions amenable to SBRT. There was no upper limit of nonprogressive lesions.
Oligoprogression was defined as Response Evaluation or Positron Emission Tomography Response Criteria in Solid Tumors documented progression ≤5 individual lesions.
Patients were randomly assigned to receive either SBRT to all progressive sites plus palliative standard of care or systemic SOC only. Systemic therapy was per physician’s discretion.
There were 58 patients with NSCLC (30 in the SBRT group) and 44 patients with breast cancer (22 in each group).
Most patients (75%) had more than one site of oligoprogression and 47% had more than 5 total metastatic lesions. About half of patients (54%) had received immunotherapy and the majority of those with NSCLC (86%) did not harbor an actionable driver mutation. About one-third (32%) of the breast cancer cohort were triple negative.
Patients were followed for a median of 45 weeks (58 weeks for living patients), by which time 78 (74%) had experienced further tumor progression and 39 (37%) had died.
Median progression-free survival for the entire cohort was 31 weeks for SBRT and 11 weeks for palliative SOC (P = .002).
In multivariable analysis that stratified for factors including age, sex, lines of systemic therapy, and change of systemic therapy, the progression-free survival benefit of SBRT continued to remain substantial in the NSCLC cohort (hazard ratio: 0.38; P = .007).
Adverse events were higher in the SBRT group. Grade 2 or higher adverse events occurred in 23 (61%) of SBRT patients, and 15 (40%) of SOC patients (P = .13).
Hoped-for results, with a few caveats
Approached for comment on the new findings, Clifford Robinson, MD, professor of radiation oncology, chief of SBRT service, and director of clinical trials and informatics at Washington University, St. Louis, said the results tie in with previous findings.
There are multiple published or presented prospective randomized phase 2 and 3 trials in various disease sites that have explored the role of local therapy, including SBRT, for patients who present with oligometastatic disease.
“These studies have nearly uniformly shown improvements in progression-free and/or overall survival with the inclusion of local therapy,” he told this news organization. Dr. Robinson was not involved with the study.
He explained that relatively few patients present with oligometastatic disease. However, many patients present with more advanced disease, but after an initial course of systemic therapy, develop oligoprogression.
“There is tremendous appeal to using local therapy at the time of oligoprogression in lieu of switching systemic therapy,” said Dr. Robinson. “It allows patients to stay on systemic therapy that is otherwise effective for the remainder of their disease.”
First-line systemic therapies are the most effective and the most tolerable, he continued, and switching systemic therapy introduces the potential for more toxicity and less efficacy. Therefore, it has become increasingly popular to offer SBRT to one or a few sites of oligoprogressive disease based on the results of oligometastatic disease.
“However, there is no established prospective data to guide this practice,” he said. “This trial is the first to examine this carefully in lung and breast cancer patients, and this trial shows what we hoped to see – that use of SBRT after oligoprogression results in improved progression-free survival as compared with standard of care alone. And this was accomplished with limited toxicity.”
There are a few caveats, though, he pointed out. “Progression-free survival is defined as time to first progression or death,” he said. “Since we don’t know what the overall survival is in this abstract, it’s entirely possible that patients live for the same length of time, but just take longer to progress.”
Another caveat is that this was a planned interim analysis. “Typically, planned interim analyses occur to see if the trial should be stopped or to adjust the study based on results,” he said. “It’s unclear what the investigators will do with this information.”
“But overall, these are very exciting data and lend support to the increasingly common practice of treating oligoprogressive disease,” Dr. Robinson added. “Since most of the serious adverse events of SBRT occur later, longer follow-up is needed, although the median survival of patients may not reach that timepoint.”
“For now, practice should not be altered based on these interim results,” he added.
Dr. Tsai reported acting as a consultant/advisor for Varian and Galera and also receiving research funding from Varian. Dr. Robinson reports stock/ownership in Radialogica, acting as a consultant/advisor for Varian, AstraZeneca, EMD Serono, Quantitative Radiology Solutions, research funding from Varian and Merck, and owning patents on systems for cardiac arrhythmias and ablation.
A version of this article first appeared on Medscape.com.
Patients treated with SBRT had a median PFS of 44 weeks, compared with 9 weeks for those who received standard care.
However, no benefit was observed in patients with metastatic breast cancer. There was no significant difference in PFS between the two groups (18 weeks with SBRT vs. 19 weeks with standard care).
“In this preplanned interim analysis, we demonstrated the benefit of SBRT to sites of oligoprogression on overall progression-free survival, which was the primary endpoint,” said lead author C. Jillian Tsai, MD, PhD, a radiation oncologist and director of metastatic disease radiation oncology research at Memorial Sloan Kettering Cancer Center in New York. “The difference was driven by the substantial response in [this] NSCLC cohort.”
There was no benefit of SBRT seen in the breast cohort, she noted, and most breast patients developed new lesions upon further progression.
Dr. Tsai and colleagues are planning to close the trial early, after the interim analysis established the benefit of SBRT. They are now investigating why SBRT was beneficial in NSCLC but not in breast cancer.
The findings were presented at the American Society for Radiation Oncology (ASTRO) annual meeting.
Dr. Tsai explained that the current standard of care for patients with oligoprogressive metastatic NSCLC is to switch to a different targeted therapy or chemotherapy following progression, but options may be limited. Efficacy for second-line therapy can be poor, with PFS ranging from about 4 months to 10 months for NSCLC, “and after second line, efficacy for third and fourth lines is even poorer,” she said.
Similarly, for breast cancer, PFS ranges from about 9 months to 20 months for estrogen-receptor positive patients. “But for triple negative patients, there really is no standard of care and PFS is poor,” Dr. Tsai said.
SBRT superior to standard of care
The authors hypothesized that there is an oligoprogressive state in metastatic cancer, in which disease control can be improved by applying local therapy to progressive lesions only.
The cohort included 102 patients with metastatic NSCLC or breast cancer who had received one or more lines of systemic therapy and had oligoprogressive lesions amenable to SBRT. There was no upper limit of nonprogressive lesions.
Oligoprogression was defined as Response Evaluation or Positron Emission Tomography Response Criteria in Solid Tumors documented progression ≤5 individual lesions.
Patients were randomly assigned to receive either SBRT to all progressive sites plus palliative standard of care or systemic SOC only. Systemic therapy was per physician’s discretion.
There were 58 patients with NSCLC (30 in the SBRT group) and 44 patients with breast cancer (22 in each group).
Most patients (75%) had more than one site of oligoprogression and 47% had more than 5 total metastatic lesions. About half of patients (54%) had received immunotherapy and the majority of those with NSCLC (86%) did not harbor an actionable driver mutation. About one-third (32%) of the breast cancer cohort were triple negative.
Patients were followed for a median of 45 weeks (58 weeks for living patients), by which time 78 (74%) had experienced further tumor progression and 39 (37%) had died.
Median progression-free survival for the entire cohort was 31 weeks for SBRT and 11 weeks for palliative SOC (P = .002).
In multivariable analysis that stratified for factors including age, sex, lines of systemic therapy, and change of systemic therapy, the progression-free survival benefit of SBRT continued to remain substantial in the NSCLC cohort (hazard ratio: 0.38; P = .007).
Adverse events were higher in the SBRT group. Grade 2 or higher adverse events occurred in 23 (61%) of SBRT patients, and 15 (40%) of SOC patients (P = .13).
Hoped-for results, with a few caveats
Approached for comment on the new findings, Clifford Robinson, MD, professor of radiation oncology, chief of SBRT service, and director of clinical trials and informatics at Washington University, St. Louis, said the results tie in with previous findings.
There are multiple published or presented prospective randomized phase 2 and 3 trials in various disease sites that have explored the role of local therapy, including SBRT, for patients who present with oligometastatic disease.
“These studies have nearly uniformly shown improvements in progression-free and/or overall survival with the inclusion of local therapy,” he told this news organization. Dr. Robinson was not involved with the study.
He explained that relatively few patients present with oligometastatic disease. However, many patients present with more advanced disease, but after an initial course of systemic therapy, develop oligoprogression.
“There is tremendous appeal to using local therapy at the time of oligoprogression in lieu of switching systemic therapy,” said Dr. Robinson. “It allows patients to stay on systemic therapy that is otherwise effective for the remainder of their disease.”
First-line systemic therapies are the most effective and the most tolerable, he continued, and switching systemic therapy introduces the potential for more toxicity and less efficacy. Therefore, it has become increasingly popular to offer SBRT to one or a few sites of oligoprogressive disease based on the results of oligometastatic disease.
“However, there is no established prospective data to guide this practice,” he said. “This trial is the first to examine this carefully in lung and breast cancer patients, and this trial shows what we hoped to see – that use of SBRT after oligoprogression results in improved progression-free survival as compared with standard of care alone. And this was accomplished with limited toxicity.”
There are a few caveats, though, he pointed out. “Progression-free survival is defined as time to first progression or death,” he said. “Since we don’t know what the overall survival is in this abstract, it’s entirely possible that patients live for the same length of time, but just take longer to progress.”
Another caveat is that this was a planned interim analysis. “Typically, planned interim analyses occur to see if the trial should be stopped or to adjust the study based on results,” he said. “It’s unclear what the investigators will do with this information.”
“But overall, these are very exciting data and lend support to the increasingly common practice of treating oligoprogressive disease,” Dr. Robinson added. “Since most of the serious adverse events of SBRT occur later, longer follow-up is needed, although the median survival of patients may not reach that timepoint.”
“For now, practice should not be altered based on these interim results,” he added.
Dr. Tsai reported acting as a consultant/advisor for Varian and Galera and also receiving research funding from Varian. Dr. Robinson reports stock/ownership in Radialogica, acting as a consultant/advisor for Varian, AstraZeneca, EMD Serono, Quantitative Radiology Solutions, research funding from Varian and Merck, and owning patents on systems for cardiac arrhythmias and ablation.
A version of this article first appeared on Medscape.com.
Patients treated with SBRT had a median PFS of 44 weeks, compared with 9 weeks for those who received standard care.
However, no benefit was observed in patients with metastatic breast cancer. There was no significant difference in PFS between the two groups (18 weeks with SBRT vs. 19 weeks with standard care).
“In this preplanned interim analysis, we demonstrated the benefit of SBRT to sites of oligoprogression on overall progression-free survival, which was the primary endpoint,” said lead author C. Jillian Tsai, MD, PhD, a radiation oncologist and director of metastatic disease radiation oncology research at Memorial Sloan Kettering Cancer Center in New York. “The difference was driven by the substantial response in [this] NSCLC cohort.”
There was no benefit of SBRT seen in the breast cohort, she noted, and most breast patients developed new lesions upon further progression.
Dr. Tsai and colleagues are planning to close the trial early, after the interim analysis established the benefit of SBRT. They are now investigating why SBRT was beneficial in NSCLC but not in breast cancer.
The findings were presented at the American Society for Radiation Oncology (ASTRO) annual meeting.
Dr. Tsai explained that the current standard of care for patients with oligoprogressive metastatic NSCLC is to switch to a different targeted therapy or chemotherapy following progression, but options may be limited. Efficacy for second-line therapy can be poor, with PFS ranging from about 4 months to 10 months for NSCLC, “and after second line, efficacy for third and fourth lines is even poorer,” she said.
Similarly, for breast cancer, PFS ranges from about 9 months to 20 months for estrogen-receptor positive patients. “But for triple negative patients, there really is no standard of care and PFS is poor,” Dr. Tsai said.
SBRT superior to standard of care
The authors hypothesized that there is an oligoprogressive state in metastatic cancer, in which disease control can be improved by applying local therapy to progressive lesions only.
The cohort included 102 patients with metastatic NSCLC or breast cancer who had received one or more lines of systemic therapy and had oligoprogressive lesions amenable to SBRT. There was no upper limit of nonprogressive lesions.
Oligoprogression was defined as Response Evaluation or Positron Emission Tomography Response Criteria in Solid Tumors documented progression ≤5 individual lesions.
Patients were randomly assigned to receive either SBRT to all progressive sites plus palliative standard of care or systemic SOC only. Systemic therapy was per physician’s discretion.
There were 58 patients with NSCLC (30 in the SBRT group) and 44 patients with breast cancer (22 in each group).
Most patients (75%) had more than one site of oligoprogression and 47% had more than 5 total metastatic lesions. About half of patients (54%) had received immunotherapy and the majority of those with NSCLC (86%) did not harbor an actionable driver mutation. About one-third (32%) of the breast cancer cohort were triple negative.
Patients were followed for a median of 45 weeks (58 weeks for living patients), by which time 78 (74%) had experienced further tumor progression and 39 (37%) had died.
Median progression-free survival for the entire cohort was 31 weeks for SBRT and 11 weeks for palliative SOC (P = .002).
In multivariable analysis that stratified for factors including age, sex, lines of systemic therapy, and change of systemic therapy, the progression-free survival benefit of SBRT continued to remain substantial in the NSCLC cohort (hazard ratio: 0.38; P = .007).
Adverse events were higher in the SBRT group. Grade 2 or higher adverse events occurred in 23 (61%) of SBRT patients, and 15 (40%) of SOC patients (P = .13).
Hoped-for results, with a few caveats
Approached for comment on the new findings, Clifford Robinson, MD, professor of radiation oncology, chief of SBRT service, and director of clinical trials and informatics at Washington University, St. Louis, said the results tie in with previous findings.
There are multiple published or presented prospective randomized phase 2 and 3 trials in various disease sites that have explored the role of local therapy, including SBRT, for patients who present with oligometastatic disease.
“These studies have nearly uniformly shown improvements in progression-free and/or overall survival with the inclusion of local therapy,” he told this news organization. Dr. Robinson was not involved with the study.
He explained that relatively few patients present with oligometastatic disease. However, many patients present with more advanced disease, but after an initial course of systemic therapy, develop oligoprogression.
“There is tremendous appeal to using local therapy at the time of oligoprogression in lieu of switching systemic therapy,” said Dr. Robinson. “It allows patients to stay on systemic therapy that is otherwise effective for the remainder of their disease.”
First-line systemic therapies are the most effective and the most tolerable, he continued, and switching systemic therapy introduces the potential for more toxicity and less efficacy. Therefore, it has become increasingly popular to offer SBRT to one or a few sites of oligoprogressive disease based on the results of oligometastatic disease.
“However, there is no established prospective data to guide this practice,” he said. “This trial is the first to examine this carefully in lung and breast cancer patients, and this trial shows what we hoped to see – that use of SBRT after oligoprogression results in improved progression-free survival as compared with standard of care alone. And this was accomplished with limited toxicity.”
There are a few caveats, though, he pointed out. “Progression-free survival is defined as time to first progression or death,” he said. “Since we don’t know what the overall survival is in this abstract, it’s entirely possible that patients live for the same length of time, but just take longer to progress.”
Another caveat is that this was a planned interim analysis. “Typically, planned interim analyses occur to see if the trial should be stopped or to adjust the study based on results,” he said. “It’s unclear what the investigators will do with this information.”
“But overall, these are very exciting data and lend support to the increasingly common practice of treating oligoprogressive disease,” Dr. Robinson added. “Since most of the serious adverse events of SBRT occur later, longer follow-up is needed, although the median survival of patients may not reach that timepoint.”
“For now, practice should not be altered based on these interim results,” he added.
Dr. Tsai reported acting as a consultant/advisor for Varian and Galera and also receiving research funding from Varian. Dr. Robinson reports stock/ownership in Radialogica, acting as a consultant/advisor for Varian, AstraZeneca, EMD Serono, Quantitative Radiology Solutions, research funding from Varian and Merck, and owning patents on systems for cardiac arrhythmias and ablation.
A version of this article first appeared on Medscape.com.
NCI mammography trial mostly a ‘waste,’ says expert
Funding for this trial is largely misspent money, it may produce misleading results, and it should be abandoned, he says.
Dr. Kopans has been an outspoken critic of the trial, describing it as a “huge waste of money” in comments made last year. Now he has set out his criticisms of the trial in an essay published in the October issue of Clinical Imaging, which outlines his objections and concerns for the first time in a peer-reviewed journal.
The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) is comparing digital breast tomosynthesis (DBT), also known as 3-D mammography, with the older 2-D technology or full-field digital mammography (FFDM).
Dr. Kopans coined the term DBT and formerly held a now-expired patent on the first version of this technology.
“It could be argued that the imaging part of TMIST is a waste of valuable resources,” he writes in the essay.
The “imaging part” of the trial refers to the primary outcome measure and driving purpose of the trial, which is designed to learn which technology is better at finding – and reducing the rate of – potentially lethal “advanced” cancers.
These cancers include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease. These malignancies correlate with breast cancer mortality, TMIST’s principal investigator Etta Pisano, MD, of the American College of Radiology, has said in the past.
However, Dr. Kopans says that this surrogate endpoint is problematic. “TMIST will only investigate whether or not digital breast tomography results in a decline in advanced cancers, ignoring the fact that many women still die from cancers that are not advanced at the time of diagnosis,” he writes.
“Clearly reducing the rate of advanced cancers is not the only way that early detection saves lives. Lives are also saved by finding cancers at a smaller size within stages,” Dr. Kopans writes. He adds that DBT has been proven in observational cohort studies to find more smaller breast cancers than FFDM.
Dr. Kopans’ opinion that TMIST is largely a waste of resources is not shared by the National Cancer Institute. “We feel strongly that TMIST is a critical study,” an NCI spokesperson told this news organization.
Study power concerns
Another concern is that TMIST “may be underpowered,” Dr. Kopans writes. That concern arises in part from a recent review of TMIST by an advisory committee (that was prompted by low patient accrual rates), which proposed reducing the size of the trial. Dr. Kopans says this would result in “a reduction of the planned power of the trial.”
The NCI says that reducing the study size has been discussed but has not yet been implemented. “Any reduction in size would, of course, have appropriate statistical considerations in mind,” according to the NCI spokesperson.
Dr. Kopans’ concern about statistical power extends beyond downsizing the trial. An advanced cancer in TMIST is counted “if it occurs at any time while the participant is on study,” according to the NCI. Dr. Kopans says that is a problem.
“Since DBT cannot have any effect on advanced cancers in the prevalence year (they are already there), data from the first year (prevalence cancers are likely the largest number) will be unusable, and if used will, inappropriately, dilute the results,” he writes.
Dr. Kopans hopes that the investigators address the statistical power issues with the trial because, if not, “its results may be grossly misleading.”
American radiology practice
Dr. Kopans praises one aspect of TMIST – the trial’s effort to create a repository of blood and oral swab specimens, along with participant genetic data. The goal, say TMIST investigators, is to individualize or optimize screening strategies by tying molecular data to clinical outcomes in the trial.
However, apart from that one aspect, Dr. Kopans is highly critical of the trial.
It is now too late to compare the two technologies, he suggests, as DBT is already replacing FFDM for breast cancer screening in the U.S.
He notes that 76% of mammography facilities in the United States have 3-D devices (as of April 2021). That percentage has climbed steadily in recent years. “By the time the TMIST study is completed, DBT will, almost certainly, have become the ‘standard of care,’” he asserts, echoing others who have commented on the trial, including some participating physicians.
The money being spent on TMIST “should not be used for looking backwards,” says Dr. Kopans.
The NCI responded to that criticism. “TMIST is looking to clarify the best screening for women based on the science and is not solely about access. We are seeking to determine which technology is better and [are] providing access to the trial across the country in diverse practices and populations,” the NCI said in an email.
In his essay, Dr. Kopans says it is time to stop TMIST and put the money into other pressing breast cancer issues and questions. “... it makes no sense to continue this flawed trial whose results will be obsolete by the time they become available,” he writes.
Dr. Kopans reports consulting with DART Imaging in China, which is developing a digital breast tomosynthesis machine.
A version of this article first appeared on Medscape.com.
Funding for this trial is largely misspent money, it may produce misleading results, and it should be abandoned, he says.
Dr. Kopans has been an outspoken critic of the trial, describing it as a “huge waste of money” in comments made last year. Now he has set out his criticisms of the trial in an essay published in the October issue of Clinical Imaging, which outlines his objections and concerns for the first time in a peer-reviewed journal.
The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) is comparing digital breast tomosynthesis (DBT), also known as 3-D mammography, with the older 2-D technology or full-field digital mammography (FFDM).
Dr. Kopans coined the term DBT and formerly held a now-expired patent on the first version of this technology.
“It could be argued that the imaging part of TMIST is a waste of valuable resources,” he writes in the essay.
The “imaging part” of the trial refers to the primary outcome measure and driving purpose of the trial, which is designed to learn which technology is better at finding – and reducing the rate of – potentially lethal “advanced” cancers.
These cancers include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease. These malignancies correlate with breast cancer mortality, TMIST’s principal investigator Etta Pisano, MD, of the American College of Radiology, has said in the past.
However, Dr. Kopans says that this surrogate endpoint is problematic. “TMIST will only investigate whether or not digital breast tomography results in a decline in advanced cancers, ignoring the fact that many women still die from cancers that are not advanced at the time of diagnosis,” he writes.
“Clearly reducing the rate of advanced cancers is not the only way that early detection saves lives. Lives are also saved by finding cancers at a smaller size within stages,” Dr. Kopans writes. He adds that DBT has been proven in observational cohort studies to find more smaller breast cancers than FFDM.
Dr. Kopans’ opinion that TMIST is largely a waste of resources is not shared by the National Cancer Institute. “We feel strongly that TMIST is a critical study,” an NCI spokesperson told this news organization.
Study power concerns
Another concern is that TMIST “may be underpowered,” Dr. Kopans writes. That concern arises in part from a recent review of TMIST by an advisory committee (that was prompted by low patient accrual rates), which proposed reducing the size of the trial. Dr. Kopans says this would result in “a reduction of the planned power of the trial.”
The NCI says that reducing the study size has been discussed but has not yet been implemented. “Any reduction in size would, of course, have appropriate statistical considerations in mind,” according to the NCI spokesperson.
Dr. Kopans’ concern about statistical power extends beyond downsizing the trial. An advanced cancer in TMIST is counted “if it occurs at any time while the participant is on study,” according to the NCI. Dr. Kopans says that is a problem.
“Since DBT cannot have any effect on advanced cancers in the prevalence year (they are already there), data from the first year (prevalence cancers are likely the largest number) will be unusable, and if used will, inappropriately, dilute the results,” he writes.
Dr. Kopans hopes that the investigators address the statistical power issues with the trial because, if not, “its results may be grossly misleading.”
American radiology practice
Dr. Kopans praises one aspect of TMIST – the trial’s effort to create a repository of blood and oral swab specimens, along with participant genetic data. The goal, say TMIST investigators, is to individualize or optimize screening strategies by tying molecular data to clinical outcomes in the trial.
However, apart from that one aspect, Dr. Kopans is highly critical of the trial.
It is now too late to compare the two technologies, he suggests, as DBT is already replacing FFDM for breast cancer screening in the U.S.
He notes that 76% of mammography facilities in the United States have 3-D devices (as of April 2021). That percentage has climbed steadily in recent years. “By the time the TMIST study is completed, DBT will, almost certainly, have become the ‘standard of care,’” he asserts, echoing others who have commented on the trial, including some participating physicians.
The money being spent on TMIST “should not be used for looking backwards,” says Dr. Kopans.
The NCI responded to that criticism. “TMIST is looking to clarify the best screening for women based on the science and is not solely about access. We are seeking to determine which technology is better and [are] providing access to the trial across the country in diverse practices and populations,” the NCI said in an email.
In his essay, Dr. Kopans says it is time to stop TMIST and put the money into other pressing breast cancer issues and questions. “... it makes no sense to continue this flawed trial whose results will be obsolete by the time they become available,” he writes.
Dr. Kopans reports consulting with DART Imaging in China, which is developing a digital breast tomosynthesis machine.
A version of this article first appeared on Medscape.com.
Funding for this trial is largely misspent money, it may produce misleading results, and it should be abandoned, he says.
Dr. Kopans has been an outspoken critic of the trial, describing it as a “huge waste of money” in comments made last year. Now he has set out his criticisms of the trial in an essay published in the October issue of Clinical Imaging, which outlines his objections and concerns for the first time in a peer-reviewed journal.
The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) is comparing digital breast tomosynthesis (DBT), also known as 3-D mammography, with the older 2-D technology or full-field digital mammography (FFDM).
Dr. Kopans coined the term DBT and formerly held a now-expired patent on the first version of this technology.
“It could be argued that the imaging part of TMIST is a waste of valuable resources,” he writes in the essay.
The “imaging part” of the trial refers to the primary outcome measure and driving purpose of the trial, which is designed to learn which technology is better at finding – and reducing the rate of – potentially lethal “advanced” cancers.
These cancers include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease. These malignancies correlate with breast cancer mortality, TMIST’s principal investigator Etta Pisano, MD, of the American College of Radiology, has said in the past.
However, Dr. Kopans says that this surrogate endpoint is problematic. “TMIST will only investigate whether or not digital breast tomography results in a decline in advanced cancers, ignoring the fact that many women still die from cancers that are not advanced at the time of diagnosis,” he writes.
“Clearly reducing the rate of advanced cancers is not the only way that early detection saves lives. Lives are also saved by finding cancers at a smaller size within stages,” Dr. Kopans writes. He adds that DBT has been proven in observational cohort studies to find more smaller breast cancers than FFDM.
Dr. Kopans’ opinion that TMIST is largely a waste of resources is not shared by the National Cancer Institute. “We feel strongly that TMIST is a critical study,” an NCI spokesperson told this news organization.
Study power concerns
Another concern is that TMIST “may be underpowered,” Dr. Kopans writes. That concern arises in part from a recent review of TMIST by an advisory committee (that was prompted by low patient accrual rates), which proposed reducing the size of the trial. Dr. Kopans says this would result in “a reduction of the planned power of the trial.”
The NCI says that reducing the study size has been discussed but has not yet been implemented. “Any reduction in size would, of course, have appropriate statistical considerations in mind,” according to the NCI spokesperson.
Dr. Kopans’ concern about statistical power extends beyond downsizing the trial. An advanced cancer in TMIST is counted “if it occurs at any time while the participant is on study,” according to the NCI. Dr. Kopans says that is a problem.
“Since DBT cannot have any effect on advanced cancers in the prevalence year (they are already there), data from the first year (prevalence cancers are likely the largest number) will be unusable, and if used will, inappropriately, dilute the results,” he writes.
Dr. Kopans hopes that the investigators address the statistical power issues with the trial because, if not, “its results may be grossly misleading.”
American radiology practice
Dr. Kopans praises one aspect of TMIST – the trial’s effort to create a repository of blood and oral swab specimens, along with participant genetic data. The goal, say TMIST investigators, is to individualize or optimize screening strategies by tying molecular data to clinical outcomes in the trial.
However, apart from that one aspect, Dr. Kopans is highly critical of the trial.
It is now too late to compare the two technologies, he suggests, as DBT is already replacing FFDM for breast cancer screening in the U.S.
He notes that 76% of mammography facilities in the United States have 3-D devices (as of April 2021). That percentage has climbed steadily in recent years. “By the time the TMIST study is completed, DBT will, almost certainly, have become the ‘standard of care,’” he asserts, echoing others who have commented on the trial, including some participating physicians.
The money being spent on TMIST “should not be used for looking backwards,” says Dr. Kopans.
The NCI responded to that criticism. “TMIST is looking to clarify the best screening for women based on the science and is not solely about access. We are seeking to determine which technology is better and [are] providing access to the trial across the country in diverse practices and populations,” the NCI said in an email.
In his essay, Dr. Kopans says it is time to stop TMIST and put the money into other pressing breast cancer issues and questions. “... it makes no sense to continue this flawed trial whose results will be obsolete by the time they become available,” he writes.
Dr. Kopans reports consulting with DART Imaging in China, which is developing a digital breast tomosynthesis machine.
A version of this article first appeared on Medscape.com.
Rituximab improves systemic sclerosis skin, lung symptoms
Rituximab effectively reduced skin sclerosis and appeared to have a beneficial effect on interstitial lung disease (ILD) for patients with systemic sclerosis (SSc) in a randomized, clinical trial.
At 24 weeks’ follow-up, there was significant improvement in total skin thickness scores among patients who received four once-weekly rituximab infusions, compared with patients who received placebo infusions. Among patients who received rituximab, there were also small but significant improvements in percentage of forced vital capacity (FVC). Among patients who received placebo, FVC worsened, reported Ayumi Yoshizaki, MD, of the University of Tokyo and colleagues.
“Systemic sclerosis is considered to have high unmet medical needs because of its poor prognosis and the lack of satisfactory and effective treatments,” he said at the virtual annual meeting of the American College of Rheumatology.
“Several clinical studies have suggested that B-cell depletion therapy with rituximab anti-CD20 antibody is effective in treating skin and lung fibrosis of SSc. However, no randomized, placebo-controlled trial has been able to confirm the efficacy of rituximab in SSc,” Dr. Yoshizaki said.
A rheumatologist who is currently conducting an investigator-initiated trial in which patients with SSC are undergoing treatment with rituximab followed by belimumab (Benlysta) said in an interview that he found the data to be “super interesting.”
“There are a lot of reasons to think that B cells might be important in systemic sclerosis, and actually that’s why our group had previously done an investigator-initiated trial with belimumab years ago,” said Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York.
Randomized trial
Dr. Yoshizaki and colleagues conducted the randomized, placebo-controlled DESIRES trial in four hospitals in Japan to evaluate the safety and efficacy of rituximab for the treatment of SSc.
In the investigator-initiated trial, patients aged 20-79 years who fulfilled ACR and European Alliance of Associations for Rheumatology classification criteria for systemic sclerosis and who had a modified Rodnan Skin Score (mRSS) of 10 or more and a life expectancy of at least 6 months were randomly assigned to receive infusions with either rituximab 375 mg/m2 or placebo once weekly for 4 weeks. Patients and clinicians were masked to treatment allocation.
The trial included 56 patients (51 women, 5 men). Of all patients enrolled, 27 of 28 who were allocated to receive rituximab and 22 of 28 who were allocated to receive placebo underwent at least one infusion and completed 24 weeks of follow-up.
The absolute change in mRSS at 24 weeks after the start of therapy, the primary endpoint, was –6.30 in the rituximab group, compared with +2.14 in the placebo group, a difference of –8.44 (P < .0001).
In a subgroup analysis, rituximab was superior to placebo regardless of disease duration, disease type (diffuse cutaneous or limited cutaneous SSc), prior receipt of systemic corticosteroids or immunosuppressants, or having C-reactive protein levels less than 0.3 mg/dL or at least 0.3 mg/dL.
However, there was no significant benefit with rituximab for patients with baseline mRSS of at least 20 or for those without ILD at baseline.
There was also evidence that rituximab reduced lung fibrosis. For patients assigned to the active drug, the absolute change in FVC at 24 weeks was +0.09% of the predicted value, compared with –3.56% for patients who received placebo (P = .044).
The researchers also observed radiographic evidence of lung improvement. The absolute change in the percentage of lung field occupied with interstitial shadows was –0.32% in the rituximab arm versus +2.39% in the placebo arm (P = .034). There was no significant between-group difference in the absolute change in diffusing capacity of lung for carbon monoxide, however.
Adverse events that occurred more frequently with rituximab included oral mucositis, diarrhea, and decreased neutrophil and white blood cell counts.
Convincing results
“What I thought the Japanese study did was to give a much more convincing proof of concept than has been out there,” Dr. Spiera said in an interview.
“There have been some preliminary experiences that have been encouraging with rituximab in scleroderma, most of which has been open label,” he said.
He also referred to a retrospective study by EUSTAR, the European Scleroderma Trials and Research group, which indicated that patients who had previously received rituximab seemed to have had better outcomes than patients who had been treated with other therapies.
Dr. Spiera added that, although he was glad to see the data from a randomized, placebo-controlled trial in this population, he was uncomfortable with the idea of leaving patients untreated for 6 months.
“From the standpoint of somebody wanting to know what strategies might be promising, this is great for us, but I would not have designed the trial that way,” he said.
The study results were previously published in the Lancet Rheumatology.
The study was supported by grants from the Japan Agency for Medical Research and Development and Zenyaku Kogyo. Dr. Yoshizaki disclosed no relevant financial relationships. Dr. Spiera has received grant/research support from and has consulted for Roche/Genentech, maker of rituximab, and has received compensation from other companies.
A version of this article first appeared on Medscape.com.
Rituximab effectively reduced skin sclerosis and appeared to have a beneficial effect on interstitial lung disease (ILD) for patients with systemic sclerosis (SSc) in a randomized, clinical trial.
At 24 weeks’ follow-up, there was significant improvement in total skin thickness scores among patients who received four once-weekly rituximab infusions, compared with patients who received placebo infusions. Among patients who received rituximab, there were also small but significant improvements in percentage of forced vital capacity (FVC). Among patients who received placebo, FVC worsened, reported Ayumi Yoshizaki, MD, of the University of Tokyo and colleagues.
“Systemic sclerosis is considered to have high unmet medical needs because of its poor prognosis and the lack of satisfactory and effective treatments,” he said at the virtual annual meeting of the American College of Rheumatology.
“Several clinical studies have suggested that B-cell depletion therapy with rituximab anti-CD20 antibody is effective in treating skin and lung fibrosis of SSc. However, no randomized, placebo-controlled trial has been able to confirm the efficacy of rituximab in SSc,” Dr. Yoshizaki said.
A rheumatologist who is currently conducting an investigator-initiated trial in which patients with SSC are undergoing treatment with rituximab followed by belimumab (Benlysta) said in an interview that he found the data to be “super interesting.”
“There are a lot of reasons to think that B cells might be important in systemic sclerosis, and actually that’s why our group had previously done an investigator-initiated trial with belimumab years ago,” said Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York.
Randomized trial
Dr. Yoshizaki and colleagues conducted the randomized, placebo-controlled DESIRES trial in four hospitals in Japan to evaluate the safety and efficacy of rituximab for the treatment of SSc.
In the investigator-initiated trial, patients aged 20-79 years who fulfilled ACR and European Alliance of Associations for Rheumatology classification criteria for systemic sclerosis and who had a modified Rodnan Skin Score (mRSS) of 10 or more and a life expectancy of at least 6 months were randomly assigned to receive infusions with either rituximab 375 mg/m2 or placebo once weekly for 4 weeks. Patients and clinicians were masked to treatment allocation.
The trial included 56 patients (51 women, 5 men). Of all patients enrolled, 27 of 28 who were allocated to receive rituximab and 22 of 28 who were allocated to receive placebo underwent at least one infusion and completed 24 weeks of follow-up.
The absolute change in mRSS at 24 weeks after the start of therapy, the primary endpoint, was –6.30 in the rituximab group, compared with +2.14 in the placebo group, a difference of –8.44 (P < .0001).
In a subgroup analysis, rituximab was superior to placebo regardless of disease duration, disease type (diffuse cutaneous or limited cutaneous SSc), prior receipt of systemic corticosteroids or immunosuppressants, or having C-reactive protein levels less than 0.3 mg/dL or at least 0.3 mg/dL.
However, there was no significant benefit with rituximab for patients with baseline mRSS of at least 20 or for those without ILD at baseline.
There was also evidence that rituximab reduced lung fibrosis. For patients assigned to the active drug, the absolute change in FVC at 24 weeks was +0.09% of the predicted value, compared with –3.56% for patients who received placebo (P = .044).
The researchers also observed radiographic evidence of lung improvement. The absolute change in the percentage of lung field occupied with interstitial shadows was –0.32% in the rituximab arm versus +2.39% in the placebo arm (P = .034). There was no significant between-group difference in the absolute change in diffusing capacity of lung for carbon monoxide, however.
Adverse events that occurred more frequently with rituximab included oral mucositis, diarrhea, and decreased neutrophil and white blood cell counts.
Convincing results
“What I thought the Japanese study did was to give a much more convincing proof of concept than has been out there,” Dr. Spiera said in an interview.
“There have been some preliminary experiences that have been encouraging with rituximab in scleroderma, most of which has been open label,” he said.
He also referred to a retrospective study by EUSTAR, the European Scleroderma Trials and Research group, which indicated that patients who had previously received rituximab seemed to have had better outcomes than patients who had been treated with other therapies.
Dr. Spiera added that, although he was glad to see the data from a randomized, placebo-controlled trial in this population, he was uncomfortable with the idea of leaving patients untreated for 6 months.
“From the standpoint of somebody wanting to know what strategies might be promising, this is great for us, but I would not have designed the trial that way,” he said.
The study results were previously published in the Lancet Rheumatology.
The study was supported by grants from the Japan Agency for Medical Research and Development and Zenyaku Kogyo. Dr. Yoshizaki disclosed no relevant financial relationships. Dr. Spiera has received grant/research support from and has consulted for Roche/Genentech, maker of rituximab, and has received compensation from other companies.
A version of this article first appeared on Medscape.com.
Rituximab effectively reduced skin sclerosis and appeared to have a beneficial effect on interstitial lung disease (ILD) for patients with systemic sclerosis (SSc) in a randomized, clinical trial.
At 24 weeks’ follow-up, there was significant improvement in total skin thickness scores among patients who received four once-weekly rituximab infusions, compared with patients who received placebo infusions. Among patients who received rituximab, there were also small but significant improvements in percentage of forced vital capacity (FVC). Among patients who received placebo, FVC worsened, reported Ayumi Yoshizaki, MD, of the University of Tokyo and colleagues.
“Systemic sclerosis is considered to have high unmet medical needs because of its poor prognosis and the lack of satisfactory and effective treatments,” he said at the virtual annual meeting of the American College of Rheumatology.
“Several clinical studies have suggested that B-cell depletion therapy with rituximab anti-CD20 antibody is effective in treating skin and lung fibrosis of SSc. However, no randomized, placebo-controlled trial has been able to confirm the efficacy of rituximab in SSc,” Dr. Yoshizaki said.
A rheumatologist who is currently conducting an investigator-initiated trial in which patients with SSC are undergoing treatment with rituximab followed by belimumab (Benlysta) said in an interview that he found the data to be “super interesting.”
“There are a lot of reasons to think that B cells might be important in systemic sclerosis, and actually that’s why our group had previously done an investigator-initiated trial with belimumab years ago,” said Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York.
Randomized trial
Dr. Yoshizaki and colleagues conducted the randomized, placebo-controlled DESIRES trial in four hospitals in Japan to evaluate the safety and efficacy of rituximab for the treatment of SSc.
In the investigator-initiated trial, patients aged 20-79 years who fulfilled ACR and European Alliance of Associations for Rheumatology classification criteria for systemic sclerosis and who had a modified Rodnan Skin Score (mRSS) of 10 or more and a life expectancy of at least 6 months were randomly assigned to receive infusions with either rituximab 375 mg/m2 or placebo once weekly for 4 weeks. Patients and clinicians were masked to treatment allocation.
The trial included 56 patients (51 women, 5 men). Of all patients enrolled, 27 of 28 who were allocated to receive rituximab and 22 of 28 who were allocated to receive placebo underwent at least one infusion and completed 24 weeks of follow-up.
The absolute change in mRSS at 24 weeks after the start of therapy, the primary endpoint, was –6.30 in the rituximab group, compared with +2.14 in the placebo group, a difference of –8.44 (P < .0001).
In a subgroup analysis, rituximab was superior to placebo regardless of disease duration, disease type (diffuse cutaneous or limited cutaneous SSc), prior receipt of systemic corticosteroids or immunosuppressants, or having C-reactive protein levels less than 0.3 mg/dL or at least 0.3 mg/dL.
However, there was no significant benefit with rituximab for patients with baseline mRSS of at least 20 or for those without ILD at baseline.
There was also evidence that rituximab reduced lung fibrosis. For patients assigned to the active drug, the absolute change in FVC at 24 weeks was +0.09% of the predicted value, compared with –3.56% for patients who received placebo (P = .044).
The researchers also observed radiographic evidence of lung improvement. The absolute change in the percentage of lung field occupied with interstitial shadows was –0.32% in the rituximab arm versus +2.39% in the placebo arm (P = .034). There was no significant between-group difference in the absolute change in diffusing capacity of lung for carbon monoxide, however.
Adverse events that occurred more frequently with rituximab included oral mucositis, diarrhea, and decreased neutrophil and white blood cell counts.
Convincing results
“What I thought the Japanese study did was to give a much more convincing proof of concept than has been out there,” Dr. Spiera said in an interview.
“There have been some preliminary experiences that have been encouraging with rituximab in scleroderma, most of which has been open label,” he said.
He also referred to a retrospective study by EUSTAR, the European Scleroderma Trials and Research group, which indicated that patients who had previously received rituximab seemed to have had better outcomes than patients who had been treated with other therapies.
Dr. Spiera added that, although he was glad to see the data from a randomized, placebo-controlled trial in this population, he was uncomfortable with the idea of leaving patients untreated for 6 months.
“From the standpoint of somebody wanting to know what strategies might be promising, this is great for us, but I would not have designed the trial that way,” he said.
The study results were previously published in the Lancet Rheumatology.
The study was supported by grants from the Japan Agency for Medical Research and Development and Zenyaku Kogyo. Dr. Yoshizaki disclosed no relevant financial relationships. Dr. Spiera has received grant/research support from and has consulted for Roche/Genentech, maker of rituximab, and has received compensation from other companies.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
Risankizumab outperforms placebo at 6 months for psoriatic arthritis
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
Patients with psoriatic arthritis (PsA) showed more improvement in symptoms at 6 months with risankizumab (Skyrizi) than with placebo in combined phase 3, randomized, controlled trials, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“Risankizumab was well tolerated and showed no new safety signals over those seen in the trial program for psoriasis,” reported Andrew Östör, MD, of Monash University and Cabrini Hospital, both in Melbourne. The results included pooled data that added KEEPsAKE 1 data to KEEPsAKE 2 results, which were presented at the 2021 congress of the European Alliance of Associations for Rheumatology.
Risankizumab received Food and Drug Administration approval in 2019 for moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The humanized monoclonal antibody inhibits interleukin-23, which is believed to be involved in the development of PsA. The FDA updated its approval in August 2021 to make it available as a 150-mg single-dose injection instead of two 75-mg doses for psoriasis treatment, but it is not yet approved for PsA.
The trials included adults with active PsA, active plaque psoriasis or nail psoriasis, and at least five swollen joints and five tender joints. All the participants had an inadequate response or intolerance to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), and KEEPsAKE 2 included participants who had an inadequate response or intolerance to at least one biologic therapy.
The majority of patients in both groups were taking anti-inflammatory drugs (58.8% with risankizumab vs. 62.1% with placebo) and methotrexate (60% vs. 59.1%, respectively), but a minority were taking oral glucocorticoids (18.2% with risankizumab vs. 15.6% with placebo). A small proportion in both groups were also taking a csDMARD besides methotrexate (11.9% with risankizumab vs. 11.3% with placebo).
Participants were randomly assigned to receive either 150 mg of subcutaneous risankizumab or placebo at baseline, 4 weeks, and 16 weeks with a double-blind protocol. The proportion of patients with 20% improvement in ACR response criteria (ACR 20) at 24 weeks was the primary endpoint. The trial is currently continuing with all participants receiving open-label risankizumab.
The 1,407 patients initially enrolled included 707 receiving risankizumab and 700 receiving placebo across both trials, with similar baseline demographic and disease characteristics in both groups. A total of 1,354 participants completed the 24-week assessments, including 688 receiving risankizumab and 666 receiving placebo. In an intent-to-treat analysis, 55.5% of patients receiving risankizumab and 31.3% of those receiving placebo achieved ACR 20 at week 24 (P < .001). Participants who received risankizumab also had more improvement in secondary clinical and patient-reported outcomes than did those who received placebo. A quarter (25.2%) of risankizumab patients versus 10.6% of placebo patients showed minimal disease activity, and significantly more participants receiving risankizumab than placebo saw resolution of enthesitis, dactylitis, and fatigue.
Adverse events of any kind occurred in 45.5% of risankizumab and 43.9% of placebo participants, with similar numbers of serious adverse events (3% vs. 4.4%, respectively). One death caused by urosepsis in an 81-year-old participant with dementia occurred in the risankizumab group and was determined to be unrelated to the drug.
David Karp, MD, PhD, chief of division of rheumatic diseases at the University of Texas Southwestern Medical Center in Dallas and ACR president, conducted a question-and-answer session with Dr. Östör following his presentation and asked whether a difference in responses was seen between patients who had failed biologic DMARDs. Dr. Östör said the response rates were similar independent of which previous therapies the participants had failed.
Regarding where risankizumab, as an IL-23 inhibitor, fits among the options for treating PsA, Dr. Östör said “the data speaks for itself” in terms of efficacy with arthritic, musculoskeletal manifestations and the patient-reported outcomes.
“One of the major benefits of these medications is their remarkable effect on skin with psoriasis,” Dr. Östör told Dr. Karp. Regarding axial response to the drug, Dr. Östör noted the statistically significant improvement in Bath Ankylosing Spondylitis Disease Activity Index, appearing to show a clinical benefit with spinal inflammatory disease. Radiologic data, however, are not currently available for the trials.
Dr. Karp noted the recent findings of a phase 2a trial published in the New England Journal of Medicine regarding risankizumab’s poor performance in patients with severe asthma, who experienced worsening symptoms sooner and more rapidly than did those who received placebo. It’s unclear whether any patients in the KEEPsAKE 1 or 2 trials had an asthma diagnosis, but any people with unstable, severe asthma would have been excluded from participation, Dr. Östör said.
The research was funded by AbbVie. Dr. Östör and colleagues have a range of financial ties to numerous pharmaceutical companies.
FROM ACR 2021