User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Abatacept shows signal to delay onset of rheumatoid arthritis
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
Early intervention with the immunomodulator abatacept (Orencia) may enable people at risk for rheumatoid arthritis but who don’t yet manifest symptomatic inflammation to either avoid or delay the onset of full-blown, symptomatic rheumatoid arthritis, early results of a European clinical trial have shown.
Early results of the ARIAA study, presented at the virtual annual meeting of the American College of Rheumatology, showed that among patients considered at-risk for RA and having arthralgia and subclinical inflammation – considered symptomatic but not having full-blown RA – 61% of those who received a 6-month course of abatacept versus 31% of the placebo group had an improvement in MRI inflammation score (P = .0043), said Juergen Rech, MD, a rheumatologist at Friedrich-Alexander University of Erlangen-Nuremberg (Germany) and University Clinic Erlangen.
“When we actually talk about early treatment, this may be not early enough or at least could be improved,” Dr. Rech said in an interview when asked what the findings add to the evidence for treating at-risk RA patients before disease onset. “It seems as if we were in the situation of delaying the development of disease or possibly even preventing it in some patients, and in our trial this approach was safe with abatacept.”
ARIAA randomized 100 patients to abatacept or placebo at 14 study sites between November 2014 and December 2019. The goal is to treat at-risk patients for 6 months with abatacept, then follow them for 12 months to determine their progression to RA. Dr. Rech noted that 8% of patients in the treatment group and 35% in the placebo group developed arthritis (P = .0025).
He noted that the safety profile of abatacept in this patient population was similar to previous trials. “No safety issues emerged,” Dr. Rech said.
The investigators used MRI to determine the patients’ status for arthralgia and subclinical inflammation before enrollment. They had no history of clinically obvious inflammation fulfilling the criteria for RA and no previous treatment with glucocorticoids or disease-modifying antirheumatic drugs.
The results showed that abatacept is superior to placebo in improving subclinical inflammation and in inhibiting the progression to RA in at-risk patients at 6 months, Dr. Rech said, but early clinical results of patients in the study who’ve had 18 months of follow-up, which were not part of the dataset he presented, revealed that time-limited treatment with the immunomodulator has a significant sustained effect on progression to RA. That “means 6 months of treatment with abatacept will delay the development of RA after 18 months,” he said.
After the complete 18-month dataset is analyzed, the next step for investigators will be to re-evaluate the ARIAA population, perhaps for genetic markers, Dr. Rech said. What would then follow, he said, could be to conduct a larger phase 3 trial, determine the risk factors that drive RA autoimmunity, see if disease progression varies among ethnic groups and people in different geographic regions, and perhaps start a head-to-head trial with rituximab (Rituxan) or an evaluation of combined time-limited abatacept and rituximab in at-risk patients.
“We should think about new strategies, new life-quality questionnaires, new biomarkers and tools for covering and understanding these RA patients at-risk in a better way,” Dr. Rech said, noting that a European Alliance of Associations for Rheumatology task force has already addressed this topic.
John D. Isaacs, MBBS, PhD, professor of rheumatology at Newcastle (England) University, said in an interview that ARIAA is the first readout from a number of studies evaluating preemptive treatment to prevent or delay RA onset. “You have to ask a question: Is this just suppressing what’s going on?” Dr. Isaacs said. “In other words, now that the treatment has been stopped, there’s great interest in what happens over the next 12 months of this study. Have we delayed the onset of rheumatoid arthritis or have we actually prevented it? I think that’s the $10 billion dollar question of this and similar studies.”
Answering that question may be difficult without a known blood biomarker. “That’s not a criticism of the trial; we just don’t have that scientifically at the moment,” Dr. Isaacs said. “Until then, it will be difficult to say we have delayed or we have prevented rheumatoid arthritis. My feeling is, even if we delay it 6 months or even a year with safe treatment, that would be worth it.”
Bristol-Myers Squibb sponsored the trial. Dr. Rech and Dr. Isaacs disclosed having financial relationships with Bristol-Myers Squibb and other pharmaceutical companies.
FROM ACR 2021
Vitamin D and omega-3 supplements reduce autoimmune disease risk
For those of us who cannot sit in the sun and fish all day, the next best thing for preventing autoimmune diseases may be supplementation with vitamin D and fish oil-derived omega-3 fatty acids, results of a large prospective randomized trial suggest.
Among nearly 26,000 adults enrolled in a randomized trial designed primarily to study the effects of vitamin D and omega-3 supplementation on incident cancer and cardiovascular disease, 5, and 5 years of omega-3 fatty acid supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases, reported Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston.
“The clinical importance of these results is very high, given that these are nontoxic, well-tolerated supplements, and that there are no other known effective therapies to reduce the incidence of autoimmune diseases,” she said during the virtual annual meeting of the American College of Rheumatology.
“People do have to take the supplements a long time to start to see the reduction in risk, especially for vitamin D, but they make biological sense, and autoimmune diseases develop slowly over time, so taking it today isn’t going to reduce risk of developing something tomorrow,” Dr. Costenbader said in an interview.
“These supplements have other health benefits. Obviously, fish oil is anti-inflammatory, and vitamin D is good for osteoporosis prevention, especially in our patients who take glucocorticoids. People who are otherwise healthy and have a family history of autoimmune disease might also consider starting to take these supplements,” she said.
After watching her presentation, session co-moderator Gregg Silverman, MD, from the NYU Langone School of Medicine in New York, who was not involved in the study, commented “I’m going to [nutrition store] GNC to get some vitamins.”
When asked for comment, the other session moderator, Tracy Frech, MD, of Vanderbilt University, Nashville, said, “I think Dr. Costenbader’s work is very important and her presentation excellent. My current practice is replacement of vitamin D in all autoimmune disease patients with low levels and per bone health guidelines. Additionally, I discuss omega-3 supplementation with Sjögren’s [syndrome] patients as a consideration.”
Evidence base
Dr. Costenbader noted that in a 2013 observational study from France, vitamin D derived through ultraviolet (UV) light exposure was associated with a lower risk for incident Crohn’s disease but not ulcerative colitis, and in two analyses of data in 2014 from the Nurses’ Health Study, both high plasma levels of 25-OH vitamin D and geographic residence in areas of high UV exposure were associated with a decreased incidence of rheumatoid arthritis (RA).
Other observational studies have supported omega-3 fatty acids for their anti-inflammatory properties, including a 2005 Danish prospective cohort study showing a lower risk for RA in participants who reported higher levels of fatty fish intake. In a separate study conducted in 2017, healthy volunteers with higher omega-3 fatty acid/total lipid proportions in red blood cell membranes had a lower prevalence of anti-cyclic citrullinated peptide (anti-CCP) antibodies and rheumatoid factor and a lower incidence of progression to inflammatory arthritis, she said.
Ancillary study
Despite the evidence, however, there have been no prospective randomized trials to test the effects of either vitamin D or omega-3 fatty acid supplementation on the incidence of autoimmune disease over time.
To rectify this, Dr. Costenbader and colleagues piggybacked an ancillary study onto the Vitamin D and Omega-3 Trial (VITAL), which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older, and 13,085 women aged 55 and older.
The study had a 2 x 2 factorial design, with patients randomly assigned to vitamin D 2,000 IU/day or placebo, and then further randomized to either 1 g/day omega-3 fatty acids or placebo in both the vitamin D and placebo primary randomization arms.
At baseline 16,956 participants were assayed for 25-OH vitamin D and plasma omega 3 index, the ratio of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to total fatty acids. Participants self-reported baseline and all incident autoimmune diseases annually, with the reports confirmed by medical record review and disease criteria whenever possible.
Results
At 5 years of follow-up, confirmed incident autoimmune diseases had occurred in 123 patients in the active vitamin D group, compared with 155 in the placebo vitamin D group, translating into a hazard ratio (HR) for vitamin D of 0.78 (P = .045).
In the active omega-3 arm, 130 participants developed an autoimmune disease, compared with 148 in the placebo omega-3 arm, which translated into a nonsignificant HR of 0.85.
There was no statistical interaction between the two supplements. The investigators did observe an interaction between vitamin D and body mass index, with the effect stronger among participants with low BMI (P = .02). There also was an interaction between omega-3 fatty acids with a family history of autoimmune disease (P = .03).
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with an HR for incident autoimmune disease of 0.68 (P = .02), omega-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03).
Dr. Costenbader and colleagues acknowledged that the study was limited by the lack of a high-risk or nutritionally-deficient population, where the effects of supplementation might be larger; the restriction of the sample to older adults; and to the difficulty of confirming incident autoimmune thyroid disease from patient reports.
Cheryl Koehn, an arthritis patient advocate from Vancouver, Canada, who was not involved in the study, commented in the “chat” section of the presentation that her rheumatologist “has recommended vitamin D for years now. Says basically everyone north of Boston is vitamin D deficient. I take 1,000 IU per day. Been taking it for years.” Ms. Koehn is the founder and president of Arthritis Consumer Experts, a website that provides education to those with arthritis.
“Agreed. I tell every patient to take vitamin D supplement,” commented Fatma Dedeoglu, MD, a rheumatologist at Boston Children’s Hospital.
A version of this article first appeared on Medscape.com.
For those of us who cannot sit in the sun and fish all day, the next best thing for preventing autoimmune diseases may be supplementation with vitamin D and fish oil-derived omega-3 fatty acids, results of a large prospective randomized trial suggest.
Among nearly 26,000 adults enrolled in a randomized trial designed primarily to study the effects of vitamin D and omega-3 supplementation on incident cancer and cardiovascular disease, 5, and 5 years of omega-3 fatty acid supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases, reported Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston.
“The clinical importance of these results is very high, given that these are nontoxic, well-tolerated supplements, and that there are no other known effective therapies to reduce the incidence of autoimmune diseases,” she said during the virtual annual meeting of the American College of Rheumatology.
“People do have to take the supplements a long time to start to see the reduction in risk, especially for vitamin D, but they make biological sense, and autoimmune diseases develop slowly over time, so taking it today isn’t going to reduce risk of developing something tomorrow,” Dr. Costenbader said in an interview.
“These supplements have other health benefits. Obviously, fish oil is anti-inflammatory, and vitamin D is good for osteoporosis prevention, especially in our patients who take glucocorticoids. People who are otherwise healthy and have a family history of autoimmune disease might also consider starting to take these supplements,” she said.
After watching her presentation, session co-moderator Gregg Silverman, MD, from the NYU Langone School of Medicine in New York, who was not involved in the study, commented “I’m going to [nutrition store] GNC to get some vitamins.”
When asked for comment, the other session moderator, Tracy Frech, MD, of Vanderbilt University, Nashville, said, “I think Dr. Costenbader’s work is very important and her presentation excellent. My current practice is replacement of vitamin D in all autoimmune disease patients with low levels and per bone health guidelines. Additionally, I discuss omega-3 supplementation with Sjögren’s [syndrome] patients as a consideration.”
Evidence base
Dr. Costenbader noted that in a 2013 observational study from France, vitamin D derived through ultraviolet (UV) light exposure was associated with a lower risk for incident Crohn’s disease but not ulcerative colitis, and in two analyses of data in 2014 from the Nurses’ Health Study, both high plasma levels of 25-OH vitamin D and geographic residence in areas of high UV exposure were associated with a decreased incidence of rheumatoid arthritis (RA).
Other observational studies have supported omega-3 fatty acids for their anti-inflammatory properties, including a 2005 Danish prospective cohort study showing a lower risk for RA in participants who reported higher levels of fatty fish intake. In a separate study conducted in 2017, healthy volunteers with higher omega-3 fatty acid/total lipid proportions in red blood cell membranes had a lower prevalence of anti-cyclic citrullinated peptide (anti-CCP) antibodies and rheumatoid factor and a lower incidence of progression to inflammatory arthritis, she said.
Ancillary study
Despite the evidence, however, there have been no prospective randomized trials to test the effects of either vitamin D or omega-3 fatty acid supplementation on the incidence of autoimmune disease over time.
To rectify this, Dr. Costenbader and colleagues piggybacked an ancillary study onto the Vitamin D and Omega-3 Trial (VITAL), which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older, and 13,085 women aged 55 and older.
The study had a 2 x 2 factorial design, with patients randomly assigned to vitamin D 2,000 IU/day or placebo, and then further randomized to either 1 g/day omega-3 fatty acids or placebo in both the vitamin D and placebo primary randomization arms.
At baseline 16,956 participants were assayed for 25-OH vitamin D and plasma omega 3 index, the ratio of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to total fatty acids. Participants self-reported baseline and all incident autoimmune diseases annually, with the reports confirmed by medical record review and disease criteria whenever possible.
Results
At 5 years of follow-up, confirmed incident autoimmune diseases had occurred in 123 patients in the active vitamin D group, compared with 155 in the placebo vitamin D group, translating into a hazard ratio (HR) for vitamin D of 0.78 (P = .045).
In the active omega-3 arm, 130 participants developed an autoimmune disease, compared with 148 in the placebo omega-3 arm, which translated into a nonsignificant HR of 0.85.
There was no statistical interaction between the two supplements. The investigators did observe an interaction between vitamin D and body mass index, with the effect stronger among participants with low BMI (P = .02). There also was an interaction between omega-3 fatty acids with a family history of autoimmune disease (P = .03).
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with an HR for incident autoimmune disease of 0.68 (P = .02), omega-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03).
Dr. Costenbader and colleagues acknowledged that the study was limited by the lack of a high-risk or nutritionally-deficient population, where the effects of supplementation might be larger; the restriction of the sample to older adults; and to the difficulty of confirming incident autoimmune thyroid disease from patient reports.
Cheryl Koehn, an arthritis patient advocate from Vancouver, Canada, who was not involved in the study, commented in the “chat” section of the presentation that her rheumatologist “has recommended vitamin D for years now. Says basically everyone north of Boston is vitamin D deficient. I take 1,000 IU per day. Been taking it for years.” Ms. Koehn is the founder and president of Arthritis Consumer Experts, a website that provides education to those with arthritis.
“Agreed. I tell every patient to take vitamin D supplement,” commented Fatma Dedeoglu, MD, a rheumatologist at Boston Children’s Hospital.
A version of this article first appeared on Medscape.com.
For those of us who cannot sit in the sun and fish all day, the next best thing for preventing autoimmune diseases may be supplementation with vitamin D and fish oil-derived omega-3 fatty acids, results of a large prospective randomized trial suggest.
Among nearly 26,000 adults enrolled in a randomized trial designed primarily to study the effects of vitamin D and omega-3 supplementation on incident cancer and cardiovascular disease, 5, and 5 years of omega-3 fatty acid supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases, reported Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston.
“The clinical importance of these results is very high, given that these are nontoxic, well-tolerated supplements, and that there are no other known effective therapies to reduce the incidence of autoimmune diseases,” she said during the virtual annual meeting of the American College of Rheumatology.
“People do have to take the supplements a long time to start to see the reduction in risk, especially for vitamin D, but they make biological sense, and autoimmune diseases develop slowly over time, so taking it today isn’t going to reduce risk of developing something tomorrow,” Dr. Costenbader said in an interview.
“These supplements have other health benefits. Obviously, fish oil is anti-inflammatory, and vitamin D is good for osteoporosis prevention, especially in our patients who take glucocorticoids. People who are otherwise healthy and have a family history of autoimmune disease might also consider starting to take these supplements,” she said.
After watching her presentation, session co-moderator Gregg Silverman, MD, from the NYU Langone School of Medicine in New York, who was not involved in the study, commented “I’m going to [nutrition store] GNC to get some vitamins.”
When asked for comment, the other session moderator, Tracy Frech, MD, of Vanderbilt University, Nashville, said, “I think Dr. Costenbader’s work is very important and her presentation excellent. My current practice is replacement of vitamin D in all autoimmune disease patients with low levels and per bone health guidelines. Additionally, I discuss omega-3 supplementation with Sjögren’s [syndrome] patients as a consideration.”
Evidence base
Dr. Costenbader noted that in a 2013 observational study from France, vitamin D derived through ultraviolet (UV) light exposure was associated with a lower risk for incident Crohn’s disease but not ulcerative colitis, and in two analyses of data in 2014 from the Nurses’ Health Study, both high plasma levels of 25-OH vitamin D and geographic residence in areas of high UV exposure were associated with a decreased incidence of rheumatoid arthritis (RA).
Other observational studies have supported omega-3 fatty acids for their anti-inflammatory properties, including a 2005 Danish prospective cohort study showing a lower risk for RA in participants who reported higher levels of fatty fish intake. In a separate study conducted in 2017, healthy volunteers with higher omega-3 fatty acid/total lipid proportions in red blood cell membranes had a lower prevalence of anti-cyclic citrullinated peptide (anti-CCP) antibodies and rheumatoid factor and a lower incidence of progression to inflammatory arthritis, she said.
Ancillary study
Despite the evidence, however, there have been no prospective randomized trials to test the effects of either vitamin D or omega-3 fatty acid supplementation on the incidence of autoimmune disease over time.
To rectify this, Dr. Costenbader and colleagues piggybacked an ancillary study onto the Vitamin D and Omega-3 Trial (VITAL), which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older, and 13,085 women aged 55 and older.
The study had a 2 x 2 factorial design, with patients randomly assigned to vitamin D 2,000 IU/day or placebo, and then further randomized to either 1 g/day omega-3 fatty acids or placebo in both the vitamin D and placebo primary randomization arms.
At baseline 16,956 participants were assayed for 25-OH vitamin D and plasma omega 3 index, the ratio of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to total fatty acids. Participants self-reported baseline and all incident autoimmune diseases annually, with the reports confirmed by medical record review and disease criteria whenever possible.
Results
At 5 years of follow-up, confirmed incident autoimmune diseases had occurred in 123 patients in the active vitamin D group, compared with 155 in the placebo vitamin D group, translating into a hazard ratio (HR) for vitamin D of 0.78 (P = .045).
In the active omega-3 arm, 130 participants developed an autoimmune disease, compared with 148 in the placebo omega-3 arm, which translated into a nonsignificant HR of 0.85.
There was no statistical interaction between the two supplements. The investigators did observe an interaction between vitamin D and body mass index, with the effect stronger among participants with low BMI (P = .02). There also was an interaction between omega-3 fatty acids with a family history of autoimmune disease (P = .03).
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with an HR for incident autoimmune disease of 0.68 (P = .02), omega-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03).
Dr. Costenbader and colleagues acknowledged that the study was limited by the lack of a high-risk or nutritionally-deficient population, where the effects of supplementation might be larger; the restriction of the sample to older adults; and to the difficulty of confirming incident autoimmune thyroid disease from patient reports.
Cheryl Koehn, an arthritis patient advocate from Vancouver, Canada, who was not involved in the study, commented in the “chat” section of the presentation that her rheumatologist “has recommended vitamin D for years now. Says basically everyone north of Boston is vitamin D deficient. I take 1,000 IU per day. Been taking it for years.” Ms. Koehn is the founder and president of Arthritis Consumer Experts, a website that provides education to those with arthritis.
“Agreed. I tell every patient to take vitamin D supplement,” commented Fatma Dedeoglu, MD, a rheumatologist at Boston Children’s Hospital.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
Antihypertensives tied to lower Alzheimer’s disease pathology
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows.
Investigators found that use of any antihypertensive was associated with an 18% decrease in Alzheimer’s disease neuropathology, a 22% decrease in Lewy bodies, and a 40% decrease in TAR DNA-binding protein 43 (TDP-43), a protein relevant to several neurodegenerative diseases. Diuretics in particular appear to be driving the association.
Although diuretics might be a better option for preventing brain neuropathology, it’s too early to make firm recommendations solely on the basis of these results as to what blood pressure–lowering agent to prescribe a particular patient, said study investigator Ahmad Sajjadi, MD, assistant professor of neurology, University of California, Irvine.
“This is early stages and preliminary results,” said Dr. Sajjadi, “but it’s food for thought.”
The findings were presented at the 2021 annual meeting of the American Neurological Association.
Autopsy data
The study included 3,315 individuals who had donated their brains to research. The National Alzheimer’s Coordinating Center maintains a database that includes data from 32 Alzheimer’s disease research centers in the United States. Participants in the study must have visited one of these centers within 4 years of death. Each person whose brain was included in the study underwent two or more BP measurements on at least 50% of visits.
The mean age at death was 81.7 years, and the mean time between last visit and death was 13.1 months. About 44.4% of participants were women, 57.0% had at least a college degree, and 84.7% had cognitive impairment.
Researchers defined hypertension as systolic BP of at least 130 mm Hg, diastolic BP of at least 80 mm Hg, mean arterial pressure of at least 100 mm Hg, and pulse pressure of at least 60 mm Hg.
Antihypertensive medications that were evaluated included antiadrenergic agents, ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, vasodilators, and combination therapies.
The investigators assessed the number of neuropathologies. In addition to Alzheimer’s disease neuropathology, which included amyloid-beta, tau, Lewy bodies, and TDP-43, they also assessed for atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, frontotemporal lobar degeneration, and hippocampal sclerosis.
Results showed that use of any antihypertensive was associated with a lower likelihood of Alzheimer’s disease neuropathology (odds ratio, 0.822), Lewy bodies (OR, 0.786), and TDP 43 (OR, 0.597). Use of antihypertensives was also associated with increased odds of atherosclerosis (OR, 1.217) (all P < .5.)
The study showed that hypertensive systolic BP was associated with higher odds of Alzheimer’s disease neuropathology (OR, 1.28; P < .5).
Differences by drug type
Results differed in accordance with antihypertensive class. Angiotensin II receptor blockers decreased the odds of Alzheimer’s disease neuropathology by 40% (OR, 0.60; P < .5). Diuretics decreased the odds of Alzheimer’s disease by 36% (OR, 0.64; P < .001) and of hippocampal sclerosis by 32% (OR, 0.68; P < .5).
“We see diuretics are a main driver, especially for lower odds of Alzheimer’s disease and lower odds of hippocampal sclerosis,” said lead author Hanna L. Nguyen, a first-year medical student at the University of California, Irvine.
The results indicate that it is the medications, not BP levels, that account for these associations, she added.
One potential mechanism linking antihypertensives to brain pathology is that with these agents, BP is maintained in the target zone. Blood pressure that’s too high can damage blood vessels, whereas BP that’s too low may result in less than adequate perfusion, said Ms. Nguyen.
These medications may also alter pathways leading to degeneration and could, for example, affect the apo E mechanism of Alzheimer’s disease, she added.
The researchers plan to conduct subset analyses using apo E genetic status and age of death.
Although this is a “massive database,” it has limitations. For example, said Dr. Sajjadi, it does not reveal when patients started taking BP medication, how long they had been taking it, or why.
“We don’t know the exact the reason they were taking these medications. Was it just hypertension, or did they also have heart disease, stroke, a kidney problem, or was there another explanation,” he said.
Following the study presentation, session comoderator Krish Sathian, MBBS, PhD, professor of neurology, neural, and behavioral sciences, and psychology and director of the Neuroscience Institute, Penn State University, Hershey, called this work “fascinating. It provides a lot of data that really touches on everyday practice,” inasmuch as clinicians often prescribe antihypertensive medications and see patients with these kinds of brain disorders.
The investigators and Dr. Sathian reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANA 2021
FFR-guided PCI falls short vs. surgery in multivessel disease: FAME 3
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
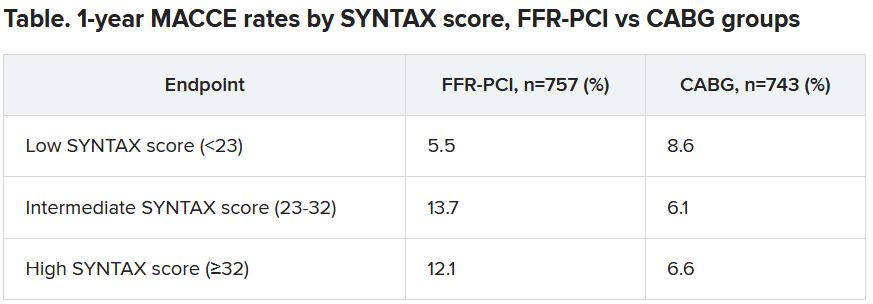
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
SUGAR trial finds superior stent for those with diabetes and CAD
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Superiority shown on TLF endpoint
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
Renal denervation remains only promising, per latest meta-analysis
Questions remain despite efficacy
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Questions remain despite efficacy
Questions remain despite efficacy
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
What are the legal risks of practicing laser cutaneous surgery?
The physician-patient relationship is a key factor in preventing litigation following cutaneous laser surgery, according to Mathew M. Avram, MD, JD.
“Numerous studies indicate that good communication and rapport are the most important means to avoid a lawsuit,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “It is helpful to say that the outcome was not optimal or what you were anticipating. Communicate your plan [for the complication] clearly and honestly to your patient. The patient may not understand the severity of the complication. If they don’t, they will either leave it alone or they will go elsewhere and may receive poor care.” He added that in New England, “we have some stoic patients who may say ‘I don’t want to bother the doctor’ or ‘It’s my fault for having the procedure done.’ ”
Establishing effective communication with patients from the outset is good practice, he continued, because 75% of physicians in low-risk specialties will face a malpractice claim by age 65. Nearly a decade ago Dr. Avram, H. Ray Jalian, MD, and Chris Jalian, JD, published results from a national legal database analysis identifying common errors and risk factors for litigation in cutaneous surgery. Their search yielded 1,807 documents with 174 unique legal claims involving injury from a cutaneous laser treatment, from 1985 to 2012. The most common litigated procedures were laser hair removal, rejuvenation (mostly related to intense pulsed-light treatments), and laser treatment of leg veins, while the most common injuries sustained were burns, scars, and pigmentary changes. The most common causes of legal action were lack of informed consent and fraud.
Among the 120 cases with public decisions, cases favored the plaintiff 51% of the time. “That’s unusual,” said Dr. Avram, president of American Society for Dermatologic Surgery. “Usually, physicians do better, but I think the fact that they’re cosmetic cases probably shades things a little bit.” The median monetary award was $350,000 and ranged from $5,000 to $2,145,000. The two largest judgments were for improper use of topical anesthesia that led to deaths of patients in laser hair removal cases.
In a separate analysis, the same authors searched an online national database to identify the incidence of medical professional liability claims resulting from cutaneous laser surgery performed by nonphysician operators (NPOs) from 1999 to 2012. Among the 175 cases identified, 43% involved an NPO. “In fact, the cases involving NPOs exploded over a 4-year period; they grew from 36% in 2008 of cases to 78% in 2011,” Dr. Avram said. “This was even more true for laser hair removal.”
The practice setting turned out to be a factor. Only 23% of NPO litigation involving laser procedures arose in medical office settings, while 77% of cases involving NPOs were performed outside of traditional medical settings such as in salons and medical spas – mostly for laser hair removal. “We updated this information by examining the setting for nonphysician operator litigation between 2012 and 2017 and found that 66% of cases involving NPOs were performed outside of a traditional medical setting, while 34% of NPO litigation arose in medical office settings,” Dr. Avram said during the meeting, which was named What’s the Truth? and sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s close to a 2 to 1 ratio.”
In an analysis of medical professional liability claims involving Mohs surgery from 1989 to 2011, 26 of the 42 cases identified involved a primary defendant who was not a Mohs surgeon. In the 26 cases, the most common reasons for lawsuits were failure or delay of diagnosis of a skin cancer, cosmetic outcome issues, lack of informed consent, and delay or failure to refer to a Mohs surgeon. Of the cases that involved Mohs surgeons, the most common causes were lack of proper informed consent and cosmetic outcome issues, but “these cases were overwhelmingly decided in favor of the surgeons,” said Dr. Avram, one of the study authors.
On a related note, Dr. Avram underscored the importance of biopsy-site photography, “because patients and physicians misidentify biopsy sites too commonly,” he said. In a single-center study of 34 biopsy sites of cutaneous head and neck malignancies, patients misidentified the biopsy site 4-7 weeks out in 29% of the cases. Blinded dermatologists and the patient misidentified the biopsy site in 12% of the cases. “Good biopsy site photography should be mandatory in your practice,” he advised.
Clinicians can avoid cutaneous laser surgery complications only by not treating patients. “Complications and side effects are inevitable; you need to know your limits,” he said. “Even in skilled hands, if you treat enough patients, you will encounter challenging side effects. Do not perform a procedure that might produce a side effect that you cannot recognize and treat.”
The best way to avoid complications is to trust your eyes – not the laser – since the same device made by the same manufacturer may produce highly different outputs at the same setting (see J Am Acad Dermatol. 2016;74[5]:807-19).
“Moreover, lasers can produce much different energies after they have been serviced,” Dr. Avram said. “Do not memorize settings. Do not blindly replicate recommended settings from a colleague or a device manufacturer,” he advised. “Some devices are not externally calibrated. Therefore, the settings on one device may not translate the same way to yours. Often, device manufacturers underplay the settings. Safe and unsafe laser endpoints and close observation are the best means to avoiding clinical complications. That means you follow clinical endpoints, not fluences. The key clinical finding is the endpoint, not the energy setting.”
Temporary and expected side effects include erythema, edema, and purpura. “With these it’s just handholding and unlikely to lead to any legal consequences,” he continued. “With temporary hyperpigmentation that can occur with laser hair removal, time is one your side, because typically this will resolve before any litigation progresses. Permanent side effects from lasers and light sources and injectables are a different issue, things like permanent hypopigmentation, depigmentation, and scarring. These are most likely to produce liability.”
In Dr. Avram’s opinion, complications are best handled with widespread communication. “There is a temptation to avoid a patient with a poor outcome or side effect,” he said. “This is bad medicine and rightfully angers your patient and increases the risk of a lawsuit. [Resist] the temptation to avoid showing a poor outcome to a colleague. Many complications can be significantly improved or cleared with timely and appropriate interventions. You should always document your efforts.”
Dr. Avram disclosed that he has received consulting fees from Allergan and Galderma. He is a member of the scientific advisory board for Allergan and Soliton, is an investigator for Endo, and holds stock options in La Jolla NanoMedical Inc.
The physician-patient relationship is a key factor in preventing litigation following cutaneous laser surgery, according to Mathew M. Avram, MD, JD.
“Numerous studies indicate that good communication and rapport are the most important means to avoid a lawsuit,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “It is helpful to say that the outcome was not optimal or what you were anticipating. Communicate your plan [for the complication] clearly and honestly to your patient. The patient may not understand the severity of the complication. If they don’t, they will either leave it alone or they will go elsewhere and may receive poor care.” He added that in New England, “we have some stoic patients who may say ‘I don’t want to bother the doctor’ or ‘It’s my fault for having the procedure done.’ ”
Establishing effective communication with patients from the outset is good practice, he continued, because 75% of physicians in low-risk specialties will face a malpractice claim by age 65. Nearly a decade ago Dr. Avram, H. Ray Jalian, MD, and Chris Jalian, JD, published results from a national legal database analysis identifying common errors and risk factors for litigation in cutaneous surgery. Their search yielded 1,807 documents with 174 unique legal claims involving injury from a cutaneous laser treatment, from 1985 to 2012. The most common litigated procedures were laser hair removal, rejuvenation (mostly related to intense pulsed-light treatments), and laser treatment of leg veins, while the most common injuries sustained were burns, scars, and pigmentary changes. The most common causes of legal action were lack of informed consent and fraud.
Among the 120 cases with public decisions, cases favored the plaintiff 51% of the time. “That’s unusual,” said Dr. Avram, president of American Society for Dermatologic Surgery. “Usually, physicians do better, but I think the fact that they’re cosmetic cases probably shades things a little bit.” The median monetary award was $350,000 and ranged from $5,000 to $2,145,000. The two largest judgments were for improper use of topical anesthesia that led to deaths of patients in laser hair removal cases.
In a separate analysis, the same authors searched an online national database to identify the incidence of medical professional liability claims resulting from cutaneous laser surgery performed by nonphysician operators (NPOs) from 1999 to 2012. Among the 175 cases identified, 43% involved an NPO. “In fact, the cases involving NPOs exploded over a 4-year period; they grew from 36% in 2008 of cases to 78% in 2011,” Dr. Avram said. “This was even more true for laser hair removal.”
The practice setting turned out to be a factor. Only 23% of NPO litigation involving laser procedures arose in medical office settings, while 77% of cases involving NPOs were performed outside of traditional medical settings such as in salons and medical spas – mostly for laser hair removal. “We updated this information by examining the setting for nonphysician operator litigation between 2012 and 2017 and found that 66% of cases involving NPOs were performed outside of a traditional medical setting, while 34% of NPO litigation arose in medical office settings,” Dr. Avram said during the meeting, which was named What’s the Truth? and sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s close to a 2 to 1 ratio.”
In an analysis of medical professional liability claims involving Mohs surgery from 1989 to 2011, 26 of the 42 cases identified involved a primary defendant who was not a Mohs surgeon. In the 26 cases, the most common reasons for lawsuits were failure or delay of diagnosis of a skin cancer, cosmetic outcome issues, lack of informed consent, and delay or failure to refer to a Mohs surgeon. Of the cases that involved Mohs surgeons, the most common causes were lack of proper informed consent and cosmetic outcome issues, but “these cases were overwhelmingly decided in favor of the surgeons,” said Dr. Avram, one of the study authors.
On a related note, Dr. Avram underscored the importance of biopsy-site photography, “because patients and physicians misidentify biopsy sites too commonly,” he said. In a single-center study of 34 biopsy sites of cutaneous head and neck malignancies, patients misidentified the biopsy site 4-7 weeks out in 29% of the cases. Blinded dermatologists and the patient misidentified the biopsy site in 12% of the cases. “Good biopsy site photography should be mandatory in your practice,” he advised.
Clinicians can avoid cutaneous laser surgery complications only by not treating patients. “Complications and side effects are inevitable; you need to know your limits,” he said. “Even in skilled hands, if you treat enough patients, you will encounter challenging side effects. Do not perform a procedure that might produce a side effect that you cannot recognize and treat.”
The best way to avoid complications is to trust your eyes – not the laser – since the same device made by the same manufacturer may produce highly different outputs at the same setting (see J Am Acad Dermatol. 2016;74[5]:807-19).
“Moreover, lasers can produce much different energies after they have been serviced,” Dr. Avram said. “Do not memorize settings. Do not blindly replicate recommended settings from a colleague or a device manufacturer,” he advised. “Some devices are not externally calibrated. Therefore, the settings on one device may not translate the same way to yours. Often, device manufacturers underplay the settings. Safe and unsafe laser endpoints and close observation are the best means to avoiding clinical complications. That means you follow clinical endpoints, not fluences. The key clinical finding is the endpoint, not the energy setting.”
Temporary and expected side effects include erythema, edema, and purpura. “With these it’s just handholding and unlikely to lead to any legal consequences,” he continued. “With temporary hyperpigmentation that can occur with laser hair removal, time is one your side, because typically this will resolve before any litigation progresses. Permanent side effects from lasers and light sources and injectables are a different issue, things like permanent hypopigmentation, depigmentation, and scarring. These are most likely to produce liability.”
In Dr. Avram’s opinion, complications are best handled with widespread communication. “There is a temptation to avoid a patient with a poor outcome or side effect,” he said. “This is bad medicine and rightfully angers your patient and increases the risk of a lawsuit. [Resist] the temptation to avoid showing a poor outcome to a colleague. Many complications can be significantly improved or cleared with timely and appropriate interventions. You should always document your efforts.”
Dr. Avram disclosed that he has received consulting fees from Allergan and Galderma. He is a member of the scientific advisory board for Allergan and Soliton, is an investigator for Endo, and holds stock options in La Jolla NanoMedical Inc.
The physician-patient relationship is a key factor in preventing litigation following cutaneous laser surgery, according to Mathew M. Avram, MD, JD.
“Numerous studies indicate that good communication and rapport are the most important means to avoid a lawsuit,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “It is helpful to say that the outcome was not optimal or what you were anticipating. Communicate your plan [for the complication] clearly and honestly to your patient. The patient may not understand the severity of the complication. If they don’t, they will either leave it alone or they will go elsewhere and may receive poor care.” He added that in New England, “we have some stoic patients who may say ‘I don’t want to bother the doctor’ or ‘It’s my fault for having the procedure done.’ ”
Establishing effective communication with patients from the outset is good practice, he continued, because 75% of physicians in low-risk specialties will face a malpractice claim by age 65. Nearly a decade ago Dr. Avram, H. Ray Jalian, MD, and Chris Jalian, JD, published results from a national legal database analysis identifying common errors and risk factors for litigation in cutaneous surgery. Their search yielded 1,807 documents with 174 unique legal claims involving injury from a cutaneous laser treatment, from 1985 to 2012. The most common litigated procedures were laser hair removal, rejuvenation (mostly related to intense pulsed-light treatments), and laser treatment of leg veins, while the most common injuries sustained were burns, scars, and pigmentary changes. The most common causes of legal action were lack of informed consent and fraud.
Among the 120 cases with public decisions, cases favored the plaintiff 51% of the time. “That’s unusual,” said Dr. Avram, president of American Society for Dermatologic Surgery. “Usually, physicians do better, but I think the fact that they’re cosmetic cases probably shades things a little bit.” The median monetary award was $350,000 and ranged from $5,000 to $2,145,000. The two largest judgments were for improper use of topical anesthesia that led to deaths of patients in laser hair removal cases.
In a separate analysis, the same authors searched an online national database to identify the incidence of medical professional liability claims resulting from cutaneous laser surgery performed by nonphysician operators (NPOs) from 1999 to 2012. Among the 175 cases identified, 43% involved an NPO. “In fact, the cases involving NPOs exploded over a 4-year period; they grew from 36% in 2008 of cases to 78% in 2011,” Dr. Avram said. “This was even more true for laser hair removal.”
The practice setting turned out to be a factor. Only 23% of NPO litigation involving laser procedures arose in medical office settings, while 77% of cases involving NPOs were performed outside of traditional medical settings such as in salons and medical spas – mostly for laser hair removal. “We updated this information by examining the setting for nonphysician operator litigation between 2012 and 2017 and found that 66% of cases involving NPOs were performed outside of a traditional medical setting, while 34% of NPO litigation arose in medical office settings,” Dr. Avram said during the meeting, which was named What’s the Truth? and sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s close to a 2 to 1 ratio.”
In an analysis of medical professional liability claims involving Mohs surgery from 1989 to 2011, 26 of the 42 cases identified involved a primary defendant who was not a Mohs surgeon. In the 26 cases, the most common reasons for lawsuits were failure or delay of diagnosis of a skin cancer, cosmetic outcome issues, lack of informed consent, and delay or failure to refer to a Mohs surgeon. Of the cases that involved Mohs surgeons, the most common causes were lack of proper informed consent and cosmetic outcome issues, but “these cases were overwhelmingly decided in favor of the surgeons,” said Dr. Avram, one of the study authors.
On a related note, Dr. Avram underscored the importance of biopsy-site photography, “because patients and physicians misidentify biopsy sites too commonly,” he said. In a single-center study of 34 biopsy sites of cutaneous head and neck malignancies, patients misidentified the biopsy site 4-7 weeks out in 29% of the cases. Blinded dermatologists and the patient misidentified the biopsy site in 12% of the cases. “Good biopsy site photography should be mandatory in your practice,” he advised.
Clinicians can avoid cutaneous laser surgery complications only by not treating patients. “Complications and side effects are inevitable; you need to know your limits,” he said. “Even in skilled hands, if you treat enough patients, you will encounter challenging side effects. Do not perform a procedure that might produce a side effect that you cannot recognize and treat.”
The best way to avoid complications is to trust your eyes – not the laser – since the same device made by the same manufacturer may produce highly different outputs at the same setting (see J Am Acad Dermatol. 2016;74[5]:807-19).
“Moreover, lasers can produce much different energies after they have been serviced,” Dr. Avram said. “Do not memorize settings. Do not blindly replicate recommended settings from a colleague or a device manufacturer,” he advised. “Some devices are not externally calibrated. Therefore, the settings on one device may not translate the same way to yours. Often, device manufacturers underplay the settings. Safe and unsafe laser endpoints and close observation are the best means to avoiding clinical complications. That means you follow clinical endpoints, not fluences. The key clinical finding is the endpoint, not the energy setting.”
Temporary and expected side effects include erythema, edema, and purpura. “With these it’s just handholding and unlikely to lead to any legal consequences,” he continued. “With temporary hyperpigmentation that can occur with laser hair removal, time is one your side, because typically this will resolve before any litigation progresses. Permanent side effects from lasers and light sources and injectables are a different issue, things like permanent hypopigmentation, depigmentation, and scarring. These are most likely to produce liability.”
In Dr. Avram’s opinion, complications are best handled with widespread communication. “There is a temptation to avoid a patient with a poor outcome or side effect,” he said. “This is bad medicine and rightfully angers your patient and increases the risk of a lawsuit. [Resist] the temptation to avoid showing a poor outcome to a colleague. Many complications can be significantly improved or cleared with timely and appropriate interventions. You should always document your efforts.”
Dr. Avram disclosed that he has received consulting fees from Allergan and Galderma. He is a member of the scientific advisory board for Allergan and Soliton, is an investigator for Endo, and holds stock options in La Jolla NanoMedical Inc.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Cannabis use common for MS-related spasticity
, new research suggests. Findings from a survey conducted through a large registry in 2020 showed that 31% of patients with MS reported trying cannabis to treat their symptoms – and 20% reported regular use.
Spasticity was reported by 80% as the reason why they used cannabis, while pain was cited as the reason by 69% and sleep problems/insomnia was cited by 61%.
Investigators noted that the new data reflect the latest patterns of use amid sweeping changes in recreational and medical marijuana laws.
“Interest in the use of cannabis for managing MS symptoms continues to increase as more data become available and access becomes easier,” co-investigator Amber Salter, PhD, associate professor, UT Southwestern Medical Center, Dallas, told attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Administration routes vary
The survey was conducted through the longitudinal North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, a voluntary, self-report registry for patients with MS. Of 6,934 registry participants invited to participate, 3,249 (47%) responded. The majority of responders were women (79%) and the mean age was 61 years. About 63% were being treated with disease-modifying therapies.
Overall, 31% of respondents reported having used cannabis to treat their MS symptoms. In addition, 20% reported regular current cannabis use, with an average use of 20 days in the past month. As many as 40% of the current users reported using cannabis daily.
“In general we saw some small differences in current users, who tended to include more males; have higher spasticity, pain, and sleep symptoms; and [were] more likely to be unemployed and younger,” Dr. Salter said.
The most common forms of cannabis administration were smoking (33%) and eating (20%). In addition, 12% reported vaporizing cannabis with a highly concentrated material, 11% administered cannabis sublingually, and 11% reported swallowing it.
Further, 8% reported vaporizing cannabis as a dried flower, 5% used it topically, and 1% reported drinking it.
Of note, the definition of “cannabis/marijuana” in the study excluded hemp cannabidiol (CBD) or products marketed as CBD only.
Consistent use
The most common reason for use by far was spasticity (80%). This was followed by for pain (69%) and sleep/insomnia problems (61%). Among users, 37% reported doing so to treat all three of those problems.
Regarding other symptoms, 36% used cannabis for anxiety, 24% for depression, 18% for overactive bladder, 17% for nausea or gastrointestinal problems, 16% for migraine or headaches, 14% for tremors, and 6% for other purposes.
The vast majority (95%) reported cannabis to be very or somewhat helpful for their symptoms.
Among the 69% of respondents who reported not using cannabis for their MS symptoms, the most commonly cited reasons were a lack of evidence on efficacy (40%) or safety (27%), concerns of legality (25%), lack of insurance coverage (22%), prohibitive cost (18%), and adverse side effects.
Surprisingly, the dramatic shift in the legalization of cannabis use in many states does not appear to be reflected in changes in cannabis use for MS, Dr. Salter said.
“We conducted an anonymous NARCOMS survey a couple of years prior to this survey, and our results are generally consistent. There’s been a small increase in the use and an acceptance or willingness to consider cannabis, but it’s relatively consistent,” she said.
“Despite the changes in access, the landscape hasn’t really changed very much in terms of evidence of the effects on MS symptoms, so that could be why,” Dr. Salter added.
Most patients appear to feel comfortable discussing their cannabis use with their physician, with 75% reporting doing so. However, the most common primary source of medical guidance for treating MS with cannabis was “nobody/self”; for 20%, the source for medical guidance was a dispensary professional.
As many as 62% of respondents reported obtaining their cannabis products from dispensaries, while other sources included family/friend (18%) or an acquaintance (13%). About 31% reported their most preferred type of cannabis to be equal parts THC and cannabidiol, while 30% preferred high THC/low cannabidiol (30%).
Mirrors clinical practice findings
Commenting on the study, Laura T. Safar, MD, vice chair of Psychiatry at Lahey Hospital and Medical Center and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings generally fall in line with cannabis use among patients with MS in her practice.
“This is [consistent] with my general experience: A high percentage of my patients with MS are using cannabis with the goal of addressing their MS symptoms that way,” said Dr. Safar, who was not involved with the research.
One notable recent change in patients’ inquiries about cannabis is their apparent confidence in the information they’re getting, she noted. This is a sign of the ever-expanding sources of information – but from sources who may or may not have an understanding of effects in MS, she added.
“What seems new is a certain level of specificity in the information patients state – regardless of its accuracy. There is more technical information widely available about cannabis online and in the dispensaries,” said Dr. Safar.
“A lot of that information may not have been tested scientifically, but it is presented with an aura of truth,” she said.
While misconceptions about cannabis use in MS may not be new, “the conviction with which they are stated and believed seems stronger,” even though they have been validated by questionably expert sources, Dr. Safar noted.
She pointed out that psychiatric effects are among her patients’ notable concerns of cannabis use in MS.
“Cannabis use, especially daily use in moderate to large amounts, can have negative cognitive side effects,” she said. “In addition, it can have other psychiatric side effects: worsening of mood and anxiety, apathy, and anhedonia, a lack of pleasure or enjoyment, and a flattening of the emotional experience.”
Countering misinformation
Dr. Safar said she works to counter misinformation and provide more reliable, evidence-based recommendations.
“I educate my patients about what we know from scientific trials about the potential benefits, including possible help with pain, excluding central pain, and with spasticity,” she said. Dr. Safar added that she also discusses possible risks, such as worsening of cognition, mood, and anxiety.
On the basis of an individual’s presentation, and working in collaboration with their neurologist as appropriate, Dr. Safar said she discusses the following issues with the patient:
- Does cannabis make sense for the symptoms being presented?
- Has the patient received benefit so far?
- Are there side effects they may be experiencing?
- Would it be appropriate to lower the cannabis dose/frequency of its use?
- If a patient is using cannabis with an objective that is not backed up by the literature, such as depression, are they open to information about other treatment options?
The study was sponsored by GW Research. Dr. Salter has conducted research for GW Pharmaceuticals companies. Dr. Safar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Findings from a survey conducted through a large registry in 2020 showed that 31% of patients with MS reported trying cannabis to treat their symptoms – and 20% reported regular use.
Spasticity was reported by 80% as the reason why they used cannabis, while pain was cited as the reason by 69% and sleep problems/insomnia was cited by 61%.
Investigators noted that the new data reflect the latest patterns of use amid sweeping changes in recreational and medical marijuana laws.
“Interest in the use of cannabis for managing MS symptoms continues to increase as more data become available and access becomes easier,” co-investigator Amber Salter, PhD, associate professor, UT Southwestern Medical Center, Dallas, told attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Administration routes vary
The survey was conducted through the longitudinal North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, a voluntary, self-report registry for patients with MS. Of 6,934 registry participants invited to participate, 3,249 (47%) responded. The majority of responders were women (79%) and the mean age was 61 years. About 63% were being treated with disease-modifying therapies.
Overall, 31% of respondents reported having used cannabis to treat their MS symptoms. In addition, 20% reported regular current cannabis use, with an average use of 20 days in the past month. As many as 40% of the current users reported using cannabis daily.
“In general we saw some small differences in current users, who tended to include more males; have higher spasticity, pain, and sleep symptoms; and [were] more likely to be unemployed and younger,” Dr. Salter said.
The most common forms of cannabis administration were smoking (33%) and eating (20%). In addition, 12% reported vaporizing cannabis with a highly concentrated material, 11% administered cannabis sublingually, and 11% reported swallowing it.
Further, 8% reported vaporizing cannabis as a dried flower, 5% used it topically, and 1% reported drinking it.
Of note, the definition of “cannabis/marijuana” in the study excluded hemp cannabidiol (CBD) or products marketed as CBD only.
Consistent use
The most common reason for use by far was spasticity (80%). This was followed by for pain (69%) and sleep/insomnia problems (61%). Among users, 37% reported doing so to treat all three of those problems.
Regarding other symptoms, 36% used cannabis for anxiety, 24% for depression, 18% for overactive bladder, 17% for nausea or gastrointestinal problems, 16% for migraine or headaches, 14% for tremors, and 6% for other purposes.
The vast majority (95%) reported cannabis to be very or somewhat helpful for their symptoms.
Among the 69% of respondents who reported not using cannabis for their MS symptoms, the most commonly cited reasons were a lack of evidence on efficacy (40%) or safety (27%), concerns of legality (25%), lack of insurance coverage (22%), prohibitive cost (18%), and adverse side effects.
Surprisingly, the dramatic shift in the legalization of cannabis use in many states does not appear to be reflected in changes in cannabis use for MS, Dr. Salter said.
“We conducted an anonymous NARCOMS survey a couple of years prior to this survey, and our results are generally consistent. There’s been a small increase in the use and an acceptance or willingness to consider cannabis, but it’s relatively consistent,” she said.
“Despite the changes in access, the landscape hasn’t really changed very much in terms of evidence of the effects on MS symptoms, so that could be why,” Dr. Salter added.
Most patients appear to feel comfortable discussing their cannabis use with their physician, with 75% reporting doing so. However, the most common primary source of medical guidance for treating MS with cannabis was “nobody/self”; for 20%, the source for medical guidance was a dispensary professional.
As many as 62% of respondents reported obtaining their cannabis products from dispensaries, while other sources included family/friend (18%) or an acquaintance (13%). About 31% reported their most preferred type of cannabis to be equal parts THC and cannabidiol, while 30% preferred high THC/low cannabidiol (30%).
Mirrors clinical practice findings
Commenting on the study, Laura T. Safar, MD, vice chair of Psychiatry at Lahey Hospital and Medical Center and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings generally fall in line with cannabis use among patients with MS in her practice.
“This is [consistent] with my general experience: A high percentage of my patients with MS are using cannabis with the goal of addressing their MS symptoms that way,” said Dr. Safar, who was not involved with the research.
One notable recent change in patients’ inquiries about cannabis is their apparent confidence in the information they’re getting, she noted. This is a sign of the ever-expanding sources of information – but from sources who may or may not have an understanding of effects in MS, she added.
“What seems new is a certain level of specificity in the information patients state – regardless of its accuracy. There is more technical information widely available about cannabis online and in the dispensaries,” said Dr. Safar.
“A lot of that information may not have been tested scientifically, but it is presented with an aura of truth,” she said.
While misconceptions about cannabis use in MS may not be new, “the conviction with which they are stated and believed seems stronger,” even though they have been validated by questionably expert sources, Dr. Safar noted.
She pointed out that psychiatric effects are among her patients’ notable concerns of cannabis use in MS.
“Cannabis use, especially daily use in moderate to large amounts, can have negative cognitive side effects,” she said. “In addition, it can have other psychiatric side effects: worsening of mood and anxiety, apathy, and anhedonia, a lack of pleasure or enjoyment, and a flattening of the emotional experience.”
Countering misinformation
Dr. Safar said she works to counter misinformation and provide more reliable, evidence-based recommendations.
“I educate my patients about what we know from scientific trials about the potential benefits, including possible help with pain, excluding central pain, and with spasticity,” she said. Dr. Safar added that she also discusses possible risks, such as worsening of cognition, mood, and anxiety.
On the basis of an individual’s presentation, and working in collaboration with their neurologist as appropriate, Dr. Safar said she discusses the following issues with the patient:
- Does cannabis make sense for the symptoms being presented?
- Has the patient received benefit so far?
- Are there side effects they may be experiencing?
- Would it be appropriate to lower the cannabis dose/frequency of its use?
- If a patient is using cannabis with an objective that is not backed up by the literature, such as depression, are they open to information about other treatment options?
The study was sponsored by GW Research. Dr. Salter has conducted research for GW Pharmaceuticals companies. Dr. Safar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Findings from a survey conducted through a large registry in 2020 showed that 31% of patients with MS reported trying cannabis to treat their symptoms – and 20% reported regular use.
Spasticity was reported by 80% as the reason why they used cannabis, while pain was cited as the reason by 69% and sleep problems/insomnia was cited by 61%.
Investigators noted that the new data reflect the latest patterns of use amid sweeping changes in recreational and medical marijuana laws.
“Interest in the use of cannabis for managing MS symptoms continues to increase as more data become available and access becomes easier,” co-investigator Amber Salter, PhD, associate professor, UT Southwestern Medical Center, Dallas, told attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Administration routes vary
The survey was conducted through the longitudinal North American Research Committee on Multiple Sclerosis (NARCOMS) Registry, a voluntary, self-report registry for patients with MS. Of 6,934 registry participants invited to participate, 3,249 (47%) responded. The majority of responders were women (79%) and the mean age was 61 years. About 63% were being treated with disease-modifying therapies.
Overall, 31% of respondents reported having used cannabis to treat their MS symptoms. In addition, 20% reported regular current cannabis use, with an average use of 20 days in the past month. As many as 40% of the current users reported using cannabis daily.
“In general we saw some small differences in current users, who tended to include more males; have higher spasticity, pain, and sleep symptoms; and [were] more likely to be unemployed and younger,” Dr. Salter said.
The most common forms of cannabis administration were smoking (33%) and eating (20%). In addition, 12% reported vaporizing cannabis with a highly concentrated material, 11% administered cannabis sublingually, and 11% reported swallowing it.
Further, 8% reported vaporizing cannabis as a dried flower, 5% used it topically, and 1% reported drinking it.
Of note, the definition of “cannabis/marijuana” in the study excluded hemp cannabidiol (CBD) or products marketed as CBD only.
Consistent use
The most common reason for use by far was spasticity (80%). This was followed by for pain (69%) and sleep/insomnia problems (61%). Among users, 37% reported doing so to treat all three of those problems.
Regarding other symptoms, 36% used cannabis for anxiety, 24% for depression, 18% for overactive bladder, 17% for nausea or gastrointestinal problems, 16% for migraine or headaches, 14% for tremors, and 6% for other purposes.
The vast majority (95%) reported cannabis to be very or somewhat helpful for their symptoms.
Among the 69% of respondents who reported not using cannabis for their MS symptoms, the most commonly cited reasons were a lack of evidence on efficacy (40%) or safety (27%), concerns of legality (25%), lack of insurance coverage (22%), prohibitive cost (18%), and adverse side effects.
Surprisingly, the dramatic shift in the legalization of cannabis use in many states does not appear to be reflected in changes in cannabis use for MS, Dr. Salter said.
“We conducted an anonymous NARCOMS survey a couple of years prior to this survey, and our results are generally consistent. There’s been a small increase in the use and an acceptance or willingness to consider cannabis, but it’s relatively consistent,” she said.
“Despite the changes in access, the landscape hasn’t really changed very much in terms of evidence of the effects on MS symptoms, so that could be why,” Dr. Salter added.
Most patients appear to feel comfortable discussing their cannabis use with their physician, with 75% reporting doing so. However, the most common primary source of medical guidance for treating MS with cannabis was “nobody/self”; for 20%, the source for medical guidance was a dispensary professional.
As many as 62% of respondents reported obtaining their cannabis products from dispensaries, while other sources included family/friend (18%) or an acquaintance (13%). About 31% reported their most preferred type of cannabis to be equal parts THC and cannabidiol, while 30% preferred high THC/low cannabidiol (30%).
Mirrors clinical practice findings
Commenting on the study, Laura T. Safar, MD, vice chair of Psychiatry at Lahey Hospital and Medical Center and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings generally fall in line with cannabis use among patients with MS in her practice.
“This is [consistent] with my general experience: A high percentage of my patients with MS are using cannabis with the goal of addressing their MS symptoms that way,” said Dr. Safar, who was not involved with the research.
One notable recent change in patients’ inquiries about cannabis is their apparent confidence in the information they’re getting, she noted. This is a sign of the ever-expanding sources of information – but from sources who may or may not have an understanding of effects in MS, she added.
“What seems new is a certain level of specificity in the information patients state – regardless of its accuracy. There is more technical information widely available about cannabis online and in the dispensaries,” said Dr. Safar.
“A lot of that information may not have been tested scientifically, but it is presented with an aura of truth,” she said.
While misconceptions about cannabis use in MS may not be new, “the conviction with which they are stated and believed seems stronger,” even though they have been validated by questionably expert sources, Dr. Safar noted.
She pointed out that psychiatric effects are among her patients’ notable concerns of cannabis use in MS.
“Cannabis use, especially daily use in moderate to large amounts, can have negative cognitive side effects,” she said. “In addition, it can have other psychiatric side effects: worsening of mood and anxiety, apathy, and anhedonia, a lack of pleasure or enjoyment, and a flattening of the emotional experience.”
Countering misinformation
Dr. Safar said she works to counter misinformation and provide more reliable, evidence-based recommendations.
“I educate my patients about what we know from scientific trials about the potential benefits, including possible help with pain, excluding central pain, and with spasticity,” she said. Dr. Safar added that she also discusses possible risks, such as worsening of cognition, mood, and anxiety.
On the basis of an individual’s presentation, and working in collaboration with their neurologist as appropriate, Dr. Safar said she discusses the following issues with the patient:
- Does cannabis make sense for the symptoms being presented?
- Has the patient received benefit so far?
- Are there side effects they may be experiencing?
- Would it be appropriate to lower the cannabis dose/frequency of its use?
- If a patient is using cannabis with an objective that is not backed up by the literature, such as depression, are they open to information about other treatment options?
The study was sponsored by GW Research. Dr. Salter has conducted research for GW Pharmaceuticals companies. Dr. Safar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021
Novel bronchoscopic interventions appear promising for patients with COPD
Several emerging bronchoscopic treatments have the potential to improve the quality of life for patients with chronic obstructive pulmonary disease, an investigator reported at the annual meeting of the American College of Chest Physicians.
Targeted lung denervation is one promising novel therapeutic option that is safe and may improve clinical outcomes according to investigator Christian Ghattas, MD.
Data from an ongoing phase 3 randomized controlled trial may provide new information on the efficacy of targeted lung denervation in patients with chronic obstructive pulmonary disease (COPD), said Dr. Ghattas, assistant professor of medicine and associate program director for the interventional pulmonary fellowship at The Ohio State University Medical Center in Columbus.
“Outcome data of longer follow-up on previously treated patients will provide us with more information on the durability and the effect of this treatment,” Dr. Ghattas said in an online presentation at the CHEST meeting, which was held virtually this year.
Meanwhile, a few compelling bronchoscopic treatment modalities for patients with chronic bronchitis are in earlier stages of clinical development. “Larger randomized, controlled trials are ongoing to confirm the available data and to evaluate treatment durability,” said Dr. Ghattas.
Targeted lung denervation
The targeted lung denervation system under study (dNerva®, Nuvaira Inc.) involves the use of a radiofrequency catheter to ablate the peribronchial branches of the vagus nerve, Dr. Ghattas said.
The goal of disrupting pulmonary nerve input is to achieve sustained bronchodilation and reduce mucous secretion, thereby simulating the effect of anticholinergic drugs, he added.
In pilot studies, the targeted lung denervation system demonstrated its feasibility and safety, while modifications to the system reduced the rate of serious adverse events, according to Dr. Ghattas.
In the AIRFLOW-1 study, which evaluated the safety of the latest generation version of the system, 30 patients with COPD were randomized to targeted lung denervation at one of two doses, 29 or 32 watts.
Of those 30 patients, 29 (96.7%) had procedural success, meaning the catheter was inserted, guided to its intended location, and removed intact with no reported in-hospital serious adverse events, according to results published in Respiration.
There was no difference between arms in the primary endpoint, which was the rate of adverse airway effects requiring intervention that were associated with targeted lung denervation, investigators reported. Four such events occurred, in 3 of 15 patients treated with 32 watts and 1 of 15 patients treated with 29 watts.
Procedural success, defined as device success without an in-hospital serious adverse event, was 96.7% (29/30). The rate of TLD-associated adverse airway effects requiring intervention was 3/15 in the 32 W versus 1/15 in the 29 W group (P = .6). However, serious gastric events were noted in five patients, prompting safety improvements and procedural enhancements that reduced both gastrointestinal and airway events, according to the study report.
Further data are available from AIRFLOW-2, a randomized, sham-controlled trial enrolling patients with symptomatic COPD.
In that study, targeted lung denervation plus optimal drug treatment led to fewer respiratory adverse events of interest, including hospitalizations for COPD exacerbation, according to a report on the study that appears in The American Journal of Respiratory and Critical Care Medicine.
Respiratory adverse events occurred in 32% of treated patients versus 71% of sham-treated patients in a predefined 3- to 6.5-month postprocedure window, the report says.
Currently underway is AIRFLOW-3, a randomized study of targeted lung denervation versus sham procedure in patients with COPD. The study has a primary outcome measure of moderate or severe COPD exacerbations over 12 months and is slated to enroll 480 patients.
To be eligible for AIRFLOW-3, patients must have had at least two moderate or one severe COPD exacerbation in the previous year, Dr. Ghattas said.
Metered cryospray
One novel intervention with the potential to benefit patients with chronic bronchitis is metered cryospray (RejuvenAir), which works by delivering liquid nitrogen to the tracheobronchial airways, according to Dr. Ghattas.
This targeted delivery ablates abnormal epithelium, facilitating the regeneration of healthy mucosa, according to investigators in a recently published single-arm prospective trial.
Metered cryospray was safe, feasible, and linked to clinically meaningful improvements in patient-reported outcomes among patients with COPD, according to authors of the study, which appears in the European Respiratory Journal.
In the study, 34 of 35 participants received three treatments 4-6 weeks apart.
Investigators reported that at 3 months there were significant reductions in the COPD Assessment Test that were durable to 6 months, and changes in the St. George’s Respiratory Questionnaire and the Leicester Cough Questionnaire that were durable to 9 months.
There were 14 serious adverse events, none of which were device- or procedure related, according to investigators.
An ongoing randomized study called SPRAY-CB is comparing metered cryospray to sham procedure in an anticipated 210 patients with COPD with chronic bronchitis.
Bronchial rheoplasty
Bronchial rheoplasty (RheOx, Gala Therapeutics), is another promising intervention under investigation for the treatment of chronic bronchitis, according to Dr. Ghattas.
This system delivers nonthermal pulsed electrical energy, Dr. Ghattas said, with the intention of ablating goblet cells in the airways.
“The preclinical studies have demonstrated epithelial ablation, followed by regeneration of normalized epithelium,” he said in his presentation.
In 12-month results of multicenter clinical trial, bronchial rheoplasty was technically feasible and safe, with reductions in goblet cell hyperplasia and changes in patient-reported quality of life seen following the procedure, investigators reported in The American Journal of Respiratory and Critical Care Medicine.
The mean goblet cell hyperplasia score was reduced by 39% from baseline to treatment, according to study results. Four procedure-related serious adverse events were observed through 6 months, and there were no procedure- or device-related serious adverse events over the next 6 months. Mild hemoptysis occurred in 47% of patients, investigators reported.
A larger randomized, double-blind prospective trial with a sham control arm is underway and will include 270 patients, according to Dr. Ghattas. “We’re going to have to wait for the results,” he said.
Dr. Ghattas reported serving as a site principal investigator for clinical trials involving the bronchoscopic interventions he discussed, including AIRFLOW-3 (evaluating the targeted lung denervation system), SPRAY-CB (metered cryospray), and RheSolve (bronchial rheoplasty).
Several emerging bronchoscopic treatments have the potential to improve the quality of life for patients with chronic obstructive pulmonary disease, an investigator reported at the annual meeting of the American College of Chest Physicians.
Targeted lung denervation is one promising novel therapeutic option that is safe and may improve clinical outcomes according to investigator Christian Ghattas, MD.
Data from an ongoing phase 3 randomized controlled trial may provide new information on the efficacy of targeted lung denervation in patients with chronic obstructive pulmonary disease (COPD), said Dr. Ghattas, assistant professor of medicine and associate program director for the interventional pulmonary fellowship at The Ohio State University Medical Center in Columbus.
“Outcome data of longer follow-up on previously treated patients will provide us with more information on the durability and the effect of this treatment,” Dr. Ghattas said in an online presentation at the CHEST meeting, which was held virtually this year.
Meanwhile, a few compelling bronchoscopic treatment modalities for patients with chronic bronchitis are in earlier stages of clinical development. “Larger randomized, controlled trials are ongoing to confirm the available data and to evaluate treatment durability,” said Dr. Ghattas.
Targeted lung denervation
The targeted lung denervation system under study (dNerva®, Nuvaira Inc.) involves the use of a radiofrequency catheter to ablate the peribronchial branches of the vagus nerve, Dr. Ghattas said.
The goal of disrupting pulmonary nerve input is to achieve sustained bronchodilation and reduce mucous secretion, thereby simulating the effect of anticholinergic drugs, he added.
In pilot studies, the targeted lung denervation system demonstrated its feasibility and safety, while modifications to the system reduced the rate of serious adverse events, according to Dr. Ghattas.
In the AIRFLOW-1 study, which evaluated the safety of the latest generation version of the system, 30 patients with COPD were randomized to targeted lung denervation at one of two doses, 29 or 32 watts.
Of those 30 patients, 29 (96.7%) had procedural success, meaning the catheter was inserted, guided to its intended location, and removed intact with no reported in-hospital serious adverse events, according to results published in Respiration.
There was no difference between arms in the primary endpoint, which was the rate of adverse airway effects requiring intervention that were associated with targeted lung denervation, investigators reported. Four such events occurred, in 3 of 15 patients treated with 32 watts and 1 of 15 patients treated with 29 watts.
Procedural success, defined as device success without an in-hospital serious adverse event, was 96.7% (29/30). The rate of TLD-associated adverse airway effects requiring intervention was 3/15 in the 32 W versus 1/15 in the 29 W group (P = .6). However, serious gastric events were noted in five patients, prompting safety improvements and procedural enhancements that reduced both gastrointestinal and airway events, according to the study report.
Further data are available from AIRFLOW-2, a randomized, sham-controlled trial enrolling patients with symptomatic COPD.
In that study, targeted lung denervation plus optimal drug treatment led to fewer respiratory adverse events of interest, including hospitalizations for COPD exacerbation, according to a report on the study that appears in The American Journal of Respiratory and Critical Care Medicine.
Respiratory adverse events occurred in 32% of treated patients versus 71% of sham-treated patients in a predefined 3- to 6.5-month postprocedure window, the report says.
Currently underway is AIRFLOW-3, a randomized study of targeted lung denervation versus sham procedure in patients with COPD. The study has a primary outcome measure of moderate or severe COPD exacerbations over 12 months and is slated to enroll 480 patients.
To be eligible for AIRFLOW-3, patients must have had at least two moderate or one severe COPD exacerbation in the previous year, Dr. Ghattas said.
Metered cryospray
One novel intervention with the potential to benefit patients with chronic bronchitis is metered cryospray (RejuvenAir), which works by delivering liquid nitrogen to the tracheobronchial airways, according to Dr. Ghattas.
This targeted delivery ablates abnormal epithelium, facilitating the regeneration of healthy mucosa, according to investigators in a recently published single-arm prospective trial.
Metered cryospray was safe, feasible, and linked to clinically meaningful improvements in patient-reported outcomes among patients with COPD, according to authors of the study, which appears in the European Respiratory Journal.
In the study, 34 of 35 participants received three treatments 4-6 weeks apart.
Investigators reported that at 3 months there were significant reductions in the COPD Assessment Test that were durable to 6 months, and changes in the St. George’s Respiratory Questionnaire and the Leicester Cough Questionnaire that were durable to 9 months.
There were 14 serious adverse events, none of which were device- or procedure related, according to investigators.
An ongoing randomized study called SPRAY-CB is comparing metered cryospray to sham procedure in an anticipated 210 patients with COPD with chronic bronchitis.
Bronchial rheoplasty
Bronchial rheoplasty (RheOx, Gala Therapeutics), is another promising intervention under investigation for the treatment of chronic bronchitis, according to Dr. Ghattas.
This system delivers nonthermal pulsed electrical energy, Dr. Ghattas said, with the intention of ablating goblet cells in the airways.
“The preclinical studies have demonstrated epithelial ablation, followed by regeneration of normalized epithelium,” he said in his presentation.
In 12-month results of multicenter clinical trial, bronchial rheoplasty was technically feasible and safe, with reductions in goblet cell hyperplasia and changes in patient-reported quality of life seen following the procedure, investigators reported in The American Journal of Respiratory and Critical Care Medicine.
The mean goblet cell hyperplasia score was reduced by 39% from baseline to treatment, according to study results. Four procedure-related serious adverse events were observed through 6 months, and there were no procedure- or device-related serious adverse events over the next 6 months. Mild hemoptysis occurred in 47% of patients, investigators reported.
A larger randomized, double-blind prospective trial with a sham control arm is underway and will include 270 patients, according to Dr. Ghattas. “We’re going to have to wait for the results,” he said.
Dr. Ghattas reported serving as a site principal investigator for clinical trials involving the bronchoscopic interventions he discussed, including AIRFLOW-3 (evaluating the targeted lung denervation system), SPRAY-CB (metered cryospray), and RheSolve (bronchial rheoplasty).
Several emerging bronchoscopic treatments have the potential to improve the quality of life for patients with chronic obstructive pulmonary disease, an investigator reported at the annual meeting of the American College of Chest Physicians.
Targeted lung denervation is one promising novel therapeutic option that is safe and may improve clinical outcomes according to investigator Christian Ghattas, MD.
Data from an ongoing phase 3 randomized controlled trial may provide new information on the efficacy of targeted lung denervation in patients with chronic obstructive pulmonary disease (COPD), said Dr. Ghattas, assistant professor of medicine and associate program director for the interventional pulmonary fellowship at The Ohio State University Medical Center in Columbus.
“Outcome data of longer follow-up on previously treated patients will provide us with more information on the durability and the effect of this treatment,” Dr. Ghattas said in an online presentation at the CHEST meeting, which was held virtually this year.
Meanwhile, a few compelling bronchoscopic treatment modalities for patients with chronic bronchitis are in earlier stages of clinical development. “Larger randomized, controlled trials are ongoing to confirm the available data and to evaluate treatment durability,” said Dr. Ghattas.
Targeted lung denervation
The targeted lung denervation system under study (dNerva®, Nuvaira Inc.) involves the use of a radiofrequency catheter to ablate the peribronchial branches of the vagus nerve, Dr. Ghattas said.
The goal of disrupting pulmonary nerve input is to achieve sustained bronchodilation and reduce mucous secretion, thereby simulating the effect of anticholinergic drugs, he added.
In pilot studies, the targeted lung denervation system demonstrated its feasibility and safety, while modifications to the system reduced the rate of serious adverse events, according to Dr. Ghattas.
In the AIRFLOW-1 study, which evaluated the safety of the latest generation version of the system, 30 patients with COPD were randomized to targeted lung denervation at one of two doses, 29 or 32 watts.
Of those 30 patients, 29 (96.7%) had procedural success, meaning the catheter was inserted, guided to its intended location, and removed intact with no reported in-hospital serious adverse events, according to results published in Respiration.
There was no difference between arms in the primary endpoint, which was the rate of adverse airway effects requiring intervention that were associated with targeted lung denervation, investigators reported. Four such events occurred, in 3 of 15 patients treated with 32 watts and 1 of 15 patients treated with 29 watts.
Procedural success, defined as device success without an in-hospital serious adverse event, was 96.7% (29/30). The rate of TLD-associated adverse airway effects requiring intervention was 3/15 in the 32 W versus 1/15 in the 29 W group (P = .6). However, serious gastric events were noted in five patients, prompting safety improvements and procedural enhancements that reduced both gastrointestinal and airway events, according to the study report.
Further data are available from AIRFLOW-2, a randomized, sham-controlled trial enrolling patients with symptomatic COPD.
In that study, targeted lung denervation plus optimal drug treatment led to fewer respiratory adverse events of interest, including hospitalizations for COPD exacerbation, according to a report on the study that appears in The American Journal of Respiratory and Critical Care Medicine.
Respiratory adverse events occurred in 32% of treated patients versus 71% of sham-treated patients in a predefined 3- to 6.5-month postprocedure window, the report says.
Currently underway is AIRFLOW-3, a randomized study of targeted lung denervation versus sham procedure in patients with COPD. The study has a primary outcome measure of moderate or severe COPD exacerbations over 12 months and is slated to enroll 480 patients.
To be eligible for AIRFLOW-3, patients must have had at least two moderate or one severe COPD exacerbation in the previous year, Dr. Ghattas said.
Metered cryospray
One novel intervention with the potential to benefit patients with chronic bronchitis is metered cryospray (RejuvenAir), which works by delivering liquid nitrogen to the tracheobronchial airways, according to Dr. Ghattas.
This targeted delivery ablates abnormal epithelium, facilitating the regeneration of healthy mucosa, according to investigators in a recently published single-arm prospective trial.
Metered cryospray was safe, feasible, and linked to clinically meaningful improvements in patient-reported outcomes among patients with COPD, according to authors of the study, which appears in the European Respiratory Journal.
In the study, 34 of 35 participants received three treatments 4-6 weeks apart.
Investigators reported that at 3 months there were significant reductions in the COPD Assessment Test that were durable to 6 months, and changes in the St. George’s Respiratory Questionnaire and the Leicester Cough Questionnaire that were durable to 9 months.
There were 14 serious adverse events, none of which were device- or procedure related, according to investigators.
An ongoing randomized study called SPRAY-CB is comparing metered cryospray to sham procedure in an anticipated 210 patients with COPD with chronic bronchitis.
Bronchial rheoplasty
Bronchial rheoplasty (RheOx, Gala Therapeutics), is another promising intervention under investigation for the treatment of chronic bronchitis, according to Dr. Ghattas.
This system delivers nonthermal pulsed electrical energy, Dr. Ghattas said, with the intention of ablating goblet cells in the airways.
“The preclinical studies have demonstrated epithelial ablation, followed by regeneration of normalized epithelium,” he said in his presentation.
In 12-month results of multicenter clinical trial, bronchial rheoplasty was technically feasible and safe, with reductions in goblet cell hyperplasia and changes in patient-reported quality of life seen following the procedure, investigators reported in The American Journal of Respiratory and Critical Care Medicine.
The mean goblet cell hyperplasia score was reduced by 39% from baseline to treatment, according to study results. Four procedure-related serious adverse events were observed through 6 months, and there were no procedure- or device-related serious adverse events over the next 6 months. Mild hemoptysis occurred in 47% of patients, investigators reported.
A larger randomized, double-blind prospective trial with a sham control arm is underway and will include 270 patients, according to Dr. Ghattas. “We’re going to have to wait for the results,” he said.
Dr. Ghattas reported serving as a site principal investigator for clinical trials involving the bronchoscopic interventions he discussed, including AIRFLOW-3 (evaluating the targeted lung denervation system), SPRAY-CB (metered cryospray), and RheSolve (bronchial rheoplasty).
FROM CHEST 2021
New consensus guideline on clinical MRI use in MS
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
The guideline represents a collaboration between the Consortium of Multiple Sclerosis Centers, the European-based Magnetic Resonance Imaging in Multiple Sclerosis, and North American Imaging in Multiple Sclerosis.
Among its recommendations for improving diagnosis and management of MS is the establishment of much-needed ways to boost protocol adherence. “The key part of these recommendations that we want to emphasize is how important it is for them to be used,” said David Li, MD, University of British Columbia, Vancouver, and cochair of the MRI guideline committee.
Dr. Li noted that there was a widespread lack of adherence among MRI centers to compliance with the 2018 CMSC guidelines in imaging for MS. This potentially compromised clinicians’ ability to identify lesions that allow for earlier and confident diagnoses and to monitor for disease changes that may necessitate the initiation or change of therapy, he said.
“The key to being able to know that brain changes have occurred in patients over time is to have scans that have been performed using standardized protocols – to be certain that the change is truly the result of a change in disease activity and progression and not erroneously due to differences resulting from different MRI scanning procedures,” he said to attendees at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The guideline was also published this summer as a position paper in Lancet Neurology.
Key recommendations
The new guideline covers a broad range of imaging topics, with key areas of focus including the use of three-dimensional imaging, when and when not to use gadolinium contrast, and spinal cord imaging.
For example, a 3 Tesla magnet strength is preferred when imaging the brain with MRI because of its increased sensitivity for detecting lesions – but a minimum magnet strength of at least 1.5 T can also be used. For the spinal cord, there is no advantage of 3 T over 1.5 T, the guideline notes.
Other recommendations include:
- Core sequences for the brain should include sagittal and axial T2-weighted 3D fluid-attenuated inversion recovery (FLAIR), along with axial T2-weighted and diffusion-weighted sequences.
- 3D acquisition, which is now available on most scanners, is preferable to 2D acquisitions.
- Use of the subcallosal plane for consistent and reproducible alignment of axial scans is again emphasized, as it allows for easier and more confident comparison of follow-up studies to detect changes over time.
- At least two of three sagittal sequences are recommended for spinal cord MRI.
- The judicious use of macrocyclic gadolinium-based contrast agents (GBCA) is reemphasized because of its invaluable role in specific circumstances.
- However, for routine follow-up monitoring for subclinical disease activity, high-quality nonenhanced scans will allow for identification of new or enlarging T2 lesions without the need for GBCA.
- A new baseline brain MRI scan without gadolinium is recommended at least 3 months after treatment initiation, with annual follow-up scans without gadolinium.
For the diagnosis of MS, imaging of the entire spinal cord, as opposed to only the cervical segments, is recommended for the detection of lesions in the lower thoracic spinal segments and conus. However, 1.5-T scans are acceptable in that imaging, as 3-T scans provide no advantage. For routine follow-up monitoring, spinal cord MRI is optional.
“The current guidelines do not recommend routine follow-up spinal cord MRI, as it remains technically challenging and would disproportionately increase the scanning time, however experienced centers have the option to do so as a small number of asymptomatic spinal cord lesions do develop on follow-up,” the authors noted.
“However, follow up spinal cord MRI is recommended in special circumstances, including unexpected disease worsening and the possibility of a diagnosis other than multiple sclerosis,” they added.
Although the central vein sign has gained significant interest as a potential biomarker of inflammatory demyelination to help distinguish between MS and non-MS lesions, the 2021 protocol does not currently recommend imaging for the feature. However, those recommendations may change in future guidelines, the authors noted.
Low protocol adherence
The ongoing lack of adherence to guidelines that has resulted in frustrating inconsistencies in imaging was documented in no less than four studies presented at the meeting. They showed compliance with standard protocols to be strikingly poor.
Among the studies was one presented by Anthony Traboulsee, MD, professor and research chair of the MS Society of Canada, and from the University of British Columbia in Vancouver. Findings showed that only about half of scans acquired in a real-world dataset satisfied 2018 CMSC Standardized Brain MRI recommendations.
“Of note was that all the scans that were compliant were acquired in 3D while none of the 2D-acquired sequences were adherent,” Dr. Li commented.
Another study assessed use of standardized MRI protocols in a pragmatic, multisite MS clinical trial, the Traditional vs. Early Aggressive Therapy in Multiple Sclerosis (TREAT-MS) trial. Results showed that, upon enrollment, only 10% of scans followed CMSC guidelines for all three structural contrasts.
In that study, when the images provided by Johns Hopkins University Medical School were excluded, that figure dropped to 2.75% of remaining scans that met the criteria.
“Despite the importance of standardization of high-quality MRIs for the monitoring of people with MS, adoption of recommended imaging remains low,” the investigators wrote.
Resistance to change?
Commenting on the research and new guideline, Blake E. Dewey, PhD student, department of electrical and computer engineering at Johns Hopkins University, Baltimore, speculated that the noncompliance is often simply a matter of resistance to change.
“There are a number of reasons that are given for the retention of older, noncompliant MRI scans at different institutions, such as timing and patient throughput; but in my mind the issue is institutional inertia,” he said.
“It is difficult in many instances to get the clinician [radiologist] and institutional buy-in to make these kinds of changes across the board,” Mr. Dewey noted.
“The most common protocol that we see acquired is a set of 2D, low-resolution images with gaps between slices. These are simply not sufficient given modern MRI technology and the needs of MS clinicians,” he added.
Importantly, Mr. Dewey noted that, through direct communication with imaging staff and practitioners in the trial, compliance increased substantially – nearly 20-fold, “indicating a real possibility for outreach, including to commonly used outpatient radiology facilities.”
The updated MAGNIMS-CMSC-NAIMS MRI protocol is beneficial in providing “simple, reasonable guidelines that can be easily acquired at almost any imaging location in the U.S., and much of the rest of the world,” he said.
“As imaging researchers, we often reach for more that is needed clinically to properly diagnose and monitor a patient’s disease,” Mr. Dewey added. “This updated protocol has ‘trimmed the fat’ and left some discretion to institutions, which should help with compliance.”
Mr. Dewey said he also encourages imaging professionals to consider performing the sequences described as “optional” as well.
“Some of these are useful in measuring potential biomarkers currently under extensive validation, such as brain volumetrics and the central vein sign, that may help patient populations that are currently underserved by more traditional imaging, such as progressive patients and patients that could be potentially misdiagnosed,” he said.
Spreading the word
In the meantime, as part of its own outreach efforts, the CMSC is providing laminated cards that detail in simplified tables the 2021 updated MRI protocol. This makes it easy for centers to access the information and patients to help improve awareness of the protocol.
“We are urging clinicians to provide the cards to their MS patients and have them present the cards to their imaging center,” Dr. Li said. “This effort could make such an important difference in helping to encourage more to follow the protocol.”
Clinicians and patients alike can download the MRI protocol card from the CMSC website.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021