User login
To vape or not to vape: Is that really a question?
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at pdnews@mdedge.com.
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at pdnews@mdedge.com.
All pediatricians are relieved that the rates of children smoking cigarettes has dropped steadily since 2011. This decline seems to be associated with education on the dangers of cigarettes and fewer parents smoking. Perhaps less modeling of cigarette use in movies (although it increased again from 2010 to 2019) and lawsuits against advertisements targeting children also has helped.
“Whew,” we may have said, “we can relax our efforts to convince children to avoid smoking.” But, as is commonly true in medicine, the next threat was right around the corner – in this case vaping or e-cigarettes, also called vapes, e-hookahs, vape pens, tank systems, mods, and electronic nicotine delivery systems. And the size of the problem is huge – over 20% of high school students report using e-cigarettes – and immediate, as vaping can kill in the short term as well as causing long-term harm.
“E-cigarette, or vaping, product use–associated Lung Injury” – EVALI for short – has killed 68 vapers and hospitalized thousands. EVALI is thought to be caused by a vitamin E acetate additive used when vaping marijuana, particularly from informal sources like friends, family, or in-person or online dealers.
Vaping increases the risk of severe COVID-19 disease
While EVALI deaths dropped in months after being explained, the COVID-19 epidemic is now a much greater threat to vapers. Vaping increases risk of severe COVID-19 disease because of its immediate paralysis of lung cilia. Sharing vape devices and touching one’s lips while using also increase the risk of virus transmission. Vaping and smoking increase the number of ACE2 receptors to which the SARS-CoV-2 virus attaches causing the characteristic cell damage, and suppresses macrophages and neutrophils, resulting in more smokers testing positive, being twice as likely to develop a severe illness and get hospitalized because of pneumonia from COVID-19, and being less likely to recover. Unfortunately, addressing this new threat to the immediate and long-term health of our patients appears to be more complicated than for addressing smoking tobacco. First of all, vaping is much more difficult to detect than smelly cigarettes sending smoke signals from behind the garage or in the school bathrooms. Many, if not most, adults do not recognize the vaping devices when they see them, as many are tiny and some look like computer thumb drives. The aerosol emitted when in use, while containing dangerous toxins, has less odor than tobacco smoke. Vaping equipment and ads have been designed to attract youth, including linking them to sports and music events. Vaping has been advertised as a way to wean off nicotine addiction, a claim that has some scientific evidence in adults, but at a lower dose of nicotine. Warning children about the dangers of marijuana vaping has been made less credible by the rapid expansion of legalization of marijuana around the United States, eliciting “I told you it was fine” reactions from youth. And the person vaping does not know what or how much of the psychoactive components are being delivered into their bodies. One Juul pod, for example, has the equivalent in nicotine of an entire pack of 20 cigarettes. They are highly addictive, especially to the developing brain, such that youth who vape are more likely to become addicted and to smoke cigarettes in the future.
Help from federal regulation has been weak
While all 50 states ban sales to youth, adults can still buy. Food and Drug Administration limitations on kid-friendly ads, and use of sweet, fruity, and mint flavorings that are most preferred by children, apply only to new producers. The FDA does not yet regulate content of vaping solutions.
So we pediatricians are on the front line for this new threat to prevent vaping or convince youth to cut down or quit. The first step in addressing vaping is being knowledgeable about its many known and emerging health risks. It may seem obvious that the dangers of vaping microscopic particles depends on the contents. Water vapor alone is not dangerous; in fact, we prescribe it in nebulizers. Unfortunately, the contents of different vaping products vary and are not well defined in different vape products. The process of using an electric current to vaporize a substance can make it more toxic than the precursor, and teens have little idea about the substances they are inhaling. The psychoactive components vary from nicotine to tetrahydrocannabinol in varying amounts. These have the well known effects of stimulation or a high, but also the potential adverse effects of poor concentration, agitation, and even psychosis. Most e-cigarettes contain nicotine, which is highly addictive and can harm adolescent brain development, which continues into the early- to mid-20s. About two-thirds of Juul users aged 15-24 years did not know that it always contains nicotine, as do 99% of all vape solutions (Centers for Disease Control and Prevention, 2020). Earlier use of nicotine is more highly associated with later addiction to tobacco products that cause lung damage, acid reflux, insulin resistance, harm to the testes, harm to fetuses, cancer, and heart disease.
E-cigarette aerosols also contain dozens of other harmful substances besides nicotine ranging from acetone, propylene glycol, and metals to formaldehyde and ethyl benzene. These same chemicals are part of familiar toxic substances such as antifreeze, paint thinner, and pesticides. These cause ear, eye and throat irritation, and impairments in the cardiovascular system reducing athletic ability – at the least. Some flavorings in vape fluids also are toxic. Even the residual left on furniture and floors is harmful to those coming in contact, including pets.
How to encourage teens not to vaping
Trying to scare youth about health hazards is not generally effective in stopping risk behaviors since adolescence is a time of perceived singularity (it does not apply to me) and even a sense of immortality. Teens also see peers who vape as being unaffected and decide on using based on this small personal sample instead of valid statistics.
But teens do pay some attention to peer models or influencers saying why they do not use. One source of such testimony you can refer to is videos of inspiring athletes, musicians, and other “cool” young adults found on the naturalhigh.org website. You may know other examples of community teens desisting you can reference.
Parent rules, and less so advice, against smoking have been shown to be effective in deterring youth cigarette smoking. Because parents are less aware of vaping and its dangers, another step we can take is educating parents in our practices about vaping, its variable forms, its effects, and dangers, supplying authoritative materials, and advising them to talk with their children. Other steps the American Academy of Pediatrics recommends regarding smoking is for parents to be a role model of not using or try to quit, designate the house and car as smoking free, avoid children viewing smoking in media, tell their children about the side effects, and encourage their children who use to quit. Parents also can encourage schools to teach and have rules about smoking and vaping (e.g., med.stanford.edu/tobaccopreventiontoolkit.html).
Another approach we have been using is to not only screen for all substance use, but also to gather information about the teen’s strengths, activities, and life goals both to enhance rapport and to reference during motivational interviewing as reasons to avoid, reduce, or quit vaping. Motivational interviewing has been shown to help patients make healthier lifestyle choices by nonjudgmentally exploring their pros and cons in a conversation that takes into account readiness to change. This fits well with the stage of developing autonomy when teens want above all to make their own decisions. The cons of using can be discussed as including the effects and side effects of vaping interfering with their favored activities and moving towards their identified goals. Praising abstinence and asking them to show you how they could decline offers to vape are valuable reinforcement you can provide.
Finally, we all know that teens hate being manipulated. Vaping education we provide can make it clear that youth are being tricked by companies – most being large cigarette producers who know the dangers of vaping – into getting addicted so these companies can get rich on their money.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication is as a paid expert to MDedge News. Email her at pdnews@mdedge.com.
A girl presents with blotchy, slightly itchy spots on her chest, back
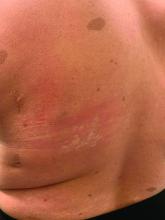
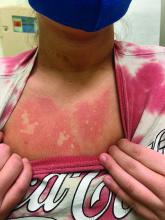
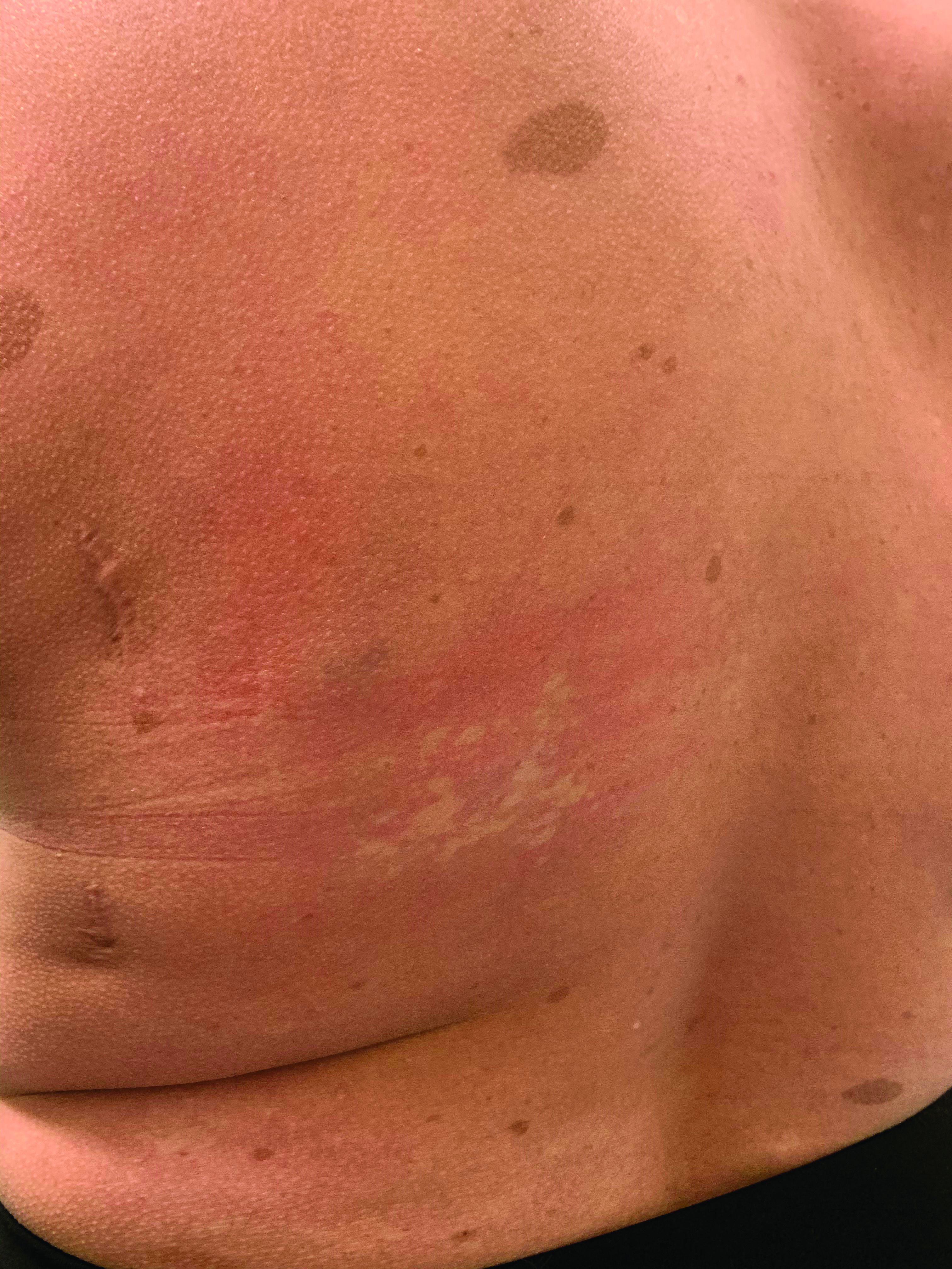
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at pdnews@mdedge.com.
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
Medicare payments could get tougher for docs
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
A shot in the arm
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
As the COVID-19 vaccine candidates have begun to roll off the production lines into the distribution networks by the millions, media coverage almost universally includes a still photo or video of someone receiving an injection. Ever observant, a retired lawyer friend of mine who learned to give shots when he was in the Army and again more recently while taking a wilderness survival course emailed me his concerns about what he felt were examples of poor injection technique. Included in his commentary was an Internet link in which a physician, who I suspect may have been a pediatrician, demonstrated what the physician considered proper intramuscular injection technique, which included a single-handed aspiration prior to giving the injection allowing the free hand to stabilize the patient’s – in this case a child’s – arm during the entire process.
I replied to my friend that I too was often troubled by what I considered to be poor injection technique. But, I said the physician in the link touting his improved technique was misguided. My understanding has been that unless the injection site is in the gluteus, there is no need aspirate prior to an intramuscular vaccine injection because the risk of intravascular injection is so small. I then confirmed this by reviewing the Centers for Disease Control and Prevention’s Vaccine Recommendations and Guidelines of the Advisory Committee on Immunization Practices, which was updated in June 2019. Included in those recommendations was the observation that the vaccine administrator does not need to wear gloves unless he or she has open lesions or is at risk from contacting the recipient’s body fluids.
Like many of the technical skills one learns in training, giving intramuscular injections is probably an example of the “see one, do one, teach one” mantra. But in the case of giving shots, I don’t recall any teaching. Do you? It was more “see a dozen and get on with it.” Or maybe you trained in an environment in which nurses gave all the injections. I hope not.
When it comes to giving immunizations to children, the art is in entering into that encounter with a calm, matter-of-fact attitude and body language, hiding the needle, firmly restraining the child, and moving quickly and smoothly. Aspirating and glove donning merely add to the drama and waste time. But how did I learn that art? No one taught me. Like many clinical skills, I watched scores of nurses and physicians, mentally logging in their tricks and mistakes that would help me craft my style.
I always felt and still feel that providing immunizations was per hour spent, the most valuable investment of my time. Doing the injecting myself was both the most efficient way to provide the service, and also emphasized the importance that I placed on the immunization. In the process of my 40-plus–year career, that included several hundred thousand patient encounters in which I gave innumerable injections. And, I egotistically assumed that I was good at it because many infants never cried, and a few children said, “That didn’t hurt.” I suspect you can make the same claim.
Injecting millions of adults with a COVID-19 vaccine, on the other hand, is a piece of cake because restraining the recipient shouldn’t factor into the scenario. However, I wonder who is going to administer all those millions of injections and who is going to train them? How many of the trainers are aware of the CDC-ACIP guidelines? Or, are they going to fall back on old techniques that lack evidence support?
From the efficiency standpoint, it probably doesn’t make much difference. The injection takes but a few seconds. Filling out the paperwork and waiting for the recipient to figure out how to expose his or her deltoid can take fifty times that long.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Advocate for legislation to improve, protect LGBTQ lives
In January in many states, the start of a new year also means the start of a new legislative session. For LGBTQ youth and their families, these sessions can create a significant amount of anxiety, as legislators in several states introduce legislation to curtail the rights of this population. In some cases, legislators have attempted to criminalize the provision of gender-affirming medical care to the trans and gender-diverse adolescents that many of us provide care to on a daily basis. As pediatricians,
2020 started on a positive note for LGBTQ children and adolescents, with Virginia becoming the 20th state to ban conversion therapy for minors. Legislation was introduced in several other states to prohibit this practice, including Kentucky, Missouri, and Ohio, and but they ultimately died in committee or were never referred. While there is not yet a nationwide ban on conversion therapy, legislation was introduced in the last three U.S. Congress sessions to ban this harmful practice. In June 2020, the Supreme Court decision in Bostock vs. Clayton County stated that employers could not fire an employee solely because of that person’s sexual orientation and/or gender identity.
However, 19 separate bills were introduced in 2020 alone in states across the United States that would prohibit gender-affirming care for adolescents under age 18.1 Many of these bills also would make the provision of gender-affirming medical care codified as felony child abuse, with loss of licensure, fines and/or jail time a possibility for physicians who prescribe hormones or puberty blockers for gender-affirming care to minors. Fortunately, these bills either died in committee or never had a hearing. However, legislation has been prefiled in several states for their 2021 session to again attempt to prohibit minors from obtaining gender-affirming medical care and/or criminalizing the provision of this care by physicians. Other bills were filed or have been prefiled again to allow various medical and mental health providers to refuse to treat LGBTQ patients because of their personal religious beliefs and/or forcing these same providers to tell a parent if a minor reveals to that provider that they are LGBTQ.
Even if this legislation does not pass or get a hearing, the fact that the bills were introduced can have a profound impact on LGBTQ patients and their families. After a bill was introduced in Texas in their 2017 legislative session that would require trans and gender-diverse (TGD) people to use the bathroom based on their sex assigned at birth, the Trevor Project reported that it had an increase of 34% in crisis calls from trans youth who were in distress.2 This was similar, but slightly less, than was reported by the Trevor Project in September 2015 when in the run-up to a vote on Houston’s Equal Rights Ordinance, advertising was run equating trans women as predators who could be lying in wait in bathrooms. On the converse, when LGBTQ youth feel supported in the media, courts, and legislatures, this can have a positive impact on their mental health. A 2017 study found that, in states who enacted same-sex marriage laws prior to the 2015 Supreme Court decision in Obergefell, compared with those who did not, there was a 7% relative reduction in the proportion of high school students who attempted suicide.3
The American Academy of Pediatrics published its policy statement in September 2018 outlining suggestions for pediatricians to provide support to TGD youth.4 In this position statement, recommendation No. 7 states “that pediatricians have a role in advocating for policies and laws that protect youth who identify as TGD from discrimination and violence.” Therefore, it is incumbent upon us to use our voices to support our LGBTQ youth. In 2020, several pediatricians from the South Dakota chapter of the AAP provided testimony – and organized public rallies – against legislation in that state which would have made gender-affirming care to minors under age 16 punishable by a fine and/or up to 10 years in prison.5
So what can you do? First, get to know your local and state legislators. While it was difficult to meet them in person for much of 2020, you can always call their district and/or Capitol offices, email them, or fill out their constituent contact form typically found on their website. Let them know that you oppose bills which introduce discrimination against your LGBTQ patients or threaten to criminalize the care that you provide to these patients.
Second, work with your state medical association or state AAP chapter to encourage them to oppose these harmful laws and support laws that improve the lives of LGBTQ patients. Third, you can write op-eds to your local newspaper, expressing your support for your patients and outlining the detrimental effects that anti-LGBTQ laws have on your patients. Lastly, you can be active on Twitter, Facebook, or other social media platforms sharing stories of how harmful or helpful certain pieces of legislation can be for your patients.
Dr. Cooper is assistant professor of pediatrics at the University of Texas, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas. He has no relevant financial disclosures. Email Dr. Cooper at pdnews@mdedge.com.
References
1. “Leglislation affecting LGBT rights across country.” www.aclu.org.
2. “Bathroom Bills Fuel Spike In Calls From Trans Youth To Suicide Hotline.” www.outsmartmagazine.com. 2017 Aug.
3. JAMA Pediatr. 2017 Apr 1. doi: 10.1001/jamapediatrics.2016.4529.
4. Pediatrics. 2018 Oct. doi: 10.1542/peds.2018-2162.
5. Wyckoff AS. “State bills seek to place limits on transgender care, ‘punish’ physicians.” AAP News. 2020 Feb 18.
In January in many states, the start of a new year also means the start of a new legislative session. For LGBTQ youth and their families, these sessions can create a significant amount of anxiety, as legislators in several states introduce legislation to curtail the rights of this population. In some cases, legislators have attempted to criminalize the provision of gender-affirming medical care to the trans and gender-diverse adolescents that many of us provide care to on a daily basis. As pediatricians,
2020 started on a positive note for LGBTQ children and adolescents, with Virginia becoming the 20th state to ban conversion therapy for minors. Legislation was introduced in several other states to prohibit this practice, including Kentucky, Missouri, and Ohio, and but they ultimately died in committee or were never referred. While there is not yet a nationwide ban on conversion therapy, legislation was introduced in the last three U.S. Congress sessions to ban this harmful practice. In June 2020, the Supreme Court decision in Bostock vs. Clayton County stated that employers could not fire an employee solely because of that person’s sexual orientation and/or gender identity.
However, 19 separate bills were introduced in 2020 alone in states across the United States that would prohibit gender-affirming care for adolescents under age 18.1 Many of these bills also would make the provision of gender-affirming medical care codified as felony child abuse, with loss of licensure, fines and/or jail time a possibility for physicians who prescribe hormones or puberty blockers for gender-affirming care to minors. Fortunately, these bills either died in committee or never had a hearing. However, legislation has been prefiled in several states for their 2021 session to again attempt to prohibit minors from obtaining gender-affirming medical care and/or criminalizing the provision of this care by physicians. Other bills were filed or have been prefiled again to allow various medical and mental health providers to refuse to treat LGBTQ patients because of their personal religious beliefs and/or forcing these same providers to tell a parent if a minor reveals to that provider that they are LGBTQ.
Even if this legislation does not pass or get a hearing, the fact that the bills were introduced can have a profound impact on LGBTQ patients and their families. After a bill was introduced in Texas in their 2017 legislative session that would require trans and gender-diverse (TGD) people to use the bathroom based on their sex assigned at birth, the Trevor Project reported that it had an increase of 34% in crisis calls from trans youth who were in distress.2 This was similar, but slightly less, than was reported by the Trevor Project in September 2015 when in the run-up to a vote on Houston’s Equal Rights Ordinance, advertising was run equating trans women as predators who could be lying in wait in bathrooms. On the converse, when LGBTQ youth feel supported in the media, courts, and legislatures, this can have a positive impact on their mental health. A 2017 study found that, in states who enacted same-sex marriage laws prior to the 2015 Supreme Court decision in Obergefell, compared with those who did not, there was a 7% relative reduction in the proportion of high school students who attempted suicide.3
The American Academy of Pediatrics published its policy statement in September 2018 outlining suggestions for pediatricians to provide support to TGD youth.4 In this position statement, recommendation No. 7 states “that pediatricians have a role in advocating for policies and laws that protect youth who identify as TGD from discrimination and violence.” Therefore, it is incumbent upon us to use our voices to support our LGBTQ youth. In 2020, several pediatricians from the South Dakota chapter of the AAP provided testimony – and organized public rallies – against legislation in that state which would have made gender-affirming care to minors under age 16 punishable by a fine and/or up to 10 years in prison.5
So what can you do? First, get to know your local and state legislators. While it was difficult to meet them in person for much of 2020, you can always call their district and/or Capitol offices, email them, or fill out their constituent contact form typically found on their website. Let them know that you oppose bills which introduce discrimination against your LGBTQ patients or threaten to criminalize the care that you provide to these patients.
Second, work with your state medical association or state AAP chapter to encourage them to oppose these harmful laws and support laws that improve the lives of LGBTQ patients. Third, you can write op-eds to your local newspaper, expressing your support for your patients and outlining the detrimental effects that anti-LGBTQ laws have on your patients. Lastly, you can be active on Twitter, Facebook, or other social media platforms sharing stories of how harmful or helpful certain pieces of legislation can be for your patients.
Dr. Cooper is assistant professor of pediatrics at the University of Texas, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas. He has no relevant financial disclosures. Email Dr. Cooper at pdnews@mdedge.com.
References
1. “Leglislation affecting LGBT rights across country.” www.aclu.org.
2. “Bathroom Bills Fuel Spike In Calls From Trans Youth To Suicide Hotline.” www.outsmartmagazine.com. 2017 Aug.
3. JAMA Pediatr. 2017 Apr 1. doi: 10.1001/jamapediatrics.2016.4529.
4. Pediatrics. 2018 Oct. doi: 10.1542/peds.2018-2162.
5. Wyckoff AS. “State bills seek to place limits on transgender care, ‘punish’ physicians.” AAP News. 2020 Feb 18.
In January in many states, the start of a new year also means the start of a new legislative session. For LGBTQ youth and their families, these sessions can create a significant amount of anxiety, as legislators in several states introduce legislation to curtail the rights of this population. In some cases, legislators have attempted to criminalize the provision of gender-affirming medical care to the trans and gender-diverse adolescents that many of us provide care to on a daily basis. As pediatricians,
2020 started on a positive note for LGBTQ children and adolescents, with Virginia becoming the 20th state to ban conversion therapy for minors. Legislation was introduced in several other states to prohibit this practice, including Kentucky, Missouri, and Ohio, and but they ultimately died in committee or were never referred. While there is not yet a nationwide ban on conversion therapy, legislation was introduced in the last three U.S. Congress sessions to ban this harmful practice. In June 2020, the Supreme Court decision in Bostock vs. Clayton County stated that employers could not fire an employee solely because of that person’s sexual orientation and/or gender identity.
However, 19 separate bills were introduced in 2020 alone in states across the United States that would prohibit gender-affirming care for adolescents under age 18.1 Many of these bills also would make the provision of gender-affirming medical care codified as felony child abuse, with loss of licensure, fines and/or jail time a possibility for physicians who prescribe hormones or puberty blockers for gender-affirming care to minors. Fortunately, these bills either died in committee or never had a hearing. However, legislation has been prefiled in several states for their 2021 session to again attempt to prohibit minors from obtaining gender-affirming medical care and/or criminalizing the provision of this care by physicians. Other bills were filed or have been prefiled again to allow various medical and mental health providers to refuse to treat LGBTQ patients because of their personal religious beliefs and/or forcing these same providers to tell a parent if a minor reveals to that provider that they are LGBTQ.
Even if this legislation does not pass or get a hearing, the fact that the bills were introduced can have a profound impact on LGBTQ patients and their families. After a bill was introduced in Texas in their 2017 legislative session that would require trans and gender-diverse (TGD) people to use the bathroom based on their sex assigned at birth, the Trevor Project reported that it had an increase of 34% in crisis calls from trans youth who were in distress.2 This was similar, but slightly less, than was reported by the Trevor Project in September 2015 when in the run-up to a vote on Houston’s Equal Rights Ordinance, advertising was run equating trans women as predators who could be lying in wait in bathrooms. On the converse, when LGBTQ youth feel supported in the media, courts, and legislatures, this can have a positive impact on their mental health. A 2017 study found that, in states who enacted same-sex marriage laws prior to the 2015 Supreme Court decision in Obergefell, compared with those who did not, there was a 7% relative reduction in the proportion of high school students who attempted suicide.3
The American Academy of Pediatrics published its policy statement in September 2018 outlining suggestions for pediatricians to provide support to TGD youth.4 In this position statement, recommendation No. 7 states “that pediatricians have a role in advocating for policies and laws that protect youth who identify as TGD from discrimination and violence.” Therefore, it is incumbent upon us to use our voices to support our LGBTQ youth. In 2020, several pediatricians from the South Dakota chapter of the AAP provided testimony – and organized public rallies – against legislation in that state which would have made gender-affirming care to minors under age 16 punishable by a fine and/or up to 10 years in prison.5
So what can you do? First, get to know your local and state legislators. While it was difficult to meet them in person for much of 2020, you can always call their district and/or Capitol offices, email them, or fill out their constituent contact form typically found on their website. Let them know that you oppose bills which introduce discrimination against your LGBTQ patients or threaten to criminalize the care that you provide to these patients.
Second, work with your state medical association or state AAP chapter to encourage them to oppose these harmful laws and support laws that improve the lives of LGBTQ patients. Third, you can write op-eds to your local newspaper, expressing your support for your patients and outlining the detrimental effects that anti-LGBTQ laws have on your patients. Lastly, you can be active on Twitter, Facebook, or other social media platforms sharing stories of how harmful or helpful certain pieces of legislation can be for your patients.
Dr. Cooper is assistant professor of pediatrics at the University of Texas, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas. He has no relevant financial disclosures. Email Dr. Cooper at pdnews@mdedge.com.
References
1. “Leglislation affecting LGBT rights across country.” www.aclu.org.
2. “Bathroom Bills Fuel Spike In Calls From Trans Youth To Suicide Hotline.” www.outsmartmagazine.com. 2017 Aug.
3. JAMA Pediatr. 2017 Apr 1. doi: 10.1001/jamapediatrics.2016.4529.
4. Pediatrics. 2018 Oct. doi: 10.1542/peds.2018-2162.
5. Wyckoff AS. “State bills seek to place limits on transgender care, ‘punish’ physicians.” AAP News. 2020 Feb 18.
Lost time amid COVID-19
At the end of my second year of medical school was what I call “The Lost Month.”
Between the end of classes and USMLE-1 we had 30 days to study for an 800-question, 2-day test that covered the entirety of the first 2 years. If you failed it once, you had to retake it. If you failed it twice you were out of medical school.
It was understandably stressful and I felt like every minute counted. I stopped shaving for the month to free up a few extra minutes each day. I unplugged my TV and put it in a closet.
Every day was the same. I was up at 7:00, had corn flakes, walked to Creighton, and found an empty library room. I took 30 minutes off at lunch and dinner to get something from the student union to eat outside (the only chance I had to enjoy sunlight), then study again until 1:00-2:00 in the morning.
The whole month become a blur. Days of the week were meaningless, only the number left until boards. Saturday or Tuesday, my life was the same. I don’t remember many specifics.
That was “The Lost Month.”
Now, somewhere in the middle of my attendinghood, I’ve come to 2020 (and likely beyond) which is, “The Lost Year.”
The days of the week have a bit more meaning now, as I still go to my office for a few hours and am home on weekends. But the weeks and months blend together. I’m home most of the time, I busy myself with working, and I have meal breaks with my family. There are no vacations or parties or movies. Even the holidays aren’t that different from the weekends—there isn’t much else to do to pass the time. And the stress is still there (in the early 90s it was academic, today it’s financial).
At least now I still try to shave regularly.
Thirty years ago I passed the boards and moved on to where I am today. My fear of failing out of medical school never materialized.
Today I try to remain optimistic. Vaccines are coming. Our learning curve on treating COVID-19 is getting better. Hopefully, The Lost Year will gradually become a memory as life goes on and normalizes.
Like the The Lost Month, I have to view 2020 as bump in the road. If this is the worst crisis I and my loved ones have to go through, I can deal with that. I know we’re fortunate compared with others. I try to remember that every time I pass a Salvation Army kettle or canned food drive, and donate.
In 1990 I had a specific date when The Lost Month would be over, and it was coming up way too fast. In 2020 no such date exists, now or in the immediate future.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
At the end of my second year of medical school was what I call “The Lost Month.”
Between the end of classes and USMLE-1 we had 30 days to study for an 800-question, 2-day test that covered the entirety of the first 2 years. If you failed it once, you had to retake it. If you failed it twice you were out of medical school.
It was understandably stressful and I felt like every minute counted. I stopped shaving for the month to free up a few extra minutes each day. I unplugged my TV and put it in a closet.
Every day was the same. I was up at 7:00, had corn flakes, walked to Creighton, and found an empty library room. I took 30 minutes off at lunch and dinner to get something from the student union to eat outside (the only chance I had to enjoy sunlight), then study again until 1:00-2:00 in the morning.
The whole month become a blur. Days of the week were meaningless, only the number left until boards. Saturday or Tuesday, my life was the same. I don’t remember many specifics.
That was “The Lost Month.”
Now, somewhere in the middle of my attendinghood, I’ve come to 2020 (and likely beyond) which is, “The Lost Year.”
The days of the week have a bit more meaning now, as I still go to my office for a few hours and am home on weekends. But the weeks and months blend together. I’m home most of the time, I busy myself with working, and I have meal breaks with my family. There are no vacations or parties or movies. Even the holidays aren’t that different from the weekends—there isn’t much else to do to pass the time. And the stress is still there (in the early 90s it was academic, today it’s financial).
At least now I still try to shave regularly.
Thirty years ago I passed the boards and moved on to where I am today. My fear of failing out of medical school never materialized.
Today I try to remain optimistic. Vaccines are coming. Our learning curve on treating COVID-19 is getting better. Hopefully, The Lost Year will gradually become a memory as life goes on and normalizes.
Like the The Lost Month, I have to view 2020 as bump in the road. If this is the worst crisis I and my loved ones have to go through, I can deal with that. I know we’re fortunate compared with others. I try to remember that every time I pass a Salvation Army kettle or canned food drive, and donate.
In 1990 I had a specific date when The Lost Month would be over, and it was coming up way too fast. In 2020 no such date exists, now or in the immediate future.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
At the end of my second year of medical school was what I call “The Lost Month.”
Between the end of classes and USMLE-1 we had 30 days to study for an 800-question, 2-day test that covered the entirety of the first 2 years. If you failed it once, you had to retake it. If you failed it twice you were out of medical school.
It was understandably stressful and I felt like every minute counted. I stopped shaving for the month to free up a few extra minutes each day. I unplugged my TV and put it in a closet.
Every day was the same. I was up at 7:00, had corn flakes, walked to Creighton, and found an empty library room. I took 30 minutes off at lunch and dinner to get something from the student union to eat outside (the only chance I had to enjoy sunlight), then study again until 1:00-2:00 in the morning.
The whole month become a blur. Days of the week were meaningless, only the number left until boards. Saturday or Tuesday, my life was the same. I don’t remember many specifics.
That was “The Lost Month.”
Now, somewhere in the middle of my attendinghood, I’ve come to 2020 (and likely beyond) which is, “The Lost Year.”
The days of the week have a bit more meaning now, as I still go to my office for a few hours and am home on weekends. But the weeks and months blend together. I’m home most of the time, I busy myself with working, and I have meal breaks with my family. There are no vacations or parties or movies. Even the holidays aren’t that different from the weekends—there isn’t much else to do to pass the time. And the stress is still there (in the early 90s it was academic, today it’s financial).
At least now I still try to shave regularly.
Thirty years ago I passed the boards and moved on to where I am today. My fear of failing out of medical school never materialized.
Today I try to remain optimistic. Vaccines are coming. Our learning curve on treating COVID-19 is getting better. Hopefully, The Lost Year will gradually become a memory as life goes on and normalizes.
Like the The Lost Month, I have to view 2020 as bump in the road. If this is the worst crisis I and my loved ones have to go through, I can deal with that. I know we’re fortunate compared with others. I try to remember that every time I pass a Salvation Army kettle or canned food drive, and donate.
In 1990 I had a specific date when The Lost Month would be over, and it was coming up way too fast. In 2020 no such date exists, now or in the immediate future.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Patient with CKD: Contrast or no contrast?
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
Leading hospitalists during a pandemic
As I write this, we are entering the third surge of the COVID-19 pandemic, with new cases, hospitalizations, and deaths from COVID-19 skyrocketing around the country. Worst of all, this surge has been most severely affecting areas of the nation least prepared to handle it (rural) and populations already marginalized by the health care system (Latinx and Black). Despite the onslaught of COVID-19, “pandemic fatigue” has begun to set in amongst colleagues, friends, and family, leading to challenges in adhering to social distancing and other infection-control measures, both at work and home.
In the face of the pandemic’s onslaught, hospitalists – who have faced the brunt of caring for patients with COVID-19, despite the absence of reporting about the subspecialty’s role – are faced with mustering the grit to respond with resolve, coordinated action, and empathy. Luckily, hospitalists are equipped with the very characteristics needed to lead teams, groups, and hospitals through the crisis of this pandemic. Ask yourself, why did you become a hospitalist? If you wanted steady predictability and control, there were many office-based specialties you could have chosen. You chose to become a hospitalist because you seek the challenges of clinical variety, problem-solving, systems improvement, and you are a natural team leader, whether you have been designated as such or not. In the words of John Quincy Adams, “if your actions inspire others to dream more, learn more, do more, and become more, you are a leader.”
As a leader, how can you lead your team through the series of trials and tribulations that this year has thrown at you? From COVID-19 to racism directed against Black and Latinx people to the behavioral health crisis, 2020 has likely made you feel as if you’re stuck in a ghoulish carnival fun house without an exit.
Yet this is where some leaders hit their stride, in what Bennis and Thomas describe as the “crucible of leadership.”1 There are many types of “crucibles of leadership,” according to Bennis and Thomas, and this year has thrown most of these at us: prejudice/bias, physical fatigue and illness, sudden elevation of responsibility to lead new processes, not to mention family stressors. Leaders who succeed in guiding their colleagues through these challenges have manifested critical skills: engaging others in shared meaning, having a distinctive and compelling voice, displaying integrity, and having adaptive capacity.
What exactly is adaptive capacity, the most important of these, in my opinion? Adaptive capacity requires understanding the new context of a crisis and how it has shifted team members’ needs and perceptions. It also requires what Bennis and Thomas call hardiness and what I call grit – the ability to face adversity, get knocked down, get up, and do it again.
There is probably no better example of a crisis leader with extraordinary adaptive capacity than Anglo-Irish explorer Sir Ernest Shackleton. Bitten by the bug of exploration, Shackleton failed at reaching the South Pole (1908-1909) but subsequently attempted to cross the Antarctic, departing South Georgia Island on Dec. 5, 1914. Depressingly for Shackleton, his ship, the Endurance, became stuck in sea ice on Jan. 19, 1915 before even reaching the continent. Drifting with the ice floe, his crew had set up a winter station hoping to be released from the ice later, but the Endurance was crushed by the pressure of sea ice and sank on Nov. 21, 1915. From there, Shackleton hoped to drift north to Paulet Island, 250 miles away, but eventually was forced to take his crew on lifeboats to the nearest land, Elephant Island, 346 miles from where the Endurance sank. He then took five of his men on an open boat, 828-mile journey to South Georgia Island. Encountering hurricane-force winds, the team landed on South Georgia Island 15 days later, only to face a climb of 32 miles over mountainous terrain to reach a whaling station. Shackleton eventually organized his men’s rescue on Elephant Island, reaching them on Aug. 30, 1916, 4½ months after he had set out for South Georgia Island. His entire crew survived, only to have two of them killed later in World War I.
You might consider Shackleton a failure for not even coming close to his original goal, but his success in saving his crew is regarded as the epitome of crisis leadership. As Harvard Business School professor Nancy F. Koehn, PhD, whose case study of Shackleton is one of the most popular at HBS, stated, “He thought he was going to be an entrepreneur of exploration, but he became an entrepreneur of survival.”2 Upon realizing the futility of his original mission, he pivoted immediately to the survival of his crew. “A man must shape himself to a new mark directly the old one goes to ground,” wrote Shackleton in his diary.3
Realizing that preserving his crew’s morale was critical, he maintained the crew’s everyday activities, despite the prospect of dying on the ice. He realized that he needed to keep up his own courage and confidence as well as that of his crew. Despite his ability to share the strategic focus of getting to safety with his men, he didn’t lose sight of day-to-day needs, such as keeping the crew entertained. When he encountered crew members who seemed problematic to his mission goals, he assigned them to his own tent.
Despite the extreme cold, his decision-making did not freeze – he acted decisively. He took risks when he thought appropriate, twice needing to abandon his efforts to drag a lifeboat full of supplies with his men toward the sea. “You can’t be afraid to make smart mistakes,” says Dr. Koehn. “That’s something we have no training in.”4 Most importantly, Shackleton took ultimate responsibility for his men’s survival, never resting until they had all been rescued. And he modeled a culture of shared responsibility for one another5 – he had once offered his only biscuit of the day on a prior expedition to his fellow explorer Frank Wild.
As winter arrives in 2020 and deepens into 2021, we will all be faced with leading our teams across the ice and to the safety of spring, and hopefully a vaccine. Whether we can get there with our entire crew depends on effective crisis leadership. But we can draw on the lessons provided by Shackleton and other crisis leaders in the past to guide us in the present.
Author disclosure: I studied the HBS case study “Leadership in Crisis: Ernest Shackleton and the Epic Voyage of the Endurance” as part of a 12-month certificate course in Safety, Quality, Informatics, and Leadership (SQIL) offered by Harvard Medical School.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. HBR’s 10 must reads on leadership. Boston: Harvard Business Review Press, 2011.
2. Lagace M. Shackleton: An entrepreneur of survival. Harvard Business School. Working Knowledge website. Published 2003. Accessed 2020 Nov 19.
3. Koehn N. Leadership lessons from the Shackleton Expedition. The New York Times. 2011 Dec 25.
4. Potier B. Shackleton in business school. Harvard Public Affairs and Communications. The Harvard Gazette website. Published 2004. Accessed 2020 Nov 19.
5. Perkins D. 4 Lessons in crisis leadership from Shackleton’s expedition. In Leadership Essentials by HarpersCollins Leadership. Vol 2020. New York: HarpersCollins, 2020.
As I write this, we are entering the third surge of the COVID-19 pandemic, with new cases, hospitalizations, and deaths from COVID-19 skyrocketing around the country. Worst of all, this surge has been most severely affecting areas of the nation least prepared to handle it (rural) and populations already marginalized by the health care system (Latinx and Black). Despite the onslaught of COVID-19, “pandemic fatigue” has begun to set in amongst colleagues, friends, and family, leading to challenges in adhering to social distancing and other infection-control measures, both at work and home.
In the face of the pandemic’s onslaught, hospitalists – who have faced the brunt of caring for patients with COVID-19, despite the absence of reporting about the subspecialty’s role – are faced with mustering the grit to respond with resolve, coordinated action, and empathy. Luckily, hospitalists are equipped with the very characteristics needed to lead teams, groups, and hospitals through the crisis of this pandemic. Ask yourself, why did you become a hospitalist? If you wanted steady predictability and control, there were many office-based specialties you could have chosen. You chose to become a hospitalist because you seek the challenges of clinical variety, problem-solving, systems improvement, and you are a natural team leader, whether you have been designated as such or not. In the words of John Quincy Adams, “if your actions inspire others to dream more, learn more, do more, and become more, you are a leader.”
As a leader, how can you lead your team through the series of trials and tribulations that this year has thrown at you? From COVID-19 to racism directed against Black and Latinx people to the behavioral health crisis, 2020 has likely made you feel as if you’re stuck in a ghoulish carnival fun house without an exit.
Yet this is where some leaders hit their stride, in what Bennis and Thomas describe as the “crucible of leadership.”1 There are many types of “crucibles of leadership,” according to Bennis and Thomas, and this year has thrown most of these at us: prejudice/bias, physical fatigue and illness, sudden elevation of responsibility to lead new processes, not to mention family stressors. Leaders who succeed in guiding their colleagues through these challenges have manifested critical skills: engaging others in shared meaning, having a distinctive and compelling voice, displaying integrity, and having adaptive capacity.
What exactly is adaptive capacity, the most important of these, in my opinion? Adaptive capacity requires understanding the new context of a crisis and how it has shifted team members’ needs and perceptions. It also requires what Bennis and Thomas call hardiness and what I call grit – the ability to face adversity, get knocked down, get up, and do it again.
There is probably no better example of a crisis leader with extraordinary adaptive capacity than Anglo-Irish explorer Sir Ernest Shackleton. Bitten by the bug of exploration, Shackleton failed at reaching the South Pole (1908-1909) but subsequently attempted to cross the Antarctic, departing South Georgia Island on Dec. 5, 1914. Depressingly for Shackleton, his ship, the Endurance, became stuck in sea ice on Jan. 19, 1915 before even reaching the continent. Drifting with the ice floe, his crew had set up a winter station hoping to be released from the ice later, but the Endurance was crushed by the pressure of sea ice and sank on Nov. 21, 1915. From there, Shackleton hoped to drift north to Paulet Island, 250 miles away, but eventually was forced to take his crew on lifeboats to the nearest land, Elephant Island, 346 miles from where the Endurance sank. He then took five of his men on an open boat, 828-mile journey to South Georgia Island. Encountering hurricane-force winds, the team landed on South Georgia Island 15 days later, only to face a climb of 32 miles over mountainous terrain to reach a whaling station. Shackleton eventually organized his men’s rescue on Elephant Island, reaching them on Aug. 30, 1916, 4½ months after he had set out for South Georgia Island. His entire crew survived, only to have two of them killed later in World War I.
You might consider Shackleton a failure for not even coming close to his original goal, but his success in saving his crew is regarded as the epitome of crisis leadership. As Harvard Business School professor Nancy F. Koehn, PhD, whose case study of Shackleton is one of the most popular at HBS, stated, “He thought he was going to be an entrepreneur of exploration, but he became an entrepreneur of survival.”2 Upon realizing the futility of his original mission, he pivoted immediately to the survival of his crew. “A man must shape himself to a new mark directly the old one goes to ground,” wrote Shackleton in his diary.3
Realizing that preserving his crew’s morale was critical, he maintained the crew’s everyday activities, despite the prospect of dying on the ice. He realized that he needed to keep up his own courage and confidence as well as that of his crew. Despite his ability to share the strategic focus of getting to safety with his men, he didn’t lose sight of day-to-day needs, such as keeping the crew entertained. When he encountered crew members who seemed problematic to his mission goals, he assigned them to his own tent.
Despite the extreme cold, his decision-making did not freeze – he acted decisively. He took risks when he thought appropriate, twice needing to abandon his efforts to drag a lifeboat full of supplies with his men toward the sea. “You can’t be afraid to make smart mistakes,” says Dr. Koehn. “That’s something we have no training in.”4 Most importantly, Shackleton took ultimate responsibility for his men’s survival, never resting until they had all been rescued. And he modeled a culture of shared responsibility for one another5 – he had once offered his only biscuit of the day on a prior expedition to his fellow explorer Frank Wild.
As winter arrives in 2020 and deepens into 2021, we will all be faced with leading our teams across the ice and to the safety of spring, and hopefully a vaccine. Whether we can get there with our entire crew depends on effective crisis leadership. But we can draw on the lessons provided by Shackleton and other crisis leaders in the past to guide us in the present.
Author disclosure: I studied the HBS case study “Leadership in Crisis: Ernest Shackleton and the Epic Voyage of the Endurance” as part of a 12-month certificate course in Safety, Quality, Informatics, and Leadership (SQIL) offered by Harvard Medical School.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. HBR’s 10 must reads on leadership. Boston: Harvard Business Review Press, 2011.
2. Lagace M. Shackleton: An entrepreneur of survival. Harvard Business School. Working Knowledge website. Published 2003. Accessed 2020 Nov 19.
3. Koehn N. Leadership lessons from the Shackleton Expedition. The New York Times. 2011 Dec 25.
4. Potier B. Shackleton in business school. Harvard Public Affairs and Communications. The Harvard Gazette website. Published 2004. Accessed 2020 Nov 19.
5. Perkins D. 4 Lessons in crisis leadership from Shackleton’s expedition. In Leadership Essentials by HarpersCollins Leadership. Vol 2020. New York: HarpersCollins, 2020.
As I write this, we are entering the third surge of the COVID-19 pandemic, with new cases, hospitalizations, and deaths from COVID-19 skyrocketing around the country. Worst of all, this surge has been most severely affecting areas of the nation least prepared to handle it (rural) and populations already marginalized by the health care system (Latinx and Black). Despite the onslaught of COVID-19, “pandemic fatigue” has begun to set in amongst colleagues, friends, and family, leading to challenges in adhering to social distancing and other infection-control measures, both at work and home.
In the face of the pandemic’s onslaught, hospitalists – who have faced the brunt of caring for patients with COVID-19, despite the absence of reporting about the subspecialty’s role – are faced with mustering the grit to respond with resolve, coordinated action, and empathy. Luckily, hospitalists are equipped with the very characteristics needed to lead teams, groups, and hospitals through the crisis of this pandemic. Ask yourself, why did you become a hospitalist? If you wanted steady predictability and control, there were many office-based specialties you could have chosen. You chose to become a hospitalist because you seek the challenges of clinical variety, problem-solving, systems improvement, and you are a natural team leader, whether you have been designated as such or not. In the words of John Quincy Adams, “if your actions inspire others to dream more, learn more, do more, and become more, you are a leader.”
As a leader, how can you lead your team through the series of trials and tribulations that this year has thrown at you? From COVID-19 to racism directed against Black and Latinx people to the behavioral health crisis, 2020 has likely made you feel as if you’re stuck in a ghoulish carnival fun house without an exit.
Yet this is where some leaders hit their stride, in what Bennis and Thomas describe as the “crucible of leadership.”1 There are many types of “crucibles of leadership,” according to Bennis and Thomas, and this year has thrown most of these at us: prejudice/bias, physical fatigue and illness, sudden elevation of responsibility to lead new processes, not to mention family stressors. Leaders who succeed in guiding their colleagues through these challenges have manifested critical skills: engaging others in shared meaning, having a distinctive and compelling voice, displaying integrity, and having adaptive capacity.
What exactly is adaptive capacity, the most important of these, in my opinion? Adaptive capacity requires understanding the new context of a crisis and how it has shifted team members’ needs and perceptions. It also requires what Bennis and Thomas call hardiness and what I call grit – the ability to face adversity, get knocked down, get up, and do it again.
There is probably no better example of a crisis leader with extraordinary adaptive capacity than Anglo-Irish explorer Sir Ernest Shackleton. Bitten by the bug of exploration, Shackleton failed at reaching the South Pole (1908-1909) but subsequently attempted to cross the Antarctic, departing South Georgia Island on Dec. 5, 1914. Depressingly for Shackleton, his ship, the Endurance, became stuck in sea ice on Jan. 19, 1915 before even reaching the continent. Drifting with the ice floe, his crew had set up a winter station hoping to be released from the ice later, but the Endurance was crushed by the pressure of sea ice and sank on Nov. 21, 1915. From there, Shackleton hoped to drift north to Paulet Island, 250 miles away, but eventually was forced to take his crew on lifeboats to the nearest land, Elephant Island, 346 miles from where the Endurance sank. He then took five of his men on an open boat, 828-mile journey to South Georgia Island. Encountering hurricane-force winds, the team landed on South Georgia Island 15 days later, only to face a climb of 32 miles over mountainous terrain to reach a whaling station. Shackleton eventually organized his men’s rescue on Elephant Island, reaching them on Aug. 30, 1916, 4½ months after he had set out for South Georgia Island. His entire crew survived, only to have two of them killed later in World War I.
You might consider Shackleton a failure for not even coming close to his original goal, but his success in saving his crew is regarded as the epitome of crisis leadership. As Harvard Business School professor Nancy F. Koehn, PhD, whose case study of Shackleton is one of the most popular at HBS, stated, “He thought he was going to be an entrepreneur of exploration, but he became an entrepreneur of survival.”2 Upon realizing the futility of his original mission, he pivoted immediately to the survival of his crew. “A man must shape himself to a new mark directly the old one goes to ground,” wrote Shackleton in his diary.3
Realizing that preserving his crew’s morale was critical, he maintained the crew’s everyday activities, despite the prospect of dying on the ice. He realized that he needed to keep up his own courage and confidence as well as that of his crew. Despite his ability to share the strategic focus of getting to safety with his men, he didn’t lose sight of day-to-day needs, such as keeping the crew entertained. When he encountered crew members who seemed problematic to his mission goals, he assigned them to his own tent.
Despite the extreme cold, his decision-making did not freeze – he acted decisively. He took risks when he thought appropriate, twice needing to abandon his efforts to drag a lifeboat full of supplies with his men toward the sea. “You can’t be afraid to make smart mistakes,” says Dr. Koehn. “That’s something we have no training in.”4 Most importantly, Shackleton took ultimate responsibility for his men’s survival, never resting until they had all been rescued. And he modeled a culture of shared responsibility for one another5 – he had once offered his only biscuit of the day on a prior expedition to his fellow explorer Frank Wild.
As winter arrives in 2020 and deepens into 2021, we will all be faced with leading our teams across the ice and to the safety of spring, and hopefully a vaccine. Whether we can get there with our entire crew depends on effective crisis leadership. But we can draw on the lessons provided by Shackleton and other crisis leaders in the past to guide us in the present.
Author disclosure: I studied the HBS case study “Leadership in Crisis: Ernest Shackleton and the Epic Voyage of the Endurance” as part of a 12-month certificate course in Safety, Quality, Informatics, and Leadership (SQIL) offered by Harvard Medical School.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. HBR’s 10 must reads on leadership. Boston: Harvard Business Review Press, 2011.
2. Lagace M. Shackleton: An entrepreneur of survival. Harvard Business School. Working Knowledge website. Published 2003. Accessed 2020 Nov 19.
3. Koehn N. Leadership lessons from the Shackleton Expedition. The New York Times. 2011 Dec 25.
4. Potier B. Shackleton in business school. Harvard Public Affairs and Communications. The Harvard Gazette website. Published 2004. Accessed 2020 Nov 19.
5. Perkins D. 4 Lessons in crisis leadership from Shackleton’s expedition. In Leadership Essentials by HarpersCollins Leadership. Vol 2020. New York: HarpersCollins, 2020.
How Twitter amplifies my doctor and human voice
When I graduated from residency in 2007, Facebook had just become “a thing,” and my cohort decided to use it to keep in touch. These days, Twitter seems to be the social media platform of choice for health care professionals.
When I started on Twitter a few years ago, it was in reaction to the current political climate. I wanted to keep track of what my favorite thinkers were writing. I was anonymous and tweeted about politics mostly. My husband was my only follower for a while.
I deanonymized when, at last year’s American College of Rheumatology meeting, I presented a poster and wanted to reach a wider audience. I could have created two different personas on Twitter, like many doctors apparently do. Initially, I resisted doing that because I am frankly too lazy to keep track of two different social media profiles, but now I resist because I see my profession as an extension of my political self, and have no problem with using my (very low) profile to amplify both my doctor voice and my human voice.
Professionally, Twitter is rewarding. It is a space for networking and for promoting one’s work. It is a fantastic learning format, as evidenced by the popularity of tweetorials. The international consortium that has worked to collect information on rheumatology patients with COVID started as an idea on Twitter. The fact that ACR Convergence 2020 abstracts are now available? I only know because of the #ACRambassadors that I follow.
But I find that I cannot separate who I am from what I do. As a rheumatologist, I build long-term relationships with patients. I cannot care for their medical conditions in isolation without also concerning myself with their nonmedical circumstances. For that reason, I have opinions that one might call humanist, and I suspect that I am not alone among rheumatologists.
I can think of three areas, broadly construed but with huge overlaps, that concern me a great deal.
First, there are things that affect all physicians: race and gender discrimination in the workplace; advancement of women in science, technology, engineering, or math; Medicare reimbursement; COVID-19 preparedness; immigration issues (an issue near and dear to me, as I am an immigrant and a foreign medical graduate); and federal funding (including funding for training programs and community health centers, funding for the National Institutes of Health, and funding for stem cell research).
Then there are the things that affect rheumatologists in particular. Access to medications and procedures is one thing. (I did say these categories hugely overlap.) If you›ve ever tried to prescribe even a drug as old as oral cyclophosphamide, you’ll have experienced the difficulty of getting it for Medicare patients. Patients who need biologics are limited by insurance contracts with pharmaceutical companies, but also by requirements such as step therapy. I am all varieties of annoyed, incredulous, and apologetic that when a patient asks me how much a treatment will cost him/her, I do not have an answer.
Speaking of pricing, don’t even get me started on pharmaceutical company price gouging. Yes, the H.P. Acthar gel may be the most egregious offender among rheumatology medications, but it’s easy to not prescribe a drug that costs $80,000 a vial and which does not do much more than prednisone does. On the other hand, I remember a time when colchicine cost $0.10 cents a pill and patients did not have to jump through hoops to get it.
And what of reproductive freedom? Our patients rely on us for advice about their childbearing options, including birth control, in vitro fertilization, and pregnancy termination.
Finally, and most important, the things that affect me most are the issues that affect patients. The lowest-hanging fruit here is the abject incompetence of the federal response to the ongoing pandemic. How many of our patients’ lives have been lost or adversely affected? And what of coverage for preexisting conditions for the vast majority of our patients, whose illnesses are chronic?
While we’re at it, the fact of health insurance being tied to employment, something that seemingly no other country in the developed world does, makes living with chronic conditions outright scary, doesn’t it? It isn’t quite so easy to remain employed when one cannot get the right medications for RA.
I could go on. Gun violence and health care disparities, vaccine denialism, coverage for mental health issues, LGBTQ rights, refugee rights, police brutality … there is a seemingly endless list of things to care about. It’s exhausting.
While I do use my Twitter account to learn from colleagues and to promote work that interests me, my primary aim is to participate in civil society as a person. Critics will use “stay in your lane” as shorthand to say x professionals should stick to x (actors to acting, musicians to music, athletes to sports). If only I could. But my humanity won’t let me. Aristotle said man is a political animal; even the venerable New England Journal of Medicine has found it impossible to keep silent.
Karmela Kim Chan, MD, is an assistant professor at Weill Cornell Medicine, New York, and an attending physician at the Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center, both in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a past columnist for MDedge Rheumatology, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice.
A version of this article originally appeared on Medscape.com.
When I graduated from residency in 2007, Facebook had just become “a thing,” and my cohort decided to use it to keep in touch. These days, Twitter seems to be the social media platform of choice for health care professionals.
When I started on Twitter a few years ago, it was in reaction to the current political climate. I wanted to keep track of what my favorite thinkers were writing. I was anonymous and tweeted about politics mostly. My husband was my only follower for a while.
I deanonymized when, at last year’s American College of Rheumatology meeting, I presented a poster and wanted to reach a wider audience. I could have created two different personas on Twitter, like many doctors apparently do. Initially, I resisted doing that because I am frankly too lazy to keep track of two different social media profiles, but now I resist because I see my profession as an extension of my political self, and have no problem with using my (very low) profile to amplify both my doctor voice and my human voice.
Professionally, Twitter is rewarding. It is a space for networking and for promoting one’s work. It is a fantastic learning format, as evidenced by the popularity of tweetorials. The international consortium that has worked to collect information on rheumatology patients with COVID started as an idea on Twitter. The fact that ACR Convergence 2020 abstracts are now available? I only know because of the #ACRambassadors that I follow.
But I find that I cannot separate who I am from what I do. As a rheumatologist, I build long-term relationships with patients. I cannot care for their medical conditions in isolation without also concerning myself with their nonmedical circumstances. For that reason, I have opinions that one might call humanist, and I suspect that I am not alone among rheumatologists.
I can think of three areas, broadly construed but with huge overlaps, that concern me a great deal.
First, there are things that affect all physicians: race and gender discrimination in the workplace; advancement of women in science, technology, engineering, or math; Medicare reimbursement; COVID-19 preparedness; immigration issues (an issue near and dear to me, as I am an immigrant and a foreign medical graduate); and federal funding (including funding for training programs and community health centers, funding for the National Institutes of Health, and funding for stem cell research).
Then there are the things that affect rheumatologists in particular. Access to medications and procedures is one thing. (I did say these categories hugely overlap.) If you›ve ever tried to prescribe even a drug as old as oral cyclophosphamide, you’ll have experienced the difficulty of getting it for Medicare patients. Patients who need biologics are limited by insurance contracts with pharmaceutical companies, but also by requirements such as step therapy. I am all varieties of annoyed, incredulous, and apologetic that when a patient asks me how much a treatment will cost him/her, I do not have an answer.
Speaking of pricing, don’t even get me started on pharmaceutical company price gouging. Yes, the H.P. Acthar gel may be the most egregious offender among rheumatology medications, but it’s easy to not prescribe a drug that costs $80,000 a vial and which does not do much more than prednisone does. On the other hand, I remember a time when colchicine cost $0.10 cents a pill and patients did not have to jump through hoops to get it.
And what of reproductive freedom? Our patients rely on us for advice about their childbearing options, including birth control, in vitro fertilization, and pregnancy termination.
Finally, and most important, the things that affect me most are the issues that affect patients. The lowest-hanging fruit here is the abject incompetence of the federal response to the ongoing pandemic. How many of our patients’ lives have been lost or adversely affected? And what of coverage for preexisting conditions for the vast majority of our patients, whose illnesses are chronic?
While we’re at it, the fact of health insurance being tied to employment, something that seemingly no other country in the developed world does, makes living with chronic conditions outright scary, doesn’t it? It isn’t quite so easy to remain employed when one cannot get the right medications for RA.
I could go on. Gun violence and health care disparities, vaccine denialism, coverage for mental health issues, LGBTQ rights, refugee rights, police brutality … there is a seemingly endless list of things to care about. It’s exhausting.
While I do use my Twitter account to learn from colleagues and to promote work that interests me, my primary aim is to participate in civil society as a person. Critics will use “stay in your lane” as shorthand to say x professionals should stick to x (actors to acting, musicians to music, athletes to sports). If only I could. But my humanity won’t let me. Aristotle said man is a political animal; even the venerable New England Journal of Medicine has found it impossible to keep silent.
Karmela Kim Chan, MD, is an assistant professor at Weill Cornell Medicine, New York, and an attending physician at the Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center, both in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a past columnist for MDedge Rheumatology, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice.
A version of this article originally appeared on Medscape.com.
When I graduated from residency in 2007, Facebook had just become “a thing,” and my cohort decided to use it to keep in touch. These days, Twitter seems to be the social media platform of choice for health care professionals.
When I started on Twitter a few years ago, it was in reaction to the current political climate. I wanted to keep track of what my favorite thinkers were writing. I was anonymous and tweeted about politics mostly. My husband was my only follower for a while.
I deanonymized when, at last year’s American College of Rheumatology meeting, I presented a poster and wanted to reach a wider audience. I could have created two different personas on Twitter, like many doctors apparently do. Initially, I resisted doing that because I am frankly too lazy to keep track of two different social media profiles, but now I resist because I see my profession as an extension of my political self, and have no problem with using my (very low) profile to amplify both my doctor voice and my human voice.
Professionally, Twitter is rewarding. It is a space for networking and for promoting one’s work. It is a fantastic learning format, as evidenced by the popularity of tweetorials. The international consortium that has worked to collect information on rheumatology patients with COVID started as an idea on Twitter. The fact that ACR Convergence 2020 abstracts are now available? I only know because of the #ACRambassadors that I follow.
But I find that I cannot separate who I am from what I do. As a rheumatologist, I build long-term relationships with patients. I cannot care for their medical conditions in isolation without also concerning myself with their nonmedical circumstances. For that reason, I have opinions that one might call humanist, and I suspect that I am not alone among rheumatologists.
I can think of three areas, broadly construed but with huge overlaps, that concern me a great deal.
First, there are things that affect all physicians: race and gender discrimination in the workplace; advancement of women in science, technology, engineering, or math; Medicare reimbursement; COVID-19 preparedness; immigration issues (an issue near and dear to me, as I am an immigrant and a foreign medical graduate); and federal funding (including funding for training programs and community health centers, funding for the National Institutes of Health, and funding for stem cell research).
Then there are the things that affect rheumatologists in particular. Access to medications and procedures is one thing. (I did say these categories hugely overlap.) If you›ve ever tried to prescribe even a drug as old as oral cyclophosphamide, you’ll have experienced the difficulty of getting it for Medicare patients. Patients who need biologics are limited by insurance contracts with pharmaceutical companies, but also by requirements such as step therapy. I am all varieties of annoyed, incredulous, and apologetic that when a patient asks me how much a treatment will cost him/her, I do not have an answer.
Speaking of pricing, don’t even get me started on pharmaceutical company price gouging. Yes, the H.P. Acthar gel may be the most egregious offender among rheumatology medications, but it’s easy to not prescribe a drug that costs $80,000 a vial and which does not do much more than prednisone does. On the other hand, I remember a time when colchicine cost $0.10 cents a pill and patients did not have to jump through hoops to get it.
And what of reproductive freedom? Our patients rely on us for advice about their childbearing options, including birth control, in vitro fertilization, and pregnancy termination.
Finally, and most important, the things that affect me most are the issues that affect patients. The lowest-hanging fruit here is the abject incompetence of the federal response to the ongoing pandemic. How many of our patients’ lives have been lost or adversely affected? And what of coverage for preexisting conditions for the vast majority of our patients, whose illnesses are chronic?
While we’re at it, the fact of health insurance being tied to employment, something that seemingly no other country in the developed world does, makes living with chronic conditions outright scary, doesn’t it? It isn’t quite so easy to remain employed when one cannot get the right medications for RA.
I could go on. Gun violence and health care disparities, vaccine denialism, coverage for mental health issues, LGBTQ rights, refugee rights, police brutality … there is a seemingly endless list of things to care about. It’s exhausting.
While I do use my Twitter account to learn from colleagues and to promote work that interests me, my primary aim is to participate in civil society as a person. Critics will use “stay in your lane” as shorthand to say x professionals should stick to x (actors to acting, musicians to music, athletes to sports). If only I could. But my humanity won’t let me. Aristotle said man is a political animal; even the venerable New England Journal of Medicine has found it impossible to keep silent.
Karmela Kim Chan, MD, is an assistant professor at Weill Cornell Medicine, New York, and an attending physician at the Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center, both in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a past columnist for MDedge Rheumatology, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice.
A version of this article originally appeared on Medscape.com.