User login
A pediatrician notices empty fields
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
How could this happen? Judge forces doctors to give ivermectin
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
When the juggling act becomes impossible
Objectivity is tough, but essential: a critical part of patient care, allowing you to make appropriate decisions based on facts and circumstances, not emotions. We’re supposed to be compassionate Vulcans – able to logically weigh possibilities and treatment options under pressure, and at the same time exhibit empathy and sensitivity.
For the most part, all of us become very good at this juggling act. But we’re only human, and once the ability to do that with a given person is lost, it’s gone for good.
Have you ever lost objectivity with a patient? I have. Generally it involves the patient being so difficult, unpleasant, or dislikable that it exceeds my ability to remain impartial and pragmatic in their care.
I don’t know any physician it hasn’t happened to. And when it does, ending the doctor-patient relationship is the only effective answer.
It’s never easy sending that letter, telling someone that they need to seek care elsewhere, and often the specific reason is harder to define. In patients who are overtly rude or noncompliant it’s easy. But often a loss in objectivity is from something less tangible, such as the vagaries of personal chemistry.
I try to get along with all my patients. I really do. That’s part of the job. But sometimes, for whatever reason, it’s just an impossible task. Too many conflicts and differences of opinion over treatments, tests, diagnosis, what they read on Facebook … whatever.
Regardless of cause, professionalism requires that it be the end of the road. If I can’t objectively weigh a patient’s symptoms and treatment options, then I’m not going to be able to do my very best for them. And my very best is what every patient deserves.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Objectivity is tough, but essential: a critical part of patient care, allowing you to make appropriate decisions based on facts and circumstances, not emotions. We’re supposed to be compassionate Vulcans – able to logically weigh possibilities and treatment options under pressure, and at the same time exhibit empathy and sensitivity.
For the most part, all of us become very good at this juggling act. But we’re only human, and once the ability to do that with a given person is lost, it’s gone for good.
Have you ever lost objectivity with a patient? I have. Generally it involves the patient being so difficult, unpleasant, or dislikable that it exceeds my ability to remain impartial and pragmatic in their care.
I don’t know any physician it hasn’t happened to. And when it does, ending the doctor-patient relationship is the only effective answer.
It’s never easy sending that letter, telling someone that they need to seek care elsewhere, and often the specific reason is harder to define. In patients who are overtly rude or noncompliant it’s easy. But often a loss in objectivity is from something less tangible, such as the vagaries of personal chemistry.
I try to get along with all my patients. I really do. That’s part of the job. But sometimes, for whatever reason, it’s just an impossible task. Too many conflicts and differences of opinion over treatments, tests, diagnosis, what they read on Facebook … whatever.
Regardless of cause, professionalism requires that it be the end of the road. If I can’t objectively weigh a patient’s symptoms and treatment options, then I’m not going to be able to do my very best for them. And my very best is what every patient deserves.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Objectivity is tough, but essential: a critical part of patient care, allowing you to make appropriate decisions based on facts and circumstances, not emotions. We’re supposed to be compassionate Vulcans – able to logically weigh possibilities and treatment options under pressure, and at the same time exhibit empathy and sensitivity.
For the most part, all of us become very good at this juggling act. But we’re only human, and once the ability to do that with a given person is lost, it’s gone for good.
Have you ever lost objectivity with a patient? I have. Generally it involves the patient being so difficult, unpleasant, or dislikable that it exceeds my ability to remain impartial and pragmatic in their care.
I don’t know any physician it hasn’t happened to. And when it does, ending the doctor-patient relationship is the only effective answer.
It’s never easy sending that letter, telling someone that they need to seek care elsewhere, and often the specific reason is harder to define. In patients who are overtly rude or noncompliant it’s easy. But often a loss in objectivity is from something less tangible, such as the vagaries of personal chemistry.
I try to get along with all my patients. I really do. That’s part of the job. But sometimes, for whatever reason, it’s just an impossible task. Too many conflicts and differences of opinion over treatments, tests, diagnosis, what they read on Facebook … whatever.
Regardless of cause, professionalism requires that it be the end of the road. If I can’t objectively weigh a patient’s symptoms and treatment options, then I’m not going to be able to do my very best for them. And my very best is what every patient deserves.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Three ‘bad news’ payment changes coming soon for physicians
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
My experience of a COVID-19 vaccine breakthrough infection
Friday, July 16, 2021, marked the end of a week on duty in the hospital, and it was time to celebrate with a nice dinner out with my wife, since COVID-19 masking requirements had been lifted in our part of California for people like us who were fully vaccinated.
We always loved a nice dinner out and missed it so much during the pandemic. Unlike 6 months earlier, when I was administering dexamethasone, remdesivir, and high-flow oxygen to half of the patients on my panel, not a single patient was diagnosed with COVID-19, much less treated for it, during the previous week. We were doing so well in Sacramento that the hospital visitation rules had been relaxed and vaccinated patients were not required to have a negative COVID-19 test prior to hospital admission.
Saturday was game 5 of the NBA finals, so we had two couples join us for the game at our house; no masks because we were all vaccinated. On Sunday, we visited our neighbors who had just had a new baby boy and made them the gift of some baby books. The new mom had struggled with the decision of whether to get vaccinated during her pregnancy, but eventually decided to complete the vaccination cycle prior to delivery. She was fully immune at the time of the baby’s birth, wisely wanting the baby to have passive immunity through her. We kept an appropriate distance, and never touched baby or mom, but since masking guidelines had been lifted for the vaccinated,we didn’t bother with them.
On Monday, I felt a little something in my nose but still pursued my usual workout. Interestingly, my performance wasn’t up to my usual standards. There was a meeting that evening that I had to prepare for, when all of a sudden I felt very fatigued. I lay down and slept for a good hour, which disrupted my preparation. I warned the participants that I was feeling a little under the weather, but they wanted to proceed. At this point, I decided it was time to start wearing a mask again.
More meetings on Tuesday morning, but I made sure that I was fully masked. That little thing in my nose had blown up into a full-scale rhinitis, requiring Kleenex and decongestants. Plus, the fatigue was hitting me very hard. “Dang!” I thought. “I haven’t had a cold since 2019. All those COVID-19 precautions not only worked against COVID-19 (which I never got) but also worked against the common cold, which I had now.”
I finished up my meetings and laid down for a good hour and a half. As the father of two, I had plenty of experience with the common cold, and I knew that plenty of rest and hydration was the key to kicking this thing. Besides, my 55th birthday was coming up, and I wanted to make sure I was fully recovered for the festivities my wife was planning for me. Nonetheless, I scheduled myself for a COVID-19 test. I knew this couldn’t be COVID-19 because I was fully vaccinated, but it was hitting me so hard. It had to be a virus that my body had never seen before; maybe the human metapneumovirus. That was my line of reasoning, anyway.
Wednesday was another day on the couch because of continued severe fatigue and myalgias. I figured another good day of rest would help me kick this cold in time for my birthday celebration. Then the COVID-19 results came back positive. “How could this be? I was vaccinated?!” Admittedly I had been more relaxed with masking, per the CDC and county guidelines, but I always wore a mask when I was seeing patients in the hospital. Yeah, I wasn’t wearing an N95 anymore, and I had given up my goggles months ago, but we just weren’t seeing much COVID-19 anymore, so a plain surgical mask was all that was required and seemed sufficient. I had been reading articles about the new Delta variant that was becoming dominant across the country, and reports were that the vaccine was still effective against the Delta variant. However, I was experiencing the COVID-19 vaccine breakthrough infection because of the remarkable talent the Delta variant has for replicating and producing high levels of viremia.
My first thoughts were for my family, of course. As my illness unfolded, I had kept checking in with them to see if they had any of these “cold” symptoms I had; none of them did. When my test came back positive, we all went into quarantine immediately and they went to get tested; all of them were negative. Next, I contacted the people I had been meeting with that week and warned them that I had tested positive. Despite my mask, and their fully vaccinated status, they needed to get tested. They did, and they were negative. I realized that I was probably contagious, though asymptomatic, on Saturday night when we had friends over to watch the NBA finals. Yeah, everyone was vaccinated, but if I could get sick from this new Delta variant, they could too. The public health department sent me a survey when they found out about my positive test, and they pinpointed Saturday as the day I started to be contagious. I told my friends that I was probably contagious when they were over for the game, and that they should get tested. They did, and everyone came back negative for COVID-19.
Wait a minute; what about Sunday night? The newborn baby and the sleep-deprived mom. Oh no! I was contagious then as well. We kept our distance, and were only there for about 10 minutes, but if I felt bad from COVID-19, I felt worse for exposing them to the virus.
I am no Anthony Fauci, and I am grateful that we have had levelheaded scientists like him to lead us through this terrible experience. I am sure there will be many papers written about COVID-19 breakthrough infections in the future, but I have many thoughts from this experience. First, my practice of wearing an N95 and goggles for all patients, not just COVID-19 patients, during the height of the pandemic was effective. Prior to getting vaccinated, my antibody tests were negative, so I never contracted the illness when I stuck to this regimen. Second, we all want to get back to something that looks like “normal,” but because there are large unvaccinated populations in the community the virus will continue to propagate and evolve, and hence everyone is at risk. While the guidelines said it was okay to ease up on our restrictions, because so many people are not vaccinated, we all must continue to keep our guard up. Third, would a booster shot have saved me from this fate? Because I was on the front lines of the pandemic as a hospitalist, I was also among the first members of my community to get vaccinated, receiving my second shot on Jan. 14, 2021. My wife was not in any risk group, was not on any vaccine priority list, and didn’t complete the series until early April. If I was going to give the infection to anyone, it would have been her. Not only did she never develop symptoms, but she also repeatedly tested negative, as did everyone else that I was in contact with when I was most contagious. The thing that was different about me from everyone else was that I had gotten the vaccine well ahead of them. Had my immunity waned over the months?
The good news is that, while I wouldn’t characterize what I had as “mild,” it certainly wasn’t protracted. Yes, I was a good boy, and did the basics: stay hydrated and get plenty of sleep. I was really bad off for about 3 days, and I hate to think what it would have been like if I had coexisting conditions such as asthma or diabetes. We all know what a bad case of COVID-19 looks like in the unvaccinated, with months in the hospital, intravenous infusions, and high-flow oxygen for the lucky ones. I had nothing remotely like that. The dominant symptom I had was incapacitating fatigue and significant body aches. The second night I had some major chills, sweats, and wild dreams. From a respiratory standpoint, I had bad rhinitis and a wicked cough for a while that tapered off. My oxygen saturations dropped into the mid 90’s, but never below 94%. But if I had been ten times sicker, I doubt I would have survived. I was on quarantine for 10 days but I highly doubt I was at all contagious by day 5, based on my symptoms and the fact that nobody around me turned COVID positive with repeat testing.
I was so relieved that none of my contacts when I was most contagious turned positive for COVID-19. Though not scientific, I find that illustrative. While I should have canceled my meetings on Monday and Tuesday, everybody knew I had a “cold” and nobody wanted to cancel. Nobody thought it possible that I had COVID-19, especially me. The Delta variant is notorious for generating high levels of viremia, yet I didn’t get anybody sick, not even my wife. That suggests to me that, while the vaccine doesn’t eliminate the risk of infection – which we already knew – it probably significantly reduced my infectivity. For that I am very grateful. Now that I can say that I had the COVID-19 experience, I can tell you it feels terrible. But I would have felt much worse if I had gotten others ill. My personal belief is that while the vaccine didn’t save me from disease, it dramatically truncated my illness, and significantly reduced my risk of passing the virus on to my friends and family.
So where did I contract the virus? We were unmasked at dinner on Friday night, which was acceptable in Yolo County at that time. By the way, I actually live in Yolo County, not YOLO (you only live once) county. You can imagine the latter would be a bit more loosey-goosey with the masking requirements. That notwithstanding, I don’t think the dinner was where I picked it up because it was too short of an incubation period. My wife and I obviously reacted differently, as I discussed, but we were both at the restaurant. She didn’t get COVID-19 and I did. I think that I probably picked it up at the hospital, because, while I was wearing a mask there, I was only wearing a surgical mask, not an N95. And I wasn’t wearing goggles anymore. While none of my patients were officially diagnosed with COVID-19, I was encountering a lot of people, getting in relatively close contact, and guidelines were being relaxed, including preadmission COVID-19 testing.
I was an outlier, as I have pointed out; none of my other close contacts contracted COVID-19. A lot of politics and public opinion is driven by outlier cases, and even pure fabrications these days; we certainly can’t create public health policy based on an outlier. I am not suggesting that my experience is any basis for rewriting the rules of COVID-19. The experience has given me pause to think through many facets of this horrible illness we have had to deal with in so many ways, however. And I have also reexamined my own practice for protecting myself in the hospital. Clearly what I was doing in the height of the pandemic was effective, and my more relaxed recent practices were not. Now that I am fully recovered after a relatively unique encounter with the condition, I look forward to seeing what the scientists and public policy makers do with COVID-19 vaccine breakthrough cases. So, between us hospitalist friends and colleagues, regardless of the policy guidelines, I say we keep on masking.
Dr. McIlraith is the founding chairman of the hospital medicine department at Mercy Medical Group in Sacramento. He received the SHM Award for Outstanding Service in Hospital Medicine in 2016.
Friday, July 16, 2021, marked the end of a week on duty in the hospital, and it was time to celebrate with a nice dinner out with my wife, since COVID-19 masking requirements had been lifted in our part of California for people like us who were fully vaccinated.
We always loved a nice dinner out and missed it so much during the pandemic. Unlike 6 months earlier, when I was administering dexamethasone, remdesivir, and high-flow oxygen to half of the patients on my panel, not a single patient was diagnosed with COVID-19, much less treated for it, during the previous week. We were doing so well in Sacramento that the hospital visitation rules had been relaxed and vaccinated patients were not required to have a negative COVID-19 test prior to hospital admission.
Saturday was game 5 of the NBA finals, so we had two couples join us for the game at our house; no masks because we were all vaccinated. On Sunday, we visited our neighbors who had just had a new baby boy and made them the gift of some baby books. The new mom had struggled with the decision of whether to get vaccinated during her pregnancy, but eventually decided to complete the vaccination cycle prior to delivery. She was fully immune at the time of the baby’s birth, wisely wanting the baby to have passive immunity through her. We kept an appropriate distance, and never touched baby or mom, but since masking guidelines had been lifted for the vaccinated,we didn’t bother with them.
On Monday, I felt a little something in my nose but still pursued my usual workout. Interestingly, my performance wasn’t up to my usual standards. There was a meeting that evening that I had to prepare for, when all of a sudden I felt very fatigued. I lay down and slept for a good hour, which disrupted my preparation. I warned the participants that I was feeling a little under the weather, but they wanted to proceed. At this point, I decided it was time to start wearing a mask again.
More meetings on Tuesday morning, but I made sure that I was fully masked. That little thing in my nose had blown up into a full-scale rhinitis, requiring Kleenex and decongestants. Plus, the fatigue was hitting me very hard. “Dang!” I thought. “I haven’t had a cold since 2019. All those COVID-19 precautions not only worked against COVID-19 (which I never got) but also worked against the common cold, which I had now.”
I finished up my meetings and laid down for a good hour and a half. As the father of two, I had plenty of experience with the common cold, and I knew that plenty of rest and hydration was the key to kicking this thing. Besides, my 55th birthday was coming up, and I wanted to make sure I was fully recovered for the festivities my wife was planning for me. Nonetheless, I scheduled myself for a COVID-19 test. I knew this couldn’t be COVID-19 because I was fully vaccinated, but it was hitting me so hard. It had to be a virus that my body had never seen before; maybe the human metapneumovirus. That was my line of reasoning, anyway.
Wednesday was another day on the couch because of continued severe fatigue and myalgias. I figured another good day of rest would help me kick this cold in time for my birthday celebration. Then the COVID-19 results came back positive. “How could this be? I was vaccinated?!” Admittedly I had been more relaxed with masking, per the CDC and county guidelines, but I always wore a mask when I was seeing patients in the hospital. Yeah, I wasn’t wearing an N95 anymore, and I had given up my goggles months ago, but we just weren’t seeing much COVID-19 anymore, so a plain surgical mask was all that was required and seemed sufficient. I had been reading articles about the new Delta variant that was becoming dominant across the country, and reports were that the vaccine was still effective against the Delta variant. However, I was experiencing the COVID-19 vaccine breakthrough infection because of the remarkable talent the Delta variant has for replicating and producing high levels of viremia.
My first thoughts were for my family, of course. As my illness unfolded, I had kept checking in with them to see if they had any of these “cold” symptoms I had; none of them did. When my test came back positive, we all went into quarantine immediately and they went to get tested; all of them were negative. Next, I contacted the people I had been meeting with that week and warned them that I had tested positive. Despite my mask, and their fully vaccinated status, they needed to get tested. They did, and they were negative. I realized that I was probably contagious, though asymptomatic, on Saturday night when we had friends over to watch the NBA finals. Yeah, everyone was vaccinated, but if I could get sick from this new Delta variant, they could too. The public health department sent me a survey when they found out about my positive test, and they pinpointed Saturday as the day I started to be contagious. I told my friends that I was probably contagious when they were over for the game, and that they should get tested. They did, and everyone came back negative for COVID-19.
Wait a minute; what about Sunday night? The newborn baby and the sleep-deprived mom. Oh no! I was contagious then as well. We kept our distance, and were only there for about 10 minutes, but if I felt bad from COVID-19, I felt worse for exposing them to the virus.
I am no Anthony Fauci, and I am grateful that we have had levelheaded scientists like him to lead us through this terrible experience. I am sure there will be many papers written about COVID-19 breakthrough infections in the future, but I have many thoughts from this experience. First, my practice of wearing an N95 and goggles for all patients, not just COVID-19 patients, during the height of the pandemic was effective. Prior to getting vaccinated, my antibody tests were negative, so I never contracted the illness when I stuck to this regimen. Second, we all want to get back to something that looks like “normal,” but because there are large unvaccinated populations in the community the virus will continue to propagate and evolve, and hence everyone is at risk. While the guidelines said it was okay to ease up on our restrictions, because so many people are not vaccinated, we all must continue to keep our guard up. Third, would a booster shot have saved me from this fate? Because I was on the front lines of the pandemic as a hospitalist, I was also among the first members of my community to get vaccinated, receiving my second shot on Jan. 14, 2021. My wife was not in any risk group, was not on any vaccine priority list, and didn’t complete the series until early April. If I was going to give the infection to anyone, it would have been her. Not only did she never develop symptoms, but she also repeatedly tested negative, as did everyone else that I was in contact with when I was most contagious. The thing that was different about me from everyone else was that I had gotten the vaccine well ahead of them. Had my immunity waned over the months?
The good news is that, while I wouldn’t characterize what I had as “mild,” it certainly wasn’t protracted. Yes, I was a good boy, and did the basics: stay hydrated and get plenty of sleep. I was really bad off for about 3 days, and I hate to think what it would have been like if I had coexisting conditions such as asthma or diabetes. We all know what a bad case of COVID-19 looks like in the unvaccinated, with months in the hospital, intravenous infusions, and high-flow oxygen for the lucky ones. I had nothing remotely like that. The dominant symptom I had was incapacitating fatigue and significant body aches. The second night I had some major chills, sweats, and wild dreams. From a respiratory standpoint, I had bad rhinitis and a wicked cough for a while that tapered off. My oxygen saturations dropped into the mid 90’s, but never below 94%. But if I had been ten times sicker, I doubt I would have survived. I was on quarantine for 10 days but I highly doubt I was at all contagious by day 5, based on my symptoms and the fact that nobody around me turned COVID positive with repeat testing.
I was so relieved that none of my contacts when I was most contagious turned positive for COVID-19. Though not scientific, I find that illustrative. While I should have canceled my meetings on Monday and Tuesday, everybody knew I had a “cold” and nobody wanted to cancel. Nobody thought it possible that I had COVID-19, especially me. The Delta variant is notorious for generating high levels of viremia, yet I didn’t get anybody sick, not even my wife. That suggests to me that, while the vaccine doesn’t eliminate the risk of infection – which we already knew – it probably significantly reduced my infectivity. For that I am very grateful. Now that I can say that I had the COVID-19 experience, I can tell you it feels terrible. But I would have felt much worse if I had gotten others ill. My personal belief is that while the vaccine didn’t save me from disease, it dramatically truncated my illness, and significantly reduced my risk of passing the virus on to my friends and family.
So where did I contract the virus? We were unmasked at dinner on Friday night, which was acceptable in Yolo County at that time. By the way, I actually live in Yolo County, not YOLO (you only live once) county. You can imagine the latter would be a bit more loosey-goosey with the masking requirements. That notwithstanding, I don’t think the dinner was where I picked it up because it was too short of an incubation period. My wife and I obviously reacted differently, as I discussed, but we were both at the restaurant. She didn’t get COVID-19 and I did. I think that I probably picked it up at the hospital, because, while I was wearing a mask there, I was only wearing a surgical mask, not an N95. And I wasn’t wearing goggles anymore. While none of my patients were officially diagnosed with COVID-19, I was encountering a lot of people, getting in relatively close contact, and guidelines were being relaxed, including preadmission COVID-19 testing.
I was an outlier, as I have pointed out; none of my other close contacts contracted COVID-19. A lot of politics and public opinion is driven by outlier cases, and even pure fabrications these days; we certainly can’t create public health policy based on an outlier. I am not suggesting that my experience is any basis for rewriting the rules of COVID-19. The experience has given me pause to think through many facets of this horrible illness we have had to deal with in so many ways, however. And I have also reexamined my own practice for protecting myself in the hospital. Clearly what I was doing in the height of the pandemic was effective, and my more relaxed recent practices were not. Now that I am fully recovered after a relatively unique encounter with the condition, I look forward to seeing what the scientists and public policy makers do with COVID-19 vaccine breakthrough cases. So, between us hospitalist friends and colleagues, regardless of the policy guidelines, I say we keep on masking.
Dr. McIlraith is the founding chairman of the hospital medicine department at Mercy Medical Group in Sacramento. He received the SHM Award for Outstanding Service in Hospital Medicine in 2016.
Friday, July 16, 2021, marked the end of a week on duty in the hospital, and it was time to celebrate with a nice dinner out with my wife, since COVID-19 masking requirements had been lifted in our part of California for people like us who were fully vaccinated.
We always loved a nice dinner out and missed it so much during the pandemic. Unlike 6 months earlier, when I was administering dexamethasone, remdesivir, and high-flow oxygen to half of the patients on my panel, not a single patient was diagnosed with COVID-19, much less treated for it, during the previous week. We were doing so well in Sacramento that the hospital visitation rules had been relaxed and vaccinated patients were not required to have a negative COVID-19 test prior to hospital admission.
Saturday was game 5 of the NBA finals, so we had two couples join us for the game at our house; no masks because we were all vaccinated. On Sunday, we visited our neighbors who had just had a new baby boy and made them the gift of some baby books. The new mom had struggled with the decision of whether to get vaccinated during her pregnancy, but eventually decided to complete the vaccination cycle prior to delivery. She was fully immune at the time of the baby’s birth, wisely wanting the baby to have passive immunity through her. We kept an appropriate distance, and never touched baby or mom, but since masking guidelines had been lifted for the vaccinated,we didn’t bother with them.
On Monday, I felt a little something in my nose but still pursued my usual workout. Interestingly, my performance wasn’t up to my usual standards. There was a meeting that evening that I had to prepare for, when all of a sudden I felt very fatigued. I lay down and slept for a good hour, which disrupted my preparation. I warned the participants that I was feeling a little under the weather, but they wanted to proceed. At this point, I decided it was time to start wearing a mask again.
More meetings on Tuesday morning, but I made sure that I was fully masked. That little thing in my nose had blown up into a full-scale rhinitis, requiring Kleenex and decongestants. Plus, the fatigue was hitting me very hard. “Dang!” I thought. “I haven’t had a cold since 2019. All those COVID-19 precautions not only worked against COVID-19 (which I never got) but also worked against the common cold, which I had now.”
I finished up my meetings and laid down for a good hour and a half. As the father of two, I had plenty of experience with the common cold, and I knew that plenty of rest and hydration was the key to kicking this thing. Besides, my 55th birthday was coming up, and I wanted to make sure I was fully recovered for the festivities my wife was planning for me. Nonetheless, I scheduled myself for a COVID-19 test. I knew this couldn’t be COVID-19 because I was fully vaccinated, but it was hitting me so hard. It had to be a virus that my body had never seen before; maybe the human metapneumovirus. That was my line of reasoning, anyway.
Wednesday was another day on the couch because of continued severe fatigue and myalgias. I figured another good day of rest would help me kick this cold in time for my birthday celebration. Then the COVID-19 results came back positive. “How could this be? I was vaccinated?!” Admittedly I had been more relaxed with masking, per the CDC and county guidelines, but I always wore a mask when I was seeing patients in the hospital. Yeah, I wasn’t wearing an N95 anymore, and I had given up my goggles months ago, but we just weren’t seeing much COVID-19 anymore, so a plain surgical mask was all that was required and seemed sufficient. I had been reading articles about the new Delta variant that was becoming dominant across the country, and reports were that the vaccine was still effective against the Delta variant. However, I was experiencing the COVID-19 vaccine breakthrough infection because of the remarkable talent the Delta variant has for replicating and producing high levels of viremia.
My first thoughts were for my family, of course. As my illness unfolded, I had kept checking in with them to see if they had any of these “cold” symptoms I had; none of them did. When my test came back positive, we all went into quarantine immediately and they went to get tested; all of them were negative. Next, I contacted the people I had been meeting with that week and warned them that I had tested positive. Despite my mask, and their fully vaccinated status, they needed to get tested. They did, and they were negative. I realized that I was probably contagious, though asymptomatic, on Saturday night when we had friends over to watch the NBA finals. Yeah, everyone was vaccinated, but if I could get sick from this new Delta variant, they could too. The public health department sent me a survey when they found out about my positive test, and they pinpointed Saturday as the day I started to be contagious. I told my friends that I was probably contagious when they were over for the game, and that they should get tested. They did, and everyone came back negative for COVID-19.
Wait a minute; what about Sunday night? The newborn baby and the sleep-deprived mom. Oh no! I was contagious then as well. We kept our distance, and were only there for about 10 minutes, but if I felt bad from COVID-19, I felt worse for exposing them to the virus.
I am no Anthony Fauci, and I am grateful that we have had levelheaded scientists like him to lead us through this terrible experience. I am sure there will be many papers written about COVID-19 breakthrough infections in the future, but I have many thoughts from this experience. First, my practice of wearing an N95 and goggles for all patients, not just COVID-19 patients, during the height of the pandemic was effective. Prior to getting vaccinated, my antibody tests were negative, so I never contracted the illness when I stuck to this regimen. Second, we all want to get back to something that looks like “normal,” but because there are large unvaccinated populations in the community the virus will continue to propagate and evolve, and hence everyone is at risk. While the guidelines said it was okay to ease up on our restrictions, because so many people are not vaccinated, we all must continue to keep our guard up. Third, would a booster shot have saved me from this fate? Because I was on the front lines of the pandemic as a hospitalist, I was also among the first members of my community to get vaccinated, receiving my second shot on Jan. 14, 2021. My wife was not in any risk group, was not on any vaccine priority list, and didn’t complete the series until early April. If I was going to give the infection to anyone, it would have been her. Not only did she never develop symptoms, but she also repeatedly tested negative, as did everyone else that I was in contact with when I was most contagious. The thing that was different about me from everyone else was that I had gotten the vaccine well ahead of them. Had my immunity waned over the months?
The good news is that, while I wouldn’t characterize what I had as “mild,” it certainly wasn’t protracted. Yes, I was a good boy, and did the basics: stay hydrated and get plenty of sleep. I was really bad off for about 3 days, and I hate to think what it would have been like if I had coexisting conditions such as asthma or diabetes. We all know what a bad case of COVID-19 looks like in the unvaccinated, with months in the hospital, intravenous infusions, and high-flow oxygen for the lucky ones. I had nothing remotely like that. The dominant symptom I had was incapacitating fatigue and significant body aches. The second night I had some major chills, sweats, and wild dreams. From a respiratory standpoint, I had bad rhinitis and a wicked cough for a while that tapered off. My oxygen saturations dropped into the mid 90’s, but never below 94%. But if I had been ten times sicker, I doubt I would have survived. I was on quarantine for 10 days but I highly doubt I was at all contagious by day 5, based on my symptoms and the fact that nobody around me turned COVID positive with repeat testing.
I was so relieved that none of my contacts when I was most contagious turned positive for COVID-19. Though not scientific, I find that illustrative. While I should have canceled my meetings on Monday and Tuesday, everybody knew I had a “cold” and nobody wanted to cancel. Nobody thought it possible that I had COVID-19, especially me. The Delta variant is notorious for generating high levels of viremia, yet I didn’t get anybody sick, not even my wife. That suggests to me that, while the vaccine doesn’t eliminate the risk of infection – which we already knew – it probably significantly reduced my infectivity. For that I am very grateful. Now that I can say that I had the COVID-19 experience, I can tell you it feels terrible. But I would have felt much worse if I had gotten others ill. My personal belief is that while the vaccine didn’t save me from disease, it dramatically truncated my illness, and significantly reduced my risk of passing the virus on to my friends and family.
So where did I contract the virus? We were unmasked at dinner on Friday night, which was acceptable in Yolo County at that time. By the way, I actually live in Yolo County, not YOLO (you only live once) county. You can imagine the latter would be a bit more loosey-goosey with the masking requirements. That notwithstanding, I don’t think the dinner was where I picked it up because it was too short of an incubation period. My wife and I obviously reacted differently, as I discussed, but we were both at the restaurant. She didn’t get COVID-19 and I did. I think that I probably picked it up at the hospital, because, while I was wearing a mask there, I was only wearing a surgical mask, not an N95. And I wasn’t wearing goggles anymore. While none of my patients were officially diagnosed with COVID-19, I was encountering a lot of people, getting in relatively close contact, and guidelines were being relaxed, including preadmission COVID-19 testing.
I was an outlier, as I have pointed out; none of my other close contacts contracted COVID-19. A lot of politics and public opinion is driven by outlier cases, and even pure fabrications these days; we certainly can’t create public health policy based on an outlier. I am not suggesting that my experience is any basis for rewriting the rules of COVID-19. The experience has given me pause to think through many facets of this horrible illness we have had to deal with in so many ways, however. And I have also reexamined my own practice for protecting myself in the hospital. Clearly what I was doing in the height of the pandemic was effective, and my more relaxed recent practices were not. Now that I am fully recovered after a relatively unique encounter with the condition, I look forward to seeing what the scientists and public policy makers do with COVID-19 vaccine breakthrough cases. So, between us hospitalist friends and colleagues, regardless of the policy guidelines, I say we keep on masking.
Dr. McIlraith is the founding chairman of the hospital medicine department at Mercy Medical Group in Sacramento. He received the SHM Award for Outstanding Service in Hospital Medicine in 2016.
Case: Patient with statin-associated muscle symptoms
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
Addressing vaccine hesitancy with patients
Breakthrough with empathy and compassion
The COVID-19 pandemic is a worldwide tragedy. In the beginning there was a lack of testing, personal protective equipment, COVID tests, and support for health care workers and patients. As 2020 came to a close, the world was given a glimpse of hope with the development of a vaccine against the deadly virus. Many world citizens celebrated the scientific accomplishment and began to breathe a sigh of relief that there was an end in sight. However, the development and distribution of the COVID-19 vaccine revealed a new challenge, vaccine hesitancy.
Community members, young healthy people, and even critically ill hospitalized patients who have the fortune of surviving acute illness are hesitant to the COVID-19 vaccine. I recently cared for a critically ill young patient who was intubated for days with status asthmaticus, one of the worst cases I’d ever seen. She was extubated and made a full recovery. Prior to discharge I asked if she wanted the first dose of the COVID-19 vaccine and she said, “No.” I was shocked. This was an otherwise healthy 30-something-year-old who was lucky enough to survive without any underlying infection in the setting of severe obstructive lung disease. A co-infection with COVID-19 would be disastrous and increase her mortality. I had a long talk at the bedside and asked the reason for her hesitancy. Her answer left me speechless, “I don’t know, I just don’t want to.” I ultimately convinced her that contracting COVID-19 would be a fate worse than she could imagine, and she agreed to the vaccine prior to discharge. This interaction made me ponder – “why are our patients, friends, and family members hesitant about receiving a lifesaving vaccine, especially when they are aware of how sick they or others can become without it?”
According to the World Health Organization, vaccine hesitancy refers to a delay in acceptance or refusal of vaccines despite availability of vaccine services. Vaccine hesitancy is complex and context specific, varying across time, place, and vaccines. It is influenced by factors such as complacency, convenience, and confidence.1 No vaccine is 100% effective. However, throughout history, the work of scientists and doctors to create vaccines saved millions of lives and revolutionized global health. Arguably, the single most life-saving innovation in the history of medicine, vaccines have eradicated smallpox, protected against whooping cough (1914), diphtheria (1926), tetanus (1938), influenza (1945) and mumps (1948), polio (1955), measles (1963), and rubella (1969), and worldwide vaccination rates increased dramatically thanks to successful global health campaigns.2 However, there was a paradox of vaccine success. As terrifying diseases decreased in prevalence, so did the fear of these diseases and their effects – paralysis, brain damage, blindness, and death. This gave birth to a new challenge in modern medicine, vaccine hesitancy – a privilege of first world nations.
Vaccines saved countless lives and improved health and wellbeing around the world for decades. However, to prevent the morbidity and mortality associated with vaccine-preventable diseases and their complications, and optimize control of vaccine-preventable diseases in communities, high vaccination rates must be achieved. Enter the COVID-19 pandemic, the creation of the COVID-19 vaccine, and vaccine hesitancy.
The question we ask ourselves as health care providers is ‘how do we convince the skeptics and those opposed to vaccination to take the vaccine?’ The answer is complicated. If you are like me, you’ve had many conversations with people – friends, patients, family members, who are resistant to the vaccine. Very often the facts are not well received, and those discussions end in argument, high emotions, and broken relationships. With the delta variant of COVID-19 on the rise, spreading aggressively among the unvaccinated, and increased hospitalizations, we foresee the reoccurrence of overwhelmed health systems and a continued death toll.
The new paradox we are faced with is that people choose to believe fiction versus fact, despite the real life evidence of the severe health effects and increased deaths related to COVID-19. Do these skeptics simply have a cavalier attitude towards not only their own life, but the lives of others? Or, is there something deeper? It is not enough to tell people that the vaccines are proven safe3 and are more widely available than ever. It is not enough to tell people that they can die of COVID-19 – they already know that. Emotional pleas to family members are falling on deaf ears. This past month, when asking patients why they don’t want the vaccine, many have no real legitimate health-related reason and respond with a simple, “I don’t want to.” So, how do we get through to the unvaccinated?
A compassionate approach
We navigate these difficult conversations over time with the approach of compassion and empathy, not hostility or bullying. As health care providers, we start by being good empathic listeners. Similar to when we have advance care planning and code status conversations, we cannot enter the dialogue with our intention, beliefs, or formulated goals for that person. We have to listen without judgement to the wide range of reasons why others are reluctant or unwilling to get the vaccine – historical mistrust, political identity, religious reasons, short-term side effects that may cause them to lose a day or two of work – and understand that for each person their reasons are different. The point is to not assume that you know or understand what barriers and beliefs they have towards vaccination, but to meet them at their point of view and listen while keeping your own emotions level and steady.
Identifying the reason for vaccine hesitancy is the first step to getting the unvaccinated closer to vaccination. Ask open ended questions: “Can you help me understand, what is your hesitancy to the vaccine?”; “What about the vaccine worries you?”; “What have you heard about/know about the COVID-19 vaccine?”; or “Can you tell me more about why you feel that way?” As meticulous as it sounds, we have to go back to the basics of patient interviewing.
It is important to remember that this is not a debate and escalation to arguments will certainly backfire. Think about any time you disagreed with someone on a topic. Did criticizing, blaming, and shaming ever convince you to change your beliefs or behaviors? The likely answer is, “No.” Avoid the “backfire effect”– which is when giving people facts disproving their “incorrect” beliefs can actually reinforce those beliefs. The more people are confronted with facts at odds with their opinions, the stronger they cling to those opinions. If you want them to change their mind, you cannot approach the conversation as a debate. You are having this vaccine discussion to try to meet the other person where they are, understand their position, and talk with them, and not at them, about their concerns.
As leaders in health care, we have to be willing to give up control and lead with empathy. We have to show others that we hear them, believe their concerns, and acknowledge that their beliefs are valid to them as individuals. Even if you disagree, this is not the place to let anger, disappointment, or resentment take a front seat. This is about balance, and highlighting the autonomy in decision making that the other person has to make a choice. Be humble in these conversations and avoid condescending tones or statements.
We already know that you are a caring health care provider. As hospitalists, we are frontline providers who have seen unnecessary deaths and illness due to COVID-19. You are passionate and motivated because you are committed to your oath to save lives. However, you have to check your own feelings and remember that you are not speaking with an unvaccinated person to make them get vaccinated, but rather to understand their cognitive process and hopefully walk with them down a path that provides them with a clarity of options they truly have. Extend empathy and they will see your motivation is rooted in good-heartedness and a concern for their wellbeing.
If someone admits to reasons for avoiding vaccination that are not rooted in any fact, then guide them to the best resources. Our health care system recently released a COVID-19 fact versus myth handout called Trust the Facts. This could be the kind of vetted resource you offer. Guide them to accredited websites, such as the World Health Organization, the Center for Disease Control, or their local and state departments of health to help debunk fiction by reviewing it with them. Discuss myths such as, ‘the vaccine will cause infertility,’ ‘the vaccine will give me COVID,’ ‘the vaccine was rushed and is not safe,’ ‘the vaccine is not needed if I am young and healthy,’ ‘the vaccine has a microchip,’ etc. Knowledge is power and disinformation is deadly, but how facts are presented will make the biggest difference in how others receive them, so remember your role is not to argue with these statements, but rather to provide perspective without agreeing or disagreeing.
Respond to their concerns with statements such as, “I hear you…it sounds like you are worried/fearful/mistrusting about the side effects/safety/efficacy of the vaccine…can we talk more about that?” Ask them where these concerns come from – the news, social media, an article, word of mouth, friends, or family. Ask them about the information they have and show genuine interest that you want to see it from their perspective. This is the key to compassionate and empathic dialogue – you relinquish your intentions.
Once you know or unveil their reasons for hesitancy, ask them what they would like to see with regards to COVID-19 and ending the pandemic. Would they like to get back to a new normal, to visit family members, to travel once again, to not have to wear masks and quarantine? What do they want for themselves, their families, communities, the country, or even the world? The goal is to find something in our shared humanity, to connect on a deeper level so they start to open up and let down walls, and find something you both see eye-to-eye on. Know your audience and speak to what serves them. To effectively persuade someone to come around to your point of view starts with recognizing the root of the disagreement and trying to overcome it before trying to change the person’s mind, understanding both the logic and the emotion that’s driving their decision making.4
Building trust
Reminding patients, friends or family members that their health and well-being means a lot to you can also be a strategy to keeping the conversation open and friendly. Sharing stories as hospitalists caring for many critically ill COVID patients or patients who died alone due to COVID-19, and the trauma you experienced as a health care provider feeling paralyzed by the limitations of modern medicine against the deadly virus, will only serve to humanize you in such an interaction.
Building trust will also increase vaccine willingness. This will require a concerted effort by scientists, doctors, and health care systems to engage with community leaders and members. To address hesitancy, the people we serve have to hear those local, personal, and relatable stories about vaccinations, and how it benefits not just themselves, but others around them in their community. As part of the #VaxUp campaign in Virginia, community and physician leaders shared their stories of hesitancy and motivation surrounding the vaccine. These are real people in the community discussing why getting vaccinated is so important and what helped them make an informed decision. I discussed my own hesitancy and concerns and also tackled a few vaccine myths.
As vaccinated health care workers or community leaders, you are living proof of the benefits of getting the COVID vaccine. Focus on the positives but also be honest. If your second shot gave you fevers, chills, or myalgias, then admit it and share how you overcame these expected reactions. Refocus on the safety of the vaccine and the fact that it is freely available to all people. Maybe the person you are speaking with doesn’t know where or how to get an appointment to get vaccinated. Help them find the nearest place to get an appointment and identify barriers they may have in transportation, child, or senior care to leave home safely to get vaccinated, or physical conditions that are preventing them from receiving the vaccine. Share that being vaccinated protects you from contracting the virus and spreading it to loved ones. Focus on how a fully vaccinated community and country can open up opportunities to heal and connect as a society, spend time with family/friends in another county or state, hold a newborn grandchild, or even travel outside the U.S.
There is no guarantee that you will be able to persuade someone to get vaccinated. It’s possible the outcome of your conversation will not result in the other person changing their mind in that moment. That doesn’t mean that you failed, because you started the dialogue and planted the seed. If you are a vaccinated health care provider, your words have influence and power, and we are obliged by our positions to have responsibility for the health of our communities. Don’t be discouraged, as it is through caring, compassionate, respectful, and empathic conversations that your influence will make the most difference in these relationships as you continue to advocate for all human life.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the Medical Executive Committee.
References
1. World Health Organization. Report of the SAGE working group on vaccine hesitancy. Oct 2014. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
2. Hsu JL. A brief history of vaccines: Smallpox to the present. S D Med. 2013;Spec no:33-7. PMID: 23444589.
3. Chiu A, Bever L. Are they experimental? Can they alter DNA? Experts tackle lingering coronavirus vaccine fears. The Washington Post. 2021 May 14. https://www.washingtonpost.com/lifestyle/2021/05/14/safe-fast-vaccine-fear-infertility-dna/
4. Huang L. Edge: Turning Adversity into Advantage. New York: Portfolio/Penguin, 2020.
Breakthrough with empathy and compassion
Breakthrough with empathy and compassion
The COVID-19 pandemic is a worldwide tragedy. In the beginning there was a lack of testing, personal protective equipment, COVID tests, and support for health care workers and patients. As 2020 came to a close, the world was given a glimpse of hope with the development of a vaccine against the deadly virus. Many world citizens celebrated the scientific accomplishment and began to breathe a sigh of relief that there was an end in sight. However, the development and distribution of the COVID-19 vaccine revealed a new challenge, vaccine hesitancy.
Community members, young healthy people, and even critically ill hospitalized patients who have the fortune of surviving acute illness are hesitant to the COVID-19 vaccine. I recently cared for a critically ill young patient who was intubated for days with status asthmaticus, one of the worst cases I’d ever seen. She was extubated and made a full recovery. Prior to discharge I asked if she wanted the first dose of the COVID-19 vaccine and she said, “No.” I was shocked. This was an otherwise healthy 30-something-year-old who was lucky enough to survive without any underlying infection in the setting of severe obstructive lung disease. A co-infection with COVID-19 would be disastrous and increase her mortality. I had a long talk at the bedside and asked the reason for her hesitancy. Her answer left me speechless, “I don’t know, I just don’t want to.” I ultimately convinced her that contracting COVID-19 would be a fate worse than she could imagine, and she agreed to the vaccine prior to discharge. This interaction made me ponder – “why are our patients, friends, and family members hesitant about receiving a lifesaving vaccine, especially when they are aware of how sick they or others can become without it?”
According to the World Health Organization, vaccine hesitancy refers to a delay in acceptance or refusal of vaccines despite availability of vaccine services. Vaccine hesitancy is complex and context specific, varying across time, place, and vaccines. It is influenced by factors such as complacency, convenience, and confidence.1 No vaccine is 100% effective. However, throughout history, the work of scientists and doctors to create vaccines saved millions of lives and revolutionized global health. Arguably, the single most life-saving innovation in the history of medicine, vaccines have eradicated smallpox, protected against whooping cough (1914), diphtheria (1926), tetanus (1938), influenza (1945) and mumps (1948), polio (1955), measles (1963), and rubella (1969), and worldwide vaccination rates increased dramatically thanks to successful global health campaigns.2 However, there was a paradox of vaccine success. As terrifying diseases decreased in prevalence, so did the fear of these diseases and their effects – paralysis, brain damage, blindness, and death. This gave birth to a new challenge in modern medicine, vaccine hesitancy – a privilege of first world nations.
Vaccines saved countless lives and improved health and wellbeing around the world for decades. However, to prevent the morbidity and mortality associated with vaccine-preventable diseases and their complications, and optimize control of vaccine-preventable diseases in communities, high vaccination rates must be achieved. Enter the COVID-19 pandemic, the creation of the COVID-19 vaccine, and vaccine hesitancy.
The question we ask ourselves as health care providers is ‘how do we convince the skeptics and those opposed to vaccination to take the vaccine?’ The answer is complicated. If you are like me, you’ve had many conversations with people – friends, patients, family members, who are resistant to the vaccine. Very often the facts are not well received, and those discussions end in argument, high emotions, and broken relationships. With the delta variant of COVID-19 on the rise, spreading aggressively among the unvaccinated, and increased hospitalizations, we foresee the reoccurrence of overwhelmed health systems and a continued death toll.
The new paradox we are faced with is that people choose to believe fiction versus fact, despite the real life evidence of the severe health effects and increased deaths related to COVID-19. Do these skeptics simply have a cavalier attitude towards not only their own life, but the lives of others? Or, is there something deeper? It is not enough to tell people that the vaccines are proven safe3 and are more widely available than ever. It is not enough to tell people that they can die of COVID-19 – they already know that. Emotional pleas to family members are falling on deaf ears. This past month, when asking patients why they don’t want the vaccine, many have no real legitimate health-related reason and respond with a simple, “I don’t want to.” So, how do we get through to the unvaccinated?
A compassionate approach
We navigate these difficult conversations over time with the approach of compassion and empathy, not hostility or bullying. As health care providers, we start by being good empathic listeners. Similar to when we have advance care planning and code status conversations, we cannot enter the dialogue with our intention, beliefs, or formulated goals for that person. We have to listen without judgement to the wide range of reasons why others are reluctant or unwilling to get the vaccine – historical mistrust, political identity, religious reasons, short-term side effects that may cause them to lose a day or two of work – and understand that for each person their reasons are different. The point is to not assume that you know or understand what barriers and beliefs they have towards vaccination, but to meet them at their point of view and listen while keeping your own emotions level and steady.
Identifying the reason for vaccine hesitancy is the first step to getting the unvaccinated closer to vaccination. Ask open ended questions: “Can you help me understand, what is your hesitancy to the vaccine?”; “What about the vaccine worries you?”; “What have you heard about/know about the COVID-19 vaccine?”; or “Can you tell me more about why you feel that way?” As meticulous as it sounds, we have to go back to the basics of patient interviewing.
It is important to remember that this is not a debate and escalation to arguments will certainly backfire. Think about any time you disagreed with someone on a topic. Did criticizing, blaming, and shaming ever convince you to change your beliefs or behaviors? The likely answer is, “No.” Avoid the “backfire effect”– which is when giving people facts disproving their “incorrect” beliefs can actually reinforce those beliefs. The more people are confronted with facts at odds with their opinions, the stronger they cling to those opinions. If you want them to change their mind, you cannot approach the conversation as a debate. You are having this vaccine discussion to try to meet the other person where they are, understand their position, and talk with them, and not at them, about their concerns.
As leaders in health care, we have to be willing to give up control and lead with empathy. We have to show others that we hear them, believe their concerns, and acknowledge that their beliefs are valid to them as individuals. Even if you disagree, this is not the place to let anger, disappointment, or resentment take a front seat. This is about balance, and highlighting the autonomy in decision making that the other person has to make a choice. Be humble in these conversations and avoid condescending tones or statements.
We already know that you are a caring health care provider. As hospitalists, we are frontline providers who have seen unnecessary deaths and illness due to COVID-19. You are passionate and motivated because you are committed to your oath to save lives. However, you have to check your own feelings and remember that you are not speaking with an unvaccinated person to make them get vaccinated, but rather to understand their cognitive process and hopefully walk with them down a path that provides them with a clarity of options they truly have. Extend empathy and they will see your motivation is rooted in good-heartedness and a concern for their wellbeing.
If someone admits to reasons for avoiding vaccination that are not rooted in any fact, then guide them to the best resources. Our health care system recently released a COVID-19 fact versus myth handout called Trust the Facts. This could be the kind of vetted resource you offer. Guide them to accredited websites, such as the World Health Organization, the Center for Disease Control, or their local and state departments of health to help debunk fiction by reviewing it with them. Discuss myths such as, ‘the vaccine will cause infertility,’ ‘the vaccine will give me COVID,’ ‘the vaccine was rushed and is not safe,’ ‘the vaccine is not needed if I am young and healthy,’ ‘the vaccine has a microchip,’ etc. Knowledge is power and disinformation is deadly, but how facts are presented will make the biggest difference in how others receive them, so remember your role is not to argue with these statements, but rather to provide perspective without agreeing or disagreeing.
Respond to their concerns with statements such as, “I hear you…it sounds like you are worried/fearful/mistrusting about the side effects/safety/efficacy of the vaccine…can we talk more about that?” Ask them where these concerns come from – the news, social media, an article, word of mouth, friends, or family. Ask them about the information they have and show genuine interest that you want to see it from their perspective. This is the key to compassionate and empathic dialogue – you relinquish your intentions.
Once you know or unveil their reasons for hesitancy, ask them what they would like to see with regards to COVID-19 and ending the pandemic. Would they like to get back to a new normal, to visit family members, to travel once again, to not have to wear masks and quarantine? What do they want for themselves, their families, communities, the country, or even the world? The goal is to find something in our shared humanity, to connect on a deeper level so they start to open up and let down walls, and find something you both see eye-to-eye on. Know your audience and speak to what serves them. To effectively persuade someone to come around to your point of view starts with recognizing the root of the disagreement and trying to overcome it before trying to change the person’s mind, understanding both the logic and the emotion that’s driving their decision making.4
Building trust
Reminding patients, friends or family members that their health and well-being means a lot to you can also be a strategy to keeping the conversation open and friendly. Sharing stories as hospitalists caring for many critically ill COVID patients or patients who died alone due to COVID-19, and the trauma you experienced as a health care provider feeling paralyzed by the limitations of modern medicine against the deadly virus, will only serve to humanize you in such an interaction.
Building trust will also increase vaccine willingness. This will require a concerted effort by scientists, doctors, and health care systems to engage with community leaders and members. To address hesitancy, the people we serve have to hear those local, personal, and relatable stories about vaccinations, and how it benefits not just themselves, but others around them in their community. As part of the #VaxUp campaign in Virginia, community and physician leaders shared their stories of hesitancy and motivation surrounding the vaccine. These are real people in the community discussing why getting vaccinated is so important and what helped them make an informed decision. I discussed my own hesitancy and concerns and also tackled a few vaccine myths.
As vaccinated health care workers or community leaders, you are living proof of the benefits of getting the COVID vaccine. Focus on the positives but also be honest. If your second shot gave you fevers, chills, or myalgias, then admit it and share how you overcame these expected reactions. Refocus on the safety of the vaccine and the fact that it is freely available to all people. Maybe the person you are speaking with doesn’t know where or how to get an appointment to get vaccinated. Help them find the nearest place to get an appointment and identify barriers they may have in transportation, child, or senior care to leave home safely to get vaccinated, or physical conditions that are preventing them from receiving the vaccine. Share that being vaccinated protects you from contracting the virus and spreading it to loved ones. Focus on how a fully vaccinated community and country can open up opportunities to heal and connect as a society, spend time with family/friends in another county or state, hold a newborn grandchild, or even travel outside the U.S.
There is no guarantee that you will be able to persuade someone to get vaccinated. It’s possible the outcome of your conversation will not result in the other person changing their mind in that moment. That doesn’t mean that you failed, because you started the dialogue and planted the seed. If you are a vaccinated health care provider, your words have influence and power, and we are obliged by our positions to have responsibility for the health of our communities. Don’t be discouraged, as it is through caring, compassionate, respectful, and empathic conversations that your influence will make the most difference in these relationships as you continue to advocate for all human life.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the Medical Executive Committee.
References
1. World Health Organization. Report of the SAGE working group on vaccine hesitancy. Oct 2014. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
2. Hsu JL. A brief history of vaccines: Smallpox to the present. S D Med. 2013;Spec no:33-7. PMID: 23444589.
3. Chiu A, Bever L. Are they experimental? Can they alter DNA? Experts tackle lingering coronavirus vaccine fears. The Washington Post. 2021 May 14. https://www.washingtonpost.com/lifestyle/2021/05/14/safe-fast-vaccine-fear-infertility-dna/
4. Huang L. Edge: Turning Adversity into Advantage. New York: Portfolio/Penguin, 2020.
The COVID-19 pandemic is a worldwide tragedy. In the beginning there was a lack of testing, personal protective equipment, COVID tests, and support for health care workers and patients. As 2020 came to a close, the world was given a glimpse of hope with the development of a vaccine against the deadly virus. Many world citizens celebrated the scientific accomplishment and began to breathe a sigh of relief that there was an end in sight. However, the development and distribution of the COVID-19 vaccine revealed a new challenge, vaccine hesitancy.
Community members, young healthy people, and even critically ill hospitalized patients who have the fortune of surviving acute illness are hesitant to the COVID-19 vaccine. I recently cared for a critically ill young patient who was intubated for days with status asthmaticus, one of the worst cases I’d ever seen. She was extubated and made a full recovery. Prior to discharge I asked if she wanted the first dose of the COVID-19 vaccine and she said, “No.” I was shocked. This was an otherwise healthy 30-something-year-old who was lucky enough to survive without any underlying infection in the setting of severe obstructive lung disease. A co-infection with COVID-19 would be disastrous and increase her mortality. I had a long talk at the bedside and asked the reason for her hesitancy. Her answer left me speechless, “I don’t know, I just don’t want to.” I ultimately convinced her that contracting COVID-19 would be a fate worse than she could imagine, and she agreed to the vaccine prior to discharge. This interaction made me ponder – “why are our patients, friends, and family members hesitant about receiving a lifesaving vaccine, especially when they are aware of how sick they or others can become without it?”
According to the World Health Organization, vaccine hesitancy refers to a delay in acceptance or refusal of vaccines despite availability of vaccine services. Vaccine hesitancy is complex and context specific, varying across time, place, and vaccines. It is influenced by factors such as complacency, convenience, and confidence.1 No vaccine is 100% effective. However, throughout history, the work of scientists and doctors to create vaccines saved millions of lives and revolutionized global health. Arguably, the single most life-saving innovation in the history of medicine, vaccines have eradicated smallpox, protected against whooping cough (1914), diphtheria (1926), tetanus (1938), influenza (1945) and mumps (1948), polio (1955), measles (1963), and rubella (1969), and worldwide vaccination rates increased dramatically thanks to successful global health campaigns.2 However, there was a paradox of vaccine success. As terrifying diseases decreased in prevalence, so did the fear of these diseases and their effects – paralysis, brain damage, blindness, and death. This gave birth to a new challenge in modern medicine, vaccine hesitancy – a privilege of first world nations.
Vaccines saved countless lives and improved health and wellbeing around the world for decades. However, to prevent the morbidity and mortality associated with vaccine-preventable diseases and their complications, and optimize control of vaccine-preventable diseases in communities, high vaccination rates must be achieved. Enter the COVID-19 pandemic, the creation of the COVID-19 vaccine, and vaccine hesitancy.
The question we ask ourselves as health care providers is ‘how do we convince the skeptics and those opposed to vaccination to take the vaccine?’ The answer is complicated. If you are like me, you’ve had many conversations with people – friends, patients, family members, who are resistant to the vaccine. Very often the facts are not well received, and those discussions end in argument, high emotions, and broken relationships. With the delta variant of COVID-19 on the rise, spreading aggressively among the unvaccinated, and increased hospitalizations, we foresee the reoccurrence of overwhelmed health systems and a continued death toll.
The new paradox we are faced with is that people choose to believe fiction versus fact, despite the real life evidence of the severe health effects and increased deaths related to COVID-19. Do these skeptics simply have a cavalier attitude towards not only their own life, but the lives of others? Or, is there something deeper? It is not enough to tell people that the vaccines are proven safe3 and are more widely available than ever. It is not enough to tell people that they can die of COVID-19 – they already know that. Emotional pleas to family members are falling on deaf ears. This past month, when asking patients why they don’t want the vaccine, many have no real legitimate health-related reason and respond with a simple, “I don’t want to.” So, how do we get through to the unvaccinated?
A compassionate approach
We navigate these difficult conversations over time with the approach of compassion and empathy, not hostility or bullying. As health care providers, we start by being good empathic listeners. Similar to when we have advance care planning and code status conversations, we cannot enter the dialogue with our intention, beliefs, or formulated goals for that person. We have to listen without judgement to the wide range of reasons why others are reluctant or unwilling to get the vaccine – historical mistrust, political identity, religious reasons, short-term side effects that may cause them to lose a day or two of work – and understand that for each person their reasons are different. The point is to not assume that you know or understand what barriers and beliefs they have towards vaccination, but to meet them at their point of view and listen while keeping your own emotions level and steady.
Identifying the reason for vaccine hesitancy is the first step to getting the unvaccinated closer to vaccination. Ask open ended questions: “Can you help me understand, what is your hesitancy to the vaccine?”; “What about the vaccine worries you?”; “What have you heard about/know about the COVID-19 vaccine?”; or “Can you tell me more about why you feel that way?” As meticulous as it sounds, we have to go back to the basics of patient interviewing.
It is important to remember that this is not a debate and escalation to arguments will certainly backfire. Think about any time you disagreed with someone on a topic. Did criticizing, blaming, and shaming ever convince you to change your beliefs or behaviors? The likely answer is, “No.” Avoid the “backfire effect”– which is when giving people facts disproving their “incorrect” beliefs can actually reinforce those beliefs. The more people are confronted with facts at odds with their opinions, the stronger they cling to those opinions. If you want them to change their mind, you cannot approach the conversation as a debate. You are having this vaccine discussion to try to meet the other person where they are, understand their position, and talk with them, and not at them, about their concerns.
As leaders in health care, we have to be willing to give up control and lead with empathy. We have to show others that we hear them, believe their concerns, and acknowledge that their beliefs are valid to them as individuals. Even if you disagree, this is not the place to let anger, disappointment, or resentment take a front seat. This is about balance, and highlighting the autonomy in decision making that the other person has to make a choice. Be humble in these conversations and avoid condescending tones or statements.
We already know that you are a caring health care provider. As hospitalists, we are frontline providers who have seen unnecessary deaths and illness due to COVID-19. You are passionate and motivated because you are committed to your oath to save lives. However, you have to check your own feelings and remember that you are not speaking with an unvaccinated person to make them get vaccinated, but rather to understand their cognitive process and hopefully walk with them down a path that provides them with a clarity of options they truly have. Extend empathy and they will see your motivation is rooted in good-heartedness and a concern for their wellbeing.
If someone admits to reasons for avoiding vaccination that are not rooted in any fact, then guide them to the best resources. Our health care system recently released a COVID-19 fact versus myth handout called Trust the Facts. This could be the kind of vetted resource you offer. Guide them to accredited websites, such as the World Health Organization, the Center for Disease Control, or their local and state departments of health to help debunk fiction by reviewing it with them. Discuss myths such as, ‘the vaccine will cause infertility,’ ‘the vaccine will give me COVID,’ ‘the vaccine was rushed and is not safe,’ ‘the vaccine is not needed if I am young and healthy,’ ‘the vaccine has a microchip,’ etc. Knowledge is power and disinformation is deadly, but how facts are presented will make the biggest difference in how others receive them, so remember your role is not to argue with these statements, but rather to provide perspective without agreeing or disagreeing.
Respond to their concerns with statements such as, “I hear you…it sounds like you are worried/fearful/mistrusting about the side effects/safety/efficacy of the vaccine…can we talk more about that?” Ask them where these concerns come from – the news, social media, an article, word of mouth, friends, or family. Ask them about the information they have and show genuine interest that you want to see it from their perspective. This is the key to compassionate and empathic dialogue – you relinquish your intentions.
Once you know or unveil their reasons for hesitancy, ask them what they would like to see with regards to COVID-19 and ending the pandemic. Would they like to get back to a new normal, to visit family members, to travel once again, to not have to wear masks and quarantine? What do they want for themselves, their families, communities, the country, or even the world? The goal is to find something in our shared humanity, to connect on a deeper level so they start to open up and let down walls, and find something you both see eye-to-eye on. Know your audience and speak to what serves them. To effectively persuade someone to come around to your point of view starts with recognizing the root of the disagreement and trying to overcome it before trying to change the person’s mind, understanding both the logic and the emotion that’s driving their decision making.4
Building trust
Reminding patients, friends or family members that their health and well-being means a lot to you can also be a strategy to keeping the conversation open and friendly. Sharing stories as hospitalists caring for many critically ill COVID patients or patients who died alone due to COVID-19, and the trauma you experienced as a health care provider feeling paralyzed by the limitations of modern medicine against the deadly virus, will only serve to humanize you in such an interaction.
Building trust will also increase vaccine willingness. This will require a concerted effort by scientists, doctors, and health care systems to engage with community leaders and members. To address hesitancy, the people we serve have to hear those local, personal, and relatable stories about vaccinations, and how it benefits not just themselves, but others around them in their community. As part of the #VaxUp campaign in Virginia, community and physician leaders shared their stories of hesitancy and motivation surrounding the vaccine. These are real people in the community discussing why getting vaccinated is so important and what helped them make an informed decision. I discussed my own hesitancy and concerns and also tackled a few vaccine myths.
As vaccinated health care workers or community leaders, you are living proof of the benefits of getting the COVID vaccine. Focus on the positives but also be honest. If your second shot gave you fevers, chills, or myalgias, then admit it and share how you overcame these expected reactions. Refocus on the safety of the vaccine and the fact that it is freely available to all people. Maybe the person you are speaking with doesn’t know where or how to get an appointment to get vaccinated. Help them find the nearest place to get an appointment and identify barriers they may have in transportation, child, or senior care to leave home safely to get vaccinated, or physical conditions that are preventing them from receiving the vaccine. Share that being vaccinated protects you from contracting the virus and spreading it to loved ones. Focus on how a fully vaccinated community and country can open up opportunities to heal and connect as a society, spend time with family/friends in another county or state, hold a newborn grandchild, or even travel outside the U.S.
There is no guarantee that you will be able to persuade someone to get vaccinated. It’s possible the outcome of your conversation will not result in the other person changing their mind in that moment. That doesn’t mean that you failed, because you started the dialogue and planted the seed. If you are a vaccinated health care provider, your words have influence and power, and we are obliged by our positions to have responsibility for the health of our communities. Don’t be discouraged, as it is through caring, compassionate, respectful, and empathic conversations that your influence will make the most difference in these relationships as you continue to advocate for all human life.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the Medical Executive Committee.
References
1. World Health Organization. Report of the SAGE working group on vaccine hesitancy. Oct 2014. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
2. Hsu JL. A brief history of vaccines: Smallpox to the present. S D Med. 2013;Spec no:33-7. PMID: 23444589.
3. Chiu A, Bever L. Are they experimental? Can they alter DNA? Experts tackle lingering coronavirus vaccine fears. The Washington Post. 2021 May 14. https://www.washingtonpost.com/lifestyle/2021/05/14/safe-fast-vaccine-fear-infertility-dna/
4. Huang L. Edge: Turning Adversity into Advantage. New York: Portfolio/Penguin, 2020.
Should magnesium be used for COPD exacerbations?
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are a major driver of disease-related morbidity. Their prevention and treatment are a focus of COPD management. Antibiotics, corticosteroids, and nebulized bronchodilators are all given to patients with AECOPD, and while the supporting data aren’t perfect, there’s little debate surrounding their use. These medications are well known to most physicians; we’re comfortable with their efficacy and aware of their side effects. They are nothing if not familiar.
What about magnesium (Mg), though? Apparently, in the emergency room (ER) it is part of the standard AECOPD cocktail. I would argue that Mg is familiar to most too; every internal medicine trainee in the United States is taught to infuse 2 g of Mg intravenously for any inpatient (ICU or otherwise) with a serum level <2.0 mg/dL. In fact, “electrolyte protocols” are part of the order sets at most hospitals where I’ve worked. Mg is infused reflexively when it drops below certain levels.
I’m less familiar with using Mg in the setting of an AECOPD, though. A recent online post by an academic ER physician (Richard Pescatore, DO) urged caution in this setting. He argues that too many in the ER are embracing the “Dutch Hypothesis” and treating asthma and COPD as the same disease. Dr. Pescatore believes that Mg works for asthma exacerbations because asthma is a disease of smooth muscle and large airways, while COPD is not. COPD, he says, is a disease of the small airways, largely resulting from parenchymal distortions due to emphysema. Therefore, Mg, which is thought to act on the smooth muscle surrounding the large airways, won’t be beneficial for AECOPD and may even cause harm.
Data are lacking
What data exist for using Mg for AECOPD? The best randomized controlled trial (RCT) I could find was published in 1995 and is cited in the reader’s rebuttal. The trial found a significant improvement in peak expiratory flow rate (PEFR) with Mg and a nonsignificant reduction in hospitalizations.
A poorly done systematic review of RCTs using Mg for AECOPD was published in 2014, and in 2020 the Agency for Healthcare Research and Quality (AHRQ) included Mg in its well-executed meta-analysis of pharmacologic treatments for AECOPD. Data across the four to five Mg RCTs included in each of the reviews (study inclusion criteria were slightly different) could not be combined. All RCTs were small, and only soft outcomes like PEFR and forced expiratory volume in 1 second (FEV1) seemed to improve with Mg. No adverse events were noted, but this should be interpreted with caution given that many studies did not report on adverse events at all.
A small RCT published this year (after both systematic reviews were completed) showed that using intravenous magnesium sulfate had no significant effect on FEV1, vital signs, or symptoms.
In summary, the data aren’t great. Mg doesn’t show up at all as a treatment option in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report on COPD, and the authors of the AHRQ review concluded that large, high-quality RCTs are needed to assess the impact of Mg in AECOPD. Although I didn’t do an extensive review of Mg for asthma exacerbations, it’s not clear that the data here are much better. Mg gets an honorable mention (add for severe exacerbations when there’s inadequate response to standard treatments) in both the 2007 National Heart, Lung, and Blood Institute (NHLBI) guideline and the 2019 Global Initiative for Asthma (GINA) guide. The 2020 update to the 2007 NHLBI guideline is more targeted in its review and does not cover Mg as a treatment option. On the basis of my anecdotal clinical experience and on networking with airway experts, I do think Mg is used more often for asthma than for AECOPD.
Final thoughts on using Mg for AECOPD
All that being said, is it reasonable to use Mg for AECOPD? I think so. I’d stick to using it for severe cases where conventional treatments have failed, just like the NHLBI and GINA advise for asthma. I’d also limit it to 2-3 g, which is the dosing range employed by several of the existing AECOPD RCTs. The assertion that Mg may be harmful in AECOPD because COPD affects the small airways, and asthma does not, is misguided. Both affect the small airways. Furthermore, none of our inhaled therapies reach the small airways, so one can’t argue against using Mg because it only targets larger airways without abandoning albuterol and ipratropium as well. I don’t think anyone would advise that. Given what we now know about asthma and COPD phenotypes and asthma-COPD overlap, I’d caution against pedantic theories about response to therapies.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He has received research grants from Fisher-Paykel and has received payments from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are a major driver of disease-related morbidity. Their prevention and treatment are a focus of COPD management. Antibiotics, corticosteroids, and nebulized bronchodilators are all given to patients with AECOPD, and while the supporting data aren’t perfect, there’s little debate surrounding their use. These medications are well known to most physicians; we’re comfortable with their efficacy and aware of their side effects. They are nothing if not familiar.
What about magnesium (Mg), though? Apparently, in the emergency room (ER) it is part of the standard AECOPD cocktail. I would argue that Mg is familiar to most too; every internal medicine trainee in the United States is taught to infuse 2 g of Mg intravenously for any inpatient (ICU or otherwise) with a serum level <2.0 mg/dL. In fact, “electrolyte protocols” are part of the order sets at most hospitals where I’ve worked. Mg is infused reflexively when it drops below certain levels.
I’m less familiar with using Mg in the setting of an AECOPD, though. A recent online post by an academic ER physician (Richard Pescatore, DO) urged caution in this setting. He argues that too many in the ER are embracing the “Dutch Hypothesis” and treating asthma and COPD as the same disease. Dr. Pescatore believes that Mg works for asthma exacerbations because asthma is a disease of smooth muscle and large airways, while COPD is not. COPD, he says, is a disease of the small airways, largely resulting from parenchymal distortions due to emphysema. Therefore, Mg, which is thought to act on the smooth muscle surrounding the large airways, won’t be beneficial for AECOPD and may even cause harm.
Data are lacking
What data exist for using Mg for AECOPD? The best randomized controlled trial (RCT) I could find was published in 1995 and is cited in the reader’s rebuttal. The trial found a significant improvement in peak expiratory flow rate (PEFR) with Mg and a nonsignificant reduction in hospitalizations.
A poorly done systematic review of RCTs using Mg for AECOPD was published in 2014, and in 2020 the Agency for Healthcare Research and Quality (AHRQ) included Mg in its well-executed meta-analysis of pharmacologic treatments for AECOPD. Data across the four to five Mg RCTs included in each of the reviews (study inclusion criteria were slightly different) could not be combined. All RCTs were small, and only soft outcomes like PEFR and forced expiratory volume in 1 second (FEV1) seemed to improve with Mg. No adverse events were noted, but this should be interpreted with caution given that many studies did not report on adverse events at all.
A small RCT published this year (after both systematic reviews were completed) showed that using intravenous magnesium sulfate had no significant effect on FEV1, vital signs, or symptoms.
In summary, the data aren’t great. Mg doesn’t show up at all as a treatment option in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report on COPD, and the authors of the AHRQ review concluded that large, high-quality RCTs are needed to assess the impact of Mg in AECOPD. Although I didn’t do an extensive review of Mg for asthma exacerbations, it’s not clear that the data here are much better. Mg gets an honorable mention (add for severe exacerbations when there’s inadequate response to standard treatments) in both the 2007 National Heart, Lung, and Blood Institute (NHLBI) guideline and the 2019 Global Initiative for Asthma (GINA) guide. The 2020 update to the 2007 NHLBI guideline is more targeted in its review and does not cover Mg as a treatment option. On the basis of my anecdotal clinical experience and on networking with airway experts, I do think Mg is used more often for asthma than for AECOPD.
Final thoughts on using Mg for AECOPD
All that being said, is it reasonable to use Mg for AECOPD? I think so. I’d stick to using it for severe cases where conventional treatments have failed, just like the NHLBI and GINA advise for asthma. I’d also limit it to 2-3 g, which is the dosing range employed by several of the existing AECOPD RCTs. The assertion that Mg may be harmful in AECOPD because COPD affects the small airways, and asthma does not, is misguided. Both affect the small airways. Furthermore, none of our inhaled therapies reach the small airways, so one can’t argue against using Mg because it only targets larger airways without abandoning albuterol and ipratropium as well. I don’t think anyone would advise that. Given what we now know about asthma and COPD phenotypes and asthma-COPD overlap, I’d caution against pedantic theories about response to therapies.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He has received research grants from Fisher-Paykel and has received payments from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are a major driver of disease-related morbidity. Their prevention and treatment are a focus of COPD management. Antibiotics, corticosteroids, and nebulized bronchodilators are all given to patients with AECOPD, and while the supporting data aren’t perfect, there’s little debate surrounding their use. These medications are well known to most physicians; we’re comfortable with their efficacy and aware of their side effects. They are nothing if not familiar.
What about magnesium (Mg), though? Apparently, in the emergency room (ER) it is part of the standard AECOPD cocktail. I would argue that Mg is familiar to most too; every internal medicine trainee in the United States is taught to infuse 2 g of Mg intravenously for any inpatient (ICU or otherwise) with a serum level <2.0 mg/dL. In fact, “electrolyte protocols” are part of the order sets at most hospitals where I’ve worked. Mg is infused reflexively when it drops below certain levels.
I’m less familiar with using Mg in the setting of an AECOPD, though. A recent online post by an academic ER physician (Richard Pescatore, DO) urged caution in this setting. He argues that too many in the ER are embracing the “Dutch Hypothesis” and treating asthma and COPD as the same disease. Dr. Pescatore believes that Mg works for asthma exacerbations because asthma is a disease of smooth muscle and large airways, while COPD is not. COPD, he says, is a disease of the small airways, largely resulting from parenchymal distortions due to emphysema. Therefore, Mg, which is thought to act on the smooth muscle surrounding the large airways, won’t be beneficial for AECOPD and may even cause harm.
Data are lacking
What data exist for using Mg for AECOPD? The best randomized controlled trial (RCT) I could find was published in 1995 and is cited in the reader’s rebuttal. The trial found a significant improvement in peak expiratory flow rate (PEFR) with Mg and a nonsignificant reduction in hospitalizations.
A poorly done systematic review of RCTs using Mg for AECOPD was published in 2014, and in 2020 the Agency for Healthcare Research and Quality (AHRQ) included Mg in its well-executed meta-analysis of pharmacologic treatments for AECOPD. Data across the four to five Mg RCTs included in each of the reviews (study inclusion criteria were slightly different) could not be combined. All RCTs were small, and only soft outcomes like PEFR and forced expiratory volume in 1 second (FEV1) seemed to improve with Mg. No adverse events were noted, but this should be interpreted with caution given that many studies did not report on adverse events at all.
A small RCT published this year (after both systematic reviews were completed) showed that using intravenous magnesium sulfate had no significant effect on FEV1, vital signs, or symptoms.
In summary, the data aren’t great. Mg doesn’t show up at all as a treatment option in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report on COPD, and the authors of the AHRQ review concluded that large, high-quality RCTs are needed to assess the impact of Mg in AECOPD. Although I didn’t do an extensive review of Mg for asthma exacerbations, it’s not clear that the data here are much better. Mg gets an honorable mention (add for severe exacerbations when there’s inadequate response to standard treatments) in both the 2007 National Heart, Lung, and Blood Institute (NHLBI) guideline and the 2019 Global Initiative for Asthma (GINA) guide. The 2020 update to the 2007 NHLBI guideline is more targeted in its review and does not cover Mg as a treatment option. On the basis of my anecdotal clinical experience and on networking with airway experts, I do think Mg is used more often for asthma than for AECOPD.
Final thoughts on using Mg for AECOPD
All that being said, is it reasonable to use Mg for AECOPD? I think so. I’d stick to using it for severe cases where conventional treatments have failed, just like the NHLBI and GINA advise for asthma. I’d also limit it to 2-3 g, which is the dosing range employed by several of the existing AECOPD RCTs. The assertion that Mg may be harmful in AECOPD because COPD affects the small airways, and asthma does not, is misguided. Both affect the small airways. Furthermore, none of our inhaled therapies reach the small airways, so one can’t argue against using Mg because it only targets larger airways without abandoning albuterol and ipratropium as well. I don’t think anyone would advise that. Given what we now know about asthma and COPD phenotypes and asthma-COPD overlap, I’d caution against pedantic theories about response to therapies.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He has received research grants from Fisher-Paykel and has received payments from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Should the starting age for CRC screening be lowered to 45?
Dear colleagues and friends,
The start age for colorectal cancer (CRC) screening in average-risk individuals has been relatively uncontroversial and unchallenged, until recent guidelines that recommended decreasing the start age from 50 to 45 years. In this edition of Perspectives, two renowned experts, Dr. Patel and Dr. Rabeneck, debate the pros and cons of this paradigm change. This will be my final Perspectives contribution as associate editor for GI & Hep News. Thank you very much for your support and feedback, and I hope you have all enjoyed and learned from the Perspectives debates as much as I have. As always, your feedback and suggestions for future topics are welcome and can be sent to ginews@gastro.org.
Charles J. Kahi, MD, MS, AGAF, is professor of medicine at Indiana University, Indianapolis.
45 is the new 50
BY SWATI G. PATEL, MD, MS
The incidence and mortality rates of CRC in individuals under the age of 50 years, or early age–onset CRC, is increasing in the United States.1 We do not fully understand the cause of this trend; however, it has been observed in multiple Westernized countries, consistent with a birth cohort effect in which generation-specific risk factors are likely responsible.1 To address this rising burden of early age–onset CRC, multiple professional societies, including the United States Preventive Services Task Force,2 now recommend decreasing the starting age for CRC screening in average-risk individuals from 50 to 45 years. These recommendations are supported by the fact that CRC incidence in 45- to 49-year-olds is now the same as that of populations already eligible for screening; the yield of screening appears to be similar among those aged 45-49 years, compared to those aged 50-59 years; and modeling studies show that the benefits outweigh the risks and costs. These factors, along with the unique benefits of early detection and prevention in young patients, support that 45 is the new 50 when it comes to CRC screening.
CRC incidence rates among 45- to 49-year-olds now match populations that are already eligible for CRC screening. The rates among 45- to 49-year-olds now is similar to the incidence rates observed in 50-year-olds in 1992, before widespread CRC screening was performed. Overall incidence rates in 45- to 49-year-olds now match incidence rates observed in 45- to 49-year-old African Americans, a population in which the American College of Gastroenterology and the U.S. Multi-Society Task Force have recommended average-risk screening beginning at age 45.
Advanced colorectal neoplasia rates in 45- to 49-year-olds are similar to rates observed in 50- to 59-year-olds. One study found that advanced colorectal neoplasia rates (including advanced colorectal polyps and adenocarcinoma) in average risk–equivalent 45- to 49-year-olds are similar rates of 50- to 54-year-old average-risk individuals.3 Similarly, a recent systematic review and meta-analysis of 17 international studies including average-risk individuals undergoing colonoscopy found no significant difference between advanced colorectal neoplasia rates in 45- to 49-years-olds compared to 50- to 59-year-olds.4 These data suggest that expanding screening to average-risk individuals aged 45-49 years will have yield similar to that of those aged 50-59.
Modeling studies show that the benefits of screening outweigh the potential harms and costs. A recent cost-effectiveness analysis showed that starting average-risk screening at age 45 years with colonoscopy would cost $33,900 per quality-adjusted life-year (QALY) or $7,700 per QALY if annual FIT is selected as the screening modality.5 These costs are less than the widely accepted willingness to pay threshold of $50,000 per QALY and less than currently accepted average-risk screening, such as annual or biennial mammograms for breast cancer screening.
Expanding screening to 45- to 49-year-olds can improve early detection of CRC and prevention of CRC. Offering screening before age 50 years provides the opportunity to detect cancers at earlier stages, which can improve overall survival. Recent data have shown an increase in CRC incidence among 50- to 54-year-olds.1 Although the etiology of the rise in this age group is likely multifactorial (low screening rates or generational risk that individuals carry with them through the birth-cohort effect), diagnosis and removal of advanced colorectal polyps among 45- to 49-year-olds can have a positive impact on reversing this trend in 50- to 54-year-olds. Furthermore, initiating conversations about CRC screening at age 45 can improve uptake of screening among 50- to 54-year-olds who may have delayed screening when first offered at age 50.
There are unique benefits of early detection and prevention of CRC in young patients. Although the absolute incidence and mortality rates associated with CRC in those aged 45-49 are significantly lower than the rates observed in those over age 50, young individuals are more likely to be in the prime of their earning and fertility potential and are more likely to be caregivers to younger and older generations. They are more likely to face material, psychological, and behavioral financial hardships, such as difficulty paying bills. Absolute incidence rates and modeling studies that do not account for these intangible effects on survivorship also do not fully reflect the societal benefit of early detection and CRC prevention in those aged 45-49 years.
There are certainly many unanswered questions about the effects of expanding CRC screening to those age 45 and older. Ongoing studies are needed to determine the cause of rising incidence, whether screening test selection and intervals should be customized for those under age 50, and how best to ensure equitable access to screening services. Finally, expanding screening to younger individuals should not detract from the critical importance of ongoing efforts to improve screening in those over age 50. As these issues are addressed by the scientific community, we cannot stand by idly when there is sufficient evidence to support that 45 is the new 50.
Dr. Patel is associate professor of medicine in the divisions of gastroenterology & hepatology at the University of Colorado and Rocky Mountain Regional Veterans Affairs Medical Center, both in Aurora, Colo. She disclosed financial relationships with Olympus America, ERBE USA, and Freenome.
References
1. Siegel RL et al. J Natl Cancer Inst. 2017. doi: 10.1093/jnci/djw322.
2. USPSTF et al. JAMA. 2021 May 18;325(19):1965-77.
3. Butterly LF et al. Am J Gastroenterol. 2021 Jan;116(1):171-9.
4. Kolb JM et al. Gastroenterology. 2021 Jun. doi: 10.1053/j.gastro.2021.06.006.
5. Ladabaum U et al. Gastroenterology. 2019 Jul;157(1):137-48.
50 is still 50
BY LINDA RABENECK, MD, MPH, FRCPC
CRC screening of women and men aged 50-75 years at average risk has contributed to the overall decrease in the incidence and mortality from the disease in the United States. In 2009, the American College of Gastroenterology recommended that CRC screening among African Americans begin at age 45 years, and a recent study from Kaiser Permanente reported favorable results from FIT screening in this group.1 In 2018 the American Cancer Society surprised the field by releasing an updated recommendation to begin CRC screening in all women and men at age 45 years (Qualified Recommendation). This spring the U.S. Preventive Services Task Force released its updated recommendation to begin screening at age 45 years (B recommendation).2 The rationale for these updated recommendations is based on new information on the epidemiology of the disease in the United States that was incorporated into updated modeling.
What was this new information? An evaluation of time trends in CRC incidence by age group showed that, although the incidence of colon and rectal cancer among those 50 years and older has been decreasing for more than 2 decades, the incidence has been increasing for those younger than 50 years. As important as these CRC incidence trends are to recognize and understand, this does not imply we should start screening everyone in the general population aged 45-49 years. We need to recognize that the absolute increase in CRC incidence in those younger than 50 years is small, that we lack empirical evidence to support such a major change in screening practice, and that we need to consider potential unintended impacts.
When the rise in incidence among younger Americans was first reported there was much discussion about what it meant. It appears that the rise in incidence is a birth cohort effect among people born in the 1960s and subsequent years. Birth cohort effects imply exposures occurring in early life or those experienced by younger generations. Sorting out what these exposures might be is an area of intense research activity currently. Regardless of the drivers of the relative increase in CRC incidence among younger persons, though, it is important to note that the absolute increase is small. What do we mean by this?
As reported by Holli Loomans-Kropp, PhD, MPH, and colleagues,3 for those under age 50 years, the age-adjusted incidence of colon cancer decreased by 0.9% annually from 1980 to 1996, and then increased by 1.3% annually from 1996 to 2016 (Figure 1). 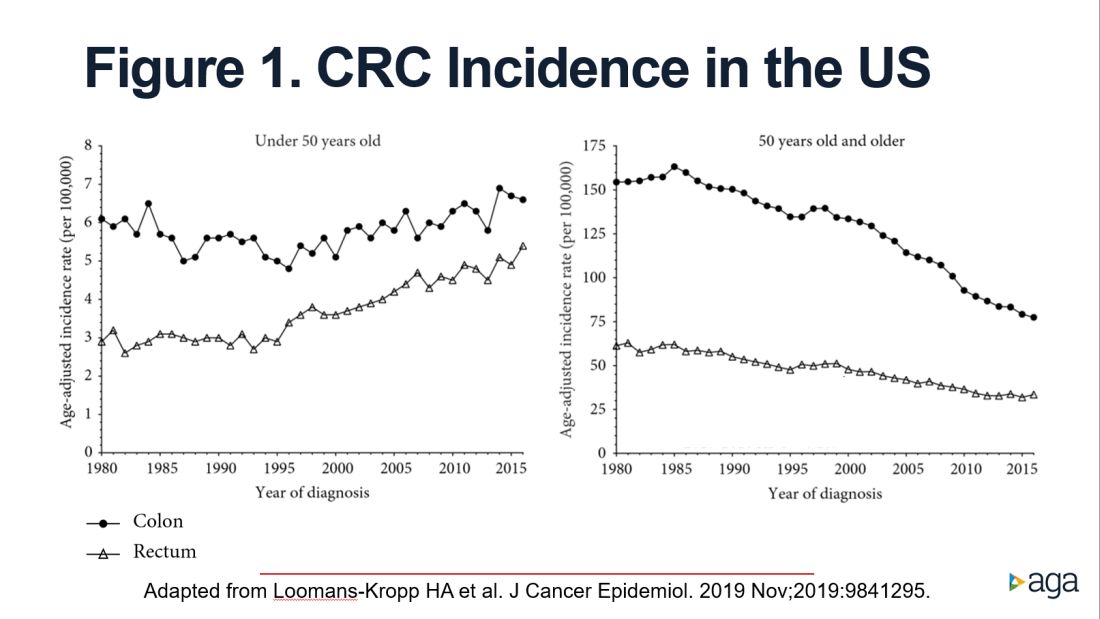
The figure shows that the age-adjusted incidence of rectal cancer among those under age 50 years increased by 2.3% annually since 1991. Figure 1 also shows declines in age-adjusted CRC incidence since 2001 for colon cancer and since 1998 for rectal cancer. However, note the differences in the scales on the Y axes between these two figures. Figure 2 shows this information plotted on the same scale (drawn by hand by author). To recap, these relative increases in incidence are real, but the absolute increases are small.
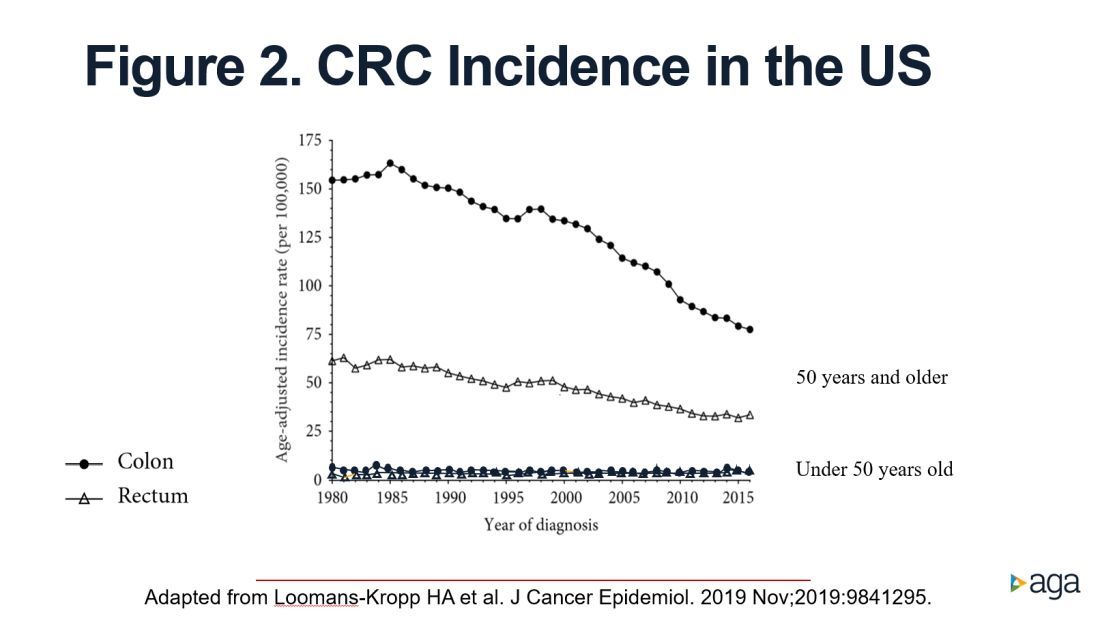
In addition, not surprisingly, we lack results from randomized controlled trials to support the change in CRC screening recommendation. We are just beginning to see results from other types of study design that seek to address this evidence gap. For example, a recent retrospective cohort study using information recorded in Florida databases evaluated the association between CRC incidence and undergoing colonoscopy at ages 45-49 versus 50-54 years. The authors reported a reduction in CRC incidence in both age groups.4
We also need to consider the resource implications. Lowering the age to start CRC screening to 45 years would expand the target population by greater than 20 million Americans, with all that this entails in terms of additional effort and resources, including colonoscopy. Modeling studies have shown that we would achieve greater clinical benefit with lower cost from directing these colonoscopy resources to those aged 50-75 years who are under/never screened and also ensuring follow-up colonoscopy among those with a positive FIT.5 For example, a study in a U.S. safety-net system reported that among 2,238 patients with a FIT+ followed over 1 year, only 55% underwent a colonoscopy.6
Finally, we need to reflect on existing disparities. If we turn our attention and resources to focus on all those aged 45-49 years in the general population, we risk worsening these disparities.
In conclusion, we need to redouble our efforts to reduce existing disparities in CRC screening participation among those aged 50-74 years and ensure colonoscopy follow-up in those with a FIT+ rather than divert our attention and resources to all those 45- to 49-year-olds who are at lower risk.
Dr. Rabeneck is professor of medicine at the University of Toronto. She has no conflicts of interest.
References
1. Levin TR et al. Gastroenterology. 2020 Nov;159(5):1695-704.e1.
2. U.S. Preventive Services Task Force et al. JAMA. 2021 May 18;325(19):1965-77.
3. Loomans-Kropp HA et al. J Cancer Epidemiol. 2019 Nov 11;2019:9841295.
4. Sehgal M et al. Gastroenterology. 2021 May;160(6):2018-28.e13.
5. Ladabaum U et al. Gastroenterology. 2019 Jul;157(1):137-48.
6. Issaka RB et al. Am J Gastroenterol. 2017 Feb;112(2):375-82.
Dear colleagues and friends,
The start age for colorectal cancer (CRC) screening in average-risk individuals has been relatively uncontroversial and unchallenged, until recent guidelines that recommended decreasing the start age from 50 to 45 years. In this edition of Perspectives, two renowned experts, Dr. Patel and Dr. Rabeneck, debate the pros and cons of this paradigm change. This will be my final Perspectives contribution as associate editor for GI & Hep News. Thank you very much for your support and feedback, and I hope you have all enjoyed and learned from the Perspectives debates as much as I have. As always, your feedback and suggestions for future topics are welcome and can be sent to ginews@gastro.org.
Charles J. Kahi, MD, MS, AGAF, is professor of medicine at Indiana University, Indianapolis.
45 is the new 50
BY SWATI G. PATEL, MD, MS
The incidence and mortality rates of CRC in individuals under the age of 50 years, or early age–onset CRC, is increasing in the United States.1 We do not fully understand the cause of this trend; however, it has been observed in multiple Westernized countries, consistent with a birth cohort effect in which generation-specific risk factors are likely responsible.1 To address this rising burden of early age–onset CRC, multiple professional societies, including the United States Preventive Services Task Force,2 now recommend decreasing the starting age for CRC screening in average-risk individuals from 50 to 45 years. These recommendations are supported by the fact that CRC incidence in 45- to 49-year-olds is now the same as that of populations already eligible for screening; the yield of screening appears to be similar among those aged 45-49 years, compared to those aged 50-59 years; and modeling studies show that the benefits outweigh the risks and costs. These factors, along with the unique benefits of early detection and prevention in young patients, support that 45 is the new 50 when it comes to CRC screening.
CRC incidence rates among 45- to 49-year-olds now match populations that are already eligible for CRC screening. The rates among 45- to 49-year-olds now is similar to the incidence rates observed in 50-year-olds in 1992, before widespread CRC screening was performed. Overall incidence rates in 45- to 49-year-olds now match incidence rates observed in 45- to 49-year-old African Americans, a population in which the American College of Gastroenterology and the U.S. Multi-Society Task Force have recommended average-risk screening beginning at age 45.
Advanced colorectal neoplasia rates in 45- to 49-year-olds are similar to rates observed in 50- to 59-year-olds. One study found that advanced colorectal neoplasia rates (including advanced colorectal polyps and adenocarcinoma) in average risk–equivalent 45- to 49-year-olds are similar rates of 50- to 54-year-old average-risk individuals.3 Similarly, a recent systematic review and meta-analysis of 17 international studies including average-risk individuals undergoing colonoscopy found no significant difference between advanced colorectal neoplasia rates in 45- to 49-years-olds compared to 50- to 59-year-olds.4 These data suggest that expanding screening to average-risk individuals aged 45-49 years will have yield similar to that of those aged 50-59.
Modeling studies show that the benefits of screening outweigh the potential harms and costs. A recent cost-effectiveness analysis showed that starting average-risk screening at age 45 years with colonoscopy would cost $33,900 per quality-adjusted life-year (QALY) or $7,700 per QALY if annual FIT is selected as the screening modality.5 These costs are less than the widely accepted willingness to pay threshold of $50,000 per QALY and less than currently accepted average-risk screening, such as annual or biennial mammograms for breast cancer screening.
Expanding screening to 45- to 49-year-olds can improve early detection of CRC and prevention of CRC. Offering screening before age 50 years provides the opportunity to detect cancers at earlier stages, which can improve overall survival. Recent data have shown an increase in CRC incidence among 50- to 54-year-olds.1 Although the etiology of the rise in this age group is likely multifactorial (low screening rates or generational risk that individuals carry with them through the birth-cohort effect), diagnosis and removal of advanced colorectal polyps among 45- to 49-year-olds can have a positive impact on reversing this trend in 50- to 54-year-olds. Furthermore, initiating conversations about CRC screening at age 45 can improve uptake of screening among 50- to 54-year-olds who may have delayed screening when first offered at age 50.
There are unique benefits of early detection and prevention of CRC in young patients. Although the absolute incidence and mortality rates associated with CRC in those aged 45-49 are significantly lower than the rates observed in those over age 50, young individuals are more likely to be in the prime of their earning and fertility potential and are more likely to be caregivers to younger and older generations. They are more likely to face material, psychological, and behavioral financial hardships, such as difficulty paying bills. Absolute incidence rates and modeling studies that do not account for these intangible effects on survivorship also do not fully reflect the societal benefit of early detection and CRC prevention in those aged 45-49 years.
There are certainly many unanswered questions about the effects of expanding CRC screening to those age 45 and older. Ongoing studies are needed to determine the cause of rising incidence, whether screening test selection and intervals should be customized for those under age 50, and how best to ensure equitable access to screening services. Finally, expanding screening to younger individuals should not detract from the critical importance of ongoing efforts to improve screening in those over age 50. As these issues are addressed by the scientific community, we cannot stand by idly when there is sufficient evidence to support that 45 is the new 50.
Dr. Patel is associate professor of medicine in the divisions of gastroenterology & hepatology at the University of Colorado and Rocky Mountain Regional Veterans Affairs Medical Center, both in Aurora, Colo. She disclosed financial relationships with Olympus America, ERBE USA, and Freenome.
References
1. Siegel RL et al. J Natl Cancer Inst. 2017. doi: 10.1093/jnci/djw322.
2. USPSTF et al. JAMA. 2021 May 18;325(19):1965-77.
3. Butterly LF et al. Am J Gastroenterol. 2021 Jan;116(1):171-9.
4. Kolb JM et al. Gastroenterology. 2021 Jun. doi: 10.1053/j.gastro.2021.06.006.
5. Ladabaum U et al. Gastroenterology. 2019 Jul;157(1):137-48.
50 is still 50
BY LINDA RABENECK, MD, MPH, FRCPC
CRC screening of women and men aged 50-75 years at average risk has contributed to the overall decrease in the incidence and mortality from the disease in the United States. In 2009, the American College of Gastroenterology recommended that CRC screening among African Americans begin at age 45 years, and a recent study from Kaiser Permanente reported favorable results from FIT screening in this group.1 In 2018 the American Cancer Society surprised the field by releasing an updated recommendation to begin CRC screening in all women and men at age 45 years (Qualified Recommendation). This spring the U.S. Preventive Services Task Force released its updated recommendation to begin screening at age 45 years (B recommendation).2 The rationale for these updated recommendations is based on new information on the epidemiology of the disease in the United States that was incorporated into updated modeling.
What was this new information? An evaluation of time trends in CRC incidence by age group showed that, although the incidence of colon and rectal cancer among those 50 years and older has been decreasing for more than 2 decades, the incidence has been increasing for those younger than 50 years. As important as these CRC incidence trends are to recognize and understand, this does not imply we should start screening everyone in the general population aged 45-49 years. We need to recognize that the absolute increase in CRC incidence in those younger than 50 years is small, that we lack empirical evidence to support such a major change in screening practice, and that we need to consider potential unintended impacts.
When the rise in incidence among younger Americans was first reported there was much discussion about what it meant. It appears that the rise in incidence is a birth cohort effect among people born in the 1960s and subsequent years. Birth cohort effects imply exposures occurring in early life or those experienced by younger generations. Sorting out what these exposures might be is an area of intense research activity currently. Regardless of the drivers of the relative increase in CRC incidence among younger persons, though, it is important to note that the absolute increase is small. What do we mean by this?
As reported by Holli Loomans-Kropp, PhD, MPH, and colleagues,3 for those under age 50 years, the age-adjusted incidence of colon cancer decreased by 0.9% annually from 1980 to 1996, and then increased by 1.3% annually from 1996 to 2016 (Figure 1). 
The figure shows that the age-adjusted incidence of rectal cancer among those under age 50 years increased by 2.3% annually since 1991. Figure 1 also shows declines in age-adjusted CRC incidence since 2001 for colon cancer and since 1998 for rectal cancer. However, note the differences in the scales on the Y axes between these two figures. Figure 2 shows this information plotted on the same scale (drawn by hand by author). To recap, these relative increases in incidence are real, but the absolute increases are small.

In addition, not surprisingly, we lack results from randomized controlled trials to support the change in CRC screening recommendation. We are just beginning to see results from other types of study design that seek to address this evidence gap. For example, a recent retrospective cohort study using information recorded in Florida databases evaluated the association between CRC incidence and undergoing colonoscopy at ages 45-49 versus 50-54 years. The authors reported a reduction in CRC incidence in both age groups.4
We also need to consider the resource implications. Lowering the age to start CRC screening to 45 years would expand the target population by greater than 20 million Americans, with all that this entails in terms of additional effort and resources, including colonoscopy. Modeling studies have shown that we would achieve greater clinical benefit with lower cost from directing these colonoscopy resources to those aged 50-75 years who are under/never screened and also ensuring follow-up colonoscopy among those with a positive FIT.5 For example, a study in a U.S. safety-net system reported that among 2,238 patients with a FIT+ followed over 1 year, only 55% underwent a colonoscopy.6
Finally, we need to reflect on existing disparities. If we turn our attention and resources to focus on all those aged 45-49 years in the general population, we risk worsening these disparities.
In conclusion, we need to redouble our efforts to reduce existing disparities in CRC screening participation among those aged 50-74 years and ensure colonoscopy follow-up in those with a FIT+ rather than divert our attention and resources to all those 45- to 49-year-olds who are at lower risk.
Dr. Rabeneck is professor of medicine at the University of Toronto. She has no conflicts of interest.
References
1. Levin TR et al. Gastroenterology. 2020 Nov;159(5):1695-704.e1.
2. U.S. Preventive Services Task Force et al. JAMA. 2021 May 18;325(19):1965-77.
3. Loomans-Kropp HA et al. J Cancer Epidemiol. 2019 Nov 11;2019:9841295.
4. Sehgal M et al. Gastroenterology. 2021 May;160(6):2018-28.e13.
5. Ladabaum U et al. Gastroenterology. 2019 Jul;157(1):137-48.
6. Issaka RB et al. Am J Gastroenterol. 2017 Feb;112(2):375-82.
Dear colleagues and friends,
The start age for colorectal cancer (CRC) screening in average-risk individuals has been relatively uncontroversial and unchallenged, until recent guidelines that recommended decreasing the start age from 50 to 45 years. In this edition of Perspectives, two renowned experts, Dr. Patel and Dr. Rabeneck, debate the pros and cons of this paradigm change. This will be my final Perspectives contribution as associate editor for GI & Hep News. Thank you very much for your support and feedback, and I hope you have all enjoyed and learned from the Perspectives debates as much as I have. As always, your feedback and suggestions for future topics are welcome and can be sent to ginews@gastro.org.
Charles J. Kahi, MD, MS, AGAF, is professor of medicine at Indiana University, Indianapolis.
45 is the new 50
BY SWATI G. PATEL, MD, MS
The incidence and mortality rates of CRC in individuals under the age of 50 years, or early age–onset CRC, is increasing in the United States.1 We do not fully understand the cause of this trend; however, it has been observed in multiple Westernized countries, consistent with a birth cohort effect in which generation-specific risk factors are likely responsible.1 To address this rising burden of early age–onset CRC, multiple professional societies, including the United States Preventive Services Task Force,2 now recommend decreasing the starting age for CRC screening in average-risk individuals from 50 to 45 years. These recommendations are supported by the fact that CRC incidence in 45- to 49-year-olds is now the same as that of populations already eligible for screening; the yield of screening appears to be similar among those aged 45-49 years, compared to those aged 50-59 years; and modeling studies show that the benefits outweigh the risks and costs. These factors, along with the unique benefits of early detection and prevention in young patients, support that 45 is the new 50 when it comes to CRC screening.
CRC incidence rates among 45- to 49-year-olds now match populations that are already eligible for CRC screening. The rates among 45- to 49-year-olds now is similar to the incidence rates observed in 50-year-olds in 1992, before widespread CRC screening was performed. Overall incidence rates in 45- to 49-year-olds now match incidence rates observed in 45- to 49-year-old African Americans, a population in which the American College of Gastroenterology and the U.S. Multi-Society Task Force have recommended average-risk screening beginning at age 45.
Advanced colorectal neoplasia rates in 45- to 49-year-olds are similar to rates observed in 50- to 59-year-olds. One study found that advanced colorectal neoplasia rates (including advanced colorectal polyps and adenocarcinoma) in average risk–equivalent 45- to 49-year-olds are similar rates of 50- to 54-year-old average-risk individuals.3 Similarly, a recent systematic review and meta-analysis of 17 international studies including average-risk individuals undergoing colonoscopy found no significant difference between advanced colorectal neoplasia rates in 45- to 49-years-olds compared to 50- to 59-year-olds.4 These data suggest that expanding screening to average-risk individuals aged 45-49 years will have yield similar to that of those aged 50-59.
Modeling studies show that the benefits of screening outweigh the potential harms and costs. A recent cost-effectiveness analysis showed that starting average-risk screening at age 45 years with colonoscopy would cost $33,900 per quality-adjusted life-year (QALY) or $7,700 per QALY if annual FIT is selected as the screening modality.5 These costs are less than the widely accepted willingness to pay threshold of $50,000 per QALY and less than currently accepted average-risk screening, such as annual or biennial mammograms for breast cancer screening.
Expanding screening to 45- to 49-year-olds can improve early detection of CRC and prevention of CRC. Offering screening before age 50 years provides the opportunity to detect cancers at earlier stages, which can improve overall survival. Recent data have shown an increase in CRC incidence among 50- to 54-year-olds.1 Although the etiology of the rise in this age group is likely multifactorial (low screening rates or generational risk that individuals carry with them through the birth-cohort effect), diagnosis and removal of advanced colorectal polyps among 45- to 49-year-olds can have a positive impact on reversing this trend in 50- to 54-year-olds. Furthermore, initiating conversations about CRC screening at age 45 can improve uptake of screening among 50- to 54-year-olds who may have delayed screening when first offered at age 50.
There are unique benefits of early detection and prevention of CRC in young patients. Although the absolute incidence and mortality rates associated with CRC in those aged 45-49 are significantly lower than the rates observed in those over age 50, young individuals are more likely to be in the prime of their earning and fertility potential and are more likely to be caregivers to younger and older generations. They are more likely to face material, psychological, and behavioral financial hardships, such as difficulty paying bills. Absolute incidence rates and modeling studies that do not account for these intangible effects on survivorship also do not fully reflect the societal benefit of early detection and CRC prevention in those aged 45-49 years.
There are certainly many unanswered questions about the effects of expanding CRC screening to those age 45 and older. Ongoing studies are needed to determine the cause of rising incidence, whether screening test selection and intervals should be customized for those under age 50, and how best to ensure equitable access to screening services. Finally, expanding screening to younger individuals should not detract from the critical importance of ongoing efforts to improve screening in those over age 50. As these issues are addressed by the scientific community, we cannot stand by idly when there is sufficient evidence to support that 45 is the new 50.
Dr. Patel is associate professor of medicine in the divisions of gastroenterology & hepatology at the University of Colorado and Rocky Mountain Regional Veterans Affairs Medical Center, both in Aurora, Colo. She disclosed financial relationships with Olympus America, ERBE USA, and Freenome.
References
1. Siegel RL et al. J Natl Cancer Inst. 2017. doi: 10.1093/jnci/djw322.
2. USPSTF et al. JAMA. 2021 May 18;325(19):1965-77.
3. Butterly LF et al. Am J Gastroenterol. 2021 Jan;116(1):171-9.
4. Kolb JM et al. Gastroenterology. 2021 Jun. doi: 10.1053/j.gastro.2021.06.006.
5. Ladabaum U et al. Gastroenterology. 2019 Jul;157(1):137-48.
50 is still 50
BY LINDA RABENECK, MD, MPH, FRCPC
CRC screening of women and men aged 50-75 years at average risk has contributed to the overall decrease in the incidence and mortality from the disease in the United States. In 2009, the American College of Gastroenterology recommended that CRC screening among African Americans begin at age 45 years, and a recent study from Kaiser Permanente reported favorable results from FIT screening in this group.1 In 2018 the American Cancer Society surprised the field by releasing an updated recommendation to begin CRC screening in all women and men at age 45 years (Qualified Recommendation). This spring the U.S. Preventive Services Task Force released its updated recommendation to begin screening at age 45 years (B recommendation).2 The rationale for these updated recommendations is based on new information on the epidemiology of the disease in the United States that was incorporated into updated modeling.
What was this new information? An evaluation of time trends in CRC incidence by age group showed that, although the incidence of colon and rectal cancer among those 50 years and older has been decreasing for more than 2 decades, the incidence has been increasing for those younger than 50 years. As important as these CRC incidence trends are to recognize and understand, this does not imply we should start screening everyone in the general population aged 45-49 years. We need to recognize that the absolute increase in CRC incidence in those younger than 50 years is small, that we lack empirical evidence to support such a major change in screening practice, and that we need to consider potential unintended impacts.
When the rise in incidence among younger Americans was first reported there was much discussion about what it meant. It appears that the rise in incidence is a birth cohort effect among people born in the 1960s and subsequent years. Birth cohort effects imply exposures occurring in early life or those experienced by younger generations. Sorting out what these exposures might be is an area of intense research activity currently. Regardless of the drivers of the relative increase in CRC incidence among younger persons, though, it is important to note that the absolute increase is small. What do we mean by this?
As reported by Holli Loomans-Kropp, PhD, MPH, and colleagues,3 for those under age 50 years, the age-adjusted incidence of colon cancer decreased by 0.9% annually from 1980 to 1996, and then increased by 1.3% annually from 1996 to 2016 (Figure 1). 
The figure shows that the age-adjusted incidence of rectal cancer among those under age 50 years increased by 2.3% annually since 1991. Figure 1 also shows declines in age-adjusted CRC incidence since 2001 for colon cancer and since 1998 for rectal cancer. However, note the differences in the scales on the Y axes between these two figures. Figure 2 shows this information plotted on the same scale (drawn by hand by author). To recap, these relative increases in incidence are real, but the absolute increases are small.

In addition, not surprisingly, we lack results from randomized controlled trials to support the change in CRC screening recommendation. We are just beginning to see results from other types of study design that seek to address this evidence gap. For example, a recent retrospective cohort study using information recorded in Florida databases evaluated the association between CRC incidence and undergoing colonoscopy at ages 45-49 versus 50-54 years. The authors reported a reduction in CRC incidence in both age groups.4
We also need to consider the resource implications. Lowering the age to start CRC screening to 45 years would expand the target population by greater than 20 million Americans, with all that this entails in terms of additional effort and resources, including colonoscopy. Modeling studies have shown that we would achieve greater clinical benefit with lower cost from directing these colonoscopy resources to those aged 50-75 years who are under/never screened and also ensuring follow-up colonoscopy among those with a positive FIT.5 For example, a study in a U.S. safety-net system reported that among 2,238 patients with a FIT+ followed over 1 year, only 55% underwent a colonoscopy.6
Finally, we need to reflect on existing disparities. If we turn our attention and resources to focus on all those aged 45-49 years in the general population, we risk worsening these disparities.
In conclusion, we need to redouble our efforts to reduce existing disparities in CRC screening participation among those aged 50-74 years and ensure colonoscopy follow-up in those with a FIT+ rather than divert our attention and resources to all those 45- to 49-year-olds who are at lower risk.
Dr. Rabeneck is professor of medicine at the University of Toronto. She has no conflicts of interest.
References
1. Levin TR et al. Gastroenterology. 2020 Nov;159(5):1695-704.e1.
2. U.S. Preventive Services Task Force et al. JAMA. 2021 May 18;325(19):1965-77.
3. Loomans-Kropp HA et al. J Cancer Epidemiol. 2019 Nov 11;2019:9841295.
4. Sehgal M et al. Gastroenterology. 2021 May;160(6):2018-28.e13.
5. Ladabaum U et al. Gastroenterology. 2019 Jul;157(1):137-48.
6. Issaka RB et al. Am J Gastroenterol. 2017 Feb;112(2):375-82.
A long look at long haulers
With the number of pediatric infections with SARS-CoV-2 rising it is not surprising that children with persistent symptoms are beginning to accumulate. Who are these pediatric “long haulers” and do they differ from their adult counterparts? The answer is far from clear because the terms “long COVID” and “long hauler” are not well defined. But, I suspect we will find that they will be similar in most respects.
In a recent Guest Essay in the New York Times, two medical school professors attempt to inject some common sense into the long hauler phenomenon. (“The Truth About Long Covid is Complicated. Better Treatment Isn’t,” Adam Gaffney and Zackary Berger, The New York Times, Aug. 18, 2021).
The authors divide the patients with long COVID into three categories. The first includes those who are complaining of persistent cough and fatigue for up to 3 months, a not unexpected course for patients recovering from a significant respiratory illness like pneumonia.
The second group comprises patients who developed acute respiratory distress syndrome during the course of their SARS-CoV-2 infection. These unfortunate individuals likely incurred lung damage that may have triggered renal damage and delirium and may never regain full function.
The third group of patients reports a wide variety of less specific symptoms including, but not limited to, severe fatigue, brain fog, shortness of breath, gastrointestinal symptoms, chronic pain, and palpitations.
The authors of the essay refer to several studies in which there was little if any correlation between these patients’ complaints and their antibody levels. In fact, one study of adolescents found that in a group with similar symptoms many of the individuals had no serologic evidence of SARS-CoV-2 infection.
Unfortunately, the lay public, the media, and some physicians make no distinction between these three groups and lump them all under the same long COVID umbrella. The resulting confusion seeds unwarranted anxiety among the first and third groups and may prevent some individuals from receiving the appropriate attention they deserve.
I suspect that like me, many of you see some similarities between this third group of long COVID patients and adolescents whose persistent symptoms don’t quite fit with their primary illness. Patients labeled as having post-concussion syndrome or “chronic Lyme disease” come immediately to mind. In both conditions, many of the patients had little if any evidence of severe insult from the initial event but continue to complain about a variety of symptoms including severe fatigue and brain fog.
We have done a very poor job of properly managing these patients. And there are a lot of them. A large part of the problem is labeling. In the old days one might have said these patients were having “psychosomatic” symptoms. But, while it may be an accurate description, like the term “retardation” it has been permanently tarnished. Fortunately, most of us are smart enough to avoid telling these patients that it is all in their heads.
However, convincing an individual that many of his symptoms may be the result of the psychological insult from the original disease compounded by other stresses and lifestyle factors can be a difficult sell. The task is made particularly difficult when there continue to be physicians who will miss or ignore the obvious and embark on therapeutic endeavors that are not only ineffective but can serve as a distraction from the real work of listening to and engaging these patients whose suffering may be just as real as that of those long haulers with structural damage.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
With the number of pediatric infections with SARS-CoV-2 rising it is not surprising that children with persistent symptoms are beginning to accumulate. Who are these pediatric “long haulers” and do they differ from their adult counterparts? The answer is far from clear because the terms “long COVID” and “long hauler” are not well defined. But, I suspect we will find that they will be similar in most respects.
In a recent Guest Essay in the New York Times, two medical school professors attempt to inject some common sense into the long hauler phenomenon. (“The Truth About Long Covid is Complicated. Better Treatment Isn’t,” Adam Gaffney and Zackary Berger, The New York Times, Aug. 18, 2021).
The authors divide the patients with long COVID into three categories. The first includes those who are complaining of persistent cough and fatigue for up to 3 months, a not unexpected course for patients recovering from a significant respiratory illness like pneumonia.
The second group comprises patients who developed acute respiratory distress syndrome during the course of their SARS-CoV-2 infection. These unfortunate individuals likely incurred lung damage that may have triggered renal damage and delirium and may never regain full function.
The third group of patients reports a wide variety of less specific symptoms including, but not limited to, severe fatigue, brain fog, shortness of breath, gastrointestinal symptoms, chronic pain, and palpitations.
The authors of the essay refer to several studies in which there was little if any correlation between these patients’ complaints and their antibody levels. In fact, one study of adolescents found that in a group with similar symptoms many of the individuals had no serologic evidence of SARS-CoV-2 infection.
Unfortunately, the lay public, the media, and some physicians make no distinction between these three groups and lump them all under the same long COVID umbrella. The resulting confusion seeds unwarranted anxiety among the first and third groups and may prevent some individuals from receiving the appropriate attention they deserve.
I suspect that like me, many of you see some similarities between this third group of long COVID patients and adolescents whose persistent symptoms don’t quite fit with their primary illness. Patients labeled as having post-concussion syndrome or “chronic Lyme disease” come immediately to mind. In both conditions, many of the patients had little if any evidence of severe insult from the initial event but continue to complain about a variety of symptoms including severe fatigue and brain fog.
We have done a very poor job of properly managing these patients. And there are a lot of them. A large part of the problem is labeling. In the old days one might have said these patients were having “psychosomatic” symptoms. But, while it may be an accurate description, like the term “retardation” it has been permanently tarnished. Fortunately, most of us are smart enough to avoid telling these patients that it is all in their heads.
However, convincing an individual that many of his symptoms may be the result of the psychological insult from the original disease compounded by other stresses and lifestyle factors can be a difficult sell. The task is made particularly difficult when there continue to be physicians who will miss or ignore the obvious and embark on therapeutic endeavors that are not only ineffective but can serve as a distraction from the real work of listening to and engaging these patients whose suffering may be just as real as that of those long haulers with structural damage.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
With the number of pediatric infections with SARS-CoV-2 rising it is not surprising that children with persistent symptoms are beginning to accumulate. Who are these pediatric “long haulers” and do they differ from their adult counterparts? The answer is far from clear because the terms “long COVID” and “long hauler” are not well defined. But, I suspect we will find that they will be similar in most respects.
In a recent Guest Essay in the New York Times, two medical school professors attempt to inject some common sense into the long hauler phenomenon. (“The Truth About Long Covid is Complicated. Better Treatment Isn’t,” Adam Gaffney and Zackary Berger, The New York Times, Aug. 18, 2021).
The authors divide the patients with long COVID into three categories. The first includes those who are complaining of persistent cough and fatigue for up to 3 months, a not unexpected course for patients recovering from a significant respiratory illness like pneumonia.
The second group comprises patients who developed acute respiratory distress syndrome during the course of their SARS-CoV-2 infection. These unfortunate individuals likely incurred lung damage that may have triggered renal damage and delirium and may never regain full function.
The third group of patients reports a wide variety of less specific symptoms including, but not limited to, severe fatigue, brain fog, shortness of breath, gastrointestinal symptoms, chronic pain, and palpitations.
The authors of the essay refer to several studies in which there was little if any correlation between these patients’ complaints and their antibody levels. In fact, one study of adolescents found that in a group with similar symptoms many of the individuals had no serologic evidence of SARS-CoV-2 infection.
Unfortunately, the lay public, the media, and some physicians make no distinction between these three groups and lump them all under the same long COVID umbrella. The resulting confusion seeds unwarranted anxiety among the first and third groups and may prevent some individuals from receiving the appropriate attention they deserve.
I suspect that like me, many of you see some similarities between this third group of long COVID patients and adolescents whose persistent symptoms don’t quite fit with their primary illness. Patients labeled as having post-concussion syndrome or “chronic Lyme disease” come immediately to mind. In both conditions, many of the patients had little if any evidence of severe insult from the initial event but continue to complain about a variety of symptoms including severe fatigue and brain fog.
We have done a very poor job of properly managing these patients. And there are a lot of them. A large part of the problem is labeling. In the old days one might have said these patients were having “psychosomatic” symptoms. But, while it may be an accurate description, like the term “retardation” it has been permanently tarnished. Fortunately, most of us are smart enough to avoid telling these patients that it is all in their heads.
However, convincing an individual that many of his symptoms may be the result of the psychological insult from the original disease compounded by other stresses and lifestyle factors can be a difficult sell. The task is made particularly difficult when there continue to be physicians who will miss or ignore the obvious and embark on therapeutic endeavors that are not only ineffective but can serve as a distraction from the real work of listening to and engaging these patients whose suffering may be just as real as that of those long haulers with structural damage.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.













