User login
Thiazide-Induced Hyponatremia Presenting as a Fall in an Older Adult
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
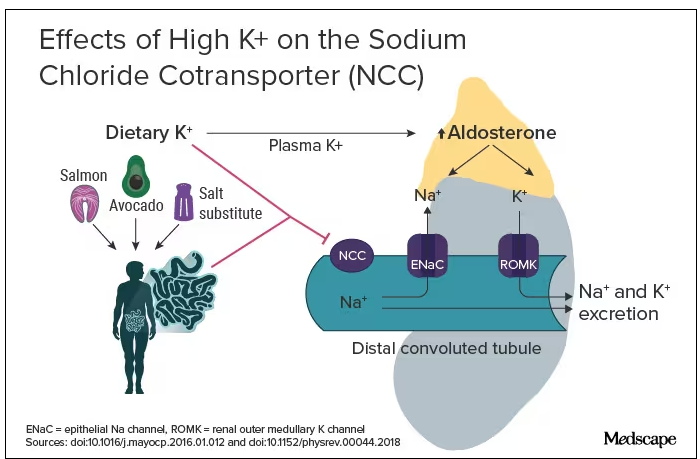
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
Reducing or Discontinuing Insulin or Sulfonylurea When Initiating a Glucagon-like Peptide-1 Agonist
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
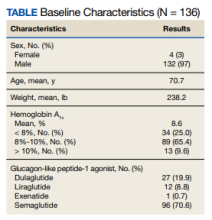
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
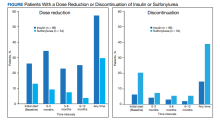
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
Hypoglycemia and weight gain are well-known adverse effects that can result from insulin and sulfonylureas in patients with type 2 diabetes mellitus (T2DM).1,2 Insulin and sulfonylurea medications can cause additional weight gain in patients who are overweight or obese, which can increase the burden of diabetes therapy with added medications, raise the risk of hypoglycemia complications, and raise atherosclerotic cardiovascular disease risk factors.3 Although increasing the insulin or sulfonylurea dose is an option health care practitioners or pharmacists have, this approach can increase the risk of hypoglycemia, especially in older adults, such as the veteran population, which could lead to complications, such as falls.2
Previous studies focusing on hypoglycemic events in patients with T2DM showed that glucagon-like peptide-1 (GLP-1) agonist monotherapy has a low incidence of a hypoglycemic events. However, when a GLP-1 agonist is combined with insulin or sulfonylureas, patients have an increased chance of a hypoglycemic event.3-8 According to the prescribing information for semaglutide, 1.6% to 3.8% of patients on a GLP-1 agonist monotherapy reported a documented symptomatic hypoglycemic event (blood glucose ≤ 70 mg/dL), based on semaglutide dosing. 9 Patients on combination therapy of a GLP-1 agonist and basal insulin and a GLP-1 agonist and a sulfonylurea reported a documented symptomatic hypoglycemic event ranging from 16.7% to 29.8% and 17.3% to 24.4%, respectively.9 The incidences of hypoglycemia thus dramatically increase with combination therapy of a GLP-1 agonist plus insulin or a sulfonylurea.
When adding a GLP-1 agonist to insulin or a sulfonylurea, clinicians must be mindful of the increased risk of hypoglycemia. Per the warnings and precautions in the prescribing information of GLP-1 agonists, concomitant use with insulin or a sulfonylurea may increase the risk of hypoglycemia, and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 According to the American College of Cardiology guidelines, when starting a GLP-1 agonist, the insulin dose should be decreased by about 20% in patients with a well-controlled hemoglobin A1c (HbA1c).12
This study aimed to determine the percentage of patients who required dose reductions or discontinuations of insulin and sulfonylureas with the addition of a GLP-1 agonist. Understanding necessary dose reductions or discontinuations of these concomitant diabetes agents can assist pharmacists in preventing hypoglycemia and minimizing weight gain.
Methods
This clinical review was a single-center, retrospective chart review of patients prescribed a GLP-1 agonist while on insulin or a sulfonylurea between January 1, 2019, and September 30, 2022, at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) in Pennsylvania and managed in a pharmacist-led patient aligned care team (PACT) clinic. It was determined by the US Department of Veterans Affairs Office of Research and Development that an institutional review board or other review committee approval was not needed for this nonresearch Veterans Health Administration quality assurance and improvement project. Patients aged ≥ 18 years were included in this study. Patients were excluded if they were not on insulin or a sulfonylurea when starting a GLP-1 agonist, started a GLP-1 agonist outside of the retrospective chart review dates, or were prescribed a GLP-1 agonist by anyone other than a pharmacist in their PACT clinic. This included if a GLP-1 agonist was prescribed by a primary care physician, endocrinologist, or someone outside the VA system.
The primary study outcomes were to determine the percentage of patients with a dose reduction of insulin or sulfonylurea and discontinuation of insulin or a sulfonylurea at intervals of 0 (baseline), 3, 6, and 12 months. Secondary outcomes included changes in HbA1c and body weight measured at the same intervals of 0 (baseline), 3, 6, and 12 months.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a locked spreadsheet. Descriptive statistics were used to analyze the data. Patient data included the number of patients on insulin or a sulfonylurea when initiating a GLP-1 agonist, the percentage of patients started on a certain GLP-1 agonist (dulaglutide, liraglutide, exenatide, and semaglutide), and the percentage of patients with a baseline HbA1c of < 8%, 8% to 10%, and > 10%. The GLP-1 agonist formulary was adjusted during the time of this retrospective chart review. Patients who were not on semaglutide were switched over if they were on another GLP-1 agonist as semaglutide became the preferred GLP-1 agonist.
Patients were considered to have a dose reduction or discontinuation of insulin or a sulfonylurea if the dose or medication they were on decreased or was discontinued permanently within 12 months of starting a GLP-1 agonist. For example, if a patient who was administering 10 units of insulin daily was decreased to 8 but later increased back to 10, this was not counted as a dose reduction. If a patient discontinued insulin or a sulfonylurea and then restarted it within 12 months of initiating a GLP-1 agonist, this was not counted as a discontinuation.
Results
This retrospective review included 136 patients; 96 patients taking insulin and 54 taking a sulfonylurea when they started a GLP-1 agonist. Fourteen patients were on both. Criteria for use, which are clinical criteria to determine if a patient is eligible for the use of a given medication, are used within the VA. The inclusion criteria for a patient initiating a GLP-1 agonist is that the patient must have atherosclerotic cardiovascular disease or chronic kidney disease with the patient receiving metformin (unless unable to use metformin) and empagliflozin (unless unable to use empagliflozin).
The baseline mean age and weight for the patient population in this retrospective chart review was 70.7 years and 238.2 lb, respectively. Ninety-six patients (70.6%) were started on semaglutide, 27 (19.9%) on dulaglutide, 12 (8.8%) on liraglutide, and 1 (0.7%) on exenatide. The mean HbA1c when patients initiated a GLP-1 agonist was 8.6%. When starting a GLP-1 agonist, 34 patients (25.0%) had an HbA1c < 8%, 89 (65.4%) had an HbA1c between 8% to 10%, and 13 (9.6%) had an HbA1c > 10% (Table).
For the primary results, 25 patients (26.0%) had a dose reduction of insulin when they started a GLP-1 agonist, and 55 patients (57.3%) had at least 1 insulin dose reduction within the year follow-up. Seven patients (13.0%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 16 patients (29.6%) had at least 1 dose reduction of a sulfonylurea within the year follow-up. Six patients (6.3%) discontinued insulin use when they initially started a GLP-1 agonist, and 14 patients (14.6%) discontinued insulin use within the year follow-up. Eleven patients (20.4%) discontinued sulfonylurea use when they initially started a GLP-1 agonist, and 21 patients (38.9%) discontinued sulfonylurea use within the year follow-up (Figure).
Fourteen patients were on both insulin and a sulfonylurea. Two patients (14.3%) had a dose reduction of insulin when they started a GLP-1 agonist, and 5 (35.7%) had ≥ 1 insulin dose reduction within the year follow-up. Three patients (21.4%) had a dose reduction of a sulfonylurea when they started a GLP-1 agonist, and 6 (42.9%) had ≥ 1 dose reduction of a sulfonylurea within the year follow-up. Seven patients (50.0%) discontinued sulfonylurea and 3 (21.4%) discontinued insulin at any time throughout the year. The majority of the discontinuations were at the initial start of GLP-1 agonist therapy.
The mean HbA1c for patients on GLP-1 agonist was 8.6% at baseline, 8.0% at 0 to 3 months, 7.6% at 3 to 6 months, and 7.5% at 12 months. Patients experienced a mean HbA1c reduction of 1.1%. The mean weight when a GLP-1 agonist was started was 238.2 lb, 236.0 lb at 0 to 3 months, 223.8 lb at 3 to 6 months, and 224.3 lb after 12 months. Study participants lost a mean weight of 13.9 lb while on a GLP-1 agonist.
Discussion
While this study did not examine why there were dose reductions or discontinuations, we can hypothesize that insulin or sulfonylureas were reduced or discontinued due to a myriad of reasons, such as prophylactic dosing per guidelines, patients having a hypoglycemic event, or pharmacists anticipating potential low blood glucose trends. Also, there could have been numerous reasons GLP-1 agonists were started in patients on insulin or a sulfonylurea, such as HbA1c not being within goal range, cardiovascular benefits (reduce risk of stroke, heart attack, and death), weight loss, and renal protection, such as preventing albuminuria.13,14
This retrospective chart review found a large proportion of patients had a dose reduction of insulin (57.3%) or sulfonylurea (29.6%). The percentage of patients with a dose reduction was potentially underestimated as patients were not counted if they discontinued insulin or sulfonylurea. Concomitant use of GLP-1 agonists with insulin or a sulfonylurea may increase the risk of hypoglycemia and reducing the dose of insulin or a sulfonylurea may be necessary.9-11 The dose reductions in this study show that pharmacists within pharmacy-led PACT clinics monitor for or attempt to prevent hypoglycemia, which aligns with the prescribing information of GLP-1 agonists. While increasing the insulin or sulfonylurea dose is an option for patients, this approach can increase the risk of hypoglycemia, especially in an older population, like this one with a mean age > 70 years. The large proportions of patients with dose reductions or insulin and sulfonylurea discontinuations suggest that pharmacists may need to take a more cautious approach when initiating a GLP-1 agonist to prevent adverse health outcomes related to low blood sugar for older adults, such as falls and fractures.
Insulin was discontinued in 20.4% of patients and sulfonylurea was discontinued in 38.9% of patients within 12 months after starting a GLP-1 agonist. When a patient was on both insulin and a sulfonylurea, the percentage of patients who discontinued insulin (21.4%) or a sulfonylurea (50.0%) was higher compared with patients just on insulin (14.6%) or a sulfonylurea (38.9%) alone. Patients on both insulin and a sulfonylurea may need closer monitoring due to a higher incidence of discontinuations when these diabetes agents are administered in combination.
Within 12 months of patients receiving a GLP-1 agonist, the mean HbA1c reduction was 1.1%, which is comparable to other GLP-1 agonist clinical trials. For semaglutide 0.5 mg and 1.0 mg dosages, the mean HbA1c reduction was 1.4% and 1.6%, respectively.9 For dulaglutide 0.75 mg and 1.5 mg dosages, the mean HbA1c reduction ranged from 0.7% to 1.6% and 0.8% to 1.6%, respectively.10 For liraglutide 1.8 mg dosage, the mean HbA1c reduction ranged from 1.0% to 1.5%.11 The mean weight loss in this study was 13.9 lb. Along with HbA1c, weight loss in this review was comparable to other GLP-1 agonist clinical trials. Patients administering semaglutide lost up to 14 lb, patients taking dulaglutide lost up to 10.1 lb, and patients on liraglutide lost on average 6.2 lb.9-11 Even with medications such as insulin and sulfonylurea that have the side effects of hypoglycemia and weight gain, adding a GLP-1 agonist showed a reduction in HbA1c and weight loss relatively similar to previous clinical trials.
A study on the effects of adding semaglutide to insulin regimens in March 2023 by Meyer and colleagues displayed similar results to this retrospective chart review. That study concluded that there was blood glucose improvement (HbA1c reduction of 1.3%) in patients after 6 months despite a decrease in the insulin dose. Also, patients lost a mean weight of 11 lb during the 6-month trial.3 This retrospective chart review at the WBVAMC adds to the body of research that supports potential reductions or discontinuations of insulin and/or sulfonylureas with the addition of a GLP-1 agonist.
Limitations
Several limitations of this study should be considered when evaluating the results. This review was comprised of a mostly older, male population, which results in a low generalizability to organizations other than VA medical centers. In addition, this study only evaluated patients on a GLP-1 agonist followed in a pharmacist-led PACT clinic. This study excluded patients who were prescribed a GLP-1 agonist by an endocrinologist or a pharmacist at one of the community-based outpatient clinics affiliated with WBVAMC, or a pharmacist or clinician outside the VA. The sole focus of this study was patients in a pharmacist-led VAMC clinic. Not all patient data may have been included in the study. If a patient did not have an appointment at baseline, 3, 6, and 12 months or did not obtain laboratory tests, HbA1c and weights were not recorded. Data were collected during the COVID-19 pandemic and in-person appointments were potentially switched to phone or video appointments. There were many instances during this chart review where a weight was not recorded at each time interval. Also, this study did not consider any other diabetes medications the patient was taking. There were many instances where the patient was taking metformin and/or sodium-glucose cotransporter-2 (SGLT-2) inhibitors. These medications along with diet could have affected the weight results as metformin is weight neutral and SGLT-2 inhibitors promote weight loss.15 Lastly, this study did not evaluate the amount of insulin reduced, only if there was a dose reduction or discontinuation of insulin and/or a sulfonylurea.
Conclusions
Dose reductions and a discontinuation of insulin or a sulfonylurea with the addition of a GLP-1 agonist may be needed. Patients on both insulin and a sulfonylurea may need closer monitoring due to the higher incidences of discontinuations compared with patients on just 1 of these agents. Dose reductions or discontinuations of these diabetic agents can promote positive patient outcomes, such as preventing hypoglycemia, minimizing weight gain, increasing weight loss, and reducing HbA1c levels.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Wilkes-Barre Veterans Affairs Medical Center in Pennsylvania.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
1. ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128-S139. doi:10.2337/dc23-S008
2. ElSayed NA, Aleppo G, Aroda VE, et al. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S216-S229. doi:10.2337/dc23-S013
3. Meyer J, Dreischmeier E, Lehmann M, Phelan J. The effects of adding semaglutide to high daily dose insulin regimens in patients with type 2 diabetes. Ann Pharmacother. 2023;57(3):241-250. doi:10.1177/10600280221107381
4. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
5. Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-160. doi:10.2337/diaspect.29.3.152
6. Castek SL, Healey LC, Kania DS, Vernon VP, Dawson AJ. Assessment of glucagon-like peptide-1 receptor agonists in veterans taking basal/bolus insulin regimens. Fed Pract. 2022;39(suppl 5):S18-S23. doi:10.12788/fp.0317
7. Chen M, Vider E, Plakogiannis R. Insulin dosage adjustments after initiation of GLP-1 receptor agonists in patients with type 2 diabetes. J Pharm Pract. 2022;35(4):511-517. doi:10.1177/0897190021993625
8. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917. doi:10.1111/j.1463-1326.2012.01618.x.
9. Ozempic (semaglutide) injection. Package insert. Novo Nordisk Inc; 2022. https://www.ozempic.com/prescribing-information.html
10. Trulicity (dulaglutide) injection. Prescribing information. Lilly and Company; 2022. Accessed December 20, 2023. https://pi.lilly.com/us/trulicity-uspi.pdf
11. Victoza (liraglutide) injection. Prescribing information. Novo Nordisk Inc; 2022. Accessed December 20, 2023. https://www.novo-pi.com/victoza.pdf
12. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi:10.1016/j.jacc.2020.05.037
13. Granata A, Maccarrone R, Anzaldi M, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657-1665. Published 2022 Mar 12. doi:10.1093/ckj/sfac069
14. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882-1894. doi:10.1161/CIRCULATIONAHA.122.059595
15. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65(12):1925-1966. doi:10.1007/s00125-022-05787-2
Piperacillin/Tazobactam Use vs Cefepime May Be Associated With Acute Decompensated Heart Failure
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
Top 5 Medications That Can Increase Blood Glucose Levels
It’s that time of the year, when social media is rife with many top 5 and top 10 lists. Let’s revisit some of the most commonly used medications known to increase glucose levels and look at some practical tips on overcoming these.
1. Glucocorticoids
Without a doubt, corticosteroids are at the top of the list when it comes to the potential for increasing blood glucose levels. High-dose glucocorticoid therapy is known to lead to new-onset diabetes (steroid-induced diabetes). Similarly, people with preexisting diabetes may notice significant worsening of glycemic control when they start on glucocorticoid therapy. The extent of glucose elevation depends on their glycemic status prior to initiation on steroids, the dose and duration of glucocorticoid therapy, and comorbid conditions, among other factors.
Management tip: For those with previously well-controlled diabetes or borderline diabetes, glucocorticoid-induced hyperglycemia may be managed by metformin with or without sulfonylurea therapy, especially if corticosteroid treatment is low-dose and for a shorter duration. However, for many individuals with preexisting poorly controlled diabetes or those initiated on high-dose corticosteroids, insulin therapy would perhaps be the treatment of choice. Glucocorticoid therapy generally leads to more pronounced postprandial hyperglycemia compared with fasting hyperglycemia; hence, the use of short-acting insulin therapy or perhaps NPH insulin in the morning might be a better option for many individuals. Dietary modification plays an important role in limiting the extent of postprandial hyperglycemia. Use of continuous glucose monitoring (CGM) devices may also be very helpful for understanding glycemic excursions and how to adjust insulin. In individuals for whom glucocorticoid therapy is tapered down, it is important to adjust the dose of medications with potential to cause hypoglycemia, such as insulin/sulfonylurea therapy, as the degree of hyperglycemia may decrease with decreased dose of the glucocorticoid therapy.
2. Antipsychotic Therapy
Antipsychotic medications can be obesogenic; between 15% and 72% of people who take second-generation antipsychotics experience weight gain of 7% or more. Increases in weight are not the only factor contributing to an elevated risk of developing type 2 diabetes. Antipsychotics are thought to cause downregulation of intracellular insulin signaling, leading to insulin resistance. At the same time, there seems to be a direct effect on the pancreatic beta cells. Antagonism of the dopamine D2, serotonin 5-HT2C, and muscarinic M3 receptors impairs beta-cell response to changes in blood glucose. In addition to the pharmacologic effects, cell culture experiments have shown that antipsychotics increase apoptosis of beta cells. Increased weight and concomitant development of type 2 diabetes is seen particularly in agents that exhibit high muscarinic M3 and histamine H1 receptor blockade. The effect on glucose metabolism is seen the most with agents such as clozapine, olanzapine, and haloperidol and the least with agents such as ziprasidone.
Management tip: Given the ongoing change in the understanding of increases in weight and their association with the risk of developing type 2 diabetes, a metabolically safer approach involves starting with medications that have a lower propensity for weight gain, and the partial agonists/third-generation antipsychotics as a family presently have the best overall data.
3. Thiazide Diuretics
Thiazide diuretics are commonly used for the management of hypertension and are associated with metabolic complications including hypokalemia; higher cholesterol, triglycerides, and other circulating lipids; and elevated glucose. It’s thought that the reduced potassium level occurring as a result of these medications might contribute to new-onset diabetes. The hypokalemia occurring from these medications is thought to lead to a decrease in insulin secretion and sensitivity, which is dose dependent. Studies show that the number needed to harm for chlorthalidone-induced diabetes is 29 over 1 year. There is believed to be no additional risk beyond 1 year.
Management tip: It’s important to monitor potassium levels for those initiated on thiazide diuretics. If hypokalemia occurs, it would be pertinent to correct the hypokalemia with potassium supplements to mitigate the risk for new-onset diabetes.
4. Statin Therapy
Statin therapy is thought to be associated with decreased insulin sensitivity and impairment in insulin secretion. The overall incidence of diabetes is pegged to be between 9% and 12% on statin therapy on the basis of meta-analysis studies, and higher on the basis of population-based studies. Overall, the estimated number needed to harm is: 1 out of every 255 patients on statin therapy for 4 years may develop new-onset diabetes. Compare this with the extremely strong evidence for number needed to treat being 39 for 5 years with statin therapy in patients with preexisting heart disease to prevent one occurrence of a nonfatal myocardial infarction.
Management tip: Although statins are associated with a small incident increase in the risk of developing diabetes, the potential benefits of using statin therapy for both primary and secondary prevention of cardiovascular disease significantly outweigh any of the potential risks associated with hyperglycemia. This is an important discussion to have with patients who are reluctant to use statin therapy because of the potential risk for new-onset diabetes as a side effect.
5. Beta-Blockers
Beta-blockers are another commonly used group of medications for managing hypertension, heart failure, coronary artery disease, and arrhythmia. Nonvasodilating beta-blockers such as metoprolol and atenolol are more likely to be associated with increases in A1c, mean plasma glucose, body weight, and triglycerides compared with vasodilating beta-blockers such as carvedilol, nebivolol, and labetalol (Bakris GL et al; Giugliano D et al). Similarly, studies have also shown that atenolol and metoprolol are associated with increased odds of hypoglycemia compared with carvedilol. People on beta-blockers may have masking of some of the symptoms of hypoglycemia, such as tremor, irritability, and palpitations, while other symptoms such as diaphoresis may remain unaffected on beta-blockers.
Management tip: Education on recognizing and managing hypoglycemia would be important when starting patients on beta-blockers if they are on preexisting insulin/sulfonylurea therapy. Use of CGM devices may be helpful if there is a high risk for hypoglycemia, especially as symptoms of hypoglycemia are often masked.
Honorable Mention
Several other medications — including antiretroviral therapy, tyrosine kinase inhibitors, mechanistic target of rapamycin (mTOR) inhibitors, immunosuppressants, and interferon alpha — are associated with worsening glycemic control and new-onset diabetes. Consider these agents’ effects on blood glucose, especially in people with an elevated risk of developing diabetes or those with preexisting diabetes, when prescribing.
A special mention should also be made of androgen deprivation therapy. These include treatment options like goserelin and leuprolide, which are gonadotropin-releasing hormone (GnRH) agonist therapies and are commonly used for prostate cancer management. Depending on the patient, these agents may be used for prolonged duration. Androgen deprivation therapy, by definition, decreases testosterone levels in men, thereby leading to worsening insulin resistance. Increase in fat mass and concomitant muscle wasting have been associated with the use of these medications; these, in turn, lead to peripheral insulin resistance. Nearly 1 out of every 5 men treated with long-term androgen deprivation therapy may be prone to developing worsening of A1c by 1% or more.
Management tip: Men on androgen deprivation therapy should be encouraged to participate in regular physical activity to reduce the burden of insulin resistance and to promote cardiovascular health.
Drug-induced diabetes is potentially reversible in many cases. Similarly, worsening of glycemic control due to medications in people with preexisting diabetes may also attenuate once the effect of the drug wears off. Blood glucose should be monitored on an ongoing basis so that diabetes medications can be adjusted. For some individuals, however, the worsening of glycemic status may be more chronic and may require long-term use of antihyperglycemic agents, especially if the benefits of continuation of the medication leading to hyperglycemia far exceed any potential risks.
Dr. Jain is Clinical Instructor, Department of Endocrinology, University of British Columbia; Endocrinologist, Fraser River Endocrinology, Vancouver, British Columbia, Canada. He disclosed ties with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk.
A version of this article appeared on Medscape.com.
It’s that time of the year, when social media is rife with many top 5 and top 10 lists. Let’s revisit some of the most commonly used medications known to increase glucose levels and look at some practical tips on overcoming these.
1. Glucocorticoids
Without a doubt, corticosteroids are at the top of the list when it comes to the potential for increasing blood glucose levels. High-dose glucocorticoid therapy is known to lead to new-onset diabetes (steroid-induced diabetes). Similarly, people with preexisting diabetes may notice significant worsening of glycemic control when they start on glucocorticoid therapy. The extent of glucose elevation depends on their glycemic status prior to initiation on steroids, the dose and duration of glucocorticoid therapy, and comorbid conditions, among other factors.
Management tip: For those with previously well-controlled diabetes or borderline diabetes, glucocorticoid-induced hyperglycemia may be managed by metformin with or without sulfonylurea therapy, especially if corticosteroid treatment is low-dose and for a shorter duration. However, for many individuals with preexisting poorly controlled diabetes or those initiated on high-dose corticosteroids, insulin therapy would perhaps be the treatment of choice. Glucocorticoid therapy generally leads to more pronounced postprandial hyperglycemia compared with fasting hyperglycemia; hence, the use of short-acting insulin therapy or perhaps NPH insulin in the morning might be a better option for many individuals. Dietary modification plays an important role in limiting the extent of postprandial hyperglycemia. Use of continuous glucose monitoring (CGM) devices may also be very helpful for understanding glycemic excursions and how to adjust insulin. In individuals for whom glucocorticoid therapy is tapered down, it is important to adjust the dose of medications with potential to cause hypoglycemia, such as insulin/sulfonylurea therapy, as the degree of hyperglycemia may decrease with decreased dose of the glucocorticoid therapy.
2. Antipsychotic Therapy
Antipsychotic medications can be obesogenic; between 15% and 72% of people who take second-generation antipsychotics experience weight gain of 7% or more. Increases in weight are not the only factor contributing to an elevated risk of developing type 2 diabetes. Antipsychotics are thought to cause downregulation of intracellular insulin signaling, leading to insulin resistance. At the same time, there seems to be a direct effect on the pancreatic beta cells. Antagonism of the dopamine D2, serotonin 5-HT2C, and muscarinic M3 receptors impairs beta-cell response to changes in blood glucose. In addition to the pharmacologic effects, cell culture experiments have shown that antipsychotics increase apoptosis of beta cells. Increased weight and concomitant development of type 2 diabetes is seen particularly in agents that exhibit high muscarinic M3 and histamine H1 receptor blockade. The effect on glucose metabolism is seen the most with agents such as clozapine, olanzapine, and haloperidol and the least with agents such as ziprasidone.
Management tip: Given the ongoing change in the understanding of increases in weight and their association with the risk of developing type 2 diabetes, a metabolically safer approach involves starting with medications that have a lower propensity for weight gain, and the partial agonists/third-generation antipsychotics as a family presently have the best overall data.
3. Thiazide Diuretics
Thiazide diuretics are commonly used for the management of hypertension and are associated with metabolic complications including hypokalemia; higher cholesterol, triglycerides, and other circulating lipids; and elevated glucose. It’s thought that the reduced potassium level occurring as a result of these medications might contribute to new-onset diabetes. The hypokalemia occurring from these medications is thought to lead to a decrease in insulin secretion and sensitivity, which is dose dependent. Studies show that the number needed to harm for chlorthalidone-induced diabetes is 29 over 1 year. There is believed to be no additional risk beyond 1 year.
Management tip: It’s important to monitor potassium levels for those initiated on thiazide diuretics. If hypokalemia occurs, it would be pertinent to correct the hypokalemia with potassium supplements to mitigate the risk for new-onset diabetes.
4. Statin Therapy
Statin therapy is thought to be associated with decreased insulin sensitivity and impairment in insulin secretion. The overall incidence of diabetes is pegged to be between 9% and 12% on statin therapy on the basis of meta-analysis studies, and higher on the basis of population-based studies. Overall, the estimated number needed to harm is: 1 out of every 255 patients on statin therapy for 4 years may develop new-onset diabetes. Compare this with the extremely strong evidence for number needed to treat being 39 for 5 years with statin therapy in patients with preexisting heart disease to prevent one occurrence of a nonfatal myocardial infarction.
Management tip: Although statins are associated with a small incident increase in the risk of developing diabetes, the potential benefits of using statin therapy for both primary and secondary prevention of cardiovascular disease significantly outweigh any of the potential risks associated with hyperglycemia. This is an important discussion to have with patients who are reluctant to use statin therapy because of the potential risk for new-onset diabetes as a side effect.
5. Beta-Blockers
Beta-blockers are another commonly used group of medications for managing hypertension, heart failure, coronary artery disease, and arrhythmia. Nonvasodilating beta-blockers such as metoprolol and atenolol are more likely to be associated with increases in A1c, mean plasma glucose, body weight, and triglycerides compared with vasodilating beta-blockers such as carvedilol, nebivolol, and labetalol (Bakris GL et al; Giugliano D et al). Similarly, studies have also shown that atenolol and metoprolol are associated with increased odds of hypoglycemia compared with carvedilol. People on beta-blockers may have masking of some of the symptoms of hypoglycemia, such as tremor, irritability, and palpitations, while other symptoms such as diaphoresis may remain unaffected on beta-blockers.
Management tip: Education on recognizing and managing hypoglycemia would be important when starting patients on beta-blockers if they are on preexisting insulin/sulfonylurea therapy. Use of CGM devices may be helpful if there is a high risk for hypoglycemia, especially as symptoms of hypoglycemia are often masked.
Honorable Mention
Several other medications — including antiretroviral therapy, tyrosine kinase inhibitors, mechanistic target of rapamycin (mTOR) inhibitors, immunosuppressants, and interferon alpha — are associated with worsening glycemic control and new-onset diabetes. Consider these agents’ effects on blood glucose, especially in people with an elevated risk of developing diabetes or those with preexisting diabetes, when prescribing.
A special mention should also be made of androgen deprivation therapy. These include treatment options like goserelin and leuprolide, which are gonadotropin-releasing hormone (GnRH) agonist therapies and are commonly used for prostate cancer management. Depending on the patient, these agents may be used for prolonged duration. Androgen deprivation therapy, by definition, decreases testosterone levels in men, thereby leading to worsening insulin resistance. Increase in fat mass and concomitant muscle wasting have been associated with the use of these medications; these, in turn, lead to peripheral insulin resistance. Nearly 1 out of every 5 men treated with long-term androgen deprivation therapy may be prone to developing worsening of A1c by 1% or more.
Management tip: Men on androgen deprivation therapy should be encouraged to participate in regular physical activity to reduce the burden of insulin resistance and to promote cardiovascular health.
Drug-induced diabetes is potentially reversible in many cases. Similarly, worsening of glycemic control due to medications in people with preexisting diabetes may also attenuate once the effect of the drug wears off. Blood glucose should be monitored on an ongoing basis so that diabetes medications can be adjusted. For some individuals, however, the worsening of glycemic status may be more chronic and may require long-term use of antihyperglycemic agents, especially if the benefits of continuation of the medication leading to hyperglycemia far exceed any potential risks.
Dr. Jain is Clinical Instructor, Department of Endocrinology, University of British Columbia; Endocrinologist, Fraser River Endocrinology, Vancouver, British Columbia, Canada. He disclosed ties with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk.
A version of this article appeared on Medscape.com.
It’s that time of the year, when social media is rife with many top 5 and top 10 lists. Let’s revisit some of the most commonly used medications known to increase glucose levels and look at some practical tips on overcoming these.
1. Glucocorticoids
Without a doubt, corticosteroids are at the top of the list when it comes to the potential for increasing blood glucose levels. High-dose glucocorticoid therapy is known to lead to new-onset diabetes (steroid-induced diabetes). Similarly, people with preexisting diabetes may notice significant worsening of glycemic control when they start on glucocorticoid therapy. The extent of glucose elevation depends on their glycemic status prior to initiation on steroids, the dose and duration of glucocorticoid therapy, and comorbid conditions, among other factors.
Management tip: For those with previously well-controlled diabetes or borderline diabetes, glucocorticoid-induced hyperglycemia may be managed by metformin with or without sulfonylurea therapy, especially if corticosteroid treatment is low-dose and for a shorter duration. However, for many individuals with preexisting poorly controlled diabetes or those initiated on high-dose corticosteroids, insulin therapy would perhaps be the treatment of choice. Glucocorticoid therapy generally leads to more pronounced postprandial hyperglycemia compared with fasting hyperglycemia; hence, the use of short-acting insulin therapy or perhaps NPH insulin in the morning might be a better option for many individuals. Dietary modification plays an important role in limiting the extent of postprandial hyperglycemia. Use of continuous glucose monitoring (CGM) devices may also be very helpful for understanding glycemic excursions and how to adjust insulin. In individuals for whom glucocorticoid therapy is tapered down, it is important to adjust the dose of medications with potential to cause hypoglycemia, such as insulin/sulfonylurea therapy, as the degree of hyperglycemia may decrease with decreased dose of the glucocorticoid therapy.
2. Antipsychotic Therapy
Antipsychotic medications can be obesogenic; between 15% and 72% of people who take second-generation antipsychotics experience weight gain of 7% or more. Increases in weight are not the only factor contributing to an elevated risk of developing type 2 diabetes. Antipsychotics are thought to cause downregulation of intracellular insulin signaling, leading to insulin resistance. At the same time, there seems to be a direct effect on the pancreatic beta cells. Antagonism of the dopamine D2, serotonin 5-HT2C, and muscarinic M3 receptors impairs beta-cell response to changes in blood glucose. In addition to the pharmacologic effects, cell culture experiments have shown that antipsychotics increase apoptosis of beta cells. Increased weight and concomitant development of type 2 diabetes is seen particularly in agents that exhibit high muscarinic M3 and histamine H1 receptor blockade. The effect on glucose metabolism is seen the most with agents such as clozapine, olanzapine, and haloperidol and the least with agents such as ziprasidone.
Management tip: Given the ongoing change in the understanding of increases in weight and their association with the risk of developing type 2 diabetes, a metabolically safer approach involves starting with medications that have a lower propensity for weight gain, and the partial agonists/third-generation antipsychotics as a family presently have the best overall data.
3. Thiazide Diuretics
Thiazide diuretics are commonly used for the management of hypertension and are associated with metabolic complications including hypokalemia; higher cholesterol, triglycerides, and other circulating lipids; and elevated glucose. It’s thought that the reduced potassium level occurring as a result of these medications might contribute to new-onset diabetes. The hypokalemia occurring from these medications is thought to lead to a decrease in insulin secretion and sensitivity, which is dose dependent. Studies show that the number needed to harm for chlorthalidone-induced diabetes is 29 over 1 year. There is believed to be no additional risk beyond 1 year.
Management tip: It’s important to monitor potassium levels for those initiated on thiazide diuretics. If hypokalemia occurs, it would be pertinent to correct the hypokalemia with potassium supplements to mitigate the risk for new-onset diabetes.
4. Statin Therapy
Statin therapy is thought to be associated with decreased insulin sensitivity and impairment in insulin secretion. The overall incidence of diabetes is pegged to be between 9% and 12% on statin therapy on the basis of meta-analysis studies, and higher on the basis of population-based studies. Overall, the estimated number needed to harm is: 1 out of every 255 patients on statin therapy for 4 years may develop new-onset diabetes. Compare this with the extremely strong evidence for number needed to treat being 39 for 5 years with statin therapy in patients with preexisting heart disease to prevent one occurrence of a nonfatal myocardial infarction.
Management tip: Although statins are associated with a small incident increase in the risk of developing diabetes, the potential benefits of using statin therapy for both primary and secondary prevention of cardiovascular disease significantly outweigh any of the potential risks associated with hyperglycemia. This is an important discussion to have with patients who are reluctant to use statin therapy because of the potential risk for new-onset diabetes as a side effect.
5. Beta-Blockers
Beta-blockers are another commonly used group of medications for managing hypertension, heart failure, coronary artery disease, and arrhythmia. Nonvasodilating beta-blockers such as metoprolol and atenolol are more likely to be associated with increases in A1c, mean plasma glucose, body weight, and triglycerides compared with vasodilating beta-blockers such as carvedilol, nebivolol, and labetalol (Bakris GL et al; Giugliano D et al). Similarly, studies have also shown that atenolol and metoprolol are associated with increased odds of hypoglycemia compared with carvedilol. People on beta-blockers may have masking of some of the symptoms of hypoglycemia, such as tremor, irritability, and palpitations, while other symptoms such as diaphoresis may remain unaffected on beta-blockers.
Management tip: Education on recognizing and managing hypoglycemia would be important when starting patients on beta-blockers if they are on preexisting insulin/sulfonylurea therapy. Use of CGM devices may be helpful if there is a high risk for hypoglycemia, especially as symptoms of hypoglycemia are often masked.
Honorable Mention
Several other medications — including antiretroviral therapy, tyrosine kinase inhibitors, mechanistic target of rapamycin (mTOR) inhibitors, immunosuppressants, and interferon alpha — are associated with worsening glycemic control and new-onset diabetes. Consider these agents’ effects on blood glucose, especially in people with an elevated risk of developing diabetes or those with preexisting diabetes, when prescribing.
A special mention should also be made of androgen deprivation therapy. These include treatment options like goserelin and leuprolide, which are gonadotropin-releasing hormone (GnRH) agonist therapies and are commonly used for prostate cancer management. Depending on the patient, these agents may be used for prolonged duration. Androgen deprivation therapy, by definition, decreases testosterone levels in men, thereby leading to worsening insulin resistance. Increase in fat mass and concomitant muscle wasting have been associated with the use of these medications; these, in turn, lead to peripheral insulin resistance. Nearly 1 out of every 5 men treated with long-term androgen deprivation therapy may be prone to developing worsening of A1c by 1% or more.
Management tip: Men on androgen deprivation therapy should be encouraged to participate in regular physical activity to reduce the burden of insulin resistance and to promote cardiovascular health.
Drug-induced diabetes is potentially reversible in many cases. Similarly, worsening of glycemic control due to medications in people with preexisting diabetes may also attenuate once the effect of the drug wears off. Blood glucose should be monitored on an ongoing basis so that diabetes medications can be adjusted. For some individuals, however, the worsening of glycemic status may be more chronic and may require long-term use of antihyperglycemic agents, especially if the benefits of continuation of the medication leading to hyperglycemia far exceed any potential risks.
Dr. Jain is Clinical Instructor, Department of Endocrinology, University of British Columbia; Endocrinologist, Fraser River Endocrinology, Vancouver, British Columbia, Canada. He disclosed ties with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk.
A version of this article appeared on Medscape.com.
Ibuprofen Fails for Patent Ductus Arteriosus in Preterm Infants
The study population included infants born between 23 weeks 0 days’ and 28 weeks 6 days’ gestation. The researchers randomized 326 extremely preterm infants with patent ductus arteriosus (PDA) at 72 hours or less after birth to ibuprofen at a loading dose of 10 mg/kg followed by two doses of 5 mg/kg at least 24 hours apart, and 327 to placebo.
The PDAs in the infants had a diameter of at least 1.5 mm with pulsatile flow.
Severe dysplasia outcome
The study’s primary outcome was a composite of death or moderate to severe bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. Overall, a primary outcome occurred in 69.2% of infants who received ibuprofen and 63.5% of those who received a placebo.
Risk of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age was not reduced by early ibuprofen vs. placebo for preterm infants, the researchers concluded. Moderate or severe bronchopulmonary dysplasia occurred in 64.2% of the infants in the ibuprofen group and 59.3% of the placebo group who survived to 36 weeks’ postmenstrual age.
‘Unforeseeable’ serious adverse events
Forty-four deaths occurred in the ibuprofen group and 33 in the placebo group (adjusted risk ratio 1.09). Two “unforeseeable” serious adverse events occurred during the study that were potentially related to ibuprofen.
The lead author was Samir Gupta, MD, of Sidra Medicine, Doha, Qatar. The study was published online in the New England Journal of Medicine.
Study limitations include incomplete data for some patients.
The study was supported by the National Institute for Health Research Health Technology Assessment Programme. The researchers had no financial conflicts to disclose.
The study population included infants born between 23 weeks 0 days’ and 28 weeks 6 days’ gestation. The researchers randomized 326 extremely preterm infants with patent ductus arteriosus (PDA) at 72 hours or less after birth to ibuprofen at a loading dose of 10 mg/kg followed by two doses of 5 mg/kg at least 24 hours apart, and 327 to placebo.
The PDAs in the infants had a diameter of at least 1.5 mm with pulsatile flow.
Severe dysplasia outcome
The study’s primary outcome was a composite of death or moderate to severe bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. Overall, a primary outcome occurred in 69.2% of infants who received ibuprofen and 63.5% of those who received a placebo.
Risk of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age was not reduced by early ibuprofen vs. placebo for preterm infants, the researchers concluded. Moderate or severe bronchopulmonary dysplasia occurred in 64.2% of the infants in the ibuprofen group and 59.3% of the placebo group who survived to 36 weeks’ postmenstrual age.
‘Unforeseeable’ serious adverse events
Forty-four deaths occurred in the ibuprofen group and 33 in the placebo group (adjusted risk ratio 1.09). Two “unforeseeable” serious adverse events occurred during the study that were potentially related to ibuprofen.
The lead author was Samir Gupta, MD, of Sidra Medicine, Doha, Qatar. The study was published online in the New England Journal of Medicine.
Study limitations include incomplete data for some patients.
The study was supported by the National Institute for Health Research Health Technology Assessment Programme. The researchers had no financial conflicts to disclose.
The study population included infants born between 23 weeks 0 days’ and 28 weeks 6 days’ gestation. The researchers randomized 326 extremely preterm infants with patent ductus arteriosus (PDA) at 72 hours or less after birth to ibuprofen at a loading dose of 10 mg/kg followed by two doses of 5 mg/kg at least 24 hours apart, and 327 to placebo.
The PDAs in the infants had a diameter of at least 1.5 mm with pulsatile flow.
Severe dysplasia outcome
The study’s primary outcome was a composite of death or moderate to severe bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. Overall, a primary outcome occurred in 69.2% of infants who received ibuprofen and 63.5% of those who received a placebo.
Risk of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age was not reduced by early ibuprofen vs. placebo for preterm infants, the researchers concluded. Moderate or severe bronchopulmonary dysplasia occurred in 64.2% of the infants in the ibuprofen group and 59.3% of the placebo group who survived to 36 weeks’ postmenstrual age.
‘Unforeseeable’ serious adverse events
Forty-four deaths occurred in the ibuprofen group and 33 in the placebo group (adjusted risk ratio 1.09). Two “unforeseeable” serious adverse events occurred during the study that were potentially related to ibuprofen.
The lead author was Samir Gupta, MD, of Sidra Medicine, Doha, Qatar. The study was published online in the New England Journal of Medicine.
Study limitations include incomplete data for some patients.
The study was supported by the National Institute for Health Research Health Technology Assessment Programme. The researchers had no financial conflicts to disclose.
Sodium vs Potassium for Lowering Blood Pressure?
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
Colchicine May Benefit Patients With Diabetes and Recent MI
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
FDA Expands Dupilumab for EoE to Younger Children
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
Robitussin Cough Syrup Recalled Nationwide Due to Fungus Concerns
The company that makes Robitussin syrups did not specify which microorganisms may be in the products. The recall announcement from the global consumer health products company Haleon stated that the contamination could lead to fungal infections or the presence of fungi or yeasts in a person’s blood. So far, the company has not received any reports of people being sickened by the recalled products.
The recall applies to bottles of Robitussin Honey CF Max Day and Robitussin Honey CF Max Nighttime. Both varieties are for adults. Affected products were sold nationwide and have specific lot numbers printed at the bottom of the back of the bottles. Consumers can view the lot numbers on the FDA’s recall webpage.
People with weakened immune systems have a higher risk of life-threatening health problems due to the cough syrup, the company warned.
“In non-immunocompromised consumers, the population most likely to use the product, life-threatening infections are not likely to occur,” the recall notice from Haleon stated. “However, the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.”
People who have affected products should stop using them immediately. The company asked that anyone with the products email Haleon at mystory.us@haleon.com, or call the company at 800-245-1040 Monday through Friday from 8 a.m. to 6 p.m. Eastern time.
A version of this article appeared on WebMD.com.
The company that makes Robitussin syrups did not specify which microorganisms may be in the products. The recall announcement from the global consumer health products company Haleon stated that the contamination could lead to fungal infections or the presence of fungi or yeasts in a person’s blood. So far, the company has not received any reports of people being sickened by the recalled products.
The recall applies to bottles of Robitussin Honey CF Max Day and Robitussin Honey CF Max Nighttime. Both varieties are for adults. Affected products were sold nationwide and have specific lot numbers printed at the bottom of the back of the bottles. Consumers can view the lot numbers on the FDA’s recall webpage.
People with weakened immune systems have a higher risk of life-threatening health problems due to the cough syrup, the company warned.
“In non-immunocompromised consumers, the population most likely to use the product, life-threatening infections are not likely to occur,” the recall notice from Haleon stated. “However, the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.”
People who have affected products should stop using them immediately. The company asked that anyone with the products email Haleon at mystory.us@haleon.com, or call the company at 800-245-1040 Monday through Friday from 8 a.m. to 6 p.m. Eastern time.
A version of this article appeared on WebMD.com.
The company that makes Robitussin syrups did not specify which microorganisms may be in the products. The recall announcement from the global consumer health products company Haleon stated that the contamination could lead to fungal infections or the presence of fungi or yeasts in a person’s blood. So far, the company has not received any reports of people being sickened by the recalled products.
The recall applies to bottles of Robitussin Honey CF Max Day and Robitussin Honey CF Max Nighttime. Both varieties are for adults. Affected products were sold nationwide and have specific lot numbers printed at the bottom of the back of the bottles. Consumers can view the lot numbers on the FDA’s recall webpage.
People with weakened immune systems have a higher risk of life-threatening health problems due to the cough syrup, the company warned.
“In non-immunocompromised consumers, the population most likely to use the product, life-threatening infections are not likely to occur,” the recall notice from Haleon stated. “However, the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.”
People who have affected products should stop using them immediately. The company asked that anyone with the products email Haleon at mystory.us@haleon.com, or call the company at 800-245-1040 Monday through Friday from 8 a.m. to 6 p.m. Eastern time.
A version of this article appeared on WebMD.com.
The Breakthrough Drug Whose Full Promise Remains Unrealized
Celebrating a Decade of Sofosbuvir for Hepatitis C
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Celebrating a Decade of Sofosbuvir for Hepatitis C
Celebrating a Decade of Sofosbuvir for Hepatitis C
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.