User login
Assessment of a Medication Deprescribing Tool on Polypharmacy and Cost Avoidance
According to the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS), the use of prescription drugs has increased in the past half century. Although prescription drugs have played an important role in preventing, controlling, and delaying onset or progression of disease, their growth in use also has posed many risks.1 One ramification of this growth is the occurrence of polypharmacy, which does not have a universal, clear definition. In general, it can be described as the concurrent use of multiple medications by a single patient to treat one or more medical ailments. Five or more medications taken simultaneously is the most common definition to date, but this is just one of many acceptable definitions and that varies from one health care facility to another.1,2
Regardless of the cutoffs established to indicate polypharmacy, its incidence can result in poor and potentially harmful health outcomes. Polypharmacy increases the risk of experiencing adverse drug events (ADEs), drug-drug interactions (DDIs), geriatric-related syndromes, falls, hospitalization, and mortality. Issues with adherence may begin to unfold secondary to increased pill burden. Both the patient and the health care system may encounter financial strain, as polypharmacy can lead to unnecessary and essentially preventable costs of care. When evaluating the likelihood of polypharmacy based on age group, NCHS found that 47.5% of patients taking ≥ 5 medications were aged ≥ 65 years.1-5 This indicates that polypharmacy is of great concern in the geriatric population, which also represents a large proportion of individuals accessing Veterans Health Administration (VHA) care.
Deprescibing
Deprescribing is the act of withdrawing or discontinuing potentially inappropriate medications (PIM), or medications used by older patients harboring ADEs that generally outweigh the clinical benefits of the drug. Deprescribing is an effective tool for managing or reducing polypharmacy. A variety of tools have been created whose sole purpose is to simplify deprescribing. Some tools explicitly identify PIM and are widely familiar in medical practice. Examples are the Beers Criteria developed in 1991 or Screening Tool to Alert Right Treatment/Screening Tool of Older Persons Prescriptions (START/STOPP) criteria created in 2003. Other tools that are less commonplace but equally as resourceful are MedStopper and Deprescribing.org. The former was launched in 2015 and is a Canadian online system that provides risk assessments for medications with guidance for tapering or stopping medications if continuation of the drug presents higher risk than benefit.5-7 The latter is a full-fledged website developed by a physician, a pharmacist, and their research teams that serves as an exchange hub for deprescribing information.
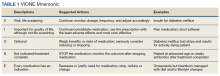
In 2016, the VIONE (Vital, Important, Optional, Not indicated/treatment complete, and Every medication has an indication) deprescribing tool was developed by Saraswathy Battar, MD, at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock, as a system that could go beyond medication reconciliation (Table 1). Health care providers (HCPs) and pharmacists evaluate each medication that a patient has been prescribed and places each medication in a VIONE category. Prescribers may then take the opportunity to deprescribe or discontinue medications if deemed appropriate based on their clinical assessments and shared decision making.8 Traditionally, medication reconciliation involves the process of obtaining a complete and accurate list of medications as reported by a patient or caregiver to a HCP. VIONE encourages HCPs and pharmacists not only to ensure medication lists are accurate, but also that each medication reported is appropriate for continued use. In other words, VIONE is meant to help implement deprescribing at opportune times. More than 14,000 medications have been deprescribed using the VIONE method, resulting in more than $2,000,000 of annualized cost avoidance after just 1 year of implementation at CAVHS.9
VIONE consists of 2 major components in the Computerized Patient Record System (CPRS): a template and a dropdown discontinuation menu. The template captured patient allergies, pertinent laboratory data, the patient’s active problem list and applicable diagnoses, and active medication list. Patient aligned care team (PACT) pharmacists used the information captured in the template to conduct medication reconciliations and polypharmacy reviews. Each medication is categorized in VIONE using data collected during reviews. A menu delineates reasons for discontinuation: optional, dose decrease, no diagnosis, not indicated/treatment complete, discontinue alternate medication prescribed, and patient reported no longer taking. The discontinuation menu allowed PACT pharmacists and physicians to choose 1 VIONE option per medication to clarify the reason for discontinuation. VIONE-based discontinuations are recorded in CPRS and identified as deprescribed.
At the time of this project, > 30 US Department of Veterans Affairs (VA) facilities had adopted VIONE. Use of VIONE at VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas has been incorporated in the everyday practices of home-based primary care pharmacists and physicians but has yet to be implemented in other areas of the facility. The purpose of this project was to determine the impact of the VIONE tool on polypharmacy and cost avoidance at VASNHS when used by primary care physicians (PCPs) and PACT primary care clinics.
Methods
Veterans receiving care at VASNHS aged ≥ 65 years with ≥ 10 active medications noted in CPRS were included in this project. PACT pharmacists and physicians were educated on the proper use of the VIONE tool prior to its implementation. Education included a 15-minute slide presentation followed by dissemination of a 1-page VIONE tool handout during a PACT all-staff clinic meeting.
Data were collected for 3 months before and after the intervention. Data were made available for assessment by the Automated Data Processing Application Coordinator (ADPAC) at VASNHS. The ADPAC created and generated an Excel spreadsheet report, which listed all medications deprescribed using the VIONE method. The primary endpoint was the total number of medications discontinued using the VIONE template and/or discontinuation menu. For the purpose of this project, appropriate discontinuation was considered any prescription deprescribed, excluding medical supplies, by pharmacists and PCPs who received VIONE education.
The secondary endpoint was the estimated annualized cost avoidance for the facility (Figure). The calculation does not include medications discontinued due to the prescription of an alternative medication or dose decreases since these VIONE selections imply that a new prescription or order was placed and the original prescription was not deprescribed. Annualized cost avoidance was determined with use of the VIONE dashboard, a database that retrospectively gathers information regarding patients at risk of polypharmacy, polypharmacy-related ADEs, and cost. Manual adjustments were made to various parameters on the Veterans Integrated Service Network 15 VIONE dashboard by the author in order to obtain data specific to this project. These parameters allowed selection of service sections, specific staff members or the option to include or exclude chronic or nonchronic medications. The annualized cost avoidance figure was then compared to raw data pulled by a VIONE dashboard correspondent to ensure the manual calculation was accurate. Finally, the 5 most common classes of medications deprescribed were identified for information purposes and to provide a better postulation on the types of medications being discontinued using the VIONE method.
Results
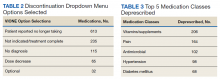
A total of 2,442 veterans met inclusion criteria, and the VIONE method was applied to 598 between late October 2018 and January 2019. The 13 PACT pharmacists contacted at least 10 veterans each, thus at least 130 were randomly selected for telephone calls to perform polypharmacy reviews using the VIONE note template. The discontinuation menu was used if a medication qualified to be deprescribed. After 3 months, 1986 prescriptions were deprescribed using VIONE; however, 1060 prescriptions were considered appropriately deprescribed (Table 2). The 13 PACT pharmacists deprescribed 361 medications, and the 29 PACT physicians deprescribed 699 medications. These prescriptions were then separated into medication categories to determine the most common discontinued classes. Vitamins and supplements were the medication class most frequently deprescribed (19.4%), followed by pain medications (15.5%), antimicrobial agents (9.6%), antihypertensive medications (9.2%), and diabetes medications (6.4%) (Table 3). The top 5 medication categories accounted for 60% of all medications appropriately deprescribed.
The estimated annualized cost avoidance for all medications deprescribed in the 3-month project period was $84,030.46. To provide the most appropriate and accurate calculation, medication classes excluded from this figure were acute or short-term prescriptions and antimicrobial agents. Medications prescribed short-term typically are not suitable to continue for an extended period, and antimicrobial agents were excluded since they are normally associated with higher costs, and may overestimate the cost avoidance calculation for the facility.
Discussion
The outcomes for the primary and secondary endpoints of this project illustrate that using VIONE in PACT primary care clinics had a notable impact on polypharmacy and cost avoidance over a short period. This outcome can be attributed to 2 significant effects of using the deprescribing tool. VIONE’s simplicity in application allowed clinicians to incorporate daily use of the tool with minimal effort. Education was all that was required to fully enable clinicians to work together successfully and exercise collaborative practice to promote deprescribing. VIONE also elicited a cascade of favorable effects that improve patient safety and health outcomes. The tool aided in identification of PIM, which helped reduce polypharmacy and medication burden. The risk for DDIs and ADEs may decrease; therefore, the incidence of falls, need for emergency department visits or inpatient care related to polypharmacy may decline. Less complex medication regimens may alleviate issues with adherence and avoid the various consequences of polypharmacy in theory. Simplified regimens can potentially improve disease management and quality of life for patients. Further studies are needed to substantiate deprescribing and its true effect on patient adherence and better health outcomes at this time.10
Reducing polypharmacy can lead to cost savings. Based on the results of this 3-month study, we expect that VASNHS would save more than $84,000 by reducing polypharmacy among its patients. Those savings can be funneled back into the health care system, and allotted to necessary patient care, prescriptions, and health care facility needs.
Limitations
There are some important limitations to this study. Definitions of polypharmacy may vary from one health care facility to another. The cutoffs for polypharmacy may differ, causing the prevalence of polypharmacy and potential costs savings to vary. Use of VIONE may be inconsistent among users if not previously educated or properly trained. For instance, VIONE selections are listed in the same menu as the standard CPRS discontinuation options, which may lead to discontinuation of medical supplies or laboratory orders instead of prescriptions.
The method of data analysis and project design used in this study may have been subject to error. For example, the list of PCPs may have been inaccurate or outdated, which would result in an over- or underrepresentation of those who contributed to data collection. Furthermore, there is some volatility in calculating the total cost avoidance. For example, medications for chronic conditions that were only taken on an as needed basis may have overestimated savings. Either under- or overestimations could occur when parameters are adjusted on the VIONE discontinuation dashboard without appropriate guidance. With the ability to manually adjust the dashboard parameters, dissimilarities in calculations may follow.
Conclusions
The VIONE tool may be useful in improving patient safety through deprescribing and discontinuing PIM. Decreasing the number of medications being taken concomitantly by a patient and continuing only those that are imperative in their medical treatment is the first step to reducing the incidence of polypharmacy. Consequently, chances of ADEs or DDIs are lessened, especially among older individuals who are considered high risk for experiencing the detrimental effects that may ensue. These effects include geriatric-related syndromes, increased risk of fall, hospital visits or admissions, or death. Use of VIONE easily promotes collaboration among clinicians to evaluate medications eligible for discontinuation more regularly. If this deprescribing tool is continuously used, costs avoided can likely be maximized within VA health care systems.
The results of this project should serve as an incentive to push for better prescribing practices and increase deprescribing efforts. It should provoke the need for change in regimens and the subsequent discontinuation of prescriptions that are not considered vital to continue. Finally, the result of this project should substantiate the positive impact a deprescribing tool can possess to avert the issues commonly associated with polypharmacy.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs. Published May 2014. Accessed May 13, 2021. https://www.cdc.gov/nchs/data/hus/hus13.pdf
2. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. Published 2017 Oct 10. doi:10.1186/s12877-017-0621-2
3. Parulekar MS, Rogers CK. Polypharmacy and mobility. In: Cifu DX, Lew HL, Oh-Park M., eds Geriatric Rehabilitation. Elsevier; 2018. doi:10.1016/B978-0-323-54454-2.12001-1
4. Rieckert A, Trampisch US, Klaaßen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract. 2018;19(1):113. Published 2018 Jul 18. doi:10.1186/s12875-018-0795-5
5. Thompson CA. New medication review method cuts veterans’ Rx load, saves millions. Am J Health Syst Pharm. 2018;75(8):502-503. doi:10.2146/news180023
6. Reeve E. Deprescribing tools: a review of the types of tools available to aid deprescribing in clinical practice. J Pharm Pract Res. 2020;50(1):98-107. doi:10.1002/jppr.1626
7. Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on Medication Communication and Deprescribing. J Am Geriatr Soc. 2017;65(10):2265-2271. doi:10.1111/jgs.15042
8. Battar S, Dickerson KR, Sedgwick C, et al. Understanding principles of high reliability organizations through the eyes of VIONE, a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
9. Battar S, Cmelik T, Dickerson K, Scott, M. Experience better health with VIONE a safe medication deprescribing tool [Nonpublic source, not verified]
10. Ulley J, Harrop D, Ali A, et al. Desprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: a systematic review. BMC Geriatr. 2019;19(15):1-13.
According to the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS), the use of prescription drugs has increased in the past half century. Although prescription drugs have played an important role in preventing, controlling, and delaying onset or progression of disease, their growth in use also has posed many risks.1 One ramification of this growth is the occurrence of polypharmacy, which does not have a universal, clear definition. In general, it can be described as the concurrent use of multiple medications by a single patient to treat one or more medical ailments. Five or more medications taken simultaneously is the most common definition to date, but this is just one of many acceptable definitions and that varies from one health care facility to another.1,2
Regardless of the cutoffs established to indicate polypharmacy, its incidence can result in poor and potentially harmful health outcomes. Polypharmacy increases the risk of experiencing adverse drug events (ADEs), drug-drug interactions (DDIs), geriatric-related syndromes, falls, hospitalization, and mortality. Issues with adherence may begin to unfold secondary to increased pill burden. Both the patient and the health care system may encounter financial strain, as polypharmacy can lead to unnecessary and essentially preventable costs of care. When evaluating the likelihood of polypharmacy based on age group, NCHS found that 47.5% of patients taking ≥ 5 medications were aged ≥ 65 years.1-5 This indicates that polypharmacy is of great concern in the geriatric population, which also represents a large proportion of individuals accessing Veterans Health Administration (VHA) care.
Deprescibing
Deprescribing is the act of withdrawing or discontinuing potentially inappropriate medications (PIM), or medications used by older patients harboring ADEs that generally outweigh the clinical benefits of the drug. Deprescribing is an effective tool for managing or reducing polypharmacy. A variety of tools have been created whose sole purpose is to simplify deprescribing. Some tools explicitly identify PIM and are widely familiar in medical practice. Examples are the Beers Criteria developed in 1991 or Screening Tool to Alert Right Treatment/Screening Tool of Older Persons Prescriptions (START/STOPP) criteria created in 2003. Other tools that are less commonplace but equally as resourceful are MedStopper and Deprescribing.org. The former was launched in 2015 and is a Canadian online system that provides risk assessments for medications with guidance for tapering or stopping medications if continuation of the drug presents higher risk than benefit.5-7 The latter is a full-fledged website developed by a physician, a pharmacist, and their research teams that serves as an exchange hub for deprescribing information.
In 2016, the VIONE (Vital, Important, Optional, Not indicated/treatment complete, and Every medication has an indication) deprescribing tool was developed by Saraswathy Battar, MD, at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock, as a system that could go beyond medication reconciliation (Table 1). Health care providers (HCPs) and pharmacists evaluate each medication that a patient has been prescribed and places each medication in a VIONE category. Prescribers may then take the opportunity to deprescribe or discontinue medications if deemed appropriate based on their clinical assessments and shared decision making.8 Traditionally, medication reconciliation involves the process of obtaining a complete and accurate list of medications as reported by a patient or caregiver to a HCP. VIONE encourages HCPs and pharmacists not only to ensure medication lists are accurate, but also that each medication reported is appropriate for continued use. In other words, VIONE is meant to help implement deprescribing at opportune times. More than 14,000 medications have been deprescribed using the VIONE method, resulting in more than $2,000,000 of annualized cost avoidance after just 1 year of implementation at CAVHS.9
VIONE consists of 2 major components in the Computerized Patient Record System (CPRS): a template and a dropdown discontinuation menu. The template captured patient allergies, pertinent laboratory data, the patient’s active problem list and applicable diagnoses, and active medication list. Patient aligned care team (PACT) pharmacists used the information captured in the template to conduct medication reconciliations and polypharmacy reviews. Each medication is categorized in VIONE using data collected during reviews. A menu delineates reasons for discontinuation: optional, dose decrease, no diagnosis, not indicated/treatment complete, discontinue alternate medication prescribed, and patient reported no longer taking. The discontinuation menu allowed PACT pharmacists and physicians to choose 1 VIONE option per medication to clarify the reason for discontinuation. VIONE-based discontinuations are recorded in CPRS and identified as deprescribed.
At the time of this project, > 30 US Department of Veterans Affairs (VA) facilities had adopted VIONE. Use of VIONE at VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas has been incorporated in the everyday practices of home-based primary care pharmacists and physicians but has yet to be implemented in other areas of the facility. The purpose of this project was to determine the impact of the VIONE tool on polypharmacy and cost avoidance at VASNHS when used by primary care physicians (PCPs) and PACT primary care clinics.
Methods
Veterans receiving care at VASNHS aged ≥ 65 years with ≥ 10 active medications noted in CPRS were included in this project. PACT pharmacists and physicians were educated on the proper use of the VIONE tool prior to its implementation. Education included a 15-minute slide presentation followed by dissemination of a 1-page VIONE tool handout during a PACT all-staff clinic meeting.
Data were collected for 3 months before and after the intervention. Data were made available for assessment by the Automated Data Processing Application Coordinator (ADPAC) at VASNHS. The ADPAC created and generated an Excel spreadsheet report, which listed all medications deprescribed using the VIONE method. The primary endpoint was the total number of medications discontinued using the VIONE template and/or discontinuation menu. For the purpose of this project, appropriate discontinuation was considered any prescription deprescribed, excluding medical supplies, by pharmacists and PCPs who received VIONE education.
The secondary endpoint was the estimated annualized cost avoidance for the facility (Figure). The calculation does not include medications discontinued due to the prescription of an alternative medication or dose decreases since these VIONE selections imply that a new prescription or order was placed and the original prescription was not deprescribed. Annualized cost avoidance was determined with use of the VIONE dashboard, a database that retrospectively gathers information regarding patients at risk of polypharmacy, polypharmacy-related ADEs, and cost. Manual adjustments were made to various parameters on the Veterans Integrated Service Network 15 VIONE dashboard by the author in order to obtain data specific to this project. These parameters allowed selection of service sections, specific staff members or the option to include or exclude chronic or nonchronic medications. The annualized cost avoidance figure was then compared to raw data pulled by a VIONE dashboard correspondent to ensure the manual calculation was accurate. Finally, the 5 most common classes of medications deprescribed were identified for information purposes and to provide a better postulation on the types of medications being discontinued using the VIONE method.
Results
A total of 2,442 veterans met inclusion criteria, and the VIONE method was applied to 598 between late October 2018 and January 2019. The 13 PACT pharmacists contacted at least 10 veterans each, thus at least 130 were randomly selected for telephone calls to perform polypharmacy reviews using the VIONE note template. The discontinuation menu was used if a medication qualified to be deprescribed. After 3 months, 1986 prescriptions were deprescribed using VIONE; however, 1060 prescriptions were considered appropriately deprescribed (Table 2). The 13 PACT pharmacists deprescribed 361 medications, and the 29 PACT physicians deprescribed 699 medications. These prescriptions were then separated into medication categories to determine the most common discontinued classes. Vitamins and supplements were the medication class most frequently deprescribed (19.4%), followed by pain medications (15.5%), antimicrobial agents (9.6%), antihypertensive medications (9.2%), and diabetes medications (6.4%) (Table 3). The top 5 medication categories accounted for 60% of all medications appropriately deprescribed.
The estimated annualized cost avoidance for all medications deprescribed in the 3-month project period was $84,030.46. To provide the most appropriate and accurate calculation, medication classes excluded from this figure were acute or short-term prescriptions and antimicrobial agents. Medications prescribed short-term typically are not suitable to continue for an extended period, and antimicrobial agents were excluded since they are normally associated with higher costs, and may overestimate the cost avoidance calculation for the facility.
Discussion
The outcomes for the primary and secondary endpoints of this project illustrate that using VIONE in PACT primary care clinics had a notable impact on polypharmacy and cost avoidance over a short period. This outcome can be attributed to 2 significant effects of using the deprescribing tool. VIONE’s simplicity in application allowed clinicians to incorporate daily use of the tool with minimal effort. Education was all that was required to fully enable clinicians to work together successfully and exercise collaborative practice to promote deprescribing. VIONE also elicited a cascade of favorable effects that improve patient safety and health outcomes. The tool aided in identification of PIM, which helped reduce polypharmacy and medication burden. The risk for DDIs and ADEs may decrease; therefore, the incidence of falls, need for emergency department visits or inpatient care related to polypharmacy may decline. Less complex medication regimens may alleviate issues with adherence and avoid the various consequences of polypharmacy in theory. Simplified regimens can potentially improve disease management and quality of life for patients. Further studies are needed to substantiate deprescribing and its true effect on patient adherence and better health outcomes at this time.10
Reducing polypharmacy can lead to cost savings. Based on the results of this 3-month study, we expect that VASNHS would save more than $84,000 by reducing polypharmacy among its patients. Those savings can be funneled back into the health care system, and allotted to necessary patient care, prescriptions, and health care facility needs.
Limitations
There are some important limitations to this study. Definitions of polypharmacy may vary from one health care facility to another. The cutoffs for polypharmacy may differ, causing the prevalence of polypharmacy and potential costs savings to vary. Use of VIONE may be inconsistent among users if not previously educated or properly trained. For instance, VIONE selections are listed in the same menu as the standard CPRS discontinuation options, which may lead to discontinuation of medical supplies or laboratory orders instead of prescriptions.
The method of data analysis and project design used in this study may have been subject to error. For example, the list of PCPs may have been inaccurate or outdated, which would result in an over- or underrepresentation of those who contributed to data collection. Furthermore, there is some volatility in calculating the total cost avoidance. For example, medications for chronic conditions that were only taken on an as needed basis may have overestimated savings. Either under- or overestimations could occur when parameters are adjusted on the VIONE discontinuation dashboard without appropriate guidance. With the ability to manually adjust the dashboard parameters, dissimilarities in calculations may follow.
Conclusions
The VIONE tool may be useful in improving patient safety through deprescribing and discontinuing PIM. Decreasing the number of medications being taken concomitantly by a patient and continuing only those that are imperative in their medical treatment is the first step to reducing the incidence of polypharmacy. Consequently, chances of ADEs or DDIs are lessened, especially among older individuals who are considered high risk for experiencing the detrimental effects that may ensue. These effects include geriatric-related syndromes, increased risk of fall, hospital visits or admissions, or death. Use of VIONE easily promotes collaboration among clinicians to evaluate medications eligible for discontinuation more regularly. If this deprescribing tool is continuously used, costs avoided can likely be maximized within VA health care systems.
The results of this project should serve as an incentive to push for better prescribing practices and increase deprescribing efforts. It should provoke the need for change in regimens and the subsequent discontinuation of prescriptions that are not considered vital to continue. Finally, the result of this project should substantiate the positive impact a deprescribing tool can possess to avert the issues commonly associated with polypharmacy.
According to the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS), the use of prescription drugs has increased in the past half century. Although prescription drugs have played an important role in preventing, controlling, and delaying onset or progression of disease, their growth in use also has posed many risks.1 One ramification of this growth is the occurrence of polypharmacy, which does not have a universal, clear definition. In general, it can be described as the concurrent use of multiple medications by a single patient to treat one or more medical ailments. Five or more medications taken simultaneously is the most common definition to date, but this is just one of many acceptable definitions and that varies from one health care facility to another.1,2
Regardless of the cutoffs established to indicate polypharmacy, its incidence can result in poor and potentially harmful health outcomes. Polypharmacy increases the risk of experiencing adverse drug events (ADEs), drug-drug interactions (DDIs), geriatric-related syndromes, falls, hospitalization, and mortality. Issues with adherence may begin to unfold secondary to increased pill burden. Both the patient and the health care system may encounter financial strain, as polypharmacy can lead to unnecessary and essentially preventable costs of care. When evaluating the likelihood of polypharmacy based on age group, NCHS found that 47.5% of patients taking ≥ 5 medications were aged ≥ 65 years.1-5 This indicates that polypharmacy is of great concern in the geriatric population, which also represents a large proportion of individuals accessing Veterans Health Administration (VHA) care.
Deprescibing
Deprescribing is the act of withdrawing or discontinuing potentially inappropriate medications (PIM), or medications used by older patients harboring ADEs that generally outweigh the clinical benefits of the drug. Deprescribing is an effective tool for managing or reducing polypharmacy. A variety of tools have been created whose sole purpose is to simplify deprescribing. Some tools explicitly identify PIM and are widely familiar in medical practice. Examples are the Beers Criteria developed in 1991 or Screening Tool to Alert Right Treatment/Screening Tool of Older Persons Prescriptions (START/STOPP) criteria created in 2003. Other tools that are less commonplace but equally as resourceful are MedStopper and Deprescribing.org. The former was launched in 2015 and is a Canadian online system that provides risk assessments for medications with guidance for tapering or stopping medications if continuation of the drug presents higher risk than benefit.5-7 The latter is a full-fledged website developed by a physician, a pharmacist, and their research teams that serves as an exchange hub for deprescribing information.
In 2016, the VIONE (Vital, Important, Optional, Not indicated/treatment complete, and Every medication has an indication) deprescribing tool was developed by Saraswathy Battar, MD, at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock, as a system that could go beyond medication reconciliation (Table 1). Health care providers (HCPs) and pharmacists evaluate each medication that a patient has been prescribed and places each medication in a VIONE category. Prescribers may then take the opportunity to deprescribe or discontinue medications if deemed appropriate based on their clinical assessments and shared decision making.8 Traditionally, medication reconciliation involves the process of obtaining a complete and accurate list of medications as reported by a patient or caregiver to a HCP. VIONE encourages HCPs and pharmacists not only to ensure medication lists are accurate, but also that each medication reported is appropriate for continued use. In other words, VIONE is meant to help implement deprescribing at opportune times. More than 14,000 medications have been deprescribed using the VIONE method, resulting in more than $2,000,000 of annualized cost avoidance after just 1 year of implementation at CAVHS.9
VIONE consists of 2 major components in the Computerized Patient Record System (CPRS): a template and a dropdown discontinuation menu. The template captured patient allergies, pertinent laboratory data, the patient’s active problem list and applicable diagnoses, and active medication list. Patient aligned care team (PACT) pharmacists used the information captured in the template to conduct medication reconciliations and polypharmacy reviews. Each medication is categorized in VIONE using data collected during reviews. A menu delineates reasons for discontinuation: optional, dose decrease, no diagnosis, not indicated/treatment complete, discontinue alternate medication prescribed, and patient reported no longer taking. The discontinuation menu allowed PACT pharmacists and physicians to choose 1 VIONE option per medication to clarify the reason for discontinuation. VIONE-based discontinuations are recorded in CPRS and identified as deprescribed.
At the time of this project, > 30 US Department of Veterans Affairs (VA) facilities had adopted VIONE. Use of VIONE at VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas has been incorporated in the everyday practices of home-based primary care pharmacists and physicians but has yet to be implemented in other areas of the facility. The purpose of this project was to determine the impact of the VIONE tool on polypharmacy and cost avoidance at VASNHS when used by primary care physicians (PCPs) and PACT primary care clinics.
Methods
Veterans receiving care at VASNHS aged ≥ 65 years with ≥ 10 active medications noted in CPRS were included in this project. PACT pharmacists and physicians were educated on the proper use of the VIONE tool prior to its implementation. Education included a 15-minute slide presentation followed by dissemination of a 1-page VIONE tool handout during a PACT all-staff clinic meeting.
Data were collected for 3 months before and after the intervention. Data were made available for assessment by the Automated Data Processing Application Coordinator (ADPAC) at VASNHS. The ADPAC created and generated an Excel spreadsheet report, which listed all medications deprescribed using the VIONE method. The primary endpoint was the total number of medications discontinued using the VIONE template and/or discontinuation menu. For the purpose of this project, appropriate discontinuation was considered any prescription deprescribed, excluding medical supplies, by pharmacists and PCPs who received VIONE education.
The secondary endpoint was the estimated annualized cost avoidance for the facility (Figure). The calculation does not include medications discontinued due to the prescription of an alternative medication or dose decreases since these VIONE selections imply that a new prescription or order was placed and the original prescription was not deprescribed. Annualized cost avoidance was determined with use of the VIONE dashboard, a database that retrospectively gathers information regarding patients at risk of polypharmacy, polypharmacy-related ADEs, and cost. Manual adjustments were made to various parameters on the Veterans Integrated Service Network 15 VIONE dashboard by the author in order to obtain data specific to this project. These parameters allowed selection of service sections, specific staff members or the option to include or exclude chronic or nonchronic medications. The annualized cost avoidance figure was then compared to raw data pulled by a VIONE dashboard correspondent to ensure the manual calculation was accurate. Finally, the 5 most common classes of medications deprescribed were identified for information purposes and to provide a better postulation on the types of medications being discontinued using the VIONE method.
Results
A total of 2,442 veterans met inclusion criteria, and the VIONE method was applied to 598 between late October 2018 and January 2019. The 13 PACT pharmacists contacted at least 10 veterans each, thus at least 130 were randomly selected for telephone calls to perform polypharmacy reviews using the VIONE note template. The discontinuation menu was used if a medication qualified to be deprescribed. After 3 months, 1986 prescriptions were deprescribed using VIONE; however, 1060 prescriptions were considered appropriately deprescribed (Table 2). The 13 PACT pharmacists deprescribed 361 medications, and the 29 PACT physicians deprescribed 699 medications. These prescriptions were then separated into medication categories to determine the most common discontinued classes. Vitamins and supplements were the medication class most frequently deprescribed (19.4%), followed by pain medications (15.5%), antimicrobial agents (9.6%), antihypertensive medications (9.2%), and diabetes medications (6.4%) (Table 3). The top 5 medication categories accounted for 60% of all medications appropriately deprescribed.
The estimated annualized cost avoidance for all medications deprescribed in the 3-month project period was $84,030.46. To provide the most appropriate and accurate calculation, medication classes excluded from this figure were acute or short-term prescriptions and antimicrobial agents. Medications prescribed short-term typically are not suitable to continue for an extended period, and antimicrobial agents were excluded since they are normally associated with higher costs, and may overestimate the cost avoidance calculation for the facility.
Discussion
The outcomes for the primary and secondary endpoints of this project illustrate that using VIONE in PACT primary care clinics had a notable impact on polypharmacy and cost avoidance over a short period. This outcome can be attributed to 2 significant effects of using the deprescribing tool. VIONE’s simplicity in application allowed clinicians to incorporate daily use of the tool with minimal effort. Education was all that was required to fully enable clinicians to work together successfully and exercise collaborative practice to promote deprescribing. VIONE also elicited a cascade of favorable effects that improve patient safety and health outcomes. The tool aided in identification of PIM, which helped reduce polypharmacy and medication burden. The risk for DDIs and ADEs may decrease; therefore, the incidence of falls, need for emergency department visits or inpatient care related to polypharmacy may decline. Less complex medication regimens may alleviate issues with adherence and avoid the various consequences of polypharmacy in theory. Simplified regimens can potentially improve disease management and quality of life for patients. Further studies are needed to substantiate deprescribing and its true effect on patient adherence and better health outcomes at this time.10
Reducing polypharmacy can lead to cost savings. Based on the results of this 3-month study, we expect that VASNHS would save more than $84,000 by reducing polypharmacy among its patients. Those savings can be funneled back into the health care system, and allotted to necessary patient care, prescriptions, and health care facility needs.
Limitations
There are some important limitations to this study. Definitions of polypharmacy may vary from one health care facility to another. The cutoffs for polypharmacy may differ, causing the prevalence of polypharmacy and potential costs savings to vary. Use of VIONE may be inconsistent among users if not previously educated or properly trained. For instance, VIONE selections are listed in the same menu as the standard CPRS discontinuation options, which may lead to discontinuation of medical supplies or laboratory orders instead of prescriptions.
The method of data analysis and project design used in this study may have been subject to error. For example, the list of PCPs may have been inaccurate or outdated, which would result in an over- or underrepresentation of those who contributed to data collection. Furthermore, there is some volatility in calculating the total cost avoidance. For example, medications for chronic conditions that were only taken on an as needed basis may have overestimated savings. Either under- or overestimations could occur when parameters are adjusted on the VIONE discontinuation dashboard without appropriate guidance. With the ability to manually adjust the dashboard parameters, dissimilarities in calculations may follow.
Conclusions
The VIONE tool may be useful in improving patient safety through deprescribing and discontinuing PIM. Decreasing the number of medications being taken concomitantly by a patient and continuing only those that are imperative in their medical treatment is the first step to reducing the incidence of polypharmacy. Consequently, chances of ADEs or DDIs are lessened, especially among older individuals who are considered high risk for experiencing the detrimental effects that may ensue. These effects include geriatric-related syndromes, increased risk of fall, hospital visits or admissions, or death. Use of VIONE easily promotes collaboration among clinicians to evaluate medications eligible for discontinuation more regularly. If this deprescribing tool is continuously used, costs avoided can likely be maximized within VA health care systems.
The results of this project should serve as an incentive to push for better prescribing practices and increase deprescribing efforts. It should provoke the need for change in regimens and the subsequent discontinuation of prescriptions that are not considered vital to continue. Finally, the result of this project should substantiate the positive impact a deprescribing tool can possess to avert the issues commonly associated with polypharmacy.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs. Published May 2014. Accessed May 13, 2021. https://www.cdc.gov/nchs/data/hus/hus13.pdf
2. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. Published 2017 Oct 10. doi:10.1186/s12877-017-0621-2
3. Parulekar MS, Rogers CK. Polypharmacy and mobility. In: Cifu DX, Lew HL, Oh-Park M., eds Geriatric Rehabilitation. Elsevier; 2018. doi:10.1016/B978-0-323-54454-2.12001-1
4. Rieckert A, Trampisch US, Klaaßen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract. 2018;19(1):113. Published 2018 Jul 18. doi:10.1186/s12875-018-0795-5
5. Thompson CA. New medication review method cuts veterans’ Rx load, saves millions. Am J Health Syst Pharm. 2018;75(8):502-503. doi:10.2146/news180023
6. Reeve E. Deprescribing tools: a review of the types of tools available to aid deprescribing in clinical practice. J Pharm Pract Res. 2020;50(1):98-107. doi:10.1002/jppr.1626
7. Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on Medication Communication and Deprescribing. J Am Geriatr Soc. 2017;65(10):2265-2271. doi:10.1111/jgs.15042
8. Battar S, Dickerson KR, Sedgwick C, et al. Understanding principles of high reliability organizations through the eyes of VIONE, a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
9. Battar S, Cmelik T, Dickerson K, Scott, M. Experience better health with VIONE a safe medication deprescribing tool [Nonpublic source, not verified]
10. Ulley J, Harrop D, Ali A, et al. Desprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: a systematic review. BMC Geriatr. 2019;19(15):1-13.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs. Published May 2014. Accessed May 13, 2021. https://www.cdc.gov/nchs/data/hus/hus13.pdf
2. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. Published 2017 Oct 10. doi:10.1186/s12877-017-0621-2
3. Parulekar MS, Rogers CK. Polypharmacy and mobility. In: Cifu DX, Lew HL, Oh-Park M., eds Geriatric Rehabilitation. Elsevier; 2018. doi:10.1016/B978-0-323-54454-2.12001-1
4. Rieckert A, Trampisch US, Klaaßen-Mielke R, et al. Polypharmacy in older patients with chronic diseases: a cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam Pract. 2018;19(1):113. Published 2018 Jul 18. doi:10.1186/s12875-018-0795-5
5. Thompson CA. New medication review method cuts veterans’ Rx load, saves millions. Am J Health Syst Pharm. 2018;75(8):502-503. doi:10.2146/news180023
6. Reeve E. Deprescribing tools: a review of the types of tools available to aid deprescribing in clinical practice. J Pharm Pract Res. 2020;50(1):98-107. doi:10.1002/jppr.1626
7. Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on Medication Communication and Deprescribing. J Am Geriatr Soc. 2017;65(10):2265-2271. doi:10.1111/jgs.15042
8. Battar S, Dickerson KR, Sedgwick C, et al. Understanding principles of high reliability organizations through the eyes of VIONE, a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
9. Battar S, Cmelik T, Dickerson K, Scott, M. Experience better health with VIONE a safe medication deprescribing tool [Nonpublic source, not verified]
10. Ulley J, Harrop D, Ali A, et al. Desprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: a systematic review. BMC Geriatr. 2019;19(15):1-13.
FDA updates label for controversial Alzheimer’s drug aducanumab (Aduhelm)
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
– the group studied in the clinical trials.
The FDA approved aducanumab in early June amid significant controversy and disregarding the recommendation by its own advisory panel not to approve the drug. The original prescribing information implied that the drug – which is administered intravenously and costs around $56,000 a year – could be used for treatment of any patient with Alzheimer’s disease.
The updated label now states that aducanumab should be initiated only in patients with mild cognitive impairment (MCI) or mild dementia stage of disease – the population in which treatment was initiated in the clinical trials leading to approval of the anti-amyloid drug.
The FDA granted accelerated approval of the drug based on data from clinical trials showing a reduction in amyloid beta plaques observed in patients with MCI or mild dementia stage of disease.
“Continued approval for the indication may be contingent upon verification of clinical benefit in confirmatory trial(s),” the label states. It emphasizes that there are no safety or effectiveness data on starting aducanumab treatment at earlier or later stages of the disease than were studied.
“Based on our ongoing conversations with prescribing physicians, FDA, and patient advocates, we submitted this label update with the goal to further clarify the patient population that was studied across the three Aduhelm clinical trials that supported approval,” Alfred Sandrock Jr., MD, PhD, Biogen’s head of research and development, said in a statement announcing the label update.
“We are committed to continue to listen to the community’s needs as clinical practice adapts to this important, first-in-class treatment option,” said Dr. Sandrock.
A version of this article first appeared on Medscape.com.
Extra COVID-19 vaccine could help immunocompromised people
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heart failure med undertreatment because of older age common, flouts evidence
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
Huge trial casts doubt on bisphosphonates for breast cancer
say researchers reporting new results from a phase 3 trial with almost 3,000 women.
Current guidelines call for 3-5 years of bisphosphonate therapy on the theory that these drugs might reduce breast cancer recurrence as well as treatment-related bone problems.
However, the new results show no difference in disease-free survival, distant disease-free survival, and overall survival – regardless of menopausal status – between the 1,540 women who received intravenous zoledronate over a 5-year period and 1,447 women who received such therapy over a 2-year period.
What they did find was a substantially higher risk for adverse events with prolonged bisphosphonate treatment, including risks for grade 3/4 events, bone pain, bone fractures, arthralgia, and jaw necrosis, a rare but well- recognized possibility with bisphosphonates.
Lead investigator Thomas Friedl, PhD, a statistician at University Hospital Ulm (Germany), and colleagues concluded that the current duration of treatment can be reduced and that, short of good reason to use bisphosphonates longer, such as decreased bone density, “treatment with zoledronate for 5 years should not be considered in patients with early breast cancer.”
The study was published online on June 24 in JAMA Oncology.
An accompanying editorial went even further, stating not only that “shorter duration of treatment is sufficient” but also that the whole idea of bisphosphonates for breast cancer is in doubt.
With “the modest outcomes of bisphosphonates, compared with no bone-targeted therapy, in historical trials” and the low rates of recurrence with modern treatment – less than 10% in the trial – “what, if any, is the benefit from adjuvant bisphosphonates? It’s time to reevaluate the guidelines,” said the editorialists, led by Alexandra Desnoyers, MD, a breast cancer fellow at the University of Toronto.
“We suggest that zoledronate or other amino-bisphosphonates should not be given as standard adjuvant therapy for unselected women with breast cancer,” they wrote.
Risk for necrosis with 5 years of zoledronate
The women in the trial had primary invasive breast cancer and were at high risk for recurrence. They had either positive nodes or high-risk features, including age (median, 53 years). They were treated at 250 centers in Germany.
The first part of the trial was to see whether use of gemcitabine improved outcomes when added to docetaxel after standard fluorouracil, epirubicin, and cyclophosphamide adjuvant therapy following surgery. It did not, and the authors reported in 2020 that adjuvant gemcitabine should not be used in the treatment of high-risk early breast cancer.
The next phase of the trial involved zoledronate. Women were randomly assigned to receive zoledronate for 2 or 5 years after surgery and after undergoing chemotherapy. Dosing was 4 mg IV every 3 months for 2 years. The women in the 5-year group went on to receive 4 mg IV every 6 months for another 3 years.
At a mean of 5 years’ follow-up after the first zoledronate dose, there was no difference in any of the survival measures between the two dosage groups.
There was also no difference in rates of bone recurrence or in circulating tumor cells, which the bisphosphonates theory would have predicted. For instance, 10.5% of women in the 5-year group had one or more circulating tumor cells on follow-up versus 7.2% in the 2-year group.
Almost half of the women in the 5-year treatment group experienced adverse events with zoledronate – including 7.6% with grade 3/4 events – versus just over a quarter in the 2-year arm and only 5.1% with grade 3/4 events.
In the 5-year group, 8.3% of patients experienced bone pain and 5.1% experienced arthralgia versus 3.7% and 3.1%, respectively, in the 2-year arm.
Atypical fractures, such as femoral spiral fractures, are another concern with bisphosphonates. Although this trial did not report on fracture type, fractures were reported in 14 women in the 5-year group but in only 3 in the 2-year arm.
Jaw necrosis, another known adverse effect of bisphosphonates, was reported in 11 women in the 5-year group and in 5 in the 2-year group.
The study was funded by several pharmaceutical companies, including Novartis, the maker of zoledronate. The investigators have numerous industry ties. Dr. Friedl has received payments from Novartis.
A version of this article first appeared on Medscape.com.
say researchers reporting new results from a phase 3 trial with almost 3,000 women.
Current guidelines call for 3-5 years of bisphosphonate therapy on the theory that these drugs might reduce breast cancer recurrence as well as treatment-related bone problems.
However, the new results show no difference in disease-free survival, distant disease-free survival, and overall survival – regardless of menopausal status – between the 1,540 women who received intravenous zoledronate over a 5-year period and 1,447 women who received such therapy over a 2-year period.
What they did find was a substantially higher risk for adverse events with prolonged bisphosphonate treatment, including risks for grade 3/4 events, bone pain, bone fractures, arthralgia, and jaw necrosis, a rare but well- recognized possibility with bisphosphonates.
Lead investigator Thomas Friedl, PhD, a statistician at University Hospital Ulm (Germany), and colleagues concluded that the current duration of treatment can be reduced and that, short of good reason to use bisphosphonates longer, such as decreased bone density, “treatment with zoledronate for 5 years should not be considered in patients with early breast cancer.”
The study was published online on June 24 in JAMA Oncology.
An accompanying editorial went even further, stating not only that “shorter duration of treatment is sufficient” but also that the whole idea of bisphosphonates for breast cancer is in doubt.
With “the modest outcomes of bisphosphonates, compared with no bone-targeted therapy, in historical trials” and the low rates of recurrence with modern treatment – less than 10% in the trial – “what, if any, is the benefit from adjuvant bisphosphonates? It’s time to reevaluate the guidelines,” said the editorialists, led by Alexandra Desnoyers, MD, a breast cancer fellow at the University of Toronto.
“We suggest that zoledronate or other amino-bisphosphonates should not be given as standard adjuvant therapy for unselected women with breast cancer,” they wrote.
Risk for necrosis with 5 years of zoledronate
The women in the trial had primary invasive breast cancer and were at high risk for recurrence. They had either positive nodes or high-risk features, including age (median, 53 years). They were treated at 250 centers in Germany.
The first part of the trial was to see whether use of gemcitabine improved outcomes when added to docetaxel after standard fluorouracil, epirubicin, and cyclophosphamide adjuvant therapy following surgery. It did not, and the authors reported in 2020 that adjuvant gemcitabine should not be used in the treatment of high-risk early breast cancer.
The next phase of the trial involved zoledronate. Women were randomly assigned to receive zoledronate for 2 or 5 years after surgery and after undergoing chemotherapy. Dosing was 4 mg IV every 3 months for 2 years. The women in the 5-year group went on to receive 4 mg IV every 6 months for another 3 years.
At a mean of 5 years’ follow-up after the first zoledronate dose, there was no difference in any of the survival measures between the two dosage groups.
There was also no difference in rates of bone recurrence or in circulating tumor cells, which the bisphosphonates theory would have predicted. For instance, 10.5% of women in the 5-year group had one or more circulating tumor cells on follow-up versus 7.2% in the 2-year group.
Almost half of the women in the 5-year treatment group experienced adverse events with zoledronate – including 7.6% with grade 3/4 events – versus just over a quarter in the 2-year arm and only 5.1% with grade 3/4 events.
In the 5-year group, 8.3% of patients experienced bone pain and 5.1% experienced arthralgia versus 3.7% and 3.1%, respectively, in the 2-year arm.
Atypical fractures, such as femoral spiral fractures, are another concern with bisphosphonates. Although this trial did not report on fracture type, fractures were reported in 14 women in the 5-year group but in only 3 in the 2-year arm.
Jaw necrosis, another known adverse effect of bisphosphonates, was reported in 11 women in the 5-year group and in 5 in the 2-year group.
The study was funded by several pharmaceutical companies, including Novartis, the maker of zoledronate. The investigators have numerous industry ties. Dr. Friedl has received payments from Novartis.
A version of this article first appeared on Medscape.com.
say researchers reporting new results from a phase 3 trial with almost 3,000 women.
Current guidelines call for 3-5 years of bisphosphonate therapy on the theory that these drugs might reduce breast cancer recurrence as well as treatment-related bone problems.
However, the new results show no difference in disease-free survival, distant disease-free survival, and overall survival – regardless of menopausal status – between the 1,540 women who received intravenous zoledronate over a 5-year period and 1,447 women who received such therapy over a 2-year period.
What they did find was a substantially higher risk for adverse events with prolonged bisphosphonate treatment, including risks for grade 3/4 events, bone pain, bone fractures, arthralgia, and jaw necrosis, a rare but well- recognized possibility with bisphosphonates.
Lead investigator Thomas Friedl, PhD, a statistician at University Hospital Ulm (Germany), and colleagues concluded that the current duration of treatment can be reduced and that, short of good reason to use bisphosphonates longer, such as decreased bone density, “treatment with zoledronate for 5 years should not be considered in patients with early breast cancer.”
The study was published online on June 24 in JAMA Oncology.
An accompanying editorial went even further, stating not only that “shorter duration of treatment is sufficient” but also that the whole idea of bisphosphonates for breast cancer is in doubt.
With “the modest outcomes of bisphosphonates, compared with no bone-targeted therapy, in historical trials” and the low rates of recurrence with modern treatment – less than 10% in the trial – “what, if any, is the benefit from adjuvant bisphosphonates? It’s time to reevaluate the guidelines,” said the editorialists, led by Alexandra Desnoyers, MD, a breast cancer fellow at the University of Toronto.
“We suggest that zoledronate or other amino-bisphosphonates should not be given as standard adjuvant therapy for unselected women with breast cancer,” they wrote.
Risk for necrosis with 5 years of zoledronate
The women in the trial had primary invasive breast cancer and were at high risk for recurrence. They had either positive nodes or high-risk features, including age (median, 53 years). They were treated at 250 centers in Germany.
The first part of the trial was to see whether use of gemcitabine improved outcomes when added to docetaxel after standard fluorouracil, epirubicin, and cyclophosphamide adjuvant therapy following surgery. It did not, and the authors reported in 2020 that adjuvant gemcitabine should not be used in the treatment of high-risk early breast cancer.
The next phase of the trial involved zoledronate. Women were randomly assigned to receive zoledronate for 2 or 5 years after surgery and after undergoing chemotherapy. Dosing was 4 mg IV every 3 months for 2 years. The women in the 5-year group went on to receive 4 mg IV every 6 months for another 3 years.
At a mean of 5 years’ follow-up after the first zoledronate dose, there was no difference in any of the survival measures between the two dosage groups.
There was also no difference in rates of bone recurrence or in circulating tumor cells, which the bisphosphonates theory would have predicted. For instance, 10.5% of women in the 5-year group had one or more circulating tumor cells on follow-up versus 7.2% in the 2-year group.
Almost half of the women in the 5-year treatment group experienced adverse events with zoledronate – including 7.6% with grade 3/4 events – versus just over a quarter in the 2-year arm and only 5.1% with grade 3/4 events.
In the 5-year group, 8.3% of patients experienced bone pain and 5.1% experienced arthralgia versus 3.7% and 3.1%, respectively, in the 2-year arm.
Atypical fractures, such as femoral spiral fractures, are another concern with bisphosphonates. Although this trial did not report on fracture type, fractures were reported in 14 women in the 5-year group but in only 3 in the 2-year arm.
Jaw necrosis, another known adverse effect of bisphosphonates, was reported in 11 women in the 5-year group and in 5 in the 2-year group.
The study was funded by several pharmaceutical companies, including Novartis, the maker of zoledronate. The investigators have numerous industry ties. Dr. Friedl has received payments from Novartis.
A version of this article first appeared on Medscape.com.
What’s best for diabetes after metformin? GRADE outdated at outset
Liraglutide and insulin glargine outperformed glimepiride and sitagliptin as single add-on agents to metformin for treating patients with type 2 diabetes in a multicenter U.S. trial that randomized just over 5,000 patients.
. Results were reported at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
The comparison included two oral medications – the sulfonylurea glimepiride and dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin – and two injectable medications – insulin glargine and glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide.
The primary endpoint was change in A1c level and overall glycemic control. Secondary endpoints include changes in weight, as well as cardiovascular, renal, gastrointestinal, and other complications.
For the primary endpoint – keeping A1c levels below 7% – liraglutide and the basal insulin glargine both did this best and were almost equivalent.
During the average 5-year follow-up, the rates of patients progressing to a confirmed A1c of 7% or higher were 67% among patients randomized to insulin glargine, 68% maintained on liraglutide, 72% taking the sulfonylurea glimepiride, and 77% taking sitagliptin, reported John M. Lachin, ScD, a biostatistician at George Washington University, Washington.
Too soon for take-aways, or are the data already obsolete?
“The ultimate goal of GRADE is to help clinicians select the therapies that will work best for individual patients, as diabetes care is not a one-size-fits all approach,” noted David M. Nathan, MD, chair of the study and director of the Diabetes Center at Massachusetts General Hospital, in an ADA press release.
Dr. Nathan, as well as several other members of the GRADE trial steering committee who presented results, repeatedly cautioned that the findings were preliminary because they represent 90% of outcomes, with the remaining 10% still to be adjudicated.
“We undertook this study to fill a gap in the guidelines,” said investigator Deborah J. Wexler, MD, clinical director of the Diabetes Center at Massachusetts General Hospital in Boston. “I would like to have all the results in ... before I comment on how the guidelines should change.”
“The metabolic data are solid, but the cardiovascular disease data are preliminary,” warned Dr. Nathan.
But that didn’t stop some from drawing their own conclusions, with Julio Rosenstock, MD, who comoderated the session but was not involved with the study, giving his own opinion.
“A pleasant surprise was the performance of basal insulin,” he said, calling the findings “a vindication” for basal insulin as a treatment for the types of patients with type 2 diabetes that enrolled in the study.
Steven E. Kahn, MB, ChB, another GRADE co-investigator agreed. “Based on the results, guidelines should say that you add insulin early on,” he observed.
A generic basal insulin and a generic sulfonylurea are both reasonable options, after metformin, for patients with limited resources, added Dr. Kahn, an endocrinologist and professor at the University of Washington, Seattle.
Dr. Rosenstock, director of the Dallas Diabetes Research Center, also saw the results as an indictment of agents in the DDP-4 inhibitor class, such as sitagliptin.
The DPP-4 inhibitors generate $9 billion a year, he said, wondering whether it “is justifiable to put them on the same level as other agents?”
Meanwhile the assigned discussant, David R. Matthews, DPhil, a professor of diabetes medicine at the University of Oxford, England – while congratulating the investigators on certain aspects of the study – said it ultimately fell short because it didn’t include an arm with an SGLT2 inhibitor.
“We should kick the authors for missing out on SGLT2 inhibitors,” Dr. Matthews said. “The omission means that the GRADE data are already obsolescent.”
In reply, Dr. Nathan admitted “we feel bad we did not include” an SGLT2 inhibitor, but he vigorously defended the dilemma faced by the trial’s organizers.
Oral SGLT2 inhibitors were not “well-established drugs” for type 2 diabetes when enrollment launched in 2013, and the researchers were wary of including what could turn out to be a problematic agent soon after controversy over the safety of agents in the thiazolidinedione drug class (such as rosiglitazone), he explained.
They also realized that adding a fifth drug to the study would necessitate doubling enrollment size, which would have undercut the funding plans already in place.
Dr. Matthews also derided GRADE as being underpowered to adequately address the impact of the tested agents on major adverse cardiovascular events (MACE) and hospitalizations for heart failure and too U.S.-centric to be generalizable elsewhere.
A study with lots of data
The roughly 5,000 patients enrolled in GRADE were an average age of 57 years old, 64% were men, 66% were White, and 20% were Black. They had had type 2 diabetes, on average, for 4.2 years. Mean body mass index at entry was about 34 kg/m2, average A1c was 7.5%, and average estimated glomerular filtration rate was 95 mL/min/1.73m2. The trial included a 6-12 week run-in period during which background metformin treatment was optimized and led to average A1c levels less than 7%.
Patients were then randomized to one of the four agents as add-on treatment.
Both liraglutide and insulin glargine performed well on many of the numerous metrics in the data-rich trial, largely funded by two branches of the National Institutes of Health, with commercial involvement limited to free supplies of the study drugs.
The secondary metabolic outcome, of disease progressing to a confirmed A1c of 7.5%, was reached by 39% of patients taking insulin glargine, significantly lower than the rate of 46% among patients taking liraglutide, and that rate, in turn, was significantly below the 50% rate among patients taking glimepiride and the 55% rate of those taking sitagliptin.
Mean doses of the second-line agents after 4 years of treatment were 38.3 units/day for glargine, 3.5 mg/day for glimepiride, 1.3 mg/day for subcutaneous liraglutide, and 82.9 mg/day for sitagliptin.
A trio of cardiovascular outcomes showed one significant benefit of liraglutide over the other three drugs for the endpoint of any cardiovascular event, which included not only major adverse cardiovascular events (MACE; cardiovascular death, myocardial infarction, or stroke), but also several other event types, including heart failure requiring hospitalization, unstable angina requiring hospitalization, revascularization or any arterial repairs, stent thrombosis, or transient ischemic attack.
For the endpoint of any cardiovascular event, the rate was 5.8% for patients taking liraglutide, significantly less than the rate of 7.6% of those taking insulin glargine, 8.0% for glimepiride, and 8.6% for sitagliptin, reported John B. Buse, MD, PhD, professor, chief of endocrinology, and director of the Diabetes Center at the University of North Carolina at Chapel Hill.
For each of the other two main cardiovascular endpoints – MACE and hospitalization for heart failure – liraglutide had a numeric advantage over the other three drugs but failed to reach significance.
Patients taking liraglutide also had a smaller but not significantly different point estimate for all-cause death, at 2.1%, compared with 3.1%-3.4% in the other three groups.
And, Dr. Nathan emphasized, the cardiovascular disease data are still considered preliminary.
Liraglutide scored a pair of additional outcome victories. Its use resulted in a significantly lower rate of patients who progressed during follow-up to either needing antihypertensive medications or having their blood pressure rise above 140/90 mm Hg compared with the other three drugs. (At baseline, average blood pressure for all patients was 128/77 mm Hg.)
And after 4 years, patients taking liraglutide lost an average of about 4 kg (8.8 lb) from their baseline weight (which averaged about 100 kg [220 lb]), roughly the same as patients taking sitagliptin but significantly better than with glimepiride or insulin glargine. Patients taking glargine gained a small amount of weight on average during their first couple of years of treatment, roughly 1 kg, but returned to around their baseline weight by the end of 4 years.
Four drugs performed equally well for some outcomes
Finally, the four drugs had similar results for some outcomes. This included their effects on renal function, distal sensory polyneuropathy, and low-density lipoprotein (LDL) cholesterol.
The four agents also had roughly similar safety profiles, with rates of serious adverse events all falling within the tight range of 33%-37%.
But the rate of severe hypoglycemic episodes that required assistance to treat showed significant separation, ranging from 2.3% for glimepiride, 1.4% for glargine, 0.9% for liraglutide, and 0.7% for sitagliptin. Gastrointestinal symptoms occurred in about 50% of patients in three of the treatment groups but were significantly higher in those taking liraglutide, affecting 60%.
GRADE received no commercial funding. Dr. Wexler has reported serving on data monitoring committees for Novo Nordisk. Dr. Buse has reported being a consultant for and holding stock in numerous companies. Dr. Rosenstock has reported being an advisor or consultant to Applied Therapeutics, Boehringer Ingelheim, Hanmi Pharmaceutical, Intarcia Therapeutics, Lilly, Novo Nordisk, Oramed, and Sanofi and has received research support from numerous companies. Dr. Kahn has reported being an advisor to or speaker on behalf of Bayer, Boehringer Ingelheim, Casma Therapeutics, Intarcia Therapeutics, Lilly, Merck, Novo Nordisk, Pfizer, and Third Rock Ventures. Dr. Matthews has reported receiving lecture and advisor fees from Merck, Novartis, Novo Nordisk, Sanofi Aventis, and Servier. Dr. Lachin and Dr. Nathan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Liraglutide and insulin glargine outperformed glimepiride and sitagliptin as single add-on agents to metformin for treating patients with type 2 diabetes in a multicenter U.S. trial that randomized just over 5,000 patients.
. Results were reported at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
The comparison included two oral medications – the sulfonylurea glimepiride and dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin – and two injectable medications – insulin glargine and glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide.
The primary endpoint was change in A1c level and overall glycemic control. Secondary endpoints include changes in weight, as well as cardiovascular, renal, gastrointestinal, and other complications.
For the primary endpoint – keeping A1c levels below 7% – liraglutide and the basal insulin glargine both did this best and were almost equivalent.
During the average 5-year follow-up, the rates of patients progressing to a confirmed A1c of 7% or higher were 67% among patients randomized to insulin glargine, 68% maintained on liraglutide, 72% taking the sulfonylurea glimepiride, and 77% taking sitagliptin, reported John M. Lachin, ScD, a biostatistician at George Washington University, Washington.
Too soon for take-aways, or are the data already obsolete?
“The ultimate goal of GRADE is to help clinicians select the therapies that will work best for individual patients, as diabetes care is not a one-size-fits all approach,” noted David M. Nathan, MD, chair of the study and director of the Diabetes Center at Massachusetts General Hospital, in an ADA press release.
Dr. Nathan, as well as several other members of the GRADE trial steering committee who presented results, repeatedly cautioned that the findings were preliminary because they represent 90% of outcomes, with the remaining 10% still to be adjudicated.
“We undertook this study to fill a gap in the guidelines,” said investigator Deborah J. Wexler, MD, clinical director of the Diabetes Center at Massachusetts General Hospital in Boston. “I would like to have all the results in ... before I comment on how the guidelines should change.”
“The metabolic data are solid, but the cardiovascular disease data are preliminary,” warned Dr. Nathan.
But that didn’t stop some from drawing their own conclusions, with Julio Rosenstock, MD, who comoderated the session but was not involved with the study, giving his own opinion.
“A pleasant surprise was the performance of basal insulin,” he said, calling the findings “a vindication” for basal insulin as a treatment for the types of patients with type 2 diabetes that enrolled in the study.
Steven E. Kahn, MB, ChB, another GRADE co-investigator agreed. “Based on the results, guidelines should say that you add insulin early on,” he observed.
A generic basal insulin and a generic sulfonylurea are both reasonable options, after metformin, for patients with limited resources, added Dr. Kahn, an endocrinologist and professor at the University of Washington, Seattle.
Dr. Rosenstock, director of the Dallas Diabetes Research Center, also saw the results as an indictment of agents in the DDP-4 inhibitor class, such as sitagliptin.
The DPP-4 inhibitors generate $9 billion a year, he said, wondering whether it “is justifiable to put them on the same level as other agents?”
Meanwhile the assigned discussant, David R. Matthews, DPhil, a professor of diabetes medicine at the University of Oxford, England – while congratulating the investigators on certain aspects of the study – said it ultimately fell short because it didn’t include an arm with an SGLT2 inhibitor.
“We should kick the authors for missing out on SGLT2 inhibitors,” Dr. Matthews said. “The omission means that the GRADE data are already obsolescent.”
In reply, Dr. Nathan admitted “we feel bad we did not include” an SGLT2 inhibitor, but he vigorously defended the dilemma faced by the trial’s organizers.
Oral SGLT2 inhibitors were not “well-established drugs” for type 2 diabetes when enrollment launched in 2013, and the researchers were wary of including what could turn out to be a problematic agent soon after controversy over the safety of agents in the thiazolidinedione drug class (such as rosiglitazone), he explained.
They also realized that adding a fifth drug to the study would necessitate doubling enrollment size, which would have undercut the funding plans already in place.
Dr. Matthews also derided GRADE as being underpowered to adequately address the impact of the tested agents on major adverse cardiovascular events (MACE) and hospitalizations for heart failure and too U.S.-centric to be generalizable elsewhere.
A study with lots of data
The roughly 5,000 patients enrolled in GRADE were an average age of 57 years old, 64% were men, 66% were White, and 20% were Black. They had had type 2 diabetes, on average, for 4.2 years. Mean body mass index at entry was about 34 kg/m2, average A1c was 7.5%, and average estimated glomerular filtration rate was 95 mL/min/1.73m2. The trial included a 6-12 week run-in period during which background metformin treatment was optimized and led to average A1c levels less than 7%.
Patients were then randomized to one of the four agents as add-on treatment.
Both liraglutide and insulin glargine performed well on many of the numerous metrics in the data-rich trial, largely funded by two branches of the National Institutes of Health, with commercial involvement limited to free supplies of the study drugs.
The secondary metabolic outcome, of disease progressing to a confirmed A1c of 7.5%, was reached by 39% of patients taking insulin glargine, significantly lower than the rate of 46% among patients taking liraglutide, and that rate, in turn, was significantly below the 50% rate among patients taking glimepiride and the 55% rate of those taking sitagliptin.
Mean doses of the second-line agents after 4 years of treatment were 38.3 units/day for glargine, 3.5 mg/day for glimepiride, 1.3 mg/day for subcutaneous liraglutide, and 82.9 mg/day for sitagliptin.
A trio of cardiovascular outcomes showed one significant benefit of liraglutide over the other three drugs for the endpoint of any cardiovascular event, which included not only major adverse cardiovascular events (MACE; cardiovascular death, myocardial infarction, or stroke), but also several other event types, including heart failure requiring hospitalization, unstable angina requiring hospitalization, revascularization or any arterial repairs, stent thrombosis, or transient ischemic attack.
For the endpoint of any cardiovascular event, the rate was 5.8% for patients taking liraglutide, significantly less than the rate of 7.6% of those taking insulin glargine, 8.0% for glimepiride, and 8.6% for sitagliptin, reported John B. Buse, MD, PhD, professor, chief of endocrinology, and director of the Diabetes Center at the University of North Carolina at Chapel Hill.
For each of the other two main cardiovascular endpoints – MACE and hospitalization for heart failure – liraglutide had a numeric advantage over the other three drugs but failed to reach significance.
Patients taking liraglutide also had a smaller but not significantly different point estimate for all-cause death, at 2.1%, compared with 3.1%-3.4% in the other three groups.
And, Dr. Nathan emphasized, the cardiovascular disease data are still considered preliminary.
Liraglutide scored a pair of additional outcome victories. Its use resulted in a significantly lower rate of patients who progressed during follow-up to either needing antihypertensive medications or having their blood pressure rise above 140/90 mm Hg compared with the other three drugs. (At baseline, average blood pressure for all patients was 128/77 mm Hg.)
And after 4 years, patients taking liraglutide lost an average of about 4 kg (8.8 lb) from their baseline weight (which averaged about 100 kg [220 lb]), roughly the same as patients taking sitagliptin but significantly better than with glimepiride or insulin glargine. Patients taking glargine gained a small amount of weight on average during their first couple of years of treatment, roughly 1 kg, but returned to around their baseline weight by the end of 4 years.
Four drugs performed equally well for some outcomes
Finally, the four drugs had similar results for some outcomes. This included their effects on renal function, distal sensory polyneuropathy, and low-density lipoprotein (LDL) cholesterol.
The four agents also had roughly similar safety profiles, with rates of serious adverse events all falling within the tight range of 33%-37%.
But the rate of severe hypoglycemic episodes that required assistance to treat showed significant separation, ranging from 2.3% for glimepiride, 1.4% for glargine, 0.9% for liraglutide, and 0.7% for sitagliptin. Gastrointestinal symptoms occurred in about 50% of patients in three of the treatment groups but were significantly higher in those taking liraglutide, affecting 60%.
GRADE received no commercial funding. Dr. Wexler has reported serving on data monitoring committees for Novo Nordisk. Dr. Buse has reported being a consultant for and holding stock in numerous companies. Dr. Rosenstock has reported being an advisor or consultant to Applied Therapeutics, Boehringer Ingelheim, Hanmi Pharmaceutical, Intarcia Therapeutics, Lilly, Novo Nordisk, Oramed, and Sanofi and has received research support from numerous companies. Dr. Kahn has reported being an advisor to or speaker on behalf of Bayer, Boehringer Ingelheim, Casma Therapeutics, Intarcia Therapeutics, Lilly, Merck, Novo Nordisk, Pfizer, and Third Rock Ventures. Dr. Matthews has reported receiving lecture and advisor fees from Merck, Novartis, Novo Nordisk, Sanofi Aventis, and Servier. Dr. Lachin and Dr. Nathan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Liraglutide and insulin glargine outperformed glimepiride and sitagliptin as single add-on agents to metformin for treating patients with type 2 diabetes in a multicenter U.S. trial that randomized just over 5,000 patients.
. Results were reported at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
The comparison included two oral medications – the sulfonylurea glimepiride and dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin – and two injectable medications – insulin glargine and glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide.
The primary endpoint was change in A1c level and overall glycemic control. Secondary endpoints include changes in weight, as well as cardiovascular, renal, gastrointestinal, and other complications.
For the primary endpoint – keeping A1c levels below 7% – liraglutide and the basal insulin glargine both did this best and were almost equivalent.
During the average 5-year follow-up, the rates of patients progressing to a confirmed A1c of 7% or higher were 67% among patients randomized to insulin glargine, 68% maintained on liraglutide, 72% taking the sulfonylurea glimepiride, and 77% taking sitagliptin, reported John M. Lachin, ScD, a biostatistician at George Washington University, Washington.
Too soon for take-aways, or are the data already obsolete?
“The ultimate goal of GRADE is to help clinicians select the therapies that will work best for individual patients, as diabetes care is not a one-size-fits all approach,” noted David M. Nathan, MD, chair of the study and director of the Diabetes Center at Massachusetts General Hospital, in an ADA press release.
Dr. Nathan, as well as several other members of the GRADE trial steering committee who presented results, repeatedly cautioned that the findings were preliminary because they represent 90% of outcomes, with the remaining 10% still to be adjudicated.
“We undertook this study to fill a gap in the guidelines,” said investigator Deborah J. Wexler, MD, clinical director of the Diabetes Center at Massachusetts General Hospital in Boston. “I would like to have all the results in ... before I comment on how the guidelines should change.”
“The metabolic data are solid, but the cardiovascular disease data are preliminary,” warned Dr. Nathan.
But that didn’t stop some from drawing their own conclusions, with Julio Rosenstock, MD, who comoderated the session but was not involved with the study, giving his own opinion.
“A pleasant surprise was the performance of basal insulin,” he said, calling the findings “a vindication” for basal insulin as a treatment for the types of patients with type 2 diabetes that enrolled in the study.
Steven E. Kahn, MB, ChB, another GRADE co-investigator agreed. “Based on the results, guidelines should say that you add insulin early on,” he observed.
A generic basal insulin and a generic sulfonylurea are both reasonable options, after metformin, for patients with limited resources, added Dr. Kahn, an endocrinologist and professor at the University of Washington, Seattle.
Dr. Rosenstock, director of the Dallas Diabetes Research Center, also saw the results as an indictment of agents in the DDP-4 inhibitor class, such as sitagliptin.
The DPP-4 inhibitors generate $9 billion a year, he said, wondering whether it “is justifiable to put them on the same level as other agents?”
Meanwhile the assigned discussant, David R. Matthews, DPhil, a professor of diabetes medicine at the University of Oxford, England – while congratulating the investigators on certain aspects of the study – said it ultimately fell short because it didn’t include an arm with an SGLT2 inhibitor.
“We should kick the authors for missing out on SGLT2 inhibitors,” Dr. Matthews said. “The omission means that the GRADE data are already obsolescent.”
In reply, Dr. Nathan admitted “we feel bad we did not include” an SGLT2 inhibitor, but he vigorously defended the dilemma faced by the trial’s organizers.
Oral SGLT2 inhibitors were not “well-established drugs” for type 2 diabetes when enrollment launched in 2013, and the researchers were wary of including what could turn out to be a problematic agent soon after controversy over the safety of agents in the thiazolidinedione drug class (such as rosiglitazone), he explained.
They also realized that adding a fifth drug to the study would necessitate doubling enrollment size, which would have undercut the funding plans already in place.
Dr. Matthews also derided GRADE as being underpowered to adequately address the impact of the tested agents on major adverse cardiovascular events (MACE) and hospitalizations for heart failure and too U.S.-centric to be generalizable elsewhere.
A study with lots of data
The roughly 5,000 patients enrolled in GRADE were an average age of 57 years old, 64% were men, 66% were White, and 20% were Black. They had had type 2 diabetes, on average, for 4.2 years. Mean body mass index at entry was about 34 kg/m2, average A1c was 7.5%, and average estimated glomerular filtration rate was 95 mL/min/1.73m2. The trial included a 6-12 week run-in period during which background metformin treatment was optimized and led to average A1c levels less than 7%.
Patients were then randomized to one of the four agents as add-on treatment.
Both liraglutide and insulin glargine performed well on many of the numerous metrics in the data-rich trial, largely funded by two branches of the National Institutes of Health, with commercial involvement limited to free supplies of the study drugs.
The secondary metabolic outcome, of disease progressing to a confirmed A1c of 7.5%, was reached by 39% of patients taking insulin glargine, significantly lower than the rate of 46% among patients taking liraglutide, and that rate, in turn, was significantly below the 50% rate among patients taking glimepiride and the 55% rate of those taking sitagliptin.
Mean doses of the second-line agents after 4 years of treatment were 38.3 units/day for glargine, 3.5 mg/day for glimepiride, 1.3 mg/day for subcutaneous liraglutide, and 82.9 mg/day for sitagliptin.
A trio of cardiovascular outcomes showed one significant benefit of liraglutide over the other three drugs for the endpoint of any cardiovascular event, which included not only major adverse cardiovascular events (MACE; cardiovascular death, myocardial infarction, or stroke), but also several other event types, including heart failure requiring hospitalization, unstable angina requiring hospitalization, revascularization or any arterial repairs, stent thrombosis, or transient ischemic attack.
For the endpoint of any cardiovascular event, the rate was 5.8% for patients taking liraglutide, significantly less than the rate of 7.6% of those taking insulin glargine, 8.0% for glimepiride, and 8.6% for sitagliptin, reported John B. Buse, MD, PhD, professor, chief of endocrinology, and director of the Diabetes Center at the University of North Carolina at Chapel Hill.
For each of the other two main cardiovascular endpoints – MACE and hospitalization for heart failure – liraglutide had a numeric advantage over the other three drugs but failed to reach significance.
Patients taking liraglutide also had a smaller but not significantly different point estimate for all-cause death, at 2.1%, compared with 3.1%-3.4% in the other three groups.
And, Dr. Nathan emphasized, the cardiovascular disease data are still considered preliminary.
Liraglutide scored a pair of additional outcome victories. Its use resulted in a significantly lower rate of patients who progressed during follow-up to either needing antihypertensive medications or having their blood pressure rise above 140/90 mm Hg compared with the other three drugs. (At baseline, average blood pressure for all patients was 128/77 mm Hg.)
And after 4 years, patients taking liraglutide lost an average of about 4 kg (8.8 lb) from their baseline weight (which averaged about 100 kg [220 lb]), roughly the same as patients taking sitagliptin but significantly better than with glimepiride or insulin glargine. Patients taking glargine gained a small amount of weight on average during their first couple of years of treatment, roughly 1 kg, but returned to around their baseline weight by the end of 4 years.
Four drugs performed equally well for some outcomes
Finally, the four drugs had similar results for some outcomes. This included their effects on renal function, distal sensory polyneuropathy, and low-density lipoprotein (LDL) cholesterol.
The four agents also had roughly similar safety profiles, with rates of serious adverse events all falling within the tight range of 33%-37%.
But the rate of severe hypoglycemic episodes that required assistance to treat showed significant separation, ranging from 2.3% for glimepiride, 1.4% for glargine, 0.9% for liraglutide, and 0.7% for sitagliptin. Gastrointestinal symptoms occurred in about 50% of patients in three of the treatment groups but were significantly higher in those taking liraglutide, affecting 60%.
GRADE received no commercial funding. Dr. Wexler has reported serving on data monitoring committees for Novo Nordisk. Dr. Buse has reported being a consultant for and holding stock in numerous companies. Dr. Rosenstock has reported being an advisor or consultant to Applied Therapeutics, Boehringer Ingelheim, Hanmi Pharmaceutical, Intarcia Therapeutics, Lilly, Novo Nordisk, Oramed, and Sanofi and has received research support from numerous companies. Dr. Kahn has reported being an advisor to or speaker on behalf of Bayer, Boehringer Ingelheim, Casma Therapeutics, Intarcia Therapeutics, Lilly, Merck, Novo Nordisk, Pfizer, and Third Rock Ventures. Dr. Matthews has reported receiving lecture and advisor fees from Merck, Novartis, Novo Nordisk, Sanofi Aventis, and Servier. Dr. Lachin and Dr. Nathan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nocturnal hypoglycemia halved with insulin degludec vs. glargine
Patients with type 1 diabetes who used insulin degludec as their basal insulin had fewer than half the number of nocturnal hypoglycemia events, compared with patients who used insulin glargine U100, in a head-to-head crossover study with 51 patients who had a history of nighttime hypoglycemia episodes.
Patients with type 1 diabetes who are “struggling with nocturnal hypoglycemia would benefit from insulin degludec treatment,” said Julie M. Brøsen, MD, at the annual scientific sessions of the American Diabetes Association.
Accumulating evidence for less hypoglycemia with insulin degludec
Results from several studies comparing insulin degludec (Tresiba), a second-generation, longer-acting insulin with more stable steady-state performance, with the first-generation basal insulin analogue glargine (Lantus), have built the case that degludec produces fewer hypoglycemia events.
The landmark SWITCH 1 crossover study published in 2017 showed in about 500 patients with type 1 diabetes and a risk factor for hypoglycemia that treatment with insulin degludec led to significantly few total hypoglycemia episodes and significantly fewer nocturnal episodes, compared with insulin glargine.
Next came similar findings from ReFLeCT, a multicenter observational study that followed 556 unselected patients with type 1 diabetes in routine practice settings who switched to insulin degludec following treatment with a different basal insulin. The results again showed a significant drop-off in total, nonsevere, severe, and nocturnal hypoglycemia events.
Homing in on higher-risk patients
The current study, HypoDeg (Insulin Degludec and Symptomatic Nocturnal Hypoglycaemia), ran at 10 Danish centers and enrolled 149 adults with type 1 diabetes who had at least one episode of severe nocturnal hypoglycemia within the prior 2 years, focusing on patients most at risk for future nocturnal hypoglycemia events. In an unusual study design, researchers identified nocturnal hypoglycemic episodes with hourly venous blood samples drawn from a subcutaneous line.
They randomized the patients to basal insulin treatment with either insulin degludec or to insulin glargine U100, allowed their treatment to stabilize for 3 months, and then tallied nocturnal hypoglycemia events for 9 months. They then crossed patients to the alternative basal insulin and repeated the process.
Results from the full study have not yet appeared in published form but were in a pair of reports at the 2020 scientific sessions of the ADA.
One report included findings based on 136 episodes of severe hypoglycemia identified clinically and showed these events occurred 35% less often during treatment with insulin degludec, a significant difference. The overall finding was primarily driven by 48% fewer episodes of severe nocturnal hypoglycemia, but this difference was not significant.
The second report identified hypoglycemia events with continuous glucose monitoring in 74 of the study participants, which identified 193 episodes of nonsevere nocturnal hypoglycemia and found that treatment with insulin degludec cut the rate by 47%, primarily by reducing asymptomatic episodes.
Hourly blood draws track overnight hypoglycemia
The current study included 51 of the 149 HypoDeg patients who agreed to undergo overnight blood sampling and had this done at least once while treated with each of the two study insulins. (The study design called for two blood sampling nights for each willing patient during each of the two treatment periods.) The 51 patients had type 1 diabetes for an average of 28 years and an average age of 58 years. Two-thirds were men, their baseline A1c was 7.8%, and on average had 2.6 episodes of severe nocturnal hypoglycemia during the prior 2 years.
The researchers drew hourly blood specimens on a total of 196 nights from the 51 participating patients and identified 57 nights when blood glucose levels reached hypoglycemia thresholds in 33 patients. One-third of the events occurred when patients were on insulin degludec treatment, and two-thirds when they were on insulin glargine, reported Dr. Brøsen.
She presented three separate analyses of the data. One analysis focused on level 1 hypoglycemia events, when blood glucose dips to 70 mg/dL or less, which occurred 54% less often when patients were on insulin degludec. A second analysis looked at level 2 events, when blood glucose falls below 54 mg/dL, and treatment with insulin degludec cut this by 64% compared with insulin glargine. The third analysis focused on symptomatic events when blood glucose was 70 mg/dL or less, and treatment with insulin degludec linked with a 62% cut in this metric. All three between-group differences were significant.
Evidence supports already-changed practice
This new evidence “supports recommending” insulin degludec over insulin glargine, commented Bastiaan E. de Galan, MD, PhD, an endocrinologist and professor at Maastrict (the Netherlands) University Medical Center. The new results “extend those from previous trials in populations with type 1 diabetes that were unselected for the risk of hypoglycemia. In clinical practice, insulin degludec is already considered for patients who reported nocturnal hypoglycemia while on insulin glargine U100, but it’s great this study provides the scientific evidence,” said Dr. de Galan in an interview.
“The lower rate of nocturnal hypoglycemia with degludec, compared with glargine U100 is well established. Inpatient assessment of hypoglycemia with measurement of hourly plasma glucose allowed HypoDeg to provide stronger evidence than prior studies. The benefit of delgudec versus glargine U100 was significant and clinically meaningful, in hypo-prone patients who would benefit the most” by using insulin degludec, commented Gian Paolo Fadini, MD, an endocrinologist at the University of Padova (Italy), and a lead investigator on the ReFLeCT study.
But insulin degludec is not a completely silver bullet. Its prolonged duration of action and stability that may in part explain why it limits hypoglycemia events can also be a drawback: “It probably offers fewer options for flexibility. Any change in dose takes at least a day or 2 to settle, which may be unfavorable in certain circumstances,” noted Dr. de Galan.
“I wouldn’t recommend insulin degludec for all patients with type 1 diabetes. It’s an individual evaluation in each patient,” said Dr. Brøsen. “We will be looking into whether some patients are better off on insulin glargine.”
Cost makes a difference
Another, potentially more consequential flaw is insulin degludec’s relative expense.
“To date, use of degludec in routine practice has been limited by its cost, compared with older basal insulins,” observed Dr. Fadini in an interview. “In several countries, including the United States, degludec is substantially more expensive than glargine.”
The ADA’s Standards of Medical Care in Diabetes–2021 includes table 9.3 that lists the costs of various insulins and shows the median average wholesale price of insulin glargine U100 follow-on products as $190/vial, compared with a $407 price for a similar vial of insulin degludec.
Insulin degludec “is clearly superior from a hypoglycemia standpoint. Patients with type 1 diabetes like the reduction because hypoglycemia is scary, and dangerous. The main issue is cost, and the extent to which it may be covered by insurance,” commented Lisa Chow, MD, an endocrinologist at the University of Minnesota, Minneapolis. “We generally won’t prescribe degludec unless it is at a price affordable to the patient. We try to use patient assistance programs sponsored by the company [that markets insulin degludec: Novo Nordisk] to try to make it more affordable.”
Dr. Chow also highlighted that a new wrinkle has been introduction of a more concentrated formulation of insulin glargine, U300, which appears to cause less hypoglycemia than insulin glargine U100. Recent study results indicated that no significant difference exists in the incidence of hypoglycemia among patients treated with insulin glargine U300 and those treated with insulin degludec, such as findings from the BRIGHT trial, which included just over 900 patients, and in the CONCLUDE trial, which randomized more than 1,600 patients.
The HypoDeg study was sponsored by Novo Nordisk, the company that markets insulin degludec. Dr. Brøsen had no personal disclosures, but several of her coauthors were either Novo Nordisk employees or had financial relationships with the company. Dr. de Galan has received research funding from Novo Nordisk. Dr. Fadini has received lecture fees and research funding from Novo Nordisk, from Sanofi, the company that markets insulin glargine, and from several other companies. Dr. Chow has received research funding from Dexcom.
Patients with type 1 diabetes who used insulin degludec as their basal insulin had fewer than half the number of nocturnal hypoglycemia events, compared with patients who used insulin glargine U100, in a head-to-head crossover study with 51 patients who had a history of nighttime hypoglycemia episodes.
Patients with type 1 diabetes who are “struggling with nocturnal hypoglycemia would benefit from insulin degludec treatment,” said Julie M. Brøsen, MD, at the annual scientific sessions of the American Diabetes Association.
Accumulating evidence for less hypoglycemia with insulin degludec
Results from several studies comparing insulin degludec (Tresiba), a second-generation, longer-acting insulin with more stable steady-state performance, with the first-generation basal insulin analogue glargine (Lantus), have built the case that degludec produces fewer hypoglycemia events.
The landmark SWITCH 1 crossover study published in 2017 showed in about 500 patients with type 1 diabetes and a risk factor for hypoglycemia that treatment with insulin degludec led to significantly few total hypoglycemia episodes and significantly fewer nocturnal episodes, compared with insulin glargine.
Next came similar findings from ReFLeCT, a multicenter observational study that followed 556 unselected patients with type 1 diabetes in routine practice settings who switched to insulin degludec following treatment with a different basal insulin. The results again showed a significant drop-off in total, nonsevere, severe, and nocturnal hypoglycemia events.
Homing in on higher-risk patients
The current study, HypoDeg (Insulin Degludec and Symptomatic Nocturnal Hypoglycaemia), ran at 10 Danish centers and enrolled 149 adults with type 1 diabetes who had at least one episode of severe nocturnal hypoglycemia within the prior 2 years, focusing on patients most at risk for future nocturnal hypoglycemia events. In an unusual study design, researchers identified nocturnal hypoglycemic episodes with hourly venous blood samples drawn from a subcutaneous line.
They randomized the patients to basal insulin treatment with either insulin degludec or to insulin glargine U100, allowed their treatment to stabilize for 3 months, and then tallied nocturnal hypoglycemia events for 9 months. They then crossed patients to the alternative basal insulin and repeated the process.
Results from the full study have not yet appeared in published form but were in a pair of reports at the 2020 scientific sessions of the ADA.
One report included findings based on 136 episodes of severe hypoglycemia identified clinically and showed these events occurred 35% less often during treatment with insulin degludec, a significant difference. The overall finding was primarily driven by 48% fewer episodes of severe nocturnal hypoglycemia, but this difference was not significant.
The second report identified hypoglycemia events with continuous glucose monitoring in 74 of the study participants, which identified 193 episodes of nonsevere nocturnal hypoglycemia and found that treatment with insulin degludec cut the rate by 47%, primarily by reducing asymptomatic episodes.
Hourly blood draws track overnight hypoglycemia
The current study included 51 of the 149 HypoDeg patients who agreed to undergo overnight blood sampling and had this done at least once while treated with each of the two study insulins. (The study design called for two blood sampling nights for each willing patient during each of the two treatment periods.) The 51 patients had type 1 diabetes for an average of 28 years and an average age of 58 years. Two-thirds were men, their baseline A1c was 7.8%, and on average had 2.6 episodes of severe nocturnal hypoglycemia during the prior 2 years.
The researchers drew hourly blood specimens on a total of 196 nights from the 51 participating patients and identified 57 nights when blood glucose levels reached hypoglycemia thresholds in 33 patients. One-third of the events occurred when patients were on insulin degludec treatment, and two-thirds when they were on insulin glargine, reported Dr. Brøsen.
She presented three separate analyses of the data. One analysis focused on level 1 hypoglycemia events, when blood glucose dips to 70 mg/dL or less, which occurred 54% less often when patients were on insulin degludec. A second analysis looked at level 2 events, when blood glucose falls below 54 mg/dL, and treatment with insulin degludec cut this by 64% compared with insulin glargine. The third analysis focused on symptomatic events when blood glucose was 70 mg/dL or less, and treatment with insulin degludec linked with a 62% cut in this metric. All three between-group differences were significant.
Evidence supports already-changed practice
This new evidence “supports recommending” insulin degludec over insulin glargine, commented Bastiaan E. de Galan, MD, PhD, an endocrinologist and professor at Maastrict (the Netherlands) University Medical Center. The new results “extend those from previous trials in populations with type 1 diabetes that were unselected for the risk of hypoglycemia. In clinical practice, insulin degludec is already considered for patients who reported nocturnal hypoglycemia while on insulin glargine U100, but it’s great this study provides the scientific evidence,” said Dr. de Galan in an interview.
“The lower rate of nocturnal hypoglycemia with degludec, compared with glargine U100 is well established. Inpatient assessment of hypoglycemia with measurement of hourly plasma glucose allowed HypoDeg to provide stronger evidence than prior studies. The benefit of delgudec versus glargine U100 was significant and clinically meaningful, in hypo-prone patients who would benefit the most” by using insulin degludec, commented Gian Paolo Fadini, MD, an endocrinologist at the University of Padova (Italy), and a lead investigator on the ReFLeCT study.
But insulin degludec is not a completely silver bullet. Its prolonged duration of action and stability that may in part explain why it limits hypoglycemia events can also be a drawback: “It probably offers fewer options for flexibility. Any change in dose takes at least a day or 2 to settle, which may be unfavorable in certain circumstances,” noted Dr. de Galan.
“I wouldn’t recommend insulin degludec for all patients with type 1 diabetes. It’s an individual evaluation in each patient,” said Dr. Brøsen. “We will be looking into whether some patients are better off on insulin glargine.”
Cost makes a difference
Another, potentially more consequential flaw is insulin degludec’s relative expense.
“To date, use of degludec in routine practice has been limited by its cost, compared with older basal insulins,” observed Dr. Fadini in an interview. “In several countries, including the United States, degludec is substantially more expensive than glargine.”
The ADA’s Standards of Medical Care in Diabetes–2021 includes table 9.3 that lists the costs of various insulins and shows the median average wholesale price of insulin glargine U100 follow-on products as $190/vial, compared with a $407 price for a similar vial of insulin degludec.
Insulin degludec “is clearly superior from a hypoglycemia standpoint. Patients with type 1 diabetes like the reduction because hypoglycemia is scary, and dangerous. The main issue is cost, and the extent to which it may be covered by insurance,” commented Lisa Chow, MD, an endocrinologist at the University of Minnesota, Minneapolis. “We generally won’t prescribe degludec unless it is at a price affordable to the patient. We try to use patient assistance programs sponsored by the company [that markets insulin degludec: Novo Nordisk] to try to make it more affordable.”
Dr. Chow also highlighted that a new wrinkle has been introduction of a more concentrated formulation of insulin glargine, U300, which appears to cause less hypoglycemia than insulin glargine U100. Recent study results indicated that no significant difference exists in the incidence of hypoglycemia among patients treated with insulin glargine U300 and those treated with insulin degludec, such as findings from the BRIGHT trial, which included just over 900 patients, and in the CONCLUDE trial, which randomized more than 1,600 patients.
The HypoDeg study was sponsored by Novo Nordisk, the company that markets insulin degludec. Dr. Brøsen had no personal disclosures, but several of her coauthors were either Novo Nordisk employees or had financial relationships with the company. Dr. de Galan has received research funding from Novo Nordisk. Dr. Fadini has received lecture fees and research funding from Novo Nordisk, from Sanofi, the company that markets insulin glargine, and from several other companies. Dr. Chow has received research funding from Dexcom.
Patients with type 1 diabetes who used insulin degludec as their basal insulin had fewer than half the number of nocturnal hypoglycemia events, compared with patients who used insulin glargine U100, in a head-to-head crossover study with 51 patients who had a history of nighttime hypoglycemia episodes.
Patients with type 1 diabetes who are “struggling with nocturnal hypoglycemia would benefit from insulin degludec treatment,” said Julie M. Brøsen, MD, at the annual scientific sessions of the American Diabetes Association.
Accumulating evidence for less hypoglycemia with insulin degludec
Results from several studies comparing insulin degludec (Tresiba), a second-generation, longer-acting insulin with more stable steady-state performance, with the first-generation basal insulin analogue glargine (Lantus), have built the case that degludec produces fewer hypoglycemia events.
The landmark SWITCH 1 crossover study published in 2017 showed in about 500 patients with type 1 diabetes and a risk factor for hypoglycemia that treatment with insulin degludec led to significantly few total hypoglycemia episodes and significantly fewer nocturnal episodes, compared with insulin glargine.
Next came similar findings from ReFLeCT, a multicenter observational study that followed 556 unselected patients with type 1 diabetes in routine practice settings who switched to insulin degludec following treatment with a different basal insulin. The results again showed a significant drop-off in total, nonsevere, severe, and nocturnal hypoglycemia events.
Homing in on higher-risk patients
The current study, HypoDeg (Insulin Degludec and Symptomatic Nocturnal Hypoglycaemia), ran at 10 Danish centers and enrolled 149 adults with type 1 diabetes who had at least one episode of severe nocturnal hypoglycemia within the prior 2 years, focusing on patients most at risk for future nocturnal hypoglycemia events. In an unusual study design, researchers identified nocturnal hypoglycemic episodes with hourly venous blood samples drawn from a subcutaneous line.
They randomized the patients to basal insulin treatment with either insulin degludec or to insulin glargine U100, allowed their treatment to stabilize for 3 months, and then tallied nocturnal hypoglycemia events for 9 months. They then crossed patients to the alternative basal insulin and repeated the process.
Results from the full study have not yet appeared in published form but were in a pair of reports at the 2020 scientific sessions of the ADA.
One report included findings based on 136 episodes of severe hypoglycemia identified clinically and showed these events occurred 35% less often during treatment with insulin degludec, a significant difference. The overall finding was primarily driven by 48% fewer episodes of severe nocturnal hypoglycemia, but this difference was not significant.
The second report identified hypoglycemia events with continuous glucose monitoring in 74 of the study participants, which identified 193 episodes of nonsevere nocturnal hypoglycemia and found that treatment with insulin degludec cut the rate by 47%, primarily by reducing asymptomatic episodes.
Hourly blood draws track overnight hypoglycemia
The current study included 51 of the 149 HypoDeg patients who agreed to undergo overnight blood sampling and had this done at least once while treated with each of the two study insulins. (The study design called for two blood sampling nights for each willing patient during each of the two treatment periods.) The 51 patients had type 1 diabetes for an average of 28 years and an average age of 58 years. Two-thirds were men, their baseline A1c was 7.8%, and on average had 2.6 episodes of severe nocturnal hypoglycemia during the prior 2 years.
The researchers drew hourly blood specimens on a total of 196 nights from the 51 participating patients and identified 57 nights when blood glucose levels reached hypoglycemia thresholds in 33 patients. One-third of the events occurred when patients were on insulin degludec treatment, and two-thirds when they were on insulin glargine, reported Dr. Brøsen.
She presented three separate analyses of the data. One analysis focused on level 1 hypoglycemia events, when blood glucose dips to 70 mg/dL or less, which occurred 54% less often when patients were on insulin degludec. A second analysis looked at level 2 events, when blood glucose falls below 54 mg/dL, and treatment with insulin degludec cut this by 64% compared with insulin glargine. The third analysis focused on symptomatic events when blood glucose was 70 mg/dL or less, and treatment with insulin degludec linked with a 62% cut in this metric. All three between-group differences were significant.
Evidence supports already-changed practice
This new evidence “supports recommending” insulin degludec over insulin glargine, commented Bastiaan E. de Galan, MD, PhD, an endocrinologist and professor at Maastrict (the Netherlands) University Medical Center. The new results “extend those from previous trials in populations with type 1 diabetes that were unselected for the risk of hypoglycemia. In clinical practice, insulin degludec is already considered for patients who reported nocturnal hypoglycemia while on insulin glargine U100, but it’s great this study provides the scientific evidence,” said Dr. de Galan in an interview.
“The lower rate of nocturnal hypoglycemia with degludec, compared with glargine U100 is well established. Inpatient assessment of hypoglycemia with measurement of hourly plasma glucose allowed HypoDeg to provide stronger evidence than prior studies. The benefit of delgudec versus glargine U100 was significant and clinically meaningful, in hypo-prone patients who would benefit the most” by using insulin degludec, commented Gian Paolo Fadini, MD, an endocrinologist at the University of Padova (Italy), and a lead investigator on the ReFLeCT study.
But insulin degludec is not a completely silver bullet. Its prolonged duration of action and stability that may in part explain why it limits hypoglycemia events can also be a drawback: “It probably offers fewer options for flexibility. Any change in dose takes at least a day or 2 to settle, which may be unfavorable in certain circumstances,” noted Dr. de Galan.
“I wouldn’t recommend insulin degludec for all patients with type 1 diabetes. It’s an individual evaluation in each patient,” said Dr. Brøsen. “We will be looking into whether some patients are better off on insulin glargine.”
Cost makes a difference
Another, potentially more consequential flaw is insulin degludec’s relative expense.
“To date, use of degludec in routine practice has been limited by its cost, compared with older basal insulins,” observed Dr. Fadini in an interview. “In several countries, including the United States, degludec is substantially more expensive than glargine.”
The ADA’s Standards of Medical Care in Diabetes–2021 includes table 9.3 that lists the costs of various insulins and shows the median average wholesale price of insulin glargine U100 follow-on products as $190/vial, compared with a $407 price for a similar vial of insulin degludec.
Insulin degludec “is clearly superior from a hypoglycemia standpoint. Patients with type 1 diabetes like the reduction because hypoglycemia is scary, and dangerous. The main issue is cost, and the extent to which it may be covered by insurance,” commented Lisa Chow, MD, an endocrinologist at the University of Minnesota, Minneapolis. “We generally won’t prescribe degludec unless it is at a price affordable to the patient. We try to use patient assistance programs sponsored by the company [that markets insulin degludec: Novo Nordisk] to try to make it more affordable.”
Dr. Chow also highlighted that a new wrinkle has been introduction of a more concentrated formulation of insulin glargine, U300, which appears to cause less hypoglycemia than insulin glargine U100. Recent study results indicated that no significant difference exists in the incidence of hypoglycemia among patients treated with insulin glargine U300 and those treated with insulin degludec, such as findings from the BRIGHT trial, which included just over 900 patients, and in the CONCLUDE trial, which randomized more than 1,600 patients.
The HypoDeg study was sponsored by Novo Nordisk, the company that markets insulin degludec. Dr. Brøsen had no personal disclosures, but several of her coauthors were either Novo Nordisk employees or had financial relationships with the company. Dr. de Galan has received research funding from Novo Nordisk. Dr. Fadini has received lecture fees and research funding from Novo Nordisk, from Sanofi, the company that markets insulin glargine, and from several other companies. Dr. Chow has received research funding from Dexcom.
FROM ADA 2021
‘Stunning’ twincretin beats semaglutide for A1c, weight reduction in T2D
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
FROM ADA 2021
FDA approves OTC antihistamine nasal spray
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Promising HER2+/HR– breast cancer survival with de-escalated therapy
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
It may not be always necessary to approach the treatment of HER2-positive, hormone receptor–negative (HER2+/HR–) early breast cancer with added chemotherapy, survival results of a prospective multicenter randomized trial suggest.
In the ADAPT-HER2+/HR– trial, comparing a de-escalated 12-week neoadjuvant regimen consisting of dual HER2 blockade with trastuzumab (Herceptin) and pertuzumab (Perjeta) with or without weekly paclitaxel, the three-drug regimen was associated with high pathologic complete response(pCR) rates and excellent 5-year survival, irrespective of whether patients received additional chemotherapy, reported Nadia Harbeck, MD, PhD, of the University of Munich.
“Chemotherapy-free regimens are promising in highly sensitive tumors with early response, but future investigation of such chemotherapy-free regimens need to be focused on selected patients, like those with HER2 3+ tumors, non–basal-like tumors, those showing early response to the de-escalated therapy, and those with predictive RNA signatures such as immune signatures,” she said in an oral abstract session during the American Society of Clinical Oncology annual meeting (Abstract 503).
Under the WGS umbrella
The ADAPT HER2+/HR– trial (NCT01779206) is one of several conducted by the West German Study Group (WGS) on therapy for intrinsic breast cancer types.
In this study, 134 patients with HER2-positive, estrogen and progesterone receptor–negative tumors with no metastatic disease and good performance status were assigned on a 5:2 basis to neoadjuvant therapy with trastuzumab at a loading dose of 8 mg/kg for the first cycle followed by 6 mg/kg for subsequent cycles every 3 weeks x 4, plus pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks x 4 (92 patients), or to trastuzumab and pertuzumab at the same dose and schedule plus paclitaxel 80 mg/m2 once weekly for 12 weeks.
Patients had surgery within 3 weeks of the end of study therapy unless they did not have a histologically confirmed pCR, in which case they went on to receive standard neoadjuvant therapy prior to surgery.
Adjuvant therapy was performed according to national guidelines, although patients with a pCR after 12 weeks of study therapy could be spared from adjuvant chemotherapy at the investigator’s discretion.
Patients underwent biopsy at 3 weeks for therapy for early response assessment, defined as either a Ki67 decrease of at least 30% from baseline, or low cellularity (less than 500 invasive tumor cells).
First survival results
The investigators previously reported the primary pCR endpoint from the trial, which showed a rate of 90% after 12 weeks in the three-drug arm, and a “substantial and clinically meaningful” pCR rate of 34% after the trastuzumab plus pertuzumab alone.
At ASCO 2021, Dr. Harbeck reported the first survival data from the trial.
After a median follow-up of 59.9 months, there were no statistically significant differences between trial arms in either overall survival, invasive disease-free survival (iDFS), or distant disease-free survival (dDFS).
The 5-year iDFS rate in the three-drug arm was 98%, compared with 87% for the dual HER2 blockade-only arm, a difference that was not statistically significant.
The 5-year dDFS rates were 98% and 92% respectively. There were only seven dDFS events during follow-up, Dr. Harbeck noted.
There were only six deaths during follow-up, with overall survival rates of 98% in the paclitaxel-containing arm, and 94% in the anti-HER2 antibodies–only arm, a difference of one overall survival event, Dr. Harbeck said.
pCR counts
However, patients who did not have pathologic complete responses at the end of first-line de-escalated therapy had worse outcomes, with a 5-year iDFS rate of 82%, compared with 98% for patients who had achieved a pCR. This translated into a hazard ratio for invasive disease in patients with pCRs of 0.14 (P = .011).
This difference occurred despite the study requirement that all patients who did not have pCR after 12 weeks of initial therapy would receive additional chemotherapy.
Looking at the tumor subtype among patients in the paclitaxel-free arm to see whether they could identify predictors of early response, the researchers found a pCR rate of 36.5% among 85 patients with nonbasal tumors, but 0% among 7 patients with basal tumors.
The investigators identified a population of patients whose tumors could be considered nonsensitive to dual HER2 blockade alone: Those with basal tumors, those tumors with low immunohistochemical HER2 expression, and those without an early response to therapy on biopsy 3 weeks into initial therapy. Among 31 of the 92 patients in the dual HER2 arm who met this description, 2 had pCRs, Dr. Harbeck noted.
The 5-year iDFS rate among patients in the dual blockade–only arm with nonsensitive tumors was 79%, compared with 93% for patients with treatment-responsive types, although there were only 13 invasive events total in this arm.
“If we look at the whole trial population, the negative prognostic impact of what we termed nonsensitive tumors was even significant regarding dDFS, with a hazard ratio of about 5,” she said.
‘A consistent theme’
Invited discussant Lisa A. Carey, MD, ScM, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, noted that the trial was underpowered for outcomes, but that results nonetheless suggest that patients with strongly HER2-driven tumors might get comparable benefits from less chemotherapy.
“This trial included only hormone receptor–negative, HER2-positive tumors, and these we know are likely to be HER2-enriched in terms of subtype, about three-quarters of them,”she said.
The previously reported pCR rate of 90% in the paclitaxel-containing arm, with 80% of patients requiring no further chemotherapy, resulted in the excellent 5-year iDFS and dDFS in this group, despite the relatively highly clinical stage, with about 60% of patients having clinical stage 2 or higher tumors, and more than 40% being node positive.
The idea that pCR itself can predict which patients could be spared from more intensive chemotherapy “is starting to look like a consistent theme,” she said.
Dr. Carey pointed out that in the KRISTINE trial comparing the combination of trastuzumab emtansine (T-DM1) and pertuzumab with standard chemotherapy in patients with HER2-positive stage I-III breast cancer, although the experimental combination was associated with lower pCR rates and worse event-free survival, rates of iDFS/dDFS were virtually identical for patients in both arms who achieved a pCR.
“So the question is can pCR mean that we can either eliminate additional therapy,” she said, noting that the question is currently being addressed prospectively in two clinical trials, COMPASS-pCR and DECRESCENDO.
ADAPT HER2+/HR- is sponsored by F. Hoffman-La Roche. Dr. Harbeck disclosed institutional research funding from Roche/Genentech, as well as honoraria and consulting/advising for multiple companies. Dr. Carey disclosed institutional research funding and other relationships with various companies.
FROM ASCO 2021