User login
Cardiac issues twice as likely with COVID plus high troponin
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Hospitalized COVID-19 patients with high troponin levels are twice as likely to have cardiac abnormalities than those with normal troponin, with or without COVID-19, a multicenter U.K. study suggests.
The causes were diverse, myocarditis prevalence was lower than previously reported, and myocardial scar emerged as an independent risk factor for adverse cardiovascular outcomes at 12 months.
“We know that multiorgan involvement in hospitalized patients with COVID-19 is common ... and may result in acute myocardial injury, detected by an increase in cardiac troponin concentrations,” John P. Greenwood, PhD, of the University of Leeds (England), told this news organization. “Elevated cardiac troponin is associated with a worse prognosis.”
“Multiple mechanisms of myocardial injury have been proposed and ... mitigation or prevention strategies likely depend on the underpinning mechanisms,” he said. “The sequelae of scar may predispose to late events.”
The study, published online in Circulation, also identified a new pattern of microinfarction on cardiac magnetic resonance (CMR) imaging, highlighting the pro-thrombotic nature of SARS-CoV-2, Dr. Greenwood said.
Injury patterns different
Three hundred and forty-two patients with COVID-19 and elevated troponin levels (COVID+/troponin+) across 25 centers were enrolled between June 2020 and March 2021 in COVID-HEART, deemed an “urgent public health study” in the United Kingdom. The aim was to characterize myocardial injury and its associations and sequelae in convalescent patients after hospitalization with COVID-19.
Enrollment took place during the Wuhan and Alpha waves of COVID-19: before vaccination and when dexamethasone and anticoagulant protocols were emerging. All participants underwent CMR at a median of 21 days after discharge.
Two prospective control groups also were recruited: 64 patients with COVID-19 and normal troponin levels (COVID+/troponin−) and 113 without COVID-19 or elevated troponin matched by age and cardiovascular comorbidities (COVID−/comorbidity+).
Overall, participants’ median age was 61 years and 69% were men. Common comorbidities included hypertension (47%), obesity (43%), and diabetes (25%).
The frequency of any heart abnormality – for example, left or right ventricular impairment, scar, or pericardial disease – was twice as great (61%) in COVID+/troponin+ cases, compared with controls (36% for COVID+/troponin− patients versus 31% for COVID−/comorbidity+ patients).
Specifically, more cases than controls had ventricular impairment (17.2% vs. 3.1% and 7.1%) or scar (42% vs. 7% and 23%).
The myocardial injury pattern differed between cases and controls, with cases more likely to have infarction (13% vs. 2% and 7%) or microinfarction (9% vs. 0% and 1%).
However, there was no between-group difference in nonischemic scar (13% vs. 5% and 14%).
The prevalence of probable recent myocarditis was 6.7% in cases, compared with 1.7% in controls without COVID-19 – “much lower” than in previous studies, Dr. Greenwood noted.
During follow-up, four COVID+/troponin+ patients (1.2%) died, and 34 (10%) experienced a subsequent major adverse cardiovascular event (MACE; 10.2%), which was similar to controls (6.1%).
Myocardial scar, but not previous COVID-19 infection or troponin level, was an independent predictor of MACE (odds ratio, 2.25).
“These findings suggest that macroangiopathic and microangiopathic thrombosis may be the key pathologic process for myocardial injury in COVID-19 survivors,” the authors conclude.
Dr. Greenwood added, “We are currently analyzing the 6-month follow-up CMR scans, the quality-of-life questionnaires, and the 6-minute walk tests. These will give us great understanding of how the heart repairs after acute myocardial injury associated with COVID-19. It will also allow us to assess the impact on patient quality of life and functional capacity.”
‘Tour de force’
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and a professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said, “This is a tour de force collaboration – obtaining this many MRIs across multiple centers in the pandemic is quite remarkable. The study highlights the multiple different processes that lead to cardiac injury in COVID patients, complements autopsy studies and prior smaller MRI studies, [and] also provides the best data on the rate of myocarditis to date among the subset of COVID patients with cardiac injury.”
Overall, he said, the findings “do support closer follow-up for patients who had COVID and elevated troponins. We need to see follow-up MRI results in this cohort, as well as longer term outcomes. We also need studies on newer, more benign variants that are likely to have lower rates of cardiac injury and even fewer MRI abnormalities.”
Matthias Stuber, PhD, and Aaron L. Baggish, MD, both of Lausanne University Hospital and University of Lausanne, Switzerland, noted in a related editorial, “We are also reminded that the clinical severity of COVID-19 is most often dictated by the presence of pre-existing comorbidity, with antecedent ischemic scar now added to the long list of bad actors. Although not the primary focus of the COVID-HEART study, the question of whether cardiac troponin levels should be checked routinely and universally during the index admission for COVID-19 remains unresolved,” they noted.
“In general, we are most effective as clinicians when we use tests to confirm or rule out the specific disease processes suspected by careful basic clinical assessment rather than in a shotgun manner among undifferentiated all-comers,” they conclude.
No commercial funding or relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Must-read acute care medicine articles from 2022
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Guidance for PCI without on-site surgical backup updated
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
FROM THE JOURNAL OF SOCIETY FOR CARDIOVASCULAR ANGIOGRAPHY AND INTERVENTIONS
Acute cardiac events common during COVID hospitalization
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
What is the psychological cost of performing CPR?
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
Long QT syndrome overdiagnosis persists
Five factors underlie the ongoing overdiagnosis and misdiagnosis of long QT syndrome (LQTS), including temporary QT prolongation following vasovagal syncope, a “pseudo”-positive genetic test result, family history of sudden cardiac death, transient QT prolongation, and misinterpretation of the QTc interval, a new study suggests.
Awareness of these characteristics, which led to a diagnostic reversal in 290 of 1,841 (16%) patients, could reduce the burden of overdiagnosis on the health care system and on patients and families, senior author Michael J. Ackerman, MD, PhD, of Mayo Clinic, Rochester, Minn., and colleagues conclude.
“The findings are a disturbing and disappointing sequel to the paper we published about LQTS overdiagnosis back in 2007, which showed that 2 out of every 5 patients who came to Mayo Clinic for a second opinion left without the diagnosis,” Dr. Ackerman told this news organization.
To date, Dr. Ackerman has reversed the diagnosis for 350 patients, he said.
The consequences of an LQTS diagnosis are “profound,” he noted, including years of unnecessary drug therapy, implantation of a cardioverter defibrillator, disqualification from competitive sports, and emotional stress to the individual and family.
By pointing out the five biggest mistakes his team has seen, he said, “we hope to equip the diagnostician with the means to challenge and assess the veracity of a LQTS diagnosis.”
The study was published online in the Journal of the American College of Cardiology.
Time to do better
Dr. Ackerman and colleagues analyzed electronic medical records on 290 of 1,841 (16%) patients who presented with an outside diagnosis of LQTS but subsequently were dismissed as having normal findings. The mean age of these patients at their first Mayo Clinic evaluation was 22, 60% were female, and the mean QTc interval was 427 ±25 milliseconds.
Overall, 38% of misdiagnoses were the result of misinterpretation of clinical factors; 29%, to diagnostic test misinterpretations; 17%, to an apparently positive genetic test in the context of a weak or absent phenotype; and 16%, to a family history of false LQTS or of sudden cardiac or sudden unexplained death.
More specifically, the most common cause of an LQTS misdiagnosis was QT prolongation following vasovagal syncope, which was misinterpreted as LQTS-attributed syncope.
The second most common cause was an apparently positive genetic test for an LQTS gene that turned out to be a benign or likely benign variant.
The third most common cause was an LQTS diagnosis based solely on a family history of sudden unexplained death (26 patients), QT prolongation (11 patients), or sudden cardiac arrest (9 patients).
The fourth most common cause was an isolated event of QT prolongation (44 patients). The transient QT prolongation was observed under myriad conditions unrelated to LQTS. Yet, 31 patients received a diagnosis based solely on the event.
The fifth most common cause was inclusion of the U-wave in the calculation of the QTc interval (40 patients), leading to an inaccurate interpretation of the electrocardiogram.
Dr. Ackerman noted that these LQTS diagnoses were given by heart-rhythm specialists, and most patients self-referred for a second opinion because a family member questioned the diagnosis after doing their own research.
“It’s time that we step up to the plate and do better,” Dr. Ackerman said. The team’s evaluation of the impact of the misdiagnosis on the patients’ lifestyle and quality of life showed that 45% had been restricted from competitive sports (and subsequently resumed sports activity with no adverse events); 80% had been started on beta-blockers (the drugs were discontinued in 84% as a result of the Mayo Clinic evaluation, whereas 16% opted to continue); and 10 of 22 patients (45%) who received an implanted cardioverter device underwent an extraction of the device without complications.
The authors conclude: “Although missing a patient who truly has LQTS can lead to a tragic outcome, the implications of overdiagnosed LQTS are not trivial and are potentially tragic as well.”
‘Tricky diagnosis’
LQTS specialist Peter Aziz, MD, director of pediatric electrophysiology at the Cleveland Clinic, agreed with these findings.
“Most of us ‘channelopathists’ who see LQTS for a living have a good grasp of the disease, but it can be elusive for others,” he said in an interview. “This is a tricky diagnosis. There are ends of the spectrum where people for sure don’t have it and people for sure do. Most clinicians are able to identify that.”
However, he added, “A lot of patients fall into that gray area where it may not be clear at first, even to an expert. But the expert knows how to do a comprehensive evaluation, examining episodes and symptoms and understanding whether they are relevant to LQTS or completely red herrings, and feeling confident about how they calculate the acute interval on an electrocardiogram.”
“All of these may seem mundane, but without the experience, clinicians are vulnerable to miscalculations,” he said. “That’s why our bias, as channelopathists, is that every patient who has a suspected diagnosis or is being treated for LQTS really should see an expert.”
Similarly, Arthur A.M. Wilde, MD, PhD, of the University of Amsterdam, and Peter J. Schwartz, MD, of IRCCS Istituto Auxologico Italiano, Milan, write in a related editorial that it “has to be kept in mind that both diagnostic scores and risk scores are dynamic and can be modified by time and by appropriate therapy.
“Therefore, to make hasty diagnosis of a disease that requires life-long treatment is inappropriate, especially when this is done without the support of adequate, specific experience.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Five factors underlie the ongoing overdiagnosis and misdiagnosis of long QT syndrome (LQTS), including temporary QT prolongation following vasovagal syncope, a “pseudo”-positive genetic test result, family history of sudden cardiac death, transient QT prolongation, and misinterpretation of the QTc interval, a new study suggests.
Awareness of these characteristics, which led to a diagnostic reversal in 290 of 1,841 (16%) patients, could reduce the burden of overdiagnosis on the health care system and on patients and families, senior author Michael J. Ackerman, MD, PhD, of Mayo Clinic, Rochester, Minn., and colleagues conclude.
“The findings are a disturbing and disappointing sequel to the paper we published about LQTS overdiagnosis back in 2007, which showed that 2 out of every 5 patients who came to Mayo Clinic for a second opinion left without the diagnosis,” Dr. Ackerman told this news organization.
To date, Dr. Ackerman has reversed the diagnosis for 350 patients, he said.
The consequences of an LQTS diagnosis are “profound,” he noted, including years of unnecessary drug therapy, implantation of a cardioverter defibrillator, disqualification from competitive sports, and emotional stress to the individual and family.
By pointing out the five biggest mistakes his team has seen, he said, “we hope to equip the diagnostician with the means to challenge and assess the veracity of a LQTS diagnosis.”
The study was published online in the Journal of the American College of Cardiology.
Time to do better
Dr. Ackerman and colleagues analyzed electronic medical records on 290 of 1,841 (16%) patients who presented with an outside diagnosis of LQTS but subsequently were dismissed as having normal findings. The mean age of these patients at their first Mayo Clinic evaluation was 22, 60% were female, and the mean QTc interval was 427 ±25 milliseconds.
Overall, 38% of misdiagnoses were the result of misinterpretation of clinical factors; 29%, to diagnostic test misinterpretations; 17%, to an apparently positive genetic test in the context of a weak or absent phenotype; and 16%, to a family history of false LQTS or of sudden cardiac or sudden unexplained death.
More specifically, the most common cause of an LQTS misdiagnosis was QT prolongation following vasovagal syncope, which was misinterpreted as LQTS-attributed syncope.
The second most common cause was an apparently positive genetic test for an LQTS gene that turned out to be a benign or likely benign variant.
The third most common cause was an LQTS diagnosis based solely on a family history of sudden unexplained death (26 patients), QT prolongation (11 patients), or sudden cardiac arrest (9 patients).
The fourth most common cause was an isolated event of QT prolongation (44 patients). The transient QT prolongation was observed under myriad conditions unrelated to LQTS. Yet, 31 patients received a diagnosis based solely on the event.
The fifth most common cause was inclusion of the U-wave in the calculation of the QTc interval (40 patients), leading to an inaccurate interpretation of the electrocardiogram.
Dr. Ackerman noted that these LQTS diagnoses were given by heart-rhythm specialists, and most patients self-referred for a second opinion because a family member questioned the diagnosis after doing their own research.
“It’s time that we step up to the plate and do better,” Dr. Ackerman said. The team’s evaluation of the impact of the misdiagnosis on the patients’ lifestyle and quality of life showed that 45% had been restricted from competitive sports (and subsequently resumed sports activity with no adverse events); 80% had been started on beta-blockers (the drugs were discontinued in 84% as a result of the Mayo Clinic evaluation, whereas 16% opted to continue); and 10 of 22 patients (45%) who received an implanted cardioverter device underwent an extraction of the device without complications.
The authors conclude: “Although missing a patient who truly has LQTS can lead to a tragic outcome, the implications of overdiagnosed LQTS are not trivial and are potentially tragic as well.”
‘Tricky diagnosis’
LQTS specialist Peter Aziz, MD, director of pediatric electrophysiology at the Cleveland Clinic, agreed with these findings.
“Most of us ‘channelopathists’ who see LQTS for a living have a good grasp of the disease, but it can be elusive for others,” he said in an interview. “This is a tricky diagnosis. There are ends of the spectrum where people for sure don’t have it and people for sure do. Most clinicians are able to identify that.”
However, he added, “A lot of patients fall into that gray area where it may not be clear at first, even to an expert. But the expert knows how to do a comprehensive evaluation, examining episodes and symptoms and understanding whether they are relevant to LQTS or completely red herrings, and feeling confident about how they calculate the acute interval on an electrocardiogram.”
“All of these may seem mundane, but without the experience, clinicians are vulnerable to miscalculations,” he said. “That’s why our bias, as channelopathists, is that every patient who has a suspected diagnosis or is being treated for LQTS really should see an expert.”
Similarly, Arthur A.M. Wilde, MD, PhD, of the University of Amsterdam, and Peter J. Schwartz, MD, of IRCCS Istituto Auxologico Italiano, Milan, write in a related editorial that it “has to be kept in mind that both diagnostic scores and risk scores are dynamic and can be modified by time and by appropriate therapy.
“Therefore, to make hasty diagnosis of a disease that requires life-long treatment is inappropriate, especially when this is done without the support of adequate, specific experience.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Five factors underlie the ongoing overdiagnosis and misdiagnosis of long QT syndrome (LQTS), including temporary QT prolongation following vasovagal syncope, a “pseudo”-positive genetic test result, family history of sudden cardiac death, transient QT prolongation, and misinterpretation of the QTc interval, a new study suggests.
Awareness of these characteristics, which led to a diagnostic reversal in 290 of 1,841 (16%) patients, could reduce the burden of overdiagnosis on the health care system and on patients and families, senior author Michael J. Ackerman, MD, PhD, of Mayo Clinic, Rochester, Minn., and colleagues conclude.
“The findings are a disturbing and disappointing sequel to the paper we published about LQTS overdiagnosis back in 2007, which showed that 2 out of every 5 patients who came to Mayo Clinic for a second opinion left without the diagnosis,” Dr. Ackerman told this news organization.
To date, Dr. Ackerman has reversed the diagnosis for 350 patients, he said.
The consequences of an LQTS diagnosis are “profound,” he noted, including years of unnecessary drug therapy, implantation of a cardioverter defibrillator, disqualification from competitive sports, and emotional stress to the individual and family.
By pointing out the five biggest mistakes his team has seen, he said, “we hope to equip the diagnostician with the means to challenge and assess the veracity of a LQTS diagnosis.”
The study was published online in the Journal of the American College of Cardiology.
Time to do better
Dr. Ackerman and colleagues analyzed electronic medical records on 290 of 1,841 (16%) patients who presented with an outside diagnosis of LQTS but subsequently were dismissed as having normal findings. The mean age of these patients at their first Mayo Clinic evaluation was 22, 60% were female, and the mean QTc interval was 427 ±25 milliseconds.
Overall, 38% of misdiagnoses were the result of misinterpretation of clinical factors; 29%, to diagnostic test misinterpretations; 17%, to an apparently positive genetic test in the context of a weak or absent phenotype; and 16%, to a family history of false LQTS or of sudden cardiac or sudden unexplained death.
More specifically, the most common cause of an LQTS misdiagnosis was QT prolongation following vasovagal syncope, which was misinterpreted as LQTS-attributed syncope.
The second most common cause was an apparently positive genetic test for an LQTS gene that turned out to be a benign or likely benign variant.
The third most common cause was an LQTS diagnosis based solely on a family history of sudden unexplained death (26 patients), QT prolongation (11 patients), or sudden cardiac arrest (9 patients).
The fourth most common cause was an isolated event of QT prolongation (44 patients). The transient QT prolongation was observed under myriad conditions unrelated to LQTS. Yet, 31 patients received a diagnosis based solely on the event.
The fifth most common cause was inclusion of the U-wave in the calculation of the QTc interval (40 patients), leading to an inaccurate interpretation of the electrocardiogram.
Dr. Ackerman noted that these LQTS diagnoses were given by heart-rhythm specialists, and most patients self-referred for a second opinion because a family member questioned the diagnosis after doing their own research.
“It’s time that we step up to the plate and do better,” Dr. Ackerman said. The team’s evaluation of the impact of the misdiagnosis on the patients’ lifestyle and quality of life showed that 45% had been restricted from competitive sports (and subsequently resumed sports activity with no adverse events); 80% had been started on beta-blockers (the drugs were discontinued in 84% as a result of the Mayo Clinic evaluation, whereas 16% opted to continue); and 10 of 22 patients (45%) who received an implanted cardioverter device underwent an extraction of the device without complications.
The authors conclude: “Although missing a patient who truly has LQTS can lead to a tragic outcome, the implications of overdiagnosed LQTS are not trivial and are potentially tragic as well.”
‘Tricky diagnosis’
LQTS specialist Peter Aziz, MD, director of pediatric electrophysiology at the Cleveland Clinic, agreed with these findings.
“Most of us ‘channelopathists’ who see LQTS for a living have a good grasp of the disease, but it can be elusive for others,” he said in an interview. “This is a tricky diagnosis. There are ends of the spectrum where people for sure don’t have it and people for sure do. Most clinicians are able to identify that.”
However, he added, “A lot of patients fall into that gray area where it may not be clear at first, even to an expert. But the expert knows how to do a comprehensive evaluation, examining episodes and symptoms and understanding whether they are relevant to LQTS or completely red herrings, and feeling confident about how they calculate the acute interval on an electrocardiogram.”
“All of these may seem mundane, but without the experience, clinicians are vulnerable to miscalculations,” he said. “That’s why our bias, as channelopathists, is that every patient who has a suspected diagnosis or is being treated for LQTS really should see an expert.”
Similarly, Arthur A.M. Wilde, MD, PhD, of the University of Amsterdam, and Peter J. Schwartz, MD, of IRCCS Istituto Auxologico Italiano, Milan, write in a related editorial that it “has to be kept in mind that both diagnostic scores and risk scores are dynamic and can be modified by time and by appropriate therapy.
“Therefore, to make hasty diagnosis of a disease that requires life-long treatment is inappropriate, especially when this is done without the support of adequate, specific experience.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Novel resuscitation for patients with nonshockable rhythms in cardiac arrest
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr Robert Glatter, medical adviser for Medscape Emergency Medicine. with a remarkable increase in neurologically intact survival. Welcome, gentlemen.
Dr. Pepe, I’d like to start off by thanking you for taking time to join us to discuss this novel concept of head-up or what you now refer to as a neuroprotective cardiopulmonary resuscitation (CPR) bundle. Can you define what this entails and why it is referred to as a neuroprotective CPR bundle?
Paul E. Pepe, MD, MPH: CPR has been life saving for 60 years the way we’ve performed it, but probably only in a very small percentage of cases. That’s one of the problems. We have almost a thousand people a day who have sudden cardiac arrest out in the community alone and more in the hospital.
We know that early defibrillation and early CPR can contribute, but it’s still a small percentage of those. About 75%-85% of the cases that we go out to see will have nonshockable rhythms and flatlines. Some cases are what we call “pulseless electrical activity,” meaning that it looks like there is some kind of organized complex, but there is no pulse associated with it.
That’s why it’s a problem, because they don’t come back. Part of the reason why we see poor outcomes is not only that these cases tend to be people who, say, were in ventricular fibrillation and then just went on over time and were not witnessed or resuscitated or had a long response time. They basically either go into flatline or autoconvert into these bizarre rhythms.
The other issue is the way we perform CPR. CPR has been lifesaving, but it only generates about 20% and maybe 15% in some cases of normal blood flow, and particularly, cerebral perfusion pressure. We’ve looked at this nicely in the laboratory.
For example, during chest compressions, we’re hoping during the recoil phase to pull blood down and back into the right heart. The problem is that you’re not only setting a pressure rate up here to the arterial side but also, you’re setting back pressure wave on the venous side. Obviously, the arterial side always wins out, but it’s just not as efficient as it could be, at 20% or 30%.
What does this entail? It entails several independent mechanisms in terms of how they work, but they all do the same thing, which is they help to pull blood out of the brain and back into the right heart by basically manipulating intrathoracic pressure and creating more of a vacuum to get blood back there.
It’s so important that people do quality CPR. You have to have a good release and that helps us suck a little bit of blood and sucks the air in. As soon as the air rushes in, it neutralizes the pressure and there’s no more vacuum and nothing else is happening until the next squeeze.
What we have found is that we can cap the airway just for a second with a little pop-up valve. It acts like when you’re sucking a milkshake through a straw and it creates more of a vacuum in the chest. Just a little pop-up valve that pulls a little bit more blood out of the brain and the rest of the body and into the right heart.
We’ve shown in a human study that, for example, the systolic blood pressure almost doubles. It really goes from 40 mm Hg during standard CPR up to 80 mm Hg, and that would be sustained for 14-15 minutes. That was a nice little study that was done in Milwaukee a few years ago.
The other thing that happens is, if you add on something else, it’s like a toilet plunger. I think many people have seen it; it’s called “active compression-decompression.” It not only compresses, but it decompresses. Where it becomes even more effective is that if you had broken bones or stiff bones as you get older or whatever it may be, as you do the CPR, you’re still getting the push down and then you’re getting the pull out. It helps on several levels. More importantly, when you put the two together, they’re very synergistic.
We, have already done the clinical trial that is the proof of concept, and that was published in The Lancet about 10 years ago. In that study, we found that the combination of those two dramatically improved survival rates by 50%, with 1-year survival neurologically intact. That got us on the right track.
The interesting thing is that someone said, “Can we lift the head up a little bit?” We did a large amount of work in the laboratory over 10 years, fine tuning it. When do you first lift the head? How soon is too soon? It’s probably bad if you just go right to it.
We had to get the pump primed a little bit with these other things to get the flow going better, not only pulling blood out of the brain but now, you have a better flow this way. You have to prime at first for a couple of minutes, and we worked out the timing: Is it 3 or 4 minutes? It seems the timing is right at about 2 minutes, then you gradually elevate the head over about 2 minutes. We’re finding that seems to be the optimal way to do it. About 2 minutes of priming with those other two devices, the adjuncts, and then gradually elevate the head over 2 minutes.
When we do that in the laboratory, we’re getting normalized cerebral perfusion pressures. You’re normalizing the flow back again with that. We’re seeing profound differences in outcome as a result, even in these cases of the nonshockables.
Dr. Glatter: What you’re doing basically is resulting in an increase in cardiac output, essentially. That really is important, especially in these nonshockable rhythms, correct?
Dr. Pepe: Absolutely. As you’re doing this compression and you’re getting these intracranial pulse waves that are going up because they’re colliding up there. It could be even damaging in itself, but we’re seeing these intracranial raises. The intracranial pressure starts going up more and more over time. Also, peripherally in most people, you’re not getting good flow out there; then, your vasculature starts to relax. The arterials are starting to not get oxygen, so they don’t go out.
With this technique where we’re returning the pressure, we’re getting to 40% of normal now with the active compression-decompression CPR plus an impedance threshold device (ACD+ITD CPR) approach. Now, you add this, and you’re almost normalizing. In humans, even in these asystole patients, we’re seeing end-title CO2s which are generally in the 15-20 range with standard CPR are now up with ACD+ITD CPR in the 30%-40% range, where we’re getting through 30 or 40 end-tidal CO2s. Now, we’re seeing even the end-tidal CO2s moving up into the 40s and 50s. We know there’s a surrogate marker telling us that we are generating much better flows not only to the rest of the body, but most importantly, to the brain.
Dr. Glatter: Ryan, could you tell us about the approach in terms of on scene, what you’re doing and how you use the device itself? Maybe you could talk about the backpack that you developed with your fire department?
Ryan P. Quinn, BS, EMS: Our approach has always been to get to the patient quickly, like everybody’s approach on a cardiac arrest when you’re responding. We are an advanced life-support paramedic ambulance service through the fire department – we’re all cross-trained firefighter paramedics. Our first vehicle from the fire department is typically the ambulance. It’s smaller and a little quicker than the fire engine. Two paramedics are going to jump out with two backpacks. One has the automated compressive device (we use the Lucas), and the other one is the sequential patient lifting device, the EleGARD.
Our two paramedics are quick to the patient’s side, and once they make contact with the patient to verify pulseless cardiac arrest, they will unpack. One person will go right to compressions if there’s nobody on compressions already. Sometimes we have a first responder police officer with an automated external defibrillator (AED). We go right to the patient’s side, concentrate on compressions, and within 90 seconds to 2 minutes, we have our bags unpacked, we’ve got the devices turned on, patient lifted up, slid under the device, and we have a supraglottic airway that is placed within 15 seconds already premade with the ITD on top. We have a sealed airway that we can continue to compress with Dr. Pepe’s original discussion of building on what’s previously been shown to work.
Dr. Pepe: Let me make a comment about this. This is so important, what Ryan is saying, because it’s something we found during the study. It’s really a true pit-crew approach. You’re not only getting these materials, which you think you need a medical Sherpa for, but you don’t. They set it up and then when they open it up, it’s all laid out just exactly as you need it. It’s not just how fast you get there; it’s how fast you get this done.
When we look at all cases combined against high-performance systems that had some of the highest survival rates around, when we compare it to those, we found that overall, even if you looked at the ones that had over 20-minute responses, the odds ratios were still three to four times higher. It was impressive.
If you looked at it under 15 minutes, which is really reasonable for most systems that get there by the way, the average time that people start CPR in any system in these studies has been about 8 minutes if you actually start this thing, which takes about 2 minutes more for this new bundle of care with this triad, it’s almost 12-14 times higher in terms of the odds ratio. I’ve never seen anything like that where the higher end is over 100 in terms of your confidence intervals.
Ryan’s system did really well and is one of those with even higher levels of outcomes, mostly because they got it on quickly. It’s like the AED for nonshockables but better because you have a wider range of efficacy where it will work.
Dr. Glatter: When the elapsed time was less than 11 minutes, that seemed to be an inflection point in the study, is that correct? You saw that 11-fold higher incidence in terms of neurologically intact survival, is that correct?
Dr. Pepe: We picked that number because that was the median time to get it on board. Half the people were getting it within that time period. The fact that you have a larger window, we’re talking about 13- almost 14-fold improvements in outcome if it was under 15 minutes. It doesn’t matter about the 11 or the 12. It’s the faster you get it on board, the better off you are.
Dr. Glatter: What’s the next step in the process of doing trials and having implementation on a larger scale based on your Annals of Emergency Medicine study? Where do you go from here?
Dr. Pepe: I’ve come to find out there are many confounding variables. What was the quality of CPR? How did people ventilate? Did they give the breath and hold it? Did they give a large enough breath so that blood can go across the transpulmonary system? There are many confounding variables. That’s why I think, in the future, it’s going to be more of looking at things like propensity score matching because we know all the variables that change outcomes. I think that’s going to be a way for me.
The other thing is that we were looking at only 380 cases here. When this doubles up in numbers, as we accrue more cases around the country of people who are implementing this, these numbers I just quoted are going to go up much higher. Unwitnessed asystole is considered futile, and you just don’t get them back. To be able to get these folks back now, even if it’s a small percentage, and the fact that we know that we’re producing this better flow, is pretty striking.
I’m really impressed, and the main thing is to make sure people are educated about it. Number two is that they understand that it has to be done right. It cannot be done wrong or you’re not going to see the differences. Getting it done right is not only following the procedures, the sequence, and how you do it, but it also has to do with getting there quickly, including assigning the right people to put it on and having well-trained people who know what they’re doing.
Dr. Glatter: In general, the lay public obviously should not attempt this in the field lifting someone’s head up in the sense of trying to do chest compressions. I think that message is important that you just said. It’s not ready for prime time yet in any way. It has to be done right.
Dr. Pepe: Bystanders have to learn CPR – they will buy us time and we’ll have better outcomes when they do that. That’s number one. Number two is that as more and more systems adopt this, you’re going to see more people coming back. If you think about what we’re doing now, if we only get back 5% of these nonshockable vs. less than 1%, it’s 5% of 800 people a day because a thousand people a day die. Several dozens of lives can be saved on a daily basis, coming back neurologically intact. That’s the key thing.
Dr. Glatter: Ryan, can you comment about your experience in the field? Is there anything in terms of your current approach that you think would be ideal to change at this point?
Mr. Quinn: We’ve established that this is the approach that we want to take and we’re just fine tuning it to be more efficient. Using the choreography of which person is going to do which role, we have clearly defined roles and clearly defined command of the scene so we’re not missing anything. Training is extremely important.
Dr. Glatter: Paul, I want to ask you about your anecdotal experience of people waking up quickly and talking after elevating their heads and going through this process. Having people talk about it and waking up is really fascinating. Maybe you can comment further on this.
Dr. Pepe: That’s a great point that you bring up because a 40- to 50-year-old guy who got saved with this approach, when he came around, he said he was hearing what people were saying. When he came out of it, he found out he had been getting CPR for about 25 minutes because he had persistent recurring ventricular fibrillation. He said, “How could I have survived that that long?”
When we told him about the new approach, he added, “Well, that’s like neuroprotective.” He’s right, because in the laboratory, we showed it was neuroprotective and we’re also getting better flows back there. It goes along with everything else, and so we’ve adopted the name because it is.
These are really high-powered systems we are comparing against, and we have the same level of return of spontaneous circulation. The major difference was when you started talking about the neurointact survival. We don’t have enough numbers yet, but next go around, we’re going to look at cerebral performance category (CPC) – CPC1 vs. the CPC2 – which were both considered intact, but CPC1 is actually better. We’re seeing many more of those, anecdotally.
I also wanted to mention that people do bring this up and say, “Well, let’s do a trial.” As far as we’re concerned, the trial’s been done in terms of The Lancet study 10 years ago that showed that the active compression-decompression had tremendously better outcomes. We show in the laboratories that you augment that a little bit. These are all [Food and Drug Administration] approved. You can go out and buy it tomorrow and get it done. I have no conflicts of interest, by the way, with any of this.
To have this device that’s going to have the potential of saving so many more lives is really an exciting breakthrough. More importantly, we’re understanding more now about the physiology of CPR and why it works. It could work much better with the approaches that we’ve been developing over the last 20 years or so.
Dr. Glatter: Absolutely. I want to thank both of you gentlemen. It’s been really an incredible experience to learn more about an advance in resuscitation that could truly be lifesaving. Thank you again for taking time to join us.
Dr. Glatter is an attending physician in the department of emergency medicine, Lenox Hill Hospital, New York. Dr. Pepe is professor, department of management, policy, and community health, University of Texas Health Sciences Center, Houston. Mr. Quinn is EMS Chief, Edina (Minn.) Fire Department. No conflicts of interest were reported.
A version of this article first appeared Jan. 26 on Medscape.com.
Is preeclampsia a cardiovascular time bomb for mothers?
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who experience preeclampsia during pregnancy are almost twice as likely to have a heart attack or stroke within 20 years of giving birth as pregnant women who did not, according to a new study published in the European Journal of Preventive Cardiology. The risks are especially high in the first decade after giving birth, the researchers found.
Preeclampsia is the onset of high blood pressure after the 20th week of pregnancy combined with signs of organ damage, such as excess protein in the urine. It can occur in up to 8% of pregnancies, and the association between preeclampsia and long-term cardiac risks is well-known. But new research suggests these risks appear much earlier in life than expected – as early as age 30 – at a time when women are often not screened for signs of heart trouble
“Targeted interventions cannot wait until women with preeclampsia become eligible for conventional screening programs in middle age,” Sara Hallum, PhD, a coauthor of the study, told this news organization.
Dr. Hallum, who was an epidemiologist at the University of Copenhagen at the time of the study, and colleagues evaluated the medical histories of more than 1.1 million women in Denmark who became pregnant once or twice between 1978 and 2017. Of this group, 3% had experienced preeclampsia. They compared rates of heart attack and stroke between the two groups over time.
While 1.2% of the entire study population had experienced a heart attack or stroke within 20 years of giving birth, 2% of the women with a history of preeclampsia had such an event. Within the first decade after delivery, women with a history of preeclampsia were four times as likely to have a heart attack and three times as likely to have a stroke as other women.
Women aged 30-39 with a history of preeclampsia were nearly five times as likely to have a heart attack and three times as likely to have a stroke as similar-aged women. And if a woman gave birth twice and had preeclampsia only during the second pregnancy, she was at especially high risk for a heart attack, the researchers found.
“Women with a history of preeclampsia should be monitored routinely for modifiable risk factors, particularly for increased blood pressure,” Dr. Hallum said.
The Danish study population is racially homogeneous, so the researchers were not able to distinguish the effects of preeclampsia by racial group. In the United States, strong evidence shows that Black women experience the effects of preeclampsia more than others.
A useful clue to cardiac risk
Ellen Seely, MD, an endocrinologist at Brigham and Women’s Hospital in Boston, who specializes in preeclampsia, said physicians are less likely to ask women who have been pregnant if they had experienced preeclampsia than to ask if they smoke or have a family history of heart attacks. As a result, they may miss a looming cardiovascular event, especially in younger women who appear healthy.
“Emerging high blood pressure shouldn’t be ignored” in a seemingly healthy young woman, Dr. Seely said, particularly if that woman has divulged a history of preeclampsia. The doctor’s first step should be to verify hypertension, Dr. Seely said. If high blood pressure is evident, immediate treatment – such as encouraging more physical activity and a healthier diet – should follow. Watchful waiting in such cases is inappropriate, she added.
Although the experience of having preeclampsia is unpleasant and scary, Dr. Seely noted that in at least one way it can prove advantageous. Some women who did not experience preeclampsia will end up having a heart attack, sometimes with no prior warning that anything was amiss. At least a history of preeclampsia provides a clue that women should take care of their hearts.
“The patient carries their history with them wherever they go,” Dr. Seely said. For now, this reality often requires women to mention their pregnancy history even if a provider doesn’t ask. Someday, Dr. Seely said, asking about that history will become just as routine for providers as asking about family history.
The study was funded by the Danish Heart Foundation. Dr. Hallum and Dr. Seely have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
AHA scientific statement on rapid evaluation for suspected TIA
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.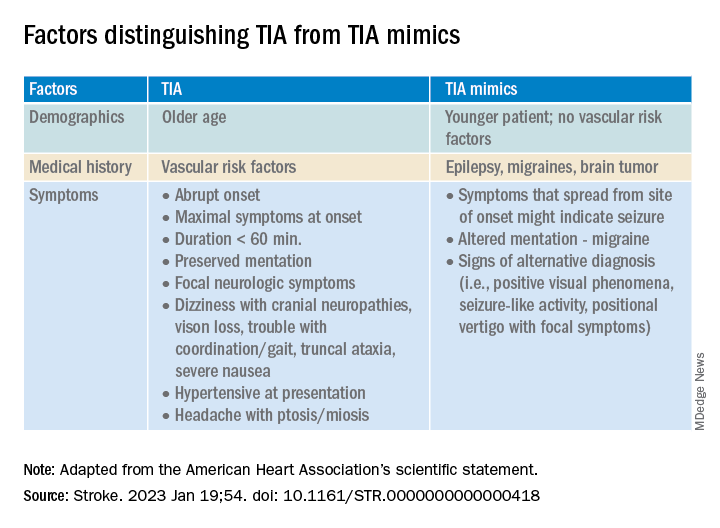
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
FROM STROKE
Damar Hamlin’s cardiac arrest: Key lessons
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.


