User login
Are docs getting fed up with hearing about burnout?
There is a feeling of exhaustion, being unable to shake a lingering cold, suffering from frequent headaches and gastrointestinal disturbances, sleeplessness and shortness of breath ...
That was how burnout was described by clinical psychologist Herbert Freudenberger, PhD, who first used the phrase in a paper back in 1974, after observing the emotional depletion and accompanying psychosomatic symptoms among volunteer staff of a free clinic in New York City. He called it “burnout,” a term borrowed from the slang of substance abusers.
It has now been established beyond a shadow of a doubt that burnout is a serious issue facing physicians across specialties, albeit some more intensely than others. But with the constant barrage of stories published on an almost daily basis, along with studies and surveys, it begs the question:
Some have suggested that the focus should be more on tackling burnout and instituting viable solutions rather than rehashing the problem.
There haven’t been studies or surveys on this question, but several experts have offered their opinion.
Jonathan Fisher, MD, a cardiologist and organizational well-being and resiliency leader at Novant Health, Charlotte, N.C., cautioned that he hesitates to speak about what physicians in general believe. “We are a diverse group of nearly 1 million in the United States alone,” he said.
But he noted that there is a specific phenomenon among burned-out health care providers who are “burned out on burnout.”
“Essentially, the underlying thought is ‘talk is cheap and we want action,’” said Dr. Fisher, who is chair and co-founder of the Ending Physician Burnout Global Summit that was held in 2021. “This reaction is often a reflection of disheartened physicians’ sense of hopelessness and cynicism that systemic change to improve working conditions will happen in our lifetime.”
Dr. Fisher explained that “typically, anyone suffering – physicians or nonphysicians – cares more about ending the suffering as soon as possible than learning its causes, but to alleviate suffering at its core – including the emotional suffering of burnout – we must understand the many causes.”
“To address both the organizational and individual drivers of burnout requires a keen awareness of the thoughts, fears, and dreams of physicians, health care executives, and all other stakeholders in health care,” he added.
Burnout, of course, is a very real problem. The 2022 Medscape Physician Burnout & Depression Report found that nearly half of all respondents (47%) said they are burned out, which was higher than the prior year. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, jumping from 43% in 2021 to 60% this year. More than half of critical care physicians (56%) also reported that they were burned out.
The World Health Organization’s International Classification of Diseases (ICD-11) – the official compendium of diseases – has categorized burnout as a “syndrome” that results from “chronic workplace stress that has not been successfully managed.” It is considered to be an occupational phenomenon and is not classified as a medical condition.
But whether or not physicians are burned out on hearing about burnout remains unclear. “I am not sure if physicians are tired of hearing about ‘burnout,’ but I do think that they want to hear about solutions that go beyond just telling them to take better care of themselves,” said Anne Thorndike, MD, MPH, an internal medicine physician at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, Boston. “There are major systematic factors that contribute to physicians burning out.”
Why talk about negative outcomes?
Jonathan Ripp, MD, MPH, however, is familiar with this sentiment. “‘Why do we keep identifying a problem without solutions’ is certainly a sentiment that is being expressed,” he said. “It’s a negative outcome, so why do we keep talking about negative outcomes?”
Dr. Ripp, who is a professor of medicine, medical education, and geriatrics and palliative medicine; the senior associate dean for well-being and resilience; and chief wellness officer at Icahn School of Medicine at Mount Sinai, New York, is also a well-known expert and researcher in burnout and physician well-being.
He noted that burnout was one of the first “tools” used as a metric to measure well-being, but it is a negative measurement. “It’s been around a long time, so it has a lot of evidence,” said Dr. Ripp. “But that said, there are other ways of measuring well-being without a negative association, and ways of measuring meaning in work – fulfillment and satisfaction, and so on. It should be balanced.”
But for the average physician not familiar with the long legacy of research, they may be frustrated by this situation. “Then they ask, ‘Why are you just showing me more of this instead of doing something about it?’ but we are actually doing something about it,” said Dr. Ripp.
There are many efforts underway, he explained, but it’s a challenging and complex issue. “There are numerous drivers impacting the well-being of any given segment within the health care workforce,” he said. “It will also vary by discipline and location, and there are also a host of individual factors that may have very little to do with the work environment. There are some very well-established efforts for an organizational approach, but it remains to be seen which is the most effective.”
But in broad strokes, he continued, it’s about tackling the system and not about making an individual more resilient. “Individuals that do engage in activities that improve resilience do better, but that’s not what this is about – it’s not going to solve the problem,” said Dr. Ripp. “Those of us like myself, who are working in this space, are trying to promote a culture of well-being – at the system level.”
The question is how to enable the workforce to do their best work in an efficient way so that the balance of their activities are not the meaningless aspects. “And instead, shoot that balance to the meaningful aspects of work,” he added. “There are enormous challenges, but even though we are working on solutions, I can see how the individual may not see that – they may say, ‘Stop telling me to be resilient, stop telling me there’s a problem,’ but we’re working on it.”
Moving medicine forward
James Jerzak, MD, a family physician in Green Bay, Wisc., and physician lead at Bellin Health, noted that “it seems to me that doctors aren’t burned out talking about burnout, but they are burned out hearing that the solution to burnout is simply for them to become more resilient,” he said. “In actuality, the path to dealing with this huge problem is to make meaningful systemic changes in how medicine is practiced.”
He reiterated that medical care has become increasingly complex, with the aging of the population; the increasing incidence of chronic diseases, such as diabetes; the challenges with the increasing cost of care, higher copays, and lack of health insurance for a large portion of the country; and general incivility toward health care workers that was exacerbated by the pandemic.
“This has all led to significantly increased stress levels for medical workers,” he said. “Couple all of that with the increased work involved in meeting the demands of the electronic health record, and it is clear that the current situation is unsustainable.”
In his own health care system, moving medicine forward has meant advancing team-based care, which translates to expanding teams to include adequate support for physicians. This strategy addressed problems in health care delivery, part of which is burnout.
“In many systems practicing advanced team-based care, the ancillary staff – medical assistants, LPNs, and RNs – play an enhanced role in the patient visit and perform functions such as quality care gap closure, medication review and refill pending, pending orders, and helping with documentation,” he said. “Although the current health care workforce shortages has created challenges, there are a lot of innovative approaches being tried [that are] aimed at providing solutions.”
The second key factor is for systems is to develop robust support for their providers with a broad range of team members, such as case managers, clinical pharmacists, diabetic educators, care coordinators, and others. “The day has passed where individual physicians can effectivity manage all of the complexities of care, especially since there are so many nonclinical factors affecting care,” said Dr. Jerzak.
“The recent focus on the social determinants of health and health equity underlies the fact that it truly takes a team of health care professionals working together to provide optimal care for patients,” he said.
Dr. Thorndike, who mentors premedical and medical trainees, has pointed out that burnout begins way before an individual enters the workplace as a doctor. Burnout begins in the earliest stages of medical practice, with the application process to medical school. The admissions process extends over a 12-month period, causing a great deal of “toxic stress.”
One study found that, compared with non-premedical students, premedical students had greater depression severity and emotional exhaustion.
“The current system of medical school admissions ignores the toll that the lengthy and emotionally exhausting process takes on aspiring physicians,” she said. “This is just one example of many in training and health care that requires physicians to set aside their own lives to achieve their goals and to provide the best possible care to others.”
A version of this article first appeared on Medscape.com.
There is a feeling of exhaustion, being unable to shake a lingering cold, suffering from frequent headaches and gastrointestinal disturbances, sleeplessness and shortness of breath ...
That was how burnout was described by clinical psychologist Herbert Freudenberger, PhD, who first used the phrase in a paper back in 1974, after observing the emotional depletion and accompanying psychosomatic symptoms among volunteer staff of a free clinic in New York City. He called it “burnout,” a term borrowed from the slang of substance abusers.
It has now been established beyond a shadow of a doubt that burnout is a serious issue facing physicians across specialties, albeit some more intensely than others. But with the constant barrage of stories published on an almost daily basis, along with studies and surveys, it begs the question:
Some have suggested that the focus should be more on tackling burnout and instituting viable solutions rather than rehashing the problem.
There haven’t been studies or surveys on this question, but several experts have offered their opinion.
Jonathan Fisher, MD, a cardiologist and organizational well-being and resiliency leader at Novant Health, Charlotte, N.C., cautioned that he hesitates to speak about what physicians in general believe. “We are a diverse group of nearly 1 million in the United States alone,” he said.
But he noted that there is a specific phenomenon among burned-out health care providers who are “burned out on burnout.”
“Essentially, the underlying thought is ‘talk is cheap and we want action,’” said Dr. Fisher, who is chair and co-founder of the Ending Physician Burnout Global Summit that was held in 2021. “This reaction is often a reflection of disheartened physicians’ sense of hopelessness and cynicism that systemic change to improve working conditions will happen in our lifetime.”
Dr. Fisher explained that “typically, anyone suffering – physicians or nonphysicians – cares more about ending the suffering as soon as possible than learning its causes, but to alleviate suffering at its core – including the emotional suffering of burnout – we must understand the many causes.”
“To address both the organizational and individual drivers of burnout requires a keen awareness of the thoughts, fears, and dreams of physicians, health care executives, and all other stakeholders in health care,” he added.
Burnout, of course, is a very real problem. The 2022 Medscape Physician Burnout & Depression Report found that nearly half of all respondents (47%) said they are burned out, which was higher than the prior year. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, jumping from 43% in 2021 to 60% this year. More than half of critical care physicians (56%) also reported that they were burned out.
The World Health Organization’s International Classification of Diseases (ICD-11) – the official compendium of diseases – has categorized burnout as a “syndrome” that results from “chronic workplace stress that has not been successfully managed.” It is considered to be an occupational phenomenon and is not classified as a medical condition.
But whether or not physicians are burned out on hearing about burnout remains unclear. “I am not sure if physicians are tired of hearing about ‘burnout,’ but I do think that they want to hear about solutions that go beyond just telling them to take better care of themselves,” said Anne Thorndike, MD, MPH, an internal medicine physician at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, Boston. “There are major systematic factors that contribute to physicians burning out.”
Why talk about negative outcomes?
Jonathan Ripp, MD, MPH, however, is familiar with this sentiment. “‘Why do we keep identifying a problem without solutions’ is certainly a sentiment that is being expressed,” he said. “It’s a negative outcome, so why do we keep talking about negative outcomes?”
Dr. Ripp, who is a professor of medicine, medical education, and geriatrics and palliative medicine; the senior associate dean for well-being and resilience; and chief wellness officer at Icahn School of Medicine at Mount Sinai, New York, is also a well-known expert and researcher in burnout and physician well-being.
He noted that burnout was one of the first “tools” used as a metric to measure well-being, but it is a negative measurement. “It’s been around a long time, so it has a lot of evidence,” said Dr. Ripp. “But that said, there are other ways of measuring well-being without a negative association, and ways of measuring meaning in work – fulfillment and satisfaction, and so on. It should be balanced.”
But for the average physician not familiar with the long legacy of research, they may be frustrated by this situation. “Then they ask, ‘Why are you just showing me more of this instead of doing something about it?’ but we are actually doing something about it,” said Dr. Ripp.
There are many efforts underway, he explained, but it’s a challenging and complex issue. “There are numerous drivers impacting the well-being of any given segment within the health care workforce,” he said. “It will also vary by discipline and location, and there are also a host of individual factors that may have very little to do with the work environment. There are some very well-established efforts for an organizational approach, but it remains to be seen which is the most effective.”
But in broad strokes, he continued, it’s about tackling the system and not about making an individual more resilient. “Individuals that do engage in activities that improve resilience do better, but that’s not what this is about – it’s not going to solve the problem,” said Dr. Ripp. “Those of us like myself, who are working in this space, are trying to promote a culture of well-being – at the system level.”
The question is how to enable the workforce to do their best work in an efficient way so that the balance of their activities are not the meaningless aspects. “And instead, shoot that balance to the meaningful aspects of work,” he added. “There are enormous challenges, but even though we are working on solutions, I can see how the individual may not see that – they may say, ‘Stop telling me to be resilient, stop telling me there’s a problem,’ but we’re working on it.”
Moving medicine forward
James Jerzak, MD, a family physician in Green Bay, Wisc., and physician lead at Bellin Health, noted that “it seems to me that doctors aren’t burned out talking about burnout, but they are burned out hearing that the solution to burnout is simply for them to become more resilient,” he said. “In actuality, the path to dealing with this huge problem is to make meaningful systemic changes in how medicine is practiced.”
He reiterated that medical care has become increasingly complex, with the aging of the population; the increasing incidence of chronic diseases, such as diabetes; the challenges with the increasing cost of care, higher copays, and lack of health insurance for a large portion of the country; and general incivility toward health care workers that was exacerbated by the pandemic.
“This has all led to significantly increased stress levels for medical workers,” he said. “Couple all of that with the increased work involved in meeting the demands of the electronic health record, and it is clear that the current situation is unsustainable.”
In his own health care system, moving medicine forward has meant advancing team-based care, which translates to expanding teams to include adequate support for physicians. This strategy addressed problems in health care delivery, part of which is burnout.
“In many systems practicing advanced team-based care, the ancillary staff – medical assistants, LPNs, and RNs – play an enhanced role in the patient visit and perform functions such as quality care gap closure, medication review and refill pending, pending orders, and helping with documentation,” he said. “Although the current health care workforce shortages has created challenges, there are a lot of innovative approaches being tried [that are] aimed at providing solutions.”
The second key factor is for systems is to develop robust support for their providers with a broad range of team members, such as case managers, clinical pharmacists, diabetic educators, care coordinators, and others. “The day has passed where individual physicians can effectivity manage all of the complexities of care, especially since there are so many nonclinical factors affecting care,” said Dr. Jerzak.
“The recent focus on the social determinants of health and health equity underlies the fact that it truly takes a team of health care professionals working together to provide optimal care for patients,” he said.
Dr. Thorndike, who mentors premedical and medical trainees, has pointed out that burnout begins way before an individual enters the workplace as a doctor. Burnout begins in the earliest stages of medical practice, with the application process to medical school. The admissions process extends over a 12-month period, causing a great deal of “toxic stress.”
One study found that, compared with non-premedical students, premedical students had greater depression severity and emotional exhaustion.
“The current system of medical school admissions ignores the toll that the lengthy and emotionally exhausting process takes on aspiring physicians,” she said. “This is just one example of many in training and health care that requires physicians to set aside their own lives to achieve their goals and to provide the best possible care to others.”
A version of this article first appeared on Medscape.com.
There is a feeling of exhaustion, being unable to shake a lingering cold, suffering from frequent headaches and gastrointestinal disturbances, sleeplessness and shortness of breath ...
That was how burnout was described by clinical psychologist Herbert Freudenberger, PhD, who first used the phrase in a paper back in 1974, after observing the emotional depletion and accompanying psychosomatic symptoms among volunteer staff of a free clinic in New York City. He called it “burnout,” a term borrowed from the slang of substance abusers.
It has now been established beyond a shadow of a doubt that burnout is a serious issue facing physicians across specialties, albeit some more intensely than others. But with the constant barrage of stories published on an almost daily basis, along with studies and surveys, it begs the question:
Some have suggested that the focus should be more on tackling burnout and instituting viable solutions rather than rehashing the problem.
There haven’t been studies or surveys on this question, but several experts have offered their opinion.
Jonathan Fisher, MD, a cardiologist and organizational well-being and resiliency leader at Novant Health, Charlotte, N.C., cautioned that he hesitates to speak about what physicians in general believe. “We are a diverse group of nearly 1 million in the United States alone,” he said.
But he noted that there is a specific phenomenon among burned-out health care providers who are “burned out on burnout.”
“Essentially, the underlying thought is ‘talk is cheap and we want action,’” said Dr. Fisher, who is chair and co-founder of the Ending Physician Burnout Global Summit that was held in 2021. “This reaction is often a reflection of disheartened physicians’ sense of hopelessness and cynicism that systemic change to improve working conditions will happen in our lifetime.”
Dr. Fisher explained that “typically, anyone suffering – physicians or nonphysicians – cares more about ending the suffering as soon as possible than learning its causes, but to alleviate suffering at its core – including the emotional suffering of burnout – we must understand the many causes.”
“To address both the organizational and individual drivers of burnout requires a keen awareness of the thoughts, fears, and dreams of physicians, health care executives, and all other stakeholders in health care,” he added.
Burnout, of course, is a very real problem. The 2022 Medscape Physician Burnout & Depression Report found that nearly half of all respondents (47%) said they are burned out, which was higher than the prior year. Perhaps not surprisingly, burnout among emergency physicians took the biggest leap, jumping from 43% in 2021 to 60% this year. More than half of critical care physicians (56%) also reported that they were burned out.
The World Health Organization’s International Classification of Diseases (ICD-11) – the official compendium of diseases – has categorized burnout as a “syndrome” that results from “chronic workplace stress that has not been successfully managed.” It is considered to be an occupational phenomenon and is not classified as a medical condition.
But whether or not physicians are burned out on hearing about burnout remains unclear. “I am not sure if physicians are tired of hearing about ‘burnout,’ but I do think that they want to hear about solutions that go beyond just telling them to take better care of themselves,” said Anne Thorndike, MD, MPH, an internal medicine physician at Massachusetts General Hospital and associate professor of medicine at Harvard Medical School, Boston. “There are major systematic factors that contribute to physicians burning out.”
Why talk about negative outcomes?
Jonathan Ripp, MD, MPH, however, is familiar with this sentiment. “‘Why do we keep identifying a problem without solutions’ is certainly a sentiment that is being expressed,” he said. “It’s a negative outcome, so why do we keep talking about negative outcomes?”
Dr. Ripp, who is a professor of medicine, medical education, and geriatrics and palliative medicine; the senior associate dean for well-being and resilience; and chief wellness officer at Icahn School of Medicine at Mount Sinai, New York, is also a well-known expert and researcher in burnout and physician well-being.
He noted that burnout was one of the first “tools” used as a metric to measure well-being, but it is a negative measurement. “It’s been around a long time, so it has a lot of evidence,” said Dr. Ripp. “But that said, there are other ways of measuring well-being without a negative association, and ways of measuring meaning in work – fulfillment and satisfaction, and so on. It should be balanced.”
But for the average physician not familiar with the long legacy of research, they may be frustrated by this situation. “Then they ask, ‘Why are you just showing me more of this instead of doing something about it?’ but we are actually doing something about it,” said Dr. Ripp.
There are many efforts underway, he explained, but it’s a challenging and complex issue. “There are numerous drivers impacting the well-being of any given segment within the health care workforce,” he said. “It will also vary by discipline and location, and there are also a host of individual factors that may have very little to do with the work environment. There are some very well-established efforts for an organizational approach, but it remains to be seen which is the most effective.”
But in broad strokes, he continued, it’s about tackling the system and not about making an individual more resilient. “Individuals that do engage in activities that improve resilience do better, but that’s not what this is about – it’s not going to solve the problem,” said Dr. Ripp. “Those of us like myself, who are working in this space, are trying to promote a culture of well-being – at the system level.”
The question is how to enable the workforce to do their best work in an efficient way so that the balance of their activities are not the meaningless aspects. “And instead, shoot that balance to the meaningful aspects of work,” he added. “There are enormous challenges, but even though we are working on solutions, I can see how the individual may not see that – they may say, ‘Stop telling me to be resilient, stop telling me there’s a problem,’ but we’re working on it.”
Moving medicine forward
James Jerzak, MD, a family physician in Green Bay, Wisc., and physician lead at Bellin Health, noted that “it seems to me that doctors aren’t burned out talking about burnout, but they are burned out hearing that the solution to burnout is simply for them to become more resilient,” he said. “In actuality, the path to dealing with this huge problem is to make meaningful systemic changes in how medicine is practiced.”
He reiterated that medical care has become increasingly complex, with the aging of the population; the increasing incidence of chronic diseases, such as diabetes; the challenges with the increasing cost of care, higher copays, and lack of health insurance for a large portion of the country; and general incivility toward health care workers that was exacerbated by the pandemic.
“This has all led to significantly increased stress levels for medical workers,” he said. “Couple all of that with the increased work involved in meeting the demands of the electronic health record, and it is clear that the current situation is unsustainable.”
In his own health care system, moving medicine forward has meant advancing team-based care, which translates to expanding teams to include adequate support for physicians. This strategy addressed problems in health care delivery, part of which is burnout.
“In many systems practicing advanced team-based care, the ancillary staff – medical assistants, LPNs, and RNs – play an enhanced role in the patient visit and perform functions such as quality care gap closure, medication review and refill pending, pending orders, and helping with documentation,” he said. “Although the current health care workforce shortages has created challenges, there are a lot of innovative approaches being tried [that are] aimed at providing solutions.”
The second key factor is for systems is to develop robust support for their providers with a broad range of team members, such as case managers, clinical pharmacists, diabetic educators, care coordinators, and others. “The day has passed where individual physicians can effectivity manage all of the complexities of care, especially since there are so many nonclinical factors affecting care,” said Dr. Jerzak.
“The recent focus on the social determinants of health and health equity underlies the fact that it truly takes a team of health care professionals working together to provide optimal care for patients,” he said.
Dr. Thorndike, who mentors premedical and medical trainees, has pointed out that burnout begins way before an individual enters the workplace as a doctor. Burnout begins in the earliest stages of medical practice, with the application process to medical school. The admissions process extends over a 12-month period, causing a great deal of “toxic stress.”
One study found that, compared with non-premedical students, premedical students had greater depression severity and emotional exhaustion.
“The current system of medical school admissions ignores the toll that the lengthy and emotionally exhausting process takes on aspiring physicians,” she said. “This is just one example of many in training and health care that requires physicians to set aside their own lives to achieve their goals and to provide the best possible care to others.”
A version of this article first appeared on Medscape.com.
Intravenous Immunoglobulin in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia: A Pharmacoeconomic Analysis
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board (research@sharp.com), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
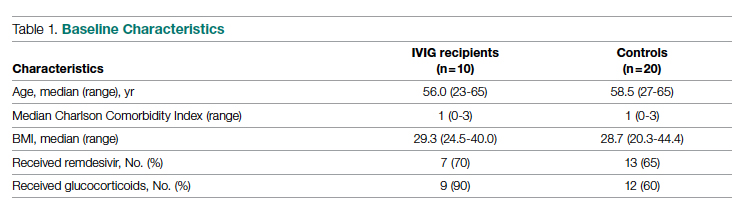
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
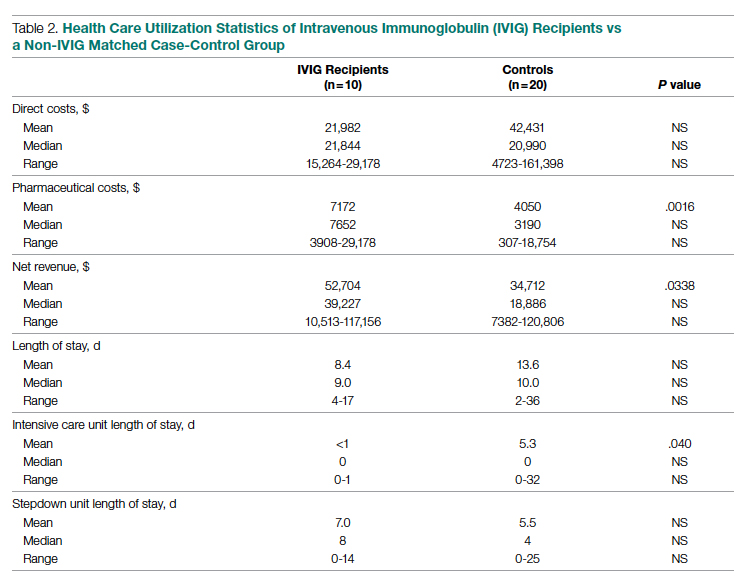
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; gsakoulas@health.ucsd.edu
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board (research@sharp.com), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; gsakoulas@health.ucsd.edu
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board (research@sharp.com), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; gsakoulas@health.ucsd.edu
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
Path to parenthood in cardiology training fraught with obstacles
The first international survey of parental benefits and policies among cardiovascular training programs shows wide variability among institutions.
Although a majority of cardiology fellows became parents during training, the survey found that family benefits and policies were not uniformly available and that knowledge about the existence of such policies was low across all institutions.
The findings are published in the Journal of the American College of Cardiology.
Such variability highlights disparities in real-world experiences, say Estefania Oliveros, MD, Temple University Hospital, Philadelphia, and colleagues.
“There are no policies to protect cardiology trainees when they become parents that are uniform across the United States or even internationally, even though, according to our survey, 61.7% become parents during training,” Dr. Oliveros told this news organization.
Dr. Oliveros said she wanted to learn more about the status of institutional practices surrounding pregnant trainees during cardiovascular fellowship, not only in the U.S., but internationally: “I wanted to study this because of my own experience.”
“I was probably the first pregnant trainee at my institution, and there were no specific policies in place, so I had to find out on my own what to do about radiation safety, where I would breastfeed, schedule changes, how that would impact my graduation time, things like that,” Dr. Oliveros said. “It would be nice if you had the resources and your institution could accommodate your needs, instead of every time you have a pregnant person on your staff, you have to reinvent the wheel.”
Dr. Oliveros and colleagues conducted an online survey during August 2020-October 2020 that was distributed via social media. Responses were made anonymous to encourage unbiased feedback.
Among the 417 completed responses, 47 (11.3%) were from training program directors, 146 (35%) from current or former pregnant trainees, and 224 (53.7%) from current or former trainees who were not pregnant during cardiology training. Two-thirds of the respondents (67.1%) were parents.
Most survey respondents said they became pregnant during the third year of general cardiology (29.1%), followed by the first year of general cardiology (26.3%), and the second year of general cardiology (23.5%).
Only 13 of the 47 training program directors (27.7%) received guidance or training on how to accommodate pregnant trainees during fellowship.
Additionally, 26% of the trainees reported their institution had readily available breastfeeding and pumping policies, 39% responded that their institution had no such policies, and 34.9% said they did not know.
Nearly one-half of the programs offered rearrangement of schedules because of radiation concerns, 27.5% did not.
The amount of parental leave varied greatly worldwide. For Europe, Central and South America, Africa, and Australia, the average parental leave was more than 4 months; for Canada, it was more than 3 months; for the United States, it was 1 to 2 months; and for Asia, it was 3 to 4 weeks.
“There is no uniformity, no policies for things like breastfeeding or places where you can pump. None of that is installed, even though by law we’re supposed to have these things,” Dr. Oliveros said.
In all countries, paternity leave was uncommon (2.6% of respondents), even though 48.5% of the programs had paternity leave.
“I would like to see associations, program directors, even trainees helping each other in finding ways to accommodate parents to promote wellness and assure that trainees can have both good training and life balance,” she added.
In an accompanying editorial, Ileana L. Piña, MD, MPH, Thomas Jefferson Institute, Philadelphia, writes: “Enough has been said about our need for a greater percentage of women cardiologists. There is no need to further debate that fact. However, it is puzzling that despite > 50% of medical students being women, the cardiology specialty is fraught with recent survey reports of hostility in the workplace, concerns of long hours, exposure to radiation, and poor work-life balance that can compel trainees to choose delaying pregnancy or taking unpaid leave, which will, in turn, delay training. Therefore, it is not surprising that only 14.9% of cardiologist specialists and 21.9% of cardiology fellows are women.”
Dr. Piña notes that while the authors understand that it’s difficult to change national policies, they issue a “call to action” for organizations and program directors to demonstrate leadership by developing fair and balanced decisions regarding parental policies.
“Those decisions are so impactful that they can change career trajectories for the better or worse ... the current status is unacceptable and must change for the benefit of all trainees, their families, and the program directors. The problem is too important and pervasive,” she adds.
Dr. Piña concludes: “Perhaps if the women who are the subjects of, and often the unwitting party to, administrative decisions about their lives, choices, and welfare were invited to contribute to the changes, we would finally see an increase in the number of women in cardiology careers. After all, aren’t we about diversity and belonging?”
“We need to normalize pregnancy and parental leave across the globe,” Laxmi S. Mehta, MD, Ohio State University Weiner Medical Center, Columbus, said in an interview.
As previously reported, Dr. Mehta recently led a study that surveyed 323 women cardiologists who were working while they were pregnant. Her study found that 75% of these women experienced discriminatory maternity leave practices, some of which were likely violations of the federal Family and Medical Leave Act.
“If we want more women to pursue a career in cardiology, then employers and health systems need to adequately support parenthood, including allowing people to spend uninterrupted time with their newborns without the fear of discrimination, retaliation, or financial burden,” Dr. Mehta said.
Limitations of the study are the small sample size, potential for bias associated with social media distribution, and the fact that 75% of respondents were women, Dr. Oliveros and colleagues write.
Dr. Oliveros, Dr. Piña, and Dr. Mehta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The first international survey of parental benefits and policies among cardiovascular training programs shows wide variability among institutions.
Although a majority of cardiology fellows became parents during training, the survey found that family benefits and policies were not uniformly available and that knowledge about the existence of such policies was low across all institutions.
The findings are published in the Journal of the American College of Cardiology.
Such variability highlights disparities in real-world experiences, say Estefania Oliveros, MD, Temple University Hospital, Philadelphia, and colleagues.
“There are no policies to protect cardiology trainees when they become parents that are uniform across the United States or even internationally, even though, according to our survey, 61.7% become parents during training,” Dr. Oliveros told this news organization.
Dr. Oliveros said she wanted to learn more about the status of institutional practices surrounding pregnant trainees during cardiovascular fellowship, not only in the U.S., but internationally: “I wanted to study this because of my own experience.”
“I was probably the first pregnant trainee at my institution, and there were no specific policies in place, so I had to find out on my own what to do about radiation safety, where I would breastfeed, schedule changes, how that would impact my graduation time, things like that,” Dr. Oliveros said. “It would be nice if you had the resources and your institution could accommodate your needs, instead of every time you have a pregnant person on your staff, you have to reinvent the wheel.”
Dr. Oliveros and colleagues conducted an online survey during August 2020-October 2020 that was distributed via social media. Responses were made anonymous to encourage unbiased feedback.
Among the 417 completed responses, 47 (11.3%) were from training program directors, 146 (35%) from current or former pregnant trainees, and 224 (53.7%) from current or former trainees who were not pregnant during cardiology training. Two-thirds of the respondents (67.1%) were parents.
Most survey respondents said they became pregnant during the third year of general cardiology (29.1%), followed by the first year of general cardiology (26.3%), and the second year of general cardiology (23.5%).
Only 13 of the 47 training program directors (27.7%) received guidance or training on how to accommodate pregnant trainees during fellowship.
Additionally, 26% of the trainees reported their institution had readily available breastfeeding and pumping policies, 39% responded that their institution had no such policies, and 34.9% said they did not know.
Nearly one-half of the programs offered rearrangement of schedules because of radiation concerns, 27.5% did not.
The amount of parental leave varied greatly worldwide. For Europe, Central and South America, Africa, and Australia, the average parental leave was more than 4 months; for Canada, it was more than 3 months; for the United States, it was 1 to 2 months; and for Asia, it was 3 to 4 weeks.
“There is no uniformity, no policies for things like breastfeeding or places where you can pump. None of that is installed, even though by law we’re supposed to have these things,” Dr. Oliveros said.
In all countries, paternity leave was uncommon (2.6% of respondents), even though 48.5% of the programs had paternity leave.
“I would like to see associations, program directors, even trainees helping each other in finding ways to accommodate parents to promote wellness and assure that trainees can have both good training and life balance,” she added.
In an accompanying editorial, Ileana L. Piña, MD, MPH, Thomas Jefferson Institute, Philadelphia, writes: “Enough has been said about our need for a greater percentage of women cardiologists. There is no need to further debate that fact. However, it is puzzling that despite > 50% of medical students being women, the cardiology specialty is fraught with recent survey reports of hostility in the workplace, concerns of long hours, exposure to radiation, and poor work-life balance that can compel trainees to choose delaying pregnancy or taking unpaid leave, which will, in turn, delay training. Therefore, it is not surprising that only 14.9% of cardiologist specialists and 21.9% of cardiology fellows are women.”
Dr. Piña notes that while the authors understand that it’s difficult to change national policies, they issue a “call to action” for organizations and program directors to demonstrate leadership by developing fair and balanced decisions regarding parental policies.
“Those decisions are so impactful that they can change career trajectories for the better or worse ... the current status is unacceptable and must change for the benefit of all trainees, their families, and the program directors. The problem is too important and pervasive,” she adds.
Dr. Piña concludes: “Perhaps if the women who are the subjects of, and often the unwitting party to, administrative decisions about their lives, choices, and welfare were invited to contribute to the changes, we would finally see an increase in the number of women in cardiology careers. After all, aren’t we about diversity and belonging?”
“We need to normalize pregnancy and parental leave across the globe,” Laxmi S. Mehta, MD, Ohio State University Weiner Medical Center, Columbus, said in an interview.
As previously reported, Dr. Mehta recently led a study that surveyed 323 women cardiologists who were working while they were pregnant. Her study found that 75% of these women experienced discriminatory maternity leave practices, some of which were likely violations of the federal Family and Medical Leave Act.
“If we want more women to pursue a career in cardiology, then employers and health systems need to adequately support parenthood, including allowing people to spend uninterrupted time with their newborns without the fear of discrimination, retaliation, or financial burden,” Dr. Mehta said.
Limitations of the study are the small sample size, potential for bias associated with social media distribution, and the fact that 75% of respondents were women, Dr. Oliveros and colleagues write.
Dr. Oliveros, Dr. Piña, and Dr. Mehta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The first international survey of parental benefits and policies among cardiovascular training programs shows wide variability among institutions.
Although a majority of cardiology fellows became parents during training, the survey found that family benefits and policies were not uniformly available and that knowledge about the existence of such policies was low across all institutions.
The findings are published in the Journal of the American College of Cardiology.
Such variability highlights disparities in real-world experiences, say Estefania Oliveros, MD, Temple University Hospital, Philadelphia, and colleagues.
“There are no policies to protect cardiology trainees when they become parents that are uniform across the United States or even internationally, even though, according to our survey, 61.7% become parents during training,” Dr. Oliveros told this news organization.
Dr. Oliveros said she wanted to learn more about the status of institutional practices surrounding pregnant trainees during cardiovascular fellowship, not only in the U.S., but internationally: “I wanted to study this because of my own experience.”
“I was probably the first pregnant trainee at my institution, and there were no specific policies in place, so I had to find out on my own what to do about radiation safety, where I would breastfeed, schedule changes, how that would impact my graduation time, things like that,” Dr. Oliveros said. “It would be nice if you had the resources and your institution could accommodate your needs, instead of every time you have a pregnant person on your staff, you have to reinvent the wheel.”
Dr. Oliveros and colleagues conducted an online survey during August 2020-October 2020 that was distributed via social media. Responses were made anonymous to encourage unbiased feedback.
Among the 417 completed responses, 47 (11.3%) were from training program directors, 146 (35%) from current or former pregnant trainees, and 224 (53.7%) from current or former trainees who were not pregnant during cardiology training. Two-thirds of the respondents (67.1%) were parents.
Most survey respondents said they became pregnant during the third year of general cardiology (29.1%), followed by the first year of general cardiology (26.3%), and the second year of general cardiology (23.5%).
Only 13 of the 47 training program directors (27.7%) received guidance or training on how to accommodate pregnant trainees during fellowship.
Additionally, 26% of the trainees reported their institution had readily available breastfeeding and pumping policies, 39% responded that their institution had no such policies, and 34.9% said they did not know.
Nearly one-half of the programs offered rearrangement of schedules because of radiation concerns, 27.5% did not.
The amount of parental leave varied greatly worldwide. For Europe, Central and South America, Africa, and Australia, the average parental leave was more than 4 months; for Canada, it was more than 3 months; for the United States, it was 1 to 2 months; and for Asia, it was 3 to 4 weeks.
“There is no uniformity, no policies for things like breastfeeding or places where you can pump. None of that is installed, even though by law we’re supposed to have these things,” Dr. Oliveros said.
In all countries, paternity leave was uncommon (2.6% of respondents), even though 48.5% of the programs had paternity leave.
“I would like to see associations, program directors, even trainees helping each other in finding ways to accommodate parents to promote wellness and assure that trainees can have both good training and life balance,” she added.
In an accompanying editorial, Ileana L. Piña, MD, MPH, Thomas Jefferson Institute, Philadelphia, writes: “Enough has been said about our need for a greater percentage of women cardiologists. There is no need to further debate that fact. However, it is puzzling that despite > 50% of medical students being women, the cardiology specialty is fraught with recent survey reports of hostility in the workplace, concerns of long hours, exposure to radiation, and poor work-life balance that can compel trainees to choose delaying pregnancy or taking unpaid leave, which will, in turn, delay training. Therefore, it is not surprising that only 14.9% of cardiologist specialists and 21.9% of cardiology fellows are women.”
Dr. Piña notes that while the authors understand that it’s difficult to change national policies, they issue a “call to action” for organizations and program directors to demonstrate leadership by developing fair and balanced decisions regarding parental policies.
“Those decisions are so impactful that they can change career trajectories for the better or worse ... the current status is unacceptable and must change for the benefit of all trainees, their families, and the program directors. The problem is too important and pervasive,” she adds.
Dr. Piña concludes: “Perhaps if the women who are the subjects of, and often the unwitting party to, administrative decisions about their lives, choices, and welfare were invited to contribute to the changes, we would finally see an increase in the number of women in cardiology careers. After all, aren’t we about diversity and belonging?”
“We need to normalize pregnancy and parental leave across the globe,” Laxmi S. Mehta, MD, Ohio State University Weiner Medical Center, Columbus, said in an interview.
As previously reported, Dr. Mehta recently led a study that surveyed 323 women cardiologists who were working while they were pregnant. Her study found that 75% of these women experienced discriminatory maternity leave practices, some of which were likely violations of the federal Family and Medical Leave Act.
“If we want more women to pursue a career in cardiology, then employers and health systems need to adequately support parenthood, including allowing people to spend uninterrupted time with their newborns without the fear of discrimination, retaliation, or financial burden,” Dr. Mehta said.
Limitations of the study are the small sample size, potential for bias associated with social media distribution, and the fact that 75% of respondents were women, Dr. Oliveros and colleagues write.
Dr. Oliveros, Dr. Piña, and Dr. Mehta report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Legislative efforts continue to revamp laws governing PAs
INDIANAPOLIS – That’s according to Phil Bongiorno, BA, senior vice president of advocacy and government relations at the American Academy of Physician Associates (AAPA), who spoke at the group’s annual meeting.
OTP refers to the AAPA’s goal of improving patient access to care and lessening administrative obligations by eliminating the legal requirement that there be a specific relationship between a PA, physician, or any other health care provider. This would allow a PA to practice to the full extent of their education, training, and experience, Mr. Bongiorno said.
The second tenet of OTP is to persuade states to create a separate majority PA board to regulate PAs. An alternative to this would be for states to add PAs and physicians who work with PAs to their medical or healing arts boards, he said.
Third, in an OTP environment, each state would authorize PAs to be eligible for direct payment by all public and private insurers. “We have seen that development at the federal level, as far as Medicare is concerned,” Mr. Bongiorno said. “Now, we’re focusing on making that happen in the individual states as well.”
According to Mr. Bongiorno, this year’s state advocacy priorities are to pursue new legislation in additional states, even as efforts continue to persuade state legislatures to act on carryover bills from the previous legislative session.
Mr. Bongiorno briefly summarized what he called “OTP successes” from 2021:
- Federal government: Authorized direct payment to PAs under Medicare
- Arkansas, Delaware, Illinois, Pennsylvania: Added one or more PAs to their medical boards
- Florida, Utah: Approved direct payment to PAs
- Tennessee, Wisconsin: Created a separate PA review board
- Utah, Wisconsin: Removed the relationship/agreement requirement (Wisconsin now requires 10,000 hours of practice to remove the relationship requirement)
North Central region
In Colorado, House Bill 1095 (HB1095) would have removed requirements for a legal relationship between a PA and a physician. Initially that would have happened after 3,000 hours of practice, although changing that to 5,000 hours has been a compromise measure. PAs changing specialties must collaborate for 2,000 hours, now negotiated to 3,000 hours.
HB1095 ultimately was not successful last year or this year, said Erika Miller, director of state advocacy and outreach for the AAPA. “But we do see it as a success, because in the 2022 session, we managed to get it passed in committee by a 10-to-1 vote,” she said. “It then moved to the full house and was not successful there.”
Ms. Miller said that South Dakota Senate Bill 134 would have removed the requirement for a legal PA/physician relationship after 1,040 hours, which is the requirement for nurse practitioners. “South Dakota had introduced similar legislation the year before, but also like Colorado, they went from not getting out of committee last year to making it to the senate floor this time,” she said.
In Wisconsin, the new PA-affiliated credentialing board began on April 1. It gives PAs the authority to license, discipline, and write regulations, Ms. Miller said.
South Central region
Arizona Senate Bill 1367 included direct pay, removed the relationship tether with a physician, and made each PA fully responsible for the care they provide. “The bill passed out of committee successfully but did not make it to a vote due to unexpected struggles between the Arizona medical society and PA chapter,” said Shannon Morey, senior director of state advocacy and outreach at the AAPA. “They are ready to go again next year.”
In Louisiana, Senate Bill 158 is a “strong” bill that addressed all the desired aspects of OTP, Ms. Morey said; “The legislation stands subject to call on the Senate floor, but it has been killed by the sponsor.”
Northeast region
Massachusetts Senate Bill 740 (S740) would remove the legal tether between PA and physician, said Carson Walker, senior director of state advocacy and outreach at the AAPA. “The committee decided to extend its time in committee until June,” he said. “By next month, we expect that the committee will schedule a hearing that includes S740, and we fully plan on submitting testimony.”
In New York, Senate Bill 9233 (S9233) would remove physician supervision after 3,600 hours of practice.
“Just about 10 days ago, sponsors were able to have S9233 introduced, which is the most succinct and, I think, the most effective OTP bill I have ever seen,” Mr. Walker said.
“S9233 says that after 3,600 hours a PA can practice without the supervision of a physician, and that’s all. There’s not a lot of time left in this session, but we are hopeful that it lays the groundwork for success next year.”
New Hampshire Senate Bill 228 has passed the legislature and is awaiting the governor’s signature. It will allow direct payment, make PAs responsible for the care they provide, and shift the physician-PA relationship from supervision to collaboration, Mr. Walker said.
Southeast region
Stephanie Radix, senior director of state advocacy and outreach at the AAPA, discussed North Carolina’s Senate Bill 345, which passed the Senate unanimously in 2021 and has been carried over to this year’s session. The bill defines team-based settings, eliminates the relationship tether, and establishes a supervised career entry interval of 4,000 clinical hours in the state.
The legislature is slated to adjourn June 30, Ms. Radix said: “We are very hopeful that we will get it across the finish line.”
In an interview, Mr. Bongiorno said that the AAPA’s overall advocacy progress is as expected.
“Optimal team practice is about allowing each practice to make that determination on how the team should work as a true collaboration,” he said. “The bottom line is that OTP would allow us to reach more patients, serve the community, and ensure that people are able to get healthcare, especially in underserved areas.”
A version of this article first appeared on Medscape.com.
INDIANAPOLIS – That’s according to Phil Bongiorno, BA, senior vice president of advocacy and government relations at the American Academy of Physician Associates (AAPA), who spoke at the group’s annual meeting.
OTP refers to the AAPA’s goal of improving patient access to care and lessening administrative obligations by eliminating the legal requirement that there be a specific relationship between a PA, physician, or any other health care provider. This would allow a PA to practice to the full extent of their education, training, and experience, Mr. Bongiorno said.
The second tenet of OTP is to persuade states to create a separate majority PA board to regulate PAs. An alternative to this would be for states to add PAs and physicians who work with PAs to their medical or healing arts boards, he said.
Third, in an OTP environment, each state would authorize PAs to be eligible for direct payment by all public and private insurers. “We have seen that development at the federal level, as far as Medicare is concerned,” Mr. Bongiorno said. “Now, we’re focusing on making that happen in the individual states as well.”
According to Mr. Bongiorno, this year’s state advocacy priorities are to pursue new legislation in additional states, even as efforts continue to persuade state legislatures to act on carryover bills from the previous legislative session.
Mr. Bongiorno briefly summarized what he called “OTP successes” from 2021:
- Federal government: Authorized direct payment to PAs under Medicare
- Arkansas, Delaware, Illinois, Pennsylvania: Added one or more PAs to their medical boards
- Florida, Utah: Approved direct payment to PAs
- Tennessee, Wisconsin: Created a separate PA review board
- Utah, Wisconsin: Removed the relationship/agreement requirement (Wisconsin now requires 10,000 hours of practice to remove the relationship requirement)
North Central region
In Colorado, House Bill 1095 (HB1095) would have removed requirements for a legal relationship between a PA and a physician. Initially that would have happened after 3,000 hours of practice, although changing that to 5,000 hours has been a compromise measure. PAs changing specialties must collaborate for 2,000 hours, now negotiated to 3,000 hours.
HB1095 ultimately was not successful last year or this year, said Erika Miller, director of state advocacy and outreach for the AAPA. “But we do see it as a success, because in the 2022 session, we managed to get it passed in committee by a 10-to-1 vote,” she said. “It then moved to the full house and was not successful there.”
Ms. Miller said that South Dakota Senate Bill 134 would have removed the requirement for a legal PA/physician relationship after 1,040 hours, which is the requirement for nurse practitioners. “South Dakota had introduced similar legislation the year before, but also like Colorado, they went from not getting out of committee last year to making it to the senate floor this time,” she said.
In Wisconsin, the new PA-affiliated credentialing board began on April 1. It gives PAs the authority to license, discipline, and write regulations, Ms. Miller said.
South Central region
Arizona Senate Bill 1367 included direct pay, removed the relationship tether with a physician, and made each PA fully responsible for the care they provide. “The bill passed out of committee successfully but did not make it to a vote due to unexpected struggles between the Arizona medical society and PA chapter,” said Shannon Morey, senior director of state advocacy and outreach at the AAPA. “They are ready to go again next year.”
In Louisiana, Senate Bill 158 is a “strong” bill that addressed all the desired aspects of OTP, Ms. Morey said; “The legislation stands subject to call on the Senate floor, but it has been killed by the sponsor.”
Northeast region
Massachusetts Senate Bill 740 (S740) would remove the legal tether between PA and physician, said Carson Walker, senior director of state advocacy and outreach at the AAPA. “The committee decided to extend its time in committee until June,” he said. “By next month, we expect that the committee will schedule a hearing that includes S740, and we fully plan on submitting testimony.”
In New York, Senate Bill 9233 (S9233) would remove physician supervision after 3,600 hours of practice.
“Just about 10 days ago, sponsors were able to have S9233 introduced, which is the most succinct and, I think, the most effective OTP bill I have ever seen,” Mr. Walker said.
“S9233 says that after 3,600 hours a PA can practice without the supervision of a physician, and that’s all. There’s not a lot of time left in this session, but we are hopeful that it lays the groundwork for success next year.”
New Hampshire Senate Bill 228 has passed the legislature and is awaiting the governor’s signature. It will allow direct payment, make PAs responsible for the care they provide, and shift the physician-PA relationship from supervision to collaboration, Mr. Walker said.
Southeast region
Stephanie Radix, senior director of state advocacy and outreach at the AAPA, discussed North Carolina’s Senate Bill 345, which passed the Senate unanimously in 2021 and has been carried over to this year’s session. The bill defines team-based settings, eliminates the relationship tether, and establishes a supervised career entry interval of 4,000 clinical hours in the state.
The legislature is slated to adjourn June 30, Ms. Radix said: “We are very hopeful that we will get it across the finish line.”
In an interview, Mr. Bongiorno said that the AAPA’s overall advocacy progress is as expected.
“Optimal team practice is about allowing each practice to make that determination on how the team should work as a true collaboration,” he said. “The bottom line is that OTP would allow us to reach more patients, serve the community, and ensure that people are able to get healthcare, especially in underserved areas.”
A version of this article first appeared on Medscape.com.
INDIANAPOLIS – That’s according to Phil Bongiorno, BA, senior vice president of advocacy and government relations at the American Academy of Physician Associates (AAPA), who spoke at the group’s annual meeting.
OTP refers to the AAPA’s goal of improving patient access to care and lessening administrative obligations by eliminating the legal requirement that there be a specific relationship between a PA, physician, or any other health care provider. This would allow a PA to practice to the full extent of their education, training, and experience, Mr. Bongiorno said.
The second tenet of OTP is to persuade states to create a separate majority PA board to regulate PAs. An alternative to this would be for states to add PAs and physicians who work with PAs to their medical or healing arts boards, he said.
Third, in an OTP environment, each state would authorize PAs to be eligible for direct payment by all public and private insurers. “We have seen that development at the federal level, as far as Medicare is concerned,” Mr. Bongiorno said. “Now, we’re focusing on making that happen in the individual states as well.”
According to Mr. Bongiorno, this year’s state advocacy priorities are to pursue new legislation in additional states, even as efforts continue to persuade state legislatures to act on carryover bills from the previous legislative session.
Mr. Bongiorno briefly summarized what he called “OTP successes” from 2021:
- Federal government: Authorized direct payment to PAs under Medicare
- Arkansas, Delaware, Illinois, Pennsylvania: Added one or more PAs to their medical boards
- Florida, Utah: Approved direct payment to PAs
- Tennessee, Wisconsin: Created a separate PA review board
- Utah, Wisconsin: Removed the relationship/agreement requirement (Wisconsin now requires 10,000 hours of practice to remove the relationship requirement)
North Central region
In Colorado, House Bill 1095 (HB1095) would have removed requirements for a legal relationship between a PA and a physician. Initially that would have happened after 3,000 hours of practice, although changing that to 5,000 hours has been a compromise measure. PAs changing specialties must collaborate for 2,000 hours, now negotiated to 3,000 hours.
HB1095 ultimately was not successful last year or this year, said Erika Miller, director of state advocacy and outreach for the AAPA. “But we do see it as a success, because in the 2022 session, we managed to get it passed in committee by a 10-to-1 vote,” she said. “It then moved to the full house and was not successful there.”
Ms. Miller said that South Dakota Senate Bill 134 would have removed the requirement for a legal PA/physician relationship after 1,040 hours, which is the requirement for nurse practitioners. “South Dakota had introduced similar legislation the year before, but also like Colorado, they went from not getting out of committee last year to making it to the senate floor this time,” she said.
In Wisconsin, the new PA-affiliated credentialing board began on April 1. It gives PAs the authority to license, discipline, and write regulations, Ms. Miller said.
South Central region
Arizona Senate Bill 1367 included direct pay, removed the relationship tether with a physician, and made each PA fully responsible for the care they provide. “The bill passed out of committee successfully but did not make it to a vote due to unexpected struggles between the Arizona medical society and PA chapter,” said Shannon Morey, senior director of state advocacy and outreach at the AAPA. “They are ready to go again next year.”
In Louisiana, Senate Bill 158 is a “strong” bill that addressed all the desired aspects of OTP, Ms. Morey said; “The legislation stands subject to call on the Senate floor, but it has been killed by the sponsor.”
Northeast region
Massachusetts Senate Bill 740 (S740) would remove the legal tether between PA and physician, said Carson Walker, senior director of state advocacy and outreach at the AAPA. “The committee decided to extend its time in committee until June,” he said. “By next month, we expect that the committee will schedule a hearing that includes S740, and we fully plan on submitting testimony.”
In New York, Senate Bill 9233 (S9233) would remove physician supervision after 3,600 hours of practice.
“Just about 10 days ago, sponsors were able to have S9233 introduced, which is the most succinct and, I think, the most effective OTP bill I have ever seen,” Mr. Walker said.
“S9233 says that after 3,600 hours a PA can practice without the supervision of a physician, and that’s all. There’s not a lot of time left in this session, but we are hopeful that it lays the groundwork for success next year.”
New Hampshire Senate Bill 228 has passed the legislature and is awaiting the governor’s signature. It will allow direct payment, make PAs responsible for the care they provide, and shift the physician-PA relationship from supervision to collaboration, Mr. Walker said.
Southeast region
Stephanie Radix, senior director of state advocacy and outreach at the AAPA, discussed North Carolina’s Senate Bill 345, which passed the Senate unanimously in 2021 and has been carried over to this year’s session. The bill defines team-based settings, eliminates the relationship tether, and establishes a supervised career entry interval of 4,000 clinical hours in the state.
The legislature is slated to adjourn June 30, Ms. Radix said: “We are very hopeful that we will get it across the finish line.”
In an interview, Mr. Bongiorno said that the AAPA’s overall advocacy progress is as expected.
“Optimal team practice is about allowing each practice to make that determination on how the team should work as a true collaboration,” he said. “The bottom line is that OTP would allow us to reach more patients, serve the community, and ensure that people are able to get healthcare, especially in underserved areas.”
A version of this article first appeared on Medscape.com.
AT AAPA 2022
$7,000 for ‘flowers’: KY doc accused in murder plot against ex
A Kentucky pediatrician accused of hiring a hitman to kill her ex-husband – and type a fake suicide text on his cell phone to disguise the plot – initially hatched the scheme 4 years ago during a custody dispute, according to court documents.
On May 19, agents with the Federal Bureau of Investigation arrested Stephanie Russell, MD, on a charge of using interstate commerce facilities in the commission of murder-for-hire, which carries a maximum 10-year sentence in federal prison.
Dr. Russell, who prosecutors said is 52, vehemently denied the plot when it was first relayed to investigators in 2020. She also dismissed suspicion from a court-appointed guardian at the time that the doctor harmed her own son, then 2, in a way “to make it appear” as if his father had hurt the child.
According to an FBI agent’s affidavit, Dr. Russell tried to recruit a killer through employees and ex-employees of Kidz Life Pediatrics, in Prospect, an upscale suburb of Louisville, Ky. She allegedly planned to time the murder during a 2-hour visitation period with her two children on the last day of the school year.
On May 24, Magistrate Judge Regina Edwards, of the U.S. District Court for the Western District of Kentucky, ordered Dr. Russell to remain in custody. A future date for the next hearing has not been set.
‘No red flags’
The case has upended the Norton Commons development in Prospect, one of Kentucky’s wealthiest communities.
“There were no red flags,” said Lance Dooley, whose two daughters had been under Dr. Russell’s care at Kidz Life. “This neighborhood was like, ‘What the hell?’ Everybody went to her and trusted and respected her judgment.”
According to prosecutors, on May 15 – after having failed to have her ex-husband murdered during the holidays – Dr. Russell contacted a person she thought she had hired to murder her ex-husband in exchange for $7,000.
On May 18, Dr. Russell placed a $3,500 down payment in a specimen drop box outside her medical office. She agreed to pay the remaining half after the murder was done, according to prosecutors. The purported hit man was an undercover FBI agent.
While making plans, Dr. Russell used several burner phones and used the word “flowers” as a code word for killing her ex-husband, Ricky Crabtree, whom she had accused of sexually abusing their children. Mr. Crabtree, a financial planner, did not return phone messages left at his office.
Family Court Judge Denise Brown had earlier appointed a guardian to represent the children and an evaluator to monitor the couple’s custodial issues.
Dr. Russell sued the judge, saying Ms. Brown acted because of allegations that Dr. Russell was “coaching” her children and inflicting “emotional harm.” Dr. Russell also objected to what she called “a vague suggestion” that previously she “‘may’ have injured the older male child in a way to make it appear that [Mr.] Crabtree had done so.”
“There wasn’t any proof of it,” said David Mour, an attorney who represented Dr. Russell in that action. The state gave custody to the father in what Mr. Mour called a “Star Chamber” action based on unsubstantiated allegations. “I don’t believe a damned thing,” he said.
In her suit against Ms. Brown, which was dismissed in 2021, Dr. Russell criticized as “preposterous” allegations that, in May 2018, she “‘attempted to hire’ a ‘hitman’ to kill [Mr.] Crabtree.”
The FBI affidavit, however, displayed numerous text messages between Dr. Russell and a former nurse, whom she thought knew a hit man, and an FBI agent posing as the purported killer. When one witness initially agreed to find an assassin who would do the job over the 2021 holiday season, Dr. Russell texted, “I am hysterically crying tears of relief.”
The witness quit Kidz Life Pediatrics and ended contact with Dr. Russell when they realized Dr. Russell was “serious” about the plot, the affidavit stated. And when Dr. Russell found a willing contractor in May, she told the hitman to write a suicide text. The killer would have to unlock Mr. Crabtree’s cell phone by having the device recognize the face of his dead body.
Mr. Dooley said Kidz Life Pediatrics was closed during business hours when he tried to retrieve his children’s medical records. He has since found another pediatrician. Dr. Russell had cared for his children for more than 4 years, he said, betraying no clue of any darkness underneath. Kidz Life Pediatrics did not return phone calls seeking comment.
“It’s very close to home,” said Mr. Dooley, who runs an advertising agency with his wife. “Dr. Russell was really good.”
A version of this article first appeared on Medscape.com.
A Kentucky pediatrician accused of hiring a hitman to kill her ex-husband – and type a fake suicide text on his cell phone to disguise the plot – initially hatched the scheme 4 years ago during a custody dispute, according to court documents.
On May 19, agents with the Federal Bureau of Investigation arrested Stephanie Russell, MD, on a charge of using interstate commerce facilities in the commission of murder-for-hire, which carries a maximum 10-year sentence in federal prison.
Dr. Russell, who prosecutors said is 52, vehemently denied the plot when it was first relayed to investigators in 2020. She also dismissed suspicion from a court-appointed guardian at the time that the doctor harmed her own son, then 2, in a way “to make it appear” as if his father had hurt the child.
According to an FBI agent’s affidavit, Dr. Russell tried to recruit a killer through employees and ex-employees of Kidz Life Pediatrics, in Prospect, an upscale suburb of Louisville, Ky. She allegedly planned to time the murder during a 2-hour visitation period with her two children on the last day of the school year.
On May 24, Magistrate Judge Regina Edwards, of the U.S. District Court for the Western District of Kentucky, ordered Dr. Russell to remain in custody. A future date for the next hearing has not been set.
‘No red flags’
The case has upended the Norton Commons development in Prospect, one of Kentucky’s wealthiest communities.
“There were no red flags,” said Lance Dooley, whose two daughters had been under Dr. Russell’s care at Kidz Life. “This neighborhood was like, ‘What the hell?’ Everybody went to her and trusted and respected her judgment.”
According to prosecutors, on May 15 – after having failed to have her ex-husband murdered during the holidays – Dr. Russell contacted a person she thought she had hired to murder her ex-husband in exchange for $7,000.
On May 18, Dr. Russell placed a $3,500 down payment in a specimen drop box outside her medical office. She agreed to pay the remaining half after the murder was done, according to prosecutors. The purported hit man was an undercover FBI agent.
While making plans, Dr. Russell used several burner phones and used the word “flowers” as a code word for killing her ex-husband, Ricky Crabtree, whom she had accused of sexually abusing their children. Mr. Crabtree, a financial planner, did not return phone messages left at his office.
Family Court Judge Denise Brown had earlier appointed a guardian to represent the children and an evaluator to monitor the couple’s custodial issues.
Dr. Russell sued the judge, saying Ms. Brown acted because of allegations that Dr. Russell was “coaching” her children and inflicting “emotional harm.” Dr. Russell also objected to what she called “a vague suggestion” that previously she “‘may’ have injured the older male child in a way to make it appear that [Mr.] Crabtree had done so.”
“There wasn’t any proof of it,” said David Mour, an attorney who represented Dr. Russell in that action. The state gave custody to the father in what Mr. Mour called a “Star Chamber” action based on unsubstantiated allegations. “I don’t believe a damned thing,” he said.
In her suit against Ms. Brown, which was dismissed in 2021, Dr. Russell criticized as “preposterous” allegations that, in May 2018, she “‘attempted to hire’ a ‘hitman’ to kill [Mr.] Crabtree.”
The FBI affidavit, however, displayed numerous text messages between Dr. Russell and a former nurse, whom she thought knew a hit man, and an FBI agent posing as the purported killer. When one witness initially agreed to find an assassin who would do the job over the 2021 holiday season, Dr. Russell texted, “I am hysterically crying tears of relief.”
The witness quit Kidz Life Pediatrics and ended contact with Dr. Russell when they realized Dr. Russell was “serious” about the plot, the affidavit stated. And when Dr. Russell found a willing contractor in May, she told the hitman to write a suicide text. The killer would have to unlock Mr. Crabtree’s cell phone by having the device recognize the face of his dead body.
Mr. Dooley said Kidz Life Pediatrics was closed during business hours when he tried to retrieve his children’s medical records. He has since found another pediatrician. Dr. Russell had cared for his children for more than 4 years, he said, betraying no clue of any darkness underneath. Kidz Life Pediatrics did not return phone calls seeking comment.
“It’s very close to home,” said Mr. Dooley, who runs an advertising agency with his wife. “Dr. Russell was really good.”
A version of this article first appeared on Medscape.com.
A Kentucky pediatrician accused of hiring a hitman to kill her ex-husband – and type a fake suicide text on his cell phone to disguise the plot – initially hatched the scheme 4 years ago during a custody dispute, according to court documents.
On May 19, agents with the Federal Bureau of Investigation arrested Stephanie Russell, MD, on a charge of using interstate commerce facilities in the commission of murder-for-hire, which carries a maximum 10-year sentence in federal prison.
Dr. Russell, who prosecutors said is 52, vehemently denied the plot when it was first relayed to investigators in 2020. She also dismissed suspicion from a court-appointed guardian at the time that the doctor harmed her own son, then 2, in a way “to make it appear” as if his father had hurt the child.
According to an FBI agent’s affidavit, Dr. Russell tried to recruit a killer through employees and ex-employees of Kidz Life Pediatrics, in Prospect, an upscale suburb of Louisville, Ky. She allegedly planned to time the murder during a 2-hour visitation period with her two children on the last day of the school year.
On May 24, Magistrate Judge Regina Edwards, of the U.S. District Court for the Western District of Kentucky, ordered Dr. Russell to remain in custody. A future date for the next hearing has not been set.
‘No red flags’
The case has upended the Norton Commons development in Prospect, one of Kentucky’s wealthiest communities.
“There were no red flags,” said Lance Dooley, whose two daughters had been under Dr. Russell’s care at Kidz Life. “This neighborhood was like, ‘What the hell?’ Everybody went to her and trusted and respected her judgment.”
According to prosecutors, on May 15 – after having failed to have her ex-husband murdered during the holidays – Dr. Russell contacted a person she thought she had hired to murder her ex-husband in exchange for $7,000.
On May 18, Dr. Russell placed a $3,500 down payment in a specimen drop box outside her medical office. She agreed to pay the remaining half after the murder was done, according to prosecutors. The purported hit man was an undercover FBI agent.
While making plans, Dr. Russell used several burner phones and used the word “flowers” as a code word for killing her ex-husband, Ricky Crabtree, whom she had accused of sexually abusing their children. Mr. Crabtree, a financial planner, did not return phone messages left at his office.
Family Court Judge Denise Brown had earlier appointed a guardian to represent the children and an evaluator to monitor the couple’s custodial issues.
Dr. Russell sued the judge, saying Ms. Brown acted because of allegations that Dr. Russell was “coaching” her children and inflicting “emotional harm.” Dr. Russell also objected to what she called “a vague suggestion” that previously she “‘may’ have injured the older male child in a way to make it appear that [Mr.] Crabtree had done so.”
“There wasn’t any proof of it,” said David Mour, an attorney who represented Dr. Russell in that action. The state gave custody to the father in what Mr. Mour called a “Star Chamber” action based on unsubstantiated allegations. “I don’t believe a damned thing,” he said.
In her suit against Ms. Brown, which was dismissed in 2021, Dr. Russell criticized as “preposterous” allegations that, in May 2018, she “‘attempted to hire’ a ‘hitman’ to kill [Mr.] Crabtree.”
The FBI affidavit, however, displayed numerous text messages between Dr. Russell and a former nurse, whom she thought knew a hit man, and an FBI agent posing as the purported killer. When one witness initially agreed to find an assassin who would do the job over the 2021 holiday season, Dr. Russell texted, “I am hysterically crying tears of relief.”
The witness quit Kidz Life Pediatrics and ended contact with Dr. Russell when they realized Dr. Russell was “serious” about the plot, the affidavit stated. And when Dr. Russell found a willing contractor in May, she told the hitman to write a suicide text. The killer would have to unlock Mr. Crabtree’s cell phone by having the device recognize the face of his dead body.
Mr. Dooley said Kidz Life Pediatrics was closed during business hours when he tried to retrieve his children’s medical records. He has since found another pediatrician. Dr. Russell had cared for his children for more than 4 years, he said, betraying no clue of any darkness underneath. Kidz Life Pediatrics did not return phone calls seeking comment.
“It’s very close to home,” said Mr. Dooley, who runs an advertising agency with his wife. “Dr. Russell was really good.”
A version of this article first appeared on Medscape.com.
Will ‘gold card’ legislation and others rein in prior authorizations?
I live in New Orleans and recently became aware of a piece of state legislation that would create a “gold card” system for prior authorizations in Louisiana. Before delving into what is a gold card and how it works, let’s take a look at the evolution of prior authorizations (PAs).
Commercial health insurance and Medicare/Medicaid had their beginnings in the 1950s and 1960s. Because the government would now be paying for medical services for seniors, there was a concern that there might be an “overutilization” of services. This concern resulted in the concepts of utilization review and “medical necessity.” These utilization reviews morphed into what are now known as utilization management tools (UMTs). The original intent of these tools was to link cost containment to quality assurance.
PAs are one of a number of UMTs, along with formulary step therapy and nonmedical switching, that are used by health insurance companies and pharmacy benefit managers to determine whether a prescribed product or service is medically necessary and cost effective. Originally, it also meant that the service/treatment would be reimbursed. That is not the case anymore.
Today, physicians face many frivolous PAs for generic medications, such as methotrexate and prednisone, and ironically sometimes higher-priced drugs are preferred over lower-priced ones.
A number of surveys, including a recent one of more than 1,000 specialty physicians by the Alliance of Specialty Medicine, show that PAs are not only a significant administrative burden on practices but also harm patients with significant delays in accessing needed treatments and diagnostic services.
The often-cited study by Zachary Wallace et al. clearly demonstrates significant harm to rheumatology patients whose treatments were delayed because of PAs. These delays caused a substantial increase in steroid dosages in patients whose PA was initially denied and even in those patients whose PAs were initially approved. These data and others support the urgent need to address the entire spectrum of PAs.
Over the last few years, we have seen many states passing laws, adding common-sense protections to mitigate the harmful consequences of UMTs. Such reforms are needed now to stop the indiscriminate use of PAs. Suggestions have included completely eliminating PAs for medications and services that are consistently approved, standardizing electronic forms across all health plans with real-time approval, and others, including “gold card” legislation. In addition to states’ efforts, Congress proposed H.R. 3173, the Improving Seniors’ Timely Access to Care Act of 2021, to protect seniors from the harm caused by PAs that are required by Medicare Advantage programs.
This brings us to the topic of gold card legislation, in which physicians would be given a gold card exempting them from PA for specific services (hopefully including prescription drugs). However, the criteria a physician needs to qualify for a gold card could vary from state to state. For example, it could be based on a physician’s PA approval rate during a specified review period, or it could be completely up to the insurance company to decide the criteria.
Texas is the only state that has passed gold card legislation thus far, although there is an active gold card bill in Louisiana (as of this writing). There are a few other states that have introduced gold card bills that have not yet passed, but there is definite interest throughout the country in this concept. In the Texas legislation, physicians would qualify for a gold card if they had a PA approval threshold of 90% for specific medications or services over a 6-month review period.
A few of the concerns about how this will be implemented and the potential unintended consequences of the legislation include:
- Would one gold card cover all drugs, a specific drug, or just a specific drug for a specific diagnosis?
- Will clinicians get bogged down appealing gold card denials/rescissions?
- Will health plans begin denying more requests up front to keep clinicians from qualifying for an exemption?
Unfortunately, the Louisiana gold card legislation has been amended from its original form to exclude “pharmacy services” and qualification for the gold card “shall be at the sole discretion of the health insurance issuer.”
Consequently, my initial excitement surrounding the Louisiana gold card legislation, for our specialty, has for the most part disappeared. Nonetheless, there is clear excitement behind the gold card concept throughout the country.
What is clear is that health insurance companies and pharmacy benefit managers have lost sight of the original purpose of UMTs, which is to ensure that patients have access to cost-effective quality care. Over the years, the aggressive use of PAs and other UMTs has led to a significant increase in administrative burden for our offices, and more importantly, a loss of disease control in many of our patients, resulting in an increase in overall health care costs.
While it is extremely disturbing that we need legislation to force health plans to keep our patients safe and ensure quality of care, it certainly proves that now, more than ever, we must make our voices be heard.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
I live in New Orleans and recently became aware of a piece of state legislation that would create a “gold card” system for prior authorizations in Louisiana. Before delving into what is a gold card and how it works, let’s take a look at the evolution of prior authorizations (PAs).
Commercial health insurance and Medicare/Medicaid had their beginnings in the 1950s and 1960s. Because the government would now be paying for medical services for seniors, there was a concern that there might be an “overutilization” of services. This concern resulted in the concepts of utilization review and “medical necessity.” These utilization reviews morphed into what are now known as utilization management tools (UMTs). The original intent of these tools was to link cost containment to quality assurance.
PAs are one of a number of UMTs, along with formulary step therapy and nonmedical switching, that are used by health insurance companies and pharmacy benefit managers to determine whether a prescribed product or service is medically necessary and cost effective. Originally, it also meant that the service/treatment would be reimbursed. That is not the case anymore.
Today, physicians face many frivolous PAs for generic medications, such as methotrexate and prednisone, and ironically sometimes higher-priced drugs are preferred over lower-priced ones.
A number of surveys, including a recent one of more than 1,000 specialty physicians by the Alliance of Specialty Medicine, show that PAs are not only a significant administrative burden on practices but also harm patients with significant delays in accessing needed treatments and diagnostic services.
The often-cited study by Zachary Wallace et al. clearly demonstrates significant harm to rheumatology patients whose treatments were delayed because of PAs. These delays caused a substantial increase in steroid dosages in patients whose PA was initially denied and even in those patients whose PAs were initially approved. These data and others support the urgent need to address the entire spectrum of PAs.
Over the last few years, we have seen many states passing laws, adding common-sense protections to mitigate the harmful consequences of UMTs. Such reforms are needed now to stop the indiscriminate use of PAs. Suggestions have included completely eliminating PAs for medications and services that are consistently approved, standardizing electronic forms across all health plans with real-time approval, and others, including “gold card” legislation. In addition to states’ efforts, Congress proposed H.R. 3173, the Improving Seniors’ Timely Access to Care Act of 2021, to protect seniors from the harm caused by PAs that are required by Medicare Advantage programs.
This brings us to the topic of gold card legislation, in which physicians would be given a gold card exempting them from PA for specific services (hopefully including prescription drugs). However, the criteria a physician needs to qualify for a gold card could vary from state to state. For example, it could be based on a physician’s PA approval rate during a specified review period, or it could be completely up to the insurance company to decide the criteria.
Texas is the only state that has passed gold card legislation thus far, although there is an active gold card bill in Louisiana (as of this writing). There are a few other states that have introduced gold card bills that have not yet passed, but there is definite interest throughout the country in this concept. In the Texas legislation, physicians would qualify for a gold card if they had a PA approval threshold of 90% for specific medications or services over a 6-month review period.
A few of the concerns about how this will be implemented and the potential unintended consequences of the legislation include:
- Would one gold card cover all drugs, a specific drug, or just a specific drug for a specific diagnosis?
- Will clinicians get bogged down appealing gold card denials/rescissions?
- Will health plans begin denying more requests up front to keep clinicians from qualifying for an exemption?
Unfortunately, the Louisiana gold card legislation has been amended from its original form to exclude “pharmacy services” and qualification for the gold card “shall be at the sole discretion of the health insurance issuer.”
Consequently, my initial excitement surrounding the Louisiana gold card legislation, for our specialty, has for the most part disappeared. Nonetheless, there is clear excitement behind the gold card concept throughout the country.
What is clear is that health insurance companies and pharmacy benefit managers have lost sight of the original purpose of UMTs, which is to ensure that patients have access to cost-effective quality care. Over the years, the aggressive use of PAs and other UMTs has led to a significant increase in administrative burden for our offices, and more importantly, a loss of disease control in many of our patients, resulting in an increase in overall health care costs.
While it is extremely disturbing that we need legislation to force health plans to keep our patients safe and ensure quality of care, it certainly proves that now, more than ever, we must make our voices be heard.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
I live in New Orleans and recently became aware of a piece of state legislation that would create a “gold card” system for prior authorizations in Louisiana. Before delving into what is a gold card and how it works, let’s take a look at the evolution of prior authorizations (PAs).
Commercial health insurance and Medicare/Medicaid had their beginnings in the 1950s and 1960s. Because the government would now be paying for medical services for seniors, there was a concern that there might be an “overutilization” of services. This concern resulted in the concepts of utilization review and “medical necessity.” These utilization reviews morphed into what are now known as utilization management tools (UMTs). The original intent of these tools was to link cost containment to quality assurance.
PAs are one of a number of UMTs, along with formulary step therapy and nonmedical switching, that are used by health insurance companies and pharmacy benefit managers to determine whether a prescribed product or service is medically necessary and cost effective. Originally, it also meant that the service/treatment would be reimbursed. That is not the case anymore.
Today, physicians face many frivolous PAs for generic medications, such as methotrexate and prednisone, and ironically sometimes higher-priced drugs are preferred over lower-priced ones.
A number of surveys, including a recent one of more than 1,000 specialty physicians by the Alliance of Specialty Medicine, show that PAs are not only a significant administrative burden on practices but also harm patients with significant delays in accessing needed treatments and diagnostic services.
The often-cited study by Zachary Wallace et al. clearly demonstrates significant harm to rheumatology patients whose treatments were delayed because of PAs. These delays caused a substantial increase in steroid dosages in patients whose PA was initially denied and even in those patients whose PAs were initially approved. These data and others support the urgent need to address the entire spectrum of PAs.
Over the last few years, we have seen many states passing laws, adding common-sense protections to mitigate the harmful consequences of UMTs. Such reforms are needed now to stop the indiscriminate use of PAs. Suggestions have included completely eliminating PAs for medications and services that are consistently approved, standardizing electronic forms across all health plans with real-time approval, and others, including “gold card” legislation. In addition to states’ efforts, Congress proposed H.R. 3173, the Improving Seniors’ Timely Access to Care Act of 2021, to protect seniors from the harm caused by PAs that are required by Medicare Advantage programs.
This brings us to the topic of gold card legislation, in which physicians would be given a gold card exempting them from PA for specific services (hopefully including prescription drugs). However, the criteria a physician needs to qualify for a gold card could vary from state to state. For example, it could be based on a physician’s PA approval rate during a specified review period, or it could be completely up to the insurance company to decide the criteria.
Texas is the only state that has passed gold card legislation thus far, although there is an active gold card bill in Louisiana (as of this writing). There are a few other states that have introduced gold card bills that have not yet passed, but there is definite interest throughout the country in this concept. In the Texas legislation, physicians would qualify for a gold card if they had a PA approval threshold of 90% for specific medications or services over a 6-month review period.
A few of the concerns about how this will be implemented and the potential unintended consequences of the legislation include:
- Would one gold card cover all drugs, a specific drug, or just a specific drug for a specific diagnosis?
- Will clinicians get bogged down appealing gold card denials/rescissions?
- Will health plans begin denying more requests up front to keep clinicians from qualifying for an exemption?
Unfortunately, the Louisiana gold card legislation has been amended from its original form to exclude “pharmacy services” and qualification for the gold card “shall be at the sole discretion of the health insurance issuer.”
Consequently, my initial excitement surrounding the Louisiana gold card legislation, for our specialty, has for the most part disappeared. Nonetheless, there is clear excitement behind the gold card concept throughout the country.
What is clear is that health insurance companies and pharmacy benefit managers have lost sight of the original purpose of UMTs, which is to ensure that patients have access to cost-effective quality care. Over the years, the aggressive use of PAs and other UMTs has led to a significant increase in administrative burden for our offices, and more importantly, a loss of disease control in many of our patients, resulting in an increase in overall health care costs.
While it is extremely disturbing that we need legislation to force health plans to keep our patients safe and ensure quality of care, it certainly proves that now, more than ever, we must make our voices be heard.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Downtime? Enjoy it
Everything in medicine, and pretty much the universe, is based on averages. Average reduction of seizures, average blood levels, average response to treatment, average insurance reimbursement, average time spent with a new consult.
Statistics are helpful in working through large amounts of data, but on a smaller scale, like my practice, statistics aren’t quite as helpful.
I see, on average, maybe 10 patients per day, consisting of new ones, follow-ups, and electromyography and nerve conduction velocity (EMG/NCV) studies. That is, by far, a smaller number of patients than my colleagues in primary care see, and probably other neurology practices as well. But it works for me.
But that’s on averages and not always. Sometimes we all hit slumps. Who knows why? Everyone is on vacation, or the holidays are coming, or they’ve been abducted by aliens. Whatever the reason, I get the occasional week where I’m pretty bored. Maybe one or two patients in a day. I start to feel like the lonely Maytag repairman behind my desk. I check to see if any drug samples have expired. I wonder if people are actually reading my online reviews and going elsewhere.
Years ago weeks like that terrified me. I was worried my little practice might fail (granted, it still could). But as years – and cycles that make up the averages – go by, they don’t bother me as much.
After 23 years I’ve learned that it’s just part of the normal fluctuations that make up an average. One morning I’ll roll the phones and the lines will explode (figuratively, I hope) with calls. At times like these my secretary seems to grow another pair of arms as she frantically schedules callers, puts others on hold, copies insurance cards, and gives the evil eye to drug reps who step in and ask her if she’s busy.
Then my schedule gets packed. My secretary crams patients in my emergency slots of 7:00, 8:00, and 12:00. MRI results come in that require me to see people sooner rather than later. My “average” of 10 patients per day suddenly doesn’t exist. I go home with a pile of dictations to do and work away into the night to catch up.
With experience we learn to take this in stride. Now, when I hit a slow patch, I remind myself that it’s not the average, and to enjoy it while I can. Read a book, take a long lunch, go home early and nap.
Worrying about where the patients are isn’t productive, or good for your mental health. They know where I am, and will find me when they need me.
. Enjoy the slow times while you can.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Everything in medicine, and pretty much the universe, is based on averages. Average reduction of seizures, average blood levels, average response to treatment, average insurance reimbursement, average time spent with a new consult.
Statistics are helpful in working through large amounts of data, but on a smaller scale, like my practice, statistics aren’t quite as helpful.
I see, on average, maybe 10 patients per day, consisting of new ones, follow-ups, and electromyography and nerve conduction velocity (EMG/NCV) studies. That is, by far, a smaller number of patients than my colleagues in primary care see, and probably other neurology practices as well. But it works for me.
But that’s on averages and not always. Sometimes we all hit slumps. Who knows why? Everyone is on vacation, or the holidays are coming, or they’ve been abducted by aliens. Whatever the reason, I get the occasional week where I’m pretty bored. Maybe one or two patients in a day. I start to feel like the lonely Maytag repairman behind my desk. I check to see if any drug samples have expired. I wonder if people are actually reading my online reviews and going elsewhere.
Years ago weeks like that terrified me. I was worried my little practice might fail (granted, it still could). But as years – and cycles that make up the averages – go by, they don’t bother me as much.
After 23 years I’ve learned that it’s just part of the normal fluctuations that make up an average. One morning I’ll roll the phones and the lines will explode (figuratively, I hope) with calls. At times like these my secretary seems to grow another pair of arms as she frantically schedules callers, puts others on hold, copies insurance cards, and gives the evil eye to drug reps who step in and ask her if she’s busy.
Then my schedule gets packed. My secretary crams patients in my emergency slots of 7:00, 8:00, and 12:00. MRI results come in that require me to see people sooner rather than later. My “average” of 10 patients per day suddenly doesn’t exist. I go home with a pile of dictations to do and work away into the night to catch up.
With experience we learn to take this in stride. Now, when I hit a slow patch, I remind myself that it’s not the average, and to enjoy it while I can. Read a book, take a long lunch, go home early and nap.
Worrying about where the patients are isn’t productive, or good for your mental health. They know where I am, and will find me when they need me.
. Enjoy the slow times while you can.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Everything in medicine, and pretty much the universe, is based on averages. Average reduction of seizures, average blood levels, average response to treatment, average insurance reimbursement, average time spent with a new consult.
Statistics are helpful in working through large amounts of data, but on a smaller scale, like my practice, statistics aren’t quite as helpful.
I see, on average, maybe 10 patients per day, consisting of new ones, follow-ups, and electromyography and nerve conduction velocity (EMG/NCV) studies. That is, by far, a smaller number of patients than my colleagues in primary care see, and probably other neurology practices as well. But it works for me.
But that’s on averages and not always. Sometimes we all hit slumps. Who knows why? Everyone is on vacation, or the holidays are coming, or they’ve been abducted by aliens. Whatever the reason, I get the occasional week where I’m pretty bored. Maybe one or two patients in a day. I start to feel like the lonely Maytag repairman behind my desk. I check to see if any drug samples have expired. I wonder if people are actually reading my online reviews and going elsewhere.
Years ago weeks like that terrified me. I was worried my little practice might fail (granted, it still could). But as years – and cycles that make up the averages – go by, they don’t bother me as much.
After 23 years I’ve learned that it’s just part of the normal fluctuations that make up an average. One morning I’ll roll the phones and the lines will explode (figuratively, I hope) with calls. At times like these my secretary seems to grow another pair of arms as she frantically schedules callers, puts others on hold, copies insurance cards, and gives the evil eye to drug reps who step in and ask her if she’s busy.
Then my schedule gets packed. My secretary crams patients in my emergency slots of 7:00, 8:00, and 12:00. MRI results come in that require me to see people sooner rather than later. My “average” of 10 patients per day suddenly doesn’t exist. I go home with a pile of dictations to do and work away into the night to catch up.
With experience we learn to take this in stride. Now, when I hit a slow patch, I remind myself that it’s not the average, and to enjoy it while I can. Read a book, take a long lunch, go home early and nap.
Worrying about where the patients are isn’t productive, or good for your mental health. They know where I am, and will find me when they need me.
. Enjoy the slow times while you can.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A psychiatric patient confesses to murder: Now what?
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
NEW ORLEANS – The patient, a 60-year-old woman who’d just tried to kill herself by overdosing on gabapentin, felt the need to make a confession. As she told a resident psychiatrist late one night at a Philadelphia crisis response center, she’d just murdered two people and buried them in her backyard. More details kept coming, including who was dead and where their bodies were.
It didn’t take long for the attending physician’s phone to ring as the resident sought guidance. This wasn’t a typical “duty to warn” case since there was no one to warn of a threat of violence. But then what kind of case was it? As Meghan Musselman, MD, and colleagues noted in a report presented at the annual meeting of the American Psychiatric Association, the law and medical ethics didn’t present a clear-cut solution to whether the patient’s claim should be reported to the authorities.
“This was much more of a gray zone case than we typically see,” said Dr. Musselman, of the department of psychiatry at Temple University in Philadelphia, in an interview. “If someone is threatening to harm someone, most states have statutes about what to do in that situation. The same doesn’t really exist for when the crime has already happened.”
Even so, might the existing “duty to warn/protect” laws be helpful as a guide to what to do? Maybe, but it’s complicated. The laws, which address the waiving of therapist-patient confidentiality when violence is threatened, are widely variable. Some don’t specifically cover psychiatrists, according to the National Conference of State Legislatures. Some simply allow – but don’t require – certain mental-health professionals to take action regarding threats of violence without getting in trouble themselves.
There are no duty to warn/protect laws in Nevada, North Dakota, North Carolina, and Maine. Pennsylvania requires “mental-health professionals” to act when there’s a “clear and immediate danger to others or to society.”
In an interview, Columbia University, New York, psychiatrist and medical law/ethics specialist Paul S. Appelbaum, MD, said that “with the exception of situations like child abuse or elder abuse, for which psychiatrists are mandatory reporters, psychiatrists generally have the same responsibilities for reporting crimes as other citizens.”
He added that there is a crime in English common law known as “misprision” that refers to failing to report a felony. “A few states still have misprision statutes, but courts have tended to interpret them to require an affirmative act to conceal a crime, not just failure to report,” he said. “Unless the patient’s confession indicates a continuing threat to other people – e.g., a serial rapist or murderer – there is probably no obligation to report a previous crime.”
In this case, Dr. Musselman said, the physicians thought they might be able to waive confidentiality because it was possible that the alleged murder victims were still alive and in need of help.
However, the patient ultimately took the decision out of the hands of the psychiatrists and agreed to confess to the police. There’s a happy ending: The patient later recanted the story, Dr. Musselman said, and there was no follow-up by the authorities.
What should psychiatrists do in a similar situation? Besides the law, Dr. Musselman said, it’s important to consider medical ethics, confidentiality, and the greater good. “Doctors may have to ask themselves: Would I rather be sued because I’m breaking confidentiality or potentially play a part in someone’s suffering?”
She recommended reaching out to attorneys for legal guidance. “There’s a saying in forensic psychiatry by [Harvard University psychiatrist] Thomas Gutheil: Never worry alone.”
Dr. Applebaum agreed, and added: “Psychiatrists should consider the credibility of the patient’s confession: Could it represent a delusion? Is it being proffered as a way of manipulating the therapist? What is the extent to which, if valid, it indicates an ongoing threat to others? Is the patient is willing to contact the police and admit to the crime or authorize the psychiatrist to do so? Only in the case of a credible confession, an ongoing threat, and a patient unwilling to contact the police themselves should the psychiatrist seriously consider breaching confidentiality to report.”
No study funding or disclosures were reported.
AT APA 2022
Roe v. Wade’s pending fall raises privacy concerns
If Roe v. Wade is overturned, can criminal prosecutors or tech companies use smartphone data against someone?
Now that the future of U.S. abortion laws hangs in the balance, many women are questioning the degree of caution needed to keep their cyber activity confidential – especially period and fertility tracking apps, smartphone location data, and social media interactions.
Cybersecurity and legal experts say the answer largely boils down to one major issue: The right to privacy.
“There’s this notion of the expectation of privacy,” said Brad Malin, PhD, professor of biomedical informatics, biostatistics, and computer science at Vanderbilt University in Nashville, Tenn.
Dr. Malin said it’s directly related to bodily privacy that a person expects they have control of as part of their own environment.
According to Dr. Malin, this is “why this whole notion of Roe v. Wade at the present moment is really relevant. The right to privacy is mentioned about a dozen times within the law for the case.
“This is why we don’t know what’s going to happen with Roe v. Wade, but it worries a lot of privacy professionals,” he said. “It leads down this slippery slope of if you don’t even have control over your own body, then with electronic communications … we might as well not even start.”
Legal protections
The Fourth Amendment of the U.S. Constitution protects people against unreasonable searches and seizures.
To acquire cyber data that could be used as evidence in courts in states where abortion is deemed a crime, prosecutors would still have to go through standard criminal procedures, said Anthony Michael Kreis, JD, a constitutional law professor at Georgia State University, Atlanta.
But the data they do get could still be used in court against someone who is suspected of having had an abortion or who “miscarried under circumstances law enforcement officers found suspicious,” Mr. Kreis said.
And there’s another possibility, he noted: States holding women who end their pregnancies criminally or civilly responsible for “leaving their jurisdiction to obtain an abortion out-of-state.”
“That legal mechanism may abridge the constitutional right to travel, but it is not out of the realm of possibilities in a post-Roe America,” said Mr. Kreis.
But while many anti-abortion groups have said that criminalizing abortion or limiting access to contraception is not the end goal, “history is not promising here,” said Ellen Wright Clayton, MD, JD, professor of pediatrics and professor of law at Vanderbilt University.
She referred to a recent proposal from lawmakers in Louisiana to classify abortion as homicide.
The bill didn’t get far in the House of Representatives, but the concern is warranted, said Dr. Clayton.
Period and fertility tracking apps
Health information privacy laws, like the Health Insurance Portability and Accountability Act (HIPAA), do not protect information on period and fertility tracking apps.
Right now, there are no signs that people plan to use period and fertility tracking data to advance a prochoice agenda, according to Adam Levin, JD, a cybersecurity expert and founder of CyberScout, a global identity and data protection company.
Still, a cycle tracking app “created by a company owned by an antiabortion activist” is totally feasible, said Mr. Levin.
If you want to ensure your data is safe from such meddling, you may want to delete your app, he said, noting that using the notepad feature on your smartphone could be a safer alternative, as could using old-fashioned pen and paper.
You don’t have to stop with period and fertility tracking apps, either.
For any apps you share personal information with, set privacy settings “as tightly as possible” – and reconsider using apps if these options are unavailable, Mr. Levin advised.
“Make sure that company is not engaging in social or political activism that does not align with your politics.”
New York State Attorney General Letitia James recently spoke on the topic, noting on May 13 that “people use fertility tracking apps and location services every day, but if they’re not careful their personal information can end up in the wrong hands.
“With abortion rights in jeopardy, it’s more important than ever that everyone take their digital privacy seriously,” she said. “I urge everyone, especially those visiting abortion clinics or seeking abortion care, to follow the tips offered by my office and be more careful of the apps and websites they use.”
The New York State Attorney General’s Office recommends women use encrypted messaging when communicating about personal health information or behaviors, and to be careful about what they share on social media posts. The office also suggests turning off location and personalized advertising options on their smartphones.
Cellphone location data
Dr. Malin said there are several ways that location services could be used to track where a woman uses her smartphone. An app could track locations if someone grants permission through the app end user agreement, for example.
A second but less likely scenario would be the service provider tracking the pings coming off cellphone towers to find a smartphone.
So what recourse does a woman have if tracked by a third-party app?
“It’s a really tricky situation there because it depends on if the individual was put expressly in harm’s way,” Dr. Malin said. What’s more, tracking someone out in public is not prohibited in general.
“There’s a big difference between documenting what an individual does within a Planned Parenthood clinic versus what they do outside of it,” he said.
Dr. Malin said it’s better that regulations protect all smartphone users rather than requiring each person to remember to turn off the location tracker and then turn it back on again. Also, it should be more of an opt-in situation – where app developers must ask permission to track app usage or location services – versus making each woman opt out.
Think before you share
Vindictive or untrustworthy partners and family members of women in abusive relationships could also be a cause of concern, said Mr. Kreis.
“Individuals within a woman’s closest circles could hold abortions over their head or threaten reporting them for reproductive health care or miscarriages,” he said.
It’s not uncommon for women to experience domestic violence after having an abortion, particularly if their partners were unaware they had the procedure, according to Dr. Clayton.
She said women should also be mindful of what they share on social media.
Dr. Clayton gave the example of a woman seeking advice on where to get a safe abortion or how to order certain medications.
“If someone goes online to look for that, that’s potentially dangerous.”
A version of this article first appeared on WebMD.com.
If Roe v. Wade is overturned, can criminal prosecutors or tech companies use smartphone data against someone?
Now that the future of U.S. abortion laws hangs in the balance, many women are questioning the degree of caution needed to keep their cyber activity confidential – especially period and fertility tracking apps, smartphone location data, and social media interactions.
Cybersecurity and legal experts say the answer largely boils down to one major issue: The right to privacy.
“There’s this notion of the expectation of privacy,” said Brad Malin, PhD, professor of biomedical informatics, biostatistics, and computer science at Vanderbilt University in Nashville, Tenn.
Dr. Malin said it’s directly related to bodily privacy that a person expects they have control of as part of their own environment.
According to Dr. Malin, this is “why this whole notion of Roe v. Wade at the present moment is really relevant. The right to privacy is mentioned about a dozen times within the law for the case.
“This is why we don’t know what’s going to happen with Roe v. Wade, but it worries a lot of privacy professionals,” he said. “It leads down this slippery slope of if you don’t even have control over your own body, then with electronic communications … we might as well not even start.”
Legal protections
The Fourth Amendment of the U.S. Constitution protects people against unreasonable searches and seizures.
To acquire cyber data that could be used as evidence in courts in states where abortion is deemed a crime, prosecutors would still have to go through standard criminal procedures, said Anthony Michael Kreis, JD, a constitutional law professor at Georgia State University, Atlanta.
But the data they do get could still be used in court against someone who is suspected of having had an abortion or who “miscarried under circumstances law enforcement officers found suspicious,” Mr. Kreis said.
And there’s another possibility, he noted: States holding women who end their pregnancies criminally or civilly responsible for “leaving their jurisdiction to obtain an abortion out-of-state.”
“That legal mechanism may abridge the constitutional right to travel, but it is not out of the realm of possibilities in a post-Roe America,” said Mr. Kreis.
But while many anti-abortion groups have said that criminalizing abortion or limiting access to contraception is not the end goal, “history is not promising here,” said Ellen Wright Clayton, MD, JD, professor of pediatrics and professor of law at Vanderbilt University.
She referred to a recent proposal from lawmakers in Louisiana to classify abortion as homicide.
The bill didn’t get far in the House of Representatives, but the concern is warranted, said Dr. Clayton.
Period and fertility tracking apps
Health information privacy laws, like the Health Insurance Portability and Accountability Act (HIPAA), do not protect information on period and fertility tracking apps.
Right now, there are no signs that people plan to use period and fertility tracking data to advance a prochoice agenda, according to Adam Levin, JD, a cybersecurity expert and founder of CyberScout, a global identity and data protection company.
Still, a cycle tracking app “created by a company owned by an antiabortion activist” is totally feasible, said Mr. Levin.
If you want to ensure your data is safe from such meddling, you may want to delete your app, he said, noting that using the notepad feature on your smartphone could be a safer alternative, as could using old-fashioned pen and paper.
You don’t have to stop with period and fertility tracking apps, either.
For any apps you share personal information with, set privacy settings “as tightly as possible” – and reconsider using apps if these options are unavailable, Mr. Levin advised.
“Make sure that company is not engaging in social or political activism that does not align with your politics.”
New York State Attorney General Letitia James recently spoke on the topic, noting on May 13 that “people use fertility tracking apps and location services every day, but if they’re not careful their personal information can end up in the wrong hands.
“With abortion rights in jeopardy, it’s more important than ever that everyone take their digital privacy seriously,” she said. “I urge everyone, especially those visiting abortion clinics or seeking abortion care, to follow the tips offered by my office and be more careful of the apps and websites they use.”
The New York State Attorney General’s Office recommends women use encrypted messaging when communicating about personal health information or behaviors, and to be careful about what they share on social media posts. The office also suggests turning off location and personalized advertising options on their smartphones.
Cellphone location data
Dr. Malin said there are several ways that location services could be used to track where a woman uses her smartphone. An app could track locations if someone grants permission through the app end user agreement, for example.
A second but less likely scenario would be the service provider tracking the pings coming off cellphone towers to find a smartphone.
So what recourse does a woman have if tracked by a third-party app?
“It’s a really tricky situation there because it depends on if the individual was put expressly in harm’s way,” Dr. Malin said. What’s more, tracking someone out in public is not prohibited in general.
“There’s a big difference between documenting what an individual does within a Planned Parenthood clinic versus what they do outside of it,” he said.
Dr. Malin said it’s better that regulations protect all smartphone users rather than requiring each person to remember to turn off the location tracker and then turn it back on again. Also, it should be more of an opt-in situation – where app developers must ask permission to track app usage or location services – versus making each woman opt out.
Think before you share
Vindictive or untrustworthy partners and family members of women in abusive relationships could also be a cause of concern, said Mr. Kreis.
“Individuals within a woman’s closest circles could hold abortions over their head or threaten reporting them for reproductive health care or miscarriages,” he said.
It’s not uncommon for women to experience domestic violence after having an abortion, particularly if their partners were unaware they had the procedure, according to Dr. Clayton.
She said women should also be mindful of what they share on social media.
Dr. Clayton gave the example of a woman seeking advice on where to get a safe abortion or how to order certain medications.
“If someone goes online to look for that, that’s potentially dangerous.”
A version of this article first appeared on WebMD.com.
If Roe v. Wade is overturned, can criminal prosecutors or tech companies use smartphone data against someone?
Now that the future of U.S. abortion laws hangs in the balance, many women are questioning the degree of caution needed to keep their cyber activity confidential – especially period and fertility tracking apps, smartphone location data, and social media interactions.
Cybersecurity and legal experts say the answer largely boils down to one major issue: The right to privacy.
“There’s this notion of the expectation of privacy,” said Brad Malin, PhD, professor of biomedical informatics, biostatistics, and computer science at Vanderbilt University in Nashville, Tenn.
Dr. Malin said it’s directly related to bodily privacy that a person expects they have control of as part of their own environment.
According to Dr. Malin, this is “why this whole notion of Roe v. Wade at the present moment is really relevant. The right to privacy is mentioned about a dozen times within the law for the case.
“This is why we don’t know what’s going to happen with Roe v. Wade, but it worries a lot of privacy professionals,” he said. “It leads down this slippery slope of if you don’t even have control over your own body, then with electronic communications … we might as well not even start.”
Legal protections
The Fourth Amendment of the U.S. Constitution protects people against unreasonable searches and seizures.
To acquire cyber data that could be used as evidence in courts in states where abortion is deemed a crime, prosecutors would still have to go through standard criminal procedures, said Anthony Michael Kreis, JD, a constitutional law professor at Georgia State University, Atlanta.
But the data they do get could still be used in court against someone who is suspected of having had an abortion or who “miscarried under circumstances law enforcement officers found suspicious,” Mr. Kreis said.
And there’s another possibility, he noted: States holding women who end their pregnancies criminally or civilly responsible for “leaving their jurisdiction to obtain an abortion out-of-state.”
“That legal mechanism may abridge the constitutional right to travel, but it is not out of the realm of possibilities in a post-Roe America,” said Mr. Kreis.
But while many anti-abortion groups have said that criminalizing abortion or limiting access to contraception is not the end goal, “history is not promising here,” said Ellen Wright Clayton, MD, JD, professor of pediatrics and professor of law at Vanderbilt University.
She referred to a recent proposal from lawmakers in Louisiana to classify abortion as homicide.
The bill didn’t get far in the House of Representatives, but the concern is warranted, said Dr. Clayton.
Period and fertility tracking apps
Health information privacy laws, like the Health Insurance Portability and Accountability Act (HIPAA), do not protect information on period and fertility tracking apps.
Right now, there are no signs that people plan to use period and fertility tracking data to advance a prochoice agenda, according to Adam Levin, JD, a cybersecurity expert and founder of CyberScout, a global identity and data protection company.
Still, a cycle tracking app “created by a company owned by an antiabortion activist” is totally feasible, said Mr. Levin.
If you want to ensure your data is safe from such meddling, you may want to delete your app, he said, noting that using the notepad feature on your smartphone could be a safer alternative, as could using old-fashioned pen and paper.
You don’t have to stop with period and fertility tracking apps, either.
For any apps you share personal information with, set privacy settings “as tightly as possible” – and reconsider using apps if these options are unavailable, Mr. Levin advised.
“Make sure that company is not engaging in social or political activism that does not align with your politics.”
New York State Attorney General Letitia James recently spoke on the topic, noting on May 13 that “people use fertility tracking apps and location services every day, but if they’re not careful their personal information can end up in the wrong hands.
“With abortion rights in jeopardy, it’s more important than ever that everyone take their digital privacy seriously,” she said. “I urge everyone, especially those visiting abortion clinics or seeking abortion care, to follow the tips offered by my office and be more careful of the apps and websites they use.”
The New York State Attorney General’s Office recommends women use encrypted messaging when communicating about personal health information or behaviors, and to be careful about what they share on social media posts. The office also suggests turning off location and personalized advertising options on their smartphones.
Cellphone location data
Dr. Malin said there are several ways that location services could be used to track where a woman uses her smartphone. An app could track locations if someone grants permission through the app end user agreement, for example.
A second but less likely scenario would be the service provider tracking the pings coming off cellphone towers to find a smartphone.
So what recourse does a woman have if tracked by a third-party app?
“It’s a really tricky situation there because it depends on if the individual was put expressly in harm’s way,” Dr. Malin said. What’s more, tracking someone out in public is not prohibited in general.
“There’s a big difference between documenting what an individual does within a Planned Parenthood clinic versus what they do outside of it,” he said.
Dr. Malin said it’s better that regulations protect all smartphone users rather than requiring each person to remember to turn off the location tracker and then turn it back on again. Also, it should be more of an opt-in situation – where app developers must ask permission to track app usage or location services – versus making each woman opt out.
Think before you share
Vindictive or untrustworthy partners and family members of women in abusive relationships could also be a cause of concern, said Mr. Kreis.
“Individuals within a woman’s closest circles could hold abortions over their head or threaten reporting them for reproductive health care or miscarriages,” he said.
It’s not uncommon for women to experience domestic violence after having an abortion, particularly if their partners were unaware they had the procedure, according to Dr. Clayton.
She said women should also be mindful of what they share on social media.
Dr. Clayton gave the example of a woman seeking advice on where to get a safe abortion or how to order certain medications.
“If someone goes online to look for that, that’s potentially dangerous.”
A version of this article first appeared on WebMD.com.
Trade-offs doctors make to become mothers: Interview study
Qualitative interviews with a group of female physicians identified several concerns about fertility and family planning and how those concerns affected their career choices.
Among the findings in a new study were that all 16 women interviewed said medical school education surrounding fertility was inadequate. Many said students should get comprehensive information about fertility’s decline with age and fertility preservation options and that information should be presented early in medical training so students can make choices. Yet, such issues are rarely discussed as part of medical training.
“[I]t would also be helpful for medical students and trainees to know what their options are, what insurance covers ... It wasn’t even touched at my orientation,” said one participant.
The findings from the hour-long interviews were used to build a survey. In a pilot test of the survey on 24 female physicians, researchers found that 71% had delayed childbearing and 67% had altered their careers to build families.
Kathryn S. Smith of Northwestern University, Chicago, led the research. Results were published online in JAMA Network Open.
In addition, 29% of survey respondents turned down career advancement opportunities; 21% chose a different specialty; and 17% changed from an academic to private practice setting to accommodate having children.
Women in the survey cited as factors in their decisions lack of support from physician peers and leadership, particularly around time off for pregnancy, maternity leave, infertility treatments, or parental responsibilities.
Results ‘alarming’
“These results are alarming, particularly in light of known gender disparities that exist within academic medicine in time to promotion, achievement of academic rank, and appointment to leadership positions,” the authors wrote.
As of 2020, women made up 43% of medical school faculty but only 21% of department chairs and 19% of medical school deans, according to Association of American Medical Colleges data.
Navigating motherhood as a physician also can take a physical and mental toll. Recent data presented at the American College of Obstetricians and Gynecologists (ACOG) 2022 annual meeting found that one in four physicians who are new mothers report struggling with postpartum depression, a rate twice that of the general population.
And one in four women in a recent survey of 600 female physicians who had attempted conception were diagnosed with infertility.
Lack of support ‘pronounced in medicine’
In an invited commentary, Ariela L. Marshall, MD, of the division of hematology-oncology at University of Pennsylvania, Philadelphia, and Arghavan Salles, MD, PhD, of the department of medicine at Stanford (Calif.) University, noted that career-family struggle is not unique to medicine but “the lack of support for pregnancy is particularly pronounced in medicine.”
The editorialists wrote that they have battled infertility and faced family-building challenges and have intimate familiarity with the struggles.
“Although other workplaces, such as Microsoft, Google, and Facebook, long ago adopted policies to support employees’ family building, including via cryopreservation, those in medicine all too frequently must pay back their parental leave, make up missed call, or even pay back money to their practice,” they wrote. “It is embarrassing that employees of tech companies have better support for reproductive health than do physicians.”
They advocate for change on the entire continuum from fertility awareness and infertility management, bringing children into a family by any method, and child care and career development support for physicians who become parents.
They urge establishing adequate paid parental leave, not just for parents who give birth but for all parents involved in rearing children. They say providing leave to only one parent sets up a discriminatory divide between the partner who continues to work and the person providing care.
Dr. Marshall and Dr. Salles wrote that lack of support is likely part of the reason that 40% of women in medicine switch to part-time positions within their first 6 years in practice.
They also note that too often fertility and family-building discussions focus on cisgendered women who are in heterosexual relationships.
They cite some “nonsensical“ policies around insurance. They give an example of coverage for fertility treatments that often requires trying to conceive before benefits are provided.
“How do two women, two men, or a single person try to conceive?” they ask.
Helen Kang Morgan, MD, clinical professor of obstetrics and gynecology at the University of Michigan, Ann Arbor, said that she, too, has made the trade-off the researchers describe.
She was in her first year as a faculty physician at the University of Michigan when she became a mom and decided to go part time.
Unlike some of the women interviewed in the Smith et al. study, she said she felt lucky to have peer support and the support of leaders in her department who made sure she wasn’t derailed from her career path because she chose part-time work for nearly 5 years.
“For some women, part-time is the right choice and for me, at the time, it was the right choice, but it should not be the only choice. It makes it so much harder for women to advance their careers if part-time is the only option,” she said.
Dr. Morgan said this work highlights that conversations about work and parenting needs in medicine have to go from informal conversations to formal conversations.
Department leaders should be asking what female physicians need and what flexibility is needed, she said.
The COVID-19 pandemic showed how bad things could get, she said.
In Ann Arbor, Dr. Morgan noted, schools were virtual until the spring of 2021, putting demands disproportionately on female physicians who absorbed much of the at-home child care responsibilities.
“That created gender inequities I think it is going to take women many, many years to catch up from,” she said.
COVID-19 also, however, forced medicine to incorporate more virtual options, something that should stay in finding solutions to ease the burden on physicians who are mothers, she said.
Reshma Jagsi, MD, deputy chair in the department of radiation oncology at the University of Michigan, said both policies and cultural norms need to change in medicine.
Hospitals must find alternative approaches to the historical reliance on residents to provide clinical service needs, she said in an interview.
“It’s not just about educating women or ensuring access to fertility services – it’s also about making it more possible and acceptable for women to combine their pursuit of a medical career and beginning a family during the peak years of fertility.”
She said the medical profession – dedicated to human well-being – seems to carve out an exception when it comes to optimizing the well-being of its future members.
“It breaks my heart to read about how hard we have made it for women to succeed in our profession,” she said.
This study was funded by the American Society for Reproductive Medicine. The authors report no relevant financial relationships. Dr. Marshall, Dr. Salles, Dr. Jagsi, and Dr. Morgan report no relevant financial relationships.
Qualitative interviews with a group of female physicians identified several concerns about fertility and family planning and how those concerns affected their career choices.
Among the findings in a new study were that all 16 women interviewed said medical school education surrounding fertility was inadequate. Many said students should get comprehensive information about fertility’s decline with age and fertility preservation options and that information should be presented early in medical training so students can make choices. Yet, such issues are rarely discussed as part of medical training.
“[I]t would also be helpful for medical students and trainees to know what their options are, what insurance covers ... It wasn’t even touched at my orientation,” said one participant.
The findings from the hour-long interviews were used to build a survey. In a pilot test of the survey on 24 female physicians, researchers found that 71% had delayed childbearing and 67% had altered their careers to build families.
Kathryn S. Smith of Northwestern University, Chicago, led the research. Results were published online in JAMA Network Open.
In addition, 29% of survey respondents turned down career advancement opportunities; 21% chose a different specialty; and 17% changed from an academic to private practice setting to accommodate having children.
Women in the survey cited as factors in their decisions lack of support from physician peers and leadership, particularly around time off for pregnancy, maternity leave, infertility treatments, or parental responsibilities.
Results ‘alarming’
“These results are alarming, particularly in light of known gender disparities that exist within academic medicine in time to promotion, achievement of academic rank, and appointment to leadership positions,” the authors wrote.
As of 2020, women made up 43% of medical school faculty but only 21% of department chairs and 19% of medical school deans, according to Association of American Medical Colleges data.
Navigating motherhood as a physician also can take a physical and mental toll. Recent data presented at the American College of Obstetricians and Gynecologists (ACOG) 2022 annual meeting found that one in four physicians who are new mothers report struggling with postpartum depression, a rate twice that of the general population.
And one in four women in a recent survey of 600 female physicians who had attempted conception were diagnosed with infertility.
Lack of support ‘pronounced in medicine’
In an invited commentary, Ariela L. Marshall, MD, of the division of hematology-oncology at University of Pennsylvania, Philadelphia, and Arghavan Salles, MD, PhD, of the department of medicine at Stanford (Calif.) University, noted that career-family struggle is not unique to medicine but “the lack of support for pregnancy is particularly pronounced in medicine.”
The editorialists wrote that they have battled infertility and faced family-building challenges and have intimate familiarity with the struggles.
“Although other workplaces, such as Microsoft, Google, and Facebook, long ago adopted policies to support employees’ family building, including via cryopreservation, those in medicine all too frequently must pay back their parental leave, make up missed call, or even pay back money to their practice,” they wrote. “It is embarrassing that employees of tech companies have better support for reproductive health than do physicians.”
They advocate for change on the entire continuum from fertility awareness and infertility management, bringing children into a family by any method, and child care and career development support for physicians who become parents.
They urge establishing adequate paid parental leave, not just for parents who give birth but for all parents involved in rearing children. They say providing leave to only one parent sets up a discriminatory divide between the partner who continues to work and the person providing care.
Dr. Marshall and Dr. Salles wrote that lack of support is likely part of the reason that 40% of women in medicine switch to part-time positions within their first 6 years in practice.
They also note that too often fertility and family-building discussions focus on cisgendered women who are in heterosexual relationships.
They cite some “nonsensical“ policies around insurance. They give an example of coverage for fertility treatments that often requires trying to conceive before benefits are provided.
“How do two women, two men, or a single person try to conceive?” they ask.
Helen Kang Morgan, MD, clinical professor of obstetrics and gynecology at the University of Michigan, Ann Arbor, said that she, too, has made the trade-off the researchers describe.
She was in her first year as a faculty physician at the University of Michigan when she became a mom and decided to go part time.
Unlike some of the women interviewed in the Smith et al. study, she said she felt lucky to have peer support and the support of leaders in her department who made sure she wasn’t derailed from her career path because she chose part-time work for nearly 5 years.
“For some women, part-time is the right choice and for me, at the time, it was the right choice, but it should not be the only choice. It makes it so much harder for women to advance their careers if part-time is the only option,” she said.
Dr. Morgan said this work highlights that conversations about work and parenting needs in medicine have to go from informal conversations to formal conversations.
Department leaders should be asking what female physicians need and what flexibility is needed, she said.
The COVID-19 pandemic showed how bad things could get, she said.
In Ann Arbor, Dr. Morgan noted, schools were virtual until the spring of 2021, putting demands disproportionately on female physicians who absorbed much of the at-home child care responsibilities.
“That created gender inequities I think it is going to take women many, many years to catch up from,” she said.
COVID-19 also, however, forced medicine to incorporate more virtual options, something that should stay in finding solutions to ease the burden on physicians who are mothers, she said.
Reshma Jagsi, MD, deputy chair in the department of radiation oncology at the University of Michigan, said both policies and cultural norms need to change in medicine.
Hospitals must find alternative approaches to the historical reliance on residents to provide clinical service needs, she said in an interview.
“It’s not just about educating women or ensuring access to fertility services – it’s also about making it more possible and acceptable for women to combine their pursuit of a medical career and beginning a family during the peak years of fertility.”
She said the medical profession – dedicated to human well-being – seems to carve out an exception when it comes to optimizing the well-being of its future members.
“It breaks my heart to read about how hard we have made it for women to succeed in our profession,” she said.
This study was funded by the American Society for Reproductive Medicine. The authors report no relevant financial relationships. Dr. Marshall, Dr. Salles, Dr. Jagsi, and Dr. Morgan report no relevant financial relationships.
Qualitative interviews with a group of female physicians identified several concerns about fertility and family planning and how those concerns affected their career choices.
Among the findings in a new study were that all 16 women interviewed said medical school education surrounding fertility was inadequate. Many said students should get comprehensive information about fertility’s decline with age and fertility preservation options and that information should be presented early in medical training so students can make choices. Yet, such issues are rarely discussed as part of medical training.
“[I]t would also be helpful for medical students and trainees to know what their options are, what insurance covers ... It wasn’t even touched at my orientation,” said one participant.
The findings from the hour-long interviews were used to build a survey. In a pilot test of the survey on 24 female physicians, researchers found that 71% had delayed childbearing and 67% had altered their careers to build families.
Kathryn S. Smith of Northwestern University, Chicago, led the research. Results were published online in JAMA Network Open.
In addition, 29% of survey respondents turned down career advancement opportunities; 21% chose a different specialty; and 17% changed from an academic to private practice setting to accommodate having children.
Women in the survey cited as factors in their decisions lack of support from physician peers and leadership, particularly around time off for pregnancy, maternity leave, infertility treatments, or parental responsibilities.
Results ‘alarming’
“These results are alarming, particularly in light of known gender disparities that exist within academic medicine in time to promotion, achievement of academic rank, and appointment to leadership positions,” the authors wrote.
As of 2020, women made up 43% of medical school faculty but only 21% of department chairs and 19% of medical school deans, according to Association of American Medical Colleges data.
Navigating motherhood as a physician also can take a physical and mental toll. Recent data presented at the American College of Obstetricians and Gynecologists (ACOG) 2022 annual meeting found that one in four physicians who are new mothers report struggling with postpartum depression, a rate twice that of the general population.
And one in four women in a recent survey of 600 female physicians who had attempted conception were diagnosed with infertility.
Lack of support ‘pronounced in medicine’
In an invited commentary, Ariela L. Marshall, MD, of the division of hematology-oncology at University of Pennsylvania, Philadelphia, and Arghavan Salles, MD, PhD, of the department of medicine at Stanford (Calif.) University, noted that career-family struggle is not unique to medicine but “the lack of support for pregnancy is particularly pronounced in medicine.”
The editorialists wrote that they have battled infertility and faced family-building challenges and have intimate familiarity with the struggles.
“Although other workplaces, such as Microsoft, Google, and Facebook, long ago adopted policies to support employees’ family building, including via cryopreservation, those in medicine all too frequently must pay back their parental leave, make up missed call, or even pay back money to their practice,” they wrote. “It is embarrassing that employees of tech companies have better support for reproductive health than do physicians.”
They advocate for change on the entire continuum from fertility awareness and infertility management, bringing children into a family by any method, and child care and career development support for physicians who become parents.
They urge establishing adequate paid parental leave, not just for parents who give birth but for all parents involved in rearing children. They say providing leave to only one parent sets up a discriminatory divide between the partner who continues to work and the person providing care.
Dr. Marshall and Dr. Salles wrote that lack of support is likely part of the reason that 40% of women in medicine switch to part-time positions within their first 6 years in practice.
They also note that too often fertility and family-building discussions focus on cisgendered women who are in heterosexual relationships.
They cite some “nonsensical“ policies around insurance. They give an example of coverage for fertility treatments that often requires trying to conceive before benefits are provided.
“How do two women, two men, or a single person try to conceive?” they ask.
Helen Kang Morgan, MD, clinical professor of obstetrics and gynecology at the University of Michigan, Ann Arbor, said that she, too, has made the trade-off the researchers describe.
She was in her first year as a faculty physician at the University of Michigan when she became a mom and decided to go part time.
Unlike some of the women interviewed in the Smith et al. study, she said she felt lucky to have peer support and the support of leaders in her department who made sure she wasn’t derailed from her career path because she chose part-time work for nearly 5 years.
“For some women, part-time is the right choice and for me, at the time, it was the right choice, but it should not be the only choice. It makes it so much harder for women to advance their careers if part-time is the only option,” she said.
Dr. Morgan said this work highlights that conversations about work and parenting needs in medicine have to go from informal conversations to formal conversations.
Department leaders should be asking what female physicians need and what flexibility is needed, she said.
The COVID-19 pandemic showed how bad things could get, she said.
In Ann Arbor, Dr. Morgan noted, schools were virtual until the spring of 2021, putting demands disproportionately on female physicians who absorbed much of the at-home child care responsibilities.
“That created gender inequities I think it is going to take women many, many years to catch up from,” she said.
COVID-19 also, however, forced medicine to incorporate more virtual options, something that should stay in finding solutions to ease the burden on physicians who are mothers, she said.
Reshma Jagsi, MD, deputy chair in the department of radiation oncology at the University of Michigan, said both policies and cultural norms need to change in medicine.
Hospitals must find alternative approaches to the historical reliance on residents to provide clinical service needs, she said in an interview.
“It’s not just about educating women or ensuring access to fertility services – it’s also about making it more possible and acceptable for women to combine their pursuit of a medical career and beginning a family during the peak years of fertility.”
She said the medical profession – dedicated to human well-being – seems to carve out an exception when it comes to optimizing the well-being of its future members.
“It breaks my heart to read about how hard we have made it for women to succeed in our profession,” she said.
This study was funded by the American Society for Reproductive Medicine. The authors report no relevant financial relationships. Dr. Marshall, Dr. Salles, Dr. Jagsi, and Dr. Morgan report no relevant financial relationships.
FROM JAMA NETWORK OPEN