User login
Attending a patient’s funeral: How psychiatrists decide
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Palliative care in the pandemic: How one hospital met the challenge
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
FROM SGIM 2021
FDA blazes path for ‘real-world’ evidence as proof of efficacy
In 2016, results from the LEADER trial of liraglutide in patients with type 2 diabetes helped jump-start awareness of the potential role of this new class of drugs, the glucagonlike peptide–1 receptor agonists, for reducing cardiovascular events. The randomized, placebo-controlled trial enrolled more than 9000 patients at more than 400 sites in over 30 countries, and took nearly 6 years from the start of patient enrollment to publication of the landmark results.
In December 2020, an independent team of researchers published results from a study with a design identical to LEADER, but used data that came not from a massive, global, years-long trial but from already-existing numbers culled from three large U.S. insurance claim databases. The result of this emulation using real-world data was virtually identical to what the actual trial showed, replicating both the direction and statistical significance of the original finding of the randomized, controlled trial (RCT).
What if research proved that this sort of RCT emulation could reliably be done on a regular basis? What might it mean for regulatory decisions on drugs and devices that historically have been based entirely on efficacy evidence from RCTs?
Making the most of a sea of observational data
Medicine in the United States has become increasingly awash in a sea of observational data collected from sources that include electronic health records, insurance claims, and increasingly, personal-health monitoring devices.
The Food and Drug Administration is now in the process of trying to figure out how it can legitimately harness this tsunami of real-world data to make efficacy decisions, essentially creating a new category of evidence to complement traditional data from randomized trials. It’s an opportunity that agency staff and their outside advisors have been keen to seize, especially given the soaring cost of prospective, randomized trials.
Recognition of this untapped resource in part led to a key initiative, among many others, included in the 21st Century Cures Act, passed in December 2016. Among the Act’s mandates was that, by the end of 2021, the FDA would issue guidance on when drug sponsors could use real-world evidence (RWE) to either help support a new indication for an already approved drug or help satisfy postapproval study requirements.
The initiative recognizes that this approach is not appropriate for initial drug approvals, which remain exclusively reliant on evidence from RCTs. Instead, it seems best suited to support expanding indications for already approved drugs.
Although FDA staff have made progress in identifying the challenges and broadening their understanding of how to best handle real-world data that come from observing patients in routine practice, agency leaders stress that this complex issue will likely not be fully resolved by their guidance to be published later this year. The FDA released a draft of the guidance in May 2019.
Can RWE be ‘credible and reliable?’
“Whether observational, nonrandomized data can become credible enough to use is what we’re talking about. These are possibilities that need to be explained and better understood,” said Robert Temple, MD, deputy director for clinical science of the FDA Center for Drug Evaluation and Research.
“Since the 1970s, the FDA has recognized historical controls as legitimate, so it’s possible [for RWE] to be credible. The big test is when is it credible and reliable enough [to assess efficacy]?” wondered Dr. Temple during a 2-day workshop on the topic held mid-February and organized by Duke University’s Margolis Center for Health Policy.
“We’re approaching an inflection point regarding how observational studies are generated and used, but our evidentiary standards will not lower, and it will be a case-by-case decision” by the agency as they review future RWE submissions, said John Concato, MD, the FDA’s associate director for real-world evidence, during the workshop.
“We are working toward guidance development, but also looking down the road to what we need to do to enable this,” said Dr. Concato. “It’s a complicated issue. If it was easy, it would have already been fixed.” He added that the agency will likely release a “portfolio” of guidance for submitting real-world data and RWE. Real-world data are raw information that, when analyzed, become RWE.
In short, the FDA seems headed toward guidance that won’t spell out a pathway that guarantees success using RWE but will at least open the door to consideration of this unprecedented application.
Not like flipping a switch
The guidance will not activate acceptance of RWE all at once. “It’s not like a light switch,” cautioned Adam Kroetsch, MPP, research director for biomedical innovation and regulatory policy at Duke-Margolis in Washington, D.C. “It’s an evolutionary process,” and the upcoming guidance will provide “just a little more clarity” on what sorts of best practices using RWE the FDA will find persuasive. “It’s hard for the FDA to clearly say what it’s looking for until they see some good examples,” Dr. Kroetsch said in an interview.
What will change is that drug sponsors can submit using RWE, and the FDA “will have a more open-minded view,” predicted Sebastian Schneeweiss, MD, ScD, a workshop participant and chief of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston. “For the first time, a law required [the FDA] to take a serious look” at observational data for efficacy assessment.
“The FDA has had a bias against using RWE for evidence of efficacy but has long used it to understand drug safety. Now the FDA is trying to wrap its arms around how to best use RWE” for efficacy decisions, said Joseph S. Ross, MD, another workshop participant and professor of medicine and public health at Yale University, New Haven, Conn.
The agency’s cautious approach is reassuring, Dr. Ross noted in an interview. “There was worry that the 21st Century Cures Act would open the door to allowing real-world data to be used in ways that weren’t very reliable. Very quickly, the FDA started trying to figure out the best ways to use these data in reasonable ways.”
Duplicating RCTs with RWE
To help better understand the potential use of RWE, the FDA sponsored several demonstration projects. Researchers presented results from three of these projects during the workshop in February. All three examined whether RWE, plugged into the design of an actual RCT, can produce roughly similar results when similar patients are used.
A generally consistent finding from the three demonstration projects was that “when the data are fit for purpose” the emulated or duplicated analyses with RWE “can come to similar conclusions” as the actual RCTs, said Dr. Schneeweiss, who leads one of the demonstration projects, RCT DUPLICATE.
At the workshop he reported results from RWE duplications of 20 different RCTs using insurance claims data from U.S. patients. The findings came from 10 duplications already reported in Circulation in December 2020 (including a duplication of the LEADER trial), and an additional 10 as yet unpublished RCT duplications. In the next few months, the researchers intend to assess a final group of 10 more RCT duplications.
Workshop participants also presented results from two other FDA demonstration projects: the OPERAND program run by the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard; and the CERSI program based at Yale and the Mayo Clinic in Rochester, Minn. Both are smaller in scale than RCT DUPLICATE, incorporate lab data in addition to claims data, and in some cases test how well RWE can emulate RCTs that are not yet completed.
Collectively, results from these demonstration projects suggest that RWE can successfully emulate the results of an RCT, said Dr. Ross, a coinvestigator on the CERSI study. But the CERSI findings also highlighted how an RCT can fall short of clinical relevance.
“One of our most important findings was that RCTs don’t always represent real-world practice,” he said. His group attempted to replicate the 5,000-patient GRADE trial of four different drug options added to metformin in patients with type 2 diabetes. One of the four options included insulin glargine (Lantus), and the attempt to emulate the study with RWE hit the bump that no relevant real-world patients in their US claims database actually received the formulation.
That means the GRADE trial “is almost meaningless. It doesn’t reflect real-world practice,” Dr. Ross noted.
Results from the three demonstration projects “highlight the gaps we still have,” summed up Dr. Kroetsch. “They show where we need better data” from observational sources that function as well as data from RCTs.
Still, the demonstration project results are “an important step forward in establishing the validity of real-world evidence,” commented David Kerr, MBChB, an endocrinologist and director of research and innovation at the Sansum Diabetes Research Institute in Santa Barbara, Calif.
‘Target trials’ tether RWE
The target trial approach to designing an observational study is a key tool for boosting reliability and applicability of the results. The idea is to create a well-designed trial that could be the basis for a conventional RCT, and then use observational data to flesh out the target trial instead of collecting data from prospectively enrolled patients.
Designing observational studies that emulate target trials allows causal inferences, said Miguel A. Hernán, MD, DrPH, a professor of biostatistics and epidemiology at the Harvard School of Public Health, Boston. Plugging real-world data into the framework of an appropriately designed target trial substantially cuts the risk of a biased analysis, he explained during the workshop.
However, the approach has limitations. The target trial must be a pragmatic trial, and the approach does not work for placebo-controlled trials, although it can accommodate a usual-care control arm. It also usually precludes patient blinding, testing treatments not used in routine practice, and close monitoring of patients in ways that are uncommon in usual care.
The target trial approach received broad endorsement during the workshop as the future for observational studies destined for efficacy consideration by the FDA.
“The idea of prespecifying a target trial is a really fantastic place to start,” commented Robert Ball, MD, deputy director of the FDA Office of Surveillance and Epidemiology. “There is still a whole set of questions once the trial is prespecified, but prespecification would be a fantastic step forward,” he said during the workshop.
Participants also endorsed other important steps to boost the value of observational studies for regulatory reviews, including preregistering the study on a site such as clinicaltrials.gov; being fully transparent about the origins of observational data; using data that match the needs of the target trial; not reviewing the data in advance to avoid cherry picking and gaming the analysis; and reporting neutral or negative results when they occur, something often not currently done for observational analyses.
But although there was clear progress and much agreement among thought leaders at the workshop, FDA representatives stressed caution in moving forward.
“No easy answer”
“With more experience, we can learn what works and what doesn’t work in generating valid results from observational studies,” said Dr. Concato. “Although the observational results have upside potential, we need to learn more. There is no easy answer, no checklist for fit-for-use data, no off-the-shelf study design, and no ideal analytic method.”
Dr. Concato acknowledged that the FDA’s goal is clear given the 2016 legislation. “The FDA is embracing our obligations under the 21st Century Cures Act to evaluate use of real-world data and real-world evidence.”
He also suggested that researchers “shy away from a false dichotomy of RCTs or observational studies and instead think about how and when RCTs and observational studies can be designed and conducted to yield trustworthy results.” Dr. Concato’s solution: “a taxonomy of interventional or noninterventional studies.”
“The FDA is under enormous pressure to embrace real-world evidence, both because of the economics of running RCTs and because of the availability of new observational data from electronic health records, wearable devices, claims, etc.,” said Dr. Kerr, who did not participate in the workshop but coauthored an editorial that calls for using real-world data in regulatory decisions for drugs and devices for diabetes. These factors create an “irresistible force” spurring the FDA to consider observational, noninterventional data.
“I think the FDA really wants this to go forward,” Dr. Kerr added in an interview. “The FDA keeps telling us that clinical trials do not have enough women or patients from minority groups. Real-world data is a way to address that. This will not be the death of RCTs, but this work shines a light on the deficiencies of RCTs and how the deficiencies can be dealt with.”
Dr. Kroetsch has reported no relevant financial relationships. Dr. Schneeweiss has reported being a consultant to and holding equity in Aetion and receiving research funding from the FDA. Dr. Ross has reported receiving research funding from the FDA, Johnson & Johnson, and Medtronic. Dr. Hernán has reported being a consultant for Cytel. Dr. Kerr has reported being a consultant for Ascensia, EOFlow, Lifecare, Merck, Novo Nordisk, Roche Diagnostics, and Voluntis. Dr. Temple, Dr. Concato, and Dr. Ball are FDA employees.
A version of this article first appeared on Medscape.com.
In 2016, results from the LEADER trial of liraglutide in patients with type 2 diabetes helped jump-start awareness of the potential role of this new class of drugs, the glucagonlike peptide–1 receptor agonists, for reducing cardiovascular events. The randomized, placebo-controlled trial enrolled more than 9000 patients at more than 400 sites in over 30 countries, and took nearly 6 years from the start of patient enrollment to publication of the landmark results.
In December 2020, an independent team of researchers published results from a study with a design identical to LEADER, but used data that came not from a massive, global, years-long trial but from already-existing numbers culled from three large U.S. insurance claim databases. The result of this emulation using real-world data was virtually identical to what the actual trial showed, replicating both the direction and statistical significance of the original finding of the randomized, controlled trial (RCT).
What if research proved that this sort of RCT emulation could reliably be done on a regular basis? What might it mean for regulatory decisions on drugs and devices that historically have been based entirely on efficacy evidence from RCTs?
Making the most of a sea of observational data
Medicine in the United States has become increasingly awash in a sea of observational data collected from sources that include electronic health records, insurance claims, and increasingly, personal-health monitoring devices.
The Food and Drug Administration is now in the process of trying to figure out how it can legitimately harness this tsunami of real-world data to make efficacy decisions, essentially creating a new category of evidence to complement traditional data from randomized trials. It’s an opportunity that agency staff and their outside advisors have been keen to seize, especially given the soaring cost of prospective, randomized trials.
Recognition of this untapped resource in part led to a key initiative, among many others, included in the 21st Century Cures Act, passed in December 2016. Among the Act’s mandates was that, by the end of 2021, the FDA would issue guidance on when drug sponsors could use real-world evidence (RWE) to either help support a new indication for an already approved drug or help satisfy postapproval study requirements.
The initiative recognizes that this approach is not appropriate for initial drug approvals, which remain exclusively reliant on evidence from RCTs. Instead, it seems best suited to support expanding indications for already approved drugs.
Although FDA staff have made progress in identifying the challenges and broadening their understanding of how to best handle real-world data that come from observing patients in routine practice, agency leaders stress that this complex issue will likely not be fully resolved by their guidance to be published later this year. The FDA released a draft of the guidance in May 2019.
Can RWE be ‘credible and reliable?’
“Whether observational, nonrandomized data can become credible enough to use is what we’re talking about. These are possibilities that need to be explained and better understood,” said Robert Temple, MD, deputy director for clinical science of the FDA Center for Drug Evaluation and Research.
“Since the 1970s, the FDA has recognized historical controls as legitimate, so it’s possible [for RWE] to be credible. The big test is when is it credible and reliable enough [to assess efficacy]?” wondered Dr. Temple during a 2-day workshop on the topic held mid-February and organized by Duke University’s Margolis Center for Health Policy.
“We’re approaching an inflection point regarding how observational studies are generated and used, but our evidentiary standards will not lower, and it will be a case-by-case decision” by the agency as they review future RWE submissions, said John Concato, MD, the FDA’s associate director for real-world evidence, during the workshop.
“We are working toward guidance development, but also looking down the road to what we need to do to enable this,” said Dr. Concato. “It’s a complicated issue. If it was easy, it would have already been fixed.” He added that the agency will likely release a “portfolio” of guidance for submitting real-world data and RWE. Real-world data are raw information that, when analyzed, become RWE.
In short, the FDA seems headed toward guidance that won’t spell out a pathway that guarantees success using RWE but will at least open the door to consideration of this unprecedented application.
Not like flipping a switch
The guidance will not activate acceptance of RWE all at once. “It’s not like a light switch,” cautioned Adam Kroetsch, MPP, research director for biomedical innovation and regulatory policy at Duke-Margolis in Washington, D.C. “It’s an evolutionary process,” and the upcoming guidance will provide “just a little more clarity” on what sorts of best practices using RWE the FDA will find persuasive. “It’s hard for the FDA to clearly say what it’s looking for until they see some good examples,” Dr. Kroetsch said in an interview.
What will change is that drug sponsors can submit using RWE, and the FDA “will have a more open-minded view,” predicted Sebastian Schneeweiss, MD, ScD, a workshop participant and chief of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston. “For the first time, a law required [the FDA] to take a serious look” at observational data for efficacy assessment.
“The FDA has had a bias against using RWE for evidence of efficacy but has long used it to understand drug safety. Now the FDA is trying to wrap its arms around how to best use RWE” for efficacy decisions, said Joseph S. Ross, MD, another workshop participant and professor of medicine and public health at Yale University, New Haven, Conn.
The agency’s cautious approach is reassuring, Dr. Ross noted in an interview. “There was worry that the 21st Century Cures Act would open the door to allowing real-world data to be used in ways that weren’t very reliable. Very quickly, the FDA started trying to figure out the best ways to use these data in reasonable ways.”
Duplicating RCTs with RWE
To help better understand the potential use of RWE, the FDA sponsored several demonstration projects. Researchers presented results from three of these projects during the workshop in February. All three examined whether RWE, plugged into the design of an actual RCT, can produce roughly similar results when similar patients are used.
A generally consistent finding from the three demonstration projects was that “when the data are fit for purpose” the emulated or duplicated analyses with RWE “can come to similar conclusions” as the actual RCTs, said Dr. Schneeweiss, who leads one of the demonstration projects, RCT DUPLICATE.
At the workshop he reported results from RWE duplications of 20 different RCTs using insurance claims data from U.S. patients. The findings came from 10 duplications already reported in Circulation in December 2020 (including a duplication of the LEADER trial), and an additional 10 as yet unpublished RCT duplications. In the next few months, the researchers intend to assess a final group of 10 more RCT duplications.
Workshop participants also presented results from two other FDA demonstration projects: the OPERAND program run by the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard; and the CERSI program based at Yale and the Mayo Clinic in Rochester, Minn. Both are smaller in scale than RCT DUPLICATE, incorporate lab data in addition to claims data, and in some cases test how well RWE can emulate RCTs that are not yet completed.
Collectively, results from these demonstration projects suggest that RWE can successfully emulate the results of an RCT, said Dr. Ross, a coinvestigator on the CERSI study. But the CERSI findings also highlighted how an RCT can fall short of clinical relevance.
“One of our most important findings was that RCTs don’t always represent real-world practice,” he said. His group attempted to replicate the 5,000-patient GRADE trial of four different drug options added to metformin in patients with type 2 diabetes. One of the four options included insulin glargine (Lantus), and the attempt to emulate the study with RWE hit the bump that no relevant real-world patients in their US claims database actually received the formulation.
That means the GRADE trial “is almost meaningless. It doesn’t reflect real-world practice,” Dr. Ross noted.
Results from the three demonstration projects “highlight the gaps we still have,” summed up Dr. Kroetsch. “They show where we need better data” from observational sources that function as well as data from RCTs.
Still, the demonstration project results are “an important step forward in establishing the validity of real-world evidence,” commented David Kerr, MBChB, an endocrinologist and director of research and innovation at the Sansum Diabetes Research Institute in Santa Barbara, Calif.
‘Target trials’ tether RWE
The target trial approach to designing an observational study is a key tool for boosting reliability and applicability of the results. The idea is to create a well-designed trial that could be the basis for a conventional RCT, and then use observational data to flesh out the target trial instead of collecting data from prospectively enrolled patients.
Designing observational studies that emulate target trials allows causal inferences, said Miguel A. Hernán, MD, DrPH, a professor of biostatistics and epidemiology at the Harvard School of Public Health, Boston. Plugging real-world data into the framework of an appropriately designed target trial substantially cuts the risk of a biased analysis, he explained during the workshop.
However, the approach has limitations. The target trial must be a pragmatic trial, and the approach does not work for placebo-controlled trials, although it can accommodate a usual-care control arm. It also usually precludes patient blinding, testing treatments not used in routine practice, and close monitoring of patients in ways that are uncommon in usual care.
The target trial approach received broad endorsement during the workshop as the future for observational studies destined for efficacy consideration by the FDA.
“The idea of prespecifying a target trial is a really fantastic place to start,” commented Robert Ball, MD, deputy director of the FDA Office of Surveillance and Epidemiology. “There is still a whole set of questions once the trial is prespecified, but prespecification would be a fantastic step forward,” he said during the workshop.
Participants also endorsed other important steps to boost the value of observational studies for regulatory reviews, including preregistering the study on a site such as clinicaltrials.gov; being fully transparent about the origins of observational data; using data that match the needs of the target trial; not reviewing the data in advance to avoid cherry picking and gaming the analysis; and reporting neutral or negative results when they occur, something often not currently done for observational analyses.
But although there was clear progress and much agreement among thought leaders at the workshop, FDA representatives stressed caution in moving forward.
“No easy answer”
“With more experience, we can learn what works and what doesn’t work in generating valid results from observational studies,” said Dr. Concato. “Although the observational results have upside potential, we need to learn more. There is no easy answer, no checklist for fit-for-use data, no off-the-shelf study design, and no ideal analytic method.”
Dr. Concato acknowledged that the FDA’s goal is clear given the 2016 legislation. “The FDA is embracing our obligations under the 21st Century Cures Act to evaluate use of real-world data and real-world evidence.”
He also suggested that researchers “shy away from a false dichotomy of RCTs or observational studies and instead think about how and when RCTs and observational studies can be designed and conducted to yield trustworthy results.” Dr. Concato’s solution: “a taxonomy of interventional or noninterventional studies.”
“The FDA is under enormous pressure to embrace real-world evidence, both because of the economics of running RCTs and because of the availability of new observational data from electronic health records, wearable devices, claims, etc.,” said Dr. Kerr, who did not participate in the workshop but coauthored an editorial that calls for using real-world data in regulatory decisions for drugs and devices for diabetes. These factors create an “irresistible force” spurring the FDA to consider observational, noninterventional data.
“I think the FDA really wants this to go forward,” Dr. Kerr added in an interview. “The FDA keeps telling us that clinical trials do not have enough women or patients from minority groups. Real-world data is a way to address that. This will not be the death of RCTs, but this work shines a light on the deficiencies of RCTs and how the deficiencies can be dealt with.”
Dr. Kroetsch has reported no relevant financial relationships. Dr. Schneeweiss has reported being a consultant to and holding equity in Aetion and receiving research funding from the FDA. Dr. Ross has reported receiving research funding from the FDA, Johnson & Johnson, and Medtronic. Dr. Hernán has reported being a consultant for Cytel. Dr. Kerr has reported being a consultant for Ascensia, EOFlow, Lifecare, Merck, Novo Nordisk, Roche Diagnostics, and Voluntis. Dr. Temple, Dr. Concato, and Dr. Ball are FDA employees.
A version of this article first appeared on Medscape.com.
In 2016, results from the LEADER trial of liraglutide in patients with type 2 diabetes helped jump-start awareness of the potential role of this new class of drugs, the glucagonlike peptide–1 receptor agonists, for reducing cardiovascular events. The randomized, placebo-controlled trial enrolled more than 9000 patients at more than 400 sites in over 30 countries, and took nearly 6 years from the start of patient enrollment to publication of the landmark results.
In December 2020, an independent team of researchers published results from a study with a design identical to LEADER, but used data that came not from a massive, global, years-long trial but from already-existing numbers culled from three large U.S. insurance claim databases. The result of this emulation using real-world data was virtually identical to what the actual trial showed, replicating both the direction and statistical significance of the original finding of the randomized, controlled trial (RCT).
What if research proved that this sort of RCT emulation could reliably be done on a regular basis? What might it mean for regulatory decisions on drugs and devices that historically have been based entirely on efficacy evidence from RCTs?
Making the most of a sea of observational data
Medicine in the United States has become increasingly awash in a sea of observational data collected from sources that include electronic health records, insurance claims, and increasingly, personal-health monitoring devices.
The Food and Drug Administration is now in the process of trying to figure out how it can legitimately harness this tsunami of real-world data to make efficacy decisions, essentially creating a new category of evidence to complement traditional data from randomized trials. It’s an opportunity that agency staff and their outside advisors have been keen to seize, especially given the soaring cost of prospective, randomized trials.
Recognition of this untapped resource in part led to a key initiative, among many others, included in the 21st Century Cures Act, passed in December 2016. Among the Act’s mandates was that, by the end of 2021, the FDA would issue guidance on when drug sponsors could use real-world evidence (RWE) to either help support a new indication for an already approved drug or help satisfy postapproval study requirements.
The initiative recognizes that this approach is not appropriate for initial drug approvals, which remain exclusively reliant on evidence from RCTs. Instead, it seems best suited to support expanding indications for already approved drugs.
Although FDA staff have made progress in identifying the challenges and broadening their understanding of how to best handle real-world data that come from observing patients in routine practice, agency leaders stress that this complex issue will likely not be fully resolved by their guidance to be published later this year. The FDA released a draft of the guidance in May 2019.
Can RWE be ‘credible and reliable?’
“Whether observational, nonrandomized data can become credible enough to use is what we’re talking about. These are possibilities that need to be explained and better understood,” said Robert Temple, MD, deputy director for clinical science of the FDA Center for Drug Evaluation and Research.
“Since the 1970s, the FDA has recognized historical controls as legitimate, so it’s possible [for RWE] to be credible. The big test is when is it credible and reliable enough [to assess efficacy]?” wondered Dr. Temple during a 2-day workshop on the topic held mid-February and organized by Duke University’s Margolis Center for Health Policy.
“We’re approaching an inflection point regarding how observational studies are generated and used, but our evidentiary standards will not lower, and it will be a case-by-case decision” by the agency as they review future RWE submissions, said John Concato, MD, the FDA’s associate director for real-world evidence, during the workshop.
“We are working toward guidance development, but also looking down the road to what we need to do to enable this,” said Dr. Concato. “It’s a complicated issue. If it was easy, it would have already been fixed.” He added that the agency will likely release a “portfolio” of guidance for submitting real-world data and RWE. Real-world data are raw information that, when analyzed, become RWE.
In short, the FDA seems headed toward guidance that won’t spell out a pathway that guarantees success using RWE but will at least open the door to consideration of this unprecedented application.
Not like flipping a switch
The guidance will not activate acceptance of RWE all at once. “It’s not like a light switch,” cautioned Adam Kroetsch, MPP, research director for biomedical innovation and regulatory policy at Duke-Margolis in Washington, D.C. “It’s an evolutionary process,” and the upcoming guidance will provide “just a little more clarity” on what sorts of best practices using RWE the FDA will find persuasive. “It’s hard for the FDA to clearly say what it’s looking for until they see some good examples,” Dr. Kroetsch said in an interview.
What will change is that drug sponsors can submit using RWE, and the FDA “will have a more open-minded view,” predicted Sebastian Schneeweiss, MD, ScD, a workshop participant and chief of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston. “For the first time, a law required [the FDA] to take a serious look” at observational data for efficacy assessment.
“The FDA has had a bias against using RWE for evidence of efficacy but has long used it to understand drug safety. Now the FDA is trying to wrap its arms around how to best use RWE” for efficacy decisions, said Joseph S. Ross, MD, another workshop participant and professor of medicine and public health at Yale University, New Haven, Conn.
The agency’s cautious approach is reassuring, Dr. Ross noted in an interview. “There was worry that the 21st Century Cures Act would open the door to allowing real-world data to be used in ways that weren’t very reliable. Very quickly, the FDA started trying to figure out the best ways to use these data in reasonable ways.”
Duplicating RCTs with RWE
To help better understand the potential use of RWE, the FDA sponsored several demonstration projects. Researchers presented results from three of these projects during the workshop in February. All three examined whether RWE, plugged into the design of an actual RCT, can produce roughly similar results when similar patients are used.
A generally consistent finding from the three demonstration projects was that “when the data are fit for purpose” the emulated or duplicated analyses with RWE “can come to similar conclusions” as the actual RCTs, said Dr. Schneeweiss, who leads one of the demonstration projects, RCT DUPLICATE.
At the workshop he reported results from RWE duplications of 20 different RCTs using insurance claims data from U.S. patients. The findings came from 10 duplications already reported in Circulation in December 2020 (including a duplication of the LEADER trial), and an additional 10 as yet unpublished RCT duplications. In the next few months, the researchers intend to assess a final group of 10 more RCT duplications.
Workshop participants also presented results from two other FDA demonstration projects: the OPERAND program run by the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard; and the CERSI program based at Yale and the Mayo Clinic in Rochester, Minn. Both are smaller in scale than RCT DUPLICATE, incorporate lab data in addition to claims data, and in some cases test how well RWE can emulate RCTs that are not yet completed.
Collectively, results from these demonstration projects suggest that RWE can successfully emulate the results of an RCT, said Dr. Ross, a coinvestigator on the CERSI study. But the CERSI findings also highlighted how an RCT can fall short of clinical relevance.
“One of our most important findings was that RCTs don’t always represent real-world practice,” he said. His group attempted to replicate the 5,000-patient GRADE trial of four different drug options added to metformin in patients with type 2 diabetes. One of the four options included insulin glargine (Lantus), and the attempt to emulate the study with RWE hit the bump that no relevant real-world patients in their US claims database actually received the formulation.
That means the GRADE trial “is almost meaningless. It doesn’t reflect real-world practice,” Dr. Ross noted.
Results from the three demonstration projects “highlight the gaps we still have,” summed up Dr. Kroetsch. “They show where we need better data” from observational sources that function as well as data from RCTs.
Still, the demonstration project results are “an important step forward in establishing the validity of real-world evidence,” commented David Kerr, MBChB, an endocrinologist and director of research and innovation at the Sansum Diabetes Research Institute in Santa Barbara, Calif.
‘Target trials’ tether RWE
The target trial approach to designing an observational study is a key tool for boosting reliability and applicability of the results. The idea is to create a well-designed trial that could be the basis for a conventional RCT, and then use observational data to flesh out the target trial instead of collecting data from prospectively enrolled patients.
Designing observational studies that emulate target trials allows causal inferences, said Miguel A. Hernán, MD, DrPH, a professor of biostatistics and epidemiology at the Harvard School of Public Health, Boston. Plugging real-world data into the framework of an appropriately designed target trial substantially cuts the risk of a biased analysis, he explained during the workshop.
However, the approach has limitations. The target trial must be a pragmatic trial, and the approach does not work for placebo-controlled trials, although it can accommodate a usual-care control arm. It also usually precludes patient blinding, testing treatments not used in routine practice, and close monitoring of patients in ways that are uncommon in usual care.
The target trial approach received broad endorsement during the workshop as the future for observational studies destined for efficacy consideration by the FDA.
“The idea of prespecifying a target trial is a really fantastic place to start,” commented Robert Ball, MD, deputy director of the FDA Office of Surveillance and Epidemiology. “There is still a whole set of questions once the trial is prespecified, but prespecification would be a fantastic step forward,” he said during the workshop.
Participants also endorsed other important steps to boost the value of observational studies for regulatory reviews, including preregistering the study on a site such as clinicaltrials.gov; being fully transparent about the origins of observational data; using data that match the needs of the target trial; not reviewing the data in advance to avoid cherry picking and gaming the analysis; and reporting neutral or negative results when they occur, something often not currently done for observational analyses.
But although there was clear progress and much agreement among thought leaders at the workshop, FDA representatives stressed caution in moving forward.
“No easy answer”
“With more experience, we can learn what works and what doesn’t work in generating valid results from observational studies,” said Dr. Concato. “Although the observational results have upside potential, we need to learn more. There is no easy answer, no checklist for fit-for-use data, no off-the-shelf study design, and no ideal analytic method.”
Dr. Concato acknowledged that the FDA’s goal is clear given the 2016 legislation. “The FDA is embracing our obligations under the 21st Century Cures Act to evaluate use of real-world data and real-world evidence.”
He also suggested that researchers “shy away from a false dichotomy of RCTs or observational studies and instead think about how and when RCTs and observational studies can be designed and conducted to yield trustworthy results.” Dr. Concato’s solution: “a taxonomy of interventional or noninterventional studies.”
“The FDA is under enormous pressure to embrace real-world evidence, both because of the economics of running RCTs and because of the availability of new observational data from electronic health records, wearable devices, claims, etc.,” said Dr. Kerr, who did not participate in the workshop but coauthored an editorial that calls for using real-world data in regulatory decisions for drugs and devices for diabetes. These factors create an “irresistible force” spurring the FDA to consider observational, noninterventional data.
“I think the FDA really wants this to go forward,” Dr. Kerr added in an interview. “The FDA keeps telling us that clinical trials do not have enough women or patients from minority groups. Real-world data is a way to address that. This will not be the death of RCTs, but this work shines a light on the deficiencies of RCTs and how the deficiencies can be dealt with.”
Dr. Kroetsch has reported no relevant financial relationships. Dr. Schneeweiss has reported being a consultant to and holding equity in Aetion and receiving research funding from the FDA. Dr. Ross has reported receiving research funding from the FDA, Johnson & Johnson, and Medtronic. Dr. Hernán has reported being a consultant for Cytel. Dr. Kerr has reported being a consultant for Ascensia, EOFlow, Lifecare, Merck, Novo Nordisk, Roche Diagnostics, and Voluntis. Dr. Temple, Dr. Concato, and Dr. Ball are FDA employees.
A version of this article first appeared on Medscape.com.
Operational changes in primary care linked with improved smoking, blood pressure outcomes
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
FROM ANNALS OF FAMILY MEDICINE
SGIM annual meeting focuses on inclusivity
In her welcome video on the opening day at the annual meeting of the Society of General Internal Medicine, meeting chair Rita Lee, MD, said she hoped that this year’s event, though virtual, will allow attendees an opportunity to “regroup, find inspiration, and celebrate the incredible strengths and diverse voices of our community.”
“We are living in an incredibly polarized world,” Dr. Lee said in an interview. “ as recent events, such as the death of George Floyd and many others, plus the disparities revealed by the COVID-19 pandemic, have brought issues of structural racism and oppression in the United States to the forefront,” she said in the interview.
“Given these circumstances, it is important now, more than ever, for generalists to move our values into action – to effect change at the health system, community, and policy levels – so our patients can achieve optimal health,” Dr. Lee emphasized.
She noted that SGIM’s vision: “A just system of care in which all people can achieve optimal health,” underlies the meeting’s sessions.
Some challenges related to adopting more antiracist training in medical education center on faculty development, Dr. Lee noted. “There are also students who don’t feel that this is part of the role of being a physician. One way to overcome these challenges is by directly linking structural competency to health outcomes for our patients,” she added. “We have evidence that structural racism impacts health and we should make that clear to our educational leaders and faculty to increase buy in. So many of our SGIM members are working on developing curricula for this.”
Two of the meeting’s workshops that addressed racism in medicine and medical education and strategies for change were “Demystifying Structural Competency – How to Develop Antiracist Training in Medical Education,” and “Combating Systemic Racism in the Health Care System – Practical Actions You Can Take Today.” Below are some details about these.
Medical education evolves to include structural competency
In the workshop “Demystifying Structural Competency – How to Develop Antiracist Training in Medical Education,” participants used interactive exercises to build structural differentials for patient cases. The workshop was based in part on the experiences of including structural competency in medical education at Albert Einstein College of Medicine, New York, and the University of Pittsburgh.
During the session, participants practiced building a structural differential diagnosis in small groups, and also practiced using a structurally competent version of the 1-minute preceptor to promote structural competency in learners.
“Structural competency represents a shift in medical education towards attention to forces that influence health outcomes at levels above individual clinical interactions and develop a provider’s capacity to recognize and respond to health and illness as downstream effects of social, political and economic structures,” presenter Iman Hassan, MD, of Albert Einstein College of Medicine and Montefiore Health System, both in New York, said in an interview.
“At the same time, structural competency incorporates structural humility, which decentralizes the provider role in addressing structural factors and emphasizes collaboration with patients and communities,” she said in the interview. “Structural competency is a useful antiracism framework because it explicitly engages learners with the broader structural forces that result in health disparities, including structural racism and its downstream effects,” Dr. Hassan explained.
Addressing structural competency is important in medical education because structural and social determinants of health contribute more than half of overall health outcomes, said Dr. Hassan.
A structural competency framework equips learners to identify, discuss, and work with patients to navigate social needs such as lack of health insurance, food, or transportation, that are preventing them from accessing needed health care services, Dr. Hassan noted.
“Importantly, training in structural competency empowers physicians to be agents of change within their clinics, health systems, and communities and to recognize the value of community-led advocacy in promoting health equity,” she said.
Structural competency training also “will also allow them to engage more fully with the body of literature that exists surrounding social determinants of health and health disparities, and the use of approaches such as critical race theory through which to view health care,” she emphasized. “Importantly, understanding of the historical and structural context of medicine allows clinicians to more readily recognize when their own clinical practices, such as use of race-based clinical prediction tools, may perpetuate disparities, and work collectively to eliminate those practices.”
Recalibrating calculators for clinical care
Another workshop, “Combating Systemic Racism in the Health Care System – Practical Actions You Can Take Today,” took on the challenge of inherent bias in clinical care caused by various factors, notably medical calculators such as those used to measure kidney function and pulmonary function.
Lamar K. Johnson, MD, of Christiana Care Hospital Partners/Christiana Care Pediatric Hospitalists in Newark, Del., and Celeste Newby, MD, of Tulane University, New Orleans, discussed the inherent biases in some calculators and how to take those biases into account. A stated goal of the workshop was to increase awareness of the origins of medical calculators in order to enhance equity and improve shared decision-making between patients and providers.
Addressing implicit bias in clinical practice is important because such bias has been shown to negatively affect physician behavior and clinical decision making, Dr. Johnson said in an interview.
“These effects can also negatively affect the doctor-patient relationship and lead to poorer health outcomes due to delays in or avoidance of care or avoidance of the health care system, and mistrust, resulting in nonadherence,” Dr. Johnson noted.
“Implicit bias training helps empower medical students and residents to recognize and address bias and advocate for patients. Such training can potentially be beneficial to faculty, too,” Dr. Johnson emphasized in the interview.
“Race is primarily a social, not a biological, construct, and we must be careful when we use it, as its use in the past has been largely inappropriate and not scientifically sound,” he said.
During the session, one of the presenters said removing specific mentions of race from clinical documentation can reduce racial bias in clinical practice.
The presenters also highlighted the estimated glomerular filtration rate (eGFR) which is used to estimate kidney function.
The eGFR “reports higher eGFR values for Blacks based on a faulty hypothesis that Black people have higher muscle mass. This higher estimated value can delay referral for specialist care or transplantation, leading to worse outcomes,” Dr. Johnson explained in the interview.
In response, “Many major institutions have eliminated the race modifier in eGFR, and a joint task force created by the National Kidney Foundation and American Society of Nephrology has recommended against using a race modifier as of March 2021,” Dr. Johnson said.
The presenters had no relevant financial conflicts to disclose.
In her welcome video on the opening day at the annual meeting of the Society of General Internal Medicine, meeting chair Rita Lee, MD, said she hoped that this year’s event, though virtual, will allow attendees an opportunity to “regroup, find inspiration, and celebrate the incredible strengths and diverse voices of our community.”
“We are living in an incredibly polarized world,” Dr. Lee said in an interview. “ as recent events, such as the death of George Floyd and many others, plus the disparities revealed by the COVID-19 pandemic, have brought issues of structural racism and oppression in the United States to the forefront,” she said in the interview.
“Given these circumstances, it is important now, more than ever, for generalists to move our values into action – to effect change at the health system, community, and policy levels – so our patients can achieve optimal health,” Dr. Lee emphasized.
She noted that SGIM’s vision: “A just system of care in which all people can achieve optimal health,” underlies the meeting’s sessions.
Some challenges related to adopting more antiracist training in medical education center on faculty development, Dr. Lee noted. “There are also students who don’t feel that this is part of the role of being a physician. One way to overcome these challenges is by directly linking structural competency to health outcomes for our patients,” she added. “We have evidence that structural racism impacts health and we should make that clear to our educational leaders and faculty to increase buy in. So many of our SGIM members are working on developing curricula for this.”
Two of the meeting’s workshops that addressed racism in medicine and medical education and strategies for change were “Demystifying Structural Competency – How to Develop Antiracist Training in Medical Education,” and “Combating Systemic Racism in the Health Care System – Practical Actions You Can Take Today.” Below are some details about these.
Medical education evolves to include structural competency
In the workshop “Demystifying Structural Competency – How to Develop Antiracist Training in Medical Education,” participants used interactive exercises to build structural differentials for patient cases. The workshop was based in part on the experiences of including structural competency in medical education at Albert Einstein College of Medicine, New York, and the University of Pittsburgh.
During the session, participants practiced building a structural differential diagnosis in small groups, and also practiced using a structurally competent version of the 1-minute preceptor to promote structural competency in learners.
“Structural competency represents a shift in medical education towards attention to forces that influence health outcomes at levels above individual clinical interactions and develop a provider’s capacity to recognize and respond to health and illness as downstream effects of social, political and economic structures,” presenter Iman Hassan, MD, of Albert Einstein College of Medicine and Montefiore Health System, both in New York, said in an interview.
“At the same time, structural competency incorporates structural humility, which decentralizes the provider role in addressing structural factors and emphasizes collaboration with patients and communities,” she said in the interview. “Structural competency is a useful antiracism framework because it explicitly engages learners with the broader structural forces that result in health disparities, including structural racism and its downstream effects,” Dr. Hassan explained.
Addressing structural competency is important in medical education because structural and social determinants of health contribute more than half of overall health outcomes, said Dr. Hassan.
A structural competency framework equips learners to identify, discuss, and work with patients to navigate social needs such as lack of health insurance, food, or transportation, that are preventing them from accessing needed health care services, Dr. Hassan noted.
“Importantly, training in structural competency empowers physicians to be agents of change within their clinics, health systems, and communities and to recognize the value of community-led advocacy in promoting health equity,” she said.
Structural competency training also “will also allow them to engage more fully with the body of literature that exists surrounding social determinants of health and health disparities, and the use of approaches such as critical race theory through which to view health care,” she emphasized. “Importantly, understanding of the historical and structural context of medicine allows clinicians to more readily recognize when their own clinical practices, such as use of race-based clinical prediction tools, may perpetuate disparities, and work collectively to eliminate those practices.”
Recalibrating calculators for clinical care
Another workshop, “Combating Systemic Racism in the Health Care System – Practical Actions You Can Take Today,” took on the challenge of inherent bias in clinical care caused by various factors, notably medical calculators such as those used to measure kidney function and pulmonary function.
Lamar K. Johnson, MD, of Christiana Care Hospital Partners/Christiana Care Pediatric Hospitalists in Newark, Del., and Celeste Newby, MD, of Tulane University, New Orleans, discussed the inherent biases in some calculators and how to take those biases into account. A stated goal of the workshop was to increase awareness of the origins of medical calculators in order to enhance equity and improve shared decision-making between patients and providers.
Addressing implicit bias in clinical practice is important because such bias has been shown to negatively affect physician behavior and clinical decision making, Dr. Johnson said in an interview.
“These effects can also negatively affect the doctor-patient relationship and lead to poorer health outcomes due to delays in or avoidance of care or avoidance of the health care system, and mistrust, resulting in nonadherence,” Dr. Johnson noted.
“Implicit bias training helps empower medical students and residents to recognize and address bias and advocate for patients. Such training can potentially be beneficial to faculty, too,” Dr. Johnson emphasized in the interview.
“Race is primarily a social, not a biological, construct, and we must be careful when we use it, as its use in the past has been largely inappropriate and not scientifically sound,” he said.
During the session, one of the presenters said removing specific mentions of race from clinical documentation can reduce racial bias in clinical practice.
The presenters also highlighted the estimated glomerular filtration rate (eGFR) which is used to estimate kidney function.
The eGFR “reports higher eGFR values for Blacks based on a faulty hypothesis that Black people have higher muscle mass. This higher estimated value can delay referral for specialist care or transplantation, leading to worse outcomes,” Dr. Johnson explained in the interview.
In response, “Many major institutions have eliminated the race modifier in eGFR, and a joint task force created by the National Kidney Foundation and American Society of Nephrology has recommended against using a race modifier as of March 2021,” Dr. Johnson said.
The presenters had no relevant financial conflicts to disclose.
In her welcome video on the opening day at the annual meeting of the Society of General Internal Medicine, meeting chair Rita Lee, MD, said she hoped that this year’s event, though virtual, will allow attendees an opportunity to “regroup, find inspiration, and celebrate the incredible strengths and diverse voices of our community.”
“We are living in an incredibly polarized world,” Dr. Lee said in an interview. “ as recent events, such as the death of George Floyd and many others, plus the disparities revealed by the COVID-19 pandemic, have brought issues of structural racism and oppression in the United States to the forefront,” she said in the interview.
“Given these circumstances, it is important now, more than ever, for generalists to move our values into action – to effect change at the health system, community, and policy levels – so our patients can achieve optimal health,” Dr. Lee emphasized.
She noted that SGIM’s vision: “A just system of care in which all people can achieve optimal health,” underlies the meeting’s sessions.
Some challenges related to adopting more antiracist training in medical education center on faculty development, Dr. Lee noted. “There are also students who don’t feel that this is part of the role of being a physician. One way to overcome these challenges is by directly linking structural competency to health outcomes for our patients,” she added. “We have evidence that structural racism impacts health and we should make that clear to our educational leaders and faculty to increase buy in. So many of our SGIM members are working on developing curricula for this.”
Two of the meeting’s workshops that addressed racism in medicine and medical education and strategies for change were “Demystifying Structural Competency – How to Develop Antiracist Training in Medical Education,” and “Combating Systemic Racism in the Health Care System – Practical Actions You Can Take Today.” Below are some details about these.
Medical education evolves to include structural competency
In the workshop “Demystifying Structural Competency – How to Develop Antiracist Training in Medical Education,” participants used interactive exercises to build structural differentials for patient cases. The workshop was based in part on the experiences of including structural competency in medical education at Albert Einstein College of Medicine, New York, and the University of Pittsburgh.
During the session, participants practiced building a structural differential diagnosis in small groups, and also practiced using a structurally competent version of the 1-minute preceptor to promote structural competency in learners.
“Structural competency represents a shift in medical education towards attention to forces that influence health outcomes at levels above individual clinical interactions and develop a provider’s capacity to recognize and respond to health and illness as downstream effects of social, political and economic structures,” presenter Iman Hassan, MD, of Albert Einstein College of Medicine and Montefiore Health System, both in New York, said in an interview.
“At the same time, structural competency incorporates structural humility, which decentralizes the provider role in addressing structural factors and emphasizes collaboration with patients and communities,” she said in the interview. “Structural competency is a useful antiracism framework because it explicitly engages learners with the broader structural forces that result in health disparities, including structural racism and its downstream effects,” Dr. Hassan explained.
Addressing structural competency is important in medical education because structural and social determinants of health contribute more than half of overall health outcomes, said Dr. Hassan.
A structural competency framework equips learners to identify, discuss, and work with patients to navigate social needs such as lack of health insurance, food, or transportation, that are preventing them from accessing needed health care services, Dr. Hassan noted.
“Importantly, training in structural competency empowers physicians to be agents of change within their clinics, health systems, and communities and to recognize the value of community-led advocacy in promoting health equity,” she said.
Structural competency training also “will also allow them to engage more fully with the body of literature that exists surrounding social determinants of health and health disparities, and the use of approaches such as critical race theory through which to view health care,” she emphasized. “Importantly, understanding of the historical and structural context of medicine allows clinicians to more readily recognize when their own clinical practices, such as use of race-based clinical prediction tools, may perpetuate disparities, and work collectively to eliminate those practices.”
Recalibrating calculators for clinical care
Another workshop, “Combating Systemic Racism in the Health Care System – Practical Actions You Can Take Today,” took on the challenge of inherent bias in clinical care caused by various factors, notably medical calculators such as those used to measure kidney function and pulmonary function.
Lamar K. Johnson, MD, of Christiana Care Hospital Partners/Christiana Care Pediatric Hospitalists in Newark, Del., and Celeste Newby, MD, of Tulane University, New Orleans, discussed the inherent biases in some calculators and how to take those biases into account. A stated goal of the workshop was to increase awareness of the origins of medical calculators in order to enhance equity and improve shared decision-making between patients and providers.
Addressing implicit bias in clinical practice is important because such bias has been shown to negatively affect physician behavior and clinical decision making, Dr. Johnson said in an interview.
“These effects can also negatively affect the doctor-patient relationship and lead to poorer health outcomes due to delays in or avoidance of care or avoidance of the health care system, and mistrust, resulting in nonadherence,” Dr. Johnson noted.
“Implicit bias training helps empower medical students and residents to recognize and address bias and advocate for patients. Such training can potentially be beneficial to faculty, too,” Dr. Johnson emphasized in the interview.
“Race is primarily a social, not a biological, construct, and we must be careful when we use it, as its use in the past has been largely inappropriate and not scientifically sound,” he said.
During the session, one of the presenters said removing specific mentions of race from clinical documentation can reduce racial bias in clinical practice.
The presenters also highlighted the estimated glomerular filtration rate (eGFR) which is used to estimate kidney function.
The eGFR “reports higher eGFR values for Blacks based on a faulty hypothesis that Black people have higher muscle mass. This higher estimated value can delay referral for specialist care or transplantation, leading to worse outcomes,” Dr. Johnson explained in the interview.
In response, “Many major institutions have eliminated the race modifier in eGFR, and a joint task force created by the National Kidney Foundation and American Society of Nephrology has recommended against using a race modifier as of March 2021,” Dr. Johnson said.
The presenters had no relevant financial conflicts to disclose.
FROM SGIM 2021
‘Malicious peer review’ destroyed doc’s career, he says
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
The problem with samples
Ubrelvy and Nurtec are the latest in acute migraine treatment, both with solid data to back them up.
As with the triptans 25 years ago, my sample cabinet (and probably everyone else’s) is loaded with them, and friendly sales reps bringing coupon cards are a frequent occurrence.
Unfortunately, samples also bring up the same conundrum I faced with the triptans earlier in my career. It’s one thing to give patients samples to see if they work. It’s quite another to get them covered if they do.
This is an ongoing issue in modern medicine. It’s hard to resist the temptation to just hand something out when it’s conveniently at hand. It saves the patient a trip to the pharmacy and a medication copay up front, which is great.
But if it works, you have a whole new set of issues. The patient wants a real prescription now. So you call it in, then get a denial back saying it isn’t covered. It tells you to call a number, or try CoverMyMeds.
You do that, but the patient has to have failed three triptans, two NSAIDs, and a partridge in a pear tree to get it approved. The “copay assistance cards” don’t help if the medication isn’t covered at all. Each of these new medications is currently listed at roughly $900/month on GoodRx.com. Inevitably, your staff gets an earful when a patient with sticker shock calls your office.
One manufacturer is now eating the cost of the first script, so the patient leaves the pharmacy with a 1-month supply, under the impression that it was covered by insurance. This only kicks the can down the road 4-6 weeks, until they call for a refill.
To the chagrin of my sales reps (who are certainly going to read this), I’ve been burned on this and similar issues many times in my career, so I don’t even bother playing the game.
Certainly, there are cases where handing out a sample of Ubrelvy or Nurtec is indicated – some patients have already failed other agents, or have medical contraindications to them – but most don’t. So I start with triptans, currently going for $15/month. That doesn’t mean I’m not open to a newer agent at some point, but leaping directly to them quickly becomes an exercise in frustration.
Which brings up another issue I’ve encountered. While I try to be aware of this sort of thing, many other docs aren’t. Especially my already overburdened colleagues in primary care, who have enough on their plate with COVID-19, insurance regulations, paperwork, and an insanely busy schedule. In the controlled chaos of a general practice, it’s often easier for the staff to just hand out a sample at the same time they refer to a neurologist. So when the patient comes to me, they’re expecting I’ll be able to get it covered. After all, I’m the specialist. Getting expensive tests and medications covered seem to be something that’s expected for the higher copay to see me.
It doesn’t work that way, either.
I have nothing against new drugs. It’s the breakthroughs that keep medicine moving forward (like the COVID-19 vaccines). Nor do I have anything against samples or sales reps.
But in many cases, the time you save handing out samples isn’t worth the time you have to spend on them down the line.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Ubrelvy and Nurtec are the latest in acute migraine treatment, both with solid data to back them up.
As with the triptans 25 years ago, my sample cabinet (and probably everyone else’s) is loaded with them, and friendly sales reps bringing coupon cards are a frequent occurrence.
Unfortunately, samples also bring up the same conundrum I faced with the triptans earlier in my career. It’s one thing to give patients samples to see if they work. It’s quite another to get them covered if they do.
This is an ongoing issue in modern medicine. It’s hard to resist the temptation to just hand something out when it’s conveniently at hand. It saves the patient a trip to the pharmacy and a medication copay up front, which is great.
But if it works, you have a whole new set of issues. The patient wants a real prescription now. So you call it in, then get a denial back saying it isn’t covered. It tells you to call a number, or try CoverMyMeds.
You do that, but the patient has to have failed three triptans, two NSAIDs, and a partridge in a pear tree to get it approved. The “copay assistance cards” don’t help if the medication isn’t covered at all. Each of these new medications is currently listed at roughly $900/month on GoodRx.com. Inevitably, your staff gets an earful when a patient with sticker shock calls your office.
One manufacturer is now eating the cost of the first script, so the patient leaves the pharmacy with a 1-month supply, under the impression that it was covered by insurance. This only kicks the can down the road 4-6 weeks, until they call for a refill.
To the chagrin of my sales reps (who are certainly going to read this), I’ve been burned on this and similar issues many times in my career, so I don’t even bother playing the game.
Certainly, there are cases where handing out a sample of Ubrelvy or Nurtec is indicated – some patients have already failed other agents, or have medical contraindications to them – but most don’t. So I start with triptans, currently going for $15/month. That doesn’t mean I’m not open to a newer agent at some point, but leaping directly to them quickly becomes an exercise in frustration.
Which brings up another issue I’ve encountered. While I try to be aware of this sort of thing, many other docs aren’t. Especially my already overburdened colleagues in primary care, who have enough on their plate with COVID-19, insurance regulations, paperwork, and an insanely busy schedule. In the controlled chaos of a general practice, it’s often easier for the staff to just hand out a sample at the same time they refer to a neurologist. So when the patient comes to me, they’re expecting I’ll be able to get it covered. After all, I’m the specialist. Getting expensive tests and medications covered seem to be something that’s expected for the higher copay to see me.
It doesn’t work that way, either.
I have nothing against new drugs. It’s the breakthroughs that keep medicine moving forward (like the COVID-19 vaccines). Nor do I have anything against samples or sales reps.
But in many cases, the time you save handing out samples isn’t worth the time you have to spend on them down the line.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Ubrelvy and Nurtec are the latest in acute migraine treatment, both with solid data to back them up.
As with the triptans 25 years ago, my sample cabinet (and probably everyone else’s) is loaded with them, and friendly sales reps bringing coupon cards are a frequent occurrence.
Unfortunately, samples also bring up the same conundrum I faced with the triptans earlier in my career. It’s one thing to give patients samples to see if they work. It’s quite another to get them covered if they do.
This is an ongoing issue in modern medicine. It’s hard to resist the temptation to just hand something out when it’s conveniently at hand. It saves the patient a trip to the pharmacy and a medication copay up front, which is great.
But if it works, you have a whole new set of issues. The patient wants a real prescription now. So you call it in, then get a denial back saying it isn’t covered. It tells you to call a number, or try CoverMyMeds.
You do that, but the patient has to have failed three triptans, two NSAIDs, and a partridge in a pear tree to get it approved. The “copay assistance cards” don’t help if the medication isn’t covered at all. Each of these new medications is currently listed at roughly $900/month on GoodRx.com. Inevitably, your staff gets an earful when a patient with sticker shock calls your office.
One manufacturer is now eating the cost of the first script, so the patient leaves the pharmacy with a 1-month supply, under the impression that it was covered by insurance. This only kicks the can down the road 4-6 weeks, until they call for a refill.
To the chagrin of my sales reps (who are certainly going to read this), I’ve been burned on this and similar issues many times in my career, so I don’t even bother playing the game.
Certainly, there are cases where handing out a sample of Ubrelvy or Nurtec is indicated – some patients have already failed other agents, or have medical contraindications to them – but most don’t. So I start with triptans, currently going for $15/month. That doesn’t mean I’m not open to a newer agent at some point, but leaping directly to them quickly becomes an exercise in frustration.
Which brings up another issue I’ve encountered. While I try to be aware of this sort of thing, many other docs aren’t. Especially my already overburdened colleagues in primary care, who have enough on their plate with COVID-19, insurance regulations, paperwork, and an insanely busy schedule. In the controlled chaos of a general practice, it’s often easier for the staff to just hand out a sample at the same time they refer to a neurologist. So when the patient comes to me, they’re expecting I’ll be able to get it covered. After all, I’m the specialist. Getting expensive tests and medications covered seem to be something that’s expected for the higher copay to see me.
It doesn’t work that way, either.
I have nothing against new drugs. It’s the breakthroughs that keep medicine moving forward (like the COVID-19 vaccines). Nor do I have anything against samples or sales reps.
But in many cases, the time you save handing out samples isn’t worth the time you have to spend on them down the line.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Pandemic took a cut of cosmetic procedures in 2020
pandemic, according to the American Society of Plastic Surgeons.
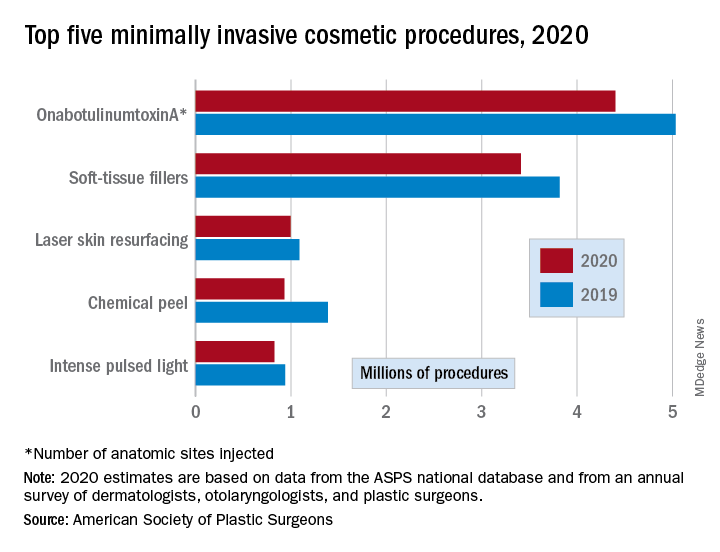
There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
Virtual APA vs. the real thing: Which is better?
Every spring, I look forward to attending the American Psychiatric Association’s annual meeting. It has become a ritual that starts many months before the actual conference.
Submissions for presentations are due in September, so the planning often starts in the late summer. Hotel and plane reservations are made in January, and the meeting itself begins in May.
The city that hosts the event changes each year but, for me, many things do not. The Clinical Psychiatry News editorial board meeting takes place on Monday morning at 7 a.m., and I scour the program for what sessions to attend. In recent years, I have made a point of writing an article for about one of the sessions while still at the meeting – in 2019 I wrote about the improv-acting workshops I attended – something that just doesn’t translate to a Zoom experience.
I go with the same friend every year, I always attend the Hopkins alumni reception, and I organize dinner at a nice restaurant for friends. I have collected so many funny stories and memories over the years that it would be hard to catalog them all. There was the time in Toronto that I set up a meal at a restaurant named Susur – a meal like no other I’ve ever had – and the check arrived with a jaw-dropping sum that I had not anticipated. In San Diego, we watched a gorgeous sunset over the Pacific Ocean from the veranda of the Hotel Coronado. There was the time I sunbathed on the beach in Waikiki with my book editor, and the notable distress when my colleague’s husband called from the airport to say he was not permitted to board his plane in Baltimore to join us in California! There are funny stories, but there is the sadness that one friend who joined us for so many of these events has died.
I always find the program options to be overwhelming: There is so much going on at once that it can be hard to decide what to go to. I try to attend a mix of sessions, some that are inspiring or entertaining, and others that will be informative for clinical issues.
The speakers have been incredible and over the years I’ve heard then-Vice President Joseph Biden, retired quarterback Terry Bradshaw, Oliver Sacks, Alan Alda, Archbishop Desmond Tutu, and perhaps my favorite – Lorraine Bracco, the actress who played Dr. Melfi on “The Sopranos” – to name just a few. And, of course, the opportunity to get the continuing medical education credits I need for licensing is just one more reason to attend.
Last year in May I was still adjusting to my “new” career from home with a computer screen. I had been scheduled to participate in several panels for the meeting in Philadelphia, but extra computer hours had no appeal. And while the fatigue of doing telemental health has eased, I still avoid extra hours interacting with my computer screen and I did not attend this year’s meeting. Without the lure of friends, fun, and the novelty of being somewhere new, my APA experience would have to wait for real life.
Virtual APA has had a drop in participation. In 2019, the last real-life convention in San Francisco, there were 700 scientific sessions and 11,000 professionals in attendance. This year’s virtual conference hosted 135 sessions with more than 7,000 attendees. Attendance was down, but so were costs associated with live conventions and
Tom Abdallah is a medical student at Weill Cornell Medicine–Qatar in Education City. He has never attended an in-person APA annual meeting, but he joined for this year’s virtual sessions. “The scientific sessions were fantastic and diverse. Networking was limited in comparison to in-person conferences. The meeting was very well organized, and it gave me the opportunity to attend without worrying about travel.”
Steven Daviss, MD, a psychiatrist in Maryland, also commented on the ease and financial benefit of attending the meeting from his home office. He calculated that the cost was much less: $350 for virtual APA, compared with approximately $3,500 for the real thing, allowing for transportation, hotels, meals out, and lost income. “But,” said Dr. Daviss, “engagement with colleagues was minimal.”
APA Assembly member Annette Hanson, MD, has continued to go into work throughout the pandemic. Still, she noted that meetings and committee work have made sure she does not miss out on the “Zoom fatigue” that everyone else is feeling. The virtual APA was tiring for her.
“It was brutal. There was the APA Assembly 1 weekend, right after evening Zoom reference committee meetings the week before. Then virtual APA the next weekend. By the end of the week, I had worked every day for 3 weeks straight, including my more-than-full-time job!”
It has been a challenging time, to say the least, and it has certainly helped that videoconferencing has allowed us to be there for our patients and for each other in so many different circumstances. Former APA President Paul Summergrad, MD, talked about how virtual meetings can be very good as educational tools, but he conveyed what I have been feeling in a sentence: “I miss the social aspect of meetings.”
Please get your vaccine, and I hope to see you in New Orleans next May!
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Every spring, I look forward to attending the American Psychiatric Association’s annual meeting. It has become a ritual that starts many months before the actual conference.
Submissions for presentations are due in September, so the planning often starts in the late summer. Hotel and plane reservations are made in January, and the meeting itself begins in May.
The city that hosts the event changes each year but, for me, many things do not. The Clinical Psychiatry News editorial board meeting takes place on Monday morning at 7 a.m., and I scour the program for what sessions to attend. In recent years, I have made a point of writing an article for about one of the sessions while still at the meeting – in 2019 I wrote about the improv-acting workshops I attended – something that just doesn’t translate to a Zoom experience.
I go with the same friend every year, I always attend the Hopkins alumni reception, and I organize dinner at a nice restaurant for friends. I have collected so many funny stories and memories over the years that it would be hard to catalog them all. There was the time in Toronto that I set up a meal at a restaurant named Susur – a meal like no other I’ve ever had – and the check arrived with a jaw-dropping sum that I had not anticipated. In San Diego, we watched a gorgeous sunset over the Pacific Ocean from the veranda of the Hotel Coronado. There was the time I sunbathed on the beach in Waikiki with my book editor, and the notable distress when my colleague’s husband called from the airport to say he was not permitted to board his plane in Baltimore to join us in California! There are funny stories, but there is the sadness that one friend who joined us for so many of these events has died.
I always find the program options to be overwhelming: There is so much going on at once that it can be hard to decide what to go to. I try to attend a mix of sessions, some that are inspiring or entertaining, and others that will be informative for clinical issues.
The speakers have been incredible and over the years I’ve heard then-Vice President Joseph Biden, retired quarterback Terry Bradshaw, Oliver Sacks, Alan Alda, Archbishop Desmond Tutu, and perhaps my favorite – Lorraine Bracco, the actress who played Dr. Melfi on “The Sopranos” – to name just a few. And, of course, the opportunity to get the continuing medical education credits I need for licensing is just one more reason to attend.
Last year in May I was still adjusting to my “new” career from home with a computer screen. I had been scheduled to participate in several panels for the meeting in Philadelphia, but extra computer hours had no appeal. And while the fatigue of doing telemental health has eased, I still avoid extra hours interacting with my computer screen and I did not attend this year’s meeting. Without the lure of friends, fun, and the novelty of being somewhere new, my APA experience would have to wait for real life.
Virtual APA has had a drop in participation. In 2019, the last real-life convention in San Francisco, there were 700 scientific sessions and 11,000 professionals in attendance. This year’s virtual conference hosted 135 sessions with more than 7,000 attendees. Attendance was down, but so were costs associated with live conventions and
Tom Abdallah is a medical student at Weill Cornell Medicine–Qatar in Education City. He has never attended an in-person APA annual meeting, but he joined for this year’s virtual sessions. “The scientific sessions were fantastic and diverse. Networking was limited in comparison to in-person conferences. The meeting was very well organized, and it gave me the opportunity to attend without worrying about travel.”
Steven Daviss, MD, a psychiatrist in Maryland, also commented on the ease and financial benefit of attending the meeting from his home office. He calculated that the cost was much less: $350 for virtual APA, compared with approximately $3,500 for the real thing, allowing for transportation, hotels, meals out, and lost income. “But,” said Dr. Daviss, “engagement with colleagues was minimal.”
APA Assembly member Annette Hanson, MD, has continued to go into work throughout the pandemic. Still, she noted that meetings and committee work have made sure she does not miss out on the “Zoom fatigue” that everyone else is feeling. The virtual APA was tiring for her.
“It was brutal. There was the APA Assembly 1 weekend, right after evening Zoom reference committee meetings the week before. Then virtual APA the next weekend. By the end of the week, I had worked every day for 3 weeks straight, including my more-than-full-time job!”
It has been a challenging time, to say the least, and it has certainly helped that videoconferencing has allowed us to be there for our patients and for each other in so many different circumstances. Former APA President Paul Summergrad, MD, talked about how virtual meetings can be very good as educational tools, but he conveyed what I have been feeling in a sentence: “I miss the social aspect of meetings.”
Please get your vaccine, and I hope to see you in New Orleans next May!
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Every spring, I look forward to attending the American Psychiatric Association’s annual meeting. It has become a ritual that starts many months before the actual conference.
Submissions for presentations are due in September, so the planning often starts in the late summer. Hotel and plane reservations are made in January, and the meeting itself begins in May.
The city that hosts the event changes each year but, for me, many things do not. The Clinical Psychiatry News editorial board meeting takes place on Monday morning at 7 a.m., and I scour the program for what sessions to attend. In recent years, I have made a point of writing an article for about one of the sessions while still at the meeting – in 2019 I wrote about the improv-acting workshops I attended – something that just doesn’t translate to a Zoom experience.
I go with the same friend every year, I always attend the Hopkins alumni reception, and I organize dinner at a nice restaurant for friends. I have collected so many funny stories and memories over the years that it would be hard to catalog them all. There was the time in Toronto that I set up a meal at a restaurant named Susur – a meal like no other I’ve ever had – and the check arrived with a jaw-dropping sum that I had not anticipated. In San Diego, we watched a gorgeous sunset over the Pacific Ocean from the veranda of the Hotel Coronado. There was the time I sunbathed on the beach in Waikiki with my book editor, and the notable distress when my colleague’s husband called from the airport to say he was not permitted to board his plane in Baltimore to join us in California! There are funny stories, but there is the sadness that one friend who joined us for so many of these events has died.
I always find the program options to be overwhelming: There is so much going on at once that it can be hard to decide what to go to. I try to attend a mix of sessions, some that are inspiring or entertaining, and others that will be informative for clinical issues.
The speakers have been incredible and over the years I’ve heard then-Vice President Joseph Biden, retired quarterback Terry Bradshaw, Oliver Sacks, Alan Alda, Archbishop Desmond Tutu, and perhaps my favorite – Lorraine Bracco, the actress who played Dr. Melfi on “The Sopranos” – to name just a few. And, of course, the opportunity to get the continuing medical education credits I need for licensing is just one more reason to attend.
Last year in May I was still adjusting to my “new” career from home with a computer screen. I had been scheduled to participate in several panels for the meeting in Philadelphia, but extra computer hours had no appeal. And while the fatigue of doing telemental health has eased, I still avoid extra hours interacting with my computer screen and I did not attend this year’s meeting. Without the lure of friends, fun, and the novelty of being somewhere new, my APA experience would have to wait for real life.
Virtual APA has had a drop in participation. In 2019, the last real-life convention in San Francisco, there were 700 scientific sessions and 11,000 professionals in attendance. This year’s virtual conference hosted 135 sessions with more than 7,000 attendees. Attendance was down, but so were costs associated with live conventions and
Tom Abdallah is a medical student at Weill Cornell Medicine–Qatar in Education City. He has never attended an in-person APA annual meeting, but he joined for this year’s virtual sessions. “The scientific sessions were fantastic and diverse. Networking was limited in comparison to in-person conferences. The meeting was very well organized, and it gave me the opportunity to attend without worrying about travel.”
Steven Daviss, MD, a psychiatrist in Maryland, also commented on the ease and financial benefit of attending the meeting from his home office. He calculated that the cost was much less: $350 for virtual APA, compared with approximately $3,500 for the real thing, allowing for transportation, hotels, meals out, and lost income. “But,” said Dr. Daviss, “engagement with colleagues was minimal.”
APA Assembly member Annette Hanson, MD, has continued to go into work throughout the pandemic. Still, she noted that meetings and committee work have made sure she does not miss out on the “Zoom fatigue” that everyone else is feeling. The virtual APA was tiring for her.
“It was brutal. There was the APA Assembly 1 weekend, right after evening Zoom reference committee meetings the week before. Then virtual APA the next weekend. By the end of the week, I had worked every day for 3 weeks straight, including my more-than-full-time job!”
It has been a challenging time, to say the least, and it has certainly helped that videoconferencing has allowed us to be there for our patients and for each other in so many different circumstances. Former APA President Paul Summergrad, MD, talked about how virtual meetings can be very good as educational tools, but he conveyed what I have been feeling in a sentence: “I miss the social aspect of meetings.”
Please get your vaccine, and I hope to see you in New Orleans next May!
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Who can call themselves ‘doctor’? The debate heats up
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.







