User login
Yale’s COVID-19 inpatient protocol: Hydroxychloroquine plus/minus tocilizumab
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
aotto@mdedge.com
This article was updated with the latest treatment algorithm on 5/6/2020.
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
aotto@mdedge.com
This article was updated with the latest treatment algorithm on 5/6/2020.
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
aotto@mdedge.com
This article was updated with the latest treatment algorithm on 5/6/2020.
Substantial very late MACE risk after PCI for SIHD
Patients with stable ischemic heart disease remain at substantial risk for major adverse cardiovascular events 1-5 years after percutaneous coronary intervention, even with contemporary second-generation drug-eluting stents, according to a pooled analysis of long-term follow-up data on 10,987 patients in 19 prospective, randomized, head-to-head metallic stent trials.
The analysis showed that, although most major adverse cardiovascular events (MACE) occurred during the first year after stenting, no plateau in MACE was reached between years 1 and 5, Mahesh V. Madhavan, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“Further studies are required to understand the mechanisms of late events and whether improvements in stent technology, revascularization technique, and adjunctive therapies may improve outcomes in patients with SIHD [stable ischemic heart disease],” said Dr. Madhavan, a cardiology fellow at Columbia University Irving Medical Center and New York–Presbyterian Hospital.
This post hoc analysis of pooled individual patient-level data from 19 randomized trials included 10,987 metallic stent recipients with SIHD. Sixty-one percent got second-generation drug-eluting stents (DES), 25% received first-generation DES, and 15% got bare metal stents (BMS). The largest prospective head-to-head RCT was SPIRIT IV, with 2,130 patients. All five TAXUS trials were also included.
The 5-year rate of the primary composite MACE endpoint composed of cardiac death, MI, or ischemia-driven target lesion revascularization was 24.1% in patients with BMS stents, 17.9% with first-gen DES, and 13.4% with second-gen DES, reflecting the advances in stent technology over time. Most of these MACE events occurred during the first year after PCI, with rates of 18%, 8.6%, and 5.3%, respectively, in the three groups. However, the MACE rate beyond the first year out through year 5 remained substantial: 10.2% with first-gen DES, 8.5% with second-gen DES, and 7.4% in the BMS group.
The cardiac death rate from PCI through year 5 was 3.8% with second-gen DES, 3.6% with first-gen DES, and 3.3% with BMS. The MI rate was 7.7% with first-gen DES, 6.1% with BMS, and 5% with second-gen DES.
Stent thrombosis occurred during the first year in 0.9% of first-gen DES and BMS recipients and in 0.7% of patients with second-gen DES. During years 1-5, the rates were 1.6% with first-gen DES, 0.9% with second-gen devices, and 0.2% with BMS.
Second-gen DES provided a big advantage in terms of lessened need for ischemia-driven target lesion revascularization through the first 5 years, with a rate of 7.3%, compared to 18.7% in patients with first-gen DES and 10.5% with BMS.
In a multivariate regression analysis, independent predictors of MACE in the first 5 years post PCI included indicators of greater lesion and/or procedural complexity, such as left main or left anterior descending disease, greater lesion length, and more than one treated lesion, as well as standard cardiovascular risk factors, including recent smoking, hypertension, and diabetes.
In contrast, hyperlipidemia was associated with a significant 15% reduction in MACE risk, which in an interview Dr. Madhavan said may have been due to aggressive lipid-lowering therapy, although he added that this is conjecture because he and his coinvestigators didn’t have access to data on the use of guideline-directed medical therapy or antiplatelet regimens.
Asked about future prospects for reducing the substantial very late risk of MACE highlighted in his study, Dr. Madhavan cited the use of adjunctive imaging during PCI as promising.
“The currently enrolling ILUMEN IV trial, among other studies, will help determine whether imaging-guided intervention can help improve intermediate and long-term rates of MACE,” he observed.
Promising medical therapies that could potentially confer benefit in terms of reducing long-term MACE in patients who’ve undergone PCI for SIHD include novel lipid-lowering drugs, tailored antithrombotic strategies, new anti-inflammatory agents, and the SGLT2 inhibitors, Dr. Madhavan continued.
In terms of advances in stent design, he cited recent evidence that ultrathin-strut stents featuring bioresorbable polymer, such as the Orsiro stent, may reduce late stent-related MACE through 3 years.
“We’ll have to see if these benefits extend to longer-term follow-up up to 5 years,” he said.
He deemed his study results “fairly consistent” with those of the ISCHEMIA trial, where ischemic events in the patients with SIHD assigned to an initial invasive strategy continued to occur in the latter years of follow-up without any clear plateau effect (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Dr. Madhavan reported no financial conflicts regarding his study, funded by an institutional research grant from the National Heart, Lung, and Blood Institute.
Shortly following Dr. Madhavan’s presentation at ACC 2020, the study results were published online (Circ Cardiovasc Interv. 2020 Apr;13[4[:e008565. doi: 10.1161/CIRCINTERVENTIONS.119.008565).
SOURCE: Madhavan MV. ACC 2020, Abstract 909-10.
Patients with stable ischemic heart disease remain at substantial risk for major adverse cardiovascular events 1-5 years after percutaneous coronary intervention, even with contemporary second-generation drug-eluting stents, according to a pooled analysis of long-term follow-up data on 10,987 patients in 19 prospective, randomized, head-to-head metallic stent trials.
The analysis showed that, although most major adverse cardiovascular events (MACE) occurred during the first year after stenting, no plateau in MACE was reached between years 1 and 5, Mahesh V. Madhavan, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“Further studies are required to understand the mechanisms of late events and whether improvements in stent technology, revascularization technique, and adjunctive therapies may improve outcomes in patients with SIHD [stable ischemic heart disease],” said Dr. Madhavan, a cardiology fellow at Columbia University Irving Medical Center and New York–Presbyterian Hospital.
This post hoc analysis of pooled individual patient-level data from 19 randomized trials included 10,987 metallic stent recipients with SIHD. Sixty-one percent got second-generation drug-eluting stents (DES), 25% received first-generation DES, and 15% got bare metal stents (BMS). The largest prospective head-to-head RCT was SPIRIT IV, with 2,130 patients. All five TAXUS trials were also included.
The 5-year rate of the primary composite MACE endpoint composed of cardiac death, MI, or ischemia-driven target lesion revascularization was 24.1% in patients with BMS stents, 17.9% with first-gen DES, and 13.4% with second-gen DES, reflecting the advances in stent technology over time. Most of these MACE events occurred during the first year after PCI, with rates of 18%, 8.6%, and 5.3%, respectively, in the three groups. However, the MACE rate beyond the first year out through year 5 remained substantial: 10.2% with first-gen DES, 8.5% with second-gen DES, and 7.4% in the BMS group.
The cardiac death rate from PCI through year 5 was 3.8% with second-gen DES, 3.6% with first-gen DES, and 3.3% with BMS. The MI rate was 7.7% with first-gen DES, 6.1% with BMS, and 5% with second-gen DES.
Stent thrombosis occurred during the first year in 0.9% of first-gen DES and BMS recipients and in 0.7% of patients with second-gen DES. During years 1-5, the rates were 1.6% with first-gen DES, 0.9% with second-gen devices, and 0.2% with BMS.
Second-gen DES provided a big advantage in terms of lessened need for ischemia-driven target lesion revascularization through the first 5 years, with a rate of 7.3%, compared to 18.7% in patients with first-gen DES and 10.5% with BMS.
In a multivariate regression analysis, independent predictors of MACE in the first 5 years post PCI included indicators of greater lesion and/or procedural complexity, such as left main or left anterior descending disease, greater lesion length, and more than one treated lesion, as well as standard cardiovascular risk factors, including recent smoking, hypertension, and diabetes.
In contrast, hyperlipidemia was associated with a significant 15% reduction in MACE risk, which in an interview Dr. Madhavan said may have been due to aggressive lipid-lowering therapy, although he added that this is conjecture because he and his coinvestigators didn’t have access to data on the use of guideline-directed medical therapy or antiplatelet regimens.
Asked about future prospects for reducing the substantial very late risk of MACE highlighted in his study, Dr. Madhavan cited the use of adjunctive imaging during PCI as promising.
“The currently enrolling ILUMEN IV trial, among other studies, will help determine whether imaging-guided intervention can help improve intermediate and long-term rates of MACE,” he observed.
Promising medical therapies that could potentially confer benefit in terms of reducing long-term MACE in patients who’ve undergone PCI for SIHD include novel lipid-lowering drugs, tailored antithrombotic strategies, new anti-inflammatory agents, and the SGLT2 inhibitors, Dr. Madhavan continued.
In terms of advances in stent design, he cited recent evidence that ultrathin-strut stents featuring bioresorbable polymer, such as the Orsiro stent, may reduce late stent-related MACE through 3 years.
“We’ll have to see if these benefits extend to longer-term follow-up up to 5 years,” he said.
He deemed his study results “fairly consistent” with those of the ISCHEMIA trial, where ischemic events in the patients with SIHD assigned to an initial invasive strategy continued to occur in the latter years of follow-up without any clear plateau effect (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Dr. Madhavan reported no financial conflicts regarding his study, funded by an institutional research grant from the National Heart, Lung, and Blood Institute.
Shortly following Dr. Madhavan’s presentation at ACC 2020, the study results were published online (Circ Cardiovasc Interv. 2020 Apr;13[4[:e008565. doi: 10.1161/CIRCINTERVENTIONS.119.008565).
SOURCE: Madhavan MV. ACC 2020, Abstract 909-10.
Patients with stable ischemic heart disease remain at substantial risk for major adverse cardiovascular events 1-5 years after percutaneous coronary intervention, even with contemporary second-generation drug-eluting stents, according to a pooled analysis of long-term follow-up data on 10,987 patients in 19 prospective, randomized, head-to-head metallic stent trials.
The analysis showed that, although most major adverse cardiovascular events (MACE) occurred during the first year after stenting, no plateau in MACE was reached between years 1 and 5, Mahesh V. Madhavan, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“Further studies are required to understand the mechanisms of late events and whether improvements in stent technology, revascularization technique, and adjunctive therapies may improve outcomes in patients with SIHD [stable ischemic heart disease],” said Dr. Madhavan, a cardiology fellow at Columbia University Irving Medical Center and New York–Presbyterian Hospital.
This post hoc analysis of pooled individual patient-level data from 19 randomized trials included 10,987 metallic stent recipients with SIHD. Sixty-one percent got second-generation drug-eluting stents (DES), 25% received first-generation DES, and 15% got bare metal stents (BMS). The largest prospective head-to-head RCT was SPIRIT IV, with 2,130 patients. All five TAXUS trials were also included.
The 5-year rate of the primary composite MACE endpoint composed of cardiac death, MI, or ischemia-driven target lesion revascularization was 24.1% in patients with BMS stents, 17.9% with first-gen DES, and 13.4% with second-gen DES, reflecting the advances in stent technology over time. Most of these MACE events occurred during the first year after PCI, with rates of 18%, 8.6%, and 5.3%, respectively, in the three groups. However, the MACE rate beyond the first year out through year 5 remained substantial: 10.2% with first-gen DES, 8.5% with second-gen DES, and 7.4% in the BMS group.
The cardiac death rate from PCI through year 5 was 3.8% with second-gen DES, 3.6% with first-gen DES, and 3.3% with BMS. The MI rate was 7.7% with first-gen DES, 6.1% with BMS, and 5% with second-gen DES.
Stent thrombosis occurred during the first year in 0.9% of first-gen DES and BMS recipients and in 0.7% of patients with second-gen DES. During years 1-5, the rates were 1.6% with first-gen DES, 0.9% with second-gen devices, and 0.2% with BMS.
Second-gen DES provided a big advantage in terms of lessened need for ischemia-driven target lesion revascularization through the first 5 years, with a rate of 7.3%, compared to 18.7% in patients with first-gen DES and 10.5% with BMS.
In a multivariate regression analysis, independent predictors of MACE in the first 5 years post PCI included indicators of greater lesion and/or procedural complexity, such as left main or left anterior descending disease, greater lesion length, and more than one treated lesion, as well as standard cardiovascular risk factors, including recent smoking, hypertension, and diabetes.
In contrast, hyperlipidemia was associated with a significant 15% reduction in MACE risk, which in an interview Dr. Madhavan said may have been due to aggressive lipid-lowering therapy, although he added that this is conjecture because he and his coinvestigators didn’t have access to data on the use of guideline-directed medical therapy or antiplatelet regimens.
Asked about future prospects for reducing the substantial very late risk of MACE highlighted in his study, Dr. Madhavan cited the use of adjunctive imaging during PCI as promising.
“The currently enrolling ILUMEN IV trial, among other studies, will help determine whether imaging-guided intervention can help improve intermediate and long-term rates of MACE,” he observed.
Promising medical therapies that could potentially confer benefit in terms of reducing long-term MACE in patients who’ve undergone PCI for SIHD include novel lipid-lowering drugs, tailored antithrombotic strategies, new anti-inflammatory agents, and the SGLT2 inhibitors, Dr. Madhavan continued.
In terms of advances in stent design, he cited recent evidence that ultrathin-strut stents featuring bioresorbable polymer, such as the Orsiro stent, may reduce late stent-related MACE through 3 years.
“We’ll have to see if these benefits extend to longer-term follow-up up to 5 years,” he said.
He deemed his study results “fairly consistent” with those of the ISCHEMIA trial, where ischemic events in the patients with SIHD assigned to an initial invasive strategy continued to occur in the latter years of follow-up without any clear plateau effect (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Dr. Madhavan reported no financial conflicts regarding his study, funded by an institutional research grant from the National Heart, Lung, and Blood Institute.
Shortly following Dr. Madhavan’s presentation at ACC 2020, the study results were published online (Circ Cardiovasc Interv. 2020 Apr;13[4[:e008565. doi: 10.1161/CIRCINTERVENTIONS.119.008565).
SOURCE: Madhavan MV. ACC 2020, Abstract 909-10.
FROM ACC 20
Survey: Hydroxychloroquine use fairly common in COVID-19
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.
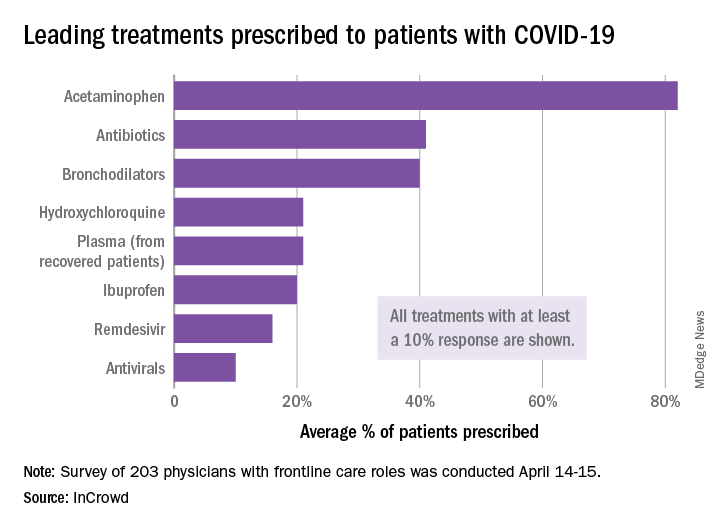
The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
SGLT2 inhibitor ertugliflozin shows no CV death or renal benefit
The sodium-glucose transporter 2 (SGLT-2) inhibitor ertugliflozin broke ranks with the other drugs in its class and failed to produce statistically significant drops in the both the combined incidence of cardiovascular (CV) death or heart failure hospitalization, and the rate of adverse renal outcomes, in the mandated CV outcomes trial run for ertugliflozin with more than 8,200 patients with type 2 diabetes and established CV disease.
Merck, one of the companies that markets the drug, announced the topline results in a quarterly financial report released on April 28, 2020.
According to the report, the results from the ertugliflozin cardiovascular outcomes trial “achieved its primary endpoint of noninferiority for major adverse CV events (MACE), compared to placebo in patients with type 2 diabetes mellitus and established atherosclerotic CV disease,” but “the key secondary endpoints of superiority” of ertugliflozin, compared with placebo, “for time to the composite of CV death or hospitalization for heart failure, CV death alone, and the composite of renal death, dialysis/transplant or doubling of serum creatinine from baseline were not met.”
However, the report added that, “while not a prespecified hypothesis for statistical testing, a reduction in hospitalization for heart failure was observed” with ertugliflozin treatment, and the report further said that the drug’s safety profile in the trial “was consistent with that reported in previous studies.” The statement closed by saying that detailed results from the trial are scheduled to be presented on June 16, 2020, at the virtual American Diabetes Association’s 80th Scientific Sessions.
These results came from the VERTIS CV (Evaluation of Ertugliflozin EffIcacy and Safety Cardiovascular Outcomes) trial, which researchers said in 2018 had administered at least one investigational dose to 8,238 randomized patients at centers in any of 34 countries during two enrollment periods in 2013-2015 and 2016-2017 (Am Heart J. 2018 Dec;206:11-23). The tested agent, ertugliflozin (Steglatro) received Food and Drug Administration marketing approval late in 2017 for the indication of improving glycemic control in patients with type 2 diabetes.
The FDA mandated cardiovascular outcomes trials for new glycemic control drugs in guidance the agency issued in 2008 (the FDA released in March 2020 a draft of updated guidance on this topic).
Other FDA-approved agents from the SGLT2 inhibitor class include canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), and all three showed evidence for a statistically significant effect on reducing the incidence of CV disease death and heart failure hospitalizations, as well as renal complications (Can J Diabetes. 2020 Feb;44[1]:61-7). The evidence showing that several SGLT2 drugs have important and consistent effects on endpoints like CV death, heart failure hospitalizations, and renal complications has helped propel this class of agents to the forefront of glycemic control treatments. More recently, one agent from this group, dapagliflozin, also significantly cut the rate of heart failure worsening or CV disease death in patients with heart failure with reduced ejection fraction but without diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). Based on this evidence, the FDA is currently considering adding a new indication for dapagliflozin that would also label it for use in patients with heart failure with reduced ejection fraction but without diabetes.
The sodium-glucose transporter 2 (SGLT-2) inhibitor ertugliflozin broke ranks with the other drugs in its class and failed to produce statistically significant drops in the both the combined incidence of cardiovascular (CV) death or heart failure hospitalization, and the rate of adverse renal outcomes, in the mandated CV outcomes trial run for ertugliflozin with more than 8,200 patients with type 2 diabetes and established CV disease.
Merck, one of the companies that markets the drug, announced the topline results in a quarterly financial report released on April 28, 2020.
According to the report, the results from the ertugliflozin cardiovascular outcomes trial “achieved its primary endpoint of noninferiority for major adverse CV events (MACE), compared to placebo in patients with type 2 diabetes mellitus and established atherosclerotic CV disease,” but “the key secondary endpoints of superiority” of ertugliflozin, compared with placebo, “for time to the composite of CV death or hospitalization for heart failure, CV death alone, and the composite of renal death, dialysis/transplant or doubling of serum creatinine from baseline were not met.”
However, the report added that, “while not a prespecified hypothesis for statistical testing, a reduction in hospitalization for heart failure was observed” with ertugliflozin treatment, and the report further said that the drug’s safety profile in the trial “was consistent with that reported in previous studies.” The statement closed by saying that detailed results from the trial are scheduled to be presented on June 16, 2020, at the virtual American Diabetes Association’s 80th Scientific Sessions.
These results came from the VERTIS CV (Evaluation of Ertugliflozin EffIcacy and Safety Cardiovascular Outcomes) trial, which researchers said in 2018 had administered at least one investigational dose to 8,238 randomized patients at centers in any of 34 countries during two enrollment periods in 2013-2015 and 2016-2017 (Am Heart J. 2018 Dec;206:11-23). The tested agent, ertugliflozin (Steglatro) received Food and Drug Administration marketing approval late in 2017 for the indication of improving glycemic control in patients with type 2 diabetes.
The FDA mandated cardiovascular outcomes trials for new glycemic control drugs in guidance the agency issued in 2008 (the FDA released in March 2020 a draft of updated guidance on this topic).
Other FDA-approved agents from the SGLT2 inhibitor class include canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), and all three showed evidence for a statistically significant effect on reducing the incidence of CV disease death and heart failure hospitalizations, as well as renal complications (Can J Diabetes. 2020 Feb;44[1]:61-7). The evidence showing that several SGLT2 drugs have important and consistent effects on endpoints like CV death, heart failure hospitalizations, and renal complications has helped propel this class of agents to the forefront of glycemic control treatments. More recently, one agent from this group, dapagliflozin, also significantly cut the rate of heart failure worsening or CV disease death in patients with heart failure with reduced ejection fraction but without diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). Based on this evidence, the FDA is currently considering adding a new indication for dapagliflozin that would also label it for use in patients with heart failure with reduced ejection fraction but without diabetes.
The sodium-glucose transporter 2 (SGLT-2) inhibitor ertugliflozin broke ranks with the other drugs in its class and failed to produce statistically significant drops in the both the combined incidence of cardiovascular (CV) death or heart failure hospitalization, and the rate of adverse renal outcomes, in the mandated CV outcomes trial run for ertugliflozin with more than 8,200 patients with type 2 diabetes and established CV disease.
Merck, one of the companies that markets the drug, announced the topline results in a quarterly financial report released on April 28, 2020.
According to the report, the results from the ertugliflozin cardiovascular outcomes trial “achieved its primary endpoint of noninferiority for major adverse CV events (MACE), compared to placebo in patients with type 2 diabetes mellitus and established atherosclerotic CV disease,” but “the key secondary endpoints of superiority” of ertugliflozin, compared with placebo, “for time to the composite of CV death or hospitalization for heart failure, CV death alone, and the composite of renal death, dialysis/transplant or doubling of serum creatinine from baseline were not met.”
However, the report added that, “while not a prespecified hypothesis for statistical testing, a reduction in hospitalization for heart failure was observed” with ertugliflozin treatment, and the report further said that the drug’s safety profile in the trial “was consistent with that reported in previous studies.” The statement closed by saying that detailed results from the trial are scheduled to be presented on June 16, 2020, at the virtual American Diabetes Association’s 80th Scientific Sessions.
These results came from the VERTIS CV (Evaluation of Ertugliflozin EffIcacy and Safety Cardiovascular Outcomes) trial, which researchers said in 2018 had administered at least one investigational dose to 8,238 randomized patients at centers in any of 34 countries during two enrollment periods in 2013-2015 and 2016-2017 (Am Heart J. 2018 Dec;206:11-23). The tested agent, ertugliflozin (Steglatro) received Food and Drug Administration marketing approval late in 2017 for the indication of improving glycemic control in patients with type 2 diabetes.
The FDA mandated cardiovascular outcomes trials for new glycemic control drugs in guidance the agency issued in 2008 (the FDA released in March 2020 a draft of updated guidance on this topic).
Other FDA-approved agents from the SGLT2 inhibitor class include canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), and all three showed evidence for a statistically significant effect on reducing the incidence of CV disease death and heart failure hospitalizations, as well as renal complications (Can J Diabetes. 2020 Feb;44[1]:61-7). The evidence showing that several SGLT2 drugs have important and consistent effects on endpoints like CV death, heart failure hospitalizations, and renal complications has helped propel this class of agents to the forefront of glycemic control treatments. More recently, one agent from this group, dapagliflozin, also significantly cut the rate of heart failure worsening or CV disease death in patients with heart failure with reduced ejection fraction but without diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). Based on this evidence, the FDA is currently considering adding a new indication for dapagliflozin that would also label it for use in patients with heart failure with reduced ejection fraction but without diabetes.
Consensus recommendations on AMI management during COVID-19
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
FDA grants Breakthrough Therapy status to sotatercept for PAH treatment
Approval for sotatercept, “a selective ligand trap for members of the TGF-beta [transforming growth factor-beta] superfamily which rebalances BMPR-II [bone morphogenetic protein receptor type II] signaling,” was based on two types of research. It was based on results of preclinical research indicating “reversed pulmonary vessel muscularization and improved indicators of right heart failure,” as well as results of the phase 2, placebo-controlled PULSAR study, in which sotatercept showed positive results, meeting primary and secondary endpoints.
Adverse events during PULSAR “were consistent with previously published data on sotatercept” in other diseases. The drug is also under investigation in the phase 2 SPECTRA trial, which includes patients with PAH.
“We believe that sotatercept has the potential to shift the current treatment paradigm and provide significant benefit to patients with PAH on top of currently available therapies. Thus, we’re thrilled that the FDA has granted this Breakthrough Therapy designation – a first for an Acceleron-discovered medicine and for a therapeutic candidate in PAH – as it supports and aligns with our mission to deliver novel therapeutic options to patients in need as quickly as possible,” Habib Dable, president and CEO of Acceleron Pharma, said in the press release.
Approval for sotatercept, “a selective ligand trap for members of the TGF-beta [transforming growth factor-beta] superfamily which rebalances BMPR-II [bone morphogenetic protein receptor type II] signaling,” was based on two types of research. It was based on results of preclinical research indicating “reversed pulmonary vessel muscularization and improved indicators of right heart failure,” as well as results of the phase 2, placebo-controlled PULSAR study, in which sotatercept showed positive results, meeting primary and secondary endpoints.
Adverse events during PULSAR “were consistent with previously published data on sotatercept” in other diseases. The drug is also under investigation in the phase 2 SPECTRA trial, which includes patients with PAH.
“We believe that sotatercept has the potential to shift the current treatment paradigm and provide significant benefit to patients with PAH on top of currently available therapies. Thus, we’re thrilled that the FDA has granted this Breakthrough Therapy designation – a first for an Acceleron-discovered medicine and for a therapeutic candidate in PAH – as it supports and aligns with our mission to deliver novel therapeutic options to patients in need as quickly as possible,” Habib Dable, president and CEO of Acceleron Pharma, said in the press release.
Approval for sotatercept, “a selective ligand trap for members of the TGF-beta [transforming growth factor-beta] superfamily which rebalances BMPR-II [bone morphogenetic protein receptor type II] signaling,” was based on two types of research. It was based on results of preclinical research indicating “reversed pulmonary vessel muscularization and improved indicators of right heart failure,” as well as results of the phase 2, placebo-controlled PULSAR study, in which sotatercept showed positive results, meeting primary and secondary endpoints.
Adverse events during PULSAR “were consistent with previously published data on sotatercept” in other diseases. The drug is also under investigation in the phase 2 SPECTRA trial, which includes patients with PAH.
“We believe that sotatercept has the potential to shift the current treatment paradigm and provide significant benefit to patients with PAH on top of currently available therapies. Thus, we’re thrilled that the FDA has granted this Breakthrough Therapy designation – a first for an Acceleron-discovered medicine and for a therapeutic candidate in PAH – as it supports and aligns with our mission to deliver novel therapeutic options to patients in need as quickly as possible,” Habib Dable, president and CEO of Acceleron Pharma, said in the press release.
Seniors with COVID-19 show unusual symptoms, doctors say
complicating efforts to ensure they get timely and appropriate treatment, according to physicians.
COVID-19 is typically signaled by three symptoms: a fever, an insistent cough, and shortness of breath. But older adults – the age group most at risk of severe complications or death from this condition – may have none of these characteristics.
Instead, seniors may seem “off” – not acting like themselves – early on after being infected by the coronavirus. They may sleep more than usual or stop eating. They may seem unusually apathetic or confused, losing orientation to their surroundings. They may become dizzy and fall. Sometimes, seniors stop speaking or simply collapse.
“With a lot of conditions, older adults don’t present in a typical way, and we’re seeing that with COVID-19 as well,” said Camille Vaughan, MD, section chief of geriatrics and gerontology at Emory University, Atlanta.
The reason has to do with how older bodies respond to illness and infection.
At advanced ages, “someone’s immune response may be blunted and their ability to regulate temperature may be altered,” said Dr. Joseph Ouslander, a professor of geriatric medicine at Florida Atlantic University in Boca Raton.
“Underlying chronic illnesses can mask or interfere with signs of infection,” he said. “Some older people, whether from age-related changes or previous neurologic issues such as a stroke, may have altered cough reflexes. Others with cognitive impairment may not be able to communicate their symptoms.”
Recognizing danger signs is important: If early signs of COVID-19 are missed, seniors may deteriorate before getting needed care. And people may go in and out of their homes without adequate protective measures, risking the spread of infection.
Quratulain Syed, MD, an Atlanta geriatrician, describes a man in his 80s whom she treated in mid-March. Over a period of days, this patient, who had heart disease, diabetes and moderate cognitive impairment, stopped walking and became incontinent and profoundly lethargic. But he didn’t have a fever or a cough. His only respiratory symptom: sneezing off and on.
The man’s elderly spouse called 911 twice. Both times, paramedics checked his vital signs and declared he was OK. After another worried call from the overwhelmed spouse, Dr. Syed insisted the patient be taken to the hospital, where he tested positive for COVID-19.
“I was quite concerned about the paramedics and health aides who’d been in the house and who hadn’t used PPE [personal protective equipment],” Dr. Syed said.
Dr. Sam Torbati, medical director of the emergency department at Cedars-Sinai Medical Center, Los Angeles, describes treating seniors who initially appear to be trauma patients but are found to have COVID-19.
“They get weak and dehydrated,” he said, “and when they stand to walk, they collapse and injure themselves badly.”
Dr. Torbati has seen older adults who are profoundly disoriented and unable to speak and who appear at first to have suffered strokes.
“When we test them, we discover that what’s producing these changes is a central nervous system effect of coronavirus,” he said.
Laura Perry, MD, of the University of California, San Francisco, saw a patient like this several weeks ago. The woman, in her 80s, had what seemed to be a cold before becoming very confused. In the hospital, she couldn’t identify where she was or stay awake during an examination. Dr. Perry diagnosed hypoactive delirium, an altered mental state in which people become inactive and drowsy. The patient tested positive for coronavirus and is still in the ICU.
Anthony Perry, MD, of the department of geriatric medicine at Rush University Medical Center in Chicago, tells of an 81-year-old woman with nausea, vomiting, and diarrhea who tested positive for COVID-19 in the emergency room. After receiving intravenous fluids, oxygen, and medication for her intestinal upset, she returned home after 2 days and is doing well.
Another 80-year-old Rush patient with similar symptoms – nausea and vomiting, but no cough, fever, or shortness of breath – is in intensive care after getting a positive COVID-19 test and due to be put on a ventilator. The difference? This patient is frail with “a lot of cardiovascular disease,” Dr. Perry said. Other than that, it’s not yet clear why some older patients do well while others do not.
So far, reports of cases like these have been anecdotal. But a few physicians are trying to gather more systematic information.
In Switzerland, Sylvain Nguyen, MD, a geriatrician at the University of Lausanne Hospital Center, put together a list of typical and atypical symptoms in older COVID-19 patients for a paper to be published in the Revue Médicale Suisse. Included on the atypical list are changes in a patient’s usual status, delirium, falls, fatigue, lethargy, low blood pressure, painful swallowing, fainting, diarrhea, nausea, vomiting, abdominal pain, and the loss of smell and taste.
Data come from hospitals and nursing homes in Switzerland, Italy, and France, Dr. Nguyen said in an email.
On the front lines, physicians need to make sure they carefully assess an older patient’s symptoms.
“While we have to have a high suspicion of COVID-19 because it’s so dangerous in the older population, there are many other things to consider,” said Kathleen Unroe, MD, a geriatrician at Indiana University, Indianapolis.
Seniors may also do poorly because their routines have changed. In nursing homes and most assisted living centers, activities have stopped and “residents are going to get weaker and more deconditioned because they’re not walking to and from the dining hall,” she said.
At home, isolated seniors may not be getting as much help with medication management or other essential needs from family members who are keeping their distance, other experts suggested. Or they may have become apathetic or depressed.
“I’d want to know ‘What’s the potential this person has had an exposure [to the coronavirus], especially in the last 2 weeks?’ ” said Dr. Vaughan of Emory. “Do they have home health personnel coming in? Have they gotten together with other family members? Are chronic conditions being controlled? Is there another diagnosis that seems more likely?”
“Someone may be just having a bad day. But if they’re not themselves for a couple of days, absolutely reach out to a primary care doctor or a local health system hotline to see if they meet the threshold for [coronavirus] testing,” Dr. Vaughan advised. “Be persistent. If you get a ‘no’ the first time and things aren’t improving, call back and ask again.”
Kaiser Health News (khn.org) is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
complicating efforts to ensure they get timely and appropriate treatment, according to physicians.
COVID-19 is typically signaled by three symptoms: a fever, an insistent cough, and shortness of breath. But older adults – the age group most at risk of severe complications or death from this condition – may have none of these characteristics.
Instead, seniors may seem “off” – not acting like themselves – early on after being infected by the coronavirus. They may sleep more than usual or stop eating. They may seem unusually apathetic or confused, losing orientation to their surroundings. They may become dizzy and fall. Sometimes, seniors stop speaking or simply collapse.
“With a lot of conditions, older adults don’t present in a typical way, and we’re seeing that with COVID-19 as well,” said Camille Vaughan, MD, section chief of geriatrics and gerontology at Emory University, Atlanta.
The reason has to do with how older bodies respond to illness and infection.
At advanced ages, “someone’s immune response may be blunted and their ability to regulate temperature may be altered,” said Dr. Joseph Ouslander, a professor of geriatric medicine at Florida Atlantic University in Boca Raton.
“Underlying chronic illnesses can mask or interfere with signs of infection,” he said. “Some older people, whether from age-related changes or previous neurologic issues such as a stroke, may have altered cough reflexes. Others with cognitive impairment may not be able to communicate their symptoms.”
Recognizing danger signs is important: If early signs of COVID-19 are missed, seniors may deteriorate before getting needed care. And people may go in and out of their homes without adequate protective measures, risking the spread of infection.
Quratulain Syed, MD, an Atlanta geriatrician, describes a man in his 80s whom she treated in mid-March. Over a period of days, this patient, who had heart disease, diabetes and moderate cognitive impairment, stopped walking and became incontinent and profoundly lethargic. But he didn’t have a fever or a cough. His only respiratory symptom: sneezing off and on.
The man’s elderly spouse called 911 twice. Both times, paramedics checked his vital signs and declared he was OK. After another worried call from the overwhelmed spouse, Dr. Syed insisted the patient be taken to the hospital, where he tested positive for COVID-19.
“I was quite concerned about the paramedics and health aides who’d been in the house and who hadn’t used PPE [personal protective equipment],” Dr. Syed said.
Dr. Sam Torbati, medical director of the emergency department at Cedars-Sinai Medical Center, Los Angeles, describes treating seniors who initially appear to be trauma patients but are found to have COVID-19.
“They get weak and dehydrated,” he said, “and when they stand to walk, they collapse and injure themselves badly.”
Dr. Torbati has seen older adults who are profoundly disoriented and unable to speak and who appear at first to have suffered strokes.
“When we test them, we discover that what’s producing these changes is a central nervous system effect of coronavirus,” he said.
Laura Perry, MD, of the University of California, San Francisco, saw a patient like this several weeks ago. The woman, in her 80s, had what seemed to be a cold before becoming very confused. In the hospital, she couldn’t identify where she was or stay awake during an examination. Dr. Perry diagnosed hypoactive delirium, an altered mental state in which people become inactive and drowsy. The patient tested positive for coronavirus and is still in the ICU.
Anthony Perry, MD, of the department of geriatric medicine at Rush University Medical Center in Chicago, tells of an 81-year-old woman with nausea, vomiting, and diarrhea who tested positive for COVID-19 in the emergency room. After receiving intravenous fluids, oxygen, and medication for her intestinal upset, she returned home after 2 days and is doing well.
Another 80-year-old Rush patient with similar symptoms – nausea and vomiting, but no cough, fever, or shortness of breath – is in intensive care after getting a positive COVID-19 test and due to be put on a ventilator. The difference? This patient is frail with “a lot of cardiovascular disease,” Dr. Perry said. Other than that, it’s not yet clear why some older patients do well while others do not.
So far, reports of cases like these have been anecdotal. But a few physicians are trying to gather more systematic information.
In Switzerland, Sylvain Nguyen, MD, a geriatrician at the University of Lausanne Hospital Center, put together a list of typical and atypical symptoms in older COVID-19 patients for a paper to be published in the Revue Médicale Suisse. Included on the atypical list are changes in a patient’s usual status, delirium, falls, fatigue, lethargy, low blood pressure, painful swallowing, fainting, diarrhea, nausea, vomiting, abdominal pain, and the loss of smell and taste.
Data come from hospitals and nursing homes in Switzerland, Italy, and France, Dr. Nguyen said in an email.
On the front lines, physicians need to make sure they carefully assess an older patient’s symptoms.
“While we have to have a high suspicion of COVID-19 because it’s so dangerous in the older population, there are many other things to consider,” said Kathleen Unroe, MD, a geriatrician at Indiana University, Indianapolis.
Seniors may also do poorly because their routines have changed. In nursing homes and most assisted living centers, activities have stopped and “residents are going to get weaker and more deconditioned because they’re not walking to and from the dining hall,” she said.
At home, isolated seniors may not be getting as much help with medication management or other essential needs from family members who are keeping their distance, other experts suggested. Or they may have become apathetic or depressed.
“I’d want to know ‘What’s the potential this person has had an exposure [to the coronavirus], especially in the last 2 weeks?’ ” said Dr. Vaughan of Emory. “Do they have home health personnel coming in? Have they gotten together with other family members? Are chronic conditions being controlled? Is there another diagnosis that seems more likely?”
“Someone may be just having a bad day. But if they’re not themselves for a couple of days, absolutely reach out to a primary care doctor or a local health system hotline to see if they meet the threshold for [coronavirus] testing,” Dr. Vaughan advised. “Be persistent. If you get a ‘no’ the first time and things aren’t improving, call back and ask again.”
Kaiser Health News (khn.org) is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
complicating efforts to ensure they get timely and appropriate treatment, according to physicians.
COVID-19 is typically signaled by three symptoms: a fever, an insistent cough, and shortness of breath. But older adults – the age group most at risk of severe complications or death from this condition – may have none of these characteristics.
Instead, seniors may seem “off” – not acting like themselves – early on after being infected by the coronavirus. They may sleep more than usual or stop eating. They may seem unusually apathetic or confused, losing orientation to their surroundings. They may become dizzy and fall. Sometimes, seniors stop speaking or simply collapse.
“With a lot of conditions, older adults don’t present in a typical way, and we’re seeing that with COVID-19 as well,” said Camille Vaughan, MD, section chief of geriatrics and gerontology at Emory University, Atlanta.
The reason has to do with how older bodies respond to illness and infection.
At advanced ages, “someone’s immune response may be blunted and their ability to regulate temperature may be altered,” said Dr. Joseph Ouslander, a professor of geriatric medicine at Florida Atlantic University in Boca Raton.
“Underlying chronic illnesses can mask or interfere with signs of infection,” he said. “Some older people, whether from age-related changes or previous neurologic issues such as a stroke, may have altered cough reflexes. Others with cognitive impairment may not be able to communicate their symptoms.”
Recognizing danger signs is important: If early signs of COVID-19 are missed, seniors may deteriorate before getting needed care. And people may go in and out of their homes without adequate protective measures, risking the spread of infection.
Quratulain Syed, MD, an Atlanta geriatrician, describes a man in his 80s whom she treated in mid-March. Over a period of days, this patient, who had heart disease, diabetes and moderate cognitive impairment, stopped walking and became incontinent and profoundly lethargic. But he didn’t have a fever or a cough. His only respiratory symptom: sneezing off and on.
The man’s elderly spouse called 911 twice. Both times, paramedics checked his vital signs and declared he was OK. After another worried call from the overwhelmed spouse, Dr. Syed insisted the patient be taken to the hospital, where he tested positive for COVID-19.
“I was quite concerned about the paramedics and health aides who’d been in the house and who hadn’t used PPE [personal protective equipment],” Dr. Syed said.
Dr. Sam Torbati, medical director of the emergency department at Cedars-Sinai Medical Center, Los Angeles, describes treating seniors who initially appear to be trauma patients but are found to have COVID-19.
“They get weak and dehydrated,” he said, “and when they stand to walk, they collapse and injure themselves badly.”
Dr. Torbati has seen older adults who are profoundly disoriented and unable to speak and who appear at first to have suffered strokes.
“When we test them, we discover that what’s producing these changes is a central nervous system effect of coronavirus,” he said.
Laura Perry, MD, of the University of California, San Francisco, saw a patient like this several weeks ago. The woman, in her 80s, had what seemed to be a cold before becoming very confused. In the hospital, she couldn’t identify where she was or stay awake during an examination. Dr. Perry diagnosed hypoactive delirium, an altered mental state in which people become inactive and drowsy. The patient tested positive for coronavirus and is still in the ICU.
Anthony Perry, MD, of the department of geriatric medicine at Rush University Medical Center in Chicago, tells of an 81-year-old woman with nausea, vomiting, and diarrhea who tested positive for COVID-19 in the emergency room. After receiving intravenous fluids, oxygen, and medication for her intestinal upset, she returned home after 2 days and is doing well.
Another 80-year-old Rush patient with similar symptoms – nausea and vomiting, but no cough, fever, or shortness of breath – is in intensive care after getting a positive COVID-19 test and due to be put on a ventilator. The difference? This patient is frail with “a lot of cardiovascular disease,” Dr. Perry said. Other than that, it’s not yet clear why some older patients do well while others do not.
So far, reports of cases like these have been anecdotal. But a few physicians are trying to gather more systematic information.
In Switzerland, Sylvain Nguyen, MD, a geriatrician at the University of Lausanne Hospital Center, put together a list of typical and atypical symptoms in older COVID-19 patients for a paper to be published in the Revue Médicale Suisse. Included on the atypical list are changes in a patient’s usual status, delirium, falls, fatigue, lethargy, low blood pressure, painful swallowing, fainting, diarrhea, nausea, vomiting, abdominal pain, and the loss of smell and taste.
Data come from hospitals and nursing homes in Switzerland, Italy, and France, Dr. Nguyen said in an email.
On the front lines, physicians need to make sure they carefully assess an older patient’s symptoms.
“While we have to have a high suspicion of COVID-19 because it’s so dangerous in the older population, there are many other things to consider,” said Kathleen Unroe, MD, a geriatrician at Indiana University, Indianapolis.
Seniors may also do poorly because their routines have changed. In nursing homes and most assisted living centers, activities have stopped and “residents are going to get weaker and more deconditioned because they’re not walking to and from the dining hall,” she said.
At home, isolated seniors may not be getting as much help with medication management or other essential needs from family members who are keeping their distance, other experts suggested. Or they may have become apathetic or depressed.
“I’d want to know ‘What’s the potential this person has had an exposure [to the coronavirus], especially in the last 2 weeks?’ ” said Dr. Vaughan of Emory. “Do they have home health personnel coming in? Have they gotten together with other family members? Are chronic conditions being controlled? Is there another diagnosis that seems more likely?”
“Someone may be just having a bad day. But if they’re not themselves for a couple of days, absolutely reach out to a primary care doctor or a local health system hotline to see if they meet the threshold for [coronavirus] testing,” Dr. Vaughan advised. “Be persistent. If you get a ‘no’ the first time and things aren’t improving, call back and ask again.”
Kaiser Health News (khn.org) is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
COVID-19 linked to large vessel stroke in young adults
In a rapid communication to be published online April 29 in the New England Journal of Medicine, investigators led by Thomas Oxley, MD, PhD, of the department of neurosurgery at Mount Sinai Health System, reported five cases of large vessel stroke over a 2-week period in COVID-19 patients under age 50 years. This represents a sevenfold increase in what would normally be expected.
The five cases had either no, or mild, COVID-19 symptoms.
“It’s been surprising to learn that the virus appears to cause disease through a process of blood clotting,” Dr. Oxley said in an interview.
The message for neurologists and other physicians is “we’re learning that this can disproportionally affect large vessels more than small vessels in terms of presentation of stroke,” he said.
Inflammation in the blood vessel walls may be driving thrombosis formation, Dr. Oxley added. This report joins other research pointing to this emerging phenomenon.
Recently, investigators in the Netherlands found a “remarkably high” 31% rate of thrombotic complications among 184 critical care patients with COVID-19 pneumonia.
Dr. Oxley and colleagues also suggested that, since the onset of the pandemic, fewer patients may be calling emergency services when they experience signs of a stroke. The physicians noted that two of the five cases in the report delayed calling an ambulance.
“I understand why people do not want to leave the household. I think people are more willing to ignore other [non–COVID-19] symptoms in this environment,” he said.
As previously reported, physicians in hospitals across the United States and elsewhere have reported a significant drop in stroke patients since the COVID-19 pandemic took hold, which suggests that patients may indeed be foregoing emergency care.
The observations from Dr. Oxley and colleagues call for greater awareness of the association between COVID-19 and large vessel strokes in this age group, they add.
One patient in the case series died, one remains hospitalized, two are undergoing rehabilitation, and one was discharged home as of April 24.
Dr. Oxley and colleagues dedicated their report to “our inspiring colleague Gary Sclar, MD, a stroke physician who succumbed to COVID-19 while caring for his patients.”
Dr. Oxley has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a rapid communication to be published online April 29 in the New England Journal of Medicine, investigators led by Thomas Oxley, MD, PhD, of the department of neurosurgery at Mount Sinai Health System, reported five cases of large vessel stroke over a 2-week period in COVID-19 patients under age 50 years. This represents a sevenfold increase in what would normally be expected.
The five cases had either no, or mild, COVID-19 symptoms.
“It’s been surprising to learn that the virus appears to cause disease through a process of blood clotting,” Dr. Oxley said in an interview.
The message for neurologists and other physicians is “we’re learning that this can disproportionally affect large vessels more than small vessels in terms of presentation of stroke,” he said.
Inflammation in the blood vessel walls may be driving thrombosis formation, Dr. Oxley added. This report joins other research pointing to this emerging phenomenon.
Recently, investigators in the Netherlands found a “remarkably high” 31% rate of thrombotic complications among 184 critical care patients with COVID-19 pneumonia.
Dr. Oxley and colleagues also suggested that, since the onset of the pandemic, fewer patients may be calling emergency services when they experience signs of a stroke. The physicians noted that two of the five cases in the report delayed calling an ambulance.
“I understand why people do not want to leave the household. I think people are more willing to ignore other [non–COVID-19] symptoms in this environment,” he said.
As previously reported, physicians in hospitals across the United States and elsewhere have reported a significant drop in stroke patients since the COVID-19 pandemic took hold, which suggests that patients may indeed be foregoing emergency care.
The observations from Dr. Oxley and colleagues call for greater awareness of the association between COVID-19 and large vessel strokes in this age group, they add.
One patient in the case series died, one remains hospitalized, two are undergoing rehabilitation, and one was discharged home as of April 24.
Dr. Oxley and colleagues dedicated their report to “our inspiring colleague Gary Sclar, MD, a stroke physician who succumbed to COVID-19 while caring for his patients.”
Dr. Oxley has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a rapid communication to be published online April 29 in the New England Journal of Medicine, investigators led by Thomas Oxley, MD, PhD, of the department of neurosurgery at Mount Sinai Health System, reported five cases of large vessel stroke over a 2-week period in COVID-19 patients under age 50 years. This represents a sevenfold increase in what would normally be expected.
The five cases had either no, or mild, COVID-19 symptoms.
“It’s been surprising to learn that the virus appears to cause disease through a process of blood clotting,” Dr. Oxley said in an interview.
The message for neurologists and other physicians is “we’re learning that this can disproportionally affect large vessels more than small vessels in terms of presentation of stroke,” he said.
Inflammation in the blood vessel walls may be driving thrombosis formation, Dr. Oxley added. This report joins other research pointing to this emerging phenomenon.
Recently, investigators in the Netherlands found a “remarkably high” 31% rate of thrombotic complications among 184 critical care patients with COVID-19 pneumonia.
Dr. Oxley and colleagues also suggested that, since the onset of the pandemic, fewer patients may be calling emergency services when they experience signs of a stroke. The physicians noted that two of the five cases in the report delayed calling an ambulance.
“I understand why people do not want to leave the household. I think people are more willing to ignore other [non–COVID-19] symptoms in this environment,” he said.
As previously reported, physicians in hospitals across the United States and elsewhere have reported a significant drop in stroke patients since the COVID-19 pandemic took hold, which suggests that patients may indeed be foregoing emergency care.
The observations from Dr. Oxley and colleagues call for greater awareness of the association between COVID-19 and large vessel strokes in this age group, they add.
One patient in the case series died, one remains hospitalized, two are undergoing rehabilitation, and one was discharged home as of April 24.
Dr. Oxley and colleagues dedicated their report to “our inspiring colleague Gary Sclar, MD, a stroke physician who succumbed to COVID-19 while caring for his patients.”
Dr. Oxley has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FDA reiterates hydroxychloroquine limitations for COVID-19
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
FROM THE FDA
COVID-19: What are the major cardiovascular issues?
Acute viral myocarditis often confounds with ischemic injury
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Acute viral myocarditis often confounds with ischemic injury
Acute viral myocarditis often confounds with ischemic injury
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.
Frontline health care workers are facing escalating challenges with rapidly spreading coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Hospitalists will often deal with various manifestations of acute cardiac injury, controversial withholding of ACE inhibitors (ACEI) or angiotensin receptor blockers (ARBs), arrhythmic toxicities from such drug therapies as hydroxychloroquine.
Presentation and cardiac risks from COVID-19
Patients with COVID-19 often have presented with noncardiac symptoms, usually a febrile illness associated with cough or shortness of breath. Recent reports from Italy and New York have suggested patients also can present with isolated cardiac involvement without any other symptoms that can portend a grim prognosis.2 Cardiac effects include myocarditis, acute coronary syndrome, malignant arrhythmias ultimately cardiogenic shock and cardiac arrest.3
The mortality rate correlates with older age, preexisting health conditions, and availability of medical resources. A recent meta-analysis including 53,000 COVID-19 patients found the most common comorbidities were hypertension (19%), diabetes (8 %) and cardiovascular disease (CVD) (3%).4 Half of the cases died from respiratory failure and one-third have died from concomitant respiratory and heart failure. Acute heart failure alone accounted for about 7% of cases.5
Overall mortality rate can be better understood with the largest case series to-date of COVID-19 in mainland China published by the Chinese Center for Disease Control and Prevention. The overall case-fatality rate was 2.3% (1,023 deaths among 44,672 confirmed cases), but the mortality reached 10.5% in patients with underlying CVD.6
Acute cardiac injuries in COVID-19
Acute cardiac injury (ACI) is defined as troponin elevation above the 99th percentile of the upper reference limit.7 A practical description of ACI in COVID-19 patients should also include broader definition with new abnormalities in ECG since not all patients with acute cardiac effects have developed troponin elevation.3 More recent reports showed up to 28% of hospitalized patients had a myocardial injury.3
It is not uncommon to see a patient with COVID-19 myocarditis as a mimicker of acute ST-elevation myocardial infarction (STEMI). The mechanism of ACI is unknown, though several hypotheses have been proposed based on case series and retrospective reviews. These include direct viral invasion into myocardial cells leading to myocarditis, oxygen demand-supply mismatch, acute coronary syndrome from plaque rupture, stress, or cytokine-mediated cardiomyopathy.3 The exact incidence of true MI from occlusive coronary disease in the COVID-19 population is yet unknown.
In some cases, troponin elevation may be a late manifestation of COVID-19. As coronavirus disease progressed slowly, a rapid rise of troponin was noted when patients developed acute respiratory failure after 10 days of illness. Among nonsurvivors, a steady rise in troponin was observed from day 4 through day 22.8
ACI is associated with ICU admission and mortality. Both troponin and BNP levels increased significantly during the course of hospitalization in those who ultimately died, but no such changes were evident in survivors.3 ACI was higher in nonsurvivors (59%) than in survivors (1%).8 ACI was higher in ICU patients (22%), compared with non-ICU patients (2%).9 Patients with CVD were more likely to exhibit elevation of troponin levels (54%), compared with patients without CVD (13%).3
Higher troponin levels and the presence of CVD are directly proportional to severe disease and death. Patients with elevated troponin developed more frequent complications including acute respiratory distress syndrome, malignant arrhythmias including ventricular tachycardia/ventricular fibrillation, acute coagulopathy, and acute kidney injury.3,8 Death was markedly higher in patients with elevated troponin, compared with normal levels: 60% versus 9%. Only 8% with no CVD and normal troponin died, whereas 69% of people with underlying CVD and elevated troponin died.3
The median duration from illness onset to death was 23 (8-41) days in the group with elevated troponin. Patients with CVD and escalation of troponin levels had the shortest survival of 1-5 days. The dynamic rise of cardiac biomarkers and increased incidence of malignant arrhythmias during the course of illness shows that myocardial injury played a greater role in the fatal outcome of COVID-19 than the presence of preexisting CVD itself.3
Management of acute cardiac issues in COVID-19
There are no established therapeutic options with randomized, clinical trials specific to the management of COVID-19 patients at this point. Standard supportive care and individualized treatment plan based on existing guidelines is probably the best approach. Disposition of cases and cardiac testing should be tailored, based on local protocols, availability of resources and expertise.10
There seems to be a consensus that baseline troponin levels should be obtained in all admitted patients. Repeat troponin levels can be obtained based on the severity of illness, for example, daily troponin checks are reasonable in ICU patients and every-other-day troponin testing may be reasonable in general inpatients. Routine troponin testing in minimally symptomatic or asymptomatic patients will likely not change any outcome.3,11,12
Daily ECG is reasonable in severe COVID-19. However, routine transthoracic ECGs are not reasonable, unless it will change further treatment plans. Transthoracic electrocardiograms (TTE) are reasonable in patients with significant troponin elevation, a decline in central venous oxygen saturation, new heart failure, shock, new persistent arrhythmias, or significant new ECG changes.12
Limited TTEs for a focused exam enough to answer the clinical question should be ordered to minimize the risk of viral exposure to the sonographers. Transesophageal echo will rarely be needed, and its use should be minimized to reduce direct contact exposure and because of anesthesia risks.13 Routine stress testing should not be ordered in active COVID-19 and should be deferred for outpatient evaluation, if clinically indicated, once the patient recovers from the infection.12
Myocarditis and pericarditis are potential manifestations of acute cardiac injury. Recent case reports have suggested evidence of myocarditis confirmed with cardiac MRI.11 Because of high fatality rates with cardiac involvement and no proven therapies yet, the role of routine advanced cardiac imaging such as cardiac CT, cardiac MRI, or cardiac biopsy is unclear.
Myocarditis can likely be caused either by the virus itself, or the body’s immune and inflammatory response (cytokine storm) to the virus.2,3 The use of anti-inflammatory drugs like colchicine, ibuprofen, steroids, or statins is not yet established.10,12 Drugs like remdesivir, lopinavir-ritonavir, hydroxychloroquine, chloroquine, and anti-interleukin-6 agents have been invariably used with some anecdotal success and randomized clinical trials for some of these drugs are presently undergoing.
Physicians may encounter situations to call a STEMI code or not in COVID-19 patients.2,11 Patients may have substernal pain, diffuse or regional ST elevations in ECG and reduced left ventricular dysfunction with regional wall motion abnormalities on ECG. These findings may be casued by myocarditis, acute type 1 MI, or stress-induced cardiomyopathy. Clinicians should make their judgment based on the overall pretest probability for type 1 MI, incorporating risk factor profiles and the presence of typical symptoms.
Treatment practice for questionable STEMI cases will likely vary across the country as we are learning more about the virus. Cath lab operators are at risk for COVID-19 infection through direct contact with patients. Few cardiologists were admitted after COVID-19 infections in the ICU at a New York hospital after they were involved in a acute MI case in a cath lab.14 Based on the Chinese experience, some have suggested the idea of lytic therapy first with follow-up cardiac CT to assess the recanalization of perfusion status, but at this point, this strategy remains controversial in the United States. In addition, if the patient has myocarditis instead, there will be a risk for pericardial effusion and hemorrhagic complications with lytic therapy.
Case examples
1. A 70-year-old male presents with fevers, chest pain, cough, shortness of breath. He has a history of metabolic syndrome and 30 pack-years of smoking. His ECG showed 1.5 mm ST elevation in inferior leads with reciprocal ST depressions in lateral leads, and his initial troponin is 2. Echocardiogram showed reduced left ventricle ejection fraction of 32% and inferior wall hypokinesis. He is suspected COVID-19 and his PCR result is pending. How would you manage this patient?
This patient presented with febrile illness and, but he had a very high pretest probability for obstructive coronary artery disease based on his age, male sex, and multiple risk factors. He may have a viral syndrome and it is a stressful situation for him. This may have precipitated plaque rupture causing acute MI.
Activating the STEMI pathway for emergent left heart catheterization is likely appropriate in this case. Coronary angiogram in this patient showed a 100% occluded mid-right coronary artery with a fresh thrombus. Delaying cardiac cath would have possibly led to malignant arrhythmias and death from ischemic injury. We need to be cognizant patients can die from non–COVID-related emergencies also.
2. An 18-year-old healthy male presents with cough and chest pain and has bilateral lung infiltrates. ECG showed anterolateral 2 mm ST elevations and no reciprocal ST changes. Stat TTE showed anterior wall hypokinesis and LV function 30% and his initial troponin are 0.6 (normal is < .05). The nasopharyngeal swab is sent out and his COVID result is pending. How would you manage this patient?
A young patient with no cardiovascular risk factors has a very low pretest probability for obstructive coronary disease and the likelihood of having a true ischemic MI is low even though he has significant new ST elevations. Especially with presumed COVID-19 and risk of virus exposure to the cath lab personnel, it will be prudent to manage this patient with supportive therapy including beta-blockers, ACEIs, etc. Repeat echo in 7 days before discharge showed improved LVEF 45%.
Controversy on ACEI/ARB
The SARS-CoV-2 virus enters via cell-entry receptor namely angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 is thought to have a higher affinity for ACE2 than other SARS-viruses.15
ACE2 is expressed in the heart, lungs, vasculature, and kidneys. ACEI and ARBs in animal models increase the expression of ACE2,16 though this has not been confirmed in human studies. This has led to the hypothesis that ACEI and ARBs might worsen myocarditis or precipitate the acute coronary syndrome. It has also been hypothesized that the upregulation of ACE2 is therapeutic in COVID-19 and that ARBs might be protective during infection.17
The increased ACE2 expression induced by ACEI or ARB would aggravate lung injury of patients with COVID-19. However, a previous study showed a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as it significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection.18
Therefore, this remains an area of investigation and it is unclear how these medications affect patients with COVID-19. In a recent review, with a limited number of patients, the mortality of those treated with or without the use of ACEI/ARB did not show a significant difference in the outcome.3
Both American and European cardiology societies recommend against routine discontinuation of ACEI and ARBs in patients with COVID-19 because of risks of uncontrolled hypertension and heart failure, stroke, or heart attack.19 However, it will be reasonable to hold off in inpatients in cases of acute kidney injury, hypotension, shock, etc.12
Cardiac concern about hydroxychloroquine and chloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug shown to have in vitro (but not yet in vivo) activity against diverse RNA viruses, including SARS-CoV-1.20 An expert consensus group from China suggests that chloroquine improved lung imaging and shortened disease course.21 HCQ was found to be more potent than chloroquine in inhibiting SARS-CoV-2 in vitro.22
Based on limited in vitro and anecdotal clinical data from other countries, the U.S. Food and Drug Administration recently authorized emergency use of chloroquine and HCQ in hopes of slowing the progression of the disease when a clinical trial is not available, or participation is not feasible for use of these drugs in hospitalized patients. However, with no clear benefit, there is a concern for possible risks with cardiac toxicity.
HCQ is known to cause cardiomyopathy in a dose-dependent manner over several years. Given the anticipated short duration in COVID-19, it is not an expected risk. QT-segment prolongation and torsades de pointes, especially if administered in combination with azithromycin, is possible even in short term use.23
Given above, frequent ECG monitoring is indicated for patients being treated with chloroquine or HCQ. All other QT-prolonging drugs should be discontinued. Continuous telemetry monitoring while under treatment is reasonable. HCQ should not be started if baseline QTc is > 500 msec and it should be stopped if the patient develops ventricular arrhythmias.12
Dr. Subedi is a noninvasive cardiologist for Wellspan Health System in Franklin and Cumberland counties in south central Pennsylvania. He is a clinical assistant professor of medicine at Penn State College of Medicine, Hershey, Pa. He is an active member of the critical care committee at Wellspan Chambersburg (Pa.) Hospital. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro Hospitals, all in Pennsylvania. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is a member of the Wellspan pharmacy and therapeutics committee. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
Key points
- Acute cardiac injury or myocarditis is common among patients infected with COVID-19. Often, COVID myocarditis can mimic acute MI or stress cardiomyopathy and will present diagnostic and therapeutic challenges. On the other hand, isolated cardiac involvement can occur, even without symptoms and signs of interstitial pneumonia.
- A most important indicator of worse prediction is the degree of myocardial injury, regardless of preexisting conditions or underlying cardiovascular disease.
- Early recognition of cardiac involvement will be helpful in targeting more aggressive supportive therapies. Commonly available clinical tools like bloodwork, ECG, or echocardiogram should be adequate to diagnose carditis in most cases.
- Advanced cardiac imaging tests or cardiac biopsy are of uncertain benefits. Meticulous evaluation is needed for possible ischemic changes before taking the patient to the cardiac cath lab in order to reduce unnecessary virus exposure to the operators.
- ACEI/ARB should be continued in most cases in COVID patients based on cardiology societies’ recommendations.
- With the widespread use of antimalarial drugs like chloroquine or hydroxychloroquine, frequent ECG and continuous telemetry monitoring is reasonable to rule out ventricular arrhythmias like torsades.
- There is no specific treatment to date for acute cardiac injuries. Since there are no specific guidelines and information about the virus is rapidly changing, it will be prudent to follow common-sense approaches outlined by institutions like the Brigham and Women’s Hospital COVID-19 Critical Care clinical guidelines, which incorporate new clinical information on a daily basis ().
References
1. Rothan HA and Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109:102433. doi: 10.1016/j.jaut.2020.102433.
2. Kolata G. A heart attack? No, it was the coronavirus. New York Times 2020 Mar 27.
3. Guo T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017.
4. Zhao X et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. MedRxIV. 2020 Mar 20. doi: 10.1101/2020.03.17.20037572.
5. Ruan Q et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3. doi: 10.1007/s00134-020-05991-x.
6. Wu Z and McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
7. Thygesen K et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018 Oct;72:2231-64.
8. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
9. Wang D et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7. doi: 10.1001/jama.2020.1585.
10. CDC: Therapeutic options for patients with COVID-19. Updated April 13, 2020.
11. Inciardi RM et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1096.
12. Brigham and Women’s Hospital COVID-19 Critical Care Clinical Guidelines.
13. American Society of Echocardiography Statement on COVID-19. 2020 Apr 1.
14. A cardiologist in Brooklyn infected with COVID-19. @jigneshpatelMD. 2020 Mar 20.
15. Paules CI et al. Coronavirus infections – more than just the common cold. JAMA. 2020 Jan 23. doi: 10.1001/jama.2020.0757.
16. Zheng YY et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17(5):259-60.
17. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656.
18. Henry C et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018 Oct 26;31(4):419-23.
19. HFSA/ACC/AHA statement addresses concerns re: Using RAAS antagonists in COVID-19. 2020 Mar 17.
20. Touret F and de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020 May;177:104762. doi: 10.1016/j.antiviral.2020.104762.
21. Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chinese journal of tuberculosis and respiratory diseases. 2020 Mar 12;43(3):185-8.
22. Yao X et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020 Mar 9. doi: 10.1093/cid/ciaa237.
23. Devaux CA et al. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12:105938. doi: 10.1016/j.ijantimicag.2020.105938.