User login
New AAD guidelines eye comorbidities in adults with atopic dermatitis
While it’s well established that atopic dermatitis (AD) in adults is associated with asthma, allergic rhinitis, and other atopic conditions, the links between AD and other comorbidities are coming into clearer focus.
, published evidence supports an association between AD and comorbidities that may not be on the radar of clinicians and patients, including substance use, attention-deficit/hyperactivity disorder (ADHD), elements of metabolic syndrome, and various cardiovascular conditions.
“There are more comorbidities with AD than we anticipated, that are supported by data in the literature,” Dawn M.R. Davis, MD, cochair and an author of the guidelines, told this news organization. “We are learning more about the interconnectivity of various medical conditions,” she continued. “Many skin diseases over time have been noted to be impactful to the whole person and not only the skin. A classic example of that is psoriasis. We now understand that psoriasis is a multisystem inflammatory disorder.”
As for AD, “we’ve always appreciated that AD patients tend to be at higher risk for other atopic diseases such as asthma, allergic rhinoconjunctivitis, and food allergies,” said Dr. Davis, of the departments of dermatology and pediatrics at the Mayo Clinic, Rochester, Minn. “With further research, we are now able to delineate those associations more intimately and have data to support our suspicions. Additionally, we’re now understanding that these inflammatory conditions can impact more than the end organ involved, such as the skin and AD. We wanted to look at how AD can affect the whole patient.”
For the guidelines, which are the first of their kind and were published online in the Journal of the American Academy of Dermatology, Dr. Davis and project cochair Robert Sidbury, MD, MPH, chief of dermatology at Seattle Children’s Hospital, led a multidisciplinary group of 12 experts to review the association between AD and selected comorbidities. They applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for prognosis approach for assessing the certainty of the evidence and provided statements of association based on the available evidence.
With respect to highlights for atopic and allergic conditions, the guideline authors found high-quality evidence that AD in adults is associated with food allergies, moderate-quality evidence that AD is associated with asthma, and low-quality evidence that AD in adults may be associated with eosinophilic esophagitis.
In the realm of mental health and substance use, ample evidence exists to support an association between AD and mental health conditions such as depression and anxiety, the guidelines state. “For many patients, low mood may be driven by the symptoms of AD, including chronic itch and poor sleep,” Dr. Davis and her coauthors wrote. “Successfully treating AD may alleviate depressive symptoms for some patients; for others, assessment and treatment specific to their mental health may be needed.”
The guidelines also state that low-quality evidence exists to suggest that AD in adults may be associated with alcohol abuse disorders and cigarette smoking.
The authors noted “limited but consistent evidence” supporting a link between AD and adverse bone health, including osteoporosis and fractures, while associations between AD and cardiovascular risk factors and comorbidities, including hypertension, myocardial infarction, and stroke, are more controversial.
“I have published on bone health and AD so that was not as surprising to me,” Dr. Davis said in the interview. “I found a lot of the evidence in the guidelines to be validating of patterns that we see in our patients. The most significant learning point for me was [the link to] cardiovascular disease and the link to specific mental health and substance use disorders. It validates how impactful AD is to the individual.”
According to the guidelines, moderate-quality evidence exists linking AD in adults to both alopecia areata and urticaria. “Because we are dermatologists and take care of both of those diseases, be mindful of that in your daily practice,” Dr. Davis advised. “I would also encourage our colleagues to remember to educate patients on the comorbidities of AD so that they are empowered, and to screen for those comorbidities in your office based on the patient and their history and physical exam, to the level that you think is appropriate for that person’s individual’s care.”
Christine Ko, MD, who was asked to comment on the guidelines, characterized some of the reported comorbidity associations as predictable, such as asthma, food allergy, allergic rhinitis, and skin infections. “As the authors comment, ‘associations between AD and other atopic and allergic conditions have been recognized for decades and even contribute to diagnostic criteria for AD,’ ” said Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn, who was not involved with the guidelines. “I was a bit surprised to see that atopic dermatitis in adults is associated with osteoporosis and fractures. As the authors suggest, this could be secondary to treatment with oral prednisone, and it is possible that use of dupilumab and JAK inhibitors may lessen this association.”
Shawn G. Kwatra, MD, of the department of dermatology at Johns Hopkins University, Baltimore, who was not involved with the guidelines, and was also asked to comment, said that the guidelines underscore the importance of informing adults with AD “of the risks of unchecked inflammation and the potential for multiple disease comorbidities.” Dr. Kwatra, who has AD, added that “these results make me want to be more proactive in treating my eczema to reduce the potential for development of these comorbidities.”
He pointed out that the guidelines did not address racial and ethnic differences in the observed comorbidities. “Unfortunately, minority populations have a greater comorbidity burden in many inflammatory skin diseases so this will be another area needing further investigation,” he said. “As an example, our group found from multicenter data that black patients with atopic dermatitis have higher levels of C-reactive protein, blood eosinophils, and other inflammatory biomarkers.”
The AAD guidelines are the first in a four-part series on AD expected to be published over the next 1-2 years, Dr. Davis said. The subsequent guidelines will address topicals, phototherapy/systemics, and pediatrics.
The study was funded by internal funds from the AAD. Dr. Davis reported having no financial disclosures. Dr. Sidbury disclosed that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, and an investigator for Brickell Biotech and Galderma. He is also a consultant for Galderma Global and Microes. Dr. Ko reported having no financial disclosures. Dr. Kwatra is a member of the board of directors of the Skin of Color Society. He is also an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron Pharmaceuticals, and Sanofi, and has served as an investigator for Galderma, Pfizer, and Sanofi.
While it’s well established that atopic dermatitis (AD) in adults is associated with asthma, allergic rhinitis, and other atopic conditions, the links between AD and other comorbidities are coming into clearer focus.
, published evidence supports an association between AD and comorbidities that may not be on the radar of clinicians and patients, including substance use, attention-deficit/hyperactivity disorder (ADHD), elements of metabolic syndrome, and various cardiovascular conditions.
“There are more comorbidities with AD than we anticipated, that are supported by data in the literature,” Dawn M.R. Davis, MD, cochair and an author of the guidelines, told this news organization. “We are learning more about the interconnectivity of various medical conditions,” she continued. “Many skin diseases over time have been noted to be impactful to the whole person and not only the skin. A classic example of that is psoriasis. We now understand that psoriasis is a multisystem inflammatory disorder.”
As for AD, “we’ve always appreciated that AD patients tend to be at higher risk for other atopic diseases such as asthma, allergic rhinoconjunctivitis, and food allergies,” said Dr. Davis, of the departments of dermatology and pediatrics at the Mayo Clinic, Rochester, Minn. “With further research, we are now able to delineate those associations more intimately and have data to support our suspicions. Additionally, we’re now understanding that these inflammatory conditions can impact more than the end organ involved, such as the skin and AD. We wanted to look at how AD can affect the whole patient.”
For the guidelines, which are the first of their kind and were published online in the Journal of the American Academy of Dermatology, Dr. Davis and project cochair Robert Sidbury, MD, MPH, chief of dermatology at Seattle Children’s Hospital, led a multidisciplinary group of 12 experts to review the association between AD and selected comorbidities. They applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for prognosis approach for assessing the certainty of the evidence and provided statements of association based on the available evidence.
With respect to highlights for atopic and allergic conditions, the guideline authors found high-quality evidence that AD in adults is associated with food allergies, moderate-quality evidence that AD is associated with asthma, and low-quality evidence that AD in adults may be associated with eosinophilic esophagitis.
In the realm of mental health and substance use, ample evidence exists to support an association between AD and mental health conditions such as depression and anxiety, the guidelines state. “For many patients, low mood may be driven by the symptoms of AD, including chronic itch and poor sleep,” Dr. Davis and her coauthors wrote. “Successfully treating AD may alleviate depressive symptoms for some patients; for others, assessment and treatment specific to their mental health may be needed.”
The guidelines also state that low-quality evidence exists to suggest that AD in adults may be associated with alcohol abuse disorders and cigarette smoking.
The authors noted “limited but consistent evidence” supporting a link between AD and adverse bone health, including osteoporosis and fractures, while associations between AD and cardiovascular risk factors and comorbidities, including hypertension, myocardial infarction, and stroke, are more controversial.
“I have published on bone health and AD so that was not as surprising to me,” Dr. Davis said in the interview. “I found a lot of the evidence in the guidelines to be validating of patterns that we see in our patients. The most significant learning point for me was [the link to] cardiovascular disease and the link to specific mental health and substance use disorders. It validates how impactful AD is to the individual.”
According to the guidelines, moderate-quality evidence exists linking AD in adults to both alopecia areata and urticaria. “Because we are dermatologists and take care of both of those diseases, be mindful of that in your daily practice,” Dr. Davis advised. “I would also encourage our colleagues to remember to educate patients on the comorbidities of AD so that they are empowered, and to screen for those comorbidities in your office based on the patient and their history and physical exam, to the level that you think is appropriate for that person’s individual’s care.”
Christine Ko, MD, who was asked to comment on the guidelines, characterized some of the reported comorbidity associations as predictable, such as asthma, food allergy, allergic rhinitis, and skin infections. “As the authors comment, ‘associations between AD and other atopic and allergic conditions have been recognized for decades and even contribute to diagnostic criteria for AD,’ ” said Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn, who was not involved with the guidelines. “I was a bit surprised to see that atopic dermatitis in adults is associated with osteoporosis and fractures. As the authors suggest, this could be secondary to treatment with oral prednisone, and it is possible that use of dupilumab and JAK inhibitors may lessen this association.”
Shawn G. Kwatra, MD, of the department of dermatology at Johns Hopkins University, Baltimore, who was not involved with the guidelines, and was also asked to comment, said that the guidelines underscore the importance of informing adults with AD “of the risks of unchecked inflammation and the potential for multiple disease comorbidities.” Dr. Kwatra, who has AD, added that “these results make me want to be more proactive in treating my eczema to reduce the potential for development of these comorbidities.”
He pointed out that the guidelines did not address racial and ethnic differences in the observed comorbidities. “Unfortunately, minority populations have a greater comorbidity burden in many inflammatory skin diseases so this will be another area needing further investigation,” he said. “As an example, our group found from multicenter data that black patients with atopic dermatitis have higher levels of C-reactive protein, blood eosinophils, and other inflammatory biomarkers.”
The AAD guidelines are the first in a four-part series on AD expected to be published over the next 1-2 years, Dr. Davis said. The subsequent guidelines will address topicals, phototherapy/systemics, and pediatrics.
The study was funded by internal funds from the AAD. Dr. Davis reported having no financial disclosures. Dr. Sidbury disclosed that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, and an investigator for Brickell Biotech and Galderma. He is also a consultant for Galderma Global and Microes. Dr. Ko reported having no financial disclosures. Dr. Kwatra is a member of the board of directors of the Skin of Color Society. He is also an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron Pharmaceuticals, and Sanofi, and has served as an investigator for Galderma, Pfizer, and Sanofi.
While it’s well established that atopic dermatitis (AD) in adults is associated with asthma, allergic rhinitis, and other atopic conditions, the links between AD and other comorbidities are coming into clearer focus.
, published evidence supports an association between AD and comorbidities that may not be on the radar of clinicians and patients, including substance use, attention-deficit/hyperactivity disorder (ADHD), elements of metabolic syndrome, and various cardiovascular conditions.
“There are more comorbidities with AD than we anticipated, that are supported by data in the literature,” Dawn M.R. Davis, MD, cochair and an author of the guidelines, told this news organization. “We are learning more about the interconnectivity of various medical conditions,” she continued. “Many skin diseases over time have been noted to be impactful to the whole person and not only the skin. A classic example of that is psoriasis. We now understand that psoriasis is a multisystem inflammatory disorder.”
As for AD, “we’ve always appreciated that AD patients tend to be at higher risk for other atopic diseases such as asthma, allergic rhinoconjunctivitis, and food allergies,” said Dr. Davis, of the departments of dermatology and pediatrics at the Mayo Clinic, Rochester, Minn. “With further research, we are now able to delineate those associations more intimately and have data to support our suspicions. Additionally, we’re now understanding that these inflammatory conditions can impact more than the end organ involved, such as the skin and AD. We wanted to look at how AD can affect the whole patient.”
For the guidelines, which are the first of their kind and were published online in the Journal of the American Academy of Dermatology, Dr. Davis and project cochair Robert Sidbury, MD, MPH, chief of dermatology at Seattle Children’s Hospital, led a multidisciplinary group of 12 experts to review the association between AD and selected comorbidities. They applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for prognosis approach for assessing the certainty of the evidence and provided statements of association based on the available evidence.
With respect to highlights for atopic and allergic conditions, the guideline authors found high-quality evidence that AD in adults is associated with food allergies, moderate-quality evidence that AD is associated with asthma, and low-quality evidence that AD in adults may be associated with eosinophilic esophagitis.
In the realm of mental health and substance use, ample evidence exists to support an association between AD and mental health conditions such as depression and anxiety, the guidelines state. “For many patients, low mood may be driven by the symptoms of AD, including chronic itch and poor sleep,” Dr. Davis and her coauthors wrote. “Successfully treating AD may alleviate depressive symptoms for some patients; for others, assessment and treatment specific to their mental health may be needed.”
The guidelines also state that low-quality evidence exists to suggest that AD in adults may be associated with alcohol abuse disorders and cigarette smoking.
The authors noted “limited but consistent evidence” supporting a link between AD and adverse bone health, including osteoporosis and fractures, while associations between AD and cardiovascular risk factors and comorbidities, including hypertension, myocardial infarction, and stroke, are more controversial.
“I have published on bone health and AD so that was not as surprising to me,” Dr. Davis said in the interview. “I found a lot of the evidence in the guidelines to be validating of patterns that we see in our patients. The most significant learning point for me was [the link to] cardiovascular disease and the link to specific mental health and substance use disorders. It validates how impactful AD is to the individual.”
According to the guidelines, moderate-quality evidence exists linking AD in adults to both alopecia areata and urticaria. “Because we are dermatologists and take care of both of those diseases, be mindful of that in your daily practice,” Dr. Davis advised. “I would also encourage our colleagues to remember to educate patients on the comorbidities of AD so that they are empowered, and to screen for those comorbidities in your office based on the patient and their history and physical exam, to the level that you think is appropriate for that person’s individual’s care.”
Christine Ko, MD, who was asked to comment on the guidelines, characterized some of the reported comorbidity associations as predictable, such as asthma, food allergy, allergic rhinitis, and skin infections. “As the authors comment, ‘associations between AD and other atopic and allergic conditions have been recognized for decades and even contribute to diagnostic criteria for AD,’ ” said Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn, who was not involved with the guidelines. “I was a bit surprised to see that atopic dermatitis in adults is associated with osteoporosis and fractures. As the authors suggest, this could be secondary to treatment with oral prednisone, and it is possible that use of dupilumab and JAK inhibitors may lessen this association.”
Shawn G. Kwatra, MD, of the department of dermatology at Johns Hopkins University, Baltimore, who was not involved with the guidelines, and was also asked to comment, said that the guidelines underscore the importance of informing adults with AD “of the risks of unchecked inflammation and the potential for multiple disease comorbidities.” Dr. Kwatra, who has AD, added that “these results make me want to be more proactive in treating my eczema to reduce the potential for development of these comorbidities.”
He pointed out that the guidelines did not address racial and ethnic differences in the observed comorbidities. “Unfortunately, minority populations have a greater comorbidity burden in many inflammatory skin diseases so this will be another area needing further investigation,” he said. “As an example, our group found from multicenter data that black patients with atopic dermatitis have higher levels of C-reactive protein, blood eosinophils, and other inflammatory biomarkers.”
The AAD guidelines are the first in a four-part series on AD expected to be published over the next 1-2 years, Dr. Davis said. The subsequent guidelines will address topicals, phototherapy/systemics, and pediatrics.
The study was funded by internal funds from the AAD. Dr. Davis reported having no financial disclosures. Dr. Sidbury disclosed that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, and an investigator for Brickell Biotech and Galderma. He is also a consultant for Galderma Global and Microes. Dr. Ko reported having no financial disclosures. Dr. Kwatra is a member of the board of directors of the Skin of Color Society. He is also an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron Pharmaceuticals, and Sanofi, and has served as an investigator for Galderma, Pfizer, and Sanofi.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Perception of atopic dermatitis severity often differs between patients, physicians
It’s no secret that atopic dermatitis (AD) is associated with a high burden of disease, with an impact on sleep disturbance, increased anxiety, depression, reduced function and productivity at work and school, and overall decreased quality of life.
But to complicate matters, , according to Zelma Chiesa Fuxench, MD, a dermatologist at the University of Pennsylvania, Philadelphia. For example, a cross-sectional study of 678 patients with AD, which assessed disease severity based on self-reports and physician-reported disease severity using components of the Eczema Area and Severity Index score, found that the level of agreement matched in about 68% of the cases. However, in about 32% of cases, there was a mismatch between how patients and physicians rated disease severity. In about 11% of the cases, patients reported a higher degree of disease severity, compared with physicians, while in about 20% of cases, patients reported lower disease severity, compared with the physician assessment.
“This has potential implications for overestimating or underestimating disease burden and could impact our treatment of AD patients,” Dr. Chiesa Fuxench said at the Revolutionizing Atopic Dermatitis symposium.
The study also found that, while the pattern of agreement was not affected by the extent of AD in terms of the body surface area, the use of immunomodulatory drugs, or the Eczema Area and Severity Index (EASI) score, increased sleep disturbance did have an influence. Also, quality of life was lower and a higher impact on work productivity was observed when patients rated their disease severity higher than the rating of physicians.
Measures to assess disease severity
“If we understand that there is mismatch between how a patient experiences their disease and how physicians rate it, what can we do to be better at assessing disease severity in AD to truly capture the full disease burden in patients with AD?” Dr. Chiesa Fuxench asked. She noted that different validated measures have been described in the literature, and objective assessment tools often used in clinical trials include the EASI and the SCORing Atopic Dermatitis (SCORAD). “These are measures that are done by the physician that take into account the extent of the body surface area involvement and also the intensity of the lesions such as how red or thick they are,” she said. “In addition, the SCORAD will also take into account the patient-reported intensity level of itch and sleep loss.”
The Patient-Oriented SCORAD (PO-SCORAD) is similar to the SCORAD except that it is completed by the patient or the patient’s caregiver. In all three outcome measures, a higher score indicates a higher level of disease severity. Other measures that have been frequently described in the literature include the Patient-Oriented Eczema Measure (POEM), which takes into account seven symptoms scored over the last week (itch, sleep, weeping/oozing, cracking, flaking, and dryness/roughness), with higher scores indicating increased disease severity, and the Dermatology Life Quality Index (DLQI), which is a generic measure to assess the burden of skin diseases including AD. The DLQI “asks 10 questions as they relate to the impact of health-related quality of life over the last week, with higher scores indicating more severe disease,” Dr. Chiesa Fuxench said.
There are also symptom-specific scales such as the Pruritus Numerical Rating Scale (Pruritus-NRS) that measures the impact of itch on a scale of 0 to 10, and the Three-Item Severity Scale (TIS) and the Validated Investigator Global Assessment (v-IGA) that are used to assess different measures in terms of intensity of the lesions.”
However, the study that looked at the discordance between AD severity reported by physicians and patients also found that awareness and use of clinical and patient-reported measures for assessing AD disease severity among physicians was low. The authors further divided their findings among primary care physicians, dermatologists, and allergists/immunologists. “While dermatologists and allergists/immunologists reported being more aware of these outcome measures, a high proportion of physicians within this group were not using these outcomes measures in daily clinical practice,” Dr. Chiesa Fuxench said.
“Is there a need for us to use more than one outcome measure instrument when trying to assess the impact of AD, understanding that many of us practice in a very busy clinical setting? The answer is probably yes. The use of multiple assessment tools that measure different domains could potentially help better capture the broad manifestations of AD, because of the complex nature of disease burden in this population. In addition, there are studies showing poor correlation between patient-reported and physician-assessed disease severity for various instruments, emphasizing the point that these measures may be capturing very different things.”
With so many measures to choose from and limited time in the office, which ones should clinicians use? Harmonizing Outcome Measures for Eczema (HOME), based at the Center of Evidence-based Dermatology, at the University of Nottingham (England), is a consortium of patients and other key stakeholders in AD aiming to develop a consensus-based core outcome set for clinical trials and clinical practice. At a consensus meeting in 2018, the consortium reported that the PO-SCORAD and the POEM could be used in the clinical setting to better capture the true level of disease severity and burden in patients with AD.
The PO-SCORAD is also available as an App. A PO-SCORAD of less than 25 is associated with mild disease; a PO-SCORAD between 25 and 50 is associated with moderate disease, and a PO-SCORAD of greater than 50 is associated with severe disease.
“It’s recommended that patients capture the PO-SCORAD once or twice a week,” Dr. Chiesa Fuxench said, noting that the newer version of the App includes photos of different skin types to make it more relevant for a larger number of patients.
Another advantage of using the App is that a patient can track their disease severity through time. They can upload photographs, or they can send you a graphical input of their disease severity either through e-mail or print it out and bring it to their office visit to share the results with you.”
A prospective observational European study of 471 adult and pediatric patients with AD found a statistically significant correlation between SCORAD and PO-SCORAD results at day 0 and day 28. A separate large study conducted in 12 countries found that PO-SCORAD was the only self-assessment score to be highly correlated with the SCORAD index and POEM (A Spearman’s correlation coefficient of greater than or equal to 0.70). In that study, PO-SCORAD also correlated most closely with the results of the DLQI (r = 0.67) and the Dermatitis Family Questionnaire Impact DFQI (r = 0.56).
A more recent study of almost 300 adults with AD that examined the correlations between PO-SCORAD, POEM, and DLQI yielded similar findings.
Other researchers are aiming to assess the full burden of AD at the patient level. Drawing from a cross-sectional study called AWARE (Adults With Atopic Dermatitis Reporting on their Experience), an international observational study, investigators sought to identify what terms AD patients were using to describe their disease. The most commonly used terms were itch (37%), embarrassed (37%), annoyed (35%), pain (25%), and frustration (22%). “Although our study did not identify all patient-reported consequences of AD, such as the known impact of AD on sexual health, our qualitative approach has provided an understanding of patient perceptions and the underlying range of physical and emotional consequences of AD, which can inform shared decision-making,” the authors wrote. “These findings suggest the need for broader assessment of the impact of AD on patients’ lives,” they added.
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
The study on AD severity reported by physicians and patients was funded by Sanofi and Regeneron Pharmaceuticals, and several authors were employees of those companies.
It’s no secret that atopic dermatitis (AD) is associated with a high burden of disease, with an impact on sleep disturbance, increased anxiety, depression, reduced function and productivity at work and school, and overall decreased quality of life.
But to complicate matters, , according to Zelma Chiesa Fuxench, MD, a dermatologist at the University of Pennsylvania, Philadelphia. For example, a cross-sectional study of 678 patients with AD, which assessed disease severity based on self-reports and physician-reported disease severity using components of the Eczema Area and Severity Index score, found that the level of agreement matched in about 68% of the cases. However, in about 32% of cases, there was a mismatch between how patients and physicians rated disease severity. In about 11% of the cases, patients reported a higher degree of disease severity, compared with physicians, while in about 20% of cases, patients reported lower disease severity, compared with the physician assessment.
“This has potential implications for overestimating or underestimating disease burden and could impact our treatment of AD patients,” Dr. Chiesa Fuxench said at the Revolutionizing Atopic Dermatitis symposium.
The study also found that, while the pattern of agreement was not affected by the extent of AD in terms of the body surface area, the use of immunomodulatory drugs, or the Eczema Area and Severity Index (EASI) score, increased sleep disturbance did have an influence. Also, quality of life was lower and a higher impact on work productivity was observed when patients rated their disease severity higher than the rating of physicians.
Measures to assess disease severity
“If we understand that there is mismatch between how a patient experiences their disease and how physicians rate it, what can we do to be better at assessing disease severity in AD to truly capture the full disease burden in patients with AD?” Dr. Chiesa Fuxench asked. She noted that different validated measures have been described in the literature, and objective assessment tools often used in clinical trials include the EASI and the SCORing Atopic Dermatitis (SCORAD). “These are measures that are done by the physician that take into account the extent of the body surface area involvement and also the intensity of the lesions such as how red or thick they are,” she said. “In addition, the SCORAD will also take into account the patient-reported intensity level of itch and sleep loss.”
The Patient-Oriented SCORAD (PO-SCORAD) is similar to the SCORAD except that it is completed by the patient or the patient’s caregiver. In all three outcome measures, a higher score indicates a higher level of disease severity. Other measures that have been frequently described in the literature include the Patient-Oriented Eczema Measure (POEM), which takes into account seven symptoms scored over the last week (itch, sleep, weeping/oozing, cracking, flaking, and dryness/roughness), with higher scores indicating increased disease severity, and the Dermatology Life Quality Index (DLQI), which is a generic measure to assess the burden of skin diseases including AD. The DLQI “asks 10 questions as they relate to the impact of health-related quality of life over the last week, with higher scores indicating more severe disease,” Dr. Chiesa Fuxench said.
There are also symptom-specific scales such as the Pruritus Numerical Rating Scale (Pruritus-NRS) that measures the impact of itch on a scale of 0 to 10, and the Three-Item Severity Scale (TIS) and the Validated Investigator Global Assessment (v-IGA) that are used to assess different measures in terms of intensity of the lesions.”
However, the study that looked at the discordance between AD severity reported by physicians and patients also found that awareness and use of clinical and patient-reported measures for assessing AD disease severity among physicians was low. The authors further divided their findings among primary care physicians, dermatologists, and allergists/immunologists. “While dermatologists and allergists/immunologists reported being more aware of these outcome measures, a high proportion of physicians within this group were not using these outcomes measures in daily clinical practice,” Dr. Chiesa Fuxench said.
“Is there a need for us to use more than one outcome measure instrument when trying to assess the impact of AD, understanding that many of us practice in a very busy clinical setting? The answer is probably yes. The use of multiple assessment tools that measure different domains could potentially help better capture the broad manifestations of AD, because of the complex nature of disease burden in this population. In addition, there are studies showing poor correlation between patient-reported and physician-assessed disease severity for various instruments, emphasizing the point that these measures may be capturing very different things.”
With so many measures to choose from and limited time in the office, which ones should clinicians use? Harmonizing Outcome Measures for Eczema (HOME), based at the Center of Evidence-based Dermatology, at the University of Nottingham (England), is a consortium of patients and other key stakeholders in AD aiming to develop a consensus-based core outcome set for clinical trials and clinical practice. At a consensus meeting in 2018, the consortium reported that the PO-SCORAD and the POEM could be used in the clinical setting to better capture the true level of disease severity and burden in patients with AD.
The PO-SCORAD is also available as an App. A PO-SCORAD of less than 25 is associated with mild disease; a PO-SCORAD between 25 and 50 is associated with moderate disease, and a PO-SCORAD of greater than 50 is associated with severe disease.
“It’s recommended that patients capture the PO-SCORAD once or twice a week,” Dr. Chiesa Fuxench said, noting that the newer version of the App includes photos of different skin types to make it more relevant for a larger number of patients.
Another advantage of using the App is that a patient can track their disease severity through time. They can upload photographs, or they can send you a graphical input of their disease severity either through e-mail or print it out and bring it to their office visit to share the results with you.”
A prospective observational European study of 471 adult and pediatric patients with AD found a statistically significant correlation between SCORAD and PO-SCORAD results at day 0 and day 28. A separate large study conducted in 12 countries found that PO-SCORAD was the only self-assessment score to be highly correlated with the SCORAD index and POEM (A Spearman’s correlation coefficient of greater than or equal to 0.70). In that study, PO-SCORAD also correlated most closely with the results of the DLQI (r = 0.67) and the Dermatitis Family Questionnaire Impact DFQI (r = 0.56).
A more recent study of almost 300 adults with AD that examined the correlations between PO-SCORAD, POEM, and DLQI yielded similar findings.
Other researchers are aiming to assess the full burden of AD at the patient level. Drawing from a cross-sectional study called AWARE (Adults With Atopic Dermatitis Reporting on their Experience), an international observational study, investigators sought to identify what terms AD patients were using to describe their disease. The most commonly used terms were itch (37%), embarrassed (37%), annoyed (35%), pain (25%), and frustration (22%). “Although our study did not identify all patient-reported consequences of AD, such as the known impact of AD on sexual health, our qualitative approach has provided an understanding of patient perceptions and the underlying range of physical and emotional consequences of AD, which can inform shared decision-making,” the authors wrote. “These findings suggest the need for broader assessment of the impact of AD on patients’ lives,” they added.
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
The study on AD severity reported by physicians and patients was funded by Sanofi and Regeneron Pharmaceuticals, and several authors were employees of those companies.
It’s no secret that atopic dermatitis (AD) is associated with a high burden of disease, with an impact on sleep disturbance, increased anxiety, depression, reduced function and productivity at work and school, and overall decreased quality of life.
But to complicate matters, , according to Zelma Chiesa Fuxench, MD, a dermatologist at the University of Pennsylvania, Philadelphia. For example, a cross-sectional study of 678 patients with AD, which assessed disease severity based on self-reports and physician-reported disease severity using components of the Eczema Area and Severity Index score, found that the level of agreement matched in about 68% of the cases. However, in about 32% of cases, there was a mismatch between how patients and physicians rated disease severity. In about 11% of the cases, patients reported a higher degree of disease severity, compared with physicians, while in about 20% of cases, patients reported lower disease severity, compared with the physician assessment.
“This has potential implications for overestimating or underestimating disease burden and could impact our treatment of AD patients,” Dr. Chiesa Fuxench said at the Revolutionizing Atopic Dermatitis symposium.
The study also found that, while the pattern of agreement was not affected by the extent of AD in terms of the body surface area, the use of immunomodulatory drugs, or the Eczema Area and Severity Index (EASI) score, increased sleep disturbance did have an influence. Also, quality of life was lower and a higher impact on work productivity was observed when patients rated their disease severity higher than the rating of physicians.
Measures to assess disease severity
“If we understand that there is mismatch between how a patient experiences their disease and how physicians rate it, what can we do to be better at assessing disease severity in AD to truly capture the full disease burden in patients with AD?” Dr. Chiesa Fuxench asked. She noted that different validated measures have been described in the literature, and objective assessment tools often used in clinical trials include the EASI and the SCORing Atopic Dermatitis (SCORAD). “These are measures that are done by the physician that take into account the extent of the body surface area involvement and also the intensity of the lesions such as how red or thick they are,” she said. “In addition, the SCORAD will also take into account the patient-reported intensity level of itch and sleep loss.”
The Patient-Oriented SCORAD (PO-SCORAD) is similar to the SCORAD except that it is completed by the patient or the patient’s caregiver. In all three outcome measures, a higher score indicates a higher level of disease severity. Other measures that have been frequently described in the literature include the Patient-Oriented Eczema Measure (POEM), which takes into account seven symptoms scored over the last week (itch, sleep, weeping/oozing, cracking, flaking, and dryness/roughness), with higher scores indicating increased disease severity, and the Dermatology Life Quality Index (DLQI), which is a generic measure to assess the burden of skin diseases including AD. The DLQI “asks 10 questions as they relate to the impact of health-related quality of life over the last week, with higher scores indicating more severe disease,” Dr. Chiesa Fuxench said.
There are also symptom-specific scales such as the Pruritus Numerical Rating Scale (Pruritus-NRS) that measures the impact of itch on a scale of 0 to 10, and the Three-Item Severity Scale (TIS) and the Validated Investigator Global Assessment (v-IGA) that are used to assess different measures in terms of intensity of the lesions.”
However, the study that looked at the discordance between AD severity reported by physicians and patients also found that awareness and use of clinical and patient-reported measures for assessing AD disease severity among physicians was low. The authors further divided their findings among primary care physicians, dermatologists, and allergists/immunologists. “While dermatologists and allergists/immunologists reported being more aware of these outcome measures, a high proportion of physicians within this group were not using these outcomes measures in daily clinical practice,” Dr. Chiesa Fuxench said.
“Is there a need for us to use more than one outcome measure instrument when trying to assess the impact of AD, understanding that many of us practice in a very busy clinical setting? The answer is probably yes. The use of multiple assessment tools that measure different domains could potentially help better capture the broad manifestations of AD, because of the complex nature of disease burden in this population. In addition, there are studies showing poor correlation between patient-reported and physician-assessed disease severity for various instruments, emphasizing the point that these measures may be capturing very different things.”
With so many measures to choose from and limited time in the office, which ones should clinicians use? Harmonizing Outcome Measures for Eczema (HOME), based at the Center of Evidence-based Dermatology, at the University of Nottingham (England), is a consortium of patients and other key stakeholders in AD aiming to develop a consensus-based core outcome set for clinical trials and clinical practice. At a consensus meeting in 2018, the consortium reported that the PO-SCORAD and the POEM could be used in the clinical setting to better capture the true level of disease severity and burden in patients with AD.
The PO-SCORAD is also available as an App. A PO-SCORAD of less than 25 is associated with mild disease; a PO-SCORAD between 25 and 50 is associated with moderate disease, and a PO-SCORAD of greater than 50 is associated with severe disease.
“It’s recommended that patients capture the PO-SCORAD once or twice a week,” Dr. Chiesa Fuxench said, noting that the newer version of the App includes photos of different skin types to make it more relevant for a larger number of patients.
Another advantage of using the App is that a patient can track their disease severity through time. They can upload photographs, or they can send you a graphical input of their disease severity either through e-mail or print it out and bring it to their office visit to share the results with you.”
A prospective observational European study of 471 adult and pediatric patients with AD found a statistically significant correlation between SCORAD and PO-SCORAD results at day 0 and day 28. A separate large study conducted in 12 countries found that PO-SCORAD was the only self-assessment score to be highly correlated with the SCORAD index and POEM (A Spearman’s correlation coefficient of greater than or equal to 0.70). In that study, PO-SCORAD also correlated most closely with the results of the DLQI (r = 0.67) and the Dermatitis Family Questionnaire Impact DFQI (r = 0.56).
A more recent study of almost 300 adults with AD that examined the correlations between PO-SCORAD, POEM, and DLQI yielded similar findings.
Other researchers are aiming to assess the full burden of AD at the patient level. Drawing from a cross-sectional study called AWARE (Adults With Atopic Dermatitis Reporting on their Experience), an international observational study, investigators sought to identify what terms AD patients were using to describe their disease. The most commonly used terms were itch (37%), embarrassed (37%), annoyed (35%), pain (25%), and frustration (22%). “Although our study did not identify all patient-reported consequences of AD, such as the known impact of AD on sexual health, our qualitative approach has provided an understanding of patient perceptions and the underlying range of physical and emotional consequences of AD, which can inform shared decision-making,” the authors wrote. “These findings suggest the need for broader assessment of the impact of AD on patients’ lives,” they added.
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
The study on AD severity reported by physicians and patients was funded by Sanofi and Regeneron Pharmaceuticals, and several authors were employees of those companies.
FROM REVOLUTIONIZING AD 2021
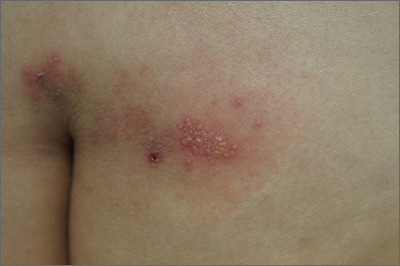
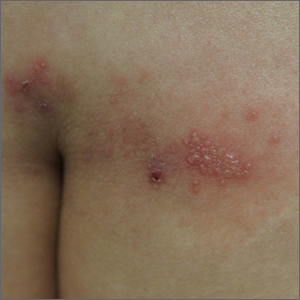
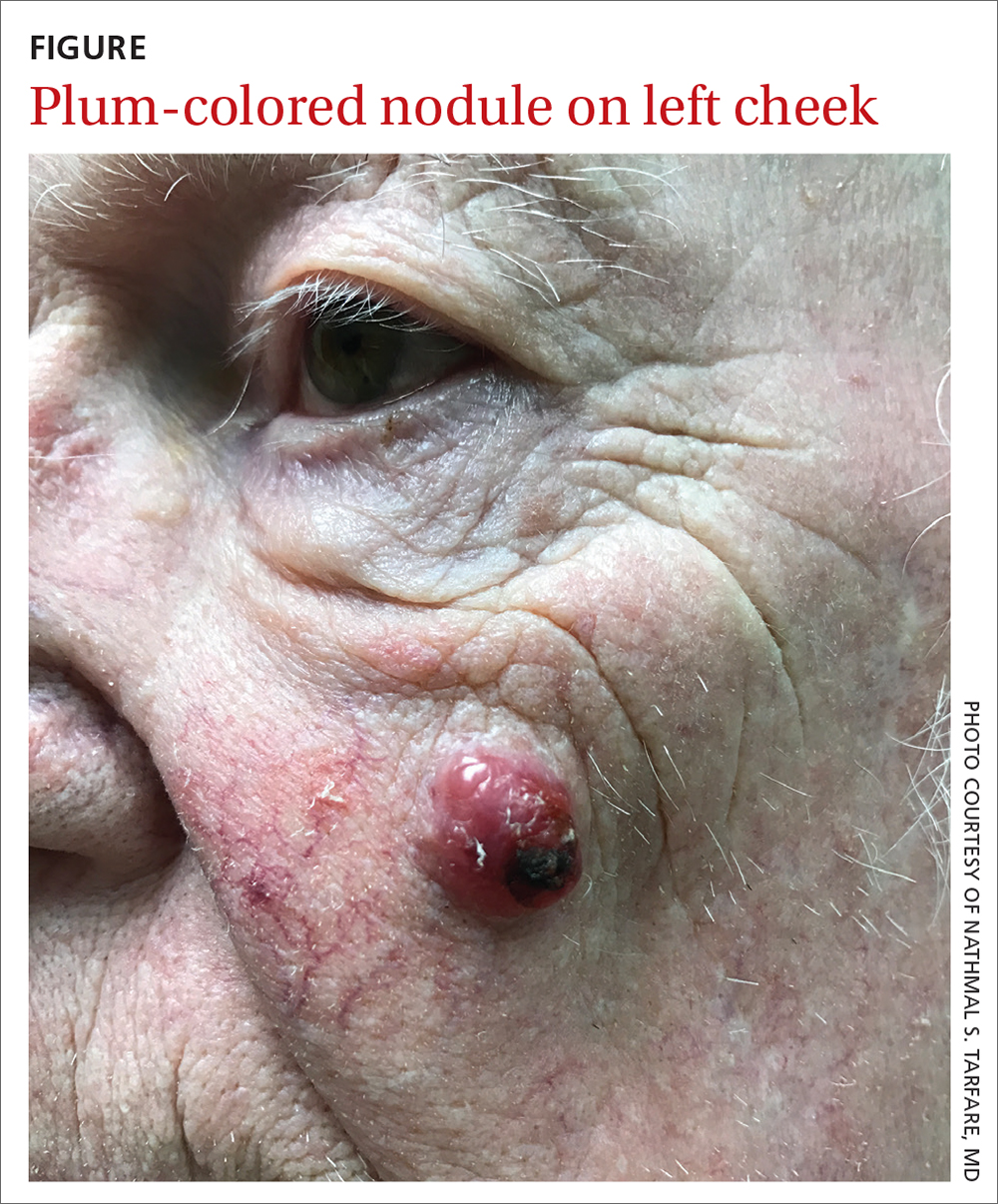
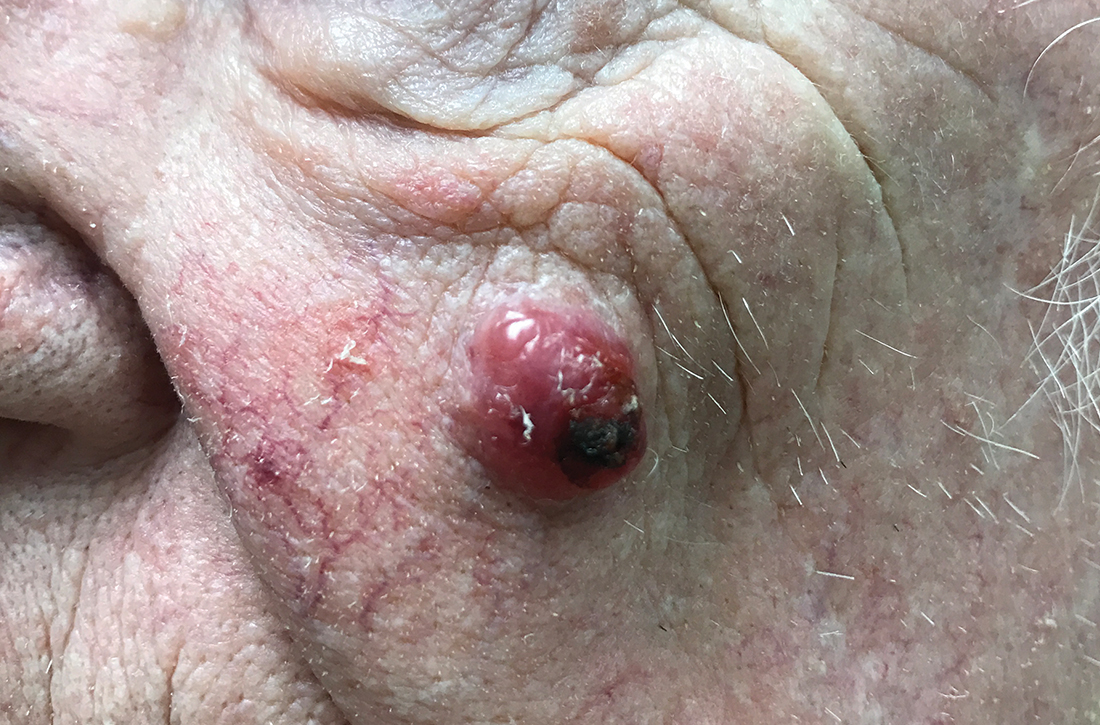
Sacral blisters
Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356
Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356
Pruritus in elderly patients: Not a diagnosis
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
that has appeared seemingly out of the blue.
“They ask: ‘What happened? Why did I get this? Everything was going so well and all of a sudden, I get this itchy rash that keeps me up every night,’ ” Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, said during the Revolutionizing Atopic Dermatitis symposium. “Is this elderly atopic dermatitis? Is that a real thing?”
But such patients often lack flexural involvement, which is a telltale sign of atopic dermatitis, “so I really struggle with making the diagnosis of new onset AD in the elderly,” he said, adding that existing medical literature on the topic is variable, with the use of terms that include chronic eczematous eruption of the elderly, chronic “eczematiform” eruption in the elderly, chronic eczematous eruption of the aged, eczematous dermatitis not otherwise specified, dermal hypersensitivity reaction, urticarial dermatitis, and eczematous drug eruptions.
“Pruritus of the elderly is not a diagnosis,” Dr. Simpson said. “That’s just a symptom with a million etiologies. Never put that as your assessment. You could put pruritic eruption or pruritus, but try to look for the cause.”
More than 50% of older patients have xerosis, according to a 2013 clinical review on pruritus in the elderly, by Timothy G. Berger, MD, and colleagues at the University of California, San Francisco, which includes advice on the evaluation and management of pruritus in this group of patients based on whether they have a rash or not. For a patient with no rash, Dr. Simpson said, the workup “includes ruling out xerosis, scabies, and effects of medications that could cause rash such as narcotics and Adderall; as well as a generalized pruritus workup including renal and hepatic function, blood count, and thyroid levels.”
In a separate analysis of pruritic elderly patients by the same authors, five rash-related diagnoses accounted for 75% of cases: eczematous dermatitis, lichen simplex/prurigo nodularis, subacute prurigo, transient acantholytic dermatosis, and neuropathic disorder. “Morphology of pruritus with rash is also important,” Dr. Simpson added. “Is it eczematous? Papular? Prurigo nodularis? This helps lead you in the right direction.”
Some case-control studies have shown that calcium channel blockers could be related to eczema in older patients.
“But there aren’t a lot of studies out there that show that when you stop your calcium channel blocker, your eczema gets better,” Dr. Simpson said. “I’m reluctant to stop medications to try to help their eczema. I haven’t had many good results doing that.”
In an abstract presented during the 2021 annual meeting of the Society of Investigative Dermatology, he and his colleagues prospectively reviewed 89 patients over age 65 who had been referred with new-onset eczema. Of these, 34 underwent drug cessation trials for 1-3 months. “Not one patient improved when they stopped medications,” Dr. Simpson said, but “multiple patients were hospitalized for discontinuing their cardiac and antihypertensive medications.” While this was a biased sample of patients coming to him with chronic eczema, “in my experience, if you have chronic eczema in an older patient, stopping medications is likely not going to help.”
Other diagnostic tips he offered included asking patients what skin products they’re using, considering patch testing, and considering biopsy to rule out cutaneous T-cell lymphoma or bullous pemphigoid. “If you’re not sure there’s a rash, you might need to do a pruritus workup,” he said. If an eczematous rash is present and no other cause is found, try treating it like AD, he added.
Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
FROM REVOLUTIONIZING AD 2021
More frequent secukinumab dosing found to benefit overweight psoriasis patients
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Sarcoidosis
THE COMPARISON
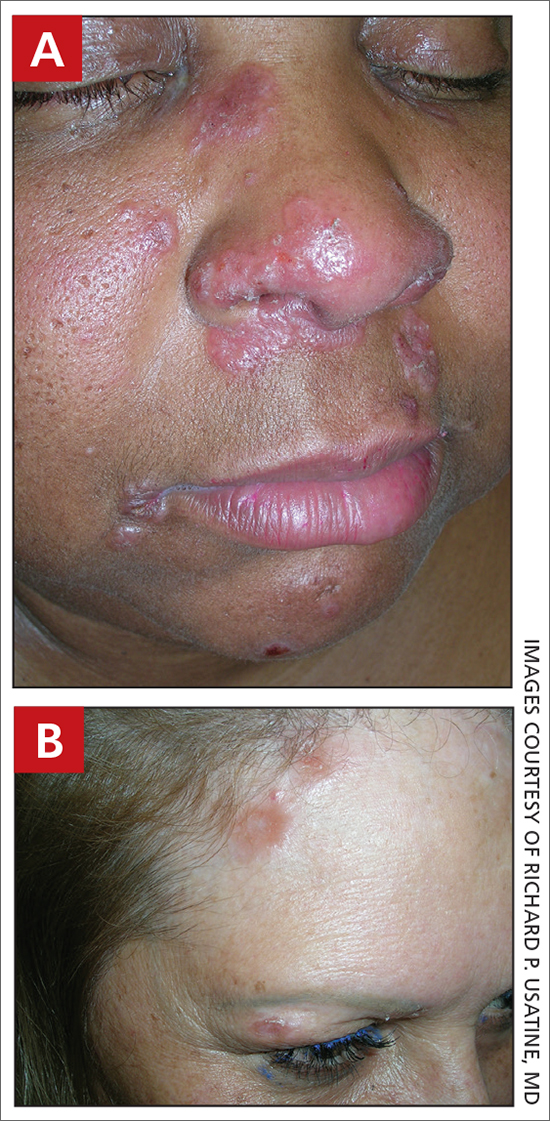
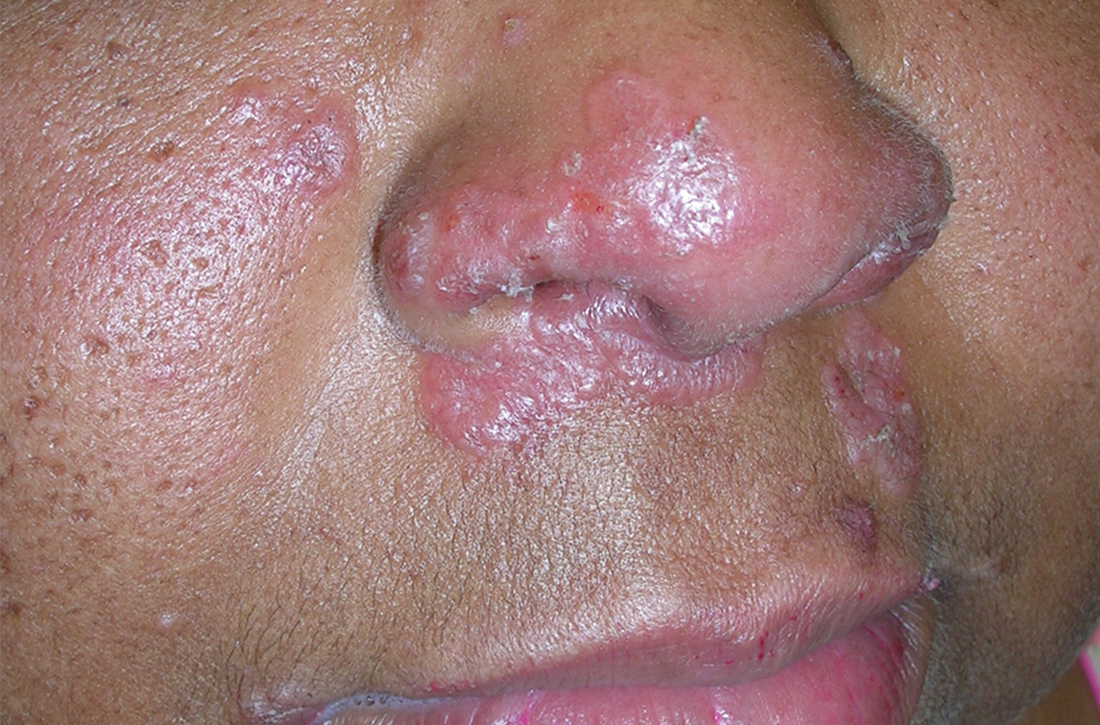
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous.
• In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjustment for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
1. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
2. Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66: 121.e1-121.e14.
3. Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/ cells10040766
4. Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/ oxfordjournals.aje.a009096
5. Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
6. Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of features in 170 patients. Respir Med. 2003;97:978-982.
7. Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
8. Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
9. Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
10. Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
11. James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
12. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
13. Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
14. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
15. Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
16. Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
17. Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
18. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
19. Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
20. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous.
• In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjustment for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
THE COMPARISON
A Pink, elevated, granulomatous, indurated plaques on the face, including the nasal alae, of a 52-year-old woman with a darker skin tone.
B Orange and pink, elevated, granulomatous, indurated plaques on the face of a 55-year-old woman with a lighter skin tone.
Sarcoidosis is a granulomatous disease that may affect the skin in addition to multiple body organ systems, including the lungs. Bilateral hilar adenopathy on a chest radiograph is the most common finding. Sarcoidosis also has a variety of cutaneous manifestations. Early diagnosis is vital, as patients with sarcoidosis and pulmonary fibrosis have a shortened life span compared to the overall population.1 With a growing skin of color population, it is important to recognize sarcoidosis as soon as possible.2
Epidemiology
People of African descent have the highest sarcoidosis prevalence in the United States.3 In the United States, the incidence of sarcoidosis in Black individuals peaks in the fourth decade of life. A 5-year study in a US health maintenance organization found that the age-adjusted annual incidence was 10.9 per 100,000 cases among Whites and 35.5 per 100,000 cases among Blacks.4
Key clinical features in people with darker skin tones:
• Papules are seen in sarcoidosis, primarily on the face, and may start as orange hued or yellow-brown and then become brown-red or pink to violaceous before involuting into faint macules.5-7
• When round or oval sarcoid plaques appear, they often are more erythematous.
• In skin of color, plaques may become hypopigmented.8
• Erythema nodosum, the most common nonspecific cutaneous lesion seen in sarcoidosis, is less commonly seen in those of African and Asian descent.9-11 This is in contrast to distinctive forms of specific sarcoid skin lesions such as lupus pernio and scar sarcoidosis, as well as papules and plaques and minor forms of specific sarcoid skin lesions including subcutaneous nodules; hypopigmented macules; psoriasiform lesions; and ulcerative, localized erythrodermic, ichthyosiform, scalp, and nail lesions.
• Lupus pernio is a cutaneous manifestation of sarcoidosis that appears on the face. It looks similar to lupus erythematosus and occurs most commonly in women of African descent.8,12
• Hypopigmented lesions are more common in those with darker skin tones.9
• Ulcerative lesions are more common in those of African descent and women.13
• Scalp sarcoidosis is more common in patients of African descent.14
• Sarcoidosis may develop at sites of trauma, such as scars and tattoos.15-17
Worth noting
The cutaneous lesions seen in sarcoidosis may be emotionally devastating and disfiguring. Due to the variety of clinical manifestations, sarcoidosis may be misdiagnosed, leading to delays in treatment.18
Health disparity highlight
Patients older than 40 years presenting with sarcoidosis and those of African descent have a worse prognosis.19 Despite adjustment for race, ethnic group, age, and sex, patients with low income and financial barriers present with more severe sarcoidosis.20
1. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
2. Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66: 121.e1-121.e14.
3. Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/ cells10040766
4. Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/ oxfordjournals.aje.a009096
5. Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
6. Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of features in 170 patients. Respir Med. 2003;97:978-982.
7. Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
8. Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
9. Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
10. Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
11. James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
12. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
13. Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
14. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
15. Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
16. Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
17. Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
18. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
19. Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
20. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
1. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
2. Heath CR, David J, Taylor SC. Sarcoidosis: are there differences in your skin of color patients? J Am Acad Dermatol. 2012;66: 121.e1-121.e14.
3. Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/ cells10040766
4. Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. doi:10.1093/ oxfordjournals.aje.a009096
5. Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venerol Leprol. 2007;73:16-21.
6. Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of features in 170 patients. Respir Med. 2003;97:978-982.
7. Olive KE, Kartaria YP. Cutaneous manifestations of sarcoidosis to other organ system involvement, abnormal laboratory measurements, and disease course. Arch Intern Med. 1985;145:1811-1814.
8. Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis. relationship to systemic disease. Arch Dermatol. 1997;133:882-888. doi:10.1001/archderm.1997.03890430098013
9. Minus HR, Grimes PE. Cutaneous manifestations of sarcoidosis in blacks. Cutis. 1983;32:361-364.
10. Edmondstone WM, Wilson AG. Sarcoidosis in Caucasians, blacks and Asians in London. Br J Dis Chest. 1985;79:27-36.
11. James DG, Neville E, Siltzbach LE. Worldwide review of sarcoidosis. Ann N Y Acad Sci. 1976;278:321-334.
12. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149-173.
13. Albertini JG, Tyler W, Miller OF III. Ulcerative sarcoidosis: case report and review of literature. Arch Dermatol. 1997;133:215-219.
14. Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
15. Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
16. Chudomirova K, Velichkva L, Anavi B. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venerol. 2003;17:360-361.
17. Kim YC, Triffet MK, Gibson LE. Foreign bodies in sarcoidosis. Am J Dermatopathol. 2000;22:408-412.
18. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153-2165.
19. Nunes H, Bouvry D, Soler P, et al. Sarcoidosis. Orphanet J Rare Dis. 2007;2:46. doi:10.1186/1750-1172-2-46
20. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889.
Phase 2 studies of novel JAK1 inhibitor for HS show promise
results from two small phase 2 studies showed.
“INCB054707 is an oral, small-molecule JAK1 inhibitor with approximately 52-fold greater selectivity for JAK1 versus JAK2,” researchers led by Afsaneh Alavi, MD, of the Mayo Clinic in Rochester, Minn., wrote in an article published recently in the British Journal of Dermatology. “Specifically targeting JAK1, a critical regulator of proinflammatory cytokine signaling implicated in several immune-related diseases, may reduce cytokine signaling involved in HS pathogenesis while limiting JAK2-mediated cytopenias.”
For the first study, 10 patients received 15 mg INCB054707 once daily for 8 weeks (NCT03569371). For the second study, 35 patients were randomized to 30, 60, or 90 mg INCB054707 once daily or placebo (3:1 within each cohort) for 8 weeks (NCT03607487). Eligibility criteria for both studies included patients with Hurley stage II/III HS who aged 18-75 years with lesions present in two or more anatomic locations, and a total abscess and inflammatory nodule count of three or more. The primary endpoint for both studies was safety and tolerability. Secondary endpoints included HS Clinical Response (HiSCR) and other efficacy measures.
The researchers reported that 30% of patients in study 1 and 42.3% of patients who received INCB054707 in study 2 experienced one or more treatment-emergent adverse event, most commonly upper respiratory tract infection. Among evaluable patients, 3 patients (42.9%) in study 1 and 17 patients in study 2 (65.4%) achieved HiSCR at week 8, compared with 57.1% of those in the placebo group. By dosing, 55.6% in the 30-mg group achieved HiSCR at week 8, compared with 55.6% in the 60-mg group and 87.5% in the 90-mg group.
“In conclusion, safety and efficacy findings from these two phase 2 studies establish proof of concept for the JAK1 inhibitor INCB054707 in the treatment of moderate to severe HS,” the authors wrote. “A phase 2, dose-ranging, placebo-controlled study exploring three dose levels and including approximately 200 patients is ongoing (NCT04476043) and expected to provide additional evidence of the safety and efficacy profile of INCB054707 in patients with HS.”
INCB054707 is being developed by Incyte Corporation. Dr. Alavi disclosed that she has received honoraria as a consultant or advisory board participant from AbbVie, Janssen, Novartis, Boehringer Ingelheim, InflaRX, and UCB, and received honoraria as an investigator for Boehringer Ingelheim and Processa.
results from two small phase 2 studies showed.
“INCB054707 is an oral, small-molecule JAK1 inhibitor with approximately 52-fold greater selectivity for JAK1 versus JAK2,” researchers led by Afsaneh Alavi, MD, of the Mayo Clinic in Rochester, Minn., wrote in an article published recently in the British Journal of Dermatology. “Specifically targeting JAK1, a critical regulator of proinflammatory cytokine signaling implicated in several immune-related diseases, may reduce cytokine signaling involved in HS pathogenesis while limiting JAK2-mediated cytopenias.”
For the first study, 10 patients received 15 mg INCB054707 once daily for 8 weeks (NCT03569371). For the second study, 35 patients were randomized to 30, 60, or 90 mg INCB054707 once daily or placebo (3:1 within each cohort) for 8 weeks (NCT03607487). Eligibility criteria for both studies included patients with Hurley stage II/III HS who aged 18-75 years with lesions present in two or more anatomic locations, and a total abscess and inflammatory nodule count of three or more. The primary endpoint for both studies was safety and tolerability. Secondary endpoints included HS Clinical Response (HiSCR) and other efficacy measures.
The researchers reported that 30% of patients in study 1 and 42.3% of patients who received INCB054707 in study 2 experienced one or more treatment-emergent adverse event, most commonly upper respiratory tract infection. Among evaluable patients, 3 patients (42.9%) in study 1 and 17 patients in study 2 (65.4%) achieved HiSCR at week 8, compared with 57.1% of those in the placebo group. By dosing, 55.6% in the 30-mg group achieved HiSCR at week 8, compared with 55.6% in the 60-mg group and 87.5% in the 90-mg group.
“In conclusion, safety and efficacy findings from these two phase 2 studies establish proof of concept for the JAK1 inhibitor INCB054707 in the treatment of moderate to severe HS,” the authors wrote. “A phase 2, dose-ranging, placebo-controlled study exploring three dose levels and including approximately 200 patients is ongoing (NCT04476043) and expected to provide additional evidence of the safety and efficacy profile of INCB054707 in patients with HS.”
INCB054707 is being developed by Incyte Corporation. Dr. Alavi disclosed that she has received honoraria as a consultant or advisory board participant from AbbVie, Janssen, Novartis, Boehringer Ingelheim, InflaRX, and UCB, and received honoraria as an investigator for Boehringer Ingelheim and Processa.
results from two small phase 2 studies showed.
“INCB054707 is an oral, small-molecule JAK1 inhibitor with approximately 52-fold greater selectivity for JAK1 versus JAK2,” researchers led by Afsaneh Alavi, MD, of the Mayo Clinic in Rochester, Minn., wrote in an article published recently in the British Journal of Dermatology. “Specifically targeting JAK1, a critical regulator of proinflammatory cytokine signaling implicated in several immune-related diseases, may reduce cytokine signaling involved in HS pathogenesis while limiting JAK2-mediated cytopenias.”
For the first study, 10 patients received 15 mg INCB054707 once daily for 8 weeks (NCT03569371). For the second study, 35 patients were randomized to 30, 60, or 90 mg INCB054707 once daily or placebo (3:1 within each cohort) for 8 weeks (NCT03607487). Eligibility criteria for both studies included patients with Hurley stage II/III HS who aged 18-75 years with lesions present in two or more anatomic locations, and a total abscess and inflammatory nodule count of three or more. The primary endpoint for both studies was safety and tolerability. Secondary endpoints included HS Clinical Response (HiSCR) and other efficacy measures.
The researchers reported that 30% of patients in study 1 and 42.3% of patients who received INCB054707 in study 2 experienced one or more treatment-emergent adverse event, most commonly upper respiratory tract infection. Among evaluable patients, 3 patients (42.9%) in study 1 and 17 patients in study 2 (65.4%) achieved HiSCR at week 8, compared with 57.1% of those in the placebo group. By dosing, 55.6% in the 30-mg group achieved HiSCR at week 8, compared with 55.6% in the 60-mg group and 87.5% in the 90-mg group.
“In conclusion, safety and efficacy findings from these two phase 2 studies establish proof of concept for the JAK1 inhibitor INCB054707 in the treatment of moderate to severe HS,” the authors wrote. “A phase 2, dose-ranging, placebo-controlled study exploring three dose levels and including approximately 200 patients is ongoing (NCT04476043) and expected to provide additional evidence of the safety and efficacy profile of INCB054707 in patients with HS.”
INCB054707 is being developed by Incyte Corporation. Dr. Alavi disclosed that she has received honoraria as a consultant or advisory board participant from AbbVie, Janssen, Novartis, Boehringer Ingelheim, InflaRX, and UCB, and received honoraria as an investigator for Boehringer Ingelheim and Processa.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
More than a month after launch, iPLEDGE glitches persist
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
Nodule on the left cheek
An 85-year-old man with a history of skin cancer presented to my dermatology practice (NT) for evaluation of a “pimple” on his left cheek that failed to resolve after 2 months (FIGURE). The patient noted that the lesion had grown, but that he otherwise felt well.
On examination, the lesion was plum colored, and the area was firm and nontender to palpation. The patient was referred to a plastic surgeon for an excisional biopsy to clarify the nature of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Merkel cell carcinoma
A biopsy performed 2 weeks after the initial visit confirmed the clinical suspicion for Merkel cell carcinoma (MCC).
MCC is a cutaneous neuroendocrine malignancy. Although its name acknowledges similarities between the tumor cells and Merkel cells, it is now considered unlikely that Merkel cells are the actual cells of origin.1
The majority of MCCs are asymptomatic despite rapid growth and are typically red or pink and occur on UV-exposed areas, as in our patient.2 A cyst or acneiform lesion is the single most common diagnosis given at the time of biopsy.2
The incidence of MCC is greatest in people of advanced age and in those who are immunosuppressed. In the United States, the estimated annual incidence rate rose from 0.5 cases per 100,000 people in 2000 to 0.7 cases per 100,000 people in 2013.3 MCC increases exponentially with advancing age, from 0.1 (per 100,000) in those ages 40 to 44 years to 9.8 in those older than 85 years.3 The growing cohort of ageing baby boomers and the increased number of immunosuppressed individuals in the community suggest that clinicians are now more likely to encounter MCC than in the past.
While UV radiation is highly associated with MCC, the major causative factor is considered to be Merkel cell polyomavirus (MCPyV).1 In fact, MCPyV has been linked to 80% of MCC cases.1,3 Most people have positive serology for MCPyV in early childhood, but the association between MCC and old age highlights the impact of immunosuppression on MCPyV activity and MCC development.1
Clinical suspicion is the first step in diagnosing MCC
The mnemonic AEIOU highlights the key clinical features of this aggressive tumor2,4:
- Asymptomatic
- Expanding rapidly (often grows in less than 3 months)
- Immune suppression (eg, chronic lymphocytic leukemia, solid organ transplant patient)
- Older than 50
- UV exposure on fair skin.
If a lesion is suspected to be MCC, the next step includes biopsy so that a definitive diagnosis can be made. A firm, nontender nodule that lacks fluctuance should raise suspicion for a neoplastic process.
Continue to: The differential is broad, ranging from cysts to melanoma
The differential is broad, ranging from cysts to melanoma
The differential diagnosis for an enlarging, plum-colored nodule on sun-exposed skin includes an abscess, a ruptured or inflamed epidermoid cyst, basal cell carcinoma, squamous cell carcinoma, and malignant melanoma.
An abscess is typically tender and expands within a matter of days rather than months.
A cyst can be ruled out by the clinical appearance and lack of an overlying pore.
Basal cell carcinoma can be characterized by a rolled border and central ulceration.
Squamous cell carcinomas often exhibit a verrucous surface with marked hyperkeratosis.
Continue to: Melanoma
Melanoma manifests with brown or irregular pigmentation and may be associated with a precursor lesion.
Tx includes excision and consistent follow-up
Complete excision is the critical first step to successful therapy. Sentinel lymph node studies are typically performed because of the high incidence of lymph node metastasis. Frequent follow-up is required because of the high risk of recurrent or persistent disease.
Local recurrence usually occurs within 1 year of diagnosis in more than 40% of patients.5 Distant metastasis can be treated with a programmed cell death ligand 1 blocking agent (avelumab) or a programmed cell death protein 1 inhibitor (nivolumab or pembrolizumab).6
Our patient was referred to a regional cancer center for sentinel lymph node evaluation, where he was found to have nodal disease. The patient was put on pembrolizumab and received radiation therapy but showed only limited response. Seven months after diagnosis, he passed away from metastatic MCC.
1. Pietropaolo V, Prezioso C, Moens U. Merkel cell polyomavirus and Merkel cell carcinoma. Cancers (Basel). 2020;12:1774. doi: 10.3390/cancers12071774
2. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381. doi: 10.1016/j.jaad.2007.11.020
3. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457-463.e2. doi: 10.1016/j.jaad.2017.10.028
4. Voelker R. Why Merkel cell cancer is garnering more attention. JAMA. 2018;320:18-20. doi: 10.1001/jama.2018.7042
5. Allen PJ, Browne WB, Jacques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309. doi: 10.1200/JCO.2005.02.329
6. D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077
An 85-year-old man with a history of skin cancer presented to my dermatology practice (NT) for evaluation of a “pimple” on his left cheek that failed to resolve after 2 months (FIGURE). The patient noted that the lesion had grown, but that he otherwise felt well.
On examination, the lesion was plum colored, and the area was firm and nontender to palpation. The patient was referred to a plastic surgeon for an excisional biopsy to clarify the nature of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Merkel cell carcinoma
A biopsy performed 2 weeks after the initial visit confirmed the clinical suspicion for Merkel cell carcinoma (MCC).
MCC is a cutaneous neuroendocrine malignancy. Although its name acknowledges similarities between the tumor cells and Merkel cells, it is now considered unlikely that Merkel cells are the actual cells of origin.1
The majority of MCCs are asymptomatic despite rapid growth and are typically red or pink and occur on UV-exposed areas, as in our patient.2 A cyst or acneiform lesion is the single most common diagnosis given at the time of biopsy.2
The incidence of MCC is greatest in people of advanced age and in those who are immunosuppressed. In the United States, the estimated annual incidence rate rose from 0.5 cases per 100,000 people in 2000 to 0.7 cases per 100,000 people in 2013.3 MCC increases exponentially with advancing age, from 0.1 (per 100,000) in those ages 40 to 44 years to 9.8 in those older than 85 years.3 The growing cohort of ageing baby boomers and the increased number of immunosuppressed individuals in the community suggest that clinicians are now more likely to encounter MCC than in the past.
While UV radiation is highly associated with MCC, the major causative factor is considered to be Merkel cell polyomavirus (MCPyV).1 In fact, MCPyV has been linked to 80% of MCC cases.1,3 Most people have positive serology for MCPyV in early childhood, but the association between MCC and old age highlights the impact of immunosuppression on MCPyV activity and MCC development.1
Clinical suspicion is the first step in diagnosing MCC
The mnemonic AEIOU highlights the key clinical features of this aggressive tumor2,4:
- Asymptomatic
- Expanding rapidly (often grows in less than 3 months)
- Immune suppression (eg, chronic lymphocytic leukemia, solid organ transplant patient)
- Older than 50
- UV exposure on fair skin.
If a lesion is suspected to be MCC, the next step includes biopsy so that a definitive diagnosis can be made. A firm, nontender nodule that lacks fluctuance should raise suspicion for a neoplastic process.
Continue to: The differential is broad, ranging from cysts to melanoma
The differential is broad, ranging from cysts to melanoma
The differential diagnosis for an enlarging, plum-colored nodule on sun-exposed skin includes an abscess, a ruptured or inflamed epidermoid cyst, basal cell carcinoma, squamous cell carcinoma, and malignant melanoma.
An abscess is typically tender and expands within a matter of days rather than months.
A cyst can be ruled out by the clinical appearance and lack of an overlying pore.
Basal cell carcinoma can be characterized by a rolled border and central ulceration.
Squamous cell carcinomas often exhibit a verrucous surface with marked hyperkeratosis.
Continue to: Melanoma
Melanoma manifests with brown or irregular pigmentation and may be associated with a precursor lesion.
Tx includes excision and consistent follow-up
Complete excision is the critical first step to successful therapy. Sentinel lymph node studies are typically performed because of the high incidence of lymph node metastasis. Frequent follow-up is required because of the high risk of recurrent or persistent disease.
Local recurrence usually occurs within 1 year of diagnosis in more than 40% of patients.5 Distant metastasis can be treated with a programmed cell death ligand 1 blocking agent (avelumab) or a programmed cell death protein 1 inhibitor (nivolumab or pembrolizumab).6
Our patient was referred to a regional cancer center for sentinel lymph node evaluation, where he was found to have nodal disease. The patient was put on pembrolizumab and received radiation therapy but showed only limited response. Seven months after diagnosis, he passed away from metastatic MCC.
An 85-year-old man with a history of skin cancer presented to my dermatology practice (NT) for evaluation of a “pimple” on his left cheek that failed to resolve after 2 months (FIGURE). The patient noted that the lesion had grown, but that he otherwise felt well.
On examination, the lesion was plum colored, and the area was firm and nontender to palpation. The patient was referred to a plastic surgeon for an excisional biopsy to clarify the nature of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Merkel cell carcinoma
A biopsy performed 2 weeks after the initial visit confirmed the clinical suspicion for Merkel cell carcinoma (MCC).
MCC is a cutaneous neuroendocrine malignancy. Although its name acknowledges similarities between the tumor cells and Merkel cells, it is now considered unlikely that Merkel cells are the actual cells of origin.1
The majority of MCCs are asymptomatic despite rapid growth and are typically red or pink and occur on UV-exposed areas, as in our patient.2 A cyst or acneiform lesion is the single most common diagnosis given at the time of biopsy.2
The incidence of MCC is greatest in people of advanced age and in those who are immunosuppressed. In the United States, the estimated annual incidence rate rose from 0.5 cases per 100,000 people in 2000 to 0.7 cases per 100,000 people in 2013.3 MCC increases exponentially with advancing age, from 0.1 (per 100,000) in those ages 40 to 44 years to 9.8 in those older than 85 years.3 The growing cohort of ageing baby boomers and the increased number of immunosuppressed individuals in the community suggest that clinicians are now more likely to encounter MCC than in the past.
While UV radiation is highly associated with MCC, the major causative factor is considered to be Merkel cell polyomavirus (MCPyV).1 In fact, MCPyV has been linked to 80% of MCC cases.1,3 Most people have positive serology for MCPyV in early childhood, but the association between MCC and old age highlights the impact of immunosuppression on MCPyV activity and MCC development.1
Clinical suspicion is the first step in diagnosing MCC
The mnemonic AEIOU highlights the key clinical features of this aggressive tumor2,4:
- Asymptomatic
- Expanding rapidly (often grows in less than 3 months)
- Immune suppression (eg, chronic lymphocytic leukemia, solid organ transplant patient)
- Older than 50
- UV exposure on fair skin.
If a lesion is suspected to be MCC, the next step includes biopsy so that a definitive diagnosis can be made. A firm, nontender nodule that lacks fluctuance should raise suspicion for a neoplastic process.
Continue to: The differential is broad, ranging from cysts to melanoma
The differential is broad, ranging from cysts to melanoma
The differential diagnosis for an enlarging, plum-colored nodule on sun-exposed skin includes an abscess, a ruptured or inflamed epidermoid cyst, basal cell carcinoma, squamous cell carcinoma, and malignant melanoma.
An abscess is typically tender and expands within a matter of days rather than months.
A cyst can be ruled out by the clinical appearance and lack of an overlying pore.
Basal cell carcinoma can be characterized by a rolled border and central ulceration.
Squamous cell carcinomas often exhibit a verrucous surface with marked hyperkeratosis.
Continue to: Melanoma
Melanoma manifests with brown or irregular pigmentation and may be associated with a precursor lesion.
Tx includes excision and consistent follow-up
Complete excision is the critical first step to successful therapy. Sentinel lymph node studies are typically performed because of the high incidence of lymph node metastasis. Frequent follow-up is required because of the high risk of recurrent or persistent disease.
Local recurrence usually occurs within 1 year of diagnosis in more than 40% of patients.5 Distant metastasis can be treated with a programmed cell death ligand 1 blocking agent (avelumab) or a programmed cell death protein 1 inhibitor (nivolumab or pembrolizumab).6
Our patient was referred to a regional cancer center for sentinel lymph node evaluation, where he was found to have nodal disease. The patient was put on pembrolizumab and received radiation therapy but showed only limited response. Seven months after diagnosis, he passed away from metastatic MCC.
1. Pietropaolo V, Prezioso C, Moens U. Merkel cell polyomavirus and Merkel cell carcinoma. Cancers (Basel). 2020;12:1774. doi: 10.3390/cancers12071774
2. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381. doi: 10.1016/j.jaad.2007.11.020
3. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457-463.e2. doi: 10.1016/j.jaad.2017.10.028
4. Voelker R. Why Merkel cell cancer is garnering more attention. JAMA. 2018;320:18-20. doi: 10.1001/jama.2018.7042
5. Allen PJ, Browne WB, Jacques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309. doi: 10.1200/JCO.2005.02.329
6. D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077
1. Pietropaolo V, Prezioso C, Moens U. Merkel cell polyomavirus and Merkel cell carcinoma. Cancers (Basel). 2020;12:1774. doi: 10.3390/cancers12071774
2. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381. doi: 10.1016/j.jaad.2007.11.020
3. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457-463.e2. doi: 10.1016/j.jaad.2017.10.028
4. Voelker R. Why Merkel cell cancer is garnering more attention. JAMA. 2018;320:18-20. doi: 10.1001/jama.2018.7042
5. Allen PJ, Browne WB, Jacques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309. doi: 10.1200/JCO.2005.02.329
6. D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077
Pioneering test predicts return of malignant melanoma
Their research, published in the British Journal of Dermatology, describes how early-stage melanomas at risk of spreading secrete transforming growth factor beta2 (TGF-beta2), which causes the reduction, or down-regulation, of the proteins AMBRA1 and loricrin, both of which are found in the skin overlaying the tumor. TGF-beta2 also causes the loss of claudin-1, which in turn leads to loss of skin integrity, facilitating ulceration.
Senior author Penny Lovat, PhD, professor of cellular dermatology and oncology at Newcastle University, and chief scientific officer at AMLo Biosciences, explained: “AMBRA1, loricrin, and claudin-1 are all proteins key to maintaining the integrity of the upper layer of the skin,” and that the loss of these proteins causes gaps to develop, allowing the tumor to spread and ulcerate – a process associated with high-risk tumors. Dr. Lovat likened the process to that of “mortar and bricks holding together a wall”, with the loss of these proteins being “like the mortar crumbling away in the wall.”
According to Cancer Research UK, there are over 16,000 new cases of melanoma skin cancer each year in the United Kingdom, with over 2,000 deaths annually. After being surgically removed, primary tumors are histologically staged, with even low-risk cases being followed up for a number of years, a process that can be time-consuming for patients and costly for the NHS.
Some reassurance for those with melanoma
The creators of the new test say that it is these low-risk patients that the test is able to identify, offering a degree of reassurance to those diagnosed with the disease, and potentially reducing the number of hospital clinic visits they require.
Dr. Lovat commented: “Our test offers a personalized prognosis as it more accurately predicts if your skin cancer is unlikely to spread.”
She added that the test will aid clinicians to identify genuinely low-risk patients diagnosed with an early-stage melanoma, reducing the number of follow-up appointments for those identified as low risk. It, therefore, offers the opportunity to save the NHS time and money.
Excellent news for those with skin cancer
Phil Brady, chief operating officer of the British Skin Foundation, echoed Dr. Lovat’s comments, saying: “The test can alleviate stress and anxiety for patients caused by this potentially deadly skin cancer, whilst increasing efficiency and reducing costs to the NHS.”
Nick Levell, MD, consultant dermatologist & British Skin Foundation spokesperson, who has not been involved in the research, commented how the arrival of the test was “excellent news,” adding that “people at low risk can be reassured and will not have to attend hospital so often for check-ups”.
The development of the new test AMBLor has been led by Dr. Lovat, in association with the university spin-out company AMLo Biosciences, and is accredited by the National Accreditation Body for the United Kingdom. The test involves tissue sections from the standard biopsy being sent in the post to the lab for analysis and costs £293 plus VAT. Currently available through a private referral service, the Newcastle team have applied for the test to be made available on the NHS.
A version of this article first appeared on Medscape UK.
Their research, published in the British Journal of Dermatology, describes how early-stage melanomas at risk of spreading secrete transforming growth factor beta2 (TGF-beta2), which causes the reduction, or down-regulation, of the proteins AMBRA1 and loricrin, both of which are found in the skin overlaying the tumor. TGF-beta2 also causes the loss of claudin-1, which in turn leads to loss of skin integrity, facilitating ulceration.
Senior author Penny Lovat, PhD, professor of cellular dermatology and oncology at Newcastle University, and chief scientific officer at AMLo Biosciences, explained: “AMBRA1, loricrin, and claudin-1 are all proteins key to maintaining the integrity of the upper layer of the skin,” and that the loss of these proteins causes gaps to develop, allowing the tumor to spread and ulcerate – a process associated with high-risk tumors. Dr. Lovat likened the process to that of “mortar and bricks holding together a wall”, with the loss of these proteins being “like the mortar crumbling away in the wall.”
According to Cancer Research UK, there are over 16,000 new cases of melanoma skin cancer each year in the United Kingdom, with over 2,000 deaths annually. After being surgically removed, primary tumors are histologically staged, with even low-risk cases being followed up for a number of years, a process that can be time-consuming for patients and costly for the NHS.
Some reassurance for those with melanoma
The creators of the new test say that it is these low-risk patients that the test is able to identify, offering a degree of reassurance to those diagnosed with the disease, and potentially reducing the number of hospital clinic visits they require.
Dr. Lovat commented: “Our test offers a personalized prognosis as it more accurately predicts if your skin cancer is unlikely to spread.”
She added that the test will aid clinicians to identify genuinely low-risk patients diagnosed with an early-stage melanoma, reducing the number of follow-up appointments for those identified as low risk. It, therefore, offers the opportunity to save the NHS time and money.
Excellent news for those with skin cancer
Phil Brady, chief operating officer of the British Skin Foundation, echoed Dr. Lovat’s comments, saying: “The test can alleviate stress and anxiety for patients caused by this potentially deadly skin cancer, whilst increasing efficiency and reducing costs to the NHS.”
Nick Levell, MD, consultant dermatologist & British Skin Foundation spokesperson, who has not been involved in the research, commented how the arrival of the test was “excellent news,” adding that “people at low risk can be reassured and will not have to attend hospital so often for check-ups”.
The development of the new test AMBLor has been led by Dr. Lovat, in association with the university spin-out company AMLo Biosciences, and is accredited by the National Accreditation Body for the United Kingdom. The test involves tissue sections from the standard biopsy being sent in the post to the lab for analysis and costs £293 plus VAT. Currently available through a private referral service, the Newcastle team have applied for the test to be made available on the NHS.
A version of this article first appeared on Medscape UK.
Their research, published in the British Journal of Dermatology, describes how early-stage melanomas at risk of spreading secrete transforming growth factor beta2 (TGF-beta2), which causes the reduction, or down-regulation, of the proteins AMBRA1 and loricrin, both of which are found in the skin overlaying the tumor. TGF-beta2 also causes the loss of claudin-1, which in turn leads to loss of skin integrity, facilitating ulceration.
Senior author Penny Lovat, PhD, professor of cellular dermatology and oncology at Newcastle University, and chief scientific officer at AMLo Biosciences, explained: “AMBRA1, loricrin, and claudin-1 are all proteins key to maintaining the integrity of the upper layer of the skin,” and that the loss of these proteins causes gaps to develop, allowing the tumor to spread and ulcerate – a process associated with high-risk tumors. Dr. Lovat likened the process to that of “mortar and bricks holding together a wall”, with the loss of these proteins being “like the mortar crumbling away in the wall.”
According to Cancer Research UK, there are over 16,000 new cases of melanoma skin cancer each year in the United Kingdom, with over 2,000 deaths annually. After being surgically removed, primary tumors are histologically staged, with even low-risk cases being followed up for a number of years, a process that can be time-consuming for patients and costly for the NHS.
Some reassurance for those with melanoma
The creators of the new test say that it is these low-risk patients that the test is able to identify, offering a degree of reassurance to those diagnosed with the disease, and potentially reducing the number of hospital clinic visits they require.
Dr. Lovat commented: “Our test offers a personalized prognosis as it more accurately predicts if your skin cancer is unlikely to spread.”
She added that the test will aid clinicians to identify genuinely low-risk patients diagnosed with an early-stage melanoma, reducing the number of follow-up appointments for those identified as low risk. It, therefore, offers the opportunity to save the NHS time and money.
Excellent news for those with skin cancer
Phil Brady, chief operating officer of the British Skin Foundation, echoed Dr. Lovat’s comments, saying: “The test can alleviate stress and anxiety for patients caused by this potentially deadly skin cancer, whilst increasing efficiency and reducing costs to the NHS.”
Nick Levell, MD, consultant dermatologist & British Skin Foundation spokesperson, who has not been involved in the research, commented how the arrival of the test was “excellent news,” adding that “people at low risk can be reassured and will not have to attend hospital so often for check-ups”.
The development of the new test AMBLor has been led by Dr. Lovat, in association with the university spin-out company AMLo Biosciences, and is accredited by the National Accreditation Body for the United Kingdom. The test involves tissue sections from the standard biopsy being sent in the post to the lab for analysis and costs £293 plus VAT. Currently available through a private referral service, the Newcastle team have applied for the test to be made available on the NHS.
A version of this article first appeared on Medscape UK.
FROM THE BRITISH JOURNAL OF DERMATOLOGY