User login
Review finds anti-staphylococcus treatments have little impact on eczema
published in Clinical and Experimental Allergy.
Eczema remains a huge disease burden worldwide, and colonization with S. aureus in eczema patients is common, but no standard intervention exists to relieve symptoms, wrote Nandini Banerjee, MD, of Addenbrooke’s Hospital, Cambridge, England. “While antibiotic treatment of clinically obvious infections such as cellulitis is beneficial, it is not clear whether antibiotic treatment of eczema influences eczema severity,” Dr. Banerjee noted.
The 41 studies included 1,753 participants and 10 treatment categories. Most of the studies were conducted in secondary care centers in Western Europe, North America, and the Far East. Twelve studies included children, four included only adults, 19 included children and adults, and in six studies, the participant age range was unclear. Among the studies with reported ages, the mean age ranged from 1.1 to 34.6 years. Eczema severity ranged from mild to severe, and treatment durations ranged from 10 minutes to 3 months.
The review presented comparisons of topical steroid/antibiotic combinations, oral antibiotics, and bleach baths. In 14 studies that compared topical steroid/antibiotic combinations to topical steroids alone, patients showed slightly greater global improvement in symptoms with the combination, but the impact on quality of life was not significantly different. Severe adverse events, including flare of dermatitis, worsening of eczema, and folliculitis, were reported by the patients who received the combination and the topical steroid–only patients. One study reported similar rates of antibiotic resistance in children treated with steroid only and with an antibiotic/steroid combination at 3 months’ follow-up.
In four studies, oral antibiotics “may make no difference in terms of good or excellent global improvement in infants and children at 14 to 28 days follow-up compared to placebo,” according to the review. The reviewers said that there was likely little or no difference in quality of life for infants and children given oral antibiotics, although they noted the low quality of evidence on this topic.
Five studies evaluated the impact of bleach baths on eczema patients with and without S. aureus infections. These studies showed no difference in global improvement measures compared with placebo and little or no difference in quality of life. Also, patients who underwent bleach baths compared with placebo patients reported similar adverse events of burning/stinging or dry skin at 2 months’ follow-up.
“Low-quality evidence, due to risk of bias, imprecise effect estimates, and heterogeneity, made pooling of results difficult,” Dr. Banerjee wrote. “Topical steroid/antibiotic combinations may be associated with possible small improvements in good or excellent signs/symptoms compared with topical steroid alone. High-quality trials evaluating efficacy, QOL, and antibiotic resistance are required,” she concluded.
In a commentary section after the review, Dr. Banerjee and colleagues noted that the United Kingdom’s NICE guidelines for managing atopic eczema in children younger than 12 years of age, published in March 2021, include evidence from the current updated Cochrane Review. The NICE guidelines emphasize that “in people who are not systemically unwell, clinicians should not routinely offer either a topical or oral antibiotic for secondary bacterial infection of eczema,” the Cochrane authors said. They added in their commentary that the use of antibiotics in cases of nonsevere infections can worsen eczema. Also, “the risk of antimicrobial resistance is high with topical antibiotics, and therefore extended doses of the same antibiotics should be avoided to prevent resistance,” they said. However, the authors acknowledged a role for antibiotics in certain situations. “In patients with systemic signs of infection such as cellulitis, systemic antibiotics have an important role in helping clear infection,” they noted.
Reasons for varying disease severity elude research
The current study is important because of the abundance of preclinical and clinical data that implicate S. aureus in atopic dermatitis pathogenesis, Brian Kim, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Dr. Kim said that he was surprised by some of the study findings but not others. “On the one hand, I thought there would be data supporting antimicrobial therapy, albeit not strong support,” he said. “However, AD is a very complex disease, and understanding what a disease modifier does to it is hard to capture across studies of various different designs,” he said.
“The data supporting antimicrobial therapy for S. aureus in AD is not as clear as our clinical impressions may indicate,” said Dr. Kim. “We need to understand the relationship better, perhaps in particular subsets of patients,” he emphasized. In addition, “We need a better understanding of why some people are colonized with S. aureus, yet with little effect on AD itself, while others experience severe exacerbation of disease,” said Dr. Kim. Therefore, a key research question for future studies is whether the exacerbation is caused by the particular strain of the bug, the host susceptibility, or both, he said.
The review received no outside funding. Dr. Banerjee and Dr. Kim have disclosed that they had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published in Clinical and Experimental Allergy.
Eczema remains a huge disease burden worldwide, and colonization with S. aureus in eczema patients is common, but no standard intervention exists to relieve symptoms, wrote Nandini Banerjee, MD, of Addenbrooke’s Hospital, Cambridge, England. “While antibiotic treatment of clinically obvious infections such as cellulitis is beneficial, it is not clear whether antibiotic treatment of eczema influences eczema severity,” Dr. Banerjee noted.
The 41 studies included 1,753 participants and 10 treatment categories. Most of the studies were conducted in secondary care centers in Western Europe, North America, and the Far East. Twelve studies included children, four included only adults, 19 included children and adults, and in six studies, the participant age range was unclear. Among the studies with reported ages, the mean age ranged from 1.1 to 34.6 years. Eczema severity ranged from mild to severe, and treatment durations ranged from 10 minutes to 3 months.
The review presented comparisons of topical steroid/antibiotic combinations, oral antibiotics, and bleach baths. In 14 studies that compared topical steroid/antibiotic combinations to topical steroids alone, patients showed slightly greater global improvement in symptoms with the combination, but the impact on quality of life was not significantly different. Severe adverse events, including flare of dermatitis, worsening of eczema, and folliculitis, were reported by the patients who received the combination and the topical steroid–only patients. One study reported similar rates of antibiotic resistance in children treated with steroid only and with an antibiotic/steroid combination at 3 months’ follow-up.
In four studies, oral antibiotics “may make no difference in terms of good or excellent global improvement in infants and children at 14 to 28 days follow-up compared to placebo,” according to the review. The reviewers said that there was likely little or no difference in quality of life for infants and children given oral antibiotics, although they noted the low quality of evidence on this topic.
Five studies evaluated the impact of bleach baths on eczema patients with and without S. aureus infections. These studies showed no difference in global improvement measures compared with placebo and little or no difference in quality of life. Also, patients who underwent bleach baths compared with placebo patients reported similar adverse events of burning/stinging or dry skin at 2 months’ follow-up.
“Low-quality evidence, due to risk of bias, imprecise effect estimates, and heterogeneity, made pooling of results difficult,” Dr. Banerjee wrote. “Topical steroid/antibiotic combinations may be associated with possible small improvements in good or excellent signs/symptoms compared with topical steroid alone. High-quality trials evaluating efficacy, QOL, and antibiotic resistance are required,” she concluded.
In a commentary section after the review, Dr. Banerjee and colleagues noted that the United Kingdom’s NICE guidelines for managing atopic eczema in children younger than 12 years of age, published in March 2021, include evidence from the current updated Cochrane Review. The NICE guidelines emphasize that “in people who are not systemically unwell, clinicians should not routinely offer either a topical or oral antibiotic for secondary bacterial infection of eczema,” the Cochrane authors said. They added in their commentary that the use of antibiotics in cases of nonsevere infections can worsen eczema. Also, “the risk of antimicrobial resistance is high with topical antibiotics, and therefore extended doses of the same antibiotics should be avoided to prevent resistance,” they said. However, the authors acknowledged a role for antibiotics in certain situations. “In patients with systemic signs of infection such as cellulitis, systemic antibiotics have an important role in helping clear infection,” they noted.
Reasons for varying disease severity elude research
The current study is important because of the abundance of preclinical and clinical data that implicate S. aureus in atopic dermatitis pathogenesis, Brian Kim, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Dr. Kim said that he was surprised by some of the study findings but not others. “On the one hand, I thought there would be data supporting antimicrobial therapy, albeit not strong support,” he said. “However, AD is a very complex disease, and understanding what a disease modifier does to it is hard to capture across studies of various different designs,” he said.
“The data supporting antimicrobial therapy for S. aureus in AD is not as clear as our clinical impressions may indicate,” said Dr. Kim. “We need to understand the relationship better, perhaps in particular subsets of patients,” he emphasized. In addition, “We need a better understanding of why some people are colonized with S. aureus, yet with little effect on AD itself, while others experience severe exacerbation of disease,” said Dr. Kim. Therefore, a key research question for future studies is whether the exacerbation is caused by the particular strain of the bug, the host susceptibility, or both, he said.
The review received no outside funding. Dr. Banerjee and Dr. Kim have disclosed that they had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published in Clinical and Experimental Allergy.
Eczema remains a huge disease burden worldwide, and colonization with S. aureus in eczema patients is common, but no standard intervention exists to relieve symptoms, wrote Nandini Banerjee, MD, of Addenbrooke’s Hospital, Cambridge, England. “While antibiotic treatment of clinically obvious infections such as cellulitis is beneficial, it is not clear whether antibiotic treatment of eczema influences eczema severity,” Dr. Banerjee noted.
The 41 studies included 1,753 participants and 10 treatment categories. Most of the studies were conducted in secondary care centers in Western Europe, North America, and the Far East. Twelve studies included children, four included only adults, 19 included children and adults, and in six studies, the participant age range was unclear. Among the studies with reported ages, the mean age ranged from 1.1 to 34.6 years. Eczema severity ranged from mild to severe, and treatment durations ranged from 10 minutes to 3 months.
The review presented comparisons of topical steroid/antibiotic combinations, oral antibiotics, and bleach baths. In 14 studies that compared topical steroid/antibiotic combinations to topical steroids alone, patients showed slightly greater global improvement in symptoms with the combination, but the impact on quality of life was not significantly different. Severe adverse events, including flare of dermatitis, worsening of eczema, and folliculitis, were reported by the patients who received the combination and the topical steroid–only patients. One study reported similar rates of antibiotic resistance in children treated with steroid only and with an antibiotic/steroid combination at 3 months’ follow-up.
In four studies, oral antibiotics “may make no difference in terms of good or excellent global improvement in infants and children at 14 to 28 days follow-up compared to placebo,” according to the review. The reviewers said that there was likely little or no difference in quality of life for infants and children given oral antibiotics, although they noted the low quality of evidence on this topic.
Five studies evaluated the impact of bleach baths on eczema patients with and without S. aureus infections. These studies showed no difference in global improvement measures compared with placebo and little or no difference in quality of life. Also, patients who underwent bleach baths compared with placebo patients reported similar adverse events of burning/stinging or dry skin at 2 months’ follow-up.
“Low-quality evidence, due to risk of bias, imprecise effect estimates, and heterogeneity, made pooling of results difficult,” Dr. Banerjee wrote. “Topical steroid/antibiotic combinations may be associated with possible small improvements in good or excellent signs/symptoms compared with topical steroid alone. High-quality trials evaluating efficacy, QOL, and antibiotic resistance are required,” she concluded.
In a commentary section after the review, Dr. Banerjee and colleagues noted that the United Kingdom’s NICE guidelines for managing atopic eczema in children younger than 12 years of age, published in March 2021, include evidence from the current updated Cochrane Review. The NICE guidelines emphasize that “in people who are not systemically unwell, clinicians should not routinely offer either a topical or oral antibiotic for secondary bacterial infection of eczema,” the Cochrane authors said. They added in their commentary that the use of antibiotics in cases of nonsevere infections can worsen eczema. Also, “the risk of antimicrobial resistance is high with topical antibiotics, and therefore extended doses of the same antibiotics should be avoided to prevent resistance,” they said. However, the authors acknowledged a role for antibiotics in certain situations. “In patients with systemic signs of infection such as cellulitis, systemic antibiotics have an important role in helping clear infection,” they noted.
Reasons for varying disease severity elude research
The current study is important because of the abundance of preclinical and clinical data that implicate S. aureus in atopic dermatitis pathogenesis, Brian Kim, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Dr. Kim said that he was surprised by some of the study findings but not others. “On the one hand, I thought there would be data supporting antimicrobial therapy, albeit not strong support,” he said. “However, AD is a very complex disease, and understanding what a disease modifier does to it is hard to capture across studies of various different designs,” he said.
“The data supporting antimicrobial therapy for S. aureus in AD is not as clear as our clinical impressions may indicate,” said Dr. Kim. “We need to understand the relationship better, perhaps in particular subsets of patients,” he emphasized. In addition, “We need a better understanding of why some people are colonized with S. aureus, yet with little effect on AD itself, while others experience severe exacerbation of disease,” said Dr. Kim. Therefore, a key research question for future studies is whether the exacerbation is caused by the particular strain of the bug, the host susceptibility, or both, he said.
The review received no outside funding. Dr. Banerjee and Dr. Kim have disclosed that they had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL AND EXPERIMENTAL ALLERGY
Male alopecia agents ranked by efficacy in meta-analysis
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While up to 90% of men experience AGA in their lifetime, only three therapies are currently approved for treatment of the condition by the Food and Drug Administration – topical minoxidil, oral finasteride 1 mg, and low-level light therapy.
However, with common use of off-label oral minoxidil, as well as oral dutasteride and higher doses of oral finasteride, the latter two being 5-alpha reductase inhibitors, Aditya K. Gupta, MD, PhD, of Mediprobe Research, in London, Ont., and colleagues sought to compare the data on the three agents. Their results were published in JAMA Dermatology.
They note that, while there have been recent comparisons between oral and topical minoxidil, “to our knowledge no study has determined the comparative effectiveness of these 2 [formulations] with that of local and systemic dutasteride and finasteride.”
For the meta-analysis, the authors identified 23 studies meeting their criteria, involving patients with mean ages ranging from 22.8 to 41.8 years.
For the primary endpoint of the greatest increases in total hair count at 24 weeks, the analysis showed the 0.5-mg/day dose of dutasteride topped the list, with significantly greater efficacy, compared with 1 mg/day of finasteride (mean difference, 7.1 hairs per cm2).
The 0.5-mg/d dutasteride dose also showed higher efficacy than oral minoxidil at 0.25 mg/day (mean difference, 23.7 hairs per cm2) and 5 mg/day (mean difference, 15.0 hairs per cm2) and topical minoxidil at 2% (mean difference, 8.5 hairs per cm2).
For the secondary endpoint of the greatest increase in terminal hair count at 24 weeks, the 5-mg/day dose of minoxidil had significantly greater efficacy compared with the 0.25-mg/day dose of the drug, as well as with minoxidil’s 2% and 5% topical formulations.
The minoxidil 5-mg/day dose was also significantly more effective than 1 mg/day of finasteride for terminal hair count at 24 weeks.
In longer-term outcomes at 48 weeks, the greatest increase in total hair count at 48 weeks was observed with 5 mg/day of finasteride, which was significantly more effective, compared with 2% topical minoxidil.
And the greatest increase in terminal hair count at 48 weeks was observed with 1 mg/day of oral finasteride, which was significantly more effective than 2% as well as 5% topical minoxidil.
Based on the results, the authors ranked the agents in a decreasing order of efficacy: 0.5 mg/day of oral dutasteride, 5 mg/day of oral finasteride, 5 mg/day of oral minoxidil, 1 mg/day of oral finasteride, 5% topical minoxidil, 2% topical minoxidil, and 0.25 mg/day of oral minoxidil.
Commenting on the analysis in an accompanying editorial, Kathie P. Huang, MD, of the department of dermatology, Brigham and Women’s Hospital, Boston, and Maryanne M. Senna, MD, of the department of dermatology, Massachusetts General Hospital, Boston, said the results, in general, are consistent with their experiences, noting that 2% minoxidil is typically not used in men.
They noted that, “although topical minoxidil ranked higher than the very-low-dose 0.25 mg oral minoxidil, our personal experience is that oral minoxidil at doses of 1.25 mg to 5 mg are far superior to topical minoxidil for treating AGA.”
Adverse event considerations important
Importantly, however, strong consideration needs to be given to adverse-event profiles, as well as patient comorbidities in selecting agents, the editorial authors asserted.
With 1 mg finasteride, for instance, potential adverse events include decreased libido, erectile dysfunction, decreased ejaculatory volume, reduction in sperm count, testicular pain, depression, and gynecomastia, they noted.
And while finasteride appears to be associated with a decreased risk of prostate cancer, those receiving the drug who do develop prostate cancer may be diagnosed with higher-grade prostate cancer; however, that “might be related to tissue sampling artifact,” the editorial authors said.
Less has been published on dutasteride’s adverse-event profile, and that, in itself, is a concern.
Overall, “as more direct-to-consumer companies treating male AGA emerge, it is especially important that the potential risks of these medications be made clear to patients,” they added.
Further commenting on the analysis to this news organization, Antonella Tosti, MD, the Fredric Brandt Endowed Professor of Dermatology and Cutaneous Surgery at the University of Miami, said the study offers some important insights – and caveats.
“I think this is a very interesting study, but you have to consider what works for your patients,” she said.
Dr. Tosti noted that the 5-mg dose of minoxidil is a concern in terms of side effects. “That dose is pretty high and could feasibly cause some hypertrichosis, which can be a concern to men as well as women.”
She agrees that the lack of data on side effects with dutasteride is also a concern, especially in light of some of the known side effects with other agents.
“That’s why I don’t use it very much in younger patients – because I’m afraid it could potentially affect their fertility,” Dr. Tosti said.
In general, Dr. Tosti said she finds a combination of agents provides the best results, as many clinicians use.
“I find dutasteride (0.5 mg/day) plus oral minoxidil (1-2.5 mg/day) plus topical 5% minoxidil is the best combination,” she said.
The authors and Dr. Tosti disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Dupilumab under FDA review for atopic dermatitis in children aged 6 months to 5 years
The and Sanofi.
If approved, dupilumab would be the first biologic approved for children in this age group in the United States, according to the statement. The proposed indication is as add-on therapy for children with moderate to severe AD not adequately controlled with topical prescription therapies or for whom topical therapies are not advised. The FDA granted breakthrough therapy designation for dupilumab for the treatment of severe AD in children aged 6 months to 11 years in 2016.
Approximately 85%-95% of atopic dermatitis patients develop symptoms before 5 years of age, and these symptoms often continue into adulthood, with an increased risk of skin infections and a significant impact on quality of life, according to the statement.
The sBLA is based on data from a phase 3 pivotal study of 162 children aged 6 months to 5 years in which dupilumab was added to standard-of-care topical corticosteroids, presented in December 2021. In the study, dupilumab plus standard of care significantly improved skin clearance and reduced overall disease severity and itch at 16 weeks compared with standard of care alone. Overall, 28% of the children randomized to dupilumab achieved the primary endpoint of clear or almost-clear skin, compared with 4% with those on standard of care alone (P < .0001), according to the manufacturers. Patients in the dupilumab group received either 200 mg (for children weighing ≥ 5 to < 15 kg) or 300 mg (for children weighing ≥ 15 to < 30 kg) every 4 weeks. Safety results were similar to those seen with dupilumab for children aged 6 years and older.
Conjunctivitis and herpes infections were among the most common adverse events associated with dupilumab in the study, according to the statement.
The target action date for the FDA decision on this application is June 9, 2022.
The and Sanofi.
If approved, dupilumab would be the first biologic approved for children in this age group in the United States, according to the statement. The proposed indication is as add-on therapy for children with moderate to severe AD not adequately controlled with topical prescription therapies or for whom topical therapies are not advised. The FDA granted breakthrough therapy designation for dupilumab for the treatment of severe AD in children aged 6 months to 11 years in 2016.
Approximately 85%-95% of atopic dermatitis patients develop symptoms before 5 years of age, and these symptoms often continue into adulthood, with an increased risk of skin infections and a significant impact on quality of life, according to the statement.
The sBLA is based on data from a phase 3 pivotal study of 162 children aged 6 months to 5 years in which dupilumab was added to standard-of-care topical corticosteroids, presented in December 2021. In the study, dupilumab plus standard of care significantly improved skin clearance and reduced overall disease severity and itch at 16 weeks compared with standard of care alone. Overall, 28% of the children randomized to dupilumab achieved the primary endpoint of clear or almost-clear skin, compared with 4% with those on standard of care alone (P < .0001), according to the manufacturers. Patients in the dupilumab group received either 200 mg (for children weighing ≥ 5 to < 15 kg) or 300 mg (for children weighing ≥ 15 to < 30 kg) every 4 weeks. Safety results were similar to those seen with dupilumab for children aged 6 years and older.
Conjunctivitis and herpes infections were among the most common adverse events associated with dupilumab in the study, according to the statement.
The target action date for the FDA decision on this application is June 9, 2022.
The and Sanofi.
If approved, dupilumab would be the first biologic approved for children in this age group in the United States, according to the statement. The proposed indication is as add-on therapy for children with moderate to severe AD not adequately controlled with topical prescription therapies or for whom topical therapies are not advised. The FDA granted breakthrough therapy designation for dupilumab for the treatment of severe AD in children aged 6 months to 11 years in 2016.
Approximately 85%-95% of atopic dermatitis patients develop symptoms before 5 years of age, and these symptoms often continue into adulthood, with an increased risk of skin infections and a significant impact on quality of life, according to the statement.
The sBLA is based on data from a phase 3 pivotal study of 162 children aged 6 months to 5 years in which dupilumab was added to standard-of-care topical corticosteroids, presented in December 2021. In the study, dupilumab plus standard of care significantly improved skin clearance and reduced overall disease severity and itch at 16 weeks compared with standard of care alone. Overall, 28% of the children randomized to dupilumab achieved the primary endpoint of clear or almost-clear skin, compared with 4% with those on standard of care alone (P < .0001), according to the manufacturers. Patients in the dupilumab group received either 200 mg (for children weighing ≥ 5 to < 15 kg) or 300 mg (for children weighing ≥ 15 to < 30 kg) every 4 weeks. Safety results were similar to those seen with dupilumab for children aged 6 years and older.
Conjunctivitis and herpes infections were among the most common adverse events associated with dupilumab in the study, according to the statement.
The target action date for the FDA decision on this application is June 9, 2022.
FROM THE FDA
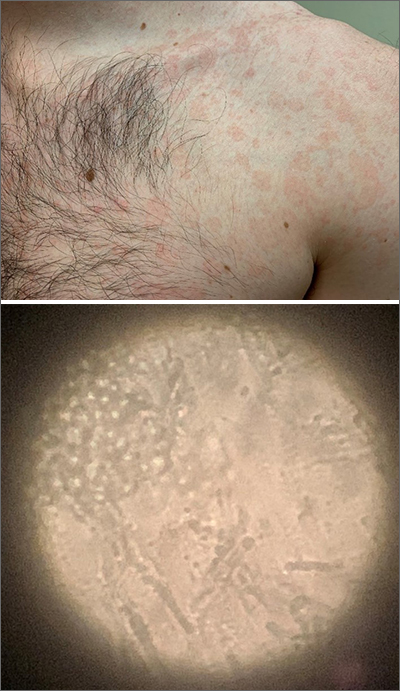
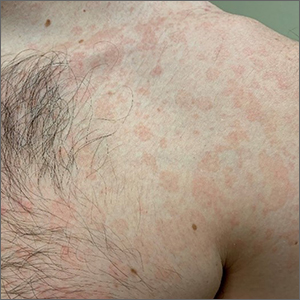
Chronic truncal rash
The patient was given a diagnosis of tinea versicolor (TV), also known as pityriasis versicolor, after a potassium hydroxide (KOH) prep test on a skin scraping confirmed the spaghetti and meatballs pattern of Malassezia Furfur (see image above). Note that in most cases, a KOH prep is not required for the diagnosis. Experienced clinicians will usually make the diagnosis based on the appearance of hyper- or hypopigmented macules or patches with fine scale on the trunk of adults. KOH prep is useful if the diagnosis is uncertain.
TV is a common fungal infection that’s seen more frequently in tropical climates and occurs equally in men and women.1M. Furfur thrives on the lipids in the skin of sebum-rich areas, which explains its truncal distribution and rare occurrence in children (who have much lower sebum production).
Usually, topical antifungal medications are considered first-line treatment, but since large areas of skin are involved, adequate amounts need to be used. One of the most common and inexpensive treatments is to apply selenium sulfide shampoo (Selsun Blue) undiluted to the entire trunk, then allow to dry and remain in place overnight before showering. A repeat application should be done 1 week later. Topical terbinafine cream applied bid for 2 weeks is another option, as is oral itraconazole in a single 400 mg dose.
This patient declined the selenium sulfide topical treatment and requested systemic therapy, so he was prescribed itraconazole 400 mg orally as a single dose. He was advised that it might take a few weeks to clear up, and to use the selenium sulfide application if the itraconazole was not effective. Follow-up was not planned due to the high success rate of these therapies.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Saunte DML, Gaitanis G, Hay RJ. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol. 2020;10:112. doi: 10.3389/fcimb.2020.00112
The patient was given a diagnosis of tinea versicolor (TV), also known as pityriasis versicolor, after a potassium hydroxide (KOH) prep test on a skin scraping confirmed the spaghetti and meatballs pattern of Malassezia Furfur (see image above). Note that in most cases, a KOH prep is not required for the diagnosis. Experienced clinicians will usually make the diagnosis based on the appearance of hyper- or hypopigmented macules or patches with fine scale on the trunk of adults. KOH prep is useful if the diagnosis is uncertain.
TV is a common fungal infection that’s seen more frequently in tropical climates and occurs equally in men and women.1M. Furfur thrives on the lipids in the skin of sebum-rich areas, which explains its truncal distribution and rare occurrence in children (who have much lower sebum production).
Usually, topical antifungal medications are considered first-line treatment, but since large areas of skin are involved, adequate amounts need to be used. One of the most common and inexpensive treatments is to apply selenium sulfide shampoo (Selsun Blue) undiluted to the entire trunk, then allow to dry and remain in place overnight before showering. A repeat application should be done 1 week later. Topical terbinafine cream applied bid for 2 weeks is another option, as is oral itraconazole in a single 400 mg dose.
This patient declined the selenium sulfide topical treatment and requested systemic therapy, so he was prescribed itraconazole 400 mg orally as a single dose. He was advised that it might take a few weeks to clear up, and to use the selenium sulfide application if the itraconazole was not effective. Follow-up was not planned due to the high success rate of these therapies.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
The patient was given a diagnosis of tinea versicolor (TV), also known as pityriasis versicolor, after a potassium hydroxide (KOH) prep test on a skin scraping confirmed the spaghetti and meatballs pattern of Malassezia Furfur (see image above). Note that in most cases, a KOH prep is not required for the diagnosis. Experienced clinicians will usually make the diagnosis based on the appearance of hyper- or hypopigmented macules or patches with fine scale on the trunk of adults. KOH prep is useful if the diagnosis is uncertain.
TV is a common fungal infection that’s seen more frequently in tropical climates and occurs equally in men and women.1M. Furfur thrives on the lipids in the skin of sebum-rich areas, which explains its truncal distribution and rare occurrence in children (who have much lower sebum production).
Usually, topical antifungal medications are considered first-line treatment, but since large areas of skin are involved, adequate amounts need to be used. One of the most common and inexpensive treatments is to apply selenium sulfide shampoo (Selsun Blue) undiluted to the entire trunk, then allow to dry and remain in place overnight before showering. A repeat application should be done 1 week later. Topical terbinafine cream applied bid for 2 weeks is another option, as is oral itraconazole in a single 400 mg dose.
This patient declined the selenium sulfide topical treatment and requested systemic therapy, so he was prescribed itraconazole 400 mg orally as a single dose. He was advised that it might take a few weeks to clear up, and to use the selenium sulfide application if the itraconazole was not effective. Follow-up was not planned due to the high success rate of these therapies.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Saunte DML, Gaitanis G, Hay RJ. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol. 2020;10:112. doi: 10.3389/fcimb.2020.00112
1. Saunte DML, Gaitanis G, Hay RJ. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol. 2020;10:112. doi: 10.3389/fcimb.2020.00112
Expert shares workup pearls for children with severe atopic dermatitis
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
FROM ODAC 2022
Questions about optimal dosing of isotretinoin persist, expert says
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
FROM ODAC 2022
Hyperpigmented plaque on back
Based on the thickness and size of this irregular lesion, a punch biopsy was performed and confirmed the diagnosis of dermatofibrosarcoma protuberans (DFSP).
DFSPs are usually found on the trunk and proximal extremities; they are most often located on the chest and shoulders. The lesion usually manifests as an asymptomatic, firm, and sometimes nodular plaque that may go undiagnosed for years. DFSP is an uncommon mesenchymal tumor with uncertain etiology. It is thought that prior injury to the affected skin may result in a translocation of chromosomes 17 and 22 in skin cells, as this molecular change characterizes the vast majority of DFSPs.
Black patients are more likely than other ethnic populations to develop DFSP and its variants; there is also a slight female predominance.1 Known variants of DFSP include violaceous plaques with telangiectatic atrophic skin, and plaques with dark brown pigmentation called Bednar tumors.1,2
DFSPs are rarely metastatic, but can be locally invasive, so primary treatment consists of wide local excision or Mohs micrographic surgery (MMS). There is a higher probability for cure when MMS is utilized to treat DFSPs with the added benefit of minimizing surgical margins and preserving healthy surrounding skin. In the rarer cases of advanced local, unresectable, or metastatic disease, inhibitor therapy with imatinib or radiation therapy may be considered.2 Due to the risk of local recurrence, patients with DFSP should have regular clinical follow-up every 6 months for 5 years, followed by annual lifelong surveillance.1
This patient was referred for MMS and has not yet returned for follow-up evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Morgan Haynes, BS, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Mendenhall WM, Scarborough MT, Flowers FP. Dermatofibrosarcoma protuberans: epidemiology, pathogenesis, clinical presentation, diagnosis, and staging. UpToDate. Updated March 31, 2021. Accessed February 2, 2022. www.uptodate.com/contents/dermatofibrosarcoma-protuberans-epidemiology-pathogenesis-clinical-presentation-diagnosis-and-staging
2. Brooks J, Ramsey ML. Dermatofibrosarcoma Protuberans. StatPearls. Updated November 14, 2021. Accessed January 27, 2022. www.ncbi.nlm.nih.gov/books/NBK513305
Based on the thickness and size of this irregular lesion, a punch biopsy was performed and confirmed the diagnosis of dermatofibrosarcoma protuberans (DFSP).
DFSPs are usually found on the trunk and proximal extremities; they are most often located on the chest and shoulders. The lesion usually manifests as an asymptomatic, firm, and sometimes nodular plaque that may go undiagnosed for years. DFSP is an uncommon mesenchymal tumor with uncertain etiology. It is thought that prior injury to the affected skin may result in a translocation of chromosomes 17 and 22 in skin cells, as this molecular change characterizes the vast majority of DFSPs.
Black patients are more likely than other ethnic populations to develop DFSP and its variants; there is also a slight female predominance.1 Known variants of DFSP include violaceous plaques with telangiectatic atrophic skin, and plaques with dark brown pigmentation called Bednar tumors.1,2
DFSPs are rarely metastatic, but can be locally invasive, so primary treatment consists of wide local excision or Mohs micrographic surgery (MMS). There is a higher probability for cure when MMS is utilized to treat DFSPs with the added benefit of minimizing surgical margins and preserving healthy surrounding skin. In the rarer cases of advanced local, unresectable, or metastatic disease, inhibitor therapy with imatinib or radiation therapy may be considered.2 Due to the risk of local recurrence, patients with DFSP should have regular clinical follow-up every 6 months for 5 years, followed by annual lifelong surveillance.1
This patient was referred for MMS and has not yet returned for follow-up evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Morgan Haynes, BS, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Based on the thickness and size of this irregular lesion, a punch biopsy was performed and confirmed the diagnosis of dermatofibrosarcoma protuberans (DFSP).
DFSPs are usually found on the trunk and proximal extremities; they are most often located on the chest and shoulders. The lesion usually manifests as an asymptomatic, firm, and sometimes nodular plaque that may go undiagnosed for years. DFSP is an uncommon mesenchymal tumor with uncertain etiology. It is thought that prior injury to the affected skin may result in a translocation of chromosomes 17 and 22 in skin cells, as this molecular change characterizes the vast majority of DFSPs.
Black patients are more likely than other ethnic populations to develop DFSP and its variants; there is also a slight female predominance.1 Known variants of DFSP include violaceous plaques with telangiectatic atrophic skin, and plaques with dark brown pigmentation called Bednar tumors.1,2
DFSPs are rarely metastatic, but can be locally invasive, so primary treatment consists of wide local excision or Mohs micrographic surgery (MMS). There is a higher probability for cure when MMS is utilized to treat DFSPs with the added benefit of minimizing surgical margins and preserving healthy surrounding skin. In the rarer cases of advanced local, unresectable, or metastatic disease, inhibitor therapy with imatinib or radiation therapy may be considered.2 Due to the risk of local recurrence, patients with DFSP should have regular clinical follow-up every 6 months for 5 years, followed by annual lifelong surveillance.1
This patient was referred for MMS and has not yet returned for follow-up evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Morgan Haynes, BS, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Mendenhall WM, Scarborough MT, Flowers FP. Dermatofibrosarcoma protuberans: epidemiology, pathogenesis, clinical presentation, diagnosis, and staging. UpToDate. Updated March 31, 2021. Accessed February 2, 2022. www.uptodate.com/contents/dermatofibrosarcoma-protuberans-epidemiology-pathogenesis-clinical-presentation-diagnosis-and-staging
2. Brooks J, Ramsey ML. Dermatofibrosarcoma Protuberans. StatPearls. Updated November 14, 2021. Accessed January 27, 2022. www.ncbi.nlm.nih.gov/books/NBK513305
1. Mendenhall WM, Scarborough MT, Flowers FP. Dermatofibrosarcoma protuberans: epidemiology, pathogenesis, clinical presentation, diagnosis, and staging. UpToDate. Updated March 31, 2021. Accessed February 2, 2022. www.uptodate.com/contents/dermatofibrosarcoma-protuberans-epidemiology-pathogenesis-clinical-presentation-diagnosis-and-staging
2. Brooks J, Ramsey ML. Dermatofibrosarcoma Protuberans. StatPearls. Updated November 14, 2021. Accessed January 27, 2022. www.ncbi.nlm.nih.gov/books/NBK513305
Dryness, conjunctival telangiectasia among ocular symptoms common in rosacea
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL OPTHALMOLOGY
Lipedema: A potentially devastating, often unrecognized disease
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
” according to C. William Hanke, MD, MPH.
“This disease is well known in Europe, especially in the Netherlands, Germany, and Austria, but in this country, I believe most dermatologists have never heard of it,” Dr. Hanke said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Clinically, patients with lipedema – also known as “two-body syndrome” – present with a symmetric, bilateral increase in subcutaneous fat, with “cuffs of fat” around the ankles. It usually affects the legs and thighs; the hands and feet are not affected.
“From the waist on up, the body looks like one person, and from the waist on down, it looks like an entirely different person,” said Dr. Hanke, a dermatologist who is program director for the micrographic surgery and dermatologic oncology fellowship training program at Ascension St. Vincent Hospital in Indianapolis. “Just think of the difficulty that the person has with their life in terms of buying clothes or social interactions. This is a devastating problem.”
Lipedema almost always affects women and is progressive from puberty. “Characteristically, patients have pain and bruise easily in the areas of lipedema,” said Dr. Hanke, who has served as president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the International Society for Dermatologic Surgery. The affected areas are painful to touch, making exercise uncomfortable for patients, he said.
Lipedema can be masked by obesity, “so, if you superimpose generalized obesity on lipedema, you have an even more difficult problem,” he added. “A physician who doesn’t understand the disease may perform standard nontumescent liposuction under general anesthesia, with cannulas, which traumatize lipedematous fat. Thereby, a patient with lipedema can then be inadvertently transformed into a patient with lympholipedema. Then you’ve got even an even worse problem.”
One might think that the rate of diabetes would be high among lipedema patients, “but diabetes is essentially nonexistent in this group,” he continued. However, patients with lipedema “may develop hypothyroidism, venous disease, joint pain, and fibrosis in the fat as the disease progresses.”
Lipedema stages, treatment
Lipedema is defined by three clinical stages: Stage one is characterized by an enlarged subcutaneous fat department, but the skin surface is smooth. In stage 2, the skin surface becomes wavy with irregularities and dents, and in stage 3, patients develop large deforming nodules and hanging flaps.
“If we can diagnose lipedema in the early stages and perform tumescent liposuction using tumescent local anesthesia, we can prevent the progression of the disease,” Dr. Hanke said. For patients who meet criteria for tumescent liposuction, three to six treatments may be required for stage 3 disease. “Tumescent local anesthesia should be used, because liposuction using tumescent local anesthesia is atraumatic to fat,” he said. “Usually, the most painful areas are treated first.”
In a single-center study from Germany that followed 85 patients who underwent tumescent liposuction for lipedema, researchers found that improvements in pain, bruising, and mobility were sustained at 4 and 8 years following the procedure. Patient quality of life and cosmetic appearance were also sustained.
In terms of liposuction’s cosmetic effects, “the goal of liposuction in lipedema patients is different,” Dr. Hanke said. “The goal is to get these people moving again, stabilize their weight, and minimize progression of the disease. Cosmetic improvement is secondary.”
A more recent follow-up study of 60 patients from the same single-center German study showed that the positive effects of liposuction lasted 12 years postoperatively without relevant progression of disease.
Following the first International Consensus Conference on Lipedema in Vienna in 2017, Dr. Hanke and colleagues published guidelines on preventing progression of lipedema with liposuction using tumescent local anesthesia.
“If patients with lipedema gain weight, the problem becomes even worse,” he said. “A sensible diet and nontraumatic exercise like water aerobics is ideal. If patients pursue yo-yo dieting, more and more fat stays in the legs after each cycle. Sometimes I’ll refer overweight patients with lipedema for a bariatric surgery consult.”
Dr. Hanke noted that Karen Herbst, MD, PhD, an endocrinologist at the University of Arizona, Tucson, who is widely considered an expert on the medical management of lipedema, has a website on lipedema care.
Dr. Hanke reported having no financial conflicts related to his presentation.
FROM ODAC 2022
A dermatologist-led model for CVD prevention in psoriasis may be feasible
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
FROM JAMA DERMATOLOGY