User login
Study aims to enhance understanding of ‘tremendously understudied’ prurigo nodularis
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
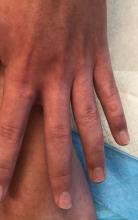
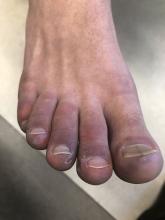
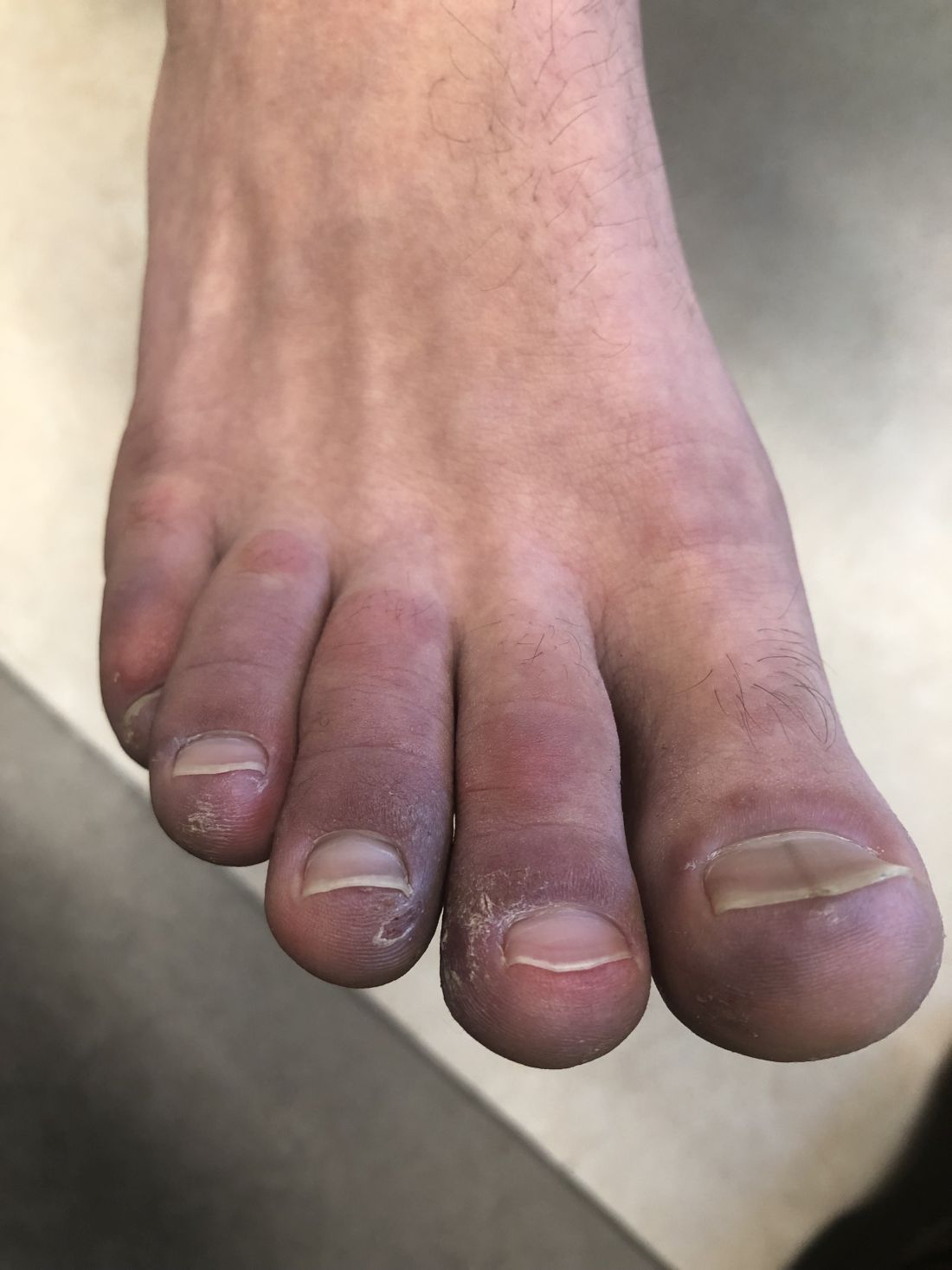
A 12-year-old male has persistent purple toes and new red lesions on his hands
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
He denied any hair loss, mouth sores, sun sensitivity, headaches, gastrointestinal complaints, joint pain, or muscle weakness.
He is not taking any medications.
He has been at home doing virtual school and has not traveled. He likes to play the piano. There is no family history of similar lesions, connective tissue disorder, or autoimmunity.
On physical exam he has purple discoloration on the toes with some violaceous and pink papules. On the fingers he has pink to violaceous papules and macules.
There is no joint edema or pain.
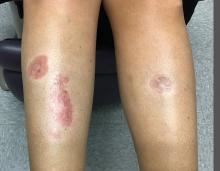
A woman with a history of diabetes, and plaques on both shins
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
. Women are often more affected than men. Patients often present in their 30s and 40s. The cause of NLD is unknown. Twenty percent of patients with NLD will have glucose intolerance or a family history of diabetes.1 The percentage of patients with NLD who have diabetes varies in reports from 11% to 65%.2 NLD may progress despite the diabetes treatment. Only 0.03% of patient with diabetes will have NLD.3
Lesions most commonly occur on the extremities, with shins being affected in most cases. They vary from asymptomatic to painful. Typically, lesions begin as small, firm erythematous papules that evolve into shiny, well-defined plaques. In older plaques, the center will often appear yellow, depressed, and atrophic, with telangiectasias. The periphery appears pink to violaceous to brown. Ulceration may be present, particularly after trauma, and there may be decreased sensation in the plaques. NLD is clinically distinct from diabetic dermopathy, which appear as brown macules, often in older patients with diabetes.
Ideally, biopsy should be taken at the edge of a lesion. Histologically, the epidermis appears normal or atrophic. A diffuse palisaded and interstitial granulomatous dermatitis consisting of histiocytes, multinucleated giant cells, lymphocytes, and plasma cells is seen in the dermis. Granulomas are often oriented parallel to the epidermis. There is no mucin at the center of the granulomas (as seen in granuloma annulare). Inflammation may extend into the subcutaneous fat. Asteroid bodies (as seen in sarcoid) are absent.
Unfortunately, treatment of NLD is often unsuccessful. Treatment includes potent topical corticosteroids for early lesions and intralesional triamcinolone to the leading edge of lesions. Care should be taken to avoid injecting centrally where atrophy and ulceration may result. Systemic steroids may be helpful in some cases, but can elevate glucose levels. Other reported medical treatments include pentoxifylline, cyclosporine, and niacinamide. Some lesions may spontaneously resolve. Ulcerations may require surgical excision with grafting.
This case and photo are provided by Dr. Bilu Martin, who is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. James WD et al. Andrews’ Diseases of the Skin: Clinical Dermatology. Philadelphia: Saunders Elsevier, 2006.
2. Hashemi D et al. JAMA Dermatol. 2019 Apr 1;155(4):455-9.
3. Bolognia JL et al. Dermatology. St. Louis, Mo.: Mosby Elsevier, 2008.
Black patients with cutaneous sarcoidosis may have more systemic and CV disease
according to a retrospective chart review of patients seen at Massachusetts General Hospital and Brigham and Women’s Hospital, both in Boston.
Black patients were also significantly more likely to have two or more organs involved and have higher rates of cardiac involvement, the latter of which is associated with worse prognosis. “Our data suggest there may be substantial variations in organ involvement between racial groups of patients presenting with cutaneous sarcoidosis,” said medical student Kylee Kus, a medical student at Oakland University, Auburn Hills, Mich., who presented the findings with Bina Kassamali, a medical student at Harvard University, Boston, at the annual Skin of Color Society scientific symposium.
Sotonye Imadojemu, MD, MBE; Avery LeChance, MD, MPH; and Ruth Anne Vleugels, MD, MPH, MBA; of Brigham and Women’s Hospital, are cosenior authors of the abstract.
The researchers identified 111 patients who were diagnosed with cutaneous sarcoidosis over a 20-year period (January 2000–December 2019), 50 of whom presented without established extracutaneous disease. They examined the charts of these 50 patients for whether subsequent work-up revealed systemic disease.
Of the 50 patients, 9 were Black. Seven of these nine patients (77.8%), were found to have systemic involvement, compared with 14 of 41 (46.3%) non-Black patients – a 31.5% higher probability (P < .05). One-third of the nine Black patients were found to have disease in one organ, and 44.4% in two or more organs. In non-Black patients, these rates were 12.2% and 34.1%, respectively.
Cardiovascular involvement was not found in any of the non-Black patients who had extracutaneous disease, but was found in 29% of the Black patients with extracutaneous disease, a statistically significant difference.
Black patients are known to be at higher risk for sarcoidosis than non-Black patients, and because “there is an association between cardiac sarcoid involvement and poor prognosis largely due to manifestations such as heart block, arrhythmias, and heart failure ... the study helps demonstrate how this organ involvement can disproportionately affect the Black population,” Ms. Kassamali said in an interview after the meeting.
A separate, recently published analysis of data from the same patient population examined the work-ups that patients received after a dermatologist’s diagnosis of sarcoidosis and found that patients with no previous systemic work-up were subsequently assessed for cardiac involvement in only 58.3% of cases. Assessment for pulmonary and ocular disease was completed more than 90% of the time.
“Crucial testing for cardiac involvement fell short,” Dr. Imadojemu, of the department of dermatology, Brigham and Women’s Hospital, and coinvestigators wrote in the research letter.
“Because the cutaneous manifestations of sarcoidosis often present at disease onset, dermatologists may be the first physicians to diagnose a patient with sarcoidosis,” they wrote. “As such, dermatologists are often responsible for initiating the appropriate evaluation of patients with sarcoidosis.”
Pulmonary involvement occurs in nearly all cases of sarcoidosis, while ocular and cardiac disease develop in approximately 25% and 10% of patients, respectively. Cardiac sarcoidosis is usually asymptomatic and accounts for 13%-25% of sarcoidosis-related deaths in the United States, they wrote.
An electrocardiogram is the appropriate initial screening tool and “is warranted in all patients with sarcoidosis,” they advised.
according to a retrospective chart review of patients seen at Massachusetts General Hospital and Brigham and Women’s Hospital, both in Boston.
Black patients were also significantly more likely to have two or more organs involved and have higher rates of cardiac involvement, the latter of which is associated with worse prognosis. “Our data suggest there may be substantial variations in organ involvement between racial groups of patients presenting with cutaneous sarcoidosis,” said medical student Kylee Kus, a medical student at Oakland University, Auburn Hills, Mich., who presented the findings with Bina Kassamali, a medical student at Harvard University, Boston, at the annual Skin of Color Society scientific symposium.
Sotonye Imadojemu, MD, MBE; Avery LeChance, MD, MPH; and Ruth Anne Vleugels, MD, MPH, MBA; of Brigham and Women’s Hospital, are cosenior authors of the abstract.
The researchers identified 111 patients who were diagnosed with cutaneous sarcoidosis over a 20-year period (January 2000–December 2019), 50 of whom presented without established extracutaneous disease. They examined the charts of these 50 patients for whether subsequent work-up revealed systemic disease.
Of the 50 patients, 9 were Black. Seven of these nine patients (77.8%), were found to have systemic involvement, compared with 14 of 41 (46.3%) non-Black patients – a 31.5% higher probability (P < .05). One-third of the nine Black patients were found to have disease in one organ, and 44.4% in two or more organs. In non-Black patients, these rates were 12.2% and 34.1%, respectively.
Cardiovascular involvement was not found in any of the non-Black patients who had extracutaneous disease, but was found in 29% of the Black patients with extracutaneous disease, a statistically significant difference.
Black patients are known to be at higher risk for sarcoidosis than non-Black patients, and because “there is an association between cardiac sarcoid involvement and poor prognosis largely due to manifestations such as heart block, arrhythmias, and heart failure ... the study helps demonstrate how this organ involvement can disproportionately affect the Black population,” Ms. Kassamali said in an interview after the meeting.
A separate, recently published analysis of data from the same patient population examined the work-ups that patients received after a dermatologist’s diagnosis of sarcoidosis and found that patients with no previous systemic work-up were subsequently assessed for cardiac involvement in only 58.3% of cases. Assessment for pulmonary and ocular disease was completed more than 90% of the time.
“Crucial testing for cardiac involvement fell short,” Dr. Imadojemu, of the department of dermatology, Brigham and Women’s Hospital, and coinvestigators wrote in the research letter.
“Because the cutaneous manifestations of sarcoidosis often present at disease onset, dermatologists may be the first physicians to diagnose a patient with sarcoidosis,” they wrote. “As such, dermatologists are often responsible for initiating the appropriate evaluation of patients with sarcoidosis.”
Pulmonary involvement occurs in nearly all cases of sarcoidosis, while ocular and cardiac disease develop in approximately 25% and 10% of patients, respectively. Cardiac sarcoidosis is usually asymptomatic and accounts for 13%-25% of sarcoidosis-related deaths in the United States, they wrote.
An electrocardiogram is the appropriate initial screening tool and “is warranted in all patients with sarcoidosis,” they advised.
according to a retrospective chart review of patients seen at Massachusetts General Hospital and Brigham and Women’s Hospital, both in Boston.
Black patients were also significantly more likely to have two or more organs involved and have higher rates of cardiac involvement, the latter of which is associated with worse prognosis. “Our data suggest there may be substantial variations in organ involvement between racial groups of patients presenting with cutaneous sarcoidosis,” said medical student Kylee Kus, a medical student at Oakland University, Auburn Hills, Mich., who presented the findings with Bina Kassamali, a medical student at Harvard University, Boston, at the annual Skin of Color Society scientific symposium.
Sotonye Imadojemu, MD, MBE; Avery LeChance, MD, MPH; and Ruth Anne Vleugels, MD, MPH, MBA; of Brigham and Women’s Hospital, are cosenior authors of the abstract.
The researchers identified 111 patients who were diagnosed with cutaneous sarcoidosis over a 20-year period (January 2000–December 2019), 50 of whom presented without established extracutaneous disease. They examined the charts of these 50 patients for whether subsequent work-up revealed systemic disease.
Of the 50 patients, 9 were Black. Seven of these nine patients (77.8%), were found to have systemic involvement, compared with 14 of 41 (46.3%) non-Black patients – a 31.5% higher probability (P < .05). One-third of the nine Black patients were found to have disease in one organ, and 44.4% in two or more organs. In non-Black patients, these rates were 12.2% and 34.1%, respectively.
Cardiovascular involvement was not found in any of the non-Black patients who had extracutaneous disease, but was found in 29% of the Black patients with extracutaneous disease, a statistically significant difference.
Black patients are known to be at higher risk for sarcoidosis than non-Black patients, and because “there is an association between cardiac sarcoid involvement and poor prognosis largely due to manifestations such as heart block, arrhythmias, and heart failure ... the study helps demonstrate how this organ involvement can disproportionately affect the Black population,” Ms. Kassamali said in an interview after the meeting.
A separate, recently published analysis of data from the same patient population examined the work-ups that patients received after a dermatologist’s diagnosis of sarcoidosis and found that patients with no previous systemic work-up were subsequently assessed for cardiac involvement in only 58.3% of cases. Assessment for pulmonary and ocular disease was completed more than 90% of the time.
“Crucial testing for cardiac involvement fell short,” Dr. Imadojemu, of the department of dermatology, Brigham and Women’s Hospital, and coinvestigators wrote in the research letter.
“Because the cutaneous manifestations of sarcoidosis often present at disease onset, dermatologists may be the first physicians to diagnose a patient with sarcoidosis,” they wrote. “As such, dermatologists are often responsible for initiating the appropriate evaluation of patients with sarcoidosis.”
Pulmonary involvement occurs in nearly all cases of sarcoidosis, while ocular and cardiac disease develop in approximately 25% and 10% of patients, respectively. Cardiac sarcoidosis is usually asymptomatic and accounts for 13%-25% of sarcoidosis-related deaths in the United States, they wrote.
An electrocardiogram is the appropriate initial screening tool and “is warranted in all patients with sarcoidosis,” they advised.
FROM SOC SOCIETY 2021
Blacks and Hispanics have higher inpatient use for mycosis fungoides
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
FROM SOC SOCIETY 2021
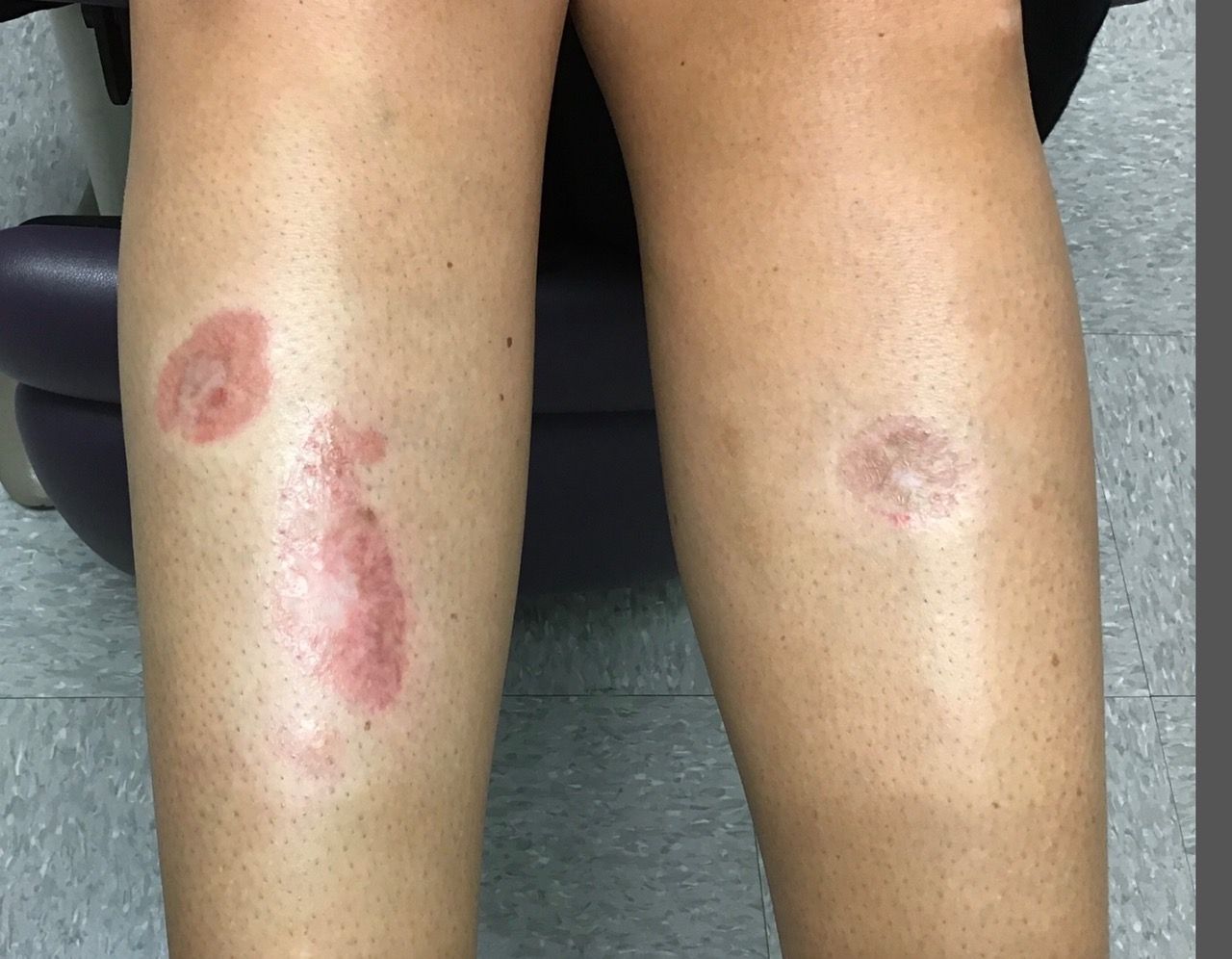
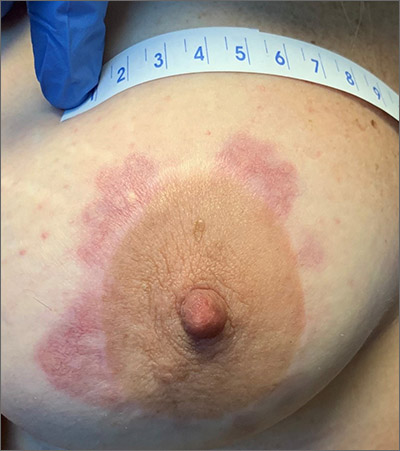
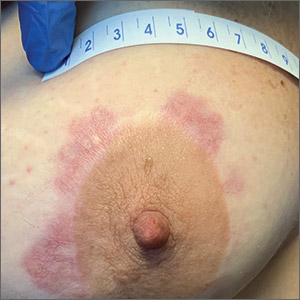
Chronic breast rash
A punch biopsy revealed that the patient had granuloma annulare (GA).
GA is usually a self-limiting disorder that manifests as a single or, less commonly, multiple nonscaly, red, annular lesions that are typically found on the extremities. It frequently starts as a papule or cluster of papules before coalescing into its classic annular pattern. Biopsy is not usually needed to make the diagnosis when annular lesions are present. In this case, the lesions displayed the Koebner phenomenon, occurring along her areolar scar, making diagnosis more difficult and necessitating the biopsy. While the cause of GA is unknown, it has been found more often in women than men, but has no predilection for race, ethnicity, or geographic areas.1
GA is typically asymptomatic and can resolve spontaneously. Treatment is often performed for cosmetic reasons. First-line therapies include topical corticosteroids, topical tacrolimus, imiquimod cream, intralesional injections into the elevated border with 2.5 to 5 mg/mL triamcinolone acetonide, or destructive methods such as cryosurgery or pulsed dye laser therapy.1
After a discussion of treatment options, this patient chose watchful waiting.
Image courtesy of Kamini Geer, MD, and text courtesy of Kamini Geer, MD, AdventHealth East Orlando Osteopathic Family Medicine Residency and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Saunders; 2015.
A punch biopsy revealed that the patient had granuloma annulare (GA).
GA is usually a self-limiting disorder that manifests as a single or, less commonly, multiple nonscaly, red, annular lesions that are typically found on the extremities. It frequently starts as a papule or cluster of papules before coalescing into its classic annular pattern. Biopsy is not usually needed to make the diagnosis when annular lesions are present. In this case, the lesions displayed the Koebner phenomenon, occurring along her areolar scar, making diagnosis more difficult and necessitating the biopsy. While the cause of GA is unknown, it has been found more often in women than men, but has no predilection for race, ethnicity, or geographic areas.1
GA is typically asymptomatic and can resolve spontaneously. Treatment is often performed for cosmetic reasons. First-line therapies include topical corticosteroids, topical tacrolimus, imiquimod cream, intralesional injections into the elevated border with 2.5 to 5 mg/mL triamcinolone acetonide, or destructive methods such as cryosurgery or pulsed dye laser therapy.1
After a discussion of treatment options, this patient chose watchful waiting.
Image courtesy of Kamini Geer, MD, and text courtesy of Kamini Geer, MD, AdventHealth East Orlando Osteopathic Family Medicine Residency and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
A punch biopsy revealed that the patient had granuloma annulare (GA).
GA is usually a self-limiting disorder that manifests as a single or, less commonly, multiple nonscaly, red, annular lesions that are typically found on the extremities. It frequently starts as a papule or cluster of papules before coalescing into its classic annular pattern. Biopsy is not usually needed to make the diagnosis when annular lesions are present. In this case, the lesions displayed the Koebner phenomenon, occurring along her areolar scar, making diagnosis more difficult and necessitating the biopsy. While the cause of GA is unknown, it has been found more often in women than men, but has no predilection for race, ethnicity, or geographic areas.1
GA is typically asymptomatic and can resolve spontaneously. Treatment is often performed for cosmetic reasons. First-line therapies include topical corticosteroids, topical tacrolimus, imiquimod cream, intralesional injections into the elevated border with 2.5 to 5 mg/mL triamcinolone acetonide, or destructive methods such as cryosurgery or pulsed dye laser therapy.1
After a discussion of treatment options, this patient chose watchful waiting.
Image courtesy of Kamini Geer, MD, and text courtesy of Kamini Geer, MD, AdventHealth East Orlando Osteopathic Family Medicine Residency and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Saunders; 2015.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Saunders; 2015.
Data about COVID-19-related skin manifestations in children continue to emerge
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Melanoma presents at later stages, but at an earlier age in Asian Americans
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
FROM SOC SOCIETY 2021
Secukinumab brings high PASI 75 results in 6- to 17-year-olds with psoriasis
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Despite new ichthyosis treatment recommendations, ‘many questions still exist’
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
FROM THE SPD PRE-AAD MEETING