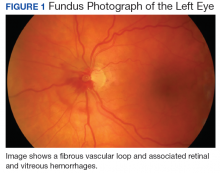
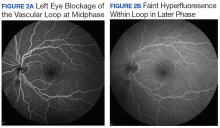
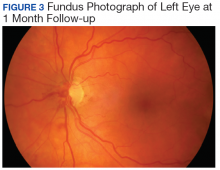
User login
Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants (FULL)
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
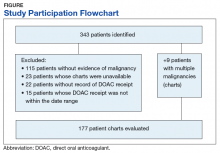
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
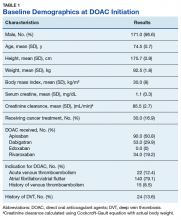
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
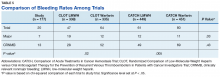
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
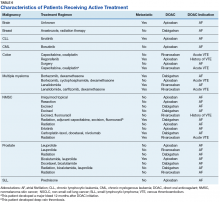
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
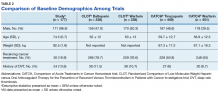
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
Phase 3 data support apixaban for cancer-associated VTE
SAN DIEGO – according to the Phase 3 ADAM VTE trial.
The rates of major bleeding and clinically relevant nonmajor bleeding in patients who received apixaban were similar to those in patients who received dalteparin. However, the rate of VTE recurrence was significantly lower with apixaban than it was with dalteparin.
“[A]pixaban was associated with very low bleeding rates and venous thrombosis recurrence rates compared to dalteparin,” said Robert D. McBane II, MD, of the Mayo Clinic in Rochester, Minn., at the annual meeting of the American Society of Hematology.
The trial included 300 adults (aged 18 years and older) with active cancer and acute VTE who were randomized to receive apixaban (n = 150) or dalteparin (n = 150). The dose and schedule for oral apixaban was 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months. Dalteparin was given subcutaneously at 200 IU/kg per day for 1 month followed by 150 IU/kg daily for 6 months. Among the patients in the study, 145 patients in the apixaban arm and 142 in the dalteparin arm ultimately received their assigned treatment.
Every month, patients completed an anticoagulation satisfaction survey and bruise survey (a modification of the Duke Anticoagulation Satisfaction Scale). They also underwent lab testing (complete blood count, liver and renal function testing) and were assessed for outcomes, medication reconciliation, drug compliance, and ECOG status on a monthly basis.
Patient characteristics
Baseline characteristics were similar between the treatment arms. The mean age was 64 years in both arms, and roughly half of patients in both arms were female. Hematologic malignancies were present in 9% of patients in the apixaban arm and 11% in the dalteparin arm. Others included lung, colorectal,
pancreatic/hepatobiliary, gynecologic, breast, genitourinary, upper gastrointestinal, and brain cancers.
Of patients in the study, 65% of those in the apixaban arm and 66% in the dalteparin arm had distant metastasis, and 74% of patients in both arms were receiving chemotherapy while on study.
Patients had the following qualifying thrombotic events:
- Any pulmonary embolism (PE) – 55% of patients in the apixaban arm and 51% in the dalteparin arm
- Any deep vein thrombosis (DVT) – 48% and 47%, respectively
- PE only – 44% and 39%, respectively
- PE with DVT – 12% in both arms
- DVT only – 37% and 35%, respectively
- Lower extremity DVT – 31% and 34%, respectively
- Upper extremity DVT – 17% and 14%, respectively
- Cerebral venous thrombosis (VT) – 1% and 0%, respectively
- Splanchnic VT – 8% and 18%, respectively.
Bleeding, thrombosis, and death
The study’s primary endpoint was major bleeding, which did not occur in any of the apixaban-treated patients. However, major bleeding did occur in two (1.4%) patients in the dalteparin arm (P = .14).
A secondary endpoint was major bleeding plus clinically relevant nonmajor bleeding. This occurred in nine (6.2%) patients in the apixaban arm and nine (6.3%) in the dalteparin arm (P = .88).
The researchers also assessed VTE recurrence. One patient in the apixaban arm (0.7%) and nine in the dalteparin arm (6.3%) had VTE recurrence (P = .03).
The patient in the apixaban arm experienced cerebral VT, and the patients with recurrence in the dalteparin arm had leg (n = 4) or arm (n = 2) VTE, PE (n = 1), or splanchnic VT (n = 2).
One patient in each arm (0.7%) had arterial thrombosis.
There was no significant difference in cumulative mortality between the treatment arms (hazard ratio, 1.40; P = .3078).
Satisfaction and discontinuation
Overall, apixaban fared better than dalteparin in the monthly patient satisfaction surveys. At various time points, apixaban-treated patients were significantly less likely to be concerned about excessive bruising, find anticoagulant treatment a burden or difficult to carry out, or say anticoagulant treatment added stress to their lives, negatively impacted their quality of life, or caused them “a great deal” of worry, irritation, or frustration.
However, apixaban-treated patients were less likely than dalteparin recipients to have confidence that their drug protected them from VTE recurrence, while the apixaban recipients were more likely than the dalteparin group to report overall satisfaction with their treatment.
In addition, premature treatment discontinuation was more common in the dalteparin group than in the apixaban group – 15% and 4%, respectively (P = .0012).
“Apixaban was well tolerated with superior patient safety satisfaction, as well as significantly fewer study drug discontinuations compared to dalteparin,” Dr. McBane said. “I believe that these data support the use of apixaban for the acute treatment of cancer-associated venous thromboembolism.”
This study was funded by BMS/Pfizer Alliance. Dr. McBane declared no other conflicts of interest.
jensmith@mdedge.com
SOURCE: McBane RD et al. ASH 2018, Abstract 421.
SAN DIEGO – according to the Phase 3 ADAM VTE trial.
The rates of major bleeding and clinically relevant nonmajor bleeding in patients who received apixaban were similar to those in patients who received dalteparin. However, the rate of VTE recurrence was significantly lower with apixaban than it was with dalteparin.
“[A]pixaban was associated with very low bleeding rates and venous thrombosis recurrence rates compared to dalteparin,” said Robert D. McBane II, MD, of the Mayo Clinic in Rochester, Minn., at the annual meeting of the American Society of Hematology.
The trial included 300 adults (aged 18 years and older) with active cancer and acute VTE who were randomized to receive apixaban (n = 150) or dalteparin (n = 150). The dose and schedule for oral apixaban was 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months. Dalteparin was given subcutaneously at 200 IU/kg per day for 1 month followed by 150 IU/kg daily for 6 months. Among the patients in the study, 145 patients in the apixaban arm and 142 in the dalteparin arm ultimately received their assigned treatment.
Every month, patients completed an anticoagulation satisfaction survey and bruise survey (a modification of the Duke Anticoagulation Satisfaction Scale). They also underwent lab testing (complete blood count, liver and renal function testing) and were assessed for outcomes, medication reconciliation, drug compliance, and ECOG status on a monthly basis.
Patient characteristics
Baseline characteristics were similar between the treatment arms. The mean age was 64 years in both arms, and roughly half of patients in both arms were female. Hematologic malignancies were present in 9% of patients in the apixaban arm and 11% in the dalteparin arm. Others included lung, colorectal,
pancreatic/hepatobiliary, gynecologic, breast, genitourinary, upper gastrointestinal, and brain cancers.
Of patients in the study, 65% of those in the apixaban arm and 66% in the dalteparin arm had distant metastasis, and 74% of patients in both arms were receiving chemotherapy while on study.
Patients had the following qualifying thrombotic events:
- Any pulmonary embolism (PE) – 55% of patients in the apixaban arm and 51% in the dalteparin arm
- Any deep vein thrombosis (DVT) – 48% and 47%, respectively
- PE only – 44% and 39%, respectively
- PE with DVT – 12% in both arms
- DVT only – 37% and 35%, respectively
- Lower extremity DVT – 31% and 34%, respectively
- Upper extremity DVT – 17% and 14%, respectively
- Cerebral venous thrombosis (VT) – 1% and 0%, respectively
- Splanchnic VT – 8% and 18%, respectively.
Bleeding, thrombosis, and death
The study’s primary endpoint was major bleeding, which did not occur in any of the apixaban-treated patients. However, major bleeding did occur in two (1.4%) patients in the dalteparin arm (P = .14).
A secondary endpoint was major bleeding plus clinically relevant nonmajor bleeding. This occurred in nine (6.2%) patients in the apixaban arm and nine (6.3%) in the dalteparin arm (P = .88).
The researchers also assessed VTE recurrence. One patient in the apixaban arm (0.7%) and nine in the dalteparin arm (6.3%) had VTE recurrence (P = .03).
The patient in the apixaban arm experienced cerebral VT, and the patients with recurrence in the dalteparin arm had leg (n = 4) or arm (n = 2) VTE, PE (n = 1), or splanchnic VT (n = 2).
One patient in each arm (0.7%) had arterial thrombosis.
There was no significant difference in cumulative mortality between the treatment arms (hazard ratio, 1.40; P = .3078).
Satisfaction and discontinuation
Overall, apixaban fared better than dalteparin in the monthly patient satisfaction surveys. At various time points, apixaban-treated patients were significantly less likely to be concerned about excessive bruising, find anticoagulant treatment a burden or difficult to carry out, or say anticoagulant treatment added stress to their lives, negatively impacted their quality of life, or caused them “a great deal” of worry, irritation, or frustration.
However, apixaban-treated patients were less likely than dalteparin recipients to have confidence that their drug protected them from VTE recurrence, while the apixaban recipients were more likely than the dalteparin group to report overall satisfaction with their treatment.
In addition, premature treatment discontinuation was more common in the dalteparin group than in the apixaban group – 15% and 4%, respectively (P = .0012).
“Apixaban was well tolerated with superior patient safety satisfaction, as well as significantly fewer study drug discontinuations compared to dalteparin,” Dr. McBane said. “I believe that these data support the use of apixaban for the acute treatment of cancer-associated venous thromboembolism.”
This study was funded by BMS/Pfizer Alliance. Dr. McBane declared no other conflicts of interest.
jensmith@mdedge.com
SOURCE: McBane RD et al. ASH 2018, Abstract 421.
SAN DIEGO – according to the Phase 3 ADAM VTE trial.
The rates of major bleeding and clinically relevant nonmajor bleeding in patients who received apixaban were similar to those in patients who received dalteparin. However, the rate of VTE recurrence was significantly lower with apixaban than it was with dalteparin.
“[A]pixaban was associated with very low bleeding rates and venous thrombosis recurrence rates compared to dalteparin,” said Robert D. McBane II, MD, of the Mayo Clinic in Rochester, Minn., at the annual meeting of the American Society of Hematology.
The trial included 300 adults (aged 18 years and older) with active cancer and acute VTE who were randomized to receive apixaban (n = 150) or dalteparin (n = 150). The dose and schedule for oral apixaban was 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months. Dalteparin was given subcutaneously at 200 IU/kg per day for 1 month followed by 150 IU/kg daily for 6 months. Among the patients in the study, 145 patients in the apixaban arm and 142 in the dalteparin arm ultimately received their assigned treatment.
Every month, patients completed an anticoagulation satisfaction survey and bruise survey (a modification of the Duke Anticoagulation Satisfaction Scale). They also underwent lab testing (complete blood count, liver and renal function testing) and were assessed for outcomes, medication reconciliation, drug compliance, and ECOG status on a monthly basis.
Patient characteristics
Baseline characteristics were similar between the treatment arms. The mean age was 64 years in both arms, and roughly half of patients in both arms were female. Hematologic malignancies were present in 9% of patients in the apixaban arm and 11% in the dalteparin arm. Others included lung, colorectal,
pancreatic/hepatobiliary, gynecologic, breast, genitourinary, upper gastrointestinal, and brain cancers.
Of patients in the study, 65% of those in the apixaban arm and 66% in the dalteparin arm had distant metastasis, and 74% of patients in both arms were receiving chemotherapy while on study.
Patients had the following qualifying thrombotic events:
- Any pulmonary embolism (PE) – 55% of patients in the apixaban arm and 51% in the dalteparin arm
- Any deep vein thrombosis (DVT) – 48% and 47%, respectively
- PE only – 44% and 39%, respectively
- PE with DVT – 12% in both arms
- DVT only – 37% and 35%, respectively
- Lower extremity DVT – 31% and 34%, respectively
- Upper extremity DVT – 17% and 14%, respectively
- Cerebral venous thrombosis (VT) – 1% and 0%, respectively
- Splanchnic VT – 8% and 18%, respectively.
Bleeding, thrombosis, and death
The study’s primary endpoint was major bleeding, which did not occur in any of the apixaban-treated patients. However, major bleeding did occur in two (1.4%) patients in the dalteparin arm (P = .14).
A secondary endpoint was major bleeding plus clinically relevant nonmajor bleeding. This occurred in nine (6.2%) patients in the apixaban arm and nine (6.3%) in the dalteparin arm (P = .88).
The researchers also assessed VTE recurrence. One patient in the apixaban arm (0.7%) and nine in the dalteparin arm (6.3%) had VTE recurrence (P = .03).
The patient in the apixaban arm experienced cerebral VT, and the patients with recurrence in the dalteparin arm had leg (n = 4) or arm (n = 2) VTE, PE (n = 1), or splanchnic VT (n = 2).
One patient in each arm (0.7%) had arterial thrombosis.
There was no significant difference in cumulative mortality between the treatment arms (hazard ratio, 1.40; P = .3078).
Satisfaction and discontinuation
Overall, apixaban fared better than dalteparin in the monthly patient satisfaction surveys. At various time points, apixaban-treated patients were significantly less likely to be concerned about excessive bruising, find anticoagulant treatment a burden or difficult to carry out, or say anticoagulant treatment added stress to their lives, negatively impacted their quality of life, or caused them “a great deal” of worry, irritation, or frustration.
However, apixaban-treated patients were less likely than dalteparin recipients to have confidence that their drug protected them from VTE recurrence, while the apixaban recipients were more likely than the dalteparin group to report overall satisfaction with their treatment.
In addition, premature treatment discontinuation was more common in the dalteparin group than in the apixaban group – 15% and 4%, respectively (P = .0012).
“Apixaban was well tolerated with superior patient safety satisfaction, as well as significantly fewer study drug discontinuations compared to dalteparin,” Dr. McBane said. “I believe that these data support the use of apixaban for the acute treatment of cancer-associated venous thromboembolism.”
This study was funded by BMS/Pfizer Alliance. Dr. McBane declared no other conflicts of interest.
jensmith@mdedge.com
SOURCE: McBane RD et al. ASH 2018, Abstract 421.
REPORTING FROM ASH 2018
Key clinical point: Apixaban is associated with a similar risk of major bleeding and a lower risk of VTE recurrence when compared with dalteparin in patients with cancer-associated VTE.
Major finding: There were no major bleeding events in the apixaban arm and two in the dalteparin arm (P = .14).
Study details: Phase 3 study of 300 patients.
Disclosures: This study was funded by BMS/Pfizer Alliance.
Source: McBane RD et al. ASH 2018, Abstract 421.
Large cohort study IDs prognostic factors in thromboangiitis obliterans
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Nonwhite ethnicity and limb infection predict poor prognosis in TAO.
Major finding: Ethnicity predicts vascular events (HR, 2.35); limb infection at diagnosis predicts vascular events and amputation (HR, 3.29 and 12.1, respectively).
Study details: A retrospective cohort study of 224 patients.
Disclosures: Dr. Le Joncour reported having no disclosures.
Source: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
AVERT: Apixaban reduced thromboembolism risk in cancer patients
Cancer patients treated with the oral anticoagulant apixaban (Eliquis) had a lower rate of venous thromboembolism but a higher rate of major bleeding, according to data from the AVERT study.
In the placebo-controlled, double-blind trial, 574 ambulatory cancer patients who were at moderate to high risk of thromboembolism (Khorana risk score of 2 or more) and were starting chemotherapy were randomized to either apixaban 2.5 mg twice daily or to placebo for 180 days. Over the 210-day study period, 12 patients (4.2%) in the apixaban group experienced a venous thromboembolism as did 28 patients (10.2%) in the placebo group, an adjusted 61% reduction in risk associated with anticoagulant therapy. The number needed to treat to prevent one venous thromboembolism was 17, Marc Carrier, MD, of the University of Ottawa, and his coauthors reported in the Dec. 4 edition of the New England Journal of Medicine.
“The treatment of venous thromboembolism with therapeutic anticoagulation is challenging in patients with cancer, because it often involves daily injections of low-molecular-weight heparin and is associated with a high risk of thromboembolism recurrence and serious bleeding complications,” they wrote. As an oral agent, apixaban offers a more convenient alternative.
The authors added that their study found more favorable benefits from anticoagulant therapy than had been seen in previous studies and suggested that this may be the result of using a different agent and a twice-daily dosing regimen.
In the AVERT study, the lower incidence of thromboembolism in the treatment arm was largely because of a reduction in pulmonary embolisms; there were 5 cases in the apixaban group, compared with 16 in the placebo group. The apixaban group experienced 7 cases of deep-vein thrombosis, and the placebo group experienced 12 cases.
During the treatment period, the placebo group had 20 venous thrombembolisms and the apixaban group had 3.
However the incidence of major bleeding was twice as high in the apixaban group: 10 patients (3.5%), compared with 5 (1.8%) in the placebo group (P = .046). The difference between the two groups was mostly based on an increased incidence of gastrointestinal bleeding, hematuria, and gynecologic bleeding among patients treated with apixaban.
None of the major bleeds affected critical organs in any patients. Most were category 2 bleeds, and three cases were judged to be clinical emergencies.
There were 62 deaths overall in the study – 35 in the apixaban group and 27 in the placebo group – and 87% of these deaths were related to the cancer.
Many patients in the study had advanced cancer, which was also the most common cause of death, the authors said. However, there was one death from pulmonary embolism in the placebo group. The dominant cancer types in the study participants were lymphoma, gynecologic, pancreatic, and lung cancers. Two-thirds of the patients in each group had a Khorana risk score of 2, and one patient in each group had a score of 5.
A different trial design and larger study would be needed to examine the impact of treatment on mortality and outcomes related to specific tumor types and chemotherapy regimens, the authors said.
They stressed that only 5.9% of patients in the study had renal dysfunction, so the study results cannot necessarily be applied to these patients more generally, especially as they are known to be at higher risk of bleeding.
The study was supported by the Canadian Institutes of Health Research and Bristol-Myers Squibb–Pfizer Alliance. Thirteen authors declared honoraria, grants or personal fees from the pharmaceutical industry unrelated to the study. Two declared grants from the study funders for the study; ten authors had no conflicts of interest to declare.
SOURCE: Carrier M et al. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468
Cancer patients treated with the oral anticoagulant apixaban (Eliquis) had a lower rate of venous thromboembolism but a higher rate of major bleeding, according to data from the AVERT study.
In the placebo-controlled, double-blind trial, 574 ambulatory cancer patients who were at moderate to high risk of thromboembolism (Khorana risk score of 2 or more) and were starting chemotherapy were randomized to either apixaban 2.5 mg twice daily or to placebo for 180 days. Over the 210-day study period, 12 patients (4.2%) in the apixaban group experienced a venous thromboembolism as did 28 patients (10.2%) in the placebo group, an adjusted 61% reduction in risk associated with anticoagulant therapy. The number needed to treat to prevent one venous thromboembolism was 17, Marc Carrier, MD, of the University of Ottawa, and his coauthors reported in the Dec. 4 edition of the New England Journal of Medicine.
“The treatment of venous thromboembolism with therapeutic anticoagulation is challenging in patients with cancer, because it often involves daily injections of low-molecular-weight heparin and is associated with a high risk of thromboembolism recurrence and serious bleeding complications,” they wrote. As an oral agent, apixaban offers a more convenient alternative.
The authors added that their study found more favorable benefits from anticoagulant therapy than had been seen in previous studies and suggested that this may be the result of using a different agent and a twice-daily dosing regimen.
In the AVERT study, the lower incidence of thromboembolism in the treatment arm was largely because of a reduction in pulmonary embolisms; there were 5 cases in the apixaban group, compared with 16 in the placebo group. The apixaban group experienced 7 cases of deep-vein thrombosis, and the placebo group experienced 12 cases.
During the treatment period, the placebo group had 20 venous thrombembolisms and the apixaban group had 3.
However the incidence of major bleeding was twice as high in the apixaban group: 10 patients (3.5%), compared with 5 (1.8%) in the placebo group (P = .046). The difference between the two groups was mostly based on an increased incidence of gastrointestinal bleeding, hematuria, and gynecologic bleeding among patients treated with apixaban.
None of the major bleeds affected critical organs in any patients. Most were category 2 bleeds, and three cases were judged to be clinical emergencies.
There were 62 deaths overall in the study – 35 in the apixaban group and 27 in the placebo group – and 87% of these deaths were related to the cancer.
Many patients in the study had advanced cancer, which was also the most common cause of death, the authors said. However, there was one death from pulmonary embolism in the placebo group. The dominant cancer types in the study participants were lymphoma, gynecologic, pancreatic, and lung cancers. Two-thirds of the patients in each group had a Khorana risk score of 2, and one patient in each group had a score of 5.
A different trial design and larger study would be needed to examine the impact of treatment on mortality and outcomes related to specific tumor types and chemotherapy regimens, the authors said.
They stressed that only 5.9% of patients in the study had renal dysfunction, so the study results cannot necessarily be applied to these patients more generally, especially as they are known to be at higher risk of bleeding.
The study was supported by the Canadian Institutes of Health Research and Bristol-Myers Squibb–Pfizer Alliance. Thirteen authors declared honoraria, grants or personal fees from the pharmaceutical industry unrelated to the study. Two declared grants from the study funders for the study; ten authors had no conflicts of interest to declare.
SOURCE: Carrier M et al. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468
Cancer patients treated with the oral anticoagulant apixaban (Eliquis) had a lower rate of venous thromboembolism but a higher rate of major bleeding, according to data from the AVERT study.
In the placebo-controlled, double-blind trial, 574 ambulatory cancer patients who were at moderate to high risk of thromboembolism (Khorana risk score of 2 or more) and were starting chemotherapy were randomized to either apixaban 2.5 mg twice daily or to placebo for 180 days. Over the 210-day study period, 12 patients (4.2%) in the apixaban group experienced a venous thromboembolism as did 28 patients (10.2%) in the placebo group, an adjusted 61% reduction in risk associated with anticoagulant therapy. The number needed to treat to prevent one venous thromboembolism was 17, Marc Carrier, MD, of the University of Ottawa, and his coauthors reported in the Dec. 4 edition of the New England Journal of Medicine.
“The treatment of venous thromboembolism with therapeutic anticoagulation is challenging in patients with cancer, because it often involves daily injections of low-molecular-weight heparin and is associated with a high risk of thromboembolism recurrence and serious bleeding complications,” they wrote. As an oral agent, apixaban offers a more convenient alternative.
The authors added that their study found more favorable benefits from anticoagulant therapy than had been seen in previous studies and suggested that this may be the result of using a different agent and a twice-daily dosing regimen.
In the AVERT study, the lower incidence of thromboembolism in the treatment arm was largely because of a reduction in pulmonary embolisms; there were 5 cases in the apixaban group, compared with 16 in the placebo group. The apixaban group experienced 7 cases of deep-vein thrombosis, and the placebo group experienced 12 cases.
During the treatment period, the placebo group had 20 venous thrombembolisms and the apixaban group had 3.
However the incidence of major bleeding was twice as high in the apixaban group: 10 patients (3.5%), compared with 5 (1.8%) in the placebo group (P = .046). The difference between the two groups was mostly based on an increased incidence of gastrointestinal bleeding, hematuria, and gynecologic bleeding among patients treated with apixaban.
None of the major bleeds affected critical organs in any patients. Most were category 2 bleeds, and three cases were judged to be clinical emergencies.
There were 62 deaths overall in the study – 35 in the apixaban group and 27 in the placebo group – and 87% of these deaths were related to the cancer.
Many patients in the study had advanced cancer, which was also the most common cause of death, the authors said. However, there was one death from pulmonary embolism in the placebo group. The dominant cancer types in the study participants were lymphoma, gynecologic, pancreatic, and lung cancers. Two-thirds of the patients in each group had a Khorana risk score of 2, and one patient in each group had a score of 5.
A different trial design and larger study would be needed to examine the impact of treatment on mortality and outcomes related to specific tumor types and chemotherapy regimens, the authors said.
They stressed that only 5.9% of patients in the study had renal dysfunction, so the study results cannot necessarily be applied to these patients more generally, especially as they are known to be at higher risk of bleeding.
The study was supported by the Canadian Institutes of Health Research and Bristol-Myers Squibb–Pfizer Alliance. Thirteen authors declared honoraria, grants or personal fees from the pharmaceutical industry unrelated to the study. Two declared grants from the study funders for the study; ten authors had no conflicts of interest to declare.
SOURCE: Carrier M et al. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Apixaban lowered the rate of venous thromboembolism to 4.2% in patients with cancer, half the rate seen in similar patients given placebo.
Major finding: The number needed to treat to prevent 1 venous thromboembolism was 17.
Study details: A placebo-controlled, double-blind, randomized trial in 574 cancer patients.
Disclosures: The study was supported by the Canadian Institutes of Health Research and Bristol-Myers Squibb–Pfizer Alliance. Thirteen authors declared honoraria, grants, or personal fees from the pharmaceutical industry unrelated to the study. Two declared grants from the study funders for the study; ten authors had no conflicts of interest to declare.
Source: Carrier M et al. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Expert panel updates guidelines on antithrombotic therapy for AF
For patients with , experts said in a comprehensive, updated guideline.
The 113-page guideline, published in the journal CHEST®, provides antithrombotic treatment recommendations for atrial fibrillation based on different levels of risk for stroke and in a variety of clinical presentations.
Altogether, the new guidelines highlight 60 key recommendations from the 12-person expert panel, chaired by Gregory Y.H. Lip, MD, of the Institute of Cardiovascular Sciences, University of Birmingham (England).
To develop the guidelines, the panel conducted a systematic literature review of relevant articles released since the 2012 publication of Thrombolytic Therapy: American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines (9th Edition).
Since that time, “there have been substantial developments in atrial fibrillation thromboprophylaxis, whether with regard to risk assessment, antithrombotic drugs, or non-drug approaches,” panelists said in their report.
The panel graded the quality of the new evidence found in the literature review, and then undertook a consensus development process. Each recommendation and statement required at least 80% consensus to pass.
Their treatment recommendations in the report are focused on three topic areas: stroke and bleeding risk assessment, antithrombotic therapy in general, and antithrombotic therapy in special situations, such as acute coronary syndrome and stenting, chronic atrial flutter, pregnancy, and chronic kidney disease.
Stroke prevention is the main priority in a “holistic approach” to management of atrial fibrillation, the panelists said in the report.
“Many of the risk factors leading to incident AF are also risk factors for ischemic stroke, and the promotion of an integrated or holistic approach to AF management is needed, incorporating stroke prevention, addressing symptoms and risk factor management,” they said.
No antithrombotic therapy is needed for patients who have atrial fibrillation without valvular heart disease, the panelists concluded.
For patients with at least one nongender CHA2DS2-VASc stroke risk factor, oral anticoagulation is recommended over aspirin, aspirin and clopidogrel, or no therapy, they said.
In high-risk patients, including males with two or more CHA2DS2-VASc risk factors and females with three or more, novel oral anticoagulants are recommended over adjusted-dose warfarin, they added.
At each patient contact, patients with atrial fibrillation should receive bleeding risk assessment starting with potentially modifiable risk factors such as uncontrolled blood pressure or excessive alcohol intake, according to the expert panel.
High-risk patients, as indicated by a HAS-BLED score of 3 or greater, should have more frequent and regular follow-up, they said.
The expert panel report concludes with a discussion on practical and patient-centered issues.
“Patient education is essential to provide patients with sufficient information to enable them to make an informed decision about whether or not they wish to take oral anticoagulants, and if they do, which oral anticoagulant they would prefer,” Dr. Lip and his colleagues said in their report.
Dr. Lip disclosed a potential conflict of interest with Boehringer Ingelheim. Expert panel members reported disclosures related to Boston Scientific, Medtronic, St. Jude Medical, Biotronik, MSD, Novartis, Pfizer, Bayer, Servier, Gilead, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Lip GYH et al. CHEST. 2018 Aug 21. pii: S0012-3692(18)32244-X.
For patients with , experts said in a comprehensive, updated guideline.
The 113-page guideline, published in the journal CHEST®, provides antithrombotic treatment recommendations for atrial fibrillation based on different levels of risk for stroke and in a variety of clinical presentations.
Altogether, the new guidelines highlight 60 key recommendations from the 12-person expert panel, chaired by Gregory Y.H. Lip, MD, of the Institute of Cardiovascular Sciences, University of Birmingham (England).
To develop the guidelines, the panel conducted a systematic literature review of relevant articles released since the 2012 publication of Thrombolytic Therapy: American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines (9th Edition).
Since that time, “there have been substantial developments in atrial fibrillation thromboprophylaxis, whether with regard to risk assessment, antithrombotic drugs, or non-drug approaches,” panelists said in their report.
The panel graded the quality of the new evidence found in the literature review, and then undertook a consensus development process. Each recommendation and statement required at least 80% consensus to pass.
Their treatment recommendations in the report are focused on three topic areas: stroke and bleeding risk assessment, antithrombotic therapy in general, and antithrombotic therapy in special situations, such as acute coronary syndrome and stenting, chronic atrial flutter, pregnancy, and chronic kidney disease.
Stroke prevention is the main priority in a “holistic approach” to management of atrial fibrillation, the panelists said in the report.
“Many of the risk factors leading to incident AF are also risk factors for ischemic stroke, and the promotion of an integrated or holistic approach to AF management is needed, incorporating stroke prevention, addressing symptoms and risk factor management,” they said.
No antithrombotic therapy is needed for patients who have atrial fibrillation without valvular heart disease, the panelists concluded.
For patients with at least one nongender CHA2DS2-VASc stroke risk factor, oral anticoagulation is recommended over aspirin, aspirin and clopidogrel, or no therapy, they said.
In high-risk patients, including males with two or more CHA2DS2-VASc risk factors and females with three or more, novel oral anticoagulants are recommended over adjusted-dose warfarin, they added.
At each patient contact, patients with atrial fibrillation should receive bleeding risk assessment starting with potentially modifiable risk factors such as uncontrolled blood pressure or excessive alcohol intake, according to the expert panel.
High-risk patients, as indicated by a HAS-BLED score of 3 or greater, should have more frequent and regular follow-up, they said.
The expert panel report concludes with a discussion on practical and patient-centered issues.
“Patient education is essential to provide patients with sufficient information to enable them to make an informed decision about whether or not they wish to take oral anticoagulants, and if they do, which oral anticoagulant they would prefer,” Dr. Lip and his colleagues said in their report.
Dr. Lip disclosed a potential conflict of interest with Boehringer Ingelheim. Expert panel members reported disclosures related to Boston Scientific, Medtronic, St. Jude Medical, Biotronik, MSD, Novartis, Pfizer, Bayer, Servier, Gilead, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Lip GYH et al. CHEST. 2018 Aug 21. pii: S0012-3692(18)32244-X.
For patients with , experts said in a comprehensive, updated guideline.
The 113-page guideline, published in the journal CHEST®, provides antithrombotic treatment recommendations for atrial fibrillation based on different levels of risk for stroke and in a variety of clinical presentations.
Altogether, the new guidelines highlight 60 key recommendations from the 12-person expert panel, chaired by Gregory Y.H. Lip, MD, of the Institute of Cardiovascular Sciences, University of Birmingham (England).
To develop the guidelines, the panel conducted a systematic literature review of relevant articles released since the 2012 publication of Thrombolytic Therapy: American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines (9th Edition).
Since that time, “there have been substantial developments in atrial fibrillation thromboprophylaxis, whether with regard to risk assessment, antithrombotic drugs, or non-drug approaches,” panelists said in their report.
The panel graded the quality of the new evidence found in the literature review, and then undertook a consensus development process. Each recommendation and statement required at least 80% consensus to pass.
Their treatment recommendations in the report are focused on three topic areas: stroke and bleeding risk assessment, antithrombotic therapy in general, and antithrombotic therapy in special situations, such as acute coronary syndrome and stenting, chronic atrial flutter, pregnancy, and chronic kidney disease.
Stroke prevention is the main priority in a “holistic approach” to management of atrial fibrillation, the panelists said in the report.
“Many of the risk factors leading to incident AF are also risk factors for ischemic stroke, and the promotion of an integrated or holistic approach to AF management is needed, incorporating stroke prevention, addressing symptoms and risk factor management,” they said.
No antithrombotic therapy is needed for patients who have atrial fibrillation without valvular heart disease, the panelists concluded.
For patients with at least one nongender CHA2DS2-VASc stroke risk factor, oral anticoagulation is recommended over aspirin, aspirin and clopidogrel, or no therapy, they said.
In high-risk patients, including males with two or more CHA2DS2-VASc risk factors and females with three or more, novel oral anticoagulants are recommended over adjusted-dose warfarin, they added.
At each patient contact, patients with atrial fibrillation should receive bleeding risk assessment starting with potentially modifiable risk factors such as uncontrolled blood pressure or excessive alcohol intake, according to the expert panel.
High-risk patients, as indicated by a HAS-BLED score of 3 or greater, should have more frequent and regular follow-up, they said.
The expert panel report concludes with a discussion on practical and patient-centered issues.
“Patient education is essential to provide patients with sufficient information to enable them to make an informed decision about whether or not they wish to take oral anticoagulants, and if they do, which oral anticoagulant they would prefer,” Dr. Lip and his colleagues said in their report.
Dr. Lip disclosed a potential conflict of interest with Boehringer Ingelheim. Expert panel members reported disclosures related to Boston Scientific, Medtronic, St. Jude Medical, Biotronik, MSD, Novartis, Pfizer, Bayer, Servier, Gilead, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Lip GYH et al. CHEST. 2018 Aug 21. pii: S0012-3692(18)32244-X.
FROM CHEST
How to identify DVT faster in pediatric osteomyelitis
MALMO, SWEDEN – Early identification of deep vein thrombosis in children with acute hematogenous osteomyelitis is critical given the need to plan anticoagulation management around the high likelihood that such patients will undergo multiple surgeries, Lawson A.B. Copley, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
He and his coinvestigators have identified a handful of risk factors helpful in expediting recognition of deep vein thrombosis (DVT) in children with suspected invasive infection of the musculoskeletal system.
“To improve the rate and timing of identification of DVT, we recommend performing early screening ultrasound on all children with these risk factors who are suspected of having acute hematogenous osteomyelitis,” declared Dr. Copley, professor of orthopaedic surgery and pediatrics at the University of Texas, Dallas.
Delayed diagnosis of DVT in the setting of acute hematogenous osteomyelitis (AHO) is common. Indeed, in a review of the experience at Children’s Medical Center Dallas during 2012-2014, the average time delay from ICU admission in patients suspected of having AHO to identification of DVT by ultrasound was 6.3 days.
“We’ve changed some things on the basis of that study in order to accelerate that timeline,” he explained.
Their major change was to identify actionable risk factors for DVT. This was accomplished by conducting a retrospective study of the EHR of nearly 902,000 Texas children during 2008-2016.
The study demonstrated that children with AHO complicated by DVT are, from the get-go, very different from AHO patients without DVT. They have higher illness severity of illness, are more likely to be admitted to the ICU, are prone to methicillin-resistant Staphylococcus aureus infection with prolonged bacteremia, and are much more likely to undergo multiple surgeries. Moreover, children with AHO and DVT differed substantially from other children with DVT: The dual diagnosis children lacked comorbid conditions, were prone to septic pulmonary emboli, didn’t develop postthrombotic syndrome marked by chronic venous stasis and ulcerations, and had invariably negative coagulopathy workups.
“There is no need, we feel, to perform a hypercoagulopathy workup in children with AHO complicated by DVT,” Dr. Copley said.
Drilling deeper into the data, he and his coinvestigators identified 224 new cases of DVT in the study population, for a prevalence of 2.5 per 10,000 children, along with 466 children with AHO. A total of 6% of children with AHO had DVT, and 12.1% of all children with DVT had AHO. The researchers then compared the demographics, laboratory parameters, and treatment in three cohorts: the 196 children with DVT without AHO, 28 with both AHO and DVT, and 438 with AHO without DVT.
Through this analysis, they came up with a list of risk factors warranting early screening ultrasound in children suspected of having AHO:
- An initial C-reactive protein level above 8 mg/dL, which was present in all 28 dual diagnosis children.
- ICU admission, which occurred in 19 of 28 (68%) children.
- A severity of illness score of at least 7 on a 10-point scale during the first several days in the hospital, present in 27 of the 28 children. The severity of illness scale was developed and validated by Dr. Copley and coworkers (J Pediatr Orthop. 2016 Oct 12. doi: 10.1097/BPO.0000000000000879).
- Bacteremia in the initial blood culture, present in 23 of 28 patients (82%).
- Just under 90% of the children with AHO and DVT had methicillin-resistant S. aureus, compared with 20% of those with AHO without DVT.
- Septic pulmonary emboli visualized on chest x-ray, a complication that occurred in 64% of the dual diagnosis group versus just 1% of patients with DVT without AHO.
- A band percentage of white blood cells greater than 1.5%, present in 86% of children with AHO and DVT.
More than 90% of children with AHO and DVT underwent surgery, with a mean of 2.7 surgeries per child, in contrast to the group with AHO without DVT, 55% of whom had surgery, with a mean of 0.7 surgeries per child.
Of note, there was no significant difference in the occurrence of pulmonary embolism between children with DVT without AHO versus those with DVT and AHO, with a rate of about 10% in both groups.
Dr. Copley reported having no relevant financial conflicts.
MALMO, SWEDEN – Early identification of deep vein thrombosis in children with acute hematogenous osteomyelitis is critical given the need to plan anticoagulation management around the high likelihood that such patients will undergo multiple surgeries, Lawson A.B. Copley, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
He and his coinvestigators have identified a handful of risk factors helpful in expediting recognition of deep vein thrombosis (DVT) in children with suspected invasive infection of the musculoskeletal system.
“To improve the rate and timing of identification of DVT, we recommend performing early screening ultrasound on all children with these risk factors who are suspected of having acute hematogenous osteomyelitis,” declared Dr. Copley, professor of orthopaedic surgery and pediatrics at the University of Texas, Dallas.
Delayed diagnosis of DVT in the setting of acute hematogenous osteomyelitis (AHO) is common. Indeed, in a review of the experience at Children’s Medical Center Dallas during 2012-2014, the average time delay from ICU admission in patients suspected of having AHO to identification of DVT by ultrasound was 6.3 days.
“We’ve changed some things on the basis of that study in order to accelerate that timeline,” he explained.
Their major change was to identify actionable risk factors for DVT. This was accomplished by conducting a retrospective study of the EHR of nearly 902,000 Texas children during 2008-2016.
The study demonstrated that children with AHO complicated by DVT are, from the get-go, very different from AHO patients without DVT. They have higher illness severity of illness, are more likely to be admitted to the ICU, are prone to methicillin-resistant Staphylococcus aureus infection with prolonged bacteremia, and are much more likely to undergo multiple surgeries. Moreover, children with AHO and DVT differed substantially from other children with DVT: The dual diagnosis children lacked comorbid conditions, were prone to septic pulmonary emboli, didn’t develop postthrombotic syndrome marked by chronic venous stasis and ulcerations, and had invariably negative coagulopathy workups.
“There is no need, we feel, to perform a hypercoagulopathy workup in children with AHO complicated by DVT,” Dr. Copley said.
Drilling deeper into the data, he and his coinvestigators identified 224 new cases of DVT in the study population, for a prevalence of 2.5 per 10,000 children, along with 466 children with AHO. A total of 6% of children with AHO had DVT, and 12.1% of all children with DVT had AHO. The researchers then compared the demographics, laboratory parameters, and treatment in three cohorts: the 196 children with DVT without AHO, 28 with both AHO and DVT, and 438 with AHO without DVT.
Through this analysis, they came up with a list of risk factors warranting early screening ultrasound in children suspected of having AHO:
- An initial C-reactive protein level above 8 mg/dL, which was present in all 28 dual diagnosis children.
- ICU admission, which occurred in 19 of 28 (68%) children.
- A severity of illness score of at least 7 on a 10-point scale during the first several days in the hospital, present in 27 of the 28 children. The severity of illness scale was developed and validated by Dr. Copley and coworkers (J Pediatr Orthop. 2016 Oct 12. doi: 10.1097/BPO.0000000000000879).
- Bacteremia in the initial blood culture, present in 23 of 28 patients (82%).
- Just under 90% of the children with AHO and DVT had methicillin-resistant S. aureus, compared with 20% of those with AHO without DVT.
- Septic pulmonary emboli visualized on chest x-ray, a complication that occurred in 64% of the dual diagnosis group versus just 1% of patients with DVT without AHO.
- A band percentage of white blood cells greater than 1.5%, present in 86% of children with AHO and DVT.
More than 90% of children with AHO and DVT underwent surgery, with a mean of 2.7 surgeries per child, in contrast to the group with AHO without DVT, 55% of whom had surgery, with a mean of 0.7 surgeries per child.
Of note, there was no significant difference in the occurrence of pulmonary embolism between children with DVT without AHO versus those with DVT and AHO, with a rate of about 10% in both groups.
Dr. Copley reported having no relevant financial conflicts.
MALMO, SWEDEN – Early identification of deep vein thrombosis in children with acute hematogenous osteomyelitis is critical given the need to plan anticoagulation management around the high likelihood that such patients will undergo multiple surgeries, Lawson A.B. Copley, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
He and his coinvestigators have identified a handful of risk factors helpful in expediting recognition of deep vein thrombosis (DVT) in children with suspected invasive infection of the musculoskeletal system.
“To improve the rate and timing of identification of DVT, we recommend performing early screening ultrasound on all children with these risk factors who are suspected of having acute hematogenous osteomyelitis,” declared Dr. Copley, professor of orthopaedic surgery and pediatrics at the University of Texas, Dallas.
Delayed diagnosis of DVT in the setting of acute hematogenous osteomyelitis (AHO) is common. Indeed, in a review of the experience at Children’s Medical Center Dallas during 2012-2014, the average time delay from ICU admission in patients suspected of having AHO to identification of DVT by ultrasound was 6.3 days.
“We’ve changed some things on the basis of that study in order to accelerate that timeline,” he explained.
Their major change was to identify actionable risk factors for DVT. This was accomplished by conducting a retrospective study of the EHR of nearly 902,000 Texas children during 2008-2016.
The study demonstrated that children with AHO complicated by DVT are, from the get-go, very different from AHO patients without DVT. They have higher illness severity of illness, are more likely to be admitted to the ICU, are prone to methicillin-resistant Staphylococcus aureus infection with prolonged bacteremia, and are much more likely to undergo multiple surgeries. Moreover, children with AHO and DVT differed substantially from other children with DVT: The dual diagnosis children lacked comorbid conditions, were prone to septic pulmonary emboli, didn’t develop postthrombotic syndrome marked by chronic venous stasis and ulcerations, and had invariably negative coagulopathy workups.
“There is no need, we feel, to perform a hypercoagulopathy workup in children with AHO complicated by DVT,” Dr. Copley said.
Drilling deeper into the data, he and his coinvestigators identified 224 new cases of DVT in the study population, for a prevalence of 2.5 per 10,000 children, along with 466 children with AHO. A total of 6% of children with AHO had DVT, and 12.1% of all children with DVT had AHO. The researchers then compared the demographics, laboratory parameters, and treatment in three cohorts: the 196 children with DVT without AHO, 28 with both AHO and DVT, and 438 with AHO without DVT.
Through this analysis, they came up with a list of risk factors warranting early screening ultrasound in children suspected of having AHO:
- An initial C-reactive protein level above 8 mg/dL, which was present in all 28 dual diagnosis children.
- ICU admission, which occurred in 19 of 28 (68%) children.
- A severity of illness score of at least 7 on a 10-point scale during the first several days in the hospital, present in 27 of the 28 children. The severity of illness scale was developed and validated by Dr. Copley and coworkers (J Pediatr Orthop. 2016 Oct 12. doi: 10.1097/BPO.0000000000000879).
- Bacteremia in the initial blood culture, present in 23 of 28 patients (82%).
- Just under 90% of the children with AHO and DVT had methicillin-resistant S. aureus, compared with 20% of those with AHO without DVT.
- Septic pulmonary emboli visualized on chest x-ray, a complication that occurred in 64% of the dual diagnosis group versus just 1% of patients with DVT without AHO.
- A band percentage of white blood cells greater than 1.5%, present in 86% of children with AHO and DVT.
More than 90% of children with AHO and DVT underwent surgery, with a mean of 2.7 surgeries per child, in contrast to the group with AHO without DVT, 55% of whom had surgery, with a mean of 0.7 surgeries per child.
Of note, there was no significant difference in the occurrence of pulmonary embolism between children with DVT without AHO versus those with DVT and AHO, with a rate of about 10% in both groups.
Dr. Copley reported having no relevant financial conflicts.
REPORTING FROM ESPID 2018
Key clinical point: Osteomyelitis patients with deep vein thrombosis are much sicker than those without DVT.
Major finding:
Study details: This was a retrospective study of the medical records of more than 900,000 Texas children.
Disclosures: Dr. Copley reported having no relevant financial conflicts.
New look at ATLAS suggests rivaroxaban may still have role in ACS
In a new analysis comparing only clinically similar outcomes in patients with acute coronary syndrome, the addition of rivaroxaban to standard antiplatelet therapy resulted in 115 fewer fatal or irreversible ischemic events per 10,000 patient-years than placebo, at the expense of only 10 additional fatal or seriously harmful events.
This new interpretation of the ATLAS ACS 2-TIMI 51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction-51) suggests that the factor Xa inhibitor may still carve out a place for itself in ACS therapy, despite Food and Drug Administration rejections for this indication.
Not only did the survival benefit of rivaroxaban appear early in postevent treatment, it continued to protect patients over time, C. Michael Gibson, MD, and colleagues reported in the Journal of the American College of Cardiology.
“Time-to-event analysis demonstrated that the risk of fatal or irreversible harm remained low and constant over time, whereas reduction in fatal or irreversible ischemic events expanded,” wrote Dr. Gibson, professor of medicine at Beth Israel Deaconess Medical Center, Boston, and his coinvestigators. “By 720 days, a net of 142 fatal or irreversible events would have been prevented by 2.5-mg oral doses twice per day of rivaroxaban. Additional time-to-event sensitivity analyses demonstrated similar results, even when TIMI major bleeding was included as a fatal or irreversible event.”
In conducting the new analysis, Dr. Gibson and his team argued that the original interpretation of the results of ATLAS ACS 2-TIMI 51 lumped both fatal and nonfatal events together in composite endpoints, resulting in an inaccurate real-life picture of rivaroxaban’s therapeutic potential. “All types of events [were] weighted equally; for example, reversible nonintracranial hemorrhage, nonfatal bleeds that can be managed with supportive care, are weighted equally with death and disabling stroke. Second, stroke can be either hemorrhagic or ischemic, and the relative contributions of hemorrhagic or ischemic stroke may not be appropriately assigned to risk-versus-benefit categories in many analyses.”
The net result was that, while rivaroxaban did reduce the risk of the composite endpoint (cardiovascular death, MI, or stroke), the 1.7% absolute difference in cardiovascular mortality was almost completely offset by a 1.3% increase in major bleeding. However, most of those bleeds were reversible and nonfatal, associated with a drop in hemoglobin and/or blood transfusion. The drug did not increase the risk of fatal bleeding.
Giving equal statistical weight to clinically equal events provides a clearer focus, the investigators said.
“In this form of analysis, only fatal or irreversible events were included so that benefit and seriously harmful events of similar clinical impact were compared,” they wrote. “This is particularly important when the endpoints and analyses do not include measurements of subjective clinical impact such as utility measurements or preference weights. This approach also uses risk differences rather than relative measurements such as hazard ratios, so the number of events prevented and caused are clearly distinguished.”
ATLAS comprised more than 15,000 patients with ST-segment elevation MI, non-STEMI, or unstable angina. They were randomized to either rivaroxaban 2.5 mg orally twice per day, 5 mg orally twice per day, or to placebo, in addition to standard of care, which included low-dose aspirin. Patients were stratified by the optional use of clopidogrel/ticlopidine.
Dr. Gibson and his team reanalyzed the data by comparing outcomes they judged as having a similar clinical impact: fatal and irreversible cardiovascular death, MI, and ischemic stroke. They also assessed all bleeding, TIMI life-threatening bleeding, and TIMI major bleeding.
In this analysis, the 2.5-mg dose was associated with 115 fewer fatal or irreversible ischemic deaths per 10,000 patient-years of exposure than placebo (548 vs. 663 nonbleeding cardiovascular deaths, MIs, or ischemic strokes).
However, the same dose was also associated with 10 more excessive, fatal, or irreversibly serious harmful events, compared with placebo per 10,000 patient years (33 fatal bleeds or intracranial hemorrhage vs. 23 for placebo).
“Considered together, there would be 105 fatal or irreversible events prevented per 10,000 patient-years of exposure to 2.5 mg of rivaroxaban taken orally twice a day, compared with placebo. An alternate interpretation of the data is that there would be 11 [10 of 115] fatal or irreversible ischemic events prevented for each fatal or irreversible harmful event caused,” Dr. Gibson and his colleagues wrote.
The benefit held when the outcomes were individually reckoned as well. If periprocedural MIs were excluded, rivaroxaban would still prevent 115 fatal or irreversible ischemic events. If only nonbleeding cardiovascular death or ischemic strokes were included, then 90 fatal or irreversible events would be prevented. And if only nonbleeding cardiovascular death was included, then 95 events would be prevented per 10,000 patient-years of exposure in the group taking rivaroxaban 2.5 mg twice daily.
“In all cases, the fatal or irreversible events prevented are 9-11 times the fatal or irreversible seriously harmful events caused,” the investigators said.
ATLAS ACS 2-TIMI 51 was supported by Johnson & Johnson and Bayer Healthcare. Dr. Gibson has received institutional funding, grants, and honoraria from those companies and from Portola Pharmaceuticals.
SOURCE: Gibson CM et al. J Am Coll Cardiol. 2018;72:129-36.
Balancing the risks and benefits of anticoagulation therapy after an acute coronary event leaves physicians on the horns of a dilemma. How do we choose the most effective and the least harmful antiplatelet and/or antithrombotic strategy?
To support decision making, a careful and thoughtful interpretation of the existing evidence is essential, with an explicit focus on the risk-versus-benefit assessment. Even the most well-designed trial can contain ambiguities, the study investigators noted, and ATLAS was one of these.
The reanalysis of ATLAS by Gibson et al. is an attempt to cut through some of these ambiguities. By comparing only serious or fatal outcomes, the investigators aimed to bring clinically meaningful insight into the picture. Such a way of reporting provides readers with an extra piece of information to assist in deciding whether a treatment should be used.
The analysis isn’t perfect. It doesn’t include less-serious bleeding events, which still may contribute to a poor prognosis. And the analysis didn’t take into about ischemia-driven revascularizations.
Although commonly successful, repeat revascularizations are not free from complications, which may include occurrence of large infarctions, stroke, and serious bleeding.
Nevertheless, the study enhances our understanding of how to best employ low-dose rivaroxaban therapy in addition to antiplatelet agents.
Although we are getting closer to therapy optimization, the final word regarding the use of low-dose rivaroxaban and other agents for secondary prevention of cardiovascular diseases has not yet been said. This is primarily because of substantial variation in the magnitude of the risks and benefits across a population. Comprehensive, individualized profiling of the patients with respect to their ischemic and bleeding risks is crucial to further improve acute coronary syndrome–related outcomes.
Eugenia Nikolsy, MD, PhD, and Freek Verheugt, MD, made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;72:137-40). Dr. Nikolsy is director of clinical research in invasive cardiology at Rambam Academic Hospital, Haifa, Israel. Dr. Verheugt is a professor of cardiology at the Heart-Lung Centre at University Medical Centre, Nijmegen, the Netherlands.
Balancing the risks and benefits of anticoagulation therapy after an acute coronary event leaves physicians on the horns of a dilemma. How do we choose the most effective and the least harmful antiplatelet and/or antithrombotic strategy?
To support decision making, a careful and thoughtful interpretation of the existing evidence is essential, with an explicit focus on the risk-versus-benefit assessment. Even the most well-designed trial can contain ambiguities, the study investigators noted, and ATLAS was one of these.
The reanalysis of ATLAS by Gibson et al. is an attempt to cut through some of these ambiguities. By comparing only serious or fatal outcomes, the investigators aimed to bring clinically meaningful insight into the picture. Such a way of reporting provides readers with an extra piece of information to assist in deciding whether a treatment should be used.
The analysis isn’t perfect. It doesn’t include less-serious bleeding events, which still may contribute to a poor prognosis. And the analysis didn’t take into about ischemia-driven revascularizations.
Although commonly successful, repeat revascularizations are not free from complications, which may include occurrence of large infarctions, stroke, and serious bleeding.
Nevertheless, the study enhances our understanding of how to best employ low-dose rivaroxaban therapy in addition to antiplatelet agents.
Although we are getting closer to therapy optimization, the final word regarding the use of low-dose rivaroxaban and other agents for secondary prevention of cardiovascular diseases has not yet been said. This is primarily because of substantial variation in the magnitude of the risks and benefits across a population. Comprehensive, individualized profiling of the patients with respect to their ischemic and bleeding risks is crucial to further improve acute coronary syndrome–related outcomes.
Eugenia Nikolsy, MD, PhD, and Freek Verheugt, MD, made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;72:137-40). Dr. Nikolsy is director of clinical research in invasive cardiology at Rambam Academic Hospital, Haifa, Israel. Dr. Verheugt is a professor of cardiology at the Heart-Lung Centre at University Medical Centre, Nijmegen, the Netherlands.
Balancing the risks and benefits of anticoagulation therapy after an acute coronary event leaves physicians on the horns of a dilemma. How do we choose the most effective and the least harmful antiplatelet and/or antithrombotic strategy?
To support decision making, a careful and thoughtful interpretation of the existing evidence is essential, with an explicit focus on the risk-versus-benefit assessment. Even the most well-designed trial can contain ambiguities, the study investigators noted, and ATLAS was one of these.
The reanalysis of ATLAS by Gibson et al. is an attempt to cut through some of these ambiguities. By comparing only serious or fatal outcomes, the investigators aimed to bring clinically meaningful insight into the picture. Such a way of reporting provides readers with an extra piece of information to assist in deciding whether a treatment should be used.
The analysis isn’t perfect. It doesn’t include less-serious bleeding events, which still may contribute to a poor prognosis. And the analysis didn’t take into about ischemia-driven revascularizations.
Although commonly successful, repeat revascularizations are not free from complications, which may include occurrence of large infarctions, stroke, and serious bleeding.
Nevertheless, the study enhances our understanding of how to best employ low-dose rivaroxaban therapy in addition to antiplatelet agents.
Although we are getting closer to therapy optimization, the final word regarding the use of low-dose rivaroxaban and other agents for secondary prevention of cardiovascular diseases has not yet been said. This is primarily because of substantial variation in the magnitude of the risks and benefits across a population. Comprehensive, individualized profiling of the patients with respect to their ischemic and bleeding risks is crucial to further improve acute coronary syndrome–related outcomes.
Eugenia Nikolsy, MD, PhD, and Freek Verheugt, MD, made these comments in an accompanying editorial (J Am Coll Cardiol. 2018;72:137-40). Dr. Nikolsy is director of clinical research in invasive cardiology at Rambam Academic Hospital, Haifa, Israel. Dr. Verheugt is a professor of cardiology at the Heart-Lung Centre at University Medical Centre, Nijmegen, the Netherlands.
In a new analysis comparing only clinically similar outcomes in patients with acute coronary syndrome, the addition of rivaroxaban to standard antiplatelet therapy resulted in 115 fewer fatal or irreversible ischemic events per 10,000 patient-years than placebo, at the expense of only 10 additional fatal or seriously harmful events.
This new interpretation of the ATLAS ACS 2-TIMI 51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction-51) suggests that the factor Xa inhibitor may still carve out a place for itself in ACS therapy, despite Food and Drug Administration rejections for this indication.
Not only did the survival benefit of rivaroxaban appear early in postevent treatment, it continued to protect patients over time, C. Michael Gibson, MD, and colleagues reported in the Journal of the American College of Cardiology.
“Time-to-event analysis demonstrated that the risk of fatal or irreversible harm remained low and constant over time, whereas reduction in fatal or irreversible ischemic events expanded,” wrote Dr. Gibson, professor of medicine at Beth Israel Deaconess Medical Center, Boston, and his coinvestigators. “By 720 days, a net of 142 fatal or irreversible events would have been prevented by 2.5-mg oral doses twice per day of rivaroxaban. Additional time-to-event sensitivity analyses demonstrated similar results, even when TIMI major bleeding was included as a fatal or irreversible event.”
In conducting the new analysis, Dr. Gibson and his team argued that the original interpretation of the results of ATLAS ACS 2-TIMI 51 lumped both fatal and nonfatal events together in composite endpoints, resulting in an inaccurate real-life picture of rivaroxaban’s therapeutic potential. “All types of events [were] weighted equally; for example, reversible nonintracranial hemorrhage, nonfatal bleeds that can be managed with supportive care, are weighted equally with death and disabling stroke. Second, stroke can be either hemorrhagic or ischemic, and the relative contributions of hemorrhagic or ischemic stroke may not be appropriately assigned to risk-versus-benefit categories in many analyses.”
The net result was that, while rivaroxaban did reduce the risk of the composite endpoint (cardiovascular death, MI, or stroke), the 1.7% absolute difference in cardiovascular mortality was almost completely offset by a 1.3% increase in major bleeding. However, most of those bleeds were reversible and nonfatal, associated with a drop in hemoglobin and/or blood transfusion. The drug did not increase the risk of fatal bleeding.
Giving equal statistical weight to clinically equal events provides a clearer focus, the investigators said.
“In this form of analysis, only fatal or irreversible events were included so that benefit and seriously harmful events of similar clinical impact were compared,” they wrote. “This is particularly important when the endpoints and analyses do not include measurements of subjective clinical impact such as utility measurements or preference weights. This approach also uses risk differences rather than relative measurements such as hazard ratios, so the number of events prevented and caused are clearly distinguished.”
ATLAS comprised more than 15,000 patients with ST-segment elevation MI, non-STEMI, or unstable angina. They were randomized to either rivaroxaban 2.5 mg orally twice per day, 5 mg orally twice per day, or to placebo, in addition to standard of care, which included low-dose aspirin. Patients were stratified by the optional use of clopidogrel/ticlopidine.
Dr. Gibson and his team reanalyzed the data by comparing outcomes they judged as having a similar clinical impact: fatal and irreversible cardiovascular death, MI, and ischemic stroke. They also assessed all bleeding, TIMI life-threatening bleeding, and TIMI major bleeding.
In this analysis, the 2.5-mg dose was associated with 115 fewer fatal or irreversible ischemic deaths per 10,000 patient-years of exposure than placebo (548 vs. 663 nonbleeding cardiovascular deaths, MIs, or ischemic strokes).
However, the same dose was also associated with 10 more excessive, fatal, or irreversibly serious harmful events, compared with placebo per 10,000 patient years (33 fatal bleeds or intracranial hemorrhage vs. 23 for placebo).
“Considered together, there would be 105 fatal or irreversible events prevented per 10,000 patient-years of exposure to 2.5 mg of rivaroxaban taken orally twice a day, compared with placebo. An alternate interpretation of the data is that there would be 11 [10 of 115] fatal or irreversible ischemic events prevented for each fatal or irreversible harmful event caused,” Dr. Gibson and his colleagues wrote.
The benefit held when the outcomes were individually reckoned as well. If periprocedural MIs were excluded, rivaroxaban would still prevent 115 fatal or irreversible ischemic events. If only nonbleeding cardiovascular death or ischemic strokes were included, then 90 fatal or irreversible events would be prevented. And if only nonbleeding cardiovascular death was included, then 95 events would be prevented per 10,000 patient-years of exposure in the group taking rivaroxaban 2.5 mg twice daily.
“In all cases, the fatal or irreversible events prevented are 9-11 times the fatal or irreversible seriously harmful events caused,” the investigators said.
ATLAS ACS 2-TIMI 51 was supported by Johnson & Johnson and Bayer Healthcare. Dr. Gibson has received institutional funding, grants, and honoraria from those companies and from Portola Pharmaceuticals.
SOURCE: Gibson CM et al. J Am Coll Cardiol. 2018;72:129-36.
In a new analysis comparing only clinically similar outcomes in patients with acute coronary syndrome, the addition of rivaroxaban to standard antiplatelet therapy resulted in 115 fewer fatal or irreversible ischemic events per 10,000 patient-years than placebo, at the expense of only 10 additional fatal or seriously harmful events.
This new interpretation of the ATLAS ACS 2-TIMI 51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction-51) suggests that the factor Xa inhibitor may still carve out a place for itself in ACS therapy, despite Food and Drug Administration rejections for this indication.
Not only did the survival benefit of rivaroxaban appear early in postevent treatment, it continued to protect patients over time, C. Michael Gibson, MD, and colleagues reported in the Journal of the American College of Cardiology.
“Time-to-event analysis demonstrated that the risk of fatal or irreversible harm remained low and constant over time, whereas reduction in fatal or irreversible ischemic events expanded,” wrote Dr. Gibson, professor of medicine at Beth Israel Deaconess Medical Center, Boston, and his coinvestigators. “By 720 days, a net of 142 fatal or irreversible events would have been prevented by 2.5-mg oral doses twice per day of rivaroxaban. Additional time-to-event sensitivity analyses demonstrated similar results, even when TIMI major bleeding was included as a fatal or irreversible event.”
In conducting the new analysis, Dr. Gibson and his team argued that the original interpretation of the results of ATLAS ACS 2-TIMI 51 lumped both fatal and nonfatal events together in composite endpoints, resulting in an inaccurate real-life picture of rivaroxaban’s therapeutic potential. “All types of events [were] weighted equally; for example, reversible nonintracranial hemorrhage, nonfatal bleeds that can be managed with supportive care, are weighted equally with death and disabling stroke. Second, stroke can be either hemorrhagic or ischemic, and the relative contributions of hemorrhagic or ischemic stroke may not be appropriately assigned to risk-versus-benefit categories in many analyses.”
The net result was that, while rivaroxaban did reduce the risk of the composite endpoint (cardiovascular death, MI, or stroke), the 1.7% absolute difference in cardiovascular mortality was almost completely offset by a 1.3% increase in major bleeding. However, most of those bleeds were reversible and nonfatal, associated with a drop in hemoglobin and/or blood transfusion. The drug did not increase the risk of fatal bleeding.
Giving equal statistical weight to clinically equal events provides a clearer focus, the investigators said.
“In this form of analysis, only fatal or irreversible events were included so that benefit and seriously harmful events of similar clinical impact were compared,” they wrote. “This is particularly important when the endpoints and analyses do not include measurements of subjective clinical impact such as utility measurements or preference weights. This approach also uses risk differences rather than relative measurements such as hazard ratios, so the number of events prevented and caused are clearly distinguished.”
ATLAS comprised more than 15,000 patients with ST-segment elevation MI, non-STEMI, or unstable angina. They were randomized to either rivaroxaban 2.5 mg orally twice per day, 5 mg orally twice per day, or to placebo, in addition to standard of care, which included low-dose aspirin. Patients were stratified by the optional use of clopidogrel/ticlopidine.
Dr. Gibson and his team reanalyzed the data by comparing outcomes they judged as having a similar clinical impact: fatal and irreversible cardiovascular death, MI, and ischemic stroke. They also assessed all bleeding, TIMI life-threatening bleeding, and TIMI major bleeding.
In this analysis, the 2.5-mg dose was associated with 115 fewer fatal or irreversible ischemic deaths per 10,000 patient-years of exposure than placebo (548 vs. 663 nonbleeding cardiovascular deaths, MIs, or ischemic strokes).
However, the same dose was also associated with 10 more excessive, fatal, or irreversibly serious harmful events, compared with placebo per 10,000 patient years (33 fatal bleeds or intracranial hemorrhage vs. 23 for placebo).
“Considered together, there would be 105 fatal or irreversible events prevented per 10,000 patient-years of exposure to 2.5 mg of rivaroxaban taken orally twice a day, compared with placebo. An alternate interpretation of the data is that there would be 11 [10 of 115] fatal or irreversible ischemic events prevented for each fatal or irreversible harmful event caused,” Dr. Gibson and his colleagues wrote.
The benefit held when the outcomes were individually reckoned as well. If periprocedural MIs were excluded, rivaroxaban would still prevent 115 fatal or irreversible ischemic events. If only nonbleeding cardiovascular death or ischemic strokes were included, then 90 fatal or irreversible events would be prevented. And if only nonbleeding cardiovascular death was included, then 95 events would be prevented per 10,000 patient-years of exposure in the group taking rivaroxaban 2.5 mg twice daily.
“In all cases, the fatal or irreversible events prevented are 9-11 times the fatal or irreversible seriously harmful events caused,” the investigators said.
ATLAS ACS 2-TIMI 51 was supported by Johnson & Johnson and Bayer Healthcare. Dr. Gibson has received institutional funding, grants, and honoraria from those companies and from Portola Pharmaceuticals.
SOURCE: Gibson CM et al. J Am Coll Cardiol. 2018;72:129-36.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Vitreous Hemorrhage in the Setting of a Vascular Loop
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Design limitations may have compromised DVT intervention trial
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
EXPERT ANALYSIS FROM THE 2018 CRT MEETING
Impaired kidney function no problem for dabigatran reversal
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
REPORTING FROM ACC 18
Key clinical point: Renal function had no impact on idarucizumab’s efficacy for dabigatran reversal.
Major finding: Complete dabigatran reversal occurred in 98% of patients with severe renal dysfunction who received idarucizumab.
Study details: Post hoc analysis of data from RE-VERSE AD, idarucizumab’s pivotal trial with 503 patients.
Disclosures: RE-VERSE AD was funded by Boehringer Ingelheim, the company that markets idarucizumab (Praxbind) and dabigatran (Pradaxa). Dr. Eikelboom has been a consultant to and has received research support from Boehringer Ingelheim, as well as from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, and Pfizer.
Source: Eikelboom JW et al. ACC 18, Abstract 1231M-11.