User login
Pronounced racial differences in HBsAg loss after stopping nucleos(t)ide
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
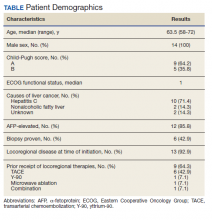
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
Vanquishing hepatitis C: A remarkable success story
One of the most remarkable stories in medicine must be the relatively brief 25 years between the discovery of the hepatitis C virus (HCV) in 1989 to its eventual cure in 2014.
HCV afflicted over 5 million Americans and was the cause of death in approximately 10,000 patients annually, the leading indication for liver transplantation, and the leading risk factor for hepatocellular carcinoma, clearly signaling it as one of the era’s major public health villains. Within that span of time, it is the work beginning in the mid-1990s until today that perhaps best defines the race for the HCV “cure.”
In the early to mid-1990s, polymerase chain reaction techniques were just becoming commonplace for HCV diagnosis, whereas HCV genotypes were emerging as major factors determining response to interferon therapy. The sustained viral response (SVR) rates were mired at around 6%-12% for a 24- to 48-week course of three-times-weekly injection therapy. Severe side effects were common and there was a relatively high relapse rate, even in patients who responded to treatment.
By 1996, the addition of ribavirin to the interferon treatment was associated with a modest but significant improvement in SVR rates to above 20%. And by 2000, the use of pegylated interferon – allowing once-weekly injection therapy – along with ribavirin, improved SVR rates to above 50% for the first time. The therapy was still poorly tolerated but was associated with better compliance.
The real breakthrough in therapy came in the early 2000s with the discovery and availability of HCV protease inhibitors: telaprevir and boceprevir. These agents could induce a more rapid decline in viral replication than interferon but could not be administered alone owing to the rapid emergence of resistant HCV variants. Therefore, these agents were administered with interferon and ribavirin as a three-drug cocktail to take advantage of interferon to prevent emergence of resistant variants. Although SVR rates improved substantially to around 75%, adverse events also increased and limited its usefulness in patients with more advanced liver disease, precisely those who were most in need of better therapies.
Nonetheless, the incredible advances in understanding the replication machinery of HCV that led to the discovery of the protease inhibitors in turn led to further elucidation and unlocking of three additional classes of HCV protein targets and inhibitors: NS5A complex inhibitors (e.g., ledipasvir), the NS5B nonnucleoside inhibitors (e.g., dasabuvir), and NS5B nucleoside inhibitors (e.g., sofosbuvir). It quickly became apparent that the use of combinations of these direct-acting antivirals (DAAs) could limit emergence of resistant variants while also providing rapid and profound viral suppression. Because HCV required viral replication to persist in the hepatocyte, it became possible to induce HCV eradication, and thus cure, with combinations of DAAs.
In addition, investigators soon learned that the duration of therapy no longer needed to be the generally accepted 24-48 weeks for SVR, but instead could be reduced eventually to 8-12 weeks. This shortened treatment duration allowed for more rapid testing of new agents and combinations, and the field took a rapid step forward between 2011 and 2017. HCV cure rates rose to 90%-95%.
The competition for Food and Drug Administration approval of new agents among several pharmaceutical companies also meant that the time-honored process of issuing treatment guidelines every 3-5 years by societies would not be adequate. Therefore, in 2013, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America joined forces to establish more nimble and responsive online HCV guidance. This important resource debuted in January 2014 just as the FDA approved the first DAA therapies.
The high cost initially associated with many of these new therapies (up to $1,000 per pill) significantly limited uptake owing to insurance and health plan cost factors. Early on, the cost was also analyzed by price per cure, seemingly to justify the high cost by the high cure rate. However, advocacy and negotiations ultimately led to marked reductions in the cost of a course of therapy (with some therapies at $225 per pill), thus making these treatments now widely available.
By 2020, the HCV field has shifted from therapeutic development to improving the care cascade by enhanced identification and testing of unsuspected but HCV infected individuals. This is our current challenge.
Moving toward noninvasive tests
While curative therapy has revolutionized HCV management, innovation in diagnostics eliminated a significant barrier in access to therapy: the liver biopsy.
Staging, or accurately identifying advanced fibrosis in persons infected with HCV, is essential for long-term follow-up. The presence of advanced disease affects drug choices, especially before the approval of all-oral therapy. Historically, a liver biopsy was obligatory before treatment. Invasive with a significant risk for complications, this requirement effectively prevented treatment in those who were unwilling to undergo the procedure and deterred those at risk from even being tested.
Over the past 25 years, numerous methods to noninvasively assess for liver fibrosis have been used. Serum biomarkers can be either indirect (based on routine tests) or direct (reflecting components of the extracellular matrix). Although highly available, they are only moderately useful for identifying advanced fibrosis and thus cannot replace liver biopsy in the care cascade. The technique of elastography dates back to the 1980s, though the role of vibration-controlled transient liver elastography in the assessment of hepatic fibrosis in patients with HCV was not recognized until around 2005 and it was not commonly used for nearly another decade.
Yet, a paradigm shift in the care cascade occurred with the release of the AASLD/IDSA guidance document in 2014. For the first time in the United States, noninvasive tests were recommended as first-line testing for the assessment of advanced fibrosis. Prior guidelines specifically stated that although noninvasive tests might be useful, they “should not replace the liver biopsy in routine clinical practice.” Current guidelines recommend combining elastography with serum biomarkers and considering biopsy only in patients with discordant results if the biopsy would affect clinical decision-making.
The last frontier
Curative therapy has also allowed the unthinkable: willingly exposing patients to the virus through donor-positive/recipient-negative solid organ transplant. Traditionally, an HCV-infected donor would be considered only for an HCV-positive recipient; however, with effective DAA therapy, the number of HCV actively infected patients in need of transplant has dwindled.
Unfortunately as a consequence of the opioid epidemic, the HCV-exposed donor population has blossomed. Given that HCV therapy is near universally curative, using organs from HCV-viremic donors can greatly expand the organ transplantation pool. Small studies[1-5] have demonstrated the safety and efficacy of this approach, both in HCV-positive liver donors as well as in other solid organs.
A disease pegged for elimination
In the past 25 years, HCV has evolved from non-A, non-B hepatitis into a disease pegged for elimination. This is a direct reflection of improved therapeutics with highly effective DAAs. Yet, without improved diagnostics, we would be unable to navigate patients through the clinical care cascade. These incredible strides in diagnostics and therapeutics allow us to push the cutting edge through iatrogenic infection of organ recipients, while recognizing that the largest hurdle to elimination remains in finding those who are chronically infected. Ultimately, the crux of elimination remains unchanged over the past 25 years and resides in screening and diagnosis with effective linkage to care.
Donald M. Jensen, MD, is a professor of medicine at Rush University Medical Center, Chicago. He was previously the director of the Center for Liver Disease at the University of Chicago until 2015. His research interest has been in newer HCV therapies. He recently received the Distinguished Service Award from the AASLD for his many contributions to the field.
Nancy S. Reau, MD, is chief of the hepatology section at Rush University Medical Center and a regular contributor to Medscape. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the AASLD and the IDSA, as well as educational chair for the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels.
References
Woolley AE et al. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606-17.
Franco A et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: A prospective study. Transpl Int. 2019;32:710-6.
Goldberg DS et al. Transplanting HCV-infected kidneys into uninfected recipients. N Engl J Med. 2017;377:1105.
Kwong AJ et al. Liver transplantation for hepatitis C virus (HCV) nonviremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380-7.
Bethea E et al. Immediate administration of antiviral therapy after transplantation of hepatitis C–infected livers into uninfected recipients: Implications for therapeutic planning. Am J Transplant. 2020;20:1619-28.
This article first appeared on Medscape.com.
One of the most remarkable stories in medicine must be the relatively brief 25 years between the discovery of the hepatitis C virus (HCV) in 1989 to its eventual cure in 2014.
HCV afflicted over 5 million Americans and was the cause of death in approximately 10,000 patients annually, the leading indication for liver transplantation, and the leading risk factor for hepatocellular carcinoma, clearly signaling it as one of the era’s major public health villains. Within that span of time, it is the work beginning in the mid-1990s until today that perhaps best defines the race for the HCV “cure.”
In the early to mid-1990s, polymerase chain reaction techniques were just becoming commonplace for HCV diagnosis, whereas HCV genotypes were emerging as major factors determining response to interferon therapy. The sustained viral response (SVR) rates were mired at around 6%-12% for a 24- to 48-week course of three-times-weekly injection therapy. Severe side effects were common and there was a relatively high relapse rate, even in patients who responded to treatment.
By 1996, the addition of ribavirin to the interferon treatment was associated with a modest but significant improvement in SVR rates to above 20%. And by 2000, the use of pegylated interferon – allowing once-weekly injection therapy – along with ribavirin, improved SVR rates to above 50% for the first time. The therapy was still poorly tolerated but was associated with better compliance.
The real breakthrough in therapy came in the early 2000s with the discovery and availability of HCV protease inhibitors: telaprevir and boceprevir. These agents could induce a more rapid decline in viral replication than interferon but could not be administered alone owing to the rapid emergence of resistant HCV variants. Therefore, these agents were administered with interferon and ribavirin as a three-drug cocktail to take advantage of interferon to prevent emergence of resistant variants. Although SVR rates improved substantially to around 75%, adverse events also increased and limited its usefulness in patients with more advanced liver disease, precisely those who were most in need of better therapies.
Nonetheless, the incredible advances in understanding the replication machinery of HCV that led to the discovery of the protease inhibitors in turn led to further elucidation and unlocking of three additional classes of HCV protein targets and inhibitors: NS5A complex inhibitors (e.g., ledipasvir), the NS5B nonnucleoside inhibitors (e.g., dasabuvir), and NS5B nucleoside inhibitors (e.g., sofosbuvir). It quickly became apparent that the use of combinations of these direct-acting antivirals (DAAs) could limit emergence of resistant variants while also providing rapid and profound viral suppression. Because HCV required viral replication to persist in the hepatocyte, it became possible to induce HCV eradication, and thus cure, with combinations of DAAs.
In addition, investigators soon learned that the duration of therapy no longer needed to be the generally accepted 24-48 weeks for SVR, but instead could be reduced eventually to 8-12 weeks. This shortened treatment duration allowed for more rapid testing of new agents and combinations, and the field took a rapid step forward between 2011 and 2017. HCV cure rates rose to 90%-95%.
The competition for Food and Drug Administration approval of new agents among several pharmaceutical companies also meant that the time-honored process of issuing treatment guidelines every 3-5 years by societies would not be adequate. Therefore, in 2013, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America joined forces to establish more nimble and responsive online HCV guidance. This important resource debuted in January 2014 just as the FDA approved the first DAA therapies.
The high cost initially associated with many of these new therapies (up to $1,000 per pill) significantly limited uptake owing to insurance and health plan cost factors. Early on, the cost was also analyzed by price per cure, seemingly to justify the high cost by the high cure rate. However, advocacy and negotiations ultimately led to marked reductions in the cost of a course of therapy (with some therapies at $225 per pill), thus making these treatments now widely available.
By 2020, the HCV field has shifted from therapeutic development to improving the care cascade by enhanced identification and testing of unsuspected but HCV infected individuals. This is our current challenge.
Moving toward noninvasive tests
While curative therapy has revolutionized HCV management, innovation in diagnostics eliminated a significant barrier in access to therapy: the liver biopsy.
Staging, or accurately identifying advanced fibrosis in persons infected with HCV, is essential for long-term follow-up. The presence of advanced disease affects drug choices, especially before the approval of all-oral therapy. Historically, a liver biopsy was obligatory before treatment. Invasive with a significant risk for complications, this requirement effectively prevented treatment in those who were unwilling to undergo the procedure and deterred those at risk from even being tested.
Over the past 25 years, numerous methods to noninvasively assess for liver fibrosis have been used. Serum biomarkers can be either indirect (based on routine tests) or direct (reflecting components of the extracellular matrix). Although highly available, they are only moderately useful for identifying advanced fibrosis and thus cannot replace liver biopsy in the care cascade. The technique of elastography dates back to the 1980s, though the role of vibration-controlled transient liver elastography in the assessment of hepatic fibrosis in patients with HCV was not recognized until around 2005 and it was not commonly used for nearly another decade.
Yet, a paradigm shift in the care cascade occurred with the release of the AASLD/IDSA guidance document in 2014. For the first time in the United States, noninvasive tests were recommended as first-line testing for the assessment of advanced fibrosis. Prior guidelines specifically stated that although noninvasive tests might be useful, they “should not replace the liver biopsy in routine clinical practice.” Current guidelines recommend combining elastography with serum biomarkers and considering biopsy only in patients with discordant results if the biopsy would affect clinical decision-making.
The last frontier
Curative therapy has also allowed the unthinkable: willingly exposing patients to the virus through donor-positive/recipient-negative solid organ transplant. Traditionally, an HCV-infected donor would be considered only for an HCV-positive recipient; however, with effective DAA therapy, the number of HCV actively infected patients in need of transplant has dwindled.
Unfortunately as a consequence of the opioid epidemic, the HCV-exposed donor population has blossomed. Given that HCV therapy is near universally curative, using organs from HCV-viremic donors can greatly expand the organ transplantation pool. Small studies[1-5] have demonstrated the safety and efficacy of this approach, both in HCV-positive liver donors as well as in other solid organs.
A disease pegged for elimination
In the past 25 years, HCV has evolved from non-A, non-B hepatitis into a disease pegged for elimination. This is a direct reflection of improved therapeutics with highly effective DAAs. Yet, without improved diagnostics, we would be unable to navigate patients through the clinical care cascade. These incredible strides in diagnostics and therapeutics allow us to push the cutting edge through iatrogenic infection of organ recipients, while recognizing that the largest hurdle to elimination remains in finding those who are chronically infected. Ultimately, the crux of elimination remains unchanged over the past 25 years and resides in screening and diagnosis with effective linkage to care.
Donald M. Jensen, MD, is a professor of medicine at Rush University Medical Center, Chicago. He was previously the director of the Center for Liver Disease at the University of Chicago until 2015. His research interest has been in newer HCV therapies. He recently received the Distinguished Service Award from the AASLD for his many contributions to the field.
Nancy S. Reau, MD, is chief of the hepatology section at Rush University Medical Center and a regular contributor to Medscape. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the AASLD and the IDSA, as well as educational chair for the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels.
References
Woolley AE et al. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606-17.
Franco A et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: A prospective study. Transpl Int. 2019;32:710-6.
Goldberg DS et al. Transplanting HCV-infected kidneys into uninfected recipients. N Engl J Med. 2017;377:1105.
Kwong AJ et al. Liver transplantation for hepatitis C virus (HCV) nonviremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380-7.
Bethea E et al. Immediate administration of antiviral therapy after transplantation of hepatitis C–infected livers into uninfected recipients: Implications for therapeutic planning. Am J Transplant. 2020;20:1619-28.
This article first appeared on Medscape.com.
One of the most remarkable stories in medicine must be the relatively brief 25 years between the discovery of the hepatitis C virus (HCV) in 1989 to its eventual cure in 2014.
HCV afflicted over 5 million Americans and was the cause of death in approximately 10,000 patients annually, the leading indication for liver transplantation, and the leading risk factor for hepatocellular carcinoma, clearly signaling it as one of the era’s major public health villains. Within that span of time, it is the work beginning in the mid-1990s until today that perhaps best defines the race for the HCV “cure.”
In the early to mid-1990s, polymerase chain reaction techniques were just becoming commonplace for HCV diagnosis, whereas HCV genotypes were emerging as major factors determining response to interferon therapy. The sustained viral response (SVR) rates were mired at around 6%-12% for a 24- to 48-week course of three-times-weekly injection therapy. Severe side effects were common and there was a relatively high relapse rate, even in patients who responded to treatment.
By 1996, the addition of ribavirin to the interferon treatment was associated with a modest but significant improvement in SVR rates to above 20%. And by 2000, the use of pegylated interferon – allowing once-weekly injection therapy – along with ribavirin, improved SVR rates to above 50% for the first time. The therapy was still poorly tolerated but was associated with better compliance.
The real breakthrough in therapy came in the early 2000s with the discovery and availability of HCV protease inhibitors: telaprevir and boceprevir. These agents could induce a more rapid decline in viral replication than interferon but could not be administered alone owing to the rapid emergence of resistant HCV variants. Therefore, these agents were administered with interferon and ribavirin as a three-drug cocktail to take advantage of interferon to prevent emergence of resistant variants. Although SVR rates improved substantially to around 75%, adverse events also increased and limited its usefulness in patients with more advanced liver disease, precisely those who were most in need of better therapies.
Nonetheless, the incredible advances in understanding the replication machinery of HCV that led to the discovery of the protease inhibitors in turn led to further elucidation and unlocking of three additional classes of HCV protein targets and inhibitors: NS5A complex inhibitors (e.g., ledipasvir), the NS5B nonnucleoside inhibitors (e.g., dasabuvir), and NS5B nucleoside inhibitors (e.g., sofosbuvir). It quickly became apparent that the use of combinations of these direct-acting antivirals (DAAs) could limit emergence of resistant variants while also providing rapid and profound viral suppression. Because HCV required viral replication to persist in the hepatocyte, it became possible to induce HCV eradication, and thus cure, with combinations of DAAs.
In addition, investigators soon learned that the duration of therapy no longer needed to be the generally accepted 24-48 weeks for SVR, but instead could be reduced eventually to 8-12 weeks. This shortened treatment duration allowed for more rapid testing of new agents and combinations, and the field took a rapid step forward between 2011 and 2017. HCV cure rates rose to 90%-95%.
The competition for Food and Drug Administration approval of new agents among several pharmaceutical companies also meant that the time-honored process of issuing treatment guidelines every 3-5 years by societies would not be adequate. Therefore, in 2013, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America joined forces to establish more nimble and responsive online HCV guidance. This important resource debuted in January 2014 just as the FDA approved the first DAA therapies.
The high cost initially associated with many of these new therapies (up to $1,000 per pill) significantly limited uptake owing to insurance and health plan cost factors. Early on, the cost was also analyzed by price per cure, seemingly to justify the high cost by the high cure rate. However, advocacy and negotiations ultimately led to marked reductions in the cost of a course of therapy (with some therapies at $225 per pill), thus making these treatments now widely available.
By 2020, the HCV field has shifted from therapeutic development to improving the care cascade by enhanced identification and testing of unsuspected but HCV infected individuals. This is our current challenge.
Moving toward noninvasive tests
While curative therapy has revolutionized HCV management, innovation in diagnostics eliminated a significant barrier in access to therapy: the liver biopsy.
Staging, or accurately identifying advanced fibrosis in persons infected with HCV, is essential for long-term follow-up. The presence of advanced disease affects drug choices, especially before the approval of all-oral therapy. Historically, a liver biopsy was obligatory before treatment. Invasive with a significant risk for complications, this requirement effectively prevented treatment in those who were unwilling to undergo the procedure and deterred those at risk from even being tested.
Over the past 25 years, numerous methods to noninvasively assess for liver fibrosis have been used. Serum biomarkers can be either indirect (based on routine tests) or direct (reflecting components of the extracellular matrix). Although highly available, they are only moderately useful for identifying advanced fibrosis and thus cannot replace liver biopsy in the care cascade. The technique of elastography dates back to the 1980s, though the role of vibration-controlled transient liver elastography in the assessment of hepatic fibrosis in patients with HCV was not recognized until around 2005 and it was not commonly used for nearly another decade.
Yet, a paradigm shift in the care cascade occurred with the release of the AASLD/IDSA guidance document in 2014. For the first time in the United States, noninvasive tests were recommended as first-line testing for the assessment of advanced fibrosis. Prior guidelines specifically stated that although noninvasive tests might be useful, they “should not replace the liver biopsy in routine clinical practice.” Current guidelines recommend combining elastography with serum biomarkers and considering biopsy only in patients with discordant results if the biopsy would affect clinical decision-making.
The last frontier
Curative therapy has also allowed the unthinkable: willingly exposing patients to the virus through donor-positive/recipient-negative solid organ transplant. Traditionally, an HCV-infected donor would be considered only for an HCV-positive recipient; however, with effective DAA therapy, the number of HCV actively infected patients in need of transplant has dwindled.
Unfortunately as a consequence of the opioid epidemic, the HCV-exposed donor population has blossomed. Given that HCV therapy is near universally curative, using organs from HCV-viremic donors can greatly expand the organ transplantation pool. Small studies[1-5] have demonstrated the safety and efficacy of this approach, both in HCV-positive liver donors as well as in other solid organs.
A disease pegged for elimination
In the past 25 years, HCV has evolved from non-A, non-B hepatitis into a disease pegged for elimination. This is a direct reflection of improved therapeutics with highly effective DAAs. Yet, without improved diagnostics, we would be unable to navigate patients through the clinical care cascade. These incredible strides in diagnostics and therapeutics allow us to push the cutting edge through iatrogenic infection of organ recipients, while recognizing that the largest hurdle to elimination remains in finding those who are chronically infected. Ultimately, the crux of elimination remains unchanged over the past 25 years and resides in screening and diagnosis with effective linkage to care.
Donald M. Jensen, MD, is a professor of medicine at Rush University Medical Center, Chicago. He was previously the director of the Center for Liver Disease at the University of Chicago until 2015. His research interest has been in newer HCV therapies. He recently received the Distinguished Service Award from the AASLD for his many contributions to the field.
Nancy S. Reau, MD, is chief of the hepatology section at Rush University Medical Center and a regular contributor to Medscape. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the AASLD and the IDSA, as well as educational chair for the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels.
References
Woolley AE et al. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med. 2019;380:1606-17.
Franco A et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: A prospective study. Transpl Int. 2019;32:710-6.
Goldberg DS et al. Transplanting HCV-infected kidneys into uninfected recipients. N Engl J Med. 2017;377:1105.
Kwong AJ et al. Liver transplantation for hepatitis C virus (HCV) nonviremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380-7.
Bethea E et al. Immediate administration of antiviral therapy after transplantation of hepatitis C–infected livers into uninfected recipients: Implications for therapeutic planning. Am J Transplant. 2020;20:1619-28.
This article first appeared on Medscape.com.
HCC rates slow in cities, continue to climb in rural areas
The incidence rate of hepatocellular carcinoma in urban areas of the United States began to slow in 2009, but the rate in rural areas of the nation continued to rise at a steady pace, especially among non-Hispanic Whites and Blacks, investigators have found.
Although overall hepatocellular carcinoma (HCC) incidence rates were consistently lower among people living in nonmetro (rural) versus metro (urban) areas, the average annual percentage change in urban areas began to slow from 5.3% for the period of 1995 through 2009 to 2.7% thereafter. In contrast, the average annual percentage change in rural areas remained steady at 5.7%, a disparity that remained even after adjusting for differences among subgroups, reported Christina Gainey, MD, a third-year resident in internal medicine at the University of Southern California Medical Center, Los Angeles.
“We found that there are striking urban-rural disparities in HCC incidence trends that vary by race and ethnicity, and these disparities are growing over time,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“Our study really highlights a critical public health issue that’s disproportionately affecting rural Americans. They already face considerable health inequities when it comes to access to care, health outcomes, and public health infrastructure and resources, and as of now we still don’t know why cases of HCC continue to rise in these areas,” she said.
Dr. Gainey noted that HCC is the fastest-growing cancer in the United States, according to the 2020 Annual Report to the Nation on the Status of Cancer, issued jointly by the Centers for Disease Control and Prevention, the North American Association of Central Cancer Registries, the American Cancer Society, and the National Cancer Institute.
Previous studies have identified disparities between urban and rural regions in care of patients with cervical cancer, colorectal cancer, and other malignancies, but there are very few data on urban-rural differences in HCC incidence, she said.
Incidence trends
To better understand whether such differences exists, the investigators compared trends in age-adjusted incidence rates of HCC in both rural and urban areas of the United States from 1995 to 2016, with stratification of trends by race/ethnicity and other demographic factors.
They drew from the NAACR database, which captures 93% of the U.S. population, in contrast to the CDC’s Surveillance, Epidemiology, and End Results (SEER) database which samples just 18% of the population.
Patients with HCC were defined by diagnostic codes, with diagnoses of intrahepatic bile duct cancers excluded.
They used 2013 U.S. Department of Agriculture Rural-Urban Continuum Codes to identify rural areas (regions of open countryside with town populations fewer than 2,500 people) and urban areas (populations ranging from 2,500 to 49,999, but not part of a larger labor market area).
The investigators identified a total of 310,635 HCC cases, 85% in urban areas and 15% in rural areas. Three-fourths of the patients (77%) were male. The median age ranged from 55-59 years.
There were notable demographic differences between the regions with non-Hispanic Whites comprising only 57% of the urban sample, but 82% of the rural sample. The urban sample included 16% non-Hispanic Blacks, 10% Asian/Pacific Islanders, and 17% Hispanics. The respective proportions in the rural areas were 8%, 2%, and 8%.
As noted before, age-adjusted incidence rates (adjusted to the year 2000 U.S. population) were lower in rural areas, at 4.9 per 100,000 population, compared with 6.9/100,000 in urban areas.
But when they looked at the average annual percentage changes using jointpoint regression, they saw that beginning in 2009 the AAPC in urban areas began to slow, from 5.3% for the period prior to 2009 to 2.7% thereafter, while the average annual percentage change in urban areas remained steady at 5.7%.
The largest increase in incidence over the course of the study was among rural non-Hispanic Whites, with an AAPC of 5.7%. Among urban non-Hispanic Blacks, the AAPC rose by 6.6% from 1995 to 2009, but slowed thereafter.
In contrast, among rural non-Hispanic Blacks the AAPC remained steady, at 5.4%.
The only group to see a decline in incidence was urban Asians/Pacific Islanders, who had an overall decline of 1%.
Among all groups, rural Hispanics had the highest age-adjusted incidence rates, at 14.9 per 100,000 in 2016.
Awareness gap?
Lewis R. Roberts, MB, ChB, PhD, a hepatobiliary cancer researcher at the Mayo Clinic in Rochester, Minn., who was not involved in the study, said in an interview that the difference in incidence rates between cities and the country may be attributable to a number of factors, including the opioid crisis, which can lead to an increase in injectable drug use or sexual behaviors resulting in increases in chronic hepatitis C infections and cirrhosis, known risk factors for HCC, as well as a lack of awareness of infections as a risk factor.
“In order for people to find these diseases, they have to be looking, and many of these are hidden diseases in our community,” he said. “What the study made me wonder was whether it just happens to be that they are in some ways more hidden in a rural community than they are in an urban community.”
He noted that clinicians in urban communities are more accustomed to treating more diverse populations who may have higher susceptibility to viral hepatitis, for example, and that screening and treatment for hepatitis C may be more common in urban areas than rural areas, he said.
No funding source for the study was reported. Dr. Gainey and Dr. Roberts reported having no conflicts of interest to disclose.
SOURCE: Gainey C et al. Liver Meeting 2020, Abstract 136.
The incidence rate of hepatocellular carcinoma in urban areas of the United States began to slow in 2009, but the rate in rural areas of the nation continued to rise at a steady pace, especially among non-Hispanic Whites and Blacks, investigators have found.
Although overall hepatocellular carcinoma (HCC) incidence rates were consistently lower among people living in nonmetro (rural) versus metro (urban) areas, the average annual percentage change in urban areas began to slow from 5.3% for the period of 1995 through 2009 to 2.7% thereafter. In contrast, the average annual percentage change in rural areas remained steady at 5.7%, a disparity that remained even after adjusting for differences among subgroups, reported Christina Gainey, MD, a third-year resident in internal medicine at the University of Southern California Medical Center, Los Angeles.
“We found that there are striking urban-rural disparities in HCC incidence trends that vary by race and ethnicity, and these disparities are growing over time,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“Our study really highlights a critical public health issue that’s disproportionately affecting rural Americans. They already face considerable health inequities when it comes to access to care, health outcomes, and public health infrastructure and resources, and as of now we still don’t know why cases of HCC continue to rise in these areas,” she said.
Dr. Gainey noted that HCC is the fastest-growing cancer in the United States, according to the 2020 Annual Report to the Nation on the Status of Cancer, issued jointly by the Centers for Disease Control and Prevention, the North American Association of Central Cancer Registries, the American Cancer Society, and the National Cancer Institute.
Previous studies have identified disparities between urban and rural regions in care of patients with cervical cancer, colorectal cancer, and other malignancies, but there are very few data on urban-rural differences in HCC incidence, she said.
Incidence trends
To better understand whether such differences exists, the investigators compared trends in age-adjusted incidence rates of HCC in both rural and urban areas of the United States from 1995 to 2016, with stratification of trends by race/ethnicity and other demographic factors.
They drew from the NAACR database, which captures 93% of the U.S. population, in contrast to the CDC’s Surveillance, Epidemiology, and End Results (SEER) database which samples just 18% of the population.
Patients with HCC were defined by diagnostic codes, with diagnoses of intrahepatic bile duct cancers excluded.
They used 2013 U.S. Department of Agriculture Rural-Urban Continuum Codes to identify rural areas (regions of open countryside with town populations fewer than 2,500 people) and urban areas (populations ranging from 2,500 to 49,999, but not part of a larger labor market area).
The investigators identified a total of 310,635 HCC cases, 85% in urban areas and 15% in rural areas. Three-fourths of the patients (77%) were male. The median age ranged from 55-59 years.
There were notable demographic differences between the regions with non-Hispanic Whites comprising only 57% of the urban sample, but 82% of the rural sample. The urban sample included 16% non-Hispanic Blacks, 10% Asian/Pacific Islanders, and 17% Hispanics. The respective proportions in the rural areas were 8%, 2%, and 8%.
As noted before, age-adjusted incidence rates (adjusted to the year 2000 U.S. population) were lower in rural areas, at 4.9 per 100,000 population, compared with 6.9/100,000 in urban areas.
But when they looked at the average annual percentage changes using jointpoint regression, they saw that beginning in 2009 the AAPC in urban areas began to slow, from 5.3% for the period prior to 2009 to 2.7% thereafter, while the average annual percentage change in urban areas remained steady at 5.7%.
The largest increase in incidence over the course of the study was among rural non-Hispanic Whites, with an AAPC of 5.7%. Among urban non-Hispanic Blacks, the AAPC rose by 6.6% from 1995 to 2009, but slowed thereafter.
In contrast, among rural non-Hispanic Blacks the AAPC remained steady, at 5.4%.
The only group to see a decline in incidence was urban Asians/Pacific Islanders, who had an overall decline of 1%.
Among all groups, rural Hispanics had the highest age-adjusted incidence rates, at 14.9 per 100,000 in 2016.
Awareness gap?
Lewis R. Roberts, MB, ChB, PhD, a hepatobiliary cancer researcher at the Mayo Clinic in Rochester, Minn., who was not involved in the study, said in an interview that the difference in incidence rates between cities and the country may be attributable to a number of factors, including the opioid crisis, which can lead to an increase in injectable drug use or sexual behaviors resulting in increases in chronic hepatitis C infections and cirrhosis, known risk factors for HCC, as well as a lack of awareness of infections as a risk factor.
“In order for people to find these diseases, they have to be looking, and many of these are hidden diseases in our community,” he said. “What the study made me wonder was whether it just happens to be that they are in some ways more hidden in a rural community than they are in an urban community.”
He noted that clinicians in urban communities are more accustomed to treating more diverse populations who may have higher susceptibility to viral hepatitis, for example, and that screening and treatment for hepatitis C may be more common in urban areas than rural areas, he said.
No funding source for the study was reported. Dr. Gainey and Dr. Roberts reported having no conflicts of interest to disclose.
SOURCE: Gainey C et al. Liver Meeting 2020, Abstract 136.
The incidence rate of hepatocellular carcinoma in urban areas of the United States began to slow in 2009, but the rate in rural areas of the nation continued to rise at a steady pace, especially among non-Hispanic Whites and Blacks, investigators have found.
Although overall hepatocellular carcinoma (HCC) incidence rates were consistently lower among people living in nonmetro (rural) versus metro (urban) areas, the average annual percentage change in urban areas began to slow from 5.3% for the period of 1995 through 2009 to 2.7% thereafter. In contrast, the average annual percentage change in rural areas remained steady at 5.7%, a disparity that remained even after adjusting for differences among subgroups, reported Christina Gainey, MD, a third-year resident in internal medicine at the University of Southern California Medical Center, Los Angeles.
“We found that there are striking urban-rural disparities in HCC incidence trends that vary by race and ethnicity, and these disparities are growing over time,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
“Our study really highlights a critical public health issue that’s disproportionately affecting rural Americans. They already face considerable health inequities when it comes to access to care, health outcomes, and public health infrastructure and resources, and as of now we still don’t know why cases of HCC continue to rise in these areas,” she said.
Dr. Gainey noted that HCC is the fastest-growing cancer in the United States, according to the 2020 Annual Report to the Nation on the Status of Cancer, issued jointly by the Centers for Disease Control and Prevention, the North American Association of Central Cancer Registries, the American Cancer Society, and the National Cancer Institute.
Previous studies have identified disparities between urban and rural regions in care of patients with cervical cancer, colorectal cancer, and other malignancies, but there are very few data on urban-rural differences in HCC incidence, she said.
Incidence trends
To better understand whether such differences exists, the investigators compared trends in age-adjusted incidence rates of HCC in both rural and urban areas of the United States from 1995 to 2016, with stratification of trends by race/ethnicity and other demographic factors.
They drew from the NAACR database, which captures 93% of the U.S. population, in contrast to the CDC’s Surveillance, Epidemiology, and End Results (SEER) database which samples just 18% of the population.
Patients with HCC were defined by diagnostic codes, with diagnoses of intrahepatic bile duct cancers excluded.
They used 2013 U.S. Department of Agriculture Rural-Urban Continuum Codes to identify rural areas (regions of open countryside with town populations fewer than 2,500 people) and urban areas (populations ranging from 2,500 to 49,999, but not part of a larger labor market area).
The investigators identified a total of 310,635 HCC cases, 85% in urban areas and 15% in rural areas. Three-fourths of the patients (77%) were male. The median age ranged from 55-59 years.
There were notable demographic differences between the regions with non-Hispanic Whites comprising only 57% of the urban sample, but 82% of the rural sample. The urban sample included 16% non-Hispanic Blacks, 10% Asian/Pacific Islanders, and 17% Hispanics. The respective proportions in the rural areas were 8%, 2%, and 8%.
As noted before, age-adjusted incidence rates (adjusted to the year 2000 U.S. population) were lower in rural areas, at 4.9 per 100,000 population, compared with 6.9/100,000 in urban areas.
But when they looked at the average annual percentage changes using jointpoint regression, they saw that beginning in 2009 the AAPC in urban areas began to slow, from 5.3% for the period prior to 2009 to 2.7% thereafter, while the average annual percentage change in urban areas remained steady at 5.7%.
The largest increase in incidence over the course of the study was among rural non-Hispanic Whites, with an AAPC of 5.7%. Among urban non-Hispanic Blacks, the AAPC rose by 6.6% from 1995 to 2009, but slowed thereafter.
In contrast, among rural non-Hispanic Blacks the AAPC remained steady, at 5.4%.
The only group to see a decline in incidence was urban Asians/Pacific Islanders, who had an overall decline of 1%.
Among all groups, rural Hispanics had the highest age-adjusted incidence rates, at 14.9 per 100,000 in 2016.
Awareness gap?
Lewis R. Roberts, MB, ChB, PhD, a hepatobiliary cancer researcher at the Mayo Clinic in Rochester, Minn., who was not involved in the study, said in an interview that the difference in incidence rates between cities and the country may be attributable to a number of factors, including the opioid crisis, which can lead to an increase in injectable drug use or sexual behaviors resulting in increases in chronic hepatitis C infections and cirrhosis, known risk factors for HCC, as well as a lack of awareness of infections as a risk factor.
“In order for people to find these diseases, they have to be looking, and many of these are hidden diseases in our community,” he said. “What the study made me wonder was whether it just happens to be that they are in some ways more hidden in a rural community than they are in an urban community.”
He noted that clinicians in urban communities are more accustomed to treating more diverse populations who may have higher susceptibility to viral hepatitis, for example, and that screening and treatment for hepatitis C may be more common in urban areas than rural areas, he said.
No funding source for the study was reported. Dr. Gainey and Dr. Roberts reported having no conflicts of interest to disclose.
SOURCE: Gainey C et al. Liver Meeting 2020, Abstract 136.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
Harnessing the HIV care continuum model to improve HCV treatment success
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Opt-out policy at a syringe service program increased HIV/HCV testing
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
FROM INTERNATIONAL JOURNAL OF DRUG POLICY
Nivolumab Use for First-Line Management of Hepatocellular Carcinoma: Results of a Real-World Cohort of Patients
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
Hepatocellular carcinoma (HCC) has a poor prognosis and remains an important cause of cancer-related morbidity and mortality.1,2 Potentially curative interventions include surgical resection, radiofrequency ablation, and liver transplantation. However, the majority of patients are not eligible for these procedures because they are diagnosed at an advanced stage, when locoregional therapies are much more limited.3,4 Although the kinase inhibitors sorafenib and lenvatinib are approved as first-line systemic treatment, at the US Department of Veterans Affairs (VA) Kansas City VA Medical Center (KCVAMC) in Missouri, nivolumab was used instead because of concerns for the tolerability of the kinase inhibitors. Locoregional therapies, resection, and transplantation options were either not appropriate or had been exhausted for these patients. The objective of this retrospective study was to determine the outcomes of those veteran patients in a small cohort.
Methods
The KCVAMC Institutional Review Board approved this retrospective chart review. Patients were selected from pharmacy records at KCVAMC. We identified all patients with a diagnosis of HCC who received nivolumab from January 2016 to December 2019. We then included only the patients that had nivolumab in the front-line setting for our final analysis. At the time of initiation of treatment, all patients were informed that immunotherapy was not approved for front-line treatment, but available evidence suggested that it would be easier to tolerate than sorafenib or lenvatinib. These patients were determined to be either ineligible for sorafenib or lenvatinib therapy or expected to tolerate it poorly, and hence they consented to the use of nivolumab. Tumor response and progression were assessed by the investigator according to iRECIST (Immune Response Evaluation Criteria in Solid Tumors) criteria.5 Data were obtained from retrospective health record review.
Results
Fourteen men received nivolumab in the front-line systemic therapy setting from January 2016 to December 2019 at KCVAMC. The median age was 63.5 years (range, 58-72 years), and the median Eastern Cooperative Oncology Group score was 1. The Table highlights patient characteristics.
Of the 14 patients included in the review, 2 patients had a response to nivolumab (14.3%) and 1 patient had a complete response (7.1%). The median duration of immunotherapy was 4.5 months. Immunotherapy was discontinued due to disease progression in 10 patients and toxicity in 3 patients.
The median progression-free survival (PFS) from initiation of immunotherapy was 4 months; median overall survival (OS) was 8 months. The median time from diagnosis to survival was 41 months. Only 1 patient received a second-line treatment.
Incidence of grade 3 or higher toxicity was 35%. Three deaths resulted from auto-immune hepatitis (grade 5 toxicity), as well as 1 grade 3 skin toxicity, and 1 grade 4 liver toxicity.
Discussion
Immunotherapy has shown promise in patients with HCC based on the results of the KEYNOTE-224 and Checkmate-040 studies,6,7 which led to an accelerated US Food and Drug Administration approval of nivolumab and pembrolizumab for HCC following failure of first-line sorafenib.8,9
Several clinical trials are evaluating front-line immunotherapy for HCC. The Checkmate 459 study demonstrated the median OS to be 16.4 months for nivolumab vs 14.7 months for sorafenib, a difference that was not statistically significant. However, tolerability of nivolumab was better than it was for sorafenib, thus positioning it as a potentially attractive first-line option.10 The GO30140 study evaluated
The results from our study differed from the previous studies and raise concern for the applicability of these trials to a real-world population. For example, both the GO30140 and IMbrave150 excluded patients with untreated varices.11,12 Both IMbrave150 and Checkmate 459 limited enrollment only to patients with a Child-Pugh A score for liver disease; 36% of the KCVAMC patients had a Child-Pugh B score. Three patients (21.4%) were homeless, 6 patients (42.8%) had substance abuse history and 5 patients (35.7%) had mental illness. Several psychosocial factors present in our patients, such as substance abuse, mental illness, and homelessness, would have excluded them from clinical trials. Our small cohort of patients, thus, represents a frail real-world population due to multiple medical and psychosocial comorbidities. Real-world experience with immunotherapy as second-line therapy after treatment with sorafenib has been reported, but this is the first reported real-world experience of immunotherapy in the front-line setting for HCC.13,14
Large differences in sociodemographic status and health status exist between the veteran population and typical clinical trial populations. Veterans are predominantly male and older than a clinical trial population. Veterans are more likely to belong to a minority group, more likely to have lower level education and more likely to be poor than a clinical trial population. They are more likely to have poorer health status with higher number of medical conditions and psychosocial conditions.15
Limitations
We acknowledge several limitations to our study, such as the small number of patients and the retrospective single center nature of this study. Patients were older men with multiple psychosocial comorbitities like mental illness, substance abuse, and homelessness. This cohort may not represent the non-VA population, but is an excellent representation of a frail, real-world veteran population.
Conclusions
Despite clinical trials showing the promise of immunotherapy as an attractive front-line systemic treatment option for HCC, our results show poor outcomes in a frail real-world population. In a cohort of patients who received immunotherapy as a front-line systemic treatment for HCC, results were poor with a response rate of 14.3%, a median PFS of 4 months, and a median OS of 8 months. We noted a significantly higher number of adverse effects, including 21% incidence of grade 5 hepatotoxicity. There remains an urgent need to develop more effective and safer therapies for this patient population as well as validation from larger real-world studies.
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. doi:10.1056/NEJMra1001683
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. doi:10.1002/ijc.29210
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi:10.1016/S0140-6736(03)14964-1
4. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl(0):S2-S6. doi:10.1097/MCG.0b013e3182872f29
5. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019 May;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152. doi:10.1016/S1470-2045(17)30074-8
6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502.doi:10.1016/S0140-6736(17)31046-2
7. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in Lancet Oncol. 2018 Sep;19(9):e440]. Lancet Oncol. 2018;19(7):940-952. doi:10.1016/S1470-2045(18)30351-6
8. US Food and Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. Updated September 25, 2017. Accessed October 7, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib.
9. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. Updated December 14, 2018. Accessed October 7, 2020. https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma.
10. Yau T, Park JW, Finn RS, et al. CheckMate 459: A randomized, multi-center phase 3 study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019. Ann Onc. 2019;30(suppl_5):v851-v934. doi:10.1093/annonc/mdz394
11. Lee M, Ryoo BY, Hsu CH, et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Presented at: ESMO 2019 Congress. Barcelona, Spain: September 27, 2019.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.doi:10.1056/NEJMoa1915745
13. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49(10):1323-1333. doi:10.1111/apt.15245
14. Yoon SE, Hur JY, Lee KK, et al. Real-world data on nivolumab treatment in Asian patients with advanced hepatocellular carcinoma. Presented at: ESMO 2018 Congress. Munich, Germany: October 21, 2018. Ann Onc. 2018;29(suppl_8):viii205-viii270. doi:10.1093/annonc/mdy282
15. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
When First-Line Systemic Treatment for Hepatocellular Carcinoma Fails, What Comes Next?
The following is a lightly edited transcript of a virtual roundtable discussion recorded in September 2020. To view the full discussion, go to www.mdedge.com/FedPrac/HCC-Roundtable.
The following is a lightly edited transcript of a virtual roundtable discussion recorded in September 2020. To view the full discussion, go to www.mdedge.com/FedPrac/HCC-Roundtable.
The following is a lightly edited transcript of a virtual roundtable discussion recorded in September 2020. To view the full discussion, go to www.mdedge.com/FedPrac/HCC-Roundtable.
Direct-acting agents cure hepatitis C in children
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at pdnews@mdedge.com.
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at pdnews@mdedge.com.
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at pdnews@mdedge.com.
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Hepatocellular carcinoma shows risk factor shift
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
FROM DIGESTIVE DISEASES: NEW ADVANCES
HPV-Mediated Head, Neck Cancers Predicted to Rise for Decades
Human papilloma virus (HPV)-mediated squamous cell carcinoma of the head and neck is on the rise, and the lack of herd immunity in young people will ensure growth for many years to come. “We’re really looking at another 30 to 40 years of HPV and oropharynx cancer growth,” said head and neck cancer surgeon Joseph Califano, MD, deputy director of the Moores Cancer Center at the University of California at San Diego, at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Califano highlighted a 2019 study that estimated the number of diagnoses of oropharynx cancer cases in the US will grow by half to 30,000 by 2030, with the wide majority (about 25,000) in men. In 2016, the annual number of oropharynx cancer cases was 20,124. “The exponential increase in oropharynx cancer incidence in young white US men has ebbed, and modest increases are occurring/anticipated in cohorts born after 1955,” the study authors wrote.
“Currently in the United States, we don't have adequate vaccine efficiency to provide herd immunity, particularly for young boys,” said Califano. He added that although HPV vaccinations may create herd immunity in 5 to 10 years, the cancers associated with HPV can take decades to develop so a dip in rates won’t come for many years.
HPV-associated head and neck squamous cell cancer (HNSCC) affects people at a younger age when compared with other head and neck cancers—a decade or 2 earlier, according to Califano. Many patients are nonsmokers and nondrinkers, he said, and tumors may be painless and asymptomatic.
It’s also becoming clear that the HPV-associated HNSCC can strike across a widespread area of the oropharynx, including the palatine and lingual tonsils, the nasal cavity, nasopharynx, and hypopharynx (the lower part of the voice box), he said. “It has an even larger footprint than we originally supposed when we realized HPV was a dominant mechanism for development of oropharyngeal cancer,” said Califano.
Describing the extent of these cancers as an “epidemic,” Califono said a turning point in the understanding of HPV’s role in oropharynx cancers came in a “definitive” 2001 study that reported that HPV-positive patients were much more likely to develop oropharynx cancer (adjusted odds ratio, 14.4). Later research found that HPV-associated oropharynx cancers were more common than HPV-associated cervical cancer. Higher lifetime numbers of vaginal sex and oral sex partners are linked to higher risk of HPV-mediated HNSCC, he said, as is prolonged daily marijuana use.
Califano emphasized the importance of counseling patients about sexual behaviors linked to the cancers, although it’s also important to consider that “the majority of patients don’t have these risk factors.”
“The diagnosis is not an indication of infidelity or promiscuity,” he added, recalling that he saw at least one marriage dissolve because of “misunderstandings” regarding how the cancer is caused.
There are multiple treatment options. Early-stage oropharynx cancers can be treated with primary excision and staging neck dissection or radiotherapy. Multimodality therapy is appropriate for late-stage cancer and can include concurrent chemotherapy and radiation, primary excision, and treatment with concurrent cisplatinum, depending on the case. Also, “patients do really benefit if they’re enrolled in clinical trials.”
The good news is that HPV-positivity is associated with improved survival in oropharynx cancer, he said. He highlighted a 2019 study that said radiotherapy and cisplatin improve survival in HPV-positive oropharynx cancer patients. “This has become the de-facto standard of care for locally advanced, low-risk HPV-positive oropharynx cancer,” he said.
Califano reported no relevant disclosures.
Human papilloma virus (HPV)-mediated squamous cell carcinoma of the head and neck is on the rise, and the lack of herd immunity in young people will ensure growth for many years to come. “We’re really looking at another 30 to 40 years of HPV and oropharynx cancer growth,” said head and neck cancer surgeon Joseph Califano, MD, deputy director of the Moores Cancer Center at the University of California at San Diego, at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Califano highlighted a 2019 study that estimated the number of diagnoses of oropharynx cancer cases in the US will grow by half to 30,000 by 2030, with the wide majority (about 25,000) in men. In 2016, the annual number of oropharynx cancer cases was 20,124. “The exponential increase in oropharynx cancer incidence in young white US men has ebbed, and modest increases are occurring/anticipated in cohorts born after 1955,” the study authors wrote.
“Currently in the United States, we don't have adequate vaccine efficiency to provide herd immunity, particularly for young boys,” said Califano. He added that although HPV vaccinations may create herd immunity in 5 to 10 years, the cancers associated with HPV can take decades to develop so a dip in rates won’t come for many years.
HPV-associated head and neck squamous cell cancer (HNSCC) affects people at a younger age when compared with other head and neck cancers—a decade or 2 earlier, according to Califano. Many patients are nonsmokers and nondrinkers, he said, and tumors may be painless and asymptomatic.
It’s also becoming clear that the HPV-associated HNSCC can strike across a widespread area of the oropharynx, including the palatine and lingual tonsils, the nasal cavity, nasopharynx, and hypopharynx (the lower part of the voice box), he said. “It has an even larger footprint than we originally supposed when we realized HPV was a dominant mechanism for development of oropharyngeal cancer,” said Califano.
Describing the extent of these cancers as an “epidemic,” Califono said a turning point in the understanding of HPV’s role in oropharynx cancers came in a “definitive” 2001 study that reported that HPV-positive patients were much more likely to develop oropharynx cancer (adjusted odds ratio, 14.4). Later research found that HPV-associated oropharynx cancers were more common than HPV-associated cervical cancer. Higher lifetime numbers of vaginal sex and oral sex partners are linked to higher risk of HPV-mediated HNSCC, he said, as is prolonged daily marijuana use.
Califano emphasized the importance of counseling patients about sexual behaviors linked to the cancers, although it’s also important to consider that “the majority of patients don’t have these risk factors.”
“The diagnosis is not an indication of infidelity or promiscuity,” he added, recalling that he saw at least one marriage dissolve because of “misunderstandings” regarding how the cancer is caused.
There are multiple treatment options. Early-stage oropharynx cancers can be treated with primary excision and staging neck dissection or radiotherapy. Multimodality therapy is appropriate for late-stage cancer and can include concurrent chemotherapy and radiation, primary excision, and treatment with concurrent cisplatinum, depending on the case. Also, “patients do really benefit if they’re enrolled in clinical trials.”
The good news is that HPV-positivity is associated with improved survival in oropharynx cancer, he said. He highlighted a 2019 study that said radiotherapy and cisplatin improve survival in HPV-positive oropharynx cancer patients. “This has become the de-facto standard of care for locally advanced, low-risk HPV-positive oropharynx cancer,” he said.
Califano reported no relevant disclosures.
Human papilloma virus (HPV)-mediated squamous cell carcinoma of the head and neck is on the rise, and the lack of herd immunity in young people will ensure growth for many years to come. “We’re really looking at another 30 to 40 years of HPV and oropharynx cancer growth,” said head and neck cancer surgeon Joseph Califano, MD, deputy director of the Moores Cancer Center at the University of California at San Diego, at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Califano highlighted a 2019 study that estimated the number of diagnoses of oropharynx cancer cases in the US will grow by half to 30,000 by 2030, with the wide majority (about 25,000) in men. In 2016, the annual number of oropharynx cancer cases was 20,124. “The exponential increase in oropharynx cancer incidence in young white US men has ebbed, and modest increases are occurring/anticipated in cohorts born after 1955,” the study authors wrote.
“Currently in the United States, we don't have adequate vaccine efficiency to provide herd immunity, particularly for young boys,” said Califano. He added that although HPV vaccinations may create herd immunity in 5 to 10 years, the cancers associated with HPV can take decades to develop so a dip in rates won’t come for many years.
HPV-associated head and neck squamous cell cancer (HNSCC) affects people at a younger age when compared with other head and neck cancers—a decade or 2 earlier, according to Califano. Many patients are nonsmokers and nondrinkers, he said, and tumors may be painless and asymptomatic.
It’s also becoming clear that the HPV-associated HNSCC can strike across a widespread area of the oropharynx, including the palatine and lingual tonsils, the nasal cavity, nasopharynx, and hypopharynx (the lower part of the voice box), he said. “It has an even larger footprint than we originally supposed when we realized HPV was a dominant mechanism for development of oropharyngeal cancer,” said Califano.
Describing the extent of these cancers as an “epidemic,” Califono said a turning point in the understanding of HPV’s role in oropharynx cancers came in a “definitive” 2001 study that reported that HPV-positive patients were much more likely to develop oropharynx cancer (adjusted odds ratio, 14.4). Later research found that HPV-associated oropharynx cancers were more common than HPV-associated cervical cancer. Higher lifetime numbers of vaginal sex and oral sex partners are linked to higher risk of HPV-mediated HNSCC, he said, as is prolonged daily marijuana use.
Califano emphasized the importance of counseling patients about sexual behaviors linked to the cancers, although it’s also important to consider that “the majority of patients don’t have these risk factors.”
“The diagnosis is not an indication of infidelity or promiscuity,” he added, recalling that he saw at least one marriage dissolve because of “misunderstandings” regarding how the cancer is caused.
There are multiple treatment options. Early-stage oropharynx cancers can be treated with primary excision and staging neck dissection or radiotherapy. Multimodality therapy is appropriate for late-stage cancer and can include concurrent chemotherapy and radiation, primary excision, and treatment with concurrent cisplatinum, depending on the case. Also, “patients do really benefit if they’re enrolled in clinical trials.”
The good news is that HPV-positivity is associated with improved survival in oropharynx cancer, he said. He highlighted a 2019 study that said radiotherapy and cisplatin improve survival in HPV-positive oropharynx cancer patients. “This has become the de-facto standard of care for locally advanced, low-risk HPV-positive oropharynx cancer,” he said.
Califano reported no relevant disclosures.