User login
Children and COVID: New cases and hospital admissions skyrocket
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.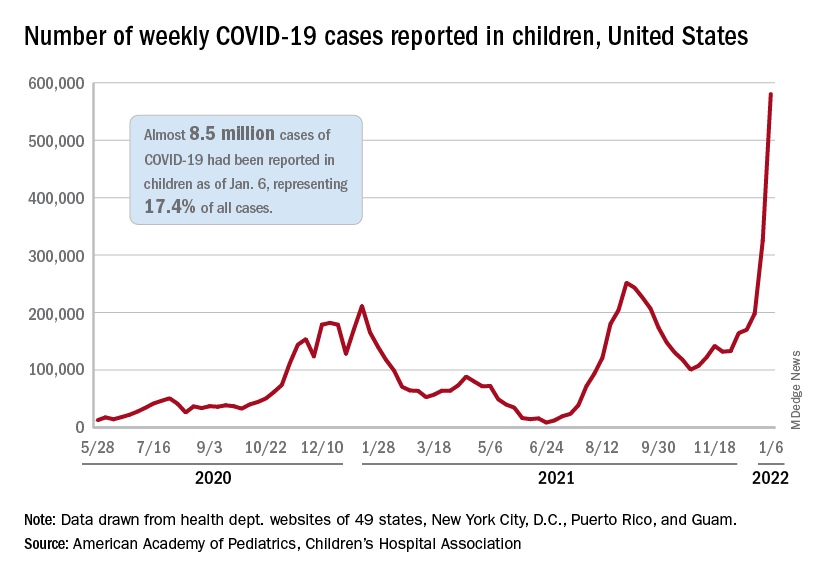
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total for the week of Dec. 31 to Jan. 6 – the highest since the pandemic began – was an increase of 78% over the previous week (325,000) and 192% higher than just 2 weeks before (199,000), the AAP and CHA said in their weekly COVID-19 report. No region of the country was spared, as all four saw at least 50,000 more cases than the week before, but the increase was largest in the West and smallest in the Midwest.
“Nearly 8.5 million children have tested positive for COVID-19 since the onset of the pandemic; nearly 11% of these cases have been added in the past 2 weeks,” the AAP said.
The situation is the same for hospitalizations. On Dec. 15, the daily rate of new admissions for children aged 0-17 years was 0.26 per 100,000, and by Jan. 7 it had more than quadrupled to 1.15 per 100,000, the Centers for Disease Control and Prevention reported. Before Omicron, the highest rate was 0.47 per 100,000 on Sept. 4, 2021.
The number of children occupying inpatient beds who had laboratory-confirmed COVID-19 went from 2,343 on Jan. 2 to 3,476 on Jan. 9, a jump of more than 48% in just 1 week. Texas had more hospitalized children (392) than any other state on Jan. 9, with California (339) and New York (313) the only other states over 300, according to data from the Department of Health & Human Services.
For vaccinations. however, the situation is definitely not the same. The number of children added to the ranks of those with at least one dose of COVID-19 vaccine was down in early 2022 (Jan. 3-9) for both 5- to 11-year-olds (–8.2%) and 16- to 17-year-olds (–12.2%) but higher among those aged 12-15 (12.2%), compared with the previous week (Dec. 27 to Jan. 2), the CDC said on its COVID Data Tracker.
Cumulative figures show that 26.3% of all children aged 5-11 had received at least one dose of vaccine and 17.2% were fully vaccinated as of Jan. 10, compared with 62.2% and 52.0% of 12- to 15-year-olds and 68.5% and 58.1% of those aged 16-17. Altogether, over 23.8 million children in those three age groups have received at least one dose and almost 18.6 million are fully vaccinated, the CDC said.
CDC: More kids hospitalized with COVID since pandemic began
Hospital admissions of U.S. children younger than 5 – the only group ineligible for vaccination – have reached their peak since the start of the pandemic, according to new data from the Centers for Disease Control and Prevention.
CDC Director Rochelle Walensky, MD, said the higher numbers show the importance of vaccination for all eligible groups.
“This is the highest number of pediatric hospitalizations we’ve seen throughout the pandemic, which we said about Delta until now,” she said at a CDC briefing Friday. “This very well may be that there are just more cases out there, and our children are more vulnerable when they have more cases surrounding them.”
Despite the skyrocketing admissions, hospitalizations are still relatively low for children, she said. The hospitalization rate for children under 5 is 4 in 100,000, and it’s about 1 in 100,000 in children 5-17.
Dr. Walensky said not all children are being hospitalized for COVID-19 – some are admitted for unrelated issues and test positive but don’t have symptoms.
“We are still learning more about the severity of Omicron in children,” she said, noting that just over 50% of children 12-18 are fully vaccinated, while only 16% of those ages 5-11 are fully vaccinated.
Friday’s teleconference was the first CDC briefing in several months and comes on the heels of recent guideline updates for testing and isolation that have left the American public dumbfounded. When asked why the briefing was held, Dr. Walensky said there had been interest in hearing more from the CDC, saying, “I anticipate this will be the first of many briefings.”
She also defended the confusing guideline changes, saying, “We’re in an unprecedented time with the speed of Omicron cases rising. … This is hard, and I am committed to continuing to improve as we learn more about the science and communicate that to you.”
A version of this article first appeared on WebMD.com.
Hospital admissions of U.S. children younger than 5 – the only group ineligible for vaccination – have reached their peak since the start of the pandemic, according to new data from the Centers for Disease Control and Prevention.
CDC Director Rochelle Walensky, MD, said the higher numbers show the importance of vaccination for all eligible groups.
“This is the highest number of pediatric hospitalizations we’ve seen throughout the pandemic, which we said about Delta until now,” she said at a CDC briefing Friday. “This very well may be that there are just more cases out there, and our children are more vulnerable when they have more cases surrounding them.”
Despite the skyrocketing admissions, hospitalizations are still relatively low for children, she said. The hospitalization rate for children under 5 is 4 in 100,000, and it’s about 1 in 100,000 in children 5-17.
Dr. Walensky said not all children are being hospitalized for COVID-19 – some are admitted for unrelated issues and test positive but don’t have symptoms.
“We are still learning more about the severity of Omicron in children,” she said, noting that just over 50% of children 12-18 are fully vaccinated, while only 16% of those ages 5-11 are fully vaccinated.
Friday’s teleconference was the first CDC briefing in several months and comes on the heels of recent guideline updates for testing and isolation that have left the American public dumbfounded. When asked why the briefing was held, Dr. Walensky said there had been interest in hearing more from the CDC, saying, “I anticipate this will be the first of many briefings.”
She also defended the confusing guideline changes, saying, “We’re in an unprecedented time with the speed of Omicron cases rising. … This is hard, and I am committed to continuing to improve as we learn more about the science and communicate that to you.”
A version of this article first appeared on WebMD.com.
Hospital admissions of U.S. children younger than 5 – the only group ineligible for vaccination – have reached their peak since the start of the pandemic, according to new data from the Centers for Disease Control and Prevention.
CDC Director Rochelle Walensky, MD, said the higher numbers show the importance of vaccination for all eligible groups.
“This is the highest number of pediatric hospitalizations we’ve seen throughout the pandemic, which we said about Delta until now,” she said at a CDC briefing Friday. “This very well may be that there are just more cases out there, and our children are more vulnerable when they have more cases surrounding them.”
Despite the skyrocketing admissions, hospitalizations are still relatively low for children, she said. The hospitalization rate for children under 5 is 4 in 100,000, and it’s about 1 in 100,000 in children 5-17.
Dr. Walensky said not all children are being hospitalized for COVID-19 – some are admitted for unrelated issues and test positive but don’t have symptoms.
“We are still learning more about the severity of Omicron in children,” she said, noting that just over 50% of children 12-18 are fully vaccinated, while only 16% of those ages 5-11 are fully vaccinated.
Friday’s teleconference was the first CDC briefing in several months and comes on the heels of recent guideline updates for testing and isolation that have left the American public dumbfounded. When asked why the briefing was held, Dr. Walensky said there had been interest in hearing more from the CDC, saying, “I anticipate this will be the first of many briefings.”
She also defended the confusing guideline changes, saying, “We’re in an unprecedented time with the speed of Omicron cases rising. … This is hard, and I am committed to continuing to improve as we learn more about the science and communicate that to you.”
A version of this article first appeared on WebMD.com.
Pediatric antibiotic prescriptions plummeted in pandemic
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
FROM PEDIATRICS
A COVID-19 Clinical Management Committee to Standardize Care in a 2-Hospital System
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
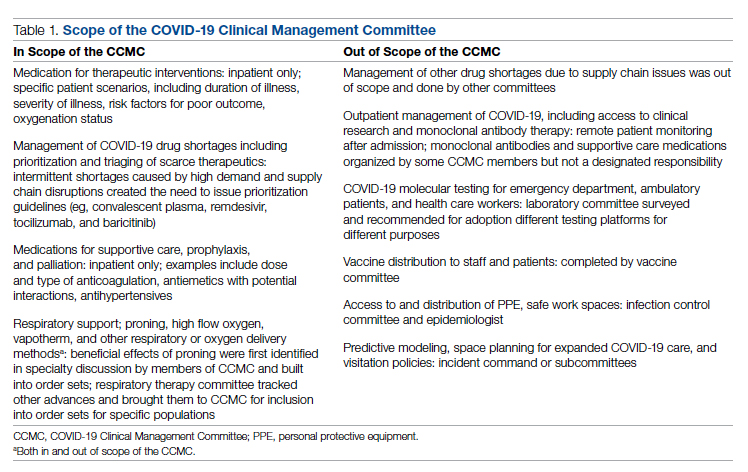
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
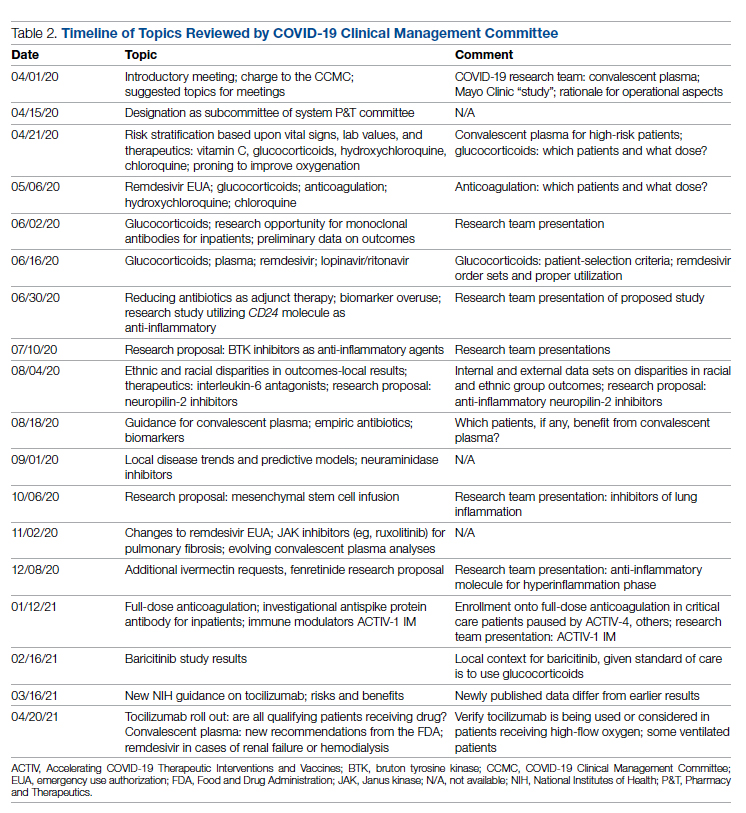
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
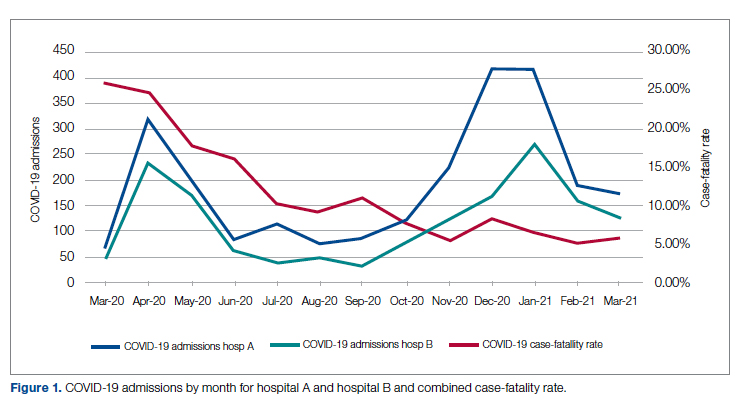
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
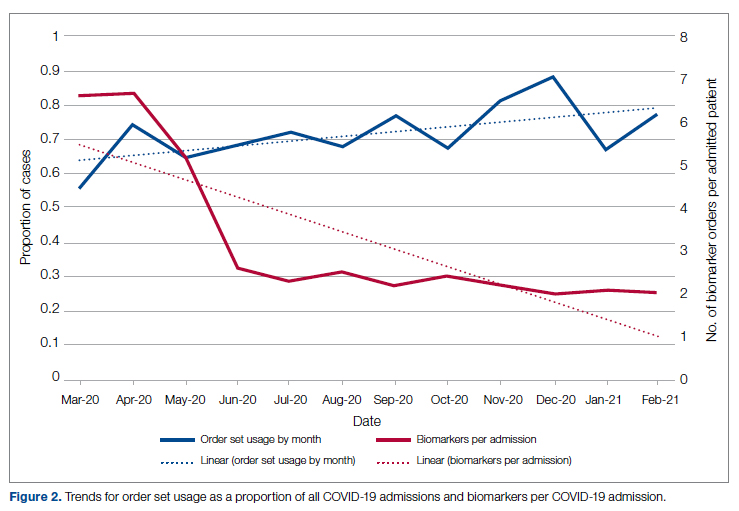
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
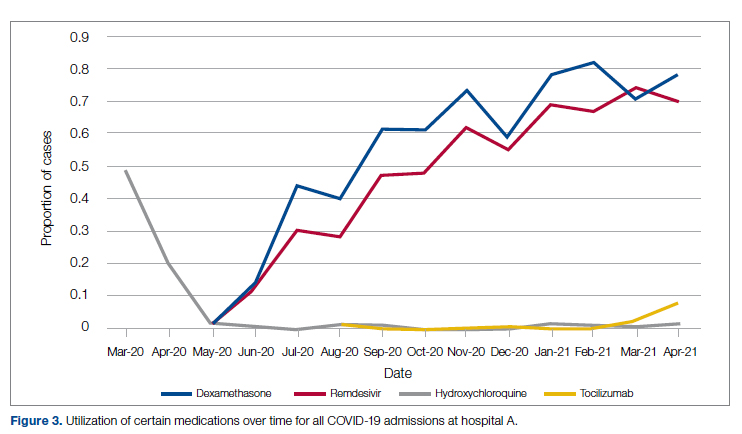
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; meisenberg@AAHS.org.
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; meisenberg@AAHS.org.
Financial disclosures: None.
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; meisenberg@AAHS.org.
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
Neonatal sepsis: WHO-recommended Rx needs a major rethink
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First-line treatment of neonatal sepsis in low- and middle-income countries (LMICs) with ampicillin-gentamicin – as recommended by the World Health Organization – needs to be reassessed, a retrospective, observational cohort study suggests. Rates of resistance to this particular antibiotic combination are extremely high in LMICs, and this treatment is unlikely to save many neonatal patients, according to the study’s results.
“The WHO guidelines are over 10 years old, and they are actually based on high-income country data, whereas data reported from low-income countries are reported by private labs, and they do not cater to the lower socioeconomic groups within these countries, which is important data to capture,” Timothy Walsh, MD, University of Oxford, United Kingdom, told this news organization.
“The main take-home message from our data is that ampicillin-gentamicin doesn’t work for most of the Gram-negative isolates we tested, and while there are alternatives, their use is confounded by [a lack of] financial support,” he added.
The study was published online in The Lancet Infectious Diseases.
BARNARDS study
In this substudy of the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study, investigators focused on the effectiveness of antibiotic therapies after taking into account the high prevalence of pathogen resistance to ampicillin-gentamicin. Participating countries included Bangladesh, Ethiopia, India, Nigeria, Pakistan, Rwanda, and South Africa.
“Blood samples were obtained from neonates presenting with clinical signs of sepsis,” the authors note, “and WGS [whole-genome sequencing] and MICs [minimum inhibitory concentrations] for antibiotic treatment were determined for bacterial isolates from culture-confirmed sepsis.” Between Nov. 2015 and Feb. 2018, 36,285 neonates were enrolled into the main BARNARDS study, of whom 9,874 had clinically diagnosed sepsis and 5,749 had antibiotic data.
A total of 2,483 neonates had culture-confirmed sepsis, and WGS data were available for 457 isolates taken from 442 neonates. Slightly over three-quarters of the 5,749 neonates who had antibiotic data received first-line ampicillin-gentamicin. The other three most commonly prescribed antibiotic combinations were ceftazidime-amikacin, piperacillin-tazobactam-amikacin, and amoxicillin-clavulanate-amikacin.
Neonates treated with ceftazidime-amikacin had a 68% lower reported mortality than those treated with ampicillin-gentamicin at an adjusted hazard ratio of 0.32 (95% confidence interval, 0.14-0.72; P = .006), the investigators report. In contrast, no significant differences in mortality rates were reported for neonates treated with amoxicillin-clavulanate-amikacin or piperacillin-tazobactam-amikacin compared to those treated with ampicillin-gentamicin.
Investigators were careful to suggest that mortality effects associated with the different antibiotic combinations might have been confounded by either country-specific effects or underreporting of mortality, as a large proportion of neonates who were treated with ampicillin-gentamicin were followed for fewer than 10 days. However, in an unreported aspect of the same study, neonatal mortality from sepsis dropped by over 50% in two federally funded sites in Nigeria that changed their treatment from the WHO-recommended ampicillin-gentamicin regimen to ceftazidime-amikacin – which Dr. Walsh suggested was an endorsement of ceftazidime-amikacin over ampicillin-gentamicin if ever there was one.
Gram-negative resistance
In looking at resistance patterns to the antibiotic combinations used in these countries, investigators found that almost all Gram-negative isolates tested were “overwhelmingly resistant” to ampicillin, and over 70% of them were resistant to gentamicin as well. Extremely high resistance rates were also found against Staphylococcus spp, which are regarded as intrinsically resistant to ampicillin, rendering it basically useless in this particular treatment setting.
Amikacin had much lower level of resistance, with only about 26% of Gram-negative isolates showing resistance. In terms of coverage against Gram-negative isolates, the lowest level of coverage was provided by ampicillin-gentamicin at slightly over 28%, compared with about 73% for amoxicillin-clavulanate-amikacin, 77% for ceftazidime-amikacin, and 80% for piperacillin-tazobactam-amikacin.
In contrast, “Gram-positive isolates generally had reduced levels of resistance,” the authors state. As Dr. Walsh noted, the consortium also did an analysis assessing how much the antibiotic combinations cost and how much payment was deferred to the parents. For example, in Nigeria, the entire cost of treatment is passed down to the parents, “so if they are earning, say, $5.00 a day and the infant needs ceftazidime-amikacin, where the cost per dose is about $6.00 or $7.00 a day, parents can’t afford it,” Dr. Walsh observed.
This part of the conversation, he added, tends to get lost in many studies of antibiotic resistance in LMICs, which is a critical omission, because in many instances, the choice of treatment does come down to affordability. “It’s all very well for the WHO to sit there and say, ampicillin-gentamicin is perfect, but the combination actually doesn’t work in over 70% of the Gram-negative bacteria we looked at in these countries,” Dr. Walsh emphasized.
“The fact is that we have to be a lot more internationally engaged as to what’s actually happening in poorer populations, because unless we do, neonates are going to continue to die,” he said.
Editorial commentary
Commenting on the findings, lead editorialist Luregn Schlapbach, MD, PhD, of University Children’s Hospital Zurich, Switzerland, pointed out that the study has a number of limitations, including a high rate of dropouts from follow-up. This could possibly result in underestimation of neonatal mortality as well as country-specific biases. Nevertheless, Dr. Schlapbach feels that the integration of sequential clinical, genomic, microbiologic, drug, and cost data across a large network in LMIC settings is “exceptional” and will serve to inform “urgently needed” clinical trials in the field of neonatal sepsis.
“At present, increasing global antibiotic resistance is threatening progress against neonatal sepsis, prompting urgency to develop improved measures to effectively prevent and treat life-threatening infections in this high-risk group,” Dr. Schlapbach and colleagues write.
“The findings from the BARNARDS study call for randomized trials comparing mortality benefit and cost efficiency of different antibiotic combinations and management algorithms to safely reduce unnecessary antibiotic exposure for neonatal sepsis,” the editorialists concluded.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With sexually transmitted infections off the charts, California pushes at-home tests
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Fossilized blood proteins from child illness may cause chalky teeth
FROM FRONTIERS IN PHYSIOLOGY
Researchers have identified a potential cause of molar hypomineralization (MH), or “chalky teeth,” an underrecognized condition affecting one in five children worldwide. The discovery could lead to preventive medical therapies to reduce dental caries and extractions, they said.
According to a team led by biochemist Michael J. Hubbard, BDS, PhD, professor in the department of medicine, dentistry, and health sciences at the University of Melbourne, the “groundbreaking” research found that the failure of enamel to adequately harden is associated with exposure to serum albumin while teeth are developing. The blood protein “poisons” the growth of mineral crystals rather than injure the cells that deposit enamel, they reported.
The investigators, including researchers from Chile, said their findings hold promise for better clinical management of MH and open a new door into research on the broader pathogenesis and causes of the condition.
“We hope this breakthrough will eventually lead to medical prevention of MH, prompting global health benefits including major reductions in childhood tooth decay,” they wrote in an article published online Dec. 21 in Frontiers in Physiology.
More than cosmetic
Chalky teeth, characterized by discolored enamel spots, are not merely a cosmetic problem. The condition can lead to severe toothache, painful eating, tooth decay, and even abscesses and extractions. Although its triggers have eluded dental research for a century, Dr. Hubbard’s group said fossilized blood proteins such as albumin in the tooth appear to be at least one cause.
Biochemical evidence indicates that serum albumin surrounding developing teeth is normally excluded from enamel, Dr. Hubbard said in an interview. “Given that albumin binds strongly to hydroxyapatite-based mineral and blocks its growth, we infer that the epithelial barrier – the enamel-forming cells termed ameloblasts and normally responsible for excluding albumin – must break down in places in response to medical triggers.”
This breach enables localized infiltration of albumin, which then blocks further hardening of soft, immature enamel, leading to residual spots or patches of chalky enamel once the tooth eventually erupts into the mouth. “In other words, we infer that chalky enamel spots coincide with localized breaches of an epithelial barrier that are triggered by yet-to-be determined systemic insults,” he said.
Joseph Brofsky, DMD, section head of pediatric dentistry at North Shore LIJ Cohen Children’s Medical Center of New York, in Queens, agreed that that the definitive cause of MH has evaded identification for a hundred years. However, he expressed skepticism about the fossilized blood protein hypothesis.
“That’s a long shot. It’s a possibility, and I’m not ruling it out, but we’re not 100% sure,” said Dr. Brofsky, who was not involved in the research.
In his experience, MH is somewhat less prevalent in the United States, affecting about 1 in 10 children here, which is about half the global rate. “But it’s a problem, and we wish it would go away, but before we know beyond a reasonable doubt what causes this condition, it’s going to be hard to stop it.”
Most cases of MH involve hypomineralization of the 6-year molars, the first adult molars to erupt, but the process starts at birth. “For 6-year molars, normal hardening of dental enamel takes place from the early postnatal period through infancy,” Dr. Hubbard said.
The 2-year and 12-year molars are affected about half as frequently as their 6-year counterparts, “so this extends the medical-risk window out to early school days, and slightly back to the perinatal period for the 12-year and 2-year molars, respectively,” he said.
A critical question is which childhood illnesses are most likely to set the stage for MH, he added. “Forty-plus years of epidemiology have failed to nail a specific cause or causal association. But given the high prevalence of MH – 20% in otherwise healthy kids – naturally we suspect some common illnesses are the culprits,” he said. “But which diseases, which medications, and which combinations?”
Dr. Hubbard’s advice to pediatricians is to be alert to MH: “If you’re inspecting a child’s throat, then why not look at their back teeth, too – particularly when they’re getting their new molars at 2, 6, and 12 years?”
The study was supported by the Melbourne Research Unit for Facial Disorders Department of Pharmacology & Therapeutics, Department of Paediatrics, and Faculty of Medicine, Dentistry, and Health Sciences at the University of Melbourne. The authors and Dr. Brofsky have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN PHYSIOLOGY
Researchers have identified a potential cause of molar hypomineralization (MH), or “chalky teeth,” an underrecognized condition affecting one in five children worldwide. The discovery could lead to preventive medical therapies to reduce dental caries and extractions, they said.
According to a team led by biochemist Michael J. Hubbard, BDS, PhD, professor in the department of medicine, dentistry, and health sciences at the University of Melbourne, the “groundbreaking” research found that the failure of enamel to adequately harden is associated with exposure to serum albumin while teeth are developing. The blood protein “poisons” the growth of mineral crystals rather than injure the cells that deposit enamel, they reported.
The investigators, including researchers from Chile, said their findings hold promise for better clinical management of MH and open a new door into research on the broader pathogenesis and causes of the condition.
“We hope this breakthrough will eventually lead to medical prevention of MH, prompting global health benefits including major reductions in childhood tooth decay,” they wrote in an article published online Dec. 21 in Frontiers in Physiology.
More than cosmetic
Chalky teeth, characterized by discolored enamel spots, are not merely a cosmetic problem. The condition can lead to severe toothache, painful eating, tooth decay, and even abscesses and extractions. Although its triggers have eluded dental research for a century, Dr. Hubbard’s group said fossilized blood proteins such as albumin in the tooth appear to be at least one cause.
Biochemical evidence indicates that serum albumin surrounding developing teeth is normally excluded from enamel, Dr. Hubbard said in an interview. “Given that albumin binds strongly to hydroxyapatite-based mineral and blocks its growth, we infer that the epithelial barrier – the enamel-forming cells termed ameloblasts and normally responsible for excluding albumin – must break down in places in response to medical triggers.”
This breach enables localized infiltration of albumin, which then blocks further hardening of soft, immature enamel, leading to residual spots or patches of chalky enamel once the tooth eventually erupts into the mouth. “In other words, we infer that chalky enamel spots coincide with localized breaches of an epithelial barrier that are triggered by yet-to-be determined systemic insults,” he said.
Joseph Brofsky, DMD, section head of pediatric dentistry at North Shore LIJ Cohen Children’s Medical Center of New York, in Queens, agreed that that the definitive cause of MH has evaded identification for a hundred years. However, he expressed skepticism about the fossilized blood protein hypothesis.
“That’s a long shot. It’s a possibility, and I’m not ruling it out, but we’re not 100% sure,” said Dr. Brofsky, who was not involved in the research.
In his experience, MH is somewhat less prevalent in the United States, affecting about 1 in 10 children here, which is about half the global rate. “But it’s a problem, and we wish it would go away, but before we know beyond a reasonable doubt what causes this condition, it’s going to be hard to stop it.”
Most cases of MH involve hypomineralization of the 6-year molars, the first adult molars to erupt, but the process starts at birth. “For 6-year molars, normal hardening of dental enamel takes place from the early postnatal period through infancy,” Dr. Hubbard said.
The 2-year and 12-year molars are affected about half as frequently as their 6-year counterparts, “so this extends the medical-risk window out to early school days, and slightly back to the perinatal period for the 12-year and 2-year molars, respectively,” he said.
A critical question is which childhood illnesses are most likely to set the stage for MH, he added. “Forty-plus years of epidemiology have failed to nail a specific cause or causal association. But given the high prevalence of MH – 20% in otherwise healthy kids – naturally we suspect some common illnesses are the culprits,” he said. “But which diseases, which medications, and which combinations?”
Dr. Hubbard’s advice to pediatricians is to be alert to MH: “If you’re inspecting a child’s throat, then why not look at their back teeth, too – particularly when they’re getting their new molars at 2, 6, and 12 years?”
The study was supported by the Melbourne Research Unit for Facial Disorders Department of Pharmacology & Therapeutics, Department of Paediatrics, and Faculty of Medicine, Dentistry, and Health Sciences at the University of Melbourne. The authors and Dr. Brofsky have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN PHYSIOLOGY
Researchers have identified a potential cause of molar hypomineralization (MH), or “chalky teeth,” an underrecognized condition affecting one in five children worldwide. The discovery could lead to preventive medical therapies to reduce dental caries and extractions, they said.
According to a team led by biochemist Michael J. Hubbard, BDS, PhD, professor in the department of medicine, dentistry, and health sciences at the University of Melbourne, the “groundbreaking” research found that the failure of enamel to adequately harden is associated with exposure to serum albumin while teeth are developing. The blood protein “poisons” the growth of mineral crystals rather than injure the cells that deposit enamel, they reported.
The investigators, including researchers from Chile, said their findings hold promise for better clinical management of MH and open a new door into research on the broader pathogenesis and causes of the condition.
“We hope this breakthrough will eventually lead to medical prevention of MH, prompting global health benefits including major reductions in childhood tooth decay,” they wrote in an article published online Dec. 21 in Frontiers in Physiology.
More than cosmetic
Chalky teeth, characterized by discolored enamel spots, are not merely a cosmetic problem. The condition can lead to severe toothache, painful eating, tooth decay, and even abscesses and extractions. Although its triggers have eluded dental research for a century, Dr. Hubbard’s group said fossilized blood proteins such as albumin in the tooth appear to be at least one cause.
Biochemical evidence indicates that serum albumin surrounding developing teeth is normally excluded from enamel, Dr. Hubbard said in an interview. “Given that albumin binds strongly to hydroxyapatite-based mineral and blocks its growth, we infer that the epithelial barrier – the enamel-forming cells termed ameloblasts and normally responsible for excluding albumin – must break down in places in response to medical triggers.”
This breach enables localized infiltration of albumin, which then blocks further hardening of soft, immature enamel, leading to residual spots or patches of chalky enamel once the tooth eventually erupts into the mouth. “In other words, we infer that chalky enamel spots coincide with localized breaches of an epithelial barrier that are triggered by yet-to-be determined systemic insults,” he said.
Joseph Brofsky, DMD, section head of pediatric dentistry at North Shore LIJ Cohen Children’s Medical Center of New York, in Queens, agreed that that the definitive cause of MH has evaded identification for a hundred years. However, he expressed skepticism about the fossilized blood protein hypothesis.
“That’s a long shot. It’s a possibility, and I’m not ruling it out, but we’re not 100% sure,” said Dr. Brofsky, who was not involved in the research.
In his experience, MH is somewhat less prevalent in the United States, affecting about 1 in 10 children here, which is about half the global rate. “But it’s a problem, and we wish it would go away, but before we know beyond a reasonable doubt what causes this condition, it’s going to be hard to stop it.”
Most cases of MH involve hypomineralization of the 6-year molars, the first adult molars to erupt, but the process starts at birth. “For 6-year molars, normal hardening of dental enamel takes place from the early postnatal period through infancy,” Dr. Hubbard said.
The 2-year and 12-year molars are affected about half as frequently as their 6-year counterparts, “so this extends the medical-risk window out to early school days, and slightly back to the perinatal period for the 12-year and 2-year molars, respectively,” he said.
A critical question is which childhood illnesses are most likely to set the stage for MH, he added. “Forty-plus years of epidemiology have failed to nail a specific cause or causal association. But given the high prevalence of MH – 20% in otherwise healthy kids – naturally we suspect some common illnesses are the culprits,” he said. “But which diseases, which medications, and which combinations?”
Dr. Hubbard’s advice to pediatricians is to be alert to MH: “If you’re inspecting a child’s throat, then why not look at their back teeth, too – particularly when they’re getting their new molars at 2, 6, and 12 years?”
The study was supported by the Melbourne Research Unit for Facial Disorders Department of Pharmacology & Therapeutics, Department of Paediatrics, and Faculty of Medicine, Dentistry, and Health Sciences at the University of Melbourne. The authors and Dr. Brofsky have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First ‘flurona’ cases reported in the U.S.
The first known case was detected in Israel, but until the first week of January no cases had been reported in the United States.
In Los Angeles, a teenaged boy tested positive for both illnesses at a COVID testing site in Brentwood, the Los Angeles Times reported. The child’s mother tested positive for COVID the next day.
“This is the first one that we’re aware of,” Steve Farzam, chief operating officer of 911 COVID Testing, told the LA Times. “In and of itself, it’s not overly concerning; however, it is concerning and can be problematic for someone who has pre-existing medical conditions, anyone who is immunocompromised.”
The teen and his family of five had just returned from vacation in Cabo San Lucas, Mexico. All said they tested negative before the trip, but they tested again when they got home because one of the children had a runny nose, Mr. Farzam said.
The boy, who had not been vaccinated for COVID or the flu, doesn’t have serious symptoms and is recovering at home.
In Houston, a 17-year-old boy, his siblings, and his father felt sick a few days before Christmas and went in for testing, TV station KTRK reported. The teen tested positive for both the flu and COVID.
“I ended up getting tested the day before Christmas for strep throat, flu and COVID,” the teenager, Alec Zierlein, told KTRK. “I didn’t think I had any of the three. It felt like a mild cold.”
Health officials reported Jan. 5 that a flurona case was detected in Hays, Kan., TV station WIBW reported. The patient was being treated in the ICU. No other details were provided. In Israel, flurona was first found in an unvaccinated pregnant woman at Rabin Medical Center in Petach Tikva, according to the Times of Israel. She tested positive for both viruses when she arrived at the medical center, and doctors double-checked to confirm her diagnosis. The woman had mild symptoms and was released in good condition, the news outlet reported.
Public health officials in Israel said they are concerned that an increase in both viruses at the same time could lead to many hospitalizations.
A version of this article first appeared on WebMD.com.
The first known case was detected in Israel, but until the first week of January no cases had been reported in the United States.
In Los Angeles, a teenaged boy tested positive for both illnesses at a COVID testing site in Brentwood, the Los Angeles Times reported. The child’s mother tested positive for COVID the next day.
“This is the first one that we’re aware of,” Steve Farzam, chief operating officer of 911 COVID Testing, told the LA Times. “In and of itself, it’s not overly concerning; however, it is concerning and can be problematic for someone who has pre-existing medical conditions, anyone who is immunocompromised.”
The teen and his family of five had just returned from vacation in Cabo San Lucas, Mexico. All said they tested negative before the trip, but they tested again when they got home because one of the children had a runny nose, Mr. Farzam said.
The boy, who had not been vaccinated for COVID or the flu, doesn’t have serious symptoms and is recovering at home.
In Houston, a 17-year-old boy, his siblings, and his father felt sick a few days before Christmas and went in for testing, TV station KTRK reported. The teen tested positive for both the flu and COVID.
“I ended up getting tested the day before Christmas for strep throat, flu and COVID,” the teenager, Alec Zierlein, told KTRK. “I didn’t think I had any of the three. It felt like a mild cold.”
Health officials reported Jan. 5 that a flurona case was detected in Hays, Kan., TV station WIBW reported. The patient was being treated in the ICU. No other details were provided. In Israel, flurona was first found in an unvaccinated pregnant woman at Rabin Medical Center in Petach Tikva, according to the Times of Israel. She tested positive for both viruses when she arrived at the medical center, and doctors double-checked to confirm her diagnosis. The woman had mild symptoms and was released in good condition, the news outlet reported.
Public health officials in Israel said they are concerned that an increase in both viruses at the same time could lead to many hospitalizations.
A version of this article first appeared on WebMD.com.
The first known case was detected in Israel, but until the first week of January no cases had been reported in the United States.
In Los Angeles, a teenaged boy tested positive for both illnesses at a COVID testing site in Brentwood, the Los Angeles Times reported. The child’s mother tested positive for COVID the next day.
“This is the first one that we’re aware of,” Steve Farzam, chief operating officer of 911 COVID Testing, told the LA Times. “In and of itself, it’s not overly concerning; however, it is concerning and can be problematic for someone who has pre-existing medical conditions, anyone who is immunocompromised.”
The teen and his family of five had just returned from vacation in Cabo San Lucas, Mexico. All said they tested negative before the trip, but they tested again when they got home because one of the children had a runny nose, Mr. Farzam said.
The boy, who had not been vaccinated for COVID or the flu, doesn’t have serious symptoms and is recovering at home.
In Houston, a 17-year-old boy, his siblings, and his father felt sick a few days before Christmas and went in for testing, TV station KTRK reported. The teen tested positive for both the flu and COVID.
“I ended up getting tested the day before Christmas for strep throat, flu and COVID,” the teenager, Alec Zierlein, told KTRK. “I didn’t think I had any of the three. It felt like a mild cold.”
Health officials reported Jan. 5 that a flurona case was detected in Hays, Kan., TV station WIBW reported. The patient was being treated in the ICU. No other details were provided. In Israel, flurona was first found in an unvaccinated pregnant woman at Rabin Medical Center in Petach Tikva, according to the Times of Israel. She tested positive for both viruses when she arrived at the medical center, and doctors double-checked to confirm her diagnosis. The woman had mild symptoms and was released in good condition, the news outlet reported.
Public health officials in Israel said they are concerned that an increase in both viruses at the same time could lead to many hospitalizations.
A version of this article first appeared on WebMD.com.
Pneumonia in infancy predicts respiratory problems in early childhood
Preschoolers who experienced community-acquired pneumonia in infancy were significantly more likely than were those with no history of pneumonia to develop chronic respiratory disorders, based on data from approximately 7,000 individuals.
“Lower respiratory tract infections (LRTI) during the first years of life cause injury to the rapidly developing lung at its most critical stage,” wrote Rotem Lapidot, MD, of Boston University, and colleagues. Previous research has linked pneumonia with subsequent chronic cough, bronchitis, and recurrent pneumonia in children, but data are needed to assess the impact of early community-acquired pneumonia (CAP) on respiratory health in otherwise healthy infants, the researchers said.
In a retrospective matched cohort study published in Respiratory Medicine , the researchers identified 1,343 infants who had CAP in the first 2 years of life, and 6,715 controls, using a large electronic health records dataset (Optum EHR dataset) for the period from Jan. 2011 through June 2018.
The primary outcomes were the development of any chronic respiratory disorders, reactive airway disease, and CAP hospitalizations between ages 2 and 5 years. Infants in the CAP group were otherwise healthy; those with congenital or other conditions that might predispose them to pneumonia were excluded. Baseline characteristics were similar between the CAP patients and controls.
Future risk
Overall, the rates per 100 patient-years for any chronic respiratory disorder were 11.6 for CAP patients versus 4.9 for controls (relative risk, 2.4). Rates for reactive airway disease and CAP hospitalization were 6.1 versus 1.9 per 100 patient-years (RR, 3.2) and 1.0 versus 0.2 per 100 patient-years (RR, 6.3) for the CAP patients and controls, respectively.
The distribution of CAP etiology of CAP in infants at the first hospitalization was 20% bacterial, 27% viral, and 53% unspecified. The relative rates of later respiratory illness were similar across etiologies of the initial hospitalization for CAP, which support the association between infant CAP and later respiratory disease, the researchers said.
Nearly all (97%) of the CAP patients had only one qualifying hospitalization for CAP before 2 years of age, and the mean age at the first hospitalization was 8.9 months. “Rates and relative rates of any chronic respiratory disorder, and our composite for reactive airway disease, increased with age at which the initial CAP hospitalization occurred,” and were highest for children hospitalized at close to 2 years of age, the researchers noted.
Persistent inflammation?
“Our findings add to the evolving hypothesis that persistent inflammation following pneumonia creates an increased risk for subsequent respiratory disease and exacerbations of underlying disease,” the researchers wrote.
The study findings were limited by several factors, including the potential for misclassification of some infants with and without underlying conditions, reliance on discharge information for etiology, and possible lack of generalizability to other populations, the researchers noted.
However, the results indicate an increased risk for respiratory illness in early childhood among infants with CAP, and support the need for greater attention to CAP prevention and for strategies to reduce inflammation after pneumonia, they said. “Further study is needed to confirm the long-term consequences of infant CAP and the underlying mechanisms that lead to such long-term sequelae,” they concluded.
Dr. Lapidot and several coauthors disclosed ties with Pfizer, the study sponsor.
A version of this article first appeared on Medscape.com.
Preschoolers who experienced community-acquired pneumonia in infancy were significantly more likely than were those with no history of pneumonia to develop chronic respiratory disorders, based on data from approximately 7,000 individuals.
“Lower respiratory tract infections (LRTI) during the first years of life cause injury to the rapidly developing lung at its most critical stage,” wrote Rotem Lapidot, MD, of Boston University, and colleagues. Previous research has linked pneumonia with subsequent chronic cough, bronchitis, and recurrent pneumonia in children, but data are needed to assess the impact of early community-acquired pneumonia (CAP) on respiratory health in otherwise healthy infants, the researchers said.
In a retrospective matched cohort study published in Respiratory Medicine , the researchers identified 1,343 infants who had CAP in the first 2 years of life, and 6,715 controls, using a large electronic health records dataset (Optum EHR dataset) for the period from Jan. 2011 through June 2018.
The primary outcomes were the development of any chronic respiratory disorders, reactive airway disease, and CAP hospitalizations between ages 2 and 5 years. Infants in the CAP group were otherwise healthy; those with congenital or other conditions that might predispose them to pneumonia were excluded. Baseline characteristics were similar between the CAP patients and controls.
Future risk
Overall, the rates per 100 patient-years for any chronic respiratory disorder were 11.6 for CAP patients versus 4.9 for controls (relative risk, 2.4). Rates for reactive airway disease and CAP hospitalization were 6.1 versus 1.9 per 100 patient-years (RR, 3.2) and 1.0 versus 0.2 per 100 patient-years (RR, 6.3) for the CAP patients and controls, respectively.
The distribution of CAP etiology of CAP in infants at the first hospitalization was 20% bacterial, 27% viral, and 53% unspecified. The relative rates of later respiratory illness were similar across etiologies of the initial hospitalization for CAP, which support the association between infant CAP and later respiratory disease, the researchers said.
Nearly all (97%) of the CAP patients had only one qualifying hospitalization for CAP before 2 years of age, and the mean age at the first hospitalization was 8.9 months. “Rates and relative rates of any chronic respiratory disorder, and our composite for reactive airway disease, increased with age at which the initial CAP hospitalization occurred,” and were highest for children hospitalized at close to 2 years of age, the researchers noted.
Persistent inflammation?
“Our findings add to the evolving hypothesis that persistent inflammation following pneumonia creates an increased risk for subsequent respiratory disease and exacerbations of underlying disease,” the researchers wrote.
The study findings were limited by several factors, including the potential for misclassification of some infants with and without underlying conditions, reliance on discharge information for etiology, and possible lack of generalizability to other populations, the researchers noted.
However, the results indicate an increased risk for respiratory illness in early childhood among infants with CAP, and support the need for greater attention to CAP prevention and for strategies to reduce inflammation after pneumonia, they said. “Further study is needed to confirm the long-term consequences of infant CAP and the underlying mechanisms that lead to such long-term sequelae,” they concluded.
Dr. Lapidot and several coauthors disclosed ties with Pfizer, the study sponsor.
A version of this article first appeared on Medscape.com.
Preschoolers who experienced community-acquired pneumonia in infancy were significantly more likely than were those with no history of pneumonia to develop chronic respiratory disorders, based on data from approximately 7,000 individuals.
“Lower respiratory tract infections (LRTI) during the first years of life cause injury to the rapidly developing lung at its most critical stage,” wrote Rotem Lapidot, MD, of Boston University, and colleagues. Previous research has linked pneumonia with subsequent chronic cough, bronchitis, and recurrent pneumonia in children, but data are needed to assess the impact of early community-acquired pneumonia (CAP) on respiratory health in otherwise healthy infants, the researchers said.
In a retrospective matched cohort study published in Respiratory Medicine , the researchers identified 1,343 infants who had CAP in the first 2 years of life, and 6,715 controls, using a large electronic health records dataset (Optum EHR dataset) for the period from Jan. 2011 through June 2018.
The primary outcomes were the development of any chronic respiratory disorders, reactive airway disease, and CAP hospitalizations between ages 2 and 5 years. Infants in the CAP group were otherwise healthy; those with congenital or other conditions that might predispose them to pneumonia were excluded. Baseline characteristics were similar between the CAP patients and controls.
Future risk
Overall, the rates per 100 patient-years for any chronic respiratory disorder were 11.6 for CAP patients versus 4.9 for controls (relative risk, 2.4). Rates for reactive airway disease and CAP hospitalization were 6.1 versus 1.9 per 100 patient-years (RR, 3.2) and 1.0 versus 0.2 per 100 patient-years (RR, 6.3) for the CAP patients and controls, respectively.
The distribution of CAP etiology of CAP in infants at the first hospitalization was 20% bacterial, 27% viral, and 53% unspecified. The relative rates of later respiratory illness were similar across etiologies of the initial hospitalization for CAP, which support the association between infant CAP and later respiratory disease, the researchers said.
Nearly all (97%) of the CAP patients had only one qualifying hospitalization for CAP before 2 years of age, and the mean age at the first hospitalization was 8.9 months. “Rates and relative rates of any chronic respiratory disorder, and our composite for reactive airway disease, increased with age at which the initial CAP hospitalization occurred,” and were highest for children hospitalized at close to 2 years of age, the researchers noted.
Persistent inflammation?
“Our findings add to the evolving hypothesis that persistent inflammation following pneumonia creates an increased risk for subsequent respiratory disease and exacerbations of underlying disease,” the researchers wrote.
The study findings were limited by several factors, including the potential for misclassification of some infants with and without underlying conditions, reliance on discharge information for etiology, and possible lack of generalizability to other populations, the researchers noted.
However, the results indicate an increased risk for respiratory illness in early childhood among infants with CAP, and support the need for greater attention to CAP prevention and for strategies to reduce inflammation after pneumonia, they said. “Further study is needed to confirm the long-term consequences of infant CAP and the underlying mechanisms that lead to such long-term sequelae,” they concluded.
Dr. Lapidot and several coauthors disclosed ties with Pfizer, the study sponsor.
A version of this article first appeared on Medscape.com.
FROM RESPIRATORY MEDICINE
HIV+ patients get good outcomes after kidney or liver transplant
in new research that represents some of the longest follow-up on these patients to date.
The findings further support the inclusion of people with HIV in transplant resource allocation, say the researchers.
“Overall, the excellent outcomes following liver and kidney transplant recipients in HIV-infected recipients justify the utilization of a scarce resource,” senior author Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program and surgical director of the Pediatric Renal Transplant Program at the University of California, San Francisco (UCSF), said in an interview.
“Many centers still view HIV as a strict contraindication [for transplantation]. This data shows it is not,” he emphasized.
The study, published in JAMA Surgery, involved HIV-positive patients who received kidney or liver transplants between 2000 and 2019 at UCSF, which has unique access to some of the longest-term data on those outcomes.
“UCSF was the first U.S. center to do transplants routinely in people with HIV, and based on the large volume of transplants that are performed, we were able to use propensity matching to address the comparison of HIV-positive and negative liver and kidney transplant recipients at a single center,” Dr. Stock explained.
“To the best of our knowledge, there are no long-term reports [greater than 10 years] on [transplant] outcomes in the HIV-positive population.”
Commenting on the study, David Klassen, MD, chief medical officer of the United Network for Organ Sharing (UNOS), noted that the findings “confirm previous research done at UCSF and reported in the New England Journal of Medicine” in 2010. “It extends the previous findings.”
“The take-home message is that these HIV-positive patients can be successfully transplanted with expected good outcomes and will derive substantial benefit from transplantation,” Dr. Klassen said.
Kidney transplant patient survival lower, graft survival similar
For the kidney transplant analysis, 119 HIV-positive recipients were propensity matched with 655 recipients who were HIV-negative, with the patients’ mean age about 52 and approximately 70% male.
At 15-years post-transplant, patient survival was 53.6% among the HIV-positive patients versus 79.6% for HIV-negative (P = .03).
Graft survival among the kidney transplant patients was proportionally higher among HIV-positive patients after 15 years (75% vs. 57%); however, the difference was not statistically significant (P = .77).
First author Arya Zarinsefat, MD, of the Department of Surgery at UCSF, speculated that the lower long-term patient survival among HIV-positive kidney transplant recipients may reflect known cardiovascular risks among those patients.
“We postulated that part of this may be due to the fact that HIV-positive patients certainly have additional comorbidities, specifically cardiovascular” ones, he told this news organization.
“When looking at the survival curve, survival was nearly identical at 5 years and only started to diverge at 10 years post-transplant,” he noted.
A further evaluation of patients with HIV who were co-infected with hepatitis C (HCV) showed that those with HIV-HCV co-infection prior to the center’s introduction of anti-HCV direct-acting antiviral (DAA) medications in 2014 had the lowest survival rate of all subgroups, at 57.1% at 5 years post-transplant (P = .045 vs. those treated after 2014).
Liver transplant patient survival similar
In terms of liver transplant outcomes, among 83 HIV-positive recipients who were propensity-matched with 468 HIV-negative recipients, the mean age was about 53 and about 66% were male.
The patient survival rates at 15 years were not significantly different between the groups, at 70% for HIV-positive and 75.7% for HIV-negative, (P = .12).
Similar to the kidney transplant recipients, the worst survival among all liver transplant subgroups was among HIV-HCV co-infected patients prior to access to HCV direct-acting antivirals in 2014, with a 5-year survival of 59.5% (P = .04).
“Since the advent of HCV direct-acting antivirals, liver transplant outcomes in HCV mono-infected patients are comparable to HCV/HIV co-infected recipients,” Dr. Stock said.
Acute rejection rates higher with HIV-positivity versus national averages
The rates of acute rejection at 1 year in the kidney and liver transplant, HIV-positive groups – at about 20% and 30%, respectively – were, however, higher than national average incidence rates of about 10% at 1 year.
Long-term data on those patients showed the acute rejection affected graft survival outcomes with kidney transplant recipients: HIV-positive kidney transplant recipients who had at least one episode of acute rejection had a graft survival of just 52.8% at 15 years post-transplant, compared with 91.8% among recipients without acute rejection.
Such differences were not observed among HIV-positive liver transplant recipients.
The authors note that the increased risk of acute rejection in HIV-positive kidney transplant patients is consistent with previous studies, with causes that may be multifactorial.
Top theories include drug interactions with protease inhibitors, resulting in some centers transitioning HIV-infected patients from those regimens to integrase-based regimens prior to transplant.
“The management and prevention of acute rejection in HIV-positive kidney transplant [patients] will therefore continue to be a key component in the care of these patients,” the authors note in their study.
The study was supported in part by the National Institutes of Health. The study authors and Dr. Klassen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in new research that represents some of the longest follow-up on these patients to date.
The findings further support the inclusion of people with HIV in transplant resource allocation, say the researchers.
“Overall, the excellent outcomes following liver and kidney transplant recipients in HIV-infected recipients justify the utilization of a scarce resource,” senior author Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program and surgical director of the Pediatric Renal Transplant Program at the University of California, San Francisco (UCSF), said in an interview.
“Many centers still view HIV as a strict contraindication [for transplantation]. This data shows it is not,” he emphasized.
The study, published in JAMA Surgery, involved HIV-positive patients who received kidney or liver transplants between 2000 and 2019 at UCSF, which has unique access to some of the longest-term data on those outcomes.
“UCSF was the first U.S. center to do transplants routinely in people with HIV, and based on the large volume of transplants that are performed, we were able to use propensity matching to address the comparison of HIV-positive and negative liver and kidney transplant recipients at a single center,” Dr. Stock explained.
“To the best of our knowledge, there are no long-term reports [greater than 10 years] on [transplant] outcomes in the HIV-positive population.”
Commenting on the study, David Klassen, MD, chief medical officer of the United Network for Organ Sharing (UNOS), noted that the findings “confirm previous research done at UCSF and reported in the New England Journal of Medicine” in 2010. “It extends the previous findings.”
“The take-home message is that these HIV-positive patients can be successfully transplanted with expected good outcomes and will derive substantial benefit from transplantation,” Dr. Klassen said.
Kidney transplant patient survival lower, graft survival similar
For the kidney transplant analysis, 119 HIV-positive recipients were propensity matched with 655 recipients who were HIV-negative, with the patients’ mean age about 52 and approximately 70% male.
At 15-years post-transplant, patient survival was 53.6% among the HIV-positive patients versus 79.6% for HIV-negative (P = .03).
Graft survival among the kidney transplant patients was proportionally higher among HIV-positive patients after 15 years (75% vs. 57%); however, the difference was not statistically significant (P = .77).
First author Arya Zarinsefat, MD, of the Department of Surgery at UCSF, speculated that the lower long-term patient survival among HIV-positive kidney transplant recipients may reflect known cardiovascular risks among those patients.
“We postulated that part of this may be due to the fact that HIV-positive patients certainly have additional comorbidities, specifically cardiovascular” ones, he told this news organization.
“When looking at the survival curve, survival was nearly identical at 5 years and only started to diverge at 10 years post-transplant,” he noted.
A further evaluation of patients with HIV who were co-infected with hepatitis C (HCV) showed that those with HIV-HCV co-infection prior to the center’s introduction of anti-HCV direct-acting antiviral (DAA) medications in 2014 had the lowest survival rate of all subgroups, at 57.1% at 5 years post-transplant (P = .045 vs. those treated after 2014).
Liver transplant patient survival similar
In terms of liver transplant outcomes, among 83 HIV-positive recipients who were propensity-matched with 468 HIV-negative recipients, the mean age was about 53 and about 66% were male.
The patient survival rates at 15 years were not significantly different between the groups, at 70% for HIV-positive and 75.7% for HIV-negative, (P = .12).
Similar to the kidney transplant recipients, the worst survival among all liver transplant subgroups was among HIV-HCV co-infected patients prior to access to HCV direct-acting antivirals in 2014, with a 5-year survival of 59.5% (P = .04).
“Since the advent of HCV direct-acting antivirals, liver transplant outcomes in HCV mono-infected patients are comparable to HCV/HIV co-infected recipients,” Dr. Stock said.
Acute rejection rates higher with HIV-positivity versus national averages
The rates of acute rejection at 1 year in the kidney and liver transplant, HIV-positive groups – at about 20% and 30%, respectively – were, however, higher than national average incidence rates of about 10% at 1 year.
Long-term data on those patients showed the acute rejection affected graft survival outcomes with kidney transplant recipients: HIV-positive kidney transplant recipients who had at least one episode of acute rejection had a graft survival of just 52.8% at 15 years post-transplant, compared with 91.8% among recipients without acute rejection.
Such differences were not observed among HIV-positive liver transplant recipients.
The authors note that the increased risk of acute rejection in HIV-positive kidney transplant patients is consistent with previous studies, with causes that may be multifactorial.
Top theories include drug interactions with protease inhibitors, resulting in some centers transitioning HIV-infected patients from those regimens to integrase-based regimens prior to transplant.
“The management and prevention of acute rejection in HIV-positive kidney transplant [patients] will therefore continue to be a key component in the care of these patients,” the authors note in their study.
The study was supported in part by the National Institutes of Health. The study authors and Dr. Klassen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in new research that represents some of the longest follow-up on these patients to date.
The findings further support the inclusion of people with HIV in transplant resource allocation, say the researchers.
“Overall, the excellent outcomes following liver and kidney transplant recipients in HIV-infected recipients justify the utilization of a scarce resource,” senior author Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program and surgical director of the Pediatric Renal Transplant Program at the University of California, San Francisco (UCSF), said in an interview.
“Many centers still view HIV as a strict contraindication [for transplantation]. This data shows it is not,” he emphasized.
The study, published in JAMA Surgery, involved HIV-positive patients who received kidney or liver transplants between 2000 and 2019 at UCSF, which has unique access to some of the longest-term data on those outcomes.
“UCSF was the first U.S. center to do transplants routinely in people with HIV, and based on the large volume of transplants that are performed, we were able to use propensity matching to address the comparison of HIV-positive and negative liver and kidney transplant recipients at a single center,” Dr. Stock explained.
“To the best of our knowledge, there are no long-term reports [greater than 10 years] on [transplant] outcomes in the HIV-positive population.”
Commenting on the study, David Klassen, MD, chief medical officer of the United Network for Organ Sharing (UNOS), noted that the findings “confirm previous research done at UCSF and reported in the New England Journal of Medicine” in 2010. “It extends the previous findings.”
“The take-home message is that these HIV-positive patients can be successfully transplanted with expected good outcomes and will derive substantial benefit from transplantation,” Dr. Klassen said.
Kidney transplant patient survival lower, graft survival similar
For the kidney transplant analysis, 119 HIV-positive recipients were propensity matched with 655 recipients who were HIV-negative, with the patients’ mean age about 52 and approximately 70% male.
At 15-years post-transplant, patient survival was 53.6% among the HIV-positive patients versus 79.6% for HIV-negative (P = .03).
Graft survival among the kidney transplant patients was proportionally higher among HIV-positive patients after 15 years (75% vs. 57%); however, the difference was not statistically significant (P = .77).
First author Arya Zarinsefat, MD, of the Department of Surgery at UCSF, speculated that the lower long-term patient survival among HIV-positive kidney transplant recipients may reflect known cardiovascular risks among those patients.
“We postulated that part of this may be due to the fact that HIV-positive patients certainly have additional comorbidities, specifically cardiovascular” ones, he told this news organization.
“When looking at the survival curve, survival was nearly identical at 5 years and only started to diverge at 10 years post-transplant,” he noted.
A further evaluation of patients with HIV who were co-infected with hepatitis C (HCV) showed that those with HIV-HCV co-infection prior to the center’s introduction of anti-HCV direct-acting antiviral (DAA) medications in 2014 had the lowest survival rate of all subgroups, at 57.1% at 5 years post-transplant (P = .045 vs. those treated after 2014).
Liver transplant patient survival similar
In terms of liver transplant outcomes, among 83 HIV-positive recipients who were propensity-matched with 468 HIV-negative recipients, the mean age was about 53 and about 66% were male.
The patient survival rates at 15 years were not significantly different between the groups, at 70% for HIV-positive and 75.7% for HIV-negative, (P = .12).
Similar to the kidney transplant recipients, the worst survival among all liver transplant subgroups was among HIV-HCV co-infected patients prior to access to HCV direct-acting antivirals in 2014, with a 5-year survival of 59.5% (P = .04).
“Since the advent of HCV direct-acting antivirals, liver transplant outcomes in HCV mono-infected patients are comparable to HCV/HIV co-infected recipients,” Dr. Stock said.
Acute rejection rates higher with HIV-positivity versus national averages
The rates of acute rejection at 1 year in the kidney and liver transplant, HIV-positive groups – at about 20% and 30%, respectively – were, however, higher than national average incidence rates of about 10% at 1 year.
Long-term data on those patients showed the acute rejection affected graft survival outcomes with kidney transplant recipients: HIV-positive kidney transplant recipients who had at least one episode of acute rejection had a graft survival of just 52.8% at 15 years post-transplant, compared with 91.8% among recipients without acute rejection.
Such differences were not observed among HIV-positive liver transplant recipients.
The authors note that the increased risk of acute rejection in HIV-positive kidney transplant patients is consistent with previous studies, with causes that may be multifactorial.
Top theories include drug interactions with protease inhibitors, resulting in some centers transitioning HIV-infected patients from those regimens to integrase-based regimens prior to transplant.
“The management and prevention of acute rejection in HIV-positive kidney transplant [patients] will therefore continue to be a key component in the care of these patients,” the authors note in their study.
The study was supported in part by the National Institutes of Health. The study authors and Dr. Klassen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.