User login
Could the Omicron surge hasten the transition from pandemic to endemic?
The record-setting surge in COVID-19 cases nationwide – including more than one million new infections reported on Jan. 3 – raises questions about whether the higher Omicron variant transmissibility will accelerate a transition from pandemic to endemic disease.
Furthermore,
Infectious disease experts weigh in on these possibilities.
An endemic eventuality?
Whether the current surge will mean the predicted switch to endemic COVID-19 will come sooner “is very hard to predict,” Michael Lin, MD, MPH, told this news organization.
“It’s an open question,” he said, “if another highly transmissible variant will emerge.”
On a positive note, “at this point many more people have received their vaccinations or been infected. And over time, repeated infections have led to milder symptoms,” added Dr. Lin, hospital epidemiologist at Rush Medical College, Chicago.
“It could end up being a seasonal variant,” he said.
COVID-19 going endemic is “a real possibility, but unfortunately ... it doesn’t seem necessarily that we’re going to have the same predictable pattern we have with the flu,” said Eleftherios Mylonakis, MD, PhD, chief of infectious diseases for Lifespan and its affiliates at Rhode Island Hospital and Miriam Hospital in Providence.
“We have a number of other viruses that don’t follow the same annual pattern,” he said.
Unknowns include how long individuals’ immune responses, including T-cell defenses, will last going forward.
A transition from pandemic to endemic is “not a light switch, and there are no metrics associated with what endemic means for COVID-19,” said Syra Madad, DHSc., MSc, MCP, an infectious disease epidemiologist at Harvard’s Belfer Center for Science and International Affairs, Boston.
“Instead, we should continue to focus on decreasing transmission rates and preventing our hospitals from getting overwhelmed,” she said.
A hastening to herd immunity?
“The short answer is yes,” Dr. Lin said when asked if the increased transmissibility and increased cases linked to the Omicron surge could get the U.S. closer to herd immunity.
“The twist in this whole story,” he said, “is the virus mutated enough to escape first-line immune defenses, specifically antibodies. That is why we are seeing breakthrough infections, even in highly vaccinated populations.”
Dr. Mylonakis was more skeptical regarding herd immunity.
“The concept of herd immunity with a rapidly evolving virus is very difficult” to address, he said.
One reason is the number of unknown factors, Dr. Mylonakis said. He predicted a clearer picture will emerge after the Omicrons surge subsides. Also, with so many people infected by the Omicron variant, immune protection should peak.
“People will have boosted immunity. Not everybody, unfortunately, because there are people who cannot really mount [a full immune response] because of age, because of immunosuppression, etc.,” said Dr. Mylonakis, who is also professor of infectious diseases at Brown University.
“But the majority of the population will be exposed and will mount some degree of immunity.”
Dr. Madad agreed. “The omicron variant will add much more immunity into our population by both the preferred pathway – which is through vaccination – as well as through those that are unvaccinated and get infected with omicron,” she said.
“The pathway to gain immunity from vaccination is the safest option, and already over 1 million doses of the COVID-19 vaccine are going into arms per day – this includes first, second, and additional doses like boosters,” added Dr. Madad, who is also senior director of the System-wide Special Pathogens Program at New York City Health and Hospitals.
A shorter, more intense surge?
The United Kingdom’s experience with COVID-19 has often served as a bellwether of what is likely to happen in the U.S. If that is the case with the Omicron surge, the peak should last about 4 weeks, Dr. Mylonakis said.
In other words, the accelerated spread of Omicron could mean this surge passes more quickly than Delta.
Furthermore, some evidence suggests neutralizing antibodies produced by Omicron infection remain effective against the Delta variant – thereby reducing the risk of Delta reinfections over time.
The ability to neutralize the Delta variant increased more than fourfold after a median 14 days, according to data from a preprint study posted Dec. 27 on MedRxiv.
At the same time, neutralization of the Omicron variant increased 14-fold as participants mounted an antibody response. The study was conducted in vaccinated and unvaccinated people infected by Omicron in South Africa shortly after symptoms started. It has yet to be peer reviewed.
Eric Topol, MD, editor-in-chief of Medscape, described the results as “especially good news” in a tweet.
The current surge could also mean enhanced protection in the future.
“As we look at getting to the other side of this Omicron wave, we will end up with more immunity,” Dr. Madad said. “And with more immunity means we’ll be better guarded against the next emerging variant.”
A version of this article first appeared on Medscape.com.
The record-setting surge in COVID-19 cases nationwide – including more than one million new infections reported on Jan. 3 – raises questions about whether the higher Omicron variant transmissibility will accelerate a transition from pandemic to endemic disease.
Furthermore,
Infectious disease experts weigh in on these possibilities.
An endemic eventuality?
Whether the current surge will mean the predicted switch to endemic COVID-19 will come sooner “is very hard to predict,” Michael Lin, MD, MPH, told this news organization.
“It’s an open question,” he said, “if another highly transmissible variant will emerge.”
On a positive note, “at this point many more people have received their vaccinations or been infected. And over time, repeated infections have led to milder symptoms,” added Dr. Lin, hospital epidemiologist at Rush Medical College, Chicago.
“It could end up being a seasonal variant,” he said.
COVID-19 going endemic is “a real possibility, but unfortunately ... it doesn’t seem necessarily that we’re going to have the same predictable pattern we have with the flu,” said Eleftherios Mylonakis, MD, PhD, chief of infectious diseases for Lifespan and its affiliates at Rhode Island Hospital and Miriam Hospital in Providence.
“We have a number of other viruses that don’t follow the same annual pattern,” he said.
Unknowns include how long individuals’ immune responses, including T-cell defenses, will last going forward.
A transition from pandemic to endemic is “not a light switch, and there are no metrics associated with what endemic means for COVID-19,” said Syra Madad, DHSc., MSc, MCP, an infectious disease epidemiologist at Harvard’s Belfer Center for Science and International Affairs, Boston.
“Instead, we should continue to focus on decreasing transmission rates and preventing our hospitals from getting overwhelmed,” she said.
A hastening to herd immunity?
“The short answer is yes,” Dr. Lin said when asked if the increased transmissibility and increased cases linked to the Omicron surge could get the U.S. closer to herd immunity.
“The twist in this whole story,” he said, “is the virus mutated enough to escape first-line immune defenses, specifically antibodies. That is why we are seeing breakthrough infections, even in highly vaccinated populations.”
Dr. Mylonakis was more skeptical regarding herd immunity.
“The concept of herd immunity with a rapidly evolving virus is very difficult” to address, he said.
One reason is the number of unknown factors, Dr. Mylonakis said. He predicted a clearer picture will emerge after the Omicrons surge subsides. Also, with so many people infected by the Omicron variant, immune protection should peak.
“People will have boosted immunity. Not everybody, unfortunately, because there are people who cannot really mount [a full immune response] because of age, because of immunosuppression, etc.,” said Dr. Mylonakis, who is also professor of infectious diseases at Brown University.
“But the majority of the population will be exposed and will mount some degree of immunity.”
Dr. Madad agreed. “The omicron variant will add much more immunity into our population by both the preferred pathway – which is through vaccination – as well as through those that are unvaccinated and get infected with omicron,” she said.
“The pathway to gain immunity from vaccination is the safest option, and already over 1 million doses of the COVID-19 vaccine are going into arms per day – this includes first, second, and additional doses like boosters,” added Dr. Madad, who is also senior director of the System-wide Special Pathogens Program at New York City Health and Hospitals.
A shorter, more intense surge?
The United Kingdom’s experience with COVID-19 has often served as a bellwether of what is likely to happen in the U.S. If that is the case with the Omicron surge, the peak should last about 4 weeks, Dr. Mylonakis said.
In other words, the accelerated spread of Omicron could mean this surge passes more quickly than Delta.
Furthermore, some evidence suggests neutralizing antibodies produced by Omicron infection remain effective against the Delta variant – thereby reducing the risk of Delta reinfections over time.
The ability to neutralize the Delta variant increased more than fourfold after a median 14 days, according to data from a preprint study posted Dec. 27 on MedRxiv.
At the same time, neutralization of the Omicron variant increased 14-fold as participants mounted an antibody response. The study was conducted in vaccinated and unvaccinated people infected by Omicron in South Africa shortly after symptoms started. It has yet to be peer reviewed.
Eric Topol, MD, editor-in-chief of Medscape, described the results as “especially good news” in a tweet.
The current surge could also mean enhanced protection in the future.
“As we look at getting to the other side of this Omicron wave, we will end up with more immunity,” Dr. Madad said. “And with more immunity means we’ll be better guarded against the next emerging variant.”
A version of this article first appeared on Medscape.com.
The record-setting surge in COVID-19 cases nationwide – including more than one million new infections reported on Jan. 3 – raises questions about whether the higher Omicron variant transmissibility will accelerate a transition from pandemic to endemic disease.
Furthermore,
Infectious disease experts weigh in on these possibilities.
An endemic eventuality?
Whether the current surge will mean the predicted switch to endemic COVID-19 will come sooner “is very hard to predict,” Michael Lin, MD, MPH, told this news organization.
“It’s an open question,” he said, “if another highly transmissible variant will emerge.”
On a positive note, “at this point many more people have received their vaccinations or been infected. And over time, repeated infections have led to milder symptoms,” added Dr. Lin, hospital epidemiologist at Rush Medical College, Chicago.
“It could end up being a seasonal variant,” he said.
COVID-19 going endemic is “a real possibility, but unfortunately ... it doesn’t seem necessarily that we’re going to have the same predictable pattern we have with the flu,” said Eleftherios Mylonakis, MD, PhD, chief of infectious diseases for Lifespan and its affiliates at Rhode Island Hospital and Miriam Hospital in Providence.
“We have a number of other viruses that don’t follow the same annual pattern,” he said.
Unknowns include how long individuals’ immune responses, including T-cell defenses, will last going forward.
A transition from pandemic to endemic is “not a light switch, and there are no metrics associated with what endemic means for COVID-19,” said Syra Madad, DHSc., MSc, MCP, an infectious disease epidemiologist at Harvard’s Belfer Center for Science and International Affairs, Boston.
“Instead, we should continue to focus on decreasing transmission rates and preventing our hospitals from getting overwhelmed,” she said.
A hastening to herd immunity?
“The short answer is yes,” Dr. Lin said when asked if the increased transmissibility and increased cases linked to the Omicron surge could get the U.S. closer to herd immunity.
“The twist in this whole story,” he said, “is the virus mutated enough to escape first-line immune defenses, specifically antibodies. That is why we are seeing breakthrough infections, even in highly vaccinated populations.”
Dr. Mylonakis was more skeptical regarding herd immunity.
“The concept of herd immunity with a rapidly evolving virus is very difficult” to address, he said.
One reason is the number of unknown factors, Dr. Mylonakis said. He predicted a clearer picture will emerge after the Omicrons surge subsides. Also, with so many people infected by the Omicron variant, immune protection should peak.
“People will have boosted immunity. Not everybody, unfortunately, because there are people who cannot really mount [a full immune response] because of age, because of immunosuppression, etc.,” said Dr. Mylonakis, who is also professor of infectious diseases at Brown University.
“But the majority of the population will be exposed and will mount some degree of immunity.”
Dr. Madad agreed. “The omicron variant will add much more immunity into our population by both the preferred pathway – which is through vaccination – as well as through those that are unvaccinated and get infected with omicron,” she said.
“The pathway to gain immunity from vaccination is the safest option, and already over 1 million doses of the COVID-19 vaccine are going into arms per day – this includes first, second, and additional doses like boosters,” added Dr. Madad, who is also senior director of the System-wide Special Pathogens Program at New York City Health and Hospitals.
A shorter, more intense surge?
The United Kingdom’s experience with COVID-19 has often served as a bellwether of what is likely to happen in the U.S. If that is the case with the Omicron surge, the peak should last about 4 weeks, Dr. Mylonakis said.
In other words, the accelerated spread of Omicron could mean this surge passes more quickly than Delta.
Furthermore, some evidence suggests neutralizing antibodies produced by Omicron infection remain effective against the Delta variant – thereby reducing the risk of Delta reinfections over time.
The ability to neutralize the Delta variant increased more than fourfold after a median 14 days, according to data from a preprint study posted Dec. 27 on MedRxiv.
At the same time, neutralization of the Omicron variant increased 14-fold as participants mounted an antibody response. The study was conducted in vaccinated and unvaccinated people infected by Omicron in South Africa shortly after symptoms started. It has yet to be peer reviewed.
Eric Topol, MD, editor-in-chief of Medscape, described the results as “especially good news” in a tweet.
The current surge could also mean enhanced protection in the future.
“As we look at getting to the other side of this Omicron wave, we will end up with more immunity,” Dr. Madad said. “And with more immunity means we’ll be better guarded against the next emerging variant.”
A version of this article first appeared on Medscape.com.
As Omicron surges, hospital beds fill, but ICUs less affected
So far, hospitalizations caused by the Omicron variant appear to be milder than in previous waves.
“We are seeing an increase in the number of hospitalizations,” Rahul Sharma, MD, emergency physician-in-chief for New York–Presbyterian/Weill Cornell Medicine, told the New York Times.
“We’re not sending as many patients to the ICU, we’re not intubating as many patients, and actually, most of our patients that are coming to the emergency department that do test positive are actually being discharged,” he said.
Most Omicron patients in ICUs are unvaccinated or have severely compromised immune systems, doctors told the newspaper.
Currently, about 113,000 COVID-19 patients are hospitalized across the country, according to the latest data from the Department of Health & Human Services. About 76% of inpatient beds are in use nationwide, with about 16% of inpatient beds in use for COVID-19.
Early data suggests that the Omicron variant may cause less severe disease. But it’s easier to catch the variant, so more people are getting the virus, including people who have some immunity through prior infection or vaccination, which is driving up hospitalization numbers.
In New York, for instance, COVID-19 hospitalizations have surpassed the peak of last winter’s surge, the newspaper reported. In addition, Maryland Gov. Larry Hogan declared a state of emergency on Jan. 4, noting that the state had more hospitalized COVID-19 patients than at any other time during the pandemic.
“We’re in truly crushed mode,” Gabe Kelen, MD, chair of the department of emergency medicine for the Johns Hopkins University, Baltimore, told the Times.
Earlier in the pandemic, hospitals faced challenges with stockpiling ventilators and personal protective equipment, doctors told the newspaper. Now they’re dealing with limits on hospital beds and staffing as health care workers test positive. The increase in COVID-19 cases has also come along with a rise in hospitalizations for other conditions such as heart attacks and strokes.
In response, some hospitals are considering cutting elective surgeries because of staff shortages and limited bed capacity, the newspaper reported. In the meantime, hospital staff and administrators are watching case numbers to see how high hospitalizations may soar because of the Omicron variant.
“How high will it go? Can’t tell you. Don’t know,” James Musser, MD, chair of pathology and genomic medicine at Houston Methodist, told the Times. “We’re all watching it, obviously, very, very closely.”
A version of this article first appeared on WebMD.com.
So far, hospitalizations caused by the Omicron variant appear to be milder than in previous waves.
“We are seeing an increase in the number of hospitalizations,” Rahul Sharma, MD, emergency physician-in-chief for New York–Presbyterian/Weill Cornell Medicine, told the New York Times.
“We’re not sending as many patients to the ICU, we’re not intubating as many patients, and actually, most of our patients that are coming to the emergency department that do test positive are actually being discharged,” he said.
Most Omicron patients in ICUs are unvaccinated or have severely compromised immune systems, doctors told the newspaper.
Currently, about 113,000 COVID-19 patients are hospitalized across the country, according to the latest data from the Department of Health & Human Services. About 76% of inpatient beds are in use nationwide, with about 16% of inpatient beds in use for COVID-19.
Early data suggests that the Omicron variant may cause less severe disease. But it’s easier to catch the variant, so more people are getting the virus, including people who have some immunity through prior infection or vaccination, which is driving up hospitalization numbers.
In New York, for instance, COVID-19 hospitalizations have surpassed the peak of last winter’s surge, the newspaper reported. In addition, Maryland Gov. Larry Hogan declared a state of emergency on Jan. 4, noting that the state had more hospitalized COVID-19 patients than at any other time during the pandemic.
“We’re in truly crushed mode,” Gabe Kelen, MD, chair of the department of emergency medicine for the Johns Hopkins University, Baltimore, told the Times.
Earlier in the pandemic, hospitals faced challenges with stockpiling ventilators and personal protective equipment, doctors told the newspaper. Now they’re dealing with limits on hospital beds and staffing as health care workers test positive. The increase in COVID-19 cases has also come along with a rise in hospitalizations for other conditions such as heart attacks and strokes.
In response, some hospitals are considering cutting elective surgeries because of staff shortages and limited bed capacity, the newspaper reported. In the meantime, hospital staff and administrators are watching case numbers to see how high hospitalizations may soar because of the Omicron variant.
“How high will it go? Can’t tell you. Don’t know,” James Musser, MD, chair of pathology and genomic medicine at Houston Methodist, told the Times. “We’re all watching it, obviously, very, very closely.”
A version of this article first appeared on WebMD.com.
So far, hospitalizations caused by the Omicron variant appear to be milder than in previous waves.
“We are seeing an increase in the number of hospitalizations,” Rahul Sharma, MD, emergency physician-in-chief for New York–Presbyterian/Weill Cornell Medicine, told the New York Times.
“We’re not sending as many patients to the ICU, we’re not intubating as many patients, and actually, most of our patients that are coming to the emergency department that do test positive are actually being discharged,” he said.
Most Omicron patients in ICUs are unvaccinated or have severely compromised immune systems, doctors told the newspaper.
Currently, about 113,000 COVID-19 patients are hospitalized across the country, according to the latest data from the Department of Health & Human Services. About 76% of inpatient beds are in use nationwide, with about 16% of inpatient beds in use for COVID-19.
Early data suggests that the Omicron variant may cause less severe disease. But it’s easier to catch the variant, so more people are getting the virus, including people who have some immunity through prior infection or vaccination, which is driving up hospitalization numbers.
In New York, for instance, COVID-19 hospitalizations have surpassed the peak of last winter’s surge, the newspaper reported. In addition, Maryland Gov. Larry Hogan declared a state of emergency on Jan. 4, noting that the state had more hospitalized COVID-19 patients than at any other time during the pandemic.
“We’re in truly crushed mode,” Gabe Kelen, MD, chair of the department of emergency medicine for the Johns Hopkins University, Baltimore, told the Times.
Earlier in the pandemic, hospitals faced challenges with stockpiling ventilators and personal protective equipment, doctors told the newspaper. Now they’re dealing with limits on hospital beds and staffing as health care workers test positive. The increase in COVID-19 cases has also come along with a rise in hospitalizations for other conditions such as heart attacks and strokes.
In response, some hospitals are considering cutting elective surgeries because of staff shortages and limited bed capacity, the newspaper reported. In the meantime, hospital staff and administrators are watching case numbers to see how high hospitalizations may soar because of the Omicron variant.
“How high will it go? Can’t tell you. Don’t know,” James Musser, MD, chair of pathology and genomic medicine at Houston Methodist, told the Times. “We’re all watching it, obviously, very, very closely.”
A version of this article first appeared on WebMD.com.
Freshwater aquarium provides source for melioidosis infection
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Herpes Zoster Following a Nucleoside-Modified Messenger RNA COVID-19 Vaccine
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
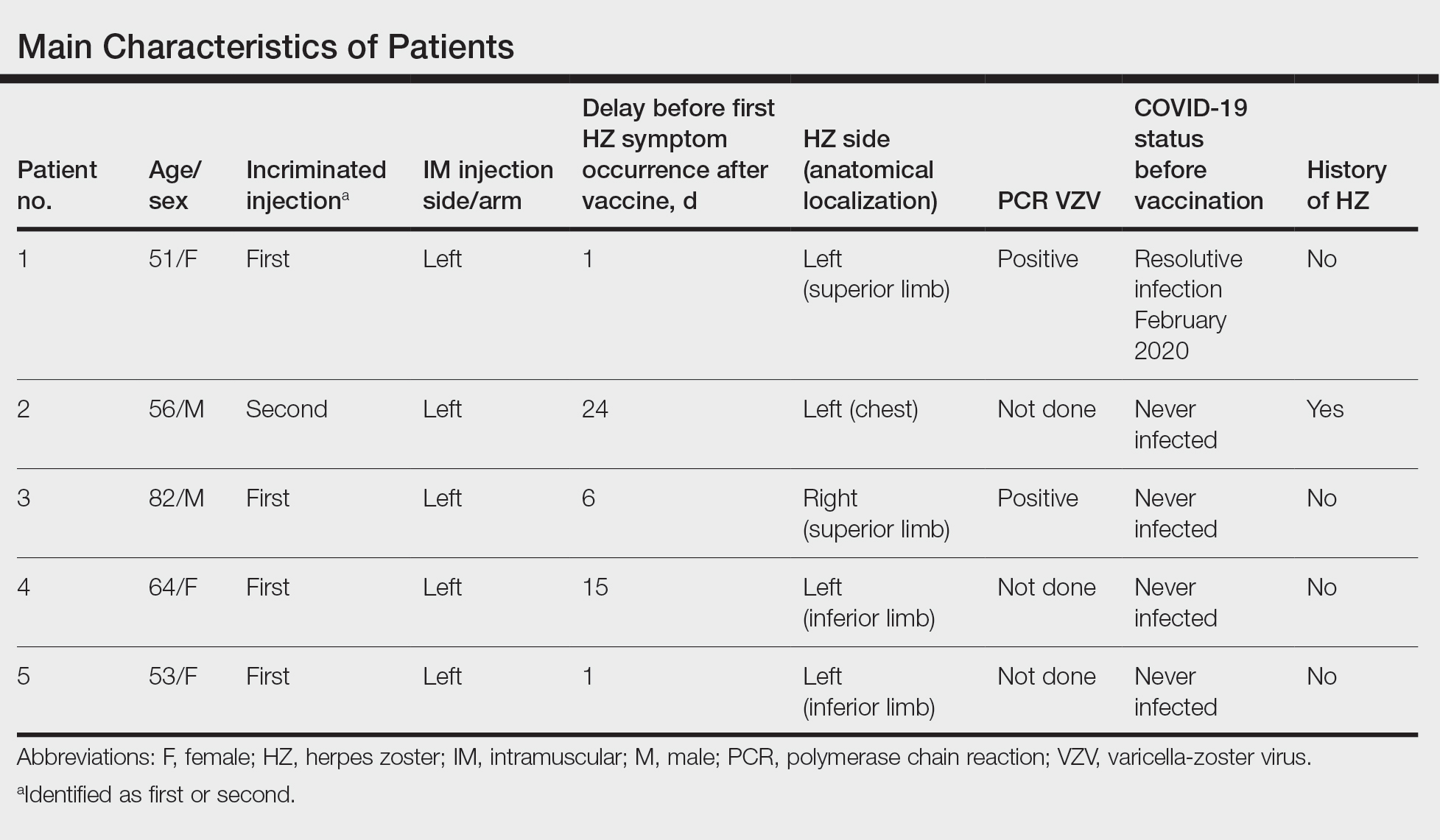
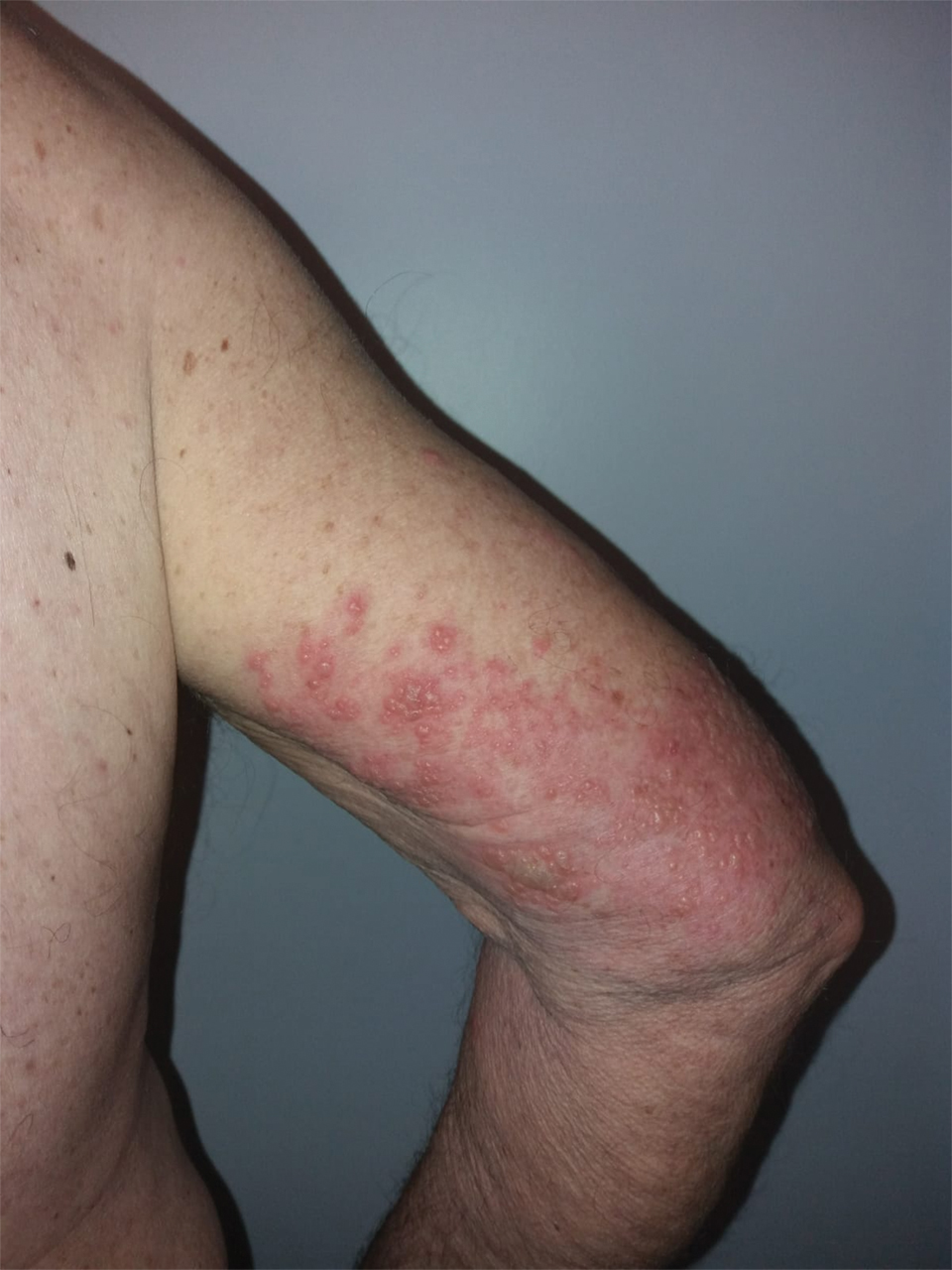
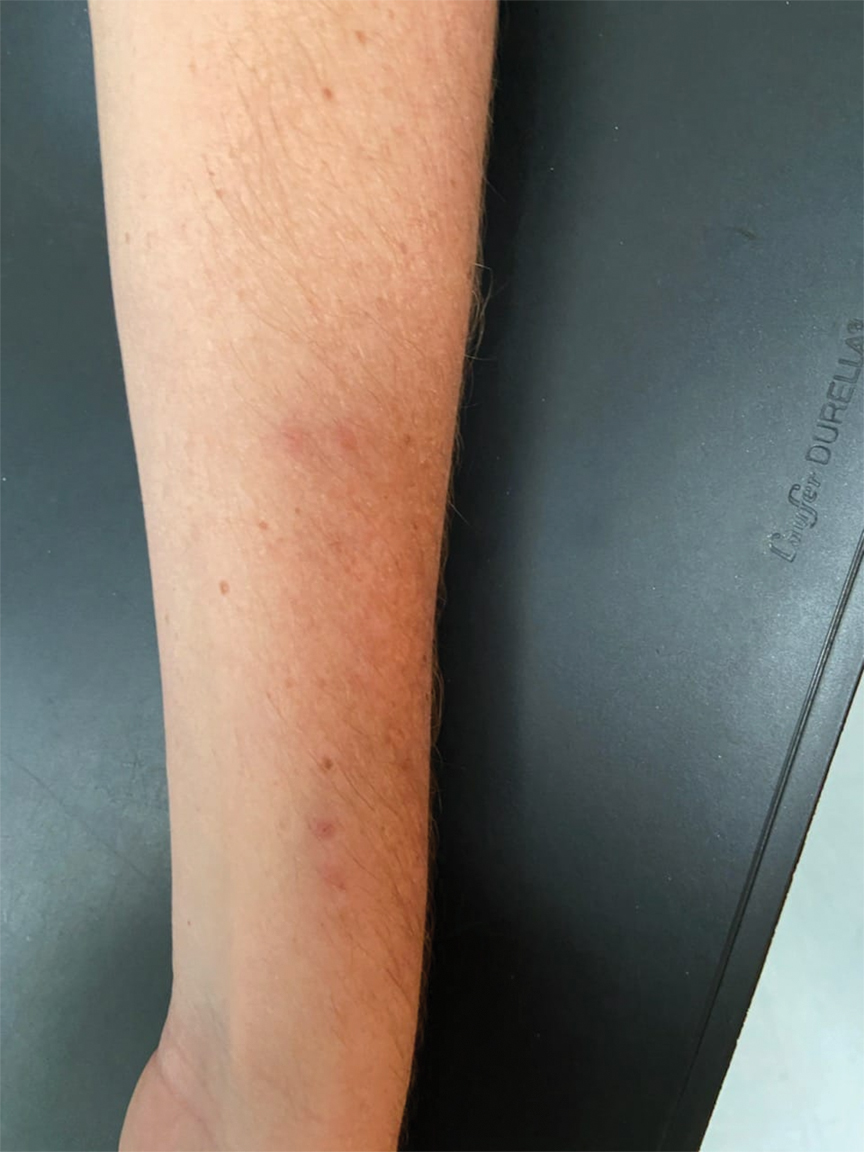
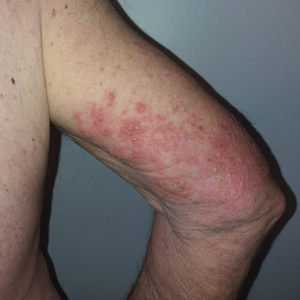
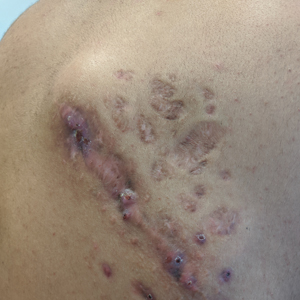
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.
Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5
Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9
In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.
Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5
Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9
In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.
Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5
Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9
In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Practice Points
- Herpes zoster (HZ) has been reported following COVID-19 vaccination.
- Postinjection pain is common with COVID-19 vaccination, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay in onset between the injection and the symptoms.
- When indicated, the second vaccine dose should not be avoided in patients who are diagnosed with HZ.
Children and COVID: New cases, admissions are higher than ever
Weekly COVID-19 cases in children passed 300,000 for the first time since the pandemic started, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The rate of new COVID-related hospital admissions also reached a new high of 0.74 per 100,000 children as of Dec. 31. The highest rate seen before the current Omicron-fueled surge was 0.47 per 100,000 in early September, data from the Centers for Disease Control and Prevention show.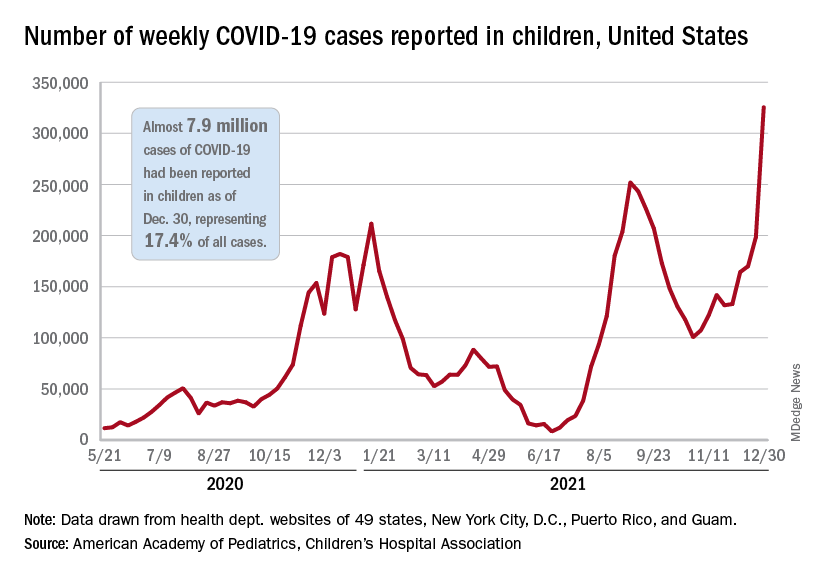
and exceeding the previous week’s count by almost 64%, the AAP and CHA said in their weekly COVID report.
New cases were up in all four regions of the United States, with the Northeast adding the most newly infected children while setting a new high for the fifth consecutive week. The South was just behind for the week but still well off the record it reached in September, the Midwest was third but recorded its busiest week ever, and the West was fourth and nowhere near its previous high, the AAP/CHA report indicated.
The total number of child cases since the pandemic began is almost 7.9 million, they said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. That figure represents 17.4% of all cases reported in the United States, and the cumulative rate of COVID infection is up to almost 10,500 per 100,000 children, meaning that 1 in 10 children have been infected.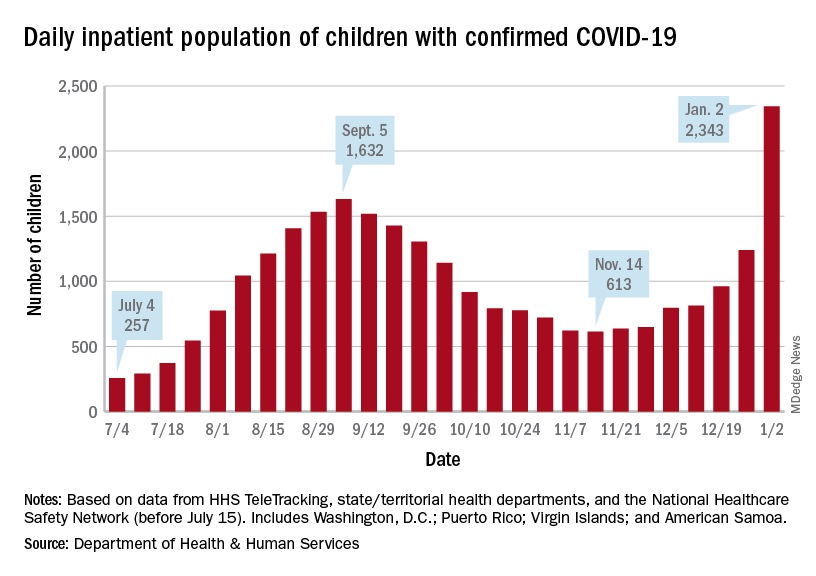
Children are still less likely to be hospitalized than adults, but the gap appears to be closing. On Jan. 2 there were 2,343 children and 87,690 adults in the hospital with confirmed COVID, a ratio of 37 adults for each child, but on Sept. 5, at the height of the previous surge, the ratio of hospitalized adults (93,647) to children (1,632) was 57:1, according to data from the Department of Health & Human Services.
New admissions show a similar pattern: The 0.74 admissions per 100,000 children recorded on Dec. 31 was lower than, for example, adults aged 30-39 years (2.7 per 100,000) or 50-59 years (4.25 per 100,000), but on Sept. 5 the corresponding figures were 0.46 (children), 2.74 (ages 30-39), and 5.03 (aged 50-59), based on the HHS data.
A look at vaccinations
The vaccination response to Omicron, however, has been more subdued and somewhat inconsistent. Vaccine initiation, not surprisingly, was down among eligible children for the week of Dec. 23-29. Before that, both the 5- to 11-year-olds and 12- to 15-year-olds were down for the second week of December and then up a bit (5.6% and 14.3%, respectively) during the third week, while the 16- to 17-year-olds, increased initiation by 63.2%, CDC’s COVID Data Tracker shows.
Less than a quarter (23.5%) of children aged 5-11 received at least one dose of the vaccine in the first 2 months of their eligibility, and only 14.7% are fully vaccinated. Among the older children, coverage looks like this: at least one dose for 61.2% of 12- to 15-year-olds and 67.4% of 16- to 17-year-olds and full vaccination for 51.3% and 57.6%, respectively, the CDC said.
At the state level, Massachusetts and Hawaii have the highest rates for children aged 12-17 years, with 86% having received a least one dose, and Vermont is highest for children aged 5-11 at 56%. The lowest rates can be found in Wyoming (38%) for 12- to 17-year-olds and in Mississippi (6%) for 5- to 11-year-olds, the AAP said in a separate report.
Weekly COVID-19 cases in children passed 300,000 for the first time since the pandemic started, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The rate of new COVID-related hospital admissions also reached a new high of 0.74 per 100,000 children as of Dec. 31. The highest rate seen before the current Omicron-fueled surge was 0.47 per 100,000 in early September, data from the Centers for Disease Control and Prevention show.
and exceeding the previous week’s count by almost 64%, the AAP and CHA said in their weekly COVID report.
New cases were up in all four regions of the United States, with the Northeast adding the most newly infected children while setting a new high for the fifth consecutive week. The South was just behind for the week but still well off the record it reached in September, the Midwest was third but recorded its busiest week ever, and the West was fourth and nowhere near its previous high, the AAP/CHA report indicated.
The total number of child cases since the pandemic began is almost 7.9 million, they said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. That figure represents 17.4% of all cases reported in the United States, and the cumulative rate of COVID infection is up to almost 10,500 per 100,000 children, meaning that 1 in 10 children have been infected.
Children are still less likely to be hospitalized than adults, but the gap appears to be closing. On Jan. 2 there were 2,343 children and 87,690 adults in the hospital with confirmed COVID, a ratio of 37 adults for each child, but on Sept. 5, at the height of the previous surge, the ratio of hospitalized adults (93,647) to children (1,632) was 57:1, according to data from the Department of Health & Human Services.
New admissions show a similar pattern: The 0.74 admissions per 100,000 children recorded on Dec. 31 was lower than, for example, adults aged 30-39 years (2.7 per 100,000) or 50-59 years (4.25 per 100,000), but on Sept. 5 the corresponding figures were 0.46 (children), 2.74 (ages 30-39), and 5.03 (aged 50-59), based on the HHS data.
A look at vaccinations
The vaccination response to Omicron, however, has been more subdued and somewhat inconsistent. Vaccine initiation, not surprisingly, was down among eligible children for the week of Dec. 23-29. Before that, both the 5- to 11-year-olds and 12- to 15-year-olds were down for the second week of December and then up a bit (5.6% and 14.3%, respectively) during the third week, while the 16- to 17-year-olds, increased initiation by 63.2%, CDC’s COVID Data Tracker shows.
Less than a quarter (23.5%) of children aged 5-11 received at least one dose of the vaccine in the first 2 months of their eligibility, and only 14.7% are fully vaccinated. Among the older children, coverage looks like this: at least one dose for 61.2% of 12- to 15-year-olds and 67.4% of 16- to 17-year-olds and full vaccination for 51.3% and 57.6%, respectively, the CDC said.
At the state level, Massachusetts and Hawaii have the highest rates for children aged 12-17 years, with 86% having received a least one dose, and Vermont is highest for children aged 5-11 at 56%. The lowest rates can be found in Wyoming (38%) for 12- to 17-year-olds and in Mississippi (6%) for 5- to 11-year-olds, the AAP said in a separate report.
Weekly COVID-19 cases in children passed 300,000 for the first time since the pandemic started, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The rate of new COVID-related hospital admissions also reached a new high of 0.74 per 100,000 children as of Dec. 31. The highest rate seen before the current Omicron-fueled surge was 0.47 per 100,000 in early September, data from the Centers for Disease Control and Prevention show.
and exceeding the previous week’s count by almost 64%, the AAP and CHA said in their weekly COVID report.
New cases were up in all four regions of the United States, with the Northeast adding the most newly infected children while setting a new high for the fifth consecutive week. The South was just behind for the week but still well off the record it reached in September, the Midwest was third but recorded its busiest week ever, and the West was fourth and nowhere near its previous high, the AAP/CHA report indicated.
The total number of child cases since the pandemic began is almost 7.9 million, they said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. That figure represents 17.4% of all cases reported in the United States, and the cumulative rate of COVID infection is up to almost 10,500 per 100,000 children, meaning that 1 in 10 children have been infected.
Children are still less likely to be hospitalized than adults, but the gap appears to be closing. On Jan. 2 there were 2,343 children and 87,690 adults in the hospital with confirmed COVID, a ratio of 37 adults for each child, but on Sept. 5, at the height of the previous surge, the ratio of hospitalized adults (93,647) to children (1,632) was 57:1, according to data from the Department of Health & Human Services.
New admissions show a similar pattern: The 0.74 admissions per 100,000 children recorded on Dec. 31 was lower than, for example, adults aged 30-39 years (2.7 per 100,000) or 50-59 years (4.25 per 100,000), but on Sept. 5 the corresponding figures were 0.46 (children), 2.74 (ages 30-39), and 5.03 (aged 50-59), based on the HHS data.
A look at vaccinations
The vaccination response to Omicron, however, has been more subdued and somewhat inconsistent. Vaccine initiation, not surprisingly, was down among eligible children for the week of Dec. 23-29. Before that, both the 5- to 11-year-olds and 12- to 15-year-olds were down for the second week of December and then up a bit (5.6% and 14.3%, respectively) during the third week, while the 16- to 17-year-olds, increased initiation by 63.2%, CDC’s COVID Data Tracker shows.
Less than a quarter (23.5%) of children aged 5-11 received at least one dose of the vaccine in the first 2 months of their eligibility, and only 14.7% are fully vaccinated. Among the older children, coverage looks like this: at least one dose for 61.2% of 12- to 15-year-olds and 67.4% of 16- to 17-year-olds and full vaccination for 51.3% and 57.6%, respectively, the CDC said.
At the state level, Massachusetts and Hawaii have the highest rates for children aged 12-17 years, with 86% having received a least one dose, and Vermont is highest for children aged 5-11 at 56%. The lowest rates can be found in Wyoming (38%) for 12- to 17-year-olds and in Mississippi (6%) for 5- to 11-year-olds, the AAP said in a separate report.
COVID-19 outbreak hits research station in Antarctica
Two-thirds of the 25 workers have tested positive at the station, despite all of them being fully vaccinated and going through several testing stages before being allowed entrance, the Belgium publication Le Soir reported.
So far, all the cases are mild at the station, which is owned by Belgium and operated by a private group: the International Polar Foundation.
The first case was discovered Dec. 14 among a group that arrived a week earlier in Antarctica, Le Soir reported. The first three people to test positive evacuated Dec. 23, Le Soir said, but the virus continued to spread among the remaining workers at the base.
Le Soir, citing a virologist, said the Omicron variant probably caused the outbreak, because the crew made its last stop in South Africa before arriving in Antarctica.
New arrivals to the station have been put on hold until the outbreak is brought under control, and one of the missions planned for the base has been postponed, Le Soir said.
“The situation isn’t dramatic,” Joseph Cheek, a project manager for the International Polar Foundation, told the BBC. “While it has been an inconvenience to have to quarantine certain members of the staff who caught the virus, it hasn’t significantly affected our work at the station overall.”
The BBC said there was another COVID outbreak in Antarctica about a year ago at the Bernardo O’Higgins research station operated by Chile.
A version of this article first appeared on WebMD.com.
Two-thirds of the 25 workers have tested positive at the station, despite all of them being fully vaccinated and going through several testing stages before being allowed entrance, the Belgium publication Le Soir reported.
So far, all the cases are mild at the station, which is owned by Belgium and operated by a private group: the International Polar Foundation.
The first case was discovered Dec. 14 among a group that arrived a week earlier in Antarctica, Le Soir reported. The first three people to test positive evacuated Dec. 23, Le Soir said, but the virus continued to spread among the remaining workers at the base.
Le Soir, citing a virologist, said the Omicron variant probably caused the outbreak, because the crew made its last stop in South Africa before arriving in Antarctica.
New arrivals to the station have been put on hold until the outbreak is brought under control, and one of the missions planned for the base has been postponed, Le Soir said.
“The situation isn’t dramatic,” Joseph Cheek, a project manager for the International Polar Foundation, told the BBC. “While it has been an inconvenience to have to quarantine certain members of the staff who caught the virus, it hasn’t significantly affected our work at the station overall.”
The BBC said there was another COVID outbreak in Antarctica about a year ago at the Bernardo O’Higgins research station operated by Chile.
A version of this article first appeared on WebMD.com.
Two-thirds of the 25 workers have tested positive at the station, despite all of them being fully vaccinated and going through several testing stages before being allowed entrance, the Belgium publication Le Soir reported.
So far, all the cases are mild at the station, which is owned by Belgium and operated by a private group: the International Polar Foundation.
The first case was discovered Dec. 14 among a group that arrived a week earlier in Antarctica, Le Soir reported. The first three people to test positive evacuated Dec. 23, Le Soir said, but the virus continued to spread among the remaining workers at the base.
Le Soir, citing a virologist, said the Omicron variant probably caused the outbreak, because the crew made its last stop in South Africa before arriving in Antarctica.
New arrivals to the station have been put on hold until the outbreak is brought under control, and one of the missions planned for the base has been postponed, Le Soir said.
“The situation isn’t dramatic,” Joseph Cheek, a project manager for the International Polar Foundation, told the BBC. “While it has been an inconvenience to have to quarantine certain members of the staff who caught the virus, it hasn’t significantly affected our work at the station overall.”
The BBC said there was another COVID outbreak in Antarctica about a year ago at the Bernardo O’Higgins research station operated by Chile.
A version of this article first appeared on WebMD.com.
Indurated Mass on the Right Central Back
The Diagnosis: Actinomycetoma
Histopathology revealed evidence of an actinomycete organism within the suppuration, consistent with actinomycosis (quiz image [inset]). Given the clinical presentation and histopathologic findings, our patient was diagnosed with actinomycetoma.
Actinomycetoma is an indolent, progressive, subcutaneous infection characterized by a well-known clinical triad of tumefaction/subcutaneous mass, draining sinuses, and an exudate containing grains on microscopy. The sinus tracts are formed from the chronic infectious process that destroys tissue, creating tunnels. This infectious disease of soft tissue is a clinical subset of mycetoma, which is categorized as eumycetoma (fungal) and actinomycetoma (bacterial). Actinomycetoma resembles the behavior of insidious and chronic fungal infections; however, most mycetoma infections are bacterial.1,2 Actinomycetoma may be confused with actinomycosis, which is caused by Actinomycoses species, commensal organisms commonly located on the teeth and oral mucosa in association with other microorganisms that may pathogenically cause cervicofacial actinomycosis.3,4 Actinomycetoma can be caused by Nocardia, Streptomyces, and Actinomadura. 2,5 The foot is the most common location of involvement followed by the thoracic region. It is more common in tropical or equatorial locations and may be contracted through exposure to soil or wood.5 Mycetoma is considered a neglected tropical disease by the World Health Organization.1 In tropical countries, this disease may go undiagnosed or untreated for so long that surgical amputation may be the only effective treatment.
Actinomycetoma commonly is identifiable by direct microscopy, Gram stain, or bacterial culture, with Gram stain being more sensitive than bacterial culture.3 It is important to indicate the suspected organism to the microbiology laboratory because common bacterial pathogens are detected within 24 to 48 hours, but the causative microorganism in actinomycetoma may require up to 4 weeks for culture,2 leading to possible false negatives due to inadequate culture time.3 Histopathology of actinomycotic infections will demonstrate granulomatous inflammation, focal suppuration, and the presence of grains (ie, a colony of filamentous bacteria in a stellate shaped mass)(quiz image [inset]).
The gold standard of treatment is trimethoprim-sulfamethoxazole for up to several years.4,5 Amoxicillin–clavulanic acid, dapsone, amikacin, streptomycin, and beta-lactams have been used successfully.2,5 The treatment course is dependent on clinical severity and location of the disease. The cure rate with appropriate antibiotics can be as high as 90%,2,5 and thus surgical intervention can be avoided.
In the differential, cutaneous tuberculosis would show tuberculoid granulomas with epithelioid histiocytes with possible caseation on histopathology, typically alongside positive tuberculosis screening. Botryomycosis has a similar clinical presentation of a swollen or indurated lesion with draining sinus tracts, but it less commonly occurs on the trunk. Histopathology also is a close mimic of actinomycetoma with a small grain inside a suppurative infiltrate; however, it has no filamentous bacteria. A foreign body reaction would not histologically present with suppuration or grains, and draining sinuses typically would not be seen on clinical presentation. Sarcoma is a neoplastic process and most commonly would show a proliferation of cells with soft tissue or bone origin on histopathology and not primarily an inflammatory cell process.6
- Verma P, Jha A. Mycetoma: reviewing a neglected disease. Clin Exp Dermatol. 2019;44:123-129.
- Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197.
- Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope. 1984;94:1198-1217.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010.
The Diagnosis: Actinomycetoma
Histopathology revealed evidence of an actinomycete organism within the suppuration, consistent with actinomycosis (quiz image [inset]). Given the clinical presentation and histopathologic findings, our patient was diagnosed with actinomycetoma.
Actinomycetoma is an indolent, progressive, subcutaneous infection characterized by a well-known clinical triad of tumefaction/subcutaneous mass, draining sinuses, and an exudate containing grains on microscopy. The sinus tracts are formed from the chronic infectious process that destroys tissue, creating tunnels. This infectious disease of soft tissue is a clinical subset of mycetoma, which is categorized as eumycetoma (fungal) and actinomycetoma (bacterial). Actinomycetoma resembles the behavior of insidious and chronic fungal infections; however, most mycetoma infections are bacterial.1,2 Actinomycetoma may be confused with actinomycosis, which is caused by Actinomycoses species, commensal organisms commonly located on the teeth and oral mucosa in association with other microorganisms that may pathogenically cause cervicofacial actinomycosis.3,4 Actinomycetoma can be caused by Nocardia, Streptomyces, and Actinomadura. 2,5 The foot is the most common location of involvement followed by the thoracic region. It is more common in tropical or equatorial locations and may be contracted through exposure to soil or wood.5 Mycetoma is considered a neglected tropical disease by the World Health Organization.1 In tropical countries, this disease may go undiagnosed or untreated for so long that surgical amputation may be the only effective treatment.
Actinomycetoma commonly is identifiable by direct microscopy, Gram stain, or bacterial culture, with Gram stain being more sensitive than bacterial culture.3 It is important to indicate the suspected organism to the microbiology laboratory because common bacterial pathogens are detected within 24 to 48 hours, but the causative microorganism in actinomycetoma may require up to 4 weeks for culture,2 leading to possible false negatives due to inadequate culture time.3 Histopathology of actinomycotic infections will demonstrate granulomatous inflammation, focal suppuration, and the presence of grains (ie, a colony of filamentous bacteria in a stellate shaped mass)(quiz image [inset]).
The gold standard of treatment is trimethoprim-sulfamethoxazole for up to several years.4,5 Amoxicillin–clavulanic acid, dapsone, amikacin, streptomycin, and beta-lactams have been used successfully.2,5 The treatment course is dependent on clinical severity and location of the disease. The cure rate with appropriate antibiotics can be as high as 90%,2,5 and thus surgical intervention can be avoided.
In the differential, cutaneous tuberculosis would show tuberculoid granulomas with epithelioid histiocytes with possible caseation on histopathology, typically alongside positive tuberculosis screening. Botryomycosis has a similar clinical presentation of a swollen or indurated lesion with draining sinus tracts, but it less commonly occurs on the trunk. Histopathology also is a close mimic of actinomycetoma with a small grain inside a suppurative infiltrate; however, it has no filamentous bacteria. A foreign body reaction would not histologically present with suppuration or grains, and draining sinuses typically would not be seen on clinical presentation. Sarcoma is a neoplastic process and most commonly would show a proliferation of cells with soft tissue or bone origin on histopathology and not primarily an inflammatory cell process.6
The Diagnosis: Actinomycetoma
Histopathology revealed evidence of an actinomycete organism within the suppuration, consistent with actinomycosis (quiz image [inset]). Given the clinical presentation and histopathologic findings, our patient was diagnosed with actinomycetoma.
Actinomycetoma is an indolent, progressive, subcutaneous infection characterized by a well-known clinical triad of tumefaction/subcutaneous mass, draining sinuses, and an exudate containing grains on microscopy. The sinus tracts are formed from the chronic infectious process that destroys tissue, creating tunnels. This infectious disease of soft tissue is a clinical subset of mycetoma, which is categorized as eumycetoma (fungal) and actinomycetoma (bacterial). Actinomycetoma resembles the behavior of insidious and chronic fungal infections; however, most mycetoma infections are bacterial.1,2 Actinomycetoma may be confused with actinomycosis, which is caused by Actinomycoses species, commensal organisms commonly located on the teeth and oral mucosa in association with other microorganisms that may pathogenically cause cervicofacial actinomycosis.3,4 Actinomycetoma can be caused by Nocardia, Streptomyces, and Actinomadura. 2,5 The foot is the most common location of involvement followed by the thoracic region. It is more common in tropical or equatorial locations and may be contracted through exposure to soil or wood.5 Mycetoma is considered a neglected tropical disease by the World Health Organization.1 In tropical countries, this disease may go undiagnosed or untreated for so long that surgical amputation may be the only effective treatment.
Actinomycetoma commonly is identifiable by direct microscopy, Gram stain, or bacterial culture, with Gram stain being more sensitive than bacterial culture.3 It is important to indicate the suspected organism to the microbiology laboratory because common bacterial pathogens are detected within 24 to 48 hours, but the causative microorganism in actinomycetoma may require up to 4 weeks for culture,2 leading to possible false negatives due to inadequate culture time.3 Histopathology of actinomycotic infections will demonstrate granulomatous inflammation, focal suppuration, and the presence of grains (ie, a colony of filamentous bacteria in a stellate shaped mass)(quiz image [inset]).
The gold standard of treatment is trimethoprim-sulfamethoxazole for up to several years.4,5 Amoxicillin–clavulanic acid, dapsone, amikacin, streptomycin, and beta-lactams have been used successfully.2,5 The treatment course is dependent on clinical severity and location of the disease. The cure rate with appropriate antibiotics can be as high as 90%,2,5 and thus surgical intervention can be avoided.
In the differential, cutaneous tuberculosis would show tuberculoid granulomas with epithelioid histiocytes with possible caseation on histopathology, typically alongside positive tuberculosis screening. Botryomycosis has a similar clinical presentation of a swollen or indurated lesion with draining sinus tracts, but it less commonly occurs on the trunk. Histopathology also is a close mimic of actinomycetoma with a small grain inside a suppurative infiltrate; however, it has no filamentous bacteria. A foreign body reaction would not histologically present with suppuration or grains, and draining sinuses typically would not be seen on clinical presentation. Sarcoma is a neoplastic process and most commonly would show a proliferation of cells with soft tissue or bone origin on histopathology and not primarily an inflammatory cell process.6
- Verma P, Jha A. Mycetoma: reviewing a neglected disease. Clin Exp Dermatol. 2019;44:123-129.
- Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197.
- Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope. 1984;94:1198-1217.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010.
- Verma P, Jha A. Mycetoma: reviewing a neglected disease. Clin Exp Dermatol. 2019;44:123-129.
- Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197.
- Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope. 1984;94:1198-1217.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010.
A 26-year-old Guatemalan man who was a former carpenter presented with an indurated, nontender, nonpruritic, subcutaneous mass on the right central back with multiple draining sinus tracts on the surface and several depressed circular atrophic scars on the periphery of the mass. He noticed that the lesion began as a pustule 1.5 years prior and gradually enlarged. He denied any trauma, insect bites, fever, chills, headaches, weight loss, or travel history (he relocated to the United States 3.5 years ago) prior to the skin eruption. A biopsy was performed by an outside dermatologist 1 year prior to the current presentation, with a diagnosis of Pityrosporum folliculitis. Throughout his clinical course, treatment with oral antifungals, oral doxycycline, and topical clindamycin all failed. The mass was removed by plastic surgery 1 year prior.
A tissue biopsy for histology and culture was obtained at presentation to our institution. Laboratory findings showed that the basic metabolic panel was within reference range. Chest radiography indicated no active disease. A tuberculosis screening was negative. A bacterial culture of the lesion identified no growth after 48 hours. Our tissue biopsy revealed fibrosing granulation tissue, but the surgical pathology from a prior mass excision revealed sinus tracts with suppuration, evidence of scarring, foreign body giant cell reaction, and a characteristic finding (inset: H&E, original magnification ×200).
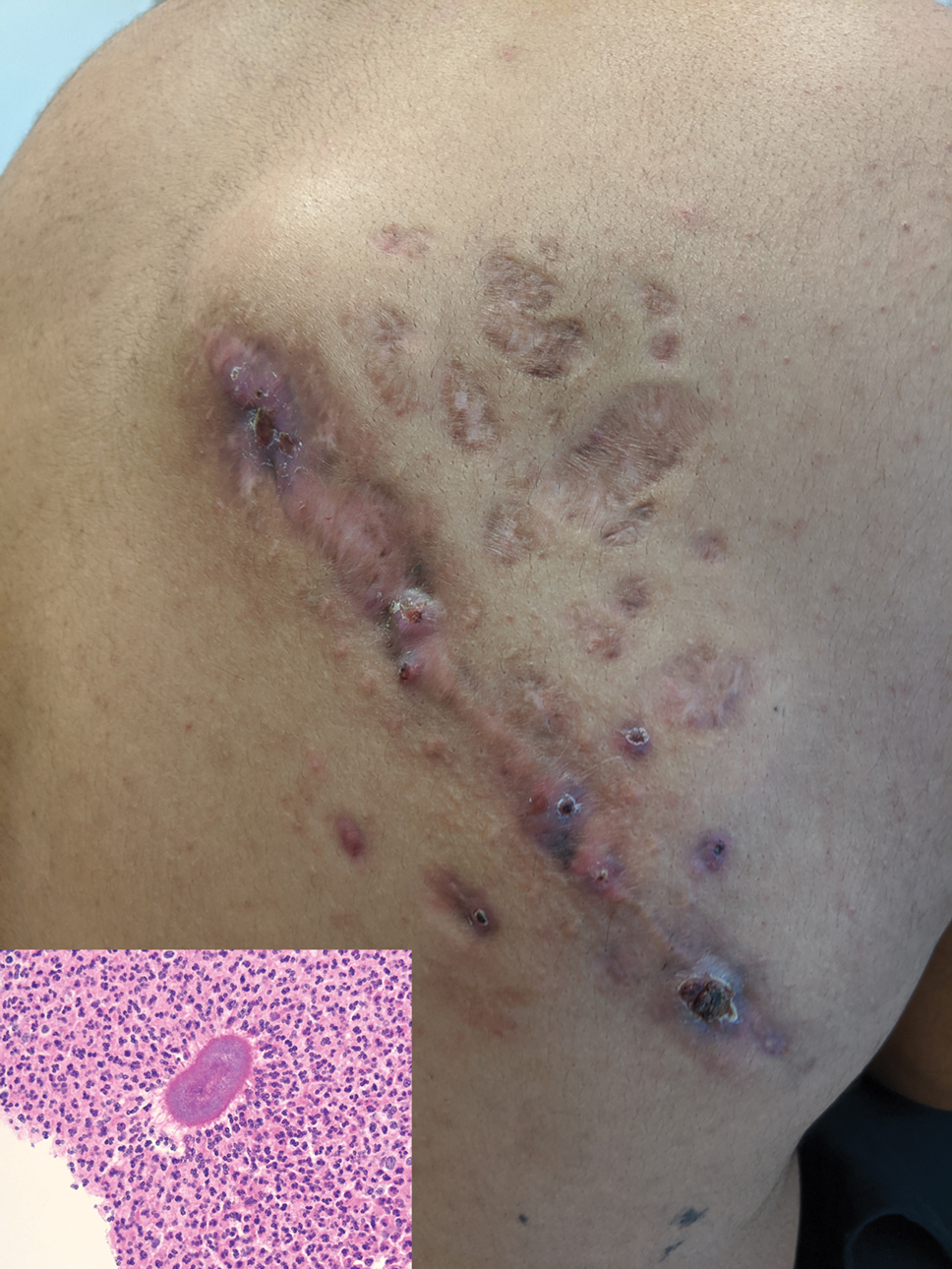
FDA backs Pfizer booster for 12- to 15-year-olds
Besides updating the authorization for the Pfizer COVID-19 vaccine, the agency also shortened the recommended time between a second dose and the booster to 5 months or more, based on new evidence. In addition, a third primary series dose is now authorized for certain immunocompromised children 5 years to 11 years old. Full details are available in an FDA news release.
The amended emergency use authorization (EUA) only applies to the Pfizer vaccine, said acting FDA Commissioner Janet Woodcock, MD.
“Just to make sure every everyone is clear on this, right now: If you got [Johnson & Johnson’s one-dose vaccine], you get a booster after 2 months. If you got Moderna, you can get a booster at 6 months or beyond,” she said during a media briefing.
What is new, she said, is “if you got Pfizer as your primary series, you can get a booster at 5 months or beyond.”
A lower risk of myocarditis?
Asked about concerns about the risk of myocarditis with vaccination in the 12- to 15-year age group, Dr. Woodcock said they expect it would be “extremely rare with the third dose.”
“We have the real-world evidence from the Israeli experience to help us with that analysis,” she said.
The data so far consistently points to a higher risk of myocarditis after a second mRNA vaccine dose among males, from teenagers to 30-year-olds, with a peak at about 16 to 17 years of age, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said during the media call.
The risk of myocarditis is about 2 to 3 times higher after a second vaccine dose, compared to a booster shot, Dr. Marks said, based on available data. It may be related to the closer dose timing of the second dose versus a third, he added.
“The inference here is that on the risk of myocarditis with third doses in the 12- to 15-year age range is likely to be quite acceptable,” he said.
Dr. Marks also pointed out that most cases of myocarditis clear up quickly.
“We’re not seeing long-lasting effects. That’s not to say that we don’t care about this and that it’s not important,” he said.
“But what it is saying is that in the setting of a tremendous number of Omicron and Delta cases in this country, the potential benefits of getting vaccinated in this age group outweigh that risk,” Dr. Marks said. “We can look at that risk-benefit and still feel comfortable.”
He said that “the really overwhelming majority of these cases, 98%, have been mild” -- shown by a 1-day median hospital stay.
Even so, the FDA plans to continue monitoring for the risk of myocarditis “very closely,” he said.
Interestingly, swollen underarm lymph nodes were seen more frequently after the booster dose than after the second dose of a two-dose primary series, the FDA said.
Reducing the time between primary vaccination with the Pfizer vaccine -- two initial doses -- and the booster shot from 6 months to 5 months is based on decreasing efficacy data that the drugmaker submitted to the FDA.
The 5-month interval was evaluated in a study from Israel published Dec. 21 in the New England Journal of Medicine .
Mixing and matching vaccines
Less clear at the moment is guidance about boosters for people who opted to mix and match their primary vaccine series.
“There was a mix-and-match study that was done which showed that in some cases, the mixing and matching … of an adenoviral record vaccine and an mRNA vaccine seem to give a very good immune response,” Dr. Marks said.
Once more data comes in on mixing and matching, “we’ll analyze them and then potentially make recommendations,” he said.
‘It’s not too late’
No federal government media briefing on COVID-19 would be complete without a plea for the unvaccinated to get immunized.
“We’re talking a lot about boosters right now, but it’s not too late for those who have not gotten a vaccine to get a vaccine,” Dr. Marks said, referring to the tens of millions of Americans who remain unvaccinated at the beginning of 2022.
“We know from our previous studies that even a single dose of the vaccine -- and probably two doses -- can help prevent the worst outcomes from COVID-19, including hospitalization and death.”
A version of this article first appeared on WebMD.com.
Besides updating the authorization for the Pfizer COVID-19 vaccine, the agency also shortened the recommended time between a second dose and the booster to 5 months or more, based on new evidence. In addition, a third primary series dose is now authorized for certain immunocompromised children 5 years to 11 years old. Full details are available in an FDA news release.
The amended emergency use authorization (EUA) only applies to the Pfizer vaccine, said acting FDA Commissioner Janet Woodcock, MD.
“Just to make sure every everyone is clear on this, right now: If you got [Johnson & Johnson’s one-dose vaccine], you get a booster after 2 months. If you got Moderna, you can get a booster at 6 months or beyond,” she said during a media briefing.
What is new, she said, is “if you got Pfizer as your primary series, you can get a booster at 5 months or beyond.”
A lower risk of myocarditis?
Asked about concerns about the risk of myocarditis with vaccination in the 12- to 15-year age group, Dr. Woodcock said they expect it would be “extremely rare with the third dose.”
“We have the real-world evidence from the Israeli experience to help us with that analysis,” she said.
The data so far consistently points to a higher risk of myocarditis after a second mRNA vaccine dose among males, from teenagers to 30-year-olds, with a peak at about 16 to 17 years of age, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said during the media call.
The risk of myocarditis is about 2 to 3 times higher after a second vaccine dose, compared to a booster shot, Dr. Marks said, based on available data. It may be related to the closer dose timing of the second dose versus a third, he added.
“The inference here is that on the risk of myocarditis with third doses in the 12- to 15-year age range is likely to be quite acceptable,” he said.
Dr. Marks also pointed out that most cases of myocarditis clear up quickly.
“We’re not seeing long-lasting effects. That’s not to say that we don’t care about this and that it’s not important,” he said.
“But what it is saying is that in the setting of a tremendous number of Omicron and Delta cases in this country, the potential benefits of getting vaccinated in this age group outweigh that risk,” Dr. Marks said. “We can look at that risk-benefit and still feel comfortable.”
He said that “the really overwhelming majority of these cases, 98%, have been mild” -- shown by a 1-day median hospital stay.
Even so, the FDA plans to continue monitoring for the risk of myocarditis “very closely,” he said.
Interestingly, swollen underarm lymph nodes were seen more frequently after the booster dose than after the second dose of a two-dose primary series, the FDA said.
Reducing the time between primary vaccination with the Pfizer vaccine -- two initial doses -- and the booster shot from 6 months to 5 months is based on decreasing efficacy data that the drugmaker submitted to the FDA.
The 5-month interval was evaluated in a study from Israel published Dec. 21 in the New England Journal of Medicine .
Mixing and matching vaccines
Less clear at the moment is guidance about boosters for people who opted to mix and match their primary vaccine series.
“There was a mix-and-match study that was done which showed that in some cases, the mixing and matching … of an adenoviral record vaccine and an mRNA vaccine seem to give a very good immune response,” Dr. Marks said.
Once more data comes in on mixing and matching, “we’ll analyze them and then potentially make recommendations,” he said.
‘It’s not too late’
No federal government media briefing on COVID-19 would be complete without a plea for the unvaccinated to get immunized.
“We’re talking a lot about boosters right now, but it’s not too late for those who have not gotten a vaccine to get a vaccine,” Dr. Marks said, referring to the tens of millions of Americans who remain unvaccinated at the beginning of 2022.
“We know from our previous studies that even a single dose of the vaccine -- and probably two doses -- can help prevent the worst outcomes from COVID-19, including hospitalization and death.”
A version of this article first appeared on WebMD.com.
Besides updating the authorization for the Pfizer COVID-19 vaccine, the agency also shortened the recommended time between a second dose and the booster to 5 months or more, based on new evidence. In addition, a third primary series dose is now authorized for certain immunocompromised children 5 years to 11 years old. Full details are available in an FDA news release.
The amended emergency use authorization (EUA) only applies to the Pfizer vaccine, said acting FDA Commissioner Janet Woodcock, MD.
“Just to make sure every everyone is clear on this, right now: If you got [Johnson & Johnson’s one-dose vaccine], you get a booster after 2 months. If you got Moderna, you can get a booster at 6 months or beyond,” she said during a media briefing.
What is new, she said, is “if you got Pfizer as your primary series, you can get a booster at 5 months or beyond.”
A lower risk of myocarditis?
Asked about concerns about the risk of myocarditis with vaccination in the 12- to 15-year age group, Dr. Woodcock said they expect it would be “extremely rare with the third dose.”
“We have the real-world evidence from the Israeli experience to help us with that analysis,” she said.
The data so far consistently points to a higher risk of myocarditis after a second mRNA vaccine dose among males, from teenagers to 30-year-olds, with a peak at about 16 to 17 years of age, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said during the media call.
The risk of myocarditis is about 2 to 3 times higher after a second vaccine dose, compared to a booster shot, Dr. Marks said, based on available data. It may be related to the closer dose timing of the second dose versus a third, he added.
“The inference here is that on the risk of myocarditis with third doses in the 12- to 15-year age range is likely to be quite acceptable,” he said.
Dr. Marks also pointed out that most cases of myocarditis clear up quickly.
“We’re not seeing long-lasting effects. That’s not to say that we don’t care about this and that it’s not important,” he said.
“But what it is saying is that in the setting of a tremendous number of Omicron and Delta cases in this country, the potential benefits of getting vaccinated in this age group outweigh that risk,” Dr. Marks said. “We can look at that risk-benefit and still feel comfortable.”
He said that “the really overwhelming majority of these cases, 98%, have been mild” -- shown by a 1-day median hospital stay.
Even so, the FDA plans to continue monitoring for the risk of myocarditis “very closely,” he said.
Interestingly, swollen underarm lymph nodes were seen more frequently after the booster dose than after the second dose of a two-dose primary series, the FDA said.
Reducing the time between primary vaccination with the Pfizer vaccine -- two initial doses -- and the booster shot from 6 months to 5 months is based on decreasing efficacy data that the drugmaker submitted to the FDA.
The 5-month interval was evaluated in a study from Israel published Dec. 21 in the New England Journal of Medicine .
Mixing and matching vaccines
Less clear at the moment is guidance about boosters for people who opted to mix and match their primary vaccine series.
“There was a mix-and-match study that was done which showed that in some cases, the mixing and matching … of an adenoviral record vaccine and an mRNA vaccine seem to give a very good immune response,” Dr. Marks said.
Once more data comes in on mixing and matching, “we’ll analyze them and then potentially make recommendations,” he said.
‘It’s not too late’
No federal government media briefing on COVID-19 would be complete without a plea for the unvaccinated to get immunized.
“We’re talking a lot about boosters right now, but it’s not too late for those who have not gotten a vaccine to get a vaccine,” Dr. Marks said, referring to the tens of millions of Americans who remain unvaccinated at the beginning of 2022.
“We know from our previous studies that even a single dose of the vaccine -- and probably two doses -- can help prevent the worst outcomes from COVID-19, including hospitalization and death.”
A version of this article first appeared on WebMD.com.
Antiretroviral pill better at suppressing HIV in children
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Why mRNA COVID vaccines are preferred (and why patients should be reassured)
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf