User login
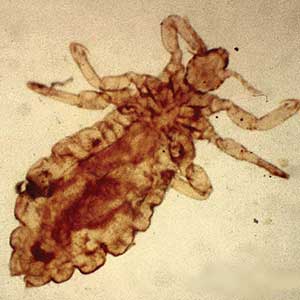
Mpox Presentation Compared in Different Racial, Ethnic Groups
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- There is limited information on the populations disproportionately affected by the recent global mpox outbreak, particularly in individuals with HIV and racial and ethnic minorities.
- To investigate morphologic and clinical presentations of mpox in diverse populations, researchers conducted a review of the records of 54 individuals (mean age, 42.4 years) diagnosed with mpox at a San Francisco clinic for patients with HIV or at high risk for HIV, between June and October 2022.
- All patients were assigned male at birth, and three identified themselves as transgender women.
- Morphologic descriptions were documented through either photographic evidence or physical examination notes.
TAKEAWAY:
- Pustules or pseudopustules were the most common morphologic finding in 57.1% of the White non-Hispanic patients and 62.5% of the patients of color (P = .72).
- White non-Hispanic patients were more likely to have no prodromal symptoms (50.0% vs 17.5%; P = .02) and were more likely to have genital lesions (78.6% vs 40.0%; P = .01) than patients of color. These differences were significant or nearly significant when White non-Hispanic patients were compared with Hispanic patients but not in other ethnic or racial groups.
- There were no differences in HIV viral loads or CD4 counts between racial and ethnic groups, and no variations in clinical presentations were observed based on CD4 counts.
- Patients with higher HIV viral loads were more likely to have concurrent sexually transmitted infections (57.1% vs 25%; P = .03).
- Symptoms resolved in all patients, regardless of medical intervention, within weeks of initial presentation, and there were no hospitalizations or deaths.
IN PRACTICE:
Considering that HIV viral burden was not significantly different between White non-Hispanic patients and patients of color, the difference in presentation of the prodrome “may indicate disparities in vulnerable populations,” the authors wrote, noting that more research in large groups is needed to confirm their results.
SOURCE:
The study, led by Richard W. Kim, BS, from the University of California San Francisco, was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Inclusion of “other” racial category in the records highlighted potential inaccuracies in data representation.
DISCLOSURES:
The study received no external funding. The authors did not declare any competing interests.
A version of this article first appeared on Medscape.com.
In the Story of the Rubella Virus as a Source of Granulomas, the Plot Is Still Thickening
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
SAN DIEGO — Approximately 10 years ago in France, high throughput .
Based on accumulating evidence, the Centers for Disease Control and Prevention (CDC) through collaborations with others also recognized this in pediatric patients with inborn errors of immunity, and it is now appropriate for clinicians to consider this etiology when no other infectious agents can be identified, according to Karolyn A. Wanat, MD, professor of dermatology, Medical College of Wisconsin, Milwaukee, who spoke about rubella as a trigger in granulomatous disease at the meeting. “This is a huge evolving area of interest,” said Dr. Wanat, who has been the first author or coauthor on several published papers, including a review article published earlier this year.
In the earliest cases, including those reported in 2014, the cutaneous granulomas presumed to be causally related to vaccine-derived rubella virus were found only in those with a primary immunodeficiency. This is no longer the case. In a collaboration among US clinics, granulomas that had persisted for years in immunocompetent adults were identified, according to Dr. Wanat, the first author of a report on these findings in four adults in 2022. In addition, it now appears that wild-type rubella virus, like vaccine-derived rubella virus, can be the source of the antigenic response that underlies the development of rubella-associated cutaneous granulomas.
The phenotype of these granulomas is comparable to granulomas associated with other infectious agents. On dermatopathology, these commonly feature a robust granulomatous inflammation with multinucleated giant cells and lymphocytic infiltrate. Necrosis and fibrosis are also common.
“These are the types of granulomas that we would be thinking infection. If tissue cultures are negative, we would probably repeat them,” she said, suggesting that suspicion of an infectious etiology would probably remain high even after multiple negative tests.
Of the cases accruing in the United States and elsewhere, most but not all have been linked to inborn errors of immunity. In a 2020 CDC review, the risk of granulomas caused by compromised immunity, such as defects in T cell function, was estimated to be in the range of 0.6% to 2.5%, Dr. Wanat said.
It is now known that primary immunodeficiency is not a prerequisite, but this should not change the perception that the rubella vaccine, which was introduced in 1979, is effective and safe, according to Dr. Wanat. The vaccine is associated with few serious adverse events and is so effective that rubella was eliminated from the United States in 2004 and from the Americas in 2015.
This makes cases of granuloma associated with wild-type rubella virus surprising, but they appear to be exceedingly rare. Whether caused by vaccine exposure or another source, the mechanism of latent development of cutaneous granulomas is consistent with other infectious sources, and is not well understood.
“Rubella is a sneaky virus that can persist in some immunoprivileged sites indefinitely,” Dr. Wanat said. These sites include the eyes, joints, and placenta.
Many initial cases of rubella-associated granulomas occurred on the arms, presumably where the vaccine was administered, despite long intervals between exposure and lesion growth. This interval is often measured in years.
With more cases, it is now understood that involvement of other organs does occur even if the skin is the most common site of antigenic response in patients with immunodeficiency. The liver and lymph nodes represent other tissues that have been affected. Even lesions in the brain have been seen on autopsy.
Based on the benefit-to-risk ratio of a highly effective and successful vaccine, however, the association with a risk of granulomas “should not raise questions” about the value of the vaccine itself, Dr. Wanat noted.
“The proportion of patients who develop these granulomas is very, very low. Yet, the vaccine provides life-long immunity,” she said.
The discovery of granulomas associated with wild type rubella infection was “shocking” based on the supposition that the rubella virus had been eliminated, but this is just one of the unexpected discoveries as the still-evolving science has traced the story of rubella-associated granulomas over the past 10 years.
Cases now include children and adults through advanced ages.
Shedding of the virus and risk of infection to others has been studied but so far, the risk — if it exists — is very low. The evidence includes the many patients who have lived with the granulomas for years, even decades, without any known spread to others.
As for ongoing work in this area, Dr. Wanat said that a histopathological case definition for rubella-associated granulomas is being developed, and she and other investigators are actively seeking new cases to better characterize the disease.
So far, optimal treatment is not well defined. A number of strategies have had limited success or are considered impractical for routine use. One example is a stem cell transplant. In a case Dr. Wanat cited, complete resolution of the skin lesions was achieved with a transplant.
“I am not suggesting that those with localized disease in the skin should undergo a transplant, but it does support the role of the immune system and the potential for a reboot to clear the skin,” she said.
Other therapies associated with benefit in at least some patients include tumor necrosis factor (TNF) inhibitors with dapsone and ribavirin. The risk of adverse events for the latter might again limit its use, Dr. Wanat said.
With awareness, the number of granulomas found to be associated with rubella virus is expected to grow. Dr. Wanat speculated that those areas of the country that not yet have documented a case will do so over time. For idiopathic cases of cutaneous granulomas, rubella should be kept in mind, she said.
Characterizing rubella-associated cutaneous granulomas as “a public health concern,” Dr. Wanat urged clinicians to consider this etiology in lesions that match the phenotype, particularly when other more common infectious agents cannot be identified.
Asked for his perspective, Jeffrey P. North, MD, managing director of the UCSF Dermatopathology, and professor of dermatology and pathology at the University of California, San Francisco, agreed that rubella should be considered as a source of granulomas with a suspected infectious etiology when a pathogen cannot be found.
“It is likely much more common than we know as it has only been recently described and testing for it is limited. I suspected there are a lot of undiagnosed patients suffering from this disease,” Dr. North said in an interview.
“One of the important points for clinicians to consider is that while this has been reported mostly in patients with some form of immunodeficiency, there have also been patients reported to have this condition with no immunodeficiency,” he added. Even though the association between rubella and granulomas was made 10 years ago, awareness is only now spreading, which means the frequency with which rubella leads to granulomas remains uncertain.
“I think we will start to get a better idea of how common this is as more people learn about and testing for it expands,” Dr. North said.
Dr. Wanat reports no potential conflicts of interest. Dr. North reports financial relationships with AdviNow and Kiniksa Pharmaceuticals.
FROM AAD 2024
New Contraindications to Coadministration of Atazanavir
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) this week recommended new contraindications on the coadministration of the protease inhibitor atazanavir (Reyataz, Bristol-Myers Squibb) with antineoplastic agents encorafenib and ivosidenib (atazanavir may significantly increase blood levels and thus side effects), and with the anticonvulsants carbamazepine, phenobarbital, and phenytoin (which may decrease serum levels of atazanavir).
The new rules alter sections 4.3 and 4.5 of the summary of product characteristics (SmPC) to reclassify drug–drug interactions with the new contraindications.
Atazanavir is an orally administered drug, used in combination with low-dose ritonavir (Norvir) to boost its pharmacokinetics. It is indicated for the treatment of HIV-1 infected adults and pediatric patients 3 months of age and older in combination with other antiretroviral medicinal products. A combination preparation boosted with cobicistat (Evotaz) is also available.
The drug is an azapeptide HIV-1 protease inhibitor (PI) that selectively inhibits the virus-specific processing of viral Gag-Pol proteins in HIV-1 infected cells, thus preventing formation of mature virions and infection of other cells. This prevents the virus from multiplying and slows the spread of infection. Based on available virological and clinical data from adult patients, no benefit is expected in patients with HIV strains resistant to multiple protease inhibitors (four or more PI mutations).
Therapy with atazanavir is intended to be initiated by a physician experienced in the management of HIV infection, with the choice of atazanavir in treatment-experienced adult and pediatric patients based on individual viral resistance testing and the patient’s treatment history. The standard dose is 300 mg atazanavir taken with 100 mg ritonavir once daily with food.
Atazanavir is already contraindicated in combination or coadministration with a wide variety of other agents:
- Coadministration with simvastatin or lovastatin [statins – risk of increased blood levels with atazanavir].
- Combination with the anti-TB antibiotic rifampicin.
- Combination with the PDE5 inhibitor sildenafil when used for the treatment of pulmonary arterial hypertension only.
- Coadministration with substrates of the CYP3A4 isoform of cytochrome P450 that have narrow therapeutic windows (eg, quetiapine, lurasidone, alfuzosin, astemizole, terfenadine, cisapride, pimozide, quinidine, bepridil, triazolam, oral midazolam, lomitapide, and ergot alkaloids).
- Coadministration with grazoprevir-containing products, including elbasvir/grazoprevir fixed dose combination (hepatitis C drug combination; atazanavir increases its blood levels).
- Coadministration with glecaprevir/pibrentasvir fixed dose combination (hepatitis C drug combination; increased hepatotoxicity due to increased bilirubin concentration).
- Coadministration with products containing St. John’s wort (Hypericum perforatum).
The EMA said detailed recommendations for the use of atazanavir will be described in the updated SmPC, which will be published in the revised European public assessment report after a decision on this change to the marketing authorization has been granted by the European Commission.
A version of this article appeared on Medscape.com.
Vaccine Against Urinary Tract Infections in Development
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Urinary tract infections are among the most common bacterial infections. They can be painful, require antibiotic treatments, and recur in 20%-30% of cases. With the risk for the emergence or increase of resistance to antibiotics, it is important to search for potential therapeutic alternatives to treat or prevent urinary tract infections.
The MV140 Vaccine
The MV140 vaccine is produced by the Spanish pharmaceutical company Immunotek. MV140, known as Uromune, consists of a suspension of whole heat-inactivated bacteria in glycerol, sodium chloride, an artificial pineapple flavor, and water. It includes equal percentages of strains from four bacterial species (V121 Escherichia coli, V113 Klebsiella pneumoniae, V125 Enterococcus faecalis, and V127 Proteus vulgaris). MV140 is administered sublingually by spraying two 100-µL doses daily for 3 months.
The vaccine is in phase 2-3 of development. It is available under special access programs outside of marketing authorization in 26 countries, including Spain, Portugal, the United Kingdom, Lithuania, the Netherlands, Sweden, Norway, Australia, New Zealand, and Chile. Recently, MV140 was approved in Mexico and the Dominican Republic and submitted to Health Canada for registration.
A randomized study published in 2022 showed the vaccine›s efficacy in preventing urinary tract infections over 9 months. In total, 240 women with a urinary tract infection received MV140 for either 3 or 6 months or a placebo for 6 months. The primary outcome was the number of urinary tract infection episodes during the 9-month study period after vaccination.
In this pivotal study, MV140 administration for 3 and 6 months was associated with a significant reduction in the median number of urinary tract infection episodes, from 3.0 to 0.0 compared with the placebo during the 9-month efficacy period. The median time to the first urinary tract infection after 3 months of treatment was 275.0 days in the MV140 groups compared with 48.0 days in the placebo group.
Nine-Year Follow-Up
On April 6 at the 2024 congress of The European Association of Urology, urologists from the Royal Berkshire NHS Foundation Trust presented the results of a study evaluating the MV140 vaccine spray for long-term prevention of bacterial urinary tract infections.
This was a prospective cohort study involving 89 participants (72 women and 17 men) older than 18 years with recurrent urinary tract infections who received a course of MV140 for 3 months. Participants had no urinary tract infection when offered the vaccine and had no other urinary abnormalities (such as tumors, stones, or kidney infections).
Postvaccination follow-up was conducted over a 9-year period, during which researchers analyzed the data from the electronic health records of their initial cohort. They queried participants about the occurrence of urinary tract infections since receiving the vaccine and about potential related side effects. Thus, the results were self-reported.
Long-Term Efficacy
In this cohort, 48 participants (59%) reported having no infections during the 9-year follow-up. In the cohort of 89 participants, the average period without infection was 54.7 months (4.5 years; 56.7 months for women and 44.3 months for men). No vaccine-related side effects were observed.
The study’s limitations included the small number of participants and the collection of self-reported data. Furthermore, all cases were simple urinary tract infections without complications.
The authors concluded that “9 years after first receiving the sublingual spray MV140 vaccine, 54% of participants remained free from urinary tract infection.” For them, “this vaccine is safe in the long-term, and our participants reported fewer urinary tract infections and, if any, they were less severe.”
Vaccination could thus be an alternative to antibiotic treatments and could help combat the emergence of antibiotic resistance. The full study results should be published by the end of 2024.
Other studies are planned to evaluate the efficacy and safety of the MV140 vaccine in older patients residing in long-term care homes, in children suffering from acute urinary tract infections, and in adults suffering from complicated acute urinary tract infections (for example, patients with a catheter or with a neurogenic bladder).
This story was translated from JIM, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FDA Approves New Antibiotic for Uncomplicated UTIs
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved a new treatment for uncomplicated urinary tract infections (UTIs).
The agency on April 24 approved pivmecillinam tablets to treat women aged 18 years or older with UTIs caused by bacteria susceptible to the drug.
The beta-lactam antibiotic already is approved in Europe and has been used for more than 40 years outside of the United States to treat infections, according to the drug’s manufacturer, Utility Therapeutics.
The drug is an aminopenicillin that rapidly converts to mecillinam, according to the company, which is marketing the medication as Pivya.
Pivmecillinam is intended to treat UTIs caused by susceptible isolates of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus.
Researchers studied the treatment in three clinical trials. One study found women who received the new antibiotic were more likely to have resolution of symptoms and a reduction in bacteria in urine compared with placebo (62% vs 10%). Similar results were seen in a trial that used ibuprofen as the comparator (66% vs 22%).
In a third study that assessed two oral antibacterial drugs, 72% of women who received pivmecillinam and 76% who received the other drug achieved resolution of symptoms and a reduction in bacteria, according to the FDA.
The most common side effects of pivmecillinam include nausea and diarrhea.
About half of all women will experience at least one UTI in their lifetime, and the infections are one the top reasons for antibiotic prescriptions, the FDA noted.
A version of this article appeared on Medscape.com.
Primary Care Shortage Reshaping How Patients Seek Care
By February of 2022, Ella, a 25-year-old behavioral interventionist in Colorado Springs, Colorado, was sick with strep-like symptoms for the third time in 3 months. She didn’t bother to call her doctor.
The first two times she had strep throat, she’d tried to schedule an appointment with her newest primary care doctor but couldn’t get in. They only had available appointments 5 and even 10 days out, but she’d already had symptoms for 3 days.
Until she graduated college, Ella had only known easy-access primary care. Her childhood family doctor and the nurse practitioners at her college clinic knew her. They anticipated her yearly allergies and knew about her predisposition for strep throat. Appointments were easy to schedule, and providers responded to her messages. But since entering the workforce and leaving her parent’s insurance, the kind of primary care she’d come to rely on was nearly impossible to find.
“I went to urgent care, and that became my primary care,” she told this news organization.
Patients Can’t Get Appointments
Primary care is in crisis. A growing number of Americans, like Ella, can’t access care when they need it. According to a 2024 report, 29% of adults and 14% of children don’t have a regular source of care. Those looking for a new primary care provider face extensive research and 6- to 9-month waits for a new patient appointment — if they can get in at all.
But even those with a primary care provider face long wait times: Days to weeks for a sick visit and months for a wellness checkup. Over one third of Medicare beneficiaries wait more than a month to see a doctor. Accessing primary care is more difficult than access to surgery, physical therapy, or rehabilitative care, according to a survey of Medicare beneficiaries by the Commonwealth Fund.
“Shortages tend to be in rural and urban underserved areas, but now, you’re hearing about primary care shortages in Boston, which is a mecca of healthcare,” said Ann Greiner, president and CEO of the Primary Care Coalition.
While retail clinics, urgent care, and telehealth help close the gap in acute needs, they miss one of primary care’s most critical benefits: A doctor who knows you. There’s strong evidence that ongoing treatment from a primary care physician (PCP) who knows your history, family, and context results in better long-term outcomes and fewer hospitalizations and emergency room visits.
If patients continue to find it too hard to break into primary care or set up an appointment, experts are concerned that they’ll stop pursuing primary care altogether.
Doctors’ Hands Are Tied
“I want to highlight that this is not an issue of primary care doctors not wanting to be accessible,” said Lisa Rotenstein, MD, MBA, a PCP and medical director of Ambulatory Quality and Safety at the University of California San Francisco Health. “These access issues are symptoms of the design of primary care in the United States.”
Across the United States, there’s a dearth of family medicine doctors, pediatricians, and internists. And without significantly more primary care providers, there’s simply no way for all Americans to get optimal primary care. The Health Resources and Services administration estimates a current shortage of 13,000 primary care providers. And that shortage will skyrocket to 68,000 by 2036 as the number of Americans needing care balloons and existing PCPs retire with too few trainees to fill their shoes.
The American Association of Medical Colleges predicts a slightly lower shortage in 2036 — between 20,000 and 40,000 primary care physicians — only if more residency positions are funded nationwide.
However, even with more positions, medical trainees see little incentive to pursue primary care. Young doctors are avoiding primary care because of the pressures, Dr. Rotenstein said. There’s incredible pressure to get reimbursement for primary care doctors. And the added administrative burden makes “the work life of these specialties not really manageable,” she said.
Continued Shortages of PCPs
“We know there’s a documented pajama time,” Ms. Greiner said. For every 1 hour spent with a patient, primary care must spend nearly 2 additional hours on electronic health records and desk work, according to a study by the American Medical Association. Even with all those additional hours devoted to getting paid, primary care doctors make an average of $103,000 less annually compared with their counterparts in surgery and oncology.
It’s not an attractive combination for a new doctor with medical debt. This year, Ms. Greiner said that residency positions in internal medicine and pediatrics went unfilled. Of those trainees who do go into a primary care specialty, many won’t last. Only half of primary care residents practice in primary care 3-5 years later. The rest choose to subspecialize or become hospitalists.
These untenable demands on a primary care provider don’t go unnoticed by patients. In Ella’s attempts to invest in a new primary care relationship, she often doesn’t feel heard and can tell the doctor is rushed. “[Urgent care is] probably not the best care because they don’t know me, but it does seem like they are able to listen to me better,” Ella said.
Patients Want to Invest in Primary Care
Primary care should work like putting money in a bank account, Dr. Rotenstein said. Young patients invest in the relationship and reap the benefits of a doctor who knows them later in life when they need more complex care. But if seeing a doctor is so difficult, many young people may stop investing in their PCP relationship.
“One thing ... that I worry about in this kind of situation where patients really have to put in a lot of work to get the care they need is in inequities of care,” Dr. Rotenstein said. “We know some of our patients are more able to undertake that work.”
Alternatively, the primary care shortage could be reshaping how patients seek care. A 2023 study showed the proportion of primary care preventative visits increased over 20 years. Policies under the Affordable Care Act were the driving force. But it’s also true that sick visits are being diverted to urgent care.
Ella told this news organization she doesn’t even consider primary care for sick visits at this point. “I can’t wait 5 days or a week and a half. Unless I have bigger issues, like I need tests, I’m not even going to go to primary care.” It’s possible that other patients also see primary care as a place for testing and wellness checks and leave sick visits to retail and urgent care.
The Road Ahead
There’s no single fix for primary care, but experts agree that the fee-for-service model is a core issue for the specialty. In a 2021 report, the National Association of Engineering and Medicine said that primary care reform needs to include higher reimbursement rates for primary care and that US primary care should be restructured so that payers “pay primary care teams to care for people, not doctors to deliver services.”
In the current model, the doctor-patient clinic time is the only income-generating part of a primary care practice. A better model would consider the communication, administration, teams, and support doctors have to fund to provide the best primary care.
“We need to change how we pay and how much we pay, so [primary care doctors] are properly incentivized to build out a team to provide the comprehensive care you need,” Ms. Greiner said.
In the meantime, primary care doctors are adapting. Some drop down to part-time to account for the additional administrative workload. Others are transitioning to concierge services to offer the quality of care they want while getting the income they need. Still, others specialize their practice, offering primary care to a subset of the population, like older adults.
Employers are also looking to improve care access for their employees, hiring in-house doctors to provide primary care on site. Ms. Greiner recently met with a group of chief medical officers from major companies to discuss expanding primary care access via the workplace.
The efforts to adapt amid a broken system are admirable, Dr. Rotenstein said. And whatever a PCP has to do to keep practicing in primary care is laudable. The only problem with these adaptations is they largely limit a doctor’s patient pool and, therefore, limit access, she said. More significant reforms that adequately reimburse primary care and incentivize new doctors are still needed.
As for Ella, she got married. Her wife is in the military, so now she has Tricare, which comes with a more streamlined process to access primary care. However, doctor shortages are just as evident in that system. The couple called to schedule new patient appointments after their recent move to Virginia. The first available ones were 6 weeks out.
A version of this article appeared on Medscape.com.
By February of 2022, Ella, a 25-year-old behavioral interventionist in Colorado Springs, Colorado, was sick with strep-like symptoms for the third time in 3 months. She didn’t bother to call her doctor.
The first two times she had strep throat, she’d tried to schedule an appointment with her newest primary care doctor but couldn’t get in. They only had available appointments 5 and even 10 days out, but she’d already had symptoms for 3 days.
Until she graduated college, Ella had only known easy-access primary care. Her childhood family doctor and the nurse practitioners at her college clinic knew her. They anticipated her yearly allergies and knew about her predisposition for strep throat. Appointments were easy to schedule, and providers responded to her messages. But since entering the workforce and leaving her parent’s insurance, the kind of primary care she’d come to rely on was nearly impossible to find.
“I went to urgent care, and that became my primary care,” she told this news organization.
Patients Can’t Get Appointments
Primary care is in crisis. A growing number of Americans, like Ella, can’t access care when they need it. According to a 2024 report, 29% of adults and 14% of children don’t have a regular source of care. Those looking for a new primary care provider face extensive research and 6- to 9-month waits for a new patient appointment — if they can get in at all.
But even those with a primary care provider face long wait times: Days to weeks for a sick visit and months for a wellness checkup. Over one third of Medicare beneficiaries wait more than a month to see a doctor. Accessing primary care is more difficult than access to surgery, physical therapy, or rehabilitative care, according to a survey of Medicare beneficiaries by the Commonwealth Fund.
“Shortages tend to be in rural and urban underserved areas, but now, you’re hearing about primary care shortages in Boston, which is a mecca of healthcare,” said Ann Greiner, president and CEO of the Primary Care Coalition.
While retail clinics, urgent care, and telehealth help close the gap in acute needs, they miss one of primary care’s most critical benefits: A doctor who knows you. There’s strong evidence that ongoing treatment from a primary care physician (PCP) who knows your history, family, and context results in better long-term outcomes and fewer hospitalizations and emergency room visits.
If patients continue to find it too hard to break into primary care or set up an appointment, experts are concerned that they’ll stop pursuing primary care altogether.
Doctors’ Hands Are Tied
“I want to highlight that this is not an issue of primary care doctors not wanting to be accessible,” said Lisa Rotenstein, MD, MBA, a PCP and medical director of Ambulatory Quality and Safety at the University of California San Francisco Health. “These access issues are symptoms of the design of primary care in the United States.”
Across the United States, there’s a dearth of family medicine doctors, pediatricians, and internists. And without significantly more primary care providers, there’s simply no way for all Americans to get optimal primary care. The Health Resources and Services administration estimates a current shortage of 13,000 primary care providers. And that shortage will skyrocket to 68,000 by 2036 as the number of Americans needing care balloons and existing PCPs retire with too few trainees to fill their shoes.
The American Association of Medical Colleges predicts a slightly lower shortage in 2036 — between 20,000 and 40,000 primary care physicians — only if more residency positions are funded nationwide.
However, even with more positions, medical trainees see little incentive to pursue primary care. Young doctors are avoiding primary care because of the pressures, Dr. Rotenstein said. There’s incredible pressure to get reimbursement for primary care doctors. And the added administrative burden makes “the work life of these specialties not really manageable,” she said.
Continued Shortages of PCPs
“We know there’s a documented pajama time,” Ms. Greiner said. For every 1 hour spent with a patient, primary care must spend nearly 2 additional hours on electronic health records and desk work, according to a study by the American Medical Association. Even with all those additional hours devoted to getting paid, primary care doctors make an average of $103,000 less annually compared with their counterparts in surgery and oncology.
It’s not an attractive combination for a new doctor with medical debt. This year, Ms. Greiner said that residency positions in internal medicine and pediatrics went unfilled. Of those trainees who do go into a primary care specialty, many won’t last. Only half of primary care residents practice in primary care 3-5 years later. The rest choose to subspecialize or become hospitalists.
These untenable demands on a primary care provider don’t go unnoticed by patients. In Ella’s attempts to invest in a new primary care relationship, she often doesn’t feel heard and can tell the doctor is rushed. “[Urgent care is] probably not the best care because they don’t know me, but it does seem like they are able to listen to me better,” Ella said.
Patients Want to Invest in Primary Care
Primary care should work like putting money in a bank account, Dr. Rotenstein said. Young patients invest in the relationship and reap the benefits of a doctor who knows them later in life when they need more complex care. But if seeing a doctor is so difficult, many young people may stop investing in their PCP relationship.
“One thing ... that I worry about in this kind of situation where patients really have to put in a lot of work to get the care they need is in inequities of care,” Dr. Rotenstein said. “We know some of our patients are more able to undertake that work.”
Alternatively, the primary care shortage could be reshaping how patients seek care. A 2023 study showed the proportion of primary care preventative visits increased over 20 years. Policies under the Affordable Care Act were the driving force. But it’s also true that sick visits are being diverted to urgent care.
Ella told this news organization she doesn’t even consider primary care for sick visits at this point. “I can’t wait 5 days or a week and a half. Unless I have bigger issues, like I need tests, I’m not even going to go to primary care.” It’s possible that other patients also see primary care as a place for testing and wellness checks and leave sick visits to retail and urgent care.
The Road Ahead
There’s no single fix for primary care, but experts agree that the fee-for-service model is a core issue for the specialty. In a 2021 report, the National Association of Engineering and Medicine said that primary care reform needs to include higher reimbursement rates for primary care and that US primary care should be restructured so that payers “pay primary care teams to care for people, not doctors to deliver services.”
In the current model, the doctor-patient clinic time is the only income-generating part of a primary care practice. A better model would consider the communication, administration, teams, and support doctors have to fund to provide the best primary care.
“We need to change how we pay and how much we pay, so [primary care doctors] are properly incentivized to build out a team to provide the comprehensive care you need,” Ms. Greiner said.
In the meantime, primary care doctors are adapting. Some drop down to part-time to account for the additional administrative workload. Others are transitioning to concierge services to offer the quality of care they want while getting the income they need. Still, others specialize their practice, offering primary care to a subset of the population, like older adults.
Employers are also looking to improve care access for their employees, hiring in-house doctors to provide primary care on site. Ms. Greiner recently met with a group of chief medical officers from major companies to discuss expanding primary care access via the workplace.
The efforts to adapt amid a broken system are admirable, Dr. Rotenstein said. And whatever a PCP has to do to keep practicing in primary care is laudable. The only problem with these adaptations is they largely limit a doctor’s patient pool and, therefore, limit access, she said. More significant reforms that adequately reimburse primary care and incentivize new doctors are still needed.
As for Ella, she got married. Her wife is in the military, so now she has Tricare, which comes with a more streamlined process to access primary care. However, doctor shortages are just as evident in that system. The couple called to schedule new patient appointments after their recent move to Virginia. The first available ones were 6 weeks out.
A version of this article appeared on Medscape.com.
By February of 2022, Ella, a 25-year-old behavioral interventionist in Colorado Springs, Colorado, was sick with strep-like symptoms for the third time in 3 months. She didn’t bother to call her doctor.
The first two times she had strep throat, she’d tried to schedule an appointment with her newest primary care doctor but couldn’t get in. They only had available appointments 5 and even 10 days out, but she’d already had symptoms for 3 days.
Until she graduated college, Ella had only known easy-access primary care. Her childhood family doctor and the nurse practitioners at her college clinic knew her. They anticipated her yearly allergies and knew about her predisposition for strep throat. Appointments were easy to schedule, and providers responded to her messages. But since entering the workforce and leaving her parent’s insurance, the kind of primary care she’d come to rely on was nearly impossible to find.
“I went to urgent care, and that became my primary care,” she told this news organization.
Patients Can’t Get Appointments
Primary care is in crisis. A growing number of Americans, like Ella, can’t access care when they need it. According to a 2024 report, 29% of adults and 14% of children don’t have a regular source of care. Those looking for a new primary care provider face extensive research and 6- to 9-month waits for a new patient appointment — if they can get in at all.
But even those with a primary care provider face long wait times: Days to weeks for a sick visit and months for a wellness checkup. Over one third of Medicare beneficiaries wait more than a month to see a doctor. Accessing primary care is more difficult than access to surgery, physical therapy, or rehabilitative care, according to a survey of Medicare beneficiaries by the Commonwealth Fund.
“Shortages tend to be in rural and urban underserved areas, but now, you’re hearing about primary care shortages in Boston, which is a mecca of healthcare,” said Ann Greiner, president and CEO of the Primary Care Coalition.
While retail clinics, urgent care, and telehealth help close the gap in acute needs, they miss one of primary care’s most critical benefits: A doctor who knows you. There’s strong evidence that ongoing treatment from a primary care physician (PCP) who knows your history, family, and context results in better long-term outcomes and fewer hospitalizations and emergency room visits.
If patients continue to find it too hard to break into primary care or set up an appointment, experts are concerned that they’ll stop pursuing primary care altogether.
Doctors’ Hands Are Tied
“I want to highlight that this is not an issue of primary care doctors not wanting to be accessible,” said Lisa Rotenstein, MD, MBA, a PCP and medical director of Ambulatory Quality and Safety at the University of California San Francisco Health. “These access issues are symptoms of the design of primary care in the United States.”
Across the United States, there’s a dearth of family medicine doctors, pediatricians, and internists. And without significantly more primary care providers, there’s simply no way for all Americans to get optimal primary care. The Health Resources and Services administration estimates a current shortage of 13,000 primary care providers. And that shortage will skyrocket to 68,000 by 2036 as the number of Americans needing care balloons and existing PCPs retire with too few trainees to fill their shoes.
The American Association of Medical Colleges predicts a slightly lower shortage in 2036 — between 20,000 and 40,000 primary care physicians — only if more residency positions are funded nationwide.
However, even with more positions, medical trainees see little incentive to pursue primary care. Young doctors are avoiding primary care because of the pressures, Dr. Rotenstein said. There’s incredible pressure to get reimbursement for primary care doctors. And the added administrative burden makes “the work life of these specialties not really manageable,” she said.
Continued Shortages of PCPs
“We know there’s a documented pajama time,” Ms. Greiner said. For every 1 hour spent with a patient, primary care must spend nearly 2 additional hours on electronic health records and desk work, according to a study by the American Medical Association. Even with all those additional hours devoted to getting paid, primary care doctors make an average of $103,000 less annually compared with their counterparts in surgery and oncology.
It’s not an attractive combination for a new doctor with medical debt. This year, Ms. Greiner said that residency positions in internal medicine and pediatrics went unfilled. Of those trainees who do go into a primary care specialty, many won’t last. Only half of primary care residents practice in primary care 3-5 years later. The rest choose to subspecialize or become hospitalists.
These untenable demands on a primary care provider don’t go unnoticed by patients. In Ella’s attempts to invest in a new primary care relationship, she often doesn’t feel heard and can tell the doctor is rushed. “[Urgent care is] probably not the best care because they don’t know me, but it does seem like they are able to listen to me better,” Ella said.
Patients Want to Invest in Primary Care
Primary care should work like putting money in a bank account, Dr. Rotenstein said. Young patients invest in the relationship and reap the benefits of a doctor who knows them later in life when they need more complex care. But if seeing a doctor is so difficult, many young people may stop investing in their PCP relationship.
“One thing ... that I worry about in this kind of situation where patients really have to put in a lot of work to get the care they need is in inequities of care,” Dr. Rotenstein said. “We know some of our patients are more able to undertake that work.”
Alternatively, the primary care shortage could be reshaping how patients seek care. A 2023 study showed the proportion of primary care preventative visits increased over 20 years. Policies under the Affordable Care Act were the driving force. But it’s also true that sick visits are being diverted to urgent care.
Ella told this news organization she doesn’t even consider primary care for sick visits at this point. “I can’t wait 5 days or a week and a half. Unless I have bigger issues, like I need tests, I’m not even going to go to primary care.” It’s possible that other patients also see primary care as a place for testing and wellness checks and leave sick visits to retail and urgent care.
The Road Ahead
There’s no single fix for primary care, but experts agree that the fee-for-service model is a core issue for the specialty. In a 2021 report, the National Association of Engineering and Medicine said that primary care reform needs to include higher reimbursement rates for primary care and that US primary care should be restructured so that payers “pay primary care teams to care for people, not doctors to deliver services.”
In the current model, the doctor-patient clinic time is the only income-generating part of a primary care practice. A better model would consider the communication, administration, teams, and support doctors have to fund to provide the best primary care.
“We need to change how we pay and how much we pay, so [primary care doctors] are properly incentivized to build out a team to provide the comprehensive care you need,” Ms. Greiner said.
In the meantime, primary care doctors are adapting. Some drop down to part-time to account for the additional administrative workload. Others are transitioning to concierge services to offer the quality of care they want while getting the income they need. Still, others specialize their practice, offering primary care to a subset of the population, like older adults.
Employers are also looking to improve care access for their employees, hiring in-house doctors to provide primary care on site. Ms. Greiner recently met with a group of chief medical officers from major companies to discuss expanding primary care access via the workplace.
The efforts to adapt amid a broken system are admirable, Dr. Rotenstein said. And whatever a PCP has to do to keep practicing in primary care is laudable. The only problem with these adaptations is they largely limit a doctor’s patient pool and, therefore, limit access, she said. More significant reforms that adequately reimburse primary care and incentivize new doctors are still needed.
As for Ella, she got married. Her wife is in the military, so now she has Tricare, which comes with a more streamlined process to access primary care. However, doctor shortages are just as evident in that system. The couple called to schedule new patient appointments after their recent move to Virginia. The first available ones were 6 weeks out.
A version of this article appeared on Medscape.com.
Metabolic Dysfunction–Associated Steatotic Liver Disease Plus HIV Ups Risk for CVD but Not Liver Disease
TOPLINE:
Metabolic dysfunction-associated steatotic liver disease (MASLD) co-occurring with HIV infection does not appear to increase the risk for cirrhosis or hepatocellular carcinoma (HCC) compared with MASLD alone. However, the incidence of major adverse cardiovascular events (MACE) is significantly increased among patients with MASLD and HIV, a large study suggested.
METHODOLOGY:
- MASLD is highly prevalent in people living with HIV, but the impact of HIV on liver and cardiovascular disease (CVD) outcomes in people with MASLD remains unclear.
- To investigate, researchers created a propensity score-matched cohort of veterans with noncirrhotic MASLD, with and without HIV (920 patients in each group).
- They evaluated the incidence of cirrhosis, HCC, and MACE, as well as overall survival, among the two groups. They also assessed these outcomes in MASLD patients with HIV on the basis of whether they were on antiretroviral therapy (ART).
TAKEAWAY:
- During a median follow-up of 10.4 years in the MASLD with HIV group and 11.8 years in the MASLD-only group, the overall incidence of cirrhosis and HCC was similar in MASLD with vs without HIV (cirrhosis: 0.97 vs 1.06 per 100 person-years, P = .54; HCC: 0.26 vs 0.17 per 100,000 person-years, P = .23), regardless of ART use.
- In contrast, the incidence of MACE was significantly higher in MASLD with vs without HIV (5.18 vs 4.48 per 100 person-years, P = .03). The incidence also was higher in patients with MASLD and HIV who were not on ART compared with those on ART (5.83 vs 4.7 per 100 person-years, P = .07).
- Compared with MASLD without HIV, the overall 5-year survival was significantly lower in MASLD with HIV (91.3% vs 85.7%). In MASLD with HIV, receipt of ART was associated with a significantly higher 5-year survival than no ART (87.4% vs 81.6%).
IN PRACTICE:
“Ensuring timely and appropriate initiation of HIV treatment is critical in patients with MASLD who have concurrent HIV infection, as well as optimizing metabolic comorbidities that may also contribute to increased risks of CVD and increased mortality,” the authors wrote.
SOURCE:
The study, led by Robert J. Wong, MD, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Palo Alto, California, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The study cohort consisted predominantly of older men, which may limit generalizability to women and younger populations. Metabolic comorbidities are more common in veterans compared with the general population, potentially affecting the generalizability of the CVD risk findings.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from Theratechnologies. Wong has received funding for his institution from Gilead Sciences, Exact Sciences, and Durect Corporation and has served as a consultant for Gilead Sciences.
A version of this article appeared on Medscape.com.
TOPLINE:
Metabolic dysfunction-associated steatotic liver disease (MASLD) co-occurring with HIV infection does not appear to increase the risk for cirrhosis or hepatocellular carcinoma (HCC) compared with MASLD alone. However, the incidence of major adverse cardiovascular events (MACE) is significantly increased among patients with MASLD and HIV, a large study suggested.
METHODOLOGY:
- MASLD is highly prevalent in people living with HIV, but the impact of HIV on liver and cardiovascular disease (CVD) outcomes in people with MASLD remains unclear.
- To investigate, researchers created a propensity score-matched cohort of veterans with noncirrhotic MASLD, with and without HIV (920 patients in each group).
- They evaluated the incidence of cirrhosis, HCC, and MACE, as well as overall survival, among the two groups. They also assessed these outcomes in MASLD patients with HIV on the basis of whether they were on antiretroviral therapy (ART).
TAKEAWAY:
- During a median follow-up of 10.4 years in the MASLD with HIV group and 11.8 years in the MASLD-only group, the overall incidence of cirrhosis and HCC was similar in MASLD with vs without HIV (cirrhosis: 0.97 vs 1.06 per 100 person-years, P = .54; HCC: 0.26 vs 0.17 per 100,000 person-years, P = .23), regardless of ART use.
- In contrast, the incidence of MACE was significantly higher in MASLD with vs without HIV (5.18 vs 4.48 per 100 person-years, P = .03). The incidence also was higher in patients with MASLD and HIV who were not on ART compared with those on ART (5.83 vs 4.7 per 100 person-years, P = .07).
- Compared with MASLD without HIV, the overall 5-year survival was significantly lower in MASLD with HIV (91.3% vs 85.7%). In MASLD with HIV, receipt of ART was associated with a significantly higher 5-year survival than no ART (87.4% vs 81.6%).
IN PRACTICE:
“Ensuring timely and appropriate initiation of HIV treatment is critical in patients with MASLD who have concurrent HIV infection, as well as optimizing metabolic comorbidities that may also contribute to increased risks of CVD and increased mortality,” the authors wrote.
SOURCE:
The study, led by Robert J. Wong, MD, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Palo Alto, California, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The study cohort consisted predominantly of older men, which may limit generalizability to women and younger populations. Metabolic comorbidities are more common in veterans compared with the general population, potentially affecting the generalizability of the CVD risk findings.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from Theratechnologies. Wong has received funding for his institution from Gilead Sciences, Exact Sciences, and Durect Corporation and has served as a consultant for Gilead Sciences.
A version of this article appeared on Medscape.com.
TOPLINE:
Metabolic dysfunction-associated steatotic liver disease (MASLD) co-occurring with HIV infection does not appear to increase the risk for cirrhosis or hepatocellular carcinoma (HCC) compared with MASLD alone. However, the incidence of major adverse cardiovascular events (MACE) is significantly increased among patients with MASLD and HIV, a large study suggested.
METHODOLOGY:
- MASLD is highly prevalent in people living with HIV, but the impact of HIV on liver and cardiovascular disease (CVD) outcomes in people with MASLD remains unclear.
- To investigate, researchers created a propensity score-matched cohort of veterans with noncirrhotic MASLD, with and without HIV (920 patients in each group).
- They evaluated the incidence of cirrhosis, HCC, and MACE, as well as overall survival, among the two groups. They also assessed these outcomes in MASLD patients with HIV on the basis of whether they were on antiretroviral therapy (ART).
TAKEAWAY:
- During a median follow-up of 10.4 years in the MASLD with HIV group and 11.8 years in the MASLD-only group, the overall incidence of cirrhosis and HCC was similar in MASLD with vs without HIV (cirrhosis: 0.97 vs 1.06 per 100 person-years, P = .54; HCC: 0.26 vs 0.17 per 100,000 person-years, P = .23), regardless of ART use.
- In contrast, the incidence of MACE was significantly higher in MASLD with vs without HIV (5.18 vs 4.48 per 100 person-years, P = .03). The incidence also was higher in patients with MASLD and HIV who were not on ART compared with those on ART (5.83 vs 4.7 per 100 person-years, P = .07).
- Compared with MASLD without HIV, the overall 5-year survival was significantly lower in MASLD with HIV (91.3% vs 85.7%). In MASLD with HIV, receipt of ART was associated with a significantly higher 5-year survival than no ART (87.4% vs 81.6%).
IN PRACTICE:
“Ensuring timely and appropriate initiation of HIV treatment is critical in patients with MASLD who have concurrent HIV infection, as well as optimizing metabolic comorbidities that may also contribute to increased risks of CVD and increased mortality,” the authors wrote.
SOURCE:
The study, led by Robert J. Wong, MD, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Palo Alto, California, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The study cohort consisted predominantly of older men, which may limit generalizability to women and younger populations. Metabolic comorbidities are more common in veterans compared with the general population, potentially affecting the generalizability of the CVD risk findings.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from Theratechnologies. Wong has received funding for his institution from Gilead Sciences, Exact Sciences, and Durect Corporation and has served as a consultant for Gilead Sciences.
A version of this article appeared on Medscape.com.
Avian Flu Threat Still Low and Vaccine Measures Are Ready
After cow-to-cow transmission of avian influenza A subtype H5N1 in US dairy herds led to a cow-to-human transmission in Texas, the Association of State and Territorial Health Officials convened a panel of experts for a scientific symposium on Thursday to talk about the public health implications.
From the sequencing data, “we can expect and anticipate that [the candidate vaccine viruses] will provide good protection,” she explained.
Establishing candidate vaccine viruses “are the precursor to moving into large-scale vaccine production,” Dr. Dugan explained. Should that be needed, the candidate viruses can be used by manufacturers to produce new vaccines.
The CDC is also actively partnering with commercial diagnostic developers and testing companies in case there is a need to scale-up testing, Dr. Dugan said.
The only current human case in the United States was reported on April 1 and confirmed by the CDC within 24 hours, reported Sonja Olsen, PhD, associate director for preparedness and response of the Influenza Division at the CDC.
The person had direct exposure to cattle and reported eye redness, consistent with conjunctivitis, as the only symptom. The person received treatment and has recovered, and there were no reports of illness among the person’s household contacts, Dr. Olsen said.
Person With the Virus Has Recovered
The only other detection of the virus in a human in the United States was in 2022 and it was associated with infected poultry exposure. That person also had mild illness and recovered, Dr. Olsen explained.
Since 1997, when the first case of human infection was reported globally, “there have been 909 [human cases] reported from 23 countries,” Dr. Olsen said. “About half [52%] of the human cases have resulted in death.” Only a small number of human cases have been reported since 2015, but since 2022, more than two dozen human cases have been reported to the World Health Organization.
Experience with the virus in the United States has been about a year behind that in Europe, said Rosemary Sifford, DVM, chief veterinary officer at the US Department of Agriculture. In the United States, the first detection — in January 2022 — was in wild birds; this was followed the next month by the first detection in a commercial poultry flock.
In March of this year, the United States had its first detection in cattle, specifically dairy cattle. But testing has shown that “it remains very much an avian virus. It’s not becoming a bovine virus,” Dr. Sifford reported.
Detected in Cattle
Earlier this week, in an effort to minimize the risk of disease spread, the USDA issued a federal order that requires the reporting of positive influenza tests in livestock and mandatory testing for influenza of dairy cattle before interstate movement.
“As of today, there are affected herds in 33 farms across eight states,” reported Dr. Olsen.
Tests are ongoing to determine how the virus is traveling, but “what we can say is that there’s a high viral load in the milk in the cattle, and it appears that the transmission is happening mostly within the lactating herds,” Dr. Sifford reported. It is unclear whether that is happening during the milking of the cows or whether contaminated milk from a cow with a high viral load is transmitting the virus to other cattle.
“We are strongly encouraging producers to limit the movement of cattle, particularly lactating cattle, as much as possible,” she says.
Milk Is Likely the Source of Transmission
“We haven’t seen anything that would change our assessment that the commercial milk supply is safe,” says Donald Prater, DVM, acting director of the Center for Food Safety and Applied Nutrition at the US Food and Drug Administration (FDA).
In the federal and state milk safety system, he explained, nearly 99% of the commercial milk supply comes from farms that participate in the Grade A program and follow the Pasteurized Milk Ordinance, which outlines pasteurization requirements.
Because detection of the virus in dairy cattle is new, there are many questions to be answered in research, he reported. Among them:
- What level of virus might be leaving the farms from shedding by apparently healthy cows?
- Does any live virus survive the pasteurization process?
- Do different methods of pasteurization and dairy production have different effects on the viability of H5N1?
- Are effects different in various forms of dairy products, such as cheese and cream?
A critical question regarding the potential risk to humans is how much milk would have to be consumed for the virus to become an established infection. That information is essential to determine “what type of pasteurization criteria” are needed to provide “acceptable public health outcomes,” Dr. Prater said.
The CDC is currently using the flu surveillance system to monitor for H5N1 activity in people. The systems show no current indicators of unusual influenza activity in people.
A version of this article appeared on Medscape.com.
After cow-to-cow transmission of avian influenza A subtype H5N1 in US dairy herds led to a cow-to-human transmission in Texas, the Association of State and Territorial Health Officials convened a panel of experts for a scientific symposium on Thursday to talk about the public health implications.
From the sequencing data, “we can expect and anticipate that [the candidate vaccine viruses] will provide good protection,” she explained.
Establishing candidate vaccine viruses “are the precursor to moving into large-scale vaccine production,” Dr. Dugan explained. Should that be needed, the candidate viruses can be used by manufacturers to produce new vaccines.
The CDC is also actively partnering with commercial diagnostic developers and testing companies in case there is a need to scale-up testing, Dr. Dugan said.
The only current human case in the United States was reported on April 1 and confirmed by the CDC within 24 hours, reported Sonja Olsen, PhD, associate director for preparedness and response of the Influenza Division at the CDC.
The person had direct exposure to cattle and reported eye redness, consistent with conjunctivitis, as the only symptom. The person received treatment and has recovered, and there were no reports of illness among the person’s household contacts, Dr. Olsen said.
Person With the Virus Has Recovered
The only other detection of the virus in a human in the United States was in 2022 and it was associated with infected poultry exposure. That person also had mild illness and recovered, Dr. Olsen explained.
Since 1997, when the first case of human infection was reported globally, “there have been 909 [human cases] reported from 23 countries,” Dr. Olsen said. “About half [52%] of the human cases have resulted in death.” Only a small number of human cases have been reported since 2015, but since 2022, more than two dozen human cases have been reported to the World Health Organization.
Experience with the virus in the United States has been about a year behind that in Europe, said Rosemary Sifford, DVM, chief veterinary officer at the US Department of Agriculture. In the United States, the first detection — in January 2022 — was in wild birds; this was followed the next month by the first detection in a commercial poultry flock.
In March of this year, the United States had its first detection in cattle, specifically dairy cattle. But testing has shown that “it remains very much an avian virus. It’s not becoming a bovine virus,” Dr. Sifford reported.
Detected in Cattle
Earlier this week, in an effort to minimize the risk of disease spread, the USDA issued a federal order that requires the reporting of positive influenza tests in livestock and mandatory testing for influenza of dairy cattle before interstate movement.
“As of today, there are affected herds in 33 farms across eight states,” reported Dr. Olsen.
Tests are ongoing to determine how the virus is traveling, but “what we can say is that there’s a high viral load in the milk in the cattle, and it appears that the transmission is happening mostly within the lactating herds,” Dr. Sifford reported. It is unclear whether that is happening during the milking of the cows or whether contaminated milk from a cow with a high viral load is transmitting the virus to other cattle.
“We are strongly encouraging producers to limit the movement of cattle, particularly lactating cattle, as much as possible,” she says.
Milk Is Likely the Source of Transmission
“We haven’t seen anything that would change our assessment that the commercial milk supply is safe,” says Donald Prater, DVM, acting director of the Center for Food Safety and Applied Nutrition at the US Food and Drug Administration (FDA).
In the federal and state milk safety system, he explained, nearly 99% of the commercial milk supply comes from farms that participate in the Grade A program and follow the Pasteurized Milk Ordinance, which outlines pasteurization requirements.
Because detection of the virus in dairy cattle is new, there are many questions to be answered in research, he reported. Among them:
- What level of virus might be leaving the farms from shedding by apparently healthy cows?
- Does any live virus survive the pasteurization process?
- Do different methods of pasteurization and dairy production have different effects on the viability of H5N1?
- Are effects different in various forms of dairy products, such as cheese and cream?
A critical question regarding the potential risk to humans is how much milk would have to be consumed for the virus to become an established infection. That information is essential to determine “what type of pasteurization criteria” are needed to provide “acceptable public health outcomes,” Dr. Prater said.
The CDC is currently using the flu surveillance system to monitor for H5N1 activity in people. The systems show no current indicators of unusual influenza activity in people.
A version of this article appeared on Medscape.com.
After cow-to-cow transmission of avian influenza A subtype H5N1 in US dairy herds led to a cow-to-human transmission in Texas, the Association of State and Territorial Health Officials convened a panel of experts for a scientific symposium on Thursday to talk about the public health implications.
From the sequencing data, “we can expect and anticipate that [the candidate vaccine viruses] will provide good protection,” she explained.
Establishing candidate vaccine viruses “are the precursor to moving into large-scale vaccine production,” Dr. Dugan explained. Should that be needed, the candidate viruses can be used by manufacturers to produce new vaccines.
The CDC is also actively partnering with commercial diagnostic developers and testing companies in case there is a need to scale-up testing, Dr. Dugan said.
The only current human case in the United States was reported on April 1 and confirmed by the CDC within 24 hours, reported Sonja Olsen, PhD, associate director for preparedness and response of the Influenza Division at the CDC.
The person had direct exposure to cattle and reported eye redness, consistent with conjunctivitis, as the only symptom. The person received treatment and has recovered, and there were no reports of illness among the person’s household contacts, Dr. Olsen said.
Person With the Virus Has Recovered
The only other detection of the virus in a human in the United States was in 2022 and it was associated with infected poultry exposure. That person also had mild illness and recovered, Dr. Olsen explained.
Since 1997, when the first case of human infection was reported globally, “there have been 909 [human cases] reported from 23 countries,” Dr. Olsen said. “About half [52%] of the human cases have resulted in death.” Only a small number of human cases have been reported since 2015, but since 2022, more than two dozen human cases have been reported to the World Health Organization.
Experience with the virus in the United States has been about a year behind that in Europe, said Rosemary Sifford, DVM, chief veterinary officer at the US Department of Agriculture. In the United States, the first detection — in January 2022 — was in wild birds; this was followed the next month by the first detection in a commercial poultry flock.
In March of this year, the United States had its first detection in cattle, specifically dairy cattle. But testing has shown that “it remains very much an avian virus. It’s not becoming a bovine virus,” Dr. Sifford reported.
Detected in Cattle
Earlier this week, in an effort to minimize the risk of disease spread, the USDA issued a federal order that requires the reporting of positive influenza tests in livestock and mandatory testing for influenza of dairy cattle before interstate movement.
“As of today, there are affected herds in 33 farms across eight states,” reported Dr. Olsen.
Tests are ongoing to determine how the virus is traveling, but “what we can say is that there’s a high viral load in the milk in the cattle, and it appears that the transmission is happening mostly within the lactating herds,” Dr. Sifford reported. It is unclear whether that is happening during the milking of the cows or whether contaminated milk from a cow with a high viral load is transmitting the virus to other cattle.
“We are strongly encouraging producers to limit the movement of cattle, particularly lactating cattle, as much as possible,” she says.
Milk Is Likely the Source of Transmission
“We haven’t seen anything that would change our assessment that the commercial milk supply is safe,” says Donald Prater, DVM, acting director of the Center for Food Safety and Applied Nutrition at the US Food and Drug Administration (FDA).
In the federal and state milk safety system, he explained, nearly 99% of the commercial milk supply comes from farms that participate in the Grade A program and follow the Pasteurized Milk Ordinance, which outlines pasteurization requirements.
Because detection of the virus in dairy cattle is new, there are many questions to be answered in research, he reported. Among them:
- What level of virus might be leaving the farms from shedding by apparently healthy cows?
- Does any live virus survive the pasteurization process?
- Do different methods of pasteurization and dairy production have different effects on the viability of H5N1?
- Are effects different in various forms of dairy products, such as cheese and cream?
A critical question regarding the potential risk to humans is how much milk would have to be consumed for the virus to become an established infection. That information is essential to determine “what type of pasteurization criteria” are needed to provide “acceptable public health outcomes,” Dr. Prater said.
The CDC is currently using the flu surveillance system to monitor for H5N1 activity in people. The systems show no current indicators of unusual influenza activity in people.
A version of this article appeared on Medscape.com.
Dermatologic Care for Refugees: Effective Management of Scabies and Pediculosis
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.
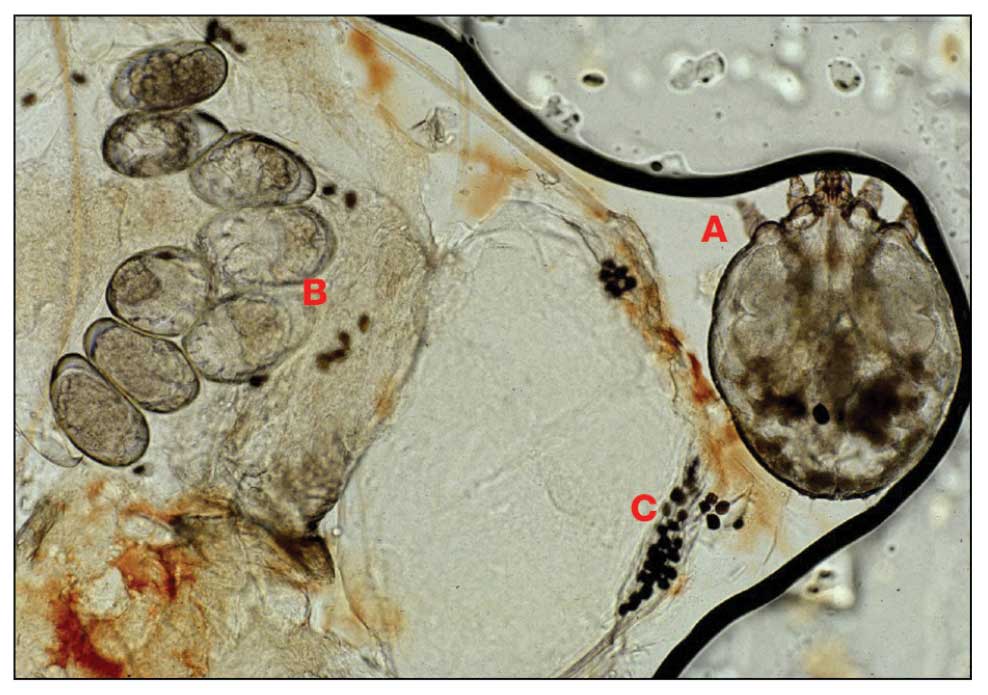
The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27
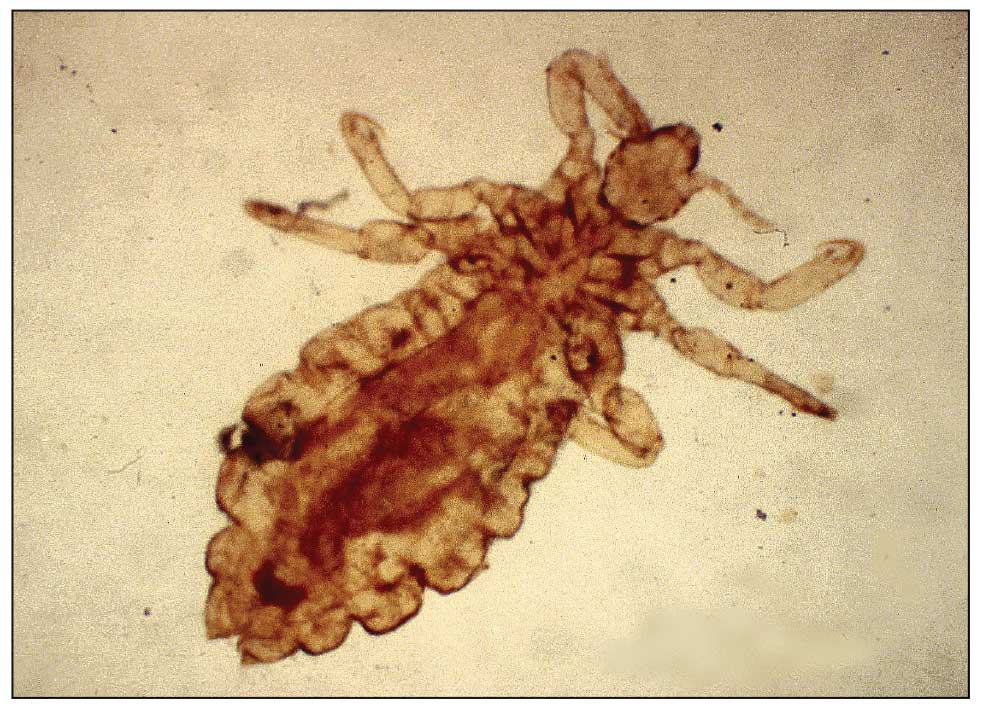
Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32
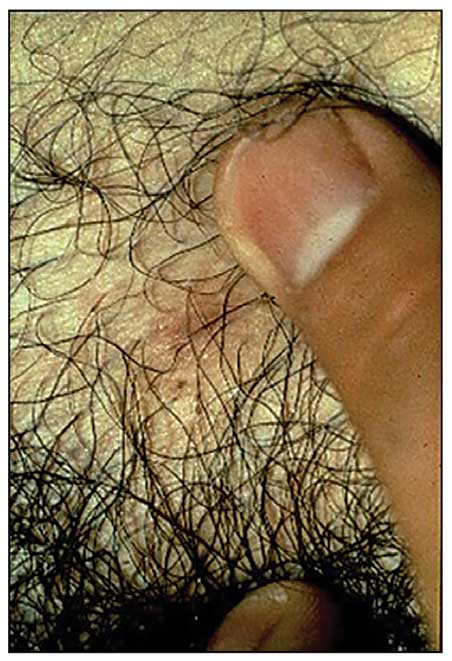
Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31
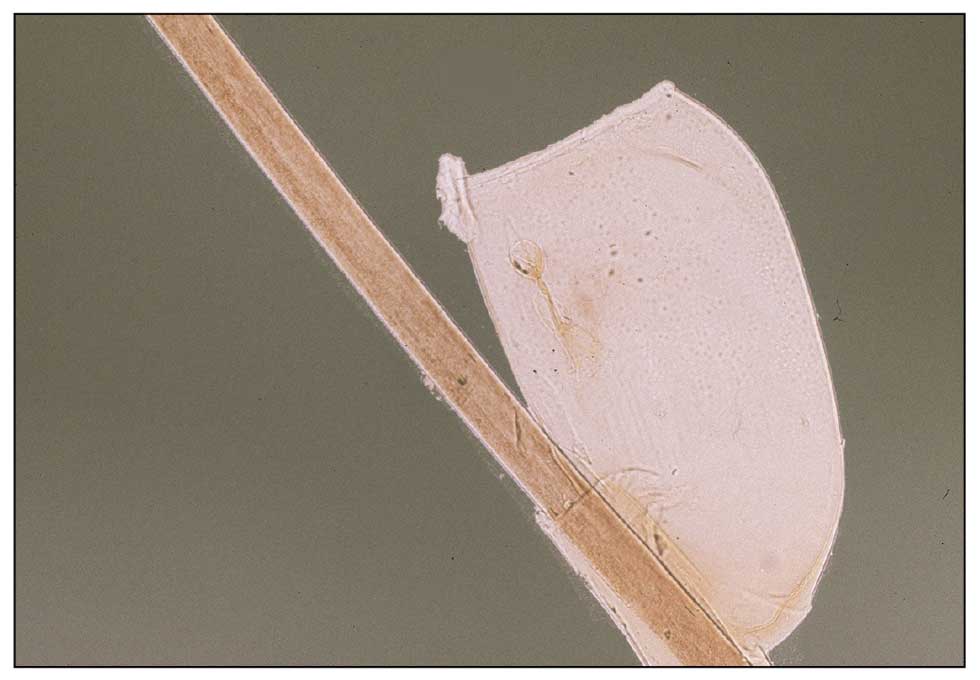
Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.
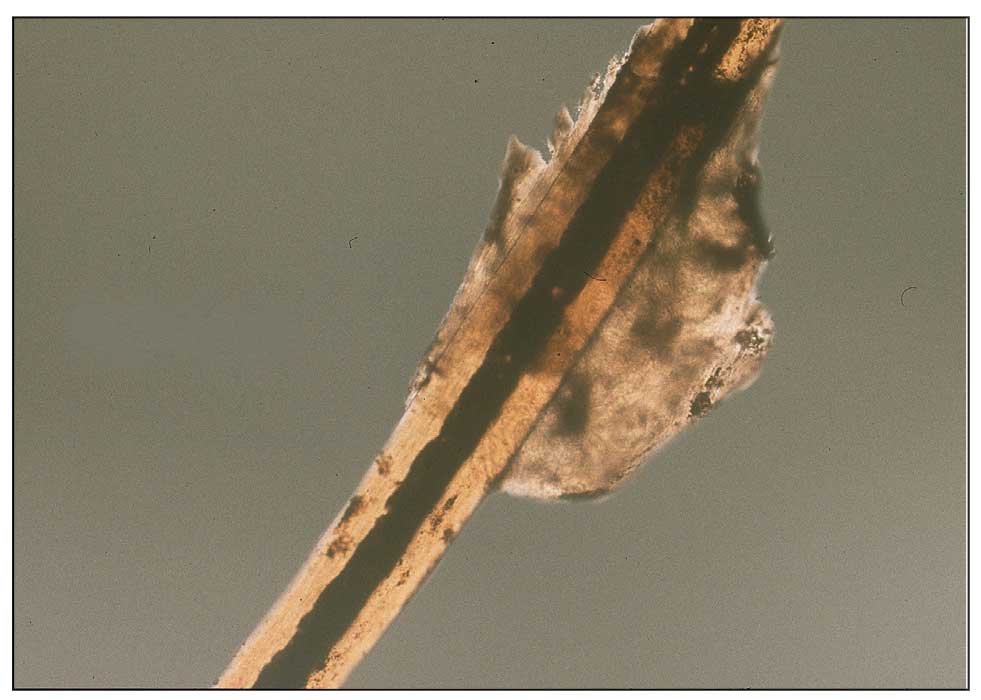
Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Practice Points
- War and natural disasters displace populations and disrupt infrastructure and access to medical care.
- Infestations and cutaneous infections are common among refugee populations, and impetigo often is a sign of underlying scabies infestation.
- Body lice are important disease vectors inrefugee populations.
Hepatitis Kills 3500 People Each Day, Says WHO
The number of deaths from viral hepatitis worldwide increased from 1.1 million in 2019 to 1.3 million in 2022. These figures equate to approximately 3500 deaths per day due to the disease, which is the second leading cause of mortality from infectious agents globally.
These data are part of the recently released Global Hepatitis Report 2024, which was published by the World Health Organization (WHO) during the World Hepatitis Summit in Lisbon, Portugal.
“This report paints a concerning picture: Despite global progress in preventing hepatitis infections, deaths are increasing because very few people with hepatitis are being diagnosed and treated,” said WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
Hepatitis B significantly is associated with the highest mortality rate. It accounted for 83% of deaths from the disease in 2022. Meanwhile, hepatitis C was responsible for 17% of deaths. The mortality of other, less common types of hepatitis was not considered in the ranking.
The report also indicates that more than 6000 people worldwide are infected with viral hepatitis every day. The 2.2 million new cases in 2022 represent a slight decrease from 2.5 million in 2019, but the WHO considers the incidence high.
The organization’s updated statistics indicate that about 254 million people had hepatitis B in 2022, while 50 million had type C.
“Besides the deaths, the number of new cases every year is also striking. These are diseases that continue to spread. In the case of hepatitis C, the spread results from lack of access to disposable or properly sterilized sharp materials,” said Thor Dantas, MD, PhD, a physician and director of the Brazilian Society of Hepatology’s Viral Hepatitis Committee.
The situation of hepatitis B is particularly problematic, given that there is a safe and effective vaccine against it, said Dantas. “It’s remarkable that we continue to have so many new cases worldwide. This shows that we are failing in access to preventive measures for control and spread.”
Half of chronic hepatitis B and C cases occur in people between ages 30 and 54 years, while 12% affect children. There are more infections among men, who represent 58% of all cases.
The WHO also drew attention to the difficulty of accessing diagnosis and treatment. Only 13% of people with chronic hepatitis B infection were diagnosed, while only 3% — equivalent to 7 million people — received antiviral therapy by the end of 2022. This result is well below the WHO’s global target, which aims to treat 80% of cases by 2030.
Brazil has a higher diagnostic rate than the global average but is still below the target. According to the report, in 2022, the country diagnosed 34.2% of all hepatitis B infections. However, treatment coverage remains low: 3.6% of the total.
For hepatitis C, the scenario is somewhat different. During the same period, Brazil diagnosed 36% of total cases, with a treatment rate of 24%.
In 2022, Brazil had 2578 deaths from hepatitis B and 2977 from hepatitis C.
Because hepatitis is a silent disease, diagnosis often comes late, when the disease is already quite advanced, said Dr. Dantas. “Viral hepatitis evolves over the years essentially asymptomatically. Malaria shows symptoms, and tuberculosis shows symptoms. Viral hepatitis does not. They are only discovered through active searching.”
The WHO report shows significant regional differences in infection rates. Almost two thirds of cases are concentrated in the following 10 countries: China, India, Indonesia, Nigeria, Pakistan, Ethiopia, Bangladesh, Vietnam, the Philippines, and Russia.
In terms of hepatitis C incidence, Brazil ranks 15th globally, with 536,000 cases in 2022, representing 1.1% of the global total. The list is led by Pakistan, with 8.8 million cases, equivalent to 17.8% of the total. Next are India, with 5.5 million (11.2%), and China, with 4 million (8.1%).
In addition to regional differences, the report also reveals profound disparities in the prices paid for major treatments.
“Price disparities between, and even within, WHO regions persist, with many countries paying above global reference values, including for nonpatented medications,” according to the report.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.
The number of deaths from viral hepatitis worldwide increased from 1.1 million in 2019 to 1.3 million in 2022. These figures equate to approximately 3500 deaths per day due to the disease, which is the second leading cause of mortality from infectious agents globally.
These data are part of the recently released Global Hepatitis Report 2024, which was published by the World Health Organization (WHO) during the World Hepatitis Summit in Lisbon, Portugal.
“This report paints a concerning picture: Despite global progress in preventing hepatitis infections, deaths are increasing because very few people with hepatitis are being diagnosed and treated,” said WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
Hepatitis B significantly is associated with the highest mortality rate. It accounted for 83% of deaths from the disease in 2022. Meanwhile, hepatitis C was responsible for 17% of deaths. The mortality of other, less common types of hepatitis was not considered in the ranking.
The report also indicates that more than 6000 people worldwide are infected with viral hepatitis every day. The 2.2 million new cases in 2022 represent a slight decrease from 2.5 million in 2019, but the WHO considers the incidence high.
The organization’s updated statistics indicate that about 254 million people had hepatitis B in 2022, while 50 million had type C.
“Besides the deaths, the number of new cases every year is also striking. These are diseases that continue to spread. In the case of hepatitis C, the spread results from lack of access to disposable or properly sterilized sharp materials,” said Thor Dantas, MD, PhD, a physician and director of the Brazilian Society of Hepatology’s Viral Hepatitis Committee.
The situation of hepatitis B is particularly problematic, given that there is a safe and effective vaccine against it, said Dantas. “It’s remarkable that we continue to have so many new cases worldwide. This shows that we are failing in access to preventive measures for control and spread.”
Half of chronic hepatitis B and C cases occur in people between ages 30 and 54 years, while 12% affect children. There are more infections among men, who represent 58% of all cases.
The WHO also drew attention to the difficulty of accessing diagnosis and treatment. Only 13% of people with chronic hepatitis B infection were diagnosed, while only 3% — equivalent to 7 million people — received antiviral therapy by the end of 2022. This result is well below the WHO’s global target, which aims to treat 80% of cases by 2030.
Brazil has a higher diagnostic rate than the global average but is still below the target. According to the report, in 2022, the country diagnosed 34.2% of all hepatitis B infections. However, treatment coverage remains low: 3.6% of the total.
For hepatitis C, the scenario is somewhat different. During the same period, Brazil diagnosed 36% of total cases, with a treatment rate of 24%.
In 2022, Brazil had 2578 deaths from hepatitis B and 2977 from hepatitis C.
Because hepatitis is a silent disease, diagnosis often comes late, when the disease is already quite advanced, said Dr. Dantas. “Viral hepatitis evolves over the years essentially asymptomatically. Malaria shows symptoms, and tuberculosis shows symptoms. Viral hepatitis does not. They are only discovered through active searching.”
The WHO report shows significant regional differences in infection rates. Almost two thirds of cases are concentrated in the following 10 countries: China, India, Indonesia, Nigeria, Pakistan, Ethiopia, Bangladesh, Vietnam, the Philippines, and Russia.
In terms of hepatitis C incidence, Brazil ranks 15th globally, with 536,000 cases in 2022, representing 1.1% of the global total. The list is led by Pakistan, with 8.8 million cases, equivalent to 17.8% of the total. Next are India, with 5.5 million (11.2%), and China, with 4 million (8.1%).
In addition to regional differences, the report also reveals profound disparities in the prices paid for major treatments.
“Price disparities between, and even within, WHO regions persist, with many countries paying above global reference values, including for nonpatented medications,” according to the report.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.
The number of deaths from viral hepatitis worldwide increased from 1.1 million in 2019 to 1.3 million in 2022. These figures equate to approximately 3500 deaths per day due to the disease, which is the second leading cause of mortality from infectious agents globally.
These data are part of the recently released Global Hepatitis Report 2024, which was published by the World Health Organization (WHO) during the World Hepatitis Summit in Lisbon, Portugal.
“This report paints a concerning picture: Despite global progress in preventing hepatitis infections, deaths are increasing because very few people with hepatitis are being diagnosed and treated,” said WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
Hepatitis B significantly is associated with the highest mortality rate. It accounted for 83% of deaths from the disease in 2022. Meanwhile, hepatitis C was responsible for 17% of deaths. The mortality of other, less common types of hepatitis was not considered in the ranking.
The report also indicates that more than 6000 people worldwide are infected with viral hepatitis every day. The 2.2 million new cases in 2022 represent a slight decrease from 2.5 million in 2019, but the WHO considers the incidence high.
The organization’s updated statistics indicate that about 254 million people had hepatitis B in 2022, while 50 million had type C.
“Besides the deaths, the number of new cases every year is also striking. These are diseases that continue to spread. In the case of hepatitis C, the spread results from lack of access to disposable or properly sterilized sharp materials,” said Thor Dantas, MD, PhD, a physician and director of the Brazilian Society of Hepatology’s Viral Hepatitis Committee.
The situation of hepatitis B is particularly problematic, given that there is a safe and effective vaccine against it, said Dantas. “It’s remarkable that we continue to have so many new cases worldwide. This shows that we are failing in access to preventive measures for control and spread.”
Half of chronic hepatitis B and C cases occur in people between ages 30 and 54 years, while 12% affect children. There are more infections among men, who represent 58% of all cases.
The WHO also drew attention to the difficulty of accessing diagnosis and treatment. Only 13% of people with chronic hepatitis B infection were diagnosed, while only 3% — equivalent to 7 million people — received antiviral therapy by the end of 2022. This result is well below the WHO’s global target, which aims to treat 80% of cases by 2030.
Brazil has a higher diagnostic rate than the global average but is still below the target. According to the report, in 2022, the country diagnosed 34.2% of all hepatitis B infections. However, treatment coverage remains low: 3.6% of the total.
For hepatitis C, the scenario is somewhat different. During the same period, Brazil diagnosed 36% of total cases, with a treatment rate of 24%.
In 2022, Brazil had 2578 deaths from hepatitis B and 2977 from hepatitis C.
Because hepatitis is a silent disease, diagnosis often comes late, when the disease is already quite advanced, said Dr. Dantas. “Viral hepatitis evolves over the years essentially asymptomatically. Malaria shows symptoms, and tuberculosis shows symptoms. Viral hepatitis does not. They are only discovered through active searching.”
The WHO report shows significant regional differences in infection rates. Almost two thirds of cases are concentrated in the following 10 countries: China, India, Indonesia, Nigeria, Pakistan, Ethiopia, Bangladesh, Vietnam, the Philippines, and Russia.
In terms of hepatitis C incidence, Brazil ranks 15th globally, with 536,000 cases in 2022, representing 1.1% of the global total. The list is led by Pakistan, with 8.8 million cases, equivalent to 17.8% of the total. Next are India, with 5.5 million (11.2%), and China, with 4 million (8.1%).
In addition to regional differences, the report also reveals profound disparities in the prices paid for major treatments.
“Price disparities between, and even within, WHO regions persist, with many countries paying above global reference values, including for nonpatented medications,” according to the report.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.