User login
Influenza vaccine may offer much more than flu prevention
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Unvaccinated 10 times more likely to be hospitalized for Omicron
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
FROM JAMA INTERNAL MEDICINE
AI and reality – diagnosing otitis media is a real challenge
Let’s pretend for a moment that you receive a call from one of your college roommates who thanks to his family connections has become a venture capitalist in California. His group is considering investing in a start-up that is developing a handheld instrument that it claims will use artificial intelligence to diagnose ear infections far more accurately than the human eye. He wonders if you would like to help him evaluate the company’s proposal and offers you a small percentage of the profits for your efforts should they choose to invest.
Your former roommate has done enough research on his own to understand that otitis media makes up a large chunk of a pediatrician’s workload and that making an accurate diagnosis can often be difficult in a struggling child. He describes his own experience watching a frustrated pediatrician attempting to remove wax from his child’s ear and eventually prescribing antibiotics “to be safe.”
You agree and review the prospectus, which includes a paper from a peer-reviewed journal. What you discover is that the investigators used more than 600 high-resolution images of tympanic membranes taken “during operative myringotomy and tympanostomy tube placement” and the findings at tympanocentesis to train a neural network.
Once trained, the model they developed could differentiate with 95% accuracy between an image of a tympanic membrane that covered a normal middle ear from one that merely contained fluid and from one that contained infected fluid. When these same images were shown to 39 clinicians, more than half of which were pediatricians and included both faculty-level staff and trainees, the average diagnostic accuracy was 65%.
The prospectus includes prediction that this technology could easily be developed into a handheld instrument similar to a traditional otoscope, which could then be linked to the operator’s smartphone, giving the clinician an instant treat or no-treat answer.
Now, remember you have nothing to lose except maybe a friendship. How would you advise your old college roommate?
My advice to your college buddy would be one of caution! Yes, there is a potential big upside because there is a real need for a device that could provide a diagnostic accuracy that this AI model promises. While I suspect that AI will always be more accurate in diagnosis using static images, I bet that most people, clinicians and nonclinicians, could improve their accuracy by linking photos with diagnoses with an hour of practice.
However, evaluating a high-resolution photograph taken through an operative scope inserted into the cerumenless ear canal of a sedated, afrebrile child is several orders of magnitude less difficult than the real-world environment in which the diagnosis of otitis media is usually made.
If the venture capitalists were still interested in getting into the otitis media marketplace, you might suggest they look into companies that have already developed image capture otoscopes. At this point I could only find one on the Internet that was portable and it certainly isn’t small-child friendly. Once we have a tool that can capture images in real-world situations, the next step is to train AI systems to interpret them using the approach these researchers have developed. I bet it can be done. It will be only a matter of time ... and money.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Let’s pretend for a moment that you receive a call from one of your college roommates who thanks to his family connections has become a venture capitalist in California. His group is considering investing in a start-up that is developing a handheld instrument that it claims will use artificial intelligence to diagnose ear infections far more accurately than the human eye. He wonders if you would like to help him evaluate the company’s proposal and offers you a small percentage of the profits for your efforts should they choose to invest.
Your former roommate has done enough research on his own to understand that otitis media makes up a large chunk of a pediatrician’s workload and that making an accurate diagnosis can often be difficult in a struggling child. He describes his own experience watching a frustrated pediatrician attempting to remove wax from his child’s ear and eventually prescribing antibiotics “to be safe.”
You agree and review the prospectus, which includes a paper from a peer-reviewed journal. What you discover is that the investigators used more than 600 high-resolution images of tympanic membranes taken “during operative myringotomy and tympanostomy tube placement” and the findings at tympanocentesis to train a neural network.
Once trained, the model they developed could differentiate with 95% accuracy between an image of a tympanic membrane that covered a normal middle ear from one that merely contained fluid and from one that contained infected fluid. When these same images were shown to 39 clinicians, more than half of which were pediatricians and included both faculty-level staff and trainees, the average diagnostic accuracy was 65%.
The prospectus includes prediction that this technology could easily be developed into a handheld instrument similar to a traditional otoscope, which could then be linked to the operator’s smartphone, giving the clinician an instant treat or no-treat answer.
Now, remember you have nothing to lose except maybe a friendship. How would you advise your old college roommate?
My advice to your college buddy would be one of caution! Yes, there is a potential big upside because there is a real need for a device that could provide a diagnostic accuracy that this AI model promises. While I suspect that AI will always be more accurate in diagnosis using static images, I bet that most people, clinicians and nonclinicians, could improve their accuracy by linking photos with diagnoses with an hour of practice.
However, evaluating a high-resolution photograph taken through an operative scope inserted into the cerumenless ear canal of a sedated, afrebrile child is several orders of magnitude less difficult than the real-world environment in which the diagnosis of otitis media is usually made.
If the venture capitalists were still interested in getting into the otitis media marketplace, you might suggest they look into companies that have already developed image capture otoscopes. At this point I could only find one on the Internet that was portable and it certainly isn’t small-child friendly. Once we have a tool that can capture images in real-world situations, the next step is to train AI systems to interpret them using the approach these researchers have developed. I bet it can be done. It will be only a matter of time ... and money.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Let’s pretend for a moment that you receive a call from one of your college roommates who thanks to his family connections has become a venture capitalist in California. His group is considering investing in a start-up that is developing a handheld instrument that it claims will use artificial intelligence to diagnose ear infections far more accurately than the human eye. He wonders if you would like to help him evaluate the company’s proposal and offers you a small percentage of the profits for your efforts should they choose to invest.
Your former roommate has done enough research on his own to understand that otitis media makes up a large chunk of a pediatrician’s workload and that making an accurate diagnosis can often be difficult in a struggling child. He describes his own experience watching a frustrated pediatrician attempting to remove wax from his child’s ear and eventually prescribing antibiotics “to be safe.”
You agree and review the prospectus, which includes a paper from a peer-reviewed journal. What you discover is that the investigators used more than 600 high-resolution images of tympanic membranes taken “during operative myringotomy and tympanostomy tube placement” and the findings at tympanocentesis to train a neural network.
Once trained, the model they developed could differentiate with 95% accuracy between an image of a tympanic membrane that covered a normal middle ear from one that merely contained fluid and from one that contained infected fluid. When these same images were shown to 39 clinicians, more than half of which were pediatricians and included both faculty-level staff and trainees, the average diagnostic accuracy was 65%.
The prospectus includes prediction that this technology could easily be developed into a handheld instrument similar to a traditional otoscope, which could then be linked to the operator’s smartphone, giving the clinician an instant treat or no-treat answer.
Now, remember you have nothing to lose except maybe a friendship. How would you advise your old college roommate?
My advice to your college buddy would be one of caution! Yes, there is a potential big upside because there is a real need for a device that could provide a diagnostic accuracy that this AI model promises. While I suspect that AI will always be more accurate in diagnosis using static images, I bet that most people, clinicians and nonclinicians, could improve their accuracy by linking photos with diagnoses with an hour of practice.
However, evaluating a high-resolution photograph taken through an operative scope inserted into the cerumenless ear canal of a sedated, afrebrile child is several orders of magnitude less difficult than the real-world environment in which the diagnosis of otitis media is usually made.
If the venture capitalists were still interested in getting into the otitis media marketplace, you might suggest they look into companies that have already developed image capture otoscopes. At this point I could only find one on the Internet that was portable and it certainly isn’t small-child friendly. Once we have a tool that can capture images in real-world situations, the next step is to train AI systems to interpret them using the approach these researchers have developed. I bet it can be done. It will be only a matter of time ... and money.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Pediatricians urge flu vaccine for children
Attention parents: The nation’s leading pediatric medical society is urging you to make sure your children get a flu shot this fall to prevent and control the spread of the illness.
The American Academy of Pediatrics recently called on parents and caregivers to seek flu vaccines for their children as soon as they are available in the fall. The group is encouraging parents to catch up on all other vaccines for their children, too.
“As a pediatrician and a parent, I consider the flu vaccine as critical for all family members,” Kristina A. Bryant, MD, said in a statement about the academy’s recommendations. “We should not underestimate the flu, especially when other respiratory viruses like COVID-19 are circulating within our communities. Besides making your child miserable and wreaking havoc on your family’s routine, influenza can also be serious and even deadly in children.”
Only 55% of children aged 6 months to 17 years had been vaccinated against influenza as of early April – down 2% from the previous April – and coverage levels were 8.1% lower for Black children compared with non-Hispanic White children, according to the CDC. In the 2019-2020 flu season, 188 children in the United States died of the infection, equaling the high mark for deaths set in the 2017-2018 season, the agency reported.
American Academy of Pediatrics guidelines recommend children aged 6 months and older be vaccinated with the flu vaccine every year. Depending on the child’s age and health, they may receive either a shot, which has an inactive version of the flu virus, or the nasal spray, which has a weakened form of the virus. The academy has more information about the different vaccines.
Children aged 6-8 months who are getting flu vaccines for the first time should receive two doses at least 4 weeks apart. Pregnant women can get the flu vaccine any time in their pregnancy. Influenza vaccines are safe for developing fetuses, according to the academy.
The group stressed the importance of flu vaccines for high-risk and medically vulnerable children and acknowledged the need to end barriers to immunizations for all people, regardless of income or insurance coverage. In 2020, an estimated 16.1% of children in the United States were living in poverty, up from 14.4% in 2019, according to the U.S. Census Bureau.
A version of this article first appeared on WebMD.com.
Attention parents: The nation’s leading pediatric medical society is urging you to make sure your children get a flu shot this fall to prevent and control the spread of the illness.
The American Academy of Pediatrics recently called on parents and caregivers to seek flu vaccines for their children as soon as they are available in the fall. The group is encouraging parents to catch up on all other vaccines for their children, too.
“As a pediatrician and a parent, I consider the flu vaccine as critical for all family members,” Kristina A. Bryant, MD, said in a statement about the academy’s recommendations. “We should not underestimate the flu, especially when other respiratory viruses like COVID-19 are circulating within our communities. Besides making your child miserable and wreaking havoc on your family’s routine, influenza can also be serious and even deadly in children.”
Only 55% of children aged 6 months to 17 years had been vaccinated against influenza as of early April – down 2% from the previous April – and coverage levels were 8.1% lower for Black children compared with non-Hispanic White children, according to the CDC. In the 2019-2020 flu season, 188 children in the United States died of the infection, equaling the high mark for deaths set in the 2017-2018 season, the agency reported.
American Academy of Pediatrics guidelines recommend children aged 6 months and older be vaccinated with the flu vaccine every year. Depending on the child’s age and health, they may receive either a shot, which has an inactive version of the flu virus, or the nasal spray, which has a weakened form of the virus. The academy has more information about the different vaccines.
Children aged 6-8 months who are getting flu vaccines for the first time should receive two doses at least 4 weeks apart. Pregnant women can get the flu vaccine any time in their pregnancy. Influenza vaccines are safe for developing fetuses, according to the academy.
The group stressed the importance of flu vaccines for high-risk and medically vulnerable children and acknowledged the need to end barriers to immunizations for all people, regardless of income or insurance coverage. In 2020, an estimated 16.1% of children in the United States were living in poverty, up from 14.4% in 2019, according to the U.S. Census Bureau.
A version of this article first appeared on WebMD.com.
Attention parents: The nation’s leading pediatric medical society is urging you to make sure your children get a flu shot this fall to prevent and control the spread of the illness.
The American Academy of Pediatrics recently called on parents and caregivers to seek flu vaccines for their children as soon as they are available in the fall. The group is encouraging parents to catch up on all other vaccines for their children, too.
“As a pediatrician and a parent, I consider the flu vaccine as critical for all family members,” Kristina A. Bryant, MD, said in a statement about the academy’s recommendations. “We should not underestimate the flu, especially when other respiratory viruses like COVID-19 are circulating within our communities. Besides making your child miserable and wreaking havoc on your family’s routine, influenza can also be serious and even deadly in children.”
Only 55% of children aged 6 months to 17 years had been vaccinated against influenza as of early April – down 2% from the previous April – and coverage levels were 8.1% lower for Black children compared with non-Hispanic White children, according to the CDC. In the 2019-2020 flu season, 188 children in the United States died of the infection, equaling the high mark for deaths set in the 2017-2018 season, the agency reported.
American Academy of Pediatrics guidelines recommend children aged 6 months and older be vaccinated with the flu vaccine every year. Depending on the child’s age and health, they may receive either a shot, which has an inactive version of the flu virus, or the nasal spray, which has a weakened form of the virus. The academy has more information about the different vaccines.
Children aged 6-8 months who are getting flu vaccines for the first time should receive two doses at least 4 weeks apart. Pregnant women can get the flu vaccine any time in their pregnancy. Influenza vaccines are safe for developing fetuses, according to the academy.
The group stressed the importance of flu vaccines for high-risk and medically vulnerable children and acknowledged the need to end barriers to immunizations for all people, regardless of income or insurance coverage. In 2020, an estimated 16.1% of children in the United States were living in poverty, up from 14.4% in 2019, according to the U.S. Census Bureau.
A version of this article first appeared on WebMD.com.
Punked By the Punctum: Domestically Acquired Cutaneous Myiasis
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
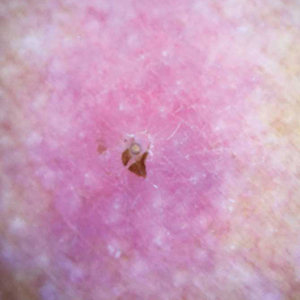
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.
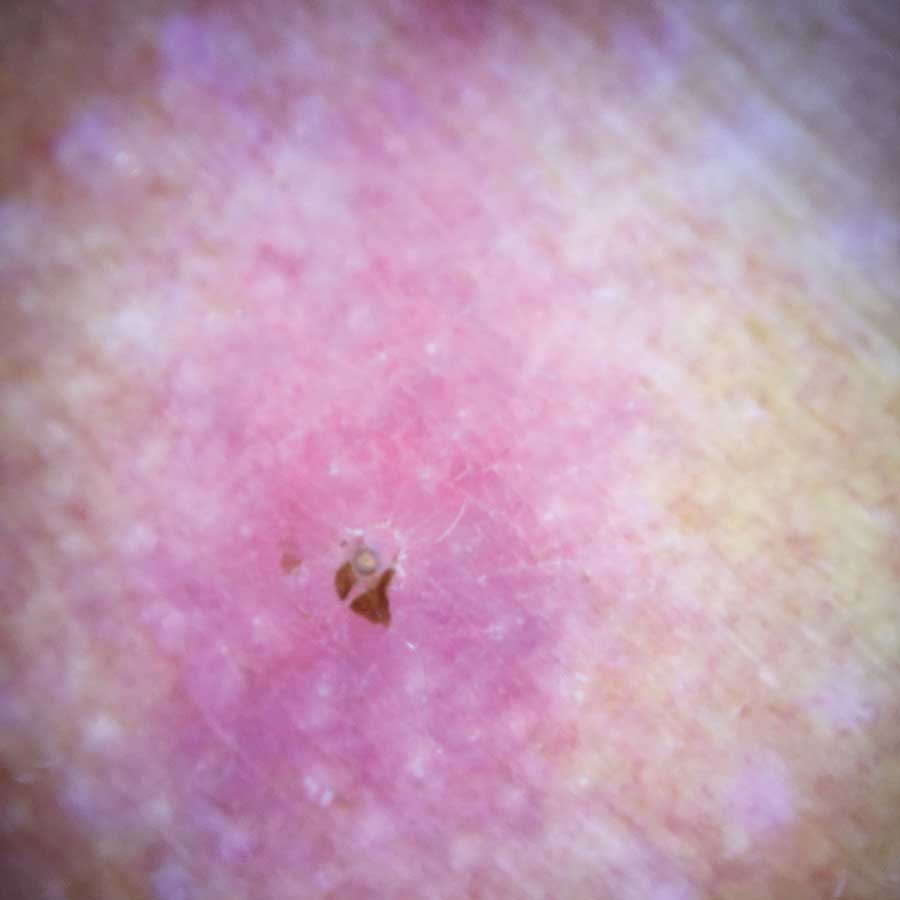
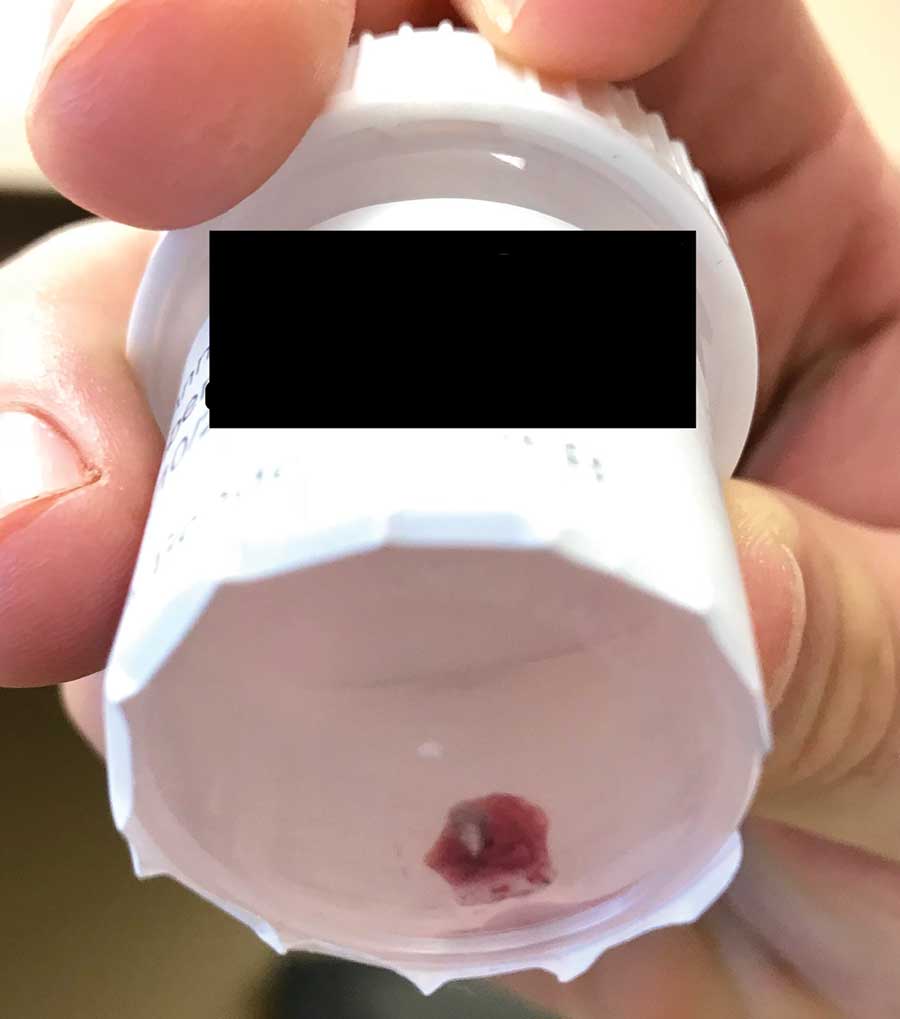
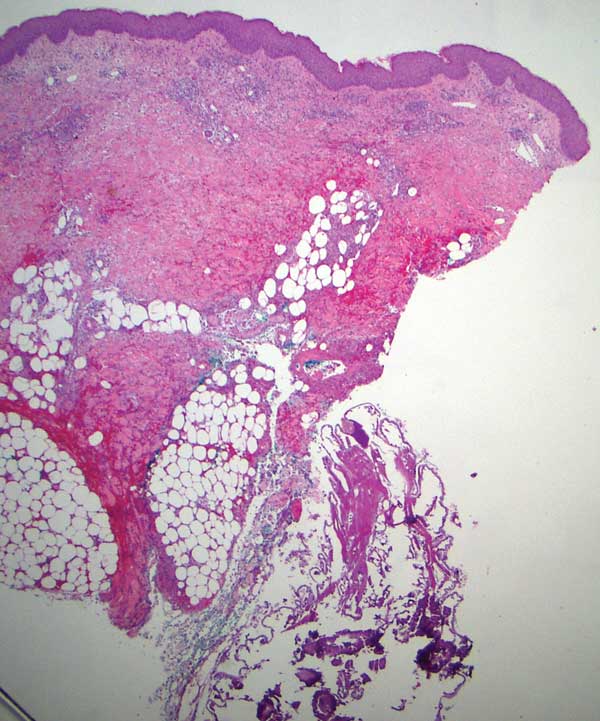
Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.
The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.
Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.

Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.

The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.

Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
To the Editor:
Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. Cutaneous myiasis, which includes furuncular, wound, and migratory types, is the most common clinical form of this condition.1 It is endemic to tropical and subtropical areas and is not common in the United States, thus it can pose a diagnostic challenge when presenting in nonendemic areas. We present the case of a woman from Michigan who acquired furuncular myiasis without travel history to a tropical or subtropical locale.
A 72-year-old woman presented to our clinic with a chief concern of a burning, pruritic, migratory skin lesion on the left arm of approximately 1 week’s duration. She had a medical history of squamous cell carcinoma, keratoacanthoma, and multiple tick bites. She reported that the lesion started on the distal aspect of the left arm as an eraser-sized, perfectly round, raised bruise with a dark pepperlike bump in the center. The lesion then spread proximally over the course of 1 week, creating 3 more identical lesions. As one lesion resolved, a new lesion appeared approximately 2 to 4 cm proximal to the preceding lesion. The patient had traveled to England, Scotland, and Ireland 2 months prior but otherwise denied leaving the state of Michigan. She reported frequent exposure to gardens, meadows, and wetlands in search of milkweed and monarch butterfly larvae that she raises in northeast Michigan. She denied any recent illness or associated systemic symptoms. Initial evaluation by a primary care physician resulted in a diagnosis of a furuncle or tick bite; she completed a 10-day course of amoxicillin and a methylprednisolone dose pack without improvement.
Physical examination revealed a 1-cm, firm, violaceous nodule with a small distinct central punctum and surrounding erythema on the proximal aspect of the left arm. Dermoscopy revealed a pulsating motion and expulsion of serosanguineous fluid from the central punctum (Figure 1). Further inspection of the patient’s left arm exposed several noninflammatory puncta distal to the primary lesion spaced at 2- to 4-cm intervals.

Gross examination of a 6-mm punch biopsy from the primary inflammatory nodule uncovered a small, motile, gray-white larval organism in the inferior portion of the specimen (Figure 2). Histopathology revealed superficial and deep eosinophil-rich inflammation, fibrosis, and hemorrhage. There was a complex wedge-shaped organism with extensive internal muscle bounded by a thin cuticle bearing rows of chitinous hooklets located at one side within the deep dermis (Figure 3). The findings were consistent with a diagnosis of cutaneous myiasis. No further treatment was required, as the organism was completely excised with the biopsy.

The most common causative agents of furuncular myiasis obtained from travelers returning from Mexico and Central and South America are Dermatobia hominis and Cordylobia anthropophaga. Cases of furuncular myiasis acquired in the United States without recent foreign travel are rare. Most of these cases are caused by larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).2 In a 2003 literature review by Safdar et al3 on 56 cases of furuncular myiasis in the United States, the median age of patients was 14 years, 87% of cases occurred in August and September, and most involved exposure in rural or suburban settings; 53% of cases presented in the northeastern United States.

Furuncular myiasis occurs when the organism’s ova are deposited on the skin of a human host by the parent organism or a mosquito vector. The heat of the skin causes the eggs to hatch and the dipteran larvae must penetrate the skin within 20 days.1 Signs of infection typically are seen 6 to 10 days after infestation.3 The larvae then feed on human tissue and burrow deep in the dermis, forming an erythematous furunculoid nodule containing one or multiple maggots. After 5 to 10 weeks, the adult larvae drop to the ground, where they mature into adult organisms in the soil.1
The most reported symptoms of furuncular myiasis include pruritus, pain, and movement sensation, typically occurring suddenly at night.4 The most common presentation is a furunclelike lesion that exudes serosanguineous or purulent fluid,1 but there have been reports of vesicular, bullous, pustular, erosive, ecchymotic, and ulcerative lesions.5Dermatobia hominis usually presents on an exposed site, such as the scalp, face, and extremities. It may present with paroxysmal episodes of lancinating pain. Over time, the lesion usually heals without a scar, though hyperpigmentation and scarring can occur. The most reported complication is secondary bacterial infection.4 Local lymphadenopathy or systemic symptoms should raise concern for infection. Staphylococcus aureus and group B Streptococcus have been cultured from lesions.6,7
The differential diagnosis for myiasis should include furuncle, insect bite, insect prurigo, pyoderma, inflamed cyst, and tungiasis. Myiasis also can present similarly to severe soft tissue infections or cellulitis. If located on the breasts, it can be mistaken for periductal mastitis, a benign mass with microcalcification, or inflammatory carcinoma. Lastly, due to pain, erythema, pruritus, small vesicles, and crusting, it may be confused for herpes simplex virus.1
Furuncular myiasis typically is diagnosed based on clinical presentation, especially in endemic regions. In nonendemic areas, the patient’s history may reveal recent travel or predisposition to myiasis. In cases where there is uncertainty, dermoscopy may be used to identify the maggot in the lesion, or ultrasonography can be used to confirm myiasis through the detection of larval movement.8 Dermoscopy will reveal a furuncular lesion with a central opening surrounded by dilated blood vessels and a yellowish structure with black barblike spines.9 Within the dermis is a fibrous cystic sinus tract containing the dipteran larva. Laboratory studies typically are unremarkable. In chronic cases, a complete blood cell count and other laboratory tests may show systemic inflammation, peripheral eosinophilia, and elevated IgE.10 Biopsies of furuncular myiasis are not necessary for diagnosis. Histopathology reveals an ulcerated epidermis with or without hyperkeratosis and an inflammatory infiltrate composed of lymphocytes and neutrophils with eosinophils, fibroblasts, histiocytes, basophils, mast cells, plasma cells, and Langerhans cells within the dermis and subcutis.11
There are various approaches to treating furuncular myiasis, with the goal of complete removal of the larva and prevention of secondary infection. One treatment option is to apply a toxic substance to the larva, effectively killing it. Another approach is to force the larva to emerge via localized hypoxia, which can be done by occluding the punctum of the lesion for at least 24 hours. A complication of this method is suffocation of the larva without migration, leading to incomplete extraction and secondary infection.1 A third method is to surgically remove the larva, which allows for debridement of necrotic tissue surrounding the lesion if present.12 Ultrasonography also can be used therapeutically to aid in the removal of the larvae. The last method is to inject lidocaine into the base of the lesion, forcing the larva out of the punctum via fluid pressure.13 Oral treatments such as ivermectin are not recommended because they can result in the death of larvae within the lesion, leading to an inflammatory response.8
Furuncular myiasis is a form of cutaneous larvae infestation not commonly seen in individuals who do not live or travel in endemic, tropical, and subtropical regions. Diagnosis is based on clinical presentation, with imaging and laboratory studies available to supplement in unclear or atypical manifestations. Treatment involves complete removal of the larva, typically through forced evacuation via hypoxia or through surgical removal. Most cases resolve without notable scarring or other sequelae; however, in those who do have complications, the most common is secondary bacterial infection. Our patient’s absence of notable travel history and frequent environmental exposure in Michigan led us to believe the organism was from a domestic source. Our case underlines the importance of a thorough history and clinical examination of furuncular lesions including the use of dermoscopy to yield an appropriate diagnosis and treatment plan.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
- Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi:10.1128/CMR.00010-11
- Schiff TA. Furuncular cutaneous myiasis caused by Cuterebra larva. J Am Acad Dermatol 1993;28:261-263.
- Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the United States: case report and literature review. Clin Infect Dis. 2003;26:73-80.
- Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominus: a case of human botfly infestation. J Emerg Med. 2012;43:618-621.
- Francesconi F, Lupi O. Myiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. Elsevier; 2006:232-239.
- Gordon PM, Hepburn NC, Williams AE, et al. Cutaneous myiasis due to Dermatobia hominis: a report of six cases. Br J Dermatol. 1995;132:811-814.
- Hubler WR Jr, Rudolph AH, Dougherty EF. Dermal myiasis. Arch Dermatol. 1974;110:109-110.
- Quintanilla-Cedillo MR, León-Ureña H, Contreras-Ruiz J, et al. The value of Doppler ultrasound in diagnosis in 25 cases of furunculoid myiasis. Int J Dermatol. 2005;44:34-37.
- Bakos RM, Bakos L. Dermoscopic diagnosis of furuncular myiasis. Arch Dermatol. 2007;143:123-124.
- Varani S, Tassinari D, Elleri D, et al. A case of furuncular myiasis associated with systemic inflammation. Parasitol Int. 2007;56:330-333.
- Grogan TM, Payne CM, Spier C, et al. Cutaneous myiasis. immunohistologic and ultrastructural morphometric features of a human botfly lesion. Am J Dermatopathol. 1987;9:232-239.
- Krajewski A, Allen B, Hoss D, et al. Cutaneous myiasis. J Plast Reconstr Aesthet Surg. 2009;62:383-386.
- Lebwohl MG, Heymann WR, Berth-Jones J, et al. Myiasis: Treatment of Skin Diseases. Comprehensive Therapeutic Strategies. 2nd ed. Elsevier-Mosby; 2006.
Practice Points
- Cutaneous myiasis is a skin infestation with dipterous larvae that feed on the host’s tissue and cause a wide range of manifestations depending on the location of infestation. It consists of 3 types: furuncular, wound, and migratory forms.
- It is uncommon in the United States and not typically seen in patients who have no history of recent travel to tropical or subtropical areas.
- The most common cause of African furuncular myiasis acquired in the United States is larvae of the Cuterebra species (also known as the rabbit botfly or rodent botfly).
Children and COVID: Weekly cases close out August with a second straight increase
New cases rose by 4.6% for the week of Aug. 26 to Sept. 1, following a week in which cases increased by almost 9%, as the second half of August basically reversed the two consecutive weeks of decreases during the first half of the month, based on the AAP/CHA data collected from state and territorial health departments.
Similar trends can be seen for emergency department visits, with the exception of children aged 0-11 years, whose ED visit rates have continued to fall since late July. Children aged 12-15, however, had a 7-day average of 4.4% of ED visits with diagnosed COVID on Aug. 25, compared with 3.1% for Aug. 12. Children aged 16-17 years were at 3.4% on Aug. 27, compared with 3.1% as late as Aug. 15, the Centers for Disease Control and Prevention reported.
Hospital admissions with confirmed COVID-19, reported only for children aged 0-17 years, also reflect the late-August trend of increased cases. New hospitalizations dropped from 0.46 per 100,000 population on July 30 to 0.40 per 100,000 on Aug. 19 but have since risen to 0.44 per 100,000 as of Aug. 27, the CDC said on its COVID Data Tracker.
Initial vaccinations, meanwhile, have declined since early August for all children, according to a separate report from the AAP. A look at CDC data for two specific days – the first and last Mondays of the month – shows that those aged under 5 received 12,982 doses on Aug. 1, compared with 5,824 doses on Aug. 29. Over that same time, initial vaccinations in 5- to 11-year-olds went from 9,058 to 2,879, while among those aged 12-17 they dropped from 4,245 to 1,226.
Cumulatively, 5.5% of all children under age 5 had received at least one dose and 1.3% were fully vaccinated by Aug. 30, compared with 38.1% and 30.7%, respectively, of those aged 5-11 and 70.7% and 60.5% of 12- to 17-year-olds, the CDC said.
New cases rose by 4.6% for the week of Aug. 26 to Sept. 1, following a week in which cases increased by almost 9%, as the second half of August basically reversed the two consecutive weeks of decreases during the first half of the month, based on the AAP/CHA data collected from state and territorial health departments.
Similar trends can be seen for emergency department visits, with the exception of children aged 0-11 years, whose ED visit rates have continued to fall since late July. Children aged 12-15, however, had a 7-day average of 4.4% of ED visits with diagnosed COVID on Aug. 25, compared with 3.1% for Aug. 12. Children aged 16-17 years were at 3.4% on Aug. 27, compared with 3.1% as late as Aug. 15, the Centers for Disease Control and Prevention reported.
Hospital admissions with confirmed COVID-19, reported only for children aged 0-17 years, also reflect the late-August trend of increased cases. New hospitalizations dropped from 0.46 per 100,000 population on July 30 to 0.40 per 100,000 on Aug. 19 but have since risen to 0.44 per 100,000 as of Aug. 27, the CDC said on its COVID Data Tracker.
Initial vaccinations, meanwhile, have declined since early August for all children, according to a separate report from the AAP. A look at CDC data for two specific days – the first and last Mondays of the month – shows that those aged under 5 received 12,982 doses on Aug. 1, compared with 5,824 doses on Aug. 29. Over that same time, initial vaccinations in 5- to 11-year-olds went from 9,058 to 2,879, while among those aged 12-17 they dropped from 4,245 to 1,226.
Cumulatively, 5.5% of all children under age 5 had received at least one dose and 1.3% were fully vaccinated by Aug. 30, compared with 38.1% and 30.7%, respectively, of those aged 5-11 and 70.7% and 60.5% of 12- to 17-year-olds, the CDC said.
New cases rose by 4.6% for the week of Aug. 26 to Sept. 1, following a week in which cases increased by almost 9%, as the second half of August basically reversed the two consecutive weeks of decreases during the first half of the month, based on the AAP/CHA data collected from state and territorial health departments.
Similar trends can be seen for emergency department visits, with the exception of children aged 0-11 years, whose ED visit rates have continued to fall since late July. Children aged 12-15, however, had a 7-day average of 4.4% of ED visits with diagnosed COVID on Aug. 25, compared with 3.1% for Aug. 12. Children aged 16-17 years were at 3.4% on Aug. 27, compared with 3.1% as late as Aug. 15, the Centers for Disease Control and Prevention reported.
Hospital admissions with confirmed COVID-19, reported only for children aged 0-17 years, also reflect the late-August trend of increased cases. New hospitalizations dropped from 0.46 per 100,000 population on July 30 to 0.40 per 100,000 on Aug. 19 but have since risen to 0.44 per 100,000 as of Aug. 27, the CDC said on its COVID Data Tracker.
Initial vaccinations, meanwhile, have declined since early August for all children, according to a separate report from the AAP. A look at CDC data for two specific days – the first and last Mondays of the month – shows that those aged under 5 received 12,982 doses on Aug. 1, compared with 5,824 doses on Aug. 29. Over that same time, initial vaccinations in 5- to 11-year-olds went from 9,058 to 2,879, while among those aged 12-17 they dropped from 4,245 to 1,226.
Cumulatively, 5.5% of all children under age 5 had received at least one dose and 1.3% were fully vaccinated by Aug. 30, compared with 38.1% and 30.7%, respectively, of those aged 5-11 and 70.7% and 60.5% of 12- to 17-year-olds, the CDC said.
COVID-19 vaccination recap: The latest developments
In recent weeks, the COVID-19 vaccine arsenal has grown more robust. Here’s what you need to know:
Variant-specific boosters. On September 1, the Advisory Committee on Immunization Practices (ACIP) adopted a recommendation for a booster of either a new bivalent Pfizer-BioNTech COVID-19 vaccine (for individuals ages 12 years and older) or bivalent Moderna COVID-19 vaccine (for individuals ages 18 years and older) at least 2 months after receipt of a primary series or prior monovalent booster dose. Both bivalent vaccines were recently approved by the Food and Drug Administration (FDA) under an Emergency Use Authorization (EUA) and offer protection against one of the more common circulating strains of SARS-COV-2 (BA.1) while boosting immunity to the original strain. Both options are approved only as booster shots, not as an original COVID vaccine series.1
Novavax vaccine. This summer, the FDA issued an EUA for the Novavax COVID-19 vaccine in adults and a later EUA for adolescents (ages 12 to 17 years).2 Novavax is the fourth vaccine available to combat COVID-19 infection. This newest addition to the COVID armamentarium consists of coronavirus protein subunits, produced using recombinant technology, and a matrix adjuvant. The primary series consists of 2 doses administered at least 3 weeks apart.3,4
A few caveats: The Novavax vaccine comes in 10-dose vials, which should be kept refrigerated until use. Once the first dose is used, the vial should be discarded after 6 hours. This may present some scheduling and logistical issues. Also, the Novavax vaccine is not currently approved for use in children younger than 12 years, or as a booster to other vaccines.3,4
The effectiveness and safety of the Novavax vaccine appears to be comparable to that of the other vaccines approved to date, although measuring vaccine effectiveness is a tricky business given the rapid mutation of the virus and changing dominant strains.3,4 The Novavax vaccine’s efficacy against currently circulating Omicron variants of the virus (eg, BA.2.12.1, BA.4, BA.5) remains to be determined.
As far as safety, preliminary studies indicate that Novavax may be associated with rare cases of myocarditis.3,4 Myocarditis can result from the COVID infection itself at an overall rate of 1 to 2 per 1000, which is 16 times the rate in adults without COVID.5
Could it provide reassurance to the hesitant? The Novavax COVID vaccine was developed using a vaccine platform and production process similar to that of other commonly administered vaccines, such as hepatitis B vaccine and human papillomavirus vaccine. This may make it an appealing option for patients who have shown hesitancy toward new vaccine technologies.
And, of course, there are the Pfizer and Moderna vaccines. Currently, there are 2 vaccines approved under the normal licensing process for adults, both of which are mRNA-based vaccines: Pfizer/BioNTech (Comirnaty) for those ages 12 years and older and Moderna (Spikevax) for those ages 18 and older. A third COVID vaccine option is manufactured by Johnson & Johnson (Janssen) and uses an adenovirus platform. The FDA revised its EUA in May to limit its use.6 The Johnson & Johnson vaccine has been associated with rare but serious reactions called thrombosis with thrombocytopenia. ACIP recommends all other vaccines in preference to the Johnson & Johnson vaccine.
For more on COVID vaccination for patients of all ages, see: www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
1. Oliver S. Evidence to recommendations framework: Bivalent COVID-19 vaccine booster doses. Presented to the Advisory Committee on Immunization Practices, September 1, 2002. Accessed September 6, 2002. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/08-COVID-Oliver-508.pdf
2. FDA. Novavax COVID-19 vaccine, adjuvanted. Updated August 19, 2022. Accessed August 23, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/novavax-covid-19-vaccine-adjuvanted
3. Dubovsky F. NVX-CoV2373 (Novavax COVID-19 vaccine) in adults (≥ 18 years of age). Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/04-covid-dubovsky-508.pdf
4. Twentyman E. Evidence to recommendation framework: Novavax COVID-19 vaccine, adjuvanted in adults ages 18 years and older. Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/05-covid-twentyman-508.pdf
5. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morbid Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
6. American Hospital Association. FDA limits J&J COVID-19 vaccine use to certain adults. Published May 6, 2022. Accessed September 6, 2022. www.aha.org/news/headline/2022-05-06-fda-limits-jj-covid-19-vaccine-use-certain-adults
In recent weeks, the COVID-19 vaccine arsenal has grown more robust. Here’s what you need to know:
Variant-specific boosters. On September 1, the Advisory Committee on Immunization Practices (ACIP) adopted a recommendation for a booster of either a new bivalent Pfizer-BioNTech COVID-19 vaccine (for individuals ages 12 years and older) or bivalent Moderna COVID-19 vaccine (for individuals ages 18 years and older) at least 2 months after receipt of a primary series or prior monovalent booster dose. Both bivalent vaccines were recently approved by the Food and Drug Administration (FDA) under an Emergency Use Authorization (EUA) and offer protection against one of the more common circulating strains of SARS-COV-2 (BA.1) while boosting immunity to the original strain. Both options are approved only as booster shots, not as an original COVID vaccine series.1
Novavax vaccine. This summer, the FDA issued an EUA for the Novavax COVID-19 vaccine in adults and a later EUA for adolescents (ages 12 to 17 years).2 Novavax is the fourth vaccine available to combat COVID-19 infection. This newest addition to the COVID armamentarium consists of coronavirus protein subunits, produced using recombinant technology, and a matrix adjuvant. The primary series consists of 2 doses administered at least 3 weeks apart.3,4
A few caveats: The Novavax vaccine comes in 10-dose vials, which should be kept refrigerated until use. Once the first dose is used, the vial should be discarded after 6 hours. This may present some scheduling and logistical issues. Also, the Novavax vaccine is not currently approved for use in children younger than 12 years, or as a booster to other vaccines.3,4
The effectiveness and safety of the Novavax vaccine appears to be comparable to that of the other vaccines approved to date, although measuring vaccine effectiveness is a tricky business given the rapid mutation of the virus and changing dominant strains.3,4 The Novavax vaccine’s efficacy against currently circulating Omicron variants of the virus (eg, BA.2.12.1, BA.4, BA.5) remains to be determined.
As far as safety, preliminary studies indicate that Novavax may be associated with rare cases of myocarditis.3,4 Myocarditis can result from the COVID infection itself at an overall rate of 1 to 2 per 1000, which is 16 times the rate in adults without COVID.5
Could it provide reassurance to the hesitant? The Novavax COVID vaccine was developed using a vaccine platform and production process similar to that of other commonly administered vaccines, such as hepatitis B vaccine and human papillomavirus vaccine. This may make it an appealing option for patients who have shown hesitancy toward new vaccine technologies.
And, of course, there are the Pfizer and Moderna vaccines. Currently, there are 2 vaccines approved under the normal licensing process for adults, both of which are mRNA-based vaccines: Pfizer/BioNTech (Comirnaty) for those ages 12 years and older and Moderna (Spikevax) for those ages 18 and older. A third COVID vaccine option is manufactured by Johnson & Johnson (Janssen) and uses an adenovirus platform. The FDA revised its EUA in May to limit its use.6 The Johnson & Johnson vaccine has been associated with rare but serious reactions called thrombosis with thrombocytopenia. ACIP recommends all other vaccines in preference to the Johnson & Johnson vaccine.
For more on COVID vaccination for patients of all ages, see: www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
In recent weeks, the COVID-19 vaccine arsenal has grown more robust. Here’s what you need to know:
Variant-specific boosters. On September 1, the Advisory Committee on Immunization Practices (ACIP) adopted a recommendation for a booster of either a new bivalent Pfizer-BioNTech COVID-19 vaccine (for individuals ages 12 years and older) or bivalent Moderna COVID-19 vaccine (for individuals ages 18 years and older) at least 2 months after receipt of a primary series or prior monovalent booster dose. Both bivalent vaccines were recently approved by the Food and Drug Administration (FDA) under an Emergency Use Authorization (EUA) and offer protection against one of the more common circulating strains of SARS-COV-2 (BA.1) while boosting immunity to the original strain. Both options are approved only as booster shots, not as an original COVID vaccine series.1
Novavax vaccine. This summer, the FDA issued an EUA for the Novavax COVID-19 vaccine in adults and a later EUA for adolescents (ages 12 to 17 years).2 Novavax is the fourth vaccine available to combat COVID-19 infection. This newest addition to the COVID armamentarium consists of coronavirus protein subunits, produced using recombinant technology, and a matrix adjuvant. The primary series consists of 2 doses administered at least 3 weeks apart.3,4
A few caveats: The Novavax vaccine comes in 10-dose vials, which should be kept refrigerated until use. Once the first dose is used, the vial should be discarded after 6 hours. This may present some scheduling and logistical issues. Also, the Novavax vaccine is not currently approved for use in children younger than 12 years, or as a booster to other vaccines.3,4
The effectiveness and safety of the Novavax vaccine appears to be comparable to that of the other vaccines approved to date, although measuring vaccine effectiveness is a tricky business given the rapid mutation of the virus and changing dominant strains.3,4 The Novavax vaccine’s efficacy against currently circulating Omicron variants of the virus (eg, BA.2.12.1, BA.4, BA.5) remains to be determined.
As far as safety, preliminary studies indicate that Novavax may be associated with rare cases of myocarditis.3,4 Myocarditis can result from the COVID infection itself at an overall rate of 1 to 2 per 1000, which is 16 times the rate in adults without COVID.5
Could it provide reassurance to the hesitant? The Novavax COVID vaccine was developed using a vaccine platform and production process similar to that of other commonly administered vaccines, such as hepatitis B vaccine and human papillomavirus vaccine. This may make it an appealing option for patients who have shown hesitancy toward new vaccine technologies.
And, of course, there are the Pfizer and Moderna vaccines. Currently, there are 2 vaccines approved under the normal licensing process for adults, both of which are mRNA-based vaccines: Pfizer/BioNTech (Comirnaty) for those ages 12 years and older and Moderna (Spikevax) for those ages 18 and older. A third COVID vaccine option is manufactured by Johnson & Johnson (Janssen) and uses an adenovirus platform. The FDA revised its EUA in May to limit its use.6 The Johnson & Johnson vaccine has been associated with rare but serious reactions called thrombosis with thrombocytopenia. ACIP recommends all other vaccines in preference to the Johnson & Johnson vaccine.
For more on COVID vaccination for patients of all ages, see: www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
1. Oliver S. Evidence to recommendations framework: Bivalent COVID-19 vaccine booster doses. Presented to the Advisory Committee on Immunization Practices, September 1, 2002. Accessed September 6, 2002. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/08-COVID-Oliver-508.pdf
2. FDA. Novavax COVID-19 vaccine, adjuvanted. Updated August 19, 2022. Accessed August 23, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/novavax-covid-19-vaccine-adjuvanted
3. Dubovsky F. NVX-CoV2373 (Novavax COVID-19 vaccine) in adults (≥ 18 years of age). Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/04-covid-dubovsky-508.pdf
4. Twentyman E. Evidence to recommendation framework: Novavax COVID-19 vaccine, adjuvanted in adults ages 18 years and older. Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/05-covid-twentyman-508.pdf
5. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morbid Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
6. American Hospital Association. FDA limits J&J COVID-19 vaccine use to certain adults. Published May 6, 2022. Accessed September 6, 2022. www.aha.org/news/headline/2022-05-06-fda-limits-jj-covid-19-vaccine-use-certain-adults
1. Oliver S. Evidence to recommendations framework: Bivalent COVID-19 vaccine booster doses. Presented to the Advisory Committee on Immunization Practices, September 1, 2002. Accessed September 6, 2002. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/08-COVID-Oliver-508.pdf
2. FDA. Novavax COVID-19 vaccine, adjuvanted. Updated August 19, 2022. Accessed August 23, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/novavax-covid-19-vaccine-adjuvanted
3. Dubovsky F. NVX-CoV2373 (Novavax COVID-19 vaccine) in adults (≥ 18 years of age). Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/04-covid-dubovsky-508.pdf
4. Twentyman E. Evidence to recommendation framework: Novavax COVID-19 vaccine, adjuvanted in adults ages 18 years and older. Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/05-covid-twentyman-508.pdf
5. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morbid Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
6. American Hospital Association. FDA limits J&J COVID-19 vaccine use to certain adults. Published May 6, 2022. Accessed September 6, 2022. www.aha.org/news/headline/2022-05-06-fda-limits-jj-covid-19-vaccine-use-certain-adults
Risk Factors Predicting Cellulitis Diagnosis in a Prospective Cohort Undergoing Dermatology Consultation in the Emergency Department
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).
Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).
Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).

Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).

Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
Cellulitis is an infection of the skin and skin-associated structures characterized by redness, warmth, swelling, and pain of the affected area. Cellulitis most commonly occurs in middle-aged and older adults and frequently affects the lower extremities.1 Serious complications of cellulitis such as bacteremia, metastatic infection, and sepsis are rare, and most cases of cellulitis in patients with normal vital signs and mental status can be managed with outpatient treatment.2
Diagnosis of cellulitis can be confounded by a number of similarly presenting conditions collectively known as pseudocellulitis, such as venous stasis dermatitis and deep vein thrombosis.1 Misdiagnosis of cellulitis is common, with rates exceeding 30% among hospitalized patients initially diagnosed with cellulitis.3,4 Dermatology or infectious disease assessment is considered the diagnostic gold standard for cellulitis4,5 but is not always readily available, especially in resource-constrained settings.
Most cases of uncomplicated cellulitis can be managed with outpatient treatment, especially because serious complications are rare. Frequent misdiagnosis leads to repeat or unnecessary hospitalization and antibiosis. Exceptions necessitating hospitalization usually are predicated on signs of systemic infection, severe immunocompromised states, or failure of prior outpatient therapy.6 Such presentations can be distinguished by corresponding notable historical or examination factors, such as vital sign abnormalities suggesting systemic infection or history of malignancy leading to an immunocompromised state.
We sought to evaluate factors leading to the diagnosis of cellulitis in a cohort of patients with uncomplicated presentations receiving dermatology consultation to emphasize findings indicative of cellulitis in the absence of clinical or historical factors suggestive of other conditions necessitating hospitalization, such as systemic infection.
Methods
Study Participants—A prospective cohort study of patients presenting to an emergency department (ED) between October 2012 and January 2017 at an urban academic medical center in Boston, Massachusetts, was conducted with approval of study design and procedures by the relevant institutional review board. Patients older than 18 years were eligible for inclusion if given an initial diagnosis of cellulitis by an ED physician. Patients were excluded if incarcerated, pregnant, or unable to provide informed consent. Other exclusion criteria includedinfections overlying temporary or permanent indwelling hardware, animal or human bites, or sites of recent surgery (within the prior 4 weeks); preceding antibiotic treatment for more than 24 hours; or clinical or radiographic evidence of complications requiring alternative management such as osteomyelitis or abscess. Patients presenting with an elevated heart rate (>100 beats per minute) or body temperature (>100.5 °F [38.1 °C]) also were excluded. Eligible patients were enrolled upon providing written informed consent, and no remuneration was offered for participation.
Dermatology Consultation Intervention—A random subset of enrolled patients received dermatology consultation within 24 hours of presentation. Consultation consisted of a patient interview and physical examination with care recommendations to relevant ED and inpatient teams. Consultations confirmed the presence or absence of cellulitis as the primary outcome and also noted the presence of any pseudocellulitis diagnoses either occurring concomitantly with or mimicking cellulitis as a secondary outcome.
Statistical Analysis—Patient characteristics were analyzed to identify factors independently associated with the diagnosis of cellulitis in cases affecting the lower extremities. Factors were recorded with categorical variables reported as counts and percentages and continuous variables as means and standard deviations. Univariate analyses between categorical variables or discretized continuous variables and cellulitis diagnosis were conducted via Fisher exact test to identify a preliminary set of potential risk factors. Continuous variables were discretized at multiple incremental values with the discretization most significantly associated with cellulitis diagnosis selected as a preliminary risk factor. Multivariate analyses involved using any objective preliminary factor meeting a significance threshold of P<.1 in univariate comparisons in a multivariate logistic regression model for prediction of cellulitis diagnosis with corresponding calculation of odds ratios with confidence intervals and receiver operating characteristic. Factors with confidence intervals that excluded 1 were considered significant independent predictors of cellulitis. Analyses were performed using Python version 3.8 (Python Software Foundation).
Results
Of 1359 patients screened for eligibility, 104 patients with presumed lower extremity cellulitis undergoing dermatology consultation were included in this study (Figure). The mean patient age (SD) was 60.4 (19.2) years, and 63.5% of patients were male. In the study population, 63 (60.6%) patients received a final diagnosis of cellulitis. The most common pseudocellulitis diagnosis identified was venous stasis dermatitis, which occurred in 12 (11.5%) patients with concomitant cellulitis and in 12 (11.5%) patients mimicking cellulitis (Table).

Univariate comparisons revealed a diverse set of historical, examination, and laboratory factors associated with cellulitis diagnosis. Diagnosis of cellulitis was associated with unilateral presentation, recent trauma to the affected site, and history of cellulitis or onychomycosis. Diagnosis of cellulitis also was associated with elevated white blood cell count, absolute neutrophil count, C-reactive protein, body mass index, hematocrit, and platelet count; age less than 75 years; and lower serum sodium and serum chloride levels. These were the independent factors included in the multivariate analysis, which consisted of a logistic regression model for prediction of cellulitis (eTable).

Multivariate logistic regression on all preliminary factors significantly associated with cellulitis diagnosis in univariate comparisons demonstrated leukocytosis, which was defined as having a white blood cell count exceeding 11,000/μL, unilateral presentation, history of onychomycosis, and trauma to the affected site as significant independent predictors of cellulitis diagnosis; history of cellulitis approached significance (eTable). Unilateral presentation and leukocytosis were the strongest predictors; having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%.

Comment
Importance of Identifying Pseudocellulitis—Successful diagnosis of cellulitis can be confounded by pseudocellulitis that can present concomitantly with or in lieu of cellulitis itself. Although cellulitis mostly affects the lower extremities in adults, pseudocellulitis also was common in this study population of patients with suspected lower extremity cellulitis, occurring both as a mimicker and concomitantly with cellulitis with substantial frequency. Notably, among patients with both venous stasis dermatitis and cellulitis diagnosed, most patients (n=10/12; 83.3%) had unilateral presentations of cellulitis as evidenced by signs and symptoms more notably affecting one lower extremity than the other. These findings suggest that certain pseudocellulitis diagnoses may predispose patients to cellulitis by disrupting the skin barrier, leading to bacterial infiltration; however, these pseudocellulitis diagnoses typically affect both lower extremities equally,1 and asymmetric involvement suggests the presence of overlying cellulitis. Furthermore, the most common pseudocellulitis entities found, such as venous stasis dermatitis, hematoma, and eczema, do not benefit from antibiotic treatment and require alternative therapy.1 Successful discrimination of these pseudocellulitis entities is critical to bolster proper antibiotic stewardship and discourage unnecessary hospitalization.
Independent Predictors of Cellulitis—Unilateral presentation and leukocytosis each emerged as strong independent predictors of cellulitis diagnosis in this study. Having either of these factors furthermore demonstrated high sensitivity and negative predictive value for cellulitis diagnosis. Other notable risk factors were history of onychomycosis, cellulitis, and trauma to the affected site. Prior studies have identified similar historical factors as predisposing patients to cellulitis.7-9 Interestingly, warmth of the affected area on physical examination emerged as strongly associated with cellulitis but was not included in the final predictive model because of its subjective determination. These factors may be especially important in diagnosing cellulitis in patients without concerning vital signs and with concomitant or prior pseudocellulitis.
Study Limitations—This study was limited to patients with uncomplicated presentations to emphasize discrimination of factors associated with cellulitis in the absence of suggestive signs of infection, such as vital sign abnormalities. Signs such as fever and tachypnea have been previously correlated to outpatient treatment failure and necessity for hospitalization.10-12 This study instead focused on patients without concerning vital signs to reduce confounding by such factors in more severe presentations that heighten suspicion for infection and increase likelihood of additional treatment measures. For such patients, suggestive historical factors, such as those discovered in this study, should be considered instead. Interestingly, increased age did not emerge as a significant predictor in this population in contrast to other predictive models that included patients with vital sign abnormalities. Notably, older patients tend to have more variable vital signs, especially in response to physiologic stressors such as infection.13 As such, age may serve as a proxy for vital sign abnormalities to some degree in such predictive models, leading to heightened suspicion for infection in older patients. This study demonstrated that in the absence of concerning vital signs, historical rather than demographic factors are more predictive of cellulitis.
Conclusion
Unilateral presentation and leukocytosis emerged as strong independent predictors of lower extremity cellulitis in patients with uncomplicated presentations. Having either of these factors had a sensitivity of 93.7% and a negative predictive value of 76.5%. Other factors such as history of cellulitis, onychomycosis, and recent trauma to the affected site emerged as additional predictors. These historical, examination, and laboratory characteristics may be especially useful for successful diagnosis of cellulitis in varied practice settings, including outpatient clinics and EDs.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? a systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med. 2018;33:1553-1560.
- Ko LN, Garza-Mayers AC, St. John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J. 2011;17:1.
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
- Björnsdóttir S, Gottfredsson M, Thórisdóttir AS, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41:1416-1422.
- Roujeau JC, Sigurgeirsson B, Korting HC, et al. Chronic dermatomycoses of the foot as risk factors for acute bacterial cellulitis of the leg: a case-control study. Dermatology. 2004;209:301-307.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med. 2007;167:709-715.
- Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for nonpurulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2019;26:51-59.
- Peterson D, McLeod S, Woolfrey K, et al. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21:526-531.
- Volz KA, Canham L, Kaplan E, et al. Identifying patients with cellulitis who are likely to require inpatient admission after a stay in an ED observation unit. Am J Emerg Med. 2013;31:360-364.
- Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12:337-343.
Practice Points
- Unilateral involvement and leukocytosis are both highly predictive of lower extremity cellulitis in uncomplicated presentations.
- Historical factors such as history of onychomycosis and trauma to the affected site are more predictive of lower extremity cellulitis than demographic factors such as age in uncomplicated presentations of cellulitis.
Acute myocarditis a possible complication of monkeypox
Clinicians in Portugal say a 31-year-old man with confirmed monkeypox developed acute myocarditis roughly 1 week after the eruption of the characteristic skin lesions of the disease.
Ana Isabel Pinho, MD, department of cardiology, São João University Hospital Centre, Porto, Portugal, said in a news release.
“We believe that reporting this potential causal relationship can raise more awareness of the scientific community and health professionals for acute myocarditis as a possible complication associated with monkeypox and might be helpful for close monitoring of affected patients for further recognition of other complications in the future,” Dr. Pinho adds.
Dr. Pinho and colleagues describe the case in a report published in JACC: Case Reports.
Case details
The patient presented with a 5-day history of malaise, myalgias, and fever followed by the eruption of multiple swollen skin lesions on his face, hands, and genitalia.
Monkeypox was confirmed by positive polymerase chain reaction assay of a swab sample from a skin lesion.
Three days later, the patient developed chest tightness that radiated through the left arm and which awoke him during the night. He was admitted to an intensive care unit with clinical suspicion of acute myocarditis.
The patient’s initial electrocardiogram showed sinus rhythm with nonspecific ventricular repolarization abnormalities.
On chest x-ray, the cardiothoracic index was normal, with no interstitial infiltrates, pleural effusion, or masses. On transthoracic echocardigraphy, biventricular systolic function was preserved, and there was no pericardial effusion.
Routine laboratory tests revealed elevated levels of C-reactive protein, creatine phosphokinase, high-sensitivity troponin I, and brain natriuretic peptide, suggesting stress injury to the heart.
Findings on cardiac magnetic resonance were consistent with myocardial inflammation and acute myocarditis.
The patient was treated with supportive care, and he made a full clinical recovery. He was discharged after 1 week. On discharge, cardiac enzymes were within the normal range. The patient showed sustained electric and hemodynamic stability, and the skin lesions had healed.
“Through this important case study, we are developing a deeper understanding of monkeypox, viral myocarditis, and how to accurately diagnose and manage this disease,” Julia Grapsa, MD, PhD, editor-in-chief of JACC: Case Reports, commented in the news release.
“I commend the authors on this valuable clinical case during a critical time as monkeypox continues to spread globally,” Dr. Grapsa added.
The researchers say further research is needed to identify the pathologic mechanism underlying monkeypox-associated cardiac injury.
By the numbers
According to the latest data, California has reported 3,629 cases, followed closely by New York with 3,367 cases, Florida with 1,957 cases, Texas with 1,698, Georgia with 1,418, and Illinois with 1,081. The other states have reported fewer than 600 cases.
The CDC says that globally, more than 52,000 monkeypox cases have been reported.
Monkeypox case counts appear to be slowing in the United States and globally.
Last week, the World Health Organization said the number of new cases worldwide declined by 21% between Aug. 15 and 21 after increasing for 4 straight weeks.
The research had no funding. Dr. Pinho and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians in Portugal say a 31-year-old man with confirmed monkeypox developed acute myocarditis roughly 1 week after the eruption of the characteristic skin lesions of the disease.
Ana Isabel Pinho, MD, department of cardiology, São João University Hospital Centre, Porto, Portugal, said in a news release.
“We believe that reporting this potential causal relationship can raise more awareness of the scientific community and health professionals for acute myocarditis as a possible complication associated with monkeypox and might be helpful for close monitoring of affected patients for further recognition of other complications in the future,” Dr. Pinho adds.
Dr. Pinho and colleagues describe the case in a report published in JACC: Case Reports.
Case details
The patient presented with a 5-day history of malaise, myalgias, and fever followed by the eruption of multiple swollen skin lesions on his face, hands, and genitalia.
Monkeypox was confirmed by positive polymerase chain reaction assay of a swab sample from a skin lesion.
Three days later, the patient developed chest tightness that radiated through the left arm and which awoke him during the night. He was admitted to an intensive care unit with clinical suspicion of acute myocarditis.
The patient’s initial electrocardiogram showed sinus rhythm with nonspecific ventricular repolarization abnormalities.
On chest x-ray, the cardiothoracic index was normal, with no interstitial infiltrates, pleural effusion, or masses. On transthoracic echocardigraphy, biventricular systolic function was preserved, and there was no pericardial effusion.
Routine laboratory tests revealed elevated levels of C-reactive protein, creatine phosphokinase, high-sensitivity troponin I, and brain natriuretic peptide, suggesting stress injury to the heart.
Findings on cardiac magnetic resonance were consistent with myocardial inflammation and acute myocarditis.
The patient was treated with supportive care, and he made a full clinical recovery. He was discharged after 1 week. On discharge, cardiac enzymes were within the normal range. The patient showed sustained electric and hemodynamic stability, and the skin lesions had healed.
“Through this important case study, we are developing a deeper understanding of monkeypox, viral myocarditis, and how to accurately diagnose and manage this disease,” Julia Grapsa, MD, PhD, editor-in-chief of JACC: Case Reports, commented in the news release.
“I commend the authors on this valuable clinical case during a critical time as monkeypox continues to spread globally,” Dr. Grapsa added.
The researchers say further research is needed to identify the pathologic mechanism underlying monkeypox-associated cardiac injury.
By the numbers
According to the latest data, California has reported 3,629 cases, followed closely by New York with 3,367 cases, Florida with 1,957 cases, Texas with 1,698, Georgia with 1,418, and Illinois with 1,081. The other states have reported fewer than 600 cases.
The CDC says that globally, more than 52,000 monkeypox cases have been reported.
Monkeypox case counts appear to be slowing in the United States and globally.
Last week, the World Health Organization said the number of new cases worldwide declined by 21% between Aug. 15 and 21 after increasing for 4 straight weeks.
The research had no funding. Dr. Pinho and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians in Portugal say a 31-year-old man with confirmed monkeypox developed acute myocarditis roughly 1 week after the eruption of the characteristic skin lesions of the disease.
Ana Isabel Pinho, MD, department of cardiology, São João University Hospital Centre, Porto, Portugal, said in a news release.
“We believe that reporting this potential causal relationship can raise more awareness of the scientific community and health professionals for acute myocarditis as a possible complication associated with monkeypox and might be helpful for close monitoring of affected patients for further recognition of other complications in the future,” Dr. Pinho adds.
Dr. Pinho and colleagues describe the case in a report published in JACC: Case Reports.
Case details
The patient presented with a 5-day history of malaise, myalgias, and fever followed by the eruption of multiple swollen skin lesions on his face, hands, and genitalia.
Monkeypox was confirmed by positive polymerase chain reaction assay of a swab sample from a skin lesion.
Three days later, the patient developed chest tightness that radiated through the left arm and which awoke him during the night. He was admitted to an intensive care unit with clinical suspicion of acute myocarditis.
The patient’s initial electrocardiogram showed sinus rhythm with nonspecific ventricular repolarization abnormalities.
On chest x-ray, the cardiothoracic index was normal, with no interstitial infiltrates, pleural effusion, or masses. On transthoracic echocardigraphy, biventricular systolic function was preserved, and there was no pericardial effusion.
Routine laboratory tests revealed elevated levels of C-reactive protein, creatine phosphokinase, high-sensitivity troponin I, and brain natriuretic peptide, suggesting stress injury to the heart.
Findings on cardiac magnetic resonance were consistent with myocardial inflammation and acute myocarditis.
The patient was treated with supportive care, and he made a full clinical recovery. He was discharged after 1 week. On discharge, cardiac enzymes were within the normal range. The patient showed sustained electric and hemodynamic stability, and the skin lesions had healed.
“Through this important case study, we are developing a deeper understanding of monkeypox, viral myocarditis, and how to accurately diagnose and manage this disease,” Julia Grapsa, MD, PhD, editor-in-chief of JACC: Case Reports, commented in the news release.
“I commend the authors on this valuable clinical case during a critical time as monkeypox continues to spread globally,” Dr. Grapsa added.
The researchers say further research is needed to identify the pathologic mechanism underlying monkeypox-associated cardiac injury.
By the numbers
According to the latest data, California has reported 3,629 cases, followed closely by New York with 3,367 cases, Florida with 1,957 cases, Texas with 1,698, Georgia with 1,418, and Illinois with 1,081. The other states have reported fewer than 600 cases.
The CDC says that globally, more than 52,000 monkeypox cases have been reported.
Monkeypox case counts appear to be slowing in the United States and globally.
Last week, the World Health Organization said the number of new cases worldwide declined by 21% between Aug. 15 and 21 after increasing for 4 straight weeks.
The research had no funding. Dr. Pinho and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dolutegravir in pregnant patients with HIV showed more viral suppression at delivery vs. other treatments
“Dolutegravir is increasingly used in pregnancy in the United States,” Kunjal Patel, DSc, one of the investigators, said in an interview. “While its effectiveness and safety in pregnancy have been compared to efavirenz in previous studies, including three randomized trials, efavirenz isn’t really used in the United States and Europe for treatment of HIV; it is mainly used in Africa,” she said. Therefore, it was important to compare dolutegravir use in pregnancy to the other antiretroviral regimens that are listed as being preferred for use in pregnancy in the U.S., including atazanavir/ritonavir, darunavir/ritonavir, and raltegravir, and others often used in the U.S. and Europe, she said.
In the study published in the New England Journal of Medicine, Dr. Patel, of Harvard T.H. Chan School of Public Health, Boston, and colleagues analyzed data from kids enrolled in the Surveillance and Monitoring for ART Toxicities Dynamic (SMARTT) cohort. This group is part of an ongoing research project focused on evaluating ART toxicities during pregnancy in children who were exposed to HIV perinatally but not infected. It included pregnancies from 2007 until January 2020 that involved use of the ARTs listed.
The study population of 1,257 pregnancies with observed birth outcomes included 120 individuals with an initial ART of dolutegravir (DTG), 464 started on atazanavir–ritonavir (ATV/r), 185 on darunavir–ritonavir (DRV/r), 243 on oral rilpivirine (RPV), 86 on raltegravir (RAL), and 159 on elvitegravir–cobicistat (EVG/c). In approximately half of the pregnancies (51%), ART was started before conception, and the initial ART was changed in 27%.
The primary outcomes were viral suppression at delivery, and adverse birth outcomes, including preterm and very preterm birth, low and very low birth weight, and neonatal death within 14 days.
The median age of the patients at conception was 29 years, and 66% were non-Hispanic Black, representative of persons with HIV of childbearing age in the United States, the researchers noted. Overall, 96.7% of the patients who received dolutegravir showed viral suppression at delivery, compared to 90.1% for darunavir–ritonavir, 89.8% for elvitegravir–cobicistat, 89.2% for raltegravir, and 84.0% for atazanavir–ritonavir.
“We expected that dolutegravir to be similar with regards to viral suppression at delivery compared to raltegravir so were surprised that we observed less viral suppression with raltegravir compared to dolutegravir,” Dr. Patel said in an interview. “Our results may be due to the higher pill burden and lower barrier to resistance with RAL compared to dolutegravir, but we did not assess adherence or resistance in our study,” she noted.
Across ART regimens, the observed risks of preterm birth ranged from 13.6% to 17.6%, risks of low birth weight ranged from 11.9% to 16.7%, and risks of being small for gestational age ranged from 9.1% to 12.5%. For the composite of any adverse birth outcome and any severe adverse birth outcome, the observed risks ranged from 22.6% to 27.9% and 0% to 4.2%, respectively.
A total of 20 very preterm births, including 15 infants with very low birth weight, occurred across patients receiving all ART regimens, and no neonatal deaths occurred. The researchers found no apparent patterns of differences in the observed risk of adverse birth outcomes across all groups related to the timing of ART initiation in pregnancy, but the risks were greater among those who began the drugs during pregnancy compared to those who began before conception.
“Our results confirm the recommendation of DTG as “preferred” in U.S. perinatal guidelines, and provide evidence suggesting ATV/r and RAL provides lower HIV viral suppression at delivery compared to DTG, and support DRV/r as a reasonable alternative when DTG use is not feasible,” Dr. Patel said in an interview.
“With regards to next steps, we are interested in comparing the effectiveness and safety of dolutegravir-based regimens that include tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF) in our U.S. setting,” she said.
The study findings were limited by several factors including the lack of data on predictors of preterm birth and low birth weight, such as previous preterm birth and prepregnancy body mass index, the researchers noted.
However, the results indicate that other common ARTs provide less HIV viral suppression at delivery than dolutegravir, with similar adverse birth outcomes; the results also support darunavir–ritonavir as a reasonable alternative when dolutegravir use is not feasible, as it showed the next highest level of viral suppression after dolutegravir, the researchers concluded.
Findings fill a key research gap
The current study is important given the limited data on effectiveness and outcomes in pregnancy with the use of contemporary HIV regimens in the United States, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Pregnancy is still among exclusion criteria for most drug studies,” said Dr. Badell, who was not involved in the current study. “Dolutegravir-based ART is first line in the U.S. today because of its effectiveness, lower side effects, and higher barrier to resistance; therefore understanding the benefits and birth outcomes in pregnancy is critical,” she explained.
Dr. Badell said she was not surprised by the study findings. “However it is very reassuring to see in a large observational study comparing the dolutegravir regimens to other contemporary regimens in pregnancy, such a high level of viral suppression and no increased risk of adverse perinatal outcomes,” she said.
The study findings will impact clinical practice by reaffirming patient counseling regarding the use of dolutegravir in pregnancy, said Dr. Badell. “The use of ART in pregnancy is complex given the number of drug choices, whether the patient was on ART prior to pregnancy or initiated during pregnancy, and the various factors other than ART that affect perinatal outcomes, such as preterm birth and congenital anomalies, she explained.
The finding that the risk of adverse outcomes was higher for those who initiated ART during pregnancy vs. those who were already on ARTs when they became pregnant contradicts some previous research, said Dr. Badell. But this is “reassuring, as we highly recommend ART with viral suppression prior to pregnancy or to start as early as possible in pregnancy.”
Adverse birth outcomes can be affected by many variables such as age, substance abuse, prior adverse birth outcome and other factors, and larger studies that control for these variables will allow better evaluation of the effect of the ART drugs, Dr. Badell added.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, along with the Office of the Director, National Institutes of Health; National Institute of Dental and Craniofacial Research; National Institute of Allergy and Infectious Diseases; National Institute of Neurological Disorders and Stroke; National Institute on Deafness and Other Communication Disorders; National Institute of Mental Health; National Institute on Drug Abuse; National Cancer Institute; National Institute on Alcohol Abuse and Alcoholism; and National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health and the Tulane University School of Medicine.
The researchers and Dr. Badell had no financial conflicts to disclose.
“Dolutegravir is increasingly used in pregnancy in the United States,” Kunjal Patel, DSc, one of the investigators, said in an interview. “While its effectiveness and safety in pregnancy have been compared to efavirenz in previous studies, including three randomized trials, efavirenz isn’t really used in the United States and Europe for treatment of HIV; it is mainly used in Africa,” she said. Therefore, it was important to compare dolutegravir use in pregnancy to the other antiretroviral regimens that are listed as being preferred for use in pregnancy in the U.S., including atazanavir/ritonavir, darunavir/ritonavir, and raltegravir, and others often used in the U.S. and Europe, she said.
In the study published in the New England Journal of Medicine, Dr. Patel, of Harvard T.H. Chan School of Public Health, Boston, and colleagues analyzed data from kids enrolled in the Surveillance and Monitoring for ART Toxicities Dynamic (SMARTT) cohort. This group is part of an ongoing research project focused on evaluating ART toxicities during pregnancy in children who were exposed to HIV perinatally but not infected. It included pregnancies from 2007 until January 2020 that involved use of the ARTs listed.
The study population of 1,257 pregnancies with observed birth outcomes included 120 individuals with an initial ART of dolutegravir (DTG), 464 started on atazanavir–ritonavir (ATV/r), 185 on darunavir–ritonavir (DRV/r), 243 on oral rilpivirine (RPV), 86 on raltegravir (RAL), and 159 on elvitegravir–cobicistat (EVG/c). In approximately half of the pregnancies (51%), ART was started before conception, and the initial ART was changed in 27%.
The primary outcomes were viral suppression at delivery, and adverse birth outcomes, including preterm and very preterm birth, low and very low birth weight, and neonatal death within 14 days.
The median age of the patients at conception was 29 years, and 66% were non-Hispanic Black, representative of persons with HIV of childbearing age in the United States, the researchers noted. Overall, 96.7% of the patients who received dolutegravir showed viral suppression at delivery, compared to 90.1% for darunavir–ritonavir, 89.8% for elvitegravir–cobicistat, 89.2% for raltegravir, and 84.0% for atazanavir–ritonavir.
“We expected that dolutegravir to be similar with regards to viral suppression at delivery compared to raltegravir so were surprised that we observed less viral suppression with raltegravir compared to dolutegravir,” Dr. Patel said in an interview. “Our results may be due to the higher pill burden and lower barrier to resistance with RAL compared to dolutegravir, but we did not assess adherence or resistance in our study,” she noted.
Across ART regimens, the observed risks of preterm birth ranged from 13.6% to 17.6%, risks of low birth weight ranged from 11.9% to 16.7%, and risks of being small for gestational age ranged from 9.1% to 12.5%. For the composite of any adverse birth outcome and any severe adverse birth outcome, the observed risks ranged from 22.6% to 27.9% and 0% to 4.2%, respectively.
A total of 20 very preterm births, including 15 infants with very low birth weight, occurred across patients receiving all ART regimens, and no neonatal deaths occurred. The researchers found no apparent patterns of differences in the observed risk of adverse birth outcomes across all groups related to the timing of ART initiation in pregnancy, but the risks were greater among those who began the drugs during pregnancy compared to those who began before conception.
“Our results confirm the recommendation of DTG as “preferred” in U.S. perinatal guidelines, and provide evidence suggesting ATV/r and RAL provides lower HIV viral suppression at delivery compared to DTG, and support DRV/r as a reasonable alternative when DTG use is not feasible,” Dr. Patel said in an interview.
“With regards to next steps, we are interested in comparing the effectiveness and safety of dolutegravir-based regimens that include tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF) in our U.S. setting,” she said.
The study findings were limited by several factors including the lack of data on predictors of preterm birth and low birth weight, such as previous preterm birth and prepregnancy body mass index, the researchers noted.
However, the results indicate that other common ARTs provide less HIV viral suppression at delivery than dolutegravir, with similar adverse birth outcomes; the results also support darunavir–ritonavir as a reasonable alternative when dolutegravir use is not feasible, as it showed the next highest level of viral suppression after dolutegravir, the researchers concluded.
Findings fill a key research gap
The current study is important given the limited data on effectiveness and outcomes in pregnancy with the use of contemporary HIV regimens in the United States, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Pregnancy is still among exclusion criteria for most drug studies,” said Dr. Badell, who was not involved in the current study. “Dolutegravir-based ART is first line in the U.S. today because of its effectiveness, lower side effects, and higher barrier to resistance; therefore understanding the benefits and birth outcomes in pregnancy is critical,” she explained.
Dr. Badell said she was not surprised by the study findings. “However it is very reassuring to see in a large observational study comparing the dolutegravir regimens to other contemporary regimens in pregnancy, such a high level of viral suppression and no increased risk of adverse perinatal outcomes,” she said.
The study findings will impact clinical practice by reaffirming patient counseling regarding the use of dolutegravir in pregnancy, said Dr. Badell. “The use of ART in pregnancy is complex given the number of drug choices, whether the patient was on ART prior to pregnancy or initiated during pregnancy, and the various factors other than ART that affect perinatal outcomes, such as preterm birth and congenital anomalies, she explained.
The finding that the risk of adverse outcomes was higher for those who initiated ART during pregnancy vs. those who were already on ARTs when they became pregnant contradicts some previous research, said Dr. Badell. But this is “reassuring, as we highly recommend ART with viral suppression prior to pregnancy or to start as early as possible in pregnancy.”
Adverse birth outcomes can be affected by many variables such as age, substance abuse, prior adverse birth outcome and other factors, and larger studies that control for these variables will allow better evaluation of the effect of the ART drugs, Dr. Badell added.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, along with the Office of the Director, National Institutes of Health; National Institute of Dental and Craniofacial Research; National Institute of Allergy and Infectious Diseases; National Institute of Neurological Disorders and Stroke; National Institute on Deafness and Other Communication Disorders; National Institute of Mental Health; National Institute on Drug Abuse; National Cancer Institute; National Institute on Alcohol Abuse and Alcoholism; and National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health and the Tulane University School of Medicine.
The researchers and Dr. Badell had no financial conflicts to disclose.
“Dolutegravir is increasingly used in pregnancy in the United States,” Kunjal Patel, DSc, one of the investigators, said in an interview. “While its effectiveness and safety in pregnancy have been compared to efavirenz in previous studies, including three randomized trials, efavirenz isn’t really used in the United States and Europe for treatment of HIV; it is mainly used in Africa,” she said. Therefore, it was important to compare dolutegravir use in pregnancy to the other antiretroviral regimens that are listed as being preferred for use in pregnancy in the U.S., including atazanavir/ritonavir, darunavir/ritonavir, and raltegravir, and others often used in the U.S. and Europe, she said.
In the study published in the New England Journal of Medicine, Dr. Patel, of Harvard T.H. Chan School of Public Health, Boston, and colleagues analyzed data from kids enrolled in the Surveillance and Monitoring for ART Toxicities Dynamic (SMARTT) cohort. This group is part of an ongoing research project focused on evaluating ART toxicities during pregnancy in children who were exposed to HIV perinatally but not infected. It included pregnancies from 2007 until January 2020 that involved use of the ARTs listed.
The study population of 1,257 pregnancies with observed birth outcomes included 120 individuals with an initial ART of dolutegravir (DTG), 464 started on atazanavir–ritonavir (ATV/r), 185 on darunavir–ritonavir (DRV/r), 243 on oral rilpivirine (RPV), 86 on raltegravir (RAL), and 159 on elvitegravir–cobicistat (EVG/c). In approximately half of the pregnancies (51%), ART was started before conception, and the initial ART was changed in 27%.
The primary outcomes were viral suppression at delivery, and adverse birth outcomes, including preterm and very preterm birth, low and very low birth weight, and neonatal death within 14 days.
The median age of the patients at conception was 29 years, and 66% were non-Hispanic Black, representative of persons with HIV of childbearing age in the United States, the researchers noted. Overall, 96.7% of the patients who received dolutegravir showed viral suppression at delivery, compared to 90.1% for darunavir–ritonavir, 89.8% for elvitegravir–cobicistat, 89.2% for raltegravir, and 84.0% for atazanavir–ritonavir.
“We expected that dolutegravir to be similar with regards to viral suppression at delivery compared to raltegravir so were surprised that we observed less viral suppression with raltegravir compared to dolutegravir,” Dr. Patel said in an interview. “Our results may be due to the higher pill burden and lower barrier to resistance with RAL compared to dolutegravir, but we did not assess adherence or resistance in our study,” she noted.
Across ART regimens, the observed risks of preterm birth ranged from 13.6% to 17.6%, risks of low birth weight ranged from 11.9% to 16.7%, and risks of being small for gestational age ranged from 9.1% to 12.5%. For the composite of any adverse birth outcome and any severe adverse birth outcome, the observed risks ranged from 22.6% to 27.9% and 0% to 4.2%, respectively.
A total of 20 very preterm births, including 15 infants with very low birth weight, occurred across patients receiving all ART regimens, and no neonatal deaths occurred. The researchers found no apparent patterns of differences in the observed risk of adverse birth outcomes across all groups related to the timing of ART initiation in pregnancy, but the risks were greater among those who began the drugs during pregnancy compared to those who began before conception.
“Our results confirm the recommendation of DTG as “preferred” in U.S. perinatal guidelines, and provide evidence suggesting ATV/r and RAL provides lower HIV viral suppression at delivery compared to DTG, and support DRV/r as a reasonable alternative when DTG use is not feasible,” Dr. Patel said in an interview.
“With regards to next steps, we are interested in comparing the effectiveness and safety of dolutegravir-based regimens that include tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF) in our U.S. setting,” she said.
The study findings were limited by several factors including the lack of data on predictors of preterm birth and low birth weight, such as previous preterm birth and prepregnancy body mass index, the researchers noted.
However, the results indicate that other common ARTs provide less HIV viral suppression at delivery than dolutegravir, with similar adverse birth outcomes; the results also support darunavir–ritonavir as a reasonable alternative when dolutegravir use is not feasible, as it showed the next highest level of viral suppression after dolutegravir, the researchers concluded.
Findings fill a key research gap
The current study is important given the limited data on effectiveness and outcomes in pregnancy with the use of contemporary HIV regimens in the United States, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Pregnancy is still among exclusion criteria for most drug studies,” said Dr. Badell, who was not involved in the current study. “Dolutegravir-based ART is first line in the U.S. today because of its effectiveness, lower side effects, and higher barrier to resistance; therefore understanding the benefits and birth outcomes in pregnancy is critical,” she explained.
Dr. Badell said she was not surprised by the study findings. “However it is very reassuring to see in a large observational study comparing the dolutegravir regimens to other contemporary regimens in pregnancy, such a high level of viral suppression and no increased risk of adverse perinatal outcomes,” she said.
The study findings will impact clinical practice by reaffirming patient counseling regarding the use of dolutegravir in pregnancy, said Dr. Badell. “The use of ART in pregnancy is complex given the number of drug choices, whether the patient was on ART prior to pregnancy or initiated during pregnancy, and the various factors other than ART that affect perinatal outcomes, such as preterm birth and congenital anomalies, she explained.
The finding that the risk of adverse outcomes was higher for those who initiated ART during pregnancy vs. those who were already on ARTs when they became pregnant contradicts some previous research, said Dr. Badell. But this is “reassuring, as we highly recommend ART with viral suppression prior to pregnancy or to start as early as possible in pregnancy.”
Adverse birth outcomes can be affected by many variables such as age, substance abuse, prior adverse birth outcome and other factors, and larger studies that control for these variables will allow better evaluation of the effect of the ART drugs, Dr. Badell added.
The study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, along with the Office of the Director, National Institutes of Health; National Institute of Dental and Craniofacial Research; National Institute of Allergy and Infectious Diseases; National Institute of Neurological Disorders and Stroke; National Institute on Deafness and Other Communication Disorders; National Institute of Mental Health; National Institute on Drug Abuse; National Cancer Institute; National Institute on Alcohol Abuse and Alcoholism; and National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T.H. Chan School of Public Health and the Tulane University School of Medicine.
The researchers and Dr. Badell had no financial conflicts to disclose.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE