User login
Children and COVID: Vaccination a harder sell in the summer
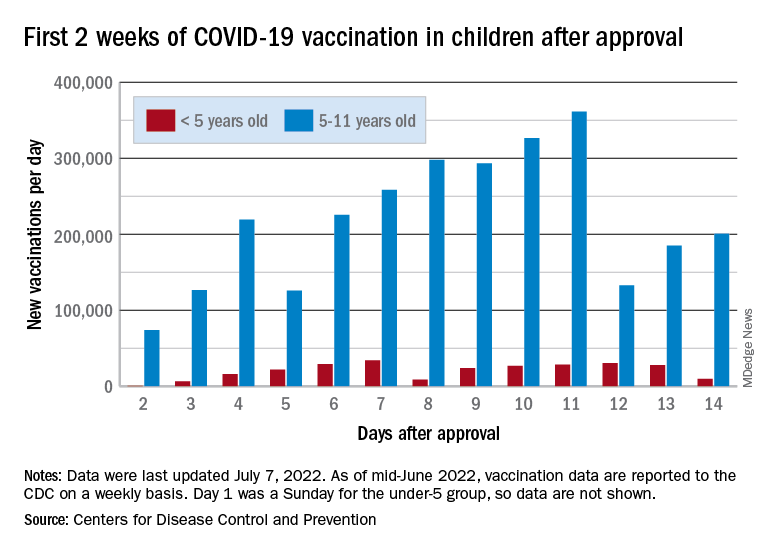
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.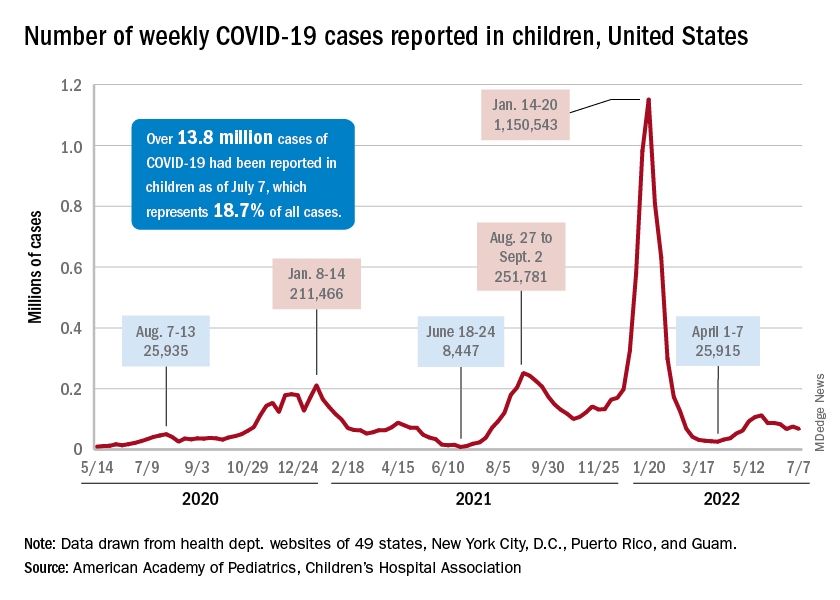
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
High residual liver cancer risk in HCV-cured cirrhosis
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Liver disease and death rates fall after hepatitis C treatment barriers are dismantled
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.
Zoster vaccination does not appear to increase flare risk in patients with immune-mediated inflammatory disease
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
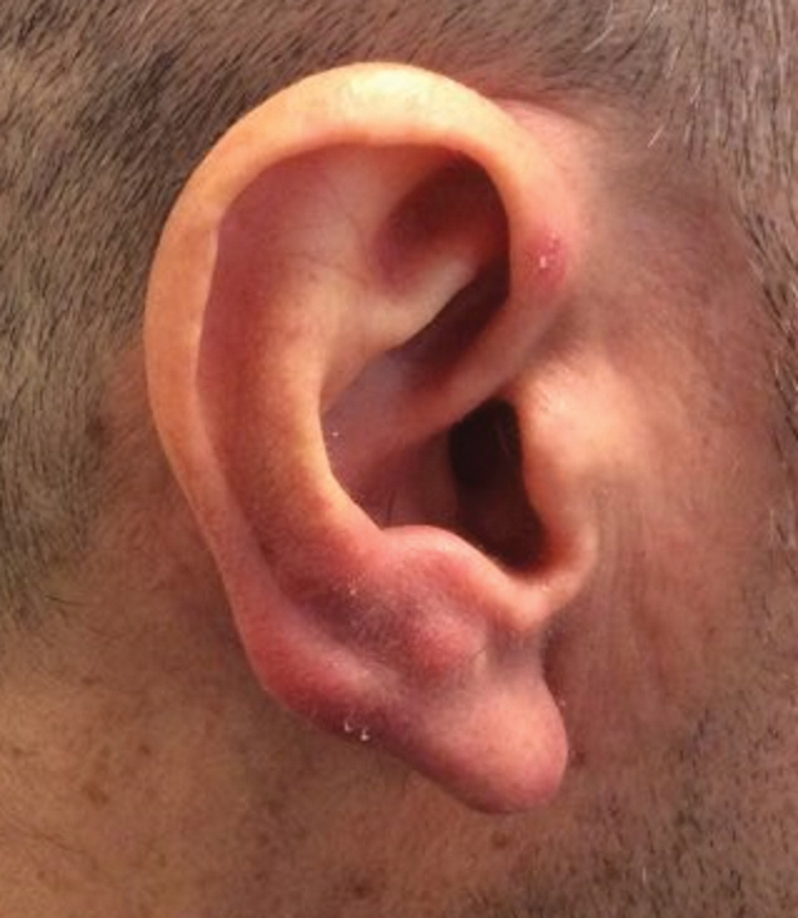
Erythematous Papules on the Ears
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
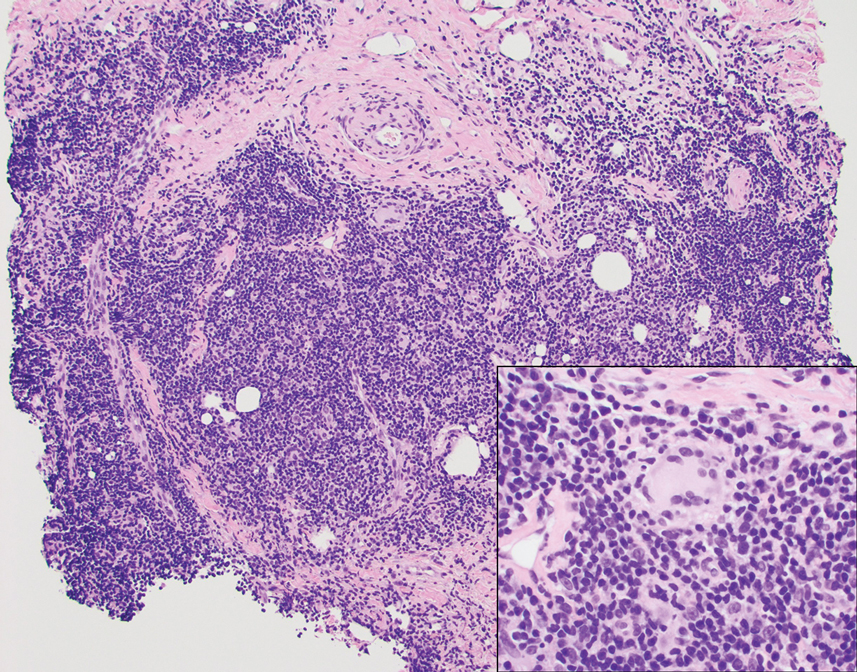
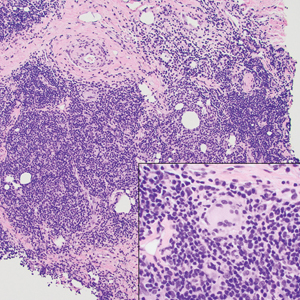
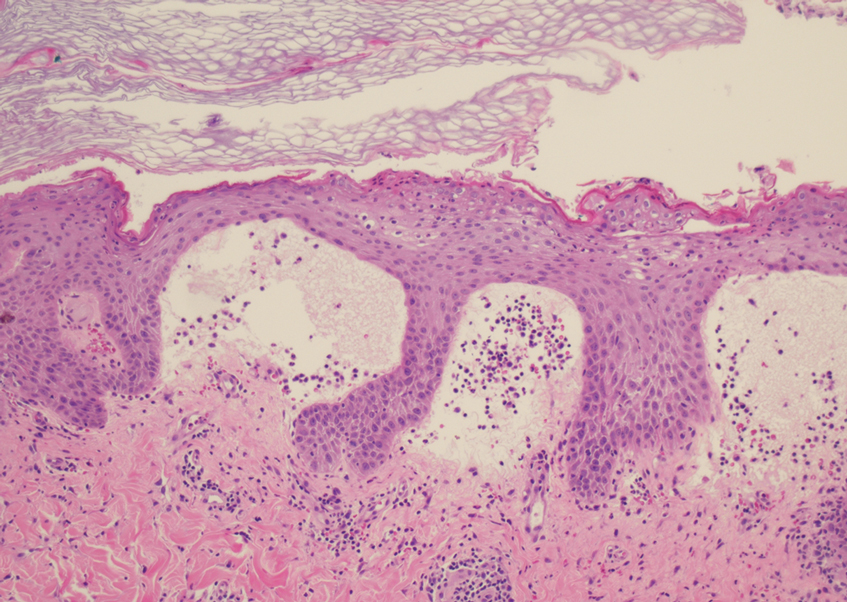
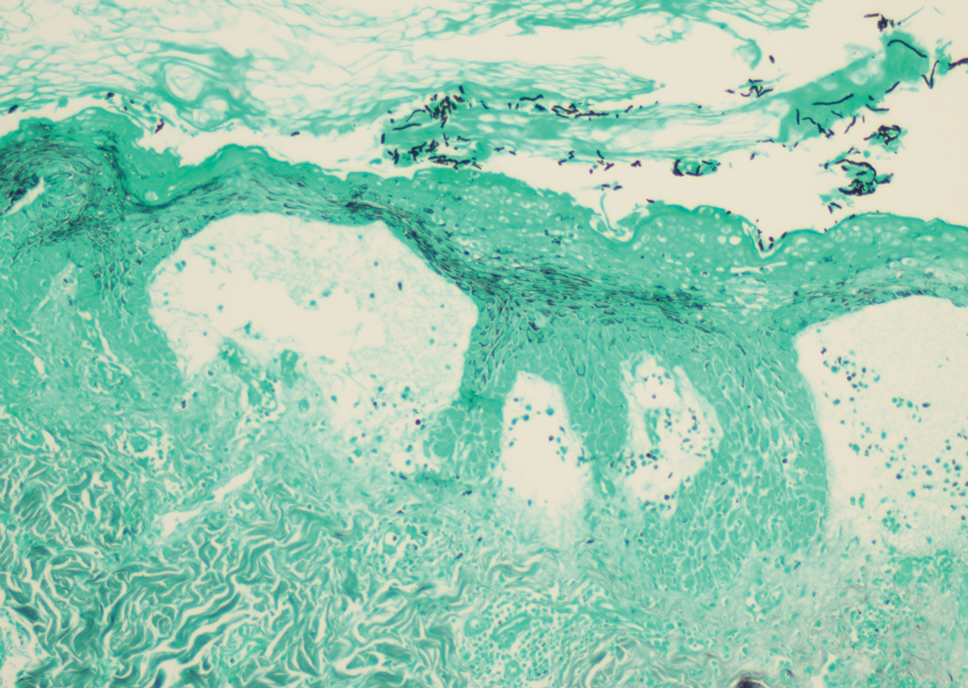
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
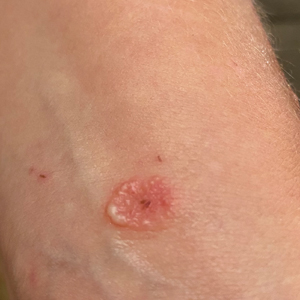
A 53-year-old man with a history of atopic dermatitis presented with pain and redness of the lobules of both ears of 9 months’ duration. He had no known allergies and took no medications. He lived in suburban Virginia and had not recently traveled outside of the region. Physical examination revealed tender erythematous and edematous nodules on the lobules of both ears (top). There was no evidence of arthritis or neurologic deficits. A punch biopsy was performed (bottom).
Orf Virus in Humans: Case Series and Clinical Review
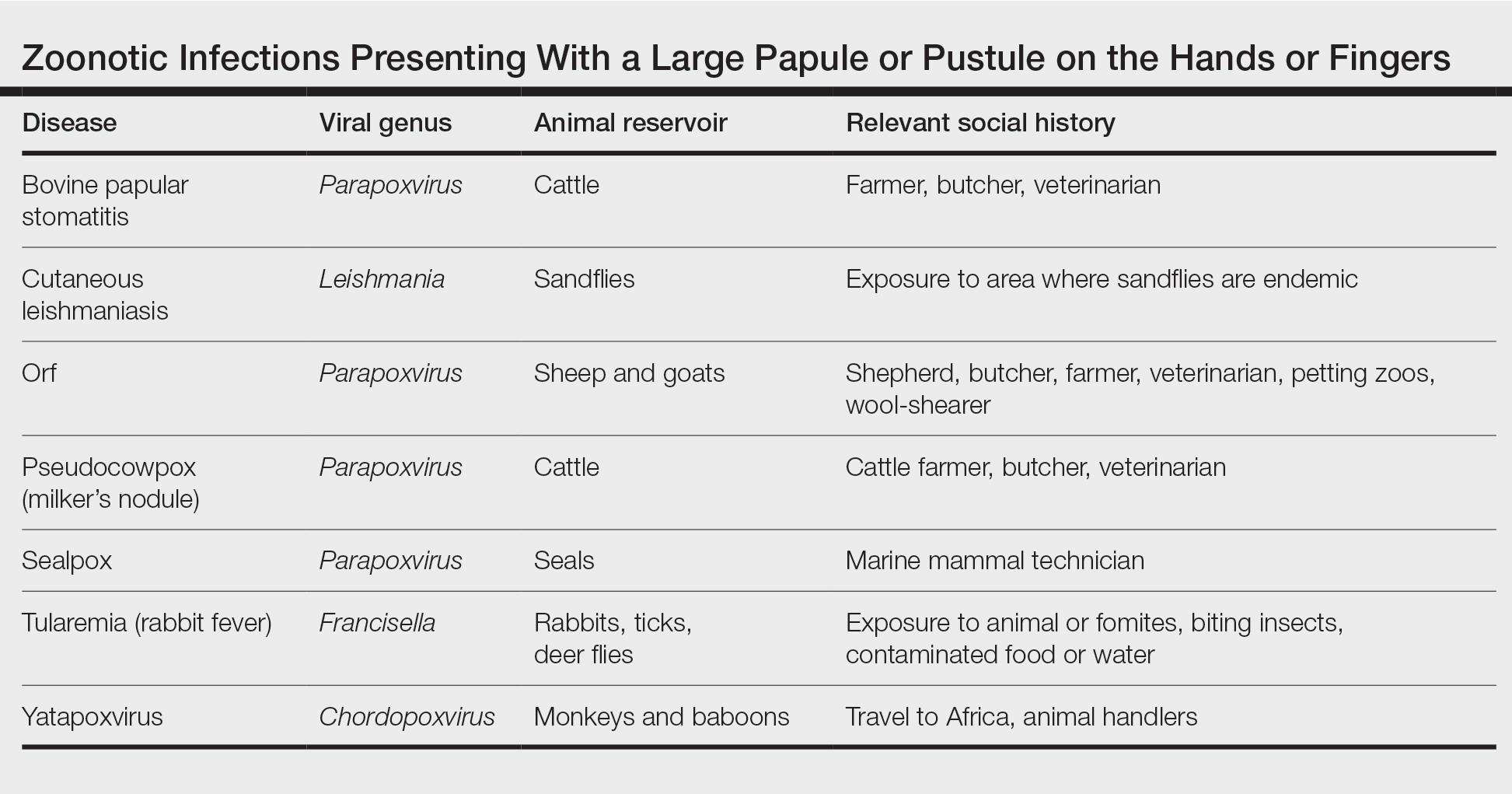
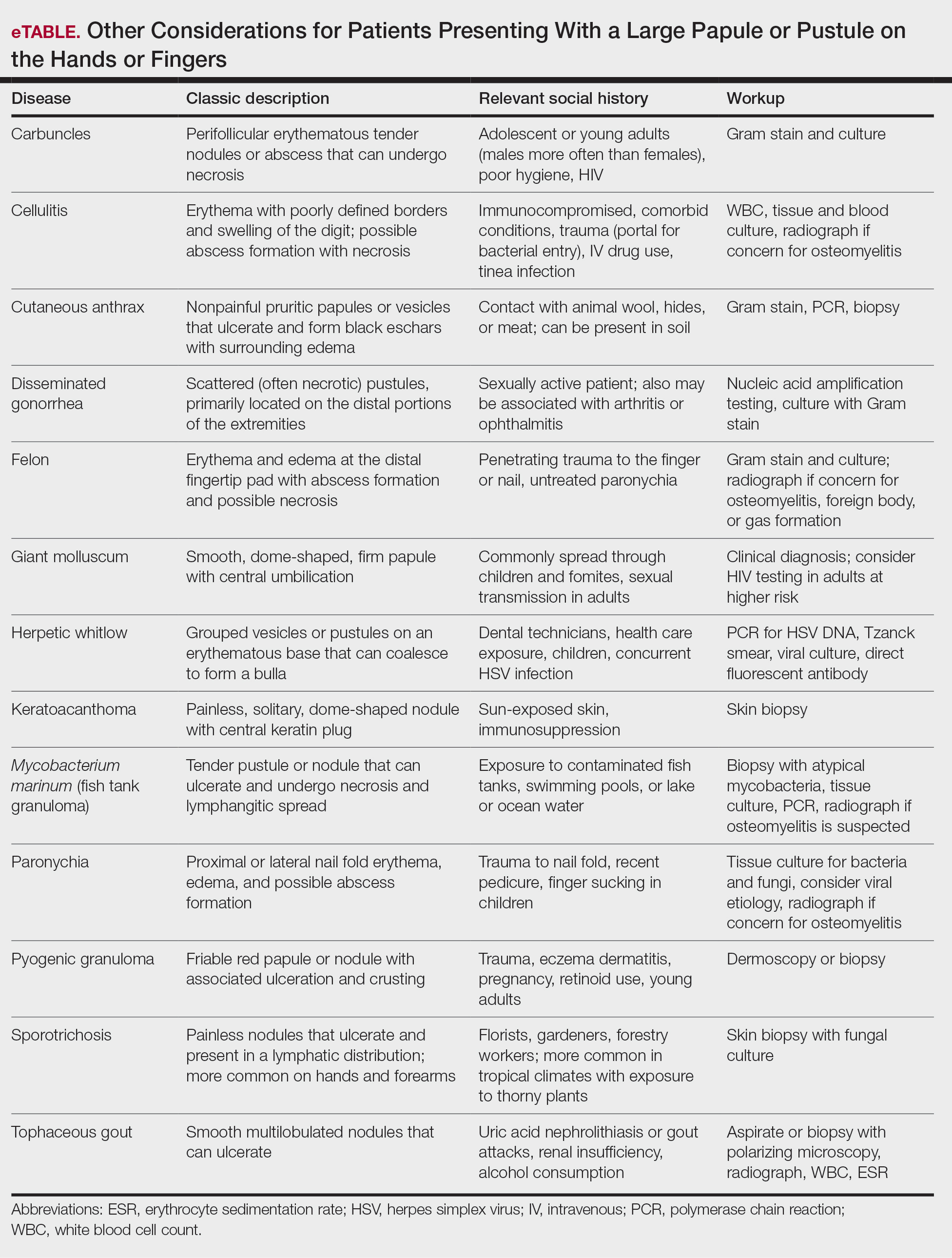
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
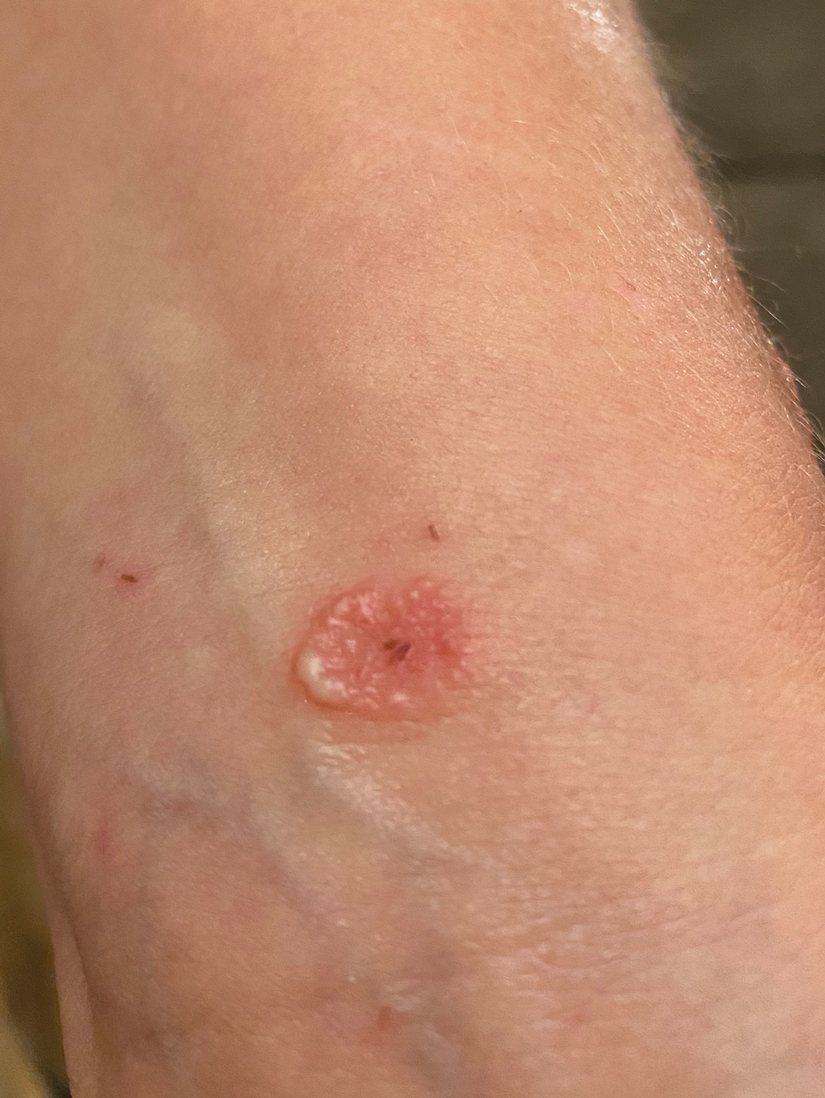
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
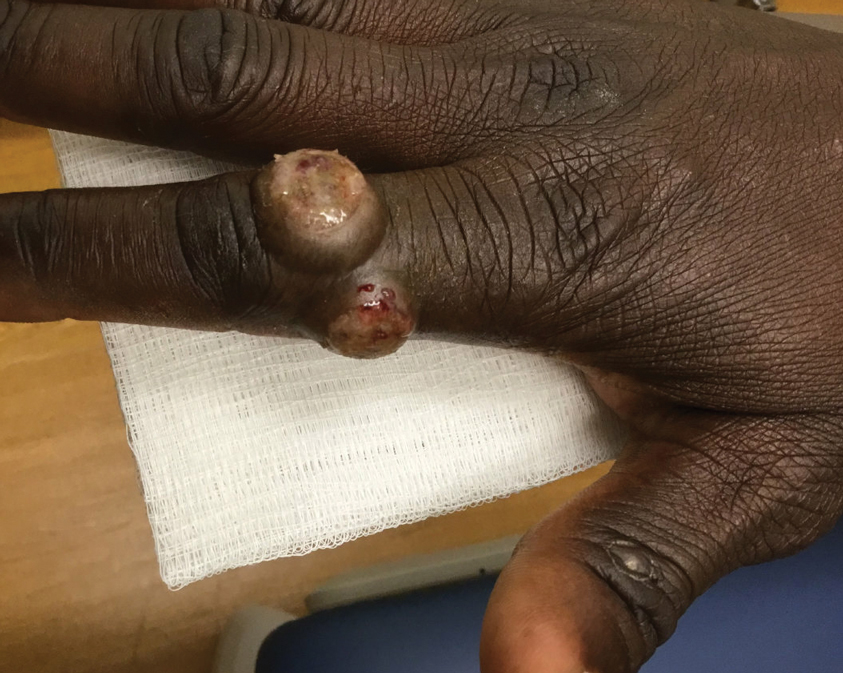
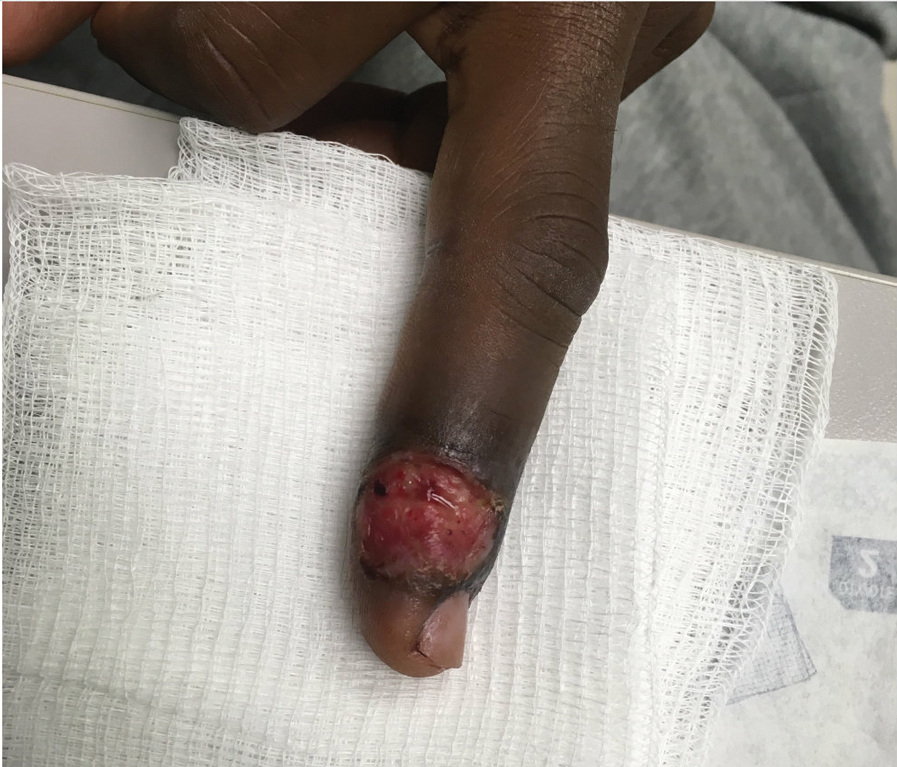
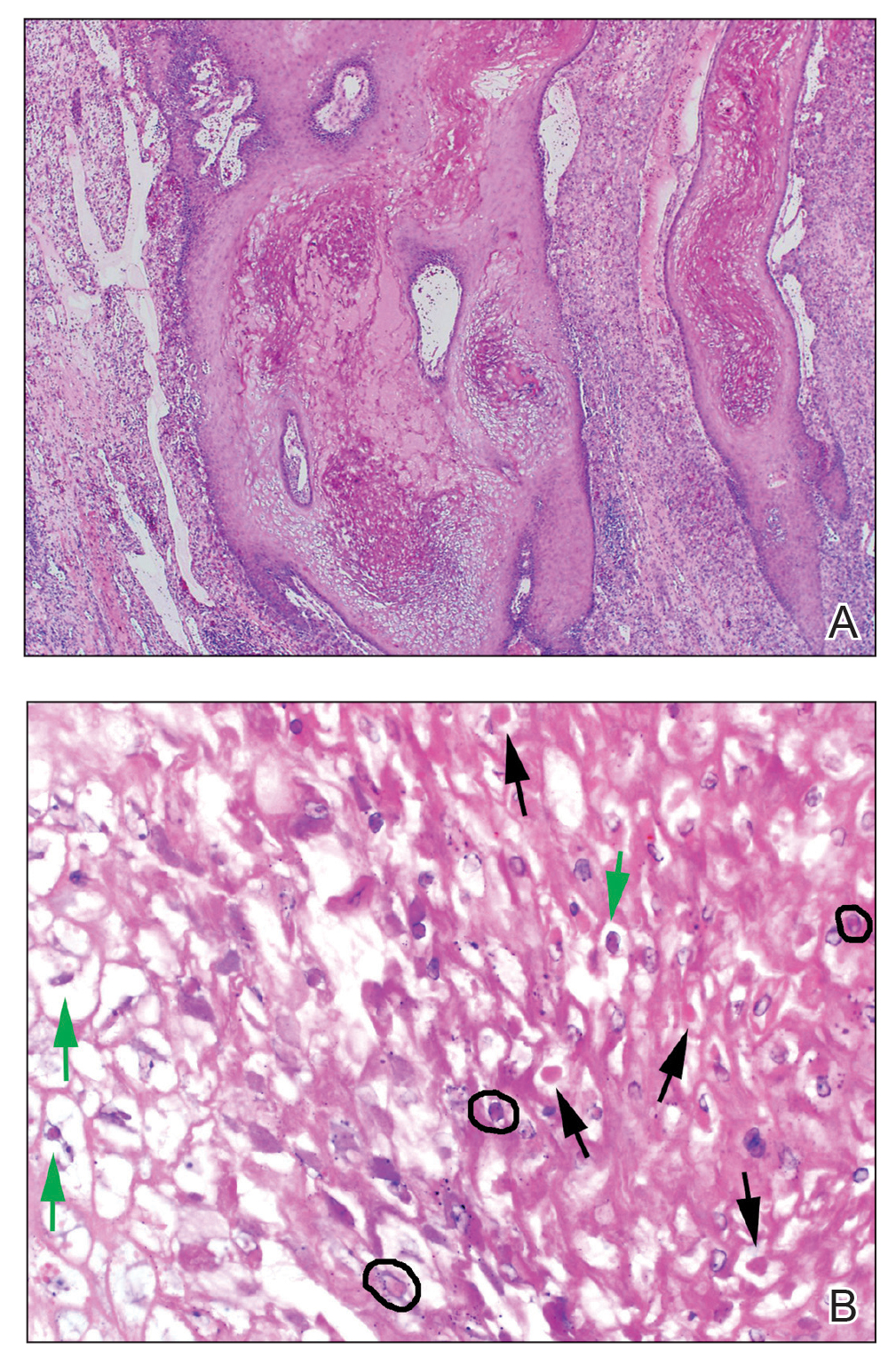
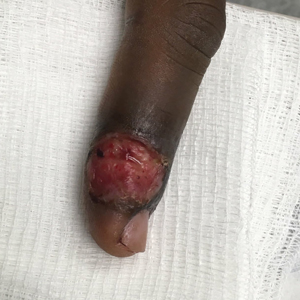
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
Practice Points
- Ecthyma contagiosum is a discrete clinical entity that occurs worldwide and demands careful attention to clinical course and social history.
- Ecthyma contagiosum is caused by orf virus, an epitheliotropic zoonotic infection that spreads from ruminants to humans.
- Early and rapid diagnosis of this classic condition is critical to prevent unnecessary biopsies or extensive testing, and determination of etiology can be important in preventing reinfection or spread to other humans by the same infected animal.
U.K. survey: Dermatologists want training in prescribing antipsychotics for delusional infestation
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
GLASGOW – that also indicated there is a clear demand for training in prescribing these drugs.
Delusional infestation is a rare disorder characterized by an individual’s belief that his or her skin, body, or immediate environment is infested by small, living pathogens, despite a lack of any medical evidence. Most of these patients require antipsychotic medication to alleviate symptoms.
The survey of almost 80 dermatologists found that almost 90% had not prescribed antipsychotics in the previous month for patients with psychodermatology conditions and that the most common barrier to prescribing was lack of experience with the drugs.
This was reflected in only 10% of survey respondents who said they were “happy to” prescribe antipsychotics without consulting either dermatology or psychiatric colleagues, and less than half having attended a related course.
Yet the research, presented at the annual meeting of the British Association of Dermatologists, indicated that more than 75% of respondents would attend such a course to increase their confidence.
This finding, said study presenter Ling Li, MD, Churchill Hospital, Oxford (England) University Hospitals NHS Foundation Trust, shows that there is a “clear demand for training, particularly among all the registrars [residents] who we surveyed.”
Dr. Li noted that the UK’s Joint Royal Colleges of Physicians Training Board’s latest curriculum for dermatology training highlights psychocutaneous medicine as a key area, and “that will include antipsychotic medication.”
The BAD also recently published guidelines for the management of adults with delusional infestation, which includes a recommendation to conduct a survey on attitudes toward antipsychotic prescribing for the condition among U.K. dermatologists.
Heeding that call, Dr. Li and colleagues sent an email containing a 10-question online survey to members of the BAD and the British Society for Medical Dermatology. Questions covered familiarity with antipsychotics and frequency of prescribing, confidence around antipsychotics, and current training and future needs. Responses were received between February through April 2021.
Among the 79 respondents, 51 (65%) were consultants and 20 (25%) were dermatology registrars, with the remainder dermatology clinical fellows, foundation doctors, or other doctors. A total of 31 respondents had an average of more than 50 visits with patients per week, 18 had an average of 41-50 patient visits, and 13 had an average of 31-40 visits per week; the remainder had an average of 11-30 visits per week.
Most of the respondents (39) said they had seen 2-5 patients with psychodermatology conditions in the last 6 months, while 17 said they had seen 1 patient, 13 said they had seen more than 10 patients, and 6 said they had seen 6-10 patients (4 had seen none and 1 could not remember).
The most commonly prescribed antipsychotics for psychodermatology patients in the past 6 months were risperidone (Risperdal; prescribed by five respondents), followed by olanzapine (Zyprexa; by four respondents). Seventy respondents had not prescribed any antipsychotics.
Asked about how confident they felt about prescribing antipsychotic medication for patients with delusional infestation, 8 (10%) said they were happy to prescribe independently, while 42 (54%) said they were not at all confident. Another 10 (13%) respondents said they would be happy to prescribe the medications after liaising with a dermatology colleague, while 17 (22%) said they would prefer to consult with the psychiatry team.
The most common barrier to prescribing antipsychotic medications was a lack of experience with the drugs, cited by 66 respondents, followed by concerns over drug monitoring, cited by 43 respondents.
In addition, 42 respondents highlighted concerns over adverse effects, 36 cited lack of experience in psychodermatology clinics, and 19 cited lack of experience in discussing psychodermatologic conditions with patients. Other barriers mentioned by the respondents included difficulties with patient acceptance of a psychiatric medication prescribed by a dermatologist.
An audience member went further, saying that clinicians have been told not to “confront” such patients and that the temptation is therefore to cloak the discussion of antipsychotics in nonthreatening language so that it is more acceptable to the patient.
However, under the U.K. system, a letter with the results of the consultation, including information that an antipsychotic has been prescribed, must be sent to the patient’s family doctor along with a copy that goes to the patient. “The situation is almost impossible,” the audience member said, adding that there “must be some arrangement where in certain circumstances dermatologists could be allowed not to write to the patient” or alternatively, “write an entirely different letter” to the family doctor.
Session cochair Susannah Baron, MD, a consultant dermatologist at St. John’s Institute of Dermatology, Guy’s and St. Thomas’ Hospital, London, said that, in these situations, it is “really helpful to talk about doses” with patients.
She explained that she uses the analogy of aspirin, which has different effects depending on the dose given, giving pain relief at high doses but primarily an antiplatelet effect at low doses.
In the case of an antipsychotic, it is helpful to explain to the patient that “you don’t think they’re psychotic, and you’re prescribing it in a very low dose, because what it can do is help with their symptoms,” Dr. Baron added. “You have to be very open because if you’re not, they go to the pharmacy, and the pharmacist says: ‘Why are you on an antipsychotic?’ ”
Further results from the survey revealed that 56 (71%) respondents did not have access to a specialist psychodermatology clinic, whereas 36 (46%) had not yet attended a psychodermatology course.
Despite these responses, 60 (77%) respondents said they would be interested in attending a training course for prescribing antipsychotics, which included all 20 of the registrars who took part in the survey. a psychodermatologist at Frimley Health Foundation Trust, Windsor, England, and lead author of the BAD guidelines, commented from the audience that the survey results were “sort of what we expected.”
She explained that the intention of the authors when developing the guidelines “was to be able to help our junior colleagues and our peers to be able to feel competent to discuss antipsychotics with patients with delusional infestation and also initiate management.”
Dr. Ahmed added: “Why we’re encouraging our colleagues to prescribe antipsychotics is the longer you leave this type of psychotic illness untreated, the worse the prognosis.”
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT BAD 2022
CDC recommends high-dose flu vaccines for seniors
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
In an online statement Fluzone High-Dose Quadrivalent, Flublok Quadrivalent, and Fluad Quadrivalent flu vaccines are among those specified in the release.
The organization says that these higher-dose vaccines may be more effective for the aging population, who often have difficulty mounting a strong enough immune response to protect themselves against the flu virus. People older than 65 years struggle the most during flu season and have the highest proportion of hospitalizations and deaths from flu, according to the release.
But the CDC believes that higher-dose vaccines have the potential to better protect against that danger. One study, from The New England Journal of Medicine, reported that high-dose/adjuvanted vaccines prevented flu in older patients 24% better than did lower-dose/nonadjuvanted vaccines.
These types of vaccines work by creating a larger immune response than a standard vaccine dose. In particular, adjuvanted vaccines contain an extra ingredient within them that helps the immune system produce a stronger reaction to the vaccine. These may be things like aluminum salts, which signal the body to respond faster. Higher-dose vaccines similarly promote a stronger immune response by having more particles of the target virus in their mixture. In theory, this means the body will create an enhanced response to the vaccine. For example, a higher-dose vaccine may quadruple the amount of antigens, compared with the standard dose.
The hope is that this recommendation may increase vaccine use across the board, says José Romero, MD, the director of the CDC’s National Center for Immunization and Respiratory Diseases. As quoted in the CDC announcement, Dr. Romero said that this may help reduce racial inequities in access to flu vaccines. A 2019 meta-analysis concluded that Black and Hispanic people are around 30%-40% less likely to get the flu vaccine. So increasing the access to this medication “could help reduce health disparities by making these vaccines more available to racial and ethnic minority groups,” said Dr. Romero.
The decision, spearheaded by CDC Director Rochelle Walensky, MD, follows recommendations from the Advisory Committee on Immunization Practices, which presented on this topic during a June 22 meeting. It is now part of official CDC policy and will continue to be developed as the 2022-2023 flu season approaches.
In addition, the organization says they’ll reveal more details for their plan later this summer, in their Morbidity and Mortality Weekly Report (MMWR). For now, seniors should know that they should try to get the recommended high-dose vaccines, but if they can’t, then a standard dose of whatever their provider has on hand will do.
At this point, there is still no specific vaccine recommendation for people aged under 65 years. The CDC historically avoids specifying one type of vaccine over another and says each should still be effective in younger patients.
A version of this article first appeared on Medscape.com.
Monkeypox mutating faster than expected
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Itchy Vesicular Rash
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
A 38-year-old woman presented with a rash of 5 days’ duration that initially appeared on the wrists after playing with her kitten, with subsequent involvement of the chest, back, abdomen, and upper and lower extremities. Physical examination revealed multiple annular plaques with raised erythematous borders, rare peripheral vesicles, and superficial central scaling. Extreme pruritus accompanied the plaques, both of which developed after playing with her kitten. The patient noted that all lesions on the upper extremities evolved in areas subject to deep puncture while more superficially excoriated areas were unaffected. She denied any other prior skin conditions and had received a 5-day course of azithromycin without improvement prior to presentation; triamcinolone ointment 0.1% had provided only temporary relief. Primary care providers prescribed a short course of oral prednisone; however, she did not start it prior to presentation.