User login
Measles outbreaks: Protecting your patients during international travel
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3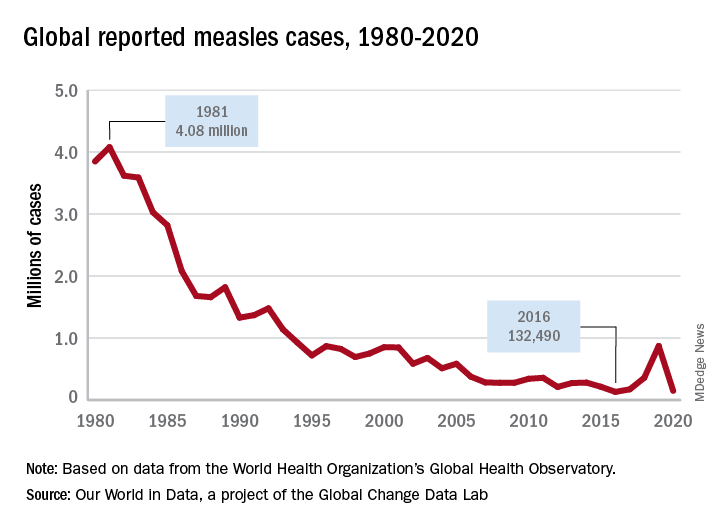
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
Severe infections often accompany severe psoriasis
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
FROM BRITISH JOURNAL OF DERMATOLOGY
FDA-cleared panties could reduce STI risk during oral sex
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
Are physician white coats becoming obsolete? How docs dress for work now
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Lyme disease may cost U.S. nearly $970 million per year
The annual cost of Lyme disease in the United States could be nearly $970 million, according to new research. But not all cases of Lyme disease are equally costly. Costs for patients with disseminated Lyme disease were more than twice as high as those for patients with localized disease.
“These findings emphasize the importance of early and accurate diagnosis to reduce both illness and its associated personal and societal costs,” the study’s authors write. The analysis was published in Emerging Infectious Diseases. The authors are from the Centers for Disease Control and Prevention, the Yale School of Public Health, and the Departments of Health of Minnesota, Maryland, and New York State.
Lyme disease is the most reported vector-borne disease in the United States; an estimated 476,000 Americans are diagnosed with the infection every year. Though the highest incidences are in the Northeast and in Minnesota and Wisconsin, the CDC has recorded cases in all 50 states. Over the past 20 years, estimates of the total cost of treating Lyme disease have varied from $203 million to nearly $1.3 billion. A major limitation to these studies, however, is that they may not accurately identify patients with Lyme disease or capture many of the nonmedical costs associated with treatment, the authors write, such as costs associated with time taken off work and travel to appointments.
To better capture the comprehensive cost of Lyme disease in the United States, researchers conducted a prospective study from September 2014 to January 2016 and followed reported cases in four states with high rates of Lyme disease: Connecticut, Maryland, Minnesota, and New York. The team conducted monthly patient surveys and queried billing codes from participants’ clinicians to estimate both the personal expenses and societal costs – such as total costs to the health care system – of Lyme disease.
Participants were variously assigned to one of three categories: those with confirmed local disease (such as patients with erythema migrans); those with confirmed disseminated disease, with symptoms including arthritis, lymphocytic meningitis, and encephalomyelitis; and those with probable cases with reported symptoms.
The researchers surveyed 901 participants with clinician-diagnosed Lyme disease: 402 with localized disease, 238 with disseminated disease, and 261 with probable cases. Nearly all participants (94%) were White, 57% were men, and 71% had an annual household income above $60,000. About 28% of participants were younger than 18 years, 16% were aged 18-45 years, 36% were aged 46-65, and 19% were older than 65 years. For most participants (68%), complete medical cost data were available. Those data were used to calculate societal costs.
Costs per patient varied dramatically, from $0.46 to $30,628. On average, patients spent $1,252 during the study period on expenses related to Lyme disease treatment, including expenses associated with travel and time taken off work. The median cost to patients was notably smaller, at $244. Patients with disseminated disease had the highest median ($358) and mean ($1,692) costs, followed by participants with probable disease (median, $315; mean, $1,277). Participants with localized disease had the lowest median ($170) and mean ($1,070) costs.
Societal costs ranged from $54 to $122,766 per patient; the median was $690, and the mean was $2,032. Multiplying these numbers by the estimated cases diagnosed nationally each year, researchers determined that the total cost of Lyme disease in the United States is between $345 million and $968 million.
The study “pointed out the tremendous variability in costs” associated with Lyme disease treatment, Brian Fallon, MD, MPH, director of the Lyme and Tick-Borne Diseases Research Center at the Columbia University Irving Medical Center, New York, told this news organization. He was not involved with the research. Some patients continued having health expenses after the end of the study, which means their costs would be even higher, he noted. Though most patients fully recovered after 1 year, about 4% still reported symptoms, and 3% were still incurring costs.
And while the calculated total cost is “significant,” it is likely an underestimate, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center, Baltimore, in an interview. Dr. Aucott is also unaffiliated with the work. The researchers included confirmed or probable Lyme disease cases, he said, but that does not capture patients who may have had Lyme disease but were initially misdiagnosed. “Those poor patients end up going to see many different doctors to try to figure out what their diagnosis is,” added Dr. Fallon. “If they do have Lyme, they may not get started on treatment until much later than those who had the classic manifestations [of the disease].” These patients are more likely to have relapsing symptoms, he said, and their cost of care would be much higher, as highlighted in the research findings.
“The take-home point is that the cost to the patient and society is much less if you make an early diagnosis,” Dr. Aucott said. “It highlights the need for rapid diagnosis and rapid identification,” added Dr. Fallon, “as well as the potential importance and value of better means of prevention.”
A version of this article first appeared on Medscape.com.
The annual cost of Lyme disease in the United States could be nearly $970 million, according to new research. But not all cases of Lyme disease are equally costly. Costs for patients with disseminated Lyme disease were more than twice as high as those for patients with localized disease.
“These findings emphasize the importance of early and accurate diagnosis to reduce both illness and its associated personal and societal costs,” the study’s authors write. The analysis was published in Emerging Infectious Diseases. The authors are from the Centers for Disease Control and Prevention, the Yale School of Public Health, and the Departments of Health of Minnesota, Maryland, and New York State.
Lyme disease is the most reported vector-borne disease in the United States; an estimated 476,000 Americans are diagnosed with the infection every year. Though the highest incidences are in the Northeast and in Minnesota and Wisconsin, the CDC has recorded cases in all 50 states. Over the past 20 years, estimates of the total cost of treating Lyme disease have varied from $203 million to nearly $1.3 billion. A major limitation to these studies, however, is that they may not accurately identify patients with Lyme disease or capture many of the nonmedical costs associated with treatment, the authors write, such as costs associated with time taken off work and travel to appointments.
To better capture the comprehensive cost of Lyme disease in the United States, researchers conducted a prospective study from September 2014 to January 2016 and followed reported cases in four states with high rates of Lyme disease: Connecticut, Maryland, Minnesota, and New York. The team conducted monthly patient surveys and queried billing codes from participants’ clinicians to estimate both the personal expenses and societal costs – such as total costs to the health care system – of Lyme disease.
Participants were variously assigned to one of three categories: those with confirmed local disease (such as patients with erythema migrans); those with confirmed disseminated disease, with symptoms including arthritis, lymphocytic meningitis, and encephalomyelitis; and those with probable cases with reported symptoms.
The researchers surveyed 901 participants with clinician-diagnosed Lyme disease: 402 with localized disease, 238 with disseminated disease, and 261 with probable cases. Nearly all participants (94%) were White, 57% were men, and 71% had an annual household income above $60,000. About 28% of participants were younger than 18 years, 16% were aged 18-45 years, 36% were aged 46-65, and 19% were older than 65 years. For most participants (68%), complete medical cost data were available. Those data were used to calculate societal costs.
Costs per patient varied dramatically, from $0.46 to $30,628. On average, patients spent $1,252 during the study period on expenses related to Lyme disease treatment, including expenses associated with travel and time taken off work. The median cost to patients was notably smaller, at $244. Patients with disseminated disease had the highest median ($358) and mean ($1,692) costs, followed by participants with probable disease (median, $315; mean, $1,277). Participants with localized disease had the lowest median ($170) and mean ($1,070) costs.
Societal costs ranged from $54 to $122,766 per patient; the median was $690, and the mean was $2,032. Multiplying these numbers by the estimated cases diagnosed nationally each year, researchers determined that the total cost of Lyme disease in the United States is between $345 million and $968 million.
The study “pointed out the tremendous variability in costs” associated with Lyme disease treatment, Brian Fallon, MD, MPH, director of the Lyme and Tick-Borne Diseases Research Center at the Columbia University Irving Medical Center, New York, told this news organization. He was not involved with the research. Some patients continued having health expenses after the end of the study, which means their costs would be even higher, he noted. Though most patients fully recovered after 1 year, about 4% still reported symptoms, and 3% were still incurring costs.
And while the calculated total cost is “significant,” it is likely an underestimate, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center, Baltimore, in an interview. Dr. Aucott is also unaffiliated with the work. The researchers included confirmed or probable Lyme disease cases, he said, but that does not capture patients who may have had Lyme disease but were initially misdiagnosed. “Those poor patients end up going to see many different doctors to try to figure out what their diagnosis is,” added Dr. Fallon. “If they do have Lyme, they may not get started on treatment until much later than those who had the classic manifestations [of the disease].” These patients are more likely to have relapsing symptoms, he said, and their cost of care would be much higher, as highlighted in the research findings.
“The take-home point is that the cost to the patient and society is much less if you make an early diagnosis,” Dr. Aucott said. “It highlights the need for rapid diagnosis and rapid identification,” added Dr. Fallon, “as well as the potential importance and value of better means of prevention.”
A version of this article first appeared on Medscape.com.
The annual cost of Lyme disease in the United States could be nearly $970 million, according to new research. But not all cases of Lyme disease are equally costly. Costs for patients with disseminated Lyme disease were more than twice as high as those for patients with localized disease.
“These findings emphasize the importance of early and accurate diagnosis to reduce both illness and its associated personal and societal costs,” the study’s authors write. The analysis was published in Emerging Infectious Diseases. The authors are from the Centers for Disease Control and Prevention, the Yale School of Public Health, and the Departments of Health of Minnesota, Maryland, and New York State.
Lyme disease is the most reported vector-borne disease in the United States; an estimated 476,000 Americans are diagnosed with the infection every year. Though the highest incidences are in the Northeast and in Minnesota and Wisconsin, the CDC has recorded cases in all 50 states. Over the past 20 years, estimates of the total cost of treating Lyme disease have varied from $203 million to nearly $1.3 billion. A major limitation to these studies, however, is that they may not accurately identify patients with Lyme disease or capture many of the nonmedical costs associated with treatment, the authors write, such as costs associated with time taken off work and travel to appointments.
To better capture the comprehensive cost of Lyme disease in the United States, researchers conducted a prospective study from September 2014 to January 2016 and followed reported cases in four states with high rates of Lyme disease: Connecticut, Maryland, Minnesota, and New York. The team conducted monthly patient surveys and queried billing codes from participants’ clinicians to estimate both the personal expenses and societal costs – such as total costs to the health care system – of Lyme disease.
Participants were variously assigned to one of three categories: those with confirmed local disease (such as patients with erythema migrans); those with confirmed disseminated disease, with symptoms including arthritis, lymphocytic meningitis, and encephalomyelitis; and those with probable cases with reported symptoms.
The researchers surveyed 901 participants with clinician-diagnosed Lyme disease: 402 with localized disease, 238 with disseminated disease, and 261 with probable cases. Nearly all participants (94%) were White, 57% were men, and 71% had an annual household income above $60,000. About 28% of participants were younger than 18 years, 16% were aged 18-45 years, 36% were aged 46-65, and 19% were older than 65 years. For most participants (68%), complete medical cost data were available. Those data were used to calculate societal costs.
Costs per patient varied dramatically, from $0.46 to $30,628. On average, patients spent $1,252 during the study period on expenses related to Lyme disease treatment, including expenses associated with travel and time taken off work. The median cost to patients was notably smaller, at $244. Patients with disseminated disease had the highest median ($358) and mean ($1,692) costs, followed by participants with probable disease (median, $315; mean, $1,277). Participants with localized disease had the lowest median ($170) and mean ($1,070) costs.
Societal costs ranged from $54 to $122,766 per patient; the median was $690, and the mean was $2,032. Multiplying these numbers by the estimated cases diagnosed nationally each year, researchers determined that the total cost of Lyme disease in the United States is between $345 million and $968 million.
The study “pointed out the tremendous variability in costs” associated with Lyme disease treatment, Brian Fallon, MD, MPH, director of the Lyme and Tick-Borne Diseases Research Center at the Columbia University Irving Medical Center, New York, told this news organization. He was not involved with the research. Some patients continued having health expenses after the end of the study, which means their costs would be even higher, he noted. Though most patients fully recovered after 1 year, about 4% still reported symptoms, and 3% were still incurring costs.
And while the calculated total cost is “significant,” it is likely an underestimate, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center, Baltimore, in an interview. Dr. Aucott is also unaffiliated with the work. The researchers included confirmed or probable Lyme disease cases, he said, but that does not capture patients who may have had Lyme disease but were initially misdiagnosed. “Those poor patients end up going to see many different doctors to try to figure out what their diagnosis is,” added Dr. Fallon. “If they do have Lyme, they may not get started on treatment until much later than those who had the classic manifestations [of the disease].” These patients are more likely to have relapsing symptoms, he said, and their cost of care would be much higher, as highlighted in the research findings.
“The take-home point is that the cost to the patient and society is much less if you make an early diagnosis,” Dr. Aucott said. “It highlights the need for rapid diagnosis and rapid identification,” added Dr. Fallon, “as well as the potential importance and value of better means of prevention.”
A version of this article first appeared on Medscape.com.
FROM EMERGING INFECTIOUS DISEASES
Most COVID-19 survivors return to work within 2 years
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
FROM THE LANCET RESPIRATORY MEDICINE
Decentralizing PrEP offers a road map for retention
Good solutions have great road maps.
For HIV preexposure prophylaxis (PrEP), the road map might just be that of contraceptive care. Once an onerous process, over time contraceptive care exploded into a range of options across a broad landscape in terms of approach and accessibility.
How then do organizations help vulnerable patients navigate their PrEP journeys using the contraceptive road map as a guide?
That’s what researchers at the University of Washington were intent on demonstrating, according to Julie Dombrowski, MD, MPH, an infectious disease specialist at the University of Washington, Seattle, and deputy director of the HIV/STD Program, Public Health for the city of Seattle and King County, Wash.
“The same sorts of things that happened with oral contraceptive pills – which initially required you to see a gynecologist and get a Pap smear – over time, became much more available,” said Dr. Dombrowski, coauthor of a new study published online in the Journal of Acquired Immune Deficiency Syndrome.
“The basic idea is that PrEP is not medically complicated; it can be easily protocolized,” she told this news organization.
Decentralizing HIV PrEP
In addition to her responsibilities at University of Washington, Dr. Dombrowski provides clinical services at the Public Health Sexual Health Clinic (PHSKC) at Seattle’s Harborview Medical Center – a dual-county center that provides confidential STI and HIV evaluation, screening, testing, and treatment on a walk-in basis for a sliding fee.
Sexual health clinics are ideal environments for reaching large numbers of patients, but strategies for integrating PrEP successfully into what are commonly one-time appointments have not been well-described or broadly adopted.
“Sexual health clinics in general are STD specialty clinics with walk-in access to care; often, patients come into a clinic, get seen, diagnosed, and treated, and they don’t necessarily come back,” said Dr. Dombrowski.
She said that, because most operations have been set up around same-day treatment, to offer PrEP and successfully change outcomes, there needs to be a shift in the current model toward one that promotes an ongoing relationship with the patients.
So, she and her colleagues decided to see what would happen if they implemented a decentralized PrEP model in their clinic over a 6-year period. They established a protocol that moves from an initial consultation with a clinician to review risk behaviors, ascertain HIV status, and acquire a PrEP prescription, to ongoing interactions with an STI and PrEP-trained disease intervention specialist (DIS).
As the clinic’s PrEP program coordinators, these specialists enroll patients in PrEP drug assistance programs, verify prescription fills, provide follow-up visits and adherence and adverse events assessments, and collect specimens.
“[Disease intervention specialists] are frontline public health workers who ensure that people diagnosed with HIV or an STI – or who’ve been exposed – get necessary testing and treatment,” explained Dr. Dombrowski. “They’re very similar to patient navigators.”
At the same time, clinicians remain the key providers for annual appointments, new symptoms, STI diagnoses, adverse drug reactions, and missed doses. Licensed medical providers review all labs.
Shifting responsibilities, better PrEP initiation, retention rates
After establishing the PrEP services protocol, the University of Washington team then assessed retention rates among PrEP patients who attended an initial visit (1,387) from October 2014 to December 2019. Follow-up continued through February 2020. (For study purposes, PrEP discontinuation was defined as either stopping PrEP after initiation or as lost to follow-up, i.e., either not attending a follow-up visit or not responding to more than three DIS calls or text messages).
Just over half of the participants were aged 20-29 years, and a third were aged 30-39. More than 9 out of 10 (93%) were men who sleep with men (MSM), 55% White, 26% Hispanic/Latinx, and 10% Black.
Over the course of the study, 6,887 PrEP visits were recorded. Quarterly visits increased concurrently with the program expansion, from 31 visits in 2014 to 623 in the fourth quarter (Q4) of 2019. Likewise, while 57% of visits overall were with a clinician, DIS visits increased from 3% in Q4 of 2014 to 45% in Q4 of 2019, an increase of 1,400% in 5 years.
Significant numbers of patients also initiated PrEP in the clinic, especially when prescribing practices were expanded to be part of routine, walk-in visits.
Retention rates also improved, with 43% (510/1,190) of patients still on PrEP at the end of the analysis period. Forty-one percent (490) discontinued PrEP, 21% within 3 months of initiation, and 72% within a year; another 16% moved, transferred care, or tested positive, and were considered “censored.” However, as of July 31, 2021, 54% (265) of the 490 patients who had discontinued PrEP returned to the clinic for a restart visit, 93% of whom refilled their restart prescription.
“This is really basic preventative care and is actually quite easy to do,” noted Sarah Schmalzle, MD, assistant professor of medicine and medical director of the Thrive Program at the Institute of Human Virology at the University of Maryland, Baltimore. Dr. Schmalzle was not involved in the study.
Dr. Schmalzle practices in inner-city Baltimore, so she and her colleagues have been thorough in terms of setting up PrEP (and postexposure prophylaxis, PEP) programs to ensure that patients access PrEP wherever they want to. But she also said that PrEP is only a part of the sexual wellness and prevention toolbox, and ideally, part of a whole prevention program.
“Focusing on how to get the prescription out is great but the rest is having ongoing and accurate sexual health conversations, healthy conversations about sex and prevention, to have [an] algorithm in place that says, ‘Here’s your PrEP, this is the next time that you need an appointment, the next time you need labs, I’m going to check your adherence, etc.’ ”
Both Dr. Dombrowski and Dr. Schmalzle emphasized that decentralization is not a one-size model; flexibility is key, especially when it comes to who is providing PrEP
“People overcomplicate PrEP and clinicians do this too,” said Dr. Dombrowski. “If we are going to successfully increase PrEP and improve the patient experience, we need to decrease the requirement for clinician involvement.”
Dr. Dombrowski has disclosed no relevant financial relationships. Dr. Schmalzle receives grant funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Good solutions have great road maps.
For HIV preexposure prophylaxis (PrEP), the road map might just be that of contraceptive care. Once an onerous process, over time contraceptive care exploded into a range of options across a broad landscape in terms of approach and accessibility.
How then do organizations help vulnerable patients navigate their PrEP journeys using the contraceptive road map as a guide?
That’s what researchers at the University of Washington were intent on demonstrating, according to Julie Dombrowski, MD, MPH, an infectious disease specialist at the University of Washington, Seattle, and deputy director of the HIV/STD Program, Public Health for the city of Seattle and King County, Wash.
“The same sorts of things that happened with oral contraceptive pills – which initially required you to see a gynecologist and get a Pap smear – over time, became much more available,” said Dr. Dombrowski, coauthor of a new study published online in the Journal of Acquired Immune Deficiency Syndrome.
“The basic idea is that PrEP is not medically complicated; it can be easily protocolized,” she told this news organization.
Decentralizing HIV PrEP
In addition to her responsibilities at University of Washington, Dr. Dombrowski provides clinical services at the Public Health Sexual Health Clinic (PHSKC) at Seattle’s Harborview Medical Center – a dual-county center that provides confidential STI and HIV evaluation, screening, testing, and treatment on a walk-in basis for a sliding fee.
Sexual health clinics are ideal environments for reaching large numbers of patients, but strategies for integrating PrEP successfully into what are commonly one-time appointments have not been well-described or broadly adopted.
“Sexual health clinics in general are STD specialty clinics with walk-in access to care; often, patients come into a clinic, get seen, diagnosed, and treated, and they don’t necessarily come back,” said Dr. Dombrowski.
She said that, because most operations have been set up around same-day treatment, to offer PrEP and successfully change outcomes, there needs to be a shift in the current model toward one that promotes an ongoing relationship with the patients.
So, she and her colleagues decided to see what would happen if they implemented a decentralized PrEP model in their clinic over a 6-year period. They established a protocol that moves from an initial consultation with a clinician to review risk behaviors, ascertain HIV status, and acquire a PrEP prescription, to ongoing interactions with an STI and PrEP-trained disease intervention specialist (DIS).
As the clinic’s PrEP program coordinators, these specialists enroll patients in PrEP drug assistance programs, verify prescription fills, provide follow-up visits and adherence and adverse events assessments, and collect specimens.
“[Disease intervention specialists] are frontline public health workers who ensure that people diagnosed with HIV or an STI – or who’ve been exposed – get necessary testing and treatment,” explained Dr. Dombrowski. “They’re very similar to patient navigators.”
At the same time, clinicians remain the key providers for annual appointments, new symptoms, STI diagnoses, adverse drug reactions, and missed doses. Licensed medical providers review all labs.
Shifting responsibilities, better PrEP initiation, retention rates
After establishing the PrEP services protocol, the University of Washington team then assessed retention rates among PrEP patients who attended an initial visit (1,387) from October 2014 to December 2019. Follow-up continued through February 2020. (For study purposes, PrEP discontinuation was defined as either stopping PrEP after initiation or as lost to follow-up, i.e., either not attending a follow-up visit or not responding to more than three DIS calls or text messages).
Just over half of the participants were aged 20-29 years, and a third were aged 30-39. More than 9 out of 10 (93%) were men who sleep with men (MSM), 55% White, 26% Hispanic/Latinx, and 10% Black.
Over the course of the study, 6,887 PrEP visits were recorded. Quarterly visits increased concurrently with the program expansion, from 31 visits in 2014 to 623 in the fourth quarter (Q4) of 2019. Likewise, while 57% of visits overall were with a clinician, DIS visits increased from 3% in Q4 of 2014 to 45% in Q4 of 2019, an increase of 1,400% in 5 years.
Significant numbers of patients also initiated PrEP in the clinic, especially when prescribing practices were expanded to be part of routine, walk-in visits.
Retention rates also improved, with 43% (510/1,190) of patients still on PrEP at the end of the analysis period. Forty-one percent (490) discontinued PrEP, 21% within 3 months of initiation, and 72% within a year; another 16% moved, transferred care, or tested positive, and were considered “censored.” However, as of July 31, 2021, 54% (265) of the 490 patients who had discontinued PrEP returned to the clinic for a restart visit, 93% of whom refilled their restart prescription.
“This is really basic preventative care and is actually quite easy to do,” noted Sarah Schmalzle, MD, assistant professor of medicine and medical director of the Thrive Program at the Institute of Human Virology at the University of Maryland, Baltimore. Dr. Schmalzle was not involved in the study.
Dr. Schmalzle practices in inner-city Baltimore, so she and her colleagues have been thorough in terms of setting up PrEP (and postexposure prophylaxis, PEP) programs to ensure that patients access PrEP wherever they want to. But she also said that PrEP is only a part of the sexual wellness and prevention toolbox, and ideally, part of a whole prevention program.
“Focusing on how to get the prescription out is great but the rest is having ongoing and accurate sexual health conversations, healthy conversations about sex and prevention, to have [an] algorithm in place that says, ‘Here’s your PrEP, this is the next time that you need an appointment, the next time you need labs, I’m going to check your adherence, etc.’ ”
Both Dr. Dombrowski and Dr. Schmalzle emphasized that decentralization is not a one-size model; flexibility is key, especially when it comes to who is providing PrEP
“People overcomplicate PrEP and clinicians do this too,” said Dr. Dombrowski. “If we are going to successfully increase PrEP and improve the patient experience, we need to decrease the requirement for clinician involvement.”
Dr. Dombrowski has disclosed no relevant financial relationships. Dr. Schmalzle receives grant funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Good solutions have great road maps.
For HIV preexposure prophylaxis (PrEP), the road map might just be that of contraceptive care. Once an onerous process, over time contraceptive care exploded into a range of options across a broad landscape in terms of approach and accessibility.
How then do organizations help vulnerable patients navigate their PrEP journeys using the contraceptive road map as a guide?
That’s what researchers at the University of Washington were intent on demonstrating, according to Julie Dombrowski, MD, MPH, an infectious disease specialist at the University of Washington, Seattle, and deputy director of the HIV/STD Program, Public Health for the city of Seattle and King County, Wash.
“The same sorts of things that happened with oral contraceptive pills – which initially required you to see a gynecologist and get a Pap smear – over time, became much more available,” said Dr. Dombrowski, coauthor of a new study published online in the Journal of Acquired Immune Deficiency Syndrome.
“The basic idea is that PrEP is not medically complicated; it can be easily protocolized,” she told this news organization.
Decentralizing HIV PrEP
In addition to her responsibilities at University of Washington, Dr. Dombrowski provides clinical services at the Public Health Sexual Health Clinic (PHSKC) at Seattle’s Harborview Medical Center – a dual-county center that provides confidential STI and HIV evaluation, screening, testing, and treatment on a walk-in basis for a sliding fee.
Sexual health clinics are ideal environments for reaching large numbers of patients, but strategies for integrating PrEP successfully into what are commonly one-time appointments have not been well-described or broadly adopted.
“Sexual health clinics in general are STD specialty clinics with walk-in access to care; often, patients come into a clinic, get seen, diagnosed, and treated, and they don’t necessarily come back,” said Dr. Dombrowski.
She said that, because most operations have been set up around same-day treatment, to offer PrEP and successfully change outcomes, there needs to be a shift in the current model toward one that promotes an ongoing relationship with the patients.
So, she and her colleagues decided to see what would happen if they implemented a decentralized PrEP model in their clinic over a 6-year period. They established a protocol that moves from an initial consultation with a clinician to review risk behaviors, ascertain HIV status, and acquire a PrEP prescription, to ongoing interactions with an STI and PrEP-trained disease intervention specialist (DIS).
As the clinic’s PrEP program coordinators, these specialists enroll patients in PrEP drug assistance programs, verify prescription fills, provide follow-up visits and adherence and adverse events assessments, and collect specimens.
“[Disease intervention specialists] are frontline public health workers who ensure that people diagnosed with HIV or an STI – or who’ve been exposed – get necessary testing and treatment,” explained Dr. Dombrowski. “They’re very similar to patient navigators.”
At the same time, clinicians remain the key providers for annual appointments, new symptoms, STI diagnoses, adverse drug reactions, and missed doses. Licensed medical providers review all labs.
Shifting responsibilities, better PrEP initiation, retention rates
After establishing the PrEP services protocol, the University of Washington team then assessed retention rates among PrEP patients who attended an initial visit (1,387) from October 2014 to December 2019. Follow-up continued through February 2020. (For study purposes, PrEP discontinuation was defined as either stopping PrEP after initiation or as lost to follow-up, i.e., either not attending a follow-up visit or not responding to more than three DIS calls or text messages).
Just over half of the participants were aged 20-29 years, and a third were aged 30-39. More than 9 out of 10 (93%) were men who sleep with men (MSM), 55% White, 26% Hispanic/Latinx, and 10% Black.
Over the course of the study, 6,887 PrEP visits were recorded. Quarterly visits increased concurrently with the program expansion, from 31 visits in 2014 to 623 in the fourth quarter (Q4) of 2019. Likewise, while 57% of visits overall were with a clinician, DIS visits increased from 3% in Q4 of 2014 to 45% in Q4 of 2019, an increase of 1,400% in 5 years.
Significant numbers of patients also initiated PrEP in the clinic, especially when prescribing practices were expanded to be part of routine, walk-in visits.
Retention rates also improved, with 43% (510/1,190) of patients still on PrEP at the end of the analysis period. Forty-one percent (490) discontinued PrEP, 21% within 3 months of initiation, and 72% within a year; another 16% moved, transferred care, or tested positive, and were considered “censored.” However, as of July 31, 2021, 54% (265) of the 490 patients who had discontinued PrEP returned to the clinic for a restart visit, 93% of whom refilled their restart prescription.
“This is really basic preventative care and is actually quite easy to do,” noted Sarah Schmalzle, MD, assistant professor of medicine and medical director of the Thrive Program at the Institute of Human Virology at the University of Maryland, Baltimore. Dr. Schmalzle was not involved in the study.
Dr. Schmalzle practices in inner-city Baltimore, so she and her colleagues have been thorough in terms of setting up PrEP (and postexposure prophylaxis, PEP) programs to ensure that patients access PrEP wherever they want to. But she also said that PrEP is only a part of the sexual wellness and prevention toolbox, and ideally, part of a whole prevention program.
“Focusing on how to get the prescription out is great but the rest is having ongoing and accurate sexual health conversations, healthy conversations about sex and prevention, to have [an] algorithm in place that says, ‘Here’s your PrEP, this is the next time that you need an appointment, the next time you need labs, I’m going to check your adherence, etc.’ ”
Both Dr. Dombrowski and Dr. Schmalzle emphasized that decentralization is not a one-size model; flexibility is key, especially when it comes to who is providing PrEP
“People overcomplicate PrEP and clinicians do this too,” said Dr. Dombrowski. “If we are going to successfully increase PrEP and improve the patient experience, we need to decrease the requirement for clinician involvement.”
Dr. Dombrowski has disclosed no relevant financial relationships. Dr. Schmalzle receives grant funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases climb slowly but steadily
The current sustained increase in COVID-19 has brought the total number of cases in children to over 13 million since the start of the pandemic, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, when cases dropped to their lowest point since last summer. The cumulative number of cases in children is 13,052,988, which accounts for 19.0% of all cases reported in the United States, the AAP and CHA said in their weekly COVID-19 report.
Other measures of incidence show the same steady rise. The rate of new admissions of children aged 0-17 with confirmed COVID-19, which had dipped as low as 0.13 per 100,000 population on April 11, was up to 0.19 per 100,000 on May 6, and the 7-day average for total admissions was 136 per day for May 1-7, compared with 118 for the last week of April, according to the Centers for Disease Control and Prevention.
At the state level, new admission rates for May 6 show wide variation, even regionally. Rhode Island came in with a 0.00 per 100,000 on that day, while Vermont recorded 0.88 admissions per 100,000, the highest of any state and lower only than the District of Columbia’s 1.23 per 100,000. Connecticut (0.45) and Massachusetts (0.33) also were in the highest group (see map), while Maine was in the lowest, CDC data show.
Nationally, emergency department visits also have been rising over the last month or so. Children aged 0-11 years, who were down to a 7-day average of 0.5% of ED visits with diagnosed COVID-19 in early April, saw that number rise to 1.4% on May 5. Children aged 12-15 years went from a rate of 0.3% in late March to the current 1.2%, as did 16- to 17-year-olds, the CDC said on its COVID Data Tracker.
The vaccination effort, meanwhile, continues to lose steam, at least among children who are currently eligible. Initial vaccinations in those aged 5-11 slipped to their lowest-ever 1-week total, 47,000 for April 28 to May 4, while children aged 16-17 continued a long-term slide that has the weekly count down to just 29,000, the AAP said in its weekly vaccination report.
Here’s how those latest recipients changed the populations of vaccinated children in the last week: 35.4% of all 5- to 11-year-olds had received at least one dose as of May 4, compared with 35.3% on April 27, with increases from 67.4% to 67.5% for 12- to 15-year-olds and 72.7% to 72.8% among those aged 16-17, the CDC reported.
The current sustained increase in COVID-19 has brought the total number of cases in children to over 13 million since the start of the pandemic, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, when cases dropped to their lowest point since last summer. The cumulative number of cases in children is 13,052,988, which accounts for 19.0% of all cases reported in the United States, the AAP and CHA said in their weekly COVID-19 report.
Other measures of incidence show the same steady rise. The rate of new admissions of children aged 0-17 with confirmed COVID-19, which had dipped as low as 0.13 per 100,000 population on April 11, was up to 0.19 per 100,000 on May 6, and the 7-day average for total admissions was 136 per day for May 1-7, compared with 118 for the last week of April, according to the Centers for Disease Control and Prevention.
At the state level, new admission rates for May 6 show wide variation, even regionally. Rhode Island came in with a 0.00 per 100,000 on that day, while Vermont recorded 0.88 admissions per 100,000, the highest of any state and lower only than the District of Columbia’s 1.23 per 100,000. Connecticut (0.45) and Massachusetts (0.33) also were in the highest group (see map), while Maine was in the lowest, CDC data show.
Nationally, emergency department visits also have been rising over the last month or so. Children aged 0-11 years, who were down to a 7-day average of 0.5% of ED visits with diagnosed COVID-19 in early April, saw that number rise to 1.4% on May 5. Children aged 12-15 years went from a rate of 0.3% in late March to the current 1.2%, as did 16- to 17-year-olds, the CDC said on its COVID Data Tracker.
The vaccination effort, meanwhile, continues to lose steam, at least among children who are currently eligible. Initial vaccinations in those aged 5-11 slipped to their lowest-ever 1-week total, 47,000 for April 28 to May 4, while children aged 16-17 continued a long-term slide that has the weekly count down to just 29,000, the AAP said in its weekly vaccination report.
Here’s how those latest recipients changed the populations of vaccinated children in the last week: 35.4% of all 5- to 11-year-olds had received at least one dose as of May 4, compared with 35.3% on April 27, with increases from 67.4% to 67.5% for 12- to 15-year-olds and 72.7% to 72.8% among those aged 16-17, the CDC reported.
The current sustained increase in COVID-19 has brought the total number of cases in children to over 13 million since the start of the pandemic, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, when cases dropped to their lowest point since last summer. The cumulative number of cases in children is 13,052,988, which accounts for 19.0% of all cases reported in the United States, the AAP and CHA said in their weekly COVID-19 report.
Other measures of incidence show the same steady rise. The rate of new admissions of children aged 0-17 with confirmed COVID-19, which had dipped as low as 0.13 per 100,000 population on April 11, was up to 0.19 per 100,000 on May 6, and the 7-day average for total admissions was 136 per day for May 1-7, compared with 118 for the last week of April, according to the Centers for Disease Control and Prevention.
At the state level, new admission rates for May 6 show wide variation, even regionally. Rhode Island came in with a 0.00 per 100,000 on that day, while Vermont recorded 0.88 admissions per 100,000, the highest of any state and lower only than the District of Columbia’s 1.23 per 100,000. Connecticut (0.45) and Massachusetts (0.33) also were in the highest group (see map), while Maine was in the lowest, CDC data show.
Nationally, emergency department visits also have been rising over the last month or so. Children aged 0-11 years, who were down to a 7-day average of 0.5% of ED visits with diagnosed COVID-19 in early April, saw that number rise to 1.4% on May 5. Children aged 12-15 years went from a rate of 0.3% in late March to the current 1.2%, as did 16- to 17-year-olds, the CDC said on its COVID Data Tracker.
The vaccination effort, meanwhile, continues to lose steam, at least among children who are currently eligible. Initial vaccinations in those aged 5-11 slipped to their lowest-ever 1-week total, 47,000 for April 28 to May 4, while children aged 16-17 continued a long-term slide that has the weekly count down to just 29,000, the AAP said in its weekly vaccination report.
Here’s how those latest recipients changed the populations of vaccinated children in the last week: 35.4% of all 5- to 11-year-olds had received at least one dose as of May 4, compared with 35.3% on April 27, with increases from 67.4% to 67.5% for 12- to 15-year-olds and 72.7% to 72.8% among those aged 16-17, the CDC reported.
COVID fallout: ‘Alarming’ dip in routine vax for pregnant women
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
FROM ACOG 2022
USPSTF recommendation roundup
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2
In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.
Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11
For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2
In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.
Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11
For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2
In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.
Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11
For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.