User login
Ukraine war likely to cause infection outbreaks that will spread beyond borders
Every day we see stark images of the war in Ukraine – bombed-out buildings, explosions, and bodies lying in the streets. But there’s another, less visible war against the bacteria and viruses that are gathering their forces together. They, too, will infect parts of the population and may spread throughout Europe. Here’s what Ukrainians, and their neighbors, are facing on the infectious disease front.
Andrey Zinchuk, MD, MHS, a pulmonary/critical care physician at Yale and a native of Ukraine who immigrated to the U.S. at the age of 14 with his family, set the background for understanding this crisis. He said that TB and HIV rates in Ukraine have long been especially high, even before the current conflict: “Part of the challenge of the health care system in Ukraine is that it’s difficult to maintain a steady policy because of political instability,” he said. “We’ve had three revolutions in the last 20 years,” not counting the current Russian invasion.
The first was the breakup of the Soviet Union, which led to “an epidemic of people with HIV, hepatitis, and opioid use.” Next was the Orange Revolution in 2004 over fraud during a presidential election. In 2014 came the Maiden Revolution, after the government chose closer ties to Russia rather than Europe. Then-president Viktor Yanukovych fled to Russia.
“That’s when Russia annexed Crimea. There was essentially infiltration in Russian propaganda in the east of the country,” Dr. Zinchuk said. “This helped the Russians manufacture uprisings there to create a separatist state (the Luhansk and Donetsk People’s Republics) which were mostly Russian-speaking parts of the country,” an area known as the Donbas. This resulted in a war in eastern Ukraine that began 2014, with more than 10,000 deaths.
After the 2014 revolution, Dr. Zinchuk said, “There was a tremendous change in the way ... medical care was provided, and tremendous growth and stability in the medical supply for those chronic medical conditions.”
Nevertheless, health care expenditures in Ukraine have been quite low. Even before the current conflict, Dr. Zinchuk noted, annual health care expenditures in Ukraine were about $600 per capita. In comparison, it’s about $4,500 per person in Germany and $12,530 in the United States.
Despite those low per-capita expenditures in Ukraine, access to medicines – such as insulin for diabetes and antibiotics for tuberculosis – was stable before the war. But now, Dr. Zinchuk said, his aunt and uncle have had to flee Kyiv for the countryside and, while safe, they have “no plumbing and have to heat the house by burning firewood.” More significantly, their supply of medicine is unstable.
Asked what infections are of most immediate concern, Sten Vermund, MD, PhD, Dean of the Yale School of Public Health, told this news organization that it was “diarrheal diseases, especially in kids ... The water supply [of Mariupol] is no longer potable, but people are drinking it anyway. And sewage systems are destroyed, and raw sewage is just released into the rivers and streams. So the whole family of diarrheal diseases and war are bedfellows. So are respiratory diseases, whenever we have mass migrations and mixing of ... homeless people and transients.”
There is one notable piece of good news that may reduce the spread of infectious diseases. Unlike the aftermath of World War II or the ongoing conflicts in the Middle East, Africa, and South Asia, refugees from the war in Ukraine are being taken into individual households throughout Poland, Germany, and other countries and are not being held in large displaced-persons camps. Dr. Vermund added, “The Syrian refugee camps in Lebanon are just tent camps with a million, 2 million people in them ... In theory, what the Poles are doing is a good thing from the point of view of preventing the spread of infection.”
One way of examining infections in war zones is by considering them based on how they are spread.
Respiratory infections
Although not as high on the list of concerns as TB or HIV, COVID-19 remains a big problem for infectious disease experts. Last fall, Ukraine ranked just behind the U.S. and Russia in deaths from COVID and in the top 10 in infections. Despite these dismal numbers, only 35% of people had completed the initial vaccination series.
The same conditions that fuel TB and COVID – crowding, especially in poorly ventilated settings – could lead to another measles outbreak. One occurred in Ukraine from 2017-2020, resulting in more than 115,000 cases. Even though the immunization rate for measles has now reached about 80%, the CDC considers Ukraine at high risk for another large outbreak since measles is so highly contagious.
According to the European Centre for Disease Prevention and Control (ECDC), Ukraine reported the second-highest number of TB cases in Europe (28,539). It is also one of the top 10 countries globally with the highest burden of multidrug-resistant tuberculosis (MDR-TB) – 27%. Equally disturbing is its ranking as having the second-highest rate of HIV/TB co-infection (26%) even before the war. Experts say war is a perfect breeding ground for TB, since starvation and overcrowding in poorly ventilated spaces encourages its spread.
Before the war, COVID had already caused severe disruptions in TB diagnosis and treatment access in Ukraine, and the World Health Organization suggested that the pandemic has set back efforts to end TB by more than a decade.
Drug-resistant TB has been one of the biggest worries. In their report on TB in Ukraine, British tuberculosis experts Tom Wingfield, MBChB, PhD, from the Liverpool School of Tropical Medicine, and Jessica Potter MBBCh, PhD, from Queen Mary University of London, pointed out that “drug resistance thrives on fractured health systems and sporadic medicine supply.”
Frederick Altice, MD, a Yale epidemiologist and addiction specialist, noted, “[if] medication for tuberculosis is discontinued, that not only causes potential recurrence of disease but multidrug-resistant TB disease,” and patients could become infectious again.
Dr. Wingfield expressed concern that people will not seek care because they see it as unaffordable, although he told this news organization that he’s impressed at the Polish government’s efforts to ensure care. Especially with the triad of HIV, TB, and opioid use, Dr. Wingfield and Dr. Potter emphasized that these problems reflect the social determinants of health – “the experiences and conditions in which people live.” These medical conditions are all quite treatable with support, and once treated they pose no risk to others.
HIV and opioid use
Before the war, an estimated 260,000 people were living with HIV in Ukraine. Their rate of new HIV diagnoses in 2017 was second highest in the world – 37 out of every 100,000, exceeded only by Russia, with 71 out of 100,000.
Dr. Vermund told this news organization that “when Crimea was seized by the Russians in 2014, there was an immediate crisis among injection drug users who were in drug treatment programs, because it’s illegal in Russia to use buprenorphine or methadone ... So immediately, those programs were shut down, and all the drug users who were holding jobs, supporting their families, were withdrawing from their addictions and searching for a replacement, which was illegal heroin.”
Dr. Altice added that of 800 patients in the region who had to go cold turkey, “ten percent were dead within 6 months. Dependent on unreliable street drugs, some overdosed or committed suicide because they could not get treatment. They went through terrible withdrawal and stress.”
And as they relapsed, the HIV rate soared. “Fifty percent of the methadone patients have got HIV,” Dr. Altice said, “and if they stop taking the methadone, they’re going to stop taking their HIV medications as well. Their lives will become chaotic and very destabilized.”
This experience may soon repeat itself. There were two methadone factories in Ukraine – in Odessa and Kharkiv – that are now shut down by the war. Although there are efforts to import methadone and many other drugs, supply chain issues are “devastating,” Dr. Altice said. “If their medication for tuberculosis is discontinued, that not only causes potential recurrence of disease but multidrug-resistant TB disease,” and they could become infectious again. “[With a] lack of medication, lack of sterile syringes, people will be sharing syringes; they’ll be desperate. So as the desperation level goes up, the risk environment goes up, so that people have decreased opportunities to protect themselves,” and there will be an explosion in HIV.
Dr. Altice observed that with the immigration to Poland and the west, many Ukrainian refugees “are relying on the kindness of strangers.” They are likely to be “fearful to disclose either their HIV or their TB treatment status,” being afraid of being regarded as modern-day lepers, even though they are likely not infectious. Both Dr. Altice and Dr. Potter emphasized the need for the governments of Poland and other receiving countries to provide the refugees with “reassurance that their health information will not be shared with others.” Dr. Altice emphasized that “this is one of the things that I would say that these other countries have to get right.”
Dr. Potter echoed that, noting that extraordinary care needs to be taken so that shared information is not used for deportation.
When refugees are housed with rural hosts, transportation problems sometimes arise, creating major barriers to accessing care and treatment. In particular, refugees with TB, HIV, and addiction who are placed in small, remote locations may have difficulty securing transportation to sites where treatments for their complex illnesses are available, including specialists and medications.
Ukrainian-born microbiologist Olena Rzhepishevska, PhD, of Umeå University in Sweden, said in an interview that a network of European TB researchers have developed a database on TBNet where patients with TB can be specifically placed with understanding and helpful hosts outside of Ukraine. They can receive housing and medication through this network.
So far, 4 million Ukrainians have fled the country and millions more have been displaced internally. Dr. Altice noted that there is an “increased vulnerability beyond the vulnerability that they already [have] just by being a refugee” that we generally don’t recognize. Additionally, Poland and Hungary are not very progressive about methadone therapy nor are those nations well-equipped to provide it.
Dr. Altice explained that even within Ukraine, those who want to move to better their chance of getting their methadone are then at risk of being conscripted. He spoke of the grave calculations men must make, choosing to become internally displaced and risk conscription or losing life-saving methadone or medicines for HIV or TB.
One other unfortunate consequence of war might be a spike in rape, sexual abuse, prostitution, unwanted pregnancies, HIV, and sexually transmitted infections.
There were an estimated 80,100 female sex workers in Ukraine in 2016, with 5.2% HIV positive. In times of war, with no home or income, some women turn to prostitution to survive. Others are victims of sex trafficking, both within Ukraine and as refugees. The Russian invasion increased the risks of a surge in HIV infections, unwanted pregnancies, and abortions. Women who find themselves pregnant due to rape (a common tool of war) or sex trafficking may also struggle to access safe abortions. Poland, for example, has severe restrictions on abortion, and Ukrainian women may turn to unsafe, back-alley abortions, with their resulting high risk of infection.
Waterborne infections
Another concern involves waterborne infections. In addition to the common diarrheal diseases such as E coli, which can be expected from poor sanitation, polio is a significant concern. In the fall of 2021, Ukraine had an outbreak of vaccine-derived polio, with two cases of paralysis and 20 additional cases. As polio only paralyzes 1 person in 200 of those infected, many other cases were likely undetected. A vaccination campaign was just beginning when the war began.
Wound infections and antimicrobial resistance
The ECDC also reports high rates of antimicrobial resistance (AMR) in Ukraine, particularly involving common gram-negative bacteria, including Escherichia coli (53% resistance to third-generation cephalosporins), Klebsiella pneumoniae (54% resistance to carbapenems), and Acinetobacter spp. (77% resistance to carbapenems). Because of this, they recommend refugees requiring hospital admission be isolated on admission and screened for AMR. These AMR often complicate traumatic injuries of war.
Prevention
Many of these potential problems stemming from the war in Ukraine and the displacement of millions of its citizens can be avoided.
Attempts are being made to immunize refugees. WHO has made working with countries receiving refugees a priority, particularly by vaccinating children against measles, rubella, and COVID. The European Union has also purchased vaccines for polio and tuberculosis.
But Russia has waged an active anti-vaccine campaign against COVID in Ukraine, while at the same time advocating for vaccines in Russia. According to UNICEF, other countries with relatively low vaccination rates and high vaccine skepticism – Moldova, Romania, and Bulgaria – are at higher risk of polio and measles than those with high vaccination levels.
The continuing war in Ukraine has exacerbated the medical challenges the citizens of Ukraine face at home and as refugees fleeing to neighboring countries. Improving communication among agencies and governments and building trust with the refugees could go a long way toward limiting the spread of preventable infectious diseases as a result of the war.
Continuing to try to keep supply chains open within Ukraine and ensuring adequate supplies of medications and vaccines to refugees will also be essential. But, of course, the better solution is to end the war.
Dr. Altice, Dr. Potter, Dr. Wingfield, Dr. Vermund, and Dr. Zinchuk all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Every day we see stark images of the war in Ukraine – bombed-out buildings, explosions, and bodies lying in the streets. But there’s another, less visible war against the bacteria and viruses that are gathering their forces together. They, too, will infect parts of the population and may spread throughout Europe. Here’s what Ukrainians, and their neighbors, are facing on the infectious disease front.
Andrey Zinchuk, MD, MHS, a pulmonary/critical care physician at Yale and a native of Ukraine who immigrated to the U.S. at the age of 14 with his family, set the background for understanding this crisis. He said that TB and HIV rates in Ukraine have long been especially high, even before the current conflict: “Part of the challenge of the health care system in Ukraine is that it’s difficult to maintain a steady policy because of political instability,” he said. “We’ve had three revolutions in the last 20 years,” not counting the current Russian invasion.
The first was the breakup of the Soviet Union, which led to “an epidemic of people with HIV, hepatitis, and opioid use.” Next was the Orange Revolution in 2004 over fraud during a presidential election. In 2014 came the Maiden Revolution, after the government chose closer ties to Russia rather than Europe. Then-president Viktor Yanukovych fled to Russia.
“That’s when Russia annexed Crimea. There was essentially infiltration in Russian propaganda in the east of the country,” Dr. Zinchuk said. “This helped the Russians manufacture uprisings there to create a separatist state (the Luhansk and Donetsk People’s Republics) which were mostly Russian-speaking parts of the country,” an area known as the Donbas. This resulted in a war in eastern Ukraine that began 2014, with more than 10,000 deaths.
After the 2014 revolution, Dr. Zinchuk said, “There was a tremendous change in the way ... medical care was provided, and tremendous growth and stability in the medical supply for those chronic medical conditions.”
Nevertheless, health care expenditures in Ukraine have been quite low. Even before the current conflict, Dr. Zinchuk noted, annual health care expenditures in Ukraine were about $600 per capita. In comparison, it’s about $4,500 per person in Germany and $12,530 in the United States.
Despite those low per-capita expenditures in Ukraine, access to medicines – such as insulin for diabetes and antibiotics for tuberculosis – was stable before the war. But now, Dr. Zinchuk said, his aunt and uncle have had to flee Kyiv for the countryside and, while safe, they have “no plumbing and have to heat the house by burning firewood.” More significantly, their supply of medicine is unstable.
Asked what infections are of most immediate concern, Sten Vermund, MD, PhD, Dean of the Yale School of Public Health, told this news organization that it was “diarrheal diseases, especially in kids ... The water supply [of Mariupol] is no longer potable, but people are drinking it anyway. And sewage systems are destroyed, and raw sewage is just released into the rivers and streams. So the whole family of diarrheal diseases and war are bedfellows. So are respiratory diseases, whenever we have mass migrations and mixing of ... homeless people and transients.”
There is one notable piece of good news that may reduce the spread of infectious diseases. Unlike the aftermath of World War II or the ongoing conflicts in the Middle East, Africa, and South Asia, refugees from the war in Ukraine are being taken into individual households throughout Poland, Germany, and other countries and are not being held in large displaced-persons camps. Dr. Vermund added, “The Syrian refugee camps in Lebanon are just tent camps with a million, 2 million people in them ... In theory, what the Poles are doing is a good thing from the point of view of preventing the spread of infection.”
One way of examining infections in war zones is by considering them based on how they are spread.
Respiratory infections
Although not as high on the list of concerns as TB or HIV, COVID-19 remains a big problem for infectious disease experts. Last fall, Ukraine ranked just behind the U.S. and Russia in deaths from COVID and in the top 10 in infections. Despite these dismal numbers, only 35% of people had completed the initial vaccination series.
The same conditions that fuel TB and COVID – crowding, especially in poorly ventilated settings – could lead to another measles outbreak. One occurred in Ukraine from 2017-2020, resulting in more than 115,000 cases. Even though the immunization rate for measles has now reached about 80%, the CDC considers Ukraine at high risk for another large outbreak since measles is so highly contagious.
According to the European Centre for Disease Prevention and Control (ECDC), Ukraine reported the second-highest number of TB cases in Europe (28,539). It is also one of the top 10 countries globally with the highest burden of multidrug-resistant tuberculosis (MDR-TB) – 27%. Equally disturbing is its ranking as having the second-highest rate of HIV/TB co-infection (26%) even before the war. Experts say war is a perfect breeding ground for TB, since starvation and overcrowding in poorly ventilated spaces encourages its spread.
Before the war, COVID had already caused severe disruptions in TB diagnosis and treatment access in Ukraine, and the World Health Organization suggested that the pandemic has set back efforts to end TB by more than a decade.
Drug-resistant TB has been one of the biggest worries. In their report on TB in Ukraine, British tuberculosis experts Tom Wingfield, MBChB, PhD, from the Liverpool School of Tropical Medicine, and Jessica Potter MBBCh, PhD, from Queen Mary University of London, pointed out that “drug resistance thrives on fractured health systems and sporadic medicine supply.”
Frederick Altice, MD, a Yale epidemiologist and addiction specialist, noted, “[if] medication for tuberculosis is discontinued, that not only causes potential recurrence of disease but multidrug-resistant TB disease,” and patients could become infectious again.
Dr. Wingfield expressed concern that people will not seek care because they see it as unaffordable, although he told this news organization that he’s impressed at the Polish government’s efforts to ensure care. Especially with the triad of HIV, TB, and opioid use, Dr. Wingfield and Dr. Potter emphasized that these problems reflect the social determinants of health – “the experiences and conditions in which people live.” These medical conditions are all quite treatable with support, and once treated they pose no risk to others.
HIV and opioid use
Before the war, an estimated 260,000 people were living with HIV in Ukraine. Their rate of new HIV diagnoses in 2017 was second highest in the world – 37 out of every 100,000, exceeded only by Russia, with 71 out of 100,000.
Dr. Vermund told this news organization that “when Crimea was seized by the Russians in 2014, there was an immediate crisis among injection drug users who were in drug treatment programs, because it’s illegal in Russia to use buprenorphine or methadone ... So immediately, those programs were shut down, and all the drug users who were holding jobs, supporting their families, were withdrawing from their addictions and searching for a replacement, which was illegal heroin.”
Dr. Altice added that of 800 patients in the region who had to go cold turkey, “ten percent were dead within 6 months. Dependent on unreliable street drugs, some overdosed or committed suicide because they could not get treatment. They went through terrible withdrawal and stress.”
And as they relapsed, the HIV rate soared. “Fifty percent of the methadone patients have got HIV,” Dr. Altice said, “and if they stop taking the methadone, they’re going to stop taking their HIV medications as well. Their lives will become chaotic and very destabilized.”
This experience may soon repeat itself. There were two methadone factories in Ukraine – in Odessa and Kharkiv – that are now shut down by the war. Although there are efforts to import methadone and many other drugs, supply chain issues are “devastating,” Dr. Altice said. “If their medication for tuberculosis is discontinued, that not only causes potential recurrence of disease but multidrug-resistant TB disease,” and they could become infectious again. “[With a] lack of medication, lack of sterile syringes, people will be sharing syringes; they’ll be desperate. So as the desperation level goes up, the risk environment goes up, so that people have decreased opportunities to protect themselves,” and there will be an explosion in HIV.
Dr. Altice observed that with the immigration to Poland and the west, many Ukrainian refugees “are relying on the kindness of strangers.” They are likely to be “fearful to disclose either their HIV or their TB treatment status,” being afraid of being regarded as modern-day lepers, even though they are likely not infectious. Both Dr. Altice and Dr. Potter emphasized the need for the governments of Poland and other receiving countries to provide the refugees with “reassurance that their health information will not be shared with others.” Dr. Altice emphasized that “this is one of the things that I would say that these other countries have to get right.”
Dr. Potter echoed that, noting that extraordinary care needs to be taken so that shared information is not used for deportation.
When refugees are housed with rural hosts, transportation problems sometimes arise, creating major barriers to accessing care and treatment. In particular, refugees with TB, HIV, and addiction who are placed in small, remote locations may have difficulty securing transportation to sites where treatments for their complex illnesses are available, including specialists and medications.
Ukrainian-born microbiologist Olena Rzhepishevska, PhD, of Umeå University in Sweden, said in an interview that a network of European TB researchers have developed a database on TBNet where patients with TB can be specifically placed with understanding and helpful hosts outside of Ukraine. They can receive housing and medication through this network.
So far, 4 million Ukrainians have fled the country and millions more have been displaced internally. Dr. Altice noted that there is an “increased vulnerability beyond the vulnerability that they already [have] just by being a refugee” that we generally don’t recognize. Additionally, Poland and Hungary are not very progressive about methadone therapy nor are those nations well-equipped to provide it.
Dr. Altice explained that even within Ukraine, those who want to move to better their chance of getting their methadone are then at risk of being conscripted. He spoke of the grave calculations men must make, choosing to become internally displaced and risk conscription or losing life-saving methadone or medicines for HIV or TB.
One other unfortunate consequence of war might be a spike in rape, sexual abuse, prostitution, unwanted pregnancies, HIV, and sexually transmitted infections.
There were an estimated 80,100 female sex workers in Ukraine in 2016, with 5.2% HIV positive. In times of war, with no home or income, some women turn to prostitution to survive. Others are victims of sex trafficking, both within Ukraine and as refugees. The Russian invasion increased the risks of a surge in HIV infections, unwanted pregnancies, and abortions. Women who find themselves pregnant due to rape (a common tool of war) or sex trafficking may also struggle to access safe abortions. Poland, for example, has severe restrictions on abortion, and Ukrainian women may turn to unsafe, back-alley abortions, with their resulting high risk of infection.
Waterborne infections
Another concern involves waterborne infections. In addition to the common diarrheal diseases such as E coli, which can be expected from poor sanitation, polio is a significant concern. In the fall of 2021, Ukraine had an outbreak of vaccine-derived polio, with two cases of paralysis and 20 additional cases. As polio only paralyzes 1 person in 200 of those infected, many other cases were likely undetected. A vaccination campaign was just beginning when the war began.
Wound infections and antimicrobial resistance
The ECDC also reports high rates of antimicrobial resistance (AMR) in Ukraine, particularly involving common gram-negative bacteria, including Escherichia coli (53% resistance to third-generation cephalosporins), Klebsiella pneumoniae (54% resistance to carbapenems), and Acinetobacter spp. (77% resistance to carbapenems). Because of this, they recommend refugees requiring hospital admission be isolated on admission and screened for AMR. These AMR often complicate traumatic injuries of war.
Prevention
Many of these potential problems stemming from the war in Ukraine and the displacement of millions of its citizens can be avoided.
Attempts are being made to immunize refugees. WHO has made working with countries receiving refugees a priority, particularly by vaccinating children against measles, rubella, and COVID. The European Union has also purchased vaccines for polio and tuberculosis.
But Russia has waged an active anti-vaccine campaign against COVID in Ukraine, while at the same time advocating for vaccines in Russia. According to UNICEF, other countries with relatively low vaccination rates and high vaccine skepticism – Moldova, Romania, and Bulgaria – are at higher risk of polio and measles than those with high vaccination levels.
The continuing war in Ukraine has exacerbated the medical challenges the citizens of Ukraine face at home and as refugees fleeing to neighboring countries. Improving communication among agencies and governments and building trust with the refugees could go a long way toward limiting the spread of preventable infectious diseases as a result of the war.
Continuing to try to keep supply chains open within Ukraine and ensuring adequate supplies of medications and vaccines to refugees will also be essential. But, of course, the better solution is to end the war.
Dr. Altice, Dr. Potter, Dr. Wingfield, Dr. Vermund, and Dr. Zinchuk all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Every day we see stark images of the war in Ukraine – bombed-out buildings, explosions, and bodies lying in the streets. But there’s another, less visible war against the bacteria and viruses that are gathering their forces together. They, too, will infect parts of the population and may spread throughout Europe. Here’s what Ukrainians, and their neighbors, are facing on the infectious disease front.
Andrey Zinchuk, MD, MHS, a pulmonary/critical care physician at Yale and a native of Ukraine who immigrated to the U.S. at the age of 14 with his family, set the background for understanding this crisis. He said that TB and HIV rates in Ukraine have long been especially high, even before the current conflict: “Part of the challenge of the health care system in Ukraine is that it’s difficult to maintain a steady policy because of political instability,” he said. “We’ve had three revolutions in the last 20 years,” not counting the current Russian invasion.
The first was the breakup of the Soviet Union, which led to “an epidemic of people with HIV, hepatitis, and opioid use.” Next was the Orange Revolution in 2004 over fraud during a presidential election. In 2014 came the Maiden Revolution, after the government chose closer ties to Russia rather than Europe. Then-president Viktor Yanukovych fled to Russia.
“That’s when Russia annexed Crimea. There was essentially infiltration in Russian propaganda in the east of the country,” Dr. Zinchuk said. “This helped the Russians manufacture uprisings there to create a separatist state (the Luhansk and Donetsk People’s Republics) which were mostly Russian-speaking parts of the country,” an area known as the Donbas. This resulted in a war in eastern Ukraine that began 2014, with more than 10,000 deaths.
After the 2014 revolution, Dr. Zinchuk said, “There was a tremendous change in the way ... medical care was provided, and tremendous growth and stability in the medical supply for those chronic medical conditions.”
Nevertheless, health care expenditures in Ukraine have been quite low. Even before the current conflict, Dr. Zinchuk noted, annual health care expenditures in Ukraine were about $600 per capita. In comparison, it’s about $4,500 per person in Germany and $12,530 in the United States.
Despite those low per-capita expenditures in Ukraine, access to medicines – such as insulin for diabetes and antibiotics for tuberculosis – was stable before the war. But now, Dr. Zinchuk said, his aunt and uncle have had to flee Kyiv for the countryside and, while safe, they have “no plumbing and have to heat the house by burning firewood.” More significantly, their supply of medicine is unstable.
Asked what infections are of most immediate concern, Sten Vermund, MD, PhD, Dean of the Yale School of Public Health, told this news organization that it was “diarrheal diseases, especially in kids ... The water supply [of Mariupol] is no longer potable, but people are drinking it anyway. And sewage systems are destroyed, and raw sewage is just released into the rivers and streams. So the whole family of diarrheal diseases and war are bedfellows. So are respiratory diseases, whenever we have mass migrations and mixing of ... homeless people and transients.”
There is one notable piece of good news that may reduce the spread of infectious diseases. Unlike the aftermath of World War II or the ongoing conflicts in the Middle East, Africa, and South Asia, refugees from the war in Ukraine are being taken into individual households throughout Poland, Germany, and other countries and are not being held in large displaced-persons camps. Dr. Vermund added, “The Syrian refugee camps in Lebanon are just tent camps with a million, 2 million people in them ... In theory, what the Poles are doing is a good thing from the point of view of preventing the spread of infection.”
One way of examining infections in war zones is by considering them based on how they are spread.
Respiratory infections
Although not as high on the list of concerns as TB or HIV, COVID-19 remains a big problem for infectious disease experts. Last fall, Ukraine ranked just behind the U.S. and Russia in deaths from COVID and in the top 10 in infections. Despite these dismal numbers, only 35% of people had completed the initial vaccination series.
The same conditions that fuel TB and COVID – crowding, especially in poorly ventilated settings – could lead to another measles outbreak. One occurred in Ukraine from 2017-2020, resulting in more than 115,000 cases. Even though the immunization rate for measles has now reached about 80%, the CDC considers Ukraine at high risk for another large outbreak since measles is so highly contagious.
According to the European Centre for Disease Prevention and Control (ECDC), Ukraine reported the second-highest number of TB cases in Europe (28,539). It is also one of the top 10 countries globally with the highest burden of multidrug-resistant tuberculosis (MDR-TB) – 27%. Equally disturbing is its ranking as having the second-highest rate of HIV/TB co-infection (26%) even before the war. Experts say war is a perfect breeding ground for TB, since starvation and overcrowding in poorly ventilated spaces encourages its spread.
Before the war, COVID had already caused severe disruptions in TB diagnosis and treatment access in Ukraine, and the World Health Organization suggested that the pandemic has set back efforts to end TB by more than a decade.
Drug-resistant TB has been one of the biggest worries. In their report on TB in Ukraine, British tuberculosis experts Tom Wingfield, MBChB, PhD, from the Liverpool School of Tropical Medicine, and Jessica Potter MBBCh, PhD, from Queen Mary University of London, pointed out that “drug resistance thrives on fractured health systems and sporadic medicine supply.”
Frederick Altice, MD, a Yale epidemiologist and addiction specialist, noted, “[if] medication for tuberculosis is discontinued, that not only causes potential recurrence of disease but multidrug-resistant TB disease,” and patients could become infectious again.
Dr. Wingfield expressed concern that people will not seek care because they see it as unaffordable, although he told this news organization that he’s impressed at the Polish government’s efforts to ensure care. Especially with the triad of HIV, TB, and opioid use, Dr. Wingfield and Dr. Potter emphasized that these problems reflect the social determinants of health – “the experiences and conditions in which people live.” These medical conditions are all quite treatable with support, and once treated they pose no risk to others.
HIV and opioid use
Before the war, an estimated 260,000 people were living with HIV in Ukraine. Their rate of new HIV diagnoses in 2017 was second highest in the world – 37 out of every 100,000, exceeded only by Russia, with 71 out of 100,000.
Dr. Vermund told this news organization that “when Crimea was seized by the Russians in 2014, there was an immediate crisis among injection drug users who were in drug treatment programs, because it’s illegal in Russia to use buprenorphine or methadone ... So immediately, those programs were shut down, and all the drug users who were holding jobs, supporting their families, were withdrawing from their addictions and searching for a replacement, which was illegal heroin.”
Dr. Altice added that of 800 patients in the region who had to go cold turkey, “ten percent were dead within 6 months. Dependent on unreliable street drugs, some overdosed or committed suicide because they could not get treatment. They went through terrible withdrawal and stress.”
And as they relapsed, the HIV rate soared. “Fifty percent of the methadone patients have got HIV,” Dr. Altice said, “and if they stop taking the methadone, they’re going to stop taking their HIV medications as well. Their lives will become chaotic and very destabilized.”
This experience may soon repeat itself. There were two methadone factories in Ukraine – in Odessa and Kharkiv – that are now shut down by the war. Although there are efforts to import methadone and many other drugs, supply chain issues are “devastating,” Dr. Altice said. “If their medication for tuberculosis is discontinued, that not only causes potential recurrence of disease but multidrug-resistant TB disease,” and they could become infectious again. “[With a] lack of medication, lack of sterile syringes, people will be sharing syringes; they’ll be desperate. So as the desperation level goes up, the risk environment goes up, so that people have decreased opportunities to protect themselves,” and there will be an explosion in HIV.
Dr. Altice observed that with the immigration to Poland and the west, many Ukrainian refugees “are relying on the kindness of strangers.” They are likely to be “fearful to disclose either their HIV or their TB treatment status,” being afraid of being regarded as modern-day lepers, even though they are likely not infectious. Both Dr. Altice and Dr. Potter emphasized the need for the governments of Poland and other receiving countries to provide the refugees with “reassurance that their health information will not be shared with others.” Dr. Altice emphasized that “this is one of the things that I would say that these other countries have to get right.”
Dr. Potter echoed that, noting that extraordinary care needs to be taken so that shared information is not used for deportation.
When refugees are housed with rural hosts, transportation problems sometimes arise, creating major barriers to accessing care and treatment. In particular, refugees with TB, HIV, and addiction who are placed in small, remote locations may have difficulty securing transportation to sites where treatments for their complex illnesses are available, including specialists and medications.
Ukrainian-born microbiologist Olena Rzhepishevska, PhD, of Umeå University in Sweden, said in an interview that a network of European TB researchers have developed a database on TBNet where patients with TB can be specifically placed with understanding and helpful hosts outside of Ukraine. They can receive housing and medication through this network.
So far, 4 million Ukrainians have fled the country and millions more have been displaced internally. Dr. Altice noted that there is an “increased vulnerability beyond the vulnerability that they already [have] just by being a refugee” that we generally don’t recognize. Additionally, Poland and Hungary are not very progressive about methadone therapy nor are those nations well-equipped to provide it.
Dr. Altice explained that even within Ukraine, those who want to move to better their chance of getting their methadone are then at risk of being conscripted. He spoke of the grave calculations men must make, choosing to become internally displaced and risk conscription or losing life-saving methadone or medicines for HIV or TB.
One other unfortunate consequence of war might be a spike in rape, sexual abuse, prostitution, unwanted pregnancies, HIV, and sexually transmitted infections.
There were an estimated 80,100 female sex workers in Ukraine in 2016, with 5.2% HIV positive. In times of war, with no home or income, some women turn to prostitution to survive. Others are victims of sex trafficking, both within Ukraine and as refugees. The Russian invasion increased the risks of a surge in HIV infections, unwanted pregnancies, and abortions. Women who find themselves pregnant due to rape (a common tool of war) or sex trafficking may also struggle to access safe abortions. Poland, for example, has severe restrictions on abortion, and Ukrainian women may turn to unsafe, back-alley abortions, with their resulting high risk of infection.
Waterborne infections
Another concern involves waterborne infections. In addition to the common diarrheal diseases such as E coli, which can be expected from poor sanitation, polio is a significant concern. In the fall of 2021, Ukraine had an outbreak of vaccine-derived polio, with two cases of paralysis and 20 additional cases. As polio only paralyzes 1 person in 200 of those infected, many other cases were likely undetected. A vaccination campaign was just beginning when the war began.
Wound infections and antimicrobial resistance
The ECDC also reports high rates of antimicrobial resistance (AMR) in Ukraine, particularly involving common gram-negative bacteria, including Escherichia coli (53% resistance to third-generation cephalosporins), Klebsiella pneumoniae (54% resistance to carbapenems), and Acinetobacter spp. (77% resistance to carbapenems). Because of this, they recommend refugees requiring hospital admission be isolated on admission and screened for AMR. These AMR often complicate traumatic injuries of war.
Prevention
Many of these potential problems stemming from the war in Ukraine and the displacement of millions of its citizens can be avoided.
Attempts are being made to immunize refugees. WHO has made working with countries receiving refugees a priority, particularly by vaccinating children against measles, rubella, and COVID. The European Union has also purchased vaccines for polio and tuberculosis.
But Russia has waged an active anti-vaccine campaign against COVID in Ukraine, while at the same time advocating for vaccines in Russia. According to UNICEF, other countries with relatively low vaccination rates and high vaccine skepticism – Moldova, Romania, and Bulgaria – are at higher risk of polio and measles than those with high vaccination levels.
The continuing war in Ukraine has exacerbated the medical challenges the citizens of Ukraine face at home and as refugees fleeing to neighboring countries. Improving communication among agencies and governments and building trust with the refugees could go a long way toward limiting the spread of preventable infectious diseases as a result of the war.
Continuing to try to keep supply chains open within Ukraine and ensuring adequate supplies of medications and vaccines to refugees will also be essential. But, of course, the better solution is to end the war.
Dr. Altice, Dr. Potter, Dr. Wingfield, Dr. Vermund, and Dr. Zinchuk all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CDC recommends hep B vaccination for most adults
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
It also added that adults aged 60 years or older without known risk factors for hepatitis B may get vaccinated.
The agency earlier recommended the vaccination for all infants and children under the age of 19 years and for adults aged 60 years or older with known risk factors.
The CDC said it wants to expand vaccinations because, after decades of progress, the number of new hepatitis B infections is increasing among adults. Acute hepatitis B infections among adults lead to chronic hepatitis B disease in an estimated 2%-6% of cases, and can result in cirrhosis, liver cancer, and death.
Among adults aged 40-49 years, the rate of cases increased from 1.9 per 100,000 people in 2011 to 2.7 per 100,000 in 2019. Among adults aged 50-59 years, the rate increased during this period from 1.1 to 1.6 per 100,000.
Most adults aren’t vaccinated. Among adults aged 19 years or older, only 30.0% reported that they’d received at least the three recommended doses of the vaccine. The rate was 40.3% for adults aged 19-49 years, and 19.1% for adults aged 50 years or older.
Hepatitis B infection rates are particularly elevated among African Americans.
Even among adults with chronic liver disease, the vaccination rate is only 33.0%. And, among travelers to countries where the virus has been endemic since 1995, only 38.9% were vaccinated.
In a 2018 survey of internal medicine and family physicians, 68% said their patients had not told them about risk factors, making it difficult to assess whether the patients needed the vaccine according to the recommendations at the time. These risk factors include injection drug use, incarceration, and multiple sex partners, experiences the patients may not have been willing to discuss.
CDC researchers calculated that universal adult hepatitis B vaccination would cost $153,000 for every quality-adjusted life-year (QALY) gained. For adults aged 19-59 years, a QALY would cost $117,000 because infections are more prevalent in that age group.
The CDC specified that it intends its new guidelines to prompt physicians to offer the vaccine to adults aged 60 years or older rather than wait for them to request it.
The Food and Drug Administration has approved both three-dose and two-dose hepatitis B vaccines, with evidence showing similar seroprotection and adverse events.
People who have already completed their vaccination or have a history of hepatitis B infection should only receive additional vaccinations in specific cases, as detailed in the CDC’s 2018 recommendations.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Skin reactions to first COVID-19 vaccine don’t justify forgoing second dose
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Pneumococcal pneumonia outcomes worse than those of Legionnaires disease
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Outcomes for patients with bacteremic Streptococcus pneumoniae were significantly worse than those for patients with Legionnaires disease (LD), based on data from 106 individuals.
Reported cases of LD in the United States have increased in recent decades, but they are likely under-reported, wrote Sima Salahie, MD, of Wayne State University School of Medicine, Detroit, and Central Michigan University College of Medicine, Grosse Pointe Woods, and colleagues.
Clinical presentations may be similar for both conditions, but different antimicrobial therapies are needed; therefore, identifying distinguishing factors can promote better management of hospitalized patients, they reported.
In a retrospective case companion study published in the American Journal of the Medical Sciences, the researchers reviewed data from 51 adults with LD and 55 with bacteremic S. pneumoniae pneumonia (SP) who were hospitalized at a single center between 2013 and 2018. Diagnoses were confirmed by laboratory and radiology results. In addition, data were collected on clinical features including body mass index, systolic and diastolic blood pressure, pulse, respiratory rate, and temperature.
Overall, patients with SP were significantly more likely than those with LD to require mechanical ventilation (P = .04), intensive care unit stay (P = .004), and to die (P = .002). Patients with SP also had higher rates of septic shock compared to LD patients, although this difference fell short of statistical significance (49.1% vs. 30.4%; P = .06).
In a multivariate analysis, male sex, diarrhea, higher body mass index, hyponatremia, and lower Charleston Weighted Index of Comorbidity (CWIC) score were significant independent predictors of LD, with odds ratios of 21.6, 4.5, 1.13, 5.6, and 0.61, respectively.
The incidence of LD peaked in summer, while the incidence of SP peaked in the winter, the researchers noted. “Seasonality is a variable that has not always been included in previous scoring systems but should be considered in future modeling,” they said.
“Noteworthy is that LD represented almost as many cases as documented bacteremic pneumococcal pneumonia,” the researchers wrote in their discussion. “This occurred at a time when there was no outbreak of L. pneumophila in our community, and as these were all community acquired, there was no evidence of a nosocomial outbreak in our institution,” they said.
The study findings were limited by several factors, including the possible underestimation of SP because of the requirement for positive blood cultures and the lack of other methods of diagnosing SP, the researchers noted.
“However, the data suggest variables to distinguish LD from SP,” they said. “Establishing reliable clinical and laboratory parameters embedded in a simple diagnostic score that can accurately identify patients with LD may be helpful in aiding physicians’ early diagnosis in distinguishing LD from SP but will need to be defined.”
The study received no outside funding. The researchers disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Polio: Five African countries vaccinating 23 million children
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychotropic med use tied to ‘striking’ post-COVID dementia risk
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN MEDICINE
Cellulitis care costly from misdiagnosis, needless hospitalizations
BOSTON – The cost of care for the more than 14 million cases of cellulitis that occur each year in the United States is in the billions of dollars, but there are multiple opportunities, many involving dermatologists, to dramatically reduce these costs, according to an outline of strategies presented at the American Academy of Dermatology 2022 annual meeting in Boston.
“Cellulitis is misdiagnosed about one-third of the time, and that cost is very high,” reported Jennifer L. Adams, MD, assistant professor of dermatology, University of Nebraska, Omaha. She sees opportunities for dermatological consults to help weed through the many cellulitis mimickers, such as venous insufficiency or psoriasiform drug reactions, to prevent unnecessary admissions and ineffective therapy.
“There is a huge need for diagnostic accuracy as a means to deliver more cost-effective care,” Dr. Adams said.
Solving misdiagnosis is only part of the story. Costs of care are also ramped up by unnecessary hospitalizations. According to Dr. Adams, published criteria to triage emergency room patients with cellulitis to outpatient care are not always followed. In one review, 14% of admitted patients had met the criteria for outpatient treatment.
Cellulitis is a common skin infection that causes redness, swelling, and pain in the infected area, most often on the legs and feet.
Unnecessary hospitalizations for misdiagnosed cellulitis, which is associated with an average 4-day hospital stay, “range from $200 million to $500 million in avoidable direct healthcare costs,” Dr. Adams said.
Even for justifiable hospitalizations, there are still opportunities for cost savings. In one study, blood cultures were ordered in 73% of patients even though only 2% produced a finding relevant to care. According to Dr. Adams, most cellulitis cases are caused by the “usual suspects” – group A beta-hemolytic streptococcus, Streptococcus pneumoniae, and Staphylococcus aureus. The exceptions stand out by clinical criteria, such as known neutropenia, history of an animal bite, signs of Systemic Inflammatory Response Syndrome (SIRS), or a purulent appearance.
“Blood cultures are not cost-effective in uncomplicated cellulitis,” Dr. Adams said. She said there are numerous published algorithms to guide clinicians on decision-making in the management of soft tissue infections, including cellulitis, including a much-cited algorithm first published more than 15 years ago and updated in 2014.
Similarly, labs and imaging are commonly ordered with no strong likelihood that they will change management, she said. These types of decisions are also covered in published algorithms.
Strategies to prevent rehospitalization are another area where there is a large opportunity to reduce health care resources consumed by cellulitis. The rehospitalization rate at 30 days is approximately 10%, but many patients have recurrent episodes over years, according to Dr. Adams. The risk factors and the preventative measures have been well described.
“Scrupulous clinical care can reduce recurrence, and it is cost-effective,” said Dr. Adams, referring to control of edema, control of underlying conditions associated with increased risk, such as diabetes, and managing dry skin and erosions with topical agents or even moisturizers. Compression socks are a simple but effective tool, she added.
For patients with repeat episodes of cellulitis over years, Dr. Adams referred to a double-blind trial that associated a twice-daily dose of 250 mg penicillin with a 45% reduction in the risk of cellulitis recurrence over 1 year. At approximately $10 a month for this treatment, she said it is very cost-effective, although she acknowledged that recurrence rates of cellulitis climb back up when the penicillin is stopped.
“I think of this as a bridge while you work on addressing the venous insufficiency or other risk factors for cellulitis,” Dr. Adams said.
For reducing the costs of cellulitis, there is evidence that dermatologists can play a role. Dr. Adams cited a study that evaluated the impact of a dermatologist consultation for suspected cellulitis in the emergency room or within 24 hours of admission. Of 34 patients already prescribed antibiotics for presumed cellulitis, discontinuation was recommended in 82%. Of 39 admissions, pseudocellulitis was identified in 51%.
Extrapolating these data to national rates of cellulitis, there was an estimated savings of up to $200 million annually without any apparent increased risk of adverse outcomes, according to Dr. Adams.
When contacted about his experience, the senior investigator of that study, Arash Mostaghimi, MD, director of the Inpatient Dermatology Consult Service, Brigham and Women’s Hospital, Boston, largely agreed with the premise of Adam’s analysis. In particular, he said, avoiding misdiagnosis of cellulitis offers a major opportunity to lower costs while possibly improving care.
True of national practice and at the local level, “misdiagnosis of noninfectious inflammatory reactions such as cellulitis has substantial cost impacts,” Dr. Mostaghimi said in an interview. Based on evidence, the savings are derived directly from “unnecessary antibiotic exposure as well as inappropriate hospitalization.”
Following publication of his study, he became involved in addressing this issue at his institution.
“At Brigham and Women’s, we collaborated with colleagues in infectious disease and in the emergency department to create cellulitis protocols that identify patients at risk for misdiagnosis and facilitate early dermatology consultation for diagnostic confirmation,” he said.
Although there are algorithms to achieve this goal, he indicated that the expertise of dermatologists can quickly and efficiently differentiate inflammatory skin reactions and expedite appropriate care.
Dr. Adams and Dr. Mostaghimi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The cost of care for the more than 14 million cases of cellulitis that occur each year in the United States is in the billions of dollars, but there are multiple opportunities, many involving dermatologists, to dramatically reduce these costs, according to an outline of strategies presented at the American Academy of Dermatology 2022 annual meeting in Boston.
“Cellulitis is misdiagnosed about one-third of the time, and that cost is very high,” reported Jennifer L. Adams, MD, assistant professor of dermatology, University of Nebraska, Omaha. She sees opportunities for dermatological consults to help weed through the many cellulitis mimickers, such as venous insufficiency or psoriasiform drug reactions, to prevent unnecessary admissions and ineffective therapy.
“There is a huge need for diagnostic accuracy as a means to deliver more cost-effective care,” Dr. Adams said.
Solving misdiagnosis is only part of the story. Costs of care are also ramped up by unnecessary hospitalizations. According to Dr. Adams, published criteria to triage emergency room patients with cellulitis to outpatient care are not always followed. In one review, 14% of admitted patients had met the criteria for outpatient treatment.
Cellulitis is a common skin infection that causes redness, swelling, and pain in the infected area, most often on the legs and feet.
Unnecessary hospitalizations for misdiagnosed cellulitis, which is associated with an average 4-day hospital stay, “range from $200 million to $500 million in avoidable direct healthcare costs,” Dr. Adams said.
Even for justifiable hospitalizations, there are still opportunities for cost savings. In one study, blood cultures were ordered in 73% of patients even though only 2% produced a finding relevant to care. According to Dr. Adams, most cellulitis cases are caused by the “usual suspects” – group A beta-hemolytic streptococcus, Streptococcus pneumoniae, and Staphylococcus aureus. The exceptions stand out by clinical criteria, such as known neutropenia, history of an animal bite, signs of Systemic Inflammatory Response Syndrome (SIRS), or a purulent appearance.
“Blood cultures are not cost-effective in uncomplicated cellulitis,” Dr. Adams said. She said there are numerous published algorithms to guide clinicians on decision-making in the management of soft tissue infections, including cellulitis, including a much-cited algorithm first published more than 15 years ago and updated in 2014.
Similarly, labs and imaging are commonly ordered with no strong likelihood that they will change management, she said. These types of decisions are also covered in published algorithms.
Strategies to prevent rehospitalization are another area where there is a large opportunity to reduce health care resources consumed by cellulitis. The rehospitalization rate at 30 days is approximately 10%, but many patients have recurrent episodes over years, according to Dr. Adams. The risk factors and the preventative measures have been well described.
“Scrupulous clinical care can reduce recurrence, and it is cost-effective,” said Dr. Adams, referring to control of edema, control of underlying conditions associated with increased risk, such as diabetes, and managing dry skin and erosions with topical agents or even moisturizers. Compression socks are a simple but effective tool, she added.
For patients with repeat episodes of cellulitis over years, Dr. Adams referred to a double-blind trial that associated a twice-daily dose of 250 mg penicillin with a 45% reduction in the risk of cellulitis recurrence over 1 year. At approximately $10 a month for this treatment, she said it is very cost-effective, although she acknowledged that recurrence rates of cellulitis climb back up when the penicillin is stopped.
“I think of this as a bridge while you work on addressing the venous insufficiency or other risk factors for cellulitis,” Dr. Adams said.
For reducing the costs of cellulitis, there is evidence that dermatologists can play a role. Dr. Adams cited a study that evaluated the impact of a dermatologist consultation for suspected cellulitis in the emergency room or within 24 hours of admission. Of 34 patients already prescribed antibiotics for presumed cellulitis, discontinuation was recommended in 82%. Of 39 admissions, pseudocellulitis was identified in 51%.
Extrapolating these data to national rates of cellulitis, there was an estimated savings of up to $200 million annually without any apparent increased risk of adverse outcomes, according to Dr. Adams.
When contacted about his experience, the senior investigator of that study, Arash Mostaghimi, MD, director of the Inpatient Dermatology Consult Service, Brigham and Women’s Hospital, Boston, largely agreed with the premise of Adam’s analysis. In particular, he said, avoiding misdiagnosis of cellulitis offers a major opportunity to lower costs while possibly improving care.
True of national practice and at the local level, “misdiagnosis of noninfectious inflammatory reactions such as cellulitis has substantial cost impacts,” Dr. Mostaghimi said in an interview. Based on evidence, the savings are derived directly from “unnecessary antibiotic exposure as well as inappropriate hospitalization.”
Following publication of his study, he became involved in addressing this issue at his institution.
“At Brigham and Women’s, we collaborated with colleagues in infectious disease and in the emergency department to create cellulitis protocols that identify patients at risk for misdiagnosis and facilitate early dermatology consultation for diagnostic confirmation,” he said.
Although there are algorithms to achieve this goal, he indicated that the expertise of dermatologists can quickly and efficiently differentiate inflammatory skin reactions and expedite appropriate care.
Dr. Adams and Dr. Mostaghimi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The cost of care for the more than 14 million cases of cellulitis that occur each year in the United States is in the billions of dollars, but there are multiple opportunities, many involving dermatologists, to dramatically reduce these costs, according to an outline of strategies presented at the American Academy of Dermatology 2022 annual meeting in Boston.
“Cellulitis is misdiagnosed about one-third of the time, and that cost is very high,” reported Jennifer L. Adams, MD, assistant professor of dermatology, University of Nebraska, Omaha. She sees opportunities for dermatological consults to help weed through the many cellulitis mimickers, such as venous insufficiency or psoriasiform drug reactions, to prevent unnecessary admissions and ineffective therapy.
“There is a huge need for diagnostic accuracy as a means to deliver more cost-effective care,” Dr. Adams said.
Solving misdiagnosis is only part of the story. Costs of care are also ramped up by unnecessary hospitalizations. According to Dr. Adams, published criteria to triage emergency room patients with cellulitis to outpatient care are not always followed. In one review, 14% of admitted patients had met the criteria for outpatient treatment.
Cellulitis is a common skin infection that causes redness, swelling, and pain in the infected area, most often on the legs and feet.
Unnecessary hospitalizations for misdiagnosed cellulitis, which is associated with an average 4-day hospital stay, “range from $200 million to $500 million in avoidable direct healthcare costs,” Dr. Adams said.
Even for justifiable hospitalizations, there are still opportunities for cost savings. In one study, blood cultures were ordered in 73% of patients even though only 2% produced a finding relevant to care. According to Dr. Adams, most cellulitis cases are caused by the “usual suspects” – group A beta-hemolytic streptococcus, Streptococcus pneumoniae, and Staphylococcus aureus. The exceptions stand out by clinical criteria, such as known neutropenia, history of an animal bite, signs of Systemic Inflammatory Response Syndrome (SIRS), or a purulent appearance.
“Blood cultures are not cost-effective in uncomplicated cellulitis,” Dr. Adams said. She said there are numerous published algorithms to guide clinicians on decision-making in the management of soft tissue infections, including cellulitis, including a much-cited algorithm first published more than 15 years ago and updated in 2014.
Similarly, labs and imaging are commonly ordered with no strong likelihood that they will change management, she said. These types of decisions are also covered in published algorithms.
Strategies to prevent rehospitalization are another area where there is a large opportunity to reduce health care resources consumed by cellulitis. The rehospitalization rate at 30 days is approximately 10%, but many patients have recurrent episodes over years, according to Dr. Adams. The risk factors and the preventative measures have been well described.
“Scrupulous clinical care can reduce recurrence, and it is cost-effective,” said Dr. Adams, referring to control of edema, control of underlying conditions associated with increased risk, such as diabetes, and managing dry skin and erosions with topical agents or even moisturizers. Compression socks are a simple but effective tool, she added.
For patients with repeat episodes of cellulitis over years, Dr. Adams referred to a double-blind trial that associated a twice-daily dose of 250 mg penicillin with a 45% reduction in the risk of cellulitis recurrence over 1 year. At approximately $10 a month for this treatment, she said it is very cost-effective, although she acknowledged that recurrence rates of cellulitis climb back up when the penicillin is stopped.
“I think of this as a bridge while you work on addressing the venous insufficiency or other risk factors for cellulitis,” Dr. Adams said.
For reducing the costs of cellulitis, there is evidence that dermatologists can play a role. Dr. Adams cited a study that evaluated the impact of a dermatologist consultation for suspected cellulitis in the emergency room or within 24 hours of admission. Of 34 patients already prescribed antibiotics for presumed cellulitis, discontinuation was recommended in 82%. Of 39 admissions, pseudocellulitis was identified in 51%.
Extrapolating these data to national rates of cellulitis, there was an estimated savings of up to $200 million annually without any apparent increased risk of adverse outcomes, according to Dr. Adams.
When contacted about his experience, the senior investigator of that study, Arash Mostaghimi, MD, director of the Inpatient Dermatology Consult Service, Brigham and Women’s Hospital, Boston, largely agreed with the premise of Adam’s analysis. In particular, he said, avoiding misdiagnosis of cellulitis offers a major opportunity to lower costs while possibly improving care.
True of national practice and at the local level, “misdiagnosis of noninfectious inflammatory reactions such as cellulitis has substantial cost impacts,” Dr. Mostaghimi said in an interview. Based on evidence, the savings are derived directly from “unnecessary antibiotic exposure as well as inappropriate hospitalization.”
Following publication of his study, he became involved in addressing this issue at his institution.
“At Brigham and Women’s, we collaborated with colleagues in infectious disease and in the emergency department to create cellulitis protocols that identify patients at risk for misdiagnosis and facilitate early dermatology consultation for diagnostic confirmation,” he said.
Although there are algorithms to achieve this goal, he indicated that the expertise of dermatologists can quickly and efficiently differentiate inflammatory skin reactions and expedite appropriate care.
Dr. Adams and Dr. Mostaghimi have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Global registry tracks COVID-19 outcomes in atopic dermatitis patients
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
AT AAD 22
Children and COVID: The long goodbye continues
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.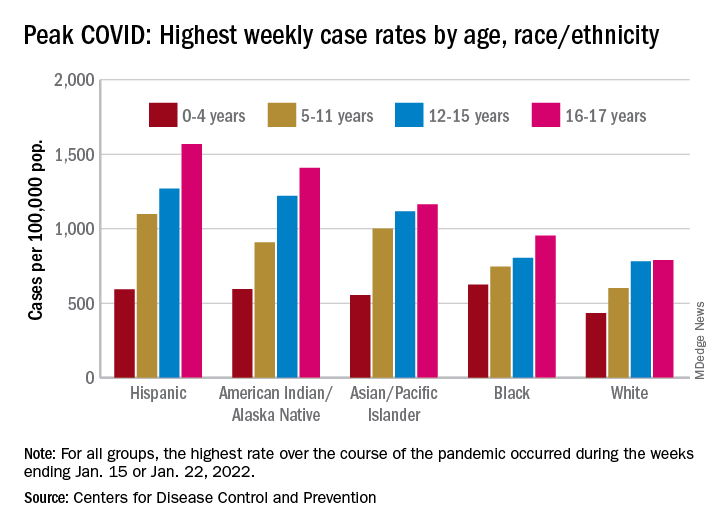
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
FDA approves HIV injectable Cabenuva initiation without oral lead-in
Initiating treatment may become easier for adults living with HIV. a combination injectable, without a lead-in period of oral tablets, according to a press release from Janssen Pharmaceuticals.
Cabenuva combines rilpivirine (Janssen) and cabotegravir (ViiV Healthcare). The change offers patients and clinicians an option for a streamlined entry to treatment without the burden of daily pill taking, according to the release.
Cabenuva injections may be given as few as six times a year to manage HIV, according to Janssen. HIV patients with viral suppression previously had to complete an oral treatment regimen before starting monthly or bimonthly injections.
The injectable combination of cabotegravir, an HIV-1 integrase strand transfer inhibitor, and rilpivirine, an HIV-1 nonnucleoside reverse transcriptase inhibitor, is currently indicated as a complete treatment regimen to replace the current antiretroviral regimen for adults with HIV who are virologically suppressed,” according to the press release.
“Janssen and ViiV are exploring the future possibility of an ultra–long-acting version of Cabenuva, which could reduce the frequency of injections even further, according to the press release.
Access may improve, but barriers persist
“Despite advances in HIV care, many barriers remain, particularly for the most vulnerable populations,” Lina Rosengren-Hovee, MD, of the University of North Carolina at Chapel Hill, said in an interview.
“Care engagement has improved with the use of bridge counselors, rapid ART [antiretroviral therapy] initiation policies, and contact tracing,” she said. “Similarly, increasing access to multiple modalities of HIV treatment is critical to increase engagement in care.
“For patients, removing the oral lead-in primarily reduces the number of clinical visits to start injectable ART,” Dr. Rosengren-Hovee added. “It may also remove adherence barriers for patients who have difficulty taking a daily oral medication.”
But Dr. Rosengren-Hovee (who has no financial connection to the manufacturers) pointed out that access to Cabenuva may not be seamless. “Unless the medication is stocked in clinics, patients are not likely to receive their first injection during the initial visit. Labs are also required prior to initiation to ensure there is no contraindication to the medication, such as viral resistance to one of its components. Cost and insurance coverage are also likely to remain major obstacles.”
Dr. Rosengren-Hovee has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initiating treatment may become easier for adults living with HIV. a combination injectable, without a lead-in period of oral tablets, according to a press release from Janssen Pharmaceuticals.
Cabenuva combines rilpivirine (Janssen) and cabotegravir (ViiV Healthcare). The change offers patients and clinicians an option for a streamlined entry to treatment without the burden of daily pill taking, according to the release.
Cabenuva injections may be given as few as six times a year to manage HIV, according to Janssen. HIV patients with viral suppression previously had to complete an oral treatment regimen before starting monthly or bimonthly injections.
The injectable combination of cabotegravir, an HIV-1 integrase strand transfer inhibitor, and rilpivirine, an HIV-1 nonnucleoside reverse transcriptase inhibitor, is currently indicated as a complete treatment regimen to replace the current antiretroviral regimen for adults with HIV who are virologically suppressed,” according to the press release.
“Janssen and ViiV are exploring the future possibility of an ultra–long-acting version of Cabenuva, which could reduce the frequency of injections even further, according to the press release.
Access may improve, but barriers persist
“Despite advances in HIV care, many barriers remain, particularly for the most vulnerable populations,” Lina Rosengren-Hovee, MD, of the University of North Carolina at Chapel Hill, said in an interview.
“Care engagement has improved with the use of bridge counselors, rapid ART [antiretroviral therapy] initiation policies, and contact tracing,” she said. “Similarly, increasing access to multiple modalities of HIV treatment is critical to increase engagement in care.
“For patients, removing the oral lead-in primarily reduces the number of clinical visits to start injectable ART,” Dr. Rosengren-Hovee added. “It may also remove adherence barriers for patients who have difficulty taking a daily oral medication.”
But Dr. Rosengren-Hovee (who has no financial connection to the manufacturers) pointed out that access to Cabenuva may not be seamless. “Unless the medication is stocked in clinics, patients are not likely to receive their first injection during the initial visit. Labs are also required prior to initiation to ensure there is no contraindication to the medication, such as viral resistance to one of its components. Cost and insurance coverage are also likely to remain major obstacles.”
Dr. Rosengren-Hovee has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initiating treatment may become easier for adults living with HIV. a combination injectable, without a lead-in period of oral tablets, according to a press release from Janssen Pharmaceuticals.
Cabenuva combines rilpivirine (Janssen) and cabotegravir (ViiV Healthcare). The change offers patients and clinicians an option for a streamlined entry to treatment without the burden of daily pill taking, according to the release.
Cabenuva injections may be given as few as six times a year to manage HIV, according to Janssen. HIV patients with viral suppression previously had to complete an oral treatment regimen before starting monthly or bimonthly injections.
The injectable combination of cabotegravir, an HIV-1 integrase strand transfer inhibitor, and rilpivirine, an HIV-1 nonnucleoside reverse transcriptase inhibitor, is currently indicated as a complete treatment regimen to replace the current antiretroviral regimen for adults with HIV who are virologically suppressed,” according to the press release.
“Janssen and ViiV are exploring the future possibility of an ultra–long-acting version of Cabenuva, which could reduce the frequency of injections even further, according to the press release.
Access may improve, but barriers persist
“Despite advances in HIV care, many barriers remain, particularly for the most vulnerable populations,” Lina Rosengren-Hovee, MD, of the University of North Carolina at Chapel Hill, said in an interview.
“Care engagement has improved with the use of bridge counselors, rapid ART [antiretroviral therapy] initiation policies, and contact tracing,” she said. “Similarly, increasing access to multiple modalities of HIV treatment is critical to increase engagement in care.
“For patients, removing the oral lead-in primarily reduces the number of clinical visits to start injectable ART,” Dr. Rosengren-Hovee added. “It may also remove adherence barriers for patients who have difficulty taking a daily oral medication.”
But Dr. Rosengren-Hovee (who has no financial connection to the manufacturers) pointed out that access to Cabenuva may not be seamless. “Unless the medication is stocked in clinics, patients are not likely to receive their first injection during the initial visit. Labs are also required prior to initiation to ensure there is no contraindication to the medication, such as viral resistance to one of its components. Cost and insurance coverage are also likely to remain major obstacles.”
Dr. Rosengren-Hovee has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.






