User login
For MD-IQ use only
Targeted Osteoporosis Program May Benefit At-Risk Older Men
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Efforts to identify older men at risk for osteoporosis and treat those who are eligible received a boost from results reported from a Veterans Affairs (VA) study that showed a significant increase in screening, treatment, and medication adherence.
The cluster randomized trial used a centralized nurse-led intervention to assess men for traditional osteoporosis risk factors, offer bone density testing, and recommend treatment for eligible men. Over 2 years, the intervention group had a higher average femoral neck bone density than patients who underwent usual care.
“We designed this study to see if a risk factor-based approach, which is what most of the guidelines use, made sense and was feasible — that men would be accepting of screening and [the approach] would yield a similar proportion of people who need osteoporosis treatment as screening in women, which is widely recommended and implemented. And sure enough, we found that about 85% of the men in the VA primary care practices in our target age range of between 65 and 85 actually met criteria for screening, and over half of them had low bone mass. They were very accepting of screening, very accepting of treatment, and had excellent compliance rates. So, our study, we believe, supports the idea of identifying men with at least one risk factor for fracture and offering them osteoporosis screening starting at age 65, similar to what we do for women,” Cathleen S. Colón-Emeric, MD, MHS, said in an interview. She is the lead author of the study, a physician in the Durham VA Health Care System, and professor of medicine at Duke University School of Medicine, Durham, North Carolina.
“We were able to see a positive effect on bone density in the bone health group, compared with the usual care group, which suggests that if we followed these folks longer and had enough of them, we would be able to show a fracture reduction benefit,” Colón-Emeric said.
There have been few randomized trials of screening interventions in men, leading to inconsistencies in guidelines, according to the authors of the new study, published online in JAMA Internal Medicine . Both the US Preventive Services Task Force and the Veterans Health Administration National Center for Health Promotion and Disease Prevention consider there to be insufficient evidence to recommend for or against screening in men who have not experienced a fracture. Some professional societies recommend such screening, but there are inconsistencies in the recommended criteria, such as age range or risk factors.
Beyond the age of 50 years, one in five men will experience an osteoporosis-related fracture at some point in their life, according to a 2009 study. Treatment is inexpensive and effective in both men and women, and economic models suggest that screening using dual-energy x-ray absorptiometry (DXA) would be cost-effective. Still, screening is rare among men, with fewer than 10% of men getting screened before having an osteoporosis-related fracture.
“It’s important to screen men at risk for osteoporosis due to the dramatically increased mortality men suffer after a fragility fracture compared with women. Within 1 year of a hip fracture, mortality is as high as 36%. Studies have also shown that osteoporosis in men is undertreated, with only 10%-50% being prescribed antifracture treatment within 1 year of a hip fracture. Most individuals do not regain their prior level of function after a hip fracture,” said Joe C. Huang, MD, who was asked for comment. He is a clinical assistant professor of gerontology and geriatric medicine at Harborview Medical Center Senior Care Clinic and Healthy Bones Clinic in Seattle.
Details of the Intervention
The bone health service (BHS) intervention employed an electronic health record case-finding tool and a nurse care manager who undertook screening and treatment monitoring. They identified potential risk factors that included hyperthyroidism, hyperparathyroidism, rheumatoid arthritis, alcohol dependence, chronic lung disease, chronic liver disease, stroke, parkinsonism, prostate cancer, smoking, diabetes, pernicious anemia, gastrectomy, or high-risk medication use in at least 3 months of the prior 2 years. These medications included traditional antiepileptics, glucocorticoids, and androgen deprivation therapy.
The BHS nurse invited eligible men to be screened using an initial letter, followed by up to three phone calls. After DXA screening, the nurse scheduled an electronic consult with an osteoporosis expert, and patients with a T-score between -1 and -2.4 and an elevated 10-year fracture risk as measured by the Fracture Risk Assessment Tool were recommended for osteoporosis medication, vitamin D, and dietary or supplemental calcium. Following the prescription, the nurse provided patient education over the phone and mailed out written instructions. The nurse also made phone calls at 1 month, 6 months, and 12 months to encourage adherence and address common treatment barriers such as forgetting to take medication or dealing with gastrointestinal effects. The researchers recruited 38 primary care physicians from two VA health systems. The study included 3112 male veterans between the ages of 65 and 85 years (40.4% Black and 56% White). Nearly all participants (85.5%) had at least one indication for screening according to VA undersecretary guidelines, and almost a third (32.1%) had been prescribed androgen deprivation therapy, traditional antiepileptic drugs, or glucocorticoids.
Over a mean follow-up of 1.5 years, there was a much higher screening rate in the BHS group (49.2% vs 2.3%; P < .001), with a similar overall yield of DXA results recommending osteoporosis treatment (22.4% vs 27.2%). In the BHS group, 84.4% of patients who had treatment recommended followed through with treatment initiation. The mean persistence over follow-up was 657 days (SD, 366 days), and adherence was high with a mean proportion of days covered of 91.7%.
It was not possible to statistically compare adherence with the usual-care group because there were too few screened patients found to be eligible for treatment in that group, but the historic mean proportion of days covered at the two participating facilities was 52%.
After 2 years, the mean femoral neck T-score tested randomly in a subset of patients was better in the BHS arm, although it did not meet statistical significance according to the Bonferroni corrected criterion of P < .025 (-0.55 vs -0.70; P = .04). Fracture rates were similar between the two groups (1.8% vs 2.0%; P = .69).
Can the Findings Be Translated Across Clinics?
It remains to be seen how well the model could translate to other healthcare settings, according to Kenny Lin, MD, MPH, who was asked for comment on the study. “Outside of the VA health system and perhaps integrated HMOs [health maintenance organizations] such as Kaiser, Geisinger, etc., it seems unlikely that most primary care docs will have access to a centralized bone health service. Who’s going to pay for it? It leaves unanswered the question of whether it’s more efficient to address [osteoporosis] screening on a practice or population level. I suspect the latter is probably superior, but this study doesn’t provide any empiric evidence that this is so,” said Lin, associate director of the Penn Medicine Lancaster General Hospital’s Family Medicine Residency Program, Lancaster, Pennsylvania. The findings could help sway recommendations to screen men for osteoporosis, according to Susan Ott, MD, who was also asked for comment. Guideline committees “have been trying to be very scientific [about it]. I think they overdo it because they only look at one or two kinds of studies, and there are more kinds of science than just a randomized clinical trial. But they’re kind of stuck on that. The fact that this study was a randomized trial maybe they will finally change their recommendation, because there really shouldn’t be any difference in screening for men and for women. The men are actually discriminated against,” said Ott, emeritus professor of medicine at the University of Washington, Seattle.
In fact, she noted that the risks for men are similar to those for women, except that men tend to develop issues 5-10 years later in life. To screen and treat men, healthcare systems can “do the same thing they do with women. Just change the age range,” Ott said.
Lin sounded a different note, suggesting that the focus should remain on improvement of screening and treatment adherence in women. “We know that up to two thirds of women discontinue osteoporosis drugs within a year, and if we can’t figure out how to improve abysmal adherence in women, it’s unlikely we will persuade enough men to take these drugs to make a difference,” he said.
The study was funded by a grant from the VA Health Systems Research. Colón-Emeric, Lin, Ott, and Huang reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
U.S. Health Chief Kennedy Targets Vaccine Injury Compensation Program
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
WASHINGTON (Reuters) - U.S. Health Secretary Robert F. Kennedy Jr. said on July 28 that he will work to “fix” the program that compensates victims of vaccine injuries, the National Vaccine Injury Compensation Program.
Kennedy, a long-time vaccine skeptic and former vaccine injury plaintiff lawyer, accused the program and its so-called “Vaccine Court” of corruption and inefficiency in a post on X. He has long been an outspoken critic of the program.
“I will not allow the VICP to continue to ignore its mandate and fail its mission of quickly and fairly compensating vaccine-injured individuals,” he wrote, adding he was working with Attorney General Pam Bondi. “Together, we will steer the Vaccine Court back to its original congressional intent.”
He said the structure disadvantaged claimants because the Department of Health & Human Services – which he now leads – is the defendant, as opposed to vaccine makers.
Changing the VICP would be the latest in a series of far-reaching actions by Kennedy to reshape U.S. regulation of vaccines, food and medicine.
In June, he fired all 17 members of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, a panel of vaccine experts, replacing them with 7 handpicked members, including known vaccine skeptics.
One of them earned thousands of dollars as an expert witness in litigation against Merck’s, Gardasil vaccine, court records show. Kennedy himself played an instrumental role in organizing mass litigation over the vaccine.
He also is planning to remove all the members of another advisory panel that determines what preventive health measures insurers must cover, the Wall Street Journal reported on July 25. An HHS spokesperson said Kennedy had not yet made a decision regarding the 16-member U.S. Preventive Services Task Force.
Kennedy has for years sown doubt about the safety and efficacy of vaccines. He has a history of clashing with the medical establishment and spreading misinformation about vaccines, including promoting a debunked link between vaccines and autism despite scientific evidence to the contrary.
He has also said the measles vaccine contains cells from aborted fetuses and that the mumps vaccination does not work, comments he made as the U.S. battles one of its worst outbreaks of measles in 25 years.
Kennedy made millions over the years from advocating against vaccines through case referrals, book sales, and consulting fees paid by a nonprofit he founded, according to ethics disclosures.
(Reporting by Ahmed Aboulenein; Additional reporting by Ryan Patrick Jones in Toronto; Editing by Doina Chiacu and Nia Williams)
A version of this article appeared on Medscape.com.
Rurality and Age May Shape Phone-Only Mental Health Care Access Among Veterans
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Patients living in rural areas and those aged ≥ 65 y had increased odds of receiving mental health care exclusively by phone.
METHODOLOGY:
- Researchers explored factors linked to receiving phone-only mental health care among patients within the Department of Veterans Affairs.
- They included data for 1,156,146 veteran patients with at least one mental health-specific outpatient encounter between October 2021 and September 2022 and at least one between October 2022 and September 2023.
- Patients were categorized as those who received care through phone only (n = 49,125) and those who received care through other methods (n = 1,107,021. Care was received exclusively through video (6.39%), in-person (6.63%), or a combination of in-person, video, and/or phone (86.98%).
- Demographic and clinical predictors, including rurality, age, sex, race, ethnicity, and the number of mental health diagnoses (< 3 vs ≥ 3), were evaluated.
TAKEAWAY:
- The phone-only group had a mean of 6.27 phone visits, whereas those who received care through other methods had a mean of 4.79 phone visits.
- Highly rural patients had 1.50 times higher odds of receiving phone-only mental health care than their urban counterparts (adjusted odds ratio [aOR], 1.50; P < .0001).
- Patients aged 65 years or older were more than twice as likely to receive phone-only care than those younger than 30 years (aOR, ≥ 2.17; P < .0001).
- Having fewer than three mental health diagnoses and more than 50% of mental health visits conducted by medical providers was associated with higher odds of receiving mental health care exclusively by phone (aORs, 2.03 and 1.87, respectively; P < .0001).
IN PRACTICE:
“The results of this work help to characterize the phone-only patient population and can serve to inform future implementation efforts to ensure that patients are receiving care via the modality that best meets their needs,” the authors wrote.
SOURCE:
This study was led by Samantha L. Connolly, PhD, at the VA Boston Healthcare System in Boston. It was published online in The Journal of Rural Health.
LIMITATIONS:
This study focused on a veteran population which may limit the generalizability of the findings to other groups. Additionally, its cross-sectional design restricted the ability to determine cause-and-effect relationships between factors and phone-only care.
DISCLOSURES:
This study was supported by the US Department of Veterans Affairs. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Searching for the Optimal CRC Surveillance Test
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert
About a third of the US population are eligible for colorectal cancer screening but aren’t up to date on screening.
Many patients are reluctant to test for colon cancer for a variety of reasons, said Jeffrey K. Lee, MD, MPH, a research scientist at the Kaiser Permanente Northern California Division of Research and an attending gastroenterologist at Kaiser Permanente San Francisco Medical Center.
“As a gastroenterologist, I strongly believe we should emphasize the importance of colorectal cancer screening. And there’s many tests available, not just a colonoscopy, to help reduce your chances of developing colorectal cancer and even dying from colorectal cancer,” said Dr. Lee.
Many patients prefer a test that’s more convenient, that doesn’t require them to take time out of their busy schedules. “We must educate our patients that there are some noninvasive screening options that are helpful, and to be able to share with them some of the benefits, but also some of the drawbacks compared to colonoscopy and allow them to have a choice,” he advised.
He is a recipient of the AGA Research Scholar Award, and has in turn supported other researchers by contributing to the AGA Research Foundation. In 2012, Dr. Lee received a grant from the Sylvia Allison Kaplan Clinical Research Fund to fund a study on long-term colorectal cancer risk in patients with normal colonoscopy results.
The findings, published in JAMA Internal Medicine, determined that 10 years after a negative colonoscopy, Kaiser Permanente members had a 46% lower risk of being diagnosed with CRC and were 88% less likely to die from disease compared with patients who didn’t undergo screening.
“Furthermore, the reduced risk of developing colorectal cancer, even dying from it, persisted for more than 12 years after the examination compared with an unscreened population,” said Dr. Lee. “I firmly believe our study really supports the ten-year screening interval after a normal colonoscopy, as currently recommended by our guidelines.”
In an interview, he discussed his research efforts to find the best detection regimens for CRC, and the mentors who guided his career path as a GI scientist.
Q: Why did you choose GI?
During medical school I was fortunate to work in the lab of Dr. John M. Carethers at UC San Diego. He introduced me to GI and inspired me to choose GI as a career. His mentorship was invaluable because he not only solidified my interest in GI, but also inspired me to become a physician scientist, focusing on colorectal cancer prevention and control. His amazing mentorship drew me to this field.
Q: One of your clinical focus areas is hereditary gastrointestinal cancer syndromes. How did you become interested in this area of GI medicine?
My interest in hereditary GI cancer syndromes stemmed from my work as a medical student in Dr. Carethers’ lab. One of my research projects was looking at certain gene mutations among patients with hereditary GI cancer syndromes, specifically, familial hamartomatous polyposis syndrome. It was through these research projects and seeing how these genetic mutations impacted their risk of developing colorectal cancer, inspired me to care for patients with hereditary GI cancer syndromes.
Q: Have you been doing any research on the reasons why more young people are getting colon cancer?
We recently published work looking at the potential factors that may be driving the rising rates of early onset colorectal cancer. One hypothesis that’s been floating around is antibiotic exposure in early adulthood or childhood because of its effect on the microbiome. Using our large database at Kaiser Permanente Northern California, we did not find an association between oral antibiotic use during early adulthood and the risk of early-onset colorectal cancer.
You have the usual suspects like obesity and diabetes, but it’s not explaining all that risk. While familial colorectal cancer syndromes contribute to a small proportion of early-onset colorectal, these syndromes are not increasing across generations. I really do feel it’s something in the diet or how foods are processed and environmental factors that’s driving some of the risk of early onset colorectal cancer and this should be explored further.
Q: In 2018, you issued a landmark study which found an association between a 10-year follow-up after negative colonoscopy and reduced risk of disease and mortality. Has there been any updates to these findings over the last 6 years?
We recently saw a study in JAMA Oncology of a Swedish cohort that showed a negative colonoscopy result was associated with a reduced risk of developing and even dying from colorectal cancer 15 years from that examination, compared to the general population of Sweden. I think there’s some things that we need to be cautious about regarding that study. We have to think about the comparison group that they used and the lack of information regarding the indication of the colonoscopy and the quality of the examination. So, it remains uncertain whether future guidelines are going to stretch out that 10-year interval to 15 years.
Q: What other CRC studies are you working on now?
We have several studies that we are working on right now. One is called the PREVENT CRC study, which is looking at whether a polygenic risk score can improve risk stratification following adenoma removal for colorectal cancer prevention and tailoring post-polypectomy surveillance. This is a large observational cohort study that we have teamed up with the Fred Hutchinson Cancer Center, Erasmus University, and Kaiser Permanente Northwest to answer this important question that may have implications for personalized medicine.
Then there’s the COOP study, funded by the Patient-Centered Outcomes Research Institute. This is looking at the best surveillance test to use among older adults 65 years and older with a history of polyps. The trial is randomizing them to either getting a colonoscopy for surveillance or annual fecal immunochemical test (FIT) for surveillance. This is to see which test is best for detecting colorectal cancer among older adults with a history of polyps.
Q: Do you think FIT tests could eventually replace colonoscopy, given that it’s less invasive?
Although FIT and other stool-based tests are less invasive and have been shown to have high accuracy for detecting colorectal cancer, I personally do not think they are going to replace colonoscopy as the most popular screening modality in the United States. Colonoscopy remains the gold standard for detecting and removing precancerous polyps and has the highest accuracy for detecting colorectal cancer.
Q: Besides Dr. Carethers, what teacher or mentor had the greatest impact on you?
Clinically it’s been Dr. Jonathan Terdiman from UCSF, who taught me everything I know about clinical GI, and the art of colonoscopy. In addition, Douglas A. Corley, MD, PhD, the Permanente Medical Group’s chief research officer, has made the greatest impact on my research career. He’s really taught me how to rigorously design a research study to answer important clinically relevant questions, and has given me the skill set to write NIH grants. I would not be here without these mentors who are truly giants in the field of GI.
Q: When you’re not being a GI, how do you spend your free weekend afternoons? Are you still a “Cal Bears” fan at your alma mater, UC Berkeley?
I spend a lot of time taking my kids to their activities on the weekends. I just took my son to a Cal Bears Game Day, which was hosted by ESPN at Berkeley.
It was an incredible experience hearing sports analyst Pat McAfee lead all the Cal chants, seeing Nick Saban from the University of Alabama take off his red tie and replace it with a Cal Bears tie, and watching a Cal student win a hundred thousand dollars by kicking a football through the goal posts wearing checkered vans.
Lightning Round
Texting or talking?
Text
Favorite breakfast?
Taiwanese breakfast
Place you most want to travel to?
Japan
Favorite junk food?
Trader Joe’s chili lime chips
Favorite season?
Springtime, baseball season
Favorite ice cream flavor?
Mint chocolate chip
How many cups of coffee do you drink per day?
2-3
Last movie you watched?
Oppenheimer
Best place you ever went on vacation?
Hawaii
If you weren’t a gastroenterologist, what would you be?
Barber
Best Halloween costume you ever wore?
SpongeBob SquarePants
Favorite sport?
Tennis
What song do you have to sing along with when you hear it?
Any classic 80s song
Introvert or extrovert?
Introvert

Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
To the Editor:
For many years, topical treatment of plaque psoriasis was limited to steroids, calcineurin inhibitors, vitamin D analogs, retinoids, coal tar products, and anthralin. In recent years, 2 new nonsteroidal treatment options with alternative mechanisms of action, roflumilast 0.3% and tapinarof 1%, have been approved by the US Food and Drug Administration.1 Roflumilast 0.3%, a topical phosphodiesterase 4 inhibitor, was shown in phase 3 clinical trials to reach an Investigator Global Assessment response of 37.5% to 42.2% in 8 weeks using once-daily application with minimal cutaneous adverse effects.1 Furthermore, it has demonstrated efficacy in treating psoriasis in intertriginous areas in subset analyses.1 Tapinarof is an aryl hydrocarbon receptor agonist that suppresses Th17 cell differentiation by downregulating IL-17, IL-22, and IL-23.1 In phase 3 clinical trials, 35% to 40% of patients who used tapinarof cream 1% once daily demonstrated improvement in psoriasis compared with 6% who used the vehicle alone.2 In these studies, 18% to 24% of patients who used tapinarof cream 1% experienced folliculitis.2
Acute generalized exanthematous pustulosis (AGEP) is a nonfollicular pustular drug reaction with systemic symptoms that typically occurs within 2 weeks of exposure to an inciting medication. Systemic antibiotics are the most commonly reported cause of AGEP.3 There are few reports in the literature of AGEP induced by topical agents.4,5 We report a case of AGEP in a young man following the use of tapinarof cream 1%.
A 23-year-old man with a history of psoriasis presented to the emergency department with fever and a pustular rash. One week prior to presentation, he developed a pustular eruption around plaques of psoriasis on the arms and legs. The patient had been prescribed tapinarof cream 1% by an outside dermatologist and was applying the medication to the affected areas once daily for 1 month prior to onset of symptoms. He discontinued tapinarof a few days prior to the eruption starting, but the rash progressed centrifugally and was associated with fevers and fatigue despite treatment with a brief course of empiric cephalexin prescribed by his primary care provider.
At presentation to our institution, the patient had widespread erythematous patches studded with pustules located on the arms, legs, and flexural areas as well as plaques of psoriasis involving approximately 20% of the body surface area (Figure 1). Furthermore, the patient was noted to have large noninflammatory bullae along the legs. The new eruption occurred on areas that were both treated and spared from the tapinarof cream 1%. Laboratory evaluation showed neutrophil-predominant leukocytosis (white blood cell count, 15.9×103/µL [reference range, 4.0-11.0×103/µL]; absolute neutrophil count, 10.3×103/µL [reference range, 1.5-8.0×103/µL]), absolute eosinophilia (1930/µL [reference range, 0-0.5×103/µL]), hypocalcemia (8.4 mg/dL [reference range, 8.5-10.5 mg/dL]), and a mild transaminitis (aspartate aminotransferase, 37 IU/L [reference range, 10-40 IU/L]; alanine aminotransferase, 53 IU/L [reference range, 7-56 U/L]). Histopathology demonstrated spongiosis with subcorneal and intraepidermal pustules and mixed dermal inflammation containing eosinophils (Figure 2). Direct immunofluorescence revealed mild granular staining of C3 at the basement membrane zone.


The patient was started on 1 mg/kg/d of prednisone tapered over 20 days, and he rapidly improved. Alanine aminotransferase levels peaked at 120 IU/L 2 weeks later. At that time, he had complete resolution of the original eruption and was transitioned to topical steroids for continued management of the psoriasis (Figure 3).

The differential diagnosis for our patient included AGEP, generalized pustular psoriasis (GPP), miliaria pustulosa, generalized cutaneous candidiasis, exuberant allergic contact dermatitis (ACD), and linear IgA bullous dermatosis (LABD). Based on the clinical manifestations, laboratory results, and histopathologic evaluation, we made the diagnosis of AGEP secondary to tapinarof with systemic absorption. Acute generalized exanthematous pustulosis has been reported with topical use of morphine and diphenhydramine, among other agents.4,5 To our knowledge, AGEP due to tapinarof cream 1% has not been reported. In the original clinical trials of tapinarof, folliculitis was contained to sites of application.2 Our patient developed pustules at sites distant to areas of application, as well as systemic symptoms and laboratory abnormalities, indicating a systemic reaction. It can be difficult to distinguish AGEP clinically and histologically from GPP. Both conditions can manifest with fever, hypocalcemia, and sterile pustules on a background of erythema that favors intertriginous areas.6 Infection, rapid oral steroid withdrawal, pregnancy, and rarely oral medications have been reported causes of GPP.6 Our patient did not have any of these exposures. There is overlap in the histology of AGEP and GPP. One retrospective series compared histologic samples to help distinguish these 2 entities. Reliable markers that favored AGEP over GPP included eosinophilic spongiosis, interface dermatitis, and dermal eosinophilia (>2/mm2).7 In contrast, the presence of CD161 positivity in the dermis with at least 10 cells favored a diagnosis of GPP.7 In our case, the presence of spongiosis with eosinophils in the dermis favored a diagnosis of AGEP over GPP.
Miliaria pustulosa is a benign condition caused by the occlusion of the epidermal portion of eccrine glands related to either high fever or hot and humid environmental conditions. While it can be present in intertriginous areas like AGEP, miliaria pustulosa can be seen extensively on the back, most commonly in immobile hospitalized patients.8 Generalized cutaneous candidiasis usually is caused by the yeast Candida albicans and can take on multiple morphologies, including folliculitis.9 The eruption may be disseminated but often is accentuated in intertriginous areas and the anogenital folds. Predisposing factors include immunosuppression, endocrinopathies, recent use of systemic antibiotics or steroids, chemotherapy, and indwelling catheters.9 Outside of recent antibiotic use, our patient did not have any risk factors for miliaria pustulosa, making this diagnosis unlikely.
Given the presence of overlapping bullae along the lower extremities, an exuberant ACD and LABD were considered. Bullae formation can occur in ACD secondary to robust inflammation and edema leading to acantholysis.10 While a delayed hypersensitivity reaction to topical tapinarof cream 1% was considered given that the patient used the medication for approximately 1 month prior to the onset of symptoms, it would be unlikely for ACD to present with a concomitant pustular eruption. Linear IgA bullous dermatosis is an autoimmune blistering disease in which antibodies target bullous pemphigoid antigen 2, and there is characteristically linear deposition of IgA at the dermal-epidermal junction that leads to subepidermal blistering.11 This often manifests clinically as widespread tense vesicles in an annular or string-of-pearls appearance. However, morphologies can vary, and large bullae may be seen. In adults, LABD typically is associated with inflammatory bowel disease, malignancy, or medications, notably vancomycin.11,12 Our patient did not have any of these predisposing factors, and his biopsy for direct immunofluorescence did not reveal the classic pattern described above.
Interestingly, there have been reports in the literature of bullous AGEP in the setting of oral anti-infectives. One report described a 62-year-old woman who developed widespread nonfollicular pustules with multiple tense serous blisters 24 hours after taking oral terbinafine.13 Another case described an 80-year-old woman with a similar presentation following a course of ciprofloxacin (although the timeline of medication administration was not described).14 In this case, patch testing to the culprit medication reproduced the response.14 In both cases, a biopsy revealed subcorneal and intraepidermal pustules with marked dermal edema.13,14 As previously described, spongiosis is a common feature of AGEP. We hypothesize that, similar to these reports, our patient had a robust inflammatory response leading to spongiosis, acantholysis, and blister formation secondary to AGEP.
Dermatologists should be aware of this case of AGEP secondary to tapinarof cream 1%, as reports in the literature are rare and it is a reminder that topical medications can cause serious systemic reactions.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848. doi:10.1016/j.jaad.2015.07.017
- Ghazawi FM, Colantonio S, Bradshaw S, et al. Acute generalized exanthematous pustulosis induced by topical morphine and confirmed by patch testing. Dermat Contact Atopic Occup Drug. 2020;31:E22-E23. doi:10.1097/DER.0000000000000573
- Hanafusa T, Igawa K, Azukizawa H, et al. Acute generalized exanthematous pustulosis induced by topical diphenhydramine. Eur J Dermatol. 2011;21:994-995. doi:10.1684/ejd.2011.1500
- Reynolds KA, Pithadia DJ, Lee EB, et al. Generalized pustular psoriasis: a review of the pathophysiology, clinical manifestations,diagnosis, and treatment. Cutis. 2022;110:19-25. doi:10.12788/cutis.0579
- Isom J, Braswell DS, Siroy A, et al. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. 2020;83:265-267. doi:10.1016/j.jaad.2020.03.015
- Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine.8th ed. McGraw-Hill; 2012:946.
- Elewski BE, Hughey LC, Marchiony Hunt K, et al. Fungal diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1329-1363.
- Elmas ÖF, Akdeniz N, Atasoy M, et al. Contact dermatitis: a great imitator. Clin Dermatol. 2020;38:176-192. doi:10.1016/j.clindermatol.2019.10.003
- Hull CM, Zone JZ. Dermatitis herpetiforms and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:527-537.
- Yamagami J, Nakamura Y, Nagao K, et al. Vancomycin mediates IgA autoreactivity in drug-induced linear IgA bullous dermatosis. J Invest Dermatol. 2018;138:1473-1480.
- Bullous acute generalized exanthematous pustulosis due to oral terbinafine. J Am Acad Dermatol. 2005;52:P115. doi:10.1016/j.jaad.2004.10.468
- Hausermann P, Scherer K, Weber M, et al. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277-280. doi:10.1159/000087024
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
Acute Generalized Exanthematous Pustulosis Secondary to Application of Tapinarof Cream 1%
PRACTICE POINTS
- Tapinarof cream 1% can be absorbed systemically and cause acute generalized exanthematous pustulosis (AGEP).
- Clinical configuration and histology can be useful to distinguish AGEP from mimickers.
- Topical application of drugs in general, particularly over large body surface areas, may lead to systemic drug eruptions.
American Hunger Games: Food Insecurity Among the Military and Veterans
American Hunger Games: Food Insecurity Among the Military and Veterans
The requisites of government are that there be sufficiency of food, sufficiency of military equipment, and the confidence of the people in their ruler.
Analects by Confucius1
From ancient festivals to modern holidays, autumn has long been associated with the gathering of the harvest. Friends and families come together around tables laden with delicious food to enjoy the pleasures of peace and plenty. During these celebrations, we must never forget that without the strength of the nation’s military and the service of its veterans, this freedom and abundance would not be possible. Our debt of gratitude to the current and former members of the armed services makes the fact that a substantial minority experiences food insecurity not only a human tragedy, but a travesty of the nation’s promise to support those who wear or have worn the uniform.
The National Defense Authorization Act for Fiscal Year 2020 charged the Secretary of Defense to investigate food insecurity among active-duty service members and their dependents.2 The RAND Corporation conducted the assessment and, based on the results of its analysis, made recommendations to reduce hunger among armed forces members and their families.3
The RAND study found that 10% of active-duty military met US Department of Agriculture (USDA) criteria for very low food security; another 15% were classified as having low food security. The USDA defines food insecurity with hunger as “reports of multiple indications of disrupted eating patterns and reduced food intake.” USDA defines low food security as “reports of reduced quality, variety, or desirability of diet. Little or no indication of reduced food intake.”4
As someone who grew up on an Army base with the commissary a short trip from military housing, I was unpleasantly surprised that food insecurity was more common among in-service members living on post. I was even more dismayed to read that a variety of factors constrained 14% of active-duty military experiencing food insecurity to seek public assistance to feed themselves and their families. As with so many health care and social services, (eg, mental health care), those wearing the uniform were concerned that participating in a food assistance program would damage their career or stigmatize them. Others did not seek help, perhaps because they believed they were not eligible, and in many cases were correct: they did not qualify for food banks or food stamps due to receiving other benefits. A variety of factors contribute to periods of food insecurity among military families, including remote or rural bases that lack access to grocery stores or jobs for partners or other family members, and low base military pay.5
Food insecurity is an even more serious concern among veterans who are frequently older and have more comorbidities, often leading to unemployment and homelessness. Feeding America, the nation’s largest organization of community food banks, estimates that 1 in 9 working-age veterans are food insecure.5 US Department of Veterans Affairs (VA) statistics indicate that veterans are 7% more likely to experience food insecurity than other sectors of the population.6 The Veterans Health Administration has recognized that food insecurity is directly related to medical problems already common among veterans, including diabetes, obesity, and depression. Women and minority veterans are the most at risk of food insecurity.7
Recognizing that many veterans are at risk of food insecurity, the US Department of Defense and VA have taken steps to try and reduce hunger among those who serve. In response to the shocking statistic that food insecurity was found in 27% of Iraq and Afghanistan veterans, the VA and Rockefeller Foundation are partnering on the Food as Medicine initiative to improve veteran nutrition as a means of improving nutrition-related health consequences of food insecurity.8
Like many federal practitioners, I was unaware of the food insecurity assistance available to active-duty service members or veterans, or how to help individuals access it. In addition to the resources outlined in the Table, there are many community-based options open to anyone, including veterans and service members.
I have written columns on many difficult issues in my years as the Editor-in-Chief of Federal Practitioner, but personally this is one of the most distressing editorials I have ever published. That individuals dedicated to defending our rights and protecting our safety should be compelled to go hungry or not know if they have enough money at the end of the month to buy food is manifestly unjust. It is challenging when faced with such a large-scale injustice to think we cannot make a difference, but that resignation or abdication only magnifies this inequity. I have a friend who kept giving back even after they retired from federal service: they volunteered at a community garden and brought produce to the local food bank and helped distribute it. That may seem too much for those still working yet almost anyone can pick up a few items on their weekly shopping trip and donate them to a food drive.
As we approach Veterans Day, let’s not just express our gratitude to our military and veterans in words but in deeds like feeding the hungry and urging elected representatives to fulfill their commitment to ensure that service members and veterans and their families do not experience food insecurity. Confucian wisdom written in a very distant time and vastly dissimilar context still rings true: there are direct and critical links between food and trust and between hunger and the military.1
Dawson MM. The Wisdom of Confucius: A Collection of the Ethical Sayings of Confucius and of his disciples. International Pocket Library; 1932.
National Defense Authorization Act for Fiscal Year 2020. 116th Cong (2019), Public Law 116-92. U.S. Government Printing Office. https://www.govinfo.gov/content/pkg/PLAW-116publ92/html/PLAW-116publ92.htm
Asch BJ, Rennane S, Trail TE, et al. Food insecurity among members of the armed forces and their dependents. RAND Corporation. January 3, 2023. Accessed September 22, 2025. https://www.rand.org/pubs/research_reports/RRA1230-1.html
US Department of Agriculture Economic Research Service. Food Security in the U.S.—Definitions of Food Security. US Department of Agriculture Economic Research Service. January 10, 2025. https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security
Active military and veteran food insecurity. Feeding America. Accessed September 22, 2025. https://www.feedingamerica.org/hunger-in-america/food-insecurity-in-veterans
Pradun S. Find access to stop food insecurity in your community. VA News. September 19, 2025. Accessed September 22, 2025. https://news.va.gov/142733/find-access-stop-food-insecurity-your-community/
Cohen AJ, Dosa DM, Rudolph JL, et al. Risk factors for veteran food insecurity: findings from a National US Department of Veterans Affairs Food Insecurity Screener. Public Health Nutr. 2022;25:819-828. doi:10.1017/S1368980021004584
Chen C. VA and Rockefeller Foundation collaborate to access food for Veterans. VA News. September 5, 2023. Accessed September 22, 2025. https://news.va.gov/123228/va-rockefeller-foundation-expand-access-to-food/
The requisites of government are that there be sufficiency of food, sufficiency of military equipment, and the confidence of the people in their ruler.
Analects by Confucius1
From ancient festivals to modern holidays, autumn has long been associated with the gathering of the harvest. Friends and families come together around tables laden with delicious food to enjoy the pleasures of peace and plenty. During these celebrations, we must never forget that without the strength of the nation’s military and the service of its veterans, this freedom and abundance would not be possible. Our debt of gratitude to the current and former members of the armed services makes the fact that a substantial minority experiences food insecurity not only a human tragedy, but a travesty of the nation’s promise to support those who wear or have worn the uniform.
The National Defense Authorization Act for Fiscal Year 2020 charged the Secretary of Defense to investigate food insecurity among active-duty service members and their dependents.2 The RAND Corporation conducted the assessment and, based on the results of its analysis, made recommendations to reduce hunger among armed forces members and their families.3
The RAND study found that 10% of active-duty military met US Department of Agriculture (USDA) criteria for very low food security; another 15% were classified as having low food security. The USDA defines food insecurity with hunger as “reports of multiple indications of disrupted eating patterns and reduced food intake.” USDA defines low food security as “reports of reduced quality, variety, or desirability of diet. Little or no indication of reduced food intake.”4
As someone who grew up on an Army base with the commissary a short trip from military housing, I was unpleasantly surprised that food insecurity was more common among in-service members living on post. I was even more dismayed to read that a variety of factors constrained 14% of active-duty military experiencing food insecurity to seek public assistance to feed themselves and their families. As with so many health care and social services, (eg, mental health care), those wearing the uniform were concerned that participating in a food assistance program would damage their career or stigmatize them. Others did not seek help, perhaps because they believed they were not eligible, and in many cases were correct: they did not qualify for food banks or food stamps due to receiving other benefits. A variety of factors contribute to periods of food insecurity among military families, including remote or rural bases that lack access to grocery stores or jobs for partners or other family members, and low base military pay.5
Food insecurity is an even more serious concern among veterans who are frequently older and have more comorbidities, often leading to unemployment and homelessness. Feeding America, the nation’s largest organization of community food banks, estimates that 1 in 9 working-age veterans are food insecure.5 US Department of Veterans Affairs (VA) statistics indicate that veterans are 7% more likely to experience food insecurity than other sectors of the population.6 The Veterans Health Administration has recognized that food insecurity is directly related to medical problems already common among veterans, including diabetes, obesity, and depression. Women and minority veterans are the most at risk of food insecurity.7
Recognizing that many veterans are at risk of food insecurity, the US Department of Defense and VA have taken steps to try and reduce hunger among those who serve. In response to the shocking statistic that food insecurity was found in 27% of Iraq and Afghanistan veterans, the VA and Rockefeller Foundation are partnering on the Food as Medicine initiative to improve veteran nutrition as a means of improving nutrition-related health consequences of food insecurity.8

Like many federal practitioners, I was unaware of the food insecurity assistance available to active-duty service members or veterans, or how to help individuals access it. In addition to the resources outlined in the Table, there are many community-based options open to anyone, including veterans and service members.
I have written columns on many difficult issues in my years as the Editor-in-Chief of Federal Practitioner, but personally this is one of the most distressing editorials I have ever published. That individuals dedicated to defending our rights and protecting our safety should be compelled to go hungry or not know if they have enough money at the end of the month to buy food is manifestly unjust. It is challenging when faced with such a large-scale injustice to think we cannot make a difference, but that resignation or abdication only magnifies this inequity. I have a friend who kept giving back even after they retired from federal service: they volunteered at a community garden and brought produce to the local food bank and helped distribute it. That may seem too much for those still working yet almost anyone can pick up a few items on their weekly shopping trip and donate them to a food drive.
As we approach Veterans Day, let’s not just express our gratitude to our military and veterans in words but in deeds like feeding the hungry and urging elected representatives to fulfill their commitment to ensure that service members and veterans and their families do not experience food insecurity. Confucian wisdom written in a very distant time and vastly dissimilar context still rings true: there are direct and critical links between food and trust and between hunger and the military.1
The requisites of government are that there be sufficiency of food, sufficiency of military equipment, and the confidence of the people in their ruler.
Analects by Confucius1
From ancient festivals to modern holidays, autumn has long been associated with the gathering of the harvest. Friends and families come together around tables laden with delicious food to enjoy the pleasures of peace and plenty. During these celebrations, we must never forget that without the strength of the nation’s military and the service of its veterans, this freedom and abundance would not be possible. Our debt of gratitude to the current and former members of the armed services makes the fact that a substantial minority experiences food insecurity not only a human tragedy, but a travesty of the nation’s promise to support those who wear or have worn the uniform.
The National Defense Authorization Act for Fiscal Year 2020 charged the Secretary of Defense to investigate food insecurity among active-duty service members and their dependents.2 The RAND Corporation conducted the assessment and, based on the results of its analysis, made recommendations to reduce hunger among armed forces members and their families.3
The RAND study found that 10% of active-duty military met US Department of Agriculture (USDA) criteria for very low food security; another 15% were classified as having low food security. The USDA defines food insecurity with hunger as “reports of multiple indications of disrupted eating patterns and reduced food intake.” USDA defines low food security as “reports of reduced quality, variety, or desirability of diet. Little or no indication of reduced food intake.”4
As someone who grew up on an Army base with the commissary a short trip from military housing, I was unpleasantly surprised that food insecurity was more common among in-service members living on post. I was even more dismayed to read that a variety of factors constrained 14% of active-duty military experiencing food insecurity to seek public assistance to feed themselves and their families. As with so many health care and social services, (eg, mental health care), those wearing the uniform were concerned that participating in a food assistance program would damage their career or stigmatize them. Others did not seek help, perhaps because they believed they were not eligible, and in many cases were correct: they did not qualify for food banks or food stamps due to receiving other benefits. A variety of factors contribute to periods of food insecurity among military families, including remote or rural bases that lack access to grocery stores or jobs for partners or other family members, and low base military pay.5
Food insecurity is an even more serious concern among veterans who are frequently older and have more comorbidities, often leading to unemployment and homelessness. Feeding America, the nation’s largest organization of community food banks, estimates that 1 in 9 working-age veterans are food insecure.5 US Department of Veterans Affairs (VA) statistics indicate that veterans are 7% more likely to experience food insecurity than other sectors of the population.6 The Veterans Health Administration has recognized that food insecurity is directly related to medical problems already common among veterans, including diabetes, obesity, and depression. Women and minority veterans are the most at risk of food insecurity.7
Recognizing that many veterans are at risk of food insecurity, the US Department of Defense and VA have taken steps to try and reduce hunger among those who serve. In response to the shocking statistic that food insecurity was found in 27% of Iraq and Afghanistan veterans, the VA and Rockefeller Foundation are partnering on the Food as Medicine initiative to improve veteran nutrition as a means of improving nutrition-related health consequences of food insecurity.8

Like many federal practitioners, I was unaware of the food insecurity assistance available to active-duty service members or veterans, or how to help individuals access it. In addition to the resources outlined in the Table, there are many community-based options open to anyone, including veterans and service members.
I have written columns on many difficult issues in my years as the Editor-in-Chief of Federal Practitioner, but personally this is one of the most distressing editorials I have ever published. That individuals dedicated to defending our rights and protecting our safety should be compelled to go hungry or not know if they have enough money at the end of the month to buy food is manifestly unjust. It is challenging when faced with such a large-scale injustice to think we cannot make a difference, but that resignation or abdication only magnifies this inequity. I have a friend who kept giving back even after they retired from federal service: they volunteered at a community garden and brought produce to the local food bank and helped distribute it. That may seem too much for those still working yet almost anyone can pick up a few items on their weekly shopping trip and donate them to a food drive.
As we approach Veterans Day, let’s not just express our gratitude to our military and veterans in words but in deeds like feeding the hungry and urging elected representatives to fulfill their commitment to ensure that service members and veterans and their families do not experience food insecurity. Confucian wisdom written in a very distant time and vastly dissimilar context still rings true: there are direct and critical links between food and trust and between hunger and the military.1
Dawson MM. The Wisdom of Confucius: A Collection of the Ethical Sayings of Confucius and of his disciples. International Pocket Library; 1932.
National Defense Authorization Act for Fiscal Year 2020. 116th Cong (2019), Public Law 116-92. U.S. Government Printing Office. https://www.govinfo.gov/content/pkg/PLAW-116publ92/html/PLAW-116publ92.htm
Asch BJ, Rennane S, Trail TE, et al. Food insecurity among members of the armed forces and their dependents. RAND Corporation. January 3, 2023. Accessed September 22, 2025. https://www.rand.org/pubs/research_reports/RRA1230-1.html
US Department of Agriculture Economic Research Service. Food Security in the U.S.—Definitions of Food Security. US Department of Agriculture Economic Research Service. January 10, 2025. https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security
Active military and veteran food insecurity. Feeding America. Accessed September 22, 2025. https://www.feedingamerica.org/hunger-in-america/food-insecurity-in-veterans
Pradun S. Find access to stop food insecurity in your community. VA News. September 19, 2025. Accessed September 22, 2025. https://news.va.gov/142733/find-access-stop-food-insecurity-your-community/
Cohen AJ, Dosa DM, Rudolph JL, et al. Risk factors for veteran food insecurity: findings from a National US Department of Veterans Affairs Food Insecurity Screener. Public Health Nutr. 2022;25:819-828. doi:10.1017/S1368980021004584
Chen C. VA and Rockefeller Foundation collaborate to access food for Veterans. VA News. September 5, 2023. Accessed September 22, 2025. https://news.va.gov/123228/va-rockefeller-foundation-expand-access-to-food/
Dawson MM. The Wisdom of Confucius: A Collection of the Ethical Sayings of Confucius and of his disciples. International Pocket Library; 1932.
National Defense Authorization Act for Fiscal Year 2020. 116th Cong (2019), Public Law 116-92. U.S. Government Printing Office. https://www.govinfo.gov/content/pkg/PLAW-116publ92/html/PLAW-116publ92.htm
Asch BJ, Rennane S, Trail TE, et al. Food insecurity among members of the armed forces and their dependents. RAND Corporation. January 3, 2023. Accessed September 22, 2025. https://www.rand.org/pubs/research_reports/RRA1230-1.html
US Department of Agriculture Economic Research Service. Food Security in the U.S.—Definitions of Food Security. US Department of Agriculture Economic Research Service. January 10, 2025. https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security
Active military and veteran food insecurity. Feeding America. Accessed September 22, 2025. https://www.feedingamerica.org/hunger-in-america/food-insecurity-in-veterans
Pradun S. Find access to stop food insecurity in your community. VA News. September 19, 2025. Accessed September 22, 2025. https://news.va.gov/142733/find-access-stop-food-insecurity-your-community/
Cohen AJ, Dosa DM, Rudolph JL, et al. Risk factors for veteran food insecurity: findings from a National US Department of Veterans Affairs Food Insecurity Screener. Public Health Nutr. 2022;25:819-828. doi:10.1017/S1368980021004584
Chen C. VA and Rockefeller Foundation collaborate to access food for Veterans. VA News. September 5, 2023. Accessed September 22, 2025. https://news.va.gov/123228/va-rockefeller-foundation-expand-access-to-food/
American Hunger Games: Food Insecurity Among the Military and Veterans
American Hunger Games: Food Insecurity Among the Military and Veterans
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.
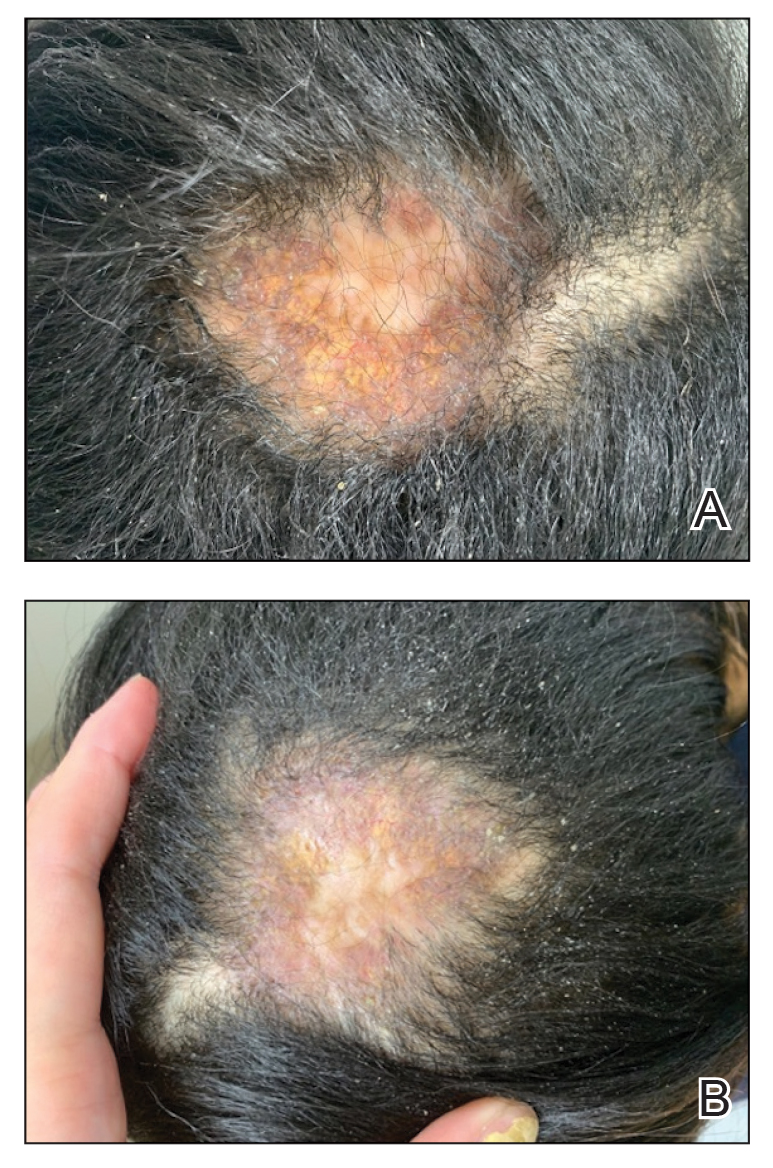
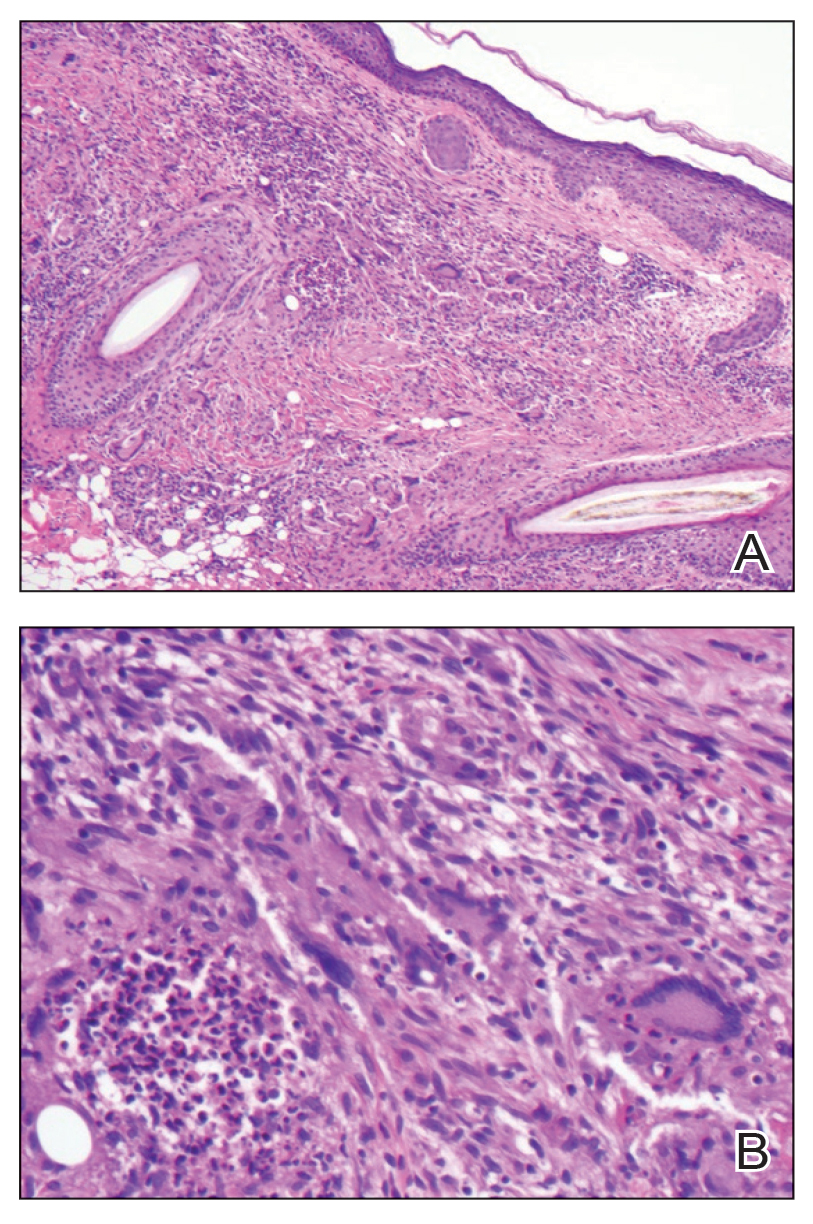
We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.


We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.


We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
PRACTICE POINTS
- In skin of color, necrobiotic xanthogranuloma can appear orange or brown compared to its yellow appearance in lighter skin types.
- When necrobiotic xanthogranuloma is suspected, a thorough malignancy workup should be conducted.
Racial, Ethnic Discrimination Tied to Psychosis Risk
TOPLINE:
Racial and ethnic discrimination was consistently associated with increased risk for psychosis in studies included in a new umbrella review, with odds nearly doubled for both psychotic symptoms and experiences.
METHODOLOGY:
- Researchers searched 5 databases and then conducted an umbrella review of 7 systematic reviews, 4 of which included meta-analyses, published between 2003 and 2023.
- The systematic reviews included 23 primary studies representing more than 40,000 participants from Europe and the US.
- Investigators assessed the potential association between perceived racial or ethnic discrimination (mostly measured using self-reported questionnaires) and risk for psychosis (measured using established questionnaires).
- They assessed the risk for bias using the 16-item A MeaSurement Tool to Assess systematic Reviews, version 2 (AMSTAR-2) checklist.
TAKEAWAY:
- All reviews that included meta-analyses showed significant associations between perceived ethnic discrimination and psychotic symptoms (adjusted odds ratio [aOR], 1.78; 95% CI, 1.3-2.5) and psychotic experiences (pooled OR, 1.9; 95% CI, 1.4-2.7).
- Perceived racial or ethnic discrimination was also strongly linked to delusional symptoms (OR, 2.5; 95% CI, 1.6-4.0) and hallucinatory symptoms (OR, 1.65; 95% CI, 1.3-2.1).
- The largest of the included studies showed a dose-response relationship between higher levels of lifetime perceived racial or ethnic discrimination and greater likelihood of psychotic experiences.
- More robust associations were found in nonclinical populations compared to clinical ones, but there were significant associations in both.
IN PRACTICE:
“Our review was only looking at the impact of a person directly perceiving racism or interpersonal racial or ethnic discrimination; it may be that systemic racism, which can go unseen but still have profound impacts, could further contribute to mental health disparities,” lead investigator India Francis-Crossley, University College London, London, UK, said in a press release.
SOURCE:
The study was published online in PLOS Mental Health.
LIMITATIONS:
The evidence was primarily based on cross-sectional studies and was limited by high heterogeneity. The reviews included showed low or critically low AMSTAR-2 quality scores, which may have affected the robustness of the findings. More robust evidence was observed for psychotic outcomes in nonclinical populations compared to clinical samples. Additionally, the study potentially exacerbated errors or misreporting in the original reviews and did not include relevant structural factors such as income, education, housing, and poverty.
DISCLOSURES:
The study was funded by the University College London-Windsor Fellowship Research Opportunities scholarship, Wellcome Trust PhD Fellowship in Mental Health Science, Mental Health Mission Early Psychosis Workstream, and UK Research and Innovation funding for the Population Mental Health Consortium. The investigators reported having no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Racial and ethnic discrimination was consistently associated with increased risk for psychosis in studies included in a new umbrella review, with odds nearly doubled for both psychotic symptoms and experiences.
METHODOLOGY:
- Researchers searched 5 databases and then conducted an umbrella review of 7 systematic reviews, 4 of which included meta-analyses, published between 2003 and 2023.
- The systematic reviews included 23 primary studies representing more than 40,000 participants from Europe and the US.
- Investigators assessed the potential association between perceived racial or ethnic discrimination (mostly measured using self-reported questionnaires) and risk for psychosis (measured using established questionnaires).
- They assessed the risk for bias using the 16-item A MeaSurement Tool to Assess systematic Reviews, version 2 (AMSTAR-2) checklist.
TAKEAWAY:
- All reviews that included meta-analyses showed significant associations between perceived ethnic discrimination and psychotic symptoms (adjusted odds ratio [aOR], 1.78; 95% CI, 1.3-2.5) and psychotic experiences (pooled OR, 1.9; 95% CI, 1.4-2.7).
- Perceived racial or ethnic discrimination was also strongly linked to delusional symptoms (OR, 2.5; 95% CI, 1.6-4.0) and hallucinatory symptoms (OR, 1.65; 95% CI, 1.3-2.1).
- The largest of the included studies showed a dose-response relationship between higher levels of lifetime perceived racial or ethnic discrimination and greater likelihood of psychotic experiences.
- More robust associations were found in nonclinical populations compared to clinical ones, but there were significant associations in both.
IN PRACTICE:
“Our review was only looking at the impact of a person directly perceiving racism or interpersonal racial or ethnic discrimination; it may be that systemic racism, which can go unseen but still have profound impacts, could further contribute to mental health disparities,” lead investigator India Francis-Crossley, University College London, London, UK, said in a press release.
SOURCE:
The study was published online in PLOS Mental Health.
LIMITATIONS:
The evidence was primarily based on cross-sectional studies and was limited by high heterogeneity. The reviews included showed low or critically low AMSTAR-2 quality scores, which may have affected the robustness of the findings. More robust evidence was observed for psychotic outcomes in nonclinical populations compared to clinical samples. Additionally, the study potentially exacerbated errors or misreporting in the original reviews and did not include relevant structural factors such as income, education, housing, and poverty.
DISCLOSURES:
The study was funded by the University College London-Windsor Fellowship Research Opportunities scholarship, Wellcome Trust PhD Fellowship in Mental Health Science, Mental Health Mission Early Psychosis Workstream, and UK Research and Innovation funding for the Population Mental Health Consortium. The investigators reported having no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Racial and ethnic discrimination was consistently associated with increased risk for psychosis in studies included in a new umbrella review, with odds nearly doubled for both psychotic symptoms and experiences.
METHODOLOGY:
- Researchers searched 5 databases and then conducted an umbrella review of 7 systematic reviews, 4 of which included meta-analyses, published between 2003 and 2023.
- The systematic reviews included 23 primary studies representing more than 40,000 participants from Europe and the US.
- Investigators assessed the potential association between perceived racial or ethnic discrimination (mostly measured using self-reported questionnaires) and risk for psychosis (measured using established questionnaires).
- They assessed the risk for bias using the 16-item A MeaSurement Tool to Assess systematic Reviews, version 2 (AMSTAR-2) checklist.
TAKEAWAY:
- All reviews that included meta-analyses showed significant associations between perceived ethnic discrimination and psychotic symptoms (adjusted odds ratio [aOR], 1.78; 95% CI, 1.3-2.5) and psychotic experiences (pooled OR, 1.9; 95% CI, 1.4-2.7).
- Perceived racial or ethnic discrimination was also strongly linked to delusional symptoms (OR, 2.5; 95% CI, 1.6-4.0) and hallucinatory symptoms (OR, 1.65; 95% CI, 1.3-2.1).
- The largest of the included studies showed a dose-response relationship between higher levels of lifetime perceived racial or ethnic discrimination and greater likelihood of psychotic experiences.
- More robust associations were found in nonclinical populations compared to clinical ones, but there were significant associations in both.
IN PRACTICE:
“Our review was only looking at the impact of a person directly perceiving racism or interpersonal racial or ethnic discrimination; it may be that systemic racism, which can go unseen but still have profound impacts, could further contribute to mental health disparities,” lead investigator India Francis-Crossley, University College London, London, UK, said in a press release.
SOURCE:
The study was published online in PLOS Mental Health.
LIMITATIONS:
The evidence was primarily based on cross-sectional studies and was limited by high heterogeneity. The reviews included showed low or critically low AMSTAR-2 quality scores, which may have affected the robustness of the findings. More robust evidence was observed for psychotic outcomes in nonclinical populations compared to clinical samples. Additionally, the study potentially exacerbated errors or misreporting in the original reviews and did not include relevant structural factors such as income, education, housing, and poverty.
DISCLOSURES:
The study was funded by the University College London-Windsor Fellowship Research Opportunities scholarship, Wellcome Trust PhD Fellowship in Mental Health Science, Mental Health Mission Early Psychosis Workstream, and UK Research and Innovation funding for the Population Mental Health Consortium. The investigators reported having no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Parental Mental Disorders May Double Offspring Mortality Risk
TOPLINE:
Offspring of parents with mental disorders had nearly double the risk for mortality, especially from unnatural causes, compared to those with parents did not have a mental disorder, a new Swedish cohort study showed. Additionally, mortality risk was highest when both parents had mental disorders but was not affected by the sex of the affected parent.
METHODOLOGY:
- A nationwide register-based cohort study in Sweden included more than 3.5 million individuals born between 1973 and 2014 (51% men); 35% had a parent with a mental disorder (13% paternal, 16% maternal, and 6% both parents).
- Mental disorder categories included alcohol or substance use, psychotic, mood, anxiety or stress-related, eating, and personality disorders and intellectual disability. Exposure timing was classified by offspring age (mean age, 15.8 years) at parental diagnosis.
- Participants were followed up from birth until death, the death of either parent, emigration (up to 2014), either parent’s emigration, or the end of 2023, whichever came first (median follow-up duration, 20.1 years).
- The main outcome was all-cause mortality; secondary outcomes were deaths from natural and unnatural causes, as well as deaths from cardiovascular disease, cancer, suicide, and unintentional injuries. Cousin comparison analyses were also conducted to account for confounding.
TAKEAWAY:
- During the follow-up, offspring exposed to parental psychiatric disorders had higher overall mortality rates than unexposed offspring (7.9 vs 3.55 per 10,000 person-years). Mortality rates due to natural causes were 4.0 vs 2.4 per 10,000 person-years and were 3.95 vs 1.1 per 10,000 person-years for mortality due to unnatural causes.
- Exposed offspring had an increased risk for mortality due to any cause (adjusted hazard ratio [aHR], 2.1), natural causes (aHR, 1.9), and unnatural causes (aHR, 2.45). Exposure was also associated with an increased risk for cardiovascular and cancer-related death, suicide, and death due to unintentional injuries. The associations remained significant, although slightly attenuated, in cousin comparison analyses.
- The highest risks for mortality were in offspring exposed at ages 1-2 years to both parents having mental disorders (HR for natural causes, 4.5; HR for unnatural causes, 5.3).
- The risk varied by the type of parental mental disorder, with HRs ranging from 1.6 for eating disorders to 2.2 for intellectual disability.
IN PRACTICE:
“Our findings highlight the need for improved surveillance, prevention, and early detection strategies to reduce the risk of premature mortality among offspring exposed to parental mental disorders. Whether additional support for families affected by mental disorders could mitigate the risk warrants further investigation,” the investigators wrote.
SOURCE:
This study was led by Hui Wang, PhD, Karolinska Institutet, Stockholm, Sweden. It was published online in JAMA Psychiatry.
LIMITATIONS:
Reliance on registry data may have led to the misclassification of parental mental disorders. The study lacked data on genetic factors, parenting quality, cohabitation, and social support, and its generalizability may have been limited. Immigration data after 2014 were unavailable, potentially leading to misclassifications of exposure and outcomes. The Patient Register did not distinguish between diagnoses made in general vs psychiatric hospital settings, and cousin comparisons remained susceptible to bias from unmeasured confounding and may have been limited in capturing temporal and familial heterogeneity.
DISCLOSURES:
This study was funded by the Swedish Research Council for Health, Working Life and Welfare and the Heart and Lung Foundation. Wang reported having no relevant financial relationships. The other investigator reported receiving grants from Forte and the Heart and Lung Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Offspring of parents with mental disorders had nearly double the risk for mortality, especially from unnatural causes, compared to those with parents did not have a mental disorder, a new Swedish cohort study showed. Additionally, mortality risk was highest when both parents had mental disorders but was not affected by the sex of the affected parent.
METHODOLOGY:
- A nationwide register-based cohort study in Sweden included more than 3.5 million individuals born between 1973 and 2014 (51% men); 35% had a parent with a mental disorder (13% paternal, 16% maternal, and 6% both parents).
- Mental disorder categories included alcohol or substance use, psychotic, mood, anxiety or stress-related, eating, and personality disorders and intellectual disability. Exposure timing was classified by offspring age (mean age, 15.8 years) at parental diagnosis.
- Participants were followed up from birth until death, the death of either parent, emigration (up to 2014), either parent’s emigration, or the end of 2023, whichever came first (median follow-up duration, 20.1 years).
- The main outcome was all-cause mortality; secondary outcomes were deaths from natural and unnatural causes, as well as deaths from cardiovascular disease, cancer, suicide, and unintentional injuries. Cousin comparison analyses were also conducted to account for confounding.
TAKEAWAY:
- During the follow-up, offspring exposed to parental psychiatric disorders had higher overall mortality rates than unexposed offspring (7.9 vs 3.55 per 10,000 person-years). Mortality rates due to natural causes were 4.0 vs 2.4 per 10,000 person-years and were 3.95 vs 1.1 per 10,000 person-years for mortality due to unnatural causes.
- Exposed offspring had an increased risk for mortality due to any cause (adjusted hazard ratio [aHR], 2.1), natural causes (aHR, 1.9), and unnatural causes (aHR, 2.45). Exposure was also associated with an increased risk for cardiovascular and cancer-related death, suicide, and death due to unintentional injuries. The associations remained significant, although slightly attenuated, in cousin comparison analyses.
- The highest risks for mortality were in offspring exposed at ages 1-2 years to both parents having mental disorders (HR for natural causes, 4.5; HR for unnatural causes, 5.3).
- The risk varied by the type of parental mental disorder, with HRs ranging from 1.6 for eating disorders to 2.2 for intellectual disability.
IN PRACTICE:
“Our findings highlight the need for improved surveillance, prevention, and early detection strategies to reduce the risk of premature mortality among offspring exposed to parental mental disorders. Whether additional support for families affected by mental disorders could mitigate the risk warrants further investigation,” the investigators wrote.
SOURCE:
This study was led by Hui Wang, PhD, Karolinska Institutet, Stockholm, Sweden. It was published online in JAMA Psychiatry.
LIMITATIONS:
Reliance on registry data may have led to the misclassification of parental mental disorders. The study lacked data on genetic factors, parenting quality, cohabitation, and social support, and its generalizability may have been limited. Immigration data after 2014 were unavailable, potentially leading to misclassifications of exposure and outcomes. The Patient Register did not distinguish between diagnoses made in general vs psychiatric hospital settings, and cousin comparisons remained susceptible to bias from unmeasured confounding and may have been limited in capturing temporal and familial heterogeneity.
DISCLOSURES:
This study was funded by the Swedish Research Council for Health, Working Life and Welfare and the Heart and Lung Foundation. Wang reported having no relevant financial relationships. The other investigator reported receiving grants from Forte and the Heart and Lung Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Offspring of parents with mental disorders had nearly double the risk for mortality, especially from unnatural causes, compared to those with parents did not have a mental disorder, a new Swedish cohort study showed. Additionally, mortality risk was highest when both parents had mental disorders but was not affected by the sex of the affected parent.
METHODOLOGY:
- A nationwide register-based cohort study in Sweden included more than 3.5 million individuals born between 1973 and 2014 (51% men); 35% had a parent with a mental disorder (13% paternal, 16% maternal, and 6% both parents).
- Mental disorder categories included alcohol or substance use, psychotic, mood, anxiety or stress-related, eating, and personality disorders and intellectual disability. Exposure timing was classified by offspring age (mean age, 15.8 years) at parental diagnosis.
- Participants were followed up from birth until death, the death of either parent, emigration (up to 2014), either parent’s emigration, or the end of 2023, whichever came first (median follow-up duration, 20.1 years).
- The main outcome was all-cause mortality; secondary outcomes were deaths from natural and unnatural causes, as well as deaths from cardiovascular disease, cancer, suicide, and unintentional injuries. Cousin comparison analyses were also conducted to account for confounding.
TAKEAWAY:
- During the follow-up, offspring exposed to parental psychiatric disorders had higher overall mortality rates than unexposed offspring (7.9 vs 3.55 per 10,000 person-years). Mortality rates due to natural causes were 4.0 vs 2.4 per 10,000 person-years and were 3.95 vs 1.1 per 10,000 person-years for mortality due to unnatural causes.
- Exposed offspring had an increased risk for mortality due to any cause (adjusted hazard ratio [aHR], 2.1), natural causes (aHR, 1.9), and unnatural causes (aHR, 2.45). Exposure was also associated with an increased risk for cardiovascular and cancer-related death, suicide, and death due to unintentional injuries. The associations remained significant, although slightly attenuated, in cousin comparison analyses.
- The highest risks for mortality were in offspring exposed at ages 1-2 years to both parents having mental disorders (HR for natural causes, 4.5; HR for unnatural causes, 5.3).
- The risk varied by the type of parental mental disorder, with HRs ranging from 1.6 for eating disorders to 2.2 for intellectual disability.
IN PRACTICE:
“Our findings highlight the need for improved surveillance, prevention, and early detection strategies to reduce the risk of premature mortality among offspring exposed to parental mental disorders. Whether additional support for families affected by mental disorders could mitigate the risk warrants further investigation,” the investigators wrote.
SOURCE:
This study was led by Hui Wang, PhD, Karolinska Institutet, Stockholm, Sweden. It was published online in JAMA Psychiatry.
LIMITATIONS:
Reliance on registry data may have led to the misclassification of parental mental disorders. The study lacked data on genetic factors, parenting quality, cohabitation, and social support, and its generalizability may have been limited. Immigration data after 2014 were unavailable, potentially leading to misclassifications of exposure and outcomes. The Patient Register did not distinguish between diagnoses made in general vs psychiatric hospital settings, and cousin comparisons remained susceptible to bias from unmeasured confounding and may have been limited in capturing temporal and familial heterogeneity.
DISCLOSURES:
This study was funded by the Swedish Research Council for Health, Working Life and Welfare and the Heart and Lung Foundation. Wang reported having no relevant financial relationships. The other investigator reported receiving grants from Forte and the Heart and Lung Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.
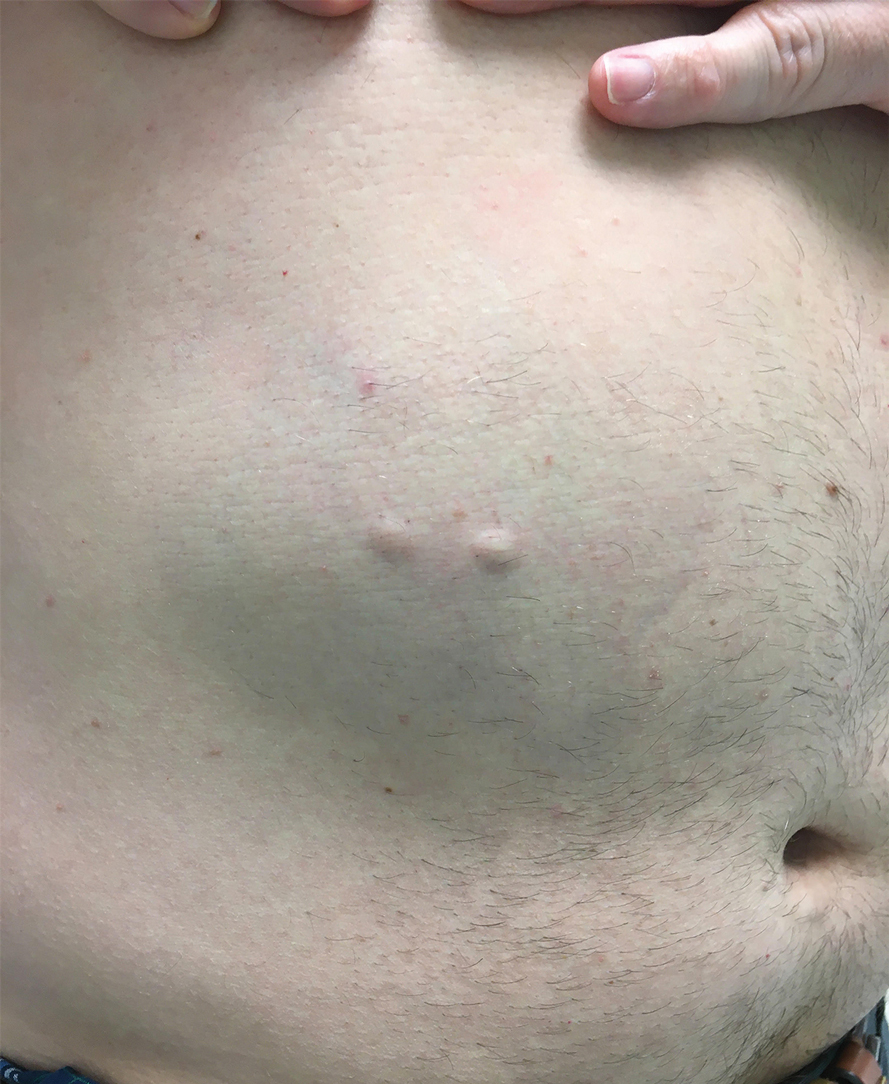
Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23
Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33
Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.
Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.

Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23


Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33

Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.


Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.

Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23


Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33

Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.


Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Practice Points
- Steatocystomas, which manifest as single or multiple flesh-colored subcutaneous cysts ranging from less than 3 mm to more than 3 cm, typically are asymptomatic and can persist indefinitely.
- Treatment options for steatocystomas include oral isotretinoin, tetracycline derivatives, and intralesional steroid injections. Minimally invasive procedures such as drainage and resection also are available, employing techniques such as blade incision, radiofrequency probes, and laser treatments to minimize scarring and recurrence.
- Conservative therapies such as watchful waiting are recommended for the simplex and multiplex variants, while more aggressive management such as surgical removal is recommended for the multiplex suppurativa variant due to its elevated risk for complications.