User login
For MD-IQ use only
What's your diagnosis?
Sigmoid colon perforation secondary to transcutaneous epicardial pacer wires.
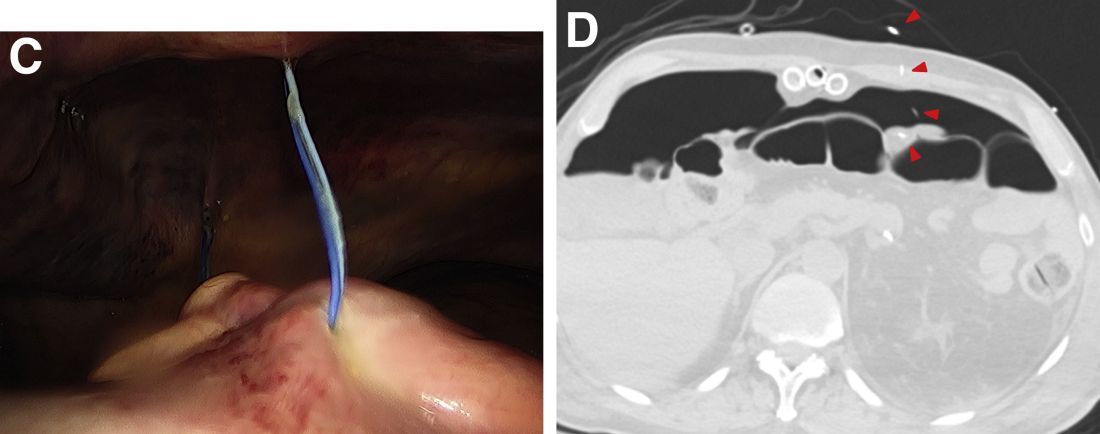
A plain film image (Figure A) shows diffusely dilated loops of bowel with subdiaphragmatic air concerning for GI viscous perforation. Dedicated cross-sectional imaging confirms intra-abdominal free air, and in representative cross section, the epicardial pacing wires can be visualized within the gastrointestinal lumen (Figure D, arrows). At the time of surgical consultation, the radiology report was notable for concern regarding possible disruption of peritoneum secondary to the difficult surgical chest tube placement in a patient with a high-riding left hemidiaphragm. Urgent laparoscopic exploration secondary to these findings unexpectedly revealed that the transcutaneous epicardial pacing wires had been inadvertently placed through the sigmoid colon (Figure C). The pacer wires were cut and removed intraoperatively. Unfortunately, 4 days after removal of pacer wires, the patient continued to have ongoing distension and was found to have sigmoid volvulus necessitating endoscopic decompression. After a prolonged hospitalization and recovery, he was discharged with a normal bowel pattern and tolerating oral intake to a skilled nursing facility.
Temporary transcutaneous epicardial pacing wires are often placed after complex cardiovascular surgical procedures. Complications from wire placement are thought to be relatively rare and are typically associated with migration into local structures after wire placement and infectious complications secondary to retained wires.1,2 Perforation of local structures during placement is less common, and GI viscous perforation in particular is not a well-characterized cause of associated morbidity.3
Our case demonstrates that, in patients with hemidiaphragm elevation, epicardial wire placement risks GI viscous perforation. Furthermore, given the frequency of concomitant surgical hardware in this patient population, identification of malpositioned epicardial wires on plain film and even cross-sectional imaging can be difficult and can delay diagnosis.
References
1. Del Nido P, Goldman BS. J Card Surg. 1989 Mar;4(1):99-103.
2. Kapoor A et al. Interact Cardiovasc Thorac Surg. 2011 Jul;13(1):104-6.
3. Haba J et al. Can Assoc Radiol J. 2013 Feb;64(1):77-80.
Sigmoid colon perforation secondary to transcutaneous epicardial pacer wires.
A plain film image (Figure A) shows diffusely dilated loops of bowel with subdiaphragmatic air concerning for GI viscous perforation. Dedicated cross-sectional imaging confirms intra-abdominal free air, and in representative cross section, the epicardial pacing wires can be visualized within the gastrointestinal lumen (Figure D, arrows). At the time of surgical consultation, the radiology report was notable for concern regarding possible disruption of peritoneum secondary to the difficult surgical chest tube placement in a patient with a high-riding left hemidiaphragm. Urgent laparoscopic exploration secondary to these findings unexpectedly revealed that the transcutaneous epicardial pacing wires had been inadvertently placed through the sigmoid colon (Figure C). The pacer wires were cut and removed intraoperatively. Unfortunately, 4 days after removal of pacer wires, the patient continued to have ongoing distension and was found to have sigmoid volvulus necessitating endoscopic decompression. After a prolonged hospitalization and recovery, he was discharged with a normal bowel pattern and tolerating oral intake to a skilled nursing facility.

Temporary transcutaneous epicardial pacing wires are often placed after complex cardiovascular surgical procedures. Complications from wire placement are thought to be relatively rare and are typically associated with migration into local structures after wire placement and infectious complications secondary to retained wires.1,2 Perforation of local structures during placement is less common, and GI viscous perforation in particular is not a well-characterized cause of associated morbidity.3
Our case demonstrates that, in patients with hemidiaphragm elevation, epicardial wire placement risks GI viscous perforation. Furthermore, given the frequency of concomitant surgical hardware in this patient population, identification of malpositioned epicardial wires on plain film and even cross-sectional imaging can be difficult and can delay diagnosis.
References
1. Del Nido P, Goldman BS. J Card Surg. 1989 Mar;4(1):99-103.
2. Kapoor A et al. Interact Cardiovasc Thorac Surg. 2011 Jul;13(1):104-6.
3. Haba J et al. Can Assoc Radiol J. 2013 Feb;64(1):77-80.
Sigmoid colon perforation secondary to transcutaneous epicardial pacer wires.
A plain film image (Figure A) shows diffusely dilated loops of bowel with subdiaphragmatic air concerning for GI viscous perforation. Dedicated cross-sectional imaging confirms intra-abdominal free air, and in representative cross section, the epicardial pacing wires can be visualized within the gastrointestinal lumen (Figure D, arrows). At the time of surgical consultation, the radiology report was notable for concern regarding possible disruption of peritoneum secondary to the difficult surgical chest tube placement in a patient with a high-riding left hemidiaphragm. Urgent laparoscopic exploration secondary to these findings unexpectedly revealed that the transcutaneous epicardial pacing wires had been inadvertently placed through the sigmoid colon (Figure C). The pacer wires were cut and removed intraoperatively. Unfortunately, 4 days after removal of pacer wires, the patient continued to have ongoing distension and was found to have sigmoid volvulus necessitating endoscopic decompression. After a prolonged hospitalization and recovery, he was discharged with a normal bowel pattern and tolerating oral intake to a skilled nursing facility.

Temporary transcutaneous epicardial pacing wires are often placed after complex cardiovascular surgical procedures. Complications from wire placement are thought to be relatively rare and are typically associated with migration into local structures after wire placement and infectious complications secondary to retained wires.1,2 Perforation of local structures during placement is less common, and GI viscous perforation in particular is not a well-characterized cause of associated morbidity.3
Our case demonstrates that, in patients with hemidiaphragm elevation, epicardial wire placement risks GI viscous perforation. Furthermore, given the frequency of concomitant surgical hardware in this patient population, identification of malpositioned epicardial wires on plain film and even cross-sectional imaging can be difficult and can delay diagnosis.
References
1. Del Nido P, Goldman BS. J Card Surg. 1989 Mar;4(1):99-103.
2. Kapoor A et al. Interact Cardiovasc Thorac Surg. 2011 Jul;13(1):104-6.
3. Haba J et al. Can Assoc Radiol J. 2013 Feb;64(1):77-80.
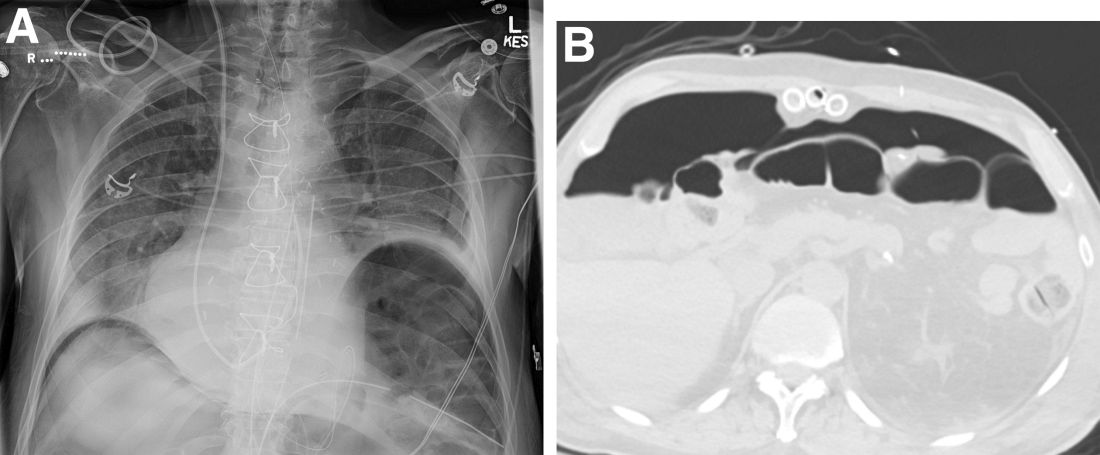
Question: An 82-year-old man was admitted for urgent coronary artery bypass and concurrent mitral valve repair. Intraoperatively, he underwent cardiopulmonary bypass, epicardial pacing, and placement of two anterior mediastinal and one pleural chest tubes. After a relatively unremarkable initial postoperative course and nonnarcotic pain control, concern for ileus developed on postoperative day 4. A nasogastric tube was placed out of concern for worsening somnolence, nausea, and the inability to safely tolerate oral intake. The patient had been passing flatus but had yet to have a bowel movement since the operation. Physical examination at the time was notable for a soft abdomen with diffuse tenderness and voluntary guarding. Subsequent plain film imaging to confirm nasogastric tube placement (Figure A) and follow-up computed tomography imaging (Figure B) are shown.
What's the diagnosis?

FDA posts new websites on accelerated approvals for cancer drugs
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
, including a public list detailing cases where accelerated approvals have been rescinded for lack of evidence.
On Oct. 29, the Food and Drug Administration posted new websites detailing the status of oncology medicines given these special clearances:
- Ongoing | Cancer Accelerated Approvals
- Verified Clinical Benefit | Cancer Accelerated Approvals
- Withdrawn | Cancer Accelerated Approvals
The FDA’s cancer center also has created a web page called Project Confirm to provide more information on the way it uses accelerated approvals.
There has been increased concern about medicines cleared by accelerated approvals in recent years, culminating in an uproar over the controversial June approval of aducanumab (Aduhelm) for Alzheimer’s disease. This drew more attention to a debate already underway about how much data supports some of the indications for some cancer drugs.
Federal and state officials and advisers are putting more pressure on pharmaceutical companies to prove that medicines that are put on the market through accelerated approval do deliver meaningful benefits for patients.
In addition, earlier this month two of the top health advisers in Barack Obama’s administration proposed a new model through which Medicare could reduce payments for certain cancer drugs cleared through accelerated approvals – and even cut off reimbursements in cases where companies fail to deliver confirmatory evidence for expected benefits.
This “Pay for Drugs That Work Model” was proposed by Richard Frank, PhD, and Ezekiel Emanuel, MD, PhD, in a recent JAMA article. In their view, the FDA’s accelerated drug approval process allows for too many delays in obtaining answers as to whether medicines cleared this way provide expected benefits.
“The proposed Pay for Drugs That Work model could test a modified approach for incentivizing rapid completion of confirmatory trials to inform clinicians and patients about the true risks and benefits of new drugs and improve the value for money of cancer drugs that receive accelerated approval,” they wrote.
Excel files, regular updates
For the FDA, accelerated approvals require balancing an estimated potential benefit for people facing serious diseases (for example, cancer) against serious risks, including potentially exposing patients to costly, toxic drugs that will later be shown not to work for their conditions.
For many years, there has been significant pressure on the FDA to lean toward speedier approvals, with members of Congress, advocacy groups, and drugmakers advocating for broad use of surrogate data in deciding on clearances. The FDA posts biannual reports on its website that highlight how quickly approvals have been granted. But these biannual reports don’t provide much information on the status of accelerated-approval drugs, other than to say if they have been given full approval or withdrawn.
The newly created websites from the FDA’s oncology division appear to reflect growing public interest in knowing what standards the agency sets for confirmatory trials and what deadlines companies face to deliver evidence of significant benefit for their drugs.
The new sortable websites also include details on trials and have links to Excel files which will help researchers and others seeking to track patterns with accelerated approvals. The FDA said in an interview that it intends to update these sites when there are developments with accelerated approvals for cancer drugs, such as new clearances of this type, conversions to regular approvals, and withdrawn approvals.
Julia Beaver, MD, chief of medical oncology at the FDA’s Oncology Center of Excellence, and acting deputy director of the Office of Oncologic Diseases of the FDA’s Center for Drug Evaluation and Research, described the new websites as part of a “commitment to preserve the integrity” of the accelerated approval program.
“These new web pages will make information on our accelerated approvals more transparent,” Dr. Beaver said in an email to this news organization.
The FDA has been able to speed many medicines to market and clear additional uses for drugs already sold through the program, giving people earlier access in many cases to critical medicines, Dr. Beaver said.
More than 165 oncology indications have received accelerated approval, with almost half converted to regular approval in a median of 3 years. Less than 10% of these indications were withdrawn, Dr. Beaver said.
“Of those accelerated approvals that were converted to regular approval, many demonstrated survival advantages to patients with several types of cancer or provided meaningful therapeutic options where none previously existed,” she said.
However, Dr. Beaver also has made public the FDA’s concerns with what she and Richard Pazdur, MD, director of the Oncology Center of Excellence, have described as “dangling” accelerated approvals.
These are cases where the required trials did not end up confirming benefit for a medicine, yet the manufacturer did not move to withdraw an accelerated approval. The FDA’s cancer center has already announced that it is doing an “industry-wide evaluation of accelerated approvals in oncology in which confirmatory trials did not confirm clinical benefit.”
This stems in part from what can be called the FDA’s “growing pains” in its efforts to manage the rapidly changing landscape for these immunotherapy checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials for an Oncologic Drugs Advisory Committee (ODAC) meeting last April on dangling accelerated approvals.
A newly posted chart on withdrawn oncology accelerated approvals, posted by the FDA’s cancer division, makes it clear that the pace of these rescinded clearances has picked up. The chart lists a total 14 withdrawn indications of oncology accelerated approvals.
Six of these withdrawals happened this year.
There were two withdrawals in 2020, including the December withdrawal of nivolumab, (Opdivo) for a form of metastatic lung cancer.
Then there was a significant gap, with no withdrawals going back to 2013 (when there was one). There were two withdrawals in 2012 and three in 2011.
A version of this article first appeared on Medscape.com.
Sunscreen, other sun-protective habits not linked with poorer bone health, fractures
Using , according to a new study that included more than 3,000 men and women.
“We have objective data for the first time, and in a large-scale representative population of the U.S. adults, to indicate sun protection is not associated with negative bone-related outcomes,” said study lead author Mohsen Afarideh, MD, MPH, a postdoctoral research fellow at the autoimmune skin diseases unit at the University of Pennsylvania, Philadelphia.
The study, published online in JAMA Dermatology, goes a step further than previous research by others that has found sunscreen use does not compromise vitamin D synthesis and has little effect on circulating 25-hydroxyvitamin D levels.
In the new study, researchers looked at three sun-protective behaviors – sunscreen use, staying in the shade, wearing long sleeves – and their effects on bone mineral density and the risk of fractures.
While the effects of sun-protective habits on blood levels of vitamin D and BMD scores are important, ‘’what we are more interested to know is if the sun-protective behaviors actually cause or increase the risk of fracture,” Dr. Afarideh said in an interview. “The answer to that is a firm ‘No.’ These data are very reassuring and will help clinicians to keep recommending sun protection to the public.”
Study details
Dr. Afarideh and his colleagues from the Mayo Clinic in Rochester, Minn., looked at data from the National Health and Nutrition Examination Survey (NHANES) from 2017 to 2018, obtaining final information on 3,403 men and women, ages 20-59, who completed a dermatology questionnaire The men and women reported on the three sun-protective habits, and noted whether they followed these practices always or most of the time, sometimes, or never or rarely.
The frequency of the three behaviors was not widespread. Frequent staying in the shade was reported by 31.6% of the sample, wearing long sleeves by 11.8%, and sunscreen use by 26.1%.
The researchers also had data on the participants’ bone mineral density (BMD) scores along with dietary information such as milk consumption, vitamin D supplement use, taking steroid drugs, and exercise activity.
“Moderate sunscreen use was linked with a slightly lower lumbar BMD score,” Dr. Afarideh said, which was “the only significant association that could be interpreted as concerning.” And this was more likely to be seen in older respondents, he said.
However, otherwise they found the practice of the three behaviors was not associated with lower total or site-specific BMD z scores, nor was it linked with an increased risk of osteoporotic fractures. (The BMD z score compares an individual’s bone density to the average bone density of someone their same age and gender.)
The focus on fracture risk is the more important outcome, Dr. Afarideh said. And they found no increased risk overall of osteoporotic fractures in those who practiced sun-protective behaviors.
Moderate to frequent staying in the shade was actually linked with a reduced prevalence of spine fractures in the multivariate model (odds ratio, 0.19; 95% confidence interval, 0.04-0.86, P = .02). The researchers say that may be attributable to these respondents also being careful in other areas of life, such as avoiding falls and not participating in high-risk activities that would increase the chance of fractures. “However, this is just an assumption,” Dr. Afarideh said.
Expert perspectives
Other dermatologists not involved in the new research said the study results provide some “real-world” information that’s valuable for clinicians to share with patients.
“I think this is an important study on multiple levels,” said Henry W. Lim, MD, a former president of the American Academy of Dermatology who is a member of the department of dermatology and senior vice president of academic affairs at Henry Ford Health System, Detroit. “It is a well-done study, involving a large number. It is a real-life situation, asking people their photo protective behaviors and then looking at their bone mineral density.” The bottom line, he said: “Bone health is not affected by photo protection habits in real life.”
The findings are important but not surprising, said Antony R. Young, PhD, emeritus professor of experimental photobiology at St. John’s Institute of Dermatology, King’s College, London, who has researched sunscreens and vitamin D status. “My study showed that correct sunscreen use, albeit with a relatively low SPF of 15, did prevent sunburn in a high UVR [ultraviolet radiation] environment but did allow very good vitamin D synthesis. I think this is because the necessary dose of UVB is very low.”
Michele Green, MD, a New York dermatologist and clinical staff member at Lenox Hill Hospital there, said she often hears concerns about bone health from patients. “Every week, patients ask, ‘Why would I wear sunblock? Don’t I need sun for bone health? Don’t I need it for vitamin D?’’’
Now, she said, ‘’Dermatologists can point to the study and say ‘Don’t worry.’ It clarifies that using sunscreen won’t cause you to have osteoporosis.’’
Dr. Afarideh, who was a postdoctoral research fellow at the Mayo Clinic, and his coauthors, Megha M. Tollefson, MD, and Julio C. Sartori-Valinotti, of the Mayo Clinic, and Dr. Green had no disclosures. Dr. Lim and Dr. Young consult for the sunscreen industry.
Using , according to a new study that included more than 3,000 men and women.
“We have objective data for the first time, and in a large-scale representative population of the U.S. adults, to indicate sun protection is not associated with negative bone-related outcomes,” said study lead author Mohsen Afarideh, MD, MPH, a postdoctoral research fellow at the autoimmune skin diseases unit at the University of Pennsylvania, Philadelphia.
The study, published online in JAMA Dermatology, goes a step further than previous research by others that has found sunscreen use does not compromise vitamin D synthesis and has little effect on circulating 25-hydroxyvitamin D levels.
In the new study, researchers looked at three sun-protective behaviors – sunscreen use, staying in the shade, wearing long sleeves – and their effects on bone mineral density and the risk of fractures.
While the effects of sun-protective habits on blood levels of vitamin D and BMD scores are important, ‘’what we are more interested to know is if the sun-protective behaviors actually cause or increase the risk of fracture,” Dr. Afarideh said in an interview. “The answer to that is a firm ‘No.’ These data are very reassuring and will help clinicians to keep recommending sun protection to the public.”
Study details
Dr. Afarideh and his colleagues from the Mayo Clinic in Rochester, Minn., looked at data from the National Health and Nutrition Examination Survey (NHANES) from 2017 to 2018, obtaining final information on 3,403 men and women, ages 20-59, who completed a dermatology questionnaire The men and women reported on the three sun-protective habits, and noted whether they followed these practices always or most of the time, sometimes, or never or rarely.
The frequency of the three behaviors was not widespread. Frequent staying in the shade was reported by 31.6% of the sample, wearing long sleeves by 11.8%, and sunscreen use by 26.1%.
The researchers also had data on the participants’ bone mineral density (BMD) scores along with dietary information such as milk consumption, vitamin D supplement use, taking steroid drugs, and exercise activity.
“Moderate sunscreen use was linked with a slightly lower lumbar BMD score,” Dr. Afarideh said, which was “the only significant association that could be interpreted as concerning.” And this was more likely to be seen in older respondents, he said.
However, otherwise they found the practice of the three behaviors was not associated with lower total or site-specific BMD z scores, nor was it linked with an increased risk of osteoporotic fractures. (The BMD z score compares an individual’s bone density to the average bone density of someone their same age and gender.)
The focus on fracture risk is the more important outcome, Dr. Afarideh said. And they found no increased risk overall of osteoporotic fractures in those who practiced sun-protective behaviors.
Moderate to frequent staying in the shade was actually linked with a reduced prevalence of spine fractures in the multivariate model (odds ratio, 0.19; 95% confidence interval, 0.04-0.86, P = .02). The researchers say that may be attributable to these respondents also being careful in other areas of life, such as avoiding falls and not participating in high-risk activities that would increase the chance of fractures. “However, this is just an assumption,” Dr. Afarideh said.
Expert perspectives
Other dermatologists not involved in the new research said the study results provide some “real-world” information that’s valuable for clinicians to share with patients.
“I think this is an important study on multiple levels,” said Henry W. Lim, MD, a former president of the American Academy of Dermatology who is a member of the department of dermatology and senior vice president of academic affairs at Henry Ford Health System, Detroit. “It is a well-done study, involving a large number. It is a real-life situation, asking people their photo protective behaviors and then looking at their bone mineral density.” The bottom line, he said: “Bone health is not affected by photo protection habits in real life.”
The findings are important but not surprising, said Antony R. Young, PhD, emeritus professor of experimental photobiology at St. John’s Institute of Dermatology, King’s College, London, who has researched sunscreens and vitamin D status. “My study showed that correct sunscreen use, albeit with a relatively low SPF of 15, did prevent sunburn in a high UVR [ultraviolet radiation] environment but did allow very good vitamin D synthesis. I think this is because the necessary dose of UVB is very low.”
Michele Green, MD, a New York dermatologist and clinical staff member at Lenox Hill Hospital there, said she often hears concerns about bone health from patients. “Every week, patients ask, ‘Why would I wear sunblock? Don’t I need sun for bone health? Don’t I need it for vitamin D?’’’
Now, she said, ‘’Dermatologists can point to the study and say ‘Don’t worry.’ It clarifies that using sunscreen won’t cause you to have osteoporosis.’’
Dr. Afarideh, who was a postdoctoral research fellow at the Mayo Clinic, and his coauthors, Megha M. Tollefson, MD, and Julio C. Sartori-Valinotti, of the Mayo Clinic, and Dr. Green had no disclosures. Dr. Lim and Dr. Young consult for the sunscreen industry.
Using , according to a new study that included more than 3,000 men and women.
“We have objective data for the first time, and in a large-scale representative population of the U.S. adults, to indicate sun protection is not associated with negative bone-related outcomes,” said study lead author Mohsen Afarideh, MD, MPH, a postdoctoral research fellow at the autoimmune skin diseases unit at the University of Pennsylvania, Philadelphia.
The study, published online in JAMA Dermatology, goes a step further than previous research by others that has found sunscreen use does not compromise vitamin D synthesis and has little effect on circulating 25-hydroxyvitamin D levels.
In the new study, researchers looked at three sun-protective behaviors – sunscreen use, staying in the shade, wearing long sleeves – and their effects on bone mineral density and the risk of fractures.
While the effects of sun-protective habits on blood levels of vitamin D and BMD scores are important, ‘’what we are more interested to know is if the sun-protective behaviors actually cause or increase the risk of fracture,” Dr. Afarideh said in an interview. “The answer to that is a firm ‘No.’ These data are very reassuring and will help clinicians to keep recommending sun protection to the public.”
Study details
Dr. Afarideh and his colleagues from the Mayo Clinic in Rochester, Minn., looked at data from the National Health and Nutrition Examination Survey (NHANES) from 2017 to 2018, obtaining final information on 3,403 men and women, ages 20-59, who completed a dermatology questionnaire The men and women reported on the three sun-protective habits, and noted whether they followed these practices always or most of the time, sometimes, or never or rarely.
The frequency of the three behaviors was not widespread. Frequent staying in the shade was reported by 31.6% of the sample, wearing long sleeves by 11.8%, and sunscreen use by 26.1%.
The researchers also had data on the participants’ bone mineral density (BMD) scores along with dietary information such as milk consumption, vitamin D supplement use, taking steroid drugs, and exercise activity.
“Moderate sunscreen use was linked with a slightly lower lumbar BMD score,” Dr. Afarideh said, which was “the only significant association that could be interpreted as concerning.” And this was more likely to be seen in older respondents, he said.
However, otherwise they found the practice of the three behaviors was not associated with lower total or site-specific BMD z scores, nor was it linked with an increased risk of osteoporotic fractures. (The BMD z score compares an individual’s bone density to the average bone density of someone their same age and gender.)
The focus on fracture risk is the more important outcome, Dr. Afarideh said. And they found no increased risk overall of osteoporotic fractures in those who practiced sun-protective behaviors.
Moderate to frequent staying in the shade was actually linked with a reduced prevalence of spine fractures in the multivariate model (odds ratio, 0.19; 95% confidence interval, 0.04-0.86, P = .02). The researchers say that may be attributable to these respondents also being careful in other areas of life, such as avoiding falls and not participating in high-risk activities that would increase the chance of fractures. “However, this is just an assumption,” Dr. Afarideh said.
Expert perspectives
Other dermatologists not involved in the new research said the study results provide some “real-world” information that’s valuable for clinicians to share with patients.
“I think this is an important study on multiple levels,” said Henry W. Lim, MD, a former president of the American Academy of Dermatology who is a member of the department of dermatology and senior vice president of academic affairs at Henry Ford Health System, Detroit. “It is a well-done study, involving a large number. It is a real-life situation, asking people their photo protective behaviors and then looking at their bone mineral density.” The bottom line, he said: “Bone health is not affected by photo protection habits in real life.”
The findings are important but not surprising, said Antony R. Young, PhD, emeritus professor of experimental photobiology at St. John’s Institute of Dermatology, King’s College, London, who has researched sunscreens and vitamin D status. “My study showed that correct sunscreen use, albeit with a relatively low SPF of 15, did prevent sunburn in a high UVR [ultraviolet radiation] environment but did allow very good vitamin D synthesis. I think this is because the necessary dose of UVB is very low.”
Michele Green, MD, a New York dermatologist and clinical staff member at Lenox Hill Hospital there, said she often hears concerns about bone health from patients. “Every week, patients ask, ‘Why would I wear sunblock? Don’t I need sun for bone health? Don’t I need it for vitamin D?’’’
Now, she said, ‘’Dermatologists can point to the study and say ‘Don’t worry.’ It clarifies that using sunscreen won’t cause you to have osteoporosis.’’
Dr. Afarideh, who was a postdoctoral research fellow at the Mayo Clinic, and his coauthors, Megha M. Tollefson, MD, and Julio C. Sartori-Valinotti, of the Mayo Clinic, and Dr. Green had no disclosures. Dr. Lim and Dr. Young consult for the sunscreen industry.
FROM JAMA DERMATOLOGY
Service animals and emotional support animals: Should you write that letter?
For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf

For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals

For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf
I have a dream … for psychiatry
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
Maria A. Oquendo, MD, PhD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
Placebo beat risankizumab in adults with severe asthma
Placebo treatment was found to be superior to treatment with risankizumab with respect to time to first asthma worsening and annualized rate of asthma worsening for adults with severe persistent asthma in a phase 2a clinical trial.
The randomized, double-blind, 24-week, parallel group, multicenter trial assessed risankizumab efficacy and safety in 214 adults with severe persistent asthma. The results were reported in The New England Journal of Medicine.
Risankizumab is a humanized, monoclonal antibody directed against subunit p19 of interleukin-23. It is approved for the treatment of moderate to severe psoriasis.
Interleukin-23 has been implicated in airway inflammation mediated by type 2 and type 17 cytokines. Noting that inhibition of interleukin-23 is effective in the treatment of psoriasis and Crohn’s disease, Christopher E. Brightling, MD, and colleagues investigated whether targeting interleukin-23 in asthma patients would improve disease control and reduce airway inflammation.
Study details
Patients received either 90 mg of risankizumab (subcutaneous) (n = 105) or placebo (n = 109) once every 4 weeks. Time to first asthma worsening was the primary endpoint. Worsening was defined as decline from baseline on 2 or more consecutive days. Deterioration was defined as a decrease of at least 30% in the morning peak expiratory flow or an increase from baseline of at least 50% in rescue medication puffs over 24 hours. In addition, a severe asthma exacerbation or an increase of 0.75 or more points on the five-item Asthma Control Questionnaire (scores range from 0 to 6, with higher scores indicating less control) were considered to be evidence of worsening. Annualized rate of asthma worsening was a secondary endpoint.
The mean age of the patients was 53 years; 66.5% of the patients were women.
Disappointing results
In the risankizumab group, median time to first asthma worsening was 40 days, significantly worse than the 86 days reported for the placebo group (hazard ratio, 1.46; 95% confidence interval, 1.05-2.04; P = .03). For annualized asthma worsening, the rate ratio for the comparison of risankizumab with placebo was 1.49 (95% CI, 1.12-1.99).
Among key secondary endpoints, the adjusted mean change in trough forced expiratory volume in 1 second (FEV1) from baseline to week 24 was –0.05 L in the risankizumab group and –0.01 L in the placebo group. The adjusted mean change in FEV1 after bronchodilator use from baseline to week 24 was –0.10 L in the risankizumab group and –0.03 L in the placebo group. Sputum transcriptomic pathway analysis showed that genes involved in the activation of natural killer cells and cytotoxic T cells and the activation of type 1 helper T and type 17 helper T transcription factors were downregulated by risankizumab. Rates of adverse events were similar among patients receiving risankizumab and those taking placebo.
Further trials unwarranted
“The findings not only failed to show benefit for any outcome but also showed asthma worsening occurred earlier and more frequently in those treated with risankizumab versus placebo,” Dr. Brightling, professor in the department of respiratory sciences at University of Leicester, England, said in an interview. “This study does not support any further trials for anti-IL23 in asthma.” Dr. Brightling speculated on the cause of accelerated asthma worsening with risankizumab.
“We found that the gene expression of key molecules involved in our response to infection was decreased in airway samples in those treated with risankizumab versus placebo. It is possible that the increased asthma worsening following risankizumab was related to this suppression of antimicrobial immunity,” he said.
He noted that risankizumab did not affect type-2/eosinophilic inflammation, which is the target for current asthma biologics, or gene expression of T2 molecules. “That suggests that this type of inflammation would have continued in the asthma patients during the trial irrespective of receiving risankizumab or placebo,” he said.
Caution with investigating biologicals
Downstream biologic responses to risankizumab were detectable, Philip G. Bardin, PhD, and Paul S. Foster, DSc, observed in an accompanying editorial, but there was no discernible clinical benefit, implying attenuation of apposite pathways. Current understanding of the basic science relevant to asthma, they stated, offers clues to the failure of risankizumab to benefit these patients with severe asthma. Although targeting the interleukin-23 and Th17 axis with risankizumab can reduce development of pathogenic Th17 cells, interleukin-23 is not critical for the development of Th17 cells.
“In contrast to pathways operated by interleukin-5 and interleukin-4R alpha, interleukin-23 has only a limited auxiliary role in amplifying type 2 responses. It is possible that the trial conducted by Brightling and colleagues failed because signaling through alternative disease pathways nullified inhibition of inter-leukin-23,” the editorialists wrote.
Dr. Bardin and Dr. Foster further speculate that because interleukin-23 is vital for effective mucosal immunity, risankizumab may have conferred to patients a predisposition to more severe or more frequent virus-induced exacerbations. They stated that generally, however, the reasons for risankizumab’s poorer outcomes compared to placebo are unclear. “Overall, these findings support a cautious approach in future research investigating biologic therapies in asthma,” they concluded.
The clinical trial was sponsored and funded by BI/AbbVie.
A version of this article first appeared on Medscape.com.
Placebo treatment was found to be superior to treatment with risankizumab with respect to time to first asthma worsening and annualized rate of asthma worsening for adults with severe persistent asthma in a phase 2a clinical trial.
The randomized, double-blind, 24-week, parallel group, multicenter trial assessed risankizumab efficacy and safety in 214 adults with severe persistent asthma. The results were reported in The New England Journal of Medicine.
Risankizumab is a humanized, monoclonal antibody directed against subunit p19 of interleukin-23. It is approved for the treatment of moderate to severe psoriasis.
Interleukin-23 has been implicated in airway inflammation mediated by type 2 and type 17 cytokines. Noting that inhibition of interleukin-23 is effective in the treatment of psoriasis and Crohn’s disease, Christopher E. Brightling, MD, and colleagues investigated whether targeting interleukin-23 in asthma patients would improve disease control and reduce airway inflammation.
Study details
Patients received either 90 mg of risankizumab (subcutaneous) (n = 105) or placebo (n = 109) once every 4 weeks. Time to first asthma worsening was the primary endpoint. Worsening was defined as decline from baseline on 2 or more consecutive days. Deterioration was defined as a decrease of at least 30% in the morning peak expiratory flow or an increase from baseline of at least 50% in rescue medication puffs over 24 hours. In addition, a severe asthma exacerbation or an increase of 0.75 or more points on the five-item Asthma Control Questionnaire (scores range from 0 to 6, with higher scores indicating less control) were considered to be evidence of worsening. Annualized rate of asthma worsening was a secondary endpoint.
The mean age of the patients was 53 years; 66.5% of the patients were women.
Disappointing results
In the risankizumab group, median time to first asthma worsening was 40 days, significantly worse than the 86 days reported for the placebo group (hazard ratio, 1.46; 95% confidence interval, 1.05-2.04; P = .03). For annualized asthma worsening, the rate ratio for the comparison of risankizumab with placebo was 1.49 (95% CI, 1.12-1.99).
Among key secondary endpoints, the adjusted mean change in trough forced expiratory volume in 1 second (FEV1) from baseline to week 24 was –0.05 L in the risankizumab group and –0.01 L in the placebo group. The adjusted mean change in FEV1 after bronchodilator use from baseline to week 24 was –0.10 L in the risankizumab group and –0.03 L in the placebo group. Sputum transcriptomic pathway analysis showed that genes involved in the activation of natural killer cells and cytotoxic T cells and the activation of type 1 helper T and type 17 helper T transcription factors were downregulated by risankizumab. Rates of adverse events were similar among patients receiving risankizumab and those taking placebo.
Further trials unwarranted
“The findings not only failed to show benefit for any outcome but also showed asthma worsening occurred earlier and more frequently in those treated with risankizumab versus placebo,” Dr. Brightling, professor in the department of respiratory sciences at University of Leicester, England, said in an interview. “This study does not support any further trials for anti-IL23 in asthma.” Dr. Brightling speculated on the cause of accelerated asthma worsening with risankizumab.
“We found that the gene expression of key molecules involved in our response to infection was decreased in airway samples in those treated with risankizumab versus placebo. It is possible that the increased asthma worsening following risankizumab was related to this suppression of antimicrobial immunity,” he said.
He noted that risankizumab did not affect type-2/eosinophilic inflammation, which is the target for current asthma biologics, or gene expression of T2 molecules. “That suggests that this type of inflammation would have continued in the asthma patients during the trial irrespective of receiving risankizumab or placebo,” he said.
Caution with investigating biologicals
Downstream biologic responses to risankizumab were detectable, Philip G. Bardin, PhD, and Paul S. Foster, DSc, observed in an accompanying editorial, but there was no discernible clinical benefit, implying attenuation of apposite pathways. Current understanding of the basic science relevant to asthma, they stated, offers clues to the failure of risankizumab to benefit these patients with severe asthma. Although targeting the interleukin-23 and Th17 axis with risankizumab can reduce development of pathogenic Th17 cells, interleukin-23 is not critical for the development of Th17 cells.
“In contrast to pathways operated by interleukin-5 and interleukin-4R alpha, interleukin-23 has only a limited auxiliary role in amplifying type 2 responses. It is possible that the trial conducted by Brightling and colleagues failed because signaling through alternative disease pathways nullified inhibition of inter-leukin-23,” the editorialists wrote.
Dr. Bardin and Dr. Foster further speculate that because interleukin-23 is vital for effective mucosal immunity, risankizumab may have conferred to patients a predisposition to more severe or more frequent virus-induced exacerbations. They stated that generally, however, the reasons for risankizumab’s poorer outcomes compared to placebo are unclear. “Overall, these findings support a cautious approach in future research investigating biologic therapies in asthma,” they concluded.
The clinical trial was sponsored and funded by BI/AbbVie.
A version of this article first appeared on Medscape.com.
Placebo treatment was found to be superior to treatment with risankizumab with respect to time to first asthma worsening and annualized rate of asthma worsening for adults with severe persistent asthma in a phase 2a clinical trial.
The randomized, double-blind, 24-week, parallel group, multicenter trial assessed risankizumab efficacy and safety in 214 adults with severe persistent asthma. The results were reported in The New England Journal of Medicine.
Risankizumab is a humanized, monoclonal antibody directed against subunit p19 of interleukin-23. It is approved for the treatment of moderate to severe psoriasis.
Interleukin-23 has been implicated in airway inflammation mediated by type 2 and type 17 cytokines. Noting that inhibition of interleukin-23 is effective in the treatment of psoriasis and Crohn’s disease, Christopher E. Brightling, MD, and colleagues investigated whether targeting interleukin-23 in asthma patients would improve disease control and reduce airway inflammation.
Study details
Patients received either 90 mg of risankizumab (subcutaneous) (n = 105) or placebo (n = 109) once every 4 weeks. Time to first asthma worsening was the primary endpoint. Worsening was defined as decline from baseline on 2 or more consecutive days. Deterioration was defined as a decrease of at least 30% in the morning peak expiratory flow or an increase from baseline of at least 50% in rescue medication puffs over 24 hours. In addition, a severe asthma exacerbation or an increase of 0.75 or more points on the five-item Asthma Control Questionnaire (scores range from 0 to 6, with higher scores indicating less control) were considered to be evidence of worsening. Annualized rate of asthma worsening was a secondary endpoint.
The mean age of the patients was 53 years; 66.5% of the patients were women.
Disappointing results
In the risankizumab group, median time to first asthma worsening was 40 days, significantly worse than the 86 days reported for the placebo group (hazard ratio, 1.46; 95% confidence interval, 1.05-2.04; P = .03). For annualized asthma worsening, the rate ratio for the comparison of risankizumab with placebo was 1.49 (95% CI, 1.12-1.99).
Among key secondary endpoints, the adjusted mean change in trough forced expiratory volume in 1 second (FEV1) from baseline to week 24 was –0.05 L in the risankizumab group and –0.01 L in the placebo group. The adjusted mean change in FEV1 after bronchodilator use from baseline to week 24 was –0.10 L in the risankizumab group and –0.03 L in the placebo group. Sputum transcriptomic pathway analysis showed that genes involved in the activation of natural killer cells and cytotoxic T cells and the activation of type 1 helper T and type 17 helper T transcription factors were downregulated by risankizumab. Rates of adverse events were similar among patients receiving risankizumab and those taking placebo.
Further trials unwarranted
“The findings not only failed to show benefit for any outcome but also showed asthma worsening occurred earlier and more frequently in those treated with risankizumab versus placebo,” Dr. Brightling, professor in the department of respiratory sciences at University of Leicester, England, said in an interview. “This study does not support any further trials for anti-IL23 in asthma.” Dr. Brightling speculated on the cause of accelerated asthma worsening with risankizumab.
“We found that the gene expression of key molecules involved in our response to infection was decreased in airway samples in those treated with risankizumab versus placebo. It is possible that the increased asthma worsening following risankizumab was related to this suppression of antimicrobial immunity,” he said.
He noted that risankizumab did not affect type-2/eosinophilic inflammation, which is the target for current asthma biologics, or gene expression of T2 molecules. “That suggests that this type of inflammation would have continued in the asthma patients during the trial irrespective of receiving risankizumab or placebo,” he said.
Caution with investigating biologicals
Downstream biologic responses to risankizumab were detectable, Philip G. Bardin, PhD, and Paul S. Foster, DSc, observed in an accompanying editorial, but there was no discernible clinical benefit, implying attenuation of apposite pathways. Current understanding of the basic science relevant to asthma, they stated, offers clues to the failure of risankizumab to benefit these patients with severe asthma. Although targeting the interleukin-23 and Th17 axis with risankizumab can reduce development of pathogenic Th17 cells, interleukin-23 is not critical for the development of Th17 cells.
“In contrast to pathways operated by interleukin-5 and interleukin-4R alpha, interleukin-23 has only a limited auxiliary role in amplifying type 2 responses. It is possible that the trial conducted by Brightling and colleagues failed because signaling through alternative disease pathways nullified inhibition of inter-leukin-23,” the editorialists wrote.
Dr. Bardin and Dr. Foster further speculate that because interleukin-23 is vital for effective mucosal immunity, risankizumab may have conferred to patients a predisposition to more severe or more frequent virus-induced exacerbations. They stated that generally, however, the reasons for risankizumab’s poorer outcomes compared to placebo are unclear. “Overall, these findings support a cautious approach in future research investigating biologic therapies in asthma,” they concluded.
The clinical trial was sponsored and funded by BI/AbbVie.
A version of this article first appeared on Medscape.com.
Accused: Doc increases patient’s penis size with improper fillers; more
, as reported in NJ.com.
The physician, Muhammad A. Mirza, MD, is a board-certified internal medicine doctor and owner of Mirza Aesthetics, which has its main New Jersey office in Cedar Grove, a township in Essex County. The practice also leases space in New York, Pennsylvania, and Connecticut, where at press time Dr. Mirza was still licensed to practice medicine.
The acting New Jersey attorney general said that Dr. Mirza had deviated from the accepted standards of medical care in at least four key areas: he practiced in ways that put his patients in bodily danger; he lacked the formal training in and an adequate knowledge of aesthetic medicine; he practiced in office settings that inspectors found to be subpar; and he failed to safely store medical supplies or maintain proper medical records.
In one instance singled out by the attorney general’s office, Dr. Mirza used an injectable dermal filler to enhance a patient’s penis. As a result of that nonsurgical procedure, the patient needed to be rushed to a nearby hospital, where he underwent two emergency surgical interventions. Contacted by the emergency department doctor, Dr. Mirza allegedly failed to disclose the name of the filler he used, thereby complicating the patient’s recovery, according to the board complaint.
Dr. Mirza’s other alleged breaches of professional conduct include the following:
- Failure to wear a mask or surgical gloves during procedures
- Failure to keep electronic medical records of any kind
- Improper, off-label use of an injectable dermal filler in proximity to patients’ eyes
- Improper, off-label use of an injectable dermal filler for breast enhancement
- Use of a certain injectable dermal filler without first testing for skin allergies
In addition, site inspections of Dr. Mirza’s offices turned up substandard conditions. On April 23, 2021, in response to numerous patient complaints, the Enforcement Bureau of the Division of Consumer Affairs inspected Dr. Mirza’s Summit, N.J. office, one of several in the state.
Among other things, the inspection uncovered the following:
- The medical office was one large room. A curtain separated the reception area and the examination/treatment area, which consisted of only chairs and a fold-away table.
- “Duffle bags” were used to store injectable fillers. No medical storage refrigerators were observed.
- COVID-19 protocols were not followed. Inspectors could identify no barrier between receptionist and patients, no posted mask mandate, no social distancing policy, and no COVID-19 screening measures.
In addition to temporarily suspending Dr. Mirza’s license, the medical board has prohibited him from treating New Jersey patients in any of the out-of-state locations where he’s licensed to practice medicine.
Prosecutors have urged other patients who believe they’ve been injured by Mirza Aesthetics to file a complaint with the State Division of Consumer Affairs.
Dr. Mirza has agreed to the temporary suspension of his medical license, pending a hearing before an administrative law judge. In addition to facing civil penalties for each of the counts against him, he could be held responsible for paying investigative costs, attorney fees, trial costs, and other costs.
Doctor’s failure to diagnose results in mega award
In what is believed to be a record verdict in a wrongful death suit in Volusia County, Fla., a jury awarded $6.46 million to the family of a woman who died from an undiagnosed heart infection after being transferred from a local hospital, according to a report in The Daytona Beach News-Journal, among other news outlets.
In March 2016, Laura Staib went to what was then Florida Hospital DeLand — now AdventHealth DeLand — complaining of a variety of symptoms. There, she was examined by a doctor who was a member of a nearby cardiology group. His diagnosis: congestive heart failure, pneumonia, and sepsis. Transferred to a long-term care facility, Ms. Staib died 4 days later.
In their complaint against the cardiologist and his cardiology group, family members alleged that the doctor failed to identify Ms. Staib’s main problem: viral myocarditis.
“This was primarily a heart failure problem and a heart infection that was probably causing some problems in the lungs,” said the attorney representing the family. “A virus was attacking her heart, and they missed it,” he said. Claims against the hospital and other doctors were eventually resolved and dismissed.
The jury’s verdict will be appealed, said the attorney representing the cardiologist.
He argues that his client “did not cause that woman’s death. She died of an overwhelming lung infection...acute respiratory distress syndrome, caused by an overwhelming pneumonia that got worse after she was transferred to a facility where [my client] doesn’t practice.”
The bulk of the award will be in compensation for family members’ future pain and suffering and for other noneconomic damages.
Botched outpatient procedure leaves woman disfigured
In early September, a patient was allegedly administered the wrong drug during an outpatient procedure on her hand. She sued the Austin, Tex., hospital and surgical center where that procedure was performed, according to a story in Law/Street.
On January 9, 2020, Jessica Arguello went to HCA Healthcare’s South Austin Surgery Center to undergo a right-hand first metacarpophalangeal arthrodesis (fusion) and neuroma excision. In her suit against the hospital, Ms. Arguello claims that while her surgeon was preparing to close the incision after having irrigated the site, he called for a syringe containing an anesthetic. He was instead handed a syringe that contained formalin, the chemical used to preserve specimens for later review.
The mistake, Ms. Arguello claims, caused her to suffer massive chemical burns and necrosis of her flesh, which required four additional surgeries. In the end, she says, her right hand is disfigured and has limited mobility.
She adds that her injuries were preventable. Standard surgical procedure typically forbids chemicals such as formalin to be included among items on the prep tray. In addition to other compensation, she seeks damages for past and future medical expenses and past and future pain and suffering.
At press time, the defendants had not responded to Ms. Arguello’s complaint.
A version of this article first appeared on Medscape.com.
, as reported in NJ.com.
The physician, Muhammad A. Mirza, MD, is a board-certified internal medicine doctor and owner of Mirza Aesthetics, which has its main New Jersey office in Cedar Grove, a township in Essex County. The practice also leases space in New York, Pennsylvania, and Connecticut, where at press time Dr. Mirza was still licensed to practice medicine.
The acting New Jersey attorney general said that Dr. Mirza had deviated from the accepted standards of medical care in at least four key areas: he practiced in ways that put his patients in bodily danger; he lacked the formal training in and an adequate knowledge of aesthetic medicine; he practiced in office settings that inspectors found to be subpar; and he failed to safely store medical supplies or maintain proper medical records.
In one instance singled out by the attorney general’s office, Dr. Mirza used an injectable dermal filler to enhance a patient’s penis. As a result of that nonsurgical procedure, the patient needed to be rushed to a nearby hospital, where he underwent two emergency surgical interventions. Contacted by the emergency department doctor, Dr. Mirza allegedly failed to disclose the name of the filler he used, thereby complicating the patient’s recovery, according to the board complaint.
Dr. Mirza’s other alleged breaches of professional conduct include the following:
- Failure to wear a mask or surgical gloves during procedures
- Failure to keep electronic medical records of any kind
- Improper, off-label use of an injectable dermal filler in proximity to patients’ eyes
- Improper, off-label use of an injectable dermal filler for breast enhancement
- Use of a certain injectable dermal filler without first testing for skin allergies
In addition, site inspections of Dr. Mirza’s offices turned up substandard conditions. On April 23, 2021, in response to numerous patient complaints, the Enforcement Bureau of the Division of Consumer Affairs inspected Dr. Mirza’s Summit, N.J. office, one of several in the state.
Among other things, the inspection uncovered the following:
- The medical office was one large room. A curtain separated the reception area and the examination/treatment area, which consisted of only chairs and a fold-away table.
- “Duffle bags” were used to store injectable fillers. No medical storage refrigerators were observed.
- COVID-19 protocols were not followed. Inspectors could identify no barrier between receptionist and patients, no posted mask mandate, no social distancing policy, and no COVID-19 screening measures.
In addition to temporarily suspending Dr. Mirza’s license, the medical board has prohibited him from treating New Jersey patients in any of the out-of-state locations where he’s licensed to practice medicine.
Prosecutors have urged other patients who believe they’ve been injured by Mirza Aesthetics to file a complaint with the State Division of Consumer Affairs.
Dr. Mirza has agreed to the temporary suspension of his medical license, pending a hearing before an administrative law judge. In addition to facing civil penalties for each of the counts against him, he could be held responsible for paying investigative costs, attorney fees, trial costs, and other costs.
Doctor’s failure to diagnose results in mega award
In what is believed to be a record verdict in a wrongful death suit in Volusia County, Fla., a jury awarded $6.46 million to the family of a woman who died from an undiagnosed heart infection after being transferred from a local hospital, according to a report in The Daytona Beach News-Journal, among other news outlets.
In March 2016, Laura Staib went to what was then Florida Hospital DeLand — now AdventHealth DeLand — complaining of a variety of symptoms. There, she was examined by a doctor who was a member of a nearby cardiology group. His diagnosis: congestive heart failure, pneumonia, and sepsis. Transferred to a long-term care facility, Ms. Staib died 4 days later.
In their complaint against the cardiologist and his cardiology group, family members alleged that the doctor failed to identify Ms. Staib’s main problem: viral myocarditis.
“This was primarily a heart failure problem and a heart infection that was probably causing some problems in the lungs,” said the attorney representing the family. “A virus was attacking her heart, and they missed it,” he said. Claims against the hospital and other doctors were eventually resolved and dismissed.
The jury’s verdict will be appealed, said the attorney representing the cardiologist.
He argues that his client “did not cause that woman’s death. She died of an overwhelming lung infection...acute respiratory distress syndrome, caused by an overwhelming pneumonia that got worse after she was transferred to a facility where [my client] doesn’t practice.”
The bulk of the award will be in compensation for family members’ future pain and suffering and for other noneconomic damages.
Botched outpatient procedure leaves woman disfigured
In early September, a patient was allegedly administered the wrong drug during an outpatient procedure on her hand. She sued the Austin, Tex., hospital and surgical center where that procedure was performed, according to a story in Law/Street.
On January 9, 2020, Jessica Arguello went to HCA Healthcare’s South Austin Surgery Center to undergo a right-hand first metacarpophalangeal arthrodesis (fusion) and neuroma excision. In her suit against the hospital, Ms. Arguello claims that while her surgeon was preparing to close the incision after having irrigated the site, he called for a syringe containing an anesthetic. He was instead handed a syringe that contained formalin, the chemical used to preserve specimens for later review.
The mistake, Ms. Arguello claims, caused her to suffer massive chemical burns and necrosis of her flesh, which required four additional surgeries. In the end, she says, her right hand is disfigured and has limited mobility.
She adds that her injuries were preventable. Standard surgical procedure typically forbids chemicals such as formalin to be included among items on the prep tray. In addition to other compensation, she seeks damages for past and future medical expenses and past and future pain and suffering.
At press time, the defendants had not responded to Ms. Arguello’s complaint.
A version of this article first appeared on Medscape.com.
, as reported in NJ.com.
The physician, Muhammad A. Mirza, MD, is a board-certified internal medicine doctor and owner of Mirza Aesthetics, which has its main New Jersey office in Cedar Grove, a township in Essex County. The practice also leases space in New York, Pennsylvania, and Connecticut, where at press time Dr. Mirza was still licensed to practice medicine.
The acting New Jersey attorney general said that Dr. Mirza had deviated from the accepted standards of medical care in at least four key areas: he practiced in ways that put his patients in bodily danger; he lacked the formal training in and an adequate knowledge of aesthetic medicine; he practiced in office settings that inspectors found to be subpar; and he failed to safely store medical supplies or maintain proper medical records.
In one instance singled out by the attorney general’s office, Dr. Mirza used an injectable dermal filler to enhance a patient’s penis. As a result of that nonsurgical procedure, the patient needed to be rushed to a nearby hospital, where he underwent two emergency surgical interventions. Contacted by the emergency department doctor, Dr. Mirza allegedly failed to disclose the name of the filler he used, thereby complicating the patient’s recovery, according to the board complaint.
Dr. Mirza’s other alleged breaches of professional conduct include the following:
- Failure to wear a mask or surgical gloves during procedures
- Failure to keep electronic medical records of any kind
- Improper, off-label use of an injectable dermal filler in proximity to patients’ eyes
- Improper, off-label use of an injectable dermal filler for breast enhancement
- Use of a certain injectable dermal filler without first testing for skin allergies
In addition, site inspections of Dr. Mirza’s offices turned up substandard conditions. On April 23, 2021, in response to numerous patient complaints, the Enforcement Bureau of the Division of Consumer Affairs inspected Dr. Mirza’s Summit, N.J. office, one of several in the state.
Among other things, the inspection uncovered the following:
- The medical office was one large room. A curtain separated the reception area and the examination/treatment area, which consisted of only chairs and a fold-away table.
- “Duffle bags” were used to store injectable fillers. No medical storage refrigerators were observed.
- COVID-19 protocols were not followed. Inspectors could identify no barrier between receptionist and patients, no posted mask mandate, no social distancing policy, and no COVID-19 screening measures.
In addition to temporarily suspending Dr. Mirza’s license, the medical board has prohibited him from treating New Jersey patients in any of the out-of-state locations where he’s licensed to practice medicine.
Prosecutors have urged other patients who believe they’ve been injured by Mirza Aesthetics to file a complaint with the State Division of Consumer Affairs.
Dr. Mirza has agreed to the temporary suspension of his medical license, pending a hearing before an administrative law judge. In addition to facing civil penalties for each of the counts against him, he could be held responsible for paying investigative costs, attorney fees, trial costs, and other costs.
Doctor’s failure to diagnose results in mega award
In what is believed to be a record verdict in a wrongful death suit in Volusia County, Fla., a jury awarded $6.46 million to the family of a woman who died from an undiagnosed heart infection after being transferred from a local hospital, according to a report in The Daytona Beach News-Journal, among other news outlets.
In March 2016, Laura Staib went to what was then Florida Hospital DeLand — now AdventHealth DeLand — complaining of a variety of symptoms. There, she was examined by a doctor who was a member of a nearby cardiology group. His diagnosis: congestive heart failure, pneumonia, and sepsis. Transferred to a long-term care facility, Ms. Staib died 4 days later.
In their complaint against the cardiologist and his cardiology group, family members alleged that the doctor failed to identify Ms. Staib’s main problem: viral myocarditis.
“This was primarily a heart failure problem and a heart infection that was probably causing some problems in the lungs,” said the attorney representing the family. “A virus was attacking her heart, and they missed it,” he said. Claims against the hospital and other doctors were eventually resolved and dismissed.
The jury’s verdict will be appealed, said the attorney representing the cardiologist.
He argues that his client “did not cause that woman’s death. She died of an overwhelming lung infection...acute respiratory distress syndrome, caused by an overwhelming pneumonia that got worse after she was transferred to a facility where [my client] doesn’t practice.”
The bulk of the award will be in compensation for family members’ future pain and suffering and for other noneconomic damages.
Botched outpatient procedure leaves woman disfigured
In early September, a patient was allegedly administered the wrong drug during an outpatient procedure on her hand. She sued the Austin, Tex., hospital and surgical center where that procedure was performed, according to a story in Law/Street.
On January 9, 2020, Jessica Arguello went to HCA Healthcare’s South Austin Surgery Center to undergo a right-hand first metacarpophalangeal arthrodesis (fusion) and neuroma excision. In her suit against the hospital, Ms. Arguello claims that while her surgeon was preparing to close the incision after having irrigated the site, he called for a syringe containing an anesthetic. He was instead handed a syringe that contained formalin, the chemical used to preserve specimens for later review.
The mistake, Ms. Arguello claims, caused her to suffer massive chemical burns and necrosis of her flesh, which required four additional surgeries. In the end, she says, her right hand is disfigured and has limited mobility.
She adds that her injuries were preventable. Standard surgical procedure typically forbids chemicals such as formalin to be included among items on the prep tray. In addition to other compensation, she seeks damages for past and future medical expenses and past and future pain and suffering.
At press time, the defendants had not responded to Ms. Arguello’s complaint.
A version of this article first appeared on Medscape.com.
Free vitamin D no better at predicting death in men than standard testing
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
German society for internal medicine reappraises its Nazi history
. The decision was made after the DGIM reappraised its own history during the Nazi period.
On the DGIM - Commemoration and Remembrance website, created in 2020, members who suffered under the Nazi regime are commemorated and those who committed crimes and caused suffering are called out.
The reappraisal began in 2012, when the DGIM commissioned two historians — Hans-Georg Hofer, PhD, from the University of Münster, and Ralf Forsbach, PhD, from the Institute for Ethics, History and Theory of Medicine at the University of Münster — to research the history of the society and its members during the periods of the National Socialism dictatorship and the young Federal Republic.
“Reappraising our own history, even at this late stage, is important and the right thing to do, although of course it cannot in any way make up for the suffering caused by individual DGIM members during that time,” Georg Ertl, MD, secretary general of the DGIM, states in a press release.
It is important, however, that the Society takes appropriate action in response to the historians’ findings, he adds.
The DGIM has done just that by retrospectively withdrawing the honorary membership status of five of its former members: Alfred Schittenhelm, Alfred Schwenkenbecher, Hans Dietlen, Siegfried Koller, and Georg Schaltenbrand.
“Out of opportunism or on the basis of Nazi beliefs, they intentionally harmed colleagues, other members of our Society, or simply other people on the basis of their ethnicity. Therefore, the DGIM can no longer accept them as honorary members,” said Markus M. Lerch, MD, chair of the DGIM and medical director of the Ludwig Maximilian University of Munich.
The board has also distanced itself from two other honorary members: Gustav von Bergmann and Felix Lommel. “More research is needed and we cannot currently make a responsible decision on withdrawal of honorary membership,” Dr. Lerch explained.
Early results from the historical reappraisal were presented to the public in 2015 at an exhibition held during the 121st DGIM Congress in Mannheim. The Society concurrently underwent a process of public self-reflection, stating that it was ashamed of having allowed 70 years to pass before it objectively examined its actions under National Socialism and acknowledged its responsibility.
The exhibition used photos, documents, and explanatory texts to show the actions, or lack of action, taken by some Society members during the Nazi regime. For example, it showed how then DGIM chair Alfred Schittenhelm — whose honorary membership has since been withdrawn — put the Society on the track to National Socialism. It also shone light on the role played by internists who consulted with the Wehrmacht in the treatment of Soviet prisoners of war and others, and on criminal experiments conducted on humans.
The exhibition also highlighted Jewish doctors who were persecuted and expelled, such as Leopold Lichtwitz, MD, who lost his position as clinic director in Berlin in 1933 and was forced to resign his chairmanship of the Society. And it presented portraits of members who loudly objected to and even actively resisted the regime, such as Wolfgang Seitz, MD, who became director of the Medical Outpatient Clinic at the University of Munich and deputy of the state parliament of the Social Democratic Party of Germany (SPD) in Bavaria after the war.
The historians focused on the years after 1945, because “1945 was no zero hour,” said Dr. Forsbach. Some culpable doctors continued to practice or became honorary DGIM members, he explained. The DGIM’s attitude toward history was characterized by suppression, denial, silence, and attempts at justification, consistent with the postwar attitude in the Federal Republic of Germany and in the medical profession as a whole.
After 1945
Behavior before 1945 is not the only source of shame. Crimes committed by doctors were never really confronted until the late 1970s, Jörg-Dietrich Hoppe, MD, former president of the German Medical Association, explained to ZEIT, a German newspaper, in 2011.
The psychoanalyst Alexander Mitscherlich, MD, and Fred Mielke were official observers from the German Commission of Physicians at the Nuremberg Doctors’ Trial who were made painfully aware that National Socialism was by no means over when the regime came to an end. They were both reviled as traitors to their country and for fouling their own nest, and, according to Mitscherlich, the behavior of the “authorities” bordered on character assassination.
As late as 1973, a renowned internist threatened that German internists would leave the room locked at the upcoming DGIM Congress if — as had been planned by congress chair Herbert Begemann — Mitscherlich gave a talk on this subject, journalist and doctor Renate Jäckle reported in her book on doctors and politics.
Even toward the end of the 1980s, Karsten Vilmar, MD, then president of the German Medical Association, reacted in an insensitive and defensive manner — during an interview — to an article in the Lancet, written by the Mainz pediatrician Hartmut M. Hanauske-Abel, MD, on the role of the German medical profession in the Third Reich and the suppression that followed after 1945.
A group of 400 doctors, at most, were culpable, and coming to terms with the past should not defame doctors collectively, Dr. Vilmar said in a statement chillingly reminiscent of declarations made by the Wehrmacht, which described itself as mainly “clean.”
Of course, the end of the Nazi regime was not the end of all barbarity, not even in Europe. “Violence will be something we have to confront in our future lives, too. Belief in the healing powers of civilization is nothing but a fairytale,” Berlin historian Jörg Barberowski wrote in a 2012 essay.
Nevertheless, as Michael Hallek, MD, from the University Hospital of Cologne, said, it is important to keep memory alive.
A version of this article first appeared on Medscape.com.
. The decision was made after the DGIM reappraised its own history during the Nazi period.
On the DGIM - Commemoration and Remembrance website, created in 2020, members who suffered under the Nazi regime are commemorated and those who committed crimes and caused suffering are called out.
The reappraisal began in 2012, when the DGIM commissioned two historians — Hans-Georg Hofer, PhD, from the University of Münster, and Ralf Forsbach, PhD, from the Institute for Ethics, History and Theory of Medicine at the University of Münster — to research the history of the society and its members during the periods of the National Socialism dictatorship and the young Federal Republic.
“Reappraising our own history, even at this late stage, is important and the right thing to do, although of course it cannot in any way make up for the suffering caused by individual DGIM members during that time,” Georg Ertl, MD, secretary general of the DGIM, states in a press release.
It is important, however, that the Society takes appropriate action in response to the historians’ findings, he adds.
The DGIM has done just that by retrospectively withdrawing the honorary membership status of five of its former members: Alfred Schittenhelm, Alfred Schwenkenbecher, Hans Dietlen, Siegfried Koller, and Georg Schaltenbrand.
“Out of opportunism or on the basis of Nazi beliefs, they intentionally harmed colleagues, other members of our Society, or simply other people on the basis of their ethnicity. Therefore, the DGIM can no longer accept them as honorary members,” said Markus M. Lerch, MD, chair of the DGIM and medical director of the Ludwig Maximilian University of Munich.
The board has also distanced itself from two other honorary members: Gustav von Bergmann and Felix Lommel. “More research is needed and we cannot currently make a responsible decision on withdrawal of honorary membership,” Dr. Lerch explained.
Early results from the historical reappraisal were presented to the public in 2015 at an exhibition held during the 121st DGIM Congress in Mannheim. The Society concurrently underwent a process of public self-reflection, stating that it was ashamed of having allowed 70 years to pass before it objectively examined its actions under National Socialism and acknowledged its responsibility.
The exhibition used photos, documents, and explanatory texts to show the actions, or lack of action, taken by some Society members during the Nazi regime. For example, it showed how then DGIM chair Alfred Schittenhelm — whose honorary membership has since been withdrawn — put the Society on the track to National Socialism. It also shone light on the role played by internists who consulted with the Wehrmacht in the treatment of Soviet prisoners of war and others, and on criminal experiments conducted on humans.
The exhibition also highlighted Jewish doctors who were persecuted and expelled, such as Leopold Lichtwitz, MD, who lost his position as clinic director in Berlin in 1933 and was forced to resign his chairmanship of the Society. And it presented portraits of members who loudly objected to and even actively resisted the regime, such as Wolfgang Seitz, MD, who became director of the Medical Outpatient Clinic at the University of Munich and deputy of the state parliament of the Social Democratic Party of Germany (SPD) in Bavaria after the war.
The historians focused on the years after 1945, because “1945 was no zero hour,” said Dr. Forsbach. Some culpable doctors continued to practice or became honorary DGIM members, he explained. The DGIM’s attitude toward history was characterized by suppression, denial, silence, and attempts at justification, consistent with the postwar attitude in the Federal Republic of Germany and in the medical profession as a whole.
After 1945
Behavior before 1945 is not the only source of shame. Crimes committed by doctors were never really confronted until the late 1970s, Jörg-Dietrich Hoppe, MD, former president of the German Medical Association, explained to ZEIT, a German newspaper, in 2011.
The psychoanalyst Alexander Mitscherlich, MD, and Fred Mielke were official observers from the German Commission of Physicians at the Nuremberg Doctors’ Trial who were made painfully aware that National Socialism was by no means over when the regime came to an end. They were both reviled as traitors to their country and for fouling their own nest, and, according to Mitscherlich, the behavior of the “authorities” bordered on character assassination.
As late as 1973, a renowned internist threatened that German internists would leave the room locked at the upcoming DGIM Congress if — as had been planned by congress chair Herbert Begemann — Mitscherlich gave a talk on this subject, journalist and doctor Renate Jäckle reported in her book on doctors and politics.
Even toward the end of the 1980s, Karsten Vilmar, MD, then president of the German Medical Association, reacted in an insensitive and defensive manner — during an interview — to an article in the Lancet, written by the Mainz pediatrician Hartmut M. Hanauske-Abel, MD, on the role of the German medical profession in the Third Reich and the suppression that followed after 1945.
A group of 400 doctors, at most, were culpable, and coming to terms with the past should not defame doctors collectively, Dr. Vilmar said in a statement chillingly reminiscent of declarations made by the Wehrmacht, which described itself as mainly “clean.”
Of course, the end of the Nazi regime was not the end of all barbarity, not even in Europe. “Violence will be something we have to confront in our future lives, too. Belief in the healing powers of civilization is nothing but a fairytale,” Berlin historian Jörg Barberowski wrote in a 2012 essay.
Nevertheless, as Michael Hallek, MD, from the University Hospital of Cologne, said, it is important to keep memory alive.
A version of this article first appeared on Medscape.com.
. The decision was made after the DGIM reappraised its own history during the Nazi period.
On the DGIM - Commemoration and Remembrance website, created in 2020, members who suffered under the Nazi regime are commemorated and those who committed crimes and caused suffering are called out.
The reappraisal began in 2012, when the DGIM commissioned two historians — Hans-Georg Hofer, PhD, from the University of Münster, and Ralf Forsbach, PhD, from the Institute for Ethics, History and Theory of Medicine at the University of Münster — to research the history of the society and its members during the periods of the National Socialism dictatorship and the young Federal Republic.
“Reappraising our own history, even at this late stage, is important and the right thing to do, although of course it cannot in any way make up for the suffering caused by individual DGIM members during that time,” Georg Ertl, MD, secretary general of the DGIM, states in a press release.
It is important, however, that the Society takes appropriate action in response to the historians’ findings, he adds.
The DGIM has done just that by retrospectively withdrawing the honorary membership status of five of its former members: Alfred Schittenhelm, Alfred Schwenkenbecher, Hans Dietlen, Siegfried Koller, and Georg Schaltenbrand.
“Out of opportunism or on the basis of Nazi beliefs, they intentionally harmed colleagues, other members of our Society, or simply other people on the basis of their ethnicity. Therefore, the DGIM can no longer accept them as honorary members,” said Markus M. Lerch, MD, chair of the DGIM and medical director of the Ludwig Maximilian University of Munich.
The board has also distanced itself from two other honorary members: Gustav von Bergmann and Felix Lommel. “More research is needed and we cannot currently make a responsible decision on withdrawal of honorary membership,” Dr. Lerch explained.
Early results from the historical reappraisal were presented to the public in 2015 at an exhibition held during the 121st DGIM Congress in Mannheim. The Society concurrently underwent a process of public self-reflection, stating that it was ashamed of having allowed 70 years to pass before it objectively examined its actions under National Socialism and acknowledged its responsibility.
The exhibition used photos, documents, and explanatory texts to show the actions, or lack of action, taken by some Society members during the Nazi regime. For example, it showed how then DGIM chair Alfred Schittenhelm — whose honorary membership has since been withdrawn — put the Society on the track to National Socialism. It also shone light on the role played by internists who consulted with the Wehrmacht in the treatment of Soviet prisoners of war and others, and on criminal experiments conducted on humans.
The exhibition also highlighted Jewish doctors who were persecuted and expelled, such as Leopold Lichtwitz, MD, who lost his position as clinic director in Berlin in 1933 and was forced to resign his chairmanship of the Society. And it presented portraits of members who loudly objected to and even actively resisted the regime, such as Wolfgang Seitz, MD, who became director of the Medical Outpatient Clinic at the University of Munich and deputy of the state parliament of the Social Democratic Party of Germany (SPD) in Bavaria after the war.
The historians focused on the years after 1945, because “1945 was no zero hour,” said Dr. Forsbach. Some culpable doctors continued to practice or became honorary DGIM members, he explained. The DGIM’s attitude toward history was characterized by suppression, denial, silence, and attempts at justification, consistent with the postwar attitude in the Federal Republic of Germany and in the medical profession as a whole.
After 1945
Behavior before 1945 is not the only source of shame. Crimes committed by doctors were never really confronted until the late 1970s, Jörg-Dietrich Hoppe, MD, former president of the German Medical Association, explained to ZEIT, a German newspaper, in 2011.
The psychoanalyst Alexander Mitscherlich, MD, and Fred Mielke were official observers from the German Commission of Physicians at the Nuremberg Doctors’ Trial who were made painfully aware that National Socialism was by no means over when the regime came to an end. They were both reviled as traitors to their country and for fouling their own nest, and, according to Mitscherlich, the behavior of the “authorities” bordered on character assassination.
As late as 1973, a renowned internist threatened that German internists would leave the room locked at the upcoming DGIM Congress if — as had been planned by congress chair Herbert Begemann — Mitscherlich gave a talk on this subject, journalist and doctor Renate Jäckle reported in her book on doctors and politics.
Even toward the end of the 1980s, Karsten Vilmar, MD, then president of the German Medical Association, reacted in an insensitive and defensive manner — during an interview — to an article in the Lancet, written by the Mainz pediatrician Hartmut M. Hanauske-Abel, MD, on the role of the German medical profession in the Third Reich and the suppression that followed after 1945.
A group of 400 doctors, at most, were culpable, and coming to terms with the past should not defame doctors collectively, Dr. Vilmar said in a statement chillingly reminiscent of declarations made by the Wehrmacht, which described itself as mainly “clean.”
Of course, the end of the Nazi regime was not the end of all barbarity, not even in Europe. “Violence will be something we have to confront in our future lives, too. Belief in the healing powers of civilization is nothing but a fairytale,” Berlin historian Jörg Barberowski wrote in a 2012 essay.
Nevertheless, as Michael Hallek, MD, from the University Hospital of Cologne, said, it is important to keep memory alive.
A version of this article first appeared on Medscape.com.