User login
For MD-IQ use only
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
Type VII Collagen Disorders Simplified
Type VII Collagen Disorders Simplified
There are 3 uncommon types of mechanobullous skin diseases caused by relative reduction or complete loss of functional type VII collagen, which is the main component of anchoring fibrils in the lamina densa of the basement membrane zone (BMZ) of the skin and mucous membrane epithelium.1 The function of the anchoring fibrils is to maintain adherence of the basement membrane of the epithelium to the connective tissue of the papillary dermis and submucosa.1 The mechanism of action of the loss of type VII collagen function is via autoimmunity in epidermolysis bullosa acquisita (EBA)2 and
Epidermolysis Bullosa
Epidermolysis bullosa consists of a heterogeneous family of 4 major genetic mechanobullous diseases that affect the skin and mucous membranes with more than 30 subtypes.1 Dystrophic EB is caused by mutations in the COL7A1 gene, which encodes for the α-1 chain of collagen type VII. Classically, EB is divided into 4 main variants based on the location of the cleavage plane or split occurring in the epithelium, which in turn helps to predict the severity of the illness.
Epidermolysis bullosa may be inherited in an autosomal-dominant or autosomal-recessive fashion, or it may occur as a spontaneous mutation. All sexes and races are affected equally. Patients present at birth or in early childhood with fragile skin and mucous membranes that may develop blisters, erosions, and ulcerations after minor trauma.7 These lesions are marked by slow healing and scar formation and often are associated with itching and pain.
Dystrophic Epidermolysis Bullosa
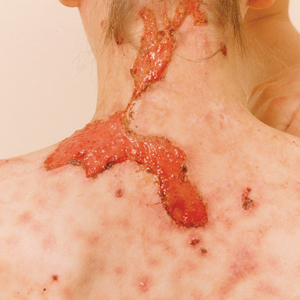
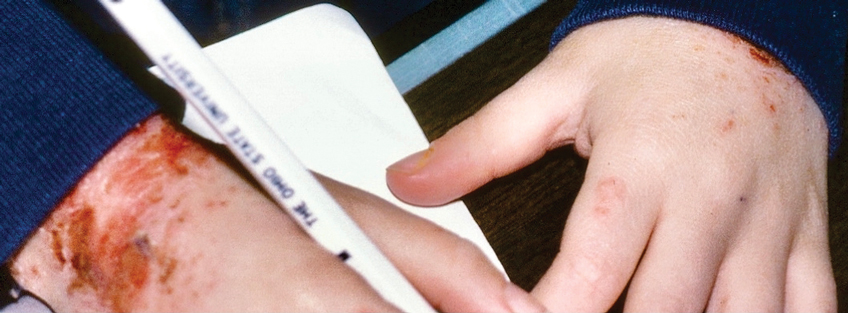
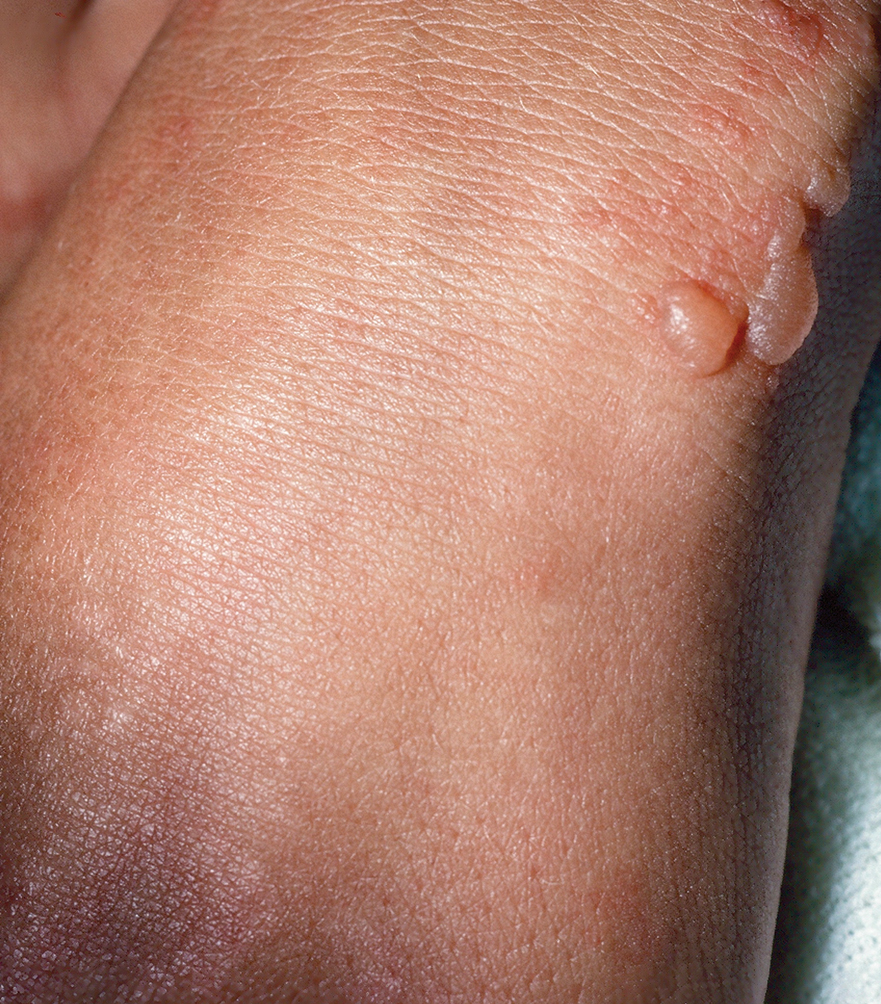
Dystrophic EB accounts for approximately 25%6 of all EB cases in the United States and may be inherited as either a dominant or recessive trait. Hundreds of different pathogenic mutations have been discovered in the COL7A1 gene in the subtypes of DEB.4,8 Dominant DEB tends to cause milder disease because the patients retain one normal COL7A1 allele and produce some type VII collagen (Figure 1), whereas patients with recessive DEB lack type VII collagen completely.9 The cleavage plane is between the lamina densa and the superficial dermis or submucosa. Severity is variable and ranges from localization to the hands and feet to severe generalized blistering and painful ulcerations depending on which of the many possible gene mutations have been inherited. Sequelae include mitten deformities, malalignment and tooth decay, and the development of early aggressive squamous cell carcinomas, which may be fatal. The most severe cases of recessive DEB also may have internal organ involvement.
Epidermolysis Bullosa Simplex
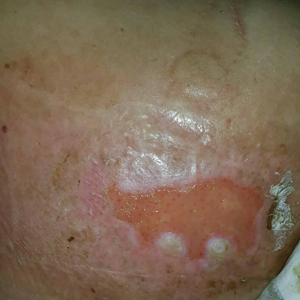
Epidermolysis bullosa simplex is the most common variant, comprising approximately 70%of EB cases in the United States.6 Epidermolysis bullosa simplex usually is inherited as autosomal-dominant mutations in the keratin 5 or keratin 14 genes,10 not COL7A1. Skin blistering results from cleavage within the basal cell layer where the keratin genes are primarily expressed. Blisters tend to occur in acral areas such as hands and feet and may heal without scarring in the localized form of epidermolysis bullosa simplex (Figure 2).
Junctional Epidermolysis Bullosa and Kindler Syndrome
Junctional epidermolysis bullosa (JEB) and Kindler syndrome11 are the rarest of the autosomal-recessive EB variants.6 The plane of cleavage in JEB is through the lamina lucida of the BMZ. Junctional epidermolysis bullosa is caused by mutations of the genes that encode for the 3 chains of laminin 332 protein and type XVII collagen,5,12 not to be confused with type VII collagen. As with DEB, there is a wide range of severity in JEB, from localized effects on the eyes, oral cavity, and tooth enamel to widespread blistering and skin cancers. In JEB cases involving newborns, nonhealing wounds on the face, buttocks, fingers, and toes may be seen, with devastating complications in the oral cavity, esophagus, and larynx. Life expectancy is limited to 2 years or less.6 There have only been approximately 40,013 cases of Kindler syndrome reported worldwide6 and there is clinical overlap with DEB. Patients also may demonstrate poikiloderma and photosensitivity. Kindler syndrome is caused by mutations in the FERMT1 gene which encodes for kindlin-1. This protein mediates anchorage between the actin cytoskeleton and the extracellular matrix.5,11 Loss of function produces variable cleavage planes around the dermoepidermal junction.
Clinical management of all EB variants, especially the severe recessive types, traditionally has been limited to the prevention of trauma to the skin and mucous membranes and supportive care, including dressing changes to erosions and ulcerations, antibiotic ointments as needed, and amelioration of pain and pruritus. Bone marrow and pluripotential stem cell transplants have been attempted.12 Complications of EB, such as deformities of the hands and feet caused by excessive scarring, esophageal strictures, poor dentition, and squamous cell carcinomas, must be addressed by a multidisciplinary team of specialists, including plastic surgery, gastroenterology, dentistry/oral surgery, ophthalmology, and dermatology/Mohs surgery.
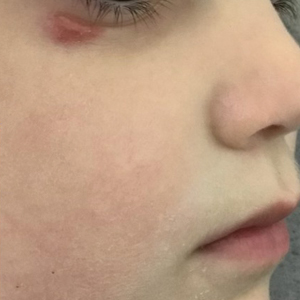
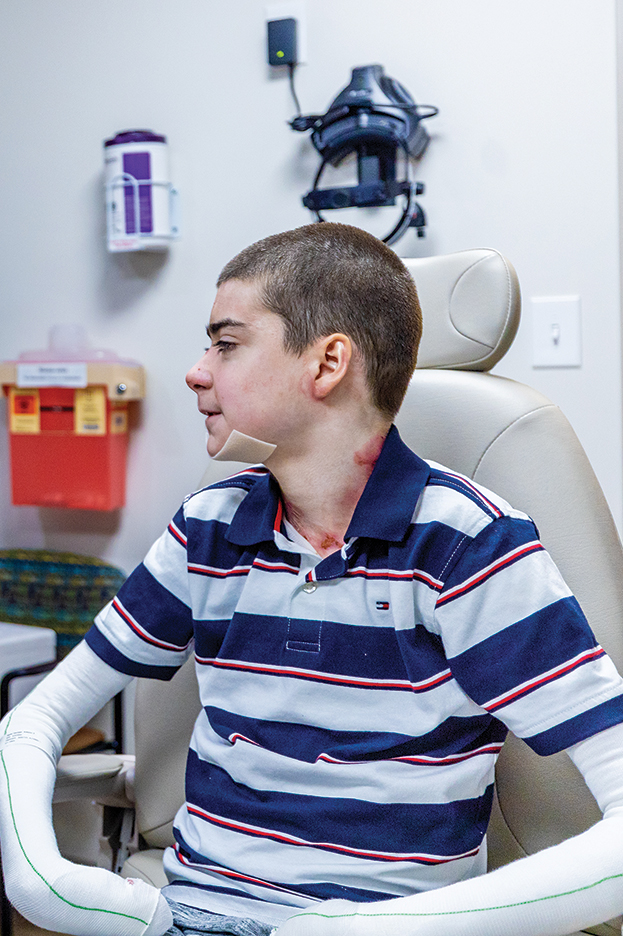
Until recently, there were no medications approved by the US Food and Drug Administration (FDA) specifically indicated for EB. In 2023, topical gene therapy was approved by the FDA for both recessive and dominant forms of DEB. Normal COL7A1 sequences are delivered by an attenuated herpes simplex virus 1 vector (beremagene geperpavec) in a gel applied directly to the wounds of patients with DEB. In a clinical trial, matching wounds on 31 patients (62 wounds total) were treated with the active agent or placebo gel. After 6 months, complete wound closure was observed in 67% (21/31) of those treated with the active agent and 22% (7/31) of those treated with placebo (P=.002).14 In a single case report, a patient with recessive DEB and cicatrizing conjunctivitis (Figure 3) was given ophthalmic beremagene geperpavec after surgery and had improved visual acuity.15 A topical gel consisting of birch triterpenes to promote healing of partial-thickness wounds also was approved for patients with DEB and JEB by the FDA and the European Commission. In a study of 223 patients, 41% of those using active gel and 29% of those using placebo gel achieved the primary end point of percentage of target wounds that had first complete closure at 45 days.16
The most recent FDA approval for DEB involves transferring the functional COL7A1 gene to the patient’s skin cells, then expanding the gene-corrected cells into sheets of keratinocytes that can be surgically applied to the chronic wound sites. In a phase 3 trial of prademagene zamikeracel (pz-cel), 11 patients with 86 matched wounds were randomized to receive pz-cel (50%) or standard wound care (50%). After 24 weeks, 35 wounds treated with pz-cel were at least 50% healed compared to 7 control wounds.17 The results for healing and reduction of pain were statistically significant (P<.0001 and P<.0002, respectively).17 Recombinant collagen VII as replacement therapy also is under study to be given by intravenous infusion to increase tissue collagen VII where it is lacking. This treatment has shown early biologic and therapeutic effects.9,18 Larger long-term follow-up studies are necessary to confirm persistence of the gene-corrected skin cells, the functionality of the replacement collagen VII, and the potential risk for the development of autoantibodies to type VII collagen.
Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita is a rare autoimmune subepithelial bullous disease that primarily affects middle-aged adults but also has been reported in children.19 Epidermolysis bullosa acquisita is caused by circulating pathogenic IgG autoantibodies that target and bind to type VII collagen in the anchoring fibrils,20-22 thereby disrupting the attachment of the epithelium to its underlying connective tissue.
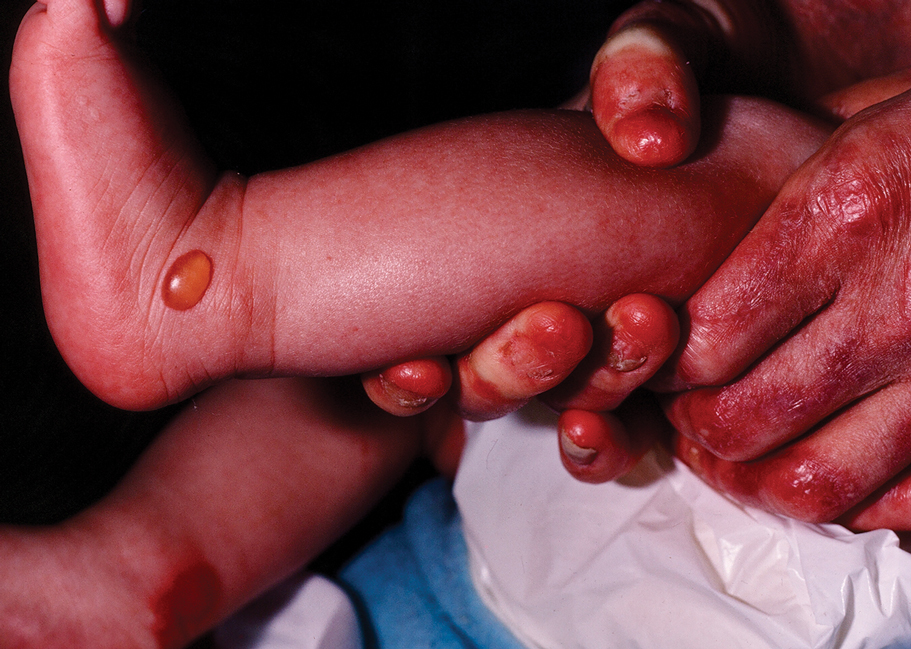
The 2 major clinical manifestations of EBA include a mechanobullous disease resembling inherited forms of DEB (Figure 4) and an inflammatory bullous pemphigoid (BP)–like disease,23 as well as a combination of both types of skin lesions (Figure 5). The skin and mucous membranes of the oral cavity, esophagus, eyes, and urogenital areas are affected in both types; scarring may cause functional disabilities. In the mechanobullous type of EBA, it is common for blisters and erosions to develop in trauma-prone areas such as the hands, feet, elbows, and knees. The blisters tend to heal with scarring and milia formation as might be seen in porphyria cutanea tarda or cicatricial pemphigoid, which are in the differential diagnosis. Dystrophy of the fingernails or complete nail loss may be observed, resembling DEB. In the BP-like presentation, tense blisters arise upon inflamed or urticarial skin and mucous membranes, which may then become generalized.
Histopathology in both forms of EBA demonstrates subepithelial separation as clefts or blisters. The mechanobullous type shows a sparse inflammatory infiltrate compared to large collections of neutrophils and eosinophils in the blister cavity and in the superficial dermis in the BP-like cases. The final diagnosis rests on the results of immunopathology testing.24 Direct immunofluorescence of perilesional skin and mucosa shows a linear-granular band of IgG and C3 and other conjugates along the BMZ. Deposits of IgA alone in EBA occur in only about 2.4% of cases and are observed more often when there is mucous membrane involvement.2 Indirect immunofluorescence of sera against salt-split skin substrates detects immunoreactants in the floor of the blister rather than in the roof, as would be seen in BP. Highly specific and sensitive enzyme-linked immunosorbent assay (ELISA) kits now are commercially available and can detect autoantibodies against the N-terminal domain of type VII collagen in more than 90% of cases of EBA.25
Inflammatory bowel disease (IBD), particularly Crohn disease (CD), precedes the onset of EBA in approximately 25% of cases.26,27 Ulcerative colitis is much less common. Type VII collagen is normally present in the basement membrane of intestinal epithelium. In a survey of patients with IBD, 68% of those with CD and 13% of those with ulcerative colitis had circulating anti–type VII collagen antibodies detected by ELISA without having symptoms of EBA.28 A case report of a patient with both well-proven EBA and CD highlighted the clinical difficulty of controlling EBA: treatment with prednisolone and sulfasalazine improved the CD but had little effect on the skin blisters.29 A variety of malignancies have been reported in association with EBA, including cancers of the uterine cervix,30 thyroid, and pancreas,31 lymphoma, and chronic lymphatic leukemia. Some of these cases have met the criteria for classification as paraneoplastic, whereas others may have been coincidental.
Treatment for chronic EBA generally has been limited.2,24 Putative antineutrophil drugs such as dapsone and colchicine combined with systemic corticosteroids may be useful in milder or juvenile cases, which tend to have a better prognosis than adult cases.19 In more severe EBA, systemic corticosteroids and/or immunosuppressive drugs such as azathioprine,23 cyclophosphamide,23 mycophenolate mofetil,31 methotrexate,23 cyclosporine,33 and infliximab23 have been used. More recently, rituximab infusion monotherapy33 and rituximab combined with intravenous immunoglobulin or
Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus is a rare and specific autoimmune skin complication that mostly is seen in patients with an established diagnosis of systemic lupus erythematosus (SLE) who are experiencing a disease flare. Although more common in women, it has been reported in all sexes and races as well as in children. Vesicles and bullae may arise on sun-exposed (Figure 6) and sun-protected areas of skin.
Histopathology shows subepidermal separation with collections of neutrophils and nuclear fragments in the blister cavity. The differential diagnosis of BSLE includes EBA, BP, dermatitis herpetiformis, and linear IgA bullous dermatosis. Direct immunofluorescence testing shows linear-granular deposits of IgG and/or IgM and IgA along the BMZ.34 When utilizing the indirect immunofluorescence split-skin assay, the autoantibody to type VII collagen would be detected in the floor of the blister if the serum titer was sufficiently high.3 Proposed criteria for the diagnosis of BSLE have been published: 1) diagnosis of SLE now based on the 2019 European League Against Rheumatism/American College of Rheumatology classification35; 2) vesicles and bullae arising upon but not limited to sun-exposed skin; 3) histopathology featuring neutrophil-rich subepithelial bullae; 4) positive indirect immunofluorescence for circulating BMZ antibodies using separated human skin as substrate; 5) and direct immunofluorescence showing IgG and/or IgM and often IgA at the BMZ.36 Using ELISA to detect circulating antibodies against type VII collagen24 should now be added to the criteria. The new criteria for SLE34 do not include BSLE, perhaps because it occurs in less than 1% of patients with SLE.37
Further investigation by Gammon et al3 confirmed that the autoantibodies in BSLE are identical to those found in EBA (ie, directed against type VII collagen in the lamina densa). Bullous systemic lupus erythematosus is not considered to be the coexistence of EBA with SLE but rather a specific entity wherein type VII collagen autoantibodies are expressed in the autoimmune spectrum of SLE. It is especially important to make the diagnosis of BSLE because it is predictive of more serious systemic complications of SLE (eg, hematologic and renal disease is found in up to 90% of cases).38
The natural course of BSLE is variable. Treatments include systemic corticosteroids, dapsone, and immunosuppressive drugs such as azathioprine, methotrexate, mycophenolate mofetil, and cyclophosphamide, especially in cases with nephritis.37 There may be spontaneous resolution of the rash as the inflammatory activity of SLE subsides. Rituximab has been used effectively in several refractory cases of BSLE that failed to respond to all other conventional treatments.39
Conclusion
Anchoring fibrils are composed primarily of type VII collagen. Their role is to maintain the attachment of epithelium to the upper dermis and submucosa. The reduction or complete loss of type VII collagen caused by mutations of the COL7A1 gene results in dominant DEB or recessive DEB, respectively. Two distinct non-heritable immunobullous diseases, EBA and BSLE, are caused by autoantibodies that target type VII collagen. A comparison of the 4 type VII collagen disorders can be found in the Table.
- Bardhan A, Bruckner-Tuderman L, Chapple ILC, et al. Epidermolysis bullosa. Nat Rev Dis Primers. 2020;6:78. doi:10.1038/s41572-020-0210-0
- Miyamoto D, Gordilho JO, Santi CG, et al. Epidermolysis bullosa acquisita. An Bras Dermatol. 2022;97:409-423. doi:10.1016/j.abd.2021.09.010.
- Gammon WR, Woodley DT, Dole KC, et al. Evidence that anti-basement membrane zone antibodies in bullous eruption of systemic lupus erythematosus recognize epidermolysis bullosa acquisita autoantigen. J Invest Dermatol. 1985;84:472-476. doi:10.1111/1523-1747.ep12272402.
- Yadav RS, Jaswal A, Shrestha S, et al. Dystrophic epidermolysis bullosa. J Nepal Med Assoc. 2018;56:879-882. doi:10.31729/jnma.3791
- Mariath LM, Santin JT, Schuler-Faccini L, et al. Inherited epidermolysis bullosa: update on the clinical and genetic aspects. An Bras Dermatol. 2020;95:551-569. doi:10.1016/j.abd.2020.05.001
- Understanding epidermolysis bullosa (EB). DEBRA website. Accessed August 17, 2025. https://www.debra.org/about-eb/understanding-epidermolysis-bullosa-eb
- Hon KL, Chu S, Leung AKC. Epidermolysis bullosa: pediatric perspectives. Curr Pediatr Rev. 2022;18:182-190. doi:10.2174/1573396317666210525161252
- Dang N, Klingberg S, Marr P, et al. Review of collagen VII sequence variants found in Australasian patients with dystrophic epidermolysis bullosa reveals nine COL7A1 variants. J Dermatol Sci. 2007;46:169-178. doi:10.1016/j.jdermsci.2007.02.006
- Payne AS. Topical gene therapy for epidermolysis bullosa. N Engl J Med. 2022;387:2281-2284. doi:10.1056/NEJMe2213203
- Khani P, Ghazi F, Zekri A, et al. Keratins and epidermolysis bullosa simplex. J Cell Physiol. 2018;234:289-297. doi:10.1002/jcp.26898
- Lai-Cheong JE, Tanaka A, Hawche G, et al. Kindler syndrome: a focal adhesion genodermatosis. Br J Dermatol. 2009;160:233-242. doi:10.1111/j.1365-2133.2008.08976.x
- Hou P-C, Wang H-T, Abhee S, et al. Investigational treatments for epidermolysis bullosa. Am J Clin Dermatol. 2021;22:801-817. doi:10.1007/s40257-021-00626-3
- Youseffian L, Vahidnezhad H, Uitto J. Kindler Syndrome. GeneReviews [Internet]. Updated January 6, 2022. Accessed August 21, 2025.
- Guide SV, Gonzalez ME, Bagci S, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387:2211-2219. doi:10.1056/NEJMoa2206663
- Vetencourt AT, Sayed-Ahmed I, Gomez J, et al. Ocular gene therapy in a patient with dystrophic epidermolysis bullosa. N Engl J Med. 2024;390:530-535. doi:10.1056/NEJMoa2301244
- Kern JS, Sprecher E, Fernandez MF, et al. Efficacy and safety of Oleogel-S10 (birch triterpenes for epidermolysis bullosa: results from the phase III randomized double-blind phase of the EASE study. Br J Dermatol. 2023;188:12-21. doi:10.1093/bjd/ljac001
- Tang JY, Marinkovich MP, Wiss K, et al. Prademagene zamikeracel for recessive dystrophic epidermolysis bullosa wounds (VIITAL): a two-centre, randomized, open-label, intrapatient-controlled phase 3 trial. Lancet. 2025;406:163-173. doi:10.1016/S0140-6736(25)00778-0
- Gretzmeier C, Pin D, Kern JS, et al. Systemic collagen VII replacement therapy for advanced recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2022;142:1094-1102. doi:10.1016/j.jid.2021.09.008
- Hignett E, Sami N. Pediatric epidermolysis bullosa acquisita. A review. Pediatr Dermatol. 2021;38:1047-1050. doi:10.1111/pde.14722
- Chen M, Kim GH, Prakash L, et al. Autoimmunity to anchoring fibril collagen. Autoimmunity. 2012;45:91-101. doi:10.1007/s12016-007-0027-6.
- Kridin K, Kneiber D, Kowalski EH, et al. Epidermolysis bullosa acquisita: a comprehensive review. Autoimmun Rev. 2019;18:786-795. doi:10.1016/j.autrev.2019.06.007
- Hofmann SC, Weidinger A. Epidermolysis bullosa acquisita. Hautarzt. 2019;70:265-270. doi:10.1007/s00105-019-4387-7
- Ishi N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what’s new? J Dermatol. 2010;37:220-230. doi:10.1111/j.1346-8138.2009.00799.x
- Iwata H, Vorobyev A, Koga H, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. 2018;13:153. doi:10.1186/s13023-018-0896-1
- Komorowski L, Muller R, Vorobyev A, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2013;68:e89-95. doi:10.1016/j.jaad.2011.12.032
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:364-390. doi:10.3390/jcm10020364
- Bezzio C, Della Corte C, Vernero M, et al. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at less frequent associations. Therap Adv Gastroenterol. 2022;15:17562848221115312. doi:10.1177/17562848221115312
- Chen M, O’Toole EA, Sanghavi J, et al. The epidermolysis acquisita antigen (type VII collagen) is present in human colon and patients with Crohn’s disease have antibodies to type VII collagen. J Invest Dermatol. 2002;118:1059-1064. doi:10.1046/j.1523-1747.2002.01772.x
- Labeille B, Gineston JL, Denoeux JP, et al. Epidermolysis bullosa acquisita and Crohn’s disease. A case report with immunological and electron microscopic studies. Arch Intern Med. 1988;148:1457-1459.
- Etienne A, Ruffieux P, Didierjean L, et al. Epidermolysis bullosa acquisita and metastatic cancer of the uterine cervix. Ann Dermatol Venereol. 1998;125:321-323.
- Busch J-O, Sticherling M. Epidermolysis bullosa acquisita and neuroendocrine pancreatic cancer-Coincidence or patho-genetic relationship? J Dtsch Dermatol Ges. 2007;5:916-918. doi:10.111/j.1610-0387.2007.06338.x
- Bevans SL, Sami N. The use of rituximab in treatment of epidermolysis bullosa acquisita: three new cases and a review of the literature. Dermatol Ther. 2018;31:e12726. doi:10.1111/j.1610-0387.2007.06338.x
- Yang A, Kim M, Craig P, et al. A case report of the use of rituximab and the epidermolysis bullosa disease activity scoring index (EBDASI) in a patient with epidermolysis bullosa acquisita with extensive esophageal involvement. Arch Dermatovenerol Croat. 2018;26:325-328.
- Burrows NP, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338. doi:10.1111/j.1365-2133.1993.tb00180.x
- Aringer M, Leuchten N, Johnson SR. New criteria for lupus. Curr Rheum Rep. 2020;22:18. doi:10.1007/s11926-020-00896-6
- Camisa C. Vesiculobullous systemic lupus erythematosus. A report of four cases. J Am Acad Dermatol. 1988;18:93-100. doi:10.1016/s0190-9622(88)70014-6
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:167064. doi:10.1155/2015/167064
- Sprow G, Afarideh M, Dan J, et al. Bullous systemic lupus erythematosus in females. Int J Womens Dermatol. 2022;8:e034. doi:10.1097/JW9.0000000000000034
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524. doi:10.1007/s40257-014-0098-0
- Fine JD, Mellerio JE. Epidermolysis bullosa. In: Bolognia JL, Jorizzo JL, Schaffer JV (eds), Dermatology (ed 3), Elsevier Saunders; 2012: 501-513.
There are 3 uncommon types of mechanobullous skin diseases caused by relative reduction or complete loss of functional type VII collagen, which is the main component of anchoring fibrils in the lamina densa of the basement membrane zone (BMZ) of the skin and mucous membrane epithelium.1 The function of the anchoring fibrils is to maintain adherence of the basement membrane of the epithelium to the connective tissue of the papillary dermis and submucosa.1 The mechanism of action of the loss of type VII collagen function is via autoimmunity in epidermolysis bullosa acquisita (EBA)2 and
Epidermolysis Bullosa
Epidermolysis bullosa consists of a heterogeneous family of 4 major genetic mechanobullous diseases that affect the skin and mucous membranes with more than 30 subtypes.1 Dystrophic EB is caused by mutations in the COL7A1 gene, which encodes for the α-1 chain of collagen type VII. Classically, EB is divided into 4 main variants based on the location of the cleavage plane or split occurring in the epithelium, which in turn helps to predict the severity of the illness.
Epidermolysis bullosa may be inherited in an autosomal-dominant or autosomal-recessive fashion, or it may occur as a spontaneous mutation. All sexes and races are affected equally. Patients present at birth or in early childhood with fragile skin and mucous membranes that may develop blisters, erosions, and ulcerations after minor trauma.7 These lesions are marked by slow healing and scar formation and often are associated with itching and pain.
Dystrophic Epidermolysis Bullosa
Dystrophic EB accounts for approximately 25%6 of all EB cases in the United States and may be inherited as either a dominant or recessive trait. Hundreds of different pathogenic mutations have been discovered in the COL7A1 gene in the subtypes of DEB.4,8 Dominant DEB tends to cause milder disease because the patients retain one normal COL7A1 allele and produce some type VII collagen (Figure 1), whereas patients with recessive DEB lack type VII collagen completely.9 The cleavage plane is between the lamina densa and the superficial dermis or submucosa. Severity is variable and ranges from localization to the hands and feet to severe generalized blistering and painful ulcerations depending on which of the many possible gene mutations have been inherited. Sequelae include mitten deformities, malalignment and tooth decay, and the development of early aggressive squamous cell carcinomas, which may be fatal. The most severe cases of recessive DEB also may have internal organ involvement.

Epidermolysis Bullosa Simplex
Epidermolysis bullosa simplex is the most common variant, comprising approximately 70%of EB cases in the United States.6 Epidermolysis bullosa simplex usually is inherited as autosomal-dominant mutations in the keratin 5 or keratin 14 genes,10 not COL7A1. Skin blistering results from cleavage within the basal cell layer where the keratin genes are primarily expressed. Blisters tend to occur in acral areas such as hands and feet and may heal without scarring in the localized form of epidermolysis bullosa simplex (Figure 2).

Junctional Epidermolysis Bullosa and Kindler Syndrome
Junctional epidermolysis bullosa (JEB) and Kindler syndrome11 are the rarest of the autosomal-recessive EB variants.6 The plane of cleavage in JEB is through the lamina lucida of the BMZ. Junctional epidermolysis bullosa is caused by mutations of the genes that encode for the 3 chains of laminin 332 protein and type XVII collagen,5,12 not to be confused with type VII collagen. As with DEB, there is a wide range of severity in JEB, from localized effects on the eyes, oral cavity, and tooth enamel to widespread blistering and skin cancers. In JEB cases involving newborns, nonhealing wounds on the face, buttocks, fingers, and toes may be seen, with devastating complications in the oral cavity, esophagus, and larynx. Life expectancy is limited to 2 years or less.6 There have only been approximately 40,013 cases of Kindler syndrome reported worldwide6 and there is clinical overlap with DEB. Patients also may demonstrate poikiloderma and photosensitivity. Kindler syndrome is caused by mutations in the FERMT1 gene which encodes for kindlin-1. This protein mediates anchorage between the actin cytoskeleton and the extracellular matrix.5,11 Loss of function produces variable cleavage planes around the dermoepidermal junction.
Clinical management of all EB variants, especially the severe recessive types, traditionally has been limited to the prevention of trauma to the skin and mucous membranes and supportive care, including dressing changes to erosions and ulcerations, antibiotic ointments as needed, and amelioration of pain and pruritus. Bone marrow and pluripotential stem cell transplants have been attempted.12 Complications of EB, such as deformities of the hands and feet caused by excessive scarring, esophageal strictures, poor dentition, and squamous cell carcinomas, must be addressed by a multidisciplinary team of specialists, including plastic surgery, gastroenterology, dentistry/oral surgery, ophthalmology, and dermatology/Mohs surgery.
Until recently, there were no medications approved by the US Food and Drug Administration (FDA) specifically indicated for EB. In 2023, topical gene therapy was approved by the FDA for both recessive and dominant forms of DEB. Normal COL7A1 sequences are delivered by an attenuated herpes simplex virus 1 vector (beremagene geperpavec) in a gel applied directly to the wounds of patients with DEB. In a clinical trial, matching wounds on 31 patients (62 wounds total) were treated with the active agent or placebo gel. After 6 months, complete wound closure was observed in 67% (21/31) of those treated with the active agent and 22% (7/31) of those treated with placebo (P=.002).14 In a single case report, a patient with recessive DEB and cicatrizing conjunctivitis (Figure 3) was given ophthalmic beremagene geperpavec after surgery and had improved visual acuity.15 A topical gel consisting of birch triterpenes to promote healing of partial-thickness wounds also was approved for patients with DEB and JEB by the FDA and the European Commission. In a study of 223 patients, 41% of those using active gel and 29% of those using placebo gel achieved the primary end point of percentage of target wounds that had first complete closure at 45 days.16

The most recent FDA approval for DEB involves transferring the functional COL7A1 gene to the patient’s skin cells, then expanding the gene-corrected cells into sheets of keratinocytes that can be surgically applied to the chronic wound sites. In a phase 3 trial of prademagene zamikeracel (pz-cel), 11 patients with 86 matched wounds were randomized to receive pz-cel (50%) or standard wound care (50%). After 24 weeks, 35 wounds treated with pz-cel were at least 50% healed compared to 7 control wounds.17 The results for healing and reduction of pain were statistically significant (P<.0001 and P<.0002, respectively).17 Recombinant collagen VII as replacement therapy also is under study to be given by intravenous infusion to increase tissue collagen VII where it is lacking. This treatment has shown early biologic and therapeutic effects.9,18 Larger long-term follow-up studies are necessary to confirm persistence of the gene-corrected skin cells, the functionality of the replacement collagen VII, and the potential risk for the development of autoantibodies to type VII collagen.
Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita is a rare autoimmune subepithelial bullous disease that primarily affects middle-aged adults but also has been reported in children.19 Epidermolysis bullosa acquisita is caused by circulating pathogenic IgG autoantibodies that target and bind to type VII collagen in the anchoring fibrils,20-22 thereby disrupting the attachment of the epithelium to its underlying connective tissue.
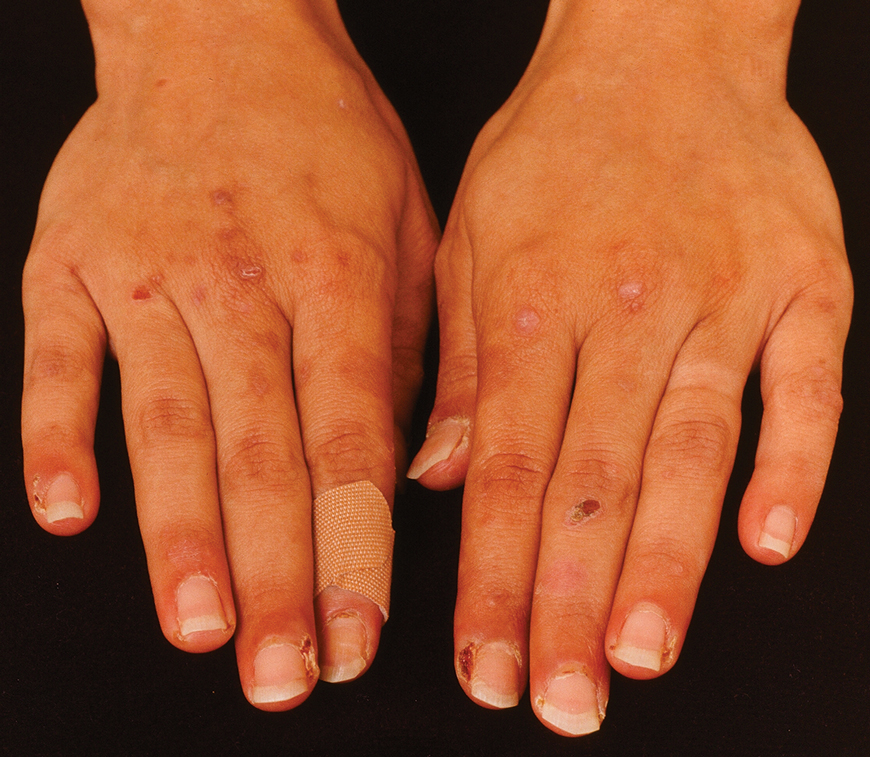
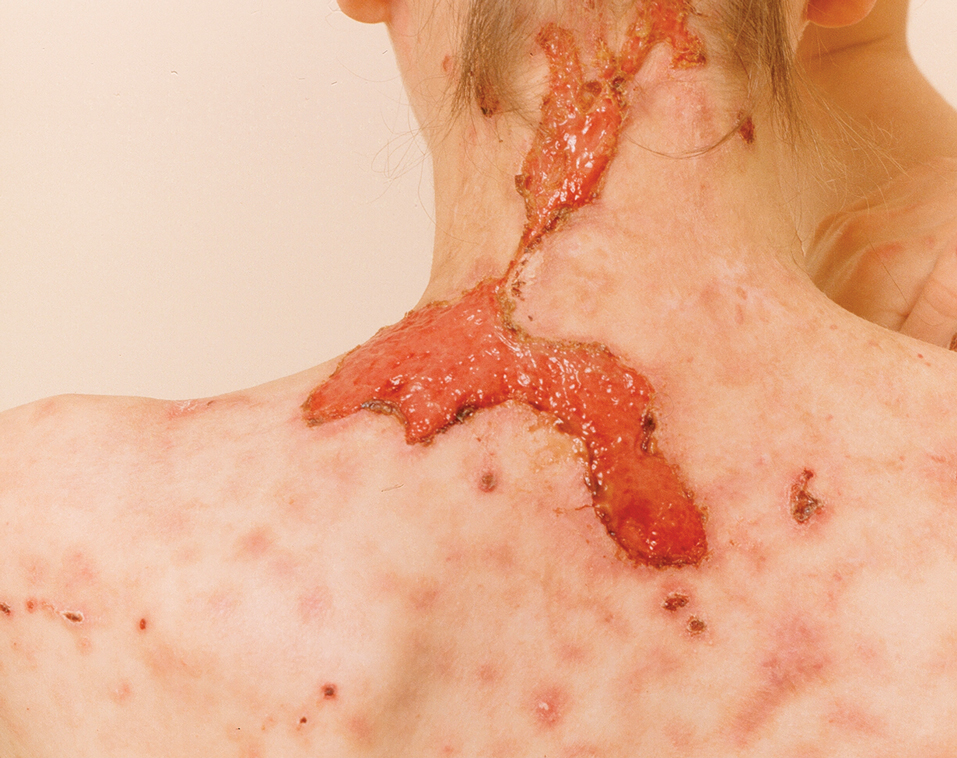
The 2 major clinical manifestations of EBA include a mechanobullous disease resembling inherited forms of DEB (Figure 4) and an inflammatory bullous pemphigoid (BP)–like disease,23 as well as a combination of both types of skin lesions (Figure 5). The skin and mucous membranes of the oral cavity, esophagus, eyes, and urogenital areas are affected in both types; scarring may cause functional disabilities. In the mechanobullous type of EBA, it is common for blisters and erosions to develop in trauma-prone areas such as the hands, feet, elbows, and knees. The blisters tend to heal with scarring and milia formation as might be seen in porphyria cutanea tarda or cicatricial pemphigoid, which are in the differential diagnosis. Dystrophy of the fingernails or complete nail loss may be observed, resembling DEB. In the BP-like presentation, tense blisters arise upon inflamed or urticarial skin and mucous membranes, which may then become generalized.


Histopathology in both forms of EBA demonstrates subepithelial separation as clefts or blisters. The mechanobullous type shows a sparse inflammatory infiltrate compared to large collections of neutrophils and eosinophils in the blister cavity and in the superficial dermis in the BP-like cases. The final diagnosis rests on the results of immunopathology testing.24 Direct immunofluorescence of perilesional skin and mucosa shows a linear-granular band of IgG and C3 and other conjugates along the BMZ. Deposits of IgA alone in EBA occur in only about 2.4% of cases and are observed more often when there is mucous membrane involvement.2 Indirect immunofluorescence of sera against salt-split skin substrates detects immunoreactants in the floor of the blister rather than in the roof, as would be seen in BP. Highly specific and sensitive enzyme-linked immunosorbent assay (ELISA) kits now are commercially available and can detect autoantibodies against the N-terminal domain of type VII collagen in more than 90% of cases of EBA.25
Inflammatory bowel disease (IBD), particularly Crohn disease (CD), precedes the onset of EBA in approximately 25% of cases.26,27 Ulcerative colitis is much less common. Type VII collagen is normally present in the basement membrane of intestinal epithelium. In a survey of patients with IBD, 68% of those with CD and 13% of those with ulcerative colitis had circulating anti–type VII collagen antibodies detected by ELISA without having symptoms of EBA.28 A case report of a patient with both well-proven EBA and CD highlighted the clinical difficulty of controlling EBA: treatment with prednisolone and sulfasalazine improved the CD but had little effect on the skin blisters.29 A variety of malignancies have been reported in association with EBA, including cancers of the uterine cervix,30 thyroid, and pancreas,31 lymphoma, and chronic lymphatic leukemia. Some of these cases have met the criteria for classification as paraneoplastic, whereas others may have been coincidental.
Treatment for chronic EBA generally has been limited.2,24 Putative antineutrophil drugs such as dapsone and colchicine combined with systemic corticosteroids may be useful in milder or juvenile cases, which tend to have a better prognosis than adult cases.19 In more severe EBA, systemic corticosteroids and/or immunosuppressive drugs such as azathioprine,23 cyclophosphamide,23 mycophenolate mofetil,31 methotrexate,23 cyclosporine,33 and infliximab23 have been used. More recently, rituximab infusion monotherapy33 and rituximab combined with intravenous immunoglobulin or
Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus is a rare and specific autoimmune skin complication that mostly is seen in patients with an established diagnosis of systemic lupus erythematosus (SLE) who are experiencing a disease flare. Although more common in women, it has been reported in all sexes and races as well as in children. Vesicles and bullae may arise on sun-exposed (Figure 6) and sun-protected areas of skin.

Histopathology shows subepidermal separation with collections of neutrophils and nuclear fragments in the blister cavity. The differential diagnosis of BSLE includes EBA, BP, dermatitis herpetiformis, and linear IgA bullous dermatosis. Direct immunofluorescence testing shows linear-granular deposits of IgG and/or IgM and IgA along the BMZ.34 When utilizing the indirect immunofluorescence split-skin assay, the autoantibody to type VII collagen would be detected in the floor of the blister if the serum titer was sufficiently high.3 Proposed criteria for the diagnosis of BSLE have been published: 1) diagnosis of SLE now based on the 2019 European League Against Rheumatism/American College of Rheumatology classification35; 2) vesicles and bullae arising upon but not limited to sun-exposed skin; 3) histopathology featuring neutrophil-rich subepithelial bullae; 4) positive indirect immunofluorescence for circulating BMZ antibodies using separated human skin as substrate; 5) and direct immunofluorescence showing IgG and/or IgM and often IgA at the BMZ.36 Using ELISA to detect circulating antibodies against type VII collagen24 should now be added to the criteria. The new criteria for SLE34 do not include BSLE, perhaps because it occurs in less than 1% of patients with SLE.37
Further investigation by Gammon et al3 confirmed that the autoantibodies in BSLE are identical to those found in EBA (ie, directed against type VII collagen in the lamina densa). Bullous systemic lupus erythematosus is not considered to be the coexistence of EBA with SLE but rather a specific entity wherein type VII collagen autoantibodies are expressed in the autoimmune spectrum of SLE. It is especially important to make the diagnosis of BSLE because it is predictive of more serious systemic complications of SLE (eg, hematologic and renal disease is found in up to 90% of cases).38
The natural course of BSLE is variable. Treatments include systemic corticosteroids, dapsone, and immunosuppressive drugs such as azathioprine, methotrexate, mycophenolate mofetil, and cyclophosphamide, especially in cases with nephritis.37 There may be spontaneous resolution of the rash as the inflammatory activity of SLE subsides. Rituximab has been used effectively in several refractory cases of BSLE that failed to respond to all other conventional treatments.39
Conclusion
Anchoring fibrils are composed primarily of type VII collagen. Their role is to maintain the attachment of epithelium to the upper dermis and submucosa. The reduction or complete loss of type VII collagen caused by mutations of the COL7A1 gene results in dominant DEB or recessive DEB, respectively. Two distinct non-heritable immunobullous diseases, EBA and BSLE, are caused by autoantibodies that target type VII collagen. A comparison of the 4 type VII collagen disorders can be found in the Table.


There are 3 uncommon types of mechanobullous skin diseases caused by relative reduction or complete loss of functional type VII collagen, which is the main component of anchoring fibrils in the lamina densa of the basement membrane zone (BMZ) of the skin and mucous membrane epithelium.1 The function of the anchoring fibrils is to maintain adherence of the basement membrane of the epithelium to the connective tissue of the papillary dermis and submucosa.1 The mechanism of action of the loss of type VII collagen function is via autoimmunity in epidermolysis bullosa acquisita (EBA)2 and
Epidermolysis Bullosa
Epidermolysis bullosa consists of a heterogeneous family of 4 major genetic mechanobullous diseases that affect the skin and mucous membranes with more than 30 subtypes.1 Dystrophic EB is caused by mutations in the COL7A1 gene, which encodes for the α-1 chain of collagen type VII. Classically, EB is divided into 4 main variants based on the location of the cleavage plane or split occurring in the epithelium, which in turn helps to predict the severity of the illness.
Epidermolysis bullosa may be inherited in an autosomal-dominant or autosomal-recessive fashion, or it may occur as a spontaneous mutation. All sexes and races are affected equally. Patients present at birth or in early childhood with fragile skin and mucous membranes that may develop blisters, erosions, and ulcerations after minor trauma.7 These lesions are marked by slow healing and scar formation and often are associated with itching and pain.
Dystrophic Epidermolysis Bullosa
Dystrophic EB accounts for approximately 25%6 of all EB cases in the United States and may be inherited as either a dominant or recessive trait. Hundreds of different pathogenic mutations have been discovered in the COL7A1 gene in the subtypes of DEB.4,8 Dominant DEB tends to cause milder disease because the patients retain one normal COL7A1 allele and produce some type VII collagen (Figure 1), whereas patients with recessive DEB lack type VII collagen completely.9 The cleavage plane is between the lamina densa and the superficial dermis or submucosa. Severity is variable and ranges from localization to the hands and feet to severe generalized blistering and painful ulcerations depending on which of the many possible gene mutations have been inherited. Sequelae include mitten deformities, malalignment and tooth decay, and the development of early aggressive squamous cell carcinomas, which may be fatal. The most severe cases of recessive DEB also may have internal organ involvement.

Epidermolysis Bullosa Simplex
Epidermolysis bullosa simplex is the most common variant, comprising approximately 70%of EB cases in the United States.6 Epidermolysis bullosa simplex usually is inherited as autosomal-dominant mutations in the keratin 5 or keratin 14 genes,10 not COL7A1. Skin blistering results from cleavage within the basal cell layer where the keratin genes are primarily expressed. Blisters tend to occur in acral areas such as hands and feet and may heal without scarring in the localized form of epidermolysis bullosa simplex (Figure 2).

Junctional Epidermolysis Bullosa and Kindler Syndrome
Junctional epidermolysis bullosa (JEB) and Kindler syndrome11 are the rarest of the autosomal-recessive EB variants.6 The plane of cleavage in JEB is through the lamina lucida of the BMZ. Junctional epidermolysis bullosa is caused by mutations of the genes that encode for the 3 chains of laminin 332 protein and type XVII collagen,5,12 not to be confused with type VII collagen. As with DEB, there is a wide range of severity in JEB, from localized effects on the eyes, oral cavity, and tooth enamel to widespread blistering and skin cancers. In JEB cases involving newborns, nonhealing wounds on the face, buttocks, fingers, and toes may be seen, with devastating complications in the oral cavity, esophagus, and larynx. Life expectancy is limited to 2 years or less.6 There have only been approximately 40,013 cases of Kindler syndrome reported worldwide6 and there is clinical overlap with DEB. Patients also may demonstrate poikiloderma and photosensitivity. Kindler syndrome is caused by mutations in the FERMT1 gene which encodes for kindlin-1. This protein mediates anchorage between the actin cytoskeleton and the extracellular matrix.5,11 Loss of function produces variable cleavage planes around the dermoepidermal junction.
Clinical management of all EB variants, especially the severe recessive types, traditionally has been limited to the prevention of trauma to the skin and mucous membranes and supportive care, including dressing changes to erosions and ulcerations, antibiotic ointments as needed, and amelioration of pain and pruritus. Bone marrow and pluripotential stem cell transplants have been attempted.12 Complications of EB, such as deformities of the hands and feet caused by excessive scarring, esophageal strictures, poor dentition, and squamous cell carcinomas, must be addressed by a multidisciplinary team of specialists, including plastic surgery, gastroenterology, dentistry/oral surgery, ophthalmology, and dermatology/Mohs surgery.
Until recently, there were no medications approved by the US Food and Drug Administration (FDA) specifically indicated for EB. In 2023, topical gene therapy was approved by the FDA for both recessive and dominant forms of DEB. Normal COL7A1 sequences are delivered by an attenuated herpes simplex virus 1 vector (beremagene geperpavec) in a gel applied directly to the wounds of patients with DEB. In a clinical trial, matching wounds on 31 patients (62 wounds total) were treated with the active agent or placebo gel. After 6 months, complete wound closure was observed in 67% (21/31) of those treated with the active agent and 22% (7/31) of those treated with placebo (P=.002).14 In a single case report, a patient with recessive DEB and cicatrizing conjunctivitis (Figure 3) was given ophthalmic beremagene geperpavec after surgery and had improved visual acuity.15 A topical gel consisting of birch triterpenes to promote healing of partial-thickness wounds also was approved for patients with DEB and JEB by the FDA and the European Commission. In a study of 223 patients, 41% of those using active gel and 29% of those using placebo gel achieved the primary end point of percentage of target wounds that had first complete closure at 45 days.16

The most recent FDA approval for DEB involves transferring the functional COL7A1 gene to the patient’s skin cells, then expanding the gene-corrected cells into sheets of keratinocytes that can be surgically applied to the chronic wound sites. In a phase 3 trial of prademagene zamikeracel (pz-cel), 11 patients with 86 matched wounds were randomized to receive pz-cel (50%) or standard wound care (50%). After 24 weeks, 35 wounds treated with pz-cel were at least 50% healed compared to 7 control wounds.17 The results for healing and reduction of pain were statistically significant (P<.0001 and P<.0002, respectively).17 Recombinant collagen VII as replacement therapy also is under study to be given by intravenous infusion to increase tissue collagen VII where it is lacking. This treatment has shown early biologic and therapeutic effects.9,18 Larger long-term follow-up studies are necessary to confirm persistence of the gene-corrected skin cells, the functionality of the replacement collagen VII, and the potential risk for the development of autoantibodies to type VII collagen.
Epidermolysis Bullosa Acquisita
Epidermolysis bullosa acquisita is a rare autoimmune subepithelial bullous disease that primarily affects middle-aged adults but also has been reported in children.19 Epidermolysis bullosa acquisita is caused by circulating pathogenic IgG autoantibodies that target and bind to type VII collagen in the anchoring fibrils,20-22 thereby disrupting the attachment of the epithelium to its underlying connective tissue.
The 2 major clinical manifestations of EBA include a mechanobullous disease resembling inherited forms of DEB (Figure 4) and an inflammatory bullous pemphigoid (BP)–like disease,23 as well as a combination of both types of skin lesions (Figure 5). The skin and mucous membranes of the oral cavity, esophagus, eyes, and urogenital areas are affected in both types; scarring may cause functional disabilities. In the mechanobullous type of EBA, it is common for blisters and erosions to develop in trauma-prone areas such as the hands, feet, elbows, and knees. The blisters tend to heal with scarring and milia formation as might be seen in porphyria cutanea tarda or cicatricial pemphigoid, which are in the differential diagnosis. Dystrophy of the fingernails or complete nail loss may be observed, resembling DEB. In the BP-like presentation, tense blisters arise upon inflamed or urticarial skin and mucous membranes, which may then become generalized.


Histopathology in both forms of EBA demonstrates subepithelial separation as clefts or blisters. The mechanobullous type shows a sparse inflammatory infiltrate compared to large collections of neutrophils and eosinophils in the blister cavity and in the superficial dermis in the BP-like cases. The final diagnosis rests on the results of immunopathology testing.24 Direct immunofluorescence of perilesional skin and mucosa shows a linear-granular band of IgG and C3 and other conjugates along the BMZ. Deposits of IgA alone in EBA occur in only about 2.4% of cases and are observed more often when there is mucous membrane involvement.2 Indirect immunofluorescence of sera against salt-split skin substrates detects immunoreactants in the floor of the blister rather than in the roof, as would be seen in BP. Highly specific and sensitive enzyme-linked immunosorbent assay (ELISA) kits now are commercially available and can detect autoantibodies against the N-terminal domain of type VII collagen in more than 90% of cases of EBA.25
Inflammatory bowel disease (IBD), particularly Crohn disease (CD), precedes the onset of EBA in approximately 25% of cases.26,27 Ulcerative colitis is much less common. Type VII collagen is normally present in the basement membrane of intestinal epithelium. In a survey of patients with IBD, 68% of those with CD and 13% of those with ulcerative colitis had circulating anti–type VII collagen antibodies detected by ELISA without having symptoms of EBA.28 A case report of a patient with both well-proven EBA and CD highlighted the clinical difficulty of controlling EBA: treatment with prednisolone and sulfasalazine improved the CD but had little effect on the skin blisters.29 A variety of malignancies have been reported in association with EBA, including cancers of the uterine cervix,30 thyroid, and pancreas,31 lymphoma, and chronic lymphatic leukemia. Some of these cases have met the criteria for classification as paraneoplastic, whereas others may have been coincidental.
Treatment for chronic EBA generally has been limited.2,24 Putative antineutrophil drugs such as dapsone and colchicine combined with systemic corticosteroids may be useful in milder or juvenile cases, which tend to have a better prognosis than adult cases.19 In more severe EBA, systemic corticosteroids and/or immunosuppressive drugs such as azathioprine,23 cyclophosphamide,23 mycophenolate mofetil,31 methotrexate,23 cyclosporine,33 and infliximab23 have been used. More recently, rituximab infusion monotherapy33 and rituximab combined with intravenous immunoglobulin or
Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus is a rare and specific autoimmune skin complication that mostly is seen in patients with an established diagnosis of systemic lupus erythematosus (SLE) who are experiencing a disease flare. Although more common in women, it has been reported in all sexes and races as well as in children. Vesicles and bullae may arise on sun-exposed (Figure 6) and sun-protected areas of skin.

Histopathology shows subepidermal separation with collections of neutrophils and nuclear fragments in the blister cavity. The differential diagnosis of BSLE includes EBA, BP, dermatitis herpetiformis, and linear IgA bullous dermatosis. Direct immunofluorescence testing shows linear-granular deposits of IgG and/or IgM and IgA along the BMZ.34 When utilizing the indirect immunofluorescence split-skin assay, the autoantibody to type VII collagen would be detected in the floor of the blister if the serum titer was sufficiently high.3 Proposed criteria for the diagnosis of BSLE have been published: 1) diagnosis of SLE now based on the 2019 European League Against Rheumatism/American College of Rheumatology classification35; 2) vesicles and bullae arising upon but not limited to sun-exposed skin; 3) histopathology featuring neutrophil-rich subepithelial bullae; 4) positive indirect immunofluorescence for circulating BMZ antibodies using separated human skin as substrate; 5) and direct immunofluorescence showing IgG and/or IgM and often IgA at the BMZ.36 Using ELISA to detect circulating antibodies against type VII collagen24 should now be added to the criteria. The new criteria for SLE34 do not include BSLE, perhaps because it occurs in less than 1% of patients with SLE.37
Further investigation by Gammon et al3 confirmed that the autoantibodies in BSLE are identical to those found in EBA (ie, directed against type VII collagen in the lamina densa). Bullous systemic lupus erythematosus is not considered to be the coexistence of EBA with SLE but rather a specific entity wherein type VII collagen autoantibodies are expressed in the autoimmune spectrum of SLE. It is especially important to make the diagnosis of BSLE because it is predictive of more serious systemic complications of SLE (eg, hematologic and renal disease is found in up to 90% of cases).38
The natural course of BSLE is variable. Treatments include systemic corticosteroids, dapsone, and immunosuppressive drugs such as azathioprine, methotrexate, mycophenolate mofetil, and cyclophosphamide, especially in cases with nephritis.37 There may be spontaneous resolution of the rash as the inflammatory activity of SLE subsides. Rituximab has been used effectively in several refractory cases of BSLE that failed to respond to all other conventional treatments.39
Conclusion
Anchoring fibrils are composed primarily of type VII collagen. Their role is to maintain the attachment of epithelium to the upper dermis and submucosa. The reduction or complete loss of type VII collagen caused by mutations of the COL7A1 gene results in dominant DEB or recessive DEB, respectively. Two distinct non-heritable immunobullous diseases, EBA and BSLE, are caused by autoantibodies that target type VII collagen. A comparison of the 4 type VII collagen disorders can be found in the Table.


- Bardhan A, Bruckner-Tuderman L, Chapple ILC, et al. Epidermolysis bullosa. Nat Rev Dis Primers. 2020;6:78. doi:10.1038/s41572-020-0210-0
- Miyamoto D, Gordilho JO, Santi CG, et al. Epidermolysis bullosa acquisita. An Bras Dermatol. 2022;97:409-423. doi:10.1016/j.abd.2021.09.010.
- Gammon WR, Woodley DT, Dole KC, et al. Evidence that anti-basement membrane zone antibodies in bullous eruption of systemic lupus erythematosus recognize epidermolysis bullosa acquisita autoantigen. J Invest Dermatol. 1985;84:472-476. doi:10.1111/1523-1747.ep12272402.
- Yadav RS, Jaswal A, Shrestha S, et al. Dystrophic epidermolysis bullosa. J Nepal Med Assoc. 2018;56:879-882. doi:10.31729/jnma.3791
- Mariath LM, Santin JT, Schuler-Faccini L, et al. Inherited epidermolysis bullosa: update on the clinical and genetic aspects. An Bras Dermatol. 2020;95:551-569. doi:10.1016/j.abd.2020.05.001
- Understanding epidermolysis bullosa (EB). DEBRA website. Accessed August 17, 2025. https://www.debra.org/about-eb/understanding-epidermolysis-bullosa-eb
- Hon KL, Chu S, Leung AKC. Epidermolysis bullosa: pediatric perspectives. Curr Pediatr Rev. 2022;18:182-190. doi:10.2174/1573396317666210525161252
- Dang N, Klingberg S, Marr P, et al. Review of collagen VII sequence variants found in Australasian patients with dystrophic epidermolysis bullosa reveals nine COL7A1 variants. J Dermatol Sci. 2007;46:169-178. doi:10.1016/j.jdermsci.2007.02.006
- Payne AS. Topical gene therapy for epidermolysis bullosa. N Engl J Med. 2022;387:2281-2284. doi:10.1056/NEJMe2213203
- Khani P, Ghazi F, Zekri A, et al. Keratins and epidermolysis bullosa simplex. J Cell Physiol. 2018;234:289-297. doi:10.1002/jcp.26898
- Lai-Cheong JE, Tanaka A, Hawche G, et al. Kindler syndrome: a focal adhesion genodermatosis. Br J Dermatol. 2009;160:233-242. doi:10.1111/j.1365-2133.2008.08976.x
- Hou P-C, Wang H-T, Abhee S, et al. Investigational treatments for epidermolysis bullosa. Am J Clin Dermatol. 2021;22:801-817. doi:10.1007/s40257-021-00626-3
- Youseffian L, Vahidnezhad H, Uitto J. Kindler Syndrome. GeneReviews [Internet]. Updated January 6, 2022. Accessed August 21, 2025.
- Guide SV, Gonzalez ME, Bagci S, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387:2211-2219. doi:10.1056/NEJMoa2206663
- Vetencourt AT, Sayed-Ahmed I, Gomez J, et al. Ocular gene therapy in a patient with dystrophic epidermolysis bullosa. N Engl J Med. 2024;390:530-535. doi:10.1056/NEJMoa2301244
- Kern JS, Sprecher E, Fernandez MF, et al. Efficacy and safety of Oleogel-S10 (birch triterpenes for epidermolysis bullosa: results from the phase III randomized double-blind phase of the EASE study. Br J Dermatol. 2023;188:12-21. doi:10.1093/bjd/ljac001
- Tang JY, Marinkovich MP, Wiss K, et al. Prademagene zamikeracel for recessive dystrophic epidermolysis bullosa wounds (VIITAL): a two-centre, randomized, open-label, intrapatient-controlled phase 3 trial. Lancet. 2025;406:163-173. doi:10.1016/S0140-6736(25)00778-0
- Gretzmeier C, Pin D, Kern JS, et al. Systemic collagen VII replacement therapy for advanced recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2022;142:1094-1102. doi:10.1016/j.jid.2021.09.008
- Hignett E, Sami N. Pediatric epidermolysis bullosa acquisita. A review. Pediatr Dermatol. 2021;38:1047-1050. doi:10.1111/pde.14722
- Chen M, Kim GH, Prakash L, et al. Autoimmunity to anchoring fibril collagen. Autoimmunity. 2012;45:91-101. doi:10.1007/s12016-007-0027-6.
- Kridin K, Kneiber D, Kowalski EH, et al. Epidermolysis bullosa acquisita: a comprehensive review. Autoimmun Rev. 2019;18:786-795. doi:10.1016/j.autrev.2019.06.007
- Hofmann SC, Weidinger A. Epidermolysis bullosa acquisita. Hautarzt. 2019;70:265-270. doi:10.1007/s00105-019-4387-7
- Ishi N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what’s new? J Dermatol. 2010;37:220-230. doi:10.1111/j.1346-8138.2009.00799.x
- Iwata H, Vorobyev A, Koga H, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. 2018;13:153. doi:10.1186/s13023-018-0896-1
- Komorowski L, Muller R, Vorobyev A, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2013;68:e89-95. doi:10.1016/j.jaad.2011.12.032
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:364-390. doi:10.3390/jcm10020364
- Bezzio C, Della Corte C, Vernero M, et al. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at less frequent associations. Therap Adv Gastroenterol. 2022;15:17562848221115312. doi:10.1177/17562848221115312
- Chen M, O’Toole EA, Sanghavi J, et al. The epidermolysis acquisita antigen (type VII collagen) is present in human colon and patients with Crohn’s disease have antibodies to type VII collagen. J Invest Dermatol. 2002;118:1059-1064. doi:10.1046/j.1523-1747.2002.01772.x
- Labeille B, Gineston JL, Denoeux JP, et al. Epidermolysis bullosa acquisita and Crohn’s disease. A case report with immunological and electron microscopic studies. Arch Intern Med. 1988;148:1457-1459.
- Etienne A, Ruffieux P, Didierjean L, et al. Epidermolysis bullosa acquisita and metastatic cancer of the uterine cervix. Ann Dermatol Venereol. 1998;125:321-323.
- Busch J-O, Sticherling M. Epidermolysis bullosa acquisita and neuroendocrine pancreatic cancer-Coincidence or patho-genetic relationship? J Dtsch Dermatol Ges. 2007;5:916-918. doi:10.111/j.1610-0387.2007.06338.x
- Bevans SL, Sami N. The use of rituximab in treatment of epidermolysis bullosa acquisita: three new cases and a review of the literature. Dermatol Ther. 2018;31:e12726. doi:10.1111/j.1610-0387.2007.06338.x
- Yang A, Kim M, Craig P, et al. A case report of the use of rituximab and the epidermolysis bullosa disease activity scoring index (EBDASI) in a patient with epidermolysis bullosa acquisita with extensive esophageal involvement. Arch Dermatovenerol Croat. 2018;26:325-328.
- Burrows NP, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338. doi:10.1111/j.1365-2133.1993.tb00180.x
- Aringer M, Leuchten N, Johnson SR. New criteria for lupus. Curr Rheum Rep. 2020;22:18. doi:10.1007/s11926-020-00896-6
- Camisa C. Vesiculobullous systemic lupus erythematosus. A report of four cases. J Am Acad Dermatol. 1988;18:93-100. doi:10.1016/s0190-9622(88)70014-6
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:167064. doi:10.1155/2015/167064
- Sprow G, Afarideh M, Dan J, et al. Bullous systemic lupus erythematosus in females. Int J Womens Dermatol. 2022;8:e034. doi:10.1097/JW9.0000000000000034
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524. doi:10.1007/s40257-014-0098-0
- Fine JD, Mellerio JE. Epidermolysis bullosa. In: Bolognia JL, Jorizzo JL, Schaffer JV (eds), Dermatology (ed 3), Elsevier Saunders; 2012: 501-513.
- Bardhan A, Bruckner-Tuderman L, Chapple ILC, et al. Epidermolysis bullosa. Nat Rev Dis Primers. 2020;6:78. doi:10.1038/s41572-020-0210-0
- Miyamoto D, Gordilho JO, Santi CG, et al. Epidermolysis bullosa acquisita. An Bras Dermatol. 2022;97:409-423. doi:10.1016/j.abd.2021.09.010.
- Gammon WR, Woodley DT, Dole KC, et al. Evidence that anti-basement membrane zone antibodies in bullous eruption of systemic lupus erythematosus recognize epidermolysis bullosa acquisita autoantigen. J Invest Dermatol. 1985;84:472-476. doi:10.1111/1523-1747.ep12272402.
- Yadav RS, Jaswal A, Shrestha S, et al. Dystrophic epidermolysis bullosa. J Nepal Med Assoc. 2018;56:879-882. doi:10.31729/jnma.3791
- Mariath LM, Santin JT, Schuler-Faccini L, et al. Inherited epidermolysis bullosa: update on the clinical and genetic aspects. An Bras Dermatol. 2020;95:551-569. doi:10.1016/j.abd.2020.05.001
- Understanding epidermolysis bullosa (EB). DEBRA website. Accessed August 17, 2025. https://www.debra.org/about-eb/understanding-epidermolysis-bullosa-eb
- Hon KL, Chu S, Leung AKC. Epidermolysis bullosa: pediatric perspectives. Curr Pediatr Rev. 2022;18:182-190. doi:10.2174/1573396317666210525161252
- Dang N, Klingberg S, Marr P, et al. Review of collagen VII sequence variants found in Australasian patients with dystrophic epidermolysis bullosa reveals nine COL7A1 variants. J Dermatol Sci. 2007;46:169-178. doi:10.1016/j.jdermsci.2007.02.006
- Payne AS. Topical gene therapy for epidermolysis bullosa. N Engl J Med. 2022;387:2281-2284. doi:10.1056/NEJMe2213203
- Khani P, Ghazi F, Zekri A, et al. Keratins and epidermolysis bullosa simplex. J Cell Physiol. 2018;234:289-297. doi:10.1002/jcp.26898
- Lai-Cheong JE, Tanaka A, Hawche G, et al. Kindler syndrome: a focal adhesion genodermatosis. Br J Dermatol. 2009;160:233-242. doi:10.1111/j.1365-2133.2008.08976.x
- Hou P-C, Wang H-T, Abhee S, et al. Investigational treatments for epidermolysis bullosa. Am J Clin Dermatol. 2021;22:801-817. doi:10.1007/s40257-021-00626-3
- Youseffian L, Vahidnezhad H, Uitto J. Kindler Syndrome. GeneReviews [Internet]. Updated January 6, 2022. Accessed August 21, 2025.
- Guide SV, Gonzalez ME, Bagci S, et al. Trial of beremagene geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med. 2022;387:2211-2219. doi:10.1056/NEJMoa2206663
- Vetencourt AT, Sayed-Ahmed I, Gomez J, et al. Ocular gene therapy in a patient with dystrophic epidermolysis bullosa. N Engl J Med. 2024;390:530-535. doi:10.1056/NEJMoa2301244
- Kern JS, Sprecher E, Fernandez MF, et al. Efficacy and safety of Oleogel-S10 (birch triterpenes for epidermolysis bullosa: results from the phase III randomized double-blind phase of the EASE study. Br J Dermatol. 2023;188:12-21. doi:10.1093/bjd/ljac001
- Tang JY, Marinkovich MP, Wiss K, et al. Prademagene zamikeracel for recessive dystrophic epidermolysis bullosa wounds (VIITAL): a two-centre, randomized, open-label, intrapatient-controlled phase 3 trial. Lancet. 2025;406:163-173. doi:10.1016/S0140-6736(25)00778-0
- Gretzmeier C, Pin D, Kern JS, et al. Systemic collagen VII replacement therapy for advanced recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2022;142:1094-1102. doi:10.1016/j.jid.2021.09.008
- Hignett E, Sami N. Pediatric epidermolysis bullosa acquisita. A review. Pediatr Dermatol. 2021;38:1047-1050. doi:10.1111/pde.14722
- Chen M, Kim GH, Prakash L, et al. Autoimmunity to anchoring fibril collagen. Autoimmunity. 2012;45:91-101. doi:10.1007/s12016-007-0027-6.
- Kridin K, Kneiber D, Kowalski EH, et al. Epidermolysis bullosa acquisita: a comprehensive review. Autoimmun Rev. 2019;18:786-795. doi:10.1016/j.autrev.2019.06.007
- Hofmann SC, Weidinger A. Epidermolysis bullosa acquisita. Hautarzt. 2019;70:265-270. doi:10.1007/s00105-019-4387-7
- Ishi N, Hamada T, Dainichi T, et al. Epidermolysis bullosa acquisita: what’s new? J Dermatol. 2010;37:220-230. doi:10.1111/j.1346-8138.2009.00799.x
- Iwata H, Vorobyev A, Koga H, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. 2018;13:153. doi:10.1186/s13023-018-0896-1
- Komorowski L, Muller R, Vorobyev A, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2013;68:e89-95. doi:10.1016/j.jaad.2011.12.032
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:364-390. doi:10.3390/jcm10020364
- Bezzio C, Della Corte C, Vernero M, et al. Inflammatory bowel disease and immune-mediated inflammatory diseases: looking at less frequent associations. Therap Adv Gastroenterol. 2022;15:17562848221115312. doi:10.1177/17562848221115312
- Chen M, O’Toole EA, Sanghavi J, et al. The epidermolysis acquisita antigen (type VII collagen) is present in human colon and patients with Crohn’s disease have antibodies to type VII collagen. J Invest Dermatol. 2002;118:1059-1064. doi:10.1046/j.1523-1747.2002.01772.x
- Labeille B, Gineston JL, Denoeux JP, et al. Epidermolysis bullosa acquisita and Crohn’s disease. A case report with immunological and electron microscopic studies. Arch Intern Med. 1988;148:1457-1459.
- Etienne A, Ruffieux P, Didierjean L, et al. Epidermolysis bullosa acquisita and metastatic cancer of the uterine cervix. Ann Dermatol Venereol. 1998;125:321-323.
- Busch J-O, Sticherling M. Epidermolysis bullosa acquisita and neuroendocrine pancreatic cancer-Coincidence or patho-genetic relationship? J Dtsch Dermatol Ges. 2007;5:916-918. doi:10.111/j.1610-0387.2007.06338.x
- Bevans SL, Sami N. The use of rituximab in treatment of epidermolysis bullosa acquisita: three new cases and a review of the literature. Dermatol Ther. 2018;31:e12726. doi:10.1111/j.1610-0387.2007.06338.x
- Yang A, Kim M, Craig P, et al. A case report of the use of rituximab and the epidermolysis bullosa disease activity scoring index (EBDASI) in a patient with epidermolysis bullosa acquisita with extensive esophageal involvement. Arch Dermatovenerol Croat. 2018;26:325-328.
- Burrows NP, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338. doi:10.1111/j.1365-2133.1993.tb00180.x
- Aringer M, Leuchten N, Johnson SR. New criteria for lupus. Curr Rheum Rep. 2020;22:18. doi:10.1007/s11926-020-00896-6
- Camisa C. Vesiculobullous systemic lupus erythematosus. A report of four cases. J Am Acad Dermatol. 1988;18:93-100. doi:10.1016/s0190-9622(88)70014-6
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:167064. doi:10.1155/2015/167064
- Sprow G, Afarideh M, Dan J, et al. Bullous systemic lupus erythematosus in females. Int J Womens Dermatol. 2022;8:e034. doi:10.1097/JW9.0000000000000034
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524. doi:10.1007/s40257-014-0098-0
- Fine JD, Mellerio JE. Epidermolysis bullosa. In: Bolognia JL, Jorizzo JL, Schaffer JV (eds), Dermatology (ed 3), Elsevier Saunders; 2012: 501-513.
Type VII Collagen Disorders Simplified
Type VII Collagen Disorders Simplified
PRACTICE POINTS
- The full complement of type VII collagen is required for the normal assembly of anchoring fibrils, whose function is to adhere the basement membrane to the underlying connective tissue of skin and mucous membranes.
- In the heritable epidermolysis bullosa (EB) family of diseases, only dominant and recessive dystrophic epidermolysis bullosa are caused by partial or total loss of type VII collagen function.
- New treatments that have been approved for EB include topical gene therapy with COL7A1, topical birch triterpene gel, and skin cells from patients that are genetically corrected with a functional COL7A1 gene.
- Epidermolysis bullosa acquisita and bullous systemic lupus erythematosus are rare distinct autoimmune subepithelial bullous diseases caused by IgG antibodies that target type VII collagen in the anchoring fibrils.
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
THE DIAGNOSIS: Impetigo Herpetiformis
Histopathology revealed epidermal acanthosis and spongiosis with overlying parakeratosis associated with subcorneal and intracorneal neutrophils, papillary dermal edema, and dermal mixed inflammation with neutrophils and eosinophils. Both direct immunofluorescence and periodic acid–Schiff studies were negative. Blood and pustule cultures were sterile and the skin flora were normal. Based on these findings, a diagnosis of impetigo herpetiformis (IH) was made. The condition improved with systemic and topical steroids, supportive care, and an intravenous infusion of infliximab 5 mg/kg. At 3 weeks’ follow-up, the patient demonstrated near-complete resolution and later delivered successfully at 40 weeks’ gestation without complications.
Impetigo herpetiformis, also known as pustular psoriasis of pregnancy, is an exceedingly rare gestational dermatosis that typically manifests in the third trimester and can be life-threatening for both the mother and fetus. The term was first used in 1872 to describe 5 pregnant women with extensive acute pustular eruptions, all in unstable condition; 4 (80%)of the cases resulted in maternal death, and all resulted in fetal death.1 Impetigo herpetiformis is characterized by pruritic and painful erythematous patches studded at the periphery with subcorneal pustules. Eruptions usually occur in the flexural areas and spread centrifugally, with extension of the lesions peripherally as the center erodes and crusts. Sparing of the face, palms, and soles is expected, and mucosal involvement is rare. Generalized involvement and exfoliation may occur in extreme cases.2 While IH typically manifests during the third trimester, it may occur any time throughout pregnancy or immediately postpartum.3 A few cases have been reported in the puerperium.2 Common symptoms include fever, chills, malaise, anorexia, nausea, vomiting, diarrhea, and arthralgias. Less common complications include hypoalbuminemia and severe hypocalcemia leading to tetany, seizures, and delirium.2,3 While maternal mortality is uncommon, fetal mortality often is a more pressing risk and is attributed to placental insufficiency.3,4 For this reason, early delivery commonly is considered in severe cases.
Whether IH is a separate entity or a variant of pustular psoriasis remains heavily debated. Although the histopathology of IH is identical to pustular psoriasis, the lack of a personal and family history of psoriasis, symptom resolution with delivery, and possible recurrence during successive pregnancies help differentiate IH from generalized pustular psoriasis.2,5 Earlier onset, diffuse involvement, faster progression, and recurrence in subsequent pregnancies all have been linked to a worse prognosis.4
The differential diagnosis for IH includes acute generalized exanthematous pustulosis, pemphigoid gestationis, dermatitis herpetiformis, and subcorneal pustular dermatosis. Acute generalized exanthematous pustulosis is an uncommon severe cutaneous drug reaction characterized by the sudden onset of numerous sterile pustules on erythematous skin within 48 hours of exposure. The most common offending medications include pristinamycin and beta-lactam antibiotics. A high fever, neutrophilic leukocytosis, and hypocalcemia often accompany acute generalized exanthematous pustulosis.6 Prompt diagnosis and withdrawal of the offending drug as well as supportive care and symptomatic treatment are crucial for disease management, as systemic symptoms and even organ involvement may occur.6
Pemphigoid gestationis, also known as gestational pemphigoid or herpes gestationis, is a rare autoimmune blistering disorder that primarily affects pregnant women. It typically manifests in the second or third trimester or shortly after delivery. Clinically, it manifests as an intensely pruritic polymorphic eruption of urticarial papules and plaques accompanied by vesicles and bullae and often is distributed on the abdomen and extends to other body regions. Although the exact etiology is unknown, pemphigoid gestationis is caused by autoantibodies targeting the BP180 and BP230 hemidesmosomal proteins.7 Treatment usually involves systemic corticosteroids and may require additional immunosuppressive therapy. In most cases, patients see resolution within 6 months of delivery.7
Dermatitis herpetiformis is a chronic autoimmune blistering skin disorder characterized by intensely pruritic, grouped vesicles and papules, often distributed symmetrically on extensor surfaces such as the elbows, knees, buttocks, and back. It is closely associated with celiac disease and is triggered by gluten ingestion in genetically predisposed individuals with human leukocyte antigen DQ2 and DQ8 haplotypes. Dermatitis herpetiformis is caused by deposition of IgA antibodies that target tissue transglutaminase 3 at the dermal papillae, leading to inflammation and blister formation. 8 Treatment typically involves a gluten-free diet and medications such as dapsone to alleviate symptoms and prevent recurrence.
Subcorneal pustular dermatosis, also known as Sneddon-Wilkinson disease, is a rare chronic relapsing pustular dermatosis characterized by sterile superficial pustules arranged in annular or circinate patterns on erythematous plaques. It predominantly affects middleaged women and often is associated with underlying conditions such as IgA gammopathy or monoclonal gammopathy of undetermined significance. The pathogenesis remains unclear, but immune dysregulation is thought to play a role. Some authors still question whether subcorneal pustular dermatosis is a distinct entity from pustular psoriasis.4,5,12 Dapsone is the preferred first-line treatment, with adjunct therapies including topical or systemic corticosteroids, other immunosuppressive agents, tumor necrosis factor inhibitors, and UV light therapy.9
Definitive management of IH is achieved through early delivery; however, systemic corticosteroids often are used in varying doses to control the disease and to extend the pregnancy period closer to term or until delivery is considered viable. Additional therapies that can be considered include infliximab, cyclosporine, and topical corticosteroids, in conjunction with fluid and electrolyte maintenance.2,4,10 If symptoms persist despite supportive care and pharmacologic intervention, induction of labor or termination of pregnancy may be indicated. In nonbreastfeeding postpartum mothers with persistent disease, therapies commonly used in generalized pustular psoriasis may be given.11
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
- Hebra F. Ueber einzelne wahrend Schwangerschaft, des wacherbette unde bei uterinal. Krankheiten der Frauen zu beobachtende Hautkrankheiten. Wien Med Wochenschr. 1872;48:1197-1202.
- Fouda UM, Fouda RM, Ammar HM, et al. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J. 2009;2:9338. doi:10.1186/1757-1626-2-9338
- Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1-22. doi:10.1067/mjd.2001.114595
- Liu J, Ali K, Lou H, et al. First-trimester impetigo herpetiformis leads to stillbirth: a case report. Dermatol Ther (Heidelb). 2022;12:1271-1279. doi:10.1007/s13555-022-00735-9
- Lotem M, Katzenelson V, Rotem A, et al. Impetigo herpetiformis: a variant of pustular psoriasis or a separate entity? J Am Acad Dermatol. 1989;20:338-41. doi:10.1016/s0190-9622(89)70042-6
- Stadler PC, Oschmann A, Kerl-French K, et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328-333. doi:10.1159/000529218
- Abdelhafez MMA, Ahmed KAM, Daud MNBM, et al. Pemphigoid gestationis and adverse pregnancy outcomes: a literature review. J Gynecol Obstet Hum Reprod. 2022;51:102370. doi:10.1016 /j.jogoh.2022.102370
- Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol. 2021;22:329-338. doi:10.1007/s40257-020-00584-2
- Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653-671. doi:10.1007 /s40257-016-0202-8
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/j.jaad.2011.01.032
- Bukhari IA. Impetigo herpetiformis in a primigravida: successful treatment with etanercept. J Drugs Dermatol. 2004;3:449-451.
- Chang SE, Kim HH, Choi JH, et al. Impetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entity. Int J Dermatol. 2003;42(9):754-755.
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
Generalized Erythematous Plaques and Pustules in a Pregnant Patient
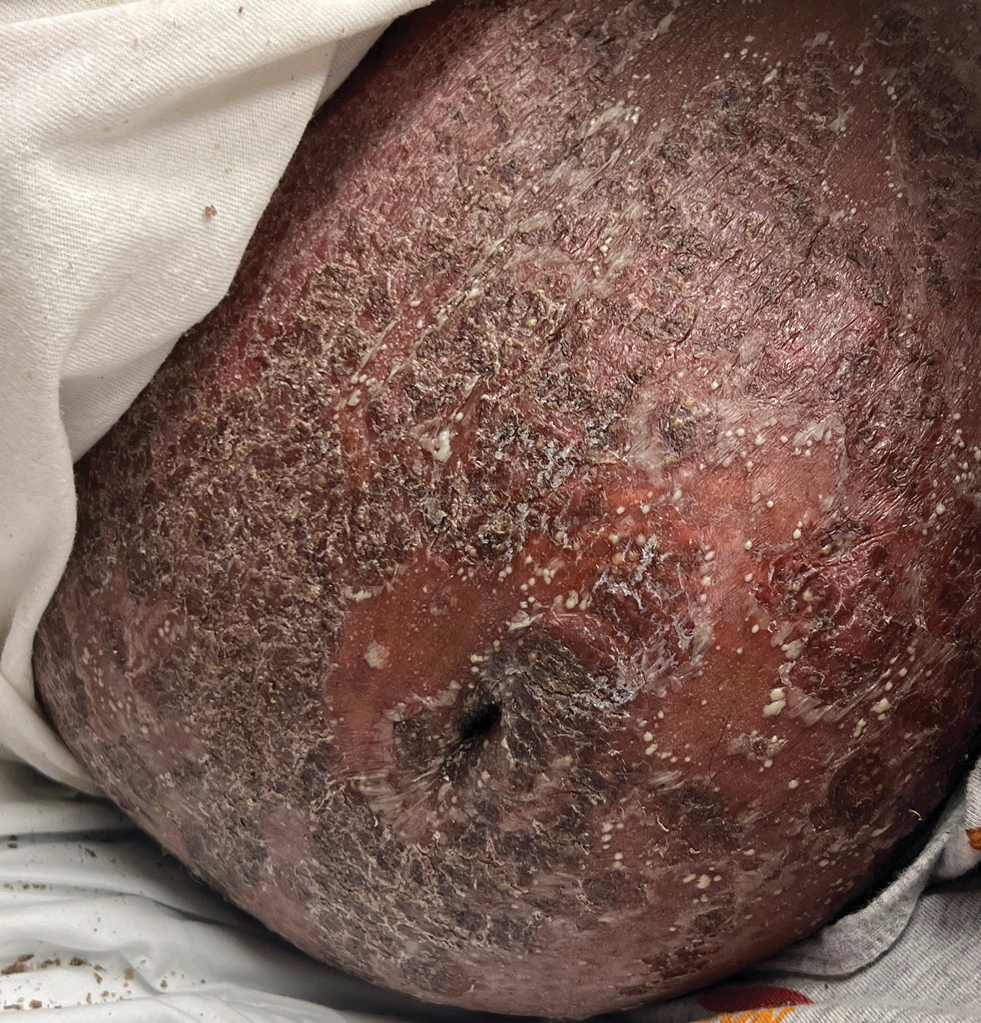
A 17-year-old girl was admitted to the hospital at 19 weeks' gestation for a widespread eruption of erythematous plaques with pustules covering more than 60% of the body and signs of sepsis. The rash initially appeared as a few small spots on the upper chest and under the breasts 5 weeks prior to hospital admission with subsequent spread to the abdomen and groin. At admission, the patient had a mild fever and tachycardia. She reported a history of eczema, herpes simplex virus, and intertrigo. Physical examination performed by dermatology revealed generalized erythematous plaques with pustule-studded margins and overlying scale involving the neck, torso, arms, and legs favoring the flexural areas. There was no involvement of the face, eyes, oral mucosa, palms, soles, or nails. Laboratory testing revealed hypoalbuminemia (2.4 g/dL [reference range, 3.5-5.5 g/dL]) and elevated inflammatory markers, including leukocytosis (15.83×103μL [reference range, 4.50- 11.00×103/μL]), absolute neutrophil count (12.87×103/μL [reference range, 1.50-8.00×103/μL]), and erythrocyte sedimentation rate (124 mm/h [reference range, 0-20 mm/h]). A culture from an abdominal pustule grew 1 colony of taphylococcus epidermidis, a suspected contaminant. A biopsy from a lesion on the right chest was performed.
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).
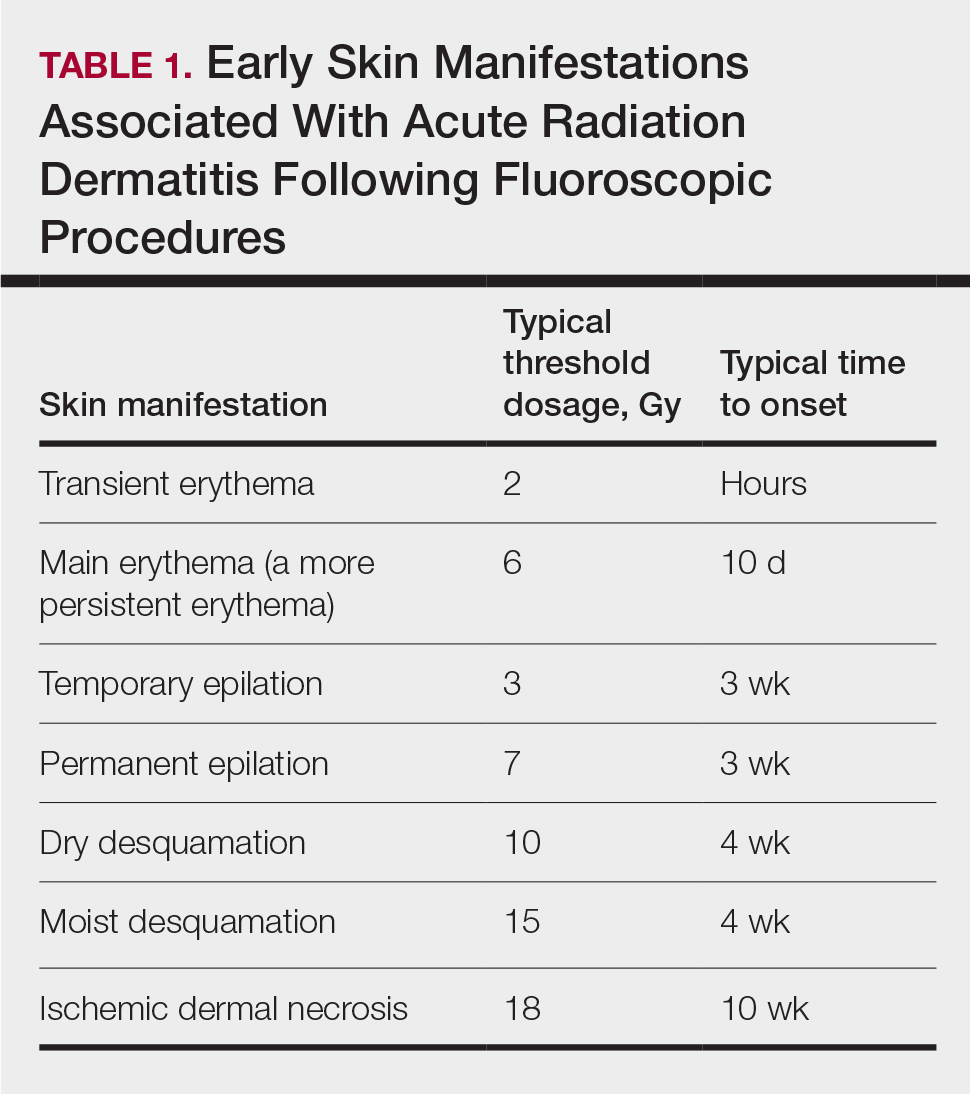
Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27

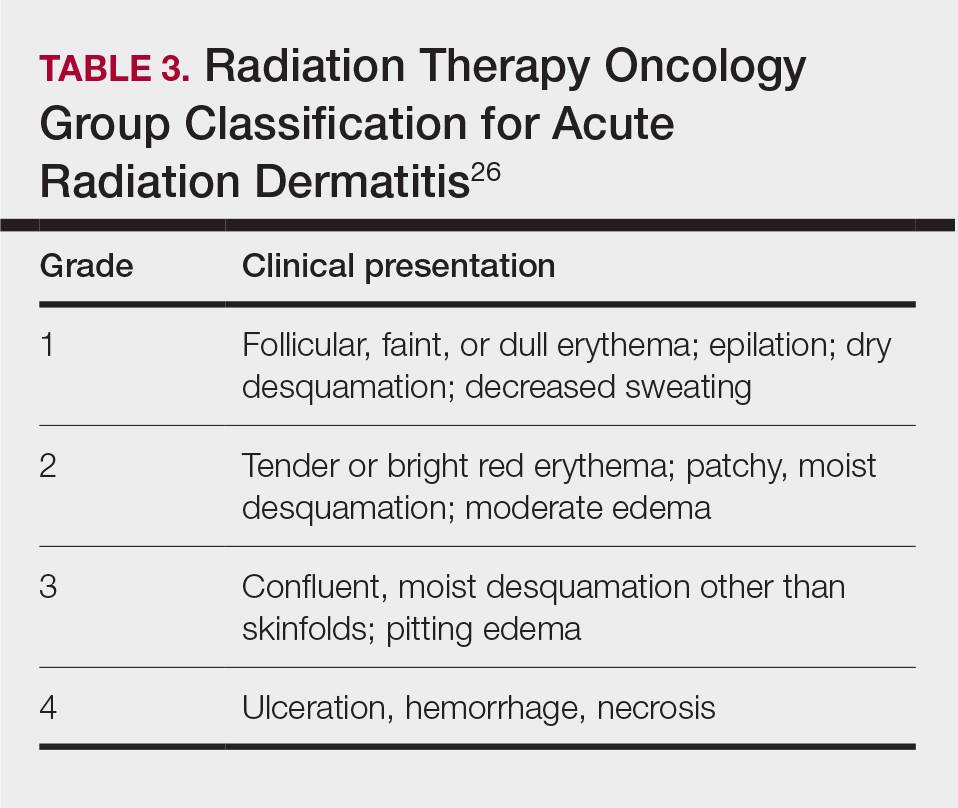
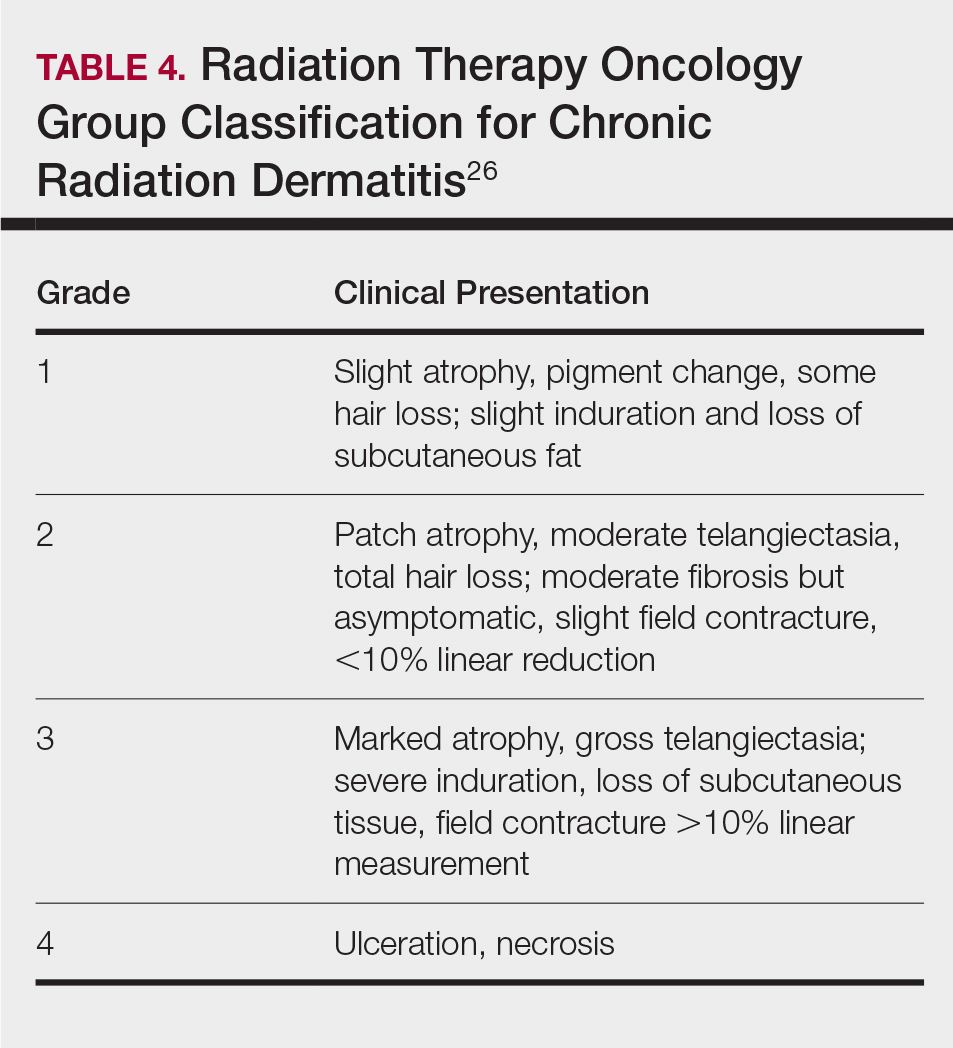
Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45
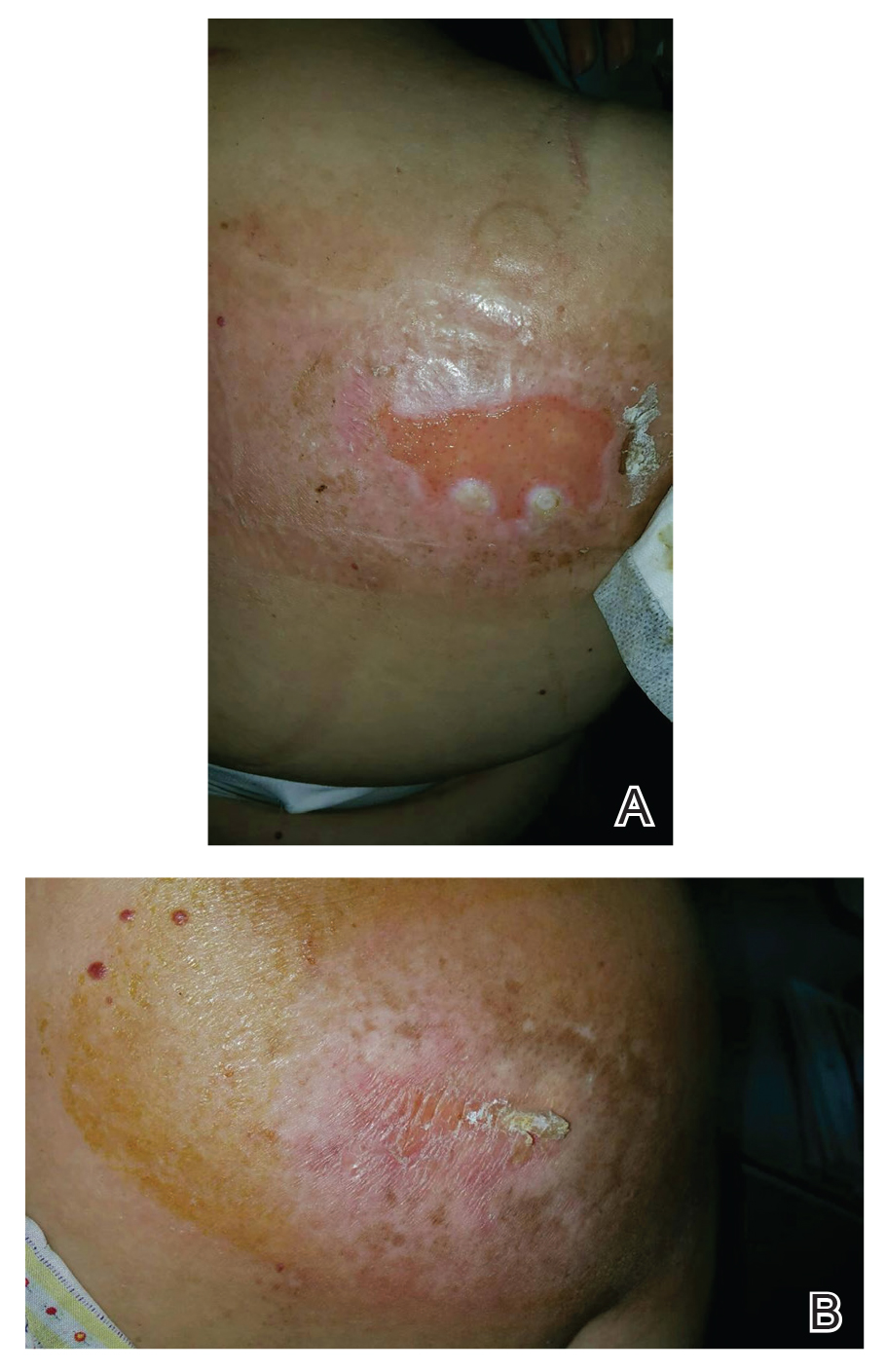
Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
PRACTICE POINTS
- Fluoroscopy-induced chronic radiation dermatitis poses diagnostic challenges, as patients often are unable to associate a history of fluoroscopic procedures with the development of skin lesions.
- Scapular and subscapular lesions as well as those on the anterolateral chest and mid back should prompt clinicians to inquire about the patient’s history of fluoroscopic procedures.
- Because lesions can remain refractory to treatment, longterm monitoring is necessary if they are not excised.
Remembering Why We Are In Medicine
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu) or Danielle Kiefer (dkiefer@gastro.org), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Weight Loss Before Military Training May Cut Injury Risk
TOPLINE:
Army recruits who lost excess weight to enter military training experienced fewer musculoskeletal injuries (MSKIs), particularly in the lower extremities, during basic combat training than those who did not lose weight to join the service.
METHODOLOGY:
- The nation’s obesity epidemic means that fewer individuals meet the US Army’s weight and body-fat standards for entering basic combat training. Only 29% of 17- to 24-year-olds in the country would have qualified to join the military in 2018, with overweight and obesity among the leading disqualifying factors.
- Researchers analyzed data from 3168 Army trainees (mean age, 20.96 years; 62.34% men; mean maximum-ever BMI, 26.71) to examine the association between weight loss before enlistment and rates of MSKI during basic combat training.
- Trainees completed a baseline questionnaire that asked whether the person lost weight to enter the Army and included follow-up questions about the amount of weight lost, duration of weight loss, methods used, and prior physical activity.
- MSKIs were classified as any injury to the musculoskeletal system and further categorized by body region (lower extremities, upper extremities, spine/back, and other areas, including the torso and head/neck).
- Researchers identified MSKIs from medical records collected throughout basic combat training and for up to 6 weeks afterward to capture injuries that occurred during training but were documented only after its completion.
TAKEAWAY:
- Overall, 829 trainees (26.16%) reported losing weight to enter the Army, and they tended to have higher mean maximum-ever BMI, body-fat percentage, and lean mass compared with those who did not lose weight to join the service. The mean weight loss was 9.06 kg at a rate of 1.27 kg/wk among the 723 trainees with complete data.
- The most commonly reported weight-loss methods were exercising more (83.7%), changing diet (61.0%), skipping meals (39.3%), and sweating using a sauna or rubber suit (25.6%).
- Trainees who lost weight to join the service had a lower risk of any MSKI (hazard ratio [HR], 0.86) and lower extremity MSKIs (HR, 0.84) during training than those who did not lose weight to enter the Army. No difference was found between the two groups in the risk of upper extremity, spine/back, or other MSKIs.
- Among trainees who lost weight to join the Army, the amount of time it took to lose weight was not associated with the risk for any MSKI or region-specific MSKIs.
IN PRACTICE:
“The findings highlight that losing excess weight before entering military training may reduce MSKI risk for incoming recruits, enforcing the benefits of healthy weight loss programs,” the authors wrote.
SOURCE:
The study, led by Vy T. Nguyen, MS, DSc, Military Performance Division, US Army Research Institute of Environmental Medicine, Natick, Massachusetts, was published online in Obesity .
LIMITATIONS:
The study did not assess whether the association between weight loss and the rate of MSKIs persisted over long-term military service. How the two most frequently reported weight loss methods — increased exercise and dietary changes — may have influenced the observed association remains unclear. Medical records may not have captured all MSKIs if trainees did not seek medical care due to concerns about graduating on time or being placed on limited duty.
DISCLOSURES:
The study was supported by the US Army Medical Research and Development Command’s Military Operational Medicine Program. Two authors received support from the funder.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Army recruits who lost excess weight to enter military training experienced fewer musculoskeletal injuries (MSKIs), particularly in the lower extremities, during basic combat training than those who did not lose weight to join the service.
METHODOLOGY:
- The nation’s obesity epidemic means that fewer individuals meet the US Army’s weight and body-fat standards for entering basic combat training. Only 29% of 17- to 24-year-olds in the country would have qualified to join the military in 2018, with overweight and obesity among the leading disqualifying factors.
- Researchers analyzed data from 3168 Army trainees (mean age, 20.96 years; 62.34% men; mean maximum-ever BMI, 26.71) to examine the association between weight loss before enlistment and rates of MSKI during basic combat training.
- Trainees completed a baseline questionnaire that asked whether the person lost weight to enter the Army and included follow-up questions about the amount of weight lost, duration of weight loss, methods used, and prior physical activity.
- MSKIs were classified as any injury to the musculoskeletal system and further categorized by body region (lower extremities, upper extremities, spine/back, and other areas, including the torso and head/neck).
- Researchers identified MSKIs from medical records collected throughout basic combat training and for up to 6 weeks afterward to capture injuries that occurred during training but were documented only after its completion.
TAKEAWAY:
- Overall, 829 trainees (26.16%) reported losing weight to enter the Army, and they tended to have higher mean maximum-ever BMI, body-fat percentage, and lean mass compared with those who did not lose weight to join the service. The mean weight loss was 9.06 kg at a rate of 1.27 kg/wk among the 723 trainees with complete data.
- The most commonly reported weight-loss methods were exercising more (83.7%), changing diet (61.0%), skipping meals (39.3%), and sweating using a sauna or rubber suit (25.6%).
- Trainees who lost weight to join the service had a lower risk of any MSKI (hazard ratio [HR], 0.86) and lower extremity MSKIs (HR, 0.84) during training than those who did not lose weight to enter the Army. No difference was found between the two groups in the risk of upper extremity, spine/back, or other MSKIs.
- Among trainees who lost weight to join the Army, the amount of time it took to lose weight was not associated with the risk for any MSKI or region-specific MSKIs.
IN PRACTICE:
“The findings highlight that losing excess weight before entering military training may reduce MSKI risk for incoming recruits, enforcing the benefits of healthy weight loss programs,” the authors wrote.
SOURCE:
The study, led by Vy T. Nguyen, MS, DSc, Military Performance Division, US Army Research Institute of Environmental Medicine, Natick, Massachusetts, was published online in Obesity .
LIMITATIONS:
The study did not assess whether the association between weight loss and the rate of MSKIs persisted over long-term military service. How the two most frequently reported weight loss methods — increased exercise and dietary changes — may have influenced the observed association remains unclear. Medical records may not have captured all MSKIs if trainees did not seek medical care due to concerns about graduating on time or being placed on limited duty.
DISCLOSURES:
The study was supported by the US Army Medical Research and Development Command’s Military Operational Medicine Program. Two authors received support from the funder.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Army recruits who lost excess weight to enter military training experienced fewer musculoskeletal injuries (MSKIs), particularly in the lower extremities, during basic combat training than those who did not lose weight to join the service.
METHODOLOGY:
- The nation’s obesity epidemic means that fewer individuals meet the US Army’s weight and body-fat standards for entering basic combat training. Only 29% of 17- to 24-year-olds in the country would have qualified to join the military in 2018, with overweight and obesity among the leading disqualifying factors.
- Researchers analyzed data from 3168 Army trainees (mean age, 20.96 years; 62.34% men; mean maximum-ever BMI, 26.71) to examine the association between weight loss before enlistment and rates of MSKI during basic combat training.
- Trainees completed a baseline questionnaire that asked whether the person lost weight to enter the Army and included follow-up questions about the amount of weight lost, duration of weight loss, methods used, and prior physical activity.
- MSKIs were classified as any injury to the musculoskeletal system and further categorized by body region (lower extremities, upper extremities, spine/back, and other areas, including the torso and head/neck).
- Researchers identified MSKIs from medical records collected throughout basic combat training and for up to 6 weeks afterward to capture injuries that occurred during training but were documented only after its completion.
TAKEAWAY:
- Overall, 829 trainees (26.16%) reported losing weight to enter the Army, and they tended to have higher mean maximum-ever BMI, body-fat percentage, and lean mass compared with those who did not lose weight to join the service. The mean weight loss was 9.06 kg at a rate of 1.27 kg/wk among the 723 trainees with complete data.
- The most commonly reported weight-loss methods were exercising more (83.7%), changing diet (61.0%), skipping meals (39.3%), and sweating using a sauna or rubber suit (25.6%).
- Trainees who lost weight to join the service had a lower risk of any MSKI (hazard ratio [HR], 0.86) and lower extremity MSKIs (HR, 0.84) during training than those who did not lose weight to enter the Army. No difference was found between the two groups in the risk of upper extremity, spine/back, or other MSKIs.
- Among trainees who lost weight to join the Army, the amount of time it took to lose weight was not associated with the risk for any MSKI or region-specific MSKIs.
IN PRACTICE:
“The findings highlight that losing excess weight before entering military training may reduce MSKI risk for incoming recruits, enforcing the benefits of healthy weight loss programs,” the authors wrote.
SOURCE:
The study, led by Vy T. Nguyen, MS, DSc, Military Performance Division, US Army Research Institute of Environmental Medicine, Natick, Massachusetts, was published online in Obesity .
LIMITATIONS:
The study did not assess whether the association between weight loss and the rate of MSKIs persisted over long-term military service. How the two most frequently reported weight loss methods — increased exercise and dietary changes — may have influenced the observed association remains unclear. Medical records may not have captured all MSKIs if trainees did not seek medical care due to concerns about graduating on time or being placed on limited duty.
DISCLOSURES:
The study was supported by the US Army Medical Research and Development Command’s Military Operational Medicine Program. Two authors received support from the funder.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).
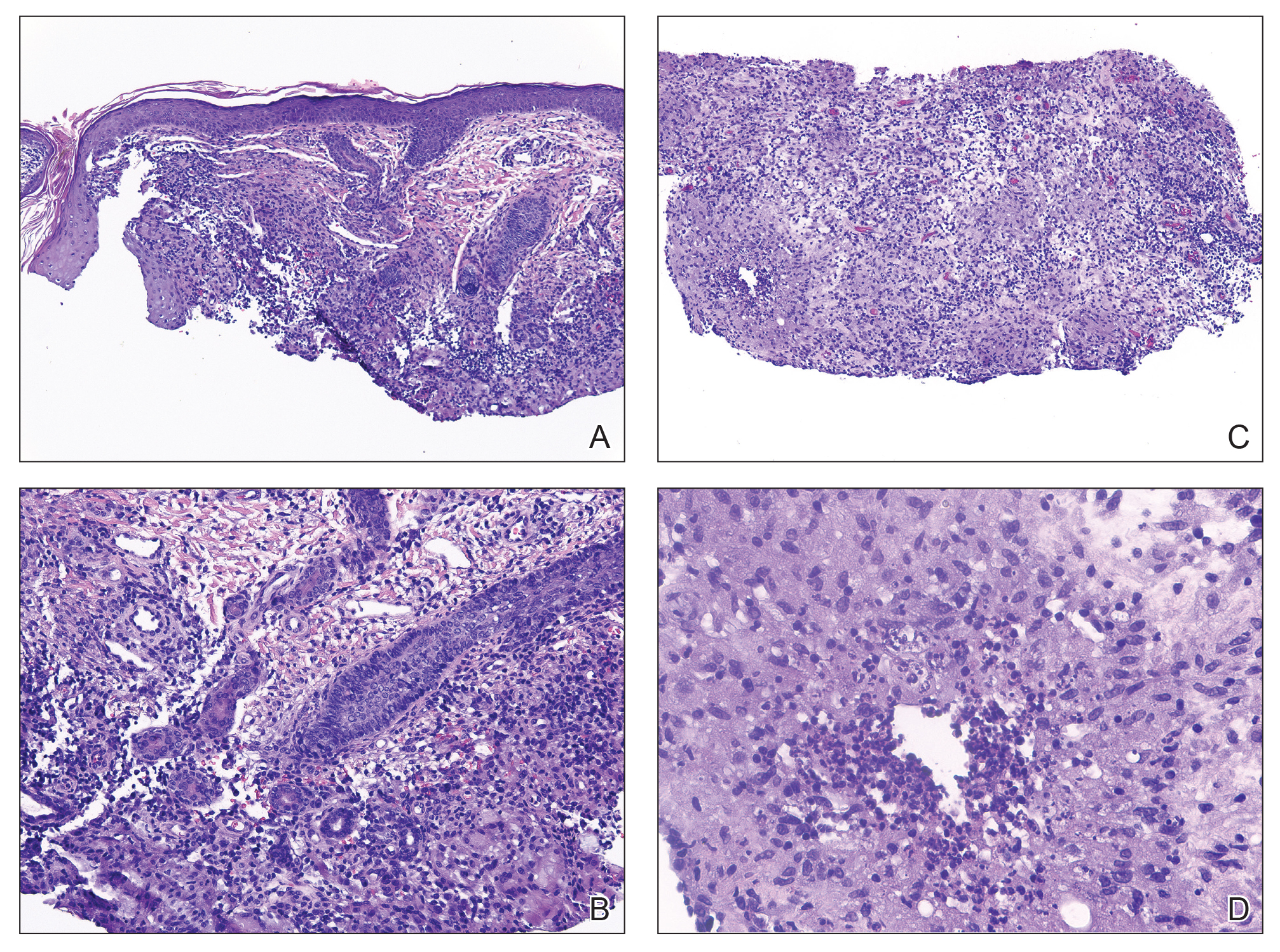
First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
A 4-year-old girl presented to the dermatology clinic with a painless, red to golden-yellowish nodule on the right lower eyelid of 4 months’ duration. The patient had no history of skin disease and was otherwise healthy. Physical examination revealed a single 1-cm, soft, erythematous and yellowish plaque on the right lower eyelid that was subtly fluctuant on palpation. She had no associated systemic symptoms or lymphadenopathy. A punch biopsy of the lesion was performed.
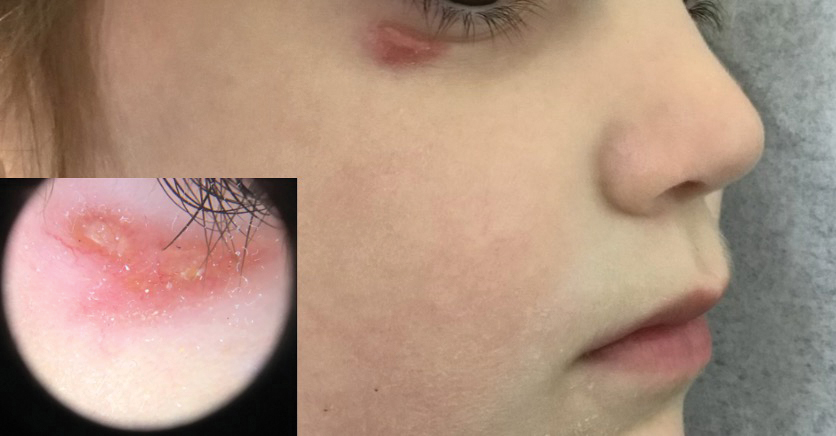
Top 5 Tips for Becoming an Effective Gastroenterology Consultant
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Military Imposters: What Drives Them and How They Damage Us All
Military Imposters: What Drives Them and How They Damage Us All
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
Military Imposters: What Drives Them and How They Damage Us All
Military Imposters: What Drives Them and How They Damage Us All
Novel Peptides Expressed in HIV Could Drive Treatment
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.