User login
For MD-IQ use only
Pilot study: Hybrid laser found effective for treating genitourinary syndrome of menopause
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
FROM ASLMS 2021
USMLE Step 1 Changes: Dermatology Program Director Perspectives and Implications
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).
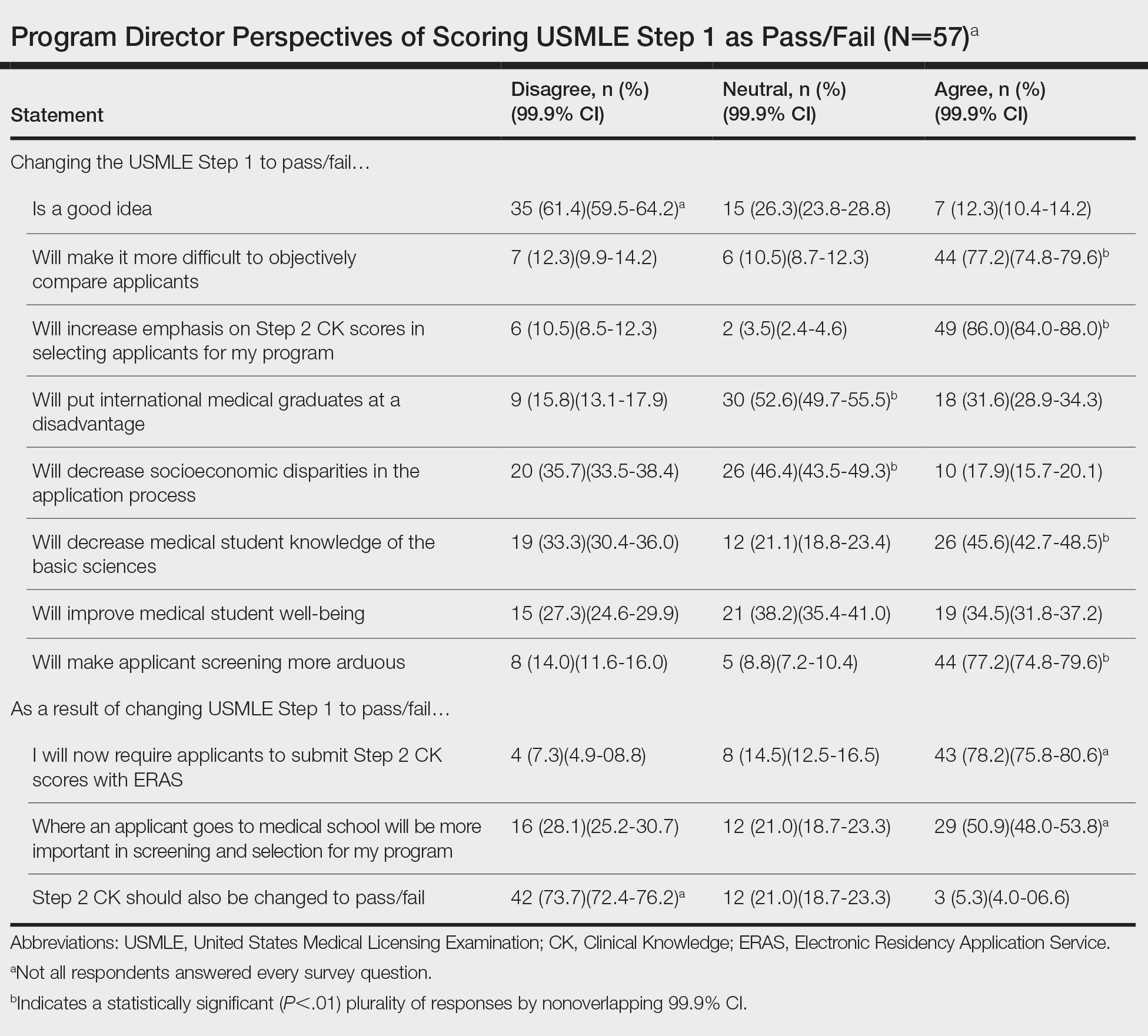
Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).
Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
To the Editor:
With a trend toward increasing pass/fail medical school curricula, residency program directors (PDs) have relied on the US Medical Licensing Examination (USMLE) Step 1 as an objective measurement of applicant achievement, which is particularly true in competitive subspecialties such as dermatology, plastic surgery, orthopedic surgery, ophthalmology, and neurosurgery, in which reported Step 1 scores are consistently the highest among matched applicants.1 Program directors in dermatology have indicated that Step 1 scores are a priority when considering an applicant.2 However, among PDs, the general perception of plans to change Step 1 scores to pass/fail has largely been negative.3 Although the impact of this change on the dermatology residency selection process remains unknown, we undertook a study to determine dermatology PDs’ perspectives on the scoring change and discuss its potential implications among all competitive specialties.
A 19-question survey was designed that assessed PD demographics and opinions of the changes and potential implications of the Step 1 scoring change (eTable). A list of current US dermatology PDs at osteopathic and allopathic programs was obtained through the 2019-2020 Accreditation Council for Graduate Medical Education list of accredited programs. Surveys were piloted at our institution to assess for internal validity and misleading questions, and then were distributed electronically through REDCap software (https://www.project-redcap.org/). All responses were kept anonymous. Institutional review board approval was obtained. Variables were assessed with means, proportions, and CIs. Results were deemed statistically significant with nonoverlapping 99% CIs (P<.01).
Of 139 surveys, 57 (41.0%) were completed. Most PDs (54.4% [31/57]) were women. The average years of service as a PD was 8.5 years. Most PDs (61.4% [35/57]) disagreed with the scoring change; 77.2% (44/57) of PDs noted that it would make it difficult to objectively assess candidates. Program directors indicated that this change would increase the emphasis they place on USMLE Step 2 Clinical Knowledge (CK) scores (86.0% [49/57]); 78.2% (43/55) reported that they would start requiring Step 2 CK results with submitted applications.
Meanwhile, 73.7% (42/57) of PDs disagreed that Step 2 CK should be changed to pass/fail. Most PDs (50.9% [29/57]) thought that binary Step 1 scoring would increase the importance of medical school reputation in application decisions. The percentage of PDs who were neutral (eTable) on whether pass/fail scoring would place international graduates at a disadvantage was 52.6% (30/57), decrease socioeconomic disparities in the application process was 46.4% (26/56), and improve student well-being was 38.2% (21/55).
Results of our survey indicate generally negative perceptions by dermatology PDs to pass/fail scoring of the USMLE Step 1. A primary goal of introducing binary scoring in both medical school grading and the USMLE was to improve student well-being, as traditional grading systems have been associated with a higher rate of medical student burnout.4-6 However, PDs were equivocal about such an impact on student well-being. Furthermore, PDs indicated that the importance of objective measures would merely shift to the USMLE Step 2 CK, which will still be graded with a 3-digit numeric score. Therefore, Step 2 likely will become the source of anxiety for medical students that was once synonymous with Step 1.
Another goal of the scoring change was to encourage a more holistic approach to applicant review, rather than focusing on numerical metrics. However, with most curricula adopting pass/fail models, there is already a lack of objective measures. Although removal of USMLE Step 1 scores could increase the focus on subjective measures, such as letters of recommendation and rank in medical school class (as indicated by our survey), these are susceptible to bias and may not be the best indicators of applicant suitability. This finding also is concerning for maintaining an equitable application process: PDs indicated that the USMLE Step 1 scoring change would not decrease socioeconomic disparities within the selection process.
In dermatology and other competitive specialties, in which USMLE Step 1 scores have become an important consideration, PDs and residency programs will need to identify additional metrics to compare applicants. Examples include research productivity, grades on relevant rotations, and shelf examination scores. Although more reliable subjective measures, such as interviews and performance on away rotations, are already important, they may become of greater significance.
The findings of our survey suggest that PDs are skeptical about changes to Step 1 and more diligence is necessary to maintain a fair and impartial selection process. Increased emphasis on other objective measurements, such as shelf examination scores, graded curricular components, and research productivity, could help maintain an unbiased approach. With changes to USMLE Step 1 expected to be implemented in the 2022 application cycle, programs may need to explore additional options to maintain reliable and transparent applicant review practices.
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
- National Resident Matching Program. Charting Outcomes in the Match: U.S Allopathic Seniors, 2018. 2nd ed. National Resident Matching Program; July 2018. Accessed May 12, 2021. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf
- Grading systems use by US medical schools. Association of American Medical Colleges. Accessed May 12, 2021. https://www.aamc.org/data-reports/curriculum-reports/interactive-data/grading-systems-use-us-medical-schools
- Makhoul AT, Pontell ME, Ganesh Kumar N, et al. Objective measures needed—program directors’ perspectives on a pass/fail USMLE Step 1. N Engl J Med; 2020;382:2389-2392. doi:10.1056/NEJMp2006148
- Change to pass/fail score reporting for Step 1. United States Medical Licensing Examination. Accessed May 12, 2021. https://www.usmle.org/incus/
- Reed DA, Shanafelt TD, Satele DW, et al. Relationship of pass/fail grading and curriculum structure with well-being among preclinical medical students: a multi-institutional study. Acad Med. 2011;86:1367-1373. doi:10.1097/ACM.0b013e3182305d81
- Summary report and preliminary recommendations from the Invitational Conference on USMLE Scoring (InCUS). United States Medical Licensing Examination. March 11-12, 2019. Accessed May 12, 2021. https://www.usmle.org/pdfs/incus/incus_summary_report.pdf
Practice Points
- The changes to US Medical Licensing Examination (USMLE) Step 1 were met with mixed reactions from dermatology program directors.
- These changes likely will increase the emphasis on USMLE Step 2 and other objective measures.
Cross-sectional study finds chronic skin conditions have highest opioid prescribing rates
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
at dermatology visits.
“Overall, opioid prescribing rates among dermatologists were low. However, dermatologists should remain aware of risk factors for long-term opioid use and consider using nonnarcotic or nonpharmacologic interventions when possible,” Sarah P. Pourali, a medical student at Vanderbilt University, Nashville, said at the annual meeting of the Society for Investigative Dermatology, where she presented the results.
Ms. Pourali said that although Mohs surgery and dermatologic procedures are the focus of “much of the literature” concerning opioid use in dermatology, there are limited data on medication prescribing patterns for other skin conditions treated by dermatologists.
She and her colleagues performed a cross-sectional study using data from the National Ambulatory Medical Care Survey (NAMCS) from 2009 to 2016 on 288,462,610 weighted dermatology visits. The researchers used International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to identify dermatologic diseases. They also identified and grouped oral pain medication into the following categories: opiate analgesics, nonsteroidal anti-inflammatory drugs, acetaminophen, and gabapentin. A linear regression analysis was used to evaluate pain medicine prescribing each year, and the researchers used a logistic regression analysis to explore how opiate prescriptions were connected to patient clinical characteristics. The analysis was adjusted for age, gender, race, ethnicity, and region.
Overall, most dermatology visits were for patients older than 65 years (36.2%) and 45-64 years old (32.1%). Over half of the dermatologist visits were for women (56.4%) and most (92.2%) visits were for patients who were White (5.1 % were for patients who were Black); most were non-Hispanic or Latino (93.5%). Most dermatology visits were in the South (35.4%) and West (25.2%), followed by the Northeast (21.9%) and Midwest (17.5%).
Opioids were prescribed in 1.3% of the visits, Ms. Pourali said. In addition, 4.7% of visits included an NSAID prescription, 0.7% an acetaminophen prescription, and 0.6% a gabapentin prescription.
Dermatologic procedure visits accounted for 43.1% of opioid prescriptions, she noted. The most common skin conditions for which opioids were prescribed included vitiligo (10.3%), hemangioma (3.8%), pemphigus (3.6%), atopic dermatitis (3.4%), and psoriasis (2.5%).
Although patients older than 65 years accounted for 36.2% of visits to dermatologists, 58.5% of opioids prescribed by dermatologists were for patients in this age group. “We hypothesize that this may be due to a higher proportion of older patients requiring skin cancer surgeries where a lot of opioids are prescribed within dermatology,” Ms. Pourali said.
The highest population-adjusted prescription rates for opiates were in the Northeast and Western regions of the United States, which “partially corroborates” previous studies that have found “higher rates of opioid prescribing in the southern and western U.S.,” she noted.
When evaluating risk-factors for long-term opiate use, Ms. Pourali and colleagues found opioids were also prescribed in 13.2% of visits where a benzodiazepine was prescribed (adjusted odds ratio, 8.17; 95% confidence interval, 5.3-12.7), 8.4% of visits where the patient had a substance abuse disorder (adjusted OR, 9.40; 95% CI, 2.0-44.4), 5.2% of visits with a patient who had depression (adjusted OR, 3.28; 95% CI, 2.0-5.4), and 2.4% of visits with a patient who used tobacco (adjusted OR, 1.09; 95% CI, 1.0-1.1).
Consider nonopioid postoperative pain management options
In an interview, Sailesh Konda, MD, associate clinical professor of dermatology and director of Mohs surgery and surgical dermatology at the University of Florida, Gainesville, who was not involved with the research, noted the finding in the study that vitiligo, hemangioma, pemphigus, AD, and psoriasis were diagnoses with the highest rates of opioid prescription was surprising. “In general, these are conditions that are not routinely managed with opioids,” he said.
NAMCS contains a primary diagnosis field and space for four additional diagnoses such as chronic conditions, as well as thirty fields for medications. “If an opioid was prescribed at a visit, it could have been prescribed for any of the diagnoses related to the visit,” Dr. Konda said. “Additionally, for those opioid prescriptions associated with dermatologic procedures, it would have been helpful to have a breakdown of the specific procedures.”
Dr. Konda compared these results to a recent study of opioid prescribing patterns in the dermatology Medicare population, which found that 93.9% of the top 1% of opioid prescribers were dermatologists working in a surgical practice.
He said that recommendations for opioid prescribing should be developed for general dermatology as they have been for Mohs surgery and dermatologic surgery. For dermatologists currently prescribing opioids, he recommended monitoring prescribing patterns and to “consider nonopioid interventions, such as acetaminophen plus ibuprofen, which has been found to effectively control postoperative pain with fewer complications.”
Ms. Pourali reports no relevant financial disclosures. Her coauthors included the principal investigator, April Armstrong, MD, MPH, professor of dermatology, University of Southern California, Los Angeles. Dr. Konda reports no relevant financial disclosures.
FROM SID 2021
LDCT lung cancer screening may ID aortic stenosis risk
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Updates in clinical practice guidelines for Lyme disease
According to the Centers for Disease Control and Prevention, Lyme disease is the fastest growing vector-borne disease, affecting approximately 300,000 Americans every year. It is caused by the spirochete, Borrelia burgdorferi which is transmitted to humans by the deer tick. Lyme disease is often an overlooked diagnosis for myriad reasons, including inaccurate test results.
Recent guidelines for the prevention, diagnosis, and treatment of Lyme disease have been developed by a panel from the Infectious Disease Society of America (IDSA), the American Academy of Neurology (AAN), and the American College of Rheumatology (ACR) using evidence-based recommendations.
Infection prevention
We all know that the best way to treat any disease is by preventing it. The following measures are recommended as tools to prevent infection: personal protective wear, repellents, and removal of the attached tick. Recommended repellents include DEET, picaridin, IR3535, oil of lemon, eucalyptus, para-Menthane-3,8-diol (PMD), 2-undecanone, and permethrin. If a tick is found, it should be removed promptly by mechanical measures, such as with tweezers. The tweezers should be inserted between the tick body and skin to ensure removal of the entire tick. Burning an attached tick or applying a noxious chemical to the tick is not recommended.
Diagnosis
Diagnosing Lyme disease is often difficult given that tests can be negative for some time after a tick bite, even when the infection is present. There is good evidence to show that submitting the removed tick for identification is good practice. However, there is no evidence supporting testing the removed tick for the presence of Borrelia burgdorferi as it does not reliably predict infection in humans. It also is recommended to avoid testing asymptomatic people following a tick bite.
Following a high-risk tick bite, adults and children can be given prophylactic antibiotics within 72 hours. It is not helpful for low-risk bites. If the risk level is uncertain, it is better to observe before giving antibiotics. For adults, a single 200-mg dose of doxycycline can be given. In children, 4.4 mg per kg of body weight, up to 200 mg max, can be used for those under 45 kg.
For patients with a tick exposure and erythema migrans, a clinical diagnosis of Lyme disease can be made without further testing. If the clinical presentation is not typical, it is recommended to do an antibody test on an acute phase serum sample followed by a convalescent serum sample in 2-3 weeks if the initial test is negative. Recommended antibiotics for treatment include doxycycline for 10 days or amoxicillin or cefuroxime for 14 days. If a patient is unable to take these, azithromycin may be used for 7 days.
The guidelines also make recommendations regarding testing for Lyme neuroborreliosis, for which neurologic presentations, for adults with psychiatric illnesses, and for children with developmental/behavioral/psychiatric disorders. They further make recommendations for treatment of Lyme disease involving the brain or spinal column, facial nerve palsy, carditis, cardiomyopathy, and arthritis, which are beyond the scope of this discussion.
As family doctors, we are often the first ones patients call upon after a tick bite. We are the ones who diagnosis and treat Lyme disease, so it is imperative that we stay up to date with current clinical guidelines and practice evidence-based medicine. These most recent guidelines from several specialty societies can provide the answers to many of our patients’ questions. They also serve as a great tool to help with our clinical decision-making regarding tick bites. Lyme disease can be a scary infection for patients but, if we offer them the recommended measures, it doesn’t have to be.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
According to the Centers for Disease Control and Prevention, Lyme disease is the fastest growing vector-borne disease, affecting approximately 300,000 Americans every year. It is caused by the spirochete, Borrelia burgdorferi which is transmitted to humans by the deer tick. Lyme disease is often an overlooked diagnosis for myriad reasons, including inaccurate test results.
Recent guidelines for the prevention, diagnosis, and treatment of Lyme disease have been developed by a panel from the Infectious Disease Society of America (IDSA), the American Academy of Neurology (AAN), and the American College of Rheumatology (ACR) using evidence-based recommendations.
Infection prevention
We all know that the best way to treat any disease is by preventing it. The following measures are recommended as tools to prevent infection: personal protective wear, repellents, and removal of the attached tick. Recommended repellents include DEET, picaridin, IR3535, oil of lemon, eucalyptus, para-Menthane-3,8-diol (PMD), 2-undecanone, and permethrin. If a tick is found, it should be removed promptly by mechanical measures, such as with tweezers. The tweezers should be inserted between the tick body and skin to ensure removal of the entire tick. Burning an attached tick or applying a noxious chemical to the tick is not recommended.
Diagnosis
Diagnosing Lyme disease is often difficult given that tests can be negative for some time after a tick bite, even when the infection is present. There is good evidence to show that submitting the removed tick for identification is good practice. However, there is no evidence supporting testing the removed tick for the presence of Borrelia burgdorferi as it does not reliably predict infection in humans. It also is recommended to avoid testing asymptomatic people following a tick bite.
Following a high-risk tick bite, adults and children can be given prophylactic antibiotics within 72 hours. It is not helpful for low-risk bites. If the risk level is uncertain, it is better to observe before giving antibiotics. For adults, a single 200-mg dose of doxycycline can be given. In children, 4.4 mg per kg of body weight, up to 200 mg max, can be used for those under 45 kg.
For patients with a tick exposure and erythema migrans, a clinical diagnosis of Lyme disease can be made without further testing. If the clinical presentation is not typical, it is recommended to do an antibody test on an acute phase serum sample followed by a convalescent serum sample in 2-3 weeks if the initial test is negative. Recommended antibiotics for treatment include doxycycline for 10 days or amoxicillin or cefuroxime for 14 days. If a patient is unable to take these, azithromycin may be used for 7 days.
The guidelines also make recommendations regarding testing for Lyme neuroborreliosis, for which neurologic presentations, for adults with psychiatric illnesses, and for children with developmental/behavioral/psychiatric disorders. They further make recommendations for treatment of Lyme disease involving the brain or spinal column, facial nerve palsy, carditis, cardiomyopathy, and arthritis, which are beyond the scope of this discussion.
As family doctors, we are often the first ones patients call upon after a tick bite. We are the ones who diagnosis and treat Lyme disease, so it is imperative that we stay up to date with current clinical guidelines and practice evidence-based medicine. These most recent guidelines from several specialty societies can provide the answers to many of our patients’ questions. They also serve as a great tool to help with our clinical decision-making regarding tick bites. Lyme disease can be a scary infection for patients but, if we offer them the recommended measures, it doesn’t have to be.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
According to the Centers for Disease Control and Prevention, Lyme disease is the fastest growing vector-borne disease, affecting approximately 300,000 Americans every year. It is caused by the spirochete, Borrelia burgdorferi which is transmitted to humans by the deer tick. Lyme disease is often an overlooked diagnosis for myriad reasons, including inaccurate test results.
Recent guidelines for the prevention, diagnosis, and treatment of Lyme disease have been developed by a panel from the Infectious Disease Society of America (IDSA), the American Academy of Neurology (AAN), and the American College of Rheumatology (ACR) using evidence-based recommendations.
Infection prevention
We all know that the best way to treat any disease is by preventing it. The following measures are recommended as tools to prevent infection: personal protective wear, repellents, and removal of the attached tick. Recommended repellents include DEET, picaridin, IR3535, oil of lemon, eucalyptus, para-Menthane-3,8-diol (PMD), 2-undecanone, and permethrin. If a tick is found, it should be removed promptly by mechanical measures, such as with tweezers. The tweezers should be inserted between the tick body and skin to ensure removal of the entire tick. Burning an attached tick or applying a noxious chemical to the tick is not recommended.
Diagnosis
Diagnosing Lyme disease is often difficult given that tests can be negative for some time after a tick bite, even when the infection is present. There is good evidence to show that submitting the removed tick for identification is good practice. However, there is no evidence supporting testing the removed tick for the presence of Borrelia burgdorferi as it does not reliably predict infection in humans. It also is recommended to avoid testing asymptomatic people following a tick bite.
Following a high-risk tick bite, adults and children can be given prophylactic antibiotics within 72 hours. It is not helpful for low-risk bites. If the risk level is uncertain, it is better to observe before giving antibiotics. For adults, a single 200-mg dose of doxycycline can be given. In children, 4.4 mg per kg of body weight, up to 200 mg max, can be used for those under 45 kg.
For patients with a tick exposure and erythema migrans, a clinical diagnosis of Lyme disease can be made without further testing. If the clinical presentation is not typical, it is recommended to do an antibody test on an acute phase serum sample followed by a convalescent serum sample in 2-3 weeks if the initial test is negative. Recommended antibiotics for treatment include doxycycline for 10 days or amoxicillin or cefuroxime for 14 days. If a patient is unable to take these, azithromycin may be used for 7 days.
The guidelines also make recommendations regarding testing for Lyme neuroborreliosis, for which neurologic presentations, for adults with psychiatric illnesses, and for children with developmental/behavioral/psychiatric disorders. They further make recommendations for treatment of Lyme disease involving the brain or spinal column, facial nerve palsy, carditis, cardiomyopathy, and arthritis, which are beyond the scope of this discussion.
As family doctors, we are often the first ones patients call upon after a tick bite. We are the ones who diagnosis and treat Lyme disease, so it is imperative that we stay up to date with current clinical guidelines and practice evidence-based medicine. These most recent guidelines from several specialty societies can provide the answers to many of our patients’ questions. They also serve as a great tool to help with our clinical decision-making regarding tick bites. Lyme disease can be a scary infection for patients but, if we offer them the recommended measures, it doesn’t have to be.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at fpnews@mdedge.com.
Benzene was found in some sunscreens. Now what?
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Just before Memorial Day, which include batches from Neutrogena, Banana Boat, CVS Health, and other brands. More than three-quarters of the products are sprays.
“We’re asking our patients to put sunscreen on from 6 months of age, telling them to do it their entire life, their whole body, multiple times a day,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said in an interview. If benzene-contaminated sunscreen proves to be a widespread problem, he said, “the benzene amounts can add up to a significant chronic exposure over a lifetime.”
In the Valisure statement announcing the findings, Dr. Bunick, who is also quoted in the petition, said that “it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
The list of products that tested positive is included in the citizen petition, and a full list of products that did not show any contamination is available in an attachment.
Benzene is not an ingredient in sunscreen, and Valisure’s petition suggests that the findings are a result of contamination somewhere in the manufacturing process, not of product degradation.
“This isn’t a sunscreen issue, it’s a manufacturing issue,” said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington. “We don’t want those things to be blurred.”
When asked to comment on Valisure’s findings, an FDA spokesperson said, “The FDA takes seriously any safety concerns raised about products we regulate, including sunscreen. While the agency evaluates the submitted citizen petition, we will continue to monitor the sunscreen marketplace and manufacturing efforts to help ensure the availability of safe sunscreens for U.S. consumers.”
Both Johnson & Johnson, Neutrogena’s parent company, and Banana Boat issued statements reiterating that benzene is not an ingredient in their products.
Assessing the risks
There is a risk of patients taking away the wrong message from these findings.
“People already have ambivalence about sunscreen, and this is just going to make that worse,” Dr. Friedman said in an interview. He pointed out that benzene is present in car exhaust, second-hand smoke, and elsewhere. Inhalation exposure has been the primary focus of toxicology investigations, as has exposure from ingesting things such as contaminated drinking water – not via topical application. “We don’t know how effectively [benzene] gets through the skin, if it gets absorbed systemically, and how that then behaves downstream,” he noted.
On the other hand, ultraviolet radiation is a well-established carcinogen. Avoiding an effective preventive measure such as sunscreen could prove more harmful than exposure to trace amounts of benzene, ultimately to be determined by the FDA.
“Just because those particular products do pose a risk, that doesn’t erase the message that sunscreens are safe and should be used,” Dr. Bunick said. “It’s not mutually exclusive.”
And then there’s the fact that the benzene contamination appears to be fairly limited. “The majority of products we tested, over 200 of them, had no detectable amounts of benzene, and uncontaminated sunscreen should certainly continue to be used,” David Light, CEO of Valisure, told this news organization.
Advising patients
With headlines blaring the news about a carcinogen in sunscreen, patients will be reaching out for advice.
“The number one question patients will have is, ‘What sunscreen do you recommend?’” said Dr. Bunick. “The answer should be to pick a sunscreen that we know wasn’t contaminated. Reassure your patient the ingredients themselves are effective and safe, and that’s not what’s leading to the contamination.”
Dr. Friedman agrees. “We need to be mindful. Dermatologists need to be armed with the facts in order to counsel patients: Sunscreen is still a very important, effective, and safe, scientifically based way to prevent the harmful effects of the sun, in addition to things like sun protective clothing and seeking shade between 10 a.m. and 4 p.m.”
As alarming as Valisure’s findings may seem initially, Dr. Bunick noted a silver lining. “The consumer, the public should feel reassured this report is out there. It shows that someone’s watching out. That’s an important safety message: These things aren’t going undetected.”
Mistrust and Mandates: COVID-19 Vaccination in the Military
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
It is June and most of us are looking forward to a more normal summer than the one we had in 2020. Many Americans have been vaccinated and states are rolling back some (or all) masking requirements and restrictions on gatherings. In many sectors, including the US Department of Defense (DoD) and the US Department of Veterans Affairs (VA), worries from public health officials about vaccine supply and how to ethically allocate demand have given way to a new set of concerns: We have the shots, but for widespread protection we have to get them into arms.
The reluctance to roll up the sleeve is known as vaccine hesitancy. The National Academies of Science comments on vaccine hesitancy in its report on COVID-19 vaccination allocation. “Potential consequences of vaccine hesitancy—which the committee views as an attitude, preference, or motivational state—are the behaviors of vaccine refusal or delay.”2
On that count, there was encouraging albeit unexpected news in waning days of May. Media reported a sharp increase in the COVID-vaccination of military personnel. Unnamed DoD officials indicated, they had seen a 55% increase in the vaccination of active-duty service members over the previous month. This news represents a dramatic turnaround in a trend of vaccine hesitancy among military members that has persisted since the vaccine became available.3 Even last month, this would have been a very different column. The DoD has not disclosed the exact number of service members who have declined COVID-19 vaccination but multiple news outlets have documented that there was widespread and significant vaccine hesitancy among military personnel. In February, Military News reported that one-third of troops who were offered the vaccine declined it; and in April, USA Today stated that 40% of Marines had refused vaccination.4,5
Still, it is worth examining the data on vaccination among active duty service members. From December 2020 through March 2021, the military conducted the first study to evaluate rates of vaccine initiation and completion in the military in general and for service members from racial/ethnic minorities in particular. Black military personnel were 28% less likely than non-Hispanic White service members to initiate vaccination against coronavirus even after adjusting for other possible confounders. Just 29% of White, 25.5% of Hispanic, and 18.7% of Black service members had initiated the vaccine process in the survey.6
The authors suggest that in part, vaccine hesitancy explains the findings.4 Vaccine hesitancy among racial and ethnic minorities is even more tragic because these same already disadvantaged cohorts have disproportionately suffered from COVID-19 throughout the pandemic with higher rates of infection, serious illness requiring hospitalization, and infection-related morbidity.7
Vaccine hesitancy, delay, or refusal in Black Americans whether military or civilian often is attributed to the historical abuses like the Tuskegee syphilis experiments or the more recent example of cancer cell lines taken from Henrietta Lacks without consent.8 Such government sponsored betrayals no doubt are the soil in which hesitancy grows but recent commentators have opined that focusing solely on these infamous examples may ignore current systemic racism that is pervasively feeding Black Americans reluctance to consider or accept COVID-19 vaccination.9 Blaming infamous research also provides a convenient excuse for confronting contemporary racial discrimination in health care and taking responsibility as health care practitioners for reversing it. “Framing the conversation about distrust in COVID vaccines in terms of everyday racism rather than historical atrocities may increase underserved communities’ willingness to be vaccinated,” Bajaj and Stanford wrote in a recent recent New England Journal of Medicine commentary. “When we hyperfocus on Sims, Lacks, and Tuskegee, we ascribe the current Black health experience to past racism, rooting our present in immovable historical occurrences and undermining efforts to combat mistrust. Everyday racism, by contrast, can be tackled in the present.”9
The study of racial/ethnic disparities in COVID-19 vaccination in active-duty service members was a work product of the Armed Forces Health Surveillance Division. The authors underscore several factors that support the connection between discrimination and vaccine hesitancy in the military. Lack of access to and ability to obtain COVID-19 vaccination continues to be a major barrier that disadvantaged populations must overcome.10 The COVID-19 vaccine is widely available, easily obtained, and free of charge for all military personnel. Yet the vaccine hesitancy in the military parallels that of the civilian sector. This led the study authors to opine that, “forces external to the U.S. Military, such as interpersonal and societal factors also contribute to vaccine hesitancy among military service members.”6
Obviously, any unvaccinated active-duty service member reduces the combat readiness of the fighting force a consideration that led some in Congress to call for mandating vaccination. The vaccine is currently being administered under an emergency use authorization (EUA), which prevents even the military from mandating it.11 Even if President Joseph Biden obtained a waiver to make the vaccine mandatory, the implications of forcing service members who have volunteered to serve their country is ethically problematic. Those problems are exponentially amplified when applied to members of ethnic and racial minorities who have a past and present of health disparities and discrimination. Respecting the decision of those in uniform to decline COVID-19 vaccination is the first and perhaps most important step to rebuilding the trust that is the most promising means of reducing vaccine hesitancy.
Part of the accountability we all bear for health care inequity and racism is to continue the work of this landmark study to better understand vaccine hesitancy among military and veteran cohorts, develop counseling and education that target those attitudes, beliefs, and motivations with education, counseling, and support. All of us can in some small measure follow the ethical mandate “to dispel rumors and provide facts to people” of Secretary Austin, a Black retired 4-star Army general.1
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
1. Garmone J. Secretary of Defense Addresses Vaccine Hesitancy in the Military. Published February 25, 2021. Accessed May 26, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military/
2. National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine . The National Academies of Science; 2020:188. doi:10.17226/25917
3. Liebermann O. US military sees 55% jump in COVID-19 vaccinations over last month. Published May 20, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/05/20/politics/us-military-covid-vaccinations/index.html
4. Kime P. Almost one-third of us troops are refusing COVID-19 vaccines, officials Say. Published February 17, 2021. Accessed May 26, 2021. https://www.military.com/daily-news/2021/02/17/almost-one-third-of-us-troops-are-refusing-covid-vaccines-officials-say.html
5. Elbeshbishi S. Nearly 40% of Marines decline COVID-19 vaccine, prompting some Democrats to urge Biden to set mandate for the military. USA Today. April 10, 2021. Accessed May 26, 2021. https://www.usatoday.com/story/news/politics/2021/04/10/covid-vaccine-nearly-forty-percent-us-marines-decline/7173918002/
6. Lang MA, Stahlman S, Wells NY, et al. Disparities in COVID-19 vaccine initiation and completion among active component service members and health care personnel, 11 December 2020-12 March 2021. MSMR. 2021;28(4):2-9.
7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA . 2020;323(24):2466-2467. doi:10.1001/jama.2020.8598
8. Kum D. Fueled by a history of mistreatment, Black Americans distrust the new COVID-19 vaccines. TIME. December 8, 2020. Accessed May 26, 2021.https://time.com/5925074/black-americans-covid-19-vaccine-distrust/
9. Bajaj SS, Stanford FC. Beyond Tuskegee - Vaccine Distrust and Everyday Racism. N Engl J Med. 2021;384(5):e12. doi:10.1056/NEJMpv2035827
10. Feldman N. Why Black and Latino people still lag on COVID-19 vaccines-and how to fix it. NPR. April 26, 2021. Accessed May 26, 2021. https://www.npr.org/sections/health-shots/2021/04/26/989962041/why-black-and-latino-people-still-lag-on-covid-vaccines-and-how-to-fix-it
11. Kaufman E. Lawmakers ask Biden to issue waiver to make COVID-19 vaccination mandatory of members of the military. Updated March 24, 2021. Accessed May 26, 2021. https://www.cnn.com/2021/03/24/politics/congress-letter-military-vaccine/index.html
Hospital acquisition had no significant change in the rate of readmission or mortality
Background: Prior studies have examined the impact of hospital system mergers on health care costs, but few studies have previously examined impact on quality and patient experience.
Study design: Retrospective, difference-in-difference analysis.
Setting: 2,232 U.S. hospitals during 2007-2016.
Synopsis: The authors identified 2,232 hospitals, including 246 hospitals that were acquired between 2009 and 2013 and 1,986 control hospitals that were not acquired during this period. They used a difference-in-difference analysis to compare hospital performance on quality and patient experience measures from before and after an acquisition to concurrent changes in control hospitals. Hospital acquisition was associated with a significant decline in measured patient experience. There was no significant differential change in 30-day readmission or mortality. Although there was an association between acquisition and significant improvement in clinical process metrics, the authors found that this improvement occurred almost entirely prior to acquisition.
Bottom line: Hospital acquisition was associated with worse experience for patients and had no significant impact on readmission or mortality rates.
Citation: Beaulieu ND et al. Changes in quality of care after hospital mergers and acquisitions. N Engl J Med. 2020 Jan 2;382:51-9.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
Background: Prior studies have examined the impact of hospital system mergers on health care costs, but few studies have previously examined impact on quality and patient experience.
Study design: Retrospective, difference-in-difference analysis.
Setting: 2,232 U.S. hospitals during 2007-2016.
Synopsis: The authors identified 2,232 hospitals, including 246 hospitals that were acquired between 2009 and 2013 and 1,986 control hospitals that were not acquired during this period. They used a difference-in-difference analysis to compare hospital performance on quality and patient experience measures from before and after an acquisition to concurrent changes in control hospitals. Hospital acquisition was associated with a significant decline in measured patient experience. There was no significant differential change in 30-day readmission or mortality. Although there was an association between acquisition and significant improvement in clinical process metrics, the authors found that this improvement occurred almost entirely prior to acquisition.
Bottom line: Hospital acquisition was associated with worse experience for patients and had no significant impact on readmission or mortality rates.
Citation: Beaulieu ND et al. Changes in quality of care after hospital mergers and acquisitions. N Engl J Med. 2020 Jan 2;382:51-9.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
Background: Prior studies have examined the impact of hospital system mergers on health care costs, but few studies have previously examined impact on quality and patient experience.
Study design: Retrospective, difference-in-difference analysis.
Setting: 2,232 U.S. hospitals during 2007-2016.
Synopsis: The authors identified 2,232 hospitals, including 246 hospitals that were acquired between 2009 and 2013 and 1,986 control hospitals that were not acquired during this period. They used a difference-in-difference analysis to compare hospital performance on quality and patient experience measures from before and after an acquisition to concurrent changes in control hospitals. Hospital acquisition was associated with a significant decline in measured patient experience. There was no significant differential change in 30-day readmission or mortality. Although there was an association between acquisition and significant improvement in clinical process metrics, the authors found that this improvement occurred almost entirely prior to acquisition.
Bottom line: Hospital acquisition was associated with worse experience for patients and had no significant impact on readmission or mortality rates.
Citation: Beaulieu ND et al. Changes in quality of care after hospital mergers and acquisitions. N Engl J Med. 2020 Jan 2;382:51-9.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
New obesity target? Dopamine circuit in brainstem affects satiety
Researchers have discovered a new dopaminergic neural circuit leading to the hindbrain that is involved in satiety (feeling full and eating cessation) in mice, which may eventually lead to new ways to treat obesity.
Moreover, when mice were given methylphenidate (Ritalin, Concerta) – a stimulant approved to treat attention deficit hyperactivity disorder (ADHD) with a well-known side effect of decreasing appetite – signals in this dopaminergic pathway were enhanced and the mice ate less.
The study by Yong Han, PhD, a postdoctoral associate at Baylor College of Medicine, Houston, and colleagues was published online May 27 in Science Advances.
“We identified a new dopamine neural circuit from the midbrain to the hindbrain (brainstem) that regulates feeding behavior through an enhanced satiation response,” senior author Qi Wu, PhD, assistant professor in pediatrics-nutrition at Baylor College of Medicine, summarized in an interview.
The findings suggest that “people with obesity have a compromised dopaminergic neural pathway, presumably in ways that delay the satiation response, which makes them eat more, have a larger meal,” he explained.
Newly identified brain circuit plays a key role in satiety response
The study is about a circuit in the brain that helps precisely regulate the size of food portion consumed, Dr. Wu emphasized in a statement from the university, adding that the satiation response is as important as appetite.
Importantly, the results also provide clues about how methylphenidate can lead to weight loss.
Regulators have deemed that methylphenidate, a controlled substance with other side effects such as anxiety and a fast heart rate, is safe and effective for ADHD, Dr. Wu noted.
He speculated that, “If researchers want to do clinical trials of methylphenidate for obesity, it ultimately could evolve to be an anti-obesity drug, alone or combined with other drugs, or possibly derivatives of methylphenidate could be tested.”
The brain circuit “we discovered is the first to be fully described to regulate portion size via dopamine signaling,” Dr. Han stressed in the statement.
“Our new study shows that a circuit connecting neurons that produce dopamine, a chemical messenger previously known for the regulation of motivation and pleasure, has a new [critical] role in the control of feeding through dynamically regulating the satiety response,” he explained.
Brain signals that control portion size
Earlier studies that investigated how the dopaminergic system may regulate food intake, appetite, and body weight, have produced conflicting results, Dr. Wu said.
The researchers performed several experiments in mice that included the use of cell-specific circuitry mapping, optogenetics, and real-time recordings of brain activity.
They identified a new dopaminergic neural circuit comprised of dopaminergic neurons in the caudal ventral tegmental area (DA-VTA neurons) in the midbrain that directly innervate dopamine receptor D1-expressing neurons within the lateral parabrachial nucleus (DRD1-LPBN neurons) in the hindbrain.
There were four main findings:
- DA-VTA neurons were activated immediately before the cessation of each feeding bout.
- Actively inhibiting DA-VTA neurons before the end of each feeding bout prolonged the feeding.
- Activating DRD1-LPBN neurons inhibited feeding.
- Mice that lacked the DRD1 gene ate much more and gained weight.
“Our study illuminates a hindbrain dopaminergic circuit that controls feeding through dynamic regulation in satiety response and meal structure,” the researchers reiterate.
The study was supported by grants from the National Institutes of Health, NIH Digestive Diseases Center, Pew Charitable Trust, American Diabetes Association, Baylor Collaborative Faculty Research Investment Program, USDA/CRIS, USDA/ARS, American Heart Association, and NIH Centers of Biomedical Research Excellence, and by Pew and Kavli scholarships. The researchers have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Researchers have discovered a new dopaminergic neural circuit leading to the hindbrain that is involved in satiety (feeling full and eating cessation) in mice, which may eventually lead to new ways to treat obesity.
Moreover, when mice were given methylphenidate (Ritalin, Concerta) – a stimulant approved to treat attention deficit hyperactivity disorder (ADHD) with a well-known side effect of decreasing appetite – signals in this dopaminergic pathway were enhanced and the mice ate less.
The study by Yong Han, PhD, a postdoctoral associate at Baylor College of Medicine, Houston, and colleagues was published online May 27 in Science Advances.
“We identified a new dopamine neural circuit from the midbrain to the hindbrain (brainstem) that regulates feeding behavior through an enhanced satiation response,” senior author Qi Wu, PhD, assistant professor in pediatrics-nutrition at Baylor College of Medicine, summarized in an interview.
The findings suggest that “people with obesity have a compromised dopaminergic neural pathway, presumably in ways that delay the satiation response, which makes them eat more, have a larger meal,” he explained.
Newly identified brain circuit plays a key role in satiety response
The study is about a circuit in the brain that helps precisely regulate the size of food portion consumed, Dr. Wu emphasized in a statement from the university, adding that the satiation response is as important as appetite.
Importantly, the results also provide clues about how methylphenidate can lead to weight loss.
Regulators have deemed that methylphenidate, a controlled substance with other side effects such as anxiety and a fast heart rate, is safe and effective for ADHD, Dr. Wu noted.
He speculated that, “If researchers want to do clinical trials of methylphenidate for obesity, it ultimately could evolve to be an anti-obesity drug, alone or combined with other drugs, or possibly derivatives of methylphenidate could be tested.”
The brain circuit “we discovered is the first to be fully described to regulate portion size via dopamine signaling,” Dr. Han stressed in the statement.
“Our new study shows that a circuit connecting neurons that produce dopamine, a chemical messenger previously known for the regulation of motivation and pleasure, has a new [critical] role in the control of feeding through dynamically regulating the satiety response,” he explained.
Brain signals that control portion size
Earlier studies that investigated how the dopaminergic system may regulate food intake, appetite, and body weight, have produced conflicting results, Dr. Wu said.
The researchers performed several experiments in mice that included the use of cell-specific circuitry mapping, optogenetics, and real-time recordings of brain activity.
They identified a new dopaminergic neural circuit comprised of dopaminergic neurons in the caudal ventral tegmental area (DA-VTA neurons) in the midbrain that directly innervate dopamine receptor D1-expressing neurons within the lateral parabrachial nucleus (DRD1-LPBN neurons) in the hindbrain.
There were four main findings:
- DA-VTA neurons were activated immediately before the cessation of each feeding bout.
- Actively inhibiting DA-VTA neurons before the end of each feeding bout prolonged the feeding.
- Activating DRD1-LPBN neurons inhibited feeding.
- Mice that lacked the DRD1 gene ate much more and gained weight.
“Our study illuminates a hindbrain dopaminergic circuit that controls feeding through dynamic regulation in satiety response and meal structure,” the researchers reiterate.
The study was supported by grants from the National Institutes of Health, NIH Digestive Diseases Center, Pew Charitable Trust, American Diabetes Association, Baylor Collaborative Faculty Research Investment Program, USDA/CRIS, USDA/ARS, American Heart Association, and NIH Centers of Biomedical Research Excellence, and by Pew and Kavli scholarships. The researchers have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Researchers have discovered a new dopaminergic neural circuit leading to the hindbrain that is involved in satiety (feeling full and eating cessation) in mice, which may eventually lead to new ways to treat obesity.
Moreover, when mice were given methylphenidate (Ritalin, Concerta) – a stimulant approved to treat attention deficit hyperactivity disorder (ADHD) with a well-known side effect of decreasing appetite – signals in this dopaminergic pathway were enhanced and the mice ate less.
The study by Yong Han, PhD, a postdoctoral associate at Baylor College of Medicine, Houston, and colleagues was published online May 27 in Science Advances.
“We identified a new dopamine neural circuit from the midbrain to the hindbrain (brainstem) that regulates feeding behavior through an enhanced satiation response,” senior author Qi Wu, PhD, assistant professor in pediatrics-nutrition at Baylor College of Medicine, summarized in an interview.
The findings suggest that “people with obesity have a compromised dopaminergic neural pathway, presumably in ways that delay the satiation response, which makes them eat more, have a larger meal,” he explained.
Newly identified brain circuit plays a key role in satiety response
The study is about a circuit in the brain that helps precisely regulate the size of food portion consumed, Dr. Wu emphasized in a statement from the university, adding that the satiation response is as important as appetite.
Importantly, the results also provide clues about how methylphenidate can lead to weight loss.
Regulators have deemed that methylphenidate, a controlled substance with other side effects such as anxiety and a fast heart rate, is safe and effective for ADHD, Dr. Wu noted.
He speculated that, “If researchers want to do clinical trials of methylphenidate for obesity, it ultimately could evolve to be an anti-obesity drug, alone or combined with other drugs, or possibly derivatives of methylphenidate could be tested.”
The brain circuit “we discovered is the first to be fully described to regulate portion size via dopamine signaling,” Dr. Han stressed in the statement.
“Our new study shows that a circuit connecting neurons that produce dopamine, a chemical messenger previously known for the regulation of motivation and pleasure, has a new [critical] role in the control of feeding through dynamically regulating the satiety response,” he explained.
Brain signals that control portion size
Earlier studies that investigated how the dopaminergic system may regulate food intake, appetite, and body weight, have produced conflicting results, Dr. Wu said.
The researchers performed several experiments in mice that included the use of cell-specific circuitry mapping, optogenetics, and real-time recordings of brain activity.
They identified a new dopaminergic neural circuit comprised of dopaminergic neurons in the caudal ventral tegmental area (DA-VTA neurons) in the midbrain that directly innervate dopamine receptor D1-expressing neurons within the lateral parabrachial nucleus (DRD1-LPBN neurons) in the hindbrain.
There were four main findings:
- DA-VTA neurons were activated immediately before the cessation of each feeding bout.
- Actively inhibiting DA-VTA neurons before the end of each feeding bout prolonged the feeding.
- Activating DRD1-LPBN neurons inhibited feeding.
- Mice that lacked the DRD1 gene ate much more and gained weight.
“Our study illuminates a hindbrain dopaminergic circuit that controls feeding through dynamic regulation in satiety response and meal structure,” the researchers reiterate.
The study was supported by grants from the National Institutes of Health, NIH Digestive Diseases Center, Pew Charitable Trust, American Diabetes Association, Baylor Collaborative Faculty Research Investment Program, USDA/CRIS, USDA/ARS, American Heart Association, and NIH Centers of Biomedical Research Excellence, and by Pew and Kavli scholarships. The researchers have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Electronic frailty index based on routine blood tests may help identify at-risk seniors
Background: Accurate identification of frail older patients at hospital admission may help target interventions; however, the extent to which risk prediction tools such as the frailty index can be utilized in the acute setting remains unclear.
Study design: Single-center prospective cohort study, during April 2015–January 2017.
Setting: A tertiary care, academic medical center in the United Kingdom.
Synopsis: This study enrolled 1,750 older adults, comprising 2,552 hospital admissions. For each admission, the authors generated a frailty index, called FI-Laboratory, based on the proportion of abnormal results from 27 of the most common admission laboratory tests. The authors found that an increase in the FI-Lab was significantly associated, independent of an existing chronic frailty score, with increased proportion of inpatient days, discharge to a higher level of care, readmission rates, and mortality. Notably, researchers were unable to calculate the FI-Lab score in 11.6% of cases because of insufficient laboratory information. The single-center design of this study may limit its generalizability.
Bottom line: The FI-Laboratory may provide information, complementary to existing frailty assessments, to help identify older adults at increased risk of inpatient adverse outcomes.
Citation: Logan Ellis H et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. CMAJ. 2020;192(1)e3-8.
Dr. Hu is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Accurate identification of frail older patients at hospital admission may help target interventions; however, the extent to which risk prediction tools such as the frailty index can be utilized in the acute setting remains unclear.
Study design: Single-center prospective cohort study, during April 2015–January 2017.
Setting: A tertiary care, academic medical center in the United Kingdom.
Synopsis: This study enrolled 1,750 older adults, comprising 2,552 hospital admissions. For each admission, the authors generated a frailty index, called FI-Laboratory, based on the proportion of abnormal results from 27 of the most common admission laboratory tests. The authors found that an increase in the FI-Lab was significantly associated, independent of an existing chronic frailty score, with increased proportion of inpatient days, discharge to a higher level of care, readmission rates, and mortality. Notably, researchers were unable to calculate the FI-Lab score in 11.6% of cases because of insufficient laboratory information. The single-center design of this study may limit its generalizability.
Bottom line: The FI-Laboratory may provide information, complementary to existing frailty assessments, to help identify older adults at increased risk of inpatient adverse outcomes.
Citation: Logan Ellis H et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. CMAJ. 2020;192(1)e3-8.
Dr. Hu is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Accurate identification of frail older patients at hospital admission may help target interventions; however, the extent to which risk prediction tools such as the frailty index can be utilized in the acute setting remains unclear.
Study design: Single-center prospective cohort study, during April 2015–January 2017.
Setting: A tertiary care, academic medical center in the United Kingdom.
Synopsis: This study enrolled 1,750 older adults, comprising 2,552 hospital admissions. For each admission, the authors generated a frailty index, called FI-Laboratory, based on the proportion of abnormal results from 27 of the most common admission laboratory tests. The authors found that an increase in the FI-Lab was significantly associated, independent of an existing chronic frailty score, with increased proportion of inpatient days, discharge to a higher level of care, readmission rates, and mortality. Notably, researchers were unable to calculate the FI-Lab score in 11.6% of cases because of insufficient laboratory information. The single-center design of this study may limit its generalizability.
Bottom line: The FI-Laboratory may provide information, complementary to existing frailty assessments, to help identify older adults at increased risk of inpatient adverse outcomes.
Citation: Logan Ellis H et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. CMAJ. 2020;192(1)e3-8.
Dr. Hu is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.