User login
For MD-IQ use only
Celebrating Excellence
As we settle back into our daily routines following another fantastic DDW, I’d like to take a moment to congratulate this year’s AGA’s Recognition Award recipients, who have made outstanding contributions to the organization and to our field, including through excellence in clinical practice, research, mentorship, and DEI.
This month’s Member Spotlight column highlights one of these remarkable individuals, Dr. Scott Ketover, president and CEO of MNGI Digestive Health, who is the recipient of this year’s AGA Distinguished Clinician Award in Private Practice. We hope you enjoy learning more about Scott, as well as the other award recipients who were recognized at a special ceremony in DC last month.
Also highlighted in our June issue is the FDA’s recent approval of subcutaneous vedolizumab for Crohn’s maintenance therapy, an exciting development that will provide us with more flexible treatment options for our patients. We also report on the 2024 AGA Tech Summit (Chicago, IL), and introduce the winners (survivors?) of its annual Shark Tank competition, Dr. Renu Dhanasekaran and Dr. Venthan Elango. Their company, Arithmedics, which developed technology that harnesses generative AI and data intelligence to streamline medical billing, was identified as the most promising among a robust field of entrants.
We also present some of the best clinically oriented content from our GI journals, including an observational study from Gastroenterology evaluating the effect of longitudinal alcohol use on risk of cirrhosis among patients with steatotic liver disease, and summarize recently released AGA Clinical Practice Updates on performance of high-quality upper endoscopy and treatment of cannabinoid hyperemesis syndrome. We hope you enjoy all the exciting content featured in this issue and take some well-deserved time to rest and recharge this summer!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
As we settle back into our daily routines following another fantastic DDW, I’d like to take a moment to congratulate this year’s AGA’s Recognition Award recipients, who have made outstanding contributions to the organization and to our field, including through excellence in clinical practice, research, mentorship, and DEI.
This month’s Member Spotlight column highlights one of these remarkable individuals, Dr. Scott Ketover, president and CEO of MNGI Digestive Health, who is the recipient of this year’s AGA Distinguished Clinician Award in Private Practice. We hope you enjoy learning more about Scott, as well as the other award recipients who were recognized at a special ceremony in DC last month.
Also highlighted in our June issue is the FDA’s recent approval of subcutaneous vedolizumab for Crohn’s maintenance therapy, an exciting development that will provide us with more flexible treatment options for our patients. We also report on the 2024 AGA Tech Summit (Chicago, IL), and introduce the winners (survivors?) of its annual Shark Tank competition, Dr. Renu Dhanasekaran and Dr. Venthan Elango. Their company, Arithmedics, which developed technology that harnesses generative AI and data intelligence to streamline medical billing, was identified as the most promising among a robust field of entrants.
We also present some of the best clinically oriented content from our GI journals, including an observational study from Gastroenterology evaluating the effect of longitudinal alcohol use on risk of cirrhosis among patients with steatotic liver disease, and summarize recently released AGA Clinical Practice Updates on performance of high-quality upper endoscopy and treatment of cannabinoid hyperemesis syndrome. We hope you enjoy all the exciting content featured in this issue and take some well-deserved time to rest and recharge this summer!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
As we settle back into our daily routines following another fantastic DDW, I’d like to take a moment to congratulate this year’s AGA’s Recognition Award recipients, who have made outstanding contributions to the organization and to our field, including through excellence in clinical practice, research, mentorship, and DEI.
This month’s Member Spotlight column highlights one of these remarkable individuals, Dr. Scott Ketover, president and CEO of MNGI Digestive Health, who is the recipient of this year’s AGA Distinguished Clinician Award in Private Practice. We hope you enjoy learning more about Scott, as well as the other award recipients who were recognized at a special ceremony in DC last month.
Also highlighted in our June issue is the FDA’s recent approval of subcutaneous vedolizumab for Crohn’s maintenance therapy, an exciting development that will provide us with more flexible treatment options for our patients. We also report on the 2024 AGA Tech Summit (Chicago, IL), and introduce the winners (survivors?) of its annual Shark Tank competition, Dr. Renu Dhanasekaran and Dr. Venthan Elango. Their company, Arithmedics, which developed technology that harnesses generative AI and data intelligence to streamline medical billing, was identified as the most promising among a robust field of entrants.
We also present some of the best clinically oriented content from our GI journals, including an observational study from Gastroenterology evaluating the effect of longitudinal alcohol use on risk of cirrhosis among patients with steatotic liver disease, and summarize recently released AGA Clinical Practice Updates on performance of high-quality upper endoscopy and treatment of cannabinoid hyperemesis syndrome. We hope you enjoy all the exciting content featured in this issue and take some well-deserved time to rest and recharge this summer!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Hypofractionated Radiotherapy Limits Toxic Effects in Cervical Cancer
TOPLINE:
results from the phase 2 POHIM-CCRT trial suggested.
METHODOLOGY:
- To date, no studies have assessed the treatment outcomes and toxic effects of hypofractionated IMRT following radical hysterectomy in patients with cervical cancer undergoing curative radiotherapy.
- The team analyzed outcomes from 79 patients undergoing hypofractionated IMRT for cervical cancer after radical hysterectomy and pelvic lymph node dissection.
- Patients were a median age of 48; 29.5% had stage IB to IIA disease, another 29.5% had stage IIB disease, and 41% had stage III disease. Patients also had at least one of the following criteria following radical hysterectomy and pelvic lymph node dissection: lymph node metastasis (39.7%), parametrial invasion (54.4%), and positive resection margin (5.1%).
- The prescribed dose to the planning target volume was 40 Gy, delivered in 16 fractions to the whole pelvis, with any type of IMRT permitted. Overall, 71 patients also underwent concurrent weekly cisplatin (40 mg/m2 of body surface area for three cycles), and eight received fluorouracil (1000 mg/m2 on days 1-5) with cisplatin (60 mg/m2 for two cycles).
- The primary endpoint was the incidence of acute grade 3 or higher gastrointestinal tract, genitourinary, and hematologic toxic effects during radiotherapy or within 3 months of completing radiotherapy.
TAKEAWAY:
- After radiotherapy, only two patients (2.5%) experienced acute grade 3 or higher toxic effects. One was hospitalized for enterocolitis on the last day of radiotherapy and developed grade 3 anemia 3 months after completing radiotherapy; the other experienced hematologic toxic effects and also developed grade 3 anemia 3 months after completing radiotherapy.
- No patients experienced late grade 3 or higher toxic effects.
- When assessing toxic effects of any grade, acute and late gastrointestinal tract toxicities occurred in 76% and 31.6% of patients, respectively; acute and late genitourinary toxicities, all grade 1, occurred in 19% and 24.1% of patients, respectively; and hematologic toxicities occurred in 29.1% and 6.3% of patients, respectively.
- Overall, at 3 years, 79.3% of patients were disease-free and 98% were alive. After a median follow-up of 43 months, 16 patients (20.3%) experienced disease recurrence, four of whom were salvaged and three of whom died.
IN PRACTICE:
“This nonrandomized controlled trial is the first prospective trial, to our knowledge, to show acceptable acute toxic effects of hypofractionated IMRT for cervical cancer in a postoperative concurrent chemoradiotherapy setting,” the authors said, adding that the rate of grade 3 or higher acute toxic effects of 2.5% reported in this study was “substantially lower than our initial hypothesis of less than 15%.”
However , in an accompanying editorial, Mark E. Bernard, MD, of the University of Kentucky College of Medicine, Lexington, highlighted caveats to the study design and raised two core questions: “Should acute toxic effects be the primary endpoint of a single-group, phase 2 study using hypofractionation with fewer cycles of concurrent chemotherapy? Should the primary endpoint rather have been a cancer control endpoint, such as disease-free survival, overall survival, or local control?”
Still, Dr. Bernard wrote, “This trial does help lay the foundation for future pelvic hypofractionated trials with concurrent chemotherapy, especially for gynecological malignant tumors.”
SOURCE:
The research, led by Won Park, MD, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, was published in JAMA Oncology.
LIMITATIONS:
The trial is a single-arm study, with a short follow-up time. In the editorial, Bernard listed several limitations, including the fact that patients received fewer cycles of concurrent chemotherapy than what’s typically given in this population.
DISCLOSURES:
No funding or relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
TOPLINE:
results from the phase 2 POHIM-CCRT trial suggested.
METHODOLOGY:
- To date, no studies have assessed the treatment outcomes and toxic effects of hypofractionated IMRT following radical hysterectomy in patients with cervical cancer undergoing curative radiotherapy.
- The team analyzed outcomes from 79 patients undergoing hypofractionated IMRT for cervical cancer after radical hysterectomy and pelvic lymph node dissection.
- Patients were a median age of 48; 29.5% had stage IB to IIA disease, another 29.5% had stage IIB disease, and 41% had stage III disease. Patients also had at least one of the following criteria following radical hysterectomy and pelvic lymph node dissection: lymph node metastasis (39.7%), parametrial invasion (54.4%), and positive resection margin (5.1%).
- The prescribed dose to the planning target volume was 40 Gy, delivered in 16 fractions to the whole pelvis, with any type of IMRT permitted. Overall, 71 patients also underwent concurrent weekly cisplatin (40 mg/m2 of body surface area for three cycles), and eight received fluorouracil (1000 mg/m2 on days 1-5) with cisplatin (60 mg/m2 for two cycles).
- The primary endpoint was the incidence of acute grade 3 or higher gastrointestinal tract, genitourinary, and hematologic toxic effects during radiotherapy or within 3 months of completing radiotherapy.
TAKEAWAY:
- After radiotherapy, only two patients (2.5%) experienced acute grade 3 or higher toxic effects. One was hospitalized for enterocolitis on the last day of radiotherapy and developed grade 3 anemia 3 months after completing radiotherapy; the other experienced hematologic toxic effects and also developed grade 3 anemia 3 months after completing radiotherapy.
- No patients experienced late grade 3 or higher toxic effects.
- When assessing toxic effects of any grade, acute and late gastrointestinal tract toxicities occurred in 76% and 31.6% of patients, respectively; acute and late genitourinary toxicities, all grade 1, occurred in 19% and 24.1% of patients, respectively; and hematologic toxicities occurred in 29.1% and 6.3% of patients, respectively.
- Overall, at 3 years, 79.3% of patients were disease-free and 98% were alive. After a median follow-up of 43 months, 16 patients (20.3%) experienced disease recurrence, four of whom were salvaged and three of whom died.
IN PRACTICE:
“This nonrandomized controlled trial is the first prospective trial, to our knowledge, to show acceptable acute toxic effects of hypofractionated IMRT for cervical cancer in a postoperative concurrent chemoradiotherapy setting,” the authors said, adding that the rate of grade 3 or higher acute toxic effects of 2.5% reported in this study was “substantially lower than our initial hypothesis of less than 15%.”
However , in an accompanying editorial, Mark E. Bernard, MD, of the University of Kentucky College of Medicine, Lexington, highlighted caveats to the study design and raised two core questions: “Should acute toxic effects be the primary endpoint of a single-group, phase 2 study using hypofractionation with fewer cycles of concurrent chemotherapy? Should the primary endpoint rather have been a cancer control endpoint, such as disease-free survival, overall survival, or local control?”
Still, Dr. Bernard wrote, “This trial does help lay the foundation for future pelvic hypofractionated trials with concurrent chemotherapy, especially for gynecological malignant tumors.”
SOURCE:
The research, led by Won Park, MD, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, was published in JAMA Oncology.
LIMITATIONS:
The trial is a single-arm study, with a short follow-up time. In the editorial, Bernard listed several limitations, including the fact that patients received fewer cycles of concurrent chemotherapy than what’s typically given in this population.
DISCLOSURES:
No funding or relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
TOPLINE:
results from the phase 2 POHIM-CCRT trial suggested.
METHODOLOGY:
- To date, no studies have assessed the treatment outcomes and toxic effects of hypofractionated IMRT following radical hysterectomy in patients with cervical cancer undergoing curative radiotherapy.
- The team analyzed outcomes from 79 patients undergoing hypofractionated IMRT for cervical cancer after radical hysterectomy and pelvic lymph node dissection.
- Patients were a median age of 48; 29.5% had stage IB to IIA disease, another 29.5% had stage IIB disease, and 41% had stage III disease. Patients also had at least one of the following criteria following radical hysterectomy and pelvic lymph node dissection: lymph node metastasis (39.7%), parametrial invasion (54.4%), and positive resection margin (5.1%).
- The prescribed dose to the planning target volume was 40 Gy, delivered in 16 fractions to the whole pelvis, with any type of IMRT permitted. Overall, 71 patients also underwent concurrent weekly cisplatin (40 mg/m2 of body surface area for three cycles), and eight received fluorouracil (1000 mg/m2 on days 1-5) with cisplatin (60 mg/m2 for two cycles).
- The primary endpoint was the incidence of acute grade 3 or higher gastrointestinal tract, genitourinary, and hematologic toxic effects during radiotherapy or within 3 months of completing radiotherapy.
TAKEAWAY:
- After radiotherapy, only two patients (2.5%) experienced acute grade 3 or higher toxic effects. One was hospitalized for enterocolitis on the last day of radiotherapy and developed grade 3 anemia 3 months after completing radiotherapy; the other experienced hematologic toxic effects and also developed grade 3 anemia 3 months after completing radiotherapy.
- No patients experienced late grade 3 or higher toxic effects.
- When assessing toxic effects of any grade, acute and late gastrointestinal tract toxicities occurred in 76% and 31.6% of patients, respectively; acute and late genitourinary toxicities, all grade 1, occurred in 19% and 24.1% of patients, respectively; and hematologic toxicities occurred in 29.1% and 6.3% of patients, respectively.
- Overall, at 3 years, 79.3% of patients were disease-free and 98% were alive. After a median follow-up of 43 months, 16 patients (20.3%) experienced disease recurrence, four of whom were salvaged and three of whom died.
IN PRACTICE:
“This nonrandomized controlled trial is the first prospective trial, to our knowledge, to show acceptable acute toxic effects of hypofractionated IMRT for cervical cancer in a postoperative concurrent chemoradiotherapy setting,” the authors said, adding that the rate of grade 3 or higher acute toxic effects of 2.5% reported in this study was “substantially lower than our initial hypothesis of less than 15%.”
However , in an accompanying editorial, Mark E. Bernard, MD, of the University of Kentucky College of Medicine, Lexington, highlighted caveats to the study design and raised two core questions: “Should acute toxic effects be the primary endpoint of a single-group, phase 2 study using hypofractionation with fewer cycles of concurrent chemotherapy? Should the primary endpoint rather have been a cancer control endpoint, such as disease-free survival, overall survival, or local control?”
Still, Dr. Bernard wrote, “This trial does help lay the foundation for future pelvic hypofractionated trials with concurrent chemotherapy, especially for gynecological malignant tumors.”
SOURCE:
The research, led by Won Park, MD, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, was published in JAMA Oncology.
LIMITATIONS:
The trial is a single-arm study, with a short follow-up time. In the editorial, Bernard listed several limitations, including the fact that patients received fewer cycles of concurrent chemotherapy than what’s typically given in this population.
DISCLOSURES:
No funding or relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
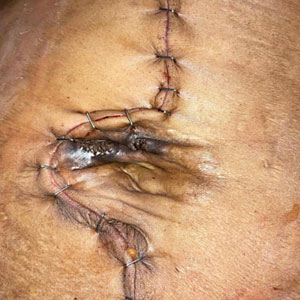
Survey Spotlights Identification of Dermatologic Adverse Events From Cancer Therapies
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology annual meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Dr. Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
Dr. LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures. Dr. Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.
A version of this article first appeared on Medscape.com.
FROM AAD 2024
New mRNA Vaccines in Development for Cancer and Infections
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Martina Prelog, MD, a pediatric and adolescent medicine specialist at the University Hospital of Würzburg in Germany, reported on the principles, research status, and perspectives for these vaccines at the 25th Travel and Health Forum of the Center for Travel Medicine in Berlin.
To understand the future, the immunologist first examined the past. “The induction of cellular and humoral immune responses by externally injected mRNA was discovered in the 1990s,” she said.
Instability Challenge
Significant hurdles in mRNA vaccinations included the instability of mRNA and the immune system’s ability to identify foreign mRNA as a threat and destroy mRNA fragments. “The breakthrough toward vaccination came through Dr. Katalin Karikó, who, along with Dr. Drew Weissman, both of the University of Pennsylvania School of Medicine, discovered in 2005 that modifications of mRNA (replacing the nucleoside uridine with pseudouridine) enable better stability of mRNA, reduced immunogenicity, and higher translational capacity at the ribosomes,” said Dr. Prelog.
With this discovery, the two researchers paved the way for the development of mRNA vaccines against COVID-19 and other diseases. They were awarded the Nobel Prize in medicine for their discovery last year.
Improved Scalability
“Since 2009, mRNA vaccines have been studied as a treatment option for cancer,” said Dr. Prelog. “Since 2012, they have been studied for the influenza virus and respiratory syncytial virus [RSV].” Consequently, several mRNA vaccines are currently in development or in approval studies. “The mRNA technology offers the advantage of quickly and flexibly responding to new variants of pathogens and the ability to scale up production when there is high demand for a particular vaccine.”
Different forms and designations of mRNA vaccines are used, depending on the application and desired effect, said Dr. Prelog.
In nucleoside-modified mRNA vaccines, modifications in the mRNA sequence enable the mRNA to remain in the body longer and to induce protein synthesis more effectively.
Lipid nanoparticle (LNP)–encapsulated mRNA vaccines protect the coding mRNA sequences against degradation by the body’s enzymes and facilitate the uptake of mRNA into cells, where it then triggers the production of the desired protein. In addition, LNPs are involved in cell stimulation and support the self-adjuvant effect of mRNA vaccines, thus eliminating the need for adjuvants.
Self-amplifying mRNA vaccines include a special mRNA that replicates itself in the cell and contains a sequence for RNA replicase, in addition to the coding sequence for the protein. This composition enables increased production of the target protein without the need for a high amount of external mRNA administration. Such vaccines could trigger a longer and stronger immune response because the immune system has more time to interact with the protein.
Cancer Immunotherapy
Dr. Prelog also discussed personalized vaccines for cancer immunotherapy. Personalized mRNA vaccines are tailored to the patient’s genetic characteristics and antigens. They could be used in cancer immunotherapy to activate the immune system selectively against tumor cells.
Multivalent mRNA vaccines contain mRNA that codes for multiple antigens rather than just one protein to generate an immune response. These vaccines could be particularly useful in fighting pathogens with variable or changing surface structures or in eliciting protection against multiple pathogens simultaneously.
The technology of mRNA-encoded antibodies involves introducing mRNA into the cell, which creates light and heavy chains of antibodies. This step leads to the formation of antibodies targeted against toxins (eg, diphtheria and tetanus), animal venoms, infectious agents, or tumor cells.
Genetic Engineering
Dr. Prelog also reviewed genetic engineering techniques. In regenerative therapy or protein replacement therapy, skin fibroblasts or other cells are transfected with mRNA to enable conversion into induced pluripotent stem cells. This approach avoids the risk for DNA integration into the genome and associated mutation risks.
Another approach is making post-transcriptional modifications through RNA interference. For example, RNA structures can be used to inhibit the translation of disease-causing proteins. This technique is currently being tested against HIV and tumors such as melanoma.
In addition, mRNA technologies can be combined with CRISPR/Cas9 technology (“gene scissors”) to influence the creation of gene products even more precisely. The advantage of this technique is that mRNA is only transiently expressed, thus preventing unwanted side effects. Furthermore, mRNA is translated directly in the cytoplasm, leading to a faster initiation of gene editing.
Of the numerous ongoing clinical mRNA vaccine studies, around 70% focus on infections, about 12% on cancer, and the rest on autoimmune diseases and neurodegenerative disorders, said Dr. Prelog.
Research in Infections
Research in the fields of infectious diseases and oncology is the most advanced: mRNA vaccines against influenza and RSV are already in advanced clinical trials, Dr. Prelog told this news organization.
“Conventional influenza vaccines contain immunogenic surface molecules against hemagglutinin and neuraminidase in various combinations of influenza strains A and B and are produced in egg or cell cultures,” she said. “This is a time-consuming manufacturing process that takes months and, particularly with the egg-based process, bears the risk of changing the vaccine strain.”
“Additionally, influenza viruses undergo antigenic shift and drift through recombination, thus requiring annual adjustments to the vaccines. Thus, these influenza vaccines often lose accuracy in targeting circulating seasonal influenza strains.”
Several mRNA vaccines being tested contain not only coding sequences against hemagglutinin and neuraminidase but also for structural proteins of influenza viruses. “These are more conserved and mutate less easily, meaning they could serve as the basis for universal pandemic influenza vaccines,” said Dr. Prelog.
An advantage of mRNA vaccines, she added, is the strong cellular immune response that they elicit. This response is intended to provide additional protection alongside specific antibodies. An mRNA vaccine with coding sequences for the pre-fusion protein of RSV is in phase 3 trials for approval for vaccination in patients aged 60 years and older. It shows high effectiveness even in older patients and those with comorbidities.
Elaborate Purification Process
Bacterial origin plasmid DNA is used to produce mRNA vaccines. The mRNA vaccines for COVID-19 raised concerns that production-related DNA residues could pose a safety risk and cause autoimmune diseases.
These vaccines “typically undergo a very elaborate purification process,” said Dr. Prelog. “This involves enzymatic digestion with DNase to fragment and deplete plasmid DNA, followed by purification using chromatography columns, so that no safety-relevant DNA fragments should remain afterward.”
Thus, the Paul-Ehrlich-Institut also pointed out the very small, fragmented plasmid DNA residues of bacterial origin in mRNA COVID-19 vaccines pose no risk, unlike residual DNA from animal cell culture might pose in other vaccines.
Prevention and Therapy
In addition to the numerous advantages of mRNA vaccines (such as rapid adaptability to new or mutated pathogens, scalability, rapid production capability, self-adjuvant effect, strong induction of cellular immune responses, and safety), there are also challenges in RNA technology as a preventive and therapeutic measure, according to Dr. Prelog.
“Stability and storability, as well as the costs of new vaccine developments, play a role, as do the long-term effects regarding the persistence of antibody and cellular responses,” she said. The COVID-19 mRNA vaccines, for example, showed a well-maintained cellular immune response despite a tendency toward a rapid decline in humoral immune response.
“The experience with COVID-19 mRNA vaccines and the new vaccine developments based on mRNA technology give hope for an efficient and safe preventive and therapeutic use, particularly in the fields of infectious diseases and oncology,” Dr. Prelog concluded.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Can a Risk Score Predict Kidney Injury After Cisplatin?
Cisplatin is a preferred treatment for a wide range of cancers, including breast, head and neck, lung, ovary, and more. However, its side effects — particularly nephrotoxicity — can be severe. Kidney injury on cisplatin is associated with higher mortality and can jeopardize a patient’s eligibility for other therapies.
Now, in a large study using data from six US cancer centers, researchers have developed a risk algorithm to predict acute kidney injury (AKI) after cisplatin administration.
A risk prediction calculator based on the algorithm is available online for patients and providers to determine an individual patient›s risk for kidney injury from cisplatin using readily available clinical data.
Other risk scores and risk prediction models have been developed to help clinicians assess in advance whether a patient might develop AKI after receiving cisplatin, so that more careful monitoring, dose adjustments, or an alternative treatment, if available, might be considered.
However, previous models were limited by factors such as small sample sizes, lack of external validation, older data, and liberal definitions of AKI, said Shruti Gupta, MD, MPH, director of onco-nephrology at Brigham and Women’s Hospital (BWH) and Dana-Farber Cancer Institute, and David E. Leaf, MD, MMSc, director of clinical and translational research in AKI, Division of Renal Medicine, BWH, Boston.
Dr. Gupta and Dr. Leaf believe their risk score for predicting severe AKI after intravenous (IV) cisplatin, published online in The BMJ, is “more accurate and generalizable than prior models for several reasons,” they told this news organization in a joint email.
“First, we externally validated our findings across cancer centers other than the one where it was developed,” they said. “Second, we focused on moderate to severe kidney injury, the most clinically relevant form of kidney damage, whereas prior models examined more mild forms of kidney injury. Third, we collected data on nearly 25,000 patients receiving their first dose of IV cisplatin, which is larger than all previous studies combined.”
‘Herculean Effort’
“We conceived of this study back in 2018, contacted collaborators at each participating cancer center, and had numerous meetings to try to gather granular data on patients treated with their first dose of intravenous (IV) cisplatin,” Dr. Gupta and Dr. Leaf explained. They also incorporated patient feedback from focus groups and surveys.
“This was truly a Herculean effort that involved physicians, programmers, research coordinators, and patients,” they said.
The multicenter study included 24,717 patients — 11,766 in the derivation cohort and 12,951 in the validation cohort. Overall, the median age was about 60 years, about 58% were men, and about 78% were White.
The primary outcome was cisplatin-induced AKI (CP-AKI), defined as a twofold or greater increase in serum creatinine or kidney replacement therapy within 14 days of a first dose of IV cisplatin.
Their simple risk score consisting of nine covariates — age, hypertension, type 2 diabetes, hemoglobin level, white blood cell count, platelet count, serum albumin level, serum magnesium level, and cisplatin dose — predicted a higher risk for CP-AKI in both cohorts.
Notably, adding serum creatinine to the model did not change the area under the curve, and therefore, serum creatinine, though also an independent risk factor for CP-AKI, was not included in the score.
Patients in the highest risk category had 24-fold higher odds of CP-AKI in the derivation cohort and close to 18-fold higher odds in the validation cohort than those in the lowest risk category.
The primary model had a C statistic of 0.75 (95% CI, 0.73-0.76) and showed better discrimination for CP-AKI than previously published models, for which the C statistics ranged from 0.60 to 0.68. The first author of a paper on an earlier model, Shveta Motwani, MD, MMSc, of BWH and Dana-Farber Cancer Institute in Boston, is also a coauthor of the new study.
Greater severity of CP-AKI was associated with shorter 90-day survival (adjusted hazard ratio, 4.63; 95% CI, 3.56-6.02) for stage III CP-AKI vs no CP-AKI.
‘Definitive Work’
Joel M. Topf, MD, a nephrologist with expertise in chronic kidney disease in Detroit, who wasn’t involved in the development of the risk score, called the study “a definitive work on an important concept in oncology and nephrology.”
“While this is not the first attempt to devise a risk score, it is by far the biggest,” he told this news organization. Furthermore, the authors “used a diverse population, recruiting patients with a variety of cancers (previous attempts had often used a homogenous diagnosis, putting into question how generalizable the results were) from six different cancer centers.”
In addition, he said, “The authors did not restrict patients with chronic kidney disease or other significant comorbidities and used the geographic diversity to produce a cohort that has an age, gender, racial, and ethnic distribution, which is more representative of the US than previous, single-center attempts to risk score patients.”
An earlier model used the Kidney Disease: Improving Global Outcomes (KDIGO) consensus definition of AKI of an increase in serum creatinine of 0.3 mg/dL, he noted. “While a sensitive definition of AKI, it captures mild, hemodynamic increases in creatinine of questionable significance,” he said.
By contrast, the new score uses KDIGO stage II and above to define AKI. “This is a better choice, as we do not want to dissuade patients and doctors from choosing chemotherapy due to a fear of insignificant kidney damage,” he said.
All that said, Dr. Topf noted that neither the current score nor the earlier model included serum creatinine. “This is curious to me and may represent the small number of patients with representative elevated creatinine in the derivation cohort (only 1.3% with an estimated glomerular filtration rate [eGFR] < 45).”
“Since the cohort is made up of people who received cis-platinum, the low prevalence of eGFRs < 45 may be due to physicians steering away from cis-platinum in this group,” he suggested. “It would be unfortunate if this risk score gave an unintentional ‘green light’ to these patients, exposing them to predictable harm.”
‘Certainly Useful’
Anushree Shirali, MD, an associate professor in the Section of Nephrology and consulting physician, Yale Onco-Nephrology, Yale School of Medicine, in New Haven, Connecticut, said that having a prediction score for which patients are more likely to develop AKI after a single dose of cisplatin would be helpful for oncologists, as well as nephrologists.
As a nephrologist, Dr. Shirali mostly sees patients who already have AKI, she told this news organization. But there are circumstances in which the tool could still be helpful.
“Let’s say someone has abnormal kidney function at baseline — ie, creatinine is higher than the normal range — and they were on dialysis 5 years ago for something else, and now, they have cancer and may be given cisplatin. They worry about their chances of getting AKI and needing dialysis again,” she said. “That’s just one scenario in which I might be asked to answer that question and the tool would certainly be useful.”
Other scenarios could include someone who has just one kidney because they donated a kidney for transplant years ago, and now, they have a malignancy and wonder what their actual risk is of getting kidney issues on cisplatin.
Oncologists could use the tool to determine whether a patient should be treated with cisplatin, or if they’re at high risk, whether an alternative that’s not nephrotoxic might be used. By contrast, “if somebody’s low risk and an oncologist thinks cisplatin is the best agent they have, then they might want to go ahead and use it,” Dr. Shirali said.
Future research could take into consideration that CP-AKI is dose dependent, she suggested, because a prediction score that included the number of cisplatin doses could be even more helpful to determine risk. And, even though the derivation and validation cohorts for the new tool are representative of the US population, additional research should also include more racial/ethnic diversity, she said.
Dr. Gupta and Dr. Leaf hope their tool “will be utilized immediately by patients and providers to help predict an individual’s risk of cisplatin-associated kidney damage. It is easy to use, available for free online, and incorporates readily available clinical variables.”
If a patient is at high risk, the clinical team can consider preventive measures such as administering more IV fluids before receiving cisplatin or monitoring kidney function more closely afterward, they suggested.
Dr. Gupta reported research support from the National Institutes of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases. She also reported research funding from BTG International, GE HealthCare, and AstraZeneca outside the submitted work. She is a member of GlaxoSmithKline’s Global Anemia Council, a consultant for Secretome and Proletariat Therapeutics, and founder and president emeritus of the American Society of Onconephrology (unpaid). Dr. Leaf is supported by NIH grants, reported research support from BioPorto, BTG International, and Metro International Biotech, and has served as a consultant. Dr. Topf reported an ownership stake in a few DaVita-run dialysis clinics. He also runs a vascular access center and has participated in advisory boards with Cara Therapeutics, Vifor, Astra Zeneca, Bayer, Renibus Therapeutics, Travere Therapeutics, and GlaxoSmithKline. He is president of NephJC, a nonprofit educational organization with no industry support. Dr. Shirali declared no competing interests.
A version of this article appeared on Medscape.com.
Cisplatin is a preferred treatment for a wide range of cancers, including breast, head and neck, lung, ovary, and more. However, its side effects — particularly nephrotoxicity — can be severe. Kidney injury on cisplatin is associated with higher mortality and can jeopardize a patient’s eligibility for other therapies.
Now, in a large study using data from six US cancer centers, researchers have developed a risk algorithm to predict acute kidney injury (AKI) after cisplatin administration.
A risk prediction calculator based on the algorithm is available online for patients and providers to determine an individual patient›s risk for kidney injury from cisplatin using readily available clinical data.
Other risk scores and risk prediction models have been developed to help clinicians assess in advance whether a patient might develop AKI after receiving cisplatin, so that more careful monitoring, dose adjustments, or an alternative treatment, if available, might be considered.
However, previous models were limited by factors such as small sample sizes, lack of external validation, older data, and liberal definitions of AKI, said Shruti Gupta, MD, MPH, director of onco-nephrology at Brigham and Women’s Hospital (BWH) and Dana-Farber Cancer Institute, and David E. Leaf, MD, MMSc, director of clinical and translational research in AKI, Division of Renal Medicine, BWH, Boston.
Dr. Gupta and Dr. Leaf believe their risk score for predicting severe AKI after intravenous (IV) cisplatin, published online in The BMJ, is “more accurate and generalizable than prior models for several reasons,” they told this news organization in a joint email.
“First, we externally validated our findings across cancer centers other than the one where it was developed,” they said. “Second, we focused on moderate to severe kidney injury, the most clinically relevant form of kidney damage, whereas prior models examined more mild forms of kidney injury. Third, we collected data on nearly 25,000 patients receiving their first dose of IV cisplatin, which is larger than all previous studies combined.”
‘Herculean Effort’
“We conceived of this study back in 2018, contacted collaborators at each participating cancer center, and had numerous meetings to try to gather granular data on patients treated with their first dose of intravenous (IV) cisplatin,” Dr. Gupta and Dr. Leaf explained. They also incorporated patient feedback from focus groups and surveys.
“This was truly a Herculean effort that involved physicians, programmers, research coordinators, and patients,” they said.
The multicenter study included 24,717 patients — 11,766 in the derivation cohort and 12,951 in the validation cohort. Overall, the median age was about 60 years, about 58% were men, and about 78% were White.
The primary outcome was cisplatin-induced AKI (CP-AKI), defined as a twofold or greater increase in serum creatinine or kidney replacement therapy within 14 days of a first dose of IV cisplatin.
Their simple risk score consisting of nine covariates — age, hypertension, type 2 diabetes, hemoglobin level, white blood cell count, platelet count, serum albumin level, serum magnesium level, and cisplatin dose — predicted a higher risk for CP-AKI in both cohorts.
Notably, adding serum creatinine to the model did not change the area under the curve, and therefore, serum creatinine, though also an independent risk factor for CP-AKI, was not included in the score.
Patients in the highest risk category had 24-fold higher odds of CP-AKI in the derivation cohort and close to 18-fold higher odds in the validation cohort than those in the lowest risk category.
The primary model had a C statistic of 0.75 (95% CI, 0.73-0.76) and showed better discrimination for CP-AKI than previously published models, for which the C statistics ranged from 0.60 to 0.68. The first author of a paper on an earlier model, Shveta Motwani, MD, MMSc, of BWH and Dana-Farber Cancer Institute in Boston, is also a coauthor of the new study.
Greater severity of CP-AKI was associated with shorter 90-day survival (adjusted hazard ratio, 4.63; 95% CI, 3.56-6.02) for stage III CP-AKI vs no CP-AKI.
‘Definitive Work’
Joel M. Topf, MD, a nephrologist with expertise in chronic kidney disease in Detroit, who wasn’t involved in the development of the risk score, called the study “a definitive work on an important concept in oncology and nephrology.”
“While this is not the first attempt to devise a risk score, it is by far the biggest,” he told this news organization. Furthermore, the authors “used a diverse population, recruiting patients with a variety of cancers (previous attempts had often used a homogenous diagnosis, putting into question how generalizable the results were) from six different cancer centers.”
In addition, he said, “The authors did not restrict patients with chronic kidney disease or other significant comorbidities and used the geographic diversity to produce a cohort that has an age, gender, racial, and ethnic distribution, which is more representative of the US than previous, single-center attempts to risk score patients.”
An earlier model used the Kidney Disease: Improving Global Outcomes (KDIGO) consensus definition of AKI of an increase in serum creatinine of 0.3 mg/dL, he noted. “While a sensitive definition of AKI, it captures mild, hemodynamic increases in creatinine of questionable significance,” he said.
By contrast, the new score uses KDIGO stage II and above to define AKI. “This is a better choice, as we do not want to dissuade patients and doctors from choosing chemotherapy due to a fear of insignificant kidney damage,” he said.
All that said, Dr. Topf noted that neither the current score nor the earlier model included serum creatinine. “This is curious to me and may represent the small number of patients with representative elevated creatinine in the derivation cohort (only 1.3% with an estimated glomerular filtration rate [eGFR] < 45).”
“Since the cohort is made up of people who received cis-platinum, the low prevalence of eGFRs < 45 may be due to physicians steering away from cis-platinum in this group,” he suggested. “It would be unfortunate if this risk score gave an unintentional ‘green light’ to these patients, exposing them to predictable harm.”
‘Certainly Useful’
Anushree Shirali, MD, an associate professor in the Section of Nephrology and consulting physician, Yale Onco-Nephrology, Yale School of Medicine, in New Haven, Connecticut, said that having a prediction score for which patients are more likely to develop AKI after a single dose of cisplatin would be helpful for oncologists, as well as nephrologists.
As a nephrologist, Dr. Shirali mostly sees patients who already have AKI, she told this news organization. But there are circumstances in which the tool could still be helpful.
“Let’s say someone has abnormal kidney function at baseline — ie, creatinine is higher than the normal range — and they were on dialysis 5 years ago for something else, and now, they have cancer and may be given cisplatin. They worry about their chances of getting AKI and needing dialysis again,” she said. “That’s just one scenario in which I might be asked to answer that question and the tool would certainly be useful.”
Other scenarios could include someone who has just one kidney because they donated a kidney for transplant years ago, and now, they have a malignancy and wonder what their actual risk is of getting kidney issues on cisplatin.
Oncologists could use the tool to determine whether a patient should be treated with cisplatin, or if they’re at high risk, whether an alternative that’s not nephrotoxic might be used. By contrast, “if somebody’s low risk and an oncologist thinks cisplatin is the best agent they have, then they might want to go ahead and use it,” Dr. Shirali said.
Future research could take into consideration that CP-AKI is dose dependent, she suggested, because a prediction score that included the number of cisplatin doses could be even more helpful to determine risk. And, even though the derivation and validation cohorts for the new tool are representative of the US population, additional research should also include more racial/ethnic diversity, she said.
Dr. Gupta and Dr. Leaf hope their tool “will be utilized immediately by patients and providers to help predict an individual’s risk of cisplatin-associated kidney damage. It is easy to use, available for free online, and incorporates readily available clinical variables.”
If a patient is at high risk, the clinical team can consider preventive measures such as administering more IV fluids before receiving cisplatin or monitoring kidney function more closely afterward, they suggested.
Dr. Gupta reported research support from the National Institutes of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases. She also reported research funding from BTG International, GE HealthCare, and AstraZeneca outside the submitted work. She is a member of GlaxoSmithKline’s Global Anemia Council, a consultant for Secretome and Proletariat Therapeutics, and founder and president emeritus of the American Society of Onconephrology (unpaid). Dr. Leaf is supported by NIH grants, reported research support from BioPorto, BTG International, and Metro International Biotech, and has served as a consultant. Dr. Topf reported an ownership stake in a few DaVita-run dialysis clinics. He also runs a vascular access center and has participated in advisory boards with Cara Therapeutics, Vifor, Astra Zeneca, Bayer, Renibus Therapeutics, Travere Therapeutics, and GlaxoSmithKline. He is president of NephJC, a nonprofit educational organization with no industry support. Dr. Shirali declared no competing interests.
A version of this article appeared on Medscape.com.
Cisplatin is a preferred treatment for a wide range of cancers, including breast, head and neck, lung, ovary, and more. However, its side effects — particularly nephrotoxicity — can be severe. Kidney injury on cisplatin is associated with higher mortality and can jeopardize a patient’s eligibility for other therapies.
Now, in a large study using data from six US cancer centers, researchers have developed a risk algorithm to predict acute kidney injury (AKI) after cisplatin administration.
A risk prediction calculator based on the algorithm is available online for patients and providers to determine an individual patient›s risk for kidney injury from cisplatin using readily available clinical data.
Other risk scores and risk prediction models have been developed to help clinicians assess in advance whether a patient might develop AKI after receiving cisplatin, so that more careful monitoring, dose adjustments, or an alternative treatment, if available, might be considered.
However, previous models were limited by factors such as small sample sizes, lack of external validation, older data, and liberal definitions of AKI, said Shruti Gupta, MD, MPH, director of onco-nephrology at Brigham and Women’s Hospital (BWH) and Dana-Farber Cancer Institute, and David E. Leaf, MD, MMSc, director of clinical and translational research in AKI, Division of Renal Medicine, BWH, Boston.
Dr. Gupta and Dr. Leaf believe their risk score for predicting severe AKI after intravenous (IV) cisplatin, published online in The BMJ, is “more accurate and generalizable than prior models for several reasons,” they told this news organization in a joint email.
“First, we externally validated our findings across cancer centers other than the one where it was developed,” they said. “Second, we focused on moderate to severe kidney injury, the most clinically relevant form of kidney damage, whereas prior models examined more mild forms of kidney injury. Third, we collected data on nearly 25,000 patients receiving their first dose of IV cisplatin, which is larger than all previous studies combined.”
‘Herculean Effort’
“We conceived of this study back in 2018, contacted collaborators at each participating cancer center, and had numerous meetings to try to gather granular data on patients treated with their first dose of intravenous (IV) cisplatin,” Dr. Gupta and Dr. Leaf explained. They also incorporated patient feedback from focus groups and surveys.
“This was truly a Herculean effort that involved physicians, programmers, research coordinators, and patients,” they said.
The multicenter study included 24,717 patients — 11,766 in the derivation cohort and 12,951 in the validation cohort. Overall, the median age was about 60 years, about 58% were men, and about 78% were White.
The primary outcome was cisplatin-induced AKI (CP-AKI), defined as a twofold or greater increase in serum creatinine or kidney replacement therapy within 14 days of a first dose of IV cisplatin.
Their simple risk score consisting of nine covariates — age, hypertension, type 2 diabetes, hemoglobin level, white blood cell count, platelet count, serum albumin level, serum magnesium level, and cisplatin dose — predicted a higher risk for CP-AKI in both cohorts.
Notably, adding serum creatinine to the model did not change the area under the curve, and therefore, serum creatinine, though also an independent risk factor for CP-AKI, was not included in the score.
Patients in the highest risk category had 24-fold higher odds of CP-AKI in the derivation cohort and close to 18-fold higher odds in the validation cohort than those in the lowest risk category.
The primary model had a C statistic of 0.75 (95% CI, 0.73-0.76) and showed better discrimination for CP-AKI than previously published models, for which the C statistics ranged from 0.60 to 0.68. The first author of a paper on an earlier model, Shveta Motwani, MD, MMSc, of BWH and Dana-Farber Cancer Institute in Boston, is also a coauthor of the new study.
Greater severity of CP-AKI was associated with shorter 90-day survival (adjusted hazard ratio, 4.63; 95% CI, 3.56-6.02) for stage III CP-AKI vs no CP-AKI.
‘Definitive Work’
Joel M. Topf, MD, a nephrologist with expertise in chronic kidney disease in Detroit, who wasn’t involved in the development of the risk score, called the study “a definitive work on an important concept in oncology and nephrology.”
“While this is not the first attempt to devise a risk score, it is by far the biggest,” he told this news organization. Furthermore, the authors “used a diverse population, recruiting patients with a variety of cancers (previous attempts had often used a homogenous diagnosis, putting into question how generalizable the results were) from six different cancer centers.”
In addition, he said, “The authors did not restrict patients with chronic kidney disease or other significant comorbidities and used the geographic diversity to produce a cohort that has an age, gender, racial, and ethnic distribution, which is more representative of the US than previous, single-center attempts to risk score patients.”
An earlier model used the Kidney Disease: Improving Global Outcomes (KDIGO) consensus definition of AKI of an increase in serum creatinine of 0.3 mg/dL, he noted. “While a sensitive definition of AKI, it captures mild, hemodynamic increases in creatinine of questionable significance,” he said.
By contrast, the new score uses KDIGO stage II and above to define AKI. “This is a better choice, as we do not want to dissuade patients and doctors from choosing chemotherapy due to a fear of insignificant kidney damage,” he said.
All that said, Dr. Topf noted that neither the current score nor the earlier model included serum creatinine. “This is curious to me and may represent the small number of patients with representative elevated creatinine in the derivation cohort (only 1.3% with an estimated glomerular filtration rate [eGFR] < 45).”
“Since the cohort is made up of people who received cis-platinum, the low prevalence of eGFRs < 45 may be due to physicians steering away from cis-platinum in this group,” he suggested. “It would be unfortunate if this risk score gave an unintentional ‘green light’ to these patients, exposing them to predictable harm.”
‘Certainly Useful’
Anushree Shirali, MD, an associate professor in the Section of Nephrology and consulting physician, Yale Onco-Nephrology, Yale School of Medicine, in New Haven, Connecticut, said that having a prediction score for which patients are more likely to develop AKI after a single dose of cisplatin would be helpful for oncologists, as well as nephrologists.
As a nephrologist, Dr. Shirali mostly sees patients who already have AKI, she told this news organization. But there are circumstances in which the tool could still be helpful.
“Let’s say someone has abnormal kidney function at baseline — ie, creatinine is higher than the normal range — and they were on dialysis 5 years ago for something else, and now, they have cancer and may be given cisplatin. They worry about their chances of getting AKI and needing dialysis again,” she said. “That’s just one scenario in which I might be asked to answer that question and the tool would certainly be useful.”
Other scenarios could include someone who has just one kidney because they donated a kidney for transplant years ago, and now, they have a malignancy and wonder what their actual risk is of getting kidney issues on cisplatin.
Oncologists could use the tool to determine whether a patient should be treated with cisplatin, or if they’re at high risk, whether an alternative that’s not nephrotoxic might be used. By contrast, “if somebody’s low risk and an oncologist thinks cisplatin is the best agent they have, then they might want to go ahead and use it,” Dr. Shirali said.
Future research could take into consideration that CP-AKI is dose dependent, she suggested, because a prediction score that included the number of cisplatin doses could be even more helpful to determine risk. And, even though the derivation and validation cohorts for the new tool are representative of the US population, additional research should also include more racial/ethnic diversity, she said.
Dr. Gupta and Dr. Leaf hope their tool “will be utilized immediately by patients and providers to help predict an individual’s risk of cisplatin-associated kidney damage. It is easy to use, available for free online, and incorporates readily available clinical variables.”
If a patient is at high risk, the clinical team can consider preventive measures such as administering more IV fluids before receiving cisplatin or monitoring kidney function more closely afterward, they suggested.
Dr. Gupta reported research support from the National Institutes of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases. She also reported research funding from BTG International, GE HealthCare, and AstraZeneca outside the submitted work. She is a member of GlaxoSmithKline’s Global Anemia Council, a consultant for Secretome and Proletariat Therapeutics, and founder and president emeritus of the American Society of Onconephrology (unpaid). Dr. Leaf is supported by NIH grants, reported research support from BioPorto, BTG International, and Metro International Biotech, and has served as a consultant. Dr. Topf reported an ownership stake in a few DaVita-run dialysis clinics. He also runs a vascular access center and has participated in advisory boards with Cara Therapeutics, Vifor, Astra Zeneca, Bayer, Renibus Therapeutics, Travere Therapeutics, and GlaxoSmithKline. He is president of NephJC, a nonprofit educational organization with no industry support. Dr. Shirali declared no competing interests.
A version of this article appeared on Medscape.com.
FROM THE BMJ
Growing Periumbilical Plaque: A Case of Perforating Calcific Elastosis
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.
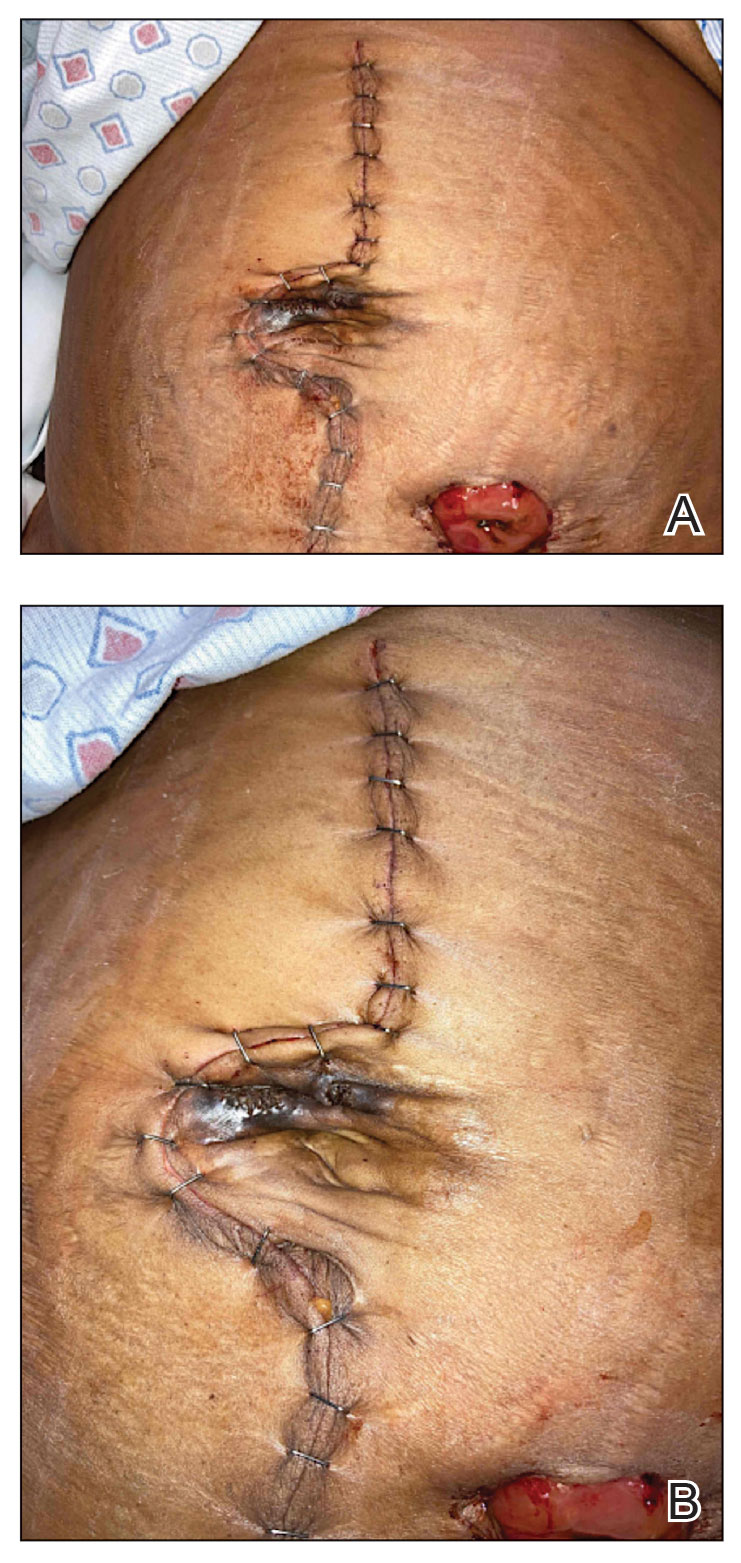
A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.
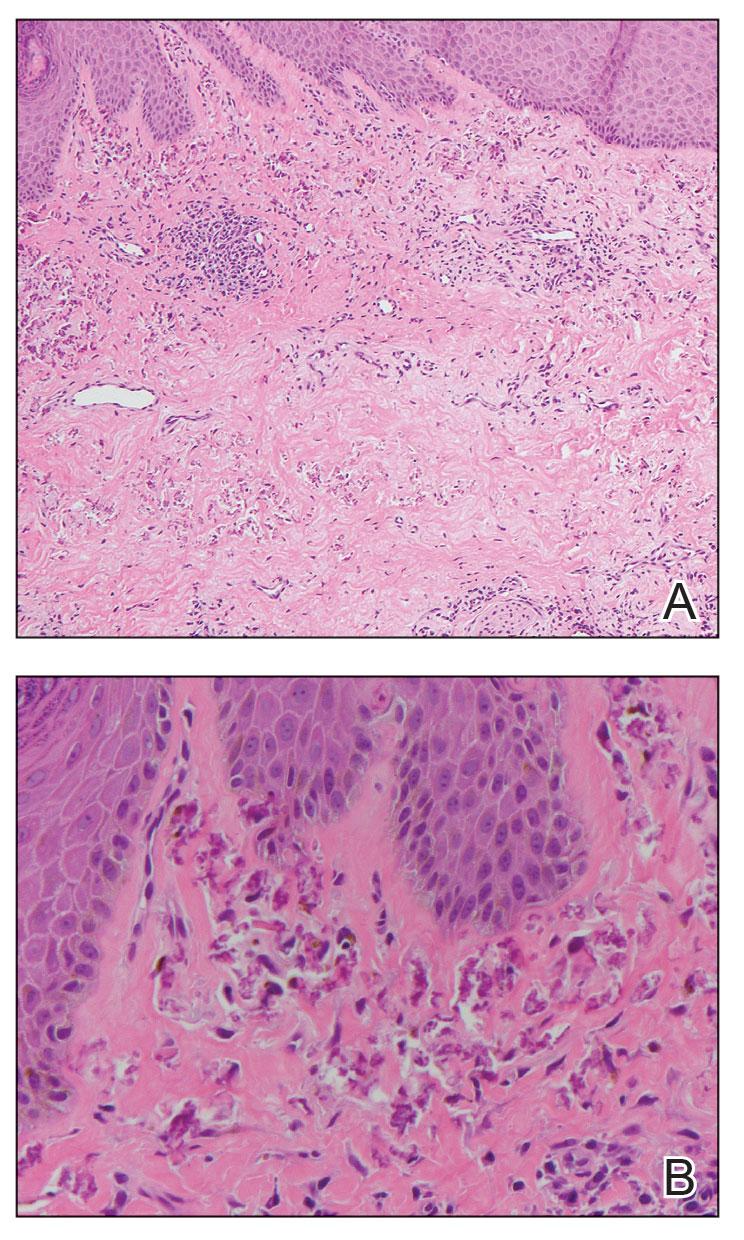
Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6
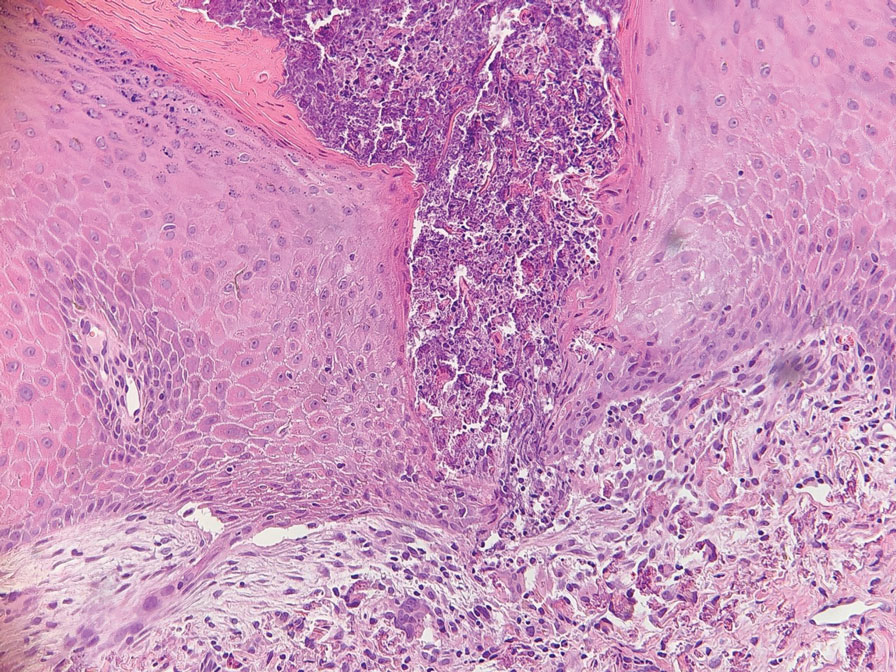
The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.

A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.

Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6

The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.

A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.

Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6

The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
PRACTICE POINTS
- Perforating calcific elastosis (PCE) is a rare, localized, acquired variant of the inherited connective tissue disorder pseudoxanthoma elasticum (PXE).
- Histopathologic findings are identical for PCE and PXE, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history.
- Although there are no definitive treatments, most cases of PCE resolve spontaneously.
- Dermatologists should be aware of the importance of clinically differentiating PCE from PXE to prevent extensive workup, which can lead to unnecessary testing and increased morbidity in patients.
Study Evaluates CVD, Mortality Risks In Patients With Prurigo Nodularis
TOPLINE:
, particularly among women and White patients.
METHODOLOGY:
- Studies have shown increased risks for cardiovascular diseases in patients with PN, but limited sample sizes have hindered further subgroup analysis. Given PN’s pronounced sex and ethnicity skew, it is important to examine underrepresented groups to accurately assess their cardiovascular risk.
- In this propensity-score matched analysis, researchers identified 64,801 patients (59.44% women) with PN using electronic health reports from the Global Collaborative Network of TriNetX and matched to individuals without PN.
- Researchers calculated risks for 15 cardiovascular endpoints and all-cause mortality within 10 years of diagnosis. Major adverse cardiovascular events (MACE) included acute cerebral and myocardial infarction (MI), heart failure, ventricular arrhythmia, and sudden cardiac death.
TAKEAWAY:
- Patients with PN showed a higher risk for death (hazard ratio [HR], 1.1243) and MACE (HR, 1.117) (P < .0001 for both).
- PN was also associated with a higher risk for heart failure (HR, 1.062), thrombotic venous disease (HR, 1.26), angina pectoris (HR, 1.096), and peripheral arterial diseases (HR, 1.082) (P < .0001 for all) and for acute MI (HR, 1.11; P = .0015) and valve disorders (HR, 1.08; P = .0018).
- White patients with PN had a significantly increased risk for MACE, death, heart failure, cardiac arrest, vascular diseases, and acute MI, but this was not observed in people of color.
- Women exhibited a higher risk for MACE, heart failure, peripheral artery disease, acute MI, conduction disease, and valve disorders, while men did not have an increased risk for major or acute cardiovascular events. Both men and women had a higher risk for death, chronic ischemic heart disease, and venous disease.
IN PRACTICE:
“Although no novel PN-specific treatment rationale can be derived from the presented data, the potential risk of subsequent cardiovascular disease should be considered in the care of patients with PN, which includes screening and optimal management of other additional cardiovascular risk factors,” the authors wrote.
LIMITATIONS:
Retrospective observational design introduced inherent biases. Misdiagnosis or false coding in electronic health records could affect the data accuracy and ethnicity-specific analyses.
SOURCE:
This work, led by Henning Olbrich, from the Department of Dermatology, University of Lübeck, Germany, was published online in eBioMedicine.
DISCLOSURES:
The study was supported by the University of Lübeck, the Deutsche Forschungsgemeinschaft, and the State of Schleswig-Holstein. One author declared financial ties outside this work, and one author is an employee of TriNetX.
A version of this article appeared on Medscape.com.
TOPLINE:
, particularly among women and White patients.
METHODOLOGY:
- Studies have shown increased risks for cardiovascular diseases in patients with PN, but limited sample sizes have hindered further subgroup analysis. Given PN’s pronounced sex and ethnicity skew, it is important to examine underrepresented groups to accurately assess their cardiovascular risk.
- In this propensity-score matched analysis, researchers identified 64,801 patients (59.44% women) with PN using electronic health reports from the Global Collaborative Network of TriNetX and matched to individuals without PN.
- Researchers calculated risks for 15 cardiovascular endpoints and all-cause mortality within 10 years of diagnosis. Major adverse cardiovascular events (MACE) included acute cerebral and myocardial infarction (MI), heart failure, ventricular arrhythmia, and sudden cardiac death.
TAKEAWAY:
- Patients with PN showed a higher risk for death (hazard ratio [HR], 1.1243) and MACE (HR, 1.117) (P < .0001 for both).
- PN was also associated with a higher risk for heart failure (HR, 1.062), thrombotic venous disease (HR, 1.26), angina pectoris (HR, 1.096), and peripheral arterial diseases (HR, 1.082) (P < .0001 for all) and for acute MI (HR, 1.11; P = .0015) and valve disorders (HR, 1.08; P = .0018).
- White patients with PN had a significantly increased risk for MACE, death, heart failure, cardiac arrest, vascular diseases, and acute MI, but this was not observed in people of color.
- Women exhibited a higher risk for MACE, heart failure, peripheral artery disease, acute MI, conduction disease, and valve disorders, while men did not have an increased risk for major or acute cardiovascular events. Both men and women had a higher risk for death, chronic ischemic heart disease, and venous disease.
IN PRACTICE:
“Although no novel PN-specific treatment rationale can be derived from the presented data, the potential risk of subsequent cardiovascular disease should be considered in the care of patients with PN, which includes screening and optimal management of other additional cardiovascular risk factors,” the authors wrote.
LIMITATIONS:
Retrospective observational design introduced inherent biases. Misdiagnosis or false coding in electronic health records could affect the data accuracy and ethnicity-specific analyses.
SOURCE:
This work, led by Henning Olbrich, from the Department of Dermatology, University of Lübeck, Germany, was published online in eBioMedicine.
DISCLOSURES:
The study was supported by the University of Lübeck, the Deutsche Forschungsgemeinschaft, and the State of Schleswig-Holstein. One author declared financial ties outside this work, and one author is an employee of TriNetX.
A version of this article appeared on Medscape.com.
TOPLINE:
, particularly among women and White patients.
METHODOLOGY:
- Studies have shown increased risks for cardiovascular diseases in patients with PN, but limited sample sizes have hindered further subgroup analysis. Given PN’s pronounced sex and ethnicity skew, it is important to examine underrepresented groups to accurately assess their cardiovascular risk.
- In this propensity-score matched analysis, researchers identified 64,801 patients (59.44% women) with PN using electronic health reports from the Global Collaborative Network of TriNetX and matched to individuals without PN.
- Researchers calculated risks for 15 cardiovascular endpoints and all-cause mortality within 10 years of diagnosis. Major adverse cardiovascular events (MACE) included acute cerebral and myocardial infarction (MI), heart failure, ventricular arrhythmia, and sudden cardiac death.
TAKEAWAY:
- Patients with PN showed a higher risk for death (hazard ratio [HR], 1.1243) and MACE (HR, 1.117) (P < .0001 for both).
- PN was also associated with a higher risk for heart failure (HR, 1.062), thrombotic venous disease (HR, 1.26), angina pectoris (HR, 1.096), and peripheral arterial diseases (HR, 1.082) (P < .0001 for all) and for acute MI (HR, 1.11; P = .0015) and valve disorders (HR, 1.08; P = .0018).
- White patients with PN had a significantly increased risk for MACE, death, heart failure, cardiac arrest, vascular diseases, and acute MI, but this was not observed in people of color.
- Women exhibited a higher risk for MACE, heart failure, peripheral artery disease, acute MI, conduction disease, and valve disorders, while men did not have an increased risk for major or acute cardiovascular events. Both men and women had a higher risk for death, chronic ischemic heart disease, and venous disease.
IN PRACTICE:
“Although no novel PN-specific treatment rationale can be derived from the presented data, the potential risk of subsequent cardiovascular disease should be considered in the care of patients with PN, which includes screening and optimal management of other additional cardiovascular risk factors,” the authors wrote.
LIMITATIONS:
Retrospective observational design introduced inherent biases. Misdiagnosis or false coding in electronic health records could affect the data accuracy and ethnicity-specific analyses.
SOURCE:
This work, led by Henning Olbrich, from the Department of Dermatology, University of Lübeck, Germany, was published online in eBioMedicine.
DISCLOSURES:
The study was supported by the University of Lübeck, the Deutsche Forschungsgemeinschaft, and the State of Schleswig-Holstein. One author declared financial ties outside this work, and one author is an employee of TriNetX.
A version of this article appeared on Medscape.com.
Do Patients Benefit from Cancer Trial Participation?
TOPLINE:
METHODOLOGY:
- The view that patients with cancer benefit from access to investigational drugs in the clinical trial setting is widely held but does necessarily align with trial findings, which often show limited evidence of a clinical benefit. First, most investigational treatments assessed in clinical trials fail to gain regulatory approval, and the minority that are approved tend to offer minimal clinical benefit, experts explained.
- To estimate the survival benefit and toxicities associated with receiving experimental treatments, researchers conducted a meta-analysis of 128 trials comprising 141 comparisons of an investigational drug and a control treatment, which included immunotherapies and targeted therapies.
- The analysis included 42 trials in non–small cell lung cancer (NSCLC), 37 in breast cancer, 15 in hepatobiliary cancer, 13 in pancreatic cancer, 12 in colorectal cancer, and 10 in prostate cancer, involving a total of 47,050 patients.
- The primary outcome was PFS and secondary outcomes were overall survival and grades 3-5 serious adverse events.
TAKEAWAY:
- Overall, the experimental treatment was associated with a 20% improvement in PFS (pooled hazard ratio [HR], 0.80), corresponding to a median 1.25-month PFS advantage. The PFS benefit was seen across all cancer types, except pancreatic cancer.
- Overall survival improved by 8% with experimental agents (HR, 0.92), corresponding to 1.18 additional months. A significant overall survival benefit was seen across NSCLC, breast cancer, and hepatobiliary cancer trials but not pancreatic, prostate, colorectal cancer trials.
- Patients in the experimental intervention group, however, experienced much higher risk for grade 3-5 serious adverse events (risk ratio [RR], 1.27), corresponding to 7.40% increase in absolute risk. The greater risk for serious adverse events was significant for all indications except prostate cancer (RR, 1.13; 95% CI, 0.91-1.40).
IN PRACTICE:
“We believe our findings are best interpreted as suggesting that access to experimental interventions that have not yet received full FDA approval is associated with a marginal but nonzero clinical benefit,” the authors wrote.
“Although our findings seem to reflect poorly on trials as a vehicle for extending survival for participants, they have reassuring implications for clinical investigators, policymakers, and institutional review boards,” the researchers said, explaining that this “scenario allows clinical trials to continue to pursue promising new treatments — supporting incremental advances that sum to large gains over extended periods of research — without disadvantaging patients in comparator groups.”
SOURCE:
Renata Iskander, MSc, of McGill University, Montreal, Quebec, Canada, led this work, which was published online on April 29, 2024, in Annals of Internal Medicine.
LIMITATIONS:
There was high heterogeneity across studies due to variations in drugs tested, comparators used, and populations involved. The use of comparators below standard care could have inflated survival benefits. Additionally, data collected from ClinicalTrials.gov might be biased due to some trials not being reported.
DISCLOSURES:
Canadian Institutes of Health Research supported this work. The authors received grants for this work from McGill University, Rossy Cancer Network, and National Science Foundation. One author received consulting fees outside this work. The other authors declared no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The view that patients with cancer benefit from access to investigational drugs in the clinical trial setting is widely held but does necessarily align with trial findings, which often show limited evidence of a clinical benefit. First, most investigational treatments assessed in clinical trials fail to gain regulatory approval, and the minority that are approved tend to offer minimal clinical benefit, experts explained.
- To estimate the survival benefit and toxicities associated with receiving experimental treatments, researchers conducted a meta-analysis of 128 trials comprising 141 comparisons of an investigational drug and a control treatment, which included immunotherapies and targeted therapies.
- The analysis included 42 trials in non–small cell lung cancer (NSCLC), 37 in breast cancer, 15 in hepatobiliary cancer, 13 in pancreatic cancer, 12 in colorectal cancer, and 10 in prostate cancer, involving a total of 47,050 patients.
- The primary outcome was PFS and secondary outcomes were overall survival and grades 3-5 serious adverse events.
TAKEAWAY:
- Overall, the experimental treatment was associated with a 20% improvement in PFS (pooled hazard ratio [HR], 0.80), corresponding to a median 1.25-month PFS advantage. The PFS benefit was seen across all cancer types, except pancreatic cancer.
- Overall survival improved by 8% with experimental agents (HR, 0.92), corresponding to 1.18 additional months. A significant overall survival benefit was seen across NSCLC, breast cancer, and hepatobiliary cancer trials but not pancreatic, prostate, colorectal cancer trials.
- Patients in the experimental intervention group, however, experienced much higher risk for grade 3-5 serious adverse events (risk ratio [RR], 1.27), corresponding to 7.40% increase in absolute risk. The greater risk for serious adverse events was significant for all indications except prostate cancer (RR, 1.13; 95% CI, 0.91-1.40).
IN PRACTICE:
“We believe our findings are best interpreted as suggesting that access to experimental interventions that have not yet received full FDA approval is associated with a marginal but nonzero clinical benefit,” the authors wrote.
“Although our findings seem to reflect poorly on trials as a vehicle for extending survival for participants, they have reassuring implications for clinical investigators, policymakers, and institutional review boards,” the researchers said, explaining that this “scenario allows clinical trials to continue to pursue promising new treatments — supporting incremental advances that sum to large gains over extended periods of research — without disadvantaging patients in comparator groups.”
SOURCE:
Renata Iskander, MSc, of McGill University, Montreal, Quebec, Canada, led this work, which was published online on April 29, 2024, in Annals of Internal Medicine.
LIMITATIONS:
There was high heterogeneity across studies due to variations in drugs tested, comparators used, and populations involved. The use of comparators below standard care could have inflated survival benefits. Additionally, data collected from ClinicalTrials.gov might be biased due to some trials not being reported.
DISCLOSURES:
Canadian Institutes of Health Research supported this work. The authors received grants for this work from McGill University, Rossy Cancer Network, and National Science Foundation. One author received consulting fees outside this work. The other authors declared no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The view that patients with cancer benefit from access to investigational drugs in the clinical trial setting is widely held but does necessarily align with trial findings, which often show limited evidence of a clinical benefit. First, most investigational treatments assessed in clinical trials fail to gain regulatory approval, and the minority that are approved tend to offer minimal clinical benefit, experts explained.
- To estimate the survival benefit and toxicities associated with receiving experimental treatments, researchers conducted a meta-analysis of 128 trials comprising 141 comparisons of an investigational drug and a control treatment, which included immunotherapies and targeted therapies.
- The analysis included 42 trials in non–small cell lung cancer (NSCLC), 37 in breast cancer, 15 in hepatobiliary cancer, 13 in pancreatic cancer, 12 in colorectal cancer, and 10 in prostate cancer, involving a total of 47,050 patients.
- The primary outcome was PFS and secondary outcomes were overall survival and grades 3-5 serious adverse events.
TAKEAWAY:
- Overall, the experimental treatment was associated with a 20% improvement in PFS (pooled hazard ratio [HR], 0.80), corresponding to a median 1.25-month PFS advantage. The PFS benefit was seen across all cancer types, except pancreatic cancer.
- Overall survival improved by 8% with experimental agents (HR, 0.92), corresponding to 1.18 additional months. A significant overall survival benefit was seen across NSCLC, breast cancer, and hepatobiliary cancer trials but not pancreatic, prostate, colorectal cancer trials.
- Patients in the experimental intervention group, however, experienced much higher risk for grade 3-5 serious adverse events (risk ratio [RR], 1.27), corresponding to 7.40% increase in absolute risk. The greater risk for serious adverse events was significant for all indications except prostate cancer (RR, 1.13; 95% CI, 0.91-1.40).
IN PRACTICE:
“We believe our findings are best interpreted as suggesting that access to experimental interventions that have not yet received full FDA approval is associated with a marginal but nonzero clinical benefit,” the authors wrote.
“Although our findings seem to reflect poorly on trials as a vehicle for extending survival for participants, they have reassuring implications for clinical investigators, policymakers, and institutional review boards,” the researchers said, explaining that this “scenario allows clinical trials to continue to pursue promising new treatments — supporting incremental advances that sum to large gains over extended periods of research — without disadvantaging patients in comparator groups.”
SOURCE:
Renata Iskander, MSc, of McGill University, Montreal, Quebec, Canada, led this work, which was published online on April 29, 2024, in Annals of Internal Medicine.
LIMITATIONS:
There was high heterogeneity across studies due to variations in drugs tested, comparators used, and populations involved. The use of comparators below standard care could have inflated survival benefits. Additionally, data collected from ClinicalTrials.gov might be biased due to some trials not being reported.
DISCLOSURES:
Canadian Institutes of Health Research supported this work. The authors received grants for this work from McGill University, Rossy Cancer Network, and National Science Foundation. One author received consulting fees outside this work. The other authors declared no competing interests.
A version of this article appeared on Medscape.com.
Do Health-Related Social Needs Raise Mortality Risk in Cancer Survivors?
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
FROM CANCER
Terminal Cancer: What Matters to Patients and Caregivers
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.