User login
For MD-IQ use only
Experience With Adaptive Servo-Ventilation Among Veterans in the Post-SERVE-HF Era
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
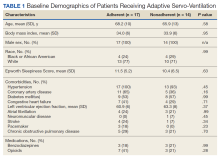
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
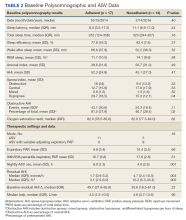
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
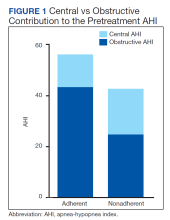
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
Sleep apnea is a heterogeneous group of conditions that may be attributable to a wide array of underlying conditions, with varying contributions of obstructive or central sleep-disordered breathing. The spectrum from obstructive sleep apnea (OSA) to central sleep apnea (CSA) includes mixed sleep apnea, treatment-emergent CSA (TECSA), and Cheyne-Stokes respiration (CSR).1 The pathophysiologic causes of CSA can be attributed to delayed cardiopulmonary circulation in heart failure, decreased brainstem ventilatory response due to stroke, blunting of central chemoreceptors in chronic opioid use, and/or stimulation of the Hering-Breuer reflex from activation of pulmonary stretch receptors after initiating positive airway pressure (PAP) for treatment of OSA.2,3 Medications are commonly implicated in many forms of sleep-disordered breathing; importantly, opioids and benzodiazepines may blunt the respiratory drive, leading to CSA, and/or impair upper airway patency, resulting in or worsening OSA.
Continuous positive airway pressure (CPAP) therapy is largely ineffective in correcting CSA or improving outcomes and is often poorly tolerated in these patients.4 Adaptive servo-ventilation (ASV) is a form of bilevel PAP (BPAP) therapy that delivers variable adjusting pressure support, primarily to treat CSA. PAP also may relieve upper airway obstructions, thereby effectively treating any comorbid obstructive component. ASV has been well documented to improve sleep-related disorders and improve apnea-hypopnea index (AHI) in patients with CSA. However, longitudinal data have demonstrated increased mortality in patients with heart failure with reduced ejection fraction (HFrEF) who were treated with ASV.5 Since the SERVE-HF trial results came to light in 2015, there has been no consensus regarding the optimal use, if any, of ASV therapy.6-8 This is partly related to the inability to fully explain the study’s major findings, which were unexpected at the time, and partly due to the absence of similar relevant mortality data in patients with CSA but without HFrEF.
TECSA may present in some patients with OSA who are new to PAP therapy. These events are frequently seen during PAP titration sleep studies, though patients can also experience significant TECSA shortly after initiating home PAP therapy. TECSA is felt to result from a combination of stimulating pulmonary stretch receptors and lowering arterial carbon dioxide below the apneic threshold. Chemoreceptors located in the medulla respond by attenuating the respiratory drive.9 Previous studies have shown most cases of mild TECSA resolve over time with CPAP treatment. However, in patients with persistent or worsening TECSA, ASV may be considered as an alternative to CPAP.
The prevalence of OSA in the veteran population is estimated to be as high as 60%, considerably higher than the general population estimation.10 Patients with more significant comorbidities may also experience a higher frequency of central events. Patients with CSA have also been shown to have a higher risk for cardiac-related hospital admissions, providing plausible justification for correcting CSA.10
In the current study, we aim to characterize the group of patients using ASV therapy in the modern era. We will assess the objective efficacy and adherence of ASV therapy in patients with primarily CSA compared with those having primarily OSA (ie, TECSA). Secondarily, we aim to identify baseline clinical and polysomnographic features that may be predictive of ASV adherence, as a surrogate for subjective benefit.11 In the wake of the SERVE-HF study, the sleep medicine community has paused prescribing ASV therapy for CSA. We hope to provide more perspective on the treatment of veterans with CSA and identify the patient groups that would benefit most from ASV therapy.
Methods
This retrospective chart review examined patients prescribed ASV therapy at the Hampton Veterans Affairs Medical Center (HVAMC) in Virginia who had therapy data between January 1, 2015, and April 30, 2020. The start date was chosen to approximate the phase-in of wireless PAP devices at HVAMC and to correspond with the release of preliminary results from the SERVE-HF trial.
Patients were initially identified through a query into commercial wireless PAP management databases and cross-referenced with HVAMC patients. Adherence and efficacy data were obtained from the most recent clinical PAP data, which allowed for the evaluation of patients who discontinued therapy for reasons other than intolerance. Clinical, demographic, and polysomnography (PSG) data were obtained from the electronic health record. One patient, identified through the database query but not found in the electronic health record, was excluded. In cases of missing PSG data, especially AHI or similar values, all attempts were made to calculate the data with other provided values. This study was determined to be exempt by the HVAMC Institutional Review Board (protocol #20-01).
Statistics
Statistical analyses were designed to compare clinical characteristics and adherence to therapy of those with primarily CSA on PSG and those with primarily OSA. Because it was not currently known how many patients would fit into each of these categories, we also planned secondary comparisons of the clinical and PSG characteristics of those patients who were adherent with therapy and those who were not. Adherence with ASV therapy was defined as device use for ≥ 4 hours for ≥ 70% of nights.
Comparisons between the means of 2 normally distributed groups were performed with an unpaired t test. Comparisons between 2 nonnormally distributed groups and groups of dates were done with the Mann-Whitney U test. The normality of a group distribution was determined using D’Agostino-Pearson omnibus normality test. Two groups of dichotomous variables were compared with the Fisher exact test. P value < .05 was considered statistically significant.
Results
Thirty-one patients were prescribed ASV therapy and had follow-up at HVAMC since 2015. All patients were male. The mean (SD) age was 67.2 (11.4) years, mean body mass index (BMI) was 34.0 (5.9), and the mean (SD) Epworth Sleepiness Scale (ESS) score was 10.9 (5.8). Patient comorbidities included 30 (97%) with hypertension, 17 (55%) with diabetes mellitus, 16 (52%) with coronary artery disease, and 11 (35%) with congestive heart failure. Three patients had no echocardiogram or other documentation of left ventricular ejection fraction (LVEF). One of these patients had voluntarily stopped using PAP therapy, another had been erroneously started on ASV (ordered for fixed BPAP), and the third had since been retitrated to CPAP. In the 28 patients with documented LVEF, the mean (SD) LVEF was 61.8% (6.9). Ten patients (32%) had opioids documented on their medication lists and 6 (19%) had benzodiazepines.
The median date of diagnostic sleep testing was January 9, 2015, and testing was completed after the release of the initial field safety notice regarding the SERVE-HF trial preliminary findings May 13, 2015, for 14 patients (45%).12 On diagnostic sleep testing, the mean (SD) AHI was 47.3 (25.6) events/h and the median (IQR) oxygen saturation (SpO2) nadir was 82% (78-84). Three patients (10%) were initially diagnosed with CSA, 19 (61%) with OSA, and 9 (29%) with both. Sixteen patients (52%) had ASV with fixed expiratory PAP (EPAP), and 15 (48%) had variable adjusting EPAP. Mean (SD) usage of ASV was 6.5 (2.6) hours and 66.0% (34.2) of nights for ≥ 4 hours. Mean (SD) titrated EPAP (set or 90th/95th percentile autotitrated) was 10.1 (3.4) cm H2O and inspiratory PAP (IPAP) (90th/95th percentile) was 17.1 (3.3) cm H2O. The median (IQR) residual AHI on ASV was 2.7 events/h (1.1-5.1), apnea index (AI) was 0.4 (0.1-1.0), and hypopnea index (HI) was 1.4 (1.0-3.2); the residual central and obstructive events were not available in most cases.
Adherence
There were no significant differences between the proportions of patients on ASV with set EPAP or the titrated EPAP and IPAP. The median (IQR) residual AHI was lower in the adherent group compared with the nonadherent group, both in absolute values (1.7 [0.9-3.2] events/h vs 4.7 [2.4-10.3] events/h, respectively [P = .004]), and as a percentage of the pretreatment AHI (3.1% [2.5-6.0] vs 10.2% [5.3-34.4], respectively; P = .002) (Figure 2).
Primarily Obstructive Sleep Apnea
Sleep apnea was a mixed picture of obstructive and central events in many patients. Only 3 patients had “pure” CSA. Thus, we were unable to define discrete comparison groups based on the sleep-disordered breathing phenotype. We identified 19 patients with primarily OSA (ie, initially diagnosed with OSA, OSA with TECSA, or complex sleep apnea). The mean (SD) age was 66.1 (12.8) years, BMI was 36.2 (4.7), and ESS was 11.4 (5.6). The mean (SD) baseline AHI was 46.9 (29.5), obstructive AHI was 40.5 (30.4), and central AHI was 0.4 (1.2); the median (IQR) SpO2 nadir was 81% (78%-84%). The mean (SD) titrated EPAP was 10.2 (3.5) cm H2O, and the 90th/95th percentile IPAP was 17.9 (3.5) cm H2O. The mean (SD) usage of ASV was 7.9 (5.3) hours with 11 patients (58%) meeting the minimum standard for adherence to ASV therapy.
No significant differences were seen between the adherent and nonadherent groups in clinical or demographic characteristics or date of diagnostic sleep testing (eAppendix, available online at doi:10.12788/fp.0374). In baseline sleep studies the mean (SD) HI was 32.3 (15.8) in the adherent group compared with 14.7 (8.8) in the nonadherent group (P = .049). In contrast, obstructive AHI was not significantly lower in the adherent group: 51.9 (30.9) in the adherent group compared with 22.2 (20.6) in the nonadherent group (P = .09). The median (IQR) residual AHI on ASV as a percentage of the pretreatment AHI was 3.0% (2.4%-6.5%) in the adherent group compared with 11.3% (5.4%-89.1%) in the nonadherent group, a statistically significant difference (P = .01). No other significant differences were seen between the groups.
Discussion
This study describes a real-world cohort of patients using ASV therapy and the characteristics associated with benefit from therapy. The patients that were prescribed and started ASV therapy most often had a significant degree of obstructive component to sleep-disordered breathing, whether primary OSA with TECSA or comorbid OSA and CSA. Moreover, we found that a higher obstructive AHI on the baseline PSG was associated with adherence to ASV therapy. Another important finding was that a lower residual AHI on ASV as a proportion of the baseline was associated with PAP adherence. Adherent patients had similar clinical characteristics as the nonadherent patients, including comorbidities, severity of sleep-disordered breathing, and obesity.
Though the results of the SERVE-HF trial have dampened the enthusiasm somewhat, ASV therapy has long been considered an effective and well-tolerated treatment for many types of CSA.13 In fact, treatments that can eliminate the central AHI are fairly limited.4,14 Our data suggest that ASV is also effective and tolerated in OSA with TECSA and/or comorbid CSA. Recent studies suggest that CSA resolves spontaneously in a majority of TECSA patients within 2 to 3 months of regular CPAP use.15 Other estimates suggest that persistent TESCA may be present in 2% of patients with OSA on treatment.16
Given the high and rising prevalence of OSA, many people are at risk for clinically significant TESCA. Another retrospective case series found that 72% of patients that failed treatment with CPAP or BPAP during PSG, met diagnostic criteria (at the time) for CSA; ASV was objectively beneficial in these patients.17 ASV can be an especially useful modality to treat OSA in patients with CSA that either prevents tolerance of standard therapies or causes clinical consequences, presuming the patient does not also have HFrEF.18 The long-term outcomes of treatment with ASV therapy remain a matter of debate.
The SERVE-HF trial remains among the only studies that have assessed the mortality effects of CSA treatments, with unfavorable findings. Treatment of OSA has been associated with favorable chronic health benefits, though recent studies have questioned the degree of benefit attributable to OSA treatment.19-24 Similar studies have not been done for comorbidities represented by our study cohort (ie, OSA with TECSA and/or comorbid CSA).
The lack of CSA diagnosis alone in our cohort may be partially attributable to changing practice patterns following the SERVE-HF trial, though it is not clear from these data why a higher baseline obstructive AHI was associated with adherence to ASV therapy. Our data in this regard are somewhat at odds with the preliminary results of the ADVENT-HF trial. In that study, adherence to ASV therapy in patients with predominantly OSA declined significantly more than in patients with predominantly CSA.25 Most of our patients were diagnosed with predominantly OSA, so a direct comparison with the CSA group is problematic; additionally, the primary brand and the pressure adjustments algorithm used in our study differed from the ADVENT-HF trial.
OSA and CSA may present with similar clinical symptoms, including sleep fragmentation, insomnia, and excessive daytime sleepiness; however, the degree of symptomatology, especially daytime sleepiness, and the response to treatment, may be less in CSA.2,26 Both the subjective report of symptoms (ESS) and PSG measures of sleep fragmentation were similar in our patients, again likely explained by the predominance of obstructive events.
The pathophysiology of CSA is more varied than OSA, which is probably relevant in this case. ASV was originally designed for the management of CSA with CSR, accomplishing this goal by stabilizing the periods of central apnea and hyperpnea characteristic of CSR.27 Although other forms of CSA demonstrate breathing patterns distinct from CSR, ASV has become an accepted treatment for most of these. It is plausible that the long-term subjective benefit and tolerance of ASV in CSA without CSR is less than for CSA with CSR or OSA. None of the patients in our study had CSA with CSR.
Ultimately, it may be the objective treatment effect that lends to adherence, as has been shown previously in OSA patients; our group of adherent patients showed a greater improvement in AHI, relative to baseline, than the nonadherent patients did.28 The technology behind ASV therapy can greatly reduce the frequencies of central apneas, yet this same treatment effectively splints the upper airway and even more effectively eliminates obstructive apneas and hypopneas. Variable adjusting EPAP devices would plausibly provide even more benefit in these patients, as has been shown in prior studies.29 To the contrary, our small sample of patients with TESCA showed a nonsignificant trend toward adherence with fixed EPAP ASV.
Opioid use was substantial in our population, without significant differences between the groups. CPAP therapy is ineffective in improving opioid-associated CSA. In a recent study, 20 patients on opioid therapy with CSA were treated with CPAP therapy; after several weeks, the average therapeutic use was 4 to 5 hours per night and CPAP was abandoned in favor of ASV therapy due to persistent central apnea. ASV treatment was associated with a considerable reduction in central apnea index, AHI, arousal index, and oxygen desaturations in a remarkable improvement over CPAP.30
Limitations and Future Directions
This retrospective, single-center study may have limited applicability to other populations. Adherence was used as a surrogate for subjective benefit from treatment, though benefit was not confirmed by the patients directly. Only patients seen in follow-up for documentation of the ASV download were identified for inclusion and data analysis. As a single center, we risk homogeneity in the treatment algorithms, though sleep medicine treatments are often decided at the time of the sleep studies. Studies and treatment recommendations were made at a variety of sites, including our sleep center, other US Department of Veterans Affairs hospitals, in the community network, and at US Department of Defense centers. Our population was homogenous in some ways; notably, 100% of our group was male, which is substantially higher than both the veteran population and the general population. Risk factors for OSA and CSA are more common in male patients, which may partially explain this anomaly. Lastly, with our small sample size, there is increased risk that the results seen occurred by chance.
There are several areas for further study. A larger multicenter study may permit these results to be generalized to the population and should include subjective measures of benefit. Patients with primarily CSA were largely absent in our group and may be the focus of future studies; data on predictors of treatment adherence in CSA are lacking. With the availability of consistent older adherence data, comparisons may be made between the efficacies of clinical practice habits, including treatment efficacy, before and after the results of the SERVE-HF trial became known.
Conclusions
In selected patients with preserved LVEF, ASV therapy appears especially effective in patients with OSA combined with CSA. Adherence to ASV treatment was associated with higher obstructive AHI during the baseline PSG and with a greater reduction in the AHI. This understanding may help guide sleep specialists in personalizing treatments for sleep-disordered breathing. Because objective efficacy appears to be important for therapy adherence, clinicians should be able to consistently determine the obstructive and central components of the residual AHI, thus taking all information into account when optimizing the treatment. Additionally, both OSA and CSA pressure requirements should be considered when developing ASV devices.
Acknowledgments
We thank Martha Harper, RRT, of Hampton Veterans Affairs Medical Center (HVAMC) for helping to identify our patients and assisting with data collection. This material is the result of work supported with resources and the use of HVAMC facilities.
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
1. Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468-475. doi:10.1093/sleep/30.4.468
2. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595-607. doi:10.1378/chest.06.2287
3. Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):372-380. doi:10.1183/20734735.042412
4. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033. doi:10.1056/NEJMoa051001
5. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-1105. doi:10.1056/NEJMoa1506459
6. Imamura T, Kinugawa K. What is the optimal strategy for adaptive servo-ventilation therapy? Int Heart J. 2018;59(4):683-688. doi:10.1536/ihj.17-429
7. Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: more questions than answers. Chest. 2016;149(4):900-904. doi:10.1016/j.chest.2015.12.021
8. Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest. 2015;148(4):848-851. doi:10.1378/chest.15-1536
9. Nigam G, Riaz M, Chang E, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
10. Ratz D, Wiitala W, Badr MS, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zsy058. doi:10.1093/sleep/zsy058
11. Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15(7):365-369. doi:10.1155/2008/534372
12. Special Safety Notice: ASV therapy for central sleep apnea patients with heart failure. American Academy of Sleep Medicine. May 15, 2015. Accessed February 13, 2023. https://aasm.org/special-safety-notice-asv-therapy-for-central-sleep-apnea-patients-with-heart-failure/
13. Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342. doi:10.1136/hrt.2005.060038
14. Randerath W, Deleanu OC, Schiza S, Pepin J-L. Central sleep apnoea and periodic breathing in heart failure: prognostic significance and treatment options. Eur Respir Rev. 2019;28(153):190084. Published 2019 Oct 11. doi:10.1183/16000617.0084-2019
15. Gay PC. Complex sleep apnea: it really is a disease. J Clin Sleep Med. 2008;4(5):403-405.
16. American Academy of Sleep Medicine. International Classification of Sleep Disorders - Third Edition (ICSD-3). 3rd ed. American Academy of Sleep Medicine; 2014.
17. Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187-195.
18. Aurora RN, Bista SR, Casey KR, et al. Updated Adaptive Servo-Ventilation Recommendations for the 2012 AASM Guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757-761. doi:10.5664/jcsm.5812
19. Martínez-García MA, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36-41. doi:10.1164/rccm.200808-1341OC
20. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi:10.1164/rccm.201203-0448OC
21. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. Published 2013 Nov 25. doi:10.1161/JAHA.113.000421
22. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157(3):858-865. doi:10.1164/ajrccm.157.3.9709042
23. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi:10.1056/NEJMoa1606599
24. Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi:10.1001/jama.2017.7967
25. Perger E, Lyons OD, Inami T, et al. Predictors of 1-year compliance with adaptive servoventilation in patients with heart failure and sleep disordered breathing: preliminary data from the ADVENT-HF trial. Eur Resp J. 2019;53(2):1801626. doi:10.1183/13993003.01626-2018
26. Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579-587. doi:10.1002/ejhf.790
27. Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619. doi:10.1164/ajrccm.164.4.9908114
28. Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21(4):419-426. doi:10.1111/j.1365-2869.2011.00969.x
29. Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS). Sleep. 2010;33(2):267-271. doi:10.1093/sleep/33.2.267
30. Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637-643. Published 2014 Jun 15. doi:10.5664/jcsm.3788
A nod to the future of AGA
CHICAGO – It’s been 125 years since the founding of the American Gastroenterological Association (AGA). It’s gone from a small organization in which gastroenterology wasn’t even a known medical specialty, to an organization that grants millions of dollars in research funding each year.
He spoke with optimism about gastroenterology’s future during his presidential address on May 8 at the annual Digestive Disease Week® (DDW) meeting in Chicago.
“I congratulate the AGA on its quasquicentennial, or 125th anniversary,” said Dr. Carethers, who is distinguished professor of medicine and vice chancellor for health sciences at the University of California, San Diego.
The AGA was founded in 1897 by Detroit-based physician Charles Aaron, MD. His passion was gastroenterology, but at that point, it wasn’t an established medical discipline. Dr. Aaron, Max Einhorn, MD, and 8 other colleagues formed the American Gastroenterological Association. Today, with nearly 16,000 members, the organization has become a driving force in improving the care of patients with gastrointestinal conditions.
Among AGA’s accomplishments since its founding: In 1940, the American Board of Internal Medicine certified gastroenterology as a subspecialty. Three years later, the first issue of Gastroenterology, the AGA’s flagship journal, was published. And, in 1971, the very first Digestive Disease Week® meeting took place.
In terms of medical advances that have been made since those early years, the list is vast: From the description of ileitis in 1932 by Burril B. Crohn, MD, in 1932 to the discovery of the hepatitis B surface antigen in 1965 and the more recent discovery of germline mutations in DNA mismatch repair genes as a cause of Lynch syndrome.
Dr. Carethers outlined goals for the future, including building a leadership team that is “reflective of our practice here in the United States,” Dr. Carethers said. Creating a culturally and gender diverse leadership team will only strengthen the organization and the practice of gastroenterology. The AGA’s first female president, Sarah Jordan, MD, was named in 1942, and since then, the AGA has been led by women and men from different ethnic backgrounds, including himself, who is AGA’s first president of African American heritage.
The AGA has committed to a number of diversity and equity objectives, including the AGA Equity Project, an initiative launched in 2020 whose goal is to achieve equity and eradicate disparities in digestive diseases with a focus on justice and equity, research and funding, workforce and leadership, recognition of the achievements of people of color, unconscious bias, and engagement with early career members.
“I am not only excited about the diversity and equity objectives within our specialty ... but also the innovation and things to come for our specialty,” Dr. Carethers said.
Securing funding for early-stage innovations in medicine can be difficult across medical disciplines, including gastroenterology. So, last year, the AGA, with Varia Ventures, launched the GI Opportunity Fund 1 to support early-stage GI-based companies. The goal is to raise $25 million for the initial fund. Through the AGA’s Center for GI Innovation and Technology and the AGA Tech Summit, early-stage companies may have new funding opportunities.
And, through the AGA Research Foundation, the organization will continue to support clinical research. Last year, $2.6 million in grants were awarded to investigators.
Dr. Carethers is a board director at Avantor, a life sciences supply company.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – It’s been 125 years since the founding of the American Gastroenterological Association (AGA). It’s gone from a small organization in which gastroenterology wasn’t even a known medical specialty, to an organization that grants millions of dollars in research funding each year.
He spoke with optimism about gastroenterology’s future during his presidential address on May 8 at the annual Digestive Disease Week® (DDW) meeting in Chicago.
“I congratulate the AGA on its quasquicentennial, or 125th anniversary,” said Dr. Carethers, who is distinguished professor of medicine and vice chancellor for health sciences at the University of California, San Diego.
The AGA was founded in 1897 by Detroit-based physician Charles Aaron, MD. His passion was gastroenterology, but at that point, it wasn’t an established medical discipline. Dr. Aaron, Max Einhorn, MD, and 8 other colleagues formed the American Gastroenterological Association. Today, with nearly 16,000 members, the organization has become a driving force in improving the care of patients with gastrointestinal conditions.
Among AGA’s accomplishments since its founding: In 1940, the American Board of Internal Medicine certified gastroenterology as a subspecialty. Three years later, the first issue of Gastroenterology, the AGA’s flagship journal, was published. And, in 1971, the very first Digestive Disease Week® meeting took place.
In terms of medical advances that have been made since those early years, the list is vast: From the description of ileitis in 1932 by Burril B. Crohn, MD, in 1932 to the discovery of the hepatitis B surface antigen in 1965 and the more recent discovery of germline mutations in DNA mismatch repair genes as a cause of Lynch syndrome.
Dr. Carethers outlined goals for the future, including building a leadership team that is “reflective of our practice here in the United States,” Dr. Carethers said. Creating a culturally and gender diverse leadership team will only strengthen the organization and the practice of gastroenterology. The AGA’s first female president, Sarah Jordan, MD, was named in 1942, and since then, the AGA has been led by women and men from different ethnic backgrounds, including himself, who is AGA’s first president of African American heritage.
The AGA has committed to a number of diversity and equity objectives, including the AGA Equity Project, an initiative launched in 2020 whose goal is to achieve equity and eradicate disparities in digestive diseases with a focus on justice and equity, research and funding, workforce and leadership, recognition of the achievements of people of color, unconscious bias, and engagement with early career members.
“I am not only excited about the diversity and equity objectives within our specialty ... but also the innovation and things to come for our specialty,” Dr. Carethers said.
Securing funding for early-stage innovations in medicine can be difficult across medical disciplines, including gastroenterology. So, last year, the AGA, with Varia Ventures, launched the GI Opportunity Fund 1 to support early-stage GI-based companies. The goal is to raise $25 million for the initial fund. Through the AGA’s Center for GI Innovation and Technology and the AGA Tech Summit, early-stage companies may have new funding opportunities.
And, through the AGA Research Foundation, the organization will continue to support clinical research. Last year, $2.6 million in grants were awarded to investigators.
Dr. Carethers is a board director at Avantor, a life sciences supply company.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – It’s been 125 years since the founding of the American Gastroenterological Association (AGA). It’s gone from a small organization in which gastroenterology wasn’t even a known medical specialty, to an organization that grants millions of dollars in research funding each year.
He spoke with optimism about gastroenterology’s future during his presidential address on May 8 at the annual Digestive Disease Week® (DDW) meeting in Chicago.
“I congratulate the AGA on its quasquicentennial, or 125th anniversary,” said Dr. Carethers, who is distinguished professor of medicine and vice chancellor for health sciences at the University of California, San Diego.
The AGA was founded in 1897 by Detroit-based physician Charles Aaron, MD. His passion was gastroenterology, but at that point, it wasn’t an established medical discipline. Dr. Aaron, Max Einhorn, MD, and 8 other colleagues formed the American Gastroenterological Association. Today, with nearly 16,000 members, the organization has become a driving force in improving the care of patients with gastrointestinal conditions.
Among AGA’s accomplishments since its founding: In 1940, the American Board of Internal Medicine certified gastroenterology as a subspecialty. Three years later, the first issue of Gastroenterology, the AGA’s flagship journal, was published. And, in 1971, the very first Digestive Disease Week® meeting took place.
In terms of medical advances that have been made since those early years, the list is vast: From the description of ileitis in 1932 by Burril B. Crohn, MD, in 1932 to the discovery of the hepatitis B surface antigen in 1965 and the more recent discovery of germline mutations in DNA mismatch repair genes as a cause of Lynch syndrome.
Dr. Carethers outlined goals for the future, including building a leadership team that is “reflective of our practice here in the United States,” Dr. Carethers said. Creating a culturally and gender diverse leadership team will only strengthen the organization and the practice of gastroenterology. The AGA’s first female president, Sarah Jordan, MD, was named in 1942, and since then, the AGA has been led by women and men from different ethnic backgrounds, including himself, who is AGA’s first president of African American heritage.
The AGA has committed to a number of diversity and equity objectives, including the AGA Equity Project, an initiative launched in 2020 whose goal is to achieve equity and eradicate disparities in digestive diseases with a focus on justice and equity, research and funding, workforce and leadership, recognition of the achievements of people of color, unconscious bias, and engagement with early career members.
“I am not only excited about the diversity and equity objectives within our specialty ... but also the innovation and things to come for our specialty,” Dr. Carethers said.
Securing funding for early-stage innovations in medicine can be difficult across medical disciplines, including gastroenterology. So, last year, the AGA, with Varia Ventures, launched the GI Opportunity Fund 1 to support early-stage GI-based companies. The goal is to raise $25 million for the initial fund. Through the AGA’s Center for GI Innovation and Technology and the AGA Tech Summit, early-stage companies may have new funding opportunities.
And, through the AGA Research Foundation, the organization will continue to support clinical research. Last year, $2.6 million in grants were awarded to investigators.
Dr. Carethers is a board director at Avantor, a life sciences supply company.
The meeting is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
AT DDW 2023
Study of environmental impact of GI endoscopy finds room for improvement
CHICAGO – according to Madhav Desai, MD, MPH, assistant professor of medicine at the University of Minnesota, Minneapolis. About 20% of the waste, most of which went to landfills, was potentially recyclable, he said in a presentation given at the annual Digestive Disease Week® meeting.
Gastrointestinal endoscopies are critical for the screening, diagnosis, and treatment of a variety of gastrointestinal conditions. But like other medical procedures, endoscopies are a source of environmental waste, including plastic, sharps, personal protective equipment (PPE), and cleaning supplies, and also energy waste.
“This all goes back to the damage that mankind is inflicting on the environment in general, with the health care sector as one of the top contributors to plastic waste generation, landfills and water wastage,” Dr. Desai said. “Endoscopies, with their numerous benefits, substantially increase waste generation through landfill waste and liquid consumption and waste through the cleaning of endoscopes. We have a responsibility to look into this topic.”
To prospectively assess total waste generation from their institution, Dr. Desai, who was with the Kansas City (Mo.) Veterans Administration Medical Center, when the research was conducted, collected data on the items used in 450 consecutive procedures from May to June 2022. The data included procedure type, accessory use, intravenous tubing, numbers of biopsy jars, linens, PPE, and more, beginning at the point of patient entry to the endoscopy unit until discharge. They also collected data on waste generation related to reprocessing after each procedure and daily energy use (including endoscopy equipment, lights, and computers). With an eye toward finding opportunities to improve and maximize waste recycling, they stratified waste into the three categories of biohazardous, nonbiohazardous, or potentially recyclable.
“We found that the total waste generated during the time period was 1,398.6 kg, with more than half of it, 61.6%, going directly to landfill,” Dr. Desai said in an interview. “That’s an amount that an average family in the U.S. would use for 2 months. That’s a huge amount.”
Most waste consists of sharps
Exactly one-third was biohazard waste and 5.1% was sharps, they found. A single procedure, on average, sent 2.19 kg of waste to landfill. Extrapolated to 1 year, the waste total amounts to 9,189 kg (equivalent to just over 10 U.S. tons) and per 100 procedures to 219 kg (about 483 pounds).
They estimated 20% of the landfill waste was potentially recyclable (such as plastic CO2 tubing, O2 connector, syringes, etc.), which could reduce the total landfill burden by 8.6 kg per day or 2,580 kg per year (or 61 kg per 100 procedures). Reprocessing endoscopes generated 194 gallons of liquid waste (735.26 kg) per day or 1,385 gallons per 100 procedures.
Turning to energy consumption, Dr. Desai reported that daily use in the endoscopy unit was 277.1 kW-hours (equivalent to 8.2 gallons of gasoline), adding up to about 1,980 kW per 100 procedures. “That 100-procedure amount is the equivalent of the energy used for an average fuel efficiency car to travel 1,200 miles, the distance from Seattle to San Diego,” he said.
“One next step,” Dr. Desai said, “is getting help from GI societies to come together and have endoscopy units track their own performance. You need benchmarks so that you can determine how good an endoscopist you are with respect to waste.”
He commented further:“We all owe it to the environment. And, we have all witnessed what Mother Nature can do to you.”
Working on the potentially recyclable materials that account for 20% of the total waste would be a simple initial step to reduce waste going to landfills, Dr. Desai and colleagues concluded in the meeting abstract. “These data could serve as an actionable model for health systems to reduce total waste generation and move toward environmentally sustainable endoscopy units,” they wrote.
The authors reported no disclosures.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
CHICAGO – according to Madhav Desai, MD, MPH, assistant professor of medicine at the University of Minnesota, Minneapolis. About 20% of the waste, most of which went to landfills, was potentially recyclable, he said in a presentation given at the annual Digestive Disease Week® meeting.
Gastrointestinal endoscopies are critical for the screening, diagnosis, and treatment of a variety of gastrointestinal conditions. But like other medical procedures, endoscopies are a source of environmental waste, including plastic, sharps, personal protective equipment (PPE), and cleaning supplies, and also energy waste.
“This all goes back to the damage that mankind is inflicting on the environment in general, with the health care sector as one of the top contributors to plastic waste generation, landfills and water wastage,” Dr. Desai said. “Endoscopies, with their numerous benefits, substantially increase waste generation through landfill waste and liquid consumption and waste through the cleaning of endoscopes. We have a responsibility to look into this topic.”
To prospectively assess total waste generation from their institution, Dr. Desai, who was with the Kansas City (Mo.) Veterans Administration Medical Center, when the research was conducted, collected data on the items used in 450 consecutive procedures from May to June 2022. The data included procedure type, accessory use, intravenous tubing, numbers of biopsy jars, linens, PPE, and more, beginning at the point of patient entry to the endoscopy unit until discharge. They also collected data on waste generation related to reprocessing after each procedure and daily energy use (including endoscopy equipment, lights, and computers). With an eye toward finding opportunities to improve and maximize waste recycling, they stratified waste into the three categories of biohazardous, nonbiohazardous, or potentially recyclable.
“We found that the total waste generated during the time period was 1,398.6 kg, with more than half of it, 61.6%, going directly to landfill,” Dr. Desai said in an interview. “That’s an amount that an average family in the U.S. would use for 2 months. That’s a huge amount.”
Most waste consists of sharps
Exactly one-third was biohazard waste and 5.1% was sharps, they found. A single procedure, on average, sent 2.19 kg of waste to landfill. Extrapolated to 1 year, the waste total amounts to 9,189 kg (equivalent to just over 10 U.S. tons) and per 100 procedures to 219 kg (about 483 pounds).
They estimated 20% of the landfill waste was potentially recyclable (such as plastic CO2 tubing, O2 connector, syringes, etc.), which could reduce the total landfill burden by 8.6 kg per day or 2,580 kg per year (or 61 kg per 100 procedures). Reprocessing endoscopes generated 194 gallons of liquid waste (735.26 kg) per day or 1,385 gallons per 100 procedures.
Turning to energy consumption, Dr. Desai reported that daily use in the endoscopy unit was 277.1 kW-hours (equivalent to 8.2 gallons of gasoline), adding up to about 1,980 kW per 100 procedures. “That 100-procedure amount is the equivalent of the energy used for an average fuel efficiency car to travel 1,200 miles, the distance from Seattle to San Diego,” he said.
“One next step,” Dr. Desai said, “is getting help from GI societies to come together and have endoscopy units track their own performance. You need benchmarks so that you can determine how good an endoscopist you are with respect to waste.”
He commented further:“We all owe it to the environment. And, we have all witnessed what Mother Nature can do to you.”
Working on the potentially recyclable materials that account for 20% of the total waste would be a simple initial step to reduce waste going to landfills, Dr. Desai and colleagues concluded in the meeting abstract. “These data could serve as an actionable model for health systems to reduce total waste generation and move toward environmentally sustainable endoscopy units,” they wrote.
The authors reported no disclosures.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
CHICAGO – according to Madhav Desai, MD, MPH, assistant professor of medicine at the University of Minnesota, Minneapolis. About 20% of the waste, most of which went to landfills, was potentially recyclable, he said in a presentation given at the annual Digestive Disease Week® meeting.
Gastrointestinal endoscopies are critical for the screening, diagnosis, and treatment of a variety of gastrointestinal conditions. But like other medical procedures, endoscopies are a source of environmental waste, including plastic, sharps, personal protective equipment (PPE), and cleaning supplies, and also energy waste.
“This all goes back to the damage that mankind is inflicting on the environment in general, with the health care sector as one of the top contributors to plastic waste generation, landfills and water wastage,” Dr. Desai said. “Endoscopies, with their numerous benefits, substantially increase waste generation through landfill waste and liquid consumption and waste through the cleaning of endoscopes. We have a responsibility to look into this topic.”
To prospectively assess total waste generation from their institution, Dr. Desai, who was with the Kansas City (Mo.) Veterans Administration Medical Center, when the research was conducted, collected data on the items used in 450 consecutive procedures from May to June 2022. The data included procedure type, accessory use, intravenous tubing, numbers of biopsy jars, linens, PPE, and more, beginning at the point of patient entry to the endoscopy unit until discharge. They also collected data on waste generation related to reprocessing after each procedure and daily energy use (including endoscopy equipment, lights, and computers). With an eye toward finding opportunities to improve and maximize waste recycling, they stratified waste into the three categories of biohazardous, nonbiohazardous, or potentially recyclable.
“We found that the total waste generated during the time period was 1,398.6 kg, with more than half of it, 61.6%, going directly to landfill,” Dr. Desai said in an interview. “That’s an amount that an average family in the U.S. would use for 2 months. That’s a huge amount.”
Most waste consists of sharps
Exactly one-third was biohazard waste and 5.1% was sharps, they found. A single procedure, on average, sent 2.19 kg of waste to landfill. Extrapolated to 1 year, the waste total amounts to 9,189 kg (equivalent to just over 10 U.S. tons) and per 100 procedures to 219 kg (about 483 pounds).
They estimated 20% of the landfill waste was potentially recyclable (such as plastic CO2 tubing, O2 connector, syringes, etc.), which could reduce the total landfill burden by 8.6 kg per day or 2,580 kg per year (or 61 kg per 100 procedures). Reprocessing endoscopes generated 194 gallons of liquid waste (735.26 kg) per day or 1,385 gallons per 100 procedures.
Turning to energy consumption, Dr. Desai reported that daily use in the endoscopy unit was 277.1 kW-hours (equivalent to 8.2 gallons of gasoline), adding up to about 1,980 kW per 100 procedures. “That 100-procedure amount is the equivalent of the energy used for an average fuel efficiency car to travel 1,200 miles, the distance from Seattle to San Diego,” he said.
“One next step,” Dr. Desai said, “is getting help from GI societies to come together and have endoscopy units track their own performance. You need benchmarks so that you can determine how good an endoscopist you are with respect to waste.”
He commented further:“We all owe it to the environment. And, we have all witnessed what Mother Nature can do to you.”
Working on the potentially recyclable materials that account for 20% of the total waste would be a simple initial step to reduce waste going to landfills, Dr. Desai and colleagues concluded in the meeting abstract. “These data could serve as an actionable model for health systems to reduce total waste generation and move toward environmentally sustainable endoscopy units,” they wrote.
The authors reported no disclosures.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
AT DDW 2023
Veterans Will Benefit if the VA Includes Telehealth in its Access Standards
The VA MISSION Act of 2018 expanded options for veterans to receive government-paid health care from private sector community health care practitioners. The act tasked the US Department of Veterans Affairs (VA) to develop rules that determine eligibility for outside care based on appointment wait times or distance to the nearest VA facility. As a part of those standards, VA opted not to include the availability of VA telehealth in its wait time calculations—a decision that we believe was a gross misjudgment with far-reaching consequences for veterans. Excluding telehealth from the guidelines has unnecessarily restricted veterans’ access to high-quality health care and has squandered large sums of taxpayer dollars.
The VA has reviewed its initial MISSION Act eligibility standards and proposed a correction that recognizes telehealth as a valid means of providing health care to veterans who prefer that option.1 Telehealth may not have been an essential component of health care before the COVID-19 pandemic, but now it is clear that the best action VA can take is to swiftly enact its recommended change, stipulating that both VA telehealth and in-person health care constitute access to treatment. If implemented, this correction would save taxpayers an astronomical sum—according to a VA reportto Congress, about $1.1 billion in fiscal year 2021 alone.2 The cost savings from this proposed correction is reason enough to implement it. But just as importantly, increased use of VA telehealth also means higher quality, quicker, and more convenient care for veterans.
The VA is the recognized world leader in providing telehealth that is effective, timely, and veteran centric. Veterans across the country have access to telehealth services in more than 30 specialties.3 To ensure accessibility, the VA has established partnerships with major mobile broadband carriers so that veterans can receive telehealth at home without additional charges.4 The VA project Accessing Telehealth through Local Area Stations (ATLAS) brings VA telehealth to areas where existing internet infrastructure may not be adequate to support video telehealth. ATLAS is a collaboration with private organizations, including Veterans of Foreign Wars, The American Legion, and Walmart.4The agency also provides tablets to veterans who might not have access to telehealth, fostering higher access and patient satisfaction.4
The VA can initiate telehealth care rapidly. The “Anywhere to Anywhere” VA Health Care initiative and telecare hubs eliminate geographic constraints, allowing clinicians to provide team-based services across county and state lines to veterans’ homes and communities.
VA’s telehealth effort maximizes convenience for veterans. It reduces travel time, travel expenses, depletion of sick leave, and the need for childcare. Veterans with posttraumatic stress disorder or military sexual trauma who are triggered by traffic and waiting rooms, those with mobility issues, or those facing the stigma of mental health treatment often prefer to receive care in the familiarity of their home. Nonetheless, any veteran who desires an in-person appointment would continue to have that option under the proposed VA rule change.
VA telehealth is often used for mental health care, using the same evidence-based psychotherapies that VA has championed and are superior to that available in the private sector.5,6 This advantage is largely due to VA’s rigorous training, consultation, case review, care delivery, measurement standards, and integrated care model. In a recent survey of veterans engaged in mental health care, 80% reported that VA virtual care via video and/or telephone is as helpful or more helpful than in‐person services.7And yet, because of existing regulations, VA telemental health (TMH) does not qualify as access, resulting in hundreds of thousands of TMH visits being outsourced yearly to community practitioners that could be quickly and beneficially furnished by VA clinicians.
Telehealth has been shown to be as clinically effective as in-person care. A recent review of 38 meta-analyses covering telehealth with 10 medical disciplines found that for all disciplines, telehealth was as effective, if not more so, than conventional care.8 And because the likelihood of not showing up for telehealth appointments is lower than for in-person appointments, continuity of care is uninterrupted, and health care outcomes are improved.
Telehealth is health care. The VA must end the double standard that has handicapped it from including telehealth availability in determinations of eligibility for community care. The VA has voiced its intention to seek stakeholder input before implementing its proposed correction. The change is long overdue. It will save the VA a billion dollars annually while ensuring that veterans have quicker access to better treatment.
1 McDonough D. Statement of the honorable Denis McDonough Secretary of Veterans Affairs Department of Veterans Affairs (VA) before the Committee on Veterans’ Affairs United States Senate on veterans access to care. 117th Cong, 2nd Sess. September 21, 2022. Accessed May 8, 2023. https://www.veterans.senate.gov/2022/9/ensuring-veterans-timely-access-to-care-in-va-and-the-community/63b521ff-d308-449a-b3a3-918f4badb805
2 US Department of Veterans Affairs, Congressionally mandated report: access to care standards. September 2022.
3 US Department of Veterans Affairs. VA Secretary Press Conference, Thursday March 2, 2023. Accessed May 8, 2023. https://www.youtube.com/watch?v=WnkNl2whPoQ
4 US Department of Veterans Affairs, VA Telehealth: bridging the digital divide. Accessed May 8, 2023. https://telehealth.va.gov/digital-divide
5 Rand Corporation. Improving the Quality of Mental Health Care for Veterans: Lessons from RAND Research. Santa Monica, CA: RAND Corporation, 2019. https://www.rand.org/pubs/research_briefs/RB10087.html.
6 Lemle, R. Choice program expansion jeopardizes high-quality VHA mental health services. Federal Pract. 2018:35(3):18-24. [link to: https://www.mdedge.com/fedprac/article/159219/mental-health/choice-program-expansion-jeopardizes-high-quality-vha-mental
7 Campbell TM. Overview of the state of mental health care services in the VHA health care system. Presentation to the National Academies’ improving access to high-quality mental health care for veterans: a workshop. April 20, 2023. Accessed May 8, 2023. https://www.nationalacademies.org/documents/embed/link/LF2255DA3DD1C41C0A42D3BEF0989ACAECE3053A6A9B/file/D2C4B73BA6FFCAA81E6C4FC7C57020A5BA54376245AD?noSaveAs=1
8 Snoswell CL, Chelberg G, De Guzman KR, et al. The clinical effectiveness of telehealth: A systematic review of meta-analyses from 2010 to 2019. J Telemed Telecare. 2021;1357633X211022907. doi:10.1177/1357633X211022907
The VA MISSION Act of 2018 expanded options for veterans to receive government-paid health care from private sector community health care practitioners. The act tasked the US Department of Veterans Affairs (VA) to develop rules that determine eligibility for outside care based on appointment wait times or distance to the nearest VA facility. As a part of those standards, VA opted not to include the availability of VA telehealth in its wait time calculations—a decision that we believe was a gross misjudgment with far-reaching consequences for veterans. Excluding telehealth from the guidelines has unnecessarily restricted veterans’ access to high-quality health care and has squandered large sums of taxpayer dollars.
The VA has reviewed its initial MISSION Act eligibility standards and proposed a correction that recognizes telehealth as a valid means of providing health care to veterans who prefer that option.1 Telehealth may not have been an essential component of health care before the COVID-19 pandemic, but now it is clear that the best action VA can take is to swiftly enact its recommended change, stipulating that both VA telehealth and in-person health care constitute access to treatment. If implemented, this correction would save taxpayers an astronomical sum—according to a VA reportto Congress, about $1.1 billion in fiscal year 2021 alone.2 The cost savings from this proposed correction is reason enough to implement it. But just as importantly, increased use of VA telehealth also means higher quality, quicker, and more convenient care for veterans.
The VA is the recognized world leader in providing telehealth that is effective, timely, and veteran centric. Veterans across the country have access to telehealth services in more than 30 specialties.3 To ensure accessibility, the VA has established partnerships with major mobile broadband carriers so that veterans can receive telehealth at home without additional charges.4 The VA project Accessing Telehealth through Local Area Stations (ATLAS) brings VA telehealth to areas where existing internet infrastructure may not be adequate to support video telehealth. ATLAS is a collaboration with private organizations, including Veterans of Foreign Wars, The American Legion, and Walmart.4The agency also provides tablets to veterans who might not have access to telehealth, fostering higher access and patient satisfaction.4
The VA can initiate telehealth care rapidly. The “Anywhere to Anywhere” VA Health Care initiative and telecare hubs eliminate geographic constraints, allowing clinicians to provide team-based services across county and state lines to veterans’ homes and communities.
VA’s telehealth effort maximizes convenience for veterans. It reduces travel time, travel expenses, depletion of sick leave, and the need for childcare. Veterans with posttraumatic stress disorder or military sexual trauma who are triggered by traffic and waiting rooms, those with mobility issues, or those facing the stigma of mental health treatment often prefer to receive care in the familiarity of their home. Nonetheless, any veteran who desires an in-person appointment would continue to have that option under the proposed VA rule change.
VA telehealth is often used for mental health care, using the same evidence-based psychotherapies that VA has championed and are superior to that available in the private sector.5,6 This advantage is largely due to VA’s rigorous training, consultation, case review, care delivery, measurement standards, and integrated care model. In a recent survey of veterans engaged in mental health care, 80% reported that VA virtual care via video and/or telephone is as helpful or more helpful than in‐person services.7And yet, because of existing regulations, VA telemental health (TMH) does not qualify as access, resulting in hundreds of thousands of TMH visits being outsourced yearly to community practitioners that could be quickly and beneficially furnished by VA clinicians.
Telehealth has been shown to be as clinically effective as in-person care. A recent review of 38 meta-analyses covering telehealth with 10 medical disciplines found that for all disciplines, telehealth was as effective, if not more so, than conventional care.8 And because the likelihood of not showing up for telehealth appointments is lower than for in-person appointments, continuity of care is uninterrupted, and health care outcomes are improved.
Telehealth is health care. The VA must end the double standard that has handicapped it from including telehealth availability in determinations of eligibility for community care. The VA has voiced its intention to seek stakeholder input before implementing its proposed correction. The change is long overdue. It will save the VA a billion dollars annually while ensuring that veterans have quicker access to better treatment.
The VA MISSION Act of 2018 expanded options for veterans to receive government-paid health care from private sector community health care practitioners. The act tasked the US Department of Veterans Affairs (VA) to develop rules that determine eligibility for outside care based on appointment wait times or distance to the nearest VA facility. As a part of those standards, VA opted not to include the availability of VA telehealth in its wait time calculations—a decision that we believe was a gross misjudgment with far-reaching consequences for veterans. Excluding telehealth from the guidelines has unnecessarily restricted veterans’ access to high-quality health care and has squandered large sums of taxpayer dollars.
The VA has reviewed its initial MISSION Act eligibility standards and proposed a correction that recognizes telehealth as a valid means of providing health care to veterans who prefer that option.1 Telehealth may not have been an essential component of health care before the COVID-19 pandemic, but now it is clear that the best action VA can take is to swiftly enact its recommended change, stipulating that both VA telehealth and in-person health care constitute access to treatment. If implemented, this correction would save taxpayers an astronomical sum—according to a VA reportto Congress, about $1.1 billion in fiscal year 2021 alone.2 The cost savings from this proposed correction is reason enough to implement it. But just as importantly, increased use of VA telehealth also means higher quality, quicker, and more convenient care for veterans.
The VA is the recognized world leader in providing telehealth that is effective, timely, and veteran centric. Veterans across the country have access to telehealth services in more than 30 specialties.3 To ensure accessibility, the VA has established partnerships with major mobile broadband carriers so that veterans can receive telehealth at home without additional charges.4 The VA project Accessing Telehealth through Local Area Stations (ATLAS) brings VA telehealth to areas where existing internet infrastructure may not be adequate to support video telehealth. ATLAS is a collaboration with private organizations, including Veterans of Foreign Wars, The American Legion, and Walmart.4The agency also provides tablets to veterans who might not have access to telehealth, fostering higher access and patient satisfaction.4
The VA can initiate telehealth care rapidly. The “Anywhere to Anywhere” VA Health Care initiative and telecare hubs eliminate geographic constraints, allowing clinicians to provide team-based services across county and state lines to veterans’ homes and communities.
VA’s telehealth effort maximizes convenience for veterans. It reduces travel time, travel expenses, depletion of sick leave, and the need for childcare. Veterans with posttraumatic stress disorder or military sexual trauma who are triggered by traffic and waiting rooms, those with mobility issues, or those facing the stigma of mental health treatment often prefer to receive care in the familiarity of their home. Nonetheless, any veteran who desires an in-person appointment would continue to have that option under the proposed VA rule change.
VA telehealth is often used for mental health care, using the same evidence-based psychotherapies that VA has championed and are superior to that available in the private sector.5,6 This advantage is largely due to VA’s rigorous training, consultation, case review, care delivery, measurement standards, and integrated care model. In a recent survey of veterans engaged in mental health care, 80% reported that VA virtual care via video and/or telephone is as helpful or more helpful than in‐person services.7And yet, because of existing regulations, VA telemental health (TMH) does not qualify as access, resulting in hundreds of thousands of TMH visits being outsourced yearly to community practitioners that could be quickly and beneficially furnished by VA clinicians.
Telehealth has been shown to be as clinically effective as in-person care. A recent review of 38 meta-analyses covering telehealth with 10 medical disciplines found that for all disciplines, telehealth was as effective, if not more so, than conventional care.8 And because the likelihood of not showing up for telehealth appointments is lower than for in-person appointments, continuity of care is uninterrupted, and health care outcomes are improved.
Telehealth is health care. The VA must end the double standard that has handicapped it from including telehealth availability in determinations of eligibility for community care. The VA has voiced its intention to seek stakeholder input before implementing its proposed correction. The change is long overdue. It will save the VA a billion dollars annually while ensuring that veterans have quicker access to better treatment.
1 McDonough D. Statement of the honorable Denis McDonough Secretary of Veterans Affairs Department of Veterans Affairs (VA) before the Committee on Veterans’ Affairs United States Senate on veterans access to care. 117th Cong, 2nd Sess. September 21, 2022. Accessed May 8, 2023. https://www.veterans.senate.gov/2022/9/ensuring-veterans-timely-access-to-care-in-va-and-the-community/63b521ff-d308-449a-b3a3-918f4badb805
2 US Department of Veterans Affairs, Congressionally mandated report: access to care standards. September 2022.
3 US Department of Veterans Affairs. VA Secretary Press Conference, Thursday March 2, 2023. Accessed May 8, 2023. https://www.youtube.com/watch?v=WnkNl2whPoQ
4 US Department of Veterans Affairs, VA Telehealth: bridging the digital divide. Accessed May 8, 2023. https://telehealth.va.gov/digital-divide
5 Rand Corporation. Improving the Quality of Mental Health Care for Veterans: Lessons from RAND Research. Santa Monica, CA: RAND Corporation, 2019. https://www.rand.org/pubs/research_briefs/RB10087.html.
6 Lemle, R. Choice program expansion jeopardizes high-quality VHA mental health services. Federal Pract. 2018:35(3):18-24. [link to: https://www.mdedge.com/fedprac/article/159219/mental-health/choice-program-expansion-jeopardizes-high-quality-vha-mental
7 Campbell TM. Overview of the state of mental health care services in the VHA health care system. Presentation to the National Academies’ improving access to high-quality mental health care for veterans: a workshop. April 20, 2023. Accessed May 8, 2023. https://www.nationalacademies.org/documents/embed/link/LF2255DA3DD1C41C0A42D3BEF0989ACAECE3053A6A9B/file/D2C4B73BA6FFCAA81E6C4FC7C57020A5BA54376245AD?noSaveAs=1
8 Snoswell CL, Chelberg G, De Guzman KR, et al. The clinical effectiveness of telehealth: A systematic review of meta-analyses from 2010 to 2019. J Telemed Telecare. 2021;1357633X211022907. doi:10.1177/1357633X211022907
1 McDonough D. Statement of the honorable Denis McDonough Secretary of Veterans Affairs Department of Veterans Affairs (VA) before the Committee on Veterans’ Affairs United States Senate on veterans access to care. 117th Cong, 2nd Sess. September 21, 2022. Accessed May 8, 2023. https://www.veterans.senate.gov/2022/9/ensuring-veterans-timely-access-to-care-in-va-and-the-community/63b521ff-d308-449a-b3a3-918f4badb805
2 US Department of Veterans Affairs, Congressionally mandated report: access to care standards. September 2022.
3 US Department of Veterans Affairs. VA Secretary Press Conference, Thursday March 2, 2023. Accessed May 8, 2023. https://www.youtube.com/watch?v=WnkNl2whPoQ
4 US Department of Veterans Affairs, VA Telehealth: bridging the digital divide. Accessed May 8, 2023. https://telehealth.va.gov/digital-divide
5 Rand Corporation. Improving the Quality of Mental Health Care for Veterans: Lessons from RAND Research. Santa Monica, CA: RAND Corporation, 2019. https://www.rand.org/pubs/research_briefs/RB10087.html.
6 Lemle, R. Choice program expansion jeopardizes high-quality VHA mental health services. Federal Pract. 2018:35(3):18-24. [link to: https://www.mdedge.com/fedprac/article/159219/mental-health/choice-program-expansion-jeopardizes-high-quality-vha-mental
7 Campbell TM. Overview of the state of mental health care services in the VHA health care system. Presentation to the National Academies’ improving access to high-quality mental health care for veterans: a workshop. April 20, 2023. Accessed May 8, 2023. https://www.nationalacademies.org/documents/embed/link/LF2255DA3DD1C41C0A42D3BEF0989ACAECE3053A6A9B/file/D2C4B73BA6FFCAA81E6C4FC7C57020A5BA54376245AD?noSaveAs=1
8 Snoswell CL, Chelberg G, De Guzman KR, et al. The clinical effectiveness of telehealth: A systematic review of meta-analyses from 2010 to 2019. J Telemed Telecare. 2021;1357633X211022907. doi:10.1177/1357633X211022907
Georgia VA Doctor Indicted on Sexual Assault Charges
A primary care physician at the Veterans Affairs Medical Center in Decatur, Georgia, has been indicted on several counts of sexual assault of veteran patients. Rajesh Motibhai Patel is accused of violating his patients’ constitutional right to bodily integrity while acting under color of law and of engaging in unwanted sexual contact.
According to US Attorney Ryan Buchanan, Patel allegedly “violated his oath to do no harm to patients under his care.” He allegedly sexually touched 4 female patients during routine examinations.
Patel’s alleged crimes were “horrific and unacceptable,” US Department of Veterans Affairs (VA) press secretary Terrence Hayes said in a statement. “As soon as VA learned of these allegations, we removed this clinician from patient care and reassigned him to a role that had no patient interaction. Whenever a patient comes to VA, they deserve to know that they will be treated with care, compassion, and respect.”
The case is being investigated by the VA Office of Inspector General. Although Patel is only charged at present, not convicted, investigators believe he may have victimized other patients as well. Anyone with information is asked to call the VA-OIG tipline at (770) 758-6646.
A primary care physician at the Veterans Affairs Medical Center in Decatur, Georgia, has been indicted on several counts of sexual assault of veteran patients. Rajesh Motibhai Patel is accused of violating his patients’ constitutional right to bodily integrity while acting under color of law and of engaging in unwanted sexual contact.
According to US Attorney Ryan Buchanan, Patel allegedly “violated his oath to do no harm to patients under his care.” He allegedly sexually touched 4 female patients during routine examinations.
Patel’s alleged crimes were “horrific and unacceptable,” US Department of Veterans Affairs (VA) press secretary Terrence Hayes said in a statement. “As soon as VA learned of these allegations, we removed this clinician from patient care and reassigned him to a role that had no patient interaction. Whenever a patient comes to VA, they deserve to know that they will be treated with care, compassion, and respect.”
The case is being investigated by the VA Office of Inspector General. Although Patel is only charged at present, not convicted, investigators believe he may have victimized other patients as well. Anyone with information is asked to call the VA-OIG tipline at (770) 758-6646.
A primary care physician at the Veterans Affairs Medical Center in Decatur, Georgia, has been indicted on several counts of sexual assault of veteran patients. Rajesh Motibhai Patel is accused of violating his patients’ constitutional right to bodily integrity while acting under color of law and of engaging in unwanted sexual contact.
According to US Attorney Ryan Buchanan, Patel allegedly “violated his oath to do no harm to patients under his care.” He allegedly sexually touched 4 female patients during routine examinations.
Patel’s alleged crimes were “horrific and unacceptable,” US Department of Veterans Affairs (VA) press secretary Terrence Hayes said in a statement. “As soon as VA learned of these allegations, we removed this clinician from patient care and reassigned him to a role that had no patient interaction. Whenever a patient comes to VA, they deserve to know that they will be treated with care, compassion, and respect.”
The case is being investigated by the VA Office of Inspector General. Although Patel is only charged at present, not convicted, investigators believe he may have victimized other patients as well. Anyone with information is asked to call the VA-OIG tipline at (770) 758-6646.
Diversity – We’re not one size fits all
The United States has often been described as a “melting pot,” defined as diverse cultures and ethnicities coming together to form the rich fabric of our nation. These days, it seems that our fabric is a bit frayed.
DEIB (diversity, equity, inclusion, and belonging) is dawning as a significant conversation. Each and every one of us is unique by age, gender, culture/ethnicity, religion, socioeconomic status, geographical location, race, and sexual identity – to name just a few aspects of our identity. Keeping these differences in mind, it is evident that none of us fits a “one size fits all” mold.
Some of these differences, such as cross-cultural cuisine and holidays, are enjoyed and celebrated as wonderful opportunities to learn from others, embrace our distinctions, and have them beneficially contribute to our lives. Other differences, however, are not understood or embraced and are, in fact, belittled and stigmatized. Sexual identity falls into this category. It behooves us as a country to become more aware and educated about this category in our identities, in order to understand it, quell our unfounded fear, learn to support one another, and improve our collective mental health.
Recent reports have shown that exposing students and teachers to sexual identity diversity education has sparked some backlash from parents and communities alike. Those opposed are citing concerns over introducing children to LGBTQ+ information, either embedded in the school curriculum or made available in school library reading materials. “Children should remain innocent” seems to be the message. Perhaps parents prefer to discuss this topic privately, at home. Either way, teaching about diversity does not damage one’s innocence or deprive parents of private conversations. In fact, it educates children by improving their awareness, tolerance, and acceptance of others’ differences, and can serve as a catalyst to further parental conversation.
There are kids everywhere who are starting to develop and understand their identities. Wouldn’t it be wonderful for them to know that whichever way they identify is okay, that they are not ‘weird’ or ‘different,’ but that in fact we are all different? Wouldn’t it be great for them to be able to explore and discuss their identities and journeys openly, and not have to hide for fear of retribution or bullying?
It is important for these children to know that they are not alone, that they have options, and that they don’t need to contemplate suicide because they believe that their identity makes them not worthy of being in this world.
Starting the conversation early on in life can empower our youth by planting the seed that people are not “one size fits all,” which is the element responsible for our being unique and human. Diversity can be woven into the rich fabric that defines our nation, rather than be a factor that unravels it.
April was National Diversity Awareness Month and we took time to celebrate our country’s cultural melting pot. By embracing our differences, we can show our children and ourselves how to better navigate diversity, which can help us all fit in.
Dr. Jarkon is a psychiatrist and director of the Center for Behavioral Health at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y.
The United States has often been described as a “melting pot,” defined as diverse cultures and ethnicities coming together to form the rich fabric of our nation. These days, it seems that our fabric is a bit frayed.
DEIB (diversity, equity, inclusion, and belonging) is dawning as a significant conversation. Each and every one of us is unique by age, gender, culture/ethnicity, religion, socioeconomic status, geographical location, race, and sexual identity – to name just a few aspects of our identity. Keeping these differences in mind, it is evident that none of us fits a “one size fits all” mold.
Some of these differences, such as cross-cultural cuisine and holidays, are enjoyed and celebrated as wonderful opportunities to learn from others, embrace our distinctions, and have them beneficially contribute to our lives. Other differences, however, are not understood or embraced and are, in fact, belittled and stigmatized. Sexual identity falls into this category. It behooves us as a country to become more aware and educated about this category in our identities, in order to understand it, quell our unfounded fear, learn to support one another, and improve our collective mental health.
Recent reports have shown that exposing students and teachers to sexual identity diversity education has sparked some backlash from parents and communities alike. Those opposed are citing concerns over introducing children to LGBTQ+ information, either embedded in the school curriculum or made available in school library reading materials. “Children should remain innocent” seems to be the message. Perhaps parents prefer to discuss this topic privately, at home. Either way, teaching about diversity does not damage one’s innocence or deprive parents of private conversations. In fact, it educates children by improving their awareness, tolerance, and acceptance of others’ differences, and can serve as a catalyst to further parental conversation.
There are kids everywhere who are starting to develop and understand their identities. Wouldn’t it be wonderful for them to know that whichever way they identify is okay, that they are not ‘weird’ or ‘different,’ but that in fact we are all different? Wouldn’t it be great for them to be able to explore and discuss their identities and journeys openly, and not have to hide for fear of retribution or bullying?
It is important for these children to know that they are not alone, that they have options, and that they don’t need to contemplate suicide because they believe that their identity makes them not worthy of being in this world.
Starting the conversation early on in life can empower our youth by planting the seed that people are not “one size fits all,” which is the element responsible for our being unique and human. Diversity can be woven into the rich fabric that defines our nation, rather than be a factor that unravels it.
April was National Diversity Awareness Month and we took time to celebrate our country’s cultural melting pot. By embracing our differences, we can show our children and ourselves how to better navigate diversity, which can help us all fit in.
Dr. Jarkon is a psychiatrist and director of the Center for Behavioral Health at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y.
The United States has often been described as a “melting pot,” defined as diverse cultures and ethnicities coming together to form the rich fabric of our nation. These days, it seems that our fabric is a bit frayed.
DEIB (diversity, equity, inclusion, and belonging) is dawning as a significant conversation. Each and every one of us is unique by age, gender, culture/ethnicity, religion, socioeconomic status, geographical location, race, and sexual identity – to name just a few aspects of our identity. Keeping these differences in mind, it is evident that none of us fits a “one size fits all” mold.
Some of these differences, such as cross-cultural cuisine and holidays, are enjoyed and celebrated as wonderful opportunities to learn from others, embrace our distinctions, and have them beneficially contribute to our lives. Other differences, however, are not understood or embraced and are, in fact, belittled and stigmatized. Sexual identity falls into this category. It behooves us as a country to become more aware and educated about this category in our identities, in order to understand it, quell our unfounded fear, learn to support one another, and improve our collective mental health.
Recent reports have shown that exposing students and teachers to sexual identity diversity education has sparked some backlash from parents and communities alike. Those opposed are citing concerns over introducing children to LGBTQ+ information, either embedded in the school curriculum or made available in school library reading materials. “Children should remain innocent” seems to be the message. Perhaps parents prefer to discuss this topic privately, at home. Either way, teaching about diversity does not damage one’s innocence or deprive parents of private conversations. In fact, it educates children by improving their awareness, tolerance, and acceptance of others’ differences, and can serve as a catalyst to further parental conversation.
There are kids everywhere who are starting to develop and understand their identities. Wouldn’t it be wonderful for them to know that whichever way they identify is okay, that they are not ‘weird’ or ‘different,’ but that in fact we are all different? Wouldn’t it be great for them to be able to explore and discuss their identities and journeys openly, and not have to hide for fear of retribution or bullying?
It is important for these children to know that they are not alone, that they have options, and that they don’t need to contemplate suicide because they believe that their identity makes them not worthy of being in this world.
Starting the conversation early on in life can empower our youth by planting the seed that people are not “one size fits all,” which is the element responsible for our being unique and human. Diversity can be woven into the rich fabric that defines our nation, rather than be a factor that unravels it.
April was National Diversity Awareness Month and we took time to celebrate our country’s cultural melting pot. By embracing our differences, we can show our children and ourselves how to better navigate diversity, which can help us all fit in.
Dr. Jarkon is a psychiatrist and director of the Center for Behavioral Health at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y.
The breathtaking effects of climate change
To see the harmful effects of climate change firsthand, you need look no farther than the nearest pulmonary clinic.
The causes and effects are unmistakable: pollen storms leading to allergy sufferers flooding into allergists’ offices; rising air pollution levels increasing risk for obstructive airway diseases, cardiopulmonary complications, and non–small cell lung cancer; melting snowpacks and atmospheric rivers inundating neighborhoods and leaving moldy debris and incipient fungal infections in their wake.
“The reason why we think climate change is going to change the type of disease patterns and the severity of illness that we see in patients with respiratory diseases is that it changes a lot of the environment as well as the exposures,” said Bathmapriya Balakrishnan, BMedSci, BMBS, from the section of Pulmonary, Critical Care, and Sleep Medicine in the department of medicine at West Virginia University, Morgantown.
“What we’re going to see is not just new diseases but also exacerbation of chronic diseases, things like asthma [and] COPD. And there’s also concern that patients who are otherwise healthy, because they now have more exposures that are due to climate change, can then develop these diseases,” she said in an interview.
Ms. Balakrishnan is the lead author of a comprehensive, evidence-based review focused on the effects of climate change and air pollution across the spectrum of pulmonary disorders. The review is published online ahead of print in the journal Chest.
“ To inform health care providers of evidence-based methods and improve patient counselling, further research regarding measures that limit exposure is needed. Empowering patients with resources to monitor air quality and minimize exposure is a key preventative measure for decreasing morbidity and mortality while improving quality of life,” Ms. Balakrishnan and colleagues write.
Similarly, in a statement on the effects of climate change on respiratory health, the American Public Health Association succinctly summarized the problem: “Warmer temperatures lead to an increase in pollutants and allergens. Poor air quality leads to reduced lung function, increased risk of asthma complications, heart attacks, heart failure, and death. Air pollution and allergens are the main exposures affecting lung and heart health in this changing climate.”
Early spring
Stanley Fineman, MD, MBA, a past president of the American College of Allergy, Asthma, & Immunology and an allergist in private practice in Atlanta, has seen firsthand how global warming and an earlier start to spring allergy season is affecting his patients.
“The season, at least in our area metro Atlanta, started earlier and has been lasting longer. The pollen counts are very high,” he told this news organization.
“In February we started seeing pollen counts over 1,000 [grams per cubic meter], which is unheard of, and in March about half the days we counted levels that were over 1,000, which is also unheard of. In April it was over 1,000 almost half the days.”
Dr. Fineman and colleagues both in Atlanta and across the country have reported sharp increases in the proportion of new adult patients and in existing patients who have experienced exacerbation of previously mild disease.
“Probably what’s happened is that they may have had some allergic sensitivity that resulted in milder manifestations, but this year they’re getting major manifestations,” Dr. Fineman said.
In a 2014 article in the journal European Respiratory Review, Gennaro D’Amato, MD, from High Speciality Hospital Antonio Cardarelli, Naples, Italy, and colleagues outlined the main effects of climate on pollen levels: “1) an increase in plant growth and faster plant growth; 2) an increase in the amount of pollen produced by each plant; 3) an increase in the amount of allergenic proteins contained in pollen; 4) an increase in the start time of plant growth and, therefore, the start of pollen production; 5) an earlier and longer pollen season; 6) change in the geospatial distribution of pollen, that is plant ranges and long-distance atmospheric transport moving polewards,” they write.
Bad air
In addition to pollen, the ambient air in many places is increasingly becoming saturated with bioallergenic proteins such as bacteria, viruses, animal dander, insects, molds, and plant species, Ms. Balakrishnan and colleagues noted, adding that “atmospheric levels of carbon dioxide have also been found to increase pollen productivity. These changes result in greater over-the-counter medication use, emergency department visits, and outpatient visits for respiratory illnesses.”
The rash of violent storms that has washed over much of the United States in recent months is also likely to increase the incidence of so-called “thunderstorm asthma,” caused when large quantities of respirable particulate matter are released before or during a thunderstorm.
Air pollution from the burning of carbon-based fuels and from wildfires sparked by hotter and drier conditions increase airborne particulate matter that can seriously exacerbate asthma, COPD, and other obstructive airway conditions.
In addition, as previously reported by Medscape, exposure to particulate matter has been implicated as a possible cause of non–small cell lung cancer in persons who have never smoked.
Critical care challenges
Among the myriad other effects of climate change postulated in evidence enumerated by Ms. Balakrishnan and colleagues are chest infections and pleural diseases, such as aspergillosis infections that occur after catastrophic flooding; increased incidence of Mycobacterium avium complex infections and hypersensitivity pneumonitis; increased demands on critical care specialists from natural disasters; pollution-induced cardiac arrest; and heat prostration and heat stroke from increasingly prevalent heat waves.
The reviewers also examined evidence suggesting links between climate change and pulmonary hypertension, interstitial lung disease, sleep disorders, and occupational pulmonary disorders.
Power to the patients
“Pulmonologists should counsel patients on ways to minimize outdoor and indoor pollution, using tight-fitting respirators and home air-purifying systems without encroaching on patients’ beliefs and choices,” the authors advise.
“Empowering patients with resources to monitor air quality daily, in inclement weather, and during disasters would help minimize exposure and thus improve overall health. The pulmonologist can play an important role in emphasizing the impact of climate change on pulmonary disorders during patient care encounters,” they write.
Ms. Balakrishan adds that another important mitigation measure that can be taken today is education.
“In medical school we don’t really learn about the impact of climate change – at least in my generation of physicians, climate change or global warming weren’t part of the medical curriculum – but now I think that there’s a lot of advocacy work being done by medical students who actually want more education on climate change and its effects on pulmonary diseases,” she said.
The study by Ms. Balakrishnan and colleagues was unfunded. Ms. Balakrishnan reports no relevant financial relationships. Co-author Mary-Beth Scholand, MD, has received personal fees from serving on advisory boards and speakers bureaus for Genentech, Boehringer Ingelheim, Veracyte, and United Therapeutics. Co-author Sean Callahan, MD, has received personal fees for serving on advisory boards for Gilead and Boehringer Ingelheim. Dr. Fineman reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
To see the harmful effects of climate change firsthand, you need look no farther than the nearest pulmonary clinic.
The causes and effects are unmistakable: pollen storms leading to allergy sufferers flooding into allergists’ offices; rising air pollution levels increasing risk for obstructive airway diseases, cardiopulmonary complications, and non–small cell lung cancer; melting snowpacks and atmospheric rivers inundating neighborhoods and leaving moldy debris and incipient fungal infections in their wake.
“The reason why we think climate change is going to change the type of disease patterns and the severity of illness that we see in patients with respiratory diseases is that it changes a lot of the environment as well as the exposures,” said Bathmapriya Balakrishnan, BMedSci, BMBS, from the section of Pulmonary, Critical Care, and Sleep Medicine in the department of medicine at West Virginia University, Morgantown.
“What we’re going to see is not just new diseases but also exacerbation of chronic diseases, things like asthma [and] COPD. And there’s also concern that patients who are otherwise healthy, because they now have more exposures that are due to climate change, can then develop these diseases,” she said in an interview.
Ms. Balakrishnan is the lead author of a comprehensive, evidence-based review focused on the effects of climate change and air pollution across the spectrum of pulmonary disorders. The review is published online ahead of print in the journal Chest.
“ To inform health care providers of evidence-based methods and improve patient counselling, further research regarding measures that limit exposure is needed. Empowering patients with resources to monitor air quality and minimize exposure is a key preventative measure for decreasing morbidity and mortality while improving quality of life,” Ms. Balakrishnan and colleagues write.
Similarly, in a statement on the effects of climate change on respiratory health, the American Public Health Association succinctly summarized the problem: “Warmer temperatures lead to an increase in pollutants and allergens. Poor air quality leads to reduced lung function, increased risk of asthma complications, heart attacks, heart failure, and death. Air pollution and allergens are the main exposures affecting lung and heart health in this changing climate.”
Early spring
Stanley Fineman, MD, MBA, a past president of the American College of Allergy, Asthma, & Immunology and an allergist in private practice in Atlanta, has seen firsthand how global warming and an earlier start to spring allergy season is affecting his patients.
“The season, at least in our area metro Atlanta, started earlier and has been lasting longer. The pollen counts are very high,” he told this news organization.
“In February we started seeing pollen counts over 1,000 [grams per cubic meter], which is unheard of, and in March about half the days we counted levels that were over 1,000, which is also unheard of. In April it was over 1,000 almost half the days.”
Dr. Fineman and colleagues both in Atlanta and across the country have reported sharp increases in the proportion of new adult patients and in existing patients who have experienced exacerbation of previously mild disease.
“Probably what’s happened is that they may have had some allergic sensitivity that resulted in milder manifestations, but this year they’re getting major manifestations,” Dr. Fineman said.
In a 2014 article in the journal European Respiratory Review, Gennaro D’Amato, MD, from High Speciality Hospital Antonio Cardarelli, Naples, Italy, and colleagues outlined the main effects of climate on pollen levels: “1) an increase in plant growth and faster plant growth; 2) an increase in the amount of pollen produced by each plant; 3) an increase in the amount of allergenic proteins contained in pollen; 4) an increase in the start time of plant growth and, therefore, the start of pollen production; 5) an earlier and longer pollen season; 6) change in the geospatial distribution of pollen, that is plant ranges and long-distance atmospheric transport moving polewards,” they write.
Bad air
In addition to pollen, the ambient air in many places is increasingly becoming saturated with bioallergenic proteins such as bacteria, viruses, animal dander, insects, molds, and plant species, Ms. Balakrishnan and colleagues noted, adding that “atmospheric levels of carbon dioxide have also been found to increase pollen productivity. These changes result in greater over-the-counter medication use, emergency department visits, and outpatient visits for respiratory illnesses.”
The rash of violent storms that has washed over much of the United States in recent months is also likely to increase the incidence of so-called “thunderstorm asthma,” caused when large quantities of respirable particulate matter are released before or during a thunderstorm.
Air pollution from the burning of carbon-based fuels and from wildfires sparked by hotter and drier conditions increase airborne particulate matter that can seriously exacerbate asthma, COPD, and other obstructive airway conditions.
In addition, as previously reported by Medscape, exposure to particulate matter has been implicated as a possible cause of non–small cell lung cancer in persons who have never smoked.
Critical care challenges
Among the myriad other effects of climate change postulated in evidence enumerated by Ms. Balakrishnan and colleagues are chest infections and pleural diseases, such as aspergillosis infections that occur after catastrophic flooding; increased incidence of Mycobacterium avium complex infections and hypersensitivity pneumonitis; increased demands on critical care specialists from natural disasters; pollution-induced cardiac arrest; and heat prostration and heat stroke from increasingly prevalent heat waves.
The reviewers also examined evidence suggesting links between climate change and pulmonary hypertension, interstitial lung disease, sleep disorders, and occupational pulmonary disorders.
Power to the patients
“Pulmonologists should counsel patients on ways to minimize outdoor and indoor pollution, using tight-fitting respirators and home air-purifying systems without encroaching on patients’ beliefs and choices,” the authors advise.
“Empowering patients with resources to monitor air quality daily, in inclement weather, and during disasters would help minimize exposure and thus improve overall health. The pulmonologist can play an important role in emphasizing the impact of climate change on pulmonary disorders during patient care encounters,” they write.
Ms. Balakrishan adds that another important mitigation measure that can be taken today is education.
“In medical school we don’t really learn about the impact of climate change – at least in my generation of physicians, climate change or global warming weren’t part of the medical curriculum – but now I think that there’s a lot of advocacy work being done by medical students who actually want more education on climate change and its effects on pulmonary diseases,” she said.
The study by Ms. Balakrishnan and colleagues was unfunded. Ms. Balakrishnan reports no relevant financial relationships. Co-author Mary-Beth Scholand, MD, has received personal fees from serving on advisory boards and speakers bureaus for Genentech, Boehringer Ingelheim, Veracyte, and United Therapeutics. Co-author Sean Callahan, MD, has received personal fees for serving on advisory boards for Gilead and Boehringer Ingelheim. Dr. Fineman reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
To see the harmful effects of climate change firsthand, you need look no farther than the nearest pulmonary clinic.
The causes and effects are unmistakable: pollen storms leading to allergy sufferers flooding into allergists’ offices; rising air pollution levels increasing risk for obstructive airway diseases, cardiopulmonary complications, and non–small cell lung cancer; melting snowpacks and atmospheric rivers inundating neighborhoods and leaving moldy debris and incipient fungal infections in their wake.
“The reason why we think climate change is going to change the type of disease patterns and the severity of illness that we see in patients with respiratory diseases is that it changes a lot of the environment as well as the exposures,” said Bathmapriya Balakrishnan, BMedSci, BMBS, from the section of Pulmonary, Critical Care, and Sleep Medicine in the department of medicine at West Virginia University, Morgantown.
“What we’re going to see is not just new diseases but also exacerbation of chronic diseases, things like asthma [and] COPD. And there’s also concern that patients who are otherwise healthy, because they now have more exposures that are due to climate change, can then develop these diseases,” she said in an interview.
Ms. Balakrishnan is the lead author of a comprehensive, evidence-based review focused on the effects of climate change and air pollution across the spectrum of pulmonary disorders. The review is published online ahead of print in the journal Chest.
“ To inform health care providers of evidence-based methods and improve patient counselling, further research regarding measures that limit exposure is needed. Empowering patients with resources to monitor air quality and minimize exposure is a key preventative measure for decreasing morbidity and mortality while improving quality of life,” Ms. Balakrishnan and colleagues write.
Similarly, in a statement on the effects of climate change on respiratory health, the American Public Health Association succinctly summarized the problem: “Warmer temperatures lead to an increase in pollutants and allergens. Poor air quality leads to reduced lung function, increased risk of asthma complications, heart attacks, heart failure, and death. Air pollution and allergens are the main exposures affecting lung and heart health in this changing climate.”
Early spring
Stanley Fineman, MD, MBA, a past president of the American College of Allergy, Asthma, & Immunology and an allergist in private practice in Atlanta, has seen firsthand how global warming and an earlier start to spring allergy season is affecting his patients.
“The season, at least in our area metro Atlanta, started earlier and has been lasting longer. The pollen counts are very high,” he told this news organization.
“In February we started seeing pollen counts over 1,000 [grams per cubic meter], which is unheard of, and in March about half the days we counted levels that were over 1,000, which is also unheard of. In April it was over 1,000 almost half the days.”
Dr. Fineman and colleagues both in Atlanta and across the country have reported sharp increases in the proportion of new adult patients and in existing patients who have experienced exacerbation of previously mild disease.
“Probably what’s happened is that they may have had some allergic sensitivity that resulted in milder manifestations, but this year they’re getting major manifestations,” Dr. Fineman said.
In a 2014 article in the journal European Respiratory Review, Gennaro D’Amato, MD, from High Speciality Hospital Antonio Cardarelli, Naples, Italy, and colleagues outlined the main effects of climate on pollen levels: “1) an increase in plant growth and faster plant growth; 2) an increase in the amount of pollen produced by each plant; 3) an increase in the amount of allergenic proteins contained in pollen; 4) an increase in the start time of plant growth and, therefore, the start of pollen production; 5) an earlier and longer pollen season; 6) change in the geospatial distribution of pollen, that is plant ranges and long-distance atmospheric transport moving polewards,” they write.
Bad air
In addition to pollen, the ambient air in many places is increasingly becoming saturated with bioallergenic proteins such as bacteria, viruses, animal dander, insects, molds, and plant species, Ms. Balakrishnan and colleagues noted, adding that “atmospheric levels of carbon dioxide have also been found to increase pollen productivity. These changes result in greater over-the-counter medication use, emergency department visits, and outpatient visits for respiratory illnesses.”
The rash of violent storms that has washed over much of the United States in recent months is also likely to increase the incidence of so-called “thunderstorm asthma,” caused when large quantities of respirable particulate matter are released before or during a thunderstorm.
Air pollution from the burning of carbon-based fuels and from wildfires sparked by hotter and drier conditions increase airborne particulate matter that can seriously exacerbate asthma, COPD, and other obstructive airway conditions.
In addition, as previously reported by Medscape, exposure to particulate matter has been implicated as a possible cause of non–small cell lung cancer in persons who have never smoked.
Critical care challenges
Among the myriad other effects of climate change postulated in evidence enumerated by Ms. Balakrishnan and colleagues are chest infections and pleural diseases, such as aspergillosis infections that occur after catastrophic flooding; increased incidence of Mycobacterium avium complex infections and hypersensitivity pneumonitis; increased demands on critical care specialists from natural disasters; pollution-induced cardiac arrest; and heat prostration and heat stroke from increasingly prevalent heat waves.
The reviewers also examined evidence suggesting links between climate change and pulmonary hypertension, interstitial lung disease, sleep disorders, and occupational pulmonary disorders.
Power to the patients
“Pulmonologists should counsel patients on ways to minimize outdoor and indoor pollution, using tight-fitting respirators and home air-purifying systems without encroaching on patients’ beliefs and choices,” the authors advise.
“Empowering patients with resources to monitor air quality daily, in inclement weather, and during disasters would help minimize exposure and thus improve overall health. The pulmonologist can play an important role in emphasizing the impact of climate change on pulmonary disorders during patient care encounters,” they write.
Ms. Balakrishan adds that another important mitigation measure that can be taken today is education.
“In medical school we don’t really learn about the impact of climate change – at least in my generation of physicians, climate change or global warming weren’t part of the medical curriculum – but now I think that there’s a lot of advocacy work being done by medical students who actually want more education on climate change and its effects on pulmonary diseases,” she said.
The study by Ms. Balakrishnan and colleagues was unfunded. Ms. Balakrishnan reports no relevant financial relationships. Co-author Mary-Beth Scholand, MD, has received personal fees from serving on advisory boards and speakers bureaus for Genentech, Boehringer Ingelheim, Veracyte, and United Therapeutics. Co-author Sean Callahan, MD, has received personal fees for serving on advisory boards for Gilead and Boehringer Ingelheim. Dr. Fineman reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CRC screening rates are higher in Medicaid expansion states
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
CHICAGO – presented on May 6 in Chicago at the annual Digestive Disease Week®.
Researchers from the University of California, Los Angeles, reported that states with expanded Medicaid coverage had significantly higher rates of colorectal cancer (CRC) screening than states where officials refused federal support for Medicaid expansion.
Led by Megan R. McLeod, MD, an internal medicine resident at the University of California, Los Angeles, researchers compared CRC screening rates in states that did not adopt Medicaid expansion in 2021 with screening rates in states that invested Medicaid expansion into 1,284 Federally Qualified Health Centers, which are nonprofit health centers or clinics that serve medically underserved areas and populations. In this study, 76% of these centers were in states that accepted Medicaid expansion. The median colorectal cancer screening rate was 42.1% in Medicaid expansion states, compared with 36.5% in nonexpansion states
“The impact of being uninsured on CRC screening participation was profound in nonexpansion states,” said Dr. McLeod, who will be a UCLA gastroenterology fellow this year.
The study adds to a growing body of evidence that shows Medicaid expansion, which increases access to health care services to previously uninsured or underinsured patients, can improve health outcomes and may reduce racial and economic disparities.
For example, a 2019 study based on electronic health record data presented at the annual meeting of the American Society of Clinical Oncology showed that, after Medicaid expansion, racial differences in timely cancer treatment effectively disappeared. Before Medicaid expansion, Black patients were 4.8% less likely than White patients to receive timely cancer treatment, which is defined as treatment starting within 30 days of the diagnosis of an advanced or metastatic solid tumor. After Medicaid expansion, however, the difference between the racial groups dwindled to 0.8% and was no longer statistically significant.
Researchers at Weill Cornell Medical Center in New York reported in 2020 at the virtual annual meeting of the American Association for the Study of Liver Diseases that, 1 year after Medicaid expansion began on Jan. 1, 2014, the rate of liver-related mortality began to decline in 18 states with expanded coverage, whereas the rate of liver-related deaths continued to climb in 14 states that did not expand Medicaid.
The U.S. Health Resources and Services Administration funds Federally Qualified Health Centers (FQHC) that serve nearly 29 million patients throughout the country, including a large proportion whose care is covered by Medicaid. Among patients cared for in these centers, one in three have incomes below the federal poverty line, and one in five are uninsured.
Screening rates compared
Dr. McLeod and colleagues sought to determine whether Medicaid expansion would have an effect on CRC screening rates at these centers. The final analysis included 6,940,879 patients (between 50 and 74 years), of whom 1.7% were unhoused and 17.6% were uninsured.
Medicaid expansion status appeared to have a direct impact on whether screenings were even offered to patients. Centers in rural areas and those with a high proportion of uninsured patients were found to have significantly higher odds for doing fewer CRC screenings. In Medicaid expansion states, CRC screening rates were significantly lower for patients who were male, Black, Hispanic, had low income, were unhoused, or were uninsured.
In a Q&A that followed the presentation, Steven Itzkowitz, MD, director of the GI fellowship program at the Icahn School of Medicine at Mount Sinai, New York, suggested the type of CRC test patients are offered is directly related to Medicaid expansion status.
“In New York, before Cologuard (a colon and rectal cancer screening test) was covered by Medicaid, it wasn’t used very much, but once it got paid for by Medicaid, rates went up,” he said.
The study was internally supported. Dr. McLeod reported no conflicts of interest. Dr. Itzkowitz has been a consultant for Exact Sciences, the maker of Cologuard.
DDW is sponsored by the American Gastroenterological Association, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
AT DDW 2023
H. pylori eradication therapy curbs risk for stomach cancer
People with H. pylori who were treated had about a 63% lower risk of developing noncardia gastric adenocarcinoma (NCGA) after 8 years of follow-up, compared with peers with H. pylori who were not treated.
The U.S. data align with previous studies, conducted mostly in Asia, that found that treating the infection can reduce stomach cancer incidence.
The KPNC study shows the “potential for stomach cancer prevention in U.S. populations through H. pylori screening and treatment,” study investigator Dan Li, MD, gastroenterologist with the Kaiser Permanente Medical Group and Kaiser Permanente Division of Research in Oakland, Calif., said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health in New York, who wasn’t involved in the research, said that the study is significant because “it is the first to show this effect in a large, diverse population in the U.S., where gastric cancer incidence is lower.”
The study was published online in Gastroenterology.
Top risk factor
About 30% of people in the United States are infected with H. pylori, which is the No. 1 known risk factor for stomach cancer, Dr. Li said.
The study cohort included 716,567 KPNC members who underwent H. pylori testing and/or treatment between 1997 and 2015.
Among H. pylori–infected individuals (based on positive nonserology test results), the subdistribution hazard ratio was 6.07 for untreated individuals and 2.68 for treated individuals, compared with H. pylori–negative individuals.
It’s not surprising that people who were treated for the infection still had a higher risk of NCGA than people who had never had the infection, Dr. Li said.
“This is likely because many people with chronic H. pylori infection had already developed some precancerous changes in their stomach before they were treated. This finding suggests that H. pylori ideally should be treated before precancerous changes develop,” he said.
When compared directly with H. pylori–positive/untreated individuals, the risk for NCGA in H. pylori–positive/treated individuals was somewhat lower at less than 8 years follow-up (sHR, 0.95) and significantly lower at 8+ years of follow-up (sHR, 0.37).
“After 7-10 years of follow-up, people with H. pylori who received treatment had nearly half the risk of developing stomach cancer as the general population,” Dr. Li said. “This is likely because most people infected with H. pylori in the general population are not screened nor treated. This highlights the impact screening and treatment can have.”
The data also show that cumulative incidence curves for H. pylori–positive/untreated and H. pylori–positive/treated largely overlapped during the first 7 years of follow-up and started to separate after 8 years.
At 10 years, cumulative NCGA incidence rates for H. pylori–positive/untreated, H. pylori–positive/treated, and H. pylori negative were 31.0, 19.7, and 3.5 per 10,000 persons, respectively (P < .0001).
This study shows that treating H. pylori reduces stomach cancer incidence in the United States, thus “filling an important research and knowledge gap,” Dr. Li said.
In the United States, Asian, Black, and Hispanic adults are much more likely to be infected with H. pylori, and they have a two- to threefold higher risk of developing stomach cancer, he noted.
“This suggests it may be reasonable to consider targeted screening and treatment in these high-risk groups. However, the optimal strategy for population-based H. pylori screening has not been established, and more research is needed to determine who should be screened for H. pylori and at what age screening should begin,” Dr. Li said.
Strong data, jury out on universal screening
For additional comment, this news organization reached out to Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y.
The study shows that the treatment of H. pylori “absolutely will decrease your risk of certain types of gastric carcinoma down the line. It does take a while to show that, 7 years, but this study shows that very clearly,” Dr. Glatt said.
“People who have definitely been shown to have H. pylori should be treated,” Dr. Glatt said.
“I don’t think this study yet supports that everybody should be screened, but it does make sense that people who have upper GI symptoms consistent with H. pylori should be checked for H. pylori and then appropriately treated, he noted.
Routine screening for H. pylori is recommended in countries with high incidence of gastric cancer, but not in the United States, Dr. Kim noted.
“Given the risk reduction of cancer with H. pylori treatment, consideration should be made in the U.S. for asymptomatic individuals with a family history of gastric cancer or immigrants from high-incidence countries,” she added.
The study was funded by the Kaiser Permanente Northern California Community Health Research Grants Program, the Permanente Medical Group Delivery Science & Applied Research Program, and the Permanente Medical Group. Dr. Li, Dr. Glatt, and Dr. Kim have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
People with H. pylori who were treated had about a 63% lower risk of developing noncardia gastric adenocarcinoma (NCGA) after 8 years of follow-up, compared with peers with H. pylori who were not treated.
The U.S. data align with previous studies, conducted mostly in Asia, that found that treating the infection can reduce stomach cancer incidence.
The KPNC study shows the “potential for stomach cancer prevention in U.S. populations through H. pylori screening and treatment,” study investigator Dan Li, MD, gastroenterologist with the Kaiser Permanente Medical Group and Kaiser Permanente Division of Research in Oakland, Calif., said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health in New York, who wasn’t involved in the research, said that the study is significant because “it is the first to show this effect in a large, diverse population in the U.S., where gastric cancer incidence is lower.”
The study was published online in Gastroenterology.
Top risk factor
About 30% of people in the United States are infected with H. pylori, which is the No. 1 known risk factor for stomach cancer, Dr. Li said.
The study cohort included 716,567 KPNC members who underwent H. pylori testing and/or treatment between 1997 and 2015.
Among H. pylori–infected individuals (based on positive nonserology test results), the subdistribution hazard ratio was 6.07 for untreated individuals and 2.68 for treated individuals, compared with H. pylori–negative individuals.
It’s not surprising that people who were treated for the infection still had a higher risk of NCGA than people who had never had the infection, Dr. Li said.
“This is likely because many people with chronic H. pylori infection had already developed some precancerous changes in their stomach before they were treated. This finding suggests that H. pylori ideally should be treated before precancerous changes develop,” he said.
When compared directly with H. pylori–positive/untreated individuals, the risk for NCGA in H. pylori–positive/treated individuals was somewhat lower at less than 8 years follow-up (sHR, 0.95) and significantly lower at 8+ years of follow-up (sHR, 0.37).
“After 7-10 years of follow-up, people with H. pylori who received treatment had nearly half the risk of developing stomach cancer as the general population,” Dr. Li said. “This is likely because most people infected with H. pylori in the general population are not screened nor treated. This highlights the impact screening and treatment can have.”
The data also show that cumulative incidence curves for H. pylori–positive/untreated and H. pylori–positive/treated largely overlapped during the first 7 years of follow-up and started to separate after 8 years.
At 10 years, cumulative NCGA incidence rates for H. pylori–positive/untreated, H. pylori–positive/treated, and H. pylori negative were 31.0, 19.7, and 3.5 per 10,000 persons, respectively (P < .0001).
This study shows that treating H. pylori reduces stomach cancer incidence in the United States, thus “filling an important research and knowledge gap,” Dr. Li said.
In the United States, Asian, Black, and Hispanic adults are much more likely to be infected with H. pylori, and they have a two- to threefold higher risk of developing stomach cancer, he noted.
“This suggests it may be reasonable to consider targeted screening and treatment in these high-risk groups. However, the optimal strategy for population-based H. pylori screening has not been established, and more research is needed to determine who should be screened for H. pylori and at what age screening should begin,” Dr. Li said.
Strong data, jury out on universal screening
For additional comment, this news organization reached out to Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y.
The study shows that the treatment of H. pylori “absolutely will decrease your risk of certain types of gastric carcinoma down the line. It does take a while to show that, 7 years, but this study shows that very clearly,” Dr. Glatt said.
“People who have definitely been shown to have H. pylori should be treated,” Dr. Glatt said.
“I don’t think this study yet supports that everybody should be screened, but it does make sense that people who have upper GI symptoms consistent with H. pylori should be checked for H. pylori and then appropriately treated, he noted.
Routine screening for H. pylori is recommended in countries with high incidence of gastric cancer, but not in the United States, Dr. Kim noted.
“Given the risk reduction of cancer with H. pylori treatment, consideration should be made in the U.S. for asymptomatic individuals with a family history of gastric cancer or immigrants from high-incidence countries,” she added.
The study was funded by the Kaiser Permanente Northern California Community Health Research Grants Program, the Permanente Medical Group Delivery Science & Applied Research Program, and the Permanente Medical Group. Dr. Li, Dr. Glatt, and Dr. Kim have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
People with H. pylori who were treated had about a 63% lower risk of developing noncardia gastric adenocarcinoma (NCGA) after 8 years of follow-up, compared with peers with H. pylori who were not treated.
The U.S. data align with previous studies, conducted mostly in Asia, that found that treating the infection can reduce stomach cancer incidence.
The KPNC study shows the “potential for stomach cancer prevention in U.S. populations through H. pylori screening and treatment,” study investigator Dan Li, MD, gastroenterologist with the Kaiser Permanente Medical Group and Kaiser Permanente Division of Research in Oakland, Calif., said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health in New York, who wasn’t involved in the research, said that the study is significant because “it is the first to show this effect in a large, diverse population in the U.S., where gastric cancer incidence is lower.”
The study was published online in Gastroenterology.
Top risk factor
About 30% of people in the United States are infected with H. pylori, which is the No. 1 known risk factor for stomach cancer, Dr. Li said.
The study cohort included 716,567 KPNC members who underwent H. pylori testing and/or treatment between 1997 and 2015.
Among H. pylori–infected individuals (based on positive nonserology test results), the subdistribution hazard ratio was 6.07 for untreated individuals and 2.68 for treated individuals, compared with H. pylori–negative individuals.
It’s not surprising that people who were treated for the infection still had a higher risk of NCGA than people who had never had the infection, Dr. Li said.
“This is likely because many people with chronic H. pylori infection had already developed some precancerous changes in their stomach before they were treated. This finding suggests that H. pylori ideally should be treated before precancerous changes develop,” he said.
When compared directly with H. pylori–positive/untreated individuals, the risk for NCGA in H. pylori–positive/treated individuals was somewhat lower at less than 8 years follow-up (sHR, 0.95) and significantly lower at 8+ years of follow-up (sHR, 0.37).
“After 7-10 years of follow-up, people with H. pylori who received treatment had nearly half the risk of developing stomach cancer as the general population,” Dr. Li said. “This is likely because most people infected with H. pylori in the general population are not screened nor treated. This highlights the impact screening and treatment can have.”
The data also show that cumulative incidence curves for H. pylori–positive/untreated and H. pylori–positive/treated largely overlapped during the first 7 years of follow-up and started to separate after 8 years.
At 10 years, cumulative NCGA incidence rates for H. pylori–positive/untreated, H. pylori–positive/treated, and H. pylori negative were 31.0, 19.7, and 3.5 per 10,000 persons, respectively (P < .0001).
This study shows that treating H. pylori reduces stomach cancer incidence in the United States, thus “filling an important research and knowledge gap,” Dr. Li said.
In the United States, Asian, Black, and Hispanic adults are much more likely to be infected with H. pylori, and they have a two- to threefold higher risk of developing stomach cancer, he noted.
“This suggests it may be reasonable to consider targeted screening and treatment in these high-risk groups. However, the optimal strategy for population-based H. pylori screening has not been established, and more research is needed to determine who should be screened for H. pylori and at what age screening should begin,” Dr. Li said.
Strong data, jury out on universal screening
For additional comment, this news organization reached out to Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y.
The study shows that the treatment of H. pylori “absolutely will decrease your risk of certain types of gastric carcinoma down the line. It does take a while to show that, 7 years, but this study shows that very clearly,” Dr. Glatt said.
“People who have definitely been shown to have H. pylori should be treated,” Dr. Glatt said.
“I don’t think this study yet supports that everybody should be screened, but it does make sense that people who have upper GI symptoms consistent with H. pylori should be checked for H. pylori and then appropriately treated, he noted.
Routine screening for H. pylori is recommended in countries with high incidence of gastric cancer, but not in the United States, Dr. Kim noted.
“Given the risk reduction of cancer with H. pylori treatment, consideration should be made in the U.S. for asymptomatic individuals with a family history of gastric cancer or immigrants from high-incidence countries,” she added.
The study was funded by the Kaiser Permanente Northern California Community Health Research Grants Program, the Permanente Medical Group Delivery Science & Applied Research Program, and the Permanente Medical Group. Dr. Li, Dr. Glatt, and Dr. Kim have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM GASTROENTEROLOGY
Scarred med student inspired by dermatologist who treated her
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”
Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”
Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”
Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.