User login
For MD-IQ use only
FDA Advisory panels consider easing isotretinoin requirements
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
JAK inhibitor ivarmacitinib shows efficacy for atopic dermatitis in a pivotal trial
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
AT AAD 2023
Acetaminophen as Renoprotective Treatment in a Patient With Severe Malaria
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
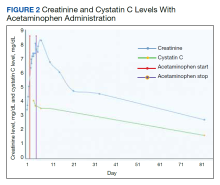
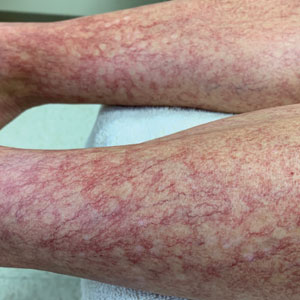
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
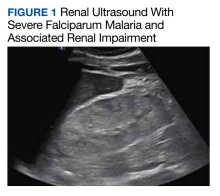
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
B-cell cancers: Sparse insight into preventing infections
Researchers found just 22 randomized controlled studies into prophylactic strategies, with several of them conducted prior to 2000. According to the report, published in Blood Advances, the studies together only evaluated a few thousand participants.
Reliable findings are so sparse that study coauthor Zoe McQuilten, MBBS, PhD, MD, a hematologist at Monash University, Melbourne, said “we simply don’t know” which preventive strategy is most effective. This is especially worrisome because more patients will survive their cancers and “be at risk of infection or have significant cytopenias and will experience impaired quality of life as a result,” she said in an interview.
The study authors launched the analysis to better understand the evidence regarding infection prevention and to guide the development of clinical trials, study coauthor Robert Weinkove, MBBS, PhD, a hematologist at Malaghan Institute of Medical Research, Wellington, New Zealand, said in an interview.
As he explained, targeted therapies have revolutionized the treatment of some B-cell cancers. They also have boosted the number of patients who survive the diseases yet still have profound hypogammaglobulinemia.
“Indeed, we may soon reach the point at which infection, and not tumor progression, is the leading cause of death for patients with certain B-cell cancers,” he said. “The evidence base for managing hypogammaglobulinemia is largely based on randomized trials of immunoglobulin replacement conducted in the 1980s and early 1990s, before the advent of B cell–targeted therapies. Immunoglobulin replacement is a costly intervention, and many countries are facing a shortage of immunoglobulin.”
The report authors identified 22 total randomized controlled trials, including one led by Dr. McQuilten: 8 studies into prophylactic immunoglobulin (n = 370; all but 1 study published prior to 2000), 5 into prophylactic antibiotics (n = 1,587), 7 into vaccination (n = 3,996), and 1 comparing immunoglobulin versus antibiotics (n = 60).
No evidence was found to support a lowering of risk by prophylactic antibiotics, although they caused adverse events.
Prophylactic immunoglobulin also caused adverse events, but a meta-analysis found that it reduced the risk of clinically documented infection by 28% (n = 2 trials; relative risk, 0.72; 95% confidence interval, 0.54-0.96). Three trials reported adverse events and found a higher risk overall (RR, 2.23; 95% CI, 1.67-2.99).
Varicella zoster virus vaccination reduced the risk of one or more infections by 63% (n = 5 trials, RR, 0.37; 95% CI, 0.30-0.45, n = 3,515). Prophylactic antibiotics did not reduce the risk.
No intervention reduced all-cause mortality.
“Our findings should be interpreted with caution, Dr. McQuilten said, “because of the low number of patients, high risk of bias in the included studies, and lack of contemporary data applicable to the current standard of care for such patients.”
The lack of useful data is surprising, she said, especially considering “how commonly these interventions are used in current clinical practice and the cost and supply constraints for immunoglobulin. Given the variation in international guidelines, rising global demand and cost of immunoglobulin, and concerns regarding antimicrobial resistance, more evidence is needed to inform infection prevention strategies for this patient population.”
More data is expected soon. One ongoing study is examining intravenous immunoglobulin versus placebo in patients with CLL. It’s expected to be completed in September 2023.
What should clinicians do for now? “Given the lack of a proven survival benefit in favor of prophylactic immunoglobulin replacement, one strategy is to maximize use of vaccination and to educate both patients and clinicians regarding the need for early treatment of infections,” Dr. Weinkove said. “For people who have recurrent or severe infections despite these measures, both immunoglobulin replacement and prophylactic antibiotics are clinical options. It would be reasonable to take account of patient preference, logistical considerations, and reimbursement and availability in deciding between these options.”
He added that, “for people with severe hypogammaglobulinemia who experience recurrent or severe infections despite prophylactic antibiotics, switching to immunoglobulin replacement would be appropriate. We advocate enrollment in clinical trials, if possible.”
In an interview, Juthaporn Cowan, MD, PhD, an infectious disease physician with the University of Ottawa, said many patients with B-cell lymphomas develop acquired hypogammaglobulinemia. “Patients tend to get prolonged colds, frequent sinusitis, bronchitis, or pneumonia. Some can end up with severe infection. Many patients told me that, even though their cancer is cured or in remission, quality of life is still quite poor due to these infections and fatigue.”
Dr. Cowan said the new report is somewhat useful, although “concluding that vaccination reduces infection is misleading. Vaccination reduces the infection that patients were vaccinated against. Patients who received Shingrix will have less shingles but will continue to have bronchitis and other infections.”
As for advice for clinicians, she said preventing acquired hypogammaglobulinemia is difficult since it can be caused by the malignancies, by treatment, or both. “The other item to consider is that we do not know how long we should continue [immunoglobulin] treatment in these patients. I have a patient post CAR [chimeric antigen receptor] T therapy who still does not have B-cell 5-6 years after CAR T, while I have lymphoma patients who could safely discontinue [immunoglobulin] treatment in a few years.”
Dr. Cowan added that patients on immunoglobulin treatment can still get opportunistic infections from cytomegalovirus or herpes simplex virus “because the mechanism of host defense against these infections is different. Antimicrobial prophylaxis should still be considered as vaccination is not available for every single potential opportunistic infection.”
Australia funded the research through the National Blood Authority. Dr. McQuilten and Dr. Weinkove reported no disclosures. Other report authors disclosed ties with Aegros, CSL Behring, Janssen, AbbVie, and BeiGene. Monash University has received funding for unrelated projects from CSL Behring. Dr. Cowan reports honoraria from Takeda, CSL Behring, Octapharma, GlaxoSmithKline, Merck, and AstraZeneca.
Researchers found just 22 randomized controlled studies into prophylactic strategies, with several of them conducted prior to 2000. According to the report, published in Blood Advances, the studies together only evaluated a few thousand participants.
Reliable findings are so sparse that study coauthor Zoe McQuilten, MBBS, PhD, MD, a hematologist at Monash University, Melbourne, said “we simply don’t know” which preventive strategy is most effective. This is especially worrisome because more patients will survive their cancers and “be at risk of infection or have significant cytopenias and will experience impaired quality of life as a result,” she said in an interview.
The study authors launched the analysis to better understand the evidence regarding infection prevention and to guide the development of clinical trials, study coauthor Robert Weinkove, MBBS, PhD, a hematologist at Malaghan Institute of Medical Research, Wellington, New Zealand, said in an interview.
As he explained, targeted therapies have revolutionized the treatment of some B-cell cancers. They also have boosted the number of patients who survive the diseases yet still have profound hypogammaglobulinemia.
“Indeed, we may soon reach the point at which infection, and not tumor progression, is the leading cause of death for patients with certain B-cell cancers,” he said. “The evidence base for managing hypogammaglobulinemia is largely based on randomized trials of immunoglobulin replacement conducted in the 1980s and early 1990s, before the advent of B cell–targeted therapies. Immunoglobulin replacement is a costly intervention, and many countries are facing a shortage of immunoglobulin.”
The report authors identified 22 total randomized controlled trials, including one led by Dr. McQuilten: 8 studies into prophylactic immunoglobulin (n = 370; all but 1 study published prior to 2000), 5 into prophylactic antibiotics (n = 1,587), 7 into vaccination (n = 3,996), and 1 comparing immunoglobulin versus antibiotics (n = 60).
No evidence was found to support a lowering of risk by prophylactic antibiotics, although they caused adverse events.
Prophylactic immunoglobulin also caused adverse events, but a meta-analysis found that it reduced the risk of clinically documented infection by 28% (n = 2 trials; relative risk, 0.72; 95% confidence interval, 0.54-0.96). Three trials reported adverse events and found a higher risk overall (RR, 2.23; 95% CI, 1.67-2.99).
Varicella zoster virus vaccination reduced the risk of one or more infections by 63% (n = 5 trials, RR, 0.37; 95% CI, 0.30-0.45, n = 3,515). Prophylactic antibiotics did not reduce the risk.
No intervention reduced all-cause mortality.
“Our findings should be interpreted with caution, Dr. McQuilten said, “because of the low number of patients, high risk of bias in the included studies, and lack of contemporary data applicable to the current standard of care for such patients.”
The lack of useful data is surprising, she said, especially considering “how commonly these interventions are used in current clinical practice and the cost and supply constraints for immunoglobulin. Given the variation in international guidelines, rising global demand and cost of immunoglobulin, and concerns regarding antimicrobial resistance, more evidence is needed to inform infection prevention strategies for this patient population.”
More data is expected soon. One ongoing study is examining intravenous immunoglobulin versus placebo in patients with CLL. It’s expected to be completed in September 2023.
What should clinicians do for now? “Given the lack of a proven survival benefit in favor of prophylactic immunoglobulin replacement, one strategy is to maximize use of vaccination and to educate both patients and clinicians regarding the need for early treatment of infections,” Dr. Weinkove said. “For people who have recurrent or severe infections despite these measures, both immunoglobulin replacement and prophylactic antibiotics are clinical options. It would be reasonable to take account of patient preference, logistical considerations, and reimbursement and availability in deciding between these options.”
He added that, “for people with severe hypogammaglobulinemia who experience recurrent or severe infections despite prophylactic antibiotics, switching to immunoglobulin replacement would be appropriate. We advocate enrollment in clinical trials, if possible.”
In an interview, Juthaporn Cowan, MD, PhD, an infectious disease physician with the University of Ottawa, said many patients with B-cell lymphomas develop acquired hypogammaglobulinemia. “Patients tend to get prolonged colds, frequent sinusitis, bronchitis, or pneumonia. Some can end up with severe infection. Many patients told me that, even though their cancer is cured or in remission, quality of life is still quite poor due to these infections and fatigue.”
Dr. Cowan said the new report is somewhat useful, although “concluding that vaccination reduces infection is misleading. Vaccination reduces the infection that patients were vaccinated against. Patients who received Shingrix will have less shingles but will continue to have bronchitis and other infections.”
As for advice for clinicians, she said preventing acquired hypogammaglobulinemia is difficult since it can be caused by the malignancies, by treatment, or both. “The other item to consider is that we do not know how long we should continue [immunoglobulin] treatment in these patients. I have a patient post CAR [chimeric antigen receptor] T therapy who still does not have B-cell 5-6 years after CAR T, while I have lymphoma patients who could safely discontinue [immunoglobulin] treatment in a few years.”
Dr. Cowan added that patients on immunoglobulin treatment can still get opportunistic infections from cytomegalovirus or herpes simplex virus “because the mechanism of host defense against these infections is different. Antimicrobial prophylaxis should still be considered as vaccination is not available for every single potential opportunistic infection.”
Australia funded the research through the National Blood Authority. Dr. McQuilten and Dr. Weinkove reported no disclosures. Other report authors disclosed ties with Aegros, CSL Behring, Janssen, AbbVie, and BeiGene. Monash University has received funding for unrelated projects from CSL Behring. Dr. Cowan reports honoraria from Takeda, CSL Behring, Octapharma, GlaxoSmithKline, Merck, and AstraZeneca.
Researchers found just 22 randomized controlled studies into prophylactic strategies, with several of them conducted prior to 2000. According to the report, published in Blood Advances, the studies together only evaluated a few thousand participants.
Reliable findings are so sparse that study coauthor Zoe McQuilten, MBBS, PhD, MD, a hematologist at Monash University, Melbourne, said “we simply don’t know” which preventive strategy is most effective. This is especially worrisome because more patients will survive their cancers and “be at risk of infection or have significant cytopenias and will experience impaired quality of life as a result,” she said in an interview.
The study authors launched the analysis to better understand the evidence regarding infection prevention and to guide the development of clinical trials, study coauthor Robert Weinkove, MBBS, PhD, a hematologist at Malaghan Institute of Medical Research, Wellington, New Zealand, said in an interview.
As he explained, targeted therapies have revolutionized the treatment of some B-cell cancers. They also have boosted the number of patients who survive the diseases yet still have profound hypogammaglobulinemia.
“Indeed, we may soon reach the point at which infection, and not tumor progression, is the leading cause of death for patients with certain B-cell cancers,” he said. “The evidence base for managing hypogammaglobulinemia is largely based on randomized trials of immunoglobulin replacement conducted in the 1980s and early 1990s, before the advent of B cell–targeted therapies. Immunoglobulin replacement is a costly intervention, and many countries are facing a shortage of immunoglobulin.”
The report authors identified 22 total randomized controlled trials, including one led by Dr. McQuilten: 8 studies into prophylactic immunoglobulin (n = 370; all but 1 study published prior to 2000), 5 into prophylactic antibiotics (n = 1,587), 7 into vaccination (n = 3,996), and 1 comparing immunoglobulin versus antibiotics (n = 60).
No evidence was found to support a lowering of risk by prophylactic antibiotics, although they caused adverse events.
Prophylactic immunoglobulin also caused adverse events, but a meta-analysis found that it reduced the risk of clinically documented infection by 28% (n = 2 trials; relative risk, 0.72; 95% confidence interval, 0.54-0.96). Three trials reported adverse events and found a higher risk overall (RR, 2.23; 95% CI, 1.67-2.99).
Varicella zoster virus vaccination reduced the risk of one or more infections by 63% (n = 5 trials, RR, 0.37; 95% CI, 0.30-0.45, n = 3,515). Prophylactic antibiotics did not reduce the risk.
No intervention reduced all-cause mortality.
“Our findings should be interpreted with caution, Dr. McQuilten said, “because of the low number of patients, high risk of bias in the included studies, and lack of contemporary data applicable to the current standard of care for such patients.”
The lack of useful data is surprising, she said, especially considering “how commonly these interventions are used in current clinical practice and the cost and supply constraints for immunoglobulin. Given the variation in international guidelines, rising global demand and cost of immunoglobulin, and concerns regarding antimicrobial resistance, more evidence is needed to inform infection prevention strategies for this patient population.”
More data is expected soon. One ongoing study is examining intravenous immunoglobulin versus placebo in patients with CLL. It’s expected to be completed in September 2023.
What should clinicians do for now? “Given the lack of a proven survival benefit in favor of prophylactic immunoglobulin replacement, one strategy is to maximize use of vaccination and to educate both patients and clinicians regarding the need for early treatment of infections,” Dr. Weinkove said. “For people who have recurrent or severe infections despite these measures, both immunoglobulin replacement and prophylactic antibiotics are clinical options. It would be reasonable to take account of patient preference, logistical considerations, and reimbursement and availability in deciding between these options.”
He added that, “for people with severe hypogammaglobulinemia who experience recurrent or severe infections despite prophylactic antibiotics, switching to immunoglobulin replacement would be appropriate. We advocate enrollment in clinical trials, if possible.”
In an interview, Juthaporn Cowan, MD, PhD, an infectious disease physician with the University of Ottawa, said many patients with B-cell lymphomas develop acquired hypogammaglobulinemia. “Patients tend to get prolonged colds, frequent sinusitis, bronchitis, or pneumonia. Some can end up with severe infection. Many patients told me that, even though their cancer is cured or in remission, quality of life is still quite poor due to these infections and fatigue.”
Dr. Cowan said the new report is somewhat useful, although “concluding that vaccination reduces infection is misleading. Vaccination reduces the infection that patients were vaccinated against. Patients who received Shingrix will have less shingles but will continue to have bronchitis and other infections.”
As for advice for clinicians, she said preventing acquired hypogammaglobulinemia is difficult since it can be caused by the malignancies, by treatment, or both. “The other item to consider is that we do not know how long we should continue [immunoglobulin] treatment in these patients. I have a patient post CAR [chimeric antigen receptor] T therapy who still does not have B-cell 5-6 years after CAR T, while I have lymphoma patients who could safely discontinue [immunoglobulin] treatment in a few years.”
Dr. Cowan added that patients on immunoglobulin treatment can still get opportunistic infections from cytomegalovirus or herpes simplex virus “because the mechanism of host defense against these infections is different. Antimicrobial prophylaxis should still be considered as vaccination is not available for every single potential opportunistic infection.”
Australia funded the research through the National Blood Authority. Dr. McQuilten and Dr. Weinkove reported no disclosures. Other report authors disclosed ties with Aegros, CSL Behring, Janssen, AbbVie, and BeiGene. Monash University has received funding for unrelated projects from CSL Behring. Dr. Cowan reports honoraria from Takeda, CSL Behring, Octapharma, GlaxoSmithKline, Merck, and AstraZeneca.
FROM BLOOD ADVANCES
Dabigatran recalled over potential carcinogen
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
Financial navigators saved about $2,500 per cancer patient
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JCO ONCOLOGY PRACTICE
The air up there: Oxygen could be a bit overrated
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Generalized Essential Telangiectasia Treated With Pulsed Dye Laser
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.
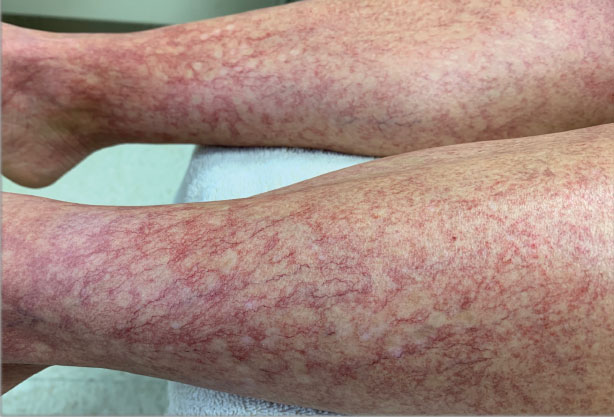
Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).
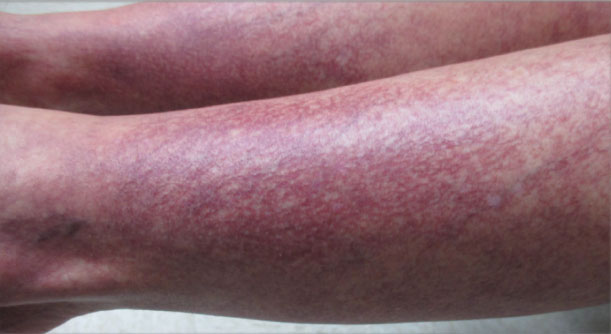
Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.

Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).

Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.

Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).

Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
Practice Points
- Generalized essential telangiectasia (GET) is a primary benign skin condition in which there is progressive development of telangiectases but a lack of systemic symptoms.
- Although patients should be assured that GET is a benign disease, its manifestation on the skin may cause negative psychologic impacts that should not be overlooked.
- Pulsed dye laser therapy does lead to improvement of the condition, but it does not prevent progression.
Cases of potentially deadly fungus jump 200%: CDC
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
Old-school printer helps scientists quickly spot bacteria in blood
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.