User login
Experts outline comprehensive preeclampsia prevention strategy
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
Preeclampsia is a leading cause of maternal mortality and premature births. The report, published in the American Journal of Obstetrics and Gynecology, developed by a working group of clinicians, researchers, patients, advocates, and payers, recommends daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, long-term follow-up, and addressing social determinants of health.
Titled “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” the report includes recommendations for providers and for patients at moderate to high risk of preeclampsia.
Top recommendations for providers include performing a risk assessment, including social determinants of health, medication recommendations (including daily aspirin and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep.)
The recommendations for patients include asking providers about aspirin use, checking blood pressure at home, and reporting any readings greater than 140/90. For those with BPs measuring 140/90 mm Hg or higher, the plan recommends antihypertensive therapy. The recommendations include making changes to diet, exercise, and sleep in consultation with providers.
Home blood pressure checks controversial
James Roberts, MD, a maternal-fetal medicine researcher at the Magee-Women’s Research Institute at University of Pittsburgh Medical Center and lead author on the paper, told this publication the home blood pressure checks may be the most controversial item in the report as not all insurers cover the at-home equipment.
In this report, the authors write that the working group “strongly advocates that payers of health care services cover the modest expense of home blood pressure determination including equipment and training.”
Dr. Roberts is the founding principal investigator of the Global Pregnancy Collaboration (CoLab), a consortium of 40 centers and one of the groups leading the creation of this report.
He said that while most of the recommendations are already recommended in guidelines, the report puts the preeclampsia plan into easy-to-read steps and downloadable checklists and compiles the evidence all in one place.
Dr. Roberts said the working group hopes this report will be adapted into guidelines developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and made part of electronic health records.
So far, the authors say, a comprehensive, integrated preeclampsia care plan has not been widely adopted.
Fewer than half of patients at risk receive aspirin
The coauthors note that “today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.”
A big part of this plan, Dr. Roberts said, calls for further educating both providers and patients.
Vesna Garovic, MD, PhD, a preeclampsia specialist at the Mayo Clinic in Rochester, Minn., who was not part of the working group, said, “This is the first comprehensive plan that provides a safe, cost-effective approach to reduce the risk of preeclampsia in individuals at moderate to high risk for this condition who qualify to receive aspirin for prevention.”
Dr. Garovic said the plan is novel in several ways, including the multispecialty input that also includes patients and advocates. Also, she says, it can be easily included in electronic health records and routine care of patients.
“The recommendations that were made, other than self-monitoring of blood pressure, are already standard of care. It will be important to understand as to which extent this comprehensive program, compared to the standard approach, would reduce further the risk of preeclampsia,” Dr. Garovic said. “A prospective, adequately powered comparative study would not only address this question, but will investigate compliance of providers and pregnant women with this shared approach, as well as patient satisfaction.”
The authors note the approach presented is for care in developed countries and that low- and middle-income countries would need to tailor the plan. The Care Plan is also meant only for prevention and is not meant to guide care for women who have developed preeclampsia.
Funding was provided to The Precia Group and the Global Pregnancy Collaboration to assemble this care plan by Mirvie, which is developing a biochemical predictor for preeclampsia. Precia and CoLab used a portion of these funds to support the time of some of the authors. Mirvie had no part in selecting authors or in the content of the manuscript.
Several authors received an honorarium for participation in the Working Group that developed the Care Plan. Two coauthors are site principal investigators overseeing sample collection on a Mirvie project. The remaining authors and Dr. Garovic report no conflicts of interest.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
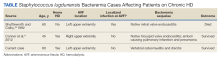
High-Grade Staphylococcus lugdunensis Bacteremia in a Patient on Home Hemodialysis
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
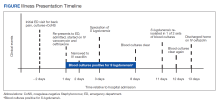
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
VA-Based Peritoneal Dialysis Program Feasibility Considerations and Process Outline
Compared with hemodialysis (HD), peritoneal dialysis (PD) offers comparable survival and superior patient-centered and health services outcomes.1,2 This has prompted repeated calls over the past 2 decades for policies to increase the use of home dialysis and, more specifically, for PD in the United States.3,4
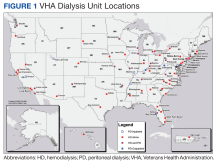
Veterans comprise nearly 10% of the population with end-stage kidney disease (ESKD) burden; > 50,000 US veterans are currently on dialysis.5,6 A majority of these veterans receive their chronic kidney disease (CKD) care through their affiliated US Department of Veterans Affairs (VA) medical centers (VAMCs).
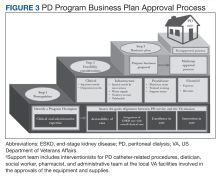
To address these needs, the VHA National Kidney Disease Program (NKDP) formed a 4-member PD workgroup in 2019. Considering the breadth of challenges involved, the PD workgroup broadly designed its approach based on the I CARE (Integrity, Commitment, Advocacy, Respect, and Excellence) VA Core Values.
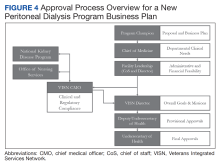
This review focuses on the initial deliberations of the PD access subgroup and provides a guide to establishing a new local VA PD program.
Step 1: Prerequisites
A functional nephrology service is a bedrock prerequisite for establishing a new PD program. A clinician champion capable of leading the effort is equally necessary. Occasionally, the prevalent ESKD economic and health care burden prompts local VAMC leadership to consider a new PD program to improve the quality or availability of services. More commonly, though, the nephrology section and the clinician champion are the first to recognize the need. In either scenario, the champion will require support and advocacy at multiple levels of local leadership, ie, the section or department chief, facility chief of staff, VAMC director, and the Veterans Integrated Service Network (VISN) director. The foremost task for the champion is to assess local clinical and infrastructure needs.
Goal Alignment
Any new VA nephrology program needs to be evaluated for its overall congruence with the local and national VA missions to improve the accessibility, integration, quality, and innovation of care for veterans. The following considerations are likely to apply to many VA systems.
Accessibility. A VHA directive recommends that all veterans be provided with the opportunity to choose and use any form of dialysis, especially home dialysis.9 Transitioning a veteran seamlessly from advanced CKD to PD requires the execution of multiple sequential processes in the pre-ESKD period, beginning with early identification of advanced CKD, timely referral to nephrology, education for shared dialysis decision making, coordination of care, and PD training and therapy.10 Splitting this sequence between VA and community-based care creates obstacles, including multiple approvals through VA Community Care Services that may substantially increase wait time and effort. This onerous process may be a significant deterrent against pursuing PD and increases the odds of emergency or inpatient initiation. Furthermore, the lack of PD availability limits the knowledge and experience among staff designated to assist veterans, which may result in inappropriate advocacy for HD or delay the transition to PD. Together, these processes can increase morbidity and health care use, and significantly delay or eliminate PD. Finally, many veterans reside in rural or remote areas where the expertise and the availability of PD may be unreliable. Establishing PD services within the local VAMC can improve access to PD, reduce the lead time needed to coordinate the transition to ESKD, and assist individual veterans in making an informed choice about dialysis. The program champion will need to identify and highlight all accessibility barriers within their business plan.
Integration. Many veterans receiving dialysis care at community-based facilities continue to receive nonnephrology care in the VA. This creates a parallel health care system with concerns for duplication of efforts and processes, suboptimal quality of care, and increased risk of medical errors. Establishing VA PD services increases access and integration of nephrology with other VA care.
Excellence. Studies of many chronic diseases have shown superior patient satisfaction and equal or superior quality of care delivered by the VA compared with that of non-VA facilities.11-14 Similarly, mortality rates for veterans receiving CKD and ESKD care in VA are lower compared with those at non-VA facilities.15-17 While these outcomes have not been examined for PD, integration of PD with VA care may lead to an improved overall quality of care and greater loyalty to the VA.
Innovation. Due to its integrated health care infrastructure, the VA is uniquely positioned to implement patient-centered and evidence-based pre-ESKD interventions that may improve outcomes. Prior studies have shown that pre-ESKD kidney disease education (KDE) improves pre- and post-ESKD outcomes, reduces health care costs, and leads to higher selection and use of home dialysis therapies.18-20 The VA recommends that all veterans with advanced CKD be provided access to pre-ESKD care and KDE. Unfortunately, KDE is uncommon among non-VA clinicians. A recent USRDS analysis reported that < 1% of patients with ESKD received pre-ESKD KDE.21 The ongoing Evaluate and Assess the effects of Comprehensive Pre-ESKD kidney disease Education on home dialysis in Veterans Trial (NCT04064086) should provide further evidence.
Step 2: Feasibility
A business plan requires the realistic projections of the costs and accounting for gains of the new clinical program. While there is limited guidance on personnel requirements when planning a PD program, we provide estimated resources needed to successfully establish and run a PD program (eAppendix 1, available online at doi:10.12788/fp.0356).
Clinical Considerations
Secondary or tertiary care VAMCs with multiple medical and surgical specialties routinely provide complex inpatient care. For these facilities, the lack of inpatient PD poses an obstacle to the provision of specialized nonnephrology care to veterans with ESKD, who are frequent users of such complex care. These considerations argue for the need for at least inpatient PD services at VAMCs that provide complex medical care for many veterans receiving PD in the community.
Deliberations for outpatient PD programs should be based on the clinical demands of ESKD care, the number of veterans likely to use PD, and growth projections. While there is no established minimum number that guarantees cost-effectiveness, most existing VA outpatient PD programs provide services for about 5 to 25 veterans. A local census can provide estimations of future PD needs. Travel considerations (ie, distance, terrain, traffic) may affect eligibility for purchased care and the decision where to receive PD. Many veterans may prefer PD from the local VAMC if it is convenient and allows them to maintain centralized VA care. Potential patients can be surveyed to gauge interest in receiving VA-based PD. Facilities providing structured pre-ESKD KDE may hold greater potential for PD growth, and it is important to highlight KDE infrastructure in the business plan.
Infrastructure
Spatial needs including clinic space and storage space for consumables, supplies, and equipment should be part of infrastructure requirements. The program champion may need to examine the available space for suitability and adequacy of the PD program early in the process. Ventilation renovations in the PD rooms should be incorporated into budget calculations. Water access for handwashing and PD effluent drainage should be confirmed, and if the program intends to establish home HD, additional considerations for the storage and water supply may be required. The VHA Handbook outlines the infrastructure requirements for a dialysis program.22 The VA has established national vendor contracts for dialysis equipment and consumables. However, a new PD program may need further guidance regarding the local agencies that provide administrative support and assist patients.
Telehealth technology has enabled many VAMCs to overcome geographical barriers for rural veterans.23 Ongoing expansion of community-based outpatient clinics (CBOCs) to include more rural locations is improving access to specialty care, while the launch of VA Video Connect (VVC) has further improved outreach. Investigators from Minneapolis have demonstrated the feasibility of multidisciplinary home-based telehealth management of veterans with CKD.24 Several existing nephrology sections across the VHA use a combination of VVC and CBOC-facilitated clinic visits to provide some pre-ESKD and ESKD care, including KDE, PD home visits and training, and comprehensive ESKD care visits. Recent changes in the clinical care pattern during the COVID-19 pandemic have further eased ESKD telehealth protocols. Integrating the projected use of telehealth in collaboration with existing resources available through the VHA NKDP can allow the local champion to improve the financial feasibility and long-term success of a new PD program.
Clinicians
Experience and expertise in managing PD vary among nephrologists. A recent survey found that only 11% of second-year nephrology trainees felt fully prepared to manage PD patients and 27% felt that they were minimally prepared.25 Thus, it is important to ensure that adequately trained nephrologists are available locally before initiating a new program, and if needed, coverage across VHS or VISN can be explored. One potential method to enhance practitioner comfort in PD is the use of existing peer-to-peer education through the VA Kidney Specialty Care Access Network-Extension for Community Health care Outcomes program that links health care professionals in rural areas with specialists at a tertiary care center.23 Nurses are a primary pillar for the success of home dialysis programs and the lack of a trained nursing workforce can be a significant limitation. Similarly, while the placement and management of complications related to PD catheters are not technically challenging, the availability of interventionists (either a surgeon or trained interventional radiologist) should be part of the business plan.
Financial Considerations
The financial considerations involving a new PD program within the VHA are complex (eAppendix 2, available online at doi:10.12788/fp.0356). ESKD is one of the most complex and costly comorbidities. It is a major determinant of the expenditure and revenue generation for facilities. The Veterans Equitable Resource Allocation system classifies ESKD on repeated dialysis as price category 10, indicating high complexity and cost. The VAMC workload and facility budget allocation is assessed annually and increases as the population of price group 10 veterans increases. VHA also provides additional Veterans Equitable Resource Allocation funds to VAMCs, which can improve the bottom line for VA-based dialysis units. Providing PD facilitates outpatient and inpatient management of comorbidities, allowing for substantial cost savings while improving the quality of nonrenal care. Outsourcing dialysis care can reduce the administrative burden, although, it deprives the VAMC of all dialysis-associated revenues while bearing the cost of all nonrenal and some renal care. The net effect is reduced facility productivity. In aggregate, establishing a local dialysis program requires greater financial resources for the capital and personnel costs; however, if captured appropriately these funds can be a major source of revenue and savings for the local VAMC.
Indirect costs are important for financial projections. Most community dialysis units operate as outpatient units, whereas all but a handful of the VA dialysis units operate within or near a VAMC. As a result, the VA units providing maintenance dialysis are regularly classified as inpatient centers while providing largely outpatient services, which negatively impacts overhead cost calculations. The predominant use of in-center HD as the default modality further sets an erroneously high baseline for the indirect cost of the VA-based PD services, especially considering that the principal savings of the home dialysis are through the reduction in the labor and capital costs. A rudimentary make-buy model for the in-center HD is available through the NKDP, and establishing a similar model for PD programs may be useful.
Cost considerations also may vary based on the model of ESKD care used locally. Of the 71 hospital-based and free-standing VA HD facilities, only 33 provide PD services, with 5 units providing only inpatient PD. The financial burden of establishing a fully operational outpatient PD program will be based on whether it is targeting a new unit or is expanding. The costs for equipment rental, disposables, and supplies vary based on the VA contract negotiations but are standardized across the nation with approved cost-of-living geographic adjustments. Caution needs to be exercised in employing a phased-hiring approach, as newer programs may require proportionally larger nursing resources due to greater needs for KDE, transitioning services, and training for PD. A target census-based hiring schedule should be negotiated with leadership before launch. If existing labor mapping does not allow for cross-coverage, part-time positions for physicians may be considered. Travel nurses, especially for PD training, can be considered to meet labor needs when long-term projections prohibit permanent full-time hires.
Finally, the balance sheet of a new program needs to account for different scenarios. In addition to nephrology costs, outsourcing veterans for PD services incurs multiple costs (eg, administrative, social work). Facilities with inpatient PD services alone are likely already bearing a component of the medications (including antibiotics) and/or surgical costs for their outsourced patients. These hidden costs are infrequently counted in projections. Facilities without inpatient PD cannot provide complex nonrenal care to ESKD patients on PD, even when the center is well equipped to provide it. These facilities also bear the cost of outsourcing even for complications related to PD. While a full estimation of these services varies, the hidden cost savings of many procedures or inpatient admissions, such as cardiovascular or musculoskeletal surgeries, can exceed those of dialysis in this complex population.
Step 3: proposal
There are no standardized formats for presenting a VHA business proposal; however, this outline provides a template. The business proposal should be designed to effectively communicate the collective data that describe the needs and requirements of a PD program to the local, regional, and national leadership. Not every rationale presented here will apply to an individual proposal and the local champion will need to tailor their rationale for their locale. A sample business plan is shown in eAppendix 3 (available online at doi:10.12788/fp.0356). VHA Handbook of dialysis requires that a PD nurse has a minimum of 12 months of nursing experience with at least 3 months of PD experience.25 Nursing training, education, and support should be discussed with nursing leadership and included in the business plan. Similarly, arrangements for laboratory, pharmacy, and prosthetics services and/or logistics to facilitate procurement of the needed devices, disposables, and supplies are essential and should be highlighted in the business plan.
Approval Process
Postapproval Process
Once approved, the champion will need to work closely with various services and managers to oversee infrastructural renovations and execute the hiring plans, establish standard operating procedures (SOPs), standardize staff proficiencies and functional statements, and finalize quality assessment parameters. Home dialysis standards have been addressed by NKDP and The Joint Commission. While PD requires home visits to assess the appropriateness of the environment, the PD program is accredited under hospital-based therapy. Standards and performance metrics should be incorporated into all the VA PD programs for standardization and assessment. Based on guidance from the VHA Handbook, quality metrics, such as dialysis adequacy, and rates of infection should be monitored and reviewed. The dialysis director may need to consider more frequent program evaluations in the first year to ensure appropriate troubleshooting. The VA infrastructure has developed the resources for a central repository for the PD SOPs and quality metrics, which can be obtained and adapted for the local program. Similarly, veteran satisfaction can be assessed through existing resources. Finally, the dialysis director can join the National VHA Dialysis Director listserv for regular updates on the existing and new VHA policies and NKDP updates.
Conclusions
Establishing a new PD program within a local federal infrastructure can appear daunting, both in terms of planning as well as approvals. However, the provision of home-based dialysis therapies may be beneficial to those in rural settings with limited access to in-center dialysis modalities as well as to those who seek autonomy and lifestyle independence in their medical care. Collaborations with the VHA NKDP or PD workgroup can help overcome many of the procedural hurdles, provide guidance about infrastructure and resource allocation and utilization, and provide easy access to established SOPs and quality parameters.
Acknowledgments
We acknowledge the late Dr. Catherine Do for her significant contribution to this manuscript. We also extend our sincere thanks to Dr. Holly Mattix-Kramer (Edward Hines Jr. Veterans Affairs Hospital and Loyola University Medical Center) for her prompt and valuable feedback on this manuscript.
1. Jung HY, Jeon Y, Park Y, et al. Better quality of life of peritoneal dialysis compared to hemodialysis over a two-year period after dialysis initiation. Sci Rep. 2019;9(1):10266. Published 2019 Jul 16. doi:10.1038/s41598-019-46744-1
2. Wong B, Ravani P, Oliver MJ, et al. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71(3):344-351. doi:10.1053/j.ajkd.2017.08.028
3. Chan CT, Collins K, Ditschman EP, et al. Overcoming barriers for uptake and continued use of home dialysis: an NKF-KDOQI Conference report. Am J Kidney Dis. 2020;75(6):926-934. doi:10.1053/j.ajkd.2019.11.007
4. Executive Order 13879: Advancing American kidney health. Fed Regist. 2019; 84(135):33817-33819. https://www.govinfo.gov/content/pkg/FR-2019-07-15/pdf/2019-15159.pdf
5. Patel TG, Pogach LM, Barth RH. CKD screening and management in the Veterans Health Administration: the impact of system organization and an innovative electronic record. Am J Kidney Dis. 2009;53(suppl 3):S78-S85. doi:10.1053/j.ajkd.2008.07.051
6. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
7. Sloan CE, Coffman CJ, Sanders LL, et al. Trends in peritoneal dialysis use in the United States after Medicare payment reform. Clin J Am Soc Nephrol. 2019;14(12):1763-1772. doi:10.2215/CJN.05910519
8. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. HR 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 9, 2023. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
9. US Department of Veterans Affairs, Veterans Health Administration. Chronic kidney disease prevention, early recognition, and management. VHA Directive 1053. March 17, 2020. Accessed February 9, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8737
10. Blake PG, Quinn RR, Oliver MJ. Peritoneal dialysis and the process of modality selection. Perit Dial Int. 2013;33(3):233-241. doi:10.3747/pdi.2012.00119
11. Stroupe KT, Hynes DM, Giobbie-Hurder A, et al. Patient satisfaction and use of Veterans Affairs versus non-Veterans Affairs healthcare services by veterans. Med Care. 2005;43(5):453-460. doi:10.1097/01.mlr.0000160377.82164.d3
12. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
13. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885. doi:10.1001/jamainternmed.2017.0605
14. Nuti SV, Qin L, Krumholz HM. Outcome after admission at Veterans Affairs vs non-Veterans Affairs hospitals--reply. JAMA. 2016;316(3):346. doi:10.1001/jama.2016.5394
15. Streja E, Kovesdy CP, Soohoo M, et al. Dialysis provider and outcomes among United States veterans who transition to dialysis. Clin J Am Soc Nephrol. 2018;13(7):1055-1062. doi:10.2215/CJN.12951117
16. Wang V, Coffman CJ, Stechuchak KM, et al. Survival among veterans obtaining dialysis in VA and non-VA settings. J Am Soc Nephrol. 2019;30(1):159-168. doi:10.1681/ASN.2018050521
17. Kurella Tamura M, Thomas IC, Montez-Rath ME, et al. Dialysis initiation and mortality among older veterans with kidney failure treated in Medicare vs the Department of Veterans Affairs. JAMA Intern Med. 2018;178(5):657-664. doi:10.1001/jamainternmed.2018.0411
18. Devins GM, Mendelssohn DC, Barré PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis. 2005;46(6):1088-1098. doi:10.1053/j.ajkd.2005.08.017
19. Devoe DJ, Wong B, James MT, et al. Patient education and peritoneal dialysis modality selection: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(3):422-433. doi:10.1053/j.ajkd.2016.02.053
20. Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLoS Med. 2018;15(3):e1002532. Published 2018 Mar 27. doi:10.1371/journal.pmed.1002532
21. Shukla AM, Bozorgmehri S, Ruchi R, et al. Utilization of CMS pre-ESRD Kidney Disease Education services and its associations with the home dialysis therapies. Perit Dial Int. 2021;41(5):453-462. doi:10.1177/0896860820975586
22. US Dept of Veterans Affairs, Veterans Health Administration. Criteria and standards for VA dialysis programs. VHA Directive 1601. 2016. May 23, 2016. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3205
23. Crowley ST, Belcher J, Choudhury D, et al. Targeting access to kidney care via telehealth: the VA experience. Adv Chronic Kidney Dis. 2017;24(1):22-30. doi:10.1053/j.ackd.2016.11.005
24. Ishani A, Christopher J, Palmer D, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68(1):41-49. doi:10.1053/j.ajkd.2016.01.018
25. Gupta N, Taber-Hight EB, Miller BW. Perceptions of home dialysis training and experience among US nephrology fellows. Am J Kidney Dis. 2021;77(5):713-718.e1. doi:10.1053/j.ajkd.2020.09.014
Compared with hemodialysis (HD), peritoneal dialysis (PD) offers comparable survival and superior patient-centered and health services outcomes.1,2 This has prompted repeated calls over the past 2 decades for policies to increase the use of home dialysis and, more specifically, for PD in the United States.3,4
Veterans comprise nearly 10% of the population with end-stage kidney disease (ESKD) burden; > 50,000 US veterans are currently on dialysis.5,6 A majority of these veterans receive their chronic kidney disease (CKD) care through their affiliated US Department of Veterans Affairs (VA) medical centers (VAMCs).
To address these needs, the VHA National Kidney Disease Program (NKDP) formed a 4-member PD workgroup in 2019. Considering the breadth of challenges involved, the PD workgroup broadly designed its approach based on the I CARE (Integrity, Commitment, Advocacy, Respect, and Excellence) VA Core Values.
This review focuses on the initial deliberations of the PD access subgroup and provides a guide to establishing a new local VA PD program.
Step 1: Prerequisites
A functional nephrology service is a bedrock prerequisite for establishing a new PD program. A clinician champion capable of leading the effort is equally necessary. Occasionally, the prevalent ESKD economic and health care burden prompts local VAMC leadership to consider a new PD program to improve the quality or availability of services. More commonly, though, the nephrology section and the clinician champion are the first to recognize the need. In either scenario, the champion will require support and advocacy at multiple levels of local leadership, ie, the section or department chief, facility chief of staff, VAMC director, and the Veterans Integrated Service Network (VISN) director. The foremost task for the champion is to assess local clinical and infrastructure needs.
Goal Alignment
Any new VA nephrology program needs to be evaluated for its overall congruence with the local and national VA missions to improve the accessibility, integration, quality, and innovation of care for veterans. The following considerations are likely to apply to many VA systems.
Accessibility. A VHA directive recommends that all veterans be provided with the opportunity to choose and use any form of dialysis, especially home dialysis.9 Transitioning a veteran seamlessly from advanced CKD to PD requires the execution of multiple sequential processes in the pre-ESKD period, beginning with early identification of advanced CKD, timely referral to nephrology, education for shared dialysis decision making, coordination of care, and PD training and therapy.10 Splitting this sequence between VA and community-based care creates obstacles, including multiple approvals through VA Community Care Services that may substantially increase wait time and effort. This onerous process may be a significant deterrent against pursuing PD and increases the odds of emergency or inpatient initiation. Furthermore, the lack of PD availability limits the knowledge and experience among staff designated to assist veterans, which may result in inappropriate advocacy for HD or delay the transition to PD. Together, these processes can increase morbidity and health care use, and significantly delay or eliminate PD. Finally, many veterans reside in rural or remote areas where the expertise and the availability of PD may be unreliable. Establishing PD services within the local VAMC can improve access to PD, reduce the lead time needed to coordinate the transition to ESKD, and assist individual veterans in making an informed choice about dialysis. The program champion will need to identify and highlight all accessibility barriers within their business plan.
Integration. Many veterans receiving dialysis care at community-based facilities continue to receive nonnephrology care in the VA. This creates a parallel health care system with concerns for duplication of efforts and processes, suboptimal quality of care, and increased risk of medical errors. Establishing VA PD services increases access and integration of nephrology with other VA care.
Excellence. Studies of many chronic diseases have shown superior patient satisfaction and equal or superior quality of care delivered by the VA compared with that of non-VA facilities.11-14 Similarly, mortality rates for veterans receiving CKD and ESKD care in VA are lower compared with those at non-VA facilities.15-17 While these outcomes have not been examined for PD, integration of PD with VA care may lead to an improved overall quality of care and greater loyalty to the VA.
Innovation. Due to its integrated health care infrastructure, the VA is uniquely positioned to implement patient-centered and evidence-based pre-ESKD interventions that may improve outcomes. Prior studies have shown that pre-ESKD kidney disease education (KDE) improves pre- and post-ESKD outcomes, reduces health care costs, and leads to higher selection and use of home dialysis therapies.18-20 The VA recommends that all veterans with advanced CKD be provided access to pre-ESKD care and KDE. Unfortunately, KDE is uncommon among non-VA clinicians. A recent USRDS analysis reported that < 1% of patients with ESKD received pre-ESKD KDE.21 The ongoing Evaluate and Assess the effects of Comprehensive Pre-ESKD kidney disease Education on home dialysis in Veterans Trial (NCT04064086) should provide further evidence.
Step 2: Feasibility
A business plan requires the realistic projections of the costs and accounting for gains of the new clinical program. While there is limited guidance on personnel requirements when planning a PD program, we provide estimated resources needed to successfully establish and run a PD program (eAppendix 1, available online at doi:10.12788/fp.0356).
Clinical Considerations
Secondary or tertiary care VAMCs with multiple medical and surgical specialties routinely provide complex inpatient care. For these facilities, the lack of inpatient PD poses an obstacle to the provision of specialized nonnephrology care to veterans with ESKD, who are frequent users of such complex care. These considerations argue for the need for at least inpatient PD services at VAMCs that provide complex medical care for many veterans receiving PD in the community.
Deliberations for outpatient PD programs should be based on the clinical demands of ESKD care, the number of veterans likely to use PD, and growth projections. While there is no established minimum number that guarantees cost-effectiveness, most existing VA outpatient PD programs provide services for about 5 to 25 veterans. A local census can provide estimations of future PD needs. Travel considerations (ie, distance, terrain, traffic) may affect eligibility for purchased care and the decision where to receive PD. Many veterans may prefer PD from the local VAMC if it is convenient and allows them to maintain centralized VA care. Potential patients can be surveyed to gauge interest in receiving VA-based PD. Facilities providing structured pre-ESKD KDE may hold greater potential for PD growth, and it is important to highlight KDE infrastructure in the business plan.
Infrastructure
Spatial needs including clinic space and storage space for consumables, supplies, and equipment should be part of infrastructure requirements. The program champion may need to examine the available space for suitability and adequacy of the PD program early in the process. Ventilation renovations in the PD rooms should be incorporated into budget calculations. Water access for handwashing and PD effluent drainage should be confirmed, and if the program intends to establish home HD, additional considerations for the storage and water supply may be required. The VHA Handbook outlines the infrastructure requirements for a dialysis program.22 The VA has established national vendor contracts for dialysis equipment and consumables. However, a new PD program may need further guidance regarding the local agencies that provide administrative support and assist patients.
Telehealth technology has enabled many VAMCs to overcome geographical barriers for rural veterans.23 Ongoing expansion of community-based outpatient clinics (CBOCs) to include more rural locations is improving access to specialty care, while the launch of VA Video Connect (VVC) has further improved outreach. Investigators from Minneapolis have demonstrated the feasibility of multidisciplinary home-based telehealth management of veterans with CKD.24 Several existing nephrology sections across the VHA use a combination of VVC and CBOC-facilitated clinic visits to provide some pre-ESKD and ESKD care, including KDE, PD home visits and training, and comprehensive ESKD care visits. Recent changes in the clinical care pattern during the COVID-19 pandemic have further eased ESKD telehealth protocols. Integrating the projected use of telehealth in collaboration with existing resources available through the VHA NKDP can allow the local champion to improve the financial feasibility and long-term success of a new PD program.
Clinicians
Experience and expertise in managing PD vary among nephrologists. A recent survey found that only 11% of second-year nephrology trainees felt fully prepared to manage PD patients and 27% felt that they were minimally prepared.25 Thus, it is important to ensure that adequately trained nephrologists are available locally before initiating a new program, and if needed, coverage across VHS or VISN can be explored. One potential method to enhance practitioner comfort in PD is the use of existing peer-to-peer education through the VA Kidney Specialty Care Access Network-Extension for Community Health care Outcomes program that links health care professionals in rural areas with specialists at a tertiary care center.23 Nurses are a primary pillar for the success of home dialysis programs and the lack of a trained nursing workforce can be a significant limitation. Similarly, while the placement and management of complications related to PD catheters are not technically challenging, the availability of interventionists (either a surgeon or trained interventional radiologist) should be part of the business plan.
Financial Considerations
The financial considerations involving a new PD program within the VHA are complex (eAppendix 2, available online at doi:10.12788/fp.0356). ESKD is one of the most complex and costly comorbidities. It is a major determinant of the expenditure and revenue generation for facilities. The Veterans Equitable Resource Allocation system classifies ESKD on repeated dialysis as price category 10, indicating high complexity and cost. The VAMC workload and facility budget allocation is assessed annually and increases as the population of price group 10 veterans increases. VHA also provides additional Veterans Equitable Resource Allocation funds to VAMCs, which can improve the bottom line for VA-based dialysis units. Providing PD facilitates outpatient and inpatient management of comorbidities, allowing for substantial cost savings while improving the quality of nonrenal care. Outsourcing dialysis care can reduce the administrative burden, although, it deprives the VAMC of all dialysis-associated revenues while bearing the cost of all nonrenal and some renal care. The net effect is reduced facility productivity. In aggregate, establishing a local dialysis program requires greater financial resources for the capital and personnel costs; however, if captured appropriately these funds can be a major source of revenue and savings for the local VAMC.
Indirect costs are important for financial projections. Most community dialysis units operate as outpatient units, whereas all but a handful of the VA dialysis units operate within or near a VAMC. As a result, the VA units providing maintenance dialysis are regularly classified as inpatient centers while providing largely outpatient services, which negatively impacts overhead cost calculations. The predominant use of in-center HD as the default modality further sets an erroneously high baseline for the indirect cost of the VA-based PD services, especially considering that the principal savings of the home dialysis are through the reduction in the labor and capital costs. A rudimentary make-buy model for the in-center HD is available through the NKDP, and establishing a similar model for PD programs may be useful.
Cost considerations also may vary based on the model of ESKD care used locally. Of the 71 hospital-based and free-standing VA HD facilities, only 33 provide PD services, with 5 units providing only inpatient PD. The financial burden of establishing a fully operational outpatient PD program will be based on whether it is targeting a new unit or is expanding. The costs for equipment rental, disposables, and supplies vary based on the VA contract negotiations but are standardized across the nation with approved cost-of-living geographic adjustments. Caution needs to be exercised in employing a phased-hiring approach, as newer programs may require proportionally larger nursing resources due to greater needs for KDE, transitioning services, and training for PD. A target census-based hiring schedule should be negotiated with leadership before launch. If existing labor mapping does not allow for cross-coverage, part-time positions for physicians may be considered. Travel nurses, especially for PD training, can be considered to meet labor needs when long-term projections prohibit permanent full-time hires.
Finally, the balance sheet of a new program needs to account for different scenarios. In addition to nephrology costs, outsourcing veterans for PD services incurs multiple costs (eg, administrative, social work). Facilities with inpatient PD services alone are likely already bearing a component of the medications (including antibiotics) and/or surgical costs for their outsourced patients. These hidden costs are infrequently counted in projections. Facilities without inpatient PD cannot provide complex nonrenal care to ESKD patients on PD, even when the center is well equipped to provide it. These facilities also bear the cost of outsourcing even for complications related to PD. While a full estimation of these services varies, the hidden cost savings of many procedures or inpatient admissions, such as cardiovascular or musculoskeletal surgeries, can exceed those of dialysis in this complex population.
Step 3: proposal
There are no standardized formats for presenting a VHA business proposal; however, this outline provides a template. The business proposal should be designed to effectively communicate the collective data that describe the needs and requirements of a PD program to the local, regional, and national leadership. Not every rationale presented here will apply to an individual proposal and the local champion will need to tailor their rationale for their locale. A sample business plan is shown in eAppendix 3 (available online at doi:10.12788/fp.0356). VHA Handbook of dialysis requires that a PD nurse has a minimum of 12 months of nursing experience with at least 3 months of PD experience.25 Nursing training, education, and support should be discussed with nursing leadership and included in the business plan. Similarly, arrangements for laboratory, pharmacy, and prosthetics services and/or logistics to facilitate procurement of the needed devices, disposables, and supplies are essential and should be highlighted in the business plan.
Approval Process
Postapproval Process
Once approved, the champion will need to work closely with various services and managers to oversee infrastructural renovations and execute the hiring plans, establish standard operating procedures (SOPs), standardize staff proficiencies and functional statements, and finalize quality assessment parameters. Home dialysis standards have been addressed by NKDP and The Joint Commission. While PD requires home visits to assess the appropriateness of the environment, the PD program is accredited under hospital-based therapy. Standards and performance metrics should be incorporated into all the VA PD programs for standardization and assessment. Based on guidance from the VHA Handbook, quality metrics, such as dialysis adequacy, and rates of infection should be monitored and reviewed. The dialysis director may need to consider more frequent program evaluations in the first year to ensure appropriate troubleshooting. The VA infrastructure has developed the resources for a central repository for the PD SOPs and quality metrics, which can be obtained and adapted for the local program. Similarly, veteran satisfaction can be assessed through existing resources. Finally, the dialysis director can join the National VHA Dialysis Director listserv for regular updates on the existing and new VHA policies and NKDP updates.
Conclusions
Establishing a new PD program within a local federal infrastructure can appear daunting, both in terms of planning as well as approvals. However, the provision of home-based dialysis therapies may be beneficial to those in rural settings with limited access to in-center dialysis modalities as well as to those who seek autonomy and lifestyle independence in their medical care. Collaborations with the VHA NKDP or PD workgroup can help overcome many of the procedural hurdles, provide guidance about infrastructure and resource allocation and utilization, and provide easy access to established SOPs and quality parameters.
Acknowledgments
We acknowledge the late Dr. Catherine Do for her significant contribution to this manuscript. We also extend our sincere thanks to Dr. Holly Mattix-Kramer (Edward Hines Jr. Veterans Affairs Hospital and Loyola University Medical Center) for her prompt and valuable feedback on this manuscript.
Compared with hemodialysis (HD), peritoneal dialysis (PD) offers comparable survival and superior patient-centered and health services outcomes.1,2 This has prompted repeated calls over the past 2 decades for policies to increase the use of home dialysis and, more specifically, for PD in the United States.3,4
Veterans comprise nearly 10% of the population with end-stage kidney disease (ESKD) burden; > 50,000 US veterans are currently on dialysis.5,6 A majority of these veterans receive their chronic kidney disease (CKD) care through their affiliated US Department of Veterans Affairs (VA) medical centers (VAMCs).
To address these needs, the VHA National Kidney Disease Program (NKDP) formed a 4-member PD workgroup in 2019. Considering the breadth of challenges involved, the PD workgroup broadly designed its approach based on the I CARE (Integrity, Commitment, Advocacy, Respect, and Excellence) VA Core Values.
This review focuses on the initial deliberations of the PD access subgroup and provides a guide to establishing a new local VA PD program.
Step 1: Prerequisites
A functional nephrology service is a bedrock prerequisite for establishing a new PD program. A clinician champion capable of leading the effort is equally necessary. Occasionally, the prevalent ESKD economic and health care burden prompts local VAMC leadership to consider a new PD program to improve the quality or availability of services. More commonly, though, the nephrology section and the clinician champion are the first to recognize the need. In either scenario, the champion will require support and advocacy at multiple levels of local leadership, ie, the section or department chief, facility chief of staff, VAMC director, and the Veterans Integrated Service Network (VISN) director. The foremost task for the champion is to assess local clinical and infrastructure needs.
Goal Alignment
Any new VA nephrology program needs to be evaluated for its overall congruence with the local and national VA missions to improve the accessibility, integration, quality, and innovation of care for veterans. The following considerations are likely to apply to many VA systems.
Accessibility. A VHA directive recommends that all veterans be provided with the opportunity to choose and use any form of dialysis, especially home dialysis.9 Transitioning a veteran seamlessly from advanced CKD to PD requires the execution of multiple sequential processes in the pre-ESKD period, beginning with early identification of advanced CKD, timely referral to nephrology, education for shared dialysis decision making, coordination of care, and PD training and therapy.10 Splitting this sequence between VA and community-based care creates obstacles, including multiple approvals through VA Community Care Services that may substantially increase wait time and effort. This onerous process may be a significant deterrent against pursuing PD and increases the odds of emergency or inpatient initiation. Furthermore, the lack of PD availability limits the knowledge and experience among staff designated to assist veterans, which may result in inappropriate advocacy for HD or delay the transition to PD. Together, these processes can increase morbidity and health care use, and significantly delay or eliminate PD. Finally, many veterans reside in rural or remote areas where the expertise and the availability of PD may be unreliable. Establishing PD services within the local VAMC can improve access to PD, reduce the lead time needed to coordinate the transition to ESKD, and assist individual veterans in making an informed choice about dialysis. The program champion will need to identify and highlight all accessibility barriers within their business plan.
Integration. Many veterans receiving dialysis care at community-based facilities continue to receive nonnephrology care in the VA. This creates a parallel health care system with concerns for duplication of efforts and processes, suboptimal quality of care, and increased risk of medical errors. Establishing VA PD services increases access and integration of nephrology with other VA care.
Excellence. Studies of many chronic diseases have shown superior patient satisfaction and equal or superior quality of care delivered by the VA compared with that of non-VA facilities.11-14 Similarly, mortality rates for veterans receiving CKD and ESKD care in VA are lower compared with those at non-VA facilities.15-17 While these outcomes have not been examined for PD, integration of PD with VA care may lead to an improved overall quality of care and greater loyalty to the VA.
Innovation. Due to its integrated health care infrastructure, the VA is uniquely positioned to implement patient-centered and evidence-based pre-ESKD interventions that may improve outcomes. Prior studies have shown that pre-ESKD kidney disease education (KDE) improves pre- and post-ESKD outcomes, reduces health care costs, and leads to higher selection and use of home dialysis therapies.18-20 The VA recommends that all veterans with advanced CKD be provided access to pre-ESKD care and KDE. Unfortunately, KDE is uncommon among non-VA clinicians. A recent USRDS analysis reported that < 1% of patients with ESKD received pre-ESKD KDE.21 The ongoing Evaluate and Assess the effects of Comprehensive Pre-ESKD kidney disease Education on home dialysis in Veterans Trial (NCT04064086) should provide further evidence.
Step 2: Feasibility
A business plan requires the realistic projections of the costs and accounting for gains of the new clinical program. While there is limited guidance on personnel requirements when planning a PD program, we provide estimated resources needed to successfully establish and run a PD program (eAppendix 1, available online at doi:10.12788/fp.0356).
Clinical Considerations
Secondary or tertiary care VAMCs with multiple medical and surgical specialties routinely provide complex inpatient care. For these facilities, the lack of inpatient PD poses an obstacle to the provision of specialized nonnephrology care to veterans with ESKD, who are frequent users of such complex care. These considerations argue for the need for at least inpatient PD services at VAMCs that provide complex medical care for many veterans receiving PD in the community.
Deliberations for outpatient PD programs should be based on the clinical demands of ESKD care, the number of veterans likely to use PD, and growth projections. While there is no established minimum number that guarantees cost-effectiveness, most existing VA outpatient PD programs provide services for about 5 to 25 veterans. A local census can provide estimations of future PD needs. Travel considerations (ie, distance, terrain, traffic) may affect eligibility for purchased care and the decision where to receive PD. Many veterans may prefer PD from the local VAMC if it is convenient and allows them to maintain centralized VA care. Potential patients can be surveyed to gauge interest in receiving VA-based PD. Facilities providing structured pre-ESKD KDE may hold greater potential for PD growth, and it is important to highlight KDE infrastructure in the business plan.
Infrastructure
Spatial needs including clinic space and storage space for consumables, supplies, and equipment should be part of infrastructure requirements. The program champion may need to examine the available space for suitability and adequacy of the PD program early in the process. Ventilation renovations in the PD rooms should be incorporated into budget calculations. Water access for handwashing and PD effluent drainage should be confirmed, and if the program intends to establish home HD, additional considerations for the storage and water supply may be required. The VHA Handbook outlines the infrastructure requirements for a dialysis program.22 The VA has established national vendor contracts for dialysis equipment and consumables. However, a new PD program may need further guidance regarding the local agencies that provide administrative support and assist patients.
Telehealth technology has enabled many VAMCs to overcome geographical barriers for rural veterans.23 Ongoing expansion of community-based outpatient clinics (CBOCs) to include more rural locations is improving access to specialty care, while the launch of VA Video Connect (VVC) has further improved outreach. Investigators from Minneapolis have demonstrated the feasibility of multidisciplinary home-based telehealth management of veterans with CKD.24 Several existing nephrology sections across the VHA use a combination of VVC and CBOC-facilitated clinic visits to provide some pre-ESKD and ESKD care, including KDE, PD home visits and training, and comprehensive ESKD care visits. Recent changes in the clinical care pattern during the COVID-19 pandemic have further eased ESKD telehealth protocols. Integrating the projected use of telehealth in collaboration with existing resources available through the VHA NKDP can allow the local champion to improve the financial feasibility and long-term success of a new PD program.
Clinicians
Experience and expertise in managing PD vary among nephrologists. A recent survey found that only 11% of second-year nephrology trainees felt fully prepared to manage PD patients and 27% felt that they were minimally prepared.25 Thus, it is important to ensure that adequately trained nephrologists are available locally before initiating a new program, and if needed, coverage across VHS or VISN can be explored. One potential method to enhance practitioner comfort in PD is the use of existing peer-to-peer education through the VA Kidney Specialty Care Access Network-Extension for Community Health care Outcomes program that links health care professionals in rural areas with specialists at a tertiary care center.23 Nurses are a primary pillar for the success of home dialysis programs and the lack of a trained nursing workforce can be a significant limitation. Similarly, while the placement and management of complications related to PD catheters are not technically challenging, the availability of interventionists (either a surgeon or trained interventional radiologist) should be part of the business plan.
Financial Considerations
The financial considerations involving a new PD program within the VHA are complex (eAppendix 2, available online at doi:10.12788/fp.0356). ESKD is one of the most complex and costly comorbidities. It is a major determinant of the expenditure and revenue generation for facilities. The Veterans Equitable Resource Allocation system classifies ESKD on repeated dialysis as price category 10, indicating high complexity and cost. The VAMC workload and facility budget allocation is assessed annually and increases as the population of price group 10 veterans increases. VHA also provides additional Veterans Equitable Resource Allocation funds to VAMCs, which can improve the bottom line for VA-based dialysis units. Providing PD facilitates outpatient and inpatient management of comorbidities, allowing for substantial cost savings while improving the quality of nonrenal care. Outsourcing dialysis care can reduce the administrative burden, although, it deprives the VAMC of all dialysis-associated revenues while bearing the cost of all nonrenal and some renal care. The net effect is reduced facility productivity. In aggregate, establishing a local dialysis program requires greater financial resources for the capital and personnel costs; however, if captured appropriately these funds can be a major source of revenue and savings for the local VAMC.
Indirect costs are important for financial projections. Most community dialysis units operate as outpatient units, whereas all but a handful of the VA dialysis units operate within or near a VAMC. As a result, the VA units providing maintenance dialysis are regularly classified as inpatient centers while providing largely outpatient services, which negatively impacts overhead cost calculations. The predominant use of in-center HD as the default modality further sets an erroneously high baseline for the indirect cost of the VA-based PD services, especially considering that the principal savings of the home dialysis are through the reduction in the labor and capital costs. A rudimentary make-buy model for the in-center HD is available through the NKDP, and establishing a similar model for PD programs may be useful.
Cost considerations also may vary based on the model of ESKD care used locally. Of the 71 hospital-based and free-standing VA HD facilities, only 33 provide PD services, with 5 units providing only inpatient PD. The financial burden of establishing a fully operational outpatient PD program will be based on whether it is targeting a new unit or is expanding. The costs for equipment rental, disposables, and supplies vary based on the VA contract negotiations but are standardized across the nation with approved cost-of-living geographic adjustments. Caution needs to be exercised in employing a phased-hiring approach, as newer programs may require proportionally larger nursing resources due to greater needs for KDE, transitioning services, and training for PD. A target census-based hiring schedule should be negotiated with leadership before launch. If existing labor mapping does not allow for cross-coverage, part-time positions for physicians may be considered. Travel nurses, especially for PD training, can be considered to meet labor needs when long-term projections prohibit permanent full-time hires.
Finally, the balance sheet of a new program needs to account for different scenarios. In addition to nephrology costs, outsourcing veterans for PD services incurs multiple costs (eg, administrative, social work). Facilities with inpatient PD services alone are likely already bearing a component of the medications (including antibiotics) and/or surgical costs for their outsourced patients. These hidden costs are infrequently counted in projections. Facilities without inpatient PD cannot provide complex nonrenal care to ESKD patients on PD, even when the center is well equipped to provide it. These facilities also bear the cost of outsourcing even for complications related to PD. While a full estimation of these services varies, the hidden cost savings of many procedures or inpatient admissions, such as cardiovascular or musculoskeletal surgeries, can exceed those of dialysis in this complex population.
Step 3: proposal
There are no standardized formats for presenting a VHA business proposal; however, this outline provides a template. The business proposal should be designed to effectively communicate the collective data that describe the needs and requirements of a PD program to the local, regional, and national leadership. Not every rationale presented here will apply to an individual proposal and the local champion will need to tailor their rationale for their locale. A sample business plan is shown in eAppendix 3 (available online at doi:10.12788/fp.0356). VHA Handbook of dialysis requires that a PD nurse has a minimum of 12 months of nursing experience with at least 3 months of PD experience.25 Nursing training, education, and support should be discussed with nursing leadership and included in the business plan. Similarly, arrangements for laboratory, pharmacy, and prosthetics services and/or logistics to facilitate procurement of the needed devices, disposables, and supplies are essential and should be highlighted in the business plan.
Approval Process
Postapproval Process
Once approved, the champion will need to work closely with various services and managers to oversee infrastructural renovations and execute the hiring plans, establish standard operating procedures (SOPs), standardize staff proficiencies and functional statements, and finalize quality assessment parameters. Home dialysis standards have been addressed by NKDP and The Joint Commission. While PD requires home visits to assess the appropriateness of the environment, the PD program is accredited under hospital-based therapy. Standards and performance metrics should be incorporated into all the VA PD programs for standardization and assessment. Based on guidance from the VHA Handbook, quality metrics, such as dialysis adequacy, and rates of infection should be monitored and reviewed. The dialysis director may need to consider more frequent program evaluations in the first year to ensure appropriate troubleshooting. The VA infrastructure has developed the resources for a central repository for the PD SOPs and quality metrics, which can be obtained and adapted for the local program. Similarly, veteran satisfaction can be assessed through existing resources. Finally, the dialysis director can join the National VHA Dialysis Director listserv for regular updates on the existing and new VHA policies and NKDP updates.
Conclusions
Establishing a new PD program within a local federal infrastructure can appear daunting, both in terms of planning as well as approvals. However, the provision of home-based dialysis therapies may be beneficial to those in rural settings with limited access to in-center dialysis modalities as well as to those who seek autonomy and lifestyle independence in their medical care. Collaborations with the VHA NKDP or PD workgroup can help overcome many of the procedural hurdles, provide guidance about infrastructure and resource allocation and utilization, and provide easy access to established SOPs and quality parameters.
Acknowledgments
We acknowledge the late Dr. Catherine Do for her significant contribution to this manuscript. We also extend our sincere thanks to Dr. Holly Mattix-Kramer (Edward Hines Jr. Veterans Affairs Hospital and Loyola University Medical Center) for her prompt and valuable feedback on this manuscript.
1. Jung HY, Jeon Y, Park Y, et al. Better quality of life of peritoneal dialysis compared to hemodialysis over a two-year period after dialysis initiation. Sci Rep. 2019;9(1):10266. Published 2019 Jul 16. doi:10.1038/s41598-019-46744-1
2. Wong B, Ravani P, Oliver MJ, et al. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71(3):344-351. doi:10.1053/j.ajkd.2017.08.028
3. Chan CT, Collins K, Ditschman EP, et al. Overcoming barriers for uptake and continued use of home dialysis: an NKF-KDOQI Conference report. Am J Kidney Dis. 2020;75(6):926-934. doi:10.1053/j.ajkd.2019.11.007
4. Executive Order 13879: Advancing American kidney health. Fed Regist. 2019; 84(135):33817-33819. https://www.govinfo.gov/content/pkg/FR-2019-07-15/pdf/2019-15159.pdf
5. Patel TG, Pogach LM, Barth RH. CKD screening and management in the Veterans Health Administration: the impact of system organization and an innovative electronic record. Am J Kidney Dis. 2009;53(suppl 3):S78-S85. doi:10.1053/j.ajkd.2008.07.051
6. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
7. Sloan CE, Coffman CJ, Sanders LL, et al. Trends in peritoneal dialysis use in the United States after Medicare payment reform. Clin J Am Soc Nephrol. 2019;14(12):1763-1772. doi:10.2215/CJN.05910519
8. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. HR 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 9, 2023. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
9. US Department of Veterans Affairs, Veterans Health Administration. Chronic kidney disease prevention, early recognition, and management. VHA Directive 1053. March 17, 2020. Accessed February 9, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8737
10. Blake PG, Quinn RR, Oliver MJ. Peritoneal dialysis and the process of modality selection. Perit Dial Int. 2013;33(3):233-241. doi:10.3747/pdi.2012.00119
11. Stroupe KT, Hynes DM, Giobbie-Hurder A, et al. Patient satisfaction and use of Veterans Affairs versus non-Veterans Affairs healthcare services by veterans. Med Care. 2005;43(5):453-460. doi:10.1097/01.mlr.0000160377.82164.d3
12. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
13. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885. doi:10.1001/jamainternmed.2017.0605
14. Nuti SV, Qin L, Krumholz HM. Outcome after admission at Veterans Affairs vs non-Veterans Affairs hospitals--reply. JAMA. 2016;316(3):346. doi:10.1001/jama.2016.5394
15. Streja E, Kovesdy CP, Soohoo M, et al. Dialysis provider and outcomes among United States veterans who transition to dialysis. Clin J Am Soc Nephrol. 2018;13(7):1055-1062. doi:10.2215/CJN.12951117
16. Wang V, Coffman CJ, Stechuchak KM, et al. Survival among veterans obtaining dialysis in VA and non-VA settings. J Am Soc Nephrol. 2019;30(1):159-168. doi:10.1681/ASN.2018050521
17. Kurella Tamura M, Thomas IC, Montez-Rath ME, et al. Dialysis initiation and mortality among older veterans with kidney failure treated in Medicare vs the Department of Veterans Affairs. JAMA Intern Med. 2018;178(5):657-664. doi:10.1001/jamainternmed.2018.0411
18. Devins GM, Mendelssohn DC, Barré PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis. 2005;46(6):1088-1098. doi:10.1053/j.ajkd.2005.08.017
19. Devoe DJ, Wong B, James MT, et al. Patient education and peritoneal dialysis modality selection: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(3):422-433. doi:10.1053/j.ajkd.2016.02.053
20. Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLoS Med. 2018;15(3):e1002532. Published 2018 Mar 27. doi:10.1371/journal.pmed.1002532
21. Shukla AM, Bozorgmehri S, Ruchi R, et al. Utilization of CMS pre-ESRD Kidney Disease Education services and its associations with the home dialysis therapies. Perit Dial Int. 2021;41(5):453-462. doi:10.1177/0896860820975586
22. US Dept of Veterans Affairs, Veterans Health Administration. Criteria and standards for VA dialysis programs. VHA Directive 1601. 2016. May 23, 2016. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3205
23. Crowley ST, Belcher J, Choudhury D, et al. Targeting access to kidney care via telehealth: the VA experience. Adv Chronic Kidney Dis. 2017;24(1):22-30. doi:10.1053/j.ackd.2016.11.005
24. Ishani A, Christopher J, Palmer D, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68(1):41-49. doi:10.1053/j.ajkd.2016.01.018
25. Gupta N, Taber-Hight EB, Miller BW. Perceptions of home dialysis training and experience among US nephrology fellows. Am J Kidney Dis. 2021;77(5):713-718.e1. doi:10.1053/j.ajkd.2020.09.014
1. Jung HY, Jeon Y, Park Y, et al. Better quality of life of peritoneal dialysis compared to hemodialysis over a two-year period after dialysis initiation. Sci Rep. 2019;9(1):10266. Published 2019 Jul 16. doi:10.1038/s41598-019-46744-1
2. Wong B, Ravani P, Oliver MJ, et al. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71(3):344-351. doi:10.1053/j.ajkd.2017.08.028
3. Chan CT, Collins K, Ditschman EP, et al. Overcoming barriers for uptake and continued use of home dialysis: an NKF-KDOQI Conference report. Am J Kidney Dis. 2020;75(6):926-934. doi:10.1053/j.ajkd.2019.11.007
4. Executive Order 13879: Advancing American kidney health. Fed Regist. 2019; 84(135):33817-33819. https://www.govinfo.gov/content/pkg/FR-2019-07-15/pdf/2019-15159.pdf
5. Patel TG, Pogach LM, Barth RH. CKD screening and management in the Veterans Health Administration: the impact of system organization and an innovative electronic record. Am J Kidney Dis. 2009;53(suppl 3):S78-S85. doi:10.1053/j.ajkd.2008.07.051
6. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
7. Sloan CE, Coffman CJ, Sanders LL, et al. Trends in peritoneal dialysis use in the United States after Medicare payment reform. Clin J Am Soc Nephrol. 2019;14(12):1763-1772. doi:10.2215/CJN.05910519
8. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. HR 5674. 115th Congress; Report No. 115-671, Part 1. May 3, 2018. Accessed February 9, 2023. https://www.congress.gov/115/bills/hr5674/BILLS-115hr5674rh.pdf
9. US Department of Veterans Affairs, Veterans Health Administration. Chronic kidney disease prevention, early recognition, and management. VHA Directive 1053. March 17, 2020. Accessed February 9, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8737
10. Blake PG, Quinn RR, Oliver MJ. Peritoneal dialysis and the process of modality selection. Perit Dial Int. 2013;33(3):233-241. doi:10.3747/pdi.2012.00119
11. Stroupe KT, Hynes DM, Giobbie-Hurder A, et al. Patient satisfaction and use of Veterans Affairs versus non-Veterans Affairs healthcare services by veterans. Med Care. 2005;43(5):453-460. doi:10.1097/01.mlr.0000160377.82164.d3
12. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
13. Blay E Jr, DeLancey JO, Hewitt DB, Chung JW, Bilimoria KY. Initial public reporting of quality at Veterans Affairs vs non-Veterans Affairs hospitals. JAMA Intern Med. 2017;177(6):882-885. doi:10.1001/jamainternmed.2017.0605
14. Nuti SV, Qin L, Krumholz HM. Outcome after admission at Veterans Affairs vs non-Veterans Affairs hospitals--reply. JAMA. 2016;316(3):346. doi:10.1001/jama.2016.5394
15. Streja E, Kovesdy CP, Soohoo M, et al. Dialysis provider and outcomes among United States veterans who transition to dialysis. Clin J Am Soc Nephrol. 2018;13(7):1055-1062. doi:10.2215/CJN.12951117
16. Wang V, Coffman CJ, Stechuchak KM, et al. Survival among veterans obtaining dialysis in VA and non-VA settings. J Am Soc Nephrol. 2019;30(1):159-168. doi:10.1681/ASN.2018050521
17. Kurella Tamura M, Thomas IC, Montez-Rath ME, et al. Dialysis initiation and mortality among older veterans with kidney failure treated in Medicare vs the Department of Veterans Affairs. JAMA Intern Med. 2018;178(5):657-664. doi:10.1001/jamainternmed.2018.0411
18. Devins GM, Mendelssohn DC, Barré PE, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis. 2005;46(6):1088-1098. doi:10.1053/j.ajkd.2005.08.017
19. Devoe DJ, Wong B, James MT, et al. Patient education and peritoneal dialysis modality selection: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(3):422-433. doi:10.1053/j.ajkd.2016.02.053
20. Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLoS Med. 2018;15(3):e1002532. Published 2018 Mar 27. doi:10.1371/journal.pmed.1002532
21. Shukla AM, Bozorgmehri S, Ruchi R, et al. Utilization of CMS pre-ESRD Kidney Disease Education services and its associations with the home dialysis therapies. Perit Dial Int. 2021;41(5):453-462. doi:10.1177/0896860820975586
22. US Dept of Veterans Affairs, Veterans Health Administration. Criteria and standards for VA dialysis programs. VHA Directive 1601. 2016. May 23, 2016. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3205
23. Crowley ST, Belcher J, Choudhury D, et al. Targeting access to kidney care via telehealth: the VA experience. Adv Chronic Kidney Dis. 2017;24(1):22-30. doi:10.1053/j.ackd.2016.11.005
24. Ishani A, Christopher J, Palmer D, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68(1):41-49. doi:10.1053/j.ajkd.2016.01.018
25. Gupta N, Taber-Hight EB, Miller BW. Perceptions of home dialysis training and experience among US nephrology fellows. Am J Kidney Dis. 2021;77(5):713-718.e1. doi:10.1053/j.ajkd.2020.09.014
SGLT2 inhibitors: Real-world data show benefits outweigh risks
Starting therapy with an SGLT2 inhibitor versus a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with more lower limb amputations, nonvertebral fractures, and genital infections, but these risks need to be balanced against cardiovascular and renoprotective benefits, according to the researchers.
The analysis showed that there would be 2.1 more lower limb amputations, 2.5 more nonvertebral fractures, and 41 more genital infections per 1,000 patients per year among those receiving SGLT2 inhibitors versus an equal number of patients receiving GLP-1 agonists, lead author Edouard Fu, PhD, explained to this news organization in an email.
“On the other hand, we know from the evidence from randomized controlled trials that taking an SGLT2 inhibitor compared with placebo lowers the risk of developing kidney failure,” said Dr. Fu, who is a research fellow in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
“For instance,” he continued, “in the DAPA-CKD clinical trial, dapagliflozin versus placebo led to 29 fewer events per 1,000 patients per year of the composite outcome (50% decline in estimated glomerular filtration rate [eGFR], kidney failure, cardiovascular or kidney death).”
In the CREDENCE trial, canagliflozin versus placebo led to 18 fewer events per 1,000 person-years for the composite outcome of doubling of serum creatinine, kidney failure, and cardiovascular or kidney death.
And in the EMPA-KIDNEY study, empagliflozin versus placebo led to 21 fewer events per 1,000 person-years for the composite outcome of progression of kidney disease or cardiovascular death.
“Thus, benefits would still outweigh the risks,” Dr. Fu emphasized.
‘Quantifies absolute rate of events among routine care patients’
“The importance of our paper,” he summarized, “is that it quantifies the absolute rate of events among routine care patients and may be used to inform shared decision-making.”
The analysis also found that the risks of diabetic ketoacidosis (DKA), hypovolemia, hypoglycemia, and severe urinary tract infection (UTI) were similar with SGLT2 inhibitors versus GLP-1 agonists, but the risk of developing acute kidney injury (AKI) was lower with an SGLT2 inhibitor.
“Our study can help inform patient-physician decision-making regarding risks and benefits before prescribing SGLT2 inhibitors in this population” of patients with CKD and diabetes treated in clinical practice, the researchers conclude, “but needs to be interpreted in light of its limitations, including residual confounding, short follow-up time, and the use of diagnosis codes to identify patients with CKD.”
The study was recently published in the Clinical Journal of the American Society of Nephrology.
Slow uptake, safety concerns
SGLT2 inhibitors are recommended as first-line therapy in patients with type 2 diabetes and CKD who have an eGFR equal to or greater than 20 mL/min per 1.73 m2, and thus are at high risk for cardiovascular disease and kidney disease progression, Dr. Fu and colleagues write.
However, studies report that as few as 6% of patients with CKD and type 2 diabetes are currently prescribed SGLT2 inhibitors in the United States.
This slow uptake of SGLT2 inhibitors among patients with CKD may be partly due to concerns about DKA, fractures, amputations, and urogenital infections observed in clinical trials.
However, such trials are generally underpowered to assess rare adverse events, use monitoring protocols to lower the risk of adverse events, and include a highly selected patient population, and so safety in routine clinical practice is often unclear.
To examine this, the researchers identified health insurance claims data from 96,128 individuals (from Optum, IBM MarketScan, and Medicare databases) who were 18 years or older (65 years or older for Medicare) and had type 2 diabetes and at least one inpatient or two outpatient diagnostic codes for stage 3 or 4 CKD.
Of these patients, 32,192 had a newly filled prescription for an SGLT2 inhibitor (empagliflozin, dapagliflozin, canagliflozin, or ertugliflozin) and 63,936 had a newly filled prescription for a GLP-1 agonist (liraglutide, dulaglutide, semaglutide, exenatide, albiglutide, or lixisenatide) between April 2013, when the first SGLT2 inhibitor was available in the United States, and 2021.
The researchers matched 28,847 individuals who were initiated on an SGLT2 inhibitor with an equal number who were initiated on a GLP-1 agonist, based on propensity scores, adjusting for more than 120 baseline characteristics.
Safety outcomes were based on previously identified potential safety signals.
Patients who were initiated on an SGLT2 inhibitor had 1.30-fold, 2.13-fold, and 3.08-fold higher risks of having a nonvertebral fracture, a lower limb amputation, and a genital infection, respectively, compared with patients who were initiated on a GLP-1 agonist, after a mean on-treatment time of 7.5 months,
Risks of DKA, hypovolemia, hypoglycemia, and severe UTI were similar in both groups.
Patients initiated on an SGLT2 inhibitor versus a GLP-1 agonist had a lower risk of AKI (hazard ratio, 0.93) equivalent to 6.75 fewer cases of AKI per 1,000 patients per year.
Patients had higher risks for lower limb amputation, genital infections, and nonvertebral fractures with SGLT2 inhibitors versus GLP-1 agonists across most of the prespecified subgroups by age, sex, cardiovascular disease, heart failure, and use of metformin, insulin, or sulfonylurea, but with wider confidence intervals.
Dr. Fu was supported by a Rubicon grant from the Dutch Research Council and has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
Starting therapy with an SGLT2 inhibitor versus a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with more lower limb amputations, nonvertebral fractures, and genital infections, but these risks need to be balanced against cardiovascular and renoprotective benefits, according to the researchers.
The analysis showed that there would be 2.1 more lower limb amputations, 2.5 more nonvertebral fractures, and 41 more genital infections per 1,000 patients per year among those receiving SGLT2 inhibitors versus an equal number of patients receiving GLP-1 agonists, lead author Edouard Fu, PhD, explained to this news organization in an email.
“On the other hand, we know from the evidence from randomized controlled trials that taking an SGLT2 inhibitor compared with placebo lowers the risk of developing kidney failure,” said Dr. Fu, who is a research fellow in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
“For instance,” he continued, “in the DAPA-CKD clinical trial, dapagliflozin versus placebo led to 29 fewer events per 1,000 patients per year of the composite outcome (50% decline in estimated glomerular filtration rate [eGFR], kidney failure, cardiovascular or kidney death).”
In the CREDENCE trial, canagliflozin versus placebo led to 18 fewer events per 1,000 person-years for the composite outcome of doubling of serum creatinine, kidney failure, and cardiovascular or kidney death.
And in the EMPA-KIDNEY study, empagliflozin versus placebo led to 21 fewer events per 1,000 person-years for the composite outcome of progression of kidney disease or cardiovascular death.
“Thus, benefits would still outweigh the risks,” Dr. Fu emphasized.
‘Quantifies absolute rate of events among routine care patients’
“The importance of our paper,” he summarized, “is that it quantifies the absolute rate of events among routine care patients and may be used to inform shared decision-making.”
The analysis also found that the risks of diabetic ketoacidosis (DKA), hypovolemia, hypoglycemia, and severe urinary tract infection (UTI) were similar with SGLT2 inhibitors versus GLP-1 agonists, but the risk of developing acute kidney injury (AKI) was lower with an SGLT2 inhibitor.
“Our study can help inform patient-physician decision-making regarding risks and benefits before prescribing SGLT2 inhibitors in this population” of patients with CKD and diabetes treated in clinical practice, the researchers conclude, “but needs to be interpreted in light of its limitations, including residual confounding, short follow-up time, and the use of diagnosis codes to identify patients with CKD.”
The study was recently published in the Clinical Journal of the American Society of Nephrology.
Slow uptake, safety concerns
SGLT2 inhibitors are recommended as first-line therapy in patients with type 2 diabetes and CKD who have an eGFR equal to or greater than 20 mL/min per 1.73 m2, and thus are at high risk for cardiovascular disease and kidney disease progression, Dr. Fu and colleagues write.
However, studies report that as few as 6% of patients with CKD and type 2 diabetes are currently prescribed SGLT2 inhibitors in the United States.
This slow uptake of SGLT2 inhibitors among patients with CKD may be partly due to concerns about DKA, fractures, amputations, and urogenital infections observed in clinical trials.
However, such trials are generally underpowered to assess rare adverse events, use monitoring protocols to lower the risk of adverse events, and include a highly selected patient population, and so safety in routine clinical practice is often unclear.
To examine this, the researchers identified health insurance claims data from 96,128 individuals (from Optum, IBM MarketScan, and Medicare databases) who were 18 years or older (65 years or older for Medicare) and had type 2 diabetes and at least one inpatient or two outpatient diagnostic codes for stage 3 or 4 CKD.
Of these patients, 32,192 had a newly filled prescription for an SGLT2 inhibitor (empagliflozin, dapagliflozin, canagliflozin, or ertugliflozin) and 63,936 had a newly filled prescription for a GLP-1 agonist (liraglutide, dulaglutide, semaglutide, exenatide, albiglutide, or lixisenatide) between April 2013, when the first SGLT2 inhibitor was available in the United States, and 2021.
The researchers matched 28,847 individuals who were initiated on an SGLT2 inhibitor with an equal number who were initiated on a GLP-1 agonist, based on propensity scores, adjusting for more than 120 baseline characteristics.
Safety outcomes were based on previously identified potential safety signals.
Patients who were initiated on an SGLT2 inhibitor had 1.30-fold, 2.13-fold, and 3.08-fold higher risks of having a nonvertebral fracture, a lower limb amputation, and a genital infection, respectively, compared with patients who were initiated on a GLP-1 agonist, after a mean on-treatment time of 7.5 months,
Risks of DKA, hypovolemia, hypoglycemia, and severe UTI were similar in both groups.
Patients initiated on an SGLT2 inhibitor versus a GLP-1 agonist had a lower risk of AKI (hazard ratio, 0.93) equivalent to 6.75 fewer cases of AKI per 1,000 patients per year.
Patients had higher risks for lower limb amputation, genital infections, and nonvertebral fractures with SGLT2 inhibitors versus GLP-1 agonists across most of the prespecified subgroups by age, sex, cardiovascular disease, heart failure, and use of metformin, insulin, or sulfonylurea, but with wider confidence intervals.
Dr. Fu was supported by a Rubicon grant from the Dutch Research Council and has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
Starting therapy with an SGLT2 inhibitor versus a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with more lower limb amputations, nonvertebral fractures, and genital infections, but these risks need to be balanced against cardiovascular and renoprotective benefits, according to the researchers.
The analysis showed that there would be 2.1 more lower limb amputations, 2.5 more nonvertebral fractures, and 41 more genital infections per 1,000 patients per year among those receiving SGLT2 inhibitors versus an equal number of patients receiving GLP-1 agonists, lead author Edouard Fu, PhD, explained to this news organization in an email.
“On the other hand, we know from the evidence from randomized controlled trials that taking an SGLT2 inhibitor compared with placebo lowers the risk of developing kidney failure,” said Dr. Fu, who is a research fellow in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston.
“For instance,” he continued, “in the DAPA-CKD clinical trial, dapagliflozin versus placebo led to 29 fewer events per 1,000 patients per year of the composite outcome (50% decline in estimated glomerular filtration rate [eGFR], kidney failure, cardiovascular or kidney death).”
In the CREDENCE trial, canagliflozin versus placebo led to 18 fewer events per 1,000 person-years for the composite outcome of doubling of serum creatinine, kidney failure, and cardiovascular or kidney death.
And in the EMPA-KIDNEY study, empagliflozin versus placebo led to 21 fewer events per 1,000 person-years for the composite outcome of progression of kidney disease or cardiovascular death.
“Thus, benefits would still outweigh the risks,” Dr. Fu emphasized.
‘Quantifies absolute rate of events among routine care patients’
“The importance of our paper,” he summarized, “is that it quantifies the absolute rate of events among routine care patients and may be used to inform shared decision-making.”
The analysis also found that the risks of diabetic ketoacidosis (DKA), hypovolemia, hypoglycemia, and severe urinary tract infection (UTI) were similar with SGLT2 inhibitors versus GLP-1 agonists, but the risk of developing acute kidney injury (AKI) was lower with an SGLT2 inhibitor.
“Our study can help inform patient-physician decision-making regarding risks and benefits before prescribing SGLT2 inhibitors in this population” of patients with CKD and diabetes treated in clinical practice, the researchers conclude, “but needs to be interpreted in light of its limitations, including residual confounding, short follow-up time, and the use of diagnosis codes to identify patients with CKD.”
The study was recently published in the Clinical Journal of the American Society of Nephrology.
Slow uptake, safety concerns
SGLT2 inhibitors are recommended as first-line therapy in patients with type 2 diabetes and CKD who have an eGFR equal to or greater than 20 mL/min per 1.73 m2, and thus are at high risk for cardiovascular disease and kidney disease progression, Dr. Fu and colleagues write.
However, studies report that as few as 6% of patients with CKD and type 2 diabetes are currently prescribed SGLT2 inhibitors in the United States.
This slow uptake of SGLT2 inhibitors among patients with CKD may be partly due to concerns about DKA, fractures, amputations, and urogenital infections observed in clinical trials.
However, such trials are generally underpowered to assess rare adverse events, use monitoring protocols to lower the risk of adverse events, and include a highly selected patient population, and so safety in routine clinical practice is often unclear.
To examine this, the researchers identified health insurance claims data from 96,128 individuals (from Optum, IBM MarketScan, and Medicare databases) who were 18 years or older (65 years or older for Medicare) and had type 2 diabetes and at least one inpatient or two outpatient diagnostic codes for stage 3 or 4 CKD.
Of these patients, 32,192 had a newly filled prescription for an SGLT2 inhibitor (empagliflozin, dapagliflozin, canagliflozin, or ertugliflozin) and 63,936 had a newly filled prescription for a GLP-1 agonist (liraglutide, dulaglutide, semaglutide, exenatide, albiglutide, or lixisenatide) between April 2013, when the first SGLT2 inhibitor was available in the United States, and 2021.
The researchers matched 28,847 individuals who were initiated on an SGLT2 inhibitor with an equal number who were initiated on a GLP-1 agonist, based on propensity scores, adjusting for more than 120 baseline characteristics.
Safety outcomes were based on previously identified potential safety signals.
Patients who were initiated on an SGLT2 inhibitor had 1.30-fold, 2.13-fold, and 3.08-fold higher risks of having a nonvertebral fracture, a lower limb amputation, and a genital infection, respectively, compared with patients who were initiated on a GLP-1 agonist, after a mean on-treatment time of 7.5 months,
Risks of DKA, hypovolemia, hypoglycemia, and severe UTI were similar in both groups.
Patients initiated on an SGLT2 inhibitor versus a GLP-1 agonist had a lower risk of AKI (hazard ratio, 0.93) equivalent to 6.75 fewer cases of AKI per 1,000 patients per year.
Patients had higher risks for lower limb amputation, genital infections, and nonvertebral fractures with SGLT2 inhibitors versus GLP-1 agonists across most of the prespecified subgroups by age, sex, cardiovascular disease, heart failure, and use of metformin, insulin, or sulfonylurea, but with wider confidence intervals.
Dr. Fu was supported by a Rubicon grant from the Dutch Research Council and has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article originally appeared on Medscape.com.
Living kidney donors should receive money for their costs of donating
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University’s Grossman School of Medicine in New York City.
We try very hard to get organ donation from those who die. That’s a commendable thing to do. I think doctors should always be discussing the opportunity to donate organs upon death, even in primary care settings.
It’s good to find out what people’s attitudes are. Let them learn about organ donation as something they can think about. Let them talk about it with family and friends and partners so that they know their wishes.
However, despite these efforts to encourage organ donation, we still have far fewer organs than we could use to transplant people, many people die on waiting lists because there are no organs to give them, and we’re in a situation where demand for organ transplant is actually increasing.
There is more capacity to do transplants both in the United States and elsewhere, and more people are living longer, so organ failure starts to become more common before, let’s say, terminal illness is really there. Now, we have more people who might benefit from organ transplant in an aging population.
One place to turn to help reduce the shortage of organs is to living donation. At least insofar as kidneys go, kidney donation from living persons has become a prominent source of organs for those who need kidneys – most of whom are surviving on dialysis, by the way, at a very high cost and often with a quality of life that they don’t find particularly easy to accept.
Transplant is far preferred, even though they have to take immunosuppression to keep those organ transplants going, and that has its own risks and side effects. They still get more mobility. They still are able to have a broader diet. They enjoy life far more than they do having to show up for dialysis three times a week for a couple of hours, every week, for every week that they live.
There is an interest in living kidney donation. One battle has been that, well, maybe we could get more kidneys if we just paid people to sell us their kidneys. That has been resisted, and I’ve been resistant to that idea, too, because I worry that it leads to exploitation.
The people who sell their kidneys are poor. They’re often in debt. They feel coerced by their circumstances, so they make a kidney sale. This happens in countries like India, where there are markets underground, and you see that it’s the poorest of the poor who do this, and they don’t really work their way out of debt. They just wind up without a kidney, help relieve their debt a little bit, and pretty soon, because they don’t have a job or an income except that sale of a kidney, they’re not much better off than they were before they started.
Also, people who sell kidneys for money are more likely not to admit to their own health problems, raising risks about the quality of organs. Then, of course, it puts doctors in a position to take out an organ for pay, even though it doesn’t benefit you, so that you can sell it. This raises some questions about whether that’s consistent with medical ethics.
A different idea has emerged. New York State Governor Kathy Hochul just signed legislation that allows living donors to be compensated for legitimate costs. That’s a little different matter. You’re not buying the organ, but you’re saying that if you experience health care problems due to complications from a donation, if you need money for transportation, if you lost money because you did this altruistically and you had to take time off from work and had expenses for a babysitter, restaurants, or other things, the state is going to try to create funds that will compensate you.
That, I think we should agree, is not a bad idea. You’re in a situation there where you don’t want to make people who are heroic, altruistic, and trying to help others by donating a kidney end up financially worse off.
I think there’s a difference between making someone financially whole after the decision to make a kidney available and creating a market where the poorest of the poor come forward to just sell because they see no other choice in terms of how to get rid of debts. I see these situations as not ethically equivalent, so I support efforts to try to compensate people who are our heroes. I don’t think we should ask them to financially suffer.
We’ll watch to see what happens as the New York state law comes into effect. By the way, New York is one of the states that really lags in the supply of organs for transplant, so this measure is particularly important for that state. Many other states should be considering this legislation as well.
It’s one thing to reward, if you will, donors by making sure they don’t suffer financial loss. It’s a very different thing to say, let’s have a free market and we’ll pay whoever it is that’s willing to sell us a kidney to do so. The former seems to me to be humane and just, whereas the latter risks exploitation.
A version of this article first appeared on Medscape.com.
Experts share early details prescribing avacopan for ANCA-associated vasculitis
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
When the Food and Drug Administration approved avacopan (Tavneos) as an adjunctive treatment for severe, active antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis (AAV) in October 2021, the oral complement C5a receptor inhibitor was hailed by its developer, ChemoCentryx, as a “new hope” for patients with the disease.
But avacopan’s novelty as a new drug for the rare diseases granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), coupled with its approval as an adjunctive to standard therapy, including glucocorticoids, rather than strictly as a glucocorticoid-sparing agent as it was tested, has so far led to little reported real-world experience with the drug.
In the phase 3 ADVOCATE trial, the pivotal trial that served as the basis for avacopan’s approval, 331 patients with active newly diagnosed or relapsing GPA or MPA received either avacopan or an oral prednisone taper over 20 weeks on a background of cyclophosphamide followed by azathioprine or rituximab. The results of the trial showed avacopan was noninferior to the group that received prednisone taper for remission at 26 weeks and superior to prednisone taper for sustained remission at 52 weeks, but the FDA was concerned that its complex design made it difficult to define the clinically meaningful benefit of avacopan and its role in the management of AAV.
The FDA noted that, in the avacopan arm of the trial, 86% of patients received glucocorticoids outside of the study protocol. Despite this, avacopan reduced the cumulative glucocorticoid dose over the trial’s 52 weeks by nearly two-thirds, compared with the prednisone group (1,349 mg vs. 3,655 mg).
The data also indicate a higher sustained remission rate at 52 weeks in patients who received induction with rituximab, compared with cyclophosphamide. But trial did not include a maintenance therapy dose of rituximab and is thereby not a good comparison against the standard of care, the FDA said. (ADVOCATE began enrolling patients prior to the FDA's 2018 approval of an expanded indication for patients with GPA or MPA who have achieved disease control after induction treatment.)
At the FDA’s Arthritis Advisory Committee meeting in May 2021, committee members were split on whether to recommend avacopan for approval. The committee voted 9-9 on whether the ADVOCATE trial showed efficacy supporting approval of avacopan, 10-8 in favor of whether the drug’s safety profile supported approval, and 10-8 in favor of the overall benefit-risk profile of avacopan for approval. But rather than give an indication to avacopan to reduce the use of glucocorticoids in adults with GPA or MPA, the agency approved avacopan as an adjunctive treatment for severe, active disease, noting in particular that avacopan “does not eliminate glucocorticoid use.”
The European Union’s marketing authorization for avacopan states its indication for use in combination with a rituximab or cyclophosphamide regimen for the treatment of adult patients with severe, active GPA or MPA and does not mention a role for reducing glucocorticoids. Avacopan will appear in forthcoming guidelines on management of AAV released by the European Alliance of Associations for Rheumatology.
In North America, the Canadian Vasculitis Research Network recently released an addendum to their guidelines on AAV specifically for avacopan, which includes recommendations to consider adding oral avacopan (30 mg twice daily) for induction of remission in patients with new or relapsing GPA or MPA who are also receiving cyclophosphamide or rituximab. The guidelines also recommend clinicians consider a glucocorticoid tapering schedule that aims for discontinuation at 4 weeks, and continuing avacopan for at least 1 year after induction therapy. The American College of Rheumatology guideline for AAV management, updated in 2021, acknowledges avacopan but did not consider its inclusion prior to FDA approval.
There have been few real-world studies of how patients with AAV are responding to avacopan, but recent studies from researchers in the Netherlands and in France have evaluated prednisone tapering and clinical outcomes.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, said those real-world studies “seemed to re-enforce the findings from the ADVOCATE study demonstrating the efficacy of avacopan in severe disease with steroid-sparing effects.”
However, Carol Langford, MD, MHS, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, emphasized caution is needed when drawing conclusions about avacopan use outside formal studies.
“We are all interested in what other settings this might be used. I think those are things that really require formal investigation to really try and understand better as far as through a study process,” she said.
Prescribing experience with avacopan
A spokesperson from Amgen, which recently acquired ChemoCentryx, said in an interview that over 800 physicians in the United States have prescribed avacopan to patients with new or relapsing ANCA-associated vasculitis as induction or maintenance treatment, and physicians have reported outcomes consistent with the ADVOCATE trial.
Many rheumatologists are likely familiar with avacopan but are not used to prescribing it, said Lindsay S. Lally, MD, a rheumatologist with Hospital for Special Surgery in New York.
“Rituximab was approved for GPA and MPA a decade ago at this point. It was a drug that we as rheumatologists were used to using. We used it for other indications. Avacopan is a totally new drug, a new mechanism of action, so there’s not a lot of extractable data that we have in terms of comfort with the drug, and so I think that’s one of the biggest hurdles,” she said.
Mehrnaz Hojjati, MD, a rheumatologist with Loma Linda (Calif.) University Health, said that, when the FDA approved avacopan, it was an “exciting time” in her practice. “I have used avacopan now in a handful of my patients with severe ANCA-associated vasculitis, and the results are similar to what [was] reported in the ADVOCATE trial.”
Amgen offers help for clinicians in obtaining avacopan for patients, financial assistance for patients, and support in navigating insurance, which several rheumatologists noted was important for patients. Dr. Langford said the process of working with the manufacturer to get avacopan while insurance information is being processed has been “fairly smooth.”
“Certainly, the ability to get a very rapid 30-day supply with the goal of trying to initiate this as early as possible in the disease process has been helpful,” she said.
In Dr. Dua’s experience, while there were “some glitches or difficulty for providers early on” in how to access and prescribe avacopan, since then “it has been much easier to obtain the medication with the first month being provided to patients free while the authorization process is managed.”
Prescribing avacopan from inpatient pharmacies has been more challenging, she said. “The inpatient side is trickier because each hospital system has their own pharmacy system and regulations that have to be navigated. For outpatients, all the provider needs to do is fill out the start form available on their website, have the patients sign it, and then have it sent in.”
Concerns about affordability, insurance approval
Another consideration is cost, with avacopan having an estimated price of $150,000-$200,000 per patient per year.
Dr. Hojjati noted that, while it is easy to prescribe, avacopan is hard to get approved through insurance. “We face the same challenge every time a new medication comes to the market on how to convince the payers to pay for it given higher prices,” she said.
Rheumatologist Michael Putman, MD, MSCI, assistant professor of medicine at the Medical College of Wisconsin, Milwaukee, also acknowledged some difficulties in prescribing the medication. “The insurance companies have no interest in spending $150,000 on a drug that they know nothing about, and patients are a little hesitant to take it because it’s just so new,” he said.
While Dr. Lally said avacopan has not been difficult to get for patients with commercial insurance, reimbursement through Medicare has been problematic. “In many of the Medicare patients it has not really been a feasible option for them to be on the drug for the year of therapy.”
Patient response
Dr. Dua said almost all her patients with new or relapsing AAV who require induction are being prescribed avacopan, and that the medication is well tolerated. “The remission and ability to wean prednisone has really paralleled the findings from the clinical trial.”
In her practice, Dr. Hojjati starts patients on avacopan immediately after discharge from the hospital after a major vasculitis flare requiring high-dose glucocorticoids. “Avacopan does not eliminate/replace GC [glucocorticoid] use but has a notable GC-sparing effect and assists in rapid tapering of the GC while treating our severe ANCA-associated vasculitis patients,” she said.
Dr. Lally said her patients are tolerating avacopan well and hasn’t seen any of the safety signals seen in the trial, including liver function abnormalities. She has treated about 20-25 patients with avacopan.
Dr. Putman noted that he has treated about five patients with avacopan but hasn’t seen dramatic efficacy or side effects in his practice, compared with standard therapy.
Unanswered questions about avacopan
A key unanswered question with avacopan is the timeline for tapering glucocorticoids once patients start treatment. “I would like to see much more data on how prednisone is being tapered in clinical practice as well as outcomes in patients who are treated with the standard of care second dose of rituximab at 6 months,” Dr. Dua said.
Dr. Lally noted she has tried to expedite the steroid taper in her patients. “That’s really where I feel this drug is going to have most relevance, is getting it started early in active disease and getting patients off of the reliance on high doses of oral steroids. I have been able to see that in practice, and I do think ultimately that’s going to lead to better outcomes and quality of life for these patients.”
Of the rheumatologists Dr. Lally has spoken to about avacopan, there is “some confusion about what type of patients are appropriate, [and] how sick or not sick the patient needs to be.”
Dr. Putman noted he is unsure which of his patients should be receiving avacopan. “I don’t totally have a sense for where avacopan stands and how often we should be using it” outside of patients with severe disease. He added that the drug is still trying to find a niche because most patients with AAV who take rituximab and steroids get better without additional treatments.
“I think we do a pretty good job treating these diseases even in the preavacopan era. But it’s really a matter of how to really optimize these outcomes, reduce damage, reduce steroid-related and treatment-related toxicity for our patients,” Dr. Lally said.
Dr. Dua reported being a consultant and serving on advisory boards for ChemoCentryx; she was also a site principal investigator for the ADVOCATE trial. Dr. Hojjati reported being on the speaker’s bureau for Amgen. Dr. Langford reported being an investigator in the ADVOCATE trial, and her institution received funding to conduct the trial. Dr. Lally reported being a consultant for Amgen on avacopan. Dr. Putman reported no relevant financial disclosures.
*This story was updated 3/15/2023.
Can SGLT2 inhibitors limit acute kidney injury in type 2 diabetes?
Adults with type 2 diabetes treated with an SGLT2 inhibitor had roughly a third fewer episodes of acute kidney injury (AKI) compared with matched people with type 2 diabetes treated with a DPP4 inhibitor, in an analysis of health insurance data from more than 100,000 Taiwan residents during 2016-2018.
The findings add to, and expand on, prior evidence that treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class cuts the incidence of AKI, say the authors of the report, which was recently published in JAMA Network Open.
The long-term risk for AKI among people with type 2 diabetes treated with an SGLT2 inhibitor “appears to be quite low” compared with adults who received an agent from the dipeptidyl peptidase 4 (DPP4) inhibitor class.
Treatment with an SGLT2 inhibitor – such as canagliflozin (Invokana), dapagliflozin (Farxiga), or empagliflozin (Jardiance) – causes a transient drop in kidney function that manifests as a temporary dip in estimated glomerular filtration rate, which caused concerns about AKI when the drugs were first introduced.
Indeed, canagliflozin and dapagliflozin had warnings strengthened 7 years ago by the Food and Drug Administration in a Drug Safety Communication for accumulating reports of AKI linked to their use.
More recent experience has calmed AKI concerns, however.
Commenting on the new study, F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn., said: “It’s a nice piece of data to demonstrate that the long-term risk from SGLT2 inhibitor treatment is low.” Dr. Wilson was not involved with the new study.
The Taiwan study found a cumulative incidence of AKI events during about 2.5 years of follow-up of 5.55 events/1,000 patient-years among adults with type 2 diabetes receiving an SGLT2 inhibitor and 7.88 events/1,000 patient-years among those taking a DPP4 inhibitor such as sitagliptin (Januvia).
Main barrier to SGLT2 inhibitor use is unfamiliarity, not AKI risk
“My impression is that the main barrier to wider use of the SGLT2 inhibitor class is not a perceived risk for causing AKI, but rather ongoing unfamiliarity with the class,” Dr. Wilson said in an interview.
Although he sees “relatively broad comfort with and enthusiasm for the class among nephrologists and cardiologists,” routine prescribing does not seem to have caught on nearly as much among primary care physicians, he said.
Clinicians in primary care “still perceive the SGLT2 inhibitor class as something of a ‘specialty drug,’ and they defer initiating it on that basis,” Dr. Wilson observed. “That’s probably not a good thing,” as many people with type 2 diabetes do not have access to a specialized clinician who might be more amenable to prescribing an SGLT2 inhibitor.
One example of the lag in SGLT2 inhibitor uptake for people with type 2 diabetes in practice was a recent report from the Centers for Disease Control and Prevention published in Annals of Internal Medicine. Researchers identified a representative U.S. sample of 1,330 adults with type 2 diabetes studied in depth during 2017-2020, of whom 82% fulfilled criteria published in 2022 for receiving treatment with an SGLT2 inhibitor. Despite this high prevalence of medical appropriateness, a scant 5.3% of those with a recommended indication actually received an agent from this class.
Early AKI concern has diminished
Results from more recent studies, such as a 2019 meta-analysis of more than 100 randomized studies and four large observational studies that together included about 180,000 people receiving SGLT2 inhibitor treatment, showed the opposite of SGLT2 inhibitor treatment triggering AKI.
In the trials, people taking an SGLT2 inhibitor had a relative 25% lower rate of AKI events, while in the observational studies, SGLT2 inhibitor treatment was linked with a 60% relative reduction in AKI. The study also found that SGLT2 inhibitor use in the trials was linked with a significant 20% relative increase in the incidence of low fluid volume.
Despite accumulated evidence exonerating AKI risk, U.S. labels for canagliflozin, dapagliflozin, and empagliflozin continue to cite AKI as a potential adverse reaction, especially in patients who undergo volume depletion while on SGLT2 inhibitor treatment.
The new Taiwan study used data from the country’s National Health Insurance Research Database. Out of more than 250,000 adults with type 2 diabetes in the system from May 2016 to December 2018, the researchers identified 52,231 propensity-score matched pairs of people where one was on treatment with an SGLT2 inhibitor and the other with a DPP4 inhibitor.
During follow-up, 856 of these people (0.8%) had an AKI event, including 102 people with AKI that required dialysis.
A logistic regression analysis that adjusted for 16 potential confounders showed that SGLT2 inhibitor treatment linked with a significant 34% reduction in AKI events compared with DPP4 inhibitor treatment, as well as with a significant 44% relative risk reduction in the incidence of AKI events requiring dialysis, reported the authors from several medical institutions in Taiwan.
The study’s main limitation was its reliance on “quite insensitive” administrative coding data to identify AKI cases, said Dr. Wilson.
He noted that although concern about AKI events secondary to SGLT2 inhibitor treatment is uncommon among U.S. clinicians they do worry about the potential risk for fungal infections, urinary tract infection, or gangrene in people with diabetes who receive an agent from this class.
The study received no commercial funding, and none of the authors had disclosures. Dr. Wilson has reported receiving research funding from AstraZeneca, Boehringer Ingelheim, Vifor, and Whoop.
A version of this article originally appeared on Medscape.com.
Adults with type 2 diabetes treated with an SGLT2 inhibitor had roughly a third fewer episodes of acute kidney injury (AKI) compared with matched people with type 2 diabetes treated with a DPP4 inhibitor, in an analysis of health insurance data from more than 100,000 Taiwan residents during 2016-2018.
The findings add to, and expand on, prior evidence that treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class cuts the incidence of AKI, say the authors of the report, which was recently published in JAMA Network Open.
The long-term risk for AKI among people with type 2 diabetes treated with an SGLT2 inhibitor “appears to be quite low” compared with adults who received an agent from the dipeptidyl peptidase 4 (DPP4) inhibitor class.
Treatment with an SGLT2 inhibitor – such as canagliflozin (Invokana), dapagliflozin (Farxiga), or empagliflozin (Jardiance) – causes a transient drop in kidney function that manifests as a temporary dip in estimated glomerular filtration rate, which caused concerns about AKI when the drugs were first introduced.
Indeed, canagliflozin and dapagliflozin had warnings strengthened 7 years ago by the Food and Drug Administration in a Drug Safety Communication for accumulating reports of AKI linked to their use.
More recent experience has calmed AKI concerns, however.
Commenting on the new study, F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn., said: “It’s a nice piece of data to demonstrate that the long-term risk from SGLT2 inhibitor treatment is low.” Dr. Wilson was not involved with the new study.
The Taiwan study found a cumulative incidence of AKI events during about 2.5 years of follow-up of 5.55 events/1,000 patient-years among adults with type 2 diabetes receiving an SGLT2 inhibitor and 7.88 events/1,000 patient-years among those taking a DPP4 inhibitor such as sitagliptin (Januvia).
Main barrier to SGLT2 inhibitor use is unfamiliarity, not AKI risk
“My impression is that the main barrier to wider use of the SGLT2 inhibitor class is not a perceived risk for causing AKI, but rather ongoing unfamiliarity with the class,” Dr. Wilson said in an interview.
Although he sees “relatively broad comfort with and enthusiasm for the class among nephrologists and cardiologists,” routine prescribing does not seem to have caught on nearly as much among primary care physicians, he said.
Clinicians in primary care “still perceive the SGLT2 inhibitor class as something of a ‘specialty drug,’ and they defer initiating it on that basis,” Dr. Wilson observed. “That’s probably not a good thing,” as many people with type 2 diabetes do not have access to a specialized clinician who might be more amenable to prescribing an SGLT2 inhibitor.
One example of the lag in SGLT2 inhibitor uptake for people with type 2 diabetes in practice was a recent report from the Centers for Disease Control and Prevention published in Annals of Internal Medicine. Researchers identified a representative U.S. sample of 1,330 adults with type 2 diabetes studied in depth during 2017-2020, of whom 82% fulfilled criteria published in 2022 for receiving treatment with an SGLT2 inhibitor. Despite this high prevalence of medical appropriateness, a scant 5.3% of those with a recommended indication actually received an agent from this class.
Early AKI concern has diminished
Results from more recent studies, such as a 2019 meta-analysis of more than 100 randomized studies and four large observational studies that together included about 180,000 people receiving SGLT2 inhibitor treatment, showed the opposite of SGLT2 inhibitor treatment triggering AKI.
In the trials, people taking an SGLT2 inhibitor had a relative 25% lower rate of AKI events, while in the observational studies, SGLT2 inhibitor treatment was linked with a 60% relative reduction in AKI. The study also found that SGLT2 inhibitor use in the trials was linked with a significant 20% relative increase in the incidence of low fluid volume.
Despite accumulated evidence exonerating AKI risk, U.S. labels for canagliflozin, dapagliflozin, and empagliflozin continue to cite AKI as a potential adverse reaction, especially in patients who undergo volume depletion while on SGLT2 inhibitor treatment.
The new Taiwan study used data from the country’s National Health Insurance Research Database. Out of more than 250,000 adults with type 2 diabetes in the system from May 2016 to December 2018, the researchers identified 52,231 propensity-score matched pairs of people where one was on treatment with an SGLT2 inhibitor and the other with a DPP4 inhibitor.
During follow-up, 856 of these people (0.8%) had an AKI event, including 102 people with AKI that required dialysis.
A logistic regression analysis that adjusted for 16 potential confounders showed that SGLT2 inhibitor treatment linked with a significant 34% reduction in AKI events compared with DPP4 inhibitor treatment, as well as with a significant 44% relative risk reduction in the incidence of AKI events requiring dialysis, reported the authors from several medical institutions in Taiwan.
The study’s main limitation was its reliance on “quite insensitive” administrative coding data to identify AKI cases, said Dr. Wilson.
He noted that although concern about AKI events secondary to SGLT2 inhibitor treatment is uncommon among U.S. clinicians they do worry about the potential risk for fungal infections, urinary tract infection, or gangrene in people with diabetes who receive an agent from this class.
The study received no commercial funding, and none of the authors had disclosures. Dr. Wilson has reported receiving research funding from AstraZeneca, Boehringer Ingelheim, Vifor, and Whoop.
A version of this article originally appeared on Medscape.com.
Adults with type 2 diabetes treated with an SGLT2 inhibitor had roughly a third fewer episodes of acute kidney injury (AKI) compared with matched people with type 2 diabetes treated with a DPP4 inhibitor, in an analysis of health insurance data from more than 100,000 Taiwan residents during 2016-2018.
The findings add to, and expand on, prior evidence that treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class cuts the incidence of AKI, say the authors of the report, which was recently published in JAMA Network Open.
The long-term risk for AKI among people with type 2 diabetes treated with an SGLT2 inhibitor “appears to be quite low” compared with adults who received an agent from the dipeptidyl peptidase 4 (DPP4) inhibitor class.
Treatment with an SGLT2 inhibitor – such as canagliflozin (Invokana), dapagliflozin (Farxiga), or empagliflozin (Jardiance) – causes a transient drop in kidney function that manifests as a temporary dip in estimated glomerular filtration rate, which caused concerns about AKI when the drugs were first introduced.
Indeed, canagliflozin and dapagliflozin had warnings strengthened 7 years ago by the Food and Drug Administration in a Drug Safety Communication for accumulating reports of AKI linked to their use.
More recent experience has calmed AKI concerns, however.
Commenting on the new study, F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn., said: “It’s a nice piece of data to demonstrate that the long-term risk from SGLT2 inhibitor treatment is low.” Dr. Wilson was not involved with the new study.
The Taiwan study found a cumulative incidence of AKI events during about 2.5 years of follow-up of 5.55 events/1,000 patient-years among adults with type 2 diabetes receiving an SGLT2 inhibitor and 7.88 events/1,000 patient-years among those taking a DPP4 inhibitor such as sitagliptin (Januvia).
Main barrier to SGLT2 inhibitor use is unfamiliarity, not AKI risk
“My impression is that the main barrier to wider use of the SGLT2 inhibitor class is not a perceived risk for causing AKI, but rather ongoing unfamiliarity with the class,” Dr. Wilson said in an interview.
Although he sees “relatively broad comfort with and enthusiasm for the class among nephrologists and cardiologists,” routine prescribing does not seem to have caught on nearly as much among primary care physicians, he said.
Clinicians in primary care “still perceive the SGLT2 inhibitor class as something of a ‘specialty drug,’ and they defer initiating it on that basis,” Dr. Wilson observed. “That’s probably not a good thing,” as many people with type 2 diabetes do not have access to a specialized clinician who might be more amenable to prescribing an SGLT2 inhibitor.
One example of the lag in SGLT2 inhibitor uptake for people with type 2 diabetes in practice was a recent report from the Centers for Disease Control and Prevention published in Annals of Internal Medicine. Researchers identified a representative U.S. sample of 1,330 adults with type 2 diabetes studied in depth during 2017-2020, of whom 82% fulfilled criteria published in 2022 for receiving treatment with an SGLT2 inhibitor. Despite this high prevalence of medical appropriateness, a scant 5.3% of those with a recommended indication actually received an agent from this class.
Early AKI concern has diminished
Results from more recent studies, such as a 2019 meta-analysis of more than 100 randomized studies and four large observational studies that together included about 180,000 people receiving SGLT2 inhibitor treatment, showed the opposite of SGLT2 inhibitor treatment triggering AKI.
In the trials, people taking an SGLT2 inhibitor had a relative 25% lower rate of AKI events, while in the observational studies, SGLT2 inhibitor treatment was linked with a 60% relative reduction in AKI. The study also found that SGLT2 inhibitor use in the trials was linked with a significant 20% relative increase in the incidence of low fluid volume.
Despite accumulated evidence exonerating AKI risk, U.S. labels for canagliflozin, dapagliflozin, and empagliflozin continue to cite AKI as a potential adverse reaction, especially in patients who undergo volume depletion while on SGLT2 inhibitor treatment.
The new Taiwan study used data from the country’s National Health Insurance Research Database. Out of more than 250,000 adults with type 2 diabetes in the system from May 2016 to December 2018, the researchers identified 52,231 propensity-score matched pairs of people where one was on treatment with an SGLT2 inhibitor and the other with a DPP4 inhibitor.
During follow-up, 856 of these people (0.8%) had an AKI event, including 102 people with AKI that required dialysis.
A logistic regression analysis that adjusted for 16 potential confounders showed that SGLT2 inhibitor treatment linked with a significant 34% reduction in AKI events compared with DPP4 inhibitor treatment, as well as with a significant 44% relative risk reduction in the incidence of AKI events requiring dialysis, reported the authors from several medical institutions in Taiwan.
The study’s main limitation was its reliance on “quite insensitive” administrative coding data to identify AKI cases, said Dr. Wilson.
He noted that although concern about AKI events secondary to SGLT2 inhibitor treatment is uncommon among U.S. clinicians they do worry about the potential risk for fungal infections, urinary tract infection, or gangrene in people with diabetes who receive an agent from this class.
The study received no commercial funding, and none of the authors had disclosures. Dr. Wilson has reported receiving research funding from AstraZeneca, Boehringer Ingelheim, Vifor, and Whoop.
A version of this article originally appeared on Medscape.com.
What do high BUN/Cr ratios mean?
He has been in good health with the only medical problem in his history being depression.
He is taking sertraline. On exam, his blood pressure is 100/60, and his pulse is 100, both while lying down. His blood pressure while standing is 90/60 and his pulse while standing is 130. The rest of his exam is normal. His lab values include hemoglobin of 10, hematocrit of 30, white blood cell of 4.6, platelet count of 175,000, sodium of 142, chloride of 100, bicarbonate of 24, potassium of 3.8, blood urea nitrogen (BUN) of 38, and creatinine clearance (Cr) of 1.1.
What is the most likely source of his bleeding?
A. Gastric ulcer
B. Meckel’s diverticulum
C. Arteriovenous malformation
D. Diverticulosis
E. Hemorrhoids
What makes the most sense
The most likely cause of this patient’s maroon stool is an upper gastrointestinal bleed, so it would make the most sense for a gastric ulcer to be the source of his bleeding. The clue here is the very high BUN/Cr ratio.
We were all taught early in our training that a high BUN/Cr ratio represented volume depletion. This is certainly the most common cause, but very high BUN/Cr ratios (over 30) can represent causes beyond volume depletion.
Witting and colleagues studied factors that predicted upper GI bleeding in patients presenting without hematemesis. They found that the three strongest predictors were black stool (odds ratio, 16.6), BUN/Cr ratio greater than 30 (OR, 10), and age greater than 50 (OR, 8.4).1
Srygley and colleagues reviewed high-quality studies of factors associated with upper GI bleeding.2 Factors that were found to increase the likelihood of an upper gastrointestinal bleed were Melenic stool on exam (likelihood ratio, 25), blood or coffee grounds on nasogastric aspiration (LR, 9.6), and BUN/Cr ratio greater than 30 (LR, 7.5).
Very high BUN/Cr ratios can indicate problems other than UGI bleeding and volume depletion. High BUN/Cr ratios are seen in patients with heart failure.
Zhang and colleagues studied if a high BUN/Cr ratio helped distinguish heart failure from asthma and chronic obstructive pulmonary disease (COPD).3 They found that, compared with those in the asthma group, the BUN/Cr ratios were significantly increased in the heart failure group (P < .05), whereas no significant differences in BUN/Cr ratios were found between the asthma and COPD groups.
Cheang and colleagues conducted their own study, as well as a meta-analysis, looking to see if high BUN/Cr ratios predicted increased mortality in patients with acute heart failure.4 In the meta-analysis of 8 studies (including their own), they found that the highest BUN/Cr ratio category was associated with an 77% higher all-cause mortality than the lowest category (hazard ratio, 1.77; 95% confidence interval, 1.52-2.07).
High dose corticosteroids can raise BUN levels, especially in patients with chronic kidney disease, and cause unexpectedly high BUN/Cr ratios.
Pearl
Very high BUN/Cr ratios (greater than 30) can signify upper GI bleeding, heart failure, or high-dose corticosteroid use.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Am J Emerg Med. 2006 May;24(3):280-5.
2. JAMA. 2012;307(10):1072-9.
3. Comput Math Methods Med. 2022 Jul 21. doi: 10.1155/2022/4586458.
4. Cardiorenal Med. 2020;10:415-28.
He has been in good health with the only medical problem in his history being depression.
He is taking sertraline. On exam, his blood pressure is 100/60, and his pulse is 100, both while lying down. His blood pressure while standing is 90/60 and his pulse while standing is 130. The rest of his exam is normal. His lab values include hemoglobin of 10, hematocrit of 30, white blood cell of 4.6, platelet count of 175,000, sodium of 142, chloride of 100, bicarbonate of 24, potassium of 3.8, blood urea nitrogen (BUN) of 38, and creatinine clearance (Cr) of 1.1.
What is the most likely source of his bleeding?
A. Gastric ulcer
B. Meckel’s diverticulum
C. Arteriovenous malformation
D. Diverticulosis
E. Hemorrhoids
What makes the most sense
The most likely cause of this patient’s maroon stool is an upper gastrointestinal bleed, so it would make the most sense for a gastric ulcer to be the source of his bleeding. The clue here is the very high BUN/Cr ratio.
We were all taught early in our training that a high BUN/Cr ratio represented volume depletion. This is certainly the most common cause, but very high BUN/Cr ratios (over 30) can represent causes beyond volume depletion.
Witting and colleagues studied factors that predicted upper GI bleeding in patients presenting without hematemesis. They found that the three strongest predictors were black stool (odds ratio, 16.6), BUN/Cr ratio greater than 30 (OR, 10), and age greater than 50 (OR, 8.4).1
Srygley and colleagues reviewed high-quality studies of factors associated with upper GI bleeding.2 Factors that were found to increase the likelihood of an upper gastrointestinal bleed were Melenic stool on exam (likelihood ratio, 25), blood or coffee grounds on nasogastric aspiration (LR, 9.6), and BUN/Cr ratio greater than 30 (LR, 7.5).
Very high BUN/Cr ratios can indicate problems other than UGI bleeding and volume depletion. High BUN/Cr ratios are seen in patients with heart failure.
Zhang and colleagues studied if a high BUN/Cr ratio helped distinguish heart failure from asthma and chronic obstructive pulmonary disease (COPD).3 They found that, compared with those in the asthma group, the BUN/Cr ratios were significantly increased in the heart failure group (P < .05), whereas no significant differences in BUN/Cr ratios were found between the asthma and COPD groups.
Cheang and colleagues conducted their own study, as well as a meta-analysis, looking to see if high BUN/Cr ratios predicted increased mortality in patients with acute heart failure.4 In the meta-analysis of 8 studies (including their own), they found that the highest BUN/Cr ratio category was associated with an 77% higher all-cause mortality than the lowest category (hazard ratio, 1.77; 95% confidence interval, 1.52-2.07).
High dose corticosteroids can raise BUN levels, especially in patients with chronic kidney disease, and cause unexpectedly high BUN/Cr ratios.
Pearl
Very high BUN/Cr ratios (greater than 30) can signify upper GI bleeding, heart failure, or high-dose corticosteroid use.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Am J Emerg Med. 2006 May;24(3):280-5.
2. JAMA. 2012;307(10):1072-9.
3. Comput Math Methods Med. 2022 Jul 21. doi: 10.1155/2022/4586458.
4. Cardiorenal Med. 2020;10:415-28.
He has been in good health with the only medical problem in his history being depression.
He is taking sertraline. On exam, his blood pressure is 100/60, and his pulse is 100, both while lying down. His blood pressure while standing is 90/60 and his pulse while standing is 130. The rest of his exam is normal. His lab values include hemoglobin of 10, hematocrit of 30, white blood cell of 4.6, platelet count of 175,000, sodium of 142, chloride of 100, bicarbonate of 24, potassium of 3.8, blood urea nitrogen (BUN) of 38, and creatinine clearance (Cr) of 1.1.
What is the most likely source of his bleeding?
A. Gastric ulcer
B. Meckel’s diverticulum
C. Arteriovenous malformation
D. Diverticulosis
E. Hemorrhoids
What makes the most sense
The most likely cause of this patient’s maroon stool is an upper gastrointestinal bleed, so it would make the most sense for a gastric ulcer to be the source of his bleeding. The clue here is the very high BUN/Cr ratio.
We were all taught early in our training that a high BUN/Cr ratio represented volume depletion. This is certainly the most common cause, but very high BUN/Cr ratios (over 30) can represent causes beyond volume depletion.
Witting and colleagues studied factors that predicted upper GI bleeding in patients presenting without hematemesis. They found that the three strongest predictors were black stool (odds ratio, 16.6), BUN/Cr ratio greater than 30 (OR, 10), and age greater than 50 (OR, 8.4).1
Srygley and colleagues reviewed high-quality studies of factors associated with upper GI bleeding.2 Factors that were found to increase the likelihood of an upper gastrointestinal bleed were Melenic stool on exam (likelihood ratio, 25), blood or coffee grounds on nasogastric aspiration (LR, 9.6), and BUN/Cr ratio greater than 30 (LR, 7.5).
Very high BUN/Cr ratios can indicate problems other than UGI bleeding and volume depletion. High BUN/Cr ratios are seen in patients with heart failure.
Zhang and colleagues studied if a high BUN/Cr ratio helped distinguish heart failure from asthma and chronic obstructive pulmonary disease (COPD).3 They found that, compared with those in the asthma group, the BUN/Cr ratios were significantly increased in the heart failure group (P < .05), whereas no significant differences in BUN/Cr ratios were found between the asthma and COPD groups.
Cheang and colleagues conducted their own study, as well as a meta-analysis, looking to see if high BUN/Cr ratios predicted increased mortality in patients with acute heart failure.4 In the meta-analysis of 8 studies (including their own), they found that the highest BUN/Cr ratio category was associated with an 77% higher all-cause mortality than the lowest category (hazard ratio, 1.77; 95% confidence interval, 1.52-2.07).
High dose corticosteroids can raise BUN levels, especially in patients with chronic kidney disease, and cause unexpectedly high BUN/Cr ratios.
Pearl
Very high BUN/Cr ratios (greater than 30) can signify upper GI bleeding, heart failure, or high-dose corticosteroid use.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at dpaauw@uw.edu.
References
1. Am J Emerg Med. 2006 May;24(3):280-5.
2. JAMA. 2012;307(10):1072-9.
3. Comput Math Methods Med. 2022 Jul 21. doi: 10.1155/2022/4586458.
4. Cardiorenal Med. 2020;10:415-28.
Experts share real-world experience prescribing voclosporin, belimumab for lupus nephritis
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Postpartum urinary retention: Intermittent catheterization may be best
Intermittent catheterization every 6 hours in postpartum women with urinary retention may be a better strategy than extended catheterization over 24 hours, a new prospective, randomized, controlled study suggests.
Patients who were catheterized every 6 hours took significantly less time to reach full relief than those who were catheterized for at least 24 hours (mean 10.2 ± 11.8 hours vs. 26.5 ± 9.0 hours, P < .001, respectively), Israeli researchers found. Their research was released at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There was no difference in hospital stay or in the rate of positive urine culture after catheter removal,” said ob.gyn. Dana Vitner, MD, of Rambam Health Care Campus in Haifa, Israel, in a presentation at the conference. “Our conclusion is that intermittent catheterization for postpartum urinary retention results in shorter time to resolution with a higher satisfaction rate and no additional complications.”
The true incidence of postpartum urinary retention is unclear, and estimates vary widely, said ob.gyn. and surgeon Lisa Hickman, MD, of the Ohio State University, Columbus, in an interview. “This is likely because many cases of covert urinary retention – when postpartum women are able to urinate but have incomplete emptying – go undiagnosed unless you are screening for it.”
According to Dr. Hickman, risk factors for postpartum urinary retention include operative vaginal births, having an epidural, obstetric anal sphincter injury, episiotomy, large newborns, first-time births, and prolonged induction of labor. Most cases resolve within 72 hours, she said, but they can lead to rare complications such as bladder injury.
For the new study, researchers defined urinary retention at the bladder holding least 150 mL more than 6 hours after vaginal delivery or removal of an in-dwelling catheter after cesarean delivery. “The treatment is catheterization,” Dr. Vitner said. “However, there is no standard protocol.”
From 2020 to 2022, researchers randomly assigned 73 women to the intermittent catheterization group and 74 to continuous catheterization. The average ages in the groups were 27.7 and 29.1 years, respectively (P = .11) and other characteristics such as body mass index, parity, infant birth weight, and mode of delivery were similar.
Most women in the intermittent catheterization group needed just one catheterization to reach resolution (75.3%); 93.2% had resolution after two, and 95.9% reached it after three. All resolved their urinary retention by 48 hours.
In the continuous catheterization group, 90.5% reached resolution at 24 hours, 97.3% at 48 hours, and 100% at 72 hours. Birth satisfaction scores were higher in the intermittent catheterization group (P < .001).
Dr. Hickman, who did not take part in the study, said the findings are helpful. Randomized, controlled trials are “important to get a better understanding of the natural history of this condition and ways to improve how we manage it clinically,” she said. Should intermittent catheterization become routine? “You need to have the staffing and the resources in order to do that, such as a bladder scanner and intermittent catheterization supplies,” Dr. Hickman said. “It can be time-intensive to continue to follow the patients to make sure they are voiding normally. And there may be many hospitals in the country that just don’t have the resources to do this, especially with all the current workforce issues.”
She added that some patients may not want the intermittent approach: “It can be uncomfortable for patients. They’ve just delivered a baby, they are likely experiencing discomfort from their delivery, and their anatomy can be distorted,” she said. “Some patients may say, ‘I would prefer you not insert a catheter into my bladder every few hours.’ They may just want to rest after having a baby.”
The best approach is to let patients make an informed choice, Dr. Hickman said. She recommended that clinicians say something like, “Because of your delivery, you are not able to empty your bladder all the way. This is typically a self-limited problem, meaning that it will likely resolve within a few days. But in the meantime, we need to let your bladder rest so that it can have time to start functioning on its own.” And then, she said, explain the catheterization options.
Dr. Vitner and Dr. Hickman have no disclosures.
Intermittent catheterization every 6 hours in postpartum women with urinary retention may be a better strategy than extended catheterization over 24 hours, a new prospective, randomized, controlled study suggests.
Patients who were catheterized every 6 hours took significantly less time to reach full relief than those who were catheterized for at least 24 hours (mean 10.2 ± 11.8 hours vs. 26.5 ± 9.0 hours, P < .001, respectively), Israeli researchers found. Their research was released at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There was no difference in hospital stay or in the rate of positive urine culture after catheter removal,” said ob.gyn. Dana Vitner, MD, of Rambam Health Care Campus in Haifa, Israel, in a presentation at the conference. “Our conclusion is that intermittent catheterization for postpartum urinary retention results in shorter time to resolution with a higher satisfaction rate and no additional complications.”
The true incidence of postpartum urinary retention is unclear, and estimates vary widely, said ob.gyn. and surgeon Lisa Hickman, MD, of the Ohio State University, Columbus, in an interview. “This is likely because many cases of covert urinary retention – when postpartum women are able to urinate but have incomplete emptying – go undiagnosed unless you are screening for it.”
According to Dr. Hickman, risk factors for postpartum urinary retention include operative vaginal births, having an epidural, obstetric anal sphincter injury, episiotomy, large newborns, first-time births, and prolonged induction of labor. Most cases resolve within 72 hours, she said, but they can lead to rare complications such as bladder injury.
For the new study, researchers defined urinary retention at the bladder holding least 150 mL more than 6 hours after vaginal delivery or removal of an in-dwelling catheter after cesarean delivery. “The treatment is catheterization,” Dr. Vitner said. “However, there is no standard protocol.”
From 2020 to 2022, researchers randomly assigned 73 women to the intermittent catheterization group and 74 to continuous catheterization. The average ages in the groups were 27.7 and 29.1 years, respectively (P = .11) and other characteristics such as body mass index, parity, infant birth weight, and mode of delivery were similar.
Most women in the intermittent catheterization group needed just one catheterization to reach resolution (75.3%); 93.2% had resolution after two, and 95.9% reached it after three. All resolved their urinary retention by 48 hours.
In the continuous catheterization group, 90.5% reached resolution at 24 hours, 97.3% at 48 hours, and 100% at 72 hours. Birth satisfaction scores were higher in the intermittent catheterization group (P < .001).
Dr. Hickman, who did not take part in the study, said the findings are helpful. Randomized, controlled trials are “important to get a better understanding of the natural history of this condition and ways to improve how we manage it clinically,” she said. Should intermittent catheterization become routine? “You need to have the staffing and the resources in order to do that, such as a bladder scanner and intermittent catheterization supplies,” Dr. Hickman said. “It can be time-intensive to continue to follow the patients to make sure they are voiding normally. And there may be many hospitals in the country that just don’t have the resources to do this, especially with all the current workforce issues.”
She added that some patients may not want the intermittent approach: “It can be uncomfortable for patients. They’ve just delivered a baby, they are likely experiencing discomfort from their delivery, and their anatomy can be distorted,” she said. “Some patients may say, ‘I would prefer you not insert a catheter into my bladder every few hours.’ They may just want to rest after having a baby.”
The best approach is to let patients make an informed choice, Dr. Hickman said. She recommended that clinicians say something like, “Because of your delivery, you are not able to empty your bladder all the way. This is typically a self-limited problem, meaning that it will likely resolve within a few days. But in the meantime, we need to let your bladder rest so that it can have time to start functioning on its own.” And then, she said, explain the catheterization options.
Dr. Vitner and Dr. Hickman have no disclosures.
Intermittent catheterization every 6 hours in postpartum women with urinary retention may be a better strategy than extended catheterization over 24 hours, a new prospective, randomized, controlled study suggests.
Patients who were catheterized every 6 hours took significantly less time to reach full relief than those who were catheterized for at least 24 hours (mean 10.2 ± 11.8 hours vs. 26.5 ± 9.0 hours, P < .001, respectively), Israeli researchers found. Their research was released at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There was no difference in hospital stay or in the rate of positive urine culture after catheter removal,” said ob.gyn. Dana Vitner, MD, of Rambam Health Care Campus in Haifa, Israel, in a presentation at the conference. “Our conclusion is that intermittent catheterization for postpartum urinary retention results in shorter time to resolution with a higher satisfaction rate and no additional complications.”
The true incidence of postpartum urinary retention is unclear, and estimates vary widely, said ob.gyn. and surgeon Lisa Hickman, MD, of the Ohio State University, Columbus, in an interview. “This is likely because many cases of covert urinary retention – when postpartum women are able to urinate but have incomplete emptying – go undiagnosed unless you are screening for it.”
According to Dr. Hickman, risk factors for postpartum urinary retention include operative vaginal births, having an epidural, obstetric anal sphincter injury, episiotomy, large newborns, first-time births, and prolonged induction of labor. Most cases resolve within 72 hours, she said, but they can lead to rare complications such as bladder injury.
For the new study, researchers defined urinary retention at the bladder holding least 150 mL more than 6 hours after vaginal delivery or removal of an in-dwelling catheter after cesarean delivery. “The treatment is catheterization,” Dr. Vitner said. “However, there is no standard protocol.”
From 2020 to 2022, researchers randomly assigned 73 women to the intermittent catheterization group and 74 to continuous catheterization. The average ages in the groups were 27.7 and 29.1 years, respectively (P = .11) and other characteristics such as body mass index, parity, infant birth weight, and mode of delivery were similar.
Most women in the intermittent catheterization group needed just one catheterization to reach resolution (75.3%); 93.2% had resolution after two, and 95.9% reached it after three. All resolved their urinary retention by 48 hours.
In the continuous catheterization group, 90.5% reached resolution at 24 hours, 97.3% at 48 hours, and 100% at 72 hours. Birth satisfaction scores were higher in the intermittent catheterization group (P < .001).
Dr. Hickman, who did not take part in the study, said the findings are helpful. Randomized, controlled trials are “important to get a better understanding of the natural history of this condition and ways to improve how we manage it clinically,” she said. Should intermittent catheterization become routine? “You need to have the staffing and the resources in order to do that, such as a bladder scanner and intermittent catheterization supplies,” Dr. Hickman said. “It can be time-intensive to continue to follow the patients to make sure they are voiding normally. And there may be many hospitals in the country that just don’t have the resources to do this, especially with all the current workforce issues.”
She added that some patients may not want the intermittent approach: “It can be uncomfortable for patients. They’ve just delivered a baby, they are likely experiencing discomfort from their delivery, and their anatomy can be distorted,” she said. “Some patients may say, ‘I would prefer you not insert a catheter into my bladder every few hours.’ They may just want to rest after having a baby.”
The best approach is to let patients make an informed choice, Dr. Hickman said. She recommended that clinicians say something like, “Because of your delivery, you are not able to empty your bladder all the way. This is typically a self-limited problem, meaning that it will likely resolve within a few days. But in the meantime, we need to let your bladder rest so that it can have time to start functioning on its own.” And then, she said, explain the catheterization options.
Dr. Vitner and Dr. Hickman have no disclosures.
FROM THE PREGNANCY MEETING