User login
Experts call for early screening for chronic kidney disease
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
Strategies for complete B-cell depletion evolve for patients with lupus nephritis
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
AT LUPUS 2023
Transplant centers often skip the top spot on the kidney waitlist
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
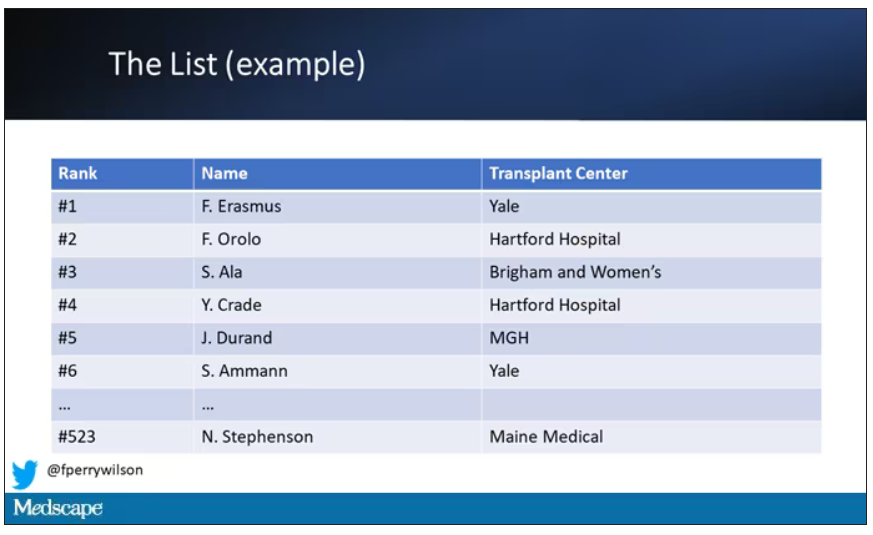
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
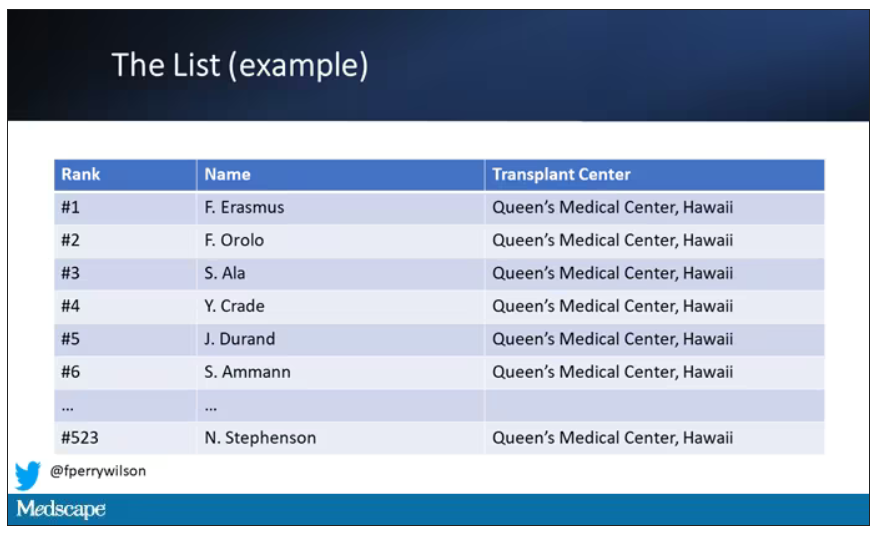
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
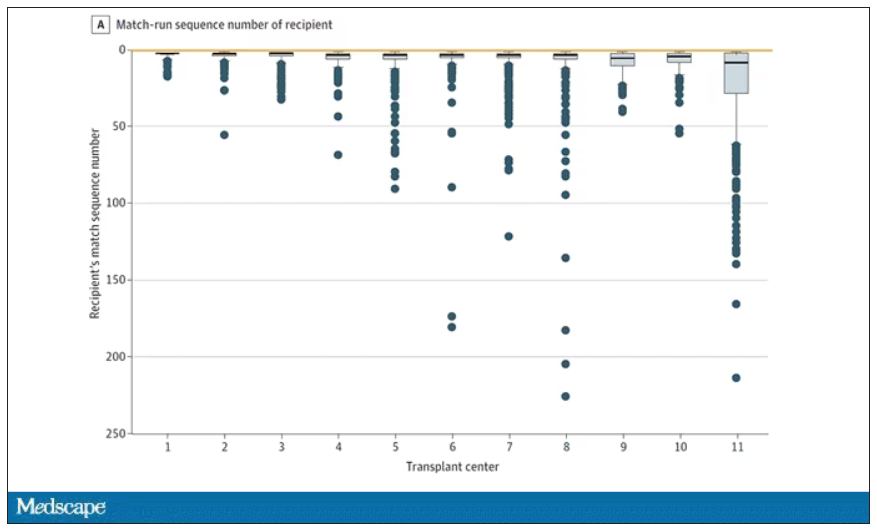
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
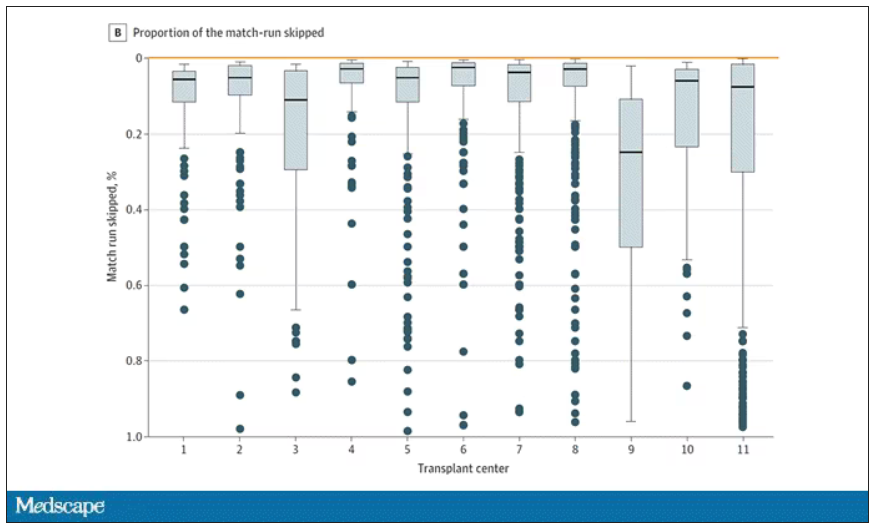
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Lupus nephritis: Hopes, questions arise for baricitinib
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
AT EULAR 2023
FDA approves new drug, sotagliflozin, for heart failure
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Early remission in lupus nephritis can still progress to advanced CKD
SEOUL, SOUTH KOREA – Nearly 8% of people with lupus nephritis who achieve complete remission of disease within 1 year of starting treatment will still go on to develop advanced chronic kidney disease (CKD), according to a presentation at an international congress on systemic lupus erythematosus.
Rheumatologist Dafna Gladman, MD, professor of medicine at the University of Toronto and codirector of the Lupus Clinic at Toronto Western Hospital, showed data from the Lupus Clinic’s prospective longitudinal cohort study in 273 patients with confirmed lupus nephritis who achieved complete remission within 12 months of baseline.
Remission was defined as less than 0.5 g proteinuria over 24 hours, inactive urinary sediment, and serum creatinine less than 120% of baseline.
Of this group, 21 (7.7%) progressed to advanced CKD during follow-up, which ranged from 0.7 to 31.7 years with a median of 5.8 years, after enrollment.
Patients who had experienced at least one flare during their first 5 years were around 4.5 times more likely to progress to advanced CKD than were those who did not experience a flare.
While the study excluded patients who already had advanced CKD, the analysis found those with evidence of impaired kidney function at baseline also had more than a fourfold higher risk of developing advanced CKD.
Other significant risk factors for progression were having low complement C3 levels at baseline and having had a longer duration of disease before enrollment.
“Those patients already have abnormal renal function, so the message is that patients who are already in trouble, you’ve got to watch them very carefully,” Dr. Gladman said in an interview.
The study also looked at whether there was a difference between patients who developed advanced CKD earlier – before the median of 5.8 years – or later. While the numbers were small, Dr. Gladman said patients who progressed earlier tended to be older and were more likely to be on antihypertensive treatment and have lower estimated glomerular filtration rate and a lower Systemic Lupus Erythematosus Disease Activity Index–2K, compared with those who progressed later. Some patients also were noncompliant and/or experienced concomitant infections; four had moderate to severe interstitial fibrosis and tubular atrophy.
“We conclude that such patients should be monitored closely despite early remission, and we also highlight the importance of maintenance therapy, which should be communicated to the patients to prevent noncompliance and subsequent flare,” Dr. Gladman told the conference.
Dr. Gladman said her clinic told patients from the very beginning of their treatment that they would need to be seen at 2- to 6-month intervals, regardless of how well their disease was doing.
Commenting on the presentation, rheumatologist Mandana Nikpour MD, PhD, of St. Vincent’s Hospital in Melbourne, said the findings showed the importance of keeping a close eye on patients with lupus nephritis, even if their disease appears to be in remission.
“If you’ve had nephritis, and you go into remission, you may already have a degree of damage in your kidneys,” said Dr. Nikpour, also from the University of Melbourne. “If there’s a degree of uncontrolled hypertension, or if a patient is noncompliant with their treatment, and there’s a degree of grumbling disease activity, that can all conspire and add up to result in long-term kidney damage and loss of renal function.”
Dr. Gladman has received grants or research support from, or has consulted for, Amgen, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Galapagos, and Gilead.
SEOUL, SOUTH KOREA – Nearly 8% of people with lupus nephritis who achieve complete remission of disease within 1 year of starting treatment will still go on to develop advanced chronic kidney disease (CKD), according to a presentation at an international congress on systemic lupus erythematosus.
Rheumatologist Dafna Gladman, MD, professor of medicine at the University of Toronto and codirector of the Lupus Clinic at Toronto Western Hospital, showed data from the Lupus Clinic’s prospective longitudinal cohort study in 273 patients with confirmed lupus nephritis who achieved complete remission within 12 months of baseline.
Remission was defined as less than 0.5 g proteinuria over 24 hours, inactive urinary sediment, and serum creatinine less than 120% of baseline.
Of this group, 21 (7.7%) progressed to advanced CKD during follow-up, which ranged from 0.7 to 31.7 years with a median of 5.8 years, after enrollment.
Patients who had experienced at least one flare during their first 5 years were around 4.5 times more likely to progress to advanced CKD than were those who did not experience a flare.
While the study excluded patients who already had advanced CKD, the analysis found those with evidence of impaired kidney function at baseline also had more than a fourfold higher risk of developing advanced CKD.
Other significant risk factors for progression were having low complement C3 levels at baseline and having had a longer duration of disease before enrollment.
“Those patients already have abnormal renal function, so the message is that patients who are already in trouble, you’ve got to watch them very carefully,” Dr. Gladman said in an interview.
The study also looked at whether there was a difference between patients who developed advanced CKD earlier – before the median of 5.8 years – or later. While the numbers were small, Dr. Gladman said patients who progressed earlier tended to be older and were more likely to be on antihypertensive treatment and have lower estimated glomerular filtration rate and a lower Systemic Lupus Erythematosus Disease Activity Index–2K, compared with those who progressed later. Some patients also were noncompliant and/or experienced concomitant infections; four had moderate to severe interstitial fibrosis and tubular atrophy.
“We conclude that such patients should be monitored closely despite early remission, and we also highlight the importance of maintenance therapy, which should be communicated to the patients to prevent noncompliance and subsequent flare,” Dr. Gladman told the conference.
Dr. Gladman said her clinic told patients from the very beginning of their treatment that they would need to be seen at 2- to 6-month intervals, regardless of how well their disease was doing.
Commenting on the presentation, rheumatologist Mandana Nikpour MD, PhD, of St. Vincent’s Hospital in Melbourne, said the findings showed the importance of keeping a close eye on patients with lupus nephritis, even if their disease appears to be in remission.
“If you’ve had nephritis, and you go into remission, you may already have a degree of damage in your kidneys,” said Dr. Nikpour, also from the University of Melbourne. “If there’s a degree of uncontrolled hypertension, or if a patient is noncompliant with their treatment, and there’s a degree of grumbling disease activity, that can all conspire and add up to result in long-term kidney damage and loss of renal function.”
Dr. Gladman has received grants or research support from, or has consulted for, Amgen, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Galapagos, and Gilead.
SEOUL, SOUTH KOREA – Nearly 8% of people with lupus nephritis who achieve complete remission of disease within 1 year of starting treatment will still go on to develop advanced chronic kidney disease (CKD), according to a presentation at an international congress on systemic lupus erythematosus.
Rheumatologist Dafna Gladman, MD, professor of medicine at the University of Toronto and codirector of the Lupus Clinic at Toronto Western Hospital, showed data from the Lupus Clinic’s prospective longitudinal cohort study in 273 patients with confirmed lupus nephritis who achieved complete remission within 12 months of baseline.
Remission was defined as less than 0.5 g proteinuria over 24 hours, inactive urinary sediment, and serum creatinine less than 120% of baseline.
Of this group, 21 (7.7%) progressed to advanced CKD during follow-up, which ranged from 0.7 to 31.7 years with a median of 5.8 years, after enrollment.
Patients who had experienced at least one flare during their first 5 years were around 4.5 times more likely to progress to advanced CKD than were those who did not experience a flare.
While the study excluded patients who already had advanced CKD, the analysis found those with evidence of impaired kidney function at baseline also had more than a fourfold higher risk of developing advanced CKD.
Other significant risk factors for progression were having low complement C3 levels at baseline and having had a longer duration of disease before enrollment.
“Those patients already have abnormal renal function, so the message is that patients who are already in trouble, you’ve got to watch them very carefully,” Dr. Gladman said in an interview.
The study also looked at whether there was a difference between patients who developed advanced CKD earlier – before the median of 5.8 years – or later. While the numbers were small, Dr. Gladman said patients who progressed earlier tended to be older and were more likely to be on antihypertensive treatment and have lower estimated glomerular filtration rate and a lower Systemic Lupus Erythematosus Disease Activity Index–2K, compared with those who progressed later. Some patients also were noncompliant and/or experienced concomitant infections; four had moderate to severe interstitial fibrosis and tubular atrophy.
“We conclude that such patients should be monitored closely despite early remission, and we also highlight the importance of maintenance therapy, which should be communicated to the patients to prevent noncompliance and subsequent flare,” Dr. Gladman told the conference.
Dr. Gladman said her clinic told patients from the very beginning of their treatment that they would need to be seen at 2- to 6-month intervals, regardless of how well their disease was doing.
Commenting on the presentation, rheumatologist Mandana Nikpour MD, PhD, of St. Vincent’s Hospital in Melbourne, said the findings showed the importance of keeping a close eye on patients with lupus nephritis, even if their disease appears to be in remission.
“If you’ve had nephritis, and you go into remission, you may already have a degree of damage in your kidneys,” said Dr. Nikpour, also from the University of Melbourne. “If there’s a degree of uncontrolled hypertension, or if a patient is noncompliant with their treatment, and there’s a degree of grumbling disease activity, that can all conspire and add up to result in long-term kidney damage and loss of renal function.”
Dr. Gladman has received grants or research support from, or has consulted for, Amgen, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Galapagos, and Gilead.
AT LUPUS 2023
CKD Screening in all U.S. adults found cost effective
(UACR) followed by confirmatory tests and treatment of confirmed cases with current standard-care medications, according to an analysis published in the Annals of Internal Medicine.
This new evidence may prove important as the U.S. Preventive Services Task Force has begun revisiting its 2012 conclusion that “evidence is insufficient to assess the balance of benefits and harms of routine screening for chronic kidney disease in asymptomatic adults.”
A big difference between 2012 and today has been that sodium-glucose cotransporter 2 (SGLT2) inhibitors arrived on the scene as an important complement to well-established treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. SGLT2 inhibitors have been documented as safe and effective for slowing CKD progression regardless of a person’s diabetes status, and have “dramatically altered” first-line treatment of adults with CKD, wrote the authors of the new study.
‘Large population health gains’ from CKD screening
“Given the high prevalence of CKD, even among those without risk factors, low-cost screening combined with effective treatment using SGLT2 inhibitors represent value,” explained Marika M. Cusick, lead author of the report, a PhD student, and a health policy researcher at Stanford (Calif.) University. “Our results show large population health gains can be achieved through CKD screening,” she said in an interview.
“This is a well-designed cost-effectiveness analysis that, importantly, considers newer treatments shown to be effective for slowing progression of CKD. The overall findings are convincing,” commented Deidra C. Crews, MD, a nephrologist and professor at Johns Hopkins University in Baltimore who was not involved in the research.
Dr. Crews, who is also president-elect of the American Society of Nephrology noted that the findings “may be a conservative estimate of the cost-effectiveness of CKD screening in certain subgroups, particularly when considering profound racial, ethnic and socioeconomic disparities in survival and CKD progression.”
The USPSTF starts a relook
The new evidence of cost-effectiveness of routine CKD screening follows the USPSTF’s release in January 2023 of a draft research plan to reassess the potential role for CKD screening of asymptomatic adults in the United States, the first step on a potential path to a revised set of recommendations. Public comment on the draft plan closed in February, and based on the standard USPSTF development steps and time frames, a final recommendation statement could appear by early 2026.
Revisiting the prior USPSTF decision from 2012 received endorsement earlier in 2023 from the ASN. The organization issued a statement last January that cited “more than a decade of advocacy in support of more kidney health screening by ASN and other stakeholders dedicated to intervening earlier to slow or stop the progression of kidney diseases.”
A more detailed letter of support for CKD screening sent to top USPSTF officials followed in February 2023 from ASN president Michelle A. Josephson, MD, who said in part that “ASN believes that kidney care is at an inflection point. There are now far more novel therapeutics to slow the progression of CKD, evidence to support the impact of nonpharmacologic interventions on CKD, and an increased commitment in public health to confront disparities and their causes.”
USPSTF recommendation could make a difference
Dr. Josephson also cited the modest effect that CKD screening recommendations from other groups have had up to now.
“Although guidance from Kidney Disease Improving Global Outcomes and the National Kidney Foundation recommends CKD screening among patients with hypertension, only approximately 10% of individuals with hypertension receive yearly screening. Furthermore, American Diabetes Association guidelines recommend yearly CKD screening in patients with diabetes, but only 40%-50% of patients receive this.”
“USPSTF recommendations tend to reach clinicians in primary care settings, where screening for diseases most commonly occurs, much more than recommendations from professional or patient organizations,” Dr. Crews said in an interview. “USPSTF recommendations also often influence health policies that might financially incentivize clinicians and health systems to screen their patients.”
“We hope [the USPSTF] will be interested in including our results within the totality of evidence assessed in their review of CKD screening,” said Ms. Cusick.
Preventing hundreds of thousands dialysis cases
The Stanford researchers developed a decision analytic Markov cohort model of CKD progression in U.S. adults aged 35 years or older and fit their model to data from the National Health and Nutrition Examination Survey (NHANES). They found that implementing one-time screening and adding SGLT2 inhibitors to treatment of the 158 million U.S. adults 35-75 years old would prevent the need for kidney replacement therapy (dialysis or transplant) in approximately 398,000 people over their lifetimes, representing a 10% decrease in such cases, compared with the status quo. Screening every 10 or 5 years combined with SGLT2 inhibitors would prevent approximately 598,000 or 658,000 people, respectively, from requiring kidney replacement therapy, compared with not screening.
Analysis showed that one-time screening produced an incremental cost-effectiveness ratio of $86,300 per quality-adjusted life-year (QALY) gained when one-time screening occurred in adults when they reached 55 years old. Screening every 10 years until people became 75 years old cost $98,400 per QALY gained for this group when adults were 35 years old, and $89,800 per QALY gained when screening occurred at 65 years old. These QALY costs are less than “commonly used” U.S. thresholds for acceptable cost-effectiveness of $100,000-$150,000 per QALY gained, the authors said.
Ms. Cusick highlighted the advantages of population-level screening for all U.S. adults, including those who are asymptomatic, compared with focusing on adults with risk factors, such as hypertension or diabetes.
“While risk-based screening can be more cost effective in some settings, risk factors are not always known, especially in marginalized and disadvantaged populations. This may lead to disparities in the use of screening and downstream health outcomes that could be avoided through universal screening policies,” she explained.
The study received no commercial funding. Ms. Cusick had no disclosures. Dr. Crews has received research grants from Somatus. Dr. Josephson has been a consultant to Exosome Diagnostics, IMMUCOR, Labcorp, Otsuka, UBC, and Vera Therapeutics, and has an ownership interest in Seagen.
(UACR) followed by confirmatory tests and treatment of confirmed cases with current standard-care medications, according to an analysis published in the Annals of Internal Medicine.
This new evidence may prove important as the U.S. Preventive Services Task Force has begun revisiting its 2012 conclusion that “evidence is insufficient to assess the balance of benefits and harms of routine screening for chronic kidney disease in asymptomatic adults.”
A big difference between 2012 and today has been that sodium-glucose cotransporter 2 (SGLT2) inhibitors arrived on the scene as an important complement to well-established treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. SGLT2 inhibitors have been documented as safe and effective for slowing CKD progression regardless of a person’s diabetes status, and have “dramatically altered” first-line treatment of adults with CKD, wrote the authors of the new study.
‘Large population health gains’ from CKD screening
“Given the high prevalence of CKD, even among those without risk factors, low-cost screening combined with effective treatment using SGLT2 inhibitors represent value,” explained Marika M. Cusick, lead author of the report, a PhD student, and a health policy researcher at Stanford (Calif.) University. “Our results show large population health gains can be achieved through CKD screening,” she said in an interview.
“This is a well-designed cost-effectiveness analysis that, importantly, considers newer treatments shown to be effective for slowing progression of CKD. The overall findings are convincing,” commented Deidra C. Crews, MD, a nephrologist and professor at Johns Hopkins University in Baltimore who was not involved in the research.
Dr. Crews, who is also president-elect of the American Society of Nephrology noted that the findings “may be a conservative estimate of the cost-effectiveness of CKD screening in certain subgroups, particularly when considering profound racial, ethnic and socioeconomic disparities in survival and CKD progression.”
The USPSTF starts a relook
The new evidence of cost-effectiveness of routine CKD screening follows the USPSTF’s release in January 2023 of a draft research plan to reassess the potential role for CKD screening of asymptomatic adults in the United States, the first step on a potential path to a revised set of recommendations. Public comment on the draft plan closed in February, and based on the standard USPSTF development steps and time frames, a final recommendation statement could appear by early 2026.
Revisiting the prior USPSTF decision from 2012 received endorsement earlier in 2023 from the ASN. The organization issued a statement last January that cited “more than a decade of advocacy in support of more kidney health screening by ASN and other stakeholders dedicated to intervening earlier to slow or stop the progression of kidney diseases.”
A more detailed letter of support for CKD screening sent to top USPSTF officials followed in February 2023 from ASN president Michelle A. Josephson, MD, who said in part that “ASN believes that kidney care is at an inflection point. There are now far more novel therapeutics to slow the progression of CKD, evidence to support the impact of nonpharmacologic interventions on CKD, and an increased commitment in public health to confront disparities and their causes.”
USPSTF recommendation could make a difference
Dr. Josephson also cited the modest effect that CKD screening recommendations from other groups have had up to now.
“Although guidance from Kidney Disease Improving Global Outcomes and the National Kidney Foundation recommends CKD screening among patients with hypertension, only approximately 10% of individuals with hypertension receive yearly screening. Furthermore, American Diabetes Association guidelines recommend yearly CKD screening in patients with diabetes, but only 40%-50% of patients receive this.”
“USPSTF recommendations tend to reach clinicians in primary care settings, where screening for diseases most commonly occurs, much more than recommendations from professional or patient organizations,” Dr. Crews said in an interview. “USPSTF recommendations also often influence health policies that might financially incentivize clinicians and health systems to screen their patients.”
“We hope [the USPSTF] will be interested in including our results within the totality of evidence assessed in their review of CKD screening,” said Ms. Cusick.
Preventing hundreds of thousands dialysis cases
The Stanford researchers developed a decision analytic Markov cohort model of CKD progression in U.S. adults aged 35 years or older and fit their model to data from the National Health and Nutrition Examination Survey (NHANES). They found that implementing one-time screening and adding SGLT2 inhibitors to treatment of the 158 million U.S. adults 35-75 years old would prevent the need for kidney replacement therapy (dialysis or transplant) in approximately 398,000 people over their lifetimes, representing a 10% decrease in such cases, compared with the status quo. Screening every 10 or 5 years combined with SGLT2 inhibitors would prevent approximately 598,000 or 658,000 people, respectively, from requiring kidney replacement therapy, compared with not screening.
Analysis showed that one-time screening produced an incremental cost-effectiveness ratio of $86,300 per quality-adjusted life-year (QALY) gained when one-time screening occurred in adults when they reached 55 years old. Screening every 10 years until people became 75 years old cost $98,400 per QALY gained for this group when adults were 35 years old, and $89,800 per QALY gained when screening occurred at 65 years old. These QALY costs are less than “commonly used” U.S. thresholds for acceptable cost-effectiveness of $100,000-$150,000 per QALY gained, the authors said.
Ms. Cusick highlighted the advantages of population-level screening for all U.S. adults, including those who are asymptomatic, compared with focusing on adults with risk factors, such as hypertension or diabetes.
“While risk-based screening can be more cost effective in some settings, risk factors are not always known, especially in marginalized and disadvantaged populations. This may lead to disparities in the use of screening and downstream health outcomes that could be avoided through universal screening policies,” she explained.
The study received no commercial funding. Ms. Cusick had no disclosures. Dr. Crews has received research grants from Somatus. Dr. Josephson has been a consultant to Exosome Diagnostics, IMMUCOR, Labcorp, Otsuka, UBC, and Vera Therapeutics, and has an ownership interest in Seagen.
(UACR) followed by confirmatory tests and treatment of confirmed cases with current standard-care medications, according to an analysis published in the Annals of Internal Medicine.
This new evidence may prove important as the U.S. Preventive Services Task Force has begun revisiting its 2012 conclusion that “evidence is insufficient to assess the balance of benefits and harms of routine screening for chronic kidney disease in asymptomatic adults.”
A big difference between 2012 and today has been that sodium-glucose cotransporter 2 (SGLT2) inhibitors arrived on the scene as an important complement to well-established treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. SGLT2 inhibitors have been documented as safe and effective for slowing CKD progression regardless of a person’s diabetes status, and have “dramatically altered” first-line treatment of adults with CKD, wrote the authors of the new study.
‘Large population health gains’ from CKD screening
“Given the high prevalence of CKD, even among those without risk factors, low-cost screening combined with effective treatment using SGLT2 inhibitors represent value,” explained Marika M. Cusick, lead author of the report, a PhD student, and a health policy researcher at Stanford (Calif.) University. “Our results show large population health gains can be achieved through CKD screening,” she said in an interview.
“This is a well-designed cost-effectiveness analysis that, importantly, considers newer treatments shown to be effective for slowing progression of CKD. The overall findings are convincing,” commented Deidra C. Crews, MD, a nephrologist and professor at Johns Hopkins University in Baltimore who was not involved in the research.
Dr. Crews, who is also president-elect of the American Society of Nephrology noted that the findings “may be a conservative estimate of the cost-effectiveness of CKD screening in certain subgroups, particularly when considering profound racial, ethnic and socioeconomic disparities in survival and CKD progression.”
The USPSTF starts a relook
The new evidence of cost-effectiveness of routine CKD screening follows the USPSTF’s release in January 2023 of a draft research plan to reassess the potential role for CKD screening of asymptomatic adults in the United States, the first step on a potential path to a revised set of recommendations. Public comment on the draft plan closed in February, and based on the standard USPSTF development steps and time frames, a final recommendation statement could appear by early 2026.
Revisiting the prior USPSTF decision from 2012 received endorsement earlier in 2023 from the ASN. The organization issued a statement last January that cited “more than a decade of advocacy in support of more kidney health screening by ASN and other stakeholders dedicated to intervening earlier to slow or stop the progression of kidney diseases.”
A more detailed letter of support for CKD screening sent to top USPSTF officials followed in February 2023 from ASN president Michelle A. Josephson, MD, who said in part that “ASN believes that kidney care is at an inflection point. There are now far more novel therapeutics to slow the progression of CKD, evidence to support the impact of nonpharmacologic interventions on CKD, and an increased commitment in public health to confront disparities and their causes.”
USPSTF recommendation could make a difference
Dr. Josephson also cited the modest effect that CKD screening recommendations from other groups have had up to now.
“Although guidance from Kidney Disease Improving Global Outcomes and the National Kidney Foundation recommends CKD screening among patients with hypertension, only approximately 10% of individuals with hypertension receive yearly screening. Furthermore, American Diabetes Association guidelines recommend yearly CKD screening in patients with diabetes, but only 40%-50% of patients receive this.”
“USPSTF recommendations tend to reach clinicians in primary care settings, where screening for diseases most commonly occurs, much more than recommendations from professional or patient organizations,” Dr. Crews said in an interview. “USPSTF recommendations also often influence health policies that might financially incentivize clinicians and health systems to screen their patients.”
“We hope [the USPSTF] will be interested in including our results within the totality of evidence assessed in their review of CKD screening,” said Ms. Cusick.
Preventing hundreds of thousands dialysis cases
The Stanford researchers developed a decision analytic Markov cohort model of CKD progression in U.S. adults aged 35 years or older and fit their model to data from the National Health and Nutrition Examination Survey (NHANES). They found that implementing one-time screening and adding SGLT2 inhibitors to treatment of the 158 million U.S. adults 35-75 years old would prevent the need for kidney replacement therapy (dialysis or transplant) in approximately 398,000 people over their lifetimes, representing a 10% decrease in such cases, compared with the status quo. Screening every 10 or 5 years combined with SGLT2 inhibitors would prevent approximately 598,000 or 658,000 people, respectively, from requiring kidney replacement therapy, compared with not screening.
Analysis showed that one-time screening produced an incremental cost-effectiveness ratio of $86,300 per quality-adjusted life-year (QALY) gained when one-time screening occurred in adults when they reached 55 years old. Screening every 10 years until people became 75 years old cost $98,400 per QALY gained for this group when adults were 35 years old, and $89,800 per QALY gained when screening occurred at 65 years old. These QALY costs are less than “commonly used” U.S. thresholds for acceptable cost-effectiveness of $100,000-$150,000 per QALY gained, the authors said.
Ms. Cusick highlighted the advantages of population-level screening for all U.S. adults, including those who are asymptomatic, compared with focusing on adults with risk factors, such as hypertension or diabetes.
“While risk-based screening can be more cost effective in some settings, risk factors are not always known, especially in marginalized and disadvantaged populations. This may lead to disparities in the use of screening and downstream health outcomes that could be avoided through universal screening policies,” she explained.
The study received no commercial funding. Ms. Cusick had no disclosures. Dr. Crews has received research grants from Somatus. Dr. Josephson has been a consultant to Exosome Diagnostics, IMMUCOR, Labcorp, Otsuka, UBC, and Vera Therapeutics, and has an ownership interest in Seagen.
FROM ANNALS OF INTERNAL MEDICINE
Biomarkers for measuring lupus nephritis treatment response gain ground
SEOUL, SOUTH KOREA – A panel of urinary biomarkers may do better than measuring proteinuria in predicting which patients with lupus nephritis are going to respond to treatment, according to a presentation at an international congress on systemic lupus erythematosus.
Physician-scientist Andrea Fava, MD, of the division of rheumatology at Johns Hopkins University, Baltimore, presented data from a study using urine proteomics to identify biomarkers present in the urine of patients with lupus nephritis at 3 months after starting treatment that were linked to better outcomes from that treatment at 1 year.
While proteinuria is the standard measure used to guide decisions about whether to do a kidney biopsy and how to treat lupus nephritis, it doesn’t always correlate with what’s actually going on inside the kidney in terms of histology and inflammation, Dr. Fava said.
He pointed to an earlier study in which researchers did kidney biopsies 6 months after patients with lupus nephritis started treatment with mycophenolate. This suggested that around half of patients who showed a clinical response to treatment – defined as proteinuria below 500 mg/day – still had significant histologic disease activity. Another study suggested that this elevated histologic disease activity is associated with a risk of flare, which can result in significant nephron loss. On the flip side, nearly two-thirds of patients in complete histologic remission still had elevated proteinuria.
Unfortunately, it’s not possible or practical to biopsy patients on a regular basis, Dr. Fava said. “So we need better biomarkers, and to do so, we need better knowledge of the pathophysiology because if we have biomarkers that reflect tissue biology in real-time, that may surely guide personalized treatments,” he said at the congress.
Dr. Fava and colleagues enrolled 225 patients with SLE who were undergoing kidney biopsy and 10 healthy controls and used proteomics to quantify the urinary levels of around 1,200 proteins at baseline, 3, 6, and 12 months after initiating treatment.
The team then analyzed these data to look for protein signatures that correlated with histologic phenotypes – particularly the amount of inflammation in the kidney – and clinical features such as response to treatment.
They found several protein biomarkers that appeared to be linked to histologic activity in the kidney, including interleukin (IL)-16, CD163, and neutrophil granule proteins.
Initially, the team looked at baseline levels of these proteins to see if they predicted who responded to treatment, but found no difference between responders and nonresponders.
However, when they looked at levels at 3 months after treatment, a pattern emerged. “We found that in patients who were not responding, there were no changes after 3 months of treatment in the urine proteome,” Dr. Fava said. Among those who did respond to treatment, the levels of these proteins – IL-16, CD163, galectin-1, and CD206 – decreased significantly.
“So the proteins that are linked to renal activity decrease only in responders, suggesting that effective immunosuppression is effective in reducing intrarenal inflammation, which eventually results in low proteinuria at 1 year.”
The decline in these biomarkers persisted at 1 year, and the study suggested it was a better predictor of which patients would respond to treatment at 1 year than proteinuria.
Dr. Fava said in an interview that better biomarkers could revolutionize the treatment and management of lupus nephritis.
“First of all, it can shift the management strategy from treatment to prevention, because at the very beginning we can nip it in the bud maybe with very gentle treatment,” he said. Different panels of urine biomarkers could identify patients at risk of treatment failure, and also help patients to taper off their immunosuppressive therapy without an increased risk of flare. “If we have a way to tell us there’s still inflammation that needs treatment, that could change the way we do it,” he said.
He acknowledged there are significant challenges to developing these biomarkers for clinical use; one is the decision of how to define disease activity without relying on proteinuria as a measure. “Why do I want a biomarker that can predict another biomarker?” he said.
Another presentation during the same session, by Huihua Ding, MD, of Shanghai Jiao Tong University in Shanghai, China, reported on the use of urinary L-selectin to assess renal disease activity and response to treatment in a multiethnic cohort.
This study, involving 474 patients with SLE with or without renal involvement in the United States and China, found levels of urinary L-selectin were elevated only in patients with active lupus nephritis and showed patterns that correlated with renal histologic characteristics.
Clinical rheumatologist Eric F. Morand, MD, PhD, and head of the School of Clinical Sciences at Monash University in Melbourne said one challenge with using urinary biomarkers was that it was not yet clear what these biomarkers reveal about the kidney. “It will be important to see whether this proteomic data actually link to renal outcomes,” Dr. Morand said in an interview. “I think predicting the response to treatment should be based around GFR [glomerular filtration rate] preservation, and I don’t think I’ve seen data yet that the urine biomarkers are going to tell us how to do that better.”
Dr. Morand is optimistic that urine biomarkers will one day be able to achieve that, but he stressed the importance of having urine biomarker tests available in the field at low cost. “You’re going to be doing the tests repeatedly, so therefore, you’re probably going to need to come down to a smaller list of proteins that you measure.”
Dr. Fava reported receiving support from Sanofi and Annexion Bio.
SEOUL, SOUTH KOREA – A panel of urinary biomarkers may do better than measuring proteinuria in predicting which patients with lupus nephritis are going to respond to treatment, according to a presentation at an international congress on systemic lupus erythematosus.
Physician-scientist Andrea Fava, MD, of the division of rheumatology at Johns Hopkins University, Baltimore, presented data from a study using urine proteomics to identify biomarkers present in the urine of patients with lupus nephritis at 3 months after starting treatment that were linked to better outcomes from that treatment at 1 year.
While proteinuria is the standard measure used to guide decisions about whether to do a kidney biopsy and how to treat lupus nephritis, it doesn’t always correlate with what’s actually going on inside the kidney in terms of histology and inflammation, Dr. Fava said.
He pointed to an earlier study in which researchers did kidney biopsies 6 months after patients with lupus nephritis started treatment with mycophenolate. This suggested that around half of patients who showed a clinical response to treatment – defined as proteinuria below 500 mg/day – still had significant histologic disease activity. Another study suggested that this elevated histologic disease activity is associated with a risk of flare, which can result in significant nephron loss. On the flip side, nearly two-thirds of patients in complete histologic remission still had elevated proteinuria.
Unfortunately, it’s not possible or practical to biopsy patients on a regular basis, Dr. Fava said. “So we need better biomarkers, and to do so, we need better knowledge of the pathophysiology because if we have biomarkers that reflect tissue biology in real-time, that may surely guide personalized treatments,” he said at the congress.
Dr. Fava and colleagues enrolled 225 patients with SLE who were undergoing kidney biopsy and 10 healthy controls and used proteomics to quantify the urinary levels of around 1,200 proteins at baseline, 3, 6, and 12 months after initiating treatment.
The team then analyzed these data to look for protein signatures that correlated with histologic phenotypes – particularly the amount of inflammation in the kidney – and clinical features such as response to treatment.
They found several protein biomarkers that appeared to be linked to histologic activity in the kidney, including interleukin (IL)-16, CD163, and neutrophil granule proteins.
Initially, the team looked at baseline levels of these proteins to see if they predicted who responded to treatment, but found no difference between responders and nonresponders.
However, when they looked at levels at 3 months after treatment, a pattern emerged. “We found that in patients who were not responding, there were no changes after 3 months of treatment in the urine proteome,” Dr. Fava said. Among those who did respond to treatment, the levels of these proteins – IL-16, CD163, galectin-1, and CD206 – decreased significantly.
“So the proteins that are linked to renal activity decrease only in responders, suggesting that effective immunosuppression is effective in reducing intrarenal inflammation, which eventually results in low proteinuria at 1 year.”
The decline in these biomarkers persisted at 1 year, and the study suggested it was a better predictor of which patients would respond to treatment at 1 year than proteinuria.
Dr. Fava said in an interview that better biomarkers could revolutionize the treatment and management of lupus nephritis.
“First of all, it can shift the management strategy from treatment to prevention, because at the very beginning we can nip it in the bud maybe with very gentle treatment,” he said. Different panels of urine biomarkers could identify patients at risk of treatment failure, and also help patients to taper off their immunosuppressive therapy without an increased risk of flare. “If we have a way to tell us there’s still inflammation that needs treatment, that could change the way we do it,” he said.
He acknowledged there are significant challenges to developing these biomarkers for clinical use; one is the decision of how to define disease activity without relying on proteinuria as a measure. “Why do I want a biomarker that can predict another biomarker?” he said.
Another presentation during the same session, by Huihua Ding, MD, of Shanghai Jiao Tong University in Shanghai, China, reported on the use of urinary L-selectin to assess renal disease activity and response to treatment in a multiethnic cohort.
This study, involving 474 patients with SLE with or without renal involvement in the United States and China, found levels of urinary L-selectin were elevated only in patients with active lupus nephritis and showed patterns that correlated with renal histologic characteristics.
Clinical rheumatologist Eric F. Morand, MD, PhD, and head of the School of Clinical Sciences at Monash University in Melbourne said one challenge with using urinary biomarkers was that it was not yet clear what these biomarkers reveal about the kidney. “It will be important to see whether this proteomic data actually link to renal outcomes,” Dr. Morand said in an interview. “I think predicting the response to treatment should be based around GFR [glomerular filtration rate] preservation, and I don’t think I’ve seen data yet that the urine biomarkers are going to tell us how to do that better.”
Dr. Morand is optimistic that urine biomarkers will one day be able to achieve that, but he stressed the importance of having urine biomarker tests available in the field at low cost. “You’re going to be doing the tests repeatedly, so therefore, you’re probably going to need to come down to a smaller list of proteins that you measure.”
Dr. Fava reported receiving support from Sanofi and Annexion Bio.
SEOUL, SOUTH KOREA – A panel of urinary biomarkers may do better than measuring proteinuria in predicting which patients with lupus nephritis are going to respond to treatment, according to a presentation at an international congress on systemic lupus erythematosus.
Physician-scientist Andrea Fava, MD, of the division of rheumatology at Johns Hopkins University, Baltimore, presented data from a study using urine proteomics to identify biomarkers present in the urine of patients with lupus nephritis at 3 months after starting treatment that were linked to better outcomes from that treatment at 1 year.
While proteinuria is the standard measure used to guide decisions about whether to do a kidney biopsy and how to treat lupus nephritis, it doesn’t always correlate with what’s actually going on inside the kidney in terms of histology and inflammation, Dr. Fava said.
He pointed to an earlier study in which researchers did kidney biopsies 6 months after patients with lupus nephritis started treatment with mycophenolate. This suggested that around half of patients who showed a clinical response to treatment – defined as proteinuria below 500 mg/day – still had significant histologic disease activity. Another study suggested that this elevated histologic disease activity is associated with a risk of flare, which can result in significant nephron loss. On the flip side, nearly two-thirds of patients in complete histologic remission still had elevated proteinuria.
Unfortunately, it’s not possible or practical to biopsy patients on a regular basis, Dr. Fava said. “So we need better biomarkers, and to do so, we need better knowledge of the pathophysiology because if we have biomarkers that reflect tissue biology in real-time, that may surely guide personalized treatments,” he said at the congress.
Dr. Fava and colleagues enrolled 225 patients with SLE who were undergoing kidney biopsy and 10 healthy controls and used proteomics to quantify the urinary levels of around 1,200 proteins at baseline, 3, 6, and 12 months after initiating treatment.
The team then analyzed these data to look for protein signatures that correlated with histologic phenotypes – particularly the amount of inflammation in the kidney – and clinical features such as response to treatment.
They found several protein biomarkers that appeared to be linked to histologic activity in the kidney, including interleukin (IL)-16, CD163, and neutrophil granule proteins.
Initially, the team looked at baseline levels of these proteins to see if they predicted who responded to treatment, but found no difference between responders and nonresponders.
However, when they looked at levels at 3 months after treatment, a pattern emerged. “We found that in patients who were not responding, there were no changes after 3 months of treatment in the urine proteome,” Dr. Fava said. Among those who did respond to treatment, the levels of these proteins – IL-16, CD163, galectin-1, and CD206 – decreased significantly.
“So the proteins that are linked to renal activity decrease only in responders, suggesting that effective immunosuppression is effective in reducing intrarenal inflammation, which eventually results in low proteinuria at 1 year.”
The decline in these biomarkers persisted at 1 year, and the study suggested it was a better predictor of which patients would respond to treatment at 1 year than proteinuria.
Dr. Fava said in an interview that better biomarkers could revolutionize the treatment and management of lupus nephritis.
“First of all, it can shift the management strategy from treatment to prevention, because at the very beginning we can nip it in the bud maybe with very gentle treatment,” he said. Different panels of urine biomarkers could identify patients at risk of treatment failure, and also help patients to taper off their immunosuppressive therapy without an increased risk of flare. “If we have a way to tell us there’s still inflammation that needs treatment, that could change the way we do it,” he said.
He acknowledged there are significant challenges to developing these biomarkers for clinical use; one is the decision of how to define disease activity without relying on proteinuria as a measure. “Why do I want a biomarker that can predict another biomarker?” he said.
Another presentation during the same session, by Huihua Ding, MD, of Shanghai Jiao Tong University in Shanghai, China, reported on the use of urinary L-selectin to assess renal disease activity and response to treatment in a multiethnic cohort.
This study, involving 474 patients with SLE with or without renal involvement in the United States and China, found levels of urinary L-selectin were elevated only in patients with active lupus nephritis and showed patterns that correlated with renal histologic characteristics.
Clinical rheumatologist Eric F. Morand, MD, PhD, and head of the School of Clinical Sciences at Monash University in Melbourne said one challenge with using urinary biomarkers was that it was not yet clear what these biomarkers reveal about the kidney. “It will be important to see whether this proteomic data actually link to renal outcomes,” Dr. Morand said in an interview. “I think predicting the response to treatment should be based around GFR [glomerular filtration rate] preservation, and I don’t think I’ve seen data yet that the urine biomarkers are going to tell us how to do that better.”
Dr. Morand is optimistic that urine biomarkers will one day be able to achieve that, but he stressed the importance of having urine biomarker tests available in the field at low cost. “You’re going to be doing the tests repeatedly, so therefore, you’re probably going to need to come down to a smaller list of proteins that you measure.”
Dr. Fava reported receiving support from Sanofi and Annexion Bio.
AT LUPUS 2023
FDA fast tracks potential CAR T-cell therapy for lupus
The U.S. Food and Drug Administration has granted Fast Track designation for Cabaletta Bio’s cell therapy CABA-201 for the treatment of systemic lupus erythematosus (SLE) and lupus nephritis (LN), the company announced May 1.
The FDA cleared Cabaletta to begin a phase 1/2 clinical trial of CABA-201, the statement says, which will be the first trial accessing Cabaletta’s Chimeric Antigen Receptor T cells for Autoimmunity (CARTA) approach. CABA-201, a 4-1BB–containing fully human CD19-CAR T-cell investigational therapy, is designed to target and deplete CD19-positive B cells, “enabling an ‘immune system reset’ with durable remission in patients with SLE,” according to the press release. This news organization previously reported on a small study in Germany, published in Nature Medicine, that also used anti-CD19 CAR T cells to treat five patients with SLE.
This upcoming open-label study will enroll two cohorts containing six patients each. One cohort will be patients with SLE and active LN, and the other will be patients with SLE without renal involvement. The therapy is designed as a one-time infusion and will be administered at a dose of 1.0 x 106 cells/kg.
“We believe the FDA’s decision to grant Fast Track Designation for CABA-201 underscores the unmet need for a treatment that has the potential to provide deep and durable responses for people living with lupus and potentially other autoimmune diseases where B cells contribute to disease,” David J. Chang, MD, chief medical officer of Cabaletta, said in the press release.
FDA Fast Track is a process designed to expedite the development and review of drugs and other therapeutics that treat serious conditions and address unmet medical needs. Companies that receive Fast Track designation for a drug have the opportunity for more frequent meetings and written communication with the FDA about the drug’s development plan and design of clinical trials. The fast-tracked drug can also be eligible for accelerated approval and priority review if relevant criteria are met.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted Fast Track designation for Cabaletta Bio’s cell therapy CABA-201 for the treatment of systemic lupus erythematosus (SLE) and lupus nephritis (LN), the company announced May 1.
The FDA cleared Cabaletta to begin a phase 1/2 clinical trial of CABA-201, the statement says, which will be the first trial accessing Cabaletta’s Chimeric Antigen Receptor T cells for Autoimmunity (CARTA) approach. CABA-201, a 4-1BB–containing fully human CD19-CAR T-cell investigational therapy, is designed to target and deplete CD19-positive B cells, “enabling an ‘immune system reset’ with durable remission in patients with SLE,” according to the press release. This news organization previously reported on a small study in Germany, published in Nature Medicine, that also used anti-CD19 CAR T cells to treat five patients with SLE.
This upcoming open-label study will enroll two cohorts containing six patients each. One cohort will be patients with SLE and active LN, and the other will be patients with SLE without renal involvement. The therapy is designed as a one-time infusion and will be administered at a dose of 1.0 x 106 cells/kg.
“We believe the FDA’s decision to grant Fast Track Designation for CABA-201 underscores the unmet need for a treatment that has the potential to provide deep and durable responses for people living with lupus and potentially other autoimmune diseases where B cells contribute to disease,” David J. Chang, MD, chief medical officer of Cabaletta, said in the press release.
FDA Fast Track is a process designed to expedite the development and review of drugs and other therapeutics that treat serious conditions and address unmet medical needs. Companies that receive Fast Track designation for a drug have the opportunity for more frequent meetings and written communication with the FDA about the drug’s development plan and design of clinical trials. The fast-tracked drug can also be eligible for accelerated approval and priority review if relevant criteria are met.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted Fast Track designation for Cabaletta Bio’s cell therapy CABA-201 for the treatment of systemic lupus erythematosus (SLE) and lupus nephritis (LN), the company announced May 1.
The FDA cleared Cabaletta to begin a phase 1/2 clinical trial of CABA-201, the statement says, which will be the first trial accessing Cabaletta’s Chimeric Antigen Receptor T cells for Autoimmunity (CARTA) approach. CABA-201, a 4-1BB–containing fully human CD19-CAR T-cell investigational therapy, is designed to target and deplete CD19-positive B cells, “enabling an ‘immune system reset’ with durable remission in patients with SLE,” according to the press release. This news organization previously reported on a small study in Germany, published in Nature Medicine, that also used anti-CD19 CAR T cells to treat five patients with SLE.
This upcoming open-label study will enroll two cohorts containing six patients each. One cohort will be patients with SLE and active LN, and the other will be patients with SLE without renal involvement. The therapy is designed as a one-time infusion and will be administered at a dose of 1.0 x 106 cells/kg.
“We believe the FDA’s decision to grant Fast Track Designation for CABA-201 underscores the unmet need for a treatment that has the potential to provide deep and durable responses for people living with lupus and potentially other autoimmune diseases where B cells contribute to disease,” David J. Chang, MD, chief medical officer of Cabaletta, said in the press release.
FDA Fast Track is a process designed to expedite the development and review of drugs and other therapeutics that treat serious conditions and address unmet medical needs. Companies that receive Fast Track designation for a drug have the opportunity for more frequent meetings and written communication with the FDA about the drug’s development plan and design of clinical trials. The fast-tracked drug can also be eligible for accelerated approval and priority review if relevant criteria are met.
A version of this article first appeared on Medscape.com.
UTI imaging falls short in some primary care settings
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
AT PAS 2023