User login
Will tirzepatide slow kidney function decline in type 2 diabetes?
The “twincretin” tirzepatide might become part of the “arsenal” against diabetic kidney disease, new research suggests. Notably, the drug significantly reduced the likelihood of macroalbuminuria, in a prespecified subanalysis of the SURPASS-4 clinical trial.
“Once-per-week tirzepatide compared to [daily] insulin glargine treatment resulted in a meaningful improvement in estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (UACR) and the risk of end stage kidney disease (ESKD) – with low risk of clinically relevant hypoglycemia in participants with type 2 diabetes at high cardiovascular risk and varying degrees of chronic kidney disease (CKD),” lead investigator Hiddo J. L. Heerspink, PhD, PharmD, summarized in an email to this news organization.
The U.S. Food and Drug Administration has just approved tirzepatide (Mounjaro, Eli Lilly) – a novel, glucose-dependent insulinotropic polypeptide (GIP) combined with a glucagonlike peptide-1 (GLP-1) receptor agonist – to treat glycemia in patients with type 2 diabetes, based on five pivotal SURPASS trials.
Dr. Heerspink presented the new findings about tirzepatide’s impact on kidney function in an oral session at the annual scientific sessions of the American Diabetes Association.
40% reduced risk of kidney function decline
The main results of SURPASS-4 were published in the Lancet in October 2021, and showed that tirzepatide appeared superior to insulin glargine in lowering hemoglobin A1c in patients with type 2 diabetes at high cardiovascular risk who were inadequately controlled on oral diabetes treatments.
Now, Dr. Heerspink has shown that patients who received tirzepatide as opposed to insulin glargine were significantly less likely to have kidney function decline that included new-onset macroalbuminuria (hazard ratio, 0.59; P < .05).
“These are very large benefits and clearly indicate the potential of tirzepatide to be a very strong kidney protective drug,” said Dr. Heerspink, from the department of clinical pharmacy and pharmacology, University Medical Center Groningen (the Netherlands).
“Based on results from the SURPASS-4 trial, tirzepatide has significant kidney-protective effects in adults with type 2 diabetes with high cardiovascular risk and largely normal kidney function,” Christine Limonte, MD, chair of the session in which the analysis was presented, agreed, in an email to this news organization.
The approximate 40% reduced risk of kidney function decline in this population “is important because it suggests that this novel agent may contribute to the growing arsenal for preventing and treating diabetic kidney disease,” added Dr. Limonte, a clinical research fellow in the division of nephrology, University of Washington, Seattle.
“Over the last several years,” she noted, “sodium glucose cotransporter-2 [SGLT2] inhibitors and GLP-1 receptor agonists have been identified as having significant kidney-protective effects in type 2 diabetes, and as such are becoming first-line agents in the treatment of diabetic kidney disease.”
Additional studies are needed, she added, to assess the impacts of tirzepatide compared to these agents (particularly GLP-1 receptor agonists, which overlap in their mechanism of action).
“With the growing number of therapeutic options for diabetic kidney disease, future research should also focus on identifying combinations of agents which benefit individuals in a ‘targeted’ manner,” according to Dr. Limonte.
“Ensuring accessibility to kidney-protective agents by promoting access to health care and reducing drug costs is essential to improving outcomes in diabetic kidney disease,” she added.
Strongest reduction seen in risk of new macroalbuminuria
One in three adults with diabetes has CKD, according to a press release issued by the ADA. Therefore, there is a need for therapies to reduce the development and progression of CKD in patients with type 2 diabetes.
The prespecified analysis of SUPRESS-4 investigated potential renoprotective effects of tirzepatide.
The trial enrolled 1,995 patients with type 2 diabetes who were at increased risk of cardiovascular disease. The patients had a mean age of 63.6 years and a mean hemoglobin A1c of 8.5%.
Most patients had normal kidney function. The mean eGFR based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was 81.3 mL/min per 1.73 m2.
Few patients (17%) had moderately or severely reduced kidney function (eGFR <60 mL/min per 1.73 m2). Around a quarter of the patients (28%) had microalbuminuria (UACR 30-300 mg/g) and 8% had macroalbuminuria (UACR >300 mg/g).
The patients were randomized to receive a weekly injection of 5, 10, or 15 mg tirzepatide or a daily individualized injection of insulin glargine starting at 10 IU/day at bedtime, titrated to a fasting blood glucose <100 mg/dL, in addition to existing oral glucose-lowering agents. The primary outcomes in the subanalysis were:
- Endpoint 1: a composite of ≥40% decline in eGFR from baseline, renal death, progression to ESKD, and new-onset macroalbuminuria.
- Endpoint 2: the same as endpoint 1 excluding new-onset macroalbuminuria.
During a median follow up of 85 weeks and up to 104 weeks, patients who received tirzepatide versus insulin glargine were significantly less likely to reach endpoint 1 but not endpoint 2.
In addition, tirzepatide “very strongly” reduced the risk of new-onset macroalbuminuria, compared to insulin glargine, by approximately 60% in the complete study cohort (hazard ratio, 0.41; P < .05), Dr. Limonte noted.
Tirzepatide also reduced the risk of a >40% decline in eGFR, but this effect was not statistically significant, possibly because this outcome was underpowered. There were also too few kidney deaths and progressions to ESKD to meaningfully assess the effects of tirzepatide on these outcomes.
Therefore, Dr. Limonte noted, “it is likely that tirzepatide’s significant benefit on composite endpoint 1 was largely driven by this agent’s impact on reducing macroalbuminuria onset [explaining why a significant benefit was not seen with composite endpoint 2, which excluded new-onset macroalbuminuria].”
The study was funded by Eli Lilly. Dr. Heerspink disclosed that he is a consultant for AstraZeneca, Bayer AG, Boehringer Ingelheim, Chinook Therapeutics, CSL Behring, Gilead Sciences, Goldfinch Bio, Janssen Research & Development, Mitsubishi Tanabe Pharma, Mundipharma, and Traveere Pharmaceuticals, and has received research support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk.
Dr. Limonte disclosed that she receives funds from the American Kidney Fund’s Clinical Scientist in Nephrology Award.
A version of this article first appeared on Medscape.com.
The “twincretin” tirzepatide might become part of the “arsenal” against diabetic kidney disease, new research suggests. Notably, the drug significantly reduced the likelihood of macroalbuminuria, in a prespecified subanalysis of the SURPASS-4 clinical trial.
“Once-per-week tirzepatide compared to [daily] insulin glargine treatment resulted in a meaningful improvement in estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (UACR) and the risk of end stage kidney disease (ESKD) – with low risk of clinically relevant hypoglycemia in participants with type 2 diabetes at high cardiovascular risk and varying degrees of chronic kidney disease (CKD),” lead investigator Hiddo J. L. Heerspink, PhD, PharmD, summarized in an email to this news organization.
The U.S. Food and Drug Administration has just approved tirzepatide (Mounjaro, Eli Lilly) – a novel, glucose-dependent insulinotropic polypeptide (GIP) combined with a glucagonlike peptide-1 (GLP-1) receptor agonist – to treat glycemia in patients with type 2 diabetes, based on five pivotal SURPASS trials.
Dr. Heerspink presented the new findings about tirzepatide’s impact on kidney function in an oral session at the annual scientific sessions of the American Diabetes Association.
40% reduced risk of kidney function decline
The main results of SURPASS-4 were published in the Lancet in October 2021, and showed that tirzepatide appeared superior to insulin glargine in lowering hemoglobin A1c in patients with type 2 diabetes at high cardiovascular risk who were inadequately controlled on oral diabetes treatments.
Now, Dr. Heerspink has shown that patients who received tirzepatide as opposed to insulin glargine were significantly less likely to have kidney function decline that included new-onset macroalbuminuria (hazard ratio, 0.59; P < .05).
“These are very large benefits and clearly indicate the potential of tirzepatide to be a very strong kidney protective drug,” said Dr. Heerspink, from the department of clinical pharmacy and pharmacology, University Medical Center Groningen (the Netherlands).
“Based on results from the SURPASS-4 trial, tirzepatide has significant kidney-protective effects in adults with type 2 diabetes with high cardiovascular risk and largely normal kidney function,” Christine Limonte, MD, chair of the session in which the analysis was presented, agreed, in an email to this news organization.
The approximate 40% reduced risk of kidney function decline in this population “is important because it suggests that this novel agent may contribute to the growing arsenal for preventing and treating diabetic kidney disease,” added Dr. Limonte, a clinical research fellow in the division of nephrology, University of Washington, Seattle.
“Over the last several years,” she noted, “sodium glucose cotransporter-2 [SGLT2] inhibitors and GLP-1 receptor agonists have been identified as having significant kidney-protective effects in type 2 diabetes, and as such are becoming first-line agents in the treatment of diabetic kidney disease.”
Additional studies are needed, she added, to assess the impacts of tirzepatide compared to these agents (particularly GLP-1 receptor agonists, which overlap in their mechanism of action).
“With the growing number of therapeutic options for diabetic kidney disease, future research should also focus on identifying combinations of agents which benefit individuals in a ‘targeted’ manner,” according to Dr. Limonte.
“Ensuring accessibility to kidney-protective agents by promoting access to health care and reducing drug costs is essential to improving outcomes in diabetic kidney disease,” she added.
Strongest reduction seen in risk of new macroalbuminuria
One in three adults with diabetes has CKD, according to a press release issued by the ADA. Therefore, there is a need for therapies to reduce the development and progression of CKD in patients with type 2 diabetes.
The prespecified analysis of SUPRESS-4 investigated potential renoprotective effects of tirzepatide.
The trial enrolled 1,995 patients with type 2 diabetes who were at increased risk of cardiovascular disease. The patients had a mean age of 63.6 years and a mean hemoglobin A1c of 8.5%.
Most patients had normal kidney function. The mean eGFR based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was 81.3 mL/min per 1.73 m2.
Few patients (17%) had moderately or severely reduced kidney function (eGFR <60 mL/min per 1.73 m2). Around a quarter of the patients (28%) had microalbuminuria (UACR 30-300 mg/g) and 8% had macroalbuminuria (UACR >300 mg/g).
The patients were randomized to receive a weekly injection of 5, 10, or 15 mg tirzepatide or a daily individualized injection of insulin glargine starting at 10 IU/day at bedtime, titrated to a fasting blood glucose <100 mg/dL, in addition to existing oral glucose-lowering agents. The primary outcomes in the subanalysis were:
- Endpoint 1: a composite of ≥40% decline in eGFR from baseline, renal death, progression to ESKD, and new-onset macroalbuminuria.
- Endpoint 2: the same as endpoint 1 excluding new-onset macroalbuminuria.
During a median follow up of 85 weeks and up to 104 weeks, patients who received tirzepatide versus insulin glargine were significantly less likely to reach endpoint 1 but not endpoint 2.
In addition, tirzepatide “very strongly” reduced the risk of new-onset macroalbuminuria, compared to insulin glargine, by approximately 60% in the complete study cohort (hazard ratio, 0.41; P < .05), Dr. Limonte noted.
Tirzepatide also reduced the risk of a >40% decline in eGFR, but this effect was not statistically significant, possibly because this outcome was underpowered. There were also too few kidney deaths and progressions to ESKD to meaningfully assess the effects of tirzepatide on these outcomes.
Therefore, Dr. Limonte noted, “it is likely that tirzepatide’s significant benefit on composite endpoint 1 was largely driven by this agent’s impact on reducing macroalbuminuria onset [explaining why a significant benefit was not seen with composite endpoint 2, which excluded new-onset macroalbuminuria].”
The study was funded by Eli Lilly. Dr. Heerspink disclosed that he is a consultant for AstraZeneca, Bayer AG, Boehringer Ingelheim, Chinook Therapeutics, CSL Behring, Gilead Sciences, Goldfinch Bio, Janssen Research & Development, Mitsubishi Tanabe Pharma, Mundipharma, and Traveere Pharmaceuticals, and has received research support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk.
Dr. Limonte disclosed that she receives funds from the American Kidney Fund’s Clinical Scientist in Nephrology Award.
A version of this article first appeared on Medscape.com.
The “twincretin” tirzepatide might become part of the “arsenal” against diabetic kidney disease, new research suggests. Notably, the drug significantly reduced the likelihood of macroalbuminuria, in a prespecified subanalysis of the SURPASS-4 clinical trial.
“Once-per-week tirzepatide compared to [daily] insulin glargine treatment resulted in a meaningful improvement in estimated glomerular filtration rate (eGFR) decline and reduced urine albumin-to-creatinine ratio (UACR) and the risk of end stage kidney disease (ESKD) – with low risk of clinically relevant hypoglycemia in participants with type 2 diabetes at high cardiovascular risk and varying degrees of chronic kidney disease (CKD),” lead investigator Hiddo J. L. Heerspink, PhD, PharmD, summarized in an email to this news organization.
The U.S. Food and Drug Administration has just approved tirzepatide (Mounjaro, Eli Lilly) – a novel, glucose-dependent insulinotropic polypeptide (GIP) combined with a glucagonlike peptide-1 (GLP-1) receptor agonist – to treat glycemia in patients with type 2 diabetes, based on five pivotal SURPASS trials.
Dr. Heerspink presented the new findings about tirzepatide’s impact on kidney function in an oral session at the annual scientific sessions of the American Diabetes Association.
40% reduced risk of kidney function decline
The main results of SURPASS-4 were published in the Lancet in October 2021, and showed that tirzepatide appeared superior to insulin glargine in lowering hemoglobin A1c in patients with type 2 diabetes at high cardiovascular risk who were inadequately controlled on oral diabetes treatments.
Now, Dr. Heerspink has shown that patients who received tirzepatide as opposed to insulin glargine were significantly less likely to have kidney function decline that included new-onset macroalbuminuria (hazard ratio, 0.59; P < .05).
“These are very large benefits and clearly indicate the potential of tirzepatide to be a very strong kidney protective drug,” said Dr. Heerspink, from the department of clinical pharmacy and pharmacology, University Medical Center Groningen (the Netherlands).
“Based on results from the SURPASS-4 trial, tirzepatide has significant kidney-protective effects in adults with type 2 diabetes with high cardiovascular risk and largely normal kidney function,” Christine Limonte, MD, chair of the session in which the analysis was presented, agreed, in an email to this news organization.
The approximate 40% reduced risk of kidney function decline in this population “is important because it suggests that this novel agent may contribute to the growing arsenal for preventing and treating diabetic kidney disease,” added Dr. Limonte, a clinical research fellow in the division of nephrology, University of Washington, Seattle.
“Over the last several years,” she noted, “sodium glucose cotransporter-2 [SGLT2] inhibitors and GLP-1 receptor agonists have been identified as having significant kidney-protective effects in type 2 diabetes, and as such are becoming first-line agents in the treatment of diabetic kidney disease.”
Additional studies are needed, she added, to assess the impacts of tirzepatide compared to these agents (particularly GLP-1 receptor agonists, which overlap in their mechanism of action).
“With the growing number of therapeutic options for diabetic kidney disease, future research should also focus on identifying combinations of agents which benefit individuals in a ‘targeted’ manner,” according to Dr. Limonte.
“Ensuring accessibility to kidney-protective agents by promoting access to health care and reducing drug costs is essential to improving outcomes in diabetic kidney disease,” she added.
Strongest reduction seen in risk of new macroalbuminuria
One in three adults with diabetes has CKD, according to a press release issued by the ADA. Therefore, there is a need for therapies to reduce the development and progression of CKD in patients with type 2 diabetes.
The prespecified analysis of SUPRESS-4 investigated potential renoprotective effects of tirzepatide.
The trial enrolled 1,995 patients with type 2 diabetes who were at increased risk of cardiovascular disease. The patients had a mean age of 63.6 years and a mean hemoglobin A1c of 8.5%.
Most patients had normal kidney function. The mean eGFR based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was 81.3 mL/min per 1.73 m2.
Few patients (17%) had moderately or severely reduced kidney function (eGFR <60 mL/min per 1.73 m2). Around a quarter of the patients (28%) had microalbuminuria (UACR 30-300 mg/g) and 8% had macroalbuminuria (UACR >300 mg/g).
The patients were randomized to receive a weekly injection of 5, 10, or 15 mg tirzepatide or a daily individualized injection of insulin glargine starting at 10 IU/day at bedtime, titrated to a fasting blood glucose <100 mg/dL, in addition to existing oral glucose-lowering agents. The primary outcomes in the subanalysis were:
- Endpoint 1: a composite of ≥40% decline in eGFR from baseline, renal death, progression to ESKD, and new-onset macroalbuminuria.
- Endpoint 2: the same as endpoint 1 excluding new-onset macroalbuminuria.
During a median follow up of 85 weeks and up to 104 weeks, patients who received tirzepatide versus insulin glargine were significantly less likely to reach endpoint 1 but not endpoint 2.
In addition, tirzepatide “very strongly” reduced the risk of new-onset macroalbuminuria, compared to insulin glargine, by approximately 60% in the complete study cohort (hazard ratio, 0.41; P < .05), Dr. Limonte noted.
Tirzepatide also reduced the risk of a >40% decline in eGFR, but this effect was not statistically significant, possibly because this outcome was underpowered. There were also too few kidney deaths and progressions to ESKD to meaningfully assess the effects of tirzepatide on these outcomes.
Therefore, Dr. Limonte noted, “it is likely that tirzepatide’s significant benefit on composite endpoint 1 was largely driven by this agent’s impact on reducing macroalbuminuria onset [explaining why a significant benefit was not seen with composite endpoint 2, which excluded new-onset macroalbuminuria].”
The study was funded by Eli Lilly. Dr. Heerspink disclosed that he is a consultant for AstraZeneca, Bayer AG, Boehringer Ingelheim, Chinook Therapeutics, CSL Behring, Gilead Sciences, Goldfinch Bio, Janssen Research & Development, Mitsubishi Tanabe Pharma, Mundipharma, and Traveere Pharmaceuticals, and has received research support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk.
Dr. Limonte disclosed that she receives funds from the American Kidney Fund’s Clinical Scientist in Nephrology Award.
A version of this article first appeared on Medscape.com.
FROM ADA 2022
AUA 2022: A report from the trenches
The annual meeting of the American Urological Association took place recently at the Ernest N. Morial Convention Center in New Orleans. Hundreds of talks and abstracts were presented over the 4 days in New Orleans; below is a summary of what I found to be the key scientific highlights.
1. Updates to the AUA’s guidelines for management of localized kidney cancer
The AUA’s recommendations for the treatment of localized kidney cancer have changed dramatically over the past few decades. Gone are the days of simply removing the entire kidney every time a mass is found. Today, a partial nephrectomy is preferred in most situations.
Our understanding that the prevalence of familial kidney cancer is much higher than previously thought has led to a change in the guidelines regarding which patients should receive genetic counseling. For the first time, the guidelines include the use of adjuvant medical treatment, such as pembrolizumab. A 2021 study in the New England Journal of Medicine showed a survival benefit for patients with high-risk disease who receive such therapies, so it›s not surprising that such treatments are now recommended.
The development of new second- and third-generation gadolinium contrast agents that spare the kidneys has dramatically increased the role for MRIs for patients with severe or even end-stage renal disease. As a result, the guidelines were updated to recommend the use of these agents. The role of a renal biopsy, which has always been limited, given the ability of cross-sectional imaging to diagnosis this disease, has further been constrained and should now be performed only when the results would clearly change a clinical decision, such as whether or not the lesion in question is a metastasis.
2. New and better ureteroscope technology
No one likes kidney stones, not the patient who deals with the incredible pain, nor the surgeon who has to remove them, given that these cases often present in the wee hours of the morning. The preferred surgical approach has changed dramatically over the past decade, moving away from extracorporeal shockwave lithotripsy toward flexible ureteroscope-based technology, which has a higher clearance rate and is more widely and more immediately available. Flexible ureteroscopy has been held back by technological barriers, including limited scope deflection and low laser power. The exceptionally high cost of repair and the tendency of the instruments to break haven’t helped, either. Although single-use ureteroscopes have been available for some time, it wasn’t until the recently introduced second-generation scopes became widely available that they have become popular. These new scopes have small external diameters, great optics, and can easily be used. Newer high-powered lasers and the change from holmium:YAG-based lasers to thulium technology is greatly increasing the size of stones that can be safely addressed ureteroscopically. The cost analysis of single-use technology versus reusable scopes tends to be site dependent but can be appealing in certain situations. Also, on the technology forefront, a new robotically assisted ureteroscope is being introduced that offers the chance for improved intrapelvic mobility and better ergonomics for the surgeon.
3. New options for the treatment of clinically localized prostate cancer
Since the guidelines were last updated in 2017, the definitive management of localized prostate cancer has changed dramatically. Although radical prostatectomy and radiotherapy remain the preferred options for men who choose treatment for their disease, the updated guidelines state that active surveillance is now the preferred approach for men with low-risk cancers.
Although the preferred surveillance protocol is still being debated, the consensus is that almost all men with low-risk disease can be safely monitored for some period. The imaging technology available to monitor patients is also radically changing with the rollout of prostate-specific membrane antigen–based PET technology. The increased sensitivity and specificity of this modality opens the door not only for better up-front staging of newly diagnosed patients with prostate cancer but also may allow clinicians to earlier identify and treat men with metastatic disease. The guidelines for the first time address the use of genetic markers to individualize treatment of men with advanced or metastatic prostate cancer. Exactly which treatments these patients need is still being debated, but the ability to use patient-specific genetic mutation information to customize treatment is potentially groundbreaking.
4. New treatment options for patients with high-grade non–muscle-invasive bladder cancer (NMIBC) refractory to bacille Calmette-Guérin (BCG) therapy
Patients with NMIBC who do not respond to BCG therapy are in a tough position. Cystectomy remains the preferred option as a second-line strategy, but the procedure has a complication rate approaching 30%. Further, many patients are not willing to have their bladder removed because of the life-altering changes that go along with having an urostomy or a neobladder. While intravesical treatments such as valrubicin, docetaxel, or gemcitabine have been available for many years, the success rates of those options are limited. The Food and Drug Administration recently approved the use of the immunotherapy-based treatment pembrolizumab. While none of these options is perfect, the fact that we now have at least some alternatives is a huge step in the right direction.
5. It’s all about the patient: Involving patients in designing the health care delivery system
Although it seems like an obvious concept, patients themselves have traditionally not been involved in designing the health care delivery system on which they rely. Research presented at the AUA shows that many health care outcomes improve when patients are actively involved in the process. For example, Angela Smith, MD, of the University of North Carolina at Chapel Hill, presented a study showing that including patients in the identification of possible research topics helps them feel engaged and more likely to participate in studies. Patients who are involved in advisory councils at the local hospital level are more likely to report having received high-quality care. And surveying patients on the goals of national health care policy helps them feel that the outcomes are more equitable.
As a small-town urologist who spends his days in the trenches of urology, I think the next time my group considers participating in new cancer research, I may talk to the local cancer support group first. If Dr. Smith’s data are correct, not only would our patients be better served, but we would also have an easier time filling the trial!
The 2023 AUA conference is going to be held in Chicago next spring. I hope to see you there!
A version of this article first appeared on Medscape.com.
The annual meeting of the American Urological Association took place recently at the Ernest N. Morial Convention Center in New Orleans. Hundreds of talks and abstracts were presented over the 4 days in New Orleans; below is a summary of what I found to be the key scientific highlights.
1. Updates to the AUA’s guidelines for management of localized kidney cancer
The AUA’s recommendations for the treatment of localized kidney cancer have changed dramatically over the past few decades. Gone are the days of simply removing the entire kidney every time a mass is found. Today, a partial nephrectomy is preferred in most situations.
Our understanding that the prevalence of familial kidney cancer is much higher than previously thought has led to a change in the guidelines regarding which patients should receive genetic counseling. For the first time, the guidelines include the use of adjuvant medical treatment, such as pembrolizumab. A 2021 study in the New England Journal of Medicine showed a survival benefit for patients with high-risk disease who receive such therapies, so it›s not surprising that such treatments are now recommended.
The development of new second- and third-generation gadolinium contrast agents that spare the kidneys has dramatically increased the role for MRIs for patients with severe or even end-stage renal disease. As a result, the guidelines were updated to recommend the use of these agents. The role of a renal biopsy, which has always been limited, given the ability of cross-sectional imaging to diagnosis this disease, has further been constrained and should now be performed only when the results would clearly change a clinical decision, such as whether or not the lesion in question is a metastasis.
2. New and better ureteroscope technology
No one likes kidney stones, not the patient who deals with the incredible pain, nor the surgeon who has to remove them, given that these cases often present in the wee hours of the morning. The preferred surgical approach has changed dramatically over the past decade, moving away from extracorporeal shockwave lithotripsy toward flexible ureteroscope-based technology, which has a higher clearance rate and is more widely and more immediately available. Flexible ureteroscopy has been held back by technological barriers, including limited scope deflection and low laser power. The exceptionally high cost of repair and the tendency of the instruments to break haven’t helped, either. Although single-use ureteroscopes have been available for some time, it wasn’t until the recently introduced second-generation scopes became widely available that they have become popular. These new scopes have small external diameters, great optics, and can easily be used. Newer high-powered lasers and the change from holmium:YAG-based lasers to thulium technology is greatly increasing the size of stones that can be safely addressed ureteroscopically. The cost analysis of single-use technology versus reusable scopes tends to be site dependent but can be appealing in certain situations. Also, on the technology forefront, a new robotically assisted ureteroscope is being introduced that offers the chance for improved intrapelvic mobility and better ergonomics for the surgeon.
3. New options for the treatment of clinically localized prostate cancer
Since the guidelines were last updated in 2017, the definitive management of localized prostate cancer has changed dramatically. Although radical prostatectomy and radiotherapy remain the preferred options for men who choose treatment for their disease, the updated guidelines state that active surveillance is now the preferred approach for men with low-risk cancers.
Although the preferred surveillance protocol is still being debated, the consensus is that almost all men with low-risk disease can be safely monitored for some period. The imaging technology available to monitor patients is also radically changing with the rollout of prostate-specific membrane antigen–based PET technology. The increased sensitivity and specificity of this modality opens the door not only for better up-front staging of newly diagnosed patients with prostate cancer but also may allow clinicians to earlier identify and treat men with metastatic disease. The guidelines for the first time address the use of genetic markers to individualize treatment of men with advanced or metastatic prostate cancer. Exactly which treatments these patients need is still being debated, but the ability to use patient-specific genetic mutation information to customize treatment is potentially groundbreaking.
4. New treatment options for patients with high-grade non–muscle-invasive bladder cancer (NMIBC) refractory to bacille Calmette-Guérin (BCG) therapy
Patients with NMIBC who do not respond to BCG therapy are in a tough position. Cystectomy remains the preferred option as a second-line strategy, but the procedure has a complication rate approaching 30%. Further, many patients are not willing to have their bladder removed because of the life-altering changes that go along with having an urostomy or a neobladder. While intravesical treatments such as valrubicin, docetaxel, or gemcitabine have been available for many years, the success rates of those options are limited. The Food and Drug Administration recently approved the use of the immunotherapy-based treatment pembrolizumab. While none of these options is perfect, the fact that we now have at least some alternatives is a huge step in the right direction.
5. It’s all about the patient: Involving patients in designing the health care delivery system
Although it seems like an obvious concept, patients themselves have traditionally not been involved in designing the health care delivery system on which they rely. Research presented at the AUA shows that many health care outcomes improve when patients are actively involved in the process. For example, Angela Smith, MD, of the University of North Carolina at Chapel Hill, presented a study showing that including patients in the identification of possible research topics helps them feel engaged and more likely to participate in studies. Patients who are involved in advisory councils at the local hospital level are more likely to report having received high-quality care. And surveying patients on the goals of national health care policy helps them feel that the outcomes are more equitable.
As a small-town urologist who spends his days in the trenches of urology, I think the next time my group considers participating in new cancer research, I may talk to the local cancer support group first. If Dr. Smith’s data are correct, not only would our patients be better served, but we would also have an easier time filling the trial!
The 2023 AUA conference is going to be held in Chicago next spring. I hope to see you there!
A version of this article first appeared on Medscape.com.
The annual meeting of the American Urological Association took place recently at the Ernest N. Morial Convention Center in New Orleans. Hundreds of talks and abstracts were presented over the 4 days in New Orleans; below is a summary of what I found to be the key scientific highlights.
1. Updates to the AUA’s guidelines for management of localized kidney cancer
The AUA’s recommendations for the treatment of localized kidney cancer have changed dramatically over the past few decades. Gone are the days of simply removing the entire kidney every time a mass is found. Today, a partial nephrectomy is preferred in most situations.
Our understanding that the prevalence of familial kidney cancer is much higher than previously thought has led to a change in the guidelines regarding which patients should receive genetic counseling. For the first time, the guidelines include the use of adjuvant medical treatment, such as pembrolizumab. A 2021 study in the New England Journal of Medicine showed a survival benefit for patients with high-risk disease who receive such therapies, so it›s not surprising that such treatments are now recommended.
The development of new second- and third-generation gadolinium contrast agents that spare the kidneys has dramatically increased the role for MRIs for patients with severe or even end-stage renal disease. As a result, the guidelines were updated to recommend the use of these agents. The role of a renal biopsy, which has always been limited, given the ability of cross-sectional imaging to diagnosis this disease, has further been constrained and should now be performed only when the results would clearly change a clinical decision, such as whether or not the lesion in question is a metastasis.
2. New and better ureteroscope technology
No one likes kidney stones, not the patient who deals with the incredible pain, nor the surgeon who has to remove them, given that these cases often present in the wee hours of the morning. The preferred surgical approach has changed dramatically over the past decade, moving away from extracorporeal shockwave lithotripsy toward flexible ureteroscope-based technology, which has a higher clearance rate and is more widely and more immediately available. Flexible ureteroscopy has been held back by technological barriers, including limited scope deflection and low laser power. The exceptionally high cost of repair and the tendency of the instruments to break haven’t helped, either. Although single-use ureteroscopes have been available for some time, it wasn’t until the recently introduced second-generation scopes became widely available that they have become popular. These new scopes have small external diameters, great optics, and can easily be used. Newer high-powered lasers and the change from holmium:YAG-based lasers to thulium technology is greatly increasing the size of stones that can be safely addressed ureteroscopically. The cost analysis of single-use technology versus reusable scopes tends to be site dependent but can be appealing in certain situations. Also, on the technology forefront, a new robotically assisted ureteroscope is being introduced that offers the chance for improved intrapelvic mobility and better ergonomics for the surgeon.
3. New options for the treatment of clinically localized prostate cancer
Since the guidelines were last updated in 2017, the definitive management of localized prostate cancer has changed dramatically. Although radical prostatectomy and radiotherapy remain the preferred options for men who choose treatment for their disease, the updated guidelines state that active surveillance is now the preferred approach for men with low-risk cancers.
Although the preferred surveillance protocol is still being debated, the consensus is that almost all men with low-risk disease can be safely monitored for some period. The imaging technology available to monitor patients is also radically changing with the rollout of prostate-specific membrane antigen–based PET technology. The increased sensitivity and specificity of this modality opens the door not only for better up-front staging of newly diagnosed patients with prostate cancer but also may allow clinicians to earlier identify and treat men with metastatic disease. The guidelines for the first time address the use of genetic markers to individualize treatment of men with advanced or metastatic prostate cancer. Exactly which treatments these patients need is still being debated, but the ability to use patient-specific genetic mutation information to customize treatment is potentially groundbreaking.
4. New treatment options for patients with high-grade non–muscle-invasive bladder cancer (NMIBC) refractory to bacille Calmette-Guérin (BCG) therapy
Patients with NMIBC who do not respond to BCG therapy are in a tough position. Cystectomy remains the preferred option as a second-line strategy, but the procedure has a complication rate approaching 30%. Further, many patients are not willing to have their bladder removed because of the life-altering changes that go along with having an urostomy or a neobladder. While intravesical treatments such as valrubicin, docetaxel, or gemcitabine have been available for many years, the success rates of those options are limited. The Food and Drug Administration recently approved the use of the immunotherapy-based treatment pembrolizumab. While none of these options is perfect, the fact that we now have at least some alternatives is a huge step in the right direction.
5. It’s all about the patient: Involving patients in designing the health care delivery system
Although it seems like an obvious concept, patients themselves have traditionally not been involved in designing the health care delivery system on which they rely. Research presented at the AUA shows that many health care outcomes improve when patients are actively involved in the process. For example, Angela Smith, MD, of the University of North Carolina at Chapel Hill, presented a study showing that including patients in the identification of possible research topics helps them feel engaged and more likely to participate in studies. Patients who are involved in advisory councils at the local hospital level are more likely to report having received high-quality care. And surveying patients on the goals of national health care policy helps them feel that the outcomes are more equitable.
As a small-town urologist who spends his days in the trenches of urology, I think the next time my group considers participating in new cancer research, I may talk to the local cancer support group first. If Dr. Smith’s data are correct, not only would our patients be better served, but we would also have an easier time filling the trial!
The 2023 AUA conference is going to be held in Chicago next spring. I hope to see you there!
A version of this article first appeared on Medscape.com.
FROM AUA 2022
Mixing BP meds with NSAID may be ‘triple whammy’ for kidneys
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
The study also looked at risk factors associated with the effect of triple therapy with these agents, which has been called “triple whammy” AKI.
“It’s not that everyone who happens to take this combination of drugs is going to have problems,” Anita Layton, PhD, University of Waterloo, Ontario, said in a statement. “But the research shows it’s enough of a problem that you should exercise caution.”
The study was published online in Mathematical Biosciences.
In an earlier study, triple therapy with a diuretic, RAS inhibitor, and NSAID was associated with a 31% increased risk for AKI, relative to diuretic and RAS inhibitor therapy only.
However, the factors that predispose some patients to develop “triple whammy” AKI are unclear.
To better understand the mechanism by which triple therapy increases risk for AKI, Dr. Layton and colleagues used computational models to gauge interactions between concurrent use of a diuretic, a RAS inhibitor, and an NSAID.
They identified dehydration and high sensitivity to drug treatment as key contributing factors to the development of triple whammy AKI.
Their model simulations suggested that low water intake, the myogenic response (that is, the reflex response of arteries and arterioles to changes in blood pressure to maintain consistent blood flow), and drug sensitivity “may predispose patients with hypertension to develop triple whammy-induced AKI,” they wrote.
“We hypothesize that individuals with an impaired myogenic response may be particularly susceptible to triple whammy AKI. Additionally, increased drug sensitivity or low water intake can predispose patients to triple whammy AKI,” they added.
In the absence of additional risk factors, there was no indication of an elevated risk for AKI when an angiotensin-converting enzyme (ACE) inhibitor and NSAID are combined, the study team said.
In contrast, when an ACE inhibitor, diuretic, and NSAID are combined, critical blood pressure and glomerular filtration rate (GFR) regulatory mechanisms are simultaneously interrupted, they reported.
“Perhaps not unexpectedly, model simulations indicate that triple treatment reduces GFR more than single or double treatments in all individuals. However, under triple treatment, urine volume and GFR have not been predicted to fall sufficiently far to indicate AKI,” they wrote. “This result is consistent with the fact that only a fraction of individuals develop AKI following triple treatment.”
They expect, therefore, that hypertensive patients who are otherwise healthy will be able to withstand triple treatment, in the absence of these aggravating factors, the researchers concluded.
Nonetheless, it’s wise to “always be careful when mixing medications,” Dr. Layton told this news organization.
She noted that “triple whammy AKI is known among kidney researchers and nephrologists. To what extent nonspecialists are aware, it isn’t clear.
“More importantly,” Dr. Layton said, “NSAIDs can be obtained over the counter, and triple whammy AKI isn’t common knowledge outside of the medical community.”
This research was supported by the Canada 150 Research Chair program and by the Natural Sciences and Engineering Research Council of Canada. The authors have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM MATHEMATICAL BIOSCIENCES
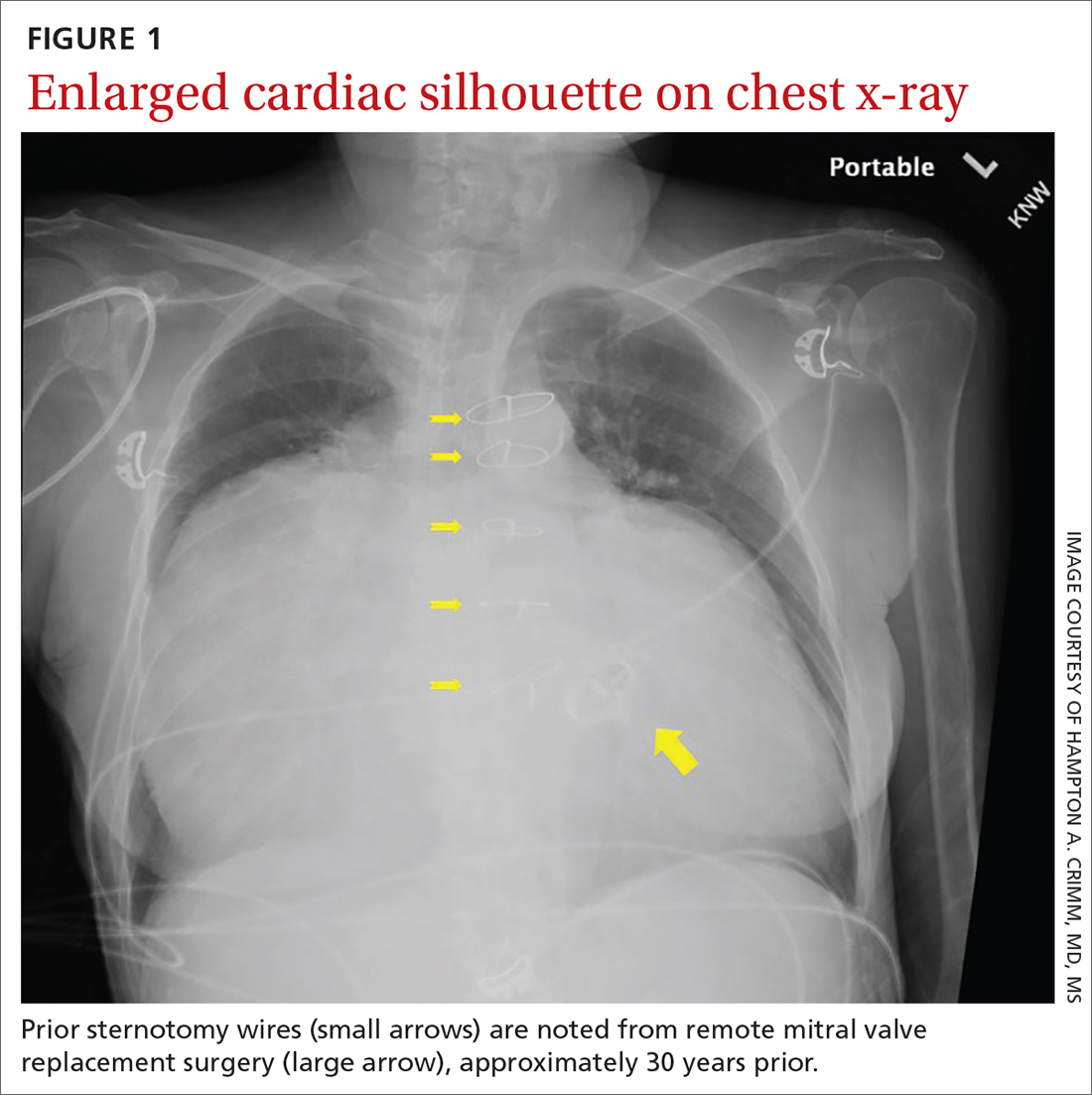
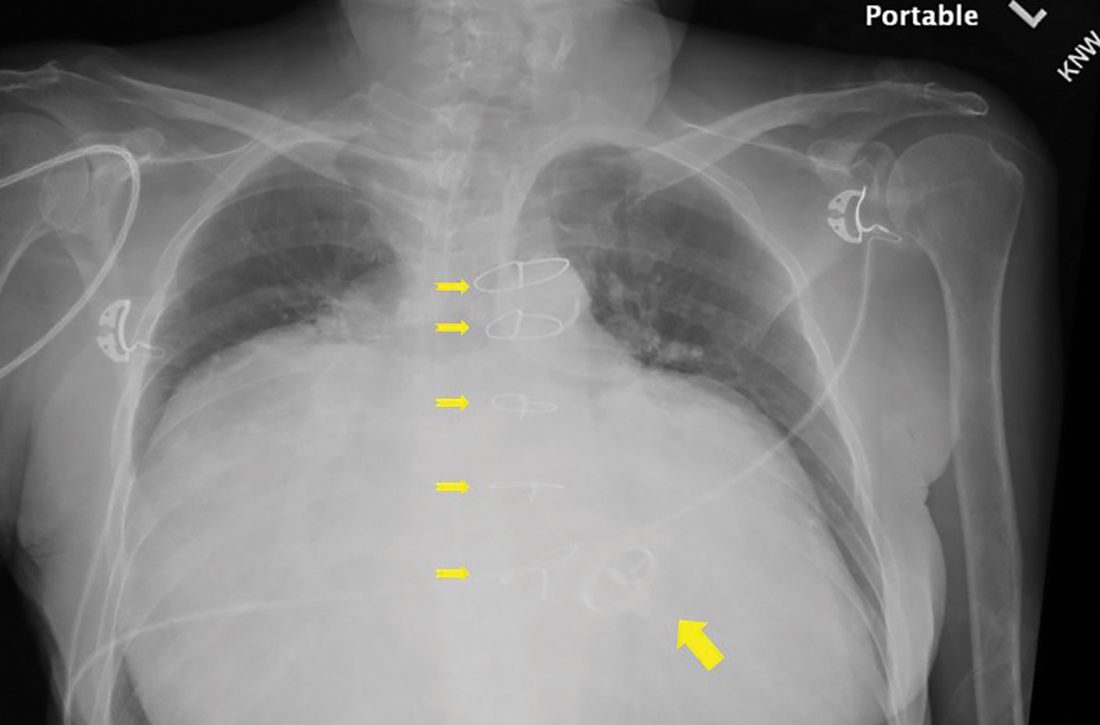
Substantially enlarged cardiac silhouette
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
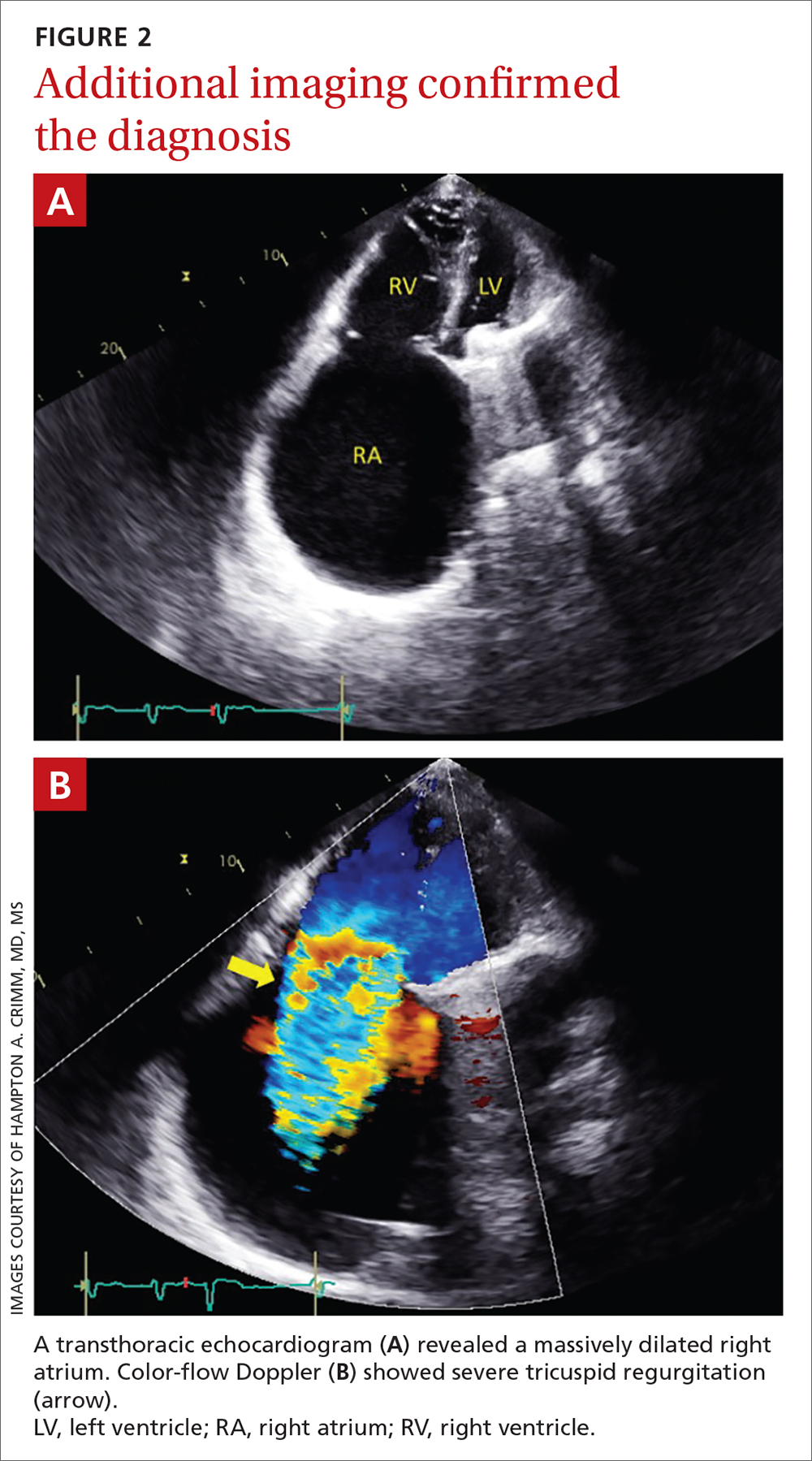
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.
RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.
RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.
RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
Lowering BP according to newest guidance would cut CV events
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Is bicarbonate therapy effective in preventing CKD progression?
Evidence summary
Bicarbonate therapy demonstrates benefit in 2 meta-analyses
Two recent meta-analyses evaluated studies of bicarbonate therapy in patients with CKD, and both found benefit.1,2
A 2020 meta-analysis included 15 RCTs (N = 2445) of adults (mean age, 61 years; range, 40.5-73.9 years) with CKD.1 Most trials enrolled patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; however, 1 study (N = 80) enrolled patients who had an eGFR of 60 to 90 mL/min/1.73 m2 and albuminuria, and another (N = 74) enrolled patients with an eGFR of 15 to 89 mL/min/1.73 m2. Four studies included patients with normal baseline bicarbonate levels, while the rest enrolled patients with metabolic acidosis. The primary outcome was CKD progression at study conclusion, which ranged from 3 to 60 months (median, 12 months).
Compared to placebo or no therapy, sodium bicarbonate (variously dosed) resulted in a small reduction in the rate of loss of kidney function (defined by eGFR or creatinine clearance) from baseline to trial completion (14 trials, N = 2073; standardized mean difference [SMD] = 0.26; 95% CI, 0.13-0.40; P = .018; I2 = 50%).1
Subgroup analysis by follow-up time found a significant preservation of eGFR only in studies with follow-up > 12 months (4 trials, N = 392; weighted mean difference = 3.71 mL/min/1.73 m2; 95% CI, 0.18-7.24; P = .042; I2 = 63%).1 Duration of therapy did not affect initiation of dialysis. Another subgroup analysis found that low- and moderate-quality studies were more likely than high-quality studies to find a change in the primary outcome. Overall, there was significant heterogeneity among the trials (control intervention, follow-up duration, methods of assessment of kidney function, dosage of sodium bicarbonate), as well as underrepresentation of female, pediatric, and elderly patients.
Another meta-analysis, published in 2019 by a different research group, analyzed 7 RCTs (N = 815) that comprised a subset of those in the newer analysis.2 The 2019 analysis similarly found that, compared to placebo or usual care, oral bicarbonate therapy resulted in statistically significantly higher eGFRs at 3 to 60 months’ follow-up (mean difference = 3.1 mL/min/1.73 m²; 95% CI, 1.3-4.9).2 The authors noted that the protective effect on eGFR was not seen in studies reporting outcomes at 1 year. Progression to end-stage renal disease or initiation of dialysis were not used as outcomes.
Significant outcomes seen in 1 large study
The largest study (N = 740) included in the 2020 meta-analysis (and discussed separately due to its size and duration) was a multicenter, unblinded, pragmatic trial investigating bicarbonate therapy in CKD.3 Patients were adults (mean age, 67.8 years) with CKD stages 3 to 5 and metabolic acidosis (serum bicarbonate level of 18-24 mmol/L); mean serum creatinine was 2.3 mg/dL, and mean serum bicarbonate was 21.5 mmol/L. Patients with severe heart failure or uncontrolled hypertension were excluded.
Researchers randomized patients to oral sodium bicarbonate (titrated to a target serum concentration of 24-28 mmol/L) or standard care for a median duration of 30 months. The primary endpoint was time to doubling of serum creatinine, and secondary endpoints included all-cause mortality, time to initiation of dialysis, hospitalization rate, and hospital length of stay.
Continue to: Patients treated with...
Patients treated with bicarbonate therapy had a 64% lower risk of doubling their serum creatinine compared to those treated with standard care (hazard ratio [HR] = 0.36; 95% CI, 0.22-0.58; P < .001; NNT = 9.6).3 Bicarbonate therapy also significantly reduced the risk of dialysis (HR = 0.5; 95% CI, 0.31-0.81; P = .005; NNT = 19); all-cause mortality (HR = 0.43; 95% CI, 0.22-0.87; P = .01; NNT = 27); hospitalization rates (34.6% vs 14.2% by end of study in standard care and bicarbonate groups, respectively; P < .001); and hospital length of stay (1160 total d/y vs 400 total d/y; P < .0001).3 Inspection of Kaplan Meier curves shows outcomes beginning to diverge after 1 to 2 years of treatment. This trial was limited by the lack of blinding, placebo control, and standardization of care protocols.
Recommendations from others
The National Kidney Foundation’s 2012 Kidney Disease Outcomes Quality Initiative guidelines for the management of CKD recommend oral bicarbonate therapy for patients with CKD and serum bicarbonate concentrations < 22 mmol/L.4 The guidelines state that serum bicarbonate levels < 22 mmol/L correlate with an increased risk of CKD progression and death, whereas high bicarbonate levels (> 32 mmol/L) correlate with increased risk of death independent of level of kidney function. These guidelines cite small studies of alkali therapy slowing progression of CKD, although it was noted that the evidence base was not strong.
Editor’s takeaway
The evidence shows a small but consistent effect of bicarbonate therapy on CKD progression. For patients with CKD stages 3 to 5 and metabolic acidosis (defined by serum bicarbonate levels < 22 mmol/L), the use of supplemental oral sodium bicarbonate, which is inexpensive and safe, can delay or prevent progression of serious disease.
1. Hultin S, Hood C, Campbell KL, et al. A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int Rep. 2020;6:695-705. doi: 10.1016/j.ekir.2020.12.019
2. Hu MK, Witham MD, Soiza RL. Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Med. 2019;8:208. doi: 10.3390/jcm8020208
3. Di Iorio BR, Bellasi A, Raphael KL, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J of Neph. 2019; 32:989-1001. doi: 10.1007/s40620-019-00656-5
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150.
Evidence summary
Bicarbonate therapy demonstrates benefit in 2 meta-analyses
Two recent meta-analyses evaluated studies of bicarbonate therapy in patients with CKD, and both found benefit.1,2
A 2020 meta-analysis included 15 RCTs (N = 2445) of adults (mean age, 61 years; range, 40.5-73.9 years) with CKD.1 Most trials enrolled patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; however, 1 study (N = 80) enrolled patients who had an eGFR of 60 to 90 mL/min/1.73 m2 and albuminuria, and another (N = 74) enrolled patients with an eGFR of 15 to 89 mL/min/1.73 m2. Four studies included patients with normal baseline bicarbonate levels, while the rest enrolled patients with metabolic acidosis. The primary outcome was CKD progression at study conclusion, which ranged from 3 to 60 months (median, 12 months).
Compared to placebo or no therapy, sodium bicarbonate (variously dosed) resulted in a small reduction in the rate of loss of kidney function (defined by eGFR or creatinine clearance) from baseline to trial completion (14 trials, N = 2073; standardized mean difference [SMD] = 0.26; 95% CI, 0.13-0.40; P = .018; I2 = 50%).1
Subgroup analysis by follow-up time found a significant preservation of eGFR only in studies with follow-up > 12 months (4 trials, N = 392; weighted mean difference = 3.71 mL/min/1.73 m2; 95% CI, 0.18-7.24; P = .042; I2 = 63%).1 Duration of therapy did not affect initiation of dialysis. Another subgroup analysis found that low- and moderate-quality studies were more likely than high-quality studies to find a change in the primary outcome. Overall, there was significant heterogeneity among the trials (control intervention, follow-up duration, methods of assessment of kidney function, dosage of sodium bicarbonate), as well as underrepresentation of female, pediatric, and elderly patients.
Another meta-analysis, published in 2019 by a different research group, analyzed 7 RCTs (N = 815) that comprised a subset of those in the newer analysis.2 The 2019 analysis similarly found that, compared to placebo or usual care, oral bicarbonate therapy resulted in statistically significantly higher eGFRs at 3 to 60 months’ follow-up (mean difference = 3.1 mL/min/1.73 m²; 95% CI, 1.3-4.9).2 The authors noted that the protective effect on eGFR was not seen in studies reporting outcomes at 1 year. Progression to end-stage renal disease or initiation of dialysis were not used as outcomes.
Significant outcomes seen in 1 large study
The largest study (N = 740) included in the 2020 meta-analysis (and discussed separately due to its size and duration) was a multicenter, unblinded, pragmatic trial investigating bicarbonate therapy in CKD.3 Patients were adults (mean age, 67.8 years) with CKD stages 3 to 5 and metabolic acidosis (serum bicarbonate level of 18-24 mmol/L); mean serum creatinine was 2.3 mg/dL, and mean serum bicarbonate was 21.5 mmol/L. Patients with severe heart failure or uncontrolled hypertension were excluded.
Researchers randomized patients to oral sodium bicarbonate (titrated to a target serum concentration of 24-28 mmol/L) or standard care for a median duration of 30 months. The primary endpoint was time to doubling of serum creatinine, and secondary endpoints included all-cause mortality, time to initiation of dialysis, hospitalization rate, and hospital length of stay.
Continue to: Patients treated with...
Patients treated with bicarbonate therapy had a 64% lower risk of doubling their serum creatinine compared to those treated with standard care (hazard ratio [HR] = 0.36; 95% CI, 0.22-0.58; P < .001; NNT = 9.6).3 Bicarbonate therapy also significantly reduced the risk of dialysis (HR = 0.5; 95% CI, 0.31-0.81; P = .005; NNT = 19); all-cause mortality (HR = 0.43; 95% CI, 0.22-0.87; P = .01; NNT = 27); hospitalization rates (34.6% vs 14.2% by end of study in standard care and bicarbonate groups, respectively; P < .001); and hospital length of stay (1160 total d/y vs 400 total d/y; P < .0001).3 Inspection of Kaplan Meier curves shows outcomes beginning to diverge after 1 to 2 years of treatment. This trial was limited by the lack of blinding, placebo control, and standardization of care protocols.
Recommendations from others
The National Kidney Foundation’s 2012 Kidney Disease Outcomes Quality Initiative guidelines for the management of CKD recommend oral bicarbonate therapy for patients with CKD and serum bicarbonate concentrations < 22 mmol/L.4 The guidelines state that serum bicarbonate levels < 22 mmol/L correlate with an increased risk of CKD progression and death, whereas high bicarbonate levels (> 32 mmol/L) correlate with increased risk of death independent of level of kidney function. These guidelines cite small studies of alkali therapy slowing progression of CKD, although it was noted that the evidence base was not strong.
Editor’s takeaway
The evidence shows a small but consistent effect of bicarbonate therapy on CKD progression. For patients with CKD stages 3 to 5 and metabolic acidosis (defined by serum bicarbonate levels < 22 mmol/L), the use of supplemental oral sodium bicarbonate, which is inexpensive and safe, can delay or prevent progression of serious disease.
Evidence summary
Bicarbonate therapy demonstrates benefit in 2 meta-analyses
Two recent meta-analyses evaluated studies of bicarbonate therapy in patients with CKD, and both found benefit.1,2
A 2020 meta-analysis included 15 RCTs (N = 2445) of adults (mean age, 61 years; range, 40.5-73.9 years) with CKD.1 Most trials enrolled patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; however, 1 study (N = 80) enrolled patients who had an eGFR of 60 to 90 mL/min/1.73 m2 and albuminuria, and another (N = 74) enrolled patients with an eGFR of 15 to 89 mL/min/1.73 m2. Four studies included patients with normal baseline bicarbonate levels, while the rest enrolled patients with metabolic acidosis. The primary outcome was CKD progression at study conclusion, which ranged from 3 to 60 months (median, 12 months).
Compared to placebo or no therapy, sodium bicarbonate (variously dosed) resulted in a small reduction in the rate of loss of kidney function (defined by eGFR or creatinine clearance) from baseline to trial completion (14 trials, N = 2073; standardized mean difference [SMD] = 0.26; 95% CI, 0.13-0.40; P = .018; I2 = 50%).1
Subgroup analysis by follow-up time found a significant preservation of eGFR only in studies with follow-up > 12 months (4 trials, N = 392; weighted mean difference = 3.71 mL/min/1.73 m2; 95% CI, 0.18-7.24; P = .042; I2 = 63%).1 Duration of therapy did not affect initiation of dialysis. Another subgroup analysis found that low- and moderate-quality studies were more likely than high-quality studies to find a change in the primary outcome. Overall, there was significant heterogeneity among the trials (control intervention, follow-up duration, methods of assessment of kidney function, dosage of sodium bicarbonate), as well as underrepresentation of female, pediatric, and elderly patients.
Another meta-analysis, published in 2019 by a different research group, analyzed 7 RCTs (N = 815) that comprised a subset of those in the newer analysis.2 The 2019 analysis similarly found that, compared to placebo or usual care, oral bicarbonate therapy resulted in statistically significantly higher eGFRs at 3 to 60 months’ follow-up (mean difference = 3.1 mL/min/1.73 m²; 95% CI, 1.3-4.9).2 The authors noted that the protective effect on eGFR was not seen in studies reporting outcomes at 1 year. Progression to end-stage renal disease or initiation of dialysis were not used as outcomes.
Significant outcomes seen in 1 large study
The largest study (N = 740) included in the 2020 meta-analysis (and discussed separately due to its size and duration) was a multicenter, unblinded, pragmatic trial investigating bicarbonate therapy in CKD.3 Patients were adults (mean age, 67.8 years) with CKD stages 3 to 5 and metabolic acidosis (serum bicarbonate level of 18-24 mmol/L); mean serum creatinine was 2.3 mg/dL, and mean serum bicarbonate was 21.5 mmol/L. Patients with severe heart failure or uncontrolled hypertension were excluded.
Researchers randomized patients to oral sodium bicarbonate (titrated to a target serum concentration of 24-28 mmol/L) or standard care for a median duration of 30 months. The primary endpoint was time to doubling of serum creatinine, and secondary endpoints included all-cause mortality, time to initiation of dialysis, hospitalization rate, and hospital length of stay.
Continue to: Patients treated with...
Patients treated with bicarbonate therapy had a 64% lower risk of doubling their serum creatinine compared to those treated with standard care (hazard ratio [HR] = 0.36; 95% CI, 0.22-0.58; P < .001; NNT = 9.6).3 Bicarbonate therapy also significantly reduced the risk of dialysis (HR = 0.5; 95% CI, 0.31-0.81; P = .005; NNT = 19); all-cause mortality (HR = 0.43; 95% CI, 0.22-0.87; P = .01; NNT = 27); hospitalization rates (34.6% vs 14.2% by end of study in standard care and bicarbonate groups, respectively; P < .001); and hospital length of stay (1160 total d/y vs 400 total d/y; P < .0001).3 Inspection of Kaplan Meier curves shows outcomes beginning to diverge after 1 to 2 years of treatment. This trial was limited by the lack of blinding, placebo control, and standardization of care protocols.
Recommendations from others
The National Kidney Foundation’s 2012 Kidney Disease Outcomes Quality Initiative guidelines for the management of CKD recommend oral bicarbonate therapy for patients with CKD and serum bicarbonate concentrations < 22 mmol/L.4 The guidelines state that serum bicarbonate levels < 22 mmol/L correlate with an increased risk of CKD progression and death, whereas high bicarbonate levels (> 32 mmol/L) correlate with increased risk of death independent of level of kidney function. These guidelines cite small studies of alkali therapy slowing progression of CKD, although it was noted that the evidence base was not strong.
Editor’s takeaway
The evidence shows a small but consistent effect of bicarbonate therapy on CKD progression. For patients with CKD stages 3 to 5 and metabolic acidosis (defined by serum bicarbonate levels < 22 mmol/L), the use of supplemental oral sodium bicarbonate, which is inexpensive and safe, can delay or prevent progression of serious disease.
1. Hultin S, Hood C, Campbell KL, et al. A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int Rep. 2020;6:695-705. doi: 10.1016/j.ekir.2020.12.019
2. Hu MK, Witham MD, Soiza RL. Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Med. 2019;8:208. doi: 10.3390/jcm8020208
3. Di Iorio BR, Bellasi A, Raphael KL, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J of Neph. 2019; 32:989-1001. doi: 10.1007/s40620-019-00656-5
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150.
1. Hultin S, Hood C, Campbell KL, et al. A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int Rep. 2020;6:695-705. doi: 10.1016/j.ekir.2020.12.019
2. Hu MK, Witham MD, Soiza RL. Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Med. 2019;8:208. doi: 10.3390/jcm8020208
3. Di Iorio BR, Bellasi A, Raphael KL, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J of Neph. 2019; 32:989-1001. doi: 10.1007/s40620-019-00656-5
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150.
EVIDENCE-BASED ANSWER:
YES. Long-term sodium bicarbonate therapy slightly slows the loss of renal function in patients with chronic kidney disease (CKD) and may moderately reduce progression to end-stage renal disease (strength of recommendation [SOR]: B, meta-analyses of lower-quality randomized controlled trails [RCTs]). Therapy duration of 1 year or less may not be beneficial (SOR: C, secondary analyses in meta-analyses).
Surgeons in China ‘are the executioners,’ procuring organs before brain death
In a deep dive into obscure Chinese language transplant journals, a pair of researchers from Australia and Israel have added a new layer of horror to what’s already known about forced organ harvesting in China.
Searching for documentation that vital organs are being harvested from nonconsenting executed prisoners, a practice that the China Tribunal confirmed “beyond any reasonable doubt” in 2020, Jacob Lavee, MD, an Israeli heart transplant surgeon, and Matthew Roberston, a PhD student at Australian National University, uncovered something even more shocking: that vital organs are being explanted from patients who are still alive.
“We have shown for the first time that the transplant surgeons are the executioners – that the mode of execution is organ procurement. These are self-admissions of executing the patient,” Dr. Lavee told this news organization. “Up until now, there has been what we call circumstantial evidence of this, but our paper is what you’d call the smoking gun, because it’s in the words of the physicians themselves that they are doing it. In the words of these surgeons, intubation was done only after the beginning of surgery, which means the patients were breathing spontaneously up until the moment the operation started ... meaning they were not brain dead.”
The research, published in the American Journal of Transplantation, involved intricate analysis of thousands of Chinese language transplant articles and identified 71 articles in which transplant surgeons describe starting organ procurement surgery before declaring their patients brain dead.
“What we found were improper, illegitimate, nonexistent, or false declarations of brain death,” Mr. Robertson said in an interview. He explained that this violates what’s known as the dead donor rule, which is fundamental in transplant ethics. “The surgeons wrote that the donor was brain dead, but according to everything we know about medical science, they could not possibly have been brain dead because there was no apnea test performed. Brain death is not just something you say, there’s this whole battery of tests, and the key is the apnea test, [in which] the patient is already intubated and ventilated, they turn the machine off, and they’re looking for carbon dioxide in the blood above a certain level.”
Mr. Robertson and Dr. Lavee have painstakingly documented “incriminating sentences” in each of the 71 articles proving that brain death had not occurred before the organ explantation procedure began. “There were two criteria by which we claimed a problematic brain death declaration,” said Mr. Robertson, who translated the Chinese. “One was where the patient was not ventilated and was only intubated after they were declared brain dead; the other was that the intubation took place immediately prior to the surgery beginning.”
“It was mind-boggling,” said Dr. Lavee, from Tel Aviv University. “When I first started reading, my initial reaction is, ‘This can’t be.’ I read it once, and again, and I insisted that Matt get another independent translation of the Chinese just to be sure. I told him, ‘There’s no way a physician, a surgeon could write this – it doesn’t make sense.’ But the more of these papers we read, we saw it was a pattern – and they didn’t come out of a single medical center, they are spread all over China.”
For the analysis, Mr. Robertson wrote code and customized an algorithm to examine 124,770 medical articles from official Chinese databases between 1980 and 2020. The 71 articles revealing cases involving problematic brain death came from 56 hospitals (of which 12 were military) in 33 cities across 15 provinces, they report. In total, 348 surgeons, nurses, anesthesiologists, and other medical workers or researchers were listed as authors of these publications.
Why would these medical personnel write such self-incriminating evidence? The researchers say it’s unclear. “They don’t think anyone’s reading this stuff,” Mr. Robertson suggests. “Sometimes it’s revealed in just five or six characters in a paper of eight pages.” Dr. Lavee wonders if it’s also ignorance. “If this has been a practice for 20 or 30 years in China, I guess nobody at that time was aware they were doing something wrong, although how to declare brain death is something that is known in China. They’ve published a lot about it.”
The article is “evidence that this barbarity continues and is a very valuable contribution that continues to bring attention to an enormous human rights violation,” said Arthur Caplan, PhD, head of the Division of Medical Ethics at New York University’s Grossman School of Medicine. “What they’ve reported has been going on for many, many years, the data are very clear that China’s doing many more transplants than they have cadaver organ donors,” he said, adding that the country’s well-documented and lucrative involvement in transplant tourism “means you have to have a donor ready when the would-be recipient appears; you have to have a matched organ available, and that’s hard to do waiting on a cadaver donor.”
Although the researchers found no incriminating publications after 2015, they speculate that this is likely due to growing awareness among Chinese surgeons that publishing the information would attract international condemnation. “We think these practices are continuing to go on,” said Dr. Lavee. He acknowledged that a voluntary organ donation program is slowly developing in parallel to this. He said, given China’s place as the world’s second largest transplant country behind the U.S., as well as its low rate of voluntary donation, it’s reasonable to conclude that the main source of organs remains prisoners on death row.
Dr. Caplan and the researchers have called for academic institutions and medical journals to resume their previous boycotts of Chinese transplant publications and speakers, but as long as China denies the practices, economic and political leaders will turn a blind eye. “In the past, I don’t think the question of China’s medical professional involvement in the execution of donors has been taken as seriously as it should have,” said Mr. Robertson. “I certainly hope that with the publication of this paper in the leading journal in the field, this will change.”
The study was supported by the Google Cloud Research Credits program, the Australian Government Research Training Program Scholarship, and the Victims of Communism Memorial Foundation. Mr. Robertson, Dr. Lavee, and Dr. Caplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a deep dive into obscure Chinese language transplant journals, a pair of researchers from Australia and Israel have added a new layer of horror to what’s already known about forced organ harvesting in China.
Searching for documentation that vital organs are being harvested from nonconsenting executed prisoners, a practice that the China Tribunal confirmed “beyond any reasonable doubt” in 2020, Jacob Lavee, MD, an Israeli heart transplant surgeon, and Matthew Roberston, a PhD student at Australian National University, uncovered something even more shocking: that vital organs are being explanted from patients who are still alive.
“We have shown for the first time that the transplant surgeons are the executioners – that the mode of execution is organ procurement. These are self-admissions of executing the patient,” Dr. Lavee told this news organization. “Up until now, there has been what we call circumstantial evidence of this, but our paper is what you’d call the smoking gun, because it’s in the words of the physicians themselves that they are doing it. In the words of these surgeons, intubation was done only after the beginning of surgery, which means the patients were breathing spontaneously up until the moment the operation started ... meaning they were not brain dead.”
The research, published in the American Journal of Transplantation, involved intricate analysis of thousands of Chinese language transplant articles and identified 71 articles in which transplant surgeons describe starting organ procurement surgery before declaring their patients brain dead.
“What we found were improper, illegitimate, nonexistent, or false declarations of brain death,” Mr. Robertson said in an interview. He explained that this violates what’s known as the dead donor rule, which is fundamental in transplant ethics. “The surgeons wrote that the donor was brain dead, but according to everything we know about medical science, they could not possibly have been brain dead because there was no apnea test performed. Brain death is not just something you say, there’s this whole battery of tests, and the key is the apnea test, [in which] the patient is already intubated and ventilated, they turn the machine off, and they’re looking for carbon dioxide in the blood above a certain level.”
Mr. Robertson and Dr. Lavee have painstakingly documented “incriminating sentences” in each of the 71 articles proving that brain death had not occurred before the organ explantation procedure began. “There were two criteria by which we claimed a problematic brain death declaration,” said Mr. Robertson, who translated the Chinese. “One was where the patient was not ventilated and was only intubated after they were declared brain dead; the other was that the intubation took place immediately prior to the surgery beginning.”
“It was mind-boggling,” said Dr. Lavee, from Tel Aviv University. “When I first started reading, my initial reaction is, ‘This can’t be.’ I read it once, and again, and I insisted that Matt get another independent translation of the Chinese just to be sure. I told him, ‘There’s no way a physician, a surgeon could write this – it doesn’t make sense.’ But the more of these papers we read, we saw it was a pattern – and they didn’t come out of a single medical center, they are spread all over China.”
For the analysis, Mr. Robertson wrote code and customized an algorithm to examine 124,770 medical articles from official Chinese databases between 1980 and 2020. The 71 articles revealing cases involving problematic brain death came from 56 hospitals (of which 12 were military) in 33 cities across 15 provinces, they report. In total, 348 surgeons, nurses, anesthesiologists, and other medical workers or researchers were listed as authors of these publications.
Why would these medical personnel write such self-incriminating evidence? The researchers say it’s unclear. “They don’t think anyone’s reading this stuff,” Mr. Robertson suggests. “Sometimes it’s revealed in just five or six characters in a paper of eight pages.” Dr. Lavee wonders if it’s also ignorance. “If this has been a practice for 20 or 30 years in China, I guess nobody at that time was aware they were doing something wrong, although how to declare brain death is something that is known in China. They’ve published a lot about it.”
The article is “evidence that this barbarity continues and is a very valuable contribution that continues to bring attention to an enormous human rights violation,” said Arthur Caplan, PhD, head of the Division of Medical Ethics at New York University’s Grossman School of Medicine. “What they’ve reported has been going on for many, many years, the data are very clear that China’s doing many more transplants than they have cadaver organ donors,” he said, adding that the country’s well-documented and lucrative involvement in transplant tourism “means you have to have a donor ready when the would-be recipient appears; you have to have a matched organ available, and that’s hard to do waiting on a cadaver donor.”
Although the researchers found no incriminating publications after 2015, they speculate that this is likely due to growing awareness among Chinese surgeons that publishing the information would attract international condemnation. “We think these practices are continuing to go on,” said Dr. Lavee. He acknowledged that a voluntary organ donation program is slowly developing in parallel to this. He said, given China’s place as the world’s second largest transplant country behind the U.S., as well as its low rate of voluntary donation, it’s reasonable to conclude that the main source of organs remains prisoners on death row.
Dr. Caplan and the researchers have called for academic institutions and medical journals to resume their previous boycotts of Chinese transplant publications and speakers, but as long as China denies the practices, economic and political leaders will turn a blind eye. “In the past, I don’t think the question of China’s medical professional involvement in the execution of donors has been taken as seriously as it should have,” said Mr. Robertson. “I certainly hope that with the publication of this paper in the leading journal in the field, this will change.”
The study was supported by the Google Cloud Research Credits program, the Australian Government Research Training Program Scholarship, and the Victims of Communism Memorial Foundation. Mr. Robertson, Dr. Lavee, and Dr. Caplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a deep dive into obscure Chinese language transplant journals, a pair of researchers from Australia and Israel have added a new layer of horror to what’s already known about forced organ harvesting in China.
Searching for documentation that vital organs are being harvested from nonconsenting executed prisoners, a practice that the China Tribunal confirmed “beyond any reasonable doubt” in 2020, Jacob Lavee, MD, an Israeli heart transplant surgeon, and Matthew Roberston, a PhD student at Australian National University, uncovered something even more shocking: that vital organs are being explanted from patients who are still alive.
“We have shown for the first time that the transplant surgeons are the executioners – that the mode of execution is organ procurement. These are self-admissions of executing the patient,” Dr. Lavee told this news organization. “Up until now, there has been what we call circumstantial evidence of this, but our paper is what you’d call the smoking gun, because it’s in the words of the physicians themselves that they are doing it. In the words of these surgeons, intubation was done only after the beginning of surgery, which means the patients were breathing spontaneously up until the moment the operation started ... meaning they were not brain dead.”
The research, published in the American Journal of Transplantation, involved intricate analysis of thousands of Chinese language transplant articles and identified 71 articles in which transplant surgeons describe starting organ procurement surgery before declaring their patients brain dead.
“What we found were improper, illegitimate, nonexistent, or false declarations of brain death,” Mr. Robertson said in an interview. He explained that this violates what’s known as the dead donor rule, which is fundamental in transplant ethics. “The surgeons wrote that the donor was brain dead, but according to everything we know about medical science, they could not possibly have been brain dead because there was no apnea test performed. Brain death is not just something you say, there’s this whole battery of tests, and the key is the apnea test, [in which] the patient is already intubated and ventilated, they turn the machine off, and they’re looking for carbon dioxide in the blood above a certain level.”
Mr. Robertson and Dr. Lavee have painstakingly documented “incriminating sentences” in each of the 71 articles proving that brain death had not occurred before the organ explantation procedure began. “There were two criteria by which we claimed a problematic brain death declaration,” said Mr. Robertson, who translated the Chinese. “One was where the patient was not ventilated and was only intubated after they were declared brain dead; the other was that the intubation took place immediately prior to the surgery beginning.”
“It was mind-boggling,” said Dr. Lavee, from Tel Aviv University. “When I first started reading, my initial reaction is, ‘This can’t be.’ I read it once, and again, and I insisted that Matt get another independent translation of the Chinese just to be sure. I told him, ‘There’s no way a physician, a surgeon could write this – it doesn’t make sense.’ But the more of these papers we read, we saw it was a pattern – and they didn’t come out of a single medical center, they are spread all over China.”
For the analysis, Mr. Robertson wrote code and customized an algorithm to examine 124,770 medical articles from official Chinese databases between 1980 and 2020. The 71 articles revealing cases involving problematic brain death came from 56 hospitals (of which 12 were military) in 33 cities across 15 provinces, they report. In total, 348 surgeons, nurses, anesthesiologists, and other medical workers or researchers were listed as authors of these publications.
Why would these medical personnel write such self-incriminating evidence? The researchers say it’s unclear. “They don’t think anyone’s reading this stuff,” Mr. Robertson suggests. “Sometimes it’s revealed in just five or six characters in a paper of eight pages.” Dr. Lavee wonders if it’s also ignorance. “If this has been a practice for 20 or 30 years in China, I guess nobody at that time was aware they were doing something wrong, although how to declare brain death is something that is known in China. They’ve published a lot about it.”
The article is “evidence that this barbarity continues and is a very valuable contribution that continues to bring attention to an enormous human rights violation,” said Arthur Caplan, PhD, head of the Division of Medical Ethics at New York University’s Grossman School of Medicine. “What they’ve reported has been going on for many, many years, the data are very clear that China’s doing many more transplants than they have cadaver organ donors,” he said, adding that the country’s well-documented and lucrative involvement in transplant tourism “means you have to have a donor ready when the would-be recipient appears; you have to have a matched organ available, and that’s hard to do waiting on a cadaver donor.”
Although the researchers found no incriminating publications after 2015, they speculate that this is likely due to growing awareness among Chinese surgeons that publishing the information would attract international condemnation. “We think these practices are continuing to go on,” said Dr. Lavee. He acknowledged that a voluntary organ donation program is slowly developing in parallel to this. He said, given China’s place as the world’s second largest transplant country behind the U.S., as well as its low rate of voluntary donation, it’s reasonable to conclude that the main source of organs remains prisoners on death row.
Dr. Caplan and the researchers have called for academic institutions and medical journals to resume their previous boycotts of Chinese transplant publications and speakers, but as long as China denies the practices, economic and political leaders will turn a blind eye. “In the past, I don’t think the question of China’s medical professional involvement in the execution of donors has been taken as seriously as it should have,” said Mr. Robertson. “I certainly hope that with the publication of this paper in the leading journal in the field, this will change.”
The study was supported by the Google Cloud Research Credits program, the Australian Government Research Training Program Scholarship, and the Victims of Communism Memorial Foundation. Mr. Robertson, Dr. Lavee, and Dr. Caplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Empagliflozin scores topline win in EMPA-KIDNEY trial
Researchers running the EMPA-KIDNEY trial that’s been testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in about 6,600 patients with chronic kidney disease (CKD) announced on March 16 that they had stopped the trial early because of positive efficacy that met the study’s prespecified threshold for early termination.
EMPA-KIDNEY is the third major trial of an agent from the sodium-glucose cotransport 2 (SGLT2) inhibitor class tested in patients with CKD to be stopped early because of positive results that met a prespecified termination rule.
In 2020, the DAPA-CKD trial of dapagliflozin (Farxiga) stopped early, after a median follow-up of 2.4 years, because of positive efficacy results. In 2019, the same thing happened in the CREDENCE trial of canagliflozin (Invokana), with the unexpected halt coming after a median follow-up of 2.62 years.
The announcement about EMPA-KIDNEY did not include information on median follow-up, but enrollment into the trial ran from May 2019 to April 2021, which means that the longest that enrolled patients could have been in the study was about 2.85 years.
The primary efficacy endpoint in EMPA-KIDNEY was a composite of a sustained decline in estimated glomerular filtration rate (eGFR) to less than 10 mL/min/1.73 m2, renal death, a sustained decline of at least 40% in eGFR from baseline, or cardiovascular death. The announcement of the trial’s early termination provided no details on the efficacy results.
EMPA-KIDNEY enrolled a wider range of patients
EMPA-KIDNEY expands the scope of types of patients with CKD now shown to benefit from treatment with an SGLT2 inhibitor. CREDENCE tested canagliflozin only in patients with type 2 diabetes and diabetic nephropathy, and in DAPA-CKD, two-thirds of enrolled patients had type 2 diabetes, and all had CKD. In EMPA-KIDNEY, 46% of the 6,609 enrolled patients had diabetes (including a very small number with type 1 diabetes).
Another departure from prior studies of an SGLT2 inhibitor for patients selected primarily for having CKD was that in EMPA-KIDNEY, 20% of patients did not have albuminuria, and for 34%, eGFR at entry was less than 30 mL/min/1.73 m2, with all enrolled patients required to have an eGFR at entry of greater than or equal to 20 mL/min/1.73 m2. Average eGFR in EMPA-KIDNEY was about 38 mL/min/1.73 m2. To be included in the trial, patients were not required to have albuminuria, except those whose eGFR was greater than or equal to 45 mL/min/1.73 m2.
In DAPA-CKD, the minimum eGFR at entry had to be greater than or equal to 25 mL/min/1.73 m2, and roughly 14% of enrolled patients had an eGFR of less than 30 mL/min/1.73 m2. The average eGFR in DAPA-CKD was about 43 mL/min/1.73 m2. In addition, all patients had at least microalbuminuria, with a minimum urinary albumin-to-creatinine ratio of 200. In CREDENCE, the minimum eGFR for enrollment was 30 mL/min/1.73 m2, and the average eGFR was about 56 mL/min/1.73 m2. All patients in CREDENCE had to have macroalbuminuria, with a urinary albumin-to-creatinine ratio of more than 300.
According to the researchers who designed EMPA-KIDNEY, the trial enrollment criteria aimed to include adults with CKD “who are frequently seen in practice but were under-represented in previous SGLT2 inhibitor trials.”
Indications for empagliflozin are expanding
The success of empagliflozin in EMPA-KIDNEY follows its positive results in both the EMPEROR-Reduced and EMPEROR-Preserved trials, which collectively proved the efficacy of the agent for patients with heart failure regardless of their left ventricular ejection fraction and regardless of whether they also had diabetes.
These results led the U.S. Food and Drug Administration to recently expand the labeled indication for empagliflozin to all patients with heart failure. Empagliflozin also has labeled indications for glycemic control in patients with type 2 diabetes and to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
As of today, empagliflozin has no labeled indication for treating patients with CKD. Dapagliflozin received that indication in April 2021, and canagliflozin received an indication for treating patients with type 2 diabetes, diabetic nephropathy, and albuminuria in September 2019.
EMPA-KIDNEY is sponsored by Boehringer Ingelheim and Lilly, the two companies that jointly market empagliflozin (Jardiance).
A version of this article first appeared on Medscape.com.
Researchers running the EMPA-KIDNEY trial that’s been testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in about 6,600 patients with chronic kidney disease (CKD) announced on March 16 that they had stopped the trial early because of positive efficacy that met the study’s prespecified threshold for early termination.
EMPA-KIDNEY is the third major trial of an agent from the sodium-glucose cotransport 2 (SGLT2) inhibitor class tested in patients with CKD to be stopped early because of positive results that met a prespecified termination rule.
In 2020, the DAPA-CKD trial of dapagliflozin (Farxiga) stopped early, after a median follow-up of 2.4 years, because of positive efficacy results. In 2019, the same thing happened in the CREDENCE trial of canagliflozin (Invokana), with the unexpected halt coming after a median follow-up of 2.62 years.
The announcement about EMPA-KIDNEY did not include information on median follow-up, but enrollment into the trial ran from May 2019 to April 2021, which means that the longest that enrolled patients could have been in the study was about 2.85 years.
The primary efficacy endpoint in EMPA-KIDNEY was a composite of a sustained decline in estimated glomerular filtration rate (eGFR) to less than 10 mL/min/1.73 m2, renal death, a sustained decline of at least 40% in eGFR from baseline, or cardiovascular death. The announcement of the trial’s early termination provided no details on the efficacy results.
EMPA-KIDNEY enrolled a wider range of patients
EMPA-KIDNEY expands the scope of types of patients with CKD now shown to benefit from treatment with an SGLT2 inhibitor. CREDENCE tested canagliflozin only in patients with type 2 diabetes and diabetic nephropathy, and in DAPA-CKD, two-thirds of enrolled patients had type 2 diabetes, and all had CKD. In EMPA-KIDNEY, 46% of the 6,609 enrolled patients had diabetes (including a very small number with type 1 diabetes).
Another departure from prior studies of an SGLT2 inhibitor for patients selected primarily for having CKD was that in EMPA-KIDNEY, 20% of patients did not have albuminuria, and for 34%, eGFR at entry was less than 30 mL/min/1.73 m2, with all enrolled patients required to have an eGFR at entry of greater than or equal to 20 mL/min/1.73 m2. Average eGFR in EMPA-KIDNEY was about 38 mL/min/1.73 m2. To be included in the trial, patients were not required to have albuminuria, except those whose eGFR was greater than or equal to 45 mL/min/1.73 m2.
In DAPA-CKD, the minimum eGFR at entry had to be greater than or equal to 25 mL/min/1.73 m2, and roughly 14% of enrolled patients had an eGFR of less than 30 mL/min/1.73 m2. The average eGFR in DAPA-CKD was about 43 mL/min/1.73 m2. In addition, all patients had at least microalbuminuria, with a minimum urinary albumin-to-creatinine ratio of 200. In CREDENCE, the minimum eGFR for enrollment was 30 mL/min/1.73 m2, and the average eGFR was about 56 mL/min/1.73 m2. All patients in CREDENCE had to have macroalbuminuria, with a urinary albumin-to-creatinine ratio of more than 300.
According to the researchers who designed EMPA-KIDNEY, the trial enrollment criteria aimed to include adults with CKD “who are frequently seen in practice but were under-represented in previous SGLT2 inhibitor trials.”
Indications for empagliflozin are expanding
The success of empagliflozin in EMPA-KIDNEY follows its positive results in both the EMPEROR-Reduced and EMPEROR-Preserved trials, which collectively proved the efficacy of the agent for patients with heart failure regardless of their left ventricular ejection fraction and regardless of whether they also had diabetes.
These results led the U.S. Food and Drug Administration to recently expand the labeled indication for empagliflozin to all patients with heart failure. Empagliflozin also has labeled indications for glycemic control in patients with type 2 diabetes and to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
As of today, empagliflozin has no labeled indication for treating patients with CKD. Dapagliflozin received that indication in April 2021, and canagliflozin received an indication for treating patients with type 2 diabetes, diabetic nephropathy, and albuminuria in September 2019.
EMPA-KIDNEY is sponsored by Boehringer Ingelheim and Lilly, the two companies that jointly market empagliflozin (Jardiance).
A version of this article first appeared on Medscape.com.
Researchers running the EMPA-KIDNEY trial that’s been testing the safety and efficacy of the SGLT2 inhibitor empagliflozin (Jardiance) in about 6,600 patients with chronic kidney disease (CKD) announced on March 16 that they had stopped the trial early because of positive efficacy that met the study’s prespecified threshold for early termination.
EMPA-KIDNEY is the third major trial of an agent from the sodium-glucose cotransport 2 (SGLT2) inhibitor class tested in patients with CKD to be stopped early because of positive results that met a prespecified termination rule.
In 2020, the DAPA-CKD trial of dapagliflozin (Farxiga) stopped early, after a median follow-up of 2.4 years, because of positive efficacy results. In 2019, the same thing happened in the CREDENCE trial of canagliflozin (Invokana), with the unexpected halt coming after a median follow-up of 2.62 years.
The announcement about EMPA-KIDNEY did not include information on median follow-up, but enrollment into the trial ran from May 2019 to April 2021, which means that the longest that enrolled patients could have been in the study was about 2.85 years.
The primary efficacy endpoint in EMPA-KIDNEY was a composite of a sustained decline in estimated glomerular filtration rate (eGFR) to less than 10 mL/min/1.73 m2, renal death, a sustained decline of at least 40% in eGFR from baseline, or cardiovascular death. The announcement of the trial’s early termination provided no details on the efficacy results.
EMPA-KIDNEY enrolled a wider range of patients
EMPA-KIDNEY expands the scope of types of patients with CKD now shown to benefit from treatment with an SGLT2 inhibitor. CREDENCE tested canagliflozin only in patients with type 2 diabetes and diabetic nephropathy, and in DAPA-CKD, two-thirds of enrolled patients had type 2 diabetes, and all had CKD. In EMPA-KIDNEY, 46% of the 6,609 enrolled patients had diabetes (including a very small number with type 1 diabetes).
Another departure from prior studies of an SGLT2 inhibitor for patients selected primarily for having CKD was that in EMPA-KIDNEY, 20% of patients did not have albuminuria, and for 34%, eGFR at entry was less than 30 mL/min/1.73 m2, with all enrolled patients required to have an eGFR at entry of greater than or equal to 20 mL/min/1.73 m2. Average eGFR in EMPA-KIDNEY was about 38 mL/min/1.73 m2. To be included in the trial, patients were not required to have albuminuria, except those whose eGFR was greater than or equal to 45 mL/min/1.73 m2.
In DAPA-CKD, the minimum eGFR at entry had to be greater than or equal to 25 mL/min/1.73 m2, and roughly 14% of enrolled patients had an eGFR of less than 30 mL/min/1.73 m2. The average eGFR in DAPA-CKD was about 43 mL/min/1.73 m2. In addition, all patients had at least microalbuminuria, with a minimum urinary albumin-to-creatinine ratio of 200. In CREDENCE, the minimum eGFR for enrollment was 30 mL/min/1.73 m2, and the average eGFR was about 56 mL/min/1.73 m2. All patients in CREDENCE had to have macroalbuminuria, with a urinary albumin-to-creatinine ratio of more than 300.
According to the researchers who designed EMPA-KIDNEY, the trial enrollment criteria aimed to include adults with CKD “who are frequently seen in practice but were under-represented in previous SGLT2 inhibitor trials.”
Indications for empagliflozin are expanding
The success of empagliflozin in EMPA-KIDNEY follows its positive results in both the EMPEROR-Reduced and EMPEROR-Preserved trials, which collectively proved the efficacy of the agent for patients with heart failure regardless of their left ventricular ejection fraction and regardless of whether they also had diabetes.
These results led the U.S. Food and Drug Administration to recently expand the labeled indication for empagliflozin to all patients with heart failure. Empagliflozin also has labeled indications for glycemic control in patients with type 2 diabetes and to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
As of today, empagliflozin has no labeled indication for treating patients with CKD. Dapagliflozin received that indication in April 2021, and canagliflozin received an indication for treating patients with type 2 diabetes, diabetic nephropathy, and albuminuria in September 2019.
EMPA-KIDNEY is sponsored by Boehringer Ingelheim and Lilly, the two companies that jointly market empagliflozin (Jardiance).
A version of this article first appeared on Medscape.com.
Opting out of dialysis not instant death sentence for kidney disease
, a new systematic review of cohort studies suggests.
“Our findings challenge the common misconception that the only alternative to dialysis for many patients with advanced chronic kidney disease is no care or death,” say Susan Wong, MD, of the Renal Dialysis Unit, Seattle, and colleagues in their review, published online March 14 in JAMA Network Open.
In an accompanying commentary, Christine Liu, MD, and Kurella Tamura, MD, MPH, note: “The decision to initiate dialysis or focus on active alleviation of symptoms, known as conservative care … is likely one of the consequential decisions [patients] will face.”
“[But] in reality, dialysis is viewed as the default treatment for kidney failure, and the option to forgo dialysis treatment is often not explicitly discussed,” they add.
“We believe it is time to broaden the scope of kidney replacement therapy registries to include persons who receive conservative treatment of kidney failure … and we need to address the conservative care information gap so that lack of awareness is no longer a barrier to informed decision-making,” Dr. Liu and Dr. Tamura, both from Stanford (Calif.) University, note.
The work by Dr. Wong and colleagues “dispels the notion that conservative care for kidney failure means a grim and near-immediate death. The study advances the idea that a conservative care approach can provide time and sustain quality of life to support patients’ life goals,” they emphasize.
Conservative care assessed in 41 studies
The review included 41 studies involving 5,102 patients with a mean age ranging from 60 to 87 years conducted in the United Kingdom, Europe, and Asia.
Median survival of cohorts ranged from 1 to 41 months as measured from a baseline mean estimated glomerular filtration rate (eGFR) ranging from 7 to 19 mL/min/1.73m2.
Younger patients between 70 and 79 years of age had a median survival of 7 to 41 months, the authors note, while cohorts consisting of patients 80 years of age and older had a median survival of 1 to 37 months despite overlapping ranges of baseline mean eGFRs.
During an observation period of 8-24 months, mental well-being improved, and physical well-being and overall quality of life were largely stable until late in the course of illness.
“Ten studies … provided information on the use of health care resources during follow-up,” the researchers say. Patients generally experienced one to two hospital admissions, 6-16 in-hospital days, seven to eight clinic visits, and two emergency department visits per person-year. Use of acute care services was “therefore common,” they note.
Not all studies provided information about end-of-life care, but those that did reported rates of hospice enrollment that ranged from 20% to 76%; hospitalization rates during the final month of life from 57% to 76%; in-hospital death rates of 27%-68%, and in-home death rates ranging from 12% to 71%.
This indicates substantial disparity in access to supportive care near the end of life across cohorts, the authors observe.
Nevertheless, “Most patients survived several years after the decision to forgo dialysis was made,” they stress.
“These findings not only suggest that conservative kidney management may be a viable and positive therapeutic alternative to dialysis, they also highlight the strengths of its multidisciplinary approach to care and aggressive symptom management.”
“Collectively, our findings demonstrate the need to implement systematic and unified research methods for conservative kidney management and to develop models of care and the care infrastructure to advance practice and outcomes of conservative kidney management,” they conclude.
Dr. Wong has no financial ties to industry. Dr. Tamura has reported receiving personal fees from the American Federation for Aging Research.
A version of this article first appeared on Medscape.com.
, a new systematic review of cohort studies suggests.
“Our findings challenge the common misconception that the only alternative to dialysis for many patients with advanced chronic kidney disease is no care or death,” say Susan Wong, MD, of the Renal Dialysis Unit, Seattle, and colleagues in their review, published online March 14 in JAMA Network Open.
In an accompanying commentary, Christine Liu, MD, and Kurella Tamura, MD, MPH, note: “The decision to initiate dialysis or focus on active alleviation of symptoms, known as conservative care … is likely one of the consequential decisions [patients] will face.”
“[But] in reality, dialysis is viewed as the default treatment for kidney failure, and the option to forgo dialysis treatment is often not explicitly discussed,” they add.
“We believe it is time to broaden the scope of kidney replacement therapy registries to include persons who receive conservative treatment of kidney failure … and we need to address the conservative care information gap so that lack of awareness is no longer a barrier to informed decision-making,” Dr. Liu and Dr. Tamura, both from Stanford (Calif.) University, note.
The work by Dr. Wong and colleagues “dispels the notion that conservative care for kidney failure means a grim and near-immediate death. The study advances the idea that a conservative care approach can provide time and sustain quality of life to support patients’ life goals,” they emphasize.
Conservative care assessed in 41 studies
The review included 41 studies involving 5,102 patients with a mean age ranging from 60 to 87 years conducted in the United Kingdom, Europe, and Asia.
Median survival of cohorts ranged from 1 to 41 months as measured from a baseline mean estimated glomerular filtration rate (eGFR) ranging from 7 to 19 mL/min/1.73m2.
Younger patients between 70 and 79 years of age had a median survival of 7 to 41 months, the authors note, while cohorts consisting of patients 80 years of age and older had a median survival of 1 to 37 months despite overlapping ranges of baseline mean eGFRs.
During an observation period of 8-24 months, mental well-being improved, and physical well-being and overall quality of life were largely stable until late in the course of illness.
“Ten studies … provided information on the use of health care resources during follow-up,” the researchers say. Patients generally experienced one to two hospital admissions, 6-16 in-hospital days, seven to eight clinic visits, and two emergency department visits per person-year. Use of acute care services was “therefore common,” they note.
Not all studies provided information about end-of-life care, but those that did reported rates of hospice enrollment that ranged from 20% to 76%; hospitalization rates during the final month of life from 57% to 76%; in-hospital death rates of 27%-68%, and in-home death rates ranging from 12% to 71%.
This indicates substantial disparity in access to supportive care near the end of life across cohorts, the authors observe.
Nevertheless, “Most patients survived several years after the decision to forgo dialysis was made,” they stress.
“These findings not only suggest that conservative kidney management may be a viable and positive therapeutic alternative to dialysis, they also highlight the strengths of its multidisciplinary approach to care and aggressive symptom management.”
“Collectively, our findings demonstrate the need to implement systematic and unified research methods for conservative kidney management and to develop models of care and the care infrastructure to advance practice and outcomes of conservative kidney management,” they conclude.
Dr. Wong has no financial ties to industry. Dr. Tamura has reported receiving personal fees from the American Federation for Aging Research.
A version of this article first appeared on Medscape.com.
, a new systematic review of cohort studies suggests.
“Our findings challenge the common misconception that the only alternative to dialysis for many patients with advanced chronic kidney disease is no care or death,” say Susan Wong, MD, of the Renal Dialysis Unit, Seattle, and colleagues in their review, published online March 14 in JAMA Network Open.
In an accompanying commentary, Christine Liu, MD, and Kurella Tamura, MD, MPH, note: “The decision to initiate dialysis or focus on active alleviation of symptoms, known as conservative care … is likely one of the consequential decisions [patients] will face.”
“[But] in reality, dialysis is viewed as the default treatment for kidney failure, and the option to forgo dialysis treatment is often not explicitly discussed,” they add.
“We believe it is time to broaden the scope of kidney replacement therapy registries to include persons who receive conservative treatment of kidney failure … and we need to address the conservative care information gap so that lack of awareness is no longer a barrier to informed decision-making,” Dr. Liu and Dr. Tamura, both from Stanford (Calif.) University, note.
The work by Dr. Wong and colleagues “dispels the notion that conservative care for kidney failure means a grim and near-immediate death. The study advances the idea that a conservative care approach can provide time and sustain quality of life to support patients’ life goals,” they emphasize.
Conservative care assessed in 41 studies
The review included 41 studies involving 5,102 patients with a mean age ranging from 60 to 87 years conducted in the United Kingdom, Europe, and Asia.
Median survival of cohorts ranged from 1 to 41 months as measured from a baseline mean estimated glomerular filtration rate (eGFR) ranging from 7 to 19 mL/min/1.73m2.
Younger patients between 70 and 79 years of age had a median survival of 7 to 41 months, the authors note, while cohorts consisting of patients 80 years of age and older had a median survival of 1 to 37 months despite overlapping ranges of baseline mean eGFRs.
During an observation period of 8-24 months, mental well-being improved, and physical well-being and overall quality of life were largely stable until late in the course of illness.
“Ten studies … provided information on the use of health care resources during follow-up,” the researchers say. Patients generally experienced one to two hospital admissions, 6-16 in-hospital days, seven to eight clinic visits, and two emergency department visits per person-year. Use of acute care services was “therefore common,” they note.
Not all studies provided information about end-of-life care, but those that did reported rates of hospice enrollment that ranged from 20% to 76%; hospitalization rates during the final month of life from 57% to 76%; in-hospital death rates of 27%-68%, and in-home death rates ranging from 12% to 71%.
This indicates substantial disparity in access to supportive care near the end of life across cohorts, the authors observe.
Nevertheless, “Most patients survived several years after the decision to forgo dialysis was made,” they stress.
“These findings not only suggest that conservative kidney management may be a viable and positive therapeutic alternative to dialysis, they also highlight the strengths of its multidisciplinary approach to care and aggressive symptom management.”
“Collectively, our findings demonstrate the need to implement systematic and unified research methods for conservative kidney management and to develop models of care and the care infrastructure to advance practice and outcomes of conservative kidney management,” they conclude.
Dr. Wong has no financial ties to industry. Dr. Tamura has reported receiving personal fees from the American Federation for Aging Research.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Antiseptic as good as antibiotics for preventing recurrent UTI
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.