User login
A review of the latest USPSTF recommendations
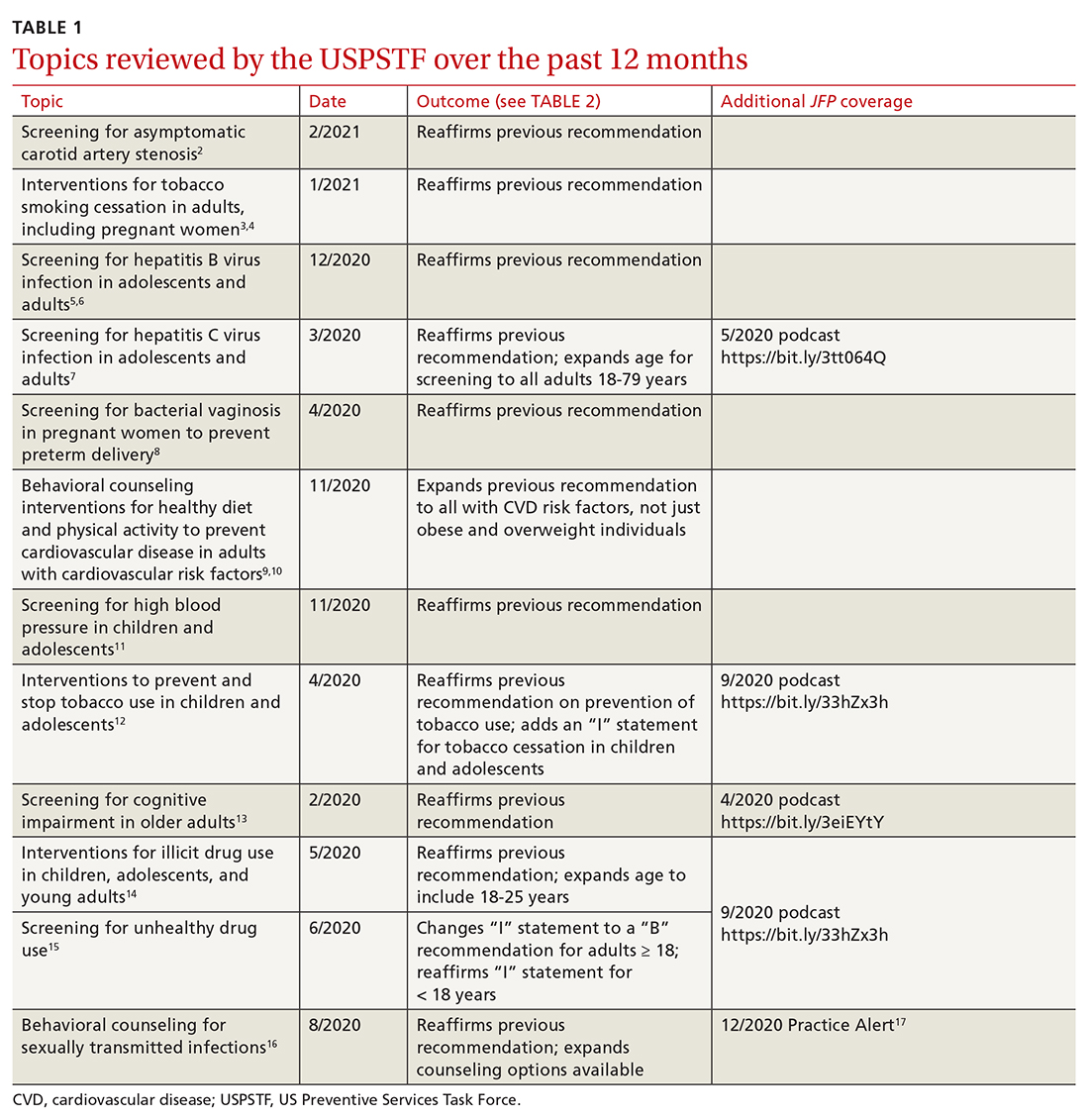
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.
Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Keep antibiotics unchanged in breakthrough UTIs
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
FROM PAS 2021
Finerenone scores second pivotal-trial success in patients with diabetic kidney disease
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
FDA approves dapagliflozin (Farxiga) for chronic kidney disease
The Food and Drug Administration has approved dapagliflozin (Farxiga, AstraZeneca) to reduce the risk for kidney function decline, kidney failure, cardiovascular death, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) at risk for disease progression.
“Chronic kidney disease is an important public health issue, and there is a significant unmet need for therapies that slow disease progression and improve outcomes,” said Aliza Thompson, MD, deputy director of the division of cardiology and nephrology at the FDA’s Center for Drug Evaluation and Research. “Today’s approval of Farxiga for the treatment of chronic kidney disease is an important step forward in helping people living with kidney disease.”
Dapagliflozin was approved in 2014 to improve glycemic control in patients with diabetes mellitus, and approval was expanded in 2020 to include treatment of patients with heart failure and reduced ejection fraction, based on results of the DAPA-HF trial.
This new approval in chronic kidney disease was based on results of the DAPA-CKD trial that was stopped early in March 2020 because of efficacy of the treatment.
DAPA-CKD randomly assigned 4,304 patients with CKD but without diabetes to receive either dapagliflozin or placebo. The full study results, reported at the 2020 annual congress of the European Society of Cardiology and simultaneously published in the New England Journal of Medicine, showed that, during a median of 2.4 years, treatment with dapagliflozin led to a significant 31% relative reduction, compared with placebo in the study’s primary outcome, a composite that included at least a 50% drop in estimated glomerular filtration rate, compared with baseline, end-stage kidney disease, kidney transplant, renal death, or cardiovascular death.
Dapagliflozin treatment also cut all-cause mortality by a statistically significant relative reduction of 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization.
“Farxiga was not studied, nor is expected to be effective, in treating chronic kidney disease among patients with autosomal dominant or recessive polycystic (characterized by multiple cysts) kidney disease or among patients who require or have recently used immunosuppressive therapy to treat kidney disease,” the FDA statement noted.
Dapagliflozin should not be used by patients with a history of serious hypersensitivity reactions to this medication, or who are on dialysis, the agency added. “Serious, life-threatening cases of Fournier’s Gangrene have occurred in patients with diabetes taking Farxiga.”
Patients should consider taking a lower dose of insulin or insulin secretagogue to reduce hypoglycemic risk if they are also taking dapagliflozin. Treatment can also cause dehydration, serious urinary tract infections, genital yeast infections, and metabolic acidosis, the announcement said. “Patients should be assessed for their volume status and kidney function before starting Farxiga.”
Dapagliflozin previously received Fast Track, Breakthrough Therapy, and Priority Review designations for this new indication.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dapagliflozin (Farxiga, AstraZeneca) to reduce the risk for kidney function decline, kidney failure, cardiovascular death, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) at risk for disease progression.
“Chronic kidney disease is an important public health issue, and there is a significant unmet need for therapies that slow disease progression and improve outcomes,” said Aliza Thompson, MD, deputy director of the division of cardiology and nephrology at the FDA’s Center for Drug Evaluation and Research. “Today’s approval of Farxiga for the treatment of chronic kidney disease is an important step forward in helping people living with kidney disease.”
Dapagliflozin was approved in 2014 to improve glycemic control in patients with diabetes mellitus, and approval was expanded in 2020 to include treatment of patients with heart failure and reduced ejection fraction, based on results of the DAPA-HF trial.
This new approval in chronic kidney disease was based on results of the DAPA-CKD trial that was stopped early in March 2020 because of efficacy of the treatment.
DAPA-CKD randomly assigned 4,304 patients with CKD but without diabetes to receive either dapagliflozin or placebo. The full study results, reported at the 2020 annual congress of the European Society of Cardiology and simultaneously published in the New England Journal of Medicine, showed that, during a median of 2.4 years, treatment with dapagliflozin led to a significant 31% relative reduction, compared with placebo in the study’s primary outcome, a composite that included at least a 50% drop in estimated glomerular filtration rate, compared with baseline, end-stage kidney disease, kidney transplant, renal death, or cardiovascular death.
Dapagliflozin treatment also cut all-cause mortality by a statistically significant relative reduction of 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization.
“Farxiga was not studied, nor is expected to be effective, in treating chronic kidney disease among patients with autosomal dominant or recessive polycystic (characterized by multiple cysts) kidney disease or among patients who require or have recently used immunosuppressive therapy to treat kidney disease,” the FDA statement noted.
Dapagliflozin should not be used by patients with a history of serious hypersensitivity reactions to this medication, or who are on dialysis, the agency added. “Serious, life-threatening cases of Fournier’s Gangrene have occurred in patients with diabetes taking Farxiga.”
Patients should consider taking a lower dose of insulin or insulin secretagogue to reduce hypoglycemic risk if they are also taking dapagliflozin. Treatment can also cause dehydration, serious urinary tract infections, genital yeast infections, and metabolic acidosis, the announcement said. “Patients should be assessed for their volume status and kidney function before starting Farxiga.”
Dapagliflozin previously received Fast Track, Breakthrough Therapy, and Priority Review designations for this new indication.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dapagliflozin (Farxiga, AstraZeneca) to reduce the risk for kidney function decline, kidney failure, cardiovascular death, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) at risk for disease progression.
“Chronic kidney disease is an important public health issue, and there is a significant unmet need for therapies that slow disease progression and improve outcomes,” said Aliza Thompson, MD, deputy director of the division of cardiology and nephrology at the FDA’s Center for Drug Evaluation and Research. “Today’s approval of Farxiga for the treatment of chronic kidney disease is an important step forward in helping people living with kidney disease.”
Dapagliflozin was approved in 2014 to improve glycemic control in patients with diabetes mellitus, and approval was expanded in 2020 to include treatment of patients with heart failure and reduced ejection fraction, based on results of the DAPA-HF trial.
This new approval in chronic kidney disease was based on results of the DAPA-CKD trial that was stopped early in March 2020 because of efficacy of the treatment.
DAPA-CKD randomly assigned 4,304 patients with CKD but without diabetes to receive either dapagliflozin or placebo. The full study results, reported at the 2020 annual congress of the European Society of Cardiology and simultaneously published in the New England Journal of Medicine, showed that, during a median of 2.4 years, treatment with dapagliflozin led to a significant 31% relative reduction, compared with placebo in the study’s primary outcome, a composite that included at least a 50% drop in estimated glomerular filtration rate, compared with baseline, end-stage kidney disease, kidney transplant, renal death, or cardiovascular death.
Dapagliflozin treatment also cut all-cause mortality by a statistically significant relative reduction of 31%, and another secondary-endpoint analysis showed a statistically significant 29% relative reduction in the rate of cardiovascular death or heart failure hospitalization.
“Farxiga was not studied, nor is expected to be effective, in treating chronic kidney disease among patients with autosomal dominant or recessive polycystic (characterized by multiple cysts) kidney disease or among patients who require or have recently used immunosuppressive therapy to treat kidney disease,” the FDA statement noted.
Dapagliflozin should not be used by patients with a history of serious hypersensitivity reactions to this medication, or who are on dialysis, the agency added. “Serious, life-threatening cases of Fournier’s Gangrene have occurred in patients with diabetes taking Farxiga.”
Patients should consider taking a lower dose of insulin or insulin secretagogue to reduce hypoglycemic risk if they are also taking dapagliflozin. Treatment can also cause dehydration, serious urinary tract infections, genital yeast infections, and metabolic acidosis, the announcement said. “Patients should be assessed for their volume status and kidney function before starting Farxiga.”
Dapagliflozin previously received Fast Track, Breakthrough Therapy, and Priority Review designations for this new indication.
A version of this article first appeared on Medscape.com.
Pregnancy increases risk for symptomatic kidney stones
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Half of patients in hospital for COVID-19 get acute kidney injury
in two independent, European case series presented recently at the International Society of Nephrology: 2021 World Congress. Many of the cases progressed to more severe, stage 3 AKI. Factors linked with incident AKI in the two reports included use of mechanical ventilation, vasopressors, or diuretics, and elevations in inflammatory markers.
The new findings confirm several U.S. reports published during the past year. In those reports, roughly a third of patients hospitalized for COVID-19 developed AKI during their hospital stay, said Jay L. Koyner, MD, during another renal conference, the National Kidney Foundation 2021 Spring Clinical Meetings.
Experience has shown it’s bad news when hospitalized COVID-19 patients develop AKI, which can prove fatal or can lead to the development or worsening of chronic kidney disease (CKD), which in some cases rapidly progresses to end-stage disease.
COVID-19 giving nephrologists an opportunity to improve AKI care
“COVID is giving us an opportunity to do a better job of taking care of patients who develop AKI, which is something that nephrologists have not often excelled at doing,” said Dr. Koyner, professor and director of the nephrology ICU at the University of Chicago.
“Many studies will look at how we can manage COVID-19 patients better after they develop AKI, because I suspect a large number of these patients will wind up with CKD,” Dr. Koyner said during his talk.
He cited several lessons from reports of AKI that occurs in patients hospitalized for COVID-19:
- Preexisting CKD, , and severe COVID-19 appear to be risk factors for developing COVID-related AKI.
- Patients who develop AKI during acutely severe COVID-19 may have slightly worse outcomes than patients without COVID-19 who develop AKI.
- Certain genetic susceptibilities may play a role in developing COVID-19–related AKI.
- Routine follow-up of AKI is generally inadequate and is not standardized, whether AKI develops while ill with COVID-19 or in other settings.
The most encouraging AKI takeaway from COVID-19’s first year is that its incidence among patients hospitalized with COVID-19 appears to have dropped from very high rates early on, possibly because of more routine use of steroids for critically ill patients with COVID-19 and a reduction in the use of ventilators, Dr. Koyner suggested.
In-hospital diuretic treatment links with AKI
One of the World Congress of Nephrology reports involved 1,248 patients admitted with confirmed COVID-19 at two tertiary care hospitals in London during March–May 2020. The average age of the patients was 69 years, 59% were men, and 17% had CKD at admission, as determined on the basis of estimated glomerular filtration rate <60 mL/min per 1.73 m2.
During hospitalization, 487 patients (39%) developed AKI, including 175 (14%) with stage 3 AKI and 109 (9%) who required renal replacement therapy (dialysis or kidney transplant). The incidence of AKI peaked 5 weeks after COVID-19 admission, Paul Jewell and associates from King’s College Hospital, London, reported in a poster.
Multivariate analysis identified several demographic and clinical variables that were significantly linked with an increased risk of developing AKI: male sex (which boosted risk by 55%), Black race (79% higher risk), CKD at admission (triple the risk), being hypertensive on admission (73% higher risk), and being administered diuretics during hospitalization (69% higher risk).
The findings of a risk linked with diuretic use “supports the cautious use of diuretics in patients hospitalized with COVID-19, especially in the presence of background renal impairment,” the authors said.
For patients with incident AKI, the 30-day mortality rate was significantly increased; mortality was 59% higher among patients who developed stage 1 AKI and was roughly triple among patients who developed stage 2 or 3 AKI.
Second report links ventilation, vasopressors with worse AKI
A separate report from clinicians at Charité Hospital, Berlin, retrospectively analyzed 223 patients admitted with symptomatic COVID-19 to three Charité sites during March–June 2020. During hospitalization, 117 patients (52%) developed AKI, including 70 (31%) with stage 3 disease; 67 (30%) required renal replacement therapy. Half the patients with stage 3 AKI required ICU admission.
Compared with patients with less severe AKI, patients who developed stage 3 AKI were more often male, older, and had a higher body mass index.
In a multivariate model, compared with patients who developed less severe AKI, those who developed stage 3 disease also were significantly more likely to have received mechanical ventilation or vasopressor drugs and were more likely to have increased levels of leukocytes or procalcitonin, two inflammatory markers, Jan-Hendrink B. Hardenburg, MD, a Charité nephrologist, and associates reported in a poster at the meeting.
Mechanical ventilation was linked with a sixfold higher rate of stage 3 AKI, and treatment with vasopressor drugs was linked with a threefold higher rate. Elevations in procalcitonin or leukocyte levels were linked with about 60% increases in rates of stage 3 AKI. For both of these risk factors, temporal relationships were tighter; increases in both values appeared just before onset of stage 3 disease.
Dr. Joyner has been a speaker on behalf of NXStage Medical; a consultant to Astute Medical, Baxter, Mallinckrodt, Pfizer, and Sphingotec; and he has received research funding from Astute, Bioporto, NxStage, and Satellite Healthcare. Mr. Jewell and Dr. Hardenburg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in two independent, European case series presented recently at the International Society of Nephrology: 2021 World Congress. Many of the cases progressed to more severe, stage 3 AKI. Factors linked with incident AKI in the two reports included use of mechanical ventilation, vasopressors, or diuretics, and elevations in inflammatory markers.
The new findings confirm several U.S. reports published during the past year. In those reports, roughly a third of patients hospitalized for COVID-19 developed AKI during their hospital stay, said Jay L. Koyner, MD, during another renal conference, the National Kidney Foundation 2021 Spring Clinical Meetings.
Experience has shown it’s bad news when hospitalized COVID-19 patients develop AKI, which can prove fatal or can lead to the development or worsening of chronic kidney disease (CKD), which in some cases rapidly progresses to end-stage disease.
COVID-19 giving nephrologists an opportunity to improve AKI care
“COVID is giving us an opportunity to do a better job of taking care of patients who develop AKI, which is something that nephrologists have not often excelled at doing,” said Dr. Koyner, professor and director of the nephrology ICU at the University of Chicago.
“Many studies will look at how we can manage COVID-19 patients better after they develop AKI, because I suspect a large number of these patients will wind up with CKD,” Dr. Koyner said during his talk.
He cited several lessons from reports of AKI that occurs in patients hospitalized for COVID-19:
- Preexisting CKD, , and severe COVID-19 appear to be risk factors for developing COVID-related AKI.
- Patients who develop AKI during acutely severe COVID-19 may have slightly worse outcomes than patients without COVID-19 who develop AKI.
- Certain genetic susceptibilities may play a role in developing COVID-19–related AKI.
- Routine follow-up of AKI is generally inadequate and is not standardized, whether AKI develops while ill with COVID-19 or in other settings.
The most encouraging AKI takeaway from COVID-19’s first year is that its incidence among patients hospitalized with COVID-19 appears to have dropped from very high rates early on, possibly because of more routine use of steroids for critically ill patients with COVID-19 and a reduction in the use of ventilators, Dr. Koyner suggested.
In-hospital diuretic treatment links with AKI
One of the World Congress of Nephrology reports involved 1,248 patients admitted with confirmed COVID-19 at two tertiary care hospitals in London during March–May 2020. The average age of the patients was 69 years, 59% were men, and 17% had CKD at admission, as determined on the basis of estimated glomerular filtration rate <60 mL/min per 1.73 m2.
During hospitalization, 487 patients (39%) developed AKI, including 175 (14%) with stage 3 AKI and 109 (9%) who required renal replacement therapy (dialysis or kidney transplant). The incidence of AKI peaked 5 weeks after COVID-19 admission, Paul Jewell and associates from King’s College Hospital, London, reported in a poster.
Multivariate analysis identified several demographic and clinical variables that were significantly linked with an increased risk of developing AKI: male sex (which boosted risk by 55%), Black race (79% higher risk), CKD at admission (triple the risk), being hypertensive on admission (73% higher risk), and being administered diuretics during hospitalization (69% higher risk).
The findings of a risk linked with diuretic use “supports the cautious use of diuretics in patients hospitalized with COVID-19, especially in the presence of background renal impairment,” the authors said.
For patients with incident AKI, the 30-day mortality rate was significantly increased; mortality was 59% higher among patients who developed stage 1 AKI and was roughly triple among patients who developed stage 2 or 3 AKI.
Second report links ventilation, vasopressors with worse AKI
A separate report from clinicians at Charité Hospital, Berlin, retrospectively analyzed 223 patients admitted with symptomatic COVID-19 to three Charité sites during March–June 2020. During hospitalization, 117 patients (52%) developed AKI, including 70 (31%) with stage 3 disease; 67 (30%) required renal replacement therapy. Half the patients with stage 3 AKI required ICU admission.
Compared with patients with less severe AKI, patients who developed stage 3 AKI were more often male, older, and had a higher body mass index.
In a multivariate model, compared with patients who developed less severe AKI, those who developed stage 3 disease also were significantly more likely to have received mechanical ventilation or vasopressor drugs and were more likely to have increased levels of leukocytes or procalcitonin, two inflammatory markers, Jan-Hendrink B. Hardenburg, MD, a Charité nephrologist, and associates reported in a poster at the meeting.
Mechanical ventilation was linked with a sixfold higher rate of stage 3 AKI, and treatment with vasopressor drugs was linked with a threefold higher rate. Elevations in procalcitonin or leukocyte levels were linked with about 60% increases in rates of stage 3 AKI. For both of these risk factors, temporal relationships were tighter; increases in both values appeared just before onset of stage 3 disease.
Dr. Joyner has been a speaker on behalf of NXStage Medical; a consultant to Astute Medical, Baxter, Mallinckrodt, Pfizer, and Sphingotec; and he has received research funding from Astute, Bioporto, NxStage, and Satellite Healthcare. Mr. Jewell and Dr. Hardenburg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in two independent, European case series presented recently at the International Society of Nephrology: 2021 World Congress. Many of the cases progressed to more severe, stage 3 AKI. Factors linked with incident AKI in the two reports included use of mechanical ventilation, vasopressors, or diuretics, and elevations in inflammatory markers.
The new findings confirm several U.S. reports published during the past year. In those reports, roughly a third of patients hospitalized for COVID-19 developed AKI during their hospital stay, said Jay L. Koyner, MD, during another renal conference, the National Kidney Foundation 2021 Spring Clinical Meetings.
Experience has shown it’s bad news when hospitalized COVID-19 patients develop AKI, which can prove fatal or can lead to the development or worsening of chronic kidney disease (CKD), which in some cases rapidly progresses to end-stage disease.
COVID-19 giving nephrologists an opportunity to improve AKI care
“COVID is giving us an opportunity to do a better job of taking care of patients who develop AKI, which is something that nephrologists have not often excelled at doing,” said Dr. Koyner, professor and director of the nephrology ICU at the University of Chicago.
“Many studies will look at how we can manage COVID-19 patients better after they develop AKI, because I suspect a large number of these patients will wind up with CKD,” Dr. Koyner said during his talk.
He cited several lessons from reports of AKI that occurs in patients hospitalized for COVID-19:
- Preexisting CKD, , and severe COVID-19 appear to be risk factors for developing COVID-related AKI.
- Patients who develop AKI during acutely severe COVID-19 may have slightly worse outcomes than patients without COVID-19 who develop AKI.
- Certain genetic susceptibilities may play a role in developing COVID-19–related AKI.
- Routine follow-up of AKI is generally inadequate and is not standardized, whether AKI develops while ill with COVID-19 or in other settings.
The most encouraging AKI takeaway from COVID-19’s first year is that its incidence among patients hospitalized with COVID-19 appears to have dropped from very high rates early on, possibly because of more routine use of steroids for critically ill patients with COVID-19 and a reduction in the use of ventilators, Dr. Koyner suggested.
In-hospital diuretic treatment links with AKI
One of the World Congress of Nephrology reports involved 1,248 patients admitted with confirmed COVID-19 at two tertiary care hospitals in London during March–May 2020. The average age of the patients was 69 years, 59% were men, and 17% had CKD at admission, as determined on the basis of estimated glomerular filtration rate <60 mL/min per 1.73 m2.
During hospitalization, 487 patients (39%) developed AKI, including 175 (14%) with stage 3 AKI and 109 (9%) who required renal replacement therapy (dialysis or kidney transplant). The incidence of AKI peaked 5 weeks after COVID-19 admission, Paul Jewell and associates from King’s College Hospital, London, reported in a poster.
Multivariate analysis identified several demographic and clinical variables that were significantly linked with an increased risk of developing AKI: male sex (which boosted risk by 55%), Black race (79% higher risk), CKD at admission (triple the risk), being hypertensive on admission (73% higher risk), and being administered diuretics during hospitalization (69% higher risk).
The findings of a risk linked with diuretic use “supports the cautious use of diuretics in patients hospitalized with COVID-19, especially in the presence of background renal impairment,” the authors said.
For patients with incident AKI, the 30-day mortality rate was significantly increased; mortality was 59% higher among patients who developed stage 1 AKI and was roughly triple among patients who developed stage 2 or 3 AKI.
Second report links ventilation, vasopressors with worse AKI
A separate report from clinicians at Charité Hospital, Berlin, retrospectively analyzed 223 patients admitted with symptomatic COVID-19 to three Charité sites during March–June 2020. During hospitalization, 117 patients (52%) developed AKI, including 70 (31%) with stage 3 disease; 67 (30%) required renal replacement therapy. Half the patients with stage 3 AKI required ICU admission.
Compared with patients with less severe AKI, patients who developed stage 3 AKI were more often male, older, and had a higher body mass index.
In a multivariate model, compared with patients who developed less severe AKI, those who developed stage 3 disease also were significantly more likely to have received mechanical ventilation or vasopressor drugs and were more likely to have increased levels of leukocytes or procalcitonin, two inflammatory markers, Jan-Hendrink B. Hardenburg, MD, a Charité nephrologist, and associates reported in a poster at the meeting.
Mechanical ventilation was linked with a sixfold higher rate of stage 3 AKI, and treatment with vasopressor drugs was linked with a threefold higher rate. Elevations in procalcitonin or leukocyte levels were linked with about 60% increases in rates of stage 3 AKI. For both of these risk factors, temporal relationships were tighter; increases in both values appeared just before onset of stage 3 disease.
Dr. Joyner has been a speaker on behalf of NXStage Medical; a consultant to Astute Medical, Baxter, Mallinckrodt, Pfizer, and Sphingotec; and he has received research funding from Astute, Bioporto, NxStage, and Satellite Healthcare. Mr. Jewell and Dr. Hardenburg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Thyroid hormone analogues can reverse NASH
Background: Fat toxicity results in inflammation of the liver and eventual hepatic fibrosis and cirrhosis. Thyroid hormones can greatly reduce this hepatic steatosis by restoring metabolic pathways in damaged liver, prevent fibrosis progression, and have broad atherogenic lipid-lowering actions by activating hepatic thyroid beta-receptors.
However, hyperthyroidism also leads to osteoporosis, tachyarrhythmias, muscle wasting, and psychiatric side effects, mediated by the alpha-thyroid receptor. Resmetirom (MGL-3196) is a novel, highly selective thyroid beta-agonist, with a minimal side-effect profile, which avoids the alpha–side effects.
Study design: Randomized, double-blind, placebo-controlled study.
Setting: 25 centers in the United States.
Synopsis: Of 125 adults with NASH fibrosis 1-3 and greater than 10% hepatic fat, 84 received resmetirom and 41 received placebo. Resmetirom resulted in a nearly 30% decrease over placebo in hepatic fat, compared with baseline, significant improvement in lipid profile, improvement in liver enzymes, fibrosis markers, and histologic resolution of NASH in some patients.
While the study showed resolution of inflammation, the 36-week study was likely not long enough to show improvement of fibrosis. The relatively small sample size also limited results. Placebo patients who lost significant weight also showed improvement and were discarded from analysis, suggesting that weight loss itself is also an excellent alternative to reverse NASH. Resmetirom use in NASH is now moving into a large phase 3 trial.
Bottom line: Resmetirom results in major liver and cardiovascular benefits in patients with NASH.
Citation: Harrison SA et al. Resmetirom (MGL-3196) for the treatment of nonalcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019 Nov 11;394(10213):2012-24.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Fat toxicity results in inflammation of the liver and eventual hepatic fibrosis and cirrhosis. Thyroid hormones can greatly reduce this hepatic steatosis by restoring metabolic pathways in damaged liver, prevent fibrosis progression, and have broad atherogenic lipid-lowering actions by activating hepatic thyroid beta-receptors.
However, hyperthyroidism also leads to osteoporosis, tachyarrhythmias, muscle wasting, and psychiatric side effects, mediated by the alpha-thyroid receptor. Resmetirom (MGL-3196) is a novel, highly selective thyroid beta-agonist, with a minimal side-effect profile, which avoids the alpha–side effects.
Study design: Randomized, double-blind, placebo-controlled study.
Setting: 25 centers in the United States.
Synopsis: Of 125 adults with NASH fibrosis 1-3 and greater than 10% hepatic fat, 84 received resmetirom and 41 received placebo. Resmetirom resulted in a nearly 30% decrease over placebo in hepatic fat, compared with baseline, significant improvement in lipid profile, improvement in liver enzymes, fibrosis markers, and histologic resolution of NASH in some patients.
While the study showed resolution of inflammation, the 36-week study was likely not long enough to show improvement of fibrosis. The relatively small sample size also limited results. Placebo patients who lost significant weight also showed improvement and were discarded from analysis, suggesting that weight loss itself is also an excellent alternative to reverse NASH. Resmetirom use in NASH is now moving into a large phase 3 trial.
Bottom line: Resmetirom results in major liver and cardiovascular benefits in patients with NASH.
Citation: Harrison SA et al. Resmetirom (MGL-3196) for the treatment of nonalcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019 Nov 11;394(10213):2012-24.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Fat toxicity results in inflammation of the liver and eventual hepatic fibrosis and cirrhosis. Thyroid hormones can greatly reduce this hepatic steatosis by restoring metabolic pathways in damaged liver, prevent fibrosis progression, and have broad atherogenic lipid-lowering actions by activating hepatic thyroid beta-receptors.
However, hyperthyroidism also leads to osteoporosis, tachyarrhythmias, muscle wasting, and psychiatric side effects, mediated by the alpha-thyroid receptor. Resmetirom (MGL-3196) is a novel, highly selective thyroid beta-agonist, with a minimal side-effect profile, which avoids the alpha–side effects.
Study design: Randomized, double-blind, placebo-controlled study.
Setting: 25 centers in the United States.
Synopsis: Of 125 adults with NASH fibrosis 1-3 and greater than 10% hepatic fat, 84 received resmetirom and 41 received placebo. Resmetirom resulted in a nearly 30% decrease over placebo in hepatic fat, compared with baseline, significant improvement in lipid profile, improvement in liver enzymes, fibrosis markers, and histologic resolution of NASH in some patients.
While the study showed resolution of inflammation, the 36-week study was likely not long enough to show improvement of fibrosis. The relatively small sample size also limited results. Placebo patients who lost significant weight also showed improvement and were discarded from analysis, suggesting that weight loss itself is also an excellent alternative to reverse NASH. Resmetirom use in NASH is now moving into a large phase 3 trial.
Bottom line: Resmetirom results in major liver and cardiovascular benefits in patients with NASH.
Citation: Harrison SA et al. Resmetirom (MGL-3196) for the treatment of nonalcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019 Nov 11;394(10213):2012-24.
Dr. Raghavan is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Patient benefits justify price of new lupus nephritis drugs
The prices of two new drugs that have been approved by the Food and Drug Administration for the treatment of lupus nephritis are in “reasonable alignment” with the drugs’ estimated benefits for patients with the disease, the Institute for Clinical and Economic Review has determined.
“Both belimumab [Benlysta] and voclosporin [Lupkynis] are important new treatment options,” Steven Pearson, MD, president of ICER, observed in a summary of the report’s findings.
“Despite remaining uncertainty about both treatments’ longer-term outcomes, their estimated net prices appear to be aligned with their anticipated clinical benefits. ... For patients and clinicians to have responsibly priced options specifically indicated for lupus nephritis is a win for patients and the entire health system,” Dr. Pearson added.
The estimated annual price of belimumab is approximately $43,000 per patient; the estimated annual price for voclosporin is approximately $92,000 per patient.
The incremental cost-effectiveness ratio for belimumab is approximately $90,0000 per quality-adjusted life-year; the corresponding value for voclosporin is higher, at approximately $149,000 per QALY, the ICER authors noted.
The report was published by ICER in April 2021.
Large unmet need for treatment of lupus nephritis
In their report, the ICER reviewed belimumab, a parenteral B-lymphocyte inhibitor, as well as voclosporin, an oral calcineurin inhibitor, as initial treatment of patients with lupus nephritis. Lupus nephritis is a serious complication of systemic lupus erythematosus (SLE).
Belimumab was first approved for the treatment of lupus in adults in the United States in March 2011. In April 2019, it was approved for use for the same indication for children aged 5 years and older. The FDA expanded the indication in December 2020 to include adults with active lupus nephritis who are receiving standard therapy.
Voclosporin was approved for the treatment of lupus nephritis in January 2021.
In the pivotal trials for the two agents, each drug was added to standard induction therapy for lupus nephritis, which consisted of high-dose corticosteroids combined with either mycophenolate mofetil (MMF) or cyclophosphamide.
Compared with standard therapy alone, belimumab increased the complete renal response and the primary efficacy renal response at 2 years. With voclosporin, complete response was nearly doubled, and there was marked increased in partial response at 1 year, compared with standard therapy alone.
Neither drug appeared to increase the adverse-event rate or the rate of discontinuations, compared with standard therapy, although the FDA did add a black box warning regarding the possible risk for serious infections and malignancies with voclosporin use.
“There is a very large unmet need for the treatment of lupus nephritis,” Chris Phillips, MD, of Paducah (Ky.) Rheumatology said in an interview.
“A very large percentage of patients who do not achieve complete remission on traditional treatments have side effects or contraindications to these treatments, so we’ve needed new ones for sure,” he stressed.
The ICER authors made it clear that there is considerable uncertainty as to how short-term assessment of each of the two drugs’ performance might translate into meaningful long-term outcomes for patients, especially given that SLE is a lifelong illness.
On the other hand, “there are a lot of attributes for both of these new drugs that suggest there is potential for kidney benefit over time,” Brad Rovin, MD, professor of medicine and pathology at the Ohio State University Wexner Medical Center, Columbus, said in an interview.
For example, data from the BLISS-LN study, reported by Dr. Rovin during a meeting last year, suggest that belimumab reduces the flare rate and appears to stabilize kidney function over time, compared with standard therapy alone.
“BLISS-LN was 2 years long, so it gave us an opportunity to look at kidney function over a longer period of time than most of our prior trials in lupus nephritis,” he explained.
“The stabilization of kidney function is important, because it suggests that belimumab has a kidney protective effect, while a decrease in lupus nephritis flares is also important, because each time the disease flares, you can accumulate chronic tissue damage, which can eventually cause end-stage renal disease [ESRD],” he said.
Dr. Rovin also pointed out that the BLISS-LN trial results indicate that patients who achieve a urine protein level less than 700 mg/d after the first year of treatment do very well on long-term follow-up – another hint that belimumab may have long-term benefits for kidney function.
Voclosporin is a calcineurin inhibitor, which are protective of podocytes. “When you start to lose too many podocytes, the kidney can again progress onto ESRD, so this is again an extra benefit of the calcineurin inhibitors in the context of kidney disease that affects the glomeruli,” he noted.
“So both of these drugs have these interesting attributes that go beyond, or at least are maybe tied to, their immunosuppressive actions, but they do offer some kidney protective effects,” he reaffirmed.
Black patients underrepresented in trials
The ICER authors voiced concern over the fact that individuals most at risk for SLE – mostly Black patients, but also patients of other racial groups – were underrepresented in clinical trials that evaluated both agents.
“We cannot stress enough that the results are highly uncertain due to the small numbers of Black patients in the available clinical trials and the lack of data on differences among subgroups in long-term outcomes,” they stated.
This is not an academic issue, Dr. Phillips pointed out. Responses to both MMF and cyclophosphamide differ among persons of different races, “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
This is not an academic issue, Dr. Phillips said, because there are racial disparities in how patients respond to both MMF and cyclophosphamide – “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
The ICER authors appear to agree. They urged the manufacturers of the two new agents to expand their research to include adequate representation of lupus nephritis patients from Black and other non-White communities.
However, it is somewhat reassuring that the pivotal voclosporin trial enrolled about 30% of Hispanic patients and that about 17% of participants in the BLISS-LN trial were also Hispanic, Dr. Rovin pointed out.
This is important because Hispanic patients can have very aggressive disease, as can Black patients, he noted. There is some evidence to suggest both drugs are effective in aggressive disease.
The ICER also pointed out that the length of time that both drugs can be used prior to tapering of treatment, after which patients receive standard maintenance therapy alone, has yet to be established.
This is important, Dr. Rovin and Dr. Phillips agreed, because calcineurin inhibitors are known to be nephrotoxic, and both drugs are immunosuppressive. At least with respect to voclosporin, there is some cause of concern regarding prolonged use of the drug for patients with kidney disease.
“We don’t want patients to be on an immunosuppressive drug forever if they don’t need to be,” Dr. Rovin emphasized.
“But we are seeing really long-term remission in the setting of other inflammatory diseases, like vasculitis with rituximab. So there is hope that we can achieve the same thing in lupus. If we use drugs that target T cells in the immune system, like voclosporin, or B cells, like belimumab, maybe we can ‘reset’ the immune system and get rid of potentially autoreactive cells that could allow long-lasting disease remission, which is an unanswered question but an intriguing possibility,” he concluded.
Dr. Rovin has served as a consultant for GlaxoSmithKline. Dr. Phillips disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prices of two new drugs that have been approved by the Food and Drug Administration for the treatment of lupus nephritis are in “reasonable alignment” with the drugs’ estimated benefits for patients with the disease, the Institute for Clinical and Economic Review has determined.
“Both belimumab [Benlysta] and voclosporin [Lupkynis] are important new treatment options,” Steven Pearson, MD, president of ICER, observed in a summary of the report’s findings.
“Despite remaining uncertainty about both treatments’ longer-term outcomes, their estimated net prices appear to be aligned with their anticipated clinical benefits. ... For patients and clinicians to have responsibly priced options specifically indicated for lupus nephritis is a win for patients and the entire health system,” Dr. Pearson added.
The estimated annual price of belimumab is approximately $43,000 per patient; the estimated annual price for voclosporin is approximately $92,000 per patient.
The incremental cost-effectiveness ratio for belimumab is approximately $90,0000 per quality-adjusted life-year; the corresponding value for voclosporin is higher, at approximately $149,000 per QALY, the ICER authors noted.
The report was published by ICER in April 2021.
Large unmet need for treatment of lupus nephritis
In their report, the ICER reviewed belimumab, a parenteral B-lymphocyte inhibitor, as well as voclosporin, an oral calcineurin inhibitor, as initial treatment of patients with lupus nephritis. Lupus nephritis is a serious complication of systemic lupus erythematosus (SLE).
Belimumab was first approved for the treatment of lupus in adults in the United States in March 2011. In April 2019, it was approved for use for the same indication for children aged 5 years and older. The FDA expanded the indication in December 2020 to include adults with active lupus nephritis who are receiving standard therapy.
Voclosporin was approved for the treatment of lupus nephritis in January 2021.
In the pivotal trials for the two agents, each drug was added to standard induction therapy for lupus nephritis, which consisted of high-dose corticosteroids combined with either mycophenolate mofetil (MMF) or cyclophosphamide.
Compared with standard therapy alone, belimumab increased the complete renal response and the primary efficacy renal response at 2 years. With voclosporin, complete response was nearly doubled, and there was marked increased in partial response at 1 year, compared with standard therapy alone.
Neither drug appeared to increase the adverse-event rate or the rate of discontinuations, compared with standard therapy, although the FDA did add a black box warning regarding the possible risk for serious infections and malignancies with voclosporin use.
“There is a very large unmet need for the treatment of lupus nephritis,” Chris Phillips, MD, of Paducah (Ky.) Rheumatology said in an interview.
“A very large percentage of patients who do not achieve complete remission on traditional treatments have side effects or contraindications to these treatments, so we’ve needed new ones for sure,” he stressed.
The ICER authors made it clear that there is considerable uncertainty as to how short-term assessment of each of the two drugs’ performance might translate into meaningful long-term outcomes for patients, especially given that SLE is a lifelong illness.
On the other hand, “there are a lot of attributes for both of these new drugs that suggest there is potential for kidney benefit over time,” Brad Rovin, MD, professor of medicine and pathology at the Ohio State University Wexner Medical Center, Columbus, said in an interview.
For example, data from the BLISS-LN study, reported by Dr. Rovin during a meeting last year, suggest that belimumab reduces the flare rate and appears to stabilize kidney function over time, compared with standard therapy alone.
“BLISS-LN was 2 years long, so it gave us an opportunity to look at kidney function over a longer period of time than most of our prior trials in lupus nephritis,” he explained.
“The stabilization of kidney function is important, because it suggests that belimumab has a kidney protective effect, while a decrease in lupus nephritis flares is also important, because each time the disease flares, you can accumulate chronic tissue damage, which can eventually cause end-stage renal disease [ESRD],” he said.
Dr. Rovin also pointed out that the BLISS-LN trial results indicate that patients who achieve a urine protein level less than 700 mg/d after the first year of treatment do very well on long-term follow-up – another hint that belimumab may have long-term benefits for kidney function.
Voclosporin is a calcineurin inhibitor, which are protective of podocytes. “When you start to lose too many podocytes, the kidney can again progress onto ESRD, so this is again an extra benefit of the calcineurin inhibitors in the context of kidney disease that affects the glomeruli,” he noted.
“So both of these drugs have these interesting attributes that go beyond, or at least are maybe tied to, their immunosuppressive actions, but they do offer some kidney protective effects,” he reaffirmed.
Black patients underrepresented in trials
The ICER authors voiced concern over the fact that individuals most at risk for SLE – mostly Black patients, but also patients of other racial groups – were underrepresented in clinical trials that evaluated both agents.
“We cannot stress enough that the results are highly uncertain due to the small numbers of Black patients in the available clinical trials and the lack of data on differences among subgroups in long-term outcomes,” they stated.
This is not an academic issue, Dr. Phillips pointed out. Responses to both MMF and cyclophosphamide differ among persons of different races, “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
This is not an academic issue, Dr. Phillips said, because there are racial disparities in how patients respond to both MMF and cyclophosphamide – “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
The ICER authors appear to agree. They urged the manufacturers of the two new agents to expand their research to include adequate representation of lupus nephritis patients from Black and other non-White communities.
However, it is somewhat reassuring that the pivotal voclosporin trial enrolled about 30% of Hispanic patients and that about 17% of participants in the BLISS-LN trial were also Hispanic, Dr. Rovin pointed out.
This is important because Hispanic patients can have very aggressive disease, as can Black patients, he noted. There is some evidence to suggest both drugs are effective in aggressive disease.
The ICER also pointed out that the length of time that both drugs can be used prior to tapering of treatment, after which patients receive standard maintenance therapy alone, has yet to be established.
This is important, Dr. Rovin and Dr. Phillips agreed, because calcineurin inhibitors are known to be nephrotoxic, and both drugs are immunosuppressive. At least with respect to voclosporin, there is some cause of concern regarding prolonged use of the drug for patients with kidney disease.
“We don’t want patients to be on an immunosuppressive drug forever if they don’t need to be,” Dr. Rovin emphasized.
“But we are seeing really long-term remission in the setting of other inflammatory diseases, like vasculitis with rituximab. So there is hope that we can achieve the same thing in lupus. If we use drugs that target T cells in the immune system, like voclosporin, or B cells, like belimumab, maybe we can ‘reset’ the immune system and get rid of potentially autoreactive cells that could allow long-lasting disease remission, which is an unanswered question but an intriguing possibility,” he concluded.
Dr. Rovin has served as a consultant for GlaxoSmithKline. Dr. Phillips disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prices of two new drugs that have been approved by the Food and Drug Administration for the treatment of lupus nephritis are in “reasonable alignment” with the drugs’ estimated benefits for patients with the disease, the Institute for Clinical and Economic Review has determined.
“Both belimumab [Benlysta] and voclosporin [Lupkynis] are important new treatment options,” Steven Pearson, MD, president of ICER, observed in a summary of the report’s findings.
“Despite remaining uncertainty about both treatments’ longer-term outcomes, their estimated net prices appear to be aligned with their anticipated clinical benefits. ... For patients and clinicians to have responsibly priced options specifically indicated for lupus nephritis is a win for patients and the entire health system,” Dr. Pearson added.
The estimated annual price of belimumab is approximately $43,000 per patient; the estimated annual price for voclosporin is approximately $92,000 per patient.
The incremental cost-effectiveness ratio for belimumab is approximately $90,0000 per quality-adjusted life-year; the corresponding value for voclosporin is higher, at approximately $149,000 per QALY, the ICER authors noted.
The report was published by ICER in April 2021.
Large unmet need for treatment of lupus nephritis
In their report, the ICER reviewed belimumab, a parenteral B-lymphocyte inhibitor, as well as voclosporin, an oral calcineurin inhibitor, as initial treatment of patients with lupus nephritis. Lupus nephritis is a serious complication of systemic lupus erythematosus (SLE).
Belimumab was first approved for the treatment of lupus in adults in the United States in March 2011. In April 2019, it was approved for use for the same indication for children aged 5 years and older. The FDA expanded the indication in December 2020 to include adults with active lupus nephritis who are receiving standard therapy.
Voclosporin was approved for the treatment of lupus nephritis in January 2021.
In the pivotal trials for the two agents, each drug was added to standard induction therapy for lupus nephritis, which consisted of high-dose corticosteroids combined with either mycophenolate mofetil (MMF) or cyclophosphamide.
Compared with standard therapy alone, belimumab increased the complete renal response and the primary efficacy renal response at 2 years. With voclosporin, complete response was nearly doubled, and there was marked increased in partial response at 1 year, compared with standard therapy alone.
Neither drug appeared to increase the adverse-event rate or the rate of discontinuations, compared with standard therapy, although the FDA did add a black box warning regarding the possible risk for serious infections and malignancies with voclosporin use.
“There is a very large unmet need for the treatment of lupus nephritis,” Chris Phillips, MD, of Paducah (Ky.) Rheumatology said in an interview.
“A very large percentage of patients who do not achieve complete remission on traditional treatments have side effects or contraindications to these treatments, so we’ve needed new ones for sure,” he stressed.
The ICER authors made it clear that there is considerable uncertainty as to how short-term assessment of each of the two drugs’ performance might translate into meaningful long-term outcomes for patients, especially given that SLE is a lifelong illness.
On the other hand, “there are a lot of attributes for both of these new drugs that suggest there is potential for kidney benefit over time,” Brad Rovin, MD, professor of medicine and pathology at the Ohio State University Wexner Medical Center, Columbus, said in an interview.
For example, data from the BLISS-LN study, reported by Dr. Rovin during a meeting last year, suggest that belimumab reduces the flare rate and appears to stabilize kidney function over time, compared with standard therapy alone.
“BLISS-LN was 2 years long, so it gave us an opportunity to look at kidney function over a longer period of time than most of our prior trials in lupus nephritis,” he explained.
“The stabilization of kidney function is important, because it suggests that belimumab has a kidney protective effect, while a decrease in lupus nephritis flares is also important, because each time the disease flares, you can accumulate chronic tissue damage, which can eventually cause end-stage renal disease [ESRD],” he said.
Dr. Rovin also pointed out that the BLISS-LN trial results indicate that patients who achieve a urine protein level less than 700 mg/d after the first year of treatment do very well on long-term follow-up – another hint that belimumab may have long-term benefits for kidney function.
Voclosporin is a calcineurin inhibitor, which are protective of podocytes. “When you start to lose too many podocytes, the kidney can again progress onto ESRD, so this is again an extra benefit of the calcineurin inhibitors in the context of kidney disease that affects the glomeruli,” he noted.
“So both of these drugs have these interesting attributes that go beyond, or at least are maybe tied to, their immunosuppressive actions, but they do offer some kidney protective effects,” he reaffirmed.
Black patients underrepresented in trials
The ICER authors voiced concern over the fact that individuals most at risk for SLE – mostly Black patients, but also patients of other racial groups – were underrepresented in clinical trials that evaluated both agents.
“We cannot stress enough that the results are highly uncertain due to the small numbers of Black patients in the available clinical trials and the lack of data on differences among subgroups in long-term outcomes,” they stated.
This is not an academic issue, Dr. Phillips pointed out. Responses to both MMF and cyclophosphamide differ among persons of different races, “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
This is not an academic issue, Dr. Phillips said, because there are racial disparities in how patients respond to both MMF and cyclophosphamide – “so it’s not unreasonable to consider that there could be racial differences in treatment responses to both drugs, and these definitely need to be investigated.”
The ICER authors appear to agree. They urged the manufacturers of the two new agents to expand their research to include adequate representation of lupus nephritis patients from Black and other non-White communities.
However, it is somewhat reassuring that the pivotal voclosporin trial enrolled about 30% of Hispanic patients and that about 17% of participants in the BLISS-LN trial were also Hispanic, Dr. Rovin pointed out.
This is important because Hispanic patients can have very aggressive disease, as can Black patients, he noted. There is some evidence to suggest both drugs are effective in aggressive disease.
The ICER also pointed out that the length of time that both drugs can be used prior to tapering of treatment, after which patients receive standard maintenance therapy alone, has yet to be established.
This is important, Dr. Rovin and Dr. Phillips agreed, because calcineurin inhibitors are known to be nephrotoxic, and both drugs are immunosuppressive. At least with respect to voclosporin, there is some cause of concern regarding prolonged use of the drug for patients with kidney disease.
“We don’t want patients to be on an immunosuppressive drug forever if they don’t need to be,” Dr. Rovin emphasized.
“But we are seeing really long-term remission in the setting of other inflammatory diseases, like vasculitis with rituximab. So there is hope that we can achieve the same thing in lupus. If we use drugs that target T cells in the immune system, like voclosporin, or B cells, like belimumab, maybe we can ‘reset’ the immune system and get rid of potentially autoreactive cells that could allow long-lasting disease remission, which is an unanswered question but an intriguing possibility,” he concluded.
Dr. Rovin has served as a consultant for GlaxoSmithKline. Dr. Phillips disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction of Dialysis-Induced Metabolic Alkalosis
Metabolic alkalosis, a disorder that causes elevations in serum bicarbonate and arterial pH, is a common metabolic abnormality found in nearly half of hospitalized patients but is rare in patients with end-stage renal disease (ESRD) on hemodialysis (HD) during the pretreatment state. The problem seems to arise due to a high rate of older patients with multiple comorbidities and malnutrition who are undergoing HD. Metabolic alkalosis is associated with increased morbidity and mortality. In this report, we present a case of metabolic alkalosis, describe an innovative approach to manage metabolic alkalosis in the dialysis population, and review the pathophysiology.
Case Presentation
A 63-year-old female with emphysema, diabetic nephropathy, and ESRD on regular HD for 2 months by a tunneled subclavian vein catheter was admitted with 2 weeks of orthopnea and leg swelling. The review of systems was negative for chest pain, cough, wheeze, or sputum production. She was a former smoker with no alcohol or drug misuse. The patient was taking carvedilol 25 mg daily, furosemide 20 mg twice daily, basal insulin premeal, lisinopril 40 mg daily, pantoprazole 40 mg daily, calcium carbonate 400 mg 3 times daily, ferrous sulphate 325 mg daily, and a vilanterol/tiotropium inhaler once daily. Her dialysate outpatient prescription included sodium 140 mEq/L, potassium 2 mEq/L, calcium 2.5 mEq/L, and bicarbonate 36 mEq/L. Our dialysis unit used NaturaLyte dry pack for bicarbonate dialysis.
The patient appeared tachypneic with 26 respirations/min, oxygen saturation of 89% on room air, which improved to 94% on a 2 L nasal cannula. Her heart rate was 89 beats/min, blood pressure was 129/72 mm Hg, and body mass index was 21.2. The physical examination revealed jugular venous distension, lung crackles, reduced air entry, and pedal edema. Muscle wasting was noted in the arms and thighs. The tunnel catheter did not appear infected.
The patient’s blood work showed sodium, 136 (reference, 132-140) mmol/L; potassium, 4.3 (reference, 3.5-5.0) mmol/L; chloride, 89 (reference, 98-111) mmol/L; total CO2, 36 (reference, 24-28) mEq/L; blood urea nitrogen, 21 (reference, 7-21) mg/dL; creatinine 3.4 (reference, 0.5-1.4) mg/dL; and albumin, 2.7 (reference, 3.7-5.0) mg/dL. Arterial gases showed pH, 7.56 (reference, 7.35-7.45), partial CO2, 47 (reference, 35-45) mm Hg; bicarbonate, 42 (reference, 22-26) mEq/L; partial O2, 54 (reference, 75 to 100) mm Hg. Brain natriuretic peptide was 2,800 (normal, < 100) pg/mL with a normal troponin. X-rays showed pulmonary congestion and bilateral pleural effusions that were transudative on fluid analysis. An echocardiogram showed ejection fraction of 20 to 25% with normal valves (baseline ejection fraction of 60%-65%). A coronary arteriogram revealed severe nonischemic cardiomyopathy.
Treatment
To reduce bicarbonate levels, 3 L of normal saline solution were infused prefilter during HD, and ultrafiltration (UF) of 4.5 L achieved a net UF of -1.5 L over 3.5 hours on lower dialysate bicarbonate (30 mEq/L). Good catheter flow was achieved with a blood flow rate of 350 mL/min and a dialysate flow of 700 mL/min. Venous blood gases and basic serum metabolic panels were obtained throughout the first HD session (Table 1). Improvement in pH from 7.5 to 7.43 and in total CO2 from 36 to 30 mEq/L were noted after the treatment. Subsequently, we used the same membrane (Optiflux F160NRe) for 2 consecutive daily treatments to remove excess fluid and prevent worsening alkalosis using the same minimal bicarbonate bath, but no further normal saline solution was given.
Outcome
Volume overload was controlled as needed with UF. The bicarbonate did not drop after the second HD session, suggesting low organic acid production in the intradialytic period. By shortening the duration of dialysis to 3 hours and improving nutritional intake, we achieved dry weight, and the patient was discharged home with a total CO2 of 25 mEq/L. Outpatient dialysis sessions were arranged to run at shorter duration (3 hours compared with 3.5 hours) and use low bicarbonate dialysate. The patient was admitted several times afterward for acute decompensated heart failure, but in all those admissions, her bicarbonate was in the normal-to-high range, between 23 and 30 mEq/L.
Discussion
Metabolic alkalosis is relatively rare in ESRD patients on HD. Particularly in the predialysis period, but with the growing number of older patients undergoing HD and the aggressive treatment of acidosis with relatively higher buffer concentrations; there has been an increase in the incidence of metabolic alkalosis in patients on HD. In the Fresenius Medical Care (FMC) prevalent HD patient study, predialysis bicarbonate levels have increased overtime from a mean (SD)22.9 (3.1) mEq/L in 2004 to a mean (SD) 24.1 (3.5) mEq/L in September 2011, with 25% of patients > 26.0 mEq/L compared with only 6% in 2004.1 The condition has been associated with cardiac arrhythmia, intradialytic hypocalcemia, hypokalemia, hypercapnia, hypoxia, accelerated hypertension, and seizure.2-4 Metabolic alkalosis may be associated with increased mortality.5-7 However, the effect dissipated after adjusting for inflammation and nutritional status.6
Our patient had primary metabolic alkalosis evident by her high pH of 7.56 and high total CO2 of 36 mEq/L. The serum total CO2 reflects the metabolic status more accurately than the blood gas bicarbonate, which is prone to calculation error by the Henderson-Hasselbalch equation. Her respiratory compensation for the metabolic alkalosis was appropriate, with an increase of arterial PaCO2 to 47 mm Hg (
In patients with ESRD on HD who have no residual urine output, causes of metabolic alkalosis are limited to loss of net acid or gain of alkali through the gastrointestinal tract; our patient had none of these. Similarly, all renal causes of metabolic alkalosis are not applicable to our patient, including mineralocorticoid excess and contraction alkalosis. In patients with preserved kidney function, loop diuretics can induce alkalosis through enhanced tubular absorption of HCO3. While acetazolamide can mitigate this scenario by blocking carbonic anhydrase in the luminal border of the collecting ducts resulting in excretion of bicarbonate in the urine, our patient had negligible urine output despite being on furosemide 20 mg twice daily, making this an unlikely cause.
Severe metabolic alkalosis in dialysis patients has been reported with cocaine use, pica ingestion, and citrate load as in plasma exchange, massive transfusions, and regional anticoagulation.2,8-11 Although calcium carbonate intake can contribute to alkalosis, her small daily dose of 1,200 mg contains approximately 12 mEq of carbonate, which is not a significant contributor to the alkalosis.
With all other causes excluded, the metabolic alkalosis in our patient is presumed to result from the bicarbonate-rich dialysate. Since the majority of patients with ESRD are acidotic before dialysis, the dialysate bicarbonate is set at a higher than normal physiologic level to bring the pH close to or even higher than normal after dialysis. The patient had been dialyzed with NaturaLyte as an outpatient, which was set at the dialysis unit default mode of 36 mEq/L. This form of alkalosis has been reported to peak immediately after treatment but in most patients returns to the predialysis acidotic state due to endogenous acid production.1,4,12 Normally, muscles play a significant role in buffering excess bicarbonate in patients with nonfunctioning kidneys; hence, malnutrition with muscle wasting tends to propagate and maintain alkalosis, as in our patient.
Managing alkalosis in patients on dialysis can be challenging and is often directed at identifying potential causes like overzealous bicarbonate dialysate and addressing comorbidities, especially malnutrition.6,7 Bicarbonate delivery can be set on dialysis machines as low as 20 mEq/L. However, the reliability of correcting serum bicarbonate by adjusting bicarbonate-based dialysis products is in question as these products deliver additional buffering capacity through mixing and metabolism of acetate, acetic acid, or citric acid (Table 2).
We infused a high volume of sodium chloride during dialysis to create hyperchloremic metabolic acidosis while removing the volume by UF, thereby eliminating more bicarbonate by convection. Normal saline has a pH of 5.5 and a chloride of 154 mmol/L. We have compensated for an inherent lack of flexibility in HD as it is currently practiced: dialysates are virtually all deliberately alkaline because most of the patients coming to HD have varying magnitudes of metabolic acidosis and acidemia. The dialysate concentrate that dilutes to a bicarbonate level of 30 mEq/L would have only a modest effect against this magnitude of metabolic alkalosis that this patient had at dialysis. We have compensated for this structural inadequacy of current HD by repairing the patient’s severe hypochloremic metabolic alkalosis by infusing a hyperchloremic sodium chloride solution and dialyzing off the excess sodium bicarbonate. This is the logical inverse of what usually happens in the severely acidotic patients seen prior to dialysis: dialyzing off an excess of normal saline and repairing the metabolic acidosis by transfer-in of sodium bicarbonate from the dialysate.
Fresenius Medical Care, which provides most HD machines and fluids in the United States, created charts to show the approximate degree that each contributes as additional buffer. That was in response to a class action lawsuit for metabolic alkalosis due to overdelivery of bicarbonate that resulted in alleged cardiac arrests in patients with HD.13 Their report cast doubt on the ability of a lower bicarbonate bath to correct metabolic alkalosis in a predictable fashion.1 We accordingly showed that normal saline delivery is a reliable option to promptly lower serum bicarbonate level. However, this is a temporary measure and long-term bicarbonate delivery during dialysis needs to be addressed.
Huber and Gennari demonstrated success in reducing severe alkalosis in patients with ESRD due to vomiting with the use of HCO3 bath of 30 mEq/L.14 In their report, the calculated bicarbonate dropped from 94 to 39 mEq/L; after 3 hours of HD, their patient also was receiving 2 L of an isotonic saline infusion daily. These observations suggest that lowering bicarbonate in the bath is effective in much more severe cases than ours, and even then, extra measures are needed to bring it down to desirable levels. In the early days, some health care providers used a specially prepared high-chloride (123 mEq/L) and low-acetate dialysate (18 mEq/L), which increased serum chloride and hydrogen ion concentrations and decreased the serum bicarbonate concentration compared with those in commercially available high-acetate dialysate (containing 37 mEq/L acetate and 104 mEq/L Cl).15 However, this method requires special preparation of dialysate. Oral potassium chloride also was used to correct metabolic alkalosis, but the risk of potassium overload precludes this approach in patients with ESRD.16
Likewise, adding oral sodium chloride risks causing volume overload, especially in patients with cardiomyopathy; it may increase thirst, resulting in interdialytic excess volume gains.17 In our patient, respiratory compensation took place by correcting pulmonary congestion by UF, and the gentle bicarbonate removal in addition to boosting chloride levels promptly improved the metabolic alkalosis.
Notably adequate volume control achieved by HD in persons with small muscle mass and severe cardiomyopathy can require longer treatment duration than required to achieve adequate clearance. Accordingly, more bicarbonate loading can take place, causing metabolic alkalosis. This problem is compounded by the potential overdelivery of bicarbonate than that entered by the physician’s order.1
Conclusions
Attention should be paid to detect elevated predialysis serum bicarbonate levels in ESRD patients on HD, especially those with values above 27 mmol/L due to higher mortality.6,7 Treatment of these patients is more challenging than for those who are acidotic predialysis, especially when alkalosis is compounded by malnutrition. Mitigation of this problem is achieved by using a lower bicarbonate bath and the shortest effective dialysis duration that achieves adequate clearance. Poor clearance also deleteriously affects patient nutrition and well-being. We have shown that normal saline solution infusion with concurrent removal by UF can correct pretreatment metabolic alkalosis when other measures are inadequate.
1. Fresenius Medical Care North America. Bicarbonate dialysis update. July 2012. Accessed May 14, 2018. http://www.renalweb.com/writings/alkalosis/FMC%20Jul%2025%202012.pdf
2. Rho M, Renda J. Pica presenting as metabolic alkalosis and seizure in a dialysis patient. Clin Nephrol. 2006;66(1):71-73. doi:10.5414/cnp66071
3. Bear R, Goldstein M, Phillipson E, et al. Effect of metabolic alkalosis on respiratory function in patients with chronic obstructive lung disease. Can Med Assoc J. 1977;117(8):900-903.
4. Javaheri S, Kazemi H. Metabolic alkalosis and hypoventilation in humans. Am Rev Respir Dis. 1987;136(4):1011-1016. doi:10.1164/ajrccm/136.4.1011
5. Yamamoto T, Shoji S, Yamakawa T, et al. Predialysis and postdialysis pH and bicarbonate and risk of all-cause and cardiovascular mortality in long-term hemodialysis patients. Am J Kidney Dis. 2015;66(3):469-478. doi:10.1053/j.ajkd.2015.04.014
6. Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1(1):70-78. doi:10.2215/CJN.00010505
7. Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(4):661-671. doi:10.1053/j.ajkd.2004.06.008
8. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Recurrent metabolic alkalosis and elevated troponins after crack cocaine use in a hemodialysis patient. Clin Exp Nephrol. 2006;10(2):156-158. doi:10.1007/s10157-006-0414-y
9. Ostermann ME, Girgis-Hanna Y, Nelson SR, Eastwood JB. Metabolic alkalosis in patients with renal failure. Nephrol Dial Transplant. 2003;18(11):2442-2448. doi:10.1093/ndt/gfg333
10. Rahilly GT, Berl T. Severe metabolic alkalosis caused by administration of plasma protein fraction in end-stage renal failure. N Engl J Med. 1979;301(15):824-826. doi:10.1056/NEJM197910113011506
11. Panesar M, Shah N, Vaqar S, et al. Changes in serum bicarbonate levels caused by acetate-containing bicarbonate-buffered hemodialysis solution: an observational prospective cohort study. Ther Apher Dial. 2017;21(2):157-165. doi:10.1111/1744-9987.12510
12. Noh U-S, Yi J-H, Han S-W, Kim H-J. Varying dialysate bicarbonate concentrations in maintenance hemodialysis patients affect post-dialysis alkalosis but not pre-dialysis acidosis. Electrolyte Blood Press. 2007;5(2):95-101. doi:10.5049/EBP.2007.5.2.95
13. Perriello B. Fresenius, plaintiffs ask for more time for $250m settlement in dialysate cases. Published March 4, 2016. Accessed May 14, 2018. https://www.massdevice.com/fresenius-askes-judge-time-250m-settlement-dialysate-cases
14. Huber L, Gennari FJ. Severe metabolic alkalosis in a hemodialysis patient. Am J Kidney Dis. 2011;58(1):144-149. doi:10.1053/j.ajkd.2011.03.016
15. Swartz RD, Rubin JE, Brown RS, Yager JM, Steinman TI, Frazier HS. Correction of postoperative metabolic alkalosis and renal failure by hemodialysis. Ann Intern Med. 1977;86(1):52-55. doi:10.7326/0003-4819-86-1-52
16. Rosen RA, Julian BA, Dubovsky EV, Galla JH, Luke RG. On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med. 1988;84(3, pt 1):449-458. doi:10.1016/0002-9343(88)90265-3
17. Hirakawa Y, Hanafusa N, Nangaku M. Correction of metabolic alkalosis and elevated calcium levels by sodium chloride in a hemodialysis patient with inadequate chloride intake. Ther Apher Dial. 2016;20(1):86-87. doi:10.1111/1744-9987.12335
Metabolic alkalosis, a disorder that causes elevations in serum bicarbonate and arterial pH, is a common metabolic abnormality found in nearly half of hospitalized patients but is rare in patients with end-stage renal disease (ESRD) on hemodialysis (HD) during the pretreatment state. The problem seems to arise due to a high rate of older patients with multiple comorbidities and malnutrition who are undergoing HD. Metabolic alkalosis is associated with increased morbidity and mortality. In this report, we present a case of metabolic alkalosis, describe an innovative approach to manage metabolic alkalosis in the dialysis population, and review the pathophysiology.
Case Presentation
A 63-year-old female with emphysema, diabetic nephropathy, and ESRD on regular HD for 2 months by a tunneled subclavian vein catheter was admitted with 2 weeks of orthopnea and leg swelling. The review of systems was negative for chest pain, cough, wheeze, or sputum production. She was a former smoker with no alcohol or drug misuse. The patient was taking carvedilol 25 mg daily, furosemide 20 mg twice daily, basal insulin premeal, lisinopril 40 mg daily, pantoprazole 40 mg daily, calcium carbonate 400 mg 3 times daily, ferrous sulphate 325 mg daily, and a vilanterol/tiotropium inhaler once daily. Her dialysate outpatient prescription included sodium 140 mEq/L, potassium 2 mEq/L, calcium 2.5 mEq/L, and bicarbonate 36 mEq/L. Our dialysis unit used NaturaLyte dry pack for bicarbonate dialysis.
The patient appeared tachypneic with 26 respirations/min, oxygen saturation of 89% on room air, which improved to 94% on a 2 L nasal cannula. Her heart rate was 89 beats/min, blood pressure was 129/72 mm Hg, and body mass index was 21.2. The physical examination revealed jugular venous distension, lung crackles, reduced air entry, and pedal edema. Muscle wasting was noted in the arms and thighs. The tunnel catheter did not appear infected.
The patient’s blood work showed sodium, 136 (reference, 132-140) mmol/L; potassium, 4.3 (reference, 3.5-5.0) mmol/L; chloride, 89 (reference, 98-111) mmol/L; total CO2, 36 (reference, 24-28) mEq/L; blood urea nitrogen, 21 (reference, 7-21) mg/dL; creatinine 3.4 (reference, 0.5-1.4) mg/dL; and albumin, 2.7 (reference, 3.7-5.0) mg/dL. Arterial gases showed pH, 7.56 (reference, 7.35-7.45), partial CO2, 47 (reference, 35-45) mm Hg; bicarbonate, 42 (reference, 22-26) mEq/L; partial O2, 54 (reference, 75 to 100) mm Hg. Brain natriuretic peptide was 2,800 (normal, < 100) pg/mL with a normal troponin. X-rays showed pulmonary congestion and bilateral pleural effusions that were transudative on fluid analysis. An echocardiogram showed ejection fraction of 20 to 25% with normal valves (baseline ejection fraction of 60%-65%). A coronary arteriogram revealed severe nonischemic cardiomyopathy.
Treatment
To reduce bicarbonate levels, 3 L of normal saline solution were infused prefilter during HD, and ultrafiltration (UF) of 4.5 L achieved a net UF of -1.5 L over 3.5 hours on lower dialysate bicarbonate (30 mEq/L). Good catheter flow was achieved with a blood flow rate of 350 mL/min and a dialysate flow of 700 mL/min. Venous blood gases and basic serum metabolic panels were obtained throughout the first HD session (Table 1). Improvement in pH from 7.5 to 7.43 and in total CO2 from 36 to 30 mEq/L were noted after the treatment. Subsequently, we used the same membrane (Optiflux F160NRe) for 2 consecutive daily treatments to remove excess fluid and prevent worsening alkalosis using the same minimal bicarbonate bath, but no further normal saline solution was given.
Outcome
Volume overload was controlled as needed with UF. The bicarbonate did not drop after the second HD session, suggesting low organic acid production in the intradialytic period. By shortening the duration of dialysis to 3 hours and improving nutritional intake, we achieved dry weight, and the patient was discharged home with a total CO2 of 25 mEq/L. Outpatient dialysis sessions were arranged to run at shorter duration (3 hours compared with 3.5 hours) and use low bicarbonate dialysate. The patient was admitted several times afterward for acute decompensated heart failure, but in all those admissions, her bicarbonate was in the normal-to-high range, between 23 and 30 mEq/L.
Discussion
Metabolic alkalosis is relatively rare in ESRD patients on HD. Particularly in the predialysis period, but with the growing number of older patients undergoing HD and the aggressive treatment of acidosis with relatively higher buffer concentrations; there has been an increase in the incidence of metabolic alkalosis in patients on HD. In the Fresenius Medical Care (FMC) prevalent HD patient study, predialysis bicarbonate levels have increased overtime from a mean (SD)22.9 (3.1) mEq/L in 2004 to a mean (SD) 24.1 (3.5) mEq/L in September 2011, with 25% of patients > 26.0 mEq/L compared with only 6% in 2004.1 The condition has been associated with cardiac arrhythmia, intradialytic hypocalcemia, hypokalemia, hypercapnia, hypoxia, accelerated hypertension, and seizure.2-4 Metabolic alkalosis may be associated with increased mortality.5-7 However, the effect dissipated after adjusting for inflammation and nutritional status.6
Our patient had primary metabolic alkalosis evident by her high pH of 7.56 and high total CO2 of 36 mEq/L. The serum total CO2 reflects the metabolic status more accurately than the blood gas bicarbonate, which is prone to calculation error by the Henderson-Hasselbalch equation. Her respiratory compensation for the metabolic alkalosis was appropriate, with an increase of arterial PaCO2 to 47 mm Hg (
In patients with ESRD on HD who have no residual urine output, causes of metabolic alkalosis are limited to loss of net acid or gain of alkali through the gastrointestinal tract; our patient had none of these. Similarly, all renal causes of metabolic alkalosis are not applicable to our patient, including mineralocorticoid excess and contraction alkalosis. In patients with preserved kidney function, loop diuretics can induce alkalosis through enhanced tubular absorption of HCO3. While acetazolamide can mitigate this scenario by blocking carbonic anhydrase in the luminal border of the collecting ducts resulting in excretion of bicarbonate in the urine, our patient had negligible urine output despite being on furosemide 20 mg twice daily, making this an unlikely cause.
Severe metabolic alkalosis in dialysis patients has been reported with cocaine use, pica ingestion, and citrate load as in plasma exchange, massive transfusions, and regional anticoagulation.2,8-11 Although calcium carbonate intake can contribute to alkalosis, her small daily dose of 1,200 mg contains approximately 12 mEq of carbonate, which is not a significant contributor to the alkalosis.
With all other causes excluded, the metabolic alkalosis in our patient is presumed to result from the bicarbonate-rich dialysate. Since the majority of patients with ESRD are acidotic before dialysis, the dialysate bicarbonate is set at a higher than normal physiologic level to bring the pH close to or even higher than normal after dialysis. The patient had been dialyzed with NaturaLyte as an outpatient, which was set at the dialysis unit default mode of 36 mEq/L. This form of alkalosis has been reported to peak immediately after treatment but in most patients returns to the predialysis acidotic state due to endogenous acid production.1,4,12 Normally, muscles play a significant role in buffering excess bicarbonate in patients with nonfunctioning kidneys; hence, malnutrition with muscle wasting tends to propagate and maintain alkalosis, as in our patient.
Managing alkalosis in patients on dialysis can be challenging and is often directed at identifying potential causes like overzealous bicarbonate dialysate and addressing comorbidities, especially malnutrition.6,7 Bicarbonate delivery can be set on dialysis machines as low as 20 mEq/L. However, the reliability of correcting serum bicarbonate by adjusting bicarbonate-based dialysis products is in question as these products deliver additional buffering capacity through mixing and metabolism of acetate, acetic acid, or citric acid (Table 2).
We infused a high volume of sodium chloride during dialysis to create hyperchloremic metabolic acidosis while removing the volume by UF, thereby eliminating more bicarbonate by convection. Normal saline has a pH of 5.5 and a chloride of 154 mmol/L. We have compensated for an inherent lack of flexibility in HD as it is currently practiced: dialysates are virtually all deliberately alkaline because most of the patients coming to HD have varying magnitudes of metabolic acidosis and acidemia. The dialysate concentrate that dilutes to a bicarbonate level of 30 mEq/L would have only a modest effect against this magnitude of metabolic alkalosis that this patient had at dialysis. We have compensated for this structural inadequacy of current HD by repairing the patient’s severe hypochloremic metabolic alkalosis by infusing a hyperchloremic sodium chloride solution and dialyzing off the excess sodium bicarbonate. This is the logical inverse of what usually happens in the severely acidotic patients seen prior to dialysis: dialyzing off an excess of normal saline and repairing the metabolic acidosis by transfer-in of sodium bicarbonate from the dialysate.
Fresenius Medical Care, which provides most HD machines and fluids in the United States, created charts to show the approximate degree that each contributes as additional buffer. That was in response to a class action lawsuit for metabolic alkalosis due to overdelivery of bicarbonate that resulted in alleged cardiac arrests in patients with HD.13 Their report cast doubt on the ability of a lower bicarbonate bath to correct metabolic alkalosis in a predictable fashion.1 We accordingly showed that normal saline delivery is a reliable option to promptly lower serum bicarbonate level. However, this is a temporary measure and long-term bicarbonate delivery during dialysis needs to be addressed.
Huber and Gennari demonstrated success in reducing severe alkalosis in patients with ESRD due to vomiting with the use of HCO3 bath of 30 mEq/L.14 In their report, the calculated bicarbonate dropped from 94 to 39 mEq/L; after 3 hours of HD, their patient also was receiving 2 L of an isotonic saline infusion daily. These observations suggest that lowering bicarbonate in the bath is effective in much more severe cases than ours, and even then, extra measures are needed to bring it down to desirable levels. In the early days, some health care providers used a specially prepared high-chloride (123 mEq/L) and low-acetate dialysate (18 mEq/L), which increased serum chloride and hydrogen ion concentrations and decreased the serum bicarbonate concentration compared with those in commercially available high-acetate dialysate (containing 37 mEq/L acetate and 104 mEq/L Cl).15 However, this method requires special preparation of dialysate. Oral potassium chloride also was used to correct metabolic alkalosis, but the risk of potassium overload precludes this approach in patients with ESRD.16
Likewise, adding oral sodium chloride risks causing volume overload, especially in patients with cardiomyopathy; it may increase thirst, resulting in interdialytic excess volume gains.17 In our patient, respiratory compensation took place by correcting pulmonary congestion by UF, and the gentle bicarbonate removal in addition to boosting chloride levels promptly improved the metabolic alkalosis.
Notably adequate volume control achieved by HD in persons with small muscle mass and severe cardiomyopathy can require longer treatment duration than required to achieve adequate clearance. Accordingly, more bicarbonate loading can take place, causing metabolic alkalosis. This problem is compounded by the potential overdelivery of bicarbonate than that entered by the physician’s order.1
Conclusions
Attention should be paid to detect elevated predialysis serum bicarbonate levels in ESRD patients on HD, especially those with values above 27 mmol/L due to higher mortality.6,7 Treatment of these patients is more challenging than for those who are acidotic predialysis, especially when alkalosis is compounded by malnutrition. Mitigation of this problem is achieved by using a lower bicarbonate bath and the shortest effective dialysis duration that achieves adequate clearance. Poor clearance also deleteriously affects patient nutrition and well-being. We have shown that normal saline solution infusion with concurrent removal by UF can correct pretreatment metabolic alkalosis when other measures are inadequate.
Metabolic alkalosis, a disorder that causes elevations in serum bicarbonate and arterial pH, is a common metabolic abnormality found in nearly half of hospitalized patients but is rare in patients with end-stage renal disease (ESRD) on hemodialysis (HD) during the pretreatment state. The problem seems to arise due to a high rate of older patients with multiple comorbidities and malnutrition who are undergoing HD. Metabolic alkalosis is associated with increased morbidity and mortality. In this report, we present a case of metabolic alkalosis, describe an innovative approach to manage metabolic alkalosis in the dialysis population, and review the pathophysiology.
Case Presentation
A 63-year-old female with emphysema, diabetic nephropathy, and ESRD on regular HD for 2 months by a tunneled subclavian vein catheter was admitted with 2 weeks of orthopnea and leg swelling. The review of systems was negative for chest pain, cough, wheeze, or sputum production. She was a former smoker with no alcohol or drug misuse. The patient was taking carvedilol 25 mg daily, furosemide 20 mg twice daily, basal insulin premeal, lisinopril 40 mg daily, pantoprazole 40 mg daily, calcium carbonate 400 mg 3 times daily, ferrous sulphate 325 mg daily, and a vilanterol/tiotropium inhaler once daily. Her dialysate outpatient prescription included sodium 140 mEq/L, potassium 2 mEq/L, calcium 2.5 mEq/L, and bicarbonate 36 mEq/L. Our dialysis unit used NaturaLyte dry pack for bicarbonate dialysis.
The patient appeared tachypneic with 26 respirations/min, oxygen saturation of 89% on room air, which improved to 94% on a 2 L nasal cannula. Her heart rate was 89 beats/min, blood pressure was 129/72 mm Hg, and body mass index was 21.2. The physical examination revealed jugular venous distension, lung crackles, reduced air entry, and pedal edema. Muscle wasting was noted in the arms and thighs. The tunnel catheter did not appear infected.
The patient’s blood work showed sodium, 136 (reference, 132-140) mmol/L; potassium, 4.3 (reference, 3.5-5.0) mmol/L; chloride, 89 (reference, 98-111) mmol/L; total CO2, 36 (reference, 24-28) mEq/L; blood urea nitrogen, 21 (reference, 7-21) mg/dL; creatinine 3.4 (reference, 0.5-1.4) mg/dL; and albumin, 2.7 (reference, 3.7-5.0) mg/dL. Arterial gases showed pH, 7.56 (reference, 7.35-7.45), partial CO2, 47 (reference, 35-45) mm Hg; bicarbonate, 42 (reference, 22-26) mEq/L; partial O2, 54 (reference, 75 to 100) mm Hg. Brain natriuretic peptide was 2,800 (normal, < 100) pg/mL with a normal troponin. X-rays showed pulmonary congestion and bilateral pleural effusions that were transudative on fluid analysis. An echocardiogram showed ejection fraction of 20 to 25% with normal valves (baseline ejection fraction of 60%-65%). A coronary arteriogram revealed severe nonischemic cardiomyopathy.
Treatment
To reduce bicarbonate levels, 3 L of normal saline solution were infused prefilter during HD, and ultrafiltration (UF) of 4.5 L achieved a net UF of -1.5 L over 3.5 hours on lower dialysate bicarbonate (30 mEq/L). Good catheter flow was achieved with a blood flow rate of 350 mL/min and a dialysate flow of 700 mL/min. Venous blood gases and basic serum metabolic panels were obtained throughout the first HD session (Table 1). Improvement in pH from 7.5 to 7.43 and in total CO2 from 36 to 30 mEq/L were noted after the treatment. Subsequently, we used the same membrane (Optiflux F160NRe) for 2 consecutive daily treatments to remove excess fluid and prevent worsening alkalosis using the same minimal bicarbonate bath, but no further normal saline solution was given.
Outcome
Volume overload was controlled as needed with UF. The bicarbonate did not drop after the second HD session, suggesting low organic acid production in the intradialytic period. By shortening the duration of dialysis to 3 hours and improving nutritional intake, we achieved dry weight, and the patient was discharged home with a total CO2 of 25 mEq/L. Outpatient dialysis sessions were arranged to run at shorter duration (3 hours compared with 3.5 hours) and use low bicarbonate dialysate. The patient was admitted several times afterward for acute decompensated heart failure, but in all those admissions, her bicarbonate was in the normal-to-high range, between 23 and 30 mEq/L.
Discussion
Metabolic alkalosis is relatively rare in ESRD patients on HD. Particularly in the predialysis period, but with the growing number of older patients undergoing HD and the aggressive treatment of acidosis with relatively higher buffer concentrations; there has been an increase in the incidence of metabolic alkalosis in patients on HD. In the Fresenius Medical Care (FMC) prevalent HD patient study, predialysis bicarbonate levels have increased overtime from a mean (SD)22.9 (3.1) mEq/L in 2004 to a mean (SD) 24.1 (3.5) mEq/L in September 2011, with 25% of patients > 26.0 mEq/L compared with only 6% in 2004.1 The condition has been associated with cardiac arrhythmia, intradialytic hypocalcemia, hypokalemia, hypercapnia, hypoxia, accelerated hypertension, and seizure.2-4 Metabolic alkalosis may be associated with increased mortality.5-7 However, the effect dissipated after adjusting for inflammation and nutritional status.6
Our patient had primary metabolic alkalosis evident by her high pH of 7.56 and high total CO2 of 36 mEq/L. The serum total CO2 reflects the metabolic status more accurately than the blood gas bicarbonate, which is prone to calculation error by the Henderson-Hasselbalch equation. Her respiratory compensation for the metabolic alkalosis was appropriate, with an increase of arterial PaCO2 to 47 mm Hg (
In patients with ESRD on HD who have no residual urine output, causes of metabolic alkalosis are limited to loss of net acid or gain of alkali through the gastrointestinal tract; our patient had none of these. Similarly, all renal causes of metabolic alkalosis are not applicable to our patient, including mineralocorticoid excess and contraction alkalosis. In patients with preserved kidney function, loop diuretics can induce alkalosis through enhanced tubular absorption of HCO3. While acetazolamide can mitigate this scenario by blocking carbonic anhydrase in the luminal border of the collecting ducts resulting in excretion of bicarbonate in the urine, our patient had negligible urine output despite being on furosemide 20 mg twice daily, making this an unlikely cause.
Severe metabolic alkalosis in dialysis patients has been reported with cocaine use, pica ingestion, and citrate load as in plasma exchange, massive transfusions, and regional anticoagulation.2,8-11 Although calcium carbonate intake can contribute to alkalosis, her small daily dose of 1,200 mg contains approximately 12 mEq of carbonate, which is not a significant contributor to the alkalosis.
With all other causes excluded, the metabolic alkalosis in our patient is presumed to result from the bicarbonate-rich dialysate. Since the majority of patients with ESRD are acidotic before dialysis, the dialysate bicarbonate is set at a higher than normal physiologic level to bring the pH close to or even higher than normal after dialysis. The patient had been dialyzed with NaturaLyte as an outpatient, which was set at the dialysis unit default mode of 36 mEq/L. This form of alkalosis has been reported to peak immediately after treatment but in most patients returns to the predialysis acidotic state due to endogenous acid production.1,4,12 Normally, muscles play a significant role in buffering excess bicarbonate in patients with nonfunctioning kidneys; hence, malnutrition with muscle wasting tends to propagate and maintain alkalosis, as in our patient.
Managing alkalosis in patients on dialysis can be challenging and is often directed at identifying potential causes like overzealous bicarbonate dialysate and addressing comorbidities, especially malnutrition.6,7 Bicarbonate delivery can be set on dialysis machines as low as 20 mEq/L. However, the reliability of correcting serum bicarbonate by adjusting bicarbonate-based dialysis products is in question as these products deliver additional buffering capacity through mixing and metabolism of acetate, acetic acid, or citric acid (Table 2).
We infused a high volume of sodium chloride during dialysis to create hyperchloremic metabolic acidosis while removing the volume by UF, thereby eliminating more bicarbonate by convection. Normal saline has a pH of 5.5 and a chloride of 154 mmol/L. We have compensated for an inherent lack of flexibility in HD as it is currently practiced: dialysates are virtually all deliberately alkaline because most of the patients coming to HD have varying magnitudes of metabolic acidosis and acidemia. The dialysate concentrate that dilutes to a bicarbonate level of 30 mEq/L would have only a modest effect against this magnitude of metabolic alkalosis that this patient had at dialysis. We have compensated for this structural inadequacy of current HD by repairing the patient’s severe hypochloremic metabolic alkalosis by infusing a hyperchloremic sodium chloride solution and dialyzing off the excess sodium bicarbonate. This is the logical inverse of what usually happens in the severely acidotic patients seen prior to dialysis: dialyzing off an excess of normal saline and repairing the metabolic acidosis by transfer-in of sodium bicarbonate from the dialysate.
Fresenius Medical Care, which provides most HD machines and fluids in the United States, created charts to show the approximate degree that each contributes as additional buffer. That was in response to a class action lawsuit for metabolic alkalosis due to overdelivery of bicarbonate that resulted in alleged cardiac arrests in patients with HD.13 Their report cast doubt on the ability of a lower bicarbonate bath to correct metabolic alkalosis in a predictable fashion.1 We accordingly showed that normal saline delivery is a reliable option to promptly lower serum bicarbonate level. However, this is a temporary measure and long-term bicarbonate delivery during dialysis needs to be addressed.
Huber and Gennari demonstrated success in reducing severe alkalosis in patients with ESRD due to vomiting with the use of HCO3 bath of 30 mEq/L.14 In their report, the calculated bicarbonate dropped from 94 to 39 mEq/L; after 3 hours of HD, their patient also was receiving 2 L of an isotonic saline infusion daily. These observations suggest that lowering bicarbonate in the bath is effective in much more severe cases than ours, and even then, extra measures are needed to bring it down to desirable levels. In the early days, some health care providers used a specially prepared high-chloride (123 mEq/L) and low-acetate dialysate (18 mEq/L), which increased serum chloride and hydrogen ion concentrations and decreased the serum bicarbonate concentration compared with those in commercially available high-acetate dialysate (containing 37 mEq/L acetate and 104 mEq/L Cl).15 However, this method requires special preparation of dialysate. Oral potassium chloride also was used to correct metabolic alkalosis, but the risk of potassium overload precludes this approach in patients with ESRD.16
Likewise, adding oral sodium chloride risks causing volume overload, especially in patients with cardiomyopathy; it may increase thirst, resulting in interdialytic excess volume gains.17 In our patient, respiratory compensation took place by correcting pulmonary congestion by UF, and the gentle bicarbonate removal in addition to boosting chloride levels promptly improved the metabolic alkalosis.
Notably adequate volume control achieved by HD in persons with small muscle mass and severe cardiomyopathy can require longer treatment duration than required to achieve adequate clearance. Accordingly, more bicarbonate loading can take place, causing metabolic alkalosis. This problem is compounded by the potential overdelivery of bicarbonate than that entered by the physician’s order.1
Conclusions
Attention should be paid to detect elevated predialysis serum bicarbonate levels in ESRD patients on HD, especially those with values above 27 mmol/L due to higher mortality.6,7 Treatment of these patients is more challenging than for those who are acidotic predialysis, especially when alkalosis is compounded by malnutrition. Mitigation of this problem is achieved by using a lower bicarbonate bath and the shortest effective dialysis duration that achieves adequate clearance. Poor clearance also deleteriously affects patient nutrition and well-being. We have shown that normal saline solution infusion with concurrent removal by UF can correct pretreatment metabolic alkalosis when other measures are inadequate.
1. Fresenius Medical Care North America. Bicarbonate dialysis update. July 2012. Accessed May 14, 2018. http://www.renalweb.com/writings/alkalosis/FMC%20Jul%2025%202012.pdf
2. Rho M, Renda J. Pica presenting as metabolic alkalosis and seizure in a dialysis patient. Clin Nephrol. 2006;66(1):71-73. doi:10.5414/cnp66071
3. Bear R, Goldstein M, Phillipson E, et al. Effect of metabolic alkalosis on respiratory function in patients with chronic obstructive lung disease. Can Med Assoc J. 1977;117(8):900-903.
4. Javaheri S, Kazemi H. Metabolic alkalosis and hypoventilation in humans. Am Rev Respir Dis. 1987;136(4):1011-1016. doi:10.1164/ajrccm/136.4.1011
5. Yamamoto T, Shoji S, Yamakawa T, et al. Predialysis and postdialysis pH and bicarbonate and risk of all-cause and cardiovascular mortality in long-term hemodialysis patients. Am J Kidney Dis. 2015;66(3):469-478. doi:10.1053/j.ajkd.2015.04.014
6. Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1(1):70-78. doi:10.2215/CJN.00010505
7. Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(4):661-671. doi:10.1053/j.ajkd.2004.06.008
8. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Recurrent metabolic alkalosis and elevated troponins after crack cocaine use in a hemodialysis patient. Clin Exp Nephrol. 2006;10(2):156-158. doi:10.1007/s10157-006-0414-y
9. Ostermann ME, Girgis-Hanna Y, Nelson SR, Eastwood JB. Metabolic alkalosis in patients with renal failure. Nephrol Dial Transplant. 2003;18(11):2442-2448. doi:10.1093/ndt/gfg333
10. Rahilly GT, Berl T. Severe metabolic alkalosis caused by administration of plasma protein fraction in end-stage renal failure. N Engl J Med. 1979;301(15):824-826. doi:10.1056/NEJM197910113011506
11. Panesar M, Shah N, Vaqar S, et al. Changes in serum bicarbonate levels caused by acetate-containing bicarbonate-buffered hemodialysis solution: an observational prospective cohort study. Ther Apher Dial. 2017;21(2):157-165. doi:10.1111/1744-9987.12510
12. Noh U-S, Yi J-H, Han S-W, Kim H-J. Varying dialysate bicarbonate concentrations in maintenance hemodialysis patients affect post-dialysis alkalosis but not pre-dialysis acidosis. Electrolyte Blood Press. 2007;5(2):95-101. doi:10.5049/EBP.2007.5.2.95
13. Perriello B. Fresenius, plaintiffs ask for more time for $250m settlement in dialysate cases. Published March 4, 2016. Accessed May 14, 2018. https://www.massdevice.com/fresenius-askes-judge-time-250m-settlement-dialysate-cases
14. Huber L, Gennari FJ. Severe metabolic alkalosis in a hemodialysis patient. Am J Kidney Dis. 2011;58(1):144-149. doi:10.1053/j.ajkd.2011.03.016
15. Swartz RD, Rubin JE, Brown RS, Yager JM, Steinman TI, Frazier HS. Correction of postoperative metabolic alkalosis and renal failure by hemodialysis. Ann Intern Med. 1977;86(1):52-55. doi:10.7326/0003-4819-86-1-52
16. Rosen RA, Julian BA, Dubovsky EV, Galla JH, Luke RG. On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med. 1988;84(3, pt 1):449-458. doi:10.1016/0002-9343(88)90265-3
17. Hirakawa Y, Hanafusa N, Nangaku M. Correction of metabolic alkalosis and elevated calcium levels by sodium chloride in a hemodialysis patient with inadequate chloride intake. Ther Apher Dial. 2016;20(1):86-87. doi:10.1111/1744-9987.12335
1. Fresenius Medical Care North America. Bicarbonate dialysis update. July 2012. Accessed May 14, 2018. http://www.renalweb.com/writings/alkalosis/FMC%20Jul%2025%202012.pdf
2. Rho M, Renda J. Pica presenting as metabolic alkalosis and seizure in a dialysis patient. Clin Nephrol. 2006;66(1):71-73. doi:10.5414/cnp66071
3. Bear R, Goldstein M, Phillipson E, et al. Effect of metabolic alkalosis on respiratory function in patients with chronic obstructive lung disease. Can Med Assoc J. 1977;117(8):900-903.
4. Javaheri S, Kazemi H. Metabolic alkalosis and hypoventilation in humans. Am Rev Respir Dis. 1987;136(4):1011-1016. doi:10.1164/ajrccm/136.4.1011
5. Yamamoto T, Shoji S, Yamakawa T, et al. Predialysis and postdialysis pH and bicarbonate and risk of all-cause and cardiovascular mortality in long-term hemodialysis patients. Am J Kidney Dis. 2015;66(3):469-478. doi:10.1053/j.ajkd.2015.04.014
6. Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1(1):70-78. doi:10.2215/CJN.00010505
7. Bommer J, Locatelli F, Satayathum S, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(4):661-671. doi:10.1053/j.ajkd.2004.06.008
8. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Recurrent metabolic alkalosis and elevated troponins after crack cocaine use in a hemodialysis patient. Clin Exp Nephrol. 2006;10(2):156-158. doi:10.1007/s10157-006-0414-y
9. Ostermann ME, Girgis-Hanna Y, Nelson SR, Eastwood JB. Metabolic alkalosis in patients with renal failure. Nephrol Dial Transplant. 2003;18(11):2442-2448. doi:10.1093/ndt/gfg333
10. Rahilly GT, Berl T. Severe metabolic alkalosis caused by administration of plasma protein fraction in end-stage renal failure. N Engl J Med. 1979;301(15):824-826. doi:10.1056/NEJM197910113011506
11. Panesar M, Shah N, Vaqar S, et al. Changes in serum bicarbonate levels caused by acetate-containing bicarbonate-buffered hemodialysis solution: an observational prospective cohort study. Ther Apher Dial. 2017;21(2):157-165. doi:10.1111/1744-9987.12510
12. Noh U-S, Yi J-H, Han S-W, Kim H-J. Varying dialysate bicarbonate concentrations in maintenance hemodialysis patients affect post-dialysis alkalosis but not pre-dialysis acidosis. Electrolyte Blood Press. 2007;5(2):95-101. doi:10.5049/EBP.2007.5.2.95
13. Perriello B. Fresenius, plaintiffs ask for more time for $250m settlement in dialysate cases. Published March 4, 2016. Accessed May 14, 2018. https://www.massdevice.com/fresenius-askes-judge-time-250m-settlement-dialysate-cases
14. Huber L, Gennari FJ. Severe metabolic alkalosis in a hemodialysis patient. Am J Kidney Dis. 2011;58(1):144-149. doi:10.1053/j.ajkd.2011.03.016
15. Swartz RD, Rubin JE, Brown RS, Yager JM, Steinman TI, Frazier HS. Correction of postoperative metabolic alkalosis and renal failure by hemodialysis. Ann Intern Med. 1977;86(1):52-55. doi:10.7326/0003-4819-86-1-52
16. Rosen RA, Julian BA, Dubovsky EV, Galla JH, Luke RG. On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med. 1988;84(3, pt 1):449-458. doi:10.1016/0002-9343(88)90265-3
17. Hirakawa Y, Hanafusa N, Nangaku M. Correction of metabolic alkalosis and elevated calcium levels by sodium chloride in a hemodialysis patient with inadequate chloride intake. Ther Apher Dial. 2016;20(1):86-87. doi:10.1111/1744-9987.12335
Helping your obese patient achieve a healthier weight
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
In 2015-2016, almost 40% of adults and 18.5% of children ages 2 to 19 years in the United States met the definition for obesity—a chronic, relapsing, multifactorial, neurobehavioral disease that results in adverse metabolic, biomechanical, and psychosocial health consequences.1,2
Tremendous resources have been invested in research, policy development, and public education to try to prevent obesity and its related complications. Despite this, the obesity epidemic has worsened. Here, we explore how to evaluate and treat obese patients in a primary care setting based on the evidence and our experience seeing patients specifically for weight management in a family medicine residency teaching clinic. Pharmacotherapy and surgery, while often helpful, are outside the scope of this article.
It begins withan obesity-friendly office
Patients may have reservations about health care interactions specific to obesity, so it is important to invite them into a setting that facilitates trust and encourages collaboration. Actively engage patients with unhealthy weight by creating an environment where they feel comfortable. Offer wide chairs without armrests, which will easily accommodate patients of all sizes, and ensure that scales have a weight capacity > 400 lb. Communicate a message to patients, via waiting room materials and videos, that focuses on health rather than on weight or body mass index (BMI).
Understand the patient’s goals and challenges
Most (although not all) family physicians will see obese patients in the context of a visit for diabetes, hypertension, or another condition. However, we feel that having visits specifically to address weight in the initial stages of weight management is helpful. The focus of an initial visit should be getting to know how obesity has affected the patient and what his or her motive is in attempting to lose weight. Explore previous attempts at weight loss and establish what the patient’s highest weight has been, as this will impact weight-loss goals. For example, if a patient has weighed > 300 lb all her adult life, it will be extremely difficult to maintain a weight loss of 150 lb.
What else to ask about. Discuss stressors that may be causing increased food intake or poor food choices, including hunger, anger, loneliness, and sleep difficulties. Multidisciplinary care including a psychologist can aid in addressing these issues. Ask patients if they keep a food diary (and if not, recommend that they start), as food diaries are often helpful in elucidating eating and drinking patterns. Determine a patient’s current and past levels of physical activity, as this will guide the fitness goals you develop for him or her.
Screen for psychosocial disorders
As noted earlier, the physical component of obesity is commonly associated with mood disorders such as anxiety and depression.2 This requires a multidisciplinary team effort to facilitate healing in the patient struggling with obesity.
Screening for depression and anxiety using standardized tools such as the Patient Health Questionnaire-9 or the Generalized Anxiety Disorder-7 is encouraged in patients who are overweight or obese. Positive screens should be addressed as part of the patient’s treatment plan, as untreated depression and anxiety can inhibit success with weight loss. Be mindful that many medications commonly used to treat these conditions can impair weight loss and even promote weight gain.
Continue to: Don't overlook binge-eating disorders
Don’t overlook binge-eating disorders. Screening specifically for binge-eating disorders is important, given the implications on treatment. The US Department of Veterans Affairs developed a single-item tool for this purpose, the VA Binge Eating Screener. The validated questionnaire asks, “On average, how often have you eaten extremely large amounts of food at one time and felt that your eating was out of control at that time?” Response options are: “Never,” “< 1 time/week,” “1 time/week,” “2-4 times/week,” and “5+ times/week.” A response of ≥ 2 times/week had a sensitivity of 88.9% and specificity of 83.2% for binge-eating disorder.3
Patients with positive screens should undergo psychotherapy and consider pharmacotherapy with lisdexamfetamine as part of their treatment plan. Caution should be used if recommending intermittent fasting for someone with binge-eating disorder.
Evaluate for underlying causes and assess for comorbidities
Review the patient’s current medication list and history. Many medications can cause weight gain, and weight loss can often be achieved by deprescribing such medications. When feasible, prescribe an alternative medication with a more favorable weight profile. A previous article in The Journal of Family Practice addresses this in more depth.4
Laboratory and other testing
Laboratory analysis should primarily be focused on determining treatment alterations specific to underlying pathophysiology. Tests to consider ordering are outlined in the Table
Diabetes and insulin resistance. The American Diabetes Association recommends screening patients who are overweight or obese and have an additional risk factor for diabetes.5 This can be done by obtaining a fasting glucose level, hemoglobin A1C, or a 2-hour oral glucose tolerance test.
Continue to: Since it is known that...
Since it is known that insulin resistance increases the risk for coronary heart disease6 and can be treated effectively,7 we recommend testing for insulin resistance in patients who do not already have impaired fasting glucose, prediabetes, type 2 diabetes, or impaired glucose tolerance. The homeostatic model assessment for insulin resistance (HOMA-IR)8 is a measure of insulin resistance and can be calculated from the fasting insulin and fasting glucose levels. This measure should not be done in isolation, but it can be a useful adjunct in identifying patients with insulin resistance and directing treatment.
If there is evidence of diabetes or insulin resistance, consider treatment with metformin ± initiation of a low-carbohydrate diet.
Hypothyroidism. Consider screening for thyroid dysfunction with a thyroid-stimulating hormone level, if it has not been checked previously.
Renal abnormalities. When serum creatinine levels and glomerular filtration rate indicate chronic kidney disease, consider recommending a protein-restricted diet and adjust medications according to renal dosing protocols, as indicated.
Liver abnormalities, including nonalcoholic fatty liver disease (NAFLD). Monitor aspartate aminotransferase and alanine aminotransferase for resolution of elevations as weight loss is achieved. If abnormalities persist, consider ordering a liver ultrasound. Traditionally, low-calorie diets have been prescribed to treat NAFLD, but evidence shows that low-carbohydrate diets can also be effective.9
Continue to: Hypertriglyceridemia and low high-density lipoprotein (HDL) levels
Hypertriglyceridemia and low high-density lipoprotein (HDL) levels. Obtain a lipid panel if one has not been completed within the past several years, as hypertriglyceridemia and low HDL can improve dramatically with specific dietary changes.7 Observe trends to assess for resolution of lipid abnormalities as weight loss is achieved.
Gout. Consider checking a uric acid level if you are thinking about recommending a low-carbohydrate diet, particularly in patients with a history of gout, as this may temporarily increase the risk of gout flare.
Hypovitaminosis D. If the patient’s vitamin D level is low, consider appropriate supplementation to support the patient’s overall health. While vitamin D deficiency is common in obesity, the role of supplementation in this population is unclear.
Cardiovascular disease. Consider ordering an electrocardiogram, particularly if you are thinking of prescribing medication therapy. Use caution with initiation of certain medications, such as phentermine or diethylproprion, in the presence of arrhythmias or active cardiovascular disease.
Obstructive sleep apnea. Sleep health is important to address, since obesity is one of the most significant risk factors for obstructive sleep apnea.10 If your patient is given a diagnosis of OSA following a sleep study, consider treatment with continuous positive airway pressure (CPAP), although there are conflicting studies regarding the effects of CPAP therapy in OSA on weight.11,12
Continue to: Provide guidance on lifestyle changes
Provide guidance on lifestyle changes
Addressing obesity with patients can be challenging in a busy primary care clinic, but it is imperative to helping patients achieve overall health. Counseling on nutrition and physical activity is an important part of this process.
There is no one-size-fits-all approach to nutrition counseling. Focus on creating individualized plans through which patients can achieve success. Some guidance follows, but also beware of common pitfalls that we have observed in clinical practice which, when addressed, can enable significant weight loss (see “Common pitfalls inhibiting weight loss”).
SIDEBAR
Common pitfalls inhibiting weight loss
On the part of the patient:
- Continuing to consume substantial amounts of high-calorie drinks.
- Taking in excessive amounts of sugar-rich foods, including cough drops.
- Using non-nutritive sweeteners (eg, aspartame, saccharin, sucralose, and erythritol). Although the mechanism is not certain, some people are able to lose weight while consuming these substances, while others are not.
On the part of the provider:
- Prescribing a diet that the patient cannot sustain long term.
- Overlooking the issue of food availability for the patient.
Choose an approach that works for the patient. Commonly prescribed diets to address obesity include, but are not limited to, Atkins, Dietary Approaches to Stop Hypertension (DASH), Glycemic Index, Mediterranean, Ornish, Paleolithic, Zone, whole food plant-based, and ketogenic. We attempt to engage patients in making the decision on what food choices are appropriate for them considering their food availability, culture, and belief systems. For patients who prefer a vegan or vegetarian whole food diet, it is important to note that these diets are generally deficient in vitamin B12 and omega 3 fatty acids, so supplementing these should be considered.
Rather than focus on a specific diet, which may not be sustainable long term, encourage healthy eating habits. Low-carbohydrate diets have been shown to promote greater weight loss compared to low-fat diets.13,14 Low-calorie diets can also be quite effective in promoting short-term weight loss. In our clinic, when weight loss is the primary goal, patients are typically encouraged to focus on either calorie or carbohydrate restriction in the initial stages of weight loss.
Eliminate sugar and refined carbohydrates. While rigorous mortality data are not available, more recent trials have demonstrated significant improvements in atherosclerotic cardiovascular disease risk markers, including weight reduction and diabetes reversal, when following a diet that markedly decreases carbohydrate intake, especially sugar and refined carbohydrates.7,14-17
Continue to: We recommend that patients focus...
We recommend that patients focus on eliminating sweetened beverages, such as soft drinks, sports drinks, energy drinks, vitamin water, sweet tea, chocolate milk, and Frappuccinos. We also recommend substantially limiting or eliminating fruit juices and fruit smoothies due to their high sugar content. For example, 8 oz of orange juice contains 26 g of carbohydrates, which is almost as much as 8 oz of soda.
Compared with eating whole fruit, consuming fruit juice has demonstrated a small amount of weight gain in young children and adults.18,19 It also has shown a higher insulin response compared with eating the same amount of carbohydrates in whole fruit.20 Better options to drink include water, unsweetened tea, and black coffee. Also, avoid ultra-processed carbohydrates from foods such as breads, cereals, and pastries, as they have similar effects on blood glucose when compared to sugar.21
Greatly restrict highly processed foods. The evidence suggests that the availability of processed food is associated with increasing obesity.22 Simple advice to offer your patients is to encourage them to shop the perimeter of the grocery store, where fresh produce, meat, and dairy products are primarily located, and avoid the inner aisles, which contain primarily processed foods. Choosing food items with 5 or fewer ingredients is a starting point when teaching patients to read labels.
Consider limiting saturated fats. In 1977, the Dietary Guidelines for Americans recommended that Americans eat no more than 30% of total energy intake from fat and less than 10% of total energy intake from saturated fat; however, no randomized controlled trials had been done that supported this recommendation and epidemiologic data supporting it were weak.23
The 2015 Dietary Guidelines continue to recommend limiting total energy intake from saturated fats.24 While there may be a small decrease in cardiovascular risk with a reduction of saturated fat intake and replacement with unsaturated fats, no overall mortality benefit has been demonstrated.24,25 More research is needed in this area to guide patients in decisions regarding consumption of saturated fats and what types of unsaturated fats are best for their health.
Continue to: Eat only 3 meals per day
Eat only 3 meals per day, but aim for fewer than that. The prescription of fasting is a modality that can be used for weight loss and improved health. Fasting has been a prescribed healing practice for thousands of years.26 It is a practice that virtually every major religion in the world embraces. Studies have demonstrated fasting to be safe and effective in the setting of obesity without significant comorbidities, and it may promote weight loss and metabolic health.26-29
There are multiple types of intermittent fasting. A practical way for patients to start is by restricting the number of hours in which they eat or drink calorie-containing beverages to 8 hours per day. In our experience, this regimen is easier for most patients to follow than alternate-day or other longer fasts. While there has been caution in the prescription of intermittent fasting due to concerns about causing eating disorders, a recent small study did not demonstrate increased risk of eating disorders with daily intermittent fasting.30
Participate in healthy exercise. Nonpharmacologic office-based strategies for treating obesity have generally focused on increasing exercise and decreasing caloric intake.31 While exercise has significant health benefits, including preventing weight regain, evidence does not support monotherapy with exercise as an effective long-term weight-loss strategy.32 There are no studies available that adequately support prescribing an exact dose of exercise.33 Generally, less than 150 minutes of exercise per week is not effective and more than that does have a dose-related response.33
Follow up to help patients stay on target
There is no ideal interval for follow-up visits. However, frequent visits—anywhere from weekly to monthly—in the initial stages of weight loss increase the patient’s sense of accountability and, in our experience, seem to be helpful.
Patients may also choose to track their progress by weighing themselves regularly. A small study published in the International Journal of Obesity found that patients who weighed themselves daily had greater and more sustained weight loss than those who didn’t.34 But the decision of whether to weigh one’s self at home should be individualized for each patient.
CORRESPONDENCE
Wesley Eichorn, DO, 1000 Oakland Drive, Kalamazoo, MI 49008; wesley.eichorn@med.wmich.edu
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016 key findings data from the National Health and Nutrition Examination Survey. NCHS Data Brief. 2017;(288):1-8.
2. Seger JC, Horn DB, Westman EC, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed March 5, 2021. www.obesityalgorithm.org. 2016-2017
3. Dorflinger LM, Ruser CB, Masheb RM. A brief screening measure for binge eating in primary care. Eat Behav. 2017;26:163-166. https://doi.org/10.1016/j.eatbeh.2017.03.009
4. Saunders KH, Igel LI, Shukla AP, et al. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65:780-788.
5. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S13-S28. https://doi.org/10.2337/dc19-S002
6. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754-1759. https://doi.org/10.1161/ATVBAHA.111.241885/-/DC1
7. Hallberg S, McKenzie A, Williams P, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9:583-612. https://doi.org/10.6084/m9.figshare
8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495.
9. Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9:e023597. https://doi.org/10.1136/bmjopen-2018-023597
10. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1217-1239. https://doi.org/10.1164/rccm.2109080
11. Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258-264. https://doi.org/10.1136/thoraxjnl-2014-205361
12. Bosworth T. CPAP use associated with greater weight loss in obese patients with sleep apnea. CHEST Physician. Published March 29, 2019. Accessed March 5, 2021. www.mdedge.com/chestphysician/article/197827/sleep-medicine/cpap-use-associated-greater-weight-loss-obese-patients
13. Tobias DK, Chen M, Manson JAE, et al. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968-979. https://doi.org/10.1016/S2213-8587(15)00367-8
14. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets: a meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
15. Bezerra Bueno N, Vieira De Melo IS, Lima De Oliveira S, et al. Very-low-carbohydrate ketogenic diet v low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. https://doi.org/10.1017/S0007114513000548
16. Santos FL, Esteves SS, da Costa Pereira A, et al. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048-1066. https://doi.org/10.1111/j.1467-789X.2012.01021.x
17. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. bioRxiv. 2018;10:348. https://doi.org/10.1101/476275
18. Auerbach BJ, Dibey S, Vallila-Buchman P, et al. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9:78-85. https://doi.org/10.1093/advances/nmx006
19. Faith MS, Dennison BA, Edmunds LS, et al. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics. 2006;118:2066-2075. https://doi.org/10.1542/peds.2006-1117
20. Bolton RP, Burroughs LF, Heaton KW. The role of dietary fiber in satiety, insulin: studies with fruit and fruit. Am J Clin Nutr. 1981;84:211-217. https://doi.org/10.1093/ajcn/34.2.211
21. Unwin D, Haslam D, Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insul Resist. 2016;1(1):a8. https://doi.org/10.4102/jir.v1i1.8
22. Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018;21:18-26. https://doi.org/10.1017/S1368980017001379
23. Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014
24. US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th edition. Published December 2015. Accessed March 5, 2021. http://health.gov/dietaryguidelines/2015/guidelines/
25. Harcombe Z, Baker JS, DiNicolantonio JJ, et al. Evidence from randomised controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Hear. 2016;3:e000409. https://doi.org/10.1136/openhrt-2016-000409
26. Fung J. The Obesity Code: Unlocking the Secrets of Weight Loss. Greystone Books; 2016.
27. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46-58. https://doi.org/10.1016/j.arr.2016.10.005
28. Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371-393. https://doi.org/10.1146/annurev-nutr-071816-064634
29. Duncan GG. Intermittent fasts in the correction and control of intractable obesity. Trans Am Clin Climatol Assoc. 1962;74:121-129.
30. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Appl Physiol Nutr Metab. 2019;44:107-109. https://doi.org/10.1139/apnm-2018-0389
31. Erlandson M, Ivey LC, Seikel K. Update on office-based strategies for the management of obesity. Am Fam Physician. 2016;94:361-368.
32. Malhotra A, Noakes T, Phinney S. It is time to bust the myth of physical inactivity and obesity: you cannot outrun a bad diet. Br J Sports Med. 2015;49:967-968. https://doi.org/10.1136/bjsports-2015-094911
33. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459-471. https://doi.org/10.1249/MSS.0b013e3181949333
34. Zheng Y, Burke LE, Danford CA, et al. Patterns of self-weighing behavior and weight change in a weight loss trial. Int J Obes (Lond). 2016;40:1392-1396. https://doi.org/10.1038/ijo.2016.68
PRACTICE RECOMMENDATIONS
› Create an office environment where patients feel comfortable discussing their weight. C
› Screen overweight and obese patients for comorbidities. B
› Focus on nutritional changes more than exercise when working with patients who want to lose weight. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series