User login
‘Stunning’ twincretin beats semaglutide for A1c, weight reduction in T2D
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
FROM ADA 2021
Post–acute kidney injury proteinuria predicts subsequent kidney disease progression
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Fact or fiction? Intravascular contrast and acute kidney injury
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.
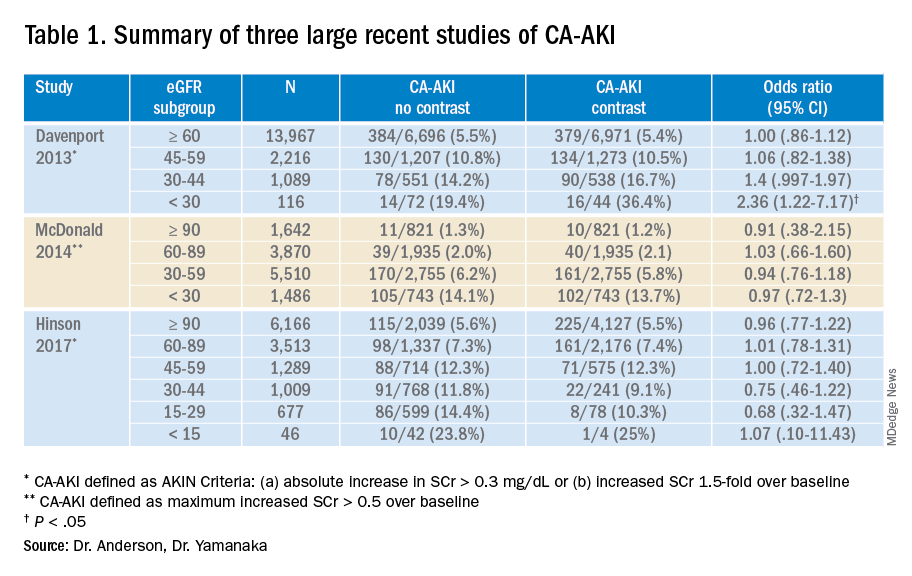
Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Withholding contrast may be the greater risk
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
The most important meal of the day, with extra zinc
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Third COVID-19 vaccine dose helped some transplant recipients
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
For patients with advanced CKD, low risk of nephrogenic systemic fibrosis with group II GBCAs
Background: With more than 500 cases of NSF reported during 1997-2007, a black box warning advises against use of all GBCAs in at-risk CKD patients. However, newer literature has shown that group II GBCAs may have lower risks of causing NSF. The risk to patients with CKD IV and V is not clear.
Study design: Systematic review and meta-analysis.
Setting: 2,700 citations were screened for eligibility, of which 16 studies were selected.
Synopsis: The authors evaluated 4,931 administrations of group II GBCAs in patients with CKD stages IV and V to determine the pooled incidence of NSF in this population. The pooled incidence of NSF was 0% (0 out of 4,931) with an upper bound of the 95% confidence interval of 0.07%. The analysis did not examine sequential group II GBCA exposures or the use of group II GBCAs in the setting of acute kidney injury. The authors advocate that the harms of withholding group II GBCAs in patients with advanced CKD (e.g., underdiagnosis or delay in diagnosis) may outweigh the risk of group II GBCA administration in this population.
Bottom line: The risk of NSF with use of group II GBCAs in patients with advanced CKD is likely less than 0.7%.
Citation: Woolen SA et al. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: A systematic review and meta-analysis. JAMA Intern Med. 2020;180(2):223-30.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
Background: With more than 500 cases of NSF reported during 1997-2007, a black box warning advises against use of all GBCAs in at-risk CKD patients. However, newer literature has shown that group II GBCAs may have lower risks of causing NSF. The risk to patients with CKD IV and V is not clear.
Study design: Systematic review and meta-analysis.
Setting: 2,700 citations were screened for eligibility, of which 16 studies were selected.
Synopsis: The authors evaluated 4,931 administrations of group II GBCAs in patients with CKD stages IV and V to determine the pooled incidence of NSF in this population. The pooled incidence of NSF was 0% (0 out of 4,931) with an upper bound of the 95% confidence interval of 0.07%. The analysis did not examine sequential group II GBCA exposures or the use of group II GBCAs in the setting of acute kidney injury. The authors advocate that the harms of withholding group II GBCAs in patients with advanced CKD (e.g., underdiagnosis or delay in diagnosis) may outweigh the risk of group II GBCA administration in this population.
Bottom line: The risk of NSF with use of group II GBCAs in patients with advanced CKD is likely less than 0.7%.
Citation: Woolen SA et al. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: A systematic review and meta-analysis. JAMA Intern Med. 2020;180(2):223-30.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
Background: With more than 500 cases of NSF reported during 1997-2007, a black box warning advises against use of all GBCAs in at-risk CKD patients. However, newer literature has shown that group II GBCAs may have lower risks of causing NSF. The risk to patients with CKD IV and V is not clear.
Study design: Systematic review and meta-analysis.
Setting: 2,700 citations were screened for eligibility, of which 16 studies were selected.
Synopsis: The authors evaluated 4,931 administrations of group II GBCAs in patients with CKD stages IV and V to determine the pooled incidence of NSF in this population. The pooled incidence of NSF was 0% (0 out of 4,931) with an upper bound of the 95% confidence interval of 0.07%. The analysis did not examine sequential group II GBCA exposures or the use of group II GBCAs in the setting of acute kidney injury. The authors advocate that the harms of withholding group II GBCAs in patients with advanced CKD (e.g., underdiagnosis or delay in diagnosis) may outweigh the risk of group II GBCA administration in this population.
Bottom line: The risk of NSF with use of group II GBCAs in patients with advanced CKD is likely less than 0.7%.
Citation: Woolen SA et al. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: A systematic review and meta-analysis. JAMA Intern Med. 2020;180(2):223-30.
Dr. Midha is a hospitalist at Beth Israel Deaconess Medical Center, instructor of medicine, Boston University, and part-time instructor in medicine, Harvard Medical School, all in Boston.
Gene therapy is bad business, and hugging chickens is just … bad
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
A guide to diagnosing and managing ascites in cirrhosis
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).
History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; faisalm@ccf.org
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).
History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; faisalm@ccf.org
Liver cirrhosis is implicated in 75% to 85% of ascites cases in the Western world, with heart failure or malignancy accounting for fewer cases.1 Among patients who have decompensated cirrhosis with ascites, annual mortality is 20%.2 Another study showed a 3-year survival rate after onset of ascites of only 56%.3 It is vital for primary care physicians (PCPs) to be alert for ascites not only in patients who have risk factors for chronic liver disease and cirrhosis—eg, a history of alcohol use disorder, chronic viral infections (hepatitis B and C), or metabolic syndrome—but also in patients with abnormal liver function tests and thrombocytopenia. In this review, we discuss the initial assessment of ascites and its long-term management, concentrating on the role of the PCP.
Pathophysiology: Vasodilation leads to a cascade
Splanchnic vasodilation is the main underlying event triggering a pathologic cascade that leads to the development of ascites.4 Initially portal hypertension in the setting of liver inflammation and fibrosis causes the release of inflammatory cytokines such as nitric oxide and carbon monoxide. This, in turn, causes the pathologic dilation of splanchnic circulation that decreases effective circulating volume. Activation of the sympathetic nervous system, vasopressin, and renin-angiotensin-aldosterone system (RAAS) then causes the proximal and distal tubules to increase renal absorption of sodium and water.5 The resulting volume overload further decreases the heart’s ability to maintain circulating volume, leading to increased activation of compensating symptoms. This vicious cycle eventually manifests as ascites.6
A complex interplay of cirrhosis-associated immune dysfunction (CAID), gut dysbiosis, and increased translocation of microorganisms into ascitic fluid is also an important aspect of the pathogenesis.7 CAID (FIGURE 1)7,8 is an immunodeficient state due to cirrhosis with reduced phagocytic activity by neutrophils and macrophages, T- and B-cell hypoproliferation, and reduced cytotoxicity of natural killer cells. In parallel, there is increased production of inflammatory cytokines due to the effects of damage-associated molecular patterns (DAMPs) from hepatocytes and pathogen-associated molecular patterns (PAMPs) from the gut microbiota on the immune system, which leads to many of the manifestations of decompensated cirrhosis including ascites.8
Key in on these elementsof the history and exam
Each step of the basic work-up for ascites provides opportunities to refine or redirect the diagnostic inquiry (TABLE).
History
Generally, patients with ascites present with weight gain and symptoms of abdominal distension, such as early satiety, nausea, and vomiting. Besides cirrhosis, rule out other causes of ascites, as treatment differs based on the cause.9 Also ask about histories of cancer and cardiac, renal, or thyroid disease.10
Patients with ascites in the setting of liver disease usually are asymptomatic in its early stages. Common complaints are vague abdominal pain, generalized weakness, malaise, and fatigue.11 Ask patients about risk factors for liver disease such as obesity, diabetes, hypertension, alcohol use, unsafe sexual practices, recent travel, and needle sharing or drug use. Due to a strong association between obstructive sleep apnea and fatty liver disease, consider screening at-risk patients for sleep apnea.12
Physical exam
When there are risk factors for liver disease, examine the patient for stigmata of cirrhosis and ascites. Signs of liver disease, aside from ascites, may include spider angiomas on the upper trunk (33% of cirrhosis patients),13 gynecomastia (44% of cirrhosis patients),14 palmar erythema, jaundice, asterixis, and abdominal wall collaterals including caput medusa.15
Continue to: We suggest a systematic...
We suggest a systematic and targeted approach to using various physical exam maneuvers described in the literature. If the patient has a full/distended abdomen, percuss the flanks. If increased dullness at the flanks is detected, check for shifting dullness, which indicates at least 1500 mL of fluid in the abdomen.16 Keep in mind that a 10% chance of ascites exists even if shifting dullness is absent.17 Maneuvers such as the puddle sign and fluid thrill are less accurate than shifting dullness, which has 83% sensitivity and 56% specificity in detecting ascites.17 Patients with cirrhosis also have a high likelihood of complications from ascites such as inguinal, umbilical, and other hernias.
Diagnostic work-up includes blood tests and ultrasound
Blood tests. The initial work-up for ascites should include complete blood count, complete metabolic panel, and prothrombin time/international normalized ratio.18
Abdominal ultrasound is recommended as the first-line imaging test.19 Aside from detecting ascites, it can give an estimate of the volume of ascites and indicate whether it is amenable to paracentesis. A vascular exam added to the standard ultrasound can detect radiologic evidence of portal hypertension such as splenomegaly, portosystemic collaterals, splenorenal shunt, patency of the paraumbilical vein, and portal vein diameter. Patients with established cirrhosis also require abdominal ultrasound every 6 months to screen for hepatocellular cancer.20
Abdominal paracentesis is the cornerstone of ascites evaluation.21 It is indicated for every patient with new-onset ascites or for any patient with known ascites and clinical deterioration. Ascitic fluid analysis can be used to easily differentiate portal hypertension from other causes of ascites. It can also be used to rule out bacterial peritonitis. The recommended sites for evaluation are in the left lower quadrant, 3 cm cranially and 3 cm medially from the anterior superior iliac spine.22 A large cohort study showed that abdominal ultrasound-guided paracentesis reduced bleeding complications by 68% following the procedure and is strongly recommended (if available).23 Generally, paracentesis is a relatively safe procedure with a low risk of complications such as abdominal wall hematoma (1%), hemoperitoneum (< 0.1%), bowel perforation (< 0.1%), and infection (< 0.1%).24
Assess all ascitic fluid samples for color, consistency, cell count and differential, albumin, and total protein. These tests are usually sufficient to provide evidence regarding the cause of ascites. If there is suspicion of infection, order a gram stain and culture (80% sensitivity for detecting an infection if obtained prior to initiation of antibiotics)25 and glucose, lactate dehydrogenase (useful to differentiate primary from secondary bacterial peritonitis),26 and amylase tests. Other tests such as cytology, acid-fast bacilli smear and culture, and triglyceride level should only be obtained if specific conditions are suspected based on high pretest probabilities.
Continue to: Calculating serum ascites albumin gradient...
Calculating serum ascites albumin gradient (SAAG) is recommended as it has been shown to better characterize ascitic fluid than total protein-based tests.27 SAAG is calculated by subtracting the level of ascitic fluid albumin from serum albumin level (SAAG = serum albumin – ascitic fluid albumin). A SAAG ≥ 1.1 g/dL is consistent with portal hypertension,28 with approximately 97% accuracy.
After calculating SAAG, look at total protein levels in ascitic fluid. Total protein concentration ≥ 2.5 g/dL with SAAG ≥ 1.1 g/dL has a 78.3% diagnostic accuracy in determining heart failure as the cause of ascites, with a sensitivity of 53.3% and specificity of 86.7%.28 On the other hand, a value of total protein < 2.5 g/dL indicates cirrhosis, liver failure, or acute hepatitis as the cause of fluid build-up.29 Stepwise evaluation of SAAG and total protein and how they can point toward the most likely cause of ascites is presented in FIGURE 2.27-29
Management
Noninvasive measures
Sodium restriction. The aim of treatment for uncomplicated clinically apparent ascites is sodium restriction and removal of fluid from the body. Dietary salt restriction is complicated, and care should be taken to properly educate patients. Salt restriction advised in the literature has shifted from a strict measure of < 2 g/d30 to more moderate strategies (described below).18
The 2 main reasons for this easing of restriction are issues with patient compliance and concerns about adverse effects with aggressive salt-restricted diets. One study assessing patient compliance with a salt-restricted diet found that more than two-thirds of the patients were noncompliant,31 and 65% of the patients incorrectly assumed they were following the plan, which suggests poor dietary education.31 Of the group that was compliant, 20% actually decreased their caloric intake, which can be detrimental in liver disease.31 Concerns have been raised that aggressive salt restriction along with diuretic use can lead to diuretic-induced hyponatremia and renal failure.32 Current European Association for the Study of the Liver (EASL) guidelines recommend salt restriction to a more moderate degree (80-120 mmol/d of sodium). This is equivalent to 4.9-6.9 g of salt (1 tablespoon is roughly equivalent to 6 g or 104 mmol of sodium).18
Diuretics. Initiation and dosage of diuretic therapy is a matter of some controversy. Historically, simultaneous administration of a loop diuretic and mineralocorticoid receptor blocker were recommended: 40 mg furosemide and 100 mg spironolactone, keeping the ratio constant with any dosage increases. This was based on a randomized controlled trial (RCT) showing that the combined diuretic therapy effectively mobilized ascites in a shorter period of time and with less frequent adverse effects (eg, hyperkalemia) compared with initial monotherapy.33
Continue to: On the other hand...
On the other hand, another study with more stable patients and relatively normal renal function showed that starting with a mineralocorticoid receptor blocker alone with sequential dose increments had equivalent benefit with no increase in adverse effects.34 Since the patient population in this study was more in line with what a PCP might encounter, we recommend following this guideline initially and keeping a close watch on serum electrolytes.
Usual maximum doses are spironolactone 400 mg/d and furosemide 160 mg/d.21,35 Adequate weight loss for patients with diffuse edema is at least 1 kg/d, per EASL guidelines.36,37 However, this might not be practical in outpatient settings, and a more conservative target of 0.5 kg/d may be used for patients without significant edema.37
It is vital to get accurate daily weights and avoid excessive diuretic use, as it has been associated with intravascular volume depletion and acute kidney injury (25%), hyponatremia (28%),38,39 and hepatic encephalopathy (30%).40 Therefore, patients with acute kidney injury, hyponatremia, acute variceal hemorrhage, or infection should also have their diuretics held until their creatinine returns to baseline.
Invasive measures
Large-volume paracentesis. Patients with extensive and tense ascites should be treated initially with large-volume paracentesis, as this has been shown to predictably remove fluid more effectively than diuretics.38 This should be accompanied by albumin administration, 8 g for every liter of ascitic fluid removed if the total amount exceeds 5 L.41 Following large-volume paracentesis, manage patients with the standard salt restriction and diuretic regimen.38 Serial large-volume paracentesis is a temporary measure reserved for a select group of patients who are intolerant to diuretics and are not candidates for a shunt.
Transjugular intrahepatic portosystemic shunt (TIPS) is another option to control refractory ascites, but its benefit should be weighed against complications such as hepatic encephalopathy. An RCT found that TIPS with covered stents improved survival in patients with cirrhosis compared with regular large-volume paracentesis.42 Patients should be referred to hepatologists to make a determination about TIPS placement. Widely accepted contraindications for the placement of TIPS are decompensated cirrhosis (Child-Pugh > 11, model for end-stage liver disease [MELD] > 18), renal failure (serum creatinine > 3 mg/dL), heart failure, porto-pulmonary hypertension, and uncontrolled sepsis.43 Recurrent or persistent hepatic encephalopathy (West Haven grade ≥ 2) is also a contraindication. The West Haven scale is widely used to measure severity of hepatic encephalopathy, grading it from 1 to 4, with 1 being mild encephalopathy characterized by lack of awareness and shorter attention span, and 4 indicating unresponsiveness or coma.44
Continue to: How to manage refractory ascites
How to manage refractory ascites
Fragile patients are those with refractory ascites that is either unresponsive to standard salt restriction and maximum-dose diuretic therapy or that results in a re-accumulation of ascitic fluid soon after paracentesis.45 Specialist care is required to improve survival and quality of life for these patients. They should be referred to a hepatologist for consideration of TIPS placement or liver transplantation.18
Long-term use of albumin was tested in 2 trials for management of decompensated cirrhosis with ascites, yielding conflicting results. The ANSWER trial from Italy showed benefit with this treatment for prolonged survival.46 The other trial, from Spain, showed no benefit from albumin and midodrine administration for survival or for improving complications of cirrhosis.47 The contradictory results are likely due to heterogeneous populations in the 2 trials and differences in dose and duration of albumin administration. Hence, no clear recommendations can be made based on the available data; further research is needed.
Getting a handle on bacterial peritonitis
Bacterial peritonitis can be divided into spontaneous bacterial peritonitis (SBP) and secondary bacterial peritonitis. SBP is a common complication in patients with cirrhosis and occurs in around 16% of hospitalized patients, based on 1 study.48 SBP is defined as a polymorphonuclear leukocyte count ≥ 250 cells/μL in the absence of a surgically treatable source of infection.49 It is believed to be caused by bacterial translocation and is treated empirically with a third-generation cephalosporin. This treatment has been shown to be effective in 85% of patients.50
Patients with SBP are at a higher risk for renal impairment, likely resulting from increased cytokine production and decreased circulatory volume.51 Concomitant albumin administration has been shown to significantly improve outcomes and to reduce rates of hepatorenal syndrome in patients with serum creatinine > 1 mg/dL, blood urea nitrogen > 30 mg/dL, or total bilirubin > 4 mg/dL.52 The recommended amount of albumin is 1.5 g/kg given within 6 hours of SBP detection and repeat administration of 1 g/kg on Day 3.52
Guidelines from the American Association for the Study of Liver Diseases and from EASL recommend the long-term use of daily norfloxacin or trimethoprim-sulfamethoxazole as secondary prophylaxis in patients who have survived an episode of SBP.18
Continue to: Avoid these medications
Avoid these medications
Commonly used medications that should be avoided in patients with cirrhosis and ascites are angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. These agents block the action of angiotensin, which is a vital vasoconstrictor, and thereby cause a drop in blood pressure. This has independently been associated with poor outcomes in patients with cirrhosis.37
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also relatively contraindicated in cirrhosis, as they can affect kidney function, induce azotemia, and reduce kidney sodium excretion. NSAIDs induce vasoconstriction of afferent arterioles in the kidneys, leading to a decreased glomerular filtration rate, further activating RAAS and sympathetic drive. This leads to increased sodium and water retention and worsening ascites.54
Improve outcomes by circling in a hepatologist
PCPs can play a vital role in the prevention, treatment, surveillance, and home care of patients with cirrhosis who are at risk for ascites.55 Referral of patients with hepatic impairment manifesting as unexplained abnormal liver function tests, new-onset ascites, and/or image findings consistent with cirrhosis to a hepatologist at least once is recommended. Such referrals have been shown to be associated with a better overall outcome.56 Patients with known cirrhosis leading to ascites can generally be managed at home with the assistance of specialists and specialized nurses.35
In a study from the University of Michigan, 69% of patients with cirrhosis had at least 1 nonelective readmission; 14% of patients were readmitted within 1 week, and 37% within 1 month.57 These are staggering statistics that highlight the gaps in care coordination and management of patients with cirrhosis in the outpatient setting. PCPs can play a vital role in bridging this gap.
A promising framework is suggested by a study from Italy by Morando et al in 2013.58 The researchers assessed a specialized health care model for cirrhotic patients and showed significant improvement in health care cost, readmission rate, and overall mortality when compared with the existing model of outpatient care.58
Continue to: This was not a blinded study...
This was not a blinded study and there were concerns raised by the scientific community about its design. Because it was conducted in Italy, the results might not be fully applicable to the United States health care setting. However, it did show that better coordination of care leads to significantly better patient outcomes and reduces health care expenditure. Therefore, a more complete understanding of the disease process and latest literature by PCPs, communication with specialists, and comprehensive coordination of care by all parties involved is vital for the management of this patient population.
CORRESPONDENCE
Muhammad Salman Faisal, MD, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195; faisalm@ccf.org
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
1. Runyon BA, Montano AA, Akriviadis EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-220.
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231.
3. Gordon FD. Ascites. Clin Liver Dis. 2012;16:285-299.
4. Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157.
5. Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946.
6. Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284.
7. Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324.
8. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396.
9. Oey RC, van Buuren HR, de Man RA. The diagnostic work-up in patients with ascites: current guidelines and future prospects. Neth J Med. 2016;74:330-335.
10. de Kerguenec C, Hillaire S, Molinié V, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. 2001;96:852-857.
11. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337.
12. Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135.
13. Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34:520-523.
14. Cavanaugh J. Gynecomastia and cirrhosis of the liver. Arch Intern Med. 1990;150:563-565.
15. Karnath B. Stigmata of chronic liver disease. Hosp Phys. 2003;7:14-16,28.
16. Schipper HG, Godfried MH. [Physical diagnosis--ascites]. Ned Tijdschr Geneeskd. 2001;145:260-264.
17. Cattau EL, Jr., Benjamin SB, Knuff TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164-1166.
18. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460.
19. Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-2107.
20. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
21. Runyon BA. Care of patients with ascites. New Engl J Med. 1994;330:337-342.
22. Sakai H, Sheer TA, Mendler MH, et al. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984-986.
23. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538.
24. Ennis J, Schultz G, Perera P, et al. Ultrasound for detection of ascites and for guidance of the paracentesis procedure: technique and review of the literature. Int J Clin Med. 2014;5:1277-1293.
25. Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351-1355.
26. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-133.
27. Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260-273.
28. Farias AQ, Silvestre OM, Garcia-Tsao G, et al. Serum B-type natriuretic peptide in the initial workup of patients with new onset ascites: a diagnostic accuracy study. Hepatology. 2014;59:1043-1051.
29. Gupta R, Misra SP, Dwivedi M, et al. Diagnosing ascites: value of ascitic fluid total protein, albumin, cholesterol, their ratios, serum-ascites albumin and cholesterol gradient. J Gastroenterol Hepatol. 1995;10:295-299.
30. Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. AASLD Practice Guideline. Accessed April 28, 2021. www.aasld.org/sites/default/files/2019-06/AASLDPracticeGuidelineAsciteDuetoCirrhosisUpdate2012Edition4_.pdf
31. Morando F, Rosi S, Gola E, et al. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515.
32. Bernardi M, Laffi G, Salvagnini M, et al. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156-162.
33. Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104.
34. Santos J, Planas R, Pardo A, et al. Spironolactone alone or in combination with furosemide in the treatment of moderate ascites in nonazotemic cirrhosis. A randomized comparative study of efficacy and safety. J Hepatol. 2003;39:187–192.
35. Grattagliano I, Ubaldi E, Bonfrate L, et al. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273-2282.
36. Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: the importance of peripheral edema. Gastroenterology. 1986;90:1827-1833.
37. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
38. Gines P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234-241.
39. Salerno F, Badalamenti S, Incerti P, et al. Repeated paracentesis and i.v. albumin infusion to treat ‘tense’ ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102-108.
40. Sola R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282-288.
41. Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172-1181.
42. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157-163.
43. Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis. 2017;49:121-137.
44. Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721.
45. Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947.
46. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429.
47. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250-1259.
48. Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229.
49. Hoefs JC, Canawati HN, Sapico FL, et al. Spontaneous bacterial peritonitis. Hepatology. 2007;2:399-407.
50. Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462.
51. Lenz K, Kapral C, Gegenhuber A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2004;39:865-866.
52. Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597-599.
53. Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824.
54. Boyer TD, Zia P, Reynolds TB. Effect of indomethacin and prostaglandin A1 on renal function and plasma renin activity in alcoholic liver disease. Gastroenterology. 1979;77:215-222.
55. Grattagliano I, Ubaldi E, Portincasa P, et al. Liver disease: early signs you may be missing. J Fam Pract. 2009;58:514-521.
56. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
57. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252.
58. Morando F, Maresio G, Piano S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257-264.
PRACTICE RECOMMENDATIONS
› Calculate the serum ascites albumin gradient and measure the total ascites protein level to distinguish cirrhotic ascites from that caused by heart failure or other disorders. C
› Recommend sodium restriction of 4.9-6.9 g for patients with established ascites secondary to cirrhosis. C
› Avoid giving angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs in cirrhosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Infants with UTI do not have an increased risk of bacterial meningitis
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
FROM JAMA NETWORK OPEN
Ultrasound renal denervation drops BP in patients on triple therapy
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
FROM ACC 2021