User login
OTC budesonide-formoterol for asthma could save lives, money
, according to a computer modeling study presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio.
Asthma affects 25 million people, about 1 in 13, in the United States. About 28% are uninsured or underinsured, and 70% have mild asthma. Many are using a $30 inhaled epinephrine product (Primatene Mist) – the only FDA-approved asthma inhaler available without a prescription, said Marcus Shaker, MD, MS, professor of pediatrics and medicine at Geisel School of Medicine at Dartmouth, and clinician at Dartmouth Health Children’s, N.H.
A new version of Primatene Mist was reintroduced on the market in 2018 after the product was pulled for containing chlorofluorocarbons in 2011, but it is not recommended by professional medical societies because of safety concerns over epinephrine’s adverse effects, such as increased heart rate and blood pressure.
Drugs in its class (bronchodilators) have long been associated with a higher risk for death or near-death.
Meanwhile, research more than 2 decades ago linked regular use of low-dose inhaled corticosteroids with reduced risk for asthma death.
More recently, two large studies (SYGMA 1 and SYGMA 2) compared maintenance therapy with a low-dose inhaled corticosteroid (budesonide) vs. on-demand treatment with an inhaler containing both a corticosteroid (budesonide) and a long-acting bronchodilator (formoterol).
“Using as-needed budesonide-formoterol led to outcomes that are almost as good as taking a maintenance budesonide dose every day,” said Dr. Shaker.
The Global Initiative for Asthma guidelines now recommend this approach – as-needed inhaled corticosteroids (ICS) plus long-acting bronchodilators – for adults with mild asthma. In the United States, however, the National Heart, Lung, and Blood Institute still suggests daily ICS plus quick-relief therapy as needed.
Dr. Shaker and colleagues used computer modeling to compare the cost-effectiveness of as-needed budesonide-formoterol vs. over-the-counter inhaled epinephrine in underinsured U.S. adults who were self-managing their mild asthma. The study randomly assigned these individuals into three groups: OTC inhaled epinephrine (current reality), OTC budesonide-formoterol (not yet available), or no OTC option. The model assumed that patients treated for an exacerbation were referred to a health care provider and started a regimen of ICS plus as-needed rescue therapy.
In this analysis, which has been submitted for publication, the OTC budesonide-formoterol strategy was associated with 12,495 fewer deaths, prevented nearly 14 million severe asthma exacerbations, and saved more than $68 billion. And “when we looked at OTC budesonide-formoterol vs. having no OTC option at all, budesonide-formoterol was similarly cost-effective,” said Dr. Shaker, who presented the results at an AAAAI oral abstract session.
The cost savings emerged even though in the United States asthma controller therapies (for example, fluticasone) cost about 10 times more than rescue therapies (for instance, salbutamol, OTC epinephrine).
Nevertheless, the results make sense. “If you’re using Primatene Mist, your health costs are predicted to be much greater because you’re going to be in the hospital more. Your asthma is not going to be well-controlled,” Thanai Pongdee, MD, an allergist-immunologist with the Mayo Clinic in Rochester, Minn., told this news organization. “It’s not only the cost of your ER visit but also the cost of loss of work or school, and loss of daily productivity. There are all these associated costs.”
The analysis “is certainly something policy makers could take a look at,” he said.
He noted that current use of budesonide-formoterol is stymied by difficulties with insurance coverage. The difficulties stem from a mismatch between the updated recommendation for as-needed use and the description printed on the brand-name product (Symbicort).
“On the product label, it says Symbicort should be used on a daily basis,” Dr. Pongdee said. “But if a prescription comes through and says you’re going to use this ‘as needed,’ the health plan may say that’s not appropriate because that’s not on the product label.”
Given these access challenges with the all-in-one inhaler, other researchers have developed a workaround – asking patients to continue their usual care (that is, using a rescue inhaler as needed) but to also administer a controller medication after each rescue. When tested in Black and Latino patients with moderate to severe asthma, this easy strategy (patient activated reliever-triggered inhaled corticosteroid, or PARTICS) reduced severe asthma exacerbations about as well as the all-in-one inhaler.
If the all-in-one budesonide-formoterol does become available OTC, Dr. Shaker stressed that it “would not be a substitute for seeing an allergist and getting appropriate medical care and an evaluation and all the rest. But it’s better than the status quo. It’s the sort of thing where the perfect is not the enemy of the good,” he said.
Dr. Shaker is the AAAAI cochair of the Joint Task Force on Practice Parameters and serves as an editorial board member of the Journal of Allergy and Clinical Immunology in Practice. He is also an associate editor of the Annals of Allergy, Asthma, and Immunology. Dr. Pongdee serves as an at-large director on the AAAAI board of directors. He receives grant funding from GlaxoSmithKline, and Mayo Clinic is a trial site for GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
, according to a computer modeling study presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio.
Asthma affects 25 million people, about 1 in 13, in the United States. About 28% are uninsured or underinsured, and 70% have mild asthma. Many are using a $30 inhaled epinephrine product (Primatene Mist) – the only FDA-approved asthma inhaler available without a prescription, said Marcus Shaker, MD, MS, professor of pediatrics and medicine at Geisel School of Medicine at Dartmouth, and clinician at Dartmouth Health Children’s, N.H.
A new version of Primatene Mist was reintroduced on the market in 2018 after the product was pulled for containing chlorofluorocarbons in 2011, but it is not recommended by professional medical societies because of safety concerns over epinephrine’s adverse effects, such as increased heart rate and blood pressure.
Drugs in its class (bronchodilators) have long been associated with a higher risk for death or near-death.
Meanwhile, research more than 2 decades ago linked regular use of low-dose inhaled corticosteroids with reduced risk for asthma death.
More recently, two large studies (SYGMA 1 and SYGMA 2) compared maintenance therapy with a low-dose inhaled corticosteroid (budesonide) vs. on-demand treatment with an inhaler containing both a corticosteroid (budesonide) and a long-acting bronchodilator (formoterol).
“Using as-needed budesonide-formoterol led to outcomes that are almost as good as taking a maintenance budesonide dose every day,” said Dr. Shaker.
The Global Initiative for Asthma guidelines now recommend this approach – as-needed inhaled corticosteroids (ICS) plus long-acting bronchodilators – for adults with mild asthma. In the United States, however, the National Heart, Lung, and Blood Institute still suggests daily ICS plus quick-relief therapy as needed.
Dr. Shaker and colleagues used computer modeling to compare the cost-effectiveness of as-needed budesonide-formoterol vs. over-the-counter inhaled epinephrine in underinsured U.S. adults who were self-managing their mild asthma. The study randomly assigned these individuals into three groups: OTC inhaled epinephrine (current reality), OTC budesonide-formoterol (not yet available), or no OTC option. The model assumed that patients treated for an exacerbation were referred to a health care provider and started a regimen of ICS plus as-needed rescue therapy.
In this analysis, which has been submitted for publication, the OTC budesonide-formoterol strategy was associated with 12,495 fewer deaths, prevented nearly 14 million severe asthma exacerbations, and saved more than $68 billion. And “when we looked at OTC budesonide-formoterol vs. having no OTC option at all, budesonide-formoterol was similarly cost-effective,” said Dr. Shaker, who presented the results at an AAAAI oral abstract session.
The cost savings emerged even though in the United States asthma controller therapies (for example, fluticasone) cost about 10 times more than rescue therapies (for instance, salbutamol, OTC epinephrine).
Nevertheless, the results make sense. “If you’re using Primatene Mist, your health costs are predicted to be much greater because you’re going to be in the hospital more. Your asthma is not going to be well-controlled,” Thanai Pongdee, MD, an allergist-immunologist with the Mayo Clinic in Rochester, Minn., told this news organization. “It’s not only the cost of your ER visit but also the cost of loss of work or school, and loss of daily productivity. There are all these associated costs.”
The analysis “is certainly something policy makers could take a look at,” he said.
He noted that current use of budesonide-formoterol is stymied by difficulties with insurance coverage. The difficulties stem from a mismatch between the updated recommendation for as-needed use and the description printed on the brand-name product (Symbicort).
“On the product label, it says Symbicort should be used on a daily basis,” Dr. Pongdee said. “But if a prescription comes through and says you’re going to use this ‘as needed,’ the health plan may say that’s not appropriate because that’s not on the product label.”
Given these access challenges with the all-in-one inhaler, other researchers have developed a workaround – asking patients to continue their usual care (that is, using a rescue inhaler as needed) but to also administer a controller medication after each rescue. When tested in Black and Latino patients with moderate to severe asthma, this easy strategy (patient activated reliever-triggered inhaled corticosteroid, or PARTICS) reduced severe asthma exacerbations about as well as the all-in-one inhaler.
If the all-in-one budesonide-formoterol does become available OTC, Dr. Shaker stressed that it “would not be a substitute for seeing an allergist and getting appropriate medical care and an evaluation and all the rest. But it’s better than the status quo. It’s the sort of thing where the perfect is not the enemy of the good,” he said.
Dr. Shaker is the AAAAI cochair of the Joint Task Force on Practice Parameters and serves as an editorial board member of the Journal of Allergy and Clinical Immunology in Practice. He is also an associate editor of the Annals of Allergy, Asthma, and Immunology. Dr. Pongdee serves as an at-large director on the AAAAI board of directors. He receives grant funding from GlaxoSmithKline, and Mayo Clinic is a trial site for GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
, according to a computer modeling study presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio.
Asthma affects 25 million people, about 1 in 13, in the United States. About 28% are uninsured or underinsured, and 70% have mild asthma. Many are using a $30 inhaled epinephrine product (Primatene Mist) – the only FDA-approved asthma inhaler available without a prescription, said Marcus Shaker, MD, MS, professor of pediatrics and medicine at Geisel School of Medicine at Dartmouth, and clinician at Dartmouth Health Children’s, N.H.
A new version of Primatene Mist was reintroduced on the market in 2018 after the product was pulled for containing chlorofluorocarbons in 2011, but it is not recommended by professional medical societies because of safety concerns over epinephrine’s adverse effects, such as increased heart rate and blood pressure.
Drugs in its class (bronchodilators) have long been associated with a higher risk for death or near-death.
Meanwhile, research more than 2 decades ago linked regular use of low-dose inhaled corticosteroids with reduced risk for asthma death.
More recently, two large studies (SYGMA 1 and SYGMA 2) compared maintenance therapy with a low-dose inhaled corticosteroid (budesonide) vs. on-demand treatment with an inhaler containing both a corticosteroid (budesonide) and a long-acting bronchodilator (formoterol).
“Using as-needed budesonide-formoterol led to outcomes that are almost as good as taking a maintenance budesonide dose every day,” said Dr. Shaker.
The Global Initiative for Asthma guidelines now recommend this approach – as-needed inhaled corticosteroids (ICS) plus long-acting bronchodilators – for adults with mild asthma. In the United States, however, the National Heart, Lung, and Blood Institute still suggests daily ICS plus quick-relief therapy as needed.
Dr. Shaker and colleagues used computer modeling to compare the cost-effectiveness of as-needed budesonide-formoterol vs. over-the-counter inhaled epinephrine in underinsured U.S. adults who were self-managing their mild asthma. The study randomly assigned these individuals into three groups: OTC inhaled epinephrine (current reality), OTC budesonide-formoterol (not yet available), or no OTC option. The model assumed that patients treated for an exacerbation were referred to a health care provider and started a regimen of ICS plus as-needed rescue therapy.
In this analysis, which has been submitted for publication, the OTC budesonide-formoterol strategy was associated with 12,495 fewer deaths, prevented nearly 14 million severe asthma exacerbations, and saved more than $68 billion. And “when we looked at OTC budesonide-formoterol vs. having no OTC option at all, budesonide-formoterol was similarly cost-effective,” said Dr. Shaker, who presented the results at an AAAAI oral abstract session.
The cost savings emerged even though in the United States asthma controller therapies (for example, fluticasone) cost about 10 times more than rescue therapies (for instance, salbutamol, OTC epinephrine).
Nevertheless, the results make sense. “If you’re using Primatene Mist, your health costs are predicted to be much greater because you’re going to be in the hospital more. Your asthma is not going to be well-controlled,” Thanai Pongdee, MD, an allergist-immunologist with the Mayo Clinic in Rochester, Minn., told this news organization. “It’s not only the cost of your ER visit but also the cost of loss of work or school, and loss of daily productivity. There are all these associated costs.”
The analysis “is certainly something policy makers could take a look at,” he said.
He noted that current use of budesonide-formoterol is stymied by difficulties with insurance coverage. The difficulties stem from a mismatch between the updated recommendation for as-needed use and the description printed on the brand-name product (Symbicort).
“On the product label, it says Symbicort should be used on a daily basis,” Dr. Pongdee said. “But if a prescription comes through and says you’re going to use this ‘as needed,’ the health plan may say that’s not appropriate because that’s not on the product label.”
Given these access challenges with the all-in-one inhaler, other researchers have developed a workaround – asking patients to continue their usual care (that is, using a rescue inhaler as needed) but to also administer a controller medication after each rescue. When tested in Black and Latino patients with moderate to severe asthma, this easy strategy (patient activated reliever-triggered inhaled corticosteroid, or PARTICS) reduced severe asthma exacerbations about as well as the all-in-one inhaler.
If the all-in-one budesonide-formoterol does become available OTC, Dr. Shaker stressed that it “would not be a substitute for seeing an allergist and getting appropriate medical care and an evaluation and all the rest. But it’s better than the status quo. It’s the sort of thing where the perfect is not the enemy of the good,” he said.
Dr. Shaker is the AAAAI cochair of the Joint Task Force on Practice Parameters and serves as an editorial board member of the Journal of Allergy and Clinical Immunology in Practice. He is also an associate editor of the Annals of Allergy, Asthma, and Immunology. Dr. Pongdee serves as an at-large director on the AAAAI board of directors. He receives grant funding from GlaxoSmithKline, and Mayo Clinic is a trial site for GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
FROM AAAAI 2023
Pulmonary hypertension: An update of Dx and Tx guidelines
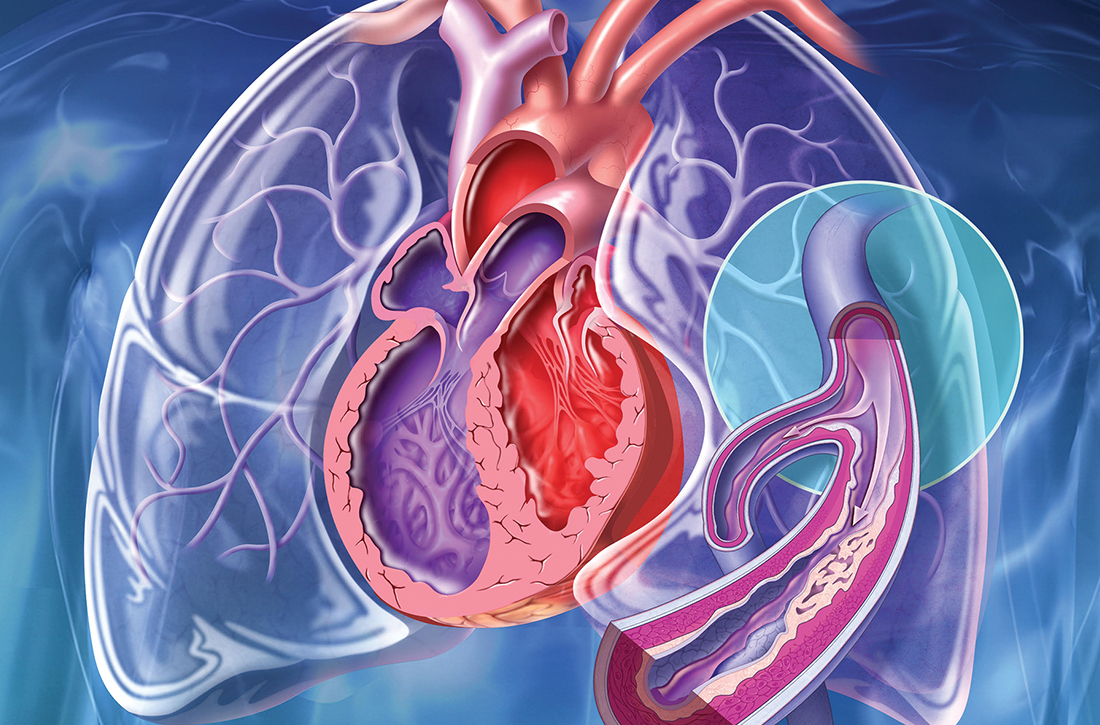
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.
Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.
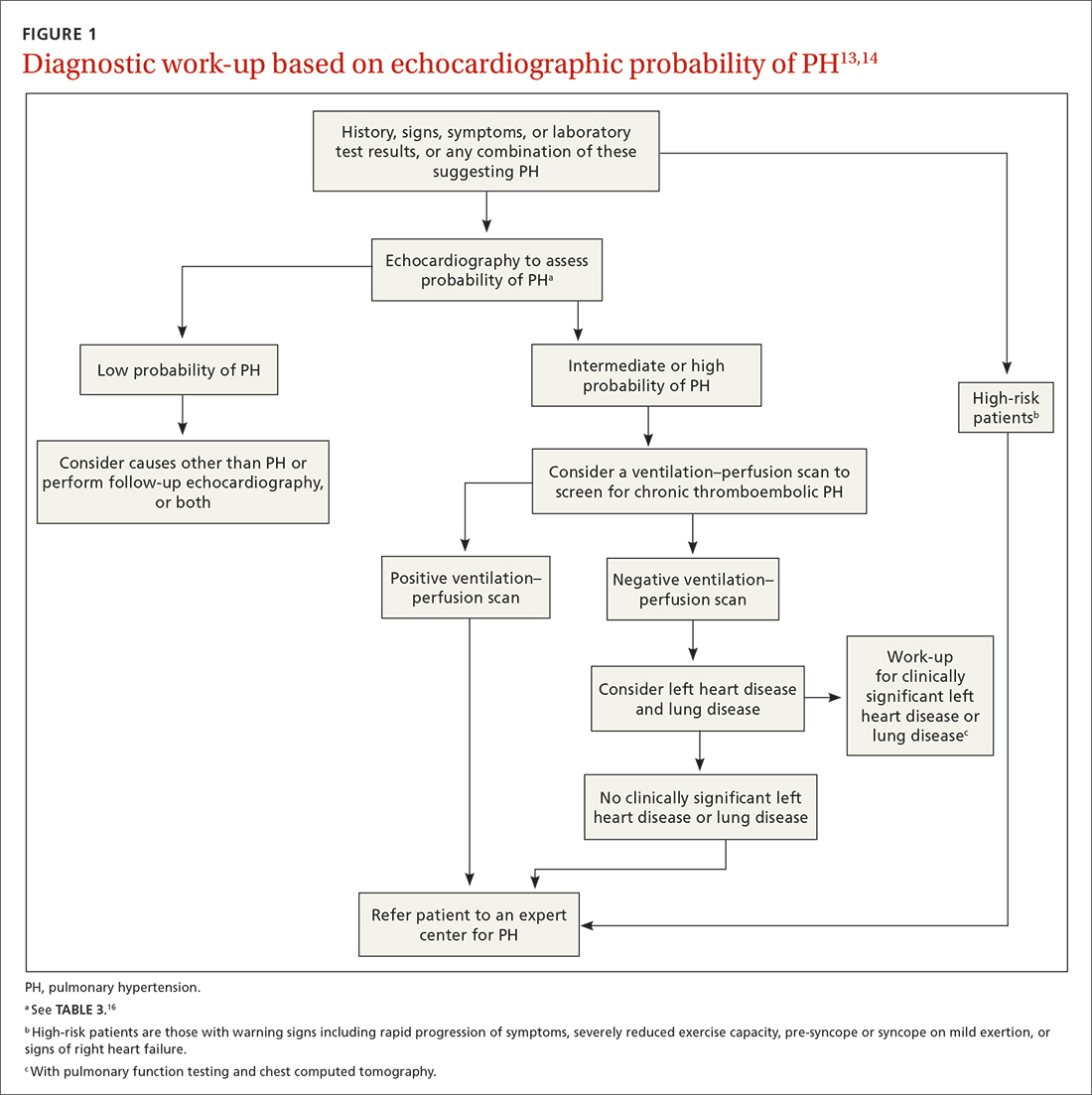
Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7
Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6
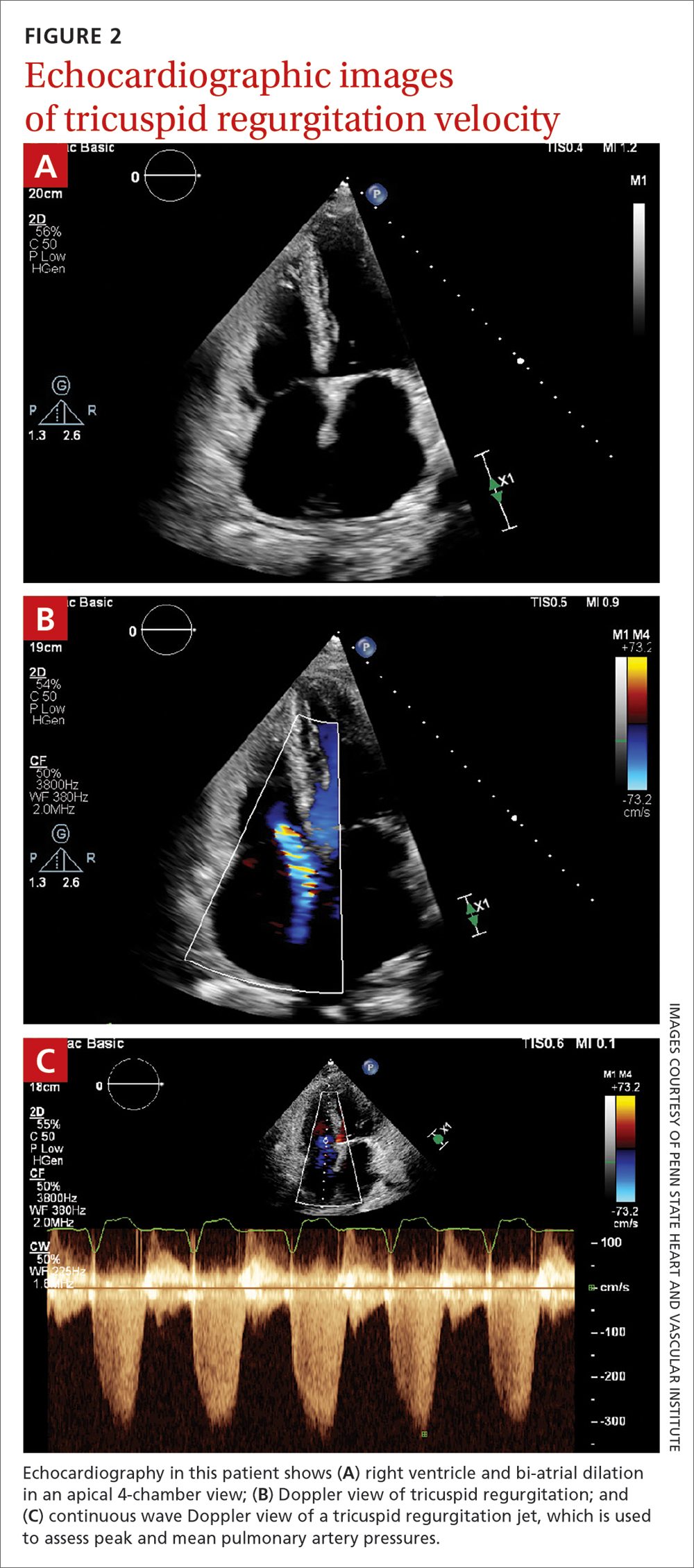
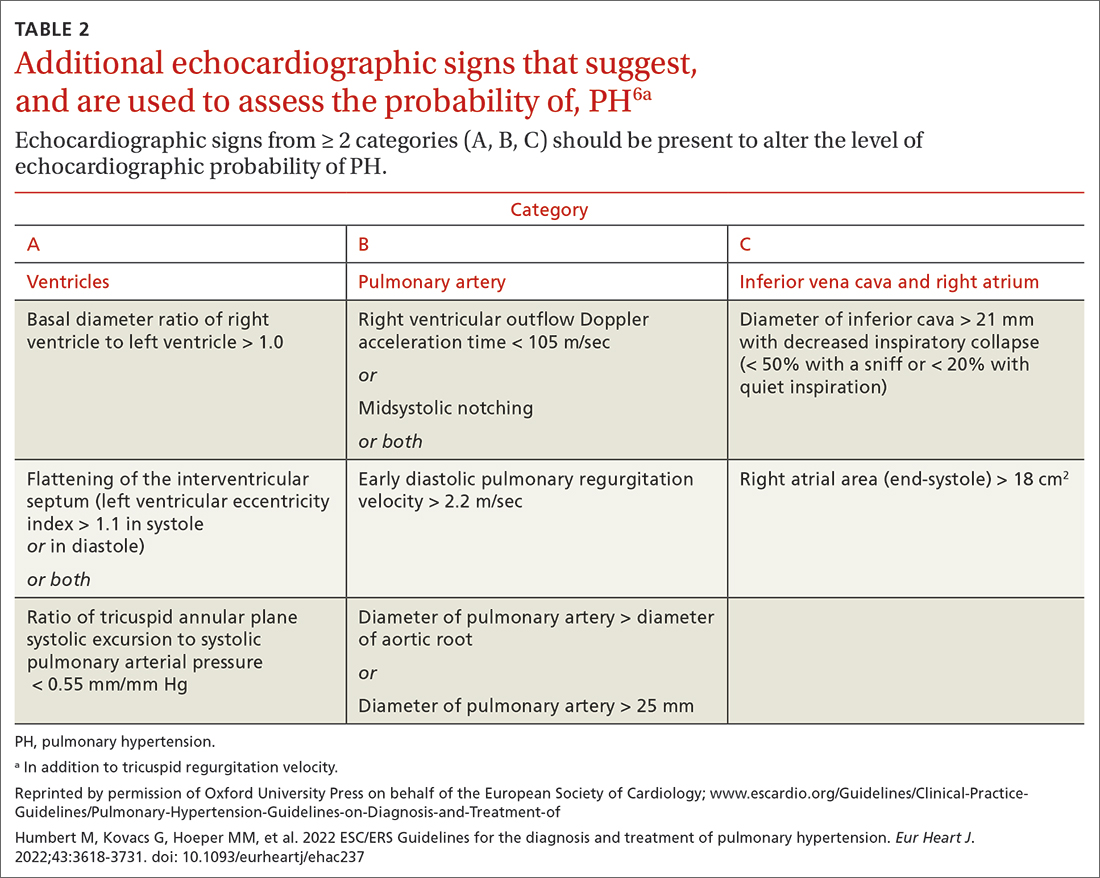
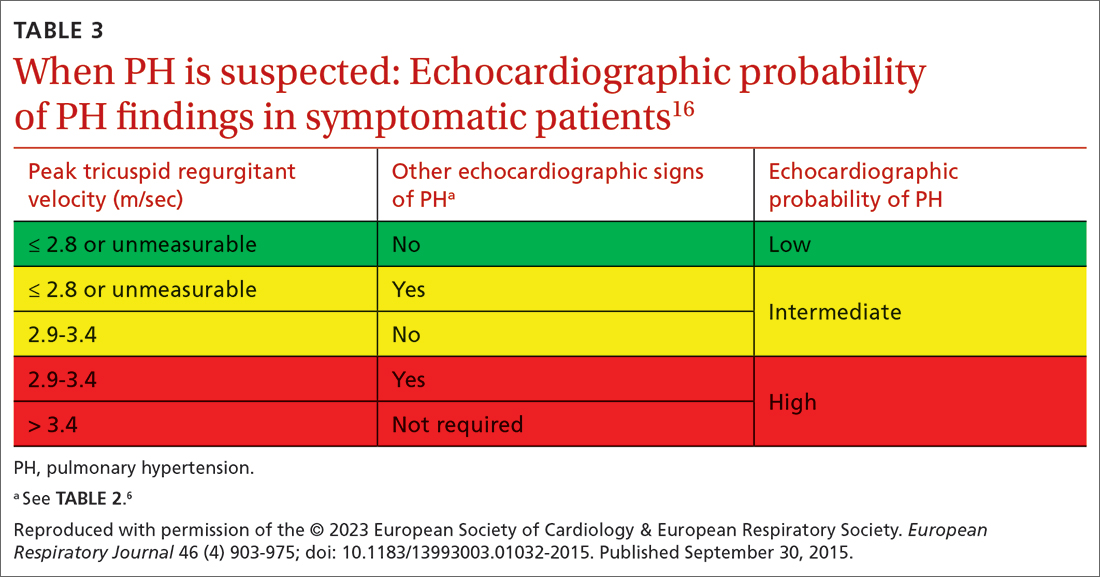
Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).
Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18
TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; msingh1@pennstatehealth.psu.edu
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; msingh1@pennstatehealth.psu.edu
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; msingh1@pennstatehealth.psu.edu
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
PRACTICE RECOMMENDATIONS
› Employ echocardiography as the first-line diagnostic test when pulmonary hypertension (PH) is suspected. C
› Order a ventilation– perfusion scan in patients with unexplained PH to exclude chronic thromboembolic PH. C
› Order lung function testing with diffusion capacity for carbon monoxide as part of the initial evaluation of PH. C
› Use right heart catheterization to confirm the diagnosis of pulmonary arterial hypertension. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Call it preclinical or subclinical, ILD in RA needs to be tracked
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Beware risk of sedatives for respiratory patients
Both asthma and chronic obstructive pulmonary disease can be challenging to diagnose, and medication-driven episodes of sedation or hypoventilation are often overlooked as causes of acute exacerbations in these conditions, according to a letter published in The Lancet Respiratory Medicine.
write Christos V. Chalitsios, PhD, of the University of Nottingham, England, and colleagues.
The authors note that exacerbations are the main complications of both asthma and COPD, and stress the importance of identifying causes and preventive strategies.
Sedatives such as opioids have been shown to depress respiratory drive, reduce muscle tone, and increase the risk of pneumonia, they write. The authors also propose that the risk of sedative-induced aspiration or hypoventilation would be associated with medications including pregabalin, gabapentin, and amitriptyline.
Other mechanisms may be involved in the association between sedatives and exacerbations in asthma and COPD. For example, sedative medications can suppress coughing, which may promote airway mucous compaction and possible infection, the authors write.
Most research involving prevention of asthma and COPD exacerbations has not addressed the potential impact of sedatives taken for reasons outside of obstructive lung disease, the authors say.
“Although the risk of sedation and hypoventilation events are known to be increased by opioids and antipsychotic drugs, there has not been a systematic assessment of commonly prescribed medications with potential respiratory side-effects, including gabapentin, amitriptyline, and pregabalin,” they write.
Polypharmacy is increasingly common and results in many patients with asthma or COPD presenting for treatment of acute exacerbations while on a combination of gabapentin, pregabalin, amitriptyline, and opioids, the authors note; “however, there is little data or disease-specific guidance on how best to manage this problem, which often starts with a prescription in primary care,” they write. Simply stopping sedatives is not an option for many patients given the addictive nature of these drugs and the unlikely resolution of the condition for which the drugs were prescribed, the authors say. However, “cautious dose reduction” of sedatives is possible once patients understand the reason, they add.
Clinicians may be able to suggest reduced doses and alternative treatments to patients with asthma and COPD while highlighting the risk of respiratory depression and polypharmacy – “potentially reducing the number of exacerbations of obstructive lung disease,” the authors conclude.
The study received no outside funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Both asthma and chronic obstructive pulmonary disease can be challenging to diagnose, and medication-driven episodes of sedation or hypoventilation are often overlooked as causes of acute exacerbations in these conditions, according to a letter published in The Lancet Respiratory Medicine.
write Christos V. Chalitsios, PhD, of the University of Nottingham, England, and colleagues.
The authors note that exacerbations are the main complications of both asthma and COPD, and stress the importance of identifying causes and preventive strategies.
Sedatives such as opioids have been shown to depress respiratory drive, reduce muscle tone, and increase the risk of pneumonia, they write. The authors also propose that the risk of sedative-induced aspiration or hypoventilation would be associated with medications including pregabalin, gabapentin, and amitriptyline.
Other mechanisms may be involved in the association between sedatives and exacerbations in asthma and COPD. For example, sedative medications can suppress coughing, which may promote airway mucous compaction and possible infection, the authors write.
Most research involving prevention of asthma and COPD exacerbations has not addressed the potential impact of sedatives taken for reasons outside of obstructive lung disease, the authors say.
“Although the risk of sedation and hypoventilation events are known to be increased by opioids and antipsychotic drugs, there has not been a systematic assessment of commonly prescribed medications with potential respiratory side-effects, including gabapentin, amitriptyline, and pregabalin,” they write.
Polypharmacy is increasingly common and results in many patients with asthma or COPD presenting for treatment of acute exacerbations while on a combination of gabapentin, pregabalin, amitriptyline, and opioids, the authors note; “however, there is little data or disease-specific guidance on how best to manage this problem, which often starts with a prescription in primary care,” they write. Simply stopping sedatives is not an option for many patients given the addictive nature of these drugs and the unlikely resolution of the condition for which the drugs were prescribed, the authors say. However, “cautious dose reduction” of sedatives is possible once patients understand the reason, they add.
Clinicians may be able to suggest reduced doses and alternative treatments to patients with asthma and COPD while highlighting the risk of respiratory depression and polypharmacy – “potentially reducing the number of exacerbations of obstructive lung disease,” the authors conclude.
The study received no outside funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Both asthma and chronic obstructive pulmonary disease can be challenging to diagnose, and medication-driven episodes of sedation or hypoventilation are often overlooked as causes of acute exacerbations in these conditions, according to a letter published in The Lancet Respiratory Medicine.
write Christos V. Chalitsios, PhD, of the University of Nottingham, England, and colleagues.
The authors note that exacerbations are the main complications of both asthma and COPD, and stress the importance of identifying causes and preventive strategies.
Sedatives such as opioids have been shown to depress respiratory drive, reduce muscle tone, and increase the risk of pneumonia, they write. The authors also propose that the risk of sedative-induced aspiration or hypoventilation would be associated with medications including pregabalin, gabapentin, and amitriptyline.
Other mechanisms may be involved in the association between sedatives and exacerbations in asthma and COPD. For example, sedative medications can suppress coughing, which may promote airway mucous compaction and possible infection, the authors write.
Most research involving prevention of asthma and COPD exacerbations has not addressed the potential impact of sedatives taken for reasons outside of obstructive lung disease, the authors say.
“Although the risk of sedation and hypoventilation events are known to be increased by opioids and antipsychotic drugs, there has not been a systematic assessment of commonly prescribed medications with potential respiratory side-effects, including gabapentin, amitriptyline, and pregabalin,” they write.
Polypharmacy is increasingly common and results in many patients with asthma or COPD presenting for treatment of acute exacerbations while on a combination of gabapentin, pregabalin, amitriptyline, and opioids, the authors note; “however, there is little data or disease-specific guidance on how best to manage this problem, which often starts with a prescription in primary care,” they write. Simply stopping sedatives is not an option for many patients given the addictive nature of these drugs and the unlikely resolution of the condition for which the drugs were prescribed, the authors say. However, “cautious dose reduction” of sedatives is possible once patients understand the reason, they add.
Clinicians may be able to suggest reduced doses and alternative treatments to patients with asthma and COPD while highlighting the risk of respiratory depression and polypharmacy – “potentially reducing the number of exacerbations of obstructive lung disease,” the authors conclude.
The study received no outside funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Insomnia, short sleep linked to greater risk for MI
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Myths about smoking, diet, alcohol, and cancer persist
FRANCE – Conducted every 5 years since 2005, the Cancer Survey documents the knowledge, perceptions, and way of life of the French people in relation to cancer. The researchers analyzed responses to telephone interviews of a representative sample of almost 5,000 individuals aged 15-85 years.
This study shows how thinking has changed over time and how difficult it is to alter preconceived notions.
Is cancer hereditary?
The report shows that 67.7% of respondents believe that cancer is a hereditary disease. Respondents were asked to explain their answer. “Data show that medical practices for cancer treatment substantiate this belief [that cancer is hereditary],” wrote the authors of the report.
“Indeed, health care professionals almost systematically ask questions about family history of breast cancer and, when a family member has been diagnosed with cancer, medical monitoring of other family members is often sought out, thus reinforcing the belief that cancer is hereditary,” they said.
Furthermore, there seems to be confusion regarding the role of genes in the development of cancer. A person can inherit cancer-predisposing genes, not cancer itself. The authors highlighted their concern that this confusion may “lead people to think that prevention measures are unnecessary because cancer is inherited.”
Misconceptions about smoking
About 41% of smokers think that the length of time one has been smoking is the biggest determining factor for developing cancer; 58.1% think the number of cigarettes smoked per day has a bigger impact.
Experts at InCA and SPF put the debate to rest, stating that prolonged exposure to carcinogenic substances is far more toxic. As for the danger threshold concerning the number of cigarettes smoked per day, respondents believed this to be 9.2 cigarettes per day, on average. They believed that the danger threshold for the number of years as an active smoker is 13.4, on average.
“The [survey] respondents clearly understand that smoking carries a risk, but many smokers think that light smoking or smoking for a short period of time doesn’t carry any risks.” Yet it is understood that even occasional tobacco consumption increases mortality.
This was not the only misconception regarding smoking and its relationship with cancer. About 34% of survey respondents agreed with the following statement: “Smoking doesn’t cause cancer unless you’re a heavy smoker and have smoked for a long time.” Furthermore, 43.3% agreed with the statement, “Pollution is more likely to cause cancer than smoking,” 54.6% think that “exercising cleans your lungs of tobacco,” and 61.6% think that “a smoker can prevent developing cancer caused by smoking if they know to quit on time.”
Overweight and obesity
Although diet and excess weight represent the third and fourth biggest avoidable cancer risk factors, after smoking and alcohol, only 30% of survey respondents knew of this link.
“Among the causes of cancer known and cited by respondents without prompting, excessive weight and obesity were mentioned only 100 times out of 12,558 responses,” highlighted the authors of the report. The explanation put forward by the authors is that discourse about diet has been more focused on diet as a protective health factor, especially in preventing cardiovascular diseases. “The link between cancer and diet is less prominent in the public space,” they noted.
Breastfeeding and cancer
About 63% of survey respondents, which for the first time included both women and men, believe that breastfeeding does not affect mothers’ risk of breast cancer, but this is a misconception. And almost 1 in 3 respondents said that breastfeeding provides health benefits for the mother.
Artificial UV rays
Exposure to UV rays, whether of natural or artificial origin, is a major risk factor for skin cancer. However, 1 in 5 people (20.9%) think that a session in a tanning bed is less harmful than sun exposure.
Daily stress
Regarding psychological factors linked to cancer, the authors noted that risk factors not supported by scientific evidence were, ironically, cited more often by respondents than proven risk factors. There is a real knowledge gap between scientific data and the beliefs of the French people. For example, “working at night” is largely not seen as a risk factor, but data show that it presents a clear risk. However, “not being able to express one’s feelings,” “having been weakened by traumatic experiences,” and “being exposed to the stress of modern life” are seen as risk factors of cancer, without any scientific evidence.
Cigarettes and e-cigarettes
About 53% of respondents agreed that “e-cigarettes are just as harmful or more harmful than traditional cigarettes.” Nicotine and the flavors in e-cigarettes are largely perceived as “very” or “extremely” harmful to the health of a person. However, the authors note that “no published study on nicotine substitutes has shown harmful effects on the health of a person, let alone determined it a risk factor for cancer. The nicotine doses in e-cigarettes are similar to traditional nicotine substitutes, and no cytotoxic effect of nicotine in its inhaled form has been found.” There seems to be confusion between dependence and risk of cancer.
Alcohol consumption
Eight of 10 respondents believe that “some people can drink a lot of alcohol all their life without ever getting cancer,” which goes against the scientific literature. The authors of the report state that the negative effects of alcohol on health seem poorly understood. Although alcohol is the second biggest cause of cancer, only a third of survey respondents cited it without having been prompted as one of the main causes of cancer. And 23.5% even think that “in terms of decreasing your risk of cancer, it’s better to drink a little wine than to drink no wine at all.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
FRANCE – Conducted every 5 years since 2005, the Cancer Survey documents the knowledge, perceptions, and way of life of the French people in relation to cancer. The researchers analyzed responses to telephone interviews of a representative sample of almost 5,000 individuals aged 15-85 years.
This study shows how thinking has changed over time and how difficult it is to alter preconceived notions.
Is cancer hereditary?
The report shows that 67.7% of respondents believe that cancer is a hereditary disease. Respondents were asked to explain their answer. “Data show that medical practices for cancer treatment substantiate this belief [that cancer is hereditary],” wrote the authors of the report.
“Indeed, health care professionals almost systematically ask questions about family history of breast cancer and, when a family member has been diagnosed with cancer, medical monitoring of other family members is often sought out, thus reinforcing the belief that cancer is hereditary,” they said.
Furthermore, there seems to be confusion regarding the role of genes in the development of cancer. A person can inherit cancer-predisposing genes, not cancer itself. The authors highlighted their concern that this confusion may “lead people to think that prevention measures are unnecessary because cancer is inherited.”
Misconceptions about smoking
About 41% of smokers think that the length of time one has been smoking is the biggest determining factor for developing cancer; 58.1% think the number of cigarettes smoked per day has a bigger impact.
Experts at InCA and SPF put the debate to rest, stating that prolonged exposure to carcinogenic substances is far more toxic. As for the danger threshold concerning the number of cigarettes smoked per day, respondents believed this to be 9.2 cigarettes per day, on average. They believed that the danger threshold for the number of years as an active smoker is 13.4, on average.
“The [survey] respondents clearly understand that smoking carries a risk, but many smokers think that light smoking or smoking for a short period of time doesn’t carry any risks.” Yet it is understood that even occasional tobacco consumption increases mortality.
This was not the only misconception regarding smoking and its relationship with cancer. About 34% of survey respondents agreed with the following statement: “Smoking doesn’t cause cancer unless you’re a heavy smoker and have smoked for a long time.” Furthermore, 43.3% agreed with the statement, “Pollution is more likely to cause cancer than smoking,” 54.6% think that “exercising cleans your lungs of tobacco,” and 61.6% think that “a smoker can prevent developing cancer caused by smoking if they know to quit on time.”
Overweight and obesity
Although diet and excess weight represent the third and fourth biggest avoidable cancer risk factors, after smoking and alcohol, only 30% of survey respondents knew of this link.
“Among the causes of cancer known and cited by respondents without prompting, excessive weight and obesity were mentioned only 100 times out of 12,558 responses,” highlighted the authors of the report. The explanation put forward by the authors is that discourse about diet has been more focused on diet as a protective health factor, especially in preventing cardiovascular diseases. “The link between cancer and diet is less prominent in the public space,” they noted.
Breastfeeding and cancer
About 63% of survey respondents, which for the first time included both women and men, believe that breastfeeding does not affect mothers’ risk of breast cancer, but this is a misconception. And almost 1 in 3 respondents said that breastfeeding provides health benefits for the mother.
Artificial UV rays
Exposure to UV rays, whether of natural or artificial origin, is a major risk factor for skin cancer. However, 1 in 5 people (20.9%) think that a session in a tanning bed is less harmful than sun exposure.
Daily stress
Regarding psychological factors linked to cancer, the authors noted that risk factors not supported by scientific evidence were, ironically, cited more often by respondents than proven risk factors. There is a real knowledge gap between scientific data and the beliefs of the French people. For example, “working at night” is largely not seen as a risk factor, but data show that it presents a clear risk. However, “not being able to express one’s feelings,” “having been weakened by traumatic experiences,” and “being exposed to the stress of modern life” are seen as risk factors of cancer, without any scientific evidence.
Cigarettes and e-cigarettes
About 53% of respondents agreed that “e-cigarettes are just as harmful or more harmful than traditional cigarettes.” Nicotine and the flavors in e-cigarettes are largely perceived as “very” or “extremely” harmful to the health of a person. However, the authors note that “no published study on nicotine substitutes has shown harmful effects on the health of a person, let alone determined it a risk factor for cancer. The nicotine doses in e-cigarettes are similar to traditional nicotine substitutes, and no cytotoxic effect of nicotine in its inhaled form has been found.” There seems to be confusion between dependence and risk of cancer.
Alcohol consumption
Eight of 10 respondents believe that “some people can drink a lot of alcohol all their life without ever getting cancer,” which goes against the scientific literature. The authors of the report state that the negative effects of alcohol on health seem poorly understood. Although alcohol is the second biggest cause of cancer, only a third of survey respondents cited it without having been prompted as one of the main causes of cancer. And 23.5% even think that “in terms of decreasing your risk of cancer, it’s better to drink a little wine than to drink no wine at all.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
FRANCE – Conducted every 5 years since 2005, the Cancer Survey documents the knowledge, perceptions, and way of life of the French people in relation to cancer. The researchers analyzed responses to telephone interviews of a representative sample of almost 5,000 individuals aged 15-85 years.
This study shows how thinking has changed over time and how difficult it is to alter preconceived notions.
Is cancer hereditary?
The report shows that 67.7% of respondents believe that cancer is a hereditary disease. Respondents were asked to explain their answer. “Data show that medical practices for cancer treatment substantiate this belief [that cancer is hereditary],” wrote the authors of the report.
“Indeed, health care professionals almost systematically ask questions about family history of breast cancer and, when a family member has been diagnosed with cancer, medical monitoring of other family members is often sought out, thus reinforcing the belief that cancer is hereditary,” they said.
Furthermore, there seems to be confusion regarding the role of genes in the development of cancer. A person can inherit cancer-predisposing genes, not cancer itself. The authors highlighted their concern that this confusion may “lead people to think that prevention measures are unnecessary because cancer is inherited.”
Misconceptions about smoking
About 41% of smokers think that the length of time one has been smoking is the biggest determining factor for developing cancer; 58.1% think the number of cigarettes smoked per day has a bigger impact.
Experts at InCA and SPF put the debate to rest, stating that prolonged exposure to carcinogenic substances is far more toxic. As for the danger threshold concerning the number of cigarettes smoked per day, respondents believed this to be 9.2 cigarettes per day, on average. They believed that the danger threshold for the number of years as an active smoker is 13.4, on average.
“The [survey] respondents clearly understand that smoking carries a risk, but many smokers think that light smoking or smoking for a short period of time doesn’t carry any risks.” Yet it is understood that even occasional tobacco consumption increases mortality.
This was not the only misconception regarding smoking and its relationship with cancer. About 34% of survey respondents agreed with the following statement: “Smoking doesn’t cause cancer unless you’re a heavy smoker and have smoked for a long time.” Furthermore, 43.3% agreed with the statement, “Pollution is more likely to cause cancer than smoking,” 54.6% think that “exercising cleans your lungs of tobacco,” and 61.6% think that “a smoker can prevent developing cancer caused by smoking if they know to quit on time.”
Overweight and obesity
Although diet and excess weight represent the third and fourth biggest avoidable cancer risk factors, after smoking and alcohol, only 30% of survey respondents knew of this link.
“Among the causes of cancer known and cited by respondents without prompting, excessive weight and obesity were mentioned only 100 times out of 12,558 responses,” highlighted the authors of the report. The explanation put forward by the authors is that discourse about diet has been more focused on diet as a protective health factor, especially in preventing cardiovascular diseases. “The link between cancer and diet is less prominent in the public space,” they noted.
Breastfeeding and cancer
About 63% of survey respondents, which for the first time included both women and men, believe that breastfeeding does not affect mothers’ risk of breast cancer, but this is a misconception. And almost 1 in 3 respondents said that breastfeeding provides health benefits for the mother.
Artificial UV rays
Exposure to UV rays, whether of natural or artificial origin, is a major risk factor for skin cancer. However, 1 in 5 people (20.9%) think that a session in a tanning bed is less harmful than sun exposure.
Daily stress
Regarding psychological factors linked to cancer, the authors noted that risk factors not supported by scientific evidence were, ironically, cited more often by respondents than proven risk factors. There is a real knowledge gap between scientific data and the beliefs of the French people. For example, “working at night” is largely not seen as a risk factor, but data show that it presents a clear risk. However, “not being able to express one’s feelings,” “having been weakened by traumatic experiences,” and “being exposed to the stress of modern life” are seen as risk factors of cancer, without any scientific evidence.
Cigarettes and e-cigarettes
About 53% of respondents agreed that “e-cigarettes are just as harmful or more harmful than traditional cigarettes.” Nicotine and the flavors in e-cigarettes are largely perceived as “very” or “extremely” harmful to the health of a person. However, the authors note that “no published study on nicotine substitutes has shown harmful effects on the health of a person, let alone determined it a risk factor for cancer. The nicotine doses in e-cigarettes are similar to traditional nicotine substitutes, and no cytotoxic effect of nicotine in its inhaled form has been found.” There seems to be confusion between dependence and risk of cancer.
Alcohol consumption
Eight of 10 respondents believe that “some people can drink a lot of alcohol all their life without ever getting cancer,” which goes against the scientific literature. The authors of the report state that the negative effects of alcohol on health seem poorly understood. Although alcohol is the second biggest cause of cancer, only a third of survey respondents cited it without having been prompted as one of the main causes of cancer. And 23.5% even think that “in terms of decreasing your risk of cancer, it’s better to drink a little wine than to drink no wine at all.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Toxic chemicals we consume without knowing it
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Life expectancy is falling precipitously. Three-fourths of Americans are overweight or obese, half have diabetes or prediabetes, and a majority are metabolically unhealthy. Furthermore, the rates of allergic, inflammatory, and autoimmune diseases are rising at rates of 3%-9% per year in the West, far faster than the speed of genetic change in this population.
Of course, diet and lifestyle are major factors behind such trends, but a grossly underappreciated driver in what ails us is the role of environmental toxins and endocrine-disrupting chemicals. In years past, these factors have largely evaded the traditional Western medical establishment; however, mounting evidence now supports their significance in fertility, metabolic health, and cancer.
Although several industrial chemicals and toxins have been identified as carcinogens and have subsequently been regulated, many more remain persistent in the environment and continue to be freely used. It is therefore incumbent upon both the general public and clinicians to be knowledgeable about these exposures. Here, we review some of the most common exposures and the substantial health risks associated with them, along with some general guidance around best practices for how to minimize exposure.
Microplastics
“Microplastics” is a term used to describe small fragments or particles of plastic breakdown or microbeads from household or personal care products, measuring less than 5 mm in length.
Plastic waste is accumulating at alarming and devastating proportions – by 2050, it is estimated that by weight, there will be more plastic than fish in the oceans. That translates into hundreds of thousands of tons of microplastics and trillions of these particles in the seas. A recent study demonstrated that microplastics were present in the bloodstream in the majority of 22 otherwise healthy participants.
Since the 1950s, plastic exposure has been shown to promote tumorigenesis in animal studies, and in vitro studies have demonstrated the toxicity of microplastics at the cellular level. However, it is not well known whether the plastic itself is toxic or if it simply serves as a carrier for other environmental toxins to bioaccumulate.
According to Tasha Stoiber, a senior scientist at the Environmental Working Group, “Microplastics have been widely detected in fish and seafood, as well as other products like bottled water, beer, honey, and tap water.” The EWG states there are no formal advisories on fish consumption to avoid exposure to microplastics at the moment.
Pressure also is mounting for a ban on microbeads in personal care products.
Until such bans are put in place, it is advised to avoid single-use plastics, favor reusable tote bags for grocery shopping rather than plastic bags, and opt for loose leaf tea or paper tea bags rather than mesh-based alternatives.
Phthalates
Phthalates are chemicals used to make plastics soft and durable, as well as to bind fragrances. They are commonly found in household items such as vinyl (for example, flooring, shower curtains) and fragrances, air fresheners, and perfumes.
Phthalates are known hormone-disrupting chemicals, exposure to which has been associated with abnormal sexual and brain development in children, as well as lower levels of testosterone in men. Exposures are thought to occur via inhalation, ingestion, and skin contact; however, fasting studies demonstrate that a majority of exposure is probably food related.
To avoid phthalate exposures, recommendations include avoiding polyvinyl chloride plastics (particularly food containers, plastic wrap, and children’s toys), which are identifiable by the recycle code number 3, as well as air fresheners and fragranced products.
The EWG’s Skin Deep database provides an important resource on phthalate-free personal care products.
Despite pressure from consumer advocacy groups, the U.S. Food and Drug Administration has not yet banned phthalates in food packaging.
Bisphenol A (BPA)
BPA is a chemical additive used to make clear and hard polycarbonate plastics, as well as epoxy and thermal papers. BPA is one of the highest-volume chemicals, with roughly 6 billion pounds produced each year. BPA is traditionally found in many clear plastic bottles and sippy cups, as well as in the lining of canned foods.
Structurally, BPA acts as an estrogen mimetic and has been associated with cardiovascular disease, obesity, and male sexual dysfunction. Since 2012, BPA has been banned in sippy cups and baby bottles, but there is some debate as to whether its replacements (bisphenol S and bisphenol F) are any safer; they appear to have similar hormonal effects as BPA.
As with phthalates, the majority of ingestion is thought to be food related. BPA has been found in more than 90% of a representative study population in the United States.
Guidance advises avoiding polycarbonate plastics (identifiable with the recycling code number 7), as well as avoiding handling thermal papers such as tickets and receipts, if possible. Food and beverages should be stored in glass or stainless steel. If plastic must be used, opt for polycarbonate- and polyvinyl chloride–free plastics, and food and beverages should never be reheated in plastic containers or wrapping. Canned foods should ideally be avoided, particularly canned tunas and condensed soups. If canned products are bought, they should ideally be BPA free.
Dioxins and polychlorinated biphenyls (PCBs)
Dioxins are mainly the byproducts of industrial practices; they are released after incineration, trash burning, and fires. PCBs, which are somewhat structurally related to dioxins, were previously found in products such as flame retardants and coolants. Dioxins and PCBs are often grouped in the same category under the umbrella term “persistent organic pollutants” because they break down slowly and remain in the environment even after emissions have been curbed.
Tetrachlorodibenzodioxin, perhaps the best-known dioxin, is a known carcinogen. Dioxins also have been associated with a host of health implications in development, immunity, and reproductive and endocrine systems. Higher levels of PCB exposure have also been associated with an increased risk for mortality from cardiovascular disease.
Notably, dioxin emissions have been reduced by 90% since the 1980s, and the U.S. Environmental Protection Agency has banned the use of PCBs in industrial manufacturing since 1979. However, environmental dioxins and PCBs still enter the food chain and accumulate in fat.
The best ways to avoid exposures are through limiting meat, fish, and dairy consumption and trimming the skin and fat from meats. The level of dioxins and PCBs found in meat, eggs, fish, and dairy are approximately 5-10 times higher than they are in plant-based foods. Research has shown that farmed salmon is likely to be the most PCB-contaminated protein source in the U.S. diet; however, newer forms of land-based and sustainable aquaculture probably avoid this exposure.
Pesticides
The growth of modern monoculture agriculture in the United States over the past century has coincided with a dramatic surge in the use of industrial pesticides. In fact, over 90% of the U.S. population have pesticides in their urine and blood, regardless of where they live. Exposures are thought to be food related.
Approximately 1 billion pounds of pesticides are used annually in the United States, including nearly 300 million pounds of glyphosate, which has been identified as a probable carcinogen by European agencies. The EPA has not yet reached this conclusion, although the matter is currently being litigated.
A large European prospective cohort trial demonstrated a lower risk for cancer in those with a greater frequency of self-reported organic food consumption. In addition to cancer risk, relatively elevated blood levels of a pesticide known as beta-hexachlorocyclohexane (B-HCH) are associated with higher all-cause mortality. Also, exposure to DDE – a metabolite of DDT, a chlorinated pesticide heavily used in the 1940s-1960s that still persists in the environment today – has been shown to increase the risk for Alzheimer’s-type dementia as well as overall cognitive decline.
Because these chlorinated pesticides are often fat soluble, they seem to accumulate in animal products. Therefore, people consuming a vegetarian diet have been found to have lower levels of B-HCH. This has led to the recommendation that consumers of produce should favor organic over conventional, if possible. Here too, the EWG provides an important resource to consumers in the form of shopper guides regarding pesticides in produce.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of fluorinated compounds discovered in the 1930s. Their chemical composition includes a durable carbon-fluoride bond, giving them a persistence within the environment that has led to their being referred to as “forever chemicals.”
PFAS have been detected in the blood of 98% of Americans, and in the rainwater of locations as far afield as Tibet and Antarctica. Even low levels of exposure have been associated with an increased risk for cancer, liver disease, low birth weight, and hormonal disruption.
The properties of PFAS also make them both durable at very high heat and water repellent. Notoriously, the chemical was used by 3M to make Scotchgard for carpets and fabrics and by Dupont to make Teflon for nonstick coating of pots and pans. Although perfluorooctanoic acid (PFOA) was removed from nonstick cookware in 2013, PFAS – a family of thousands of synthetic compounds – remain common in fast-food packaging, water- and stain-repellent clothing, firefighting foam, and personal care products. PFAS are released into the environment during the breakdown of these consumer and industrial products, as well as from dumping from waste facilities.
Alarmingly, the EWG notes that up to 200 million Americans may be exposed to PFAS in their drinking water. In March 2021, the EPA announced that they will be regulating PFAS in drinking water; however, the regulations have not been finalized. Currently, it is up to individual states to test for its presence in the water. The EWG has compiled a map of all known PFAS contamination sites.
To avoid or prevent exposures from PFAS, recommendations include filtering tap water with either reverse osmosis or activated carbon filters, as well as avoiding fast food and carry-out food, if possible, and consumer products labeled as “water resistant,” “stain-resistant,” and “nonstick.”
In a testament to how harmful these chemicals are, the EPA recently revised their lifetime health advisories for PFAS, such as PFOA, to 0.004 parts per trillion, which is more than 10,000 times smaller than the previous limit of 70 parts per trillion. The EPA also has proposed formally designating certain PFAS chemicals as “hazardous substances.”
Dr. Goel, clinical assistant professor of medicine at Weill Cornell Medicine, New York, has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Physicians and clinicians should be required to get flu shots: Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University’s Grossman School of Medicine, where I’m the director.
I’ve long believed that every health care institution – nursing homes, hospitals, clinics, home care, hospice – should require flu shots for all doctors and all nurses because it is the easiest, cheapest, and most ethical way to protect the workforce, who you need to be in there when flu outbreaks take place, and to protect patients against getting the flu when they come into hospital settings and get exposed to health care workers who may have the flu already but don’t know it.
In a recent poll, I was happy to see that the majority of physicians surveyed agreed with me: 65% said they supported mandatory flu vaccination in hospitals and only 23% said they did not. I think flu vaccination is something that has already been shown to be useful and important, not only in stopping people from getting the flu but also in making sure that they don’t get as sick when they get the flu.
Just like COVID-19 vaccination, it doesn’t always prevent somebody from getting infected, but if you get it, it keeps you from winding up sick at home, or worse – from dying and winding up in the morgue. Flu kills many, many people every year. We don’t want that to happen. A flu vaccine will really help prevent deaths, help prevent the number of symptoms that somebody gets, and will get people back to work. The benefits are pretty clear.
Does the flu vaccine work equally well every year? It does not. Some years, the strains that are picked for the vaccine don’t match the ones that circulate, and we don’t get as much protection as we hoped for. I think the safety side is so strong that it’s worth making the investment and the effort to promote mandatory flu vaccination.
Can you opt out on religious grounds? Well, some hospitals permit that at New York University. You have to go before a committee and make a case that your exemption on religious grounds is based on an authentic set of beliefs that are deeply held, and not just something you thought up the day before flu vaccine requirements went into effect.
There may be room for some exemptions – obviously, for health reasons. If people think that the flu vaccine is dangerous to them and can get a physician to agree and sign off that they are not appropriate to vaccinate, okay.
On the other hand, if you’re working with an especially vulnerable population – newborns, people who are immunosuppressed – then I think you’ve got to be vaccinated and you shouldn’t be working around people who are at huge risk of getting the flu if you refuse to be vaccinated or, for that matter, can’t be vaccinated.
Would I extend these mandates? Yes, I would. I’d extend them to COVID-19 vaccination and to measles vaccination. I think physicians and nurses should be good role models. They should get vaccinated. We know that the best available evidence says that vaccination for infectious disease is safe. It is really the best thing we can do to combat a variety of diseases such as the flu and COVID-19.
It seems to me that, in addition, the data that are out there in terms of risks from flu and COVID-19 – deaths in places like nursing homes – are overwhelming about the importance of trying to get staff vaccinated so they don’t bring flu into an institutionalized population. This is similar for prison health and many other settings where people are kept close together and staff may move from place to place, rotating from institution to institution, spreading infectious disease.
I’m going to go with the poll. Let’s keep pushing for health care workers to do the right thing and to be good role models. Let’s get everybody a flu vaccination. Let’s extend it to a COVID-19 vaccination and its boosters.
Let’s try to show the nation that health care is going to be guided by good science, a duty to one’s own health, and a duty to one’s patients. It shouldn’t be political. It should be based on what works best for the interests of health care providers and those they care for.
I’m Art Caplan at the New York University Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape. A version of this article originally appeared on Medscape.com.
Prone positioning curbs need for intubation in nonintubated COVID-19 patients
as indicated by data from a new meta-analysis of more than 2,000 individuals.
The use of prone positioning for nonintubated patients (so-called “awake prone positioning”) has been common since the early days of the COVID-19 pandemic. Prone positioning is more comfortable for patients, and it entails no additional cost. Also, awake prone positioning is less labor intensive than prone positioning for intubated patients, said Jie Li, PhD, in a presentation at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
However, data on the specific benefits of prone positioning are lacking and contradictory, said Dr. Li, a respiratory care specialist at Rush University, Chicago.
Dr. Li and colleagues from a multinational research group found that outcomes were improved for patients who were treated with awake prone positioning – notably, fewer treatment failures at day 28 – but a pair of subsequent studies by other researchers showed contradictory outcomes.
For more definitive evidence, Dr. Li and colleagues conducted a systematic review and meta-analysis of 11 randomized, controlled trials and one unpublished study of awake prone positioning for patients with COVID-19. The studies were published between Jan. 1, 2020, and July 1, 2022, and included a total of 2,886 adult patients.
The primary outcome was the reported cumulative risk of intubation among nonintubated COVID-19 patients. Secondary outcomes included mortality, the need for escalating respiratory support, length of hospital length of stay, ICU admission, and adverse events.
Overall, awake prone positioning significantly reduced the intubation risk among nonintubated patients compared to standard care (risk ratio, 0.85).
A further subgroup analysis showed a significant reduction in risk for intubation among patients supported by high-flow nasal cannula or noninvasive ventilation (RR, 0.83).
However, no additional reduction in intubation risk occurred among patients who received conventional oxygen therapy (RR, 1.02).
Mortality rates were similar for patients who underwent awake prone positioning and those who underwent supine positioning (RR, 0.96), as was the need for additional respiratory support (RR, 1.03). The length of hospital stay, ICU admission, and adverse events were similar between the patients who underwent prone positioning and those who underwent supine positioning.
The findings were limited by several factors. There was a potential for confounding by disease severity, which may have increased the use of respiratory support devices, Li said in her presentation.
“Another factor we should not ignore is the daily duration of prone positioning,” said Dr. Li. More research is needed to identify which factors play the greatest roles in treatment success.
The current study was important in that it evaluated the current evidence of awake prone positioning, “particularly to identify the patients who benefit most from this treatment, in order to guide clinical practice,” Dr. Li said in an interview.
“Since early in the pandemic, awake prone positioning has been broadly utilized to treat patients with COVID-19,” she said. “In 2021, we published a multinational randomized controlled trial with over 1,100 patients enrolled and reported lower treatment failure. However, no significant differences of treatment failure were reported in several subsequent multicenter randomized, controlled trials published after our study.”
Dr. Li said she was not surprised by the findings, which reflect those of her team’s previously published meta-analysis. “The increased number of patients helps confirm our previous finding, even with the inclusion of several recently published randomized controlled trials,” she said.
For clinicians, “the current evidence supports the use of awake prone positioning for patients with COVID-19, particularly those who require advanced respiratory support from high-flow nasal cannula or noninvasive ventilation,” Dr. Li said.
The study received no outside funding. Dr. Li has relationships with AARC, Heyer, Aeorgen, the Rice Foundation, and Fisher & Paykel Healthcare.
A version of this article first appeared on Medscape.com.
as indicated by data from a new meta-analysis of more than 2,000 individuals.
The use of prone positioning for nonintubated patients (so-called “awake prone positioning”) has been common since the early days of the COVID-19 pandemic. Prone positioning is more comfortable for patients, and it entails no additional cost. Also, awake prone positioning is less labor intensive than prone positioning for intubated patients, said Jie Li, PhD, in a presentation at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
However, data on the specific benefits of prone positioning are lacking and contradictory, said Dr. Li, a respiratory care specialist at Rush University, Chicago.
Dr. Li and colleagues from a multinational research group found that outcomes were improved for patients who were treated with awake prone positioning – notably, fewer treatment failures at day 28 – but a pair of subsequent studies by other researchers showed contradictory outcomes.
For more definitive evidence, Dr. Li and colleagues conducted a systematic review and meta-analysis of 11 randomized, controlled trials and one unpublished study of awake prone positioning for patients with COVID-19. The studies were published between Jan. 1, 2020, and July 1, 2022, and included a total of 2,886 adult patients.
The primary outcome was the reported cumulative risk of intubation among nonintubated COVID-19 patients. Secondary outcomes included mortality, the need for escalating respiratory support, length of hospital length of stay, ICU admission, and adverse events.
Overall, awake prone positioning significantly reduced the intubation risk among nonintubated patients compared to standard care (risk ratio, 0.85).
A further subgroup analysis showed a significant reduction in risk for intubation among patients supported by high-flow nasal cannula or noninvasive ventilation (RR, 0.83).
However, no additional reduction in intubation risk occurred among patients who received conventional oxygen therapy (RR, 1.02).
Mortality rates were similar for patients who underwent awake prone positioning and those who underwent supine positioning (RR, 0.96), as was the need for additional respiratory support (RR, 1.03). The length of hospital stay, ICU admission, and adverse events were similar between the patients who underwent prone positioning and those who underwent supine positioning.
The findings were limited by several factors. There was a potential for confounding by disease severity, which may have increased the use of respiratory support devices, Li said in her presentation.
“Another factor we should not ignore is the daily duration of prone positioning,” said Dr. Li. More research is needed to identify which factors play the greatest roles in treatment success.
The current study was important in that it evaluated the current evidence of awake prone positioning, “particularly to identify the patients who benefit most from this treatment, in order to guide clinical practice,” Dr. Li said in an interview.
“Since early in the pandemic, awake prone positioning has been broadly utilized to treat patients with COVID-19,” she said. “In 2021, we published a multinational randomized controlled trial with over 1,100 patients enrolled and reported lower treatment failure. However, no significant differences of treatment failure were reported in several subsequent multicenter randomized, controlled trials published after our study.”
Dr. Li said she was not surprised by the findings, which reflect those of her team’s previously published meta-analysis. “The increased number of patients helps confirm our previous finding, even with the inclusion of several recently published randomized controlled trials,” she said.
For clinicians, “the current evidence supports the use of awake prone positioning for patients with COVID-19, particularly those who require advanced respiratory support from high-flow nasal cannula or noninvasive ventilation,” Dr. Li said.
The study received no outside funding. Dr. Li has relationships with AARC, Heyer, Aeorgen, the Rice Foundation, and Fisher & Paykel Healthcare.
A version of this article first appeared on Medscape.com.
as indicated by data from a new meta-analysis of more than 2,000 individuals.
The use of prone positioning for nonintubated patients (so-called “awake prone positioning”) has been common since the early days of the COVID-19 pandemic. Prone positioning is more comfortable for patients, and it entails no additional cost. Also, awake prone positioning is less labor intensive than prone positioning for intubated patients, said Jie Li, PhD, in a presentation at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
However, data on the specific benefits of prone positioning are lacking and contradictory, said Dr. Li, a respiratory care specialist at Rush University, Chicago.
Dr. Li and colleagues from a multinational research group found that outcomes were improved for patients who were treated with awake prone positioning – notably, fewer treatment failures at day 28 – but a pair of subsequent studies by other researchers showed contradictory outcomes.
For more definitive evidence, Dr. Li and colleagues conducted a systematic review and meta-analysis of 11 randomized, controlled trials and one unpublished study of awake prone positioning for patients with COVID-19. The studies were published between Jan. 1, 2020, and July 1, 2022, and included a total of 2,886 adult patients.
The primary outcome was the reported cumulative risk of intubation among nonintubated COVID-19 patients. Secondary outcomes included mortality, the need for escalating respiratory support, length of hospital length of stay, ICU admission, and adverse events.
Overall, awake prone positioning significantly reduced the intubation risk among nonintubated patients compared to standard care (risk ratio, 0.85).
A further subgroup analysis showed a significant reduction in risk for intubation among patients supported by high-flow nasal cannula or noninvasive ventilation (RR, 0.83).
However, no additional reduction in intubation risk occurred among patients who received conventional oxygen therapy (RR, 1.02).
Mortality rates were similar for patients who underwent awake prone positioning and those who underwent supine positioning (RR, 0.96), as was the need for additional respiratory support (RR, 1.03). The length of hospital stay, ICU admission, and adverse events were similar between the patients who underwent prone positioning and those who underwent supine positioning.
The findings were limited by several factors. There was a potential for confounding by disease severity, which may have increased the use of respiratory support devices, Li said in her presentation.
“Another factor we should not ignore is the daily duration of prone positioning,” said Dr. Li. More research is needed to identify which factors play the greatest roles in treatment success.
The current study was important in that it evaluated the current evidence of awake prone positioning, “particularly to identify the patients who benefit most from this treatment, in order to guide clinical practice,” Dr. Li said in an interview.
“Since early in the pandemic, awake prone positioning has been broadly utilized to treat patients with COVID-19,” she said. “In 2021, we published a multinational randomized controlled trial with over 1,100 patients enrolled and reported lower treatment failure. However, no significant differences of treatment failure were reported in several subsequent multicenter randomized, controlled trials published after our study.”
Dr. Li said she was not surprised by the findings, which reflect those of her team’s previously published meta-analysis. “The increased number of patients helps confirm our previous finding, even with the inclusion of several recently published randomized controlled trials,” she said.
For clinicians, “the current evidence supports the use of awake prone positioning for patients with COVID-19, particularly those who require advanced respiratory support from high-flow nasal cannula or noninvasive ventilation,” Dr. Li said.
The study received no outside funding. Dr. Li has relationships with AARC, Heyer, Aeorgen, the Rice Foundation, and Fisher & Paykel Healthcare.
A version of this article first appeared on Medscape.com.
FROM SCCM 2023
COVID vs. flu: Which is deadlier?
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN