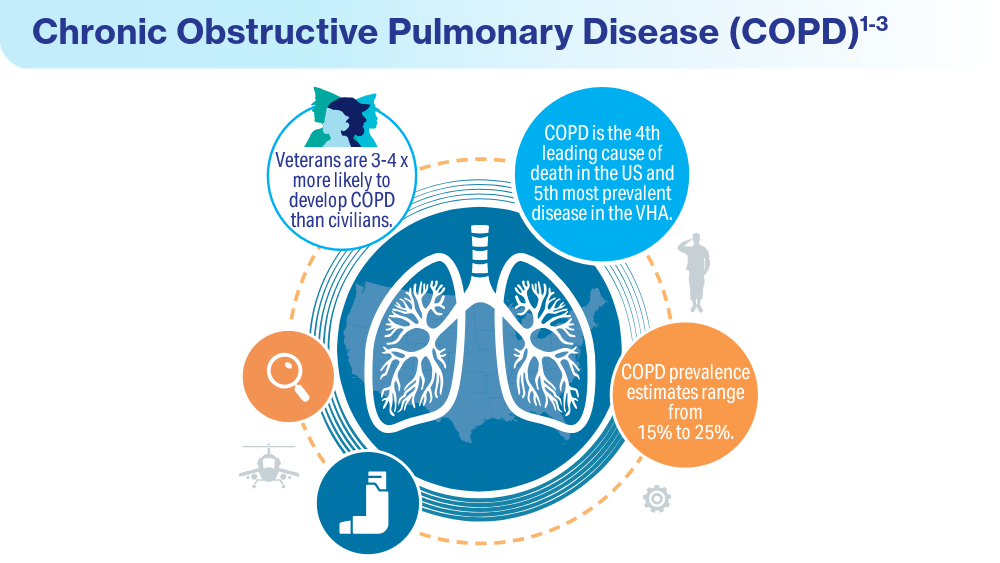
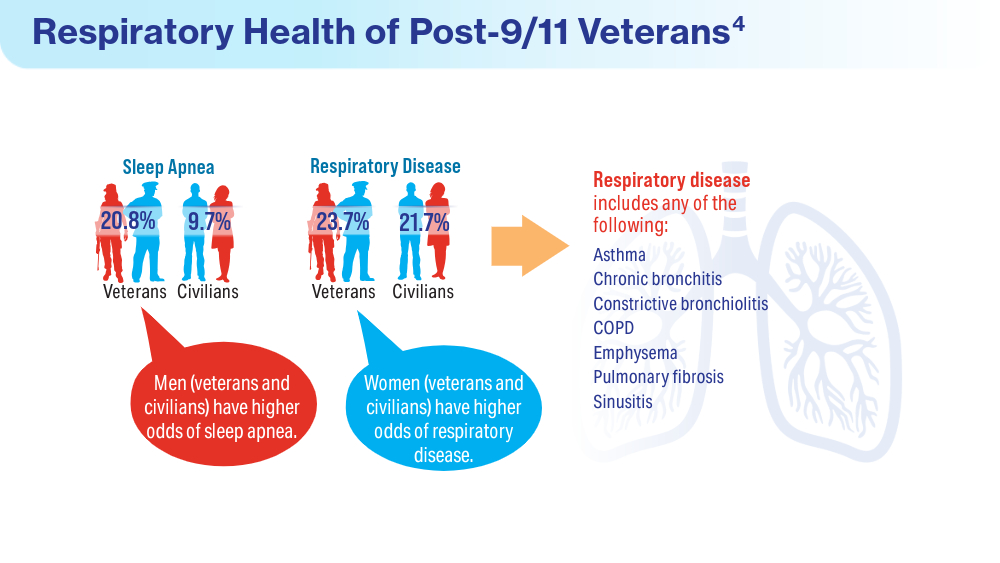
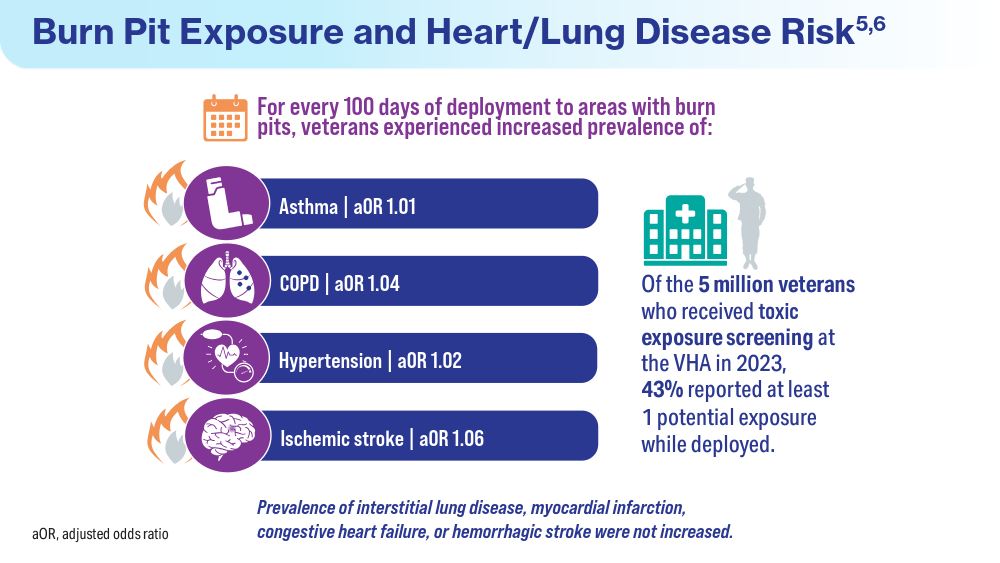
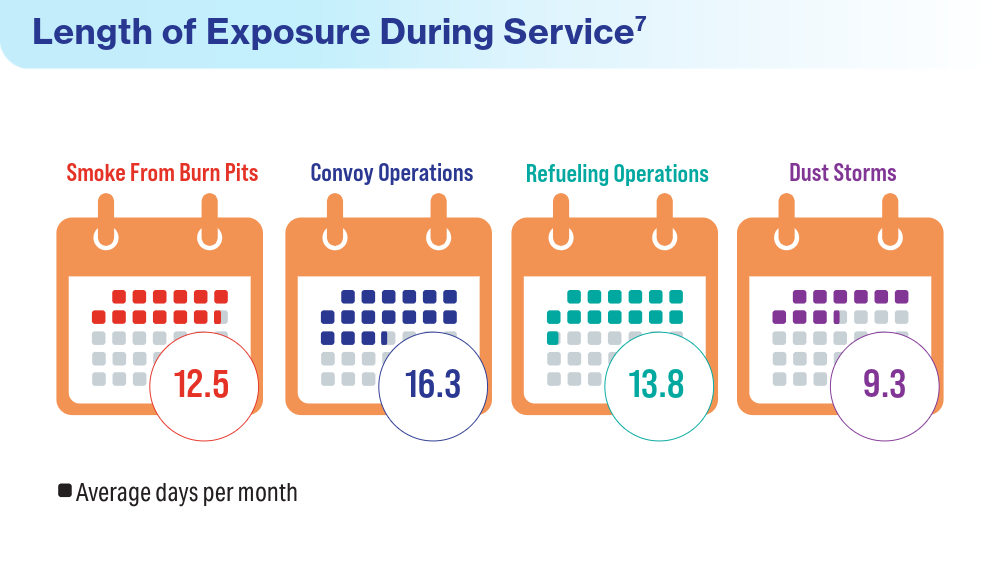
User login
Data Trends 2024: Respiratory Health
- McVeigh C, Reid J, Carvalho P. Healthcare professionals’ views of palliative care for American war veterans with non-malignant respiratory disease living in a rural area: a qualitative study. BMC Palliat Care. 2019;18(1):22. doi:10.1186/s12904-019-0408-7
- US Department of Veterans Affairs. Study finds uptick in lung disease in recent veterans. May 23, 2016. Accessed April 15, 2024. https://www.research.va.gov/currents/0516-3.cfm
- Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
- Trupin L, Schmajuk G, Ying D, Yelin E, Blanc PD. Military service and COPD risk. Chest. 2022;162(4):792-795. doi:10.1016/j.chest.2022.04.016
- Bastian LA. Pain-related anxiety intervention for smokers with chronic pain: a comparative effectiveness trial of smoking cessation counseling for veterans. Veteran’s Health Systems Research, IIR 15-092-HSR Study. Published January 2022. Accessed April 2024. https://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141704797
- Rivera AC, Powell TM, Boyko EJ, et al; for the Millennium Cohort Study Team. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144. doi:10.1093/aje/kwy112
- Savitz DA, Woskie SR, Bello A, et al. Deployment to Military Bases With Open Burn Pits and Respiratory and Cardiovascular Disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- McVeigh C, Reid J, Carvalho P. Healthcare professionals’ views of palliative care for American war veterans with non-malignant respiratory disease living in a rural area: a qualitative study. BMC Palliat Care. 2019;18(1):22. doi:10.1186/s12904-019-0408-7
- US Department of Veterans Affairs. Study finds uptick in lung disease in recent veterans. May 23, 2016. Accessed April 15, 2024. https://www.research.va.gov/currents/0516-3.cfm
- Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
- Trupin L, Schmajuk G, Ying D, Yelin E, Blanc PD. Military service and COPD risk. Chest. 2022;162(4):792-795. doi:10.1016/j.chest.2022.04.016
- Bastian LA. Pain-related anxiety intervention for smokers with chronic pain: a comparative effectiveness trial of smoking cessation counseling for veterans. Veteran’s Health Systems Research, IIR 15-092-HSR Study. Published January 2022. Accessed April 2024. https://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141704797
- Rivera AC, Powell TM, Boyko EJ, et al; for the Millennium Cohort Study Team. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144. doi:10.1093/aje/kwy112
- Savitz DA, Woskie SR, Bello A, et al. Deployment to Military Bases With Open Burn Pits and Respiratory and Cardiovascular Disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- McVeigh C, Reid J, Carvalho P. Healthcare professionals’ views of palliative care for American war veterans with non-malignant respiratory disease living in a rural area: a qualitative study. BMC Palliat Care. 2019;18(1):22. doi:10.1186/s12904-019-0408-7
- US Department of Veterans Affairs. Study finds uptick in lung disease in recent veterans. May 23, 2016. Accessed April 15, 2024. https://www.research.va.gov/currents/0516-3.cfm
- Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan). Am J Trop Med Hyg. 2005;73(4):713-719.
- Trupin L, Schmajuk G, Ying D, Yelin E, Blanc PD. Military service and COPD risk. Chest. 2022;162(4):792-795. doi:10.1016/j.chest.2022.04.016
- Bastian LA. Pain-related anxiety intervention for smokers with chronic pain: a comparative effectiveness trial of smoking cessation counseling for veterans. Veteran’s Health Systems Research, IIR 15-092-HSR Study. Published January 2022. Accessed April 2024. https://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141704797
- Rivera AC, Powell TM, Boyko EJ, et al; for the Millennium Cohort Study Team. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187(10):2136-2144. doi:10.1093/aje/kwy112
- Savitz DA, Woskie SR, Bello A, et al. Deployment to Military Bases With Open Burn Pits and Respiratory and Cardiovascular Disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
Federal Health Care Data Trends 2024
Could Mobile Tech Help to Minimize COPD Exacerbations?
Could mobile technology help patients with chronic obstructive pulmonary disease (COPD) who may not seek care until they experience an exacerbation?
Self-management interventions for COPD can potentially improve quality of life and reduce hospitalizations, wrote Robert Wu, MD, associate professor in the Department of Medicine at the University of Toronto, and colleagues. However, data on the use of devices and apps to manage COPD by providing reminders for self-care, predicting early exacerbations, and facilitating communication with healthcare providers are limited, they said.
In a study published in COPD: Journal of Chronic Obstructive Pulmonary Disease, the researchers reported details from interviews with 26 adult patients with COPD who used a wearable device and app for 6 months to help manage their condition. The interviews were part of a larger cohort study.
“The motivation for this study was to understand the patient perspective on using wearables to help support their chronic lung condition,” Dr. Wu said in an interview. “People with COPD can be at high risk of being admitted to hospital, so it is important to see if innovative technology like wearables or remote monitoring can help them,” he said.
Individuals with COPD tend to be older and less technologically adept, and they may be less willing to adopt new technology, he added. “We wanted to understand what would make people use a self-management app,” he said.
On enrollment in the study, patients received a smartwatch and a smartphone with a preinstalled app for COPD management. The app included daily reminders to take medication, perform guided breathing sessions, check blood oxygen on the smartwatch or an oximeter, and complete a symptom questionnaire. The app also allowed participants to record when they exercised and provided feedback on heart rate and daily activity, including passive step counts. Participants earned stars for meeting daily exercise goals of active minutes and total steps.
Participants received training in the use of the app from members of the research team and completed semi-structured interviews after using the items for 6 months.
The researchers divided their findings into four main themes: information, support and reassurance; barriers to adoption; impact on communication with healthcare providers; and opportunities for improvement.
Overall, most patients reported that the feedback they received through the app was useful. In particular, participants reported that the app and smartwatch provided reassurance and feedback about stable vitals during exercise, which encouraged some to adhere to regular exercise routines. Approximately two thirds (65%) said that the daily exercise reminders were motivational. In addition, 20% reported that they interpreted vital data, including heart rate, as a signal to slow down.
Participants rated medication reminders and the option to create an action plan for COPD management as the least useful features; 69% said that they already had medication reminders in place.
A total of four patients experienced technical difficulties with the app that kept it from impacting their disease management. Some of the suggestions from participants for improvement included adding information about food intake, weight, blood pressure, and temperature to the health information being tracked, as well as restoring the oxygen saturation measure, which had been disabled because of accuracy concerns. Barriers to use of the device and app included the bulkiness of the device as well as the reported technical malfunctions.
The findings were limited by several factors, including the small sample size and likely focus on early adopters of technology, which may not represent most patients with COPD, the researchers noted. Other limitations included the recruitment of most patients after the start of the COVID-19 pandemic, which may have affected their experience and also limited the assessment of the app on communication with healthcare providers, the researchers noted. The study also did not address financial or social barriers.
However, the results suggest that patients with COPD identified the potential value of wearable devices for disease management and that improved technology could promote patient empowerment and lifestyle changes, the researchers concluded.
Technology Can Augment Care and Connections
“As clinicians and researchers, we have ideas about what patients would want, but it is always better to get their feedback of what they really want and what they would use,” Dr. Wu told this news organization. “We thought older adults with COPD would be less likely to engage with the technology. We found that many wanted to have their data to help make connections with their condition, and some purchased smartwatches after the study to make these connections,” he said.
The takeaway message from the current study is that people with COPD may benefit from self-management apps, but they would like to use them in collaboration with their healthcare team, said Dr. Wu. “Clinicians may see more of their patients bringing in data from wearables and apps,” he noted.
Concerns persist that using technology to help support people with COPD could increase the “digital divide” and that those with lower digital literacy, financial insecurity, or English as a second language could be left behind, and it is important to remain attentive to equity in pursuing the use of devices and apps, Dr. Wu told this news organization.
Looking ahead, research involving self-management, remote monitoring, and wearable devices has focused on other conditions such as heart failure and diabetes, and more work is needed to examine how these technologies can improve care for patients with COPD, said Dr. Wu. “We see this study as one important step — to understand what will motivate people to use self-management apps and wearables,” he said.
“Acute exacerbations of COPD are very important events that can alter quality of life, lung function, and even mortality in COPD,” said Nathaniel Marchetti, DO, medical director of the Respiratory Intensive Care Unit at Temple University Hospital, Philadelphia, in an interview.
“Many of these exacerbations are not recognized by clinicians or even patients until they present late and end up in an urgent office visit with a physician or in the emergency room [ER], so addressing exacerbations earlier has the potential to avoid ER visits or hospitalizations,” he said.
The study identified areas for further research, Dr. Marchetti said. “More information would be needed to determine if the use of an app to monitor heart rate, symptoms, and oxygen saturation could alter important outcomes in COPD such as exacerbations,” he noted.
As for limitations, “no one wants to carry two smartphones,” said Dr. Marchetti. “Future devices need to be easy to use and available on the patient’s own phone,” he said. Patients should be able to choose a smartwatch or possibly a bracelet that can be synced to a smartphone, he added. The current study also failed to address what would be done with collected data, such as link them to health professionals who would offer treatment when needed, he said.
Overall, the data from the current study suggest that patients with COPD would like some device that monitors symptoms and vital signs and offers suggestions/incentives to exercise and take medications, Dr. Marchetti told this news organization. “A larger study will be needed that compares how such a device could improve outcomes of COPD; outcomes could include admissions/ER visits, exercise performance, or compliance with medication,” he said. In addition, clinical algorithms for the identification and treatment of acute exacerbations of COPD would be needed, Dr. Marchetti noted. These algorithms would determine whether treatment decisions would be initiated by a clinical team of health professionals or whether clinicians would provide medications that the patients would then decide to take based on data collected on the app, using the investigator-provided algorithms, he said.
The study was supported in part by Samsung Research America (SRA) and was initiated by Dr. Wu with input from SRA, but the company had no role in the methods or results. The study also was supported by grants from the National Natural Science Foundation of China.
Dr. Marchetti had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Could mobile technology help patients with chronic obstructive pulmonary disease (COPD) who may not seek care until they experience an exacerbation?
Self-management interventions for COPD can potentially improve quality of life and reduce hospitalizations, wrote Robert Wu, MD, associate professor in the Department of Medicine at the University of Toronto, and colleagues. However, data on the use of devices and apps to manage COPD by providing reminders for self-care, predicting early exacerbations, and facilitating communication with healthcare providers are limited, they said.
In a study published in COPD: Journal of Chronic Obstructive Pulmonary Disease, the researchers reported details from interviews with 26 adult patients with COPD who used a wearable device and app for 6 months to help manage their condition. The interviews were part of a larger cohort study.
“The motivation for this study was to understand the patient perspective on using wearables to help support their chronic lung condition,” Dr. Wu said in an interview. “People with COPD can be at high risk of being admitted to hospital, so it is important to see if innovative technology like wearables or remote monitoring can help them,” he said.
Individuals with COPD tend to be older and less technologically adept, and they may be less willing to adopt new technology, he added. “We wanted to understand what would make people use a self-management app,” he said.
On enrollment in the study, patients received a smartwatch and a smartphone with a preinstalled app for COPD management. The app included daily reminders to take medication, perform guided breathing sessions, check blood oxygen on the smartwatch or an oximeter, and complete a symptom questionnaire. The app also allowed participants to record when they exercised and provided feedback on heart rate and daily activity, including passive step counts. Participants earned stars for meeting daily exercise goals of active minutes and total steps.
Participants received training in the use of the app from members of the research team and completed semi-structured interviews after using the items for 6 months.
The researchers divided their findings into four main themes: information, support and reassurance; barriers to adoption; impact on communication with healthcare providers; and opportunities for improvement.
Overall, most patients reported that the feedback they received through the app was useful. In particular, participants reported that the app and smartwatch provided reassurance and feedback about stable vitals during exercise, which encouraged some to adhere to regular exercise routines. Approximately two thirds (65%) said that the daily exercise reminders were motivational. In addition, 20% reported that they interpreted vital data, including heart rate, as a signal to slow down.
Participants rated medication reminders and the option to create an action plan for COPD management as the least useful features; 69% said that they already had medication reminders in place.
A total of four patients experienced technical difficulties with the app that kept it from impacting their disease management. Some of the suggestions from participants for improvement included adding information about food intake, weight, blood pressure, and temperature to the health information being tracked, as well as restoring the oxygen saturation measure, which had been disabled because of accuracy concerns. Barriers to use of the device and app included the bulkiness of the device as well as the reported technical malfunctions.
The findings were limited by several factors, including the small sample size and likely focus on early adopters of technology, which may not represent most patients with COPD, the researchers noted. Other limitations included the recruitment of most patients after the start of the COVID-19 pandemic, which may have affected their experience and also limited the assessment of the app on communication with healthcare providers, the researchers noted. The study also did not address financial or social barriers.
However, the results suggest that patients with COPD identified the potential value of wearable devices for disease management and that improved technology could promote patient empowerment and lifestyle changes, the researchers concluded.
Technology Can Augment Care and Connections
“As clinicians and researchers, we have ideas about what patients would want, but it is always better to get their feedback of what they really want and what they would use,” Dr. Wu told this news organization. “We thought older adults with COPD would be less likely to engage with the technology. We found that many wanted to have their data to help make connections with their condition, and some purchased smartwatches after the study to make these connections,” he said.
The takeaway message from the current study is that people with COPD may benefit from self-management apps, but they would like to use them in collaboration with their healthcare team, said Dr. Wu. “Clinicians may see more of their patients bringing in data from wearables and apps,” he noted.
Concerns persist that using technology to help support people with COPD could increase the “digital divide” and that those with lower digital literacy, financial insecurity, or English as a second language could be left behind, and it is important to remain attentive to equity in pursuing the use of devices and apps, Dr. Wu told this news organization.
Looking ahead, research involving self-management, remote monitoring, and wearable devices has focused on other conditions such as heart failure and diabetes, and more work is needed to examine how these technologies can improve care for patients with COPD, said Dr. Wu. “We see this study as one important step — to understand what will motivate people to use self-management apps and wearables,” he said.
“Acute exacerbations of COPD are very important events that can alter quality of life, lung function, and even mortality in COPD,” said Nathaniel Marchetti, DO, medical director of the Respiratory Intensive Care Unit at Temple University Hospital, Philadelphia, in an interview.
“Many of these exacerbations are not recognized by clinicians or even patients until they present late and end up in an urgent office visit with a physician or in the emergency room [ER], so addressing exacerbations earlier has the potential to avoid ER visits or hospitalizations,” he said.
The study identified areas for further research, Dr. Marchetti said. “More information would be needed to determine if the use of an app to monitor heart rate, symptoms, and oxygen saturation could alter important outcomes in COPD such as exacerbations,” he noted.
As for limitations, “no one wants to carry two smartphones,” said Dr. Marchetti. “Future devices need to be easy to use and available on the patient’s own phone,” he said. Patients should be able to choose a smartwatch or possibly a bracelet that can be synced to a smartphone, he added. The current study also failed to address what would be done with collected data, such as link them to health professionals who would offer treatment when needed, he said.
Overall, the data from the current study suggest that patients with COPD would like some device that monitors symptoms and vital signs and offers suggestions/incentives to exercise and take medications, Dr. Marchetti told this news organization. “A larger study will be needed that compares how such a device could improve outcomes of COPD; outcomes could include admissions/ER visits, exercise performance, or compliance with medication,” he said. In addition, clinical algorithms for the identification and treatment of acute exacerbations of COPD would be needed, Dr. Marchetti noted. These algorithms would determine whether treatment decisions would be initiated by a clinical team of health professionals or whether clinicians would provide medications that the patients would then decide to take based on data collected on the app, using the investigator-provided algorithms, he said.
The study was supported in part by Samsung Research America (SRA) and was initiated by Dr. Wu with input from SRA, but the company had no role in the methods or results. The study also was supported by grants from the National Natural Science Foundation of China.
Dr. Marchetti had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Could mobile technology help patients with chronic obstructive pulmonary disease (COPD) who may not seek care until they experience an exacerbation?
Self-management interventions for COPD can potentially improve quality of life and reduce hospitalizations, wrote Robert Wu, MD, associate professor in the Department of Medicine at the University of Toronto, and colleagues. However, data on the use of devices and apps to manage COPD by providing reminders for self-care, predicting early exacerbations, and facilitating communication with healthcare providers are limited, they said.
In a study published in COPD: Journal of Chronic Obstructive Pulmonary Disease, the researchers reported details from interviews with 26 adult patients with COPD who used a wearable device and app for 6 months to help manage their condition. The interviews were part of a larger cohort study.
“The motivation for this study was to understand the patient perspective on using wearables to help support their chronic lung condition,” Dr. Wu said in an interview. “People with COPD can be at high risk of being admitted to hospital, so it is important to see if innovative technology like wearables or remote monitoring can help them,” he said.
Individuals with COPD tend to be older and less technologically adept, and they may be less willing to adopt new technology, he added. “We wanted to understand what would make people use a self-management app,” he said.
On enrollment in the study, patients received a smartwatch and a smartphone with a preinstalled app for COPD management. The app included daily reminders to take medication, perform guided breathing sessions, check blood oxygen on the smartwatch or an oximeter, and complete a symptom questionnaire. The app also allowed participants to record when they exercised and provided feedback on heart rate and daily activity, including passive step counts. Participants earned stars for meeting daily exercise goals of active minutes and total steps.
Participants received training in the use of the app from members of the research team and completed semi-structured interviews after using the items for 6 months.
The researchers divided their findings into four main themes: information, support and reassurance; barriers to adoption; impact on communication with healthcare providers; and opportunities for improvement.
Overall, most patients reported that the feedback they received through the app was useful. In particular, participants reported that the app and smartwatch provided reassurance and feedback about stable vitals during exercise, which encouraged some to adhere to regular exercise routines. Approximately two thirds (65%) said that the daily exercise reminders were motivational. In addition, 20% reported that they interpreted vital data, including heart rate, as a signal to slow down.
Participants rated medication reminders and the option to create an action plan for COPD management as the least useful features; 69% said that they already had medication reminders in place.
A total of four patients experienced technical difficulties with the app that kept it from impacting their disease management. Some of the suggestions from participants for improvement included adding information about food intake, weight, blood pressure, and temperature to the health information being tracked, as well as restoring the oxygen saturation measure, which had been disabled because of accuracy concerns. Barriers to use of the device and app included the bulkiness of the device as well as the reported technical malfunctions.
The findings were limited by several factors, including the small sample size and likely focus on early adopters of technology, which may not represent most patients with COPD, the researchers noted. Other limitations included the recruitment of most patients after the start of the COVID-19 pandemic, which may have affected their experience and also limited the assessment of the app on communication with healthcare providers, the researchers noted. The study also did not address financial or social barriers.
However, the results suggest that patients with COPD identified the potential value of wearable devices for disease management and that improved technology could promote patient empowerment and lifestyle changes, the researchers concluded.
Technology Can Augment Care and Connections
“As clinicians and researchers, we have ideas about what patients would want, but it is always better to get their feedback of what they really want and what they would use,” Dr. Wu told this news organization. “We thought older adults with COPD would be less likely to engage with the technology. We found that many wanted to have their data to help make connections with their condition, and some purchased smartwatches after the study to make these connections,” he said.
The takeaway message from the current study is that people with COPD may benefit from self-management apps, but they would like to use them in collaboration with their healthcare team, said Dr. Wu. “Clinicians may see more of their patients bringing in data from wearables and apps,” he noted.
Concerns persist that using technology to help support people with COPD could increase the “digital divide” and that those with lower digital literacy, financial insecurity, or English as a second language could be left behind, and it is important to remain attentive to equity in pursuing the use of devices and apps, Dr. Wu told this news organization.
Looking ahead, research involving self-management, remote monitoring, and wearable devices has focused on other conditions such as heart failure and diabetes, and more work is needed to examine how these technologies can improve care for patients with COPD, said Dr. Wu. “We see this study as one important step — to understand what will motivate people to use self-management apps and wearables,” he said.
“Acute exacerbations of COPD are very important events that can alter quality of life, lung function, and even mortality in COPD,” said Nathaniel Marchetti, DO, medical director of the Respiratory Intensive Care Unit at Temple University Hospital, Philadelphia, in an interview.
“Many of these exacerbations are not recognized by clinicians or even patients until they present late and end up in an urgent office visit with a physician or in the emergency room [ER], so addressing exacerbations earlier has the potential to avoid ER visits or hospitalizations,” he said.
The study identified areas for further research, Dr. Marchetti said. “More information would be needed to determine if the use of an app to monitor heart rate, symptoms, and oxygen saturation could alter important outcomes in COPD such as exacerbations,” he noted.
As for limitations, “no one wants to carry two smartphones,” said Dr. Marchetti. “Future devices need to be easy to use and available on the patient’s own phone,” he said. Patients should be able to choose a smartwatch or possibly a bracelet that can be synced to a smartphone, he added. The current study also failed to address what would be done with collected data, such as link them to health professionals who would offer treatment when needed, he said.
Overall, the data from the current study suggest that patients with COPD would like some device that monitors symptoms and vital signs and offers suggestions/incentives to exercise and take medications, Dr. Marchetti told this news organization. “A larger study will be needed that compares how such a device could improve outcomes of COPD; outcomes could include admissions/ER visits, exercise performance, or compliance with medication,” he said. In addition, clinical algorithms for the identification and treatment of acute exacerbations of COPD would be needed, Dr. Marchetti noted. These algorithms would determine whether treatment decisions would be initiated by a clinical team of health professionals or whether clinicians would provide medications that the patients would then decide to take based on data collected on the app, using the investigator-provided algorithms, he said.
The study was supported in part by Samsung Research America (SRA) and was initiated by Dr. Wu with input from SRA, but the company had no role in the methods or results. The study also was supported by grants from the National Natural Science Foundation of China.
Dr. Marchetti had no relevant financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Viral Season 2024-2025: Try for An Ounce of Prevention
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
Rheumatoid Arthritis May Raise Lung Cancer Risk, Particularly in Those With ILD
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Rheumatoid arthritis (RA) is linked with over a 50% increased risk for lung cancer, with those having RA-associated interstitial lung disease (RA-ILD) being particularly vulnerable, facing nearly a threefold higher risk.
METHODOLOGY:
- Researchers conducted a retrospective matched cohort study to evaluate the risk for lung cancer in participants with RA, including those with RA-ILD, within Veterans Affairs (VA) from 2000 to 2019.
- A total of 72,795 participants with RA were matched with 633,937 participants without RA on the basis of birth year, sex, and VA enrollment year.
- Among those with RA, 757 had prevalent RA-ILD and were matched with 5931 participants without RA-ILD.
- The primary outcome was incident lung cancer, assessed using the VA Oncology Raw Domain and the National Death Index.
TAKEAWAY:
- Over a mean follow-up of 6.3 years, 2974 incidences of lung cancer were reported in patients with RA, and 34 were reported in those with RA-ILD.
- The risk for lung cancer was 58% higher in patients with RA than in those without RA (adjusted hazard ratio [aHR], 1.58; 95% CI, 1.52-1.64), with this association persisting even when only never-smokers were considered (aHR, 1.65; 95% CI, 1.22-2.24).
- Participants with prevalent RA-ILD had 3.25-fold higher risk for lung cancer than those without RA (aHR, 3.25; 95% CI, 2.13-4.95).
- Both patients with prevalent and those with incident RA-ILD showed a similar increase in risk for lung cancer (aHR, 2.88; 95% CI, 2.45-3.40).
IN PRACTICE:
“Our results highlight RA and RA-ILD as high-risk populations that may benefit from enhanced lung cancer screening,” the authors wrote.
SOURCE:
The study was led by Rebecca T. Brooks, MD, Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota. It was published online on July 28, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The study included a predominantly male population, which may have affected the generalizability of the study. Although the study considered smoking status, data on the duration and intensity of smoking were not available. Restriction to never-smokers could not be completed for comparisons between patients with RA-ILD and those without RA because of insufficient sample sizes.
DISCLOSURES:
This study did not receive funding from any source. Some authors reported receiving research funding or having ties with various pharmaceutical companies and other sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Immunotherapy and Survival in Advanced NSCLC: Does Obesity Matter?
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Overall, however, compared with low body mass index (BMI), overweight or obesity was associated with a lower risk for mortality among patients receiving either therapy.
METHODOLOGY:
- The association between BMI and overall survival in patients with cancer who receive immunotherapy or conventional chemotherapy in the frontline remains unclear. Patients with cancer and obesity are generally considered to have a worse prognosis, but some data suggest an obesity paradox, where patients with cancer and a higher BMI demonstrate better overall survival following immunotherapy or chemotherapy.
- To clarify whether (or how) BMI affects overall survival outcomes and the optimal frontline treatment choice, researchers evaluated 31,257 patients with advanced NSCLC from Japan who received immune checkpoint inhibitors (n = 12,816) or conventional chemotherapy (n = 18,441).
- Patient outcomes were assessed according to weight categories and frontline therapy type (immune checkpoint inhibitors or conventional chemotherapy), with overall survival as the primary outcome.
- A BMI < 18.5 was considered underweight, 18.5-24.9 was considered normal weight, 25.0-29.9 was considered overweight, and ≥ 30.0 was considered obese.
TAKEAWAY:
- In the overall population, regardless of weight, patients who received chemotherapy had a higher mortality rate than those who received immunotherapy — 35.9% vs 28.0%, respectively — over a follow-up of 3 years.
- However, overweight or obesity was associated with a lower risk for mortality compared with a lower BMI among patients with advanced NSCLC, regardless of whether they received immune checkpoint inhibitor therapy or conventional chemotherapy.
- Among patients who received immunotherapy, the risk for mortality decreased steadily as BMI increased from 15 to 24 and then increased at higher BMIs, indicating a U-shaped association.
- Immunotherapy was associated with a significant improvement in overall survival compared with conventional chemotherapy among patients with a BMI < 28; however, researchers observed no difference in overall survival between the two therapies in those with a BMI ≥ 28.
IN PRACTICE:
Overall, “these results support the presence of the obesity paradox in patients with [advanced] NSCLC who underwent either therapy,” the authors concluded.
But when focused on patients in the higher BMI group, there was no overall survival benefit with the frontline immunotherapy vs the conventional chemotherapy. “Immunotherapy therapy may not necessarily be the optimal first-line therapy for patients with overweight or obesity,” the authors wrote, adding that “the use of conventional chemotherapy should also be considered.”
SOURCE:
The study, led by Yasutaka Ihara, PharmD, Osaka Metropolitan University, Osaka, Japan, was published online in JAMA Network Open.
LIMITATIONS:
Retrospective design has inherent bias. PD-L1 status was not known, and the inclusion of Japanese population may have limited the generalizability of the findings.
DISCLOSURES:
This study received funding from the Graduate School of Medicine, Osaka Metropolitan University. Several authors reported receiving personal fees from various pharmaceutical sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Cannabis Overuse Linked to Increased Risk for Head and Neck Cancer
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The study analyzed data from over four million patients, highlighting the potential carcinogenic effects of the substance.
METHODOLOGY:
- Researchers analyzed data from a globally federated health research network TriNetX, which included over 90 million men and women from 64 health care organizations in the United States.
- More than 4.1 million patients were included in the analysis, including 116,076 individuals diagnosed with cannabis-related disorder and 3.9 million without the disorder. Cannabis-related disorders involve the excessive use of cannabis with associated psychosocial symptoms, such as impaired social and/or occupational functioning.
- Patients with cannabis-related disorder were matched with those without the disorder based on demographic characteristics, alcohol-related disorders, and tobacco use.
- The primary outcome was the diagnosis of head and neck cancer, including subsites such as oral, oropharyngeal, nasopharyngeal, laryngeal, hypopharyngeal, and salivary gland malignancies.
- Propensity score matching and Poisson regression analysis were used to compare the incidence of head and neck cancers between the groups.
TAKEAWAY:
- According to the researchers, patients with a cannabis-related disorder had a higher risk for any head and neck cancer (relative risk [RR], 3.49; 95% CI, 2.78-4.39) than those without the disorder.
- The risk for specific cancers was also higher in the group with cannabis-related disorders, including oral (RR, 2.51; 95% CI, 1.81-3.47) and oropharyngeal malignancies (RR, 4.90; 95% CI, 2.99-8.02).
- The RR for laryngeal cancer was significantly higher in the patients with a cannabis-related disorder (RR, 8.39; 95% CI, 4.72-14.90).
- The findings suggest that cannabis use disorder is associated with an increased risk for head and neck cancers, highlighting the need for further research to understand the mechanisms involved.
IN PRACTICE:
“In this cohort study, cannabis disorder diagnosis was independently associated with greater risk of subsequent development of any [head or neck cancer] as well as cancers in various subsites of the head and neck among US adults. When limited to cases of [such cancers] occurring greater than 1 year after cannabis use disorder diagnosis, many of the associations increased, demonstrating additional strength in the association,” the authors of the study wrote.
“The association of cannabis and head and neck cancer in this study spanned 2 decades during a rapid growth in use. If this association is causative, the burden of [head and neck cancers] attributable to cannabis will continue to increase, and perhaps dramatically,” said the authors of an editorial accompanying the journal article. “Given that cannabis is now a $20 billion industry in the US alone with expanding availability, use, and popularity, this may be “déjà vu, all over again” without appropriate research to understand the potential carcinogenic and salutatory effects of cannabis. Or, in the words of Yogi Berra, “If you don’t know where you are going, you might wind up someplace else.”
SOURCE:
The study was led by Tyler J. Gallagher and Niels C. Kokot, MD, at the Keck School of Medicine of the University of Southern California in Los Angeles. It was published online in JAMA Otolaryngology–Head & Neck Surgery.
LIMITATIONS:
The study had limited information about cohort composition and length of follow-up, which may affect the generalizability of the findings. The lack of direct exposure duration, intensity, and dosage information limits the ability to analyze dose-response relationships. Potential inconsistency of diagnosis and reliance on medical record codes may introduce bias. Cannabis use is likely underreported, which could decrease the relative risks discovered. The study was further limited by the lack of information on dosage and frequency of cannabis use, as well as some controls, including alcohol and tobacco use.
DISCLOSURES:
Gallagher disclosed receiving grants from the Keck School of Medicine of the University of Southern California, Los Angeles. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
How Safe is Anti–IL-6 Therapy During Pregnancy?
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
PPI Prophylaxis Prevents GI Bleed in Ventilated Patients
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
according to a randomized trial and a systematic review led by researchers at McMaster University, Hamilton, Ontario, Canada.
Patients in the intensive care unit (ICU) who need mechanical ventilation typically are given a PPI, such as pantoprazole, to prevent upper GI bleeding caused by stress-induced stomach ulcers, but some evidence suggested that their use might increase the risk for pneumonia and death in the most severely ill patients.
As a result, recent guidelines have issued only weak recommendations for stress ulcer prophylaxis, especially with PPIs, in critically ill patients at a high risk for bleeding, Deborah Cook, MD, professor of medicine at McMaster University, and colleagues noted.
To address clinical questions, they investigated the efficacy and safety of PPIs to prevent upper GI bleeding in critically ill patients.
Both the randomized trial in The New England Journal of Medicine and the systematic review in NEJM Evidence were published online in June.
Significantly Lower Bleeding Risk
The REVISE trial, conducted in eight countries, compared pantoprazole 40 mg daily with placebo in critically ill adults on mechanical ventilation.
The primary efficacy outcome was clinically important upper GI bleeding in the ICU at 90 days, and the primary safety outcome was death from any cause at 90 days.
A total of 4821 patients in 68 ICUs were randomly assigned to the pantoprazole group or placebo group.
Clinically important upper GI bleeding occurred in 25 patients (1%) receiving pantoprazole and in 84 patients (3.5%) receiving placebo. At 90 days, 696 patients (29.1%) in the pantoprazole group died, as did 734 (30.9%) in the placebo group.
No significant differences were found on key secondary outcomes, including ventilator-associated pneumonia and Clostridioides difficile infection in the hospital.
The authors concluded that pantoprazole resulted in a significantly lower risk for clinically important upper GI bleeding than placebo, and it had no significant effect on mortality.
Disease Severity as a Possible Factor
The systematic review included 12 randomized controlled trials comparing PPIs with placebo or no prophylaxis for stress ulcers in a total of 9533 critically ill adults. The researchers performed meta-analyses and assessed the certainty of the evidence. They also conducted a subgroup analysis combining within-trial subgroup data from the two largest trials.
They found that PPIs were associated with a reduced incidence of clinically important upper GI bleeding (relative risk [RR], 0.51, with high certainty evidence) and may have little or no effect on mortality (RR, 0.99, with low-certainty evidence).
However, the within-trial subgroup analysis with intermediate credibility suggested that the effect of PPIs on mortality may differ based on disease severity. The results also raised the possibility that PPI use may decrease 90-day mortality in less severely ill patients (RR, 0.89) and increase mortality in more severely ill patients (RR, 1.08). The mechanisms behind this possible signal are likely multifactorial, the authors noted.
In addition, the review found that PPIs may have no effect on pneumonia, duration of ICU stay, or duration of hospital stay, and little or no effect on C difficile infection or duration of mechanical ventilation (low-certainty evidence).
“Physicians, nurses, and pharmacists working in the ICU setting will use this information in practice right away, and the trial results and the updated meta-analysis will be incorporated into international practice guidelines,” Dr. Cook said.
Both studies had limitations. The REVISE trial did not include patient-reported disability outcomes, and the results may not be generalizable to patients with unassisted breathing. The systematic review included studies with diverse definitions of bleeding and pneumonia, and with mortality reported at different milestones, without considering competing risk analyses. Patient-important GI bleeding was available in only one trial. Other potential side effects of PPIs, such as infection with multidrug-resistant organisms, were not reported.
In an editorial accompanying both studies, Samuel M. Brown, MD, a pulmonologist and vice president of research at Intermountain Health, Salt Lake City, Utah, said that the REVISE trial was “well designed and executed, with generalizable eligibility criteria and excellent experimental separation.” He said the researchers had shown that PPIs “slightly but significantly” decrease the risk of important GI bleeding and have a “decent chance” of slightly decreasing mortality in less severely ill patients during mechanical ventilation. At the same time, he noted, PPIs “do not decrease — and may slightly increase — mortality” in severely ill patients.
Dr. Brown wrote that, in his own practice, he intends to prescribe prophylactic PPIs to patients during mechanical ventilation “if they have an APACHE II score of less than 25” or a reasonable equivalent. The APACHE II scoring system is a point-based system that estimates a patient’s risk of death while in an ICU.
“For sicker patients, I would probably reserve the use of proton-pump inhibitors for those who are being treated with antiplatelet agents, especially in the presence of therapeutic anticoagulants,” he added.
REVISE was supported by numerous grants from organizations in several countries. No funding was specified for the systematic review. Author disclosures and other supplementary materials are available with the full text of the article.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Can Addressing Depression Reduce Chemo Toxicity in Older Adults?
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of a randomized controlled trial to evaluate whether greater reductions in grade 3 chemotherapy-related toxicities occurred with geriatric assessment-driven interventions vs standard care.
- A total of 605 patients aged 65 years and older with any stage of solid malignancy were included, with 402 randomized to the intervention arm and 203 to the standard-of-care arm.
- Mental health was assessed using the Mental Health Inventory 13, and chemotherapy toxicity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
- Patients in the intervention arm received recommendations from a multidisciplinary team based on their baseline GA, while those in the standard-of-care arm received only the baseline assessment results.
- The study was conducted at City of Hope National Medical Center in Duarte, California, and patients were followed throughout treatment or for up to 6 months from starting chemotherapy.
TAKEAWAY:
- According to the authors, patients with depression had increased chemotherapy toxicity in the standard-of-care arm (70.7% vs 54.3%; P = .02) but not in the GA-driven intervention arm (54.3% vs 48.5%; P = .27).
- The association between depression and chemotherapy toxicity was also seen after adjustment for the Cancer and Aging Research Group toxicity score (odds ratio, [OR], 1.98; 95% CI, 1.07-3.65) and for demographic, disease, and treatment factors (OR, 2.00; 95% CI, 1.03-3.85).
- No significant association was found between anxiety and chemotherapy toxicity in either the standard-of-care arm (univariate OR, 1.07; 95% CI, 0.61-1.88) or the GA-driven intervention arm (univariate OR, 1.15; 95% CI, 0.78-1.71).
- The authors stated that depression was associated with increased odds of hematologic-only toxicities (OR, 2.50; 95% CI, 1.13-5.56) in the standard-of-care arm.
- An analysis of a small subgroup found associations between elevated anxiety symptoms and increased risk for hematologic and nonhematologic chemotherapy toxicities.
IN PRACTICE:
“The current study showed that elevated depression symptoms are associated with increased risk of severe chemotherapy toxicities in older adults with cancer. This risk was mitigated in those in the GA intervention arm, which suggests that addressing elevated depression symptoms may lower the risk of toxicities,” the authors wrote. “Overall, elevated anxiety symptoms were not associated with risk for severe chemotherapy toxicity.”
SOURCE:
Reena V. Jayani, MD, MSCI, of Vanderbilt University Medical Center in Nashville, Tennessee, was the first and corresponding author for this paper. This study was published online August 4, 2024, in Cancer.
LIMITATIONS:
The thresholds for depression and anxiety used in the Mental Health Inventory 13 were based on an English-speaking population, which may not be fully applicable to Chinese- and Spanish-speaking patients included in the study. Depression and anxiety were not evaluated by a mental health professional or with a structured interview to assess formal diagnostic criteria. Psychiatric medication used at the time of baseline GA was not included in the analysis. The study is a secondary analysis of a randomized controlled trial, and it is not known which components of the interventions affected mental health.
DISCLOSURES:
This research project was supported by the UniHealth Foundation, the City of Hope Center for Cancer and Aging, and the National Institutes of Health. One coauthor disclosed receiving institutional research funding from AstraZeneca and Brooklyn ImmunoTherapeutics and consulting for multiple pharmaceutical companies, including AbbVie, Adagene, and Bayer HealthCare Pharmaceuticals. William Dale, MD, PhD, of City of Hope National Medical Center, served as senior author and a principal investigator. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of a randomized controlled trial to evaluate whether greater reductions in grade 3 chemotherapy-related toxicities occurred with geriatric assessment-driven interventions vs standard care.
- A total of 605 patients aged 65 years and older with any stage of solid malignancy were included, with 402 randomized to the intervention arm and 203 to the standard-of-care arm.
- Mental health was assessed using the Mental Health Inventory 13, and chemotherapy toxicity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
- Patients in the intervention arm received recommendations from a multidisciplinary team based on their baseline GA, while those in the standard-of-care arm received only the baseline assessment results.
- The study was conducted at City of Hope National Medical Center in Duarte, California, and patients were followed throughout treatment or for up to 6 months from starting chemotherapy.
TAKEAWAY:
- According to the authors, patients with depression had increased chemotherapy toxicity in the standard-of-care arm (70.7% vs 54.3%; P = .02) but not in the GA-driven intervention arm (54.3% vs 48.5%; P = .27).
- The association between depression and chemotherapy toxicity was also seen after adjustment for the Cancer and Aging Research Group toxicity score (odds ratio, [OR], 1.98; 95% CI, 1.07-3.65) and for demographic, disease, and treatment factors (OR, 2.00; 95% CI, 1.03-3.85).
- No significant association was found between anxiety and chemotherapy toxicity in either the standard-of-care arm (univariate OR, 1.07; 95% CI, 0.61-1.88) or the GA-driven intervention arm (univariate OR, 1.15; 95% CI, 0.78-1.71).
- The authors stated that depression was associated with increased odds of hematologic-only toxicities (OR, 2.50; 95% CI, 1.13-5.56) in the standard-of-care arm.
- An analysis of a small subgroup found associations between elevated anxiety symptoms and increased risk for hematologic and nonhematologic chemotherapy toxicities.
IN PRACTICE:
“The current study showed that elevated depression symptoms are associated with increased risk of severe chemotherapy toxicities in older adults with cancer. This risk was mitigated in those in the GA intervention arm, which suggests that addressing elevated depression symptoms may lower the risk of toxicities,” the authors wrote. “Overall, elevated anxiety symptoms were not associated with risk for severe chemotherapy toxicity.”
SOURCE:
Reena V. Jayani, MD, MSCI, of Vanderbilt University Medical Center in Nashville, Tennessee, was the first and corresponding author for this paper. This study was published online August 4, 2024, in Cancer.
LIMITATIONS:
The thresholds for depression and anxiety used in the Mental Health Inventory 13 were based on an English-speaking population, which may not be fully applicable to Chinese- and Spanish-speaking patients included in the study. Depression and anxiety were not evaluated by a mental health professional or with a structured interview to assess formal diagnostic criteria. Psychiatric medication used at the time of baseline GA was not included in the analysis. The study is a secondary analysis of a randomized controlled trial, and it is not known which components of the interventions affected mental health.
DISCLOSURES:
This research project was supported by the UniHealth Foundation, the City of Hope Center for Cancer and Aging, and the National Institutes of Health. One coauthor disclosed receiving institutional research funding from AstraZeneca and Brooklyn ImmunoTherapeutics and consulting for multiple pharmaceutical companies, including AbbVie, Adagene, and Bayer HealthCare Pharmaceuticals. William Dale, MD, PhD, of City of Hope National Medical Center, served as senior author and a principal investigator. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of a randomized controlled trial to evaluate whether greater reductions in grade 3 chemotherapy-related toxicities occurred with geriatric assessment-driven interventions vs standard care.
- A total of 605 patients aged 65 years and older with any stage of solid malignancy were included, with 402 randomized to the intervention arm and 203 to the standard-of-care arm.
- Mental health was assessed using the Mental Health Inventory 13, and chemotherapy toxicity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
- Patients in the intervention arm received recommendations from a multidisciplinary team based on their baseline GA, while those in the standard-of-care arm received only the baseline assessment results.
- The study was conducted at City of Hope National Medical Center in Duarte, California, and patients were followed throughout treatment or for up to 6 months from starting chemotherapy.
TAKEAWAY:
- According to the authors, patients with depression had increased chemotherapy toxicity in the standard-of-care arm (70.7% vs 54.3%; P = .02) but not in the GA-driven intervention arm (54.3% vs 48.5%; P = .27).
- The association between depression and chemotherapy toxicity was also seen after adjustment for the Cancer and Aging Research Group toxicity score (odds ratio, [OR], 1.98; 95% CI, 1.07-3.65) and for demographic, disease, and treatment factors (OR, 2.00; 95% CI, 1.03-3.85).
- No significant association was found between anxiety and chemotherapy toxicity in either the standard-of-care arm (univariate OR, 1.07; 95% CI, 0.61-1.88) or the GA-driven intervention arm (univariate OR, 1.15; 95% CI, 0.78-1.71).
- The authors stated that depression was associated with increased odds of hematologic-only toxicities (OR, 2.50; 95% CI, 1.13-5.56) in the standard-of-care arm.
- An analysis of a small subgroup found associations between elevated anxiety symptoms and increased risk for hematologic and nonhematologic chemotherapy toxicities.
IN PRACTICE:
“The current study showed that elevated depression symptoms are associated with increased risk of severe chemotherapy toxicities in older adults with cancer. This risk was mitigated in those in the GA intervention arm, which suggests that addressing elevated depression symptoms may lower the risk of toxicities,” the authors wrote. “Overall, elevated anxiety symptoms were not associated with risk for severe chemotherapy toxicity.”
SOURCE:
Reena V. Jayani, MD, MSCI, of Vanderbilt University Medical Center in Nashville, Tennessee, was the first and corresponding author for this paper. This study was published online August 4, 2024, in Cancer.
LIMITATIONS:
The thresholds for depression and anxiety used in the Mental Health Inventory 13 were based on an English-speaking population, which may not be fully applicable to Chinese- and Spanish-speaking patients included in the study. Depression and anxiety were not evaluated by a mental health professional or with a structured interview to assess formal diagnostic criteria. Psychiatric medication used at the time of baseline GA was not included in the analysis. The study is a secondary analysis of a randomized controlled trial, and it is not known which components of the interventions affected mental health.
DISCLOSURES:
This research project was supported by the UniHealth Foundation, the City of Hope Center for Cancer and Aging, and the National Institutes of Health. One coauthor disclosed receiving institutional research funding from AstraZeneca and Brooklyn ImmunoTherapeutics and consulting for multiple pharmaceutical companies, including AbbVie, Adagene, and Bayer HealthCare Pharmaceuticals. William Dale, MD, PhD, of City of Hope National Medical Center, served as senior author and a principal investigator. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.